
Presented by

FEBRUARY 25 - 28

#RAREDC 2024 @RAREADVOCATES
To Our Generous 2024 Rare Disease Week on Capitol Hill Sponsors
Table of Contents



Leadership

Ranking






This
Presidential Champion
3 Welcome Letter
4 Board of Directors
5-6 EveryLife Team
7-8 Schedule of Events
Sunday
9 Documentary Screening & Reception
Monday
10-13 Legislative Conference Agenda
Wednesday






Advocate
















14 Rare Disease DEIA Discussion & Congressional Caucus Briefing
15 Rare Artist Reception
16 Notes
Legislative Asks & Tips
17 Ask #1: Interagency Coordinating Committee on Rare Disease
18 Ask #2: Accelerating Kids’ Access to Care Act
19 Ask #3: Creating Hope Reauthorization Act
20 Ask #4: Safe Step Act
21 Ask #5: Ask your Member of Congress to Join the Caucus
22

Grassroots
handbook is generously underwritten by: Co-Sponsor Introduction
Rare Disease Congressional Caucus Members List
23 Congressional Meeting Tips 24 Social Media Advocacy Tips Glossary
Resources
Maps
Process
Accessibility Resources
Capitol Hill Resources
Capitol Hill Map
Metro Map
Reagan Building Map
Ads EVERY VOICE MATTERS #RAREDC 2024 1 2
25 Congressional Terms 26 Government Agencies
&
27 Legislative
28
29
30
31
32
33-42
Welcome
to Rare Disease Week on Capitol Hill hosted by the EveryLife Foundation for Rare Diseases.
Unified. Amplified. Stronger than ever.
This week marks the 15th anniversary of the EveryLife Foundation and the 13th year of Rare Disease Week on Capitol Hill.
15 years of impact. 15 years of landscape-changing policy. 15 years of ensuring the roar of the rare community is heard. 15 years of life-changing innovation. This progress is only possible thanks to your dedication and passion for rare disease advocacy.
And we are just getting started. Our community’s needs are greater than ever.
Together, we must ensure that the promise of today’s innovations and development pipelines are transformed into life-changing diagnostics and therapies that can be accessed by all who need them.
We are thrilled to welcome attendees from all 50 states, Washington, D.C., and Puerto Rico. We recognize and appreciate the sacrifice and effort required to join us in Washington this week. Your stories and influence as rare disease ambassadors hold immense importance this year. Rare diseases pose a significant public health crisis, with the cost reaching a staggering $1 trillion dollars in 2019. Prioritizing and accelerating access to treatments and timely diagnoses is crucial in saving lives and mitigating this crisis.
As we celebrate the 15th anniversary of the EveryLife Foundation for Rare Diseases, we reflect on the incredible progress made possible by your unwavering dedication and advocacy. Together, we form an extraordinary team united in our mission to drive positive change for the rare disease community, and we have so much important work ahead of us.
As we embark on this week of advocacy and collaboration, let us recognize the power of our collective efforts. On behalf of the EveryLife Team, our volunteers, and partners, we wish you an exceptional and impactful week in Washington, DC.



EveryLife Foundation for Rare Diseases Board of Directors
The EveryLife Foundation Board of Directors is comprised of a diverse group of individuals who are both personally and professionally dedicated to the development of treatment and diagnostic opportunities for the 30 million Americans living with a rare disease. With decades of experience in the government, nonprofit, finance, science, medicine, industry, and academic sectors, EveryLife board members provide valuable guidance to the Foundation toward achieving its mission. In addition, several of our board members are the parents of children with a rare disease, enabling them to offer firsthand knowledge of the challenges facing the rare disease community.




Jennifer



 Cristina Casanova Might, BS, MBA CEO, Welcomed Co. Patient and Parent Advocate
Merrill Friedman Regional Vice President, Inclusive Policy & Advocacy
Lisa Carlton, PhD Lisa Carlton Consulting Parent Advocate
Abbey Hauser Young Adult Rare Disease Patient Advocate
Frank Sasinowski, MS, MPH, JD - Vice Chair Director, Hyman, Phelps & McNamara P.C. Patient and Parent Advocate
Vicki Seyfert-Margolis, PhD Chair Founder and CEO, Respond Health Family Advocate
Bernstein Secretary Executive Vice President, Horizon Government Affairs Parent Advocate
Stephen C. Groft Treasurer Pharm.D
Cristina Casanova Might, BS, MBA CEO, Welcomed Co. Patient and Parent Advocate
Merrill Friedman Regional Vice President, Inclusive Policy & Advocacy
Lisa Carlton, PhD Lisa Carlton Consulting Parent Advocate
Abbey Hauser Young Adult Rare Disease Patient Advocate
Frank Sasinowski, MS, MPH, JD - Vice Chair Director, Hyman, Phelps & McNamara P.C. Patient and Parent Advocate
Vicki Seyfert-Margolis, PhD Chair Founder and CEO, Respond Health Family Advocate
Bernstein Secretary Executive Vice President, Horizon Government Affairs Parent Advocate
Stephen C. Groft Treasurer Pharm.D
EVERY VOICE MATTERS #RAREDC 2024 3 4
EveryLife Foundation for Rare Diseases Team
Communications & Marketing



Policy, Advocacy & Patient Engagement



Development



Operations











The EveryLife Foundation’s team works every day to improve the lives of the 30 million Americans living with one of 10,000 known rare diseases. We do this by educating and activating the patient community to ensure they are heard by policy makers in government and the healthcare industry.

 Ted Brasfield Vice President of Corporate Giving
Stephanie Siddiqi Associate Director of Development
Collin Sovie Alliance Development Manager
Brenda Colmenares Associate Director of Communications
Margo Metzger Senior Director of Marketing and Public Relations
Lindsey Cundiff Associate Director of Policy Programs
Shannon von Felden Senior Director of Advocacy
Courtney Felle Young Adult Rare Representatives Program Manager
Kendly Jones State Advocacy Manager
Chrissy Lanham Event Coordinator
Annie Kennedy Chief of Policy, Advocacy & Patient Engagement
Katelyn Laws Associate Director of RDLA
Baillie McGowan Associate Director of Policy & Research
Priscilla Rodriguez Diversity Inclusion Advocacy Manager
Dylan Simon Director of Policy
Emily Stauffer State Policy Manager Jamie Sullivan Vice President of Policy
Lina Arslanian Associate Director of Finance & Salesforce
Megan Pinegar Chief Operating Officer
Alyssa Terwall Senior Director of Operations & Events
Stephanie Riordan Director of Patient Programs
Ted Brasfield Vice President of Corporate Giving
Stephanie Siddiqi Associate Director of Development
Collin Sovie Alliance Development Manager
Brenda Colmenares Associate Director of Communications
Margo Metzger Senior Director of Marketing and Public Relations
Lindsey Cundiff Associate Director of Policy Programs
Shannon von Felden Senior Director of Advocacy
Courtney Felle Young Adult Rare Representatives Program Manager
Kendly Jones State Advocacy Manager
Chrissy Lanham Event Coordinator
Annie Kennedy Chief of Policy, Advocacy & Patient Engagement
Katelyn Laws Associate Director of RDLA
Baillie McGowan Associate Director of Policy & Research
Priscilla Rodriguez Diversity Inclusion Advocacy Manager
Dylan Simon Director of Policy
Emily Stauffer State Policy Manager Jamie Sullivan Vice President of Policy
Lina Arslanian Associate Director of Finance & Salesforce
Megan Pinegar Chief Operating Officer
Alyssa Terwall Senior Director of Operations & Events
Stephanie Riordan Director of Patient Programs
EVERY VOICE MATTERS #RAREDC 2024 5 6
Mariela Romero Executive Assistant
Thank you to the Presidential Sponsor of Rare Disease Week on Capitol Hill
Schedule of Events




SUN 25 MON
26
• Rare Disease Documentary Screening and Reception
5:15 p.m. - 8:30 p.m. EST
Ronald Reagan Building and International Trade Center
1300 Pennsylvania Ave, NW, Concourse Level, Washington DC, 20004
Metro Stop: Metro Center and Federal Triangle
• Legislative Conference


9:00 A.M. - 5:00 P.M. EST
Ronald Reagan Building and International Trade Center
Metro Stop: Metro Center and Federal Triangle
Young Adult Rare Representatives (YARR) Meetup
5:00 P.M. - 6:30 P.M. EST
Ronald Reagan Building and International Trade Center


Presented by:

Presented by:



1300 Pennsylvania Ave, NW, Concourse Level, Washington DC, 20004
Presented by:

TUE 27 WED 28
• Meetings with Members of Congress


10:00 a.m. - 5:00 p.m. EST
Capitol Hill, Washington DC, 20004
Metro Stop: Union Station (Senate side) or Capitol South (House side)
• Rare Disease Week 2024 DEIA Discussions


10:00 a.m. - 12:00 p.m. EST
Capitol Hill, Cannon House Office Building, Room 390
Metro Stop: Union Station (Senate side) or Capitol South (House side)
• Rare Disease Congressional Caucus Briefing


1:00 p.m. - 2:30 p.m. EST
Capitol Hill, Cannon House Office Building, Room 390
Metro Stop: Union Station (Senate side) or Capitol South (House side)
• Group Photo on Capitol Hill Steps
3:00 p.m. EST
Capitol Hill, Washington D.C 20004
East Steps
Energy Guide Key

Physical
Low: Little movement, ample seating available

Medium: Prolonged standing, some walking and some seating
Mental

Low: Optional participation, minimal to no participation

Medium: Might require participation, note taking or cognitive effort

High: Ample walking and standing, seating not guaranteed

High: Requires participation, note taking, and/or cognitive effort



Metro Stop: Union Station (Senate side) or Capitol South (House side)
• Rare Artist Reception
3:45 p.m. - 5:45 p.m. EST


Russell Senate Office Building, Kennedy Caucus Room (SR-325)
2 Constitution Ave NE, Washington, DC 20002
Metro Stop: Hill Center is two blocks east of the Eastern Market Metro stop on the Blue, Orange, and Silver lines.
Presented by:

Presented by:

Presented by:

Metro Stop: Metro Center and Federal Triangle EVERY VOICE MATTERS #RAREDC 2024 7 8
Rare Disease Week on Capitol Hill Every Voice Matters
Rare Disease
Documentary Screening
• Sunday, February 25
5:15 p.m. - 8:30 p.m. EST
Ronald Reagan Building and International Trade Center 1300 Pennsylvania Ave, NW, Concourse Level Washington DC, 20004
Chris Bombardier has never let severe hemophilia stop him from climbing some of the world’s tallest mountains. In 2017, Chris partnered with hemophiliac filmmaker Patrick James Lynch and his award-winning production team to film his journey through Nepal to summit the world’s tallest peak, Mount Everest. Bombardier Blood, highlights the physical, psychological and emotional struggle of acclimating to the mountain and preparing for the greatest challenge of Chris’ life.
• 5:15 p.m. - 6:30 p.m. EST: Reception
• 6:30 p.m. - 6:45 p.m. EST: Opening Remarks
• 6:45 p.m. - 8:05 p.m. EST: Documentary Screening
• 8:05 p.m. - 8:30 p.m. EST: Panel Discussion
Introduction Sponsor Remarks



Founder and CEO, Respond Health
Alison Clifford Director, Therapeutic Policy & Advocacy, Takeda Pharmaceuticals America, Inc.

Patrick James Lynch
Filmmaker and CEO, Believe Limited
Presented by:


Discussion Panel Featuring:

Chris Bombardier Executive Director, Save One Life

Jessica Bombardier
Scientist and Rare Disease Advocate
Legislative Conference Agenda
• Monday, February 26
Ronald Reagan Building and International Trade Center 1300 Pennsylvania Ave, NW, Concourse Level Washington DC, 20004
8:00 - 9:00 a.m. Registration and Breakfast
Atrium
9:00 - 9:45 a.m. Welcome
Atrium Hall and via Livestream
Annie Kennedy, EveryLife Foundation for Rare Diseases
Brett McReynolds, Amgen

Dylan Simon Director of Policy, EveryLife Foundation for Rare Diseases

Connie Montgomery
Patient and Family Advisor, PFCC Partners
Presented by:

Registration Presented by: Breakfast Presented by:


Frank Sasinowski, Hyman, Phelps, & McNamara and Vice Chair, EveryLife Foundation for Rare Diseases
Keynote
Abbey Hauser, Advocacy Chair of Rare Disease Week on Capitol Hill, EveryLife Foundation for Rare Diseases
Logistics Overview
Shannon von Felden, EveryLife Foundation for Rare Diseases
9:45 - 10:15 a.m. Legislative Outlook
Atrium Hall and via Livestream
Hear from experts on the 118th Congress and how Congress will impact healthcare policy in 2024.
Moderator: Ryan Shay, Faegre Drinker
Josh Trent, Leavitt Partners
Caitlin Van Sant, Mehlman Consulting
10:15 - 10:30 am Break

Family Space
Ballroom A is available for social distancing with livestream
Presented by:

Ronald Reagan Building, Oculus, Ground Level. The Family Space will be available for families all day except from 1:45-2:45, when families are required to attend the “Preparing for Successful Meetings” session. We also encourage families to eat in the Atrium with their state teams. Thank you to our Family Space sponsor, Biogen.


Watch Trailer
Vicki Seyfert-Margolis, PhD Chair, EveryLife Foundation for Rare Diseases
EVERY VOICE MATTERS #RAREDC 2024 9 10
Legislative Conference Agenda
• Monday, February 26 - Continued
10:30 - 11:30 a.m. Deep Dive Policy Ask #1:
Interagency Coordinating Committee on Rare Disease
Atrium Hall and via Livestream
Moderator: Annie Kennedy, EveryLife Foundation for Rare Diseases
Matthew Ellinwood, Ph.D., National MPS Society
Stephen Groft, PharmD, NIH/NCATS, EveryLife Foundation Board of Directors
Marsha Henderson, Foundation for Sarcoidosis Research
Nisha Quasba, Faegre Drinker
11:30 - 12:00 p.m. Deep Dive Policy Ask #2:
Accelerating Kids’ Access to Care Act
Atrium Hall and via Livestream
Moderator: Paloma Juarez, RDLA Advisory Committee
Nicholas Manetto, Faegre Drinker
Kyle Underwood, RDLA Advisory Committee
12:05 pm Group Photo
Atrium Stairs

12:15 - 1:15 pm Networking Lunch
Atrium
Lunch
Presented by:

1:15 - 1:45 pm Deep Dive Policy Ask #3: Pediatric Priority Review
Voucher (PRV) Reauthorization
Atrium Hall and via Livestream
Moderator: Jamie Sullivan, EveryLife Foundation for Rare Diseases
Chris Jones, Office of Congressman Gus Bilirakis
Melissa Kennedy, International Rett Syndrome Foundation
1:45 - 2:45 pm Preparing for Successful Meetings*
Atrium Hall, Ballroom A & B, and via Livestream
*Mandatory for advocates participating in Hill Day (family room will be closed at this time). Advocates receive information on their Hill meeting schedule and their meetings and strategize with their teams to make the most of their meetings on Capitol Hill.
Katelyn Laws, EveryLife Foundation for Rare Diseases
Accessibility and Inclusion
Closed captioning, Spanish translation materials and important event updates via text are possible thanks to our Accessibility and Inclusion sponsor, Travere Therapeutics.


Reagan WiFi Network Access
Legislative Conference Agenda
Network Name: Ronald Reagan Building
WiFi Username: RareDC2024
Password: RDLA
2:45 - 3:15 pm Snack Break
Atrium
3:15 - 4:00 p.m. Breakout Sessions
Track 1:
Deep Dive Policy Ask #4: Safe Step Act
Atrium Hall and via Livestream
Moderator: Nisha Trivedi, RDLA Advisory Committee
Sarah Buchanan, National Psoriasis Foundation
Dylan Simon, EveryLife Foundation for Rare Diseases
Anita Wilkerson, HAE advocate
Track 2:
Advocacy for Young Adults
Atrium Ballroom B
This session is for young adults (ages 16-30) to learn more about the Young Adult Rare Representatives (YARR) community legislative ask.
Moderator: Courtney Felle, EveryLife Foundation for Rare Diseases
Rachelle Cook, YARR Member
Sophie Melancon, YARR Member

YOUNG A DULTRAR E ESERPER N SEVITAT YARR YARR EVERY VOICE MATTERS #RAREDC 2024 11 12
Legislative Conference Agenda
• Monday, February 26 - Continued
4:05 - 4:50 p.m. Breakout Sessions
Track 1:
Emerging Policy Issues for the Rare Disease Community
Atrium Hall and via Livestream
This session will share updates and information on policy issues impacting rare disease including the PROTECT Rare Act and newborn screening and how rare disease advocates can become involved.
Introductions: Duane Clark, Sanofi
Moderator: Jennifer Bernstein, Horizon Government Affairs
Amy Gaviglio, MS, CGC, Genetics and Public Health Consultant
Sarah Gilbert, Office of Representative
Neal Dunn
Brian Fahey, Office of Representative
Brett Guthrie
Track 2:
Practice Your Pitch
Atrium Ballroom B
*This is a must-attend session for those individuals who are new to advocacy. Learn how to develop and make your legislative ask during your meetings. The session will offer new advocates an opportunity to practice making their ask and telling their story with other advocates. This is a great opportunity to get questions answered by experienced rare disease advocates.
Moderator: Stephanie Riordan, EveryLife Foundation for Rare Diseases
4:50 - 5:00 p.m. Closing Remarks
Atrium Hall and Atrium Ballroom B

*Sessions and speakers are subject to change.



Rare Disease DEIA Discussion & Congressional Caucus Briefing
• Wednesday, February 28
Rare Disease Week 2024 DEIA discussion
10:00 a.m. - 12:00 p.m. EST
Capitol Hill, Cannon House Office Building, Room 390
Session 1: Access & Utilization of Healthcare Services
Access to Genetic and Genomic Testing in Hispanic/Latinx Communities, Amanda de Leon, Providence St. Jude Medical Center
Prior Authorization and Other Utilization Management Practice Challenges in Rural Communities, Michael Ward, Alliance for Aging Research
The Intersectionality of LGBTQIA+ and Rare Disease, William Romero, Disability Advocate
Session 2: Cell and Gene Therapy Disparities:
Development, Approval and Access, Ashley Valentine, Sick Cells
• Wednesday, February 28
Rare Disease Congressional Caucus Briefing
1:00 p.m. - 2:30 p.m. EST
Capitol Hill, Cannon House Office Building, Room 390
The Rare Disease Community & Congress –Changing the Status Quo
Rare Disease Legislative Advocates and the Rare Disease Congressional Caucus invite you to a rare disease briefing.
Guest Panel
Moderator: Ryan Fischer, Foundation for Angelman Syndrome Therapeutics
Introductions: Mary-Lacey Reuther, Head of North America Policy, Advocacy Government Affairs for CSL Behring
Presented by:

Presented by:
Additional support from:


Panelists:
Brigid Brennan, Friedreich’s Ataxia Research Alliance
Billy Cline, Rare Disease Patient Community Representative
Brian Denger, Parent Project Muscular Dystrophy
EVERY VOICE MATTERS #RAREDC 2024 13 14

EVERY VOICE MATTERS #RAREDC 2024 15 16
Notes
Hill Day Issue Information
Legislative Ask #1
Support the Creation of an Interagency Coordinating Committee for Rare Diseases
Background
Agencies across the federal government have long implemented policies and programs to support rare disease patients and their families. The expansion of newborn screening to include rare diseases, the FDA’s Accelerating Rare Disease Cures Program, and the recently announced CMS Cell and Gene Therapy Access demonstration project are all examples of federal initiatives that focus on the rare disease community. Beyond specific initiatives like these, federal agencies provide services, make policies, and prioritize resources on a host of issues that are relevant to the health and quality of life for people living with rare diseases. Considering the expansive scope of federal initiatives and the substantial unmet needs within the rare disease community, it is imperative to establish a mechanism that fosters optimal coordination and collaboration and ensures resources are efficiently utilized.
Interagency Coordinating Committee for Rare Diseases
• Interagency committees elevate the awareness of the associated issues, provide forums for collaboration across agencies and even departments and ensure effective and efficient resource utilization. Interagency coordinating committees also provide invaluable opportunities to engage non-governmental stakeholders.
• HHS can use its authorities under the Federal Advisory Committee Act to establish an interagency coordinating committee for rare diseases. The committee should include leaders from agencies within HHS including the National Institutes of Health (NIH), especially the National Center for Advancing Translational Sciences (NCATS), the Food and Drug Administration (FDA) and relevant offices and centers, the Centers for Medicare & Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), the HHS office of the Secretary, and many more. Departments and agencies outside of HHS, such as the Department of Education, the Social Security Administration, the Department of Veterans Affairs, and the Department of Defense’s medical research function, serve vital roles that affect rare disease patients and should be included as well.
• An interagency coordinating committee must include a role for external rare disease stakeholders including rare disease patient community representatives, clinicians, researchers, and social service providers who can share their experiences and expertise.
• The Committee should meet periodically, undertake an assessment of rare disease initiatives and services that acutely impact Americans living with rare diseases, identify opportunities for improvements in existing efforts and community needs yet to be met in the existing efforts and provide regular reports with outcomes to assess progress.
Hill Day Issue Information

Legislative Ask #2
Cosponsor the Accelerating Kids’ Access to Care Act (AKACA), H.R. 4758 / S. 2372
Talking Points
✓ Many children live in states where specialized care is not available locally. Even some states with leading medical centers lack health providers with expertise in some rare conditions.
✓ Children with complex medical needs like cancer often must travel outside their states to receive treatment.
✓ More than half of children in the U.S. rely on Medicaid and the Children’s Health Insurance Program (CHIP) for their health insurance coverage. But bureaucratic rules in these programs often limit kids’ ability to get timely treatment outside their home state.
✓ This process needlessly results in delays in treatment that can cause a child’s condition to worsen.
Accelerating Kids’ Access to Care Act (AKACA)
• Enact H.R. 4758 / S. 2372 to allow pediatric providers to enroll more efficiently in multiple state Medicaid programs for a five-year period, positioning them to provide essential, time-sensitive care to children who need it.
• The Accelerating Kids’ Access to Care Act will ensure better, faster healthcare for kids who need it most by reducing the burden on the doctors who treat them.


Scan for Legislative Asks One Pagers Scan for Legislative Asks One Pagers
EVERY VOICE MATTERS #RAREDC 2024 17 18
Hill Day Issue Information

Legislative Ask #3 Cosponsor the Creating Hope Reauthorization Act (H.R. 7384)
Talking Points
✓ Developing drugs for rare pediatric diseases is challenging due to the small populations affected, difficulties associated with conducting clinical trials for children, delays in diagnosis and more.
✓ About 70% of rare diseases are exclusively pediatric onset and overall, 95% of rare diseases have no approved treatments.
✓ The Creating Hope Act expanded the Rare Pediatric Disease Priority Review Voucher (PRV) program to include drugs that treat rare pediatric diseases as part of the Food and Drug Administration Safety and Innovation Act (FDASIA) in 2012.
✓ To be eligible for a PRV, the treatment must obtain Rare Pediatric Designation from the FDA, be eligible for priority review, and it must be the first approval for the drug’s active ingredient.
✓ After an eligible treatment is approved by the FDA, the company is issued a PRV. The opportunity to obtain a PRV is an important incentive in pediatric rare disease therapy development because:
• Earlier Review: a company who gets a PRV can use it on a future treatment that wouldn’t otherwise qualify for priority review, leading to about a 4-month reduction in review time.
• Revenue Generation: some companies chose to sell the PRV to another company, generating revenue for the seller that is often used to continue and expand their rare disease research and development programs.
✓ The program was last reauthorized in 2020 for a 4-year period. Without Congressional action, companies will have to receive a Rare Pediatric Designation by September 30, 2024, to be eligible. After September 30, 2026, FDA will no longer be able to award Rare Pediatric PRVs.
The Creating Hope Reauthorization Act
• H.R. 7384 will reauthorize the Rare Pediatric Disease Priority Review Voucher (PRV) program for four years, ensuring this vital incentive that has no cost to taxpayers can continue to spur innovation in rare diseases that disproportionately affect children.

Scan
Hill Day Issue Information

Talking Points
✓ A medication step therapy protocol establishes a specific sequence in which a group health plan or a health insurance issuer covers prescription drugs.
✓ Step therapy protocols may require patients to try and fail an insurer preferred medication before being covered by the physician-prescribed medication.
✓ Many insurers have instituted this practice to help control the costs of expensive medications. However, while this practice may initially reduce insurer costs, it can have devastating health consequences for patients and ultimately lead to more expensive health care costs in the long run.
✓ Patients who are denied first coverage of medications recommended by their physicians can end up with poor health outcomes due to adverse health events, which can lead to costly hospitalizations.
Safe Step Act
• Amends the Employee Retirement Income Security Act of 1974 (ERISA) to require a group health plan to establish an exception process to medication step therapy protocol:
• When the treatment is contraindicated;
• Expected to be ineffective;
• Likely to cause an adverse reaction;
• Expected to decrease the individual’s ability to perform daily activities or occupational responsibilities; or
• The individual is stable based on the prescription drugs already selected.
• This legislation would require that requests be granted in a timely manner — within 72 hours after receipt of the request or 24 hours when the protocol jeopardizes the life or health of the individual.

Legislative Ask #4 Cosponsor and Advance the Safe Step Act (S. 652/H.R. 2630) Scan for Legislative Asks One Pagers
Legislative Asks One Pagers EVERY VOICE MATTERS #RAREDC 2024 19 20
for
Hill Day Issue Information

Legislative Ask #5
Talking Points
✓ The Rare Disease Caucus is a bipartisan, bicameral caucus that works to raise awareness of rare diseases.
✓ Rare diseases affect more than 30 million Americans and their families.
✓ More than 95% of the estimated 10,000 rare diseases do not have an FDA approved treatment.
✓ Rare or orphan diseases are defined as diseases affecting fewer than 200,000 people in the U.S.
✓ Rare diseases include rare cancers, tropical or neglected diseases, genetic diseases and many pediatric diseases including cancers. Many of these diseases are life-threatening and have no treatment options.
✓ The Rare Disease Congressional Caucus helps bring public and Congressional awareness to the unique needs of the rare disease community (including patients, physicians, scientists, and industry), and creates opportunities to address barriers to the development of and access to life-altering treatments.
✓ The Caucus gives a permanent voice to the rare disease community.

Co-Sponsors



Rare Disease Congressional Caucus Member List
House
• Mark Amodei NV-2
• Jake Auchincloss MA-4
• Don Bacon, NE-2
• Andy Barr KY-6
• Joyce Beatty OH-3
• Ami Bera CA-7
• Donald Beyer, Jr. VA-8
• Gus Bilirakis* FL-12
• Sanford Bishop, Jr. GA-2
• Lisa Blunt Rochester DE
• Suzanne Bonamici OR-1
• Julia Brownley CA-26
• Vern Buchanan FL-16
• Michael Burgess TX-26
• Ken Calvert CA-42
• Salud Carbajal CA-24
• Tony Cardenes CA-29
• Andre Carson IN-7
• John Carter TX-31
• Sean Casten IL-6
• Kathy Castor FL-14
• Yadira Caraveo, M.D CO-8
• Judy Chu CA-27
• Emanuel Cleaver, II MO-5
• Ben Cline VA-6
• Steve Cohen TN-9
• Jenniffer Gonzalez-Colon PR-AL
• James Comer KY-1
• Gerald Connolly VA-11
• Jason Crow CO-6
• Sharice Davids KS-3
• Diana DeGette CO-1
• Rosa DeLauro CT-3
• Suzan DelBene WA-1
• Mark DeSaulnier CA-10
• Debbie Dingell MI-12
• Lloyd Doggett TX-37
• Tom Emmer MN-6
• Anna Eshoo CA-18
• Brian Fitzpatrick PA-8
• Lizzie Fletcher TX-7
• Bill Foster IL-11
• Ruben Gallego AZ-7


• John Garamendi CA-3
• Josh Gottheimer N-J5
• Garret Graves LA-6
• Raul Grijalva AZ-3
• Glenn Grothman WI-6
• Brett Guthrie KY-2
• Kevin Hern OK-1
• Jim Himes CT-4
• Richard Hudson NC-8
• Jared Huffman CA-2
• Hank Johnson GA-4
• Dusty Johnson SD-AL
• David P. Joyce OH-14
• Marcy Kaptur OH-9
• Tom Kean NJ-7
• Ro Khanna CA-17
• Derek Kilmer WA-6
• Andy Kim NJ-3
• Raja Krishnamoorthi IL-8
• Darin LaHood IL-18
• John Larson CT-1
• Bob Latta OH-5
• Susie Lee NV-3
• Teresa Leger Fernandez NM-3
• Debbie Lesko AZ-8
• Mike Levin CA-49
• Ted Lieu CA-33
• Zoe Lofgren CA-19
• Blaine Luetkemeyer MO-3
• Stephen Lynch MA-8
• Nancy Mace SC-1
• Nicole Malliotakis NY-11
• Kathy Manning NC-6
• Brian Mast FL-18
• Doris Matsui* CA-6
• Michael McCaul TX-10
• Jim McGovern MA-2
• Cathy McMorris Rodgers WA-5
• Grace Meng NY-6
• Mariannette Miller-Meeks IA-2
• Barry Moore AL-2
• Seth Moulton MA-6
• Kevin Mullin CA-15
• Richard Neal MA-1
• Joe Neguse CO-2
• Donald Norcross NJ-1
• Ralph Norman SC-5
• Eleanor Holmes Norton DC
• Frank Pallone NJ-6
• Jimmy Panetta CA-20
• Chris Pappas NH-1
• Bill Pascrell NJ-9
• Donald Payne, Jr. NJ-10
• Scott Peters CA-52
• Dean Phillips MN-3
• Chellie Pingree ME-1
• Katie Porter CA-45
• Bill Posey FL-8
• Mike Quigley IL-5
• Jamie Raskin MD-8
• Deborah Ross NC-2
• David Rouzer NC-7
• C.A. Dutch Ruppersberger MD-2
• John Rutherford FL-4
• Maria Elvira Salazar FL-27
• Mary Gay Scanlon PA-5
• Jan Schakowsky IL-9
• Brad Schneider IL-10
• David Scott GA13
• Mikie Sherrill NJ-11
• Mike Simpson ID-2
• Elissa Slotkin MI-8
• Adam Smith WA-9
• Chris Smith NJ-4
• Jason Smith MO-8
• Lloyd Smucker PA-16
• Darren Soto FL-9
• Abigail Spanberger VA-7
• Melanie Stansbury NM-1
• Haley Stevens MI-11
• Marilyn Strickland WA-10
• Eric Swalwell CA-15
• Glenn Thompson PA-5
• Rashida Tlaib MI-13
• Paul Tonko NY-20
• Lori Trahan MA-3
• Jeff Van Drew NJ-2
• Juan Vargas CA-51
Caucus: 171 Members
• Nydia Velazquez NY-7
• Ann Wagner MO-2
• Debbie WassermanSchultz FL-23
• Bonnie Watson Coleman NJ-12
• Bruce Westerman AR-4
• Jennifer Wexton VA-10
• Susan Wild PA-7
• Joe Wilson SC-2
• Robert Wittman VA-1
Senate
• John Barrasso WY
• John Boozman AR
• Mike Braun IN
• Maria Cantwell WA
• Shelley Moore Capito WV
• Christopher Coons DE
• Tom Cotton AR
• Steve Daines MT
• Charles Grassley IA
• Cindy Hyde-Smith MS
• James Inhofe OK
• John Kennedy LA
• Angus King ME
• Amy Klobuchar* MN
• Edward Markey MA
• Jeff Merkley OR
• Jerry Moran KS
• Markwayne Mullin OK
• Alex Padilla CA
• Gary Peters MI
• James Risch ID
• Kyrsten Sinema AZ
• Jeanne Shaheen NH
• Tina Smith MN
• Debbie Stabenow MI
• Chris Van Hollen MD
• Raphael Warnock GA
• Roger Wicker* MS
House: 144 Members Senate: 27 Members
Caucus Co-Chairs*: Sen. Amy Klobuchar (MN), Sen. Roger Wicker (MS), Rep. Gus Bilirakis (FL), and Rep. Doris Matsui (CA)
Sen. Amy Klobuchar (MN) Sen. Roger Wicker (MS)
Rep. Gus Bilirakis (FL) Rep. Doris Matsui (CA)
Scan for Legislative Asks One Pagers
EVERY VOICE MATTERS #RAREDC 2024 21 22
for up to date Caucus members
Scan
Ask
Your Members of Congress to Join the Rare Disease Congressional Caucus
Congressional Meeting Tips
• Start each meeting by thanking the Member/staffer for meeting with you.
• Share your personal story and explain why a specific issue is important to you. Explain the problem and how your “ask” can improve or solve it.
• Make a specific “ask.” Give Congress the solution.
• You don’t have to be an expert on legislation.
• If you are asked a question that you are not sure how to answer, write it down and be sure to follow up.
• Respect the time of the Member, staffer and fellow advocates by limiting your story to no more than a minute or two.
• Typical meetings will last 15 minutes in total.
• Email the Congressional staffer with a one-pager on your asks as well as your contact information.
• Remember rare disease issues are nonpartisan. Don’t talk about partisan politics in your meetings.
• Report back to RDLA on how the meeting went by filling out the Online Meeting Feedback Form.
• Follow-up with a thank you note/email reinforcing your asks.

RDLA Congressional Scorecard
Tip: Encourage your legislator to join the Rare Disease Caucus and cosponsor rare disease legislation to improve their score. Scan to view your state specific scorecard.



Social Media Advocacy Tips
• How does a “#” work?
On Facebook, Instagram, LinkedIn, and X (formerly Twitter) the pound sign (#) turns any word or group of words that directly follow it into a searchable link. This allows you to organize content and track discussion topics based on those keywords. For instance, if you want to post about Rare Disease Week on Capitol Hill, you would include #RareDC2024 to join the conversation. You could then click the hashtag to see other posts on Rare Disease Week on Capitol Hill.
• How do I ‘mention’ someone on Twitter, Facebook or Instagram? “@”
Many Congressional offices have social media accounts to keep in touch with constituents. If you know your legislator’s handle, you can mention them in your post about #RareDC2024 using the “@” symbol before the name. If you don’t know your legislator’s social media handle, check his or her official website.

Tip: Look for a blue checkmark on the Instagram or X account to make sure the account’s identity has been verified.
• Before your meeting:
Create a post tagging the Member’s office and the issue you will be talking about. Example post shown at right. This is a good way to introduce yourself and your issue to the staff. This will add a face to the upcoming meeting and will help them remember you.
• During the meeting:
Ask to take a photo, preferably towards the end of the meeting. Write down any notes that might make for good tweets or quotes for your social post.
• After the meeting:
Post your picture with a thank you note on Facebook, Instagram, LinkedIn, or X to re-emphasize the ask or any key points you discussed during the meeting. Example post shown at right.
Share Your #RareDC2024 Experience
Follow us and share your Rare Disease Week experience for a chance to win a $100 Gift Card of your choice.
Tip: Be Creative!
@RareAdvocates #RareDC2024

“We are excited to meet with @RepGusBilirakis for #RareDC2024 to talk about ways to bring more treatments to #RareDisease patients.”
Thank you @RepGusBilirakis for joining the Rare Disease Congressional Caucus and supporting #RareDisease legislation! #RareDC2024.
EVERY VOICE MATTERS #RAREDC 2024 23 24
Glossary Congressional Terms
Scan to access full glossary

• Act: A bill that has passed both house of congress and has been enacted into a law.
• Appropriation: The allocation of funds for a specific purpose within government. Allows for funds to be spent but is not an actual expenditure.
• Bill Sponsor: A Representative or Senator who introduces a bill.
• Bill Cosponsor: Representative or Senator who formally signs on to support a bill. Only the first-named Member is the sponsor. All others are cosponsors, even those whose names appeared on the measure at the time it was submitted.
• Bicameral Bill: A bill that has been introduced in both the House and Senate.
• Bipartisan Bill: A bill that has at least one cosponsor from both parties.
• Congressional Budget Office (CBO): Agency within the legislative branch that produces independent analyses of budgetary and economic issues to support the Congressional process. Often calculates the cost or savings from enacting a specific bill. This is referred to as a “score”.
• Committee: A panel with members from the House or Senate tasked with conducting hearings, examining and developing legislation, and conducting oversight. The Senate and House have separate versions of each committee, but occasionally a joint committee is made of members from both chambers.
• Subcommittee: A subpanel of a committee with a more specific jurisdiction. For example, the House Energy and Commerce Committee has a Health Subcommittee.
• Chair: The member of the majority party on a committee or subcommittee who has formal responsibility over the panel’s agenda and resources, presides at its meetings, and can, in some circumstances, act on the committee’s behalf.
• Caucus: An informal meeting of members of a body of government (typically belonging to the same political party and/or another common interest such as the Rare Disease Congressional Caucus).
• Enacted: When a bill is passed by both chambers and signed into law by the President.
• Legislator: An elected official of a legislative body.
• Lobbyist: A person who attempts to influence legislation on behalf of a specific interest group.
• Markup: Meeting by a committee or subcommittee during which committee members offer, debate, and vote on amendments to a bill or other measure.
• Nonpartisan: Not associated with a single political party or caucus.
• Partisan: Associated with a single political party or caucus.
Glossary Government Agencies
• Department of Health and Human Services (HHS)
A cabinet-level department of the U.S. federal government with the goal of protecting the health of all Americans and providing essential human services. This Department includes the below agencies, among others (12 total).
• Centers for Disease Control and Prevention (CDC)
Tasked with protecting the nation from health, safety and security threats, both foreign and domestic. Monitors reported disease and maintains information databases on prevalence, region, and other factors.
• Centers for Medicare and Medicaid Services (CMS)
Administers healthcare/reimbursement programs including Medicare, Medicaid, and the Children’s Health Insurance Program (CHIP).
• Food and Drug Administration (FDA)
Responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.
• Health Resources and Services Administration (HRSA)
The primary federal agency for improving access to health care services for people who are uninsured, isolated or medically vulnerable. This agency administers several newborn screening programs.
• National Institutes of Health (NIH)
The nation’s medical research agency tasked with making discoveries that improve health and save lives. Comprised of 27 institutes and centers.



EVERY VOICE MATTERS #RAREDC 2024 25 26
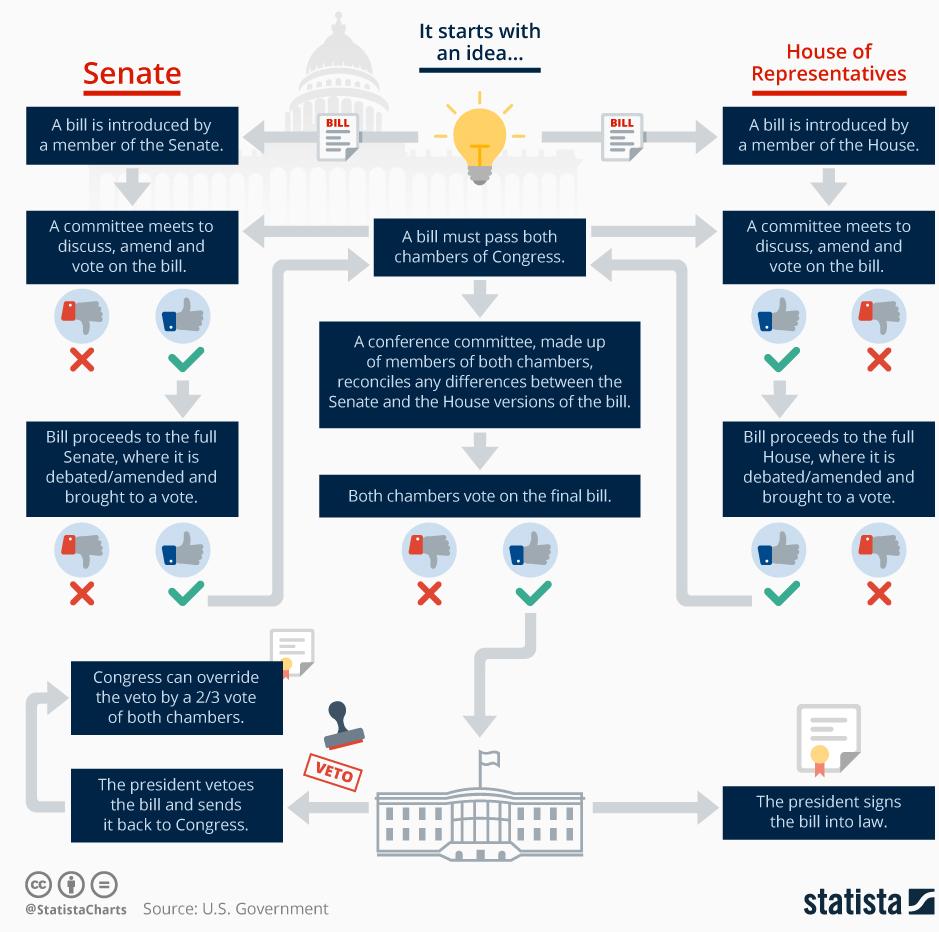
U.S. Legislative Process
Accessibility Resources
General Travel Links
• Visit the Capitol
www.visitthecapitol.gov/plan-visit/visitors-disabilities
• Wheelchair Travel
www.wheelchairtravel.org/washington-dc/
• Washington.org
www.washington.org/dc-information/washington-dcdisability-information
Capitol Shuttle
For your convenience, the Capitol Visitor Center provides an on-demand shuttle service for individuals who use wheelchairs or who need mobility assistance. The shuttles run from the bus drop-off and pick-up areas on the West side of the Capitol to the Capitol Visitor Center entrance at the center of the Capitol’s East Plaza. Shuttles operate continuously, as needed, from 8:30am – 4:30pm, Monday – Saturday.
Transportation Options in DC
• Metro (Subway)
https://www.wmata.com/service/accessibility/ #main-content
A list of out-of-service elevators are available at the information booth of every metro station. Check the WMATA website or call 202-637-7000 for outages before leaving.
• UberWAV
https://www.uber.com/us/en/ride/uberwav/
• Lyft Access
https://help.lyft.com/hc/en-us/articles/ 115013081668-Accessible-vehicle-dispatch
Medications
• Cannabis on Federal Property
https://mpdc.dc.gov/marijuana

https://www.acludc.org/en/know-your-rights/know-yourrights-marijuana-laws-district-columbia
Wheelchair Accessible Van Rentals and Sales
• Mobility Works – Service (877) 275-4912 Rentals (877) 275-4915
• Accessible Vehicles – (301) 838-9700 119 Taft Street, Rockville, MD
For easy access to links scan here

Scooters and Wheelchair Rentals
• Scootaround – (888) 441-7575
Scooter and wheelchair rentals are available daily, weekly, or for longer periods of time. Take a tour of DC and the National Mall on a mobility scooter.
• Bike and Roll – (202) 842-BIKE
Electric scooters and manual wheelchairs available. Two-hour, half-day, daily, and multi-day rentals.
• Lenox Medical – (202) 387-1960
Provides short-term scooter, wheelchair, and knee walker rentals to tourists and residents
Rare Disease Week Event Buildings, Entrances and Services on Capitol Hill
To enter the Capitol and the office buildings, all visitors are screened by a magnetometer and all personal items are screened by x-ray. Some items are prohibited from entering the Capitol such as liquid, including water. Capitol Police can make exceptions if a prohibited item is determined to be necessary for childcare, medical, or other special needs.
• For more information, go to:
https://www.visitthecapitol.gov/visit/know-before-yougo/prohibited-items
• For questions on accessibility contact the Office of Congressional Accessibility Services (OCAS) on Capitol Hill – (202) 224-4048
https://www.aoc.gov/accessibility-services
House Office Buildings Accessible Entrances:
• Cannon House Office Building: Entrance on New Jersey Avenue, SE, south of the terrace at the intersection with Independence Avenue.
• Rayburn House Office Building: Main entrance, horseshoe drive off South Capitol Street.
• Longworth House Office Building: Main entrance, Independence, and New Jersey Avenues.
Senate Office Buildings Accessible Entrances
• Dirksen Senate Office Building: First Street and C Street entrance.
• Russell Senate Office Building: Delaware entrance on ground level closest to Constitution Avenue.
• Hart Senate Office Building: Second Street entrance.
Resources Section
Presented by:

EVERY VOICE MATTERS #RAREDC 2024 27 28
Capitol Hill Resources
Food Service Options on Capitol Hill buildings
• Longworth Cafeteria - B223
Basement level of Longworth House Office Bldg.
• Rayburn Cafeteria - 2063
Ground level of Rayburn House Office Bldg.
• Dirksen Cafeteria - SD-G26
Ground level of Dirksen Senate Office Bldg.
Understanding Room Numbers in the House and Senate Office
• Rooms with three digits:
The first digit in the number indicates the floor level of the room.
Example: 231 Cannon House Office Building.
The room is located on the 2nd floor
Example: 104 Hart Senate Office Building.
The room is located on the 1st floor
• Rooms with four digits:
The first digit indicates the building. Rayburn and Longworth are the only building with four-digit room numbers.
1 is the first number for all Longworth rooms.
Example: 1365 Longworth House Office Building
2 is the first number for all Rayburn rooms.
Example: 2145 Rayburn House Office Building
The second digit in these room numbers indicates the appropriate floor level.
Example: 1365 Longworth House Office Room is located on the 3rd floor
Example: 2145 Rayburn House Office Room is located on the 1st floor
Resources Section
Presented by:

• The Coffee Shop - SD-BR8
Basement level of Dirksen Senate Office Bldg.
• Southside Buffet - SD-BR8
Basement level of Dirksen Senate Office Bldg.
• Dirksen North Cafe - SD-BR7
Basement level of Dirksen Senate Office Bldg.



House of Representatives
Office Buildings
Network Name: HousePublic
Password: HousePublic
Senate Office Buildings:
Network Name: Senate_Guest
Password: 118congress






U.S. Capitol
Map
Public WiFi Network Access
Accessible Rout e MassachusettsAvenue Visitor Services Shut tle Bus Pick-U p Bus Dr op-O ff Circulator Bus Stop Madison Drive NW F our th S treet , NW F our th S treet , SW Jeffer son Drive SW Accessible Entrance Legend CVC 19-004
EVERY VOICE MATTERS #RAREDC 2024 29 30
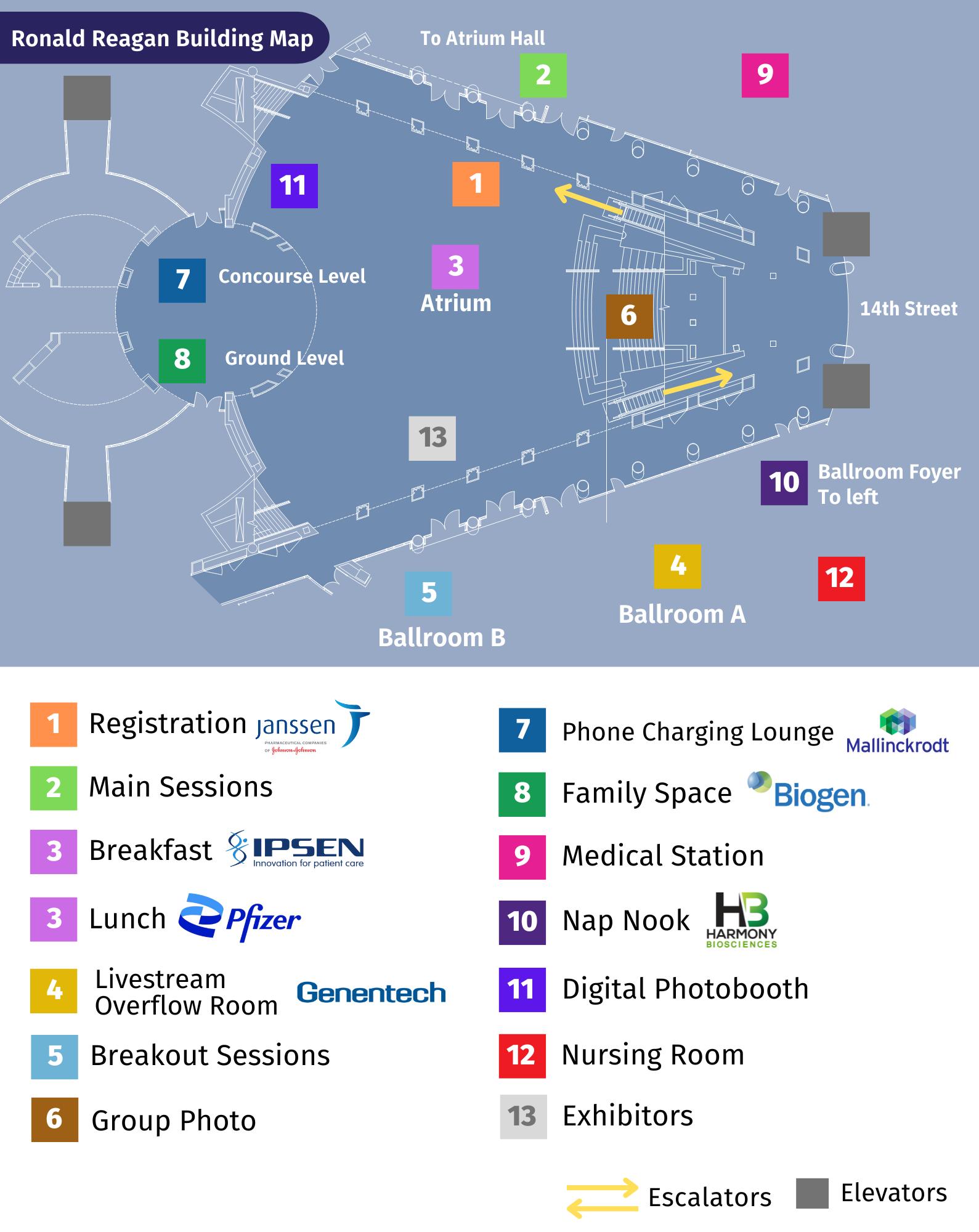
Metro System Map Ronald Reagan Building Map PotomacRiver AnacostiaRiver FairfaxCoArlingtonCo LoudounCoFairfaxCo MontgomeryCoPrinceGeorge’sCo DistrictPrinceGeorge’sCo ofColumbia Districtof Columbia PrinceGeorge’sCo FairfaxArlingtonCo Co Alexandria Fairfax Co Alexandria MontgomeryCo Districtof Columbia No Dangerous or Flammable Items No Littering or Spitting No Audio (without earphones) No Animals (except service animals) No Smoking No Eating or Drinking Legend wmata.com Information: 202-637-7000 TTY: 202-962-2033 Metro Transit Police: 202-962-2121 Text: MYMTPD (696873) System Map Station in Service TransferStation Silver Line • Ashburn Downtown Largo Yellow Line • Huntington / Greenbelt Green Line • Branch Ave / Greenbelt Blue Line • Franconia-Springfield Downtown Largo Orange Line • New Carrollton / Vienna Red Line • Glenmont Shady Grove Connecting Rail Systems Station Features Airport Parking Hospital Future Station — Map is not to scale Metro is accessible. WestFallsChurchVT Shaw-HowardU Glenmont Wheaton Forest Glen Silver Spring Waterfront NavyYard-Ballpark Anacostia CongressHeightsSouthernAveNaylorRdSuitland EasternMarketFederalCapitolSouth CenterSW JudiciarySq Arlington Cemetery Pentagon Pentagon City CrystalCity FarragutWest MetroCenter FederalTriangle Smithsonian Archives NavyMem’l-PennQuarter MtVernonSq7thSt-ConventionCenter GalleryPlace Chinatown CourtHouse Ronald Reagan Washington National Airport Braddock Rd Potomac Yard (Future Station) VT King St-Old Town Eisenhower Ave Huntington Columbia Heights Tenleytown-AUVanNess-UDC GeorgiaAve-Petworth VanDornSt Franconia-Springfield PotomacStadium-Armory Ave Brookland-CUA Union Station NoMa-Gallaudet U Greenbelt College Park-U of Md Hyattsville Crossing West Hyattsville Fort Totten Rhode Island Ave Brentwood U St African-Amer Civil War Mem’l/Cardozo EastFallsChurch Vienna Fairfax-GMUDunnLoringMerrifield Ballston-MUVirginiaSq-GMUClarendon MorganBlvdBenningCapitolHeights Rd SeatAddisonRd Pleasant Landover New Carrollton Cheverly Deanwood Minnesota Ave SpringHill Wiehle-RestonEastGreensboroTysonsMcLean Friendship Heights North Bethesda Twinbrook Rockville Shady Grove Cleveland Park Zoo/Adams Morgan Woodley Park Bethesda Medical Center G osveno - Strathmore Takoma Rosslyn L’EnfantPlaza Foggy Bottom-GWU McPherson Sq DowntownLargo Dupont Circle Farragut North BranchAve KennedyCenter NationalMall WashingtonHerndonRestonTownCenter Dulles InternationalAirport LoudounInnovationCenter Gateway Ashburn EVERY VOICE MATTERS #RAREDC 2024 31 32




EVERY VOICE MATTERS #RAREDC 2024 33 34




EVERY VOICE MATTERS #RAREDC 2024 35 36

Notes
Our Mission
We empower the rare disease patient community to advocate for impactful, science-driven legislation and policy that advances the equitable development of and access to lifesaving diagnoses, treatments, and cures.
We Must Act Now


More than 30 million Americans live with one or more rare diseases.
The economic impact of 379 rare diseases reached nearly $1 trillion in the U.S. in 2019.

95% of the more than 10,000 known rare diseases have no FDA-approved treatment.

Rare disease patients wait an average of 6.3 years and 17 healthcare encounters before receiving a rare disease diagnosis.

It takes an average of 15 years for a drug to be developed and approved by the FDA.
Delays in receiving a diagnosis result in an average of $220,000 of avoidable costs per person.


everylifefoundation.org @EveryLifeOrg Check out our latest work and stay tuned for three new studies Coming Soon! EveryLife Foundation for Rare Diseases is committed to producing studies and evidence that informs policymakers and advances our mission.
EVERY VOICE MATTERS #RAREDC 2024 37 38


We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives.
In Specialty Care, our mission is to help people with debilitating and complex conditions in rare diseases, rare blood disorders, neurology, oncology, and immunology. These conditions are often difficult to diagnose and treat. But we aren’t afraid of challenges. They just push us to work harder, to chase new potential therapies that help patients to live their lives.
 MAT-US-2204557-v.1.0-06/2022
Sanofi speciality Care_RareDisease - US _8x4.93.indd 1
1/25/2024
MAT-US-2204557-v.1.0-06/2022
Sanofi speciality Care_RareDisease - US _8x4.93.indd 1
1/25/2024
EVERY VOICE MATTERS #RAREDC 2024 39 40
10:29:53 AM


At Travere
Therapeutics,
we are in rare for life.
Transforming Lives. Every Day.

At Alexion, our mission is to transform the lives of people affected by rare diseases and devastating conditions through the development and delivery of innovative medicines, as well as through supportive technologies and healthcare services. alexion.com
We come together every day to help patients, families, and caregivers of all backgrounds as they navigate life with a rare disease. On this path, we know the need for treatment options is urgent - that is why our global team works with the rare disease community to identify, develop, and deliver life-changing therapies. travere.com


ALL NEW UNTOLD STORIES!
The compelling podcast series, Untold Stories: Life with a Severe Autoimmune Condition, is back with a second season! Previously highlighting the experiences of real people living with MG, this season is expanding to share the stories of people living with other rare autoimmune conditions, such as CIDP. Host Martine Hackett continues to speak with a variety of guests, as they share their trials, tribulations, and triumphs.
MG=myasthenia gravis
�CIDP=chronic inflammatory demyelinating polyneuropathy LISTEN NOW

Host Martine Hackett Associate Professor, Director of Public Health Programs Hofstra University

Tip: Be Creative! @RareAdvocates #RareDC2024
We are proud to support Rare Disease Week on Capitol Hill 2024
@TravereRare #lnRareForlife For U.S. audiences only. ©2024 argenx US-NON-24-00051 V1 01/2024
DONNAN WITH HIS FAMILY LIVING WITH a HUS
EVERY VOICE MATTERS #RAREDC 2024 41 42
share
Follow us and
your Rare Disease Week experience for a chance to win a $100 Gift Card of your choice.
Share
#RareDC2024 Experience
Your







everylifefoundation.org Every Voice Matters #RAREDC 2024 @RAREADVOCATES @EveryLifeOrg POWERED BY THE EVERYLIFE FOUNDATION 1012 14th Street NW, Suite 500, Washington, D.C. 20005 Office: (202) 697-RARE(7273) rareadvocates.org The EveryLife Foundation for Rare Diseases is a 501(c)(3) nonprofit, nonpartisan organization dedicated to empowering the rare disease patient community to advocate for impactful, science-driven legislation and policy that advances the equitable development of and access to lifesaving diagnoses, treatments and cures.











































 Cristina Casanova Might, BS, MBA CEO, Welcomed Co. Patient and Parent Advocate
Merrill Friedman Regional Vice President, Inclusive Policy & Advocacy
Lisa Carlton, PhD Lisa Carlton Consulting Parent Advocate
Abbey Hauser Young Adult Rare Disease Patient Advocate
Frank Sasinowski, MS, MPH, JD - Vice Chair Director, Hyman, Phelps & McNamara P.C. Patient and Parent Advocate
Vicki Seyfert-Margolis, PhD Chair Founder and CEO, Respond Health Family Advocate
Bernstein Secretary Executive Vice President, Horizon Government Affairs Parent Advocate
Stephen C. Groft Treasurer Pharm.D
Cristina Casanova Might, BS, MBA CEO, Welcomed Co. Patient and Parent Advocate
Merrill Friedman Regional Vice President, Inclusive Policy & Advocacy
Lisa Carlton, PhD Lisa Carlton Consulting Parent Advocate
Abbey Hauser Young Adult Rare Disease Patient Advocate
Frank Sasinowski, MS, MPH, JD - Vice Chair Director, Hyman, Phelps & McNamara P.C. Patient and Parent Advocate
Vicki Seyfert-Margolis, PhD Chair Founder and CEO, Respond Health Family Advocate
Bernstein Secretary Executive Vice President, Horizon Government Affairs Parent Advocate
Stephen C. Groft Treasurer Pharm.D





















 Ted Brasfield Vice President of Corporate Giving
Stephanie Siddiqi Associate Director of Development
Collin Sovie Alliance Development Manager
Brenda Colmenares Associate Director of Communications
Margo Metzger Senior Director of Marketing and Public Relations
Lindsey Cundiff Associate Director of Policy Programs
Shannon von Felden Senior Director of Advocacy
Courtney Felle Young Adult Rare Representatives Program Manager
Kendly Jones State Advocacy Manager
Chrissy Lanham Event Coordinator
Annie Kennedy Chief of Policy, Advocacy & Patient Engagement
Katelyn Laws Associate Director of RDLA
Baillie McGowan Associate Director of Policy & Research
Priscilla Rodriguez Diversity Inclusion Advocacy Manager
Dylan Simon Director of Policy
Emily Stauffer State Policy Manager Jamie Sullivan Vice President of Policy
Lina Arslanian Associate Director of Finance & Salesforce
Megan Pinegar Chief Operating Officer
Alyssa Terwall Senior Director of Operations & Events
Stephanie Riordan Director of Patient Programs
Ted Brasfield Vice President of Corporate Giving
Stephanie Siddiqi Associate Director of Development
Collin Sovie Alliance Development Manager
Brenda Colmenares Associate Director of Communications
Margo Metzger Senior Director of Marketing and Public Relations
Lindsey Cundiff Associate Director of Policy Programs
Shannon von Felden Senior Director of Advocacy
Courtney Felle Young Adult Rare Representatives Program Manager
Kendly Jones State Advocacy Manager
Chrissy Lanham Event Coordinator
Annie Kennedy Chief of Policy, Advocacy & Patient Engagement
Katelyn Laws Associate Director of RDLA
Baillie McGowan Associate Director of Policy & Research
Priscilla Rodriguez Diversity Inclusion Advocacy Manager
Dylan Simon Director of Policy
Emily Stauffer State Policy Manager Jamie Sullivan Vice President of Policy
Lina Arslanian Associate Director of Finance & Salesforce
Megan Pinegar Chief Operating Officer
Alyssa Terwall Senior Director of Operations & Events
Stephanie Riordan Director of Patient Programs


































































































 MAT-US-2204557-v.1.0-06/2022
Sanofi speciality Care_RareDisease - US _8x4.93.indd 1
1/25/2024
MAT-US-2204557-v.1.0-06/2022
Sanofi speciality Care_RareDisease - US _8x4.93.indd 1
1/25/2024











