
24 minute read
SCIENCE AND THE SENSES
Science and the Senses: Pigments
Jasmine Chan (Year 12, Wu)
Pigments are everywhere. They can be found in the fabric of clothes that you are currently wearing, the hair on top of your head and even the food that you consume. As a person who loves colour, the realm of pigments fascinates me. In this article, I am going to introduce you to the basics of pigments, how they are classified and their biological applications.
1 INTRODUCTION TO PIGMENTS
1.1 What are pigments? Pigments are chemical compounds that are coloured, black or white [1]. In nature they usually have specific functions, but we make extensive use of pigments in many industries to add colour to objects. Pigments are usually insoluble solids but in order to change the surface colour of an object easily, a liquid form would be desired. Due to their insolubility, a solution cannot be formed and therefore, a mixture containing solid particles in a liquid is used. When the pigments are incorporated, they remain unaffected, chemically and physically [2, 3]. However, there are some cases where pigments are used in their solid form, such as eyeshadows in the cosmetics industry.
1.2 Organic and Inorganic Pigments Organic pigments are made from natural sources, such as plants. Organic means that one or more carbon atoms are present in the pigment molecules, whereas inorganic products do not contain any carbon atoms. Organic pigments also usually contain small amounts of sulphur and nitrogen atoms [4]. There are three types of organic pigments: ‘carbon’ pigments (eg. lamp black pigment which is made from the products of incomplete combustion of oils), ‘lake’ pigments (combining anionic dyes with metallic salts) and ‘non-ionic’ pigments (eg. azo pigments) [5]. The largest group of organic pigments is azo pigments which contain one or more azo groups (-N=N-) which form red, orange and yellow pigments [6].
Inorganic pigments are usually made from the oxidation of chemicals that are not sourced from plants. One example is ‘titanium white’; these white pigments are known as white extenders which not only provide opacity and lighten the colour of other substances but also improve their properties, such as durability, and strength [3, 4].
When comparing organic and inorganic pigments, inorganic pigments have a much larger average particle size than organic pigments which allows inorganic pigments to be more opaque. As organic pigments have a larger surface area to volume ratio, they provide higher colour strength (better ability to provide a colour of higher intensity to other Figure 1: List of organic and inorganic pigments [7]
materials). [7, 8] However, inorganic pigments are longer-lasting than organic pigments. In addition, organic pigments are much safer to use, as inorganic pigments may cause serious side effects on the human body [3, 4].
1.3 Synthetic Pigments The vast majority of pigments, both organic and inorganic, are synthetic. This means they are manufactured or processed from raw materials. Pigments are either produced by processing chemicals such as acids and petroleum compounds under intense heat/pressure or are manufactured using other minerals to mimic the natural form of pigments [9]. Organic pigments are derived from natural substances on earth (eg. plants) which means that the colour of the pigments are earthy and nature-like. However, due to the processing of these pigments, unnatural colours (which cannot be seen in nature) can be created.
One example of organic and synthetic pigment would be ultramarine blue and synthetic ultramarine blue. Natural ultramarine blue is derived from a gemstone known as lapis lazuli (Fig. 2). However, synthetic ultramarine blue is made by combining alumina, silica, sulphur and soda under high temperatures. Since the ingredients are the main elements of lapis lazuli, it makes the synthetically produced pigment almost chemically identical to the natural one. In this case, since lapis lazuli has a crystalline structure, the pigment creates more depth than the one that is synthetically made [10]. Figure 2: A picture of lapis lazuli and ultramarine blue pigment (Source: eBay)
1.4 What gives a pigment its specific colour? Pigments are chemical substances that only reflect a small range of wavelengths of the visible light spectrum. This gives the pigment its specific colour, as the wavelengths of light reflected determines what colour humans perceive (Fig. 3) [46]. Different pigments have different substances in them to give their colour [3]. For black pigments, due to their organic nature, they contain carbon. As carbon is black, it gives the pigments a black shade. For white pigments, a variety of substances can give them a white shade such as titanium dioxide (TiO 2 ), calcium carbonate (CaCO 3 ), calcium sulphate (CaSO 4 ) and diatomaceous earth (SiO 2 ). Iron oxides such as ochres, siennas and umbers create brown pigments which have hints of yellows and oranges. Different compounds of chromium are used to create chrome yellows (lead chromate - PbCrO 4 ) [11], oranges (mixture of lead chromate and lead molybdate - PbMoO 4 ) [12] and greens (chromium sesquioxide - Cr 2 O 3 ) [13], whereas different compounds of cadmium are used to create brilliant reds and oranges (mixture of cadmium sulphide - CdS and cadmium selenide sulphide - Cd 2 SeS) [14, 15] and yellows (mixture of cadmium sulphide and zinc sulphide - ZnS). [16] Blues such as Prussian blue (iron (II, III) hexacyanoferrate (II, III) - Fe 7 C 18 N 18 ) [17] and ultramarine blue (sodium sulphide aluminosilicate - Na 2 OSAl 2 O 3 SiO 2 ) [18, 19] are made using compounds of iron.
Figure 3: A Visual Representation of Wavelengths of Light and Its Corresponding Colour (Source: VectorStock)

2 NAMING PIGMENTS
Pigments have a specialised naming system called the Colour Index. This system is regulated and standardised by the Society of Dyers and Colourists and the American Association of Textile Chemists and Colorists [20]. This is very important for people working with colour, especially for artists that want to know the appearance of the colour in the paint tube and how they mix with other colours [21]. This Colour Index system is necessary as paint companies tend to name the same pigment with different names. For example, phthalocyanine blue can be called phthalo blue, Winsor blue, or bocour blue even though they are the same pigment [22].
The colour index uses a dual classification system. One system is called the Colour Index Generic Name (CIGN) which is more common and easier to remember, and the other system is called Colour Index Constitution Number (CICN) which links back to the chemical structure of the pigments [23].
2.1 Colour Index Generic Name (CIGN) Before this system was created, pigments were named after their technical chemical name. One example would be quinophthalone yellow. However, with the CIGN, it is now known as PY138 [24].
The alphabets at the beginning of CIGN are to describe the general colour of the pigment (Fig. 4). The P represents the word ‘pigment’ and the rest of the alphabets represents the colour. The number(s) in the pigment code is the individual pigment identifier which is assigned for commercial use. However, if the pigment is no longer manufactured in the industry, the number would be withdrawn. Currently, PY40 refers to a yellow pigment called aureolin as it is the 40th entry in the list of yellow pigments.
However, due to the deletion of particular pigments throughout the years, the numbering of these pigments may not be consecutive [22]. Figure 4: Pigment codes and the corresponding colour [22] 2.2 Colour Index Constitution Number (CICN) The Colour Index Constitution Number originally started with five-digit numbers. In the beginning, the numbering of these pigment colours was in multiples of five to ensure space for future pigments that are chemically similar. However, since 1997, there have been more pigments which are added to the CICN than anticipated. To alleviate congestion, new pigments have six-digit numbers [25].
The numbers in the CICN correspond to a main chemical class (Fig. 5). Colon numbers are used to further divide both CIGNs and CICNs for more identification of the pigment’s chemical properties and structure [23]. For example, PR48 (C.I. 15865) uses sodium salt whereas PR 48:1 (C.I. 15865:1) uses barium salt during formation.
3. CHARACTERISTICS OF PIGMENTS
3.1 Lightfastness Lightfastness (also known as the permanence of colour due to light) is the chemical stability of a pigment under the exposure to light for a long duration of time. Light is a source of energy which can cause the colour to alter due to chemical changes in pigments. For example, over time, the pigments may become desaturated, tinted (whitened), shaded (darkened) or even completely disappear.
Lightfastness testing was first developed by the American Society for Testing and Materials (ASTM) which conducted these tests with various lights such as sunlight, fluorescent UV lamps, cool white
Figure 5: Table of CIGNs and the corresponding main chemical class [26]




fluorescent etc. However, these experiments were inaccurate as these tests often exposed paint samples to an intense radiation of light which caused a quick change in the appearance of the pigments. Moreover, when testing, the amount of light was unknown or was not kept constant.
In order to solve this problem, the Association of Textile Chemists and Colorists (AATCC) developed a ‘blue wool scale’ (Fig. 6). The scale uses two blue wool textile fading cards which each consist of eight strips of blue wool that fade at different rates [20]. Ultraviolet radiation in light causes the pigment in blue wool to fade. All of the blue pigments selected for the scale differ as each pigment takes 2-3 times longer to begin fading compared to the pigment selected above. This means that the strip on the top has the least permanence and the strip on the bottom has the most permanence (Fig. 7) [27].
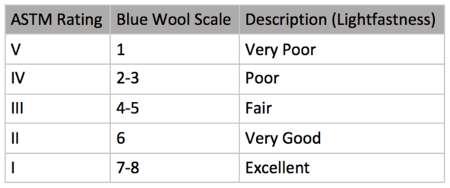

Figure 7: Strips on the Blue Wool Scale Corresponding to the Time Taken to Fade [20] Figure 8: Blue Wool Scale. Left: exposed to 800 hours of UV Right: exposed to no light [28]
One cardscale is exposed to a specific wavelength of ultraviolet and the another is exposed to no light [20] (Fig. 8).
Two samples are also made for the pigment that wants to be tested. One of the samples is also placed in the dark and the other exposed to ultraviolet light at the same time as the blue wool scale. When a reference pigment in the blue wool scale begins to discolour, all the other pigments that have started to discolour after that point are rated as having the level of lightfastness as the reference pigment. The pigment is then given an ASTM (American Society of Testing and Materials) rating (Fig. 6).
3.2 Tinting Strength Tinting strength (or known as tinting power) is the measure of the relative power of colouring of a pigment in a form of a paste [30].
A tint test is used to show the ability of a pigment (in paste form) to maintain its strength when mixed with a white pigment paste. The test reveals the ability of the pigment to maintain its strength (its intense colour) while using the same volume of white paste. The darker/more vibrant the colour is at the end of the test, the higher the tinting strength of the pigment (see Fig. 11). For example, when comparing phthalocyanine blue with ultramarine blue, phthalocyanine blue has 40 times higher tinting strength than ultramarine blue. This means that if phthalocyanine blue were to be used in high concentrations, it would completely overpower a larger variety of colours mixed with it compared to ultramarine blue [31, 32].

Figure 10: Visual Example of Phthalocyanine (Top) and Ultramarine Blue (Bottom) (Source: Pigment Tokyo) Tinting strength is always determined relatively, meaning that there is no absolute scale. Hence, when a tint test is performed, information of the medium used must be recorded. A reference pigment would be selected and it is labelled as 100%. Other pigments compared to the reference pigment can have a higher or lower percentage than 100% depending on the tinting strength (the lower the number, the lower the tinting strength) [33].
The tinting strength values for two similar pigments can also be used to calculate the concentration of the pigment required to give the same intensity. If the pigments have a high tinting strength such as phthalocyanine, companies would most likely reduce the concentration within the application substance as a high concentration is not required [30].

Figure 11: Example of a Tint Test with 12 Cadmium Reds from Different Brands [32]

Figure 12: An Example of Refraction [34]
3.3 Hiding Strength Hiding Strength (or known as opacity) is the covering power of pigment when applied to another substance. The opacity of pigment varies due to its chemical structure and size of particles [33]. The hiding strength is heavily influenced by the refractive index and particle size of the pigment.
3.3.1 Refractive Index Different pigments can absorb different wavelengths of light to create ‘colour’. When light travels through a substance, its velocity is slower, causing the beam of light to be refracted. The refractive index is calculated by the formula of n = (sin i) / (sin r). n represents the refractive index, i is the angle of incidence (the angle between the light beam entering the substance and the line perpendicular to the surface of the substance) and r is the angle of refraction (the angle between the refracted beam of light and the line perpendicular to the surface) (Fig. 12).

As the refractive index increases, more of the light is scattered, meaning that opacity increases. Though this is the case for many pigments, there is one special case for this relationship which are white pigments. White pigments have little to no absorption of light. This means that the hiding strength of white pigments completely relies on the scattering of the incident light.
As mentioned in 1.2, inorganic pigments have a higher hiding strength than organic pigments. This is because inorganic pigments have a higher refractive index [35].
3.3.2 Particle Size The particle size of a pigment is the average size of the particles in the pigment. Most synthetic pigments are manufactured in a range of particle sizes to ensure they can be used for different applications. As the particle size decreases, the tinting strength increases. This is because small particle sizes have a large surface area to volume ratio which produces a more intense colour [32].

3.3.3 Dispersibility Dispersibility is the measure of how easy a pigment can spread/distribute across an area when in a liquid medium. Some pigments are more susceptible to clumping due to the electrostatic cling of their particles. Due to the clumping, the hiding strength decreases, since the dispersity increases [32].
4 TOXICITY OF PIGMENTS
Pigments must be handled with care during usage as some are extremely poisonous and may cause serious damage to the health of the user [36].
4.1 Exposure to Pigments The toxicity of pigments varies depending on the type of exposure: inhalation, ingestion and absorption or through the skin.
4.1.1 Inhalation All pigments in solid form are hazardous as they can travel into and irritate the lungs. When handling pigments, it is recommended to use a respirator mask designed to prevent toxic dust for safety measures. In addition, if the user smokes while handling the pigments, the pigments may be inhaled through the cigarettes which can be extremely dangerous [37].
4.1.2 Ingestion While working with pigments, traces of the pigments may end up on the skin of the user. If the pigment is not washed away properly, the residue of pigments may be ingested while eating. In order to lower the chance of consuming pigments, food or drink should not be in the same room as pigments to ensure not even a trace of pigment can land on the food or drink. It is also highly recommended to wash hands thoroughly after handling pigments [37].
4.1.3 Absorption Through Skin Pigments can enter the skin through cuts and scratches in the skin. Some pigments can cause inflammation (dermatitis) if in contact or other allergic reactions. In order to protect the entry of pigments to exposed wounds, it is recommended to use a bandage or any seals to cover the cuts [37].
4.2 Toxicity Levels Toxicity of pigments is evaluated by the Art and Creative Materials Institute (ACMI) alongside various independent toxicologists. Tested pigments would have either an ‘AP’ (approved product) or a ‘CL’ (toxic) label [36, 37]. Pigments can be classified as ‘highly toxic’ (serious injury/death), ‘moderately toxic’ (permanent minor injury), ‘slightly toxic’ (temporary minor injury) or ‘non-toxic’ (no detectable injury) [36, 38].
4.3 What causes the pigments to be toxic? Some pigments contain elements/substances that are toxic, which causes the pigment itself to be toxic as well [36, 38].
4.3.1 Antimony (Sb) Antimony is a toxin that has a moderate to high toxicity. It causes the eyes and respiratory tract to be irritated when in contact. It also affects the functioning of enzymes which often causes indigestion in the user. In extreme exposures, the worst-case scenario would be respiratory failure which may lead to death.
4.3.2 Arsenic (As) Arsenic is an element that has high toxicity and is a suspected carcinogen. It is corrosive and it can affect the peripheral nervous system. In addition, in large quantities, it may cause lung cancer and kidney damage.
Figure 12: Pictures of metals in Toxic Pigments [Source: periodictable.com]


Figure 13: Table of Highly Toxic Pigments [40, 41]
4.3.3 Cadmium (Cd) Cadmium is a suspected carcinogen and has high toxicity. It also causes the respiratory tract and eyes to be irritated when in contact. When ingested, symptoms such as abdominal cramps and extreme nausea can happen. Overexposure to cadmium is associated with lung and prostate cancer.
4.3.4 Chromium (Cr) Chromium is a suspected carcinogen as well as an element that has moderate to high toxicity. Symptoms include dermatitis, respiratory irritation, severe enteritis (inflammation of the intestine).
4.3.5 Lead (Pb) Lead has a high toxicity and is a reproductive toxin. It can cause anaemia, gastroenteritis, nervous system damage and many more serious effects. If a pregnant woman has been in contact with lead, it may affect the neurological development of the foetus.
4.3.6 Mercury (Hg) Mercury has a high toxicity. When a large amount of mercury is inhaled, it leads to respiratory irritation and pulmonary edema (excess fluids in the lungs).
5 BIOLOGICAL PIGMENTS
Apart from pigments that are used to add colour to objects such as paints, there are a variety of pigments that are located in plants and animals to aid specific function in these organisms. Biological pigments, as known as biochromes, can be produced in the organism or introduced from the environment. [42]
5.1 In Plants Some plant biochromes are used to provide colour, such as the colour on the petals of flowers, but the most important pigments in plants are the ones controlling photosynthesis. Photosynthesis is a vital process in plants for growth and development. These specific pigments are known as photosynthetic pigments [1, 43].
5.1.1 Photosynthetic Pigments Photosynthesis is the process in which plants (and some autotrophs such as algae) capture the energy of sunlight to convert carbon dioxide and water into glucose and oxygen. However, since pigments can only absorb a narrow range of wavelengths, there is more than one type of pigment needed in order to maximise the light absorbed from the sun. [1]
5.1.1.1 Chlorophyll Chlorophylls are green pigments that are found in chloroplasts. Chlorophylls are visually green as when light travels towards it, green light is reflected whereas the other wavelengths of light are absorbed. This makes plants appear green to the eye. The pigment molecule has a central magnesium atom which is surrounded by a porphyrin ring (a structure which contains nitrogen). The porphyrin ring allows electrons to be free to move around due Figure 14: Structure of Chlorophyll [Source: Wikipedia] to its shape. The ring has the ability to gain or lose electrons which can provide electrons to other substances. This enables the chlorophyll to trap light energy from the sun to allow the plant to photosynthesise for growth [1, 44].
5.1.1.2 Carotenoids Carotenoids are red, orange or yellow pigments that are synthesised in plastids in the plant cells. Carotenoids cannot convert light energy into chemical energy like chlorophyll. Instead, they pass on the absorbed energy to chlorophyll and are hence known as accessory pigments [1, 45]. Carotenoids also help to protect plants from excess light, since overexposure of light can destroy proteins in the cells [47].
During fall, leaves start to turn into a warm reddish colour. This is because chlorophyll breaks down and allows the carotenoids to be more visible [45]. Figure 15: General Structure of Carotenoids (Source: Wikipedia) 5.1.2 Other Plant Biochromes Aside from photosynthetic pigments, there are other pigments present in plants.
5.1.2.1 Flavonoids Flavonoids are vibrant pigments that are found in plants and fruits. [48] These pigments are a visual signal for pollinators such as bees to locate the plant or for organisms who consume fruits to disperse the seeds, both for reproductive reasons [49]. Figure 16: General Structure of
5.1.2.2 Betalain Betalain also plays an important role in pollination, just like flavonoids. However, they are only found in a niche group of plants such as beetroot. Betalain has two subgroups: betacyanin and betaxanthin. Betacyanin gives a deep red-violet colour, whereas betaxanthin gives a yelloworange colour [49].
5.2 In Humans There is a variety of pigments which are vital in order to aid humans in but some can have no function in the body.
daily activities. Some pigments are extremely important for survival, Flavonoids (Source: Wikipedia)


5.2.1 Melanin Melanin is the main pigment found in the skin of humans. It is a yellowbrown pigment made by melanocytes in order to protect humans from overexposure to the sun. Melanin is found near the surface of the skin to absorb dangerous ultraviolet wavelengths from penetrating the skin layer. Without melanin, skin cancer can develop.

Melanin is also found in the hair and eyes of humans. The different proportions of melanin in the hair and the iris of the eyes determine its colour. As the concentration of melanin increases, the darker the hair/ iris colour would be [49].
Figure 18: Structure of Melanin (Source: Wikipedia)
5.2.2 Haemoglobin Haemoglobin is a red pigment that is found inside red blood cells to carry oxygen for respiration. Respiration is a vital process in humans for energy. Blood in humans has a red colour due to the presence of haemoglobin. Each haemoglobin has four polypeptide chains: two alpha chains and two beta chains. Each chain contains a haem group which has an iron ion. The iron allows the haemoglobin to bind with oxygen [49].

Figure 19: 3D Image of Haemoglobin (Source: ANSTO)
5.2.3 Pigments in the Eye There are pigments present in the eye which are essential for humans to see. This includes rhodopsin and iodopsin.

5.2.3.1 Rhodopsin Rhodopsin is a pigment found in the photoreceptive rod cells which is in the retina of the eye. It allows humans to see in dim light and see shades of gray by converting light into an electrical signal. In a bright area, rhodopsin undergoes structural changes, resulting in electrical signals being sent to the brain, and is regenerated when the area is dark [49, 50].
Figure 20: Diagram of the Eye (Source: everdaypsych.com)
5.2.3.2 Iodopsin Iodopsin is a pigment that is also located in the retina of the eye, but in the photoreceptive cone cells (instead of rods) to perceive colour. In the human eye, there are three types of cone cells: S cones, M cones and L cones. S cones are sensitive to short wavelengths of light (eg. blue), M cones are most responsive to medium wavelengths of light (eg. green) and L cones are most sensitive to long wavelengths of light (eg. red) [49]. Figure 21: Visual Imagery of a Rod and Different Types of Cones (Source: NCBI)

CONCLUSION
Pigments are fascinating substances. They are so ingrained in our lives that they often go unnoticed, but we would not be able to see or perceive colour if they did not exist. Pigments are extremely important to the survival of all organisms, as well as to the beauty of our surroundings, making our lives more lively and exciting. To conclude this article, I would like to thank pigments for all they have done to make our lives easier and better.
[1] “Photosynthetic Pigments.” UC Museum of Paleontology, UCMP Berkeley, 9 July 1997, ucmp.berkeley.edu/glossary/gloss3/pigments.html [2] “Difference between Organic Pigments and Inorganic Pigments.” Koel Colours Blog, Koel Colours Private Limited, 8 May 2018, www. koelcolours.com/blog/pigments/difference-organic-pigments-inorganic-pigments/ [3] The Editors of Encyclopaedia Britannica. “Pigment.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 25 Mar. 2019, www.britannica. com/technology/pigment [4] “Understanding Organic and Inorganic Pigments and Their Areas of Applications.” Vipul Dye-Chem Ltd., 24 May 2017, vipulorganics.com/ blog/2017/05/24/understanding-organic-and-inorganic-pigments-and-their-areas-of-applications/ [5] “Colouration with Pigments.” Fundamentals and Practices in Colouration of Textiles, Second Edition, by J. N. Chakraborty, WPI Publishing, 2015, pp. 202–213 [6] “Additives.” Handbook of Thermoplastic Elastomers, by Jiri George Drobny, Elsevier, 2014, pp. 17–32 [7] “Pigments.” Edited by Jennifer Moore-Braun, BASF SE, www.dispersions-pigments.basf.com/portal/basf/ien/dt.jsp?setCursor=1_561069 [8] NeelimaNarendra. “Computation of Colour Strength.” Chromatic Notes, 19 May 2013, drnsg.wordpress.com/2013/05/19/computation-ofcolor-strength/ [9] Saitzyk, Steven. “Types of Pigments.” True Art Information, 30 June 2013, www.trueart.info/?page_id=520 [10] MacEvoy, Bruce. “Synthetic Organic Pigments.” Handprint, 8 Jan. 2015, www.handprint.com/HP/WCL/pigmt1d.html [11] O'Hanlon, George. “Artists Materials - Chrome Yellow: A Primary Color with a Brief History.” Natural Pigments Inc., 23 Sept. 2013, www. naturalpigments.com/artist-materials/chrome-yellow-paint/ [12] “Orange Molybdate Pigment.” Natural Pigments Inc., www.naturalpigments.com/orange-molybdate-pigment.html [13] “Chromium Oxide Green.” Natural Pigments Inc., www.naturalpigments.com/chromium-oxide-green.html [14] “Cadmium Red Pigment.” Natural Pigments Inc., https://www.naturalpigments.com/cadmium-red-pigment.html [15] “Cadmium Orange Pigment.” Natural Pigments Inc., https://www.naturalpigments.com/cadmium-orange-pigment.html [16] “Cadmium Yellow Pigment” Natural Pigments Inc., https://www.naturalpigments.com/cadmium-yellow-pigment.html [17] “Prussian Blue Pigment” Natural Pigments Inc., https://www.naturalpigments.com/prussian-blue-pigment.html [18] “Ultramarine Blue (Red Shade) Pigment” Natural Pigments Inc., https://www.naturalpigments.com/ultramarine-blue-red-shade.html [19] “Ultramarine Blue (Green Shade) Pigment” Natural Pigments Inc., https://www.naturalpigments.com/ultramarine-blue-green-shade.html [20] MacEvoy, Bruce. “Labeling, Lightfastness & Toxicity.” Handprint, 8 Jan. 2015, www.handprint.com/HP/WCL/pigmt6.html#lightfast [21] Caves, Julie. “Pigments and Colour Names.” Jackson's Art Blog, 9 Mar. 2016, www.jacksonsart.com/blog/2015/01/14/pigments-colour-names/ [22] Myers, David. “Paint and Pigment Reference Table Key.” Artiscreation, www.artiscreation.com/pigment_key.html [23] GH. “Introduction to the Colour Index™ : Classification System and Terminology.” Society of Dyers and Colourists, Apr. 2013, colour-index. com/introduction-to-the-colour-index [24] “Paliotol™ Yellow K 0961 HD.”, BASF SE, www2.basf.us/additives/pdfs/Paliotol_Yellow_K0961HD.pdf [25] GH. “Chemical Constitutions in the Colour Index.” Society of Dyers and Colourists, Sept. 2013, colour-index.com/cicn-explained [26] “CICN Groups & Sub-Groups.” Society of Dyers and Colourists, colour-index.com/cicn-groups-sub-groups [27] “The Blue Wool Scale.” Materials Technology Limited, www.drb-mattech.co.uk/uv%20blue%20wool.html [28] Johnson, Nicholas. “Blue Wool Lightfastness Standard References.” Tanguay Photo Mag, 10 Apr. 2019, www.tanguayphotomag.biz/digitalprinting/blue-wool-lightfastness-standard-references.html [29] Jürgens, Martin C. ASTM and Lightfastness of Media. Getty Publications, 2009, www.artcons.udel.edu/mitra/Documents/ASTM-andLightfastness.pdf [30] Briggs, T. R. “The Tinting Strength of Pigments.” The Journal of Physical Chemistry, ACS Publications, 1918, p. 1 [31] Schadler, Koo. “Learn the Characteristics of Pigments.” Artists Network, 31 Aug. 2011, www.artistsnetwork.com/art-mediums/watercolor/ learning-the-characteristics-of-pigments/ [32] MacEvoy, Bruce. “The Material Attributes of Paints.” Handprint, 8 Jan. 2015, www.handprint.com/HP/WCL/pigmt3.html [33] Teichmann, Günther. “Practical Methods for Determining the Tinting Strength of Pigments in Concrete.” Technical Service Department, www. sept.org/techpapers/40.pdf [34] Hodgkins, Leila. “Refraction.” Schoolphysics, 2013, www.schoolphysics.co.uk/age16-19/Optics/Refraction/text/Refraction_/index.html [35] O'Hanlon, George. “Why Some Paints Are Transparent and Others Opaque.” Natural Pigments Inc., 6 Dec. 2013, www.naturalpigments.com/ artist-materials/transparent-opaque-paints/ [36] Saitzyk, Steven. “Characteristics of Pigments.” True Art Information, 30 June 2013, www.trueart.info/?page_id=513 [37] “Toxicity of Pigments.” The Notebook, 1993, www.noteaccess.com/MATERIALS/ToxicityPigmt.htm [38] Kinnally, Edward. “Painting Safety; Painting Hazards.” Art Prints, 2007, www.pixelatedpalette.com/artmaterialssafety.html [39] Babin, Angela, and Diane Johnson. “Metal Pigments Used in Paints and Inks.” Nontoxicprint, 2019, www.nontoxicprint.com/metalpigments. htm [40] “Toxicity of Paint Pigments.” Captain Packrat, captainpackrat.com/furry/toxicity.htm [41] Babin, Angela. “Pigment Safety.” NontoxicHub, Nontoxicprint, 2019, www.nontoxichub.com/pigment-safety [42] http://www.gopetsamerica.com/substance/biological-pigments.aspx [43] https://www.encyclopedia.com/science/encyclopedias-almanacs-transcripts-and-maps/plant-pigment-0#D [44] The Editors of Encyclopaedia Britannica. “Chlorophyll.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 17 Mar. 2020, www. britannica.com/science/chlorophyll [45] “Carotenoid Pigments: Definition & Structure.” STUDY.COM, study.com/academy/lesson/carotenoid-pigments-definition-structure.html [46] “Biological Pigments in Plants - Types of Plant Pigments: Uses of Pigments.” BYJUS, BYJU'S, 23 Dec. 2019, byjus.com/biology/pigments/ [47] “Plant Pigment.” The Gale Encyclopedia of Science, Encyclopedia.com, 23 Apr. 2020, www.encyclopedia.com/science/encyclopediasalmanacs-transcripts-and-maps/plant-pigment-0#D [48] “Plants: Causes of Color.” Plants | Causes of Color, www.webexhibits.org/causesofcolor/7H.html [49] “Plant Pigment - Flavonoids.” Flavonoids - Anthocyanins, Color, Flavonols, and Occur - JRank Articles, science.jrank.org/pages/5304/PlantPigment-Flavonoids.html [50] Rogers, Kara. “Rhodopsin.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 6 Feb. 2018, www.britannica.com/science/rhodopsin











