











































Welcome, Advocates, to the 12th annual Rare Disease Week on Capitol Hill!
We are so honored that you are sharing your time so generously, using your vacation, braving COVID and flu season, and spending your own funds to travel to join us in Washington, DC. The EveryLife team is in awe of your courage, dedication and passion for rare disease advocacy. We are grateful to have you here with us. For the first time, our Rare Disease Week registrants represent every state in the country and the sovereign Cherokee Nation. This year, your stories and influence as rare disease ambassadors matter more than ever. Here’s why:
In 2021, EveryLife released a first-of-its-kind study that found the cost of rare disease in 2019 was a devastating $1 trillion dollars. By prioritizing, incentivizing and accelerating rare disease treatments access to those treatments and more timely diagnoses, we can alleviate the crisis and save lives.
We are welcoming in a new Congress. There are many newcomers to the Hill who may not be familiar with historical issues affecting rare disease patients and their families. Helping them understand your needs as a valued constituent is vital to cultivating their support for legislation that can advance drug development and the policies that make new treatments accessible and affordable.
We are observing the 40th anniversary of the Orphan Drug Act. Since it was enacted with bipartisan support in 1983, this law has saved or improved countless lives. On the 40th anniversary of this truly life-changing legislation, we must urge our lawmakers to protect, strengthen and grow the ODA to serve rare patients in today’s fast-changing scientific landscape.
On behalf of the EveryLife Team and our volunteers and partners, we wish you an exceptional week in Washington, DC.
Rare Disease Week is important to me because advocates from across the country are collectively raising awareness about rare diseases and the challenges this community faces. Our stories, one after another, can impact legislation and better health outcomes for rare disease patients and families.”
Sarita Edwards, Advocacy Chair Rare Disease Week on Capitol Hill
Rare Disease Week on Capitol Hill
2023
I love Rare Disease Week because I get to witness advocates harness the unique power of their stories into conversations about impactful policy changes.”
Abbey Hauser, Advocacy Vice ChairRare Disease Week on Capitol Hill 2023












The EveryLife Foundation Board of Directors is comprised of a diverse group of individuals who are both personally and professionally dedicated to the development of treatment and diagnostic opportunities for the 30 million Americans living with a rare disease. With decades of experience in the government, nonprofit, finance, science, medicine, industry, and academic sectors, EveryLife board members provide valuable guidance to the Foundation toward achieving its mission. In addition, several of our board members are the parents of children with a rare disease, enabling them to offer firsthand knowledge of the challenges facing the rare disease community.

The knowledge, skills, network, and experience I gained through Rare Disease Week made a major impact on my family’s rare disease journey. Participation in Rare Disease Week is my top recommendation to new advocates, and I am amazed at how much I still manage to learn and take away from the experience! It is empowering and transformative for rare disease advocates of all experience levels.”
– Cristina Casanova MightJulia Jenkins Board President Frank Sasinowski MS, MPH, JD, EveryLife Foundation Chair, Director, Hyman, Phelps & McNamara P.C. Patient and parent advocate Vicki Seyfert-Margolis PhD, EveryLife Foundation Vice-Chair, Founder and CEO, MyOwnMed Family advocate Jennifer Bernstein Secretary, Horizon Government Affairs Parent advocate Stephen C. Groft Treasurer, Pharm.D Ritu Baral Managing Director/Senior Biotechnology Analyst, Cowen and Company Patient advocate Emil Kakkis MD, PhD, EveryLife Foundation Founder, President/CEO, Ultragenyx Amrit Ray MD, MBA, Biopharmaceuticals R&D Executive, and Corporate Board Director Parent advocate Abbey Hauser Young Adult Rare Disease Advocate Patient advocate Lisa Carlton Vice President, Global Regulatory Affairs at REGENXBIO Parent advocate Cristina Casanova Might B.S., M.B.A. CEO at Welcomed Co. Patient and parent advocate Merrill Friedman Regional Vice President, Inclusive Policy & Advocacy at Elevance Health























TUESDAY, FEBRUARY 28
12:00 p.m. EST
Rare Artist Virtual Gallery Opens

PRESENTED BY
6:00 p.m. to 9:00 p.m. EST
Rare Disease Documentary Screening and Reception

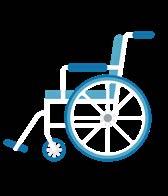
Ronald Reagan Building and International Trade Center
1300 Pennsylvania Ave NW, Concourse Level, Washington DC, 20005
Metro Stop: Metro Center and Federal Triangle
WEDNESDAY, MARCH 1
9:15 a.m. to 4:45 p.m. EST
Legislative Conference
Ronald Reagan Building and International Trade Center
1300 Pennsylvania Ave NW, Concourse Level, Washington DC, 20005
Metro Stop: Metro Center and Federal Triangle
6:00 p.m. to 7:00 p.m. EST
YARR Leadership Academy Graduation
In-person ceremony, by invitation only
7:00 p.m. to 8:00 p.m. EST

Young Adult Rare Representatives (YARR) Meetup
Rare Hub, 1012 14th Street NW, Suite 500, Washington, DC 20005

Metro Stop: McPherson Square or Farragut North
PRESENTED BY
PRESENTED BY
PRESENTED BY




THURSDAY, MARCH 2
9:00 a.m. to 10:00 a.m. EST
Rare Disease Congressional Caucus Briefing
Capitol Hill, Russell Senate Office Building, Room 325
Washington, DC 20004
Metro Stop: Union Station (Senate side) or Capitol South (House side)
4:30 p.m. EST
Group Photo on Capitol Hill Steps
Capitol Hill, Washington DC 20004
10:00 a.m. to 5:00 p.m. EST

Hill Meetings
Capitol Hill, Washington DC 20004
Metro Stop: Union Station (Senate side) or Capitol South (House side)
5:00 p.m. to 7:00 p.m. EST
Rare Artist Reception
Hart Senate Office Building, Room 902
Washington, DC 20004 (Use “North” elevators)
Metro Stop: Union Station (Senate side) or Capitol South (House side)
Share your experience during Rare Disease Week by taking a selfie in our Virtual Photobooth and posting it on social media with the hashtag #RareDC2023 THANK YOU TO OUR


Tuesday, February 28
6:00 p.m. to 9:00 p.m. EST
Ronald Reagan Building and International Trade Center
1300 Pennsylvania Ave NW, Concourse Level, Washington DC, 20005
Special Blood highlights the stories of four patients with a lifethreatening rare disease called hereditary angioedema (HAE), who learn to find strength in each other and their small, but strong community.



6:00 p.m. EST – 7:00 p.m. EST: Reception
7:00 p.m. – 8:15 p.m. EST: Introductions and Film Screening
8:15 p.m. – 9:00 p.m. EST: Panel Discussion

Introduction Frank Sasinowski
EveryLife Chair, MS, MPH, JD, Director, Hyman, Phelps & McNamara P.C.

PRESENTED BY

Special Remarks: Natalie Metzger Special Blood Director and rare disease patient

The film screening will be followed by a panel discussion featuring:
Moderator: Cristina Casanova-Might Board of Directors, EveryLife Foundation for Rare Diseases; CEO at Welcomed Co.


Ballroom A is available for social distancing with livestream
WEDNESDAY, MARCH 1
8:00 a.m. – 4:45 p.m.
Ronald Reagan Building and International Trade Center - Atrium
1300 Pennsylvania Ave NW, Washington, DC 20004
Registration and Breakfast (Atrium)
8:00 – 9:15 am

Welcome
9:15 – 9:45 am
Atrium Hall and via Livestream
• Brett McReynolds, Horizon Therapeutics
• Shannon von Felden, EveryLife Foundation for Rare Diseases.
• Keynote: Sarita Edwards, Advocacy Chair of Rare Disease Week on Capitol Hill
9:45 – 10:45 am
Atrium Hall and via Livestream
PRESENTED BY
REGISTRATION PRESENTED BY

BREAKFAST SPONSORED BY
Hear from experts on the new 118th Congress and how Congress will impact healthcare policy in 2023 and the latest statistics on rare diseases. Then hear how you can impact policy change through the legislative asks for 2023. We encourage advocates to make one specific “ask” during their meetings with Members of Congress. This panel highlights some potential “asks” that are priorities for the rare disease patient community.

• Introductions: Stephen Meunier, Sanofi
• Moderator: Shannon von Felden, EveryLife Foundation for Rare Diseases
• Nicholas Manetto, Faegre Drinker
• Jamie Sullivan, EveryLife Foundation for Rare Diseases
• Chris Jones, Office of Representative Gus Bilirakis (FL)

• Dylan Simon, EveryLife Foundation for Rare Diseases
• Dave Zook, Faegre Drinker
• Jackie Weinrich, Office of Representative Doris Matsui (CA)
REAGAN WIFI ACCESS
Network Name: Ronald Reagan Building
Wifi Username: RareDC2023
Password: RDLA
Break
10:45 – 11:00 am
Deep Dive Policy Ask #1: FDA Task Force on Rare Disease Activities
11:00 am – 11:45 am
Atrium Hall and via Livestream
• Moderator: Vicki Seyfert-Margolis, Ph. D., My Own Med, Inc. and EveryLife Foundation Board Member
• Annie Kennedy, EveryLife Foundation for Rare Diseases

• Isabelle Lousada, Amyloidosis Research Consortium

• Matthew Ellinwood, Ph. D., National MPS Society
Deep Dive Policy Ask #2: Appropriations for Critical Rare Disease Programs
11:45 am—12:30 pm
Atrium Hall and via Livestream
• Moderator: Megan O’Boyle, RARE-X
• PJ Brooks, MA, Ph. D., National Center for Translations Sciences, NIH
• Kathy Needleman, MS, Ph.D., Office of Orphan Product Development, FDA
• Emily Henkle, MPH, Ph.D., Oregon Health and Science University
• Dylan Simon, EveryLife Foundation for Rare Diseases
Group Photo

12:35 pm
Atrium Stairs
FAMILY SPACE
Networking Lunch
12:45 – 1:30 pm Atrium
We encourage families to eat with their state team. Look at the poster board to find the location of your state meeting.
Preparing for Successful Meetings*
1:30 – 2:30 pm
Atrium Hall & via Livestream. Teams may utilize Atrium Ballroom A during the breakout time if extra room is needed.
*Mandatory for advocates participating in Hill Day (family room will be closed at this time). Advocates receive information on their Hill meetings and scheduled and strategize with their teams to make the most of their time on Capitol Hill.
• Katelyn Laws, EveryLife Foundation for Rare Diseases
Snack Break
2:30 – 3:00 pm Atrium
#RAREis Scholarship Recipient Alumni Gathering
2:45 – 3:00 pm Ballroom B, Invitation only

Breakout Sessions
3:00 – 3:45 pm
Track 1: Deep Dive Policy Ask #3: The BENEFIT Act
Atrium Hall and via Livestream
• Moderator: Jamie Sullivan, EveryLife Foundation for Rare Diseases
• Lauren Sanford, Parent Project Muscular Dystrophy
• Brian Denger, Rare disease advocate
• Ron Bartek, Friedreich’s Ataxia Research Alliance (FARA)

Track 2: Advocacy for Young Adults
Atrium Ballroom B
This session is for young adults (ages 16-30) to learn more about the YARR community legislative ask.
• Moderator: Courtney Felle, EveryLife Foundation for Rare Diseases
• Alexis Levine, Young Adult Rare Representative
• Kyle Underwood, Young Adult Rare Representative
Did you know, it only takes about 30 social media posts about an issue to get a legislator’s attention? Stop by our social media lounge to meet with digital leaders and discuss social media opportunities, ways to get the attention of legislators and 1:1 tutorials and information about best practices.
All Day: Brenda Colmenares: Social Media 101: Hashtags, Handles and Tracking Your Impact
8:30– 9:15 a.m.: Ella Balasa: A Deep Dive on Instagram and LinkedIn
12:45 – 1:30 p.m.: Jonathan Cappiello: So, You Want to Start a Rare Disease Podcast?
2:30 – 3:00 p.m.: Abbey Hauser: Building Your Advocacy Brand

Breakout Sessions
3:50 – 4:35 pm
Track 1: Achieving Equitable Access and Utilization of Genetic Testing, Counseling, and Research

Atrium Hall and Livestream
• Introductions: Christina Hochul, Alexion Pharmaceuticals
• Moderator: Teneasha Washington, MPH, MBA, Ph.D., RARE-X
• Barbara Harrison, MS, CGC, Howard University
• Tshaka Cunningham, Ph.D., Polaris Genomics
• Natasha Bonhomme, Expecting Health
Track 2: Practice Your Pitch
Atrium Ballroom B
*This is a must-attend session for those individuals who are new to advocacy. Hear from a professional lobbyist on how to be most impactful in Congressional meetings and the do’s and donts. Learn how to develop and make your legislative ask during your meetings. The session will offer new advocates an opportunity to practice making their ask and telling their story with other advocates. This is a great opportunity to get questions answered by experienced rare disease advocates.
• Stephanie Riordan, EveryLife Foundation for Rare Diseases
• Ryan Shay, Faegre Drinker
• Abbey Hauser, Board of Directors, EveryLife Foundation for Rare Diseases
• Shannon Hudson, Rare disease advocate
• Ali SP, Rare disease advocate
• Emily Stauffer, EveryLife Foundation for Rare Diseases
• Nisha Trivedi, Rare disease advocate

Closing Remarks
4:35 – 4:45 pm
Atrium Hall and Atrium Ballroom B
Thursday, March 2
9:00 a.m. to 10:00 a.m. EST
Russell Senate Office Building, Room 325
Strengthening the Novel Therapy Development Ecosystem for Rare Diseases


Rare Disease Legislative Advocates and the Rare Disease Congressional Caucus invite you to a rare disease briefing

Guest Panel
Moderator: Esther Krofah, FasterCures, Milken Institute
Introductions: William Rote, Ph. D., Senior Vice President, Research and Development, Travere Therapeutics
Annie Kennedy, Chief of Policy, Advocacy, and Patient Engagement, EveryLife Foundation for Rare Diseases
Emil Kakkis, MD, Ph.D., CEO and President, Ultragenyx Pharmaceuticals

Pat Furlong, President and CEO, Parent Project Muscular Dystrophy
Malkia White, Ambassador, IgAN Foundation
PRESENTED BY
ADDITIONAL SUPPORT FROM
FDA Task Force on Rare Disease Activities
• Ask your Member of Congress to join a Congressional sign-on letter to the FDA requesting the formation of an internal FDA task force to review agency-wide rare disease activities.
*More information regarding this ask will be provided during the Legislative Conference
Appropriations Requests:
• Support increased funding for rare disease research at both the FDA and NIH:
• Fully fund the Orphan Products Grant Program to help support the critical programs that fund clinical trials and natural history studies to aid in the development of new rare disease therapies.
• Increase funding to help NCATS leverage its robust rare disease research program to accelerate the development of new treatments.
• Support the National Academy of Medicine to facilitate a scientific process around defining ultra-rare diseases and explore the opportunities and implications around such a definition. The process would include a consensus-based approach, convening the rare disease community, regulators, and researchers.
• Investments into rare disease research benefits everyone.
• A 2022 study showed that the conservatively estimated impact of rare diseases in the U.S. in 2019 was nearly $1 trillion. Discovery of treatments for the more than 95% of rare diseases with no FDA approved therapy – and investments into opportunities to improve health outcomes -- is a societal priority.
• For many rare diseases, treatments are now within reach.
• Advances in the understanding of rare diseases and novel technology platforms have transformed many disease pipelines. Yet we must ensure that incentives and regulatory pathways exist for all rare diseases to make development financially viable for companies.
• Natural history studies are essential for powering rare disease therapeutic development.
• Robust natural history studies and very small population clinical research are critical to development programs, yet funding sources are extremely limited.
• The ODA reshaped the rare disease landscape, yet more remains to be done.
• The incentives within the Orphan Drug Act helped to exponentially increase the number of FDAapproved rare disease therapies.
• Small, rare disease populations (ultra-rare diseases) still face significant challenges in the development of new therapies and may require additional policy and regulatory considerations to address the unique complexities of therapy development.
• While much progress has been made in driving forward policies and procedures to ensure the patient perspective is considered by FDA, reviewers evaluating candidate drugs and other medical products, some significant gaps remain.
• One such gap is the lack of any requirement in law today that the FDA include patient experience or patient-focused drug development (PFDD) data as a part of its risk-benefit framework.
• This means that the agency’s signature tool for evaluating risk-benefit does not have data from the patient perspective that could be critical to informing the agency’s evaluation and, ultimately, decision on whether or not to approve a product.
• The BENEFIT Act will enhance an important transparency and accountability provision included in the 21st Century Cures Act by requiring the FDA to share how such patient experience and PFDD data was considered within the risk-benefit assessment for any approved therapies.
• This will provide additional learnings to all stakeholders, particularly patients, and help further refine and develop such tools going forward.
• This legislation will amend the Food, Drug and Cosmetic Act (FDCA) to ensure that patient experience, PFDD and related data including information developed by a product sponsor or a third party such as a patient advocacy organization or academic institution – be considered as part of the risk-benefit assessment.
• Many significant statutory and regulatory advances have been made in the past decade to ensure that patient experience is meaningfully incorporated into product development and regulatory review processes.
• The BENEFIT Act represents the evolution, following on critical provisions coming out of PDUFA V, VI, and the 21st Century Cures Act that have been implemented by the FDA and embraced by stakeholders.
Co-Chairs:
Representative Gus Bilirakis (FL)

Representative Doris Matsui (CA)

Senator Amy Klobuchar (MN)

Senator Roger Wicker (MS)

• The Rare Disease Caucus is a bipartisan, bicameral caucus that works to raise awareness of rare diseases.
• Rare diseases affect more than 30 million Americans and their families.
• More than 95% of the estimated 10,000 rare diseases do not have an FDA approved treatment.
• Rare or orphan diseases are defined as diseases affecting fewer than 200,000 people in the U.S.
• Rare diseases include rare cancers, tropical or neglected diseases, genetic diseases and many pediatric diseases including cancers. Many of these diseases are life-threatening and have no treatment options.
• The Rare Disease Congressional Caucus helps bring public and Congressional awareness to the unique needs of the rare disease community (including patients, physicians, scientists, and industry), and creates opportunities to address barriers to the development of and access to lifealtering treatments.
• The Caucus gives a permanent voice to the rare disease community.
Mark Amodei NV-2
Jake Auchincloss MA-4
Don Bacon NE-2
Andy Barr KY-6
Joyce Beatty OH-3
Ami Bera CA-7
Donald Beyer, Jr. VA-8
Gus Bilirakis* FL-12
Sanford Bishop, Jr. GA-2
Lisa Blunt Rochester DE-At-Large
Suzanne Bonamici OR-1
Julia Brownley CA-26
Vern Buchanan FL-16
Michael Burgess TX-26
Ken Calvert CA-42
Salud Carbajal CA-24
Andre Carson IN-7
John Carter TX-31
Sean Casten IL-6
Judy Chu CA-27
David Cicilline RI-1
Emanuel Cleaver, II MO-5
Ben Cline VA-6
Steve Cohen TN-9
Jenniffer González Colón PR-AL
James Comer KY-1
Gerald Connolly VA-11
Jason Crow CO-6
Sharice Davids KS-03
Diana DeGette CO-1
Rosa DeLauro CT-3
Suzan DelBene WA-1
Mark DeSaulnier CA-11
Debbie Dingell MI-12
Lloyd Doggett TX-35
Tom Emmer MN-6
Anna Eshoo CA-18
Brian Fitzpatrick PA-1
Lizzie Fletcher TX-07
Bill Foster IL-11
Ruben Gallego AZ-7
John Garamendi CA-3
Josh Gottheimer NJ-5
Garret Graves LA-6
Raul Grijalva AZ-3
Glenn Grothman WI-6
Brett Guthrie KY-2
Kevin Hern OK-1
Jim Himes CT-4
Richard Hudson NC-08
Jared Huffman CA-2
Dusty Johnson SD-AL
Hank Johnson GA-4
David P. Joyce OH-14
Marcy Kaptur OH-9
Ro Khanna CA-17
Derek Kilmer WA-6
Andy Kim NJ-3
Raja Krishnamoorthi IL-8
Darin LaHood IL-18
John Larson CT-1
Bob Latta OH-5
Susie Lee NV-3
Teresa Leger Fernández NM-3
Debbie Lesko AZ-08
Mike Levin CA-49
Ted Lieu CA-33
Zoe Lofgren CA-19
Blaine Luetkemeyer MO-9
Stephen Lynch MA-8
Nancy Mace SC-1
Nicole Malliotakis NY-11
Kathy Manning NC-6
Brian Mast FL-18
Doris Matsui* CA-6
Michael McCaul TX-10
Jim McGovern MA-2
Cathy McMorris Rodgers WA-5
Grace Meng NY-6
Mariannette Miller-Meeks IA-2
Barry Moore AL-02
Seth Moulton MA-6
Richard Neal MA-1
Joe Neguse CO-2
Donald Norcross NJ-1
Ralph Norman SC-5
Eleanor Norton DC
Frank Pallone NJ-6
Jimmy Panetta CA-20
Chris Pappas NH-01
Bill Pascrell NJ-9
Donald Payne, Jr. NJ-10
Scott Peters CA-52
Dean Phillips MN-03
Chellie Pingree ME-1
Katie Porter CA-45
Bill Posey FL-8
Mike Quigley IL-5
Jamie Raskin MD-8
Mike Rogers AL-3
Deborah Ross NC-2
David Rouzer NC-7
C.A. Dutch Ruppersberger MD-2
John Rutherford FL-4
Maria Elvira Salazar FL-27
Mary Gay Scanlon PA-5
Jan Schakowsky IL-9
Brad Schneider IL-10
David Scott GA-13
Mikie Sherrill NJ-11
Mike Simpson ID-2
Elissa Slotkin MI-8
Chris Smith NJ-4
Adam Smith WA-9
Jason Smith MO-8
Lloyd Smucker PA-11
Darren Soto FL-9
Melanie Stansbury NM-1
Haley Stevens MI-11
Chris Stewart UT-2
Marilyn Strickland WA-10
Eric Swalwell CA-15
Glenn Thompson PA-15
Rashida Tlaib MI-13
Paul Tonko NY-20
Lori Trahan MA-3
David Trone MD-6
Jeff Van Drew NJ-2
Juan Vargas CA-51
Nydia Velazquez NY-7
Ann Wagner MO-2
Debbie Wasserman-Schultz FL-23
Bonnie Watson Coleman NJ-12
Bruce Westerman AR-4
Jennifer Wexton VA-10
Susan Wild PA-7
Joe Wilson SC-2
Robert Wittman VA-1
John Barrasso WY
John Boozman AR
Mike Braun IN
Maria Cantwell WA
Shelley Moore Capito WV
Christopher Coons DE
Tom Cotton AR
Steve Daines MT
Chuck Grassley IA
John Hoeven ND
Cindy Hyde-Smith MS
John Kennedy LA
Angus King ME
Amy Klobuchar* MN
Edward Markey MA
Jeff Merkley OR
Jerry Moran KS
Markwayne Mullin OK
Alex Padilla CA
Gary Peters MI
James Risch ID
Kyrsten Sinema AZ
Jeanne Shaheen NH
Tina Smith MN
Debbie Stabenow MI
Chris Van Hollen MD
Raphael Warnock GA
Roger Wicker* MS
166 Members
Democrats: 15
House: 138
Republicans: 12
Senate: 28
Independent: 1
You can find the most up-to-date list of Caucus Members on the website here: www.rarecaucus.org

• Start each meeting by thanking the Member/staffer for meeting with you.
• Share your personal story and explain why a specific issue is important to you. Explain the problem and how your “ask” can improve or solve it.
• Make a specific “ask”. Give Congress the solution.

• You don’t have to be an expert on legislation.
• If you are asked a question that you are not sure how to answer, write it down and be sure to follow up.
• Respect the time of the Member, staffer and fellow advocates by limiting your story to no more than a minute or two.
• Typical meetings will last 15 minutes in total.
• Email the Congressional staffer with a one-pager on your asks as well as your contact information.
• Remember rare disease issues are nonpartisan. Don’t talk about politics in your meetings.
• Report back to RDLA on how the meeting went by filling out the Online Meeting Feedback Form.
• Follow-up with a thank you note/ email reinforcing your asks.
TIP! Encourage your legislator to join the Rare Disease Caucus and cosponsor rare disease legislation to improve their score.



View your state specific scorecard:

On Twitter, Facebook, Instagram and LinkedIn the pound sign (#) turns any word or group of words that directly follow it into a searchable link. This allows you to organize content and track discussion topics based on those keywords. For instance, if you want to post about Rare Disease Week on Capitol Hill, you would include #RareDC2023 to join the conversation. You could then click the hashtag to see other posts on Rare Disease Week on Capitol Hill.
Many Congressional offices have Social Media accounts to keep in touch with constituents. If you know your legislator’s handle, you can mention them in your post about #RareDC2023 using the “@” symbol before the name. If you don’t know your legislator’s Social Media handle, check his or her official website.
Tip: Look for a blue checkmark on their Twitter/Instagram account to make sure their account’s identity has been verified.
Before your meeting:
Create a post tagging the Member’s office and the issue you will be talking about, for example:

“We are excited to meet with @dorismatsui for #RareDC2023 to talk about ways to bring more treatments to #Raredisease patients.”
This is a good way to introduce yourself and your issue to the staff. This will add a face to the upcoming meeting and will help them remember you.
During the meeting:
Ask to take a photo, preferably towards the end of the meeting. Write down any notes that might make for good tweets or quotes for your social post.

After the meeting:
Post your picture with a thank you note on Twitter, Facebook, Instagram or LinkedIn re-emphasizing the ask or any key points you discussed during the meeting, for example:
“Thank you @dorismatsui for joining the Rare Disease Congressional Caucus and supporting #RareDisease legislation! #RareDC2023”.
Share your Rare Disease Week experience and include how has RDW changed your life or if new to the event, why are you attending? Include #RareDC2023 for a chance to win a $100 Gift Card of your choice!

Tip: Be Creative! #RareDC2023
SOCIAL MEDIA LOUNGE
Stop by our social media lounge for expert tips and support to boost your impact as a rare disease advocate.
Bill Sponsor – A Representative or Senator who introduces a bill.
Bill Cosponsor – A Representative or Senator who formally signs on to support a bill. Only the first-named Member is the sponsor, all others are cosponsors, even those whose names appeared on the measure at the time it was submitted.
Bicameral bill – A bill that has been introduced in both the House and Senate.
Bipartisan bill – A bill that has at least one cosponsor from both parties.
Congressional Budget Office (CBO) – Agency within the legislative branch that produces independent analyses of budgetary and economic issues to support the Congressional process. Often calculates the cost or savings from enacting a specific bill. This is referred to as a “score”.
Committee – A panel with members from the House or Senate tasked with conducting hearings, examining and developing legislation, and conducting oversight.
The Senate and House have separate versions of each committee, but occasionally a joint committee is made of members from both chambers. The Energy and Commerce Committee, Ways and Means Committee, and Appropriations Committee in the House and Health, Education, Labor and Pensions Committee (HELP), Finance Committee, and Appropriations Committee in the Senate have most of the jurisdiction over healthcare issues.
Subcommittee – A subpanel of a committee with a more specific jurisdiction. For example, the House Energy and Commerce Committee has a Health Subcommittee.
Chair – The member of the majority party on a committee or subcommittee who has formal responsibility over the panel’s agenda and resources, presides at its meetings, and can, in some circumstances, act on the committee’s behalf.
Ranking Member – The most senior (though not necessarily the longest-serving) member of the minority party on a committee or subcommittee. The ranking member typically oversees minority committee staff and may coordinate involvement of the minority party members in committee activities.
Passed – When a bill is approved in one chamber by a majority vote.
Enacted – When a bill is passed by both chambers and signed into law by the President.
Hearing – A formal meeting of a congressional committee (or subcommittee) to gather information from witnesses for use in its activities.
Markup – Meeting by a committee or subcommittee during which committee members offer, debate, and vote on amendments to a bill or other measure.

Secretary Xavier Becerra
A cabinet-level department of the U.S. federal government with the goal of protecting the health of all Americans and providing essential human Services. This Department includes the below agencies, among others.
Lawrence A. Tabak, DDS, Ph.D. (performing the duties of the NIH Director)
The nation’s medical research agency, tasked with making discoveries that improve health and save lives.
Commissioner Robert Califf, MD
Responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.
Administrator Carole Johnson
The primary federal agency for improving access to health care services for people who are uninsured, isolated or medically vulnerable. This agency administers a number of newborn screening programs.
Director Rochelle Walensky, MD, MPH
Tasked with protecting the nation from health, safety and security threats, both foreign and in the U.S. Monitors reported disease and maintains information databases on prevalence, region, etc.
Administrator Chiquita Brooks-LaSure
Administers healthcare/ reimbursement programs including Medicare, Medicaid, and the Children’s Health Insurance Program (CHIP).


www.visitthecapitol.gov/plan-visit/visitors-disabilities www.wheelchairtravel.org/washington-dc/ www.washington.org/dc-information/washington-dc-disabilityinformation
Metro (Subway) – https://www.wmata.com/ service/accessibility/#main-content
• Alist of out-of-service elevators are available at the information booth of every metro station. You can also check the WMATA website or call 202-637-7000 for outages before leaving.
UberWAV – https://www.uber.com/us/en/ride/ uberwav/

Lyft Access – https://help.lyft.com/hc/en-us/ articles/115013081668-Accessible-vehicledispatch
Capitol Shuttle – For your convenience, the Capitol Visitor Center provides an on-demand shuttle service for individuals who use wheelchairs or who need mobility assistance. The shuttles run from the bus drop-off and pick-up areas on the West side of the Capitol to the Capitol Visitor Center entrance at the center of the Capitol’s East Plaza. They operate continuously, as needed, from 8:30am – 4:30pm, Monday –Saturday.

To enter the Capitol and the office buildings, all visitors are screened by a magnetometer and all personal items are screened by x-ray. Some items are prohibited from entering the Capitol such as liquid, including water. Capitol Police can make exceptions if a prohibited item is determined to be necessary for childcare, medical, or other special needs. For more information, go to: https://www.visitthecapitol.gov/visit/knowbefore-you-go/prohibited-items
• For questions on accessibility contact the Office of Congressional Accessibility Services (OCAS) on Capitol Hill https://www.aoc.gov/accessibility-services Phone: 202-224-4048
• House Office Buildings Accessible Entrances:
• Cannon House Office Building: Entrance on New Jersey Avenue, SE, south of the terrace at the intersection with Independence Avenue.
• Rayburn House Office Building: Main entrance, horseshoe drive off South Capitol Street.
• Longworth House Office Building: Main entrance, Independence, and New Jersey Avenues.
• Senate Office Buildings Accessible Entrances:
• Dirksen Senate Office Building: First Street and C Street entrance.
• Russell Senate Office Building: Delaware entrance on ground level closest to Constitution Avenue.
• Hart Senate Office Building: Second Street entrance.
Cannabis on Federal property:
• https://mpdc.dc.gov/marijuana
• https://www.acludc.org/en/know-your-rights/know-yourrights-marijuana-laws-district-columbia
Mobility Works – Service (877) 275 – 4912, Rentals (877) 275-4915
Accessible Vehicles – 1119 Taft Street, Rockville, MD (301) 838-9700
Scootaround – (888) 441-7575. Scooter and wheelchair rentals are available daily, weekly, or for longer periods of time. Take a tour of DC and the National Mall on a mobility scooter.
Bike and Roll – (202) 842-BIKE. Electric scooters and manual wheelchairs available. Two-hour, half-day, daily, and multi-day rentals.
Lenox Medical – (202) 387-1960. Provides short-term scooter, wheelchair, and knee walker rentals to tourists and residents.

• Longworth Cafeteria – B223—Basement level of Longworth House Office Building
• Rayburn Cafeteria – 2063—Ground level of Rayburn House Office Building
• Dirksen Cafeteria – SD-G26—Ground level of Dirksen Senate Office Building
• The Coffee Shop – SD-BR8—Basement level of Dirksen Senate Office Building
• Southside Buffet – SD-BR8—Basement level of Dirksen Senate Office Building
• Dirksen North Cafe – SD-BR7—Basement level of Dirksen Senate Office Building
Rooms with 3 digits:
The first digit in the number indicates the floor level of the room.
• Example: 231 Cannon House Office Building. The room is located on the 2nd floor
• Example: 104 Hart Senate Office Building. The room is located on the 1st floor
Rooms with 4 digits:
The first digit indicates the building. Rayburn and Longworth are the only building with 4-digit room numbers.
• 1 is the first number for all Longworth rooms.
• Example: 1365 Longworth House Office Building
• 2 is the first number for all Rayburn rooms.
• Example: 2145 Rayburn House Office Building
• The second digit in these room numbers indicates the appropriate floor level.
• Example: 1365 Longworth House Office Building is located on the 3rd floor
• Example: 2145 Rayburn House Office Building is located on the 1st floor
Public Wifi Network Access
House of Representatives Office Buildings: Network Name: HousePublic Password: HousePublic
Senate Office Buildings: Network Name: Senate_Guest Password: wethepeople




Horizon is proud to support Rare Disease Week on Capitol Hill 2023.
Horizon is a global biotechnology company focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our pipeline is purposeful: We apply scientific expertise and courage to bring clinically meaningful therapies to patients. We believe science and compassion must work together to transform lives. horizontherapeutics.com











REGENXBIO is committed to developing gene therapies that improve treatment options for people with serious diseases. The personal stories of patients and families help guide our work. We earn their trust through our actions and our words.


›
visit REGENXBIO.com

At Alexion, our mission is to transform the lives of people affected by rare diseases and devastating conditions through the development and delivery of innovative medicines, as well as through supportive technologies and healthcare services. We believe it is our responsibility to listen to, understand, and change the lives of patients and those who work tirelessly to help them. Our passion drives us to continuously innovate and create meaningful value in all we do. In doing so, we change lives for the better – ours, people affected by rare diseases and devastating conditions, and the communities we serve. Every day. alexion.com

We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives.

In Specialty Care, our mission is to help people with debilitating and complex conditions in rare diseases, rare blood disorders, neurology, oncology, and immunology. These conditions are often difficult to diagnose and treat. But we aren’t afraid of challenges. They just push us to work harder, to chase new potential therapies that help patients to live their lives.


















