








We spoke to Prof. Marianna Kruithof-de Julio and her team at the University of Bern about their research which involves using patient-derived organoids to improve bladder cancer treatment. Their work aims to tailor therapies based on individual tumor profiles, addressing the disease’s high heterogeneity and recurrence rates.
Bladder cancer ranks as the tenth most prevalent cancer worldwide, with approximately 550,000 new cases and 200,000 deaths each year, making it a substantial public health challenge. Despite advancements in treatment, its heterogeneity and high recurrence rate pose ongoing difficulties, highlighting the demand for more effective and personalised treatments. Dr. Marianna Kruithof-de Julio and her team at the University of Bern are developing patientderived organoids from bladder cancer tumors to tackle these issues. These small clusters of cells mimic the genetic and molecular traits of the original tumors, providing a promising path for personalised cancer treatment. Bladder cancer is characterised by a high heterogeneity between patients which often contributes to treatment failures. Molecular and histological differences highlight the need for personalised treatment approaches. Pathologically, the disease is classified into two main types: non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). NMIBC accounts for around 70% of cases and is typically treated with transurethral resection of the bladder (TUR-B) and intravesical instillations of chemotherapy, often in combination with the Bacillus Calmette–Guérin (BCG) vaccine. However, many of these tumors will recur, and about 10% will progress to MIBC, a highly aggressive tumor with a high mortality rate. MIBC is usually treated with systemic cisplatin-based chemotherapy followed by radical cystectomy, a highly invasive procedure that is only beneficial for 30-40% of patients.
Patient-derived organoids have been successfully used to predict drug response in vitro for various cancers such as ovarian, pancreatic, and gastrointestinal cancers.
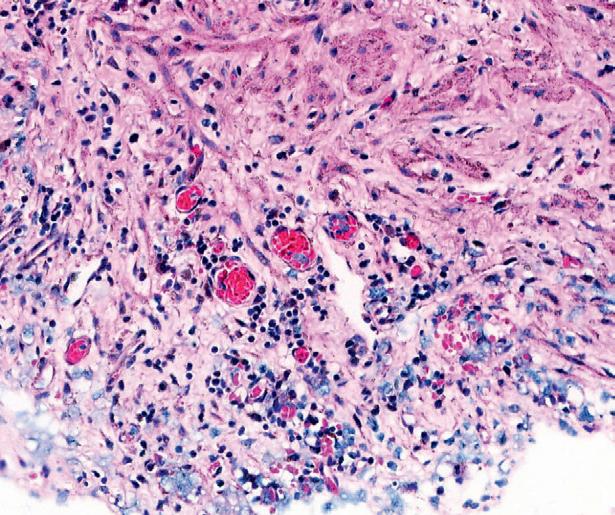
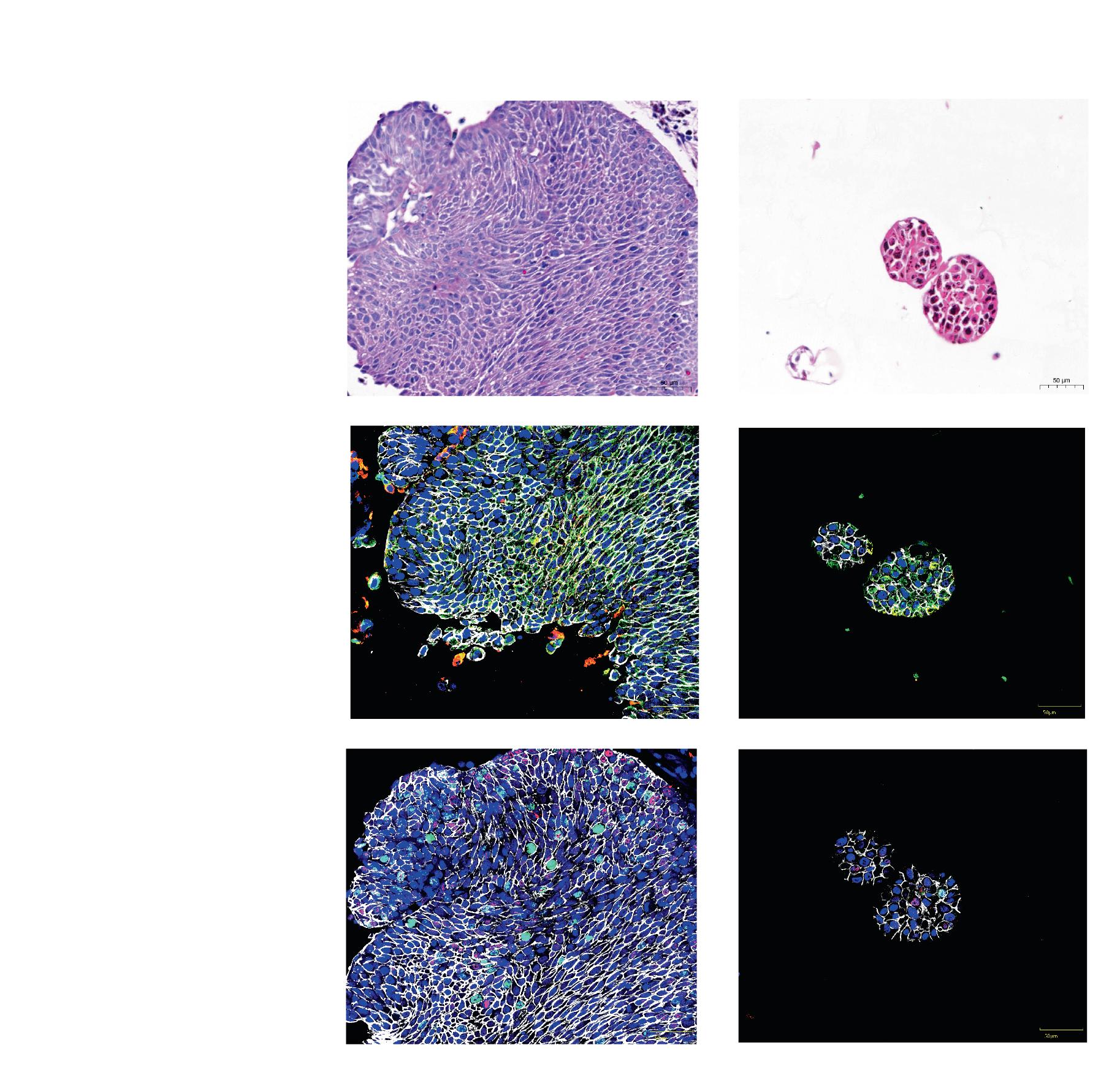
Prof. Kruithof-de Julio and her research team have successfully grown organoids from various bladder cancer stages and grades that preserve the histological and molecular heterogeneity of the original tumors. In their study, the team created the organoids from specimens obtained through transurethral resection, cystectomy. The organoids were successfully cultured
biomarkers that could forecast therapy response and resistance. This approach aids in understanding how mutations render therapies ineffective and how tumors develop resistance to treatment over time. It can also determine which treatments might be effective based on the genetic composition of a patient’s tumor. However, before organoids can be broadly implemented in clinical settings, they must undergo further validation through clinical trials. Dr. Kruithof-de Julio highlights that the success achieved with organoids in cancer treatment can potentially be applied to human patients.
“With the use of this technology, we can evaluate a broad spectrum of drugs to identify the most promising options for each individual patient.”
regardless of the tumor’s stage, grade, or histological pattern. The organoids maintained a similar level of cellular proliferation compared to the original tumors and mirrored the tumor’s evolution over time, allowing researchers to observe how the cancer changes and adapts.
The researchers have created a drug screening method to evaluate both standard-care drugs and FDA-approved cancer drugs on patient-derived organoids. By examining how these organoids react to the drugs and comparing their drug response profiles with their genetic information, the researchers have identified
One of the most significant findings is the heterogeneity in organoid drug responses. For example, in few NMIBC organoids tested, the standard chemotherapy drug mitomycin C showed low significant effect, whereas epirubicin was effective in 60% of the samples tested. In contrast, MIBC organoids displayed a higher sensitivity to the cisplatin/gemcitabine combination, which is a common treatment regimen for this aggressive form of cancer. “These findings underscore the significance of personalised treatment plans. Identifying which drugs are
most effective for particular genetic profiles can enhance patient outcomes and minimise the trial-and-error method that currently dominates cancer treatment”, explains Prof. Kruithof-de Julio.
The researchers analysed the genetic mechanisms behind drug resistance. They found that organoids derived from tumors with high genomic burdens, such as those from high-grade invasive cancers, showed different responses than those from low-grade tumors. In one case, the team analysed organoids from a patient who experienced multiple recurrences of NMIBC. By comparing organoids from different stages of the disease, they could track how the tumor evolved and became resistant to treatment. This longitudinal analysis provides a deeper understanding of the disease and its evolution. In another case, organoids from a patient diagnosed with pan-urothelial disease were tested with various drugs. The organoids showed a high
sensitivity to lapatinib, a drug targeting the EGFR/ERBB2 pathway, which is frequently altered in bladder cancer. This finding suggests that lapatinib could be a viable option for patients with similar genetic profiles, which highlights the potential for repurposing existing drugs for more personalised treatments.
“Organoids allow us to test a wide array of drugs and pinpoint the most promising options for each patient,” says Prof. Kruithofde Julio. “This approach not only saves time and resources but also reduces the burden on patients who would otherwise endure multiple treatment trials.” The research team is now intent on further refining their organoid models and increasing their drug screening capabilities. They aim to include a wider range of drugs and investigate new therapeutic targets. Additionally, they are working on enhancing the scalability of organoid cultures to make this technology more accessible for clinical use.
Project Objectives
The research project focuses on cultivating patient-derived organoids from bladder cancer tumors to develop personalised treatment plans. By growing organoids replicating the original tumors’ genetic and molecular characteristics, the team aims to identify effective drug therapies for individual patients. The study also seeks to understand the genetic mechanisms behind drug resistance and the evolution of bladder cancer, ultimately aiming to translate these findings into clinical practice for improved patient outcomes.
Project Funding
Development of a platform for GU cancer patient-derived organoids OC-2019003 3RCC / InoSwiss 101.951 IP-LS awarded to Marianna Kruithof-de Julio.
Project Partners
The team includes Prof. Marianna Kruithof-de Julio, Prof. Bernhard Kiss, Prof Beat Roth, and Prof. George Thalmann, from the Department of Urology of the University Hospital Bern, Prof. Roland Seiler-Blarer from the Department of Urology of the Spitalzentrum Biel-Bienne and Dr. Marta De Menna, Deputy Director of the Translational Organoid Resource Core at the Department for BioMedical Research of the University of Bern. Their combined expertise drives this innovative research in personalised cancer treatment and precision oncology.

Contact Details
Professor Marianna Kruithof-de Julio, PhD Head of the Urology Research Laboratory Department of Urology & Department for BioMedical Research
Director
Organoid CORE facility
University of Bern
Murtenstrasse 24, 3008 Bern, Switzerland e: marianna.kruithofdejulio@unibe.ch

Professor Kruithof-de Julio is a researcher at the University of Bern, specializing in precision medicine within urology. She leads the Urology Research Laboratory and the Organoid CORE facility, focusing on advanced tools for precision medicine. Her team has successfully developed patient-derived organoids for bladder, prostate, and pancreatic cancers, providing critical models for better understanding these diseases. They also utilize microvascular on-chip chambers to study the functional potential of single cells. What distinguishes her research group is their holistic approach, examining tumor cells, stroma, immune cells, and vasculature together to gain a comprehensive view of cancer and its progression.


