ONE-TO-ONE
I DIDN'T SEE THE SKY
A Ukrainian biomedical scientist discusses life in a war zone: p14


ONE-TO-ONE
I DIDN'T SEE THE SKY
A Ukrainian biomedical scientist discusses life in a war zone: p14

OPINION
THE BIG QUESTION
How worried should we still be about COVID-19? p.16
ACCREDITATION
ISO 15189:2022
An update from UKAS on transitioning to the new standard: p.22
THEBIOMEDICALSCIENTIST.NET MARCH 2023ID NOW ™ PLATFORM



NOW
IMPROVED
patient swab for COVID-19 and influenza A & B IDNOW.ABBOTT
5 Biomedical scientists have a lot to offer outside the laboratory


NEWS
7 News in numbers
8 Research, funding, developments and clinical updates
13 Product advances and launches
14 One-to-one: Inesa Iefimova, a Ukrainian biomedical scientist, talks about her experiences working in a war zone and moving to the UK

16 The big question: This month we ask three experts: “How worried should we still be about COVID-19?”
18 One Health: seeing the whole ecosystem: With One Health gaining increasing prominence in research and the media, we look at the driving factors behind the concept and ask what action may need to be taken

22 ISO 15189:2022 transition: UKAS with an update for labs
24 Autoimmune diseases revisited pt.1: A review of historical developments
EDITOR
Rob Dabrowski
SENIOR DESIGNER
Gary Hill
PICTURE EDITOR
Akin Falope
PUBLISHING DIRECTOR
Aaron Nicholls
PRODUCTION
PUBLISHED BY Redactive Publishing Ltd
9 Dallington Street, London EC1V 0LN redactive.co.uk
RECRUITMENT
27 Congress 2023: The full IBMS Biomedical Science Congress programme revealed
41 How to… inspire change: Navigating the complex worlds of leadership and management
43 Institute news: The latest from the IBMS
45 Can I be a career woman?: Supporting women in the workforce
46 Journal-based learning: CPD exercises based on journal articles
49 Here to help: A look at becoming HCPC registered
50 Robyn Wilson gives a guided tour of POCT for NHS Tayside
PRINTED BY Warners Midlands plc Bourne, Lincolnshire PE10 9PH
SUBSCRIPTIONS Subscriptions are available by calling 01580 883844
Neither the publisher nor the IBMS is able to take responsibility for any views or opinions expressed in this publication. Readers are advised that while the contents are believed to be accurate, correct and complete, no reliance should be placed upon its contents being applicable to any particular circumstances. Any advice or information published is done so without the Institute, its servants or agents and any contributors having liability in respect of its content.
• Innovative technology advance

Fluorescent Immunoassay based on lateral flow
• Objective
Unlike skin test, AFIAS IGRA-TB is a controlled and objective assay



• Single visit test


AFIAS IGRA-TB requires only one visit for patient

• Effective in BCG-Vaccinated patients
Unaffected by BCG vaccination
• On-demand testing
1 patient, 1 test, 1 result
• Ready-to-use reagents
Affordable and Accessible Test for Latent TB diagnosis
Standard ~ 24 hour incubation with IGRA incubation tubes
No ELISA and calibration software required
Quick results in 15 min
The HCPC recently published the retention rates from across their professional registers and it seems that biomedical scientists have bucked the overall trend of the last few years by remaining consistent. Once we get into our profession, we tend to stay here.
The most interesting difference was seeing that 70% of newly registered biomedical scientists are now female. Given that only 66% of our current membership are female, it follows that the future of the profession will be even more female. I think diverging from most STEM careers in this area gives our profession a wonderful opportunity – to lead the way and set the standards in professional progress for women in science.
However, the career path of a biomedical scientist after registration is often not as hard to navigate as getting there in the first place. That’s why our “Routes to Registration” project is underway – spreading information about routes into the profession to college and sixth-form students, careers advisors, undergraduates and graduates across the UK.
We have started by making sure that every IBMS Accredited university across the country has appropriate leaflets and information to hand out to their prospective students this year – informing them about IBMS Accredited degrees, registration training and HCPC requirements before they make any choices that might negatively impact their career paths.

We are now in the process of scripting and commissioning four short videos that will be used on our website and on social media to give accessible and clear information about the choices and routes available to the people in our key demographics who might not be directly within our reach.
We will follow this up with campaign posters and further leaflets for careers advisors and careers fairs, and attempts to get these into the hands of those who will benefit from understanding the quickest and easiest routes to HCPC registration as a biomedical scientist. I hope you will find them useful in your outreach work and share them with your wider network.
Alongside accrediting course content and providing training systems that meet HCPC requirements, we want to provide the means to make sure that more people than ever understand that their education choices are also career choices. This will help our members to recruit the skilled and qualified staff they need, at the level they need them. BMS
David Wells Chief ExecutiveDavid Wells, IBMS Chief Executive, on the Institute helping members recruit skilled and qualified staff.





More than 1.6 million adults aged 50 and over are unable to work because of long-term sickness, according to analysis of Office for National Statistics data.
The number has increased 20% in three years, states the analysis by Rest Less, a digital community for over fifties. It showed that of the 2.8 million people out of work due to long-term sickness, nearly 60% were aged over 50.
More than 1000 NHS buildings across London and Scotland still contain asbestos, the Trades Union Congress has warned. Its research revealed that the substance was present in at least 451 NHS premises in London and 695 in Scotland. Two-thirds of these, including hospitals, health centres and GP surgeries, were open to the public, it added. Asbestos-related diseases kill around 4500 people a year across England, Scotland and Wales, according to the Health and Safety Executive.
On 24 January no hospital admissions of patients with COVID-19 were recorded in Wales for the first time since the start of the pandemic. Cases of seasonal flu in Wales were also at their lowest since October, while only one patient was being treated primarily for COVID in critical care.
1146 41% 30 , 000 0
A Jersey government report says 41% of people over the age of 50 on the island have not received a COVID autumn booster. A total of 26,959 boosters have been administered since the scheme was launched in September .
The report also stated that 93% of the population aged 18 and over had received their first dose, 92% had been double vaccinated, and 75% had received a third dose.
At the public inquiry into the infected blood scandal, the trust said it recognised the harm, hurt and distress that had been caused Up to 30,000 people were given contaminated blood. In September, modelling commissioned by the public inquiry estimated that 26,800 people were infected after being given contaminated transfusions between and
Immunity from COVID-19 appears to gather strength with more time between vaccination and infection, a new laboratory study from researchers at Oregon Health & Science University (OHSU) suggests. The findings carry implications for vaccine recommendations as the pandemic transitions to an endemic state.
Researchers measured the antibody response in blood samples for a group of people who gained so-called “hybrid immunity” through two means: either vaccination followed by a breakthrough infection, or by
vaccination after contracting COVID-19. They measured the immune response in blood samples of 96 generally healthy OHSU employees and found that the immune response was uniformly stronger the longer the time period between vaccination and infection. The longest interval was 404 days.
Their findings suggest that vaccine boosters should be spaced no more frequently than a year apart, at least among healthy people.
bit.ly/3HEA74W
A new biosensor chip that boasts an accurate and inexpensive design may increase accessibility to high-quality diagnostics, it is claimed. It identifies biomarkers by measuring how binding occurs between DNA strands and the device. Its modular design lowers costs by making it easier to mass produce and allowing the most expensive components to be reused.
Like other DNA biosensors, the device takes advantage of the fact that a single DNA strand, when not paired with another within the familiar double helix, is primed for chemical bonding. Part of the device is coated with single strands of DNA.
When these “probes” encounter DNA biomarkers that have a corresponding, or complementary, genetic sequence, the two strands bind, sending a signal that is picked up by the device.
bit.ly/3DsF3qW
ARTIFICIAL
More than one-third of all people admitted to hospital, and as many as % of all patients in an intensive care unit (ICU), develop delirium, it is reported.
This is a type of brain dysfunction marked by sudden bouts of confusion, inattention, paranoia, or even agitation and hallucinations.
A team from Johns Hopkins Medicine has now developed artificial intelligence (AI) algorithms that can detect the
early warning signs of delirium and can predict – at any time during an ICU stay – a high risk of delirium for a significant number of patients.
“Being able to differentiate between patients at low and high risk of delirium is incredibly important in the ICU because it enables us to devote more resources toward interventions in the high-risk population,” says Robert Stevens, senior author.
The team applied AI algorithms
to a publicly available dataset covering more than , ICU stays at hospitals around the country.
Once the researchers developed the AI models, they tested them on two other sets of data from a Boston hospital, collectively covering more than , ICU stays. The area under the receiver operating characteristic curve ( % CI) for the first -hour model was , meaning that it was able to
predict which patients would get delirium .% of the time. The dynamic model performed even better, predicting delirium-prone patients up to % of the time. bit.ly/3HGCQux


Researchers publishing in the Journal of Medicinal Chemistry have identified a new candidate molecule that could become an effective non-hormonal contraceptive for many people who produce sperm.
New research has established a blood-based test that could be used to predict the risk of Alzheimer’s disease up to 3.5 years before clinical diagnosis.
The study supports the idea that components in the human blood can modulate the formation of new brain cells, a process termed neurogenesis.
Neurogenesis occurs in an important part of the brain called the hippocampus that is involved in learning and memory.


While Alzheimer’s disease affects the formation of new brain cells in the hippocampus during the early stages of the disease, previous studies have only been able to study neurogenesis in its later stages through autopsies.


To understand the early changes, King’s College London researchers collected blood samples over several years from 56 individuals with mild cognitive impairment (MCI), a condition where someone will begin to experience a worsening of their memory or cognitive ability.
Of the 56 participants in the study, 36 went on to receive a diagnosis of Alzheimer’s disease.
Dr Aleksandra Maruszak, study joint first author, said: “We treated brain cells with blood taken from people with MCI, exploring how those cells changed in response to blood as Alzheimer’s disease progressed.”
In studying how blood affected the brain cells, the researchers made several key discoveries. The blood samples collected from participants over the years who subsequently deteriorated and developed Alzheimer’s disease promoted a decrease in cell growth and division and an increase in apoptotic cell death (the process by which cells are programmed to die). However, the researchers noted that these samples also increased the conversion of immature brain cells to hippocampal neurons. bit.ly/3WQKiaV
3.5YRS
NEW RESEARCH HAS ESTABLISHED A BLOODBASED TEST THAT COULD BE USED TO PREDICT THE RISK OF ALZHEIMER’S DISEASE UP TO 3.5 YEARS BEFORE CLINICAL DIAGNOSIS.
A combination of proteins and antioxidants doubles the anti-inflammatory properties in immune cells, claim University of Copenhagen scientists looking at how polyphenol reacts with an amino acid.

Scientists have created an artificial intelligence system capable of generating artificial enzymes from scratch. In laboratory tests, some of these enzymes worked as well as those found in nature.


Fathers being exposed to chemicals in plastics could affect the metabolic health of their offspring for two generations, a University of California, Riverside mouse study reports.
The 2018 UK implementation of a levy that taxed soft drinks that are high in sugar at a higher level is associated with an 8% reduction in obesity among 10–11-year-old girls, according to a new study.
A new study shows significant improvements in sleep quality and improved autonomic nervous system function using closed-loop, acoustic stimulation neurotechnology.

DIABETES
A US study examining the use by older adults of continuous glucose monitors and other wearable devices found issues with wearability and use.
In an initial trial of continuous glucometer use, faulty device adhesive and patient di culty in interpreting graphs produced by the device, were noted.
In a subsequent trial, older adults with diabetes wore glucometers and activity monitors, and used smartphones and electronic medication bottles to track and manage blood sugar over two weeks.
Nearly three-quarters of the study participants experienced low blood sugar levels, often serious in magnitude.
Study lead Michael Weiner said: “Although a smartphone is very commonly used with continuous glucometers, we found that when we issued smartphones to study participants, they often didn’t carry the phones with them, contributing to the fact that, during the study, one-third of daytime phone prompts about medications, behaviours, and symptoms were ignored.”
bit.ly/3DqiELc
ANTIBODIES
Functionalised nanoparticles could soon revolutionise point-of-care diagnostics, it is claimed.
Researchers have developed a method for binding specific molecules in samples and serums, such as antibodies in the blood, to the surface of iron oxide particles thus allowing them to be identified using an inexpensive and compact detector.
This method is based on magnetic nanoparticles – particles of iron oxide that are a few hundred nanometers in size to which specific surfaces have been applied.
Binding nanoparticles with specific antibodies or antigens creates molecules that have a unique movement pattern,
I’ve not heard of “pharmabiome”, what is it?
Don’t worry, you won’t be alone. The term has only just been coined. It refers to the genetic signatures found in medicines.
Sounds intriguing. Tell me more.
It is from an international study investigating whether bacterial, plant, fungal, animal and human DNA traces, or environmental DNA (eDNA), could be found in the ingredients of falsified (also known as counterfeit) antimalarial medicines.
Are falsified medicines a big issue?
The WHO estimates at least 10% of medicines sold in lowand middle-income countries are either substandard or falsified – with falsified antimalarials
for example in rotating magnetic fields.
The researchers have demonstrated that it is possible to make highly sensitive measurements in a very short space of time with little technical outlay.
For example, they were able to reliably detect SARS-CoV-2 antibodies within a few seconds using a rapid test. Since the diagnostic device fits into a small case and the materials only cost a few hundred pounds, the technology set could soon become part of the standard equipment for point-of-care diagnosis and become a genuine alternative to complex and costly methods of analysis such as ELISA or flow cytometry.

bit.ly/3DqbypE
prevalent in disease hotspots where access, affordability and corruption supports their distribution.
What did the researchers find?
“We found a much greater diversity of eDNA in the falsified tablets, with differences between the diverse falsified packaging types, as well as traces of human DNA,” said lead scientist Dr Jennifer Young, who led the DNA analysis.
What are the implications?
Poor-quality medicines are a serious
threat to global public health, but there has been little research into new techniques to provide actionable evidence as to where they are coming from, who is making them and what their illegal trade routes are. This pilot research on the pharmabiome states that with the falling cost of genetic analysis it could offer a cost-effective tool to help investigators understand much more objectively where falisified medicines originate.”
Where can I read more?
Go to the link – bit.ly/3XTJBPp
More people in the US died from cardiovascular-related causes in 2020, the first year of the COVID-19 pandemic, than in any year since 2003, according to the American Heart Association’s 2023 update.
The biggest increases in deaths were seen among Asian, Black and Hispanic people, it reported.
While the pandemic’s effects on death rates may not be noticed for several years, lessons learned offer major opportunities to address structural and societal issues that drive health disparities, said Association leaders.
Michelle A Albert, a Cardiology Chair,said: “COVID-19 has both direct and indirect impacts on cardiovascular health.
As we learned, the virus is associated with new clotting and inflammation.
“We also know that many people who had new or existing heart disease and stroke symptoms were reluctant to seek medical care, particularly in the early days of the pandemic. This resulted in people presenting with more advanced stages of cardiovascular conditions and needing more acute or urgent treatment for what may have been manageable chronic conditions.” bit.ly/3WTHQQW
One Day Workshops in Non-Gynaecological Cytology – Preparation and Cytology of Urinary Tract, Respiratory Tract & Serous Cavities
This course covers sample collection and preparation techniques, serous uids, urine and respiratory cytology and will include lectures, workshops, multi-header sessions and self-assessment elements.
10th, 11th and 12th May 2023
Non-Gynaecological Cytology Updates for Experienced Staff
This course comprises of three one-day courses covering serous uids, urine and respiratory cytology. All three full days are ideal for anyone wishing to further their experience in nongynae cytology. and are suitable for both nonmedical and medical staff. These courses are also suitable for those preparing for the DEP and ASD in non-gynaecological cytology.
8th, 9th and 10th November 2023
Three-day Update Course for Consultant Biomedical Scientists
This three-day course is aimed at Consultant Biomedical Scientists but is also open to those in training for that role and to medical staff. It includes sessions on histopathology, developments of HPV testing and invasive cancer audit.
14th, 15th and 16th June 2023
Two-Day Course for Cervical Screening Provider Leads (CSPL)
These one-day events have been put together in association with the NHSCSP Cervical Cancer Audit Management Group to help guide both new and experienced CSPLs through the various aspects of their role.
27th & 28th February 2023
Exam Practice for the IBMS Advanced Specialist Diploma in Cervical Cytology
This two-day course is ideal for anyone intending to sit the Advanced Diploma in Cervical Cytology.
3rd & 4th April 2023
Course for the Expert Role in Specimen Dissection
Suitable for BMSs who intend to train as histological tissue specimen dissectors, in particular those undertaking the RCPath/IBMS Diploma. It covers all the mandatory modules. Specialist Modules scheduled throughout 2023
Update in the Theory and Principles of Immunocytochemistry and DEP Mock
This course is aimed at those interested in taking the IBMS DEP in Immunocytochemistry but also useful as a refresher.
8th & 9th June 2023
Tissue Recognition, Section Quality and Clinical Consequences
This is aimed at BMSs preparing for their specialist portfolios and involved in the preparation of routine histology samples.
28th July 2023
Haematological Morphology Study Days
This course is aimed at those studying for their specialist portfolio but is also suitable as a refresher or an update.
25th & 26th May 2023
For further information contact our Admin Team: sht-tr.nepsec@nhs.net Tel: 0113 2466330 www.nepsec.org.uk











A new biomedical science lab, which will be used to carry out research into diseases including cancer and diabetes, as well as looking at antimicrobial resistance, has been
University. The lab, which is based at the School of Health, City Campus houses specialist equipment that will enable undergraduate and postgraduate students to engage with cutting-edge research. This includes the latest technologies, such as fl real-time PCR.


leedsbeckett.ac.uk
The Swedish medtech company Capitainer AB announced a research collaboration with AstraZeneca. AstraZeneca will use a novel device from Capitainer to develop protocols for biomarkers relevant to AstraZeneca’s clinical drug programmes. The collaboration aims to develop protocols for biomarkers relevant to AstraZeneca’s clinical drug programmes based on Capitainer’s novel selfsampling product delivering cell-free blood. This allows new patient-centric sampling solutions. capitainer.se
• Mutations in NUDT15 are associated with poor metabolism of thiopurines and increased risk of myelosuppression
Wastewater testing labs can now scale up their infectious disease surveillance using two reliable technologies in a single kit. The new system released by Promega Corporation pairs the company’s Maxwell HT chemistry with Ceres Nanosciences’ Nanotrap particles, an established technology for capturing and concentrating low-abundance analytes, such as viral particles. Together, these technologies empower labs to purify pathogen nucleic acids from wastewater samples in fl that enables concentration of microbes from wastewater that is unique to the market,” said Brandon Krueger, Promega Field Support Scientist. promega.co.uk
• c.415C>T mutation associated with NUDT15*2 and NUDT15*3 haplotypes
• Increased prevalence of c.415C>T mutation in Asian populations
• Recommended that NUDT15 genotyping is performed prior to initiation of thiopurine drugs (ALLtogether guidelines)
• Analysis performed by real-time polymerase chain reaction (RT-PCR)

rajvindergarcha@nhs.net
www.bcpathology.org.uk
0121 507 5348
Clinical Biochemistry, City Hospital Dudley Road, Birmingham B18 7QH
@BCPathology BCPathology
Black Country Pathology TV News
 IMAGE: ©LEEDS BECKETT UNIVERSITY
LEEDS BECKETT UNIVERSITY
PROMEGA
IMAGE: ©LEEDS BECKETT UNIVERSITY
LEEDS BECKETT UNIVERSITY
PROMEGA


to the invasion “all the media were describing Russia threatening Ukraine, but we didn’t think anything would happen”. At the time, she was working as Head of the Clinical Diagnostics Laboratory (CDL), heading up a team of 16 at Kharkiv Regional Clinical Trauma Hospital – a 260-bed hospital located 19 miles from the Russian border.
“It was a Thursday and at about 4am I heard the sound of shelling,” says Inesa Iefimova. “From this moment, for three months, I was within the hospital without going outside. It was a very frightening situation. Before this I had never heard the sound of shelling, or rockets exploding. For a week my organs refused me – I was unable to eat and I could only have small drinks.”
Inesa, a 60-year-old Ukrainian biomedical scientist, is remembering 24 February 2022 – the day Russia launched a full-scale assault on Ukraine. Since that moment, over seven thousand civilians (including 438 children) have been killed, while nearly 17.7 million people have fled the country, according to UN statistics.
Inesa says that in the week leading up

In the coming weeks, while treating injured citizens and soldiers from the Kharkiv region, the hospital was also under attack. It was shelled three times, damaging sections of the roof and the top two levels of the building and destroying a technical area, an operating theatre and the X-ray department. More than 150 hospital windows were blown out by the force of the explosions.





“I gathered myself. I had 16 lab staff behind me and I needed to support and encourage them,” says Inesa. “It was terrible, but I needed to look after my staff – they were my responsibility. Sometimes I would cry, but it would be at night, so no one could see my weakness.”
Inesa had been the Head of CDL since 1995, a position that she says
is very different from the UK equivalent. “In the UK, it is a managerial position, but in Ukraine, it is many jobs in one – I was providing and supervising the implementation of lab quality control and systems, training staff, assisting laboratory staff in interpreting abnormal laboratory tests and working with the doctors.” She also maintained contact with all suppliers to ensure that the laboratory was continuously supplied with everything necessary for smooth operation.
By March, her job had become far harder. “Many people in my lab were frightened, so we let them go. It was just two of us in the lab – me and my subordinate. I would rest during the day while she worked, and then she would rest at night while I worked. We would sleep on the floor. We had the blinds down and during this time I didn’t see the sky. I didn’t want to see what was out there.”
The only time Inesa would leave the hospital was when she needed to change clothes. She would go home to get them between 11am and 2pm, “when it was usually quiet”. Before the invasion, the hospital had about 400 staff, but this number was reduced to about 150 who were sleeping and working at the
A Ukrainian biomedical scientist talks about her experiences working in a war zone and moving to the UK.
hospital. At the same time, the Director organised deliveries of food, water and humanitarian aid packages.

Since the early 2000s, Inesa had wanted to move to England and had been studying English in Ukraine with a private teacher and flying over twice a year to complete language courses in London. She even completed an IBMS Specialist Diploma and became an IBMS member – the only member in Ukraine.

“The IBMS got in touch and asked what kind of support they could provide for me,” says Inesa. “I thanked them very much, but I didn’t know what kind of support I needed or how this support could reach me – transport, post offices, shops… everything was closed.”
It was not until weeks later in May, when the fighting in and around Kharkiv started to die down that Inesa changed her mind. “At first, we thought that the situation would end in a month, but the situation continued and continued and became harder and harder – it was at the start of May that I wrote to the IBMS and asked if they could help me move to the UK.”
The IBMS Council was consulted, and
a vacancy was found working for Black Country Pathology in The Royal Wolverhampton NHS Trust. The training was organised, accommodation was lined up, and they were able to arrange a position for six months. Inesa had a sponsor whom she had kept in touch with after meeting on an IBMS course in the UK in 2020 – Elena Cohen, an Advanced Biomedical Scientist working at the Biochemistry Department of Royal Oldham Hospital, Northern Care Alliance NHS Foundation Trust. Elena continues to provide assistance support to Inesa in any matters.
“In the summer, the situation became quieter – people started returning home to Ukraine and it became safe to travel, so I went to the UK,” she says. “I had been in the UK many times as a visitor, but now I was going to be here as a resident.”
After pulling together all her paperwork, qualifications and accreditations and getting a hepatitis vaccine (which is not needed in Ukraine) Inesa started in the Haematology department at Manor Hospital in Walsall. “I went to the hospital and I started to learn how to use the new equipment. The differences between a UK and Ukrainian lab are the equipment and the processes of how you authorise analysis. But everything was changing for me – how to buy food, what food to buy, where to find things. All the lab staff tried to help me and if I had any questions, I could ask,” she says.
“The first three months were very difficult and sometimes I still struggle to understand the accents of some people, but most people I understand.”
Now that she is settled into her new life and laboratory, does she miss friends and family in Ukraine? “I am alone,” she says. “I have an older sister with two sons, and the youngest grew up with me and is like my son. He has a sponsor in Ipswich and is waiting to see if he gets his visa. But yes, some friends and colleagues I miss.”
Would she ever consider going back to Ukraine when the war is over? “Before coming to the UK, I made the decision that if I was going to do it, then I would do it forever,” says Inesa. “Ukraine is becoming a different country to the country I knew before the war. People have become different. War changed minds and values and so many people lost their homes and became homeless. People lost everything. To return to Ukraine makes no sense.
“I have lost in Ukraine all that I have. Here in the UK, I have to start life from scratch, but it is easier than living and working under constant shelling.” BMS
“Sometimes I would cry, but it would be at night, so no one could see my weakness”
THIS MONTH WE ASK






“How worried should we still be about COVID-19?”
Senior Lecturer in Biomedical Science



Nottingham Trent University
Perception on how worried we need to be about COVID-19 depends on your point of view.
On the one hand, the number of cases has dramatically fallen and is currently low. On 3 January 2022, 193,615 cases were reported, with a seven-day average of 166,268 cases per day being seen. The latest data as of 17 January 2023 showed that 1964 cases were reported with a seven-day average of 1776. This is still significantly higher than the level of circulating influenza, which saw 203 samples testing positive for all lineages of influenza virus in the week four National Influenza and COVID Report.
COVID-19 vaccination rates remain high. Of the over 50s and those eligible for an autumn booster, 64.4% were boosted. Higher booster rates are seen in the older age groups, with 82.4% of the over 80s having received their autumn dose.
Whilst still possible, the risk of catching, and becoming ill with COVID-19 is low. However, this must be balanced with the fact that testing has significantly reduced, with lab tests peaking at 701,933 per day on January 2022 and being reduced to 35,825 on 14 January 2023. This can skew data. At the time of writing, 5697 patients were in hospital with COVID-19, leading to 100 deaths daily.
With the almost complete suspension of COVID-19 measures and increased social mixing, it would seem that society’s worries about COVID-19 have disappeared. However, this may be premature.

If you get infected (or reinfected), chances are you won’t get seriously ill, and the chief concern would be the chance of getting long COVID. Remember, there is a dose–response relationship between the number of times you get infected and your chances of getting long COVID. However, stay home if you have cold or flu-like symptoms, or at least wear a high-quality face mask when out and about to protect others. If you are older and/or have underlying health issues, then you should really take care to protect yourself. Make sure you are fully vaccinated and wear a good-quality face mask when going out.
Be careful around elderly or vulnerable family members. Don’t visit if you are feeling unwell and consider wearing a face mask to protect them. Consider taking a lateral flow test before visiting.
COVID-19 is still causing a lot of sickness. This is putting pressure on our health system, both in terms of services being taken up by COVID patients and health staff off sick with COVID.
We will have better vaccines and antivirals this year. The new subvariants like CH.1.1 and XBB.1.5 are more transmissible than their predecessors, but the wall of immunity built up from vaccination and previous infections should ensure future waves of infection are smaller, and no more severe than previous ones. The only major concern is if a new variant emerges that is more severe than Omicron – let’s hope it doesn’t!
From the beginning of the new year 2023 there has been less emphasis on COVID-19, particularly from the media. Also, only around one in four adults (26%) in Great Britain reported the coronavirus pandemic as an important issue facing the UK today, between 11 and 22 January 2023, according to the Office for National Statistics.
However, I do believe we should still be worried as laboratories and the NHS service are still facing staff sickness absences related to COVID-19, which has increased towards the end of January 2023. This is impacting our ability to process increased workload as services are trying to clear the backlog experienced from the pandemic. From what I have observed, all health service providers, such as GPs and hospitals, are continuing with the mask mandate. Staff are often debating about when this will be dropped as they are not mandatory to wear outside of work within the local community. Some argue there is no justifiable reason why staff are still having to wear them, due to the uncertainty about the effects of the mask. The continued wearing of masks is also a significant barrier to effective verbal and non-verbal communication and could impact sustainability within the environment. However, the most vulnerable patients need to be protected, so I do not envisage use of masks changing. As a profession we should still be cautious surrounding COVID-19 and ensure other measures, such as hand hygiene, are reiterated to modestly reduce the burden of respiratory illness.

The philosophy at the heart of “One Health” is far from new but it has acquired a fresh impetus as the fallout from the COVID-19 pandemic continues to be picked over. Was it triggered by an animal virus crossing over to humans? Could better food safety have prevented its spread? Did environmental destruction make it more likely? As critical as these lines of inquiry are individually, the greater significance, when viewed from the perspective of One Health, is that they cannot be separated from one another.
As a concept, One Health has its roots in the 1960s, when Calvin Schwabe, a veterinarian and public health expert, wrote about the close relationship between human and animal medicine. Leading on from this observation, the concept suggested that human medicine, particularly in relation to public health, doesn’t occur in isolation, and that not only does the interaction between humans and animals need to be taken
into account, but also the interaction between humans and animals and the environment.

This holistic view of medicine began to take a firmer shape during the first decade of the 21st century, with the emergence of new and threatening infectious diseases, such as H5N1 influenza (bird flu), severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The origins of these of outbreaks could be traced to the breakdown of the traditional barriers between animals and humans, allowing the virus to jump from one to the other.
These events were the impetus to establish a formal approach for One Health. A big step came in 2008 with the launch of the One Health Framework, known as the FAO-OIE-WHO Collaboration, by the Food and Agricultural Organization of the United Nations (FAO), the World Organisation for Animal Health (OIE) and the World Health Organization. This set
With One Health gaining increasing prominence in research and the media, we look at the driving factors behind the concept and ask what action may need to be taken.
out a vision of “a world capable of preventing, detecting, containing, eliminating, and responding to animal and public health risks attributable to zoonoses and animal diseases with an impact on food security through multi-sectoral cooperation and strong partnerships”.

Then in 2009 the One Health Commission was set up. The objective of this joint initiative by the American Medical Association, American Veterinary Medical Association and American Public Health Association was to bring experts together from across all three disciplines and to “raise awareness and to educate all audiences about the importance of transcending institutional and disciplinary boundaries to transform the way that human, animal, plant, and ecosystem health professionals work together for the health of all living things and the planet”.
In the years that followed, more organisations dedicated to understanding and promoting the tenets of One Health were established, international crossdiscipline conferences were held, research papers written and strategies drawn up. Ultimately none of this did much to help prevent the emergence or soften the impact of the SARS-CoV-2 virus and the global pandemic; even so, one of the lessons coming out of the pandemic has been an evident need to sharpen the focus on a coherent and more intent response to the underlying issues.
“The emergence of the SARS-CoV-2 virus that causes COVID-19 has underlined the need to strengthen the One Health approach, with a greater emphasis on connections to animal health and the environment,” says WHO. “Attempting to save money by neglecting environmental protection, emergency preparedness, health systems, water and sanitation infrastructure, and social safety nets has proven to be a false economy, and the bill is now being paid many times over.”
Today, WHO’s definition of One Health
emphasises vigorous action, describing it as: “an approach to designing and implementing programmes, policies, legislation and research in which multiple sectors work together to achieve better public health outcomes”. The areas where it believes a One Health approach is especially relevant include food safety, zoonotic diseases, climate change, environmental health and antimicrobial resistance.

Here in the UK, a proponent of the One Health approach is Dr Sarah Pitt, Principal Lecturer in the School of Applied Sciences at the University of Brighton. She says a key step is to remove some of the barriers between human and animal medicine: “They’re taught separately, you are either a vet or a doctor, and they don’t necessarily talk to each other. But there’s a lot of overlap in learning and understanding anatomy and physiology, particularly for mammals. What we learn from or know about one thing we can extrapolate to another. It doesn’t always translate but it’s often a very useful starting point.”
In essence it’s about not looking at
“Resistant bacteria can be transmitted between companion animals, such as dogs, and their owners”
“Perhaps it has generated more interest in the wider world outside of nerdy microbiologists like me”
things in isolation. But it’s about taking that step further and taking a much wider view. “One Health sees the whole ecosystem, which includes humans and animals, but also includes the climate, and the environments we live in, whether that’s a city or a village. These things are all part of our health,” says Sarah.
Climate change and its associated extreme weather events in particular have in recent years rammed home this point. “On the one hand people might be worried about mosquitoes appearing in Surrey, but on the other there is a lot more to it than that. We have to ask, with the very hot temperatures and the extreme flooding we had all across the world last year, how does that affect people’s health and wellbeing? It leaves them susceptible to all sorts of diseases, and if gets too extreme they are likely to want to migrate somewhere not quite as hot, and they might be carrying those infectious diseases with them. If they decide to stay where they are, they will have to live in a much more restricted environment, so how would that affect their health?”
Another complicating factor is deforestation, which has only accelerated over the past couple of decades, bringing humans and animals into much closer contact in ways that previously never occurred. The potential ramifications of this for global health could be enormous.
Along with COVID, has all this had the effect of raising awareness of One Health and the many intertwined issues?
“Perhaps it has generated more interest in the wider world outside of nerdy microbiologists like me,” says Sarah. “That has got to be a good thing, hasn’t it?”
A further key concern under the One Health banner is the well-documented problem of antimicrobial resistance. With the misuse and overuse of antibiotics among humans and animal populations throughout the late 20th and early 21st
centuries, the rise of drug-resistant microbes is now one of the major challenges facing medical science.
In a recent paper, “One Health interprofessional stewardship to combat antimicrobial resistance”, published in Nature Medicine, a research team at Tufts University in Massachusetts found that the threat of resistance is not limited to animals in agriculture: “studies increasingly recognise that resistant bacteria can be transmitted between companion animals, such as dogs and cats, and their human owners, and that pets can act as a reservoir for multidrug-resistant organisms that affect human health”. As with COVID-19, the issues are far closer to home than suspected.

One of authors, Dr Claire Fellman, Assistant Professor in the School of
Acknowledging the growing importance and awareness in the wake of the COVID- pandemic of the need for a balanced and sustainable approach to the health of humans, animals and ecosystems, the integrated approach of One Health will be the subject of a special edition of the British Journal of Biomedical Science later this year.
Manuscript submissions are being welcomed on perspectives, challenges and recent advances in the fields of One Health, diagnostics, pathology and epidemiology of existing and emerging zoonotic diseases. The deadline date for submission is April .

For more details visit: frontierspartnerships.org/researchtopics/ /zoonoses-and-one-health

Veterinary Medicine at Tufts, says that the One Health approach underpins much of the school’s work. “It’s a really important concept for us. There’s a lot of expertise on the human side of medicine for which we need to build analogous expertise on the veterinary side. The exciting thing is that it’s not just them helping us. On occasions we’ve been able to help them with health issues related to zoonosis.”
Whether they are in the doctor’s office or the veterinarian’s office, notes Claire, people are becoming more receptive to the message that antimicrobials need to be protected and preserved. “Of course One Health applies much more broadly,” she says, “but I do think antimicrobial stewardship is a good example of how it can work.” The job is far from finished, though: “We still need to gain traction within the medical fields of the concrete benefits and I also think we’ve been a little slow to loop in the environmental aspects.”
She adds that, from a US perspective at least, there is now a firm expectation of collaborative science. “I think that within 10 years collaboration is going to be extensive. And as we realise the interconnections and the expertise that could be found, even more collaboration will be expected, and even greater benefits will be realised.”
As the issues that feed into One Health become yet more pressing, raising uncomfortable questions about the robustness of global health, strenuous and systematic collaboration might appear to be the best bet for avoiding the next pandemic and similar devastating events. BMS
In December last year, ISO 15189:2022 Medical Laboratories – Requirements for Quality and Competence was published. This initiated a three-year transition period for all organisations accredited to ISO 15189:2012, including those also accredited to ISO 22870:2016 (Point of care testing (POCT) – Requirements for Quality and Competence). At the end of the transition period, all organisations accredited to ISO 15189:2012, with or without ISO 22870:2016, must have been assessed and accredited to ISO 15189:2022.

UKAS has already sent out information to accredited laboratories, and to UKAS technical assessors, regarding the transition process, all of which is also available on the UKAS website (ukas.com/ accreditation/iso-15189-transition). Customers should periodically check the website hub for updates, as additional information will be added throughout the transition period.


Although a three-year transition sounds like a long time, the time will fly by. UKAS is asking customers to spend 2023 reviewing and implementing the requirements of ISO 15189:2022. Transition assessments to ISO 15189:2022 will be mandatory from January 2024 and run alongside each customer’s annual surveillance or reassessment visit. This will allow sufficient time for all laboratories to clear findings and for UKAS to complete the administrative side of the transition and grant any remaining accreditations to ISO 15189:2022 prior to the deadline towards the end of 2025.
It is possible for accredited laboratories to undergo their transition assessment from April 2023 if they have discussed this with their UKAS assessment manager, have implemented the requirements of ISO 15189:2022, and provide their completed gap analysis to UKAS at least one month before their assessment (more about the gap analysis later).






The transition process requires each accredited laboratory to review the ISO 15189:2022 standard (available from knowledge.bsigroup.com) and complete a gap analysis (template available online on the UKAS ISO 15189 transition arrangements hub). The completed gap analysis, and associated evidence, must be returned to UKAS at least one month before the surveillance/reassessment visit is due to take place. The gap analysis and evidence will be reviewed by the customer’s UKAS assessment manager, and any areas for follow-up will be identified. These will then be assessed




during the surveillance/reassessment visit. Following the surveillance/ reassessment visit, each accredited laboratory will be issued with two UKAS reports: one for the surveillance/ reassessment to ISO 15189:2012, and one for the transition assessment to ISO 15189:2022. The report of the transition assessment will be each customer’s original gap analysis form, annotated by the UKAS assessment team to include comments on compliance or noncompliance with ISO 15189:2022. It will also include an executive summary of the transition assessment. Each report will contain a

Alyson Bryant, Healthcare Accreditation Specialist at the United Kingdom Accreditation Service (UKAS), with an update for laboratories.
recommendation: one for renewal/ maintenance of accreditation to ISO 15189:2012, and one for transition to ISO 15189:2022. In this way, laboratories that demonstrate compliance to ISO 15189:2012 but have not been able to demonstrate compliance to the requirements of ISO 15189:2022 are able to maintain accreditation to ISO 15189:2012 while they work towards compliance with ISO 15189:2022.
When each customer clears any mandatory findings raised against ISO 15189:2012 and ISO 15189:2022, they will be granted accreditation to ISO 15189:2022 and their UKAS schedule
and accreditation certificate will be updated. The four-year accreditation cycle will be unaffected, i.e. if the transition assessment is performed alongside Surveillance Assessment 2 (SU2) the first assessment post-transition will be Surveillance Assessment 3 (SU3).
Key differences between ISO 15189:2012 and ISO 15189:2022 relate to the greater focus on clinical risk and the impact of services on patients. The first line of the introduction states that “the objective of this document is to promote the welfare of patients and satisfaction of laboratory users through confidence in the quality and competence of medical laboratories”. Readers of the ISO 15189:2022 standard need to be aware of this change in focus; even where the wording of individual clauses remains unchanged between the 2012 and 2022 versions, the emphasis on clinical risk and patient care may mean accredited laboratories need to implement new or revised processes in order to demonstrate compliance. Accredited organisations need to consider their end-to-end service and the impact this may have on patients. Has the laboratory engaged with its users to ensure the right tests are being requested in the first place?
Have the tests provided been validated/ verified for the clinical scenario in which they are being used? Consider the faecal immunochemical test (FIT), which may be used as part of the bowel cancer screening programme or for testing symptomatic patients, and have different clinical considerations for the different pathways. Has the laboratory ensured their test results are getting to the right people in the right timeframe? This may include results sent to GPs, hospital clinical teams or directly to patients. Delays may
impact patient treatment and delay patients being released from hospital.
POCT services must review the requirements of ISO 15189:2022. By bringing the requirements of POCT services into this standard, it is clear that POCT services must function and be managed like any lab-based testing in terms of quality assurance and service delivery.

UKAS, the IBMS, the Royal College of Pathologists and The Association for Clinical Biochemistry and Laboratory Medicine are working together to support accredited laboratories through this transition. Throughout the course of the transition there will be numerous articles published and webinars/conferences held to ensure all organisations can access relevant transition information.
The UKAS Training Academy is developing a suite of courses, including bite-sized e-learning modules, to support customers in understanding and implementing the requirements of ISO 15189:2022. More information can be found on the UKAS website. The relationship between UKAS customers and their assessment manager will be key throughout this transition process. If customers have any questions about the transition process or timelines, their UKAS assessment manager is the first point of contact. BMS
“Has the laboratory engaged with its users to ensure the right tests are being requested?”
Autoimmune diseases develop when the autoreactive B lymphocytes produce autoantibodies and autoreactive T lymphocytes cause damage to the cells, tissues or organs that display specific target autoantigens to cause inflammation, cell abnormalities or cell destruction. In autoimmune diseases autoreactive lymphocytes expand polyclonally because critical control measures fail, so many different types of autoreactive lymphocytes are produced against a greater range of target antigens increasing their potential to cause more severe pathological damage.
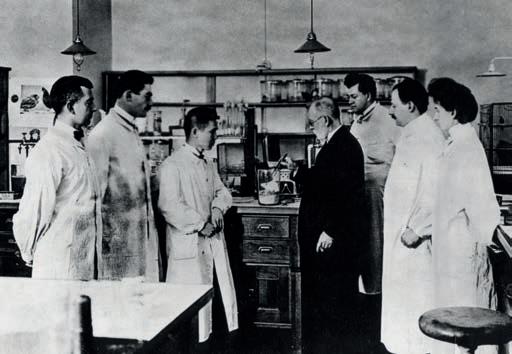
Medical historians identify the foundation of immunology with the Ukrainian zoologist and microbiologist Elie Metchnikoff and German scientist Paul Ehrlich, towards the end of the 19th Century. Metchnikoff studied starfish mesenchymal cells and observed phagocytosis by macrophages and microphages. This was seen as a critical first-line defence mechanism, now known as innate cellular immunity. Meanwhile in 1897 Paul Ehrlich proposed “the side-chain” theory in which antibodies are produced that bind to antigens, such as toxins via “side-chains”, which he later called receptors, to nullify the potential harm caused. This is now seen as the basis of humoral immunity. During this time period, German bacteriologist Emil von Behring performed research on the identification of antibodies and passive immunity with serum therapy, which proved effective in the treatment of diphtheria.
In time it was accepted that innate and acquired were complementary and mutually beneficial processes. It is, however, relevant that in 1901 Ehrlich coined the term “horror autotoxicus” that immunological self-harm and destruction could not occur. Just three years later an
autohaemolysin responsible for cold haemoglobinuria was described, but the concept of autoimmunity was not developed further until 1940s.
The concept and mechanism of immunotolerance was developed following the first description in 1945 by US biologist Ray Owen in dizygotic cattle twin studies sharing blood and tissue and that “self” was learned in development. In 1949, this principle was further developed by Australian virologist Frank Macfarlane Burnet and his colleague Frank Fenner who predicted acquired immunological tolerance by self-markers developed during embryogenesis. Brazilian-born British biologist Peter Medawar and his research
team at the University of Birmingham validated Burnet’s hypothesis in 1953 by extracting cells from mice embryos and inoculating other mice strains; when the mice became adults, skin grafts from the original mice strain showed no rejection.
In 1957 Burnet proposed his theory of clonal selection in that antibody production by each B lymphocyte is unique with receptors for a particular antigen. This creates a vast diversity of antibodies to have the potential to respond to the equally diverse antigens that may be encountered. When binding occurs, the lymphocyte is activated to produce a clone of antibodies with the same binding characteristics to amplify the response. This was a modification of Danish immunologist Niels Jerne’s proposals in 1955 that soluble antibodies
Following articles on Addison’s, Crohn’s and Graves, this review describes some significant historical developments in our understanding of autoimmunity.
exist and the immune system would select the specific B cell and antibody for the antigen. Burnet’s theory is considered central to immunology.
In 1959 Burnet suggested that removal of autoreactive lymphocytes, termed clonal deletion, was required for immune tolerance. This involves the reliable removal of B and T cells with expressed receptors for self before maturation, a defence against autoimmunity. Around 2–5% of T cells are considered to develop autoreactive receptors and undergo clonal deletion. Clonal deletion is a negative selection process, which may be imperfect and requires passive and active regulation. Passive regulation can be achieved if there is an absence of non-antigen signals or mode of antigen presentation. Active regulation can be achieved by specialised T cells (Tregs) naturally occurring or induced, first reported by Sakaguchi and colleagues in Osaka, Japan in 1995 as specialised T cells that control peripheral tolerance to suppress immune response by inhibition of T cell proliferation and cytokine production. Enhancement of Treg cells has been proposed as a possible therapeutic option in autoimmune diseases.
During the 1950s significant progress was achieved in understanding autoimmune diseases and brief notes are
presented for three outstanding pioneers during this period:
Noel Rose (1927–2020) was a US immunologist, pathologist and medical microbiologist who demonstrated that rabbits could develop an immune response against their own tissues, using thyroid gland extract in research studies. He performed research on Hashimoto’s disease and in 1956 showed the production of autoantibodies in this condition. In 1971 he demonstrated that susceptibility to an autoimmune disease of the thyroid in mice is determined by the murine major histocompatibility complex. He has been hailed as the “Father of Autoimmunity”.
Henry Kunkel (1916–1983) was a US immunologist who performed groundbreaking research on rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and primary biliary cholangitis. All these conditions are now recognised as autoimmune diseases. In patients with RA, Kunkel and his team demonstrated the presence of a 19S antibody (rheumatoid factor) in the sera of these patients, which reacted with other antibodies as an autoantibody; this was the first autoantibody to be described in 1957. Rheumatoid factor binds with the Fc component of IgG to form immune complexes, which may deposit in joints to cause inflammation, swelling and stiffness. Kunkel turned his attention to SLE, the most common form of lupus, and found that sera from patients contained several autoantibodies towards host mitochondria, histones and DNA to form circulating immune complexes to deposit and cause tissue injury, notably inflammation. Kunkel also found that deposits in the glomerular basement
membrane may cause acute or chronic renal impairment.
Kunkel had a long-term interest in liver disease and in 1949 reported changes in serum lipids and occurrence of xanthomata in primary biliary cirrhosis (now cholangitis).

Deborah Doniach (1912–2004) was a Swiss-born, British clinical immunologist who, with her research team at the Middlesex Hospital, pioneered research into autoimmune diseases and inspired the introduction of clinical immunology into clinical pathology laboratories. In 1958 Doniach and colleagues Ivan Roitt and Peter Campbell showed that Hashimoto’s thyroiditis was caused by an organ-specific autoimmunity with circulating autoantibodies to thyroglobulin and thyroid peroxidase. Doniach also studied the autoimmune nature of pernicious anaemia with autoantibodies to gastric parietal cells, and in type 1 diabetes mellitus autoantibodies formed to pancreatic islet cells. Doniach also identified an antigen reacting with complement fixing antibody in the sera of patients with primary biliary cholangitis and located the antigen to the mitochondrial fraction of tissue homogenates by immunofluorescence.
It is highly significant to the importance of immunology that pioneers described, such as Metchnikoff, Burnet and Medawar, were recognised for their achievements by Nobels in Physiology and Medicine. BMS
Stephen Clarke is a retired IBMS fellow. To read this article with full references, visit thebiomedicalscientist.net
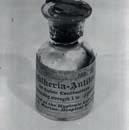
Left. German physician, with Sahachiro Hata and other assistants in the laboratory, Georg-Speyer-House, Frankfurt am Main, circa 1909. Left-below. Bottle of diphtheria antitoxin (1895). Right-below. Emil von Behring in his laboratory.“In the 1950s significant progress was made in understanding autoimmune diseases ”
The HM-JACKarc automated, quantitative FIT system, provides a rapid and consistent, high throughput solution, for both screening and symptomatic Faecal Occult Blood testing.

Designed to maximise sample throughput with minimal hands-on time, it ensures easy workflows so your laboratory
Timely Results
Load up to 80 samples
Time to first result: 5.6 minutes
Further results every 18 seconds, 200/hour
Accurate, Sensitive and Reliable



High hook capacity >200,000 μg Hb/g faeces
Wide linear dynamic assay range:







(7μg Hb/g faeces to 400μg Hb/g faeces)
Limit of Detection 0.6μg Hb/g Faeces
Easy to Use
Light, compact bench top analyser





Innovative software with touch screen



Easy to use interface with quick access menus

Minimal training required
Recommended by NICE DG30
www.nice.org.uk/guidance/dg30/chapter/
1-Recommendations
Complete FIT service support - Contact us today


T: 023 8048 3000














I am proud to welcome you to the launch of Congress
It’s good to be back on more stable ground after the pressures of COVID that
Debra Padgett, IBMS President

post-COVID Congress 2022 was almost



 Sarah May, Deputy Chief Executive
Sarah May, Deputy Chief Executive
Congress is reintroducing its molecular pathology programme on molecular pathology and genomics as a specialism in its own right. The programme has been designed by our new Molecular Pathology Advisory Panel and it represents all that is new and cutting edge in the subject.
To increase the opportunity for people to hear the most popular programmes we will be running the Quality Management programme on both Monday 25 September and again on Thursday 28 September, with a special programme on Tuesday 26 September run by UKAS, primarily dedicated to the new ISO 15189 standards.





Conclude your Monday afternoon at Congress by joining us at the ever-popular Brasshouse Welcome Evening.
13.00 Understanding and evidencing the new HCPC SoPs
Dr Sue Jones, IBMS Executive Head of Education
13.30 Specialist Portfolios achievable

Donna Torrance, IBMS Head of Learning and Development
14.00 The tyranny of learning outcomes
Revd Dr Gordon Sinclair, London Metropolitan University
14.30 A project with a purpose – the challenge of identifying and supporting research projects in the workplace
15.45 Practice educators – their role in helping to develop the laboratory workforce
Louise Jeffries, NHS England, SW Region
16.15 Use of apprenticeships at different levels as a route into the Biomedical Science profession
Gemma Durbridge, Health Education England
16.45 Using digital technology to revolutionise how we learn Dr Jim Taylor, Head of Digital Education, IBMS
Sponsored by:
Chaired by: Andrew Blann
13.00 Molecular Pathology: An expanding discipline
Dr Andrew Blann, Chair of the IBMS Molecular Pathology Advisory Panel
multi-omic approaches (SARSCoV-2)

Dr Matthew Pugh, University of Birmingham
14.00 The role of the liquid biopsy in cancer diagnosis and monitoring
Claire Swift, The Royal Marsden NHS Foundation Trust
14.30 Bioinformatics

Chaired by: Dr Gary Reynolds
15.45 EQA – keeping pace with Molecular Pathology
Dr Jennie Fairely, GenQA
16.15 Optical genome mapping: A game changer in the detection of genomic aberrations

Tasmiya Wahed, GenQA
16.45 Solid tumours: Biomarkers and treatments
Amy Newman, Synnovis
13.00 Quality in POCT merging to ISO 15189 John Ringrow, Senior Assessment Manager, UKAS
13.30 Change Management
14.00 Quality in IT procurement
Hannah Cox, Northumbria Healthcare NHS Foundation Trust
14.30 Regulation of in vitro diagnostic medical devices in the UK

Stephen Lee, Association of British HealthTech Industries
15.45 Development & Introduction of ISO 15189:2022
Dr David Ricketts, Health Services Laboratories LLP
16.05 Institute view of ISO 15189:2022
Debra Padgett, President, IBMS
16.25 Introduction to the UKAS ISO 15189:2022 transition
Al Bryant, Healthcare Accreditation Specialist, UKAS
16.45 ISO 15189:2022 Q&A
Chaired by: Dr Guy Orchard
13.00–17.15 Tissue recognition workshop
Sponsored by:
Tuesday 26 September – Afternoon
14.00 Albert Norman Keynote Opening Address
Debra Padgett, President, IBMS

14.20 Tackling health inequality – a lesson learned from COVID-19 pandemic
14.50 The challenges of health reporting
15.20 Delivering our strategy

David Wells, Chief Executive, IBMS

16.00 Precision medicine and its impact on health and health delivery
16.20 A national strategy for POCT and the workforce needs for its delivery



16.40 Meeting the global challenge of dementia
Thursday 28 September
Disasters and mass fatalities feature all too frequently in the news, as accidents, the consequences of terrorist activity or the casualties of war.
aftermath of the event or it may be weeks, months or years later when their names and deaths are discovered.
who help to uncover what really happened and help to restore dignity to those whose lives were brutally ended.
the subject of disaster victim identity and is an important reminder of a duty of care that extends way beyond what most people could ever imagine.
the tough and complex health
Congress Keynote Plenary Sponsored by:Digital Pathology and
9.00 Spatial Transcriptomics: A novel tool to elucidate cell populations in nonmelanoma skin cancer
Jeyrroy Gabriel, Synnovis Analytics
9.30 Introduction of Digital Image Analysis into EQA Assessments

Andrew Dodson, UK NEQAS ICC & ISH
10.30 The Introduction of Digital Pathology EQA
Will Davies, UK NEQAS Cellular Pathology
Technique
11.00 The Reality of Digital Pathology Implementation

Louise Dolan, Pathlinks, Northern Lincolnshire & Goole NHS Trust
11.30 Quality Assurance
Pathology
Prof David Brettle, Leeds Teaching Hospitals NHS Trust
Sponsored by:
9.00 Transplant Assessment and Relative Opportunity Tool (TAROT) for Renal Transplantation: Improving the chance of transplant for immunologically complex patients.
Pamela Hughes, The Leeds Teaching Hospitals NHS Trust
9.30 Narcolepsy and its association with HLA type Dr Lizzie Hill, University of Oxford


10.30 Controlled Human Infection Models: Anti-viral immunity in the respiratory tract
Dr Stephanie Ascough, Imperial College London
11.00 Complement testing
Dr Ashleigh Ross, Newcastle upon Tyne Hospitals NHS Foundation Trust
11.30 Complement genetic testing
Dr Adrian Heaps, North Bristol NHS Trust
Sponsored by:
Chaired by: Victoria Moyse
9.00 Making Science Sustainable – Laboratory
Framework (LEAF) and Clinical Laboratories
Mr Martin Farley, University College London
9.30 Transgender Reference
Ranges
Tamsin Glenwright, VH BIO Ltd
10.30 Metabolic complications posttraumatic brain injury (TBI)
Dr Tony Goldstone, Imperial College
Healthcare NHS Trust
11.00 Clinical and economic evaluation of the clinical utility of UCH-L1 and GFAP in mild TBI


Dr Hayley Sharrod-Cole, Royal Wolverhampton NHS Trust
11.30 The headache of CSF bilirubin analysis
Lisa Garrison, Royal Surrey NHS Foundation Trust
12.45 Your career in Clinical Biochemistry: IBMS
the Advisory Panel
Tony Dedman, Lee Peters, Sheri Scott, Chris Ward, Victoria Moyse, IBMS Advisory Panel

Sponsored by:
9.00 Is phenotypic visualisation of resistance needed to guide antimicrobial stewardship Francis Yongblah, Great Ormond Street Hospital for Children NHS Foundation Trust
9.30 AMR/CSO Diagnostic pathway change –Biomedical Scientist Perspective
10.30 Rapid AST using Impedance Cytometry
11.00 Rapid ID from blood cultures
11.30 Sepsis and systemic or disseminated infections
Dr Rinna Richardson, Manchester University NHS Foundation Trust
Sponsored by:
9.00 Digital/AI in Cellular Pathology including Cytology
9.30 Digital interpretive EQA in cytology or use of digital in clinic

10.30 Clinical Andrology: A Urology Surgeon’s Perspective
Miss Odunayo Kalejaiye, North Bristol NHS Trust
11.00 Genetics and the Fertility Clinic
Graham Fews, West Midlands Regional Genetics Laboratory
11.30 Teratozoospermia Index (TZI): The debate Dr Bryan Woodward, X&Y Fertility, Dr Gulam Bahdur, North Middlesex University Hospital NHS Trust

9.00 Major haemorrhage guidelines
9.30 What’s in the new precompatibility guidelines?
Richard Haggas
10.30 Highlights from the 2022 Annual SHOT Report: lessons learnt and learning from excellence

Nicola Swarbrick and Victoria Tuckley, Serious Hazards Of Transfusion (SHOT)

11.00
Incident Management –MHRA expectations
Chris Robbie, MHRA
11.30 UKTLC Standards and survey
Kerry Dowling, University Hospital Southampton NHS Foundation Trust
Sponsored by:
9.00 Preparing for Professional Practice –ensuring your graduate has the knowledge and skills they need
9.30 Improving employability through simulation learning Tahmina Hussain, University of Salford
10.30 What are the barriers to an inclusive curriculum and learning experience?
Glenn Hussey, Keele University
11.00 Motivating, training and developing on multiple sites – the SHYPS workforce journey
11.30 Ensuring fairness in training opportunities
Sponsored by:
9.00 Introduction to the UKAS ISO 15189:2022 transition
UKAS
9.30 Using accreditation to support the validity of test results: pre-examination requirements of ISO
15189:2022
Alison Benson, Assessment Manager, UKAS
10.30 Using accreditation to support the validity of test results: validation and
ISO 15189:2022

Mark Prescott, Assessment Manager, UKAS
11.00 Pathology Network
Accreditation
Al Bryant, Healthcare Accreditation Specialist, UKAS
11.30 Point of care accreditation under ISO
15189:2022
John Ringrow, Senior Assessment Manager, UKAS
Parasitology
9.00 LAMP and the Future of Malaria Detection
Morphology
9.30 Morphology Training
Nicki Lawrence, University Hospitals of North Midlands NHS Trust
10.30 Morphology Quiz
Dr Michelle Brereton & Dr John Burthem, Manchester University NHS Foundation Trust
11.30 The Blood Film to the Rescue
Prof Barbara Bain, Imperial College Healthcare NHS Trust
Sponsored by:
9.00 Post-pandemic respiratory viral infections in hospitals
9.30 Post-pandemic respiratory viral infections in the community
10.30 Emerging arthropodborne viral threats to the UK
Dr Nicholas Johnson, Animal and Plant Health Agency
11.00 Tick-borne encephalitis in the UK
11.30 Changing epidemiology of CCHF
Sponsored by:
Support staff delegates can attend rate or can book a full Congress day





Tuesday 26 September – Afternoon

Dr Jim Taylor, Head of Digital Education, IBMS



13.30 The purpose and value of level 2 and 4 apprenticeships
14.00 Expanding your role into POCT – a career opportunity to consider

14.30 Workshop: First impressions last the longest – how to be the best public face of your laboratory
16.00 Key skills for a supervisory role

16.30 A role with a difference – anatomical pathology technologist in a mortuary
Helpline: 01892 779990
In memory of Council Member Mary Macdonald, the Institute is again awarding 20 free places for non-HCPC-registered IBMS members to attend the Biomedical Support Staff programme at Congress. Successful applicants will also receive £60.00 towards their travelling expenses. Applicants must be members of the IBMS and working in a support staff role. For further information and to apply, please visit www.ibms.org/congress. The deadline for applications is 31 March 2023. Visit www.ibms.org/congress
Wednesday 27 September – Morning
Histological Staining and Quality Assurance
Chaired by: Cassandra Cooklynn
9.00 Designing and Automating a Modern Mohs Laboratory
Mohammad Shams, Synnovis Analytics, London
9.30 Complex and Unusual Case Studies in Mohs
Dr Guy Orchard, Synnovis Analytics, London
10.30 Application and Imaging of Direct
Dr John Mee, Synnovis Analytics, London
11.00 Transmission Electron Microscopy (TEM)
11.30 Amyloid: Rigour is Essential for Diagnosis
Janet Gilbertson, National Amyloidosis Centre, Royal Free NHS Foundation Trust
Sponsored by:
9.00 Reducing the carbon footprint of pathology samples
Andrew Turner, GP Liaison Service
9.30 Gender: lets talk about sex... and the implications for pathology
Alexandra Liversidge, Leeds Beckett University
10.30 HR and OH departments – how to work with them to achieve the best results
Wendy Leversuch, Health Services Laboratories
11.00 Managing change with compassion
Steve Singer, The Royal Marsden NHS Foundation Trust
working and workingfrom-home requests
Sponsored by:
Chaired by: Martin McFadden
from Trainee Biomedical Scientist to Consultant Clinical Scientist
Dr Andrew Teggert, South Tees NHS Foundation Trust
9.30 Moving from a Biomedical Scientist to research and PhD and Clinical Scientist
Dr Nigel Brown, Northumbria Healthcare NHS Foundation Trust
10.30 A basic overview of Gonadotropins –perspective from a fertility clinician
Dr Aruna Manivasagam, Newcastle upon Tyne Hospitals NHS Foundation Trust
11.00 Ovarian hyperstimulation syndrome (OHSS) – how does the clinical biochemistry laboratory inform clinical decisions?
Dr Rekha Pillai, Newcastle upon Tyne Hospitals NHS Foundation Trust
11.30 The role of a Regional Newborn Screening Laboratory
Dr Ben Sholademi, Foundation Trust
12.45 Your career in Clinical Biochemistry: IBMS
the Advisory Panel
Tony Dedman, Lee Peters, Sheri Scott, Chris Ward, Victoria Moyse, IBMS Advisory Panel
Sponsored by:
9.00 Head & Neck Clinic –Sonographer’s perspective
Sarah Martyn, Royal Cornwall Hospitals NHS Trust
9.30 Pathology of Head & Neck – Histology & Cytology
Dr Kris Leung, Royal Devon University Healthcare NHS Foundation Trust
10.30 Specialist portfolios: achievable
Donna Torrence, Head of Learning and Development, IBMS
11.00 New ROSE specialist module
Leonie Wheeldon, Royal Cornwall Hospitals NHS Trust
11.30 Changing & expanding roles in Cellular Pathology
Paul Hawkins, Royal Devon University Healthcare NHS Foundation Trust
9.00 Clinical Liaison Role in Wales Public Health Wales

9.30 The advantages and disadvantages of automation in the laboratory.
Dr Daniel Bailey, UK Health Security Agency
10.30 The ringworm turns: The emergence of in the UK
Dr Richard Barton, The Leeds Teaching Hospitals NHS Trust
11.00 FMT/NICE guidance
11.30 Recent increases in diphtheria cases in England
Dr David Litt, UK Health Security Agency
Sponsored by:
9.00 vWF Guideline Update
Dr Annette Bowyer, Hospitals Foundation Trust
9.30 Thrombophilia
Guideline Update
Professor Mike Laffan, Imperial College London
10.30 The effects of long COVID on coagulopathy
11.00 DOACs
11.30 Best Practice
Haemostasis Laboratory
ISO 15189
12.45 Meet your Haematology Portfolio examiners
Ian Jennings & Nicki
Lawrence, IBMS Specialist Advisory Panel
Sponsored by:
9.00 Myeloma screening
– Best practice and new developments
Dr Ross Sadler, Oxford University Hospitals NHS Foundation Trust
9.30 EQA: What’s happening?
Dina Patel, UK NEUK NEQAS Immunology,
10.30 Autoimmune serology in systemic sclerosis
Dr Liz Furrie, NHS Tayside, Dundee
11.00 Myositis
Dr Sarah Tansley, Royal National Hospital for Rheumatic Diseases, Bath
11.30 Idiopathic Membranous Nephropathy: PLA2 and beyond Chris Scott, Barts Health NHS Trust (ESEL Partnership)
Sponsored by:
9.00 Nuisance Antibodies
9.30 Kell Blood Group System
Dr Tom Bullock, NHS Blood and Transplant – Red Cell Immunohaematology
10.30 Help, nothing compatible
11.00 What the F? –Explaining anti-f, with case studies (HSD Presentation)
Richard Ulyatt, Manx Care
11.30 International
Blood Group Reference Laboratory (IBGRL) –Finding New Blood Groups
Shane Grimsley, NHS Blood and Transplant
12.45 Antibody Workshop
Maley and Catherine Lorenzen, IBMS Transfusion Science Specialist Advisors
Sponsored by:
9.00 Performance of a new molecular point-of-care system for respiratory conditions
Dr Jürgen Becker, QuidelOrtho
9.30 Role of AI in viral diagnostics
10.30 Early career - career progression HSST
10.50 Early career - Point of care
11.10 Early careerOutreach activities
11.30 Near-patient testing - (self-collected samples; breath test to bloods)
Sponsored by:
Wednesday 27 September – Afternoon
Molecular Techniques and Advanced Diagnostics
Chaired by: Suzanne Parry
14.00 Pre-Analytical – Tissue Requirements/Fixation
– To Enable Molecular Pathology
University Hospitals
Birmingham NHS Foundation Trust
14.30 Analytical – focus on Next-Generation Sequencing
Dr Phillipe Taniere, University Hospitals
Birmingham NHS Foundation Trust
15.00 How do molecular diagnostic techniques make a difference to the patient pathway?
Prof Dean Fennell, University Hospitals of Leicester
16.00 Mismatch Repair (MMR): Ten years of EQA experience
Dr Ian Frayling, University of Wales, Cardiff
16.30 UK NEQAS ICC: PDL1 and EQA
Dawn Wilkinson, UK
NEQAS, ICC & ISH
Sponsored by:
14.00 The metabolic role of Vitamin B12
Dr Peter Timms, The Scientists Laboratory
14.30 Lipid disorders
Hospitals NHS Foundation Trust
15.00 Molar pregnancy and gestational trophoblastic disease – why the hCG assay matters
Teaching Hospitals NHS Foundation Trust
16.00 Implementing the GIRFT national recommendations to reduce unwarranted variation in pathology

Dr Martin Myers MBE, Lancashire Teaching Hospitals NHS Foundation Trust
16.30 Reporting direct to the patient
Division of Clinical Laboratory Sciences
Sponsored by:
Intelligence
Dr Graeme Wild, Hospitals NHS Foundation Trust
14.30 Peanut Immunotherapy
Dr Eleanor Minsall, Foundation Trust
15.00 Can a laboratory investigate allergic reactions to COVID vaccines?
Teaching Hospitals NHS Foundation Trust
16.00 Interpretation of ISAC
Dr Graeme Wild, Hospitals NHS Foundation Trust
16.30 Vaccines and PID testing
Sponsored by:
14.00 Use of cell blocks in cytology – Technical Aspects
Anna Patterson, UK NEQAS for Cellular Pathology Technique (CPT)
14.30 The cellular pathology of clots and cell blocks
Dr Anthony Maddox, West Hertfordshire Teaching Hospitals NHS Trust
15.00 EQA pilot update for cytology cell blocks
Chantell Hodgson, UK NEQAS for Cellular Pathology Technique (CPT)
16.00 Ask the Expert – GYN & NG (issues with EQA, IQC, Quality, UKAS etc.) Tracey Stevenson, Leonie Wheeldon, Gary Player IBMS Specialist Advisors
14.00 Continuity planning –building resilience into your workforce and service
Chris Elliott, South Tees Hospitals NHS Foundation Trust
14.30 Generation X,Y,Z: Ageism in the workplace and its impact on service delivery
Sarah May, Deputy Chief Executive, IBMS
15.00 Workforce and sustainability – getting the equation to balance Denise Cook, Synnovis LLP
16.00 CE and UKCA mean and the implications for pathology
Ashleigh Batchen, BIVDA
16.30 Restore and recover: Be like bamboo and bend with the wind Sue Alexander, The Royal Marsden NHS Foundation Trust
Sponsored by:
14.00 Managing blood stocks during an amber alert
Matthew Bend, Blood Stocks Management Scheme, NHS Blood and Transplant
14.30 CRYOSTAT 2 trial
Prof Simon Stanworth, NHS Blood and Transplant
15.00 Production of platelets and red cell in vitro for human transfusion
Prof Cedric Ghevaert, University of Cambridge and NHS Blood and Transplant
16.00 Coagulation for Blood Bankers: Help! I’ve lost the clot…. Elisabeth Davies, Welsh Blood Service
16.30 Managing obstetric haemorrhage
Prof Peter Collins, University Hospital of Wales, Cardiff
Sponsored by:
14.00 It’s life, but not as we know it. Emerging parasitic diseases
Prof Peter Chiodini, University College London Hospitals NHS Foundation Trust
14.30 Neonatal Meningitis
Paola Espin, Nottingham University Hospitals NHS Trust
14.50 Rat Bite Fever
Meerani Munasinghe, East Suffolk and North Essex Foundation Trust
of Acanthamoeba species directly from Ocular Tissue Lisa Connelly, Scottish Microbiology, Scottish Microbiology Reference Laboratory
16.00 Anaerobe update
16.30 Being Patient: Patient experiences of infection and why we should be listening
Dr Jane Freeman, Leeds Teaching Hospitals NHS Trust
Sponsored by:
Polycythaemia: A Clinical Case Study
Elizabeth Handley, Nottingham University Hospitals NHS Trust
14.20 Leukaemia associated with Down’s syndrome: A case study
Dan Pennick, Cambridge University Hospitals NHS Foundation Trust
14.40 Introduction of New D-dimer Assay in Response to the COVID-19 Pandemic (HSD Case Study)
Eva Nkansah, Princess Alexandra Hospital, Harlow
15.00 Haemoglobinopathy case study – new agent Quality
Unsatisfactory Performance
Dr Barbara De La Salle, West Herts Teaching Hospitals NHS Trust
16.30 Overview and Escalation of EQA Revd Dr Gordon Sinclair, London Metropolitan University
Sponsored by:
14.00 Polio – why has it reappeared?
14.30 COVID – current issues
15.00 Monkeypox (mpox) case management/infection control
16.00 What is new in hepatitis?
16.30 HIV – is a cure on the horizon?
Sponsored by:
Specialist Practices, Diagnostics and Education/Learning
Chaired by: David Muskett
9.00 Troubleshooting specialist demonstration techniques
UK NEQAS Cellular Pathology Technique
9.30 Supporting Daily Quality and Compliance
Julie Coaker, UK NEQAS Cellular Pathology Technique
10.30 Success Stories in Dermatopathology
11.00 New ASD in Histopathology Reporting
Chris Ward, Head of Examinations, IBMS
11.30 Training of individuals undertaking dissection
Cassandra Cooklynn, St Helens and Knowsley Teaching Hospitals NHS Trust
12.45 Meet the Examiners Session
Sponsored by:
9.00 CF Microbiome
Prof Chris van der Gast, Manchester Metropolitan University
9.30 Shiga toxin-producing O26 (STEC O26)
Dr Claire Jenkins, UK Health Security Agency
10.30 Rapid Diagnostics –
11.00 New drugs for bad bugs: What’s in the pipeline?
Dr Katie Hopkins, UK Health Security Agency
11.30 EUCAST – striving towards a complete system
Prof Gunnar Kalhmeter, EUCAST
12.45 Meet the Microbiology Experts IBMS Advisory Panel
Sponsored by:
Chaired by: Karen Brazier
9.00 Fetal alcohol spectrum disorder
Dr Helen Howlett, Senior Commissioner for Children, Young People and Maternity, North Cumbria
9.30 Androgens and Sports: Conventional urine and modern dried blood sample testing methodologies
Dr Shobha Ahi, Department of Analytical, Environmental and College London
10.30 Heroin markers
Dr Nigel Brown, Northumbria Healthcare
NHS FT
11.00 Dimorphine-assisted treatment programme
Dr Daniel Ahmad, Clinical Partner, Foundations, Middlesbrough
11.30 The public health side of drug misuse

Rachael Hope, Public Health, Newcastle-UponTyne City Council
Sponsored by:
Chaired by: David Ryder
9.00 Setting up a POCT service to support the virtual frailty ward: The Leeds experience
Rebecca Kift, The Leeds Teaching Hospitals NHS Trust
9.30 Delivery of a virtual ward in East Kent: A clinical overview
Anthony Perryman, East Kent Community Fraility Team
10.30 Embedding sustainability in the clinical laboratory - partnerships, POCT and GIRFT
Sheri Scott, Nottingham Trent University
11.00 Getting it Right First Time
Dr Martin Myers MBE, Lancashire Teaching Hospitals NHS Foundation Trust
11.30 The revised ISO 15189 standard and the impact upon delivery and maintenance of effective quality management within point-of-care testing
John Ringrow, UKAS
Sponsored by:
9.00 NHS Digital Work on Gynae Cytology Laboratory Experience
9.30 Digital cervical cytology: The Monklands experience
Professor Allan Wilson, University Hospital Monklands, NHS Lanarkshire
10.30 Perspectives on the Borderline Endocervical Cell
Angela Brown, Royal Wolverhampton NHS Trust
11.00 Benign glandular histology as potential for BLEC
Gary Player, Newcastle upon Tyne Hospitals NHS Foundation Trust
11.30 Update on endocervical adencarcinomas
9.00 Patient Safety Incident Response Framework NHCT Perspective
Rachel Carter, Northumbria Healthcare NHS Foundation Trust
9.30 Rudeness costs lives Shauna McAuley, Belfast Health and Social Care Trust
10.30 Development & Introduction of ISO 15189:2022
Dr David Ricketts, Health Services Laboratories LLP
10.50 Institute view of ISO 15189:2022
Debra Padgett, President, IBMS
11.10 Introduction to the UKAS ISO 15189:2022 transition
Al Bryant, Healthcare Accreditation Specialist, UKAS
11.30 ISO 15189:2022 Q&A
12.45 Validation and Dr Mairiead MacLennan, NHS Fife/Gary Collins, NHS Ayrshire & Arran
Guidance
9.00 How to report staining
EQA exercises
Liam Whitby, UK
Teaching Hospitals NHS Foundation Trust
9.30 CAR T Cell Therapy
Dr Paul Ferguson, University Hospitals
Birmingham NHS Foundation Trust
10.30 Immunophenotyping
Case Studies
Dr Fiona Clark, University Hospitals Birmingham
NHS Foundation Trust
11.00 Gene therapy
11.30 Evaluation of Measurement Uncertainty –Where we were and where we are going
Dr Stephen MacDonald, Cambridge University
Hospitals NHS Foundation Trust
Sponsored by:
9.00 Biomedical Scientist Empowerment, Education and Discussion Group: Improving access to CPD for hospital transfusion staff
Danny Gaskin, NHS Blood and Transplant
9.30 Blood Stocks Management Scheme –My role as a Biomedical Scientist
Stocks Management Scheme, NHS Blood and Transplant
10.30 Crossroads to a Compatible Career: From Pipette to Patient Blood Management
Anwen Davies, NHS Blood and Transplant
11.00 RCI – the Biomedical Scientist Role
Zoe Stone & Rebecca Hills, NHS Blood and Transplant, Filton Centre
11.30 Transfusion outside the NHS – jobs for Biomedical Scientists
Nicola Polley, Haemonetics Ltd
12.45 Meet the Examiner Session
Members of the Panel
Sponsored by:
9.00 A practical guide to serum free light chains –technical challenges and clinical utility
Joanne Morris, South West London Pathology
9:30 EQA of Autoimmunity
Carol Stanley & Dina Patel, UK NEQAS
10.30 Immunology
online Specialist Diploma programme
Ellen Roberts, North Bristol NHS Trust & Kanki Kavi, North West London Pathology
11.00 HSD in Immunology – A case study (Multiple Myeloma)
Emily Gallimore, Manchester University NHS Foundation Trust
11.30 A dual positive PLA2r and GBM
Sam Blacknell, Nottingham University Hospital Trust
Sponsored by:
9.00 What’s new in antivirals? (Antivirals & Mabs as therapeutics)
9.30 Advances in vaccinology
10.30 Pets to pandemics – viral zoonoses. Food, environmental and veterinary. (Hep E and Hep A)
11.00 Nature’s mixing pot for new/emerging viruses (bat-borne viruses)
11.30 In-house tests to CE marked – impact on the laboratory (Debate –impact on laboratory vs commercial company) Dr Andy Rutter, QuidelOrtho
Sponsored by:
Thursday 28 September – Afternoon
14.00 Mastectomy specimen for multifocal invasive ductal carcinoma (11115/20)
Ashley Gilchrist, The Royal Marsden NHS Foundation Trust
14.20 Stented adenocarcinoma in a young female
Rebecca Renouf, Portsmouth Hospitals
NHS Trust
14.40 Gestational Trophoblastic Disease: What’s all the fuss about?
Sandie Iles, NWLP, hosted by Imperial College NHS Foundation Trust
15.00 The Lundy Murders – the role and reliability of immunohistochemistry in forensic neuropathology practice
Dr Daniel du Plessis, Salford Royal Hospital NHS Foundation Trust
Sponsored by:
14.00 Emerging Issues in Medical Mycology
Professor Andy Borman, UKHSA, Mycology Reference Laboratory
14.30 Biomarkers, disease severity and triaging: The role of mid-regional proadrenomodulin



Dr Kordo Saeed, University Hospital
Southampton NHS Foundation Trust
15.00 A year in the life of an HSST: Cases of interest
Phillipa Burns, SHYPS
14.00 From the laboratory to headlines. How to validate laboratory methods to screen and measure novel drugs
Dr Alexander Lawson, Heartlands Hospital, University Hospitals
Birmingham NHS Foundation Trust
14.30 EQA development
Gareth Davies, WEQAS
15.00 Decision support in clinical chemistry – progress and pitfalls ?

Dr Ian Godber, QEUH, NHS Greater Glasgow & Clyde
Sponsored by:
Chaired by: Sarah Glover/ Nicky Hollowood
14.00 High Sensitivity
Troponin in the Community
Roanna George, University Hospitals
Plymouth NHS Trust
14.30 POCT in the Community
15.00 National Point-of-Care Strategy
David Wells, Chief Executive, IBMS
Sponsored by:
14.00 Self-sampling for cervical cytology
14.30 Role of MDT – Clinical Perspective; Colposcopy for patients with learning
Jilly Goodfellow & Jill Fozzard, Newcastle upon Tyne Hospitals NHS Foundation Trust
14.00 Competences for Managers
Nigel Coles, Birmingham
NHS Foundation Trust
14.30 Managing the HTA inspection and selected case studies
Dr Brian Herron, Belfast Health & Social Care Trust
15.00 Quality in IT Procurement
Jonathan Boxshall, Northumbria Healthcare NHS Foundation Trust
Quality
14.00 Discussion from UK
NEQAS Parasitology
14.30 The Advanced Digital Morphology Programme
Nicki Lawrence, University Hospitals of North Midlands NHS Trust
15.00 Digital Morphology for CPD. What can we learn about morphology reporting from the past 15 years?
Dr Michelle Brereton, Manchester University NHS Foundation Trust
Sponsored by:
14.00 Implementation of Blood on Board: A laboratory perspective
Chloe Wilkes, Nottingham University Hospitals NHS Trust
14.30 Blood on Board
Laurie Philipson, Essex and Herts Air Ambulance
15.00 Resuscitation with Pre-Hospital Blood Products (RePHILL) trial
Dr Nick Crombie, University Hospitals
Birmingham NHS Foundation Trust
Sponsored by:
14.00 Myositis
14.30 Scleroderma
15.00 aHUS
Sponsored by:
14.00 Pandemics – Past (polio; smallpox; Ebola)
14.30 Pandemics – Present
CoV-2)
15.00 Pandemics – Future – preparedness planning & what might come next (measles resurgence; polio; mpox)
Sponsored by:
This programme was accurate as at the time of printing. Please visit www.ibms.org/congress for the latest developments at Congress and to view the most up-to-date lecture programme and list of exhibitors.
BIOHIT HealthCare
HCA Healthcare
Sarstedt

Serosep
SpeeDx
Starlab
Intersystems

BIVDA
Blood Academy
Copan
Mark Reed, Chairman, IBMS Company Members GroupHelpline: 01892 779990
Your delegate fee includes:


lunch (not 25 September), morning and afternoon refreshments and a copy of the Biomedical Science Congress Handbook.
•Premium Early Booking Discount (book by 30 April 2023)
•Discounted rates for retired members
•All rates include VAT
How to calculate your delegate fee:
1.Choose the day(s) you wish to attend the Biomedical Science on 26, 27 and 28 September plus the option to add the afternoon programme of Monday 25 September).
2.You can add the Monday programme to your 1, 2 or 3 day registration. Simply add the relevant rate to your booking to arrive at your total delegate fee (including 25 September) – see the Congress Delegate Fees table.
3.It is also possible to attend the programme for Monday 25 September 2023 only – see the Congress Delegate Fees table.
Principal Sponsors
BOOK BY 30 APRIL
IBMS Members
1 day £145£180£220
2 days £280£340£420
3 days £385£470£575
Mon PM + 1 day£220£270£330
Mon PM + 2 days£355£430£530
Mon PM + 3 days£460£560£685
Mon PM only £90£105£130
Lab Support Staff ½ day £75£90£110
Non-Members
1 day £185£225£260
2 days £370£440£515
3 days £485£585£685
Mon PM + 1 day£295£355£410
Mon PM + 2 days£480£570£665
Mon PM + 3 days£595£715£835
Mon PM only £125£150£180
Lab Support Staff ½ day £90£110£130
01892 779990
The IBMS Congress Helpline is here to assist with any delegate issue:


Delegate Rates & Discounts
Booking Deadlines
Multiple/Group Bookings
Booking Conditions
Exhibition-Only Registration

Or any other question regarding registration.

A grant of up to £1000 each to help more IBMS members attend Congress

The ICC is within easy reach of Birmingham International Airport and in walking distance of Birmingham New Street station.
If travelling by road please use the postcode B1 2EA for your satnav. There is a car park at the venue.
Act early to take advantage of discounted delegate rates:
Premium Early Deadline: 30 April 2023
Early Booking Deadline:
Helpline: 01892 779990
www.ibms.org/congress
Continuing Professional Development
The full programme of Congress lectures has IBMS recognition for CPD and can be added to your CPD e-Portfolio online.



exists to help more members have the opportunity to attend Congress, the event she was so passionate about. For Congress 2023, the Bursary will provide 20 successful applicants a grant of up to £1000 (including VAT) to help them attend IBMS Congress.
Bursary, applicants must:
•Be a current IBMS member in the grades of Licentiate, Member or Fellow.
at the time of application (see T&C point 9).
•Use the Bursary towards Congress 2023 delegate fees, accommodation and travel.
•Submit a 500-word supporting statement outlining their motivations for attending Congress their team/workplace.


An application form and information on how to apply are available on the IBMS website.
Visit www.ibms.org/congress
Leadership and management theories attempt to explain some of the most complex workplace dynamics. When we read “management”, we imagine an executive silhouette creating rules for the workforce with the aim of increasing productivity and corporate value. Management, therefore, is a unilateral process that directs achievement of objectives through the efficient use of resources. However, when we imagine a leader, our perspectives begin to diverge and become much more creative. A leader is someone, an individual or a group, held in high regard and for reasons that are mutually beneficial. A quick Google, and activists such as Mahatma Gandhi and Martin Luther King Jr
can be found. These are rare examples of leadership in action. Look closer to home: parents, siblings, carers, and volunteers all display various aspects of leadership.

It is a multi-dimensional phenomenon, focusing on motivation and inspiration, as opposed to organisation and delegation. Leaders can be thought of as the catalyst of organisational and social change. If we combine both concepts, we conclude that an organisation may only achieve true operational effectiveness when leaders and managers work together to achieve the same outcome.

An additional concept is that of
organisational culture. A phenomenon steeped in psychological and social theory that can be simply defined as “the way we do things here”. It is the day-to-day working environment. We spend so much of our time in work that we settle into our own comfort zones. When an organisation undergoes a period of change, this cultural paradigm begins to shift. The change could be the successful recruitment of a new biomedical support worker, the promotion of a laboratory scientist or introducing a new process. It is fair to say that the cultural movement may be positive, negative or both, depending on the observer. The magnitude of cultural
disruption also depends on the nature of the internal or external drivers influencing the need for change. If we look at the Lord Carter of Coles reports over the past 10 years, this aspect becomes quite clear – the shift to amalgamate regional laboratories into “hub-andspoke” models is beyond the scope of this writing, but will instil a sense of insecurity about the future of laboratory services. Another factor to consider is the amount of resistance to change. This could range from informal workplace protests to union-based industrial action. The image projected here is often found through management instigation of an unfavourable change with the opposing leaders conferring resistance, but this isn’t always the case. Those who support, promote, or instigate organisational change are referred to as “agents of change”. Often, these are not managerial executive-types, but any individual that can demonstrate a very specific set of characteristics. They must be able to integrate, innovate, grow and continuously develop. They also need to be able to quickly generate relationships and impart credibility. I believe that these attributes impart fluidity to the agents, enabling them to transcend existing cultural challenges while generating good working relationships within the organisational hierarchy. These are prime examples of effective leadership and ones that we should look to empower within our workforce.
When it comes to optimising organisational performance, there is vast empirical evidence to support strong collaborative cultures being the key differentiator between success and failure. When the right style of leadership is present, these cultures convey greater stakeholder value. This means when people work together, we get a much better result. If we consider the characteristics of a change agent as desirable for an
organisation to successfully adapt to change, then we need to identify and empower these individuals. This can be accomplished by developing a “learning organisation”, a concept coined by Peter Senge in 1990. Here, employees are encouraged to expand their capacity to innovate by being free to discuss concerns in a safe and open environment. This translates to staff providing solutions to problems and feeling able to action them. They should be empowered by management to instigate changes. Compare this to the above description of change agents, who require the freedom, autonomy, and accountability to generate the momentum required to facilitate change. We then arrive at an organisation
that is capable of improvement strategies that are self-perpetuating. What we need to develop are cultures based on education and development, with supportive and collaborative leadership, and an environment free of reprisal. To achieve this, we must accept that learning and teaching are an integral component of normal working practices and should not be treated as an entirely separate entity. For management, this will mean putting aside time and resources for team building and work-based learning. By doing this we remove barriers and open avenues for natural development to take place. This should be extended across educational and professional boundaries to facilitate shared learning and better provide the means for cross-discipline support.
We are all, at one time, followers and it takes a good follower to be a great leader. My aim is to highlight the importance of creating a sustainable workplace culture, with education and development at its core. Knowledge should be appreciated as a key strategic resource and management need to recognise the role and value of our learners. When this happens we can use it to better inform laboratory processes and provide a much better service to our users. A leader who acknowledges the work of employees and acts on their suggestions is pivotal to enabling successful collaboration. By doing this we can increase workforce resilience through times of uncertainty. Everyone within the organisation is a potential change agent, capable of directing us to the next best thing. If only we knew how to provide the right opportunities. Making the most of our clinical colleagues is a good start and, so far, I’ve never known a clinical setting to say “no ” to shared learning experiences. From my point of view it seems we are the only ones missing out. BMS
Phil Cummings is an Advanced Biomedical Scientist and Training Manager at Leeds Teaching Hospitals NHS Trust.
“Agents of change must be able to integrate, innovate, grow and continuously develop”
Congratulations to the IBMS President’s Prize Winners .
Each year an IBMS eStudent member who achieves academic distinction graduating from an IBMS Accredited BSc Hons programme is awarded the President’s Prize.
One award is made per university, with the winner receiving a certificate, IBMS Licentiate membership for a year and a cheque for £ Presentation usually takes place at graduation and, where possible, is awarded by a member of the IBMS who is closely involved in the programme.
Students must have either renewed their membership or joined the IBMS as an eStudent by January to be eligible for next year.
A couple of examples of this year’s winners now follow.
Hannah Boyd – Glasgow Caledonian University
The IBMS is encouraging members to get involved in celebrating Healthcare Science Week 2023, which takes place this month.
Healthcare Science Week, which is on 13–19 March, is held annually to raise awareness of the many careers in healthcare science.
The event shines a light on the incredible work of biomedical scientists, support staff, and students.
It is an invaluable opportunity to promote our work to the communities we serve and other healthcare professionals and inspire the next generation of biomedical scientists.

Members can run activities, give presentations, host workshops, shoot videos for social media or even sign up to become healthcare science ambassadors – there are many ways to get involved with the celebrations this year.
For those who are running events or public engagement activities, the IBMS is able to supply a range of materials, including magazines, comics for schoolaged events, promotional items and online resources.
In order to ensure that packs of promotional materials can be delivered on time, the IBMS must receive requests for items by Monday 6 March.
You can tag the IBMS in your social media posts @IBMScience using the hashtag in your tweets #AtTheHeartOfHealthcare.
You can also follow the weeklong events on Twitter using the hashtag #HCSweek23 and use these to promote your events.
For more information and for details on ordering IBMS promotional materials, visit bit.ly/HSW_2023
Hannah graduated with first-class honours in Applied Biomedical Science from Glasgow Caledonian University. She completed her placement in the clinical laboratories at St John’s Hospital, Livingston. Hannah has secured a Biomedical Scientist post in Clinical Chemistry at Forth Valley Royal Infirmary.
Iqra Ellahi – Manchester Metropolitan University
Iqra graduated with first-class honours in BSc (Hons) Applied Biomedical Science from Manchester Metropolitan University (MMU). In her final year, she completed her dissertation on the “Evaluation of VITEK AND ETEST for temocillin MIC and susceptibility determination of Enterobacterales”.
Iqra thoroughly enjoyed all aspects of her degree programme, in particular her one-year clinical laboratory placement in microbiology at Manchester Royal Infirmary, where she completed and passed her IBMS Registration Training Portfolio.
For more information and to see the full list of winners, keep an eye on the IBMS website
#IBMSCHAT
For the February Twitter Space edition of #IBMSChat, there was a lively discussion about how to get your work published as an IBMS member.
The live online audio conversation featured Rob Dabrowski, editor of The Biomedical Scientist magazine, Cherie Beckett, Senior Biomedical Scientist and IBMS member, and Simon Hoggart, Partner Journal Development Manager for the British Journal of Biomedical Science
The conversation covered the benefits of publishing, how to select a publication that is right for you and your work and how best to pitch features or articles to be published.
To listen back to the recording, visit bit.ly/3JP6K1k
The IBMS is holding a COVIDsymposium with a range of expert speakers this month.
The event will take place on March am– pm and members can attend in person at the Institute of Biomedical Science, Coldbath Square, London, or online via Microsoft Teams.
The morning will be chaired by Dr Andrew Blann, Deputy Editor of the British Journal of Biomedical Science and IBMS Molecular Pathology Advisory Panel Chair.
It is set to include sessions on the history of epidemics and pandemics; testing for SARS-CoV- in the laboratory and the community; and the UK’s response to the pandemic – establishing a national testing programme.
The afternoon will be chaired by the IBMS Chief Executive David Wells. Topics covered will include the response to COVIDand the RECOVERY trial, among others.
For information on the event, keep an eye on the news section of the IBMS website and IBMS social media feeds.
UPDATES
NHS England and the UK Standards for Microbiology Investigations (UK SMI) have developed two co-aligning documents to tackle the growing issue of antimicrobial resistance.
The UK Health Security Agency (UKHSA) is responsible for protecting the public from the impact of infectious diseases, nuclear incidents and all other health threats. Under the UKHSA umbrella, the UK SMI acts as a referenced collection of procedures and algorithms for clinical microbiology.
A recent UK SMI document has been developed, UK SMI 12, detailing procedures on investigating sepsis and systemic or disseminated infections.
This document highlights the changes needed in both clinical and laboratory
MEMBERSHIP
practice to improve the sensitivity and utility of blood cultures.
Co-aligning with the SMI document, NHS England’s Blood Culture Implementation Task and Finish Group has updated the UK’s national standards for collecting and processing blood cultures as well.
Importantly, the two documents aim to tackle the growing public health issue of antimicrobial resistance and inappropriate use of antimicrobials.
Debra Padgett, IBMS President, said: “We welcome the update to the UK SMI, taking into account the recently published NHSE Improving the blood culture pathway guidelines.”
To see the full UK SMI 12 document, visit bit.ly/3RTfPbu
The IBMS is sad to report it has been informed of the deaths of the following members and retired members:
IWD, marked on 8 March, celebrates social, economic, cultural, and political achievements of women worldwide. It is a call for women’s equality, the right to be free of bias, stereotypes, and discrimination. A reminder to create and action awareness for diversity, inclusion, and equity for females across the globe.
The first official IWD gathering took place in 1911, women and men campaigned for women’s rights to work, vote, train, and end discrimination. Today there are women in leadership roles acting as role models and providing mentorship to a new generation so they can reach their goals and aspirations – something I am grateful for. However, there is a reinforced culture for women to stay small – a career block, where dreams and talents are kept to themselves. It takes a strong mindset to break the cycle and to create a workplace for bright and ambitious individuals to shine in an environment that is safe, rewarding and provides equal opportunities for all.
Create a supportive work environment where individuals are valued and feel heard. Be open to discuss limitations and barriers. Identify skills and knowledge gaps that affect career progression and enable further
education, resources, and opportunities. Personal circumstances shouldn’t be a limitation. Be open to listen and acknowledge what change is required. This could be accommodating women’s health, carer/childcare responsibilities and allowing flexible working.
Dare to dream and achieve. Have a vision. Have the urge to explore. To allow yourself to feel confident and accomplished, create a vision of what you want for yourself. Discuss personal goals with your appraiser and ask for support from the experts in the field. Seek guidance when you feel stuck. It is easy to assume help is not available, have the courage to speak up.
Recognise imposter syndrome (selfdoubt, overthinking, lack of trust, stress, and anxiety) is common in women today. There are courses and health and wellbeing facilities available to equip you with skills to overcome these.
Don’t be afraid of failure. Be proactive, the grass is green where you water it. Seek mentorship or guidance especially if you are breaking stereotypes or overcoming barriers. Find and attend courses beneficial to you. Identify and build experiences, skills are always an asset to career progression!
Be adaptable and patient, especially in the current industry. Keep a positive mindset and outlook. Lastly but not finally, do not downplay your success – know your self-worth.
Career women today have limitations but have the unity to overcome these. Change is present but more is to come. I hope to see representation in leadership roles with a healthy work–life balance. BMS
To mark International Women’s Day (IWD), Lavanya Kanapathypillai celebrates and acknowledges the women in biomedical science and addresses the ways in which we can provide support.
SARS-CoV-2 and COVID-19: A Narrative Review. Blann AD, Heitmar R. Br J Biomed Sci. 2022 Sep 6; 79: 10426.
01
Being of Black, Asian or Minority Ethnic background is a nominally greater risk factor for admission and death in men than in women.
11
One of the first variants was characterised by a change of glutamic acid to glycine at position of the spike protein. 02
The weekly rate of case referral around week of the pandemic was small compared to that around week , a trend that was reversed in terms of the number of deaths.
Compared to deaths from all causes in the equivalent period in


– , there have been consistently more deaths during the pandemic.
Certain immune response genes, particularly those of HLA and cytokines, are linked to the severity of COVID-
12
The Beta and Gamma variants failed to make a big impact on public health in the UK. 03
13
14
On the whole, more people vaccinated with the Moderna product reported a side effect than did those vaccinated with the Pfizer/BioNtech or Johnson & Johnson products.
In young people, the BA. variant of Omicron brought a lower hazard ratio for hospitalisation than the BA. variant.
05
Monoclonal antibodies to the SARS-CoV- spike protein may be used widely as treatments for COVID-



15 The Alpha, Beta, Delta and Omicron variants had sequentially lower and lower rates of deaths per case. 06
In those aged or older, the Oxford/AstraZeneca product brought a much lower rate of events than the Pfizer/BioNtech product.
The incidence of myocarditis in a COVID- infection in those unvaccinated exceeds the rate in those who are vaccinated by over -fold.
16
17
Microvascular damage is one potential mechanism that explains long COVID.
Fatigue and anosmia are more common in acute COVID- than in long COVID.
It is estimated that there are million people in the UK who are at high risk of COVID- .
By September , it was estimated that vaccination prevented around , deaths, , hospitalisations and million infections.
18
COVID- is likely to exceed tuberculosis as the leading fatal infectious micropathogen. 09
19
Of , COVID- deaths in March-June , only % occurred in those free of any co-morbidity.
In the last two weeks of May and the first three weeks of June , there were as many deaths due to COVID- as were due to influenza and pneumonia.
The international WAO/EAACI guideline for the management of hereditary angioedema – The 2021 revision and update.
Maurer M, Magerl M, Betschel S et al Allergy. 2022 Jul; 77 (7): 1961–90. doi: 10.1111/all.15214 (https://onlinelibrary.wiley.com/ doi/10.1111/all.15214). Assessment No: 030523
01
The most common cause of hereditary angioedema (HAE) involves either a deficiency (type ) or dysfunction (type ) of C inhibitor (C–INH).
11
HAE with normal C inhibitor can currently only be diagnosed by genetic testing. 02
Angioedema is characterised by a transient vascular reaction of deep dermal/subcutaneous tissues or mucosal/submucosal tissues with localised increased permeability of blood vessels resulting in tissue swelling.
The clinical course of HAE attacks is unpredictable. Mortality due to laryngeal angioedema occurs, and extreme caution is essential.
The primary mediator of swelling in HAE-/ is bradykinin, a high molecular weight pentapeptide that is generated when active plasma kallikrein cleaves high molecular weight kininogen (HMWK).
05
06
07
08
09
The authors advise the use of antifibrinolytics (eg tranexamic acid) or androgens (eg danazol) for on-demand treatment of HAE attacks, as these drugs show good benefits.
Tests for C–INH, by many laboratories, are performed infrequently, which runs the risk of false-positive and false-negative results, which is mitigated by repeat testing.
HAE-/ is caused by a mutation in the SERPING gene, which codes for C–INH.
Cq is nearly always normal in HAE. Cq is elevated in % of patients with AAE-C-INH.
HAE with normal C–INH (HAE-nC-INH) is a group of very rare diseases. The clinical appearance of HAE-nC-INH is very different from that of HAE-
In approximately – % of patients, a de novo mutation of SERPING is responsible for the disease.
10
01
12
HAE-/ is a rare autosomal dominant disease that is estimated to affect, globally, in , individuals. 03
13
14
15
16
The offspring of a patient with HAE-/ stands a % chance of inheriting the disease.
HAE-/ should be suspected when a patient presents with a history of recurrent swelling of the skin (extremities, face, genitals), gastrointestinal attacks (painful abdominal cramps), and laryngeal oedema.
Measurements of serum/plasma levels of C–INH function, C–INH protein and C are used to diagnose HAE-/.
For HAE-/, icatibant, ecallantide and intravenous C–INH are the recommended on-demand treatments of choice.


17 The recommendation to repeat testing for C–INH function, C–INH protein and C refers only to the initial diagnosis of HAE.
18 There is no indication for repeated testing once the diagnosis has been established.
19
20
C–INH is a serine protease inhibitor (SERPIN) and the major inhibitor of several complement proteases (Cr, Cs, MASP).


Patients who are diagnosed with ACEI-AE should be tested for HAE-/, as the occurrence of angioedema attacks after the initiation of treatment with an ACE-inhibitor may point to previously asymptomatic HAE.
Compare your testing algorithm for suspected HAE and its compliance with the recommendations in this paper.
IBMS RESOURCES
02
My CPD Members can enhance their professional practice and development with the IBMS CPD scheme. The scheme offers members a flexible system of recording CPD that
is easy to use and meets the requirements for achieving and maintaining professional registration. The scheme is now electronic, so recording, amending and validating are all carried out online.
C inhibitor levels can be tested by a variety of laboratory methods. Compare the relative advantages and disadvantages of each method.
IBMS JBL involves reading and answering questions based on articles in scientific journals. It is an excellent way to learn about scientific
advances and techniques as part of CPD.
IBMS reading lists, textbooks and journals support learning and development.

Sonic Healthcare UK are leaders in consultant-led diagnostics, innovation, value, and long-term investment in healthcare provision. With over 30 years’ experience in the UK pathology market, working in partnership with the NHS for over 20 years with laboratories on private and NHS hospital sites across the UK, they continue to provide acute and routine services across all pathology disciplines, including specialist referral services. One of these services has undergone significant changes with the introduction of a new system that will transform the networked facility.
Patient safety is at the heart of the organisation’s UK-wide blood transfusion service, which comprises 13 hub BT laboratories servicing 39 hospitals that span the country from Canterbury to Glasgow. However, with newer technology available and rising volumes, the time had come to replace the legacy remote blood tracking and blood issue system.

Being a diverse and widespread facility required a clear transformation strategy and rollout approach. 33 of the hospital sites had to replace their blood fridge and tracking system kiosk, meaning the old system had to be shut down for three months. This also meant not only maintaining business continuity, but installing and validating the new system, while training both their own and hospital staff on the new technology.

“The project was a really good example of how the teams within the organisation operated to support each other,” said Anna, TDL Blood Transfusion Technical Lead.

“Installing a completely new system not only involved coordinating teams from IT, QA and Facilities Management, but because the automated system was effectively down, we relied on a manual version. Our couriers played a crucial role to deliver all the extra transfers that were needed to ensure blood stocks were always available and replenished. Lots of physical equipment testing and report writing was carried out by the lab staff - a testament to their efforts.”
Another aspect that was key to the success of the project was a coordinated training programme for hospital staff. A train-the-trainer method was developed that involved weekly online training sessions. Two dedicated training labs were also set

up in their Ealing and Manchester facilities for in-person training.
Members of the PoCT training team provided all the resources - delivering around 1,000 training sessions over a short nine-week period. Anna’s laboratory team then had to update the considerable number of training records needed for lab and hospital teams to operate the new system. Now, the new BloodTrack system, supplied by Haemonetics, is up and running successfully and means Sonic Healthcare UK can ensure the efficient and timely delivery of blood to the patients who rely on it.
If you are interested in working for an organisation that continues to invest in and develop new cutting-edge technologies, scan the QR code on the right or visit: www.sonicukjobs.com to view the opportunities available.

To register with the HCPC as a biomedical scientist, you must have met both the HCPC Standards of Education and Training (SETs) and Standards of Proficiency (SOPs).
In the UK, the SETs are met either through completion of an IBMS Accredited undergraduate degree programme, or by completion of a related BSc degree programme, plus the supplementary education identified following a non-accredited degree assessment. More details can be found at ibms.org/degree-assessment.
To meet the HCPC SOPs, everyone wishing to register as a biomedical scientist must complete the IBMS Registration Training Portfolio in an IBMS-approved training laboratory and be successfully verified.
Below is a list of the IBMS Accredited degrees that contain an integrated placement completed in an IBMSapproved training laboratory: Biomedical Science (ibms.org/accredited-degrees)
Applied Biomedical Science
Healthcare Science PTP programme, or Level 6 Healthcare Science
Practitioner degree apprenticeship.
When you graduate with a degree from an accredited
provider, your university will send your details to the IBMS education team who will issue your Certificate of Competence. They will then send your details to the HCPC to allow your registration as a biomedical scientist.
If your degree programme with the integrated clinical pathology placement is directly HCPC approved, your university may send your details directly to the HCPC once you have completed your degree and registration portfolio verification successfully.
If you took a sandwich placement and completed the IBMS Registration Training Portfolio during your placement, at the end of your final year you will need to send a copy of your degree certificate (or an official transcript of your completed degree programme) to the IBMS education team. They will then issue your Certificate of Competence to you and inform the
HCPC that you have completed an IBMS Accredited degree programme and have been issued your Certificate of Competence.
You can then apply directly to the HCPC for registration as a biomedical scientist. Under these circumstances, you must cite the IBMS as the education provider on Section 3 of the application form (Education and Training), NOT your university – the IBMS is the HCPCapproved education provider for this route to registration.
BMSFor info on registering your application with the HCPC, visit hcpc-uk.org/registration/ getting-on-the-register. If you have already applied for registration and are waiting for confirmation from the HCPC, you can check the HCPC register at hcpc-uk. org/check-the-register. Additional info and resources to support the different routes to registration as an HCPC-registered biomedical scientist can be found on our website at ibms.org/accredited
Sue Jones, IBMS Executive for Education, and Tom Mason, IBMS Education Manager, set out the process for graduates to become HCPC registered as biomedical scientists.
The point-of-care testing (POCT) team at NHS Tayside has developed dramatically over the past five years. We have a wonderful team consisting of a Department Manager, Deputy Manager, Specialist Biomedical Scientist, a rotational Biomedical Scientist and one Medical Laboratory Assistant.

We work incredibly hard to deliver the best service possible for our users and day to day we provide training on our devices, perform audits, distribute and process EQA, and complete verifications for departments within the hospital setting that are providing new POCT services within their clinical area. We cover a huge geographical area, which consists of Tayside, Angus, North Fife and Perth and Kinross. One of the biggest challenges of serving such a far-reaching area is ensuring that our users in remote areas receive the same service as our local users. We manage this challenge by regularly scheduling time to visit these areas – during which we will provide refresher training, perform troubleshooting and take the time to build working relationships with users so they feel they can reach out should issues arise.
The biggest challenge in POCT is user
engagement, but we alleviate this by ensuring that users know who we are and how to find us. We are friendly, helpful and approachable and each member of our team strives to go above and beyond for our users. It is demonstrated in the lovely feedback we receive.
Our strengths are found in our ability to work as a team, our excellent communication and the fact that even when our days are incredibly busy we are still able to have camaraderie, which keeps morale high and improves job satisfaction as we are genuinely contributing positively towards patient care. We have a great skill mix and bring the best out in each other, which ensures we are able to manage our workload effectively and keep progressing forward.
Currently, we are responsible for 18 blood gas analysers, 250 glucose meters,
75 ketone meters, 11 HbA1c devices, 15 haemoglobin devices and 30 international normalised ratio (INR) devices. However, our repertoire is always growing as clinical areas need to employ POCT devices to improve patient management and contribute to better clinical outcomes.
In the interest of continuing professional development, members of our team have created a consulting business and website with the primary aim of building a national collaborative network within the POCT community to improve quality and contribute more effectively to the management of patient care. At www. poct-for-scot.com you can read blogs written by our POCT team at NHS Tayside, which detail our journey to POCT ISO accreditation, among other POCT developments.
Our ethos in POCT at NHS Tayside is: “Vision Statement: Point of care; here for you. Aim: Working together to produce the right result for the benefit of the patient.” This is at the core of everything we do and it is what inspires us to come into work every day and do the very best that we possibly can. BMS
Robyn Wilson is a Senior Biomedical Scientist working in Point of Care Testing at Ninewells Hospital, NHS Scotland. Senior Biomedical Scientist Robyn Wilson gives a guided tour of point-of-care testing for NHS Tayside.

The right diagnostic test, at the right time, can change the course of someone’s healthcare experience – and their life.
Diagnostics inform the countless decisions which need to be made along every step of a person’s health, wellness and disease journey.
We are committed to developing diagnostic solutions that support healthcare professionals in making critical decisions for their patients.
roche.co.uk