Policy for the Management of Meticillin Resistant Staphylococcus aureus (MRSA)
(including screening for Podiatric Surgery)
Version: V10
Ratified by:

Infection Prevention Group
Date ratified: 21/05/2024
Job Title of author: Infection Prevention and Control
Reviewed by Committee or Expert Group Infection Prevention Group
Equality Impact Assessed by: Head of infection Prevention and Control
Related procedural documents
IPPOL3 Hand Hygiene Policy and Procedure
IPPOL9 Decontamination of Medical Equipment Policy and Procedure
MM30 Medicines Management Policy
IPPOL18 Management and Safety of Sharps Policy
IPPRO22 Ward cleaning guidelines, Community hospital wards
IPPOL20 Isolation Policy
IPPOL16 Community hospital wards outbreak policy
IPPOL19 MRSA Screening for Podiatric Day Case Surgery
IPPOL12 Healthcare Waste Policy
IPGUI02 Infection Prevention Guidelines
Review date: 21/05/2027

It is the responsibility of users to ensure that you are using the most up to date document template – i.e. obtained via the intranet.

In developing/reviewing this policy Provide Community has had regard to the principles of the NHS Constitution.
Version Control Sheet
Version Date

Author Status Comment
V2 July 2007 Director of Corporate Development & Governance Director of Infection Prevention and Control Approved New
V3 March 2009 Director of Corporate Development & Governance Director of Infection Prevention and Control Approved Reviewed
V4 March 11 Julia Shields Approved Reviewed
V4.1 February 12 Julia Shields Approved Inclusion of HII MRSA audit tool
V4.2 June 2012 Julia Shields Approved Change to screening regimen
V4.3 June 2013 Julia Shields Slight change to Appendix 1.
V4.4 August 2013 Steph Schuster –Quality & Safety Administrator No change to review date Updated in line with re-structure and Organisational name change
V5 January 2014 Julia Shields Review
V6 August 14 Julia Shields Approved Review and updated protocol for post infection review, removal of appendices and change to MRSA screening protocol in line with MEHT regimen
V7 September2016 Head of Infection Prevention Approved 2- Year review. Previously IPPOL1
V8 July 2017 Head of Infection Prevention Routine revision & new community provision
V9 May 2022 Head of Infection Prevention Approved Routine review and update combined policy withIPOL19MRSA screening for Podiatric Day case surgery.
V10 May 2024 Head of Infection Prevention Approved Change to MRSA pathway for community wards in line with MSE FT and MSE Community providers EPUT and NELFT.

1. Introduction
Meticillin Resistant Staphylococcus aureus (MRSA) is a strain of Staphylococcus aureus that has developed resistance to Flucloxacillin and other related antibiotics. MRSA infection can lead to serious complication resulting in additional mortality and morbidity for patients as well as contributing to increased healthcare costs.
The main source of MRSA transmission in healthcare is via the hands of healthcare workers and remains the principal route for patients acquiring MRSA By its very nature MRSA is an opportunistic bacterium which can colonise skin and spread easily in the right conditions MRSA colonization occurs when bacteria can reside on an individual but there are no signs of infection. MRSA can colonize patients’ skin and subsequently be dispersed into the environment when skin scales are shed. MRSA can survive in dry and dusty environments or on contaminated surfaces and equipment. Hands become contaminated when touching surfaces and through their interactions. This contamination becomes the main route of spread by healthcare workers hence the emphasis on effective hand decontamination at the point of care to minimise spread.
2. Purpose
The purpose of this policy is to minimise risk and maximise best practice in the prevention and management of MRSA.
Good infection control is essential to ensure that people who use our health and care services receive safe and effective care. If basic principles of infection control are followed, the risks to our patients of acquiring MRSA or any other Health Care Associated Infection (HCAI) will be effectively minimised in all types of community settings.
3. Definitions
Term Definition
MRSA

Meticillin resistant Staphylococcus aureus are bacteria identified as resistant to the Meticillin antibiotic group.
MSSA Meticillin sensitive Staphyloccus aureus are bacteria that can be treated with Meticillin antibiotics.
MRSA Colonisation
The presence of MRSA on mucosal and skin surfaces, without the presence of clinical manifestations of illness or infection.
DIPC Director of infection Prevention and Control
PIR Post infection review
Bacteraemia The presence of MRSA in the blood stream. This type of MRSA infection can lead to septicaemia and has a high mortality rate.
MRSA infection The presence of MRSA in a body site, where there is clinical evidence of infection
MRSA screening The process of obtaining microbiological swabs to identify the presence of MRSA
Decolonisation
The process of eradicating or reducing asymptomatic skin carriage of MRSA, through the use of topical anti-bacterial applications
Elective admission

A patient admitted following a planned period of waiting. This may be from a waiting list, from being “booked” (i.e. given a date at the time the decision to admit was made) or following any other plan for delayed admission
Direct contact is the main route of transmission of direct human to human contact The hands of healthcare workers have the potential to transfer MRSA from one person to another or from one body site to another if hand hygiene is not performed.
Indirect contact Transmission of MRSA can occur when there is no direct human to human contact but contact with environmental surfaces and equipment that are colonised with MRSA
Endogenous spread is when a person transfers organisms from one part of the body to another. This can be done by the patient themselves or by a member of staff who fails to carry out correct hand hygiene between different episodes of care on the same person.
MRSA care pathway
SICP’s
Terminal or deep clean
An MRSA care pathway is a prescriptive risk assessment template to follow to ensure that patients are screened for MRSA and treated until the screening results are known.
Standard Infection control Precautions are infection prevention practices used to avoid the transmission of infectious agents. Precautions are used with any patient, regardless of known or suspected infection status
A high level clean of the environment and equipment which is required once a patient with a known infection has vacated a room or bed space.
4. Duties
Specific Responsibilities
Infection Prevention and control is everyone’s responsibility and depends upon members of staff maintaining high standards of care This includes contract, temporary or visiting staff working for Provide who must familiarise themselves with this policy
Chief Executive
The Chief Executive has overall statutory responsibility for the prevention and control of infections including MRSA and ensuring that appropriate management systems for infection prevention & control are in place.
The Chief Executive delegates responsibility to the Director of Infection Prevention and Control (DIPC) who reports directly to the Board (Health &Social Care Act, 2008, 2015).
Director of Infection Prevention and Control (DIPC)
• Is responsible for overseeing the development and implementation of infection prevention and control best practice across the organisation inc. prevention and management of MRSA infections
The IPCT possesses expert knowledge of IPC and provides specialist support with the provision of IPC services throughout the organisation.
• The IPCT supports and aims to reduce the risks of Healthcare Acquired Infections
• The IPCT advises, informs and ensures appropriate control measures are put in place to stop or minimise the spread of infections.

Assistant Directors
• It is the responsibility of the Assistant Directors to ensure that all staff read this policy
• That there is consistent compliance with MRSA screening, reducing risks of HCAIs and completion of related IPC audits
• Are aware of exceptions found during audit and provide actions to resolve any issues
• Are aware of any cases of MRSA bacteraemia in their services and have overall responsibility for ensuring non-compliance issues identified during are rectified
Ward managers/clinical manager/community team managers
• Ensure that staff adhere to policy and there is consistent compliance with MRSA screening, reducing risks of HCAIs and the completion of related IPC audits
• Are aware of exceptions found during audit and provide actions to resolve any issues
• Participate in and contribute to infection reviews of MRSAs bacteraemia and any hospital acquired infections
• Ensures all samples sent to the laboratory are followed up in a timely manner and results obtained to enable treatment (if required)
• Ensures all MRSA screens are reported monthly to the infection prevention team (where appropriate)

Ward staff and all clinical staff
• Ward staff must ensure all patients are screened on admission within 2 hours and placed on MRSA care pathway. If delayed a datix must be completed and recorded.
• All clinical staff should ensure the patient (or their next of kin) is informed and given a full explanation of any positive result
• Make patients aware of the reasons for MRSA screening and decolonisation
• Inform patients of their screening result as soon as it is available
• Use patient MRSA leaflets to supplement information regarding MRSA colonisation/infection
• Be consistent in the use of protective equipment whilst caring for any patients with MRSA inc. in isolation (where appropriate)
• Advise visitors about the need for hand hygiene whilst visiting and the location of facilities
• Inform any receiving ward/unit/care home and patient transport services that the patient is colonised/infected with MRSA (ensuring patient confidentiality at all times)
• Staff are aware that MRSA colonisation should not restrict the transfer/discharge of a patient to another health care setting, their home or residential care
• Clean and disinfect shared items of equipment used in the delivery of patient care after each use; ideally utilise single use or single patient use equipment where possible
• All staff should be familiar with their specific responsibilities for cleaning and decontaminating the clinical environment and the equipment used in patient care
• All clinical staff should ensure that MRSA status has been clearly recorded in the patient record (HCAI SystmOne template on clinical tree)
• Clinical staff should be aware of their responsibility to report MRSA positive cases to the IP team by completing an HCAI alert form which should be e-mailed to: provide.infectionpreventionteam@nhs.net
Pre-Assessment Nursing Team (Podiatric Surgery)
Clinical Lead for Podiatric Surgery
• Strategic responsibility for ensuring systems are in place to facilitate staff awareness of the pre elective MRSA screening service and to ensure appropriate support is given to enable staff in delivering the required practice
Microbiologists (MSE)

• Responsible for reporting on all the screening samples and liaising with the preassessment team and Matron.
5. Consultation and Communication
This document was formulated after reviewing local and national policies and after discussion and review of the Infection Prevention team / IPC Group at Provide
6. Monitoring
This policy will be reviewed by the Infection Prevention Team every three years and updated with any national or local changes to practice.
7. Process
A risk assessment must be undertaken on all inpatients to minimise the risk and reduce transmission of MRSA in patients being transferred or admitted with colonisation or MRSA infection.
National and local evidence shows that certain people/patients will be at increased risk of acquiring MRSA, including (but not restricted to) those who:
• Have been MRSA positive in the past
• Have had an admission to a healthcare facility in the last six months
• All patients with a wound or a wound that has been present for longer than two weeks (excluding leg ulcers)
• Has a wound that is showing clinical signs of infection
• Live in residential settings i.e., nursing, or residential homes
• Have an in-dwelling medical device e.g, urinary catheter, intravenous device, enteral feed tube or tracheostomy tube etc.
• Have a chronic skin condition i.e., eczema or psoriasis
• People who inject drugs (PWID)
• People whose immune system is compromised by existing disease
• Healthcare workers
• Direct inter-hospital or ward transfers
Inpatient risk assessment.
• An MRSA care pathway must be completed on admission for patients admitted to inpatient area– (template on SystmOne)
• If patients are moved between hospitals an Inter-healthcare infection control transfer form should be completed (staff intranet/template on SystmOne)
• If patients are found to have MRSA infection or colonisation a Healthcare associated infection alert form must be completed – (staff intranet/ template on SystmOne
8. Inpatient admission screening and decolonisation for community ward

An MRSA care pathway must be completed for all patients admitted to a community hospital ward.
The MRSA care pathway must be started on day 1 of the patient’s admission:
• All patients admitted to the ward are risk assessed and put on the MRSA care pathway. If the patient is positive, they will continue the pathway for 5 days receiving decolonisation treatment. The pathway can be found on SystemOne (See SystemOne MRSA template)
• All patients are screened on admission within 2 hours
• This must be evidenced in the variance section of the MRSA care pathway and recorded on SystemOne.
• All patients admitted to the ward from the community should be checked on SystmOne to identify if they a record of a previous MRSA positive result
MRSA Screen
MRSA screen consists of the following:
• Nose (both anterior nares)
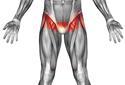
• Perineum / groin
• Wounds, indwelling devices and if cough present obtain sputum
Microbiology request forms should state:
MRSA admission screen or pre admission if elective screen.
Please take swabs from:
Nose – use one swab (blue-topped swab) to screen both nostrils (nose). Moisten the swab in the transport medium before sampling both inner nostrils. Place swab (0.5-1cm) into the front of each nostril and rotate to collect sample. There is no need to introduce the swab further into the nose. 1 swab for both nostrils.
Perineum or groin – use one swab for both left and right groin areas (See Appendix 1 How to take an MRSA screen)
MRSA screen swabs from MSET will contain one tube, place both swabs inro tube. Ensure the buds of the swabs are immersed in the transport medium.
N.B In some incidences dry swabs without medium maybe required for screens that are sent to services outside Mid and South Essex such as East Suffolk North Essex Foundation Trust (ESNEFT). To use dry swabs, moisten swab with sterile saline or sterile water. one swab for both nostrils and one swab for left and right groin area
If the patient has the following, take further swabs (1 from each site) to complete the full screen:
• Invasive device (i.e., PEG, peripheral line): swab
• Skin lesions and wounds: swab

• Catheterised patient: CSU sample
• Productive cough: Sputum sample
Screening results should be back within 24/48hrs with 7-day microbiology service MRSA results can be checked using pathology system ‘ICE’. In the event MRSA screens have been sent to ESNEFT for patients in Northeast Essex staff must contact the microbiology department to gain results.
Following a positive MRSA result (community hospital)
• Record result on MRSA pathway
• Inform patient of their positive result. Explain status to patient and give patient an MRSA leaflet. Document /record this action on SystemOne
• If possible, isolate patient inside a single room within 2 hours and refer to isolation policy
• If, unable to isolate please complete risk assessment and document in variance section of MRSA pathway
• Complete HCAI alert form: email to provide.infectionpreventionteam@nhs.net
• Continue 5-day decolonisation regimen: record this on the MRSA care pathway
• Ask the ward GP to review any current antibiotics the patient is taking considering the MRSA result. GPs can contact MSEFT microbiologist for advice if required
• Inform the cleaning team, place signage & isolation precautions on door as per isolation policy and risk assessment.
A negative MRSA results
• If result is negative - Inform patient of their negative result and document action.
MRSA 5- day decolonisation regimen:
• Mupirocin (Bactroban) 2% nasal ointment applied to both anterior nares three times daily for 5 days (apply a match head size amount each time)
• Daily washes with antibacterial body wash (Octenisan) for 5 days. If excessive skin drying occurs, consider Oilatum Plus (not ordinary Oilatum) as a bath / shower additive. Ensure Octenisan is clearly labelled with patient’s name. This is a single patient use product
• Hair wash with antibacterial body wash (Octenisan) on day 2 and day 4 of the treatment regimen
• For bed-bound / decreased-mobility patients, Octenisan wash mitts can be used in place of the body wash. No rinsing off is required. Hair can be washed on day 2 and 4 using the Octenisan wash cap.
• Encourage daily change of flannel, towel, personal clothing and bedding.
• After day 5 of treatment patients will return to normal soap for washing. A further MRSA screen is required on day 8.
• If the MRSA screen result is NEGATIVE no further treatment is required, and isolation precautions can be discontinued.
• Where the day 8 MRSA screen result is POSITIVE the IPCT must be contacted to discuss further treatment. MRSA
Antibacterial (Octenisan) body wash
Antibacterial (Octenisan) hair wash
Mupirocin (Bactroban ointment 2%)

Treatment of Mupirocin-resistant strains
Where Mupirocin resistant strains are identified treatment will be advised by the reporting laboratory or Consultant Microbiologist or the Infection Prevention team. Alternative antimicrobials may be advised such as Naseptin cream or Polyfax ointment. Both require application to anterior nares. Naseptin contains peanut oil so must not be used on patients with a nut allergy.
Post treatment screening
No further screening is required once MRSA regimen is completed for community hospital wards, unless requested by the microbiologist, medical doctor
The patient may become recolonised over time especially if they have a chronic wound/skin condition. Previously colonised patients are always considered a risk for MRSA carriage on subsequent admissions to hospital.
Consent
The procedures should be discussed with the patient, so they are aware of the treatment process and can give informed consent
9. Management of MRSA: Community Patients
MRSA in community patient (when to suspect & swab)

Patients in their own homes do not usually require MRSA swabbing as part of routine admission onto a new caseload. However, some patients may be discharged back into the community still undergoing treatment/decolonisation for MRSA. This should be continued as per discharge instructions/transfer letter. This may vary in accordance with the recommendations from the hospital i.e., Addenbrookes, MEHT and ESNEFT. If you are unclear contact the ward to confirm instructions.
It is essential to check the MRSA history of all patients and record on SystmOne.
It is important to establish if the patient has risk factors for / previous history of MRSA colonisation and swab for MRSA if appropriate
Management of Community MRSA positive patient
• If an MRSA positive result is received for a patient with MRSA infection/colonisation contact the GP and inform the GP to ensure that they are aware of the result. GP’s will follow their local MRSA guidelines
• Report on SystmOne HCAI template and email electronic HCAI alert form to provide.infectionpreventionteam@nhs.net
• Monitor for signs of sepsis, cellulitis, pneumonia, urinary tract infection, wound infection
• Ensure MRSA status is fully explained to patient and a MRSA information leaflet is given to patient/family/carers
• Reassure the person that MRSA infection/or colonisation does not present a risk to healthy people in the community
• Advise the person (and/or their carer) to keep wounds, cuts, and abrasions covered until they are healed and dry
• Advise the person (and/or their carer) to maintain good hygiene with regular hand –washing/washing and washing of clothing/ bed linen
• Advise the person not to share personal items where possible in order to prevent possible contamination e.g. towels, clothing, bedding
• If possible, try to see people who are known to be colonised with MRSA at the end of your clinic/surgery list to prevent possible transmission to others
• Observe good hand hygiene as per hand hygiene policy
Post treatment screening
No further screening is required once MRSA regimen is completed.
10. Waste
Waste generated by MRSA patient in their own home

Where a patient in their own home has been diagnosed with MRSA and is not being cared for by a healthcare worker (HCW), their waste is not classed as infectious waste.
However, when a patient is receiving care from Provide staff, a waste risk assessment must be completed to accurately assess whether the waste generated is infectious The risk assessment template is on SystmOne and must be completed for all patients at the initial assessment.
Risk assessment should be based on professional assessment inc. clinical signs and symptoms, and any prior knowledge of the patient. For example, if a wound assessment indicates that the wound is infected, all associated contaminated dressings should be classified as infectious waste and discarded into a clinical waste bag (orange) and arrangements made for collection as per local policy.
11. Podiatric Surgery
Some elective surgical procedures have been identified as procedures that require patients to be screened for MRSA prior to procedure. Podiatric surgery falls into this group
Screening
Screening is required in the 12 weeks prior to admission. If the date of admission falls outside this timeframe the patient requires further screening
Results
Details of the date and time of the screen should be recorded in the patient’s notes/care pathway by the person performing the screen. A result will be available in 3 working days
Results are imported directly in to SystmOne by the lab and should be viewed and filed in a timely manner. The extension number to chase or query results is 5019.
Negative MRSA result
The surgery can go ahead as planned if the patient has a negative screen from the preceding 12 weeks prior to the date of admission. The result must be recorded in the patients care pathway. If surgery is delayed beyond twelve weeks, screening should be repeated
Positive MRSA result
Pre assessment clinic/ nurses
The team will inform the patient that the result is positive either by phone or letter. Any concerns that the patient may have will be addressed. The GP will be informed and advised of decolonisation required for the patient.
The patient will be asked to contact their GP for the 5-day decolonisation protocol. If antibiotics are required for prophylaxis, these must include cover for MRSA (i.e. in the hour before the first operative incision.)
• On admission to Day stay unit Surgeon and Anaesthetist must be informed

• Podiatrist with independent prescribing is responsible for ensuring correct use of antibiotics on induction, depending on type of surgery
Where a patient on list to come in does not have MRSA screen result
• If the screen has not been processed on time / or cannot be found, the operation date may require to be postponed and the patient informed.
• The patient should be directed to pre assessment for MRSA pre-screening Consent
• The need for testing should be explained to all patients and verbal consent obtained.
• Verbal consent must be obtained for screening.
• If a patient initially refuses to be screened, they should be informed that it is procedure and be given a patient information leaflet. If they continue to refuse to be screened the pre assessment nurse will inform the Clinical Lead for the service. This must be documented on the patient’s consent form. This patient will be risk assessed and will be put at the end of the operating list and treated as a patient who is MRSA positive.
• If a patient lacks the mental capacity to consent to screening refer to the Mental Health Capacity Act 2005 and inform Clinical Lead for the service and Safeguarding Lead.
12.References
Coia, J., Duckworth, G., Edwards, D., Farrington, M., Fry, C., Humphreys, H., Mallaghan,C.andTucker,D.,2022. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities
Implementation of modified admission MRSA admission screening guidance for NHS 2014 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/345 144/Implementation_of_modified_admission_MRSA_screening_guidance_for_N HS.pdf
DepartmentofHealthImplementationofmodifiedadmissionMRSAScreening GuidanceforNHS(2014).DepartmentofHealthexpertadvisorycommitteeon antimicrobialresistanceandhealthcareassociatedinfection(ARHCAI)
The Health and Social Care Act 2008: Code of Practice for the NHS on the prevention and control of healthcare associated infections and related guidance DH
NICE Clinical Guideline 125 (2019) Surgical site infections: prevention and treatment
Guidanceonthereportingandmonitoringarrangementandpostinfectionreview processforMRSAbloodstreaminfectionsfromApril2014(version2). https://www.england.nhs.uk/wp-content/uploads/2014/04/mrsa-pir-guidapril14.pdf

Appendix 1 How to take an MRSA Screen
Sites

Nose & Groin