CLINICAL INITIATIVES, RESEARCH AND CURRENT UPDATES IN TREATMENT

Would you like to receive Circuit as an e-newsletter?
Scan here to sign up to our mailing list


CLINICAL INITIATIVES, RESEARCH AND CURRENT UPDATES IN TREATMENT

Would you like to receive Circuit as an e-newsletter?
Scan here to sign up to our mailing list

Penny Lee, Pharmacy Manager, Epic Pharmacy Lismore
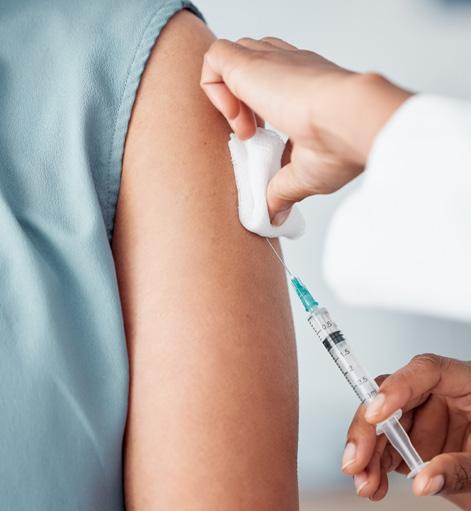
In November 2023, Shingrix® replaced Zostavax® on the National Immunisation Program (NIP), for the prevention of reactivation of the varicella-zoster virus (VZV) in a person who has previously been infected with VZV (i.e. chickenpox).1,2 VZV remains dormant in dorsal root ganglia nerve cells and may reactivate in some people as herpes zoster, commonly known as shingles.
Shingles generally presents as a unilateral, painful, vesicular rash. Other symptoms include tingling, headache, malaise and itch.1-4 Although self-limiting, shingles can lead to long term complications such as post-herpetic neuralgia.1,2
Unlike Zostavax, Shingrix does not contain live virus.1,2,4 Therefore Shingrix is suitable for use in people who are immunocompromised.1,2 Shingrix also contains an immunostimulant adjuvant system to help improve the body’s immune response to the vaccine over a longer period when compared to Zostavax.1,3,4
Shingrix is administered as a two-dose schedule (refer to Table 1 for NIP eligibility criteria and schedule).1,2,4 Those who do not qualify for the NIP free vaccination program and and are aged over 18 years can still be vaccinated by private prescription. It has been shown that from the age of 50, the burden of shingles dramatically increases, therefore, anyone over the age of 50 is encouraged to be vaccinated.1,2
Patients should be counselled on potential common side effects when administered Shingrix including pain, swelling and redness at the injection site. General post-vaccination care, such as paracetamol for pain or applying a cool, damp cloth to injection site, provides relief to most patients.1,2 Approximately 10% of people may experience generalised symptoms such as fatigue, muscle ache and headache, that may be severe enough to disrupt normal daily activities for 1-3 days; these typically go away without treatment.1,2
Table 1: Eligibility criteria for free shingles vaccination under the NIP1
Eligibility criteria Dosing
People aged 65 years and over
Aboriginal and Torres Strait Islander people aged 50 years and over
Immunocompromised people aged 18 years and over with the following medical conditions:
¬ Haematopoietic stem cell transplant
¬ Solid organ transplant
¬ Haematological malignancy (blood cancer)
¬ Advanced or untreated human immunodeficiency virus (HIV)
Two doses administered 2-6 months apart
Two doses administered 2-6 months apart
Two doses administered 1-2 months apart
Shingrix can be co-administered with other live inactivated vaccines if given at different injection sites.1,2
If the second vaccination is not completed within the six-month recommended schedule, it can be attended to as soon as practical.1,2
Once the initial two-dose vaccination schedule is completed, patients are not required to have further booster doses of Shingrix.1,2
Patients are ineligible for Shingrix vaccination on the NIP if vaccinated with Zostavax within the past 5 years.1,2
Forrest Tang, Clinical Pharmacist, Slade Pharmacy Maitland
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a class of medication that has become increasingly popular over the last few years for the management of diabetes and obesity. There are currently four TGA-approved products, with limited intermittent availability in Australia: semaglutide (Ozempic®), dulaglutide (Trulicity®) and liraglutide (Saxenda® and Victoza®). Tirzepatide (Mounjaro®), a GLP-1 RA as well as an agonist at the glucose-dependent insulinotropic polypeptide (GIP), is also available.1,2 Dulaglutide, semaglutide and tirzepatide are considered long-acting agents, and liraglutide is short-acting (Table 1).3
Semaglutide (Ozempic®)
Tirzepatide (Mounjaro®)
Dulaglutide (Trulicity®)
Type 2 diabetes4
Type 2 diabetes5
Type 2 diabetes6
Liraglutide (Saxenda®) Weight loss7
Liraglutide (Victoza®) Type 2
Glucagon-like peptide-1 (GLP-1) is a type of incretin hormone that is secreted in response to food intake.9 GLP-1 receptors, which can be found in the brain, gastrointestinal tract, and pancreas, have numerous actions.9 GLP-1 can increase the release of insulin and suppress the secretion of glucagon, leading to lowered glucose levels.9 GLP-1 also acts at the hypothalamus to enhance satiety and slows gastrointestinal motility/emptying, which also increases the sensation of fullness.9 All these actions lead to improved glucose management and weight loss.9,10
Common side effects experienced with GLP-1 RAs are gastrointestinal and include nausea, vomiting, and diarrhoea.10,11 These adverse effects are especially common when starting treatment or when there is an increase in the dose.10,11 In fact, nausea and/or vomiting are experienced in 50% of patients; however, with continued use, these symptoms usually improve.10,11
As the use of GLP-1 RAs increases, there have been recent concerns that the delayed gastric emptying from GLP-1 RAs may increase the risk of regurgitation and pulmonary aspiration of gastric contents during general anaesthesia and deep sedation.3 Several case reports described of patients taking GLP-1 RAs arriving for elective surgery with a full stomach, sometimes after fasting as long as 18 hours.12,13 In a retrospective study consisting of 404 patients undergoing elective upper esophagogastroduodenoscopies, it was observed that there was an increase in residual gastric content in patients using semaglutide compared to patients not using semaglutide.14 Similarly, a prospective study consisting of 20 volunteers showed that 90% of volunteers on semaglutide versus 20% of control volunteers had the presence of solid contents identified on gastric ultrasound, after an 8-hour fast.15
There are currently limited guidelines or evidence available on the preoperative management of these drugs to prevent regurgitation and pulmonary aspiration of gastric contents. The American Society of Anesthesiologists (ASA) released a consensus guideline in June 2023, with the following suggestions for patients undergoing elective surgery. For patients on daily doses of a GLP-1 RAs, such as liraglutide, consider withholding the dose on the day of the procedure/ surgery, and for patients on a weekly dosing regimen, such as semaglutide or dulaglutide, consider withholding the GLP-1 RA one week prior to the procedure/surgery.3 This guidance
Approximately 7 days3,4
Approximately 5 days5
Approximately 5 days3,6
is regardless of the indication for GLP-1 RAs. The ASA also advises that if GLP-1 RAs prescribed for diabetes management are withheld for longer than the dosing schedule, then an endocrinologist should be consulted for bridging the antidiabetic therapy to avoid hyperglycaemia.3 The guidelines also recommend the presence of gastrointestinal symptoms should be considered when assessing risk on the day of the procedure, and that patients taking GLP-1 RAs who require urgent surgery should be treated as though they have a full stomach, with appropriate airway protection.3
The Medical Journal of Australia (MJA) speculates that due to the long half-life of long-acting GLP-1 RAs, it is likely that withholding for one week may not be enough to prevent or minimise the effects of gastric delay.16 It is currently unclear when gastric emptying returns to normal after cessation of a GLP-1 RA, and more evidence is needed to understand the potential role of withholding these agents during the perioperative period.13 Additionally, when higher doses of the drug are used for weight loss management, gastric stasis may be prolonged.16 Furthermore, delayed gastric emptying is a common complication of diabetes itself, with around 30-50% having abnormally slow gastric emptying.13 Withholding GLP-1 RAs for several days or weeks prior to surgery to achieve normalisation of gastric function may not be an appropriate option with unknown impact of aspiration rates, and detrimental effect of poorer glycaemic control.16 Gastric ultrasound may be considered as a tool to ascertain volume and consistency of gastric contents, to guide further management.13
GLP-1 RAs slow gastric emptying, which may create safety implications for patients undergoing surgery. The perioperative healthcare team should be aware of the potential increased risk of gastric aspiration in these patients when planning and managing their surgery. Further large-scale studies are required to better estimate the risk of pulmonary aspiration in order to develop a rational perioperative management plan for patients taking GLP-1 RAs.
Note: Following publication of this article, Australian consensus recommendations endorsed by ANZCA, and peak diabetes, obesity and gastroenterological bodies have been released. For further information access here: ‘Periprocedural GLP-1/GIP receptor agonist – clinical practice guide’.

Andexanet alfa is provisionally approved for the reversal of anticoagulation in adult patients treated with apixaban or rivaroxaban (direct factor Xa (FXa) inhibitors) in life threatening or uncontrolled bleeding.1 Direct FXa inhibitors are oral anticoagulants that have fixed dosing regimens and predictable pharmacokinetics without the need for routine anticoagulation monitoring, however severe bleeding events can occur.1 Treatment of life threatening bleeding is often managed with replacement of clotting factors, such as prothrombin complex concentrates (an off-label indication).1 The Therapeutic Goods Administration (TGA) granted provisional approval for adenxanet alfa in July 20232 based on haemostasis efficacy and targeted reversal of anticoagulation.3 One trial reported that at 12 hours post-administration of andexanet alfa, anti-FXa activity decreased by 94% and haemostatic efficacy was achieved in 80% of patients.4 Other studies have demonstrated andexanet alfa’s pharmacological efficacy5 however more clinically relevant measures such as mortality benefits have only been reported in observational studies 2,6,7 which lack high-quality evidence.2 Continued approval by the TGA will depend on the full, peer reviewed results from the Andexxa Phase IV trial and description of benefit in a confirmatory trial.3
Andexanet alfa is an inactive recombinant form of FXa which competes with the bodies native FXa to bind apixaban or rivaroxaban.8 The andexanet alfa sequesters the direct FXa inhibitor reducing free plasma concentrations of the drug, allowing the native FXa to be active in the formation of thrombin.8
Andexanet alfa also binds to another protein (tissue factor pathway inhibitor) inhibiting its action and causing a procoagulant effect.1 Thromboembolic events were reported in 10.4% of patients in one clinical study, however this cohort had high intrinsic thrombotic risk at baseline.4 Other side effects
include infusion reactions1 and treatment-emergent adverse events have reported urinary tract infections (10.5%) and pneumonia in 8.2% of patients.8
The dose of andexanet alfa is administered as a low or high dose regimen depending on the patient’s dose of apixaban or rivaroxaban, and the timing of the last dose.8 A bolus dose is administered which is then followed by a continuous infusion.8 Reintroduction of the patient’s anticoagulant should occur promptly, when indicated, after major bleeding in highly prothrombotic patients.1
The following indications have not been approved by the TGA for andexanet alfa: reversal of direct FXa inhibitors prior to surgery; treating FXa inhibitor overdoses; or reversing anticoagulation of indirect FXa inhibitors such as enoxaparin.1
Australian organisations such as the Council of Australian Therapeutic Advisory Groups2 advises that, “Andexanet alfa is not routinely recommended for use in the management of patients treated with a FXa inhibitor presenting with a severe and life-threatening bleed.” This recommendation is based on the high cost of andexanet alfa (over $20,000) and uncertain benefit for patients in terms of mortality and functional outcomes, as well as clear evidence to identify patients most likely to benefit or suffer harm.2 Exceptional use of andexanet alfa may be considered appropriate in certain situations, on a case-by-case basis, for example in trauma patients.9
Andexanet alfa is a novel agent with an uncertain risk-benefit trade-off, significant cost, and varied access and availability in hospitals and health services. Where andexanet alfa is approved on a formulary, the health facility is recommended to have procedures that advise staff on patient criteria, approval and adverse reporting processes.
