


SUMMER 2023 PMMI Media Group | www.HealthcarePackaging.com + Annual OTC Package Design Gallery + 5 Tips for Qualifying Large Thermal Shippers + Automation Boosts Cannabis Oil Filling + Startup Pilots Sustainable Rx Packaging Takeback Schemes Drivers and healthcare-specific hurdles Renew Today! Stay Connected







↑ pp. 20

Annual
16 Sustainability
Healthcare Packaging Takeback Programs



101
At Pharmapack Europe, a principal consultant offered a primer on takeback and recycling schemes for pharmaceutical packaging and medical devices, offering core principles, business benefits, and healthcare-specific hurdles.
20 Package Design
Annual Package Design Gallery
Healthcare Packaging evaluates an array of over-the-counter product packaging designs, assessing the pros and cons from a user perspective.

26 OEM Application Note
Automation Lets Loud Labs’ Cannabis Oil Go National
A seemingly simple filling application shows that handling cannabis oils requires an understanding of their unique properties.
31 Traceability/Serialization The Simple Email Issue Holding Up DSCSAImplementation Activity
Employees come and go—a consistent contact will be necessary for data exchange implementation and tracing requests.
32 Package Design Smart Packaging Illustrates Ongoing Industrial

Revolution
The AIPIA World Congress proved smart packaging holds promise for brand objectives like traceability, recyclability, and supply chain logistics. Can tech suppliers band together to deliver total solutions?
CONTENTS Summer 2023 • Healthcare Packaging | 3
COLUMNS AND DEPARTMENTS 05 KEREN SOOKNE’S PERSPECTIVE 06 QUICK HITS 07 QUOTABLES/BY THE NUMBERS 08 NEWS 10 COLD CHAIN CORNER 13 MATERIAL DEVELOPMENTS 35 NEW PRODUCTS
OTC Package Design Gallery
www.healthcarepackaging.com
Editorial EDITOR-IN-CHIEF Keren Sookne ksookne@pmmimediagroup.com
SENIOR DIRECTOR, CONTENT & BRAND GROWTH Mike Prokopeak
CONTRIBUTING EDITOR Melissa Griffen
CONTRIBUTING EDITOR Tim Hayes
Art
ART DIRECTOR Jonathan Fleming
CREATIVE DIRECTOR Dave Bacho
Publishing
MANAGER, CUSTOMER EXPERIENCE & SALES SUPPORT Courtney Nichols
AD SERVICES/PRODUCTION MANAGER George Shurtleff
Audience & Digital VICE PRESIDENT, DIGITAL Elizabeth Kachoris DIRECTOR, DIGITAL MEDIA Jen Krepelka
Advertising
VICE PRESIDENT, SALES Wendy Sawtell wsawtell@pmmimediagroup.com
ACCOUNT EXECUTIVE Elizabeth Tierney
SENIOR DIRECTOR, CLIENT SUCCESS & MEDIA OPERATIONS Kelly Greeby
PMMI Media Group PRESIDENT Dave Newcorn DIRECTOR, MARKETING Sharon Taylor
SENIOR MARKETING MANAGER Amber Miller
FINANCIAL SERVICES MANAGER Janet Fabiano
FOUNDING PARTNER AND EXECUTIVE VP, INDUSTRY OUTREACH, PMMI Joseph Angel FOUNDING PARTNER Lloyd Ferguson
PMMI Media Group 401 N. Michigan Ave., Suite 1700 Chicago, IL 60611 p: 312.222.1010 | f: 312.222.1310 www.pmmimediagroup.com
PMMI The Association for Packaging and Processing Technologies 11911 Freedom Drive, Suite 600, Reston, VA 20190 p: 703.243.8555 | f: 703.243.8556 | www.pmmi.com
PLEASE RECYCLE THIS MAGAZINE Remove inserts or samples before recycling.
Healthcare Packaging® (ISSN # 21543666) is a registered trademark of PMMI, The Association for Packaging and Processing Technologies. Healthcare Packaging® is published bi-monthly by PMMI with its publishing office, PMMI Media Group, located at 401 N. Michigan Ave, Suite 1700, Chicago, IL 60611; 312.222.1010; Fax: 312.222.1310. Periodicals postage paid at Chicago, IL, and additional mailing offices. Copyright 2023 by PMMI. All rights reserved. Materials in this publication must not be reproduced in any form without written permission of the publisher. Applications for a free subscription may be made online at www.healthcarepackaging.com/ subscribe. Paid subscription rates per year are $55 in the U.S., $80 Canada and Mexico by surface mail; $130 Europe, $200 in all other areas. Single copy price in U.S. is $20. Free digital edition available to qualified individuals outside the United States. POSTMASTER; Send address changes to Healthcare Packaging®, 401 N. Michigan Avenue, Suite 1700, Chicago, IL 60611-3789. PRINTED IN USA by Quad. Volume 17, Number 2 The opinions expressed in articles are those of the authors and not necessarily those of PMMI. Comments, questions and letters to the editor are welcome and can be sent to: editors@healthcarepackaging.com. Mailing List: We make a portion of our mailing list available to reputable firms. If you would prefer that we don’t include your name, please write us at the Chicago, IL address.
PUBLICATIONS MAIL AGREEMENT NO. 40064408 • USPS
Takeback Schemes Emerge in Healthcare
There are many healthcare-specific hurdles related to the collection, recycling, or proper disposal of medical waste, be it packaging or the medical device itself.

But as Ellen Struthers said in her talk at Pharmapack Europe, companies in healthcare are beginning to tackle takebacks, via mail or store/ pharmacy drop-off (pp. 16). Early programs and pilots focus mostly on the collection of autoinjectors and inhalers. A lot goes into the decision to run a takeback scheme— it’s not something to implement on a whim. As we highlight, there must be a net environmental benefit to the program, and it must be ethical and legal. Finding ways to sort and collect waste is also an important step toward making advanced recycling possible, and
deriving value from packaging at its tradidional “end of life.”
In material developments, Melissa Griffen covers a pilot implementing Parcel Health’s paper-based packaging at ten pharmacies, discussing pharmacist and customer reception (pp. 13).
On the logistics side, have you ever felt that one of your shippers was underperforming? On pp. 10, we feature tips for large shipper qualification, including an overlooked test on shipper-to-shipper variability. Not only does this test alert you to shipper performance, but it’s also beneficial (1) as a baseline for testing degradation down the road and (2) for evaluating cool down time, i.e. how long it takes the shipper to cool after you load dry ice.
In package design, for a real-world look at what’s on shelves today, check out our annual package design gallery (pp. 20)!
KEREN SOOKNE is the Editor-in-Chief of Healthcare Packaging She may be reached at ksookne@pmmimediagroup or at linkedin. com/in/kerensookne

PERSPECTIVE
Once relegated to the consumer packaged goods sector, companies are initiating takeback and recycling schemes in life sciences.
Using Urine Tests to Find Brain Tumors
According to a Medgadget article, researchers at Japan’s Nagoya University have developed a urine test for earlier, non-invasive brain tumor detection using a nanowire assay. The technique uses a well plate coated with zinc oxide nanowires that attract extracellular vesicles with their surface electrical charge. Fluorescently-labeled antibodies then detect tumor-specific extracellular vesicles. The technology is based on the concept that extracellular vesicles shed by brain tumors into the bloodstream are ultimately excreted in the urine and provide tumor hallmarks, from RNA and DNA to protein markers.
2 Blood Sugar Could Power Medical Implants
An ETHzürich article discussed a new method of generating electricity using enzymes that break down glucose in the blood. Researchers have developed a biofuel cell that uses glucose oxidase and bilirubin oxidase to generate electricity from glucose. The biofuel cell has the potential to power implantable medical devices, such as pacemakers, using the patient’s own blood glucose as a fuel source. In vitro testing showed promise, but several challenges remain before the technology can be used in humans.
3 Bacterial Nanosyringe Shows Potential for Gene Therapy Delivery
A Scientific American article reported on a “nanosyringe” study that could potentially deliver gene therapies to human cells. The nanosyringe is a bacterial protein called SdeA, which forms a needle-like structure that can puncture the membranes of cells and deliver molecules such as DNA or RNA. Testing on human cells in the lab found that the nanosyringe could deliver genetic material without causing significant damage to the cells. Hurdles must be overcome before testing in humans.
4
Tiny Cylinders Control Drug Release
According to a Medgadget article, “nanocylinders” made of a biocompatible polymer have been developed by UCLA researchers. These can be loaded with drugs and injected into the body to slowly release over a period of days to weeks. The nanocylinders could potentially be used to deliver drugs to specific sites in the body or in steady doses over an extended period of time, including difficult-to-deliver drugs. Advantages include being able to bypass the body’s immune system and avoid the liver, which break down and remove drugs from the body.
5 Packaging Issue Leads to Massive Pfizer Recall
According to a recent FiercePharma article, Pfizer is recalling 4.2 million units of its migraine therapy Nurtec ODT because its blister packaging is not child-resistant. The recall is being carried out in conjunction with the U.S. Consumer Product Safety Commission (CPSC). No injuries have been reported, and a remedy has already been identified. Pfizer has instructed pharmacists to place the implicated blister cards in a child-resistant vial before administering to patients. The products were sold at pharmacies across the U.S. between December 2021 and March 2023, and were manufactured by Biohaven and Pfizer.
6 Police Union Exec Busted for Fentanyl Smuggling
According to a Justice.gov article, Joanne Marian Segovia, Vice President of the San Jose Police Union, has been charged with attempted illegal importation of fentanyl, a powerful synthetic opioid. The executive allegedly used WhatsApp for communication— messages discussed details for shipping and payment of pills and contained hundreds of pictures of tablets, shipping labels, packaging, and more. The charges carry a maximum penalty of 20 years in prison and a $250,000 fine. The San Jose police department issued a statement condemning the alleged actions, noting she has been placed on administrative leave pending the outcome of the case.
To keep up with the latest news bits from around the world visit healthcarepackaging.com to subscribe and get Quick Hits sent right to your inbox.

QUICK HITS
TIM HAYES, CONTRIBUTING EDITOR, HCP 6 | Healthcare Packaging • Summer 2023
1
$5.01 MILLION
THE AVERAGECOST of a data breach in the pharmaceutical industry in 2022, per IBM Security’s Cost of a Data Breach Report 2022.
$966 BILLION
THE ESTIMATED ECONOMIC BURDEN of rare disease in the U.S. in 2019. Approx. 30 million people are affected by rare disease in the U.S.—over 50% are pediatric onset cases.
Source: EveryLife Foundation for Rare Diseases 38
THE NUMBER OF STATES with legal medical marijuana programs, plus the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands.
Source: National Conference of State Legislatures 5
THE NUMBER of months until DSCSA requirements come into full force on November 27, 2023, when trading partners will be required to add serialized product data to the transaction information provided when a DSCSA-covered product changes ownership.
Summer 2023 • Healthcare Packaging | 7 QUOTABLES
“The challenge really is the lack of standardization of data around what constitutes a [drug] shortage. Many countries actually do report shortages, but it’s very hard to compare shortages across countries. And not all countries report shortages—there are total blind spots.”
—VIMALA RAGHAVENDRAN, MBA, VP, INFORMATICS PRODUCT
DEVELOPMENT,
U.S.
PHARMACOPEIA
“When you look at factory automation, space is... the last thought of when you're putting the line together. It's always making the product first. And then, when you look at the secondary or tertiary packaging side, there seem to be issues. So there are always space constraints.”
—SEAN SPEES, MARKET SEGMENT MANAGER, BOSCH REXROTH
“Just because something technically does its job doesn’t mean that people will be happy to use it. In fact, 83% of people who are prescribed sleep apnea machines take them off halfway through the night.”
BY THE NUMBERS
—OSCAR DAWS, CO-FOUNDER, TONE PRODUCT DESIGN
New Features at PACK EXPO Las Vegas 2023;

Registration Open
Registration for PACK EXPO Las Vegas 2023 (Sept. 11–13, 2023; Las Vegas Convention Center) is open. Produced by PMMI, The Association for Packaging and Processing Technologies, the show is the most comprehensive packaging and processing event in North America with over 2,000 suppliers showcasing diverse innovations for more than 40 vertical markets. Attendees will gain insight from 100+ educational sessions and have access to more solutions under one roof than available anywhere else in North America. A multitude of familiar and new features make the show a must-see for all packaging and processing professionals.
The newSustainability Central is an interactive destination that takes an expansive look into what sustainability means and provides actionable sustainable solutions in manufacturing, materials, and design. On the newSustainability Stage, attendees will hear from experts on a range of packaging sustainability topics and learn how to make brands more sustainable in the future.
The Industry Speaks Stage will feature experts from the PACK EXPO Partner Program, covering multiple industry verticals, addressing hot topics and industry trends like sustainability, remote access, supply chain solutions, augmented reality, and operational efficiency.
With the boom in e-commerce, the Logistics Pavilion is an important addition to the show that will feature targeted solutions related to supply chains, including warehousing, fulfillment, distribution logistics services, and transportation providers.
To learn more about these programs and destinations, and to register, visit: packexpolasvegas.com
—Casey Flanagan
Scan from a Distance, Enlarge Product Information for Visually Impaired

At the AIPIA (Smart Packaging Association) World Congress held in November 2022, Zappar debuted Zapvision, a new scanning technology, alongside the company’s usual AR fare. It builds upon a standard QR code to enable scanning from a distance, and to deliver relevant and personalized content that adapts to smartphone accessibility settings for partially sighted or nearly blind people. The scanning tech uses a “D3 QR code” named D3 for its “dot-dotdash” pattern placed around the corner of a standard QR code.
The company says brands can easily and affordably implement this D3 QR code on packaging, at scale, without having to create any new code scheme, use up any additional on-pack space, or introduce new printing processes. That means it is easy for brands to implement while also making it easier for partially sighted customers to find their products, either in-store or on the shelf at home, and instantly access the product information they need.
According to Zappar, “There are currently 285 million people in the world with visual impairment and 39 million registered as blind. Two billion people globally wear corrective glasses. For many, the simple task of identifying products on a shelf and accessing relevant product information is a daily challenge.”
For more smart packaging insights, visit pp. 32.
—Matt Reynolds
NEWS 8 | Healthcare Packaging • Summer 2023
Coding
Cottoning
Counting
Decontamination
Feeding
Labeling

Serialization








Sterilization
Washing
©2023 ProMach Inc. 5600 Kieran Montreal, QC H4S 2B5 PHONE 514-337-6990 EMAIL info@NJMPackaging.com WEB NJMPackaging.com • Individual machines to • Full production lines The single-source pharmaceutical packaging systems provider with over a century of customer satisfaction • Oral solid dose • Liquid dose / Aseptic fill finish • Powders • Gummies • Personal care products • In-house manufacturing • Exclusive representation of partner brands • Full line integration
Fill Finish
Packing
Aseptic
Capping Case/Tray
Feeding
Filling Insert
Isolation Technology
Line Integration
Robotic Palletizing
Excellence in: For: Visit Us in booth 6501 and in the ProMach Metroplex.
Merck Expert Shares 5 Tips for Qualifying Pallet-Sized Thermal Shippers
Find out why Lee Menszak advocates for shipper-to-shipper variability testing from his talk at ISTA’s TempPack event.
KEREN SOOKNE, EDITOR-IN-CHIEF
At ISTA’s TempPack in Houston (May 1-3, 2023), Lee Menszak, Associate Director, Engineering, at Merck, shared tips and considerations for qualifying pallet-sized shippers, where the risk is often much greater than that of a small parcel-sized shipper.

“Large shippers are different than small shippers for a variety of reasons. The ones that I would point out the most are the payloads are larger, they are expensive, and they would be difficult and time-consuming to replace. So it’s important that you get it right the first time, and that you have a robust solution,” said Menszak. He also highlighted that large shippers have longer packout durations, larger temperature gradients within the payload space, and a greater number of closure points during assembly, among other factors. Here are five of Menszak’s tips to reduce risk.
1. Don’t ignore regional user requirements (including safety)
Menszak showed attendees a picture in which an operator is inside the shipper loading dry ice while wearing a full backpack respirator. “The reason is in the Netherlands, it is a requirement you must do this. You need to know the specifics of the individual country. Don’t think about your perspective from your desk when you’re qualifying the shipper,” he said, adding that you must consider safety during loading, as dry ice is regulated as a dangerous good. IATA PI 954 requires specific labeling and adequate ventilation so that pressure build-up doesn’t rupture the packaging.
2. Thou shalt know thy shipper
“My number one rule of qualification is ‘Thou shalt know thy shipper,’” he said. “When you get done with the qualification, you should know it as well as—or better—than the manufacturer, you need to become the SME. The earlier you do it the better.”
If the space for dry ice in the shipper is 2.75 inches, you can’t load a 3.5-inch block of dry ice. If the dry ice block is 1.5 inches, it will rattle
and won’t fill the space. He added, “My question to you is what good is qualification if when you give it to a user site, the dry ice they have available isn’t going to work?”
3. TOCS is a user requirement
Time out of controlled storage (TOCS) is the time duration to go from where the product is stored to where it’s packed out. Prior to the packout, it’s expected there will be a delay while preparing the payload for loading, with time for quality checks, securing the payload onto the pallet with banding or strapping, and other routine operational handling requirements.
COLD CHAIN CORNER 10 | Healthcare Packaging • Summer 2023
↑ Lee Menszak addressed the crowd at the ISTA Forum’s TempPack in Houston.
TOCS must be controlled and minimized, but it also needs to (1) be quantified and (2) be representative of what is truly needed. “It is for the user to define, so you need to talk to those responsible for doing the packout,” he noted.
Menszak said he has found TOCS is typically 30 minutes, but he encountered one site that needed one hour from removal from -40°C to the shipper door close. This user requirement specification needs to be included in the qualification testing, so it’s important to let your qualification lab know.
Max Load and Worst Case
“What’s the worst case? In my experience—not to be taken as law—max load, max mass is the worst case. I have seen it the opposite way where dry ice sublimates, CO₂ gas sinks, and the hot air rises. Unlike small shippers, convection dominates here [in pallet-sized shippers]. You need to test to find out what your worst-case scenario is.”
Lee Menszak
4. Rationale is a neglected area
As he explained, “It’s one thing to say that you’re going to use this profile or this configuration, but the rationalities need to be included. Why? For the benefit of the approvers—they need to understand why you’re doing what you’re doing. There’s a better reason, though, to be honest. Two, three years down the road when you get audited on the shippers, you may have forgotten.”
Also, it may be another person presenting your protocol who is in the dark about why you did what you did. Documenting the rationale will clarify why you made those choices—whether it’s you or another person in the audit.
Some parameters may be obvious for inclusion in your rationale such as ambient profile, duration, payload and thermal mass, and refrigerant loading. But there are other activities to consider which may actually precede qualification, such as CO₂ sensitivity, cyro temperature tolerance, container closure integrity, and re-icing. “Dry ice sublimates… CO₂ gas doesn’t know it is not allowed to interact with the product. If you’re using dry ice, you need to have a CO₂ sensitivity test in advance of qualification concluding that CO₂ ingress is not an issue with your product,” Menszak said. Additionally, plastics, tubing, and clamps can be sensitive to cracking at low temperatures, so testing primary packaging early on is critical.
5. Shipper-to-shipper variation
While it’s not a requirement, Menszak highly advocates you test shipper-to-shipper variability. If you fly a fleet of a dozen hardshell shippers, how do you know that they are all performing equally?
While the vendor provides an analysis, the only way to find out is to actually test. “And it’s a simple test. Load them up with dry ice, put the temperature monitors in at minimum load, and you let them run. It doesn’t require a laboratory; it can be in the warehouse. At the end of testing, you may, as I have, find one that’s an underperformer. So from Day One, you have a troublesome shipper. Here’s your justification. Go back to the vendor and say this one’s bad, we need a replacement. And they also know they better give you a good one back because you’re going to test it to find out,” he said.
He explained there’s another good reason for doing this test: when years go by, suddenly you get a shipment that didn’t go as expected, without a good explanation. “You can repeat this test. So you can test shipper #4 that’s a problem today and compare the results with how it performed when it was brand new,” Menszak said. “There may be degradation. What is the life expectancy of the shipper? My experience is that the manufacturers will give you a vague answer because it depends on the usage, the number of turns.
So it’s a good way, five years down the road to say, ‘We’re starting to see some problems. Let’s run through the same test we did when they were new and see what’s going on.’” This can help you determine if you’ll need to replace certain shippers in the near future.
This test is also beneficial for evaluating cool down time—how long does it take the shipper to cool down after you load the dry ice? Following the loading of the payload into the shipper, one can expect that there will be a cool down time where the product temperature will exceed the allowable storage and shipping temperature. Whether or not this is acceptable depends on your stability data, hence why stability data is a predecessor to performing shipping qualification.
Cool down time is important information to include in the qualification, and it’s important to the user. He shared an example showing that a shipper took approximately two hours to arrive at -40°C. “The user, for example, loaded the shipper in the morning at 8am. And the question is ‘When can I load the product?’ This tells you your spec. You load it, you wait two hours. From 10am up to 2pm is your window of opportunity. You would qualify for the worst case which would be a six-hour delay before loading the product,” he said. “Inevitably you get the question, ‘Can I use it the next day since we didn’t get to load it today?’ My answer is ‘No.’ The reason being that you’re then sacrificing shipper duration. You’re losing what we’re setting out to achieve: maximum duration. So my answer is to start all over. Empty it out and load the dry ice again the next day.”
For the latest in logistics and supply chain solutions, check out the new Logistics Pavilion at PACK EXPO Las Vegas (Sept. 11-13, 2023). To register, visit packexpolasvegas.com
COLD CHAIN CORNER Summer 2023 • Healthcare Packaging | 11








































































































Discover your next packaging or processing solution fast on the free PMMI ProSource directory. Find validated suppliers by using the Package Type lter, such as Flexible Bags or Rigid Bottles. Browse categories, such as Filling, Capping & Closing or Processing Equipment. Narrow down your search using lters within a category, such as Capabilities or Fill Type www.prosource.org Search by package types: LiquidFillers (11companies) Entry-level/budget models available Hot Fill r LiquidFillers (11companies) Fill Type Capabilities Fill by time (6) Fill byweight (7) Hot fill (11) Volumetric (9) Entry-level/budget models available (11) IIoT ready (6) Tool-lesschangeover (8) Entry-level/budget models available Hot Fill
Women-founded Medication Packaging Startup Pilots Sustainable Prescription Packaging
Ten nationwide, independent pharmacies pilot Parcel Health’s 100% paper-based, FSC-certified, compostable medication packaging. Third-party child-resistance testing is underway.
MELISSA GRIFFEN, EDITOR, CONTRACT MANUFACTURING & PACKAGING
The healthcare industry, especially in pharmaceuticals, generates a significant amount of global CO₂ emissions. An example of the industry’s impact is through the billions of single-use, amber pill bottles (also known as amber vials) that stock the shelves of every pharmacy across the United States.
Parcel Health, a women-founded sustainable medication packaging startup located in Pittsburgh, PA, has set its eye on minimizing pharmaceutical packaging waste, starting with its first product, a pill bottle replacement called the Phill Box™. The system, which is best suited for oral solid doses, is designed to be functional while also being recyclable, compostable, water-resistant, and an easy-touse alternative to traditional packaging.

Parcel Health has a short and robust supply chain contained within the U.S. that starts with sustainable paper, a manufacturing partner, and an assembly and fulfillment center in Pittsburgh.
“The healthcare industry is a very carbon-intensive service sector, representing 4 to 5% of global emissions. With eight billion plastic pill bottles used in the United States every year, this will be one of the easiest ways for any healthcare provider to lower their carbon footprint,” says Melinda Lee, PharmD, Co-Founder and CEO of Parcel Health. “The Phill Box is made with Forest Stewardship Council (FSC)-certified sustainably sourced paper and requires 30% less carbon emissions to produce compared to plastic bottles. Every part of the Phill Box has been made and designed with care to the environment, patients, and pharmacies.”
The Phill Box is also Programme for the Endorsement of Forest Certification (PEFC)-certified, according to Mallory Barrett, Co-Founder and President of Parcel Health. The box is coated with a sustainable and proprietary coating that gives it water-resistant qualities to withstand accidental spills, and it also has a slight grease-resistance quality to make peeling off labels easy. When asked about moisture barrier, Lee explains that along with the blend of coatings, it was designed as two boxes that fit together, hence
↑ The system, which is best suited for oral solid doses, is designed to be functional while also being recyclable, compostable, coated for water-resistance, and an easy-to-use alternative to traditional packaging.

↑ Patient perception has been positive overall, according to the PharmDs, though the geriatric population has taken a bit longer to warm up and sometimes struggles to understand the opening mechanism.
providing two layers of coating and two paperboard barriers of protection against the aforementioned accidental water splashes or
MATERIAL DEVELOPMENTS Summer 2023 • Healthcare Packaging | 13
spills. [Editor’s note: many pharmaceuticals require barrier protection against moisture and oxygen. Healthcare Packaging has not verified testing for barrier properties of the system compared to existing pharmacy packaging options.]
“After a brief wash in the sink, the Phill Box biodegrades completely if buried under soil in warm conditions, such as in the summer heat. Additionally, it can be recycled in any municipal recycling program that accepts paper or paperboard products,” says Barrett.
The Phill Box is currently being piloted by ten independently-owned pharmacies around the country. Many of these pharmacies offer quick prescription transfer and free delivery, making the switch easy and convenient for patients.
Successful pilot program
“This is the next generation of medication packaging,” notes Kyle McCormick, PharmD, of Blueberry Pharmacy. “It is a fantastic product that’s leading the industry towards a sustainable future.”
Blueberry Pharmacy, also located in Pittsburgh, PA, was one of the first pharmacies to join the pilot program over a year ago and has been a strong marketing source to spread the word to other pharmacies.
“I don’t know if I saw on social media or she happened to stop in or how that connection happened at first. But the idea of reducing the ridiculous amount of plastic produced by pharmacy specifically, really resonated,” McCormick says.
After signing up for the pilot program, McCormick shared it on social media and created videos displaying the Phill Box and its purpose, which hooked Carlee Wilmot, PharmD, and Pharmacy Manager at Health Park Pharmacy/Isabella Citizens for Health, Inc. in Michigan and Kristin Proffit and husband Chris Proffit, PharmD, of Uptown Drug and Home Medical in Arizona.
Wilmot and Proffit expressed their interest in reducing the amount of single-use plastic in their field, which would provide additional benefits in reducing the storage of plastic pill bottles and their associated costs.
All three PharmDs note that the Phill Box delivery process was seamless and that Parcel Health was easy to communicate with and very open to answering the pharmacies’ questions. After a quick form fill-out and a web meeting with Barrett, the Phill Box packages were sent along with marketing displays and social media advertising assistance.
Proffit says her pharmacy trained staff briefly on the new packaging and how to engage with the patients on the phone and in person to promote the Phill Box. Once a patient opted to use this paperbased option, the pharmacies would demonstrate how to open and
close the box.
McCormick explains that in the beginning, the opening-closure feature could get caught and may have seemed counterintuitive to many patients. Parcel Health took the feedback and was able to improve the feature and “iterated every design flaw that we could think of out of them,” he says.
Patient perception and acceptance
The pharmacists reported that the majority of first users were very much on the same page as far as environmental awareness goes—realizing that each time they came in to refill a prescription, that was another plastic bottle in the garbage—and stuck with the packaging through its various iterations. Other patients, especially the geriatric patients, warmed up to the packaging at a slower pace, and some struggled to understand how the box functioned.
“We learned that older patients need more instruction when using the Phill Box,” says Barrett. “One pilot pharmacy provided Phill Boxes to their older patient population and received critical feedback of it. We subsequently launched a study to better understand this and found that, once given a 5- to 10-second demonstration of the Phill Box mechanism, eight out of 10 older adults (above age 65) actually prefer the Phill Box compared to their standard orange pill bottles due to our unique opening mechanism.”
McCormick says patient perception was broadly positive, though Wilmot noted that it was not well received by all, which could be due to misconception that the Phill Box would contain notoriously hardto-open foil blister packs. However, Wilmot also expresses that her pharmacy’s main form of marketing was word of mouth—including through its patients—and says people actually transferred to Health Park Pharmacy/Isabella Citizens for Health, Inc. for the Phill Boxes.
The Phill Box design and working out the quirks
According to McCormick, his patients “overwhelmingly” liked the design of the Phill Box. But it’s not only the patients who like the look.
“From the appearance, it’s a nice clean look. And the instructions are printed on there. One of the most exciting things is the customizability of the display where it can be white labeled, essentially. And so when I saw the holiday design being printed, that was very exciting. One thing that we currently have with our vials is that the lids have customized messaging on them for our pharmacy. But imagine, with the box, you can almost have anything printed on it. And you can design a label now to fit on a box to where you can read everything in a single panel, I think that’s very intriguing as well. So there’s just some benefits there from being box shaped versus cylindrical,” says McCormick.
MATERIAL DEVELOPMENTS 14 | Healthcare Packaging • Summer 2023
Wilmot’s pharmacy also makes use of customizable labeling by changing the appearance of the paper bags that the prescriptions are dispensed in throughout the year, the most recent being a breast cancer awareness design. She comments that this customizability along with the clean, sleek look of the box are part of what attracts patients to the Phill Box.
Each pharmacy provided invaluable feedback to Parcel Health. Current issues that are being addressed are:




• Tightening the box so that tiny tablets can’t slip between the pieces of paperboard (now fixed as of press time)
• Developing a bigger Phill Box for patients with larger prescriptions
• Getting the Phill Box child resistant-certified—Barrett confirms that third-party testing is underway













Pharmacists have also asked about making the boxes more water-resistant, which may be addressed in the future.
“The pilot was very successful,” says Barrett. “We learned that pharmacies and their patients are much more receptive and enthusiastic about environmentally friendly packaging than we previously anticipated. We thought that our pending child-resistant certification would be a huge barrier to adoption, but found that since pharmacies utilized many non-child resistant packages such as adherence packaging, they were ready to purchase the Phill Box as a non-child resistant and sustainable product to stand out in the crowded pharmacy market.”
Barrett also affirms that running the pilot helped Parcel Health to build strong relationships with its pharmacy partners. Parcel Health has developed a Starter Kit and has plans to expand its
sustainability packaging offerings for prescriptions and other medications—such as injectable packaging, liquid packaging, shippers, and secondary packaging, etc. within pharmacy— and reduce plastic in single-dose packaging





within healthcare systems and hospitals.
“There is also a huge need for non-plastic sustainable packaging in the OTC and vitamin space. We hope to touch and impact each of these areas in due time,” says Barrett.
Partner With the Product Inspection Experts

Su 2023 • Healthcare Packaging | 15 MATERIAL DEVELOPMENTS Y �;¤é �� ¹ÀºÒÙÎ{Ù¤ÀºÒ ¤º��ÀÀÙ¡�ŠŧŠţ�
Metal Detection� �X-Ray Inspection �Checkweighing Vision Inspection �Track & Trace� �Serialization Solutions Customized Material Handling �Global Field-based Service �www.mt.com/pi rÀÝÎ�Ë{ÎÙº Î�ê¤Ù¡�{� ݳ³�Òݤ٠�À � Ù ¡ºÀ³À ¤ Ò�ÙÀ� ÀÀÒÙ�ËÎÀ Ý Ù¤é¤Ùíű� ¤º Î {Ò �ËÎÀ Ý Ù�Ò{ Ùíű�ËÎÀÙ Ù� Î{º � Î ËÝÙ{Ù¤Àºű�{º �Î Ý �Î {³³ÒŰ


SUSTAINABILITY 16 | Healthcare Packaging • Summer 2023 Healthcare Packaging Takeback Programs 101 KEREN SOOKNE, EDITOR-IN-CHIEF
TOP THREE TAKEAWAYS
Whether via store drop-off or shipping from home, a number of packaging material reuse or takeback schemes have been implemented for consumer packaged goods. But the landscape is changing for healthcare, too.
Ellen Struthers is a principal consultant at Anthesis Group, a global sustainability consultancy that works across a variety of areas of environmental sustainability—carbon, green design, sustainable sourcing, life cycle analyses, circularity, and more. With 1,100 experts operating in 40 countries, the certified B Corp has clients across all industry sectors ranging from start-ups to corporate multinationals such as Reckitt, Cisco, Tesco, Nestlé, and Target.
At Pharmapack Europe 2023, she discussed that takeback case studies are emerging for life sciences product waste. (If you attended the[PACK]out in 2022, you may have seen Janssen’s safe returns device recovery program). Struthers discussed why a company might want to implement a takeback scheme, the challenges her clients in healthcare face, and common principles for success.

Defining takeback/returns schemes
While there may not be a globally agreed upon definition for takeback programs at this point, Struthers said they are typically initiatives funded and organized by the private sector to collect and treat consumer products and packaging. She said a scheme might not necessarily be restricted to a brand’s own products, as there are companies that will accept multiple brands’ products if they’re similar in type.
Takeback schemes are operationally complex, but generally consist of three core parts:
• The user interface is commonly a postal or courier return service in which the consumer sends materials back to a processor or consolidation site. Installed drop-off points—such as collection at a pharmacy or health/beauty store—or other types of public collection points at reuse and recycling centers or community centers may play a role. “Sometimes companies will use a combination of these different types of methods to capture back materials,” she said.
• Collection and consolidation typically take place if the materials aren’t sent directly back to the processor. Some schemes use reverse logistics where materials are taken back on delivery vehicles and consolidated at a transport depot, while others involve a separate collection done by a specialized waste management company.
• Treatment is where materials are (ideally) either reused or
recycled, but in some cases, companies are collecting materials for controlled disposal—usually incineration with energy recovery—where this still provides an overall environmental benefit.
Many factors determine the right-fit approach. “One of the critical factors is the type and amount of material that’s being collected, and particularly how hazardous it’s deemed to be under local regulation, as this will influence how tightly it needs to be controlled and managed at each stage of the process,” said Struthers. “The available infrastructure is also a big driver: whether there is a processor within the country [to process materials], or whether it needs to be taken across borders for treatment. The amount of funding that’s available to deliver the scheme from the lead organization and any partners that it’s collaborating with [is a factor]. Ideally, companies will be thinking about how different options for scheme delivery compare in terms of overall environmental benefit.”
Struthers highlighted the following examples in healthcare, and with planned expansions, she said more will follow. (Editor’s note: Anthesis has strict confidentiality agreements for clients—these are publicly available schemes.)
• Chiesi—inhaler takeback schemes with regional pilots operating in the UK and Italy
• Teva—inhaler recycling pilot in the Republic of Ireland
• Novartis—inhaler recycling in Switzerland
• Novo Nordisk—injection pen schemes in Denmark and the UK
• Janssen—Safe Returns scheme for injection pens in Switzerland (and the Janssen SimponiOne® Safe Returns program)
• Superdrug—blister pack recycling in the UK
• Acuvue—contact lens recycling in the UK run through opticians
SUSTAINABILITY Summer 2023 • Healthcare Packaging | 17
↑ Ellen Struthers, Principal Consultant at Anthesis Group, presented at Pharmapack Europe 2023.
1. Takeback schemes, once only applicable in the consumer packaged goods space, are emerging among healthcare manufacturers.
2. The available infrastructure is a big driver— can material be processed domestically or must it be taken across borders treatment?
3. Healthcare-specific hurdles include multiple materials in one device and risk of infection if a pen is not presented properly.
Key drivers
Any packaging engineer knows that sustainability is more than solely recycling, but there are a number of social and business drivers around cutting waste. “One of the reasons that I got interested in a career in waste in the first place is just how tangible it is, you can hold it in your hand, and you can see the visual impact that it has on the environment. The products and packaging that companies put on the market form a really powerful interface with the consumer, and particularly at the point where they become waste,” Struthers said. “When you’re holding your end-of-life product or packaging in your hand, whether you can dispose of that into a recycling system or you need to send it into residual waste, sends a really powerful message about the company and its commitment to the environment.”
While a multi-faceted approach to sustainability is always a good idea, waste doesn’t just affect the health of the environment, but it also affects human health. She explained, “It’s particularly an issue for the pharmaceutical sector when we think about active pharmaceutical ingredients, and the impact that they can have on human health if they are incorrectly managed and leak into the environment.”
In Europe/UK, the U.S., Canada, and Australia, she noted, “We’re very lucky we have really tightly controlled and well-managed systems for collecting waste. But that’s not reflected in the rest of the world. And many countries are still relying on informal collection systems with lots of waste leaking into the environment and being mismanaged. So, it’s a global issue of equality. In those countries, people are a lot more affected by waste issues in terms of their health and in terms of equality.”
Public awareness of waste continues to increase. One of the top three environmental concerns for the public in Europe, people are making the link between their activities and the activities of the companies that they buy from, and the impact on the local and the wider environment. 68% of Europeans citizens surveyed agree their consumption habits adversely affect the environment in Europe and the rest of the world (2020 Eurobarometer). Additionally, 53% indicated in a 2021 GWI report that they reduced their plastic use in the past 12 months, and 73% and 82% of U.S. and UK consumers, respectively, choose “greener” packaging.
This leads to pressure from the public demanding companies be more “sustainable” beyond the traditional value chain to include sourcing and material end-of-life. There is also increased scrutiny from the media—particularly copious messaging that’s sent to the public when things go wrong, she said—as well as from NGOs that are active in this space and driving change. Pressure also comes from regulators, particularly led by “polluter pays” principles in the EU that have impacts around the globe.
Beyond these pressures, Struthers said Anthesis finds that compa-
nies that show strong determination to make a sustainable change reap business benefits from it including:
• Attracting more investment
• Making higher sales in sustainable products
• Attracting more talent (71% of employees and job seekers said environmentally sustainable companies are “more attractive employers” per a 2021 survey from the IBM Institute for Business Value)
• Potentially creating operational savings through efficiencies (in one case, Unilever reported 1.2 billion euro in estimated costs avoided via energy, water, and materials efficiencies from 2010-2020)
Healthcare-specific difficulties
If you’re part of the life science community, you know there are difficulties with transferring over concepts that work readily in consumer packaged goods. Struthers noted that with pharmaceutical packaging and devices, challenges include:

• Multiple materials, such as an inhaler that has five or six different types of plastic as well as metal elements and paper
• At times, pressurized gases which are difficult to handle and capture
• “Problematic” plastics for recycling, such as PVC
• Residues of active pharmaceutical ingredients which need to be handled correctly
• Risk of infection from devices such as injection pens if they’re not presented correctly by the consumer at the end of life
• Non-standard shapes, which will not to travel reliably through a materials recovery facility
Above all, Struthers said that the scheme must provide an overall environmental benefit, and it’s “an absolute requirement that whatever you do, you’ve got to make sure that it is legally compliant, and it’s ethically operated.” She shared five core principles for a successful takeback scheme, and also shared a link to register for a free upcoming guide. Scroll to ‘Core principles for success (the CliffsNotes!)’ at: hcpgo.to/takeback101
SUSTAINABILITY 18 | Healthcare Packaging • Summer 2023































































































































Annual Package Design Gallery
Each year, Healthcare Packaging evaluates an array of over-the-counter product packaging designs, assessing the pros and cons from a user perspective.
KEREN SOOKNE, EDITOR-IN-CHIEF
Wonderbelly Antacid Chewable Heartburn Relief Tablets (Ginger Health Company)
PROS
+ Metal canister with screw-top lid features retro font and graphics in matte pastels printed directly on the can.


+ Colorful containers match modern flavors among established calcium carbonate offerings: watermelon mint, fruity cereal, strawberry milkshake, and lemon sorbet.
+ Recycling symbol—stream presumably aluminum—is on the can itself and the tamper evidence sticker.


+ The label makes use of clear print and graphics to convey that the product does not contain talc, dyes, or artificial sweeteners.

CONS

- The tamper-evidence sticker left silver residue over two of the graphics—we were able to rub the residue away and see the plastic-free and recycle symbol under (importantly, the Drug Facts remained clear).
From child-resistance to a portability loop, WILL PERFORM (co-founded by Serena Williams) shared details on their packaging journey. Get the story at hcpgo.to/will

OTC PACKAGING 20 | Healthcare Packaging • Summer 2023
Allegra Hives Non-Drowsy 24HR Hive Reduction & Itch Relief (Sanofi Consumer Healthcare)


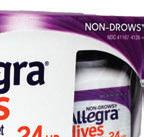


PROS
+ For transparency, the front panel features a window to the bottle and actual size image of the tablet.
+ The recycle-ready HDPE bottle features a purple child-resistant cap that is relatively easy to open and a dual-layer label which peeled effortlessly.
+ Eye-catching and clean front panel design let the user know this product both reduces hives and relieves associated itching.


NOTES/CONS
• While the carton is nearly double the width of the bottle it houses, this is presumably to be able to include Drug Facts on the carton. The carton features a recycling symbol on the bottom but it’s not clear whether the user would need to remove the window material first.
• The on-bottle directions are small, and are hard to find amid a lot of text.


OTC PACKAGING
Safe. Reliable. Customizable. 252-446-6177 • www.ossid.com Healthcare and medical packaging solutions for your products. From entry level flow wrappers to highly engineered and customizable horizontal form fill seal machines, Ossid has a solution to suit your product needs.
8000M
Performance, Packaged
Ossid
Nasonex 24HR

Allergy Nasal Spray (Perrigo)



PROS
+ A thermoform tray with paper backing (that wraps around the front) allows for a clear view of the spray bottle and attractive blue cap.
+ Number of sprays and symptoms addressed are clearly noted on the front panel.

+ The How2Recycle symbol clears up confusion by letting users know how to dispose of each component.
+ A thin, paper instruction leaflet is attached to the inside of the tray. Instructions A,B, and C are clear and concise with diagrams and sub-steps.
NOTES/CONS
• As with the Allegra Hives medication, consumers may question the size of the thermoform tray, with so much airspace compared to the bottle itself (again, likely due to Drug Facts or loss prevention).
Unisom Simple Slumbers ReturnTo-Sleep Mint Strips (Chattem, Inc.)




PROS
+ Quick dissolving strips offer a unique delivery mechanism among established sleep tablets and gummies.
+ The carded blister packaging with hang tab lets the user see the small container, which is slim for portability.
+ The strips are accessed via a door on the white plastic container—in tests, it was easy to open and remove a single strip with a fingertip.
+ Front panel is colorful and informative on the “return to sleep” claim, while still featuring whitespace and nighttime graphics.
CONS
- Serving size/dosing are clearly stated, but could the other suggested usage notes, such as the warning not to drive, be organized in a more readable way?

- We also noticed that the bottom edge of the white plastic is slightly jagged (but was not sharp to the touch).

OTC PACKAGING 22 | Healthcare Packaging • Summer 2023
Preparation H Soothing

Relief Spray (Haleon)

PROS
+ No-touch spray offers targeted application in a portable aerosol container.


+ Clear tamper-evidence sleeve peels off to reveal a cap-free design with easy-to-press nozzle.
+ The sturdy top twists left or right with a double-click to open or close the spray, respectively, allowing for portability without a cap.
+ Blue bottle with misty graphics conveys cooling.
NOTES
• There are no instructions visible on the bottle itself to operate the twist top, however there is an arrow symbol on the cap itself.
Obviously, there’s more over-the-counter packaging innovation than can fit on several pages in print. If you’re a brand owner in pharma, med device, or nutraceuticals with a package launch you’re proud of, reach out to Keren Sookne to tell your story at: ksookne@pmmimediagroup.com
PHARMACEUTICAL INSPECTION & FOREIGN OBJECT DETECTION

• CEIA high sensitivity metal detection; ferrous, non-ferrous, stainless steel



• Ishida X-ray for foreign object and product defects
• Ishida precision checkweighing for [IMKLX�ZIVMƼ�GEXMSR


• Powders, capsules, tablets, liquids

Heat and Control® offers a complete line of metal detectors, checkweighers, and X-ray inspection systems for pharmaceutical products from the leading manufacturers, Ishida and CEIA®. Technical support, demonstration, and testing is available in North, Central and South America.

Sep. 11-13, 2023
Booth C-1623, Central Hall Las Vegas Convention Center Las Vegas, NV USA

OTC PACKAGING Su 2023 • Healthcare Packaging | 23 92 91 97 98 34 108 94 6 116 17 20 33 32 99 36 26 42 0842 ~2 info@heatandcontrol.com | heatandcontrol.com 3 Ensure your products match the prescription. Quality Control. Inspection and foreign object detection systems that offer unparalleled sensitivity for pharmaceutical products to protect your consumer and your brand. LOOKING BACK. PRESSING FORWARD. ALWAYS INNOVATING.
New-Skin Kids’ Sting-Free Liquid Bandage Paint in






Purple (Advantice Health, LLC.)
PROS
+ Paperboard carton with hang tab features kid-friendly font and active graphics.
+ The glass bottle fits snugly in the carton, which also shows the brush and paint color.
+ Backing touts “100 fewer bandages in landfill” and clear bulleted instructions on back.

NOTES/CONS
• The checklist of facts on the back is helpful, but appears in a font that may be difficult to read at the smaller size.


• The addition of disposal instructions may’ve helped close the loop on environmental claims. Presumably the system is recycle-ready in the paper and glass
Aveeno Eczema Therapy Rescue Relief Treatment Gel Cream (J&J Consumer Inc.)






PROS
+ The wide dispenser on the pump is visible through a translucent cap.

+ A soft color palette offers a calming appearance in a portable size.
+ While the package itself is not recycle-ready, the use of a How2Recycle symbol to prevent consumers from wish-cycling is appreciated.
NOTES/CONS
• The dual-layer label on the bottle maximizes space for Drug Facts and warnings. When we peeled it back, some ink from the top layer remained on the bottom layer’s QR code, however, it did not prevent the phone from picking up the QR code properly.


OTC PACKAGING 24 | Healthcare Packaging • Summer 2023
Gainful Performance Nutrition Supplements



PROS
+ Supplement system is designed for customization by choosing an unflavored protein base and adding a flavor powder and goal boost powder (right) for muscle building, recovery, and more.
+ Hydration Drink Mix (left) is a paperboard tube housing 10 sachets for portability.

+ Recycle-ready matte paperboard tubes feature minimal graphics and stand out with whitespace and easy-to-read instructions for mixing on the tubes and sachets.




+ Tubes housing loose powder contain a recycle-ready metal inner lid that fits snugly. that fits snugly.






In flexible packaging, check out the seven health and personal care packages that won FPA awards, from UV blocking pouches to pet health packaging. A common theme was the ability to strike a balance between eye-catching aesthetics and performance in product protection. Visit hcpgo.to/fpa23

OTC PACKAGING Su 2023 • Healthcare Packaging | 25
MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD





OEM APPLICATION NOTE 26 | Healthcare Packaging • Summer 2023
Lets Loud Labs’ Cannabis Oil Go National
Automation
TOP THREE TAKEAWAYS
In 2015, Jake Berry and Coley Walsh founded Pyramid Pens, which now operates under the Loud Labs umbrella, a brand selling various formulations of cannabis oil packaged in cartridges that could be used in a host of vaping devices.

Using the well-regarded CO₂ extraction process, the partners began formulating unique strains and flavors of THC and CBD oils for vaping. In fact, the brand’s innovative attitude toward packaging caught our eye back in 2019. Read about what they were doing then, and see how far they’ve come at: hcpgo.to/cannabisblisterpacks
Today Loud Labs markets its Pyramid Pens line of cannabis formulated oils packed in cartridges and pods in Colorado and Michigan and is setting the stage for future expansion into other states. Expansion is a complex process of navigating each state’s individual laws and sales environments. The company offers a total of six oil formulations, each with its own characteristics of potency and flavor, concentrates, distillates, and CBD/THC combinations. The company also offers lines of infused pre-rolls and edibles.
Vape devices, which come in many different shapes, sizes, and technologies, all rely on cartridges filled with oil. Cartridges typically feature 0.3-, 0.5-, or 1-g of oil, depending on the type of device. Filling needs to be accurate for the optimum allocation of an expensive oil.
“We would have kilos of compounds coming out of the extractor,” says Berry, CEO. “The compounds would then be mixed into our various formulations to create our unique offerings. And then we sat with small syringes to laboriously draw the oil out of flasks and dispense a specified volume into a cartridge.”
As cannabis oil cools it becomes more viscous and harder to draw and accurately dispense. This oil is sticky, and difficult to work with and clean up. The process of drawing and dispensing via syringe is physically and mentally taxing, not to mention slow and wasteful. Plus, each formulation has a different viscosity that changes the force on drawing and dispensing. Barry says that at best 100 to 200 cartridges per hour can be filled by a diligently working team member. As popularity for the Loud Labs’ formulations increased, the speed at which orders were fulfilled decreased. There was simply too much filling needed in too short a time.
“We wanted to grow a business utilizing our best insights into product development, the market, and customer needs, not spend a big part of the workday filling cartridges by hand,” Berry says.
Loud Labs needed a better way to produce a competitive and affordable product, while maintaining high quality. Automating the process seemed a potential solution. It is important to note, however, that since the industry was in its infancy, automation solutions, good ones at any rate, were not as common as they are in established industries.
In 2018, Berry and Walsh became familiar with Thompson Duke Industrial of Portland, OR, a wholly owned company of Portland Engineering that’s dedicated to the manufacture and support of automated solutions for filling and capping cannabis-based vape cartridges and pods.
“One thing about designing a cannabis cartridge filling machine that we knew was extremely important was solving the issue of variable viscosity of the oil,” says Chris Gardella, CTO, Thompson Duke Industrial. “Cannabis oil does not behave like any other
See
OEM APPLICATION NOTE Summer 2023 • Healthcare Packaging | 27
1. A seemingly simple filling application shows that handling cannabis oils requires an understanding of their unique properties.
2. Loud Labs sought to automate the laborious filling process for a variety of oils with different viscosities, with precise temperature control.
3. They selected a machine that can fill 1,000 cartridges/hr, capable of changeover from one oil to the next in less than 60 seconds.
↑ Printed pouches are the secondary packaging for vape cartridges by Pyramid Pens, a Loud Labs brand.
Festo’s technology in action at their booth at PACK EXPO Las Vegas 2023! Join 30,000 attendees at the Las Vegas Convention Center from Sept. 11-13. For info and registration, visit packexpolasvegas.com
fluid. Each oil formula will have a different native viscosity. Some formulations may be so thick that the oil will not pour out of the jar at room temperature.”
Gardella says that to facilitate the flow of oil, the material must be heated. The temperature has to be controlled precisely, however, as too high a temperature may damage key components of the oil, while too low of a temperature reduces the flow. Another consideration is that some formulations must be dispensed gently, or they can be damaged.
The Thompson Duke cartridge filling machine features an oil path that involves a heated reservoir and short tube running to a stationary dispense head. In this path, a pneumatically controlled actuator raises a
→ Once filled by hand, a time-consuming and wasteful process, hundreds of cartridges are now processed at Loud Labs in a matter of minutes cleanly and without waste with Thompson Duke automatic machines powered by Festo.

↓ Heated cannabis oil pours easily into the heated reservoir of the Thompson Duke IZR high-capacity automatic filling machine. On the machine, tooling holding fill-ready cartridges is secured to a Festo EXCM X-Y table. A touchscreen HMI allows the operator to control and optimize the process through a simple menu of commands.

OEM APPLICATION NOTE 28 | Healthcare Packaging • Summer 2023
syringe plunger, drawing in a specific volume of oil. A second actuator lowers the syringe to an empty cartridge, and the plunger is depressed by the actuator. An automated X-Y table, which holds a matrix of hundreds of cartridges, accurately positions each cartridge in turn under the dispense head. Thompson Duke standardized on Festo pneumatic and electric components and systems for its machine based on part availability, quality, and support.
“Another design consideration is that each oil formulation will dispense at a different speed and as the oil warms it can be dispensed faster, which means speeding up and coordinating the X-Y table with the dispense head,” Gardella says. “Complicating this already complex process is the fact that the vaporizer device industry is moving toward many different cartridge configurations.”
Knowing the handling properties of Loud Labs’ formulations as well as they did and listening to the Thompson Duke personnel describe the design characteristics of the company’s patented IZR automated filling machine, Berry and Walsh thought they were talking with a vendor that understood their needs.
They were excited about the potential of an industrial grade system capable of filling 1,000 cartridges per hour, which would mean a single machine could do the work of at least four employees with higher accuracy and less waste. That level of throughput would be a game changer for the company not only in the number of cartridges filled and rapid response to orders, but also in labor savings. The business owners learned that Thompson Duke machines offer changeover from one oil to the next in less than 60 seconds, a benefit for a company like

Loud Labs with multiple formulations. Thompson Duke added two additional facts to the discussion. The company was dedicated to technical support. Customers were assured of world-class support after the sale.




Furthermore, Thompson Duke’s software made operation of a complex process simple for operators. Berry and Walsh purchased a Thompson Duke IZR automated filler as quickly as they could.
We believe every project we work on is a way to create a dynamic customer experience. That’s why we design customized packaging solutions to fit exactly what your customer needs. To
OEM APPLICATION NOTE Su 2023 • Healthcare Packaging | 29
find out
we can help, visit us at mrpsolutions.com
We
how
We make possibilities.
don’t just make caps.
Expansion and diversification
“In the cannabis industry, consumers look for trustworthy brands—brands that deliver consistency in product quality and a range of options,” Berry says. “Today Pyramid Pens offers six different pure, potent, and clean cannabis oils packaged in cartridges compatible with any 510-battery vape device. It offers five different types of Pax Era pods, and three different reloads and disposable vapes. All of these are filled by state-of-the-art Thompson Duke automatic filling machines.”
Further, Loud Labs has streamlined its manufacturing by automating the process of moving empty cartridges from shipping cartons to the tooling used on a machine’s X-Y indexing table. The company has also added a Thompson Duke LFP press for capping cartridges.

Automation removed the physical constraints associated with manual processes, allowed for rapid turnaround times on orders, and provided precise quality control. Prior to implementation, a large order might take up to a month to fulfill, whereas now large orders can be filled within days.
“By partnering with Thompson Duke Industrial, Loud Labs has realized a rapid return on investment, bringing speed, efficiency, quality control, and cost-effective solutions to its manufacturing
floors,” says Berry.
“There are three takeaways from Loud Labs automation experience,” adds Walsh. “Cannabis is a material with unique properties. The vendor community must develop automation and packaging solutions specifically for cannabis or at a minimum be prepared to significantly modify systems to accommodate the performance characteristics of the materials.
“The second takeaway is that this is a new industry. Cannabis companies will benefit from ease of use and high levels of support. And lastly, electronic record keeping, traceability, and Good Manufacturing Practices that conform to FDA-level regulations in equipment and process may, in the not-too-distant future, be required. Suppliers and end users must be ready for this.”
In the meantime, both Berry and Walsh say they are continuing product development, looking for ways to automate, exploring expansion into new states, and most importantly focusing on giving their retailers and consumers a quality brand, one they can rely on.
on G&K–Vijuk MV outsert systems!



ï 4UBSU�TNBMM
�GPMEJOH�VQ�UP����QBOFMTyPS�HP�CJH
�GPMEJOH�VQ�UP�����QBOFMTyPS�B�TJ[F�JO�CFUXFFO��
ï 'PME�MBSHFS�TJ[F�PVUTFSUT�PS�NFEJDBUJPO�HVJEFT�CZ�TQFDJGZJOH�PS�BEEJOH�UIF�OFX�(6,�'"���'PMEFS � GPMEJOH�TIFFUT�VQ�UP�����NN�XJEF�
PO�UIF�(�,o7JKVL�$PEJOH�BOE�4FSJBMJ[JOH�4UBUJPO�
ï�1SJOU�B�VOJRVF�DPEF�PO�FBDI�PVUTFSU�GPS�USBDFBCJMJUZ�XJUI� QSJOU�BOE�EJNFOTJPOBM�WFSJöDBUJPO�
ï�Log all veri cation results, good and bad, for an accurate SFDPSE�PG�FYJTUJOH�OVNCFST�GPS�USBDLJOH�BOE�DPNQMJBODF�
ï�*O�MJOF�PS�Pò�MJOF�
OEM APPLICATION NOTE ����$IVSDI�3PBE
�&MNIVSTU
�*-��������t����������������t� www.guk-vijuk.com
Check out the full story for more on the anatomy of a vaporizer device filling machine: hcpgo.to/loudlabs
The Simple Email Issue Holding Up DSCSA-Implementation Activity
KEREN SOOKNE, EDITOR-IN-CHIEF
TOP THREE TAKEAWAYS
At HDA’s Distribution Management Conference in Indianapolis, multiple panelists and attendees hammered home how critical it is to ensure open communication with trading partners as the pharma manufacturing and distribution community hurtles toward November 27, 2023—the compliance deadline when DSCSA requirements come into full force.
Pam Clements, GM/Director of Operations at Smith Drug Company, highlighted a major hindrance that manufacturers can take steps to tackle: the simple act of a distributor trying to reach the appropriate contact at the manufacturer by email. [Editor’s note: Email may get some bad press these days, but it’s still the most ubiquitous communication tool for intercompany messaging.]
Clements said she’s run into issues where she communicates with her contact at the manufacturer regarding tracing data exchange via the person’s email at the company. “But people come and go. Someone leaves the job, and often there’s no auto-reply letting you know who to follow up with. It can take weeks just to find out who’s taken over the role,” she explained. “My advice to manufacturers is to make a generic email like DSCSA@[companyname].com to keep that continuity for your trading partner.”
Max Peoples, Owner at RxScan, LLC & Uptown Pharmacy LLC, echoed this sentiment to panelists later during a Q&A session related to exceptions reporting: “From a dispenser perspective, it would be really nice if the industry could come up with a common email address, like DSCSAexceptions@pfizer.com or DSCSAexceptions@valuedrugco.com, for example, so when my system identifies the exceptions, it’s built in there, I hit a button, and it’d be really nice to be able to just email off the exceptions list to that particular address. Maybe the industry could solidify around how the dispensers are going to report exceptions. I doubt you’d want to get phone calls on these if you could get an email over to a common place—
not to the representative’s email address, but a consistent address.”
As Heather Cooley, Project Lead, Strategic Operations and Initiatives at McKesson, added, the second part of this advice is to ensure someone is actually checking these messages and responding.



It’s not just dispensers and wholesalers requesting consistent contacts. As Mike Mazur, Director, Trade Operations at Pfizer Inc., said, it can be a struggle for the pharmaceutical manufacturer to find the right person to engage with at the trading partner. It may not be such an issue with big trading partners or large regional chains, but he noted that when you work with mid-tier and smaller customers, it’s a challenge to identify who the individual is that (a) understands the DSCSA and (b) knows what’s ahead and what needs to happen in terms of the connections for data exchange, or can articulate how they’re planning to handle DSCSA—whether that’s via EPCIS files or not.

TRACEABILITY/SERIALIZATION Summer 2023 • Healthcare Packaging | 31
1. Email remains a common tool for trading partners to reach manufacturers regarding DSCSA data exchange.
2. Experts expressed concern that it can take weeks to find out whom to email in the event that their contact leaves the job.
3. Manufacturers should consider setting up a generic DSCSA email address to avoid putting all of their eggs in one employee’s basket.
Smart Packaging Illustrates Ongoing Industrial Revolution
 MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
Packaging World was in attendance for the first live AIPIA (Smart Packaging Association) World Congress since 2019. Given the longer-than-usual threeyear interval between installments of a typically annual event (thanks, pandemic), we expected to see some pronounced vaults forward in the technology. We weren’t disappointed.
While the world was mired in the pandemic, creators and suppliers in the active and intelligent packaging space kept pushing the envelope on technologies. This has kept many of them roughly following Moore’s Law, with quality and capabilities improving, and costs dropping. For instance, we’re seeing a shift away from the need for expensive, material-heavy batteries for RFID functions. Wiliot’s light, batteryfree Pixels, for instance, need only ambient energy, propagating in space through radio frequency waves, to power them. Also, the stalwart QR code, which was a basic building block technology of active and intelligent packaging three years ago, emerged in 2022 capable of carrying multiple loads at once in ways it couldn’t before, including consumer engagement, insight gathering for brands, traceability, security for all, and achieving that pleasing GTIN “beep” at a checkout counter.
But smart packaging adoption undoubtedly experienced pandemic-related delays. While the suppliers kept moving the ball down the field technologically, brands had to shift their focus away from R&D and toward more practical realities—like simply keeping production facilities procuring, producing, packaging, and meeting orders.
Brand packagers know that precious little packaging line space could be spared for R&D or new product testing; you were struggling just to keep up. While brand owners did the hard work of navigating the pandemic, many active and intelligent technologies that seemed to be just on the cusp of scalable adoption in 2019 were shelved for a few years.
But there were some silver linings in the interim that lead AIPIA delegates to believe active and intelligent packaging is primed to come roaring back. For one, many of the underlying supply chain
PACKAGE DESIGN 32 | Healthcare Packaging • Summer 2023
1. The implementation of smart packaging experienced pandemic-related delays.
2. In the AIPIA Packaging Challenge, tech suppliers pitched solutions to Haleon and GSK.
3. Suppliers need to be open about what a smart technology takes to implement.
TOP
THREE TAKEAWAYS
↑ Brand owner Haleon, which judged smart packaging pitches in the Active & Intelligent Packaging Challenge, recently teamed with Microsoft to launch an enhanced version of the software giant’s Seeing AI app to improve accessibility for people with limited sight.
inefficiencies that the pandemic revealed could have been far better managed with active and intelligent packaging. Having navigated their own pandemic supply chains, brands see this now more clearly than ever. Consider all the supply chain efficiencies afforded by digital printing, digital watermarks, digital twins, and sensors reading those marks. Brands now know they could be following individual packs through the chain, collecting actionable data, and responding to it in real time.
Another result of the pandemic is that consumers are (at least generally) better acquainted with QR codes in bars and restaurants when traditional menus were deemed unhygienic.
Apple first cleared the way by making QR codes readable to the native camera in iPhones in 2017. The pandemic accelerated adoption, and by the Super Bowl in 2022, we saw a QR code dancing enticingly on the TV screen in an unimaginably expensive 30-second commercial spot. No brand or messaging was revealed, just a 2D barcode. Did you scan it? Did one of your friends at the Super Bowl party?
Despite setbacks based on a pandemic pause in R&D, AIPIA Director Andrew Manly says, “We’re getting much more of a feel that there’s a lot more interest from the brand owners now. They’re much more focused on it. Digitization is becoming the key issue.”
Bottom line? Most of the technologies and connected packaging capabilities being discussed at AIPIA are inevitable, and our industry will be a major vector in implementing them. Speaker Anita Etrati of consultancy Accenture posed this question, “What does an industrial revolution feel like?” answering, “Just like this, like this moment, right now; it doesn’t feel momentous until we see it
in the rearview mirror.”
What remains to be seen is the relative speed and ease of scaling active, intelligent, connected packaging in the global economy.
Brands seek ‘pre-connected’ solution among disparate providers
A coordination challenge currently vexes many leading-edge suppliers seeking to get brands on board with their smart packaging tech. Most end-to-end active or intelligent packaging solutions require several layers of disparate technologies and capabilities, and they may involve a network of software providers, hardware suppliers, and Application Programming Interfaces (APIs) to accomplish. That means layers of suppliers working together—an ecosystem that isn’t yet fully formed. There is some consolidation occurring in the industry that may alleviate this disconnect over time—for instance the sale of EVRYTHNG to Digimarc—but it remains a sore spot for brands. This dynamic was evident in the Active & Intelligent Packaging Challenge. But first, more on the challenge itself.
Long a fixture of this event, the AIPIA Packaging Challenge gives technology suppliers the opportunity to pitch their smart packaging solutions to a major brand owner in a “lightning round” of tailored presentations, each only three minutes.
Challenging these suppliers was a duo of consumer health giants: brand owner Haleon, a new primarily OTC entity holding brands such as Sensodyne, Aquafresh, Advil, Theraflu, and Centrum, and brand owner GSK, from whom Haleon was spun off (demerged) in early 2022. At the show, Haleon’s Alex Orchard and GSK’s Anu Gadhiraju viewed product pitches from nearly a dozen active and

PACKAGE DESIGN Summer 2023 • Healthcare Packaging | 33
Tom Quinten (left) with anti-counterfeiting specialist Ennoventure receives recognition from Alex Orchard with home health brand owner Haleon. Orchard suggests smart packaging providers present entire ‘pre-connected’ ecosystems, rather than disparate, piecemeal parts of a total active or intelligent packaging solution.
intelligent solution providers to see if there were any fits between technology and brand.
It’s worth first mentioning that Haleon recently made a splash on the smart packaging front, teaming with Microsoft to launch an enhanced version of its Seeing AI app, which it says will advance inclusivity and improve accessibility. This new collaboration aims to help people who are blind, have low vision, or have difficulty reading packaging labels due to low literacy. On World Sight Day in October, Haleon launched its Always Read the Label campaign, hoping consumers will be able to access more detailed labeling information directly from Haleon by scanning the product barcode and using the app. In short, Haleon is no stranger to this space.
In advance of the event, the Haleon/GSK duo instructed technology providers that it was seeking a “trusted, circular, inclusive solution with an advanced UX (user experience).” More plainly, the request was for safety, security, sustainability, accessibility, and consumer engagement features. Ticking all five boxes would be a stretch for any single smart packaging solution provider, which brings into sharp relief the coordination problem between brand owners and the many disparate packaging tech companies that seek
to supply them.
Suppliers presented their cases, pitching digital temperature indicators to avoid temperature exclusions, individualized interactive experiences that could boost adherence, reusable packaging systems that are sustainable and engaging, QR-adorned tamper-evident labels for safety, and more. It came down to three winners: Ennoventure (anti-counterfeit solutions), Securikett (cloud platform for non-transferable, paper-based, tamper-evident labels), and AlmaScience (paper- and bio-based electronics), that will continue their conversations with Haleon.
Why did the new brand owner select these three companies? The winners had demonstrated the fullest end-to-end solutions, the most complete ecosystems, with clear paths to implementation.
Alex Orchard at Haleon had this to say at the awards soiree after the winners were announced: “Someone this morning brought up the notion of it taking a village [to get smart packaging adoption off the ground], and we talk a lot about ecosystems, but [in the real world], many of the pitches are still happening where everyone has individual solutions. I think it’s very, very powerful if you are pre-connecting as an ecosystem of solution providers and instead come into a pitch with that pre-connected vision and set of solutions that are more integrated, as far as facing the brand owners. Some brand owners do have resources internally, where they can start to sort that out, but many times they don’t. So, it’s much more powerful if we can see your value chain and know who the other players are that are needed to make an integrated proposal. And then for you to bring that pre-connected, integrated proposal to the table. That would be quite powerful in the future.”
+ Food waste prevention technologies (with potential life science applications), including temperature indicators, edible coatings, antimicrobial active packaging technologies, smart inks, and more
+ Smart packaging and sustainability, including how EU regulations are driving brands toward digital product passport (DPP) models where the packaging’s plastic resin information travels with each individual product through the supply chain and is easily readable by sensors at a material recovery facility (MRF) for sortation
+ The new standard launched in November called GS1 Digital Link URI format for retail, allowing a single 2D QR code accomplish the tasks of many
+ How augmented reality (AR) and consumer engagement drive consumer insight feedback loops

Another misstep smart packaging tech suppliers make in pitching to brand owners is getting ahead of themselves. They know their own products so well, and have so deeply thought about their potential, that they are prone to think several steps down the road, pitching an ultimate solution or “endgame” without describing what it might take to make it happen. Orchard invoked Maslow’s hierarchy of needs as a pyramid-shaped model to help tech suppliers approaching a pitch—you need to cover the basics, the structure, and the roadmap, before you promise a complete solution.
“You really need to help us understand what you believe are those foundation stones, the value-add, and [only] then the endgame and the aggregate effect of the topic you’re bringing to the table. We need to see the direction of travel, so we can make the endgame come alive, and then can we build the stepping stones towards that,” Orchard advised at the packaging challenge. “You must understand that with some of these topics, some of the brand-owning companies are very early stages. We need to understand how to learn to walk before we run, before we fly.”
PACKAGE DESIGN 34 | Healthcare Packaging • Summer 2023
This article is an abridged version of the original article—here we focused on healthcare-specific technologies. The full article delves into:
1 Phase Change Material



BIOLIFE SOLUTIONS
+ BioLife Solutions’ UltraguardTM non-toxic, non-flammable -70°C phase change material provides backup cooling in ULT freezers and a dry ice alternative for benchtop biologic material storage.


+ Its form factor is an ergonomic-friendly plastic bottle or “brick” that is “charged” or conditioned by freezing below -80°C.
2 Tablet Press

KORSCH AMERICA
+ Offering a dedicated single-layer capability, the Korsch America XL 4004 SFP tablet press includes an integrated electrical cabinet, torque drive, and fully sealed machine design.








+ With a maximum press speed of 120 rpm and an extended feeder length, it features a maximum output of over 300,000 tablets/hr.





3 Capsule Filler
MG AMERICA
+ Capable of producing up to 100,000 capsules/ hr, the MG America Essentia 100 capsule filler can handle powder or pellet dosing, with quality assurance provided through a statistical weight control system.
+ The filler is user-friendly, with intuitive recipe and parameters selection via a 15-in. human-machine interface (HMI).

NEW PRODUCTS Double Check with 0.01g Accuracy WeightCheQ 0-250 10x More Accurate Than Other Check Weighers High Performance Check Weigher Check weigh all your high value products for the tightest quality control Q 1.833.4PAXIOM | info@paxiom.com ¿ƝŪ PaxiomGroup } PaxiomXperience Learn more at paxiom.com NEW Our Automation Solutions
PMMI, the association for packaging and processing technologies and the producer of the PACK EXPO portfolio of trade shows, is celebrating our 90th anniversary with a focus on the resources we offer to ensure our industry’s strength for years to come.
















To nd out how you can bene t, go to pmmi.org.

4 Multifunctional Label
SCHREINER MEDIPHARM
+ Wrapping around an entire cap and syringe barrel, the Schreiner MediPharm Syringe-Closure-Wrap multifunctional label for prefilled syringes provides clear first-opening indication.

+ The label is sealed using an ultrasonic welding process without heat, making it suitable even for syringes containing sensitive active ingredients.
5 Label Inspection System

SOBEL IMAGING SYSTEMS



+ The Sobel Imaging Systems complete label inspection solution includes a contact image sensor that integrates lens, light, and image acquisition into a single small head.
+ It features easy-to-use software for setting up desired label inspections, image acquisition, a controller, and operator interface terminal—all in a compact design.
6 Blister Machine
ROTZINGER GROUP



+ Able to process up to 400 blisters/ min, the Rotzinger TLT blister machine has a clear separation of the production and drive areas.
+ It can be used for packaging a variety of pharmaceutical products, such as tablets, capsules, and dragées, using standard thermoforming and aluminum foil.

NEW PRODUCTS
AD INDEX ESS Technologies, Inc. www.esstechnologies.com 37 G&K-Vijuk Intern. Corp. www.guk-vijuk.com 30 Heat and Control, Inc. www.heatandcontrol.com 23 METTLER TOLEDO www.mt.com/pi.com 15 MRP Solutions www.mrpsolutions.com 29 Multivac, Inc. www.mulrivac.com/us 2 NJM Packaging www.NJMPackaging.com 9 Ossid www.ossid.com 21 Paxiom Automation, Inc. www.paxiom.com 35 PMMI ProSource www.prosource.org 12 PMMI 90th Anniversary www.pmmi.org 36 2023 PACK EXPO Las Vegas www.packexpolasvegas.com 39 ProSys Fill LLC www.prosysfill.com 25 Starview Packaging Machinery Inc. www.starviewpackaging.com 5 Visiott Track & Trace Solutions www.visiott.com 40 Weiler Engineering, Inc. www.asep-tech.com 19 38 | Healthcare Packaging • Summer 2023
to be wowed
Explore Every Possible Solution
In the life sciences industry, you’re challenged by multiple market forces, so attend the show that offers comprehensive solutions. PACK EXPO Las Vegas is where you can delve into innovations from specialized suppliers and discover surprising crossover solutions from other industries.

2,000 exhibitors 40+ vertical markets 100+ free show floor sessions
Endless networking opportunities
packexpolasvegas.com prepare
REGISTER NOW at the $30 Early Bird Rate!
Featuring








































































































































































































































































































 MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD
MATT REYNOLDS, CHIEF EDITOR, PACKAGING WORLD






































