IMMpress Magazine
Beyond nourishment:
Breastfeeding versus formula and its immunological effects
Vaccinating children: Evidently not a walk in the park
Navigating the hormonal highway: Can puberty act as a trigger for autoimmunity?
Magazine of the Department of Immunology, University of Toronto 2024 | vol.12 no.1 9 772291 239001 ISSN 2291-2398

Innovation in every reagent
For over two decades, our scientists have focused on creating unique products for the scientific community—empowering you with antibodies and reagents to find answers for your immunology research. We are constantly innovating to ensure you have the tools needed to make legendary discoveries.
Discover how our 30,000 reagents can make a difference in your lab:
• Fluorophore-antibody conjugates: dyes for conventional and spectral cytometry, including Spark PLUS UV™ 395.
• TotalSeq™: reagents for multiomic applications.
• LEGENDplex™: bead-based immunoassays for cytokine detection.
Discover the difference at biolegend.com
• MojoSort™: magnetic separation system for cell isolation and enrichment.
• Ultra-LEAF™ & GoInVivo™ antibodies: ideal for functional assays like blocking or stimulation assays.
• GMP recombinant proteins: for research and ex vivo cell bioprocessing applications.
World-Class Quality | Superior Customer Support | Outstanding Value BioLegend products are manufactured in an ISO 13485:2016-certifi ed facility to ensure the highest quality standards. BioLegend is a registered trademark of BioLegend, Inc. All other trademarks are the property of their respective owners. 08-0116-10 biolegend.com
About the Cover
A baby’s first playground begins in the womb, marking the start of their immunological journey that defines their future health. The immune system, acting as an interface between a person and the world, is greatly influenced by both the environment and genetics. These dynamic interactions continually shape a child’s health, with each event laying down the essential building blocks.
This developmental window is crucial as it is also when disorders and diseases can emerge, underscoring its importance. Advances in research and understanding of early-life development not only emphasize the significance of this period but also present opportunities for early interventions during this critical time. In a full-circle manner, it is in the beginning of life where we may find answers to the future.
In the cover of this issue, pediatric immunology is depicted within the playful confines of a nursery. The colourful toy building blocks represent various factors that, during early stages in life, set the base for a child’s health. This imagery harmonizes with a wallpaper backdrop showcasing some of the many immunological elements that contribute to a child’s well-being. With a design reminiscent of childhood innocence, we invite you to explore this issue with the same boundless curiosity that defines early discovery.
Design notes
In this issue of IMMpress, our team of talented designers have embraced their inner child, delivering a remarkably lively issue. From the use of playful colour palettes to imaginative imagery, they skillfully conveyed the diverse topics within pediatric immunology. As I come to the end of my journey as the Design Director at IMMpress, I would like to thank everyone for making this experience unforgettable. It has been an honour witnessing the growth of our incredible designers, particularly Jennifer Ahn who will be the new Design Director. Though she may still be in her “infancy,” her creative vision has shown exceptional promise. We look forward to the creative direction in which she will lead us in!
- Kitty Liu-
Copyright © 2013 IMMpress Magazine. All rights reserved. Reproduction without permission is prohibited.
IMMpress Magazine is a student-run initiative. Any opinions expressed by the author(s) do not necessarily reflect the opinions, views or policies of the Department of Immunology or the University of Toronto.
EDITORS-IN-CHIEF
James Pollock
Karen Yeung
DESIGN DIRECTOR
Kitty Liu
SOCIAL MEDIA COORDINATOR
Tianning Yu
SENIOR EDITORS
Jennifer Ahn
Baweleta Isho
Manjula Kamath
James Pollock
Jinny Tsang
Sila Usta
Deeva Uthayakumar
Karen Yeung
Tianning Yu
DESIGN ASSISTANTS
Daniah Alkassab
Leon Baronijan
Baweleta Isho
Meggie Kuypers
Kitty Liu
Sophie Sun
Jinny Tsang
Nasana Vaidya
Diana Yang
Tianning Yu
Adriana Zutic
CONTENT CONTRIBUTORS
Jennifer Ahn
Leon Baronijan
Zi Yan Chen
Arwa Hilal
Baweleta Isho
Manjula Kamath
Rafeed Khan
Jennifer Sahadeo
Alara Tuncer
Sila Usta
Nasana Vaidya
Tianning Yu
FOUNDING EDITORS
Yuriy Baglaenko
Charles Tran

Beyond nourishment: Breastfeeding versus formula and its immunological effects Vaccinating children: Evidently not a walk in the park Navigating the hormonal highway: Can puberty act as a trigger for autoimmunity?
Magazine of the Department of Immunology, University of Toronto 2024 vol.12 no.1 9 772291 239001 ISSN 2291-2398 IMMpress
IMMpress Magazine
P LEASERE-READ, RE-GIFTAND I F Y UO ,TSUMYLLAER ELCYCER


Breastfeeding versus formula and its immunological effects
Vaccinating children
Evidently not a walk in the park
Navigating the hormonal highway
Can puberty act as a trigger for autoimmunity?







Cultivating early immune system ontogeny across a shifting landscape
Pediatric immunology: a journey of discovery with Dr. Dilan Dissanayake
Immunology in utero - the immunology of pregnancy
Impact of the microbiome on immune system development - caesarean vs vaginal birth
On guard with Gardasil: HPV vaccines against cancer
Unlocking hope: progress in understanding and treating inborn errors of immunity
The invisible scars of childhood adversity
Your fur baby: an early immune system’s best friend?
Book review - The Baby and the Biome: Nurturing Nature’s
@immpressmag facebook.com/IMMpressMagazine
CONTENTS VOL 12. NO 1. [2024] › CONNECT WITH US! › SUBMIT FEEDBACK We would love to hear your thoughts! Comment on our articles online or send us an e-mail at editor@immpressmagazine.com
immpressmagazine.com
8 16 18 20 22 24 25 26 27 Beyond nourishment
Connection
› FEATURE ON [ISSUE] 10 12 14 8 18 20 22 24 25 26 27$100 $100 $100
LETTER FROM THE EDITORS

From the moment a baby is born, their immune system embarks on a remarkable journey of development and adaptation. At this stage, every tiny triumph and defeat of the immune system can shape a child’s health for decades to come. In this issue of IMMpress Magazine, we follow this journey from toddler to teenager, highlighting recent findings from the world of pediatric immunology.
We showcase many of the hallmarks of immune development from infancy to childhood in an infographic (p8). Before delving into pediatrics, though, we cover obstetrics, with articles on the immunological interactions between mother and child (p18) and the genetic conditions that often affect a newborn’s immune function (p24). Next, we discuss factors such as breastfeeding vs. formula milk practices (p10) and Caesarean section vs. vaginal birth (p20) to understand how they affect immunity at the macro level.
First steps, first words, first day at school; childhood is a sequence of one milestone after the other. We delve into the importance of some of these milestones with articles on the influence of early pet ownership on allergy development later in life (p15), and the declining receipt of routine vaccinations due to vaccine hesitancy (p12). To learn more about pediatric immunology, we interviewed Department of Immunology alumnus Dr. Dilan Dissanayake to discuss his latest research on autoimmune diseases in children (p16).
And then of course arrives the tumultuous adolescent phase. We explore the interplay of puberty with autoimmune diseases such as lupus (p14), the impact of psychological stress in youth on adult immune health (p25), and the success of the HPV vaccine against different types of cancers (p22). Finally, we conclude this issue with a book review of The Baby and the Biome, in which a mother, drawing on her scientific background, searches for solutions to her son’s severe allergies by sifting through decades of microbiome research (p27).
We hope you feel young at heart while reading this overview of the field of pediatric immunology. This issue of IMMpress Magazine would not have been possible without our wonderful team of writers, editors, and designers. We extend our heartfelt gratitude to everyone on the team, with special thanks to Kitty Liu for her years of exceptional leadership and boundless creativity as she steps down from her role as Design Director. In her place we are excited to welcome Jennifer Ahn as our new Design Director!
As always, stay safe and healthy!

 In order of left to right: James Pollock (Co-Editor-in-Chief), Kitty Liu (Design Director), Karen Yeung (Co-Editor-in-Chief), and Tianning Yu (Social Media Coordinator)
In order of left to right: James Pollock (Co-Editor-in-Chief), Kitty Liu (Design Director), Karen Yeung (Co-Editor-in-Chief), and Tianning Yu (Social Media Coordinator)
IMMpress Vol. 12 No. 1 2024 5
James Pollock Karen Yeung

The Graduate Peer Support Network (GPSN) Mentorship Program is looking for motivated individuals to join our mentorship team as a peer mentor. Scan to fill out our recruitment form.


Join our Join our mentorship team mentorship team Twitter: GPSN _UofT Instagram: gpsn uoft Website: uoftgpsn.ca
FROM THE CHAIR

“One of the luckiest things that can happen to you in life is, I think, to have a happy childhood.”
- Agatha Christie
Surely we are all convinced of that. But this issue of IMMpress Magazine should also convince us that a childhood with the right pre- and post-natal exposures is key to a happy immune system.
I learned so much about the burgeoning field of pediatric immunity from this stellar issue. Zi Yan Chen gives us some terms of reference on this interesting area of research before we hear from Baweleta Isho on how the immune system handles the maternal fetal interface. We learn about vertical transmission of pathogens, but also of the microbiota and how this can be different based on mode of birth (Tianning Yu). We also hear from Rafeed Khan about the advantages of breastmilk for early life immunity, and a book review on The Baby and the Biome: Nurturing Nature’s Connection by Alara Tuncer provides a peak at the parent’s perspective on the impact of the environment on a child’s health. As a self-confessed cat lady, one of my favourite articles was by Sila Usta “Your fur baby: an early immune system’s best friend” where she argues that early introduction of pets can provide some protection against the development of dander allergies. But let’s not stop at childhood - Leon Baronijan reminds us that puberty is more than acne and angst – sex hormones have a tremendous impact on the immune system which has implications for autoimmunity risk.
Although we are learning a great deal about how early life environments shape the immune system, let us not forget the impact of genetics. Jennifer Sahadeo does a great job at explaining inborn errors of immunity and advances for diagnosing and treating them. Moreover, Jennifer Ahn reminds us about the importance of childhood vaccination and an alarming drop in vaccination rates due in part to the COVID-19 pandemic and increasing vaccine hesitancy among parents. While vaccines have allowed us to nearly forget the scourge of preventable childhood illnesses such as Polio and Diptheria, Nasana Vaidya explains the additional benefits of vaccination against Human Papilloma Virus for prevention of some anogenital and oropharyngeal cancers.
Lastly, it was so nice to hear from Dr. Dilan Dissanayake. Interviewed by Arwa Hilal, we catch up with what Dilan is up to as a staff rheumatologist at SickKids. I was lucky to be a co-author on Dilan’s paper “NFkB controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells” (Nature Medicine 2011) which he published while he was in Dr. Pam Ohashi’s lab. Our students certainly go on to do some great things – like looking after the immune systems of Canadian children.
Go ahead and channel your inner child as you enjoy this latest issue of IMMpress. Congratulations to the team for such stellar work!
 Jen Gommerman, PhD
Canada Research Chair in Tissue Specific Immunity Professor and Chair, Department of Immunology
Jen Gommerman, PhD
Canada Research Chair in Tissue Specific Immunity Professor and Chair, Department of Immunology
LETTER
IMMpress Vol. 12 No. 1 2024 7
Cultivating Early Immune System
The human body has been bombarded by environmental insults from conception. The prenatal immune system facilitates the generation of tissues and organs and is a versatile medium for communication with the outside world, which largely constitute endogenous maternally-derived solutes. As the fetus matures, an elaboration of physical and chemical barriers occurs, preparing for the postnatal exposure to exogenous environmental antigens.
Prenatal exposures, including maternal nutrition, illnesses, and immune factors can impact fetal development. Deficiencies in essential nutrients like zinc, iron, and retinoic acid can disrupt immune development. Maternal infections, including ToRCH viruses, and recently, Zika and Dengue viruses, also pose significant risks to fetal development. Additionally, maternal antibodies and their transfer across the placenta contribute to the developing fetal immune responses and overall health.


Development of the immune system begins in waves, with the earliest macrophages observed at 4 weeks of gestation, followed by T cell development between 8-12 weeks of gestation. Mature neutrophils are present at the end of the first trimester, and increase in number until shortly before birth. Most immune cells have functional defects in early life, for instance, reduced adherence and chemotaxis, that will mature as they encounter new antigens and stimuli postnatally.

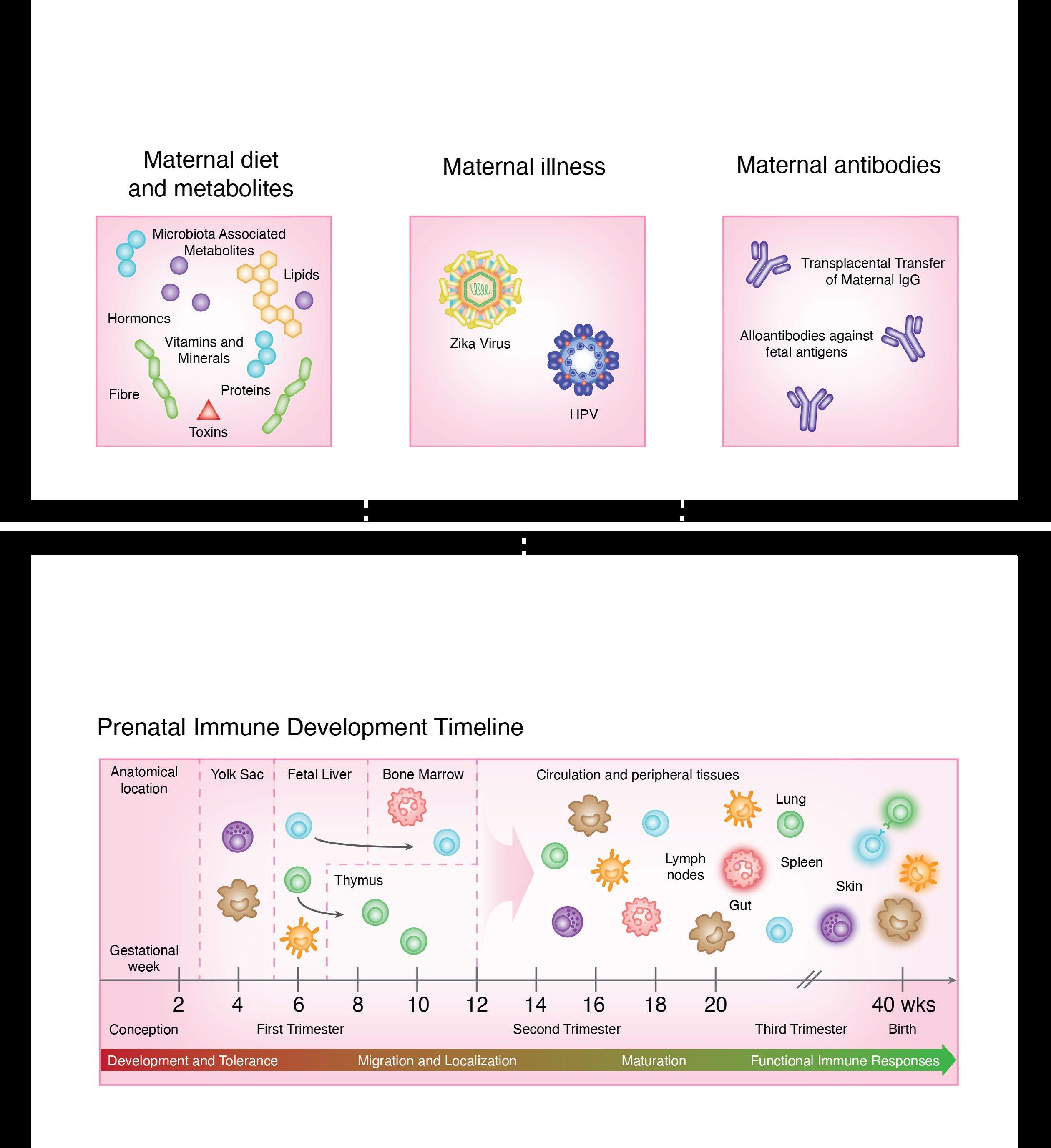
8 IMMpress Vol. 12 No. 1 2024
Ontogeny across a Shifting Landscape
These early life impressions leave epigenetic footprints that may dictate how the immune system senses and responds to pathogens later in adult life. Here, we illustrate the differences between immune system components in prenatal versus postnatal life, and the impact of the environmental transformation in this critical developmental process.
Postnatal exposures, such as breast milk, environmental antigens, and vaccination play a key role in postnatal immune development. Breast milk provides essential bioactive molecules, bacteria, and antibodies that shape humoral immunity and gut colonization while oral feeding and exposure to food antigens aid in mucosal immune maturation. Furthermore, childhood vaccinations are crucial for acquiring immunity against infectious diseases.

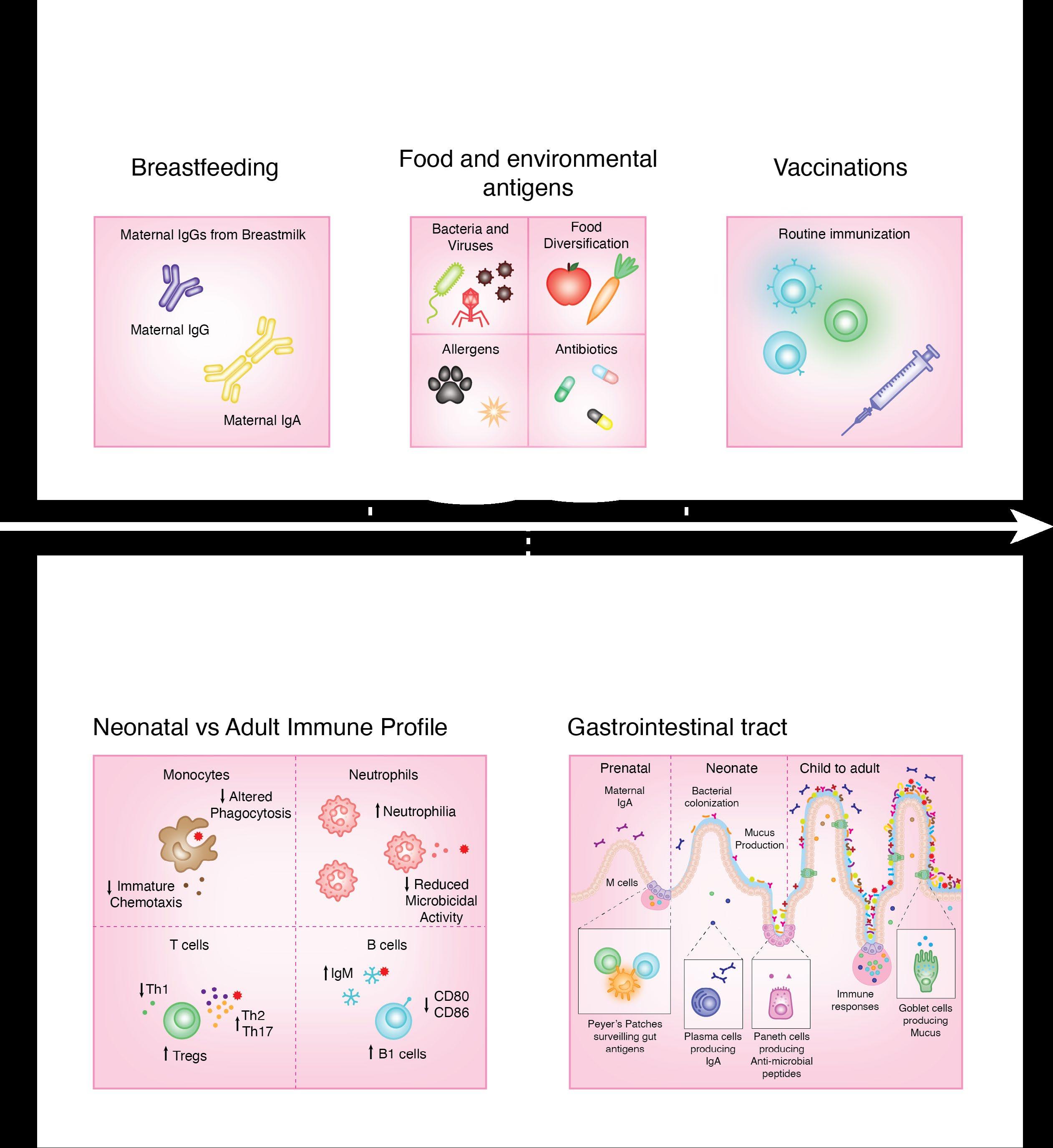



The immune system undergoes profound changes across all tissues from neonate to adulthood. Neonates initially exhibit high white blood cell counts and neutrophilia, with impaired immune responses and altered cytokine profiles characterized by increased anti-inflammatory cytokine production. As mucosal membranes develop prenatally and mature postnatally, the mucosal barrier continues to evolve, with ongoing specialization and adaptation in response to increased microbial exposure.
IMMpress Vol. 12 No. 1 2024 9
- Zi Yan Chen
Beyond Nourishment
Since the beginning of Homo sapiens as a species, breastfeeding has been the main feeding practice for newborns. The earliest documented cases of neonatal breastfeeding date as far back as 2000 BC, and the importance of this practice has been well understood for centuries as a primary source of nutrition for infants. Alternative feeding practices have an equally long history, with evidence of animal milk found in vessels buried with newborn infants also dating back to 2000 BC. When various forms of milk were first analyzed for their health benefits during the 18th century, scientists realized the potential health benefits of human milk and tried to replicate its components in a synthesized formula. Thus birthed the invention of formula milk, which represented a new chapter in infant feeding that has since become a common choice of supplementation or replacement for breastfeeding, particularly for mothers who are unable to breastfeed due to health, cultural, or societal reasons.
The health benefits of breastfeeding extend beyond nourishment, and is crucial to the development of a healthy immune system. To many, the nutrients found in breast milk and formula are one and the same, a notion that is, in fact, far from the truth. Apart from the vitamins and macromolecules gained from breast milk, the immunological factors and healthy microbes that are transferred help reduce the risk of numerous diseases such as asthma, obesity, type 1 diabetes, as well as respiratory and gastrointestinal disorders. Dietary guidelines provided globally by the World Health Organization (WHO) typically recommend feeding for 6 consecutive months with only breast milk, followed by an additional 1.5 years of breast milk alongside the gradual introduction of age-appropriate food.
The first two years after birth also encompass a physiologically vulnerable and malleable period that can be significantly affected by diet. Breast milk is packed with rich bioactive nutrients, bacteria, and immune cells and their secreted molecules, which can safely promote infant gut health and immune system development.
While still in the womb, a fetus’ immune system is under development, and the first B and T cells begin to form as early as the second or third month of pregnancy. However, since the mother’s womb is sterile, these immune cells have never been tested against any environmental threat and remain inactivated. In addition to these inexperienced cells, the fetus also receives antibodies from the mother through the placenta. These antibodies are referred to as immunoglobulin Gs (IgGs), which are a class of antibodies commonly found in circulation and are the only ones capable of passing through the placental barrier. Upon birth, the infant is exposed to an entirely new environment, and the passive immune protection provided by the mother’s IgGs will stick around until the infant’s immune system activates and can take control. This passive immune protection can protect the infant for a short period following birth, but is one class of antibodies enough?
The short answer: sort of, but the more classes of antibodies present, the broader the scope of protection and the more this passive immune system can “cover its bases”. Breast milk includes another important antibody, IgA, a common antibody type found near mucosal tissues. Other classes of antibodies can also be present, such as IgM and IgG, depending on the mother’s environment and history of infection. Additionally, breast milk contains other immunomodulatory factors, such as nucleotides, amino acids, polyunsaturated fatty
10 IMMpress Vol. 12 No. 1 2024
Breastfeeding vs Formula and its immunological effects
acids, soluble immune effector proteins, antibacterial proteins/peptides, and even immune cells, such as macrophages and neutrophils. All these modulators are crucial for immunity at birth, as the infant’s immune system, at this stage, is highly limited. The mother’s immune cells and effector molecules not only help protect the baby from foreign particles but also help to form tolerance to harmless food components.
The microbiota, a diverse community of bacteria that covers the body, inside and out, is largely implicated in human health and interacts with the host immune system to protect against invading pathogens. With regards to microbiota, a fetus’ gut is a blank slate, upon which different microorganisms will colonize based on the circumstances of birth. This first introduction of an infant’s gut to healthy microbiota is typically in the vaginal canal during birth; if birthed through a different method, then the first time is often when fed breast milk. Like immune components, breast milk is packed with beneficial bacteria that can promote healthy infant gut colonization and maturation. Studies have shown that two major groups of bacteria in breast milk, Bifidobacteria and Lactobacilli , can metabolize oligosaccharides present in the milk to help promote intestinal barrier integrity and inhibit the settlement of pathogenic bacterial species. Early gut microbial colonizers will eventually occupy their niche in the gut; through competition for nutrients and space, they limit further colonization by other microbes, eventually reaching homeostasis to help maintain a balance between the immune system and the external gut environment. Commensal bacteria are known to play key roles in stimulating sub-populations of immune cells, such as promoting the development of T cells with anti-inflammatory functions, and IgA-producing B cells, that help integrate the immune system.
Given the rich source of immune cells and immunomodulatory components in breast milk, simply breastfeeding for the first 6 to 24 months of an infant’s life can greatly reduce the risk of hospitalization due to severe infections; inflammation-related symptoms such as diarrhea, acute otitis media, and asthma; and other health concerns such as obesity.
Despite the benefits that breastfeeding provides over other methods, formula feeding is still a popular choice among new parents. The advantage of anyone being able to feed your child, along with less frequent feeding times, increased satiation rates, and excess amounts of nutrients, make it out to be a very easy and reliable feeding method during what is, undeniably, a very stressful time for new parents. However, while formula milk has provided an accessible option for mothers who are unable or choose not to breastfeed - for example due to biological issues related to lactation, the social norms of their culture, or economic issues related to the lack of paid family leave - breastfeeding remains the best option to support both nutritional and developmental needs of growing infants.
- Rafeed-Uz-Zaman Khan
IMMpress Vol. 12 No. 1 2024 11

EVIDENTLY NOT A WALK IN THE PARK ACCINATING HILDREN
Four years ago in March 2020, the World Health Organization (WHO) officially declared coronavirus disease 2019 (COVID-19) a global pandemic. Nations scrambled to develop and secure vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) –competing for supplies, intensifying political rivalries, and hoarding in the name of nationalism. As adults engaged in this frenetic scramble, millions of children were missing out on basic vaccines due to halted immunization services. The pandemic had inevitably given rise to lockdowns, social distancing measures, and economic struggles that disrupted essential healthcare services across the globe. Consequently, by the end of 2020, 23 million children had not received routine vaccinations, increasing their risk of contracting fatal, yet vaccine-preventable, diseases such as measles, polio, and diphtheria.
After birth, most infants around the world receive a standard set of vaccines that protect them from viral and bacterial infections. These vaccines target MMR (measles, mumps, rubella), DPT (diphtheria, pertussis, tetanus), rotavirus, poliovirus, pneumococcus, and more. During the first year of the COVID-19 pandemic, there were 17 million “zero-dose” children – those who had not received a single dose of any vaccine. Importantly, 98% of zero-dose children were from low- and middle-income countries. In some cases, children failed to complete all the required doses in a vaccine series. For instance, three doses of the DPT vaccine are usually administered within 12 months of birth. In 2020, the number of children who did not complete the 3-dose DPT series increased by 20% compared to the previous year – with the greatest increases in South-East Asian and Eastern Mediterranean regions.
Similarly, polio vaccination programs were significantly disrupted by the COVID-19 pandemic. In 2020, 46 poliovirus immunization campaigns were suspended in 38 countries. Coincidentally, a newly mutated strain of poliovirus was reported in more than 30 countries and polio outbreaks emerged during the same year. In Pakistan, 40 million children did not receive polio vaccines when immunization programs were suspended in April 2020. While vaccinations gradually resumed thereafter, there may be long-term consequences to face in the future – considering Pakistan is one of the few countries in the world where polio has not been eliminated.
It is undeniable that childhood vaccinations took a severe hit in 2020. In low- and middle-income coun-
~ ~ ~
12 IMMpress Vol. 12 No. 1 2024
tries, pre-existing challenges, such as lack of infrastructure, political instability, and fragile healthcare systems, made it even more challenging to maintain immunization services during the pandemic. Additionally, vaccine hesitancy had been on the rise long before the SARS-CoV-2 outbreak, threatening childhood vaccination efforts worldwide.
In fact, the WHO had highlighted “vaccine hesitancy –the reluctance or refusal to vaccinate – despite the availability of vaccines” as one of the top 10 threats to global health in 2019. While drivers of vaccine hesitancy are multifaceted with geopolitical and cultural nuances, the dissemination of false and sensationalizing information through the Internet has certainly played a role. In 2005, a study conducted by Johns Hopkins Bloomberg School of Public Health reported that in the United States, parents who exempt their children from vaccinations were more likely to have consulted anti-vaccination websites compared to parents of vaccinated children. Supporting studies have shown these websites discuss vaccine safety concerns, alternative medicines, civil liberties, and conspiracy theories that proport collusion between doctors, pharmaceutical industries, and the government.
12 years of age. Fueling such doubts were studies reporting that the risk of severe COVID-19 was significantly lower in infected, healthy children compared to adults. In addition, there were reports of rare, transient myocarditis (inflammation of the heart) occurring in adolescent and young adult males within a week of receiving their second dose of the COVID-19 vaccine that were sensationalized in the media.
By the end of 2023, 23 million chil- dren had not received routine vaccinations, increasing their risk of contracting fatal, yet vaccine- preventable, diseases.
As of March 2024, all children 6 months of age and older are eligible for COVID-19 vaccines in Canada. Interestingly, a recent 2023 survey conducted by the Government of Canada revealed that 44% of parents with children below 18 years of age are reluctant to vaccinate their children against SARS-CoV-2. Among the 44%, the majority (72%) stated their reluctance stemmed from lack of COVID-19 vaccine research conducted in children. Altogether, it appears that national and global strategies are required to not only improve vaccine accessibility but also educate the public on vaccine efficacy, while gaining its trust with rigorous and transparent clinical research.
- Jennifer Ahn
As a corollary to vaccine hesitancy, vaccine-preventable diseases had begun to re-emerge prior to 2020. For example, the Centers for Disease Control and Prevention reported that measles incidence had increased in all six WHO regions during 2017-2019. In an attempt to eliminate this disease worldwide, vaccination efforts had decreased annual incidence rates by 88% from the year 2000 to 2016. The lowest incidence had occurred in 2016 with 18 cases per population of 1 million. Yet by 2019, measles incidence had risen to 120 cases per million, suggesting difficulties in the vaccination campaign, improving surveillance, and closing immunity gaps.
Finally, as COVID-19 vaccines were being approved amidst the recent pandemic, vaccine hesitancy brewed among experts and parents alike. Specifically, there were doubts about the necessity and safety of COVID-19 vaccines for children under
IMMpress Vol. 12 No. 1 2024 13
NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HIGHWAY HORMONAL HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HIGHWAY HORMONAL HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HIGHWAY HORMONAL HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HORMONAL HIGHWAY NAVIGATING THE HORMONAL NAVIGATING THE HORMONAL HIGHWAY HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY NAVIGATING THE HORMONAL HIGHWAY
What has puberty taught us about the sex-bias in autoimmunity?
Growing pains, painful acne, menstruation, pubic hair, and voice cracks – these are some of the physical changes many of us can remember as we unavoidably stepped into teenhood. Puberty marks a crucial period in sexual maturation characterized by alterations in the composition and levels of our sex hormones which drive sex-specific changes in physical appearance. Androgens, primarily found in men, and estrogens, predominant in women, constitute two major classes of sex hormones. Testosterone is the primary androgen, whereas estradiol is the predominant form of estrogen. During puberty, testosterone levels surge dramatically, escalating 250-fold in boys aged 6 to 20. Similarly, estradiol levels undergo a significant increase, multiplying by 15-fold in girls aged 7 to 23. Although testosterone and estrogen are often considered male and female-specific hormones due to their sex-specific effects, they are found in both sexes.
Beneath the physical changes we tend to associate with puberty, sex hormones have also been shown to regulate components of the immune system. For instance, estrogen has been shown to trigger immune cells to release pro- and anti-inflammatory molecules known as cytokines. Evidence suggests that estrogen stimulates the production of the pro-inflammatory cytokine interferon gamma (IFNγ) – a key player in the immune response to viruses – and adolescent girls produce higher levels of IFNγ than boys after 12 years of age. It has also been reported that women produce higher levels of antibodies in response to vaccination during enhanced states of estrogen production, such as pregnancy. Estrogen directs the macrophage – a type of immune cell – to help in the development of breast mammary ducts in females, whereas macrophages in males safeguard fertility by suppressing inadvertent or harmful immune responses in the testes during puberty. Clearly, sex hormones regulate multiple facets of the immune system in healthy individuals – but could this suggest their involvement in immune-related disease?


Autoimmunity refers to a type of pathological immune response in which the body attacks and damages its own tissues. For reasons which remain unclear, numerous autoimmune diseases show a sex bias towards women. Given the wide-ranging effects of sex hormones on the immune system, the presence of an autoimmune sex-bias suggests the involvement of sex hormones in the development of autoimmunity. Indeed, the incidence of autoimmune disease in women specifically increases during and after puberty.
Self-antigens are molecules the body normally recognizes as its own, but are mistakenly targeted in autoimmunity. The immune system employs many different checkpoints to prevent the development and persistence of auto-reactive immune cells, or immune cells that target self-antigens. Collectively, these checkpoints confer tolerance to self-antigens. A breakdown in tolerance refers to an autoimmune response where the body, due to auto-reactive immune cells escaping tolerance checkpoints, is no longer tolerant to self-antigen. For example, auto-reactive B cells can produce auto-antibodies that wreak havoc within the body by attacking self-antigens, leading to tissue damage and chronic inflammation.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that presents as widespread inflammation in the joints, lungs, kidneys, skin, brain, and blood vessels. Patients with SLE tend to experience waves of symptoms, or flare-ups. Approximately 90% of patients diagnosed with SLE are women. Could such a strong sex-bias have something to do with estradiol levels alone? It’s likely not that simple, since some studies suggest that women with SLE have higher plasma levels of estradiol compared to healthy women, while others find no significant differences. Auto-antibody levels have been shown to increase in prevalence during puberty in adolescent girls, suggesting that puberty plays a role in autoimmune development. Still, the extent to which puberty-associated sex hormones contribute to auto-antibody production remains poorly understood. A study from Brazil tracked cytokine levels

14 IMMpress Vol. 12 No. 1 2024



in teen SLE patients, in which 92% of patients were adolescent girls with an average age of 15. When compared to healthy participants, the study found increased levels of IL-10 in SLE patients, a cytokine known to stimulate the production of auto-antibodies. Interestingly, treating immune cells taken from SLE patients with estradiol provokes higher levels of IL-10 and auto-antibody production. This suggests a complex relationship between sex hormones, cytokine production, and auto-antibodies in SLE development during puberty.
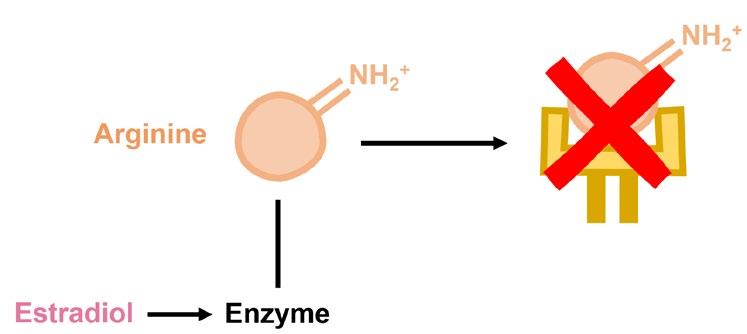
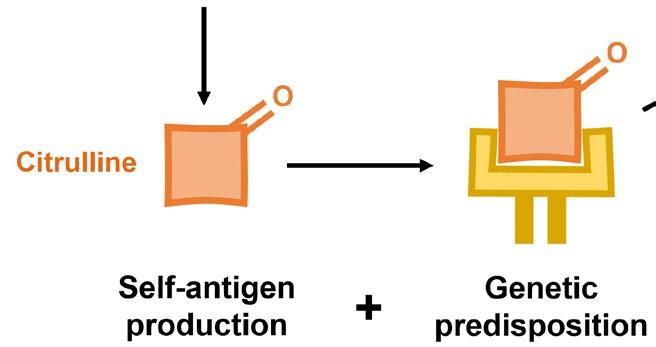
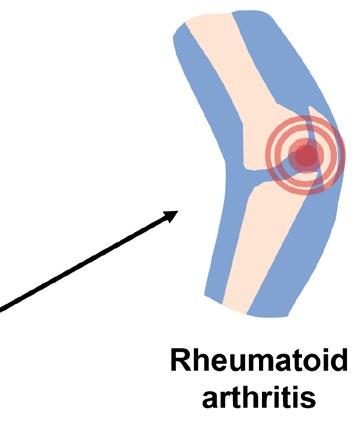
Rheumatoid arthritis (RA) is another sex-biased autoimmune disorder that specifically causes inflammation in the joints. Predisposition to RA is associated with specific variants in genes essential for recognizing pathogens, likely because these variants encode proteins that mistakenly target self-antigens rather than foreign ones. One of the more well-characterized self-antigens known to cause RA are proteins that contain citrulline, a modified form of the amino acid arginine. The conversion of arginine to citrulline is catalyzed by a distinct group of enzymes, which happen to be controlled by estradiol. This exemplifies how two distinct genetic factors can cooperate to cause disease whereby a predisposition to recognizing self-antigens and a sex-biased increase in the level of self-antigens conspire to cause autoimmunity. However, many questions remain to be answered: Do males have lower levels of enzymes that convert arginine to citrulline? Do other sex hormones control the levels of these enzymes? Are the levels of these enzymes increased during puberty in either sex?

Yet another interesting aspect of sex-biased autoimmunity lies in chromosomal inactivation. Unlike males, females have two X chromosomes. To properly balance the expression of genes on X chromosomes, female cells “silence” one of two X chromosomes at random – but this may not always be the case. Evidence suggests that some cells escape X-chromosome inactivation, leading to increased expression of genes. In particular, it has been shown that X-chromosome inactivation in female B cells leads to increased expression of the X-chromosome gene encoding Toll-like receptor 7 (TLR7) – an intracellular “sensor” which normally recognize parts of viruses but can also recognize self-antigens. Female B cells that have increased TLR7 expression appear to have a survival advantage, lending to the idea that auto-reactive B cells can outcompete healthy B cells. Upon TLR7 activation, these auto-reactive B cells can go on to produce auto-antibodies. Strikingly, expression of TLR7 increases in females after puberty, but this increase was not shown to be related to estradiol. Could this effect be independent of sex hormones or perhaps hormones altogether?
It has become increasingly clear that sex hormones play a role in the development of female-biased autoimmunity, particularly during puberty. The extent to which estrogens can be attributed to autoimmune development during puberty remains unclear, since evidence suggests they are not the only contributors to autoimmunity in predisposed individuals. Sex-biased autoimmunity instead appears to be a complex waltz between sex hormones and various compartments of the immune system. Even this may be an oversimplified explanation, as other puberty-associated hormonal and non-hormonal factors seem to be at play. As we continue to unravel the role of sex hormones on the immune system, puberty emerges not only as a rite of passage but also as a means to better understand sex-biased autoimmunity.
- Leon Baronijan
IMMpress Vol. 12 No. 1 2024 15
pediatric immunology: a journey of discovery with DR. DILAN DISSANAYAKE
For many families and their children, the discipline of immunology offers the potential of innovative therapeutics in the ever-changing environment of pediatric healthcare. From deciphering the intricacies of the immune system, to harnessing its power for therapeutic interventions, researchers in pediatric immunology are at the forefront of changing the lives of young patients suffering with immune-mediated illnesses. Dr. Dilan Dissanayake is an example of such a researcher, having graduated from the Immunology PhD program at the University of Toronto in 2012, before finishing his MD in 2014 as part of the MD-PhD program. As an alum of our program, his trajectory from PhD to pioneering research in pediatric immunology is nothing short of inspirational. Despite his busy schedule, Dilan was able to put aside some time to chat about his past and present research, as well as the many lessons he’s learned along the way.
Dilan is currently a staff rheumatologist at SickKids hospital, a transition clinician-scientist at SickKids Research Institute, and an assistant professor at the University of Toronto. Prior to taking on this position, however, Dilan completed his PhD under the supervision of Dr. Pamela Ohashi where his research interests revolved around dendritic cell biology. Through the use of adoptive cell transfer, Dilan focused on identifying cell-intrinsic factors of dendritic cells that affect their maturation. However, his learning did not stop at dendritic cells. When asked about the most valuable lessons learned during his PhD journey, Dilan emphasized the importance of resiliency and mentorship in navigating the complexities of immunology research.
“I think persistence and sustained motivation is extremely important during your graduate studies, because it can be a long road with lots of ups and downs… being able to keep an eye on the bigger picture is something that I learned during my PhD, and I think this really helped when it came to my subsequent clinical training in terms of how I approached challenging situations.”

Dr. Dilan Dissanayake, MD, PhD, FRCPC Staff Rheumatologist and Transitional ClinicianScientist, The Hospital for Sick Children Toronto Assistant Professor, University of Toronto
He also stressed the need for interdisciplinary collaboration and the invaluable role of mentors in nurturing both scientific growth and personal development, stating how important it is to identify “mentors
who can not only
help
your
but also your growth as a scientist.”
science,
16 IMMpress Vol. 12 No. 1 2024
Following his PhD, Dilan shifted from studying basic immunology to a more specialized area of research; that being pediatric rheumatology. Despite the differences in his past and present work, Dilan was quick to acknowledge how the two areas of research tie together.
“I think the understanding of basic immunology is absolutely essential to rheumatology, but even more so when it comes to some of these auto inflammatory disorders where it’s a single dysfunctional protein… Understanding how we can use targeted treatment approaches to reverse the dysfunction is really impossible without a basic understanding of immune cells and the main pathways involved.”
Beyond rheumatology, the pediatric arm of Dilan’s current work was largely inspired by his clinical experiences, in which he found that he connected well with kids and enjoyed working with them and their families. Upon the completion of his medical degree, Dilan merged his passion for immunology and pediatrics by specializing in pediatric rheumatology, where he was able to delve into the intricate world of autoinflammatory diseases. With an interest in bridging the gap between bench and bedside, Dilan took on the role of a clinician-scientist at SickKids – participating in a translational pioneering program that blends mentored research with clinical practice. By being enrolled in an initiative that offers research mentorship alongside a staff clinical appointment, the Transition Clinician Scientist Program provided Dilan with the platform to delve deeper into the mechanisms underlying autoinflammatory disorders under the supervision of Dr. Rae Yeung. At the moment, his research interests center around understanding the dysregulation of the inflammasome and its implications for pediatric autoimmune diseases. Dilan’s work, spanning from genetic analysis to mechanistic studies, holds the promise of unlocking novel therapeutic approaches and revolutionizing the treatment landscape for young patients battling immune-mediated disorders.
Professional timeline
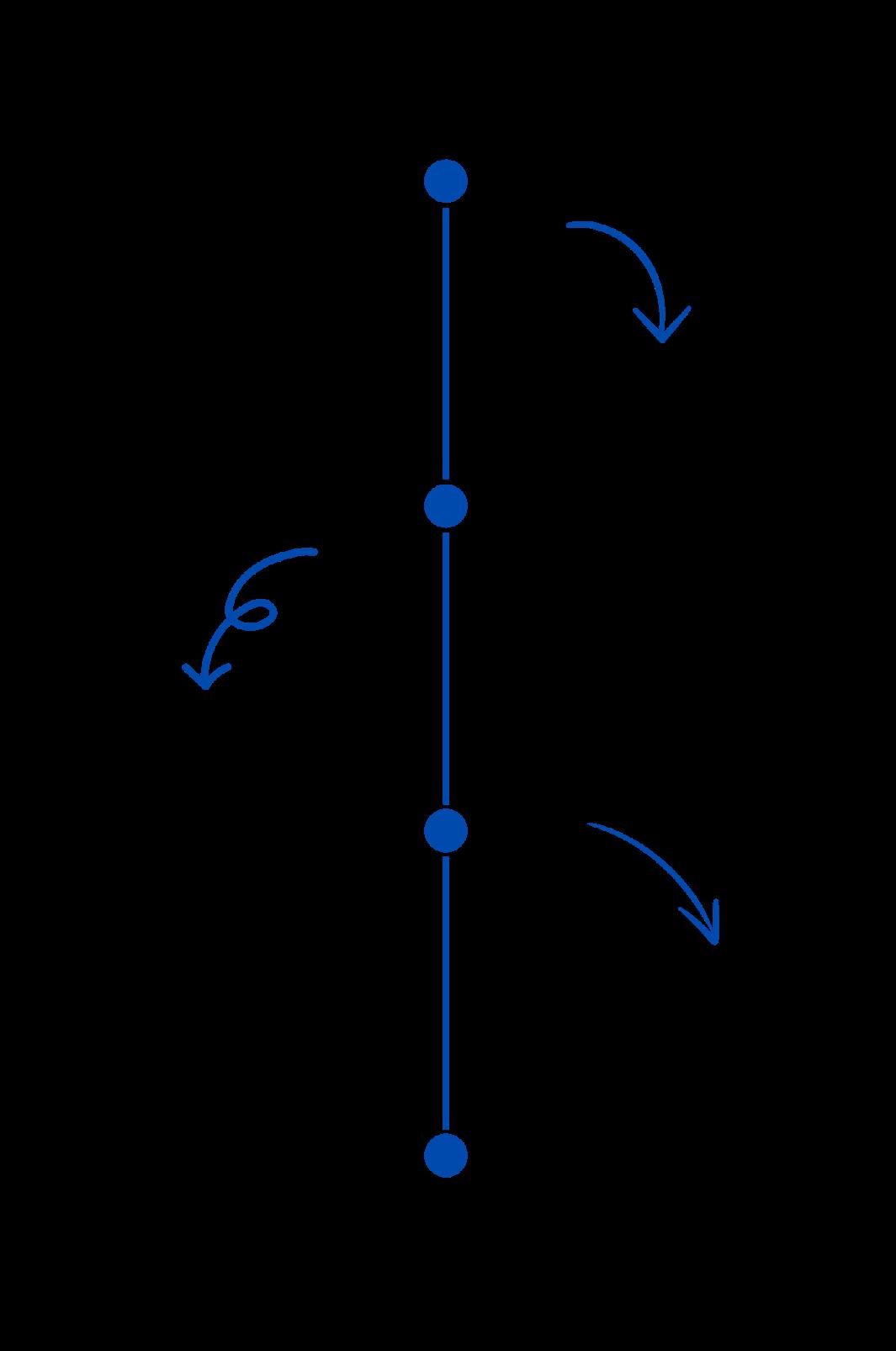

Looking ahead, Dilan envisions establishing his own lab dedicated to unravelling the complexities of pediatric autoimmune diseases. With a strong interest in continuing to reside in Toronto, he aspires to contribute to the vibrant research community that has shaped his journey, while continuing to advocate for the well-being of pediatric patients. As a final piece of advice to the immunologists-in-training that make up our Toronto research community, Dilan emphasized the importance of perseverance:
“There will be lots of ups and downs as I said before, but you just need to make the most of it and the ups will be worth it.”
As we celebrate the achievements of our alumni, Dilan’s journey stands as a shining example of the transformative impact of pediatric immunology research. His immense dedication to advancing the field reminds us of the profound impact that scientific inquiry can have on the lives of children and families around the world.
- Arwa Hilal
IMMpress Vol. 12 No. 1 2024 17
immunology in utero the immunology of pregnancy
Historically, it was thought that successful pregnancies require a dampening of the maternal immune system for fetal growth and survival, not unlike the need to suppress host immunity following organ transplant. However, we now understand that this is not exactly accurate. Although not often considered, the immune system is crucial in the protection of both the mother and fetus from embryonic implantation through pregnancy. It is now appreciated that intricate interactions and responses between the maternal immune system and fetus characterize the immunology of pregnancy, particularly at the maternal-fetal interface.
Thematernal-fetal interface facilitates vital nutrient transfer and waste removal between the pregnant person and fetus while also protecting the fetus from the extrauterine environment. Understanding the components of the maternal-fetal interface is crucial to fully grasp the dynamics of the immune system during pregnancy. These components of the maternal-fetal interface are necessary in ensuring the successful growth of a fetus during pregnancy.
Decidua: Derived from the uterine endometrium, the decidua serves as a transitional barrier which shields the embryo from maternal immune cells and provides early nutritional support prior to the development of the placenta. Decidual cells, made up of stromal and immune cells, are some of the first that interact with fetal antigens, ensuring immune tolerance to the fetus. The decidua also contributes to the invasion of trophoblasts (i.e., the main cells of the placenta and the first to develop from the fertilized egg), which is pivotal for placental development and remodeling of the spiral arteries, enabling the exchange of waste and nutrients through the placenta.
Fetal-derived Placenta: Formed from fetal trophoblast cells, the trophectoderm acts as a cellular barrier that protects the embryo from infection. Several morphological changes to the developing placenta rapidly take place following this, including the development of chorionic villi which establish primary contact between the placenta and maternal blood
supply. The placenta is fully formed at the end of the first trimester.
Leukocytes: Leukocytes play a crucial role at the maternal-fetal interface and are recruited through cytokine signals from decidual stromal cells and trophoblasts. The three main leukocytes that that contribute to the interface are decidual natural killer (NK) cells, decidual macrophages, and regulatory T cells (Treg). Decidual NK cells are important for the formation of the decidua, implantation of the embryo, and remodeling of the spiral arteries. These cells also promote trophoblast invasion during placental development through the production of IL-8. Similarly, decidual macrophages contribute to tissue remodelling and blood vessel growth by producing soluble immune factors including vascular endothelial growth factor and matrix metalloproteinases. They also play a key role in the clearance of apoptotic trophoblasts and for protection of the fetus from infections. Finally, Treg cells foster maternal tolerance to fetal antigens by downregulating inflammatory responses, allowing for successful implantation by the embryo. These cells ultimately help maintain an environment conducive to fetal survival.
The Immune System During Pregnancy
As the field of reproductive immunology progresses new insights into the immunology of pregnancy have emerged, shedding light on the innate and adaptive immune systems.
18 IMMpress Vol. 12 No. 1 2024
Innate Immune System: Numerous aspects of the innate immune system are modified during pregnancy, a subset of which are discussed below:
The complement system is involved in enhancing the function of phagocytic immune cells and antibodies to clear microbes and cellular debris in an organism. Several studies have reported increased complement activity during pregnancy. This activity is balanced by high levels of regulatory proteins, such as the complement inhibitor Decay-Accelerating Factor (DAF) in peripheral blood mononuclear cells, which functions by inhibiting the effects of complement activation. The balance of complement activation is necessary to prevent cases of preterm birth or pre-eclampsia (disorder of high blood pressure during pregnancy).
Neutrophils, cells that respond against microorganisms through production of toxic granules and reactive oxygen species, are also elevated during pregnancy, with a notable increase observed from the first trimester. During pregnancy, neutrophils undergo significant alterations in their functionality and exhibit retrograde transport of metabolic enzymes that ultimately limits their metabolic output which impacts their overall function. This functional change is likely necessary for a successful pregnancy, as increased neutrophil activity has been shown to impact placental development and can lead to pre-eclampsia.
The role of monocytes during pregnancy has also been extensively studied. In particular, numbers of intermediate monocytes, which are cells that display both inflammatory and phagocytic capacity, are elevated during pregnancy. It is hypothesized that the increase in numbers and activation markers of intermediate monocytes may be influenced by placenta-secreted molecules and overall maternal hormone changes. As pregnancy progresses, these activation patterns and cellular dynamics evolve, with studies supporting the upregulation of genes encoding for IL-10, a regulatory marker, and downregulation of genes encoding for IL-8, an activation marker.
Adaptive Immune System: As with the innate system, various changes occur within the adaptive immune system that protects both the fetus and mother throughout pregnancy.
During pregnancy, a reduction in T cell frequency aligns with changes in T cell subsets, though the implications of these changes remain elusive. Interestingly, Treg populations were higher than helper and cytotoxic T cells during the first trimester, likely to facilitate successful embryo implantation, placental development, and early fetal growth. Although debated in the field, studies on Type 1 T helper (Th1) and Type 2 T helper (Th2) cells during pregnancy suggest that pregnancy maintains a prevailing Th2 state, supported by a rise in anti-inflammatory cytokines, a reduction in Th1-type autoimmune diseases, and increased incidences of Th2-type diseases. Despite this, the function of T cells during pregnancy is not entirely defined nor is it consistent between studies, underscoring the need for further research to understand the mechanistic role of T cell changes during pregnancy.
Maternal antibodies are required to protect the neonate immediately after birth. Yet, less known is that maternal B cells produce non-cytotoxic antibodies against paternal proteins that may be critical for a successful pregnancy, as these antibodies are absent in women who experience spontaneous miscarriage. Moreover, B cells have been shown to migrate to the placental decidua during the third trimester of pregnancy, hinting at their potential involvement in maintaining tolerance at the maternal-fetal interface. Analogous to Tregs, regulatory B cell (Breg) populations peak in the first trimester of pregnancy, presumably to suppress maternal Th1 responses, thereby fostering tolerance to fetal antigens. Research is ongoing to elucidate the mechanism behind the activation and expansion of Bregs during pregnancy.
Vertical Pathogen Transmission
Despite a highly protective immune system and physical barriers that develop during pregnancy to protect the fetus, vertical transmission, the transmission of infectious agents from an infected mother to her fetus, poses significant risks to fetal development. Vertical pathogen transmission can occur either before or after birth, however prenatal transmissions often lead to severe morbidity and mortality.
Toxoplasma gondii, Rubella, Cytomegalovirus, Herpesviruses, Parvovirus, HIV, and more recently Zika virus are collectively known as TORCH pathogens - a subset of microbes which have the capacity of causing teratogenic effects when transmitted from a mother to her developing fetus. These microbes have the capacity to access the intra-amniotic compartment where the fetus resides via placental damage or disruption, direct transmission through the placenta, sexual transmission and/or ascending infections. Infections can adversely affect the mother, fetus, and the placenta, potentially resulting in maternal sepsis, respiratory distress or even death. Importantly, vertically transmitted infections are known to cause birth defects (i.e., blindness, microcephaly, deafness), growth restriction, or death. TORCH pathogens further impact the placenta and can impair nutrient transport, exacerbating adverse outcomes for the pregnancy.
Despite our understanding of the outcomes of vertical transmission, the molecular mechanisms underlying microbial pathogeneses remain unknown, largely due to difficulties in modeling the complex architecture and immunology of the maternal-fetal interface. Current animal models fail to fully replicate human pregnancy. However, organoid models that contain both maternal and fetal cell types offer a promising technical model to explore the mechanism behind microbial pathogenesis and its impact on human pregnancies. Despite the complexity associated with mimicking the environment of pregnancy, ongoing research into the impact of infections at the maternal-fetal interface holds promise for advancing maternal and fetal health during pregnancy and beyond.

IMMpress Vol. 12 No. 1 2024 19
- Baweleta Isho
IMPACT of the Microbiome on

The development of a newborn’s immune system is a complex process influenced significantly by the early-life microbiome. This critical phase of immune system maturation is affected by various factors, including the maternal immune system, microbial metabolites during pregnancy, microbial transfer at birth, and the transfer of microorganisms, maternal immune factors, and metabolites via breastfeeding. Among these, the mode of birth delivery plays a pivotal role in shaping the neonatal microbiome and, subsequently, the development of the immune system. The two methods of birth
delivery are vaginal birth and Cesarean birth (C-section), which consists of delivering a baby through a surgical incision made in the mother’s abdomen and uterus.
Birth mode is a main driver of compositional differences in the infants’ gut microbiota. Infants born via C-section acquire more oral and skin microbes due to reduced exposure to their mother’s vaginal and fecal microbiota. Whether or not most vaginal and skin bacteria can successfully establish themselves in the infant gut is still debated, a study has shown that infants born by C-section share 41% of gut microbes with the mother, whereas infants born by vaginal delivery share 72% of gut microbes with the mother. Moreover, different birth modes influence the colonization of other microbes. Studies have shown that infants born by C-section have less intestinal colonization by probiotic microorganisms, whereas the colonization of C. difficile, a type of bacteria that causes diarrhea and colitis, was increased at one month of age. Interestingly, a study has shown that maternal antibiotic administration before C-section does not further impair microbiome colonization of the infants, suggesting that the initial lack of exposure to maternal vaginal and gut microbiota is the primary concern. Moreover, C-section has been associated with an increased risk for immune disorders, such as asthma, allergic rhinitis and celiac disease.
How does delivery mode affect the development of immune cells? Microbes shape the infant immune landscape by engaging molecular receptors in various gut immune cells and activat ing signalling cascades that control cell differentiation and inflammation. For example, immune cells, such as regulartory T cells (Treg) and invariant natural killer T (iNKT) cells, can only be “educat-
20 IMMpress Vol. 12 No. 1 2024
Immune System Development

ed” by microbes during a specific postnatal time window called the “neonatal window of opportunity”. This critical time period in a newborn’s life is when environmental factors drive immune and tissue maturation and influence susceptibility to diseases in adult life, helping the body develop tolerance to subsequent environmental exposures. Thus, the immune cell repertoire established during this critical period under the influence of the gut microbiota can potentially affect host immunity and disease vulnerability. In studies with mice, those born via C-section had a different microbiota composition which impacted their immune systems in various ways, including having fewer Treg and dendritic cells that help control immune responses. Therefore, C-section can potentially have lasting effects on the body’s ability to regulate immune responses.
The process by which the intestines become accustomed to bacteria shortly after birth is also affected by delivery method. For example, intestinal epithelial cells (IECs), which line the intestine and are important for protection against microbial infections, need to be critically regulated to maintain intestinal homeostasis. During vaginal birth, IECs are quickly activated by bacteria-associated molecules such as lipopolysaccharides (LPS), which helps prepare the body to defend against harmful microbes. The activation of IECs by LPS is only transient and is lost hours after birth, with no detectable inflammation, suggesting that tolerance towards microbes is acquired to facilitate microbial colonization of the fetal intestine. This activation is a delicate balance that, if disrupted, could lead to higher risk of inflammatory diseases. The reduced bacterial exposure from C-section might impair this critical early-life immune training, leading to decreased tolerance. This evidence points to the complex inter-
play between the mode of delivery, microbial exposure, and the developing immune system, underscoring the importance of understanding the long-term health implications of C-sections.
C-section significantly influences both the gut microbiota and immune system development, and these effects may lead to various long-term immune health issues.
C-section significantly influences both the gut microbiota and immune system development, and these effects may lead to various long-term immune health issues. To address potential dysbiosis associated with C-sections, new treatments, such as fecal microbiota transplantation (FMT), are being investigated for restoring neonatal microbiota following C-section births. A recent proof-of-concept study has demonstrated that the gut microbiota of infants born via C-section can be restored postnatally by maternal FMT. However, careful microbiological screening is mandatory to eliminate harmful bacteria. Further research is needed to understand the complex interactions and the long-term impacts of C-section delivery on immune system development. Such mechanistic understanding may provide the basis for the development of preventative approaches for negative health effects in C-section infants in the future.
- Tianning Yu
IMMpress Vol. 12 No. 1 2024 21
On Guard with Gardasil
Whilea “cure” for cancer remains elusive, prevention of some cancers is within our grasp. It is estimated that 30 – 50% of cancer cases are preventable through risk-reducing measures. One simple method involves just two shots of the human papillomavirus (HPV) vaccine during childhood. This vaccine not only protects against HPV infections, but also reduces the risk of developing cancers caused by HPV.
In the late 1960s, the herpes simplex virus type 2 (HSV-2) was a prime suspect as the cause of cervical cancer. Virologist Harald zur Hausen attempted to find HSV-2 DNA in cervical cancer biopsies, but to no avail. Upon learning that genital warts, previously shown to contain papillomavirus, could become cancerous, Dr. zur Hausen connected the dots. His research group was able to identify DNA from HPV in cervical cancer biopsies and characterize the integration of viral genes into the host cells. His pivotal work in discovering HPV as a causal agent of cervical cancer was recognized in 2006 with a Nobel Prize in Medicine.
HPV is now established as a cancer-causing, or oncogenic, pathogen that is the primary cause for anogenital (cervical, anal, vulvar, vaginal, and penile) cancers and oropharyngeal (throat) cancers. It represents a group of related viruses with double-stranded DNA, encompassing more than 100 different types. The virus is transmitted by skin-to-skin contact and commonly contracted through sexual intercourse.
HPV infections are, in fact, very common. The majority of sexually active individuals become infected at some point in their lives. Most infections resolve on their own, often without any symptoms. Yet, HPV accounts for a staggering 90% of cervical and anal cancer cases.
HPV accounts for 90% of cervical and anal cancer cases
It all comes down to the type of HPV: While an infection with a low-risk type may be harmless, a long-persisting infection with specific oncogenic HPV variants can transform normal cells into cancerous ones. HPV types 16 and 18 are highly oncogenic and responsible for approximately 72% of HPV-attributable cancer cases. The rest are associated with HPV types 31, 33, 45, 52, and 58.
33, 45, 52, 58
The research linking HPV to cervical cancer catapulted the development of vaccines for HPV. Immunologist Ian Frazer and virologist Jian Zhou developed virus-like particles (VLP) of proteins from the surface layer of HPV. VLPs closely resemble the virus but cannot mount an infection. HPV vaccines based on VLPs train the immune system to recognize and clear the virus in subsequent encounters – before it leads to a persistent infection that could trigger the oncogenic process.
There are two different HPV vaccines available in Canada: HPV9 vaccine (Gardasil-9) by Merck and HPV2 vaccine (Cervarix) by GlaxoSmithKline. Both VLP-based vaccines protect against oncogenic HPV types 16 and 18, which cause most HPV-attributable cancers. Gardasil-9 protects against an additional five oncogenic HPV types (31, 33, 45, 52, and 58) along with two genital wart-causing types (6 and 11). Currently, only Gardasil-9 is authorized for use in both sexes in Canada.
HPV types 16, 18 Ce ra vix
Gardasil-9
HPV types 16, 18, 31, 33, 45, 52, 58, + 6, 11
HPVattributable cancer cases HPV
31,
types 16,
72% 28%
types
HPV
18
22 IMMpress Vol. 12 No. 1 2024
Vaccines against cancers caused by HPV
Both vaccines have demonstrated their safety in large-scale clinical trials involving 15 000 – 30 000 participants and in continued safety surveillance. Cervarix and Gardasil-9 have a >90% efficacy against HPV type 16 and 18-related cervical cancer. Likewise, immunization with HPV4 vaccine (Gardasil), an early predecessor to Gardasil-9, showed 84-100% efficacy against HPV-associated genital lesions in men. Vaccine efficacy against vaccine-type specific oral HPV infections has been reported to be similarly high, ranging from 88% to 93% depending on the sampled population. Moreover, studies following vaccinated individuals for more than 10 years provide evidence that the protection is sustained long-term.
As a preventative measure, the HPV vaccine is most effective when administered before exposure to the virus or start of sexual activity. Further research on vaccination of girls and young women between the ages of 14 and 26 years reveal the additional benefit of improving herd immunity, which provides indirect protection to unvaccinated individuals through immunization of a significant portion of the population.
In Canada, as part of routine childhood immunization, two doses of HPV vaccines 6 to 12 months apart are recommended for children (girls and boys) aged 9 through 14 years. Subsequently, ‘catch-up’ vaccinations with three doses are recommended for youths and young adults between the ages of 15 to 26 years and remain available for adults between the ages of 26 to 45 years.
As of 2017, all Canadian provinces and territories have school-based immunization programmes that offer publicly funded HPV vaccination, most commonly, in Grades 6 or 7. In 2017, the rate of fulldose HPV vaccination within the target adolescent demographic ranged by province/territory from 57% to 91%. These figures markedly declined amidst the COVID-19-related disruptions of schools, emphasizing the importance of school-based delivery of vaccination.
of full-dose HPV vaccination within target adolescent demographics in 2017
Publicly funded HPV vaccinations continue to be accessible for individuals eligible for catch-up programs through healthcare providers. However, if ineligible, the three-dose HPV vaccine series can cost over $600. Other barriers to vaccination include lack of information or awareness about the vaccine and inequities in accessing care, which disproportionately impact Indigenous peoples, rural and remote communities, people with low income, and 2SLGBTQI+ individuals.
While vaccination against HPV has now been adopted by national vaccination programmes in more than 130 countries, the World Health Organization estimates that only 21% of girls worldwide have had their first dose. Limited or lack of access to affordable vaccination poses a major barrier in many lowand middle-income countries, which face the highest burden of deaths from cervical cancer. To address the disparities in childhood immunization, UNICEF is working to supply HPV vaccines to 52 countries and to introduce them into routine vaccination programmes of more countries.
The advent of HPV vaccine marks the culmination of many breakthroughs in research. It has given us the opportunity to control a known preventable risk factor for many types of cancers. However, much work remains to done in improving vaccine coverage so that more people can benefit from this life-saving vaccine.
- Nasana Vaidya
$100 $100 $100 $100 $100 $100
rate
57% 91%
IMMpress Vol. 12 No. 1 2024 23
Unlocking Hope Progress in Understanding and Treating Inborn Errors of Immunity
David Vetter, popularly known as the “boy in the bubble”, was born with Severe Combined Immune Deficiency (SCID). As one of the most severe cases of primary immunodeficiency, SCID impairs the growth and operation of immune cells that protect against infection. Although seemingly healthy initially, infants born with SCID are at heightened risk of severe infections and usually do not survive beyond two years of age without treatment. These infants may experience frequent infections, particularly viral ones, which can lead to serious conditions like pneumonia and chronic diarrhea. Additionally, yeast infections in the mouth and diaper area are common indicators of SCID. Despite seeming healthy when he was born, David had to be kept in a sterile environment to avoid exposure to germs. Otherwise, he risked developing various severe illnesses.
Hematopoietic Stem Cell Transplantation (HSCT) is the standard form of treatment for infants with SCID, which often only partially restores immunity. When David was born in 1971, the sole treatment available for SCID was a bone marrow transplant. This procedure involved replacing the compromised immune system with a healthy one by providing a source of hematopoietic stem cells (HSCs). This is achievable because HSCs are robust blood-forming cells capable of maturing into various types of immune cells, contributing to the establishment of the immune system. Unfortunately for David and his family, despite receiving a bone marrow transplant he tragically succumbed to lymphoma, a cancer originating from abnormal white blood cells in the lymph glands, just four months after receiving the bone marrow transfusion.
SCID is one of the many diseases that is a result of inborn abnormalities of immunity. Monogenic immune system abnormalities, referred to as inborn errors of immunity (IEI), are mutations in single genes that result in specific impairment to the normal development and function of the immune system. IEI can lead to various health issues such as increased vulnerability to infections, problems with the immune system, inflammation within the body, autoimmune conditions, allergies, and even certain types of cancers. Although rare, the aggregated number of individuals with an IEI represents a significant health burden.

gene therapy. This breakthrough led to further research, particularly with lentivirus-based vectors, to address other types of IEI through gene transfer.
The development of safe and effective gene therapy techniques throughout the previous 25 years has resulted in long-term clinical benefits in four IEI’s: SCID-X1, ADA SCID, WAS (Wiskott-Aldrich syndrome), and CGD (chronic granulomatous disorders). Researchers have made significant scientific and technological strides, enhancing the effectiveness and safety of treatments for IEIs. Traditional HSCT has advanced, leading to higher cure rates for IEIs, allowing researchers to compare gene therapy’s new approach with HSCT.
Overall, the steady advancement of science and technology has greatly enhanced the efficacy of both traditional HSCT and gene therapy in treating IEIs. Through the study of these conditions, researchers have gained invaluable insights into immunology, driving ongoing improvements in therapeutic strategies. As our understanding continues to deepen, we can anticipate further progress in the treatment and management of immune-related disorders, offering hope for better outcomes and quality of life for those affected by IEIs.
- Jennifer Sahadeo
In the 1970s, scientists made significant strides in understanding genetic diseases and how genes work. This led to the development of genomic cell modification as a potential treatment approach. Thanks to progress in virology, researchers figured out how to create vectors, which are delivery vehicles that carry healthy copies of genes into cells. This technology showed promise as a treatment for people with IEI. One of the first successes was in treating SCID using




24 IMMpress Vol. 12 No. 1 2024


The Invisible Scars of Childhood Adversity


The impact of early childhood experiences and parent-child relationships on our neurodevelopment and psychological health is popularly known. However, research has shown that there is a deeper link between the nature of our early childhood life and adult immune function.
Early life stress or childhood adversity can include child abuse, parental conflict or neglect, low socioeconomic conditions, and poor parent-child relationships among a multitude of other scenarios that might put undue stress on a child. Researchers investigating the physiological implications of such early-life stress found that these children grew up to have elevated levels of pro-inflammatory immune biomarkers. Adults diagnosed with depression who experienced childhood adversity have higher levels of C-reactive protein (CRP) than those who did not.
Early life stress can increase sympathetic activity and decrease parasympathetic activity in the nervous system. These processes in early life can cause increased hypothalamic-pituitary-adrenal (HPA)-axis activity in the body upon facing stress, either physiological or psychological. This can cause an increase in cortisol levels to enormous amounts, leading to insensitivity to glucocorticoids that are important for regulating the effects of stress, such as the production of messenger molecules that promote inflammation, including interleukin 6, TNF-alpha, and CRP. Early life stress can also cause certain immune cells such as monocytes and macrophages to have an excessive inflammatory response to microbial antigens. Another mechanism is increased systemic inflammation due to elevated transcription of NF-kB, a molecule that regulates the expression of pro-inflammatory molecules. This is proposed to occur through epigenetic processes.

Childhood adversity-related systemic, chronic inflammation is associated with increased morbidity, mortality, and lower quality of adult life. Our DNA is packed into dense structures called chromosomes. These chromosomes have protective caps at their ends called ‘telomeres’. Telomere shortening is a direct indication of aging and a predictor of lifespan. Adults who reported having faced childhood adversity had shorter telomeres than the adults who did not. While childhood adversity was correlated with shorter telomere lengths in adults, current social adversity was not. Moreover, researchers estimate a 7 to 15-year reduction in lifespan in older adults if they faced early life stress or any form of childhood adversity.
The beginnings of these studies were in animal models where scientists found that rats that were lifted, held, and/or touched by scientists before weaning had slower development of tumors and showed better anti-microbial responses upon challenge. It has also been shown that the negative effects of childhood adversity due to low socioeconomic status can be ameliorated to a significant extent by the presence of maternal warmth. Interestingly, some studies show strong associations between childhood adversity and inflammatory biomarkers in girls but no association in boys. This hints towards complex sex effects in different facets of this psychosomatic link.
The long-lasting impact of childhood adversity on the immune landscape and inflammatory status of the adult sheds light on the lesser-known impacts of pediatric psychological health and urges the implementations of better systems in our society that help children feel seen, heard, and above all, safe.
IMMpress Vol. 12 No. 1 2024 25
- Manjula Kamath
Haveyou ever met a devoted cat-owner that is simultaneously allergic to that same cat? While 10-20% of the world’s population is allergic to cats or dogs, a 2020 survey of cat owners by the Human Animal Bond Research Institute found 23% of cat owners lived with an allergy to cats. 84% of those allergic owners preferred to seek alternatives
Allergies are a broad group of hypersensitivities to seemingly harmless foreign substances. The most common symptom of these hypersensitivities is allergic rhinitis which consists of a runny nose, sneezing, hives, watery eyes, or itchiness around the face or area of contact.
Studies have reported correlations between increased pet exposure in the first
Theexistence of allergies to common food items such as peanuts or eggs, and domesticated cats or dogs has stumped immunologists for many years. This went against the understanding of the immune system’s ability to distinguish harmful vs harmless foreign substances and react appropriately. That is, until epidemiologist Dr. David Strachan published his observations about the prevalence of asthma and eczema in 1989, leading to the development of a theory later called the hygiene hypothesis.
Briefly, the hygiene hypothesis suggests that reduced exposure to various microorganisms leads to deficient education of the immune system and a hypersensitive immune system later in life.
The foundation of the hypothesis is that changes in living conditions, such as better sanitation, improved barriers dividing the inside world from the outside world, and a reduction in the average family size, have led to less frequent
to physician-recommended surrendering of their cats, such as through allergy management. While the reason behind pet ownership despite its obvious health challenges may be a testament to emotional bonds between humans and pets, it raises the question about the development of pet allergies.

year of life and reduced development of pet allergies. Additional studies have demonstrated decreased sensitivity to certain animals and general allergens in children living in a household with pets in the first two years of life compared to those who had never lived with pets. Further work studying the strength of the immune system of children living with pets is underway to understand whether these observations are causative. In the meantime, there is an absence of evidence suggesting that exposure to these harmless environmental agents could contribute towards allergy development.
exposure and infection by viruses, bacteria and parasites during one’s early years (0-3 yrs). While regular exposure to harmful parasites, and bacteria have been eliminated, exposure to harmless environmental substances such as pollen, and pet dander has also been reduced. Through mechanisms still being studied, the reduced repertoire of substances whose harmfulness was to be “learned” earlier in development has led to an ‘underdeveloped’ immune system. Later, when the immune system is exposed to these ordinary substances, it mounts very specific immune responses that are designed to target parasites (ex: histamine production). It is noteworthy that allergies are highly skewed to countries that are highly industrialized and are most impacted by the changes in living conditions.
Although not entirely irrefutable, the evidence for the hygiene hypothesis highly supports pet ownership during childhood and may provide parents with something to think about regarding the health of their children.
- Sila Usta
26 IMMpress Vol. 12 No. 1 2024
Book Review: The Baby and the Biome: Nurturing Nature’s Connection
In her book The Baby and the Biome, Meenal Lele, a mother of two children, invites readers on a deeply personal journey as she navigates the complexities of caring for her son Leo, who suffers from severe allergies. Drawing from her own maternal experiences and extensive research, Lele goes beyond the conventional scope of managing symptoms to delve into the web of factors influencing her son’s health. Through the lens of Leo’s journey, Lele sheds light on the crucial role of the microbiome and environmental exposures in shaping the development of immune diseases, allergies, environmental sensitivities, and eczema.
Lele’s narrative not only highlights the challenges she faced in deciphering the root causes of her son’s conditions but also underscores the broader disconnect between conventional medical approaches and a more holistic understanding of health and wellness. By connecting the dots between Leo’s complex symptoms, alterations in his microbiome, and environmental triggers, Lele encourages readers to consider a more integrative approach to healthcare—one that addresses not just the symptoms of disease but also its underlying causes and preventive measures.
“Almost all chronic diseases are largely caused by external exposures,” said Lele, though genetics can certainly also contribute. Nevertheless, this serves as a poignant reminder of the pivotal role of preventive healthcare choices highlighted throughout the book.
Lele debunks the common belief that food allergies are solely genetic, emphasizing the multifaceted influences of diet, medication, pathogens, and environment on allergic responses. This reframing emphasizes the importance of mitigating these factors to safeguard children’s immune systems against allergic reactions.


“We have the ability to prevent most of the diseases, but we have to make the right choices. We could make far better choices about our food, the chemicals we use and even makeup.”
The book also sheds light on the crucial symbiotic relationship between infants and the microbial world, stressing how disruptions can have detrimental effects. From the moment of birth, babies are not merely isolated beings, but rather intimately intertwined with the vast ecosystem of microorganisms that inhabit their bodies and surroundings. This symbiotic relationship shapes every aspect of a baby’s life, from their immune system to their cognitive development. A disruption in this relationship can quickly turn from beneficial to detrimental.
What sets this book apart is its message of empowerment. Rather than succumbing to fearmongering or alarmism, Lele offers practical strategies for parents and caregivers to support the development of a robust infant microbiome. Her evidence-based recommendations include promoting breastfeeding and encouraging outdoor play to foster a healthy microbial environment for babies. “Food allergy or immune diseases are about an accumulation of insults. So, what you’re trying to do is limit the number of insults that there are. And even go after the insults that are easiest for you.”
The Baby and the Biome is a thought-provoking read that challenges conventional wisdom and inspires readers to rethink their approach to healthcare. Whether you’re a parent grappling with a child’s health issues, a healthcare professional seeking new insights, or simply curious about the intricate interplay between human biology and the environment, this book offers valuable perspectives and guidance. Lele’s journey serves as a powerful reminder to nurture our own microbiomes and foster connections with the natural world for optimal health and well-being.
- Alara Tuncer

IMMpress Vol. 12 No. 1 2024 27
Department of Immunology
University of Toronto
1 King’s College Circle
Toronto, ON M5S 1A8
Canada

Campus views - Roberts Street Rink (1972)
Credits: University of Toronto Archives.
Photo





















 In order of left to right: James Pollock (Co-Editor-in-Chief), Kitty Liu (Design Director), Karen Yeung (Co-Editor-in-Chief), and Tianning Yu (Social Media Coordinator)
In order of left to right: James Pollock (Co-Editor-in-Chief), Kitty Liu (Design Director), Karen Yeung (Co-Editor-in-Chief), and Tianning Yu (Social Media Coordinator)




 Jen Gommerman, PhD
Canada Research Chair in Tissue Specific Immunity Professor and Chair, Department of Immunology
Jen Gommerman, PhD
Canada Research Chair in Tissue Specific Immunity Professor and Chair, Department of Immunology



















































