NORTH AMERIC AN EDITION
MEDICAL PLASTICS + news LSR — A CURE FOR ENGINEERING AILMENTS? AUTHENTICATION TECHNOLOGY STERILIZATION AGENTS
Killing Germs Inside You, On You & Around You
TridAnt® prevents, protects & progresses antimicrobial technology ISSUE 18
July / Aug / Sept 2021
WWW.MEDICALPLASTICSNEWS.COM
ADVANCING MEDICAL PLASTICS
BIOMEDevice
December 8-9, 2021 SAN JOSE, CA, SAN JOSÉ MCENERY CONVENTION CENTER
JOIN
NorCal Biotech Community’s Regional Event
Biomedevicesanjose.com/MPN
18316_BIO_SJ21
REGISTER NOW at
CONTENTS MPN North America | Issue 18 | July/Aug/Sept 2021
Regulars 3 Comment Corrine Lawrence talks about sustainability and its importance in the medical plastics industry. 4 Digital spy 10 Cover story BioInteractions’ TridAnt® protects and prevents germs inside, outside, and around you. 20 Back to the future
Features 6 Adhesives: Surface control BTG Labs’ Rose Roberts and Matthew Nichols explain how controlling surface cleanliness can safeguard the future of medical diagnostic testing.
12 Joining technologies: Just what the doctor ordered? Liquid silicone rubber may be the cure for current medical device engineering ailments, suggests Trelleborg Sealing Solutions’ Ursula Nollenberger. 14 Anticounterfeiting & serialization: Visible authentication with zero consumables Morphotonix explains how its lithography-based nanoengraving technology provides manufacturers with a safe and reliable method to protect brands and lives. 17 Sterilization: EtO go home Ethylene oxide’s days are numbered as the medical devices industry looks set to embrace alternative sterilizing agents, offers Emily Lorcheim of ClorDiSys.
8 Adhesives: Don’t get stuck! James Darlucio of NuSil provides key tips on selecting the right silicone adhesives for medical device manufacturing.
WWW.MEDICALPLASTICSNEWS.COM
1
BPA- and DEHP-free: CYROLITE® acrylics
have just what it takes to save lives.
It’s nice when new life can rely on CYROLITE®: our BPA- and DEHP-free high-performance acrylics are safe to use in medical devices, especially in prenatal and neonatal applications. Additionally, CYROLITE® can be reliably sterilized and is resistant to medical fluids and disinfectants. This has impressed newborns and health-care professionals alike: CYROLITE® easily meets the requirements of USP Class VI, ISO 10993-1, and REACH. We give you all the reasons you need at www.cyrolite.com.
CREDITS editor | corrine lawrence corrine.lawrence@rapidnews.com advertising | caroline jackson caroline.jackson@rapidnews.com
Editor’s Comment
head of media sales plastics & life sciences | lisa montgomery
C O R R I N E L AW R E N C E
head of studio & production | sam hamlyn graphic design | matt clarke publisher | duncan wood
Medical Plastics News NA Print subscription - qualifying criteria US/Canada – Free UK & Europe – £249 ROW – £249 Medical Plastics News Europe Print subscription - qualifying criteria UK & Europe – Free US/Canada – £249 ROW – £249 FREE on iOS and Android devices Subscription enquiries to subscriptions@rapidnews.com
Medical Plastics News is published by: Rapid Life Sciences Ltd, Carlton House, Sandpiper Way, Chester Business Park, Chester, CH4 9QE T: +44(0)1244 680222 F: +44(0)1244 671074
© 2021 Rapid Life Sciences Ltd While every attempt has been made to ensure that the information contained within this publication is accurate the publisher accepts no liability for information published in error, or for views expressed. All rights for Medical Plastics News are reserved. Reproduction in whole or in part without prior written permission from the publisher is strictly prohibited.
ISSN No: 2632 - 3818 (Print) 2632 - 3826 (Digital)
W
How green does your garden company grow?
hether you’re a consumer or a business, sustainability is front and center of many agendas. Even if you believe the world is flat and global warming is merely the brainchild of pot-smoking treehuggers, choosing to ignore it is, at the very least, a high-risk business strategy. If you think sustainability doesn’t apply to your business and the view looks more appealing with your head in the sand, I heavily suspect it’s only a (short) matter of time before you realize your customers have deserted you, choosing instead to work with green or ‘greening’ companies.
‘nice to have’ folder laying in the bottom of filing cabinets — they need dragging out, the dust blowing off and, to be honest, they probably need updating. Although many large companies, such as Mitsubishi Chemical Advanced Materials, have the capital or financial backing to invest in such programs, smaller businesses can and are able to contribute. Global warming is not a local or parochial concern; it’s global. We’ve already experienced and are still dealing with one crisis. Unlike COVID, an antidote to global warming can’t be assembled in a lab and rolled out over a few months.
Sustainability has come to encompass many separate yet interrelated issues. We can’t get too far into a day before we hear such words as greenhouse gases, carbon dioxide emissions, recycling, reusing, biodegradable, biobased, circular economy, biomass, carbon neutral, net zero and so on. For the purposes of removing all doubt, I am particularly passionate about supporting green initiatives.
As ambassadors of medical plastics, we must keep innovating and collaborating to make our products and facilities kinder to the world. Throughout the COVID crisis the industry has demonstrated that collaboration delivers impressive results; it also delivers them quicker than any unilateral approach.
Although sustainability isn’t a new concept, businesses have, by and large, been slow to respond and adopt new ways of thinking and working. Where previously companies could appease themselves with token gestures, possibly as a marketing tactic, only now are many treating this issue with the importance it deserves, as demonstrated by the big bucks they’re putting behind internal programs and the wholesale changes they’re making to their business. Try searching “sustainability” on the MPN website for inspiration.
Please continue to keep me informed about how your company is making strides, or steps, to becoming more sustainable by dropping me a line at corrine.lawrence@rapidnews.com. Similarly, if you’re experiencing issues with your sustainability programs, do get in touch — the MPN community is likely to have a solution.
As members of the “dirty” plastics industry, it is uncomfortable when fingers point to us as culprits. But we know plastics have value, particularly in the medical industry, and it’s our responsibility to clean up their image: plastics don’t need to equate with “bad”. No longer can sustainability plans live out their days in the
WWW.MEDICALPLASTICSNEWS.COM
3
DIGITAL SPY
DIGITAL
spy
www.pulsetechnologies.com Improved electrode performance in implantable and diagnostic medical devices
PROCESS UPDATE
www.brownmachinegroup.com THE FIRST SECURE CUSTOMER DATA PORTAL FOR THE THERMOFORMING MARKET
B
rown Machine Group (BMG) has launched the BMG Advanced Digital Readiness (BMG ADR) platform, a weband app-based portal to access important process data in thermoforming and product handling applications. Available with new installations and as a retrofit for most BMG machinery, the platform enables operators, maintenance personnel, operations teams, and executive leadership to interface with equipment securely and in real-time. The BMG ADR platform is highly customizable to meet individual customer requirements, with unlimited custom alerts by text message or email, for a wide range of possible alarm conditions.
TECHNOLOGY UPDATE
By customizing the ADR tool to their specific needs, users can review how critical assets are running, analyze unplanned downtime, and improve operations through notifications when user-defined parameters are out of range. The BMG ADR platform features enhanced security with automatic updates and allows customer-defined digital access to enable remote service sessions. The platform, the company claims, is the first of its kind in BMG’s markets and heralds a new age of secure connectivity and IIoT-based information to ensure maximum uptime and efficiency.
P
ulse Technologies’ newly patented Hierarchical Surface Restructuring (HSR) technology enables medical device manufacturers to achieve greater performance, efficiency and longevity in their neural interfacing devices. Long-term implantable and diagnostic medical devices such
MACHINE UPDATE
www.azcocorp.com
A
MAKE YOUR OWN TEST STRIPS
ZCO Corp. systems is enabling manufacturers to make their own test strips or improve their current test strip making process. Available options include unwinds and rewinds, lamination, coating and drying, and a cut-to-length module. All systems can be customized to meet specific needs. Companies can manufacture their own test strips from raw material with AZCO’s multi-station reel-toreel laminator system with cutto-length knife assembly. Rolls of material are laminated onto a base material, and then strips are cut to a set length. Included is an unwind and rewind system, lamination station, print area, and cut to length module. Another product option for making test strips is a membrane
4
as pacemakers and various types of neurostimulators function via sensing, recording and stimulating between their electrodes and surrounding tissue in the biological environment in order to deliver effective treatment for patients. Generally speaking, higher electrode surface area improves electrochemical performance of the electrode. Electrodes and microelectrode arrays treated and optimized by HSR’s proprietary process can assist device manufacturers in producing higher-performing and more efficient implantable and diagnostic medical devices with enhanced performance and longevity in their neural interfacing devices.
WWW.MEDICALPLASTICSNEWS.COM
coating and drying station. One or multiple rolls of material are unwound, travel through an adjustable marking system, and then serpentine through a solution bath. The material is then dried by a forced hot air system and rewound onto a motorized rewind.
DIGITAL SPY
CERTIFICATION UPDATE
www.technimark.com
Technimark earns MedAccred Certification for El Paso Facility
T
echnimark’s production facility in El Paso, Texas, received its MedAccred certification from the Performance Review Institute. The facility, which is also ISO 13485:2016 certified and 21 CFR 820 compliant, specializes in medical contract manufacturing, including services
such as custom injection molding, assembly and logistics operations for the medical device industry. MedAccred is managed by the medical device OEMs, contract manufacturers and suppliers who collaborate to provide the sector with a trustworthy, evidence-based tool to ensure the suppliers’ processes and products meet the highest standards and improve product quality. Kris Peavy, president of Technimark’s Healthcare division, said: “The significant growth of our medical device business around the world, and specifically in our El Paso facility, made MedAccred certification a perfect fit. We’re currently pursuing accreditation at all of our medical device facilities, and we expect them to be certified in the near future.”
METHOD UPDATE
www.polyplastics.com
NEW TESTING METHOD TO IDENTIFY GAS FORMATION DURING INJECTION MOLDING
P
olyplastics has developed a testing method that identifies gas formation during the injection molding process and reduces mold deposits. The company’s Gas Investigation Method in Injection Molding (GIMIM) facilitates continuous molding and improves production efficiency. The company’s proprietary method captures and evaluates the gases formed during molding and identifies the mechanism by which pyrolysis gas forms during injection molding. The innovative method traps gases according to mold-based methods, uses gas chromatography mass spectrometers (GC/MS) to qualitatively and quantitatively analyze their composition, identifies the gases that are formed, and makes fundamental
improvements to the sources of their formation. This simple system configuration is divided into the three stages of plasticization, metering, and injection, and each unit is fitted with gas traps to seclude the gases that form within each part of the process. GIMIM can reflect the actual circumstances during molding by directly trapping and analyzing the gases formed during molding.
WWW.MEDICALPLASTICSNEWS.COM
talking
POINT
www.pyramidplastics.com
Andrew Peterson, Chief Operating Officer, Pyramid Plastics Why has Pyramid Plastics recently made a $2 million investment? We need to grow to be a better supplier to our current customers, as we have recently been turning away work due to capacity constraints. We already have a really good team who all share a passion for quality and exceeding customer expectations, and a great relationship with our customers; we now just need the additional equipment. The investment will also add about seven full-time jobs. How, exactly is the company going to spend the funds? By year-end we will have installed two additional new 1000T LS Mtron presses and one 550T press, as well as robotics and auxiliary equipment. We have also added an overhead crane to convert warehousing space to manufacturing space and plan an electrical upgrade for the new equipment. In Q1 2022 we intend to add another two presses — 1000T and 550T. Does making such investments make you nervous? Not at all. We have been turning away too many opportunities, which was frustrating. How will the investment benefit customers? The investment will increase our capacity and flexibility and improve quality because the new presses deliver more consistency shot to shot. The extra capacity will allow us to grow with our customers. We anticipate the investment will improves cycle times and scrap, which will result in lower costs for our customers.
5
ADHESIVES
ROSE ROBERTS, SENIOR CUSTOM APPLICATIONS & MATERIALS ENGINEER, AND MATTHEW NICHOLS, MATERIALS & APPLICATIONS ENGINEER OF BTG LABS EXPLAIN HOW CONTROLLING SURFACE CLEANLINESS CAN SAFEGUARD THE FUTURE OF MEDICAL DIAGNOSTIC TESTING.
SURFACE CONTROL
C
OVID-19 has highlighted how infectious diseases can spread at alarming rates and paralyze entire societies. Diagnostic testing is one of many effective tools that can be used to help prevent disease proliferation. Diagnostic tests depend on reliable medical devices that produce consistent results for each test. CONTROLLING MEDICAL DEVICE PRODUCTION PROCESSES Manufacturing process control is especially critical when producing medical devices. Incorporating surface quality specifications for materials used in medical device design enables manufacturers to make products that perform consistently in the field and provide reliable test results.
which are used to test for COVID-19, are one type of LFA. ELISA tests depend on capillary flow between two closely spaced surfaces. Reliable capillary flow requires surfaces that are reproducibly clean on a molecular level. Even a monomolecular layer of a contaminant can impede this capillary flow and cause device failure. CAPILLARY FLOW AND VACUUM DEPOSITED COATINGS Hydrophilic coatings facilitate successful capillary flow. A common method of applying these coatings to diagnostic plates is through vacuum deposition processes. Physical vapor deposition (PVD) and chemical vapor deposition (CVD) are two of the vacuum deposition methods used to apply these molecularly thin coatings on surfaces. Consistently applied coatings drive consistent wetting behaviors and, therefore, achieve reliable testing results. MICROPLATE WELL DENSITY A single 3.25” x 4.5” microplate may contain more than 1500 wells. Individual wells can have diameters of 3 mm and depths of 10 mm. These wells are frequently coated in batch vacuum processes. The miniscule cell size and submicron coating thickness makes testing the uniformity of these coatings extremely challenging.
Polymeric testing devices such as diagnostic slides or microplates are handled, cut, cleaned, and coated during their assembly. Each of these steps has the potential to affect the caliber of products; quantitative control of these steps during the manufacturing process ensures the quality and reliability of these devices.
Accurate and rapid validation of medical device surface properties has been made possible through new and innovative technology
CONSISTENT SURFACE QUALITY IS KEY FOR RELIABLE DIAGNOSTIC TEST RESULTS Effective diagnostic testing methodologies are predicated on consistent, predictable behavior of body fluids as they interact with the surface of a device. This frequently requires cleaning, surface treatment, and perhaps coating. These surfaces need to be prepared to a consistent level of chemical cleanliness and composition. Lateral flow assays (LFAs) are an example of products that need consistent, predictable wetting properties. Enzyme-linked immunosorbent assays (ELISAs),
6
WWW.MEDICALPLASTICSNEWS.COM
ADHESIVES
types of medical diagnostic devices. Integration of the Surface Analyst with an automated collaborative robot allows measurement of the surface quality inside individual microplate culture wells. The Surface Analyst takes these measurements by depositing a tiny drop of purified water straight down into the culture well. Figure 1: Clean, high energy surfaces pull liquids to the surface, creating a low contact angle. Contaminated, poorly treated, low energy surfaces cause the liquid to bead up, resulting in high contact angles. Few appropriate techniques exist for quantitatively ensuring quality and consistency of important device surface properties. The century-old technique of contact angle measurement, however, can be adapted to provide an excellent quantitative interrogation of these properties. WATER CONTACT ANGLES Contact angle measurements provide vital insight into the chemical state of a material surface. When a drop of liquid is deposited on a surface, the surface will repel or attract the droplet, causing it to bead up or flatten out, respectively. The attraction between the surface and the water is quantified by measuring the angle formed between the substrate surface and the drop edge. A surface that will be coated or bonded will be chemically clean and have a high surface energy. This is characterized by a water droplet that spreads to form a low contact angle across the surface. This indicates that there are many active chemical groups on the surface pulling the water droplet to the surface (Figure 1). The way that these chemical groups attract the water droplet indicates how a coating or adhesive will spread. If the water droplet does not spread, neither will the coating. A high contact angle indicates the presence of a surface contaminant or the need for additional surface preparation before bonding or coating. When a coating has been homogenously applied, contact angles will be uniform across the surface. Various types of coatings are used in the medical industry. As described above, when capillary action is required for diagnostic devices and a hydrophilic coating is in place, contact angles should be low (high surface energy). Alternatively, hydrophobic coatings, like those used for catheter applications, require high contact angles (low surface energy). In either case, contact angles provide a direct method for quantifying the surface wetting properties both before and after applying a coating or adhesive. VALIDATION OF MEDICAL DIAGNOSTICS COATINGS For general applications there are handheld devices available that enable rapid contact angle measurement at any location on the production floor and on any material surface. This mobile ability to measure contact angles is highly adaptable and of great value to many manufacturers. Rapid water contact angle testing performed by the Surface Analyst line of products provides an effective method to validate surface state including cleanliness, treatment level, coating presence and uniformity.1 The Surface Analyst is used to validate surface quality on many
Robotic deployment ensures precise positioning of the camera and the water droplet. Automation of rapid water contact angle measurements allows manufacturers to validate coating presence and uniformity on medical device surfaces by producing reliable and consistently repeatable contact angles. For microplate applications, statistical sampling techniques are used to establish how many individual wells need to be evaluated to generate statistically valid results. Once these statistical models have been established, manufacturers can develop “pass/fail” parameters for the production process. Accurate and rapid validation of medical device surface properties has been made possible through new and innovative technology such as the Surface Analyst line of products. With the support of materials science experts, medical device manufacturers can now achieve statistical process control of their coating, printing, bonding, sealing, and cleaning operations. This statistical process control helps ensure that medical diagnostic devices will consistently perform as expected. Note: Surface Analyst is a Trademark of BTG Labs. REFERENCE 1. https://www.btglabs. com/products?utm_ campaign=Publication%20 Articles&utm_source=mpn-futureof-medical-diagnostic-testing-relieson-surface-cleanliness-article&utm_ medium=june-2021-product-pg
WWW.MEDICALPLASTICSNEWS.COM
7
ADHESIVES
JAMES DARLUCIO, APPLICATIONS ENGINEER, NUSIL, PROVIDES KEY TIPS ON SELECTING THE RIGHT SILICONE ADHESIVES FOR MEDICAL DEVICE MANUFACTURING
M
edical device manufacturers often use silicone adhesives to bond parts together when assembling products such as catheters, pacemakers, cochlear implants, aesthetic implants, and gastric balloons, due to their biocompatibility and versatility.
t ’ n o D get k c u st
Selecting the appropriate adhesive can ensure a proper, long-lasting bond, protecting the integrity and performance of the medical device. Manufacturing considerations such as cure rates, adhesive application factors, and surface preparation requirements also affect adhesive selection. By understanding these factors, selecting the optimum silicone adhesive solution can help medical device manufacturers improve their products, increase manufacturing productivity, and gain a competitive edge. SILICONE ADHESIVE OPTIONS The main types of silicone adhesives available for use with medical devices include condensation cure systems and addition cure systems. Condensation cure silicone adhesives Traditional silicone adhesives cure at room temperature upon exposure to atmospheric moisture and are available in one- or two-part formulations.
Addition cure silicone adhesives Two-part silicone adhesives offer a high degree of versatility and device assembly efficiency because they do not require ambient moisture to cure. These silicones can meet unique assembly requirements, such as forming bonds at interfaces that have little or no access to air and thick cross sections. Two-part adhesives are also ideal for products where a relatively low temperature must be maintained. They adhere well to a variety of substrates and can be used for sealing, potting, or encapsulating.
One-part adhesives, which are the most widely used medical device silicone adhesives, do not require heat to cure, making them ideal for temperature-sensitive components, such as electronics and thermoplastics. They are also wellsuited for bonding silicone to other silicones, metals, some plastics, and glass. • Cure characteristics: One-part adhesive cure rates are influenced by several factors, including humidity levels (20–60% relative humidity recommended), cure temperature (25–50 °C recommended), crosssectional thickness and the amount of surface area exposed to air.
8
• Application considerations: One-part adhesives tend to have slower cure rates compared with two-part adhesives. In addition, liberation of byproducts of the curing process, such as mild acids, alcohols, or other volatile substances, can cause these adhesives to shrink during cure, typically between 3–6% of its original volume. • Specialty applications: In certain applications, adhesives can be supplied in a compatible solvent to help facilitate a specific process or application of the adhesive. This method is especially useful in conformal coating applications, which require thin sections or small intricate areas that might otherwise be hard to access.
• Cure characteristics: With two-part adhesives, curing starts by mixing two components together in a 1:1 mix ratio. The cure rate is influenced by temperature, and unlike one-part adhesives, practically no byproducts or leaving groups are released. • Application considerations: The typical work times range from 15 minutes to 2 hours, depending on temperature, and the cure rate can be accelerated by increasing temperature. Some leading silicone adhesive suppliers can provide the product in ready-to-use cartridges, where the material is dispensed through a static mix tip where air is not introduced. • Specialty applications: High temperature vulcanizing silicone adhesives are formulated to cure with the application of heat; if a manufacturing or device assembly process requires very fast curing after the adhesive is applied, the cure rate can be accelerated by increasing temperatures. There are assembly operations where manufacturers may need a prolonged period from when the adhesive is applied until it is cured, yet they also need very rapid curing once the assembly process calls for it, so they apply heat for rapid curing. For example, high-temperature addition cured adhesives can
WWW.MEDICALPLASTICSNEWS.COM
ADHESIVES
be fully cured at temperatures and times ranging from 10 minutes at 116 °C to 2 minutes at 150 °C. MEDICAL DEVICE SUBSTRATES AND SURFACE PREPARATION Most medical devices, whether implanted or used externally, are made of one or more of the following types of materials: • Silicone • Metals such as aluminum, stainless steel, and titanium • A wide range of plastics, including polytetrafluorethylene (PTFE), polycarbonate, polyurethane, and polyimide. Increasingly, device manufacturers are discovering that plastic substrate materials such as PTFE can be particularly difficult, if not impossible, to bond using silicone adhesives without first applying some type of surface treatment. Materials such as polyethylene do not allow a liquid adhesive to easily ‘wet out’ or spread outward across its surface. Proper preparation of the substrate surface is essential to forming a long-lasting bond. Cleaning, activating and/or priming the surface can maximize the surface area’s available bond sites and wettability to improve long-term adhesion. When developing a surface preparation procedure, there are several variables to consider: • Substrate cleaning: Certain chemical elements and compounds can reduce or inhibit the adhesive’s curing process. Proper cleaning methods remove contaminants such as finger oils, dust particles, mold release agents, and machine oils on metal parts. Surface cleaning can be done by mechanically or manually wiping the surface with an appropriate solvent (for example, isopropyl alcohol or heptanes). • Surface treatments: To help increase the substrate’s surface energy for better adhesion, several methods can be considered. Plasma treatment Image below: Whether the application is a biocompatible adhesive for assembling a medical device used in the body or for an external application such as this insulin pump, there is a broad range of both off-the-shelf and customized silicone adhesives to serve virtually every application.
Selecting the optimum silicone adhesive solution can help medical device manufacturers improve their products, increase manufacturing productivity, and gain a competitive edge bombards the substrate surface with ions of gas such as oxygen or argon. Another treatment method is the corona discharge technique, which uses increasing voltage cyclically to generate a plasma known as ‘corona discharge’. The type of surface treatment used is typically dependent on the type of substrate and to what degree that substrate needs modification of its surface energy. • Adhesive primers: Primers can be applied to substrate surfaces that have particularly low surface energy to ensure proper silicone adhesive joining and sealing. Primers act as coupling agents, increasing and strengthening the covalent bonds between the adhesive and substrate. They have also been shown to greatly increase the silicone adhesive’s ability to wet out the substrates. BIOCOMPATIBILITY AND PURITY Medical-grade silicone adhesive applications are biocompatible and conform with applicable ISO and USP testing protocols. Consequently, silicone adhesives are resistant to chemical attack, oxidation, and shear stresses. They can be readily sterilized by ethylene oxide, dry heat, or other standard techniques without degradation. Device designers should be sure to consider high-purity, medical-grade silicone adhesives with extensive regulatory support and Master Files submitted to the FDA and international authorities. It is also important to select medical-grade silicone adhesives manufactured to strict purity standards with products that use carefully selected raw materials and utilize advanced purification technologies to protect the safety and integrity of the medical devices using those adhesives.
WWW.MEDICALPLASTICSNEWS.COM
9
COVER STORY
ARJUN LUTHRA, COMMERCIAL DIRECTOR, BIOINTERACTIONS, DISCUSSES AN ENHANCED MULTI-ACTION BIOCOMPATIBLE COATING IS ENHANCING BIOCOMPATIBILITY AND ANTIMICROBIAL PROTECTION ON MEDICAL DEVICES FOR EXTENSIVE PERIODS OF TIME.
Killing germs inside you, on you & around you
T
he case for revolutionary technologies and solutions that greatly enhance the standards of our health services plays a significant role in our quality of living. This has never been more important than now. Innovations which protect and enhance the standard routines used by healthcare services are in constant demand and are especially critical considering the global Covid-19 pandemic. As the pandemic has persisted, our healthcare services and innovative technologies battle to keep up with the viral evolutions as well as mitigate impact to core areas of healthcare services. This highlights the importance and the necessity for preventative technologies to be used at global scale to effectively prevent infections, reduce antibiotic resistance, and enhance healthcare infrastructure. The prevention measures and standards must focus on efficacy against a broad spectrum of pathogens equal to medical device requirements, durable protection to minimise costs of implementation and safe variations which are biocompatible to allow for large scale application.
Materials play a critical role in improving the performance of the technology and can improve the implementation and application into a range of environments and industries. The antimicrobial efficacy of materials involves critical interactions between the pathogen and surface interface. The successes of applications are dependent on a variety of biological events occurring at this interface and these events intensify the complications of procedures. This must be all addressed during innovation and development. The challenge of infection prevention will help us move toward long-term prevention mechanisms and enhanced medical devices as well as industrial & consumer products. The biological events which occur at the pathogensurface interface is critical to improving the prevention technology against pathogens. Biological responses are complex processes which are governed by a variety of factors. These factors range from surface properties to protein interactions which includes the chemistry, topography, wettability, as well as the composition of a surface and the biological entities present at the interface Infection and pathogens on surfaces have been long studied as it is a significant challenge which has impeded improvement of long-term medical devices & a range of industrial applications. A variety of techniques including silver, copper, chlorhexidine, and other leaching chemistries used on medical devices have not proven to be significantly effective and highlight the need for an innovative solution. It has been seen in a randomized study of surface treatments to prevent infections that silver surface treatments have failed to reduce infection rates. It has also been seen that the use of silver-impregnated collagen cuffs can impede catheter fixation due to the killing of fibroblasts and cause the catheter to dislodge. Therefore, highlighting the limits and toxic impacts of similar leaching chemistries to silver technology on eukaryotic cells. Furthermore, it has been recommended that any catheter which has caused bacteremia should be immediately removed and only replaced once results of blood cultures normalised. This highlights the difficulty previous technologies experience when combating infections safely on a surface over long periods of time. These approaches have had limited success, whilst additionally requiring the consistent use of antibiotics, leading to resistance and further complications. The biological events which occur on a surface are a complex result of interactions between the surface and the proteins and cells which are present at the pathogen-surface interface. These complex challenges require a multi-action material which considers all these varying factors in one straightforward technology. Multi-action materials provide a highperformance surface which are the most effective at preventing pathogen growth, as well as the longest durability, all whilst improving safety to the patient in one straight-forward solution.
10
WWW.MEDICALPLASTICSNEWS.COM
COVER STORY
TridAnt® technology is the only antimicrobial coating that kills 99.999% of bacteria ... for up to 100 days without leaching or use of toxic components ...
BioInteractions Ltd. have utilised their multi-action materials to develop TridAnt®, the most effective and advanced antimicrobial technology for medical devices. The technology enhances the protection against a broad spectrum of pathogens which includes gram-positive and gram-negative bacteria as well as yeast. TridAnt® is the only medical device coating which kills 99.999% (5 log reduction) of bacteria and yeast as for up to 100 days. We utilise advanced multi-action materials which are biocompatible and non-leaching to ensure a consistent level of efficacy on medical devices for extensive periods of time inside a patient. TridAnt® biocompatible, antimicrobial technology is the only medical device coating to: • Kills 99.999% of bacteria (gram positive and gram-negative) as well as yeast • Consistently protects medical devices which ensures devices maintain their protection for 100 days • Contact-Kill technology provides a highly effective mechanism to kills broad spectrum of pathogens as they interact with the device surface • Multi-Action material uses active and passive components to prevent deposition, protect against adhesion and kill pathogens on contact • Biocompatible and non-leaching coating assists in patient safety whilst targeting microbes TridAnt® technology has been derived specifically to enhance the stateof-the-art in antimicrobial technology on medical devices. The technology utilises innovative features developed from over 30 years in the medical device industry to provide revolutionary benefits to the healthcare professional as well as the patient. The technology is the first of its kind to provide both market leading efficacy as well as the longest duration of an antimicrobial coating on a device. This is achieved by utilising multi-action materials to ensure the highest efficacy seen on a medical device to date. The non-leaching mechanisms ensure that antimicrobial efficacy stays on the surface and doesn’t flow through the blood. This also ensure that the amount of active component remains constant throughout the duration of the device to ensure a complete and consistent effect whilst implanted in a patient. The combination of highest efficacy, against a broad spectrum of pathogens for the longest duration seen on a medical device provides a platform for new procedures and products to be innovated. These benefits allow for devices to remain in the patient for longer, whilst also aiding in reducing the amount of antibiotics they are required to consume. WWW.MEDICALPLASTICSNEWS.COM
TridAnt® has been further optimised to provide the World’s Most Advanced Infection Prevention Infrastructure. The prevention technology is the first line of defence and is a straightforward, long-term solution to protecting surfaces, preventing transmission, and controlling pathogens. The infrastructure provides enhanced protection against a broad spectrum of bacteria, viruses and fungi on surfaces ranging from stainless steel to skin. The infrastructure comprises of products which utilise TridAnt® technology to enhance protection and provide further long-term benefits to the healthcare and wider industrial environment. The use of TridAnt® as a daily tool provide a viable method to improve hygiene and healthcare standards whilst reducing maintenance costs as well as reliance on single-use items. The various products reduce the level of consumption of harsh chemicals as well as provides enhanced hygiene infrastructure which prevents future pathogens. The use of TridAnt® items actively disinfects surfaces on contact, therefore actively maintaining a healthy and hygienic environment. This helps us to move into a better normal where we maintain health and hygiene conveniently as well as reducing the costs of maintain the environment . This complete infrastructure can be applied safely into schools, restaurants, hotels and hospitals to allow a swift recovery to our normal routines. TridAnt® technology uses hygiene of the future to bring back the good phoelings (“feelings) of the past.
11
JOINING TECHNOLOGIES
URSULA NOLLENBERGER, GLOBAL PRODUCT LINE DIRECTOR, HEALTHCARE & MEDICAL AND LIQUID SILICONE RUBBER COMPONENTS, TRELLEBORG SEALING SOLUTIONS SUGGESTS LIQUID SILICONE RUBBER MAY BE THE CURE FOR CURRENT MEDICAL DEVICE ENGINEERING AILMENTS.
T
Just what the doctor ordered?
oday’s medical device designers and manufacturers operate in a challenging landscape. Stricter regulations and the need for biocompatibility are making development and manufacturing more demanding. Add to that, user requirements for devices that suit their lifestyle choices, such as wearable products and home monitoring solutions, and you can understand why engineers are looking for novel component options. THE CURE One solution is liquid silicone rubber (LSR) molding and multicomponent manufacturing. LSR is a stable and adaptable material that’s accelerating innovations in medical device design, facilitating to make devices more robust, efficient, and adaptable to patients’ needs.
Figure 1: Multicomponent example of selfbonding LSR with polycarbonate that combines a flexible relief area with a rigid backbone in one piece, reducing assembly and performance risk in the final device.
12
Silicone is ideal for medical devices and equipment, not only because it’s inert, biostable and biocompatible, but also because it can be processed in many ways (Figure 1). It can be molded on its own, but the real magic happens when it is combined during the molding process with engineered plastics and other substrates in what is termed ‘multicomponent manufacturing’. This technology produces a single component, instead of separate parts that require assembly, and offers the medical device manufacturer several advantages. It reduces production costs and supply chain expenses associated with stocking and handling multiple parts. The single, integrated device has greater integrity and, for example, allows the elimination of undesirable spaces for bacteria growth, increasing patient safety. For new multicomponent LSR applications, it is important to involve the component manufacturer as early as possible in the development process, ideally from the concept stage. Some molders, including Trelleborg, take a black box approach in which the designer specifies the component’s function, performance, and regulatory requirements, along with the available design window. The molder then develops a proposal that includes the benefits of LSR processing. MINIATURIZATION The LSR molding process is well-suited for miniature parts. It can produce micro- and nano-sized components below 10 mg through specialized micro and needle-point injection technology. An example of a small piece manufactured is a septum — the membrane in the cap of a medicine bottle through which a syringe is inserted and withdrawn. This typically weighs just 0.003 g. At that size, you can hardly pick the part up … and standard molding burrs are larger than the part itself. Manufacturing a microcomponent such as this requires extreme accuracy in tool construction, control of shot weight and the molding process. After molding, automatic handling of the product is performed by a specially developed robot gripper arm. The process ensures reliability and accuracy for millions of shots. AUTOMATION AND QUALITY The example of the septum highlights the importance of automation for medical device component manufacturing; it makes the high-volume production of extremely complex multicomponent LSR geometries possible. Automation can also help ensure cleanliness requirements are met by reducing the risk of contamination during the production process Quality is paramount for medical devices. The ‘holy grail’ here is to ensure quality in process rather than to conduct post-production quality checks; thus, certified quality systems and process controls are built into the production process.
WWW.MEDICALPLASTICSNEWS.COM
JOINING TECHNOLOGIES
Current applications for LSR technologies range from drug delivery, such as primary drug packaging or wearable smart drug pump systems, to fluid management, diagnostics, shortand long-term implantable medical devices, and biotechnology. With so many advantages, it’s no surprise that LSR technology is seeing exponential growth.
CASE STORY: Patient benefits When a customer’s medical valve was leaking and generating too much friction, a multicomponent LSR solution was the answer. Figure 2: In an ISO Class 7 cleanroom, only 352,000 particles of 0.5 μm or larger are permitted per m³ of space. The ability to segregate suspect products effectively with minimal disruption is crucial to minimizing downtime in a high-volume, rapid production process. Ideally, in-line quality checks should be electronically recorded to allow full traceability. Any issue can therefore be isolated to just a small number of components and separated by a cavity. CLEANLINESS Though standards vary, cleanliness in medical device manufacturing is always vital. For some medical devices, production in an ‘uncontrolled environment’ is clean enough. Due to the nature and positioning of LSR moldings within a medical device, however, they may need to be manufactured and packed in a fully ‘controlled’ cleanroom of class 100,000, ISO 8 or class 10,000, ISO 7, or even higher. Although they’re not generally a sterile environment, cleanrooms control a specified number of particles per cubic meter, at a maximum specified particle size. This includes environmental pollutants, such as dust, airborne microbes, aerosol particles and chemical vapors. The ambient air in a typical urban environment contains 35 million particles per cubic meter of a diameter of 0.5 μm or larger. By comparison, in an operating ISO Class 7 cleanroom, only 352,000 particles of size 0.5 μm and larger are permissible per cubic meter of space (Figure 2).
Silicone is ideal for medical devices and equipment, not only because it’s inert, biostable and biocompatible, but also because it can be processed in many ways WWW.MEDICALPLASTICSNEWS.COM
The control valve was made up of three separate parts — a piston sealed with two silicone O-Rings. Finite Element Analysis (FEA) simulations found that the plastic piston was misaligned, causing the leak. High friction between mating surfaces and assembled O-Rings was a second concern. A new valve was developed using multicomponent technology, consisting of a single component with a compressive inner seal that was pressurized on both sides. It also had a deflective outer seal to reduce friction, creating a pressure-energized seal. The new design was subjected to FEA simulations and a Design for Manufacturing (DfM) analysis, including material flow simulation, to ensure manufacturing feasibility and to prove the intended tool concept. The new LSR part was a reliable solution to leakage and friction issues. The integration of three individual components into one, streamlined the manufacturer’s supply chain and production process, increasing the company’s product quality and reliability, while also reducing production risk and overall costs.
13
ANTICOUNTERFEITING & SERIALIZATION
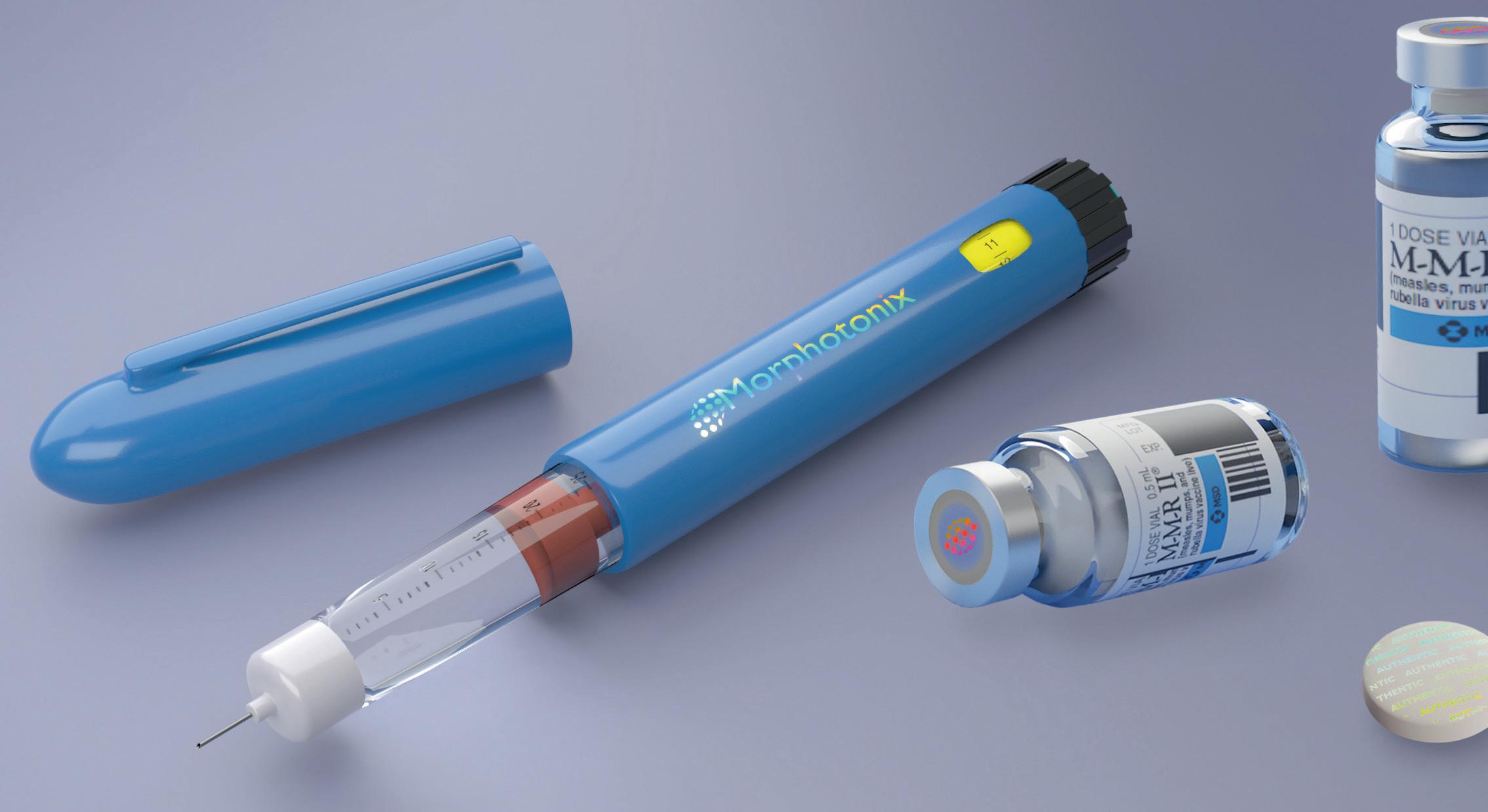
HOW CAN MANUFACTURERS HELP CONSUMERS TO DISTINGUISH REAL FROM FAKE? SWISS START-UP MORPHOTONIX PROVIDES A SAFE AND RELIABLE WAY FORWARD TO PROTECT BRANDS AND LIVES.
VISIBLE AUTHENTICATION
W
WITH ZERO CONSUMABLES
hether a simple prefilled syringe, an auto-injector, or high-tech equipment for an examining room, plastic components within the medical industry must maintain strict standards to ensure patient safety, product reliability, and regulatory compliance. Unfortunately, it is far too easy to produce counterfeit products that cannot be distinguished from the genuine article by the naked eye alone. With the global medical device market estimated at over $450 billion in 2021 and the World Health Organization stating that 8% of devices on the market in 2010 were fake, the size of the problem and the resulting risk to patient safety cannot be underestimated.1,2 MOLD NANO-ENGRAVING TECHNOLOGY To ensure the authenticity of rigid plastic materials against counterfeiting, Morphotonix has designed, developed, and patented a unique advance in lithographybased nano-engraving. The technology is integrated without any extra steps in the manufacturing process and does not affect product integrity. Security features, which can be anything from complex diffractive designs to forensic signatures, are engraved directly into steel production molds. These custom markings are then replicated on the surface of each plastic part without using labels, ink, or additional post-processing (Figure 1). The technology is so safe manufacturers can apply it to medical plastics and even directly onto pharmaceuticals such as medicine pills — the company, in fact, launched with proof-of-concept holographic designs on gourmet Swiss chocolate. The technology has already found many uses in a variety of industries,
14
Figure 1: Overt Morphotonix security used on three different substrates: polymer, rubber, and pharmaceutical pills.
from auto parts to nutraceutical packaging to high-tech components.³ Overt, covert, and machine-readable features are nano-engraved with a precision of 130,000 dpi. New legislation introduced into US and Europe, and measures such as serialization and unique device identification is insufficient to prevent counterfeiting.⁴,⁵ Traditional security measures involve labels, direct printing or taggants. In most products, these usual approaches add complexity and can have high investment costs to adapt manufacturing lines. In the medical industry, these approaches may have additional failure points under standard usage conditions, such as sterilization or exposure to UV or chemical interactions. Furthermore, the anticounterfeit measures may be on outer packaging, leaving the device itself unprotected. By comparison, the Morphotonix solution appears directly on the device component, dramatically increasing the level of protection. The Morphotonix solution is intrinsically embedded within the products themselves. The process does not involve any additives or post-processing that increase the production cost, face concerns about health interactions, or require additional detection machinery. There is no change to the material or its processing, and therefore no need to perform expensive and time-consuming process and material revalidation. With a lifecycle of more than a million units per engraving, the process is extremely cost-effective at industrial scale.⁶ Features are designed according to the custom needs of each client, including functional and branding considerations — from instant authentication by consumers and in-field investigations to differentiated authentication channels for multiple stakeholders. The logistics to securely send out the mold inserts to Morphotonix is as simple as sending a shoe box-size package. The inserts are engraved in a nanotechnology cleanroom in Switzerland. Once they are marked with the custom design, they are returned to the customer’s
WWW.MEDICALPLASTICSNEWS.COM
SilcoTek CVD Coatings for Medical Devices and Molding
CASE STUDY A Swiss market leader in the global breastfeeding products industry integrated the Morphotonix security solution for a device selling millions of units, where authenticity is crucial to consumer confidence. Given the babies’ extreme sensitivity, being able to authenticate the highquality, regulatory-compliant products, the customer decided to pre-emptively include Morphotonix highly secure technology which does not use any extra additives. To secure a smooth manufacturing process for a delicate product, Morphotonix merged its nanoengraving with Wild & Kupfer’s expertise in complex and highprecision molding.7
• • • • •
Increase Surface Release Improve corrosion resistance Prevent contamination Eliminate frequent maintenance Excellent adhesion properties
This successful collaboration led Wild & Küpfer to further recommend Morphotonix technology to their clients, contributing an effective advance to their product portfolio.
SilcoTek Corporation Game-Changing Coatings www.SilcoTek.com TechService@SilcoTek.com +1(814) 353-1778
production line. This entire process usually takes 6–8 weeks. FUTURE DEVELOPMENT Morphotonix is developing its technology so that it can be applied to other substrates.³ Each substrate has characteristics that present unique industrial manufacturing requirements. The company is currently looking at rubber so that vial septums for vaccines can be authenticated to prevent counterfeiting. REFERENCES 1. https://www.fortunebusinessinsights.com/industry-reports/medical-devices-market-100085 2. https://www.who.int/medical_devices/global_forum/3rd_gfmd/ againstcounterfittingforgingdocuments.pdf 3. https://bit.ly/3h7k95v 4. https://www.congress.gov/ bill/113th-congress/house-bill/3204 5. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0745-20200424 6. https://bit.ly/3DkPAmz 7. https://bit.ly/3zlVO39
Webinars offer a multi-layered marketing outcome, enabling you to tell your story to a global audience, define your organisation as a thought- leader and simultaneously deliver a healthy number of leads for your sales team to get to work on. To find out more contact Caroline Jackson t: +44 (0) 1244 952 383 | e: caroline.jackson@rapidnews.com www.mpnmagazine.com/webinars
STERILIZATION
AS A STERILIZING AGENT, ETHYLENE OXIDE’S DAYS ARE NUMBERED, AS THE MEDICAL DEVICES INDUSTRY LOOKS SET TO EMBRACE ALTERNATIVE STERILIZING AGENTS SUCH AS CHLORINE DIOXIDE, SAYS EMILY LORCHEIM, PROJECT MANAGER, CLORDISYS SOLUTIONS, INC.
ET0
M
Go Home
edical device sterilization has seen few advancements compared with most other industries. Even as ethylene oxide (EtO) faces increasing pressure due to potential environmental health hazards, its market share has held steady since the mid-1990s. Much of this is due to the lack of a clear industry standard to innovate and accept new technologies, prolonging the potentially dangerous status quo. Recent events, however, have shown chlorine dioxide (CD) gas to offer promise as a viable low temperature sterilization method, and the first commercially available medical device product sterilized with CD gas has just hit the US market.1 ETO ISSUES In 2006, and after reviewing studies by the National Institute of Occupational Safety and Health (NIOSH) studies, the Environmental Protection Agency (EPA) released a draft of its review of EtO and determined it is a human carcinogen (as published in its Final Report).² In 2018, the EPA released its latest National Air Toxics Assessment based on industry supplied emissions data from 2014.³ The report showed that 109 of the 73,057 census tracts within the US faced cancer risks due to exceeding EtO emission levels according to EPA guidelines. One area with very high EtO levels was Willowbrook, IL. Illinois Attorney General and the County Attorney General sued the main sterilization
company who performs EtO services on medical devices within the city.⁴ Despite attempts to add extra pollution control measures, the city of Willowbrook experienced exceedingly high EtO levels in the air for nearly a year. In February 2019, the Governor ultimately decided to ban the sterilization firm from using EtO at the plant. A legal settlement eventually allowed the company to resume operations in Willowbrook once they installed new equipment to reduce EtO emissions. ADDRESSING THE ROADBLOCKS TO INNOVATION One reason why the use of EtO was allowed to continue was the lack of a suitable alternate method. Guidance existed for the use of established medical device
WWW.MEDICALPLASTICSNEWS.COM
17
STERILIZATION
sterilization methods in the form of ISO standards and guidance documents from the Association for the Advancement of Medical Instrumentation (AAMI). There was no roadmap on how to implement and validate alternate sterilization methods. Limiting the use of EtO was deemed a risk to the global medical device supply chain, as shortages to these essential products could have fatal effects.
cannot be sterilized by EtO, CD gas has been shown to be compatible, as it doesn’t pose the same explosive risk as EtO. With many medical devices becoming even more complex and technical, companies commonly have devices that cannot be autoclaved, irradiated, or treated with EtO due to incompatibilities. This results in CD gas being the only viable solution for device sterilization for many manufacturers and engineers.
To address the situation, the US Food and Drug Administration (FDA) and General Hospital and Personal Use Devices Panel of the Medical Device Advisory Committee met to discuss the topic of industrial EtO sterilization and its impact on public health in November 2019. Alternative methods of sterilization were presented to spur innovation within the industry. One sterilization method presented at the meeting was CD gas. CHLORINE DIOXIDE GAS CD gas has been recognized and used as a sterilizing agent since the 1980s. As a non-carcinogenic, residue-free sterilizing agent with high material compatibility, it has found many uses across multiple industries. The idea for CD gas to be used a medical device sterilant is not a new one. In fact, its first applications were for the sterilization of artificial joints, suture products, and interocular lenses in the late 1990s. As a true gas at low temperatures, CD follows gas laws to evenly and completely fill the chamber that it is injected into. Although devices containing embedded batteries
18
Although CD gas can provide sterilization at ambient pressure, use within vacuum pressure chambers allows for the sterilization of complex devices and those within bulk packaging. CD gas also offers a vast reduction in cycle time and complexity of the sterilization process. Items can be loaded into a single sterilization chamber where the entire cycle, including aeration, occurs. Cycle lengths vary depending on each device’s requirements, but typically last from 2 to 8 hours, inclusive of aeration time. PROGRESSION Furthering the progression away from EtO, the FDA newly approved a contract sterilization facility for medical devices utilizing CD gas in late 2020.1 This breakthrough advancement was awarded to ClorDiSys Solutions. The company was established in 2001, beginning as an adoption of technology the founders originally created within Johnson and Johnson. There, medical devices such as interocular contact lenses, sutures, artificial hip joints and more were sterilized internally using CD gas. This facility recently processed the first commercially sold medical device sterilized with CD gas that has US approval. A draft Reference File for the use of CD gas has been created, which is developed from ISO and AAMI EtO guidelines. This serves to aid in submission packages to both the FDA and EU regulatory agencies. These tools provide a regulatory roadmap for validating the sterilization process outside of the typical EtO, Gamma irradiation, E-Beam/X-ray, steam, and dryheat methods. As regulations regarding the use of EtO tighten, the ability to pursue effective alternatives is becoming crucial. CD gas’s increasing prevalence within the market marks the closest process to EtO without the environmental concerns, giving hope to a safer future for medical device sterilization. REFERENCES 1. ClorDiSys Solutions, Inc, Branchburg, NJ, (Registration #3013115071). 2. U S EPA. Evaluation of the Inhalation Carcinogenicity of Ethylene Oxide (Final Report). US Environmental Protection Agency, Washington, DC, EPA/635/R-16/350F, 2016. 3. https://www.epa.gov/national-air-toxics-assessment/nata-overview 4. https://www.chicagotribune.com/news/ct-sterigenics-eto-timelinehtmlstory.html
WWW.MEDICALPLASTICSNEWS.COM
Qosina offers a wide selection of stock and custom tubing solutions for medical and bioprocessing applications. Choose from over 100 tubing options available in a variety of brands, types and materials.
Brands
Stock Materials
Tygon ® C-Flex ® SaniPure ™ BDF ™ PharMed ® BPT PharmaFluor ® TuFlux ®
DEHP-free PVC TPE HDPE FEP Platinum- and peroxide-cured silicone Multi-layer
Qosina is a leading global supplier of thousands of stock components to the medical and pharmaceutical industries. Qosina Corp.: Qosina Europe:
2002-Q Orville Drive North, Ronkonkoma, NY 11779 USA
qosina.com
Viale Giacomo Matteotti, 26, 20095 - Cusano Milanino (MI) - Italy
+1 (631) 242-3000
+39 02 66401337
info@qosina.com info@qosinaeurope.com
Dynamic Extrusion for Today’s Medical Industry Your medical tubing operation deserves precision and profitability. From microbore to multi-lumen, catheter to radio-opque, we’re ready to engineer a versatile, tighttolerance solution for your cleanroom process. Call +1 860-934-0575 to optimize your medical tubing!
Visit us at booth #4202
davis-standard.com
New company launches to aid industrial digital transformation 1
ASME has launched a new company to accelerate digital transformation in engineering in a variety of industries, including medical.
2
Metrix’s purpose is to build a network for crossindustry and interdisciplinary collaboration.
3
The company will host in-person and virtual events, expert content, networking, and collaboration opportunities for the engineering community.
‘Hyper glue’ adhesive forges new bonds at the molecular level XlynX Materials has created a new class of adhesives they are calling “molecular glues”. These make it possible to permanently adhere difficult-to-bond polymers such as polyethylene and polypropylene to themselves, and to other materials, through exceptionally strong chemical bonds. Unlike conventional adhesives BondLynx employs bis-diazirine chemistry to create covalent chemical bonds between polymer chains, permanently
20
crosslinking them together through strong carbon-carbon bonds. This is the same type of joinery found between carbon atoms in the polymer chains themselves. Once BondLynx has been applied to a polymer, the crosslinking process can be initiated by heat, ultraviolet/ visible light, or an electric field depending on the specific demands of the manufacturing process. “What’s really amazing about BondLynx is that it can glue virtually any plastic to any other plastic. It acts by inserting itself into the carbon-hydrogen bonds that are present in almost every commodity polymer. The potential applications are limitless,” stated Jeremy Wulff, Professor of Organic Chemistry at the University of Victoria.
4
Metrix launches with eight established event brands including AM Medical, Oct. 27–28; and Robotics for Inspection & Maintenance, Dec. 8–9.
5
The events will focus on creating enduring, immersive experiences where technology insights, expert advice, and resources.
Partnership produces world’s first 3D-printed connected stethoscope Nexa3D and Henkel have has partnered to produce the world’s first additively manufactured connected SKOP stethoscope for WeMed. Based on biomimicry design and produced on the NXE400 ultrafast 3D printer using performance matched Henkel materials, the WeMed SKOP is the world’s first connected stethoscope to be additively manufactured in its entirety at scale with production volumes to exceed 100,000 units per annum. Born during the COVID-19 health crisis, French start-up, WeMed saw an opportunity to quickly respond to accelerating demand for new diagnostic medical devices that support teleconsulting and remote monitoring. Nexa3D and Henkel came together with French contract manufacturing provider
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Copyright WeMed
Third, to help WeMed develop, manufacture and launch its new product. The SKOP is a medical device for remote auscultation. It provides excellent listening quality, essential for emergency situations and for isolated patients. SKOP takes inspiration from nature, using a biomimetic design based on the human ear to maximize performance. The SKOP geometry can only be additively manufactured using ultrafast 3D printing.
MEET MEDICAL REGULATIONS AND
MITIGATE RISK With MEVOPUR™ Compliant Colors When it comes to plastics used in medical applications, the need for compliant and consistent colorants and additives has never been more important. With robust raw material pre-testing and an unparalleled global change control process, MEVOPUR medical-grade concentrates offer peace of mind and help you deliver safe, reliable treatments.
For more idea inspiration, visit avient.com COV3