





• Discuss the roles of epithelial cytokine, including thymic stromal lymphopoietin (TSLP), in the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP)
• Comprehensively assess patients with CRSwNP for symptom burden, disease phenotypes, and effects on health-related quality of life
• Describe the clinical profiles of current and emerging biologic therapies for patients with CRSwNP
• Individualize treatment regimens for patients with CRSwNP based on symptoms, endotype, treatment goals, and comorbidities





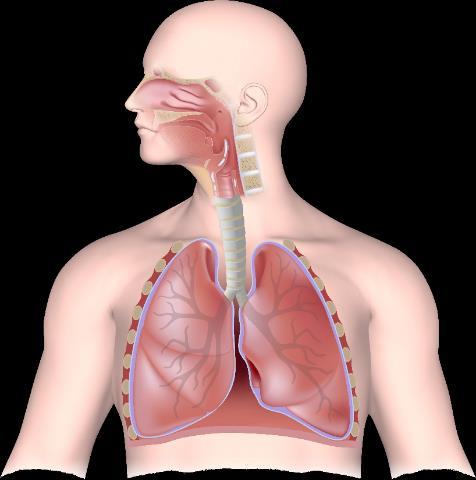


Nasal polyps Asthma
Microbiome imbalance
Allergens, viruses, and irritants
Biofilm S aureus Allergens Tight junction Goblet cell

Airway epithelium


IL, interleukin; S aureus, Staphylococcus aureus; TSLP, thymic stromal lymphopoietin. Maspero J, et al. ERJ Open Res. 2022;8:00576-2021.

Airway Injury Triggers



• Allergens
• Smoke
• Cytokines
• Microorganisms
• Tryptase



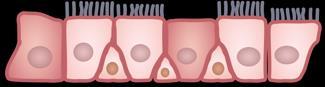

Th, helper T cell; Treg, regulatory T cell; R, receptor.
Bagnasco D, et al. Int J Mol Sci. 2024;25(11):5972.
Mast cells
Eosinophils

Keratinocytes
Fibroblasts
Dendritic cells
TSLP-Related Proinflammatory Signaling



Cellular Target Immune Cells


• Maturation

B lymphocytes
• Survival, maturation, chemokine production
Dendritic cells
T lymphocytes
• Treg development
• Th2 differentiation
• CD4+ T-cell homeostasis
• Activation
Mast cells, eosinophils, macrophages
Bidirectional provocation with allergens

• AR
• CRSwNP
• CRSsNP
• Nonallergic rhinitis
• Mixed rhinitis
• Otitis media
• AERD/NSAID-ERD


• Asthma
• Eosinophilic COPD



Upper and lower airway diseases reflect a single pathologic process manifesting in different locations within the airway.
AERD, aspirin-exacerbated respiratory disease; AR, allergic rhinitis; COPD; chronic obstructive pulmonary disease; CRSsNP; chronic rhinosinusitis without nasal polyps; NSAID-ERD, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease.
Bachert C, et al. J Allergy Clin Immunol Pract. 2023;11(9):2630-2641.
Epidemiologic Evidence
In patients with CRSwNP
• Up to 70% have comorbid asthma
In patients with asthma
• 40% have comorbid CRSwNP




Pathophysiologic Evidence
Severe eosinophilic asthma, CRSwNP, and NSAID-ERD are commonly characterized by eosinophilia and elevated levels of IL-4, IL-5, and IL-13


Functional and Pathophysiologic Evidence
The upper and lower airways share common cell types and immune interactions













ILC, innate lymphoid cell; NK, natural killer.
Bachert C, et al. J Allergy Clin Immunol Pract. 2023;11(9):2630-2641.
Higher rates of nasal polyp recurrence and corticosteroid dependence

More difficult-to-treat asthma/CRSwNP symptoms
Are more prone to asthma exacerbations

Worse outcomes


…compared with patients with only upper OR lower airway disease.
et al. J Allergy Clin Immunol Pract. 2021;9:1133-1141.


• ~40% of patients with diagnosed CRS had premorbid allergic rhinitis1 CRS

• NSAID-ERD/AERD is one of the most serious and treatment-resistant comorbidities in patients with CRSwNP. It is reported in up to 26% of patients2


CRS and Asthma

• Asthma and CRSwNP occur together in 40% to 70% of patients3
• Greater asthma severity has been linked to more radiologic evidence of CRS and higher risk of nasal polyps and allergic sensitization4

• GERD coexists in approximately 30% of patients with CRSwNP5

GERD, gastroesophageal reflux disease.
1. Tan BK, et al. J Allergy Clin Immunol. 2013;131(5):1350-1360; 2. Hannikainen P, et al. Otolaryngol Clin North Am. 2024;57(2):171-178; 3. Bachert C, et al. J Allergy Clin Immunol Pract. 2023;11(9):2630-2641; 4. Lin DC, et al. Am J Rhinol Allergy. 2011;25(4):205-208; 5. Orlandi RR, et al. Int Forum Allergy Rhinol. 2021;11(3):213-739.

Nasal polyps




• Alarmins
Asthma





–IL-25, IL-33, TSLP
–Produced by epithelial cells
• T2-high cytokines
–IL-4, IL-5, IL-13
–Produced by Th2 and ILC2 cells

AR, asthma, and CRSwNP are predominantly driven by chronic T2 inflammation, sustained by close interactions between the innate and adaptive immune responses.







Irritants, pollutants, microbes, and viruses




















































• Non-T2 cytokines1-3 –IL-1β, IL-6, IL-8, IL-17, and TNF-α –Produced by Th17 and Th1 cells













• Neutrophilic or paucigranulocytic1-3
CXCR2, C-X-C motif chemokine receptor 2; GM-CSF, granulocyte–macrophage colony-stimulating factor; TNF, tumor necrosis factor.
1. Kalchiem-Dekel O, et al. Chest. 2020;157(1):26-33; 2. Hinks TSC, et al. Eur Respir J. 2021;57(1):2000528; 3. Lambrecht BN, et al. Immunity. 2019;50(4):975-991.
Image adapted from Corren J. J Allergy Clin Immunol Pract. 2019;7(5):1394-1403 and Israel E, Reddel HK. N Engl J Med. 2017;377(10):965-976.

• Mucus hypersecretion is apparent in both upper and lower airway diseases1
• Airway hyperresponsiveness, traditionally characterized in the lower airways, frequently occurs in patients with CRSwNP and AR1
• Vasodilation and edema are the result of sinonasal inflammation and cause
2
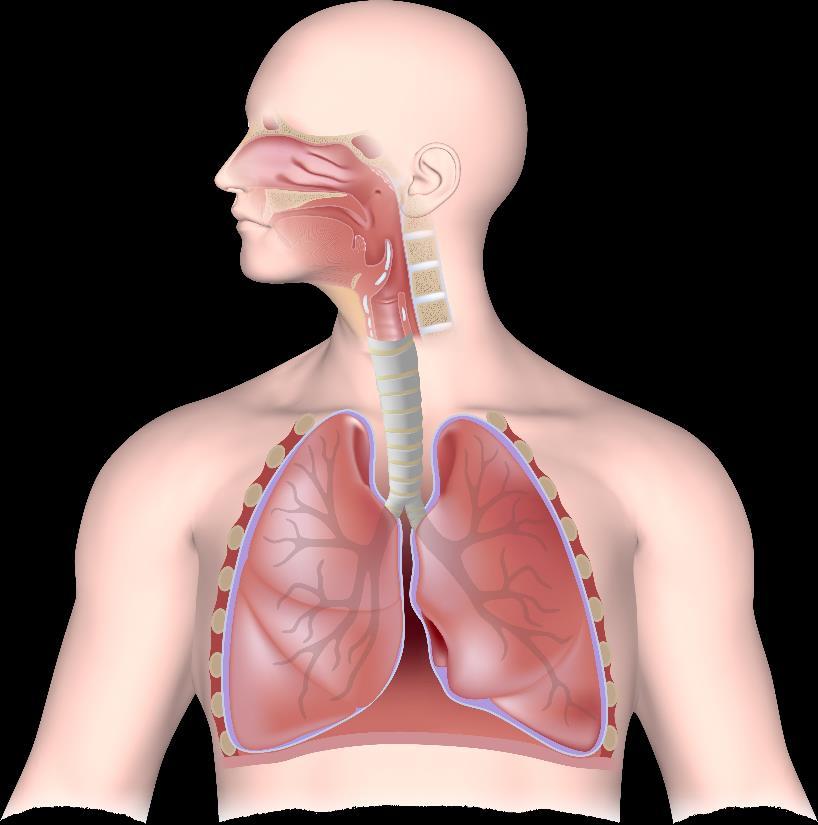

•Inflammation causes smooth muscle contraction in the lower airway, leading to bronchial hyperresponsiveness1 •Nasal polyp formation can be associated with many inflammatory cells, including eosinophils, neutrophils, plasma cells, and lymphocytes2


1
• Sinonasal inflammation persisting for ≥12 weeks, with a combination of ≥2 of the following symptoms, confirmed by endoscopic or radiographic findings:
– Nasal obstruction/congestion/blockage
– Anterior or posterior (mucopurulent) nasal drainage
– Lost or decreased sense of smell
– Facial pressure/pain/fullness


• Persistent or recurring despite long-term INCSs and having received ≥1 course of OCSs in the preceding 2 years and/or previous sinonasal surgery
– One course of OCSs refers to a minimum of 5 days at a dose of 0.5 to 1 mg/kg/day or more
– Previous sinonasal surgery refers to any surgical procedure, from resection of polyps to conventional ESS to extended approaches


• Bilateral CRSwNP with an NPS of ≥4 and persistent symptoms with the need for add-on treatment with INCSs
– Bilateral polyposis (nasal endoscopy)
– NPS ≥4 out of 8
– Presence of persistent symptoms assessed by:
• Loss of smell score (0-3): ≥2 points
• SNOT-22: ≥35 points
• Total symptoms on VAS: ≥5 out of 10 cm


• Head and neck assessment of mouth and nasal breathing
• Anterior rhinoscopy


hpf, high-powered field.

• Source mediums from which to obtain CRS biomarkers or investigate the microbiome include peripheral blood draws, tissue biopsies, nasal secretions, and nasally exhaled breath2
• The importance of identifying T2 inflammation cannot be overstated
– Evidence includes tissue eosinophils ≥10 cells/hpf OR blood eosinophils ≥250 cells/µL OR total IgE ≥100 IU/mL3
1. Australasian Society of Clinical Immunology and Allergy. https://www.allergy.org.au/images/stories/pospapers/ASCIA_HP_Position_Paper_CRSwNP_2021.pdf; 2. Workman AD, et al. Immunol Allergy Clin North Am. 2018;38(4):679-692; 3. Fokkens WJ, et al. Rhinology. 2020;58(suppl 29):1-464.
• CT scans
– Lund-Mackay Score is a CT scan scoring system (range 0-24) that assesses the severity of CRS based on the degree of opacification in 6 bilateral sinus areas and assigns each sinus group a numeric grade: 0=no abnormality; 1=partial opacification; and 2=total opacification
• MRI may be indicated when more detailed information is needed

• Nasal endoscopy permits visualization of the entire nasal cavity, including the 3 meatuses and the postnasal space
– Endoscopy is also useful when assessing response to treatment, tissue biopsy, or sinus cultures


CT, computed tomography; MRI, magnetic resonance imaging. Australasian Society of Clinical Immunology and Allergy. https://www.allergy.org.au/images/stories/pospapers/ASCIA_HP_Positio n_Paper_CRSwNP_2021.pdf.








Ig, immunoglobulin; PGD2, prostaglandin D2; TARC, thymus and activation-regulated chemokine; Th0, naive T cell. Maspero J, et al. ERJ Open Res. 2022;8:00576-2021. Nasal polyps
Allergens, viruses, and irritants
Microbiome imbalance


Eosinophil activation in bone marrow


Upregulation of chemokines (eg, eotaxin, TARC)



Inflammatory cell trafficking to the tissue



























• Clinical phenotypes do not reflect the underlying inflammatory mechanisms1
• Endotypes represent the most prominent molecular components and can be useful in guiding therapeutic decision-making1

• ILC1/Th1 mediated
• Key cytokine: IFN-γ
Endotype CRSwNP or CRSsNP


• Neutrophilic



• ILC2/Th2 mediated
• Key cytokines: IL-4, IL-5, IL-13
• Eosinophilic

• ILC3/Th17 mediated
• Key cytokine: IL-17
• Neutrophilic


• Associated with asthma comorbidity, nasal polyps, anosmia, and allergic mucin2
• Comorbid CRS and asthma demonstrating T2 inflammation has the poorest outcome and most severe clinical manifestations2




IFN, interferon; T1, type 1; T3, type 3. Fokkens WJ, et al. Rhinology. 2020;58(suppl S29):1-464; 2. Hannikainen P, et al. Otolaryngol Clin North Am. 2024;57(2):171-178.

Although the majority of patients with CRSwNP exhibit T2 inflammation, ~one-third have mixed or non-T2 inflammatory components, which may have implications for biologic selection.
Stevens WW, et al. J Allergy Clin Pract. 2019;7(8):2812-2820.e3.
• Multiple studies have demonstrated that ≥1/day saline irrigations reduce symptoms and improve QoL in patients with CRS
• Low-pressure, high-volume (240 mL) saline irrigation may significantly reduce sinonasal symptoms in 50% of patients (NNT=2) and is superior to nasal saline spray in reducing sinonasal symptoms (ARR=0.2; NNT=5)
• Isotonic saline irrigations are currently recommended because hypertonic saline irrigations may lead to burning and patient discomfort

• Many RCTs have demonstrated that INCSs improve sinonasal symptoms and endoscopic findings in CRS
• INCSs should be administered by bending the head down to look at the floor and spraying laterally (away from the nasal septum)
• INCSs are first-line therapy for CRS, usually in conjunction with isotonic nasal saline irrigation
• Antibiotic therapy for CRS can be short term (≤3 weeks) or long term, but there is little evidence to support their use
• When there is evidence of infection (eg, mucopurulent drainage on endoscopy), short-term antibiotic therapy can be used with selection guided by endoscopic culture1,2
• Macrolides may be used long term for their anti-inflammatory properties, but high-quality studies are needed to support their benefit in patients with CRS3,4
• Multiple RCTs have shown that short courses (≤3 weeks) of OCSs, alone or as an adjunct to standard maintenance therapy, improve sinonasal symptoms and endoscopic findings in the short term for patients with CRSwNP5-8
• Two Cochrane reviews showed no long-term benefit with short courses of OCSs, but reported short-term improvement in sinonasal symptoms and QoL in patients with CRSwNP9,10
• OCSs should be used only after weighing the possible benefits and harms of therapy

1. Head K, et al. Cochrane Database Syst Rev. 2016(4):CD011994; 2. Barshak MB, Durand ML. Laryngoscope Investig Otolaryngol. 2017;2(1):36-42; 3. Luo Q, et al. ORL J Otorhinolaryngol Relat Spec. 2011;73(4):206-211; 4. Yamada T, et al. Am J Rhinol. 2000;14(3):143-148; 5. Alobid I, et al. Laryngoscope. 2014;124(1):50-56; 6. Hissaria P, et al. J Allergy Clin Immunol. 2006;118(1):128-133; 7. Vaidyanathan S, et al. Ann Intern Med. 2011;155(4):277-278; 8. Kirtsreesakul V, et al. Rhinology. 2011;49(5):525-532; 9. Head K, et al. Cochrane Database Syst Rev. 2016(4):CD011992; 10. Head K, et al. Cochrane Database Syst Rev. 2016(4):CD011991.




















T2DM, type 2 diabetes mellitus; VTE, venous thromboembolism.















1. Sweeney J, et al. Thorax. 2016;71(4):339-346; 2. Sullivan PW, et al. J Allergy Clin Immunol. 2018;141(1):110-116.e7; 3. Price DB, et al. J Asthma Allergy. 2018;11:193-204; 4. Dalal AA, et al. J Manag Care Spec Pharm. 2016;22(7):833-847.

Nasal polyps can obstruct the sinuses and nasal passages and are associated with significant impact on QoL.1-5


As many as half of affected patients suffer from uncontrolled symptoms on a daily basis, in addition to long-term adverse effects from repeated courses of OCSs.6,7



The presence of nasal polyps elevates the cost of care, increases the frequency of emergency room visits and hospitalizations, and doubles the risk of revisional interventions within the first year after treatment with initial FESS.8,9


1. Deal RT, Kountakis SE. Laryngoscope. 2004;114(11):1932-1935; 2. Bhattacharyya N. Laryngoscope. 2007;117(10):1834-1838; 3. Toros SZ, et al. Eur Arch Otorhinolaryngol. 2007;264(9):1003-1008; 4. Epperson MV, et al. Laryngoscope. 2021;131(6):1206-1211; 5. Bachert C, et al. J Asthma Allergy. 2021;14:127-134; 6. Chen S, et al. Curr Med Res Opin. 2020;36(11):1897-1911; 7. Seys SF, et al. Allergy. 2020;75(11):2867-2878; 8. Palmer JN, et al. Allergy Asthma Proc. 2019;40(1):48-56; 9. Hunter TD, et al. J Med Econ. 2018;21(6):610-615.














• Preoperative symptom score (eg, SNOT-22) is a good predictor of postoperative outcome1
• When loss of smell is a major symptom, improvement in olfactory function with OCS use predicts positive outcome of surgery1
• Primary surgery has better outcomes than revision surgery1

• Comorbid asthma and AERD2
• S aureus superantigen2
• Eosinophilic infiltration2
• Biofilms and neutrophilic infiltrate2
• Lack of adherence to inhaled nasal corticosteroids2
Dupilumaba IL-4Rα

Mepolizumaba IL-5
•Moderate to severe asthma, eosinophilic phenotype OR OCSdependent; aged ≥6 years
•Inadequately controlled CRSwNP; aged ≥12 years
•Severe asthma, eosinophilic phenotype; aged ≥6 years
•CRSwNP; aged ≥18 years
Omalizumaba IgE
FDA, US Food and Drug Administration.
aFDA approved for other diseases besides CRSwNP and asthma. Drugs@FDA. https://www.accessdata.fda.gov/scripts/cder/daf/.
•Moderate to severe, persistent asthma inadequately controlled with ICS; (+) skin test or in vitro reactivity to aeroallergen; aged ≥6 years
•CRSwNP inadequately controlled on INCS; aged ≥18 years

POLYP-1/placebo (n=66)
POLYP-1/omalizumab (n=72)
POLYP-2/placebo (n=65) POLYP-2/omalizumab (n=62)
POLYP-1/placebo (n=66)
POLYP-1/omalizumab (n=72)
POLYP-2/placebo (n=65)
POLYP-2/omalizumab (n=62)









































LS, least squares. aP<0.0001 vs placebo. N=276 patients (LIBERTY NP SINUS-24) and N=448 patients (LIBERTY NP SINUS-52) aged ≥18 years with bilateral CRSwNP and uncontrolled symptoms despite INCS use, OCSs in the prior 2 years, or past sinonasal surgery. LIBERTY NP SINUS-24 randomized patients to either dupilumab 300 mg SQ Q2W or placebo for 24 weeks. LIBERTY NP SINUS-52 randomized patients to dupilumab 300 mg SQ Q2W for 52 weeks, dupilumab 300 mg SQ Q2W for the first 24 weeks followed by Q4W until week 52, or placebo for 52 weeks. Bachert C, et al. Lancet. 2019;394(10209):1638-1650.































There is still no FDA-approved biologic to treat AR or one specifically indicated in non-T2 CRS or asthma.

Despite our current therapies, patients with AR and CRS are still experiencing1-3:
• Fatigue, mood changes, impaired cognitive function, depression, and anxiety
And a substantial proportion of patients with asthma continue to experience uncontrolled disease, in which they have4-8:
• High levels of medical and mental distress
• Decreased productivity due to missing work/school
• Increased risk for death

1. Dierick BJH, et al. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):437-453; 2. Kim JY, et al. J Allergy Clin Immunol Pract. 2020;8(2):721-727; 3. Nam JS, et al. Clin Otolaryngol. 2022;47(1):167-173; 4. Reibman J, et al. Ann Allergy Asthma Immunol. 2021;127(3):318-325.e2; 5. Heaney LG, et al. Chest. 2021;160(3):814-830; 6. Haselkorn T, et al. J Allergy Clin Immunol Pract. 2020;8(7):2243-2253; 7. Mullerova H, et al. J Asthma. 2021;58(4):459-470; 8. Trevor J, et al. Ann Allergy Asthma Immunol. 2021;127(5):579-587.
Absence of Symptoms and Signs of Active Disease

REMISSION
No symptoms/no endoscopic signs of active disease for ≥12 months

Sustained remission for >5 years
CONTROL
Absence of symptoms in the last month
On Treatment Off Treatment
. Fokkens WJ, et al. Rhinology. 2024;62(3):287-298.
Persistent Treatment


Evaluation of 5 criteriaa:
• Reduced nasal polyp size (≥1)
• Reduced need for OCS/salvage surgery
• Improved quality of life (SNOT-22 <40 + >MCID)
• Improved sense of smell (hyposmic by semi-objective smell test)
• Reduced impact of comorbidities (defined by MCID for specific T2 disease test)


Evaluate treatment response
After 6 months





Evaluate treatment response
After 12 months





MCID, minimum clinically important difference.
aCutoff values for research purposes only.
Fokkens WJ, et al. Rhinology. 2024;62(3):287-298.



Good-Excellent Response 4-5 criteria Moderate Response 2-3 criteria No-Poor Response 0-1 criteria


Re-evaluate before continuing:
• Reconsider diagnosis
• Identify treatment traits





Consider:
• Discontinuing/Switching biologic
• Salvage surgery (covered by biologic)










Maspero J, et al. ERJ Open Res. 2022;8:00576-2021. Nasal polyps
Allergens, viruses, and irritants

Eosinophil activation in bone marrow










macrophage polarization


Inflammatory cell trafficking to the tissue Eosinophil Upregulation of chemokines (eg, eotaxin, TARC)











deposition



polyp formation








Mucus layer
Mucosal barrier

Inflammatory cells
Normal nasal mucosa and colonization with microbes
Nasal airway

Submucosal gland
Recruitment and expansion of inflammatory cells, tissue swelling, inflammation, and deposition of cross-linked fibrin
Nasal airway

Image adapted from: Stevens WW, et al. J Allergy Clin Immunol. 2016;4(4):565-572.
Nasal airway

Nasal polyp
Loss of barrier, with increased abundance and decreased diversity of microbes

Nasal airway
Tissue remodeling, with loss of submucosal glands in polyp and profound inflammatory cell expansion
Analyze Possible Therapeutic Options and Factors Influencing Decision
• Evaluate patient perspectives and expectations
• Involve the patient in the decision

Factors Orienting Toward Surgery
• Good long-term disease control after previous surgery
• Patient refusing long-term biologic therapy
• Previous surgical sequelae that require revision surgery (eg, synechiae, mucocele, persistent septal deviation limiting penetration of topical steroids)


• Educate the patient






Revision Surgery May Be Proposed


• Define goal of surgery
• Define approach based on the endotype
• Personalize follow-up
• Provide adequate postoperative medical therapy

In case of uncontrolled disease or recurrence


GA, general anesthesia. De Corso E, et al. Acta Otorhinolaryngol Ital. 2023;43(suppl 1):S3-S13.




Severe Asthma No Severe Asthma

Factors Orienting Toward Biologic
• Recurrence <3 years from previous surgery
• Inadequate control of symptoms after previous surgery, in particular, in case of smell impairment


Discuss the clinical case in a multidisciplinary board Choose biologics tailored to the patient’s endotype


Possibility of salvage surgery or shift to another biologic, in case of uncontrolled disease
• Multiple previous surgeries
• Severe complications (eg, CSF leak)
• Clear predictors of failure (eg, NSAID-ERD)



• Patient not fit for surgery (eg, coagulation disorders, contraindications to GA)


3 Criteria Are Required
Criteria Cutoff Points
Tissue eosinophils ≥10/hpf OR


Evidence of T2 inflammation
Need for OCS or contraindication to OCS
Blood eosinophils ≥250 cells/µL OR
Total IgE ≥100 IU/mL
≥2 courses/year OR
Long-term (>3 months) low-dose OCS
Significantly impaired QoL SNOT-22 ≥40
Significant loss of smell
Anosmic on smell test
Diagnosis of comorbid asthma Regular need for ICS
Fokkens WJ, et al. Rhinology. 2020;58(suppl 29):1-464.

CRL, complete response letter; sBLA, supplemental biologics license.
aFDA issued a CRL regarding the sBLA for benralizumab for patients with inadequately controlled CRSwNP based on the OSTRO phase 3 trial results and requested additional clinical data. 1. NCT04157335. https://clinicaltrials.gov/study/NCT04157335?cond=Chronic%20Rhinosinusitis%20With%20Nasal%20Polyps&term=benra lizumab&rank=3; 2. NCT05436275. https://clinicaltrials.gov/study/NCT05436275; 3. NCT05274750. https://clinicaltrials.gov/study/NCT05274750?cond=Chronic%20Rhinosinusitis%20With%20Nasal%20Polyps&intr=Depem okimab&rank=1; 4. NCT05281523.
https://clinicaltrials.gov/study/NCT05281523?cond=Chronic%20Rhinosinusitis%20With%20Nasal%20Polyps&intr=Depemokimab&rank=2; 5 . NCT06338995.
https://clinicaltrials.gov/study/NCT06338995?cond=Chronic%20Rhinosinusitis%20With%20Nasal%20Polyps&intr=lebrikizumab&rank=1; 6. NCT04851964. https://clinicaltrials.gov/study/NCT04851964?cond=Chronic%20Rhinosinusitis%20With%20Nasal%20Polyps&term=tezepleumab&rank=1.



























• Phase 3–WAYPOINT study1
– Efficacy and Safety of Tezepelumab in Participants With Severe Chronic Rhinosinusitis With Nasal Polyposis
–Coprimary endpoints: change from baseline in total NPS and biweekly mean NCS evaluated as part of the Nasal Polyposis Symptom Diary at week 52
• Phase 3–NAVIGATOR study2
–Post hoc analysis of a subset of patients with asthma and CRSwNP on tezepelumab found clinically meaningful improvement from baseline in SNOT-22 total score compared with patients on placebo (week 28; LS mean difference: -12.57 points), sustained over 52 weeks

1. Clinicaltrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT04851964; 2. Laidlaw TM, et al. J Asthma Allergy. 2023;16:915-932.
In March 2022, the FDA issued a CRL regarding benralizumab’s sBLA based on the OSTRO phase 3 results, requesting additional clinical data.1
• Phase 3 OSTRO study2 – Coprimary endpoints: benralizumab significantly improved NPS and NBS compared to placebo at week 40a
• Phase 3 ORCHID study3
– Efficacy and Safety Study of Benralizumab in Patient With Eosinophilic Chronic Rhinosinusitis With Nasal Polyps2 – Coprimary endpoints: change from baseline in endoscopic NPS and mean NBS at week 56

1. News release. https://www.astrazeneca.com/media-centre/press-releases/2022/update-on-us-review-of-fasenra-in-nasal-polyps.html; 2. Bachert C, et al. J Allergy Clin Immunol. 2022;149(4):1309-1317; 3. Clinicaltrials.gov. https://clinicaltrials.gov/study/NCT04157335.

• Phase 2–CROWNS-1 study1
– Demonstrated improvement in the coprimary efficacy endpoints of change from baseline in NPS and NCS at week 16 in severe eosinophilic CRSwNP
• Phase 3–CROWNS-2 study2
– A Study of CM310 in Patients With Chronic Rhinosinusitis With Nasal Polyposis
– Coprimary endpoints: change from baseline in NPS and NCS at week 24

• Phase 3–ANCHOR-2 study3
– Efficacy and Safety of Depemokimab (GSK3511294) in Participants With Chronic Rhinosinusitis With Nasal Polyps
– Coprimary endpoints: change from baseline in total endoscopic NPS at week 52 and mean NOS using verbal response scale from weeks 49 to 52

NOS, nasal obstruction score.
1. Zhang Y, et al. eClinicalMedicine. 2023;61:102076; 2. Clinicaltrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05436275?term=CM310&cond=Chronic+Rhinosinusitis+with+Nasal+Polyps&draw=2&rank=3; 3. Clinicaltrial.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05281523?term=depemokimab&cond=Chronic+Rhinosinusitis+with+ Nasal+Polyps&draw=2&rank=2.

• Upper and lower airway inflammatory diseases are closely related and frequently coexist in the same patients
• Patients who suffer from comorbid upper and lower airway diseases suffer from more-severe disease that is difficult to treat compared to those with upper OR lower airway disease
• Biologic therapy has demonstrated efficacy and safety in the treatment of severe asthma and CRSwNP, and new targeted biologics show promise in improving patient outcomes even more
• A multidisciplinary approach that combines the expertise of allergists/immunologists, otolaryngologists, and pulmonologists is critical to caring for patients with chronic inflammatory airway disease