Think
6
12
14
18
30
34
35
36
24
40
44
48
49
50
51
52
negis.e@ethosmedia.eu
56
58
60
KEY ACCOUNT MANAGER charalampakis.c@ethosmedia.eu
krouli.c@ethosmedia.eu
62
64
κοκαΐνη, συνθετικές καθινόνες, φάρμακα υποκατάστασης, νέες ψυχοδραστικές
ανιχνεύονται σε υπολείμματα στις σύριγγες, συχνά σε συνδυασμό, αυξάνοντας και τον κίνδυνο υπερδοσολογίας. Αυτό προκαλεί ανησυχία,
1.
τα οποία μόνο 221 ήταν νέα καινο-
σκευάσματα (Διάγραμμα 1 και 2).
Από αυτά τα 221 νέα καινοτόμα φάρμα-
μόνο 43 (19%) είναι σήμερα διαθέσι-
(Διάγραμμα 3).
Μόνο 5 από τα 35 (14%)
Tο Accelerating Clinical Trials in the EU (ACT
Φαρμάκων (EMA).
Με γνώμονα τους στόχους καινοτομίας των κλινικών
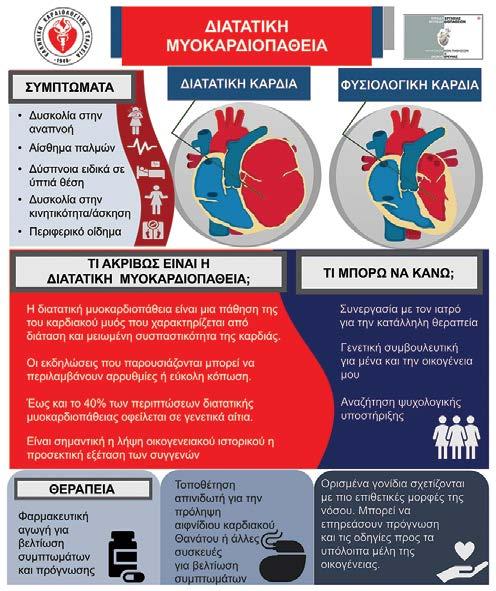
διερεύνηση
του συγκεκριμένου γενετικού τύπου της νό-
σου, καθώς και ο έλεγχος της οικογένειας
του ασθενούς που πρωτοδιαγιγνώσκεται. Τις τελευταίες δεκαετίες, η ανάπτυξη της
MYO Health and Agora Labs
Synthetic Data and how they revolutionize clinical and pharmacoeconomic research in Europe
Access to clinical and pharmacoeconomic data at large scale
The healthcare sector finds itself at a pivotal juncture, where the convergence of multi-omics, personalized care and AI-enabled digital health solutions promises to reshape the landscape of healthcare diagnosis and delivery. Access to large-scale health data emerges as a crucial need in research, technology development and market access roadmaps. With an exponential growth forecasted, artificial intelligence is set to revolutionize the data ecosystem.
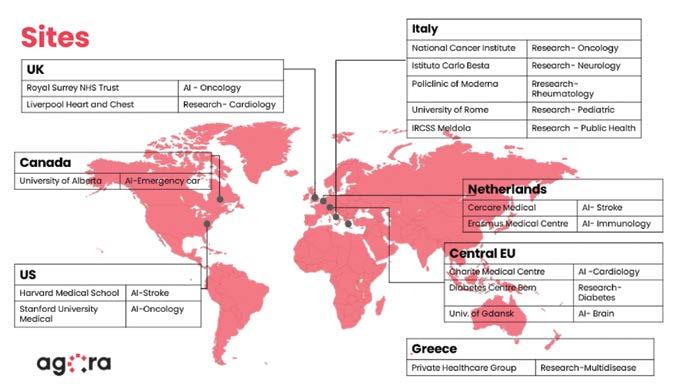
Industry pioneers, MYO Health and Agora Labs have partnered to provide the life sciences industry with access to clinical and pharmacoeconomic data sourcing and research services on a large scale, performing studies nationally as well as internationally across multiple sites (Figure 1). The rapid developments they are bringing to the field are discussed at Ph.B by the founders of the two companies. Both of them are medical doctors with studies at Harvard (in Health Policy & Economics, Dr. Myrsini Ouzounelli, CEO MYO Health) and at MIT (in Health Data Science, Dr. Davide Zaccagnini, CEO Agora Labs) and with extensive prior work in the field both in Europe and in the United States. The two scientists and entrepreneurs explain the importance of synthetic data, contributing to clinical and pharmacoeconomic research by enabling access to data and evidence sets.
They secure valuable datasets for generating realworld evidence, for designing and conducting clinical and pharmacoeconomic studies.
Figure 1
Q_What is synthetic data and what role can it play for the pharma industry?
R_Dr. Davide Zaccagnini: Synthetic data are high-fidelity datasets created to precisely replicate real-world data for their statistical properties, information structure and record-level characteristics. They allow to fully meet GDPR and HIPAA regulations in Europe and the United States, respectively, addressing privacy limitations at their root and enabling compliant research at large scale. Organizations increasingly choose synthetic data when real data can’t be used, can’t be shared or can’t be moved, which is what describes the present situation in many European counties, including Greece. In that sense, synthetic data is a great, revolutionary AI enabler that will transform clinical and pharmacoeconomic research in Europe.
Q_What was your starting point and how did the collaboration come about? What are you aiming for?
R_Dr. Myrsini Ouzounelli: Agora Labs was born from EU-funded research on privacy-preserving technologies and spawn-off of Athena Hi-Tech Research Center. The founders, Dr. Davide Zaccagnini and PhD George Pikramenos and Professor Minos Garofalakis bring together clinical and computer science expertise that led to the development of the platform. The system has been installed and is in use in more than 30 sites internationally. In MYO Health we have more than two decades of experience subcontracting pharmacoeconomic research and HEOR services for market access and reimbursement for US partners, as well as in generating post-market comparative effectiveness and/ or safety studies, using data from the large US datahouses, such as Optum, Symphony, or CMS. In the last five years MYO Health has added operations in Greece and in the United Kingdom. The design, implementation and publication of studies based on the secondary use of healthcare


data are central within MYO Health’s spectrum of consulting activities. The two companies first cooperated within the framework of MYO Health’s innovation management work; soon their natural synergies led to a partnership agreement, which is exclusive in Greece and some other geographies, when it comes to the secondary use of data for clinical/ pharmacoeconomic research. With this partnership we combine our capabilities, to generate valuable, privacy-guaranteed datasets for RealWorld Evidence generation, to then design and conduct clinical and pharmacoeconomic studies. This supports optimal product positioning and price negotiations for our clients. It also provides a very robust support to new pharma products, both in terms of informing clinical trial design, but
Dr. Myrsini Ouzounelli
Dr. Davide Zaccagnini
In Greece, we have finalized contracting and are installing nodes and we are ready to conduct research on more than 5 million patient records, across a range of disease areas, which is unprecedented.
primarily for post-marketing safety and comparative effectiveness studies. We have already activated datasets in oncology, neurology and cardiology and are quickly augmenting datasets in other clinical areas.
Q_What is your common vision for your international market expansion?
R_Dr. Davide Zaccagnini: The partnership is rapidly expanding both inside and outside of Europe, starting with the UK, Canada and soon the Gulf countries. In Canada, for instance, we are powering the development of AI for emergency care over multiple Emergency Room (ER) facilities in the Alberta region, while in Italy we are supporting large scale training and validation of medical AI for the early diagnosis of breast and prostate cancer, and we are generating real-world evidence in neurology and oncology. Further, regional healthcare systems in Italy are using the platform to compare centralized and distributed delivery systems in their efforts to reduce waste and improve quality of care. Greece has been among the first countries in which we’ve activated research on data at large scale. This has been by virtue of the relationship between Agora Labs and the Athena High-Tech incubator, as well as of MYO Health’s established presence in the country leading all research operations. We
have activated research on more than 5 million patient records, spanning several disease areas, which is unprecedented. We expect that this can bring Greece at the forefront of these game-changing developments, next to the UK, France, Italy and Spain.
Q_What innovation has the cooperation between your companies introduced?
R_Dr. Myrsini Ouzounelli: Until recently, other than the UK’s CPRD database, we didn’t have, in Europe, an effective way to use largescale healthcare data in support of market access, in the same way we did in the United States. The CMS (Medicare/Medicaid) database alone generates hundreds of studies per year (Figure 2), and for most of the HTA submissions we prepare in the US with our partner Strategic Health Resources, we’ve been generating studies that have made a very substantial difference in negotiations and pricing. The synthetic datasets recently created by the UK’s NHS (CPRD synthetic datasets) and by Germany’s Clarite Lab for AI in Medicine (CLAIM) are the first such initiatives, using nation-wide data in Europe, that can enable similar access to large-scale data in Europe and we are proud to be among the first to be bringing this capability to our clients through our exclusive partnership agreement with Agora Labs. •••
Figure 2