FLUID THERAPY BOOK





Welcome
This booklet has been produced as a guide only and is not intended to replace official training and education.
Fluid therapy should only be undertaken when there is a clinical indication to do so and under the supervision and guidance of a veterinary surgeon.
Whilst every precaution has been taken in the preparation of this booklet, Millpledge Ltd do not assume any responsibility for errors or omissions, neither is any liability assumed for damages resulting from the use of the information contained within.
Written and compiled by Karen Dean RVN, Helen Stephens VN, DipAVN (surg), Jack Newton RVN Cert TESOL.
Edited by Meryl Lang, Angela de-Zille RVN, Melissa Matthews RVN & Sarah Cornall RVN.
Thanks to Sarah Shipley RVN, Lonnie Dorrill SVN, & Ellie Gillron at Midsummer Vets, Milton Keynes for their valued assistance and allowing us use of their practice for many of the photographs within this publication. Thanks also to Gemma Blundell for allowing us to use her rough collie Logan, and to him for being so patient and understanding!
First published 2011 ©Millpledge Ltd. This edition ©2025

Working in partnership with industry experts, Lantra has developed the Level 3 Work-based Diploma in Veterinary Nursing, which has full accreditation from RCVS, as well as the end point assessment apprenticeship standard (equivalent to a Modern Apprenticeship in Scotland and Wales).
Lantra is very pleased to be associated with the Millpledge Fluid Therapy Book. ‘A great resource for all those in clinical practice’


Distribution Of Body Fluids
60% of an adult healthy body weight is made up of water or fluid and this is called Total Body Water or TBW. This TBW can be split into two areas; Intracellular fluid and extracellular fluid.
The Intracellular fluid equals 40% of the animal’s bodyweight / approx 66% of TBW. This is the fluid that is found inside the cell wall ( cytoplasm) and is responsible for transporting nutrients and gases within the cell to keep it alive.
The remaining 20% of body weight / 33% of TBW is found in the extracellular fluid and this can be further split into 3 different areas.
• 8% of TBW is found in the intravascular space as plasma, the liquid portion of blood.
• 24% of TBW is found in the interstitial space (the areas between the cells) and this bathes the cells and provides nutrients.
• 1% of TBW is found in the transcellular fluid which is manufactured by specialist cells to produce fluid such as saliva, tears, digestive fluid, urine, synovial fluid, cerebrospinal fluid, and is also found in the epithelial lining of the pericardium, peritoneum and pleural space.
Fluid is distributed to the areas that require it on a regular basis. The body achieves this by monitoring the levels of sodium in the Intracellular and extra cellular compartments. Normally, the level of sodium is equal, but when the levels are not of equal amounts, fluid will pass via a semi permeable membrane from the area of lower concentration to the area of higher concentration until an equilibrium has been reached. This process is called Osmosis. Osmosis is therefore responsible for the movement of water from the interstitial fluid into the cells and vice versa as required.
The pH of blood is dependent upon the concentration of hydrogen ions in the blood.
The normal pH of blood is 7.35–7.45; this must be maintained for normal cell function to occur. The levels of hydrogen ions in the blood are determined by the amount of bicarbonate and carbon dioxide, which is regulated by the kidneys and respiration. Carbon dioxide is carried in the blood as carbonic acid. A change in pH occurs when a disease process causes a decrease or increase in either hydrogen or bicarbonate.
Acidosis happens when the blood pH is below 7.35. It may be due to a loss of bicarbonate or an increase in hydrogen ions. Acidosis can be metabolic, being caused by acute renal failure, diabetic ketoacidosis, vomiting, diarrhoea and shock. In addition Acidosis can also be respiratory and caused by the animal hypoventilating.
Alkalosis is also broken down into metabolic and respiratory. Alkalosis occurs when the pH of the blood goes above 7.45, and is caused by a rise in bicarbonate or a loss of hydrogen ions. Metabolic alkalosis occurs when an animal has been vomiting, or receives an over infusion of solutions that contain bicarbonate. Respiratory alkalosis is causedby hyperventilation.
The aim of fluid therapy should be to treat the underlying cause and then in turn the acidosis or alkalosis will also be corrected.
Arterial blood gas analysis will detect acidemia or alkalemia and can help differentiate between metabolic or respiratory.
Distribution Of Body Fluids
(Acid Base Balance)
CO2 in the body is converted into Carbonic acid which can quickly be turned into bicarbonate therefore maintaining the neutral pH (equilibrium).
Generally the CO2 levels and the bicarbonate levels effect each other i.e. if CO2 level is high the bicarbonate level is low.

Respiratory Acidosis Metabolic Acidosis
Meaning CO2 Bicarbonate Bicarbonate CO2
(Hypoventilation)
Poor ventilation so get increase in CO2 i.e. pulmonary disease. Due to acute renal failure, diabetic ketoacidosis, vomiting, diarrhoea, shock. Can be due to bicarbonate overdose or prolonged vomiting. Due to Hyperventilation.
Secondary Effect
Mode of Compensation
Possible Treatments
Kidneys will retain bicarbonate therefore increasing the pH.
Need to ensure breathing is adequate i.e. treat the cause of the decreased ventilation.
Lungs ‘blow off’ CO2 (increase respiratory rate) to decrease the level of CO2 and therefore increase bicarbonate levels.
Kidneys will decrease the amount of bicarbonate they excrete.
Some cases require IV administration of sodium bicarbonate solutions.
• One or a combination can occur at any given time.
Lungs will retain CO2 (slow the respiratory rate) thereby decreasing the bicarbonate levels.
Kidneys will increase the amount of bicarbonate they excrete.
Treat with antiemetics if persistent vomiting. Possible IV administration of an isotonic sodium chloride solution.
Kidneys will excrete more bicarbonate thereby decreasing the pH.
Encourage slowing of respiration rate, or maybe breathe into bag/mask to induce rebreathing of CO2
• The lungs can make rapid changes to the pH levels, the kidneys can take days to make changes.
The processes involved in the body to maintain acid base balance are very complex, the table above demonstrates it in a simplified manner.
Reasons For Fluid Therapy
Fluid intake must be sufficient to meet body needs and prevent dehydration.
The normal healthy animal is able to maintain normal function by matching the output of fluids, with the intake of water, which is obtained from two main sources.
• Ingestion – Food and fluids
• Metabolism – Fats and carbohydrates


Animals can lose fluid through 4 main routes:
• Kidneys – The animal can regulate the amount of fluid lost via the kidneys / urine by concentrating the urine to reduce fluid loss when water availability is limited.
• Gastrointestinal tract – Loss occurs via normal faeces and from vomiting and diarrhoea.
• Respiratory tract – The animal loses fluid through breathing and panting, because air is humidified as it passes through the respiratory system.
• Skin – Fluid is lost via sweating.

The animal will lose water via natural means and these are called insensible and sensible losses.
Insensible loss
This relates to fluid lost via natural means but where the animal is unaware of its loss, nor is there a method to calculate the amount of fluid lost in this way. In times of water deprivation, the body has no ability to conserve fluid lost in this way and it will occur, no matter the condition of the patient.
• Respiratory/skin loss – 20 ml/kg.
Sensible loss
A sensible loss is a loss of fluid via natural means. The animals senses this loss and is aware it has occurred. In times of water deprivation, the body can employ systems to conserve the amount of fluid lost this way, to be utilised elsewhere. It is also possible, in the case of urination, to measure the amount of fluid lost this way.
• Normal urinary loss – 20 ml/kg. • Normal faecal loss – 10–20 ml/kg.


Sweating
Many medical or surgical conditions and injuries can upset the normal water balance of an animal:
• Dehydration due to vomiting or diarrhoea. For the purposes of calculations the amount of fluid lost by the animal per episode of vomiting or diarrhoea is 4 ml/ kg bodyweight.
• Significant blood loss.
• Hypovolaemia.
• Shock
Animals can also lose water via:
• Metabolic and endocrine disorders
• Acidosis and alkalosis
• Urinary obstruction
• Renal failure
• Hepatic disease
• Prolonged surgical procedures
• Medication
• Medication – Some types of medications such as diuretics can cause an animal to lose fluid.
• Non-medical/surgical conditions:
Situations that cause an animal to be stressed i.e. travel, stress, environmental changes, change in diet, could all cause conditions such as diarrhoea or excessive panting, which in turn could affect the water balance of an animal.
Assessing Dehydration
It is important to assess how dehydrated an animal is before you commence any fluid therapy. There are several clinical and laboratory methods used to assess how much fluid an animal has lost. We will look at a couple of the more simple ones.
History
Allows you to assess how much fluid the animal has lost. The owner should be asked:
1. Has the pet been eating and drinking normally recently?
2. Has there been any vomiting or diarrhoea, and how many episodes have there been?
3. Has the animal been passing urine normally?
4. Has the animal had any abnormal discharge?
Laboratory Analysis
There are some simple lab tests which can help estimate fluid loss:
1. Packed Cell Volume Test (PCV) – Inexpensive and quite revealing. For each 1% increase in PCV a fluid loss of 10 ml/kg has occurred. Care should be taken if blood loss has occurred as the patient will have lost both blood and fluid, therefore the PCV result will be uneliable.
2. Haemoglobin – Dehydration results in an increase in haemoglobin values, but care should be taken when interpreting results from an anaemic animal.
3. Total Plasma Protein (TPP) – Dehydration causes a rise in values. This can be measured from either a biochemistry test or from your refractometer. Measuring the total plasma protein is done in a similar way as measuring the specific gravity of urine.
Clinical Signs
These are useful but not always accurate when used alone.
% Dehydration Values
< 5 %
5 – 8 %
8 – 10 %
10 – 12 %
> 12 %
Clinical Signs Shown
No clinical signs
Slight skin tenting Slight increase in CRT (capillary refill time) Tacky MM
3rd eyelid visible
Deficient or no tear production resulting in dry eyes
Obvious skin Tenting Sunken eyes Prolonged CRT
Skin stands in place Oliguria (< 0.5 ml/kg/hr) Shock
Progressive shock Coma Death

Packed Cell Volume Test (PCV)
The PCV is the percentage of whole blood that is taken up by the red blood cells. The PCV will fall in patients with anaemia and may be elevated in patients, that are dehydrated.
Normal PCV
The Packed Cell Volume or PCV is measured by spinning a capillary tube of whole blood in a centrifuge at very high speeds. This enables the whole blood to be separated into its different components, from which the measurement of the red blood cells or RBC’s can be taken. To carry out a PCV, a blood sample should be obtained from your patient in the usual way, using an anticoagulant such as EDTA or Heparin. Heparinised capillary tubes are also available to enable a blood sample to be transferred directly from the patient, into the capillary tube. A haematocrit test or HCT is the same test as a PCV but is carried out and measured completely by a specified laboratory machine (often the terms PCV and HCT are interchangeable).
How to determine the PCV
1. Invert the blood tube to ensure that sample is thoroughly mixed.
2. Wearing gloves, three-quarters fill two capillary tubes by holding both the blood tube and capillary tube at an angle to enhance the flow. Most capillary tubes will have a ‘fill’ mark.
3. Place a finger at the top end of the capillary tube to prevent blood flowing out.
4. Wipe the capillary tubes clean of excess blood with a piece of lint-free tissue.
5. Plug opposite end of the tube with soft clay or haematocil™.
6. Place both capillary tubes on opposite sides of the centrifuge, with the plug against the rubber rim of the centrifuge.
7. Close and lock the centrifuge lid. Centrifuge at 10,000rpm for 5 minutes.
8. Remove the capillary tubes and measure PCV using a microhaematocrit reader or, with a ruler. The sample will be divided into three layers.
i) Plasma.
ii) White blood cells (buffy coat).
iii) Red cells.
How to measure your PCV with a ruler
The PCV is calculated by:
Measuring the length of the red cell layer and dividing it by the total length of all 3 layers, this figure is then multiplied by 100 to give the PCV as a percentage.
For each 1% increase in PCV, a fluid loss of 10 ml/kg bodyweight has occurred. Combined interpretation of PCV and Total Protein (TP)

The above diagram and photo displays how whole blood is separated after being centrifuged.
Routes For Fluid Therapy
The route chosen to administer fluids will depend on the species, and size of the patient, the reason for fluid therapy and the fluid to be administered.
1. Oral – Inexpensive to the practice as no specialised equipment is needed. The fluid does not need to be sterile. This method is not recommended for acute or emergency cases and should not be used for cases where the patient is vomiting. Manually administered oral fluids, via syringe, can be highly stressful and could potentially result in aspiration pneumonia.



2. Peripheral Intravenous – Cephalic (foreleg, pic 2a), saphenous (Hind leg, lateral pic 2b or medial pic 2c), marginal ear vein (commonly used in rabbits pic 2d) or the jugular vein may be used but specialised equipment is required for this. Used for all cases of dehydration and shock, where rapid replacement of circulating blood volume and electrolyte adjustment is required.
The peripheral route is the only route that is chosen for hypertonic solutions. Surgical preparation of site is required. Cannulae should be checked at least once daily and changed routinely every 3-5 days or sooner if required.





3. Subcutaneous – Only appropriate for mild dehydration. Multiple sites over the shoulders, back and hindquarters can be used. Only use non-irritant and isotonic fluids. Give 10ml / kg, this is absorbed over 6 – 8 hours so not appropriate for emergency or acute conditions (Pic 3a & 3b).
4. Intraperitoneal – Fluid can be injected intra-abdominally. Useful in small mammals and very young animals. Not appropriate for emergency or acute situations. Surgical preparation of the site is required (Pic 4) and care needs to be taken to avoid puncturing an internal organ.
Routes For Fluid Therapy
5. Intraosseous –Very useful in small mammals, reptiles, young animals or in animals where severe shock has made peripheral venous access difficult. Using an intraosseous or spinal needle, sites for access are medial aspect of trochanteric fossa of the femur, flat medial surface of proximal tibia 1 –2 cm distal to tibial tuberosity, cranial aspect of greater tubercle of humerus or the wing of the ilium. Absorption rate is excellent and similar to that of a peripheral intravenous cannula. Ensuring good skin preparation of the site is vital, as risk of infection is high.
Greater tubercle of humerus
Sites for intraosseous fluid therapy
Tibial tuberosity
Wing of ilium
Trochanteric fossa
Calculating Fluid Requirements
In order to estimate the fluid requirement for an animal, you must first calculate how much the animal has already lost (fluid deficit) and how much the animal needs on a daily basis (maintenance).
We can use the PCV to tell us how much as a percentage the animal is dehydrated and we can also use clinical signs.
For every 1% increase in PCV or dehydration, the animal has lost 10 ml/kg bodyweight.
Every time the animal vomits or has diarrhoea, we must add 4ml/kg bodyweight/per episode to the calculation.
Maintenance fluids
Rabbits
0 – 10kg
100 mls/kg/24 hours
Cats & small Dogs 0 – 10kg
ml/kg/24 hours Medium Dogs
ml/kg/24 hours Large Dogs
These calculations refer to the administration of Crystalloid fluids only.
Example calculation
– 50kg+ 40 ml/kg/24 hours
You are presented with a 30kg Dog, which has vomited once. After running a haematology blood test the PCV result is 50%.
How much fluid does the patient require?
Calculating Fluid Requirements
1. Work out the fluid deficit:
PCV of 50% (normal PCV is 45%) equals a 5% increase in PCV.
The animal has vomited once. So...
2. Work out maintenance requirement:
3. Total Fluid Requirement:
This fluid should be given over 24 hours unless the animal is showing signs of severe shock and hypovolaemia. In this case the deficit should be replaced over 4 – 6 hours and then the remaining maintenance fluid given over 24 hours.
Calculating The Drip Rate
Once you have calculated how much fluid the animal needs you must then calculate the drip rate.
1. Calculate ml per hour:
If you are using an infusion pump or if using an Aniset™ Dial-a-flow (see page 30) this may be as far as you need to take the calculation as most infusion pumps require the mls per hour to be input into the machine
2. Calculate ml per Minute:
3. Calculate the drops per minute:
You will need to know how many drops your giving set delivers per ml. Standard Aniset™ sets are usually 20 drops/ml and paediatric are usually 60 drops/ml, but check the packaging. An Aniset™ (standard 20 drops/ml giving set) is being used in this case.
4. Calculate drip rate:
High Performance Cohesive Bandages. Provides protection and support for tendons & ligaments whilst being practical, hardwearing and cost effective. Rapz Eazy Tear™ bandages are available in a wide variety of vibrant prints and colours.








Cohesive ‘Chewy NoNo™’ Safety Bandage
Identify patients who have an IV cannula in situ or those who are recently out of surgery. The addition of the bad taste ‘Chewy NoNo™’ flavour deters biting, chewing and licking of the bandage.

Fluid Therapy Chart
Fluid should be given over 24 hours unless the animal is showing signs of severe shock and Hypovolaemia. In this case the deficit should be replaced over 4 - 6 hours and then the remaining maintenance fluid given over 24 hours. These calculations refer to the administration of Crystalloid fluids only.
Rabbits
Cats/Small Dogs
Always ensure that all luer lock connections are secure during fluid administration.
*This value should NEVER be rounded up as this will lead to over-infusion. **Using a 20 drops/ml giving set.
Cats/Small Dogs 60ml/kg/24 hours
Medium Dogs 50ml/kg/24 hours
Large Dogs 40ml/kg/24 hours
Types Of Fluid
The type of fluid chosen, will be dependent upon the patient condition, the route of administration, and the desired outcome. The choice of fluids may be crystalloids, colloids, whole blood and blood products and all are prescribed by the attending veterinarian.
Crystalloid solutions are most often the fluids of choice and are the “ saline” type of fluids. Crystalloids are isotonic plasma volume expanders that contain solutes in the form of electrolytes in a similar quantity to blood. An isotonic fluid can increase the circulatory volume within the vascular spaces and when infused, they expand the intracellular fluid and extracellular fluid equally, maintaining osmolality between the two fluid compartments.
The most frequently used crystalloid fluid is sodium chloride 0.9%, more commonly known as normal saline 0.9%. Other crystalloid solutions are compound sodium lactate solutions (Ringer’s lactate solution, Hartmann’s solution) and glucose solutions. Some crystalloid preparations containing additives such as potassium or glucose are used in specific circumstances, for example, in hypokalaemia and hypoglycaemia (Joint Formulary Committee, 2017).
Crystalloids may be used for replacement fluids and maintenance fluids.
As crystalloids have the same electrolyte makeup as plasma; this means that large quantities can be given quickly. Replacement fluids are indicated in cases where fluid and electrolytes have been lost, for example, in vomiting, diarrhoea, polyuria, effusions and haemorrhage.
Common replacements fluids: Normal saline ;0.9% sodium chloride (No 1), Ringers solution, (No 9) Hartmann’s (No 11).
The hypertonic saline (7.2% sodium chloride) may also be required.
These same fluids may also be used to address normal maintenance fluids requirement. Maintenance fluids are used once an animal is rehydrated and ongoing losses are being controlled. It may be necessary to change the animal to a fluid with lower sodium content; this lessens the risk of hypernatremia (elevated blood sodium level) and peripheral oedema (swelling of tissues due to fluid retenion). Low sodium fluids are useful when managing patients with congestive heart failure (CHF) and renal disease. Patients with renal disease are less able to filter a high sodium fluid and excrete large amounts of potassium, in such cases a low sodium fluid may be more suitable.

Types of Crystalloids
Normal Saline – 0.9% sodium chloride *
This is the oldest type of infusion solution, containing only sodium chloride and water. It does not contain the same balance of electrolytes as plasma and so should not be used as a universal fluid. Patients that are suffering from metabolic acidosis should not be placed on normal saline as it lowers the blood plasma bicarbonate. Animals suffering from vomiting and animals on Furosemide BP (Vet) may benefit from being on normal saline. Normal saline can be used for animals with hyperkalemia, as it does not contain any potassium, but Hartmann’s is probably a better choice as the potassium level in Hartmann’s is quite low, and as Hartmann’s contains bicarbonate it may be beneficial as hyperkalaemic patients are often acidotic as well. 0.9% sodium chloride can be useful in hypercalcaemic patients because the high sodium levels compete with calcium for re-absorption by the kidney and the calcium is excreted in the urine.
Ringer’s Solution *
Ringer’s solution is a solution that contains potassium, calcium and has a sodium level of 147 mmol/l, it is isotonic in cats and dogs but the chloride level is much higher than that of the plasma. Ringer’s has no source of bicarbonate and high levels of chloride and therefore it is not as effective at treating metabolic acidosis, in cases of metabolic acidosis Hartmann’s should be used instead. Ringer’s may be used to correct volume deficit, which has been a result of shock or gastrointestinal disease. Ringer’s is especially useful in cases of hypokaleamia resulting from prolonged vomiting.
Both Hartmann’s and Ringer’s should not be infused through the same catheter as blood products, the combination can cause thrombi to form which can then pass into the blood stream.
Hartmann’s Solution *
Hartmann’s solution is a solution containing sodium chloride, sodium lactate, and phosphates of calcium and potassium. It is isotonic, so can be used intravenously and also via subcutaneous injection. It is used to replace body fluid and mineral salts that may be lost for a variety of medical reasons. Hartmann’s solution is ideal for use in patients with hypovolaemia. Care should be taken with patients with (or potential for) cerebral oedema (brain swelling) as fluid will move from the extra cellular space into the intra cellular space because Hartmann’s is slightly hypotonic. Hartmann’s Solution should also be used in patients with metabolic acidosis, gastroenteritis, renal insufficiency, diabetic ketoacidosis, GDV, Pyometra and shock. Patients with alkalosis should not be given Hartmann’s due to the bicarbonate; they should be given normal saline instead. Metronidazole should never be added to a bag of Hartmann’s due to a reaction causing crystallisation of particles.
Hypertonic Saline: Sodium Chloride 7.2% *
This is a special type of replacement fluid used for acute fluid resuscitation only. Giving a fluid with a high sodium level (approx 8 times normal plasma level) causes any free water to move into the intravascular space from the interstitial and intracellular space. This happens rapidly, within 5 minutes. The downside to this type of fluid is as soon as equilibrium is reached, the fluid returns to the interstitial space, and its effects are short lived.
The recommended dose for hypertonic saline is 4 – 8 ml/kg and a rate of 60 ml/kg/per hour should not be exceeded. It should be delivered over 5 minutes. If administration is too quick then bronchoconstriction (narrowing of the respiratory tract) and bradycardia (slow heart rate) can occur. Its effect is short lived at approximately 30 – 60 minutes.
Hypertonic Saline has the advantage in that a small dose can be administered quickly, and is more cost effective than colloids, especially when dealing with giant dogs. Hartmann’s or Saline (0.9% sodium chloride) should be started as a follow-up treatment, and water should be offered unless water is contraindicated due to the animal’s condition. Hypertonic Saline (7.2% sodium chloride) is useful where hypovolaemia is not caused by normal bleeding such as in Gastric Dilation Volvulus (GDV). Hypertonic saline should not be used in animals that are dehydrated.
* NOTE: These type of fluids are POM-V and the data sheet should be read fully before admisistration.
Fluid Therapy Equipment
Intravenous cannulisation
Over-the-needle cannulae (Anicath™ Winged IV Cannulae, Anicath™ Non-Winged IV Cannulae).
These are the most commonly used cannulae in veterinary practice to administer intravenous fluids. They are generally short, being 25 – 50 mm long depending on the size of animal they are being placed into. They consist of polyurethane or Teflon outer lumen with a metal stylet running through the centre of the cannula, this stylet/needle is only used to introduce the cannula into the vein, and then it is removed and discarded. The cannula can be winged or wingless. They may be left in place for 3 – 5 days.



Butterfly cannulae (Anicath™ IV Wing Set).
These consist of a metal needle and plastic wings and sometimes a short piece of tubing with an injection port is also attached and they can be used for subcutaneous fluids. The wings help secure the cannulae in place. This type of cannulae are generally not used for IV placement but are very useful for introducing subcutaneous fluids.

Through-the-needle cannulae.
These are usually longer than the over-theneedle cannulae and are most commonly used to cannularise the jugular vein. They must be placed in a sterile manner using sterile gloves and drapes. They can be left in place for up to 7 days, and are often used to measure central venous pressure. They can also be used to administer fluids or drugs and collection of blood samples. The disadvantage of these cannulae is that they are much more expensive and are more difficult to place.



Burettes (Aniflow™ Burette).
This is a large graduated chamber and is used when a very small amount of fluid needs to be given, i.e in the case of small patients such as small cats or kittens, or when a certain amount of drug has to be given. The burette is usually placed between the fluid bag and the drip chamber. They can come as separate pieces of infusion equipment attached to a standard giving set or are part of the infusion system. The burette is filled by opening the roller clamp between the fluid bag and burette allowing the fluid to fill the burette to the desired amount or up to 150ml. The clamp below the burette should be closed, to prevent air entering the rest of the giving set.
T-Connectors (Aniset™ T-Connectors).
T-Connectors are placed between the cannula and the giving set. They usually include an injection port and a plastic clamp. This allows the cannula to be flushed with saline without removing the giving set and can also be used to administer intravenous drugs. One of the

Ensure the T-Connector is primed with the fluid

Extension sets (Anitube-S Standard Flow Extension, Aniset™ High Flow Extension).

These are additional lengths of tubing that are placed between the cannula or Aniset™ T-Connector and the original giving set thus making it longer.
This is useful for medium to large dogs who require bigger kennels and therefore need more tubing to be able to move around their kennels. The extension sets can either be straight or coiled.
Fluid Therapy Equipment
Giving sets (Aniset™)
This is the link between the fluid bag and the IV cannula. A giving set is made up of a long piece of tubing with a drip chamber and spike at one end and a luer lock connector at the other. A flow regulator in the form of a dial (in the case of dial-a-flow giving sets) or roller clamps are positioned below the drip chamber, which allows the fluid rate to be controlled. There may be one or more injection ports to allow for the administration of drugs or to facilitate the removal of air bubbles from the set. Burettes are also ideal for use in the absence of an infusion pump.
Types of administration set:
REGULAR REGULAR

• Standard sets that deliver 20 drops/ml.
• Paediatric/Microbore sets that deliver 60 drops/ml.
• Available with standard or needle-free injection ports.
No pumps or too many patients? Use the Dial-A-Flow.
Aniset™ Dial-A-Flow sets, can deliver 20 and 60 drops/ml. The advantage of the dial a flow sets over standard administration sets it that the dial allows you to control the rate that the fluid is given, usually in ml/hr. This lessens the chance of overinfusion and potentially serious pulmonary edema.
20 drop/ml suitable for use during CT/MRI Scans.

SAFE
Aniset™ UV IV sets can deliver 20 and 60 drops/ml and have a light refractive, laminated bronze coloured anti-kink tubing, maintaining drug potency and limpidity. This reduces the potential harmful effects that light has on drugs administered via transparent, pellucid IV sets.

Anti-kink® IV administration set that incorporates a veterinary designed, Y-Style, safer Needle-Free (NF) injection valve. Designed specifically for veterinary use. Allows the introduction of fluid to the IV line without the use of needles, removing the incidence of needle stick related injuries, accidents and costs. Also allows other IV accessories, extensions and secondary IV lines to be connected seamlessly without the need for needles, clamps or ancillary joining devices. Swabable with a protective cap. Needle-Free IV Sets are also available with Dial-A-Flow Regulator for controlling fluid during surgery.

Administration sets that are used with volumetric infusion pumps have been specially adapted to be used with certain infusion pumps. It is recommended that you check with the manufacturer of the infusion pump to ensure it is compatible with your set.
Aniset Anti-Kink®
IV Giving Set Components
Drip chamber with vented spike:
• Allows for rapid and controlled infusion.
• Assists in priming.
• Squeezable chamber with 15 Micron particle filter to prevent contaminants entering the fluid line.
Roller clamp with IV line dock: Park your excesstubing here.
Dial-A-Flow regulator: Control fluid volume by simply turning the dial to required flow rate.
Baxter Set slide clamp: Removable/reusable slide clamp enables use with Baxter fluid pump.
Flip covered bacterial air vent: Open when using non collapsible bottles.
Needle-Free port:
• Entirely removes the risk of needle injury.
• NF allows for sets to be ‘piggy-backed’ easily.
Proximal injection port with finger guard: Reduces the risk of needle related injuries when adding meds to the line.
Quick fluid clamp: Allows for a quick on/off control of fluids.

Anti-Kink® tubing:
• Continuous fluid flow.
• No tube occlusions.
Self priming end cap:
• Allows the set to be primed, expelling all air-bubbles.
• No fluid loss, waste or mess –Keeps the treatment area and patient clean and dry.
S/L connector: Joins to an indwelling cannula with a push fit connection.
L/L connector:
• Secure connection to any IV line accessory or cannula.
• Can be slid back if required creating an S/L connection.
Anti-Kink® spiral tubing:
• Allows for a longer length.
• Ideal for patients in larger recovery areas and runs.
Slide clamp: Allows end of tube to remain primed with fluid if break point is used and patient needs to be moved.
Extention tubing:
• Built-in extention tubing –Allows patients to be easily disconnected from fluids for exercise purposes.
Distal injection port:
• For immediate effect of additional medication.
How To Place A Cephalic










The saphenous vein can also be used where access to the cephalic is not possible, i.e. if the animal has already had cannula placement in of both front legs, or if there are wounds on the legs etc. However, you should consider the possibility of infection from contamination if the animal is suffering from diarrhoea.
1. Over-the-needle intravenous cannulae (Anicath™ Winged IV Cannulae, Anicath™ Non-Winged IV Cannulae).
2. Aniset™ T-Connector.
3. Clippers.
4. Fluid of choice.
5. Surgical scrub solution suitable for full skin preparation.
6. Surgical spirit.
7. Millswabs™, Zorbs™ Gauze Balls, Millsoft™ cotton wool.
8. Adhesive tape, ideally hypoallergenic (Anifilm™, Anipore™, Anisilk™).
9. Orthoband™ bandage.
10. Rapz Eazy Tear™ or Co-form™ bandage.
Optional:
• Local anaesthetic cream.
How To Place A Cephalic Intravenous Cannula continued
Placing your cannula
Ideally, an aseptic technique using sterile gloves should be adopted, but in reality this isn’t always possible. If you are not using surgical gloves then your hands should be washed thoroughly and normal examination gloves should be used instead.

1. Gather the equipment and keep within easy reach. Flush the Aniset™ T-Connector with saline.
2. Ask an assistant to restrain the patient following correct handling and restraining techniques.


3. Clip the hair over the vein to be catheterised and ideally, also around the entire leg, taking care not to introduce infection by causing clipper rash. Identify the vein visually and by feel.

4. Clean the area with a surgical scrub solution suitable for full skin preparation.

5. Wipe the area with a Millswab™ dampened with surgical spirit.

7. Replace your gloves with a fresh pair, the surgical area should now not be touched. Introduce the cannula into the vein.


6. Ask your assistant to raise the vein. It is important your assistant does not twist the vein as this may lead to problems with cannula placement once your assistant releases the vein.

8. Insert the cannula and stylet tip into the visible vein. Once blood returns into the cannula hub, hold the stylet still and advance the cannula into the vein.
How To Place A Cephalic Intravenous Cannula
9. Ask your assistant to stop raising the vein and press over the vein a few centimetres above the cannula entry site, this stops the blood flowing out of the cannula when the stylet is completely removed.
10. Use a length of tape to secure the cannula. Start by placing the tape underneath the cannula directly on the patient’s skin and then wrap around the leg and over the cannula hub.





11. Remove the stylet and attach the Aniset™ T-Connector. Secure the Aniset™ T-Connector in the same manner as the cannula using tape. The assistant can now release the vein.



12. Flush the cannula with some more saline to check placement. There should be very little resistance when injecting and there should be no swelling around the cannula.
13. The animal can now have its leg bandaged using the padding bandage and cohesive bandage to protect the cannula and Aniset™ T-Connector.
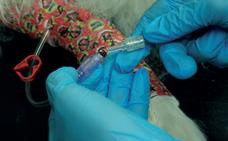
The IV cannula can now be attached to a giving set to receive fluids.
How To Place A Cannula In The Marginal Ear Vein Of A Rabbit
1. Over-the-needle intravenous cannulae (Anicath™ Winged IV Cannulae, Anicath™ Non-Winged IV Cannulae).
2. Local anaesthetic cream.
3. Clippers.
4. Fluid bag of choice.
5. Surgical scrub solution suitable for full skin preparation.
6. Surgical spirit.
7. Millswabs™, Zorbs™ Gauze Balls, Millsoft™ cotton wool.
8. Adhesive tape, ideally hypoallergenic (Anifilm™ , Anipore™, Anisilk™).
9. Orthoband™ bandage.



10. Rapz Eazy Tear™ or Co-form™ bandage.







1. Gather the equipment and keep within easy reach.
2. Ask your assistant to restrain the rabbit. Ensure correct handling and restraint techniques are followed.
3. Clip the back/dorsal aspect of the ear using the clippers and apply some local anaesthetic cream to the area over the vein to be used and leave as per manufacturer’s instructions.
4. Ask your assistant to raise the vein by applying pressure to the base of the ear. The marginal ear veins are the veins that run around the outside of the ear. The blood vessel that runs up the middle should never be used.
5. After the recommended contact time of the numbing agent, using dilute Surgical skin scrub, remove any remaining local anaesthetic cream and clean the area.
How To Place A Cannula In The Marginal Ear Vein Of A Rabbit
continued
6. Wipe with surgical spirit.
9. Ask your assistant to stop raising the vein and apply gentle pressure to the area just below the cannula entry site to prevent blood escaping the cannula when the stylet is removed.
7. Identify the vein and insert the cannula through the skin into the vein. The marginal ear vein is very shallow, extremely delicate and care should be taken not to perforate the other side of the vein and ‘blow’ the vein, causing an aural haematoma.
8. Once blood returns into the hub of the cannula, gently advance the cannula into the vein whilst holding the stylet still.
10. Apply tape around the ear, starting underneath the cannula and coming around the ear and back over the cannula. Do not pull the tape too tight, the ear should stay a natural shape.
Use a small, folded piece of Orthoband™ on the inner aspect of the pinna to help maintain natural pinna shape.
If an IV line is not tolerated, the fluids can be given as a regular bolus via the IV cannula
11. Remove the stylet and flush the cannula with saline, the assistant can release the vein. You will be able to see the fluid running around the ear veins and it will be obvious if the cannula is not in the vein.
12. An Anisite injection bung or giving set can now be attached to the cannula. More tape will be needed to secure a giving set to the ear to prevent it pulling the cannula out. It is the author’s experience that a little trial and error is needed to find the best technique to securing the giving set to the ear.
13. An Elizabethan collar should then be put on the rabbit to prevent it scratching out the cannula and giving set.
How To Set Up A Drip
Equipment
1. Giving set (Aniset™).
2. Saline flush.
3. Intravenous fluids.



1. Collect together equipment and flush Aniset™ T-Connector with saline or prime with fluid of choice.

2. Ensure that the cannula has been placed, flushed and is patent. Attach the primed T-Connector to patient if one is not already present (See pages 40-41).

3. Check the packaging of the giving set to ensure that it is not damaged and/ or out of date. Also check that the fluids are in date, are not discoloured or have any sediment present.
4. Remove the giving set from the package and close the flow regulating clamp.
5. Remove the cap from the spike on the giving set and push it in to the port of the fluid bag. Keep your hands clear of the spike and ensure you insert it straight to prevent piercing of the tubing.





6. Half fill the drip chamber by squeezing it.

7. Open the flow regulating clamp slowly to allow the fluid to flow through the giving set until it is full, this is known as ‘priming’. Fully close the flow regulator.
8. Check that there are no air bubbles in the line.
9. Invert the line at the injection ports during priming to allow the dead space to be replaced with the fluid.
10. Suspend the fluid bag from a drip stand.
11. Connect the end of the giving set to the Aniset™ T-Connector, open the clamp on the Aniset™ T-Connector (See page 41).
12. Open the flow regulator so the fluid is delivered at the correct infusion rate (See pages 18-19).
Monitoring The Fluid Therapy Patient
It is very important that once a patient is receiving fluid therapy that it is monitored very closely and vital signs and any comments are recorded and then reported to the veterinary surgeon.
Parameters that should be monitored throughout the fluid therapy are:
Cardiovascular system
• Pulse-rate, rhythm and strength.
• Mucus membrane colour.
• Capillary refill time.
• Check for any jugular distension.
• Auscultation of the Thorax – Cardiac arrhythmias.
Respiratory system
• Respiratory rate, depth and effort.
• Thorax auscultation – Pulmonary oedema.
Body Temperature Normal body temperature ranges:
Urine output-normal
• 1 – 2 ml/kg/hr.
Urine specific gravity
Normal urine SG ranges:
General checks
• Peripheral oedema – Hocks, over the dorsum. Oedema of the scruff and the area above the bandage is quite easy to spot.
• Chemosis – The mucus membranes of the eye become puffy.
• Skin turgor – Rate at which the skin rapidly returns to its normal position.
• Bodyweight.
Normal blood serum levels of Na+, K, Cl-, and Bicarbonate
It is highly advisable you should record the patients intake of fluid ( food, water and IV fluids) and record the patients output (urine, faeces, vomit and diarrhoea). The best way to do this is via a hospital sheet or fluid therapy monitoring sheet page 50, then the veterinary surgeon can build up an entire picture of how the animal is progressing.
Monitoring the input of fluids:
All food and water should be measured before it is given to the patient. This may mean finding out exactly how much water is in a tin of dog food. It is then easy to measure what is left after an allocated amount of time and this can tell us what the animal has taken in. You should also include what the animal receives in the way of intravenous fluids.
Monitoring the output of fluids:
Estimate volume of vomit and diarrhoea. The easiest way to monitor urine output is via a urinary catheter and urine collection bag, and this is fine if you have a recumbant patient, that requires an indwelling urinary catheter. If your patient is more stable then they may not have one in situ. In this case you need to weigh the wet bedding against the dry bedding. Weigh all the animal bedding before it goes into the kennel, use non-absorbent cat litter, nappies or cage liners (P-Rugz™). After an allotted time weigh it all again. You can safely assume that 1ml of urine weighs 1g. The increase in weight will tell you how much urine the animal has passed.
The input and output should be measured and recorded every 4 hours. ideally, the output should be approximately 10% less that the input.
If the animal is excreting more than is being given, the fluid rate should be increased. If the animal is taking in a lot more than is being excreted then this should be investigated, and the intravenous fluid rate decreased. It may be that the animal has not been on intravenous fluids long enough and you are still correcting the dehydration. Therefore, in this situation it is reasonable to expect the animal to still be retaining some fluid. This should be checked again in a couple of hours and your findings recorded.
Fluid Overload
This is when the patient receives too much fluid in a given period of time. This could be due to an error in calculating the amounts or in setting up the infusion equipment. It may also be due to the patient moving after an infusion has been set up causing a bolus to be given inadvertently. If the patient is suffering from an underlying condition, such as heart disease, he may not be able to process the fluid he is being given, which will also lead to over perfusion.
Fluid overload can lead to peripheral and pulmonary oedema, respiratory distress, heart failure, hypothermia and death.
Fluid Therapy Monitoring Chart
DATE:
OWNER: PATIENT: AGE:
SEX:
SPECIES/BREED: ADMISSION WEIGHT:
DAILY WEIGHT AM:
DAILY WEIGHT PM:
REASON FOR FLUIDS:
CATHETER IN PLACE: Y / N
LOCATION: CEPHALIC / SAPHENOUS / OTHER
SIZE:
DATE PLACED:
Contraindications
There are certain patients where over infusion is more likely. In these cases fluid therapy should still be used if needed but extra, special attention needs to be paid to monitoring their vital signs, as discussed in the chapter on monitoring.
Patients with chronic heart failure:
These patients retain fluid due to decreased cardiac output. The heart is less efficient at pumping fluid around the tissues.
Patients with acute renal failure:
The kidney is less efficient at controlling the amount of fluid that is excreted.
Patients with bladder rupture or urethral obstruction:
The urine cannot leave the body.
Aniset™ Transfusion
Set Instructions
Administering a blood transfusion
On rare occasions it may be necessary to administer blood to a patient. The description below explains the correct use of the Aniset™ Transfusion blood giving set, and also contains the correct calculation for the administration of blood.
We have included a quick reference table to help you decide how much blood you give initially, and how quickly to give the remaining blood (assuming that you haven’t had any problems).
• Close the roller clamp.
• Remove protector from piercing spike and insert into bag of blood that is to be transfused.
• Gently squeeze the drip chamber until it is half full of blood.
• Whilst holding the end of the Aniset™ Transfusion set, slowly open the roller clamp until the blood has reached the priming cap. Close the roller clamp.
• Remove priming cap and attach the Aniset™ Transfusion set to the indwelling IV cannula of the transfusion recipient.
• Blood should be initially administered at a rate of 0.25ml/kg/hr, and the recipient closely observed for signs of transfusion reaction.
• After 15 minutes and if no problems have been observed, increase the infusion rate so that the remaining blood is given over 4 hours (see pagse 53 - 57).
In cases where severe blood loss has occurred it may be necessary to infuse the blood more rapidly to save the animal’s life

Blood Infusion - a General Guide
A blood transfusion may be needed as a result of a lack of red blood cells (anaemia) in the circulatory system. This could be as a result of a disease process or condition such as haemolytic anaemia or as a result of blood loss following surgery or trauma.
In the circumstances of a condition, a blood transfusion will be given to replace the red bloods cells in the circulatory system to give the patient the best chance of recovery. In the situation of actual blood loss, you can calculate blood loss and replace what has been lost.
Normal circulatory volume in dogs is 80 – 90 ml/kg
Normal circulatory volume in cats is 50 – 70 ml /kg
To calculate blood loss following surgery
Know the weight of a dry swab and multiply this by the number of swabs used. This equals the total weight of the dry swabs.
Weigh the number of blood-soaked swabs used. Deduct the weight of the dry swabs from the weight of the soaked swabs.
The remaining weight will be the weight of the blood. 1g = 1ml of blood
If suction has been used, deduct the amount of the blood in the suction machine from any lavage that has been used and add this figure to the blood loss collected by the swabs.
If the blood loss is 10% of the total blood volume, use crystalloids such as Hartman’s Solution (Lactated Ringers USA/Canada) to replace lost volume x 4.
If blood loss is 10 to 15% use colloids such as Haemocoel at 5ml / kg rate and 1.5 x volume lost in a bolus and then continue with crystalloids at 4 x volume lost.
If blood loss is 20% plus, your patient requires a blood transfusion.
Prior to transfusion
• A blood sample should be taken from your donor patient and recipient.
• The donor patient should have a PCV taken and temperature taken.
• Perform cross matching and blood typing and an agglutination test to check for suitability.
• A maximum of 16ml per kg can be taken from the canine patients.
• A maximum of 10ml per kg be taken from feline patients up to a maximum of 50mls in total.
Calculating the amount of blood required from the donor patient
As a general guide , 2mls of whole blood per kg body weight will raise the recipients PCV by 1%.
So for example, if the recipient patient weighs 40kg , 80mls of whole blood will raise the PCV by 1%.
To raise the PCV by 5% would mean that 400mls of whole blood will be required (80 x 5 = 400mls)
To be a bit more accurate you can calculate the amount of blood required by using the following formula:
Volume of donor blood required in ml =
DOGS – 80 x bodyweight (bw) in kg x desired PCV increase ÷ PCV of donor blood.
CATS – 60 x bodyweight (bw) in kg x desired PCV increase ÷ PCV of donor blood.
So for example if a 40kg dog needs to have his PCV raised by 5% and the donor blood has a PCV of 47%:
Blood typing
Dogs and cats have a number of blood groups.
Blood typing determines the presence or absence of species-specific inherited antigens which are present on the surface of the RBC’s.
These antigens are responsible for inciting devasting transfusion reactions. In 98% of the population, these antigens are not present.
The first mismatched transfusion will only result in sensitisation of the immune system to the antigen leading to the development of the development of antibodies over the course of 4-5 days.
Cats should always be blood typed as they have natural occurring antibodies to the antigens occurred with other blood types.
In dogs, there are very low levels of naturally occurring antibodies, but these can occur as a result of a transfusion making a potential reaction to a subsequent transfusion quite high.
Blood types in dogs
Canine blood typing kits are testing for the presence of DEA 1 antigen. It will determine if the patient is DEA 1 negative or positive.
DEA 1 negative dogs are viewed as being universal donors as they can be used successfully as donors for both DEA 1 negative and Positive dogs.
DEA 1 Positive dogs can only be used with another positive dog.
Blood typing in cats
Blood typing for cats uses the AB system. Testing kits will determine if they are A, B, or AB. They can only be matched with a cat with the same blood type. If no other donor is available, an A cat can be used in an emergency for an AB cat but this is not really recommended.
Cross matching
Cross matching is performed to determine the compatibility of blood between donor or recipient. Cross matching is recommended even if blood typing has been done.
Cross matching should always be done
• if the recipient has already received a blood transfusion
• has had a previous transfusion reaction
• has an unknown previous history
• has been pregnant in the past
Cross matching can be done in house using a test kit or can be performed by the Pet Blood Bank who will test the blood with the recipient’s sample prior to sending out the best match. If there is a reaction between the recipient and the donor the blood should not be transfused.
A cross match should ALWAYS be done for every subsequent transfusion even if the same donor blood is used.
Donors
• Dogs need to be above 25kg in weight and cats need to be above 4kg in body weight.
• Both dogs and cats need to be between 1 to 8 years of age.
• Both dogs and cats need to be vaccinated but more than 2 weeks ago.
• Worming needs to be current but more than 2 weeks ago.
• Ideally cats should be indoors only if possible.
• General health needs to be good with a biochemistry and haematology blood screen being performed.
• No more than 3 to 4 donations per year per donor dog.
• No more than 4 to 5 times per year per donor cat.
• Sedation in dogs best avoided.

• Sedation in cats is recommended with a suitable protocol.
• IVFT should be done for 1 hour for dogs if sedation has been used.
• IVFT should always be used for cats.
• Food and water to be offered to the donor.
• Gentle exercise for dogs for 72 hours and cats to be kept indoors for 24 hours to 48 hours.
Preparation of recipient patient
• Record baseline PCV and TPR.
• Take patient outside to urinate and defaecate.
• Place a fresh dedicated iv cannula.
• Only use SALINE to flush. DO NOT use Hartman’s or colloids at all.
• Do not give any other medications via the cannula used for blood transfusions.
Preparation of blood
• Collect blood using commercially available collection sets, containing a suitable anticoagulant.
• Gently tip the bag from side to side to encourage the anticoagulant to thoroughly mix. Do not shake or agitate.
• Use collected blood as soon as possible to maintain the heat. Must be used within 4 to 6 hours.
• Whole blood should ideally not be warmed and given at room temperature.
• Over warming will cause clotting and haemolysis and can also encourage any bacterial activity.
• If warming the blood is needed, use a water bath and always protect the blood bag with a zip lock bag to prevent contamination of the ports.
• Never heat blood or fluids in a microwave.
Equipment needed for transfusion
• Use a commercially filtered blood administration line.
• Check the delivery rate of your transfusion line as some deliver at 15 drops per ml (Aniset US) rather than 20 drops per ml (Aniset UK).
• Alternatively, use an in-line blood filter 170um to 200um to filter blood clots (suitable for transfusions of 250mls or less).
• Few infusion pumps and syringe drivers can be used but do check with manufacturers first.
• Connect the blood product to the transfusion line in the same aseptic way you would connect any other fluids to a giving set.
• Prime the transfusion line with the donor blood, eliminated any air bubbles.
Transfer rates
• 0.25ml / kg/ hr for 15minutes to check tolerance.
• 1ml per kg per hour for 15 minutes.
• Increase to 2ml per kg per hour for a further 15 minutes.
• TPR should be monitored carefully every 10 minutes until completion.
• If no adverse effects, can increase to 5 to 10ml per kg per hour for the duration
• The Transfusion should not exceed 4 hours due to risk of bacterial activity.
• This 4 hour time frame starts at the point the unit of blood is breached with the transfusion line.
• A new transfusion set should be used for each unit of blood given
Monitoring the patient during transfusion
• A TPR and baseline PCV should be taken prior to blood transfusion.
• TPR, CRT, MM and blood pressure should be taken throughout.
• The transfusion should not be stopped unless a reaction occurred, or a MRCVS has requested this.
• Offer water throughout but no food.
• Take a TPR and PCV upon completion of the transfusion.
• Consider an indwelling urinary catheter to monitor for haematuria.
Possible reactions
Reactions are rare if blood typing and cross matching have been done (recommended). A reaction occurs if the blood becomes haemolysed. Haemolysis can occur as the cells react to each other in mis-matched blood hence the reason for typing and cross matching prior to collection / administering.
Haemolysis can also occur as a result of result of incorrect physical collection, handling and delivery of the of the blood transfusion hence the importance for using the correct equipment.
TPR Monitoring: call the vet if the patient becomes tachycardic, dyspnoeic, tachypnoeic, abnormal heart or pulse rhythm, elevated temperature.
General demeanour: call the vet if the patient becomes weak, ataxic, depressed, vomiting, hypersalivation, vocalising, muscle tremors or convulsions.
Appearance: Urticaria, facial oedema, jaundice.
Urination: Haematuria.
If changes are subtle it can be hard to determine if the changes are due to the blood transfusion or as a result of the original condition. The blood transfusion can be slowed to 0.5ml per kg per hour. Care should be taken if considering the use of steroids or antihistamines as you may be masking a reaction, which would lead to a delay in stopping a transfusion that is causing harm. All drugs should therefore be considered with caution and only under the direction of the attending Veterinary surgeon.
Legalities
• The details of the donor have to be recorded.
• The date the blood was collected.
• The blood type.
• The PCV at the time of collection and temperature at time of collection.
• The name of the clinician.
• How much blood was collected.
• Complete paperwork for your recipient patient detailing exactly how the blood was administered.
Glossary And Prefixes
Acute: Sudden onset.
Albumin: A protein in the blood responsible for the maintenance of osmotic (water) pressure in the blood; also binds (attaches) to large molecules in the blood and serves to transport them;produced by the liver; also called ‘serum albumin’.
Anaemia: A condition in which the number of viable red blood cells present in the blood is lower than normal.
Anaphylactic: A severe, potentially life-threatening allergic reaction.
Aspiration pneumonia: Pneumonia (disease of the lungs characterised by inflammation and consolidation of lung tissue) caused by inhalation of fluid into the lungs.
Ataxic: Refers to a patient displaying a lack of co ordination and imbalance in gait.
Auscultation: The act of listening to the thorax typically with a stethoscope.
Bolus: A single, usually large volume of liquid or medication given as a single amount.
Bradycardia: Slow heart rate.
Bronchoconstriction: The narrowing of the Bronchioles.
Ca2+: = Calcium.
Cardiac arrhythmia: A variation from normal heart rhythm.
Caudal: A directional term used to refer to an area more toward the cauda, or tail region; opposite of cranial.
Central venous pressure: Describes the pressure of blood in the thoracic vena cava, near the right atrium of the heart. CVP reflects the amount of blood returning to the heart and the ability of the heart to pump the blood into the arterial system.
Cephalic: Large vein in the foreleg.
Chemosis: Swelling of the conjunctiva with fluid.
Chronic: Long term i.e. not sudden onset.
Cl-: Chloride.
Congestive heart failure: Inability of the heart to supply sufficient blood flow to meet the needs of the body.
Cranial: Towards the head of the body.
Crea: Relates to creatinine, a by-product that helps to supply the muscle with energy and is excreted from the body via the kidneys.
CRT: Capillary refill time, the time it takes for the blood to return to the tissues after pressure has been applied.
Crystalloid: Saline type fluid.
Distal: Situated away from the centre of the body.
Dyspnoeic / Dyspnoea: Difficulty in breathing.
EDTA: Ethylenediaminetetraacetic acid, an anticoagulant.
Equilibrium: A state of balance.
Extracellular fluid: Fluid found outside the cells.
GDV: Gastric dilation volvulus, condition where the stomach twists and occludes and causes a greatly distended abdomen.
Globulin: A group of proteins that are produced by the liver and in the immune system. They play an important part in liver function, clotting function and fighting infection.
Haematuria: Blood in the urine
Haemoglobin: A protein inside of red blood cells, responsible for the binding and transport of oxygen to the body tissues (Hb).
Haemolysis: Destruction of red Blood cells ( Erythrocytes)
HCO3: = Bicarbonate
Hepatic: Relating to the liver.
Hyperglobulinaemia: An abnormally large amount of globulins in the circulating blood plasma.
Hyperkaleamia: High blood potassium.
Hypernatraemia: Elevated blood sodium level.
Hypertonic: An intravenous fluid that has a higher concentration of solutes than blood.
Hypertonic saline: Has a higher concentration of sodium (approximately 8 times the normal plasma concentration of sodium) which causes free water to migrate into the intravascular space from the interstitial and intracellular spaces.
Hyperventilating: An increase in the rate and/or depth of respiration.
Hypocalcaemia: Low blood calcium.
Hypoproteinaemia: A condition where there is an abnormally low level of protein in the blood.
Hypotension: Low blood pressure.
Hypotonic: an intravenous fluid that has a lower concentration of solutes than blood.
Hypoventilating: A decrease in the rate and/or depth of respiration.
Hypovolaemia: Low circulating blood volume.
Immunogenic: A substance that is able to illicit an immune response.
Interstitial: Space that surrounds the cells of a given tissue.
Intracellular fluid: Fluid found inside the cells.
Intraosseous: Inside the bone.
Intraperitoneal: Inside the peritoneal cavity.
Intravascular: Within the blood vessel.
Isotonic: Intravenous fluid that has a similar concentration of solutes as blood.
Jugular distention: Protruding of the jugular vein.
Glossary And Prefixes
K+: Potassium.
Medial: Towards the midline.
MM: Specialised membrane which covers various passages and cavities exposed to the air such as the mouth, nose, inner portion of the eyelids, vagina. Examination of the mucous membranes can provide important information: if they are dry, the animal is likely dehydrated; pale, and the animal may be anaemic or in shock; yellow, and the animal is said to be jaundiced due to accumulation of waste products which should be eliminated by the liver.
Na+: Sodium.
Oedema: Accumulation of fluid in one or more body cavities or tissues.
Oliguria: Reduced excretion of urine.
PCV: Packed cell volume. A laboratory test to monitor the relative number of red blood cells present in the blood.
Peripheral: Near the surface or outside of; external.
Peritonitis: Inflammation of the peritoneum (lining of the abdomen).
Plasma: The fluid part of the blood.
Polycythemia: Refers to an increase in either the number or concentration of red blood cells in circulation. Polycythemia is also called erythrocytosis, because red blood cells are medically referred to as ‘erythrocytes’.
Polyuria: Excessive urination.
Proximal: The end of limb nearest the body.
Pulmonary: Pertaining to the lungs.
Pyometra: Pus filled uterus.
Recumbent: Laying down.
Renal: Relating to the kidneys.
Right sided heart failure: The right ventricle loses its pumping function, and blood may back up into other areas of the body, producing congestion. Congestion affects the liver, the gastrointestinal tract, and the limbs. In addition, the right ventricle may be unable to pump blood efficiently to the lungs and to the left ventricle.
Saphenous: Large superficial veins in the hind limb, can be lateral (outer aspect of the hind leg) or medial (on the inner aspect of the hind leg).
Sepsis: The presence of toxins in the blood or other tissues; the toxins are produced by bacteria or other microorganisms.
Splenic contraction: When the spleen contracts, it injects large numbers of red blood cells into circulation, thereby increasing the relative ratio of red cells to fluid in the blood.
Subcutaneous: Under the skin; often called ‘sub Q’.
Tachycardia / Tachycardic: Elevated heart rate.
Tachypnoea: Elevated / rapid respiration.
Thrombi: A blood clot.
Umbilicus: The area of the body where the umbilical cord is attached; the belly button.
Urea: Waste product of protein metabolism that is removed from the body by the kidneys.
Urethral obstruction: Obstruction of the urethra with calculi/sediment causing the animal to be unable to urinate.
Urine specific gravity: Urine specific gravity is a laboratory test that measures the concentration of all chemical particles in the urine.
Urticaria: An allergic reaction typically with intense rash / irritation and raised welts.

FLUID THERAPY BOOK
Peace of Mind for the Veterinary Professional
Freephone: 08000 11 22 88
Copyright Millpledge 2024.
Brand names mentioned are all copyright of respective owners.
Duplication and use of images or text is by prior written consent only.
We are continually updating and improving our products for the veterinary professional. Due to this commitment it may be necessary to alter some products and therefore goods received may differ slightly from those shown within this catalogue.
No part of this booklet may be reproduced or transmitted in any form or other means, electronic or mechanical, including photocopying, recording or otherwise for any purpose except for the practice’s own use without the express written permission of Millpledge Ltd. Diagrams are the copyright of Millpledge ©2024. Millpledge Logo ©2024. Due to the commitment of updating and developing our products, goods received may differ from those shown within this publication.
Duplication and use of images or text is by prior written consent only.
