

www.CFRjournal.com
Intended for
Promotional information developed and funded by AstraZeneca

FORXIGA (dapagliflozin) is indicated in adults for the treatment of: chronic kidney disease (CKD); symptomatic chronic heart failure (HF); and insufficiently controlled type 2 diabetes (T2D).1
THERE’S A NEW REASON TO RETHINK HOW YOU TREAT HEART FAILURE
Dapagliflozin is the only SGLT2i proven to reduce the risk of CV death across the LVEF range, on top of SoC, vs placebo.2*
Dapagliflozin is now the first SGLT2i recommended by NICE for the treatment of symptomatic chronic HF with LVEF >40%.3
Click Here for GB Prescribing Information
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App store. Adverse events should also be reported to AstraZeneca by visiting https://contactazmedical.astrazeneca.com or by calling 0800 783 0033.
*A patient-level pooled meta-analysis of two trials testing dapagliflozin in participants with heart failure and different ranges of left ventricular ejection fraction (≤40% and >40%). The pre-specified endpoints were: death from cardiovascular causes; death from any cause; total hospital admissions for heart failure; and the composite of death from cardiovascular causes, myocardial infarction or stroke (major adverse cardiovascular events (MACEs)). A total of 11,007 participants with a mean ejection fraction of 44% (s.d. 14%) were included. Dapagliflozin reduced the risk of death from cardiovascular causes (hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.76–0.97; P = 0.01), death from any cause (HR 0.90, 95% CI 0.82–0.99;P = 0.03), total hospital admissions for heart failure (rate ratio 0.71, 95% CI 0.65–0.78;P < 0.001) and MACEs (HR 0.90, 95% CI 0.81–1.00;P = 0.045).2
Abbreviations:
CI, confidence interval; CKD, chronic kidney disease; CV, Cardiovascular; GB, great britain; HF, heart failure; HR, hazard ratio; MACEs, major adverse cardiovascular events; LVEF, left ventricular ejection fraction; NICE, National Institute for Health and Care Excellence; SD, standard deviation; SoC, Standard of Care, SGLT2i, sodium-glucose cotransporter 2 inhibitor; T2D, type 2 diabetes.
References:
1. Forxiga (dapagliflozin) Summary of Product Characteristics.
2. Jhund PS et al. Nat Med. 2022;28,1956-64.
3. ©NICE TA902 [2023] Dapagliflozin for treating chronic heart failure with preserved or mildly reduced ejection fraction. Available from https://www.nice.org.uk/guidance/ta902/resources/dapagliflozin-for-treating-chronic-heart-failure-with-preserved-or-mildly-reducedejection-fraction-pdf-82615423312069. Accessed July 2023. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
GB
Discover dapagliflozin. Visit forxiga.co.uk ©2023 AstraZeneca. All rights reserved. GB-44403 | July 2023
Healthcare Professionals
FORXIGA (dapagliflozin) 10 mg is indicated in adults for the treatment of: chronic kidney disease (CKD); insufficiently controlled type 2 diabetes (T2D); and symptomatic chronic heart failure (HF).1
FORXIGA INITIATION CHECKLIST
Checklist for Healthcare Professionals in Great Britain only. Not to be used in Northern Ireland.
❑ This checklist is intended to aid patient consultation following the decision to prescribe Forxiga for your CKD, T2D and symptomatic chronic HF patients.
DOSING
❑� Remind patients that Forxiga 10 mg can be taken orally once daily at any time of day with or without food, and that tablets are to be swallowed whole.1


FOLLOW-UP
❑� Blood pressure, urea and electrolytes will be monitored as needed by the individual requirements of the patient and their coexisting medications1,2
If patient has a history of diabetic ketoacidosis (DKA), consider discussion with diabetes team prior to initiation
Symptoms of diabetic ketoacidosis (DKA)1,2
Dapagliflozin initiation checklist
❑� DKA is a rare (≥ 1/10,000 to < 1/1,000) but serious side effect. In a number of DKA cases reported with sodium-glucose cotransporter-2 (SLGT2) inhibitors, presentation was atypical with only moderately increased blood glucose values (below 14 mmol/L)
SGLT2i dosing considerations by eGFR reference table
PATIENT COUNSELLING FOR POTENTIAL SIDE EFFECTS
Genital/perineal hygiene
❑� With Forxiga, fungal genital infections (thrush) are common (≥1/100 to <1/10) in patients with T2D1
❑� Most cases are mild to moderate and can be managed with a short course of antifungal cream or oral treatment1
❑� Those with prior history are more likely to have recurring infection1
❑ Counsel the patient on risk/symptoms and encourage personal hygiene
❑� Awareness is important. Symptoms include:
• Feeling and/or being sick
• Drowsiness/ confusion
• Sweet-smelling breath (like pear drops or acetone)
• Thirst
This checklist has been developed to assist in communicating key initiation information to patients, following the decision to prescribe dapagliflozin.
• Stomach pain
• Rapid weight loss
• Deep sighing breaths
This resource provides information on dosing across the SGLT2i class according to eGFR, when treating T2D, CKD, and HF.
❑� Higher risk if dehydrated, fasting, have an infection or have alcohol dependency
❑� DKA should be treated in hospital as soon as possible
Your patients prescribed dapagliflozin
Scan this QR code to visit Forxiga.co/uk/resources for more useful resources for you and your patients prescribed Forxiga. YOUR
YOUR GUIDE TO FORXIGA® (DAPAGLIFLOZIN)
This checklist was produced and fully funded by AstraZeneca. This checklist should not be forwarded to non-consenting HCPs. Prescribing information can be found on the next page.
©2022 AstraZeneca GB-41011 December 2022
❑� Due to the risk of DKA, advise patients with T2D to follow ‘sick day rules’ if they have an acute dehydrating illness, infection or are undergoing surgery Temporarily withhold Forxiga for this period then RESTART when patient feeling better (eating and drinking normally)
❑� Routine ketone monitoring is not required (monitoring is recommended in patients undergoing major surgical procedures or acute serious medical illnesses)
IN HEART FAILURE FOR PATIENTS WITH TYPE 2 DIABETES
WITHOUT TYPE 2 DIABETES
GUIDE TO FORXIGA® (DAPAGLIFLOZIN) IN HEART FAILURE FOR PATIENTS
Dapagliflozin initiation resources for you
HF with T2D Patient Booklet
HF without T2D Patient Booklet
Click here to download Click here to download Click here to download Click here to download
Editor-in-Chief
Andrew JS Coats
Monash University, Melbourne, Australia, and University of Warwick, Coventry, UK
Deputy Editor-in-Chief
Giuseppe Rosano
IRCCS San Raffaele, Rome, Italy, and St George’s Hospitals NHS Trust, University of London, UK
Associate Editor
Cristiana Vitale
Department of Medical Sciences, IRCCS San Raffaele, Rome, Italy
Section Editors
Case Reports and Clinical Cases
Josip A Borovac
University of Split, Split, Croatia
Advanced Heart Failure
Ersilia M DeFilippis
Columbia University Irving Medical Center, New York, NY, US
Acute Heart Failure
Ovidiu Chioncel University of Medicine Carol Davila, Bucharest, Romania
William T Abraham
Ohio State University College of Medicine, Columbus, OH, US
Ali Ahmed
Washington DC VA Medical Center, Washington DC, US
Fozia Ahmed
Manchester University NHS Foundation Trust, Manchester, UK
Amod Amritphale
University of South Alabama, Mobile, AL, US
John J Atherton
Royal Brisbane and Women’s Hospital, Brisbane Australia
Feras Bader
Heart and Vascular Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates
Michael Böhm
University of Saarland, Homburg, Germany
Eugene Braunwald Harvard Medical School, Boston, MA, US
Javed Butler
University of Mississippi Medical Center, Jackson, MS, US
Vijay Chopra
Heart Failure Programme and Research, Max Super Specialty Hospital, New Delhi, India
Alain Cohen-Solal
Université de Paris, Lariboisière Hospital, Paris, France
Kevin Damman
University of Groningen, University Medical Center
Groningen, Groningen, the Netherlands
Carmine De Pasquale
Flinders University, Adelaide, Australia
Frank Edelmann
Charité University Medicine, Berlin, Germany
Giuseppe Galati
San Raffaele Hospital and Scientific Institute (IRCCS), Milan, Italy
Emerging Technologies
Sean Lal
Royal Prince Alfred Hospital and University of Sydney, Sydney, Australia
Cardiogenic Shock
Finn Gustafsson University of Copenhagen, Copenhagen, Denmark
Editorial Board
Julia Grapsa
Digital Health
Maurizio Volterrani
IRCCS San Raffaele Pisana, Rome, Italy
Critical Care Cardiology
Aniket S Rali
Vanderbilt University, Nashville, TN, US
St Bartholomew’s Hospital and King’s College
London, London, UK
David L Hare
University of Melbourne, Melbourne, Australia
Sivadasanpillai Harikrishnan
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India
Loreena Hill
School of Nursing and Midwifery, Queen’s University Belfast, Belfast, Northern Ireland
Tiny Jaarsma
Linköping University, Linköping, Sweden
Ewa Jankowska
Centre for Heart Diseases, Faculty of Health Sciences, Wrocław Medical University, Wrocław, Poland
Prathap Kanagala
Liverpool University Hospital NHS Foundation Trust, University of Liverpool and Liverpool Centre for Cardiovascular Science Liverpool, UK
Dipak Kotecha
University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
Bernard Kwok Farrer Park Hospital, Singapore
Ekaterini Lambrinou
Cyprus University of Technology, Limassol, Cyprus
Lars H Lund
Karolinska Insitutet and Karolinska University Hospital, Stockholm, Sweden
Alexander Lyon
Royal Brompton Hospital, London, UK
Francesco Maisano University Hospital, Zurich, Switzerland
Mamas A Mamas University of Keele, Keele, Staffordshire, UK
Theresa A McDonagh King’s College Hospital, London, UK
Kenneth McDonald
St Vincent’s University Hospital, Dublin, Ireland
Ana Olga Mocumbi
Mozambique Institute for Health Education and Research, Maputo, Mozambique
Ileana L Piña
Wayne State University, Detroit, MI, US
Kian Keong Poh
National University Heart Center, Singapore
Amina Rakisheva
Scientific Research Institute of Cardiology and Internal Medicine, Almaty, Kazakhstan
Clara Saldarriaga
Cardiovascular Clinic Santa Maria, University of Antioquia, Medellín, Colombia
Simon Stewart
Torrens University, Adelaide, Australia
David Thompson
Queen’s University Belfast, Belfast, Northern Ireland, UK
Izabella Uchmanowicz
Wrocław Medical University, Wrocław, Poland
Harriette Van Spall
McMaster University, Hamilton, Canada
Raymond Wong
National University Heart Centre, National University Hospital, Singapore
Yuhui Zhang
Fuwai Hospital and National Center for Cardiovascular Diseases, Beijing, China
Shelley Zieroth
Max Rady College of Medicine, University of Manitoba, Winnipeg, Canada
Robert Zuckermann
Rambam Medical Health Center, Haifa, Israel
Volume 9 • 2023 © RADCLIFFE GROUP 2023 www.CFRjournal.com
Editorial
Publisher Ola Wisniewska
Production Editors Aashni Shah, Bettina Vine | Senior Graphic Designer Lewis Allen
Peer Review Editor Nicola Parsons | Editorial Coordinator Liam McKnight
Contact ola.wisniewska@radcliffe-group.com
Marketing
Marketing Manager Emily King
Marketing Executive Calum Barlow
Radcliffe Medical Media
Managing Director Jonathan McKenna
Agency Sales Director Gary Swanston
Senior Account Managers William Cadden, Brad Wilson
Contact jonathan.mckenna@radcliffe-group.com
Radcliffe Medical Education
Managing Director Rob Barclay
Sales Director Carrie Barclay
Contact carrie.barclay@radcliffe-group.com
Leadership
Chief Executive Officer David Ramsey
Chief Operations Officer Liam O’Neill
Published by Radcliffe Cardiology.
All information obtained by Radcliffe Cardiology and each of the contributors from various sources is as current and accurate as possible. However, due to human or mechanical errors, Radcliffe Cardiology and the contributors cannot guarantee the accuracy, adequacy or completeness of any information, and cannot be held responsible for any errors or omissions, or for the results obtained from the use thereof. Published content is for information purposes only and is not a substitute for professional medical advice. Where views and opinions are expressed, they are those of the author(s) and do not necessarily reflect or represent the views and opinions of Radcliffe Cardiology. Radcliffe Cardiology, Unit F, First Floor, Bourne End Business Park, Cores End Road, Bourne End, Buckinghamshire SL8 5AS, UK © 2023 All rights reserved • ISSN: 2057-7540 • eISSN: 2057-7559
www.CFRjournal.com
© RADCLIFFE GROUP 2023
www.CFRjournal.com
Aims and Scope
• Cardiac Failure Review is an international, English language, peer-reviewed, open access journal that publishes articles continuously on www.CFRjournal.com
• Cardiac Failure Review aims to assist time-pressured physicians to stay abreast of key advances and opinions in heart failure.
• Cardiac Failure Review publishes balanced and comprehensive articles written by leading authorities.
• Cardiac Failure Review provides comprehensive updates on a range of salient issues to support physicians in continuously developing their knowledge and effectiveness in day-to-day clinical practice.
Structure and Format
• Cardiac Failure Review publishes review articles, original research, expert opinion pieces, guest editorials and letters to the editor.
• The structure and degree of coverage assigned to each category of the journal is the decision of the Editor-in-Chief, Deputy Editor and Section Editors, with the support of the Editorial Board.
Abstracting and Indexing
Cardiac Failure Review is abstracted, indexed and listed in PubMed, Crossref, Scopus, Google Scholar and Directory of Open Access Journals. All articles are published in full on PubMed Central a month after publication. Radcliffe Group is an STM member publisher.
Editorial Expertise
Cardiac Failure Review is supported by various levels of expertise:
• Overall direction from an Editor-in-Chief, supported by the Deputy Editor, Section Editors and Editorial Board, comprising leading authorities from a variety of related disciplines.
• Invited contributors who are recognised authorities in their fields.
• Peer review – conducted by experts appointed for their experience and knowledge of a specific topic.
• An experienced team of editors and technical editors.
Submissions and Instructions to Authors
• Contributors are identified by the Editor-in-Chief, with the support of the Deputy Editor, Section Editors, Editorial Board and Publisher.
• Following acceptance of an invitation, the author(s) and Publisher, in conjunction with the Editor-in-Chief, Deputy Editor and Section Editors, formalise the working title and scope of the article.
• Instructions for authors and additional submission details are at www.radcliffecardiology.com/guideline/author-guidelines
• Leading authorities wishing to discuss potential submissions should contact the Publisher, Ola Wisniewska, ola.wisniewska@radcliffe-group.com.
• Articles may be submitted directly at www.editorialmanager.com/cfr
Ethics and Conflicts of Interest
The journal follows guidance from the International Committee of Medical Journal Editors and the Committee on Publication Ethics. We expect all parties involved in the journal’s publication to follow these guidelines. All authors must declare any conflicts of interest.
Open Access, Copyright and Permissions
Articles published in this journal are gold open access, which means the version of record is freely available, immediately upon publication, without charge. Articles may be published under a CC-BY-NC or CC-BY licence.
CC-BY-NC: Allows users to read, download, copy, redistribute and make derivative works for non-commercial purposes. The author retains all non-commercial rights for articles published herein under the CC-BY-NC 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/ legalcode). To support open access publication costs, Radcliffe charges an article publication charge upon acceptance of an unsolicited paper: £1,500 UK | €1,770 Eurozone | $1,970 all other countries. Waivers are available, as specified in the ‘For authors’ section on www.CFRjournal. com. Permission to reproduce an article published under CC-BY-NC for commercial purposes, either in full or part, should be sought from the Publisher.
CC-BY: Allows users to read, download, copy, redistribute and make derivative works for any purpose, including commercially. Radcliffe offers publication under the CC-BY 4.0 License (https://creativecommons.org/ licenses/by/4.0/legalcode) to authors funded by UK Research Councils (UKRI) or The Wellcome Trust. The article publication charge is £1,750 | €2,069 Eurozone | $2,299 all other countries. The author retains all rights under this option.
Peer Review
• On submission, all articles are assessed by the Editor-in-Chief to determine their suitability for inclusion.
• Suitable manuscripts are sent for double-blind peer review.
• The Editor-in-Chief reserves the right to accept or reject any proposed amendments.
• Once a manuscript has been amended in accordance with the reviewers’ comments, it is assessed.
• The manuscript is sent to the Editor-in-Chief for final approval.
Distribution and Readership
Cardiac Failure Review is an online publication. Articles are published continuously on www.CFRjournal.com. The journal is free to read online and PDF downloads are available for registered users.
Online
All published manuscripts are free to read at www.CFRjournal.com They are also available at www.radcliffecardiology.com, along with articles from the other journals in Radcliffe Cardiology’s cardiovascular portfolio – Arrhythmia & Electrophysiology Review, European Cardiology Review, Interventional Cardiology, Journal of Asian Pacific Society of Cardiology and US Cardiology Review.
Reprints
All articles included in Cardiac Failure Review are available as reprints. Please contact Rob Barclay rob.barclay@radcliffe-group.com
© RADCLIFFE GROUP 2023 Access at: www.CFRjournal.com
Implications of Extra-cardiac Disease in Patient Selection for Heart Transplantation: Considerations in Cardiac Amyloidosis
Lynn Raju Punnoose, Hasan Siddiqi, Julie L Rosenthal, Michelle Kittleson, Ronald Witteles and Kevin Alexander
DOI: https://doi.org/10.15420/cfr.2022.24
Remote Patient Monitoring for Patients with Heart Failure: Sex- and Race-based Disparities and Opportunities
Ioannis Mastoris, Ersilia M DeFilippis, Trejeeve Martyn, Alanna A Morris, Harriette GC Van Spall and Andrew J Sauer
DOI: https://doi.org/10.15420/cfr.2022.22
Pharmacy Challenges in Cardiac Patient Care During the COVID-19 Pandemic: Lessons Learnt For the Future
Andrew McRae
DOI: https://doi.org/10.15420/cfr.2022.20
Imaging in Heart Failure with Preserved Ejection Fraction: A Multimodality Imaging Point of View
Serkan Ünlü, Özge Özden and Ahmet Çelik
DOI: https://doi.org/10.15420/cfr.2022.27
Treatment of Persistent Left Atrial Appendage Thrombus in Patients with Atrial Fibrillation on Adequate Oral Anticoagulation: Pathways of Care for All-comers and Heart Failure Patients
Josip Katic and Josip Andelo Borovac
DOI: https://doi.org/10.15420/cfr.2022.28
Epicardial Fat in Heart Failure with Preserved Ejection Fraction: Bad Actor or Just Lying Around?
Mary-Tiffany Oduah, Varun Sundaram and Yogesh NV Reddy
DOI: https://doi.org/10.15420/cfr.2022.25
Effects of Sacubitril/Valsartan on Exercise Capacity in Patients with Heart Failure with Reduced Ejection Fraction and the Role of Percentage of Delayed Enhancement Measured by Cardiac Magnetic Resonance in Predicting Therapeutic Response: A Multicentre Study
Cinzia Nugara, Francesco Giallauria, Giuseppe Vitale, Silvia Sarullo, Giovanni Gentile, Francesco Clemenza, Annamaria Lo Voi, Antonino Zarcone, Elio Venturini, Gabriella Iannuzzo, Andrew JS Coats and Filippo M Sarullo
DOI: https://doi.org/10.15420/cfr.2022.13
Role of Imaging in Cardiomyopathies
Vincenzo Castiglione, Alberto Aimo, Giancarlo Todiere, Andrea Barison, Iacopo Fabiani, Giorgia Panichella, Dario Genovesi, Lucrezia Bonino Alberto Clemente, Filippo Cademartiri, Alberto Giannoni, Claudio Passino, Michele Emdin and Giuseppe Vergaro
DOI: https://doi.org/10.15420/cfr.2022.26
Pathophysiological Rationale and Clinical Evidence for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction
Vincenzo Castiglione, Francesco Gentile, Nicolò Ghionzoli, Martina Chiriacò, Giorgia Panichella, Alberto Aimo, Giuseppe Vergaro, Alberto Giannoni, Claudio Passino and Michele Emdin
DOI: https://doi.org/10.15420/cfr.2022.23
Defining Heart Failure Based on Imaging the Heart and Beyond
Fraser J Graham, Antonio Iaconelli, Piotr Sonecki, Ross T Campbell, David Hunter, John GF Cleland and Pierpaolo Pellicori
DOI: https://doi.org/10.15420/cfr.2022.29
Global Public Health Burden of Heart Failure: An Updated Review
Bahira Shahim, Chris J Kapelios, Gianluigi Savarese and Lars H Lund
DOI: https://doi.org/10.15420/cfr.2023.05
Cardiovascular Involvement in Fabry’s Disease: New Advances in Diagnostic Strategies, Outcome Prediction and Management
Emanuele Monda, Luigi Falco, Giuseppe Palmiero, Marta Rubino, Alessia Perna, Gaetano Diana, Federica Verrillo, Francesca Dongiglio, Annapaola Cirillo, Adelaide Fusco, Martina Caiazza and Giuseppe Limongelli
DOI: https://doi.org/10.15420/cfr.2023.06
e02
e03
e04
e08
e09
e10
e11
e12
© RADCLIFFE GROUP 2023 www.CFRjournal.com Contents www.CFRjournal.com
e01
e05
e06
e07
Implications of Extra-cardiac Disease in Patient Selection for Heart Transplantation: Considerations in Cardiac Amyloidosis
Lynn Raju Punnoose , 1 Hasan Siddiqi,1 Julie L Rosenthal,2 Michelle Kittleson,3 Ronald Witteles4
Alexander4
Abstract
Disease-modifying therapies in both light chain and transthyretin amyloidosis have improved patient functional status and survival. Conceivably, as heart failure may progress despite amyloid therapies, more patients may be considered for heart transplantation. In earlier eras, extra-cardiac amyloid deposits significantly reduced post-heart transplant patient survival and functional status compared to the non-amyloid population. In the modern era, transplant centres have reported improved outcomes in amyloidosis as patient selection has grown more stringent. Importantly, systematic candidate evaluation should assess the degree of extra-cardiac involvement, the effectiveness of disease-modifying therapies and downstream effects on patients’ nutrition and frailty. This review outlines such an overall approach while also considering that organ-specific selection criteria may vary between individual transplant centres. A methodical approach to patient evaluation will promote better understanding of the prevalence and severity of extra-cardiac disease in amyloidosis patients referred for heart transplantation and of any disparities in decision outcomes in this population.
Keywords
Cardiac amyloidosis, heart transplantation, transthyretin, light chain
Disclosure: JLR reports speaker fees from Scripps Research. RW has received financial support from Alnylam, BridgeBio, Novo Nordisk and Pfizer, and is an advisory board participant for Alnylam, BridgeBio, Ionis, Janssen, Pfizer, Regeneron and Novo Nordisk. KA reports grants and/or consulting fees from Alnylam, Arbor Biotechnologies, Eidos, Ionis, Novo Nordisk and Pfizer, outside this work. All other authors have no conflicts of interest to declare.
Funding: KA is supported by the American Heart Association-Amos Medical Faculty Development Program (19AMFDP34990036) and the National Center for Advancing Translational Sciences of the National Institutes of Health (award number KL2TR003143).
Received: 17 July 2022 Accepted: 26 October 2022 Citation: Cardiac Failure Review 2023;9:e01. DOI: https://doi.org/10.15420/cfr.2022.24
Correspondence: Lynn Punnoose, 5th Floor MCE, Vanderbilt University Medical Center, 1215 Medical Center Drive, Nashville, TN 37232, US. E: lynn.r.punnoose@vumc.org
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Certain patients with cardiac amyloidosis (CA) and advanced heart failure may be considered for heart transplantation. However, extra-cardiac amyloidosis can significantly diminish the functional and survival benefits of such advanced therapies. As transplant teams carefully assess the severity and prognosis of organ-specific disease in heart transplant candidates, they must also consider systemic processes such as frailty and malnutrition.
As we review outcomes associated with extra-cardiac amyloidosis, we present a granular approach to identifying patients with CA and advanced heart failure who may benefit from heart transplantation.
Rise in Transplant Candidates
Survival outcomes in patients with transthyretin (ATTR) and light chain (AL) CA are influenced by not only progressive heart failure but also extracardiac disease.1 2 This natural history guides patients’ candidacy for advanced strategies such as heart transplantation.
Modern therapies for both ATTR and AL amyloidosis have improved patient survival and functional status, potentially rendering heart
transplantation feasible for more CA patients with advanced heart failure. Examples of such drugs include ATTR stabilisers such as tafamidis, ATTR silencers including small interfering RNAs such as patisiran and vutrisiran, antisense oligonucleotide inotersen and AL therapies such as bortezomib and daratumumab.3–10
Several mechanisms may contribute to progressive cardiomyopathy despite targeted precursor protein treatment. For example, among wildtype and variant ATTR amyloidosis patients treated with tafamidis in the ATTR-ACT trial and the subsequent open-label extension study, over a median follow-up of 58.5 months, heart failure worsened in 2% and 10%, respectively, requiring heart transplantation, and mortality rates were 38% and 45%, respectively.3
In vitro examinations of neural cells and cardiomyocytes have demonstrated that ATTR monomers, ATTR oligomers and light chains are cytotoxic. ATTR silencer therapies produce an 85% reduction in circulating TTR; the remaining misfolded protein can, conceivably, continue to be deposited in patients with variant ATTR. In addition, wild-type ATTR can continue to accumulate in variant amyloid deposits, as has been
REVIEW New Advances in Heart Failure © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com
and Kevin
1. Division of Cardiovascular Medicine, Vanderbilt University School of Medicine, Nashville, TN, US; 2. Department of Cardiovascular Medicine, Mayo Clinic, Phoenix, AZ, US; 3. Department of Cardiology, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, US; 4. Division of Cardiovascular Medicine, Stanford University School of Medicine, Palo Alto, CA, US
Table 1: Significant Clinical Presentations and Mechanisms of Extra Cardiac Amyloidosis
Neurological AL, variant ATTR
Peripheral neuropathy and autonomic dysfunction54–58
Endoneural deposition, sometimes perivascular54
Myelinated fibre loss, axonal degeneration54
Affect large and small myelinated fibres, small non-myelinated fibres59
Gastrointestinal tract AL
ATTR
Hepatic AL
Renal AL
Pulmonary AL
Nausea, vomiting48 Diarrhoea, malabsorption, bleeding, pseudoobstruction48
Early satiety, nausea, vomiting, diarrhoea, constipation, faecal incontinence, weight loss60 In variant ATTR, malabsorption50
Hepatomegaly and abnormal alkaline phosphatase63
Proteinuria, end-stage renal disease and dialysis dependence40
Coughing, dyspnea, reduced diffusion capacity on pulmonary function tests64
Tracheobronchial deposits66
Nodular lung deposits65, 66 Pleural effusions66
demonstrated in patients with variant ATTR who underwent liver transplantation.4 11–15
In the modern context of effective therapies that reduce precursor protein production, heart transplantation is an important strategy for certain patients with progressive cardiac disease. Indeed, the proportion of heart transplants in the US performed for CA has increased from 0.3% of all heart transplants between 1987 and 2007 to 1.2% between 2008 and 2013 and 1.7% between 2010 and 2019.16,17
Extra-cardiac Manifestations and Severity Evidence of Extra-cardiac Manifestations
As patients with ATTR or AL CA are considered for heart transplantation, transplant team members must consider that extra-cardiac disease can adversely affect outcomes after heart transplantation. Amyloidosis can affect multiple organ systems, including the nervous system, the gastrointestinal (GI) tract and liver, the respiratory tract and the kidneys (Table 1).18
However, while amyloid deposition can be associated with symptoms, the extent of deposition does not correlate perfectly with disease severity. In addition to amyloid deposition, toxicity from transthyretin fibrils or light chains may contribute to organ dysfunction. Finally, the distribution of organ involvement may vary depending on amyloid subtype.
In earlier reports of heart transplantation in CA patients with advanced heart failure, extra-cardiac disease at the time of transplant was described through clinical assessment, tissue diagnosis at transplant and imaging.
For example, among 11 AL CA patients undergoing heart transplantation between 1994 and 2005, one patient was noted to have peripheral neuropathy, macroglossia and GI involvement, and another had renal involvement identified at transplantation.19
In a UK series of CA patients undergoing heart transplantation between 1982 and 2002, extra-cardiac involvement was assessed using serum
Direct amyloid infiltration or dysmotility, autonomic neuropathy48
In variant ATTR, loss of interstitial cells of Cajal61 and bile acid malabsorption62
Sinusoidal and perivascular amyloid deposition63
Light chain mediated toxicity, deposition disease and cast nephropathy40
Amyloid tubular and glomerular deposition40
Diffuse parenchymal deposition64, 65 Light chain deposition also possible66
amyloid P component scintigraphy. Six of seven non-AL patients underwent imaging: one with ApoA1 amyloidosis had liver, kidney and splenic involvement; and another with variant ATTR had renal involvement. Of 13 out of 17 AL patients similarly assessed, 10 had renal, splenic, hepatic and/or bone marrow involvement.20
Renal insufficiency, as defined by proteinuria, renal biopsy or estimated glomerular filtration rate (eGFR), has been reported in recent case series of AL CA patients selected for heart transplantation. Comparing the transplant eras of 2002–2007 and 2008–2017, one centre demonstrated that, while patients selected for heart transplant had proteinuria, the average degree of this decreased from 0.28 g/day (25th–75th percentile 0.13–1.43 g/day; n=16) to 0.10 g/day (0.10–0.20 g/day; n=16) between these periods.21
At another transplant centre, two of 13 AL CA patients transplanted between 2004 and 2017 were found to have renal involvement by biopsy and neither had proteinuria ≥1 g/day. One of these recipients had progressive renal failure and required dialysis seven years after a heart transplant.22
In 13 AL amyloidosis patients transplanted at a third centre between 2010 and 2018, the average eGFR before transplant was 47.7 ± 27.6 ml/ min/1.73 m2 and improved to 60 ± 61.2 22 ml/min/1.73 m2 over the 3-year follow-up period. Notably, one-third of patients underwent simultaneous heart and kidney transplantation and none had proteinuria ≥500 mg/day before transplant. The need for dialysis after heart transplantation was not reported.23
Neuropathy was reported in 77% of AL CA patients undergoing transplant at one centre, but categorisation by autonomic and peripheral systems was not available.23
Another analysis reported autonomic neuropathy in one of 13 AL CA patients and one of 18 ATTR CA patients undergoing heart transplant, and peripheral neuropathy in six of 18 ATTR CA patients undergoing heart transplant.22
Patient Selection for Heart Transplantation in Cardiac Amyloidosis CARDIAC FAILURE REVIEW www.CFRjournal.com
Organ System Amyloid Subtype Symptoms and/or Imaging Findings Pathophysiology
AL = light chain; ATTR = transthyretin.
Patient Selection for Heart Transplantation in Cardiac Amyloidosis
Lastly, amyloid deposition in the oesophagus or stomach was reported in 11 of 13 AL CA and one of 18 ATTR CA patients transplanted between 2004 and 2017; deposition in the colon was reported in all 13 AL CA and one of 18 ATTR CA patients in the same series.22
Impact of Extra-cardiac Manifestations
In earlier eras of heart transplantation, extra-cardiac manifestations were associated with poor survival. United Network of Organ Sharing analyses of heart transplantation in cardiac amyloidosis over 1987–2010 (n=142) and 1987–2002 (n=69) demonstrated 1- and 5-year survival rates of 75–79% and 47%, respectively.24 25
However, larger registry analyses do not provide further comparisons by amyloid subtype, degree or type of extra-cardiac involvement, or cause of death. Older, smaller case series describe mode of death and the contribution of extra-cardiac disease.
For example, among 24 amyloidosis patients undergoing heart transplantation in the UK over 1982–2002, survival at 1 and 5 years after a heart transplant ranged between 50% and 20% in AL patients who did not receive chemotherapy; 71% and 36% in AL patients who did receive chemotherapy; and 86% and 64% in non-AL patients. Seven of the 10 deaths in the AL cohort were attributed to gastrointestinal, pulmonary or renal amyloidosis, while neither of the two non-AL patient deaths were due to amyloidosis.20
Extra-cardiac Manifestations and Outcomes in AL Amyloidosis
Light-chain control is key to improving survival outcomes after heart transplantation in AL amyloidosis recipients and has improved in the modern era of AL therapies.
In an older series of 11 patients who underwent heart transplantation and stem cell transplant (SCT) but no routine maintenance chemotherapy between 1994 and 2005, Lacy et al. reported that nine had >50% decrease in serum immunoglobulin free light chains, but only four remained in continued response during follow-up.19 Three died from progressive amyloidosis, one had a renal relapse and two died from SCTrelated mortality, including infection, renal and hepatic failure.19
Recent single-centre case series of highly selected patients have reported robust longer-term light chain control after heart transplant, using strategies such as proteasome inhibitors, daratumumab and SCT. For example, in their series describing 13 AL amyloidosis patients transplanted between 2004 and 2017, Barrett et al. reported no cases of relapse, with five patients undergoing SCT and six requiring therapies such as proteasome inhibitors or daratumumab. No patients died of amyloidosis.22 In a more recent series of 13 AL amyloidosis heart transplant recipients between 2010 and 2018, six patients underwent SCT after heart transplant, with four of them requiring chemotherapy because they had a relapse. None of the four deaths were attributed to amyloidosis.23
Extra-cardiac Manifestations and Outcomes in ATTR Amyloidosis
TTR stabilisers and silencers will likely modify the natural history of progressive ATTR amyloidosis after heart transplant, but little data have been reported in the heart transplant population to date.23,26
Certainly, as heart transplantation improves survival in ATTR patients with CA, transplant teams will increasingly encounter extra-cardiac
manifestations of amyloidosis. For example, an older report of seven wildtype ATTR cardiac amyloidosis patients undergoing heart transplantation between 2007 and 2015 did not report the use of ATTR-specific therapies, but only two of these patients remained free of extra-cardiac disease, such as dysmotility, autonomic or peripheral neuropathy, or carpal tunnel syndrome, after transplant.27
Another analysis of 12 patients with wild-type and variant ATTR cardiac amyloidosis describes amyloidosis progression after heart transplantation between 2002 and 2019.26 Before transplant, five patients had amyloidosis causing carpal tunnel syndrome and/or peripheral neuropathy. At a median of 4 years after transplant, eight patients had lumbar spinal stenosis, carpal tunnel syndrome, neuropathy and/or biopsy-proven GI amyloidosis. Severity of symptoms was assessed only after transplant, using the self-reported Composite Autonomic Symptom Score (COMPASS-31) and a simple clinician staging system, the polyneuropathy disability score (PND), which categorises patients by neuropathy and ambulatory ability.28 Two of the eight patients were on tafamidis and one on patisiran. At this centre, of an additional eight patients without amyloidosis surveillance who died after heart transplant, five died because of progressive GI amyloidosis or neuropathy. Two of the five had neuropathy or GI symptoms before transplant.
Earlier case series of CA heart transplants in patients with variant ATTR over 1982–2002 (n=3) and 2002–11 (n=9) highlight the use of either simultaneous or sequential liver transplantation in 100% and 66% of patients, respectively, as an attempt to reduce further amyloid deposition and progressive disease including neuropathy.20 26
However, after liver transplant, wild-type TTR can continue to accumulate in variant amyloid deposits, leading to progressive peripheral neuropathy and autonomic dysfunction among other problems.15 29 30
Moreover, in the modern era, therapies such as TTR silencers can significantly reduce circulating TTR without the additional morbidity from having a dual organ transplant. Recent reports of heart transplantation in patients with variant ATTR have described the use of heart-liver transplant infrequently, and this has primarily been in patients with concurrent cardiac cirrhosis.22 26 23
Extra-cardiac Disease, Downstream Effects and Patient Selection
Malnutrition and Frailty
Neuropathy and GI involvement in ATTR and AL amyloidosis can, conceivably, lead to a high prevalence of malnutrition and frailty in patients with CA. As in the overall population of patients referred for heart transplantation, these markers may prove useful as predictors of outcome in CA for those being considered for transplantation. 31 32
Among patients with amyloidosis, nutrition and frailty have been quantified using symptom assessments and scores based on laboratory values and weight. For example, among patients with AL CA, dysphagia occurs in 6–25%, diarrhoea in 29% and early satiety in 23%. Weight loss of 9 kg (20 lb) or greater is reported in 17–70% of patients.33
As measured using modified BMI, malnutrition occurs in up to 64% of variant ATTR cardiac amyloidosis patients and 59% of those with wild-type ATTR cardiac amyloidosis.34 35 Frailty is estimated to occur in 33–50% of patients with wild-type ATTR cardiac amyloidosis, in domains including (among others) autonomy, balance and muscle weakness.36
CARDIAC FAILURE REVIEW www.CFRjournal.com
Table 2: Assessment Approach and Concerning Red Flags in Multisystem Evaluation for Heart Transplantation
Organ System Assessment Tools
Frailty
Nutritional status
Modified Fried frailty phenotype31
Modified BMI (BMI kg/m2 x albumin gm/L)34
Nutritional risk index32
1.519 × serum albumin (g/l) + 41.7 × (actual body weight [kg]/ideal body weight [kg])
Red Flags
Patients who meet ≥3 criteria
Modified BMI <600
Nutritional risk index <97.5
Autonomic neuropathy
Peripheral neuropathy
Pulmonary disease
Gastrointestinal tract disease
Orthostatic blood pressures
COMPASS-31 questionnaire28
Formal autonomic function tests
Variant ATTR: Polyneuropathy disability score28
EMG
Hepatic disease
Renal disease
Haematologic disease
AL: EMG
Chest X ray/CT—interstitial thickening, consolidations or nodules, pleural effusions, airway thickening51
Symptom screen48
Nutritional status assessment68
Assessment of fat malabsorption50
Routine esophagogastroduodeonoscopy and colonoscopy with random biopsies22 (unclear role – performed at some centres)
Poor correlation of symptoms with functional studies49
Consider liver biopsy if hepatomegaly or serum alkaline phosphatase
>1.5 times normal.52
Consider renal biopsy if proteinuria present to distinguish causes, such as amyloidosis, light chain deposition disease and cast nephropathy40 from diabetes or other intrinsic processes.
Examine light chain response to pre-heart transplant therapies
Review cytogenetics with oncologists
AL = light chain; ATTR = transthyretin; EMG = electromyography.
Such estimates are generally comparable to the prevalence of moderateto-severe malnutrition and frailty among all patients evaluated for heart transplantation, but their prevalence after heart transplantation in cardiac amyloidosis has been reported only occasionally.23 32 31 Their effect on survival in heart transplant recipients with amyloidosis has not been ascertained.16,22,23,26
Organ-specific Selection Criteria
Organ-specific selection criteria for heart transplantation in patients with cardiac amyloidosis have been generally described in consensus guidelines and single-centre reports as part of efforts to improve posttransplant outcomes in this population in the modern era, but precise, acceptable thresholds for extra-cardiac disease severity and symptoms differ between centres.19,22,23,37 There are several reasons for this variation.
Transplant centres may differ in the extent of resources they can devote to post-transplant amyloidosis management, which requires close collaboration between multiple medical specialists and pharmacists.38 In addition, programmatic risk tolerance will vary depending on the consensus established at each centre among treating cardiologists, haematologists, nephrologists and neurologists.
As an example, for patients with AL amyloidosis, a recent American Society of Transplantation consensus statement proposed that in collaboration with oncologists, solid organ transplant physicians consider only candidates with no high-risk cytogenetics, good functional status with single organ involvement, a robust light-chain response to therapy, haematologic remission for >6 months and who are good candidates for eventual stem cell transplant.39
Orthostasis
Modified BMI <600
Nutritional risk index <97.5
Disability score ≥ III28, 47
Symptoms severe enough to limit ambulation22
AL: Exudative pleural effusions67
Modified BMI <600
Nutritional risk index <97.5
GI bleeding
Interstitial amyloid deposition, not vascular deposits48
Total bilirubin > 2mg/dl63
Proteinuria ≥500mg/day23
Glomerular amyloid deposits, not vasculature40
Light chains not responsive to therapy
High-risk cytogenetics
Active myeloma
However, recent single-centre case series variably rely on only some of these factors in deciding candidacy for heart transplantation and, in the modern era of improved chemotherapy and immunotherapy, it is unclear that stem cell transplantation eligibility, in particular, should be a criterion.22 23
Transplant centres also vary in their patient selection criteria for simultaneous heart and kidney transplants in AL amyloidosis patients. Renal insufficiency in this population extends from proteinuria to anuria and dialysis dependence.40
Among patients with renal AL amyloidosis, severe proteinuria (defined either as >5 g/day and eGFR<50 ml/min/1.732 or as 24 hours’ protein: eGFR ratio <30 mg/ml/min/1.73m2) is associated with a significant risk of progression to dialysis dependence.41,42 The risks of acute and chronic nephrotoxicity associated with immunosuppressive regimens after heart transplantation alone may be perceived as prohibitive for heart-kidney transplantation in such patients, but individual centre volumes are too small to accurately describe post-transplant outcomes. 43 44
For AL amyloidosis patients with end-stage renal disease, 65% of US renal transplant clinicians recently surveyed reported a lack of consensus on which patients were appropriate for renal transplant, with the majority expressing concern about long-term survival.45
Common Principles for Organ System Evaluation
Patient selection for heart transplant must be informed by close discussion and coordination among multiple specialties at the transplanting centre, both before and after transplant. Importantly, specific extra-cardiac,
Patient Selection for Heart Transplantation in Cardiac Amyloidosis CARDIAC FAILURE REVIEW www.CFRjournal.com
Patient Selection for Heart Transplantation in Cardiac Amyloidosis
organ-system-based assessments should be accompanied by a thorough appraisal of nutritional status and frailty.
Relying on prognostic markers assessed in larger populations of heart transplant candidates and in patients with amyloidosis, we propose assessment approaches and red-flag benchmarks for organ-specific disease; adequate response to light-chain suppressive therapy in AL amyloidosis; and systemic markers, including functional and nutritional status in CA patients being considered for heart transplantation (Table 2).31,32,34,46,47
In many organ systems, amyloid deposition alone is not a contraindication to transplantation, as it does not predict dysfunction and poor outcomes after heart transplantation. This is particularly true for amyloidosis of the GI tract. Symptoms and nutritional status do not correlate perfectly with amyloid deposition, as autonomic neuropathy can also lead to diarrhoea, constipation, weight loss and early satiety.48 Therefore, the role of routine gastrointestinal tract biopsies is not clear, although they are performed at some centres.22 While certain functional studies, such as gastric emptying, do not correlate well with symptoms, a thorough symptom screen and objective measures of fat malabsorption may prove more useful.48–50
Assessing patient-reported symptoms in addition to certain functional measurements similarly facilitates the evaluation of amyloidosis-related peripheral and autonomic neuropathy. Such a strategy may include patient-completed questionnaires such as COMPASS-31, provider assessments such as PND and objective measures such as electromyography.28
While neurologic and GI involvement can be seen in both AL and ATTR amyloidosis, certain other organ systems are affected only in AL amyloidosis, for example, the pulmonary, hepatic and renal systems (Table 1). In such patients, invasive testing such as tissue biopsy can be
1. Barrett CD, Dobos K, Liedtke M, et al. A changing landscape of mortality for systemic light chain amyloidosis. JACC Heart Fail 2019;7:958–66. https://doi.org/10.1016/j.jchf.2019.07.007; PMID: 31606365.
2. Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019;140:16–26. https://doi. org/10.1161/CIR.0000000000000714; PMID: 31356127.
3. Elliott P, Drachman BM, Gottlieb SS, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail 2022;15:e008193. https://doi. org/10.1161/CIRCHEARTFAILURE.120.008193; PMID: 34923848.
4. Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. https://doi. org/10.1056/NEJMoa1716153; PMID: 29972753
5. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/ NCT04153149 (accessed 24 November 2022).
6. Karam C, Brown D, Yang M, et al. Long-term treatment effects of inotersen on health-related quality of life in patients with hATTR amyloidosis with polyneuropathy: analysis of the open-label extension of the NEURO-TTR trial. Muscle Nerve 2022;66:438–46. https://doi.org/10.1002/ mus.27675; PMID: 35799473.
7. Venner CP, Lane T, Foard D, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood 2012;119:4387–90. https:// doi.org/10.1182/blood-2011-10-388462; PMID: 22331187.
8. Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood 2012;119:4391–4. https:// doi.org/10.1182/blood-2011-11-390930; PMID: 22331188.
9. Kastritis E, Palladini G, Minnema MC, et al. Daratumumabbased treatment for immunoglobulin light-chain amyloidosis.
considered if screening chest imaging or laboratory values such as alkaline phosphatase are abnormal.51,52
Renal biopsy should be considered in AL CA patients with proteinuria, particularly to distinguish between renal amyloidosis or light chain disease and other processes such as diabetes.40 In these patients, assessment of renal function using serum creatinine and eGFR may be challenging because of malnutrition; however, alternative measures such as cystatin C clearance have not been studied in this population. 53
Transplant programmes should couple these systematic evaluations with mechanisms to track decision outcomes in all CA patients referred for heart transplantation. Not all transplant centres may be able to offer advanced therapies to this population based on programmatic risk tolerance. Therefore, data on patient evaluation and decision-making, collected in multi-centre registries, may delineate geographic and socioeconomic disparities in access to heart transplantation for CA patients.
Conclusion
Outcomes for heart transplantation in cardiac amyloidosis have improved in the recent era of transplantation, due in part to successful diseasemodifying therapies and more stringent patient selection.
At the time of evaluation for heart transplantation, teams must pay specific attention to the severity and natural history of extra-cardiac disease. We propose a systematic organ-based approach, with a particular focus on nutrition and frailty, to identify patients who could most benefit from heart transplantation in terms of functional and survival outcomes.
At individual transplant centres, tracking patient-specific data and decision outcomes in cardiac amyloidosis will help the transplant community ascertain the prevalence and severity of extra-cardiac amyloidosis in patients with advanced heart failure.
N Engl J Med 2021;385:46–58. https://doi.org/10.1056/ NEJMoa2028631; PMID: 34192431.
10. Sanchorawala V, Sarosiek S, Schulman A, et al. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase 2 study. Blood 2020;135:1541–7. https://doi.org/10.1182/blood.2019004436; PMID: 31978210.
11. Reixach N, Deechongkit S, Jiang X, et al. Tissue damage in the amyloidoses: transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci U S A 2004;101:2817–22. https://doi. org/10.1073/pnas.0400062101; PMID: 14981241.
12. McWilliams-Koeppen HP, Foster JS, Hackenbrack N, et al. Light chain amyloid fibrils cause metabolic dysfunction in human cardiomyocytes. PloS One 2015;1:0e0137716. https:// doi.org/10.1371/journal.pone.0137716; PMID: 26393799.
13. Imperlini E, Gnecchi M, Rognoni P, et al. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci Rep 2017;7:15661. https://doi.org/10.1038/s41598-017-15424-3; PMID: 29142197.
14. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. https://doi. org/10.1056/NEJMoa1716793; PMID: 29972757.
15. Pomfret EA, Lewis WD, Jenkins RL, et al. Effect of orthotopic liver transplantation on the progression of familial amyloidotic polyneuropathy. Transplantation 1998;65:918–25. https://doi.org/10.1097/00007890-199804150-00010; PMID: 9565095.
16. Davis MK, Lee PH, Witteles RM. Changing outcomes after heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant 2015;34:658–66. https://doi.org/10.1016/j.healun.2014.09.006; PMID: 25444369.
17. Akintoye E, Salih M, Aje K, et al. Trends and outcomes of patients with amyloid cardiomyopathy listed for heart
transplantation. Can J Cardiol 2022;38:1263–70. https://doi. org/10.1016/j.cjca.2022.04.023; PMID: 35525397.
18. Varr BC, Liedtke M, Arai S, et al. Heart transplantation and cardiac amyloidosis: approach to screening and novel management strategies. J Heart Lung Transplant 2012;31:325–31. https://doi.org/10.1016/j.healun.2011.09.010; PMID: 22051505.
19. Lacy MQ, Dispenzieri A, Hayman SR, et al. Autologous stem cell transplant after heart transplant for light chain (AL) amyloid cardiomyopathy. J Heart Lung Transplant 2008;27:823–9. https://doi.org/10.1016/j. healun.2008.05.016; PMID: 18656793
20. Dubrey SW, Burke MM, Hawkins PN, Banner NR. Cardiac transplantation for amyloid heart disease: the United Kingdom experience. J Heart Lung Transplant 2004;23:1142–53. https://doi.org/10.1016/j.healun.2003.08.027; PMID: 15477107
21. Kristen AV, Kreusser MM, Blum P, et al. Improved outcomes after heart transplantation for cardiac amyloidosis in the modern era. J Heart Lung Transplant 2018;37:611–8. https:// doi.org/10.1016/j.healun.2017.11.015; PMID: 29217108.
22. Barrett CD, Alexander KM, Zhao H, et al. Outcomes in patients with cardiac amyloidosis undergoing heart transplantation. JACC Heart Fail 2020;8:461–8. https://doi. org/10.1016/j.jchf.2019.12.013; PMID: 32387068.
23. Vaidya GN, Patel JK, Kittleson M, et al. Intermediate-term outcomes of heart transplantation for cardiac amyloidosis in the current era. Clin Transplant 2021;35:e14308. https://doi. org/10.1111/ctr.14308; PMID: 33825224.
24. DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012;31:1269–75. https://doi. org/10.1016/j.healun.2012.09.018; PMID: 23079066.
25. Kpodonu J, Massad MG, Caines A, Geha AS. Outcome of heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant 2005;24:1763–5. https://doi.org/10.1016/j.healun.2004.08.025;
CARDIAC FAILURE REVIEW www.CFRjournal.com
Patient Selection for Heart Transplantation in Cardiac Amyloidosis
PMID: 16297778.
26. Griffin JM, Baughan E, Rosenblum H, et al. Surveillance for disease progression of transthyretin amyloidosis after heart transplantation in the era of novel disease modifying therapies. J Heart Lung Transplant 2022;41:199–207. https:// doi.org/10.1016/j.healun.2021.10.007. PMID: 34922822.
27. Rosenbaum AN, AbouEzzeddine OF, Grogan M, et al. Outcomes after cardiac transplant for wild type transthyretin amyloidosis. Transplantation 2018;102:1909–13. https://doi. org/10.1097/TP.0000000000002240; PMID: 29677073.
28. Conceição I, Coelho T, Rapezzi C, et al. Assessment of patients with hereditary transthyretin amyloidosis –understanding the impact of management and disease progression. Amyloid 2019;26:103–11. https://doi.org/10.1080/ 13506129.2019.1627312; PMID: 31339362.
29. Saelices L, Chung K, Lee JH, et al. Amyloid seeding of transthyretin by ex vivo cardiac fibrils and its inhibition. Proc Natl Acad Sci U S A 2018;115:e6741–50. https://doi.org/10.1073/ pnas.1805131115; PMID: 29954863.
30. Banerjee D, Roeker LE, Grogan M, et al. Outcomes of patients with familial transthyretin amyloidosis after liver transplantation. Prog Transplant 2017;27:246–50. https://doi. org/10.1177/1526924817715463; PMID: 29187090.
31. Jha SR, Hannu MK, Chang S, et al. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation 2016;100:429–36. https://doi.org/10.1097/ TP.0000000000000991; PMID: 26516676.
32. Barge-Caballero E, García-López F, Marzoa-Rivas R, et al. Prognostic value of the nutritional risk index in heart transplant recipients. Rev Esp Cardiol (Engl Ed) 2017;70:639–45. https://doi.org/10.1016/j.rec.2017.01.005; PMID: 28209304.
33. Grigoletti SS, Zuchinali P, Lemieux-Blanchard É, et al. Focused review on nutritional status of patients with immunoglobulin light chain amyloidosis. Curr Probl Cancer 2022;46:100833. https://doi.org/10.1016/j. currproblcancer.2021.100833; PMID: 35101705.
34. Suhr O, Danielsson A, Holmgren G, Steen L. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med 1994;235:479–85. https://doi.org/10.1111/j.1365-2796.1994. tb01106.x; PMID: 8182405.
35. Driggin E, Helmke S, De Los Santos J, et al. Markers of nutritional status and inflammation in transthyretin cardiac amyloidosis: association with outcomes and the clinical phenotype. Amyloid 2020;27:73–80. https://doi.org/10.1080/1 3506129.2019.1698417; PMID: 31825676.
36. Broussier A, David JP, Kharoubi M, et al. Frailty in wild-type transthyretin cardiac amyloidosis: the tip of the iceberg. J Clin Med 2021;10:3415. https://doi.org/10.3390/jcm10153415; PMID: 34362197.
37. Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1–23. https://doi.org/10.1016/j. healun.2015.10.023; PMID: 26776864.
38. Chen H, Chandrashekar P, Fischer K, et al. Navigating the complex web of prescribing amyloidosis therapeutics: a primer. J Am Heart Assoc 2022;11:e023895. https://doi. org/10.1161/JAHA.121.023895; PMID: 35301856.
39. Al-Adra DP, Hammel L, Roberts J, et al. Preexisting melanoma and hematological malignancies, prognosis, and timing to solid organ transplantation: a consensus expert opinion statement. Am J Transplant 2021;21:475–83. https:// doi.org/10.1111/ajt.16324; PMID: 32976703.
40. Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol 2006;17:3458–71. https://doi.org/10.1681/ ASN.2006050460; PMID: 17093068.
41. Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124:2325–32. https://doi.org/10.1182/blood-2014-04-570010; PMID: 25115890.
42. Kastritis E, Gavriatopoulou M, Roussou M, et al. Renal outcomes in patients with AL amyloidosis: prognostic factors, renal response and the impact of therapy. Am J Hematol 2017;92:632–9. https://doi.org/10.1002/ajh.24738; PMID: 28370245.
43. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009;4:481–508. https://doi.org/10.2215/CJN.04800908; PMID: 19218475.
44. Eisen HJ. CAVEAT mTOR: you’ve heard about the benefits of using mTOR inhibitors, here are some of the risks. Am J Transplant 2021;21:449–50. https://doi.org/10.1111/ajt.16225; PMID: 32715588.
45. Lam R, Lim MA, Dember LM. Suitability for kidney transplantation in AL amyloidosis: a survey study of transplant and amyloidosis physicians. Kidney360 2021;2:1987–97. https://doi.org/10.34067/KID.0004232021; PMID: 35419526.
46. Adams D, Polydefkis M, González-Duarte A, et al. Long-term safety and efficacy of patisiran for hereditary transthyretinmediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol 2021;20:49–59. https://doi.org/10.1016/S14744422(21)00004-1; PMID: 33484655.
47. González-Duarte A, Conceição I, Amass L, et al. Impact of non-cardiac clinicopathologic characteristics on survival in transthyretin amyloid polyneuropathy. Neurol Ther 2020;9:135–49. https://doi.org/10.1007/s40120-020-00183-7; PMID: 32232748.
48. Rosenzweig M, Comenzo RL. Liver and gastrointestinal involvement. Hematol Oncol Clin North Am 2020;34:1081–90. https://doi.org/10.1016/j.hoc.2020.09.001; PMID: 33099425.
49. Wixner J, Karling P, Rydh A, et al. Gastric emptying in hereditary transthyretin amyloidosis: the impact of autonomic neuropathy. Neurogastroenterol Motil 2012;24:1111–e568. https://doi.org/10.1111/j.1365-2982.2012.01991.x; PMID: 22897426.
50. Obici L, Suhr OB. Diagnosis and treatment of gastrointestinal dysfunction in hereditary TTR amyloidosis. Clin Auton Res 2019;29(Suppl 1):55–63. https://doi. org/10.1007/s10286-019-00628-6; PMID: 31452022.
51. Czeyda-Pommersheim F, Hwang M, Chen SS, et al. Amyloidosis: modern cross-sectional imaging. Radiographics 2015;35:1381–92. https://doi.org/10.1148/rg.2015140179; PMID: 26230754.
52. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005;79:319–28. https://doi.org/10.1002/ajh.20381; PMID: 16044444.
53. Inker LA, Titan S. Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis 2021;78:736–49. https://doi.org/10.1053/j.ajkd.2021.04.016; PMID: 34518032.
54. Qian M, Qin L, Shen K, et al. Light-chain amyloidosis with peripheral neuropathy as an initial presentation. Front Neurol 2021;12:707134. https://doi.org/10.3389/fneur.2021.707134; PMID: 34650504.
55. Conceição I, De Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve 2007;35:116–8. https://doi.org/10.1002/mus.20644; PMID: 16969832.
56. Sekijima Y, Ueda M, Koike H, et al. Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan: red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis 2018;13:6. https:// doi.org/10.1186/s13023-019-1092-7; PMID: 31113447.
57. Dispenzieri A, Coelho T, Conceição I, et al. Clinical and genetic profile of patients enrolled in the transthyretin Amyloidosis Outcomes Survey (THAOS): 14-year update. Orphanet J Rare Dis 2022;17:236. https://doi.org/10.1186/ s13023-022-02359-w; PMID: 35717381.
58. Gonzalez-Duarte A. Autonomic involvement in hereditary transthyretin amyloidosis (hATTR amyloidosis). Clin Auton Res 2019;29:245–51. https://doi.org/10.1007/s10286-018-0514-2; PMID: 29511897.
59. Koike H, Nakamura T, Nishi R, et al. Common clinicopathological features in late-onset hereditary transthyretin amyloidosis (Ala97Gly, Val94Gly and Val30Met). Amyloid 2019;26(Suppl 1):24–5. https://doi.org/10.1080/13506 129.2019.1582495; PMID: 31343348.
60. Wixner J, Mundayat R, Karayal ON, et al. THAOS: gastrointestinal manifestations of transthyretin amyloidosis – common complications of a rare disease. Orphanet J Rare Dis 2014;9:61. https://doi.org/10.1186/1750-1172-9-61; PMID: 24767411.
61. Wixner J, Obayashi K, Ando Y, et al. Loss of gastric interstitial cells of Cajal in patients with hereditary transthyretin amyloidosis. Amyloid 2013;20:99–106. https:// doi.org/10.3109/13506129.2013.787985; PMID: 23642163.
62. Suhr O, Danielsson A, Steen L. Bile acid malabsorption caused by gastrointestinal motility dysfunction? An investigation of gastrointestinal disturbances in familial amyloidosis with polyneuropathy. Scand J Gastroenterol 1992;27:201–7. https://doi. org/10.3109/00365529208999949; PMID: 1502482.
63. Park MA, Mueller PS, Kyle RA, et al. Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Med (Baltim) 2003;82:291–8. https://doi. org/10.1097/01.md.0000091183.93122.c7; PMID: 14530778.
64. Liu Y, Jin Z, Zhang H, et al. Diffuse parenchymal pulmonary amyloidosis associated with multiple myeloma: a case report and systematic review of the literature. BMC Cancer 2018;18:802. https://doi.org/10.1186/s12885-018-4565-5; PMID: 30089469.
65. Smith RR, Hutchins GM, Moore GW, Humphrey RL. Type and distribution of pulmonary parenchymal and vascular amyloid. Correlation with cardiac amyloid. Am J Med 1979;66:96–104. https://doi.org/10.1016/00029343(79)90488-1; PMID: 420256.
66. Milani P, Basset M, Russo F, et al. The lung in amyloidosis. Eur Respir Rev 2017;26:170046. https://doi. org/10.1183/16000617.0046-2017; PMID: 28877975.
67. Berk JL. Pleural effusions in systemic amyloidosis. Curr Opin Pulm Med 2005;11:324–8. https://doi.org/10.1097/01. mcp.0000162378.35928.37; PMID: 15928500.
68. Adams D, Suhr OB, Hund E, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol 2016;29:S14–26. https://doi.org/10.1097/ WCO.0000000000000289; PMID: 26734952.
CARDIAC FAILURE REVIEW www.CFRjournal.com
Remote Patient Monitoring for Patients with Heart Failure: Sex- and Race-based Disparities and Opportunities
Ioannis Mastoris , 1 Ersilia M DeFilippis , 2 Trejeeve Martyn,3 Alanna A Morris,4 Harriette GC Van Spall 5,6 and Andrew J Sauer7,8
1. Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, US; 2. Department of Medicine, Columbia University Irving Medical Center, New York, NY, US; 3. Department of Cardiovascular Medicine, Heart, Vascular, and Thoracic Institute, Kaufman Center for Heart Failure Treatment and Recovery, Cleveland Clinic, Cleveland, OH, US; 4. Department of Medicine, Emory University School of Medicine, Atlanta, GA, US; 5. Department of Medicine, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada; 6. Population Health Research Institute and Research Institute of St Joseph’s, Hamilton, Ontario, Canada; 7. Saint Luke’s Mid America Heart Institute, Kansas City, MO, US; 8. University of Missouri-Kansas City, Kansas City, MO, US
Abstract
Remote patient monitoring (RPM), within the larger context of telehealth expansion, has been established as an effective and safe means of care for patients with heart failure (HF) during the recent pandemic. Of the demographic groups, female patients and black patients are underenrolled relative to disease distribution in clinical trials and are under-referred for RPM, including remote haemodynamic monitoring, cardiac implantable electronic devices (CIEDs), wearables and telehealth interventions. The sex- and race-based disparities are multifactorial: stringent clinical trial inclusion criteria, distrust of the medical establishment, poor access to healthcare, socioeconomic inequities, and lack of diversity in clinical trial leadership. Notwithstanding addressing the above factors, RPM has the unique potential to reduce disparities through a combination of implicit bias mitigation and earlier detection and intervention for HF disease progression in disadvantaged groups. This review describes the uptake of remote haemodynamic monitoring, CIEDs and telehealth in female patients and black patients with HF, and discusses aetiologies that may contribute to inequities and strategies to promote health equity.
Keywords
Remote patient monitoring, heart failure, racial disparity, health equity, sex difference.
Disclosure: EMDF is a section editor and HGCVS is on the Cardiac Failure Review editorial board; this did not influence peer review. EMDF owns stock in Abbott Laboratories. TM receives research support from Ionis Therapeutics and is an advisor to Recora Health. AAM receives research support from the American Heart Association and the Association of Black Cardiologists, and consulting fees from Abbott, Acorai, BI Lilly, Edwards Lifesciences, Ionis Therapeutics and Merck. HGCVS is funded by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. AJS receives research funding and consulting fees from Abbott, Biotronik, Boston Scientific, General Prognostics, Impulse Dynamics, Medtronic and Story Health. IM has no conflicts of interest to declare.
Received: 22 May 2022
Accepted: 16 September 2022 Citation: Cardiac Failure Review 2023;9:e02. DOI: https://doi.org/10.15420/cfr.2022.22
Correspondence: Ioannis Mastoris, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, US, E: imastoris@mgh.harvard.edu
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The recent COVID-19 pandemic has highlighted the need for more timely, dependable and remote access to medical care. Consequently, the pandemic has dramatically shifted the approach to patient care on multiple levels. This transformation includes a rapid transition to a virtual physician–patient encounter environment (telehealth) and an increasing investment in remote patient monitoring technology.1 With increasing implementation and experience in remote patient monitoring (RPM), challenges have become more apparent when addressing the needs of vulnerable populations such as women and African-Americans. These challenges include applicability across the broad spectrum of heart failure (HF) phenotypes, disadvantages for those with limited access to the internet, affordability due to frequent non-coverage by payers and poor uptake by patients who are less proficient with technology overall.2 These challenges may represent barriers to the uniform application of RPM and may inadvertently perpetuate disparities in disadvantaged populations.
From risk factors to different pathophysiological processes and clinical phenotypes in HF, sex
differences have been well documented.3 4 Traditional risk factors such as hypertension, diabetes, obesity and smoking confer a comparatively higher risk for HF in female patients.5,6 In addition, female patients are subject to sex-specific risk factors such as autoimmune disease, breast cancer therapy, pregnancy and coronary syndromes without atherosclerotic disease.3 Furthermore, they frequently present with pronounced symptoms and generally later in their HF trajectory.7 8 Plasma concentrations of HF biomarkers differ between sexes, which is only partially explained by differences in hormone status.9 As in sex-based differences in the cardiovascular research field, racial disparities have also been described. For example, African-American patients are more likely to die from HF compared with white patients, particularly in the younger age groups (35–64 years) and regardless of sex.6 10 In addition, compared with men and white patients, African-
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Treatment
Table 1: Proportion of Women, Patients of Colour and Sex/Race-specific Outcomes in Haemodynamic Monitoring Studies
GUIDE-HF
all-cause mortality or HF events at 1 year for women (HR 0.64; 95%CI [0.47–0.87], p for interaction=0.01) and black patients (HR 0.68; 95%CI [0.48–0.97], p for interaction=0.095)
CRT-D = cardiac resynchronisation therapy–defibrillator; EF = ejection fraction; HF = heart failure; NR = not reported; NYHA = New York Heart Association.
American female patients are less likely to receive appropriate medical therapy, ICDs or cardiac resynchronisation therapy with a defibrillator, or be included in clinical trials.11–14
Whether founded in biology or other factors, these differences may lead to inequities and disparities related to sex and race. While some of these factors are modifiable through better trial design, others require investment in research infrastructure and communities. Here, we review disparities related to sex and race in remote monitoring, elucidate aetiologies that contribute to inequities in black patients and suggest potential ways to mitigate them.
Enrolment of Women and Black Patients in Remote Monitoring Trials
Remote Haemodynamic Monitoring
Small pulmonary artery (PA) pressure monitoring has gained significant importance in managing patients with HF. The only PA pressure monitor (CardioMEMS Heart Sensor; Abbott) currently approved by the Food and Drug Administration has recently received expanded indication for patients with HF and New York Heart Association (NYHA) class II–III symptoms. Most studies that have evaluated the effectiveness of PA pressure monitoring, such as the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes) randomised trial, the observational CardioMEMS post-approval study (PAS) and MEMS-HF, have enrolled only a modest proportion of women and black patients.15–17 There was no evidence of effect modification of PA pressure monitoring by sex or race, with results following consistently the overall decrease in HF hospitalisations observed in all studies.18 Subgroup analyses on race or ethnicity were not reported in MEMS-HF. The percentages of women and black patients enrolled are listed in Table 1
Published in 2021, the GUIDE-HF randomised trial assessed the composite of all-cause mortality and HF-related events in 1,000 patients with NYHA
class II–IV symptoms.19 The study results were significantly affected by changing event rates during the COVID-19 pandemic. The two groups (remote monitoring vs. conventional treatment) did not differ in clinical outcomes during the study period; however, a pre-COVID analysis demonstrated significant benefit in the treatment group (HR 0.81; 95% CI [0.66–1.00]; p=0.049) primarily due to reductions in hospitalisations (Table 1). In contrast to CHAMPION, more participants were women (38%). Racial diversity was modest but comparable to the general population of 11–13%; only 17% of enrolled patients were black, while white patients comprised the majority (81%).20 Nevertheless, prespecified subgroup analysis indicated significant interactions for sex (p for interaction=0.01) and race (p for interaction=0.095; level of significance at 0.15), suggesting a more substantial treatment effect of remote PA pressure-guided management in women (HR 0.64; 95% CI [0.47–0.87]) and black patients (HR 0.68; 95% CI [0.48–0.97]) compared with men and non-black patients, respectively. No such finding has been previously demonstrated in similar studies. It has been suggested that such differences may represent treatment bias (implicit bias) and the possibility that these two populations are disproportionately affected by HF, present later in disease progression, and thus are more likely to experience significant benefits with PA pressure-guided treatment. The findings of the subgroup analyses are both hypothesis-generating and encouraging. They could provide the foundation for mitigating disparities in HF treatment and give an outline for future intervention targets. In broader terms, through a protocol-driven clinic intervention and the absence of direct patient–clinician interaction, RPM could facilitate a form of ‘community single blinding’ outside of the clinical trials context that could reduce bias.
Left atrial (HOMEOSTASIS, LAPTOP-HF) and right ventricular (COMPASSHF) pressure monitoring trials have evaluated the relationship between haemodynamic monitoring and HF-related outcomes.15 21–23 Female participation ranged from 22% to 35%. No information was provided regarding racial or ethnic diversity.
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
and COMPASS-
While HOMEOSTASIS
Study (Year) Target Population Location Women n (%) Patients of Colour n (%) Sex/race-specific Subgroup Analyses for the Primary Outcome (p-value for interaction) HOMEOSTASIS (2007, 2010)21 22 NYHA III–IV HF with a HF event treated with IV diuretics within 12 months Australia, New Zealand, US 9 (22.0) NR NR COMPASS-HF (2008)23 NYHA III–IV HF with a HF event treated with IV diuretics within 6 months US 96 (35.0) 64 (23.4) NR CHAMPION (2011, 2016)59 NYHA III HF with recent hospitalisation US 150 (27.0) 149 (27.0) NR LAPTOP-HF (2016)15 NYHA III HF with recent hospitalisation in 12 months or elevated natriuretic peptide US 122 (25.0) NR NR CardioMEMS post-approval study (2020)17 NYHA III HF with hospitalisation within 12 months US 452 (37.7) 202 (14.8) Reduced hospitalisations for women (HR 0.39; 95%CI [0.33–0.46], p for interaction=NR) and black patients (HR 0.49; 95%CI [0.39–0.62], p for interaction=NR) MEMS-HF (2020)16 NYHA III HF with hospitalisation within 12 months Germany, the Netherlands, Ireland 51 (21.8) NR Reduced hospitalisations
women
at 1 year for
(HR 0.51; CI [0.32–0.8], p for interaction=NR)
(2021)19 NYHA II–IV HF with hospitalisation
12 months or
brain natriuretic peptides within 1 month US 375 (37.5) 193 (19.3) Reduced
within
elevated
Table 2: Proportion of Women, Patients of Colour and Sex/Race-specific Outcomes in CIED-related Physiologic Parameter Monitoring Studies
HF failed to show significant effectiveness in the overall population, LAPTOP-HF was prematurely discontinued due to perceived excess in procedure-related complications. None of these devices has been commercially available for use.
Cardiac Implantable Electronic Devices
Although landmark trials have demonstrated that ICDs reduce the risk of sudden death in HF with reduced ejection fraction (HFrEF), women were vastly underrepresented.24 Similar observation exists for black patients who are less likely to receive an ICD or cardiac resynchronisation therapy (CRT) despite a clinical indication.13 Consequently, controversy remains regarding the effectiveness of ICDs as primary prevention in women, with one meta-analysis (in which only 19.7% of the enrolled patients were female) that combined five landmark primary prevention ICD trials (DEFINITE, SCD-HeFT, DINAMIT, MUSTT and MADIT II) notably suggesting a lack of significant survival benefit for women randomised to ICD (HR 1.01; 95% CI [0.76–1.33]) while there was a 22% reduction in mortality for men (HR 0.78; 95% CI [0.70–0.87]).25 Additional studies suggest significant under-utilisation of CRT in female patients and black patients, with these disparities increasing over time in the US despite an observed benefit of CRT in the female population.6 26–29 Although the primary goal of these studies was not to evaluate implantable remote monitoring technology, differences seen in cardiac implantable electronic device (CIED)-related remote monitoring may be attributable to the lack of representative inclusion of women and black patients in these initial studies.
The rising prevalence of CIEDs in patients with HFrEF has spurred the evolution of the technology, providing an array of diagnostic measures and leveraging proprietary algorithms that may be used to guide the management of HF in a more individualised manner. Different CIED manufacturers provide variable single or multiple physiologic parameter monitoring, collectively known as HF diagnostics. The sensitivity and effectiveness of intrathoracic impendence monitoring (CorVue; Abbott)
were evaluated in the DEFEAT-PE and LIMIT-CHF studies (Table 2).30 31 There was no observed difference by sex or race in the reduction of emergency treatment of HF. Three different studies, TRUST, ECOST and IN-TIME, evaluated the Biotronik Home Monitoring System in reducing allcause mortality and hospitalisation for worsening HF.32 33 Pooled data from these studies have shown a reduction in all-cause mortality and the composite of all-cause mortality or worsening HF hospitalisations.34 Finally, the DOT-HF and PARTNERS HF trials evaluated the use of intrathoracic impedance in combination with other parameters in Medtronic ICDs and CRTs in reducing HF events.35,36 Although a positive combined HF diagnostics algorithm conferred a higher risk of future HF hospitalisation, it did not reduce all-cause mortality or HF hospitalisations.
Two studies have evaluated the sensitivity of integrated multiparameter algorithms using device diagnostics for future HF events in intermediate to high-risk patients: MultiSENSE and SELENE-HF.37 The MultiSENSEderived algorithm had a sensitivity of 70% for HF-related events (HFEs) at a nominal threshold of 16, paired with a low unexplained alert rate and a median lead time of 34 days. Alternatively, the proprietary algorithm derived and validated in SELENE-HF had a sensitivity of 66% for predicting the primary endpoint of HF hospitalisation (95% CI: [45.7–82.1%]) with a median alert lead time of 42 days.38 Neither of the two trials described sex- or race-specific interactions (Table 2). Accordingly, the generalisability of the results of these studies, as it pertains to female patients and black patients, remains unclear. In addition, European studies systematically underreport the racial/ethnic background of patients enrolled.
Telemonitoring and Wearable Devices
Telemonitoring, unlike RPM, generally uses a multifaceted monitoring approach consisting of patient-derived data from self-reported symptoms, external devices (weight, electrocardiogram, oxygen saturation), bloodwork and more involvement of the patient’s primary clinician to improve HF outcomes.39 Compared with RPM, telemonitoring is dependent
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
Study Target Population Location Women n (%) Patients of Colour n (%) Sex/race-specific Subgroup Analyses for the Primary Outcome (p-value for interaction) TRUST (2010)92 Prior ICD implantation per guidelines US 368 (27.5) NR NR PARTNERS HF (2010)36 Prior CRT-D, EF ≤ 35%, NYHA III–IV, QRS ≥ 130 ms US 227 (32.7) 102 (14.7) NR DOT-HF (2011)35 Prior ICD or CRT-D, EF < 35%, NYHA II–IV, last HF hospitalisation within 12 months Europe, Africa, Middle East, Asia 47 (14.0) NR NR SENSE-HF (2011)93 Prior to ICD or CRT-D, the last HF hospitalisation within 12 months Europe, China, UK 79 (16.0) NR NR ECOST (2013)32 Prior ICD implantation per guidelines and NYHA II–III France 51 (11.8) NR Risk of major adverse events in women (HR 0.98; 95%CI [0.45–2.13]) DEFEAT-PE (2014)31 Prior ICD or CRT-D with 1 HF-related episode within 6 months US 42 (29.2) 61 (42.4) NR IN-TIME (2014)34 Prior ICD or CRT-D, EF < 35%, NYHA II–III Australia, Europe, Israel 128 (19.2) NR Worsening clinical score at 12 months (death, HF hospitalisation, change in clinical status) in women (HR 0.54; 95%CI [0.23–1.17]) LIMIT-CHF (2016)30 Prior ICD or CRT-D with previous HF admission, EF < 50%, and NYHA III UK 5 (6.3) NR NR MultiSENSE (2017)37 Prior CRT-D with prior HF event within 12 months, NYHA II–IV within 6 months US, Europe 273 (28.0) 322 (33.0) NR CRT-D = cardiac resynchronisation therapy–defibrillator; EF = ejection fraction; HF = heart failure; NYHA = New York Heart Association.
Table 3: Proportion of Women, Patients of Colour and Sex/Race-specific Outcomes in Remote Management Strategies Studies
within 6 months, or EF ≤ 35% with HF decompensation within 24 months
on the active engagement and participation of patients to collect data. Several randomised clinical trials have evaluated this approach of remote HF management, including TELE-HF, TIM-HF, TIM-HF2 and BEAT-HF, using a combination of strategies (automated phone systems) or integrated tools (a three-lead ECG, a blood pressure device and a weighing scale).40–43 Enrolment of female patients and black patients was significantly higher than in other remote monitoring studies (Table 3). Of those studies, only TIM-HF2 successfully demonstrated the benefit of remote monitoring in reducing HF hospitalisation days and all-cause mortality (4.88 versus 6.64%; HR 0.80; 95% CI [0.65–1.00]; p=0.046). Nevertheless, this benefit was driven by a primary outcome reduction in men (HR 0.72; 95% CI [0.56–0.95]), while no benefit was observed in women (HR 1.02; 95% CI [0.71–1.49]).
Expanding on wearable technologies for remote monitoring, commercial wearables are used for tracking activity, sleep, ECG, heart rate, oxygen saturation and so on. These devices are increasingly prevalent, especially in younger populations. Still, their accuracy (peak VO2, 6-minute walk test) or effectiveness in improving health outcomes has not been well-studied in large-scale trials and is currently under investigation in the HF population.44 The Apple Heart Study was a large study evaluating the efficacy of the Apple Watch in detecting AF in healthy participants.45 Patients with notification of AF by the watch underwent further testing with an ECG patch. A positive predictive value of 0.84 for observing AF on the ECG was shown for patients notified of an irregular pulse by the watch. Of the total study population of 419,297 participants, 42% were women, but only 12% and 7.7% identified as Latino or black, respectively. Notably, the percentage of women who received a notification for abnormal heart rhythm was almost half of that observed in enrolled men, suggesting a difference in either occurrence or detection of AF in women compared with men (21% versus 77%, respectively). However, real-world data show that although AF is more prevalent in men, the difference seen in the Apple study notifications between women and men suggests possible under-detection in women.46
The LINK-HF study examined the effectiveness of a personalised analytical platform using longitudinal data integrating vital signs to predict future HF hospitalisations via a disposable multisensor chest patch in the Veteran Administration (VA) population (Table 3).47 This was one of the first studies of wearable devices to use machine learning to predict clinical HF
deterioration. Of the 100 individuals enrolled, female participants constituted a minute 2% of the entire population. Despite the encouraging findings of the study showing a 76–88% sensitivity in detecting precursors of clinical deterioration, the small sample size limits the generalisability of these findings. Other devices have focused on surrogate measures of pulmonary oedema such as thoracic impedance. In the IMPEDANCE-HF study, which recruited 256 patients (15% female, race not reported) with chronic HFrEF and a recent hospitalisation, and randomly assigned patients to receive standard care or standard care plus monitoring with the Edema Guard Monitor (RS Medical Monitoring), monthly assessments of lung impedance resulted in a 55% reduction in HF hospitalisation, 48% reduction in all-cause mortality and 70% in HF-related deaths.48
Finally, important lessons were learned during the COVID-19 pandemic that forced health systems and individual health providers to offer video and telephone telehealth visits to complement in-person visits. Although this strategy has provided significant benefits for patient health safety, it may have exacerbated health inequities as they pertain to digital health literacy, language proficiency, access as well as proficiency with internet technology, and low socioeconomic status.49
Female patients were 30% less likely to complete a cardiovascular telehealth visit in a recent retrospective study of 2,940 patients in a large urban academic centre.50 Black patients were 17% more likely to achieve a telehealth visit than white patients, although they were more likely to use their telephone instead of a video call.50 In addition, younger patients, patients with commercial insurance, higher income and access to broadband internet were more likely to complete a video telehealth visit, as shown in three recent studies (Supplementary Material Table 1).51,52 Overall, persistent gaps are observed in randomised trials and the community as it relates to telemedicine care in female patients and black patients.
Barriers to Remote Patient Monitoring for Women
A multitude of causes could explain the persistent gaps in sex-specific research (Figure 1). The lack of sex and racial and/or ethnic diversity in principal investigators and local research staff may discourage women from participating.53 Similarly, sex-specific inclusion criteria, intervention trials (drug, device and surgery), and the location of trial coordinating sites may lead to under-enrolment.53 In addition, female patients may be more
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
Study Target Population Location Women n (%) Patients of Colour n (%) Sex/race-specific Findings TELE-HF (2010)50 Prior HF hospitalisation within 1 month US 695 (40.6) 838 (50.7) Rehospitalisation or death in women (HR 0.87; 95%CI [0.7–1.07]) and black patients (HR 1.03; 95%CI [0.83–1.28]) TIM-HF (2020) Prior HF, NYHA II–III, EF ≤ 25%
Germany 126 (17.7) NR NR TIM-HF2 (2018)42 Prior HF hospitalisation
12 months, NYHA II–III,
Germany 468 (30.4) NR All-cause mortality or unplanned rehospitalisation in women (HR 1.02; 95%CI [0.71–1.48]) BEAT-HF (2016)43 Prior documented treatment for HF US 673 (46.8) 654 (45.5) The 3-month readmission rate was not different in female patients and black patients LINK-HF (2020)47 NYHA II–IV with acute HF exacerbation in hospital US 2 (2.0) 21 (21.0) NR CRT-D =
within
EF ≤ 45%
cardiac resynchronisation therapy–defibrillator; EF = ejection fraction; HF = heart failure; NR = not reported; NYHA = New York Heart Association.
risk-averse than men under stress, which could be pertinent in healthcare decisions.54

Despite these well-described contributors, the US government authorised the National Institutes of Health (NIH) Revitalization Act in 1993 to increase the enrolment of women. However, no specific goal has been mandated.55 The limited funding for cardiovascular trials may indirectly limit the conduct of sex-specific studies and indirectly affect the care of women.
Other less tangible causes for the low rate of female participation in trials may pertain to the lack of awareness of available cardiovascular studies among women, traditional sex-specific barriers, such as caregiving responsibilities and long work hours, and decreased referrals for appropriate speciality care.55 Female subjects are overrepresented among those living in poverty and consequently are disproportionately affected by the disparities in the distribution of wealth, income and access to health-related resources.56 Thus, the underrepresentation of female participants in cardiovascular studies may be partly explained by the lack of access to healthcare and social determinants of health.57 58
Barriers to Remote Patient Monitoring for African-Americans

Technical Factors

Technical considerations may occasionally affect the enrolment of minorities in clinical trials. For example, in the CHAMPION and GUIDE-HF trials, patients were required to have a BMI <35 kg/m2 19 59 Data from the National Center for Health Statistics have demonstrated higher rates of obesity for non-Hispanic African-American patients and women in the US between 2017 and 2018.60 Recent data underscore the importance of central obesity over BMI alone in adversely impacting cardiac function, especially in women.61 Moreover, due to limitations of photoplethysmographic green light signalling, PRM devices using such technology may be accessible only to people with lighter skin tones.62 Green light lacks precision and accuracy and may not read when measuring heart rate in darker skin types.63 This becomes even more important as large-scale trials using commercial wearables become available.45 The results of these studies may not generalise to people of colour and may introduce further bias in the interpretation of the results.



Historical and Societal Factors

The low participation of African-American patients in clinical trials may be influenced by underlying mistrust of the healthcare system. AfricanAmericans have the ongoing experience of racism and segregation, which has contributed to the development of behavioural patterns and beliefs that may keep African-American patients from feeling comfortable in accessing needed diagnostic and therapeutic medical care and in clinical research studies.64 Many black Americans cite the infamous Tuskegee Syphilis Study, the obtainment of Henrietta Lacks’ cervical cells without consent, and James Marion Sims’ experiments as a few examples contributing to their medical mistrust.64 A common perception among African-American patients is that they will be used for experiments in clinical trials. The underlying mistrust is seen not only in research but also in the limited dissemination of RPM.
Furthermore, patient–provider concordance has been shown to enhance adherence to and trust in medical recommendations, particularly among African-American men.65 However, racial diversity is often lacking in the healthcare workforce (Figure 1).66 The lack of diversity may be attributed to various factors resulting from structural racism, such as low-quality secondary education, limited financial support, lack of mentorship and
Figure 1: Factors Impeding Dissemination of Remote Patient Monitoring in Women and African-Americans
role models, and unreceptive educational environments.67 In addition, patients of colour may be more often perceived as non-adherent, and physicians are less likely to discuss new therapies and clinical trials, indicating a residual bias.68 Adherence is the common endpoint of a complex and multifaceted issue that includes the inability to afford medication, fear of adverse effects, misunderstanding of the need for drugs and an inability to attend medical appointments due to work duties. Consequently, and possibly due to residual implicit bias, there is systemic under-enrolment of African-American patients in clinical trials.69

Socioeconomic Factors

Economic and racial segregation has resulted in lower socioeconomic status among racial and ethnic minority patients, including poor access to and quality of healthcare. These inequalities are likely to contribute to disparities in access to remote monitoring.70 Lower socioeconomic status is associated with an increased incidence of cardiovascular disease even after adjusting for traditional cardiovascular risk factors such as hypertension, diabetes and obesity.71 Lack of exercise facilities, healthy food outlets and institutional resources such as healthcare facilities may explain some of the increased risks for cardiovascular disease and HF seen in people living in deprived neighbourhoods. However, there appears to be a residual risk even when many of these factors are controlled for.72 Multiple socially determined vulnerabilities, including low educational attainment, low annual household income, ZIP code poverty, poor public health infrastructure and lack of health insurance, tend to cluster in the same individuals and increase future HF hospitalisations.73 These social determinants of health account for poor access to organised healthcare structures and new and innovative therapies.
In this context, remote monitoring may be at the forefront of mitigating disparities related to socioeconomic status by providing healthcare access in populations and areas with limited resources. However, this goal may prove challenging due to the lack of appropriate infrastructure in segregated or rural areas and the need for a multifaceted approach in tackling these closely intertwined factors.
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
Medical nonadherence Study technical considerations Lack of diversity in trials leadership and workforce Lack of awareness of new technology Physician inertia due to scepticism Poor socioeconomic status Implicit bias Women and black patients Gaps in knowledge in
di erences Insurance coverage
heart failure-related sex
2: Pathways to Reduce Disparities for Women and African-Americans in Remote Monitoring

at bias and racism reduction has increased diversity among healthcare professionals and may improve clinical care delivery.80,81

Policy Changes to Address the Social Determinants of Health
The social determinants of health consist of multiple dynamic components, including insurance coverage, health literacy, education, housing and wealth. To that extent, structural-level and multilevel interventions guided by city- or state-wide policies are more likely to achieve equity and eliminate disparities than individual-level interventions.82 Ensuring policy support for funding of the community health workers is critical to the sustainability of culturally appropriate interventions. Although access to insurance is necessary for providing primary healthcare, it may be insufficient in improving cardiovascular health and eliminating disparities for RPM.83 Ostensible market and health policy changes, as well as payment models focused on longer-term episodes of care and population management strategies, may serve as an impetus for the expansion of RPM in selected patients. This is particularly true if the efficacy of the RPM is around reduction in acute usage (HF hospitalisations, emergency department visits etc.).39
How to Improve Equitable Access to Remote Patient Monitoring


Tailored RPM Devices
Addressing these previously described factors is paramount (Figure 2). Careful designing of these devices in direct consultation with female leaders on industry steering committees, female patient advocacy groups, tech companies and physicians may be warranted. This process should be designed to address the specific monitoring accuracy, needs and comfort of female participants. Sex-specific design projects have been implemented in different fields of remote monitoring, such as antepartum and perinatal care.74 With regard to cardiac monitoring, radiolucent bralike garments have been used during exercise stress testing.75 These designs could culminate in the use of bra-like multisensor monitors specifically designed for women.
Eliminating Distrust
In addition, specific disparities for both female patients and AfricanAmerican patients may pertain to underlying distrust of the medical system. Improving diversity among healthcare staff is crucial in eliminating distrust. Evidence shows that diversification of the healthcare workforce may lead to improved patient outcomes and increased hospital revenue.76 77 The diversification process must disseminate through all levels and layers of healthcare professionals, including physicians, nursing staff, scientists and research coordinators.78 For example, the Faculty Institutional Recruitment for Sustainable Transformation initiative through the NIH aspires to enhance diversity and inclusion among the biomedical faculty.79 The process of workforce diversification is lengthy and would require the contribution of many key stakeholders, including medical schools, nursing colleges, technical schools, hospital administrations and healthcare professionals themselves. Training aiming
A data-proven clinical benefit of RPM in underrepresented populations would further incentivise commercial and public payers to cover the cost of wearable devices and RPM.84 However, data are lacking, and further efforts to include these newer devices in clinical trials are needed. A few digital health companies are targeting low-income and Medicaid populations as potential markets for expanding their reach.85 Innovative platforms providing wrap-around, community-based services for dual eligible and Medicaid populations may be rapid adopters of efficacious RPM and provide a bridge for existing gaps in RPM coverage.86 A better distribution of medical services in underserved neighbourhoods is also required in addition to insurance access.87 To improve vulnerable patients’ awareness of available medical services, community clinic engagement has been recognised as an effective means of increasing involvement and understanding of underrepresented patients.88
Disparity Reduction Through Technological Innovation and Patient Engagement

RPM is centrally positioned to reduce sex and racial disparities in multiple ways. First, it can theoretically mitigate implicit sex and racial bias through a protocol-driven clinic intervention and the absence of direct patient–clinician interactions, to result in a form of community single blinding outside of the clinical trials context. For example, providers are prompted to treat data directly, reducing potential bias sources. This, in turn, may lead to earlier identification and treatment of subclinical conditions that affect female patients and African-American patients.89 90 Second, RPM may improve healthcare access in underserved areas with poor healthcare structures, eliminating isolation and perceived borders in segregated neighbourhoods. However, the latter may prove challenging given the concurrent absence of other vital infrastructure needed for RPM, such as access to broadband internet. Third, automated patient communication between visits may improve their health engagement and guide them in adhering to their medical care plan. Voice prompts to measure one’s weight or remind one to take their prescribed medication may encourage patients that their condition is professionally managed and reinforce the need to follow essential steps to maintain their well-being. Finally, the delivery of interactive digital material for patient education on their condition could improve outcomes and intensify guideline-directed medical therapies through a sense of patient engagement and selfempowerment in underserved populations.91

Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure
Improving workforce and leadership diversity
Accurately assessing the impact of heart failure on women and black patients
Increasing
Avoiding
Reducing implicit bias by blinded interventions Increasing awareness of gaps and/or inequalities among stakeholders
access to healthcare and health innovation
delays in intervention
Reducing disparities by:
Workforce diversification and accurate assessment of the impact of heart failure on vulnerable populations will culminate in reduction in implicit bias and increased access to remote patient monitoring, thereby avoiding delays in appropriate healthcare delivery.
Conclusion
As in other cardiovascular diagnostics and therapeutics fields, disparities in the representation and access of women and African-American patients to RPM for the management of HF persist. The research enterprise’s complex technical, socioeconomic, cultural, educational and systemic factors contribute to this phenomenon. The engagement of key stakeholders (female and African-American associations, policymakers) will create the premises for policy transformation targeting equitable
1. Pronovost PJ, Cole MD, Hughes RM. Remote patient monitoring during COVID-19. JAMA 2022;327:1125–6. https:// doi.org/10.1001/jama.2022.2040; PMID: 35212725.
2. Kennel PJ, Rosenblum H, Axsom KM, et al. Remote cardiac monitoring in patients with heart failure: a review. JAMA Cardiol 2022;7:556–64. https://doi.org/10.1001/ jamacardio.2021.5090; PMID: 34964805.
3. Lala A, Tayal U, Hamo CE, et al. Sex differences in heart failure. J Card Fail 2022;28:477–98. https://doi.org/10.1016/j. cardfail.2021.10.006; PMID: 34774749.
4. Defilippis EM, Beale A, Martyn T, et al. Heart failure subtypes and cardiomyopathies in women. Circ Res 2022;130:436–54. https://doi.org/10.1161/ CIRCRESAHA.121.319900; PMID: 35175847.
5. Cesaroni G, Mureddu GF, Agabiti N, et al. Sex differences in factors associated with heart failure and diastolic left ventricular dysfunction: a cross-sectional population-based study. BMC Public Health 2021;21:415. https://doi.org/10.1186/ s12889-021-10442-3; PMID: 33639910.
6. Sullivan K, Doumouras BS, Santema BT, et al. Sex-specific differences in heart failure: pathophysiology, risk factors, management, and outcomes. Can J Cardiol 2021;37:560–71. https://doi.org/10.1016/j.cjca.2020.12.025; PMID: 33383166.
7. Stolfo D, Uijl A, Vedin O, et al. Sex-based differences in heart failure across the ejection fraction spectrum: phenotyping, and prognostic and therapeutic implications. JACC Heart Fail 2019;7:505–15. https://doi.org/10.1016/j. jchf.2019.03.011; PMID: 31146874.
8. Dewan P, Rørth R, Raparelli V, et al. Sex-related differences in heart failure with preserved ejection fraction. Circ Heart Fail 2019;12:e006539. https://doi.org/10.1161/ CIRCHEARTFAILURE.119.006539; PMID: 31813280.
9. Lew J, Sanghavi M, Ayers CR, et al. Sex-based differences in cardiometabolic biomarkers. Circulation 2017;135:544–55. https://doi.org/10.1161/CIRCULATIONAHA.116.023005; PMID: 28153991.
10. Glynn P, Lloyd-Jones DM, Feinstein MJ, et al. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol 2019;73:2354–5. https://doi. org/10.1016/j.jacc.2019.02.042; PMID: 31072580.
11. Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol 2010;56:8–14. https:// doi.org/10.1016/j.jacc.2010.03.043; PMID: 20620710.
12. Shah RU, Klein L, Lloyd-Jones DM. Heart failure in women: epidemiology, biology and treatment. Womens Health (Lond) 2009;5:517–27. https://doi.org/10.2217/WHE.09.50; PMID: 19702451.
13. Farmer SA, Kirkpatrick JN, Heidenreich PA, et al. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009;6:325–31. https://doi.org/10.1016/j. hrthm.2008.12.018; PMID: 19251206.
14. Sullivan LT, Randolph T, Merrill P, et al. Representation of black patients in randomized clinical trials of heart failure with reduced ejection fraction. Am Heart J 2018;197:43–52. https://doi.org/10.1016/j.ahj.2017.10.025; PMID: 29447783.
15. Abraham WT, Adamson PB, Costanzo MR, et al. Hemodynamic monitoring in advanced heart failure: results from the LAPTOP-HF trial. J Card Fail 2016;22:940. https:// doi.org/10.1016/j.cardfail.2016.09.012
16. Angermann CE, Assmus B, Anker SD, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European monitoring study for heart failure. Eur J Heart Fail 2020;22:1891–901. https://doi.org/10.1002/ejhf.1943; PMID: 32592227.
17. Shavelle DM, Desai AS, Abraham WT, et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail 2020;13:e006863. https://doi. org/10.1161/CIRCHEARTFAILURE.119.006863; PMID: 32757642.
access in RPM. Diversification of medical workforce and leadership through a multifaceted involvement (medical and nursing schools, hospital administrations etc.) may improve cultural diversity and improve the inclusion milieu. Adopting alternative pathways of funding with a specific aim to bridge existing gaps will require the demonstration of objective clinical benefit through clinical trials in these populations. Overall, RPM has the unique potential to eliminate disparities through a combination of bias elimination and health equity propagation.
18. Defilippis EM, Henderson J, Axsom KM, et al. Remote hemodynamic monitoring equally reduces heart failure hospitalizations in women and men in clinical practice: a sex-specific analysis of the CardioMEMS post-approval study. Circ Heart Fail 2021;14:e007892 https://doi.org/10.1161/ CIRCHEARTFAILURE.120.007892; PMID: 34129363.
19. Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamicguided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991–1001. https://doi.org/10.1016/S0140-6736(21)01754-2; PMID: 34461042.
20. Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 2017;70:2476–86. https://doi. org/10.1016/j.jacc.2017.08.074; PMID: 29141781.
21. Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 2007;116:2952–9. https://doi.org/10.1161/ CIRCULATIONAHA.107.702191; PMID: 18056531.
22. Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010;121:1086–95. https:// doi.org/10.1161/CIRCULATIONAHA.108.800490; PMID: 20176990.
23. Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51:1073–9. https://doi.org/10.1016/j.jacc.2007.10.061; PMID: 18342224.
24. Hsich EM. Sex differences in advanced heart failure therapies. Circulation 2019;139:1080–93. https://doi. org/10.1161/CIRCULATIONAHA.118.037369; PMID: 30779645.
25. Ghanbari H, Dalloul G, Hasan R, et al. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:1500–6. https:// doi.org/10.1001/archinternmed.2009.255; PMID: 19752408.
26. Chatterjee NA, Borgquist R, Chang Y, et al. Increasing sex differences in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator. Eur Heart J 2017;38:1485–94. https://doi.org/10.1093/eurheartj/ ehw598; PMID: 28065904.
27. Sridhar ARM, Yarlagadda V, Parasa S, et al. Cardiac resynchronization therapy: US trends and disparities in utilization and outcomes. Circ Arrhythm Electrophysiol 2016;9:e003108. https://doi.org/10.1161/CIRCEP.115.003108; PMID: 26921376.
28. Lund LH, Braunschweig F, Benson L, et al. Association between demographic, organizational, clinical, and socioeconomic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail 2017;19:1270–9. https://doi. org/10.1002/ejhf.781; PMID: 28176416.
29. Arshad A, Moss AJ, Foster E, et al. Cardiac resynchronization therapy is more effective in women than in men: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol 2011;57:813–20. https://doi. org/10.1016/j.jacc.2010.06.061; PMID: 21310317.
30. Domenichini G, Rahneva T, Diab IG, et al. The lung impedance monitoring in treatment of chronic heart failure (the LIMIT-CHF study). Europace 2016;18:428–35. https://doi. org/10.1093/europace/euv293; PMID: 26683599.
31. Heist EK, Herre JM, Binkley PF, et al. Analysis of different device-based intrathoracic impedance vectors for detection of heart failure events (from the Detect Fluid Early from Intrathoracic Impedance Monitoring study). Am J Cardiol 2014;114:1249–56. https://doi.org/10.1016/j. amjcard.2014.07.048; PMID: 25150135.
32. Guedon-Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–14. https://doi.org/10.1093/eurheartj/
ehs425; PMID: 23242192.
33. Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–90. https://doi.org/10.1016/S01406736(14)61176-4; PMID: 25131977.
34. Hindricks G, Varma N, Kacet S, et al. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38:1749–55. https://doi.org/10.1093/eurheartj/ehx015; PMID: 29688304.
35. Van Veldhuisen DJ, Braunschweig F, Conraads V, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719–26. https://doi.org/10.1161/ CIRCULATIONAHA.111.043042; PMID: 21931078.
36. Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J Am Coll Cardiol 2010;55:1803–10. https://doi.org/10.1016/j.jacc.2009.11.089; PMID: 20413029.
37. Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices. JACC Heart Fail 2017;5:216–25. https:// doi.org/10.1016/j.jchf.2016.12.011; PMID: 28254128.
38. D’Onofrio A, Solimene F, Calò L, et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: results from the SELENE HF study. Europace 2022;24:234–44. https://doi.org/10.1093/europace/euab170; PMID: 34392336.
39. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail 2019;7:922–32. https://doi.org/10.1016/j.jchf.2019.08.008; PMID: 31672308.
40. Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–9. https://doi.org/10.1056/NEJMoa1010029; PMID: 21080835.
41. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873–80. https://doi.org/10.1161/ CIRCULATIONAHA.111.018473; PMID: 21444883.
42. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallelgroup, unmasked trial. Lancet 2018;392:1047–57. https://doi. org/10.1016/S0140-6736(18)31880-4; PMID: 30153985.
43. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition – Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med 2016;176:310–8. https://doi.org/10.1001/ jamainternmed.2015.7712; PMID: 26857383.
44. Apple. Using Apple Watch to Estimate Cardio Fitness with VO2 max. Cupertino, CA: Apple, 2021. htttps://www.apple.com/ healthcare/docs/site/Using_Apple_Watch_to_Estimate_ Cardio_Fitness_with_VO2_max.pdf (accessed 26 March 2022).
45. Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. https://doi.org/10.1056/ NEJMoa1901183; PMID: 31722151.
46. Westerman S, Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev 2019;15:136–44. https://doi.org/10 .2174/1573403X15666181205110624; PMID: 30516110.
47. Stehlik J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF Multicenter Study. Circ Heart Fail 2020;13:e006513. https://doi.org/10.1161/
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
CIRCHEARTFAILURE.119.006513; PMID: 32093506.
48. Shochat MK, Shotan A, Blondheim DS, et al. Non-invasive lung IMPEDANCE-guided preemptive treatment in chronic heart failure patients: a randomized controlled trial (IMPEDANCE-HF trial). J Card Fail 2016;22:713–22. https://doi. org/10.1016/j.cardfail.2016.03.015; PMID: 27058408.
49. Crawford A, Serhal E. Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health. J Med Internet Res 2020;22:e19361. https://doi. org/10.2196/19361; PMID: 32452816.
50. Eberly LA, Khatana SAM, Nathan AS, et al. Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation 2020;142:510–2. https://doi.org/10.1161/ CIRCULATIONAHA.120.048185; PMID: 32510987.
51. Rodriguez JA, Betancourt JR, Sequist TD, Ganguli I. Differences in the use of telephone and video telemedicine visits during the COVID-19 pandemic. Am J Manag Care 2021;27:21–6. https://doi.org/10.37765/ajmc.2021.88573; PMID: 33471458.
52. Sammour Y, Spertus JA, Shatla I, et al. Comparison of video and telephone visits in outpatients with heart failure. Am J Cardiol 2021;158:153–6. https://doi.org/10.1016/j. amjcard.2021.08.008; PMID: 34470705.
53. Whitelaw S, Sullivan K, Eliya Y, et al. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur J Heart Fail 2021;23:15–24. https://doi.org/10.1002/ejhf.2034; PMID: 33118664.
54. Mather M, Lighthall NR. Both risk and reward are processed differently in decisions made under stress. Curr Dir Psychol Sci 2012;21:36–41. https://doi.org/10.1177/0963721411429452; PMID: 22457564.
55. Tobb K, Kocher M, Bullock-Palmer RP. Underrepresentation of women in cardiovascular trials: it is time to shatter this glass ceiling. Cardiol Res Pract 2022;13:100109. https://doi. org/10.1016/j.ahjo.2022.100109
56. Kenkre TS, Malhotra P, Johnson BD, et al. Ten-year mortality in the WISE study (Women’s Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes 2017;10:e003863. https://doi. org/10.1161/CIRCOUTCOMES.116.003863; PMID: 29217675.
57. Wei S, Le N, Zhu JW, et al. Factors associated with racial and ethnic diversity among heart failure trial participants: a systematic bibliometric review. Circ Heart Fail 2022;15:e008685. https://doi.org/10.1161/ CIRCHEARTFAILURE.121.008685; PMID: 34911363.
58. Whitelaw S, Thabane L, Mamas MA, et al. Characteristics of heart failure trials associated with under-representation of women as lead authors. J Am Coll Cardiol 2020;76:1919–30. https://doi.org/10.1016/j.jacc.2020.08.062; PMID: 33092727.
59. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66. https://doi.org/10.1016/S01406736(11)60101-3; PMID: 21315441.
60. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;360:1–8. PMID: 32487284.
61. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. https://doi.org/10.1016/j.jacc.2013.02.092; PMID: 23684677.
62. Fallow BA, Tarumi T, Tanaka H. Influence of skin type and wavelength on light wave reflectance. J Clin Monit Comput 2013;27:313–7. https://doi.org/10.1007/s10877-013-9436-7;
PMID: 23397431.
63. Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation:
the effects of oximeter probe type and gender. Anesth Analg 2007;105(6 Suppl):S18–23. https://doi.org/10.1213/01. ane.0000285988.35174.d9; PMID: 18048893.
64. Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers 2007;14:56–60. PMID: 19175244.
65. Takeshita J, Wang S, Loren AW, et al. Association of racial/ ethnic and gender concordance between patients and physicians with patient experience ratings. JAMA Netw Open 2020;3:e2024583. https://doi.org/10.1001/ jamanetworkopen.2020.24583; PMID: 33165609.
66. Salsberg E, Richwine C, Westergaard S, et al. Estimation and comparison of current and future racial/ethnic representation in the US health care workforce. JAMA Netw Open 2021;4:e213789. https://doi.org/10.1001/ jamanetworkopen.2021.3789; PMID: 33787910.
67. Bajaj SS, Stanford FC. Beyond Tuskegee: vaccine distrust and everyday racism. N Engl J Med 2021;384:e12. https://doi. org/10.1056/NEJMpv2035827; PMID: 33471971.
68. Breathett K, Yee E, Pool N, et al. Does race influence decision making for advanced heart failure therapies? J Am Heart Assoc 2019;8:e013592. https://doi.org/10.1161/ JAHA.119.013592; PMID: 31707940.
69. Chen S, Li J. Participation of Black US residents in clinical trials of 24 cardiovascular drugs granted FDA approval, 2006–2020. JAMA Netw Open 2021;4:e212640. https://doi. org/10.1001/jamanetworkopen.2021.2640; PMID: 33755163.
70. Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137:2166–78. https://doi.org/10.1161/ CIRCULATIONAHA.117.029652; PMID: 29760227.
71. Akwo EA, Kabagambe EK, Harrell FE, et al. Neighborhood deprivation predicts heart failure risk in a low-income population of blacks and whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes 2018;11:e004052. https://doi.org/10.1161/CIRCOUTCOMES.117.004052; PMID: 29317456.
72. Diez Roux AV. Neighborhoods and health: what do we know? What should we do? Am J Public Health 2016;106:430–1. https://doi.org/10.2105/AJPH.2016.303064; PMID: 26885960.
73. Pinheiro LC, Reshetnyak E, Sterling MR, et al. Multiple vulnerabilities to health disparities and incident heart failure hospitalization in the REGARDS study. Circ Cardiovasc Qual Outcomes 2020;13:e006438. https://doi.org/10.1161/ CIRCOUTCOMES.119.006438; PMID: 32703013.
74. Ryu D, Kim DH, Price JT, et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc Natl Acad Sci USA 2021;118:e2100466118. https://doi.org/10.1073/ pnas.2100466118; PMID: 33972445.
75. CardioBra. 2022. https://cardiobra.com/#intro (accessed 26 March 2022).
76. Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc 2019;111:383–92. https://doi. org/10.1016/j.jnma.2019.01.006; PMID: 30765101.
77. Alsan M, Garrick O, Graziani G. Does diversity matter for health? Experimental evidence from Oakland. Am Econ Rev 2019;109:4071–111. https://doi.org/10.1257/aer.20181446
78. Rotenstein LS, Reede JY, Jena AB. Addressing workforce diversity: a quality-improvement framework. N Engl J Med 2021;384:1083–6. https://doi.org/10.1056/NEJMp2032224; PMID: 33764706.
79. National Institute on Minority Health and Health Disparities. Faculty institutional recruitment for sustainable transformation (FIRST). 2022. https://www.nimhd.nih.gov/ programs/collab/first (accessed 26 March 2022).
80. Capers Q, Clinchot D, McDougle L, Greenwald AG. Implicit racial bias in medical school admissions. Acad Med
2017;92:365–9. https://doi.org/10.1097/ ACM.0000000000001388; PMID: 27680316.
81. Devine PG, Forscher PS, Cox WTL, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol 2017;73:211–5. https://doi.org/10.1016/j.jesp.2017.07.002; PMID: 29249837.
82. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–98. https://doi. org/10.1161/CIR.0000000000000228; PMID: 26240271.
83. Breathett K, Allen LA, Helmkamp L, et al. The Affordable Care Act Medicaid expansion correlated with increased heart transplant listings in African-Americans but not Hispanics or Caucasians. JACC Heart Fail 2017;5:136–47. https://doi.org/10.1016/j.jchf.2016.10.013; PMID: 28109783.
84. Joynt KE. Health policy and cardiovascular medicine: rapid changes, immense opportunities. Circulation 2015;131:1098–105. https://doi.org/10.1161/CIRCULATIONAHA.114.013606; PMID: 25802255.
85. Kim SE, Castro Sweet CM, Cho E, et al. Evaluation of a digital diabetes prevention program adapted for low-income patients, 2016–2018. Prev Chronic Dis 2019;16:E155. https:// dx.doi.org/10.5888/pcd16.190156; PMID: 31775010.
86. Olsen E. Cityblock rakes in $400M for platform focused on Medicaid and low-income populations and other digital health fundings. Mobile Health News 7 September 2021. https://www.mobihealthnews.com/news/cityblock-rakes400m-platform-focused-medicaid-and-low-incomepopulations-and-other-digital (accessed 26 March 2022).
87. Sims M, Kershaw KN, Breathett K, et al. Importance of housing and cardiovascular health and well-being: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes 2020;13:e000089. https://doi. org/10.1161/HCQ.0000000000000089; PMID: 32673512.
88. Johnson DA, Joosten YA, Wilkins CH, Shibao CA. Case study: community engagement and clinical trial success: outreach to African American women. Clin Transl Sci 2015;8:388–90. https://doi.org/10.1111/cts.12264; PMID: 25752995.
89. Devon HA, Burke LA, Nelson H, et al. Disparities in patients presenting to the emergency department with potential acute coronary syndrome: it matters if you are black or white. Heart Lung 2014;43:270–7. https://doi.org/10.1016/j. hrtlng.2014.04.019; PMID: 24992880.
90. Walsh MN, Joynt KE. Delays in seeking care: a women’s problem? Circ Cardiovasc Qual Outcomes 2016;9(2 Suppl 1):S97–9. https://doi.org/10.1161/CIRCOUTCOMES.116.002668; PMID: 26908868.
91. Allen LA, Venechuk G, McIlvennan CK, et al. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction: the EPIC-HF trial. Circulation 2021;143:427–37. https://doi.org/10.1161/ CIRCULATIONAHA.120.051863; PMID: 33201741.
92. Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverterdefibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–32. https://doi.org/10.1161/ CIRCULATIONAHA.110.937409; PMID: 20625110.
93. Conraads VM, Tavazzi L, Santini M, et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J 2011;32:2266–73. https://doi.org/10.1093/eurheartj/ehr050; PMID: 21362703.
Disparities in Heart Failure Remote Monitoring CARDIAC FAILURE REVIEW www.CFRjournal.com
Pharmacy Challenges in Cardiac Patient Care During the COVID-19 Pandemic: Lessons Learnt For the Future
Andrew McRae
Department of Pharmacy, Vanderbilt University Medical Center, Nashville, TN, US
Keywords
COVID-19, drug management, pharmacy, pharmacist, multidisciplinary, cardiology
Disclosure: The author has no conflicts of interest to declare
Received: 11 May 2022 Accepted: 19 Oct 2022 Citation: Cardiac Failure Review 2023;9:e03. DOI: https://doi.org/10.15420/cfr.2022.20
Correspondence: Andrew McRae, Department of Pharmacy, Vanderbilt University Medical Center, 1211 Medical Center Drive, B-131 VUH, Nashville, TN 32732, US.
E: Andrew.s.mcrae@vumc.org
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The COVID-19 pandemic created many unexpected challenges for pharmacists in providing patient care. Some of the issues discussed here have presented as a single challenge in the past, but rarely – if ever – has there been a situation that brought so many challenges simultaneously. Several articles have described the incredible stress that was placed on community pharmacists and hospital pharmacy operations, as well as pharmacists in positions of infectious disease and hospital policy development.1–3 This editorial will focus on unique challenges that were encountered in caring for cardiac patient populations.
Early in the pandemic – before transmission and communicability were well known – there was tremendous concern about virus exposure in our procedural areas, such as the cardiac catheterisation laboratory. Because of this, many primary percutaneous coronary intervention (PCI) centres were faced with the challenge of managing COVID-19 patients who presented with concern for acute coronary syndrome (ACS). The American College of Cardiology’s Interventional Council and Society for Cardiovascular Angiography and Interventions released a statement to help assist in assessing the risk and benefits for both the patient and staff involved.4 In turn, this led to patients receiving thrombolytic therapy at a much higher frequency.
This was the first time that I personally had a patient who presented to a primary PCI centre undergo lytic therapy for an ST-elevation MI instead of immediately being taken to the catheterisation laboratory. Many community and rural hospital systems that do not offer 24-hour PCI availability often use thrombolytic therapy before transporting the patient to a PCI centre.5 Staff at these centres have a much higher level of practice experience with these medications compared with primary PCI centres.
At our centre, we did not have drug files for alteplase built into our pump infusion libraries for the indication of MI. Additionally, cardiology providers and nursing staff had to be educated about the contraindications for thrombolytic therapy and the monitoring that is required during and after the infusion. This experience emphasised the importance of monitoring drug medication files and their indications continually within a hospital
system. Additionally, institutions must be able to act quickly to update these files in instances when an absence is discovered or when a medication is approved for a new indication. Because we had these review processes and protocols in place before the pandemic, we were able to quickly address this issue without having to administer a high-risk medication without the appropriate safety guardrails in the infusion pump. The pharmacists in the emergency department and cardiac intensive care unit (ICU) have a close relationship with the nursing educators for these units. An education effort was quickly implemented to update staff on the administration and monitoring procedures for these patients.
Another unique challenge related to protecting staff was how to respond to in-hospital codes. Because this was still early in the pandemic, as described above, it was imperative to protect our staff as much as possible. At our institution, we have a pharmacist on our multidisciplinary cardiopulmonary resuscitation committee. Through that committee, a plan was implemented to help reduce the number of staff entering a patient’s room when COVID-19 was suspected or confirmed, as well as to reduce the number of times that staff would leave the room. We developed code bags that could be accessed from our medication dispensing cabinets to avoid having to take the entire code cart into the patient’s room. These bags contained two code syringes of epinephrine, two vials of amiodarone and an atropine syringe. The initial code team would take this bag into the room at the initiation of the code. The code cart with other medications would then be stationed just outside the patient’s room. When a new person would enter the room to relieve the person doing compressions, they would then bring in a new bag of medications. This was accomplished through great collaboration with all the different provider groups involved in hospital code response. Additionally, pharmacy operations played a vital role in developing these code bags and in keeping them stocked on the floors.
Monitoring of outpatient heart failure patients and warfarin anticoagulation was another unique challenge presented by the COVID-19 pandemic. Goal-directed medical therapy and the related pharmacological regimen is the backbone for patient survival and quality of life.6 Patients with heart
EDITORIAL Patient care during pandemic and beyond © 2023 The Author(s). Published by Radcliffe Group Ltd. www.CFRjournal.com
failure are at a very high risk of serious complications from infection with SARS-CoV-2.7 Consequently, many patients expressed fear about coming to clinic appointments or presenting to the emergency department when experiencing an exacerbation of heart failure because of fear of exposure to the virus. During the initial lockdown period of the pandemic, our institution had many days on which most of our heart-failure clinic patients would cancel or not attend. These are important appointments, particularly for medication optimisation; it is essential that patients are up-titrated as tolerated and that they are being maintained on an appropriate diuretic regimen. Furthermore, many medical centres rely on pharmacist-led warfarin anticoagulation monitoring.
In response to these issues, telehealth and telemedicine opportunities expanded exponentially.8,9 Providers were now able to connect to patients in the safety of their own homes. There are challenges in implementing these technologies into patient care.8 9 However, faced with the alternative of limited or no medical follow-up, many patients embraced this opportunity and it provided a very good way to help these high-risk patients. Without the pandemic, it would be difficult to envision an expansion to implement this technology at the rate that was seen during these past 2 years. Unfortunately, this did not solve all the issues as there was still difficulty in reaching many of our patients, emphasising the importance of communication with our patients in the community and finding other unique ways to reach them.
There were other very difficult challenges during the pandemic. Identifying drug–drug and drug–disease interactions and their appropriate monitoring was especially complicated. At the very beginning of the pandemic there were early reports of hydroxychloroquine having antiviral properties that would help to prevent or treat COVID-19. Although clinical trials later demonstrated that this was not an effective treatment, many patients were prescribed or self-administered hydroxychloroquine.10 Because of QT prolongation that this medication can cause, there were many reports of patients having serious adverse effects because of an interaction with either an underlying prolonged QT or medications they were already taking that prolonged their QT.11 12
Once Food and Drug Administration (FDA)-approved medications were introduced, new challenges with drug–drug interactions arose. The antiviral regimen nirmatrelvir/ritonavir is a very strong cytochrome P450 and P-glycoprotein inhibitor that can substantially change the pharmacokinetics of vital medications. Tacrolimus, clopidogrel, ticagrelor, rivaroxaban, apixaban, dabigatran, edoxaban and amiodarone are just a few examples of medications that should be co-administered only under close supervision due to significant dose adjustments or increased therapeutic drug monitoring that may be required.13,14 Because these treatment regimens are so new, it is very easy to not identify a key interaction when prescribing or dispensing, in particular when the patient is prescribed and dispensed the medication outside their normal hospital care network.
As of this publication, many of these medications have only been available in limited quantities. As they become more widely available, hopefully, there will be a better system to help flag these interactions. There has been substantial improvement with the electronic medical record having access to insurance claims and third-party prescriptions; however, there is a great opportunity to use it at its full potential. Admittedly, as a pharmacist using these resources daily, they are not intuitive, and it can be very difficult to access some major health centres such as the Veterans Affairs system. I hope we can use this pandemic as a way to continue to push for
better development of more transparent medical records systems. This also highlights the importance of a thorough patient interview in the clinic or upon patient admission to screen for these serious drug–drug interactions. Finally, it is important to educate patients receiving critical medications such as tacrolimus to check with their transplant pharmacist or transplant clinic to ensure there are no dangerous interactions if they are started on a new medication.
An additional key area of patient monitoring where the COVID-19 pandemic created a challenge was anticoagulation management. The partial thromboplastin time (PTT) is a crucial measurement of clotting time that is used for the management of many cardiac conditions. Patientspecific coagulation has been shown to be greatly variable in patients positive for COVID-19.15 Many hospital systems use the assistance of their pharmacy department to monitor and titrate anticoagulation therapies. This could be for indications such as ACS, AF, deep venous thrombosis and pulmonary embolisms, all of which carry a risk for further cardiac complications. Because of this variability in patient’s baseline PTT before anticoagulation initiation, alternative monitoring strategies had to be quickly implemented. These changes were reviewed and approved by our institution’s anticoagulation committee – a multidisciplinary group comprising haematologists, emergency department physicians, clinical pharmacists, pharmacy informatics specialists and administrators. Hospital protocol changes that would normally take months to change were modified within days to permit monitoring and adjustments based on unfractionated heparin levels (anti-Xa) or thromboelastography.15
One health-system issue further exacerbated by the pandemic was supply chain problems. In the area of pharmaceutical manufacturing, the medication supply chain has become increasingly reliant on single manufacturers of medications. Third-party supply of packaging, containers and materials has led to drug manufacturers being unable to keep up with the demands of production. However, according to data from the FDA, the number of new drug shortages has declined every year since 2019.16
The past 2 years have introduced unprecedented challenges to healthcare providers. The collaboration and work done by groups from all over the world have been inspiring. Among the lessons learned that can hopefully continue in the future is the close communication between intradepartment and multidisciplinary teams. During the height of the pandemic, there were daily emails sent to our entire pharmacy department. These emails would outline, for example, patient census information (ICU expansion and new COVID-19 intake locations), drug updates (treatment and shortages) and policy changes (visitor policies, code responses). This was very helpful in keeping the staff updated on a constantly changing landscape and also allowed pharmacists to give updates to the multidisciplinary committees they worked in. Additionally, in these committees, there would be questions that would arise about possible drug shortages or successful treatment regimens that the pharmacist could then relay to their management or the appropriate team so we could anticipate changes. This level of communication contributed to the implementation of changes to our infusion pumps, code response and drug inventory at a better rate, which hopefully led to better patient care.
Another change that will hopefully be continued is the use of telehealth. This can be a significant way to maintain meaningful relationships with patients that can lead to medication optimisation and prevention of readmissions. The convenience of telehealth can reach our more rural patient populations and prevent appointment cancellations and patients lost to follow-up if implemented properly. There are drawbacks that will
Pharmacy Challenges in Cardiac Care During COVID-19: Lessons Learnt CARDIAC FAILURE REVIEW www.CFRjournal.com
need to be addressed, but, with continued support, these changes can be made so that other patients are not left out. A third lesson that was learnt was to help and support the healthcare community. It was moving to see how – at a moment’s notice – colleagues from different departments, different specialities and in different parts of the world did everything they
1. Bhamra SK, Parmar J, Heinrich M. Impact of the coronavirus pandemic (COVID-19) on the professional practice and personal well-being of community pharmacy teams in the UK. Int J Pharm Pract 2021;29:556–65. https://doi. org/10.1093/ijpp/riab062; PMID: 34605895
2. Bookwalter CM. Challenges in community pharmacy during COVID-19: the perfect storm for personnel burnout. US Pharm 2021;46:28–31.
3. Herzik KA, Bethishou L. The impact of COVID-19 on pharmacy transitions of care services. Res Social Adm Pharm 2021;17:1908–12. https://doi.org/10.1016/j. sapharm.2020.10.017; PMID: 33162381
4. Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC’s Interventional Council and SCAI. J Am Coll Cardiol 2020;75:2372–5. https://doi.org/10.1016/j. jacc.2020.03.021; PMID: 32199938
5. O’gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–e140. https://doi.org/10.1016/j.jacc.2012.11.019; PMID: 23256914
6. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/ HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American
could do to help. Many of these colleagues are now having difficulty with burnout and have lost the desire to be in medicine. I feel that we are starting to realise the impact that this pandemic had on our healthcare community and it is going to take a lot of work and support for it to recover.
Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–421. https://doi. org/10.1016/j.jacc.2021.12.012; PMID: 35379503
7. Kong KA, Jung S, Yu M, et al. Association between cardiovascular risk factors and the severity of coronavirus disease 2019: nationwide epidemiological study in Korea. Front Cardiovasc Med 2021;8:1-10. https://doi.org/10.3389/ fcvm.2021.732518; PMID: 34568465
8. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. 2020. https://www.cdc.gov/ coronavirus/2019-ncov/hcp/telehealth.html (accessed 13 January 2023).
9. Doraiswamy S, Jithesh A, Mamtani R, et al. Telehealth use in geriatrics care during the COVID-19 pandemic—a scoping review and evidence synthesis. Int J Environ Res Public Health 2021;18:1755. https://doi.org/10.3390/ijerph18041755; PMID: 33670270
10. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA 2020;324:2165–76. https://doi.org/10.1001/ jama.2020.22240; PMID: 33165621
11. Jankelson L, Karam G, Becker ML, et al. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19:
a systematic review. Heart Rhythm 2020;17:1472–9. https:// doi.org/10.1016/j.hrthm.2020.05.008; PMID: 32438018
12. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 2020;17:1425–33. https://doi.org/10.1016/j. hrthm.2020.05.014; PMID: 32407884
13. Norvir (ritonavir) [prescribing information]. North Chicago, IL, US: AbbVie, 2020. https://www.accessdata.fda.gov/ drugsatfda_docs/label/2017/209512lbl.pdf (accessed 13 January 2023).
14. Marsousi N, Daali Y, Fontana P, et al. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet 2018;57:1347–54. https://doi.org/10.1007/ s40262-018-0637-6; PMID: 29453687
15. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the Anticoagulation Forum. J Thromb Thrombolysis 2020;50:72–81. https://doi. org/10.1007/s11239-020-02138-z; PMID: 32440883
16. U.S. Food & Drug Administration. Report to Congress: Drug Shortages for Calendar Year 2021. FDA, 2021. https://www.fda. gov/media/159302/download (accessed 13 January 2023).
Pharmacy Challenges in Cardiac Care During COVID-19: Lessons Learnt CARDIAC FAILURE REVIEW www.CFRjournal.com
Imaging in Heart Failure with Preserved Ejection Fraction: A Multimodality Imaging Point of View
Serkan Ünlü , 1 Özge Özden 2 and Ahmet Çelik 3
1. Department of Cardiology, Gazi University, Ankara, Turkey; 2. Cardiology Department, Memorial Bahçelievler Hospital, Istanbul, Turkey; 3. Department of Cardiology, Mersin University, Mersin, Turkey.
Abstract
Heart failure with preserved ejection fraction (HFpEF) is an important global health problem. Despite increased prevalence due to improved diagnostic options, limited improvement has been achieved in cardiac outcomes. HFpEF is an extremely complex syndrome and multimodality imaging is important for diagnosis, identifying its different phenotypes and determining prognosis. Evaluation of left ventricular filling pressures using echocardiographic diastolic function parameters is the first step of imaging in clinical practice. The role of echocardiography is becoming more popular and with the recent developments in deformation imaging, cardiac MRI is extremely important as it can provide tissue characterisation, identify fibrosis and optimal volume measurements of cardiac chambers. Nuclear imaging methods can also be used in the diagnosis of specific diseases, such as cardiac amyloidosis.
Disclosure: The authors have no conflicts of interest to declare.
Received: 15 September 2022 Accepted: 18 October 2022 Citation: Cardiac Failure Review 2023;9:e04. DOI: https://doi.org/10.15420/cfr.2022.27
Correspondence: Ahmet Çelik, Mersin University Medical Faculty, Department of Cardiology, Mersin, 33343, Turkey. E: ahmetcelik39@hotmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure (HF) with preserved ejection fraction (HFpEF) is an important global health problem. HFpEF, which is mainly common in older patients with hypertension and/or obesity, is closely associated with left ventricular (LV) hypertrophy (LVH). Patients with HFpEF usually present with symptomatic HF despite a normal LV ejection fraction (LVEF) but have similar morbidity and mortality as patients with HF with reduced ejection fraction (HFrEF). An increased risk of first-onset AF and a higher incidence of stroke is also common in patients with HFpEF.1 HFpEF involves a complex interplay of pathophysiological changes involving diastolic dysfunction, remodelling of the LV and left atrium (LA), pulmonary vascular haemodynamics and noncardiac factors, and it is associated with a poor prognosis.2–4
Diastolic properties are difficult to examine and have many determinants. It is a complex phenomenon with several phases involving both relaxation and subsequent filling of the ventricle.1 Physical examination, electrocardiography (ECG), chest radiographs, laboratory findings and multi-modality imaging methods should be used together for proper evaluation of diastolic function.1 However, no reliable and reproducible single method has been defined that can lead to a diagnosis. Invasive measurements of LV diastolic properties and pressures are impractical on a broad scale. Evaluation of the type and extent of LV diastolic dysfunction currently relies on assessment of LV filling pattern and determination of myocardial deformation with imaging tools.2–4 Although the use of multimodality imaging is increasing – including nuclear imaging, CT and MRI –echocardiography is the first-line method for evaluation of diastolic dysfunction.2–4
Echocardiography in HFpEF
Transthoracic echocardiography (TTE) has been the standard first-line imaging modality for the evaluation of HFpEF as it is a non-invasive,
widely available low-cost tool providing many strong predictors of poor prognosis.5
Transthoracic Echocardiography
A comprehensive 2D echocardiographic examination should be performed to assess diastolic properties including LV and LA dimensions, right ventricular (RV) and LV contractility, spectral Doppler properties of mitral and tricuspid valve and pulmonary vein flow, estimated pulmonary artery pressure (PAP) and tissue Doppler imaging (TDI) properties of the mitral valve annulus.5
Assessment of LV Filling
LV diastolic properties and LV filling are associated with elastic recoil, LV relaxation, LV and LA compliance, mitral valve function, viscoelasticity, LVRV interaction, atrial contraction, the electrical system and pericardial constraint. LV filling can be basically evaluated with continuous wave (CW) and pulsed-wave (PW) Doppler techniques. LV isovolumic relaxation time (IVRT), early peak mitral flow velocity in diastole (E wave) and atrial contraction (A wave), mitral deceleration time (mitral DT) and duration of mitral A wave velocity (Adur) are commonly used Doppler parameters to assess LV filling.6
LV relaxation rate and LV compliance are substantial in assessing LV filling and pressures that indicate LV diastolic function. Doppler assessment of transmitral flow is graded as normal with impaired relaxation and pseudonormal and restrictive filling (Figure 1). Transmitral Doppler findings have a U-shaped inter-relationship with LV filling pressure. Thus, determining normal and pseudonormal patterns requires the assessment of additional echocardiographic parameters.3 Estimation of LV filling pressures is summarised in Figure 2
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Imaging in HF
Figure 1: Spectral Doppler Examples of Mitral Inflow and Tissue Doppler Examples of Mitral Annulus For Diastolic Filling Patterns
Most patients with an impaired relaxation filling pattern usually have normal filling pressures and are asymptomatic.7 8 With the progression of diastolic dysfunction, LV compliance during the atrial contraction phase decreases along with impaired LV relaxation. Decreased compliance increases mean LA pressure and dimensions. The increased LA pressure causes early mitral valve opening and higher transmitral flow velocity despite the slower LV relaxation rate, thus the LV filling pattern would be observed as normal. Patients with this pseudonormal LV filling may experience symptoms of HF and decreased exercise capacity.7–9 Significant elevation in LV pressure with severe deterioration in LV compliance causes LA dilatation and HF. Early diastolic filling becomes prominent and significant. Late diastolic filling is reduced and its duration is shortened – a restrictive filling pattern. Patients with restrictive filling usually have poor prognosis and reduced functional capacity due to HF symptoms.
Assessment of Tissue Doppler
Imaging of Mitral Annulus
TDI assessment of mitral annulus is used to distinguish between normal and impaired LV filling. The ratio of the peak mitral E wave velocity to the mitral annulus velocity (E/e’) is the most used parameter.5 In addition, the mitral annulus e’/a’ ratio could also be assessed and would be >1 in most of the patients with impaired relaxation. On the other hand, the e’/a’ ratio would be <1 in patients with a pseudo-normal filling patten.10,11 The E/e’ ratio has been shown to be associated with cardiovascular endpoints. However, the relation with LV pressures has been questioned in a recent meta-analysis which reported only a moderate correlation with the invasively measured resting filling pressures.12 Still E/e’ is a guideline recommended strong echocardiographic parameter that has a prognostic value in cases of HFpEF.1
Assessment of Pulmonary Venous Flow
Pulmonary venous flow velocity assessment is performed by placing the sample of PW Doppler to the right upper pulmonary vein in apical-four chamber view. The pulmonary venous flow assessment can be used to
reflect the filling haemodynamics of the LA. Doppler assessment of pulmonary venous flow is considered to be problematic in daily practice due to reproducibility and image quality, although high-quality PW Doppler transthoracic recordings can be obtained in about 85% of patients.5,13 The haemodynamic waves of PV flow rate include the peak forward flow rate in early systole (PVs1), late systole (PVs2), early diastole (PVd) and peak reverse flow rate and duration (PVa stop) in atrial contraction (PVa).14–16
Assessment of Left Atrial Function and Remodelling
Increased LA size is usually associated with high LA pressure and abnormal filling patterns. LA volume is shown to be strongly associated with adverse cardiac events and its measurement is recommended in clinical guidelines.17 Detection of normal LA dimensions usually indicates normal LA mean pressure whereas minimal volume of the LA has been observed to correlate with the pulmonary wedge pressure.5 18 19
Atrial volume and compliance are directly related to LV diastolic function which would cause LA remodelling and dysfunction. Increased LA volume occurs as a result of the chronic LV end-diastolic pressure elevation. LA volume should be calculated from the apical 4 and 2 cavity views and indexed to body surface area (LAVi). Although maximum LA volume is used more frequently, minimal LA volume can also provide important prognostic and diagnostic information.17 The upper normal limit for LAVi with 2D echocardiography is defined as 34 ml/m2. LA volumes >34 ml/m2 can be detected in 10% of the healthy population.3 The threshold for LAVi in patients with AF is >40 ml/m2 in recent guidelines.1 Enlarged LA volume is commonly observed in patients with HFpEF and is associated with increased cardiovascular risk. Therefore, LAVi should be measured in all patients with definite or suspected HFpEF.3 17
LA strain also plays an important role in the diagnosis and prognosis of HFpEF patients. Current recommendations say to evaluate LA functions by using LAVi together with LA reservoir or contractile strain for the diagnosis and prognosis of HFpEF patients.3 The novel approaches of using LA strain parameters could be fruitful in estimating LV end-diastolic
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Normal Delayed relaxation Pseudonormal Restrictive Mitral flow Rest Valsalva Tissue Doppler Rest Valsalva 1<E/A ratio <2 150 <DT <200 ms 50 <IVRT <100 ms 1 <e’/a’ E/A ratio <0.8 200 ms <DT 100 ms <IVRT e’/a’ <1 0.8 <E/A ratio <1.5 e’/a’ <1 2 <E/A ratio DT <160 ms IVRT <80 ms e’/a’ <1 DT = deceleration time; IVRT = isovolumetric relaxation time.
pressure and implementation to conventional criteria would improve the diagnostic efficiency. LA reservoir strain should not be used for patients with poor image quality and patients with AF.5 20–23
Assessment of Tricuspid Valve Flow and Pulmonary Artery Pressure
Estimation of systolic and diastolic PAP by echocardiography is helpful when determining LV diastolic dysfunction. The end-diastolic pulmonary regurgitation rate is used to estimate diastolic PAP and the maximum velocity of the tricuspid valve regurgitation jet is used to estimate systolic PAP. It is important to evaluate and add an estimate of central venous pressure. Central venous pressure assessment is performed by right atrial pressure estimation. Inferior vena cava size and degree of respiratory collapse, hepatic venous Doppler pattern are used to assess right atrial pressure. Diastolic PAP may reflect the mean LA pressure in the absence of pulmonary vascular disease. Patients with impaired relaxation LV filling are expected to have normal or mildly increased systolic PAP whereas patients with more serious dysfunction would have increased systolic PAP associated with high LA pressure.1 2 5 24 25 Moreover, recent studies show that patients with HFpEF are at risk of developing pulmonary vascular disease which is mainly characterised by increment in pulmonary vascular resistance and reduced pulmonary arterial compliance.26 Pulmonary vascular disease can result in reduced exercise capacity and be associated with adverse outcomes.27–29 Obese patients with HFpEF are shown to have pulmonary vascular disease more often which can be sometimes manifested only during exercise.30 Mid-systolic notching in the RV outflow Doppler profile could be of value for diagnosing pulmonary vascular disease.5
Assessment of LV Dimensions and Systolic Function
Concentric or eccentric hypertrophy are common in patients with HFpEF. Both concentric remodelling and hypertrophy are associated with increased cardiovascular morbidity and mortality and commonly observed in patients with HFpEF along with eccentric hypertrophy.17 Although echocardiography is used as the first-line imaging tool, cardiac MRI (CMRI) is also useful for demonstrating fibrosis and making a differential diagnosis.3 In general, the most common cause of LV hypertrophy is hypertension, but other causes of hypertrophy should be kept in mind. When the aetiology of LV hypertrophy is unclear – and especially when echocardiographic images are insufficient to conclude the underlying pathology – CMRI is very useful for a more accurate assessment of LV structure and a more precise diagnosis. It should be remembered that LV hypertrophy in HFpEF patients cannot be an exclusion criterion because it is highly specific (88%) but weakly sensitive (26%) for the diagnosis of HFpEF.1 3 5 24 31 32
Strain Imaging in HFpEF
Deformation imaging has evolved as a promising, reproducible and valuable tool, which enables additional and better prognostic information in patients with HFpEF.33 Global longitudinal strain (GLS) is found to be reduced in more than half of the patients with HFpEF.34 35 In patients with LVH, circumferential strain and apical rotation have been shown to be increased as a compensatory mechanism to retain systolic function.17 Despite being vendor-dependent, the cut-off value would be 16%. Moreover, speckle tracking strain imaging is an excellent method for assessing diastolic function by evaluating early and late filling phasic diastolic strain rates. It reflects myocardial elongation and untwisting rate, which are closely related to diastolic function. Strain imaging can be combined with stress echocardiography and provides additional
prognostic information. Pattern analysis of LV deformation can also be useful in differential diagnosis. Apical sparing is commonly observed in patients with cardiac amyloidosis, whereas longitudinal dysfunction of the septum may indicate asymmetric septal hypertrophic cardiomyopathy.34,36,37
Diastolic Stress Test by Echocardiography
Echocardiographic parameters might fail to show increased LV filling pressure signs at rest in some patients with HFpEF, however, changes in diastolic haemodynamics during exercise can lead to more sensitive assessment for diagnosing HFpEF.38 Although diastolic stress echocardiography can be performed using the supine bike or treadmill exercise protocol, supine bike is the recommended method for diastolic stress echocardiography because it allows continuous Doppler recordings throughout the exercise test.39 The regular stress test starts with 25 W workload and increases by 25 W every 3 minutes. Mitral flow, tricuspid regurgitation jet velocities and mitral annular velocities are recorded and evaluated at the start of the test, during exercise and during the recovery phase.40
A decrease in the E/A ratio or increased deceleration time of E wave is typical for mild-diastolic dysfunction or impaired myocardial relaxation. Shortening of the diastole during exercise causes an increased rate of myocardial relaxation and LV filling to provide adequate cardiac output.40 TR max velocity and E/e’ values measured during exercise were found to have high sensitivity for diastolic dysfunction and were correlated with invasive measurements.39 Stress echocardiography is also extremely useful in patients with borderline GLS. In healthy subjects, the E/e’ ratio does not change significantly with exercise due to proportional increases in mitral flow and annular velocities. In contrast, an increase in the E/e’ ratio and/or systolic PAP exercise has been shown to be correlated with increases in LV diastolic pressures.41 Septal E/e’<10 and peak tricuspid
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Left ventricular filling pressure estimation E/A E/A ≤0.8 + E ≤50 cm/s E/A ≤0.8 + E >50 cm/s Or 0.8 <E/A <2 E/A ≥2 ≥2 negative • E/e’ (mean) >14 • TR vel. (m/s) >2.8 • LA vol. index (ml/m2) >34 ≥2 positive 2 negative 1 positive 1 negative 2 positive Normal
Indeterminate ↑ LAP,
dysfunction ↑ LAP,
dysfunction Negative LA reservoir strain <18% Positive DT = deceleration time; IVRT = isovolumetric relaxation time; LA = left atrium; LAP = left atrial pressure; TR = tricuspid regurgitation; vel= velocity; vol = volume.
Figure 2: The Evaluation of LV Filling Pressures with Transthoracic Echocardiography
LAP, Grade I diastolic dysfunction
Grade II diastolic
Grade III diastolic
regurgitation velocity <2.8 m/s at rest and during exercise are normal findings. Septal E/e’ ratio over 15, mean E/e’ over 14 and peak TR velocity over 2.8 m/s with exercise indicates the presence of impairment in diastolic function.42 Despite being useful in evaluating increased LV filling pressures, it is difficult to record echocardiographic images with good image quality during exercise. This situation becomes more difficult with increased heart rate, but most patients with diastolic function can present diagnostic findings even at a moderately increased heart rate. If the assessment of mitral flow and annular velocities is not optimal because of immediate tachycardia, assessment of mentioned parameters during the recovery period is necessary. Stress echocardiography is one of the main methods recommended by the guidelines in the evaluation of patients with unexplained dyspnoea and subclinical LV diastolic dysfunction.21,38,41,43
CMRI in HFpEF
Given the latest criteria for the definition of HFpEF, it is obvious that to predict LV dimensions and haemodynamics with sole clinical data in HFpEF patients is impossible and performing an imaging study is inevitable.1
The aetiological work-up of patients with HFpEF would be better performed by using CMRI which is the gold standard cardiovascular imaging modality for the atrial and ventricular volume assessment and ejection fraction quantification. It is also the best alternative imaging modality in patients with suboptimal echocardiographic image quality that doesn’t only make morphological, functional evaluations but also gives information about perfusion, viability and tissue characterisation which may provide a better understanding of the pathophysiological mechanism of the HFpEF. It allows assessment of LA enlargement, LV hypertrophy, permanent replacement fibrosis and dynamic interstitial fibrosis using late gadolinium enhancement (LGE) and T1 mapping. By providing tissue characterisation with T1 and T2 mapping, CMRI is the best imaging modality in the differential diagnosis of myocarditis, infiltrative disorders, such as Fabry’s disease, sarcoidosis, both systemic and amyloid transthyretin amyloidosis, non-compaction cardiomyopathy, arrhythmogenic RV cardiomyopathy, hypertrophic cardiomyopathy, or Chagas disease.44–46 CMRI has high reproducibility and good spatial and temporal resolution. CMRI has emerged as one of the most useful techniques by minimising geometric assumptions and being less operatordependent than other cardiovascular imaging modalities. Moreover, it is radiation-free and thus safe. New methods such as feature tracking enables myocardial strain analysis as well.
CMRI plays a pivotal role particularly in patients with obesity and lung diseases with non-diagnostic echocardiographic examinations secondary to suboptimal image quality.44 On the other hand, CMRI requires expertise in scanning and interpreting the images in the clinical context. Moreover, it is not portable and not as practical as echocardiography. Claustrophobia is one of the leading patient-related limitations of the technique. Regrettably, achieving high-resolution CMRI cine images in patients with supraventricular and ventricular arrhythmia still represents a clinical challenge. Gadolinium-based contrast agents should be used carefully in people with a glomerular filtration rate <30 ml/min/1.73 m2. However, according to recent radiology recommendations, delaying group II gadolinium-based contrast agent (GBCA) for CMRI which is clinically needed in a patient with acute kidney failure or an estimated glomerular filtration rate <30 ml/min/1.73 m2 may be more harmful than the risk of nephrogenic systemic fibrosis. Thus, the safety of using group II GBCA should be evaluated against the potential harm of delayed diagnosis.47
Assessment of LV Systolic Function
Although the LVEF is preserved, LV systolic function is not always normal in HFpEF. Impairments in LV systolic performance can be detected at rest by TTE. It has been shown that worse longitudinal strain despite preserved LVEF is a strong prognostic factor for worse outcomes in HFpEF patients. Several studies have been published showing that many patients with HFpEF have abnormal longitudinal systolic function, which can be detected by reduced mitral annulus systolic ejection velocity, mitral annulus plane systolic descent and longitudinal strain. Myocardial strain and torsion can be acquired by CMRI as well. However, most centres do not perform strain analysis routinely. This measurement is primarily a research tool which can give insights into LV systolic function. Feature tracking analysis helps to detect anatomical features of interest in the LV subendocardium and subepicardium on segmented steady-state free precession (SSFP) cine images similar to echocardiographic speckle tracking. CMRI feature tracking method provides LA strain and strain rate calculation. These measures have been demonstrated to be impaired and associated with exercise intolerance in HFpEF patients. CMRI is known to be the gold standard technique to assess biventricular morphology and systolic function, especially in patients with non-diagnostic echocardiographic studies due to bad image quality. It is the preferred imaging tool for volume and ejection fraction estimation in heart failure patients, due to its 3D approach for non-symmetrical ventricles and superior image quality which is less user-dependent and has a higher reproducibility.24 48–51 Volumes are measured from a cine stack of short-axis biventricular contiguous slices. Modern cine sequences use breath hold, electrocardiographic-gated and segmented SSFP to produce images with high-spatial/temporal resolution, which are superior to other cardiac imaging modalities. Myocardial mass is also measured from the same short axis slices and CMRI is considered the ideal method for the assessment of LV mass without geometric assumptions for the same reasons. Biventricular function is evaluated at the same time and CMRI is considered the goldstandard imaging modality for global and regional LV function as well.1 3
Assessment of LV Diastolic Function
Diastolic dysfunction is generally considered a key component for the diagnosis of HFpEF. Diastolic dysfunction diagnosis requires demonstration of elevated filling pressures. Given the invasive nature of cardiac catheterisation, it is not feasible for routine clinical use and non-invasive techniques are used for the assessment of LV diastolic function.52 LV relaxation and compliance are evaluated by measuring the transmitral inflow and pulmonary venous flow data mostly by TTE which may have several limitations such as limited field of view, cosine errors and an inadequate acoustic window.45 CMRI is another non-invasive technique which has an excellent image quality with high spatial and temporal resolution as well as great accuracy and reproducibility which may provide other various LV diastolic function parameters such as LA size and function, LV hypertrophy and mass, and myocardial deformation imaging with strain method, which are the most useful parameters for the assessment of patients with HFpEF.45,52,53
Assessment of LA
Atrial volume and function are two important measures of ventricular diastolic performance and are shown to be reliable indicators of the duration and severity of diastolic dysfunction independent from loading conditions.54 They provide significant prognostic information not only in the general population but also in patients with heart disease.55 56 LA has a poor compliance, thus LA dilation is an indicator of diastolic dysfunction with elevation of LV filling pressure in the diastolic phase. LA volume should be measured at the end of the systolic phase of the LV before the mitral valve opening. It can be measured using two methods. First, the
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
bi-plane area-length method with manually drawn endocardial contours in 2- and 4-CH views with exclusion of pulmonary veins. Second is Simpson’s method on the short axis slices encompassing the whole left atrium. The study reported normal reference values in 108 healthy volunteers as 103 ± 30 ml for men and 89 ± 21 ml for women by arealength method.57 There is a large amount of data supporting the maximal LA volume use. However, there are also several studies demonstrating minimal LA volume to be of prognostic significance. In 140 HFpEF patientsLA emptying fraction correlated inversely with LA volumes and plasma natriuretic peptides and resulted in an independent prognostic predictor of all-cause death or hospitalisation for HF.18 58 59
Assessment of Mitral Inflow Pattern
Quantitative CMRI-derived flow measurement with phase contrast CMRI (PC-CMRI) has been used for the past four decades. It is a potentially useful alternative to echocardiographic-PW Doppler and can be used for evaluation of mitral valve flow and velocity quantification.60 An encoding velocity should be optimised to match the peak velocity as closely as possible without aliasing, which is typically 100 to 150 cm/s for the mitral inflow. After a cine-phase contrast ECG-gated CMRI sequence is performed, the slice is precisely selected using multiplanar localisation to transverse the tips of the leaflets of the mitral valve and is placed perpendicular to the LV inflow.61 This generates short-axis cine-phase contrast images. A graphical contour of the mitral valve orifice is then drawn and automatically propagated (with manual override) to all timeframes of the cine loop to calculate the velocity, peak velocity and flow plots over time.61 The E-wave peak, A-wave peak, DT and E/A ratio are calculated afterwards. Data is retrospectively ECG gated. It can be acquired using either free breathing or with a breath hold. However, the acquisition plane remains fixed during the cardiac cycle and does not accompany the cyclical motion of the mitral annulus.52 Acquisition techniques have been introduced using moving slice velocity mapping. Recently, it has been demonstrated that three-directional 3D velocity-encoded MRI with retrospective valve tracking showed better agreement with echo Doppler when differentiating a restrictive filling pattern from other patterns.52 61–63
Assessment of Pulmonary Venous Flow
Waveform analysis of pulmonary venous flow is a helpful way to assess LV diastolic dysfunction. Pulmonary vein velocity-encoded MRI is an alternative method to PW Doppler to investigate atrial filling pattern.45 2D one-directional velocity encoding at the pulmonary vein and 3D threedirectional velocity-encoding approach of the intra-atrial blood flow field are two different ways of obtaining a pulmonary venous time-velocity curve. The pulmonary vein flow is sampled 1 cm into the pulmonary vein ostium similar to Doppler echocardiography. The velocity sensitivity of the acquisition should be adjusted to a maximal velocity of 80 cm/s to optimise the signal-to-noise ratio, and the acquisition plane should be perpendicular to pulmonary vein flow.45 The temporal resolution that determines the accuracy of waveform is inferior for velocity-encoded MRI when compared to echo Doppler. Moreover, it has a longer acquisition time. The use of pulmonary venous flow for the LV diastolic dysfunction assessment is limited in conditions such as sinus tachycardia, first-degree atrioventricular block and AF.60 Despite all limitations, velocity-encoded MRI offers a useful alternative to PW Doppler for pulmonary venous flow assessment and several studies have shown good correlations when compared with Doppler echocardiography.45 60
Assessment of Tissue Characterisation
Several diseases which have similar clinical presentations may have the same clinical phenotype as HFpEF and should be considered
‘phenocopies’ which confuses the diagnostic process.64 It is of utmost importance to recognise other alternative cardiac diseases, which should be considered in the differential diagnosis for HFpEF.49 Those diseases are mainly restrictive cardiomyopathy, constrictive pericarditis and severe tricuspid regurgitation. Typical CMRI findings of restrictive cardiomyopathy are biatrial dilatation and increased LV thickness.49 Sometimes small pericardial effusion can be seen as well. Diastolic function parameters are also typically impaired. Specific findings with LGE include subendocardial LV, RV free wall and septum hyperenhancement.49 Amyloidosis, cardiac sarcoidosis and haemochromatosis are the primary restrictive cardiomyopathies that are encountered in daily practice.65 CMRI is the only non-invasive technique for quantifying myocardial iron overload. Introduced in 1999, the T2* technique is a robust, fast, reproducible method that is the method of choice for cardiac iron quantification which is transferable among different CMRI scanners. T2* values in the myocardium are directly associated with iron levels in the tissue. Decreased T2* levels are associated with systolic and diastolic ventricular dysfunction; values lower than 20 ms show iron overload, while T2* lower than 10 ms indicate severe iron overload. 66–70
Cardiac amyloidosis is characterised by progressive diastolic dysfunction followed by systolic dysfunction and arrhythmia. Cardiac amyloidosis is usually diagnosed in the late stages of the disease.1 3 71
Constrictive pericarditis is another clinical scenario which can be misdiagnosed as HFpEF.49 Features of constrictive pericarditis that will be shown using CMRI include pericardial thickening, septal bounce and delayed hyperenhancement of the pericardium in patients with active inflammation.49
Tissue characterisation plays a pivotal role in the context of HFpEF in identifying specific patterns of fibrosis and scarring in most of the cardiomyopathies that could be a differential diagnosis for HFpEF (Figure 3). The identification of ‘phenocopies’ in HFPEF may allow an individualised approach to molecular targets and functional abnormalities, such as the use of some important drugs in senile amyloidosis, and ß-blockers and/or calcium channel antagonists in patients with hypertrophic cardiomyopathy.49 50 72
Novel Approaches
Although it is well known that myocardial stiffness plays an important role in cardiac function and increased stiffness may cause restrictive diastolic filling, there is still no conventional imaging method to measure myocardial stiffness directly in vivo 73,74 High-resolution magnetic resonance elastography is a novel technique based on a stiffness map produced by an external vibrating source that generates shear waves inside a tissue of interest. Arani et al. have demonstrated the feasibility of 3D highfrequency cardiac MR elastography diagnostic imaging technique for quantitatively measuring myocardial stiffness in vivo which does not require a contrast agent.74
Obesity is common in HFpEF disease and has numerous cardiovascular effects. Obokata et al. compared cardiovascular structure, function and reserve capacity in people with HFpEF and obesity and those without obesity and control subjects using echocardiography and showed that epicardial adipose tissue has a direct mechanical effect caused by increased pericardial restraint and enhanced ventricular interdependence.75 Another recent study showed the relationship between epicardial fat tissue volume and LV diastolic function, using multidetector CT and TTE, finding a significant correlation between
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 3: Coronary and Non-coronary Late Gadolinium Enhancement Patterns and Some Cardiac MRI Features of Different Phenotypes of Heart Failure with Preserved Ejection Fraction
usion
Hypertensive left ventricle hypertrophy
• Pleural e usion
• Increased T1 and ECV valves
• Di culty in T1 nulling
• Basal and mid-interventricular septum but also patchy LGE
• Hilar lymphadenopathy
Occasionally patchy LGE pattern or focal (mid-wall) LGE of RV insertion points (hinge points)
T2* contours: ROIs are placed in the ventricular septum and the liver
liver
Iron overload cardiomyopathy
T2* value assessment in the septum due to less artefact T2* value and native T1 mapping values correlate well (both decrease)
• Basal septum hypertrophy is common in elderly and hypertensive patients
• LVOT obstruction is uncommon
• Non-dilated ventricles, preserved systolic function, diastolic dysfunction, enlarged atria
• Abnormally dark liver
Heart failure development risk
• T2* >20 ms: low
• T2* 10–20 ms: intermediate
• T2* <10 ms: high
Multiple myocardial crypts may help to define HCM mutation carriers without LV hypertrophy
ECV = extracellular volume; HCM = hypertrophic cardiomyopathy; LGE = late-gadalinium enhancement; LV = left ventricle, LVOT = left ventricular outflow tract; ROIs = region(s) of interest; RV =right ventricle.
Multimodality Imaging Chart in HFpEF
The presence of HF signs and symptoms +
Elevated natriuretic peptides
• LV diastolic dysfunction/ raised LV filling pressures (Figure 2)
• LVEF ≥50%
Tissue characterisation for specific conditions (Figure 3)
Suspected ATTR cardiac amyloidosis
• Transthoracic echocardiography
Considering CMRI and its ability to study anatomical structure and myocardial perfusion precisely, it may have a robust role in searching the significance of epicardial adipose tissue in the pathogenesis of HFpEF.76
• Cardiac MRI
Stress and exercise CMRI have emerged as important tools in HFpEF diagnosis during the past few years. Considering that patients with HFpEF develop symptoms on exertion, an exercise test for the diagnosis becomes inevitable. The gold standard for the diagnosis of the effects of HFpEF is right heart catheterisation during exercise.77 However, exercise stress echocardiography is used more frequently in daily practice because it is non-invasive.39 Additionally, stress perfusion CMRI may help identify patients at higher cardiovascular risk in HFpEF patients with no known coronary artery disease.78 Additional to that, in a study by Backhaus et al., real time-CMRI bicycle exercise stress testing showed high accuracy in the diagnosis of HFpEF, and LA longitudinal shortening during the exercise was demonstrated as the best independent predictor of HFpEF proven with an invasive method.79
• Nuclear scanning (bone scintigraphy)
ATTR = transthyretin amyloidosis; HFpEF = feart failure with preserved ejection fraction; LV = left ventricle; LVEF = left ventricular ejection fraction
diastolic dysfunction and increased epicardial adipose tissue.76 Epicardial adipose tissue could potentially have a pathophysiological inflammatory role in HFpEF patients, which necessitates further investigation.
4D flow is another new method which can quantitively assess LV 3D blood flow over the cardiac cycle.80 It is possible to identify and monitor diastolic dysfunction with 4D flow. High isotropic spatial resolution and low operator dependency and lack of imaging plane restriction are the principal advantages of this technique. Analysis of flow components and the kinetic energy and momentum will provide a precise assessment of
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Ischaemic LGE pattern Non-ischaemic LGE pattern Subendocardial LGE Transmural LGE Amyloidosis Sarcoidosis Anderson Fabry disease Hypertrophic cardiomyopathy Global subendocardial LGE Subepicardial/ transmural/ midmyocardial LGE Midmyocardial or subepicardial LGE of the mid to basal inferolateral wall Focal, midmyocardial LGE of anterior and inferior RV insertion points (hinge points) and of hypertrophied segments Patchy subendocardial or transmural LGE Multiple, patchy midmyocardial LGE Low ECV and low native T1 value of septum Thickened interatrial septum Biatrial dilatation Thickened valves Pericardial e
Figure 4: Multimodality Imaging Chart in HFpEF
the dynamic of ventricular filling and ejection.44 It can differentiate restrictive diastolic filling and normal diastolic filling patterns, and can also evaluate kinetic energy, vorticity, or particle tracing-based metrics.81,82 Machine learning and artificial intelligence (AI) are gaining importance in the medical imaging field and are expected to transform clinical practice. CMRI can use machine learning to help guide diagnosis and therapy management as it relies on complex acquisition strategies.84,85 AI is associated with radiomics which is a novel image analysis technique. Digital images are converted into numeric data which can then be analysed to obtain multiple numerical quantifiers of shape and tissue character and it has been demonstrated that disease conditions or clinical outcomes may be identified with high accuracy with this new method.86
Cardiac Computed Tomography in HFpEF
It has been already shown that cardiac CT (CCT) may provide varying parameters for the assessment of diastolic dyfunction.3 87 88 Similar to echocardiography, CCT has been applied to measure early and late diastolic filling rates for the assessment of diastolic function. However, LV filling rates are not recommended as the only indices of diastolic function due to their dependence on LV relaxation and LV filling pressure.49 Although CCT is not the first-line imaging method for comprehensive differential diagnosis of HFpEF, it may still be used as a part of multimodality imaging. One of the most useful usages of CCT is the diagnosis of constrictive pericarditis by detecting pericardial thickening. A normal EF with a concentric hypertrophy, LA enlargement and coronary artery disease (CAD) can be detected as indicators of HFpEF with CCT.49 Similar to CMRI, CCT can provide extracellular volume measurements for the further assessment of HFpEF.89
Nuclear Imaging in HFpEF
Radionuclide assessment of the systolic and diastolic function of the LV can be performed by creating an activity time plot during the cardiac cycle to obtain volumes at specific cardiac phases as the time-activity plot can be expressed as volume changes over time (end-diastolic volume (EDV/s)). This plot would provide rapid ventricular filling (peak filling rate – PFR), time to PFR, normalised PFR over to EDV/sec is normally >2.5. PFR is a sensitive marker of diastolic function; however, it can be present in young, healthy people and can show variability depending on sex, heart rate and
LVEF. On the other hand, time to PFR is a more robust measure of diastolic function. Time to PFR should be normally <180 ms. Another parameter is the percentage of accumulated volume during rapid filling period. The cut-off has been shown to be 69%. Any values lower than 69% of EDV/sec during rapid filling phase would suggest diastolic dysfunction. Transmitral flow waves can also be acquired by nuclear imaging. An A/E ratio of <0.25 is suggested to be normal. Radionuclide studies are not performed for diastolic dysfunction assessment solely. However, diastolic assessment with radionuclide techniques can be added to perfusion studies. The most important radionuclide criteria suggestive of diastolic dysfunction can be listed as the peak filling rate, time to peak filling rate and transmitral flow wave ratio.90–94 Similar to CCT, nuclear methods may be helpful for the definitive diagnosis of the underlying aetiology of HFpEF. Transthyretintype cardiac amyloidosis is common among HFpEF patients and nuclear imaging carries substantial importance for its diagnosis with high sensitivity and specifity.64 95–97 Suspected patients should be evaluated by CMRI and consequent imaging with bone scintigraphy (Figure 4). Sarcoidosis is another multisystemic disease that can involve the heart and be diagnosed with nuclear methods such as 18F-FDG-PET/CT (PET) and 123I-BMIPP/201TlCl dual myocardial single-photon emission computerized tomography.98
In summary, multimodality imaging plays a key role for defining HFpEF and establishing specific aetiology. Although echocardiography is the first-line imaging modality in patients with HFpEF, CMRI has been a cornerstone in the work-up. A simplified algorithm for the use of multimodality imaging is presented in Figure 5
Conclusion
HFpEF is an important global health problem with increasing incidence. Diagnosis mainly depends on showing evidence of LV diastolic function and excluding other cardiac and non-cardiac pathologies. Echocardiography is the first-line imaging modality to diagnose diastolic LV dysfunction and define cardiac structure and function. Diastolic dysfunction is graded by filling pattern and filling pressure. In cases of suspected diastolic dysfunction in patients with normal filling pressures or non-conclusive echocardiographic findings, stress echocardiography should be performed. However, other clinical features should be
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 5: Diagnostic Work-Up in Patients with HfpEF
A: Transthoracic echocardiography four-chamber view showing thickened left ventricle, interatrial septum and valves, mild pericardial effusion. B: Transthoracic echocardiography speckle tracking bull’s eye image showing apical sparing of left ventricle. C: Cardiac MRI four-chamber CINE image showing thickened left ventricle, interatrial septum and valves, mild pericardial effusion. D: Cardiac MRI short axis CINE image showing thickened left ventricle, interatrial septum and valves, mild pericardial effusion. E: Cardiac MRI parametric T1 mapping showing increased T1 values. F: Cardiac MRI T2-PSIR imaging showing extensive subendocardial LGE in the anterior wall. G–H: 99mTc-PYP scintigraphy showing myocardial uptake of radiotracer. LGE = late gadolinium enhancement.
considered as echocardiographic estimates of LV filling pressure have moderate accuracy.
CMRI is very useful for unmasking underlying pathologies in HFpEF patients. Size and function of the cardiac chambers can be easily
1. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https:// doi.org/10.1093/eurheartj/ehab368; PMID: 34447992.
2. Pieske B, Tschöpe C, De Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–317. https:// doi.org/10.1093/eurheartj/ehz641; PMID: 31504452.
3. Smiseth OA, Morris DA, Cardim N, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2022;23:e34–61. https://doi.org/10.1093/ehjci/ jeab154; PMID: 34729586.
4. Sorrentino R, Esposito R, Santoro C, et al. Practical impact of new diastolic recommendations on noninvasive estimation of left ventricular diastolic function and filling pressures. J Am Soc Echocardiogr 2020;33:171–81. https://doi. org/10.1016/j.echo.2019.08.013; PMID: 31619369.
5. Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin 2019;15:241–56. https://doi.org/10.1016/j.hfc.2018.12.004; PMID: 30832815.
6. Lancellotti P, Galderisi M, Edvardsen T, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 2017;18:961–8. https://doi.org/10.1093/ehjci/jex067; PMID: 28444160.
7. Andersen OS, Smiseth OA, Dokainish H, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017;69:1937–48. https://doi.org/10.1016/j. jacc.2017.01.058; PMID: 28408024.
8. Smiseth OA. Evaluation of left ventricular diastolic function: state of the art after 35 years with Doppler assessment. J Echocardiogr 2018;16:55–64. https://doi.org/10.1007/s12574017-0364-2; PMID: 29236226.
9. Shah AM, Claggett B, Sweitzer NK, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circ Heart Fail 2014;7:740–51. https://doi.org/10.1161/ CIRCHEARTFAILURE.114.001583; PMID: 25122186.
10. Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–80. https://doi.org/10.1016/S07351097(97)88335-0; PMID: 9247521.
11. Lam CSP, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007;115:1982–90. https://doi.org/10.1161/CIRCULATIONAHA.106.659763; PMID: 17404159.
12. Nauta JF, Hummel YM, van der Meer P, et al. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2018;20:1303–11. https://doi.org/10.1002/ ejhf.1220; PMID: 29877602.
13. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788–94. https://doi.org/10.1161/01. cir.102.15.1788; PMID: 11023933.
14. Appleton CP. Hemodynamic determinants of doppler pulmonary venous flow velocity components: new insights from studies in lightly sedated normal dogs. J Am Coll Cardiol 1997;30:1562–74. https://doi.org/10.1016/S07351097(97)00354-9; PMID: 9362417.
15. Buffle E, Kramarz J, Elazar E, et al. Added value of pulmonary venous flow Doppler assessment in patients with preserved ejection fraction and its contribution to the diastolic grading paradigm. Eur Heart J Cardiovasc Imaging
evaluated by CMRI which provides important prognostic information. LGE and extracellular volume can add to the diagnostic work-up and prognosis by being the only imaging modality to assess myocardial fibrosis. Nuclear imaging is very useful for the diagnosis of cardiac amyloidosis. CCT and CMRI can also identify constrictive pericarditis.
2015;16:1191–7. https://doi.org/10.1093/ehjci/jev126; PMID: 26034092.
16. Klein AL, Abdalla I, Murray RD, et al. Age independence of the difference in duration of pulmonary venous atrial reversal flow and transmitral A-wave flow in normal subjects. J Am Soc Echocardiogr 1998;11:458–65. https://doi. org/10.1016/S0894-7317(98)70026-4; PMID: 9619618.
17. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. https://doi.org/10.1016/j.echo.2016.01.011; PMID: 27037982.
18. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. https://doi.org/10.1016/j. jacc.2013.10.055; PMID: 24291276.
19. Tsang TSM, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284–9. https://doi.org/10.1016/S00029149(02)02864-3; PMID: 12480035.
20. Kanagala P, Arnold JR, Cheng ASH, et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging 2020;36:101–10. https://doi.org/10.1007/s10554-019-01684-9; PMID: 31401742.
21. Lundberg A, Johnson J, Hage C, et al. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol 2019;108:703–15. https://doi.org/10.1007/s00392-018-1399-8; PMID: 30536044.
22. Von Roeder M, Rommel KP, Kowallick JT, et al. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017;10:e005467. https://doi. org/10.1161/CIRCIMAGING.116.005467; PMID: 28360259.
23. Singh A, Carvalho Singulane C, Miyoshi T, et al. Normal values of left atrial size and function and the impact of age: results of the world alliance societies of echocardiography study. J Am Soc Echocardiogr 2022;35:154–164.e3. https://doi. org/10.1016/j.echo.2021.08.008; PMID: 34416309.
24. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019;124:1598–617. https://doi.org/10.1161/ CIRCRESAHA.119.313572; PMID: 31120821.
25. Lam WC, Pennell DJ. Imaging of the heart: historical perspective and recent advances. Postgrad Med J 2016;92:99–104. https://doi.org/10.1136/ postgradmedj-2015-133831; PMID: 26647305.
26. Guzzi M, Gio S, Adir Y. Pulmonary hypertension in HFpEF and HfrEF: JACC review topic of the week. J Am Coll Cardiol 2020;76:1102–11. https://doi.org/10.1016/j.jacc.2020.06.069. PMID: 32854845.
27. Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J 2017;38:2874–8. https://doi.org/10.1093/eurheartj/ehx184; PMID: 28431020.
28. Vanderpool RR, Saul M, Nouraie M, et al. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018;3:298–306. https://doi. org/10.1001/jamacardio.2018.0128; PMID: 29541759.
29. Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470–9. https://doi.org/10.1161/ CIRCULATIONAHA.112.000667; PMID: 24060943.
30. Gorter TM, Obokata M, Reddy YNV, et al. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–35. https://doi.org/10.1093/ eurheartj/ehy331; PMID: 29947750.
31. Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:833–40. https://doi.org/10.1093/ehjci/jex140; PMID: 28637227.
32. Cardim N, Galderisi M, Edvardsen T, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular
Imaging endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 2015;16:280. https://doi.org/10.1093/ ehjci/jeu291; PMID: 25650407.
33. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. J Am Coll Cardiol Imaging 2018;11:260–74. https://doi.org/10.1016/j. jcmg.2017.11.017; PMID: 29413646.
34. Morris DA, Ma XX, Belyavskiy E, et al. Left ventricular longitudinal systolic function analysed by 2D speckletracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart 2017;4:e000630. https://doi.org/10.1136/ openhrt-2017-000630; PMID: 29018535.
35. Onishi T, Saha SK, Delgado-Montero A, et al. Global longitudinal strain and global circumferential strain by speckle-tracking echocardiography and feature-tracking cardiac magnetic resonance imaging: comparison with left ventricular ejection fraction. J Am Soc Echocardiogr 2015;28:587–96. https://doi.org/10.1016/j.echo.2014.11.018; PMID: 25577185.
36. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost 2015;113:943–51. https://doi. org/10.1160/TH14-12-1080; PMID: 25789661.
37. Ünlü S, Özden Tok Ö, Avcı Demir F, et al. Differential diagnosis of apical hypertrophic cardiomyopathy and apical displacement of the papillary muscles: a multimodality imaging point of view. Echocardiography 2021;38:103–13. https://doi.org/10.1111/echo.14895; PMID: 33067903.
38. Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol 2006;47:1891–900. https://doi.org/10.1016/j.jacc.2006.02.042; PMID: 16682317.
39. Obokata M, Kane GC, Reddy YNV, et al. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasiveechocardiographic study. Circulation 2017;135:825–38. https://doi.org/10.1161/CIRCULATIONAHA.116.024822; PMID: 28039229.
40. Ha JW, Oh JK, Pellikka PA, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 2005;18:63–8. https://doi.org/10.1016/j.echo.2004.08.033; PMID: 15637491.
41. Belyavskiy E, Morris DA, Url-Michitsch M, et al. Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study. ESC Heart Fail 2019;6:146–53. https://doi.org/10.1002/ ehf2.12375; PMID: 30451399.
42. Huis in ’t Veld AE, de Man FS, van Rossum AC, Handoko ML. How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J 2016;24:244–51. https://doi.org/10.1007/s12471-016-0811-0; PMID: 26914917.
43. Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging 2020;13:272–82. https://doi.org/10.1016/j.jcmg.2019.01.037; PMID: 31202741.
44. Webb J, Fovargue L, Tøndel K, et al. The emerging role of cardiac magnetic resonance imaging in the evaluation of patients with HFpEF. Curr Heart Fail Rep 2018;15:1–9. https:// doi.org/10.1007/s11897-018-0372-1; PMID: 29404975.
45. Barison A, Aimo A, Todiere G, et al. Cardiovascular magnetic resonance for the diagnosis and management of heart failure with preserved ejection fraction. Heart Fail Rev 2022;27:191–205. https://doi.org/10.1007/s10741-02009998-w; PMID: 32572736.
46. Kanagala P, Cheng ASH, Singh A, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction –implications for clinical trials. J Cardiovasc Magn Reson 2018;20:4. https://doi.org/10.1186/s12968-017-0424-9; PMID: 29321034.
47. Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
2021;298:28–35. https://doi.org/10.1148/radiol.2020202903;
PMID: 33170103.
48. Kinno M, Nagpal P, Horgan S, Waller AH. Comparison of echocardiography, cardiac magnetic resonance, and computed tomographic imaging for the evaluation of left ventricular myocardial function: part 2 (diastolic and regional assessment). Curr Cardiol Rep 2017;19:6. https://doi. org/10.1007/s11886-017-0816-3; PMID: 28116679.
49. Nagueh SF, Chang SM, Nabi F, et al. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017;10:e006547. https://doi. org/10.1161/CIRCIMAGING.117.006547; PMID: 28838962.
50. Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, society for cardiovascular magnetic resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2011;24:473–98. https://doi.org/10.1016/j.echo.2011.03.006; PMID: 21514501.
51. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. https://doi.org/10.1093/ejechocard/jep007; PMID: 19270053.
52. Rathi VK, Doyle M, Yamrozik J, et al. Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: a practical approach. J Cardiovasc Magn Reson 2008;10:36. https://doi.org/10.1186/1532-429X-10-36;
PMID: 18611254.
53. Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387–96. https://doi. org/10.1053/euhj.2000.2011; PMID: 10952828.
54. Simek CL, Feldman MD, Haber HL, et al. Relationship between left ventricular wall thickness and left atrial size: comparison with other measures of diastolic function. J Am Soc Echocardiogr 1995;8:37–47. https://doi.org/10.1016/S08947317(05)80356-6; PMID: 7710749.
55. Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol 2002;40:1425. https://doi. org/10.1016/S0735-1097(02)02305-7; PMID: 12392832.
56. Rossi A, Cicoira M, Florea VG, et al. Chronic heart failure with preserved left ventricular ejection fraction: diagnostic and prognostic value of left atrial size. Int J Cardiol 2006;110:386–92. https://doi.org/10.1016/j. ijcard.2005.08.049; PMID: 16325283.
57. Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. https://doi.org/10.1186/s12968-015-0111-7; PMID: 25928314.
58. Hudsmith LE, Petersen SE, Francis JM, et al. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 2005;7:775–82. https://doi. org/10.1080/10976640500295516; PMID: 16353438.
59. Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr 2008;9:356–62. https://doi.org/10.1016/j. euje.2007.06.002; PMID: 17689293.
60. Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the evaluation of diastolic dysfunction and phenotyping of HFpEF: current role and future perspectives. JACC Cardiovasc Imaging 2020;13:283–96. https://doi.org/10.1016/j. jcmg.2019.02.031; PMID: 31202753.
61. Pandey T, Jambhekar K. Evaluation of diastolic dysfunction using cardiac magnetic resonance imaging. Eur Cardiol 2010;6:21–5. https://doi.org/10.15420/ecr.2010.6.1.21.
62. Westenberg JJM. CMR for assessment of diastolic function. Curr Cardiovasc Imaging Rep 2011;4:149–58. https://doi. org/10.1007/s12410-011-9070-z; PMID: 21475412.
63. Brandts A, Bertini M, Van Dijk EJ, et al. Left ventricular diastolic function assessment from three-dimensional threedirectional velocity-encoded MRI with retrospective valve tracking. J Magn Reson Imaging 2011;33:312–9. https://doi. org/10.1002/jmri.22424; PMID: 21274972.
64. Rapezzi C, Longhi S, Milandri A, et al. Cardiac involvement in hereditary-transthyretin related amyloidosis. Amyloid 2012;19(Suppl 1):16–21. https://doi.org/10.3109/13506129.201 2.673185; PMID: 22494034.
65. Nojima Y, Ihara M, Kurimoto T, Nanto S. Amyloid light-chain amyloidosis manifesting as heart failure with preserved ejection fraction in a patient with hyper-immunoglobulin E-emia. Am J Case Rep 2016;17:235–40. https://doi. org/10.12659/AJCR.896839; PMID: 27064109.
66. Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22:2171–9. https:// doi.org/10.1053/euhj.2001.2822; PMID: 11913479.
67. Pennell DJ, Udelson JE, Arai AE, et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation 2013;128:281–308. https://doi.org/10.1161/ CIR.0b013e31829b2be6; PMID: 23775258.
68. Westwood MA, Anderson LJ, Firmin DN, et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging 2003;18:616–20. https://doi.org/10.1002/jmri.10396; PMID: 14579406.
69. Pepe A, Lombardi M, Positano V, et al. Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol 2006;76:183–92. https://doi.org/10.1111/j.1600-0609.2005.00587.x; PMID: 16451393.
70. Pennell DJ. T2* magnetic resonance and myocardial iron in thalassemia. Ann N Y Acad Sci 2005;1054:373–8. https://doi. org/10.1196/annals.1345.045.
71. Quarta G, Gori M, Iorio A, et al. Cardiac magnetic resonance in heart failure with preserved ejection fraction: myocyte, interstitium, microvascular, and metabolic abnormalities. Eur J Heart Fail 2020;22:1065–75. https://doi.org/10.1002/ ejhf.1961; PMID: 32654354.
72. Mörner S, Hellman U, Suhr OB, et al. Amyloid heart disease mimicking hypertrophic cardiomyopathy. J Intern Med 2005;258:225–30. https://doi. org/10.1111/j.1365-2796.2005.01522.x; PMID: 16115295.
73. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure –abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953–9. https://doi. org/10.1056/NEJMoa032566. PMID: 15128895.
74. Arani A, Arunachalam SP, Chang ICY, et al. Cardiac MR elastography for quantitative assessment of elevated myocardial stiffness in cardiac amyloidosis. J Magn Reson Imaging 2017;46:1361–7. https://doi.org/10.1002/jmri.25678; PMID: 28236336.
75. Obokata M, Reddy YNV, Pislaru SV, et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. https://doi.org/10.1161/ CIRCULATIONAHA.116.026807; PMID: 28381470.
76. Vural M, Talu A, Sahin D, et al. Evaluation of the relationship between epicardial fat volume and left ventricular diastolic dysfunction. Jpn J Radiol 2014;32:331–9. https://doi. org/10.1007/s11604-014-0310-4; PMID: 24687226.
77. Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126:2261–74. https://doi. org/10.1161/CIR.0b013e31826fb946; PMID: 22952317.
78. Pezel T, Hovasse T, Sanguineti F, et al. Long-term prognostic value of stress CMR in patients with heart failure and preserved ejection fraction. JACC Cardiovasc Imaging 2021;14:2319–33. https://doi.org/10.1016/j.jcmg.2021.03.010; PMID: 34419409.
79. Backhaus SJ, Lange T, George EF, et al. Exercise stress realtime cardiac magnetic resonance imaging for noninvasive characterization of heart failure with preserved ejection fraction: the HFpEF-Stress trial. Circulation 2021;143:1484–98. https://doi.org/10.1161/CIRCULATIONAHA.120.051542; PMID: 33472397.
80. Pica S, Piatti F, Milani P, et al. 4D flow CMR for diastolic function assessment in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2019;20:ii408–9. https://doi.org/10.1093/ ehjci/jez124.008.
81. Ashkir Z, Myerson S, Neubauer S, et al. Four-dimensional flow cardiac magnetic resonance assessment of left
ventricular diastolic function. Front Cardiovasc Med 2022;9:866131. https://doi.org/10.3389/fcvm.2022.866131; PMID: 35935619.
82. Alattar Y, Soulat G, Gencer U, et al. Left ventricular diastolic early and late filling quantified from 4D flow magnetic resonance imaging. Diagn Interv Imaging 2022;103:345–52. https://doi.org/10.1016/j.diii.2022.02.003; PMID: 35227634.
83. Miller DD, Brown EW. Artificial intelligence in medical practice: the question to the answer? Am J Med 2018;131:129–33. https://doi.org/10.1016/j. amjmed.2017.10.035; PMID: 29126825.
84. Krittanawong C. The rise of artificial intelligence and the uncertain future for physicians. Eur J Intern Med 2018;48:e13–4. https://doi.org/10.1016/j.ejim.2017.06.017; PMID: 28651747.
85. Leiner T, Rueckert D, Suinesiaputra A, et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson 2019;21:61. https://doi.org/10.1186/s12968-019-0575-y; PMID: 31590664.
86. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441–6. https://doi.org/10.1016/j.ejca.2011.11.036; PMID: 22257792.
87. van der Veen HA, Lessick J, Abadi S, Mutlak D. Accuracy of diastolic function by cardiac computed tomography relative to Echo-Doppler: additive clinical and prognostic value. J Comput Assist Tomogr 2021;45:242–7. https://doi.org/10.1097/ RCT.0000000000001136; PMID: 33661156.
88. Ghersin I, Ghersin E, Abadi S, et al. Assessment of diastolic function in hypertrophic cardiomyopathy by computed tomography-derived analysis of left ventricular filling. J Comput Assist Tomogr 2017;41:339–43. https://doi.org/10.1097/ RCT.0000000000000533; PMID: 27798446.
89. Nacif MS, Liu Y, Yao J, et al. 3D left ventricular extracellular volume fraction by low-radiation dose cardiac CT: assessment of interstitial myocardial fibrosis. J Cardiovasc Comput Tomogr 2013;7:51–7. https://doi.org/10.1016/j. jcct.2012.10.010; PMID: 23333188.
90. Mitra D, Basu S. Equilibrium radionuclide angiocardiography: its usefulness in current practice and potential future applications. World J Radiol 2012;4:421–30. https://doi. org/10.4329/wjr.v4.i10.421; PMID: 23150766.
91. Matsuo S, Nakajima K, Kinuya S. Clinical use of nuclear cardiology in the assessment of heart failure. World J Cardiol 2010;2:344–56. https://doi.org/10.4330/wjc.v2.i10.344; PMID: 21160612.
92. Levy WC, Cerqueira MD, Abrass IB, et al. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation 1993;88:116–26. https://doi.org/10.1161/01.CIR.88.1.116; PMID: 8319324.
93. Thomas JD, Weyman AE. Echocardiographic Doppler evaluation of left ventricular diastolic function: physics and physiology. Circulation 1991;84:977–90. https://doi. org/10.1161/01.CIR.84.3.977; PMID: 1884473.
94. Johannessen KA, Cerqueira M, Veith RC, Stratton JR. The relation between radionuclide angiography and Doppler echocardiography during contractile changes with infusions of epinephrine. Int J Cardiol 1991;33:149–57. https://doi. org/10.1016/0167-5273(91)90163-J; PMID: 1937970.
95. Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:1–11. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006075; PMID: 31480867.
96. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–94. https://doi.org/10.1093/eurheartj/ehv338; PMID: 26224076.
97. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation 2020;142:e7–e22. https://doi.org/10.1161/ CIR.0000000000000792; PMID: 32476490.
98. Kataoka S, Momose M, Fukushima K, et al. Regional myocardial damage and active inflammation in patients with cardiac sarcoidosis detected by non-invasive multi-modal imaging. Ann Nucl Med 2017;31:135–43. https://doi. org/10.1007/s12149-016-1136-1; PMID: 27804054.
Imaging HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Treatment of Persistent Left Atrial Appendage Thrombus in Patients with Atrial Fibrillation on Adequate Oral Anticoagulation: Pathways of Care for All-comers and Heart Failure Patients
Josip Katic 1 and Josip Andelo Borovac 1,2
Abstract
In patients with AF, the presence of left atrial/left atrial appendage (LA/LAA) thrombus is related to an increased risk of thromboembolic events. Anticoagulation therapy, either with vitamin K antagonists or novel oral anticoagulants (NOACs) is therefore mandatory in AF with LA/LAA thrombus in order to lower the risk of stroke or other systemic embolic events. Despite the efficacy of these treatments, some patients will have persistent LAA thrombus remaining or may have contraindications to oral anticoagulation. Currently, little is known about the occurrence, risk factors and resolution rate of LA/LAA thrombus in patients who are already under optimal chronic oral anticoagulation, including vitamin K antagonists or NOACs. The common action in clinical practice in this scenario is switching from one to another anticoagulant drug exhibiting a different mechanism of action. Repeated cardiac imaging is then advised within several weeks to visually verify thrombus dissolution. Finally, there is a substantial scarcity of data on the role and optimal use of NOACs after LAA occlusion. The aim of this review is to critically evaluate data and provide up-to-date information on the best antithrombotic strategies in this challenging clinical scenario.
Keywords
AF, left atrial appendage, non-vitamin K oral anticoagulant, novel oral anticoagulants, persistent thrombus, thrombosis, warfarin
Disclosure: JAB is Section Editor for Cardiac Failure Review; this did not influence peer review. JK has no conflicts of interest to declare.
Received: 30 September 2022 Accepted: 27 January 2023 Citation: Cardiac Failure Review 2023;9:e05. DOI: https://doi.org/10.15420/cfr.2022.28
Correspondence: Josip A Borovac, Cardiovascular Diseases Department, University Hospital of Split (KBC Split), Spinciceva 1, 21000 Split, Croatia. E: jborovac@mefst.hr
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Non-valvular AF is the most prevalent sustained cardiac arrhythmia linked to a high risk of stroke, systemic embolism (SE), heart failure (HF) and allcause death.1 Without oral anticoagulation, the age-adjusted risk of AFrelated stroke increases fivefold.2 For decades, oral anticoagulants (OAC) with vitamin K antagonists (VKA) were the standard therapy for AFassociated stroke and SE, with a 64% relative risk decrease in stroke.3 Because of the narrow therapeutic window of VKAs, it is mandatory for warfarin therapy to stay within adequate therapeutic range as reflected by tests of haemostasis, such as prothrombin time normalised by the international normalised ratio (INR). The time that patients spend in the VKA therapeutic range (TTR) of 65% is rare, even in large randomised trials, while drug compliance and TTR, as expected, are even worse in real life than in randomised controlled trials.4–6
Meta-analysis of all four novel OACs (NOACs) reveals a 19% reduction in the incidence of stroke or SE compared to VKA.7 Left atrial/left atrial appendage (LA/LAA) thrombus is found in 13–19% of AF patients without anticoagulation.8 9 The EMANATE trial reported 7.1% thrombus formation in anticoagulation-naive AF patients and 3.5–17.8% under VKA treatment.10 A recent retrospective cohort study showed that, despite anticoagulation for the recommended 3 weeks before cardioversion, a significant proportion of patients (40%) were found to have LA/LAA thrombus (LAT), especially those on warfarin who had a much higher incidence of this finding compared with on NOACs.11
In patients with nonvalvular AF, LAA thrombosis increases the risk of thromboembolic events.12 13 Implications for long-term stroke and thromboembolism risks due to persistent LAT to long-term anticoagulation are poorly understood. Such refractory LAT may become organised over time and pose a lower embolisation risk than newly generated LAT. This theory is reinforced by the fact that, despite reported rates of LAT detection of up to 3.6% among patients on continuous anticoagulation, recorded rates of thromboembolic events after cardioversion are much lower.14,15 In conclusion, regarding stroke risk, current evidence reveals that both fresh and organised thrombus might be the embolic source. However, because organised thrombus may be challenging to distinguish from the endocardium, a high degree of suspicion might be needed to diagnose an organised thrombus.16
Predictors of Left Atrial Appendage
Thrombosis Despite Oral Anticoagulation
Factors impacting the occurrence of LA or LAA thrombus despite therapeutic anticoagulation with VKA or NOACs among patients with AF are mainly unexplored. In a recent study, Angelini et al. reported that 7.7% of patients with AF referred for catheter ablation or electrical cardioversion had LA/LAA thrombus verified by transoesophageal echocardiography (TOE), despite receiving a guideline-recommended daily dose of NOAC for the purpose of thromboembolic prevention.17 Moreover, 5.1% of all patients had an echocardiographic finding of a dense LA/LAA spontaneous echo
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Treatment
1. Cardiovascular Diseases Department, University Hospital of Split, Split, Croatia; 2. Department of Pathophysiology, University of Split School of Medicine, Split, Croatia
contrast, which may precipitate thrombus formation. Finally, they found that this population’s significant predictors of LA/LAA thrombus were CHA 2 DS2-VASc score >3 and obesity, providing an OR for thrombus presence of 4.54 and 6.01, respectively. This is concordant with previous data from Bertaglia et al., reporting that 3.6% of patients with AF treated with NOAC for at least 3 weeks had LAT visualised by TOE, and all were located in the LAA.18 They also found that this finding was not dependent on NOAC type, while patients with LAA thrombus tended to have a mean CHA 2 DS2-VASc score of ≥3, thus suggesting that preprocedural TOE in this group might be considered.
Despite anticoagulant therapy, similar findings were reported elsewhere, confirming the association of high CHA2DS2-VASc score and LAT presence and its link to future cerebrovascular events.19,20 However, this relationship is not that simple because even in patients with non-valvular AF and low CHA 2 DS2-VASc score, elevated plasma homocysteine levels were predictive of LA/LAA thrombus.21 Similarly, in two Polish cohorts enrolling consecutive AF patients of whom the majority or all were receiving oral anticoagulation, the presence of LA/LAA thrombus was 5.7% and 7.5%, respectively, while persistent and permanent AF, renal dysfunction (estimated glomerular filtration rate <56 ml/min/1.73m2), lower mean LAA flow velocity and history of vascular disease were established as solid independent predictors of LA/LAA thrombus formation.22 23
Interestingly, data derived from the retrospective registry of 820 consecutive patients with AF undergoing TOE who were anticoagulated with apixaban for at least 4 weeks before imaging demonstrated that no thrombi were detected in patients with CHA2DS2-VASc score of ≤1.24
Furthermore, LAA morphological architecture and function may differentially impact on thrombogenesis of LA in patients with AF.25 For example, complex LAA morphology characterised by the increased number of LAA lobes was independently associated with LAT, spontaneous echo contrast and stroke in patients with non-valvular AF.26 27 Similarly, non-chicken wing LAA morphology, according to TOE, was associated with an 11.5-fold higher likelihood of LA/LAA thrombosis in patients with non-valvular AF compared to those having a chicken wing LAA formation.28 Decreased LAA flow velocity propagates blood stasis within LAA and this phenomenon occurs in AF; thus, it might independently enhance the risk of thrombogenesis.29 30 Echocardiographic and morphological parameters, such as decreased a-wave rate, increased LA dimensions, atrial sphericity and the degree of atrial fibrosis quantified by late gadolinium enhancement cardiac MRI, were shown to be independently associated with appendage thrombus, thromboembolic events and spontaneous echo contrast in several studies.31–35 The degree of LA dysfunction in non-valvular AF, such as LA emptying fraction <30% in addition to CHA2DS2-VASc score, was a crucial enhancing risk factor for LAT or dense spontaneous contrast in patients with AF.36 Similarly, contrast retention during the LAA occlusion procedure, LAA cauliflower morphology and reduced left ventricular ejection fraction (LVEF) were independently associated with LA/LAA thrombosis.37
Similarly, inappropriately reduced daily dosages of NOACs are likely to enhance the potential for LAA thrombus formation, thus emphasising the need to critically evaluate the pros and cons of NOAC dose reduction in each patient with AF.38 Finally, some drugs concomitantly used with NOACs, such as antiepileptic medications (phenobarbital, phenytoin and carbamazepine), might reduce the therapeutic efficacy of NOACs, thus facilitating the formation of LAT despite guideline-recommended continuous oral anticoagulation.39
Characteristics and Presence of Left Atrial Appendage Thrombus Depending on the LVEF and Presence of Heart Failure
It is recognised that the LA or LAA thrombus can be an essential source of thromboembolism in patients with HF, especially those with a dilated cardiomyopathy phenotype. The anatomical shape of the LAA facilitates haemostasis, which is even more enhanced in cases of poor systolic function and slow flow. Thus, it is a common site for thrombus formation among patients with HF.40 In the subanalysis of the multicentre, prospective, observational LATTEE registry, it was found that the prevalence of LAT was nearly three-fold higher in patients with HF compared to non-HF patients (12.8% versus 4.4%).41 As expected, the LAT presence increased as the systolic dysfunction decreased, meaning that HF with reduced ejection fraction was associated with a significantly 4.1fold greater likelihood of LAT presence (95% CI [3.13–5.46]) compared to non-HF patients. At the same time, this relationship was insignificant in patients with mildly reduced or preserved systolic function. The multivariable regression analysis within the same study revealed that lower LVEF was an independent predictor of LAT formation, whereas LVEF ≤48% was associated with an increased risk of LAT presence. Of note, this study employed chronic anticoagulation in 88% of patients before TOE; 1.5% were using transient anticoagulation, while only 10% were naive to oral anticoagulation. Age ≥75 years and HF were strongly associated with the presence of LAT among patients with non-valvular AF enrolled in the ENSURE-AF trial.42 Similar findings were validated in a significant metaanalysis pooling 56,660 patients with AF that underwent catheter ablation or electrical cardioversion (ECV). The presence of LAT was 1.3% and 4.9% among those adequately taking OAC, respectively. This study showed that HF was an essential predictor of LAT presence: OR 4.3 among AF patients undergoing ablation and OR 2.8 for those undergoing ECV.43 Interestingly, the OR for LAT was nearly identical for congestive HF patients in the study by Wu et al. (OR 4.4; 95% CI [1.6–12]).15
Novel echocardiographic parameters such as peak LA longitudinal strain (PALS) for LAA thrombus have been recently evaluated among HF patients. Concordantly, in a study that included CHF patients with severely depressed systolic function (LVEF <25%) and sinus rhythm, it was found that LAA thrombus was present in nearly one-third of patients (31.7%), while global PALS was a strong predictor of LAAT (OR 30.4; 95% CI [7.2–128]) for LAAT presence if the measured PALS value was <8%. This study also showed that the tendency for thrombus formation in LAA is significantly enhanced in HF patients with severely depressed systolic function, even in the absence of AF.44
Risk factors, predictors or markers of LA/LAA thrombus are summarised in Figure 1
Oral Anticoagulant Strategies in Persistent Left Atrial Appendage Thrombus Among Patients Already on Continuous Oral Anticoagulation
The management strategy in patients with verified LAA thrombus despite therapeutic oral anticoagulation is unclear and mainly based on expert consensus statements or limited case series reports. The recent European Heart Rhythm Association (EHRA) survey conducted among 54 hospital centres showed that in cases of persistent thrombus. In contrast, regarding VKA, most centres would switch VKA in eligible patients to NOAC (42.5% of cases); some would reassess the quality and adherence to VKA in 23.4% of cases. In contrast, 17% would remain on VKA and aim for the higher INR values (2.5–3.5).45 Similarly, about 6.4% of centres would switch from VKA to low-molecular-weight heparin (LMWH).
LA/LAA
CARDIAC FAILURE REVIEW www.CFRjournal.com
Persistent
Thrombus in Patients on Chronic Anticoagulation
Concerning antiplatelet therapies, the same survey showed that adding antiplatelet agents was infrequent, while none of the centres opted for dual antiplatelet therapy (DAPT). Similarly, the switch to unfractionated heparin among enrolled centres was highly uncommon.
On the other hand, when thrombus was present despite chronic NOAC treatment, the EHRA study showed that the most common strategy was to switch from NOAC (regardless of type) to VKA with a target INR of 2.5–3.5 or to switch to VKA with a target INR of 2–3; these two strategies accounted for about half of all management scenarios. Switch from NOAC to LMWH was used among 6.4–12.8% of participating centres, depending on the NOAC type, with the highest switching rate registered for apixaban and lowest for edoxaban. When NOAC to NOAC substitution was opted for, apixaban and dabigatran were the most commonly tried replacement NOACs. Similarly, the EHRA survey showed that the timing of repeated imaging after the change in OAC remains heterogeneous across centres.45 Nearly half of the centres would repeat imaging 3–4 weeks after the antithrombotic switch, while one-third would repeat imaging after 5–6 weeks. About 11% of centres would opt for delayed imaging arranged >2 months following the antithrombotic switch.
Real-world data might help shed light on the practical use of antithrombotics to resolve refractory LATA thrombus in patients with AF. For example, Faggiano et al. analysed data from 8,888 consecutive patients with AF who underwent TOE in two high-volume clinical centres. Most patients with identified LAA thrombus (3% of the total cohort) were on OAC for at least 3 weeks before index imaging. Their study showed that a VKA for LAA thrombus resolution was prescribed in 52%, NOAC in 27%, and LMWH in 18.5% of patients. Two-thirds of these patients received repeat TOE within a median time of 39 days, while one-third did not receive any follow-up imaging study. Importantly, thrombus resolution was achieved in 67% of all patients who underwent repeated TOE, while no significant difference in efficacy was established between VKA and NOACs.
Kolakowski et al. specifically reported on chronically anticoagulated patients for AF or atrial flutter and still had LAA thrombus detected by the TOE.46 They showed that nearly 52% of patients had LAA thrombus dissolution regardless of the number of treatment cycles employed. In contrast, any change in treatment (switch to a different OAC) was associated with increased odds of success. However, it is unclear whether any particular treatment strategy is more effective than the other. Additionally, the authors showed that several anticoagulation treatment cycles and the left atrium area were adversely related to thrombus resolution. Nelles et al. performed a similar study with their retrospective single-centre registry analysis, including 78 patients with AF. In that patient cohort, a large proportion of participants were diagnosed with solid LAT despite being treated with NOAC (45% of patients) or VKA (41% of patients).47 Their data show how thrombus resolution was achieved in almost half the enrolled patients during the mean follow-up time of 1 year, without a significant difference in efficacy between NOACs and VKAs. However, among those patients that responded to therapy with visualised thrombus resolution, there was a significantly shorter mean time to achieve that with NOACs versus VKA (81 versus 129 days; p=0.03).
Harada et al. previously showed how administering 300 mg of dabigatran (150 mg twice daily) in patients with LAAT and AF while on continuous NOAC therapy was effective in achieving thrombus resolution. However, this finding was obtained in a small sample size, and previous adherence to NOACs was not carefully evaluated.48 Similar results were obtained in a
Figure 1: Risk Factors and Clinical Determinants of Left Atrial Appendage Thrombus in Patients With Non-valvular AF
Patient-related factors
• Age
• Obesity
• Non-paroxysmal AF
• CHA2DS2-VASc score ≥3
• HFrEF
• Chronic renal failure

Structural heart characteristics

• Reduced LVEF
• LA remodelling
• Non-chicken wing
LAA morphology
• Decreased LAA blood flow velocity
Anticoagulantrelated factors
• Inappropriate or reduced VKA or NOAC doses
• Patient adherence to oral anticoagulant therapy
• Drug–drug interactions
Biochemical characteristics
• Higher D-dimer values
• Increased circulating homocysteine levels
HFrEF = heart failure with reduced ejection fraction; LA = left atrial; LAA = left atrial appendage; LVEF = left ventricular ejection fraction; NOAC = novel oral anticoagulant; VKA = vitamin K antagonist. Created with BioRender.com and reproduced with permission.
small-sized study by Yilmaz et al., including 17 patients with AF and LAA thrombus who also completed baseline and follow-up TOE examinations after initiating or switching their anticoagulation regimen.49 Patients in their study were treated with 300 mg dabigatran daily. Thrombus resolution was achieved in 87% of patients (7/8, all paroxysmal or persistent AF). At the same time, it was ineffective in only one patient with long-standing continuous AF. In another report, two patients with LAT resistant to rivaroxaban had thrombus resolution after starting dabigatran.50 Dabigatran is the only OAC that serves as a direct thrombin inhibitor and a prodrug. In contrast, the others (rivaroxaban, apixaban and edoxaban) act as factor Xa inhibitors in their active forms, thus reflecting different mechanisms of action. They concluded that dabigatran given twice daily was more efficient than a factor Xa inhibitor given once daily at dissolving existing thrombi and preventing the creation of new ones.50 The RIVA-TWICE prospective open-label study declared that when standard rivaroxaban therapy fails, rivaroxaban 15 mg twice daily appears as a safe therapeutic option and may dissolve LAA thrombus, with a resolution rate of LA/LAA thrombosis of 46.7%.51
A recent systematic review and meta-analysis comparing the use of NOAC versus warfarin for the treatment of LA thrombosis in patients with nonvalvular AF showed that NOAC use was associated with a 2.2-fold increased probability of LAT resolution and this was not offset with higher risks of bleeding or stroke/transient ischaemic attack (TIA).52 However, cautious interpretation of this analysis is advised since previous/current anticoagulation varied greatly across included trials. Some trials did not report previous anticoagulant exposure; some had all patients covered by NOACs or VKAs, while some enrolled patients were not previously treated with OACs.
The formal approach and management strategy are laid out in the recent EHRA 2021 practical guide on using NOACs in patients with AF.53 This document recommends that the management be individually tailored to each patient with AF with the persistent thrombus regardless of good adherence to NOAC treatment. Some general principles to consider are provided in this document – patients might be switched to a NOAC with a different mechanism of action (for example, switching from factor Xa
Persistent LA/LAA Thrombus in Patients on Chronic Anticoagulation CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 2: Treatment Pathway for Resistant Left Atrial/Left Atrial Appendage Thrombus
its marker aligns with the LAA’s ostial plane, which may increase the risk of distal contact and embolisation.55 In this patient subgroup, the lobe and disc devices might be a better option for LAA closure.
Herein, we propose a management scheme to patients with persistent LA/LAA thrombus despite full-dose anticoagulation for non-valvular AF (Figure 2).
Adding Antiplatelet to Anticoagulation Drugs for the Pharmacological Resolution of Persistent Left Atrial Appendage Thrombus
Assess adherence and consider increasing NOAC dose or switch to a di erent NOAC Preferably Rivaroxaban 15 mg twice daily or Dabigatran 150 mg twice daily for 8 weeks duration

Perform imaging Arrange TOE in 6–8 weeks Resolution of thrombus? Yes
To date, no randomised data or studies show the superiority or increased effectiveness of adding an antiplatelet agent to the existing or switched anticoagulant regimen for this indication. In a retrospective work by Kolakowski et al., it was suggested that keeping the same anticoagulant medication but adding an antiplatelet agent was associated with a numerically greater efficacy compared to several other strategies for LAA thrombus resolution (e.g. switch to an anticoagulant with a different mechanism, switch to an anticoagulant with a similar mechanism of action, switch to another anticoagulant with an added antiplatelet agent, adding a second anticoagulant drug or deliberate no change in treatment).46 However, combining an OAC and antiplatelet failed to show a statistical advantage in efficacy over any other antithrombotic regimen. It can be concluded that the role of antiplatelet addition for this indication is highly limited and currently not supported by the evidence except in cases in which a patient has another indication, such as concomitant coronary artery disease.
Continuous Oral Anticoagulant Regimen Following Left Atrial Appendage Occlusion Device Implantation
Perform imaging Arrange TOE in 6–8 weeks Resolution of thrombus?

Switch to VKA with targeted INR of 2.5–3.5 or LAAO implantation if distal thrombus
A proposed management pathway in the clinical scenario of persistent left atrial/appendage thrombus among patients already receiving full-dose continuous anticoagulant therapy for the purpose of stroke prevention in non-valvular AF. INR = international normalised ratio; LAAO = left atrial appendage occluder; NOAC = novel oral anticoagulant; TOE = transoesophageal echocardiography; TTR = time in therapeutic range; VKA = vitamin K antagonist. Source: Created with BioRender.com and reproduced with permission.
inhibitor to direct thrombin inhibitor or vice versa) or to VKA therapy with a customised INR target. Similarly, non-pharmacological alternative strategies such as LAA closure with dedicated devices might be considered in particular clinical scenarios. However, the authors clearly state the lack of prospective evidence in this setting.
Therefore, it becomes clear that all management decisions should be carefully balanced by estimating each individual patient’s bleeding and thrombotic risks. Only a few options have been available regarding the results of LAA closure in patients with AF and LAA thrombus. A recent review that comprised 35% of patients whose LAA thrombosis was persistent and distally situated demonstrated that LAA occlusion (LAAO) was possible in these individuals.54 The WATCHMAN device (Boston Scientific) requires the delivery sheath to be progressed into the LAA until
While oral anticoagulation therapy is effective in mitigating thromboembolic risks in non-valvular AF, for some patients bleeding risks and nonadherence to therapy present important barriers in effective anticoagulation. For these patients, surgical and percutaneous LAAO devices are important non-pharmacological strategies to overcome the challenges of anticoagulant pharmacotherapy.56 LAAO is also a feasible and safe therapeutic option for those patients that suffered a cerebrovascular event despite being on adequate anticoagulant treatment.57
The recent meta-analysis of observational data showed no difference in stroke, major bleeding, device-related thrombosis, and all-cause mortality rates in patients receiving antiplatelet versus anticoagulant agents following LAAO.58
However, whether patients after LAAO should still receive anticoagulants and, if yes, for how long and at what dose remains an open question in clinical practice.59 The results of the real-world prospective study in which 41% of patients did not receive OAC while 59% received OAC after LAAO with the LARIAT device (SentreHEART Inc) showed that there was no difference between the two groups in relevant outcomes such as rates of ischaemic stroke/TIA, thromboembolic events, bleeding, lifethreatening, disabling or significant events, and annual mortality rate.60 Cepas-Guillen et al. recently conducted a study in which a low-dose strategy with apixaban (2.5 mg twice daily) was tested against single antiplatelet therapy (SAPT; low-dose aspirin 100 mg once daily) and DAPT (aspirin 100 mg + clopidogrel 75 mg once daily) in patients with nonvalvular AF who underwent LAAO.61 The authors concluded that a strategy with low-dose apixaban following LAAO might be a feasible and effective alternative to DAPT and SAPT concerning combined efficacy and safety

Persistent LA/LAA Thrombus in Patients on Chronic Anticoagulation CARDIAC FAILURE REVIEW www.CFRjournal.com
Switch to VKA with targeted INR of 2.5–3.5 or LAAO implantation if distal thrombus Clinical scenario: persistent left atrium/appendage thrombus while on oral anticoagulant regimen for AF Current
anticoagulant type VKA Patient adherent to VKA treatment (adequate TTR, INR)? Optimise and continue VKA No 1 1 2 2 Switch to NOAC Yes Preferably
twice daily
Dabigatran
Alternative NOAC
weeks duration
No
oral
Rivaroxaban 15 mg
or
150 mg twice daily or
for 8
Yes
Continue initiated treatment NOAC
No Continue initiated reatment
endpoints. However, this study was not randomised and enrolled a limited number of patients. In the ADRIFT trial, strategies of two doses of rivaroxaban were compared versus DAPT consisting of 75 mg aspirin and 75 mg clopidogrel in patients implanted with Amplatzer Amulet (Abbott) and WATCHMAN devices for LAAO.62 This study showed that the circulating levels of prothrombin fragments 1 and 2 reflecting thrombin generation following the LAOO procedure were higher among patients treated with DAPT than 10 or 15 mg rivaroxaban. However, it remains unclear whether this effect can reduce adverse post-procedural events such as device-related thrombosis or other thromboembolic events. In line with this, Tjoe et al. showed, in a retrospective analysis of 213 patients, that use of DOAC with or without aspirin had similar safety and efficacy profile post-WATCHMAN device implantation when compared to warfarin and aspirin use.63
Furthermore, robust nationwide data on oral anticoagulation following LAAO became recently available from the LAAO Registry of the National Cardiovascular Data Registry that enrolled patients implanted with the WATCHMAN device in the US.64 This extensive analysis of 31,994 patients who underwent successful LAAO showed that the most significant deviations from implantation protocol were observed in post-discharge antithrombotic medications. This analysis showed that the postimplantation discharge on warfarin or DOAC, compared to DOAC + aspirin
1. Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and metaanalysis. Eur Heart J 2016;37:1591–602. https://doi. org/10.1093/eurheartj/ehw007; PMID: 26888184.
2. Stewart S, Hart CL, Hole DJ, McMurray JJV. A populationbased study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–64. https://doi.org/10.1016/s00029343(02)01236-6; PMID: 12401529.
3. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation meta-analysis. Ann Intern Med 1999;131:492–501. https://doi.org/10.7326/0003-4819-131-7-19991005000003; PMID: 10507957.
4. Giugliano RP, Ruf CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. https://doi.org/10.1056/ NEJMoa1310907; PMID: 24251359.
5. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. https://doi.org/10.1056/NEJMoa0905561; PMID: 19717844.
6. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. https://doi.org/10.1056/ NEJMoa1107039; PMID: 21870978.
7. Chen YY, Liu Q, Liu L, et al. Effect of metabolic syndrome on risk stratification for left atrial or left atrial appendage thrombus formation in Patients with nonvalvular atrial fibrillation. Chin Med J (Engl) 2016;129:2395–402. https://doi. org/10.4103/0366-6999.191744; PMID: 27748329.
8. Cohoon KP, McBane RD, Ammash N, et al. Relationship between body mass index and left atrial appendage thrombus in nonvalvular atrial fibrillation. J Thromb Thrombolysis 2016;41:613–8. https://doi.org/10.1007/s11239015-1266-7; PMID: 26282111.
9. Ezekowitz MD, Pollack CV, Halperin JL, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–71. https://doi.org/10.1093/ eurheartj/ehy148; PMID: 29659797.
10. Kitkungvan D, Nabi F, Ghosn MG, et al. Detection of LA and LAA thrombus by CMR in patients referred for pulmonary vein isolation. JACC Cardiovasc Imaging 2016;9:809–18. https://doi.org/10.1177/1747493018778713; PMID: 29786478.
11. Erickson M, Yadav H, Sneij E, et al. Incidence of left atrial appendage thrombus desthreeite three weeks of anticoagulation and the need for precardioversion echocardiography. Ann Noninvasive Electrocardiol 2022;27:e12989. https://doi.org/10.1111/anec.12989; PMID: 35802810.
12. Stoddard MF, Singh P, Dawn B, Longaker RA. Left atrial
or DAPT alone, was associated with a significant reduction in the composite endpoint of adverse outcomes.
Conclusion
Taken together, it seems that in case of LAA thrombus presence despite chronic anticoagulation treatment, most centres would practice switching to another anticoagulant drug with a different mechanism of action. In contrast, repeated imaging for LAA thrombus would be performed within 3–6 weeks in over 80% of cases. It is also evident that several essential questions in the scenario of LAA thrombus – despite apparently adherent chronic OAC treatment – remain unanswered. These are which anticoagulation drug should be selected in these cases, for how long treatment should be initiated, which dosing regimen should be selected and when should the follow-up imaging be arranged. As previously elaborated, relevant metaanalysis suggests increased efficacy with NOACs than warfarin, and there is limited clinical evidence that 300 mg of dabigatran might be particularly effective. However, these observations need to be confirmed in a prospective randomised fashion. In summary, it becomes evident that the optimal choice, dosing and duration of antithrombotic and anticoagulation treatment following LAAO is unclear and that high-quality large randomised trials adequately powered for relevant clinical outcomes are warranted. The role of continuous anticoagulant use following LAAO implantation would need to be prospectively validated by such studies.
thrombus predicts transient ischemic attack in patients with atrial fibrillation. Am Heart J 2003;145:676–82. https://doi. org/10.1067/mhj.2003.91; PMID: 12679765.
13. Mügge A, Kühn H, Nikutta P, et al. Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with nonrheumatic atrial fibrillation: identification of a subgroup of patients at increased embolic risk. J Am Coll Cardiol 1994;23:599–607. https://doi.org/10.1016/0735-1097(94)90743-9; PMID: 8113541.
14. Frenkel D, D’Amato SA, Al-Kazaz M, et al. Prevalence of left atrial thrombus detection by transesophageal echocardiography a comparison of continuous non–vitamin K antagonist oral anticoagulant versus warfarin therapy in patients undergoing catheter ablation for atrial fibrillation. JACC Clin Electrophysiol 2016;2:295–303. https://doi. org/10.1016/j.jacep.2016.01.004; PMID: 29766887.
15. Wu M, Gabriels J, Khan M, et al. Left atrial thrombus and dense spontaneous echocardiographic contrast in patients on continuous, direct oral anticoagulant therapy undergoing catheter ablation of atrial fibrillation: comparison of dabigatran, rivaroxaban, and apixaban. Heart Rhythm 2018;15:496–502. https://doi.org/10.1016/j.hrthm.2017.12.005; PMID: 29605015.
16. Yamaji K, Fujimoto S, Yutani C, et al. Is the site of thrombus formation in the left atrial appendage associated with the risk of cerebral embolism? Cardiology 2002;97:104–10. https://doi.org/10.1159/000057681; PMID: 11978958.
17. Angelini F, Bocchino PP, Peyracchia M, et al. Prevalence and predictors of left atrial thrombosis in atrial fibrillation patients treated with non-vitamin K antagonist oral anticoagulants. Acta Cardiol 2021. https://doi.org/10.1080/000 15385.2021.2005307; PMID: 34821203; online ahead of press.
18. Bertaglia E, Anselmino M, Zorzi A, et al. NOACs and atrial fibrillation: incidence and predictors of left atrial thrombus in the real world. Int J Cardiol 2017;249:179–83. https://doi. org/10.1016/j.ijcard.2017.07.048; PMID: 29121724.
19. Durmaz E, Karpuz MH, Bilgehan K, et al. Left atrial thrombus in patients with atrial fibrillation and under oral anticoagulant therapy; 3-D transesophageal echocardiographic study. Int J Cardiovasc Imaging 2020;36:1097–103. https://doi.org/10.1007/s10554-02001811-x; PMID: 32140812.
20. Springer A, Schleberger R, Oyen F, et al. Genetic and clinical predictors of left atrial thrombus: a Single Center case-control study. Clin Appl Thromb Hemost 2021;27:1-7. https://doi.org/10.1177/10760296211021171; PMID: 34184557.
21. Yao Y, sheng SM, et al. Elevated homocysteine increases the risk of left atrial/left atrial appendage thrombus in nonvalvular atrial fibrillation with low CHA2DS2-Vasc score. Europace 2017;20:1093–8. https://doi.org/10.1093/europace/ eux189; PMID: 28637244.
22. Karwowski J, Rekosz J, Mączyńska-Mazuruk R, et al. Left atrial appendage thrombus in patients with atrial fibrillation who underwent oral anticoagulation. Cardiol J 2022. https:// doi.org/10.5603/CJ.a2022.0054; PMID: 35703043; online ahead of press.
23. Kapłon-Cieślicka A, Budnik M, Gawałko M, et al. Atrial fibrillation type and renal dysfunction as essential predictors of left atrial thrombus. Heart 2019;105:1310–5. https://doi. org/10.1136/heartjnl-2018-314492; PMID: 31040170.
24. Whiteside HL, Nagabandi A, Brown K, et al. Prevalence and clinical characteristics associated with left atrial thrombus detection: apixaban. World J Cardiol 2019;11:84–93. https:// doi.org/10.4330/wjc.v11.i2.84; PMID: 30820278; PMCID: PMC6391620.
25. Anselmino M, Gili S, Castagno D, et al. Do left atrial appendage morphology and function help predict thromboembolic risk in atrial fibrillation? J Cardiovasc Med (Hagerstown) 2016;17:169–76. https://doi.org/10.2459/ JCM.0000000000000305; PMID: 26556443.
26. Wang F, Zhu M, Wang X, et al. Predictive value of left atrial appendage lobes on left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord 2018;18:153. https://doi.org/10.1186/ s12872-018-0889-y; PMID: 30064363.
27. Yamamoto M, Seo Y, Kawamatsu N, et al. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ Cardiovasc Imaging 2014;7:337–43. https://doi.org/10.1161/ CIRCIMAGING.113.001317; PMID: 24523417.
28. Du H, Bi K, Xu L, et al. Analysis of risk factors for thrombosis of the left atrium/left atrial appendage in patients with non-valvular atrial fibrillation. Cardiovasc J Afr 2021;32:116–22. https://doi.org/10.5830/CVJA-2019-071; PMID: 33950066.
29. Zuo K, Sun L, Yang X, et al. Correlation between cardiac rhythm, left atrial appendage flow velocity, and CHA2DS2vasc score: study based on transesophageal echocardiography and 2-dimensional speckle tracking. Clin Cardiol 2017;40:120–5. https://doi.org/10.1002/clc.22639; PMID: 28075503.
30. Clark CB, Telles Garcia NA, Hackett Renner C, Ryan SM. Correlation of left atrial appendage ejection velocities with the CHADS2 and CHA2DS2-vasc scores. Echocardiography 2016;33:1195–201. https://doi.org/10.1111/echo.13228; PMID: 27060690.
31. Akoum N, Fernandez G, Wilson B, et al. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J CardioVasc Electrophysiol 2013;24:1104–9. https://doi. org/10.1111/jce.12199; PMID: 23844972.
32. Watanabe A, Suzuki S, Kano H, et al. Left atrial remodeling assessed by transthoracic echocardiography predicts left
Persistent LA/LAA Thrombus in
CARDIAC FAILURE REVIEW www.CFRjournal.com
Patients on Chronic Anticoagulation
atrial appendage flow velocity in patients with paroxysmal atrial fibrillation. Int Heart J 2016;57:177–82. https://doi. org/10.1536/ihj.15-345; PMID: 26973273.
33. Bisbal F, Gómez-Pulido F, Cabanas-Grandío P, et al. Left atrial geometry improves risk prediction of thromboembolic events in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:804–10. https://doi.org/10.1111/ jce.12978; PMID: 27027899.
34. Dudzińska-Szczerba K, Zalewska M, Niemiro W, et al. Association of left atrial sphericity with risk of stroke in patients with atrial fibrillation. Sub-analysis of the Assam study. Cardiovasc Eng Technol 2022;13:419–27. https://doi. org/10.1007/s13239-021-00587-y; PMID: 34750781.
35. Uziębło-Życzkowska B, Krzesiński P, Jurek A, et al. Left ventricular ejection fraction is associated with the risk of thrombus in the left atrial appendage in patients with atrial fibrillation. Cardiovasc Ther 2020;2020:3501749. https://doi. org/10.1155/2020/3501749; PMID: 32411299.
36. Kim MN, Kim SA, Choi JI il, et al. Improvement of predictive value for thromboembolic risk by incorporating left atrial functional parameters in the CHADS2 and CHA2DS2-vasc scores. Int Heart J 2015;56:286–92. https://doi.org/10.1536/ ihj.14-380; PMID: 25912904.
37. Lu X, Chen T, Liu G, et al. Relations between left atrial appendage contrast retention and thromboembolic risk in patients with atrial fibrillation. J Thromb Thrombolysis 2022;53:191–201. https://doi.org/10.1007/s11239-021-024908; PMID: 34128199.
38. Lee WC, Fang CY, Chen YL, et al. Left atrial or left atrial appendage thrombus resolution after adjustment of oral anticoagulant treatment. J Stroke Cerebrovasc Dis 2019;28:90–6. https://doi.org/10.1016/j. jstrokecerebrovasdis.2018.09.015; PMID: 30301596.
39. Vazquez SR. Drug-drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Hematology Am Soc Hematol Educ Program 2018;2018:339–47. https://doi.org/10.1182/ asheducation-2018.1.339; PMID: 30504330.
40. Ellis CR, Kanagasundram AN. Atrial fibrillation in heart failure left atrial appendage management. Cardiol Clin 2019;37:241–9. https://doi.org/10.1016/j.ccl.2019.01.009; PMID: 30926025.
41. Wybraniec MT, Mizia-Szubryt M, Cichoń M, et al. Heart failure and the risk of left atrial thrombus formation in patients with atrial fibrillation or atrial flutter. ESC Heart Fail 2022;9:4064–76. https://doi.org/10.1002/ehf2.14105; PMID: 36039813.
42. Merino JL, Lip GYH, Heidbuchel H, et al. Determinants of left atrium thrombi in scheduled cardioversion: an ENSUREAF study analysis. Europace 2019;21:1633–8. https://doi. org/10.1093/europace/euz213; PMID: 31436835.
43. Noubiap JJ, Agbaedeng TA, Ndoadoumgue AL, et al. Atrial thrombus detection on transoesophageal echocardiography in patients with atrial fibrillation undergoing cardioversion or catheter ablation: a pooled analysis of rates and predictors. J Cardiovasc Electrophysiol 2021;32:2179–88. https://doi. org/10.1111/jce.15082; PMID: 33969568.
44. Kurzawski J, Janion-Sadowska A, Zandecki L, et al. Global peak left atrial longitudinal strain assessed by transthoracic echocardiography is a good predictor of left atrial appendage thrombus in patients in sinus rhythm with heart failure and very low ejection fraction – an observational study. Cardiovasc Ultrasound 2020;18:7. https://doi.org/10.1186/ s12947-020-00188-0; PMID: 32061249.
45. Farkowski MM, Jubele K, Marín F, et al. Diagnosis and management of left atrial appendage thrombus in patients with atrial fibrillation undergoing cardioversion or percutaneous left atrial procedures: results of the European Heart Rhythm Association survey. Europace 2019;22:162–9. https://doi.org/10.1093/europace/euz257; PMID: 31501852.
46. Kołakowski K, Farkowski MM, Pytkowski M, et al. The comparative effectiveness and safety of different anticoagulation strategies for treatment of left atrial appendage thrombus in the setting of chronic anticoagulation for atrial fibrillation or flutter. Cardiovasc Drugs Ther 2023;37:159-68. https://doi.org/10.1007/s10557021-07278-9; PMID: 34669102.
47. Nelles D, Lambers M, Schafigh M, et al. Clinical outcomes and thrombus resolution in patients with solid left atrial appendage thrombi: results of a single-center real-world registry. Clin Res Cardiol 2021;110:72–83. https://doi. org/10.1007/s00392-020-01651-8; PMID: 32307589.
48. Harada M, Koshikawa M, Motoike Y, et al. Left atrial appendage thrombus prior to atrial fibrillation ablation in the era of direct oral anticoagulants. Circ J 2018;82:2715–21. https://doi.org/10.1253/circj.CJ-18-0398; PMID: 30101809.
49. Yilmaz KC, Ciftci O, Ozin B, Muderrisoglu H. Anticoagulants in left atrial thrombus resolution. Ann Med Res 2020;27:1908–12. https://doi.org/10.5455/ annalsmedres.2020.03.284
50. Watanabe T, Shinoda Y, Ikeoka K, et al. Dabigatran therapy resulting in the resolution of Rivaroxaban-resistant left atrial appendage thrombi in patients with atrial fibrillation. Intern Med 2017;56:1977–80. https://doi.org/10.2169/ internalmedicine.56.8508; PMID: 28768967.
51. Piotrowski R, Zaborska B, Pilichowska-Paszkiet E, et al. Rivaroxaban TWICE daily for lysis of thrombus in the left atrial appendage in patients with non-valvular atrial fibrillation: the RIVA-TWICE study. Arch Med Sci AMS 2019;16:289–96. https://doi.org/10.5114/aoms.2019.86616; PMID: 32190138.
52. Dong SJ, Luo CY, Xiao CL, et al. Efficacy and safety profile of novel oral anticoagulants in the treatment of left atrial thrombosis: a systematic review and meta-analysis. Curr Ther Res Clin Exp 2022;96:100670. https://doi.org/10.1016/j. curtheres.2022.100670; PMID: 35515958.
53. Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of nonvitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. https://doi. org/10.1093/europace/euab065; PMID: 33895845.
54. Sharma SP, Cheng J, Turagam MK, et al. Feasibility of left atrial appendage occlusion in left atrial appendage
thrombus a systematic review. JACC Clin Electrophysiol 2020;6:414–24. https://doi.org/10.1016/j.jacep.2019.11.017; PMID: 32327075.
55. Jalal Z, Iriart X, Dinet ML, et al. Extending percutaneous left atrial appendage closure indications using the AMPLATZERTM cardiac plug device in patients with persistent left atrial appendage thrombus: the thrombus trapping technique. Arch Cardiovasc Dis 2016;109:659–66. https://doi.org/10.1016/j.acvd.2016.02.012; PMID: 27402154.
56. Collado FMS, von Buchwald CML, Anderson CK, et al. Left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation. J Am Heart Assoc 2021;10:e022274. https://doi.org/10.1161/JAHA.121.022274; PMID: 34668395.
57. Falasconi G, Gaspardone C, Godino C, et al. Left atrial appendage closure: a new strategy for cardioembolic events despite oral anticoagulation. Panminerva Med 2021. https://doi.org/10.23736/S0031-0808.21.04446-3; PMID: 34664480; epub ahead of press.
58. Osman M, Busu T, Osman K, et al. Short-term antiplatelet versus anticoagulant therapy after left atrial appendage occlusion a systematic review and meta-analysis. JACC Clin Electrophysiol 2020;6:494–506. https://doi.org/10.1016/j. jacep.2019.11.009; PMID: 32439033.
59. Chew DS, Piccini JP. Postprocedural antithrombotic therapy following left atrial appendage occlusion: no longer adrift in uncertainty? Circ Cardiovasc Interv 2020;13:e009534. https:// doi.org/10.1161/CIRCINTERVENTIONS.120.009534; PMID: 32674680.
60. Litwinowicz R, Filip G, Sobczyk D, et al. Long-term effect of anticoagulation following left atrial appendage occlusion with the LARIAT device in patients with nonvalvular atrial fibrillation: impact on thromboembolism, bleeding and mortality. Real-life data. Postepy Kardiol Interwencyjnej 2020;16:89–96. https://doi.org/10.5114/aic.2020.93916; PMID: 32368241.
61. Cepas-Guillen PL, Flores-Umanzor E, Regueiro A, et al. Low dose of direct oral anticoagulants after left atrial appendage occlusion. J Cardiovasc Dev Dis 2021;8:142. https://doi. org/10.3390/jcdd8110142; PMID: 34821695.
62. Duthoit G, Silvain J, Marijon E, et al. Reduced rivaroxaban dose versus dual antiplatelet therapy after left atrial appendage closure: ADRIFT a randomized pilot study. Circ Cardiovasc Interv 2020;13e008481. https://doi.org/10.1161/ CIRCINTERVENTIONS.119.008481; PMID: 32674675.
63. Tjoe B, Nguyen H, Mandava S, et al. Use of direct oral anticoagulation therapy following implantation of the Watchman left atrial appendage occlusion device. Struct Hear 2021;5:295–301. https://doi.org/10.1080/24748706.202 1.1890286
64. Freeman JV, Higgins AY, Wang Y, et al. Antithrombotic therapy after left atrial appendage occlusion in patients with atrial fibrillation. J Am Coll Cardiol 2022;79:1785–98. https:// doi.org/10.1016/j.jacc.2022.02.047; PMID: 35512858.
LA/LAA Thrombus in Patients on Chronic Anticoagulation CARDIAC FAILURE REVIEW www.CFRjournal.com
Persistent
Epicardial Fat in Heart Failure with Preserved Ejection Fraction: Bad Actor or Just Lying Around?
Mary-Tiffany Oduah , 1 Varun Sundaram2 and Yogesh NV Reddy 1
Abstract
Heart failure with preserved ejection fraction (HFpEF) is increasingly recognised to be strongly associated with obesity and abnormalities in fat distribution. Epicardial fat has been associated with abnormal haemodynamics in HFpEF, with potential for direct mechanical effects on the heart causing constriction-like physiology and local myocardial remodelling effects from secretion of inflammatory and profibrotic mediators. However, patients with epicardial fat generally have more systemic and visceral adipose tissue making determination of causality between epicardial fat and HFpEF complex. In this review, we will summarise the evidence for epicardial fat being either directly causal in HFpEF pathogenesis or merely being a correlate of worse systemic inflammatory and generalised adiposity. We will also discuss therapies that directly target epicardial fat and may have potential for treating HFpEF and elucidating the independent role of epicardial fat in its pathogenesis.
Keywords
Heart failure with preserved ejection fraction, obesity, epicardial fat
Disclosure: YNVR is supported by grants from Sleep Number, Bayer Accelerated Pulmonary Hypertension Award, United Jenesis Award and a competitive internal grants programme from the Mayo Clinic Cardiovascular Division and Circulatory Failure. All other authors have no conflicts of interest to declare.
Received: 4 August 2022
Accepted: 4 November 2022 Citation: Cardiac Failure Review 2023;9:e06. DOI: https://doi.org/10.15420/cfr.2022.25
Correspondence: Yogesh NV Reddy, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, US E: Reddy.Yogesh@mayo.edu
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure with preserved ejection fraction (HFpEF) is becoming the most common cause of heart failure worldwide, in part, driven by a rising prevalence of obesity.1 Since the first description of obesity-related HFpEF, there has been widespread recognition of this being a unique phenotype with its pathophysiology driven directly by excess adiposity.2–4 Notably, obesity is the predominant driver of an increasing prevalence of HFpEF in younger people.5,6 Although generalised and visceral adiposity is important in the pathogenesis of obesity-related HFpEF, there is increasing recognition of the potential role of epicardial adipose tissue (EAT) in disease pathogenesis. EAT is metabolically active tissue located directly on the surface of the myocardium underneath the visceral pericardium. By virtue of its anatomical interface with the heart and the lack of fascial separation between the underlying myocardium and epicardial fat, locally secreted adipokines directly bathe the surface of the heart and result in underlying myocardial remodelling.7 Its position on the surface of the myocardium allows EAT to directly contribute to an increase in total heart size with stretch of the pericardium and results in relative pericardial restraint with constrictive physiology.3 8 9 EAT is most commonly measured by echocardiography in the parasternal long axis view perpendicular to the right ventricle (RV) to quantify epicardial fat thickness and this has been correlated with worse haemodynamic derangements and adverse outcomes in HFpEF.9 10 Alternatively, cardiac MRI or CT can provide a more complete volumetric assessment of epicardial fat volume and has also demonstrated associations with adverse outcomes and functional metrics in most but not all HFpEF studies (Table 1).11–15
Epicardial fat has been independently associated with abnormal cardiac structural changes and incidence of HFpEF in community-based studies further supporting its potential causal role.16,17 Notably, these studies have demonstrated independent associations between baseline epicardial fat with risk of heart failure in both men and women, which persisted even after adjustment for various anthropometric measures, including overall BMI, visceral fat and biomarkers of inflammation.17 There is, however, a strong collinear relationship between generalised obesity and epicardial fat, making the relative contributions of epicardial fat compared to overall adiposity in the pathogenesis of HFpEF less clear even after statistical adjustment.11,12 Therefore, it remains unclear if EAT truly independently contributes to obesity-related HFpEF or is just a marker of severe obesity. In this review, we will discuss the potential pathophysiology of epicardial fat as it relates to HFpEF, potential therapies targeting EAT with implications for HFpEF and the arguments for and against its causal role.
Does Epicardial Adipose Tissue Have a Causal Contribution to Obesity-related HFpEF?
Epicardial, visceral and overall adiposity are clearly linked with incident HFpEF but also with AF and atrial myopathy, which are important components of the HFpEF syndrome.18–23 Recent studies focusing on EAT have elucidated some of its key roles in obesity-related HFpEF. EAT can theoretically be a contributor to obesity-related HFpEF by either mechanically enhancing pericardial restraint and/or through local secretory effects from its direct contact with the underlying myocardium causing adjacent ventricular and atrial remodelling.2 9 24–26
REVIEW © 2023 The Author(s). Published by Radcliffe Group Ltd. www.CFRjournal.com Special Focus on Patients with HFpEF
1. Department of Cardiovascular Disease, Mayo Clinic, Rochester, MN, US; 2. Division of Cardiovascular Diseases, Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Cleveland, OH, US
Table 1: Studies Evaluating Epicardial Adipose Tissue in HFpEF
Study Sample
EAT Differences Between Groups EAT Association with Clinical Parameters
Obokata et al. 20172 195 HFpEF, 71 controls EAT increased in obese compared to non-obese HFpEF
Wu et al. 201742 63 HFpEF, 59 controls EAT in HFpEF similar to control group
Van Woerden et al. 201813 64 HFpEF, 20 controls EAT increased in HFpEF
Haykowsky et al. 201811 100 HFpEF, 61 controls EAT lower in HFpEF compared to controls
Gorter et al. 202012 75 HFpEF N/A
Koepp et al. 20209 77 HFpEF with high EAT 92 HFpEF with lower EAT N/A
Wu C et al. 202041 163 HFpEF, 108 controls EAT increased in HFpEF
Tromp et al. 202115 47 HFpEF, 113 controls EAT increased in HFpEF compared to controls
Lin J et al. 202143 51 HFpEF, 40 controls EAT increased in HFpEF
Pugliese et al. 202110 205 HFrEF, 188 HFpEF, 44 controls EAT highest in HFpEF compared to both controls and HFrEF
Obese HFpEF had worse pericardial restraint and higher epicardial fat compared to non-obese HFpEF
EAT associated with worse ECV
EAT associated with AF, diabetes, higher troponin, lower LV GLS, lower EF, higher atrial volumes
In contrast to all other studies, higher EAT was lower in HFpEF and paradoxically associated with higher peak VO2
EAT associated with RV end diastolic pressure and reduced peak VO2 After adjustment for obesity, EAT was only associated with reduced peak VO 2 and not RV end diastolic pressure
HFpEF with higher EAT had worse resting and exercise biventricular filling pressures, pulmonary hypertension, pericardial restraint and peak VO2
EAT associated with LV volume, LV mass, LA size and ECV in HFpEF
Potential greater impact of epicardial fat in men compared to women with HFpEF
EAT associated with impaired LV GLS and greater ECV in HFpEF
EAT associated with impaired LV GLS, higher adipocyte fatty acid-binding protein and increased risk of HF hospitalisation
After adjustment for BMI and waist circumference, EAT in HFpEF was associated with lower peak VO2, lower arteriovenous O2 difference, higher troponin levels and higher C-reactive protein levels
Higher EAT level in HFpEF was also associated with cardiovascular death and HF hospitalisation
Jin X et al. 2022111 149 controls, 99 HFpEF, 366 HFrEF
Van Woerden et al. 202214 50 HFpEF with high EAT, 52 HFpEF with lower EAT
EAT higher in HFpEF and controls compared to HFrEF
N/A
EAT associated with worse LA and LV function
HFpEF with high EAT had larger RV end diastolic volume, RV mass, LA/RA volume and impaired LA strain
HFpEF with high EAT associated with HF hospitalisation and mortality even after adjustment for BMI
EAT = epicardial adipose tissue; ECV = extracellular volume; EF = ejection fraction; GLS = global longitudinal strain; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LA = left atrial; LV = left ventricular; RA = right atrial; RV = right ventricular; VO2 = oxygen consumption.
The pericardium normally exerts a compressive contact force on the heart that helps couple left and right ventricular preload to try and maintain a constant stroke volume in the face of changing venous return. As with any chamber in the heart, the pericardium has its own curvilinear pressure–volume relationship with an initial flat portion followed by an exponential increase when stretched to the steep portion of its pressure-volume relationship.8,27 In patients with substantial obesity with cardiomegaly and increased epicardial fat volume, the pericardial sac is stretched to a steeper pressure-volume relationship and there is enhanced diastolic ventricular interaction and pericardial restraint (Figures 1 and 2). This exerts an exaggerated compressive force on the heart resulting in higher left- and right-sided filling pressures for any degree of left ventricular (LV) end diastolic volume – the true LV preload.
Although gradual cardiac enlargement can allow some degree of pericardial remodelling and improved pericardial compliance, eventually such reserve is exhausted, particularly during exercise when there is a rapid bolus of venous return to an already overstretched pericardial sac.28,2 Since EAT lies between the visceral pericardium and underlying myocardium, its expansion can result in a stretched pericardium with resultant compressive contact force on the heart, independent of the underlying heart size and intrinsic pericardial properties. This compressive force increases pulmonary capillary wedge pressure (PCWP) for any LV end diastolic volume and is associated with higher right atrial (RA) pressure (which closely approximates pericardial pressure in man) (Figure 3).29 Functionally, this results in a heightened RA/PCWP ratio
during rest and exercise and an increased eccentricity index on echocardiography (D shaped LV), which compromises LV filling and cardiac output response during exercise despite a pathologically elevated PCWP.8 This is further reinforced by the finding that filling pressures in obesity-related HFpEF are higher in patients with HFpEF and greater EAT.9 Even if the PCWP does rise primarily due to constrictive physiology, this still contributes to pulmonary congestion and dyspnoea during exercise, and in fact patients with higher RA pressures, such as those with pericardial restraint, have the greatest pulmonary congestion during exertion resulting from impeded lung lymphatic drainage into a systemic venous system with high venous pressure.30–33 Multiple other studies have also correlated epicardial fat with peak oxygen consumption (VO2) and haemodynamic derangements in HFpEF consistent with a potential contributory role (Table 1).9 13 15
Epicardial fat has also been associated with AF even in HFpEF patients, with biological plausibility for a causal relationship through underlying myocardial injury, inflammation, and adjacent atrial and ventricular remodelling.13,23,25,26,34–39 This atrial and ventricular remodelling may relate to direct secretion of adipokines and inflammatory profibrotic markers from the epicardial fat, thereby affecting the underlying myocardium through activin-A, angiopoietin-2, interleukins, tumour necrosis factor α and infiltration of inflammatory cells such as monocytes and macrophages.24 26 35 36 Meta-analyses have demonstrated an association between EAT and diastolic dysfunction.40 A number of HFpEF studies have also demonstrated a correlation between EAT with worse exercise
Epicardial Fat in HFpEF: Correlation or Causation? CARDIAC FAILURE REVIEW www.CFRjournal.com
capacity, myocardial injury, impaired myocardial structure and function, extracellular volume suggestive of fibrosis and impaired resting and exercise haemodynamics (Table 1).9 13–15 41 42
A study that specifically looked at exercise haemodynamics in HFpEF patients with and without increased EAT demonstrated worse resting and exercise biventricular filling pressures, pulmonary hypertension and pericardial restraint along with impaired exercise capacity.9 Longitudinal studies have demonstrated an independent relationship between EAT and incident heart failure, even after adjusting for overall BMI, fat distribution and sex suggesting a causal relationship.17 Similarly, EAT in overt HFpEF patients appears to be associated with hospitalisation for heart failure and mortality risk, even after adjusting for BMI.10,14,43 EAT in community participants is also associated with preclinical abnormalities in atrial and RV strain and a worse 6-minute walk distance which further suggests the potential to predispose to early HFpEF.16 Additionally, epicardial fat appears to be associated with atherosclerosis progression and is linked with incident HFpEF even in patients with coronary disease suggesting that coronary endothelial dysfunction and atherosclerotic changes may also mediate some of the links between HFpEF and epicardial fat.44,45 Thus, there is a body of evidence supporting a potential independent role for epicardial fat in the pathogenesis of HFpEF and specific therapies directed towards EAT require further investigation to both prevent and treat HFpEF.
Is EAT Merely a Marker of Functionally Severe Obesity in HFpEF?
Although EAT appears to be associated with abnormal haemodynamics that are pathognomonic of HFpEF, the presence of EAT is also associated with diabetes and greater visceral and overall adiposity, which have also been associated with HFpEF and this makes a direct determination of causality with HFpEF difficult.9,13,14,18,20,46,47 Therefore, while there is a growing body of literature to suggest a strong association between EAT and obese HFpEF, there remains difficulty in establishing causality given the strong collinear associations between epicardial fat, visceral fat and total body fat.11 12 In other words, it remains unclear if the presence of EAT is merely a correlate of the generalised adiposity-related inflammation that drives the obese HFpEF syndrome, or if excess EAT directly contributes to HFpEF pathogenesis independent of visceral and systemic adiposity.3 48 49 Patients with increased EAT and HFpEF generally are more obese with larger blood volumes and greater potential for generalised obesity-related HFpEF haemodynamic derangements.2 9 12 Notably, although epicardial fat appears to be associated with worse pericardial restraint in HFpEF, there are multiple other reasons for increased pericardial restraint in obese HFpEF, including global cardiomegaly, mediastinal restraint, increased blood volume, abdominal compression from visceral adipose tissue and pulmonary hypertension with abnormal RV-pulmonary artery coupling (Figure 4).9 High filling pressures in obesityrelated HFpEF are proportional to body mass and associated plasma volume, all of which distend the heart and pericardium exacerbating relative pericardial restraint.2 50
EAT is also clearly associated with visceral adipose tissue and tends to track visceral adipose changes, as seen with bariatric surgery where substantial weight loss results in regression of both fat depots.51 Furthermore, many patients still develop HFpEF despite having normal amounts of EAT. Therefore the presence of EAT is not essential for the manifestation of exertional pulmonary venous hypertension that is pathognomonic of HFpEF. 9 52 53 In addition, the only randomised trial of weight loss in HFpEF demonstrated that symptoms and exercise capacity
Figure 1: Haemodynamics in a Patient with Obesity-related Heart Failure With Preserved Ejection Fraction and Epicardial Fat Demonstrating Relative Pericardial Restraint
This shows the haemodynamics of a typical middle-aged patient with obesity-related heart failure with preserved ejection fraction who presented with 2 years of exertional dyspnoea and normal natriuretic peptide levels with a BMI of 34 kg/m2. Baseline echocardiogram revealed normal left ventricular (LV) function, increased LV myocardial mass, biatrial enlargement, increased epicardial adipose tissue and subtle ventricular interdependence. LV (blue), right ventricular (RV; green) and right atrial pressure (purple) are shown. There is subtle ventricular interdependence with an increase in RV pressure and a decrease in LV pressure during inspiration (inspiratory beats with interdependence denoted by vertical black arrows). There is also evidence of a square root sign in the LV and RV pressure tracing due to relative pericardial restraint during inspiration with equalisation with right atrial pressure. Normally there is less blood entering the LV during inspiration, so the development of a square root pattern in the LV reflects a decrease in effective operating compliance of the LV (despite there being less blood filling the LV) as a result of right–left diastolic ventricular interaction from increased inspiratory venous return to the right heart compressing the LV from relative pericardial restraint.
could be improved with modest weight loss in obese HFpEF and this clinical improvement was associated with a regression in visceral and subcutaneous adipose tissue but with no measurable change in epicardial fat by MRI.54 Large weight loss results in improved haemodynamics in obese patients, which may be only partially related to epicardial fat regression, but, regardless of the mechanism, weight loss through bariatric surgery clearly prevents incident heart failure that is likely to be HFpEF.51,55,56 Therefore, epicardial fat does not appear essential for HFpEF in all patients and obese HFpEF patients still experience clinical improvement with weight loss in the absence of epicardial fat modulation.2,9 Although there may be subsets of obese HFpEF patients where epicardial fat is directly causal in part of their pathophysiology, it remains difficult to conclusively delineate the independent causal contribution of EAT to obesity-related HFpEF. Regardless of causality, the mere presence of epicardial fat in a patient with HFpEF is a biomarker associated with worse exercise haemodynamics, pericardial restraint, exercise tolerance and prognosis and is an important clinical feature to identify.9 14
Therapies Targeting Epicardial Fat Lifestyle Modification
Substantial weight loss, such as that associated with bariatric surgery, has been associated with reduced epicardial fat thickness along with regression of visceral and generalised adiposity.51 57 In contrast to this data with bariatric surgery, modest weight loss just through diet change and exercise in obese HFpEF in the SECRET trial did not decrease epicardial fat volume.54 Apart from general recommendations for patients to lose weight (which are generally ineffective), the CENTRAL randomised trial demonstrated that a Mediterranean-style diet high in unsaturated fats with low carbohydrate intake decreased epicardial fat, compared to a low-fat diet, independent of
Epicardial Fat in HFpEF: Correlation or Causation? CARDIAC FAILURE REVIEW www.CFRjournal.com
Subtle interdependence Inspiratory pericardial restraint 160 140 120 100 80 60 40 20 0 Pressure (mmHg)
Figure 2: Exercise Echocardiogram and Right Heart Catheterisation in Obesity-related Heart Failure with Preserved Ejection Fraction with Epicardial Fat
The same patient from Figure 1 underwent exercise right heart catheterisation, which revealed abnormal elevations in pulmonary capillary wedge pressure (PCWP) and right atrial (RA) pressure during rest and exercise. There was additional evidence of left atrial non-compliance with large PCWP V waves during rest and exercise. As suggested by echocardiography, there was subtle ventricular interdependence with relative pericardial restraint (constrictive physiology). Left ventricular (LV), PCWP and right atrial (RA) pressure are shown at baseline (left). With exercise (upper right), there is an increase in PCWP along with larger PCWP V waves due to atrial myopathy associated with obesity-related HFpEF, and with inspiration there is a square root pattern in the LV and equalisation with RA pressure due to relative pericardial restraint. Simultaneous echocardiography showed a small D-shaped and underfilling LV despite the high PCWP due to relative pericardial restraint. Notably, there was an improvement in haemodynamics and pericardial restraint after administering sodium nitroprusside. Simultaneous exercise echocardiogram revealed a D-shaped underfilled LV during rest which worsened with exercise and improved with nitroprusside. With nitroprusside (lower right), there is both venous and arterial dilation with a reduction in RA pressure, PCWP and LV end diastolic pressure (LVEDP). Despite the reduction in PCWP there is an increase in LV transmural pressure filling the LV due to greater reduction in RA pressure from the venodilation (LV transmural pressure = PCWP-RA pressure). As a result, there is better LV filling despite the lower PCWP and LVEDP with a more circular and better filled LV on echocardiogram with an improvement in cardiac output. There is also decreased inspiratory pericardial restraint with no square root sign seen on the LV tracing. These findings are classic for obesity-related HFpEF with associated atrial myopathy and ventricular interdependence with relative pericardial restraint. Although epicardial fat could explain many of these features, including underlying atrial myopathy and pericardial restraint, it remains unclear if epicardial fat is directly contributing to these changes or is a correlate for greater overall adiposity, which then causes underlying HFpEF physiology and pericardial restraint.
absolute weight loss achieved.58 However, physical activity alone failed to achieve epicardial fat reduction similar to findings from the SECRET trial.54 Since a Mediterranean-style diet has also been shown to reduce adverse cardiovascular outcomes in the large PREDIMED randomised trial, this may represent an attractive dietary intervention, specifically in obese HFpEF patients, for cardiovascular risk reduction and for its direct effects on epicardial fat and central haemodynamics.59 In the CENTRAL trial, although physical activity by itself failed to affect epicardial fat, it did appear to enhance the effect of the Mediterranean and low-carbohydrate diet on epicardial fat loss, in addition to contributing to loss of visceral adiposity with all the associated metabolic benefits, and therefore the combination may be the most efficacious.58
The SECRET trial in obese HFpEF, as discussed above, used a lowcalorie diet without the Mediterranean component and this failed to affect epicardial fat, which suggests that it may be the Mediterranean diet and increased unsaturated fats that specifically modulate epicardial
fat reduction.54 Even without epicardial fat reduction, a low-calorie diet in obese HFpEF was associated with reduced overall adipose mass, improved quality of life, peak VO2, 6-minute walk distance, decreased serum inflammatory markers and reduced LV mass, and an increased E/A ratio suggestive of improved diastolic filling, with less impressive results seen with exercise training only.54 The combination of diet and exercise again appeared to be additive. These results contrast previous prospective observational research demonstrating reductions in epicardial fat with a low-calorie diet where more weight loss was achieved.60 Notably, weight loss was modest in the SECRET trial and generally <10 kg, which is much lower than that achieved with bariatric surgery. Additional research is needed to understand the relative benefit of specific diet interventions in obesity-related HFpEF, the relationship between the complex reduction in epicardial fat (as opposed to visceral and subcutaneous fat deposits) and epicardial heart size, and how this relates to an improvement in symptoms, exercise capacity and pericardial restraint.
Epicardial Fat in HFpEF: Correlation or Causation? CARDIAC FAILURE REVIEW www.CFRjournal.com
80 70 60 50 40 30 20 10 0 80 70 60 50 40 30 20 10 0 80 70 60 50 40 30 20 Exercise Nitroprusside 10 0
Given the emerging role of obesity and potentially epicardial fat in the pathogenesis of AF, the observation of dramatic decreases in AF recurrence with weight loss are of great interest in obesity-related HFpEF. 13 23 26 34 38 39 61–64 Although these studies did not specifically include patients with HFpEF, the diagnosis of HFpEF in the presence of symptomatic AF is particularly challenging in the presence of obesity.2,52,65 It is likely that many patients in these studies had underlying occult HFpEF, which would have been confirmed with exercise testing, particularly since HFpEF is extremely common in symptomatic patients with obesity and AF.52,53,65–69 How much of the reported weight loss benefit on AF recurrence is related to improvements in haemodynamics and the local inflammatory secretory profile from loss of epicardial fat remains to be determined. Permanent AF is also liable to further worsen relative pericardial restraint from progressive severe biatrial enlargement based on animal and human studies providing further rationale for prevention of AF progression through regression in epicardial fat, but direct confirmation of these results is lacking.70–72
Bariatric Surgery

Bariatric surgery is the most effective weight loss therapy currently available but also has independent benefits on epicardial fat deposits, which may have advantages in terms of relieving pericardial restraint.51,57 Independent of these changes in epicardial fat, there are also reductions in blood volume, LV mass, cardiac output and LA size, all of which would be expected to further improve relative pericardial restraint through a reduction in total epicardial heart size.73 These reductions in blood volume and heart size are associated with reduced biventricular filling pressures suggestive of the alleviation of pericardial restraint.55 The reduction in epicardial fat deposits may also provide the independent benefit of reducing proinflammatory and profibrotic signalling pathways affecting the underlying adjacent myocardium.24 26 47 Since epicardial fat is associated with the future risk of HFpEF and heart failure hospitalisation, it is likely that some of the observed benefits of bariatric surgery on improving haemodynamics and decreasing incident heart failure are mediated by the observed reduction in epicardial fat.14,17,51,55–57
Drug Therapies Targeting Epicardial Fat and Implications for HFpEF
Very little is understood about the impact of medical modulation of epicardial fat in HFpEF. The first proven agents to improve heart failure hospitalisation and quality of life in HFpEF are the sodium–glucose cotransporter-2 inhibitors (SGLT2i).74–76 Although the mechanisms of benefit of these drugs are uncertain, they have demonstrated a reduction in epicardial fat despite only minimal weight loss suggesting a direct lipolytic effect on epicardial fat.77–79 The use of SGLT2i has also been associated with reduced incident AF, which may, in part, be due to the reduction in epicardial fat.80 81 The diuretic effect of SGLT2i may facilitate a reduction in plasma volume and mechanistic studies have shown that they also promote ventricular mass regression, which may cumulatively decrease pericardial restraint.77,82,83 Likely, in part, through these mechanisms, SGLT2i have demonstrated haemodynamic benefits on PCWP at rest and exercise in systolic heart failure and HFpEF (NCT04730947).
Thiazolidinediones, glucagon-like peptide 1 receptor (GLP-1) agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors and statins have also shown efficacy in targeting epicardial fat. The thiazolidinediones, such as pioglitazone, have an effect on epicardial fat, but given their propensity to cause fluid retention, their use is contraindicated in patients with HFpEF.84 GLP-1 agonists improve cardiovascular outcomes in people with diabetes with a normal EF who are at risk of HFpEF.85–87 Liraglutide, a GLP-1 agonist,
Figure 3: Theoretical Effects of Epicardial Fat
Left ventricular (LV) end diastolic pressure (LVEDP) is determined by the sum of (1) the outwardly distending LV transmural pressure (LVTMP) related to the volume of blood in the LV and material properties of LV myocardium and (2) the inwardly compressive pericardial pressure related to intrinsic pericardial compliance and the position on the pericardial pressure-volume relationship due to stretch on the pericardium by the total heart size. Pericardial pressure can be approximated by right atrial (RA) pressure such that LVEDP = LVTMP + RA pressure. LV = left ventricular; LVEDP = left ventricular end diastolic pressure; LVTMP = left ventricular transmural pressure; RA = right atrial.
Figure 4: Mechanisms of Pericardial Restraint in Obesity-related Heart Failure with Preserved Ejection Fraction
Although epicardial fat may directly contribute to pericardial restraint by occupying space within the pericardial cavity compromising pericardial compliance, there are a number of other potential contributors to the increased pericardial restraint seen in obese HFpEF. Blood volume expansion, increased atrial and ventricular volumes all may increase total epicardial heart size and increase pericardial restraint. Pulmonary hypertension exacerbates pericardial restraint and abnormal right ventricular-pulmonary artery coupling. Additionally, there may be extrinsic restraint on the heart and pericardium that can increase ventricular interdependence from either mediastinal restraint due to chest wall adiposity or increased abdominal pressure and restraint from visceral adiposity. HFpEF = heart failure with preserved ejection fraction; LV = left ventricular; PA = pulmonary artery; RV = right ventricular.
Epicardial Fat in HFpEF: Correlation or Causation? CARDIAC FAILURE REVIEW www.CFRjournal.com
LVEDP = Since pericardial pressure ≈ RA pressure LVEDP ≈ LV 35 30 25 20 15 10 5 0 40 LVTMP + pericardial pressure LVTMP + RA pressure LVTMP Pericardial pressure Potential contributors to pericardial restraint in obesity-related HFpEF Increased left and right atrial volume Blood volume expansion Mediastinal/ abdominal extrinsic restraint Increased LV and RV volume Increased epicardial fat volume Pulmonary hypertension with abnormal RV-PA coupling
failed to improve outcomes in systolic heart failure with a possible signal of harm in heart failure hospitalisation with consistent signals towards increasing heart rates, which may explain the detriment in systolic heart failure.88–90 However, the pathophysiology of HFpEF differs profoundly from systolic heart failure and these drugs have proven benefits for cardiovascular outcomes and excellent safety data in patients with a normal EF.85–87 With the known presence of GLP receptors on epicardial fat, the effect of GLP-1 agonists on reducing epicardial fat and the potential contributory role of epicardial fat in HFpEF pathogenesis, targeting obese HFpEF patients with GLP-1 agonists may represent a high yield investigational target and provide insight into the role of epicardial fat in HFpEF.91–94 There has been recent data that higher doses than are used for diabetes of the GLP-1 agonists liraglutide and particularly semaglutide, and the novel dual glucose-dependent insulinotropic polypeptide and GLP-1 agonist tirzepatide induce profound weight loss with improved quality of life even in people without diabetes, and these drugs are being actively investigated in HFpEF (NCT05371496). 95–97
There is controversy about the safety of DPP-4 inhibitors in heart failure (leading to a Food and Drug Administration warning for saxagliptin and alogliptin) though this risk is not uniform across the drug class.89,98 Sitagliptin has demonstrated cardiovascular safety with no increased risk for heart failure and experimental evidence suggests its use can reduce epicardial fat.99–101 Therefore, further investigation of sitagliptin in obese HFpEF and its effect on epicardial fat may be warranted. Finally, statins
1. Ward ZJ, Bleich SN, Cradock AL, et al. Projected US statelevel prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–50. https://doi.org/10.1056/ NEJMsa1909301; PMID: 31851800.
2. Obokata M, Reddy YNV, Pislaru SV, et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. https://doi.org/10.1161/ CIRCULATIONAHA.116.026807; PMID: 28381470.
3. Reddy YNV, Lewis GD, Shah SJ, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc 2019;94:1199–209. https://doi.org/10.1016/j. mayocp.2018.11.037; PMID: 31272568.
4. Reddy YNV, Obokata M, Testani JM, et al. Adverse renal response to decongestion in the obese phenotype of heart failure with preserved ejection fraction. J Card Fail 2020;26:101–7. https://doi.org/10.1016/j.cardfail.2019.09.015; PMID: 31618698.
5. Tromp J, MacDonald MR, Tay WT, et al. Heart failure with preserved ejection fraction in the young. Circulation 2018;138:2763–73. https://doi.org/10.1161/ CIRCULATIONAHA.118.034720; PMID: 30565987.
6. Tromp J, Shen L, Jhund PS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2019;74:601–12. https:// doi.org/10.1016/j.jacc.2019.05.052; PMID: 31370950.
7. Van Wagoner DR. Paracrine signals modulate atrial epicardial progenitor cells and development of subepicardial adiposity and fibrosis implications for atrial fibrillation. Circ Res 2020;126:1343–5. https://doi.org/10.1161/ CIRCRESAHA.120.317007; PMID: 32379572.
8. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 2019;7:574–85. https://doi.org/10.1016/j. jchf.2019.03.021; PMID: 31248569.
9. Koepp KE, Obokata M, Reddy YNV, et al. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:657–66. https://doi.org/10.1016/j.jchf.2020.04.016; PMID: 32653449.
10. Pugliese NR, Paneni F, Mazzola M, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–71. https://doi.org/10.1002/ejhf.2337; PMID: 34427016.
11. Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail
have prominent effects on reducing epicardial fat and given their safety and proven cardiovascular efficacy in patients at risk for adverse cardiovascular outcomes and observational data supporting benefit in HFpEF, these drugs may also have a role in modulating epicardial fat in obese HFpEF.84,102–108 Pragmatically, a randomised trial testing statins in obese HFpEF would be difficult to perform as most of these patients independently meet criteria for statin therapy, especially with the outcome benefit of statin therapy in the HOPE-3 trial for which most middle-aged obesity-related HFpEF patients would meet entry criteria.106
Conclusion
Although there is a clearly demonstratable association between epicardial fat and HFpEF, the association remains highly confounded by comorbid conditions and the greater generalised obesity seen in patients with more epicardial fat. Ongoing studies testing different methods of weight loss, SGLT2i and GLP-1 agonists will provide novel insight into the potential contributory role and effect of therapies on epicardial fat in HFpEF. Further study of how epicardial fat affects cardiovascular haemodynamics and pericardial restraint in obesity-related heart failure is urgently needed given the increasing prevalence of obesity in the western world, particularly among patients with heart failure.1,2,18,109,110 For now, what is clear is that regardless of causality, the mere presence of epicardial fat is a useful biomarker for the presence of worse haemodynamics, pericardial restraint, exercise tolerance, myocardial remodelling and heart failure risk in patients with or at risk for HFpEF.
2018;6:640–9. https://doi.org/10.1016/j.jchf.2018.06.002; PMID: 30007558.
12. Gorter TM, van Woerden G, Rienstra M, et al. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:667–76. https://doi.org/10.1016/j.jchf.2020.06.003; PMID: 32653444.
13. van Woerden G, Gorter TM, Westenbrink BD, et al. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 2018;20:1559–66. https:// doi.org/10.1002/ejhf.1283; PMID: 30070041.
14. van Woerden G, van Veldhuisen DJ, Manintveld OC, et al. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ Heart Fail 2022;15:e009238. https://doi.org/10.1161/ CIRCHEARTFAILURE.121.009238; PMID: 34935412.
15. Tromp J, Bryant JA, Jin X, et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail 2021;23:835–8. https://doi.org/10.1002/ejhf.2156; PMID: 33724596.
16. Min J, Putt ME, Yang W, et al. Association of pericardial fat with cardiac structure, function, and mechanics: the multiethnic study of atherosclerosis. J Am Soc Echocardiogr 2022;35:579–587.e5. https://doi.org/10.1016/j. echo.2022.01.005; PMID: 35063614.
17. Kenchaiah S, Ding J, Carr JJ, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol 2021;77:2638–52. https://doi.org/10.1016/j.jacc.2021.04.003; PMID: 34045020.
18. Savji N, Meijers WC, Bartz TM, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–9. https://doi.org/10.1016/j. jchf.2018.05.018; PMID: 30007554.
19. Rao VN, Bush CG, Mongraw-Chaffin M, et al. Regional adiposity and risk of heart failure and mortality: the Jackson Heart study. J Am Heart Assoc 2021;10:e020920. https://doi. org/10.1161/JAHA.121.020920; PMID: 34238024.
20. Rao VN, Zhao D, Allison MA, et al. Adiposity and incident heart failure and its subtypes: MESA (multi-ethnic study of atherosclerosis). JACC Heart Fail 2018;6:999–1007. https:// doi.org/10.1016/j.jchf.2018.07.009; PMID: 30316935.
21. Kim MS, Kim WJ, Khera AV, et al. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur Heart J 2021;42:3388–403. https://doi.org/10.1093/eurheartj/ehab454; PMID: 34333589.
22. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. https:// doi.org/10.1001/jama.292.20.2471; PMID: 15562125.
23. Wong CX, Sun MT, Odutayo A, et al. Associations of epicardial, abdominal, and overall adiposity with atrial
fibrillation. Circ Arrhythm Electrophysiol 2016;9:e004378. https://doi.org/10.1161/CIRCEP.116.004378; PMID: 27923804.
24. Greulich S, Maxhera B, Vandenplas G, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012;126:2324–34. https://doi.org/10.1161/ CIRCULATIONAHA.111.039586; PMID: 23065384.
25. Shaihov-Teper O, Ram E, Ballan N, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation 2021;143:2475–93. https://doi.org/10.1161/ CIRCULATIONAHA.120.052009; PMID: 33793321.
26. Venteclef N, Guglielmi V, Balse E, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2015;36:795–805. https://doi.org/10.1093/eurheartj/eht099; PMID: 23525094.
27. Spadaro J, Bing OH, Gaasch WH, Weintraub RM. Pericardial modulation of right and left ventricular diastolic interaction. Circ Res 1981;48:233–8. https://doi.org/10.1161/01. res.48.2.233; PMID: 7460199.
28. LeWinter MM, Pavelec R. Influence of the pericardium on left ventricular end-diastolic pressure-segment relations during early and later stages of experimental chronic volume overload in dogs. Circ Res 1982;50:501–9. https:// doi.org/10.1161/01.res.50.4.501; PMID: 7067057.
29. Tyberg JV, Taichman GC, Smith ER, et al. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32. https://doi. org/10.1161/01.cir.73.3.428; PMID: 3948353.
30. Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–30. https://doi.org/10.1093/eurheartj/ehz713; PMID: 31609443.
31. Burrage MK, Hundertmark M, Valkovič L, et al. Energetic basis for exercise-induced pulmonary congestion in heart failure with preserved ejection fraction. Circulation 2021;144:1664–78. https://doi.org/10.1161/ CIRCULATIONAHA.121.054858; PMID: 34743560.
32. Melenovsky V, Andersen MJ, Andress K, et al. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 2015;17:1161–71. https://doi.org/10.1002/ejhf.417; PMID: 26467180.
33. Pugliese NR, Mazzola M, Madonna R, et al. Exerciseinduced pulmonary hypertension in HFpEF and HFrEF: different pathophysiologic mechanism behind similar functional impairment. Vascul Pharmacol 2022;144:106978. https://doi.org/10.1016/j.vph.2022.106978; PMID: 35301117.
34. Thanassoulis G, Massaro JM, O’Donnell CJ, et al. Pericardial fat is associated with prevalent atrial fibrillation: the
Epicardial Fat in HFpEF: Correlation or Causation? CARDIAC FAILURE REVIEW www.CFRjournal.com
Framingham Heart Study. Circ Arrhythm Electrophysiol 2010;3:345–50. https://doi.org/10.1161/CIRCEP.109.912055; PMID: 20558845.
35. Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–45. https://doi.org/10.1038/oby.2009.343; PMID: 19875999.
36. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–6. https://doi.org/10.1161/01. CIR.0000099542.57313.C5
37. Pugliese NR, Pellicori P, Filidei F, et al. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovasc Res 2022:cvac133. https://doi.org/10.1093/cvr/ cvac133; PMID: 36004819.
38. Mahajan R, Lau DH, Brooks AG, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. https://doi.org/10.1016/j.jacc.2015.04.058; PMID: 26139051.
39. Mahajan R, Nelson A, Pathak RK, et al. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol 2018;4:1529–40. https://doi.org/10.1016/j.jacep.2018.08.014; PMID: 30573116.
40. Nerlekar N, Muthalaly RG, Wong N, et al. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc 2018;7:e009975. https://doi.org/10.1161/JAHA.118.009975; PMID: 30571602.
41. Wu CK, Lee JK, Hsu JC, et al. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:445–54. https:// doi.org/10.1002/ejhf.1617; PMID: 31696627.
42. Wu CK, Tsai HY, Su M-YM, et al. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J Clin Lipidol 2017;11:1421–31. https://doi.org/10.1016/j.jacl.2017.08.018; PMID: 29050981.
43. Lin JL, Sung KT, Lai YH, et al. Epicardial adiposity in relation to metabolic abnormality, circulating adipocyte FABP, and preserved ejection fraction heart failure. Diagnostics (Basel) 2021;11:397. https://doi.org/10.3390/diagnostics11030397; PMID: 33652956.
44. Mahabadi AA, Lehmann N, Kälsch H, et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging 2014;7:909–16. https://doi. org/10.1016/j.jcmg.2014.07.002; PMID: 25190138.
45. Mahabadi AA, Anapliotis V, Dykun I, et al. Epicardial fat and incident heart failure with preserved ejection fraction in patients with coronary artery disease. Int J Cardiol 2022;357:140–5. https://doi.org/10.1016/j. ijcard.2022.04.009; PMID: 35395282.
46. Pugliese NR, Pieroni A, De Biase N, et al. Impact of diabetes on cardiopulmonary function: the added value of a combined cardiopulmonary and echocardiography stress test. Heart Fail Rev 2021. https://doi.org/10.1007/s10741-02110194-7; PMID: 34820732; online ahead of press.
47. Gaborit B, Kober F, Jacquier A, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes (Lond) 2012;36:422–30. https://doi.org/10.1038/ijo.2011.117; PMID: 21730964.
48. Sabbah MS, Fayyaz AU, de Denus S, et al. Obeseinflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail 2020;13:e006414. https://doi. org/10.1161/CIRCHEARTFAILURE.119.006414; PMID: 32809874.
49. Schiattarella GG, Altamirano F, Tong D, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–6. https://doi.org/10.1038/s41586-0191100-z
50. Reddy YNV, Melenovsky V, Redfield MM, et al. High-output heart failure: a 15-year experience. J Am Coll Cardiol 2016;68:473–82. https://doi.org/10.1016/j.jacc.2016.05.043; PMID: 27470455.
51. Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol 2012;60:1381–9. https://doi.org/10.1016/j.jacc.2012.06.016; PMID: 22939560.
52. Reddy YNV, Carter RE, Obokata M, et al. A simple, evidencebased approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–70. https://doi.org/10.1161/CIRCULATIONAHA.118.034646; PMID: 29792299.
53. Reddy YNV, Kaye DM, Handoko ML, et al. Diagnosis of heart
failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol 2022;7:891–9. https:// doi.org/10.1001/jamacardio.2022.1916; PMID: 35830183.
54. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. https://doi.org/10.1001/ jama.2015.17346; PMID: 26746456.
55. Reddy YNV, Anantha-Narayanan M, Obokata M, et al. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail 2019;7:678–87. https://doi.org/10.1016/j.jchf.2019.04.019; PMID: 31302042.
56. Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation 2021;143:1468–80. https://doi.org/10.1161/CIRCULATIONAHA.120.052386; PMID: 33813836.
57. Willens HJ, Byers P, Chirinos JA, et al. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol 2007;99:1242–5. https://doi. org/10.1016/j.amjcard.2006.12.042; PMID: 17478151.
58. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018;137:1143–57. https://doi. org/10.1161/CIRCULATIONAHA.117.030501; PMID: 29142011.
59. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. https://doi.org/10.1056/NEJMoa1800389; PMID: 29897866.
60. Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–7. https://doi.org/10.1038/oby.2008.251; PMID: 18451775.
61. Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. https://doi.org/10.1093/ europace/euy117; PMID: 29912366.
62. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. https://doi.org/10.1001/jama.2013.280521; PMID: 24240932.
63. Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. https://doi.org/10.1016/j. jacc.2015.03.002; PMID: 25792361.
64. Jamaly S, Carlsson L, Peltonen M, et al. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol 2016;68:2497–504. https://doi. org/10.1016/j.jacc.2016.09.940; PMID: 27931605.
65. Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation 2018;137:534–5. https://doi.org/10.1161/ CIRCULATIONAHA.117.030093; PMID: 29378762.
66. Reddy YNV, Borlaug BA. Dyspnea in paroxysmal atrial fibrillation: when perception falls out of rhythm with reality. J Card Fail 2017;23:563–5. https://doi.org/10.1016/j. cardfail.2017.05.006; PMID: 28522240.
67. Pugliese NR, Biase DEN, Balletti A, et al. Characterization of hemodynamic and metabolic abnormalities in the heart failure spectrum: the role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol Angiol 2022;70:370–384. https://doi.org/10.23736/S27245683.21.05743-4; PMID: 34137244.
68. Pugliese NR, De Biase N, Gargani L, et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol 2021;28:1650–61. https://doi.org/10.1093/ eurjpc/zwaa129; PMID: 33624088.
69. Obokata M, Kane GC, Reddy YNV, et al. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasiveechocardiographic study. Circulation 2017;135:825–38. https://doi.org/10.1161/CIRCULATIONAHA.116.024822; PMID: 28039229.
70. Linderer T, Chatterjee K, Parmley WW, et al. Influence of atrial systole on the Frank-Starling relation and the enddiastolic pressure-diameter relation of the left ventricle. Circulation 1983;67:1045–53. https://doi.org/10.1161/01.
cir.67.5.1045; PMID: 6831669.
71. Maruyama Y, Ashikawa K, Isoyama S, et al. Mechanical interactions between four heart chambers with and without the pericardium in canine hearts. Circ Res 1982;50:86–100. https://doi.org/10.1161/01.res.50.1.86; PMID: 7053880.
72. Reddy YNV, Obokata M, Verbrugge FH, et al. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol 2020;76:1051–64. https://doi.org/10.1016/j.jacc.2020.07.009; PMID: 32854840.
73. Aggarwal R, Harling L, Efthimiou E, et al. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg 2016;26:1030–40. https://doi.org/10.1007/s11695-015-1866-5; PMID: 26328532.
74. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038; PMID: 34449189.
75. Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 2022;28:809–13. https://doi.org/10.1038/s41591-022-01703-8; PMID: 35228753.
76. Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 2021;27:1954–60. https://doi.org/10.1038/s41591-02101536-x; PMID: 34711976.
77. Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail 2021;9:578–89. https://doi.org/10.1016/j. jchf.2021.04.014; PMID: 34325888.
78. Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018;17:6. https://doi.org/10.1186/s12933-017-0658-8; PMID: 29301516.
79. Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2inhibitors on epicardial adipose tissue: a meta-analysis. Cells 2021;10:2150. https://doi.org/10.3390/cells10082150; PMID: 34440918.
80. Reddy YNV, Borlaug BA, Gersh BJ. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation 2022;146:339–57. https://doi.org/10.1161/ CIRCULATIONAHA.122.057444; PMID: 35877831.
81. Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 tirla. Circulation 2020;141:1227–34. https://doi.org/10.1161/ CIRCULATIONAHA.119.044183; PMID: 31983236.
82. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–74. https://doi.org/10.1038/s41591-021-01659-1; PMID: 35228754.
83. Omar M, Jensen J, Frederiksen PH, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2020;76:2740–51. https://doi.org/10.1016/j.jacc.2020.10.005; PMID: 33272368.
84. Grosso AF, de Oliveira SF, Higuchi MdL, et al. Synergistic anti-inflammatory effect: simvastatin and pioglitazone reduce inflammatory markers of plasma and epicardial adipose tissue of coronary patients with metabolic syndrome. Diabetol Metab Syndr 2014;6:47. https://doi. org/10.1186/1758-5996-6-47; PMID: 24684779.
85. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. https://doi.org/10.1056/ NEJMoa1603827; PMID: 27295427.
86. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. https://doi.org/10.1056/ NEJMoa1607141; PMID: 27633186.
87. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–51. https://doi. org/10.1056/NEJMoa1901118; PMID: 31185157.
88. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316:500–8. https://doi.org/10.1001/ jama.2016.10260; PMID: 27483064.
89. Reddy YNV, Borlaug BA, O’Connor CM, Gersh BJ. Novel approaches to the management of chronic systolic heart failure: future directions and unanswered questions. Eur Heart J 2020;41:1764–74. https://doi.org/10.1093/eurheartj/
Epicardial
in HFpEF:
CARDIAC FAILURE REVIEW www.CFRjournal.com
Fat
Correlation or Causation?
ehz364; PMID: 31199474.
90. Robinson LE, Holt TA, Rees K, et al. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013;3:e001986. https://doi.org/10.1136/ bmjopen-2012-001986; PMID: 23355666.
91. Iacobellis G, Camarena V, Sant DW, Wang G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res 2017;49:625–30. https:// doi.org/10.1055/s-0043-109563; PMID: 28514806.
92. Dutour A, Abdesselam I, Ancel P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 2016;18:882–91. https:// doi.org/10.1111/dom.12680; PMID: 27106272.
93. Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 2017;25:311–6. https://doi.org/10.1002/ oby.21718; PMID: 28124506.
94. Berg G, Barchuk M, Lobo M, Nogueira JP. Effect of glucagon-like peptide-1 (GLP-1) analogues on epicardial adipose tissue: a meta-analysis. Diabetes Metab Syndr 2022;16:102562. https://doi.org/10.1016/j.dsx.2022.102562; PMID: 35816950.
95. Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med 2021;384:1719–30. https://doi. org/10.1056/NEJMoa2028198; PMID: 33951361.
96. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002. https://doi.org/10.1056/ NEJMoa2032183; PMID: 33567185.
97. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205–16. https://doi.org/10.1056/NEJMoa2206038;
PMID: 35658024.
98. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. https://doi. org/10.1056/NEJMoa1307684; PMID: 23992601.
99. McGuire DK, Van de Werf F, Armstrong PW, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:126–35. https://doi.org/10.1001/jamacardio.2016.0103; PMID: 27437883.
100. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. https://doi.org/10.1056/ NEJMoa1501352; PMID: 26052984.
101. Lima-Martínez MM, Paoli M, Rodney M, et al. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: a pilot study. Endocrine 2016;51:448–55. https://doi.org/10.1007/s12020-015-0710-y; PMID: 26233684.
102. Cho KI, Kim BJ, Cha TJ, et al. Impact of duration and dosage of statin treatment and epicardial fat thickness on the recurrence of atrial fibrillation after electrical cardioversion. Heart Vessels 2015;30:490–7. https://doi.org/10.1007/s00380014-0505-8; PMID: 24691701.
103. Soucek F, Covassin N, Singh P, et al. Effects of atorvastatin (80 mg) therapy on quantity of epicardial adipose tissue in patients undergoing pulmonary vein isolation for atrial fibrillation. Am J Cardiol 2015;116:1443–6. https://doi.org/10.1016/j.amjcard.2015.07.067; PMID: 26372211.
104. Park JH, Park YS, Kim YJ, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound 2010;18:121–6. https://doi.org/10.4250/
jcu.2010.18.4.121; PMID: 21253360.
105. Alexopoulos N, Melek BH, Arepalli CD, et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol 2013;61:1956–61. https://doi.org/10.1016/j.jacc.2012.12.051; PMID: 23500254.
106. Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31. https://doi.org/10.1056/ NEJMoa1600176; PMID: 27040132.
107. Fukuta H, Goto T, Wakami K, Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol 2016;214:301–6. https://doi.org/10.1016/j.ijcard.2016.03.186; PMID: 27082778.
108. Nochioka K, Sakata Y, Miyata S, et al. Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ J 2015;79:574–82. https://doi. org/10.1253/circj.CJ-14-0865; PMID: 25746542.
109. Joyce E, Lala A, Stevens SR, et al. Prevalence, profile, and prognosis of severe obesity in contemporary hospitalized heart failure trial populations. JACC Heart Fail 2016;4:923–31. https://doi.org/10.1016/j.jchf.2016.09.013; PMID: 27908391.
110. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. https://doi. org/10.1056/NEJMoa020245; PMID: 12151467.
111. Jin X, Hung CL, Tay WT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved (HFpEF) versus reduced and mildly reduced ejection fraction (HFrEF/HFmrEF). Eur J Heart Fail 2022;24:1346–56. https://doi.org/10.1002/ejhf.2513; PMID: 35475591.
in HFpEF:
CARDIAC FAILURE REVIEW www.CFRjournal.com
Epicardial Fat
Correlation or Causation?
Effects of Sacubitril/Valsartan
Cinzia Nugara , 1 Francesco Giallauria , 2 Giuseppe Vitale , 1 Silvia Sarullo , 3 Giovanni Gentile , 4,5 Francesco Clemenza , 4,5 Annamaria Lo Voi,1 Antonino Zarcone,1 Elio Venturini , 6 Gabriella Iannuzzo , 7 Andrew JS Coats 8,9, 10 and Filippo M Sarullo 1
1. Cardiovascular Rehabilitation Unit, Buccheri La Ferla Fatebenefratelli Hospital, Palermo, Italy; 2. Department of Translational Medical Sciences, Federico II University of Naples, Naples, Italy; 3. School of Sport Medicine and Physical Exercise Medicine, Department of Biomedicine, Neurosciences and Advances Diagnostic, University of Palermo, Palermo, Italy; 4. Diagnostic and Therapeutic Services, Radiology Unit, IRCCS-ISMETT, Palermo, Italy; 5. Department for the Treatment and Study of Cardiothoracic Diseases and Cardiothoracic Transplantation, IRCCS-ISMETT, Palermo, Italy; 6. Cardiac Rehabilitation Unit, AUSL Toscana Nord-Ovest,
Abstract
Background: This study aims to evaluate the cardiopulmonary effects of sacubitril/valsartan therapy in patients with heart failure with reduced ejection fraction (HFrEF), investigating a possible correlation with the degree of myocardial fibrosis, as assessed by cardiac magnetic resonance. Methods: A total of 134 outpatients with HFrEF were enrolled. Results: After a mean follow-up of 13.3 ± 6.6 months, an improvement in ejection fraction and a reduction in E/A ratio, inferior vena cava size and N-terminal pro-B-type natriuretic peptide levels were observed. At follow-up, we observed an increase in VO 2 peak of 16% (p<0.0001) and in O2 pulse of 13% (p=0.0002) as well as an improvement in ventilatory response associated with a 7% reduction in the VE/VCO2 slope (p=0.0001). An 8% increase in the ΔVO2/Δ work ratio and an 18% increase in exercise tolerance were also observed. Multivariate logistic regression analysis showed that the main predictors of events during follow-up were VE/VCO2 slope >34 (OR 3.98; 95% CI [1.59–10.54]; p=0.0028); ventilatory oscillatory pattern (OR 4.65; 95% CI [1.55–16.13]; p=0.0052); and haemoglobin level (OR 0.35; 95% CI [0.21–0.55]; p<0.0001). In patients who had cardiac magnetic resonance, when delayed enhancement >4.6% was detected, a lower response after sacubitril/valsartan therapy was observed as expressed by improvement in ΔVO2 peak, O2 pulse, LVEF and N-terminal pro-B-type natriuretic peptide. No significant differences were observed in ΔVO2/Δ work and VE/VCO2 slope. Conclusion: Sacubitril/valsartan improves cardiopulmonary functional capacity in HFrEF patients. The presence of myocardial fibrosis on cardiac magnetic resonance is a predictor of response to therapy.
Keywords
Heart failure, sacubitril/valsartan, cardiopulmonary test, cardiac magnetic resonance, myocardial fibrosis
Disclosure: FG has received speaker fees from Impulse Dynamics. AJSC has received speaker fees from Abbott, Actimed, Arena, Astra Zeneca, Boehringer Ingelheim, Cardiac Dimensions, Cleer, Corvia, CVRx, Enopace, ESN, Faraday, Impulse Dynamics, Menarini, Novartis, Respicardia, Servier, Vifor and Viatris in the past 36 months; and is the Editor-in-chief of Cardiac Failure Review; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgements: CN and FG contributed equally.
Authors’ contributions: Conceptualisation: CN, FG, FMS; data curation: FG, GV, SS, GG, ALV, AZ; formal analysis: CN, FG, GV, FMS; investigation: CN, FMS; methodology: CN; project administration: FMS; resources: GG; supervision: FMS; validation: FG, GV, SS, FC, ALV, AZ, EV, GI, AJSC, FMS; visualisation: SS, FC, ALV, AZ, EV, GI, AJSC; writing – original draft preparation: CN, FG, FMS; writing – review & editing: AJSC.
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics: The Ethics Committee of Buccheri La Ferla FBF Hospital approved the study protocol before the study commenced. The study respected the Declaration of Helsinki and current Italian regulations for observational studies.
Consent: All patients have given written informed consent
Received: 21 March 2022 Accepted: 15 August 2022 Citation: Cardiac Failure Review 2023;8:e07. DOI: https://doi.org/10.15420/cfr.2022.13
Correspondence: Filippo M Sarullo, Cardiovascular Rehabilitation Unit, Buccheri La Ferla Fatebenefratelli Hospital, Via Messina Marine 197, 90123 Palermo, Italy. E: sarullo.filippo@fbfpa.it/fsarullo@libero.it
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Sacubitril/valsartan (S/V) therapy in patients with heart failure (HF) with reduced ejection fraction (HFrEF) has been shown to be superior to enalapril in reducing the risk of death and hospitalisation for HF.1
Cardiopulmonary exercise testing (CPET) is a powerful predictor of mortality in HF patients. Recent studies have demonstrated a significant symptomatic and functional improvement following the initiation of S/V
ORIGINAL RESEARCH
Therapy
© The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com
on Exercise Capacity in Patients with Heart Failure with Reduced Ejection Fraction and the Role of Percentage of Delayed Enhancement Measured by Cardiac Magnetic Resonance in Predicting Therapeutic Response: A Multicentre Study
Cecina Civil Hospital, Livorno, Italy; 7. Department of Clinical Medicine and Surgery, Federico II University of Naples, Naples, Italy; 8. Monash University, Australia; 9. University of Warwick, UK; 10. IRCCS San Raffaele Pisana, Rome, Italy
Figure 1: Patient Disposition
186 outpatients with HFrEF followed from January 2018 to May 2020
52 excluded for not meeting the inclusion criteria
maximum speed peak of the tricuspid regurgitation) and pressure in the right atrium (estimated based on the size and collapsibility of the inferior vena cava). Tricuspid annular plane systolic excursion was interpreted according to current guidelines.9
134 enrolled patients performed at least one CPET during follow-up
54 patients (who had not yet received a cardiac device) underwent CMR
therapy.1–6 Moreover, S/V treatment was reported to attenuate cardiac remodelling and dysfunction, inhibit fibrosis and reduce hypertrophy in a rat model of HF after MI.7 S/V treatment also prevented maladaptive cardiac fibrosis and dysfunction in a mouse model of left ventricular (LV) pressure overload.8 However, the molecular mechanism of this pleiotropic effect is not fully understood. The present study aims to evaluate the effects of S/V on functional capacity in HFrEF patients and whether myocardial fibrosis, assessed by cardiac magnetic resonance (CMR), influences cardiopulmonary functional capacity responses in HFrEF patients after S/V therapy.
Methods
This was an observational, prospective study conducted in patients with HFrEF. All patients signed informed consent at enrolment. The inclusion criteria were as follows: New York Heart Association functional class (NYHA) class II–III, ejection fraction <35%, treatment with an optimal dose of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor antagonists (ARBs) in the previous 6 months, systolic blood pressure >100 mmHg, serum potassium level <5.4 mEq/l, estimated glomerular filtration rate (eGFR)>30 ml/min/1.73m2, absence of severe hepatic insufficiency (Child-Pugh class C) and a negative history of angioedema. The exclusion criteria were as follows: hospitalisation for HF in the 90 days prior to the outpatient evaluation, myocardial revascularisation in the 180 days before the outpatient evaluation, concomitant initiation of cardiac resynchronisation therapy (CRT) and/or percutaneous mitral valve treatment or within the previous 6 months, congenital heart disease and inability to perform CPET.
The following demographic, anthropometric and clinical data were collected in an anonymised database for every patient: date of birth; race; sex; weight; height; BMI; body surface area (BSA); comorbidities (e.g. arterial hypertension, dyslipidaemia, chronic obstructive pulmonary disease, coronary artery disease, chronic renal failure); and previous pacemaker, ICD or CRT device implantation; Echocardiographic and CMR data (when available); LV end-diastolic diameter (measured in parasternal long axis projection) and the ejection fraction measured by the Simpson biplane method were evaluated on the echocardiogram. Diastolic function was assessed by pulsed and tissue Doppler. Left atrium volume and area were evaluated in four apical chambers projection. Pulmonary systolic arterial pressure was obtained from the sum of the pressure gradient between the right atrium and the right ventricle (calculated from the
CMR was performed in a subset of study patients who had not yet undergone ICD/CRT implantation using a 1.5-T scanner (TwinSpeed EXCITE, GE Healthcare). Data relating to LV mass, maximum wall thickness, biventricular and biatrial volumes and ejection fraction were collected and evidence of delayed enhancement (DE) was evaluated after administration of paramagnetic contrast medium intravenously (0.2 ml/kg of gadolinium). The presence and distribution of late gadolinium enhancement (LGE) were determined by signal-intensity-based quantitative analysis. The extent of LGE, defined by a signal intensity >5 SDs above the mean of the reference region of interest (ROI), was quantified on the short-axis contrast images. The extent of LGE was expressed as a weight of tissue and as a percentage of LV mass (indexed LGE extent).
S/V was prescribed according to current European guidelines.10 Drug titration was performed every 4 weeks (if tolerated by the patient). Changes in the dosage of diuretics during follow-up were made only when clinically necessary.
A baseline CPET was performed prior to initiation of S/V therapy and was repeated at 3, 6, 12 and 24 months. All CPETs were conducted on a cycle ergometer with a mean pedalling frequency of 60 rpm. A ramp protocol was performed in all patients, with a base workload of 10 Watts for 2 minutes and an increment of 10 W every 60 seconds. CPET was performed using the VMax2900 device (SensorMedics). The 12-lead ECG and oxygen saturation (measured by pulse oximeter) were constantly monitored during the test. Patients were encouraged to continue exercising until the onset of muscle exhaustion and/or dyspnoea. The anaerobic threshold was measured by V-slope analysis, based on carbon dioxide production (VCO2) and oxygen consumption (VO2), and confirmed by ventilatory equivalents and end-expiratory pressures of CO2 and O2. The percentage at which VO 2 increased per Watt of work (ΔVO2/Δ work) was calculated for the progressive increase in exercise, starting 1 minute after the percentage of work began to increase. Finally, the relationship between minute ventilation and carbon dioxide production slope (VE/VCO2 slope) was calculated from 1 minute before the beginning of the workload to the end of the isocapnic breathing period.
For the calculation of the ratio between dead space volume and tidal volume (RV/VT), Jones’s prediction equation was used.11 Values for VO2, ventilation, tidal volume and RV/VT ratio at peak exercise (calculated through a non-invasive assessment of blood pressure of carbon dioxide) were extrapolated as an average greater than the 30 seconds in which the examination was carried out.
Statistical Analysis
Statistical analysis was performed using SAS JMP pro 9.0 software version. Continuous variables are described as mean ± SD and as a median and interquartile interval in the case of non-normal distribution. Categorical variables were expressed in numbers (percentages). The baseline (at time zero) and on-going follow-up parameters of the cardiopulmonary test were compared using the Mann-Whitney U-test for continuous variables and the Fisher’s exact test for categorical variables, respectively. McNemar’s paired t-test was used for analysing changes from baseline. Univariate and multivariate logistic regression
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF CARDIAC FAILURE REVIEW www.CFRjournal.com
CMR = cardiac magnetic resonance; CPET = cardiopulmonary exercise testing; HFrEF = heart failure with reduced ejection fraction.
analyses were performed to identify the predictive role of clinical variables for S/V therapy undergoing CMR. All cut-offs for continuous variables were obtained through the analysis of the receiver operating characteristic (ROC) curves. A p<0.05 was considered statistically significant.
Results
From 1 January 2018 to 31 May 2020, 186 outpatients with HFrEF were evaluated in the centres involved in the study. Fifty-two patients were excluded: 16 treated with ACEI for <6 months, 10 with eGFR <30 ml/ min/1.73 m2, five with severe hepatic insufficiency, 11 with a history of recent hospitalisation for acute HF, eight unable to perform CPET and two who underwent MitraClip surgery in the previous 6 months (Figure 1). Data from 134 patients were analysed. All patients underwent at least one follow-up visit with CPET at 3, 6, 12 or 24 months.
Table 1 shows the demographic, clinical, echocardiographic and pharmacological data of the population under examination. The average age of the studied sample was 57.9 ± 9.5 years and 13% of the subjects were female. Seventy-seven patients (57%) had chronic ischaemic heart disease. At the time of enrolment, 78 patients (58%) were in NYHA class II and 56 (42%) in NYHA class III. The mean LV ejection fraction (LVEF) was 28 ± 5.7% (Table 1). The starting dose of S/V was 24/26 mg twice daily in 72% of patients. After titration of S/V, during the follow-up (mean follow-up 13.3 ± 6.6 months), 29% of patients received the 24/26 mg dose, 35% the 49/51 mg dose and 36% the 97/103 mg dose.
During follow-up there was improvement in LVEF(p=0.0003), a significant reduction in systolic blood pressure (from 117 ± 16 mmHg to 103.1 ± 13 mmHg; p<0.0001), a reduction in E/A ratio (p=0.007) and in the size of the inferior vena cava (p=0.009).
Table 2 shows the CPET data at baseline and at follow-up. At baseline, most patients were classified into the second ventilatory class and Weber class C. During follow-up there was an increase of 16% in VO2 peak (Δ = +2.5 ml/kg/min; p<0.0001) and of 13% in O2 pulse (Δ = +1.7 ml/beat; p=0.0002), as well as an improvement in ventilatory response associated with a 7% reduction in the VE/VCO2 slope (Δ =-2.5; p=0.0009). VO2 at the anaerobic threshold (AT-VO2) changed from 11.5+2.6 to 12.5+3.3 ml/kg/min (p=0.021); in addition, an increase of 8% in the ΔVO2/Δ work ratio (Δ = +0.8 ml/beat; p<0.0001) and of 18% in exercise tolerance (Δ = +16 Watt; p<0.0001) were registered.
During follow-up visits, significant reduction in mean N-terminal pro-Btype natriuretic peptide (NT-proBNP) levels (from 1021 to 570 pg/ml; p=0.007) was observed.
Table 3 shows the CPET data in baseline conditions and during the followup at 3, 6, 12 and 24 months. CPET parameters were assessed during follow-up by stratifying patients according to S/V dose. An improvement in VO 2 peak and in predicted VO2 max, plus a reduction in VE/VCO2 was observed in all patient categories. In patients in whom S/V was administered at dosages of 97/103 mg twice daily and 49/51 mg twice daily, the observed increase in O2 pulse and in VO2 /work was greater than the group of patients taking the lowest dose (Table 4).
Patients were observed for a mean follow-up of 13.3 ± 6.6 months. Fiftysix events were observed during the follow-up: 43 hospitalisations for HF (32%), two LV assist device implants (1.4%), two heart transplants (1.4%) and nine deaths from cardiovascular disease (6.7%).
Table 1: Baseline Characteristics of the Study Population (n=134)
ACEIs = angiotensin-converting enzyme inhibitors; ARBs = angiotensin II receptor antagonists; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronisation therapy; DBP = diastolic blood pressure; DT = deceleration time; E/A ratio = early filling (E) and early diastolic mitral annular velocity (E’) ratio; E/E’= peak mitral inflow velocity during early diastole/ early diastolic velocity; EF = ejection fraction; eGFR = estimated glomerular filtration rate; IQR = interquartile range; IVC = inferior vena cava; LA volume = left atrial volume; LVEDD = left ventricular end-diastolic diameter; LVEDVi = left ventricular end-diastolic volume index; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association functional class; RA area = right atrial area; SBP = systolic blood pressure; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspidal regurgitation.
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Baseline Characteristics n (%)/mean (SD) Demographic Data Age (years) 57.9 ± 9.5 Women 18 (13) SBP (mmHg) 117 ± 16 DBP (mmHg) 72 ± 10 Heart rate (BPM) 68 ± 11 BMI (kg/m2) 28.2 ± 4.5 Clinical Data Arterial hypertension 73 (54) Type 2 diabetes 45 (33) AF 23 (17) COPD 21 (15) eGFR (ml/min/1.73 m2) 70 ± 21 NT-proBNP (pg/dl), median (IQR) 1,021 (446–2,000) Ischaemic cardiomyopathy 77 (57) NYHA II 78 (58) NYHA III 56 (42) Echocardiographic Data EF (%) 28 ± 5.7 LVEDD (mm) 63 ± 6.7 LVEDVi (ml/m2) 112 ± 28 E/A ratio 1.6 ± 1.3 DT (ms) 170 ± 5.6 E/E’ 15 ± 7 LA volume (ml/m2) 49.5 ± 18 TR velocity (m/s) 2.7 ± 0.5 TAPSE (mm) 18 ± 4.3 RA area (cm2) 21.6 ± 8.4 IVC (mm) 17.9 ± 4.7 Pharmacological Therapy Furosemide 112 (83) Furosemide dose (mg), median (IQR) 50 (25–125) ACEIs 94 (70) ARBs 40 (30) Mineralocorticoid antagonists 111 (83) β-blockers 126 (94) Ivabradine 25 (18) Digoxin 7 (5) ICD 80 (59) CRT 43 (32)
Table 2: Cardiopulmonary Exercise Stress Testing Data at Baseline and at Follow-up
Table 3: Cardiopulmonary Test Parameters in Patients Stratified during Follow-up
VO 2 peak (ml/kg/min), mean ± SD 3 months (48 patients) 6 months (110 patients)
12 months (98 patients)
24 months (22 patients)
VO 2 peak predict (%), mean ± SD
3 months (48 patients)
6 months (110 patients)
12 months (98 patients)
24 months (22 patients)
VE/VCO2 slope, mean ± SD
3 months (48 patients) 6 months (110 patients) 12 months (98 patients)
months (22 patients)
(ml/beat), mean ± SD
= anaerobic threshold; AT-VO2 = anaerobic threshold at oxygen consumption peak; CPET = cardiopulmonary exercise testing; RQ = respiratory quotient; RR peak = respiratory exchange ratio calculated as VCO 2 /VO2 ; VD/VT = dead space to tidal volume ratio; VE/VCO2 slope = minute ventilation/carbon dioxide production ratio slope; VO2 peak = oxygen consumption peak; ΔVO2 /Δ work = Δ oxygen consumption (VO2 )/Δ workrate relationship.
In the multivariate logistic regression analysis, the main predictors of events during follow-up were VE/VCO2 slope >34 (OR 3.98; 95% CI [1.59–10.54]; p=0.0028), a ventilatory oscillatory pattern (OR 4.65; 95% CI [1.55–16.13]; p=0.0052), and haemoglobin (OR 0.35; 95% CI [0.21–0.55]; p<0.0001).
The CMR study was conducted in 54 patients who had not yet undergone ICD or CRT implantation at the time of enrolment. The demographic, clinical, anamnestic and instrumental features of the studied subgroups were comparable to those of the general population. Analysing the magnetic resonance data, the ejection fraction was comparable to that calculated by the echocardiogram (28 ± 6% versus 28 ± 5.7%); the indexed mass of the LV had an average value of 83 ± 22 g; the mass of DE in g was equal to 8 (0–25.7); the % of DE was 10.9 ± 14.4% and the DE index was 3.9 (0–12.8). Supplementary Material Table 1 shows the CMR data.
ROC curve analysis showed that a DE cut-off of >4.6% is the best predictor of events during follow-up (AUC 0.65; sensitivity 71%; specificity 63%).
In patients with a DE>4.6%, a lower response to S/V therapy was observed, showing lower improvement in Δ VO2 peak (+2.1 l/min versus +4.7 l/min), O 2 pulse (+1.4 ml/beat versus+4.2 ml/beat), ejection fraction (+4.1% versus +10%), and NT-pro BNP (760 versus 810 pg/ml), whereas no significant differences were observed in ΔVO2/Δ work and VE/VCO2 slope (Supplementary Material Table 2).
In the cohort of patients undergoing CMR, 20 events (four deaths and 16 hospitalisations) were recorded during a mean follow-up of 14 ± 7 months. Both univariate and multivariate logistic regression analyses were also performed in the subgroup of patients undergoing CMR. In the multivariate
ΔVO2/Δ work (ml/min/W), mean ± SD
VE/VCO2 slope = minute ventilation/carbon dioxide production ratio slope; VO2 peak = oxygen consumption peak; ΔVO2 /Δ work = Δ oxygen consumption (VO2 )/Δ work rate relationship.
analysis, the main predictors of cardiovascular events during follow-up were VE/VCO2slope (OR 1.42; 95% CI [1.19–1.82]; p<0.0001) and the % of DE (OR 1.13; 95% CI [1.02–1.36]; p=0.0087).
Discussion
The present study showed that S/V improves cardiopulmonary functional capacity by 16% despite the presence of myocardial fibrosis at CMR in a substantial proportion of patients. The CPET is a useful diagnostic and prognostic tool in patients with HF with both reduced and preserved ejection fraction.12–15 In the PARADIGM-HF trial, S/V was shown to reduce the risk of death and hospitalisation in HFrEF patients compared to enalapril therapy alone.1 However, the effects of S/V therapy on cardiopulmonary function and cardiac remodelling have not been fully elucidated. In our observational, prospective and multicentre study, we evaluated the early and late effects of S/V therapy on the functional capacity of patients with HFrEF as assessed by CPET parameters. During follow-up, we observed a significant improvement in the main CPET parameters in the short, medium and long term. At the same time, we demonstrated an improvement in the indexes of LV systolic and diastolic function, in line with previous findings.
Palau et al. observed an increase in VO 2 peak and a decrease in the VE/ VCO 2 slope in a small cohort of HFrEF patients over a mean follow-up of 30 days.15 In our study, conducted in a population of 134 patients with a mean follow-up of 13.7 ± 6.6 months, a significant increase in VO2 peak, predicted VO2, O2 pulse and VO2 was confirmed. A reduction of the VE/ VCO 2 slope was already observed after 3 months of treatment, persisting during long-term follow-up (24 months). Swank et al. showed that for each 6% increase in VO 2 peak there is an 8% reduction in cardiovascular mortality and hospitalisation for HF and a 7% reduction in mortality from
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF CARDIAC FAILURE REVIEW www.CFRjournal.com
CPET Data Baseline Follow-up p-value VO 2 peak (ml/kg/min), mean ± SD 15.1 ± 3.7 17.6 ± 4.7 <0.0001 VO 2 peak (% del predicted), mean ± SD 55.5 ± 14.1 65.5 ± 16.9 <0.0001 VE/VCO2 slope, mean ± SD 33.2 ± 6.1 30.7 ± 6.1 0.0009 VE/VCO2 slope >34, n (%) 58 (43) 38 (28) 0.015 RQ, mean ± SD 1.13 ± 0.11 1.14 ± 0.10 0.45 Watt (peak), mean ± SD 74 ± 25 90 ± 32 <0.0001 AT-VO2, mean ± SD 11.5 ± 2.6 12.5 ± 3.3 0.021 AT-VO 2 predicted, mean ± SD 42.8 ± 12 47 ± 13.4 0.020 AT not achieved, n (%) 33 (25) 24 (18) 0.23 O 2 pulse (ml/beat), mean ± SD 11.7 ± 3.1 13.4 ± 3.8 0.0002 ΔVO2/Δ work (ml/min/W), mean ± SD 9.1 ± 1.5 9.9 ± 1.6 <0.0001 VD/VT mean ± SD 0.21 ± 0.04 0.19 ± 0.05 0.010 Peak ventilation (l/min), mean ± SD 48 ± 12.5 57.8 ± 17 <0.0001 Tidal peak volume (l), mean ± SD 1.58 ± 0.42 1.76 ± 0.51 0.001 RR peak (b/m), mean ± SD 31.1 ± 6.4 33.3 ± 6.4 0.006 Ventilatory oscillation, n (%) 33 (25) 11 (8) 0.0004 AT
Parameter Baseline Follow-up p-value
14.1 ± 3.4 14.8 ± 3.3 14.7 ± 3.3 15.9 ± 4.7 16.2 ± 4 17.5 ± 4.3 17.6 ± 4.5 19.1 ± 6 <0.0001 <0.0001 <0.0001 <0.0001
52.7 ± 15.6 55.5 ± 14.4 53.7 ± 14.1 56.8 ± 16.5 60.9 ± 15.7 65.6 ± 16.8 65 ± 17.5 70 ± 22 <0.0001 <0.0001 <0.0001 <0.0001
24
34.1 ± 6.9 33.1 ± 5.9 33.5 ± 6.1 33 ± 5.5 32.2 ± 6.4 31.4 ± 5.8 30.4 ± 5.8 29.6 ± 5.4 0.009 0.002 <0.0001 0.011 O 2 pulse
3 months (48
6 months (110 patients) 12 months (98 patients) 24 months (22 patients) 11.7 ± 3.1 11.7 ± 3.1 11.6 ± 3 11.7 ± 3.2 13.2 ± 3.8 13.6 ± 4.1 13.3 ± 3.7 13.2 ± 3.5 <0.0001 <0.0001 <0.0001 0.0003
3 months
6 months
12 months (98 patients) 24 months (22 patients) 9 ± 1.6 9.1 ± 1.5 9.1 ± 1.6 9.2 ± 1.8 9.9 ± 1.5 10.3 ± 2.1 10 ± 1.6 9.9 ± 1.8 0.0008 <0.0001 <0.0001 0.062
patients)
(48 patients)
(110 patients)
Table 4: Stratified Cardiopulmonary Exercise Testing Parameters According to Sacubitril/Valsartan
exercise capacity and VO2 peak was shown in patients treated with both drugs compared to patients on monotherapy.11, 25
Accordingly, the present data confirm an improvement in exercise tolerance during follow-up both in terms of VO2/work (Δ = +0.8 ml/beat, p=0.0002) and VO2 at the anaerobic threshold. (Δ = +1 ml/kg/min, p=0.021).
slope = minute ventilation/carbon dioxide production ratio
all causes.16 Arena et al. observed that patients with a VE/VCO2 slope >34 showed an increase in cardiac mortality at 1 year (survival 83.1% versus 99.2% in those with slope <34; p<0.0001) and an increase in emergency hospitalisation for cardiac causes compared to patients who had a value of this ratio <34.17 Furthermore, numerous pieces of evidence confirm the prognostic importance of the VE/VCO2 slope in patients with HF.18–23 The results of our study show that patients treated with S/V had a significant reduction in VE/VCO2 in the short, medium and long term, regardless of the S/V dosage used.
In a post hoc analysis of the PARADIGM-HF study, Vardeny et al. showed that – even at low doses – S/V leads to clinical benefits compared to enalapril; however, patients taking low doses have a higher risk of primary events.24 In our study, patients taking low doses of S/V had less improvement in oxygen pulse and in VO2/work than those taking high or intermediate doses. It could be argued that these results are linked to the greater fragility of patients treated with lower doses of the drug; the latter, in fact, had lower systolic blood pressure values both at baseline and during the follow-up, higher levels of NT-pro BNP, higher prevalence of NYHA III class, higher doses of furosemide, reduced values of eGFR and higher ratios of VE/VCO2 at baseline.
In a recent study, Guazzi et al. demonstrated that subjects treated with enalapril had higher peak VO2 values and lower VE/VCO2 slope values compared to those treated with placebo.11 25 These results could be due to an improvement in pulmonary alveolar-capillary diffusion mediated by an increase in bradykinins and, consequently, by prostaglandin-mediated vasodilation. These authors observed a statistically significant increase in lung diffusion capacity of carbon monoxide (DLCO) and an inverse relationship between DLCO and VD/VT. The same authors demonstrated that treatment with losartan was associated with a significant increase in the ΔVO2/Δ work ratio (p<0.05 in patients treated with losartan versus enalapril), probably due to improved peripheral muscle perfusion. Furthermore, a synergistic effect between enalapril and losartan on
The improvement of exercise tolerance during therapy with S/V is likely because of the combination of the effect of ARBs and neprilysin inhibitors: Valsartan is an angiotensin II type I (AT1) receptors antagonist (that antagonises the effects of angiotensin II, causing vasodilation and volume reduction), while sacubitril is an inhibitor of the enzyme neprilysin (a neutral endopeptidase responsible for the degradation of vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. By this mechanism, the levels of these substances increase causing vasodilation and natriuresis). As a consequence of these medications’ actions, an amplification of the system of natriuretic peptides and other vasoactive peptides (such as bradykinin, adrenomedullin, endothelin-1, substance P and angiotensin II) is observed.26 27 Therefore, based on the results of our study, it seems that S/V can improve pulmonary diffusion through an increase in bradykinin and can exert a positive effect on muscle efficiency. This results in a clear improvement in exercise tolerance and muscle performance. In particular, the early improvement in VO2 peak could lead to an increase in pulmonary diffusion, and the simultaneous reduction in the VE/VCO2 ratio could lead to a lasting reduction over time in the LV volume overload with consequent reverse remodelling of the LV. Recently published data demonstrate an improvement in LVEF and effects on reverse remodelling of the LV in patients receiving S/V.28 29
An increase in peak ventilatory response was also observed, which could be secondary to the improved cardiac performance and reduced congestion, allowing patients to increase ventilation without increasing the VE/VCO2 slope. In agreement with these data, an improvement in the ejection fraction, plus a reduction in the E/A ratio and in the size of the inferior vena cava after S/V treatment, was confirmed in our study population.
In the subgroup of patients who underwent CMR, the clinical and demographic characteristics were comparable to those of the entire study population. The morpho-volumetric data obtained by CMR confirmed the echocardiographic data. The mass of DE was also quantified in g and in % related to the mass of the LV. In this subgroup of patients, we observed that patients with DE>4.6% reached a minor improvement in cardiorespiratory function parameters.
Recent studies have suggested a possible role of S/V therapy in the remodelling and deposition of fibrotic material at the myocardial level through an activating action on cardiac fibroblasts.8 30 Few data are available regarding ventricular remodelling, and other mechanisms of benefit are unclear, including reduction of myocardial injury and fibrosis; nonetheless, given that the benefit of S/V appeared to be related to improvement in rates of progressive pump failure or sudden cardiac death, it is reasonable to hypothesise significant reverse remodelling related to therapy.
Even in patients with ischaemic heart disease, there are data in the literature demonstrating the role of S/V in reducing cardiac remodelling and LV dysfunction.7 Therefore, S/V therapy may reduce cardiac remodelling in ischaemic and non-ischaemic patients. The results of our analysis suggest that, in patients with myocardial fibrosis, the effects of
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Dosage Parameter Baseline Follow-up p-value VO 2 peak (ml/kg/min), (mean ± SD) S/V 24/26 mg (39 patients) S/V 49/51 mg (47 patients) S/V 97/103 mg (48 patients) 14.2 ± 3.3 15.4 ± 3.8 15.6 ± 3.8 16.6 ± 4.4 17.5 ± 4.7 18.6 ± 4.9 0.008 0.017 0.001 Predicted VO 2 peak (%), (mean ± SD) S/V 24/26 mg (39 patients) S/V 49/51 mg (47 patients) S/V 97/103 mg (48 patients) 52.9 ± 13.8 57.8 ± 13.8 55.4 ± 14.6 62.9 ± 15.2 66 ± 16.4 67 ± 18.8 0.003 0.009 0.001 VE/VCO2 slope,
S/V 24/26 mg (39 patients) S/V 49/51 mg (47 patients) S/V 97/103 mg (48 patients) 35 ± 6.4 32.6 ± 6.4 32.4 ± 5.2 32 ± 5.6 30.6 ± 5.9 29.8 ± 6.6 0.032 0.110 0.036 O 2 pulse (ml/beat), (mean ± SD) S/V 24/26 mg (39 patients) S/V 49/51 mg (47 patients) S/V 97/103 mg (48 patients) 11.1 ± 2.9 11.5 ± 3 12.4 ± 3.2 12.3 ± 3.6 13.2 ± 3.7 14.3 ± 3.8 0.13 0.013 0.011 ΔVO2/Δ work (ml/min/W), mean ± SD S/V 24/26 mg (39 patients) S/V 49/51 mg (47 patients) S/V 97/103 mg (48 patients) 8.8 ± 1.7 9.1 ± 1.4 9.3 ± 1.5 9.5 ± 1.9 10.2 ± 1.4 10 ± 1.5 0.11 0.004 0.035 S/V = sacubitril/valsartan;
VO2
Δ
(VO2 )/Δ
(mean ± SD)
VE/VCO2
slope;
peak = oxygen consumption peak; ΔVO2 /Δ work =
oxygen consumption
work-rate relationship.
the drug on functional capacity and cardiorespiratory parameters, even if still evident, are reduced in extent. Finally, in the total population multivariate analysis, the main predictors of major cardiovascular events during follow-up were VE/VCO2>34, the presence of ventilatory oscillation and the haemoglobin value. These results are in accordance with the data present in the literature. In the subgroup of patients undergoing CMR, the main prognostic predictors during follow-up were VE/VCO2 and % DE. To date, the present study represents the largest series of HFrEF patients treated with S/V in whom functional capacity has been assessed through CPET parameters with both short- and long-term follow-up. Further studies are needed to better understand the mechanism of action of the drug and its effects on cardiac remodelling.
Study Limitations
The main limitation of this study is the absence of a control group. However, the study was initiated after the publication of the current guidelines of the recommendation for the use of S/V in patients with HFrEF.10 Having demonstrated a significant reduction in mortality compared to enalapril therapy alone, it would not have been ethically correct to deny treatment to the control group. It was also considered methodologically incorrect to select a control group with contraindications to S/V therapy (e.g. patients with arterial systolic hypotension, class IV–V chronic renal failure), because the two populations under examination would have been highly heterogeneous.
In the subgroup of patients undergoing CMR, the limitations of the small size of the sample and lack of an MRI examination during follow-up should be noted. However, these patients underwent primary preventive ICD implantation in the study and were unable to undergo CMR after initiation
1. McMurray JJ, Packer M, Desai AS, et al. Angiotensinneprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. https://doi.org/10.1056/ NEJMoa1409077; PMID: 25176015.
2. Vitale G, Romano G, Di Franco A, et al. Early effects of sacubitril/valsartan on exercise tolerance in patients with heart failure with reduced ejection fraction. J Clin Med 2019;8:262. https://doi.org/10.3390/jcm8020262; PMID: 30791533.
3. Chandra A, Lewis EF, Claggett BL, et al. Effects of sacubitril/ valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol 2018;3:498–505. https:// doi.org/10.1001/jamacardio.2018.0398; PMID: 29617523.
4. Beltran P, Palau P, Dominguez E, et al. Sacubitril/valsartan and short-term changes in the 6-minute walk test: a pilot study. Int J Cardiol 2018;252:136–9. https://doi.org/10.1016/j. ijcard.2017.10.074; PMID: 29249422.
5. Lau CW, Martens P, Lambeets S, et al. Effects of sacubitril/ valsartan on functional status and exercise capacity in realworld patients. Acta Cardiol 2019;74:405–12. https://doi.org/1 0.1080/00015385.2018.1521054; PMID: 30474478.
6. Gonçalves AV, Pereira-da-Silva T, Galrinho A, et al. Maximal oxygen uptake and ventilation improvement following sacubitril-valsartan therapy. Arq Bras Cardiol 2020;115:821–7. https://doi.org/10.36660/abc.20190443; PMID: 33084746.
7. Von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015;8:71–8. https://doi.org/10.1161/ CIRCHEARTFAILURE.114.001785; PMID: 25362207.
8. Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail 2019;12:1–15. https://doi. org/10.1161/CIRCHEARTFAILURE.118.005565; PMID: 30998392.
9. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging
of S/V treatment. Finally, a further limitation of the study is that patients undergoing CMR presented a recent diagnosis of HF and for this reason they had not yet been implanted with a device.
Conclusion
In patients with HFrEF treated with S/V in the short, medium and long-term follow-up, a significant improvement in cardiovascular, muscle and ventilatory efficiency was demonstrated. The presence of DE in the myocardium influences the response to therapy with S/V, suggesting that myocardial fibrosis might attenuate the effects of the drug on functional capacity and cardiorespiratory parameters. Further studies are eagerly awaited in order to elicit the drug’s mechanism of action and its effects on cardiac remodelling.
Clinical Perspective
• This study aimed to evaluate the cardiopulmonary effects of sacubitril/valsartan (S/V) therapy in patients with heart failure with reduced ejection fraction (HFrEF) and investigating the role of myocardial fibrosis, assessed with cardiac magnetic resonance (CMR), in predicting response to S/V therapy.
• At the end of our study we concluded that:
1. The CPET is confirmed as the best evaluating test for the patients’ setting treated with S/V. During the follow-up, by CPET, we were able to evaluate how S/V improved functional capacity in HFrEF patients.
2. A percentage delayed enhancement cut-off (<4,6%) can be established as predictive for a better response to therapy.
2015;16:233–70. https://doi.org/10.1093/ehjci/jev014; PMID: 25712077.
10. Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128; PMID: 27206819.
11. Guazzi M, Marenzi GC, Assanelli E, et al. Evaluation of the dead space/tidal volume ratio in patients with chronic congestive heart failure. J Card Fail 1995;1:401–8. https://doi. org/10.1016/s1071-9164(05)80009-0; PMID: 12836715.
12. Corrà U, Agostoni PG, Anker SD, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:3–15. https://doi.org/10.1002/ejhf.979; PMID: 28925073.
13. Contini M. Cardiopulmonary test as a tool to choose therapy in heart failure. Ann Am Thorac Soc 2017;14:S67–73. https:// doi.org/10.1513/AnnalsATS.201611-887FR; PMID: 28375655.
14. Myers J, Arena R, Cahalin LP, et al. Cardiopulmonary exercise testing in heart failure. Curr Probl Cardiol 2015;40:322–72. https://doi.org/10.1016/j. cpcardiol.2015.01.009; PMID: 26096801.
15. Palau P, Mollar A, Dominguez E, et al. Early sacubitril/ valsartan-driven benefit on exercise capacity in heart failure with reduced ejection fraction: a pilot study. Rev Esp Cardiol (Engl Ed) 2019;72:167–9. https://doi.org/10.1016/j. rec.2017.11.025; PMID: 29373254.
16. Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO 2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012;5:579–85. https://doi.org/10.1161/ CIRCHEARTFAILURE.111.965186; PMID: 22773109.
17. Arena R, Myers J, Aslam SS, et al. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J 2004;147:354–60. https://doi. org/10.1016/j.ahj.2003.07.014; PMID: 14760336.
18. Francis DP, Shamim W, Davies LC. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO. Eur Heart J 2000;21:154–61. https://doi.org/10.1053/ euhj.1999.1863; PMID: 10637089.
19. Robbins M, Francis G, Pashkow FJ, et al. Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation 1999;100:2411–7. https://doi.org/10.1161/01.cir.100.24.2411; PMID: 10595953.
20. Corra U, Mezzani A, Bosimini E, et al. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J 2002;143:418–26. https://doi.org/10.1067/ mhj.2002.120772; PMID: 11868046.
21. Kleber FX, Vietzke G, Wernecke KD, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation 2000;101:2803–9. https://doi.org/10.1161/01. cir.101.24.2803; PMID: 10859285.
22. Sarullo FM, Fazio G, Brusca I, et al. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J 2010;4:127–34. https://doi.org/10.2174/ 1874192401004010127; PMID: 20657715.
23. MacGowan GA, Janosko K, Cecchetti A, Murali S. Exerciserelated ventilatory abnormalities and survival in congestive heart failure. Am J Cardiol 1997;79:1264–6. https://doi. org/10.1016/s0002-9149(97)00097-0; PMID: 9164901.
24. Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/ valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail 2016;18:1228–34. https://doi.org/10.1002/ ejhf.580; PMID: 27283779.
25. Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016;133:e694–711. https://doi.org/10.1161/ CIR.0000000000000406; PMID: 27143685.
26. Bayés-Genís A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol 2016;68:639–53. https://doi.org/10.1016/j. jacc.2016.04.060; PMID: 27491909.
27. D’Elia E, Iacovoni A, Vaduganathan M, et al. Neprilysin
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Effects of S/V Therapy on Exercise Capacity in Patients with HFrEF
inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail 2017;19:710–7. https://doi.org/10.1002/ejhf.799; PMID: 28326642.
28. Martens P, Beliën H, Dupont M, et al. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther
2018;36:e12435. https://doi.org/10.1111/1755-5922.12435; PMID: 29771478.
29. Almufleh A, Marbach J, Chih S, et al. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis 2017;7:108–13. PMID: 29348971.
30. Schmieder RE, Wagner F, Mayr M, et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J 2017;38:3308–17. https:// doi.org/10.1093/eurheartj/ehx525; PMID: 29029087.
CARDIAC FAILURE REVIEW www.CFRjournal.com
Role of Imaging in Cardiomyopathies
Abstract
Imaging has a central role in the diagnosis, classification, and clinical management of cardiomyopathies. While echocardiography is the firstline technique, given its wide availability and safety, advanced imaging, including cardiovascular magnetic resonance (CMR), nuclear medicine and CT, is increasingly needed to refine the diagnosis or guide therapeutic decision-making. In selected cases, such as in transthyretin-related cardiac amyloidosis or in arrhythmogenic cardiomyopathy, the demonstration of histological features of the disease can be avoided when typical findings are observed at bone-tracer scintigraphy or CMR, respectively. Findings from imaging techniques should always be integrated with data from the clinical, electrocardiographic, biomarker, genetic and functional evaluation to pursue an individualised approach to patients with cardiomyopathy.
Keywords
Imaging, cardiomyopathies, echocardiography, cardiac magnetic resonance, nuclear medicine
Disclosure: The authors have no conflicts of interest to declare.
Received: 31 August 2022
Accepted: 7 November 2022 Citation: Cardiac Failure Review 2023;9:e08. DOI: https://doi.org/10.15420/cfr.2022.26
Correspondence: Giuseppe Vergaro, Scuola Superiore Sant’Anna and Fondazione Toscana Gabriele Monasterio, Via Moruzzi, 1 56124 Pisa, Italy.
E: giuseppe1.vergaro@santannapisa.it
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Imaging and Cardiomyopathy Phenotype
Cardiomyopathies (CMPs) are a group of diseases affecting the cardiac muscle, heterogeneous in terms of aetiology and clinical presentation. In 2008, a European Society of Cardiology (ESC) position statement defined CMPs as myocardial disorders “in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality”.1
CMPs were grouped into specific morphological and functional phenotypes (hypertrophic, dilated, arrhythmogenic, restrictive, or other CMPs), and each phenotype was sub-classified into familial/genetic and non-familial/non-genetic forms.1 CMPs present variable expressions and symptoms, which may change over time.
CMPs may represent a challenge for cardiologists and imaging specialists, but an in-depth knowledge of aetiologies and characteristic features can lead to the correct diagnosis. The diagnostic workup of patients with suspected CMP includes clinical history and physical examination, a 12lead ECG, specific laboratory tests, and often an endomyocardial biopsy and genetic testing. Cardiac imaging plays a pivotal role in the diagnosis of CMPs and their differentiation from mimicking conditions, such as athlete’s heart. Transthoracic echocardiography (TTE) is a safe, readily available technique that may raise the suspicion of CMP or allow a first assessment of patients with suspected CMP. Other imaging modalities, such as cardiac magnetic resonance (CMR), cardiac CT (CCT) and nuclear
imaging, may help come to the correct diagnosis. In some cases, imaging findings alone are sufficient to diagnose specific disorders with no need for histological demonstration. The most notable case is amyloid transthyretin (ATTR) cardiac amyloidosis (CA), which can be diagnosed when there is an intense myocardial uptake of bone tracers on scintigraphy, and no monoclonal protein is found.2
Besides aetiological diagnosis, imaging techniques may help detect cardiac involvement in an earlier stage, characterise the remodelling pattern, perform an accurate functional assessment and risk stratification, and finally guide therapeutic decisions. TTE also offers the possibility of serial examinations over time. When possible, basic TTE examination should be integrated by speckle-tracking imaging of the left ventricle (LV), and, ideally, also of other cardiac chambers. CMR provides additional anatomical and functional information and enables tissue characterisation, which has important prognostic implications. Other techniques, such as CCT, scintigraphy or PET, may provide additional information to predict future disease trajectories and guide patient management.3 Table 1 provides an overview of the utility of different imaging modalities for the assessment of CMPs.
Overall, multimodality cardiac imaging plays an important role in clinical decision-making and helps to improve patients’ management and outcomes. In this review, we provide an overview of the current applications and some future perspectives on the use of imaging techniques in the diagnosis and treatment of patients with CMPs.
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Imaging
Vincenzo Castiglione,1,2 Alberto Aimo,1,2 Giancarlo Todiere,1 Andrea Barison,1,2 Iacopo Fabiani,1 Giorgia Panichella,1 Dario Genovesi,1 Lucrezia Bonino,1 Alberto Clemente,1 Filippo Cademartiri,1 Alberto Giannoni,1,2 Claudio Passino,1,2 Michele Emdin1,2 and Giuseppe Vergaro 1,2
1. Cardiothoracic Department, Fondazione Toscana Gabriele Monasterio, Pisa, Italy; 2. Health Science Interdisciplinary Center, Scuola Superiore Sant’Anna, Pisa, Italy
Table 1: Utility of Different Imaging Modalities for the Assessment of Cardiomyopathies
Main applications
• Main imaging technique for the initial assessment of patients with suspected CMP
• Risk stratification
• Main imaging technique during follow-up
• Gold standard for chamber quantification
• Differential diagnosis
• Risk stratification
• Non-biopsy diagnosis of ATTR-CA with 99mTc-labelled bone tracers
• Detecting inflammatory process (18F-FDG PET for sarcoidosis diagnosis and treatment monitoring)
• Rule out coronary artery disease
Main limitations
• Acoustic window limitation
• Operator dependency
• Availability
• Metallic implants
• Use of contrast
• Low quality in arrhythmias or poor breath-holding ability
• Radiation exposure
• Attenuation artefacts
• Radiation exposure
• Availability
• Cost
• Radiation exposure
• Low quality in arrhythmias
Number of crosses (+) indicates how helpful each test is in assessing the index parameter; while a minus sign (–) means not helpful. ATTR-CA = amyloid transthyretin cardiac amyloidosis; CCT = cardiac CT; CMP = cardiomyopathy; CMR = cardiac magnetic resonance; FDG = fluorodeoxyglucose; SPECT = single photon emission CT; TTE = transthoracic echocardiography. Adapted from: Mitropoulou et al. 2020.120 Reproduced from Frontiers Media under a Creative Commons CC-BY licence.
Echocardiography
TTE is the first-line imaging modality and is of unquestionable value in the diagnosis and characterisation of CMPs, as it can identify both structural and functional abnormalities. The main strengths of TTE are availability, low cost, non-invasiveness and possibility of serial testing; possible drawbacks are the reliance on the operator’s experience and the acoustic window, as well as the inter- and intra-observer variability.
Conventional Echocardiography
Conventional echocardiography is an essential tool to characterise LV geometry and function in dilated cardiomyopathy (DCM), and the response to pharmacological and non-pharmacological interventions in terms of reverse remodelling. In hypertrophic cardiomyopathy (HCM), M-mode imaging can identify the systolic anterior motion of the mitral valve, a common, though non-specific, feature, and a determinant of LV outflow tract obstruction (LVOTO).4–6 Continuous wave (CW) Doppler is used to quantify the severity of LVOTO in HCM: the Doppler envelope appears as a “dagger-shaped” and late-peaking curve.5 In obstructive HCM, the peak intraventricular gradient is >30 mmHg at rest or after provocative manoeuvres.7 Dynamic LVOTO is also identifiable in the acute phase of takotsubo syndrome (intraventricular gradient >25 mmHg), as a result of basal hypercontractility in the LV cavity with an asymmetrical and hypertrophic interventricular septum.8 Pulsed wave (PW) Doppler can be used in HCM patients to sample the left anterior descending artery, leading to the frequent finding of a reduced coronary flow reserve (CFR).9 Further, abnormal tissue Doppler imaging (TDI) data, such as lateral s’<13 cm/s and reduced e’ velocities, can be suggestive of an early prehypertrophic phenotype.7 In restrictive cardiomyopathy (RCM), the change of E/A ratio during the Valsalva manoeuvre is used to define the restrictive filling as reversible (>0.5) or fixed (<0.5, a more severe form).10 11 The discrepancy between LV mass and ECG voltages is an important clue to the diagnosis of CA (Figure 1). 2D echocardiography is also useful in arrhythmogenic cardiomyopathy (ACM), as it allows the study of right ventricular (RV) motion and the measurement of RV outflow tract diameter and right ventricle longitudinal systolic function and fractional area change.12 13 Contrast agents can be used in rest and stress echocardiography to improve image quality (especially in patients with suboptimal image quality).14–16
3D Echocardiography
3D echocardiography provides real-time images of the heart in motion that can be displayed at full or cropped volume, allows a more accurate study of cardiac geometry (LV and RV volumes, LV mass) and function (LV and RV ejection fraction, LV regional wall motion, dyssynchrony, and dyssynchrony index). Measurements of linear dimensions and areas can be based on 3D-guided 2D, which ensures higher accuracy than 2D measurements, or on volumetric rendered images that highly depend on image processing.7
Speckle Tracking Echocardiography
Speckle tracking echocardiography (STE) enables the calculation of motion, velocity, strain and strain rate by identifying a specific image feature, following it frame after frame, and analysing its displacement over time. Good image quality is necessary for STE.7 Global longitudinal strain (GLS) is one of the most studied parameters to detect preclinical disease, especially in DCM.17 Studies on HCM have focused on LA peak strain during the reservoir phase, corresponding to LV systole, while in ACM RV GLS can be calculated by averaging RV peak systolic longitudinal strain values from six RV segments.12 18 Conversely, RV free wall longitudinal strain is to be preferred to RV GLS in takotsubo syndrome.19 In patients with suspected CA, peak LA longitudinal strain and peak atrial contraction strain have independent diagnostic value (Figure 1).20 LV and RV strain, as well as LA enlargement, RV dilatation and RV contractile dysfunction, are important prognostic markers in DCM.7 Longitudinal myocardial function can be studied also in cardiac sarcoidosis with 2D STE or TDI-derived strain, allowing the identification of the disease at early stages, when other 2D echo features may be absent.21
Stress Echocardiography
Stress echocardiography dynamically evaluates myocardial structure and function under a stress condition induced by physical exercise (using a treadmill or a bicycle) or a pharmacological agent (inotrope or vasodilator). Images are acquired at baseline, at a low workload and at peak exercise.7 Myocardial contractile reserve, studied with stress echocardiography, helps distinguish ischaemic from non-ischaemic disease and holds prognostic importance.22 23 Moreover, a limited coronary flow and a reduced or absent contractile reserve are often present in subclinical
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
TTE CMR SPECT PET CCT Morphologic assessment ++ +++ + + ++ Systolic and diastolic function +++ +++ + + + Valve disease +++ ++ + Tissue characterisation + +++ +
CMPs.24–26 Exercise echocardiography is used to quantify mitral regurgitation and to study inducible LVOTO (which is typically increased at the beginning of the recovery phase) in HCM patients.24
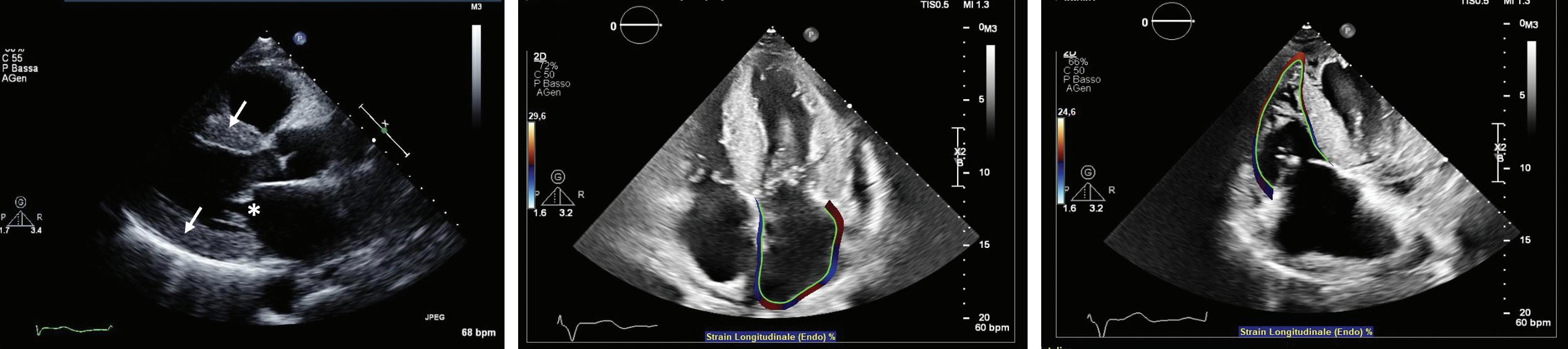
Cardiac Magnetic Resonance
CMR is a multi-parametric, highly reproducible, non-invasive imaging technique with a relatively high spatial, temporal and contrast resolution, which has become an essential tool for the evaluation of CMPs. Indeed, CMR allows not only the quantification of biventricular volumes, mass, wall thickness, systolic- and diastolic function, intra- and extracardiac flows, but also the detection of myocardial oedema, fibrosis, and the accumulation of other intra/extracellular substances (such as fat, iron, or amyloid), thus providing unique information for disease characterisation.27 28 It has only a few contraindications, mostly related to MR-unsafe metal implants, severe renal dysfunction (which limits the use of several gadolinium-based contrast agents), patient discomfort (claustrophobia), tachyarrhythmias or poor breath-holding ability (which might significantly degrade image quality).29
In patients with non-ischemic DCM, CMR is the gold standard technique for the quantification of biventricular volumes, mass, and EF with cine steady-state free-precession (SSFP) sequences, and for the assessment of myocardial fibrosis with late gadolinium enhancement (LGE) sequences.30 LGE can be found in about 30–50% of DCM patients, typically in a patchy, midwall or subepicardial distribution (Figure 2). Since the first study by Assomull et al. in 2006, midwall fibrosis detected by LGE has emerged as a predictor of adverse prognosis, including all-cause and cardiovascular mortality, sudden cardiac death (SCD), appropriate ICD therapy and ventricular arrhythmias (VA), independently from left ventricular ejection fraction (LVEF).31–33 Interestingly, while the relationship between LVEF and VA or SCD is weak in DCM patients, a strong, significant association has been demonstrated between LGE and VA or SCD, even in patients with LVEF >35%. Different LGE location, pattern or extent have different prognostic impacts, although further larger investigations are needed.34 35 The absence of LGE predicts the occurrence of reverse remodelling, while the presence and transmural pattern of LGE in the LV lateral wall predicts a poor response to cardiac resynchronisation therapy (CRT).36–39 Native myocardial T1 and extracellular volume (ECV) mapping track myocardial fibrosis and interstitial remodelling, and have recently emerged as independent markers of poor outcome.40–42 Recently, GLS measured by feature-tracking analysis of cine SSFP images was found to correlate better than LVEF and B-type natriuretic peptide with the composite outcome of cardiac death, heart transplantation and appropriate ICD shock.43 CMR plays a crucial role also in the detection of LV noncompaction (LVNC), whose diagnosis is particularly challenging,
especially due to the overlap with other CMPs and normal LV trabeculation. Besides several geometric diagnostic criteria with limited diagnostic and prognostic utility, the most powerful prognostic parameters in LVNC are the presence of LV systolic dysfunction and LGE.44–48
In patients with HCM, CMR allows the morphological assessment of cardiac chambers, including unusual patterns of hypertrophy (lateral, apical or RV distribution), myocardial crypts, papillary muscle abnormalities, elongated mitral valve leaflets and apical aneurysms, which are not always easily visualised by echocardiography.49–52 Myocardial fibrosis detected by LGE imaging is a common finding in HCM, occurring in up to 80% of patients (Figure 3), so that only LGE extent is predictive of outcome: an LGE threshold of 10–15% of the LV mass identifies patients at high risk of VA, even in the absence of other major risk factors, and has been listed among the criteria to be considered for the selection of patients to be referred for ICD in the recently updated American Heart Association/American College of Cardiology (AHA/ACC) guidelines.53–57 Even scar heterogeneity (expressed as ‘dispersion map of LGE’), scar channels (assessed with an advanced post-processing analysis to differentiate the scar core and the border zone of LGE images), longitudinal strain, myocardial oedema (assessed through T2-weighted imaging), native T1 and ECV mapping are emerging as prognostic markers in HCM patients.58–64
CMR is of utmost importance for the differential diagnosis of hypertrophic phenocopies. In particular, CA is characterised by diffuse subendocardialto-transmural LGE with variable biventricular and biatrial involvement, which tracks amyloid deposition and patient prognosis: while only subtle LGE areas might be present in the very early disease stages, LGE imaging can be challenging in advanced stages, due to the diffuse nature of LGE and to the equalisation of myocardial and blood pool nulling point.65 An increased native T1 demonstrated high diagnostic accuracy in patients with suspected CA, but ECV represents the best parameter to assess amyloid burden, patient outcome and response to treatment.66–71
CMR holds strong potential for diagnostic, prognosis and therapeutic monitoring in Anderson-Fabry disease (AFD), particularly because intracellular glycosphingolipids accumulation causes a shortening of native T1, while ECV is normal compared to other CMPs characterised by interstitial infiltration.72
CMR represents an essential tool for the diagnosis and risk stratification of iron overload CMPs, including genetic haemochromatosis and hereditary anaemias: myocardial iron deposits reduce both T2* and native T1 relaxation times, thus allowing an accurate non-invasive diagnosis of cardiac siderosis and treatment monitoring.73 74
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
B C
Figure 1: Echocardiography in Cardiac Amyloidosis
A
A: Parasternal long axis view showing increased left ventricular wall thickness (arrows) and thickened mitral leaflets (asterisk) in amyloid light-chain amyloidosis; B: Apical four-chamber view showing left atrial strain in transthyretin amyloidosis (ATTR); C: Right ventricle global longitudinal strain in ATTR.
ACM is a genetically determined heart muscle disease characterised by myocardial fibro-fatty replacement with variable RV and LV involvement, clinically associated with malignant ventricular arrhythmias and SCD.75 CMR has always been regarded as a tool for the non-invasive detection of major morpho-functional abnormalities (biventricular dilation, dysfunction and regional wall motion abnormalities) (Figure 4). In the recently published Padua criteria, CMR has gained further importance, with the inclusion of LGE as a major structural criterion besides endomyocardial biopsy.76 The presence of circumferential LV subepicardial LGE in short axis view (ring pattern) has been consistently reported in left-dominant forms with a specific genotype.77 Similarly to DCM, LV LGE identifies patients with a high arrhythmic risk who are candidates to ICD implantation regardless of the severity of systolic dysfunction.78 CMR can also detect myocardial fatty infiltration as hyperintense areas on T1- or proton density (PD)-weighted images, or as India ink artefacts at conventional cine-SSFP images, which nonetheless have limited sensitivity and specificity in the context of a low spatial resolution.79 80 T2-weighted images might allow the depiction of myocardial oedema in case of an ACM presentation with chest pain and

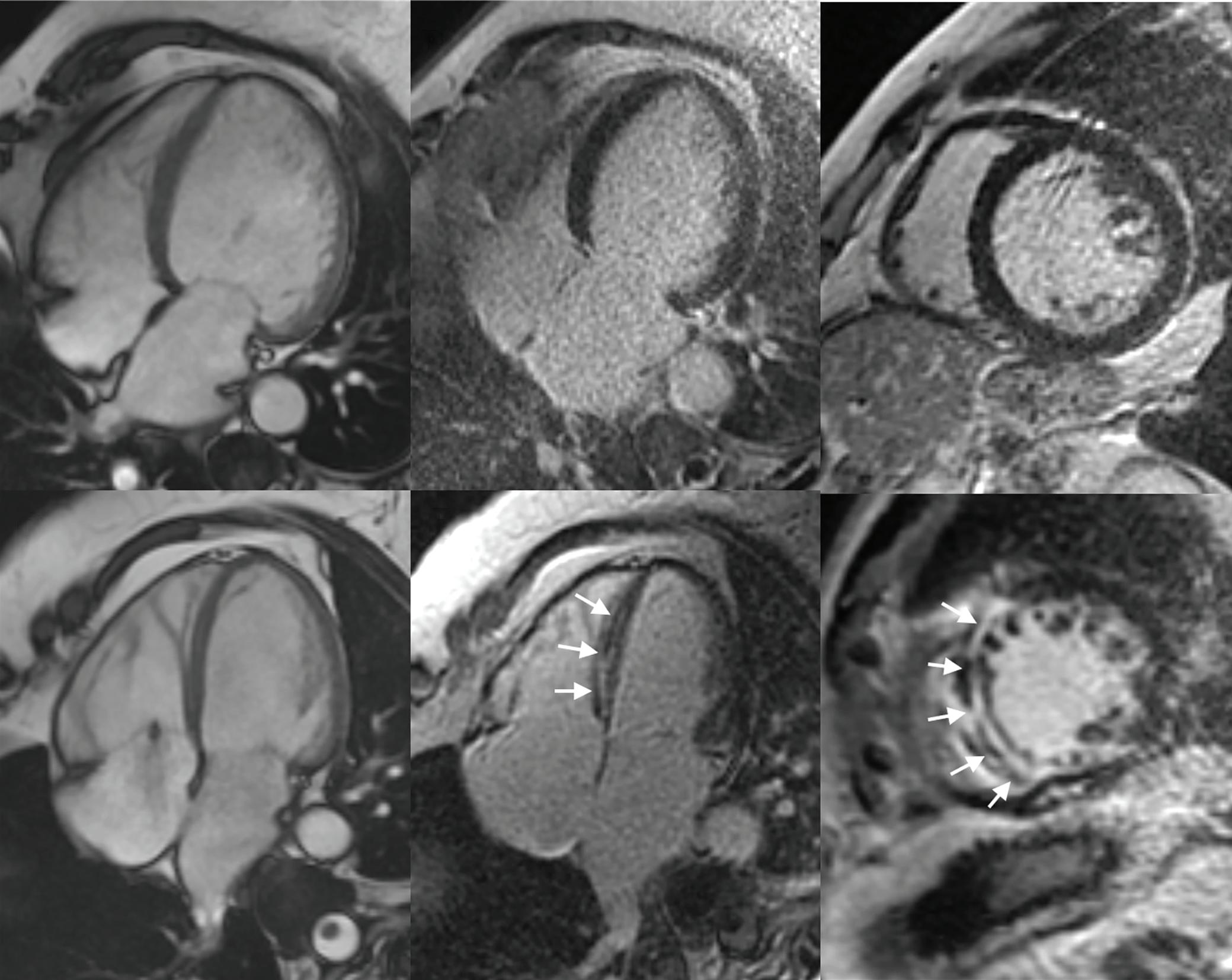
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
troponin release, which might be encountered in paediatric
A B C D E F
Figure 2: Cardiac Magnetic Resonance in Dilated Cardiomyopathy Left Ventricular Dilation in a Patient with Dilated Cardiomyopathy
A: Four-chamber steady-state free-precession diastolic frame, without late gadolinium enhancement; B: Four chamber; C: Short axis view. A similar left ventricular dilation in a different dilated cardiomyopathy patient; D: Four-chamber steady-state free-precession diastolic frame, associated with midwall late gadolinium enhancement in the interventricular septum; E: Four chamber; F: Short axis view (white arrows).
Figure 3: Cardiac Magnetic Resonance in Hypertrophic Cardiomyopathy Disproportionate Apical Hypertrophy in a Patient with Hypertrophic Cardiomyopathy
A B
A: Four-chamber steady-state free-precession diastolic frame, associated with midwall late gadolinium enhancement in the left ventricular apex. B: Four chambers (white arrows).
patients and carriers of DSP (desmoplakin) gene mutations with a clinical presentation similar to a recurrence of myocarditis.81 82
Nuclear Medicine
Nuclear medicine offers imaging tools to evaluate myocardial perfusion, innervation and metabolism induced by inflammatory processes as in myocarditis and cardiac sarcoidosis, or in CA. In the last decade, the use of specific single-photon emission CT (SPECT) radiopharmaceuticals has allowed the identification of the presence of ATTR-CA, while the use of new PET radiopharmaceuticals could help to achieve an early diagnosis of immunoglobulin light-chain (AL) CA without the need for biopsy confirmation.

Perfusion and Innervation SPECT Tracers
SPECT with tracers such as 201Tl, sestamibi or tetrofosmin labelled with 99mTc allows the exclusion of the presence of ischaemia, particularly in patients with HCM who present with chest pain or who have LV systolic dysfunction. Using dedicated software, it is possible to estimate the systolic and diastolic function of the LV from a myocardial perfusion study.83,84 In cardiac sarcoidosis, the assessment of LV contraction dyssynchrony by myocardial perfusion-gated SPECT can be useful for prognostic stratification; furthermore, the assessment of perfusion recovery after corticosteroid therapy seems to be associated with a lower risk of major adverse cardiac events.85 86
SPECT with 123I-labeled metaiodobenzylguanidine (MIBG) allows the evaluation of the integrity of myocardial adrenergic innervation. In AFD, the extent of impairment of adrenergic innervation correlates with the degree of fibrosis, but autonomic dysfunction appears to precede myocardial fibrosis, so the innervation scintigraphy could allow an earlier diagnosis of this disease.87 A reduction in the density of adrenergic endings has also been reported in CA, probably as a consequence of the toxic effect of the amyloid fibrils.88,89 The use of 123I-MIBG for the evaluation of adrenergic damage has also been described in patients with cardiac sarcoidosis, but it is not routinely adopted.90 91
PET Tracers Assessing Myocardial Inflammation
18F-labelled fluorodeoxyglucose (FDG) is the main radiopharmaceutical used in PET. Although its main use is oncological, it is not tumour-specific, and it allows the visualisation of areas of leukocyte infiltration during
inflammatory processes. The degree of glucose uptake in the vital myocardium correlates directly with the circulating levels of insulin and inversely with the level of circulating free fatty acids, while the degree of glucose metabolism of the inflammatory tissue is relatively independent of insulin. For this reason, the use of 18F-FDG for the study of myocarditis requires an adequate dietary preparation that suppresses the myocardial glucose uptake.92–94 PET with 18F-FDG can be used to characterise the inflammatory response after acute myocardial infarction, to assess the extent of the disease and the response to therapy in the case of myocarditis and in sarcoidosis.95–98 Lymphocytes involved in the granulomatous process of sarcoidosis express membrane receptors for somatostatin; new PET radiopharmaceuticals with an affinity for somatostatin receptors, such as the peptides DOTATOC, DOTATATE and DOTANOC labelled with 68Ga, have been shown to have a greater diagnostic accuracy than 18F-FDG for cardiac sarcoidosis.99
Nuclear Medicine in Cardiac Amyloidosis
Some SPECT tracers originally developed to study bone metabolism and labelled with 99mTc have been successfully used to identify ATTR-CA. 99mTc3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) cardiac uptake in patients with ATTR-CA has been known since the 1970s; thanks to the evidence collected in the last 15 years, bone tracer scintigraphy has been a fundamental tool in the diagnostic path of patients with suspected ATTR-CA.100 18F-NaF is a PET radiopharmaceutical for bone imaging that also accumulates in myocardial ATTR deposits.101 102 However, a lower sensitivity of PET compared to scintigraphy has been reported.103 104 In the last decade, PET radiopharmaceuticals developed for brain amyloid substance have shown binding affinity also for myocardial amyloid, both in the context of a systemic amyloidosis with cardiac involvement and in the case of isolated CA.105–110 The most promising of these tracers is 18F-florbetaben, which has proven able not only to identify CA, but also to discriminate between AL-CA and other mimicking conditions and to evaluate the response to therapy (Figure 5).111,112
Cardiac CT
The latest generation of CCT equipment, with the implementation of temporal resolution, the use of lower kV values, high volume coverage, iterative reconstruction algorithms and low radiation exposure, allows the assessment of ventricular and atrial size, morphology, function, density,
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
A B C
Figure 4: Cardiac Magnetic Resonance in Arrhythmogenic Cardiomyopathy Right Ventricular Arrhythmogenic Cardiomyopathy, Manifesting with Right Ventricular Free Wall Hypo/Akinesia
A: Four-chamber steady-state free-precession diastolic frame. B: Subepicardial fatty infiltration at black-blood proton-density spin-echo imaging. C: Late gadolinium enhancement.
Figure 5: Nuclear Medicine in Cardiac Amyloidosis Scans from 18F-Florbetaben PET and from 99mTc-HDP Scintigraphy in a Patient with Light Chain Cardiac Amyloidosis
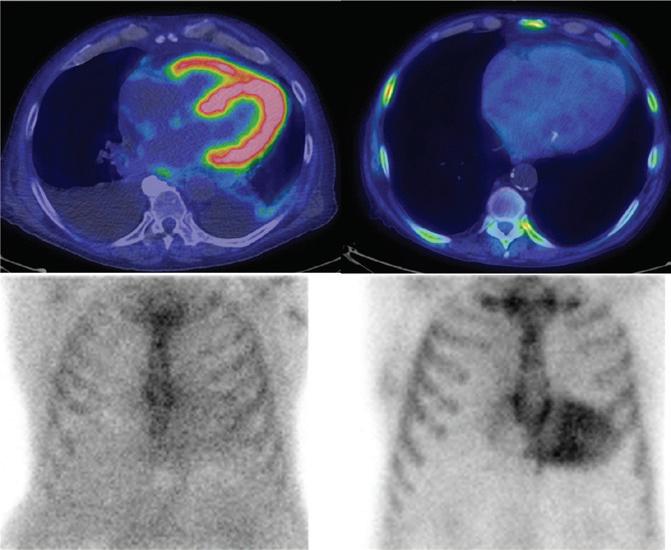
Further research is required to integrate imaging findings from different techniques with clinical data and circulating biomarkers to create a multifactorial algorithm for the diagnosis and prognostic assessment, as well as for the individualisation of therapy in each specific CMP.119
Perspectives
first pass perfusion defects and delayed-enhancement pattern in submillimetric multiplanar reconstructions with a short acquisition time.113 In the setting of CMPs, pre-contrast CCT can easily depict myocardial areas with adipose density as well as calcifications. After contrast enhancement, in addition to providing information on the causes of ischaemia and on the risk of major cardiac events, CCT can qualitatively and quantitatively assess myocardial perfusion and the presence of myocardial muscle alterations, discriminating between ischaemic and non-ischemic aetiology, disclosing myocardial phenotype, the presence of fat, as well as complications such as intracavitary thrombosis.114 115 CCT may therefore be considered as an option in patients with contraindications to CMR or when coronary artery disease must be ruled out.116 Next challenges will concern the evaluation of myocardial metabolism with CCT photon counting and the assessment of myocardial oedema and fibrosis.117 118
Multimodality Imaging and Integration with Circulating Biomarkers
The integration of different imaging techniques may help overcome the different limitations and weaknesses of single techniques.17,119 For example, echocardiographic findings may be non-specific, therefore, have a limited role in identifying the underlying aetiology.119 CMR is generally performed after echocardiography and has a primary role in assessing the aetiology of CMPs.120 CMR findings, namely LGE and parametric imaging (T1 and T2 values), allow an accurate characterisation of myocardial tissue in terms of fibrosis, scar, inflammation, oedema, or infiltration.119 120 CCT is highly valuable to exclude significant epicardial coronary artery disease in DCM.17 Additionally, the good spatial resolution and ease of navigation make cardiac CCT an option in the presence of suboptimal echocardiographic findings and contraindications to CMR.17
The integration of imaging with circulating biomarkers allows an even more accurate characterisation of the different types of CMPs.121 For example, although the role of nuclear imaging is generally limited in the evaluation of patients with suspected CMPs, ATTR-CA is diagnosed with a 100% specificity in the presence of a positive bone scintigraphy combined with the absence of a monoclonal protein or abnormal light chains in blood samples.122 In addition, specific imaging markers provide additional information to biomarkers and refine risk prediction in AL-CA.123
The growing body of knowledge on the pathophysiology and natural history of CMPs, and the availability of disease-modifying therapies for some conditions have made early diagnosis more crucial than ever. However, some CMPs may show milder phenotypes in early disease stages, which can overlap between different diseases or even physiological conditions. Emblematic cases are represented by DCM versus ACM with prevalent LV involvement, or HCM versus athlete’s heart.17,124 The integration of additional information from different imaging modalities can maximise the diagnostic performance.17 For inherited CMPs with adolescent-onset, such as HCM and some forms of ACM, early diagnosis presents additional challenges, particularly for family screening. First, the diagnostic criteria have been mostly validated on adult subjects with well-differentiated phenotypes, therefore, their diagnostic performance at a younger age may be lower.125 Second, echocardiography is the preferred tool for screening, but is unable to identify subtler structural abnormalities, so it currently remains to be defined which methods and timing are the most appropriate for family screening of inherited CMPs. For example, the 2014 ESC guidelines on the diagnosis and management of HCM recommend clinical evaluation with ECG and echocardiography and long-term follow-up in first-degree relatives who have the same defined pathological mutation as the proband; when no definite genetic mutation is identified in the proband, ECG and echocardiography should be considered in first-degree adult relatives and repeated every 2–5 years (or 6–12 months if nondiagnostic abnormalities are present).126 Similar recommendations are provided by the 2020 AHA/ACC Guidelines, which also mention the possibility of employing CMR for family screening when echocardiography is inconclusive.57 As for ACM, the 2019 Heart Rhythm Society (HRS) expert consensus statement recommends clinical evaluation by ECG and cardiac imaging every 1–3 years starting at 10–12 years of age in first-degree relatives. However, the precise imaging technique to be employed is not specified.127
Another future perspective is the validation of new diagnostic methods and their integration into the diagnostic flow charts of CMPs. CMR mapping techniques have proven to be very reliable in supporting the diagnosis of CA and predicting prognosis, yet CMR currently has a secondary role in the diagnostic flow chart of CA proposed by the ESC.2 In many cases of suspected CMP, endomyocardial biopsy is still crucial.128 However, in the near future, new imaging tools might limit the need for endomyocardial biopsy in specific cases. For example, recent studies have shown that delayed cardiac uptake of 18F-florbetaben on PET/CT imaging is able to accurately discriminate AL-CA from either ATTR-CA or other phenocopies.111
Besides diagnosis, imaging tools can be used to highlight collateral structural abnormalities of some CMPs that might have clinical relevance. For example, it has been demonstrated that some forms of ACM, such as those caused by DSP mutations, can have a clinical course that passes through “hot phases” characterised by myocardial inflammation and the release of biomarkers of myocardial damage, which sometimes are misdiagnosed as acute myocarditis.81 These phases hold prognostic significance and can be identified both by CMR as the presence of oedema T2-weighted sequences and by 18F-FDG PET.124 129 Myocardial
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
99mTc-HDP SPECT 18F-Florbetaben PET-CT AL-CA ATTR-CA
Myocardial uptake at PET imaging and negative bone-tracer scintigraphy, and in a patient with transthyretin cardiac amyloidosis, with negative PET scan and myocardial uptake at bone-tracer scintigraphy. AL-CA = immunoglobulin light-chain cardiac amyloidosis. ATTR-CA = amyloid transthyretin cardiac amyloidosis.
oedema detected through CMR can also be a hallmark of cardiac remodelling in HCM, and is associated with more advanced disease and increased arrhythmic load.61
Finally, the role of advanced imaging modalities in monitoring the response to therapy remains to be defined. One study on patients with ATTR-CA has demonstrated that serial ECV quantification by CMR can be used to monitor the effectiveness of reducing the cardiac amyloid burden by patisiran, a small interfering RNA able to knock down the expression of transthyretin in the liver.71 Similarly, it has been recently demonstrated that ECV quantification by CMR can be used to monitor cardiac amyloid regression following chemotherapy in AL amyloidosis. Notably, a deep haematologic response is not sufficient per se to grant cardiac amyloid regression, while changes in ECV over time independently predict prognosis, making it a unique marker of treatment response.130
1. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–6. https://doi. org/10.1093/eurheartj/ehm342; PMID: 17916581.
2. Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021;23:512–26. https://doi.org/10.1002/ejhf.2140; PMID: 33826207.
3. Casas G, Rodríguez-Palomares JF. Multimodality cardiac imaging in cardiomyopathies: from diagnosis to prognosis. J Clin Med 2022;11:578. https://doi.org/10.3390/jcm11030578; PMID: 35160031.
4. Rankin K, Thampinathan B, Thavendiranathan P. Imagingspecific cardiomyopathies a practical guide. Heart Fail Clin 2019;15:275–95. https://doi.org/10.1016/j.hfc.2018.12.007; PMID: 30832818.
5. Cardim N, Galderisi M, Edvardsen T, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 2015;16:280. https://doi.org/10.1093/ ehjci/jeu291; PMID: 25650407.
6. Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2022;35:533–69. https://doi.org/10.1016/j.echo.2022.03.012; PMID: 35659037.
7. Lancellotti P, Cosyns B, eds. The EACVI echo handbook Oxford: Oxford University Press, 2016.
8. Chockalingam A, Xie GY, Dellsperger KC. Echocardiography in stress cardiomyopathy and acute LVOT obstruction. Int J Cardiovasc Imaging 2010;26:527–35. https://doi.org/10.1007/ s10554-010-9590-7; PMID: 20119847.
9. Cortigiani L, Rigo F, Gherardi S, et al. Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol 2008;102:1718–23. https://doi.org/10.1016/j. amjcard.2008.08.023; PMID: 19064030.
10. Habib G, Bucciarelli-Ducci C, Caforio ALP, et al. Multimodality imaging in restrictive cardiomyopathies: an EACVI expert consensus document in collaboration with the “Working Group on Myocardial and Pericardial Diseases” of the European Society of Cardiology endorsed by the Indian Academy of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:1090–121. https://doi.org/10.1093/ehjci/jex034;
PMID: 28510718.
11. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. https://doi.org/10.1016/j.echo.2016.01.011;
PMID: 27037982.
12. Haugaa KH, Basso C, Badano LP, et al. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy – an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–53. https://doi.org/10.1093/
Conclusion
Recent advances in cardiac imaging and its increased accessibility allow a precise assessment of ventricular dimension and function, as well as a non-invasive tissue characterisation of the myocardium. However, the growing knowledge from imaging studies should always be interpreted based on all clinical elements (i.e. clinical assessment, electrocardiographic findings, biomarkers, genetic and functional assessment). While echocardiography remains the first-line imaging tool for the management of patients with CMP and can be implemented with advanced analyses (e.g. STE, stress echocardiography, contrast-enhanced echocardiography), CMR represents the gold standard technique for myocardial anatomy and tissue characterisation, including novel tools (e.g. T1/T2/T2*/ECV mapping, quantitative perfusion, diffusion tension imaging and feature tracking). An integrated clinical and imaging approach seems essential to characterise the CMP phenotype and identify the underlying aetiologies, to predict disease prognosis and to ensure a tailored therapeutic management.
ehjci/jew229; PMID: 28069601.
13. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–14. https://doi.org/10.1093/eurheartj/ehq025; PMID: 20172912.
14. Mansencal N, Pellerin D, Lamar A, et al. Diagnostic value of contrast echocardiography in Tako-Tsubo cardiomyopathy. Arch Cardiovasc Dis 2010;103:447–53. https://doi.org/10.1016/j. acvd.2010.08.001; PMID: 21074123.
15. Nunes MCP, Badano LP, Marin-Neto JA, et al. Multimodality imaging evaluation of Chagas disease: an expert consensus of Brazilian Cardiovascular Imaging Department (DIC) and the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging 2018;19:459–460n. https://doi.org/10.1093/ehjci/jex154; PMID: 29029074.
16. Viotti RJ, Vigliano C, Laucella S, et al. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart 2004;90:655–60. https://doi.org/10.1136/hrt.2003.018960; PMID: 15145872.
17. Donal E, Delgado V, Bucciarelli-Ducci C, et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2019;20:1075–93. https://doi.org/10.1093/ehjci/jez178; PMID: 31504368.
18. Paraskevaidis IA, Panou F, Papadopoulos C, et al. Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: a tissue Doppler imaging and two-dimensional strain study. Heart 2009;95:483–9. https:// doi.org/10.1136/hrt.2008.146548; PMID: 18765436.
19. Heggemann F, Hamm K, Brade J, et al. Right ventricular function quantification in takotsubo cardiomyopathy using two-dimensional strain echocardiography. PLoS One 2014;9:e103717. https://doi.org/10.1371/journal.pone.0103717; PMID: 25089702.
20. Aimo A, Fabiani I, Giannoni A, et al. Multi-chamber speckle tracking imaging and diagnostic value of left atrial strain in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2022;24:130–41. https://doi.org/10.1093/ehjci/jeac057; PMID: 35292807.
21. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine. Eur Heart J Cardiovasc Imaging 2017;18:1073–89. https://doi.org/10.1093/ehjci/jex146; PMID: 28984894.
22. Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement – executive summary. Eur Heart J 2009;30:278–89. https://doi. org/10.1093/eurheartj/ehn492; PMID: 19001473.
23. Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007;20:1021–41. https://doi. org/10.1016/j.echo.2007.07.003; PMID: 17765820.
24. Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2008;9:415–37. https://doi. org/10.1093/ejechocard/jen175; PMID: 18579481.
25. Naqvi TZ, Goel RK, Forrester JS, Siegel RJ. Myocardial contractile reserve on dobutamine echocardiography predicts late spontaneous improvement in cardiac function in patients with recent onset idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1999;34:1537–44. https:// doi.org/10.1016/s0735-1097(99)00371-x; PMID: 10551704.
26. Lee JH, Yang DH, Choi WS, et al. Prediction of improvement in cardiac function by high dose dobutamine stress echocardiography in patients with recent onset idiopathic dilated cardiomyopathy. Int J Cardiol 2013;167:1649–50. https://doi.org/10.1016/j.ijcard.2012.11.021; PMID: 23312406.
27. Grigoratos C, Todiere G, Barison A, Aquaro GD. The role of MRI in prognostic stratification of cardiomyopathies. Curr Cardiol Rep 2020;22:61. https://doi.org/10.1007/s11886-02001311-3; PMID: 32562090.
28. Merlo M, Gagno G, Baritussio A, et al. Clinical application of CMR in cardiomyopathies: evolving concepts and techniques: a position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail Rev 2023;28:77–95. https://doi.org/10.1007/s10741-022-10235-9; PMID: 35536402.
29. Barison A, Baritussio A, Cipriani A, et al. Cardiovascular magnetic resonance: what clinicians should know about safety and contraindications. Int J Cardiol 2021;331:322–8. https://doi.org/10.1016/j.ijcard.2021.02.003; PMID: 33571560.
30. Barison A, Grigoratos C, Todiere G, Aquaro GD. Myocardial interstitial remodelling in non-ischaemic dilated cardiomyopathy: insights from cardiovascular magnetic resonance. Heart Fail Rev 2015;20:731–49. https://doi. org/10.1007/s10741-015-9509-4; PMID: 26423909.
31. Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977–85. https:// doi.org/10.1016/j.jacc.2006.07.049; PMID: 17112987.
32. Di Marco A, Anguera I, Schmitt M, et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy. JACC Heart Fail 2017;5:28–38. https://doi.org/10.1016/j.jchf.2016.09.017; PMID: 28017348.
33. Guaricci AI, Masci PG, Muscogiuri G, et al. Cardiac magnetic resonance for prophylactic implantable-cardioverter defibrillator therapy in non-ischaemic dilated cardiomyopathy: an international registry. Europace 2021;23:1072–83. https://doi.org/10.1093/europace/euaa401; PMID: 33792661.
34. Halliday BP, Baksi AJ, Gulati A, et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 2019;12:1645–55. https://doi.org/10.1016/j.jcmg.2018.07.015; PMID: 30219397.
35. Barison A, Aimo A, Mirizzi G, et al. The extent and location of late gadolinium enhancement predict defibrillator shock and cardiac mortality in patients with non-ischaemic dilated cardiomyopathy. Int J Cardiol 2020;307:180–6. https://doi. org/10.1016/j.ijcard.2020.02.028; PMID: 32067833.
36. Ikeda Y, Inomata T, Fujita T, et al. Cardiac fibrosis detected by magnetic resonance imaging on predicting time course diversity of left ventricular reverse remodeling in patients with idiopathic dilated cardiomyopathy. Heart Vessels 2016;31:1817–25. https://doi.org/10.1007/s00380-016-08052; PMID: 26843195.
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
37. Barison A, Aimo A, Ortalda A, et al. Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non-ischemic dilated cardiomyopathy. Int J Cardiol 2018;250:195–200. https://doi.org/10.1016/j.ijcard.2017.10.043; PMID: 29107357.
38. Leyva F, Foley PW, Chalil S, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13:29. https://doi.org/10.1186/1532-429X-13-29; PMID: 21668964.
39. Taylor RJ, Umar F, Panting JR, et al. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: a feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm 2016;13:481–9. https://doi.org/10.1016/j.hrthm.2015.10.024; PMID: 26498258.
40. Puntmann VO, Carr-White G, Jabbour A, et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging 2016;9:40–50. https://doi.org/10.1016/j.jcmg.2015.12.001; PMID: 26762873.
41. Barison A, Del Torto A, Chiappino S, et al. Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2015;16:681–7. https://doi.org/10.2459/ JCM.0000000000000275; PMID: 26090916.
42. Vita T, Gräni C, Abbasi SA, et al. Comparing CMR mapping methods and myocardial patterns toward heart failure outcomes in nonischemic dilated cardiomyopathy. JACC Cardiovasc Imaging 2019;12:1659–69. https://doi.org/10.1016/j. jcmg.2018.08.021; PMID: 30448130.
43. Buss SJ, Breuninger K, Lehrke S, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:307–15. https://doi.org/10.1093/ehjci/jeu181; PMID: 25246506.
44. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101–5. https://doi.org/10.1016/j.jacc.2005.03.045; PMID: 15992642.
45. Jacquier A, Thuny F, Jop B, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular noncompaction. Eur Heart J 2010;31:1098–104. https://doi. org/10.1093/eurheartj/ehp595; PMID: 20089517.
46. Grothoff M, Pachowsky M, Hoffmann J, et al. Value of cardiovascular MR in diagnosing left ventricular noncompaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 2012;22:2699–709. https://doi.org/10.1007/s00330-012-2554-7; PMID: 22772366.
47. Grigoratos C, Barison A, Ivanov A, et al. Meta-analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc Imaging 2019;12:2141–51. https://doi.org/10.1016/j. jcmg.2018.12.029; PMID: 30878415.
48. Macaione F, Meloni A, Positano V, et al. The prognostic role of CMR using global planimetric criteria in patients with excessive left ventricular trabeculation. Eur Radiol 2021;31:7553–65. https://doi.org/10.1007/s00330-02107875-0; PMID: 33821336.
49. Maron MS, Rowin EJ, Maron BJ. How to image hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2017;10:e005372. https://doi.org/10.1161/CIRCIMAGING.116.005372; PMID: 28701526.
50. Harrigan CJ, Appelbaum E, Maron BJ, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2008;101:668–73. https://doi.org/10.1016/j. amjcard.2007.10.032; PMID: 18308018.
51. Captur G, Lopes LR, Mohun TJ, et al. Prediction of sarcomere mutations in subclinical hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2014;7:863–71. https://doi.org/10.1161/CIRCIMAGING.114.002411; PMID: 25228707.
52. Reant P, Captur G, Mirabel M, et al. Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2015;17:64. https://doi.org/10.1186/s12968-015-0160-y;
PMID: 26219660.
53. Aquaro GD, Masci P, Formisano F, et al. Usefulness of delayed enhancement by magnetic resonance imaging in hypertrophic cardiomyopathy as a marker of disease and its severity. Am J Cardiol 2010;105:392–7. https://doi. org/10.1016/j.amjcard.2009.09.045; PMID: 20102955.
54. Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic
resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484–95. https://doi.org/10.1161/ CIRCULATIONAHA.113.007094; PMID: 25092278.
55. Todiere G, Nugara C, Gentile G, et al. Prognostic role of late gadolinium enhancement in patients with hypertrophic cardiomyopathy and low-to-intermediate sudden cardiac death risk score. Am J Cardiol 2019;124:1286–92. https://doi. org/10.1016/j.amjcard.2019.07.023; PMID: 31447011.
56. Freitas P, Ferreira AM, Arteaga-Fernández E, et al. The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson 2019;21:50. https://doi.org/10.1186/s12968-019-0561-4; PMID: 31412875.
57. Ommen SR, Mital S, Burke MA, et al. AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2020;76:3022–55. https:// doi.org/10.1016/j.jacc.2020.08.044; PMID: 33229115.
58. Aquaro GD, Grigoratos C, Bracco A, et al. Late gadolinium enhancement–dispersion mapping: a new magnetic resonance imaging technique to assess prognosis in patients with hypertrophic cardiomyopathy and lowintermediate 5-year risk of sudden death. Circ Cardiovasc Imaging 2020;13:e010489. https://doi.org/10.1161/ CIRCIMAGING.120.010489; PMID: 32539460.
59. Sánchez-Somonte P, Quinto L, Garre P, et al. Scar channels in cardiac magnetic resonance to predict appropriate therapies in primary prevention. Heart Rhythm 2021;18:1336–43. https://doi.org/10.1016/j.hrthm.2021.04.017; PMID: 33892202.
60. Negri F, Muser D, Driussi M, et al. Prognostic role of global longitudinal strain by feature tracking in patients with hypertrophic cardiomyopathy: the STRAIN-HCM study. Int J Cardiol 2021;345:61–7. https://doi.org/10.1016/j. ijcard.2021.10.148; PMID: 34728259.
61. Todiere G, Pisciella L, Barison A, et al. Abnormal T2-STIR magnetic resonance in hypertrophic cardiomyopathy: a marker of advanced disease and electrical myocardial instability. PLoS One 2014;9:e111366. https://doi.org/10.1371/ journal.pone.0111366; PMID: 25356653.
62. Avanesov M, Münch J, Weinrich J, et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol 2017;27:5136–45. https://doi.org/10.1007/s00330-0174869-x; PMID: 28616729.
63. Li Y, Liu X, Yang F, et al. Prognostic value of myocardial extracellular volume fraction evaluation based on cardiac magnetic resonance T1 mapping with T1 long and short in hypertrophic cardiomyopathy. Eur Radiol 2021;31:4557–67. https://doi.org/10.1007/s00330-020-07650-7; PMID: 33449190.
64. Treibel TA, Fridman Y, Bering P, et al. Extracellular volume associates with outcomes more strongly than native or postcontrast myocardial T1. JACC Cardiovasc Imaging 2020;13:44–54. https://doi.org/10.1016/j.jcmg.2019.03.017; PMID: 31103587.
65. Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging 2020;13:1368–83. https://doi.org/10.1016/j.jcmg.2019.07.015; PMID: 31607664.
66. Baggiano A, Boldrini M, Martinez-Naharro A, et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:69–80. https://doi.org/10.1016/j.jcmg.2019.03.026; PMID: 31202744.
67. Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging 2020;13:1299–310. https://doi. org/10.1016/j.jcmg.2020.03.010; PMID: 32498919.
68. Banypersad SM, Fontana M, Maestrini V, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J 2015;36:244–51. https://doi.org/10.1093/eurheartj/ehu444; PMID: 25411195.
69. Martinez-Naharro A, Treibel TA, Abdel-Gadir A, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017;70:466–77. https://doi.org/10.1016/j. jacc.2017.05.053; PMID: 28728692.
70. Martinez-Naharro A, Abdel-Gadir A, Treibel TA, et al. CMRverified regression of cardiac AL amyloid after chemotherapy. JACC Cardiovasc Imaging 2018;11:152–4. https://doi.org/10.1016/j.jcmg.2017.02.012; PMID: 28412427.
71. Fontana M, Martinez-Naharro A, Chacko L, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging 2021;14:189–99. https://doi.org/10.1016/j.jcmg.2020.07.043;
PMID: 33129740.
72. Sado DM, White SK, Piechnik SK, et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging 2013;6:392–8. https://doi.org/10.1161/ CIRCIMAGING.112.000070; PMID: 23564562.
73. Sado DM, Maestrini V, Piechnik SK, et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J Magn Reson Imaging 2015;41:1505–11. https://doi.org/10.1002/jmri.24727; PMID: 25104503.
74. Meloni A, Martini N, Positano V, et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: a sensitive approach that correlates with cardiac complications. J Cardiovasc Magn Reson 2021;23:70. https://doi.org/10.1186/s12968-021-00765-w; PMID: 34120634.
75. Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res 2017;121:784–802. https://doi. org/10.1161/CIRCRESAHA.117.309345; PMID: 28912183.
76. Corrado D, Perazzolo Marra M, Zorzi A, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–14. https://doi.org/10.1016/j. ijcard.2020.06.005; PMID: 32561223.
77. Augusto JB, Eiros R, Nakou E, et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. Eur Heart J Cardiovasc Imaging 2020;21:326–36. https://doi. org/10.1093/ehjci/jez188; PMID: 31317183.
78. Aquaro GD, De Luca A, Cappelletto C, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2020;75:2753–65. https://doi.org/10.1016/j. jacc.2020.04.023; PMID: 32498802.
79. Aquaro GD, Barison A, Todiere G, et al. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2016;118:1730–6. https://doi. org/10.1016/j.amjcard.2016.08.056; PMID: 27825581.
80. Tandri H, Castillo E, Ferrari VA, et al. Magnetic resonance imaging of arrhythmogenic right ventricular dysplasia: sensitivity, specificity, and observer variability of fat detection versus functional analysis of the right ventricle. J Am Coll Cardiol 2006;48:2277–84. https://doi.org/10.1016/j. jacc.2006.07.051; PMID: 17161260.
81. Bariani R, Cipriani A, Rizzo S, et al. ‘Hot phase’ clinical presentation in arrhythmogenic cardiomyopathy. Europace 2021;23:907–17. https://doi.org/10.1093/europace/euaa343; PMID: 33313835.
82. Smith ED, Lakdawala NK, Papoutsidakis N, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872–84. https://doi.org/10.1161/ CIRCULATIONAHA.119.044934; PMID: 32372669.
83. Germano G, Kiat H, Kavanagh PB, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995;36:2138–47. PMID: 7472611
84. Gimelli A, Liga R, Pasanisi EM, et al. Evaluation of left ventricular diastolic function with a dedicated cadmiumzinc-telluride cardiac camera: comparison with Doppler echocardiography. Eur Heart J Cardiovasc Imaging 2014;15:972–9. https://doi.org/10.1093/ehjci/jeu037; PMID: 24618658.
85. Koyanagawa K, Naya M, AIkawa T, et al. Prognostic value of phase analysis on gated single photon emission computed tomography in patients with cardiac sarcoidosis. J Nucl Cardiol 2021;28:128–36. https://doi.org/10.1007/s12350-01901660-9; PMID: 30815835.
86. Koyanagawa K, Naya M, AIkawa T, et al. The rate of myocardial perfusion recovery after steroid therapy and its implication for cardiac events in cardiac sarcoidosis and primarily preserved left ventricular ejection fraction. J Nucl Cardiol 2021;28:1745–56. https://doi.org/10.1007/s12350-01901916-4; PMID: 31605274.
87. Imbriaco M, Pellegrino T, Piscopo V, et al. Cardiac sympathetic neuronal damage precedes myocardial fibrosis in patients with Anderson-Fabry disease. Eur J Nucl Med Mol Imaging 2017;44:2266–73. https://doi.org/10.1007/s00259017-3778-1; PMID: 28733764.
88. Gimelli A, Aimo A, Vergaro G, et al. Cardiac sympathetic denervation in wild-type transthyretin amyloidosis. Amyloid 2020;27:237–43. https://doi.org/10.1080/13506129.2020.176 9059; PMID: 32441155.
89. Noordzij W, Glaudemans AWJM, van Rheenen RWJ, et al. 123I-labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis.
Imaging in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
Eur J Nucl Med Mol Imaging 2012;39:1609–17. https://doi. org/10.1007/s00259-012-2187-8; PMID: 22806059.
90. Hoitsma E, Faber CG, Van Kroonenburgh MJ, et al. Association of small fiber neuropathy with cardiac sympathetic dysfunction in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:43–50. PMID: 15881279.
91. Imai E, Kaminaga T, Takada K, et al. Radioactive defect on I-123 MIBG myocardial SPECT imaging in a patient with cardiac sarcoidosis. Clin Nucl Med 2002;27:729–30. https:// doi.org/10.1097/00003072-200210000-00011; PMID: 12352118.
92. Soussan M, Brillet PY, Nunes H, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol 2013;20:120–7. https://doi.org/10.1007/s12350012-9653-3; PMID: 23188627.
93. Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 2017;24:86–99. https://doi.org/10.1007/s12350-016-0502-7; PMID: 27277502.
94. Kobayashi Y, Kumita S, Fukushima Y, et al. Significant suppression of myocardial 18F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet. J Cardiol 2013;62:314–9. https://doi. org/10.1016/j.jjcc.2013.05.004; PMID: 23810066.
95. Wollenweber T, Roentgen P, Schäfer A, et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging 2014;7:811–8. https://doi.org/10.1161/ CIRCIMAGING.114.001689; PMID: 25049056.
96. Moriwaki K, Dohi K, Omori T, et al. A survival case of fulminant right-side dominant eosinophilic myocarditis. Int Heart J 2017;58:459–62. https://doi.org/10.1536/ihj.16-338; PMID: 28496024.
97. Nensa F, Kloth J, Tezgah E, et al. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol 2018;25:785–94. https://doi.org/10.1007/s12350016-0616-y; PMID: 27638745.
98. Ning N, Guo HH, Iagaru A, et al. Serial cardiac FDG-PET for the diagnosis and therapeutic guidance of patients with cardiac sarcoidosis. J Card Fail 2019;25:307–11. https://doi. org/10.1016/j.cardfail.2019.02.018; PMID: 30825644.
99. Saric P, Young KA, Rodriguez-Porcel M, Chareonthaitawee P. PET imaging in cardiac sarcoidosis: a narrative review with focus on novel PET tracers. Pharmaceuticals (Basel) 2021;14:1286. https://doi.org/10.3390/ph14121286; PMID: 34959686.
100. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/ HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2 – evidence base and standardized methods of imaging. J Nucl Cardiol 2019;26:2065–123. https://doi.org/10.1007/ s12350-019-01760-6; PMID: 31468376.
101. Trivieri MG, Dweck MR, Abgral R, et al. 18F-sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 2016;68:2712–4. https://doi.org/10.1016/j. jacc.2016.09.953; PMID: 27978955.
102. Morgenstern R, Yeh R, Castano A, et al. 18Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol 2018;25:1559–67. https://doi.org/10.1007/s12350-017-0799-x; PMID: 28176254.
103. Ng QKT, Sethi P, Saunders TA, et al. Discordant findings on 18F-NaF and 99mTc-HDP bone scans in a patient with ATTR
cardiac amyloidosis. Clin Nucl Med 2018;43:e89–92. https:// doi.org/10.1097/RLU.0000000000001933; PMID: 29261619.
104. Zhang LX, Martineau P, Finnerty V, et al. Comparison of 18F-sodium fluoride positron emission tomography imaging and 99mTc-pyrophosphate in cardiac amyloidosis. J Nucl Cardiol 2022;29:1132–40. https://doi.org/10.1007/s12350020-02425-5; PMID: 33146862.
105. Dorbala S, Vangala D, Semer J, et al. Imaging cardiac amyloidosis: a pilot study using ¹⁸F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41:1652–62. https://doi.org/10.1007/s00259-014-27876; PMID: 24841414.
106. Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010;68:319–29. https://doi.org/10.1002/ana.22068; PMID: 20687209.
107. Lhommel R, Sempoux C, Ivanoiu A, et al. Is 18F-flutemetamol PET/CT able to reveal cardiac amyloidosis? Clin Nucl Med 2014;39:747–9. https://doi.org/10.1097/ RLU.0000000000000492; PMID: 24978329.
108. Dietemann S, Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: a pilot study using 18F-flutemetamol positron emission tomography. Ann Nucl Med 2019;33:624–8. https:// doi.org/10.1007/s12149-019-01372-7; PMID: 31140154.
109. Möckelind S, Axelsson J, Pilebro B, et al. Quantification of cardiac amyloid with [18F]flutemetamol in patients with V30M hereditary transthyretin amyloidosis. Amyloid 2020;27:191–9. https://doi.org/10.1080/13506129.2020.1760237; PMID: 32400202.
110. Law WP, Wang WY, Moore PT, et al. Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J Nucl Med 2016;57:1733–9. https://doi.org/10.2967/jnumed.115.169870; PMID: 27307344.
111. Genovesi D, Vergaro G, Giorgetti A, et al. Florbetaben PET/ CT for differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging 2021;14:246–55. https:// doi.org/10.1016/j.jcmg.2020.05.031; PMID: 32771577.
112. Kircher M, Ihne S, Brumberg J, et al. Detection of cardiac amyloidosis with 18F-florbetaben-PET/CT in comparison to echocardiography, cardiac MRI ad DPD-scintigraphy. Eur J Nucl Med Mol Imaging 2019;46:1407–16. https://doi. org/10.1007/s00259-019-04290-y; PMID: 30798427.
113. Cademartiri F, Clemente A, Nistri S, Maffei E. Cardiac computed tomography as a complete functional tool. Eur Heart J Cardiovasc Imaging 2022;23:485–6. https://doi. org/10.1093/ehjci/jeab288; PMID: 34986224.
114. Seitun S, Clemente A, De Lorenzi C, et al. Cardiac CT perfusion and FFRCTA: pathophysiological features in ischemic heart disease. Cardiovasc Diagn Ther 2020;10:1954–78. https://doi.org/10.21037/cdt-20-414; PMID: 33381437.
115. Aziz W, Claridge S, Ntalas I, et al. Emerging role of cardiac computed tomography in heart failure. ESC Heart Fail 2019;6:909–20. https://doi.org/10.1002/ehf2.12479; PMID: 31400060.
116. Lee HJ, Im DJ, Youn JC, et al. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: a prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2017;33:577–84. https://doi. org/10.1007/s10554-016-1024-8; PMID: 27873128.
117. Dewey M, Siebes M, Kachelrieß M, et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat Rev Cardiol 2020;17:427–50. https://doi.
org/10.1038/s41569-020-0341-8; PMID: 32094693.
118. Scully PR, Bastarrika G, Moon JC, Treibel TA. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep 2018;20:15. https://doi.org/10.1007/s11886-018-0961-3; PMID: 29511861.
119. Ederhy S, Mansencal N, Réant P, et al. Role of multimodality imaging in the diagnosis and management of cardiomyopathies. Arch Cardiovasc Dis 2019;112:615–29. https://doi.org/10.1016/j.acvd.2019.07.004; PMID: 31607558.
120. Mitropoulou P, Georgiopoulos G, Figliozzi S, et al. Multimodality imaging in dilated cardiomyopathy: with a focus on the role of cardiac magnetic resonance. Front Cardiovasc Med 2020;7:97. https://doi.org/10.3389/fcvm.2020.00097; PMID: 32714942.
121. Schulz-Menger J, Maisch B, Abdel-Aty H, Pankuweit S. Integrated biomarkers in cardiomyopathies: cardiovascular magnetic resonance imaging combined with molecular and immunologic markers – a stepwise approach for diagnosis and treatment. Herz 2007;32:458–72. https://doi. org/10.1007/s00059-007-3046-4; PMID: 17882371.
122. Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging 2011;38:470–8. https://doi.org/10.1007/s00259-010-1642-7; PMID: 21069320.
123. Salinaro F, Meier-Ewert HK, Miller EJ, et al. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2017;18:1057–64. https://doi. org/10.1093/ehjci/jew298; PMID: 27965280.
124. Galderisi M, Cardim N, D’Andrea A, et al. The multi-modality cardiac imaging approach to the athlete’s heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:353. https:// doi.org/10.1093/ehjci/jeu323; PMID: 25681828.
125. Malik N, Mukherjee M, Wu KC, et al. Multimodality imaging in arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging 2022;15:e013725. https://doi.org/10.1161/ CIRCIMAGING.121.013725; PMID: 35147040.
126. Authors/Task Force members, Elliott PM, Anastasakis A, et al.2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. https://doi.org/10.1093/ eurheartj/ehu284; PMID: 25173338.
127. Towbin JA, McKenna WJ, Abrams DJ, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm 2019;16:e373–407. https://doi. org/10.1016/j.hrthm.2019.09.019; PMID: 31676023.
128. Porcari A, Baggio C, Fabris E, et al. Endomyocardial biopsy in the clinical context: current indications and challenging scenarios. Heart Fail Rev 2023;28:123–35. https://doi. org/10.1007/s10741-022-10247-5; PMID: 35567705.
129. Wang W, Murray B, Tichnell C, et al. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. Europace 2022;24:268–77. https://doi.org/10.1093/europace/ euab183; PMID: 34352074.
130. Protonotarios A, Wicks E, Ashworth M, et al. Prevalence of 18F-fluorodeoxyglucose positron emission tomography abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol 2019;284:99–104. https://doi.org/10.1016/j.ijcard.2018.10.083; PMID: 30409737.
in Cardiomyopathies CARDIAC FAILURE REVIEW www.CFRjournal.com
Imaging
Pathophysiological Rationale
Abstract
and Clinical
Evidence
for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome resulting from the interaction between cardiac diseases, comorbidities and ageing. HFpEF is characterised by the activation of neurohormonal axes, namely of the renin-angiotensin-aldosterone system and the sympathetic nervous system, although to a lesser extent compared with heart failure with reduced ejection fraction. This provides a rationale for neurohormonal modulation as a therapeutic approach for HFpEF. Nonetheless, randomised clinical trials have failed to demonstrate a prognostic benefit from neurohormonal modulation therapies in HFpEF, with the sole exception of patients with left ventricular ejection fraction in the lower range of normality, for whom the American guidelines suggest that such therapies may be considered. In this review, the pathophysiological rationale for neurohormonal modulation in HFpEF is summarised and the clinical evidence on pharmacological and nonpharmacological approaches backing current recommendations discussed.
Keywords
Heart failure with preserved ejection fraction, neurohormonal modulation, β-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, mineralocorticoid receptor antagonist, sacubitril/valsartan, soluble guanylate cyclase stimulator.
Disclosure: The authors have no conflicts of interest to declare.
Received: 15 September 2022 Accepted: 2 March 2023 Citation: Cardiac Failure Review 2023;9:e09. DOI: https://doi.org/10.15420/cfr.2022.23
Correspondence: Michele Emdin, Scuola Superiore Sant’Anna, Via G. Moruzzi 1, 56124, Pisa, Italy. E: michele.emdin@santannapisa.it
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure (HF) with preserved ejection fraction (HFpEF) is defined as ‘a clinical syndrome characterised by prior or current symptoms and/or signs secondary to a cardiac structural or functional abnormality, corroborated by the presence of elevated natriuretic peptides (NPs) levels or objective evidence of cardiogenic pulmonary or systemic congestion’ in the presence of a left ventricular (LV) ejection fraction (LVEF) ≥50%.1
While the prevalence of HF with reduced ejection fraction (HFrEF) (LVEF ≤40%) is stable or decreasing, that of HFpEF is rising worldwide, probably due to various factors, including the increased awareness of clinicians in recognising this HF phenotype; the ageing population; and the increasing number of patients with HF and improved LVEF (i.e. patients with a prior diagnosis of HFrEF whose LVEF improves over time mostly because of guideline-directed medical therapy).2 3 Compared with patients with other types of LVEF, those with HFpEF have similar rehospitalisation and mortality risks, albeit with a higher proportion of non-cardiac causes, which highlights the role of comorbidities.2–5
Therapies targeting the neurohormonal systems, such as β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), mineralocorticoid receptor antagonists (MRAs) and angiotensin receptor-neprilysin inhibitors (ARNIs) are the mainstay of treatment for HFrEF and HF with mildly reduced ejection fraction (EF).
Moreover, it has been observed that, although to a lesser extent than in patients with HFrEF, those with HFpEF show a significant activation of neurohormonal systems, further supporting a potential benefit from their modulation.6 However, in the last two decades, various randomised clinical trials (RCTs) have failed to demonstrate a significant prognostic benefit of neurohormonal modulation therapies in HFpEF.7 The phenotypical and pathophysiological heterogeneity of HFpEF, together with flaws in study design, might partly explain such disappointing results.8 Nonetheless, subgroup and post-hoc analyses of RCTs suggest that some subsets of HFpEF patients (e.g. those with LVEF in the lower range of normality) might derive a benefit from those therapies.7
This review explains the pathophysiological rationale of neurohormonal modulation in HFpEF, the results of clinical trials and guideline recommendations as well as future perspectives on optimising the use of neurohormonal modulation therapies in this clinical scenario.
Pathophysiology of HFpEF
HFpEF derives from the interaction of various cardiac and extracardiac conditions, resulting in a heterogeneous phenotypic spectrum (Figure 1).2
At the cardiac level, increased LV filling pressures are a landmark finding in HFpEF patients, being responsible for HF signs and symptoms.9 Although diastolic dysfunction secondary to increased LV stiffness and
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Treatment
Vincenzo Castiglione , 1,2 Francesco Gentile , 2 Nicolò Ghionzoli , 3 Martina Chiriacò , 4 Giorgia Panichella , 1 Alberto Aimo , 1,5 Giuseppe Vergaro , 1,5 Alberto Giannoni , 1,5 Claudio Passino 1,5 and Michele Emdin 1,5
1. Interdisciplinary Research Center Health Science, Scuola Superiore Sant’Anna, Pisa, Italy; 2. Cardiology Division, Pisa University Hospital, Pisa, Italy; 3. Department of Medical Biotechnologies, Division of Cardiology, University of Siena, Siena, Italy; 4. Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy; 5. Fondazione Toscana Gabriele Monasterio, Pisa, Italy
Approaches for Neurohormonal Modulation

Several cardiovascular and non-cardiovascular factors contribute to the development of heart failure with preserved ejection fraction (HFpEF) syndrome. Albeit to a lower degree compared to heart failure with reduced ejection fraction, HFpEF is characterised by neurohormonal activation along with its cardiovascular and renal consequences. Some of the possible pharmacological strategies for neurohormonal modulation and the corresponding therapeutic targets in HFpEF are reported. ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor/neprilysin inhibitor; COPD = chronic obstructive pulmonary disease; CSR = Cheyne-Stokes respiration; HCM = hypertrophic cardiomyopathy; HFpEF = heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonists; OSA = obstructive sleep apnoea.
impaired relaxation usually plays a major role, subtle systolic dysfunction can also be found in many patients through strain analysis.10
Historically, systemic hypertension has been considered the main cause of diastolic dysfunction in HFpEF, being associated with LV concentric hypertrophy and increased myocardial stiffness.11 Nevertheless, growing evidence suggests that microvascular dysfunction and cardiomyocyte metabolic/energetic inefficiency may also play a role.12,13


Finally, infiltrative disorders (e.g. cardiac amyloidosis) or other determinants of myocardial damage (e.g. related to valvular, metabolic, toxic and inflammatory conditions) can cause diastolic dysfunction in specific patient subsets.14–17 Left atrial enlargement is common in HFpEF patients, and has long been considered a direct consequence of increased LV filling pressures.18 However, as recently disclosed, left atrial dysfunction is often observed in HFpEF from an early stage, and directly contributes to disease progression.18 Similarly, post-capillary pulmonary hypertension and right ventricular dysfunction can be observed in some HFpEF patients.19 20
Compared with HFrEF, extracardiac comorbidities seem to be a major determinant in HFpEF pathophysiology.5 For example, obesity, diabetes and chronic kidney disease are highly prevalent in patients with HFpEF, promoting cardiovascular damage by activating proinflammatory and profibrotic cascades.21–23
Similarly, respiratory disorders (e.g. chronic pulmonary obstructive disease, obstructive sleep apnoeas and Cheyne-Stokes respiration) may
trigger inflammatory and oxidant pathways as well as autonomic imbalance and pulmonary hypertension.23 24
Rationale for Neurohormonal Modulation in HFpEF
Both cardiovascular (e.g. hypertension, microvascular disease and cardiomyopathies) and non-cardiovascular (e.g. diabetes, renal disease and respiratory disorders) determinants of HFpEF are associated with increased activity of the renin-angiotensin-aldosterone system (RAAS) and autonomic imbalance, characterised by an enhanced sympathetic drive and vagal withdrawal. Nevertheless, compared with HFrEF, the pathophysiological role and clinical implications of neurohormonal activation have been poorly investigated in HFpEF patients.2
The degree of neurohormonal activation, as expressed by circulating biomarkers (namely plasma renin activity, aldosterone, catecholamines and N-terminal pro-B-type NP (NT-proBNP), has recently been evaluated in a well-treated HF cohort, including 189 patients for each LVEF class.6 A significant subset of HFpEF patients (67%) had elevated concentrations of at least one biomarker, while 10% of patients showed increased values in all of them (plasma renin activity, aldosterone and norepinephrine), after adjustment for the underlying HF therapy.6
The prognostic significance of RAAS activation was tested in different observational studies. Among 873 HFpEF patients, aldosterone concentrations were associated with LV concentric remodelling and independently predicted the risk of all-cause mortality and HF

Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Cardiovascular determinants Hypertension HFpEF syndrome Neurohormonal activation Hypertrophy, fibrosis and apoptosis Endothelial dysfunction/ perivascular fibrosis Reduced diuresis and natriuresis Arrhythmias • ACEI/ARB • MRA • ARNI • ACEI/ARB • MRA • ARNI • β-blockers • ACEI/ARB • ARNI • β-blockers • ARNI Microvascular dysfunction Valvular disease Ischaemic heart disease Cardiomyopathies (e.g. HCM,
AF
Figure 1: Pathophysiology of Heart Failure with Preserved Ejection Fraction and Pharmacological
amyloidosis)
Ageing Obesity Diabetes
(COPD, OSA, CSR)
Non-cardiovascular determinants
Breathing disorders
(chemotherapy)
Chronic kidney disease Inflammation Cardiotoxicity
hospitalisation. Median aldosterone concentrations in patients with and without events were 124.22 pmol/l (48.62–256.20) and 96.33 pmol/l (37.33–215.76), respectively, (p=0.023).25 Similarly, increased plasma renin activity was identified as an independent predictor of all-cause death among 150 HFpEF patients, regardless of RAAS antagonist therapy.26 Although the specific mechanisms relating RAAS activation to poor prognosis in HFpEF have not been specifically investigated in such works, the well-known contributions of the RAAS to cardiovascular stiffness and fibrosis may be implicated here.22
Considering the pivotal pathophysiological role of the sympathovagal imbalance in HFrEF and the prognostic implications of this, various studies have investigated the possible contribution of autonomic dysregulation to the HFpEF syndrome, but have yielded contradictory results.27
Although the evaluation of heart rate variability and baroreflex sensitivity, mirroring vagal activity, was often hampered by the presence of atrial arrhythmias, patients with HFpEF seemed to show an intermediate phenotype between that of HFrEF and healthy controls.27,28 Similarly, sympathetic nervous system (SNS) activation, as estimated by norepinephrine concentrations or microneurography, increases from healthy controls to patients with HFpEF to HFrEF.27
Such observations were further confirmed in a study in which cardiac norepinephrine spillover from the heart (measured at the coronary sinus) was significantly higher in HFpEF patients than in healthy controls, and was independently correlated with wedge pressure.29 Furthermore, among 278 HFpEF patients, plasma norepinephrine concentration also retained independent prognostic significance.30
The possible determinants of sympathovagal imbalance in HFpEF are still to be clarified. Whereas age, sex and cardiac and extracardiac comorbidities may act as possible confounders, the contribution of visceral feedbacks modulating autonomic function that are often dysregulated in HFrEF (e.g. baroreflex, chemoreflex and ergoreflex) is yet to be evaluated in HFpEF patients.31 32
On the other hand, the importance of increased filling pressure and left atrial enlargement/dysfunction in HFpEF may mean cardiopulmonary reflexes have a potential role in these patients.27
Neurohormonal Modulation in HFpEF: Pharmacological Therapies
Drugs blunting RAAS and SNS activation and improving NP activity target key pathophysiological mechanisms of HFrEF and have greatly improved the prognosis for patients with this condition. Based on the evidence of neurohormonal activation in HFpEF, targeted therapies have also been tested in HFpEF (Supplementary Material Table 1).
β-blockers
β-blockers slow the heart rate, reduce myocardial contractility and increase the duration of diastolic filling. No phase II/III RCT specifically tested β-blockers in HFpEF, hence evidence of their use in this clinical setting comes mainly from observational studies and subgroup/post-hoc analyses of RCTs.
A propensity score-matched cohort study on β-blocker use among HFpEF patients from the Swedish Heart Failure Registry showed that β-blocker therapy reduced all-cause mortality but not the composite of all-cause death or HF hospitalisations.33
In a subgroup analysis of the SENIORS trial, in which randomised patients aged ≥70 years with an HF hospitalisation within the previous year or an LVEF ≤35% to nebivolol or placebo, treatment with nebivolol significantly reduced the primary composite outcome of all-cause death or cardiovascular hospitalisation regardless of LVEF class (≤35% versus >35%; p for interaction=0.42).34,35 However, the broad definition of preserved EF (namely LVEF >35%) adopted in this trial does not allow a definite conclusion to be drawn on the eventual beneficial role of nebivolol in HFpEF. Moreover, a post-hoc analysis of this trial showed that, unlike in the LVEF ≤35% subgroup, nebivolol did not improve systolic or diastolic parameters in patients with LVEF >35%.36
Notably, in a subgroup analysis of the CIBIS-ELD trial, which assessed the tolerability and clinical effects of bisoprolol versus carvedilol in HF patients aged >65 years, HFpEF patients (LVEF >45%; n=250; 29%) showed higher rates of β-blocker dose-escalation delays and treatment-related sideeffects and less improvement in New York Heart Association (NYHA) class than HFrEF patients (LVEF ≤45%).37
Additionally, a post hoc analysis of the TOPCAT trial showed that patients with LVEF ≥50% treated with β-blockers at baseline had a higher risk of HF hospitalisations but not cardiovascular mortality than those not receiving β-blockers.38
Although β-blockers might limit some negative effects of SNS activation in HFpEF, some authors have suggested that their beneficial effects are offset by their negative action on the chronotropic response. Indeed, chronotropic incompetence appears to be a major driver of exercise functional capacity limitation in HFpEF.39 The recently published PRESERVE-HR study showed that β-blocker withdrawal improved peak VO 2 and peak VO2% in HFpEF patients with chronotropic incompetence (defined as a chronotropic index of <0.62).39
Taken together, these data suggest that β-blocker treatment might not be suitable for all patients with HFpEF and requires re-evaluation in appropriately designed, large RCTs.
Angiotensin-Converting Enzyme Inhibitors
RAAS activation promotes myocardial hypertrophy and fibrosis, which are two key aspects of HFpEF pathophysiology, providing a rationale for testing RAAS antagonists in HFpEF.
The ACEI perindopril has been tested in the phase III RCT PEP-CHF.40 This study randomised patients aged ≥70 years with LV wall motion index ≥1.4 (roughly equivalent to an LVEF ≥40%), evidence of diastolic dysfunction, symptomatic HF treated with diuretics and hospitalisation for cardiovascular reasons within 6 months to either perindopril 4 mg daily or placebo. Treatment with perindopril did not result in a lower primary composite endpoint of all-cause mortality or HF hospitalisation at 1-year follow-up (p=0.055). Nonetheless, enrolment and event rates were lower than expected, but the study was underpowered so could not detect a statistically significant difference in the primary endpoint between the two groups.
When the individual components of the primary endpoints were assessed, perindopril was found to be effective in preventing HF hospitalisations (HR 0.63; 95% CI [0.41–0.97]; p=0.033). Improvements in NYHA class (p<0.030) and 6 minute walking distance (p=0.011) were also observed in the perindopril group. According to subgroup analysis, younger patients (≤75 years) and those with a previous history of hypertension or MI received a greater benefit from perindopril.40
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Angiotensin Receptor Blockers
The CHARM-Preserved trial enrolled patients with LVEF >40%, NYHA class II–IV and a history of hospitalisation for cardiac reasons to receive either candesartan 32 mg daily or placebo. Treatment with candesartan almost met statistical significance for the primary composite endpoint of cardiovascular death or HF hospitalisation (HR 0.86; 95% CI [0.74–1.00]; p=0.051).
The number of cardiovascular deaths was equal in the two groups (170 versus 170), but fewer patients in the candesartan group were hospitalised for HF (230 versus 279; p=0.017).41 A post-hoc analysis showed that candesartan reduced the rate of recurrent HF hospitalisations to a greater extent than was evident from the assessment of first hospitalisations alone.42
Another post-hoc analysis demonstrated that the rates of the primary outcome declined with increasing LVEF. Specifically, candesartan significantly reduced the primary outcome in patients with LVEF up to 50%, and recurrent HF hospitalisations in patients with LVEF up to 60%.43
In the I-PRESERVE trial, patients aged ≥60 years with LVEF ≥45%, NYHA class III–IV or NYHA class II with HF hospitalisation within the previous 6 months were randomly assigned to receive either irbesartan 300 mg daily or placebo. After a median follow-up of 49.5 months, no significant differences were observed concerning the composite outcome of allcause mortality or hospitalisation for a cardiovascular cause (HF, MI, unstable angina, arrhythmia or stroke).44
These results in part conflict with those from the CHARM-Preserved trial, although several factors may explain this divergence: a different definition of HFpEF (LVEF >40% versus ≥45%); a different primary endpoint, with that of I-PRESERVE including outcomes less sensitive to the effect of treatment with ARBs, such as all-cause death; a high percentage of patients concomitantly taking another RAAS inhibitor (nearly 40% received an ACEI and nearly 29% a MRA) in both study arms of the I-PRESERVE trial possibly reducing the additive benefit from irbesartan; and the presence of some imbalances in baseline variables that might have affected outcomes and/or the response to irbesartan not being adjusted for in the I-PRESERVE trial.45
In a post-hoc analysis of the I-PRESERVE trial, after adjusting for key prognostic baseline covariates such as age, sex, medical history, physiological and laboratory variables, treatment with irbesartan resulted in a HR of 0.89 (95% CI [0.80–0.99]; p=0.033) for the primary composite outcome. Similar findings were observed for the composite of cardiovascular death or HF hospitalisation.45
Another post-hoc analysis from the I-PRESERVE trial showed that patients with baseline NT-proBNP values above the median of 339 ng/l had an increased risk of cardiovascular hospitalisations (p=0.001), all-cause mortality (p=0.001) and a composite of HF events, including death due to worsening HF or sudden death or hospitalisation because of worsening HF (p=0.001). Treatment with irbesartan was associated with improved outcomes in patients with NT-proBNP values below but not above the median, even after adjusting for 20 baseline covariates.46 This suggests that the effect of RAAS antagonism in HFpEF may be more relevant in early, lower-risk stages of the disease.
Mineralocorticoid Receptor Antagonists
In the Aldo-DHF trial, the investigators randomised patients aged ≥50 years with LVEF ≥50%, NYHA class II–III, peak VO2<25 ml/min/kg, and
echocardiographic evidence of diastolic dysfunction or AF to either spironolactone 25 mg daily or placebo. Treatment with spironolactone for 12 months improved LV diastolic function (assessed through a change in E/e’ ratio) and significantly reduced LV mass index and NT-proBNP, but did not affect maximal exercise capacity (assessed through a change in peak VO2), patient symptoms or quality of life.47
Several subsequent post-hoc analyses tried to identify the specific subsets of HFpEF patients who received the greatest benefit from spironolactone treatment. Ravassa et al. measured serum levels of biomarkers associated with myocardial collagen deposition (carboxyterminal propeptide of procollagen type I) and cross-linking (ratio of serum carboxy-terminal telopeptide of collagen type I to serum matrix metalloproteinase-1) in 381 patients from the Aldo-DHF trial, finding that a biochemical phenotype of high collagen cross-linking identified patients resistant to the beneficial effects of spironolactone on diastolic dysfunction.48
Another study reported that, among patients receiving a placebo, carriers of the rs5522 G allele of the NR3C2 gene, which encodes for spironolactone’s target protein, showed a greater increase in E/e’ ratio over 12 months compared to non-carriers. Conversely, treatment with spironolactone seemed to attenuate the progression of diastolic dysfunction associated with the NR3C2 rs5522 G allele.49
A further study aimed to identify a biomarker profile associated with spironolactone effect in HFpEF. Among 92 biomarkers tested, 13 proteins including renin, growth hormone, adrenomedullin and inflammatory peptides (e.g. tumour necrosis factor receptor superfamily member 11A, interleukin-18 and interleukin-4 receptor subunit alpha) showed a significantly different expression change between spironolactone and placebo from baseline to 12-month follow-up.50
Following the positive results of the Aldo-DHF trial, the TOPCAT trial tested spironolactone (up to 45 mg daily) versus placebo in 3,445 patients from six countries (the US, Argentina, Brazil, Canada, Georgia and Russia) with an LVEF ≥45% and clinically overt HF (defined as the presence of at least one sign and at least one symptom of HF plus a history of HF hospitalisation within 12 months or elevated NP levels). Over a mean follow-up of 3.3 years, treatment with spironolactone did not significantly reduce the incidence of the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest or hospitalisation for the management of HF (HR 0.89; 95% CI [0.77–1.04]; p=0.14).51
However, issues regarding patient enrolment in certain locations (namely Russia and Georgia) were raised. In particular, patients enrolled on the basis of the hospitalisation criterion had a lower event rate than those enrolled on the basis of the NP criterion and were younger and had fewer coexisting conditions.
The majority of patients from Russia and Georgia were enrolled according to the hospitalisation criterion and were possibly at lower risk.52 In a posthoc analysis, treatment with spironolactone met the primary endpoint when the patients from these countries were excluded.53
Angiotensin Receptor–Neprilysin Inhibitors
Sacubitril/valsartan is a first-in-class angiotensin receptor–neprilysin inhibitor with proven prognostic benefit in HFrEF. Sacubitril/valsartan combines the positive effects of RAAS antagonism with those of NP enhancement, making it an actual neurohormonal modulator.
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
The PARAMOUNT trial randomised 149 patients with LVEF ≥45%, symptomatic HF (NYHA class II–III), and NT-proBNP >400 ng/l to either sacubitril/valsartan (target dose of 97/103 mg twice daily) or valsartan (target dose 160 mg twice daily). Treatment with sacubitril/valsartan produced a greater reduction in NT-proBNP from baseline to week 12 compared to valsartan (primary outcome; p=0.005). However, at 36 weeks, the difference between the two treatment groups was no longer significant. Nonetheless, at 36 weeks, patients taking sacubitril/valsartan showed a greater reduction in left atrial size and a significant improvement in NYHA class compared to those taking valsartan, although overall quality of life assessed through the Kansas City Cardiomyopathy Questionnaire (KCCQ) did not differ between the two arms.54
After PARAMOUNT, the phase III PARGON-HF trial was intended to demonstrate a beneficial effect of sacubitril/valsartan over valsartan in terms of cardiovascular events. All patients had symptomatic chronic HF (NYHA class II–IV), LVEF ≥45% and NT-proBNP levels >300 ng/l (or >900 ng/l if in AF at screening). If a hospitalisation for HF had occurred in the previous 9 months, NT-proBNP cut-offs were 200 ng/l and 600 ng/l for sinus rhythm and AF, respectively. Patients were randomised 1:1 to sacubitril/valsartan or valsartan, with the same target doses as in the PARAMOUNT trial. At a median follow-up of 35 months, the study did not demonstrate a significant reduction in the composite primary endpoint of total HF hospitalisations and cardiovascular death between the two groups (p=0.06).
However, subgroup analysis showed that sacubitril/valsartan reduced the primary endpoint in patients with an LVEF below the median (57%), those with a reduced baseline estimated glomerular filtration rate (<60 ml/ min/1.73 m2) and women.55 A study pooling data from the PARADIGM-HF and the PARAGON-HF trials confirmed these results, showing that the prognostic advantage of treatment with sacubitril/valsartan over ACEI/ARB persisted in patients with LVEF up to values just below the normal range.56
In the PARAGON-HF trial, sacubitril/valsartan demonstrated a greater improvement in NYHA class and a lower decline in KCCQ Clinical Summary Score (KCCQ-CSS) compared to valsartan at 8 months.55 Building on this evidence, the PARALLAX trial investigated the effect of sacubitril/valsartan versus either placebo or an ACEI/ARB (enalapril or valsartan) in patients with LVEF ≥40% and impaired quality of life (KCCQ-CSS <75 points [range 0–100]). Although treatment with sacubitril/valsartan was associated with a greater reduction in NT-proBNP levels compared to the control arm, it did not lead to significant improvements in 6 minute walk distance, KCCQCSS or NYHA class.57
Cyclic Guanosine Monophosphate Modulators
Besides sacubitril, which increases NP levels by inhibiting their degradation, there have been pharmacological attempts to enhance the beneficial effects of NPs by acting downstream of their cellular target, i.e. the nitric oxide (NO)-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway.58
The NEAT-HFpEF trial assigned patients with LVEF >50% to a 6-week dose-escalation regimen of isosorbide mononitrate (ISMN; from 30 mg to 60 mg to 120 mg once daily) or placebo, with subsequent crossover to the other group for 6 weeks. Patients receiving ISMN were less active as measured by an accelerometer, with activity levels decreasing progressively with increased doses of ISMN. Moreover, there were no significant between-group differences in 6 minute walking distance, quality-of-life scores or NT-proBNP levels.59
Sildenafil is a phosphodiesterase 5 (PDE5) inhibitor, with proven efficacy in pulmonary artery hypertension. PDE5 converts second messenger cGMP into GMP and is upregulated in cardiac hypertrophy and HF.58 A small clinical trial randomised patients with HFpEF (LVEF ≥50%) and pulmonary artery systolic pressure >40 mmHg to receive sildenafil (50 mg thrice daily) or placebo. At 6 and 12 months, sildenafil significantly improved mean pulmonary artery pressure and vasomotility, right ventricular function and dimension, LV relaxation and distensibility, and lung interstitial water metabolism.60
Following these positive results, the phase III RELAX trial randomised HFpEF patients (LVEF ≥50%) with peak VO2 ≤60% of the age- and sexspecific normal value, NT-proBNP ≥400 ng/l or high LV filling pressures to either sildenafil (20 mg thrice daily uptitrated to 60 mg twice daily) or placebo. However, after 24 weeks, sildenafil did not significantly reduce peak VO2 (primary outcome), 6 minute walking distance or clinical status.61
sGC stimulators enhance sGC sensitivity to endogenous NO. sGC stimulators are a mainstay of therapy for pulmonary artery hypertension, and the sGC stimulator vericiguat has recently gained a lot of attention following the positive results of the VICTORIA trial, which led to its approval for use in patients with HFrEF.7,62
As for HFpEF, the first sGC stimulator to be investigated was riociguat, which was tested at a dose of 0.5–2 mg against placebo in the DILATE-1 trial, which included patients with LVEF >50%, mean pulmonary artery pressure ≥25 mmHg and pulmonary capillary wedge pressure >15 mmHg. After 6 hours, riociguat 2 mg failed to reduce mean pulmonary artery pressure compared to placebo (primary outcome), although it improved some exploratory haemodynamic and echocardiographic parameters, such as stroke volume, systolic blood pressure and right ventricular enddiastolic area.63
Vericiguat has been tested in two phase II RCTs on patients with HFpEF. The SOCRATES-PRESERVED trial randomised patients with LVEF ≥45% and a recent (<4 weeks) HF decompensation to receive vericiguat at various doses (1.25 mg to 10 mg daily) or placebo for 12 weeks. Treatment with vericiguat did not meet the two primary endpoints (change from baseline in NT-proBNP and left atrial volume) compared to placebo. Nonetheless, the highest vericiguat dose (10 mg daily) significantly improved the KCCQ-CCS score compared to placebo (mean difference: 9.2 points).64
This result was confirmed in a post-hoc analysis showing that both the KCCQ score and the generic health-related quality of life measure EQ-5D score had dose-dependent relationships with vericiguat.65
However, the VITALITY-HFpEF trial, which randomised patients with LVEF ≥45%, within 6 months of a recent HF decompensation and with elevated NPs to either vericiguat (10 mg or 15 mg) or placebo, failed to demonstrate an effect of vericiguat on measures of physical limitation (KCCQ-Physical Limitation Score and 6 minute walking distance) after 24 weeks of treatment.66
Neurohormonal Modulation in HFpEF: Nonpharmacological Interventions
Despite the contrasting findings of the clinical trials testing neurohormonal modulation drugs in HFpEF patients, a growing body of evidence from preclinical and clinical studies suggests that at least a subset of these patients may benefit from neuromodulation.27
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
The use of nonpharmacological strategies to modulate the autonomic nervous system (ANS) has therefore been proposed and tested in ancillary studies enrolling HFpEF patients, yielding encouraging results.
Renal Denervation
Considering the well-known interplay between the SNS, the RAAS, hypertension and HFpEF pathophysiology, the disruption of this link through renal denervation has been proposed as a possible therapeutic approach in HFpEF patients. Widely tested in patients with resistant hypertension, transcatheter renal denervation aims to reduce or interrupt the sympathetic innervation of renal arteries by the intravascular delivery of energy (most commonly through radiofrequency).67 68
In the RDT-PEF trial, 25 patients with HFpEF were randomised (2:1) to renal denervation versus medical therapy alone. Although renal denervation was associated with beneficial effects at 3-month follow-up, no significant differences in terms of quality of life, exercise capacity, B-type NP concentration and echocardiographic parameters were observed at 12 months.69 The underpowering of the study and the possible ineffectiveness of the ablation procedure were identified by the authors as possible explanations for the findings.69
Subsequently, Kresoja et al. compared the effects of renal denervation in patients with uncontrolled arterial hypertension with (n=99) or without (n=65) a diagnosis of HFpEF. Despite a similar efficacy in lowering blood pressure, renal denervation was associated with a greater improvement in NYHA class, NT-proBNP concentration and haemodynamic parameters (including systolic and diastolic function, aortic distensibility and myocardial work) in patients with HFpEF.70
Given these findings, renal denervation may prove valuable in the treatment of HFpEF patients. Nevertheless, optimising patient selection (e.g. focusing on those with a hypertensive phenotype) and improving ablation strategies seem necessary goals to be pursued by future clinical trials.71
Splanchnic Nerve Denervation
Inappropriate control of blood volume distribution, at rest and during physical effort, has been recently identified as a key pathophysiological determinant of HFpEF syndrome.72
Considering the key role of the autonomic modulation of visceral vessel capacitance, targeting the greater splanchnic nerve (GSN) has emerged as a potential therapeutic strategy in these patients. The surgical resection of the right GSN in 11 HFpEF patients resulted in a sustained improvement in functional capacity and quality of life by reducing cardiac filling pressure during exercise.73
More recently, the preliminary findings of the ongoing REBALANCE-HF trial have shown that, among the 18 HFpEF patients enrolled (89% in NYHA class III), the transvenous ablation of the right GSN was associated with a significant improvement in exercise filling pressures, NYHA class and quality of life in the absence of serious device/procedure-related adverse events.74 The results of the ongoing randomised, sham-controlled portion of the trial are thus expected to confirm the efficacy and the safety of this promising therapy for HFpEF patients.
Vagus Nerve Stimulation
Beyond sympathetic hyperactivity, vagal withdrawal has also been documented in HFpEF patients, potentially contributing to disease
progression and poor outcomes.27 Vagus nerve stimulation (VNS) may thus provide beneficial effects in these patients.
In this regard, the ongoing ANTHEM-HFpEF study will provide information about the potential role of a device for cervical VNS in symptomatic HFpEF patients.75 However, since VNS has major drawbacks of an invasive implantation procedure and possible device-related complications, transcutaneous VNS (tVNS), carried out through the noninvasive stimulation of the auricular branch of the vagus nerve at the tragus level, is emerging as an alternative.76
In a prospective, randomised, double-blind, 2x2 crossover study, 1-hour tVNS improved heart rate variability and global longitudinal strain in the 24 HFpEF patients enrolled.77 Subsequently, in a pilot RCT including 52 HFpEF patients, 1-hour daily tVNS for 3 months improved global longitudinal strain and quality of life. Furthermore, tVNS reduced the concentration of inflammatory cytokines, underlining the important physiological link between the ANS and the immune system.78
Other Nonpharmacological Approaches in HFpEF
Beyond the strategies aimed at direct modulation of neurohormonal systems, other bioelectronic/interventional approaches have been recently tested in HFpEF.
Cardiac contractility modulation (CCM) delivers electrical impulses to the heart during the absolute refractory period, yielding beneficial structural and functional cardiac improvement, probably by increasing intracellular calcium.79 The CCM-HFpEF pilot study recently tested the effects of CCM in 47 symptomatic HFpEF patients, and CCM implantation was associated with a significant improvement in quality of life at 24 weeks, with no safety concerns arising.80 Future studies are expected to confirm these preliminary findings and evaluate the impact of CCM on hard outcomes in HFpEF patients.
An increase in left atrial filling pressure is a key pathophysiological determinant of HFpEF, contributing to poor exercise tolerance and disease progression by promoting pathological cascades.79 Lowering these pressures by creating a small shunt between the left and right atria through dedicated devices has therefore been proposed as a possible therapeutic approach. However, despite promising results in preliminary studies, the RCT REDUCE LAP-HF II has recently failed to demonstrate the clinical efficacy of this approach in a large cohort of HF patients with LVEF ≥40%, questioning the rationale behind this device.81
Neurohormonal Modulation in HFpEF: Recommendations from International Guidelines
The reasons why most trials on neurohormonal modulation therapies in HFpEF led to disappointing results are partly unclear. The intrinsic heterogeneity of HFpEF phenotype as well as flaws in study design (such as inadequate diagnostic criteria or low statistical power) might be some of the more reliable explanations.
International HF guidelines reflect this heterogeneous evidence, providing different recommendations for the management of HFpEF.7 62 82 This is due both to the different weight that the guidelines attribute to the evidence from RCTs and post-hoc analyses as well as to the different timing of their publication.
As a consequence, the 2021 European Society of Cardiology Guidelines recommend to screen for and treat underlying aetiologies and
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
cardiovascular and non-cardiovascular comorbidities (e.g. hypertension, coronary artery disease, amyloidosis, AF and valvular heart disease) in HFpEF.7 They also recommend using diuretics to relieve HF symptoms and signs, without mentioning neurohormonal modulation therapies, stating that ‘none of the large RCTs conducted in HFpEF [had] achieved their primary endpoints’ at the time of guideline publication.7
The 2022 American College of Cardiology/American Heart Association/ Heart Failure Association of America (ACC/AHA/HFSA) guidelines also underscore the importance of treating comorbidities, such as hypertension and AF, as well as the usefulness of diuretics to relieve congestion.62 However, they value more the evidence from RCTs approaching statistical significance for their primary endpoints and from post-hoc analyses showing that neurohormonal modulation therapies might be effective in specific HFpEF subgroups, especially in patients with LVEF approaching the lower boundary of HFpEF definition. Therefore, they state that ARB, MRA and sacubitril/valsartan may be considered to decrease hospitalisations in patients with LVEF ‘on the lower end of [HFpEF] spectrum’.62
This recommendation is in line with the Food and Drug Administration decision to broaden the sacubitril/valsartan indication to all patients with ‘LVEF below normal’.83 Conversely, the 2022 ACC/AHA/HFSA guidelines give an overall negative weight to the discordant results of studies on cGMP modulators, stating that ‘routine use of nitrates or phosphodiesterase-5 inhibitors to increase activity or quality of life is ineffective’ in HFpEF.62
Although a strict application of HF guidelines might create significant differences between Europe and the US, this is partly mitigated by the fact that, even without specific recommendations for HFpEF, a great proportion of HFpEF patients already receive neurohormonal modulation therapies for the treatment of underlying comorbidities.82,7 For example, in the PARAGON-HF trial, 80% of patients were receiving β-blockers at baseline – 86% received ACEI/ARBs and 26% received MRAs.55
As for non-pharmacological neuromodulation therapies, current evidence from clinical studies is still limited in patients with HFpEF, so international HF guidelines do not provide specific recommendations for their use.7 62
Neurohormonal Modulation and Sodium-glucose Cotransporter 2 Inhibitors: Future Perspectives
Numerous molecular mechanisms, including neurohormonal activation, contribute to HFpEF pathophysiology. Despite most major RCTs testing neurohormonal modulation therapies not reaching their primary endpoints, these treatments should not be definitively labelled as ineffective in HFpEF. Evidence from post-hoc analyses suggests that at least a subgroup of HFpEF patients, namely those with LVEF below normal, might derive prognostic benefit from pharmacological neurohormonal modulation, as also per the 2022 ACC/AHA/HFSA guidelines.
Although this certainly applies to ACEIs, ARBs, ARNIs and MRAs, RCT data
1. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure
on the use of β-blockers in HFpEF are still lacking, hence the role of this drug class in HFpEF deserves further investigation. Similarly, nonpharmacological neuromodulation therapies for HFpEF are still in their infancy and need further validation before entering the clinical arena. Conversely, cGMP modulators have been tested in several RCTs, mainly with disappointing results, casting a shadow on their efficacy in HFpEF.
Another area of uncertainty concerns the relationship between neurohormonal modulation therapies and sodium-glucose cotransporter-2 inhibitors (SGLT2Is) in HFpEF. SGLT2Is, from being antidiabetic drugs, have recently emerged as remarkable treatments with cardio- and nephroprotective effects in recent years.84
The EMPEROR-Preserved and the DELIVER trials recently demonstrated that empagliflozin and dapagliflozin reduce the combined risk of worsening HF or cardiovascular death in patients with LVEF >40%, proving to be the first drugs with a positive impact on hard cardiovascular outcomes in HFpEF.85,86 The 2022 ACC/AHA/HFSA guidelines have already endorsed the positive results of the EMPEROR-Preserved trial, recommending SGLT2Is for the treatment of HFpEF.62
Nonetheless, the mechanisms underlying SGLT2Is’ cardioprotective effects are still to be clarified. It has been proposed that SGLT2Is act as smart diuretics, promoting electrolyte-free water clearance, especially concerning the interstitial fluid space; however, this can explain only in part their positive impact on the cardiovascular system.87
In a recently published review, Packer summarised all available evidence from preclinical studies investigating the effects of SGLT2I therapy on the cardiovascular system, and concluded that, at the cellular level, SGLT2 acts as a nutrient surplus sensor, thus its inhibition through an SGLT2I causes simultaneous upregulation of nutrient-deprivation signalling and downregulation of nutrient-excess signalling, resulting in increased autophagic flux, which underlies several cytoprotective effects, such as reductions in oxidative and endoplasmic reticulum stress, restoration of mitochondrial health and improvement of mitochondrial biogenesis, and a decrease in proinflammatory and profibrotic pathways.88 A few preclinical studies have also reported an interplay between SGLT2 and the SNS, particularly at the renal level.89
Nevertheless, a direct neurohormonal modulation effect of SGLT2Is has not been demonstrated yet, hence, strictly speaking, this drug class cannot be considered a neurohormonal modulation therapy. In light of this, future studies should evaluate whether a combination of neurohormonal modulators and SGLT2Is offers any advantage over the exclusive use of the latter. While most HFpEF patients already receive β-blockers, ACEI/ARBs and MRAs to treat underlying comorbidities (for example, in the EMPEROR-Preserved trial, 86% of patients were on β-blockers at baseline, 81% were on ACEI/ARBs/ARNIs and 37% were on MRAs), sacubitril/valsartan has no indication other than HF treatment, hence the effect of the combination of SGLT2I and ARNI is still largely unexplored.85
Association. Eur J Heart Fail 2021;23:352–80. https://doi. org/10.1002/ejhf.2115; PMID: 33605000.
2. Gentile F, Ghionzoli N, Borrelli C, et al. Epidemiological and clinical boundaries of heart failure with preserved ejection fraction. Eur J Prev Cardiol 2022;29:1233–43. https://doi. org/10.1093/eurjpc/zwab077; PMID: 33963839.
3. Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of
epidemiology. Cardiovasc Res 2023;118:3272–87. https://doi. org/10.1093/cvr/cvac013; PMID: 35150240.
4. Vergaro G, Ghionzoli N, Innocenti L, et al. Noncardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc 2019;8:e013441. https://doi.org/10.1161/JAHA.119.013441; PMID: 31587602.
5. Aimo A, Barison A, Castiglione V, Emdin M. The unbearable
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
underreporting of comorbidities in heart failure clinical trials. Eur J Heart Fail 2020;22:1043–4. https://doi.org/10.1002/ ejhf.1846; PMID: 32351008.
6. Vergaro G, Aimo A, Prontera C, et al. Sympathetic and reninangiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol 2019;296:91–7. https://doi.org/10.1016/j. ijcard.2019.08.040; PMID: 31443984.
7. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https:// doi.org/10.1093/eurheartj/ehab368; PMID: 34447992.
8. Borlaug BA, Olson TP, Lam CSP, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. https://doi. org/10.1016/j.jacc.2010.03.077; PMID: 20813282.
9. Borlaug BA, Jaber WA, Ommen SR, et al. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 2011;97:964–9. https://doi.org/10.1136/hrt.2010.212787; PMID: 21478380.
10. Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–56. https://doi.org/10.1016/j.jacc.2013.09.052; PMID: 24184245.
11. Tsioufis C, Georgiopoulos G, Oikonomou D, et al. Hypertension and heart failure with preserved ejection fraction: connecting the dots. Curr Vasc Pharmacol 2017;16:15–22. https://doi.org/10.2174/15701611156661704141 20532; PMID: 28413968.
12. Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–50. https://doi.org/10.1093/eurheartj/ ehy531; PMID: 30165580.
13. Empel VPM Van, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 2014;3:e001293. https:// doi.org/10.1161/JAHA.114.001293; PMID: 25468660.
14. Liao R, Jain M, Teller P, et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 2001;104:1594–7. https://doi.org/10.1161/circ.104.14.1594; PMID: 11581134.
15. Fan Y, Pui-Wai A. Valvular disease and heart failure with preserved ejection fraction. Heart Fail Clin 2021;17:387–95. https://doi.org/10.1016/j.hfc.2021.02.005; PMID: 34051971.
16. Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res 2021;117:423–34. https://doi.org/10.1093/cvr/cvaa217; PMID: 32666082.
17. Aimo A, Castiglione V, Borrelli C, et al. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol 2020;27:494–510. https://doi.org/10.1177/2047487319870344; PMID: 31412712.
18. Smiseth OA, Baron T, Marino PN, et al. Imaging of the left atrium: pathophysiology insights and clinical utility. Eur Heart J Cardiovasc Imaging 2021;23:2–13. https://doi.org/10.1093/ ehjci/jeab191; PMID: 34601594.
19. Vanderpool RR, Saul M, Nouraie M, et al. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018;3:298–306. https://doi. org/10.1001/jamacardio.2018.0128; PMID: 29541759.
20. Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–62. https://doi.org/10.1093/ eurheartj/ehu193; PMID: 24875795.
21. Tadic M, Cuspidi C. Obesity and heart failure with preserved ejection fraction: a paradox or something else? Heart Fail Rev 2019;24:379–85. https://doi.org/10.1007/s10741-018-09766-x; PMID: 30610456.
22. McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-theart review. J Am Coll Cardiol 2019;73:602–11. https://doi. org/10.1016/j.jacc.2018.11.033; PMID: 30732715.
23. Iorio A, Senni M, Barbati G, et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail 2018;20:1257–66. https://doi.org/10.1002/ejhf.1202; PMID: 29917301.
24. Borrelli C, Gentile F, Sciarrone P, et al. Central and obstructive apneas in heart failure with reduced, mid-range and preserved ejection fraction. Front Cardiovasc Med 2019;6:125. https://doi.org/10.3389/fcvm.2019.00125; PMID: 31555667.
25. Ke B, Tan X, Ren L, et al. Aldosterone dysregulation predicts the risk of mortality and rehospitalization in heart failure
with a preserved ejection fraction. Sci China Life Sci 2022;65:631–42. https://doi.org/10.1007/s11427-021-1945-6; PMID: 34258711.
26. Binder C, Poglitsch M, Duca F, et al. Renin feedback is an independent predictor of outcome in HFpEF. J Pers Med 2021;11:370. https://doi.org/10.3390/jpm11050370; PMID: 34063595.
27. Badrov MB, Mak S, Floras JS. Cardiovascular autonomic disturbances in heart failure with preserved ejection fraction. Can J Cardiol 2021;37:609–20. https://doi. org/10.1016/j.cjca.2020.12.006; PMID: 33310140.
28. Arora R, Krummerman A, Vijayaraman P, et al. Heart rate variability and diastolic heart failure. Pacing Clin Electrophysiol 2004;27:299–303. https://doi. org/10.1111/j.1540-8159.2004.00431.x; PMID: 15009853.
29. Kaye DM, Nanayakkara S, Wang B, et al. Characterization of cardiac sympathetic nervous system and inflammatory activation in HFpEF patients. JACC Basic Transl Sci 2022;7:116–27. https://doi.org/10.1016/j.jacbts.2021.11.007; PMID: 35257038.
30. Jimenez-Marrero S, Moliner P, Rodríguez-Costoya I, et al. Sympathetic activation and outcomes in chronic heart failure: does the neurohormonal hypothesis apply to midrange and preserved ejection fraction patients? Eur J Intern Med 2020;81:60–6. https://doi.org/10.1016/j. ejim.2020.07.008; PMID: 32718877.
31. Aimo A, Saccaro LF, Borrelli C, et al. The ergoreflex: how the skeletal muscle modulates ventilation and cardiovascular function in health and disease. Eur J Heart Fail 2021;23:1458–67. https://doi.org/10.1002/ejhf.2298; PMID: 34268843.
32. Alberto G, Francesco G, Francesco B, et al. Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure. JACC Heart Fail 2022;10:662–76. https://doi.org/10.1016/j.jchf.2022.02.006; PMID: 36049816.
33. Lund LH, Benson L, Dahlström U, et al. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014;312:2008–18. https://doi.org/10.1001/jama.2014.15241; PMID: 25399276.
34. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–25. https:// doi.org/10.1093/eurheartj/ehi115; PMID: 15642700.
35. Veldhuisen DJ van, Cohen-Solal A, Böhm M, et al. Betablockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with heart failure). J Am Coll Cardiol 2009;53:2150–8. https:// doi.org/10.1016/j.jacc.2009.02.046; PMID: 19497441.
36. Ghio S, Magrini G, Serio A, et al. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. Eur Heart J 2006;27:562–8. https://doi.org/10.1093/eurheartj/ehi735; PMID: 16443607.
37. Edelmann F, Musial-Bright L, Gelbrich G, et al. Tolerability and feasibility of beta-blocker titration in HFpEF versus HFrEF: insights from the CIBIS-ELD trial. JACC Heart Fail 2016;4:140–9. https://doi.org/10.1016/j.jchf.2015.10.008; PMID: 26682793.
38. Silverman DN, Plante TB, Infeld M, et al. Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open 2019;2:e1916598. https://doi.org/10.1001/ jamanetworkopen.2019.16598; PMID: 31800067.
39. Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol 2021;78:2042–56. https://doi.org/10.1016/j.jacc.2021.08.073; PMID: 34794685.
40. Cleland JGF, Tendera M, Adamus J, et al. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study. Eur Heart J 2006;27:2338–45. https://doi.org/10.1093/ eurheartj/ehl250; PMID: 16963472.
41. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARMPreserved trial. Lancet 2003;362:777–81. https://doi. org/10.1016/S0140-6736(03)14285-7; PMID: 13678871.
42. Rogers JK, Pocock SJ, McMurray JJV, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARMPreserved. Eur J Heart Fail 2014;16:33–40. https://doi. org/10.1002/ejhf.29; PMID: 24453096.
43. Lund LH, Claggett B, Liu J, et al. Heart failure with midrange ejection fraction in CHARM: characteristics, outcomes
and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–9. https://doi. org/10.1002/ejhf.1149; PMID: 29431256.
44. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. https://doi.org/10.1056/ NEJMoa0805450; PMID: 19001508.
45. Ferreira JP, Dewan P, Jhund PS, et al. Covariate adjusted reanalysis of the I-Preserve trial. Clin Res Cardiol 2020;109:1358–65. https://doi.org/10.1007/s00392-02001632-x; PMID: 32215700.
46. Anand IS, Rector TS, Cleland JG, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569–77. https://doi.org/10.1161/ CIRCHEARTFAILURE.111.962654; PMID: 21715583.
47. Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781–91. https://doi.org/10.1001/jama.2013.905; PMID: 23443441.
48. Ravassa S, Trippel T, Bach D, et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the AldoDHF trial. Eur J Heart Fail 2018;20:1290–9. https://doi. org/10.1002/ejhf.1194; PMID: 29709099.
49. Dumeny L, Vardeny O, Edelmann F, et al. NR3C2 genotype is associated with response to spironolactone in diastolic heart failure patients from the Aldo-DHF trial. Pharmacotherapy 2021;41:978–87. https://doi.org/10.1002/ phar.2626; PMID: 34569641.
50. Schnelle M, Leha A, Eidizadeh A, et al. Plasma biomarker profiling in heart failure patients with preserved ejection fraction before and after spironolactone treatment: results from the Aldo-DHF trial. Cells 2021;10:2796. https://doi. org/10.3390/cells10102796; PMID: 34685778.
51. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. https://doi.org/10.1056/NEJMoa1313731; PMID: 24716680.
52. McMurray JJV, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med 2014;370:1453–4. https://doi.org/10.1056/ NEJMe1401231; PMID: 24716685.
53. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 2015;131:34–42. https://doi. org/10.1161/CIRCULATIONAHA.114.013255; PMID: 25406305.
54. Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. https://doi.org/10.1016/S0140-6736(12)61227-6
55. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–20. https://doi. org/10.1056/NEJMoa1908655; PMID: 31475794.
56. Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/ valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020;141:352–61. https://doi.org/10.1161/ CIRCULATIONAHA.119.044586; PMID: 31736342.
57. Pieske B, Wachter R, Shah SJ, et al. Effect of sacubitril/ valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA 2021;326:1919–29. https://doi.org/10.1001/jama.2021.18463; PMID: 34783839.
58. Emdin M, Aimo A, Castiglione V, et al. Targeting cyclic guanosine monophosphate to treat heart failure: JACC review topic of the week. J Am Coll Cardiol 2020;76:1795–807. https://doi.org/10.1016/j.jacc.2020.08.031;
PMID: 33032741.
59. Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–24. https://doi.org/10.1056/ NEJMoa1510774; PMID: 26549714.
60. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011;124:164–74. https://doi. org/10.1161/CIRCULATIONAHA.110.983866; PMID: 21709061.
61. Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–77.
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
https://doi.org/10.1001/jama.2013.2024; PMID: 23478662.
62. Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2022;145:e895–1032. https://doi.org/10.1161/ CIR.0000000000001063; PMID: 35363499.
63. Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebocontrolled, single-dose study. Chest 2014;146:1274–85. https://doi.org/10.1378/chest.14-0106; PMID: 24991733.
64. Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATESPRESERVED) study. Eur Heart J 2017;38:1119–27. https://doi. org/10.1093/eurheartj/ehw593; PMID: 28369340.
65. Filippatos G, Maggioni AP, Lam CSP, et al. Patient-reported outcomes in the SOluble guanylate cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (Socrates-PRESERVED) study. Eur J Heart Fail 2017;19:782–91. https://doi.org/10.1002/ejhf.800; PMID: 28586537.
66. Armstrong PW, Lam CSP, Anstrom KJ, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 2020;324:1512–21. https:// doi.org/10.1001/jama.2020.15922; PMID: 33079152.
67. Kiuchi MG, Esler MD, Fink GD, et al.. Renal denervation update from the International Sympathetic Nervous System Summit: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:3006–17. https://doi.org/10.1016/j.jacc.2019.04.015; PMID: 31196459.
68. Chiriacò M, Masi S, Pugliese NR, Taddei S. Renal denervation for the treatment of refractory hypertension: where are we?. G Ital Cardiol (Rome) 2020;21:961–8 [in Italian]. https://doi.org/10.1714/3472.34550; PMID: 33231215.
69. Patel HC, Rosen SD, Hayward C, et al. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail 2016;18:703–12. https://doi.org/10.1002/ejhf.502; PMID: 26990920.
70. Kresoja KP, Rommel KP, Fengler K, et al. Renal sympathetic denervation in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2021;14:e007421. https://doi.
org/10.1161/CIRCHEARTFAILURE.120.007421; PMID: 33706547.
71. Fudim M, Sobotka PA, Piccini JP, Patel MR. Renal denervation for patients with heart failure: making a full circle. Circ Heart Fail 2021;14:e008301. https://doi.org/10.1161/ CIRCHEARTFAILURE.121.008301; PMID: 33706548.
72. Fudim M, Ponikowski PP, Burkhoff D, et al. Splanchnic nerve modulation in heart failure: mechanistic overview, initial clinical experience, and safety considerations. Eur J Heart Fail 2021;23:1076–84. https://doi.org/10.1002/ejhf.2196; PMID: 33886137.
73. Málek F, Gajewski P, Zymliński R, et al. Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: first-in-human clinical trial. Eur J Heart Fail 2021;23:1134–43. https://doi. org/10.1002/ejhf.2209; PMID: 33932262.
74. Fudim M, Fail PS, Litwin SE, et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: early results of the REBALANCEHF trial roll-in cohort. Eur J Heart Fail 2022;24:1410–4. https:// doi.org/10.1002/ejhf.2559; PMID: 35598154.
75. DiCarlo LA, Libbus I, Kumar HU, et al. Autonomic regulation therapy to enhance myocardial function in heart failure patients: the ANTHEM-HFpEF study. ESC Heart Fail 2018;5:95–100. https://doi.org/10.1002/ehf2.12241; PMID: 29283224.
76. Giannoni A, Gentile F, Passino C. Bioelectronic medicine and its applications in cardiology. Eur Heart J 2022;43:4453–5. https://doi.org/10.1093/eurheartj/ehac343; PMID: 35751532.
77. Tran N, Asad Z, Elkholey K, et al. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res 2019;12:221–30. https:// doi.org/10.1007/s12265-018-9853-6; PMID: 30560316.
78. Stavrakis S, Elkholey K, Morris L, et al. Neuromodulation of inflammation to treat heart failure with preserved ejection fraction: a pilot randomized clinical trial. J Am Heart Assoc 2022;11:e023582. https://doi.org/10.1161/JAHA.121.023582; PMID: 35023349.
79. Fudim M, Abraham WT, Bardeleben RS von, et al. Device therapy in chronic heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:931–56. https://doi.org/10.1016/j. jacc.2021.06.040; PMID: 34446165.
80. Linde C, Grabowski M, Ponikowski P, et al. Cardiac contractility modulation therapy improves health status in patients with heart failure with preserved ejection fraction: a pilot study (CCM-HFpEF). Eur J Heart Fail 2022;24:2275–84.
https://doi.org/10.1002/ejhf.2619; PMID: 35855646.
81. Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet 2022;399:1130–40. https://doi.org/10.1016/S0140-6736(22)00016-2; PMID: 35120593.
82. Bayés-Genís A, Aimo A, Metra M, et al. Head-to-head comparison between recommendations by the ESC and ACC/AHA/HFSA heart failure guidelines. Eur J Heart Fail 2022;24:916–26. https://doi.org/10.1002/ejhf.2542; PMID: 35579428.
83. Gandotra C, Clark J, Liu Q, et al. Heart failure population with therapeutic response to sacubitril/valsartan, spironolactone and candesartan: FDA perspective. Ther Innov Regul Sci 2022;56:4–7. https://doi.org/10.1007/s43441021-00347-z; PMID: 34699047.
84. Chiriacò M, Tricò D, Solini A. Mechanisms of cardio-renal protection of sodium-glucose cotransporter-2 inhibitors. Curr Opin Pharmacol 2022;66:102272. https://doi.org/10.1016/j. coph.2022.102272; PMID: 35964531.
85. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038; PMID: 34449189.
86. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–98. https://doi. org/10.1056/NEJMoa2206286; PMID: 36027570.
87. Fontes-Carvalho R, Santos-Ferreira D, Raz I, et al. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J Prev Cardiol 2022;29:1352–60. https://doi.org/10.1093/eurjpc/zwab034; PMID: 33659986.
88. Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 2022;146:1383–405. https://doi. org/10.1161/CIRCULATIONAHA.122.061732; PMID: 36315602.
89. Matthews VB, Elliot RH, Rudnicka C, et al. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens 2017;35:2059–68. https://doi.org/10.1097/HJH.0000000000001434; PMID: 28598954.
Neurohormonal Modulation in HFpEF CARDIAC FAILURE REVIEW www.CFRjournal.com
Defining Heart Failure Based on Imaging the Heart and Beyond
1 and Pierpaolo Pellicori 1
1. School of Cardiovascular and Metabolic Health, University of Glasgow, Glasgow, UK; 2. Queen Elizabeth University Hospital, Glasgow, UK
Abstract
Water and salt retention, in other words congestion, are fundamental to the pathophysiology of heart failure and are important therapeutic targets. Echocardiography is the key tool with which to assess cardiac structure and function in the initial diagnostic workup of patients with suspected heart failure and is essential for guiding treatment and stratifying risk. Ultrasound can also be used to identify and quantify congestion in the great veins, kidneys and lungs. More advanced imaging methods might further clarify the aetiology of heart failure and its consequences for the heart and periphery, thereby improving the efficiency and quality of care tailored with greater precision to individual patient need.
Keywords
Congestion, ultrasound, inferior vena cava, jugular vein, heart failure
Disclosure: FJG has received financial support from Pharmacosmos for travel to international meetings. RTC has received grants and honoraria from AstraZeneca and has participated in an advisory board for Bayer HealthCare Pharmaceuticals LLC. JGFC has received funding from British Heart Foundation; personal fees from Abbott, Amgen, Novartis, Medtronic, Idorsia, Servier, AstraZeneca, Innolife, Torrent and Respicardia; grants and personal fees from Bayer, Bristol Myers Squibb, Vifor, Johnson & Johnson, Myokardia and Viscardia; personal fees and non-financial support from Boehringer Ingelheim and NI Medical; and grants from Pharma Nord and Pharmacosmos. PP has received consultancy honoraria and/or sponsorship support from Boehringer Ingelheim, Pharmacosmos, Novartis, Vifor, AstraZeneca and Caption Health; and research support from Bristol Myers Squibb. All other authors have no conflicts of interest to declare.
Acknowledgments: FJG and AI contributed equally. The current manuscript summarises the content of discussions started by this group in July 2022, during the masterclass ‘Relevance and Identification of Congestion in Heart Failure’, which was sponsored by Heart Research UK (https://heartresearch.org.uk/), a national charity. We thank Dr Jeroen Dauw (Genk, Belgium) for providing images of discontinuous venous renal flow.
Received: 26 October 2022
Accepted: 19 February 2023 Citation: Cardiac Failure Review 2023;9:e10. DOI: https://doi.org/10.15420/cfr.2022.29
Correspondence: Pierpaolo Pellicori, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
E: Pierpaolo.pellicori@glasgow.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure is an umbrella term for a variety of common and serious problems that are often overlooked in clinical practice.1 Two universal definitions of heart failure have recently been proposed; both highlight the central role of congestion in the pathophysiology and presentation of heart failure.2 3 One definition requires symptoms (such as exertional breathlessness) and clinical signs (such as peripheral oedema) of congestion to make a diagnosis of heart failure.2 However, symptoms and signs are late manifestations of disease and lack specificity until they are severe. Indeed, for many patients, symptoms and signs of heart failure go unrecognised until they are so severe that admission to hospital is required.4 Intervening earlier might delay disease progression more effectively.5 6
Higher plasma concentrations of natriuretic peptides reflect increases in intra-cardiac pressures and transmural myocardial wall tension due to cardiac dysfunction; they are associated with an adverse prognosis even in the absence of obvious symptoms. However, natriuretic peptides provide little-to-no information on the aetiology of heart failure.
Imaging, particularly by ultrasound, provides information on cardiac structure and function to guide introduction of appropriate treatments,
and can also be used to identify and quantify congestion – both haemodynamic or vascular – and water excess in the tissues of other organs, as we will discuss in this review (Figure 1).7
Echocardiography
Echocardiography is fundamental in the assessment of myocardial structure and function. Through a complex process involving the transmission and subsequent reflection of ultrasound waves, dynamic motion imaging of the heart can be obtained and interpreted in real time via echocardiography. Compared with other complex imaging modalities, echocardiography is widely accessible, free from radiation, relatively affordable and highly versatile; it can provide detailed information on cardiac haemodynamics and valvular function, either at patient’s bedside or in the outpatient setting.8 As a result, it remains the most useful initial imaging diagnostic tool for the vast majority of patients with suspected or diagnosed heart failure.9
The Left Ventricle
The first question that an echocardiographer is asked to answer in a patient with heart failure is usually: “what is the left ventricular ejection fraction?” In its simplest sense, the left ventricular ejection fraction (LVEF) is the percentage of blood that the left ventricle (LV) ejects during systole,
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Diagnosis
Fraser J Graham , 1 Antonio Iaconelli , 1 Piotr Sonecki,2 Ross T Campbell , 1 David Hunter , 1 John GF Cleland
relative to end-diastole. It reflects the systolic contractility of the LV and – although influenced by changes in loading conditions (volume status, blood pressure) and underlying cardiac rhythm (for instance AF) – its accurate assessment is pivotal in determining the heart failure ‘phenotype’. This guides eligibility for medical or device therapies, inclusion in clinical trials and the assessment of recovery of function in optimally treated patients. Assessment of LVEF can be qualitative (subjective) or quantitative (objective). Cardiologists and sonographers should strive towards attainment of objective measures of ejection fraction over subjective ‘eyeball’ measurement; the latter is usually the result of poor echocardiographic windows, suboptimal training or insufficient scan time.10 Simpson’s biplane method is the day-to-day standard for LVEF measurement. In cases of poor imaging quality, LV contrast agents can be used to opacify the LV, allowing more accurate interpretation of LVEF.11 Newer methods for assessing LV volumes using 3D applications have improved accuracy compared with cardiac magnetic resonance (CMR) as a gold-standard.12 The use of 3D echocardiography also aids in the interpretation and quantification of valvular disease, such as mitral regurgitation, which is common in patients with heart failure, but it is reliant on good image quality.13
Speckle tracking echocardiography tracks multiple specific regions, or speckles, of the myocardium and can evaluate global or regional longitudinal, circumferential and radial myocardial deformation.14 15 A worsening (less negative/more positive value) global longitudinal strain (GLS), in particular, correlates with increasing natriuretic peptides and might facilitate identification of those with heart failure and preserved LVEF.16 GLS can also be useful in the longitudinal follow-up of patients being treated with cardio-toxic agents, to identify those in whom a change

in oncological and/or cardio-protective therapy might be warranted, or in gauging response to therapy for cardiac amyloidosis.11,17

The Left Atrium

Current diagnostic algorithms proposed to identify elevated cardiac filling pressure are time consuming with limited applicability in routine clinical practice. The left atrium (LA) is a thin-walled chamber that acts as a reservoir, conduit and pump for oxygenated blood prior to transport to the muscular, sub-systemic LV. The LA dilates in response to chronic elevations in pressure, which may be due to LV or mitral valve dysfunction or both. A dilated LA is associated with elevated natriuretic peptide levels and with an increased risk of a broad range of cardiovascular events both in patients with heart failure and the general population, particularly AF, a common precipitant of heart failure symptoms and signs.18–21 Transition from dilation to LA dysfunction and failure might lead to poorer prognosis; treatments that decrease LA size might delay onset of heart failure or progression of disease.22–25
The Right Side of the Heart
The failure to control elevated LV filling pressures eventually leads to right ventricular (RV) dysfunction, development of venous hypertension and poorer prognosis.26 In clinical practice, pulmonary artery systolic pressure can be estimated from the peak velocity of the tricuspid regurgitant jet via Doppler. When elevated, it identifies patients with heart failure with more severe symptoms and greater risk of death; however, reliability of this method is questionable in the presence of severe tricuspid regurgitation or substantial RV dysfunction.27 The inferior vena cava (IVC) diameter and its changes with respiratory manoeuvres can be easily assessed in the vast majority of patients with heart failure to estimate fluid status and right
Imaging of Congestion in Heart Failure CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 1: Imaging of Venous, Pulmonary and Renal Congestion by Ultrasound
The internal jugular vein ratio
B-lines
Renal venous flow
Valsalva/rest >4
Valsalva/rest <4
No B-lines
Some B-lines
Several B-lines +/ pleural e usion
Discontinuous
Continuous
With increasing severity of congestion (green to red/left to right; bottom arrow), the ratio between the diameter of the jugular vein during Valsalva to that at rest decreases (top panel), the number of B-lines increases and eventually pleural effusion develops (central panel) and the renal venous flow becomes discontinuous (bottom panel); Source: Created using Servier Medical Art. Used with permission under a Creative Commons CC BY licence.
atrial (RA) pressure.28 Almost 20% of outpatients with heart failure who are clinically free from signs or symptoms of congestion have a dilated IVC; interestingly, a dilated IVC can be also identified in >10% of those with cardiovascular risk factors only, such as diabetes or hypertension.29,30 An increasing IVC diameter is associated with a larger LA, higher natriuretic peptides and a greater risk of premature cardiovascular events, regardless of LVEF. Conversely, in patients with chronic heart failure, a non-dilated IVC that collapses >50% with inspiration is associated with better prognosis.31 For those who are hospitalised with heart failure, serial assessment of IVC diameter might be used to monitor response to diuretic therapy; for those in whom the IVC remains dilated at discharge, there is a high risk of an early readmission.32 On-going clinical trials are evaluating whether treatment guided by changes in IVC diameter improves management of congestion in patients hospitalised with heart failure (NCT04549701 and NCT03140566).
Jugular Vein Assessment
When assessment of the IVC is difficult, either because not tolerated or limited by the patient’s body habitus, RA pressures or intravascular volume can be estimated by ultrasonic evaluation of the internal jugular vein (IJV). The IJV lies in the carotid sheath, close to the carotid artery and is covered – superficially – by the sternocleidomastoid muscle in the neck. Using a high frequency transducer, the IJV can be easily imaged in every person.
With the patient’s head and neck reclined at 45–60º degrees, in normal haemodynamic conditions, the IJV is almost collapsed; its distension can be provoked by asking the patient to perform a Valsalva manoeuvre, causing a rapid increase in venous pressures. The ratio between the maximal IJV diameter during Valsalva to that at rest or the percentage of cross-sectional area change correlate with natriuretic peptides and RV function.33 34 An already distended jugular vein at rest will only marginally increase in size during a Valsalva: in that case, intravascular congestion is likely to be substantial, as is the risk of hospitalisation or death.35 36
Lung Ultrasound
Frank pulmonary oedema may be triggered by an acute event and usually presents as a symptomatic crisis. However, many patients with heart failure have persistent pulmonary congestion that is not clinically or radiologically obvious in routine clinical practice. Ultrasound is a quick and simple test that can be performed in minutes at the bedside to identify de-aerated segments of the lung, the consequence of which will be vertical artefacts, originating from the pleura that traverse the ultrasound screen, called B-lines. In a breathless patient, multiple (i.e. three or more in a single intercostal space, usually called ‘chest zone’), diffuse (i.e. in more than one chest zone) and bilateral B-lines suggest a diagnosis of heart failure.37 However, other conditions – for instance, adult respiratory distress syndrome or pulmonary fibrosis – may produce lung B-lines and distinguishing among them always requires a global assessment of the patient.38
Different protocols for the assessment of B-lines exist.39 Overall, an increasing number of B-lines is associated with a faster respiratory rate, higher natriuretic peptides and a decreasing BMI; therefore, additional care must be taken when interpreting their number and distribution in obese patients.40 41 Not surprisingly, in patients with either acute or chronic heart failure, an elevated number of B-lines is associated with a greater risk of cardiovascular events and there is accumulating evidence suggesting that it could be a therapeutic target.42,43 The use of lung ultrasound might also facilitate identification of other frequent
complications of heart failure, such as pleural effusions, or concurrent lung diseases, such as pneumonia.44
Renal Ultrasound
Heart failure is, in essence, a cardio-renal syndrome: assessment of blood flow within the kidney by ultrasound provides important clinical information. While a few studies suggest that impaired renal arterial flow correlates with higher natriuretic peptides and a poorer outcome, in recent years Doppler assessment of the interlobar veins has received particular attention to estimate central venous pressure (CVP) and intraparenchymal congestion of the kidneys.45,46 With a normal CVP, there is continuous renal venous flow during the whole cardiac cycle; as renal and intravascular congestion worsen, the flow pattern becomes discontinuous with two (systolic and diastolic; biphasic) or one (diastolic; monophasic) component in more severe cases, suggesting the need for urgent therapeutic action and a poor prognosis.47–49 The use of a convex probe might facilitate the study of the kidney venous flow; however, visualisation of the interlobar veins can be difficult at times, particularly in those with severe renal dysfunction or who are unable to hold their breath for long periods to avoid diaphragmatic movements that interfere with correct renal evaluation by ultrasound.
Other Cardiac Imaging Modalities
Coronary artery disease (CAD) is one of the most common causes of heart failure; its identification is important for risk stratification and – particularly in younger patients – therapeutic decisions.50 Although the STICH trial did not demonstrate that coronary artery bypass graft surgery reduced allcause mortality in patients with an LVEF ≤35%, extended follow-up suggested a survival benefit, at least for younger patients.51 Excluding CAD in patients with heart failure with a reduced LVEF might also be useful in selecting patients for an ICD.52 Patients with CAD and wellcontrolled heart failure might also benefit from the addition of rivaroxaban 2.5 mg twice daily and statins.53,54 In order to exclude important CAD, guidelines now recommend that CT coronary angiography may be considered.55 For those who are symptomatic, either due to angina or to ventricular arrhythmias, invasive coronary angiography is currently recommended to assess severity and extent of CAD. CMR imaging can also be used to assess for the presence of significant CAD with stress perfusion imaging, or for evidence of scar from a prior MI with late gadolinium enhancement (LGE).56 57
CMR is also considered the gold standard test for assessing cardiac chamber volumes and, therefore, ejection fraction. CMR is angleindependent and not limited by factors that may have an impact on the quality of an echocardiographic exam such as a poor acoustic window or body habitus.58 CMR has the unique ability among cardiac imaging techniques to provide detailed tissue characterisation, through the use of LGE and non-contrast tissue characterisation with cardiac mapping. Specifically, CMR can identify areas and patterns of myocardial fibrosis or scar, inflammation, fatty infiltration or iron overload, as well as quantifying extracellular volume. CMR’s ability to characterise tissue can be particularly useful in determining the aetiology of heart failure, to improve risk stratification, or to guide individualised treatments in those with suspected sarcoidosis, amyloidosis or other infiltrative or inflammatory cardiomyopathies.56–59 CMR shows that a surprisingly high number of people have myocardial scar consistent with a previous MI despite having no history of such an event. Patients with unrecognised (silent) MI have a similar prognosis to those with a recognised event, suggesting that the gadolinium-enhancement is not merely an artefact.60 Of note (see below), patients with heart failure also have a high prevalence of unrecognised
Imaging of Congestion in Heart Failure CARDIAC FAILURE REVIEW www.CFRjournal.com
(silent) cerebral infarctions. CMR does have limitations in terms of availability as the scanners are expensive; arrhythmia can reduce the accuracy of image quality and some implantable devices are not CMR conditional. For those with heart failure with cardiac or extracardiac red flags of transthyretin amyloidosis, 99mTc-DPD scintigraphy has a very high specificity and positive predictive diagnostic value for diagnosis.61 An endomyocardial biopsy is usually reserved for selected cases, even in those with myocarditis presenting as heart failure.62
Other Organs
The Bowel
Heart failure may be associated with loss of appetite that – combined with hepatic and intestinal congestion – may lead to iron malabsorption, malnutrition and cachexia.63 There is some evidence that patients with chronic heart failure have increased colonic wall thickness, perhaps reflecting oedema and reduced intestinal blood flow. This, in turn, may result in changes in the gut microbiota, triggering a systemic inflammatory response that may accelerate heart failure progression and death.64,65 Bowel-wall thickness can be assessed by ultrasound and, when increased, is associated with greater congestion and poorer outcomes in patients with heart failure.63,66
The Brain
The relationship between heart and brain dysfunction is complex. Many patients with heart failure report a variety of neurological symptoms, ranging from cognitive impairment and loss of attention to anxiety and depression.67 68 Advanced age and hypoperfusion secondary to a reduced cardiac output or hypotension and atherosclerotic disease of the cerebral vessels might be key drivers of degenerative brain changes associated with heart failure.69 However, micro- or macroembolic events, in the context of atherosclerotic disease in the carotids or an intracardiac thrombus, and/or the presence of other comorbidities common in heart failure such as hypertension, diabetes or AF, might also contribute to and accelerate brain damage. MRI scans suggest that patients with heart failure have several structural cerebral abnormalities, including white matter hyper-intensities, lacunar and cortical infarcts and cortical atrophy, even in the absence of a prior history of neurological symptoms or disease, similar to the high prevalence of silent MI noted above.70 In
1. Pellicori P, Fitchett D, Kosiborod MN, et al. Use of diuretics and outcomes in patients with type 2 diabetes: findings from the EMPA-REG OUTCOME trial. Eur J Heart Fail 2021;23:1085–93. https://doi.org/10.1002/ejhf.2220; PMID: 34031968.
2. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021;27:387–413. https://doi. org/10.1016/j.cardfail.2021.01.022; PMID: 33663906.
3. Cleland JGF, Pfeffer MA, Clark AL, et al. The struggle towards a universal definition of heart failure-how to proceed? Eur Heart J 2021;42:2331–43. https://doi. org/10.1093/eurheartj/ehab082; PMID: 33791787.
4. Taylor CJ, Ordóñez-Mena JM, Roalfe A, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ 2019;364:l223. https://doi.org/10.1136/bmj.l223; PMID: 30760447.
5. Cleland JGF, Ferreira JP, Mariottoni B, et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the Heart “OMics” in AGEing (HOMAGE) randomized clinical trial. Eur Heart J 2021;42:684–96. https://doi. org/10.1093/eurheartj/ehaa758; PMID: 33215209.
6. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98. https://doi.org/10.1056/
patients with heart failure, an inverse correlation has been reported between the density of cerebral grey matter in the hippocampus – a region implicated in development of cognitive dysfunction and memory loss – and N-terminal pro-B-type natriuretic peptide concentration; however, for those with less advanced heart failure, progression of hippocampal atrophy over time is minimal and perhaps not different from that expected due to ageing.71–73
Skeletal Muscle
A loss of muscle mass, quality and/or strength – sarcopaenia – occurs in patients with advanced heart failure but, as with so many other aspects of heart failure, its presence and severity often go unrecognised until it is extreme and the patient is overtly cachectic.74 Sarcopaenia, even when not clinically obvious, is associated with adverse outcomes. In clinical practice and research, several imaging methods are available to quantify muscle mass. MRI provides detailed information on muscle quantity and is a non-invasive gold standard tool for research in this setting but is not suitable for clinical use on a large scale due to high costs and limited access.75 Dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) are widely available, affordable and easy to use in an ambulatory setting even by non-medical personnel.76 Although there is a strong correlation between muscle mass measured with these two techniques (correlation coefficient >0.9), some studies suggest that BIA might produce higher readings for muscle mass compared with DEXA. In patients with heart failure, muscle mass measured with both techniques is only weakly associated with age or biomarkers of cardiac stress or inflammation but more closely related to other measures of body size, such as BMI or waist or hip circumference.77
Conclusion
Modern imaging methods enable congestion – both haemodynamic/ vascular and in tissues – to be identified and quantified objectively. Patients with cardiac dysfunction who have evidence of congestion on imaging are at increased risk of disease progression, heart failure decompensation and death, even if their symptoms and clinical signs appear adequately controlled. Whether early identification and treatment of congestion will improve outcomes is controversial, but accumulating evidence suggests that this might be the case.
NEJMoa0801369; PMID: 18378519.
7. Pellicori P, Platz E, Dauw J, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail 2021;23:703–12. https://doi.org/10.1002/ ejhf.2032; PMID: 33118672.
8. Marwick TH. The role of echocardiography in heart failure. J Nucl Med 2015;56(Suppl 4):31S–8S. https://doi.org/10.2967/ jnumed.114.150433; PMID: 26033901.
9. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/ HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2022;79:1757–80. https://doi.org/10.1016/j.jacc.2021.12.011; PMID: 35379504.
10. Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol 2007;50:381–96. https://doi. org/10.1016/j.jacc.2007.03.048; PMID: 17662389.
11. Marwick TH. Ejection fraction pros and cons: JACC state-ofthe-art review. J Am Coll Cardiol 2018;72:2360–79. https:// doi.org/10.1016/j.jacc.2018.08.2162; PMID: 30384893.
12. Benameur N, Arous Y, Ben Abdallah N, Kraiem T. Comparison between 3D echocardiography and cardiac magnetic resonance imaging (CMRI) in the measurement of left ventricular volumes and ejection fraction. Curr Med Imaging Rev 2019;15:654–60. https://doi.org/10.2174/1573405 614666180815115756; PMID: 32008513.
13. Poon J, Leung JT, Leung DY. 3D echo in routine clinical practice - state of the art in 2019. Heart Lung Circ 2019;28:1400–10. https://doi.org/10.1016/j.hlc.2019.04.003;
PMID: 31047786.
14. Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:833–40. https://doi.org/10.1093/ehjci/jex140; PMID: 28637227.
15. Nishikage T, Nakai H, Mor-Avi V, et al. Quantitative assessment of left ventricular volume and ejection fraction using two-dimensional speckle tracking echocardiography. Eur J Echocardiogr 2009;10:82–8. https://doi.org/10.1093/ ejechocard/jen166; PMID: 18490270.
16. Pellicori P, Kallvikbacka-Bennett A, Khaleva O, et al. Global longitudinal strain in patients with suspected heart failure and a normal ejection fraction: does it improve diagnosis and risk stratification? Int J Cardiovasc Imaging 2014;30:69–79. https:// doi.org/10.1007/s10554-013-0310-y; PMID: 24150723.
17. Cohen OC, Ismael A, Pawarova B, et al. Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis. Eur Heart J 2022;43:333–41. https://doi.org/10.1093/eurheartj/ehab507; PMID: 34472567.
18. Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation 1995;92:835–41. https://doi. org/10.1161/01.cir.92.4.835; PMID: 7641364
19. Laukkanen JA, Kurl S, Eränen J, et al. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med 2005;165:1788–93. https://doi.org/10.1001/ archinte.165.15.1788; PMID: 16087829.
20. Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial
Imaging of Congestion in Heart Failure CARDIAC FAILURE REVIEW www.CFRjournal.com
structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278–85. https://doi. org/10.1093/eurheartj/ehs188; PMID: 22782941.
21. Rossi A, Temporelli PL, Quintana M, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009;11:929–36. https://doi.org/10.1093/eurjhf/hfp112; PMID: 19789395.
22. Pellicori P, Zhang J, Lukaschuk E, et al. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J 2015;36:733–42. https://doi. org/10.1093/eurheartj/ehu405; PMID: 25336215.
23. Inciardi RM, Claggett B, Minamisawa M, et al. Association of left atrial structure and function with heart failure in older adults. J Am Coll Cardiol 2022;79:1549–61. https://doi. org/10.1016/j.jacc.2022.01.053; PMID: 35450571.
24. Reddy YNV, Obokata M, Egbe A, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891–900. https://doi.org/10.1002/ejhf.1464; PMID: 30919562.
25. Ravassa S, López B, Ferreira JP, et al. Biomarker-based assessment of collagen cross-linking identifies patients at risk of heart failure more likely to benefit from spironolactone effects on left atrial remodelling. Insights from the HOMAGE clinical trial. Eur J Heart Fail 2022;24:321–31. https://doi.org/10.1002/ejhf.2394; PMID: 34841615.
26. Pellicori P, Cleland JG, Zhang J, et al. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther 2016;30:599–609. https://doi.org/10.1007/s10557-016-6697-7; PMID: 27819111.
27. Iaconelli A, Pellicori P, Caiazzo E, et al. Implanted haemodynamic telemonitoring devices to guide management of heart failure: a review and meta-analysis of randomised trials. Clin Res Cardiol 2022:1–13. https://doi. org/10.1007/s00392-022-02104-0; PMID: 36241896.
28. Elzeneini M, Gupta S, Li Y, et al. Estimation of right atrial pressure using a portable handheld ultrasound device. Am J Med 2022;135:1378–81. https://doi.org/10.1016/j. amjmed.2022.05.018; PMID: 35636478.
29. Pellicori P, Shah P, Cuthbert J, et al. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019;21:904–16. https://doi.org/10.1002/ejhf.1383; PMID: 30666769.
30. Cuthbert JJ, Pellicori P, Flockton R, et al. The prevalence and clinical associations of ultrasound measures of congestion in patients at risk of developing heart failure. Eur J Heart Fail 2021;23:1831–40. https://doi.org/10.1002/ ejhf.2353; PMID: 34632680.
31. Pellicori P, Carubelli V, Zhang J, et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging 2013;6:16–28. https://doi.org/10.1016/j.jcmg.2012.08.012;
PMID: 23328557.
32. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging 2008;1:595–601. https://doi.org/10.1016/j.jcmg.2008.06.005;
PMID: 19356487.
33. Simon MA, Schnatz RG, Romeo JD, Pacella JJ. Bedside ultrasound assessment of jugular venous compliance as a potential point-of-care method to predict acute decompensated heart failure 30-day readmission. J Am Heart Assoc 2018;7:e008184. https://doi.org/10.1161/ JAHA.117.008184; PMID: 30371245.
34. Pellicori P, Kallvikbacka-Bennett A, Zhang J, et al. Revisiting a classical clinical sign: jugular venous ultrasound. Int J Cardiol 2014;170:364–70. https://doi.org/10.1016/j. ijcard.2013.11.015; PMID: 24315339.
35. Pellicori P, Kallvikbacka-Bennett A, Dierckx R, et al. Prognostic significance of ultrasound-assessed jugular vein distensibility in heart failure. Heart 2015;101:1149–58. https:// doi.org/10.1136/heartjnl-2015-307558; PMID: 26006717.
36. Pellicori P, Clark AL, Kallvikbacka-Bennett A, et al. Noninvasive measurement of right atrial pressure by nearinfrared spectroscopy: preliminary experience. A report from the SICA-HF study. Eur J Heart Fail 2017;19:883–92. https:// doi.org/10.1002/ejhf.825; PMID: 28387033.
37. Gargani L. Ultrasound of the lungs: more than a room with a view. Heart Fail Clin 2019;15:297–303. https://doi. org/10.1016/j.hfc.2018.12.010; PMID: 30832819.
38. Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 2011;9:6. https://doi.org/10.1186/14767120-9-6; PMID: 21352576.
39. Platz E, Jhund PS, Girerd N, et al. Expert consensus document: reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure.
Eur J Heart Fail 2019;21:844–51. https://doi.org/10.1002/ ejhf.1499; PMID: 31218825.
40. Palazzuoli A, Ruocco G, Beltrami M, et al. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 2018;107:586–96. https://doi.org/10.1007/s00392-018-1221-7; PMID: 29532155.
41. Palazzuoli A, Ruocco G, Franci B, et al. Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin Res Cardiol 2020;109:1423–33. https:// doi.org/10.1007/s00392-020-01642-9; PMID: 32296972.
42. Platz E, Merz AA, Jhund PS, et al. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017;19:1154–63. https://doi. org/10.1002/ejhf.839; PMID: 28557302; PMCID: PMC5731779
43. Rastogi T, Bozec E, Pellicori P, et al. Prognostic value and therapeutic utility of lung ultrasound in acute and chronic heart failure: a meta-analysis. JACC Cardiovasc Imaging 2022;15:950–2. https://doi.org/10.1016/j.jcmg.2021.11.024; 35033496.
44. Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med 2021;47:444–54. https:// doi.org/10.1007/s00134-021-06373-7; PMID: 33743018.
45. Ciccone MM, Iacoviello M, Gesualdo L, et al. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 2014;16:210–6. https://doi. org/10.1002/ejhf.34; PMID: 24464953.
46. Tang WH, Kitai T. Intrarenal venous flow: a window into the congestive kidney failure phenotype of heart failure? JACC Heart Fail 2016;4:683–6. https://doi.org/10.1016/j. jchf.2016.05.009; PMID: 27395345.
47. Pugliese NR, Pellicori P, Filidei F, et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur Heart J Cardiovasc Imaging 2023:jeac254. https://doi.org/10.1093/ ehjci/jeac254; PMID: 36595324.
48. Nijst P, Martens P, Dupont M, et al. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail 2017;5:672–81. https://doi.org/10.1016/j.jchf.2017.05.006; PMID: 28711449.
49. Iida N, Seo Y, Sai S, et al. Clinical implications of intrarenal hemodynamic evaluation by doppler ultrasonography in heart failure. JACC Heart Fail 2016;4:674–82. https://doi. org/10.1016/j.jchf.2016.03.016; PMID: 27179835.
50. Rastogi T, Ho FK, Rossignol P, et al. Comparing and contrasting risk factors for heart failure in patients with and without history of myocardial infarction: data from HOMAGE and the UK biobank. Eur J Heart Fail 2022;24:976–84. https://doi.org/10.1002/ejhf.2495; PMID: 35365899.
51. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20. https://doi.org/10.1056/ NEJMoa1602001; PMID: 27040723.
52. Yafasova A, Butt JH, Elming MB, et al. Long-term follow-up of Danish (the Danish study to assess the efficacy of ICDs in patients with nonischemic systolic heart failure on mortality). Circulation 2022;145:427–36. https://doi.org/10.1161/ CIRCULATIONAHA.121.056072; PMID: 34882430.
53. Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease. Circulation 2019;140:529–37. https://doi.org/10.1161/ CIRCULATIONAHA.119.039609; PMID: 31163978.
54. Cleland JG, Squire I, Ng L. Interpretation of amino-terminal pro-brain natriuretic peptide levels in the HPS and the CORONA study. J Am Coll Cardiol 2008;52:1104–5. https://doi. org/10.1016/j.jacc.2008.04.070; PMID: 18848145.
55. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. https://doi. org/10.1002/ejhf.2333; PMID: 35083827.
56. Karamitsos TD, Arvanitaki A, Karvounis H, et al. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging 2020;13:1221–34. https://doi.org/10.1016/j. jcmg.2019.06.030; PMID: 31542534.
57. McDiarmid AK, Pellicori P, Cleland JG, Plein S. Taxonomy of segmental myocardial systolic dysfunction. Eur Heart J 2017;38:942–54. https://doi.org/10.1093/eurheartj/ehw140; PMID: 27147609.
58. Peterzan MA, Rider OJ, Anderson LJ. The role of cardiovascular magnetic resonance imaging in heart failure. Card Fail Rev 2016;2:115–22. https://doi.org/10.15420/
cfr.2016.2.2.115; PMID: 28785465.
59. Menghoum N, Vos JL, Pouleur AC, et al. How to evaluate cardiomyopathies by cardiovascular magnetic resonance parametric mapping and late gadolinium enhancement. Eur Heart J Cardiovasc Imaging 2022;23:587–9. https://doi. org/10.1093/ehjci/jeac051; PMID: 35262680.
60. Acharya T, Aspelund T, Jonasson TF, et al. Association of unrecognized myocardial infarction with long-term outcomes in community-dwelling older adults: the ICELAND MI study. JAMA Cardiol 2018;3:1101–6. https://doi.org/10.1001/ jamacardio.2018.3285; PMID: 30304454.
61. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. https://doi.org/10.1161/ CIRCULATIONAHA.116.021612; PMID: 27143678.
62. Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70:1964–76. https://doi.org/10.1016/j. jacc.2017.08.050; PMID: 29025553.
63. Valentova M, von Haehling S, Bauditz J, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J 2016;37:1684–91. https://doi.org/10.1093/ eurheartj/ehw008; PMID: 26865478.
64. Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 2014;64:1092–102. https://doi.org/10.1016/j. jacc.2014.06.1179; PMID: 25212642.
65. Pellicori P, Zhang J, Cuthbert J, et al. High-sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes, and mode of death. Cardiovasc Res 2020;116:91–100. https://doi.org/10.1093/cvr/cvz198; PMID: 31350553.
66. Ikeda Y, Ishii S, Maemura K, et al. Association between intestinal oedema and oral loop diuretic resistance in hospitalized patients with acute heart failure. ESC Heart Fail 2021;8:4067–76. https://doi.org/10.1002/ehf2.13525; PMID: 34323025.
67. Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: systematic review and metaanalysis. J Card Fail 2017;23:464–75. https://doi.org/10.1016/j. cardfail.2017.04.007; PMID: 28433667.
68. Sokoreli I, Pauws SC, Steyerberg EW, et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail 2018;20:689–96. https://doi.org/10.1002/ejhf.1112; PMID: 29314447.
69. Sherwi N, Wong K, Zhang J, et al. The prevalence of extracranial carotid artery disease in chronic heart failure. Cardiol Angiol Int J 2014;3:17–26. https://doi.org/10.9734/ CA/2015/10584
70. Vogels RL, van der Flier WM, van Harten B, et al. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 2007;9:1003–9. https://doi. org/10.1016/j.ejheart.2007.07.006; PMID: 17719270.
71. Mueller K, Thiel F, Beutner F, et al. Brain damage with heart failure: cardiac biomarker alterations and gray matter decline. Circ Res 2020;126:750–64. https://doi.org/10.1161/ CIRCRESAHA.119.315813; PMID: 31969053.
72. Woo MA, Ogren JA, Abouzeid CM, et al. Regional hippocampal damage in heart failure. Eur J Heart Fail 2015;17:494–500. https://doi.org/10.1002/ejhf.241; PMID: 25704495.
73. Frey A, Homola GA, Henneges C, et al. Temporal changes in total and hippocampal brain volume and cognitive function in patients with chronic heart failure-the COGNITION. MATTERS-HF cohort study. Eur Heart J 2021;42:1569–78. https://doi.org/10.1093/eurheartj/ehab003; PMID: 33496311.
74. Zhang Y, Zhang J, Ni W, et al. Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail 2021;8:1007–17. https://doi.org/10.1002/ehf2.13255; PMID: 33576177.
75. Kumar A, Ansari BA, Kim J, et al. Axial muscle size as a strong predictor of death in subjects with and without heart failure. J Am Heart Assoc 2019;8:e010554. https://doi. org/10.1161/JAHA.118.010554; PMID: 30755074.
76. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 2017;8:187–9. https://doi.org/10.1002/jcsm.12159; PMID: 28145079.
77. Shah P, Abel AAI, Boyalla V, et al. A comparison of noninvasive methods of measuring body composition in patients with heart failure: a report from SICA-HF. ESC Heart Fail 2021;8:3929–34. https://doi.org/10.1002/ehf2.13402; PMID: 34216106.
of Congestion
CARDIAC FAILURE REVIEW www.CFRjournal.com
Imaging
in Heart Failure
Global Public Health Burden of Heart Failure: An Updated Review
Bahira Shahim , 1,2 Chris J Kapelios , 3 Gianluigi Savarese 1,2 and Lars H Lund 1,2
Abstract
Heart failure (HF) is a rapidly growing public health issue with an estimated prevalence of 64 million people globally. Although the incidence of HF has stabilised worldwide and seems to be declining in developed countries, the prevalence is increasing due to the ageing of the population, improved survival after MI and improved treatment and survival of patients with HF. Yet, HF remains associated with high mortality and morbidity, poor quality of life and functional capacity, and confers a substantial burden to the healthcare system. The prevalence, incidence, mortality and morbidity rates reported show geographical variations, depending on the different aetiologies and clinical characteristics observed among patients with HF. In this review, we provide an overview of the global epidemiology of HF with updated data on prevalence, incidence, mortality and morbidity worldwide.
Keywords
Heart failure, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, heart failure with mildly reduced ejection fraction, prevalence, incidence, mortality, morbidity, epidemiology
Disclosures: GS has received funding from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Merck, Cytokinetics, Novartis, Boston Scientific, Pharmacosmos, Bayer and Horizon; consulting fees from Teva, MedEd Global Solutions, Genesis Medical, Agence Nationale de la Recherche, Muir Health, Atheneum and Vifor Pharma; honoraria from Servier, Cytokinetics, Medtronic, Dynamicom Education, Vifor Pharma, Roche, TMA Healthcare Ltd, MedEd Global Solutions, AstraZeneca, Novartis; and has participated on advisory boards for AstraZeneca, Uppsala Clinical Research Center, Servier, Edwards and Vifor Pharma. LHL has received grants from AstraZeneca, Vifor Pharma, Boston Scientific, Boehringer Ingelheim, Novartis; consulting fees from Merck, Vifor Pharma, AstraZeneca, Bayer, Pharmacosmos, MSD, Medscape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, Servier, Edwards Life Sciences and Alleviant Medical; speaker’s honoraria from Abbott, Orion Pharma, Medscape, Radcliffe, AstraZeneca, Novartis, Boehringer Ingelheim and Bayer; has stock ownership in AnaCardio; is a board member for the European Society of Cardiology Heart Failure Association and the Swedish Society of Cardiology; and is on the Cardiac Failure Review editorial board; this did not influence peer review. All other authors have no conflicts of interest to declare.
Received: 15 March 2023 Accepted: 10 May 2023 Citation: Cardiac Failure Review 2023;9:e11. DOI: https://doi.org/10.15420/cfr.2023.05
Correspondence: Lars H Lund, Karolinska Institutet, Solna, S1:02, 171 76 Stockholm, Sweden. E: lars.lund@ki.se
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure (HF) is a clinical syndrome with different underlying aetiologies rather than being a specific disease. Traditionally, HF has been defined as a condition where there is a reduced ability of the heart to pump and/or fill with blood, or alternatively an inadequate cardiac output caused by a structural or functional abnormality, or adequate cardiac output secondary to compensatory neurohormonal activation and increased left ventricular filling pressure. Despite different definitions of HF, left ventricular ejection fraction (LVEF) has generally been viewed as the cornerstone of HF diagnosis, characterisation, prognosis and treatment selection.1–3 Natriuretic peptides, which are produced by the heart in response to increased wall inflammation and stress, also provide diagnostic and prognostic information for patients with HF.4 The risk of adverse outcomes is also predicted by elevated natriuretic peptides in patients without HF.5
A universal definition and classification of HF was proposed in 2021. HF was defined as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.4 The stages of HF were also revised as at risk for HF (stage A), pre-HF (stage B), symptomatic HF (stage C) and advanced HF
(stage D). Finally, HF classification based on ejection fraction (EF) ranges was revised, including HF with reduced ejection fraction (HFrEF; EF ≤40%), mildly reduced ejection fraction (HFmrEF; EF 41–49%), and HF with preserved ejection fraction (HFpEF; EF ≥50%). Additionally, a new entity, HF with improved EF (HFimpEF; baseline LVEF ≤40%, a ≥10 point increase from baseline LVEF, and a second measurement of LVEF >40%), was introduced to account for the dynamic and changing clinical trajectories of HF syndrome and the increasingly common scenario where EF improves substantially with treatment.6
HF is considered a pandemic affecting an estimated 64 million people worldwide.7 It is projected that the prevalence of HF will increase due to the ageing of the population. Most recent projections for the US suggest an increase in the prevalence of HF by about 46% from 2012 to 2030, with a corresponding increase in healthcare costs of about 127%.8
Epidemiology studies on HF have many limitations related to differences in definitions, population selection and lack of data from some geographical areas. In addition, most studies rely on administrative data, including International Classification of Disease (ICD) codes, which may be lacking for a significant proportion of HF patients and does not include
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com Clinical Syndromes
1. Division of Cardiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; 2. Heart and Vascular Theme, Karolinska University Hospital, Stockholm, Sweden; 3. Department of Cardiovascular Medicine, University of Utah Health Sciences Center, Salt Lake City, UT, US
Worldwide
Portugal Spain Germany Sweden UK Meta-analysis* US HongKongIndonesiaPhilippinesSouthKorea
EF data, self-reported data, which requires that the patients know their diagnosis, or hospital records, which cannot capture patients receiving care in outpatient settings.9 However, despite these limitations, we aim to provide a contemporary assessment of the burden of HF, providing data about its prevalence, incidence and outcomes.
Prevalence Europe and Northern America
A summary of estimated prevalence of HF across different countries is shown in Figure 1. In the 2019 European Society of Cardiology (ESC) Heart Failure Association (HFA) atlas, data on prevalence were available for 13 (31%) of the participating countries. The median crude prevalence of HF per 1,000 people was estimated at 17, ranging from ≤12 in Greece and Spain to >30 in Lithuania and Germany.10 The estimated prevalence of HF in Germany based on healthcare claims data of over 3 million inhabitants from 2009 to 2014 was 4%.11 In a population-based study of 4 million individuals from the UK, the age- and sex-adjusted prevalence of HF was 1.6% from 2002 to 2014.12 Although this prevalence was stable between 2002 and 2014, the absolute number of patients with HF increased by 23%. The 2021 American Heart Association Heart Disease and Stroke Statistics (NHANES) reported a prevalence of HF of about 2.5% based on self-reported data.8
A meta-analysis based on echocardiographic screening studies from 1997 to 2014 in the general population from developed countries reported a prevalence of any HF of 11.8% in people ≥60 years and about 1% among
Taiwan Thailand China India AustraliaSouthAmerica Africa
those <60 years.13 Previously unrecognised cases were also included. Considering that in developed countries about 30% of the adult population is estimated to be 60 years or older, extrapolation of these findings would result in an estimated prevalence of HF of 4.2% (11.8 × 0.30 + 1.0 × 0.70 = 4.2) in the adult population.14 This prevalence is about twice as high as the often-reported estimate of 2% for HF in the adult population at large. Furthermore, the difference between 4% and 2% illustrates that HF may remain undetected in over half of all cases.
The prevalence of HF also varies depending on LVEF-based phenotypes. In the ESC Long-Term Registry (ESC-HF-LT), 60% of patients were classified as HFrEF (EF <40%), 24% as HFmrEF (EF 40–49%) and 16% as HFpEF (HF ≥50%).15 Among patients enrolled in the nationwide Swedish HF registry in 2005–18, 53% had reduced EF, 23% mildly reduced EF, and 24% preserved EF.16 In the OPTIMIZE-HF registry from the US, 49% had HFrEF (EF <40%), 17% had HFmrEF (EF 40–50%), and 24% HFpEF (EF >50%).17 In the GWTG-HF study, including the Medicare population, 39% had HFrEF (EF <40%), 14% HFmrEF (EF 40–50%), and 47% HFpEF (EF ≥50%).18
Interestingly, studies indicate an increase in the prevalence of HFpEF while the prevalence of HFrEF seems to be stable or even declining. In a study of consecutive patients hospitalised with HF at Mayo Clinic Hospitals in Olmsted County, Minnesota, the proportion of the 6,076 patients represented by HFpEF increased from 38% to 54% between 1987 and 2001.19 In the Swedish HF Registry, the overall proportion of HFrEF in
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
ESCHFAAtlas Prevalence (%) 0 1 2 3 4 5 6 7 8
Figure 1: Prevalence of Heart Failure
1.7 4.4 0.9–6.8 3.9 1.8–2.2 1.6 2.5 4.2 2.0–3.0 5.0 1.0–2.0 0.6 6.0 0.4 1.3 1.0 1.0–2.0 1.0 Data missing
*Meta-analysis of studies from developed countries using echocardiographic case validation. ESC = European Society of Cardiology; HFA = Heart Failure Association.
2000–2004 versus 2013–2016 decreased (60% versus 49%) whereas the overall proportion of HFpEF increased (20% versus 30%).20
Asia, Australia, South America and Africa
A summary of the estimated prevalence of HF across different countries is shown in Figure 1. There is a concerning lack of epidemiological data from countries outside Europe and North America, especially from lower and middle-income countries, even though these are estimated to carry 80% of the cardiovascular disease burden.21 HF prevalence has been estimated to be 2–3% in Hong Kong, 5% in Indonesia, 1–2% in the Philippines, 0.6% in South Korea, 6% in Taiwan and 0.4% in Thailand.22 In the China Hypertension Survey, prevalence of HF was 1.3%, of whom 40% had HFrEF (EF <40%), 23% had HFmrEF (EF 40–49%) and 36% had HFpEF (EF ≥50%).23 There are no population-based studies in Japan on the epidemiology of HF but one report estimated a prevalence of left ventricular dysfunction of 0.8% among outpatients.24 In a multicentre cohort study from Japan including patients hospitalised for HF between 2013 and 2014, 36% had HFrEF (EF <40%), 21% had HFmrEF (EF 40–49%), and 43% had HFpEF (EF ≥50%).18 The prevalence of HF in India is estimated to be about 1%.25 Prevalence estimates in Australia range between 1% and 2%.26
In a systematic review, including studies between 1994–2014 from Latin American and Caribbean populations (most studies had been conducted in South America [92%] and mainly in Brazil [64%]), the prevalence of HF was estimated at 1%.27 To date, there are no population-based studies estimating prevalence and incidence in northern and sub-Saharan Africa. In a study from a hospital in Soweto, South Africa, serving 1.1 million people, 1,960 patients presented with HF in 2006 (163 per month), of whom 43% had newly diagnosed HF and 48% of these had HFpEF defined as a LVEF ≥45%.28
Incidence Europe and Northern America
A summary of the estimated incidence of HF across different countries is shown in Figure 2. The reported incidence of HF in European countries and the US ranges widely from one to nine cases per 1,000 person-years depending on the population studied and the diagnostic criteria used. In developed countries, incidence rates have stabilised between 1970 and 1990 and are now thought to be decreasing. Crude incidence statistics were available for 12 (29%) of the participating countries in the ESC HF atlas.10 The median annual incidence of HF per 1,000 person-years was 3.20 cases (ranging from <2 in Italy to ≥6 in Estonia and Germany). In the PREVEND study of 8,592 people in a Dutch community, the incidence rate of HF between 1998–2010 was 3.7 per 1,000 person-years in men and 2.4 per 1,000 person-years in women.29 Among these, 34% were classified as HFpEF (LVEF ≥50%) and 66% as HFrEF (LVEF <40%). Of note, only eight patients had LVEF 41–49%; hence, this category was excluded from the analysis. In a population-based study from the UK including more than 4 million people, a decline of 7% in the incidence of HF was observed between 2002 and 2014 from 3.6 to 3.3 per 1,000 person-years.12 These findings seemed to be largely driven by a decline in the incidence of HF in people aged 60–84 years. However, the incidence remained stable or increased in younger people (<55 years) and the very old (>85 years). In a national sample of hospitalised patients in Denmark, similar trends were observed in the incidence of HF between 1995 and 2012.30
In an analysis from the Cardiovascular Lifetime Risk Pooling Project, the incidence of HF was 7.9 per 1,000 person-years in the Chicago Heart
Association detection project in industry and 6.0 per 1,000 person-years in the ARIC study after the index age of 45 years.31–34 However, the incidence of HF was much higher – 21.1 per 1,000 person-years – after the index age of 65 years as observed in the Cardiovascular Health Study.35 In another pooled analysis of the Cardiovascular Health Study and the Framingham Study for participants who were ≥60 years of age and free of HF, the age- and sex-standardised HF incidence rates for 1990–99 and 2000–09 were overall similar at 19.7 and 18.9 per 1,000 person-years, respectively.36 However, divergent trends of decreasing HFrEF and increasing HFpEF incidence were observed. Although HFrEF incidence declined more in men than in women, men had a higher incidence of HFrEF than women in each decade, whereas incident HFpEF increased in both men and women. In contrast, a decline in the incidence of HF was observed in the Olmsted County cohort where the age- and sex-adjusted incidence of HF declined from 3.2 to 2.2 cases per 1,000 person-years between 2000 and 2010.37 The decline was greater in women (43%) than in men (29%) and greater in HFrEF (45%) than in HFpEF (28%).
Asia, Australia, South America and Africa
A summary of estimated incidence of HF across different countries is shown in Figure 2. The incidence of HF in India is estimated to be at least between 0.5 and 1.7 cases per 1,000 person-years, for a total of 492,000 to 1.8 million new cases per year.38 However, the age-specific incidence for India is unknown. In a study including 43 Australian general practices between 2013 and 2018, the age-standardised annual incidence of HF was 3.5 cases per 1,000 person-years.39 The estimated incidence rate of HF was 1.9 per 1,000 person-years in South America.27 Studies on the incidence of HF in Africa are lacking. A summary of estimated incidence of HF across different countries is shown in Figure 1
Demographic and Clinical Characteristics
Demographics and clinical characteristics in HF have been shown to differ considerably between LVEF-based HF categories (Table 1). Patients with HFpEF compared with HFrEF are more likely older, women, have a higher prevalence of hypertension, higher mean pulse pressure, obesity, AF and anaemia and suffer more often from comorbidities, such as chronic kidney disease, chronic pulmonary disease,
disease and
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
valvular heart
cancer,
ESCHFAAtlas SpainGermanySwedenDenmarkScotland UK US Canada SouthAmerica India Africa 3.2 Incidence (per 1,000 person-years) 2.9–3.9 3.3 3.8 3.1 1.0–1.2* 3.3–3.6 2.2–2.6 4.5* 0.5–1.7 1.9 Data missing 5 4 3 2 1 0
Figure 2: Incidence of Heart Failure Worldwide
*Per 1,000 population. ESC = European Society of Cardiology; HFA = Heart Failure Association.
Table 1. Characteristics and Prognosis of HFrEF, HFmrEF and HFpEF
south Asia (India), south-east Asia (Thailand, Malaysia, the Philippines, Indonesia, Singapore) and north-east Asia (South Korea, Japan, Taiwan, Hong Kong, China), striking regional differences were observed in patient characteristics.66 South-east Asians had the highest burden of comorbidities, particularly diabetes and chronic kidney disease, despite being younger than north-east Asian participants. However, the 23,000-patient global CHF registry (G-CHF), suggests that quality of life in HF is universally poor across the globe and that poor quality of life is a predictor of increased risk of HF hospitalisation and all-cause mortality.67
Outcomes Europe and North America
Although outcomes in HF in Europe and North America have been extensively studied, estimates of the mortality of HF vary considerably depending on the age and comorbidity profile of the population studied, definitions of HF and among inpatients and outpatients. For instance, mortality rates are higher in observational studies in contrast to clinical trials, which have included outpatients who are younger with a lower comorbidity burden. Outcomes also differ across LVEF-based HF phenotypes.
higher or more common and ↓ denotes lower or less common than in an age-matched control population, respectively. HFmrEF = heart failure with mildly reduced ejection fraction;
= heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
whereas coronary artery disease is the main determinant of HFrEF.15 40–49 In recent years, HFmrEF has been recognised as a potentially distinct entity sharing features with both HFpEF, such as hypertension, milder HF symptoms, lower levels of natriuretic peptides and accordingly lower CV event rates, including HF, and worsening of function and HFrEF, such as a higher prevalence of coronary artery disease and less frequent chronic kidney disease.42,46,48,50–52 The totality of the evidence suggests that in terms of pathophysiology, clinical characteristics, and response to therapy, HFmrEF resembles, on average, more HFrEF than HFpEF.3 4 53
Although valvular heart disease is shown to be common in HFpEF, most available data are from single-centre studies, clinical trials with narrowly selected populations or population-based studies in which patients were not yet diagnosed with HF.54–62 In addition, they lack within-cohort comparisons of the characteristics and consequences of all valvular heart diseases with regards to LVEF-based categories. However, in two studies from the ESC-HF-LT registry with echocardiographic data on LVEF and moderate-to-severe valvular heart diseases, HFpEF seemed to be distinct from HFmrEF and HFrEF with isolated tricuspid regurgitation and aortic stenosis being more prevalent in HFpEF, mitral regurgitation more prevalent with HFrEF and aortic regurgitation having a similar distribution across all HF categories.63,64
Most studies describing HF characteristics have been performed in North America and Europe, although regional differences in phenotypes of HF patients are likely to exist due to different aetiologies, comorbidities, economic and healthcare systems. In the INTER-CHF study, patients with HF in Africa and Asia were younger than in other regions and more likely to be men.65 Ischaemic heart disease was the most common HF aetiology in all regions except Africa where hypertensive heart disease was most common. In the prospective ASIAN HF registry recruiting patients from
In the ESC-HF-LT registry enrolling 12,440 patients from 21 European and/ or Mediterranean countries between 2011 and 2013, 1-year mortality rates differed between acute and chronic HF (23.6% versus 6.4%) and across countries (21–36.5% for acute HF and 6.9–15.6% for chronic HF).68 In addition, 1-year mortality rates in outpatients differed between HFrEF and HFpEF (8.8% versus 6.3%), while patients with HFmREF experienced intermediate rates (7.6%).15 In acute HF, HFrEF is more severe and has greater in-hospital mortality. Post-discharge, HFrEF has greater CV risk, HFpEF greater non-CV risk, and HFmrEF lower overall risk.41 In the nationwide Swedish HF Registry, crude 1-year mortality rates among 42,061 outpatients were 15.4% in HFrEF, 17.4% in HFpEF and 14.2% in HFmrEF.69 However, the covariate-adjusted risk of 1-year mortality was higher in HFrEF compared with HFpEF (HR 1.26, 95% CI [1.17–1.35]). Similarly, the large MAGGIC study, pooling data from 30 observational studies and clinical trials, reported that patients with HFpEF had a 32% lower adjusted risk of 3-year mortality compared to their HFrEF counterparts. In the ECHOES study including 6,162 patients at a mean age of 64, 10-year survival was 27% for those with definitive HF and 75.4% for those without HF.70 Stratifying by LVEF category, 10-year survival was 76% for patients with LVEF >50%, 48% for LVEF 40–50% and only 31% for those with LVEF <40%. In the GWTG-HF registry of 39,982 patients hospitalised for HF between 2005 and 2009 in the US, a 5-year mortality rate of 75% was reported and mortality rates were similar in patients with HFrEF (75.3%) and HFpEF (75.7%).71 The OPTIMIZE-HF study enrolling 20,118 patients with HFrEF and 21,149 with HFpEF (EF ≥40%) reported a higher in-hospital mortality in HFrEF (3.9%) than in HFpEF patients (2.9%). However, the 30–60-day mortality rates were similar (9.8% versus 9.5%) in HFrEF compared to HFpEF. No differences in outcomes were observed when analysis was stratified according to HFpEF (EF >50%) and HFmrEF (EF 40–50%).17
In a recent systematic review of 60 studies across high-income countries including 1.5 million patients, pooled survival rates at 1 month, 1, 2, 5 and 10 years were 95.7%, 86.5%, 72.6%, 56.7% and 34.9%, respectively.72 The 5-year survival rates improved between 1970–79 and 2000–09 from 29.1% to 59.7%, likely reflecting improved treatment of acute MI and evidence-based and effective treatment options for HF. In the Olmsted County study, survival rates after HF diagnosis improved during the early 1990s and early- to mid-2000s but seemingly levelled off thereafter,
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
HFrEF HFmrEF HFpEF Characteristics Age ↑ ↑↑ ↑↑↑ Women ↓↓ ↓ ↑ Ischaemic heart disease ↑↑↑ ↑↑↑ ↑ AF ↑ ↑↑ ↑↑↑ Hypertension ↑ ↑↑ ↑↑↑ Diabetes ↑↑↑ ↑↑↑ ↑↑↑ Chronic kidney disease ↑↑ ↑↑ ↑↑↑ Natriuretic peptide levels ↑↑↑ ↑ ↑ Valvular heart disease Mitral regurgitation ↑↑↑ ↑↑ ↑ Tricuspid regurgitation ↑ ↑ ↑↑ Aortic stenosis ↑ ↑ ↑↑ Aortic regurgitation ↑ ↑ ↑ Prognosis Cardiovascular risk ↑↑↑ ↑ ↑ Non-cardiovascular risk ↑ ↑ ↑↑ ↑
HFpEF
denotes
possibly reflecting the transition from HFrEF to HFpEF for which effective evidence-based strategies are still largely lacking, and the increasing comorbidity burden in HF.37
Outcomes in HF may fluctuate and change over time. It has been reported that after the initial months following diagnosis of HF, outcomes might improve due to implementation of guideline-directed medical therapy.73 Therefore, repeat assessment of the risk of death from HF should be considered for optimal patient care.
Asia, Australia, South America, and Africa
In the prospective ASIAN HF registry, all-cause mortality for the whole population was 9.6% at 1 year and higher in patients with HFrEF (10.6%) than in those with HFpEF (5.4%).66 One-year, all-cause mortality was significantly higher in south-east Asian patients (13.0%), compared with south Asian (7.5%) and north-east Asian patients (7.4%). In the prospective China-HF registry, in-hospital mortality was 4.1% and significantly higher in patients with HFrEF (4.0%) versus HFpEF (2.4%).74 In the Japanese Cardiac Registry of Heart Failure in Cardiology, in-hospital mortality was higher in HFpEF patients (6.5%) compared with HFrEF patients (3.9%).75 Similarly, 1-year mortality rates were also higher in HFpEF (11.6%) than in HFrEF (8.9%). In a meta-analysis of 12 studies enrolling 67,255 patients hospitalised for HF between 1990 and 2016 in Australia, the pooled estimated 30-day and 1-year mortality rates were 8% and 25%, respectively.76
In the INTER-CHF prospective cohort study of 5,823 patients, overall mortality was 16.5%: highest in Africa (34%) and India (23%), intermediate in south-east Asia (15%), and lowest in China (7%), South America (9%) and the Middle East (9%).65 Notably, these regional differences remained even after multivariable adjustment. Patients in Africa, India and south-east Asia were on average 10 years younger than those in South America and China but had higher mortality rates. In the REPORT-HF, a global registry enrolling patients during hospitalisation for acute HF from 44 countries on six continents, patients from eastern Europe had the lowest 1-year mortality (16%) and those from eastern Mediterranean and Africa (22%) and Latin America (22%) had the highest.77 A large inter-country variation was observed, ranging from 10% in Bulgaria to 32% in Indonesia. Ageadjusted and HF diagnosis-adjusted mortality (new onset versus chronic HF) were higher in patients from lower-income countries (26%) compared with middle-income (20%) and higher-income (17%) countries. Patients from regions with greater income inequality had worse mortality.
Causes of Death
As HF is a syndrome of many underlying causes or conditions leading to cardiac impairment, estimating the number of deaths attributable to HF as the actual cause of death is difficult. Also, cause-specific death and readmission in most registries are obtained from International Classification of Diseases codes or death certificates, which are inherently subject to misclassification.
In the ESC HF-LT registry, mortality at 1 year was mainly due to CV death which was more frequent in HFrEF (53.5%) versus HFmrEF (50.6%) versus HFpEF (47.2%). Conversely, non-CV mortality at 1 year was lower in HFrEF (20.1%) versus HFmrEF (27.8%) versus HFpEF (30.7%).78 In the ECHOES study of causes of death at 10 years follow-up, 44% of deaths were due to CV or cerebrovascular disease, 21% were due to respiratory disease, 21%, due to cancer and 14% due to other causes.70 Definitive HF was the cause of death in 32% of those who had HF with LVEF <40%, 19% of those who had HF with LVEF >40% and 10% of those who had neither HF nor left ventricular systolic dysfunction. In the GWTG-HF registry, between 2005
and 2008, patients with HFrEF had the greatest percentage of deaths caused by CVD (66%) as compared with HFpEF (53%).71 In competing risk analysis, patients with HFrEF had a 26% increased risk of CV death at 1-year follow-up compared with patients with HFpEF. However, the percentage of death attributed to HF was similar in HFrEF and HFpEF (11% versus 10%).
In a subset of patients from the Framingham Heart Study, causes of death were adjudicated by an expert panel for 463 participants with HF who died between 1974 and 2004 and for whom LVEF and detailed death reports were available.79 Overall, 62% of underlying causes of death were CV, with a large proportion of underlying causes attributable to CHD (25%). Progressive pump failure was the major non-CHD cause of cardiovascular death (16% of all underlying causes). Respiratory disease (infectious and non-infectious) was the leading underlying cause of nonCV death (10%), followed by cancer (9%). The underlying causes of death were, to a greater extent, cardiovascular in subjects with HFrEF (70%) than HFpEF (45%). HFrEF increased the odds of CV death more than threefold in men and twofold in women. In the INTER-CHF study, cardiac deaths (46%) were more common than non-cardiac deaths (16%) and deaths from an unknown cause (38%).80
Some studies have reported a shift in the distribution of causes of death over time. In Olmsted County, the proportion of deaths occurring within 5 years of incident HF that were categorised as CV decreased from 74% in 1979–84 to 51% in 1997–2002.81 When stratifying by preserved or reduced LVEF, the proportion of CV deaths decreased mainly in patients with HFpEF.82 In contrast, in a Spanish cohort of 1,876 patients with LVEF <50%, CV deaths decreased significantly over time in patients from 83% in 2002 to 34% in 2018. The decrease in CV death was mainly explained by the decrease in risk of sudden cardiac death, without any change in deaths from MI or stroke and an increase in cancer as a mode of death in HF. In a recent analysis of patients with HFrEF (LVEF ≤40%) from 12 clinical trials spanning the period from 1995 to 2014, a 44% decline in the rate of sudden cardiac death was observed, which paralleled the increasing use of evidence-based medical therapy known to reduce the incidence of sudden death. In a population-based retrospective study from the UK of patients with a first diagnosis of HF between 2002 and 2013, the risk of CV death declined by 27% over time, which was offset by an increase in risk of nonCV death by 22%, with cancer, respiratory conditions and infections being the major non-CV causes of death.83
Hospitalisations
In an analysis from the US National Inpatient Sample Database, HF was consistently among the three most common causes of hospitalisation between 2005 and 2018.84 Also, there was a trend towards increase in hospitalisations for HF during this time, with HF becoming the second most common cause of hospitalisations in 2018. In patients aged >65 years, HF is the most common cause of hospitalisation.85 Patients with HF have the highest 30-day readmission rates (20–25%) compared to patients with other diagnoses. Within 5 years from the initial HF diagnosis, 83.1% of the subjects in the Olmsted County cohort were hospitalised at least once and 66.9% were hospitalised more than twice.86 HF and other CV causes contributed to 16.5% and 21.6% of the hospitalisations, while non-CV causes contributed to most of the hospitalisations (61.9%). Total hospitalisation rates were similar regardless of LVEF, with some evidence of a higher rate of CV hospitalisations among HFrEF offset by a higher rate of non-CV hospitalisations among HFpEF patients.37 In contrast, the rates of total hospitalisation rates at 1 year in the ESC-HF-LT registry were significantly higher in HFrEF patients (31.9%) compared with HFpEF
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 3: Summary of Trends in Global Burden of Heart Failure
CV = cardiovascular; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
(23.5%) and HFmrEF patients (22%). A similar pattern was observed for 1-year HF-hospitalisation rates, which were higher in HFrEF than HFpEF and HFmrEF (14.6%, 9.7% and 8.7%, respectively).15 Similarly, in the CHARM programme, after adjustment for baseline differences, HFrEF was associated with a 42% increased risk of HF hospitalisations when compared with HFpEF.34 These differentiations may possibly reflect the highly selected populations followed in specialised HF centres and clinical trials, respectively. However, since the case mix of HF is changing, with a larger proportion of patients with HFpEF compared to HFrEF, the proportion of HF hospitalisations for HFpEF seems to be increasing as well. In the GWTG-HF cohort, the proportion of patients admitted for HF who had HFpEF, increased from 33% in 2005 to 39% in 2010.71
During the 1990s, a peak in the number of HF hospitalisations was observed in several developed countries followed by a decline in recent decades. In Denmark, age-adjusted hospitalisation rates decreased between 1983 and 2012 by 25% for women and by 14% for men.87 The decrease reflected an average annual 1% increase between 1983 and
1. Khan MS, Shahid I, Fonarow GC, Greene SJ. Classifying heart failure based on ejection fraction: imperfect but enduring. Eur J Heart Fail 2022;24:1154–7. https://doi. org/10.1002/ejhf.2470; PMID: 35239210.
2. Lund LH, Pitt B, Metra M. Left ventricular ejection fraction as the primary heart failure phenotyping parameter. Eur J Heart Fail 2022;24:1158–61. https://doi.org/10.1002/ejhf.2576; PMID: 35703027.
3. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol 2022;19:100–16. https://doi.org/10.1038/s41569-02100605-5; PMID: 34489589.
4. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–80. https://doi. org/10.1002/ejhf.2115; PMID: 33605000.
5. York MK, Gupta DK, Reynolds CF, et al. B-type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol 2018;71:2079–88. https://doi. org/10.1016/j.jacc.2018.02.071; PMID: 29747827.
6. Savarese G, Vedin O, D’Amario D, et al. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail 2019;7:306–17. https://doi.org/10.1016/j.jchf.2018.11.019; PMID: 30852236.
7. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study
2000 and a 3.5% decline thereafter. In an analysis of the National Inpatient Sample from the US, age-adjusted HF hospitalisation rate decreased by 30.8% from 2002 to 2013 but substantial variations were observed across different races and ethnicities, such as black patients having a 2.5-fold higher HF hospitalisation rate versus white patients.88 In contrast to these findings, a recent report from the ARIC study showed a substantial increase in rates of hospitalisation for acute decompensated HF driven by primarily HFpEF.89 Over 10 years between 2005 and 2014, the average annual percentage increase was +4.3% for black women, +3.7% for black men, +1.9% for white women and +2.6% for white men. In the Olmsted County study, hospitalisation rates did not change significantly during 2000–10.37 An increase in non-CV hospitalisations was paralleled with a decrease in CV hospitalisations, particularly among HFrEF cases. There is a trend toward outpatient treatment of worsening HF, which also likely affects hospitalisation rates for HF over time.90,91
Conclusion
The HF epidemic is changing (Figure 3). Although age-adjusted incidence has stabilised and seems to be declining, the total number of patients living with HF is increasing. Also, the case mix of HF is shifting from HFrEF to a larger proportion of patients with HFpEF, which may become the most common form of HF in the future. HFpEF seems to be distinct from HFrEF, and therapeutic options for HFpEF are only beginning to emerge. HFmrEF has been increasingly well characterised and appears more like HFrEF than HFpEF and medications effective in HFrEF may also be effective in HFmrEF, although this requires further study. Over the past decades, prognosis of HF has slightly improved, but mortality and hospitalisation rates remain high, and many patients progress to advanced HF with few treatment options. CV death is still the major underlying cause of death in HF. However, CV death has been decreasing over time while non-CV deaths have been increasing, particularly in HFpEF. Lastly, very little is known about HF epidemiology in countries outside Europe and North America, but scarce literature suggests the prevalence of HF is rapidly increasing in these regions and that HF is more often prevalent in the young.
2017. Lancet 2018;392:1789–858. https://doi.org/10.1016/ S0140-6736(18)32279-7; PMID: 30496104.
8. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics – 2021 update: a report from the American Heart Association. Circulation 2021;143:e254–743. https://doi. org/10.1161/CIR.0000000000000950; PMID: 33501848.
9. Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43:1424–41. https://doi. org/10.1111/j.1475-6773.2007.00822.x; PMID: 18756617.
10. Seferovic PM, Vardas P, Jankowska EA, et al. The Heart Failure Association atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906–14. https://doi.org/10.1002/ejhf.2143; PMID: 33634931.
11. Stork S, Handrock R, Jacob J, et al. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 2017;106:913–22. https://doi.org/10.1007/s00392-0171137-7; PMID: 28748265.
12. Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–80. https://doi. org/10.1016/S0140-6736(17)32520-5; PMID: 29174292.
13. van Riet EE, Hoes AW, Wagenaar KP, et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18:242–52. https://doi.org/10.1002/ ejhf.483; PMID: 26727047.
14. Eurostat: Statistics Explained (2023). https://ec.europa.eu/ eurostat/statistics-explained/index.php?title=Ageing_ Europe_-_statistics_on_population_developments#Older_ people_.E2.80.94_population_overview (accessed 6 June 2023).
15. Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an
analysis of the ESC heart failure long-term registry. Eur J Heart Fail 2017;19:1574–85. https://doi.org/10.1002/ejhf.813; PMID: 28386917.
16. Stolfo D, Lund LH, Benson L, et al. Persistent high burden of heart failure across the ejection fraction spectrum in a nationwide setting. J Am Heart Assoc 2022;11:e026708. https://doi.org/10.1161/JAHA.122.026708; PMID: 36326055.
17. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol 2007;50:768–77. https://doi.org/10.1016/j.jacc.2007.04.064; PMID: 17707182.
18. Shiga T, Suzuki A, Haruta S, et al. Clinical characteristics of hospitalized heart failure patients with preserved, midrange, and reduced ejection fractions in Japan. ESC Heart Fail 2019;6:475–86. https://doi.org/10.1002/ehf2.12418; PMID: 30829002.
19. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. https://doi. org/10.1056/NEJMoa052256; PMID: 16855265.
20. Canepa M, Kapelios CJ, Benson L, et al. Temporal trends of heart failure hospitalizations in cardiology versus noncardiology wards according to ejection fraction: 16-year data from the SwedeHF registry. Circ Heart Fail 2022;15:e009462. https://doi.org/10.1161/ CIRCHEARTFAILURE.121.009462; PMID: 35938444.
21. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–27. https://doi.org/10.1056/ NEJMoa1311890; PMID: 25162888.
22. Reyes EB, Ha JW, Firdaus I, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol 2016;223:163–7. https://doi.org/10.1016/j. ijcard.2016.07.256; PMID: 27541646.
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
Outcomes CV death HFrEF Non-CV death HFpEF Hospitalisations for HF Prevalence Overall prevalence 1–3% Prevalence in HFpEF Prevalence in HFrEF Incidence Overall incidence 1–20 cases per 1,000 population Incidence in HFrEF Incidence in HFpEF
23. Hao G, Wang X, Chen Z, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China hypertension survey, 2012–2015. Eur J Heart Fail 2019;21:1329–37. https://doi.org/10.1002/ejhf.1629; PMID: 31746111.
24. Konishi M, Ishida J, Springer J, et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail 2016;3:145–51. https://doi. org/10.1002/ehf2.12103; PMID: 27840692.
25. Huffman MD, Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India 2010;23:283–8. PMID: 21250584.
26. Sahle BW, Owen AJ, Mutowo MP, et al. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord 2016;16:32. https://doi.org/10.1186/s12872-016-0208-4; PMID: 26852410.
27. Ciapponi A, Alcaraz A, Calderon M, et al. Burden of heart failure in Latin America: a systematic review and metaanalysis. Rev Esp Cardiol (Engl Ed) 2016;69:1051–60. https:// doi.org/10.1016/j.rec.2016.04.054; PMID: 27553287.
28. Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the heart of Soweto study cohort: emerging challenges for urban African communities. Circulation 2008;118:2360–7. https://doi.org/10.1161/ CIRCULATIONAHA.108.786244; PMID: 19029467.
29. Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424–31. https://doi.org/10.1093/eurheartj/eht066; PMID: 23470495.
30. Christiansen MN, Kober L, Weeke P, et al. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation 2017;135:1214–23. https://doi.org/10.1161/CIRCULATIONAHA.116.025941; PMID: 28174193.
31. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–9. https://doi.org/10.1056/NEJMoa1012848; PMID: 22276822.
32. Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013;61:1510–7. https://doi.org/10.1016/j.jacc.2013.01.022; PMID: 23500287.
33. Stamler J, Dyer AR, Shekelle RB, et al. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term followup of Chicago cohorts. Cardiology 1993;82:191–222. https:// doi.org/10.1159/000175868; PMID: 8324780.
34. The ARIC investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. https://doi.org/10.1093/ oxfordjournals.aje.a115184; PMID: 2646917.
35. Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol 1991;1:263–76. https://doi.org/10.1016/1047-2797(91)90005-w; PMID: 1669507.
36. Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018;6:678–85. https://doi.org/10.1016/j.jchf.2018.03.006; PMID: 30007560.
37. Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. https://doi.org/10.1001/jamainternmed.2015.0924; PMID: 25895156.
38. Martinez-Amezcua P, Haque W, Khera R, et al. The upcoming epidemic of heart failure in South Asia. Circ Heart Fail 2020;13:e007218. https://doi.org/10.1161/
CIRCHEARTFAILURE.120.007218; PMID: 32962410.
39. Liew D, Audehm RG, Haikerwal D, et al. Epidemiology of heart failure: study of heart failure in the Australian primary care setting (SHAPE). ESC Heart Fail 2020;7:3871–80. https:// doi.org/10.1002/ehf2.12979; PMID: 32902206.
40. Chen X, Savarese G, Dahlstrom U, et al. Age-dependent differences in clinical phenotype and prognosis in heart failure with mid-range ejection compared with heart failure with reduced or preserved ejection fraction. Clin Res Cardiol 2019;108:1394–405. https://doi.org/10.1007/s00392-01901477-z; PMID: 30980205.
41. Kaplon-Cieslicka A, Benson L, Chioncel O, et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction – insights from the ESC-HFA EORP heart failure long-term registry. Eur J Heart Fail 2022;24:335–50. https:// doi.org/10.1002/ejhf.2408; PMID: 34962044.
42. Lofman I, Szummer K, Dahlstrom U, et al. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail 2017;19:1606–14. https://doi. org/10.1002/ejhf.821; PMID: 28371075.
43. Rosano GMC, Moura B, Metra M, et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2021;23:872–81. https://doi.org/10.1002/ejhf.2206; PMID: 33932268.
44. Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail 2017;5:565–74. https://doi. org/10.1016/j.jchf.2017.05.001; PMID: 28711451.
45. Savarese G, Jonsson Å, Hallberg AC, et al. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int J Cardiol 2020;298:59–65. https://doi.org/10.1016/j. ijcard.2019.08.049; PMID: 31521440.
46. Savarese G, Settergren C, Schrage B, et al. Comorbidities and cause-specific outcomes in heart failure across the ejection fraction spectrum: a blueprint for clinical trial design. Int J Cardiol 2020;313:76–82. https://doi.org/10.1016/j. ijcard.2020.04.068; PMID: 32360702.
47. Teng TK, Tay WT, Dahlstrom U, et al. Different relationships between pulse pressure and mortality in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol 2018;254:203–9. https://doi.org/10.1016/j. ijcard.2017.09.187; PMID: 29407092.
48. Vedin O, Lam CSP, Koh AS, et al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 2017;10. https://doi.org/10.1161/ CIRCHEARTFAILURE.117.003875; PMID: 28615366.
49. Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14,964 patients in the European Society of Cardiology heart failure long-term registry. Eur Heart J 2018;39:4277–84. https://doi. org/10.1093/eurheartj/ehy626; PMID: 30325423.
50. Faxen UL, Lund LH, Orsini N, et al. N-terminal pro-B-type natriuretic peptide in chronic heart failure: the impact of sex across the ejection fraction spectrum. Int J Cardiol 2019;287:66–72. https://doi.org/10.1016/j.ijcard.2019.04.023; PMID: 31005415.
51. Lofman I, Szummer K, Evans M, et al. Incidence of, associations with and prognostic impact of worsening renal function in heart failure with different ejection fraction categories. Am J Cardiol 2019;124:1575–83. https://doi. org/10.1016/j.amjcard.2019.07.065; PMID: 31558270.
52. Savarese G, Orsini N, Hage C, et al. Utilizing NT-proBNP for eligibility and enrichment in trials in HFpEF, HFmrEF, and HFrEF. JACC Heart Fail 2018;6:246–56. https://doi. org/10.1016/j.jchf.2017.12.014; PMID: 29428439.
53. Lund LH, Claggett B, Liu J, et al. Heart failure with midrange ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–9. https://doi. org/10.1002/ejhf.1149; PMID: 29431256.
54. Abdurashidova T, Monney P, Tzimas G, et al. Non-severe aortic regurgitation increases short-term mortality in acute heart failure with preserved ejection fraction. ESC Heart Fail 2020;7:3901–9. https://doi.org/10.1002/ehf2.12983; PMID: 33026164.
55. Verbrugge FH, Reddy YNV, Eleid MF, et al. Mild aortic valve disease and the diastolic pressure-volume relationship in heart failure with preserved ejection fraction. Open Heart 2021;8:e001701. https://doi.org/10.1136/openhrt-2021-001701; PMID: 34670831.
56. Kao DP, Lewsey JD, Anand IS, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015;17:925–35. https:// doi.org/10.1002/ejhf.327; PMID: 26250359.
57. Shah AM, Cikes M, Prasad N, et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2019;74:2858–73. https://doi.org/10.1016/j.jacc.2019.09.063; PMID: 31806129.
58. Chieffo A, Van Mieghem NM, Tchetche D, et al. Impact of mixed aortic valve stenosis on VARC-2 outcomes and postprocedural aortic regurgitation in patients undergoing transcatheter aortic valve implantation: results from the international multicentric study PRAGMATIC (pooled Rotterdam-Milan-Toulouse in collaboration). Catheter Cardiovasc Interv 2015;86:875–85. https://doi.org/10.1002/ ccd.25975; PMID: 26032764.
59. Hahn RT, Pibarot P, Stewart WJ, et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves). J Am Coll Cardiol 2013;61:2514–21. https://doi.org/10.1016/j. jacc.2013.02.087; PMID: 23623915.
60. Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic
regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 registry. Circulation 2014;129:1415–27. https://doi.org/10.1161/CIRCULATIONAHA.113.002677; PMID: 24566199.
61. Marciniak A, Glover K, Sharma R. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ Open 2017;7:e012240. https://doi.org/10.1136/bmjopen-2016-012240; PMID: 28131996.
62. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. https://doi.org/10.1016/S01406736(06)69208-8; PMID: 16980116.
63. Shahim B, Shahim A, Adamo M, et al. Prevalence, characteristics and prognostic impact of aortic valve disease in patients with heart failure and reduced, mildly reduced, and preserved ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2023. https://doi.org/10.1002/ejhf.2908; PMID: 37210639; epub ahead of press.
64. Adamo M, Chioncel O, Benson L, et al. Prevalence, clinical characteristics and outcomes of heart failure patients with or without isolated or combined mitral and tricuspid regurgitation: insight from the ESC-HFA EORP Heart Failure Long-Term Registry. Eur J Heart Fail 2023. https://doi. org/10.1002/ejhf.2929; PMID: 37365841; epub ahead of press.
65. Dokainish H, Teo K, Zhu J, et al. Heart failure in Africa, Asia, the Middle East and South America: the INTER-CHF study. Int J Cardiol 2016;204:133–41. https://doi.org/10.1016/j. ijcard.2015.11.183; PMID: 26657608.
66. MacDonald MR, Tay WT, Teng TK, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc 2020;9:e012199. https://doi. org/10.1161/JAHA.119.012199; PMID: 31852421.
67. Johansson I, Joseph P, Balasubramanian K, et al. Healthrelated quality of life and mortality in heart failure: the global congestive heart failure study of 23,000 patients from 40 countries. Circulation 2021;143:2129–42. https://doi. org/10.1161/CIRCULATIONAHA.120.050850; PMID: 33906372.
68. Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology heart failure long-term registry (ESCHF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–25. https://doi. org/10.1002/ejhf.566; PMID: 27324686.
69. Koh AS, Tay WT, Teng THK, et al. A comprehensive population-based characterization of heart failure with midrange ejection fraction. Eur J Heart Fail 2017;19:1624–34. https://doi.org/10.1002/ejhf.945; PMID: 28948683.
70. Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail 2012;14:176–84. https://doi.org/10.1093/ eurjhf/hfr170; PMID: 22253455.
71. Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. https://doi.org/10.1161/ CIRCULATIONAHA.111.080770; PMID: 22615345.
72. Jones NR, Roalfe AK, Adoki I, et al. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019;21:1306–25. https:// doi.org/10.1002/ejhf.1594; PMID: 31523902.
73. Codina P, Zamora E, Levy WC, et al. Mortality risk prediction dynamics after heart failure treatment optimization: repeat risk assessment using online risk calculators. Front Cardiovasc Med 2022;9:836451. https://doi.org/10.3389/ fcvm.2022.836451; PMID: 35498033.
74. Zhang Y, Zhang J, Butler J, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart failure (China-HF) registry. J Card Fail 2017;23:868–75. https://doi.org/10.1016/j.cardfail.2017.09.014; PMID: 29029965.
75. Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). Circ J 2009;73:1893–900. https:// doi.org/10.1253/circj.cj-09-0254; PMID: 19644216.
76. Al-Omary MS, Davies AJ, Evans TJ, et al. Mortality and readmission following hospitalisation for heart failure in Australia: a systematic review and meta-analysis. Heart Lung Circ 2018;27:917–27. https://doi.org/10.1016/j.hlc.2018.01.009; PMID: 29519691.
77. Tromp J, Bamadhaj S, Cleland JGF, et al. Post-discharge prognosis of patients admitted to hospital for heart failure
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health 2020;8:e411–22. https://doi.org/10.1016/S2214109X(20)30004-8; PMID: 32087174.
78. Papadaki A, Martinez-Gonzalez MÁ, Alonso-Gomez A, et al. Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. Eur J Heart Fail 2017;19:1179–85. https://doi.org/10.1002/ejhf.750; PMID: 28133855.
79. Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail 2011;4:36–43. https://doi. org/10.1161/CIRCHEARTFAILURE.110.957480; PMID: 21071547.
80. Dokainish H, Teo K, Zhu J, et al. Global mortality variations in patients with heart failure: results from the international congestive heart failure (INTER-CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–72. https://doi. org/10.1016/S2214-109X(17)30196-1; PMID: 28476564.
81. Henkel DM, Redfield MM, Weston SA, et al. Death in heart failure: a community perspective. Circ Heart Fail 2008;1:91–7. https://doi.org/10.1161/CIRCHEARTFAILURE.107.743146; PMID: 19300532.
82. Moliner P, Lupon J, de Antonio M, et al. Trends in modes of
death in heart failure over the last two decades: less sudden death but cancer deaths on the rise. Eur J Heart Fail 2019;21:1259–66. https://doi.org/10.1002/ejhf.1569; PMID: 31359563.
83. Conrad N, Judge A, Canoy D, et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86,000 individuals. JAMA Cardiol 2019;4:1102–11. https://doi.org/10.1001/jamacardio.2019.3593; PMID: 31479100.
84. Salah HM, Minhas AMK, Khan MS, et al. Causes of hospitalization in the USA between 2005 and 2018. Eur Heart J Open 2021;1:oeab001. https://doi.org/10.1093/ ehjopen/oeab001; PMID: 35919090.
85. Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol 2014;11:329–37. https:// doi.org/10.11909/j.issn.1671-5411.2014.04.008; PMID: 25593582.
86. Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009;54:1695–702. https://doi.org/10.1016/j. jacc.2009.08.019; PMID: 19850209.
87. Schmidt M, Ulrichsen SP, Pedersen L, et al. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide
cohort study. Eur J Heart Fail 2016;18:490–9. https://doi. org/10.1002/ejhf.486; PMID: 26868921.
88. Ziaeian B, Kominski GF, Ong MK, et al. National differences in trends for heart failure hospitalizations by sex and race/ ethnicity. Circ Cardiovasc Qual Outcomes 2017;10:e003552. https://doi.org/10.1161/CIRCOUTCOMES.116.003552; PMID: 28655709.
89. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): ARIC study community surveillance. Circulation 2018;138:12–24. https:// doi.org/10.1161/CIRCULATIONAHA.117.027551; PMID: 29519849.
90. Greene SJ, Bauersachs J, Brugts JJ, et al. Worsening heart failure: nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol 2023;81:413–24. https://doi.org/10.1016/j.jacc.2022.11.023; PMID: 36697141.
91. Kristjansdottir I, Thorvaldsen T, Lund LH. Congestion and diuretic resistance in acute or worsening heart failure. Card Fail Rev 2020;6:e25. https://doi.org/10.15420/cfr.2019.18; PMID: 33042585.
Global Heart Failure Burden CARDIAC FAILURE REVIEW www.CFRjournal.com
Cardiovascular Involvement in Fabry’s Disease: New Advances in Diagnostic Strategies, Outcome Prediction and Management
Italy; 2. Institute of Cardiovascular Science, University College London, London, UK
Abstract
Cardiovascular involvement is common in Fabry’s disease and is the leading cause of morbidity and mortality. The research is focused on identifying diagnostic clues suggestive of cardiovascular involvement in the preclinical stage of the disease through clinical and imaging markers. Different pathophysiologically driven therapies are currently or will soon be available for the treatment of Fabry’s disease, with the most significant benefit observed in the early stages of the disease. Thus, early diagnosis and risk stratification for adverse outcomes are crucial to determine when to start an aetiological treatment. This review describes the cardiovascular involvement in Fabry’s disease, focusing on the advances in diagnostic strategies, outcome prediction and disease management.
Keywords
Fabry’s disease, hypertrophic cardiomyopathy, diagnosis, outcome, management
Disclosure: The authors have no conflicts of interest to declare.
Received: 28 April 2023 Accepted: 22 May 2023 Citation: Cardiac Failure Review 2023;9:e12. DOI: https://doi.org/10.15420/cfr.2023.06
Correspondence: Giuseppe Limongelli, Inherited and Rare Cardiovascular Disease Clinic, Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Via L. Bianchi 1 c/o Monaldi Hospital, AORN Colli, Naples, Italy. E: limongelligiuseppe@libero.it
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Fabry’s disease (FD) is a multisystem X-linked disease caused by pathogenic variants in the galactosidase-α (GLA) gene, leading to reduced α-galactosidase A (α-Gal A) enzyme activity.1 This reduction is responsible for the progressive lysosomal storage of globotriaosylceramide (Gb3) and related globotriaosylsphingosine (lyso-Gb3) in several tissues and organs.1
The clinical manifestations of FD depend on the residual α-Gal A enzyme activity, and two different subtypes of FD have been described, named the early-onset and later-onset phenotypes.1 2 Affected patients with the early-onset phenotype have low or no functional α-Gal A enzyme activity, with marked Gb3 and lysoGb3 accumulation and symptoms onset in childhood or adolescence, while those with the later-onset phenotype have residual α-Gal A enzyme activity, which is responsible for a milder phenotype and a later clinical presentation (rarely before the third decade).1
Cardiac involvement is the main determinant of adverse outcomes in patients with FD.3 It is largely variable in patients and ranges from uncomplicated asymptomatic disease to severe left ventricular hypertrophy (LVH), leading to disease complications, including heart failure and lifethreatening arrhythmias, and premature death.3 4 The heterogeneous and often subtle presentation of FD can be responsible for a significant diagnostic delay, requiring active screening of high-risk patients.5,6
It is common for patients with the later-onset disease to present with single-organ involvement, mainly of the heart (usually manifesting as LVH
or arrhythmias), central nervous system (manifesting as cryptogenic ischaemic stroke) or kidneys (manifesting as proteinuria or progressive chronic kidney dysfunction).1 Therefore, identifying specific clinical, laboratory or imaging findings in these patients may prompt a diagnostic work-up for FD.6–8 The main diagnostic steps are the identification of reduced α-Gal A enzyme activity, which is usually measured in plasma, leukocytes or dried blood spots, and confirmation of the disease-causing mutation in GLA 1 However, given that α-Gal A enzyme activity could be in the normal range in female patients, identification of a GLA mutation is required for the diagnosis.9
Different pathophysiologically driven therapies are currently or will soon be available to treat FD, with the most significant benefit observed in the early stages of the disease.10 Thus, the early diagnosis and risk stratification for the adverse outcome is crucial to determine when to start an aetiological treatment.11
This review describes the cardiovascular involvement in FD, focusing on the advances in diagnostic strategies, outcome prediction and disease management.
When to Suspect Fabry’s Disease
The high variability in the clinical manifestation of FD, with the different possible ages of onset and symptoms onset, can lead to delayed diagnosis and treatment.5 Therefore, given that FD is a multisystem disease, cardiologists, neurologists, dermatologists, nephrologists,
REVIEW © The Author(s) 2023. Published by Radcliffe Group Ltd. www.CFRjournal.com New Advances in HF
Emanuele Monda , 1,2 Luigi Falco,1 Giuseppe Palmiero , 1 Marta Rubino,1 Alessia Perna,1 Gaetano Diana,1 Federica Verrillo,1 Francesca Dongiglio,1 Annapaola Cirillo,1 Adelaide Fusco,1 Martina Caiazza 1 and Giuseppe Limongelli 1,2
1. Inherited and Rare Cardiovascular Diseases, Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples,
Figure 1: When to Suspect and Screen for Fabry’s Disease
Family history of CKD or stroke
Family history of LVH with X-linked transmission
Neuropathic pain
Gastrointestinal symptoms
Angiokeratomas
Cornea verticillata
Hypohidrosis
Juvenile or cryptogenic
Table 1: Imaging Features of Cardiac Involvement According to Fabry’s Disease Stage
Early Stage Advanced Stage
ECG Short PR interval
Echocardiography No LVH
Reduced GLS in basal posterolateral segment
Radial strain impairment
LVH ≥12 mm +
≥1 of the following red flags
TIA or stroke
Dolichoectasia of the basilar artery on brain MRI
Chronic white matter hyperintensities on brain MRI
Sensorineural deafness
Proteinuria
Chronic kidney disease
Short PR interval
Chronotropic incompetence
Atrioventricular block
Disproportionate papillary muscle hypertrophy
Reduced GLS in the posterolateral basal segment
LGE in the posterolateral basal segment
Low native T1 values
Screen for Fabry’s disease
In the presence of left ventricular hypertrophy (LVH) with a maximum left ventricular wall thickness ≥12 mm and one or more additional diagnostic features it is recommended to screen for Fabry’s disease. CKD = chronic kidney disease; GLS = global longitudinal strain; LGE = late gadolinium enhancement; LVH = left ventricular hypertrophy; TIA = transient ischaemic attack. Created with BioRender.com.
paediatricians and ophthalmologists should all be aware of the possibility of FD, depending on the patient’s clinical presentation.6
In the cardiology environment, a diagnosis of FD should be systematically considered in the case of unexplained LVH, especially when it is concentric, symmetric, homogeneous and non-obstructive (Figure 1).3 All patients with LVH should be screened for additional extracardiac or cardiac manifestations associated with FD.7 8 12 13
Extracardiac Red Flags
The clinical manifestations generally vary according to the age of presentation.
In patients with early-onset disease, the first symptoms include chronic neuropathic pain and episodic pain crises that generally emerge during childhood.14 Other common symptoms manifesting during childhood are gastrointestinal symptoms, including abdominal pain, diarrhoea, nausea or vomiting.15 However, these symptoms are common in the general population and are not specific to FD. Angiokeratoma is the most common dermatological abnormality and is a specific clinical feature of early-onset FD.16 Finally, hypohidrosis and corneal opacity (i.e. cornea verticillata) are other common manifestations during the first two decades of life.1
Cardiac, kidney and central nervous system involvement generally appear from the third decade and may be preceded by the other described clinical manifestations (in early-onset disease) or present as an isolated feature (in later-onset disease). For example, kidney involvement presents with progressive proteinuria and reduction in glomerular filtration rate, while cerebrovascular manifestations include transient ischaemic attack, stroke or isolated neuroradiological findings such as chronic white matter
Atrioventricular block
High QRS voltages
Negative T waves
LVH
Reduced GLS
Papillary muscles hypertrophy
Diastolic dysfunction
CMR Reduced native T1 values Normal native T1 value
Diffuse LGE
CMR = cardiac MRI; GLS = global longitudinal strain; LGE = late gadolinium enhancement; LVH = left ventricular hypertrophy.
hyperintensities or basilar artery dolichoectasia.17–19 These features usually occur at an early age compared with the general population.
Cardiac Red Flags
In patients with LVH, several ECG, echocardiographic and cardiac MRI (CMR) findings may suggest FD.3,4 Typical ECG findings are short PR interval, atrioventricular block and diffuse repolarisation abnormalities.20 Common imaging findings are papillary muscle hypertrophy, right ventricular hypertrophy, advanced diastolic dysfunction, late gadolinium enhancement (LGE) in the basal inferolateral wall, and mitral and aortic regurgitation due to leaflet involvement.21
In addition, it is common to observe the so-called ‘binary sign’, characterised by a bright endocardial layer and a hypoechogenic intraventricular septum. The binary sign, previously considered specific for FD, has been observed in several other causes of LVH and is now considered to have low sensitivity and specificity for FD.22
All of the above-mentioned cardiac features have generally appeared in patients with advanced cardiac involvement, in whom the initiation of an aetiological treatment was not associated with significant benefit. Therefore, efforts have recently been directed to improve the identification of patients with early cardiac involvement.
Detection of Preclinical Cardiac Involvement
Given that the most significant benefit of aetiological treatment has been observed in patients with the mild disease phenotype, it is crucial to identify patients during the preclinical phase of the disease. Several ECG and imaging markers could be identified prior to LVH development (Table 1).
ECG Parameters
Several ECG abnormalities have been described in patients with FD and they generally correlate with the myocardial involvement phenotype.3 The typical ECG abnormality is the presence of a short PR interval, caused by the shortening of the P wave, and lacking the typical pre-excitation pattern.23 This sign is common in the early stages of the disease. In contrast, signs of LVH, negative T waves, and atrioventricular and intraventricular conduction delays generally appear in advanced stages.24
Thus, in the pre-hypertrophic phase, in which patients do not show signs of LVH or impaired left ventricular function, ECG abnormalities may represent the only signs of left ventricular involvement.25 Moreover, a significant correlation between ECG abnormalities and the detection of
Cardiovascular Involvement in Fabry’s Disease CARDIAC FAILURE REVIEW www.CFRjournal.com
low native T1 values at CMR has been observed.26 Based on this observation, an ECG-based score to estimate the probability of detecting FD cardiac involvement at CMR has been proposed.26 This score was based on four different parameters: the Sokolow–Lyon index, the ratio between the P wave and PR segment duration, the QRS duration, and the QT duration, and can potentially improve the early detection of FD cardiac involvement.26
Echocardiographic Deformation-based Markers
Deformation-based parameters in strain analysis are superior to standard echocardiographic measurements in demonstrating left ventricular involvement.27 Left ventricular involvement in patients with FD shows a typical regional distribution from the early stages. Compared with the general population, FD patients have a significant reduction in average global longitudinal strain (GLS), and basal and mid-ventricular longitudinal strain.28 Moreover, patients with higher lysoGb3 values had lower apical–ventricular longitudinal strain values, suggesting an early basal and midventricular left ventricular involvement. In contrast, the apical involvement was observed in the advanced stages of the disease.28
The left ventricular segments most affected in FD are the basal lateral and posterior segments. A correlation has been seen between the reduction of GLS and the amount of LGE at CMR.29 Indeed, the GLS reduction in the basal posterolateral segments was an independent predictor of LGE.29 Even in the absence of left ventricular involvement on standard echocardiography (i.e. normal left ventricular mass and preserved systolic function), GLS is reduced in FD patients compared with controls, especially in the basal segments. In addition, basal longitudinal strain reduction in FD was associated with major adverse cardiovascular events.30
The Gb3 and lysoGb3 accumulation process shows a typical myocardial mural involvement. Thus, in 33 newly diagnosed FD patients, layerspecific myocardial deformation analysis on 2D-speckle tracking (2D-ST) echocardiography showed a significant reduction in subepicardial compared with subendocardial longitudinal strain, with a consequent significant strain gradient. Furthermore, the subepicardial longitudinal strain was the best parameter for discriminating FD patients from healthy subjects.31
In addition, left ventricular radial and circumferential strain analysis has been investigated in recent years. Spinelli et al. showed that FD patients with preserved left ventricular ejection fraction (LVEF) had lower longitudinal, radial and circumferential strain than controls, regardless of left ventricular geometry.32 Moreover, in patients without LVH, the radial strain was significantly impaired, suggesting the possible role of radial strain as an early marker of left ventricular involvement in FD.32
Right ventricular (RV) involvement in FD on strain imaging has been studied more recently. In a recent study, RV systolic function assessed using non-deformation parameters was normal in approximately 92% of patients, while RV GLS and free wall strain (RV-FWS) were impaired in approximately 40%.33 Both strain-derived parameters were significantly more impaired in patients with LVH than in patients with normal cardiac mass, suggesting a possible association of RV involvement with the accumulation burden and the stage of the disease.33 Moreover, RV function assessed on strain analysis provides a better prognostic assessment than other RV echocardiographic parameters.
In a recent study of 56 patients with FD, RV-FWS was associated with adverse cardiovascular outcomes during a median follow-up of
47 months.34 However, RV-FWS did not retain a significant association with outcomes when adjusted for left ventricular GLS or left atrial volume index (LAVI), demonstrating the superior prognostic power of echocardiographic left-sided parameters in FD patients, and underscoring the fact that the prognosis is mainly driven by the severity of left ventricular cardiomyopathy.34
Cardiac MRI Features
CMR has a dominant role in the diagnosis, risk stratification and detection of preclinical myocardial involvement in patients with FD.11 Moreover, it offers the possibility to identify several diagnostic clues suggestive of FD, even in the pre-hypertrophic phase.35 With the use of gadolinium contrast agents, it is possible to observe in many patients with FD the presence of mid-myocardial LGE in the basal inferolateral wall. This typical pattern is helpful for the differential diagnosis of LVH.35 Moreover, there is increasing evidence that LGE may also be observed before LVH development, enabling early identification of patients who may benefit from aetiological therapies, especially with regard to female patients.36
In addition, CMR can provide myocardial tissue characterisation. Low native T1 values appear to be sensitive for identifying patients with FD, correlate with intracellular Gb3 accumulation, and represent an early marker of the disease in the pre-hypertrophic phase. Three stages of cardiac involvement have been proposed using three CMR features: native T1 value; LGE; and LVH.24 In the first stage (the accumulation phase), patients have low native T1 and do not present LGE or LVH. In this phase it is common to observe ECG abnormalities. In the second stage (the inflammation and myocyte hypertrophy phase), patients have low native T1 and LGE, with or without LVH. Finally, in the third stage (the fibrosis and impairment phase), patients have LVH, extensive LGE and normal T1.
Finally, CMR can potentially improve the risk stratification of patients with FD. It can be used to identify risk factors, such as diffuse LGE, extreme LVH and left ventricular dysfunction, which are associated with the increased risk of adverse events. Moreover, an internally validated model for predicting outcomes in patients with FD has been proposed.37 Three different clinical parameters, that is, age, left ventricular mass index and T1 dispersion, are incorporated into the model, showing the potential for it to be applied without the need for gadolinium contrast agents.37
Major Cardiovascular Disease Complications
The hypertrophic cardiomyopathy phenotype is the most common cardiovascular manifestation of FD.38 However, evolution to restrictive cardiomyopathy may occur in advanced stages of the disease.39 Therefore, FD should be considered in the differential diagnosis of patients presenting with heart failure (HF) with preserved ejection fraction (HFpEF).10 Moreover, cardiac involvement may eventually progress to left ventricular systolic dysfunction and HF with reduced ejection fraction (HFrEF) in 6–8% of patients (especially in the absence of enzyme replacement therapy) and confers a high risk of HF-related mortality. Patients with FD have been shown to have a significant risk of developing overt HF, which was observed in 23% of patients, usually between the third and the fifth decades of life.40 Furthermore, progression to advanced HF has been observed in 10% of patients over a median period of 7.1 years, and increased levels of cardiac biomarkers (i.e. troponin T, N-terminal proB-type natriuretic peptide) and a greater extent of fibrosis have been associated with a reduction in LVEF during the follow-up.40 However, published studies are largely heterogeneous regarding the definition of adverse outcomes. Therefore, prognostic models that accurately predict HF development or HF-related death are lacking.
Cardiovascular Involvement in Fabry’s Disease CARDIAC FAILURE REVIEW www.CFRjournal.com
Figure 2: Therapeutic Options for Patients with Fabry’s Disease
• Agalsidase-α
• Agalsidase-β
• Pegunigalsidase-α
• Moss-GalA
Infused enzymes are mostly taken up by the liver, while cardiomyocytes and podocytes have low intracellular levels.55

• mRNA nanoparticles
• Lentivirus-based HSPC-GT

• AAV encoding GalA
• Lucerastat
• Venglustat
Possible aetiological therapies include enzyme replacement therapy, chaperone therapy, substrate reduction therapy and gene therapy. AAV = adeno-associated virus; GalA = galactosidase A; HSPC-GT = haematopoietic stem/progenitor cell gene therapy. Created with BioRender.com.
Treatment
The current treatment options for FD include enzyme replacement therapy (ERT) and oral chaperone therapy with migalastat. However, several different therapeutic options will be available soon (Figure 2). The decision to start an aetiological treatment varies according to the sex and clinical manifestation of the disease.
According to the current expert recommendations, the treatment of FD should be considered in all male patients, regardless of the clinical manifestation.2 6 41 In contrast, female patients should receive an aetiological therapy after identifying any signs of organ involvement, while those without signs of organ involvement will require only serial follow-up. Thus, after a diagnosis of FD, a multidisciplinary team should be consulted for further evaluation and treatment tailoring.
Enzyme Replacement Therapy
ERT has been the cornerstone of FD management since the early 2000s.42 43 Two formulations are currently available: agalsidase-α 0.2 mg/ kg bodyweight and agalsidase-β 1.0 mg/kg body weight. Both are given as biweekly infusions for lifelong therapy and are effective in clearing Gb3. Nearly every aspect of FD is addressed by ERT, with several studies reporting stabilisation of renal function, slowed progression of myocardial fibrosis and hypertrophy, reduction in thromboembolic episodes, and improvements in symptoms and quality of life.44–51 Early therapy is required to prevent or minimise disease development.47,52,53 Patients who begin ERT at a younger age have better results. Conversely, as the disease advances, ERT efficacy declines.
However, ERT has some drawbacks. Frequent infusion reactions, high cost, and the burden of a lifelong regimen are important issues that need to be addressed. Moreover, a significant proportion of patients, especially male patients lacking native α-Gal A, develop antidrug antibodies (ADAs). ADAs may work at different levels, limiting the effects of infused enzymes. These neutralising antibodies may bind different sites, preventing cellular uptake and the intracellular conformational changes required for enzymatic function.54
Despite these limitations, research on ERT has continued. Various attempts to refine the pharmacokinetic properties of infused enzymes have paved the way for next-generation ERT, with a focus on biodistribution.


Pegunigalsidase-α (PegA) (previously PRX-102) is manufactured in modified tobacco cells.56 PEGylation is a chemical modification that offers attractive benefits. It hides molecules from the immune system, delays clearance and increases half-life.57 These features may help counteract ERT’s immunogenicity and lengthen the intervals between infusions.57 Preclinical studies have shown greater heart and kidney uptake than firstgeneration ERT.55 This potentially ground-breaking preclinical research was followed by three randomised clinical trials (RCTs).58–60 First, patients with worsening renal failure despite long-term ERT with agalsidase-β were enrolled in the BALANCE (NCT02795676) trial for the first head-tohead comparison between first- and second-generation ERT.58 Second, the feasibility of monthly 2 mg/kg PegA infusion was evaluated in the BRIGHT (NCT03180840) study.59 This regimen was well-tolerated in FD patients previously treated with standard ERT. Finally, the safety and effectiveness of switching from agalsidase-α to 1 mg/kg biweekly PegA infusions were tested in the BRIDGE (NCT03018730) study.60 After only 6 months from ERT transition, an improvement in renal function was seen.
Moss-GalA is another potential second-generation ERT. According to experimental models, it is taken up by mannose receptors, especially in the kidney. Moreover, it is stripped of immunogenic potential.61 The safety and pharmacokinetics were assessed in female patients not receiving ERT for at least 3 months.62 However, other studies are expected to corroborate these findings.
Chaperone Therapy
The only approved chaperone therapy is migalastat, an oral pharmacological chaperone that enhances native α-Gal A activity.63 This effect is provided by a specific and reversible interaction with the catalytic sites of amenable mutant forms of α-Gal A. More than 1,000 mutations have been identified.2 Some of them have minimal residual activity and are amenable to migalastat therapy.64 Once migalastat binds to α-Gal A, the enzymatic structure is stabilised, degradation in the endoplasmic reticulum is avoided, and appropriate lysosomal trafficking is promoted. Then, lower lysosomal pH levels lead to migalastat detachment, enabling α-Gal A to clear Gb3. Finally, migalastat is swiftly shuttled out of the cell and eliminated.2
The efficacy and safety of oral 123 mg migalastat, given every other day, has been evaluated in two pivotal Phase III RCTs: FACETS and ATTRACT.64,65 ERT-naive and ERT-treated patients, respectively, were enrolled. Besides the oral treatment route, other advantages include a lack of immunogenicity and the small size, which can potentially enable the crossing of the blood–brain barrier.
Substrate Reduction Therapy
Substrate reduction therapy (SRT) represents a paradigm shift in FD management. Unlike ERT and migalastat, SRT operates by preventing the accumulation of metabolites that cannot be broken down because of the underlying enzyme deficiency.66 SRT inhibits glucosylceramide synthase (GCs), the enzyme responsible for catalysing the first step in glycosphingolipid biosynthesis.66 Moreover, SRT may be given orally, regardless of genotype.
Gaucher’s disease was the first lysosomal storage disease to be treated with SRT.67 There are two approved SRTs for Gaucher’s disease: miglustat, a glucose-based iminosugar, and eliglustat, a ceramide analogue.68 These
Cardiovascular Involvement in Fabry’s Disease CARDIAC FAILURE REVIEW www.CFRjournal.com
Migalastat
Enzyme replacement therapy Chaperone therapy Substrate reduction therapy Gene therapy
Cardiovascular Involvement in Fabry’s Disease
two therapies laid the groundwork for novel FD compounds, which are currently being investigated in RCTs.
Lucerastat, the galactose derivative of miglustat, significantly reduced Gb3 levels in mice and FD patient-derived fibroblasts.66,69 A phase III RCT, MODIFY (NCT03425539), was conducted to investigate the effects on neuropathic pain.70 Unfortunately, no differences in the primary endpoint were observed. However, most patients entered the open-label extension (NCT03737214), and a recent interim analysis showed further reductions in Gb3, and slowing of estimated glomerular filtration rate decline and cardiac hypertrophy, encouraging long-term evaluation.
Venglustat is a ceramide-derived iminosugar with the unique property of being able to cross the blood–brain barrier.71 A Phase IIa study (NCT02228460) assessed the safety profile and pharmacological features and explored efficacy outcomes in ERT-naive classic-FD patients. After 26 weeks, patients could choose to participate in an open-label extension (NCT02489344) of the study.72 Daily intake of 15 mg of venglustat reduced several biomarkers (such as Gb3, GC, GM3 ganglioside, and lyso-Gb3), suggesting that both synthetic and catabolic glycolipid pathways are affected. Moreover, it prevented the progression of FD with an acceptable rate of adverse events. Indeed, light microscopy evaluation of skin biopsies indicated significantly lower Gb3 scores after venglustat treatment. Additionally, a quantitative analysis of Gb3 inclusions in superficial skin capillary endothelium corroborated the light microscopy results. Two phase III RCTs, PERIDOT (NCT05206773) and CARAT (NCT05280548), are ongoing and are investigating the effects of venglustat on neuropathic pain and LVH, respectively.
Despite the established efficacy of approved medications and promising results of novel treatments, none is curative. Therefore, a substantial unmet medical need exists with regard to FD patients.
Gene Therapy
Many gene delivery strategies, including viral and non-viral approaches and in vivo and ex vivo methods, have been studied. This field of research is based on the idea that the treated cells would overexpress and produce α-Gal A, which will be picked up by other cells via the mannose-6phosphate receptor.73 Endogenous enzymatic expression in specific tissues may be achieved using DNA and RNA-based delivery systems. Efficacy was demonstrated for mRNA nanoparticles. Interestingly, a dosedependent trend was noted. Regrettably, owing to the short half-life of mRNA and its non-genomic action, repeated injection is needed.74 75
Haematopoietic stem/progenitor cell gene therapy (HSPC-GT) has the potential to permanently cure FD, avoiding or at least reducing the need for repeated treatment. Transduction of autologous haematopoietic stem and progenitor cells (HSPCs) can be achieved using a lentivirus with an active integrase enabling ex vivo insertion of a transgene. After insertion, engineered HSPCs are then infused and start to replicate. In this way, the inserted transgene is expressed by the progeny of the HSPCs. In addition,
1. Germain DP. Fabry disease. Orphanet J Rare Dis 2010;5:30. https://doi.org/10.1186/1750-1172-5-30; PMID: 21092187.
2. Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab 2018;123:416–27. https://doi. org/10.1016/j.ymgme.2018.02.014; PMID: 29530533.
3. Rubino M, Monda E, Lioncino M, et al. Diagnosis and management of cardiovascular involvement in Fabry disease. Heart Fail Clin 2022;18:39–49. https://doi. org/10.1016/j.hfc.2021.07.005; PMID: 34776082.
4. Pieroni M, Moon JC, Arbustini E, et al. Cardiac involvement
transgene products can be taken up and used by neighbouring cells. Although lentivirus-based strategies are safer than retrovirus approaches, concerns about potential insertional mutagenesis still exist.76
A first-in-human Phase I clinical study (NCT02800070) is testing autologous lentivirus-transduced HSPCs injection in male patients with classical FD.76 In 1 week, all patients had normalised production of α-Gal A. To date, no adverse effects have been reported. The first attempts at in vivo gene therapy used adenovirus because of its versatility.77 However, toxicity reports led to the withdrawal of this approach. Hepatocellular necrosis emerged as a relevant drawback. Indeed, the hepatotropic nature of the vectors used led to an inflammatory response that triggered both innate and adaptive immunity. As a result, transduced cells were targeted and destroyed.78 Thus, the focus has moved toward the adenoassociated virus (AAV).
Four AAV experimental therapies are being studied: FLT-190, ST-920, AMT-191 and 4D-310. Preclinical data on FLT190 showed safety and a steady rise in α-Gal A activity in mice and non-human primate models.79 These results led to the Phase I/II MARVEL-1 study (NCT04040049). Untreated and previously treated adult male patients with classic FD were recruited. After hopeful results from the first dosage group, in which the medication was well tolerated and provided a persistent and dosedependent increase in α-Gal A plasma level, the study is now recruiting patients for the second dose group.
A Phase I/II dose-ranging multicentre study, STAAR (NCT04046224), is evaluating isaralgagene civaparvovec (ST-920) in adult patients. Five patients were on ERT at the beginning of the study. Four of them managed to cease the enzyme infusions without a relapse in Gb3 levels. AMT-191 increases the concentration of N-acetylgalactosaminidase (NagA). This enzyme enables Gb3 clearance while escaping from the antibody response. Finally, despite the potential additional benefits of a cardiacdirected capsid, three patients treated with 4D-310 developed atypical haemolytic-uraemic syndrome. Therefore, 4D Molecular Therapeutics ceased recruitment.
The potential benefit of gene therapies should be evaluated in the context of issues and concerns regarding current gene-editing techniques. Safety is still the major concern in gene editing given its potential to produce off-target effects, which could lead to severe genotoxic effects. Moreover, the cost of treatment is expected to be extremely high. However, it represents a unique opportunity to provide long-term treatment of FD, thereby removing the dependence on ERT or chaperone therapy.
Conclusion
The importance of early suspicion and diagnosis, multidisciplinary evaluation and management, and targeted therapy in FD demonstrates the need for precision medicine, from diagnosis to therapy, in this rare disease. Future developments are expected in preclinical diagnosis with novel biomarkers and effective therapeutic approaches to each stage of the disease.
in Fabry disease: JACC review topic of the week. J Am Coll Cardiol 2021;77:922–36. https://doi.org/10.1016/j. jacc.2020.12.024; PMID: 33602475.
5. Reisin R, Perrin A, García-Pavía P. Time delays in the diagnosis and treatment of Fabry disease. Int J Clin Pract 2017;71:e12914. https://doi.org/10.1111/ijcp.12914; PMID: 28097762.
6. Limongelli G, Adorisio R, Baggio C, et al. Diagnosis and management of rare cardiomyopathies in adult and paediatric patients. A position paper of the Italian Society of Cardiology (SIC) and Italian Society of Paediatric Cardiology
(SICP). Int J Cardiol 2022;357:55–71. https://doi.org/10.1016/j. ijcard.2022.03.050; PMID: 35364138.
7. Limongelli G, Monda E, Tramonte S, et al. Prevalence and clinical significance of red flags in patients with hypertrophic cardiomyopathy. Int J Cardiol 2020;299:186–91. https://doi. org/10.1016/j.ijcard.2019.06.073; PMID: 31303393.
8. Rapezzi C, Arbustini E, Caforio ALP, et al. Diagnostic workup in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:1448–58. https://doi.
CARDIAC FAILURE REVIEW www.CFRjournal.com
Cardiovascular Involvement in Fabry’s Disease
org/10.1093/eurheartj/ehs397; PMID: 23211230.
9. Amodio F, Caiazza M, Monda E, et al. An overview of molecular mechanisms in Fabry disease. Biomolecules 2022;12:1460. https://doi.org/10.3390/biom12101460; PMID: 36291669.
10. Monda E, Bakalakos A, Rubino M, et al. Targeted therapies in pediatric and adult patients with hypertrophic heart disease: from molecular pathophysiology to personalized medicine. Circ Heart Fail 2023;21:e010687. https://doi. org/10.1161/CIRCHEARTFAILURE.123.010687; PMID: 37477018.
11. Monda E, Limongelli G. A roadmap to predict adverse outcome in Fabry disease. J Am Coll Cardiol 2022;80:995–7. https://doi.org/10.1016/j.jacc.2022.06.027; PMID: 36049807.
12. Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. https://doi.org/10.1093/eurheartj/ehu284; PMID: 25173338.
13. Monda E, Limongelli G. Heart failure with preserved ejection fraction: the need for a better genetic characterization. Eur Heart J Cardiovasc Imaging 2023;24:jead043. https://doi. org/10.1093/ehjci/jead043; PMID: 36935444.
14. Politei JM, Bouhassira D, Germain DP, et al. Pain in Fabry disease: practical recommendations for diagnosis and treatment. CNS Neurosci Ther 2016;22:568–76. https://doi. org/10.1111/cns.12542; PMID: 27297686.
15. Hopkin RJ, Jefferies JL, Laney DA, et al. The management and treatment of children with Fabry disease: a United States-based perspective. Mol Genet Metab 2016;117:104–13. https://doi.org/10.1016/j.ymgme.2015.10.007; PMID: 26546059.
16. Zampetti A, Orteu CH, Antuzzi D, et al. Angiokeratoma: decision-making aid for the diagnosis of Fabry disease. Br J Dermatol 2012;166:712–20. https://doi. org/10.1111/j.1365-2133.2012.10742.x; PMID: 22452439.
17. Silva CAB, Moura-Neto JA, Dos Reis MA, et al. Renal manifestations of Fabry disease: a narrative review. Can J Kidney Health Dis 2021;8:2054358120985627. https://doi. org/10.1177/2054358120985627; PMID: 33786192.
18. Rubino M, Monda E, Caiazza M, et al. Diagnosis of Fabry disease in a patient with a surgically repaired congenital heart defect: when clinical history and genetics make the difference. Cardiogenetics 2022;12:102–8. https://doi. org/10.3390/cardiogenetics12010010.
19. Kolodny E, Fellgiebel A, Hilz MJ, et al. Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke 2015;46:302–13. https://doi.org/10.1161/ STROKEAHA.114.006283; PMID: 25492902.
20. Antezana-Chavez E, Cianciulli TF, Hadid CL, et al. Value of electrocardiography to distinguish Fabry disease from sarcomeric hypertrophic cardiomyopathy. Am J Cardiol 2022;178:131–6. https://doi.org/10.1016/j. amjcard.2022.05.021; PMID: 35810008.
21. Tower-Rader A, Jaber WA. Multimodality imaging assessment of Fabry disease. Circ Cardiovasc Imaging 2019;12:e009013. https://doi.org/10.1161/ CIRCIMAGING.119.009013; PMID: 31718277.
22. Mundigler G, Gaggl M, Heinze G, et al. The endocardial binary appearance (‘binary sign’) is an unreliable marker for echocardiographic detection of Fabry disease in patients with left ventricular hypertrophy. Eur J Echocardiogr 2011;12:744–9. https://doi.org/10.1093/ejechocard/jer112; PMID: 21857019.
23. Namdar M, Steffel J, Vidovic M, et al. Electrocardiographic changes in early recognition of Fabry disease. Heart 2011;97:485–90. https://doi.org/10.1136/hrt.2010.211789;
PMID: 21270075.
24. Nordin S, Kozor R, Medina-Menacho K, et al. Proposed stages of myocardial phenotype development in Fabry disease. JACC Cardiovasc Imaging 2019;12:1673–83. https:// doi.org/10.1016/j.jcmg.2018.03.020; PMID: 29778854.
25. Nordin S, Kozor R, Baig S, et al. Cardiac phenotype of prehypertrophic Fabry disease. Circ Cardiovasc Imaging 2018;11:e007168. https://doi.org/10.1161/
CIRCIMAGING.117.007168; PMID: 29853467.
26. Figliozzi S, Camporeale A, Boveri S, et al. ECG-based score estimates the probability to detect Fabry disease cardiac involvement. Int J Cardiol 2021;339:110–7. https://doi. org/10.1016/j.ijcard.2021.07.022; PMID: 34274410.
27. Shanks M, Thompson RB, Paterson ID, et al. Systolic and diastolic function assessment in Fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J Am Soc Echocardiogr 2013;26:1407–14. https://doi.org/10.1016/j. echo.2013.09.005; PMID: 24125876.
28. Esposito R, Galderisi M, Santoro C, et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naïve Anderson-Fabry disease
patients. Eur Heart J Cardiovasc Imaging 2019;20:438–45. https://doi.org/10.1093/ehjci/jey108; PMID: 30085001.
29. Krämer J, Niemann M, Liu D, et al. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur Heart J 2013;34:1587–96. https://doi.org/10.1093/eurheartj/eht098; PMID: 23520186.
30. Zada M, Lo Q, Boyd AC, et al. Basal segmental longitudinal strain: a marker of subclinical myocardial involvement in Anderson-Fabry disease. J Am Soc Echocardiogr 2021;34:405–413. https://doi.org/10.1016/j.echo.2020.11.009; PMID: 33242609.
31. Esposito R, Santoro C, Sorrentino R, et al. Layer-specific longitudinal strain in Anderson-Fabry disease at diagnosis: a speckle tracking echocardiography analysis. Echocardiography 2019;36:1273–81. https://doi.org/10.1111/ echo.14399; PMID: 31246327.
32. Spinelli L, Giugliano G, Imbriaco M, et al. Left ventricular radial strain impairment precedes hypertrophy in AndersonFabry disease. Int J Cardiovasc Imaging 2020;36:1465–76. https://doi.org/10.1007/s10554-020-01847-z; PMID: 32306159.
33. Lillo R, Graziani F, Panaioli E, et al. Right ventricular strain in Anderson-Fabry disease. Int J Cardiol 2021;330:84–90. https://doi.org/10.1016/j.ijcard.2021.02.038; PMID: 33600844.
34. Meucci MC, Lillo R, Mango F, et al. Right ventricular strain in Fabry disease: prognostic implications. Int J Cardiol 2023;374:79–82. https://doi.org/10.1016/j.ijcard.2022.12.047; PMID: 36586515.
35. Monda E, Palmiero G, Lioncino M, et al. Multimodality imaging in cardiomyopathies with hypertrophic phenotypes. J Clin Med 2022;11:868. https://doi.org/10.3390/jcm11030868; PMID: 35160323.
36. Niemann M, Herrmann S, Hu K, et al. Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment. JACC Cardiovasc Imaging 2011;4:592–601. https://doi.org/10.1016/j. jcmg.2011.01.020; PMID: 21679893.
37. Orsborne C, Bradley J, Bonnett LJ, et al. Validated model for prediction of adverse cardiac outcome in patients with Fabry disease. J Am Coll Cardiol 2022;80:982–94. https://doi. org/10.1016/j.jacc.2022.06.022; PMID: 36049806.
38. Seferović PM, Polovina M, Bauersachs J, et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:553–76. https://doi.org/10.1002/ejhf.1461; PMID: 30989768.
39. Rapezzi C, Aimo A, Barison A, et al. Restrictive cardiomyopathy: definition and diagnosis. Eur Heart J 2022;43:4679–93. https://doi.org/10.1093/eurheartj/ ehac543; PMID: 36269634.
40. Patel V, O’Mahony C, Hughes D, et al. Clinical and genetic predictors of major cardiac events in patients with Anderson–Fabry disease. Heart 2015;101:961–6. https://doi. org/10.1136/heartjnl-2014-306782; PMID: 25655062.
41. Linhart A, Germain DP, Olivotto I, et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur J Heart Fail 2020;22:1076–96. https://doi.org/10.1002/ejhf.1960; PMID: 32640076.
42. Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 2001;345:9–16. https://doi.org/10.1056/NEJM200107053450102; PMID: 11439963.
43. Schiffmann R, Kopp JB, Austin HA, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 2001;285:2743–9. https://doi. org/10.1001/jama.285.21.2743; PMID: 11386930.
44. Beck M, Hughes D, Kampmann C, et al. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: a Fabry Outcome Survey analysis. Mol Genet Metab Rep 2015;3:21–7. https://doi.org/10.1016/j. ymgmr.2015.02.002; PMID: 26937390.
45. Germain DP, Waldek S, Banikazemi M, et al. Sustained, longterm renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 2007;18:1547–57. https://doi.org/10.1681/ASN.2006080816; PMID: 17409312.
46. Schiffmann R, Ries M, Timmons M, et al. Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant 2006;21:345–54. https://doi.org/10.1093/ndt/ gfi152; PMID: 16204287.
47. Germain DP, Weidemann F, Abiose A, et al. Analysis of left ventricular mass in untreated men and in men treated with agalsidase-β: data from the Fabry Registry. Genet Med 2013;15:958–65. https://doi.org/10.1038/gim.2013.53; PMID: 23703683.
48. Kampmann C, Perrin A, Beck M. Effectiveness of agalsidase
alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis 2015;10:125. https://doi.org/10.1186/s13023-015-0338-2; PMID: 26416388.
49. Lenders M, Karabul N, Duning T, et al. Thromboembolic events in Fabry disease and the impact of factor V Leiden. Neurology 2015;84:1009–16. https://doi.org/10.1212/ WNL.0000000000001333; PMID: 25663229.
50. Watt T, Burlina AP, Cazzorla C, et al. Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: findings from the Fabry Registry. Genet Med 2010;12:703–12. https://doi.org/10.1097/ GIM.0b013e3181f13a4a; PMID: 20885332.
51. Hoffmann B, Garcia de Lorenzo A, Mehta A, et al. Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: data from FOS (Fabry Outcome Survey). J Med Genet 2005;42:247–52. https://doi.org/10.1136/jmg.2004.025791; PMID: 15744039.
52. Weidemann F, Niemann M, Störk S, et al. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 2013;274:331–41. https://doi. org/10.1111/joim.12077; PMID: 23586858.
53. Warnock DG, Ortiz A, Mauer M, et al. Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant 2012;27:1042–9. https://doi.org/10.1093/ndt/ gfr420; PMID: 21804088.
54. Stappers F, Scharnetzki D, Schmitz B, et al. Neutralising antidrug antibodies in Fabry disease can inhibit endothelial enzyme uptake and activity. J Inherit Metab Dis 2020;43:334–47. https://doi.org/10.1002/jimd.12176; PMID: 31587315.
55. Kizhner T, Azulay Y, Hainrichson M, et al. Characterization of a chemically modified plant cell culture expressed human α-galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metab 2015;114:259–67. https://doi.org/10.1016/j. ymgme.2014.08.002; PMID: 25155442.
56. Ma JKC, Drossard J, Lewis D, et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J 2015;13:1106–20. https://doi.org/10.1111/pbi.12416; PMID: 26147010.
57. Schiffmann R, Goker-Alpan O, Holida M, et al. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: a 1-year Phase 1/2 clinical trial. J Inherit Metab Dis 2019;42:534–44. https://doi.org/10.1002/jimd.12080; PMID: 30834538.
58. Warnock D, Eric W, Schiffmann R, et al. Analysis of the baseline characteristics of Fabry disease patients screened for the pegunigalsidase alfa phase III BALANCE study. Mol Genet Metab 2019;126:S150–1. https://doi.org/10.1016/j. ymgme.2018.12.389.
59. Holida MD, Bernat J, Longo N, et al. Once every 4 weeks –2 mg/kg of pegunigalsidase alfa for treating Fabry disease: preliminary results of a phase 3 study. Mol Genet Metab 2019;126(Suppl 73):S73. https://doi.org/10.1016/j. ymgme.2018.12.176.
60. Linhart A, Nicholls K, West M, et al. Pegunigalsidase alfa for the treatment of Fabry disease: preliminary results from a phase III open label, switch over study from agalsidase alfa. Mol Genet Metab 2019;126(Suppl 94):S94. https://doi. org/10.1016/j.ymgme.2018.12.234.
61. Shen JS, Busch A, Day TS, et al. Mannose receptormediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metab Dis 2016;39:293–303. https://doi.org/10.1007/s10545015-9886-9; PMID: 26310963.
62. Hennermann JB, Arash-Kaps L, Fekete G, et al. Pharmacokinetics, pharmacodynamics, and safety of mossαGalactosidase A in patients with Fabry disease. J Inherit Metab Dis 2019;42:527–33. https://doi.org/10.1002/ jimd.12052; PMID: 30746723.
63. McCafferty EH, Scott LJ. Migalastat: a review in Fabry disease. Drugs 2019;79:543–54. https://doi.org/10.1007/ s40265-019-01090-4; PMID: 30875019.
64. Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet 2017;54:288–96. https://doi.org/10.1136/ jmedgenet-2016-104178; PMID: 27834756.
65. Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 2016;375:545–55. https://doi. org/10.1056/NEJMoa1510198; PMID: 27509102.
66. Welford RWD, Mühlemann A, Garzotti M, et al. Glucosylceramide synthase inhibition with lucerastat lowers globotriaosylceramide and lysosome staining in cultured
CARDIAC FAILURE REVIEW www.CFRjournal.com
Cardiovascular Involvement in Fabry’s Disease
fibroblasts from Fabry patients with different mutation types. Hum Mol Genet 2018;27:3392–403. https://doi.org/10.1093/ hmg/ddy248; PMID: 29982630.
67. Cox T, Lachmann R, Hollak C, et al. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet 2000;355:1481–5. https://doi.org/10.1016/S0140-6736(00)02161-9; PMID: 10801168.
68. Van Rossum A, Holsopple M. Enzyme replacement or substrate reduction? A review of Gaucher disease treatment options. Hosp Pharm 2016;51:553–63. https://doi.org/10.1310/ hpj5107-553; PMID: 27559188.
69. Porubsky S, Jennemann R, Lehmann L, Gröne HJ. Depletion of globosides and isoglobosides fully reverts the morphologic phenotype of Fabry disease. Cell Tissue Res 2014;358:217–27. https://doi.org/10.1007/s00441-014-1922-9; PMID: 24992926.
70. Wanner C, Kimonis V, Politei J, et al. Understanding and modifying Fabry disease: rationale and design of a pivotal Phase 3 study and results from a patient-reported outcome validation study. Mol Genet Metab Rep 2022;31:100862. https://doi.org/10.1016/j.ymgmr.2022.100862;
PMID: 35782623.
71. Peterschmitt MJ, Crawford NPS, Gaemers SJM, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of oral venglustat in healthy volunteers. Clin Pharmacol Drug Dev 2021;10:86–98. https://doi.org/10.1002/ cpdd.865; PMID: 32851809.
72. Deegan PB, Goker-Alpan O, Geberhiwot T, et al. Venglustat, an orally administered glucosylceramide synthase inhibitor: assessment over 3 years in adult males with classic Fabry disease in an open-label phase 2 study and its extension study. Mol Genet Metab 2023;138:106963. https://doi. org/10.1016/j.ymgme.2022.11.002; PMID: 36481125.
73. Medin JA, Tudor M, Simovitch R, et al. Correction in trans for Fabry disease: expression, secretion and uptake of alphagalactosidase A in patient-derived cells driven by a hightiter recombinant retroviral vector. Proc Natl Acad Sci USA 1996;93:7917–22. https://doi.org/10.1073/pnas.93.15.7917; PMID: 8755577.
74. DeRosa F, Smith L, Shen Y, et al. Improved efficacy in a Fabry disease model using a systemic mRNA liver depot system as compared to enzyme replacement therapy. Mol Ther 2019;27:878–89. https://doi.org/10.1016/j.
ymthe.2019.03.001; PMID: 30879951.
75. Zhu X, Yin L, Theisen M, et al. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wildtype mice, Fabry mouse model, and wild-type non-human primates. Am J Hum Genet 2019;104:625–37. https://doi. org/10.1016/j.ajhg.2019.02.003; PMID: 30879639.
76. Khan A, Barber DL, Huang J, et al. Lentivirus-mediated gene therapy for Fabry disease. Nat Commun 2021;12:1178. https:// doi.org/10.1038/s41467-021-21371-5; PMID: 33633114.
77. Poletto E, Pasqualim G, Giugliani R, et al. Effects of gene therapy on cardiovascular symptoms of lysosomal storage diseases. Genet Mol Biol 2019;42(Suppl 1):261–85. https://doi. org/10.1590/1678-4685-GMB-2018-0100; PMID: 31132295.
78. Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002;9:979–86. https://doi.org/10.1038/sj.cgt.7700539; PMID: 12522437.
79. Jeyakumar JM, Kia A, Tam LCS, et al. Preclinical evaluation of FLT190, a liver-directed AAV gene therapy for Fabry disease. Gene Ther 2023;30:487–502. https://doi. org/10.1038/s41434-022-00381-y; PMID: 3663154.
CARDIAC FAILURE REVIEW www.CFRjournal.com






















































