
7 minute read
Synthesis and Preliminary Characterization of Histidine-Metal Complexes
SYNTHESIS AND PRELIMINAR CHARACTERIZATION OF HISTIDINE-METAL COMPLEXES Cayla Guillory, Dr. Gina Chiarella, and Dr. Huajun Fan Department of Chemistry Prairie View A&M University

Abstract
In this research, Synthesis Characterization and Computation Modeling of Metal Histidine Complexes, we are to prepare transitional metals complexes using the Amino Acid L- Histidine. Only focusing on 4/38 transition metals and that is Cobalt (ll), Chromium (lll), Copper (l), and Manganese (ll). It is the preparation of transition metal complexes with especial interest in compounds with potential catalytic activity and resemblance with biological molecular structures.
Introduction
Amino acids binding transition metals are very common in biological systems; they are present in enzymes playing powerful role in metabolic processes, detoxification and preservation of different living entities; however the nature and structure of the Metal-amino acid or metal peptide bond is not totally clear, as well as, the mechanism that allow their formation; this information would be crucial in the prevention of some diseases and in the preparation of new medicines. This project will try to contribute to the knowledge of the L-histidinetransition metal connection, and their chemical and physical properties for posterior uses.
Goals
My research has two main objectives: •To synthesize the metal complexes of the Amino Acid L-Histidine and the metals Chromium (lll), Copper (l), Manganese (ll),and Cobalt (ll). •To perform the instrument analysis to characterize those examples and make the computation calculations of the structure
Experimental procedure
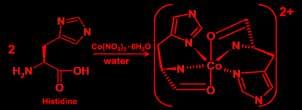
Preparation of Cobalt(II) Histidine Complex: Aqueous solution of L-histidine is mixed with aqueous solution of Cobalt(II) nitrate in a 100-mL beaker in mole ratio of 2:1, the mixture was stirred for half an hour until the solution change from pink-reddish to a very dark orange, latter the product was evaporated and analyzed.
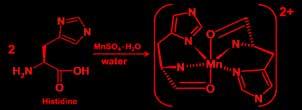
Preparation of Manganese (ll) Histidine: Aqueous solution of L-histidine is mixed with aqueous solution of Manganese(II) sulfate in a 100-mL beaker in mole ratio of 2:1, the mixture was stirred for half an hour until the appearance change from cloudy withe to clear colorless, latter the product was evaporated and analyzed.
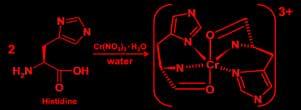
Preparation of Chromium (lll) Histidine: Aqueous solution of L-histidine is mixed with aqueous solution of Chromium(III) nitrate in a 100-mL beaker in mole ratio of 2:1, the mixture was stirred for half an hour until the solution change from dark green to a creamy light green, latter the product was evaporated and analyzed.
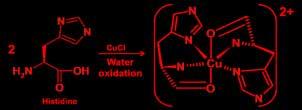
Preparation of Copper (ll) Histidine: Aqueous solution of L-histidine is mixed with aqueous solution of Copper(II) nitrate in a 100-mL beaker in mole ratio of 2:1, the mixture was stirred for half an hour until the solution change from bright green to a dark blue color, latter the product was evaporated and analyzed Synthesis of Histidine –Chromium (III) complex
Synthesis of Histidine –Cobalt (II) complex
Synthesis of Histidine –Manganese (II) complex
Synthesis of Histidine –Copper (II) complex
Results
A preliminary analysis of the products was executed by infrared and UV-visible spectroscopy, the respective spectra were studied, indicating that products and reactants presented different peaks, which indicate that the products are different compounds; additional analysis will be required to determine the structure of those compounds.

Infrared spectra in solid state of Histidine and the product Histidine-Metal complexes
UV-vis spectra in aqueous solution of Histidine and the product Histidine-Metal complexes

Summary
The metals Copper (ll) Histidine, Chromium (lll) Histidine, Manganese(ll) Histidine, and Cobalt (ll) Histidine complexes has been synthesized.
References
•Richard J. Sundberg and R. Bruce Martin. Chemical Reviews, 1974, 74, 471 - 517 •Georgia C. Boles, Rebecca A. Coates, Giel Berden, Jos Oomens, and P. B. Armentrout. J. Phys. Chem. B, 2016, 120, 12486−12500 •Lei Zhou, Shenhui Li, Yongchao Su, Xianfeng Yi, Anmin Zheng, and Feng Deng. J. Phys. Chem. B, 2013, 117, 8954−8965
•Acknowledgements R&I’s Office of Undergraduate Research (OUR and Chemistry Department,, Prairie View A&M University.
Introduction
Amino acids binding transition metals are prevalent in biological systems; they are present in enzymes playing a dominant role in metabolic processes, detoxification, and preservation of different living entities; however, the nature and structure of the Metal-amino acid or metal peptide bond is not precise, as well as, the mechanism that allows their formation; this information would be crucial in the prevention of some diseases and the preparation of new medicines. This project will try to contribute to the knowledge of the L-histidine-transition metal connection, and their chemical and physical properties for posterior uses. The objective of this research project is to synthesize, isolate, purify and characterize transition metal complexes of L-histidine with Nickle (II), Cobalt (II), Chromium (II), Copper (II), Iron(II), and Manganese(II) cations. The primary purpose of our research group is to prepare compounds with structure and properties analogous to biological catalysts, known mostly as enzymes. Histidine is an amino acid frequently present in those biochemical compounds. Due to the presence of three nitrogen and one oxygen donor atoms in its structure, L-histidine forms a very stable complex with those metal-ions. The nature of the interaction of L- Histidine – Metal is still inconclusive, and most of the analytical data and properties of these complexes are incomplete in the scientific literature. An essential goal in this research is to determine the molecular structure of and the redox activity of the metal derivatives of this amino acid. In this project it has been achieved the synthesized of the transition metal complexes with Histidine in two different solvents, water, and methanol; the UV-visible and infrared spectroscopic studies have been obtained, the proton and carbon NMR spectra were recorded when it was possible to do, all these results have been contrasted with the data previously obtained from the pure L-histidine. Electrochemical studies have also been collected, and computational calculations have been performed.
Materials and Methods
Preparation of Cobalt(II) Histidine Complex: An aqueous solution of L-histidine is mixed with aqueous solution of Cobalt (II) nitrate in a 100-mL beaker in a mole ratio of 2:1, and the mixture was stirred for half an hour until the solution change from pink-reddish to very dark orange, latter the product was evaporated and analyzed.
Preparation of Manganese (ll) Histidine: An aqueous solution of L-histidine is mixed with aqueous solution of Manganese(II) sulfate in a 100-mL beaker in a mole ratio of 2:1, and the mixture was stirred for half an hour until the appearance change from cloudy withe to clear colorless, latter the product was evaporated and analyzed.
Preparation of Chromium (lll) Histidine: An aqueous solution of L-histidine is mixed with aqueous solution of chromium(III) nitrate in a 100-mL beaker in a mole ratio of 2:1; the mixture was stirred for half an hour until the solution change from dark green to creamy light green, latter the product was evaporated and analyzed.
Preparation of Copper (ll) Histidine: An aqueous solution of L-histidine is mixed with aqueous solution of Copper(II) nitrate in a 100-mL beaker in a mole ratio of 2:1; the mixture was stirred for half an hour until the solution change from bright green to a dark blue color, latter the product was evaporated and analyzed
Results and Discussion
A preliminary analysis of the products was performed by infrared and UV-visible spectroscopy; the respective spectra were studied, indicating that products and reactants presented different peaks, which indicated that the products are different compounds; additional analysis will be required to determine the structure of those compounds.
Conclusion(s) or Summary
A preliminary analysis of the products was executed by infrared and UV-visible spectroscopy, and the respective spectra were studied, indicating that products and reactants presented different peaks, which indicate that the products are different compounds; additional analysis will be required to determine the structure of those compounds. The metals Copper (ll) Histidine, Chromium (lll) Histidine, Manganese(ll) Histidine, and Cobalt (ll) Histidine complexes have been synthesized.
References
Richard J. Sundberg and R. Bruce Martin. Chemical Reviews, 1974, 74, 471- 517 Georgia C. Boles, Rebecca A. Coates, Giel Berden, Jos Oomens, and P. B. Armentrout. J. Phys. Chem. B, 2016, 120, 12486-12500 Lei Zhou, Shenhui Li, Yongchao Su, Xianfeng Yi, Anmin Zheng, and Feng Deng. J. Phys. Chem. B, 2013, 117, 8954- 8965
Cayla J. Guillory is a senior, majoring in Chemical Engineering. Dr. Gina Chiarella is an Assistant Professor in Chemistry with research interst in medicinal bio-chemistry










