The Official Publication of The Tennessee Nursery and Landscape Association

From Chasing to Solving Labor Scarcity
Part II: Advances in Automation within Tasks for Field Production
Plus, Save the Date for TNGRO
September 28 – 29

The Official Publication of The Tennessee Nursery and Landscape Association

Part II: Advances in Automation within Tasks for Field Production
Plus, Save the Date for TNGRO
September 28 – 29


YEAR WARRANTY 6 INDUSTRY LEADING











The Tennessee Nursery and Landscape Association serves its members in the industry through education, promotion and representation. The statements and opinions expressed herein are those of the individual authors and do not necessarily represent the views of the association, its staff, or its board of directors, Tennessee Greentimes, or its editors. Likewise, the appearance of advertisers, or their identification as Tennessee Nursery and Landscape Association members, does not constitute an endorsement of the products or services featured in this, past or subsequent issues of this quarterly publication. Copyright ©2023 by the Tennessee Nursery and Landscape Association. Tennessee Greentimes is published quarterly. Subscriptions are complimentary to members of the Tennessee Nursery and Landscape Association. Third-class postage is paid at Jefferson City, MO. Printed in the U.S.A. Reprints and Submissions: Tennessee Greentimes allows reprinting of material. Permission requests should be directed to the Tennessee Nursery and Landscape Association. We are not responsible for unsolicited freelance manuscripts and photographs. Contact the managing editor for contribution information. Advertising: For display and classified advertising rates and insertions, please contact Leading Edge Communications, LLC, 206 Bridge Street, Suite 200, Franklin, TN 37064, (615) 790-3718, Fax (615) 794-4524.






























TNLA would like to thank the following companies for being
Barky Beaver Mulch & Soil Mix, Inc.
BASF
Blankenship Farms and Nursery
Bobcat of McMinnville
Botanico, Inc.
BWI of Memphis
Cam Too Camellia Nursery, Inc.
Cherry Springs Nursery
Delta Mulch and Materials, LLC
Flower City Nurseries
Gravely NYP Corp.
Putnals Premium Pine Straw, Inc.
Randall Walker Farms
Riverbend Nurseries, LLC
Super-Sod
Swafford Nursery, Inc. Tennessee 811
Tennessee Valley Nursery, Inc.
Warren County Nursery, Inc.
Youngblood Farms, LLC
Bert Driver Nursery
Dayton Bag & Burlap Co.
Mid-South Nursery
Mike Brown’s Wholesale Nursery, LLC
Old Courthouse Nursery
Rusty Mangrum Nursery
Samara Farms
Turf Mountain Sod
Itis always a challenge in this industry when Mother Nature doesn’t go the direction we want. We have always faced these difficulties head on with gusto and the recent events are no different. I think of a great quote from a man who faced extreme challenges providing and protecting his country in a dark time. Winston Churchill once said, “Success is stumbling from failure with no loss in enthusiasm.” With the freeze and late frost, we have had no easy road, and everyone has stumbled through the failure of crops due to weather conditions. We as an industry are always looking to “green times” and good growing weather is now upon us.
As we look forward to good weather for growing, we can also look forward to the opportunity to come out to Field Day on June 27th. This will be a great opportunity to network with other growers and learn about new products and services in our industry. The University of Tennessee will be hosting this event on campus at the UT Garden and Brehm Animal Science Building. Another opportunity to network with other growers and customers will be at our trade show TNGRO held in Lebanon, TN. The board and committee are working diligently to ensure we have a successful show on September 28th and 29th. We also have our golf tournament coming up in October at the McMinnville Country Club. This is a great opportunity to relax from our day-to-day hustle and fellowship with other members and community supporters.
With a great list of events ahead of us, it is hard to look back at the things that make us stumble. We are sure to be successful in our green industry with involvement in activities TNLA provides for us. Being involved in these events and participating in the building up of our association is a great way to ensure prosperity for our industry. I look forward to seeing all of you at these events.
Sincerely,
Terri E. Turner TNLA President











 By Ashley Kite-Rowland, Urban and Community Forestry Partnership Coordinator
By Ashley Kite-Rowland, Urban and Community Forestry Partnership Coordinator

Doyou feel better after a walk in the woods? Less stressed when walking down a tree lined street? If so, you are experiencing what a growing body of research has verified — trees are good for human health and well-being.

Healthy Trees, Healthy Lives, a national campaign to raise awareness of the connections between human health and trees, will officially launch in Tennessee in June 2023. This Tennessee Division of Forestry initiative will highlight the numerous mental, physical, economic, and financial benefits healthy trees offer, not only to individuals who experience them firsthand, but to the entire community. These benefits include decreased asthma rates, increased physical activity, lower rates of depression, greater community connectedness, healthier waterways, lower crime rates, and more.
One key audience of Healthy Trees, Healthy Lives initiative are health care providers. The initiative asks health care providers to recommend spending time outside among trees as a supplement to other care their patients may need, to advocate for tree-filled spaces around clinics and hospitals, and to see community forests as a key component of public
health. By making this connection between trees and health known and having time among trees “prescribed” to patients, community leaders will hopefully begin to consider trees and greenspace to be initial aspects of infrastructure and provide easy access to these health promoting environments to all community members. By increasing access to trees and greenspace through the state from big cities to small towns, we can increase the health and well-being of Tennesseans and the communities in which they live.
To kick off the Tennessee Healthy Trees, Healthy Lives initiative, materials that promote spending more time outdoors among trees, caring for community forests, and ensuring equitable access to greenspace will be shared in medical offices, health departments, counseling centers, schools, and universities throughout the state. If interested in this campaign or helping to spread the word, please reach out to Ashley Kite-Rowland at Ashley.Kite-Rowland@tn.gov. Also, please visit the national website https://healthytreeshealthylives.org. The website provides links to research on the benefits of trees and free resources for you to share the Healthy Trees, Healthy Lives message in your community.

Fulya Baysal-Gurel 1, Dr. Prabha Liyanapathiranage 2 , Dr. Janna Beckerman 3, Dr. Tom Creswell 4 , and Dr. John Bonkowski 5
1 Interim Associate Dean for Research and Research Associate Professor, Otis L. Floyd Nursery Center, Tennessee State University
2 Post-doctoral Researcher, Otis L. Floyd Nursery Center, Tennessee State University
3 Professor of Plant Pathology, Department of Botany and Plant Pathology, Purdue University
4 Director, the Purdue University Plant and Diagnostic Laboratory
5 Plant Disease Diagnostician, the Purdue University Plant and Diagnostic Laboratory
Vascular streak dieback (VSD) describes plants that exhibit a constellation of symptoms including stunted growth, declining trees with water sprouts/epicormic shoots below the dead branches, chlorosis, necrosis, scorching on leaves, tip dieback that may continue into the main stem or stems of the tree and ultimately cause tree death, poor root development, and brown- to- gray vascular streaking of infected branches (Figures 1 – 4). In recent years, multiple laboratories throughout the country have received numerous plant samples with symptoms similar to VSD on multiple hosts. This issue has been detected in seedlings, grafted plants, and older nursery stock produced in container and field
nursery production settings, along with established landscape plants. It is possible that this issue has been in the United States for some time but has been overlooked and/or misdiagnosed.
Positively identifying the causal agent is the first step in diagnosing what is causing VSD in woody ornamentals. Ceratobasidium theobromae (Ct) [synonym, Rhizoctonia theobromae], identified by both molecular tests and isolations, has been consistently found in association with redbud exhibiting vascular discoloration and other symptoms of VSD, in Virginia, North Carolina and Tennessee. However, it has not yet been determined if this fungus is causing the observed symptoms or if it is simply present within the affected plants as wood-inhabiting endophytes or mycorrhizal associates.
To determine if Ct is causing dieback, we must first re-create the disease in the laboratory after inoculating plants with Ct, something that has so far been unsuccessful. The fastidious nature of this fungus (hard to isolate, grow and maintain in culture) presents challenges to obtaining sufficient material for DNA analysis and for inoculation work. Future work needs to be conducted to identify if VSD is caused by Ct alone or if is it caused by multiple organisms, including cankercausing pathogens that may make host plants more susceptible to Ct.
Many redbuds with VSD diagnoses also are infected with common canker pathogens, like Botryosphaeria dothidea, Didymella spp., and Diaporthe spp. Finally, multiple diagnostic tests were used to check for other diseases that cause similar symptoms, including Verticillium wilt, bacterial leaf scorch, and other vascular diseases - all of which were ruled out as causing the VSD symptoms.




Recently, additional VSD-symptomatic tree and shrub species have been found (Table 1). Detections have occurred primarily in nurseries in Virginia, North Carolina, and Tennessee, but individual detections have occurred in nurseries in Indiana, Florida, and Oklahoma. VSD has also been found in newly planted landscape plants, a botanic garden, and in a natural setting in North Carolina.

Acer rubrum, A. xfreemanii, A. griseum
COMMON NAME
Red maple, Freeman maple, Paperbark maple
Amelanchier canadensis Serviceberry
Calycanthus floridus
Sweetshrub
Catalpa speciosa, C. bignonioides Northern and Southern catalpa
Cercis canadensis Eastern redbud
Cornus florida, C. kousa Flowering dogwood, Kousa dogwood
Craetaegus viridis Green hawthorn
Fothergilla spp.
Hamamelis virginiana
Witch alder
Witchhazel
Lindera benzoin Spicebush
Liriodendron tulipifera
Tulip poplar
Magnolia tripetala Umbrella magnolia
Myrica cerifera
Nyssa sylvatica
Wax myrtle
Blackgum
Prunus salicina Chinese plum
Rhus aromatica
Syringa reticulata
Fragrant sumac
Giant tree lilac
According to research conducted by the Tennessee State University (TSU) team in 2022, redbud cultivars with yellow-colored foliage and papery leaf texture were more susceptible to VSD than other tested cultivars. The more VSD-susceptible cultivars began to present leaf-scorching symptoms in late May. VSD-tolerant cultivars that possess dark green and purple foliage and thick leathery leaves exhibited VSD-associated leaf-scorching symptoms in late August. None of the tested cultivars in this trial exhibited 100% resistance to VSD-associated symptoms during the growing season. The TSU team will be continuing with the cultivar screening this year as well, including more redbud species, cultivars, and hybrids received from different regions to identify VSD-resistance. Susceptibility screening information will be beneficial in initiating breeding programs to obtain hybrids with promising horticultural characteristics and increased resistance to VSD.
Since the first detection, grower concern for VSD has increased, leading to rejection of plant shipments, cancellation of redbud orders, and destruction of symptomatic plant material prior to sale by nurseries. As Tennessee is the #1 redbud producer in the United States, the economic impact has been severe, with redbud producers in other states in the southern region, including North Carolina and Virginia, also experiencing economical losses. To identify the extent of economic losses that occurred due to VSD in the southern U.S., Tennessee State University is currently conducting a multi-state survey and the findings of this survey will be crucial in understanding the dimensions of the issue. If you are a redbud producer in the southern U.S., please contact Dr. Fulya Baysal-Gurel (fbaysalg@tnstate.edu) or follow the QR code below to participate in this survey.
Regular scouting of susceptible plants (Table 1) for VSD-related symptoms is highly recommended. Remove symptomatic plants and remove all associated debris associated with symptomatic plants from crop production areas. Have plant material tested by reaching out to a plant and pest diagnostic laboratory regarding their submission forms and shipping recommendations.
Recently, Dr. Baysal-Gurel’s team at TSU was able to develop a novel molecular tool for the accurate detection of Ct from potentially infected host plant material. These real-time polymerase chain reaction primers are currently being tested further by several other institutions. With demonstrated success, these primers may soon be released for use as a diagnostic tool enabling efficient and accurate diagnosis of Ctinfected plant materials.
The use of healthy rootstock and asymptomatic plant chips or buds for propagation is recommended to prevent the spread of the disease. If a rootstock is already infected, it is unlikely that the subsequent grafted plant will be marketable. Infected plant rootstocks will also pose a threat to healthy plants located nearby. Frequently sanitizing tools, blades, and pruners during propagation and pruning practices can also reduce the risk of plant-to-plant disease transmission.
If growers and landscape managers have only incomplete information, what can be done to prevent VSD or limit the spread and severity of VSD?





Pruning provides injuries that may serve as avenues of infection for the Ct fungus. Until we properly understand the epidemiology of VSD, we advise growers to:
1. Minimize pruning to reduce cut surfaces that are receptive to infection.
2. Sanitize pruners with disinfectants.
3. Remove pruned material and debris from the fields and destroy.
4. If pruning is required, the growers can apply protectant fungicide within 24 h of pruning to protect pruning wounds from pathogens.
If you experienced losses due to VSD in a particular area of your nursery, it is advisable to avoid replanting redbuds or other known susceptible host plants (Table 1) into that area for the next few growing seasons.
Excess nitrogen fertilization has been implicated in increasing plant susceptibility to several plant pathogens. Nitrogen fertility should be on the lean side, 50–100 ppm nitrate when plants are actively growing. The TSU team observed that based on foliar nutrient analyses, some of the yellow foliage cultivars that were exhibiting higher susceptibility to VSD also had higher foliar levels of nitrogen, sulfur, and phosphorus, and low levels of micronutrients such as boron, iron, and aluminum. Elemental accumulation conditions in foliage may be a trait associated with the cultivars, yet the results we observed could also be associated with the high degree of susceptibility observed in these cultivars. Future experiments will help determine what is occurring during seasonal growth and how fertilization may be affecting disease development. In the meantime, however, we advise that growers make wise use of fertilizers especially if they are growing susceptible cultivars. It is also helpful to understand that redbud is tolerant of a wide pH range, yet trees grow best in soils in which the pH is above 7.5.
Plants should be planted at the appropriate site, correct depth and be properly spaced in production rows. Trees that are planted too deeply will have less uniform and effective root development, and these trees will have increased exposure to crown and root pathogens (Figure 5).

Redbuds are intolerant of flooding and/or poorly aerated soils. Flooding results in oxygen deficiency and increases the risk of root rot and attacks by ambrosia beetles. Drought stress should also be avoided, as it is associated with increased canker disease severity.
Redbuds are highly susceptible to phenoxy/2,4-D herbicides and other post-emergent herbicides and care should be taken whenever these are being used around young trees to avoid injury. Avoid the use of aminopyralid, triclopyr, and clopyralid herbicides that are known to damage redbud in nursery plantings.
At this time, there are no chemical treatment recommendations as a specific pathogen has not been identified. The TSU team was able to conduct fungicide efficacy trials during 2022 using redbud plants that were naturally exhibiting VSD-related symptoms. Results indicate that foliar application of Postiva (FRAC 3 + 7) at 20 fl oz/100 gal and Mural (FRAC 7 + 11) at 7 oz/100 gal in 14-day application interval were the most effective treatments in reducing leaf scorching associated with VSD on eastern redbud seedling, in 3-year-old plants, and in several 2-year-old budded cultivars that were growing in field soil and container settings. These treatments were also effective in reducing the population levels of canker-causing pathogens such as Botryosphaeria spp. and Didymella spp., yet did not protect plants from new infections nor do treatments cure already-infected plants. Until more information regarding the causal agents(s) becomes available, we can advise that growers focus on management practices that improve the plant’s health while avoiding the practices that create plant stress.
Proper management of soilborne pathogens such as Phytophthora spp., Pythium spp., Rhizoctonia spp., and Fusarium spp. might be beneficial in reducing the damage caused by vascular streak dieback. Management for these pathogens would provide protection against other opportunistic root rots while improving plant health. If using preventative fungicide drenches to protect roots against Rhizoctonia and other soilborne pathogens, fungicides with different modes of action should be applied in rotation. Some recommended rotations include:
• Empress or Heritage (FRAC 11), rotated with Prostar (FRAC 7) or Medallion (FRAC 12) or Terraguard (FRAC 3). OR
• Mural or Orkestra (both FRAC 7+11) rotated with Terraguard (FRAC 3) or Medallion (FRAC 12).
If you encounter redbud plants in nurseries or managed landscapes with VSD symptoms, contact Dr. Fulya Baysal-Gurel at fbaysalg@tnstate.edu.





Athree-part series of articles was developed to provide information to the Tennessee nursery industry and the automation manufacturers who serve the green industry that could guide them in the development, commercialization, and adoption of automation options. The overall goal is to help prepare nurseries for an increasingly labor-scarce future. In Part I “Current Automation Adoption by the U.S. Nursery Industry,” use of a range of container- and field-production automation was described. In Part II “Advances in Automation within Tasks for Field Production” we evaluate the portion of a given task that is automated, focusing on field production. In Part III “Outcomes from Adopting Automation and Perceived Helpfulness Analysis,” we will examine nursery producer perceptions of helpfulness associated with specific pieces of automation and outcomes from using automation. In Part II, like in Part I, we use automation to refer to both automation and mechanization interchangeably.
The portion of a given nursery task that was automated varied by task. The most automated field production task was harvesting, which was 56% automated (Table 1). Even harvesting bare-root liners can be very physical labor, especially when the soil is wetter than desired (Figure 1). Considering the physical labor required to dig a tree by hand and the finished weight of a balled and burlapped tree, it is not surprising to see automation incorporated into this task. Yet there is room for increased automation, considering that roughly half of the task is still accomplished with manual labor. The next most automated task was weed management, which was 51% automated. Tractors and ATVs are already in use in field production, and a wide production block spacing is already established to accommodate these vehicles. These wider aisles also accommodate the use of mechanical weed control and sprayers making post emergence herbicide applications.
Monitoring for pests and making pesticide applications were collectively 47% automated (Table 1). The reported percent of task automated likely reflects the use of air-blast sprayers, other tractor PTOoperated sprayers, and the Enviromist post-emergence herbicide applicator (Micron Group, Bromyard, Herefordshire, England). Mixing and filling tanks and the need to operate tractors and ATVs that pull sprayers likely explain the 53% of this task that is performed by workers.


Currently, there is no commercialized system for automated pest scouting in nursery crops. Although technological advances, such as image recognition and machine learning, make this a possibility in the near future. For example, Dr. Mahmud, a TSU agricultural engineer, recently helped develop an experimental automated fireblight detection system. In Part I, we saw that sprayers equipped with Smart-Apply® Intelligent Spray Control System™ (Smart Apply Inc., Indianapolis, IN) and SmartSpray (Durand Wayland, LaGrange, GA) already are in use at just 3% of nurseries. These technologies, therefore, represent a commercially available but under-adopted technology. An automated herbicide applicator for nursery production also is currently in development at Carnegie Mellon in partnership with Hale and Hines Nursery.
Planting and fertilizer application were both 49% automated in field nurseries in 2020. While a mechanical planter is often used to move soil, many laborers are still required to plant a crop. Workers cut bundles of bare root liners and prune roots or remove liners from containers to prepare them for planting. Other workers transport liners to the planting site, hand prepare liners for workers on the setter, place the liner in the furrow, straighten liners and firm the soil around them. One additional worker operates the tractor that pulls the setter. Some growers are adopting larger planters to achieve greater efficiency; however, these planters require more people working to prepare and simultaneously plant the increased number of liners (Figure 2). Therefore, while efficiency is gained and more plants can be planted in the same amount of time, the reliance on labor is ever-present. Even an applicator that broadcasts or applies a band of fertilizer requires an operator to drive and refill the hopper. Depending on the choice of fertilizer selection and its potential expense, for example high-cost controlled release field fertilizers, the application method may rely entirely on manual labor to provide a per-plant application made with a spoon or cup, similar to applications in container production.
Transporting plant material was 46% automated. Tractors pull wagons, however, wagons are usually loaded and unloaded by hand. Tree Boss® (Tree Equipment Design Inc, New Ringgold, PA) and other articulating ball-handling equipment may expand the plant handling tasks that can be automated. Portable conveyers have been adopted rapidly in container production and may have a place in transporting bareroot liners considering their weight is not unlike container crops (See side bar).
An Ohio, U.S. nursery producing bare-root nursery crops is using telescoping conveyers (MaxxReach®, FMH Conveyers, Jonesboro, AR) at their docks to eliminate the need for workers to walk each armload of liners to the front of the shipping container, making this task more efficient. In much the same way that portable racks have improved labor efficiency required to move groups of small container crops, telescoping conveyers and similar technologies have the potential to dramatically improve loading efficiency of field-grown crops. Sources: McClellan, 2018a and Spirgen, 2019
Turner and Sons Nursery is a bareroot and container operation in Tennessee, where labor is at a premium and for which labor costs recently nearly doubled. That caused John, Terri, and sons Lee-Allen and John Adam, to start thinking about more efficient ways to complete tasks. They recently acquired four tying machines (Figures 3 a and b) at a cost of $7,300 each that bundle trees approximately four times as fast as their workers could bundle by hand. The tying machines allowed the business to re-allocate 2-3 workers from their limited workforce to assist with grading or harvesting trees. While expensive, the tying machines have helped Turner and Sons Nursery extend their limited workforce and complete tasks on time.
An Oklahoma nursery now uses a custom root pruner instead of hand pruning bare root liners that has helped their operation reduce the number of employees required for that task from roughly 27 employees to three. At Hale and Hines Nursery, a single employee with a gantry-style pruning machine (Figure 4, page 22) is now able to prune as many plants as four workers. After adopting this gantry-style pruner, pruning expense was reduced by approximately $0.10/plant. This pruner had a pay-back-period of two years based on use on just one of the two crops it is used to prune. These pruning machines demonstrate the effectiveness of automation and mechanization in addressing the nursery labor shortage. In both cases, nurseries were able to re-allocate workers to other, non-automated tasks. On a recent visit to Cherrylake Tree Farm in Groveland, Florida, nursery workers were using battery operated pruners with a protective glove to simultaneously eliminate hand and wrist strain while also protecting the worker from severing fingers. Workers also stated they felt they could prune faster and make a cleaner, better, more controlled cuts with the electric pruners. Similar pruners include Infaco Electrocoup Battery Pruner F3015, and Bahco Electronic Pruner BCL22, and Zenport EP2. The Infaco wired model costs approximately $1,300 and requires charging the batteries and sharpening blades about every three days.






Pruning is 23% automated at field nurseries in the U.S. (Table 1), similar to container nurseries (25% automated). Most nurseries have crews of several workers who prune blocks of shrubs at a time. Many crops must be pruned multiple times per season. With a reduced workforce, there is an opportunity to increase the portion of this task that is automated and then reallocate that labor to tasks that are not easily automated. Additionally, in listening sessions, growers listed pruning as a particularly helpful task to automate given their expectation that improved efficiency could increase timeliness in completing the task, aid in improving crop uniformity, and enhance predictability in crop scheduling (e.g., to promote flowering during a targeted sales window). Some nurseries have experimented with automated pruning systems. For example, motorized, gantry-style mechanized pruners require two workers: a driver and a worker walking behind with shears to remove any missed branches (Figure 4). Other growers have custommade or modified large combine-style multi-row pruners that better meet their production needs.

Employee training, labelling, pulling orders, and inventory tracking tasks each are less than 21% automated. Some online programs exist for training employees, including various pesticide certification trainings, and programs such as the University of Tennessee Original and Advanced Tennessee Master Nursery Production programs www. tnmasternursery.com. Labeling is a highly repetitive, low skill task. Automated labelling could allow workers to be placed in more enriching tasks that add more value to the crops, possibly aiding in worker retention. Inventory management requires years of experience to correctly identify plants, gauge their health, and assess additional growth that will occur before harvest. Repeated counts and the changing status of individual plants due to sales and changes in health and size make inventory assessments a time-intensive task. Recently, container producers have experimented with sensors connected to a monitor that detects and accounts for each plant as it is potted (ScoreBoard System,
AgroNomix, Oberlin, OH). This technology could be adapted for field production and include GPS location of crops. Currently, Smart Guided Systems (Indianapolis, IN) offers a laser-based inventory system that can be mounted on an ATV or tractor and detects individual plants in the field. Other image-based technologies that may set the stage for counting the number of plants at a nursery or in a given production block include research by Dr. Hao Gan, UT Agricultural Engineer, and collaborators who developed experimental systems that detected both fruit and flowers. Pulling orders is just 13% automated and, like inventory, represents a task that integrates plant identification skills, discerning plant quality and grade, calculating anticipated future growth, and tagging. The judgment-based skills may be harder to automate. Collectively, the overall percentage of automation for all field production tasks is low at 35%, an amount similar to automation in container production (33%). Laborers are needed in nurseries to ensure the quality of the plants produced and perform tasks that require a significant amount of judgment or are otherwise difficult or expensive to automate. Likewise, tasks may be impractical to adopt due, for example, to infrastructure constraints. However, automation offers an opportunity to alleviate the labor strain and aid in production efficiencies, reduce employee injuries and physical strain, improve worker satisfaction, and maintain quality standards of the end products. This opportunity has never been greater because more technological developments and automated products are being introduced each year. University scientists, Extension personnel, and engineers are working with industry stakeholders to better integrate automation technologies in nursery production and ultimately help nurseries remain sustainable for the long-term. This is an exciting time to be in the nursery industry!
LEAP Labor, Efficiency, Automation and Production. Virtual Nursery Automation Tours. https://www.nurseryleap.com/virtual-nurseryautomation-tours.html
McClellan, M. 2018a. Ahead of the curve. Nursery Mgt.: GIE Media, Inc. https://www.nurserymag.com/article/willowbend-nurseries-automation/
McClellan, M. 2018b. Don’t wait, automate. Nursery Mgt.: GIE Media, Inc. https://www.nurserymag.com/article/five-tips-automation/ McClellan, M. 2021. Money matters. Nursery Mgt.: GIE Media, Inc. https://www.nurserymag.com/article/how-are-nurseries-spending-cash/
Spirgen, K. 2019. Rack ‘em up. Nursery Management. https://www. nurserymag.com/article/racks-instead-of-boxes-cultivate-19/.
The authors acknowledge Lauren Fessler and Lilia Provoda for technical assistance, Dr. Jim Brosnan, Mr. Heath Nokes, and Dr. Annette Wszelaki for review of an earlier version of this article. The coauthors also acknowledge the LEAP Advisory Board, Cherrylake Tree Farm, Decker’s Nursery, Turner and Sons Nursery, and Hale and Hines Nursery for their generous contribution to the LEAP project and for sharing their nursery’s experiences with automation. USDA NIFA SCRI award 202051181-32137 and USDA NIFA Hatch Projects TEN 00575.








Hello Tennessee! My name is Midhula Gireesh and I have recently been hired as a faculty member in the Entomology and Plant Pathology Department at The University of Tennessee. As an Extension Entomologist, I will be involved in diagnosing and managing arthropod pests of ornamental plants, nursery plants, and turfgrass. I am looking forward to meeting as many of you as possible during my initial months in this position. My official start date is June 1, 2023, and will be based at the Soil, Plant, and Pest Center in Nashville.

Until we get to meet in person, here is a little of my background. I grew up in Kerala, a southwestern coastal state of India, which is known as “God’s Own Country” for its natural beauty and vibrant culture. During high school days, the concept of “hybridization” taught in a biology class grabbed my attention. Moreover, I was curious to learn about plants. This compelled me to take a double major in Botany and Biotechnology for pursuing my BS. For my post-graduate studies, I chose MS in Genomic Science, which deals with the advancements in genomics and genetics. After completing Bachelors and Masters from India, I moved to the US to pursue my doctoral degree in Entomology at the University of Georgia in 2018.
During my doctoral program, I was exposed to the world of insects and agriculture and took a 180° turn in my career goals. My doctoral research involved developing Integrated Pest Management (IPM) strategies for hunting billbugs in the turfgrass production (sod) farms of central Georgia. My initial research goals were to record the major billbug species, their seasonal occurrence, abundance, and movement activity in the turfgrass. Later, my research focused on determining the influence of abiotic factors on the movement of hunting billbugs under field, semi-field, lab conditions and the spatial distribution of hunting billbugs. Ultimately, the aim was to improve the sampling plan and develop an IPM strategy for hunting billbug problems in sod farms. Additionally, I have conducted a survey to determine the major turfgrass pests and current management practices adopted by golf course superintendents and sod producers.
Soon after my graduation, I received the opportunity to work as postdoctoral research associate at the Gulf Coast Research and
Education Center, University of Florida. In September 2021, I joined the Small Fruit Entomology lab where my research focused on developing integrated pest management strategies for thrips pests of strawberry in Florida. My applied research involved manipulation of available management strategies including cultural control, biological control, and chemical control to suppress chilli thrips (Scirtothrips dorsalis Hood) and twospotted spider mites (Tetranychus urticae Koch) populations in conventional and organic strawberry fields. Besides research responsibilities, I have utilized opportunities to foster my skills in extension through face-to-face interaction with growers, field visits, extension meetings, presentation, and publications. I have been an active member of the Entomological Society of America since 2019. Music and tea are my favorite stressbusters. In my free time, I love to sing while having the perfect cup of tea. Also, I catch up with my family and friends back home, and I enjoy cooking.
When I arrive in Nashville in June, I am looking forward to meeting with Green Industry professionals to identify your specific IPM needs. Once specific IPM needs are identified, I will be integrating my academic knowledge and research expertise to study various aspects of IPM. I truly believe in face-to-face interactions with the growers and will arrange as many farm visits as possible and work closely with the county Extension agents. My broader interests are to conduct problemsolving research that eventually develops into an effective, sustainable IPM program while benefiting Tennessee’s clientele. Through collaboration with turf, ornamental and nursery specialists and industrial partners, I will make sure that all clientele have access to and benefit from my research and extension program.
From my experience in UF IFAS, and before that in Georgia, I believe that identifying specific needs from the grower is critical in developing a successful program. Through my doctoral and postdoctoral programs, I have had the opportunity to collaborate with entomologists, horticulturists, sod producers, golf course superintendents, strawberry growers, and industries. I believe these experiences will be valuable in developing insect management programs, and I hope to utilize my skills and expertise as an extension entomologist serving the needs of the nursery, ornamentals and turfgrass industries in Tennessee.

Hello, Tennessee! I am excited to introduce myself as a newly appointed faculty member of the Entomology and Plant Pathology Department at The University of Tennessee. My name is Nar Ranabhat, and I will be serving as an Extension Plant Pathologist specializing in disease management of landscape plants, nursery plants and turf. I begin at UT on July 1 and I will be based at the UT Soil, Plant, and Pest Center in Nashville. I am eager to meet with all of you during my initial months and to learn about your businesses, production systems, and disease problems. I look forward to working with you in the upcoming growing season.
Prior to the official start date, I would like to share a little bit about of my academic background with readers of “Tennessee Greentimes” and “Tennessee Turfgrass” magazines. My passion for plant pathology was ignited during a school trip to the breathtaking Rhododendron Forest in the mountains of my hometown, Pokhara, Nepal. While on this trip, my high school teacher spoke to us about the importance of protecting these stunning flowers from pests and pathogens.
Before coming to the USA, I studied the many benefits that insects provide. As an undergraduate student, I conducted a survey of pests and predators of honeybees. After graduating, I taught for a few years and then moved to the University of Hohenheim in Stuttgart, Germany where I worked on a research project exploring the suitability of pollen as an alternative food for predatory mites, which are biocontrol agents of small arthropods such as spider mites and thrips, in high-value ornamental crop systems.
I began my career in the field of plant pathology at Montana State University (MSU), where I earned my master’s degree. My research projects focused on utilizing integrated management practices to manage viral and fungal pathogens, such as Rhizoctonia, Fusarium, Pythium, and Sclerotinia, which also cause major diseases in turf.

During my time at MSU, I conducted on-farm studies with local growers, which provided opportunities to interact with them and share our research findings during field days.

Later, I pursued my doctoral degree at Kansas State University (KSU), where I delved into using advanced pathogen identification tools to identify wheat viral pathogens. These viruses have a complex disease cycle, can live in grassy alternative hosts, and are transmitted by mites, making viruses difficult to manage. Accurate identification helps developing disease resistant varieties. I collaborated with multi-state breeders and private companies to develop disease-resistant wheat varieties that would yield better under Kansas growing conditions. During my post-doctoral research at KSU, my research has been focused on the development of advanced tools to accurately identify bacterial pathogens both in field conditions and in the lab. These advanced diagnostic techniques can be used to identify diseases of great importance to the Tennessee Green Industry.
When I arrive in Tennessee, I am looking forward to learning about the diseases that challenge your production systems. During my initial months, I will closely work with growers and county extension agents and plan to conduct a needs assessment to prioritize extension and research program efforts. I will initiate collaborative research of the major diseases problems and share research results with stakeholders to provide them with options.
I am excited about the opportunities to work with nursery growers and landscape industries on plant disease management, and I look forward to knowing you and your high-risk pathogen(s). Together we will find solutions that fit with your production system. Stay tuned for more updates, and I cannot wait to meet you all!
For more information, please contact Department of Entomology and Plant Pathology, Knoxville TN and UT Soil, Plant and Pest Center, Nashville, TN.

Brute 65
•1200 lb. capacity
•Cart size 34”x61” easily fits through gates
•Up to 65 gal containers/32” ball
Easy Anchoring:


•Helical flights cut through the soil 3” or 4” for every 360° rotation
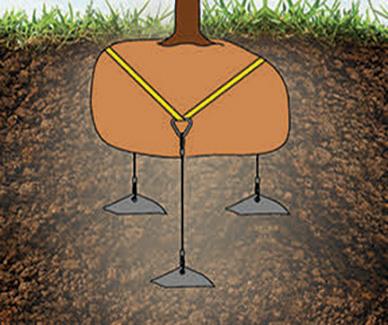

•Solid steel shaft with chiseled lead edge and welded closed eye

•High holding capacity: up to 3500 lbs
•Easy to remove and reuse
•No power tools needed to install
*3 models available: 15” to 30”
Brute 100
•1600 lb. capacity
•Cart sizes 45”x61”
•Up to 100 gal containers/44” ball
•Multi use: boulders, bags, timbers
Diablo B&B Tree Ball/ Container Cart

•1200 lb. capacity
•Cart size 34” x 61” easily fits through gates
•Up to 32” ball, 23” depth on lip
•Tow/lifting loop
























