
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072
Mr. K. KARTHI-1, Mr. R. KAVI PRAKASH 2, Mr. R. PARTHIBAN 3 , Mr. A. JANARTHANAN 4
1, 2, 3, 4 - Assistant Professors, Department of Mechanical Engineering, Sri Sai Ranganathan Engineering College Coimbatore, Tamil Nadu, India ***
Abstract - The goal of this project is to create a straightforward yet inventive HHO producing system using water electrolysis and assess the impact of adding hydroxyl gas HHO to petrol, which is utilised as fuel in 4stroke engines. The HHO cell is designed to produce the most HHO gas per unit of input power. As the ideal system, potassium hydroxide is used to increase the electrical conductivity to separate the hydrogen in water. The outcome of the project HHO produced 8.4 L/hr Using 6g of KOH with 1litre of water used. This hydrogen is performed with IC engine to achieve more fuel efficiency and to reduce toxic gases.
Key Words: Analysis, Hydrogen Production, IC Engines, Electrolysis, KOH.
1.Introduction
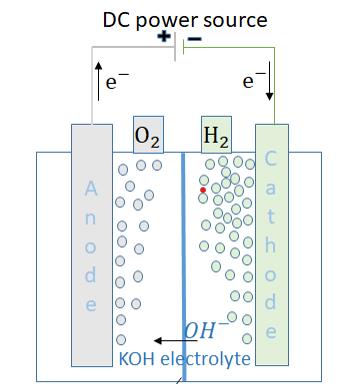
In the modern world, fossil fuels are the in the modern world, fossil fuelsare the mainenergysource forpower plants, autos, etc. When engines burn using fossil fuel, they release noxious gases such carbon dioxide, carbon monoxide,nitrogenoxides,andunburnedhydrocarbons. These pollutants have a number of negative effects, including acid rain, global warming, and several health problems. Recently, a number of alternative fuels have beeninvestigatedinanefforttolessonrelianceonfossil fuels and cut emissions. The state of the art for HHO petrol, a renewable alternative fuel with a number of advantages over fossil fuels, is presented in this study. Water is electrolyzed to create HHO gas using KOH chemical. After adding an electrolyte, a direct current is run through water to initiate the electrolysis process. It causes the solution to become electrically conductive. Wateris broken downinto otherelementsasa resultof the ensuing ionisation reactions Many names for approximate stoichiometric mixture of hydrogen and oxygen gas that electrolyser produces including the hydrogengas,oxy-hydrogen,andHHO.
2. Materials
The stainless-steel plate is used as electrode plates. Dimensions of electrode plates 30×60×2 mm. The hydrogen production shell is made of PVC (polyvinyl chloride)material.Theproductionshellisfullyshieldto avoid leakage of hydrogen gas during production
process. KOH (potassium hydroxide) is a chemical used. KOH is mixed water and the electricity is passed into it. The KOH increases the electrical conductivity for hydrogenproduction.
PU (polyurethane) tube is used to transfer hydrogen from one place to another. Here a plastic can acts as storagecan.Thecanisfilledwithwater,thiswaterhelps topreventbackfirewhileperformingtestinaICengine.


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072
3. Engine specification
Table - 1: ICenginespecification.
Engine type 4 stroke single cylinder
Displacement 97.2cc
Maximumpower 8.02bhp@8000rpm
Maximumtorque 8.05Nm@6000rpm
Boreandstroke 50×49.5mm
Idlespeed 1100rpm
No.ofcylinders 1
Coolingsystem Aircooled
The produced hydrogen is transferred to the engine and calculating the efficiency at a particular millilitre of petrol with and without hydrogen to calculate the efficiencycorrespondingly.
(Note: - before performing ensure the safety measurementsandcheckforleakageofhydrogen,petrol andengineconditionandstarttheexperiment.)
4. Methodology
4.1 Hydrogen production process
The 1lire of water is mixed with 6g of KOH and poured into the production shell. The According to the faradays rule 1.24 volt minimum was based on the useof battery acid. The KOH minimum voltage is1.67 voltage drop. So, theelectrolytesolutionisaffectedbythedrop.Duetothe low voltage drop KOH was chosen. It can be calculated fromoneplate,theproductionofH2 is0.035lit/minand O2 is0.017lit/min.
Electrodes plates anode and cathode are immersed into themixtureofwaterandKOH.Thepotassiumhydroxide helpstoincreasetheelectricalconductivityandhelpsthe produce hydrogen quick and easy. The DC (direct current)isusedthroughouttheexperimentbecauseitis more efficient and has high power comparing to AC (alternatingcurrent).ThehydrogeniscollectedusingPU (polyurethane)tubeandpassedtoastoragecan.Thecan whichactsasastoragereservoirisfilledwithwater.The water in reservoir because when the experiment is performing engine may miss fire and leads to back fire. This back fire can lead to burn and explosive into the productionshell.Accordingtothefaradaysrule1.24volt minimumwasbasedontheuseofbatteryacid.TheKOH minimum voltage is1.67 voltage drop. So, the electrolyte solution is affected by the drop. Due to the low voltage drop KOH was chosen. It can be calculated from one
plate, the production of H2 is 0.035 lit/min and O2 is 0.017lit/min.
4.2 Calculation
Totalproductionofgasfromoneplate(H2O)
Hydrogen=0.035lit/min
Oxygen =0.017lit/min
So,byaddingthehydrogenandoxygenweget, =0.035+0.017
Totalgas=0.052lit/min.
Theamountofhydrogengeneratedfromtheexperiment isabout,
Table – 2: Parametersforproducinghydrogengas.
=N×totalamountofgasgenerated
HereN=noofelectrodeplates =2×0.052
Amountofhydrogengenerated=0.104lit/min.
Calculationfortheparametersintable2;
Thevoltageandamperewerecalculatedusingthedigital multi-meter and amp meter. The amount of 104 ml of hydrogen in seconds is using the stopwatch. The KOH grams are varied like 2g, 4g, 5g, 6g and 8g mixture with water and measuring it at which time it achieves 104ml using stopwatch. Here the measure values are in milli litres.
Weknowthat the total amountof hydrogengenerated = 0.104litre Totalamountofhydrogen×1000

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072
=0.104×1000 =104ml.
This hydrogen gas is held into the IC engine and measured the difference between hydrogen with petrol andwithouthydrogeniscalculatedforthemeasurement ofefficiency.
5. Literature survey
[1] The Water electrolysis method consists of decomposingwatermoleculesintohydrogenandoxygen. We choose alkaline electrolysis which uses hydroxide aqueoussolutionofpotassium(KOH)aselectrolyte
[2] This one can maximize the conversion of solar radiation into chemical energy in the form of hydrogen by water electrolysis hybridizing the solar hydrogen production system, namely using both electrical energy aswellasthermalenergyintheformsteam.
[3] Green hydrogen is booming and the preferred Technology is solar photovoltaic pulse electrolysis. The solar concentrators may produce thermal energy at a costlessthanthecostofnuclearthermalenergy.
[4]Thisisbecauseofitsadvantagesfortheenvironment, theelectrolysisofwatertoproducehydrogen(HHO)has drawninterestasapossiblealternativefuel source.HHO addition to petrol may enhance engine performance, resulting in higher fuel economy and lower emissions, according to research. To completely comprehend the impact of HHO addition on engine performance and emissions,moreresearchisnecessary.
[5] promise of HHO petrol as an alternative fuel is highlighted in this literature review, which also emphasises its production methods, thermodynamics, and chemical kinetics. Additionally, it talks about how HHO petrol affects the performance of IC engines, demonstratingincreasesintorque,power,and efficiency as well as a decrease in pollutants. But obstacles includingsystemcomplexity,cost,safety,andelectrolysis efficiencycontinuetobemajorconcernsforbroaderuse.
6. Performance test
The hydrogen isheld with into the IC 4stroke engine to calculate the efficiency that how long the engine is running at constant idle speed of 1100 rpm with and withouthydrogenismeasuredinthetablebelow.
Table - 3: PerformanceMeasure
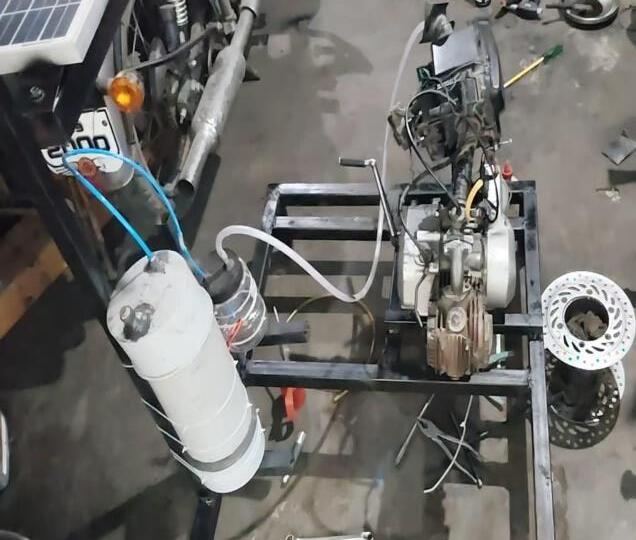
7. Fabrication
The base frame is made and the IC engine, production shell,reservoirismounted.Theproductionshellisshield withoutleakageandtheexperimenthasheldbyrunning theengineusingpetrolandhydrogengas.


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072
Engine Modification: Install a hydrogen fuel injection system and make sure the engine's parts are hydrogencompatible. To account for the altered air-fuel ratio neededforhydrogencombustion,theengine'sairintake systemmustbemodified.
8. Result
Thedifferencewhilethe4strokeICenginerunswithand without hydrogen gas is plotted into a bar chart. This chartsaystheincreasein efficiency ofanIC engine with hydrogen.
(Note: - the use of hydrogen in a normal air-cooled engine causes more heat. So, to rectify this problem the cooling system like oil cooled and liquid cooled can be employed. The tail pipe emission is reduced by varying theair–fuel mixtureinthe carburettorbyadjustingthe fuelscrewintoleanmixture.)
The hydrogen production using electrolysis method is very easy and quick to produce hydrogen. This can be madeintoasmallcompactsizethatsuitsinaautomobile engine to run the engine using h2 gas which gives even more efficient running and lowtail pipe emission. Apart from air cooled engine it is easy to employ it into liquid and oil cooled engines which has a several temperature rangesandcoolthe enginetemperatureaccordinglyand reduce the heating issues. This can be connected in the vehicle battery for generation process. The vehicle battery will automatically charge while the vehicle is in motion.
[1]. Boubekeur Dokkar, Belkhir Negrou, Noureddine Settou, Omar Imine, Nasreddine Chennouf, Abdsslem Benmhidi,OptimizingofPEMFuelCellsforPV-Hydrogen PowerSystem.36(2013),798-807,2013.
[2]. Gheorghe Badea, George Sebestian Naghiu, Ioan Giurca, Ioan Aschilean, Emanuel Megyesi, Hydrogen Production using Solar Energy- technical Analysis, International conference on sustainable Solution for EnergyandEnvironment.112,418-425,2016.
[3]. Alberto boretti, jamal nayfeh, ayman al-maaitah, hydrogen production by solar thermochemical watersplittingcycleviaabeamdownconcentration.9,2021.
[4]Shingane,S.,Dorababu, C.H.,Kumar, P. S.,Naidu,P. L. N., Subramanian, K. R. V., & Rao, T. N. The electrolysis of water to generate hydrogen (hho) and a study of the effect of addition of hho to gasoline as an engine.International Journal of Mechanical and Production Engineering Research and Development (IJMPERD),8,181-186.
[5] Subramanian, B., & Ismail, S. (2018). Production and use of HHO gas in IC engines.International Journal of HydrogenEnergy,g43(14),7140-7154.
[6]Alotaibi,B.,Fan,S.,Nguyen,H.P.T.,Zhao,S.,Kibria,M. G., & Mi, Z. (2014, July). Photoelectrochemical water splitting and hydrogen generation using InGaN/GaN nanowire arrays. In2014 IEEE Photonics Society SummerTopicalMeetingSeries(pp.206-207).
[7] Yoshii, M., Murata, Y., Nakabayashi, Y., Ikeda, T., Fujishima, M., & Tada, H. (2016). Coverage control of CdSe quantum dots in the photodeposition on TiO2 for the photoelectrochemical solar hydrogen generation.Journal of colloid and interface science,474, 34-40.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 07 | July 2024 www.irjet.net p-ISSN:2395-0072
[8] Boretti, A., Nayfeh, J., & Al-Maaitah, A. (2021). Hydrogen production by solar thermochemical watersplittingcycleviaabeamdownconcentrator.Frontiersin EnergyResearch,9,116.
[9] Hu, Y., Yan, H., Liu, K., Cao, H., & Li, W. (2015). Hydrogen production using solar grade wasted silicon.International Journal of Hydrogen Energy,40(28),8633-8641.
[9] Zhang, Y. H., Jia, Z. C., Yuan, Z. M., Yang, T., Qi, Y., & Zhao, D. L. (2015). Development and application of hydrogen storage.Journal of Iron and Steel Research International,22(9),757-770.
[10] Hassani, H., Rekioua, D., Aissou, S., Bacha, S., & Zaouche, F. (2018, December). Hybrid stand-alone photovoltaic/batteries/fuel cells system for green cities. In2018 6th International Renewable and Sustainable EnergyConference(IRSEC)(pp.1-6).
[11] Brayek, M., Jemni, M. A., Driss, Z., Kantchev, G., & Abid, M. S. (2019). Study of spark-ignition engine fueled with hydrogen produced by the reaction between aluminumandwaterinpresenceofKOH.ArabianJournal forScienceandEngineering,44(2),695-705.
[12]Morales,J.G.,Bobadilla,M.C.,Escobar-Jimenez,R.F., Gomez-Aguilar, J. F., García-Beltran, C. D., & OlivaresPeregrino, V. H. (2016). Control scheme formulation for the production of hydrogen on demand to feed an internalcombustionengine.Sustainability,9(1),7.
[13] Vinoth Kanna, I., Vasudevan, A., & Subramani, K. (2020). Internal combustion engine efficiency enhancer by using hydrogen.International Journal of Ambient Energy,41(2),237-240.
[14]Subramanian,B.,&Ismail,S.(2018).Productionand use of HHO gas in IC engines.International Journal of HydrogenEnergy,43(14),7140-7154.
[15]Paparao,J.,&Murugan,S.(2021).Oxy-hydrogengas as an alternative fuel for heat and power generation applications-Areview.International Journal of Hydrogen Energy,46(76),37705-37735.
[16] Subramanian, B., Lakshmaiya, N., Ramasamy, D., & Devarajan, Y. (2022). Detailed analysis on engine operating in dual fuel mode with different energy fractions of sustainable HHO gas.Environmental Progress&SustainableEnergy,41(5),e13850.
[17]Bui, V.G., Tran, V.N.,Hoang,A.T.,Bui,T.M.T., &Vo, A. V. (2020). A simulation study on a port-injection SI engine fueled with hydroxy-enriched biogas.Energy Sources,PartA:Recovery,Utilization,andEnvironmental Effects,1-17.
[18] Manigandan, S., Gunasekar, P., Poorchilamban, S., Nithya,S.,Devipriya,J.,&Vasanthkumar,G.(2019).Effect of addition of hydrogen and TiO2 in gasoline engine in various exhaust gas recirculation ratio.International JournalofHydrogenEnergy,44(21),11205-11218.
[19] Salvi, B. L., & Subramanian, K. A. (2022). A novel approach for experimental study and numerical modeling of combustion characteristics of a hydrogen fuelled spark ignition engine.Sustainable Energy TechnologiesandAssessments,51,101972.
[20] Duenow, J. N., Gessert, T. A., Wood, D. M., Egaas, B., Noufi,R.,&Coutts,T.J.(2008,May).ZnO:Aldopinglevel and hydrogen growth ambient effects on CIGS solar cell performance.In200833rdIEEEPhotovoltaicSpecialists Conference(pp.1-5).
[21] Noro, Y., & Uchiyama, S. (2021). Use of hydrogen storage in an off-grid system for implementing a renewable-energysystem.CleanEnergy,5(4),704-712.
[22] Zhang, W., Liu, S., Gandhi, O., Rodríguez-Gallegos, C. D., Quan, H., & Srinivasan, D. (2021). Deep-learningbased probabilistic estimation of solar PV soiling loss.IEEE Transactions on Sustainable Energy,12(4), 2436-2444.
[23]Wei,W.E.N.,Shuangqi,L.I.,Fei,Z.H.O.U.,Mingte,L. I., Qi, X. I. E., & Chen, S. (2021, March). Stain detection methodofsolarpanelbasedonspotelimination.In2021 IEEE2ndInternationalConferenceonBigData,Artificial Intelligence and Internet of Things Engineering (ICBAIE)(pp.820-824).IEEE.
[24] Holmes-Gentle, I., Tembhurne, S., Suter, C., & Haussener, S. (2023). Kilowatt-scale solar hydrogen production system using a concentrated integrated photoelectrochemicaldevice.NatureEnergy,1-11.
2024, IRJET | Impact Factor value: 8.226 | ISO 9001:2008