





• Explain the underlying pathophysiology of nonadvanced SM, including the effects of driver mutations on MC hyperactivation and the rationale for new targeted therapies
• Use WHO criteria to differentially diagnose nonadvanced SM and classify subtype based on symptoms, biomarkers, and genetic testing results
• Describe recent clinical trial evidence for new and emerging targeted treatment options, including FDA-approved therapies
• Create individualized treatment plans reflecting clinical evidence for treatment efficacy and safety, patient-reported outcomes, and shared decision-making
• Educate patients with nonadvanced SM on support resources available, including those provided by TMS



• SM is characterized by the abnormal expansion and accumulation of MCs in 1 or more ECO systems1
• SM affects approximately 1 in 10,000 people1
• No apparent gender bias2
Nonadvanced SM (~88%-95%)3-5
• Bone marrow mastocytosis (BMM)
• Indolent SM (ISM; 88% of cases)3
• Smoldering SM (SSM)
Advanced SM (~5%-12%)3-5
• SM with associated hematologic neoplasm (SM-AHN)
• Aggressive SM (ASM)
• Mast cell leukemia (MCL)
1. Brockow K. Immunol Clin North Am. 2014;34(2):283-295; 2. American Society of Clinical Oncology (ASCO). https://www.cancer.net/cancer-types/mastocytosis/statistics; 3. Cohen SS, et al. Br J Haematol. 2014;166(4):521-528; 4. Ungerstedt J, et al. Cancers (Basel). 2022;14(16):3942; 5. Mesa RA, et al. Cancer. 2022;128(20):3700-3708.










KIT D816V causes constitutive activation of the KIT receptor and downstream signaling

Infiltration of multiple organ systems
SCF binds KIT and activates signaling pathways that control MC differentiation, maturation, migration, proliferation, survival, and cytokine production.
~95% of patients with nonadvanced SM have a KIT D816V mutation.
Adapted from Gilreath JA, et al. Clin Pharmacol. 2019;11:77-92; Tracy George, MD, personal communication.





Environmental stimuli

Preformed mediators (found in granules)





















• Histamine
• Tryptase
• Heparin

Lipid mediators (synthesized from arachidonic acid)
• Leukotrienes
• Prostaglandins

Chemokines/Cytokines
• IL-4
• IL-6
FcεR1, high-affinity IgE receptor 1; GPCR, G protein-coupled receptor; IgE, immunoglobulin E; IL, interleukin; MRGPRX2, mas-related G-protein coupled receptor member X2; TNF, tumor necrosis factor.
1. Giannetti MP. Ann Allergy Asthma Immunol. 2021;127(4):412-419; 2. Moon TC, et al. Front Immunol. 2014;14(5):569.


CRH, chymase, histamine, IL-6, PAF, renin, TNF, tryptase


Histamine, IL-6, CysLTs, PAF, PGD2



Allergens, bacteria, cytokines, drugs, fungi, peptides, toxins, viruses




CRH, histamine, IL-6, IL-8, IL-33, PAF, PGD2, TNF, tryptase



IL-6, PGD2, RANKL,TNF, tryptase
CRH, histamine, IL-6, neurotensin, PAF, PGD2, TNF


CRH, histamine, IL-6, neurotensin, PAF, PGD2, serotonin, TNF, tryptase, VIP


CRH, histamine, IL-6, TNF


CRH, corticotropin-releasing hormone; CysLT, cysteinyl leukotriene; PAF, platelet -activating factor; PGD2, prostaglandin D2, RANKL, receptor activator of nuclear factor kappa-β ligand; VIP, vasoactive intestinal peptide. Theoharides TC, et al. N Engl J Med. 2015;373(2):163-172.
Venoms
IgE Mediated

Food
Allergens
MRGPRX2 Medications
Cyclooxygenase
Inhibition
Stressors
Medications
Acute infection
Pain
Environment
Fatigue
Physical triggers
Surgery
Procedures
Vaccinations
Bee, wasp,3,a mixed vespids, fire ants
Alcohol consumption
Pollen, pet dander, dust mites
Opioids, some antibiotics (eg, vancomycin), contrast dyes
NSAIDs
Viral, bacterial, fungal
Emotional, physical
Heat, cold, sudden changes in temperature, sun/sunlight
Lack of sleep/sleep deprivation
Mechanical irritation, friction, vibration, exercise
Anesthesia (eg, atracurium)
Colonoscopy, endoscopy, interventional radiologyb
Anaphylaxis is noted in up to 50% of patients with ISM.4
NSAID, nonsteroidal anti-inflammatory drug. aHymenoptera venom was recently identified as the most common trigger in ISM; bPerioperative management should be considered.
1. Adapted from National Comprehensive Cancer Network (NCCN ®). https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; 2. Rama TA, Castells M. Curr
Treat Options Allergy. 2023;10:442-457; 3. Niedoszytko M, et al. Allergy. 2024;79(9):2470-2481; 4. Farmer I, Radha DH. Curr Hematol Malig Rep. 2024;19(5):197-207.





• Anxiety
• Decreased concentration and memory
• Cognitive dysfunction/brain fog
• Depression
• Headaches/Migraines
• Insomnia
• Aches
• Bone pain
• Joint pain
• Muscle pain
• Stiffness
• Angioedema
• Generalized itching
• Shortness of breath/wheezing
• Chest pain
• Abdominal pain
• Bloating
• Diarrhea
• Nausea
• Vomiting
• Bladder pain, urgency and frequency

• Nasal congestion/itching
• Runny nose
• Difficulty swallowing
• Oral itching and swelling
• Flushing (of the face, neck, and upper chest)
• Hypotension/Hypertension/ Blood pressure instability
• Syncope/Near syncope
• Tachycardia
• Osteopenia
• Osteoporosis
• Gastroesophageal reflux
• Hives
• Other skin rashes
• Pruritus
Jennings SV, et al. Ann Allergy Asthma Immunol. 2023;127(4):407-409.
• Fatigue
• Malaise
• Weakness
• Weight loss
• ANAPHYLAXIS Females
• Uterine cramps and/or bleeding Males
• Prostatitis
• Sexual dysfunction
• Urticaria Generalized systemic symptoms


2,b
SM-related employment changes
54% reduced work hours
27% voluntarily quit
• QoL: Mastocytosis Quality-of-Life
Questionnaire (MQLQ)1
• HRQoL: Mastocytosis Quality of Life
Questionnaire (MC-QoL)2
• Symptom assessment:
– Direct questions about symptom response to treatment
– Mastocytosis Symptom Assessment Form (MSAF)1
– Mastocytosis Activity Score (MAS)3
– ISM Symptom Assessment Form (ISM-SAF©)4
• Validated tool used in clinical trials
• Patient Global Impression of Change/Severity (PGIC/PGIS)
HRQoL, health-related quality of life; QoL, quality of life; TSS, Total Symptom Score.
aDiarrhea frequency is scored separately but contributes to total score.
Abdominal pain
Diarrheaa Nausea Spots
Itching
Flushing
Brain fog
Headache
Dizziness
Bone pain

Symptoms scored daily over a 24-hour recall period
0 = no symptom
10 = worst imaginable symptom
Fatigue TSS (0-110)
≥28 indicates moderate to severe symptoms
1. National Comprehensive Cancer Network (NCCN). https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; 2. Siebenhaar F, et al. Allergy. 2016;71(6):869-877; 3. Siebenhaar F, et al. Allergy. 2018;73(7):1489-1496; 4. Taylor F, et al. Orphanet J Rare Dis. 2021;16(1):414.







• Monomorphic MPCM (maculopapular cutaneous mastocystosis): diffuse pink and tan maculopapular rash with predilection for the upper extremities and trunk
• Darier’s sign positive






Figure from Hartmann K, et al. J Allergy Clin Immunol. 2016;137(1):35-45.

• History of flushing
• GI symptoms: recurrent abdominal bloating and diarrhea; diagnosed with IBS 2 years prior
• History of anaphylaxis: 1 ED visit for anaphylactic response to wasp sting at age 22 years
• History of fractures: 1 vertebral fracture (waterskiing)





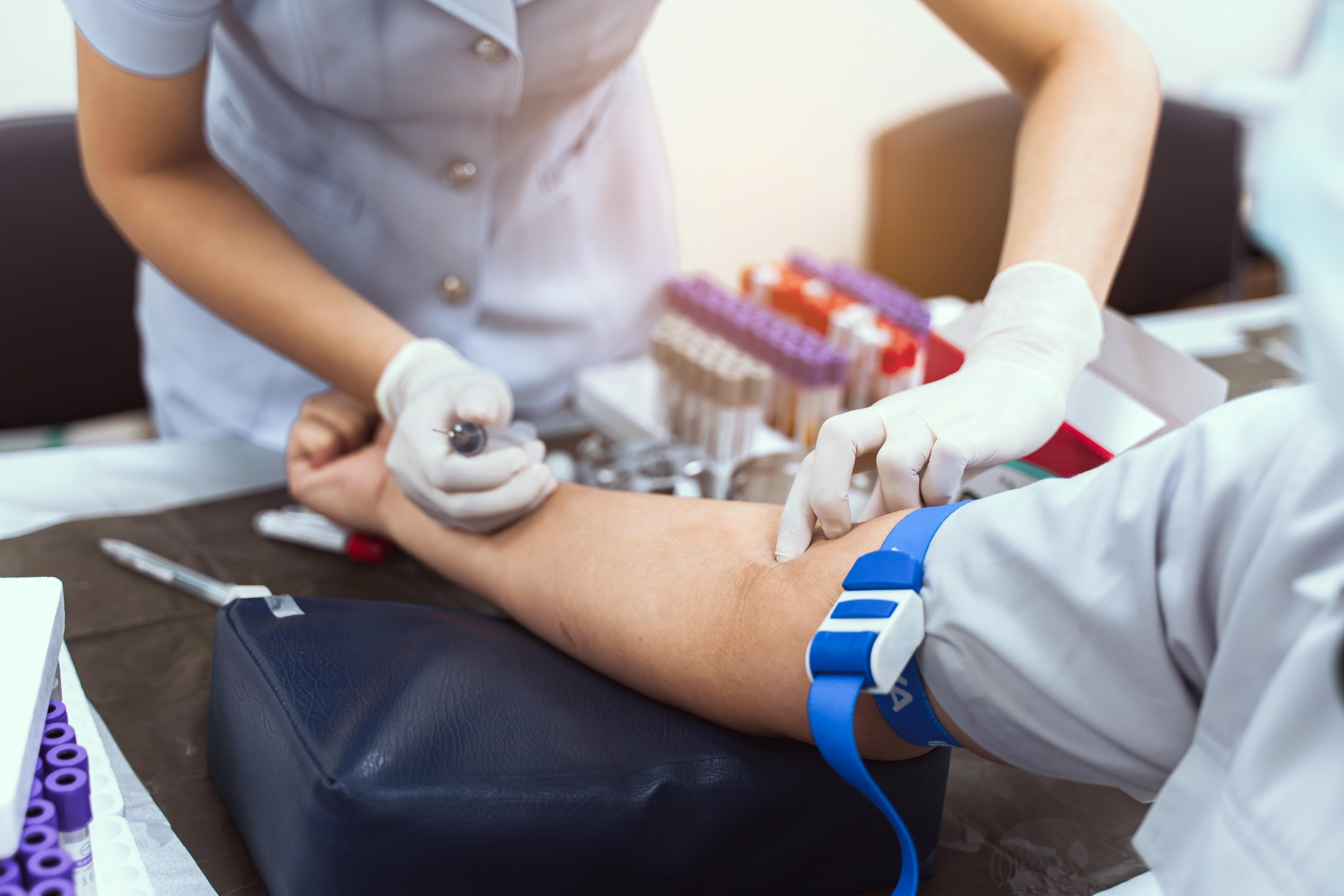
• CBC with differential
• CMP
• Basal serum tryptase (BST)
• High-sensitivity KIT D816V mutation analysis (eg, ddPCR, ASO-qPCR)

ASO-qPCR, allele-specific oligonucleotide-quantitative PCR; CBC, complete blood count; CMP, comprehensive metabolic panel; ddPCR, droplet digital PCR; PCR, polymerase chain reaction.
NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf.


• Most reliable for KIT D816V analysis, especially with a low VAF
– 0.01% to 0.03% sensitivity vs 3% to 5% sensitivity of NGS1-3
• ddPCR detected KIT D816V mutations in 95% of PB samples vs 28% by NGS vs 80% by local detection2
– Consider testing BM samples if the PB is negative in a patient with a high suspicion of disease4
– Recommended by NCCN and ICC Guidelines to avoid false negatives4,5
• 80% of patients positive for KIT D816V in PROSPECTOR had BST <20 ng/mL6 NGS, next-generation sequencing; PB, peripheral blood; VAF, variant allele fraction.
1. Greiner G, et al. Clin Chem. 2018;64(3):547-555; 2. George T, et al. Blood. 2020;136(suppl 1):7-8; 3. Boggs NA, et al. Blood Adv. 2023;7(13):3150-3154; 4. NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; 5. Arber DA, et al. Blood. 2022;140(11):1200-1228; 6. Hartmann K, et al. Presented at: European Academy of Allergy and Clinical Immunology Congress; May 31-June 3, 2024; Valencia, Spain. Abstract 403.

• BST: 29.4 ng/mL
• CBC with differential: normal
• CMP: normal
• PB: KIT D816V+; VAF: 0.09%
• Biopsy of skin lesions: increased number of dermal CD117+/tryptase+ MCs
• No organomegaly
• Colonoscopy results: no lesions identified; random biopsies show no increase in MCs











• Bone symptoms or osteoporosis +1
• Constitutional or cardiovascular symptoms +1











• Sex: male +1; female −1
• Clinical symptoms: – Presyncope or syncope +3
– Urticaria/Pruritic/Angioedema −1
– No urticaria/pruritic/angioedema +1


• BST: – <15 ng/mL −1 – >25 ng/mL +2









1 major criterion + 1 minor criterion
HαT, hereditary alpha tryptasemia.



Serum tryptase level >20 ng/mL (in the case of known HαT, tryptase levels should be adjusted)

>25% of MC are immature or atypical in BM smears or spindle-shaped in MC infiltrates detected in sections of BM or ECO


KIT D816V (or any other mutation causing ligand-independent activation of KIT)

CD2 and/or CD25 and/or CD30 detected on MCs in BM, blood, or other ECO

aThe ICC defines the major criterion as multifocal infiltrates of MCs that are tryptase- and/or CD117+ . Arber DA, et al. Blood. 2022;140(11):1200-1228; Khoury JD, et al. Leukemia. 2022;36(7):1703-1719; NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf. Images courtesy of Tracy George, MD, and Anton Rets, MD, PhD.

• MCs slightly increased (4%), 70% spindle shaped, 30% mature round/oval forms


Biopsy
• Few, dense multifocal aggregates with >15 MCs
• MC in aggregates, CD25+, CD117+, tryptase+ , CD30−, abnormal, spindle-shaped morphology
• Burden of MCs,10%






No additional findings
• Absence of skin lesions
• BM involvement
• Serum tryptase <125 ng/mL






No additional findings
• <2 B-findings
• Skin lesions may be present



SM diagnostic criteria met

≥2 B-findings, No C-findings
• MCs in BM ≥30% and/or serum tryptase ≥200 ng/mL and/or KIT D816V with VAF ≥10% in BM or PB leukocytes
• BM hypercellularity or dysplasia or myeloproliferation in a non-MC lineage
• Organomegaly (without organ impairments)
• Does not meet criteria for AHN
≥20% MCs on BM aspirate smear



MCLa



≥1 C-finding
• Cytopenia(s) present (ANC <1.0 × 109/L, hemoglobin <10 g/dL, or platelets <100 × 109/L)
• Hepatomegaly with portal hypertension/ascites
• Splenomegaly with hypersplenism
• Osteolytic lesions (≥2 cm) or pathologic fractures
• Malabsorption with weight loss with hypoalbuminemia


ANC, absolute neutrophil count; CMML, chronic myelomonocytic leukemia. aB- and C-findings possible.
WHO criteria for associated hematologic neoplasm met (eg, dysplasia, monocytosis, eosinophilia)

Adapted from Mannelli F. Ann Hematol. 2021;100(2):337-344; Khoury JD, et al. Leukemia. 2022;36(7):1703-1719; NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; Zanotti R, et al. Leukemia. 2022;36(2):516-524.


SM-AHNa
• SM-acute myeloid leukemia (AML)
• SM-myelodysplastic syndrome (MDS)
• SM-myeloproliferative neoplasm (MPN)
• SM-MDS/MPN (eg, SMCMML)

• ~6% of general population, accounting for 90% of cases with elevated BST levels1
• Amplification at the TPSAB1 locus (encodes alphatryptase) that increases BST levels (>8 ng/mL)2,3 – ~9- to 10-ng/mL increase in tryptase for every extra copy of TPSAB11 – Symptoms consistent with MC activation4
Chromosome 163

TPSAB1, tryptase alpha/beta 1; TPSB2, tryptase beta 2; TPSD1, tryptase delta 1; TPSG1, tryptase gamma 1. 1. Glover SC, et al. Ann Allergy Asthma Immunol. 2021;127(6):638-647; 2. Lyons JJ, et al. Nat Genet. 2016;48(12):1564-1569; 3. Lyons JJ. Immunol Allergy Clin North Am.
495; 4. Giannetti MP, et al. J Allergy Clin Immunol. 2022;150(5):1225-1227.
Significant association with greater risk of severe anaphylaxis in patients with mastocytosis1
Affects ~12%-17% of patients with SM2



• Cardiovascular: coronary hypersensitivity,a labile hypertension, orthostatic hypotension, paroxysmal arrhythmias
• Digestive: adverse food reaction,a celiac disease, GERD, gluten enteropathy, IBS
• Endocrinologic: carcinoid syndrome, insulinoma, parathyroid/thyroid carcinoma, pheochromocytoma, thyrotoxicosis
• Immunologic: autoinflammatory disorders,a familial hyper-IgE syndrome, vasculitisa
• Neurologic: anxiety, chronic fatigue syndrome, depression, fibromyalgia, headaches/migraines, hyperventilation, multiple sclerosis, panic attacks, postural orthostatic tachycardia syndrome, seizure disorder, somatization disorder, stroke
• Skina: angioedema, atopic dermatitis, chronic urticaria
• MMAS: – KIT D816V present or CD25+
– Symptoms consistent with MC activation and evidence of clonal population
GERD, gastroesophageal reflux disease; MMAS, monoclonal mast cell activation syndrome.
aLocalized MC activation can occur.
Castells M, Butterfield J. J Allergy Clin Immunol Pract. 2019;7(4):1097-1106; Picard M, et al. Clin Ther. 2013;35(5):548-562; Scherber RM, Borate U. Br J Haematol. 2018;180(1):11-23; Theoharides TC, et al. N Engl J Med. 2015;373(2):163-172.

• BST variability1:
– HαT affects BST levels, so adjustments should be made
– Tryptase is not reliable when AHN is present
– Tryptase <20 ng/mL does not rule out SM
• Negative KIT D816V in PB does not rule out SM (often negative even by high-sensitivity methods in low MC burden)2
• Be aware of overlapping findings between MMAS and SM3
1. Boggs NA, et al. Blood Adv. 2023;7(13):3150-3154; 2. Arock M, et al. Leukemia. 2015;29(6)1223-1232; 3. Jackson CW, et al. Int J Mol Sci. 2021;22(20):11270.
• Delayed diagnosis due to the rarity of SM and the broad spectrum of signs and symptoms that are shared by other, more-common disorders1,2
– Puts patients at risk of life-threatening anaphylaxis, organ dysfunction, and severe osteoporosis
• Prior to diagnosis, patients had seen an average of 6 HCPs3

The median time from symptom onset to diagnosis of SM is 5 to 6 years.3,4



HCP, health care provider.

1. Ungerstedt J, et al. Cancers (Basel). 2022;14(16)3942; 2. Mikkelsen CS, et al. Dermatol Reports. 2014;6(1):5199; 3. Mesa RA, et al. Cancer. 2022;128(20):3691-3699; 4. Tse KY, et al.
J Allergy Clin Immunol. 2024;3(4):100316.
• Disease subtype1
• Age: >60 years1
• Hemoglobin: <10 g/dL1
• Platelets: <100/150 × 109/L1,a
• BST level2
• ≥6% VAF of KIT D816V in PB3

Nonadvanced SM Subtype2 Risk of Progression
• Elevated serum alkaline phosphatase (≥100 U/L)4
• Other genetic mutations2 (eg,ASXL1, RUNX1, SRSF2)
• Splenomegaly1
• High symptom burden5
ASXL1, Additional Sex Combs Like 1; RUNX1, Runt-related Transcription Factor 1; SRSF2, Serine/Arginine -rich Splicing Factor 2.
aDependent on whether Mutation-Adjusted Risk Score (MARS) for advanced SM or Mayo Alliance Prognostic System (MAPS) for mastocytosis is used. 1. NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; 2. Valent P, et al. J Allergy Clin Immunol Pract. 2022;10(8):1999-2012; 3. Maurer M, et al. J Allergy Clin Immunol. 2024;153(2):AB238; 4. Mukherjee, et al. Blood. 2023;142(suppl 1):6406. 5. Reiter A, et al. Blood. 2020;135(16):1365-1376.



Skin: pruritus, flushing, urticaria, angioedema dermatographism

GI: abdominal pain, cramping, diarrhea, heartburn, nausea, vomiting
2. Leukotriene receptor antagonist
H1R antagonist and H2R antagonist
H2R antagonist
Neurologic: headache, cognitive impairment (eg, brain fog, poor concentration and memory), depression
Cardiovascular/Pulmonary: presyncope, tachycardia, wheezing, throat swelling
Hypotensive episodes/anaphylaxis
Osteopenia/Osteoporosis
H1R and H2R antagonist
H1R and H2R antagonist
3. Aspirin
4. Ketotifen
5. Cromolyn sodium ointment
2. Cromolyn sodium
3. Proton pump inhibitor
4. Leukotriene receptor antagonist
5. Ketotifen
2. Cromolyn sodium
3. Aspirin
4. Ketotifen
2. Corticosteroids
3. Omalizumab
Epinephrine IM (acutely), supine positioning Prevention: VIT, Rush desensitization, omalizumab
Supplemental calcium and vitamin D, bisphosphonate; bone mineral density assessment
Denosumab, interferon-α inhibitor, cytoreductive agent; vertebroplasty/kyphoplasty

More than half of patients take ≥3 prescription medications and ≥3 OTC medications. 2
IM, intramuscular; OTC, over the counter; VIT, venom immunotherapy. 1. NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf; 2. Mesa RA, et al. Cancer. 2022;128(20):3691-3699.

• Anti-IgE monoclonal antibody FDA approved to treat chronic persistent spontaneous urticaria, chronic rhinosinusitis with nasal polyps, allergic asthma, and IgE-mediated food allergy1
• Effective on all vasomotor symptoms, including anaphylaxis and urinary symptoms2
• Safety and efficacy study of omalizumab in SM (2018)3
– Demonstrated significant reduction in symptoms and patient-reported QoL:
• 38.5% had complete symptom control
• 23% had major response, 23% had partial response
– Most effective for recurrent anaphylaxis and skin symptoms, less for GI, musculoskeletal, and neuropsychiatric
– No significant changes in tryptase levels or KIT VAF
– No SAEs SAE, serious adverse event.
aN=14 adult patients with SM who received omalizumab for a median duration of 17 months.
1. US Food and Drug Administration (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/103976s5245lbl.pdf; 2. Buonomo A, et al. Mediterr J Hematol Infect Dis. 2022;14(1):e2022040; 3. Broesby-Olsen S, et al. Allergy. 2018;73(1):230-238; 4. Valent P, et al. J Allergy Clin Immunol Pract. 2022;10(8):1999-2012.
What would you recommend for Mark’s initial treatment plan?


• MPCM; Darier’s sign, positive

• History of GI symptoms, anaphylaxis, and fractures
• BST: 29.4 ng/mL
• Skin lesions: CD117+, tryptase+
• Aspirate: MCs slightly increased (4%), 70% spindle-shaped
• Biopsy: 10% MC burden, dense aggregates >15 MCs are spindle-shaped, CD25+, CD117+, tryptase+
• Usually target active site of the kinase1
• Classified based on type of binding (eg, competitive, allosteric)1
• KIT inhibitors2,3:
– Imatinib: inhibits WT or non-D816V mutations
• Inhibits ABL1, FLT3, PDGFRA, PDGFRB, CSFR1; FIP1L1PDGFRA
– Midostaurina: inhibits WT and D816V
– Avapritinib,b elenestinib, bezuclastinib: selectively inhibit D816V
• There can be overlap in inhibition (eg, avapritinib inhibits KIT and PDGFRA mutants4)








aNot indicated for nonadvanced disease, and utility limited by GI toxicity; bAvapritinib is the only TKI currently FDA approved to treat ISM. 1. Pottier C, et al. Cancers. 2020;12(3):731; 2. Akin C. Immunol Allergy Clin North Am. 2023;43(4):743-750; 3. Akin C, et al. J Allergy Clin Immunol. 2022;149(6):1912-1918; 4. Trullas-Jimeno A, et al. ESMO Open. 2021;6(3):100159. ATP binding domain

ABL, ABL proto-oncogene 1, non-receptor tyrosine kinase; ATP, adenosine triphosphate; FLT3, FMS -like tyrosine kinase 3; PDGFRB, platelet-derived growth factor receptor β; CSFR1, colony-stimulating factor 1 receptor; WT, wild-type.

Avapritinib Targets the KIT
Receptor on the MC1,2


aP<0.003.
Avapritinib Significantly Improved TSS Score3


















































BSC, best supportive care; C, complement; IgG, immunoglobulin G; OLE, open -label extension. N=212 adults with confirmed ISM with inadequately controlled symptoms (TSS ≥28) despite receiving BSC with ≥2 antimediator dr ugs were randomly assigned 2:1 to receive 25 mg avapritinib daily + BSC (n=141) or placebo + BSC (n=71) for 24 weeks. Patients could then continue to an OLE, in which they received 25 mg avapritinib daily for up to 5 years. 1. Figure adapted from Castells M, Akin C. Nat Med. 2021;27(12):2081-2082; 2. Nicolosi M, et al. Medicina (Kaunas). 2021;57(11):1135; 3. Gotlib J, et al. NEJM Evid. 2023;2(6):EVIDoa2200339; 4. Drugs@FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/212608s013lbl.pdf.
Avapritinib Significantly Improved Clinical Markers and Symptoms

Avapritinib Improved on a 30% Reduction in Symptoms vs Placebo
• 25 mg orally QD on an empty stomach
– (ASM: 200 mg orally QD)
– Not recommended for patients with platelet count <50 × 109/L
• Avoid coadministration with strong and moderate CYP3A inhibitors and inducers
• AEs: peripheral and periorbital edema, dizziness, flushing, photosensitivity
• Increased bleeding has not been reported in patients with ISM who are taking avapritiniba
– Ensure patient does not have a history of intracranial hemorrhage before prescribing
• Avapritinib was well tolerated in patients receiving concomitant omalizumab, similar to overall avapritinib-treated PIONEER part 2 population2
• Monitoring: lab monitoring (CMP, CBC with differential) and visit at 3 and 6 months after starting medication, then every 6 months
AE, adverse event; CYP, cytochrome P450; QD, once daily.
aAs of February 20, 2025.
Annual Meeting; October 24-28, 2024; Boston, MA. Abstract R291.

1. Drugs@FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/212608s013lbl.pdf; 2. Akin C, et al. Presented at: American College of Allergy, Asthma, and Immunology




• Diagnosed with ISM 7 years ago
• Splenomegaly (15 cm) but no hepatomegaly
• QoL severely impacted by
– History of osteoporotic fractures
– Frequent nausea, vomiting, abdominal pain after eating
– MPCM
• Baseline BSC: famotidine 20 mg QD, levocetirizine
5 mg BID, hydroxyzine 25 mg PRN, and cromolyn sodium 200 mg QID
• Osteoporosis managed by an endocrinologist with oral vitamin D and calcium, bisphosphonates, and monthly denosumab
• Baseline BM: 33% MC burden
• Serum tryptase: 170 ng/mL
• KIT D816V VAF: 3%

BID, 2 times a day; PRN, as needed.









































2,3










2 Phase 2/3 (HARBOR)
2 Phase 3
BTK, Bruton tyrosine kinase; siglec-8, sialic acid-binding immunoglobulin-like lectin-8.
1. Figure adapted from Castells M, Akin C. Nat Med. 2021;27(12):2081-2082; 2. Nicolosi M, et al. Medicina (Kaunas). 2021;57(11):1135; 3. Akin C, et al. Blood. 2022;140(suppl 1):68386839; 4. ClinicalTrials.gov identifier: NCT04655118, Updated May 10, 2024. Accessed February 20, 2024. https://clinicaltrials.gov/study/NCT04655118; 5. Siebenhaar F, et al. Br J Dermatol. 2023;189(5):511-519.
• Minimal CNS penetration1
• High selectivity: does not inhibit PDGFRA, PDGFRB, FLT3, CSFR12
• Part 1a: primary endpoints: safety, PK, MC biomarkers, symptom improvement at 12 weeks1
– No treatment-related SAEs; majority of TEAEs at 100 to 200 mg QD were low grade and reversible
– No bleeding or cognitive impairment events reported
• Part 2: 24-week treatment (OLE)3
• Enrollment closed4

NA, not available; PK, pharmacokinetics; TEAE, treatment-emergent AE.
aN=54 adult patients with ISM or SSM per 2016 WHO criteria with moderate to severe symptoms (on ≥2 antimediator therapies), wh o were randomly assigned 1:1:1 to BSC + bezuclastinib or BSC + placebo for 12 weeks, followed by OLE: part 1a, N=20, 100 mg QD or 200 mg QD or placebo; part 1b, N=34, 100 mg QD (n =11), 150 mg QD (n=11), placebo (n=12); OLE: N=36.
bP=0.046.
1. Bose P, et al. Blood. 2023;142(suppl 1):77; 2. Akin C. Immunol Allergy Clin North Am. 2023;43(4):743-750; 3. Modena B, et al. Presented at: American Academy of Allergy, Asthma, and Immunology Annual Meeting; February 23-26, 2024; Washington, DC. Poster 694; 4. Cogent Biosciences. https://smpathways.com/. Accessed February 20, 2024; 5. Triggiani M, et al. EMJ Allergy Immunol. 2024;9(10):26-36; 6. Rein LAM, et al. Blood. 2024;144(suppl 1):4556-4558.
• Limited CNS penetration1
• Selectively inhibits KIT D816V; does not inhibit WT KIT1
• Primary endpoints: safety, PK, PD1
– Well tolerated at all dose levels for 35 weeks (median)
– No treatment-related SAEs or AEs that led to drug discontinuation
• Part 2 actively enrolling
CNS, central nervous system; PD, pharmacodynamics.

Significant Dose-Dependent Improvements After 12 Weeks of Elenestinib1,2
Mean Percentage Change From Baselinea
aN=39 adult patients with ISM per WHO criteria with moderate to severe symptoms (ISM -SAF TSS ≥28), who were randomly assigned to BSC + elenestinib 25 mg, 50 mg, 100 mg, or BSC + placebo. N=83 additional open-label PK cohorts enrolled in parallel to further characterize PK and safety: n=21 at
the






As compared to before diagnosis, after diagnosis, patients with SM had an increase in1:
• Specialty provider visits
– Greatest increases noted for hematologist/oncologist, allergist/immunologist, dermatologist, and gastroenterologist
• Urgent care visits
• ED visits
• Number of hospital admissions

Allergist/Immunologist General practitioner
Hematologist/Oncologist Other

• Patients with stable SSMa should be monitored every 6 months and those with stable ISMa every 12 months, for changes in:
– Serum tryptase level
– KIT D816V VAF
– CBC with differential
– CMP
– Weight
– Physical examination, including skin/lesions
• Regression of MPCM lesions does not correlate with changes in underlying ISM
– QoL/Symptom burden: ability to participate in daily activities, ability to eat, respiratory symptoms under control, sleep
• Use MSAF or MQLQ
• DEXA scan every 1 to 3 years for patients with osteopenia/osteoporosis

If laboratory results or symptoms change, therapeutic adjustment should be considered.
DEXA, dual x-ray absorptiometry.
aPatients with unstable SSM or ISM should be seen more frequently until stabilized.
Brockow K, et al. Arch Dermatol. 2002;138(6):785-790; NCCN. https://www.nccn.org/professionals/physician_gls/pdf/mastocytosis.pdf.
Perioperative Triggers Examples
• Tourniquet use

Mechanical
Pharmacologic
Temperature Changes
• Mild trauma of the skin
• Surgery
• Medications (eg, atracurium, mivacurium, nefopam)
• Hypothermia
• Hyperthermia
Main Corresponding Symptoms
Skin: pruritis, flushing, erythema, urticaria
Cardiovascular signs: tachycardia, hypotension

Medication Type

General Medications
Avoid or Use With Caution
• Alcohol
• Amphotericin B
• Dextran
• Dextromethorphan
• Polymyxin B
Pain Medications
General Anesthetics
• Opioid narcotics (may be tolerated by some individuals)
• Ketorolac
• Atracurium
• Doxacurium
• Benzocaine
Local Anesthetics
Intraoperative Induction Medications
• Chloroprocaine
• Quinine
• Vancomycin IV
• Alpha-adrenergic blockers
• Beta-adrenergic blockers
• NSAIDs (unless the patient is already taking a drug from this class)
• Rocuronium
• Mivacurium
• Procaine
• Tetracaine
Medications That Are Typically Tolerated
• Calcium channel blockers
• Centrally-acting alpha 2 adrenergic stimulants
• Aldosterone antagonists
• Fentanyl (may require adjunctive treatment with ondansetron)
• Tramadol
• Pancuronium
• Vecuronium
• Bupivacaine
• Lidocaine
• Levobupivacaine
• Ketamine
• Midazolam
• Propofol
• Mepivacaine
• Prilocaine
• Ropivacaine
Inhaled Anesthetics
IV, intravenous.
TMS. https://tmsforacure.org/wp-content/uploads/2023/03/TMS_Full-Patient-Guide_r6.pdf.
• Sevoflurane
• Trigger avoidance
– Identification of potential triggers
– Unpredictability of response
• Periprocedural precautions
• Anaphylaxis action plan
• ED response plan
• Medication adherence








• Education and resources on conditions/treatment
• Peer-to-peer support groups and opportunities for connection
• Help connect to experts, assistance, and clinical trials
• Fund and conduct research


• Bridge knowledge gaps between patients and medical professionals
• Expand awareness of disease to non-expert HCPs
• Advocate for patient-centric drug development by sharing patient experiences and needs during clinical trials and treatment development
• Advocate for changes in health care laws, ensuring patient rights and access to necessary treatments



• Website: https://tmsforacure.org
• Medication: https://tmsforacure.org/treatments/ medications-treat-mast-cell-diseases
• Patient Guide: https://tmsforacure.org/patient-guide
• Patient Assistance: https://tmsforacure.org/patientassistance
• Clinical Trials: https://tmsforacure.org/clinical-trials
• Facebook: https://www.facebook.com/TMSforacure
• Instagram: @mcdiseasesunite
• LinkedIn: https://www.linkedin.com/company/ 74058179/admin/feed/posts
• Inspire Forum: https://www.inspire.com/groups/mastcell-diseases-unite
• Nurse Line: NURSES@tmsforacure.org


















NCCN Guidelines for Patients®
Systemic Mastocytosis, 2022
GARD helps patients find information, services, experts, financial aid, and support groups.
NORD is a patient advocacy organization committed to the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services.
AIM is a group dedicated to advancing the research, education, and treatment of mastocytosis and related mast cell diseases.
ECNM (European Competence Network on Mastocytosis) is a group dedicated to improving disease recognition, diagnosis, and therapy in patients with mastocytosis in Europe.

Visit Our Clinical Resource Center at ExchangeCME.com/SMRoundTableResources.

• The underlying mechanisms of nonadvanced SM are complex, with symptoms that can differ significantly between patients
• High-sensitivity assays are the most reliable for detection of KIT D816V, particularly in cases with a low VAF
• Traditional management of nonadvanced SM primarily focuses on prevention and symptom management
• A collaborative, multidisciplinary approach is crucial for effective management
• Avapritinib is FDA approved to specifically address the symptoms and underlying pathophysiology of ISM
– Other selective TKIs are under investigation and may soon be available as additional therapeutic options
• Patient education (on triggers, anaphylaxis, and ED action plans) is vital to reduce the significant burdens associated with SM