VOL. 33, NO. 2, Summer 2024 10 Meet the New Society Officers 61 Electrochemically Induced Deposition 47 Advancements in Electrodeposition for Precise Manufacturing and Sustainability 26 ECS Celebrates the Life of Allen J. Bard ELECTRODEPOSITION SPECIAL ISSUE: Frontiers of Electrodeposition (Present and Future) The Electrochemical Society
ECS, a prestigious nonprofit professional society, has led the world in electrochemistry, solid state science and technology and allied subjects since 1902, providing a rigorous and high-quality home for the whole community.
ECS is dedicated to moving science forward by empowering researchers globally to leave their mark on science. The Society connects a diverse and representative constituency of members and nonmembers to accelerate scientific discovery, facilitate the engagement of an inclusive network, and champion the dissemination of research to support a sustainable future.
For more information on becoming a member, or publishing in ECS publications, visit electrochem.org
BioLogic Potentiostats
Instruments for Electrochemists

SP-50e
1 channel
±1 A to 20 nA
Up to 1 MHz

SP-300
2 channels
±10 A to 100 fA
Up to 7 MHz
VMP-3e
16 channels
±1 A to 20 nA
Up to 1 MHz


VMP-300 16 channels
±150 A to 100 fA
Up to 7 MHz
From 1 to 16 channels, BioLogic offers a range of modular single and multichannel potentiostats.
Our range of Premium and Essential Potentiostats boast some of the most powerful specifications available: including up to 7 MHz EIS measurements with EIS Quality Indicators and up to 800 A with boosters.
So whether your area of expertise lies in energy or corrosion, material characterization or bio-molecular electrochemistry – we have a potentiostat to match your specific needs.
Shaping the future. Together. biologic.net


Artificial Intelligence?
Iadmit that I struggle with the issues surrounding artificial intelligence (AI). As a technologist, my knee-jerk reaction is that how it works is cool, and its potential for good amazing. As a pessimist, I can foresee an AI machine taking over writing these editorials and doing a better job of it. The more I read about it the more excited and scared I get. At its core, most AI is based on making mathematical correlations or connections among many, many pieces of information. Although this description is an oversimplification of only one part of AI, it helps a simple guy like me conceptualize what it does. Correlation is not causation, of course, but we of natural intelligence use it all the time. Sometimes, believing in the power of correlation can lead us astray. When a large language model is asked to write papers based on spurious correlations, the results can be hysterical. Paging through Spurious Scholar shows more than 4,000 fake papers that were generated by AI after being given a misleading correlation. My current favorite is one that links the number of B.A. degrees awarded in literature with the number of Google searches for “how to delete browsing history,” which has an R2 of 0.958. I have always been suspicious of my friends in the humanities, truth be told. Going beyond text, generative AI can use a tremendous amount of information to create images, although that does not stop it from producing nonsense at times, including the physically impossible, but hilarious, rendition of a mouse that was a figure in a published scientific article in a journal with an IF of 5.5. Google it (thus using AI to see its failures).
As with every new technology since the discovery of fire, people find ways to use it for evil. Technology is agnostic with respect to its use (but is that true for AI…). Using AI to create deep fakes or to amplify stupidity to the point of acceptance is an ongoing problem, although amplifying stupidity has been a cottage industry for some people for a long time. The deep fakes are the ones that scare me. In the absence of being in the physical presence of someone, it could get to the point where we won’t be sure of anything. With the stakes so high in elections, the use of AI to create and boost misinformation has its perfect customer. The good news is that there are white hats who are using AI tools to catch AI deep fakes. The bad news is that AI is getting better every day as a natural condition. The constant struggle between good and evil plays out in silico.
Having picked on AI’s foibles, let’s turn to its successes (at least partial). The use of AI in medical image analysis may eventually fundamentally change the way some diseases and conditions are detected from the gamut of medical images that are collected. That said, it still has a long way to go to be better than a human radiologist. Of course, I am thankful for AI when it alerts me when someone is using my credit card without authorization; it amazes me how fast it does it, and how rarely it is wrong. It is also being used to improve forecasting of weather and climate, assist in decision making, and for process optimization (thank you, Google Maps and Waze).
The optimist in me says that maybe this technological revolution will create more free time, as John Maynard Keynes predicted almost 100 years ago when he said that in 100 years, people would be working only 15 hours a week. My friends say that it has been successfully achieved already by university faculty, so maybe it wasn’t that wild a prediction. People can be so cruel.
Many years ago, I received an unsigned card. On the cover was a cartoon of a man holding a toaster and a butter knife. The caption on the front of the card is “Sometimes we are too smart for our own good.” The inside of the card reads “You don’t look to be in any trouble with that at this time.” So if you were looking for an answer to the question posed by the title, I am sorry to disappoint. But honestly, if you have read even one of my earlier epistles you should have known.
Until next time, be safe and happy.
 Rob Kelly Editor
Rob Kelly Editor

Published by:
The Electrochemical Society (ECS) 65 South Main Street Pennington, NJ 08534-2839, USA
Tel 609.737.1902, Fax 609.737.2743
www.electrochem.org
Editor: Rob Kelly
Guest Editors: Philippe Vereecken, Luca Magagnin, and Natasa Vasiljevic
Contributing Editors: Christopher L. Alexander, Christopher G. Arges, Scott Cushing, Ahmet Kusolgu, Donald Pile, Alice Suroviec
Director of Publications: Adrian Plummer
Director of Community Engagement: Shannon Reed
Production Editor: Kara McArthur
Graphic Design & Print Production Manager: Dinia Agrawala
Staff Contributors: Frances Chaves, Genevieve Goldy, Mary Hojlo, Christopher J. Jannuzzi, John Lewis, Anna Olsen, Fern Oram, Jennifer Ortiz, Francesca Di Palo, JaneAnn Wormann
Advisory Board: Brett Lucht (Battery Division) Dev Chidambaram (Corrosion Division)
Uroš Cvelbar (Dielectric Science and Technology Division)
Luca Magagnin (Electrodeposition Division) Qiliang Li (Electronics and Photonics Division) Katherine Ayers (Energy Technology Division) Cortney Kreller (High-Temperature Energy, Materials, & Processes Division)
Maria Inman (Industrial Electrochemistry and Electrochemical Engineering Division) Eugeniusz Zych (Luminescence and Display Materials Division)
Jeff Blackburn (Nanocarbons Division)
Shelley Minteer (Organic and Biological Electrochemistry Division)
Stephen Paddison (Physical and Analytical Electrochemistry Division)
Larry Nagahara (Sensor Division)
Publications Subcommittee Chair: Francis D'Souza
Society Officers: Colm O'Dwyer, President; James (Jim) Fenton, Senior Vice President; Francis D'Souza, 2nd Vice President; Robert Savinell, 3rd Vice President; Gessie Brisard, Secretary; Elizabeth J. PodlahaMurphy, Treasurer; Christopher J. Jannuzzi, Executive Director & CEO
Statements and opinions given in The Electrochemical Society Interface are those of the contributors, and ECS assumes no responsibility for them.
Authorization to photocopy any article for internal or personal use beyond the fair use provisions of the Copyright Act of 1976 is granted by The Electrochemical Society to libraries and other users registered with the Copyright Clearance Center (CCC). Copying for other than internal or personal use without express permission of ECS is prohibited. The CCC Code for The Electrochemical Society Interface is 1064-8208/92.
ISSN : Print: 1064-8208 Online: 1944-8783
The Electrochemical Society Interface is published quarterly by The Electrochemical Society (ECS), at 65 South Main Street, Pennington, NJ 08534-2839 USA. Subscription to members is part of membership service. © Copyright 2024 by The Electrochemical Society. *“Save as otherwise expressly stated.”
The Electrochemical Society is an educational, nonprofit 501(c)(3) organization with more than 8,500 scientists and engineers in over 75 countries worldwide who hold individual membership. Founded in 1902, the Society has a long tradition in advancing the theory and practice of electrochemical and solid state science by dissemination of information through its publications and international meetings.


https://orcid.org/0000-0002-7354-0978
EDITOR
EDITOR The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 3
FROM THE
FROM THE




4 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Advanced Features & Capabilities Are One Click Away! 2005
2007
LEADING ENEGRY CONVERSION AND STORAGE TECHNOLOGIES SINCE 1980 Fuel Cell Test | Electrolyzer Test | Membrane Conductivity Test | Redox Flow Cell Test
LET’S TALK UPGRADES
Model
Model


61 67
A Bright Future for Electrodeposition
by Philippe Vereecken, Natasa Vasiljevic, Luca Magagnin, J. X. Kent Zheng, and Martin Leimbach
Advancements in Electrodeposition for Precise Manufacturing and Sustainability
by Anar Badalbayli, Nicholas Sinclair, Roberto Bernasconi, Natalia Borisenko, Krishna Venkatesh, Adriana Ispas, Rohan Akolkar, and Luca Magagnin
Engineering Electrodeposition for Nextgeneration Batteries
by Stephen T. Fuller, Yonglin Huang, Ruixin Wu, Fudong Han, J. X. Kent Zheng, and Natasa Vasiljevic
Electrochemically Induced Deposition (ECiD): A Versatile Method that Greatly Extends the Portfolio of Surface Coatings and Materials Fabricated by Electrochemical Deposition
by Sai Gourang Patnaik, Genis Vanheusden, Ali Amir Saleh, and Philippe M. Vereecken
Electrochemically Induced Templated Sol-gel Deposition of Mesoporous Silica and Nanocomposites: The New Kid on the ECiD Block
by Genis Vanheusden and Philippe M. Vereecken
Vol. 33, No. 2 Summer 2024
3 From the Editor: Artificial Intelligence?
7 From the President: ECS United
10 Meet the New Society Officers
16 Society News
24 Websites of Note
26 ECS Celebrates the Life of Allen J. Bard
28 People News
32 Reports from the Frontier
38 Electrochemistry in Action
44 Tech Highlights
73 Section News
75 Awards Program
77 New Members
81 Student News
This issue's cover is an interpretation of an illustration and an SEM image of electrodeposition (Figs. 4 and 5), from Patnaik et al.'s article, “Electrochemically Induced Deposition (ECiD): A Versatile Method that Greatly Extends the Portfolio of Surface Coatings and Materials Fabricated by Electrochemical Deposition” (see p. 61).
Cover design: Dinia Agrawala
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 5


gamry.com Ph: +1 215 682 9330 INTRODUCING THE RxE 10K ROTATING ELECTRODE NEW RDE/RCE/RRDE Corrosion Catalyst Development Fuel Cell Research Kinetic Studies SELECT SPECIFICATIONS 50-10500RPM +/-1RPMAccuracy AnalogorDigitalControl

IECS United
t gives me great pleasure to write to you for the first time since becoming ECS President. I assumed the role following the 245th ECS Meeting in San Francisco, CA, which I certainly hope you attended. That amazing event featured 49 symposia with 439 sessions presenting more than 2,290 talks, 3,022 abstracts, and 701 posters by authors from 68 countries. Of these, 575 were invited talks, 25 ECS award and keynote talks, and 1,240 student abstracts, 842 oral talks, and 388 posters! If you were unable to attend, complete coverage of the meeting’s highlights and key moments is forthcoming in the next edition of Interface I would like to share with you my vision as President of this esteemed Society, and what I see are the main challenges and opportunities facing ECS in the year ahead.
ECS has led the forefront of electrochemical and solid state science and technology education and dissemination for over 120 years. The need for a cleaner, better, and more sustainable future has focused the Society’s thinking in the 21st century. Electrochemistry and the solid state disciplines are more important now than ever—and they have greater impact when united. Energy conversion and storage are the lungs, fuel, and muscles of the sustainable and mobile electric future; displays, processors, and semiconductor devices are the heart, brain, and nervous system. All are made possible by multiple forms of material science, electrochemical processes, optoelectronics, and more. ECS’s mission, in the round, is a driver for the sustainable electric future.
the US CHIPS and Science Act and the EU Chips Act. This will be part of a long-term vision to develop educational initiatives across major disciplines in ECS. It is important that the Society expands its leadership in providing industryfacing opportunities for re-skilling in the electrochemical and solid state science and technology disciplines that are central to workforce development needs—for all the technologies we use now and need in the future.
The Society has successfully launched ECS Sensors Plus and ECS Advances, new journals that open the door to full open-access publication. I commit to continuing these ongoing initiatives, including ECS for Sustainability launched by Past President Gerri Botte, and to foster new ones to maximize their impact, attractiveness, and accessibility to all authors across academia and industry R&D.
Of course, underlying the growth of our meetings, publications, education initiatives, and beyond are the people who make all that ECS does possible. Within the Society and throughout our worldwide membership, all forms of diversity are our strength. During this year, as the organization grows and expands in size and influence, the Ad-Hoc Committee on Diversity’s recommendations will be embedded in ECS’s future primary leadership and society-wide activities.
“Electrochemistry and the solid state disciplines are more important now than ever—and they have greater impact when united.”
Like many of you, I have made the Society my go-to place for presenting and publishing my work since my first conference presentation as a graduate student at the 199th ECS Meeting in Washington, DC. As an ECS member for the past 23 years, and active in electrochemical and solid state science research, I have seen how this scientific society tracks the ebb and flow in research priorities, new technologies, and international policies, and how they are mirrored in our meeting and journal content. Through uniting electrochemical and solid state science, ECS has core scientific expertise that is arguably the most critical and most relevant to the cutting edge of technologies we see today.
As ECS President, I will elevate our STEM education and outreach by ensuring the creation of new programs like our battery workforce development and upskilling education programs. I plan to work with the leadership team and divisions to scope out parallel opportunities articulated in
Continuing to support ECS Student Chapters around the globe is central to growing our international community and fostering diversity within ECS membership. Therefore, I call on ECS members to consider founding an ECS student chapter at their institutions. Doing so provides students with a direct pipeline to ECS. All students in the chapter receive complimentary ECS membership and the Society provides up to $1,000 annually to support chapter activities and events. Only six students are required to launch a chapter, so if you are interested, please click here
I very much look forward to working with you all as we advance the ECS mission in education and scientific impact. The quality and breadth of our core disciplines, united, will put ECS front and center in a sustainable future.

Colm O’Dwyer ECS President


http://orcid.org/0000-0001-7429-015X
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 7 FROM THE PRESIDENT
FROM THE PRESIDENT

ACTIVATE WITH ECS PUBLICATIONS

ECS TOPICAL INTEREST AREAS
Electrochemical
• Batteries and Energy Storage
• Corrosion Science and Technology
• Electrochemical/Electroless Deposition
• Electrochemical Engineering
• Fuel Cells, Electrolyzers, and Energy Conversion
• Organic and Bioelectrochemistry
• Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
• Sensors (Electrochemical)
Solid State
• Carbon Nanostructures and Devices
• Dielectric Science and Materials
• Electronic Materials and Processing
• Electronic and Photonic Devices and Systems
• Luminescence and Display Materials, Devices, and Processing
• Sensors (Solid State)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 9
LEARN MORE
Meet the New Society Officers
On May 31, 2024, the newly elected officers of The Electrochemical Society assumed their posts. We are pleased to welcome Colm O’Dwyer as President, Robert Savinell as Third Vice President, and Gessie Brisard as Secretary.
Colm O’Dwyer

Colm O’Dwyer ECS President, 2024–2025
Colm O’Dwyer is Professor of Chemical Energy in the University College Cork (UCC) School of Chemistry, and Principal Investigator at the Environmental Research Institute, Tyndall National Institute, and Advanced Materials and BioEngineering Research Centre. His research interests include 3D printed energy storage devices, materials science, and operando photonics for examining optoelectronic materials and energy storage materials.
Colm joined the Society as a graduate student in 2001 after attending the 199th ECS Meeting in Washington, DC. He has served ECS in numerous roles, including as Chair of the ECS Electronic and Photonics Division, and, with many colleagues, he has organized or co-organized about 40 ECS symposia since 2007.
As an award-winning advocate for open-access publication and open science, Colm has guest edited several JES and JSS special focus issues. What excites him most about ECS and its future are the many possibilities in education and engagement with society and industry, and how important electrochemistry and semiconductor science are in today’s world.
Colm completed his PhD in Semiconductor Electrochemistry and Physics at the University of Limerick (UL) in 2003. From 2008 to 2012, he was a Science Foundation Ireland Stokes Lecturer on Nanomaterials at UL; he then joined UCC in 2012. He has coauthored, with talented students, postdocs, and collaborators, ~300 peer-reviewed articles, book chapters, and ECS Transactions articles. Colm was one of the recipients of the 2017 Bell Labs Prize and is a Fellow of the Institute of Physics.
Colm’s life revolves around his family, his wife Gillian and children Caera and Aaron. They especially enjoy time outdoors in the hills and coastlines of Ireland—luckily, it’s a short trip to cliffs, caves, beaches, and mountains. He is adamant that the water feels warmer when swimming in the rain and wind (when you have no other choice). Rare cloudless nights are spent imaging nebulae and galaxies in the night sky.




10 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
2024
Members of the research family tree (students and advisors) together at the 236th ECS Meeting in Atlanta in 2019, with ECS Past President D. Noel Buckley (center) and David McNulty, University of Limerick (right).
Photo: Colm O'Dwyer
Colm hikes on a ridge in the Coomloughra Reeks with his wife Gillian and friends. Photo: Colm O'Dwyer
In December 2023, Colm captured the famous Orion Nebula (M42), Running Man Nebula (NGC 1977), and surrounding molecular clouds from a back garden in Cork.
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Photo: Colm O'Dwyer
Colm spends rainy Sunday afternoons indoors with family. Photo: Colm O'Dwyer

Robert (Bob) Savinell
Robert (Bob) Savinell is Distinguished University Professor and George S. Dively Professor of Engineering at Case Western Reserve University (CWRU). He is also PI and Director of the DOE Emerging Frontiers Research Center on Breakthrough Electrolytes for Energy Storage (BEES). His research focuses on fundamental science and mechanistic issues of electrochemical processes, and device design and development. Bob co-invented the first high-temperature proton-conducting polymer membrane based on phosphoric acid doping of PBI and co-authored the first experimental paper reporting results of the all-iron flow battery.
Bob joined the Society in 1978 and has served the ECS Industrial Electrochemistry and Electrochemical Engineering Division in all its officer positions, including Chair, as well as on many ECS committees and subcommittees. He has been Editor-in-Chief of the Journal of The Electrochemical Society since 2013. Bob is a Fellow of The Electrochemistry Society and received the 2022 ECS Vittorio de Nora Award.
After receiving his PhD in Chemical Engineering from the University of Pittsburg, Bob joined CWRU in 1986 and was Director of the Ernest B. Yeager Center for Electrochemical Sciences for 10 years and Dean of Engineering for seven. He has been a visiting professor at the Massachusetts Institute of Technology, Yamanashi University, and Danmarks Tekniske Universitet. He is a Fellow of The Electrochemical Society, the American Institute of Chemical Engineers, and the International Society of Electrochemistry, and received the 2020 CWRU Frank and Dorothy Hummel Prize.
Bob and Coletta Savinell will celebrate their 50th wedding anniversary this August with their three children and six grandchildren. They often host holiday get-together dinners with 45 siblings, spouses, children, and grandchildren. Avid sailors, the Savinells race their 34-foot sailboat on Lake Erie during the summer with crews composed mostly of students and former students. Bob enjoys riding his motorcycle on day or multiday trips in the country.



The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 11
Robert (Bob) Savinell ECS Third Vice President, 2024–2025
Bob and Coletta with children, spouses, and grandchildren.
Photo: Francis D'Souza
Bob, colleague Prof. Betar Galant, senior racing crew Nick Sinclair (left) and Evan Guarr (right), and former students prepare to race his 34-foot sailboat.
Photo: Robert Savinell
Bob pictured on a four-day road trip to the 2023 BMW rally in Virginia.
• Summer 2023 • 11
Photo: Robert Savinell
Bob and Coletta.
Photo: Robert Savinell

ECS Secretary, 2024–2028
Gessie Brisard
Gessie Brisard is Professor of Chemistry at the Université de Sherbrooke (UdeS). Her research focuses on analytical electrochemistry, electrocatalysis, and energy production and storage.
Gessie joined ECS as a student in 1986 and has served on the Society’s Sections Council, Meeting Committee, Interface Advisory Board, and Institutional Engagement Committee, as well as on the ECS Board of Directors in various positions, including Treasurer. She has also chaired the ECS Physical and Analytical Electrochemistry Division. Gessie has organized many ECS meeting symposia, including co-founding the electrocatalysis symposium. Gessie is a long-standing member of the ECS Canada Section, including service on the executive committee. The section received the ECS Gwendolyn B. Wood Section Excellence Award during her term as Chair. Gessie has organized multiple ECS Canada Section symposia and received the 1989 ECS Canada Section Student Award. After completing a PhD in Electrochemistry at UdeS, Gessie was a postdoc at Lawrence Berkeley National Laboratory. She joined UdeS in 1992, where she was Assistant Professor then Full Professor in the Chemistry Department, and Vice Dean of Academia and Secretary of the Faculty of Science. She developed research programs in electrocatalysis and worked on cathode material for lithium batteries, carbon dioxide reduction, and electrodeposition in ionic liquids. She collaborated with the Institut de recherche d’Hydro-Québec and Alcan-Rio Tinto on electrochemistry in nonaqueous solvents of the interfacial behavior of non-aqueous electrolytes, with application to lithium batteries and metal deposition in nonconventional media.
Gessie never misses an opportunity to mix business with pleasure, combining her passions with international business travel. An avid cyclist, she has admired hundreds of kilometers of breathtaking views from California to Italy on cycling trips with friends and students. She loves symphony and opera, attending performances at the prestigious Cleveland Orchestra, Munich Symphony Orchestra, New York Metropolitan Opera, and others. She always packs fabulous, elegant attire for these occasions (shopping is another great passion!).




 Gessie Brisard
Gessie visits New York City with her sister, Nadia. Photo: Gessie Brisard
Gessie attends a performance at the Metropolitan Opera in New York City. Photo: Gessie Brisard
Shopping in New York City is a favorite pastime.
Gessie Brisard
Gessie visits New York City with her sister, Nadia. Photo: Gessie Brisard
Gessie attends a performance at the Metropolitan Opera in New York City. Photo: Gessie Brisard
Shopping in New York City is a favorite pastime.
12 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Photo: Gessie Brisard
Gessie and Hubert Gasteiger on a 2018 bike trip in Italy.
Photo: Gessie Brisard
Gessie and friends bicycling through Italy in 2019
Photo: Gessie Brisard







The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 13 ECS Advances ECS Journal of The Electrochemical Society ECS Journal of Solid State Science and Technology GOLD OPEN ACCESS >3 MILLION downloads OPEN ACCESS Average of 467 downloads per article Average of >1,000 downloads per article ECS Sensors Plus NEW LEGACY publications 2022 Impact Factor 3.9 2022 Impact Factor 2.1 120+ YEARS OF CHAMPIONING THE ACCELERATION OF SCIENTIFIC DISCOVERY
Journal of The Electrochemical Society
JES is the flagship journal of The Electrochemical Society. Published continuously from 1902 to the present, JES remains one of the most highly cited journals in electrochemistry and solid state science and technology.
ECS Journal of Solid State Science and Technology
and
and devices.
14 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
We are Family!
solid state
SUBMIT TODAY! SUBMIT TODAY! Visit Visit www.ecsdl.org www.electrochem.org/focusissues Review the amazing research published by ECS • Calls for upcoming focus issues • Links to published focus issues • Future focus issue proposals
JSS is a peer-reviewed journal covering fundamental
applied areas of
science and technology, including experimental and theoretical aspects of the chemistry and physics of materials
ECS Sensors Plus
ECS Sensors Plus is a one-stop shop journal for sensors. This multidisciplinary, Gold Open Access journal provides an international platform for publishing high-quality impactful articles and promoting scholarly communication and interactions among scientists, engineers, and technologists whose primary interests focus on materials, structures, properties, performance, and characterization of sensing and detection devices and systems, including sensor arrays and networks.
ECS Advances is a multidisciplinary, Gold Open Access forum of peer-reviewed, high-quality content covering all technical areas supported by the Society. ECS Advances publishes full-length original work, brief communicationstyle papers, perspectives, review articles, and special issues.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 15
ECS Advances Visit www.electrochem.org/oa Learn more about our open access initiatives • Read articles published for free in 2023 in ECS Advances and ECS Sensors Plus • Our new Gold Open Access journals! SUBMIT TODAY! SUBMIT TODAY!
SOCIETY NEWS SOCIETY NEWS
From the Publications Director՚s Desk
In store for 2024
by Adrian Plummer, MPA, PMP, Director of Publications

The ECS Publications family started off 2024 celebrating another year of growth in our readership across the globe, with more than nine million downloads, driven by the continued growth of our open access content. As we move into 2024, ECS will celebrate two full years of the newest members of our journal family, ECS Sensors Plus and ECS Advances. Following two full years of consistent and quality publication of the best articles in electrochemical and solid state science, the applications for indexing with Web of Science and SCOPUS have been submitted. We are anxiously awaiting the indexing of these two amazing journals—making their mark in the scholarly record. The success of these two new journals thus far is a testament to the strength, dedication, and commitment of our amazing editorial teams and the support of our valued reviewers.
We are also excited to welcome a new Editor-in-Chief to our ECS Publications leadership team, Rohan Akolkar, who will be leading our ECS Advances gold open access journal. As a long-time member of ECS, a stalwart supporter of the ECS mission, a fiveyear member of the ECS Joint Journal Editorial Board, and as an Associate Editor for the Journal of The Electrochemical Society, in the Electrochemical/Electroless Deposition topical interest area, Rohan is uniquely suited to grow ECS Advances. As ECS Advances
is the only ECS publication that hosts all ECS topics, Rohan will need the support of the entire ECS community in his editorial vision. Let’s help Rohan in his effort to make ECS Advances a continued success story for ECS!
As a society publisher that carries a broader organizational mission to advance theory and practice at the forefront of electrochemical and solid state science, and to encourage research, discussion, critical assessment, and dissemination of knowledge in these fields, it is central to this mission that we are consistently assessing our publications, and how they are serving the scholarly record. Sadly, after an extended period of assessment, The Electrochemical Society Board of Directors, at the recommendation of the Technical Affairs Committee and the Publications Subcommittee, have decided to sunset The Electrochemical Society Transactions (ECST) proceedings publication after 18 years of publishing proceedings manuscripts for ECS and sponsored ECS Meetings. ECST will publish its final volume with the PRiME 2024 meeting. Presenters of work in select symposiums included in the 245th ECS Meeting and PRiME 2024 meeting will continue to have the opportunity to submit a full manuscript to ECS Transactions. In addition, ECS will continue to publish the extended abstracts of authors who present at ECS meetings, via the ECS Meeting Abstracts publication. Subscribers of ECST will continue to be able to access, read, and cite articles published in ECST from the ECS digital library on IOP Science. Starting with the 247th ECS Meeting, authors of select symposiums will have a new opportunity to publish proceedings manuscripts: Stay tuned! But as always, we look forward to publishing the highest quality, novel, and groundbreaking research in our family of journals!
Editorial Updates Summer 2024
Reappointments



Ariel Furst
Reappointed as Associate Editor, ECS Sensors Plus, for the term March 1, 2024 –February 28, 2025
Harshini Mukundan
Reappointed as Associate Editor, ECS Sensors Plus, for the term January 24, 2024 –January 23, 2025
Thomas Thundat
Reappointed as Associate Editor, ECS Sensors Plus, for the term April 10, 2024 –April 9, 2025


John Staser
Reappointed as Associate Editor, Journal of The Electrochemical Society, for the term February 20, 2024 –February 19, 2025
Peter Mascher
Reappointed as Technical Editor, ECS Journal of Solid State Science and Technology, Dielectric Science and Materials topical interest area, for the term May 1, 2024 – April 30. 2027
Thank You to our Departing Editorial Board Members
The Electrochemical Society wishes to express its gratitude to the Editorial Board members whose service on the Joint Journal Editorial Board ended as we started 2024. Thank you for your commitment and dedication to ECS and its mission to advance scientific discovery. We wish you the best in your future endeavors.

Amanda Clifford
Associate Editor, ECS Sensors Plus (ECSSP), March 1, 2023 –February 22, 2024
16 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
SOCIETY NEWS SOCIETY NEWS
ECS Appoints
Rohan Akolkar as Inaugural Editor-in-Chief of ECS
Advances

The ECS Board of Directors has appointed Professor Rohan Akolkar of Case Western Reserve University as the inaugural Editor-in-Chief (EiC) of ECS Advances (ECSA). ECSA is the only gold open access journal in the ECS publications portfolio that spans ECS’s full range of topical interest areas (TIAs). Rohan has been a member of the ECS Joint Journal Editorial Board for more than four years, following appointment by the ECS Publications Subcommittee as an Associate Editor for the Journal of The Electrochemical Society Electrochemical and Electroless Deposition TIA on September 1, 2019.
Rohan is the Milton and Tamar Maltz Professor of Energy Innovation at Case Western Reserve University (CWRU). He is an Ohio Eminent Scholar in Advanced Energy Research, serves as Faculty Director of CWRU’s Great Lakes Energy Institute, and holds a joint appointment as Chief Scientist at Pacific Northwest National Laboratory. His research spans many areas of electrochemical engineering: electrodeposition, electrometallurgy, and electrochemical materials development for applications in nanoelectronics, batteries, sensors, and in sustainable extraction and refining of critical materials. He has made important contributions to fundamental and applied electrochemistry, including patented electrochemical processes and materials which have enabled highperformance interconnects in advanced semiconductor devices; novel electrowinning and electrorefining processes for the sustainable extraction and recycling of metals; fundamental studies unraveling mechanisms of dendrite formation in batteries; and a novel patented sensor concept for detecting heavy-metal contaminants in water.
Rohan received a PhD in Chemical Engineering from CWRU in 2004. He worked in industrial research and development at Intel
Corporation for eight years before returning to CWRU as a faculty member in 2012. His research has been recognized by CWRU’s School of Engineering Innovation and Research Awards, the Norman Hackerman Young Author Award (2004) and the Electrodeposition Division Research Award (2023) of The Electrochemical Society, and numerous industry awards during his tenure at Intel. In 2021, he was elected Senior Member of the National Academy of Inventors. Over his 22 years of association with ECS, he has served as Associate Editor of the Journal of the Electrochemical Society (2019–2024), as a member of the ECS Honors and Awards Committee (2016–2019), and as Secretary of the ECS Electrodeposition Division. He is cofounder of a small business (Galvanix Inc.) in Ohio that aims to commercialize his invention of a novel electrolytic neodymium metal production process.
Please join us in welcoming Rohan to the Publications leadership team, and in cooperating and collaborating with him as he works to implement a new vision for ECSA that aims to make a stronger and lasting impact on the scholarly record.
ECS Advances
ECS Advances is a gold open access journal that covers all the technical areas supported by The Electrochemical Society. ECS Advances carries the broadest dissemination of electrochemical and solid state science and technology content among all journals in the field, coupled with a rigorous peer review. Enabling open access to scientific research is imperative to ECS in our mission to disseminate the best research in our technical fields as widely as possible. In the spirit of maintaining the ECS standard of excellence and quality scholarly publications, all papers submitted to ECS Advances are rigorously peer reviewed before acceptance.
ECS Board of Directors Report
The ECS Board of Directors held its spirited 90-minute March video conference meeting on the 22nd, with board members joining from locations around the globe.
ECS President Gerri Botte called the meeting to order and kicked off the 2024 governance year by thanking the Board for their continued leadership, support, and dedication. She then gave an important update on the work of the two Presidential Ad Hoc Committees launched at the start of her term, one dealing with diversity, equity, access, and inclusion, and the other addressing sustainability. Since the 244th ECS Meeting in May in Sweden, both committees have been hard at work advancing their respective goals, with motions expected at the next board meeting.
The Ad Hoc Committee on Diversity seeks to establish diversity oversight in our volunteer governance structure and create a new travel grant program aimed at increasing participation from underrepresented groups in the ECS community. The Ad Hoc Committee on Sustainability focuses on engaging the ECS community in exploring how electrochemical technologies can drive the transition to clean and renewable energy sources; protect the environment; mitigate climate change; and foster ecological, human, and economic well-being. Look for more to come from these critical working groups later this year.
Next, ECS Secretary Marca Doeff presented the previous board meeting minutes and had the pleasure of announcing the newly elected board members: incoming President Colm O’Dwyer, incoming Third Vice President Robert Savinell, and incoming Secretary Gessie Brisard. Congratulations to Colm, Bob, and Gessie!
Following the Secretary’s report, ECS Executive Director Chris Jannuzzi provided a brief overview of the past year, 2023, and updated the Board on new initiatives launched in 2024. Highlights included:
• Sustained growth and engagement across meetings, membership, and publications;
• Increased net assets, owing to strong revenue and significant gains in the Society’s investment portfolio;
• Over $700k in investment in new content and products to serve the ECS community and provide future resources to advance the Society’s mission;
• New initiatives for 2024, including a redefined membership model and a new education platform to help train technical professionals to work in ECS-related fields, beginning with batteries.
The meeting concluded with a report from ECS Treasurer Lisa Podlaha-Murphy, who outlined a proposal to revise the ECS division funding model. Now in its final stages, this 18-month long project is designed to streamline and simplify the process for providing financial resources for ECS divisions. A final vote on the revised funding plan is slated for the May board meeting.
Last, a motion to close the meeting was made, seconded, and unanimously approved.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 17
Rohan Akolkar
SOCIETY NEWS SOCIETY NEWS
The 2024 Neal R. Amundson’s Lecture at the University of Guadalajara by Professor Gerardine G. Botte
by Norberto Casillas Santana
The Neal R. Amundson’s Lecture, held annually by the Department of Chemical Engineering at the University of Guadalajara, pays tribute to the distinguished professor Neal R. Amundson. It is the most prestigious and traditional scientific event in its field at the University of Guadalajara. It was established in 1999 thanks to the initiative of Professors Héctor Antonio Rodriguez Sanchez and Jorge Emilio Puig Arevalo, both distinguished alumni of the master’s and doctoral programs in Chemical Engineering and Materials Science at the University of Minnesota, with the approval of Professor Neal R. Amundson.
Since its establishment, the Neal R. Amundson’s Lecture has had as its primary goal to disseminate the latest developments in Chemical Engineering made by the most eminent professionals in the field worldwide through two keynote lectures. Additionally, it seeks to promote and strengthen collaborative links with other Chemical Engineering departments and scientific research institutions around the world.
For two decades, Professor Neal R. Amundson led the Department of Chemical Engineering and Materials Science at the University of Minnesota, earning the title of “Chief” and later considered by many the “Father of Modern Chemical Engineering.” Beyond his renowned academic career, Professor Amundson stood out for his generosity toward his former home, the Faculty of Chemical Sciences at the University of Guadalajara. He greatly supported the program by providing scholarships for master’s and doctoral degrees, so that students from the former Faculty of Chemical Sciences, now known as the Department of Chemical Engineering at the University of Guadalajara, could pursue graduate studies.
This support was a decisive factor that allowed for the elevation of the academic level of the faculty at the former Faculty of Chemical Sciences, at a time when the Department of Chemical Engineering and Materials Science at the University of Minnesota was considered the most prominent in the world. In return, the University of Guadalajara awarded Professor Amundson an honorary doctorate in 1999.
The University of Minnesota granted a total of 16 scholarships to students from the former Faculty of Chemical Sciences at the University of Guadalajara. Among the beneficiaries of this program, prominent figures stand out, such as two Presidents of the University Center for Exact Sciences and Engineering (CUCEI), a General President of the University of Guadalajara, a National Science Award winner, a director of a worldwide corporation in Latin America, a director of the national researchers’ system, a Secretary of the Interior for the State of Jalisco, as well as several members of the national system of researchers and distinguished professors from the Departments of Chemical Engineering and and of Chemistry at the University of Guadalajara, and from the Metropolitan Autonomous University in Iztapalapa.
Throughout its 25-year history, the Neal R. Amundson’s Lecture has seen the participation of more than 38 professors and researchers of the highest international level in the field of Chemical Engineering and related disciplines, including a Nobel Prize laureate.
The distinguished academics who have participated in the Neal R. Amundson’s Lecture in the field of electrochemistry include, in 2003, Professor Allen J. Bard, recognized as a “father of modern electrochemistry” from the University of Texas at Austin; in 2009, Professor Richard Compton of the University of Oxford in the United Kingdom, recognized as one of the most prominent researchers in the field of electrochemistry in the world; in 2018, Professor Jorge Ibáñez Cornejo from the Universidad Iberoamericana,

a distinguished Mexican scientist and humanist in the field of environmental and microscale electrochemistry; and, in this edition, the participation of the renowned Professor Gerardine (Gerri) G. Botte from the Texas Tech University, Fellow of the Electrochemical Society and current President. Prof. Botte is recognized for her innovations in electrochemical processes toward sustainability and circularity. Dr. Botte is the founder and director of the National Science Foundation Engineering Research Center for Advancing Sustainable and Distributed Fertilizer Production, CASFER. She is also the founding Director of the Institute for Sustainability and Circular Economy at Texas Tech University.
In her participation in the Neal R. Amundson’s Lecture, Professor Gerri Botte presented the keynote lecture entitled, “Electrochemical Engineering towards an Environmentally Viable Economy” and led a “Roundtable Discussion on Sustainability and Circular Economy.” This event was attended by graduate students from various disciplines taught at the University of Guadalajara, such as Chemical Engineering, Chemistry, Materials Science, and Water Technology from different university centers within the University of Guadalajara’s university network. The participating students had the opportunity to present parts of their research in a dynamic questionand-answer session directly with Professor Botte.
Following the conclusion of the event, a social gathering was organized with undergraduate and graduate students, providing the opportunity to interact directly with Professor Botte. Additionally, a working meeting was held with university authorities to explore the establishment of collaborative research projects between the University of Guadalajara and the Institute for Sustainability and Circular Economy.
Attendees were invited to be part of the upcoming Neal R. Amundson’s Lecture series, scheduled for the first week of October 2024. This event is part of the Chemical Engineering Week SIC 2024 and will commemorate the 25th anniversary of the Neal R. Amundson´s Lecture in the Department of Chemical Engineering at the University of Guadalajara.
Professor Norberto Casillas Santana is the Chair of the Mexico Section of The Electrochemical Society and Coordinator of the Amundson’s Lecture series at the University of Guadalajara.
18 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
The 2024 Neal R. Amundson’s Lecture at the University of Guadalajara included a roundtable discussion on sustainability and the circular economy.
SOCIETY NEWS SOCIETY NEWS
ECS Division Contacts

Battery
Brett Lucht, Chair
University of Rhode Island
Jie Xiao, Vice Chair
Jagjit Nanda, Secretary
Xiaolin Li, Treasurer
Doron Aurbach, Journals Editorial Board Representative

Corrosion
Dev Chidambaram, Chair
University of Nevada, Reno
Eiji Tada, Vice Chair
Rebecca Schaller, Secretary/Treasurer
Sannakaisa Virtanen, Journals Editorial Board Representative

Dielectric Science and Technology
Sreeram Vaddiraju, Chair
Texas A&M University
Eva Kovacevic, Vice Chair
Zhi David Chen, Secretary
Thorsten Lill, Treasurer
Peter Mascher, Journals Editorial Board Representative

Electrodeposition
Luca Magagnin, Chair
Politecnico di Milano
Andreas Bund, Vice Chair
Rohan Akolkar, Secretary
Adriana Ispas, Treasurer
Takayuki Homma, Journals Editorial Board Representative

Electronics and Photonics
Qiliang Li, Chair
George Mason University
Vidhya Chakrapani, Vice Chair
Zia Karim, 2nd Vice Chair
Helmut Baumgart, Secretary
Travis Anderson, Treasurer
Khanna Aniruddh Jagdish, Journals Editorial Board Representative
Fan Ren, Journals Editorial Board Representative

Energy Technology
Katherine Ayers, Chair
Nel Hydrogen
Minhua Shao, Vice Chair
Hui Xu, Secretary
Iryna Zenyuk, Treasurer
Minhua Shao, Journals Editorial Board Representative

High-Temperature Energy, Materials, and Processes
Cortney Kreller, Chair
Los Alamos National Laboratory
Xingbo Liu, Senior Vice Chair
Teruhisa Horita, Junior Vice Chair
Dong Ding, Secretary/Treasurer
Minhua Shao, Journals Editorial Board Representative

Industrial Electrochemistry and Electrochemical Engineering
Paul Kenis, Chair
University of Illinois at Urbana-Champaign
Elizabeth Biddinger, Vice Chair
Chockalingam Karuppaiah, Secretary/Treasurer
Paul Kenis, Journals Editorial Board Representative

Luminescence and Display Materials
Eugeniusz Zych, Chair
Uniwersytet Wrocławski
Chong-Geng Ma, Vice Chair
Marco Bettinelli, Secretary/Treasurer
Im Won Bin, Journals Editorial Board Representative

Nanocarbons
Jeff L. Blackburn, Chair
National Renewable Energy Laboratory
Ardemis Boghossian, Vice Chair
Yan Li, Secretary
Hiroshi Imahori, Treasurer
Dirk M. Guldi, Journals Editorial Board Representative

Organic and Biological Electrochemistry
Shelley Minteer, Chair University of Utah
Jeffrey Halpern, 1st Vice Chair
Sabine Kuss, 2nd Vice Chair
Ariel Furst, Secretary/Treasurer
Janine Mauzeroll, Journals Editorial Board Representative

Physical and Analytical Electrochemistry
Stephen Paddison, Chair University of Tennessee, Knoxville
Anne Co, Vice Chair
Svitlana Pylypenko, Secretary
Iwona Rutkowska, Treasurer
David Cliffel, Journals Editorial Board Representative

Sensor
Larry Nagahara, Chair
John Hopkins University
Praveen Kumar Sekhar, Vice Chair
Dong-Joo Kim, Secretary
Leyla Soleymani, Treasurer
Netz Arroyo, Journals Editorial Board Representative
Stefano Cinti, Journals Editorial Board Representative
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 19
SOCIETY NEWS SOCIETY NEWS
New Division Officers

Chair
Dielectric Science and Technology Division
Sreeram Vaddiraju, Texas A&M University
Vice Chair
Eva Kovacevic, GREMI/Université d’Orléans
Secretary
Zhi David Chen, University of Kentucky
Treasurer
Thorsten Lill, Lam Research Corporation
Members at Large
Gautam Banerjee, IBM Research
Vimal H. Chaitanya, New Mexico State University
Stefan De Gendt, imec
Dennis Hess, Georgia Institute of Technology
Hemanth Jagannathan, IBM Corporation Research Center
Zia Karim, Yield Engineering Systems
Steve Kilgore, NXP Semiconductor
Dong-Kyun Ko, New Jersey Institute of Technology
Paul Kohl, Georgia Institute of Technology
Hiroki Kondo, Nagoya University
Sunghwan Lee, Purdue University
Oana Leonte, Berkeley Polymer Technologies, Inc.
Durga Misra, New Jersey Institute of Technology
Yaw Obeng, National Institute of Standards and Technology
Neelakandan Santhosh Marath, Jožef Stefan Institute
Kay Song, Yield Engineering Systems
Kalpathy Sundaram, University of Central Florida
Mahendra Sunkara, University of Louisville

Chair
Industrial Electrochemistry and Electrochemical Engineering
Paul Kenis, University of Illinois at Urbana-Champaign
Vice Chair
Elizabeth Biddinger, City College of New York
Division Secretary/Treasurer
Chockalingam Karuppaiah, Vetri Labs
Members at Large
Christopher Arges, The Pennsylvania State University
Saket Bhargava, Dow Chemical Company
Gerardine Botte, Texas Tech University
Fikile Brushett, Massachusetts Institute of Technology
Damilola Daramola, Northeastern University
Luis Diaz Aldana, Idaho National Laboratory
James Fenton, University of Central Florida
Taylor Garrick, General Motors Holdings, LLC
Matthew Graaf, Corteva Agriscience
John Harb, Brigham Young University
Marta Hatzell, Georgia Institute of Technology
Shrisudersan Jayaraman, Corning, Inc.
Wenzhen Li, Iowa State University
Juan Lopez-Ruiz, Pacific Northwest National Laboratory
Trung Van Nguyen, University of Kansas
Mark E. Orazem, University of Florida
Tyler Petek, Lubrizol Corporation
Doug Riemer, TDK Ventures
Shrihari Sankarasubramanian, University of Texas at San Antonio
Robert Savinell, Case Western Reserve University
John Staser, Ohio University
Xiao Su, University of Illinois at Urbana-Champaign
Venkat Subramanian, University of Texas at Austin
William Tarpeh, Stanford University
Santosh Vijapur, Faraday Technology, Inc.
John Weidner, University of Cincinnati

Chair
Nanocarbons
Jeff L. Blackburn, National Renewable Energy Laboratory
Vice Chair
Ardemis Boghossian, École Polytechnique Fédérale de Lausanne
Secretary
Yan Li, Peking University
Treasurer
Hiroshi Imahori, Kyoto University
Members at Large
Noe Alvarez, University of Cincinnati
Mike Arnold, University of Wisconsin–Madison
Delphine Bouilly, Université de Montréal
Tatiana Da Ros, Università degli Studi di Trieste
Francis D’Souza, University of North Texas
Andrew Ferguson, National Renewable Energy Laboratory
Daniel Heller, Memorial Sloan Kettering Cancer Center
Mark Hersam, Northwestern University
Nicole Iverson, University of Nebraska–Lincoln
Markita Landry, University of California, Berkeley
Fernando Langa, Universidad de Castilla–La Mancha
Richard Martel, Université de Montréal
Nazario Martín, Universidad Complutense de Madrid
Shigeo Maruyama, University of Tokyo
Elisa Miller-Link, National Renewable Energy Laboratory
Anton Naumov, Texas Christian University
Roberto Paolesse, Università di Roma Tor Vergata
Slava V. Rotkin, The Pennsylvania State University
Ángela Sastre-Santos, Universidad Miguel Hernández de Elche
Steve Stevenson, Purdue University
Tomás Torres, Universidad Autónoma de Madrid
R. Bruce Weisman, Rice University
Yoko Yamakoshi, Laboratorium für Organische Chemie –ETH Zürich
Shangfeng Yang, University of Science and Technology of China
Ming Zheng, National Institute of Standards and Technology
20 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
2024-2025 ECS Committees
Executive Committee of the Board of Directors
Colm O’Dwyer
James Fenton
President, Spring 2025
Senior Vice President, Spring 2025
Francis D’Souza 2nd Vice President, Spring 2025
Robert Savinell
Gessie Brisard
Elizabeth Podlaha-Murphy
3rd Vice President, Spring 2025
Secretary, Spring 2028
Treasurer, Spring 2026
Christopher Jannuzzi Executive Director, Term as ED
Audit Committee
Gerardine Botte, Chair
Immediate Past President, Spring 2025
Colm O’Dwyer President, Spring 2025
James Fenton
Robert Micek
Senior Vice President, Spring 2025
Nonprofit Financial Professional, Spring 2025
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
Education Committee
Alice Suroviec, Chair Spring 2025
Damilola Daramola Spring 2027
Samantha Gateman Spring 2026
David Hall Spring 2025
Stephen Maldonado Spring 2025
Elif Selin Sahin Spring 2025
Wen Shen Spring 2026
Maureen Tang Spring 2027
Fernando Garzon Spring 2028
Roseann Warren Spring 2028
New Student Member Spring 2026
Gessie Brisard
Secretary, Spring 2028
E. Jennings Taylor Chair, Individual Membership Committee, Spring 2026
Ethical Standards Committee
Gerardine Botte, Chair
Gessie Brisard
Immediate Past President, Spring 2025
Secretary, Spring 2028
Peter Fedkiw Past Officer, Spring 2026
Elizabeth Podlaha-Murphy
Treasurer, Spring 2026
Daniel Steingart Past Officer, Spring 2027
Finance Committee
Elizabeth Podlaha-Murphy, Chair
Gessie Brisard
Treasurer, Spring 2026
Secretary, Spring 2028
Paul Kenis Spring 2026
Dong-Joo Kim Spring 2025
Thorsten Lill Spring 2026
Robert Micek
Nonprofit Financial Professional, Spring 2025
Tim Gamberzky Chief Operating Officer, Term as COO
Honors and Awards Committee
Adam Weber, Chair Spring 2027
Elizabeth Biddinger Spring 2027
Stanko Brankovic Spring 2027
Mikhail Brik Spring 2028
Dev Chidambaram Spring 2026
Uroš Cvelbar Spring 2028
Alanah Fitch Spring 2025
Andrew Hoff Spring 2026
Shigeo Maruyama Spring 2025
Shirley Meng Spring 2026
Jean St-Pierre Spring 2025
Thomas Thundat Spring 2027
Siegfried Waldvogel Spring 2028
Colm O’Dwyer
Individual Membership Committee
President, Spring 2025
E. Jennings Taylor, Chair Spring 2026
Jedidian Adjetey Adjei Spring 2025
Uroš Cvelbar Spring 2026
Jiaxin Duan Spring 2025
Joshua Gallaway Spring 2027
Hussain Abdul Jabbar Spring 2027
Qizhi Liu Spring 2025
Shuthi T. Kumar Raj Spring 2025
Kent Jingxu Zheng Spring 2026
Gessie Brisard Secretary, Spring 2028
Alex Peroff Chair, Institutional Engagement Committee, Spring 2025
Institutional Engagement Committee
Alex Peroff, Chair Spring 2025
Vimal Chaitanya Spring 2026
Hanping Ding Spring 2026
Hemanth Jagannathan Spring 2026
Yaw Obeng Spring 2025
Santosh Vijapur Spring 2025
Yoko Yamakoshi Spring 2025
Jacob Ketter Spring 2027
Nominating Committee
Gerardine Botte, Chair
Immediate Past President, Spring 2025
Robert Savinell 3rd Vice President, Spring 2025
Mark Allendorf Spring 2025
Brett Lucht Spring 2025
Peter Mascher Spring 2025
Christopher Jannuzzi Executive Director, Term as ED
Technical Affairs Committee
James Fenton, Chair
Gerardine Botte
Senior Vice President, Spring 2025
Immediate Past President, Spring 2025
Francis D’Souza Chair, Publications Subcommittee, Spring 2025
Turgut Gür 2nd Immediate Past President, Spring 2025
Jennifer Hite Chair, Interdisciplinary Science and Technology Subcommittee, Spring 2025
Colm O’Dwyer President, Spring 2025
Robert Savinell Chair, Meetings Subcommittee, Spring 2025
Christopher Jannuzzi Executive Director, Term as ED
Publications Subcommittee of the Technical Affairs Committee
Francis D’Souza, Chair
Robert Savinell, Vice Chair
Robert Kelly
Vice President, Spring 2025
, Spring 2025
Editor-in-Chief (EIC), 5/31/2025
Ajit Khosla ECSSP, 10/21/2024
Krishnan Rajeshwar
Interdisciplinary Science and Technology Subcommittee of the Technical Affairs Committee
Jennifer Hite, Chair
Jeff L. Blackburn
Vidhya Chakrapani
Huyen
Rangachary Mukundan
James Noël Spring 2025
Alok Srivastava Spring 2027
Sreeram Vaddiraju Spring 2026
Symposium Planning Advisory Board of the Technical Affairs Committee
Robert Savinell, Chair
3rd Vice President, Spring 2025
Kathryn Ayers Chair, Energy Technology Division, Spring 2025
Jeff Blackburn Chair, Nanocarbons Division, Spring 2026
Dev Chidambaram Chair, Corrosion Division, Fall 2024
Jennifer Hite Chair, Interdisciplinary Science and Technology Subcommittee, Spring 2025
Paul Kenis Chair, Industrial Electrochemistry and Electrochemical Engineering Division, Spring 2026
Cortney Kreller Chair, High-Temperature Energy, Materials, & Processes Division, Fall 2025
Qiliang Li Chair, Electronics and Photonics Division, Spring 2025
Brett Lucht Chair, Battery Division, Fall 2024
Luca Magagnin Chair, Electrodeposition Division, Fall 2025
Shelley Minteer Chair, Organic and Biological Electrochemistry Division, Spring 2025
Larry Nagahara Chair, Sensor Division, Fall 2024
Stephen Paddison Chair, Physical and Analytical Electrochemistry Division, Spring 2025
Sreeram Vaddiraju Chair, Dielectric Science and Technology Division, Spring 2026
Eugeniusz Zych Chair, Luminescence and Display Materials Division, Fall 2025
Other Representatives
Society Historian
Roque Calvo Spring 2025
American Association for the Advancement of Science
Christopher Jannuzzi Term as Executive Director
National Inventors Hall of Fame
Adam Weber
Chair, Honors & Awards Committee, Spring 2027
John Muldoon Spring 2027
Bill Cohen Spring 2027
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
E. Jennings Taylor Chair, Individual Membership Committee, Spring 2026
2nd
3rd Vice President
Interface
JSS EIC, 12/31/2024 Daniel Schwartz Spring 2025 Chunsheng Wang Spring 2025 TBD Fall 2024 Xingfan Jin Spring 2026 Marc Secanell Spring 2026 Meetings Subcommittee of the Technical Affairs Committee
Savinell, Chair 3rd Vice President, Spring 2025 Francis
Chair 2nd Vice President, Spring 2025 Xiaolin Li Spring 2026
Mascher Spring 2025 Xiao Su Spring 2027
Robert
D’Souza, Vice
Peter
Spring 2025
Spring 2025
Spring 2026
Spring 2026
Dinh
Spring 2026
Spring 2027
Jackson Spring 2025 Christopher Johnson Spring 2025
Spring 2027
Spring 2025
Alanah Fitch
David Hickey
Greg
Chockkalingam Karuppaiah
Luca Magagnin
Spring 2027
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 21 SOCIETY NEWS SOCIETY NEWS
2024 Leadership Circle Award Winners
The Electrochemical Society has honored long-term supporters with Leadership Circle Awards since the fall of 2002. These awards recognizing our partners in electrochemistry and solid state science are granted in the year an institutional partner reaches an anniversary milestone level. Congratulations to the ECS Institutional Partners who reached milestone levels in 2024. These companies have been tremendous partners with the Society over the years. Thank you for supporting our mission to advance theory and practice at the forefront of electrochemical and solid state science and technology, and allied subjects.
Gold Partner – 25 years
Teledyne Energy Systems, Inc.
Learn more about Teledyne Energy Systems at https://www.teledynees.com/ en-us
Silver Partners – 10 years
El-Cell GmbH
Learn more about El-Cell at http://www. el-cell.com/
Ford Motor Corporation
Learn more about Ford at http://corporate. ford.com/homepage.html
Ion Power, Inc.
Learn more about Ion Power at http://www. ion-power.com/







Metrohm USA, Inc.
Learn more about Metrohm at http://www. metrohmusa.com/
Western Digital Corporation
Learn more about Western Digital at https://www.sandisk.com/


Bronze Partner – 5 years
Pacific Northwest National Laboratory (PNNL)
Learn more about PNNL at https://www.pnnl.gov/ energy-storage

To learn more about the ECS Institutional Partnership Program, contact Anna Olsen, Senior Manager, Corporate Programs, at Anna. Olsen@electrochem.org
Creating Powerful Partnerships
ECS welcomes these great new additions to the institutional partnership program.
PalmSens BV
At PalmSens, we are committed to making electrochemistry easier, portable, and accessible for researchers and entrepreneurs. We provide a comprehensive range of instruments for electrochemistry, including the smallest potentiostat (EIS) module, the EmStat Pico, and our PalmSens4, which is one of the most versatile and compact EIS-capable devices in the market.
Learn more about PalmSens at https://www.palmsens.com/
Sensolytics GmbH
Sensolytics GmbH specializes in developing and manufacturing analytical systems for characterizing local electrochemical properties. Besides our highly modular scanning electrochemical microscopes
NEXT ISSUE OF IN THE
The fall issue of Interface will be a special issue on battery safety, guest edited by Paul Shearing and Thomas Barrera Fall 2023 will also include 245th ECS Meeting highlights, the 2024 Toyota Young
(SECM) with the first commercialized shear-force-based distance control unit, Sensolytics offers individual scanning droplet cell systems (SDC) for high throughput screening applications in academia and industry. In addition to tailored instrument solutions, developed in close cooperation with our customers and marketed through a worldwide distribution network, Sensolytics provides the widest range of SECM tips on the market, various accessories such as measuring cells or polishing equipment, and sample preparation devices.
Learn more about Sensolytics GmbH at https://www.sensolytics.de/ en/
ECS’s Institutional Partnership Program opens doors to a network that helps organizations meet business goals and objectives. Contact sponsorship@electrochem.org for more information.
Investigator recipients, Pennington Corner, features favorites like EChem Education, Tech Highlights, and Looking at Patent Law, updates on ECS journal impact factors, and the latest news about people, students, and the Society.
22 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS
The Electrochemical Society book series provides authoritative, detailed accounts on specific topics in electrochemistry and solid state science and technology. These titles are sponsored by ECS and published in cooperation with Wiley.

ECS members receive 20% off all Wiley books. ECS members use promo code ECS18 at checkout.











New

uly
practical, single-source reference on the unique nature of seawater as a corrosive environment. Explains practical corrosion control solutions via design, proper materials selection, and implementation of good corrosion control engineering practices.

21 - CD5827 ON THE MOST TRUSTED RESOURCES IN ELECTROCHEMISTRY SAVE 20%
INTERESTED
PUBLISHING WITH US?
IN
LaQue’s Handbook on
David
ISBN
SECOND EDITION ATMOSPHERIC CORROSION
Please contact publications@electrochem.org to discuss your idea.
Marine Corrosion, 2nd Edition
A. Shifler
978-1-119-78883-6 | J
2022 A
EDITED BY DAVID A. SHIFLER LAQUE’S HANDBOOK OF MARINE CORROSION THE ELECTROCHEMICAL SOCIETY SERIES SECOND EDITION
Shop the Latest ECS Titles at wiley.com
Christofer Leygraf Inger Odnevall Wallinder Johan Tidblad Thomas Graedel
Websites of Note
Selected for you
by Alice H. Suroviec
On The Line

A podcast hosted by Products Finishing for individuals in the world of finishing. This monthly podcast interviews experts about the latest trends in electroplating, electrocoating, and mass finishing. Recent topics include wastewater management, trivalent chromium processes, and automation trends in the industry.
https://www.pfonline.com/podcast
Chemistry for Your Life

Chemistry for Your Life is a chemistry podcast for those who are curious about how the world around them works. This weekly podcast, which has covered topics from PFAS to mosquito bites to the chemistry of kombucha, is wide ranging and entertaining.
https://chemforyourlife.transistor.fm/episodes
About the Author The Finish Line

The Finish Line podcast covers all things related to surface finishing, including electroplating, anodizing, powder and liquid coatings, electrocoat, PVD, pretreatment, and wastewater treatment. The podcast is a hosted by the Editorin-Chief of Finishing and Coating (www.finishingandcoating. com) an e-zine updated daily with the latest news in surface finishing.
https://finishingandcoating.com/index.php/podcasts

Alice Suroviec is a Professor of Bioanalytical Chemistry and Dean of the School of Mathematical and Natural Sciences at Berry College. She earned a BS in Chemistry from Allegheny College in 2000. She received her PhD from Virginia Tech in 2005 under the direction of Dr. Mark R. Anderson. Her research focuses on enzymatically modified electrodes for use as biosensors. She is a Fellow of the Electrochemical Society and Associate Editor of the PAE topical interest area for the Journal of the Electrochemical Society. She welcomes feedback from the ECS community. https://orcid.org/0000-0002-9252-2468



UPCOMING ECS SPONSORED MEETINGS
ECS, its divisions, and its sections sponsor meetings and symposia of interest to the technical audience ECS serves as well as ECS biannual meetings and ECS satellite conferences. A partial list of upcoming sponsored meetings follows. Consult the ECS website for a complete list.
2025
19th International Symposium on Solid Oxide Fuel Cells (SOFC-XIX)
July 13–18, 2025 | Stockholm, Sweden
The Brewery Conference Center
To request ECS sponsorship of your technical event or to learn what ECS sponsorship can do for your meeting, contact ecs@electrochem.org.
24 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS
2024 ECS Officer Election Results and 2025 Slate of Officers
The results of the 2024 ECS officers’ election are:
PRESIDENT: Colm O’Dwyer, University College Cork
3RD VICE PRESIDENT: Robert Savinell, Case Western Reserve University
SECRETARY: Gessie Brisard, Université de Sherbrooke
TREASURER Elizabeth Podlaha-Murphy of Clarkson University’s term of office was not affected by the election.
At the May 30 Board of Directors meeting, members voted to approve the ECS Nominating Committee’s recommended 2025 ECS Officers Election slate of candidates. The candidates on the ballot for the election taking place from January to March 2025 are:
PRESIDENT: James Fenton, University of Central Florida
3RD VICE PRESIDENT: Marca Doeff, Lawrence Berkeley National Laboratory E. Jennings (EJ) Taylor, Faraday Strategies, LLC
Full biographies and candidate statements will be included in the Interface winter 2024 issue

SOCIETY NEWS SOCIETY NEWS Your Lab in a Box. Integrated Temperature Chamber (10 to 80°C) No additional external devices needed for battery cycling Fully featured Potentiostat, Galvanostat and EIS
independent test channels, no multiplexing Ideally suited for High-Precision Coulometry Excellent accuracy and signal-to-noise ratio Small Footprint, Easy to Setup and Operate
cell wiring required, full remote control via LAN sales@el-cell.com Discover the PAT-Tester-i-16, our All-in-One Solution for Multichannel Battery Testing! +49 40 79012 734 Contact us for more information: www.el-cell.com
Sixteen
No
ALLEN J. BARD
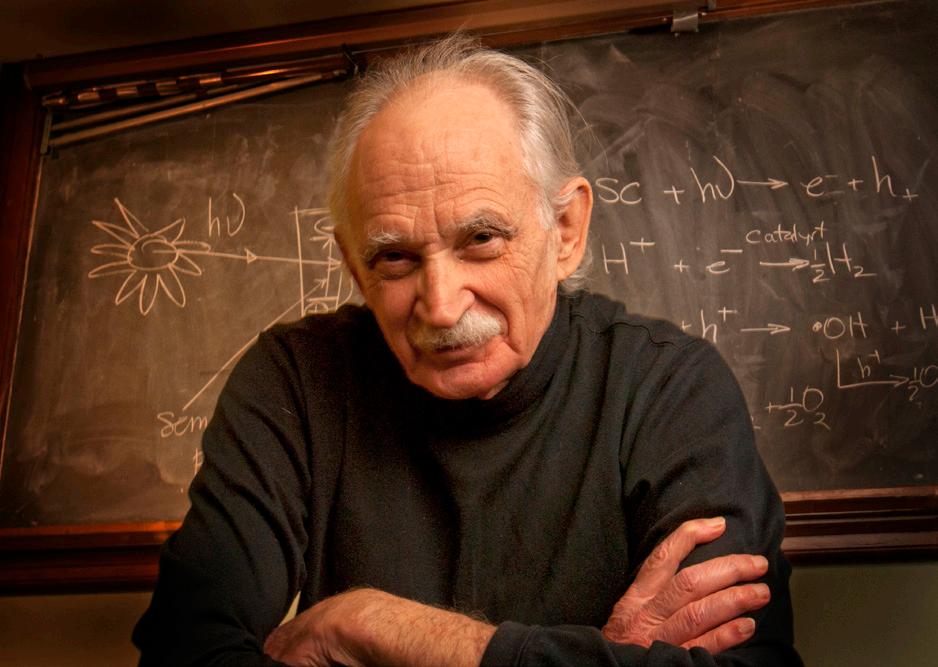 by Frances N. Chaves
by Frances N. Chaves
50+ YEAR SOCIETY MEMBER PIONEERED
The ECS community is mourning the passing of our colleague and friend, Allen (Al) J. Bard, on February 11, 2024. Throughout a distinguished career that brought him worldwide recognition for his pioneering scientific achievements, Al was a beloved and respected member of the ECS community as an author, Fellow, awardee, editor, meeting participant and organizer, and more.
“Al’s contributions to the advancement of electrochemistry and science are extraordinary, and his dedication to the advancement of many generations of younger scientists is legendary. If there is a common theme to his work, it is the pursuit of

fundamental scientific knowledge and discovery; Al was a fearless scientist, encouraging young scientists in his laboratory to constantly create and pursue their own ideas and experiments off the beaten path,” said Shelley D. Minteer and Henry White, co-editors of the Journal of The Electrochemical Society Focus Issue in Honor of Allen J. Bard
“Professor Bard was a humble genius who insisted that his students were his most important contribution. Certainly, ECS benefited largely from Bard’s commitment to teaching. Three of my fellow ECS presidents were his students: Larry Faulkner, ECS president from 1991 to 1992; Paul Kohl, ECS president from 2014 to 2015; and Johna Leddy, ECS president from 2017 to 2018,” said current ECS President Gerardine Botte. ECS Executive Director Christopher Jannuzzi added, “Professor Bard became an ECS member in 1965, setting a great example of service to the Society as a journal associate editor, chair of the ElectroOrganic Division (now the Organic and Biological Electrochemistry Division), and member-at-large of the ECS Texas Section. He enjoyed working with the Society’s governance and was especially proud to help establish organic/biological electrochemistry as an essential topical interest area for the Society, and, as a member of the

26 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
ECS CELEBRATES THE LIFE OF
Bard Group picture from 1999 (shared by Shigeru Amemiya).
Allen J. Bard in 2011
ELECTROCHEMISTRY

What I am building is my students, and their students after them. And they will help build the edifice of science. That’s the most important thing that I do.”
Committee on the Free Dissemination of Research (2014–2015), he helped establish the Free the Science initiative.”
Life and Science
Al was born in New York City in 1933. In an ECS Masters Interview at the 227th ECS Meeting in May 2015, Al says he always knew he wanted to be a scientist and describes playing with his older brother’s chemistry set when he was only four or five years old! He attended the prestigious Bronx High School of the Sciences, then obtained his BS in Chemistry at City College of New York in 1955. After a brief stint in industry, he went on to Harvard University. There he was introduced to electrochemistry, which he said he “took to like a fish to water.” Under the supervision of renowned electroanalytical chemist James J. Lingane, Al received an MSc in 1956 and PhD in 1958. Norman Hackerman, University of Texas at Austin (UT-Austin) Chemistry Department Chairperson (and later president of the university), offered Al a teaching job with a salary of $5,200 a year and $5,000 for equipment. Although the New York native had never been to Texas, which he thought of as the Wild West filled with cowboys, he accepted the job. He taught there for 63 years—from 1958 until his retirement in 2021 as the Hackerman-Welch Regents Chair Professor and Director of the Center for Electrochemistry.
Al mentored more than 75 PhD students and 150 postdoctoral fellows. Their combined contributions to the field of electrochemistry are legendary, including electroanalytical techniques for evaluating electrode reaction mechanisms, simultaneous electrochemistry electron spin resonance (SEESR) techniques, nonaqueous solvents for investigating energetic species, electrogenerated chemiluminescence (ECL), polymer modified electrodes, semiconductor photoelectrochemistry, photocatalysis, scanning electrochemical microscopy (SECM), and single-particle collision electrochemistry.
The co-author of more than 1,000 publications (including 125 in the Journal of The Electrochemical Society) covering all areas of modern electrochemistry, Al produced 88 book chapters and other publications, and holds more than 30 patents. His 1980 book, Electrochemical Methods: Fundamentals and Applications, written with former student (and former ECS president) Larry Faulkner, is still the most widely used electrochemistry textbook. He served as Editor-in-Chief of the Journal of the American Chemical Society from 1982 to 2001. When asked in the Masters Interview how he produced this extraordinary volume of inventions and publications, he said, “I was very fortunate to have a lot of very good students.”
Awards and Accolades
The Electrochemical Society named Al an Honorary Member in 2013 and Fellow of The Electrochemical Society in 1990. He received top ECS awards that include the 2007 Europe Section Heinz Gerischer Award, 1987 Olin Palladium Award, 1986 Henry B. Linford Award for Distinguished Teaching, and 1981 Carl Wagner Memorial Award.
ECS founded the Allen J. Bard Award in 2013 to honor the professor’s extensive contributions in the field of electrochemistry. The Bard award recognizes paradigm-shifting contributions in the field of electrochemical science and exceptionally creative

experimental or theoretical studies that have opened new directions in electroanalytical chemistry or electrocatalysis.
ECS published a focus Issue of the Journal of The Electrochemical Society in honor of Allen J. Bard in 2016. The 24 research articles in this special issue, many from former students and postdocs, covered the many electrochemistry topics impacted by Al during his career.
In 2013, President Barack Obama presented him with a National Medal of Science for Chemistry. At the awards ceremony, Obama said of Al and his colleague John B. Goodenough, “I am proud to honor these inspiring American innovators. They represent the ingenuity and imagination that has long made this nation great—and they remind us of the enormous impact a few good ideas can have when these creative qualities are unleashed in an entrepreneurial environment.”
Among the many awards Al received through his distinguished career are the 2019 King Faisal International Prize in Chemistry, 2014 Enrico Fermi Award, 2008 Wolf Prize in Chemistry, 2002 Priestley Medal, 1984 Charles N. Reilly Award in Electroanalytical Chemistry, and 1984 ACS Fisher Award in Analytical Chemistry. He was elected a Fellow of the American Academy of Arts and Sciences in 1990.
After Al
Al was asked about potentially significant areas of electrochemistry in the future. He replied, “I still think that eventually photoelectrochemistry will be a solution to the energy problem… It will take discoveries. But what I learned in my career is that discoveries do happen. You have to have faith; discoveries happen… and electrochemistry is the best way to get there.”
Al dismissed the notion that he is the “father of electrochemistry.” Rather, his primary focus was teaching and mentoring future generations of scholars and scientists. “What I am building is my students, and their students after them. And they will help build the edifice of science. That’s the most important thing that I do.”
The images in this article were contributed by ECS members touched by Al. More remembrances are available in a collection curated by his former student, Xiaole (Joy) Chen. ©The Electrochemical Society. DOI: 10.1149/2.F03242IF

The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 27
Dr. Bard’s picture with President Obama in 2013 (shared by Dick Crooks).
Dr. Bard’s picture with graduate student Brent (shared by David Eisenberg, former postdoc, currently Technion faculty).
In Memoriam ... William
E. O’Grady
1939–2024

Dr. William (Bill) E. O’Grady began his research career at Shell Chemical Co. in Denver, Colorado after graduating from the Colorado School of Mines with a BS degree in chemical engineering in 1964. While at Shell, he worked on developing a process which included a chlorination step, and thus began his career of contributions to the field of electrochemistry. Bill married Stephanie (Stevie) in April 1966 before leaving for graduate school at the University of Pennsylvania. After a year of course work Bill joined the Bockris group and began his thesis studies in electrochemistry in the fall of 1967. As Bill described it, “Following another year of courses and a number of lengthy (i.e., raucous) discussions with Prof. Bockris we came to the decision that I would study the structure of the passive film on iron using Mossbauer spectroscopy.” Bill was the first to use Mossbauer spectroscopy to study passive oxide films, observing passive film structures that did not coincide with any of those of the bulk iron oxides. As would become customary in Bill’s research for the remainder of his career, this insightful data led to the creation of a new model to describe the complex electrochemical interface. This model suggested that the passive layer was made up of a hydrous layer of iron coordinated with water and held together by hydroxide ions. He completed his PhD in 1972. Bill then joined Professor Ernest Yeager and Dr. Boris Cahan at Case Western Reserve University (CWRU) as a postdoctoral fellow. After one year as a post-doc, he was appointed to the research faculty at CWRU. In October of 1977, Bill joined the scientific research staff at Brookhaven National Laboratory where he became one of the earliest researchers to use synchrotron radiation to study electrochemical interfaces and phosphoric acid fuel cell catalysis. In 1987 Bill joined the US Naval Research Laboratory in Washington DC as Section Head for Electrochemical Interfaces, later joining the Corrosion Science Section in the Center for Corrosion Science and Engineering. He retired from government service at NRL in 2012, and subsequently consulted to the Navy on special electrochemistry topics for a few years while continuing to mentor fellow scientists.
Bill was among the earliest scientists to use surface analytical techniques to study electrochemical interfaces. While at Case Western, he developed an ultra-high vacuum LEED, Auger, XPS system coupled to an electrochemical cell in order to use electron spectroscopy to characterize the metal electrolyte interface. He obtained the first cyclic voltammograms on the well-defined (111), (100), and (110) single crystal planes of Pt. He was a pioneer and became an internationally recognized leader in the development and application of surface analytical techniques to probe electrochemical interfaces, and he is especially well known for his work on the application of synchrotron radiation to electrochemical systems. His groundbreaking contributions on interpreting X-ray absorption spectra were the subject of an article in the January 21, 2002 issue of C&E News entitled “Hiding in the Background: Ignored region of X-ray absorption spectrum now scrutinized for chemical information.” This work was also highlighted in his keynote presentation at the 2002 International Society of Electrochemistry (ISE) meeting in Düsseldorf, Germany. Bill’s research programs have provided significant insights into electrochemical processes in such diverse areas as electrocatalysis, batteries and fuel cells, adsorption
at interfaces, corrosion and passivity, and organic waste remediation. His research has resulted in 151 referreed publications with 3,979 citations The interest in this work can, in part, be seen by his 75 invited lectures at major meetings and universities and the citations of his work. He was inducted as Fellow of The Electrochemical Society in 2001 and he received the 2000 William Blum Award from the National Capital Section of the ECS.
Bill joined The Electrochemical Society in 1975, and became an ECS Emeritus Member in 2005. He showed great leadership in the scientific community and in his service to ECS. Bill began his service as an Executive Committee member of the Cleveland Section in 1974, became Section President in 1976, and went on to serve on numerous committees, including the Publications Committee, ECS Transactions Charter Committee, and the executive committee of the National Capital Section; he was a Divisional Editor of JES (1982–1992).
Bill’s research contributions to various fields of electrochemistry were groundbreaking and internationally respected. But, to Bill, enjoying the journey was more important than any individual scientific accomplishment. He had a wonderful sense of humor and loved goodhearted jocularity. Morning coffee was an important part of the day for Bill and everyone he mentored. The light conversations quickly gave way to discussions about electrochemical mechanisms, recent journal articles, and current research efforts. “What if” discussions were common with Bill. Inevitably these conversations produced new data analysis approaches, improvements on research methods, and the groundwork for ensuing proposals. On some occasions after the discussion, it would be decided “this will never work; what were we thinking?”—followed by a laugh. There was never any such thing as a “bad idea” when talking with Bill O’Grady—only things that wouldn’t work. Under Bill’s tutelage, the best learning moments came when he asked, “Of all the things you could do, why are you doing THAT?” An unsuccessful defense of the reasoning behind an experimental approach would then result in the real teaching moment: “OK, now that neither one of us can defend that approach, let’s do something that probes the questions we really want to ask.”
Bill was at the same time encouraging, challenging, and inspiring— always with an abundance of enthusiasm. The atmosphere that Bill created provided fertile, non-judgmental ground for discussions. He played a critical role in shaping the professional careers and personal lives of undergraduate and graduate students, post-docs, and colleagues. We joked at times that if you wanted a successful research career, all you had to do was follow Bill around and pick up on the ideas he didn’t have time to pursue.
Although Bill loved research, it was not his whole life. He was an incredibly creative and giving person. Bill wrote poetry, loved dancing, practiced meditation, and worked with hospice and visited those in hospice care. Above all, Bill loved his family. You could see his chest swell when he talked about Stevie and her art, his children, and grandchildren. Bill was a colleague and a mentor but most of all a wonderful friend; he enriched our lives and made the world a better place. We as well as many others feel blessed to have had Bill in our lives. He will be greatly missed and fondly remembered. Bill is survived by his wife, Stevie, his children Katy, Elizabeth (Liz), and Eamon, and his grandchildren.
This notice was contributed by P. M. Natishan, E. J. Taylor, and F. J. Martin
28 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS
In Memoriam ... Ralph Brodd
1928–2024
Ralph J. Brodd passed away February 5, 2024 in Broadview Heights, Ohio after a long illness. He was born in Moline, Illinois in 1928, where he spent his childhood. He received his BA degree in 1950 at Augustana College in Rock Island, IL, part of the quad cities area of Illinois and Iowa. Ralph and his wife Dorothy were married at this time. He then enrolled in the University of Texas, Austin where he received an MA in 1953 and a PhD degree in 1955 in Physical Chemistry under the direction of Prof. Norman Hackerman. Hackerman at the time was Chairman of the Department of Chemistry and soon to become the President of The Electrochemical Society. Ralph won the Corrosion Division Student Essay Contest in 1954, which carried a cash award. This was a significant fact as Dorothy had just given birth to their second child and the award paid for the hospital bill. His thesis work involved the study of the double layer of various metals in aqueous electrolytes.
Ralph then took a position at the National Bureau of Standards (now known as the National Institute of Standards and Testing –NIST) and began his study of batteries, which was to become his lifelong interest. His approach was new to the battery field, as he applied impedance techniques to the full battery at various stages of life to better understand time and discharge effects on the Leclanché primary battery, which was the dominant commercial battery at the time. After six years, Ralph took a position at Ling-Temco-Vought to develop a group to study fuel cells and batteries. With this management experience, Ralph then moved to the Battery Products Division of Union Carbide Corp. in Parma, Ohio in 1963. In this position he managed the exploratory work of the Laboratory at the time of the rapid development of lithium primary batteries and the beginning work with lithium rechargeable batteries. Many lithium batteries were explored, resulting in many patents and to the development of the ambient temperature lithium-iron disulfide cell which has become a major product of Energizer Batteries (the successor company to Union Carbide). This period also saw the development of alkaline zinc primary batteries, now a commodity in small batteries for electronics. During this period, Ralph and a Union Carbide colleague, Akiya Kozawa, established the International Battery Materials Association (IBA), an organization devoted to establishing collaborations between US and Japanese battery researchers, which continues to this day and includes battery researchers worldwide.

Starting in 1978, Ralph took several different positions: Director of Technology at ESB (INCO Electroenergy Inc.) in Pennsylvania; Development Manager of a unique rechargeable lithium-sulfur dioxide battery at AMOCO; Battery Manager at Gould, Inc. in Ohio; and finally, from 1992 to 1998 as Vice President at Valence Technology in Nevada, where he was responsible for developing a strong patent position in lithium-ion batteries as well as marketing. During this period, he was president of his own consulting company, Broddarp Inc. His consulting was broad—covering government, academia and private industry around the world—giving him a unique perspective on the battery industry, in which lithium-ion rechargeable batteries and primary lithium batteries were both developing to dominate the marketplace in personal electronics and large motive power batteries. Under the auspices of NIST and the Advanced Technology Program of the US Department of Commerce, he published a seminal report in 2006 entitled “Factors Affecting US Production Decisions: Why Are There No Volume Lithium-Ion Battery Manufacturers in the United States?” which has had a profound effect on developing government policy to ensure that US companies participate in battery production, particularly in large batteries for vehicles of all types. Ralph was a member of the National Research Council, “Transitions to Alternative Vehicles and Fuels” in 2013, which was equally influential in setting national policy on batteries and fuel cells.
Ralph joined ECS in 1954 and was a member of the Battery Division. He was elected Vice President and then served as Society president from 1981 to 1982. Ralph was made an Honorary Member of the Society in 1987 and received the designation Fellow of The Electrochemical Society in 2014. In recognition of his important contributions to the battery field and to The Electrochemical Society, Ralph was given the Edward Goodrich Acheson Award of the Society in 2014. His service to ECS spanned decades and included membership in the Council of Past Presidents, Financial Policy Advisory Committee, and Development Committee. His ECS Masters Series Interview is available on the ECS website. Ralph was a member of the board of many international groups in battery technology and received many additional awards for his service.
He is survived by his wife of 73 years, his 3 children, 8 grandchildren and 11 great-grandchildren.
This notice was contributed by Jim Akridge and George Blomgren
From Jim Akridge, EnPower:
Ralph Brodd was a significant presence for many decades in the battery and overall electrochemical field. He traveled the globe bringing his expertise targeting the younger generations of chemists with encouragement to push the boundaries of technology in electrochemistry. Ralph’s most significant contribution was his generosity of time and patient and gentle but firm pressure for advances in science. His wife, Dorothy, who survives Ralph, was a capable and generous contributor to the Society as well. Those who knew and worked closely with Ralph and Dorothy are grateful for their many decades of offering encouragement and assistance.
P.S. I spend decades working closely with Ralph and Dorothy. I communicated with her frequently as Ralph’s health declined. We had many, many evenings together over decades throughout the globe in discussions of how to improve the battery world and what to do next to push the technology. Dorothy was a participant and capable of generating ideas to help the younger people. We should not forget the wives.
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 29 SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS
Ralph Brodd during the IBA2013 meeting in Barcelona with M. Rosa Palacin (primary meeting organizer) who is indebted to him for his trustworthy advice and help in making it such a memorable event.
(continued from previous page)
SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS
From Christopher Johnson, Argonne National Laboratory
Ralph was an excellent mentor for me early in my career and for that I will always be grateful for his help. I have been missing his smiling face at our IBA meetings in recent years. Rest in peace, Ralph Brodd. We will miss your energy, jovial nature, and battery acumen.
From Boryann Liaw, Fellow of ECS:
Ralph will be remembered always as a role model and great person by me and, I trust, by this community. So sad that we lost a friend and colleague. No words can express how nice a person he was.
From Shirley Meng, Past Chair of the ECS Battery Division: Ralph made an impact in our community that lasts beyond his lifetime—his passion for electrochemical sciences, kindness to younger generations, and devotion to The Electrochemical Society will always be remembered.
In Memoriam ...
Ugo Bertocci
1926–2024

Dr. Ugo Bertocci, ECS Fellow and former Associate Editor, passed away March 7, 2024 at the age of 98. Ugo was born in Tripoli, Libya on January 31, 1926 and received his Doctorate in Industrial Chemistry from the University of Milan, Italy in 1950. He then spent eight years as a young professor at the Institute of Technology in Milan, among the first institutes in the world to study the electrochemical behavior of metal single crystals and the influence of crystal structure on the kinetics of electrochemical processes.
He then moved on to Harwell in England from 1958 to 1961 and served as Chemistry Group Leader representing the Italian Atomic Energy Committee while working on solvent extraction for reprocessing nuclear fuel. Moving to the United States in 1961, Ugo joined Oak Ridge National Laboratory where he continued his singlecrystal work, focusing on a variety of issues related to Cu etching and deposition.
He was one of the first to study single-crystal Cu at equilibrium, a condition met only after rigorous scrubbing of oxygen and preconditioning. Ugo’s work clearly showed that some of the singlecrystal data appearing in the literature at the time was driven by kinetic phenomena and did not describe thermodynamic equilibrium. This set the stage for some very elegant work on the influence of defect structure, adsorption, and nucleation processes in electrodeposition. Among other accomplishments, he provided the first demonstration of the independence of the reversible potentials for Cu/Cu+/Cu2+ ion transfer reaction from the orientation of the Cu crystal surface, in contrast to its well-known anisotropy of the pzc and double layer charging. In addition to this cutting-edge experimental work, Ugo was the first to conduct Monte Carlo simulations of the influence of step motion and two-dimensional island nucleation on single crystal growth. The importance of his contributions did not go unnoticed, as Evgeni Budevski, a recognized leader in electrocrystallization, soon invited him for a one-month visit to his laboratory in Sofia, Bulgaria.
Ugo moved to NIST (then NBS) in 1971, and his focus changed from metal deposition to corrosion processes. Although he made several seminal contributions to the more traditional corrosion
From Martin Winter, Chairman of the Board, IBA: We are mourning a great personality, a persistent advocate of young scientists, a primary rock of the battery community, and a long-term chairman of IBA.
From Jie Xiao, Pacific Northwest National Laboratory: Ralph will always be remembered for his smile when he talked. When I was still a postdoc, I had the chance to know Ralph and discussed battery research with him when he visited Pacific Northwest National Laboratory in 2009. Ralph was very knowledgeable but also humble. He knew almost everything but was still very patient to hear from others first before he provided his comments. Ralph never hesitated to praise others’ good work or to encourage people when they met challenges. Ralph will be dearly missed by the scientific community.
disciplines such as stress corrosion cracking, underground corrosion, and passivity, he is best known for his development of new electrochemical methods, such as impedance spectroscopy, random fluctuation or noise analysis, and their application to corrosion problems. Following his retirement from NIST in 1992, Ugo spent a year with the Federal Highway Administration, examining the effectiveness of corrosion inhibitors added to commercial highway deicing salts. He was then offered a one-year sabbatical at the Université Pierre et Marie Curie, Paris, France to conduct experimental and theoretical work on electrochemical noise. This collaboration with Francois Huet resulted in a series of papers that established the close connection between electrochemical noise and electrochemical impedance spectroscopy, and developed the theoretical framework for electrochemical noise that has contributed to its acceptance as a practical technique for corrosion monitoring both in the laboratory and in the field.
Following his sabbatical in Paris, Ugo returned to NIST as a guest researcher in the Metallurgy Division and was a major contributor to projects ranging from stress in thin films to electrodeposition of catalytic platinum alloys. Despite having formally retired from NIST in 1992, over 20% of his publications describe work performed after returning as a guest researcher in 1996. He continued to be an active contributor until the arrival of the Covid-19 pandemic in 2020. Over his career, Ugo authored or coauthored more than 120 technical papers and his work has been cited over 2500 times. He received both the Bronze (1980) and Silver (1987) Medal of the US Department of Commerce, and in 1986 he received the Blum Award from the DC section of The Electrochemical Society. In 2009, Ugo was elected Fellow of both ECS and AAAS, the latter for “pioneering contributions to experimental and theoretical studies of metal surfaces and corrosion properties.”
Over several generations, Ugo’s colleagues have described him as super smart, self-effacing, and a mentor to many, whether the work was fundamental or applied. Although he was very passionate about his science, he shunned the limelight and had an aversion to selfpromotion. At the same time, Ugo’s interests reached well beyond the lab. He was a student of languages and fluent in many, an avid bird watcher, history buff, and an accomplished sailor, which included, amongst other adventures, a voyage across the Atlantic Ocean with a small group of friends. In his life choices and professionally, he served as a perfect example of the career advice generally given to
30 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS
young students—find something that you have a passion for, then figure out how to make a living doing it.
Ugo is survived by his wife of 70 years, Carla, his sons Bruno and Guido, their wives Theresa and Susan, grandchildren Lucy, Simon, Emilia, Cristina, and Laura, a great grandchild Maddalena (Frankie), and Italy-based nephews and nieces Francesco, Paolo,
In Memoriam ...
Vernon H. (Bud) Branneky
1927–2024

Vernon H. (Bud) Branneky, the former Executive Secretary of The Electrochemical Society (ECS), passed away on March 30, 2024, at the age of 97. He had a distinguished career working primarily in the nonprofit association industry, spending his last 22 years with ECS before retiring in 1991. Bud was appointed as only the third Executive Secretary (now Executive Director) of ECS and shepherded the Society into the modern age.
V. H. Branneky, generally known as Bud, was born in St. Charles, MO, in 1927. After graduating from high school, he enlisted in the Army Specialized Training Program and was sent to the Pacific Theater where he served as Sergeant Major of the 5th Replacement Depot in Luzon, Philippines. Upon his return in late 1946, he entered Elmhurst College and graduated with a BA cum laude in June of 1949. The following June, Bud received his MBA in Financial and Marketing Management from Washington University.

Beatrice, Renato, Valentina, and Silvia. A memorial website has been established by the family at https://www.mykeeper.com/profile/ UgoBertocci/ where photos, comments, and stories can be shared.
This remembrance was contributed by Gery Stafford and Thomas P. Moffat, National Institute of Standards and Technology.
In April 1969, Bud joined the staff of The Electrochemical Society as Assistant Executive Secretary. On September 1, 1975, upon Ernest G. Enk’s retirement, he was appointed Executive Secretary. During his term of service, Bud led initiatives that grew the worldwide reputation of ECS meetings and publications. Applying his early career financial experience, he was responsible for securing the Society’s long-term financial stability.
A leader in the association management industry, Bud served in leadership positions with the New Jersey Society of Association Executives, the Federation of Materials Societies, and the Council of Engineering and Scientific Society Executives (CESSE). In 1981, he was elected to the Board of Directors of CESSE where, in 1985, he was elected Vice President and then from 1986 to 1987 served as President.
“Bud was an astute observer of people. He also understood and defended the values that kept the Society dynamic through periods of deep change. You could depend on Bud to make the common-sense remark that would often become the seedbed for solving the thorny problem at hand. ˮ
Former ECS President (1985–1986) Richard Alkire said this about Bud’s service at ECS, “Bud was an astute observer of people. He also understood and defended the values that kept the Society dynamic through periods of deep change. You could depend on Bud to make the common-sense remark that would often become the seedbed for solving the thorny problem at hand.”
Bud’s early professional career was spent in the Saint Louis area where he worked in the financial and cost analysis field for 10 years. Before changing his career direction, he became a Senior Financial Planner for McDonnell Aircraft. After 1960, Bud worked exclusively with nonprofit organizations. He was Controller of the United Church Board for World Ministries; Secretary-Treasurer of the Radio Free Europe Fund; and Assistant Executive Secretary of the Metallurgical Society of the American Institute of Mining, Metallurgical, and Petroleum Engineers (AIME).
Roque Calvo, former ECS Executive Director (1991–2018), expressed his gratitude, “Bud hired me in 1980 right out of college to work as the ECS Accounting Supervisor. It was the beginning of a relationship that started with him as my boss, then my mentor, and finally, it evolved into a lifetime friendship. His support led to my appointment as the fourth Executive Director of ECS. Bud’s mentoring was the foundation that aided my 27 years of service in that position. He brought me to ECS and launched me on a wonderful 38-year career path at the Society. I was very fortunate to have Bud as a mentor and friend and will miss him very much.”
In 1991, Bud was awarded Honorary Membership in The Electrochemical Society in recognition of his outstanding contributions and substantial service to the Society.
Genelle (nee Phillips), Bud’s wife of 66 years, predeceased him. He is survived by his two daughters, Jane Branneky and SuAnne Aune, both of whom pursued business careers like their father.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 31
Genelle Branneky, Bud’s wife of 66 years


Reports from the Frontier
edited by Scott Cushing, Interface Contributing Editor
This feature is intended to let ECS award-winning students and post-docs write primary author perspectives on their field, their work, and where they believe things are going.
This month we highlight the work of Sanjay Choudhary, the 2023 winner of the Corrosion Division’s Morris Cohen Graduate Student Award, and KyuJung Jun, the 2023 Battery Division Student Award Winner.
The Need for a Better Understanding of Passive Films
by Sanjay Choudhary
Passive film is a cornerstone that enables the utilization of metallic materials across myriad technological applications in our society.1 However, the breakdown of this protective layer can result in localized corrosion, imperiling the ability of metals and alloys to fulfill their intended functions, which can lead to catastrophic failures. Metallic materials like Fe-based stainless steels and Ni-based Inconel have emerged as crucial structural components in critical settings owing to their exceptional corrosion resistance, attributed to the presence of nanometer-thin Cr2O3 surface films. However, the development of most corrosion-resistant alloys has largely been empirical rather than stemming from a comprehensive understanding of the underlying phenomenon. For instance, the critical concentration of Cr in stainless steels for achieving passivity was stumbled upon during investigations into its effects on wear resistance.2 Similarly, the beneficial effects of secondary alloying elements, including Mo and N, on Cr-based passivity were realized serendipitously through curiosity-driven experiments. These unforeseen discoveries led to the development of a diverse array of corrosion-resistant alloys that have profoundly shaped our metal-based civilization. However, despite decades of research, several fundamental aspects of Cr-based passivity, including its growth and breakdown mechanisms, remain elusive, highlighting the ongoing need for deeper exploration and understanding in the field of passivity.
From the aerospace and automotive to the electronics and energy sectors, there is a relentless pursuit of alloys that offer superior strength, corrosion resistance, thermal stability, and other tailored properties. This demand stems from the desire to optimize efficiency, durability, and sustainability in manufacturing processes and end products. The push toward green technologies and stringent environmental regulations necessitates the development of alloys that minimize resource consumption, reduce emissions, and enhance recyclability. Recent advances in metallurgy have opened new avenues for alloy design, enabling the creation of novel compositions and microstructures with unprecedented properties. With the development of advanced metallic materials such as multiprincipal element alloys3 and additive-manufactured alloys,4 which boast intricate compositions and microstructures, understanding the nuances of passive film formation and breakdown becomes paramount.
A crucial pathway to better understanding involves research aimed at uncovering how the properties of passive films contribute
to the passivity and eventual breakdown. This article delves into significant advances in grasping the processes of nucleation, growth, and breakdown of passive films, employing conventional electrochemical techniques alongside ex-situ and advanced in-situ film characterization methodologies. By highlighting the unresolved queries, a perspective on modern tools that hold promise in addressing these lingering questions is presented.
Passivity is inherently a kinetic phenomenon, yet it is imperative to acknowledge its thermodynamic aspects as they provide crucial insights into the conditions conducive to passivation. A fundament of corrosion science, the potential-pH diagram, also known as the Pourbaix diagram, serves as a valuable tool delineating theoretical domains for corrosion, immunity, passivation, and transpassivation.5 Fig. 1a depicts the potential-pH diagram of Cr, outlining the regions where a corrosion product film (i.e., Cr2O3) forms; however, the protective nature of this film hinges on its physical and chemical
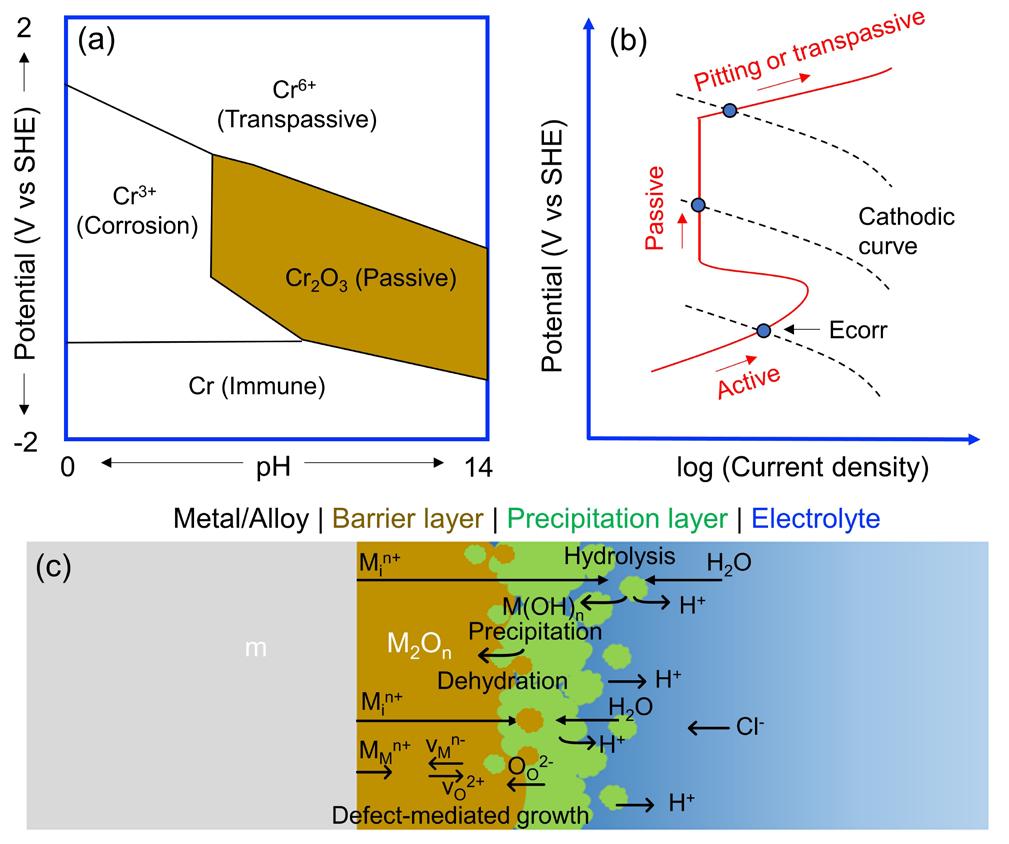
32 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Fig. 1. Schematic representation of (a) potential-pH diagram of Cr, (b) potentiodynamic polarization curves including anodic (red line) and cathodic (black dashed lines) domains, and (c) a typical structure and growth mechanisms of passive film.
properties. Assessment of the film’s protectiveness against an electrolyte typically involves anodic potentiodynamic polarization. Fig. 1b illustrates an ideal anodic polarization curve (red line), indicating an initial active domain at lower anodic potential, followed by the onset of passivity marked by a sudden decrease in anodic current density that stabilizes at a low value despite a further increase in anodic potential. Nevertheless, at sufficiently high applied potentials, the passive film may experience localized breakdown (pitting) or transpassive dissolution (transition into soluble species). The free corrosion potential (Ecorr) is determined by the balance between anodic and cathodic kinetics; thus, while a material may be capable of exhibiting passivity, its corrosion rate depends on the intersection of the cathodic polarization curve with the anodic curve. In essence, therefore, both the existence and the stability of the passive film are contingent upon the cathodic kinetics at the surface.
The passive current density and the pitting/breakdown potential serve as decisive parameters for evaluating the barrier properties of passive films, which are then correlated with their chemical, physical, and electronic characteristics. Consequently, electrochemical testing, in conjunction with equilibrium thermodynamics, is employed alongside a variety of ex-situ and often in-situ film-characterization techniques to rationalize the recorded electrochemical response. Across various methodologies, it has been consistently confirmed that passive films typically exhibit a bi-layer structure comprising an outer precipitation layer enriched with hydroxides/oxyhydroxides and an inner oxide-rich barrier layer that is solely credited for the passivity in most cases.6 The barrier layer is commonly reported to be nano-crystalline and can consist of multiple oxides depending on the metal and formation conditions.6,7
Among the key properties studied, the average composition and thickness of the film have been extensively investigated, followed by electronic properties, whereas crystallinity remains relatively less explored (Fig. 2a). Film composition is predominantly analyzed using X-ray photoelectron spectroscopy (XPS), with less utilization of other spectroscopic tools such as auger electron spectroscopy (AES), ion scattering spectroscopy, and time-of-flight secondary ion mass spectroscopy.6,8,9 Film thickness is estimated through methods that include ellipsometry, coulometry, electrochemical impedance spectroscopy, sputtering treatments in spectroscopic methods, and transmission electron microscopy (TEM).8,10,11 Electronic properties are assessed using techniques like Mott-Schottky analysis12 and photo-electrochemistry methods,13,14 while crystallinity has been investigated through scanning tunnelling microscopy (STM),15,16 atomic force microscopy (AFM),17 and TEM. Nevertheless, most studies conducted to date involve ex-situ film characterization, performed after the sample is withdrawn from the solution, thereby exposing the film to air and halting electrochemical control. Consequently, the film may undergo changes before, and often during, characterization. For instance, ex-situ characterization
often reveals a reduced fraction of hydroxide/water content in the film due to dehydration following exposure to air.18 Furthermore, highly dynamic interfacial phenomena, including the onset of passivity and localized breakdown of the film, cannot be adequately examined using ex-situ methods. Hence, to comprehensively study the formation mechanisms and characteristics of passive films, in-situ investigations under controlled atmospheric conditions have been conducted for various metals and alloys.19
In-situ TEM analysis of the early air oxidation of Al has unveiled that atomic terraces with high surface energy play a pivotal role in fostering the heterogeneous nucleation of oxide islands, which subsequently grow laterally toward each other, culminating in the formation of a 3D amorphous oxide layer. Conversely, in aqueous media, in-situ studies conducted using STM demonstrate that the adsorption of hydroxide ions precedes the 3D growth of passive films, generating 2D overlayer structures of adsorbed hydroxyl groups that serve as structural precursors for the growth of a nano-crystalline barrier layer.7 The film growth phenomenon in aqueous media is elucidated through two mechanisms: the dissolution-precipitation mechanism and the defect-mediated growth mechanism.21 The former involves the hydrolysis of metal cation interstitials being drawn out through the film due to a high electric field, followed by the precipitation of hydroxides, oxyhydroxides, or oxides at the barrier layer/electrolyte interface, ultimately governed by chemical equilibrium. On the other hand, the defect-mediated growth of the barrier layer requires the generation, migration, and annihilation of ionic defects such as cationic and anionic vacancies, intertwined with the semiconducting behavior of the film, typically explored using the Mott-Schottky method.21 However, despite its utility, the notion of the dynamic nature of the film-electrolyte interface has prompted criticism of the method,22 leading to its decreased utilization in recent years (Fig. 2a). Nevertheless, both ex-situ and in-situ methods confirm the linear increase in film thickness with increasing applied anodic potential in the passive domain, with the oxidation rate of metals and alloys exponentially decreasing as film thickness increases during potentiostatic conditions. This relationship is often explained by physicochemical models focusing on defect-mediated growth, although the transition of hydroxide into oxide has been observed using XPS and STM during film electrochemical growth.5 Recent findings using atomic spectroelectrochemistry (ASEC) have unveiled that the dissolution rate, although minor compared to the oxidation rate during oxide growth, decreases linearly with a decreasing oxidation rate.11 Consequently, there is a continual evolution of metal concentration at the film-electrolyte interface until it reaches a steady state during potentiostatic conditions. This underscores the need for a comprehensive growth model to incorporate non-equilibrium thermodynamics and the interplay between the precipitation layer and evolving film properties.
(continued on next page)
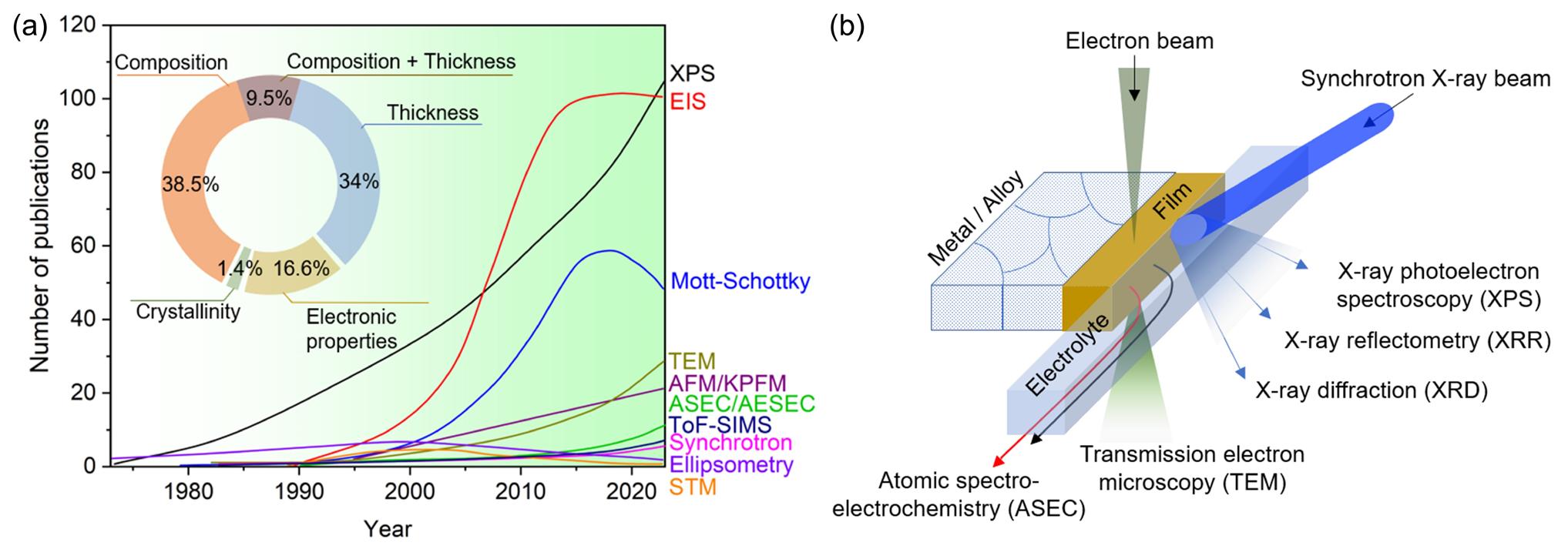
Fig. 2 (a) Research publications on passive film utilizing both traditional and modern characterization methodologies as extracted from Scopus®, for the period of 1973 to 2023, and (b) Illustrative depiction of a few contemporary in-situ film characterization techniques, chosen for their potential to enhance comprehension of passive films.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 33
Choudhary and Jun
(continued from previous page)
The evolution of film properties, encompassing chemical heterogeneity and defects during growth, holds critical importance in understanding the breakdown mechanism.7 Various film breakdown theories, such as the Cl- penetration,24 localized thinning,25 dielectric breakdown,26 and the point defect model,27 emphasize specific defects as key contributors. However, there remains a scarcity of research and comprehensive understanding regarding defects and heterogeneities within the passive film. Studies conducted using insitu STM and atomic force microscopy (AFM) have revealed that the barrier layer of passive films exhibits a nanocrystalline structure with grain sizes ranging from 30 nm to 100 nm.7 This variation in grain sizes contributes to lateral variations in film thickness, resulting in even a single crystal surface displaying a polycrystalline film with numerous crystallographic defects such as grain boundaries and voids. For alloys, passive films demonstrate compositional heterogeneity both laterally and vertically, as observed in the case of stainless-steel surfaces where clusters of Fe2O3 were found within the Cr2O3 nanocrystalline matrix. Additionally, study utilizing conductive-AFM has revealed variations in electronic properties among Cr(III)-based oxide grains within the film,28 likely due to the formation of Cr(III)-depleted oxide (FeCr2O4) phases. Similar lateral variations in electronic properties have been observed in passive films on pure Ni using scanning tunnelling spectroscopy,29 which have been attributed to the differing surface density of states at grain boundaries induced by oxygen vacancies. A comprehensive theory of the breakdown of nano-matrix passive films remains elusive due to a lack of understanding regarding the severity of each heterogeneity and defect.
While STM and AFM provide valuable nanostructural insights, their “scanning” nature limits their ability to capture extremely dynamic events associated with pit initiation. Hence, alongside high spatial resolution, time-resolution information is crucial for understanding breakdown events at the nanoscale. In this context, in situ liquid-cell TEM holds promise, although further development is necessary to study the growth and breakdown of nanometric films.30 Recent applications of in-situ TEM have captured dielectric breakdown at the metal-oxide interface under high electric fields,31 which are also expected in passive films on metals and alloys during anodic polarization in aqueous media.32 TEM could facilitate elemental mapping in the film during its growth, providing critical insights to validate breakdown localization.
While research efforts continue, the understanding of the susceptibility of passive films has fostered a notion that localized breakdown is inevitable, emphasizing the significance of repassivation for maintaining passivity. It is now widely acknowledged that the so-called “pitting” potential does not denote the critical potential required for localized film breakdown; rather, it signifies the condition where a damaged site cannot undergo repassivation.11 Despite this recognition, many studies on corrosion-resistant alloys still correlate the pitting potential with the overall composition of the passive film rather than considering the kinetics of film regeneration. There is a pressing need for a deeper comprehension of how alloy composition and microstructure influence the development and distribution of chemical heterogeneities and crystallographic defects within these films. Concurrently, investigation into film composition should prioritize understanding the thermodynamic stability and dissolution kinetics of individual film constituents, particularly in the context of changes occurring at defective or ruptured film sites, such as acidification and the accumulation of aggressive anions like Cl-. Notably, the migration of Cl- increases with metal dissolution,33 underscoring the importance of oxides that rapidly form but dissolve slowly in acidic solutions, such as single-cationic oxides like MoO3, WO3, and TiO2, which are known to facilitate repassivation due to their stability in acidic environments.34 Recently, certain oxide compounds have demonstrated relatively higher thermodynamic stability and slower dissolution kinetics than their parent singlecationic oxides in specific environments,35 suggesting avenues for further exploration of alloy chemistries. A refined understanding of the interplay between the growth and dissolution kinetics of oxides
concerning applied potential, pH, and exposure duration could pave the way for a “passive film-by-design” paradigm. Within the realm of complex multi-elemental alloys, the barrier layer is believed to adopt a non-stoichiometric solid solution,36 a composite of multiple oxides (including single-element oxides37 and/or oxide compounds), or infiltrated with unoxidized metals38 and non-equilibrium solutes,39 dictated by the alloy composition and the conditions under which it forms. In this scenario, conducting in-situ film composition analysis is crucial, as exposure to air and dehydration have the potential to induce alterations in composition via phase transformations. Therefore, in-situ methods, including synchrotron X-ray techniques for film composition analysis,40,41 ASEC for dissolution kinetics,42,43 and liquid-cell TEM for spatially resolved compositional and structural insights,30 employed either individually or in combination, hold promise for advancing corrosion science beyond its current boundaries (Fig. 2b).
©The Electrochemical Society. DOI: 10.1149/2.F04242IF
About the Author

Sanjay Choudhary, Postdoctoral Research Associate, Department of Materials Science and Engineering, University of Virginia
Research Interests: Passivity and passivity breakdown of metallic materials
Education: BTech in Metallurgical Engineering (National Institute of Technology, Raipur), MTech (Indian Institute of Technology, Kanpur) and PhD (Monash University) in Materials Science and Engineering
Work Experience: Researcher at TATA Steel India Limited, Jamshedpur (India), 2016 – 2018; Postdoctoral Research Associate at the University of Virginia, May 2022 – present Pubs & Patents: 23 peer-reviewed publications, 2 granted patents, 6 conference presentations (including 1 award talk and 1 invited talk), 1 invited webinar; h-index: 13
Awards: 2023 ECS Morris Cohen Graduate Student Award
Work with ECS: ECS Early Career Member Website: https://engineering.virginia.edu/labs-groups/robert-kellyresearch-group/people https://orcid.org/0000-0003-0296-9811


References
1. D. D. Macdonald, Pure Appl Chem, 71, 951 (1999).
2. H. M. Cobb, The Life of harry Brearley (1871–1948), in: History of Stainless Steel, ASM Internation (2010).
3. N. Birbilis, S. Choudhary, J. R. Scully, and M. L. Taheri, npj Mater Degrad, 5, 14 (2021).
4. G. Sander, J. Tan, P. Balan, et al., Corrosion, 74, 1318 (2018).
5. M. Pourbaix, Corros Sci, 14, 25 (1974).
6. H. H. Strehblow, Electrochim Acta, 212, 630 (2016).
7. V. Maurice and P. Marcus, Prog Mater Sci, 95, 132 (2018)
8. D. Landolt, S. Mischler, A. Vogel, and H. J. Mathieu, Corros Sci, 31, 431 (1990).
9. A. Rossi, C. Calinski, H.‐W Hoppe, and H. H. Strehblow, Surf Interface Anal, 18, 269 (1992).
10. N. Sato and M. Cohen, J Electrochem Soc., 111, 512 (1964).
11. S. Choudhary, R. G. Kelly, and N. Birbilis, Corros Sci, 230, 111911 (2024).
12. K. Azumi, J Electrochem Soc, 134, 1352 (1987).
13. J. S. Kim, H. S. Kwon, Mater Sci Forum, 289, 1119 (1998).
14. A. Seyeux, V. Maurice, L. H. Klein, and P. Marcus, J Electrochem Soc, 153, B453 (2006).
15. A. Machet, A. Galtayries, S. Zanna, et al., Electrochim Acta, 49, 3957 (2004).
16. Y. Shi, L. Collins, N. Balke, P.K. Liaw, and B. Yang, Appl Surf Sci, 439, 533 (2018).
17. W. E. O’Grady, J Electrochem Soc, 127, 555 (1980).
34 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
18. G. G. Long, J. Kruger, D. R. Black, and M. Kuriyama, J Electrochem Soc, 130, 240 (1983).
19. L. Nguyen, T. Hashimoto, D. N. Zakharov, et al., ACS Appl Mater Interfaces, 10, 2230 (2018)
20. D. D. Macdonald and M. Urquidi-Macdonald, J Electrochem Soc, 137, 2395 (1990).
21. D. D. Macdonald, Electrochim Acta, 56, 1761 (2011).
22. Y. Ren and G. Zhou, J Electrochem Soc, 164, C182 (2017).
23. S. Choudhary, S. Thomas, D. D. Macdonald, and N. Birbilis, J Electrochem Soc, 168, 051506 (2021).
24. U. R. Evans, J Chem Soc, 1020 (1927).
25. P. Marcus, V. Maurice, and H. H. Strehblow, Corros Sci, 50, 2698 (2008)
26. N. Sato, Corros Sci, 3, 1 (1990).
27. L. F. Lin, C. Y. Chao, and D. D. Macdonald, J Electrochem Soc, 128, 1194 (1981).
28. T. Massoud, V. Maurice, L. H. Klein, and P. Marcus, J Electrochem Soc, 160, C232 (2013).
29. V. Maurice and P. Marcus, Electrochim Acta, 84, 129 (2012)
30. A. Kosari, H. Zandbergen, F. Tichelaar, et al., Corrosion, 76, 4 (2020).
31. W. A. Hubbard, J. J. Lodico, H. L. Chan, M. Mecklenburg, B. C. Regan, Adv Funct Mater, 32, 2102313 (2022).
32. Z. Szklarska-Smialowska, Corros Sci, 44, 1143 (2002).
33. J. R. Galvele, J Electrochem Soc, 123, 464 (1976).
34. P. Marcus, Corros Sci, 36, 2155 (1994).
35. S. Choudhary, S. O’Brien, Y. Qiu, et al., Electrochem Commun, 125, 106989 (2021).
36. K. F. Quiambao, S. J. McDonnell, D. K. Schreiber, et al., Acta Mater, 164, 362 (2019).
37. S. Choudhary, Y. Qiu, S. Thomas, and N. Birbilis, Electrochim Acta, 362, 137104 (2020).
38. Y. Qiu, S. Thomas, M. A. Gibson, et al., Corros Sci, 133, 386, (2018).
39. X. X. Yu, A. Gulec, Q. Sherman, et al., Phys Rev Lett, 121, 145701 (2018).
40. J. Eidhagen, A. Larsson, A. Preobrajenski, et al., J Electrochem Soc, 170, 021506 (2023).
41. M. Långberg, C. Örnek, F. Zhang, et al., J Electrochem Soc, 166, C3336 (2019).
42. S. Choudhary, K. Ogle, O. Gharbi, S. Thomas, and N. Birbilis, Electrochem Sci Adv, e2100196 (2021).
43. K. Ogle, Corrosion, 75,1398 (2019).
Understanding Complex Correlations in Lithium Superionic Conductors
by KyuJung Jun
Over the past decade, all-solid-state batteries have emerged as one of the most promising next-generation battery technologies for improving safety and energy density.1 All-solid-state batteries provide a safer alternative to conventional lithium-ion batteries by replacing the flammable organic liquid electrolytes with inorganic solid electrolytes. One main challenge in adopting solid electrolytes is that lithium-ion diffusion in an inorganic solid medium is typically slower than that in a liquid medium, which translates to higher internal resistance in the battery. One of the most important criteria for solid electrolytes is to exhibit high ionic conductivities comparable to those of their liquid counterparts.
Various mechanisms have been proposed for enhancing the lithiumion conductivity of inorganic materials.2,3 An important consideration has been to understand how the motion of lithium can be accelerated by the motion of other lithium ions, cations, anions, or anion groups. The concerted motion of ions has frequently been discussed as a mechanism for lowering the activation barrier for the hopping of Liions.4 The correlation between the motion of anion groups such as PS4, PO4, and SO4 has been of specific interest. Although these anion groups do not diffuse, it is possible that their rotational motion assists the migration of Li ions.5 This has been metaphorically referred to as a paddlewheel effect. Although the definition of the paddlewheel effect varies among researchers, it can be summarized as follows: the broad definition refers to the beneficial effect of rotation of anion groups on lithium-ion diffusion, whereas the literal definition refers to the lithium-ion transport mechanism, where each lithium-ion hop is supported by the rotation of nearby anion groups, which resembles the motion of a paddlewheel (Fig. 1a).
In this article, we provide a pedagogical overview of the discrete event-detection-based analysis of correlations in lithium-ion conductors and how they were used in a recent article6 to analyze the existence of the paddlewheel effect in lithium superionic conductors. We present our perspective on how such an analysis can be extended to rigorously understand the various atomistic motions that are intertwined to affect lithium-ion transport in these technologically important materials.
Discrete Event Detection Algorithm
Detecting lithium-ion hop events in molecular dynamics (MD) trajectories can be simply performed by searching for instantaneous displacements over a minimum distance (to distinguish them from vibrations), for example, 3 Å within a picosecond timescale. However, detecting the rotational motion of anion groups (e.g., PS4 groups) from the MD trajectory is more complicated. An elegant approach is to harness the fact that these anion groups behave as rigid bodies up to high temperatures and use a four-dimensional physical quantity, called quaternions, to track their orientations at each MD snapshot.7 According to Euler’s rotation theorem, any rotational displacement of a rigid body can be reduced to a single rotation angle with respect to some axis. Once the orientation of each anion group at each snapshot is represented as a quaternion, we can search for any instantaneous change in the quaternions within a short timescale,8 which corresponds to a rotation event. This method naturally provides a complete description of a rotation event, including the angle, time, and axis.
Comparison of Rotation and Hop Frequency
With quaternion-based detection of the rotational motion of anion groups, the most straightforward analysis is to compare the absolute frequency of the rotational motion and the frequency of lithium-ion hops. In Fig. 1b, the frequency of the lithium-ion hops (circles) and the frequency of anion-group rotations as a function of the rotation angle (squares) are compared for amorphous Li3PS4 (blue) and hightemperature (HT) Li2SO4 (gray). In amorphous Li3PS4, large-angle rotation events over 60° occurred orders of magnitude less frequently than lithium-ion hopping, directly indicating that the paddlewheel effect in a literal definition is not the Li-ion transport mechanism in play.
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 35
Choudhary and Jun
(continued from previous page)

fullrotationofPS4 (paddlewheeleffect)
P(rotation),Poisson
P(rotation)
P(rotation|hop)
P(rotation|no-hop)
amorphousLi3PS4 (1.8g/cm3)
as a function of the time window between the hop and the rotation. Even though 120° rotation events occur as frequently as lithiumion hops, P(rot | hop) is less than 0.1. This means that more than 90% of the lithium-ion hops occurred without any temporally or spatially adjacent SO4 group rotation, clearly proving that the large paddlewheel-like rotation is not the lithium-ion diffusion mechanism in HT-Li2SO4
Remarks and Perspectives
Such discrete event detection algorithms applied to MD trajectories can be used to analyze other important correlations in superionic conductors. For example, the correlated, cooperative, or concerted motion of lithium ions has been suggested as a characteristic feature of various lithium superionic conductors.4 The Van Hove correlation function9 or Onsager transport coefficients10 provide information on the correlations between different particles, but they are averaged over space and time. Using discrete event-based analysis, it is possible to compute the fraction of concerted multi-ion hops and single-ion hops with spatial and temporal considerations.
In some systems where two types of cations are mobile, the diffusion of one species may trigger the accelerated motion of the other species. For example, while it is challenging to find materials where multivalent Ca2+ are mobile,11 it has been suggested that in oxides with both Na+ and Ca2+, the existence of Na+ in the diffusion channel may facilitate the diffusion of Ca2+12,13. The correlation between two different mobile cations in such systems can be rationalized using discrete event-detection algorithms. Finally, this algorithm can be utilized to detect the phase transitions between polymorphs that occur via the collective rotation of the anion groups. For example, it has been reported that Li3PS4 polymorphs undergo phase transitions accompanied by a collective rotation of the PS4 groups.14
As it is challenging to achieve Å-level spatial resolution with pslevel temporal resolution in characterization methods, experimental methods that aim to provide insight into such correlations inherently incorporate either temporal or spatial averaging or both. Consequently, it is challenging to experimentally demonstrate the atomistic mechanism of correlated motions. We anticipate that atomistic simulations will continue to serve as an invaluable tool to capture both temporal and spatial correlations without averaging, providing a clear picture of the correlation effects in lithium-ion transport.
Acknowledgement
The author gratefully acknowledges support from the Kwanjeong Educational Foundation scholarship.
Fig. 1. (a) Illustration of literal paddlewheel effect. (b) Event frequency of hops (circles) and rotations (squares) as a function of rotation angle for amorphous Li3PS4 (1.8 g/cm3) (blue) and high-temperature Li2SO4 (gray).
(c) Probability of 120° rotation of an SO4 group under the condition that its nearest neighboring lithium underwent a hop at dt = 0. (Adapted from Ref. 6)
Capturing Temporal and Spatial Correlation Between Distinct Events
In HT-Li2SO4 (Fig. 1b, gray), the frequency of rotational motion and lithium-ion hops are similar in magnitude, suggesting that a paddlewheel effect following its literal definition may be possible. However, it is possible that both rotational motion and lithium-ion hop events occur at similar frequencies and activation energies, but with little correlation between them. To clarify the atomistic correlation between these rotational motions and lithium-ion hops, it is crucial to consider the temporal and spatial correlations between these events.
This can be achieved by calculating the conditional probabilities that connect lithium-ion hop events and SO4 group rotation events. In Fig. 1c, the probability of rotation of an SO4 group under the condition that its nearest-neighbor lithium underwent a hop at dt = 0, that is, P(rot | hop) is plotted. The spatial correlation is captured by considering only the nearest neighboring Li and SO4 groups, and the temporal correlation is captured by computing the probability
©The Electrochemical Society. DOI: 10.1149/2.F05242IF
About the Author

KyuJung Jun, Postdoctoral Associate, Massachusetts Institute of Technology
Education: BS in Nuclear Engineering (Seoul National University) with a minor degree in Materials Science and Engineering, PhD in Materials Science and Engineering (University of California, Berkeley).
Research Interests: His PhD work involved a computational understanding of lithiumion diffusion in superionic conductors using first-principles calculations, large-scale molecular dynamics, and machine-learning interatomic potentials. He discovered various structural features that allow fast Li-ion transport, which led to the discovery of numerous novel structural frameworks for superionic conductors. He also elucidated the types of anion-group rotational motions that exist in lithium-ion conductors and revealed how they may be related to lithium-ion diffusion.
Awards: ECS Battery Division Student Research Award (2023), Materials Research Society (MRS) Graduate Student Award, Silver (2022 fall).


https://orcid.org/0000-0003-1974-028X
36 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Hop 20406080 100 120 RotationAngle(°) 10 3 10 2 10 1 100 Frequency(1/ps/atom)
HT-Li2SO4 a b c
HT-Li2SO4 phaseat800K 120° Probabilityofrotationin±dt -2 0 2 1 -1 dtw.r.t.event(ps) 0.0 0.2 0.4 0.6 0.8 1.0
800K
References
1. J. Janek and W. G. Zeier, Nat Energy 1, 16141 (2016).
2. Y. Xiao, K. Jun, Y. Wang, et al., Adv Energy Mater 11, 2101437 (2021).
3. K. Jun, Y. Sun, Y. Xiao, et al., Nat Mater 1 (2022).
4. X. He, Y. Zhu, and Y. Mo, Nat Commun 8, ncomms15893 (2017).

About the Editor
Scott Cushing, Assistant Professor of Chemistry, Caltech Education: BS in Physics, emphasis in Material Science and Chemistry and PhD in Physics, under Nick Wu and Alan Bristow (West Virginia University).



W E B I N A R S E R I E S S H O W C A S I N G D I S T I N G U I S H E D S P E A K E R S A N D E C S C O M M U N I T Y M E M B E R S T H E E L E C T R O C H E M I C A L S O C I E T Y ELECTROCHEM.ORG/WEBINARS Co-sponsored by ECS and partners at PhysicsWorld.com Upcoming and past webinars are at
Electrochemistry in Action:
edited by Christopher L. Alexander, Interface Contributing Editor
Iron and Steel Manufacturing
by Trevor Braun, Colleen Wallace, Quoc Pham, and Sandeep Nijhawan
Steel is one of the most manufactured materials in modern society, with 1.9 billion metric tons produced annually for use in building materials, vehicles, wind-turbines, and appliances, among many other applications. As you might expect for something so ubiquitous, the technology used to manufacture steel is also quite mature, having been first identified over 4,000 years ago and heavily industrialized in the 19th century. The general approach is to mine iron ore from the earth’s crust and refine that ore to metallic iron (i.e., ironmaking) which is then combined with carbon and other elements to make steel products (i.e., steelmaking). However, conventional steel manufacturing relies primarily on carbon-based fuels, such as coal, to create the high temperatures (≈ 1600°C) required for the process and can emit as much as 2.2 tons of CO2 per ton of crude steel produced.1 The iron ore reduction step accounts for 90% of CO2 emissions associated with steel production. The heightened effort to decarbonize industrial process and reverse climate change is putting pressure on this 600+ year-old technology to shift to lowcarbon alternatives, especially considering that the steel industry is responsible for ≈ 7% of all global CO2 emissions annually.1 Fig. 1 summarizes the global market share and CO2 emissions profile for the leading steelmaking technologies discussed in the next section.
Current State of Steel Manufacturing
Blast furnace-basic oxygen furnace – Traditional steel manufacturing is performed by the two-step blast furnace-basic oxygen furnace (BF-BOF) process (Fig. 2), which accounts for 73% of global steel production.2 Here, mined iron ore FexOy is converted
to metallic iron Fe0 in the blast furnace using coal/coke as a reductant. Impurities present in the ore are removed via a molten mixture called “slag,” a byproduct of smelting that contains a mixture of metal oxides. The molten iron is then fed into the basic oxygen furnace along with scrap metal and oxygen, reducing the carbon content of the fed iron (a process which emits CO and CO2) while producing the final steel product. This steel manufacturing route emits 2.2 tons of CO2 per ton of crude steel.1
Electric arc furnace (EAF) steelmaking – The most mature technology for lower emission steelmaking involves recycling steel “scrap” in an electric arc furnace (EAF), which currently accounts for 22% of the global steel output1 (70% of the US steel output1). This process emits approximately 0.3 tons of CO2 per ton of steel. One challenge with EAF steel manufacturing is that scrap steel has several impurities (such as Cu) that are difficult to remove in the EAF and detrimental to the steel product. Thus, up to 0.4 tons of fresh ore-based-metallic (OBM) iron per ton of steel produced is necessary to dilute these impurities.3 While an EAF operating on renewable electricity can manufacture green steel and the circularity of scrap steel recycling is highly attractive for sustainability, the OBM input has a significant carbon footprint, up to 0.8 tons of CO2 per ton of EAF steel if the OBM source is iron from a blast furnace.1 Therefore, innovation toward a high iron purity and low carbon footprint ironmaking process is necessary to shift the industry into even lower emission manufacturing.
Direct reduced ironmaking (DRI) – As an alternative to the blast furnace ironmaking approach, natural gas–based direct reduced iron (NG-DRI) was developed in the 1970s and now accounts for about 5% of global steel production.4 This pyrometallurgical method
38 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
methods BF-BOF = blast
Fig. 1. (Left) market breakdown of global steel production and (right) CO2 emissions profile for primary steelmaking
furnace-basic
oxygen furnace. EAF = electric arc furnace. NG-DRI = natural gas–based direct reduced iron.
Fig. 2. Schematic of the traditional blast-furnace (BF) + basic oxygen furnace (BOF) method for ironmaking and steelmaking.
replaces coal with methane as the reductant fuel, reducing the overall carbon footprint, and relies on a newer furnace known as a direct reduced iron shaft. First, natural gas is reformed to syngas (CO and H2), which is then used to reduce iron ore to iron metal at 900 °C. The NG-DRI product is then fed into an EAF to produce steel, emitting approximately 1.40 tons of CO2 per ton of steel produced if no scrap steel is used, or, 0.74 tons of CO2 per ton of steel if the NG-DRI OBM accounts for 40% of the EAF input.1 A spin on NG-DRI for even cleaner ironmaking involves using green hydrogen (electrolytic hydrogen produced from renewable electricity) as the reductant fuel (H2-DRI). The carbon footprint of this ironmaking pathway is estimated to be as low as 0.3 tons of CO2 per ton of steel if truly 100% green hydrogen is used; the remaining emissions cost is associated with pretreatment of the iron ore.4 A significant challenge with this approach is the availability, cost, and storage of green hydrogen. Overcoming these challenges is a major effort that many in the ECS community are currently working toward.
Another challenge for direct reduced ironmaking is the diminishing availability of high-grade ores (> 67% Fe or > 95.8% Fe2O3 content by weight); a recent report by the International Iron and Metallics Association forecasts shortages in high-grade ore by the early 2030s.5 All iron ore contains some amount of gangue, primarily SiO2 and Al2O3, with higher concentrations of those impurities in lower grades of ore. The DRI furnace cannot separate out these impurities from reduced iron via slag like a blast furnace can and consequently requires higher ore grades. Otherwise, the DRI process passes the impurity problem of low-grade ores along to the EAF steelmakers. While EAFs can handle some impurities through slagging, they were not designed to handle the levels of gangue associated with lowergrade ores without significant cost and alteration to their process.
Innovation Challenges in Iron and Steel Manufacturing
The steelmaking industry, in general, has four significant challenges worth addressing:
1. Decarbonization: Reducing steelmaking’s carbon footprint must rely on electrical energy in place of carbon-based fuels, whether through DRI-H2 or direct electrolysis, and the energy input must be renewable.
2. Access to green energy: Electrifying global iron production requires 7,000 TWh of green energy annually. To put that number into context, the total amount of energy produced by renewables in 2021 was 8,300 TWh.6 Among all renewable energy resources (solar, wind, nuclear, and hydro), only intermittent renewable resources, like solar and wind energy, have the required cost, geographic availability, and ability to scale to TWhs to decarbonize the steel industry.
3. Utilization of low-grade ores: As referenced above, the known reserves of high-grade ore are dwindling and cannot support the transition away from blast furnace ironmaking. This requires new technologies that can tap into lower-grade ores and, ideally, already mined waste feedstock.
4. Production of high-purity OBMs: The good news is that EAFs are a mature technology that is already more sustainable than the traditional BOF. However, lower-purity OBM inputs increase the energy and cost of producing steel through this path. Production of high-purity OBMs makes the EAF process more efficient and therefore more sustainable.
Emerging Electrochemical Methods for Iron and Steel Manufacturing
Several recent electrochemical pathways for decarbonizing steelmaking also attack the first step of iron ore reduction to iron metal. They can broadly be categorized into three areas: (1) molten electrolysis,7,8 (2) alkaline electrolysis,9 and (3) acidic electrolysis.10 In molten oxide electrolysis (MOE), a current is passed through a solid oxide mixture (predominantly Fe2O3 with additional oxides to reduce the melting point),7 eventually liquifying the ore at 1600 °C at which point the cathode produces liquid iron and the anode produces oxygen gas. For alkaline electrolysis, the most well-developed technology involves direct electrochemical reduction of finely milled hematite ore particles in sodium hydroxide solution, producing metallic iron at the cathode and oxygen gas at the anode.9 An example of acidic electrolysis will be detailed in the next section.
Like H2-DRI technology requiring green hydrogen to truly be considered clean ironmaking, all electrochemical pathways also must rely on renewable electricity to be regarded as low emission. Currently, the US electrical grid, on average, is about 40% renewable energy (including nuclear).11 The remaining 60% of grid energy has an emissions profile of 0.334 tons CO2/MWh12 (primarily coal and NG). Assuming 5 MWh of energy is required to produce 1 ton of iron (approximately the energy usage of the NG-DRI route13), then electrochemical ironmaking from grid energy would have a carbon footprint of 1.67 tons of CO2 per ton of Fe. This carbon footprint is only a marginal reduction relative to the BF approach (≈ 2.0 tons of CO2 per ton of Fe), and thus, electrochemical iron decarbonization technologies require access to renewable energy to drive carbon emissions closer to zero.
Electra – Clean Iron for Green Steel
Electra is a start-up company that has developed and patented an acid-electrolysis ironmaking process capable of dissolving iron ore in acid and extracting iron metal in a manner most similar to conventional copper or zinc electrowinning (EW) at around 60 °C.
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 39
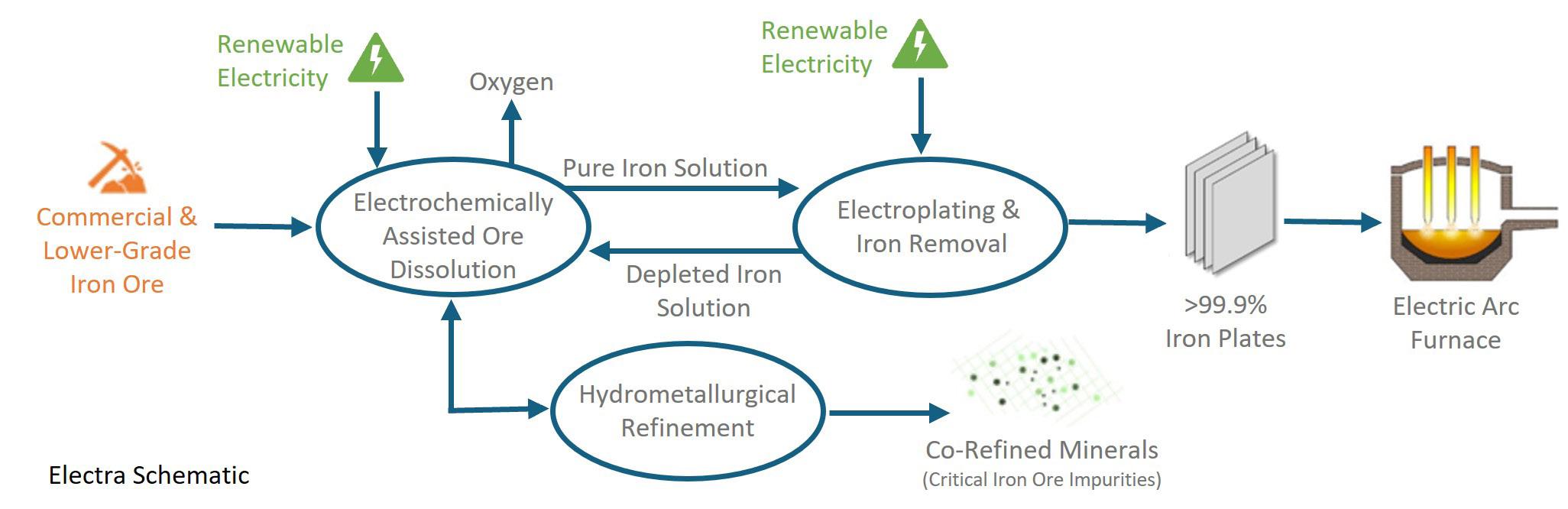
3. Schematic of Electra’s hydrometallurgical + electrochemical ironmaking process. Low-grade iron ore and electricity are combined in a two-step process to produce > 99.9% iron plates and oxygen. Co-dissolved impurities are further refined for value added products. Electra’s iron is utilized as a highpurity ore-based metallic (OBM) in a conventional electric arc furnace steelmaking process.
This overall process (shown in Fig. 3) addresses all the challenges identified above by employing a combined hydrometallurgical + electrochemical approach to refining iron ore.
Electra’s approach addresses the decarbonization challenge by implementing a low-temperature electrochemical process (60 °C) that permits intermittent operation of the ironmaking plant to sync energy consumption with renewables generation, resulting in a much lower carbon emissions profile (as clean as the input energy) than technologies that are reliant on grid energy. The use of intermittent generation and the ability to adjust energy consumption readily based on the cost of electricity (commonly referred in the utility industry as demand response) also lowers the energy operating cost for this approach. By processing low-grade ores via hydrometallurgy (instead of pyrometallurgy), the principal impurities (Al2O3, SiO2, and P) can be selectively removed from the leached ore solution with conventional and scalable separation techniques such as solvent extraction or solids precipitation routes. If separated with enough selectivity, these byproducts can be valorized, reducing the carbon footprint of mining new minerals and creating additional value from waste. Given the magnitude of ironmaking and steelmaking, refining alumina impurities in iron ore could eliminate the need for mining and refining bauxite ore and generating red mud waste associated with conventional aluminum making. Lastly, the impurity removal process also proves critical for achieving > 99.9% iron via EW, addressing the challenge of producing high-purity OBMs for EAFs.
While Electra’s technology has proved capable of addressing each of the previously stated challenges to steelmaking, three major technical hurdles first had to be solved before applying copper or zinc acid EW technology to iron:
1. Most iron ore exists as hematite (Fe2O3), which has kinetically slow dissolution in acid.
2. Soluble iron has multiple oxidation states that are stable in aqueous solution (FeO4 2-, Fe3+, and Fe2+), whereas copper and zinc exist primarily as divalent cations.
3. Unlike copper, iron and zinc are less noble than hydrogen evolution (HER) and, unlike zinc, iron has favorable kinetics for HER.14 As a result, copper and zinc EW technologies operate efficiently in highly acidic environments whereas high-efficiency iron EW requires lower acidity.
Electra has developed propriety methods to address the first challenge for accelerating the dissolution of hematite and other low-grade iron ores that are discussed elsewhere.10 The solution to the other two challenges involves separating the electrochemical reduction process into two steps, outlined in the schematic in Fig. 4. In the first step (acid regeneration cell), dissolved ore (primarily as Fe3+) is fed to the cathode and reduced to Fe2+ while oxygen evolution (OER) occurs on the anode via the following half-cell reactions:
Fe3+ + e- Fe2+ E0 = 0.77 V
2H2O O2 + 4H+ + 4e- E0 = 1.23 V
Charge is balanced by moving protons generated at the anode through a separator to the catholyte. The acidified catholyte then dissolves more iron ore in a cascading sequence that consumes acid and concentrates dissolved iron. Electra has also demonstrated use of a low-cost, earth-abundant, and non-precious metal material for OER that is stable in acidic and oxidizing environments. In the second step (plating cell), electrolyte containing Fe2+ is split and fed into both the catholyte and anolyte compartments of the cell. On the anode, Fe2+ is oxidized back to Fe3+ and returned to the acid regeneration cell and, on the cathode, Fe2+ is reduced to iron metal typical of standard industrial electrowinning via the following half-cell reactions:
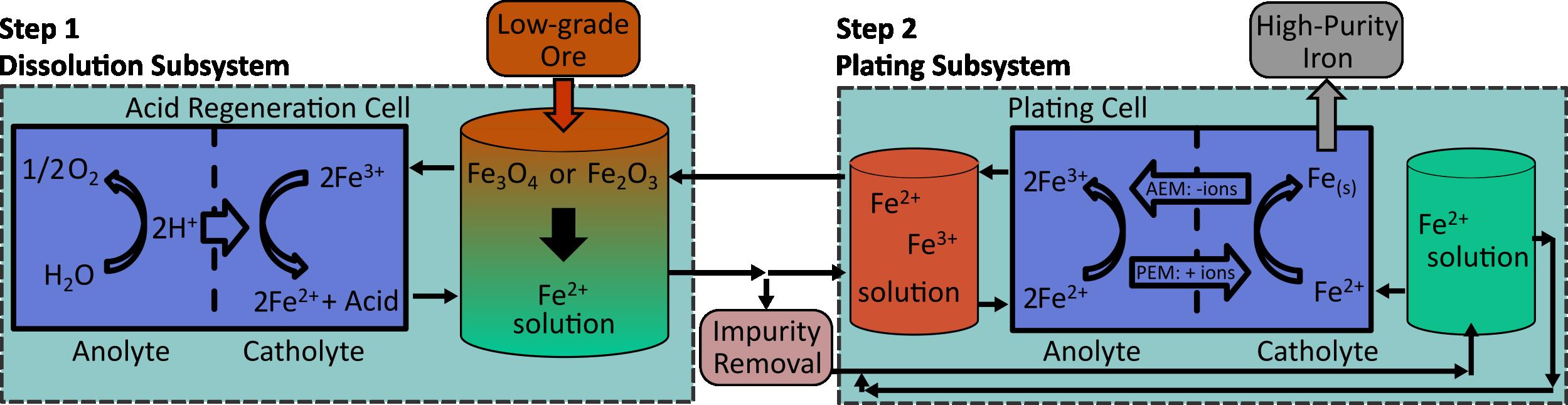
40 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Braun et al. (continued from previous page)
Fig.
electrochemical process consisting of an “Acid Regeneration Cell” in Step 1 and a “Plating Cell” in Step 2.
Fig. 4. Schematic of Electra’s two step
Fe2+ + 2e- Fe(s) Eo = -0.44 V Fe2+ Fe3+ + e- Eo = 0.77 V
This configuration is equivalent to an all-iron flow battery except it only operates in the charge direction and has a cell design such that iron is harvestable. Here, the charge is balanced by moving cations through a proton exchange membrane (PEM) or anions through an anion exchange membrane (AEM). The electrolyte fed to the cathode is first treated to remove impurities in the ore that are dissolved along with iron and detrimental to iron plating.
Electra’s two-step process solves Challenge 2 identified above by supplying only Fe2+ to the plating cell and using a separator to keep Fe3+ generated at the anode from interacting with the cathode, where it could discharge plated iron and/or reduce the faradaic efficiency of the cell. Challenge 3 is addressed by moving the acid-producing OER to an entirely different electrochemical cell, permitting the operation of the plating cell at a higher catholyte pH level than would be possible if a more traditional EW configuration were used.
There are further benefits to decoupling the three electrons necessary for iron electrowinning into a two-step process. First, electrodeposition reactions are most effective at current densities of 10 to 100 mA/cm2, whereas gas-evolving reactions, such as OER, have been commercially demonstrated at rates greater than 1000 mA/ cm2. Thus, an acid regeneration type cell can lean on engineering design aspects typical of electrolyzers and/or flow-batteries, which can reduce capital costs by running at higher currents. Additionally, the overall energy costs of operation are anticipated to be lower in the two-step configuration than if a one-step process were capable of fully reducing Fe3+ to Fe(s) against an OER anode. Thermodynamically, the minimum energy penalty is the same for either pathway; for every mole of iron plated, 0.75 moles of O2 must be evolved by OER (Fig. 5). The tradeoffs derive from the kinetic and
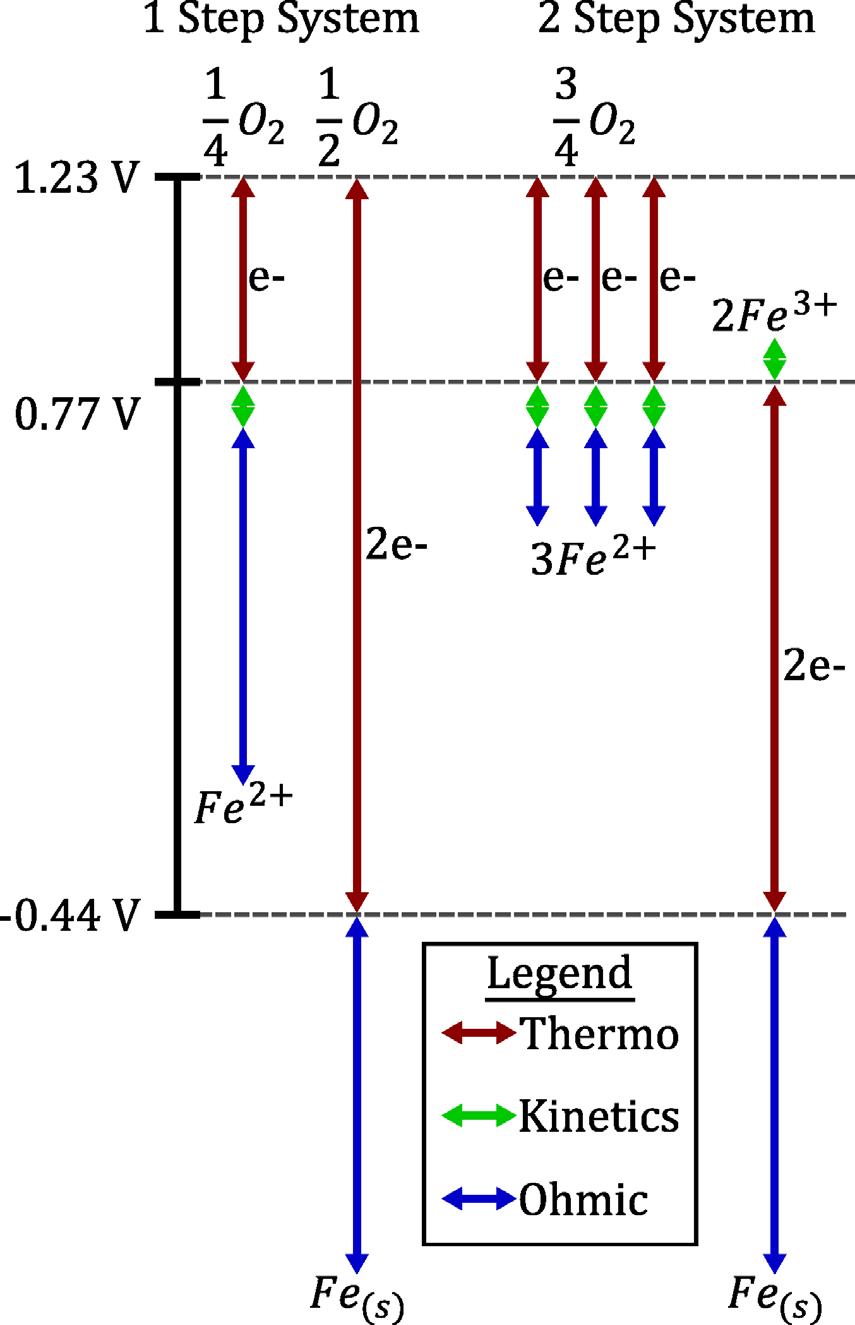
Fig. 5. Schematic qualitatively illustrating relevant overpotentials for a one-step reduction process (3 electrons transferred) from Fe3+ to Fe(s) versus a two-step process (5 electrons transferred). Kinetic overpotentials for OER and Fe deposition are excluded as they are the same for either scenario. Standard thermodynamic potentials are shown on the left axis and products from each half cell reaction are listed.
and plating tank.
ohmic overpotential differences between the two routes. The two-step process adds three kinetic overpotentials via the additional Fe3+/Fe2+ reactions; however, these overpotentials can be minimal.14 In terms of ohmic contributions, traditional plating cells require a significant anode-to-cathode gap (approximately 30–40 mm) to allow space for removing cathodes for metal harvesting, which would add substantial unnecessary ohmic losses to the reduction of Fe3+ to Fe2+ in a singlestep process. Meanwhile, the acid regeneration cell can be designed as a zero-gap cell, significantly reducing the ohmic contribution for the one-electron reduction of Fe3+ .
Electra has recently demonstrated the process described above at a pilot scale (Fig. 6). The pilot operation utilizes an already mined ore with too much phosphorus for conventional BF or DRI methods, removing the carbon and energy costs associated with mining new ores until that supply runs out. The pilot operation produces approximately two square meters of high-purity iron (60 kg per cathode), offering significant value for use in EAF steelmaking while substantially improving the sustainability and decarbonization of the ore-to-metal value chain.
In its effort to electrify and decarbonize ironmaking, Electra generally encounters challenges similar to those faced by many other industries attempting electrochemical decarbonization methods. Specifically, this these challenges include:
● Lowering the OER overpotential for non-precious metal electrodes that are also stable in acidic and oxidizing environments.
● Robust, long-lasting, and selective separators.
● Dendrite suppression and morphological control of metal electrodeposition reactions.
● Scaling systems to industrially relevant formats to make a true impact on industrial decarbonization.
The team at Electra is energized by the technological strides made since it was founded three years ago and are excited to continue scaling our process beyond the 1 m2 scale we are currently operating at in our Boulder, Colorado facility. We are also excited to continue learning from the ECS community and begin to share our progress at future meetings and publication opportunities.
© The Electrochemical Society. DOI:10.1149/2.F06242IF
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 41
Fig. 6. Photo of Electra’s pilot demonstration. In view are the plating subsystem with 1m2 cathode

About the Authors
Trevor Braun, Sr. Manager of Electrochemical Development & Test, Electra
Education: BS (Colorado School of Mines) and PhD in Chemical Engineering (University of Washington)
Research Interests: Bipolar electrochemistry for indirect deposition of materials, scanning probe electrochemical methods, characterization of electrodeposited thin films, and decarbonization of industrial processes for a sustainable future.
Work Experience: National Research Council Postdoctoral Fellow at the National Institute of Standards and Technology (NIST) before transitioning to a position as a Research Chemical Engineer. At NIST, Dr. Braun developed new electroanalytical measurement methods and computational models to describe electrodepositing systems for electronics applications. With Electra, Dr. Braun leads development of electrochemical systems and components critical to Electra’s clean iron technology.
Work with ECS: Member-at-Large, Electrodeposition Division.

Colleen Wallace, Manager of Electrowinning Development, Electra Education: BS in Physics (James Madison University)
Research Interests: Developing technologies for early-stage electrochemical start-ups spanning iron battery chemistries, green hydrogen electrolysis, and decarbonization of ironmaking.
Work Experience: Colleen has been with Electra since the seed-fund phase and has been heavily involved in developing Electra’s core electrochemical technology. Currently, she leads the development of Electra’s electrowinning system.

Quoc Pham, Co-Founder & Chief Technology Officer, Electra
Education: PhD in Solid State Chemistry (University of Caen, France)
Research Interests: Quoc has been at the forefront of clean tech for 30 years, with extensive experience in multidisciplinary hydrometallurgical and electrochemical projects, including fuel cell design, electrolyzer development, flow battery design, and membrane optimization.
Work Experience: Prior to co-founding Electra, Quoc was VP of Technology for AquaHydrex, which developed an alkaline water electrolyzer for the hydrogen economy, and Staq Energy, which developed a sealed nickel-iron battery for distributed use in energy storage systems. Earlier in his career, he was VP of Technology for EnerVault and CTO and co-founder of Evogy.

Sandeep Nijhawan, Co-Founder & Chief Executive Officer, Electra
Education: PhD in Mechanical Engineering (University of Minnesota)
Research Interests: Novel deepdecarbonization technologies
Work Experience: Sandeep has been at the center of deep-technology and venture investments for over 20 years, leading electrochemical and energy distribution projects from concept to commercialization. Prior to starting Electra, he was an operating partner of True North Venture Partners and president of two electrochemistry-focused cleantech start-ups, AquaHydrex
and Staq Energy. Sandeep has held leadership positions at Intermolecular, Siorah Incorporated, and Applied Materials. Website: https://www.electra.earth
About the Editor

Christopher L. Alexander, Assistant Professor of Civil & Environmental Engineering and Affiliate faculty of Chemical Biological and Materials Engineering, University of South Florida Education: BS and ME in Civil Engineering and PhD in Chemical Engineering (University of Florida).
Research Interests: Unraveling the mechanisms controlling corrosion damage evolution with the goal to revolutionize the diagnosis and mitigation of corrosion, ultimately extending the lifespan of critical components. I aim to inform the future design of materials that are not only highly sustainable but also optimized for reliability considering various applications.
Work Experience: Postdoctoral fellow at Sandia National Laboratories within the Materials Reliability Center where he studied atmospheric stress corrosion cracking as it relates to the aging and lifetime of nuclear waste interim storage containers. Honors & Awards: NSF Career Award 2024, McKnight Junior Faculty Fellowship 2023, Susan and William Bracken Faculty Fellowship 2021.
Website: https://usfcorrosion.wixsite.com/mysite
References
1. “Iron and Steel Technology Roadmap,” IEA, Paris, License: CC BY 4.0 (2020).
2. J. Suer, M. Traverso, and N. Jäger, Sustainability, 14, 14131 (2022).
3. International Iron Metallics Association. (Accessed Feb 2024).
4. Z. Fan and S. J. Friedmann, Joule, 5, 829 (2021).
5. “Lack of high-quality iron ore supply threatens steel’s green push.” T. Kuykendall, S&P Global Insights. (Accessed Feb 2024).
6. “Global Energy Review 2021 – Renewables.” (Accessed Feb 2024).
7. H. Kim, J. Paramore, A. Allanore, and D. R. Sadoway, J Electrochem Soc, 158 (10), E101 (2011).
8. R. Akolkar (2023) System and Process for Sustainable Electrowinning of Metal. US Patent Office No. US20230279572A1
9. A. Allanore, H. Lavelaine, G. Valentin, J. P. Birat, and F. Lapicque, J Electrochem Soc, 154 (12), E187 (2007).
10. A. Pham, et al. (2022) 2-Step Iron Conversion System. US Patent Office No. US11767604B2.
11. “2023 US Energy Information Administration Electric Power Data” (Accessed Feb 2024).
12. “Carbon Dioxide Emissions Coefficients by Fuel,” US Energy Information Administration. (Accessed Feb 2024).
13. E. Worrell, L. Price, M. Neelis, C. Galitsky, and Z. Nan, “World Best Practice Energy Intensity Values for Selected Industrial Sectors,” LBL, US Department of Energy (2008).
14. A. Zeradjanin, J.-P. Grote, G. Polymeros, K. J. J. Mayrhofer, Electroanalysis, 28, 2256 (2016).
15. A. Manohar, K. M. Kim, E. Plitchta, et al., J Electrochem Soc, 163, A5118 (2016).
42 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org Braun et al. (continued from previous page)
2024 ECS INSTITUTIONAL PARTNERS
BENEFACTOR PATRON
BioLogic USA/BioLogic SAS (16)*
Duracell US Operations, Inc. (67)
Gamry Instruments (17)
Gelest Inc. (15)
PalmSens BV (1)
Hydro-Québec (17)
Pine Research Instrumentation (18)
Energizer Battery (79)
Faraday Technology, Inc. (18)
GE Global Research Center (72)
Lawrence Berkeley National Laboratory (LBNL) (20)
Scribner, LLC (28)
Toyota Research Institute of North America (TRINA) (16)
SPONSORING SUSTAINING
BASi (9)
Center for Solar Energy and Hydrogen Research Baden-Wurttemberg (ZSW) (20)
Central Electrochemical Research Institute (31)
Corteva Agriscience (2)
DLR – Institute of Engineering Thermodynamics (16)
EL-CELL GmbH (10)
Electrosynthesis Company, Inc. (28)
Ford Motor Company (10)
GS Yuasa International Ltd. (44)
Honda R&D Co., Ltd. (17)
Medtronic, Inc. (44)
Nel Hydrogen (2)
Nissan Motor Co., Ltd. (17)
NSF Center for Synthetic Organic Electrochemistry (3)
Pacific Northwest National Laboratory (PNNL) (5)
Panasonic Energy Corporation (29)
Permascand AB (21)
Plug Power, Inc. (3)
Teledyne Energy Systems, Inc. (25)
UL Research Institutes (3)
Current Chemicals (2)
General Motors Holdings LLC (72)
Giner, Inc. (38)
Ion Power, Inc. (10)
Kanto Chemical Co., Inc. (12)
Los Alamos National Laboratory (LANL) (16)
Metrohm USA, Inc. (10)
Microsoft Corporation (7)
Occidental Chemical Corporation (82)
Sandia National Laboratories (48)
Sensolytics GmbH (1)
Sherwin-Williams (3)
Spectro Inlets ApS (2)
Technic, Inc. (28)
United Mineral & Chemical Corporation (3)
Western Digital GK Corporation (10)
Westlake Corporation (29)
Please help us continue the vital work of ECS by joining as an institutional partner today.
To renew, join, or discuss institutional partnership options please contact Anna Olsen, Sr. Manager, Corporate Programs, sponsorship@electrochem.org
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 43
*Years of membership as of April 16, 2024
TECH HIGHLIGHTS TECH HIGHLIGHTS
Transition Metal Dissolution from Single
Crystal Li[Ni1-x-yMnxCoy]O2 and Li[Ni1-xMnx]O2 Positive Electrodes Subjected to Aggressive Conditions
There is an increasing need from cost and sustainability perspectives to reduce cobalt content in layered nickel manganese cobalt oxide–based (NMC) cathode materials. Cobalt-free nickel-manganese (NM) cathode materials, especially those with singlecrystal form, draw attention since they show comparable performance to their NMC competitors. Transition metal (TM) dissolution, particularly disproportionation reaction of Mn3+ and subsequent deposition on the anode materials, can be catalytic to the adverse electrolyte decomposition. In this work, the authors performed systematic studies to learn TM dissolution from singlecrystal NMC811, NMC622, NMC532, LiNi0.75Mn0.25O2 (NM7525), NM73, and NM64. To accelerate TM dissolution and deposition, high temperature at 70 oC was applied during the cell formation phase followed by cycling (C/3) and storage (holding at higher cutoff voltage for different periods) testing. Capacity retention results after 200 cycles at 70 oC showed that NM64 exhibited the highest retention, followed by NMC532 and NM73. Through examination using scanning micro X-ray fluorescence (μ-XRF), all NM species had less Mn deposition on the anode compared to all the NMC species. A similar trend was observed after storage (calendar aging) testing. With these advantages, cobalt-free NM materials like NM64 can be optimized with longer lifetime.
From: B. Tang, N. Zhang, E. Alter, et al., J. Electrochem. Soc., 171, 010518 (2024)
Corrosion Resistance, Composition, and Stratification of Passive Films: Ni-22Cr and Ni-22Cr-6Mo Alloys Passivated and Exposure Aged in Acidic Chloride Solutions Ni-Cr based super alloys possess superior corrosion resistance and mechanical properties, even at elevated temperatures, and are widely used in a variety of industries, including aerospace and oil and gas. The corrosion resistance of these alloys is due to the formation of a Cr2O3 passive layer. The incorporation of minor alloying elements (MAEs) is known to further enhance corrosion resistance. It is less well understood how passive layer formation kinetics affect the composition and electrochemical performance of the formed passive layer on a Ni-Cr alloy with an added MAE, such as Ni-22Cr-6Mo. Researchers at the University of Virginia, Sorbonne Université, and Chimie ParisTech recently investigated this experimentally through electrochemical and materials characterization of passive layers grown under a variety of conditions (e.g., fast and slow) on both Ni-22Cr and Ni-22Cr-6Mo alloys. Their work demonstrated that while generally the addition of Mo enhances corrosion resistance,
a single compositional parameter is not the sole indicator of performance. Passive layer growth kinetics must also be considered in determination of the complex passive layer chemistry and corresponding performance. These results are valuable in the continued development of more corrosion resistant alloys.
From: K. Orson, E. Romanovskaia, A. Costine, et al., J. Electrochem. Soc., 171, 011505 (2024).
Performance Enhancement of a Membrane Electrochemical Cell for CO2 Capture
The reduction of specific energy consumed per mole of CO2 is critical for the capture and utilization of CO2. Electrochemical pathways can enable a reduction in energy used (both fossil fuels and renewable energy). Key parameters that impact capital expenditure and operating expenditure are current density and faradaic efficiency, respectively. Researchers at the Electrochemistry Laboratory at Paul Scherrer Institut in Switzerland carried out a detailed engineering study of a hydrogenlooping anion exchange membrane cell. After finalizing the specific geometry of the spacer to reduce the mass transport loss, the team evaluated three types of cathode structure, with no gap, a small gap, and a large gap between the cathode electrode layers. Cyclic voltammetry was used to characterize the actual electrochemical surface area. Cell-level characterization of the different electrodes included polarization curve, faradaic efficiency, and specific energy. Despite the reduction in area, the large gap showed improved faradaic efficiency. The team was able to demonstrate an energy consumption in the range of 246 to 325 kJ/mol of CO2 and increased current density to 100 mA/cm2, which enables the consideration of this type of approach for commercialization.
From: A. P. Muroyama, D. Abu-Arja, B. K. Rogerio, et al., J. Electrochem. Soc., 171, 013504 (2024).
Potential Modulation and Control of Redox Reactions at Bipolar Electrodes Using an Ion-Selective Membrane
A bipolar electrochemical system consists of two types of electrodes. The first is the driving electrodes, a pair of electrodes connected to an external power source to form a potential field across the electrolyte solution(s) in which they are immersed. The other is the bipolar electrode (BPE), a single piece of metal or other conductors, immersed in the electrolyte solution(s) without electrical connection to any external power source. Anodic and cathodic reactions can occur at the two ends, or poles, of the BPE, due to the potential gradient in the solution(s) generated by the driving electrodes. A key advantage of the system is that multiple BPEs (thousands have been reported) can be studied simultaneously without connecting to any potentiostat. However, this very feature carries a price: independent control of the interface potentials at the two BPE poles cannot
be achieved. A recent report by Mutalib et al. provided a clever solution to this problem by putting an ion selective membrane (ISM) over part of the BPE to fix its potential. The authors demonstrated multiple ways to control the BPE potentials by varying the ISM locations on the BPE, the concentrations of ions in solution, as well as the BPE shapes.
From: N. A. A. Mutalib, A.-J. Hsueh, Y. Deng, et al., J. Electrochem. Soc., 171, 027502 (2024).
Novel Surface-Enriched Spiky Ball with Spines NiCo2S4@GO/CNT Electrode
Material for a High-Performance Flexible Asymmetric Supercapacitor
Supercapacitors are essential components in modern energy storage systems due to their ability to efficiently store and release energy in short bursts, making them ideal for applications such as regenerative braking in electric vehicles. Various classes of materials have been considered for use in supercapacitors, including metal oxides, metal hydroxides, and MXenes. In recent years, metal sulfides have gained a lot of attention due to their high abundance, facile synthesis methods, and high theoretical capacities. Spinel-type NiCo2S4 has stood out due to its high theoretical specific capacitance of 2534 F g−1. However, there are challenges associated with increasing the capacity retention of NiCo2S4. To this end, a team of researchers from India have reported on the synthesis of composite materials consisting of NiCo2S4 nanoparticles and carbon nanotubes (CNTs) on graphene oxide (GO) sheets. The NiCo2S4/GO/CNT composite delivered a relatively high specific capacitance of 845 F g−1 when cycled at a current density of 2 A g−1, approximately double the value obtained when NiCo2S4 nanoparticles were cycled on their own. This report offers valuable insights into methods to enhance the electrochemical performance of metal sulfidebased supercapacitor electrode materials.
From: D. Ravisankar, D. Geetha, et al., J. Solid State Sci. Technol., 13, 011001 (2024).
Tech Highlights was prepared by Zenghe Liu of Abbott Diabetes Care, Mara Schindelholz of Sandia National Laboratories, David McNulty of University of Limerick, Chock Karuppaiah of Vetri Labs and Ohmium International, Chao (Gilbert) Liu of Shell, and Donald Pile of EnPower, Inc. Each article highlighted here is available free online. Go to the online version of Tech Highlights in each issue of Interface, and click on the article summary to take you to the fulltext version of the article.
44 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
A Bright Future for Electrodeposition
by Philippe M. Vereecken, Natasa Vasiljevic, Luca Magagnin, J. X. Kent Zheng, and Martin Leimbach
The Electrodeposition Division (ELDP), which was founded in 1922 as the second ECS division, celebrated their centennial anniversary at the 242nd ECS Fall meeting in Atlanta, two years ago this October. For the occasion, the division organized several sessions with invited contributions to honor the achievements of 100 years of electrodeposition, but also to take a closer look at the present trendsetters and give a perspective on future challenges and opportunities in this thriving field. Our then newly established Electrodeposition Early Career Forum (ECF, founded in spring 2022) organized a full-day symposium with contributions from outstanding early-career researchers involved in cutting-edge research across a broad range of areas of active electrodeposition research. It turned out to be a fantastic day with invigorating talks full of ideas.
The ELDP division decided to share some of the excitement with the ECS community and asked our ECF members to suggest topics and participate in the articles for this summer edition of Interface dedicated to electrochemical and electroless deposition. Dr. Kent Zheng, assistant professor at the University of Texas, and Dr. Martin Leimbach, postdoc at Technische Universität Ilmenau, Germany, took up the challenge. Together with us, humble guest editors, four articles have been selected that center around three important topics: (1) electrodeposition for manufacturing and sustainability, edited by Prof. Luca Magagnin; (2) electrodeposition for energy applications, edited by Prof. Natasa Vasiljevic; and (3) new electrodeposition approaches extending the material library, edited by Prof. Philippe Vereecken. The articles have been authored largely by the members of the Electrodeposition Early Career Forum with the guidance and support of more experienced co-authors.
The first article, “Advancements in Electrodeposition for Precise Manufacturing and Sustainability,” discusses how advanced electrodeposition techniques help contribute to a more sustainable world. Electrodeposition from molten salts can be used for
sustainable steel fabrication. Electrodeposition from ionic liquids is used for recovering of rare earth elements from electronic waste or in synthetizing novel nanostructured materials. Hybrid approaches combining wet metallization and additive manufacturing can be employed to build functional structures in an energy efficient way and with limited materials waste. The second article, “Engineering Electrodeposition for Next-generation Batteries,” deals with the metal electrodeposition process inside batteries which use elemental metals such as Li, Na, Al, Mn, and Fe as anodes. The desired reversibility of the plating and striping process, essential for battery cycle life, can be enhanced by 3D structured current collector architectures. Also, the effect of the electrolyte being liquid, solid, or hybrid is discussed. Electrodeposition from molten salts can also be used for the fabrication of thick metal oxide cathodes. The third article, “Electrochemically Induced Deposition (ECiD): A Versatile Method that Greatly Extends the Portfolio of Surface Coatings and Materials Fabricated by Electrochemical Deposition,” introduces the principle of the ECiD process for the electrodeposition of oxides, hydroxides, and compounds such as metal phosphates and metal-organic frameworks. The paper discusses the mechanisms of these electrochemically induced deposition reactions as well as the pro-bases used to trigger the deposition. The fourth paper, “Electrochemically Induced Templated Sol-gel Deposition of Mesoporous Silica and Nanocomposites: The New Kid on the ECiD Block,” goes a bit more into the details of the potential of the ECiD method for the deposition of compounds via electrochemically induced sol-gel reactions. Specifically, the example of gel ECiD of mesoporous silica films and silica nanocomposite films on planar and 3D electrodes is discussed. The paper concludes with the introduction of electro-blading as a new technique, a hybrid of electrochemical and sol-gel coating. Enjoy!
©The Electrochemical Society. DOI: 10.1149/2.F08242IF
(continued on next page)

The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 45
The Electrodeposition ECF Luncheon at the 244th ECS Meeting in Gothenburg, 2023.
Vereecken et al.
(continued from previous page)
About the Authors
Philippe Vereecken was chair of the Electrodeposition Division from October 2019 to October 2021.

Philippe Vereecken, Fellow, imec, program line coordinator P2M, EnergyVille, and professor, KU Leuven Education: PhD in Physical Chemistry (Ghent University)
Research Interests: The combination of electrochemistry and nanomaterials and specifically in its use in semiconductors, energy conversion, electrochemical storage, and nanomaterials synthesis
Work Experience: Postdoctoral Associate in the Department of Materials Science and Engineering at The Johns Hopkins University (1998–2001) and Research Staff Member (RSM) at IBM T.J. Watson Research Center (2001–2005). He joined imec in 2005 and KU-Leuven in 2009.
Work with ECS: ELDP Division executive committee member (2008-2023)
Luca Magagnin became chair of the Electrodeposition Division in October 2023.

Luca Magagnin, Professor, Surface Engineering & Applied Electrochemistry, Politecnico di Milano
Education: MSc in nuclear engineering and PhD in electrochemical engineering (Politecnico di Milano)
Research Interests: Include electrodeposition and electroless plating, MEMS and development, fabrication and testing of innovative functional materials and coatings
Pubs + Patents: Authored or coauthored >250 publications


https://orcid.org/0000-0003-4115-0075
Natasa Vasiljevic is currently past-chair of the Electrodeposition Division and was chair of the division from October 2021 to October 2023.

Natasa Vasiljevic, Senior Lecturer, School of Physics, University of Bristol Education: BS in Physics (University of Belgrade); PhD in Science and Engineering of Materials (Arizona State University)
Research Interests: Electrochemical surface science, electrodeposition of thin films and functional nanomaterials for electrocatalysis, magnetics and optoelectronics.
Work Experience: Lecturer at the School of Physics, University of Bristol (since 2011); Research Associate, Sandia National Laboratories, Albuquerque, New Mexico, US (2004–2008)
Pubs: 50 + papers, 1 book chapter
Awards: Great Western Research Fellowship, University of Bristol, UK (2008–2011)
Work with ECS: Member of the ECS ISTS Subcommittee (2019–2023), ELDP Division executive committee member (2011–2023), ELDP Division Chair (2021–2023).
Website: https://research-information.bris.ac.uk/en/persons/natasavasiljevic


https://orcid.org/0000-0002-7515-9708


Work with ECS: Chair of the Electrodeposition Division of ECS https://orcid.org/0000-0001-5553-6441
J. X. Kent Zheng is a member of ECS Electrodeposition Division Early Career Forum Organizing Committee.

J. X. Kent Zheng, Assistant Professor, McKetta Department of Chemical Engineering, Department of Physics, and Texas Materials Institute, UT Austin
Education: PhD (Cornell University)
Research Interests: Understanding and controlling the physicochemical processes involved in electro-crystallization
Pubs: 60+ papers, 4000+ citations
Awards: Recipient of the ECS Electrodeposition
Early Career Investigator Award
Work with ECS: Member of ECS and ECS Electrodeposition Division Early Career Forum Organizing Committee
Website: https://che.utexas.edu/people/faculty/zheng https://orcid.org/0000-0002-0673-0560


Martin Leimbach is a member of ECS Electrodeposition Division Early Career Forum Organizing Committee.

Martin Leimbach, Postdoctoral Research Fellow, TU Ilmenau
Education: Undergraduate, master’s, and PhD degrees in Materials Science and Engineering (Technische Universität Ilmenau), completing his PhD under the supervision of Prof. Dr. Andreas Bund in the Electrochemistry and Electroplating Group.
Research Interests: Functional coatings for water electrolysis, corrosion protection, alloy plating, electroless deposition processes, and pretreatment of metal surfaces.
Awards: Schwäbisch Gmünd Prize for Young Scientists (2020), DGO Nasser Kanani Prize (2022)
Work with ECS: President of the ECS Ilmenau Student Chapter. https://orcid.org/0000-0002-9548-7653


46 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Advancements in Electrodeposition for Precise Manufacturing and Sustainability
by Anar Badalbayli, Nicholas Sinclair, Roberto Bernasconi, Natalia Borisenko, Krishna Venkatesh, Adriana Ispas, Rohan Akolkar, and Luca Magagnin
The term circular economy is often found in literature references such as scientific articles1 and socioeconomic reports.2 Simply expressed, the circular economy implies that the people living on Earth should reuse and recycle the products that are currently in use as long as possible and reduce the waste produced, thus reducing CO2 emissions. The latter goal is fundamental from the perspective of mitigating the well-known greenhouse effect and the consequent global warming observed at the planetary scale. Under these conditions, advanced electrodeposition processes can play a fundamental role in the optimization of materials use and in the reduction of the energetic footprint for a wide variety of industrial processes. The aim of the present paper is precisely to suggest how this is possible, showing readers the potential that electrodeposition holds for efficient manufacturing of many different products that have a huge significance for industry. The first part of the present article focuses on the use of molten salts in the context of iron and steel production, one of the most energy intensive and CO2 impactful industries. It shows how electrodeposition from molten salts can be used to refine iron from the corresponding ores. The second part of the paper focuses on the use of ionic liquids for advanced applications like urban mining, which is a part of this concept of circular economy. Urban mining involves the recovery of compounds, materials, and elements from objects, products or waste generated by people, which are originally derived from conventional mining.3 Among the many materials that can be recovered in this manner, the focus in this article is placed on rare earth elements (REEs). In addition to this application, the paper also demonstrates the applicability of ionic liquids to the fabrication of nanometric 3D structures, to the improvement of 3D printing processes, and to the realization of aerospace products. The last part of the article is a brief discussion of the implementation of wet metallization techniques (electrolytic and electroless deposition) in the manufacturing of 3D printed parts. A wealth of approaches is described, with a special focus on the technologies that are able to deposit metals selectively following specified patterns.
Molten Salts–based Technology for Iron and Steel Production
Iron and steel production, constituting approximately 95% of global metal output annually,4 is fundamental for modern industrialization, with steel serving as a critical material across a wide variety of sectors, including the construction and automotive industries. However, the environmental impact of this production is considerable, as indicated by escalating CO2 emissions over the last decade. Presently, the steel sector ranks among the top contributors to CO2 emissions, accounting for 7% of the global total.5
In 2022, worldwide steel production reached 1885 million tons, primarily generated by three main processes.6 The blast furnace–basic oxygen furnace (BF-BOF) method, constituting 71% of steel production, involves the reduction of iron ore to pig iron in a blast furnace, emitting approximately 70% of CO2 in integrated iron-steel production plants. Secondary steel production, mainly utilizing steel scrap in electric arc furnaces (EAF), represents 24% of global steel production. Direct reduced iron (DRI) production accounts for 5% and is on the rise.6 Due to this massive environmental burden, efforts to develop cleaner alternatives through the electrolytic production of iron have gained interest. The molten oxide electrolysis (MOE) being developed by Boston Metal,7 and the aqueous-based iron
electrowinning being pursued by ULCOS/Siderwin8 and Electra9 (see “EChem in Action” in this issue) hold promise. These processes can electrolyze iron salts to iron and oxygen, eliminating CO2 emissions. Aqueous electrowinning processes are attractive because they can be operated at ambient temperatures, but MOE operates at temperatures around 1600°C in a molten oxide electrolyte and offers the advantage of high rates. An ideal process would combine these advantages (low temperature yet high rates) to create an efficient, CO2-free process.
Moderate temperature, chloride molten salt electrolysis (MSE) of iron and other metals, being developed by Akolkar’s lab at CWRU, offers a clean, cost-effective, and practical solution for sustainable iron metal production. During chloride MSE, iron is electrodeposited from a binary (NaCl+FeCl3) or ternary (LiCl+KCl+FeCl3) chloride mixture at moderate temperatures (300-500 °C). Electrowinning in these molten salts leverages their several attractive properties such as low viscosity, high ionic conductivity, high diffusivity, high solubility for electroactive species, electrochemical stability over a wide potential range, and fast electrochemical kinetics.10 These properties make chloride MSE a promising method for energyefficient, selective, and scalable industrial metal extraction processes. Fig. 1 illustrates the overall process flow of iron production via chloride MSE, including the upstream non-carbothermic chlorination of ore to FeCl3 (resulting in separation of ore impurities), and the subsequent electrolytic deposition process. Initial findings indicate promising outcomes for sustainable iron electrowinning at lower operational costs, attributed to the moderate temperatures (300–500 °C), high electrolyte conductivity (1–2 S/cm), and adequate FeCl3 concentrations (>2 M), which allow high current efficiencies (>85%) at high electrodeposition rates (approaching 1 A/cm2). Additionally, the chloride MSE process allows the incorporation of a proprietary dimensionally stable anode (DSA) to catalyze the chlorine evolution reaction at the anode. This innovation significantly lowers cell voltage, thereby enhancing overall process energy efficiency.9
Ionic Liquids as Alternative Electrolytes for Advanced Electrodeposition
Binnemans and Jones recently wrote a critical article on why electrolytes such as ionic liquids (ILs) and deep eutectic solvents (DES), despite being long considered possible game changers, have never made any significant commercial breakthrough in industrialscale metallurgical applications, such as electrowinning.1 The authors identified several factors contributing to this issue, including their high viscosity that renders them challenging to be pumped and stirred, their relative high price, administrative and licensing issues, lack of demonstrated unit processes on a pilot scale, and difficulties with recycling and reuse. Despite some of the drawbacks mentioned, we strongly believe that ILs and DES still have the potential to be applied on a large scale in different industrial aspects. In the 2014 spring edition of Interface, 11 two co-authors of this article presented various applications and properties of ILs. In the present work, we focus on recent trends in the usage of ILs. One of the applications of ILs is for the electrodeposition of metals and alloys that have highly negative reduction potentials. This renders aqueous electrolytes useless for their electrodeposition, making non-aqueous, aprotic solvents necessary. These electrolytes show a significantly larger electrochemical window and, therefore, offer the necessary stability for the deposition of materials such as rare earth elements (REEs), different metals, and (continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 47
Badalbayli et al.
(continued from previous page)
electrodeposition of Sm and SmCo from ILs21,22 and deep eutectic solvents (DES).23,24 SmCo alloys are used in permanent magnets. In addition to these reports, it is possible to find several accounts in the recent literature on recovery/ electrodeposition of other REEs, showing the importance and current relevance of this theme.17,25 Fig. 2 shows the cyclic voltammetry obtained on a Au electrode at 100 °C from a 0.1 M Dysprosium(III)-triflate (Dy(OTf)3) solution in 1-ethyl3-methylimidazolium trifluoromethanesulfonate ([EMIm][OTf]) and the morphology of a deposited Dy thin film.



alloys. This application is interesting because REEs, consisting of scandium, yttrium, and the 15 lanthanides,12 do not occur in ores in metallic form, but rather only in mixed and scattered form and, due to their similar physico-chemical properties, they are difficult to separate from one another, making their extraction extremely uneconomical. REEs are mostly associated with applications in high-tech industries such as in miniature nuclear batteries, laser repeaters, superconductors, and permanent magnets. They are also used extensively in the field of new “green technology,” for example in batteries for hybrid cars, wind turbines, and solar cells.12,13 There are fewer than 30 countries in the world with substantial REE reserves, most of them in Asia and Africa,14 with China having the largest REE deposits. This situation also makes China the largest producer and exporter of rare earth oxides. Given the disproportionate concentration of these deposits across the aforementioned regions, the need to recycle REEs to meet the growing demand and mitigate regional dependence is more crucial than ever.15,16 As a result, the European community has recognized REEs as a critical resource and underlined the necessity of their recovery.13 REEs can be recovered from electronic waste such as cell phones, laptops, flatscreen TVs, and car catalytics using ionic liquids17 and molten salts.18–20 Some of the authors of this article have already published works on the
ILs can also be successfully employed to produce materials with morphologies that can be controlled down to the nanoscale. In recent years nanostructured materials, especially nanowires and nanotubes, and ordered macroporous materials, have attracted great research interest for a wide range of applications such as electrode materials in batteries, catalysis, photocatalysis, sensors, solar cells, and photonic crystals. A common advantage of these materials is the increased surface area compared to bulk materials. In general, high vacuum and high temperature techniques, such as chemical vapor deposition (CVD), physical vapor deposition (PVD), molecular beam epitaxy (MBE), and hydrothermal techniques, have been applied for the synthesis of nanostructures and macroporous materials. Compared to these techniques, electrochemical deposition is advantageous due to a low-cost and relatively simple experimental requirements. The growth process and the properties of the deposited structures can be controlled by changing the deposition parameters, such as current density, temperature, or electrolyte composition. To date, the electrodeposition of nanowires and macroporous structures has been reported in aqueous, organic, and ionic liquid electrolytes.26 However, as already mentioned, aqueous electrolytes exhibit a limited electrochemical window (due to hydrogen evolution) that limits the electrodeposition of some metals and semiconductors. Ionic liquids have significant advantages over aqueous solutions and organic solvents, such as wide electrochemical windows (up to 6 V), high thermal stability, high conductivity, and negligible vapor pressure. Most ionic liquids are also nonflammable. These properties make them promising electrolytes for the electrodeposition of metals and semiconductors in the form of nanostructures and macroporous structures at or near room temperature.27 Over the last two decades, various metals, semiconductors, and core-shell nanostructures have been obtained electrochemically in ionic liquids using both template-assisted and template-free methods.28 Template-assisted electrodeposition has been applied to produce nanostructures and macroporous structures with controlled size and shape.28,29 Germanium,30-36 gallium,37 lithium,38 tin,39 zinc,40,41 aluminium,42,43 copper,44 silicon,33-35,45 niobium,46 SixGe1-x,34,37 GaSb,48 and CuSn49 nanostructures and macroporous structures have all been obtained via template assisted electrodeposition in ionic liquids. Commercially available anodic aluminum oxide (AAO) and track-etched polycarbonate (PC) membranes50 have been used as templates for the synthesis of nanostructures serving as a working electrode during the deposition as well. For this purpose, one side of the membrane was sputtered by a thin metal layer (Cu, Au, Pt)

2. Left: Cyclic Voltammetry at 20mV/s of a 0.1 M Dy[OTf]3 solution in [EMIm][OTf] at 100°C, on a Au electrode, with Pt plate and a Pt wire as counter and reference electrode, respectively. Right: Dy film electrodeposited at E = -1,25 V vs Pt at 100°C for 2 h from a 0.1M Dy(OTf)3 solution in [EMIm][OTf] on Au.
at E = -1,25 V vs Pt at 100°C for 2 h from a 0.1M Dy(OTf)3 solution in [EMIm][OTf] on Au.
48 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Fig. 1. Iron metal production from ore via a chloride molten salt electrolysis (MSE) process.
Fig. 2. Left: Cyclic Voltammetry at 20mV/s of a 0.1 M Dy[OTf]3 solution in [EMIm][OTf] at 100°C, on a Au electrode, with Pt plate and a Pt wire as counter and reference electrode, respectively. Right: Dy film electrodeposited
-3-2-101 -20 -15 -10 -5 0 i [mA/cm²] E/VvsPtquasi-ref. 1stcyc 2ndcyc
FIG.
Fe 3+ Fe 2+ Fe 0 x Cathode: Fe Electrowinning “DSA” Anode: Cl 2 Evolution Porous Separator Molten Salt Electrolysis Process Noncarbothermic Chlorination Process Iron Metal Fe2O3 Iron Ore + Impurities FeCl3 Cl2 Fe ( - ) (+) + impurities
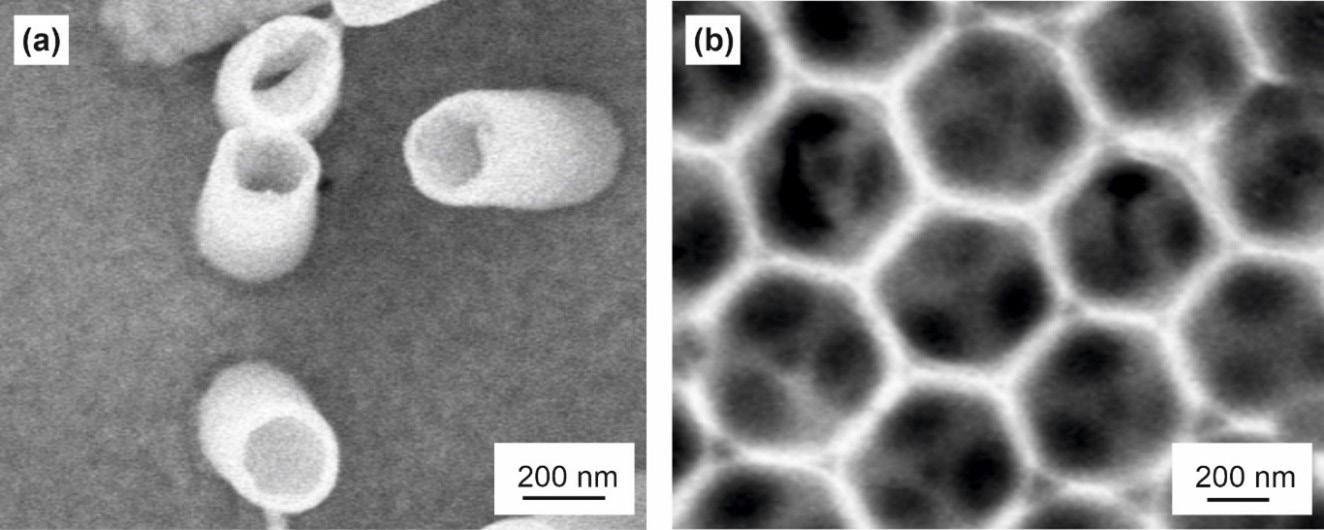
prior to electrodeposition to provide the required conductivity during the deposition process. The nanostructure growth occurs in the pores or channels of membranes, which results in the formation of nanostructures with an average diameter corresponding to the diameter of the pores in the membrane. After removing the template, free-standing or randomly distributed nanowires and nanotubes as well as core-shell structures of various metals and semiconductors have been produced.28 Fig. 3a shows SEM images of Ga nanostructures of 200 nm in diameter grown electrochemically at -1.6 V for 1 h from 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide ([Py1,4]Tf2N) containing 0.2 M GaCl3 at room temperature. The length of the nanowires can be varied simply by changing parameters like GaCl3 concentration, applied potential, and deposition time. Interestingly, some nanowires can be obtained in ionic liquids without any external template, which opens a new perspective for the synthesis of nanostructures. It has been reported that Zn,51,52 Sn,52 Te,52,53 TeBi,54 Al,55,56 AlFe,57 and semiconducting SnSi58 nanowires can be template-free electrodeposited in ionic liquids. Ionic liquids exhibit several remarkably strongly adherent solvation layers at the solid surfaces.59 Depending on the applied electrode potential, ionic liquid ions show a preferable orientation with respect to solid surfaces. The adsorbed IL’s ions may, in principle, act as a template for the synthesis of various nanostructures. However, the exact growth mechanism of such nanostructures is far from being understood. Similar to nanostructures, ordered macroporous structures can be prepared by template-assisted electrodeposition in ionic liquids.29 For this purpose, the required templates are typically produced by the self-assembling of monodisperse polystyrene (PS), polymethylmethacrylate (PMMA), or silica spheres into close-packed three-dimensional (3D) arrays on conductive surfaces (e.g., Cu, Au, Pt, or indium tin oxide (ITO)-coated glass). The electrodeposition occurs in the vacancies of the matrix leading to the formation of dense deposits because the filling process occurs from the bottom to the top. As the 3D colloidal crystals resemble naturally occurring opals, the resulting macroporous structure can be regarded as inverse replicas of the template arrays or inversed opals after removing the template. Using this method, 3D-ordered macroporous structures of various metals and semiconductors have been obtained.29 Fig. 3b shows a SEM image of a Ga macroporous structure obtained electrochemically at -1.6 V for 1 h in [Py1,4]Tf2N containing 0.2 M GaCl3 at room temperature. The average pore size of the macroporous structure is about 600 nm, which corresponds to the sphere’s size of the employed template.
Ionic liquids can also find application in additive manufacturing (AM), or 3D printing. Reichert et al. recently demonstrated a possible future application of ILs as additives in stereolithography, which is a type of 3D printing.60 An acoustic levitator was used to hold the monomer resin-IL mixture in place at the so-called node or lower atmospheric density region formed by the standing sound wave arising from the combination of constructive and destructive interference. A laser beam then initiated photopolymerization of the
monomer resin-IL mixture. Reichert et al. showed that incorporating various ILs into the monomer resin, predominantly consisting of commercial methyl methacrylate (MMA), reduced the maximum temperature of polymerization and enhanced the flexibility, durability, and stability of the 3D-printed object. A review of the use of polymeric ionic liquids (PILs), in 3D printing has recently been presented.61 PILs have the ability to stabilize molecular and nanostructured materials, thus opening the door for a variety of possible future applications in optoelectronics, antimicrobial and biomedical devices, and for catalysis and waste water treatment, to name just a few. Customized PILs can be produced by simply changing the anions of ILs, thus making them either hydrophilic or hydrophobic, thereby changing their stability or their ability to dissolve in nonpolar solvents. PILs can also be combined with a variety of different materials such as carbon nanotubes, conducting polymers, and Ag nanoparticles. These combinations could be the basis for next-generation devices produced by AM. Using ILs in fully printed devices offers the advantage of operating at higher voltages compared to those using traditional electrolytes, while also avoiding heavy corrosion of the devices.61
.
Hybrid materials produced by combining imidazolium-based ILs of different alkyl chain lengths with PVDF (poly (vinylidene fluoride)) have been reported.62 The electromechanical and magnetoionic response of such materials can be tuned by changing the IL. Through AM, these new generation multifunctional materials could find widespread applications that include sensors, actuators, and biomedical devices. The use of self-assembling IL monomers in a 3D printing process was proved63 to generate high resolution structures/pores of 2 to 4 nm within micrometer scale features of polymers builds. These features could, for example, be used as filtration membranes for water purification or in electronics where structures in the order of few nanometers are needed.
The last example of ILs discussed here relates to the aerospace industry. Shipping materials and tools from Earth to the Moon and Mars implies high costs and presents significant logistical challenges.64,65 However, the soil of both Mars and the Moon, the so-called regolith, a fine porous rock resembling volcanic rocks from Earth, contains oxides of Fe, Si, Al, Ti and other elements that could be selectively recovered. Globally, efforts are already underway in this direction. For instance, Al or Fe could be recovered from the regolith by electrodeposition using ILs.66 Such obtained materials can further be used to produce tools and structures directly where needed, on Mars or the Moon. At the same time, the additional benefit of performing the metal deposition/recovery in ILs or molten salts in space is the generation of oxygen on the anodes. This oxygen could be collected and utilized by the inhabitants living in these places. The successful use of a Fe-IL solution as feedstock for a LB-PBF (laser beam power bed fusion) AM machine is reported in the literature.65 The huge potential of obtaining complex geometry tools in space through AM without the need for machining or post-processing using the regolith and ILs as starting materials is very promising. Consequently, energy consumption and waste production can be minimized.
Wet Metallization for Additive Manufacturing
As already partially discussed, AM is one of the key enabling technologies for advanced precise manufacturing. In the context of the 4th industrial revolution, it is considered a vital element to overcome the limitations of traditional manufacturing by favoring the (continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 49
Fig. 3. SEM images of (a) Ga nanostructures and (b) Ga macroporous structure electrodeposited at -1.6 V for 1 h in [Py1,4]Tf2N containing 0.2 M GaCl3. These unpublished images were obtained within the framework of the PhD thesis of Janine Zahlbach.
(continued from previous page)
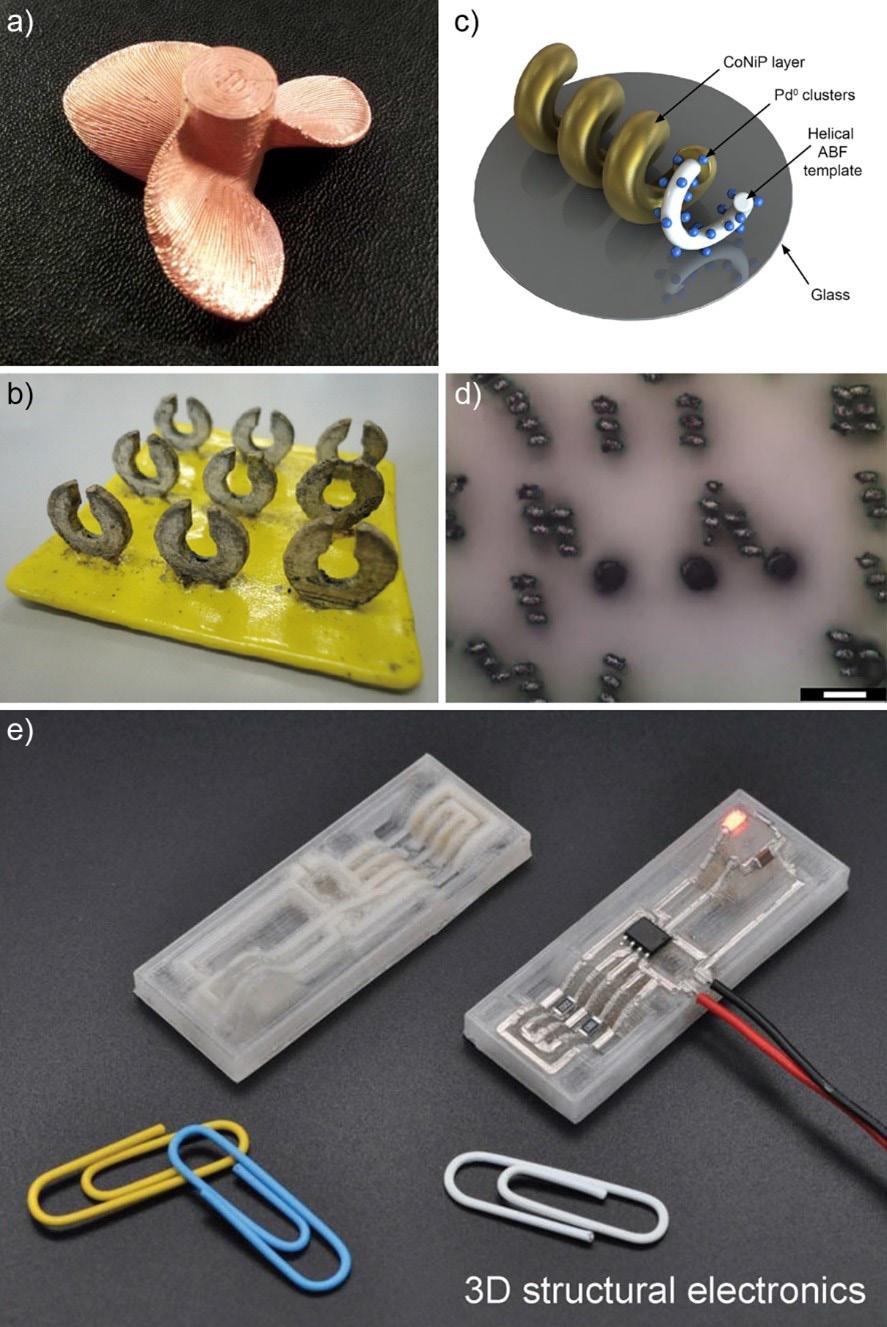
Fig. 4. a) ELES plated FDM part (reprinted with permission from70); b) selective metallization of FDM parts (reprinted with permission from79); c) and d) ELES metallization of DLW micrometric architectures (reprinted with permission from,73 scale bar = 10 µm); e) selective plating of circuitry on AM substrates (reprinted with permission from78).
integration of smart production systems and digital technologies.67
More precisely, AM is a family of techniques sharing a common working principle: the multiscale fabrication of complex objects in a layer-by-layer fashion. Exploiting this approach, a wealth of advantages over traditional subtractive approaches can be introduced: excellent scalability and customizability, shapes that are impossible to obtain with conventional subtractive manufacturing, optimal use of materials and energy, and the opportunity to optimize parts design. AM also offers the possibility of working at the microscale, with technologies like stereolithography and direct laser writing that are fully capable of reaching sub-micrometric resolutions.
For some applications, like with conventionally fabricated parts, it can be useful to apply functional coatings to AM parts in order to impart specific properties. These properties may include conductivity (in the case of insulators), magnetic properties, and wear or corrosion protection. Many technologies can in principle be used, including vapor deposition, but the most convenient in terms of cost and productivity are wet metallization techniques like electrolytic deposition (ELDP) or electroless deposition (ELES). Their implementation constitutes the most obvious complement for the realization of high performance AM parts68 at acceptable costs. The application of functional layers to AM metallic parts is relatively easy and, as certified by a general lack of literature, does not pose significant scientific questions. The metallization of polymeric and non-conductive parts in general, on the contrary, presents interesting challenges and peculiarities and it currently constitutes a major research topic in surface engineering.
The most straightforward approach to wet metallization of non-conductive AM parts is ELES. By carefully selecting surface pretreatments and autocatalytic processes, ELES can be successfully employed to metallize parts manufactured using different AM techniques like fused deposition modelling (FDM, Fig. 4a),69,70 stereolithography (SLA),71,72 and direct laser writing (DLW, Figs. 4c and 4d).73 The research on the ELES plating of AM parts is particularly active in the investigation of innovative processes, like supercritical carbon dioxide assisted ELES74 or deposition under magnetic field,75 in the optimization of the adhesion between the metal and the coating,76 and in the development of novel activation strategies.77
Besides generalized surface metallization, wet techniques also offer the attractive possibility of selectively depositing the material of interest. This approach, made possible in the case of ELES by carefully controlling the activation step, is probably the most interesting from the point of view of applications, as it makes possible transferring conductive metallic patterns onto non-conductive substrates. Different approaches have been developed, including the manipulation of surface wettability to selectively adsorb the catalyzer (Figure 4e)78 or the controlled removal of the same exploiting differentially soluble materials (Figure 4b).79 Also light, in the form of UV80 or laser radiation,81 can be used to selectively activate AM parts. In the first case, UV light selectively induces the reduction of Ag ions to the catalytically active 0-valent state. In the case of laser radiation, it creates the catalytically active sites on selected areas of the substrate. Interestingly, a laser has been also used in one of the few available examples of selective ELES metallization on AM ceramic parts.82 It is also possible to selectively ELES plate the surface of polymeric parts by cold spraying the activator.83
In the framework of selective ELES plating on AM pieces, one of the most promising approaches is the use of self-activating materials. These materials enclose, directly during the AM step, the activator for ELES, either as precursor or as metal particles. Typical precursors include Ag,84,85 Cu,86 and Pd (Fig. 5b),87–89 which have been implemented into parts manufactured via FDM, SLA, and direct ink writing (DIW). An example of a precursor is ZnO, which can be loaded into an SLA printed part and used for selective laser activation.90 Regarding metal particles, the use of Cu91,92 or Ni (Fig. 5a)93 with techniques like FDM or SLA has been reported.
ELES is a viable metallization technique for most AMmanufactured, non-conductive parts. However, in some limited cases, it is also possible to apply ELDP directly. The evident discriminating element is that the substrate must be conductive, and this is possible only in a few limited cases. The most common is AM materials loaded with conductive fillers like Cu,94 carbon nanowires,95 or graphene (Fig. 5c).95,96 Besides these specific materials, all ELES metallized parts can be metallized with ELDP,97 which constitutes the most viable way to impart conductivity to AM parts. Once made conductive via ELES, ELDP with alloys, composites, or conducting polymer becomes possible.
Like with ELES, the most attractive ELDP application for conductive AM materials is selective metallization. Indeed, by alternating non-conductive and conductive regions via multimaterial AM, it is possible to pattern metals and alloys (Fig. 5d).98 Alternatively, it is possible to laser pattern conductive graphene regions on AM parts that can then be metallized using ELDP.99
Considering the literature presented so far, it appears evident that wet metallization is generally intended as a postprocessing step for AM parts. In virtually all the approaches described, manufacturing and metallization are two well-separated stages. However, there is a highly unconventional technology that makes possible the direct realization of 3D microstructures via ELDP, thus directly merging AM and wet metallization. This technique, known as maskless localized electrochemical deposition (MLED), relies on the spatial localization of metal deposition in a small area100,101 by using microanodes,102 microcapillaries,103 or modified AFM probes (Fig. 5e).104 This methodology enables a remarkable tridimensionality with pure metals,105 alloys,106 and even biopolymers.107 In addition, it is one of the few techniques, together with DLW, that can be used to manufacture objects at the few-microns scale. The approaches
50 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Badalbayli
et al.
discussed so far can be applied in a large range of applications requiring functional coatings. Beside aesthetic applications, wet metallized AM parts can be used to build sensors, both physical108,109 and chemical,110 or for the realization of highly customized electronic devices.111,112 The intrinsic dimensional flexibility of some AM techniques, like SLA and DLW, can be combined with ELES and ELDP to manufacture microrobotic devices (Fig. 5f).113,114 Finally, tailored AM metallized electrodes can be used in batteries,115 or for the realization of electrocatalytic devices (Fig. 5g).116–118
Conclusions and Perspectives
The material discussed here shows that electrodeposition in ionic liquids and molten salts has the potential to open a new perspective for the synthesis of materials that are difficult to produce using conventional techniques. Ionic liquids and molten salts are thus suitable electrolytes for the electrochemical synthesis of nanostructures and ordered macroporous structures of metals, alloys, semiconductors, and REEs, which cannot be obtained in aqueous solutions. The quality of the obtained materials can be controlled by varying the deposition parameters, allowing the synthesis of the materials with the required properties. Ionic liquids also have the potential to be integrated into AM techniques, thus boosting their applicability. It is natural to wonder about the real industrial potential of hybrid wet metallization-AM technologies. Undoubtedly, they are among the most promising techniques to address the goals of the 4th industrial revolution and the quest for environmental sustainability. AM, thanks to its significant reduction of materials and energy usage with respect to traditional manufacturing methods, is considered a “green” manufacturing method.119 For this reason, especially if coupled with green wet deposition processes,120 it holds the potential of significantly impacting carbon emissions. Despite these undeniable advantages, there are many challenging aspects that must be addressed before full industrial implementation. Aspects like parts durability, mechanical properties, and coatings adhesion are still critical. In addition, the limited productivity that is intrinsic to some techniques, makes hybrid wet metallization-AM more suitable for small and highly customized productions. In general, wet metallization-AM technologies are currently approaching the critical step of industrial implementation and the next decade will be critical to understanding if they will find full applicability in global production chains.
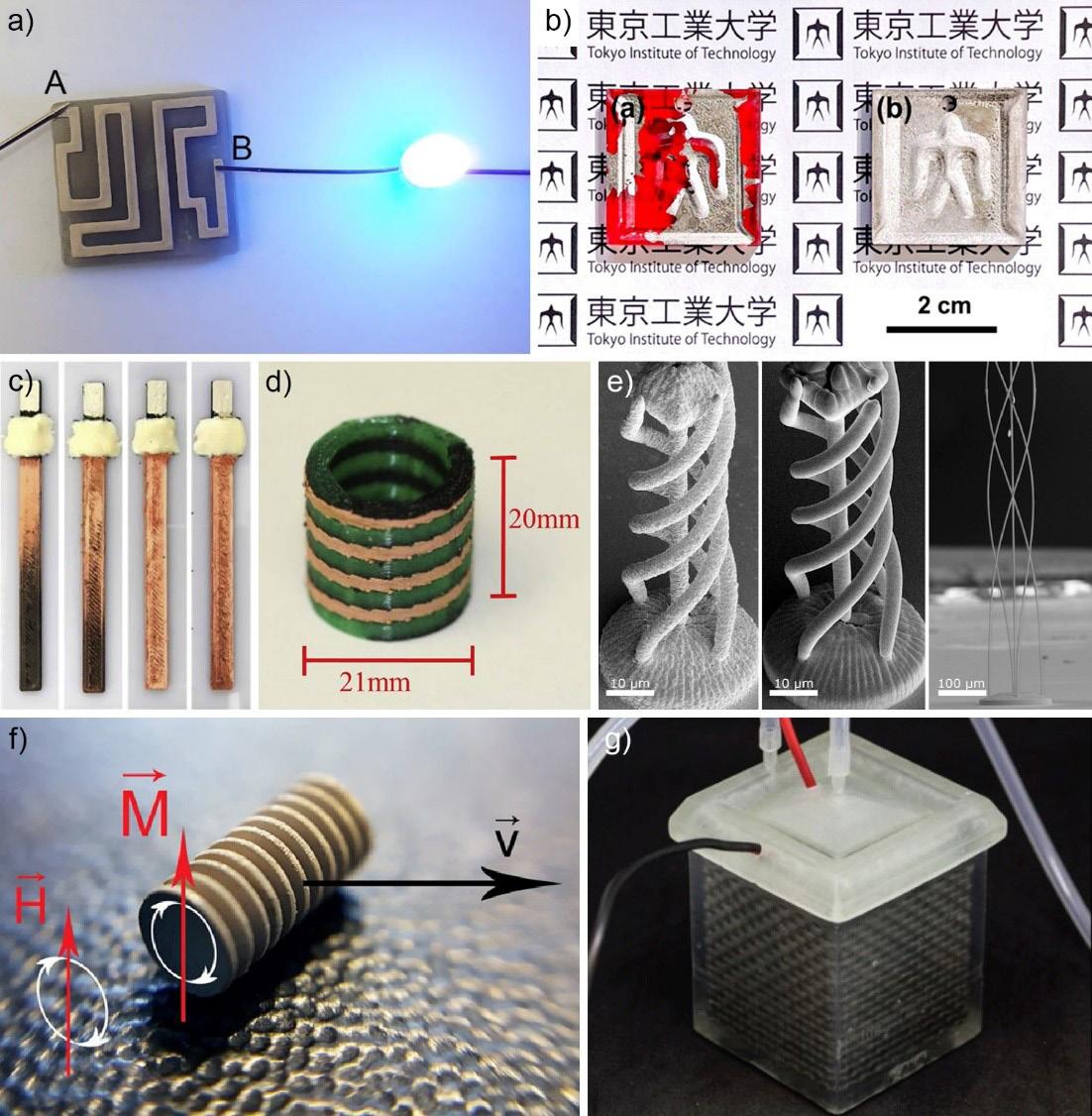
Fig. 5. a) conductive metallic track plated on a self-activating SLA composite (reprinted with permission from93); b) self-activating composite containing Pd functionalized carbon nanotubes (reprinted with permission from87); c) Cu ELDP on a FDM graphene composite (reprinted with permission from95); d) selective ELDP on FDM parts (reprinted with permission from98); e) MLED manufacturing of 3D structures at the microscale (reprinted with permission from104); f) magnetically guidable microrobot for environmental control (reprinted with permission from114); g) 3D printed water splitting setup (reprinted with permission from116).
About the Authors

Acknowledgements
The work on nanostructured materials in ILs was financially supported by the Deutsche Forschungsgesellschaft (DFG) within Priority Program SPP 1708 (grant number: BO 4290/1-1). Authors AB, NS, and RA acknowledge support from the US Department of Energy’s Office of Energy Efficiency and Renewable Energy, Award Number DE-EE0010846
© The Electrochemical Society. DOI:10.1149/2.F09242IF
Anar Badalbayli, PhD Student, Case Western Reserve University (CWRU) Education: BS in Chemical Engineering (Heriot Watt University), MBA with a specialization in Project Management (Azerbaijan State University of Economics) Research Interests: With a focus on sustainable manufacturing, electrochemical engineering and electrodeposition; iron metal electrodeposition through molten salt electrolysis while designing novel electrodes and reactor cells for high-temperature molten salt processes.
Work Experience: Prior to joining the EMF lab as a graduate research assistant, he worked as a process engineer at bp. He is an Associate Member (AMIChemE) of the Institution of Chemical Engineers (IChemE).


https://orcid.org/0009-0006-4370-3322
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 51
Badalbayli et al.
(continued from previous page)

Nick Sinclair, Staff Scientist & Adjunct Instructor, CWRU
Education: PhD in Chemical Engineering (CWRU)
Research Interests: Advanced energy storage systems based on redox organics in deep eutectic solvent electrolytes
Work Experience: As a staff engineer, researched electrochemical technologies from bench-top proof-of-concept to scaledup systems leading to successful tech-transfer to an industrial licensee of a DOE ARPA-E sponsored energy storage concept utilizing Fe electrochemistry. Experienced with electrolysis cell design and scale-up, as well as manufacturing for a variety of industrial applications. Most recently, he has been involved in the development of a molten salt electrolysis process for the sustainable production of rare-earth metals.

Adriana Ispas, Institute of Chemistry and Bioengineering, TU Ilmenau
Education: MSc in Applied Physics (“Alexandru I. Cuza” University), PhD in Physical Chemistry (Technische Universität Dresden) under Prof. Wadfried Plieth Research Interests: Fundamental and applied aspects of electrochemistry, such as electroplating in aqueous electrolytes and in ionic liquids
Work Experience: Carl Zeiss Postdoctoral Fellowship
Work with ECS: Member since 2004; Secretary, ECS Europe Section; Treasurer, Electrodeposition Division


https://orcid.org/0000-0002-8268-966X

Roberto Bernasconi, Assistant Professor of Chemistry, Materials & Chemical
Engineering “Giulio Natta,” Politecnico di Milano
Education: BSc, MSc and PhD in Materials Engineering (Politecnico di Milano)
Research Interests: General and applied electrochemistry, with particular interest in applications in the field of microfabrication; innovative materials for energy applications
Work with ECS: Member since 2015
Website: https://www.cmic.polimi.it/persone/docenti-e-ricercatori/ bernasconi-roberto/ https://orcid.org/0000-0003-2193-8017



Natalia Borisenko, Privatdozentin (Private Professor), Institute of Electrochemistry, Clausthal University of Technology
Education: MS in electrochemistry & physical chemistry (St. Petersburg State Polytechnical University), PhD under Prof. Endres (Clausthal University of Technology)
Research Interests: Electrochemistry of ionic liquids, processes at the ionic liquid/solid interfaces, electrodeposition of metals and semiconductors, and materials synthesis in ionic liquids.
Pubs + Patents: >70 ISI-refereed journal articles
Website: https://www.iec.tu-clausthal.de/ueber-uns/team

Krishna Venkatesh, PhD Student, Electrochemistry and Electroplating Group, TU Ilmenau
Education: BE in Mechanical Engineering (VTU Belagavi), MSc in Material Science and Engineering (CAU Kiel)
Research Interests: Electrodeposition of metals and alloys, ionic liquids, and secondary batteries.
Work with ECS: Student member of the Ilmenau Chapter of ECS.


Website: https://www.tu-ilmenau.de/wt-ecg https://orcid.org/0009-0007-6557-3482.


Website: https://www.tu-ilmenau.de/chemie https://orcid.org/0000-0001-8203-7752

Rohan Akolkar, Milton and Tamar Maltz Professor of Energy Innovation, CWRU Research Interests: Span many areas of electrochemical engineering, including batteries, semiconductors, and electrometallurgy.
Work Experience: An Ohio Eminent Scholar, Faculty Director of CWRU’s Great Lakes Energy Institute, Director of the CWRU Electronic Design Center (EDC), and Chief Scientist at the Pacific Northwest National Laboratory. Prior to joining the CWRU faculty in 2012, he conducted R&D at Components Research Division of Intel Corporation.
Honors & Awards: 2023 Electrodeposition Division Research Award of ECS, Norman Hackerman Young Author Award of ECS, and recent election as Senior Member of the National Academy of Inventors (NAI).
Work with ECS: Associate Editor of the Journal of the Electrochemical Society https://orcid.org/0000-0002-9865-5704



Luca Magagnin, Professor, Surface Engineering & Applied Electrochemistry, Politecnico di Milano
Education: MSc in nuclear engineering and PhD in electrochemical engineering (Politecnico di Milano)
Research Interests: Include electrodeposition and electroless plating, MEMS and development, fabrication and testing of innovative functional materials and coatings
Pubs + Patents: Authored or coauthored >250 publications


Work with ECS: Chair of the Electrodeposition Division of ECS https://orcid.org/0000-0001-5553-6441
References
1. K. Binnemans and P.T. Jones, J Sustain Metall, 9, 423 (2023).
2. https://www.europarl.europa.eu/topics/en/topic/circulareconomy (accessed 2024-03-13)
3. L. T. Espinoza, L. Rostek, A. Loibl and D. Stijepic, Fraunhofer ISI Report 2020, The promise and limits of Urban Mining.
4. Iron and Steel Statistics and Information, U.S. Geological Survey.
5. International Energy Agency. CO2 Emissions in 2022. IEA.
6. CGEP, C., Low-Carbon Production of Iron & Steel: Technology Options, Economic Assessment, and Policy. Center on Global Energy Policy at Columbia University, SIPA.
7. Boston Metal, A world with no pollution from metals production.
8. SiderWin Public Final Test Report (2023) (accessed 2024-0315).
9. B. Holcombe et al., ACS Sustainable Chem Eng, 12 (10), 4186 (2024).
52 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Photo:
CWRU
10. D. Shen and R. Akolkar, J Electrochem Soc,164(8) H5292 (2017)
11. A. Bund and F. Endres, Electrochem Soc Interface, 23, 45 (2014)
12. G. A. Moldoveanu and V. G. Papangelakis, Hydrometallurgy, 117, 71 (2012).
13. E. Commission, Commun. from Comm. to Eur. Parliam. Counc. Eur. Econ. Soc. Comm. Comm. Reg. (2020).
14. C. Zhanheng, J Rare Earths, 29, 1 (2011).
15. K. Binnemans, P. T. Jones, B. Blanpain, et al., J Clean Prod, 51, 1 (2013).
16. D. T. Buechler, N. N. Zyaykina, C. A. Spencer, E. Lawson, N. M. Ploss, and I. Hua, Waste Manag, 103, 67 (2020).
17. V. Kaim, J. Rintala, and C. He, Sep Purif Technol, 306, 122699 (2023).
18. T. Nohira, H. Kambara, K. Amezawa, and Y. Ito, J Electrochem Soc, 152, C183 (2005).
19. H. Konishi, H. Ono, T. Nohira, and T. Oishi, ECS Trans, 50, 463 (2013).
20. Y. Ito, T. Nishikiori, and H. Tsujimura, Electrochemistry, 86, 21 (2018).
21. A. Ispas, M. Buschbeck, S. Pitula, et al., ECS Trans, 16, 119 (2009).
22. M. Manjum, N. Serizawa, A. Ispas, A. Bund, and Y. Katayama, J Electrochem Soc, 167, 42505 (2020).
23. P. Cojocaru, L. Magagnin, E. Gomez, and E. Valles, Mater Lett, 65, 3597 (2011).
24. G. Panzeri, M. Tresoldi, C. Rinaldi, and L. Magagnin, J Electrochem Soc, 164, D930 (2017).
25. C. Andrew and M. Jayakumar, J Electrochem Soc, 169, 92517 (2022).
26. A. Lahiri and F. Endres, J Electrochem Soc, 164, D597 (2017).
27. F. Endres, A. Abbott, and D. MacFarlane, Electrodeposition from Ionic Liquids, John Wiley & Sons, (2017).
28. I. Sun and P. Chen, in Electrodeposition from Ionic Liquids, F. Endres, A. Abbott, and D. MacFarlane, Eds., p. 289, Wiley VCH, Weinheim, Germany, (2017).
29. Y. Li and J. Zhao, in Electrodeposition from Ionic Liquids, 2nd ed., F. Endres, A. Abbott, and D. MacFarlane, Eds., p. 278, Wiley VCH, Weinheim, Germany, (2017).
30. J. Hao, J. Zhao, Y. Zhang, et al., J Nanosci Nanotechnol, 16, 777 (2016).
31. X. Liu, J. Hao, X. Liu, et al., Chem Commun, 51, 2064 (2015).
32. A. Lahiri, A. Willert, S. Zein. El Abedin, and F. Endres, Electrochim Acta, 121, 154 (2014).
33. A. Willert, S. Zein. El Abedin, and F. Endres, Aust J Chem, 67, 875 (2014).
34. R. Al-Salman, X. Meng, J. Zhao, et al., Pure Appl Chem, 82, 1673 (2010).
35. R. Al-Salman, J. Mallet, M. Molinari, et al., Phys Chem Chem Phys, 10, 6233 (2008).
36. X. Meng, R. Al‐Salman, J. Zhao, et al., Angew Chem Int Ed, 48, 2703 (2009).
37. M. Al Zoubi, R. Al‐Salman, S. Zein. El Abedin, et al., ChemPhysChem, 12, 2751 (2011).
38. A. Willert, S. Zein. El Abedin, and F. Endres, Electrochem Commun, 48, 91 (2014).
39. A. M. R. Elbasiony, S. Zein El Abedin, and F. Endres, J Solid State Electrochem, 18, 951 (2014).
40. Z. Liu, A. Prowald, S. Zein El Abedin, and F. Endres, J Solid State Electrochem, 17, 1185 (2013)
41. M. Shapouri Ghazvini, G. Pulletikurthi, Z. Liu, et al., J Solid State Electrochem, 19, 1453 (2015).
42. S. Zein El Abedin, and F. Endres, ChemPhysChem, 13, 250 (2012).
43. L. H. S. Gasparotto, A. Prowald, N. Borisenko, et al., J Power Sources, 196, 2879 (2011).
44. S. Zein. El Abedin, A. Prowald, and F. Endres, Electrochem Commun, 18, 70 (2012).
45. S. Thomas, J. Mallet, F. Martineau, H. Rinnert, and M. Molinari, ACS Photonics, 5, 2652 (2018).
46. K. Blagg, T. Greymountain, W. Kern, and M. Singh, Electrochem Commun, 101, 39 (2019).
47. R. Al-Salman and F. Endres, J Mater Chem, 19, 7228 (2009).
48. A. Lahiri, N. Borisenko, M. Olschewski, et al., Angew Chem Int Ed, 54, 11870 (2015).
49. A. M. Elbasiony, A. Prowald, S. Zein. El Abedin, and F. Endres, J Solid State Electrochem, 26, 783 (2022).
50. I. Kazeminezhad, A. C. Barnes, J. D. Holbrey, K. R. Seddon, and W. Schwarzacher, Appl Phys A, 86, 373 (2007).
51. G. Pulletikurthi, M. Shapouri Ghazvini, A. Prowald, S. Zein El Abedin, and F. Endres, ChemElectroChem, 2, 1366 (2015).
52. R. Al-Salman, H. Sommer, T. Brezesinski, and J. Janek, Chem Mater, 27, 3830 (2015).
53. L. Thiebaud, S. Legeai, and N. Stein, Electrochim Acta, 197, 300 (2016).
54. L. Thiebaud, S. Legeai, J. Ghanbaja, and N. Stein, Electrochem Commun, 86, 30 (2018).
55. H. Wang and B. Li, J Electrochem Soc, 165, D641 (2018).
56. H. Wang, G.-M. Li, B. Li, and J.-L. You, Nanomaterials, 12, 1390 (2022)
57. G. Chen, Y. Chen, Q. Guo, H. Wang, and B. Li, Faraday Discuss, 190, 97 (2016).
58. A. M. Elbasiony, M. Olschewski, S. Z. El Abedin, and F. Endres, ChemElectroChem, 2, 1361 (2015).
59. H. Li, T. Carstens, A. Elbourne, N. Borisenko, R. Gustus, F. Endres, and R. Atkin, in Electrodeposition from Ionic Liquids, 2nd ed., F. Endres, A. Abbott, and D. MacFarlane, Eds., p. 321, Wiley VCH, Weinheim, Germany, (2017).
60. B. M. Dobyns, W. M. Reichert, and E. Duranty, J Electrochem Soc, 170, 66507 (2023).
61. S. Miralles-Comins, M. Zanatta, and V. Sans, Polymers (Basel), 14, 5121 (2022).
62. L. C. Fernandes, D. M. Correia, E. Fernández, et al., ACS Appl Mater Interfaces, 12, 42089 (2020).
63. B. G. Ndefru, B. S. Ringstrand, S. I.-Y. Diouf, et al., Mol Syst Des Eng, 4, 580 (2019).
64. G. S. Jackson, S. Elangovan, and P. E. Hintze, Electrochem Soc Interface, 29, 65 (2020).
65. B. C. Stewart, H. R. Doude, S. Mujahid, et al., J Mater Eng Perform, 31, 6060 (2022).
66. A. Dietz and E. Moustafa, in Electrochemical Society Meeting Abstracts 236, p. 2442, The Electrochemical Society, Inc. (2019).
67. U. M. Dilberoglu, B. Gharehpapagh, U. Yaman, and M. Dolen, Procedia Manuf, 11, 545 (2017).
68. T. M. Braun and D. T. Schwartz, Electrochem Soc Interface, 25, 69 (2016).
69. R. Bernasconi, G. Natale, M. Levi, and L. Magagnin, ECS Meet Abstr, MA2015-01, 1141 (2015).
70. R. Bernasconi, G. Natale, M. Levi, and L. Magagnin, J Electrochem Soc, 163, D526 (2016).
71. R. Bernasconi, C. Credi, M. Tironi, M. Levi, and L. Magagnin, J Electrochem Soc, 164, B3059 (2017).
72. K. Kołczyk, W. Zborowski, D. Kutyła, et al., Arch Metall Mater, 63, 1031 (2018).
73. R. Bernasconi, G. Prioglio, M. C. Angeli, et al., J Manuf Process, 78, 11 (2022).
74. P. W. Cheng, C. Y. Chen, T. Ichibayashi, et al., MRS Commun, 11, 278 (2021).
75. K. Kołczyk-Siedlecka, K. Skibińska, D. Kutyła, et al., Arch Metall Mater, 64, 17 (2019).
76. G. A. L. Andreatta, N. R. Hendricks, A. Grivel, and M. Billod, ACS Appl Electron Mater, 4, 1864 (2022).
77. K. Moraczewski, A. Trafarski, T. Karasiewicz, et al., Mater Today Commun, 34 (2023).
78. J. Li, Y. Wang, G. Xiang, H. Liu, and J. He, Adv Mater Technol, 4, 1 (2019).
79. A. Ishikawa, T. Kato, N. Takeyasu, K. Fujimori, and K. Tsuruta, Appl Phys Lett, 111 (2017).
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 53
Badalbayli et al.
(continued from previous page)
80. A. Ryspayeva, T. D. A. Jones, S. R. Khan, M. N. Esfahani, et al., IEEE Access, 7, 104947 (2019).
81. I. Shin and M. Park, Phys Status Solidi Appl Mater Sci, 214 (2017).
82. F. Zhao, C. Jiao, D. Xie, et al., RSC Adv, 10, 44015 (2020).
83. S. Akin, C. Nath, and M. B. G. Jun, ACS Appl Electron Mater, 5, 5164 (2023).
84. A. Ryspayeva, A. Zhakeyev, M. P. Y. Desmulliez, and J. Marques-Hueso, Adv Eng Mater, 24, 1 (2022).
85. S. Lee, M. Wajahat, J. H. Kim, et al., ACS Appl Mater Interfaces, 11, 7123 (2019).
86. P. Wang, J. Li, L. Deng, et al., Adv Mater Technol, 8, 1 (2023).
87. C. Y. Chen, P. W. Cheng, T. Ichibayashi, et al., ACS Appl Nano Mater, 6, 4584 (2023)
88. A. T. K. Perera, K. Wu, W. Y. Wan, et al., Addit Manuf 61
100. J. Xu, W. Ren, Z. Lian, P. Yu, and H. Yu, Int J Adv Manuf Technol, 110, 1731 (2020).
101. S. Hu, X. Huan, Y. Liu, S. Cao, Z. Wang, and J. T. Kim, Int J Extrem Manuf, 5, 032009 (2023).
102. Y. T. Tseng, G. X. Wu, J. C. Lin, et al., J. Alloys Compd, 885, 160873 (2021).
103. S. K. Seol, D. Kim, S. Lee, J. H. Kim, W. S. Chang, and J. T. Kim, Small, 11, 3896–3902 (2015)
104. G. Ercolano, T. Zambelli, C. van Nisselroy, D. Momotenko, et al., Adv Eng Mater, 22, 1 (2020).
105. C. van Nisselroy, C. Shen, T. Zambelli, and D. Momotenko, Addit Manuf, 53, 102718 (2022).
106. C. Wang, M. E. Hossain Bhuiyan, S. Moreno, and M. MinaryJolandan, ACS Appl Mater Interfaces, 12, 18683 (2020).
107. W. Shang, Y. Liu, W. Wan, et al., Biofabrication, 9 (2017).
108. V. Zega, M. Invernizzi, R. Bernasconi, et al., IEEE Sens J, 19, 9131 (2019).





MARK THE DATE! Registration opens in June! H O N O L U L U , H I O c t o b e r 6 - 1 1 , 2 0 2 4 Joint International Meeting
Engineering Electrodeposition for Nextgeneration Batteries
by Stephen T. Fuller, Yonglin Huang, Ruixin Wu, Fudong Han, J. X. Kent Zheng, and Natasa Vasiljevic
Electro-Plating/Stripping: The “Holy Grail” Anode Chemistries for Batteries
The redox reaction M ne M n+ (sol.) (s) has been utilized since ancient times.1 This utilization is due, in part, to the simplicity of the reaction and the ubiquity of the materials involved. Recently, there has been a resurgence of interest from the battery research community in the development of metal anodes that rely solely on this type of simple metal redox reaction, also known as the plating/ stripping or, interchangeably, the deposition/dissolution reaction in the electrochemistry context. The metal anodes feature simple redox chemistry by design and remarkably improved energy density (Table I). Metal electrodes of this kind, especially Li, have been dubbed the “Holy Grail” for battery anodes.
Li metal was first discovered as a possible anode for batteries over a century ago by Lewis and Keyes.2 Once an organic electrolyte was developed that prevented the direct reaction of lithium with the electrolyte, primary Li metal batteries began to be commercialized in the late 1960s using various cathodes.3 Initially, the transition to secondary Li batteries was focused on finding a cathode material that could reversibly intercalate and de-intercalate Li+. Li metal batteries with TiS2 and MoS2 cathodes were deployed commercially in the late 1970s and 80s but were quickly recalled due to the discovery of fire incidents due to metal shorting from dendrite formation on the anode.4
The academic community then turned to the graphite anode to develop the rocking chair battery. A rocking chair battery uses a host material that allows reversible intercalation and de-intercalation of the metal cations in the anode (Fig. 1A). This process is in stark contrast to a plating/stripping metal anode where a new solid phase is created through nucleation and growth processes (Fig. 1B and Fig. 2). Driven by multiple possible kinetic heterogeneities,5 such processes oftentimes result in non-compact deposits that are prone to aggressive outward propagation in battery cells.
Conversely, examples of electrodeposition of metals were first reported at the turn of the 19th century, and significant developments were made in the field in the latter half of the 20th century when
industrial electrodeposition became important.6 The morphological evolution of metal electrodeposits turned out to be a critical issue for using metal anodes in batteries. The non-compact growth modes create a variety of fading mechanisms and safety risks (Fig. 2B). As far as we know, some of the first reports on using electrodeposition principles in batteries were in the early 1960s, which focused on limiting dendritic growth for the purpose of secondary zinc alkaline batteries.7 Since then, there have been other works focused on tuning the electrodeposition morphology to improve battery cyclability.5 A demand for higher energy density batteries than conventional intercalation-type rechargeable batteries has sparked further interest in electrodeposition (plating/stripping anodes) over the last decade. In addition to the interest in raising energy density by utilizing a lithium metal anode, there has been renewed interest in the expanding library of batteries where the anode can be operated based on a metal plating/stripping mechanism to reduce the manufacturing cost of the anode. For example, in addition to the more reactive alkaline and alkali-earth metals, the plating/stripping reaction of metals such as Al, Mn, Fe, Zn, Cu, and Sn have all attracted research interest aimed at integrating them into next-generation batteries as anode materials (Table I).8
The Challenge of Attaining “Close-to-Unity” Reversibility
Coulombic efficiency is a key metric to determine the feasibility of these batteries, and it has been calculated that this metric must be above 99.9% for metal anode batteries to have serious commercial viability.9 The deviation of this metric from 100% is known to result from two possibilities: (1) chemical degradation, which involves the reaction of the metal anode to form a solid electrolyte interphase (SEI); or (2) metal orphaning, which involves physical disconnection of fragments of the metal anode from the current collector. The academic community is beginning to accept that the latter possibility plays the most important role in coulombic efficiencies below 100%.10 The past decade of research has focused primarily on limiting the dendritic growth of the batteries during the charging process through (continued on next page)
*Metal anodes outperform the current intercalation-based graphite anode in almost every aspect, except for the extraordinary cycling stability of the graphite intercalation chemistry. Other metals emerge as promising energy storage materials complementing the dominant Li-based technology that is limited by Li’s relatively low earth-crust abundance. Li sets itself apart by its gravimetric energy density a few times to one order of magnitude higher than other metal anodes. This explains Li’s key role in high-end portable energy storage, where mass is the main design consideration. By contrast, other metals, in general, exhibit comparable or even higher volumetric energy density than Li. This means these alternative chemistries may find applications in stationary energy storage or portable storage where volume is more important than mass.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 55
ANODE CHEMISTRY LOG ABUNDANCE (ppm) CAPACITY (Ah/mL) CAPACITY (Ah/g) CELL VOLTAGE EST. (V) ENERGY DENSITY (Wh/mL) ENERGY DENSITY (Wh/g) Graphite N/A 0.8 0.3 4 3.2 1.2 Li 1 2.0 3.9 4 8.0 16 Na 4 1.2 1.2 3 3.6 3.6 Al 5 8.0 3.0 2 16 6.0 Mn 3 7.4 1.0 2 15 2.0 Fe 5 7.9 1.0 1 7.9 1.0 Zn 2 5.8 0.8 1.5 8.7 1.2
Table I. Key Theoretical Figures-of-Merit for Battery Anode Chemistries*
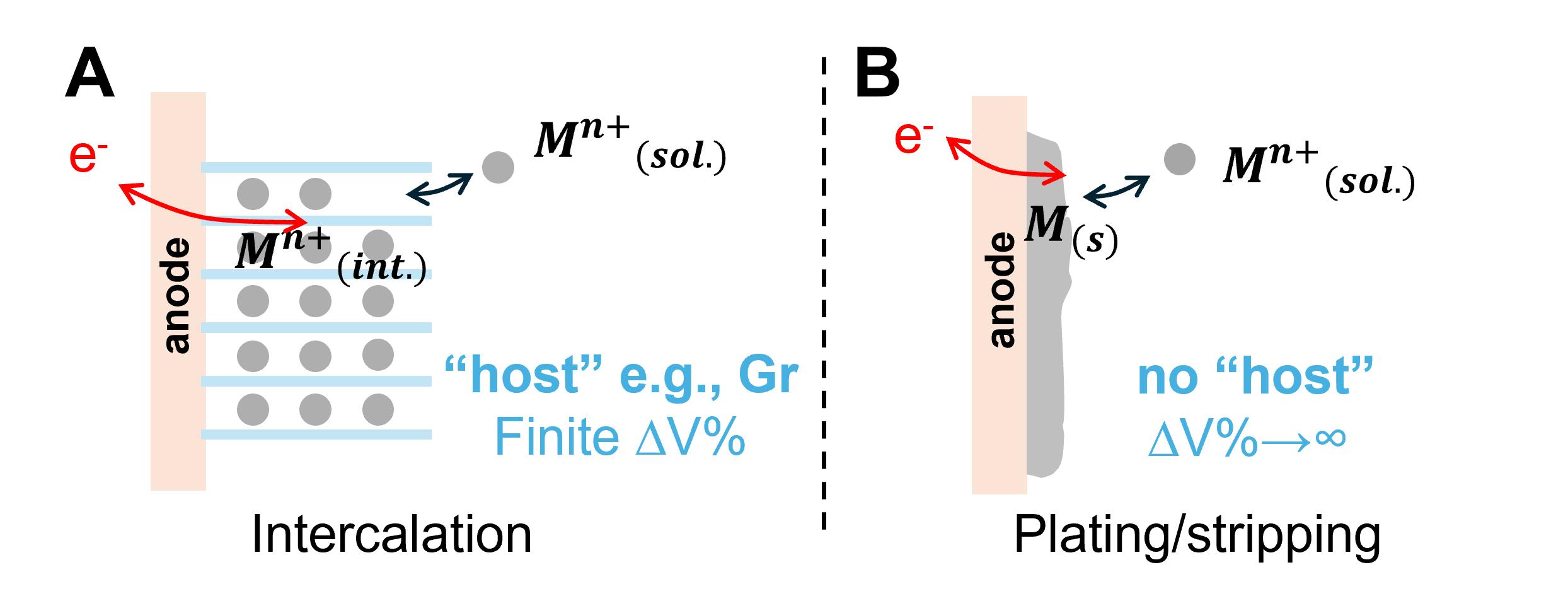
Fig. 1. Electrochemical mechanisms of rechargeable battery electrodes. (A) An intercalation electrode: charged ions are reversibly inserted into / de-inserted from a host material (e.g., graphite, whose layered structure facilitates ion storage and transport) during discharge-charge cycles. This reversible reaction (e.g., Li + 6C ↔ LiC6) is associated with a finite volume change ∆V%. (B) A metal electrode utilizing the plating/stripping reaction. In the absence of the “host,” the charged ions (i.e., normally metal cations dissolved in a liquid solution) are reduced and converted to a solid phase upon charging. The dissolution of the metallic solid phase happens upon discharging. The plating/stripping reaction (e.g., Li+ + e- ↔ Li) is, in theory, associated with an infinite percentage volume change ∆V%. While a significant amount of mass/volume can be saved by removing the host, the nature of the plating/stripping reaction creates the challenge of precisely controlling such dynamic plating/stripping processes inside battery electrodes.
a variety of processes including electrolyte engineering, artificial SEI, and even modifications to the structure of the bulk metal foil.
The fundamental aspects involved in understanding and controlling the electro-plating/stripping morphology have been extensively discussed in a few prior reviews.5,11-13 Here, we highlight two future
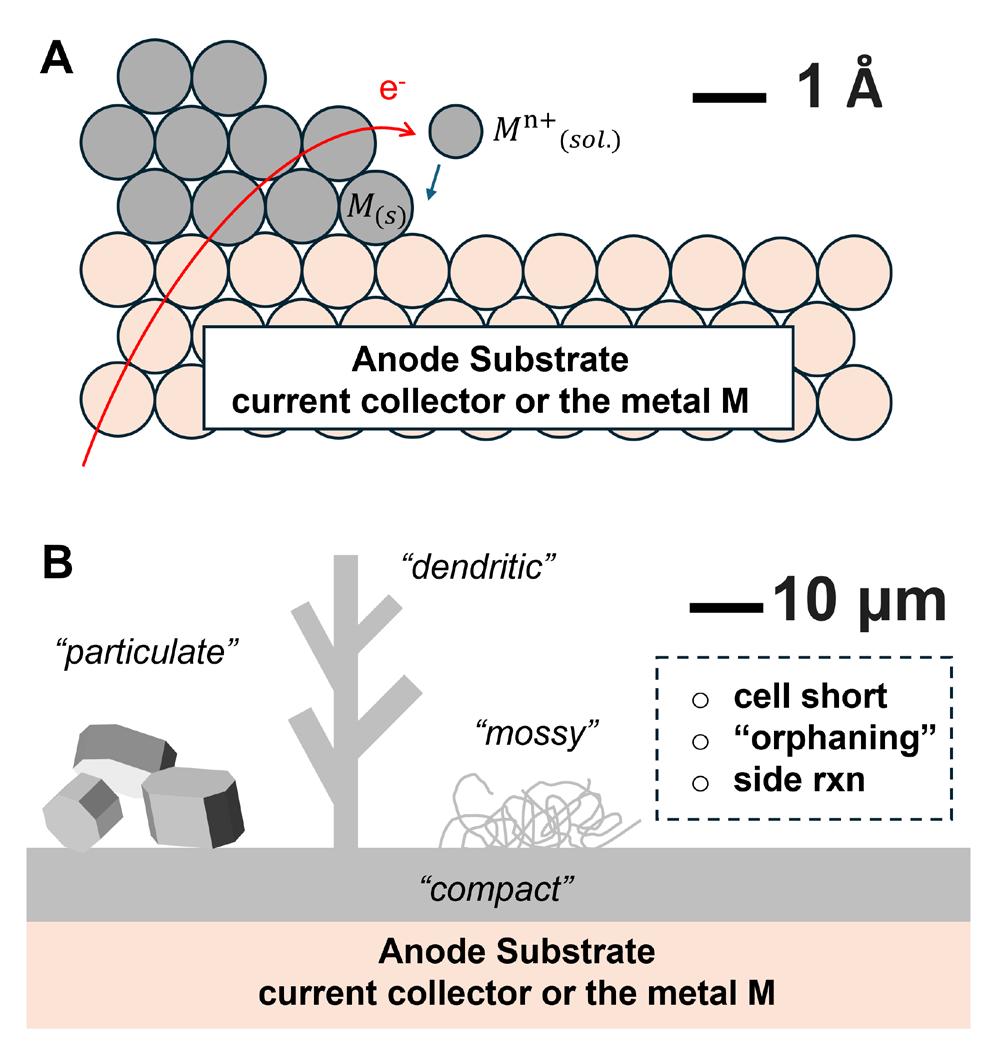
Fig. 2 Nucleation and growth of electrodeposits. (A) Electrons flow from the electrode surface into the orbitals of metal cations. The chargeneutral metal atoms nucleate—either epitaxially (shown in the schematic diagram) or not—on the electrode surface. (B) As the deposition proceeds, the nuclei develop into various possible morphologies or a mixture of them. It is desirable that the morphology stays compact and uniform during the plating/stripping. A porous morphology results in multiple issues causing irreversibility in plating/stripping (e.g., metal “orphaning,” side reactions, etc.) and even fatal battery shorts.
research avenues that could be vital to advancing the viability of metal anode batteries in addition to the ongoing research on limiting dendritic growth during battery charging.
Use of a three-dimensional, nonplanar current collector has been shown to increase the coulombic efficiency of the metal battery to >99% at high areal capacities ~10 mAh/cm2. Rational design (e.g., focusing on the electrical conductivity and interfacial chemistry of the nonplanar architecture) is essential to achieving high reversibility.14 Although these current collectors decrease the potential energy density, they could serve as a short-term solution for Li metal anode batteries. More research is needed on finding nonplanar current collector networks that are optimized for the length scale of the metal electrodeposits to maximize coulombic efficiency.
The other aspect that will be important for batteries is understanding electrodeposition and stripping in new solvent systems, such as waterin-salt electrolyte systems, ionic liquids, and deep eutectic solvents.15 These electrolytes are nonflammable, have high ionic conductivity, and have higher electrochemical stability windows than conventional aqueous or organic electrolytes. Understanding how the properties of the deposits change as a function of the solvent system will have large implications for metal anodes outside of Li.
Electrodeposition in the Solid State
Lithium metal anodes (LMAs) are considered the ultimate solution for high-energy batteries.16,17 While LMAs have already been successfully applied to thin-film solid-state batteries,18 the utilization of LMAs in high-areal-capacity, bulk-type solid-state cells faces several challenges such as interfacial instability, dendritic growth, and morphological instability.19,20 As Li electrodeposition is the very first process on the anode during the initial charge of solid-state Li metal batteries and occurs every subsequent charge/discharge cycle, it plays a critical role in governing the performance and degradation of batteries, especially for anode-free batteries where there is no Li on the anode prior to Li plating. In this section, we will briefly overview the physicochemical concepts that describe the electrodeposition of Li in inorganic solid electrolytes with a focus on the growth behavior. While the discussions are primarily framed in terms of Li, the electrodepositions of Ag21 and Na22,23 in inorganic solid electrolytes were also extensively studied decades ago and many of the underlying core concepts are essentially transferable.
56 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org Fuller et al. (continued from previous page)
During Li plating, a homogenous layer-by-layer deposition between the anode and solid electrolyte (Fig. 3A) is desired for developing highly reversible solid-state batteries. The layer-bylayer electrodeposition of Li with bulk-type Li2S-P2S5 glass24 and Li7La3Zr2O12 25 solid electrolytes has been demonstrated. Nevertheless, it is shown that this type of deposition can occur only at small current densities (i.e., low deposition rates). As the current density increases, the growth mechanism deviates significantly from the layer-by-layer mode and Li penetration through the solid electrolyte has been widely observed, causing cell shorting.26-28 Borrowing from the concept in liquid-electrolyte lithium metal batteries,29 the formation of metallic Li inside the solid electrolyte is usually called dendritic growth, although the morphology of Li formed in solid electrolyte can be sharply different from the dendritic feature.19,26 The conventional theories to explain the dendrite formation in organic electrolytes, based on transport limitation of the salt30 and mechanics of electrolyte,31 cannot be applied to solid-state Li metal cells.
Based on the previous study on Na plating in Na-β-Alumina solid electrolytes,22 the growth of metallic Li through the solid electrolyte is divided into two modes.19 Mode I describes the Li growth at the interface of anode and solid electrolytes (Fig. 3B). This growth mode is caused primarily by current focusing (i.e., current hotspots) at the anode/electrolyte interface due to the existence of cracks, pores, impurities, and grain boundaries at the surface of solid electrolytes. The significantly enhanced current density at these local positions can easily exceed the self-diffusion limit of Li in the bulk, leading to the growth of Li toward the solid electrolyte through crack propagation.19 On the other hand, Mode II describes the internal deposition of Li directly inside the solid electrolyte (Fig. 3C).28 Internal deposition in solid electrolyte has also been experimentally
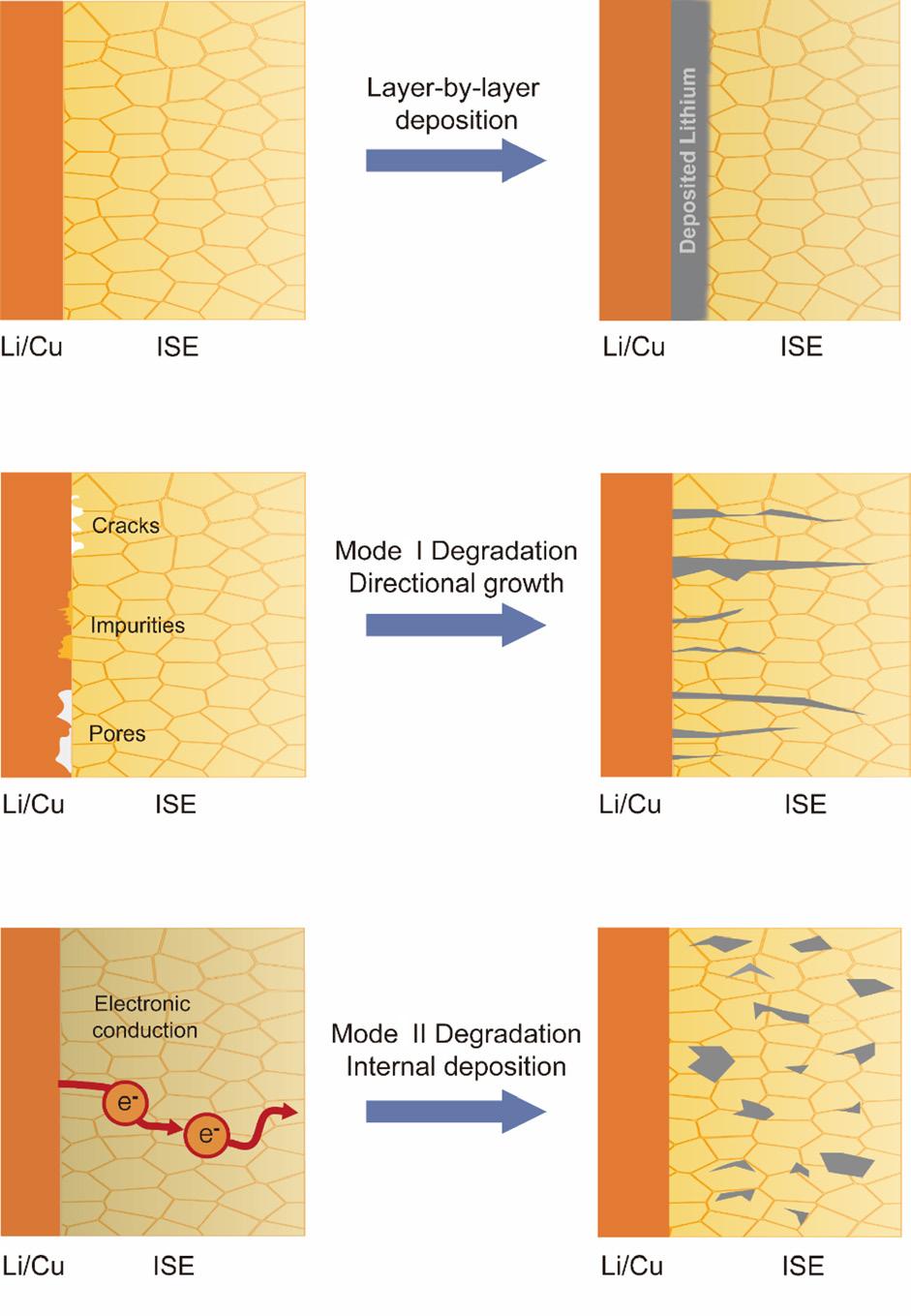
observed in the electrodeposition of Na and Ag.21,22 The root cause of this growth mode is the residual electronic conduction in solid electrolytes.21,22 While electronic transport in Li solid electrolytes has not been carefully studied,32 inhomogeneous distribution of electronic conductivity inside solid electrolytes, possibly due to the surface electronic conduction of solid electrolyte,33 reduction of solid electrolyte,22 or enhanced conductivity at local defects such as grain boundaries,34 can result in a gradient of transference number, leading to an overshot in the Li chemical potential as the driving force for direct formation of Li inside the solid electrolyte.23
It is still a matter of intense debate in the community as to which mode dominates the Li growth in inorganic solid electrolytes. Due to the much slower self-diffusion of Li versus the large localized current densities, Mode I growth has been considered as a faster process than Mode II. Nevertheless, one should note that these two modes cannot be considered as separate processes. The directional growth of Li through Mode I can increase the electronic conductivity of solid electrolytes and promote Mode II growth, while internal deposition of Li can extend crack formation and accelerate directional growth. The key information for determining which mode is dominant is to identify where the Li first nucleates at the first deposition, but this information has not been revealed due to challenges in characterizing the nucleation behavior of Li in solid electrolytes. Nevertheless, current understanding of the mechanism of electrodeposition of Li can provide some insights into the strategies to suppress Li penetration into solid electrolytes. To suppress Mode I growth, the surface of inorganic solid electrolytes should be carefully treated to remove cracks, pores, and other defects as much as possible prior to plating. Designing an additional interlayer, based on polymeric, metallic, or ceramic materials,35 at the anode/electrolyte interface may also be helpful in achieving homogeneous current distribution, but these interlayers should sustain during repeated cycles. Another less-mentioned strategy is to improve the self-diffusion coefficient of Li in the substrate Li anode, for example by alloying or microstructure engineering.36 On the other hand, more careful studies of electronic transport in solid electrolytes are still needed to understand the voltage dependence, charge carrier, and root causes of electronic conductivity in solid electrolyte to eventually design strategies to suppress the internal deposition of Li.
Future Inquiries Toward the “Holy Grail” and Beyond
In addition to playing a critical role in anodes, electrodeposition has found important applications in enabling high-areal-capacity, thick batteries cathodes. A variety of transition metal oxides can be directly electroplated—at elevated temperatures from their corresponding molten salts—onto planar Al current collectors.37,38 In particular, these electroplated electrodes manifest extraordinary rate capabilities at areal capacities (i.e., 20 mAh/cm2) that are a few times higher than normal commercial Li-ion electrodes. This type of dense electroplated cathode—possible because of the continuous ion and electron conduction network formed during electroplating—can be seamlessly integrated into solid state batteries. Future research might further enable electroplating of cathode materials that are less intrinsically electrically conductive.
The reverse reaction that occurs during battery discharge, electrodissolution, has been only rarely investigated in contemporary battery literature. A pioneering work by Shi et al. demonstrated that the electro-dissolution process plays an equally, if not more, important role in determining the morphological evolution and reversibility of Li metal anodes through mechanisms that have been underexplored to date.39 While dendritic growth is mostly seen in mass transport controlled conditions during deposition, electro-stripping at the mass transport limit produces a bright, micro-finished surface.40 More research is needed on this fundamental phenomenon, as well as on the interrelation between the stripping morphology and subsequent deposition morphology onto the stripped surface. This research will require borrowing past results in industries such as surface finishing
Fig. 3. Electrodeposition from a solid-state electrolyte. (A) Electrodeposition of Li in solid-state lithium metal batteries: Layer-bylayer deposition under low current densities; (B) Directional growth of Li from the anode/electrolyte interface due to current focusing and Li flux imbalance; (C)Internal deposition of Li due to electronic conductivity of solid electrolyte. (continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 57
(continued from previous page)
that rely on electro-stripping and applying these in the context of batteries.
©The Electrochemical Society. DOI: 10.1149/2.F10242IF
About the Authors




Stephen T. Fuller, PhD Student, Chemical Engineering, University of Texas at Austin (UT Austin)
Education: PhD student with Prof. Kent Zheng; BS in Chemical Engineering (Georgia Tech).
Research Interests: Include controlling crystallographic growth for highly reversible plating and stripping in metal anodes, and understanding the structure and composition of the solid-electrolyte interphase in batteries.
Yonglin Huang, PhD Student in Mechanical Engineering, Rensselaer Polytechnic Institute (RPI)
Education: PhD student with Prof. Fudong Han Research Interests: Development of anode materials for solid-state batteries

Natasa Vasiljevic, Senior Lecturer, School of Physics, University of Bristol
Education: BS in Physics (University of Belgrade); PhD in Science and Engineering of Materials (Arizona State University)
Research Interests: Electrochemical surface science, electrodeposition of thin films and functional nanomaterials for electrocatalysis, magnetics and optoelectronics.
Work Experience: Lecturer at the School of Physics, University of Bristol (since 2011); Research Associate, Sandia National Laboratories, Albuquerque, New Mexico, US (2004–2008)
Pubs: 50 + papers, 1 book chapter
Awards: Great Western Research Fellowship, University of Bristol, UK (2008–2011)
Work with ECS: Member of the ECS ISTS Subcommittee (2019–2023), ELDP Division executive committee member (2011–2023), ELDP Division Chair (2021–2023).
Ruixin Wu, PhD Student in Mechanical Engineering, RPI
Education: PhD student with Prof. Fudong Han Research Interests: lithium and lithium alloy anode for solid-state batteries
Fudong Han, Assistant Professor of Mechanical, Aerospace, and Nuclear Engineering, RPI
Education: PhD (University of Maryland College Park)
Research Interests: Advanced materials for electrochemical energy storage systems, particularly solid-state batteries Pubs: 80+ papers, 18000+ citations
Honors & Awards: Recipient of the ECS Electrodeposition Early Career Investigator Award
Work with ECS: Member of the ECS Electrodeposition Division Early Career Forum Organizing Committee.
Website: https://faculty.rpi.edu/fudong-han http://orcid.org/ 0000-0003-2507-4340



J. X. Kent Zheng, Assistant Professor, McKetta Department of Chemical Engineering, Department of Physics, and Texas Materials Institute, UT Austin
Education: PhD (Cornell University)
Research Interests: Understanding and controlling the physicochemical processes involved in electro-crystallization
Pubs: 60+ papers, 4000+ citations
Awards: Recipient of the ECS Electrodeposition Early Career Investigator Award
Work with ECS: Member of ECS and ECS Electrodeposition Division Early Career Forum Organizing Committee


Website: https://che.utexas.edu/people/faculty/zheng https://orcid.org/0000-0002-0673-0560


Website: https://research-information.bris.ac.uk/en/persons/natasavasiljevic https://orcid.org/0000-0002-7515-9708
References
1. T. N. Lung, Hydrometallurgy, 17(1), 113 (1986).
2. G. N. Lewis and F. G. Keyes, F. G., J Am Chem Soc, 35(4), 340 (1913).
3. M. V. Reddy, A. Mauger, C. M. Julien, et al., Materials, 13(8), 1884 (2020).
4. B. Scrosati, J Solid State Electrochem, 15 (7), 1623 (2011).
5. J. Zheng and L. A. Archer, Sci Adv, 7 (2), eabe0219 (2021).
6. C. Raub, The history of electroplating. In Metal Plating and Patination, Elsevier: 1993; pp 284-290.
7. Higgins, T. W., The Causes and Prevention of Dendritic Growth in Zinc Electrodeposition. Polytechnic Institute of Brooklyn: 1962.
8. J. X. K. Zheng, J Electrochem Soc, 169 (10), 100532 (2022).
9. J. Zheng, T. Tang, Q. Zhao, et al., ACS Energy Lett, 4 (6), 1349 (2019).
10. C. Fang, J. Li, M. Zhang, et al., Nature, 572 (7770), 511 (2019).
11. J. Zheng and L. A. Archer, Chem Rev, 122 (18), 14440 (2022).
12. J. Zheng, M. S. Kim, Z. Tu, et al., Chem Soc Rev, 49 (9), 2701 (2020)
13. J. Zheng, R. Garcia-Mendez, and L. A. Archer, ACS Nano, 15 (12), 19014 (2021).
14. T. Tang, J. X. K. Zheng, and L. A. Archer, JACS Au (2024).
15. R. Puttaswamy, C. Mondal, D. Mondal, and D. Ghosh, Sustain Mat Technol, 33, e00477 (2022).
16. J. Janek and W. G. Zeier, Nat Energy, 1, 16141 (2016).
17. P. Albertus, S. Babinec, S. Litzelman, and A. Newman, Nat Energy, 3 (1), 16 (2018).
18. B. J. Neudecker, N. J. Dudney, and J. B. Bates, J Electrochem Soc, 147 (2), 517 (2000).
19. T. Krauskopf, F. H. Richter, W. G. Zeier, and J. Janek Jr, Chem Rev, 120 (15), 7745 (2020).
20. F. Han, J. Yue, X. Zhu, and C. Wang, Adv Energy Mat (2018).
21. K. Peppler, M. Poelleth, S. Meiss, M. Rohnke, and J. Janek, Zeitschrift für Physikalische Chemie, 220 (10), 1507 (2006).
22. L. C. De Jonghe, L. Feldman, and A. Beuchele, J Mater Sci, 16 (3), 780 (1981).
23. L. C. De Jonghe, J Electrochem Soc, 129 (4), 752 (1982)
24. M. Nagao, A. Hayashi, M. Tatsumisago, et al., Phys Chem Chem Phys, 15 (42), 18600 (2013).
25. M. J. Wang, E. Carmona, A. Gupta, et al., Nat Comm, 11 (1), 5201 (2020).
26. Y. Y. Ren, Y. Shen, Y. H. Lin, and C. W. Nan, Electrochem Commun, 57, 27 (2015).
58 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Fuller et al.
27. L. Porz, T. Swamy, B. W. Sheldon, et al., Adv Energy Mat (2017).
28. F. D. Han, A. S. Westover, J. Yue, et al., Nat Energy, 4 (3), 187 (2019).
29. J. Xiao, Science, 366 (6464), 426 (2019).
30. C. Brissot, M. Rosso, J. N. Chazalviel, and S. Lascaud, J Power Sources, 81, 925 (1999).
31. C. Monroe and J. Newman, J Electrochem Soc, 152 (2), A396 (2005).
32. B. Shao, Y. Huang, and F. Han, Adv Energy Mat, 13 (16), 2204098 (2023).
33. H.-K. Tian, Z. Liu, Y. Ji, L.-Q. Chen, and Y. Qi, Chem Mat, 31 (18), 7351 (2019).
34. X. Liu, R. Garcia-Mendez, A. R. Lupini, et al., Nat Mater, 20 (11), 1485 (2021).
35. Y. G. Lee, S. Fujiki, C. Jung, et al., Nature Energy, 5, 299 (2020).
36. T. Krauskopf, B. Mogwitz, C. Rosenbach, W. G. Zeier, and J. Janek, Adv Energy Mat, 9 (44) (2019).
37. A. Patra, J. Davis III, J.; S. Pidaparthy, et al., PNAS, 118 (22), e2025044118 (2021).
38. H. Zhang, H. Ning, J. Busbee, et al., Sci Adv, 3 (5), e1602427 (2017).
39. F. Shi, A. Pei, D. T. Boyle, et al., PNAS, 115 (34), 8529 (2018).
40. M. Datta and D. Landolt, J Electrochem Soc, 122 (11), 1466 (1975).


The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 59
Exhibit Sponsor Advertise Contact Anna.Olsen@electrochem.org today! CONNECT WITH THE ECS COMMUNITY











60 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org MichaelAdachi–Simon Fra s e r ytisrevinU 120+ YEARS OF CHAMPIONING THE ACCELERATION OF SCIENTIFIC DISCOVERY T h omasThundat–University a t B u f f la o Vish a l yrahduahChleDfoytisrevinU–i ItthiponJeerapan–Prince o f S ytisrevinUalkgno neevarP S e khar –Washington StateUnive r s i yt TrishaAndrew–University o f M sttesuhcassa tsrehmA leirA uF r st –Massachusetts Institute o fTe c h lonygo sraH h i ni Mukundan–LawrenceBerkele y NbaLlanoita NEW >73,000 downloads since launching in 2022 Average of >1,000 downloads per article GOLD OPEN ACCESS Associate Editors Associate Editors MAKE THE ECS FAMILY OF JOURNALS YOUR RESEARCH HOME www.electrochem.org/publications ECS Sensors Plus A j i tKhosla–Editor-in-Chief Netz Arroyo–Technic a l E rotid Johns Hopkins University School of Medicin
Electrochemically Induced Deposition (ECiD): A Versatile Method that Greatly Extends the Portfolio of Surface Coatings and Materials
Fabricated by Electrochemical Deposition
by Sai Gourang Patnaik, Genis Vanheusden, Ali Amir Saleh, and Philippe M. Vereecken
Electrochemical deposition captures a wide variety of materials, ranging from the electroplating of metals, alloys, and semiconductors to the electro-polymerization of polymers. Deposition can be done from both aqueous and non-aqueous solutions, but also from ionic liquids and molten salts. In more general terms, electrochemical deposition comprises all processes of (electro)chemical conversion of species initially dissolved in an electrolyte solution into a poorly soluble or insoluble compound, leading to their deposition onto an electrode surface. In the case of direct electrodeposition, this conversion is the direct result of an electrochemical change (i.e., by electroreduction or electro-oxidation of the dissolved species). The best-known examples are cathodic electrodeposition of metals from metal ion solutions such as copper plating from an aqueous solution of CuSO4 and sulfuric acid, where the Cu2+ ions are reduced by direct electron transfer of two electrons (e2 reaction) at the copper metal cathode:
Cu2+(aq) + 2e- → Cu(s) (1)
The obtained metal deposits are dense and smooth and are, for example, used as surface decoration coatings on shaped surfaces or to fill small cavities, such as for copper interconnects in computer chips.1,2 As the metal product is a perfect electronic conductor, there is in principle no limit on its thickness.
Electrolytic manganese dioxide or EMD is a good example of electrodeposition reactions where direct electron transfer at the electrode goes together with a chemical precipitation reaction. EMD is used in alkaline MnO2 batteries, and it is typically made by anodic electrodeposition from an aqueous solution of dissolved MnSO4 with sulfuric acid on titanium electrodes.3
Mn2+(aq) + 2H2O → MnO2(s) + 4H+(aq) + 2e- (2)
The deposition of MnO2 happens by the electro-oxidation of Mn2+ to Mn4+ which is insoluble and in the form MnO2. The actual reaction mechanism is somewhat more complex, with Mn3+ as the intermediate which can either precipitate as MnOOH and then oxidize further to MnO2 (ece reaction path 1) or first oxidize further to Mn4+ before it precipitates directly to MnO2 (eec reaction path 2)4:
(e1) Mn2+(aq) - e- ⇌ Mn3+(aq) (3)
Reaction path 1:
(c1) Mn3+(aq) + 2H2O ⇌ MnOOH(s) + 3H+(aq) (e2) MnOOH(s) + 2H+(aq) - e- ⇌ MnO2(s)
Reaction path 2:
(e2’) Mn3+(aq) - e- ⇌ Mn4+(aq) (c2’) Mn4+(aq) + 2H2O ⇌ MnO2(s) + 4H+(aq)
Both reaction paths do occur in parallel where the dominant mechanism determines the properties of the MnO2, morphology, and phase, which can be steered by the choice of solution composition and pH, deposition conditions, and even the nature of the substrate.4,5
Unlike copper metal, MnO2 is a poor electronic conductor and thus one would expect that the thickness is limited, as the formation of a thin, dense oxide film would inhibit the electron transfer steps and thus effectively passivate the electrode. However, despite the poorly conducting properties, micron thick layers can be grown with time as the deposited oxide films are mesoporous. In fact, the films seem to be constructed of tiny nanoparticles (~10nm) leaving openings of about the same pore size as shown in Fig. 1d. As such, the soluble precursor species for the reaction can still be transported through this porous deposit, allowing the electrochemical reactions to continue at the electrode surface, as illustrated in Fig. 1. The Mn2+ travels from the solution through the porous layer toward the electrode surface where it is oxidized with consequent precipitation reactions at the oxide/electrode interface, with the hydrated proton products traveling out of the layer.4,5 Hence, in contrast to the deposition of conductive metals which grow on the top of the deposited surface, these oxide films instead grow up from the MnO2/electrode interface (i.e., from the bottom up). This example demonstrates the role of film porosity during the electrodeposition of poorly conducting and insulating materials. Important, however, is that the diffusion through the mesoporous EMD is significantly slower than in the bulk solution, so that the growing film thickness creates an additional resistance that allows conformal coatings over high aspect ratio features such as the pillar array shown in Fig. 1c.
A special case in the group of redox precipitation reactions is the anodic deposition of metal-organic frameworks or MOFs. These are polymeric coordination structures consisting of metal ion centers coordinated by organic linkers and are typically crystalline with an ordered microporous structure. Anodic deposition entails that metal ions are introduced in a solution of linker by anodic dissolution of the metal anode, whereby the MOF forms on the surface. For example, HKUST-1 with composition [Cu3BTC2]n is a MOF with Cu2+ centers coordinated by 1,3,5-benzenetricarboxylate (BTC3-) ligands. Upon anodic polarization of a copper electrode in a solution of trimesic acid in an ethanol:water mixture, electronically insulating Cu-BTC clusters are formed on the copper surface:
Cu(s) - 2e- → Cu2+(solv.) (4)
3n Cu2+(solv.) + 2n BTC3- (solv.) ⇌ [Cu3BTC2]n (s) (5)
N. Campagnol et al.7 showed that the Cu-BTC clusters are formed according to a progressive nucleation and growth-like behavior where initially formed small clusters grow while more are being formed on a copper surface, as shown in Fig. 2. Hence, as opposed to EMD where a dense, mesoporous, conformal film is formed, here large crystallites are piled up on the surface. Using markers, the authors were able to show that the MOF crystals grow by diffusion of Cu2+ through the microporous MOF which grows by its coordination with BTC3- at the perimeters of the crystals. Upon extended growth time, the copper surface under-etches the MOF crystals, resulting in detachment.7
A third group of ECiD processes comprises indirect electrochemical deposition reactions (i.e., where the species being (continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 61
(continued from previous page)
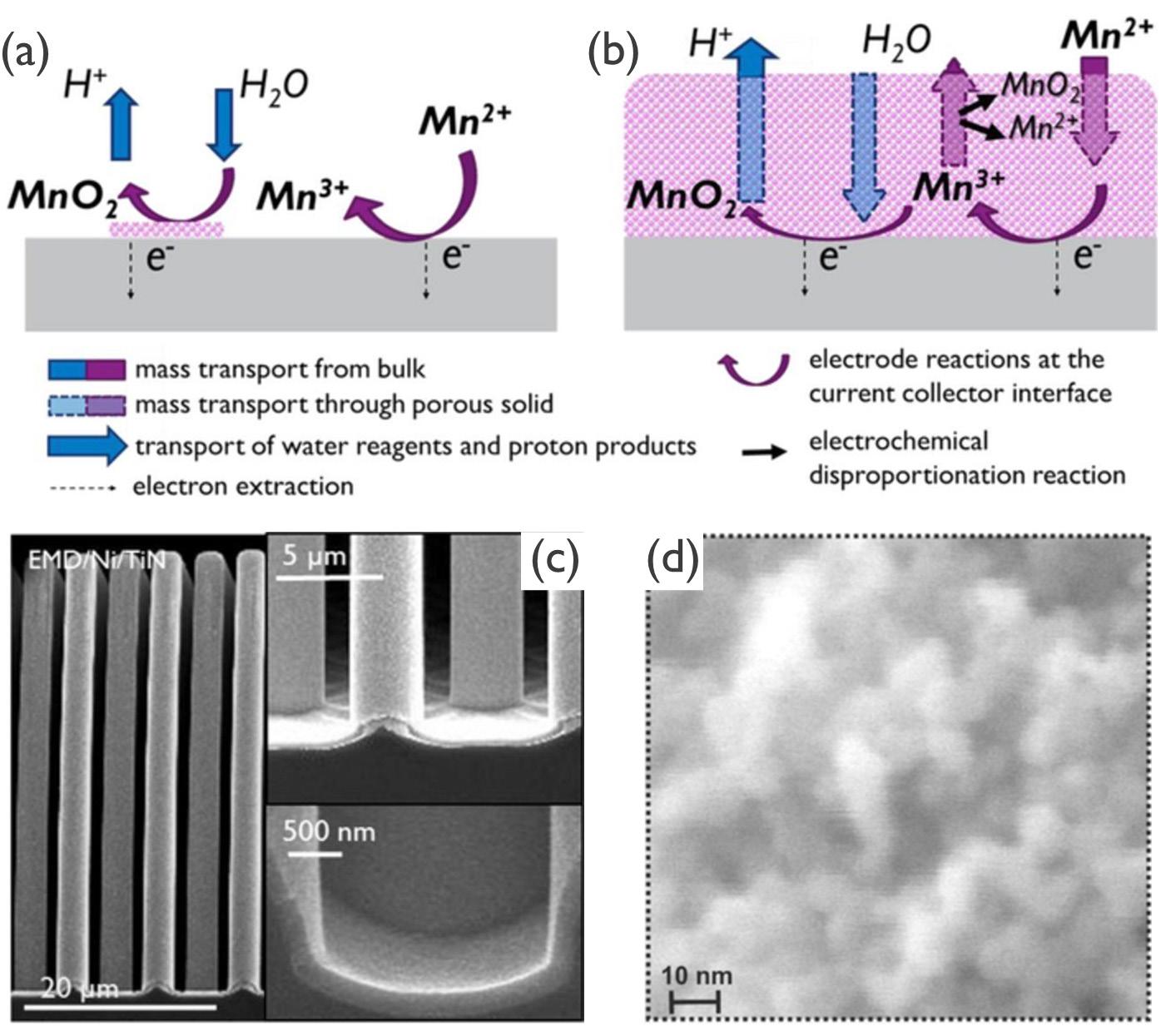
1. (a–b) Schematic of the electrodeposition of MnO2, which involves the transport of the reactant and product species through the porous deposited layer. (c) SEM images of conformal thick EMD films electrodeposited on ALD TiN/Si pillars coated with Ni by electrodeposition. EMD coating is ~250 nm, the pillars are 2 µm wide with 2 µm spacing and 50 µm height. (d) High resolution SEM image of the porosity of a 470 nm thick EMD layer grown by galvanostatic deposition at 0.5 mA/cm² for 600s on C/TiN. Reprinted with permission from references4 and6
deposited do not actually undergo a redox conversion, but instead another mediating species is electrochemically reduced or oxidized at the electrode). The first and best known examples are the deposition of oxides and hydroxides such as Cd(OH)2, Ni(OH)2, and ZnO, as a result of a local pH increase (e.g., by the electrochemical reduction of water or nitrates at the electrode).8,9
2H2O(l) + 2e- ⇌ H2(g/aq) + 2OH-(aq) (6) U0 = 0.00V vs. RHE
NO3-(aq) + 2e- + H2O ⇌ NO2-(aq) + 2OH-(aq) (7) U0 = +0.97V vs. RHE
When the OH- concentration near the electrode rises above the value of the solubility product of the metal hydroxide, the dissolved metal ions precipitate and deposit on the electrode surface. For example, Ni(OH)2 will precipitate from a 0.1M Ni2+ solution when the surface pH goes above 7.
Ni2+(aq) + 2OH-(aq) ⇌ Ni(OH)2 (s) (8) Ksp=5.5 x10-16 (mol/L)3
Most often nanocrystals are formed, sometimes with particular shapes, such as the sheet and nanopillar morphologies for ZnO shown
in Fig. 3.9 The crystalline morphologies leave sufficient porosity for, for example, the water reduction reaction to proceed with growth and nucleation of new crystals. The electrodeposition of oxides has been suggested for use in batteries and photoelectrochemical cells and as electrocatalysts.9,10
Early reports exploring electrodeposition of hydroxides for alkaline batteries date back to the 1980s. Palanisamy et al. reported the electrodeposition of Cd(OH)2 from aqueous Cd(NO3)2 solutions in 1980, followed by a similar process for the ECiD of Ni(OH)2 in 1987 by Ho et al.8,11 Although the reduction of nitrate ions was postulated as one of the possible mechanisms, judging from the very negative potentials needed, the local pH change was likely predominantly from the reduction of water. In 2000, O’Regan et al. used an allelectrodeposition process to fabricate ZnO/CuSCN heterojunctions for dye-sensitized solar cell applications.10 In this case, propylene carbonate was used as the solvent with the addition of small amounts of water (< 0.2%) such that the nanocrystalline ZnO was now deposited indirectly via the electroreduction of nitrate. Interestingly, the CuSCN was electrodeposited directly on top, filling the opening in the porous ZnO for an optimal interfacial heterojunction area. Note that the CuSCN is electrodeposited by an ec-mechanism from the
62 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Patnaik et al.
Fig.
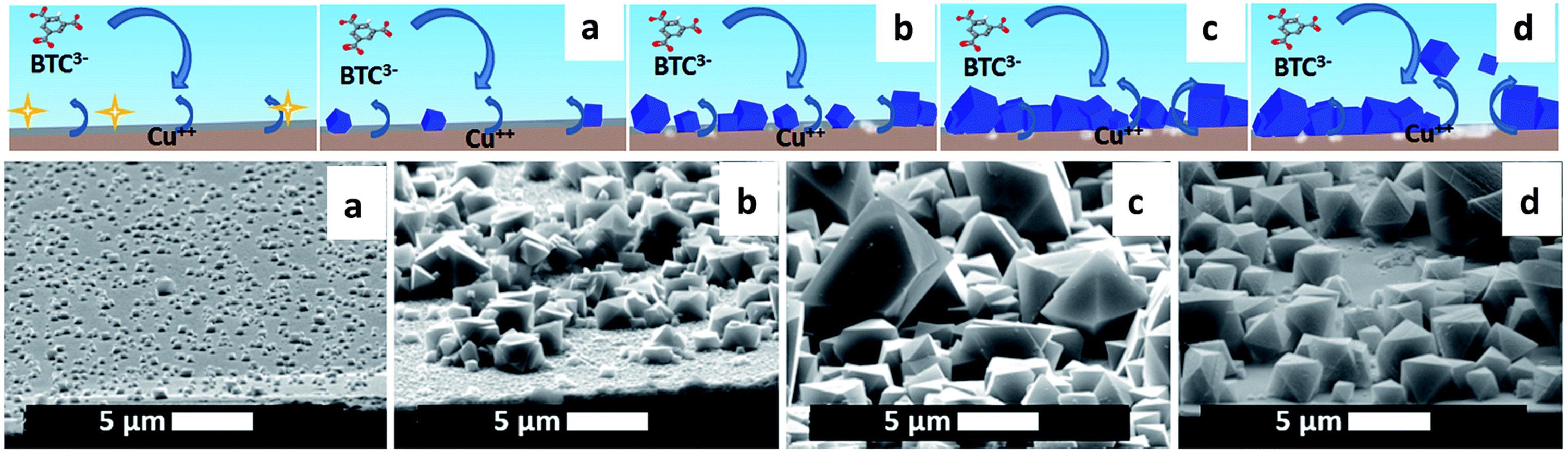
2. Proposed mechanism of MOF anodic electrodeposition showing four phases: (I) initial nucleation (a), (II) growth of islands (b), (III) intergrowth (c), and (IV) detachment (d) for HKUST-1 or [Cu3BTC2]n being a crystalline coordination polymer of Cu2+ metal ion center coordinated by BTC3- ligands from 48mM trimesic acid in ethanol/water 67:33 vol%. Reproduced with permission from reference7
second group described above where dissolved Cu2+ ions are reduced to Cu(I) and form insoluble CuSCN.
Because of the success of these early examples of electrochemically induced precipitation by a change in the near-surface pH, many more materials and electrochemical mediators, known as pro-bases for acid-base reactions, have been developed over the past decade or so. Along with oxides and hydroxides, other compounds such as phosphates have also been explored. Cathodic MOF deposition relies on a similar principle for the deprotonation of the ligands at the surface. Electrochemically induced deposition has now been
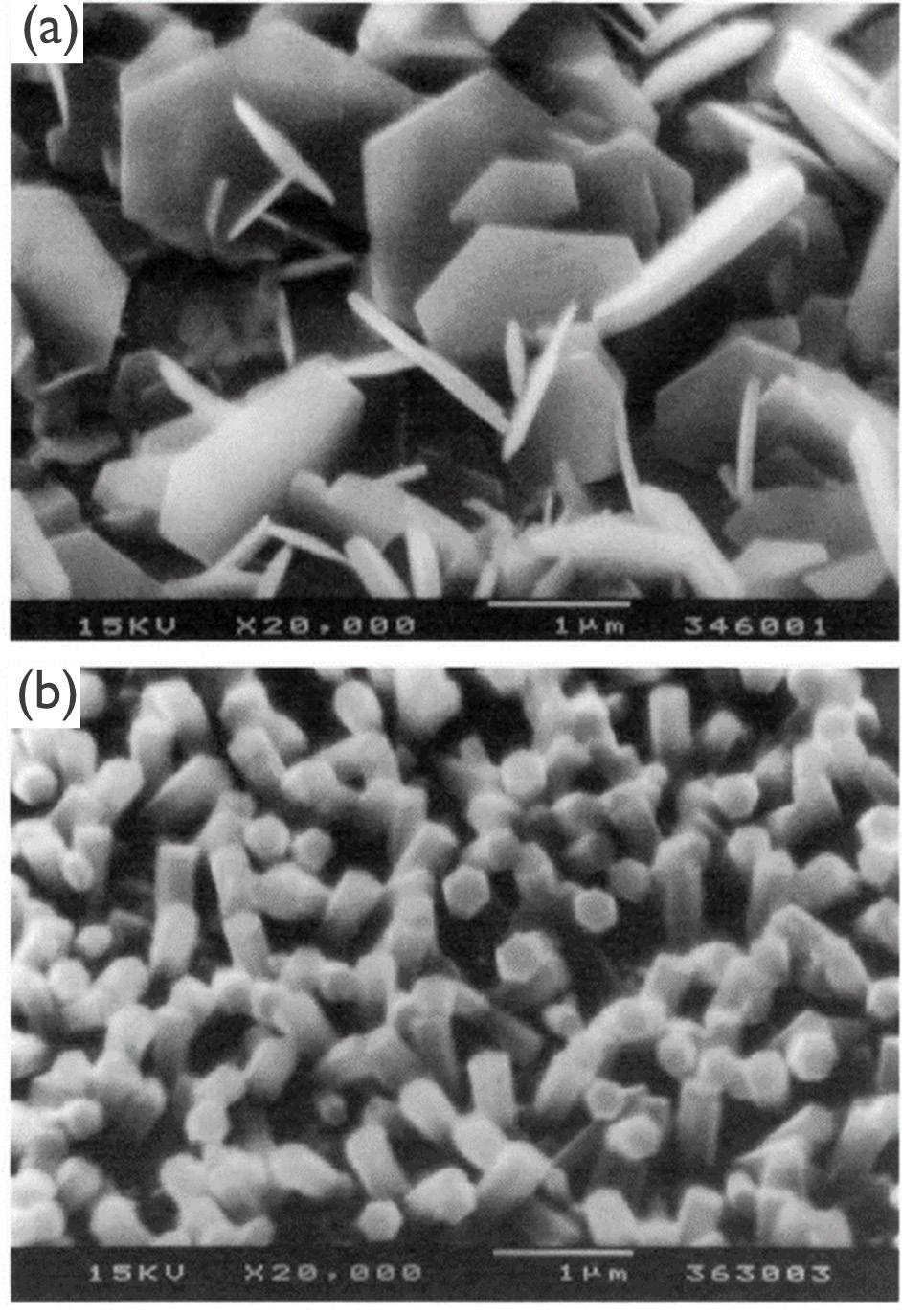
extended even to sol-gel reactions (see the next article in this issue), biochemical synthesis, and the deposition of soft biological coatings.12–16
Advances in Electrochemically induced Deposition
Electrochemically induced deposition or ECiD can be defined as the deposition of compound films on a surface, as the result of chemical reactions in the near surface region between a precursor present in the bulk solution and an electrochemically generated precursor, mediator, or catalyst near that surface (Fig. 4). The electrochemically modified zone is characterized by a diffusion layer with a concentration gradient of the electrochemically generated or consumed mediator. Interestingly, such concentration gradients can also be built up over a membrane, inducing deposition on the membrane instead.17–19
Pro-bases for ECiD
The term pro-base refers to a chemical entity that can undergo electrochemical alteration near the working electrode to facilitate specific reactions on/near the working electrode surface. Bronsted pro-bases consume H+ or generate OH- depending on the pH during electro-reduction or electro-oxidation. Typical examples of Bronsted pro-bases are: H2O (including H3O+ hydronium cations and OHhydroxyl anions), NO3- , H2O2, and dissolved O2, but in principle any electrochemical half-reaction that involves H+ and OH- (and that is the majority) could work. The purpose is to initiate a Bronsted acidbase reaction locally, near the electrode surface.
Water is the most straightforward pro-base to use for aqueous chemistry (reaction (6)). In an electrochemically induced precipitation reaction, the electrochemical reaction triggers the (de)protonation and subsequent precipitation of an electrochemically inactive species as the solubility limit is locally exceeded. For the electrochemical deposition of oxides and hydroxides, the electrogenerated OH- is used directly in the precipitation reaction. However, the OH- can also be used for an acid-base reaction to form another anion, such as phosphate, for its precipitation with a cation. The deposition of hydroxyapatite (Ca10(PO4)6(OH)2) was reported by Eliaz et al.20 in 2005, and the deposition of Li3PO4 by Liu et al.21 in 2006. The precipitation of these metal phosphates is also induced by a pH increase at the surface due to water reduction which then regulates the phosphate ion equilibrium and thus indirectly the solubility product of the metal phosphates:
H2PO4-(aq) + OH- ⇌ HPO42-(aq) + H2O(l) (9) pKa = 7.2
HPO42-(aq) + OH- ⇌ PO43-(aq) + H2O(l) (10) pKa = 12.7
3Li+(aq) + PO43-(aq) ⇌ Li3PO4(s) (11) Ksp = 2.37*10-11 ref22
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 63
Fig.
Fig. 3. SEM images of ECiD of ZnO from (a) 5mM zinc chloride solution on a gold substrate with -1 V applied, and from (b) 20mM zinc chloride solution on a tin oxide substrate with -1.3 V applied. Reproduced with permission from reference9
(continued from previous page)
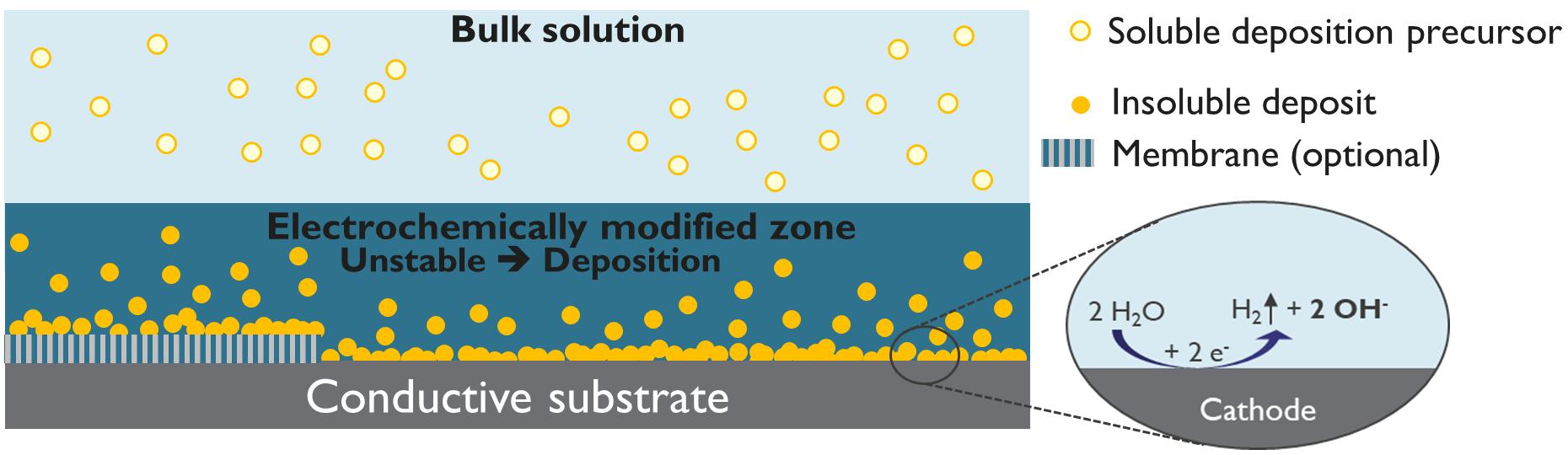
Note that all reactions are written from the point of view of increasing OH- concentration for ease of explanation. However, pH change can also start from mild acid solutions and precipitation can also happen at pH<7, depending on the targeted anion and the solubility product.
The precipitation reactions induced by water electroreduction have hydrogen gas evolution going on during the deposition reaction. The formation of gas bubbles does affect the film morphology and intrinsically ensures porosity. In the case of hydroxyapatite, that is a favorable quality, as porosity in the coating is needed for biocompatibility.20 However, for Li3PO4 to be used in battery applications as thin-film solid electrolyte, a dense, pinhole-free film is desired. Therefore, other pro-bases than water are considered.
Peroxide, which has a higher reduction potential than water, has been suggested as a pro-base to avoid hydrogen evolution:
H2O2(aq) + 2 e ⇌ 2OH- (aq) (12) U0=1.78V vs. RHE
However, due to its high oxidative nature, peroxide severely limits the availability of stable metal substrates. Copper, for example, etches in peroxide solutions. Also, the chemical instability of the peroxide solution itself can lead to oxygen gas formation, which is especially an issue when using catalytic surfaces such as platinum. Fig. 5 shows Li3PO4 deposits obtained from reduction of H2O2 on TiN substrates, which were passivated by a thin TiO2 film formed by oxidation.23 Note the hollow igloo-like structures, which are due to gas bubbles on the
surface. The oxidative properties can also be used as an advantage. For example, the cathodic deposition of Cu-BTC MOF is not possible at negative potentials due to the co-deposition of metallic copper.24 In the presence of H2O2, the potential can be kept less negative and any copper still co-deposited electrochemically will be chemically oxidized again by H2O2 as long as the H2O2 electroreduction is not driven under diffusion limitation.
The pro-base principle can indeed also be used for deprotonation of a ligand to launch its attachment to metal nodes during cathodic MOF electrodeposition.18,25 The local pH change leads to deprotonation of ligands and precipitation of metal organic frameworks.
mOH- + Hm(Ligand) ⇌ (Ligand)m- + mH2O (13) n(Ligand)m- + mMn+ ⇌ [MmLigandn] (14)
In conventional solution-based solvothermal/hydrothermal synthesis, metal salts and organic linkers are dissolved in a solvent with some water, which acts as a weak acid for deprotonating the linkers and autoclaved at elevated temperatures to achieve the desired MOFs. However, for specific applications like membrane fabrication, catalytic coatings, and micro patterning for electronic devices, compact and uniform MOF thin films are required on various substrates for integration in devices.26 Cathodic deposition provides excellent synthetic knobs for tuning the thickness, porosity, and morphology of MOF coatings. Already today, MOFs are industrially synthesized via electrochemical routes, for example by BASF.27,28

64 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Patnaik et al.
Fig. 5. SEM images showing the effect of H2 bubble formation during Li3PO4 ECiD from (a) 0.5M LiNO3 and 0.02M NH4H2PO4 aqueous solution and from (b) 0.48M LiH2PO4 in 1% H2O2 solution. Note the “igloo” structure where the deposit seems to have grown around a gas bubble. (a) reproduced from reference21
Fig. 4. Illustration of an indirect electrodeposition by a local pH increase, either directly on an electrode or on a membrane on the electrode.
Water can be used for MOFs that are water stable. For example, Wei et al.29 have shown all-aqueous cathodic deposition of ZIF-8 type MOFs. However, as many MOF materials are not stable in pure water, their synthesis is done with organic solvents. As discussed above for the ZnO nanocrystals, nitrate anions are very well suited as pro-bases for ECiD in organic solutions with low water content. Nitrate was also used as the pro-base for the first demonstration of cathodic deposition of MOF-5 or Zn4O(BDC)3 by Dinca et al.25 Since then, this principle has been successfully used for the deposition of a range of MOFs (Cu3BTC27, FeBTC30, and zeolitic imidazolate frameworks or ZIFs31). Cathodic deposition of MOFs in the presence of small amounts of water still holds a risk of precipitating oxides/ hydroxides and hence such synthesis is usually carried out in organic solvents like dimethyl formamide (DMF). However, because metal nitrates have limited solubility in organic solvents, alternative probases to nitrates had to be sought. In this regard, trimethyl ammonium is a pro-base which is compatible with non-aqueous solutions which has already shown to be useful for MOF deposition32:
2Et3NH+ + 2e - ⇌ 2Et3N + H2 (15) U0= -0.63V vs. SHE Et3N + H-Ligand ⇌ (Ligand)- + Et3NH+ (16)
Molecular oxygen (O2) can be electrochemically reduced to form its superoxide O2- radical in aprotic polar solvents like DMF.33
O2 + e- ⇌ O2 (17) U0 = 0.05V vs. SHE
In the presence of metal ions, oxide deposition occurs with excellent electrode coverage as demonstrated by the quick electrode passivation, which is of particular interest for thin catalyst coatings. The reaction for the example of a bivalent cation is:
2O 2 (solv.) + 2M 2+ (solv.) → 2MO(s) + O 2 (g) (18)
The deposition reaction (18) is a redox reaction and more particularly the disproportionation of the superoxide radical in molecular O2 oxidation state 0 and O (II) in the form of the precipitated metal oxide.33 Hence, as no acid-base reaction is involved, molecular oxygen is not actually a Bronsted pro-base. Yet, it is often indicated as a pro-base because it induces a precipitation reaction. Synthesis of many metal oxides and mixed oxides has been demonstrated this way (e.g., CoO, FeO, NiO, MnO2,, CdO, ZnO and In2O3).33–36 Note that the superoxide radical would react with the solvent in the absence of metal ions, unless it is stabilized with complexing agents such as quaternary ammonia cations like tertiary butyl ammonium (TBA+)33:
O 2 + TBA + + e ⇌ [TBA + − O 2 ˙ ] (19)
The stabilization of the superoxide radical has been used also to deposit MOFs.31,37
When designing an ECiD, in principle any half reaction that involves H+ (or OH-) is a candidate, as long as it does not interfere with the chemical deposition reaction and is compatible with the chemical environment. An example is the p-benzoquinone/1,4dihydroxybenzene redox couple, which is often used for electrochemically assisted processes in biological devices such as for synthesis of DNA microarrays by localized acid generation at microelectrode arrays.38
Conclusions
The ECiD process has evolved from an odd curiosity into a thriving new branch in electrodeposition science. The library of materials synthesized by ECiD has been greatly expanded, along with the number of precipitation triggering knobs or tricks. Together with the breadth of possibilities and applications, the science behind the process has also accelerated; however, many open questions remain. The moving boundary problem of supersaturation into the solution phase for confined interfacial deposition means a complex description of the problem which triggers more and more the scientific interest of
our electrodeposition community. The fabrication of closed coatings by electrochemically induced deposition is not evident following the inherent flocculating nature of a precipitation reaction. Nucleation and growth studies which are commonplace in direct electrodeposition are still rare for these indirect deposition mechanisms.7,39 For a complete description, such studies not only call for a good description of the supersaturation of species near the surface but one also needs to be able to describe the kinetics and place of nucleation, as initial precipitates and complexes can be formed in the solution region above and near the surface. Furthermore, the growth of these insulators needs to be understood and controlled. If diffusion of the precursors through the material is inhibited, the growth will stop. If the products such as dissolved hydrogen cannot be removed sufficiently, gas formation will occur with potential or eventual breakage of the films. The field of electrochemically induced deposition is growing with new material systems, processes, and methodologies to tackle also coating properties such as morphology and composites. The theorical understanding still lags behind, but with the prospect of exciting new science emerging in the coming decades.
©The Electrochemical Society. DOI: 10.1149/2.F11242IF
About the Authors

Sai Gourang Patnaik, Researcher, Electrochemical Storage and Conversion Group, imec
Education: PhD in Material Science (Japan Advanced Institute of Science and technology, JAIST)
Research Interests: Electrochemistry, material synthesis, and interfacial engineering
Work Experience: Japanese government MEXT Fellow during graduate school, working on synthesis of imine-based polymers for electrochemical applications. Postdoctoral training at the Laboratory for Analysis and Architecture of Systems (LAAS-CNRS, Toulouse) and as an Alexander von Humboldt Fellow at Humboldt University of Berlin



Genis Vanheusden, Researcher, Electrochemical Storage and Conversion Group, imec
Education: PhD in Bioscience Engineering (University of Leuven (KU Leuven) and imec)
Research Interests: Include electrochemically induced sol-gel depositions for energy applications
Ali Amir Saleh, PhD student, KU-Leuven and Electrochemical Storage and Conversion Group, imec
Education: Master’s degree in Chemistry (KULeuven)
Research Interests: Include material synthesis, electrochemistry, and physical chemistry
Philippe Vereecken, Fellow, imec, program line coordinator P2M, EnergyVille, and professor, KU Leuven
Education: PhD in Physical Chemistry (Ghent University)
Research Interests: The combination of electrochemistry and nanomaterials and specifically in its use in semiconductors, energy conversion, electrochemical storage, and nanomaterials synthesis
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 65
(continued from previous page)
Work Experience: Postdoctoral Associate in the Department of Materials Science and Engineering at The Johns Hopkins University (1998–2001) and Research Staff Member (RSM) at IBM T.J. Watson Research Center (2001–2005). He joined imec in 2005 and KU-Leuven in 2009.
18. S. Xie, Z. Zhou, X. Zhang, and J. Fransaer, Chem Soc Rev, 52 (13), 4292 (2023).
19. P. Vereecken, S. Zankowski, N. Hendrickx, et al., Method for the Fabrication of a Thin-Film Solid-State Battery with Ni(OH)2 Electrode, Battery Cell, and Battery. US 10,115,961 B2, 2018.
20. N. Eliaz, T. M. Sridhar, U. Kamachi Mudali, and B. Raj, Surf Eng, 21 (3), 238 (2005).
21. H. C. Liu and S. K. Yen, J Power Sources, 159 (1 SPEC. ISS.), 245 (2006).


Work with ECS: ELDP Division executive committee member (2008-2023) https://orcid.org/0000-0003-4115-0075
References
1. P. C. Andricacos, C. Uzoh, J. O. Dukovic, J. Horkans, and H. Deligianni, IBM J Res Dev 42 (5), 567 (1998).
2. P. M. Vereecken, R. A. Binstead, H. Deligianni, and P. C. Andricacos, IBM J Res Dev, 49 (1), 3 (2005).
3. A. J. Gibson, B. Johannessen, Y. Beyad, J. Allen, and S. W. Donne, J Electrochem Soc, 163 (5), H305 (2016).
4. M. Y. Timmermans, N. Labyedh, F. Mattelaer, et al., J Electrochem Soc, 164 (14), D954 (2017).
5. N. Labyedh, F. Mattelaer, C. Detavernier, and P. M. Vereecken, J Mater Chem A, 7 (32), 18996 (2019).
6. S. Deheryan, Y. Zargouni, R. Sinha, et al., J Electrochem Soc, 164 (2), A538 (2017)
7. N. Campagnol, T. R. Van Assche, M. Li, et al., J Mater Chem A, 4 (10), 3914 (2016).
8. T. Palanisamy, Y. K. Kao, D. Fritts, and J. T. Maloy, J Electrochem Soc, 127 (12), 2535 (1980).
9. S. Peulon and D. Lincot, J Electrochem Soc, 145 (3), 864 (1998).
10. B. O’Regan, D. T. Schwartz, S. M. Zakeeruddin, and M. Grätzel, Adv Mater, 12 (17), 1263 (2000).
11. K. Ho, J Electrochem Soc, 134 (2), 52C (1987).
12. J. Li, S. Wu, E. Kim, et al., Biofabrication, 11 (3), 032002 (2019).
13. J. Li, E. Kim, K. M. Gray, et al., Adv Funct Mater, 30 (30), 1 (2020).
14. J. Li, S. P. Wang, G. Zong et al., Adv Mater, 33 (18), 1 (2021).
15. K. Yan, Y. Liu, J. Zhang, et al., Biomacromolecules, 19 (2), 364 (2018).
16. M. Lei, X. Qu, H. Liu, et al., Adv Funct Mater, 29 (18), 1 (2019).
17. S. Xie, X. Zhang, X. Tan, et al., ACS Mater Lett, 4 (9), 1721 (2022).

22. W. M. Haynes, CRC Handbook of Chemistry and Physics, 95th ed., Hoboken: CRC Press, 2014.
23. A. A. Saleh, Electrochemically Induced Li3PO4 Precipitation, Master’s thesis, KU Leuven, 2022.
24. S. Xie, W. Monnens, K. Wan, et al., Angew Chemie Int Ed, 60 (47), 24950 (2021).
25. M. Li and M. Dincă, J Am Chem Soc, 133 (33), 12926 (2011).
26. A. J. Cruz, I. Stassen, M. Krishtab, Chem Mater, 31 (22), 9462 (2019).
27. I. Stassen, N. Campagnol, J. Fransaer, P. Vereecken, D. De Vos, and R. Ameloot, CrystEngComm, 15 (45), 9308 (2013).
28. C. Kiener, U. Müller, and M. Schubert, Method of Using a Metal Organic Frameworks Based on Aluminum Fumarate. US 8,518.264 B2, 2013.
29. R. Wei, H. Chi, X. Li, et al., Adv Funct Mater, 30 (7), 1 (2020).
30. B. Zhang, P. Huang, J. Chen, et al., Appl Surf Sci, 504 (June 2019), 144504 (2020).
31. Q. Zhang, Z. Wu, Y. Lv, et al., Angew.Chemie Int Ed, 58 (4), 1123 (2019).
32. M. Li and M. Dincă, Chem Sci, 5 (1), 107 (2014).
33. R. A. Prato M., J. Fransaer, and X. Dominguez-Benetton, J Mater Chem A, 11 (38), 20824 (2023).
34. S. Sawatani, S. Ogawa, T. Yamada, et al., Trans Mater Res Soc Jpn, 28, 381 (2003).
35. R. Jayakrishnan and G. Hodes, Thin Solid Films, 440 (1–2), 19 (2003).
36. G. Vanhoutte, M. Wu, S. Schaltin, et al., J Mater Chem, 4 (35), 13555 (2016).
37. I. Liberman, R. Ifraemov, R. Shimoni, and I. Hod, Adv Funct Mater, 32 (19) (2022).
38. K. Maurer, J. Cooper, M. Caraballo, et al., PLoS One, 1 (1), e34 (2006).
39. N. Eliaz, W. Kopelovitch, L. Burstein, E. Kobayashi, and T. Hanawa, J Biomed Mater Res Part A, 89A (1), 270 (2009). Patnaik et al.

247th ECS MEETING
Montréal, Canada May 18-22, 2025
Palais des Congrès de Montréal
66 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
electrochem . o rg/meetings Abstract Submission Opens in August
Electrochemically Induced Templated Sol-gel Deposition of Mesoporous Silica and Nanocomposites: The New Kid on the ECiD Block
by Genis Vanheusden and Philippe M. Vereecken
Electrochemically Induced Sol-gel Deposition of Silica Thin-films
A relatively new member of the group of electrochemically induced deposition is that of templated sol-gel deposition. Uniform and smooth mesoporous silica thin films were first fabricated by an ECiD process nicknamed electrochemically assisted self-assembly or EASA, introduced by Walcarius et al. (Fig. 1).1 The addition of cetyltrimethylammonium bromide or CTAB as organic template molecules to the tetraethyl orthosilicate (TEOS) sol-gel precursor solution resulted in silica thin films with highly ordered, hexagonal arrays of vertical mesopores of 2–3 nm in diameter. These films have been proposed for applications in electroanalysis2–4 and sensors,5–7 where effects such as pre-concentration or perm selectivity have been exploited. Their synthesis combines the well-established field of templated sol-gel synthesis with an electrochemically induced deposition process. To prevent spontaneous gel formation, the silica sol-gel precursor solution has a mildly acidic pH which kinetically inhibits the gelation reaction. Only when a cathodic potential/current is applied to electrogenerate OH- ions from the pro-base (water mostly), the gelation reaction is catalyzed by the local increase in pH, resulting in the growth of silica gel films on the electrode. We will first review the principles of sol-gel reactions, before proceeding to details of ECiD of sol-gel films.
Hydrolysis-condensation Reaction: From Sol to Gel
Fig. 2 shows a gelation scheme for the hydrolysis-condensation reactions of tetraethyl orthosilicate (TEOS) to form a silica gel. The TEOS, added to a water-alcohol mixture, undergoes a hydrolysis
reaction with the release of alcohol as the alkoxy groups are replaced by –OH or silanol groups8,9:
Hydrolysis reactions:
In parallel, the hydrolyzed species undergo condensation reactions with their silanol groups, releasing water or alcohol, resulting in the interlinking of the silanol monomers by forming Si–O–Si networks:
Condensation reactions:
These reactions initially lead to the formation of small, branched clusters or nanoparticles fully dispersed in the solvent (the sol), which link further to eventually form an interlinked silica network (the gel). The kinetics of both hydrolysis and condensation reactions are highly sensitive to the reaction conditions, particularly to the pH, defining the network structure and gelation times.10 The condensation and subsequent gelation is catalyzed by alkaline conditions, thus allowing a “two-step” process,9,11 which is exploited by electrochemically induced gelation. Other oxides such as TiO212,13 or ZrO214 can also be prepared through similar routes. Generally, SiO2 is considered to have the most favorable kinetics to enable process control, while also being the most well-studied sol-gel system.15–18
Templated Sol-gel
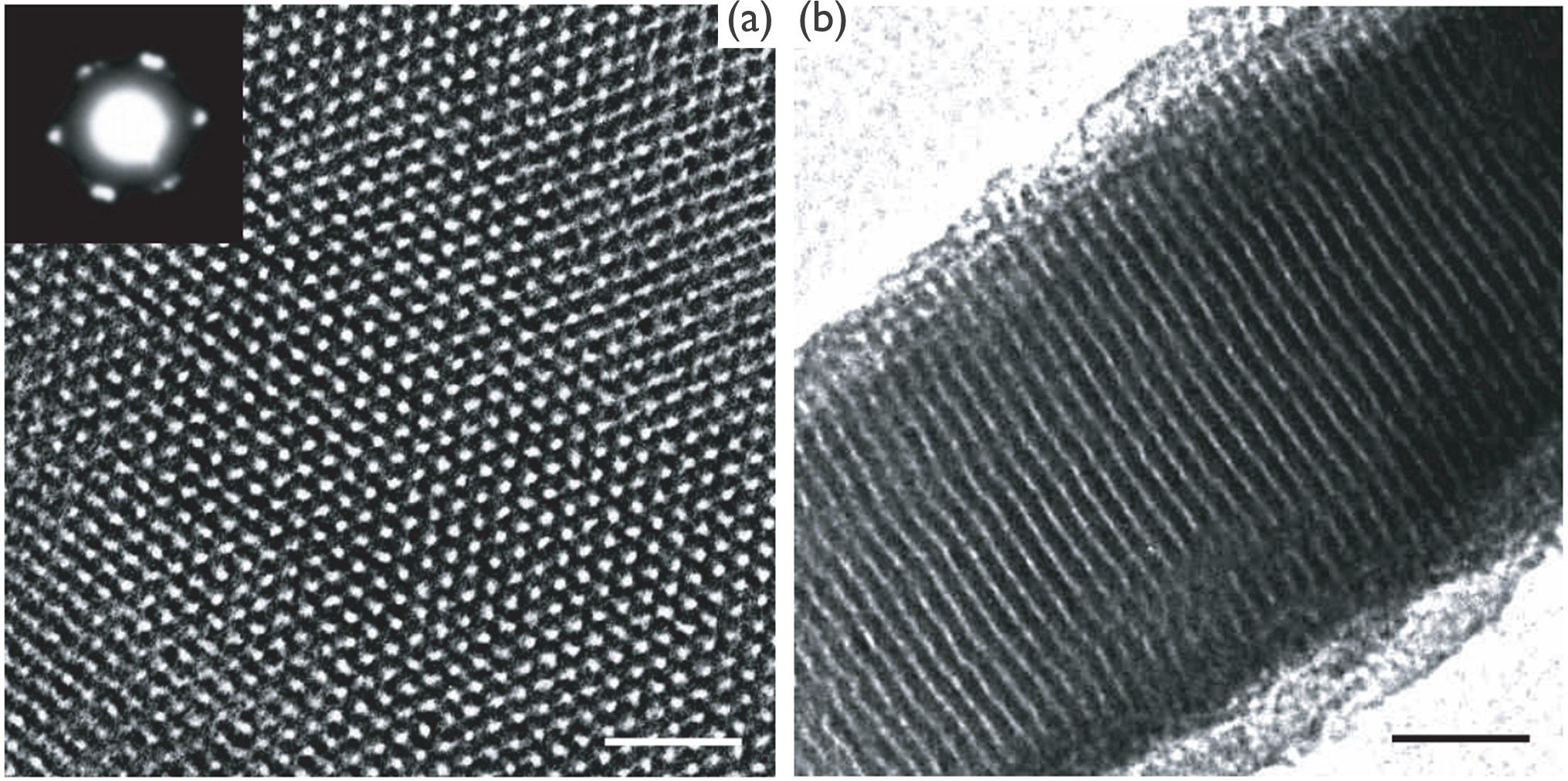
The addition of templates or structure-directing agents to the solgel process enables the formation of pores, the structure of which can be tuned by the choice of template and synthesis conditions (Fig. 3). The first reports on templated sol-gel synthesis date back to 1992 from Mobil19,20 with a family of mesoporous silica materials, designated as M41S. Fig. 3(A) shows MCM-41, which possesses an ordered, hexagonally packed array of cylindrical pores resulting from the micelle-forming characteristic of CTAB surfactant and template. The pore size and pore direction are determined by the micelle size and their interaction and can be tailored by the length of the surfactants, by adding so-called “swelling agents,” and by the synthesis conditions.9,21–25
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 67
≡ Si – OR + H2O ⇌ ≡ Si – OH + ROH (1)
≡ Si – OH + HO – Si ≡ ⇌ ≡ Si – O – Si ≡ + H2O (2) ≡ Si – OH + RO – Si ≡ ⇌ ≡ Si – O – Si ≡ + ROH (3)
from reference.1
Fig. 1. TEM images of mesoporous silica deposited through the EASA process on glassy carbon surfaces. (a) topdown view and (b) cross-section view. Scale bars correspond to (a) 50 and (b) 20 nm. Modified
(continued from previous page)
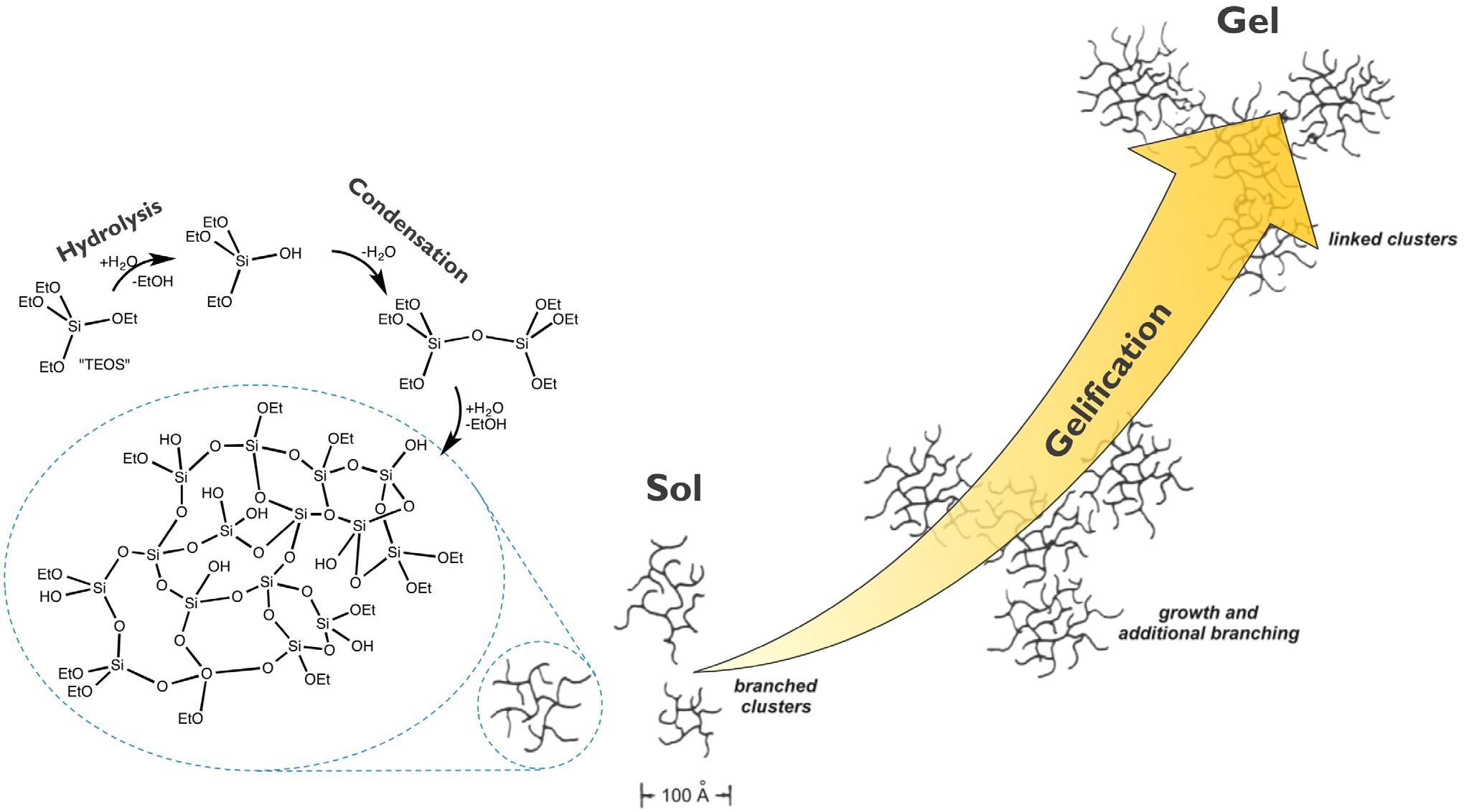
Electrochemically Induced Sol-gel Deposition
The process of silica electrodeposition from sol-gel solution was first reported by Woo et al.27 in 1993. The principle was further explored by Shacham et al., first for SiO2 28 and later extended to zirconia29 in 1999 and 2004, respectively. Meanwhile, other oxides such as Al2O330 and ZnO31,32 were demonstrated, also including combinations of oxides33,34 and composites.35 In electrochemically induced sol-gel deposition, the gelation reaction is triggered by the
electrochemical generation of OH- anions (for alkaline catalyzed condensation such as for silica and zirconia) or of H+ cations (for acid catalyzed condensation such as ZrO2) at the electrode surface, typically from water as a pro-base (for more detail on pro-bases in electrochemically induced deposition or ECiD, we refer to the previous article in this issue). Templated electrodeposition was first introduced in 2007 by Walcarius and colleagues.1 They added the CTAB surfactant, and thus obtained the typical cylindrical hexagonally packed nanochannels (similar to those in “traditional” MCM-41 in Fig. 3(A), but interestingly with the pores oriented normal to the electrode substrate (Fig. 1). The vertical pore orientation was
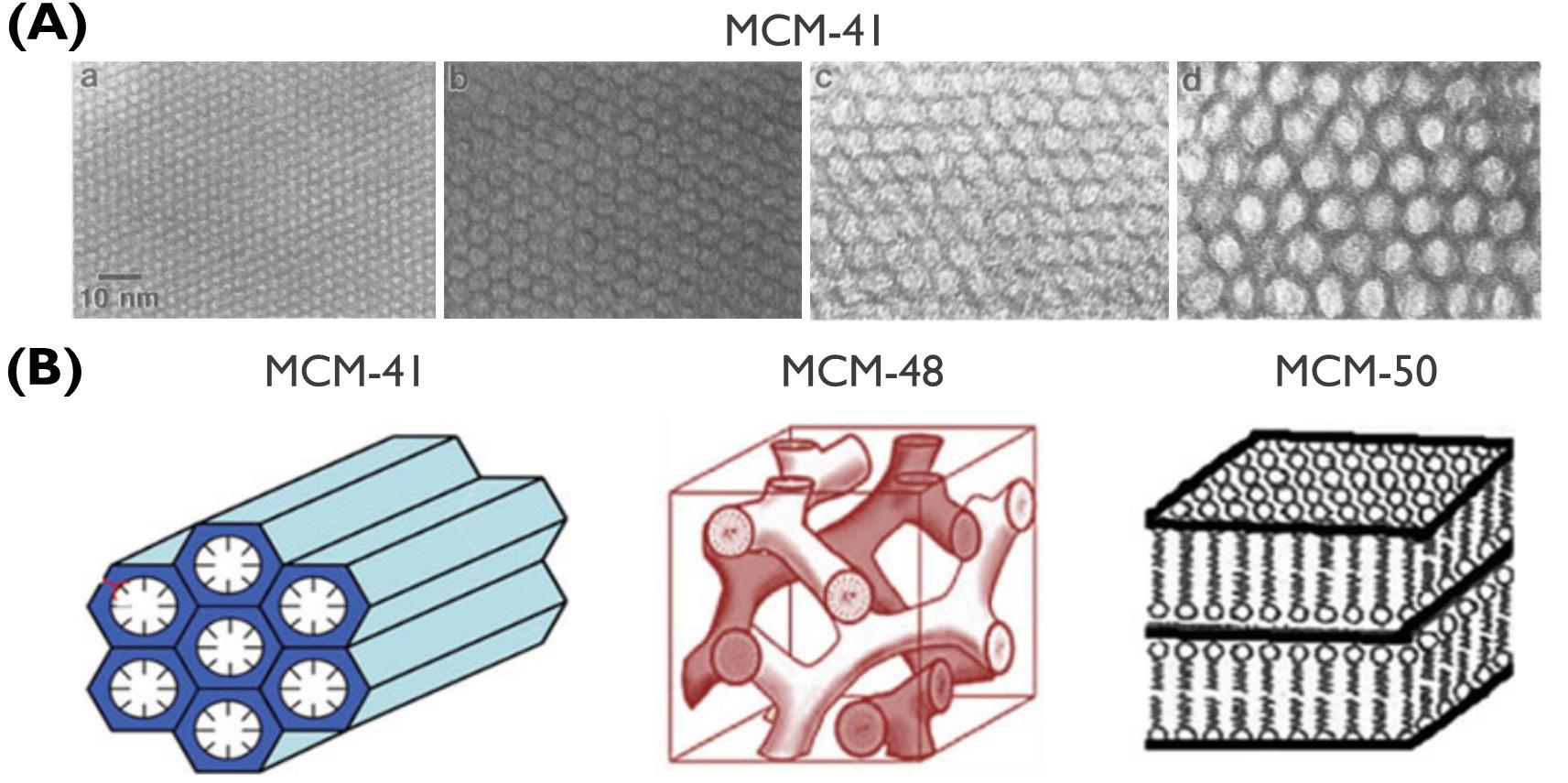
68 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org Vanheusden
and Vereecken
Fig. 2. Scheme showing the gelation through hydrolysis-condensation reactions of TEOS to form SiO2
Fig. 3. (A) Transmission electron microscopy (TEM) images of the MCM-41 materials prepared with varying pore sizes of 2 (a), 4 (b), 6.5 (c), and 10 (d) nm diameter. (B) Schematic showing the pore structure of several of the M41S-class materials. Adapted with permission from references19 (A) and26 (B).

Fig. 4. SEM images ((a) and (b)) of typical mesoporous silica thin films on TiN-coated Si wafers, prepared via the electrochemically induced templated sol-gel deposition procedure using (a) a stationary electrode without diffusion layer control and (b) a rotating disk electrode rotating at 500 RPM with a controlled diffusion layer. (c) Potential – time transients of the applied potential necessary to maintain a -1 mA/cm² current density during the deposition on a TiN substrate at 500 RPM. Different regions in the deposition are indicated, with the initial phase of a thin film with negligible resistance (white zone), followed by the growth of thicker layers (> 200 nm) with an ohmic resistance (gray zone), and finally the breaking of the gel film after prolonged deposition time (red zone). (d) Calculated final resistance of the deposited layers as a function of final film thickness. Resistance was calculated based on the potential difference between start (10–12s) and end of the deposition. (e) SEM images of the micropillars after growing mesoporous silica thin films. (f) Cross-section SEM images of the coated pillars after FIB milling was used to cut open the pillars and reveal the deposited silica coatings. Figure modified from references 42 and 38
explained by the surface adsorption of the long-tailed CTA+ cations.
The electrochemically induced sol-gel deposition features several advantages over traditional sol-gel processes. Firstly, the process is faster than classical Stöber-like gelation36 with the deposition of hundred-nanometers thick films requiring seconds/minutes instead of hours. Nor does sol-gel ECiD require the high-temperature treatments that are often required in evaporation-based methods.37 Additionally, in the electrochemical process, gelation is limited to the environment close to the electrode substrate (i.e., inside the OH- (H+) diffusion layer), rather than being throughout the whole precursor solution, giving better control over the deposition rate and film thickness. Another major advantage is the ability to coat 3D structured electrodes, as shown by us recently.38,39
How Thick Can the Mesoporous Oxide Be?
Using stationary electrodes, mesoporous silica thin films are limited to 50–150 nm in thickness. Extending the deposition time led to the formation of aggregated particles (Fig. 4a), making the growth of thicker layers difficult. As the alkaline diffusion layer penetrates deeper in the solution over time, with a thickness of √πDt (with D the OH- diffusion coefficient, and t the deposition time), the gelation is no longer confined to the surface and particles are formed deeper in the solution which flocculate on the surface. Sequential depositions with rinsing steps in-between was shown as a strategy to grow thicker films up to 400 nm.40 In 2021, we showed that thick films up to 2.5 microns thick can be grown simply by using a rotation disk electrode, as now the diffusion layer thickness remains constant and growth is confined to the surface.38 Moreover, the thickness of the layer can be deduced directly from the potential transient as the rate is now limited by diffusion-migration of OHanions through the gel, in a similar fashion as for EMD layers discussed in the previous article. The potential transient in Fig. 4(c) shows the different stages of silica-gel film growth. After an initial few hundred seconds for build-up of the OH- diffusion layer and gelation growth front (more detail below and in Fig. 5), a steady-state growth regime is achieved where the potential now scales linearly with time as the ionic resistance through the film increases with thickness. Evidence for this is seen in Fig. 4(d) where the resistance obtained from potential difference (Vt – Vinitial) over the current density (i=1 mA/ cm2) from the transients after different current densities have passed is plotted versus the film thickness obtained from SEM inspection. From the found linear relationship, a specific resistivity of 2.7 MΩ∙cm is determined, corresponding to a field of 2.7 kV/cm over the silica gel layer. Hence, a significant ion migration contribution to the OH- transport is expected. Also, the hydrogen is removed through the gel, most probably as dissolved hydrogen as the hydrogen solubility is higher in the gel with water-ethanol mixture.41 In fact, the maximum film thickness was limited by cracking of the film, most likely due to onset of formation of hydrogen gas bubbles and identifiable in the potential transient by a sudden increase and further noise in the transient (red zone in Fig. 4 (c)). The resistive nature of OH- transport through the coating to maintain the pH above the critical gelation pH also lends itself perfectly to the conformal coating of 3D structures. An example of 130–350 nm of silica coatings is shown in Fig. 4(e)-(f) for the same 3D silicon pillar array as those shown from EMD (similar to EMD in Fig. 1 of the preceding article which relies similarly on resistive drop over the coating, but in this case Mn2+ through the mesoporous oxide to the electrode surface).
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 69
(continued from previous page)

Fig. 5. Schematic overview of the mechanism of electrochemically induced sol-gel depositions. Left side shows typical OH- and H+ concentration profiles obtained by simulation models. Film growth occurs in the alkaline part of the profile (��pH>7). The middle part of the schematic shows the transport of relevant species during extended depositions; H2O goes into the deposited gel, reduction happens at the gel/electrode interface, and electrogenerated OH- is transported out of the gel layer. Film growth occurs at the gel layer/liquid interface, where the pH is highest, through sol-gel reactions illustrated on the right.
Mechanistic Insights for Sol-gel ECiD of Mesoporous Silica Coatings
The key to understanding the mechanism driving the electrochemically induced sol-gel process is the build-up of the combined diffusion layer for OH- and H+ that forms the alkaline region near the electrode wherein the sol-gel reactions are catalyzed once above the critical gelation pH. The OH- and H+ concentration profiles are controlled by the starting or bulk pH of the solution (typically pH 3 in our experiments) and by the applied current density and solution convection (RDE rotation rate). Fig. 5 shows a typical concentration profile obtained from the Nernst-Planck equation by modelling a galvanostatic deposition using the acidic bulk solution.42 The profile consists of an alkaline domain (��pH>7) near the electrode and an acidic domain (��pH<7) toward the bulk solution where CH+(x) < CH+,Bulk. The sum of both regions is the diffusion layer thickness (��Total). Regarding SiO2 sol-gel deposition, the alkaline region (��pH>7) is the most relevant part of the profile to understand. In this region, the OH- concentration can be approximated by a linear profile. The most alkaline region forms at the electrode/liquid interface, resulting in the OH- catalyzed
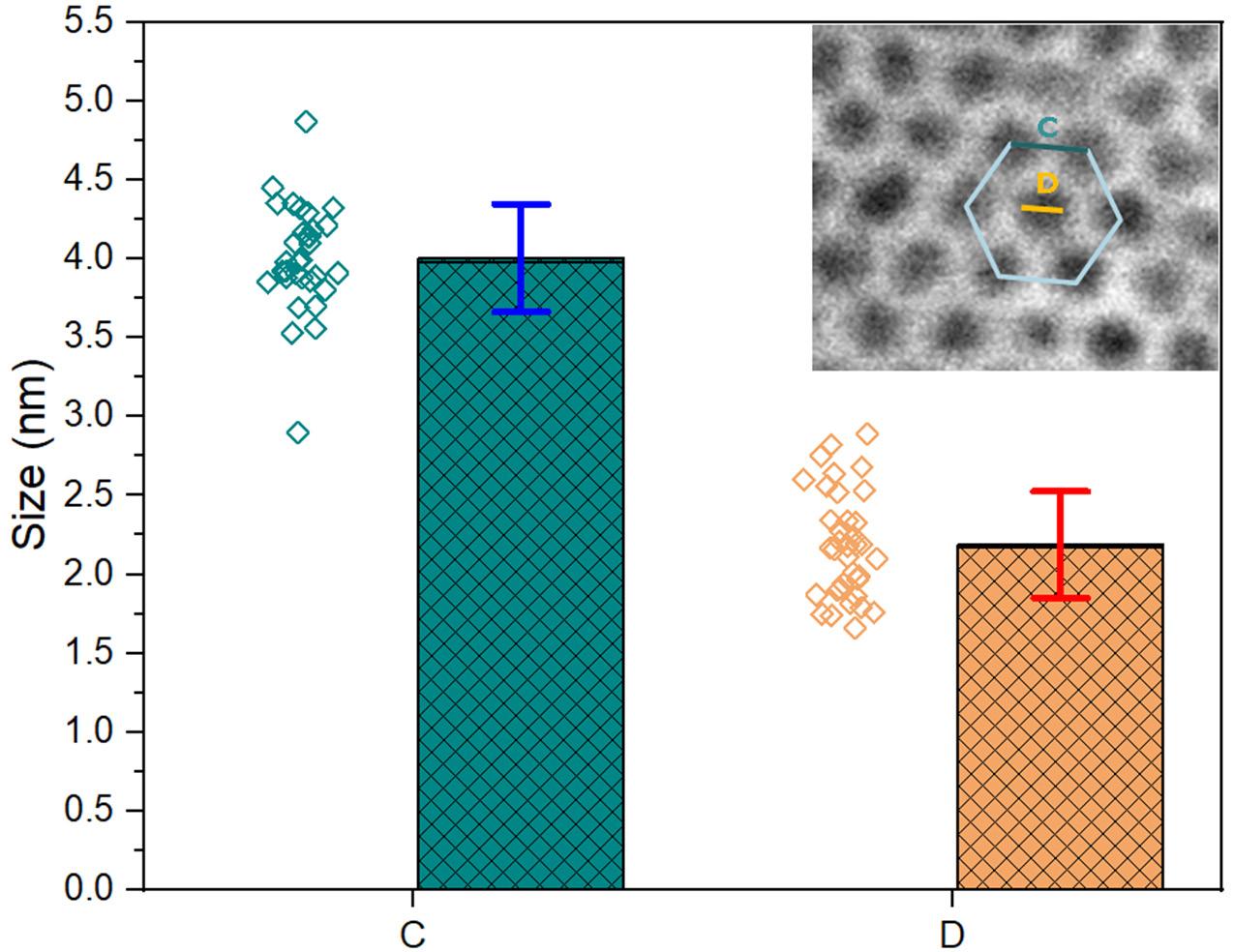
growth of the mesoporous films in the gelation growth front. The gelation reactions in this zone lead to the growth of the silica gel coating on the electrode. Despite SiO2 being an electronic insulator, extended SiO2 deposition is possible due to the porosity of the gel layer, with slower diffusion of reagents and products through this layer as for the MnO2 (EMD) deposition discussed in the preceding article. The deposition continues through reduction of the water in the gel film at the electrode/hydrated silica film interface. The formed OH- ions are transported through the gel film, thus maintaining the formed pH profile and gelation growth front at the hydrated silica film/ liquid interface. Here, film growth occurs through the deprotonation of silica precursor molecules and subsequently reacting with the gel film (dR1 and dR2 in Fig. 5) through heterogeneous growth. Homogeneous growth (dR3) can also occur deeper in the solution, leading to the aggregated particle formation observed with absence of solution convection. By confining the pH profile close to the surface by using an RDE, the homogeneous gelation reaction deeper in the solution is prevented and gelation occurs only at the electrode surface through the kinetically favored heterogeneous growth.42
Mesopore Structure of the Sol-gel ECiD Templated Silica Coatings
Fig. 6 presents a TEM image showing the typical honeycomb pore structure for a ~100 nm silica layer deposited on a planar TiN substrate from TEOS precursor solution with CTAB. In such a hexagonally packed structure, two main spatial parameters can be defined: the inter-pore spacing (C), which is the distance between the centers of two neighboring pores and the pore diameter (D). These parameters were determined from TEM images at different spots on two different planar samples. Fig. 6 shows the distances measured for the (C) and (D) parameters, and they were determined to be C = 4.00 + 0.34 and D = 2.19 + 0.34 nm. From these geometrical parameters, the pore density was estimated to be approximately 7x1012 pores/cm2 which translates to a porosity of ~26%, which is in good agreement with other literature reports on CTAB-templated electrochemically deposited silica.1,43
Electrochemically Induced Sol-gel Deposition of Thick Nanocomposite Coatings
In addition to surfactant templates, electrochemically induced solgel deposition was also recently demonstrated using ionic liquids as templates. This was first done using ionic liquid surfactants or so-
70 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Vanheusden and Vereecken
Fig. 6. Scatter plot showing multiple measurements of the pore spacing (C) and diameter (D). TEM image (inset) of the mesoporous structure of the silica deposited by EASA on a planar substrate. The pore spacing (C) and diameter (D) were measured across multiple positions on two different samples.
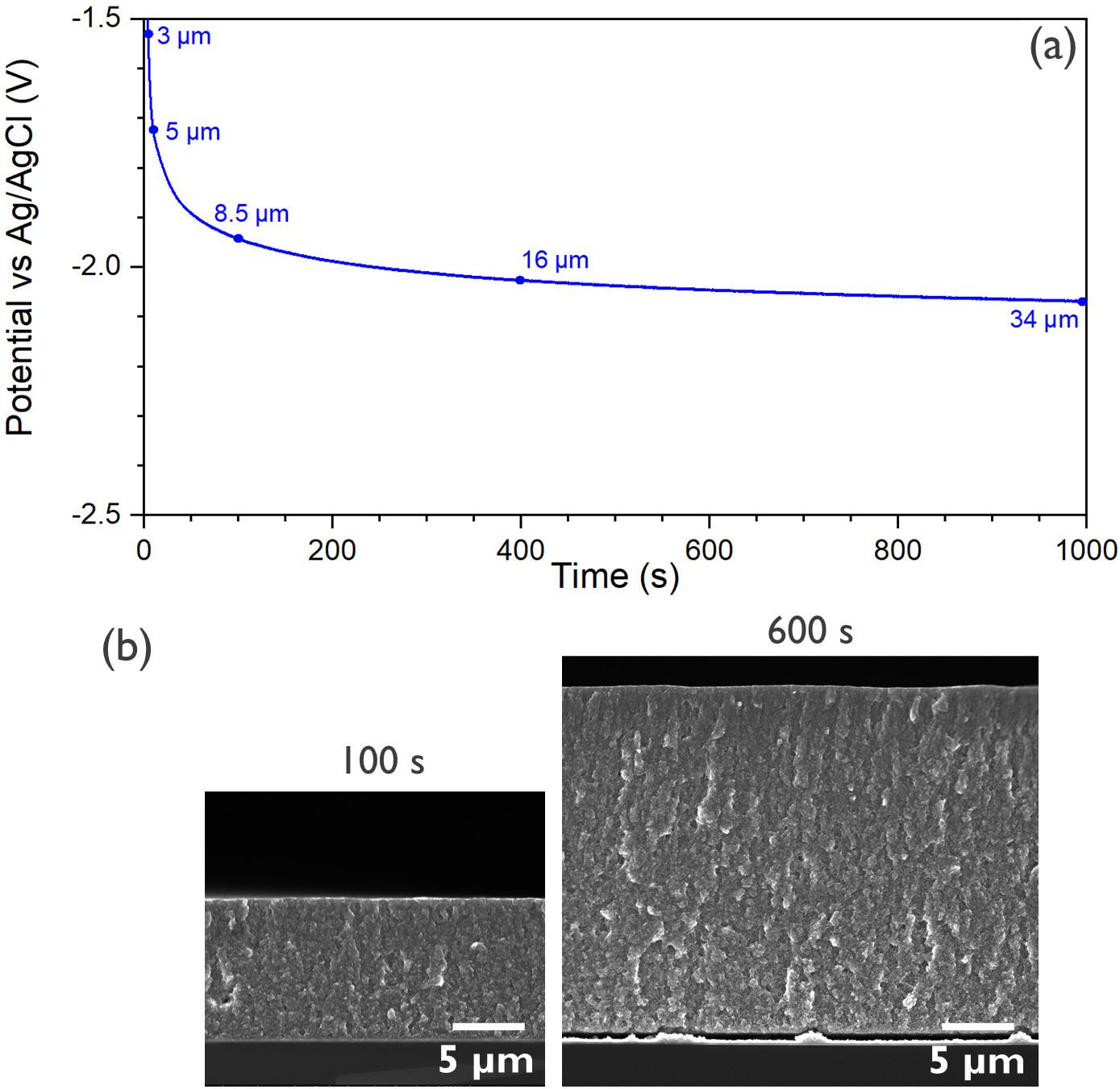
7. (a) Typical deposition transient of electrochemically deposited ionicliquid-templated silica solid electrolyte at -1 mA/cm² on TiN substrate while rotating at 500 RPM using a mixture of 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (EMIM-TFSI) and Li-TFSI as the template, with TEOS as silica precursor. The thickness of the deposited layer is indicated by the points. (b) Cross-section SEM images of electrodeposited nanocomposite electrolyte layers deposited for 100 and 600 seconds.
called surface-active ionic liquids or SAIL by Vavra et al. in 2020.44,45 By using a SAIL with similarly long alkyl chain lengths as the CTAB template used above, the ionic liquid-based films retained the characteristic vertically aligned nanopore structures: 1-hexadecyl-3methylimidazolium chloride (C16MIMCl) has an alkyl chain “tail” that is 16 carbons long, which is the same as CTAB. Using this SAIL, films in the range of around 70 nm in thickness were shown. The growth of thicker layers was again restricted by the formation of aggregated particles at these stationary electrodes.
Recently, we extended the methodology further to include functional ionic liquids such as Li-ion electrolytes for the electrochemically induced templated sol-gel deposition of nanocomposite silica-gel electrolytes which can be used for supercapacitors, batteries, and sensors.39,46,47 Solid nanocomposite electrolytes which consist of an ionic liquid electrolyte (ILE) that is nanoconfined in an inert silica matrix have been synthesized using a similar silica sol-gel procedure to that outlined above, with
TEOS as the silica precursor and using the ionic liquid electrolyte as the template.48–50 Due to the nanoconfinement of the ionic liquid in the mesoporous channels of the silica matrix, the monolithic structure becomes solid, while the Li+ ions retain liquid-like high conductivities even exceeding that of bulk ILE.48 In the initial reports, the solidification of the electrolyte typically requires several days. Using electrochemically induced sol-gel deposition, we were able to grow coatings of 1 to 35 micrometers in thickness on electrode surfaces in seconds to minutes.39 Interestingly, the potential transient for deposition of the ion-conductive ionic liquid-templated silica gel was quite different from that for the resistive silica hydrogel from the TEOS-CTAB system described above. Now, a voltage drop of -0.5V corresponds to coatings of over 10 micrometer in thickness with the incorporated ionic-liquid electrolyte, whereas -0.8V was needed for a quarter of the thickness with CTAB. Hence, the intrinsic ion conductivity of the ionic liquid-templated silica gel, together with its high water content uptake, allows for good ionic and mass transport of OH- ions through the nanocomposite coating.
Extending the Manufacturing Options with Electroblading and Electro-slot-die
The electrochemically induced deposition method offers an industrially relevant path for the fast and controlled synthesis of compounds and nanocomposite coatings from solution, both via precipitation as discussed in the preceding article and via the solgel synthesis described here. The ECiD can be implemented in standard electroplating tools, which should be straightforward, especially for aqueous-based solutions which are stable over time. For precursor solutions, such as sol-gel solutions, which may have a limited shelf life, large tanks with electroplating solutions might be more problematic. In traditional sol-gel synthesis, coatings are often applied by blade-coating or slot-die coating. Inspired by this, we have investigated a novel technique which we called electroblading and electro-slot-die for the practical and scalable manufacturing of sol-gel layers.51 The proposed techniques combine the application of precursor and the electrochemical triggering under the blade by applying a potential between the foil current collector substrate and the blade. In this way, the triggered process allows for sheet-to-sheet or roll-to-roll processing. Fig. 8 shows the electroblading setup in the lab and a SEM of an electrobladed ILE-silica nanocomposite coating (compare with the coatings obtained at an RDE in Fig. 7). The electroblading process works like conventional blade-coating, where the deposition mixture (sol-gel precursor solution) is added to the front of the blade-coater as it moves across the current collector foil. An additional parameter to control here is the speed of the moving blade, which provides agitation but should be tailored to the growth rate.
(continued on next page)
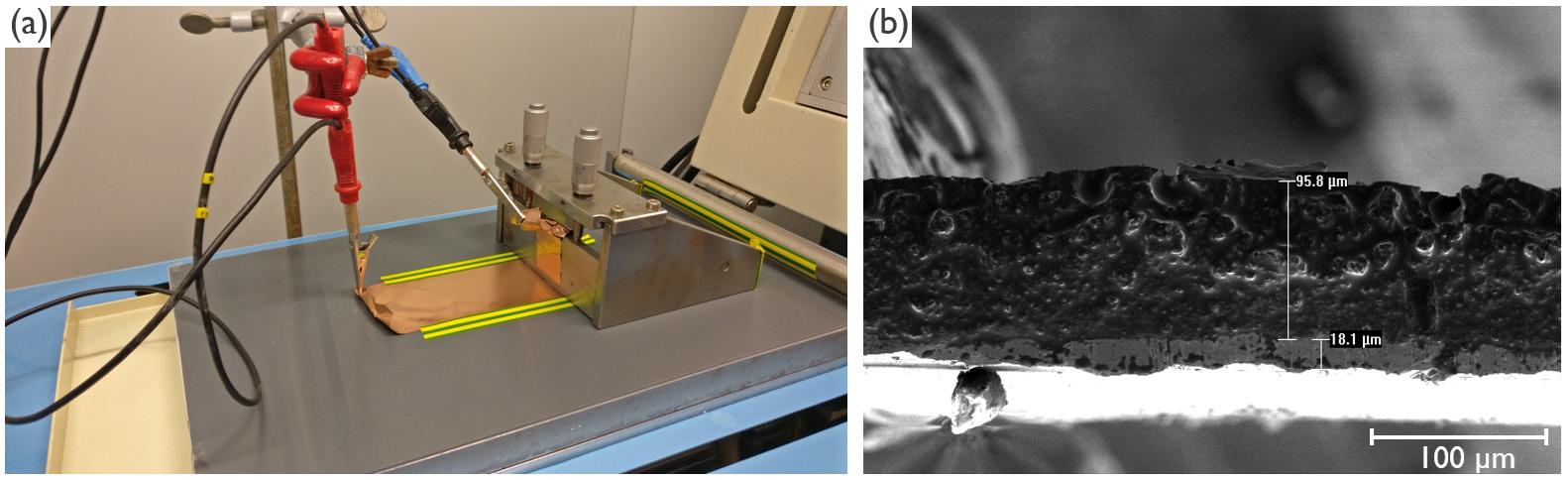
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 71
Fig. 8. (a) Lab-scale prototype electroblade setup used to electrodeposit templated silica layers from sol-gel precursor solution. (b) Cross-section SEM image of ionic liquid-templated silica nanocomposite layer deposited on a Cu foil by the electroblading setup.
Fig.
Vanheusden and Vereecken
(continued from previous page)
Conclusions
The electrochemically induced sol-gel deposition framework opens opportunities for yet more material compositions and composites to the growing list already discussed for ECiD in previous article. The gels can be dried and converted to compounds and films, similar to what is done for sol-gel deposition by, for example, spin-, dip-, spray-, blade-, and slot-die coating. Interestingly, polarization can be used in combination with some of these coating techniques, as demonstrated in this paper. The inclusion of templates allows for the fabrication of mesoporous materials. Additionally, the resistive nature of the coatings also allows for conformal coating on 3D structured electrodes.
©The Electrochemical Society. DOI: 10.1149/2.F12242IF
About the Authors


Genis Vanheusden, Researcher, Electrochemical Storage and Conversion Group, imec
Education: PhD in Bioscience Engineering (University of Leuven (KU Leuven) and imec)
Research Interests: Include electrochemically induced sol-gel depositions for energy applications
Philippe Vereecken, Fellow, imec, program line coordinator P2M, EnergyVille, and professor, KU Leuven
Education: PhD in Physical Chemistry (Ghent University)
Research Interests: The combination of electrochemistry and nanomaterials and specifically in its use in semiconductors, energy conversion, electrochemical storage, and nanomaterials synthesis
Work Experience: Postdoctoral Associate in the Department of Materials Science and Engineering at The Johns Hopkins University (1998–2001) and Research Staff Member (RSM) at IBM T.J. Watson Research Center (2001–2005). He joined imec in 2005 and KU-Leuven in 2009.
Work with ECS: ELDP Division executive committee member (2008-2023) Website: https://orcid.org/0000-0003-4115-0075
13. M. E. Simonsen and E. G. Søgaard, J Sol-Gel Sci Technol, 53 (3), 485 (2010).
14. B. Tyagi, K. Sidhpuria, B. Shaik, and R. V. Jasra, Ind Eng Chem Res, 45 (25), 8643 (2006).
15. K. Kajihara, J Asian Ceram Soc, 1 (2), 121 (2013).
16. L. L. Hench and J. K. West, Chem Rev, 90 (1), 33 (1990).
17. A. C. Pierre, Introduction to Sol-Gel Processing; Springer International Publishing: Cham, 2020.
18. J. Šefčik and A. V. McCormick, Catal Today, 35 (3), 205 (1997).
19. J. S. Beck, J. C. Vartuli, W. J. Roth, et al., J Am Chem Soc, 114 (27), 10834 (1992).
20. C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, and J. S. Beck, Nature, 359 (6397), 710 (1992).
21. I. Díaz, J. Pérez-Pariente, and O. Terasaki, J Mater Chem, 14 (1), 48 (2004).
22. V. Meynen, P. Cool, and E. F. Vansant, Microporous Mesoporous Mater, 125 (3), 170 (2009).
23. G. J. de A. A. Soler-Illia, C. Sanchez, B. Lebeau, and J. Patarin, Chem Rev, 102 (11), 4093 (2002).
24. A. Corma, Chem Rev, 97 (6), 2373 (1997).
25. Y. Wan and D. Zhao, Chem Rev, 107 (7), 2821 (2007).
26. P. Srivastava, S. K. Hira, and P. P. Manna, Newly Emerged 2D Mesoporous Silica Nanoparticles: Role in Target-Setting Biomedicines. In 2D Nanomaterials for Energy and Environmental Sustainability; Khanam, Z., Gogoi, N., Srivastava, D., Eds.; 2022; 197.
27. H. Woo, P. J. Reucroft, and R. J. Jacob, J Adhes Sci Technol, 7 (7), 681 (1993).
28. R. Shacham, D. Avnir, and D. Mandler, Adv Mater, 11 (5), 384 (1999).
29. R. Shacham, D. Mandler, and D. Avnir, Chem - A Eur, 10 (8), 1936 (2004).
30. R. Chaim, G. Stark, L. Gal-Or, and H. Bestgen, J Mater Sci, 29 (23), 6241 (1994).
31. B. O’Regan, D. T. Schwartz, S. M. Zakeeruddin, and M. Grätzel, Adv Mater, 12 (17), 1263 (2000).
32. P. Steegstra and E. Ahlberg, Appl Surf Sci, 289, 345 (2014).
33. G. Giordano, C. Durante, N. Michieli, et al., J Sol-Gel Sci Technol, 89 (1), 196 (2019).
34. L. Liu and D. Mandler, Electrochim Acta, 102, 212 (2013).
35. R. Toledano, R. Shacham, D. Avnir, and D. Mandler, Chem Mater, 20 (13), 4276 (2008).
36. Z. Teng, G. Zheng, Y. Dou, Angewandte, 2173 (2012).
37. D. Grosso, F. Cagnol, G. J. D. A. A. Soler-Illia, et al., Adv Funct Mater, 14 (4), 309 (2004).


References
1. A. Walcarius, E. Sibottier, M. Etienne, and J. Ghanbaja, Nat Mater, 6 (8), 602 (2007).
2. E. Sibottier, S. Sayen, F. Gaboriaud, and A. Walcarius, Langmuir, 22 (20), 8366 (2006).
3. A. Walcarius and A. Kuhn, TrAC Trends Anal Chem, 27 (7), 593 (2008).
4. D. Basnig, N. Vilá, G. Herzog, and A. Walcarius, J Electroanal Chem, 872 (113993), 1572 (2020).
5. T. Nasir, N. A. Vodolazkaya, G. Herzog, and A. Walcarius, Electroanalysis, 31 (2), 202 (2019).
6. T. Nasir, G. Herzog, M. Hébrant, C. Despas, L. Liu, and A. Walcarius, ACS Sensors, 3 (2), 484 (2018).
7. M. Etienne, A. Quach, D. Grosso, L. Nicole, C. Sanchez, and A. Walcarius, Chem Mater, 19 (4), 844 (2007).
8. P. Innocenzi, The Sol-to-Gel Transition, 2nd ed.; SpringerBriefs in Materials; Springer International Publishing: Cham, 2019.
9. C. J. Brinker, Sol-Gel Science; Elsevier, 1990.
10. C. J. Brinker, J Non Cryst Solids, 100 (1–3), 31 (1988).
11. C. J. Brinker, T. L. Ward, R. Sehgal, et al., J Memb Sci, 77 (2–3), 165 (1993).
12. D.P. Macwan, P. N. Dave, and S. Chaturvedi, J Mater Sci, 46 (11), 3669 (2011).
38. G. Vanheusden, H. Philipsen, S. J. F. Herregods, and P. M. Vereecken, Chem Mater, 33 (17), 7075 (2021).
39. G. Vanheusden, Electrochemically Induced Deposition of Functional Templated Oxide Coatings for Energy Applications, PhD Thesis, KU Leuven, 2023.
40. G. Giordano, N. Vilà, E. Aubert, I. Ghanbaja, and A. Walcarius, Electrochim Acta, 237, 227 (2017).
41. C. L. Young, Solubility Data Series; Pergamon P. for the International Union of Pure and Applied Chemistry, 1981; Vol. 5–6.
42. G. Vanheusden, L. De Taeye, M. J. W. Blom, M. Jobbagy, and P. M. Vereecken, J Electrochem Soc, 171 (3), 032508 (2024).
43. A. Goux, M. Etienne, E. Aubert, C. Lecomte, J. Ghanbaja, and A. Walcarius, Chem Mater, 21 (4), 731 (2009).
44. S. Vavra, N. Vilà, A. Lotsari, and A. Walcarius, Microporous Mesoporous Mater, March, 110407 (2020).
45. S. Vavra and A. Martinelli, J Mol Liq, 353, 118686 (2022).
46. K. Takada, Acta Mater, 61 (3), 759 (2013).
47. Y.-S. Hu, Nat Energy, 1 (4), 16042 (2026).
48. X. Chen, B. Put, A. Sagara, et al., Sci Adv, 6 (2), eaav3400 (2020).
49. A. Sagara, X. Chen, K. B. Gandrud, et al., J Electrochem Soc, 167 (7), 070549 (2020).
50. A. Sagara, H. Yabe, X. Chen, et al., ACS Appl Mater Interfaces, 13 (34), 40543 (2021).
51. P. Vereecken, S. Herregods, G. Vanheusden, and F. Barde, Electrochemical Process for Forming a Solid Electrolyte. US 2023/0411677 A1, 2023.
72 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
SECTION
ECS Mexico Section
The ECS Mexico Section Executive Committee election concluded on March 1. The newly elected committee is composed of:
• President Dr. Norberto Casillas, Professor in the Department of Chemistry, University of Guadalajara, working in electrochemistry and corrosion;
• Vice President Dr. Erika Bustos-Bustos, expert in environmental electrochemistry, Centro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ);
• Secretary Dr. Erika Méndez-Albores, Professor, Benemérita Universidad Autónoma de Puebla, specializing in environmental electrochemistry;
• Treasurer Dr. José Cabral-Miramontes, Professor and researcher, Universidad Autónoma of Nuevo León, expert in the field of corrosion;
Members at Large:
o Dr. Bernardo Frontana-Uribe, Professor, Universidad Nacional Autónoma de México (UNAM), working in organic electrochemistry;
o Dr. Marcelo Videa-Vargas, Professor, Department of Sciences, Tecnológico de Monterrey (ITESM), specializing in electrocatalysis;
o Dr. Ricardo Galván-Martínez, Professor, Universidad Veracruzana, working in the field of corrosion;
o Dr. Citlalli Gaona-Tiburcio, Professor, Universidad Autónoma de Nuevo León (UANL), expert in corrosion.
The ECS Mexico Section Executive Committee defined its objectives, tasks, and future plans at its first working meeting, held virtually on March 21st, 2024. The meeting included an informative talk by ECS Director of Community Engagement Shannon Reed and ECS Executive Director and CEO Christopher Jannuzzi The former spoke about opportunities to strengthen the community of electrochemists by encouraging their participation and fostering collaborative networks. He also outlined regulations and provisions necessary for the proper functioning of the ECS Mexico Section. Chris provided information about the administrative functioning and treasury of the ECS Mexico Section Executive Committee.
The new ECS Mexico Section Executive Committee set the task of reviewing the section’s bylaws and regulations. It also defined

as a priority establishing more student chapters in Mexico and strengthening those already existing at the Tecnológico de Monterrey, Centro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ) in Querétaro, Universidad Nacional Autónoma de México (UNAM), and Universidad Autónoma de Nuevo León (UANL). As part of the agreements reached, it was recommended that ECS Mexico Section Executive Committee members promote the chartering of student chapters at their universities. In cases where student chapters are already established, efforts should be made to increase the number of members.
It was also proposed that an agenda for upcoming meetings be set, events be planned to promote electrochemistry in Mexico, and ties of work and friendship with ECS be strengthened. One proposal is to hold the CIDETEQ student chapter’s first congress in 2024. Additionally, there is a plan to organize a special event recognizing the scientific career of Prof. Allen J. Bard from the University of Texas during the XXXIX Congreso Nacional de la Sociedad Mexicana de Electroquímica (SMEQ) and the 17th Meeting of the ECS Mexico Section, which will take place in San Francisco de Campeche, Campeche, Mexico, October 7–11, 2024. Professors from the ECS Mexico Section who had the opportunity to interact and share memorable moments with Prof. Bard as students, postdoctoral fellows, or when he held the Neal R. Amundson Chair at the Universidad de Guadalajara in 2003, will be invited to participate. Scientific achievements will be analyzed, and anecdotes from interactions with Prof. Bard shared. A keynote lecture by a former student of Prof. Bard who has had a successful career in electrochemistry is planned. In addition, the ECS Mexico Section Executive Committee’s role in organizing a joint SMEQ and ECS conference on sustainability and circular economy, scheduled to take place in Guadalajara, Mexico in 2025, was defined.
The work meeting concluded with good wishes from ECS leaders for the success of the ECS Mexico Section. New executive committee members were assigned tasks required to achieve work plan objectives.
We take this opportunity to express our gratitude to the outgoing board of the ECS Mexico Section, led by Dr. Carlos Frontana, for their achievements and support in establishing the new board.
Contributed by Norberto Casillas Santana.
ECS Sections
ECS sections serve a fundamental role within the Society. Sections advance ECS’s mission by supporting activities in electrochemistry and solid state science while offering excellent networking opportunities.
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 73
NEWS SECTION NEWS
Not yet a section member? Email customer.service@electrochem.org now to join your region’s ECS section!
Section Name
Section Leadership
Section Chair
Arizona Section Candace K. Chan
Brazil Section Raphael Nagao
Canada Section Steen B. Schougaard
Chile Section José H. Zagal
China Section Open
Detroit Section Erik Anderson
Europe Section Jan Macak
Georgia Section Faisal Alamgir
India Section Sinthai Ilangovan
Israel Section Daniel Mandler
Japan Section Yasushi Idemoto
Korea Section Won-Sub Yoon
Mexico Section Norberto Casillas Santana
Mid-America Section Nosang Myung
National Capital Section Chunsheng Wang
New England Section Sanjeev Mukerjee
Pacific Northwest Section Corie Cobb
Pittsburgh Section Open
San Francisco Section Gao Liu
Singapore Section Zhichuan J. Xu
Taiwan Section Chi-Chang Hu
Texas Section Jeremy P. Meyers
Twin Cities Section Victoria Gelling




74 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
NEWS
NEWS
SECTION
SECTION
Learn more about ECS sections at www.electrochem.org/sections.
ORCID Connecting research and researchers to register. www.orcid.org Visit 4 GET YOUR ORCID ID Add your ID to your ECS profile

ECS Awards, Fellowships, Grants
ECS recognizes outstanding technical achievements in electrochemistry, solid state science, and related technologies, along with exceptional service to the Society. ECS award opportunities are provided in the categories of Society Awards, Division Awards, Section Awards, and Student Awards.
Recognizing that today’s emerging scientists are the next generation of leaders in our field, ECS offers competitive fellowships and grants that enable students and young professionals to make discoveries and shape science long into the future.
Society Awards

ECS Carl Wagner Memorial Award: Established in 1980, the award recognizes mid-career achievement, excellence in research areas of interest to the Society, and significant contributions in the teaching or guidance of students or colleagues in education, industry, or government. The award consists of a sterling medal, wall plaque, ECS Life Membership, complimentary meeting registration, and travel assistance up to $1,000.*
Nomination deadline: October 1, 2024

ECS Olin Palladium Award: Distinguished contributions to the fields of electrochemical or corrosion science are recognized by this award established in 1950. The award consists of a palladium medal; wall plaque; $7,500; ECS Life Membership; and complimentary meeting registration.
Nomination deadline: October 1, 2024
ECS Toyota Young Investigator Fellowship: Established in partnership with the Toyota Research Institute of North America in 2015, the award encourages young professionals and scholars to pursue research on batteries, fuel cells, hydrogen, and future sustainable technologies. Each year, at least one candidate receives the fellowship restricted grant of no less than $50,000 to conduct the proposed research within one year, and a one-year complimentary ECS membership. Recipients present at a Society biannual meeting and publish their research in a relevant ECS journal within 24 months of receiving the award.
Materials are due by January 31 of each year.
Leadership Circle Awards: Established in 2002 to honor and thank our electrochemistry and solid state science partners, these awards are granted in the year that an institutional member reaches a milestone level. Awardees receive a commemorative plaque and recognition on the ECS website and in ECS Interface magazine.
Nominations are not accepted
Section Awards
Pacific Northwest Section Electrochemistry Research Award Sponsored by Gamry Instruments: Established in 2021, the award recognizes excellence in electrochemistry and solid state science and technology research by an independent scientist or engineer working in Washington, Oregon, or Idaho. The award consists of a framed certificate and $1,000.
Nomination deadline: July 15, 2024
Student Awards
Biannual Meeting Travel Grants: Many ECS divisions and sections offer travel grants to undergraduates, graduate students, postdoctoral researchers, young professionals, and faculty presenting papers at ECS biannual meetings. The awards consist of financial support ranging from complimentary meeting registration to luncheon/reception tickets, travel support, and more. Each division and section has its own application requirements.
Materials deadline: The abstract deadline for the ECS Meeting in which the talk will be presented
Colin Garfield Fink Fellowship: First awarded in 1962, the fellowship supports research from June through August in a field of interest to the Society by a postdoctoral scientist/researcher. The award consists of $5,000 and publication of a summary report in the award year’s ECS Interface winter issue.
Materials deadline: January 15, annually
ECS General Student Poster Session Awards: This forum enables undergraduate and graduate students to present research results of general interest to ECS. Established in 1993, the session fosters and promotes work in electrochemical and solid state science and technology and stimulates active student interest and participation in ECS. Accepted posters are eligible for General Student Poster Awards: $1,500 for 1st place, $1,000 for 2nd place, and $500 for 3rd place. To be considered for awards, authors must submit their abstracts to the Z01 General Student Poster Session; be accepted for inclusion in the session; upload a digital poster; and be present during in-person judging.
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 75 AWARDS AWARDSAWARDSPROGRAM AWARDS PROGRAM
Visit www.electrochem.org/awards for more information.
(continued from previous page)
AWARDS PROGRAM AWARDS PROGRAM
Materials deadline: The abstract deadline for the ECS meeting in which the poster will be presented (December 6, 2024, for the 247th ECS Meeting in Montréal, Canada)
ECS Outstanding Student Chapter Award: The award recognizes distinguished student chapters that demonstrate active participation in the Society’s technical activities; establish community and outreach activities in electrochemical and solid state science and engineering education; and create and maintain a robust membership base. The Outstanding Student Chapter receives a recognition plaque and certificates; $1,000; and award recognition and chapter group photo in ECS Interface or other electronic communications. Up to two Chapters of Excellence are also awarded.
Materials deadline: April 15, annually
ECS Summer Fellowships: Established in 1928, up to four fellowships are awarded each year to support students’ research from June through August in a field of interest to ECS. Edward G. Weston Fellowship, Joseph W. Richards Fellowship, F. M. Becket Fellowship, and H. H. Uhlig Fellowship winners receive $5,000 and publication of a summary report in the award year’s ECS Interface winter issue.
Materials deadline: January 15, annually
Georgia Section Outstanding Student Achievement Award: The $500 prize established in 2011 recognizes academic accomplishments by students pursuing PhDs at universities in the ECS Georgia Section region in any area of science or engineering in which electrochemical and/or solid state science and technology is the central consideration.
Nomination deadline: August 15, 2024
*All awards are in US dollars.

76 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
BioLogic 1 ECS Transactions 245th ECS Meeting inside back cover El-Cell ............................................................................ 25 Gamry 6 Ion Power ........................................................................ 8 IOP Publishing ...................................... inside front cover PalmSens BV 13 Pine Research Instrumentation ....................................... 2 Scribner LLC 4 Wiley............................................................................... 23 Advertisers Index
NEW MEMBERS NEW MEMBERS
ECS is proud to announce the following new members for January, February, and March 2024 (Members are listed alphabetically by family/last name.)
Members
A
Eric Anderson, Santa Clara, CA, USA
B
Jarid Barthold-Robinson, Columbia, MD, USA
Ilenia Battiato, Stanford, CA, USA
Thierry Belmonte, Nancy, Grand Est, France
Prasad Bhagavatula L V, Bangalore, KA, India
Amrtha Bhide, Karaikal, PY, India
Brian Bloom, Pittsburgh, PA, USA
Moritz Brehm, Linz, Upper Austria, Austria
Tom Breugelmans, Nijlen, Antwerp, Belgium
Giovanna Bucci, Livermore, CA, USA
Megan Butala, Gainesville, FL, USA
Ravinder Butreddy, Hyderabad, TG, India
C
Gerard Carroll, Golden, CO, USA
Ai-Lin Chan, Golden, CO, USA
Vishal Chaudhary, New Delhi, DL, India
Pochun Chen, Taipei, Taipei, Taiwan
Yu-Ming Chen, Fremont, CA, USA
Manjeet Chhetri, Los Alamos, NM, USA
Jong Hyun Choi, West Lafayette, IN, USA
Sang-Il Choi, Daegu, Gyeongsangbuk-do, ROK
D
Nick Daems, Wilrijk, Antwerp, Belgium
Dipankar Das Sarma, Bengaluru, KA, India
Govinda Devkota, South Bend, IN, USA
Dan (Annie) Du, Pullman, WA, USA
Robert Durante, Landenberg, PA, USA
E
Kathrin Ebner, München, BY, Germany
F
Omar Faruk, Binghamton, NY, USA
Fathima Fasmin, Kozhikode, KL, India
Liang Feng, Durham, NC, USA
Xiaofeng Feng, Orlando, FL, USA
David Flaherty, Atlanta, GA, USA
G
Rajeshkhanna Gaddam, Warangal, TG, India
Sergi Garcia-Segura, Tempe, AZ, USA
Seval Gunduz, Columbus, OH, USA
H
Dominik Haering, Avalon, CA, USA
Stefanie Harvey, Sparks, NV, USA
Xiang He, Melbourne, FL, USA
Ming-Che Ho, Penn Yan, NY, USA
Hsiao-Ying Shadow Huang, Raleigh, NC, USA
Michael Huang, Hsinchu, Hsinchu, Taiwan
Tae Seon Hyun, Ann Arbor, MI, USA
JUmamaheswari Janakiraman, Troy, MI, USA
Haifeng Ji, Philadelphia, PA, USA
Yanzhou Ji, Columbus, OH, USA
Anurag Jindal, Boise, ID, USA
Sang Hoon Joo, Seoul, Gyeonggi-do, ROK
KDmitri Kilin, Fargo, ND, USA
Jaehoon Kim, Seongnam-si, Gyeonggi-do, ROK
LAlaeddine Lakraychi, Houston, TX, USA
Ambra Lattanzi, Rome, LZ, Italy
Jungmin Lee, Suwon City, Gyeonggi-do, ROK
Chokkakula Leela Pydi Pavithra, Hyderabad, TG, India
Kevin Leonard, Lawrence, KS, USA
Timothy Lichtenstein, Lemont, IL, USA
Itai Lieberman, Leuven, Flemish Brabant, Belgium
Yen-Hsuan Lin, Taipei, Taipei, Taiwan
Bowen Liu, Birmingham, West Midlands, UK
Te-I Liu, Taipei, Taipei, Taiwan
Alberto Lucas, München, BY, Germany
M
Priyanka Makkar, Orlando, FL, USA
William Martinez, Albuquerque, NM, USA
Drew Martino, Somerset, NJ, USA
Maricris Mayes, North Dartmouth, MA, USA
Tom Miller, London, England, UK
Andrea Monforti Ferrario, London, England, UK
Stelios Mores, Milton Keynes, Buckinghamshire, UK
Hichem M’Saad, Phoenix, AZ, USA
Peter Mueller-Buschbaum, Garching bei München, BY, Germany
Subhasish Mukerjee, Horsham, England, UK
N
Hiroshi Naito, Tokyo, Tokyo, Japan
Hideo Nakamura, Inuyama, Aichi, Japan
Koji Nakano, Koganei, Tokyo, Japan
Zachariah Norman, Somerville, MA, USA
OJun Ogawa, Yonezawa, Yamagata, Japan
P
Sudeshna Patra, Hannover, NI, Germany
Michelle Personick, Charlottesville, VA, USA
Natalia Pimenova, Cranberry Township, PA, USA
R
Marco Raabe, Taipei, Taipei, Taiwan
Ronald Radzilowski, Canton, MI, USA
Rocio Ranchal, Madrid, MAD, Spain
Barry Rand, Princeton, NJ, USA
Brian Rawls, Amsterdam, North Holland, Netherlands
Jose Romo-Herrera, Ensenada, Baja California, México
Alice Ruini, Modena, EM, Italy
Izabela Rzeznicka, Saitama, Honshu, Japan
S
Bryce Sadtler, Saint Louis, MO, USA
Masaaki Sato, Hirakata, Osaka, Japan
Manikoth Shaijumon, Thiruvananthapuram, KL, India
Surbhi Sharma, Churchill, Victoria, Australia
Devon Shipp, Potsdam, NY, USA
Mohana Shivanna, Livermore, CA, USA
Anjan Sil, Roorkee, UT, India
Glaura Silva, Belo Horizonte, Minas Gerais, Brazil
Hyebeen Son, Daejeon, South Chungcheong, ROK
Chi Cheung Su, Westmont, IL, USA
Seiho Sugawara, Machida, Tokyo, Japan
T
Ming Tang, Salt Lake City, UT, USA
Alan Taub, Ann Arbor, MI, USA
Howard Tu, Rochester, NY, USA
W
Gang Wan, Stanford, CA, USA
Lucun Wang, Idaho Falls, ID, USA
Jianzhong Wu, Chino Hills, CA, USA
X
Hongliang Xin, Blacksburg, VA, USA
Guoping Xiong, Richardson, TX, USA
Y
Jenny Yang, Irvine, CA, USA
Z
Xinyu Zhang, Auburn, AL, USA
Hong Zhao, Foshan, Guangdong, China
Frank Zimone, Cherry Hill, NJ, USA
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 77
(continued from previous page)
Student Members
ANayab Abdul Karim, Garching bei München, BY, Germany
Kareem Abo Gamra, Garching bei München, BY, Germany
Samuel Adesanoye, Iowa City, IA, USA
Parisa Akhtari, Tuscaloosa, AL, USA
Abdullah Al Harthy, Athens, OH, USA
Abdulmohsin Al Isaee, Athens, OH, USA
Ali Alenezi, College Station, TX, USA
Alessandro Alleva, Legnano, LM, Italy
Omar Almutairi, Cary, NC, USA
Nadiah Alyamni, Hyattsville, MD, USA
Sara Alzahrani, Gainesville, FL, USA
Chinna Amgothu, Durgi, AP, India
Nafiseh Amiri, Houston, TX, USA
Seyed Mostafa Amoozadeh, Tehran, Tehran, Iran
Ethan Anderson, Montréal, QC, Canada
Manuel Ank, Garching bei München, BY, Germany
Sreenivasan Annadurai, Potsdam, NY, USA
Godfred Appiah, Kongens Lyngby, Hovedstaden, Denmark
Maura Appleberry, La Jolla, CA, USA
Fatma Aras, Dresden, SN, Germany
Mehran Arzani, Chicago, IL, USA
Sogol Asaei, Cleveland, OH, USA
Gaukhar Askarova, Flushing, NY, USA
BIsabel Baca, Euclid, OH, USA
Ridge Bachman, State College, PA, USA
Anar Badalbayli, Cleveland, OH, USA
Julius Bahrke, München, BY, Germany
Ifra Bashir, Lahore, Punjab, Pakistan
Shaghayegh Bashiri, Mansfield Center, CT, USA
Sreehari Batni Ravindranath, Melbourne, VIC, Australia
Mariah Batool, Willimantic, CT, USA
Favour Bawa, Athens, OH, USA
Juri Becker, Gießen, HE, Germany
Mayuresh Bhattu, Stanford, CA, USA
Shaikh Al Mahmud Bhuiyan, Lexington, Kentucky, USA
Christopher Blackwell, Saint Clairsville, OH, USA
Moritz Bock, Garching bei München, BY, Germany
Emmanuel Bourret, Montréal, QC, Canada
Willa Brenneis, Evanston, IL, USA
Brennon Brown, Wilmington, NC, USA
Emily Butler, Halifax, NS, Canada
C
Laura Cahuantzi, Chiautempan, Tlaxcala, México
Xinyin Cai, Shanghai, Shanghai, China
Luca Camuti, Stuttgart, BW, Germany
Zachary Carroll, Dunrobin, ON, Canada
Sara Chavez-Vera, Toluca, Estado de México, México
NEW MEMBERS NEW MEMBERS
Yimei Chen, Edmonton, AB, Canada
Yupeng Chen, Hachioji, Tokyo, Japan
Joseph Chiong, Saint-Hubert, QC, Canada
Yu-Sheng Chiou, Taoyuan City, Taoyuan, Taiwan
Nikita Vasudev Chitre, Mumbai, MH, India
Jagoda Chmielarz, Stuttgart, BW, Germany
Rubi Choudhary, Chennai, TN, India
Euihyuk Chung, Sendai, Miyagi, Japan
Nathan Churcher, Richardson, TX, USA
Sarah Cole, Meridian, ID, USA
Emily Crum, New York, NY, USA
D
Roya Damircheli, Washington, DC, USA
Smita Shivraj Dasari, Bryan, TX, USA
Marco De Sousa, College Station, TX, USA
Sayyam Deshpande, Bryan, TX, USA
Pranuti Devisetty, West Lafayette, IN, USA
Darshani Hansamani Dewage Dona, Stillwater, OK, USA
Germany Diaz De la Cruz, Los Angeles, CA, USA
Felix Diller, Garching bei München, BY, Germany
Sara Domenici, Torino, PM, Italy
Siddharth Doshi, Stanford, CA, USA
Susomon Dutta, Kanjikode, KL, India
Endy Dwiputra, Ulm, BW, Germany
Shyamli Dwivedi, La Jolla, CA, USA
EJay Easter, Severn, MD, USA
Eran Eini, Ra’anana, Israel, Israel
Abdussalam Elbanna, Cairo, Cairo, Egypt
Franziska Engel, München, BY, Germany
Luis Esquivel, Santa Rosa, CA, USA
Johnathan Evans, Boulder, CO, USA
F
Pramahadi Febriyanto, Bandung, West Java, Indonesia
Lena Fiedler, Erlangen, BY, Germany
Meriem Fikry, Taufkirchen, BY, Germany
Nicolas Fontaine, Thetford Mines, QC, Canada
Federico Frezza, Prague, Prague, Czech Republic
Lydia Fries, Princeton, NJ, USA
Hinata Fujimura, Yokohama, Kanagawa, Japan
Shun Furuhata, Yokohama, Kagawa, Japan
GPragati Gaire, Athens, OH, USA
Jessica Gallawa, Fort Collins, CO, USA
Hongpeng Gao, La Jolla, CA, USA
Tanner George, Halifax, NS, Canada
Masoomeh Ghasemi, Columbia, SC, USA
Arindam Ghosh, Bengaluru, KA, India
Florian Gollmann, München, BY, Germany
Abigail Gonzalez Zea, San Juan del Rio, Queretaro de Arteaga, México
Keerthana Govindaraj, Pittsburgh, PA, USA
Duygu Gumus, Stjordal, Trondelag, Norway
Akhilesh Gupta, Omaha, NE, USA
Richa Gupta, Chennai, TN, India
Varun Gupta, La Jolla, CA, USA
Eugene Gyasi Agyemang, Fayetteville, AR, USA
HLuis Hagner, Freiburg, BW, Germany
Hannah Halstead, Seattle, WA, USA
Louis Hamlet, Montréal, QC, Canada
Mitchell Hansen, Golden, CO, USA
Alyssa Hash, Bryan, TX, USA
Zimad Hashmi, New Delhi, DL, India
Emad Hatami, Guelph, ON, Canada
Darius Hayes, Denver, CO, USA
Amir Hegazy, Rensselaer, NY, USA
Michael Hehemeku, Athens, OH, USA
Johanna Hemauer, Garching bei München, BY, Germany
Niklas Hensle, Freiburg, BW, Germany
Kate Hermiller, Athens, OH, USA
Robert Hinz, Münster, NRW, Germany
Nozomi Hirakuni, Yokohama, Kanagawa, Japan
Binh Hoang, Silver Spring, MD, USA
John Hoerauf, College Park, MD, USA
Benjamin Holcombe, Shaker Heights, OH, USA
G M Mehedi Hossain, Edinburg, TX, USA
Elliot Howell, Montréal, QC, Canada
Alex Hughes, Broomfield, CO, USA
Chi-Kai Hung, Toronto, ON, Canada
I
Darlington Imhanzuaria, Fayetteville, AR, USA
Aoi Inoue, Kumamoto, Kyushu, Japan
Md Najmul Islam, Tyler, TX, USA
Md Sirajul Islam, Cleveland, OH, USA
J
Abdoulie A Jallow, College Station, TX, USA
Bryn Jenkins, College Station, TX, USA
Pawan Kumar Jha, Bengaluru, KA, India
Yangxin Jin, Kowloon, Hong Kong, China
Aashish Joshi, Delhi, DL, India
K
Gurunathan K, Karaikudi, TN, India
Bhaskar Kakoty, Bangalore, KA, India
Liyaqat Ali Kamran, Stillwater, OK, USA
Al Kasani, Storrs Mansfield, CT, USA
Kapiamba Kashala Fabrice, Coral Gables, FL, USA
Raphael Kempf, München, BY, Germany
Gaelle Khalil, Paris, Île-de-France, France
Vedasri Khavala, Braunschweig, NI, Germany
Ernest Konadu-Yiadom, Golden, CO, USA
Autumn Kudlack, Hudson, NY, USA
Anushan Kulendran, Morwell, VIC, Australia
Neelesh Kumar, Bethlehem, PA, USA
Rahul Pandit Kumarchinchole, Stillwater, OK, USA
Tejaswini Kumbharde, Erlangen, BY, Germany
Po-Yu Kung, Ann Arbor, MI, USA
78 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
L
Somtochukwu Lambert, Bryan, TX, USA
Ryan Lamkin, San Diego, CA, USA
Adina Landstrom, Stockholm, Uppland and Södermanland, Sweden
Helene Langli, Heimdal, Sør-Trøndelag, Norway
Hanh Vi Le, Rennes, Brittany, France
Huy Le, Halifax, NS, Canada
Kyle Leatt, Amherst, NY, USA
Tanmaee Ledalla, West Lafayette, IN, USA
Dong Ju Lee, San Diego, CA, USA
Jaejun Lee, La Jolla, CA, USA
Jordan Lee, Palo Alto, CA, USA
Dev Lekhadia, West Lafayette, IN, USA
Wei Li, San Diego, CA, USA
Xingjun Li, Aalborg, North Jutland, Denmark
Xinyi Li, Pullman, WA, USA
Yunxing Li, Corvallis, OR, USA
Apichanont Limsukhon, Wangchan, Rayong, Thailand
Yilei Lin, München, BY, Germany
Øyvind Lindgård, Trondheim, SørTrøndelag, Norway
Jiahao Liu, Macomb, MI, USA
Shengqin Liu, Kowloon, Hong Kong, China
Jiayi Lu, Shanghai, Shanghai, China
Alexis Luglio, Ann Arbor, MI, USA
MMacgregor Macintosh, Halifax, NS, Canada
Filip Mackowicz, Brea, CA, USA
Mika Malmer, Trondheim, Sør-Trøndelag, Norway
Fourth Manaanuntakul, La Jolla, CA, USA
Nandapriya Manivelan, Busan, Gyeongsangnam-do, ROK
Yasaman Masoudi, Windsor, ON, Canada
Christopher Mauersberger, München, BY, Germany
Jack McGrath, Cincinnati, OH, USA
Michael McKee, Silver Spring, MD, USA
Bharath Krupa Teja Mekala, Storrs Mansfield, CT, USA
Caio Miliante, Hamilton, ON, Canada
Yuka Miyaoka, Yokohama, Kanagawa, Japan
Kazuki Mizumura, Higashiura, Aichi, Japan
Somayeh Mohammadi, El Paso, TX, USA
Ryan Moore, College Park, MD, USA
José Morales Martinez, Cuernavaca, Morelos, México
Marisa Moss, Troy, NY, USA
Peter Mwinisin, Vergato, EM, Italy
Vinit Nagda, Stockholm, Uppland and Södermanland, Sweden
NSiddhartha Nanda, Austin, TX, USA
Somen Nandi, Palaiseau, Île-de-France, France
Santiago Navarro, München, BY, Germany
Douglas Nelson, Lilburn, GA, USA
Vaishnavi Nemaniwar, Bryan, TX, USA
Jonas Neumeyer, Dornstadt, BW, Germany
Aoife Newman, Trim, Leinster, Ireland
Hung Nguyen, Trondheim, Trøndelag, Norway
Huyen Nguyen, Austin, TX, USA
Navid Noor, Hamilton, ON, Canada
O
Yui Obara, Kumamoto, Kyushu, Japan
Connor O’Dea, Austin, TX, USA
Taekeon Oh, Daejeon, South Chungcheong, ROK
Teppei Ono, Yokohama, Kanagawa, Japan
Miguel Orozco, Goleta, CA, USA
Haley Otting, Golden, CO, USA
Okoroike Ozoemena, Guelph, ON, Canada
PRavichandar P, Karaikudi, TN, India
Jeffrey Page, Stafford Springs, CT, USA
Daniel Lwanzo Paluku, Princeton, NJ, USA
Tanmay Panchpor, Greenwood, IN, USA
Ravi Pandey, Motihari, BR, India
Biplab Patra, Bangalore, KA, India
Pratikshya Paudel, Stillwater, OK, USA
Joyce Paula, Athens, OH, USA
Fnu Payal, Mumbai, MH, India
Sara Pedram, Storrs Mansfield, CT, USA
Juliette Pelletier, Outremont, QC, Canada
Quoc Phan, West Lafayette, IN, USA
Daranphop Pikulrat, Payubnai, Rayong, Thailand
Albert Pimenov, Cranberry Township, PA, USA
Kayla Poling, Cleveland, OH, USA
QJianting Qin, San Diego, CA, USA
Carlos Quiroz Reyes, Taipei, Taipei, Taiwan
Mo Rizwan Qureshi, Gandhinagar, GJ, India
RShomitha R, Chennai, TN, India
Kazi Rifat Bin Rafiq, Golden, CO, USA
Vikram Raghuraman, Gobichettipalayam, TN, India
Sapna Ramesh, Evanston, IL, USA
Nathanael Ramos, Boulder, CO, USA
Natcha Rasitanon, Hat Yai, Songkhla, Thailand
Abhishek Rawat, Arlington, TX, USA
Jeygeerthika Reddy, Busan, Gyeongsang, ROK
Alexander Rex, Hannover, NI, Germany
Maria Reynoso, Chula Vista, CA, USA
Phillip Ridley, La Jolla, CA, USA
Mahmudul Hassan Riyad, Lubbock, TX, USA
Eric Roman, Ann Arbor, MI, USA
Jackson Rose, Bountiful, UT, USA
Alana Rossen, Antwerp, East Flanders, Belgium
Erik Rottinghaus, West Lafayette, IN, USA
S
Habibollah Safari, Lincoln, NE, USA
Armance Sagot, Caen, Normandie, France
Takuya Sakurai, Nagoya, Aichi, Japan
Yonathan Salazar Lara, Santa Rosa Jauregui, Querétaro, México
Fereshteh Salimi Nanadegani, Athens, OH, USA
Ghazaleh Salmanian, Fayetteville, AR, USA
Rodrigo Sanchez, West Lafayette, IN, USA
Oluwafemi Sanumi, Willimantic, CT, USA
Azeem Sarwar, Storrs Mansfield, CT, USA
VItaly Savvin, München, BY, Germany
Survanshu Saxena, Hamilton, ON, Canada
Christian Schneemann, Braunschweig, NI, Germany
Jan Schoeberl, Garching bei München, BY, Germany
Kelby Schrader, Owasso, OK, USA
Charles Schreiber, Tuscaloosa, AL, USA
Markus Schreiber, München, BY, Germany
Coby Scrudder, Coppell, TX, USA
Becca Segel, Newtown, PA, USA
Felix Setiawan, Vancouver, BC, Canada
Mahmoud Shaban, Lincoln, NE, USA
Yasmin Shabeer, Waterloo, ON, Canada
Md Mahmudul Hasan Shagar, Cleveland, OH, USA
Sameep Rajubhai Shah, West Lafayette, IN, USA
Muntasir Shahabuddin, Worcester, MA, USA
Hessam Shahbazi, Chicago, IL, USA
Farhanaaz Shaik, Chennai, TN, India
Shan Shao, Hong Kong, China
Parul Sharma, Delhi, DL, India
Alexander Shearer, Stanford, CA, USA
Liya Sherly Leo, Trondheim, Trøndelag, Norway
Vadim Shipitsyn, Charlotte, NC, USA
Resham Shrestha, Stillwater, OK, USA
James Sieffert, Montréal, QC, Canada
Kaan Şimşek, İstanbul, İstanbul, Turkey
Rifael Snitkoff-Sol, Byniamina, Israel
Jakub Sochor, Blovice, Plzeň-South District, Czech Republic
Nicholas Solan, La Jolla, CA, USA
Simran Sony, Oxford, MS, USA
Patricia Isabel Soriano, Muntinlupa City, Metro Manila, Philippines
Noémie Sorrentinella, München, BY, Germany
Shaswat Srivastava, Bangalore, KA, India
Michael Steffe, West Lafayette, IN, USA
Lukas Stein, Hannover, NI, Germany
Siddhartha Subramanian, Delft, South Holland, Netherlands
Lawnardo Sugiarto, Hong Kong, Sha Tin, Hong Kong
Kimaya Suryarao, Cambridge, MA, USA
Harshada Suryawanshi, Ann Arbor, MI, USA
Susana Suttor, Unterföhring, BY, Germany
TNene Takao, Kumamoto, Kyushu, Japan
Babak Tavana, Burlington, ON, Canada
Robel Mehari Tesfaye, Merced, CA, USA
Pranav Thacker, Austin, TX, USA
Shreya Thakkar, Pittsburgh, PA, USA
Sai Praneeth Thota, Trois-Rivieres, QC, Canada
(continued on next page)
The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 79 NEW MEMBERS NEW MEMBERS NEW MEMBERS NEW MEMBERS
(continued from previous page)
Yeshvi Tomar, Los Angeles, CA, USA
Kaouther Touidjine, Jülich, NRW, Germany
Duc Tran, San Jose, CA, USA
Cheng-Hung Tsai, Taipei, Taipei City, Taiwan
Wei-Shiang Tsai, Hsinchu, Hsinchu County, Taiwan
Yang Tu, London, England, UK
Liam Twight, Eugene, OR, USA
USeçil Ünsal, London, England, UK
VA Varsha, Trichy, TN, India
Chandana Sai Velagaleti, Chennai, TN, India
Krishna Venkatesh, Ilmenau, TH, Germany
Marta Vicencio, San Diego, CA, USA
Jaya Vikeswara Rao Vajja, Lafayette, IN, USA
David Villalva Mejorada, Miguel Hidalgo, Ciudad de México, México
Raja Viswanathan, Madurai, TN, India
Emily Volk, Lakewood, CO, USA
Phat Vu, Ho Chi Minh, HCM, Vietnam
WZhiwen Wan, Ann Arbor, MI, USA
Luqi Wang, Halifax, NS, Canada
Shao-Che Wang, Keelung, Keelung City, Taiwan
Yanyu Wang, Montréal, QC, Canada
Yinyu Wang, Storrs, CT, USA
Zixuan Wang, College Park, MD, USA
Patrick Wargo, West Lafayette, IN, USA
Christina Wark, Okemos, MI, USA
Christopher Warsch, Taufkirchen, BY, Germany
Oliver Waszkiewicz, London, England, UK
Aidan Weber, Ann Arbor, MI, USA
Shudan Wei, Leeds, England, UK
Daniel Weindl, Garching bei München, BY, Germany
Kaden Wheeler, Santa Barbara, CA, USA
Nathan Wichert, Athens, OH, USA
Leon Wickert, Essen, NRW, Germany
Warren Wilczewski, Rocky River, OH, USA
Franziska Wilfinger, Eching, BY, Germany
Jan Witte, Clausthal-Zellerfeld, NI, Germany
Jaina Wollowitz, New York, NY, USA
Ho Kun Woo, Champaign, IL, USA
Eric Wornyo, Tuscaloosa, AL, USA
Chun-Huang Wu, Dongshi, Chiayi County, Taiwan
XYing Xia, Richland, WA, USA
Yiying Xiao, Singapore, West Region, Singapore
Jiale Xing, Storrs, CT, USA
Weijie Xu, Halifax, NS, Canada
Y
Himanshu Yadav, Detroit, MI, USA
Cheng Yan, Shanghai, Shanghai, China
Wenlin Yan, La Jolla, CA, USA
Chen-Hsun Yang, Hsinchu, Hsinchu, Taiwan
Gang Yang, Stafford Springs, CT, USA
Zilan Yang, Auburn, AL, USA
Sabina Yasmin, Halifax, NS, Canada
Ecem Yelekli Kirici, Burlington, ON, Canada
Kyeong-Rim Yeo, Seoul, Gyeonggi-do, ROK
Sarah York, Fayetteville, AR, USA
Li Yu, Mong Kok, Kowloon City, Hong Kong
Seungju Yu, Seoul, Gyeonggi-do, ROK
Weilai Yu, Palo Alto, CA, USA
Z
Federica Zammillo, Turin, PM, Italy
Ardavan Zanganeh, Melrose, MA, USA
Briar Zapalac, Lubbock, TX, USA
Lanshuang Zhang, La Jolla, CA, USA
Luqi Zhang, San Diego, CA, USA
Muhan Zhang, Hong Kong, Kowloon City, Hong Kong
WeIran Zhang, College Park, MD, USA
Zhuldyz Zhigulina, Athens, OH, USA
Qi Zhu, Hong Kong, Hong Kong
Shang Zhu, Ann Arbor, MI, USA
Zilong Zhuang, Madison, WI, USA
Australia Belgium Brazil Canada China Czech Republic Denmark Egypt France Germany Hong Kong India Indonesia Iran Ireland Israel Italy Japan Mexico NetherlandsNorway Pakistan Philippines Singapore South KoreaSpain Sweden Taiwan Thailand Turkey UK USA Vietnam New Members by Country Look who joined ECS in the First Quarter of 2024 Australia. 4 Belgium 4 Brazil. 1 Canada 30 China. 6 Czech Republic. 2 Denmark 2 Egypt. 1 France 5 Germany . 45 Hong Kong 5 India . 34 Indonesia 1 Iran. 1 Ireland 1 Israel. 2 Italy . 6 Japan 19 Mexico . 7 Netherlands 2 Norway. 6 Pakistan 1 Philippines . 1 Singapore 1 South Korea. 11 Spain 1 Sweden . 2 Taiwan . 12 Thailand 3 Turkey. 1 UK 9 USA . 245 Vietnam 1
80 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org NEW MEMBERS NEW MEMBERS
STUDENT NEWS STUDENT NEWS
ECS Case Western Reserve University Student Chapter
Electrifying Excellence: The Chapter’s Journey
Electrochemistry is a cornerstone of scientific exploration and innovation at Case Western Reserve University (CWRU). CWRU has a rich history in electrochemistry and electrochemical engineering, disciplines initiated through Professor Ernest B. Yeager’s leadership. CWRU’s dedication to excellence in this field is evident through its robust community of researchers, scholars, and students who continuously push the boundaries of knowledge and discovery. From fundamental studies of electrode processes to developing novel electrochemical devices and materials, electrochemists and electrochemical engineers at CWRU tackle diverse challenges creatively and rigorously. A vibrant and supportive community fostering collaboration, mentorship, and knowledge exchange, driving the collective pursuit of scientific excellence forward, is central to CWRU’s success in electrochemistry.
A once-vibrant ECS Case Western Reserve University Student Chapter struggled over recent years as the electrochemical industry around Northeast Ohio changed and the COVID-19 pandemic forced the chapter into inactivity. One year ago, a dedicated executive committee—comprising President Oğuz Kağan Coşkun, Vice President Desiree Mae Prado, Secretary Saurabh Pathak, Treasurer


Zeynep Bagbudar, Social Media Liaison Yuanman Ma, and Faculty Advisor Prof. Robert Savinell—reestablished the chapter and launched it on a journey to revitalize its presence and impact within the university’s electrochemistry community.
The revived chapter quickly gained momentum, in a short time attracting over 25 members, including postdocs, graduate students, and undergraduates. With a renewed sense of purpose, plans were made to fulfill ECS’s mission to advance theory and practice at the forefront of electrochemical and solid state science and technology and allied subjects—a mission that aligns perfectly with CWRU’s commitment to research excellence and innovation. The chapter embarked on various initiatives to promote research, foster discussion, encourage critical assessment, and disseminate knowledge in the electrochemistry field. Seminars and workshops were organized to engage members and the broader community in meaningful discussions and collaborations.
Throughout the year, these educational opportunities provided chapter members with valuable insights into various aspects of electrochemistry. The inaugural electrochemistry gathering on
(continued on next page)


The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 81
The ECS Case Western Reserve University Student Chapter organized numerous activities during the academic term. a) CWRU’s Prof. Jesse Wainright discusses electrochemical impedance spectroscopy. b) Dr. Alex Peroff from Pine Research elucidates the functionality of potentiostats. c) Chapter executive committee members engage with Dr. Zhimin Zhong from NASA over lunch. d) Student chapter members host Prof. Hubert Gasteiger, Technische Universität München (back row, third from the left), and Prof. Gessie Brisard, Université de Sherbrooke (back row, fifth from the left), during the CRWU Eltech Lecture Series.
a) c) b) d)
(continued from previous page)
STUDENT NEWS STUDENT NEWS
September 21, 2023 featured CWRU Professors Rohan Akolkar and Dr. Nicholas Sinclair reporting on reference electrodes and electrode kinetics. On November 20, 2023, Prof. Jesse Wainright (CWRU) delivered a comprehensive session on electrochemical impedance spectroscopy, offering participants a deeper understanding of this powerful analytical technique. Dr. Zhimin Zhong from NASA shared insights on electroplating and PEM fuel cells on January 25, 2024, shedding light on cutting-edge developments in these fields.
On February 15, Dr. Alex Peroff from Pine Research elucidated the inner workings of potentiostats, providing attendees with a clearer understanding of this essential electrochemical instrument. On February 27, the chapter was honored to host two distinguished guests, Prof. Hubert Gasteiger from the Technische Universität München (who delivered two insightful Eltech Series Lectures during his visit to CWRU) and Prof. Gessie Brisard from Université de Sherbrooke. On March 22, Prof. Daniel Scherson (CWRU) engaged participants in an intriguing discussion on bioelectrochemistry, exploring the innovative concept of utilizing enzymatic fuel cells to harness the sugar in an insect’s blood and power electronics to deliver commands to these cyborg insects.
Additional engaging and informative sessions include two Interface Sessions led by CRWU professors Robert Warburton and Burcu Gurkan on crucial aspects of interfacial simulation by fundamental approaches and developing interfacial models to describe the electrode-electrode interface in complex electrolytes, respectively. The semester culminated with a lecture by Prof. Rohan Akolkar on cyclic voltammetry simulations, offering members a practical insight into this essential electrochemical technique. These ongoing seminars and workshops continue to foster a vibrant community of electrochemistry enthusiasts, enriching its members’ academic and professional experiences.
Looking ahead, the chapter aspires to be recognized as vibrant, engaged, and contributing to advancements that positively impact society and the electrochemical community. Our members were stimulated by attending and presenting their research results at the 245th ECS Meeting and look forward to PRiME in Hawaii. Our dedicated leadership, enthusiastic membership, and supportive faculty have electrified the revived chapter and will propel us into an impactful future.
Contributed by Oğuz Kağan Coşkun, Desiree Mae Prado, Saurabh Pathak, Zeynep Bagbudar, Yuanman Ma, and Robert Savinell
ECS Central Electrochemical Research Institute Student Chapter
ECS recently chartered a student chapter at the Council of Scientific and Industrial Research-Central Electrochemical Research Institute (CSIR-CECRI). Dr. S. A. Ilangovan, the Former Deputy Director of the Vikram Sarabhai Space Centre (VSSC) inaugurated the chapter on February 28, 2024, unveiling the chapter’s logo and website, and delivering the inaugural lecture, “Powering the Batteries: A Promising Approach.” His illuminating lecture discussed current challenges in advancing battery technology, especially the technical difficulties of engineering batteries and supercapatteries for futuristic applications requiring high power and high energy. Participants’ active interaction with the speaker reflected their curiosity and enthusiasm about electrochemistry. In his presidential address, Dr. K. Ramesha, Director, CSIR-CECRI, thanked ECS for this interesting initiative, which will serve as a launching pad for students’ many inspiring and innovative ideas. The chapter’s faculty advisor, Dr. N. Lakshminarasimhan, Senior Principal Scientist, CSIR-CECRI, informed the gathering about ECS’s mission and the chapter’s future plans. The event, which attracted about 150 participants, will serve as a catalyst for future endeavors, providing a platform for learning, collaboration, and growth within the electrochemical community.


82 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Students and professors gather for the inauguration of the ECS Central Electrochemical Research Institute Student Chapter.
Dr. S. A. Ilangovan, Former Deputy Director, Vikram Sarabhai Space Centre, inaugurated the recently chartered ECS Central Electrochemical Research Institute Student Chapter
STUDENT NEWS STUDENT NEWS
ECS Indian Institute of Technology Madras Student Chapter
The ECS Indian Institute of Technology Madras Student Chapter organized a week-long, hands-on training session from January 12 to 17 featuring distinguished solid state chemist Prof. Werner Paulus of the Institut Charles Gerhardt, Université de Montpellier. This part of our lecture series was restricted to a limited number of participants and provided a comprehensive one-on-one learning experience on structure analysis and the application of solid state chemistry in electrochemistry.
To broaden the horizons of electrochemistry education beyond our local community, the chapter collaborated with the National Institute of Technology Tiruchirappalli (NITT) to host the “Electrochemical Characterization of Batteries” workshop on January 22–23. This event attracted more than 100 participants from the region. A hands-on session, followed by a quiz based on the hands-on topics, complemented lectures by esteemed faculty members from the Indian Institute of Technology Madras (IITM), Prof. Kothandaraman R and Prof. Ramanathan S, and from NITT, Prof. R. Karvembu, Prof. L. Cindrella, and Dr. Sunandan Sarkar
The chapter and IITM Energy Consortium co-hosted a twoday symposium titled “Symposium and Hands-on on Batteries,” honoring the remarkable contributions to electrochemistry of Prof.
(continued on next page)

At their Atria University-Bengaluru “Battery Characterization Techniques” workshop, chapter members present a hands-on demonstration of assembling a DIY Redox battery kit and conducting galvanostatic charge-discharge.


The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 83
The ECS Indian Institute of Technology Madras Student Chapter collaborated with the National Institute of Technology Tiruchirappalli (NITT) to host the “Electrochemical Characterization of Batteries” workshop.
ECS Indian Institute of Technology Madras Student Chapter committee members co-organized a two-day “Symposium and Hands-on on Batteries” with the IITM Energy Consortium.
(continued from previous page)
STUDENT NEWS STUDENT NEWS
A. K. Shukla of the Indian Institute of Science, Bengaluru. Prof. Shukla is a Fellow of The Electrochemistry Society and former editor of the Journal of The Electrochemical Society. The event was a blend of insightful talks by luminary professors Shukla, Werner Weppner (Christian-Albrechts-Universität zu Kiel), and Venkataraman Thangadurai (University of Calgary), and 10 invited speakers. A hands-on session took place on the second day. To encourage trying different redox systems and to ignite a passion for electrochemistry, all participants received redox battery kits and were taught how to assemble them independently. The event was sponsored in part by Renault Nissan Technology & Business Centre India and IITM Pravartak.
The chapter was invited to conduct a three-day “Battery Characterization Techniques” workshop at Atria UniversityBengaluru. This workshop, the chapter’s second initiative outside IITM in Chenai, provided an end-to-end understanding of electrode making and battery fabrication. From preparing the slurry to mastering cell performance evaluation techniques, participants were meticulously guided through each step of the process.
In July, the chapter ran a one-week summer school program at the Vellore Institute of Technology-Vellore. A special lecture by Prof. Archita Patnaik is scheduled for Teacher’s Day on September 5, 2024.

ECS Massachusetts Institute of Technology Student Chapter
The ECS Massachusetts Institute of Technology (MIT) Student Chapter is thrilled to announce the addition of new members to our executive committee. The chapter is led by Katelyn Ripley and Trent Weiss, Co-Chairs; Yash Samantaray, Treasurer; Lia Bu, Secretary; Katherine Steinberg, Secretary; and Ethan Benderly-Kremen, Nicholas Matteucci, Anish Sukumar, Cindy Wong, and Siqi Wu, Event Planners. Committee members are associated with a variety of departments across MIT, including Chemical Engineering, Materials Science and Engineering, and Mechanical Engineering.
The chapter successfully hosted various events, including student mixers, a joint summer barbeque with the ECS Harvard University Student Chapter, many speaker sessions, and industry/start-up panels with local industry representatives. The group hosted Dr. Jerome Babauta from Gamry Instruments in April for an event covering potentiostat operation, experimental troubleshooting, and a demonstration on assembling, troubleshooting, and operating an aqueous electrochemical cell. Other speakers included, from MIT, professors Martin Bazant, Yogesh Surendranath, and Ju Li; from Stanford University, Prof. William Tarpeh; and from Harvard, Prof. Zachary Schiffer
In the coming academic year, the chapter looks forward to hosting more seminar speakers and electrochemistry experts sharing their most recent and upcoming work in the field of electrochemistry!

ECS Munich Student Chapter
ECS Munich Student Chapter members and 20 students visited Varta Microbattery GmbH this past spring. In a series of exciting talks, the company provided insights into its R&D activities and the industrialization of micro batteries. The visit concluded with a guided tour of the production line for high-power batteries in cylindrical and coin cell format, from the mixing of the slurry overcoating, calendering, electrode cutting, coil winding, and electrolyte filling up, to the formation of batteries.
In March, the chapter hosted a continuation of its lecture series. Invited speakers Prof. Yang ShaoHorn (Massachusetts Institute of Technology) and Prof. Jeff Dahn (Dalhousie University) gave exciting talks about their research in the fields of energy materials and batteries, then answered participating students’ numerous questions. The event, which

84 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
IITM Prof. Kothandaraman R explains the transport number calculation in polymer electrolytes to Atria University students.
The ECS Massachusetts Institute of Technology Student Chapter hosted a speaker seminar featuring Prof. Zachary Schiffer, Harvard University.
Photo: Nicholas Matteucci
The ECS Munich Student Chapter Lecture Series features Prof. Yang Shao-Horn (Massachusetts Institute of Technoloy, bottom) and Prof. Jeff Dahn (Dalhousie University, top).
attracted around 60 people, was open to all interested Technische Universität München students, including undergraduates, graduate and PhD students, postdocs, and professors.
Further 2024 chapter plans include visiting the BMW Group battery pilot line and hosting a conference to connect students and university chairs working in electrochemistry in the Munich area.

ECS Texas Tech University Student Chapter
With the kickoff of the spring semester, the ECS Texas Tech University Student Chapter arranged an engaging inaugural meeting where we celebrated the start of a new term, shared ideas, and set goals and plans for the months ahead. Each member had the opportunity to voice their thoughts and contribute to our plan, ensuring the agenda was dynamic and diverse.
In the spirit of diversity and equity, the chapter also conducted its officer selection meeting. The chapter demonstrated its dedication to involving younger students and incorporating their viewpoints by electing an undergraduate member to represent undergrads. This new addition to the chapter will help connect the chapter to the Society’s goals and newer generations.
Beyond its regular activities, the chapter emphasizes cultivating professional and academic relationships. Upholding this commitment, we successfully organized a trio of webinars, fostering a rich exchange of ideas and igniting lively discussions. In the first webinar, Prof. Miguel A. Modestino from New York University presented “Engineering Electrochemical Reactions for Sustainable Chemical Manufacturing,” seamlessly tying academic theory to practicalities for sustainable solutions. Prof. Karthish Manthiram from the California Institute of Technology explored the vital topic of “Electrification and Decarbonization of Chemical Synthesis” in the second webinar. Chris Starr from Metrohm USA presented “The Interdisciplinary Future of Electrochemistry—The Experimental” in the third webinar. His talk provided a compelling look into the experimental and commercial potentials of electrochemistry.
Moving forward, the ECS Texas Tech University Student Chapter aims to deepen engagements with leading experts, do more webinar series, organize further seminar series, and establish connections with other ECS Student Chapters. In the coming academic year, the chapter looks forward to hosting more seminar speakers and electrochemistry experts sharing their most recent and upcoming work in the field of electrochemistry!


The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 85 STUDENT NEWS STUDENT NEWS
The ECS Texas Tech University Student Chapter is delighted to introduce their new undergraduate representative. Pictured (from left to right:) Vice President Zenifar Haque, President Nathan Wilson, Secretary Jessica Ortega, Treasurer Dipu Saha, and newly recruited Undergrad Officer, Archer Montgomery
Chris Starr, Karthish Manthiram, and Miguel Modestino present three engaging talks in the spring ECS Texas Tech Student Chapter webinar series.
ECS Munich Student Chapter members visit Varta Microbattery GmbH.
ECS University of Michigan Student Chapter
The ECS University of Michigan Student Chapter welcomes the newly appointed External Relations Chair Christopher Woodley, Publicity Chair Davy Zeng, Outreach Chair Hongyi Lin, and new Faculty Mentor Assistant Professor Joshua Jack! With the help of its new executive committee members, the chapter had an eventful start to the year.
The chapter hosted four invited speakers. First was Dr. Stephen J. Harris, Scientific Engineering Associate at Lawrence Berkeley National Laboratory, who gave an informative talk on “Statistical and Machine Learning-Based Durability Testing Strategies for Energy” and provided further insight on industry vs. academic career paths. Principal Engineer at Mercedes-Benz Research & Development North America and Vice Chair of the ECS Detroit Section Tobias Glossmann presented “Advanced Batteries Technologies— Opportunities Revisited” on the future possibilities and challenges for battery usage in electric powertrains, from fast charging to battery manufacturing. Dr. Owen (Zijie) Lu, Research Engineer for Cell Technology at Ford Motor Company, delivered an exciting lecture on the future of sulfide-based solid state batteries titled “Challenges and Opportunities in Developing Sulfide-Based Solid-State Batteries for Electric Vehicle Application.”

Research Engineer for Cell Technology at Ford Motor Company Dr. Owen (Zijie) Lu presents “Challenges and Opportunities in Developing SulfideBased Solid-State Batteries for Electric Vehicle Application.”
Jinhong Min



“Advanced

86 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
NEWS
STUDENT NEWS STUDENT
Invited Speaker Dr. Stephen J. Harris, Scientific Engineering Associate at Lawrence Berkeley National Laboratory, presents “Statistical and Machine Learning-Based Durability Testing Strategies for Energy.”
Photo: Joshua P. Hazelnis
The ECS University of Michigan Student Chapter Treasurer, Jinhong Min, hosts a Journal Club meeting.
Photo: Jinhong Min
ECS University of Michigan Student Chapter officers (from left to right): President Daniel Liao, External Relations Chair Christopher Woodley, and Secretary Joshua P. Hazelnis prepare to dish out pizza for a chapter social event.
Photo: Ryan Van Daele.
Invited Speaker Tobias Glossmann, Principal Engineer at Mercedes-Benz Research & Development North America and Vice Chair of the ECS Detroit Section, delivers a talk on
Batteries Technologies—Opportunities Revisited.”
Photo:
STUDENT
To reach a broader body of students and host larger events, the chapter has started collaborating with other student organizations across the University of Michigan (UofM). “Electric Mobility Transition Pathways: Comparing US and India Experience,” a seminar co-hosted with the UofM Battery Club, featured Dr. Rahul Walawalkar, President and Managing Director of Customized Energy Solutions (India). Professors, post-doctoral students, and graduate students across a multitude of engineering and STEM disciplines attended the seminars. To continue providing our students with diverse experiences, the chapter will invite a range of local and international speakers from industry, national labs, and professors.
The chapter collaborated with the Mechanical Engineering Graduate Council (MEGC) to present a “Lunch and Learn” with industry and academic professionals Dr. Jeff Lowe from General Motors, Shaurya Sarna from Our Next Energy, Dr. Javier Parrondo from Nissan Motor Corporation, and UofM Assistant Professors Joshua Jack and Yiyang Li. Small group discussions were facilitated by providing each speaker with a table where attendees could enjoy a provided lunch while learning about the professional’s individual career path.

Photo: Davy Zeng
The ECS University of Nebraska–Lincoln Student Chapter held its annual executive officers’ election on February 28, 2024, in Othmer Hall on the university’s campus. Chapter President Sahand Serajian ran the meeting and was unanimously reelected for his second term. From among all the nominees, the chapter elected Oghenetega Obewhere as Vice President, Sarang Ismail as Treasurer, and Micah Quirie as Secretary.
With its new advisory board in place, the chapter is planning future meetings and events.
The club hosts journal clubs and social events regularly to help inform interested electrochemists on current literature research topics and promote conversations among colleagues in a comfortable small-group setting. Chapter Treasurer Jinhong Min, External Relations Chair Christopher Woodley, and Secretary Joshua P. Hazelnis hosted three journal club social events, each filled with fruitful discussions, even better refreshments, and pizza! The chapter participated in Student Organization Fairs to connect with students beyond the Engineering and STEM disciplines. Thank you to the University of Michigan’s Electric Vehicle Center, Chemistry, Mechanical Engineering, and Material Science Departments, and The Electrochemical Society for their generous support and funding of chapter events.
As the chapter approaches its one-year anniversary, we are excited to keep growing and hosting larger events around campus. For more information and additional details about activities and initiatives, please visit the chapter’s website

ECS University of Nebraska—Lincoln Student Chapter

The Electrochemical Society Interface • Summer 2024 • www.electrochem.org 87
NEWS
NEWS STUDENT
Dr. Javier Parrondo (far left) from Nissan Motor Corporation is one of five industry and academic professionals featured in the ECS University of Michigan Student Chapter “Lunch and Learn” series presented in collaboration with the UofM Mechanical Engineering Graduate Council (MEGC).
The chapter collaborates with the UofM Battery Club to present “Electric Mobility Transition Pathways: Comparing US and India Experience,” a seminar by Dr. Rahul Walawalkar, President and Managing Director of Customized Energy Solutions (India).
Photo: Daniel Liao
Meet the members of the ECS University of Nebraska–Lincoln Student Chapter!
STUDENT NEWS STUDENT NEWS
ECS University of Texas at Austin Student Chapter
In the spring semester, the ECS University of Texas at Austin Student Chapter focused on building partnerships with other student chapters in Texas. In March, we visited our friends at Texas A&M University in College Station. The ECS Texas A&M University Student Chapter organized tours of the laboratories of Professors Lane Baker, Abdoulaye Djire, Emily Pentzer, and Jodie Lutkenhaus. We learned about exciting electrochemical research such as titanium nitride 2D materials for electrocatalysis, organic energy storage devices, and novel battery electrolytes. Afterward, we shared a nice lunch and discussed research questions and social activities with ECS Texas A&M Student Chapter members. We also toured the university campus and learned about its history. More information about the visit is on the chapter website
The University of Texas at Austin (UT)’s long tradition of electrochemistry research began with the late Prof. Allen Bard. He founded the Center for Electrochemistry (CEC) at UT to promote excellence in electrochemistry research and foster collaboration. The chapter actively collaborates with CEC on events and seminars. A CEC Fellowship was awarded to chapter Vice Chair Tushar Telmasre for the spring 2024 cohort. On February 2, Telmasre delivered a talk on “Simulation Strategies for Measuring Impedance Response of Lithium-Ion Batteries” at the CEC seminar.
We continue hosting our Chalk Talks showcasing chapter members’ research in their areas of expertise. We were lucky to host Dr. Manan Pathak, former member of Prof. Venkat Subramanian’s research group, as part of the series. Dr. Pathak cofounded BattGenie Inc., a company that provides efficient battery management solutions for lithium-ion batteries. His talk provided valuable insight into using graduate research to start a company.
We are excited to announce that research collaborations and publications have resulted from students interacting at our Chalk Talks! Chapter members Raul A. Marquez and Jay T. Bender published “Transition metal incorporation: electrochemical, structure, and chemical composition effects on nickel oxyhydroxide oxygenevolution electrocatalysts” in Energy and Environmental Science after meeting at a Chalk Talk last year. Chalk Talk recordings can be viewed on our YouTube channel

Jay
Throughout the rest of the year, we are committed to building a strong community of electrochemistry researchers at UT Austin and engaging with scientists in the ECS community beyond our campus!
Hook ‘em Horns!
and Anthony C.



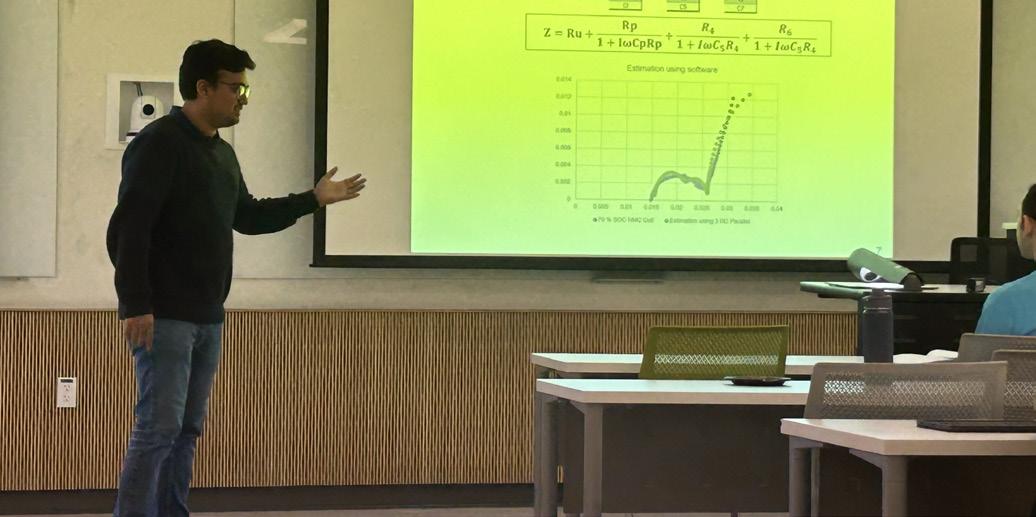
88 The Electrochemical Society Interface • Summer 2024 • www.electrochem.org
Contributed by
T. Bender
Concepción, Chairs; Tushar Telmasre, Vice Chair; Jeremy Brinker, Treasurer; and Raul A. Marquez, Secretary.
Dr. Segun Wahab describes nanoscale electrochemical characterization equipment during ECS University of Texas at Austin Student Chapter members’ visit to Lane Baker’s lab.
Houston Smith explains synthetic chemistry and electrolyte development at the Emily Pentzer lab during ECS University of Texas at Austin Student Chapter members’ visit to Texas A&M University.
All smiles! UT Austin chapter members gather with Texas A&M student chapter members for a last selfie before heading back to Austin.
(From left to right:) ECS UT Austin Student Chapter Vice Chair Tushar Telmasre introduces the chapter’s Chalk Talk series speaker, Dr. Manan Pathak, Cofounder and CEO of BattGenie Inc.
ECS UT Austin Student Chapter Vice Chair Tushar Telmasre delivers a talk at a UT Austin Center for Electrochemistry seminar.

Full issues now available for purchase and download from the ECS Online Store: www.electrochem.org/online-store Volume 113: 245th Meeting of The Electrochemical Society 20% ECS Members’ Discount ENHANCE YOUR MEETING EXPERIENCE


PRiME 2024
Joint International Meeting of ECS, ECSJ, and KECS
Honolulu, HI
October 6-11, 2024
Hawaii Convention Center & Hilton
Hawaiian Village
247th ECS Meeting
Montréal, Canada
May 18-22, 2025
Palais des Congrès de Montréal



248th ECS Meeting
Chicago, IL
October 12-16, 2025
Hilton Chicago
249th ECS Meeting
Seattle, WA
May 24-28, 2026
Washington State Convention Center
electrochem.org/meetings
UPCOMING ECS MEETINGS







 Rob Kelly Editor
Rob Kelly Editor

























 Gessie Brisard
Gessie visits New York City with her sister, Nadia. Photo: Gessie Brisard
Gessie attends a performance at the Metropolitan Opera in New York City. Photo: Gessie Brisard
Shopping in New York City is a favorite pastime.
Gessie Brisard
Gessie visits New York City with her sister, Nadia. Photo: Gessie Brisard
Gessie attends a performance at the Metropolitan Opera in New York City. Photo: Gessie Brisard
Shopping in New York City is a favorite pastime.



















































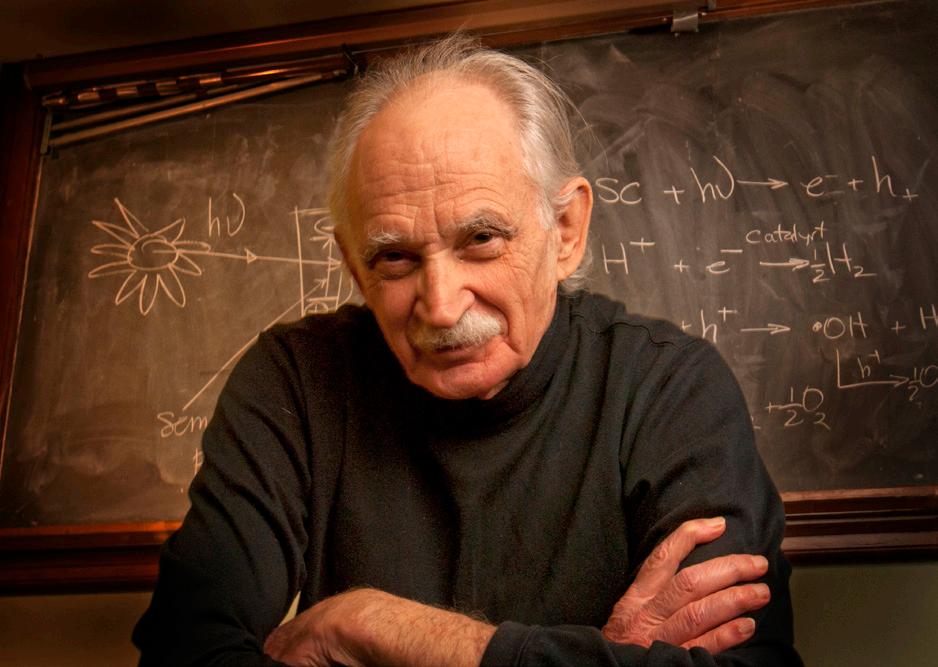 by Frances N. Chaves
by Frances N. Chaves




































































































































