EXTRACTABLES LEACHABLES M edical P lastics NEWS | INTERNATIONAL WuXi AppTec shares the importance of extractables and leachables testing. + PREVENT AND PROTECT ARE BIOPLASTICS THE PHONYANSWER?PHARMA EXTRACTING THE TRUTH ISSUE 22 July/Aug/Sept 2022 WWW.MEDICALPLASTICSNEWS.COMADVANCINGMEDICALPLASTICS NORTH AMERICAN EDITION
1 CONTENTS MPN North America | Issue 22 | July/Aug/Sept 2022 Regulars 3 Comment Olivia Friett reflects on how technology has changed the medical game 4 Digital Spy Sharing some of the latest news in the healthcare market 12 Cover story WuXi shares the importance of complete chemical characterisation 32 Q&A Enteral Access Technologies shares the inspiration for DoubleCHEK Features 7 Bioplastics Tecman outlines why bioplastics are the choice for PPE 11 Coatings BioInterations discusses the significance of infection prevention 18 Injection molding ENGEL delves into the world of ophthalmic production 23 andAnticounterfeitingserialization Origin explains how to stay ahead of the fake pharma market EXTRACTABLES LEACHABLES WWW. M EDICALPLASTICSNEWS .COM
Additionally, CYROLITE® can be reliably sterilized and is resistant to medical fluids and disinfectants. This has impressed newborns and health-care professionals alike: CYROLITE® easily meets the requirements of USP Class VI, ISO 10993-1, and REACH. We give you all the reasons you need at www.cyrolite.com.
BPA- and CYROLITE®DEHP-free:acrylicshavejustwhatittakestosavelives.
It’s nice when new life can rely on CYROLITE®: our BPA- and DEHP-free high-performance acrylics are safe to use in medical devices, especially in prenatal and neonatal applications.
WWW. M EDICALPLASTICSNEWS .COM
3 Medical Plastics News NA Print subscription - qualifying US/Canadacriteria– Free UK & Europe – £249 ROW – £249 Medical Plastics News Europe Print subscription - qualifying criteria UK & Europe – Free US/Canada – £249 ROW – £249 FREE on iOS and Android devices Subscription enquiries subscriptions@rapidnews.comto © 2022 Rapid Life Sciences Ltd While every attempt has been made to ensure that the information contained within this publication is accurate the publisher accepts no liability for information published in error, or for views expressed. All rights for Medical Plastics News are reserved. Reproduction in whole or in part without prior written permission from the publisher is strictly prohibited. ISSN No: 2047 - 4741 (Print) 2047 - 475X (Digital) editor | olivia victoria.dunsmore@rapidnews.comcaroline.jackson@rapidnews.comadvertisingolivia.friett@rapidnews.comfriett|carolinejacksonadvertising|christinejoinsonchristine.joinson@rapidnews.comadvertising|victoriadunsmorevp,sales&salestalent|juliebalmforthjulie.balmforth@rapidnews.comheadofstudio&production|samhamlyngraphicdesign|robertwoodpublisher|duncanwoodMedicalPlasticsNewsispublishedby:RapidLifeSciencesLtd,CarltonHouse,SandpiperWay,ChesterBusinessPark,Chester,CH49QET:+44(0)1244680222F:+44(0)1244671074
OLIVIA FRIETT THE Worldwide Membership necessarily mean the newest and trendiest devices, it’s thinking about the patient and understanding what they need. George Gallagher, the founder and CEO of Enteral Access Technologies, was able to make a medical device for a family member in hospital from materials in an air freshener factory and eventually developed this into a professional, functional device which now helps thousands of patients in the NHS (you can read the full story in the Q&A section). Knowing your target audience, knowing what the issue is and knowing how to fix it –this is innovation. At Med-Tech Innovation Expo, there was a conference called “The PITCH” where up and coming innovators talked on stage about their new products and how they can affect the market. It was inspiring seeing so many peoplesome new to the sector - wanting to create devices that could potentially help patients. Oxford Heartbeat, a company that is trying to make successful high-risk brain stenting surgery a norm, was just one example of the medical plastics innovation at the show. If the last century is something to go by, then no one knows what the future of healthcare will look like, but the anticipation invigorating.is
While I do find myself feeling a lot more comfortable in my position as editor, I am still learning so much – which I think is the best thing about this sector; there will always be new ideas and designs or new ways to improve. I am in awe of the medical field; whilst the world has been changing so vastly over the last couple of years, this sector seems to be thriving.
Editor’s Comment
The past few months have been very UK centric – with Med-Tech Innovation Expo at the center of it all, and I have gone on a few road trips for other expos and meetings.
I’ve also recently been able to experience my very first (and second) factory tour; learning about new innovative devices and technology is one thing but being able to see the manufacturing and development process in a factory is very insightful.
If we look back to 100 years ago – or even 20 years ago, technology has changed immensely. And as technology is evolving, so is healthcare. The perfect example of this is the COVID-19 vaccine; it took scientists less than two years to find a successful vaccine due to the advanced technology we have now - in comparison to the Spanish Influenza just over a century ago which didn’t have an effective vaccine until the 1940s.
LOOKINGFUTURE’SBRIGHT BPA
Hello, I’d like to welcome you to the Q3 issue of Medical Plastics News International. This is now my second issue of Medical Plastics News and I’m still seeing and learning new things every day.
The advancements we’re seeing in technology is nothing short of incredible, however while innovation is at the forefront of this sector, it’s important to remember that innovation doesn’t
www.aquapakpolymers.com
CONMED agreementannouncestoacquireBiorez,Inc.
SUSTAINABILITY UPDATE Aquapak partners with Industrial Physics to help customers transition to sustainable packaging
ACQUISITION UPDATE
www.conmed.com
NEW 800 SERIES HYBRID EXTRUSION TOOLING Aquapak Polymers has partnered with Industrial Physics to create a harmonized set of WVTR (Water Vapor Transmission Rate) testing methods for its Hydropol biodegradable polymer. Hydropol offers all the benefits of traditional polymer plastics yet is water soluble and completely biodegradable as well as non-toxic and UV resistant. It also offers multiple end-of-life options such as anaerobiccompatibilitycompostabilityrecyclability,andwithdigestion plants. Establishing a reliable and repeatable test method for WVTR is an industry-wide challenge. By using pollutionhelpingtraditionalrealrangecustomersultimatelyPhysics,partneringtoInc.,andincludingPhysics’designedequipmentbyIndustrialproductlines,SystechIllinois,TestingMachinesAquapakresolvedfindawayforwardbywithIndustrialwhichwillprovideitsacrossawideofsectorswithaalternativetousingpolymers,themcutplasticintheprocess.
into our existing suite of products, and we are excited to advance the next generation of healing in sports medicine.”KevinRocco, chief executive officer of Biorez added, “I am proud of the Biorez team for developing an innovative healing solution that provides a new treatment option for surgeons and patients. We are thrilled to join Conmed to accelerate the growth of our scale.”ontechnologyaglobal
Conmed announcedCorporationanagreement to acquire privately held Biorez. Biorez is a medical device start-up based in New Haven, Connecticut and is focused on advancing the healing of soft tissue using its BioBrace Implant technology. The BioBrace implant is an innovative bioinductive scaffold that is intended to reinforce soft tissue where weakness exists and facilitate healing. BioBrace is cleared for use by the FDA in multiple product sizes. “The addition of Biorez and its BioBrace platform represents an important step forward for our sports medicine portfolio,” commented Curt R. Hartman, Conmed’s chair of the board, President, and chief executive officer. “BioBrace fits seamlessly for a way to incorporate this technology into an updated version of the 800 Series. This led to the creation of the 800 Series Hybrid. The benefits of the 800 Series are retained, including compact design, low residence time and a common deflector bore that
DIGITAL spy
Guill announces the introduction of a new version of its popular 800 series, known as 800 Series Hybrid. In some extrusion applications that utilize crossheads and inlines, layers of the exact same material are applied multiple times, using a single die. This method is used to reduce the propensity for errors caused by gels breaking through a thin wall, weld lines, inconsistent wall thickness, plus material and process variations. Additional errors include difficult-to-process materials and demanding applications where there is zero fault tolerance.Seeking to design the next generation multi-layer die to overcome these challenges, the engineers at Guill looked www.guill.com
WWW. M EDICALPLASTICSNEWS .COM4
EXTRUSION UPDATE GUILL ANNOUNCES
stacktoleranceeliminatesup.forawaytoincorporate stacktoleranceeliminatesup.
Protection for healthcare staff from surgical smoke secures agreement www.eakinsurgical.com
PROVIDING QUALITY INSPECTION WITH LEAK DETECTION SYSTEM
www.tantec.com
TESTING UPDATE
Leak detection using LeakTEC is the process of using a non-destructive electrical current to detect any holes, defects, or abnormalities in nonconductive materials. Tantec UK & Ireland’s premier leak detection system – LeakTEC – is a tool to provide the user with 100% quality inspection. Dozens of parts can be checked every second, giving you a repeatable operation without the need for processed gasses or consumables. In many cases, it is a standard procedure to surface test molding defects, it can also be used for bonded and sealed sections. This can be used on applications including medical devices, caps, and closures; indeed, any item where the contained product will spoil when exposed to the atmosphere.
LeakTEC can make a definitive decision on the quality of each part presented, deciding whether they can progress, be scrapped, or require rework. When coupled with automation, individual problem parts can be entirescraphavingwithoutprocessfromremovedautomaticallythetoanbatch.
Plasmaformation.cleaning can be applied as an in-line solution e.g., prior to encapsulation, or as a batch processing step, with bespoke frame loading arrangements. Batch processing involves parts being loaded into a plasma chamber. The process is quick and effective, taking no more than five minutes to complete the treatment.
5 DIGITAL SPYSURGICAL UPDATE BONDING UPDATE
Plasma treatment for wire improvementbond www.plasmatreatment.co.uk
Asmoke evacuation system developed to keep operating theaters smoke free has successfully won a place on the NHS Welsh framework agreement. Cardiff-based manufacturer Eakin Surgical is one of the suppliers appointed by NHS Wales Shared Services Partnership to the national framework agreement. The contract award means the company – a distributor for the CIMPAX C-PURE 750 Smoke Evacuation System – can help the NHS across Wales to better protect its staff from the dangers of surgical smoke. Developed by Danish manufacturer CIMPAX ApS, the CIMPAX C-PURE 750 Smoke Evacuation System is widely recognized as the gold standard in smoke evacuation. use high-frequency current to cut and coagulate tissue are the most common at producing surgical smoke plumes. One study found that on average the smoke produced daily was equivalent to the amount produced by 27-30 cigarettes.
WWW. M EDICALPLASTICSNEWS .COM
Henniker Plasma has announced the latest and most reliable solutions for reducing wire bonding failures. Wire bonding is a technique used when creating electrical interconnections between semiconductors, integrated circuits and silicone chips using fine wires. Wire bond failure can occur when the fine wire bonds are unable to connect efficiently to a bond pad. Halogen and silicone contamination can cause corrosion and impede adhesion.
The lead frames used in the semiconductor device assembly process need to be completely contaminant free. Contamination on the contact surfaces of lead frames can cause a reduction in the quality and provide insufficient bond
K 2022 I 19-26 October 2022 I Hall 15 HIGH PERFORMANCE SOLUTIONS FOR LEADINGAPPLICATIONS.MEDICAL Precision meets speed and cleanliness. The all-electric ELION is the ideal injection molding machine for the cost-effective mass production of consumables for in-vitro diagnostics, primary packaging and drug delivery systems, as well as healthcare and medical disposables. • Clamping force range 800-2800 kN • Cleanroom equipment up to class ISO 5 • Perfect precision and reproducibility • Excellent overall equipment effectiveness • Added productivity boost with NETSTAL Smart Operation (available as an option) • Leading price-performance ratio • Worldwide customer service More details on www.netstal.com NEW SERVICE Machine calibration according to ISO/IEC 17025:2017 on-site at customer’s premises
Arethebioplasticsanswer? WWW. M EDICALPLASTICSNEWS .COM
BARRIERS TO BIOPLASTICS
From a manufacturing point of view, the use of bioplastic materials is undoubtedly better for the planet but is not without its challenges. Recycling in healthcare works differently to other industries because of the need to safely manage hazardous waste. This complication to what can be processed is further challenged by the fact that some Trusts recycle themselves, whereas others outsource it. This results in a myriad of different regional and local approaches to what is and what is not sustainable. One reason there has not been uptake of more bioplastic-based products is because of confusion by officials as to whether bioplastic products are biodegradable. The Department for Health and Social Care argues biodegradable products can’t be recycled because of the difficulty involved in separating them within existing waste streams, but it is important to point out that biobased products are not the same as biodegradable products and can be disposed of within regular waste streams.
7 BIOPLASTICS
Bioplastic is plastic manufactured from plant or other biological material. It could be created from corn starch derivatives, trees, cellulose, or another raw material and has a very low carbon footprint compared to traditional oil-derived alternatives. In our own field, face shields play an important role in a variety of healthcare settings. They offer greater flexibility and comfort, and an additional layer of protection atop of facemasks. But, no surprise, they are almost entirely made of plastic.
I’ve long been fascinated by how we can use new materials and exploit their properties to do things better and more sustainably across the healthcare sector. At Tecman, we focus on product design and advanced material technology, so we regularly undertake R&D activity to design and develop more sustainable medical products. The challenge in healthcare is to ensure that innovation that improves product sustainability does not compromise functionality or result in a loss of quality, which many are concerned is usually the case.
KEVIN PORTER, TECHNICAL DIRECTOR OF TECMAN ADVANCED HEALTHCARE PRODUCTS, CLEARS THE AIR ON BIOPLASTICS MISCONCEPTIONS.
BIOPLASTICS WWW. M EDICALPLASTICSNEWS .COM8
SEIZING THE OPPORTUNITY Bioplastics offer a third way between single use products and biodegradable ones. They are more sustainable and less carbon intensive than the former, but unlike many of the latter, can be recycled and disposed of via existing waste streams. One major way to make plastic products more sustainable is through material innovation. Selecting the right material given a specific design challenge is difficult, but by adopting new technologies and processes and exploiting the properties of excellent materials like bioplastic, manufacturers can help bring down carbon emissions significantly and ultimately the commercial elements improve too. In something as straightforward as a face shield, the use of biomaterials can reduce plastic use by 90%, so the opportunity to make a seismic difference if this was used across the medical device arena is there for the Preventingtaking.hospital acquired infections (HAIs) is a major reason for the use of disposable products in healthcare, and so we are unlikely to see a move to most items becoming reusable. The World Health Organization estimates that high-income countries generate an average of 0.5kg of hazardous waste per hospital bed, per day. The focus going forward for many healthcare organizations will be on implementing reusable products where possible, but otherwise looking to sourcing more sustainable single use products, particularly where HAIs are a present risk. Bioplastics aren’t appropriate in every situation, with molding behavior, moisture stability issues and higher cost often preventing them from likefor-like replacing all existing plastic injection molded devices. But they are incredibly versatile and should always be a key consideration for new product developments. I am confident that in the near future we will see the adoption and use of more biomaterial products, so that we can continue improving health outcomes in an increasingly sustainable way.
One major way to make plastic products more sustainable is through material innovation. Selecting the right material given a specific design challenge is difficult, but by adopting new technologies and processes, manufacturers can help bring down carbon emissions significantly.
Transportation of Category B biological samples requires a multi-layered packaging system to meet the legal requirements of the UN3373 regulation. These include: ■ Leak proof secondary packaging ■ Inclusion of adequate absorbent material ■ Separation of multiple tubes Choose from two secondary sample packaging solutions that provide these features, helping you achieve compliance with ease, speed and reliability. ® Rigid, clam protectsconstructionshellsamples Built in absorbent gel prevents leaks Multi-vial options with tube separation Leak-proof pouch for individual vials Fixed materialabsorbentwithin Easy to open 95kPa optionscompliant Available from Find out more about our range of UN3373 compliant packaging solutions at www.UN3373.co.uk Simple Solutions for Secondary Packaging Making UN3373 Compliance Quick and Easy T: 023 8048 3000 W: www.alphalabs.co.uk Medical Plastics News provides essential information on materials and engineering processes for professionals involved in the design, supply and manufacture of plastic medical devices. THE LEADING VOICEIN POLYMER INNOVATION FOR THE MEDICAL DEVICE SECTOR SUBSCRIBE TODAY
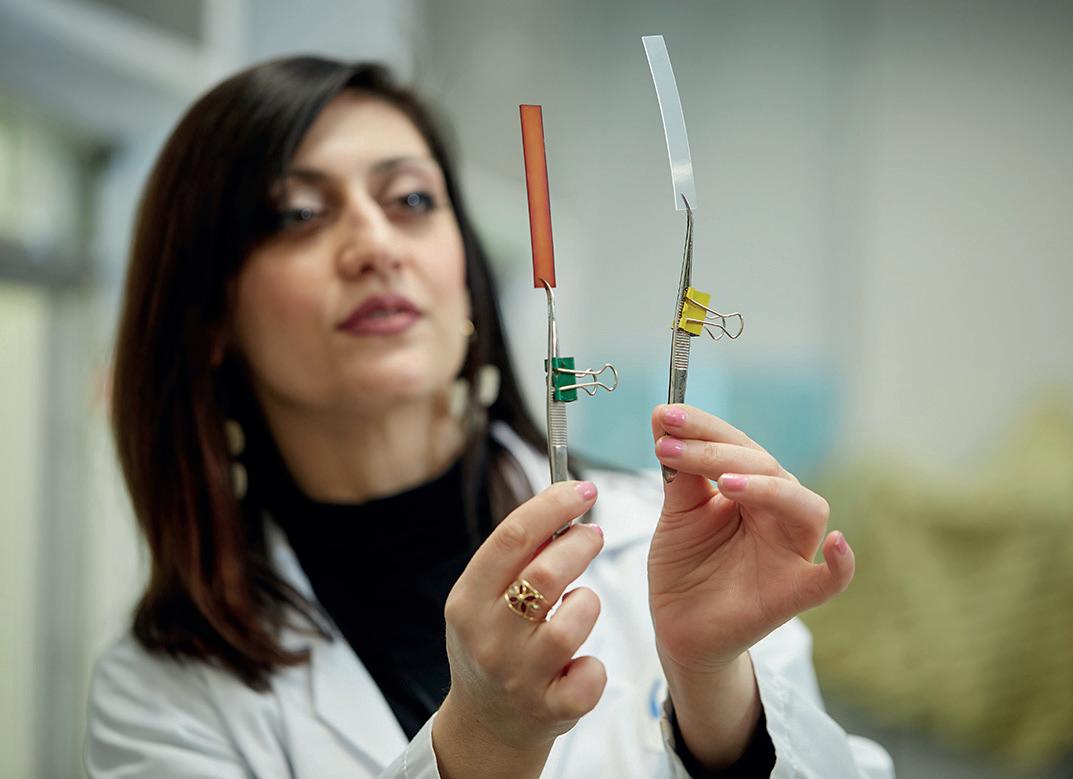
In a diverse and ever changing medical device industry, PC technology continues to demonstrate its viability as a durable, hemocompatible, anti-thrombogenic, clinically-proven coating for medical devices.
The most appreciated and widely deployed use of PC technology in recent years is in Extracorporeal Life Support (ECLS) devices. ECLS devices are used for cardiac and/or pulmonary support before, during and after surgical procedures.
Terms such as biomimicry and bio-inspired have long been used to describe systems that are designed on principles existing in nature. PC Technology is one such surface modification technology, encompassing synthetic biomaterials whose chemical compositions are founded upon the structure of the cell membrane.
PC Technology, a proprietary platform of methacrylate polymers incorporating phosphorylcholine, is synthetic, allowing precise control over the purity profile, the molecular structure and avoiding any of the risks associated with animal-derived material.
KUMAR, STRATEGIC
COATINGS 10 WWW. M EDICALPLASTICSNEWS .COM
WHAT IS PC TECHNOLOGY Short for phosphorylcholine, PC is the chemical name of a polar head group found in many phospholipids, particularly those that form the bi-layers that make up cell membranes. PC plays a key role in determining how a cell interacts with its surrounding environment and is attributed to be one of the primary natural materials responsible for the biocompatibility that exists between most cells.
WHAT SEPARATES PC TECHNOLOGY FROM OTHER HEMOCOMPATIBLE COATINGS?
The zwitterionic nature of PC confers the PC group with high polarity and, consequently, a natural affinity for water. As a result, materials incorporating the PC group are surrounded by molecular layers of water that effectively mask the substrate to which it is applied, providing a biological friendly surface that resists protein and cell adhesion. Essentially the surface layer of water that is bound to the PC acts as a camouflage and disguises the surface such that the protein does not respond to the foreign body. PC Technology helps the performance of medical devices and materials through reduced protein
Furthermore, the application of PC Polymers onto the device surface does not require any elaborate surface pre-treatments, expensive coating equipment, or significant changes in the device manufacturing value-chain. PC Technology can be deployed seamlessly into existing processes with minimal changes and investment.
NEELAM MARKETING MANAGER FOR MEDICAL AT VERTELLUS, EXPLAINS HOW PC TECHNOLOGY IS DIFFERENT TO OTHER HEMOCOMPATIBLE COATINGS.
surfacemakingdecreasedfideposition,reduceddeposition/activation,bacterialadhesion,biofilminflammatoryresponse,brouscapsuleformation,andbloodactivation,itatrulymultifunctionaltechnology.
PC Technology has been used to successfully coat a broad range of substrates including a variety of metal, plastic, rubber, glass and ceramic.
In addition, medical devices coated with a bio-passive agent such as PC enjoy a relatively favorable medical device rating compared to ones using a bio-active agent such as Heparin or Albumin, or other types of combination devices.
APPLICATIONS OF PC TECHNOLOGY
During clinical use, blood is exposed to the artificial tubing and membrane surfaces. Despite the routine anticoagulation treatments, deposition of blood proteins onto the artificial ECLS surfaces may still occur, leading to inefficient membrane functioning, insufficient gas transfer, and potentially device failure. It is therefore of critical importance that device surfaces be managed to appropriately handle the issues of hemocompatibility and thrombogenesis, and to avoid such potential modes of device failure due to extracorporeal blood activation and resulting negative impacts on patient health outcomes.
WHAT WILL HAPPEN IN THE FUTURE?
Currently, there are many companies in this industry that have turned their resources to the development of effective antimicrobial coatings, another option for medical devices to prevent infection and protect against pathogens. But unfortunately, most of these products are still far from perfect. The perfect antimicrobial coating needs to prevent growth on surfaces as well as confront active infections that are within the patient. A new innovation has achieved this by incorporating both active and passive components to create a non-leaching, effective, safe, and highly durable solution for even the most chronic medical devices and implants. This solution that has already been tested and is fully compliant with current medical device regulations, has been independently tested to international standards (ISO, EN, PAS) and proven to provide monoclonal protection which kills a broad spectrum of gram-positive and gram-negative bacteria as well as enveloped and non-enveloped viruses, including E.Coli, MRSA, Influenza, Norovirus and SARS-Cov-2 for 365 days without any reduction in efficacy over time. The combination of components provides a contact-kill mechanism which provides an evolutionary step forward in antimicrobial technologies. The coating prevents growth of pathogens and biofilm under all conditions (acidic, neutral and basic) as well as confronts active infections that interact with the device surface to further reduce infections within the patient.
There is an identified need to protect surfaces from germs and microbes. Everything is susceptible to microbes and bacteria, which find their way to humans. It is not always possible to clean, disinfect or use strong chemicals on surfaces to prevent the growth of germs in regular intervals throughout the busy day. An antimicrobial coating is an application of a chemical agent on a surface that can stop the growth of disease-causing microorganisms. Apart from increasing the surface’s durability, appearance and corrosion resistance, these coatings also protect from harmful disease-causing microbes. Antimicrobial coatings stick to the surface they are applied on and remain effective for a limited period, defining them as a critical option to fight bacteria in this environment.
In the USA, the use of antimicrobial coatings is more widespread than in the EU. The US Food and Drug Administration allows the use of coatings, regardless of them using silver or leaching and reducing their effectiveness overtime. Thorough testing and aiming for perfection in all medical-adjacent products are especially important not only for patient care, but also to bring about a continued cycle of technology innovation and enhanced procedures for the professionals. This current method of testing helps hospitals, patients and healthcare professionals to be certain that antimicrobial coatings are effective against antibioticresistant bacteria as well as viruses.
All we have to do now is choose to develop it and apply it in effective ways for both the professionals as well as the patient.
One of the main factors stunting the widespread use of antimicrobial coatings are the current regulations in place. Under today’s European Union (EU) regulations, medical devices are considered medicines and are therefore tested by the European Medicines Agency (EMA) following the same tests and approval processes that drugs do.
WHY ISN’T THEIR USE MORE WIDESPREAD?
HOW DO WE ELEVATE THE STATUS OF ANTIMICROBIAL COATINGS?
PREVENT PROTECT
11 ARJUN LUTHRA, COMMERCIAL DIRECTOR FOR BIOINTERACTIONS, EXPLAINS HOW IT’S POSSIBLE TO AVOID INFECTIONS. COATINGS
WWW. M EDICALPLASTICSNEWS .COM
The prospect of a biocompatible technology which can enhance the function of medical devices through eliminating existing microbes, actively confronting existing infections within a patient and also preventing the formation of new colonies represents a paradigm shift in prevention and treatment of Healthcare Associated Infections. The future brings an opportunity to raise medical standards. The innovation already exists.
EXTRACTABLES LEACHABLES
SANDI SCHAIBLE, SENIOR DIRECTOR OF ANALYTICAL CHEMISTRY AT WUXI APPTEC, IMPORTANCE OF COMPLETE CHEMICAL CHARACTERIZATION.
SHARES THE
Plastics are everywhere in medical device manufacturing. Whether we’re talking about a filter, bag, container, delivery system or implantable device, these days, chances are, plastic is part of the equation. While plastics and polymeric materials can enhance performance and pricing, these materials do carry risk of their own. Manufacturers need to understand these risks as they develop their products, plan for production and identify both materials and component suppliers as well as laboratory testing partners to support their submission and lifecycle management of their products. They need to understand the key tenet that not all plastics are created equally. Plastic and polymeric products, parts and components can include significant numbers of chemical constituents. Some of these constituents are expected, but many are unexpected and can be potentially harmful. Additives, excipients, and release agents along with impurities, can pose significant differences in plastics that, at surface level, appear to be equivalent. It is these chemical constituents that can leach from the products and compromise patient safety. Extractables and leachables (E&L) testing is the most effective way to extract, detect and identify these potentially problematic compounds so that a thorough risk/safety assessment can be conducted to determine the risks. Extractables testing challenges products using aggressive solvents under exaggerated conditions. The point of extractables testing is to generate “worst-case” data to create a conservative approach to safety. Leachables testing or simulated use extractions investigates compounds that have migrated from a plastic component under clinically relevant or “normal” conditions utilizing drug products, placebos, or more clinically relevant extraction Regulatorysolutions.bodiesare becoming more and more interested in understanding complete chemical characterisation, looking for extractables and leachables data. Because even common materials or those with a long history of clinical use can contain chemicals of concern. Per ISO 10993-1, most medical devices require extractables or exaggerated extractables studies and a toxicological risk assessment, with potentially additional chemistry to mitigate risks to fulfi materials characterisation endpoints. For drug manufacturers, the release of USP <1665> and <665> signals a keen interest in the extractables and leachables in the plastic components of the biomanufacturing process in addition to the extractables and leachables work that has been conducted for years with regards to container closure and stability studies.
COVER STORY EXTRACTINGTHETRUTH WWW. M EDICALPLASTICSNEWS .COM12
● What support do you provide after analytical testing concludes? What if regulators have
● What is your on-time delivery record? And, when does the clock start? Upon receipt of the test article?
● Is complete identification included in the price and timeline that you provided? Or is this extra?
The point of extractables testing is to generate “worst-case” data to create a conservative approach to safety. Leachables testing investigates compounds that have migrated from a plastic component under clinically relevant or “normal” conditions utilizing drug products or more clinically relevant extraction solutions.
There are no commercial databases of LC-MS compounds, and even the most comprehensive private databases contain only a fraction of possible chemicals derived from E&L studies. So it takes the effort of expert chemists doing compound elucidation to get to identification. But, without the identification, an E&L program is incomplete and often, unusable for a toxicologist doing a risk/safety assessment.
For example, liquid chromatography-mass spectrometry (LC-MS) is a highly sensitive platform used to detect semi to non-volatile compounds. When it comes to plastic medical products, typically the most chemicals are found using LC-MS. And this is often when chemicals of concern are found.
● How long has the laboratory been conducting E&L studies? How many programs have you run?
ASK THE RIGHT QUESTIONS
Robust, well conducted E&L studies require a commitment by the laboratories to provide complete characterisation; they must identify the unknowns. Many chemistry laboratories advertise E&L capabilities, but a lab designed specifically for them is different. It means building an infrastructure that includes the instrumentation, cross-functional skill sets and staff to generate and interpret the data in meaningful ways.
COVER STORY WWW. M EDICALPLASTICSNEWS .COM
To reiterate, manufacturers need to know how committed their laboratory testing partners are to identifying all potential hazards. Complete identification is complex and resource intensive. Working with a lab that specializes in complete chemical characterisation will yield the best results.
Thoroughly vetting potential partners can save manufacturers from unexpected charges and unanticipated problems down the road. Understanding your lab testing partner’s method of identifying chemicals of concern and level of rigor is critical. To this end, manufacturers should dig deeply into any potential partner’s experience. Helpful questions include:
A FINAL WORD ON E&L TESTING
Plastic and polymers have become the dominant materials in medical products, but several factors contribute to their safe use. Manufacturers cannot assume that just because a plastic or polymer has been used before or is commonly used that there will not be potential safety concerns. E&L testing helps manufacturers find the unexpected chemicals that may, or may not, potentially impact safety. A robust E&L program should detect and identify all chemical constituents and provide a clear picture to assess risk. Manufacturers are right to question any laboratory testing partner that cannot deliver complete data about their container, delivery system or device.
● E&L studies often yield unique situations. Can you commit to proactive communications when these arise?
The goal of an E&L study is to detect and identify all the chemical constituents above an analytical threshold, including chemicals that are unexpected. It takes a committed laboratory to do the work needed to identify compounds, a report with “unknowns” can cost delays and require retesting.
Successful partnerships require more than experienced practitioners, so it is essential to also ask about project management and communication. Helpful questions include:
SHIFT YOUR MINDSET ON E&L TESTING
13
Finally,questions?manufacturers must understand the services they are purchasing—and those they are not. Some laboratories charge extra for identification or identification beyond what can be found in a commercial database. Other laboratories claim they have the capacity to take on a new E&L program but fail to deliver on time when the process proves more complex than anticipated. Asking the right questions when vetting partners allows manufacturers to make informed decisions, achieve regulatory success and market safe products.
● Can you commit to the elucidation and complete identification of all components?
Medical applicationsequipmentdiagnostic&biotech
©2022 The Lubrizol Corporation, all rights reserved. All marks are the property of The Lubrizol Corporation. The Lubrizol Corporation is a Berkshire Hathaway company. 22-0119902 9911 Brecksville Road Cleveland, OH 44141-3201 USA Learn more at www.lubrizol.com/Health/Medical CHOOSING THE RIGHT PARTNER FOR YOUR NEXT MEDICAL DEVICE.
ALBIS offers the medical industry an unparalleled choice of standard, technical and high-performance polymers from renowned producers. This offering is complemented by customized polymer compound solutions tailored to customer’s needs and made by ALBIS’ sister company, MOCOM. Our products comply with the requirements of the VDI 2017 Medical Grade Plastics Directive. We support numerous projects in the medical sector, pharmaceutical packaging and diagnostic equipment and biotechnology, putting technical and regulatory requirements at the heart of our work, so that we can define the most appropriate material for each application. Our regional experts draw on in-depth knowledge of the medical and pharmaceutical industry to provide you with a powerful service offering that integrates technical support, product safety and risk management. Contact us to learn more about our solutions and assortment of medical polymers and compounds: healthcare@albis.com albis.com
The mission for the next decade is to optimize the interactions between the implants and the human body, reducing the risk of rejection, infection and capsular formation.
15 TILMANN PETERSON, HEAD OF BUSINESS DEVELOPMENT MEDTECH & CORPORATE PLANNING, AMSILK, SHARES HOW TO OPTIMIZE INTERACTIONS BETWEEN THE HUMAN BODY AND IMPLANTS. INNOVATION IN IMPLANTS
Over the last decade, we have seen a lot of improvements and developments made within the implants industry, whether it be in the quality of silicone or the improved texture of implant surface.
AMSilk is a biotechnology company developing a range of vegan silk biopolymers that can be applied in various medical devices. The overall aim is to enhance the biocompatibility of medical devices. The properties of such coated implants include anti-adhesion and increased hydrophilicity. This results in reduced inflammation and capsular formation, less invasive stress and better handling during surgical procedure. One problem with implants is in some cases the body can identify it as a foreign object and cause the immune system to respond and reject it. If the body attacks the implant, it can result in serious complications such as pain, infection or capsular formation, all of which can require further surgery.
Engineered spider silk coatings work by creating a ‘bioshield’, a non-stick surface which has an anti-adhesive effect on bacteria so that it does not adhere to the implant shielding it from bacteria and thus infection. This unique silk technology is safe and bioharmonic for the human body, and is also non-immunogenic, non-inflammatory and non-toxic.
In the near future, we will need new beyondhaveinfections,ficoncerningdirectionstheghtagainstwhichtobefarthesimple use of antibiotics. In this respect, coatings made of silk proteins are an important step towards a new generation of biocompatible surfaces and bioharmonic solutions to better patient outcomes after surgery.
The multiple benefits of silk polymers can be adapted to the needs of various market segments, enhanced skin comfort whilst also improving bacteriostatic and hygienic properties. These individually developed solutions will enable the medical sector to address industry challenges in the best possible way.
WWW. M EDICALPLASTICSNEWS .COM
As well as reducing the risk of implants being rejected, spider silk coatings can prevent infections by forming a surface on which bacteria cannot grow.
Spider insider
Biofabricated coatings have the potential to become a powerful tool in the fight against antimicrobial resistance (AMR). AMR is a major concern, with medical procedures such as surgery becoming extremely difficult, or even impossible, due to an increase in resistant infections. AMR has the potential to affect people at any stage of life with resistance to even one antibiotic causing serious problems. Many medical advances are dependent on the ability to fight infections using antibiotics. Current pharmaceutical coatings available on the market can have high antibacterial effects however, this can create an environment that’s difficult to control. Non-active ingredients, such as AMSilk’s, promote a positive acceptance of implants and limit negative body reactions in a controlled way. The unique biofabrication process reprograms microbes enabling them to produce silk proteins. The microbes are grown in large-scale stainless-steel vessels, fed with natural and renewable raw materials such as sugar. Upon a trigger, they start to produce the silk proteins. The biofabricated silk polymers are vegan, completely biodegradable and produced only with renewable resources, and thus are truly environmentally friendly making a contribution to a future zero-waste society.
Spider silk coatings have the potential to prevent infection-caused inflammation and to reduce post-operative issues caused by implants, minimizing the risk of further medical intervention. Together with its biocompatibility and biodegradability properties, biofabricated spider silk has the potential to be part of a strategy to reduce complications of implant applications.
ALAN HUTCHINSON, PRINCIPAL SCIENTIST AT BROUGHTON, DISCUSSES AND TRENDS WITH EXTRACTABLES AND LEACHABLES.
EXTRACTABLES AND LEACHABLES
TRENDS
One of the major challenges with performing leachables studies is the common requirement to meet lower detection limits, which challenges both the instrument vendors and the laboratory scientists. Highly skilled and experienced analysts are required to develop instrument methods and sample preparation techniques to meet these low analytical evaluation thresholds, and state-of-the-art mass spectrometry instruments and software are needed to detect these low levels and process the data.
THE FUTURE In the future, it would be helpful to see the further alignment of processes and methods for performing E&L projects, and this would benefit spectral libraries for identifying leachables. The development of response factor libraries for determining uncertainty factors would help in determining improved analytical evaluation thresholds for methods. We are starting to see the use of automation for the sample preparation methods required for sample clean-up and enrichment. More use of automation can only help with the consistency of data and reduce the time taken. The improvement of knowledge around E&L processes and materials, and the standardization of materials used for products, will mean that E&L assessments become a more desk-based activity. For pharmaceuticals, we are already seeing this with common industry-grade materials being used; within companies, the same materials are being used across product ranges. ENDS products will likely follow this path and in the future, cleaner, bettergrade plastics and rubbers will also become common across that industry.
Extractables and Leachables (E&L) testing aims to reduce the safety risk to end-users of products such as pharmaceutical medicines or Electronic Nicotine Delivery Systems (ENDS), from the container closure system of the E&Lproduct.testingin the ENDS community is a recent requirement, with new regulations introduced as part of US Premarket Tobacco Product Application (PMTA) guidance. In contrast, in the pharmaceutical industry, E&L testing has been part of the required safety evaluation of products for a while. As pharmaceutical requirements are more developed, the materials used for these products are more consistent and understood. For the ENDS industry, the hope is that the use of grades of materials like those used in the pharmaceutical industry will become more prevalent over time.
Another challenge is a historical lack of clarity regarding the guidelines for designing and performing E&L testing. This has been a slow process and alignment of approaches would be valuable.
CHALLENGES
The formulation of the product can contribute to this issue of sensitivity in so-called dirty matrices such as e-liquids, as their natural flavors and biological samples can make it increasingly problematic to detect lower levels in a forest of peaks due to formulation interference.
The greater emphasis on performing risk assessments as a first step of the E&L process is a main trend. This helps focus the studies and determine where effort is best placed to quickly and efficiently de-risk any concerns from the container closure system. To perform a valuable risk assessment, the knowledge and information, as well as the expertise of the Subject Matter Experts (SMEs) performing the risk assessment that inputs into a Failure Mode and Effects Analysis (FMEA) style process, is essential.
This helps to focus effort and can reduce testing if previous data can be used to bridge to past studies. E&L studies are expensive and timeconsuming, and using previous relevant data will reduce costs and accelerate E&L projects.
The use of high-resolution accurate mass instruments for the analysis of E&L samples is becoming more widespread with E&L projects, helping to identify leachables and reduce the number of unknown compounds reported. Accurate mass instruments can help target leachables in complicated matrices, which can reduce the sensitivity burden by removing interferences from the formulation.
THE CHALLENGES
Up for challengethe 16 WWW. M EDICALPLASTICSNEWS .COM
rapid3devent.com North America’s largest and most influential Additive Manufacturing event.
Connected to a cleanroom, Aptar Radolfzell produces an innovative multiple dosing system for the administration of eye drops. The dosing system is microbiologically sealed and does not require any preservatives. In production, high precision needs to be combined with efficiency. For series production, ENGEL supplied two new production cells, each consisting of e-victory injection molding machines and integrated easix articulated robots.
In Germany alone, millions of applicators for eye drops are used. The symptoms for which liquid medications are dripped into patients’ eyes are manifold. What all eye drops used to have in common was unavoidable contact with bacteria after opening. The systems commonly seen on the market are therefore often single applicators whose contents can only be used for a few hours after opening. Aptar Radolfzell, an Aptar Group Inc. company, produces a sustainable alternative: a multiple-use dosing system with bottles of 5 and 10 ml capacities. As a self-contained system, it provides the necessary protection against microbiological contamination that reliably prevents premature expiration of the drug. “Our applicator delivers the drug drop by drop and is free from preservatives. That is the added value of the system,” says Ingo Korherr, production manager at Aptar Pharma, summing up. “The advantages here are that this is not a one-off application, which is not ecological and produces a lot of waste. The multiple dosage system can be used for several weeks, and it is more ergonomic to apply at the same time,” adds Ralf Fichtner, site manager at Aptar Pharma in Eigeltingen in southern Germany. In ophthalmology, one of the Aptar Group’s product lines, the strategy of manufacturing all plastic parts in-house, has been successfully implemented. This has largely been achieved with ENGEL manufacturing systems.
WWW. M EDICALPLASTICSNEWS .COM
AND HOW AUTOMATED PRODUCTION OF OPHTHALMIC PRODUCTS CAN BE DONE IN THE SMALLEST POSSIBLE WORK AREA.
18 INJECTION MOLDING ENGEL DISCUSSES HOW INTEGRATED SOLUTION CAN BOOST EFFICIENCY
Polyolefins with this precision are certainly not standard, and this is exactly what we specialize in. The challenge is to achieve this precision in a cost-effective way in multi-cavity molding with a high level of process stability.”
After all, the company name, Aptar, is derived from the Latin word aptare. And that means to adapt, which reflects the company’s philosophy in thepractice.company’s The injection molding machines and automation are encapsulated in cleanroom-compatible production cells in compliance with ISO 7 requirements.
“Let’s not forget the very strict demands on the mold and the part, which necessitate a high level of precision – requirements which the e-victory series meets in full. The servo-hydraulic clamping unit side from ENGEL runs like clockwork,” says Andreas Gräber, manager of injection molding services at Aptar Pharma.
The production process is fully automated and 100% monitored. SPC checks of the parts are also an integral part of the process. After completing the respective injection molding cycle, the parts are removed by ENGEL easix articulated robots, including separation of the cavities, which is important for traceability. In other applications at Aptar, ENGEL linear robots handle the parts.
The fact that the control unit for the ENGEL robots is fully integrated into the control CC300 control unit of the ENGEL injection molding machines, made the change to articulated robots particularly easy for Aptar. “One major advantage is that I can see everything on the machine display and do not have to walk around the machine. Of course, I have the hand-held unit for the process settings and teach-in, but that is a one-off thing, otherwise I can control everything from the machine, and it is very easy. The employees are familiar with the user interface and do not have to readjust or learn anything new, but can apply what they know 1:1,” says Andreas Gräber, summing up.
The manual robot panel and the smart ENGEL CC300 control unit access one and the same database. Another benefit is that both systems coordinate their motion sequences with each other. This reduces the handling time in some applications.
19 INJECTION MOLDING
The dosing systems consist of eight parts all told, seven of which are made of polymer materials.
WWW. M EDICALPLASTICSNEWS .COM
The division of the company focuses on injection molding technology. “We buy in other processing technologies strategically,” explains Ralf Fichtner: “The answer to the question as to what we do in-house, and what we don’t, is driven by the complexity of the parts to be produced.”
Ralf Fichtner adds: “We process polyolefins, especially PP and PE. There are certainly more dimensionally stable plastics. The tolerance range with two decimal places in which we manufacture our parts, requires very high precision of the mold, the injection molding machine, and the entire process.
The applicator with a diameter of 15 millimeters is produced from polypropylene (PP) in a 32-cavity mold equipped with a partial hot runner on an ENGEL e-victory 740/220 injection molding machine with a clamping force of 220 tonnes. The total shot weight is 30.5 grams. An ENGEL e-victory 50/90 with a clamping force of 90 tonnes produces the spray pin from TPE with a total shot weight of 1.97 grams in a 16-cavity full hot runner mold. Both parts are productcarriers, i.e., they come into contact with the medication. Where the plastic parts are highly filigree, complex at the same time, and are also produced in a multi-cavity mold, hybrid e-victory injection molding machine with an electric injection unit and servo-hydraulic clamping unit often offer benefits. The reason for this is the tie-bar-less clamping unit, which provides sufficient space for large multi-cavity molds even on machines with comparatively low clamping forces. Patented force dividers distribute the clamping force evenly across the mold mounting platens, ensuring consistently high molding precision across all cavities. These were the factors that tipped the scales at Aptar in favor of capital expenditure on machines from the e-victory series. As ENGEL sales representative Jürgen Fridrich adds: “What was required up front was very high repeatability, shot for shot, and very high availability of the entire production cell in each case.”
The ophthalmic squeeze dispenser (OSD) consists of a total of seven plastic parts with varying degrees of complexity and one metal part. To produce the applicator and the spray pins, capital was invested in two new production cells for the Eigeltingen location; this is Aptar Radolfzell’s second production location in addition to Radolfzell.
INTEGRATED CONTROL UNIT FACILITATES CHANGE TO ARTICULATED ROBOT
The applicator (right top) and spray pin (right bottom) are produced on the two new ENGEL e-victory injection molding machines.
500 MILLION DOSING UNITS A YEAR As an expert for innovative medication delivery systems and packaging solutions, Aptar pursues a two-supplier strategy in-house. Of the total of 85 injection molding machines with clamping forces of 35 to 250 tonnes, ENGEL machines account for more than 60 percent. Some 850 employees produce more than 500 dosing units annually at both locations. With several global good manufacturing practice (GMP) production sites, Aptar Pharma ensures both a reliable supply chain and local support for its customers.
TIE BAR-LESS CLAMPING UNIT ENSURES HIGH-PRECISION MOLDING
A great way of staying ahead is being out there and meeting potential customers. Shawpak stays busy with medical expos around the globe; the company exhibited at Med-Tech Innovation Expo in 2022 and has signed up for 2023, as well as MD&M West, Compamed and more.
Tony Crofts, sales director explained: ‘’To compliment our range of medical packaging machinery we are now developing a rigid blister making machine which forms the blister, die cuts the outer profile and stacks them ready for use in production. “This along with our pouch making machine gives our customer the full flexibility in making their packaging on demand from roll stock rather than relying on their supply chain to deliver the packaging they require and keeping months of stock to ensure supply doesn’t run out. The tooling can be quickly changed to give the customer full flexibility and control, it allows you to make the blisters and pouches you require just ahead of time and not keep pallets of stock taking up space and tying up cash.‘’
The start of Shawpak began in 2013 when David Shaw, CEO, thought that the size of a long linear thermoforming machine - which was six meters long - could be condensed into a smaller machine, but with the same input.
Shawpak is a UK manufacturer of a range of packaging equipment for medical devices, including rotary thermoforming machinery.
Shawpak’s newest addition to the range, the Shuttle Machine, is also coming soon.
Shawpak is now developing their own rigid blister making machine.
Not only do Shawpak exhibit at expos, but the company also held its own in-house expo over three days, in July, called Shawmed. The expo consisted of showcasing many of their own machines, a factory tour of Riverside and opportunities to speak to their related companies – Amaco Flexible Print Solutions, SP Automation and Robotics and Sterimed Infection Control.
THERMOFORMING
The Shawpak Pouch Machine launched at the start of 2021, Shawpak says it represents the best solution for packaging flat medical products.
Shawpak’s range also includes the Pouch Machine and the Pouch Sealer.
“We started developing the first machine and took it to the first trade show, Medica, in Dusseldorf. The machine received such a great response that we knew we had to promote the machine commercially.’’
ALAN WADE, OPERATIONS DIRECTOR AT SHAWPAK, SHARES HOW THE COMPANY PLANS TO KEEP AHEAD OF THE THERMOFORMING COMPETITION.
IT’S HEATING UP WWW. M EDICALPLASTICSNEWS .COM20
The shawpak thermoforming machine uses a rotary drum to form, fill and seal the medical devices unlike the traditional linear thermoformers. The benefit of wrapping this around a drum reduces expensive clean room space, as it’s so compact, reduces waste on blister packs and also increases flexibility to change tools Riversideover.Medical Packaging has played a huge role in developing the shawpak machine. With their experience in packaging, they were able to assist in finding the advantages that the shawpak machine provides and overcoming many of the disadvantages that thermoforming machines generally have. While thermoforming is a huge industry, Shawpak intends to stay ahead of the game. Wade discussed several ideas that could benefit the growth of the company, including making assemblies in advance to put into stock, so when a customer wants something, they can pull the assembly and build, rather than manufacture from new, which can reduce time significantly.
Alan Wade commented: “Our CEO, David Shaw had an idea at the end of 2013, to make a smaller rotary thermoforming machine.
Form/Cut/Stack systems are usually enclosed in a protective envelope protecting the process and the product from airborne particulates and ambient temperature and humidity, and they can be more fully automated and therefore more precisely monitored and controlled, especially machines with 100% servo motors and drives.
BRIAN GOLDEN, SALES DIRECTOR, AMERICAS, GN THERMOFORMING EQUIPMENT, SHARES HOW THE MEDICAL PACKAGING INDUSTRY LEADS THE TREND TOWARDS THERMOFORMING AUTOMATION.
The most challenging aspect of medical packaging design is often the complex geometries required to isolate individual items into separate compartments and to lock each item in place. When properly designed and manufactured, undercuts allow each part to be snapped into place and held securely.
21 THERMOFORMING
ELIMINATION OF AIRBORNE PARTICULATES
INCREASING AUTOMATION
It is imperative that measures are taken to eliminate static that will attract particulates and to ensure that the cutting process does not generate particulates that can migrate to the product.
These various special requirements for medical packaging have led to the wide-scale adoption of Form/Cut/Stack thermoforming systems. Simpler and less costly Contact Heat systems are suitable for only a small fraction of medical packaging applications because they lack plug assist capabilities required for complex geometries and the higher clamping forces that Form/Cut/Stack systems offer.
Automation of the thermoforming process has been embraced in the medical packaging industry more quickly than in other markets. Originally, this trend was driven by stringent quality requirements and the need for high levels of repeatability. Automation has also brought other benefits of strategic importance to medical packaging producers, including facilitating higher and more predictable throughput, as well as helping to address the challenges of attracting, training, and retaining a quality manufacturing workforce.
Instead of requiring one or more operators per production line, a single operator can cover multiple lines. First of all, this requires that the machines autonomously perform the forming, cutting, and stacking processes with little or no hands-on engagement by the operator. For high volume production, robotics are increasingly employed to automatically perform downstream packaging and palletising functions. But the autonomous operation of these functions is only one element of Theautomation.procedures for changing tooling between SKUs and for replacing roll stock also need to minimize human error and effort. The right tooling needs to be
WWW. M EDICALPLASTICSNEWS .COM
Enclosing the machinery is important not only for protection against particulates but also for isolating the system from ambient air and temperature that cause fluctuations in the heating of materials and in the air pressure of pneumatic components.
Investment in automation offers many advantages in medical packaging manufacturingmore precision and control result in much higher repeatability, which means higher quality products as well as fewer defects and less waste. The process improvements achieve faster cycle times and predictable output, for higher throughput and scalability. Precise control and repeatability are also critical to the calibration and production consistency required by the strict standards and government regulations for medical packaging, such as ISO 11607:2019. Automation may also include vision systems and other inspection technology that provide automatic, continuous real-time quality control. In general, the more automation, the greater the opportunity to collect data for process improvement and for traceability.
EASE OF OPERATION
The shape of the packaging typically differs for medical products. The entire package must withstand the temperature and pressure extremes of the sterilization process. The flange must have the required thickness and rigidity, and smooth surface, to accept and maintain a hermetic seal to the Tyvek lid. The sidewalls must also have adequate and consistent strength and thickness to prevent cracking and leakage during transport and handling.
STRONG FLANGE AND COMPLEX GEOMETRIES
Most medical packaging applications call for enclosing the thermoforming machinery in a protective enclosure to minimize exposure to airborne particulates that can cause gaps during the hermetic sealing process.
The Emerson logo is a trademark and a service mark of Emerson Electric Co. © 2022 Emerson Electric Co.
Latest Plastic Welding Technologies
LOOKING FORWARD
Plastic Material Changes Require
Market pressures are driving medical device designers away from PVC and PC, yet replacements like PE and PP do not respond well to traditional plastic joining methods. Reliable assembly requires advanced BransonTM ultrasonic and laser welding systems from Emerson. They produce ultra-clean, aesthetically superior welds for today’s preferred materials while also delivering design flexibility, process efficiency, energy saving and sustainability advantages. Learn more at: Emerson.com/Branson Come and speak with an expert at: K-Show, Hall 11 Booth F55, 19-26th Oct 2022. installed in exactly the right way, for every production run, and roll stock can weigh 1500 lbs. Thermoforming equipment should be designed with procedures and tools for streamlining these processes and preventing errors and with ergonomic aids for lifting and correctly positioning heavy objects.
THERMOFORMING
The whole concept of a Form/ Cut/Stack thermoforming machine is to integrate and automate these multiple functions in a single system. Medical packaging manufacturers have been at the forefront of the trend toward automation, initially driven by the need for high quality, repeatability, and traceability. Automation also increased scalability and throughput. The third driver of automation is the need to address labor shortages and high turnover by making processes more productive and less labor-intensive, and easier to learn. Form/Cut/Stack systems can be fully automated and therefore more precisely monitored and controlled, especially machines with 100% servo motors and drives.
RICH QUELCH, GLOBAL HEAD OF MARKETING AT ORIGIN, SHARES THE CHALLENGES WITH THE ‘FAKE’ PHARMA MARKET. agents across
PHARMA
A CAT AND MOUSE GAME
THE DIFFERENCE BETWEEN ‘COUNTERFEIT’ AND ‘FALSIFIED’ To understand the scope of the challenge facing the pharmaceutical market and the threat to public health, it’s important to distinguish between the terms ‘counterfeit’ and ‘falsified’ medicines.
Anti-counterfeiting is a cat and mouse game, with criminals usually cracking today’s systems in two to three years. This means continuous improvements and advancements are needed to stay one step ahead.
Pharmaceuticals are the world’s most counterfeited consumer product, with the latest figures from the World Customs Organisation estimating the global ‘fake’ pharma market to be worth upwards of $200 billion.
23 ANTICOUNTERFEITING AND SERIALIZATION
The global trade in counterfeit and falsified medicines has always been a very large and real threat to public health – and pharma companies’ reputation. But the COVID-19 pandemic heightened this risk, creating a ‘perfect storm’ which made it easier and quicker for counterfeiters to circulate and sell fake products, thanks to supply chain disruption and complexities. In the early emergency phase of the pandemic, it was clear that criminals would take advantage of the situation and put profit over safety. In fact, by March 2020, Interpol’s Operation Pangea XIII seized counterfeit pharmaceuticals worth more than $14 million worldwide, just months after the COVID-19 virus was first discovered. So, what are the latest anti-counterfeiting strategies being actioned by the industry, regulators and governments? And how are packaging technologies aiding the fight against the fakes?
PHONY
A significant advancement in worldwide standards for mass serialization on packaging came in 2019 when two important regulations came into force – the EU’s False Medicines Directive and the US’ anti-counterfeiting protocol, ‘The Drug Quality and Security Act’. Both regulations focus on connected approaches to authentication, with all
The European Medicines Agency defines counterfeit medicine as being “made by someone other than the genuine manufacturer, by copying or imitating an original product without authority or rights and [infringing] trademark law”. These products are disguised as legitimate branded medicines, putting their reputations at risk. There is also the challenge of falsified medicines –the fake, unauthorized medical products that make their way into the market. These may be mislabelled or produced in fake packaging and, most dangerously, there is no regulation around their manufacturing. Falsified medicines may contain the wrong ingredients or low levels of the active ingredient. Both counterfeit and falsified medicines pose risks to public health and threaten to undermine the healthcare system and pharmaceutical industry.
WWW. M EDICALPLASTICSNEWS .COM
Packaging technologies remain the first line of defense against counterfeit and falsified medicines. For pharmaceutical manufacturers, the primary solution has long been to build anti-counterfeiting technology directly into medical packaging – providing a convenient method for tracking products across supply chains as well as visual authenticity for the consumer.
This is invaluable information for anti-tampering and wider commercial strategies, allowing companies to locate and interrogate a product anywhere in the supply chain. For example, the geographical location of a product and the route it took to arrive there can all be captured and stored, thus revealing any unauthorized journey routes, interventions or delays.
The challenge, however, is that criminals are only ever one step behind. And each new development in product-level coding only remains a barrier for a few years before fraudulent manufacturers produce counterfeit copies and bypass security protocols.
Anti-counterfeitingproviders.solutions are becoming smarter every year. But so are criminal networks. There is a real opportunity here for pharma companies with the foresight to invest in Supply Chain 4.0, and for governments to remove any red tape holding back its development, to set the stage for new business models that can create value in many ways – and limit counterfeit activity in the process, too.
Advanced tracking systems, built into primary and secondary packaging, are an exciting area of innovation. By managing and recording all the typical activities that occur in the supply chain or designed to cover special requirements, tracking chips can log events or raise queries that occur across a product’s lifespan remotely.
They are also making it easier to spot and eliminate weak links in supply chains that criminals are quick to exploit. They have the added benefit of creating value in other ways too, helping to increase end-to-end visibility and efficiencies across the pharma supply chain by gathering data that can be analyzed and acted upon, often in Bigreal-time.data, provided by smart packaging in part, will be key to giving pharma companies a more granular, real-time picture of events taking place along the supply chain, from manufacture to healthcare settings.
As a result, the development of advanced packaging-level tokens has led to watermarking techniques – invisible, encoded data that requires specialist verification software. This technology proves difficult to replicate as it is invisible to the human eye, and its unique data is required throughout tracing and decommission to verify against interference.
the supply chain expected to contribute to the tracking of legitimate products. The EU’s FMD requires complete product traceability from manufacturing to decommissioning, rather than placing the burden of authentication on any single stage of the process. The Drug Quality and Security Act also requires authentication at every supply chain juncture, including wholesalers.
Collected data can also highlight inefficiencies and bottlenecks, which can be addressed to streamline processes and drive cost-savings.
The development of advanced packaging level tokens has led to watermarking techniques - invisible, encoded data that requires specialist verification.
ANTICOUNTERFEITING AND SERIALIZATION WWW. M EDICALPLASTICSNEWS .COM24
Drug companies won’t necessarily have to build their own anticounterfeiting ecosystems, either. Manufacturers can avoid the high up-front costs of developing an anti-counterfeiting system by handing off all or part of the work to external
SUPPLY CHAIN 4.0 Pharmaceutical supply chains are increasingly complex, introducing a greater risk of exploitation and making it harder for stakeholders to monitor the flow of products. Enhancedmethods,anti-counterfeitingincludingcloud-basedtrackingandperennialencryptiontechnologies,areextendingthelifecycleofprotectionformanufacturerssofrequentandcostlyoverhaulsareavoided.
FOR FURTHER INFORMATION ABOUT
Vyon PTFE is a unique, high-performance porous plastic material manufactured from 100% pure polytetrafluoroethylene. Through sintering, Porvair produces porous PTFE structures composed of tortuous interconnected pathways with minimal dead-end pores. Combining the unique porous structure and tight pore distribution, Vyon PTFE offers enhanced controlled flow of liquids and gasses making it ideal for filtration, separation, and retention of biological and chemical materials. Developed to cope with the most demanding applications, Vyon PTFE is extremely durable offering superior chemical resistance to most aggressive media and corrosive solvents. In addition, Vyon PTFE components are temperature resistant up to 260°C, naturally hydrophobic, exhibit minimal flex fatigue and contaminants do not easily adhere to components made from this advanced engineering plastic. Sheets of advanced Vyon PTFE plastic materials can be manufactured into a variety of shapes and sizes to suit your specific application. Tightly controlled manufacturing processes ensure that Vyon PTFE porous plastic components for your product are produced with consistent, reproducible, and controlled critical properties such as thickness, diameter, and porosity.
Vyon PFTE is a naturally hydrophobic porous plastic that is ideal for applications such as chromatographic separations where longer term solvent impermeability (e.g., acetonitrile can be held up for 24hrs) is important. VYON PTFE VISIT orpolytetraflhttps://www.vyonporousplastics.com/draft/vyon-uoroethylene-ptfe/contactPorvairSciencesLtdon+44-1978-661144
before after www.vyonporousplastics.com Work in peace with Vyon® • Significant noise reduction • Minimal flow loss • Choice of screw or push-in formats • Range of precision engineered sizes available Designed to reduce noise with no compromise on air flow, the Vyon® range of pneumatic silencers are quick, easy to install and made from materials built to last. EU/RoW: enquiries@porvairsciences.com
/ enquiries@porvairsciences.com. SPONSORED
PLEASE
Porous PTFE Components for Demanding Applications
VALUE CHAIN COOPERATION
WHY IS THE CHOICE OF STERILIZATION SO IMPORTANT?
The validation of the recently developed Bormed BJ868MO is the result of close cooperation among Premix Oy (a leading manufacturer of electrically conductive and high frequency plastics), and a leading provider of in-vitro diagnostic solutions. These electrically conductive compounds enable extremely accurate liquid level detection and are widely used in in-vitro diagnostic consumables to ensure precise measurement. Bormed BJ868MO functions as a base for such an electrically conductive compound used in the production of high precision pipette tips.
Choosing the right material for IVDs, consumables and labware often turns out to be crucial for the end performance of the product itself. dosage of irradiation, rather than the mean full dosage is not delivered
WWW. M EDICALPLASTICSNEWS .COM26 DIAGNOSTICS
Grade A
An increasingly important trend in the healthcare industry in recent years has been the stringent regulatory demands on the entire value chain with the end goal to ensure patient safety. High level of quality, protection and health for the end users as well as supporting innovation and making sure that the new innovative medical and diagnostic devices reach patients in a timely manner have been at the heart of the transition to Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) in Europe.
WHAT ROLE DOES THE RAW MATERIAL PLAY? Along with the changes in classification system for IVDs, the IVDR requires clinical studies and evidence in many IVD applications where previously self-assessment was accepted. In practice, this highlights the need for assurance that the material and the IVD that you qualify for today remains unchanged so that the validity of the clinical study is intact. This also safeguards the investment in material qualification and ensures that any final item testing maintains its Onrelevance.top,project timelines during new healthcare product developments are critical; often a quick access to advanced raw material technical data that goes beyond the product data sheet is needed. Additionally, a rapid confirmation from the supplier on substances of concern or even a composition disclosure and extractable data may be crucial for accelerating time-to-market. Fast technical and regulatory reaction globally and peace of mind for the healthcare value chain is at the heart of the Borealis Bormed medical grades service package. Choosing the right material for IVDs, consumables and labware often turns out to be crucial for the end performance of the product itself. Polyolefins are frequently being selected due to their combination of good property profiles and value in use as well as often offering a better sustainability profile by being lighter, easier or safer to use, chemically inert and recyclable.
PAULO CAVACAS, BUSINESS DEVELOPMENT MANAGER, HEALTHCARE, BOREALIS HIGHLIGHTS THE TRENDS IN MEDICAL GRADE PLASTICS FOR IN-VITRO DIAGNOSTIC APPLICATIONS.
Sterilizing IVD and consumables is a common requirement in the industry and can be carried out by several different approaches with chemicals e.g. ethylene oxide (EtO) and irradiation (e-beam or gamma) being the most common ones for such applications. Gamma rays have high energy and penetration capability and as such are frequently used for items that are bulk packaged. Extensive safety measures are required for both operators and additionally the radioactive isotopes from where the gamma rays originate. Some polymers, especially PP, are sensitive to degradation (formation of radicals) due to the high energy used. As a result of the radiation the final product can become brittle and/or yellow either directly after radiation or after a certain undefined time. Additionally, it is important to consider the ‘delivered’ dosage of irradiation, rather than the ‘emitted’ level as bulk irradiation will mean full dosage is not delivered to every part. In order to mitigate the effects of the irradiation, PP resins can be specially added.
MEGAN MUROSKI, PHD, SENIOR PRODUCT MANAGER, MILLIPORESIGMA, THE U.S. AND CANADA LIFE SCIENCE BUSINESS OF MERCK, SHARES HOW GOLD NANOPARTICLES CONTINUE TO OFFER PROMISE IN DIAGNOSTIC INNOVATION. IS GOLD Gold nanoparticles are easy to synthesize, are commercially available and considered biocompatible.
The extensive research in gold nanoparticle lateral flow tests has resulted in significant improvements to their sensitivity, specificity, and overall performance. Despite the significant progress, further challenges remain for GNP-LFAs, including improving the reproducibility of gold nanoparticle synthesis and the lack of regulatory protocols for the development and characterisation of nanomaterials, which has limited mainstream integration of assays. This is a tremendously challenging task, as global regulations can vary, and the number of diverse nanomaterials is constantly expanding. However, it is important to understand, assess, and manage risks to ensure quality and regulatory standards are met. Companies, such as MilliporeSigma, have developed the M-Clarity system to help industry researchers choose materials with the correct compliance to standards. Despite these challenges, gold nanoparticles are becoming increasingly commercially available with strict characterisation and quality controls. This may pave the way for a universal rapid testing solution that is readily available and accepted worldwide.
27 Nanoparticles offer great promise for applications in biomedical research and clinical therapies. Nanoparticles, characterized as under 100nm in size, have unique physicochemical properties, including high surface area to volume ratio, strong signal intensities, and tuneable surface chemistries. Due to their large surface areas, nanomaterials can load a variety of molecules, such as antibodies, DNAs, and organic dyes, making them useful to detect low concentrations of analytes. They are also easily functionalized, allowing for multiple targets to be measured Thesimultaneously.useofmaterials, such as iron oxide and gold, has been extensively explored for the field of nanomedicine as they offer desirable and unparalleled characteristics for chemical and biological detection methods. Gold nanoparticles are easy to synthesize, are commercially available, and biocompatible,consideredwhichmake them ideal reagents for use in many applications, including bioimaging, nanomedicine, and diagnostics. In addition, gold nanoparticles have a localized surface plasmon resonance that results in their characteristic ruby red color, that can shift purple, depending on the size of the particle; this makes them particularly suitable for colorimetric readouts, such as lateral flow assays (LFAs). In addition, gold nanoparticles have excellent biocompatibility and stability which is crucial for diagnostic testing. Due to the rise in demand for rapid testing during the COVID-19 pandemic, gold nanoparticles were catapulted into the mainstream of testing materials.
The use of gold nanoparticle (GNP)-based lateral flow assays for detection of diseases like COVID-19 has demonstrated a fine balance of complexity and high sensitivity. In lateral flow development, the sensitivity of the reporter in the assay is dependent on the visual readout. There are many factors to consider during development, as reporters need to generate the largest possible signal, but also be small enough to travel through the membrane and bind to molecular targets. Other limitations of rapid testing include low sensitivity and cross-reactivity. Gold nanoparticles can overcome these limitations, as they can be made to be approximately the same size as antibodies, allowing for a 1:1 readout with a large signal with an easily visible testing line. Furthermore, gold nanoparticles can be easily modified, allowing for a host of applications based on their surface chemistry, such as the detection of the host antibody (serological) or antigen. Their suitability and stability provide additional value for often price-sensitive point-of-care diagnostics. Rapid testing diagnostics need to be inexpensive, easy to operate, and instrument-free.
DIAGNOSTICS WWW. M EDICALPLASTICSNEWS .COM
THIS
28
MARFRAN, PRODUCER OF TPE/TPO COMPOUNDS FOR FOOD CONTACT AND MEDICAL USE, SHARES ITS FOCUS FOR THE PRESENT AS WELL AS FOR THE FUTURE. T NE with the times
When selecting TPE for medical applications, manufacturers can either choose food contact or medical grades. The current trend seems to be swinging towards medical grades, but awareness about the similarities and differences between both grades is necessary to make the right choices.
SPONSOREDSince their first appearance on the market, TPEs have mainly been used in injection molding and have experienced some difficulties gaining ground in the extrusion world, as this has traditionally been dominated by vulcanized rubber and plasticised PVC. However, in recent years there has been a strong tendency to reconsider TPEs as suitable candidates for several extrusion applications - a trend favored mainly in the fields of drinking water and medicine because of more stringent hygienic requirements. In the medical field, the dominant position of plasticised PVC as a raw material to produce medical devices stands as a point of reference to all market newcomers like TPEs, which must conform to many common processing techniques applicable to PVC or enable new applications/alternative processes to be created to carve out their own market space. Marfran is committed to develop and produce new TPE solutions for strongly regulated applications for both present and future challenges.
The company’s most recent developments have been focused on extrusion grades for many applications in the medical field, for drinking water and other relevant industrial applications. Our current projects are dedicated first and foremost to Marfran TPEs for drinking water applications and to medical compounds, with a particularly strong focus on solvent-bonding products - a complete revolutionary solution for a TPE that until only recently could not be bonded with a solvent. By working closely with raw material suppliers and investing in new process technologies, Marfran uses the latest innovations to identify new material combinations and develop new possibilities.
In the medical industry, the demand for safe and halogen-free polymers such as Styrenic block copolymer-based materials is currently on the rise. The safe and non-toxic properties of TPEs make them an ideal component in the design of medical products, for which superior performance levels and safety are required. TPEs combine flexibility with high performance, while also complying with many food and skin contact regulations due to their inherent low toxicity.
MEDICAL COMPOUNDS Medical applications are a high-growth TPE sector with major applications in tubing, catheters, intravenous systems, resealable membranes and films.
IN
WWW. M EDICALPLASTICSNEWS .COM
In 2015 Marfran registered a patent on the use of usnic acid in TPE compounds as an antibacterial (WO 2016/020774 A1). The effectiveness of usnic acid has been proven by numerous tests conducted on various compounds, however its organic nature makes it “sustainable” but it also introduces limits on temperatures both for processing and operation. The increase in the temperatures of the sterilization cycles requires the use of TPE based on polymers with an average higher molecular weight which, therefore, requires an average higher processing temperature. Thanks to the collaboration with some suppliers, Marfran has developed solutions based on bacteriostatic substances which, due to their inorganic nature, can be used at higher processing temperatures more suitable for TPE compounds with higher molecular weight. The bacteriostatic effect prevents the formation of bacterial biofilm on the surface of the artifacts, facilitating sanitation even with common detergents and making autoclaving more effective and lasting.
times WWW. M EDICALPLASTICSNEWS .COM
29 SPONSORED
The “bacteriostatic” solution can be applied both on materials in contact with food and in the medical field, having no effect of cytotoxicity, unlike other bactericidal substances. The effectiveness of the bacteriostatic treatment of TE compounds was confirmed by evaluating the antibacterial activity conducted according to ISO 22196: 2011. The results obtained on a Marfran.Med 40A M compound are quite positive, considering the hardness of the chosen compound.
Numerous studies have highlighted the ability of COVID-19 to survive on the surfaces of various materials, even for several days. An antiviral activity measurement test, conducted in accordance with the ISO 21702:2019, highlights a significant improvement in the reduction of COVID-19 on the surface of the Marfran.Med 40A M when treated with bacteriostatic treatments.
The transformation processes for Marfran.Med are carried out in a dedicated cleanroom, which is compliant with ISO 13485; ISO 7 class, according to ISO 14644-1, and Class 10,000 equivalent, according to US FEL STD 209E.
The most stringent requirements relating to sanitation and sterilization force us to reconsider the materials and identify new solutions to allow a more efficient and safer sterilization of the products.
Food contact raw materials must be cleared by Regulation (EU) 10-2011, stating whether they are a substance with specific migration limits (SML) or a dual use substance. Medical raw materials have to be compliant with European Pharmacopeia and have their biocompatibility certified according to ISO 10993 and USP class VI. With rapidly advancing technologies and increasing regulatory requirements, today’s customers are asking for more material knowledge and consistent processes in order to minimize variation and improve performance.
The Cleanroom is highly automated, subject to strict cleaning procedures and optical checks for quality control. That means more extensive clean down and line clearance procedures, with the guarantee of process change control and that production only takes place on dedicated lines. As a leader in the production of compounds based on thermoplastic elastomer compounds (TPE/TPO based on SBS and SEBS), Marfran has a full range of TPE Marfran.Med solutions for medical applications. TPEs do not easily bond to other materials, such as the substrates polycarbonate (PC) or ABS connectors that are used in standard intravenous tubing sets. Recent developments allow Marfran to propose Marfran.Med grades suitable for both molding and extrusion, that allow solvent bonding with cyclohexanone or THF. The challenge of the pandemic and the sanitation of TPE surfaces. The pandemic has forced all health authorities to reconsider all the criteria for sanitizing the environments and therefore the surfaces of all the artifacts used in the medical field.
Systems design, wireless design, cloud architecture, usability engineering, integrated artificial intelligence (AI) and machine learning (ML), cybersecurity - the list of technical requirements to consider seems endless when it comes to designing a connected medical device. While an existing product might look the same as a connected device, the product design is fundamentally more complicated. Connectivity requirements launch the design process into a larger network of associated products and processes in a wider and interdependent ecosystem.
THE FUTURE PROMISES INNOVATION As AI and ML technologies continue to mature from consumer to medical platforms, corporate partnerships and incentive subsidies will be desirable to share in the substantial design/development overhead necessary to build, test, and validate robust and reliable software algorithms required to guide and control medical devices. Additionally, support is needed from governments, related industries, and clinical and insurance communities. Without this support, the return on investment (ROI) for manufacturers is difficult to predict and may stall the pace of new technology integration into the design process.
WWW. M EDICALPLASTICSNEWS .COM30
Further complicating device design and development for connected devices is that wireless telemetry protocols and hardware are not standardized internationally, making it virtually impossible to develop a single device that will be able to operate globally. Regional differences in radio standards and institutional infrastructure require multiple design versions or duplicated components, further complicating R&D for device manufacturers. And various design versions need to not only meet regional standards but also must be created to meet specific global regulatory requirements.
CONNECTED DEVICES
PHILIP REMEDIOS, PRINCIPAL AND DIRECTOR OF DESIGN AND DEVELOPMENT, BLACKHÄGEN DESIGN, DISCUSSES THE DEVELOPMENT PROCESS FOR CONNECTED DEVICE DESIGN. for the Real World
ACCELERATION OF DISTANCE CARE PRACTICES COVID certainly is an accelerant to instigate more focus on the needs of patient-operated healthcare. In fact, the practice of in-home monitoring and drug delivery has become so widespread that “distance care” is now a common term that includes everything from wearables to in-home devices for real-time monitoring, therapy, and chronic drug delivery applications. Secure data collection and transfer to professional healthcare providers for oversight and interaction are powerful features in these new devices. Many also include sophisticated embedded intelligence that not only provides optimized performance but also feeds vital data into remote databanks for analysis and AI advancement. The development of more sophisticated and even smarter devices for patient self-care is expected to continue. But, in the design and development processes, manufacturers must be particularly cognisant of the added usability requirements for the patient. Just the demographic and psychographic variations among patients present numerous design challenges to overcome comorbidity-driven limitations such as strength, vision, dexterity, and social/cultural variabilities to assure predictable operability and user safety.
USABILITY ENGINEERING PROVIDES THE PLATFORM FOR DESIGN Usability engineering requires several steps – discovery research, design confirmation, and risk-based validation through user studies. This approach provides an opportunity to identify and mitigate potential end-user challenges, which may be demographic. It also considers patient safety if potential or actual comorbidities are involved. The environment in which the device will be used is also identified if, for instance, hygiene or hazardous situations cannot be predicted. The usability approach also looks at how the device can be designed to blend inconspicuously into a home setting or, if wearable, can be non-intrusive or perhaps worn comfortably under a patient’s clothing. It also considers regulatory requirements upfront so that specific issues can be adequately addressed during the design process.
Design
With appropriate concurrent commitments from all these entities, the advancement of this new and exciting medtech paradigm can absolutely elevate opportunities for patients to interact with their clinicians to improve healthcare at reduced operating costs.
OVERCOMING CHALLENGES WITH TECHNOLOGY STANDARDS
Because IPA dries very slowly, the assembly cycle time increases while manufacturers wait to move to the next stage of production. Just like oil, IPA can negatively affect the rigidity of thin-structured tubing, making it difficult to work with.
silicone is a popular material choice for tubing, due to its high level of biocompatibility, durability and flexible structure, it generally does not expand or stretch without Siliconeassistance.also has a high coefficient of friction, or a tacky surface. Therefore, sliding a silicone tube onto a barbed or textured fitting often requires some force which can result in stress-cracks, splitting and ultimately scrapped materials.
CONNECTED DEVICES
Medical device manufacturers require small, light and flexible tubing that meets tight tolerances. This often means reduced inner and outer diameters, thinner wall thickness, and multi-lumen tube
ENGINEERED SWELLING FLUIDS
Ultra-pure isopropyl alcohol (IPA) is another popular fluid when assembling devices that use silicone tubing. Although IPA is cost-effective and easy to obtain, it does have problems.
A better option is an engineered silicone swelling fluid. It quickly and evenly swells tubing simply by soaking one end of the tube in the fluid. Depending on how much expansion is required, the longer tubing stays immersed in the fluid allowing it to increase to the desired dimension. For example, if the tubing only needs to expand by 1–2% for assembly, the entire swelling process is often completed in less than a minute.
Silicone has been widely used in the medical sector for decades. Thanks to its unique chemical and physical properties, highly-versatile medical-grade silicone can be found in everything from diagnostic and monitoring implements to surgical implants and device tubing. With developing countries undergoing rapid changes and rising aging populations, the need for silicone medical tubing in the healthcare and medical device industries is increasing. However, as medical devices become smaller and more complex, the use of this biocompatible material becomes more Advancedproblematic.swelling fluids assist with this challenge by providing greater design flexibility. They are an effective way to easily and successfully incorporate silicone tubing in today’s cutting-edge devices. DESIGN FLEXIBILITY
After the tube is fitted to the connector, the engineered swelling fluid evaporates quickly. The tubing returns to its original size, shape, durometer, compression and strength to form a tight, sealed fit over the fixture, regardless of its geometry. This makes it particularly useful when using multi-lumen tubes.
Althoughconstruction.medical-grade
Unlike hexane or toluene, swelling fluids are specifically engineered for the job. They do not change the physical properties of the tubing. It is not aggressive, so it has excellent materials capability and is safe to use on most elastomers and plastics including neoprene, EDPM, polyethylene and polypropylene. It is especially useful for swelling tubing with thin, soft walls or those with larger diameters.
The swelling fluid does not cause long-term change to the mechanical properties of the tubing material. Furthermore, it will not weld or bond the tubing onto the fitting, so it can be easily removed later, if needed.
A CLOSE FIT Assisting medical device manufacturers in this assembly challenge are fluids that allow silicone tubes to slide and fit more quickly and easily. Oil, alcohol, and elastomer swelling agents are the three most common types.
A TIGHT FIT Engineered silicone-based swelling fluids are helping advance the design and production of complex medical devices. Tubing that was once difficult or impossible to join to other components can now be securely fitted with ease to ensure the safety and reliability of the medical device. Thanks to their specific properties, swelling agents make medical device assembly efficient and effective and do so in the safest, most sustainable way.
JAY TOURIGNY, SENIOR VICE PRESIDENT AT MICROCARE MEDICAL, EXPLAINS WHY SILICONE SWELLING FLUIDS HELP MEDICAL DESIGNERS TO MAKE A CONNECTION. CONNECTED DEVICES 31WWW. M EDICALPLASTICSNEWS .COM
The NHS is just littered with innovation and people with good ideas, but they don’t have access to the resources, skills, and the knowledge to be able to bring that to market. On top of this, there’s very few of us who have walked the road from concept through to commercially available lifesaving products - it’s quite rare because most of them fall over in the first two years. Mentoring and investment is key – that is one of the most valuable lessons I’ve learned on my journey and would encourage others to make sure those two things are in place.
How DoubleCHEKdoes work? DoubleCHEK is a device designed to help clinicians and carers to safely place
DoubleCHEK is UK designed and made - all of our injection molding is done in Newcastle and the product is assembled in Stevenage. Britain is a great place to design and develop medical devices – we have a great innovation landscape, access to capital and world leading regulators.
We’re also thinking big. We’ve already filed with the FDA and have a US based team exploring the North American market and have also received interest from European distributors. A big part of what the company is trying to do is to make sure that UK innovation that’s developed and seeded into the NHS is then introduced to the rest of the world so we can improve patient safety for as many people as possible.
We’re also looking at expanding our product range in the medium term –DoubleCHEK has received a fantastic reception so far from the clinical and business communities, and my vision is to use the knowledge, expertise, and capabilities we have at Enteral Access Technologies to solve other challenges in our sector.
Q&A
READ ABOUT THE MEDICAL DEVICE THAT REDUCES THE CHANCE OF FEEDING TUBES BEING MISPLACED INTO THE LUNGS. GEORGE GALLAGHER, THE FOUNDER AND CEO OF ENTERAL ACCESS TECHNOLOGIES, EXPLAINS HOW THE DEVICE WORKS AND WHY IT’S SO IMPORTANT.
I started back in 1997. The first device I developed was an IV drip monitor. I had no idea how to develop medical devices at the time but wanted to provide a solution to a problem. A family member was in the hospital, and needed medication, so I took some technology that I was using in a factory and adapted it to try and solve an issue the hospital was having. This led me to refining the product further and becoming CEO of Zi Medical PLC, raising £8m to develop an intravenous drop monitor and grow the business. Quite the baptism in medical devices! I then moved into confirmation devices for nasogastric feeding and have been in this sector ever since. What other factors are you considering?
MisplacementmedicationthecorrectlyfeedingItfeedingnasogastrictubes.checksthatatubeisplacedintostomachtoadministerandfood.ofafeeding tube into the lungs should not happen –it’s what the NHS terms a ‘Never Event’. But it happens multiple times every year. Our device prevents feeding tubes from ever going into the lung by ‘DoubleCHEK’ing for the presence of both carbon dioxide and stomach acid through a pH test. Conventional products only check for pH, so this double lock significantly reduces the chance of misplacement and is something nurses are recognizing as a real Thereinnovation.isnoother product on the market that does what we do. As a result, we’ve been able to get excellent access to hospitals, which is unusual given the impact Coronavirus has had on medical device company visits. We’re already working with multiple hospitals in the UK, after having only launched our product in October last year! What was the inspiration for this? My backstory to this begins when I was a baby. I was born with my esophagus and trachea fused together, a condition known as a trachea-oesophageal fistula that allowed food and stomach acid to enter my lungs. I had a procedure at Alder Hey to correct this that saved my life but left me with a big scar from my armpit to my waist to tell the story! I was then on a feeding tube for three or four months in intensive care and as an adult became aware of my condition and decided we needed to do something about it. How long have you been developing medical devices?
DoubleCHEK THIS OUT!
WWW. M EDICALPLASTICSNEWS .COM32
Medtech | Digital HealthTech | Medical Plastics | Manufacturing | Software | Inspection and Metrology Regulation | Design | Early-Stage | Innovation | Pharmaceutical | Manufacturing 7-8 JUNE 2023 NEC | BIRMINGHAM | UK Medical Device Supply Chain Intelligence MED-TECH INNOVATION Exhibit with us in 2023