

Breakout Sessions #1: Treatment Approaches in Myeloma
An Approach to Relapsed Myeloma
Angela Dispenzieri, MD, Mayo Clinic
– Rochester, MN
An Approach to relapsed myeloma
IMF Patient education - LA
Angela Dispenzieri, M.D.
The Serene M. and Frances C. Durling Professor of Medicine and of Laboratory Medicine
August 16, 2024
DISCLOSURES
Companies Role
Janssen Advisory board and independent review committee
HaemaLogiX
Alynlam, Pfizer, Takeda, BMS, AbbVie
Advisory board
Research dollars
The presentation includes off-label information on treatment regimens
An approach to relapsed myeloma
1. Background
2. Let’s talk immunotherapy
3. Back to general principles
4. mSMART recommendations
1. BACKGROUND
77 Years of Myeloma Treatment: Representative Changes

Pillars of Myeloma

Bortezomib
Elotuzumab
Daratumumab
General principles
1. Therapies change over time—clinical trials make this possible
2. Outcomes (e.g. survival) changes over time, as new therapies emerge
3. If one therapy did not work well, it doesn’t mean another won’t
4. Terms like “overall survival” and “progression free survival” typically refer to statistical probabilities for GROUPS of people and do not seal the fate of an INDIVIDUAL
5. At a given point in time, there may not be a known “right answer;” hence many opinions. Again, think clinical trials
y.o. woman with t(11;14)
Continued in complete response for 4 years (no maintenance)
10/9/200711/2020072/4/20083/10/20086/3/20087/9/20088/12/200811/6/20082/19/2008

2. LET’S TALK IMMUNOTHERAPY
Retrain the immune system to kill mm

Emerging immunotherapies in multiple myeloma


Carvykti
Talquetamab (CPRC5D)
Cevostamab (FcRH5)
Naked monoclonal antibody
NAKED ANTIBODIES
Examples:
1. Daratumumab (Darzalex) — recognizes CD38
2. Isatuximab (Sarclisa) — recognizes CD38
3. Elotuzumab (Empliciti) — recognizes SLAMF7
Cancer cell

ANTIBODY DRUG CONJUGATE
Examples:
Belantamab mafodotin
— recognizes
DREAMM 7: Velcade-Dex with Belantamab or daratumumab
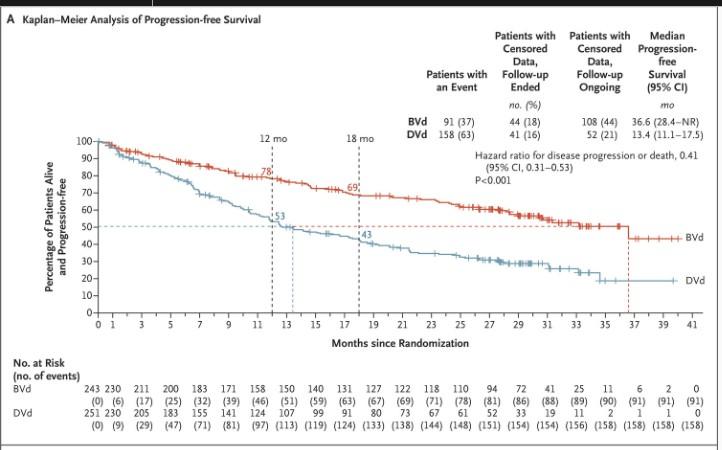
Progression free Survival Overall Survival

Hungria V. NEJM; 391(5): 393-407.
DREAMM 8: POM-DEX WITH BELANTAMAB
OR VELCADE
Progression free Survival

Dimopoulos MA. NEJM 2024; 391(5): 408-421.

Overall Survival
BISPECIFIC ANTIBODY
Bispecific MM target Brand name
Teclistamab BCMA Tecvayli
Talquetama b GPRC5D Talvey
Elranatamab BCMA Elrexfio
Cevostama b FcRH5
Livoseltamab BCMA
Plasma cell aka myeloma cell
T-cell
Bispecific antibody
Fc domain
• Cytokine release
• T cell activation
• Perforin/Granzymes
Summary points about Bispecific antibodies (AKA T-cell engagers)
• Overall response rate ~ 60% with most of the responses being VGPR or better
• If patients respond, duration of response is about 18 months
• Less CRS and neurologic toxicity than CAR-T
• Serious infection in about 1/3 of patients, but improved with IVIG
• Handy because right out of the box; approved for after 4 lines of therapy
• Cumbersome because weekly (though this is starting to improve to every other week and even every 4 weeks) 1
• Note: Talquetamab can be given either weekly or every other week, has lower infection rate, overall response rate of about 70%, but issues with skin, nails and taste
Bispecific antibodies (AKA t-cell engagers)
• Major questions
• Induction, consolidation, maintenance?
• Sequence relative to similar target but different modality or relative to different target but same modality (another TCE)?
• In combination with other agents?
CAR-T CELLS
CAR-T cell
Cilta-cel CARVYKTI (BCMA)
CAR T-cell
Therapy
Benefits
• High response rates (~ 75% of patients)
• No maintenance
• No steroids
• Effective even in heavily pretreated or previously refractory patients
Remove blood from patient to get T cells
T cell
Antigens
CAR T cells bind to cancer cells and kill them
CAR T cell
Make CAR T cells in the lab
Insert gene for CAR

Chimeric antigen receptor (CAR)
Grow millions of CAR T cells
Lymphodepleting chemotherapy
• Cytokine release syndrome (CRS)
• Fevers, chills
• Low blood pressure
• Low oxygen levels
• Multi-system organ damage
• Neurotoxicity
• Delirium
• Loss of ability to speak
• Inability to write
• Decreased alertness (obtundation)
• seizures
• Prolonged cytopenias
CAR T cells – Risks
May occur within minutes or hours but generally appears within days or weeks Coincides with
earlier line vs later line CAR-T
Real world experience (Sidana ASH 2023)
• Neurologic toxicity 27%
• Prolonged neutropenia 13%
• Prolonged thrombocytopenia 25%
• Secondary primary malignancies 4.5%
Neurologic toxicities in 24% (69/285)
• ICANS 13%
• Peripheral neuropathy 7%
• Cranial nerve palsy in 7%
• Parkinsonism 3%
• Immune mediated 1%
Secondary primary malignancies
CAR-T
• Major questions
• Induction, consolidation, maintenance?
• Sequence relative to similar target but different modality or relative to different target but same modality (another TCE)?
• In combination with other agents, i.e use maintenance after?
• Waiting on faster production, better products, products from stored cells, new targets
• Data so far suggest that CAR-T likely better to do first, but data aggregating
3. BACK TO GENERAL PRINCIPLES
Overall response rate and PFS of recently approved therapies in RRMM
Richardson Blood 2014; 123:1826-32
Siegel Blood 2012; 120:2817-25
Lonial Lancet 2016; 387:1551-60
Rasche EHA 2024, P915
Huang ASCO 2024; 7511 > 4 LOT and triple refractory
Van de Donk ASCO 2023; abs 8011
Lesohkin Nat Med 2023; 29:2259-67
Munshi NEJM 2021; 384:705-16
Munshi EHA 2023; S202

The good news... The bad news.. ..there are many treatment options ..there are many treatment options
Pillars of Myeloma
Opportunities to kill myeloma
CLONAL TIDES

Keats Blood 2012:120:1067-76
Tolerability?
Sequencing?
Duration? Dosing?
SYNERGY
SENSITIVITY
1 + 1 > 3 !
4. MSMART.ORG

First Relapse (2nd Line) Off-Study
Not Refractory to Lenalidomide* Refractory to Lenalidomide*#
Not refractory to Anti-CD38 monoclonal antibody
Refractory to or relapse while on AntiCD38 monoclonal antibody
Not refractory to Anti-CD38 monoclonal antibody
Refractory to or relapse while on AntiCD38 monoclonal antibody
Anti-38 moAB plus PI-dex or Anti-38 moAB plus Pd
*Consider salvage ASCT in patients eligible for ASCT who have not had transplant before # CART may be an option for triple class refractory patients at first relapse or early relapse after quadruplet induction and ASCT
PI, proteasome inhibitor; Preferred PI is bortezomib or carfilzomib moAB, monoclonal antibody: daratumumab or isatuximab PI plus Rd PI plus Cd or PI plus Pd

Second or later Relapse (≥3rd line)
Not Plasma Cell Leukemia (PCL) or Similar extramedullary disease (EMD)
Triple Class Refractory, Type 1*
Refractory to:
• Bortezomib
• Lenalidomide
• Anti-CD38 moAB
Off-Study Treatment Options
Triple Class Refractory, Type 2*
Refractory to:
• Bortezomib and Carfilzomib
• Lenalidomide
• Anti-CD38 moAB
Triple Class Refractory, Type 3*
Refractory to:
• Bortezomib & Carfilzomib
• Lenalidomide & Pomalidomide
• Anti-CD38 moAB


Venetoclax
# Listed regimens are not in the order of preference
Venetoclax-based therapy if t(11;14)

Bispecific Antibody
Venetoclax-based therapy if t(11;14)
*Auto transplant is an option, if transplant candidate and feasible; **If known to be refractory to Daratumumab as single agent, use elotuzumab instead

Refractory MM
Refractory to IMIDs (Lenalidomide and Pomalidomide), PIs
Bortezomib and Carfilzomib), Alkylators, CD38, and BCMA
Options
• Talquetamab
• Another anti-BCMA treatment approach
• Other non-BCMA immunotherapy (eg., cevostamab on clinical trial)
• Selinexor-based regimen
*CVAD or similar regimen can be used in place of VDT-PACE in older patients or patients with poor functional status
• VDT-PACE
v8 //last reviewed May 2024; Dingli et al. Mayo Clin Proc 2017;92(4):578-59
• Alkylator or Bendamustine-based regimens

Secondary PCL or extensive EMD
VDT-PACE or similar to debulk x 1-2 cycles;*
Then: Auto transplant if transplant candidate, or anti-BCMA approach, or Venetoclax-based therapy for t(11;14)
*CVAD or similar regimen can be used in place of VDT-PACE in older patients or patients with poor functional status
Future is bright

Many more novel therapies on the horizon
https://www.dreamstime.com/stock-photos-golden-path-bright-future-eps-image5580023
