
The Advantage of Using Pet Acoustics to Reduce Canine Stress
Could Re-use of Data
Be State-of-the-art for Authorisation Processes?
Inter-laboratory Agreement of Swine Influenza
Serology Proficiency Testing
How to Avoid Sticky Situations Official







The Advantage of Using Pet Acoustics to Reduce Canine Stress
Could Re-use of Data
Be State-of-the-art for Authorisation Processes?
Inter-laboratory Agreement of Swine Influenza
Serology Proficiency Testing
How to Avoid Sticky Situations Official





Do you need a contract research organisation for large multi-disciplinary studies?
Royal GD’s experts specialise in veterinary clinical trials with world-class animal facilities that meet global quality standards. We excel in controlled environment studies involving poultry, pigs, cattle and small ruminants.
Partner with us to enhance your multidisciplinary research and discover new opportunities!
Get in touch with our contract research project team
One of the largest veterinary laboratories in the world
In vitro models for virology, bacteriology, parasitology
Expertise teams for poultry, cattle, swine
Animal models for infectious diseases
MANAGING DIRECTOR
Mark A. Barker
EDITORIAL MANAGER
Chloe Euripides chloe@senglobalcoms.com
RESEARCH AND CIRCULATION
Chloe Euripides chloe@senglobalcoms.com
DESIGNER
Jana Sukenikova www.fanahshapeless.com
BUSINESS DEVELOPMENT
Jerome D’Souza info@senglobalcoms.com
ADMINISTRATOR
Jessica Chapman info@senglobalcoms.com
FRONT COVER © istockphoto
PUBLISHED BY
Senglobal Ltd.
Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0) 2045417569
Email: info@senglobalcoms.com www.international-animalhealth.com
International Animal Health Journal – ISSN 2752-7697 is published quarterly by Senglobal Ltd.
04 FOREWORD
REGULATORY & MARKETPLACE

06
One of the foundational elements of a successful CDMO partnership is the alignment of strategic investments and technological capabilities. Companies in the animal health sector seek CDMOs that are not only equipped with state-of-the-art facilities but also committed to continuous improvement and innovation. Alex Del Priore at Syngene International, discuss why, advanced technologies such as data analytics, artificial intelligence (AI), and machine learning are no longer optional but are integral to staying competitive and meeting the complex demands of the market.
The new Great Britain (GB) veterinary medicines laws are largely pro-innovation and generally have been welcomed by industry following close engagement with the regulator during the consultation period. While the laws mostly align with new standards introduced in the EU (and Northern Ireland), thereby helping companies to implement similar changes across the region, they diverge in some favourable ways from EU law. Chris Boyle of Sidley Austin LLP show how the new GB laws create the possibility for valuable additional periods of data protection for existing active substances.
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
Volume 11 Issue 2 Summer 2024
Senglobal Ltd.
10
Everyone is talking about sustainability and the will to replace animal testing with alternative methods, such as New Approach Methodologies (NAMs). Can both issues be realised at once by re-using test data and data trading between science and the major regulatory authorities can be harmonised. Dr. Regina Ohlmann at 4ReValue GmbH summarises how the European authorities EFSA, ECHA and EMA publicly present how they establish the use of NAMs and the topic of data sharing in their assessment processes.


RESEARCH AND DEVELOPMENT
14 Development of Livestock and Aquaculture Infectious Disease Models
The development of new disease challenge models is required where new pathogens, pathogen strains or serotypes emerge. It may also be necessary where pathogen strains have developed resistance to existing licensed products. David Reddick and Bill Roy at Moredun Scientific assess the efficacy of veterinary medicinal products (VMPs) against these pathogens, if it is necessary to have validated disease models available for conducting tests under controlled conditions.
APPLICATION NOTE – COMPANION ANIMALS
18 Experts Discuss the Benefits of a More ‘Cat-centric’ View on Parasitology
In March 2024, over thirty global leaders in parasitology, pharmacology, zoonoses and feline medicine came together to discuss the latest developments in feline parasitology, and debate where the focus needs to be to improve cat care most effectively. Katrin Blazejak and Norbert Mencke of Vetoquinol, reviews the 3rd Scientific Roundtable, hosted by Vetoquinol in Lisbon, Portugal, delivered a packed three-day agenda that facilitated knowledge sharing, lively discussion and even the promise of exciting collaboration to further existing research programmes.
22 The Advantage of Using Pet Acoustics to Reduce Canine Stress
Understanding and addressing auditory stress is vital for promoting canine welfare. By recognising the sources and impacts of auditory stress, and implementing strategies to mitigate it, we can improve the quality of life for dogs, particularly those in highstress environments like shelters and kennels. Janet Marlow of Pet Acoustics Inc. shows that biometric study shows the advantage of
Pet Acoustics Music in reducing canine stress compared to classical music and no-music in a kennel environment.
LIVESTOCK AND DISEASES
28 Inter-laboratory Agreement of Swine Influenza Serology Proficiency Testing
Influenza diagnosis, surveillance and monitoring, is currently often based on detection and characterisation of influenza virus by molecular tests. However, in some cases, serological analysis become the preferred option due to lack of access to samples from acutely affected animals. Lucía Dieste Pérez, Lianneke and Huub van de of Royal GD, discuss the limited harmonisation of diagnostic protocols across labs and countries, all adjusted to the characteristics of regional circulating strains, challenging the cross-country, sero-surveillance of swine influenza.
FOOD & FEED
32 Unveiling Insights: Meta-Analysis on Mycosorb and Layer Performance
Mycotoxins are naturally occurring, toxic secondary metabolites produced by fungi that can be found in feedstuffs such as cereal grains and their byproducts. The consumption of mycotoxins by poultry animals can result in several effects, both direct and indirect. Dr. Alexandra Weaver at Alltech explores the impact of yeast cell wall extract on laying hens facing mycotoxin challenges.
MANUFACTURING
34 How to Avoid Sticky Situations
Sticking is often described as the Achilles’ heel of tablet production. It is one of the most common tablet tooling problems and can have a profound effect on production, leading to reduced output and increased costs. Rob Blanchard of IHolland explains why sticking occurs and proposes several solutions.



Efficacy Studies
• Broad portfolio of infectious disease models in livestock and fish
• New model and protocol development
• VICH-GCP compliant studies
Target Animal Safety Testing
• GLP compliant studies
• All species of livestock
• Aquaculture species
• All types of biological & pharmaceutical products
Animal Health Facilities
• GLP accredited animal facilities
• Conventional farm animal accommodation
• Category 3 containment
• Specific Pathogen Free
• Gnotobiotic units
• GLP accredited lab facilities
Aquaculture Facilities
• Seawater
• Freshwater
• GLP compliant small scale
• Larger scale biocontainment
• Commercial scale
• Warm water for fish and shrimp


Another quarter, another great selection of developments in the world of animal health. In this years’ summer issue of IAHJ we gain an insight into the positive impact Pet Acoustics music can have on the canine ear, we receive an update on the new laws of veterinary medicine, and we conclude with packaging solutions that can help you avoid “sticky situations”.
We kick off our Regulatory and Market Place section with Syngene International’s Alex Del Priore highlighting the importance of strategic investments and technological advancements. In this article Alex takes time to explain why investing in the correct strategies and technologies is integral to the evolution of the animal health sector’s growth. He explains that it is essential to the development of the market that we take time in assessing and investing in the flexibility, scalability and reliability of these processes and, doing this successfully, ultimately allows us the best possible opportunity to thrive at keeping up with complex demands of the market while remaining competitive too.
Katrin Blazejak and Norbert Mencke of Vetoquinol bring light to the disparity in research carried out between cats and dogs, urging that more is done to improve attitudes toward cat health. They specifically draw focus on the lack of research collected on feline parasitology, discussing how this is not something that has been prioritised in the same regard as other animal species, and as parasitic infections prove to be
constantly changing, we create greater risks by leaving this to the unknown.
A particular favourite of mine, which falls under Research and Development, is Janet Marlow of Pet Acoustics’ in-depth research on reducing canine stress through playing them species-specific music. In this study, Janet explains the importance of recognising and addressing auditory stress to best improve canine welfare. Not only does she report the positive impacts on stress and anxiety levels, but she also records that canine sleep quality and behaviour amongst its species, as well as human-dog interactions, drastically improve too. A piece that showcases the importance and ease of bettering canine welfare.
The Manufacturing section concludes our summer issue, offering several solutions to the sticking problems that arise in the pill-packaging process. Rob Blanchard of I Holland shares tips and tricks on how to avoid the different types of sticking within the manufacturing process so to help increase efficiency and reduce company costs.
A thrillingly diverse issue this summer, spanning across the animal health industry in its entirety. As we further our knowledge and understanding of the animal world, we enable the industry to progress and evolve, and thus I do hope you enjoy reading Volume 11, Issue 2.
Chloe Euripides, Editorial Manager

EDITORIAL ADVISORY BOARD
Amanda Burkardt, MSc, MBA – CEO of Nutripeutics Consulting
Germán W. Graff – Principal, Graff Global Ltd
Fereshteh Barei – Health Economist & Strategy Advisor, Founder of BioNowin Santé Avenue Association
Carel du Marchie Sarvaas Executive Director Health For Animals
Kimberly H. Chappell – Senior Research Scientist & Companion Animal Product Development Elanco Animal Health
Dr. Sam Al-Murrani – Chief Executive Officer Babylon Bioconsulting & Managing Director at Bimini LLC
Sven Buckingham – Buckingham QA Consultancy Ltd.
Dan Peizer – Director Animal Health at Catalent Pharma Solutions
Dawn Howard – Chief Executive of the National Office of Animal Health (NOAH)
Jean Szkotnicki – President of the Canadian Animal Health Institute (CAHI)
Dr. Kevin Woodward – Managing Director KNW Animal Health Consulting
Norbert Mencke – VP Global Communications & Public Affairs Bayer Animal Health GmbH


Lyophilization and Sterile Manufacturing
Driving development and connecting commercialization of sterile and lyophilized drug products, we are dedicated to your success in bringing life-changing therapies to patients.
talkfuture@pci.com Your
www.pci.com
OUR END-TO-END BIOLOGIC SOLUTIONS INCLUDE:
• Sterile Formulation & Lyophilization Cycle Development
• Lyophilization and Sterile Fill-Finish Manufacturing
• Aseptic Robotic Technologies
• Analytical Support
• Clinical & Commercial Labeling & Packaging
• Refrigerated/Frozen Storage & Distribution

One of the foundational elements of a successful CDMO partnership is the alignment of strategic investments and technological capabilities. Companies in the animal health sector seek CDMOs that are not only equipped with state-of-the-art facilities but also committed to continuous improvement and innovation. Advanced technologies such as data analytics, artificial intelligence (AI), and machine learning are no longer optional; they are integral to staying competitive and meeting the complex demands of the market.
For instance, leading CDMOs invest significantly in new manufacturing facilities designed for flexibility and scalability. These facilities are capable of handling diverse production needs, including those of the animal health industry. The modular nature of such infrastructures allows for rapid expansion, ensuring that CDMOs can quickly adapt to new projects and scaling requirements. This adaptability is crucial in a field where the ability to respond swiftly to changing market conditions can provide a significant competitive advantage.
Establishing Trust Through Quality and Compliance
Trust is the cornerstone of any successful partnership, especially in the regulated world of animal health. CDMOs must demonstrate a robust governance framework, including stringent quality management systems, comprehensive information sharing protocols, and reliable supply chain mechanisms. These elements are vital in ensuring that products meet the highest standards of quality and safety.
A CDMO's ability to deliver consistent quality is a key determinant of trust. This involves not just meeting but exceeding regulatory requirements and client expectations. Regular audits, transparent communication, and a commitment to continuous improvement are practices that help build and maintain this trust. For animal health companies, knowing that their manufacturing partner adheres to the highest standards of quality and compliance provides peace of mind and allows them to focus on other aspects of their business.
The Role of Transparency and Communication
Effective communication is another critical factor in building strong CDMO partnerships. Transparency in operations, progress updates, and problem-solving processes ensures that clients are kept informed at every stage of the manufacturing cycle. This openness not only fosters trust but also allows for collaborative problem-solving, which is essential in managing the complexities of biopharmaceutical production.
For example, regular updates on the status of production, potential issues, and timelines help manage expectations and prevent misunderstandings. This level of communication is particularly important in the animal health sector, where delays or quality issues can have significant impacts on market supply and, ultimately, animal health outcomes.
One of the most challenging aspects of CDMO partnerships is the process of technology transfer. This involves moving a product's manufacturing process from one facility to another or scaling it up for larger production runs. Successful tech transfer requires not only the right infrastructure but also the expertise to absorb and implement new technologies effectively.
A CDMO's ability to handle tech transfers efficiently is a significant value proposition. This capability ensures that products can be manufactured consistently and reliably, regardless of the geographic location or initial development conditions. A structured and well-documented tech transfer process minimises risks and ensures that the transition is smooth and successful. For animal health companies, this means their products can reach the market faster and with fewer disruptions, a critical factor in maintaining a competitive edge.
The animal health sector is characterised by its dynamic nature, with fluctuating demands and evolving regulatory landscapes. Successful CDMO partnerships are those that offer flexibility and agility in their operations. This means having the capacity to scale production up or down based on market needs, as well as the ability to quickly adapt to new regulations or client requirements.
CDMOs that prioritise operational excellence and have robust systems in place for process improvement and problem-solving are better positioned to meet these challenges. By focusing on continuous improvement and leveraging advanced manufacturing technologies, these organisations can provide their clients with the flexibility needed to navigate an ever-changing market.
A prime example of a successful CDMO partnership in the animal health sector is the collaboration between Zoetis and Syngene. This partnership, which spans over a decade, highlights the critical components of successful collaborations in this field. Zoetis, a global leader in animal health, has relied on Syngene's state-of-the-art infrastructure, technical capabilities, and robust governance procedures to meet its production needs.
Several factors have contributed to the longevity and success of this partnership. Firstly, Syngene's ability to deliver consistent quality and meet the stringent requirements of Zoetis has been crucial. The company's investment in advanced technologies and flexible manufacturing facilities has ensured that it can scale production to meet Zoetis's needs efficiently.
Secondly, the transparency and communication between the two organisations have fostered a strong sense of trust and collaboration. Regular updates, open communication channels, and a shared commitment to quality have ensured that both parties are aligned in their goals and expectations.


The small molecule animal health space offers a diverse array of opportunities, and Syngene is uniquely positioned to take advantage of them with its extensive capabilities in development and manufacturing. Syngene can develop processes with a focus on speed and costeffectiveness without sacrificing quality, depending on the stage of development. The company can safely, optimally, and competitively accommodate chemical processes in its dedicated animal health facility. Syngene's capabilities span across large and small-scale manufacturing, as well as new areas such as ADC for animals.
Lastly, Syngene's expertise in tech transfer has been a significant advantage. The company's structured and welldocumented tech transfer processes have ensured that new products can be brought to market quickly and efficiently, minimising risks and maximising efficiency.
As the animal health sector continues to grow and evolve, the importance of strong CDMO partnerships will only increase. Companies in this space will continue to seek out CDMOs that can offer advanced technological capabilities, flexibility, and reliability. By focusing on strategic investments, quality and compliance, transparency, and tech transfer excellence, CDMOs can position themselves as valuable partners in this dynamic industry.
In conclusion, the future of animal health CDMO partnerships looks promising. By building on the principles of trust, innovation, and collaboration, CDMOs can help drive the next wave of advancements in animal health, ensuring that high-quality products are available to meet the needs of veterinarians and pet owners around the world. As exemplified by the partnership between Zoetis and Syngene, these collaborations are not just about manufacturing products but about creating value and fostering innovation in the animal health sector.

Alex Del Priore
Alex has three decades of experience in developing, commercialising and life-cycle management of products in various life science industries. Holding positions in both the US and Europe at Syngene International, his experience includes senior roles with global P&L responsibility. As a member of the Executive Committee, Alex plays a technocommercial role, providing technical expertise to the API plant at Mangalore while building a sustainable client base for the business in collaboration with the commercial and business development teams. In addition, Alex is also responsible for biologics operation at Syngene International.

The new Great Britain (GB) veterinary medicines laws are largely pro-innovation and generally have been welcomed by industry following close engagement with the regulator during the consultation period. While the laws mostly align with new standards introduced in the EU (and Northern Ireland), thereby helping companies to implement similar changes across the region, they diverge in some favourable ways from EU law, e.g. the new GB laws create the possibility for valuable additional periods of data protection for existing active substances.
The new laws will nevertheless require stakeholders to make further investment and to implement changes to their processes in GB. For example, anyone advertising veterinary medicines, medicated feed or feed additives will have to comply with new advertising laws; manufacturers will need to comply with new labelling requirements; active substance manufacturers/ importers/ distributers will have to become registered and now have an explicit requirement to comply with good manufacturing practices (GMP); wholesalers will have to comply with good distribution practices (GDP); retailers will have to comply with storage and audit requirements; and veterinary groups will need to ensure they have adequate processes to enable their prescribers to comply with additional recordkeeping requirements.
The new laws apply from 17 May 2024, but transition periods are available for some requirements, e.g. advertisers have up to 17 August 2024 to implement the new requirements, and manufacturers have up to 1 April 2029 to implement certain labelling requirements (provided conditions are met).
All stakeholders should familiarise themselves with the requirements that will affect their businesses and prioritise changes in accordance with the applicable transition periods.
Below are the non-exhaustive key takeaways closely focusing on topics of innovation, compliance, regulatory and antimicrobial resistance (AMR):
Pro-Innovation Takeaways
• Increased periods of data protection: This will help protect manufacturers’ investments in research and development by preventing generic companies from relying on the innovator’s data for the period of protection.
• New data protection periods for variations: New and separate periods of protection will be available where an existing product is varied to add a new pharmaceutical formulation or a new species, and where the new product is packaged separately and accorded its own marketing authorisation number. This is an innovative law that supports investment and moves away from a 20-year trend to limit the available data protection to a single ‘global marketing authorisation’ principle in the EU.
• Closing the parallel import route into GB: The new laws remove the ability for distributers to obtain ‘Marketing Authorisations for Parallel Import’, closing the parallel
import supply channel into GB. The change means manufacturers will no longer have to compete with ‘parallel traded’ products (versions of their own products purchased in a lower price country and re-distributed to GB). Veterinary surgeons will still be able to prescribe and import small volumes of medicines for animals under their care in accordance with the ‘specials’ scheme
• Prohibition on compounded products that are a "pharmaceutical equivalent" of an authorised product: This measure, together with stricter controls on the promotion of the 'prescription cascade', will help ensure that licensed products are used where available rather than unlicensed compounded products. Compounded products must also make state on their label "this veterinary medicinal product does not hold a marketing authorisation".
• Labelling and packaging: The new laws aim to ensure full alignment with the labelling and packaging requirements in the EU and Northern Ireland, with the option of providing an electronic package information leaflet.
• Location of marketing authorisation holder: The marketing authorisation holders can either be established in the UK or in a country demonstrated to have equivalent standards, e.g. the EU. In such instances, having a 'local representative' in GB will remain voluntary.
• New advertising laws include a requirement to state "prescription decisions are for the person issuing the prescription alone" in adverts for prescription medicines.
• New restrictions on inducements and hospitality, namely the provision of gifts, pecuniary advantages or benefits to persons qualified to prescribe or supply veterinary medicines.
• Hospitality, including travel and accommodation, may potentially be provided to 'animal health professionals' if it is subordinate to the main purpose of the meeting or event.
• Trade practices relating to prices, margins or discounts in existence as of 17 May 2024 are exempted from these restrictions.
• Promotional samples of antimicrobials are prohibited, and additional controls have been introduced for other promotional samples.
• New record-keeping requirements for all stakeholders, including manufacturers, wholesalers, retailers, veterinarians and animal keepers. Businesses will need to ensure their processes and software enable the capture of the requisite data.
• Increased regulatory enforcement powers include new powers for the authorities to seize goods, suspend marketing authorisations, prohibit supply and impose temporary restrictions for breach of the Regulations.


• Active substance manufacturers, importers and distributers must comply with GMP and must be registered.
• Wholesalers must comply with a range of new requirements, including compliance with GDP, and must have the services of technically competent staff (including a Wholesale Qualified Person).
• Manufacturers must obtain a separate wholesale dealing license to wholesale.
• Manufacturers must report shortages "as soon as reasonably practicable".
• Additional pharmacovigilance system and personnel requirements have been introduced.
• Retailers must comply with new audit, record-keeping, and storage requirements.
• Online retailers of veterinary medicines must be registered.
• Prescribers must record the reason for prescribing veterinary medicines supplied pursuant to an oral prescription.
• Medicated feed must meet manufacturing and labelling requirements.
• Post-authorisation studies may be required for antimicrobials to ensure that the benefit-risk balance remains positive.
• Marketing authorisations may be refused on the basis of a negative benefit-risk balance taking into consideration risk of AMR. However, refusal of the authorisation on the grounds that the antibiotic is reserved for human use has been removed.
• Restrictions on preventative (prophylactic) use of antibiotics (rather than antimicrobials more generally) although no specific restrictions on metaphylactic use.
• Continued ban on using antimicrobials as growth promoters
• Stakeholders must provide data on usage of antibiotics on request.
• The new laws are consistent with UK Government's 5-year antimicrobial resistance action plan.
Disclaimer - The views expressed in this article are exclusively those of the author and do not necessarily reflect those of Sidley Austin LLP and its partners. This article has been prepared for informational purposes only and does not constitute legal advice. This information is not intended to create, and receipt of it does not constitute, a lawyer-client relationship. Readers should not act upon this without seeking advice from professional advisers.

Dr. Chris Boyle is a life sciences lawyer and qualified veterinarian at the global law firm Sidley Austin LLP, where he works as counsel. Dr. Boyle advises animal health companies across a broad range of legal and regulatory issues and is the founder or Sidley's Animal Health Legal Forum.
Email: cboyle@sidley.com


Everyone is talking about sustainability and the will to replace animal testing with alternative methods, such as New Approach Methodologies (NAMs). But couldn't both issues be realised at once by re-using test data? What if data trading between science and the major regulatory authorities could be harmonised? Would authorisation processes suddenly be simplified and much quicker? In this article, we have summarised how the European authorities EFSA, ECHA and EMA publicly present how they establish the use of NAMs and the topic of data sharing in their assessment processes.
Looking at the progress for alternative animal testing, one can see strong efforts at all levels. In the scientific community, a great deal of focus has recently been placed on so-called NAMs (New Approach Methodologies). NAMs are computer or lab-based research approaches intended to more accurately model animal or human biology to replace or sometimes at least complement traditional research models. Numerous research funds are offered as prizes (SET Foundation, BMEL Animal Welfare Research Prize, NIH Common Fund Complement-ARIE programme and many others) to promote research into replacement methods or to recognise successful efforts. In addition, databases are created that make it possible to find alternative methods (e.g. NAMs Network). On the NAMs Network homepage, e.g. 257 different NAMS for hazard characterisation can now be found. These are categorised into organ/systems, guidance, regulations and many more.
The authorities are also recognising more and more alternative methods for authorisation work. Whereas not so long ago, all toxicological tests were still carried out in-vivo, tests such as ‘the eye irritation test on reconstructed human cornea-like epithelium’ have become established as in-vitro tests for the safety assessment of feed additives, among other things. The EFSA (European Food Safety Authority) has a favourable attitude towards NAMS. The recognition of alternative methods is also laid down in Regulation (EC) 429/2008 Annex 2, Article 3: “In-vitro methods or procedures are recommended which refine or replace the usual studies on laboratory animals or reduce the number of animals used in these studies. Such methods shall have the same quality and validity as the methods they are intended to replace”. In addition, EFSA published an activity proposition of the Development of a Roadmap for Action on New Approach Methodologies in Risk Assessment.[1] The roadmap is as follows: EFSA will develop a “guidance on the integration of NAM data into feed and food risk assessment and incorporate the concepts of a weight of evidence approach (as used in traditional human risk assessment) into NAM approaches”. They mention that the alternatives to animal testing “have the potential to provide sound, cost-effective, timely and reliable information, but their regulatory acceptance has not yet been established”.1 This guidance might bring more information on that topic.
The ECHA (European Chemicals Agency) also runs workshops on the implementation of NAMs and has
established NAMs in various areas of the assessment of chemicals. But at the moment they only accept alternative methods as a direct replacement for animal testing for acute and short-term effects. Examples of this are eye irritation, skin sensitisation or bioaccumulation tests. The ECHA still “considers animal testing to be indispensable, especially for the assessment of long-term effects such as organ damage, weakening of the immune system, development of allergies or asthma or reproductive problems and birth defects”.2
The EMA (European Medicines Agency) is also open to NAMS. They offer “scientific advice to support the qualification of innovative development methods for a specific intended use in the context of research and development into pharmaceuticals”. The EMA also publishes their opinion on various NAMs that have been shown to be successful in the context of science and drug development.
But what about the obvious, the direct re-use of data again. With everyone talking about sustainability, wouldn't that be the non-plus-ultra? Not only does it save animals from experiments, but it also protects the environment, considering the material cost of a new experiment, whether in vivo or in vitro. Not forgetting the time aspect. Every experiment takes time to plan and time again to carry out. If the experiment fails, it has to be planned and carried out again. Let’s have a look at three different authorities (EFSA, ECHA and EMA) and how they handle the re-use of data.
Chapter IV, Article 20 of Regulation (EC) 1831/2003 states that scientific data and other information for application dossiers are protected from re-use by others for at least 10 years, unless two applicants agree to trade their data between each other. This data trading is particularly welcome for data from toxicological tests on vertebrates so that they do not have to be carried out again. “However, if no agreement is reached on the sharing of the data, the Commission may decide to disclose the information in order to avoid the repetition of toxicological tests on vertebrates (...)”. After 10 years, the data is available to the authority for evaluation in favour of another applicant. The following guideline has also been in place since 2008: “Common guidelines on practical arrangements for the sharing of scientific data between the scientific committees and panels of European agencies and the scientific committees of the commission”. This states that “the exchange of data/dossiers between scientific committees/panels will take place when the necessity arises, on an ad-hoc and on-request basis, (...)”. The data exchange will then be regulated between EMEA, EFSA, ECHA and the Commission. It is not a guideline how applicants can exchange data with each other. There is also no extra guideline on this. If you look at the Open. EFSA portal under Questions (https:// open.EFSA.europa.eu) you can see individual applications publicly available in various categories and at various stages of the process. If you then go to the “Administrative data” section of the individual dossiers, you will see the sub-item “Data sharing agreement in place”. After we have been monitoring the “data sharing agreement in place” topic since the introduction of the Transparency Regulation (EU) 2019/1381 in March 2021, on July 17th 2024 we saw for the first time that “yes” was clicked and the data sharing agreement
was publicly available. It is obviously possible to say “yes”, but it is common knowledge that clicking “no” does not involve any major action or consequences. The applicant can simply say "no" without any further questions (Graph 1 and 2). It is questionable whether this could change in the near future and what will be changed then.
A call for data sharing and a demand that the initiation of animal testing is seen as the last possible resource to prove the efficacy and safety of a substance does not seem to exist on the part of EFSA for the time being.


In comparison to EFSA, the topic of “data sharing” has long been established at ECHA. There is not only explicit guidance on the topic of data sharing and the so-called REACH Regulation (Regulation (EU) 2016/9), but also a notice directly on the homepage to all applicants:

“Registrants must make every effort to share data on intrinsic substance properties in a fair, transparent and nondiscriminatory manner. This applies in particular to information on tests on vertebrate animals. In this way, registrants reduce registration costs and avoid unnecessary testing, especially on vertebrate animals. If the parties cannot reach an agreement, ECHA can assist in resolving data sharing disputes as a last resort”.1
Article 2 of Regulation (EU) 2016/9 establishes the transparency of data sharing: “Where multiple registrants of the same substance or participants in a Substance Information Exchange Forum (SIEF) are obliged to share information in accordance with their duties under Regulation (EC) No 1907/2006, they shall make every effort to reach an agreement on the sharing of the information (...)”. 1, In addition, Article 3 also regulates the “one substance, one assessment” policy. The Agency should ensure that there is only one application for a specific substance. Without ever having submitted a separate application to the ECHA, this gives the impression that the registrants could already have the option of planning animal tests together, should they be necessary. This obviously saves animal testing, resources and time for everyone.
How all this is and can be implemented in practice needs to be examined elsewhere. However, the efforts are recognisable and the fact that this direction is being taken can only be welcomed.
The EMA uses so-called catalogues and has replaced the old register (European Union electronic register of post-



authorisation studies (EU PAS Register®)) with the publication of the HMA-EMA catalogue.
“The HMA-EMA Catalogues are repositories of metadata collected from real-world data (RWD) sources and RWD studies. They are intended to help regulators, pharmaceutical companies and researchers to identify and use such data when investigating the use, safety and effectiveness of medicines”.5
The following purpose shall be fulfilled with the catalogue:
• “Facilitate the discoverability of adequate data sources to generate real-world evidence for regulatory purposes (e.g., identification of RWD data sources suitable for investigating a specific research question);
• Aid in the suitability assessment of data sources by providing clear and easy access to information from the study protocol and study report;
• Improve interoperability between studies and data sources;
• Improve transparency“
The Catalogues have the FAIR (Findable, Accessible, Interoperable and Reusable) data principles. With that those data are fully searchable, and it is even possible to export the data. The EMA states that “the Catalogues implement globally unique and persistent identifiers, ensuring data accessibility and interoperability”.
The following types of studies should be included in this catalogue:
• “Non-interventional post-authorisation safety studies (PASS)
• Required non-interventional PASS
• Any other real-world data (RWD) study conducted by MAHs, regulators, academia or research organisations”
The extent to which an actual re-use of data is guaranteed later on was not pursued further at this point. The fact is that the catalogue at least provides a tool that the industry can use to obtain marketing authorisations and possible PASSs certainly help to assess the potential risks of a new medicinal product.
Summary
Each of the authorities has its own provisions, regulations and
approaches to establishing new products and prove their individual safety. A harmonisation of these regulations is not apparent, at least at a first glance. After all, data sharing among the authorities seems to be established. As a citizen, it is difficult to judge what this looks like in reality, at least at the moment. While the industry is trying to close the gap of missing databases for commercial issues and set up databases themselves (e.g. 4ReValue GmbH), the consortia have always helped their members to exchange data internally. It will be interesting to see what further developments emerge on the part of the authorities.
1. Escher SE, Partosch F, Konzok S, Jennings P, Luijten M, Kienhuis A, de Leeuw V,Reuss R, Lindemann K-M, Hougaard Bennekou S, 2022. Development of a Roadmap for Action on NewApproach Methodologies in Risk Assessment. 153 pp. doi:10.2903/ sp.efsa.2022.EN-7341
2. Miccoli A, Marx-Stoelting P and Braeuning A, 2022. The use of NAMs and omics data in risk assessment. EFSA Journal 2022; 20(S2):e200908, 9 pp.
3. https://Alternatives to animal testing under REACH – ECHA (europa.eu), visited on 14th July 2024
4. https://Data sharing – ECHA (europa.eu), visited on 17th July 2024
5. Homepage | HMA-EMA Catalogues of real-world data sources and studies (europa.eu), visited on 15th July 2024

The veterinarian Dr. Regina Ohlmann was working as a consultant in the feed industry, when she realised that the introduction of the Transparency Regulation not only meant more work for the EU approval of feed additives, but also an opportunity to introduce data sharing. With that in mind, Dr. Ohlmann founded the 4ReValue GmbH together with Dr. Schreiner. She is enthusiastic about the idea of reducing animal testing through her work.
Email: ohlmann@4Revalue.com

Where reliability meets advanced animal health packaging
Nordson EFD Dial-A-Dose™ and Posi-Dose® disposable dosing syringes allow users to tailor multiple, accurate doses based on treatment requirements.
• Molded from FDA-approved resins
• Wide range of nozzles for varied applications
• Oral, topical, intramammary, and rectal uses
• Self-venting feature prevents air entrapment
• Reliable, accurate, repeatable dosing
• Global customer support nordsonefd.com/ReliableAH



The development of new disease challenge models is required where new pathogens (or pathogens of new significance), pathogen strains or serotypes emerge. It may also be necessary where pathogen strains have developed resistance to existing licensed products. In order to assess the efficacy of veterinary medicinal products (VMPs) against these pathogens, it is necessary to have validated disease models available for conducting tests under controlled conditions.
Validated experimental models of livestock and aquaculture infectious disease are important tools for the generation of efficacy data for candidate veterinary medicinal products (VMPs) or feed products / supplements. For efficacy data to be submitted for registration purposes in Europe, the US and Japan, pivotal efficacy studies must be conducted to the standards defined in the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products, Good Clinical Practice (VICH-GCP).
While it is widely acknowledged that the number and scale of in vivo assays should be reduced, replaced and or refined (the 3Rs) most of the dossiers submitted contain data from in vivo challenge model efficacy studies. It is unlikely that the requirements for animal challenge models will reduce markedly in the near future, since in most cases it is not possible to produce the same quality of efficacy data using in vitro assays. There is, however, a responsibility to ensure the in vivo models used to generate registration data are industry relevant, biologically valid and statistically robust.
Challenge model development can be complex and costly. It is generally undertaken by animal health companies, aquaculture companies, contract research organisations or academic groups. Prior to undertaking any development, it is necessary to have a clear idea of what will be required in the development, the resources required and the likely problems that may occur. In some cases, it is possible to review the scientific literature for similar models which can help to determine appropriate model design, however, such information is not always available and, even with well-established model designs, there can be considerable variability in clinical signs, pathology and other outputs for different isolates for the same pathogen species.
There are 5 main steps involved in the development of a new disease model:
1. Sourcing of pathogen isolates
2. Identification of challenge titre, volume and route
3. Identification of target species parameters (eg age range)
4. In vitro validation
5. In vivo validation
This article will review the critical success factors for each of these steps.
Once the need for a disease model for a particular pathogen has been identified, it should also be considered whether

there is a particular serotype, subtype or toxin production profile of the pathogen that is of particular significance in the geographic region(s) where the VMP will be marketed. This will increase the likelihood that data generated from the use of the model will be acceptable to the regulatory authorities. With changing epidemiology of disease worldwide, this selection can prove challenging, but sufficient information is generally available within the literature and through contacts with industry to allow an informed choice to be made. With improvements in the speed and cost for DNA sequencing in recent years, the genotype of different isolates can now also be used to assist in determining the most appropriate strains to use, and identification of particular pathogenic gene sequences can aid in accurate selection of pathogen strains.
Generally, it is suggested that selection of isolates should be based on a number of factors, taking into account:
• Geographical location
• Where the isolate was sourced
• The year of isolation – (as often the regulators require the use of field isolates which are <5 years old for regulatory studies)
• The age of the animal – and, in fish, the development stage and production environment, from which it was sourced (selecting isolates from animals similar to the intended target for the model)
• The clinical history of the isolate (i.e. what clinical signs were observed in the host animal and what pathology was observed at postmortem, if applicable).
This can be particularly important where pathogens can cause a range of different clinical signs – offering a wide range of parameters where statistical significance can be evaluated.
Where possible, isolates should be selected from cases where a single isolate was recovered. This is important since with multifactorial infections it is difficult, if not impossible, to attribute clinical signs to a single pathogen. Secondly, opportunistic species may or may not be disease-causing under certain conditions.
An additional factor to be considered for bacterial pathogens is antimicrobial sensitivity. For bacterial challenge models to be used in antimicrobial efficacy studies, the sensitivity of the pathogen against the common veterinary antimicrobial families in use should be established. For some studies, it may be appropriate to use a bacterial challenge isolate with high or low minimum inhibitory concentration (MIC). This is increasingly important as the incidence of resistant bacterial populations grows. The use of challenge isolates with higher MICs against a particular antimicrobial product provides the option of monitoring field effectiveness of the product against increasingly resistant pathogens.
Challenge models should be designed to produce clinical disease to a level that allows for a stringent test of the VMP but without being overly severe. Models producing very mild disease, or which are sub-clinical in nature, and models that are acute or severe, are unlikely to be wholly representative of the field situation and may result in an unrealistic assessment








of the product efficacy. Models should be designed to produce a moderate level of clinical disease where appropriate, with clear clinical signs that are consistent and reproducible and with significant differences in the measured parameters between challenge and control animals. Large differences between control and challenged animals and low variability of clinical signs in the challenged group, means that a smaller number of animals are likely to be required to give statistical significance to the study. This is ethically more acceptable and also reduces development costs.
An example of the clinical signs that can be generated during challenge model development is shown in Figure 1. This data shows the clear difference in mean group respiratory scores for calves challenged with a respiratory bacteria and a control (unchallenged) group.
Other considerations include the route of administration of the challenge (e.g. intravenous, intranasal, subcutaneous, oral, intra-tracheal or in fish, intra-peritoneal injection, immersion in a suspension of pathogen or co-habitation with infected animals, or topical application e.g. of a pathogen suspension directly onto the gills), the volume of the challenge to be administered, the challenge concentration, and also the number of occasions and/or period over which the challenge has to be administered. The route of administration should mimic the route of entry of the pathogen in the field where possible. For example, the administration of respiratory bacteria by intravenous injection is unlikely to produce field-type symptoms, whereas the use of an intranasal delivery method is likely to produce disease more consistent with field infections. The volume of the challenge is important as in some cases, due to specific pathogen characteristics, it is not possible to use a small volume of challenge material. For example, the respiratory pathogen Mannheimia haemolytica challenge can require a large volume to be used, which is made up of a diluted neat broth culture. M. haemolytica produces endotoxins which, when concentrated, will produce acute endotoxic reactions resulting in rapid death of animals. The use of a large, diluted, volume of challenge material dilutes the concentration of the endotoxin and negates, or at least reduces, the toxic effect. The challenge titre is very important as this normally determines the resulting clinical signs. Too low a titre and no disease will occur; too high and the disease may be too acute and therefore, ineffective as a model. Finally, the number of doses of challenge and their frequency should be determined. Some challenges work well with only a single challenge occasion; others require multiple doses over a period of time. For example, some Mycoplasma bovis challenge models are most successful when a low volume / high titre is administered daily over a number of days.
Another example is that of a sea lice challenge model for farmed Atlantic salmon which uses seawater adapted ‘postsmolt’ salmon. Sea lice copepodid larvae are introduced into a tank containing fish, attaching and developing into the more harmful, mobile stages. The number of copepodids must be carefully controlled to ensure that the number of mobile lice is sufficient to enable valid statistical comparisons between treated and untreated groups, whilst avoiding damaging levels of parasite infestation. This is achieved by use of copepodids from a standard source and age, and careful control of environmental conditions, particularly water temperature and light, which influence settlement success. With careful control of the challenge two or more cohorts can be established on one group of fish which provides efficiencies in animal numbers and costs without causing undue harm to the experimental animals. For VMPs with preventative action, fish may be treated and then challenged, adult lice manually removed at sampling, and fish allowed to recover and challenged again at a later date in order to assess the duration of efficacy. For therapeutic products, fish may be infected with two cohorts of sea lice at an interval of several weeks in order to be able to measure effects on different parasite stages in a single study.
These factors need to be carefully considered and it is not uncommon during new model development for a range of different routes, volumes and concentrations to be tested. Literature searches can provide clues to possible challenge procedures for different species, however, with strain-to-strain variability the model that works in one situation may not be suitable for a situation with a different isolate of the same species.
Following pathogen selection, the next stage in the development of a model is to consider the nature of the VMPs that will be tested with the model to ensure that the age range, development stage, production environment, gender, breed or reproductive status of the target species reflects the planned use of the product. Should it be developed in weaning age animals to support antimicrobial product development, or is the model to be used for vaccine development, where older animals will be the target?
An example of this would be Streptococcus suis, which can cause lameness, septicemia and/or meningitis in piglets where the models for testing vaccines or therapeutic products are generally quite different with regard to challenge volume, titre and route of administration. On the other hand, for pig respiratory bacterial disease models, it is possible in many
cases to set up one model with a wider range of possible uses, such as challenge in weaning age (four-week-old) piglets for use with therapeutic VMPs or with 10-12-week-old animals for vaccine studies. The only differences in these models are likely to be either a higher volume or higher concentration of the challenge material.
In farmed Atlantic salmon, vaccines tend to be administered to juvenile fish prior to transfer to seawater, whereas therapeutics for control of several economically important diseases are administered to larger fish in the seawater phase. Ideally, the model should be able to service a number of different requirements (e.g. both preventative and therapeutic VMPs), but this may require multiple models to be developed with the same pathogen.
Once isolates are sourced, the growth in the laboratory must be validated to determine the optimal conditions required to produce the pre-determined challenge titre and determine the reproducibility of the culture conditions. This is necessary as most challenge models use a fresh challenge culture prepared on the day of challenge, and in these situations it is generally not possible to confirm the titre accurately prior to use, therefore, there must be considerable confidence in the growth conditions and challenge titre produced.
Retrospective confirmation is possible following titration of the material, however, the results will take at least 24 hours to become available and the challenge will have already been administered before the titre is known. It is necessary to show that in the laboratory an isolate can be produced to the required titre, within the required timeframe, on a number of occasions, before it can be confirmed as suitable for use. For all isolates, large seed stocks should be prepared and maintained to ensure that testing is always carried out from the same basic stock material, and stability checks should be carried out before any use of the isolates in challenge efficacy work to ensure viability is retained during the freezing / storage process. All seed stocks must be confirmed as free from contamination and tested to confirm that they are pure before use, since the presence of other organisms may result in unexpected clinical disease or increased severity of disease.
However, in vitro culture is not possible for all pathogens and alternative approaches may be required. For example, in vitro culture conditions for replication of Piscine Myocarditis Virus (which is responsible for Cardiomyopathy Syndrome in farmed Atlantic salmon), have not been successfully established and in this case, the challenge model uses a pool of tissue homogenate prepared from heart, spleen or kidney reservoir tissue from affected fish. The material is screened for other pathogens and purified by filtration and centrifugation. QPCR is used to confirm consistency of virus load and viability is confirmed by assessment of cytopathic effect in cell culture.
Once the laboratory validation is complete, isolates should be selected for in vivo model development. Generally, isolates are selected based on meeting different criteria relating to growth level and growth rate, however, successful growth in the laboratory does not necessarily mean that the isolates will be successful at producing clinical disease. While isolates are selected from clinical cases, it is not possible to ensure that they are capable of causing clinical disease prior to use. Unknown factors may be present in the environment of the source farms where clinical cases were observed, which allowed previously benign organisms to become pathogenic and may not be present or reproducible in research facilities.

These unknown factors could be related to stress from high stocking densities, poor quality feed or previous disease outbreaks, as well as other factors. The high animal care standards in disease research facilities means that these stressors are normally reduced in comparison to some commercial farms.
Selected isolates should be administered to the target species using the recommended route, volume and titre. During validation, animals must be observed at routine intervals to track the progress of any disease and to monitor the welfare of the animals. This information is vital in determining appropriate parameters of disease for use in regulatory efficacy studies, as well as allowing data to be collected for use in determining ethical endpoints for veterinary intervention. Many in vivo model development studies will include a range of different routes, volumes and concentrations, however, it is generally recommended that only one parameter is assessed at any one time. For example, for a bovine respiratory model, the concentration of the challenge may be assessed at three different titres but the route and volume should remain constant for all. In vivo studies should include sufficient numbers of animals to allow identification of variability of the model, but ethical use of animals will limit the maximum number used. In general, groups of between five and ten animals are sufficient for this purpose, although this can be disease and species dependent. A control group is normally also included to provide baseline species data for comparison.
It would also be expected as a minimum that a repeat of the model following the optimised procedures is carried out. This allows identification of any variability and, only once this has been assessed and confirmed to be of an appropriate level, can the model be considered as fully validated.
Summary
Experimental disease challenge models are essential for the generation of efficacy data for VMPs for regulatory submission. Model development is a complex and timeconsuming process incorporating biological, financial and logistical factors. Companies developing VMPs or novel feed products must take these factors into consideration when deciding between in-house development and potential outsourcing to a contract research organisation.


David Reddick is Head of Animal Health.
Email: dreddick@moredun-scientific.com
Bill Roy
Bill Roy is Head of Aquaculture. Email: wroy@moredun-scientific.com


In March 2024, over thirty global leaders in parasitology, pharmacology, zoonoses and feline medicine came together to discuss the latest developments in feline parasitology, and debate where the focus needs to be to improve cat care most effectively. The 3rd Scientific Roundtable, hosted by Vetoquinol in Lisbon, Portugal, delivered a packed three-day agenda that facilitated knowledge sharing, lively discussion and even the promise of exciting collaboration to further existing research programmes.
Many topics were covered, following four key pillars of focus – ‘Science and innovation’, ‘Feline parasitology’, ‘Feline medicine and behaviour’, and ‘One Health and zoonoses’. One of the key outtakes is that while lots of important work is being done, there could be huge benefits for both feline and human health if feline parasitology was placed higher on the agenda at multiple levels of the veterinary and scientific sector.
Science & Innovation
Despite the wealth of feline-specific research activity presented at the Roundtable event, wider evidence indicates that cats are generally neglected as a species within scientific literature. Cats outnumber dogs in many regions – there are 127 million cats and 104 million dogs living in Europe for example1 – yet there is a clear mismatch when it comes to the number of published scientific articles specifically related to each of the species.2
This species gap may be reflected in clinical practice too, with anecdotal reports from multiple Roundtable delegates that fewer parasitological diagnostic procedures are performed on cats compared to dogs. This could be due to several factors – cats may not be presented for regular veterinary examination (the possible reasons for which are numerous), there may be feline-specific barriers to proceeding with diagnostic investigation, or veterinarians are not placing parasites as high on differential diagnostic
lists for their feline as for their canine patients. Again, the root causes of this could be varied, including lack of awareness or confidence.
“Cats are just less parasitically investigated. For every cat faecal sample that is submitted to our diagnostic laboratory at the University of Adelaide, Australia there are 9 canine samples.”
Dr. Ryan O’Handley, University of Adelaide, Australia
The Roundtable attendees commonly agreed that cats need to be studied more if we are to comprehend the true extent and nature of the feline parasite challenges that must be addressed. A big part of achieving this is understanding the barriers to feline-specific research projects being made possible, something that Vetoquinol is keen to facilitate.
To truly understand parasitological risks, research relating to feline parasites is equally important at both micro- and macroscopic levels – from genome analysis to broader consideration of regional and global parasite distribution.
One example of the benefits of a research focus on the genetic make-up of parasites came from Dr. Jeba Jesudoss Chelladurai, Kansas State University, USA, who shared how genome sequencing research has uncovered that there are likely two species of Dipylidium caninum which demonstrate host specificity. She postulated that the praziquantel resistance experienced in dogs in some regions is likely not an issue for the feline-specific species of the flea tapeworm, meaning that the risk-benefit analysis of treatment for cats could be very different to dogs.
At the other end of the scale, taking a much more ‘zoomed out’ view of parasite distribution allows shifting trends to be identified - essential for us all to try and stay one step ahead in protecting both feline and human health from parasitic risks. There was lively discussion at the Roundtable about how encounters between parasites, domestic animals and
humans are changing in nature and frequency, with the proposed reasons behind this being varied.
Time was spent analysing this from the parasite’s perspective – for example discussing the impact that urbanisation has on the local climate of a particular area. However, shifting patterns of parasite distribution were also attributed to their host species, with many delegates noting in their observations that the presence of wildlife species – known to be a significant source of parasite and vectorborne disease (VBD) infection risk to domestic species – is increasing in urban areas.
“Towns are typically warmer than rural locations, and this may favour the development of tick populations. The presence of wildlife species in urbanised areas is also growing, increasing crossover between wild and domestic animal populations… and their parasites.”
Prof. Ezio Ferroglio, University of Turin, Italy
The reality is that these changes are likely the result of a combination of many factors, but the important end outcome is the same – parasite infection risks are constantly changing. This is already manifesting in many regions as emerging disease risks for pets and veterinarians must stay informed to ensure they are giving the best advice to pet owners.
In some instances, perceptions need to change too –one example highlighted at the meeting being the range of respiratory worms that can affect cats. With some species almost disregarded as an incidental finding in certain regions, established attitudes were challenged with the presentation of evidence demonstrating surprising pathogenesis and reasoned discussion of the challenges that these parasites can present.
Prof. Angela Di Cesare, University of Teramo – Italy, explained how Capillaria aerophila can cause lung parenchyma damage and chronic bronchitis in cats and Prof. Manuela Schnyder, University of Zurich – Switzerland shared case studies of Aelurostrongylus abstrusus which demonstrated that even mild or inapparent infections can lead to substantial damage to lung tissue. The difficulties of diagnosis were acknowledged as a compounding factor in identifying and managing these infections, but the takeout message remained the same – it is important for veterinarians to remain vigilant, open-minded and unbiased when it comes to parasite risks in cats.
“All cats with outdoor access are at risk of lungworm infection, therefore lungworm treatment cover should be considered for them.”
Prof. Manuela Schnyder, University of Zurich, Switzerland
Feline Medicine & Behaviour
To deliver the best parasitological care, veterinarians must first overcome another key barrier, getting cats to veterinary clinics in the first place. It’s a challenge that has been identified in many regions – for example, in the UK, 3.9 million cats are only taken to the veterinarian when they are ill.3
“The difficulties associated with bringing a cat to a clinic or hospital often presents a massive barrier for owners and results in failed attempts and less veterinary visits compared to their canine counterparts”.
Dr. Rachel Korman, Veterinary Specialist Services, Feline Specialist, Underwood, Qld. Australia


“Cat owners will be much more likely to bring their cats to a clinic where they feel their cat's welfare is prioritised, both physical and mental wellbeing, by respecting their need to avoid dogs, be handled gently and kindly and kept in a quiet space with somewhere to hide. Simple clinic changes can make a huge difference to the cat's, and therefore the owner’s, experience at the veterinary clinic.”
Dr. Samantha Taylor, International Society of Feline Medicine (ISFM) Academy Lead and Specialist Veterinary Advisor to ISFM, UK
“Educational opportunities are being missed when owners can purchase parasite products over the counter – veterinarians have to work hard to proactively encourage cat owners into the clinic”.
Dr. Ryan O’Handley, University of Adelaide, Australia
So, the question remains as to how best we reach these owners who have little veterinarian contact and encourage better engagement with their cat’s health?
It was here that Dr. Korman, and Dr. Taylor had some excellent advice for clinical veterinarians: Dr. Korman has developed ‘The 4 C’s’ – an easy way for clinicians to consider all the ways that stress can be minimised for cats, and therefore their owners, when making a veterinary visit.
Multiple sensory assaults, which occur on a trip to the veterinary clinic, result in ‘stressor stacking’, and when paired with the inability to exhibit the normal flight response, lead to fear.
Remembering and addressing the 4 C’s can help reduce some of the stressors:
• Catching
• Carrier
• Courier
• Clinic
Further to this, Dr. Taylor explained how to improve the clinic experience for cats via many of ISFM ‘Cat Friendly Clinic’ (CFC) principles, stressing that any aspects that can be implemented from this scheme – however small – will reap benefits for everyone. It has been shown that cats visit CFC clinics more frequently and more diagnostic tests are carried out, improving patient care and increasing clinic revenue. Applying CFC principles has also been shown to have practical benefits too, such as reducing staff injury.

Protecting feline health is clearly a compelling reason to strive for constant improvement in parasitological care, but for the many parasites that have zoonotic potential, human health is a concern too.
One fascinating insight into how animal parasite studies could benefit human health relates to Toxocara. In her presentation looking at neurotoxocarosis, Prof. Christina Strube, Veterinary University Hannover – Germany, shared the results of mice model studies that demonstrate how T.cati and T.canis have a preference for differing regions of the brain. What’s more, these differences translate into marked differences in neurobehavioural signs. This raises many questions and exciting opportunities for further research. Toxocara seroprevalence in humans has increased across Europe in the past few decades and it was noted that cats are more likely to defecate, and the faeces remain unremoved, in areas frequented by at-risk groups such as children. In Hannover, Toxocara was found to be present in up to 41% of playgrounds.4
Research like this underlines the importance of the role veterinarians have in ensuring the successful implementation of preventative parasite protocols in pet cats. Effective products exist but compliance can be a key barrier with cats and about more than just the product and the owner. Good compliance for cats starts long before a prescription event, including consideration at product development and through owner education.
“Compliance is a daily challenge in feline medicine. All the best research and pharmacological R&D in the world is wasted if that medication does not make it into the cat.”
Dr. Rachel Korman, Veterinary Specialist Services, Feline Specialist, Underwood, Qld. Australia
Poor disease awareness amongst owners was cited as a major contributing factor to the struggles faced in controlling zoonotic parasitic diseases. The importance of public health education programmes to help combat this was highlighted at the meeting, as well as the need for these efforts to be ongoing over decades. This is to prevent these programmes from becoming victims of their own success - when public awareness becomes high, communication urgency is reduced, and important information is therefore not conveyed to the next generation.
“The general public tends to assume direct host-tohost transmission of parasites, but lifecycles can obviously be much more complicated. The public not knowing this can lead to inadvertent introduction of infection risk – for example, via BARF diets.
Dr. Ryan O’Handley, University of Adelaide, Australia
The principle of ‘contextualised care’ was also proposed as a key part of ensuring better compliance with parasite prevention protocols. This is where importance is placed on empathetic and considered clinical decisions which take into account the realistic expectations of the owner. Balancing clinical requirements and owner capabilities is one part of this. For example, can the number of prescribed medications be reduced perhaps to improve the chances of the cat receiving the most critical ones? Is there an alternative formulation that could be prescribed that will aid compliance?
Proactivity and Collaboration is Key
The Roundtable discussions highlighted that changing and
increasing infection risk, a shortage of research relating specifically to cats and potential lack of awareness are all factors that could contribute to inadvertent and risky complacency when it comes to feline parasites.
“Parasitism of cats is increasing in the USA, with positivity highest in samples from young cats. In the human world, this would set off alarm bells.”
Dr. Cassan Pulaski, University of Georgia, USA
With veterinarians being such a vitally important part of ensuring the delivery of the best parasitological care for cats, their proactive involvement in taking on parasite risks will subsequently have a significant positive impact. This can be as simple as placing parasites higher on the differential diagnosis list and remaining open-minded when investigating clinical disease. Straightforward diagnostics can reveal a lot and it is always prudent to remember that if parasites aren’t looked for, they won’t be found!
Another key component is knowledge sharing and awareness, so that veterinarians can remain vigilant and informed as to their local parasite risks. One of the primary objectives of this Roundtable event has always been to facilitate the dissemination of the latest developments beyond the meeting room for exactly this reason. Many of the discussions from this year are already being translated into practical and accessible resources for clinical veterinarians, to help them deliver better parasitological care, protecting feline and human health.
Ultimately, a collaborative approach from across the veterinary sector such as clinicians, the pharmaceutical industry, and wider scientific community, is essential to facilitate the development of solutions that are meaningful and robust. This is why events like this are so important, bringing together the full spectrum of stakeholders within the veterinary sector – from research through to delivery of frontline clinical care. Only then can a multi-perspective view of the challenges be achieved, and true progress be made.
“Sharing knowledge across countries can improve our impact in our world; parasites have no borders, neither should we.”
“Sharing science on feline parasitology will help to find answers faster together, everything for the sake of our clients but also for the environment.”
“There is an incredible wealth of knowledge in the veterinary world and a great number of exciting research projects occurring. Events that enable this information to be distributed and further collaboration between scientists and veterinarians is welcomed.”
Prof. Norbert Mencke & Dr. Katrin Blazejak, Veterinary Parasitologists Vetoquinol during 3rd Scientific Roundtable (2024)
About Vetoquinol
Vetoquinol is a leading global animal health company that supplies drugs and non-medicinal products for the livestock (cattle and pigs) and pet (dogs and cats) markets.
As an independent pure player, Vetoquinol designs, develops and sells veterinary drugs and non-medicinal products in Europe, the Americas and the Asia Pacific region. Since its foundation in 1933, Vetoquinol has been pursuing a strategy combining innovation with geographical


diversification. The Group's hybrid growth is driven by the reinforcement of its product portfolio coupled with acquisitions in high-potential growth markets.
Vetoquinol is committed to advancing feline parasitology, working with leading parasitology organisations, ESCCAP, CAPC and WAAVP. We support key parasitology conferences across the globe to encourage progress and The Vetoquinol Scientific Roundtable in Parasitology is just one example of this.
REFERENCES
1. European Pet Food Industry Federation (FEDIAF)
2. Image adapted from how cat-astrophic are the knowledge gaps in feline parasitology. Prof. Andrei Mihalca. April 2024
3. CATS (Cats and their stats) 2022 UK Report
4. Kleine A, Springer A, & Strube C. (2017). Seasonal variation in the prevalence of Toxocara eggs on children’s playgrounds in the city of Hanover, Germany. Parasites & Vectors. 10:1-8 doi:https://doi.org/10.1186/s13071-017-2193-6
Additional Reading
Morelli S, Diakou A, Colombo M, Di Cesare A, Barlaam A, Dimzas D, & Traversa D. (2021) Cat respiratory nematodes: Current knowledge, novel data and warranted studies on clinical features, treatment and control. Pathogens, 10 (4), 454. doi: https://doi. org/10.3390/pathogens10040454
• https://catfriendlyclinic.org/vets-nurses/applicationsupporting-documents/
• Rousseau J, Castro A, Novo T, Maia C. (2022) Dipylidium caninum in the twenty-first century: epidemiological studies and reported cases in companion animals and humans. Parasit Vectors. 15(1):131. doi: https://doi.org/10.1186/s13071022-05243-5
• Bonilla-Aldana JL, Espinosa-Nuñez AC, Bonilla-Aldana DK, Rodriguez-Morales AJ. (2024) Toxocara cati Infection in
Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals (Basel). 14(7):1022. doi: https://doi.org/10.3390/an

Katrin Blazejak studied Veterinary Medicine at the University of Veterinary Medicine, Hannover, Germany. After graduation in 2015, she commenced her specialisation in parasitology with a doctoral degree (Dr. med. vet.), and obtained a German veterinary specialisation degree as a certified Veterinarian for Parasitology (Fachtierarzt für Parasitologie) in 2020. In September 2021, she joined Vetoquinol as Global Medical Manager Parasitology and is based in Paris, France.

Norbert Mencke studied Veterinary Medicine at the University of Veterinary Medicine, Hannover, Germany. After graduation in 1987, he commenced his PhD studies at the Department of Agriculture in Adelaide, Australia. In 1995 he became a certified Veterinarian for Parasitology, and in 2003 a European Veterinary Specialist in Parasitology. He has lectured in veterinary parasitology and tropical veterinary medicine at the University of Hannover since 2003. In 2020, he joined Vetoquinol and holds the position of Global Medical Manager Parasitology, Paris France.


Biometric Study Shows the Advantage of Pet Acoustics Music in Reducing Canine Stress Compared to Classical Music and No-Music in a Kennel Environment.
Understanding and addressing auditory stress is vital for promoting canine welfare. By recognising the sources and impacts of auditory stress, and implementing strategies to mitigate it, we can improve the quality of life for dogs, particularly those in highstress environments like shelters and kennels. Reducing auditory environmental stress not only enhances the well-being of dogs but also fosters better human-animal relationships and supports overall animal welfare.
Auditory stress in dogs can originate from various sources, including loud noises, sudden sounds, and continuous background noise, all of which can be prevalent in highdensity environments. Chronic exposure to these stressors can lead to behavioural issues, anxiety, and even physical health problems in dogs. Recognising these stressors and their impacts is the first step towards mitigating them. Strategies such as soundproofing kennels, introducing calming music, and providing quiet spaces for rest can significantly alleviate stress. Implementing these measures not only improves the immediate well-being of the dogs but also aids in their longterm emotional and physical health, making them more adaptable and well-adjusted. Managing auditory stress ensures a more humane and supportive environment, ultimately leading to stronger bonds between humans and their canine companions.
Introduction
The purpose of this six-week study was to support canine welfare by helping canines to manage auditory and behavioural stress through the most effective sound intervention for calming.
Dogs can hear frequencies ranging from approximately 40 Hz to 60,000 Hz, which is significantly broader than the human hearing range of 20 Hz to 20,000 Hz. This heightened sensitivity allows dogs to detect sounds, such as high-frequency noises emitted by electronic and ultrasonic devices, and other animals. However, it also means that dogs are more vulnerable to auditory stress from loud, sudden, or continuous noises as well as ultra-high and sub-low frequencies not being audible by humans.
Environmental noise is a common source of auditory stress for dogs. Sounds such as traffic, construction, fireworks, thunderstorms, and appliances can cause significant stress and anxiety in dogs. Studies have shown that noise phobia, particularly fear of thunderstorms and fireworks, is a prevalent issue among dogs, leading to behaviours such as trembling, hiding, barking, and destructive behaviour (Blackwell et al., 2013).
Dogs housed in kennels and shelters are often exposed to high levels of noise from barking dogs, cleaning activities, and
human interactions. This constant auditory stimulation can lead to increased stress levels, manifesting in behaviours like restlessness, barking, and aggression. Research by Coppola et al. (2006) found that shelter dogs exposed to high noise levels exhibited elevated cortisol levels, indicating increased stress.
Playing calming music specifically designed for dogs can help reduce stress and anxiety. Research by Kogan et al. (2012) and Wells et al. (2002) has shown that classical music can have a calming effect on dogs, reducing barking and promoting restful behaviour.
This convenient sample study employed a repeated measures design to gain biometric understanding of canine responses to music/sound interventions to diminish stress using three conditions: Pet Acoustics canine-designed music, classical music selections and no-music.
Participants A convenience sample of canines participated in the study. Canine study participants were recruited from Educated Canines Assisting with Disabilities (ECAD, Torrington, CT, US), an Assistance Dog International (ADI) accredited service dog organisation. ECAD reviewed the study for animal welfare and safety considerations and based on the noninvasive biometric monitoring parameters and non-invasive music/ sound interventions agreed to have 12 service dogs in training participate in the study.
The kennel site used for the study was a large kennel room in the E.C.A.D. facility where the service dogs in training are placed for quiet time. Each dog rested in a hard rubber crate with a large metal door grill opening, commonly used in kennels.
Study Tools
To accurately measure and monitor the physiological responses of dogs in this study, several specialised tools and techniques were employed. These tools ensured precise data collection and analysis of auditory and physiological parameters.
• Pet Tunes Pro Speaker: Pet Acoustics Pet Tunes Pro speaker was set up in the center of the kennel room, four feet off the ground to disburse the 360-degree sound interventions for the dogs resting.
• Decibel and Frequency Monitoring: The volume level of the speaker was set between fifty and eighty decibels (dB) which is considered safe and non-stressful for dogs. The frequencies were monitored with a frequency app during each intervention.
• Response Indicators: Non-Invasive biometric collars to record each dogs' responses to auditory stimuli (PetPace™ Smart collars) were placed on six dogs at a time, during the data collection phase of the study.
• Software for Data Analysis: Response patterns as biometric graphs for each dog were supplied with Tableau


Data Software. This provided a visual data platform which analysed pulse rates, Heart Rate Variability (HRV), activity level, posture and respiration rates. (Sample Chart)
Video Documentation: Video was taken throughout the six-week study, capturing visual observations of the twelve dogs' behavioural responses to the three interventions. The footage was compiled into a ten-minute documentary, which can be seen on YouTube. https://www.youtube.com/ watch?v=L3lRE0ZeBf8
The combination of these monitoring tools allowed for comprehensive data collection on the auditory and physiological responses of the dogs. This multi-faceted approach provided checks and balances to support the accuracy and reliability of the study's findings, providing valuable insights into the relationship between kennel stress and hearing responses of the twelve dogs.
The Interventions
The biometric study was conducted in two, three week periods. The first group of dogs wore the PetPace™ Smart collars while resting and listening in their crates during each of the three interventions; No-music, Pet Acoustics music, and classical music. With the second group of dogs, the sequence of
Tool
PetPace Collar Data collected from each dog
Speaker Setup
Time Logs
Intervention Metrics
interventions was rearranged to minimise data bias. During the second testing, the original six dogs returned to wearing their regular collars, while the new group of six dogs wore the PetPace™ biometric collars.
Data Collection
Data Analysis
Descriptive statistics were calculated and reported as aggregate data for a group of 12 dogs over six weeks.
Results
For each biometric measure, overall trends and significant differences were analysed. A key finding revealed that the absence of music in the kennel led to significantly higher stress levels in all twelve dogs compared to when music was present. Among the music interventions, Pet Acoustics music was the most effective in calming the dogs, followed by classical music.
Discussion – What is learned from the study is that Pet Acoustics music offers a consistent, positive impact across all the biometrics tested. This supports canines in receiving a steady benefit that may contribute to longterm improvements in their welfare. While the immediate
Data Collected
Pulse, HRV, Posture, Activity, Respiration
Placed speaker in the center of Kennel
Start and end times for each task
Week 1/6 No Music, Week 2/4 Pet Acoustics music, Week 3/5 classical music
Notes
Ensure data was collected every 2 to 5 minutes
Music decibel and frequency levels monitored
Start and end times for each task
Each intervention followed by three days of no testing to reset behaviour.



Pulse
A lowering of the pulse reflects a calmer state in a canine. The lowest average score for pulse in the Pet Acoustics condition was 67.3, with the classical music condition at 68.0, and the no music condition at 69.5.


HRV
An increase in HRV reflects a calmer state and less stress in a canine. The highest average score for HRV in the Pet Acoustics condition was 11.46, followed by the classical music condition at 11.45, and the no music condition at 11.40.
improvements are modest, regular exposure to Pet Acoustics music could lead to significant long-term benefits in reducing chronic stress and improving overall health in canines. Inherent in the design of Pet Acoustics music are controlled volumes, tones and frequencies contoured for canine hearing comfort. When a frequency or volume creates pressure in a dog’s ear, this triggers stress and agitation. Therefore, music that accommodates canine hearing sensitivities can provide measurable and repeatable calming results.
The highest stress data points occurred when no music was played in the kennel. This demonstrates that the absence of music can significantly increase stress in dogs, concluding that music is a valuable calming tool for kennel environments.
Classical music can have varying effects depending on the composition and the individual dog's response. The dynamics of the classical music was not controllable and the music was sometimes over ninety decibels and then below fifty decibels indicated on a decibel reader during the testing. Classical music inherently has a wide variety of sonorities and volume levels that create inconsistencies of sound levels. Instrument timbres and voice dynamics produced high frequencies that became pressure in some of the dog's ears which interrupted rest. It was observed that some dogs were standing and circling in their crate without resting during both classical music testing sessions.
Although classical music studies have been conducted on dogs in kennels and shelters and have shown calming benefits, this study marks the first instance where classical music is being compared to music specifically designed for canines, such as Pet Acoustics canine-designed music, revealing through biometrics, an advantage in reducing stress.
Conclusion
Intentional music and sound interventions are a powerful tool

Respiration
A lowering of the respiratory rate reflects a calmer state in a canine. The lowest average respiratory score was seen in the Pet Acoustics condition was 16.0, followed by the classical music condition at 16.2, and the no music condition at 16.6.

Posture
A lowering of the posture score reflects a calmer state in a canine. The lowest average posture score was seen in the Pet Acoustics music condition at 51.4, followed by the classical music condition at 52.0, and the no music condition at 53.5.
in supporting canine welfare. By reducing stress and anxiety, improving physiological health, and creating a more serene environment, music interventions can enhance the overall quality of life for dogs in kennels, shelters, and other care settings such as puppy training and general behavioural modification.
Reduced Stress and Anxiety: Calming music can help reduce stress and anxiety levels in dogs, which is especially beneficial in a kennel environment where dogs might feel unsettled due to unfamiliar surroundings and separation from their owners.
Improved Sleep Quality: Music can create a soothing environment that encourages dogs to relax and sleep more soundly. Better sleep contributes to overall well-being and health.
Behavioural Improvement: Playing calming music can help decrease barking, restlessness, and other anxious behaviours in a kennel. This can lead to a more peaceful and quieter nvironment for all the dogs.
Enhanced Recovery: For dogs recovering from surgery or illness, a calm environment facilitated by music can promote faster healing and recovery.
Easing Separation Anxiety: Music can provide comfort and a sense of familiarity, helping dogs cope with the absence of their owners and reducing separation anxiety.
Enrichment and Mental Stimulation: Different types of music can serve as a form of enrichment, providing mental stimulation and preventing boredom.
Improved Human-Dog Interaction: A calm and relaxed environment created by music can enhance interactions

between kennel staff and dogs, making handling and care easier and more pleasant.
About Pet Acoustics: Pet Acoustics is a global award-winning company that specialises in creating music specifically designed for animals. Recognising that animals have different auditory sensitivities compared to humans, Pet Acoustics tailors its products to meet these unique needs. The company's offerings include a range of music products designed to calm and soothe various types of pets, such as dogs, cats, horses, birds, rabbits, and small animals.
These products are often compatible with SD cards preloaded with specific types of calming music, ensuring that pets are exposed to sounds that are harmonious and non-threatening to their sensitive ears. Scientific studies and findings have supported the effectiveness of such music in reducing anxiety and promoting relaxation in pets for longterm health. (www.petacoustics.com)
The classical music intervention selections played in the study were: Johann Sebastian Bach – "Air on the G String" , Wolfgang Amadeus Mozart – "Piano Sonata No. 11 in A Major, K. 331, Frédéric Chopin – "Nocturne in E-flat Major, Ludwig van Beethoven – "Moonlight Sonata" Antonio Vivaldi – "The Four Seasons" (the slower movements) Franz Schubert – "Ave Maria" Debussy, La Plus Que Lente.

About Janet Marlow – Janet Marlow is an accomplished composer and researcher who is widely recognised for her pioneering work in the field of animal acoustics. She is the founder of Pet Acoustics, a company dedicated to creating music and sound products designed specifically for the auditory sensitivities of animals. Marlow's work focuses on understanding how sound affects the behaviour and wellbeing of pets, and she has developed specialised music to help reduce stress and anxiety in animals.
Her research has led to the creation of products such as Pet Tunes and Pet Tunes Pro which are tailored to the unique hearing ranges of different animals, including dogs, cats, horses, birds and rabbits. Marlow's innovative approach combines her extensive background in music with her passion for animal welfare, resulting in practical solutions that improve the quality of life for pets and their carers. Through Pet Acoustics, Janet Marlow continues to advance the understanding of how sound influences animal behaviour and health.
PetPace, Ltd. is a company that specialises in creating innovative solutions to improve pets' quality of life, health, and overall well-being. Founded by veterinarians, this company utilises machine learning algorithms to design a smart collar capable of monitoring pets' daily vitals and habits. The PetPace™ Smart collar 2.0 is a patented, non-invasive, collar equipped with an array of sensors and backed by sophisticated algorithms that can accurately monitor a range of animals’ biometrics, including vital signs, behaviour, and location. Multiple non-invasive, all-passive sensors are incorporated inside each PetPace collar, including:
• Thermometers – for temperature detection
• Acoustic sensors – for pulse, HRV, and respiration acquisition
• 6-D accelerometers – for activity, calories, and posture calculation
• GPS – for location tracking (www.PetPace.com)
Educated Canines Assisting with Disabilities (E.C.A.D.) a nonprofit organisation, provides service dogs to individuals with disabilities to help them gain greater independence and mobility. Founded by Lu and Dale Picard, the organisation offers various programs tailored to different groups, including veterans, children with autism, and individuals residing in facilities like hospitals and nursing homes. E.C.A.D. trains these dogs to perform various tasks that aid in daily living, such as retrieving items, turning on lights, and providing physical support. (www.ecad1.org)
1. Bowman, A., et al. (2015). "Four Seasons" in an animal rescue center; classical music reduces environmental stress in kennelled dogs. Physiology & Behavior.
2. Wells, D. (2002). Influence of various types of auditory stimulation, including classical music, on dogs in animal shelters. Belfast, Ireland.
3. Brayley, C., & Montrose, V. (2016). The effects of audiobooks on the behavior of dogs at a rehoming kennel. Applied Animal Behavior Science.
4. Bains, M., et al. (2017). Effect of different types of classical music played at a veterinary hospital on dog behavior and owner satisfaction. Journal of the American Veterinary Medical Association (JAVMA).
5. Bowman, A., et al. (2017). The effect of different genres of music on the stress levels of kennelled dogs. Physiology & Behavior.


1. Blackwell, E. J., Bradshaw, J. W. S., & Casey, R. A. (2013). Fear responses to noises in domestic dogs: Prevalence, risk factors, and co-occurrence with other fear-related behavior. Applied Animal Behaviour Science, 145(1-2), 15-25.

3. Kogan, L. R., Schoenfeld-Tacher, R., & Simon, A. A. (2012). Behavioral effects of auditory stimulation on kenneled dogs. Journal of Veterinary Behavior, 7(5), 268-275.
2. Coppola, C. L., Grandin, T., & Enns, R. M. (2006). Human interaction and cortisol: Can human contact reduce stress for shelter dogs? Physiology & Behavior, 87(3), 537-541.
PET ACOUSTICS PUBLISHED STUDIES
1. Biometric Study Proves Pet Acoustics Canine-Specific Music Mitigates Stress Levels in dogs. (2022). International Animal Health Journal, 9(2).
2. Clinical Study Proves Benefits of Feline-Specific Music Through Biometric Data. (2021). International Animal Health Journal, 8(2).
3. Audiometric Study Reveals Patterns of Age-Related Hearing Loss in Dogs and Cats. (2024). International Animal Health Journal, 10(4).
4. Evaluation of the Behavioral and Productive Effect of FrequencyModified Music in Piglets. (2022). International Animal Health Journal, 9(1).
5. Massage or Music Meant to be Relaxing, Results in Lowering Salivary Cortisol Concentration in Race Horses. (2021). International Animal Health Journal, 8(4).

Janet Marlow, M.A., Founder of Pet Acoustics Inc., Dr. Asaf Dagan, Chief Veterinary Scientist and Founder of PetPace, Prof. Joanne Singleton, PhD, Pace University, Alan Brennan, Pet Acoustics Inc., Lu and Dale Picard, Founders of E.C.A.D., Brandi Lebel, Kennel Manager, E.C.A.D
“Our gratitude to the esteemed Dr. Temple Grandin, Distinguished Professor, renowned animal behaviourist and autism advocate for her encouragement and support in the creation of this study.” Janet Marlow
Email: janetmarlow@petacoustics.com

Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.
www.international-pharma.com

Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.
www.journalforclinicalstudies.com
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.
www.international-animalhealth.com
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.

www.international-biopharma.com
Pharma Nature Positive, is a platform for all stakeholders in this industry to influence decision making by regulators, governments, investors and other service providers to achieve Nature Net Positive Results. This journal will enable pharma the ability to choose the right services to attain this goal.
www.pharmanaturepositive.com
‘Putting science into conversation, and conversation into science.’Join some of the most esteemed and integral members of the Drug Discovery & Development world as they give insights & introspect into the latest movements, discoveries and innovations within the industry. senglobalcoms.com



Introduction
Influenza diagnosis, surveillance and monitoring, is currently often based on detection and characterisation of influenza virus by molecular tests. However, in some cases, serological analysis become the preferred option due to lack of access to samples from acutely affected animals, due to convenient access to blood samples collected and used for the screening of other diseases or, for seroprevalence studies. Serological diagnostic approach usually consists of antibody detection by Enzyme-Linked Immunosorbent Assay (ELISA) usually targeting the nucleoprotein of influenza A virus (IAV) and/or Hemagglutination Inhibition (HI) test targeting hemagglutinin (HA)-antigen. ELISA is a highly sensitive test and several kits are commercially available. HI allows for subtyping based on HA but it is highly dependent on the antigen used in the test. ELISA is therefore mainly applied for testing freedom of infection and prevalence determination purposes, while HI is recommended for the determination of the immune status in individual animals or populations post-vaccination (WOAH, 2023. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 12th edition). Due to the known high diversity and constant evolution of IAV strains, reference antigens used in the HI test aiming for antigens with broad cross reactivity and reflecting contemporary circulating field strains should be ideally updated. A logic consequence is the limited harmonisation of diagnostic protocols across labs and countries, all adjusted to the characteristics of regional circulating strains, challenging the crosscountry sero-surveillance of swine influenza.
Proficiency testing schemes (PTS) are inter-laboratory testing schemes to determine the performance of individual laboratories for specific tests and to monitor laboratories’ continuing performance. These programs are of special interest for viruses such as Influenza or Porcine Respiratory and Reproductive Syndrome that evolve so rapidly. In these cases, it is essential to assess the availability of the tests to properly detect new emerged strains. Royal GD (GD) is accredited according to NEN-EN-ISO/IEC 17043:2010 to perform PTS. Every year, GD organises a PTS for swine influenza antibody detection for both ELISA and HI. The aim of this study was to analyse the results of the participating laboratories in the PTS organised by GD for the ELISA and HI of the last six years in order to get insights into the performance of the serological tests throughout the years, as well as to detect potential challenges on the detection of swine influenza infections.
Methods:
Anonymised data from GD PTS Swine Influenza Virus antibody detection in serum results from 2018 to 2023 were used. Annually, eight lyophilized samples (selected from a set of 12 available samples) were sent to participating laboratories to be tested in duplicate. All samples originated from animal experiments with known infection and or vaccination moment. The average participation rate, overall agreement of results between laboratories and laboratory performance were

assessed. In addition, the qualitative agreement between ELISA and HI were determined. Sample-agreement was determined separately for screening ELISAs and HI subtype-specific (H1N1, H1N2 and H3N2) individually. The sample-agreement (%) was assessed by determining the qualitative sample status (e.g. positive or negative), number of participants with true qualitative results and total of reported qualitative results for that sample. The sample-agreement (%) was then calculated by dividing the number of participants with true qualitative results by the total of reported qualitative results by test system for that sample. Best-case sample-agreement (%) where samples reported as ‘suspected’ were evaluated according to the true qualitative value of the sample and worst-case agreement where samples reported as ‘suspected’ were defined as false.
On average, 23 laboratories participated per year in the period 2018–2023. Thirteen laboratories participated at least four PTS using ELISA, five laboratories participated at least in four PTS using HI and 4 laboratories participated in at least four PTS using both ELISA and HI during this period.
Different influenza screening ELISAs kits were used, both commercial and in-house tests. Best-case and worst-case annual sample-set inter-lab agreement was on average 98.0% and 97.5% respectively for laboratories using ELISAs. For eight selected samples which were included for at least four years during that period, best-case and worst-case sampleagreement was on average 99.0% and 98.7% respectively.
Yearly, on average six laboratories participated using HI assays. In contrast to ELISAs results, the average inter-lab agreements of laboratories using HI assays were lower, being 73.5% and 68.2% for H1N1, 82.5% and 77.2%for H1N2 and 91.7 and 85.3% for H3N2 for the best and worst case, respectively, for selected samples included for at least four years during the period of 2018 and 2023 (H1N2 HI results of 2020 could not be retrieved).
Interestingly, almost all laboratories using ELISA reported qualitative correct results for serum samples positive for one
Less interactions of drug molecules and formulations with the inner glass surface
• Optimized lyophilization process (less fogging)
• Reduced risk of glass delamination

Water for injection | Aqueous NaCl solution Lower pH shift
• Reduced risk of glass delamination


influenza virus subtype (e.g. H1N1, H1N2 or H3N2) and serum samples positive for all three influenza virus subtypes after vaccination including all three subtypes. However, laboratories performing HI were more likely to report false negative results for serum samples positive for one influenza virus subtype (e.g. H1N1) when testing with subtype specific antigens (e.g. H1N1). In addition, inconsistent results were reported for serum samples obtained after vaccination including all three subtypes with laboratories reporting positive results using the HI with H1N2antigen and H3N2-antigen while reporting negative results using the HI with H1N1-antigen.
Laboratories participating with commercial ELISAs showed overall good performance. Only one laboratory participated using both an in-house ELISA and commercial ELISA showing more ‘suspected’ or doubtful results with the in-house ELISA compared to the commercial ELISA. Laboratories using HI showed, however, considerable variation in both qualitative and quantitative results among labs and throughout the years following the reused samples. No laboratories used similar subtype-specific antigens for the HI tests, supporting the not only subtype-, but also antigen-specific sensitivity of the HI test.


Lucía Dieste Pérez, DVM, MSc, PhD graduated from the University of Zaragoza with a DVM and MSc in Veterinary Science Research in 2009 and 2010 respectively. After working for 5 years as a research assistant in the animal health department at the Agrifood Research and Technology Centre of Aragon (CITA), Spain, she obtained her PhD in 2015 and came to the Netherlands to work at the Faculty of Veterinary Medicine in Utrecht, where she was involved in research and education on swine health and management. Since 2017, she's been working at the Swine Department of the Royal GD, currently as a Senior Veterinary Researcher, where she is performing applied research projects specially on swine infectious diseases.

Lianneke Bosma, MSc graduated from Wageningen University & Research with a MSc in Animal Sciences in 2020. Since 2020 she’s been working at the R&D Department of Royal GD, currently as a Junior Researcher, where she is involved in immunodiagnostics of infectious diseases of swine and poultry.

Huub van de Sande, since 1982 working at Royal GD. With a background in virology and immunology, now product specialist Proficiency testing schemes and diagnostics.
CPHI Milan connects you to the global pharma community to level up your business at the heart of our industry.
Learn more


8-10 October 2024
Fira Milano, Italy



Mycotoxins are naturally occurring, toxic secondary metabolites produced by fungi that can be found in feedstuffs such as cereal grains and their byproducts. The consumption of mycotoxins by poultry animals can result in several effects, both direct and indirect.
A recently published meta-analysis titled “Meta-Analysis of the Effects of Yeast Cell Wall Extract Supplementation during Mycotoxin Challenges on the Performance of Laying Hens,” authored by Dr. Alexandra Weaver, Dr. Daniel Weaver, Nick Adams and Dr. Alexandros Yiannikouris, explores the impact of yeast cell wall extract (YCWE, Mycosorb®, Alltech) on laying hens facing mycotoxin challenges.
Understanding the Study
A meta-analysis is a statistical technique used in research to combine and analyse results from multiple independent studies on a particular topic. The process involves literature reviews, the use of inclusion and exclusion criteria, data extraction, statistical analysis, and interpretation of the results. Meta-analysis is particularly useful when individual studies may have limitations, or when there is a need for a more robust, reliable and comprehensive understanding.
25 trials investigating the use of YCWE in hens were found following the literature search, with 8 trials being selected that met selection inclusion. These selected trials represent 12 treatments involving 1,774 laying hens. The average length of the trials was 9.5 weeks. Treatments included control (CTRL), challenge with mycotoxins (MT), and use of YCWE during a mycotoxin challenge (YCWE+MT).
A total of 8 mycotoxin types were identified:
• Aflatoxin (AF): Produced by Aspergillus flavus mold, typically during warm and dry field conditions or in the presence of high moisture levels during crop storage. AF causes a variety of effects in animals, including organ damage, poor growth or efficiency, and higher mortality.
• Ochratoxin A (OTA): Produced by Penicillium molds. Poultry that ingest this toxin may show reduced growth and immunity, damage to kidneys or other internal organs, dehydration, or higher mortality.
• Deoxynivalenol (DON)/15-acetyl-DON (15ADON)/ DON-3-glucoside (DON-3-G): Members of the type B trichothecenes family, produced by Fusarium molds. When consumed by poultry, these toxins can cause digestive disorders, lower feed intake and growth, reduced immunity, and damage to internal organs.
• T2-toxin (T2): A member of the type A trichothecenes family, T2 is produced by Fusarium molds. This mycotoxin can have a strong impact on the digestive system, causing oral and gut lesions as well as hemorrhagic and other digestive disorders. Affected birds may also have poor growth or feed intake, damage to internal organs, and increased mortality.
• Fumonisins (FUM): Produced by Fusarium moulds, often when dry and warm conditions during crop flowering are followed by wet and warm temperatures closer to harvest. FUM can cause digestive disorders, alter immune response, and damage internal organs.
• Zearalenone (ZEA): Produced by Fusarium species, ZEA mimics the action of hormones as an oestrogen analogue. This can lead to reproductive challenges. ZEA can also hamper immunity.
To compare the mycotoxin scenarios included in the research to real-life challenges, we can look at the results of extensive Alltech 37+® laboratory analysis over the past 3 years. A total of 848 samples of finished feeds for layers/ breeders, gathered from locations around the world, showed an average of 8.2 mycotoxins per sample, with 97% of samples containing 2 or more mycotoxins. This is significant because multiple-mycotoxin challenges can increase the overall risk to the bird, combining to cause even greater damage than each toxin would cause on its own.
Additionally, some of the most prevalent mycotoxin groups in these feed samples were similar to those used in the studies in this meta-analysis, including the type B trichothecenes (found in 86% of samples), fumonisins (79%) and zearalenone (41%). As such, the results of the meta-analysis could be applied to the typical laying/breeding hen.
Other notable mycotoxins of high occurrence in feed samples collected globally included emerging mycotoxins (89%) and fusaric acid (85%), though these were not included in the majority of tests conducted for research trials.
The meta-analysis revealed that layers fed mycotoxins experienced lower body weight, decreased egg production, and reduced egg weight compared to control-fed birds. However, birds supplemented with YCWE during mycotoxin challenges showed significant improvements in performance, with increased egg production and egg weight. Through greater egg production and weight, total output per hen increases, resulting in enhanced profitability. In fact, this meta-analysis indicated that YCWE inclusion offered a 4.65:1 return on investment (ROI) when used during mycotoxin challenges.
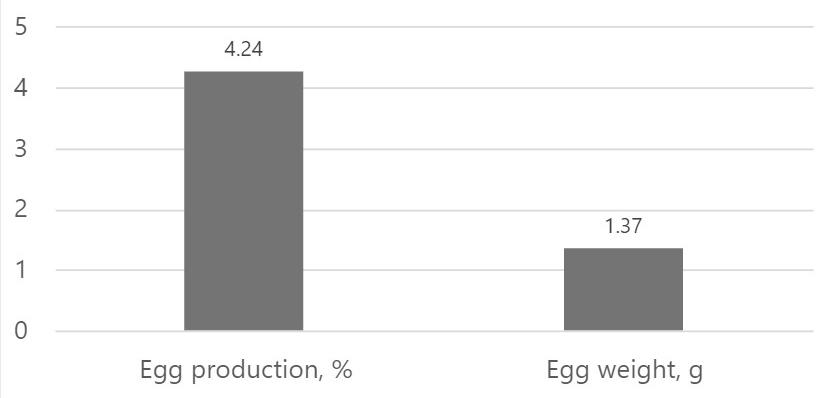

These results demonstrate that YCWE, in the form of Mycosorb, offers direct and significant reduction of the impact of mycotoxins within the animal. This is because it is rich in complex insoluble carbohydrates, enabling it to interact with and mitigate the effects of a wide range of mycotoxins.
These results are significant not only for producers but for human health and environmental sustainability. Egg production is estimated to require less land and energy use than many other animal agriculture types, and it has a lower global warming potential. As such, eggs are a particularly sustainable way to provide multiple nutrients – including the protein increasingly needed to support a growing global population. When mycotoxins were consumed by hens, total edible protein production decreased by 43.9 grams per hen housed over 9.5 weeks, but the inclusion of YCWE during these mycotoxin challenges increased edible protein output by a full 29.7 grams.


To the researchers’ knowledge, this is the first time a metaanalysis has been conducted with laying hens to evaluate the influence not only of mycotoxins alone, but also of a mycotoxin mitigation strategy, on key performance parameters. These findings underscore the importance of addressing mycotoxin


challenges in layer production and highlight the potential role of YCWE in doing so.
It is clear from these findings that efforts to minimise the risk of mycotoxins are crucial to supporting poultry health and performance. As such, proactive measures should be introduced to better understand and control mycotoxin risk.
A proper mycotoxin management program should include:
1. Testing, to determine the current mycotoxin risk
2. Risk assessment, to better understand the mycotoxin challenge level and the potential outcomes of mycotoxin consumption
3. Mitigation, to reduce the challenge to the animal
4. Monitoring, to continue assessing the mycotoxin challenge over time
Conclusion
This meta-analysis on the effects of yeast cell wall extract (YCWE) supplementation during mycotoxin challenges on the performance of laying hens, sheds light on the intersection of mycotoxin management and layer production. The findings underscore the significant impact of mycotoxin exposure on body weight, egg production and egg weight in laying hens, while also highlighting the potential benefits of YCWE supplementation in mitigating these adverse effects.
By revealing the positive outcomes associated with YCWE supplementation, including increased egg production and egg weight, alongside a potential return on investment, this study offers actionable insights for poultry producers seeking to optimise their operations. Furthermore, it underscores the importance of proactive mycotoxin management strategies, including testing, risk assessment, mitigation and monitoring, in safeguarding the health and productivity of laying hens.

Dr. Alexandra Weaver earned her master’s and Ph.D. in animal science and nutrition from North Carolina State University, where she conducted her research under the guidance of Dr. Sung Woo Kim. Her dissertation, “The Impact of Mycotoxins on Growth and Health of Swine”, explored the effects of mycotoxins on swine performance, immunity, oxidative stress, gut health, and reproductive capacity. Alexandra recently assumed a new role providing global technical support for the core Technology Group at Alltech. Prior to this, she spent over 10 years as a member of the Alltech Mycotoxin Management team, where she developed a special interest in creating software to track mycotoxin risk and evaluate their physical and financial impacts on animals. Alexandra has published numerous research articles in peer-reviewed journals and is dedicated to helping producers and nutritionists manage mycotoxins across various animal species.

Sticking is often described as the Achilles’ heel of tablet production. It is one of the most common tablet tooling problems and can have a profound effect on production, leading to reduced output and increased costs. This article explains why sticking occurs and proposes several solutions.
Sticking can be confused with picking, which is another common manufacturing problem. However, they are fundamentally different. Sticking is when granule builds up on the punch tip face or die bore. Picking is compressed granule that has adhered to the embossing detail on the punch face, resulting in the “picking out” of parts from the tablet face.
Both issues have a negative effect on tablet appearance and can become so serious that production is interrupted. In extreme cases, punches need to be removed and cleaned. This is disruptive, labour intensive, reduces output and increases production costs.
There are many reasons why sticking can occur, and while it can feel like a challenge to resolve, there are solutions available.
There is no simple answer to this question. It can be due to any number of reasons, from the physiochemical properties of the formulation to the surface characteristics of the punch face. It can also be related to the machinery, for example the compression force and speed, as well as the environmental conditions like temperature and humidity.
The granule within a formulation can be highly influential when it comes to sticking. Simply put, sticking is the battle between the cohesive forces holding a tablet together and the adhesive forces between the ingredients in the formulation. When the difference in force is significant and adhesive forces are higher, sticking occurs. There are three factors to consider when understanding the cohesive forces. The first is Van der Waals. This is the attraction of intermolecular forces between molecules and is affected by surface roughness and the effective contact area. Essentially, molecules can attract each other at moderate distances and repel at close range. Although these forces are very small, in mass they combine not only increasing cohesive forces within a tablet but also creating adhesive forces (when interacting with the elements in the tools steel or coating), which can cause sticking.
Capillary action is the second cohesive force created when moisture condenses into the gap between a particle and the surface, causing a capillary bridge. The strength of these forces can depend upon relative humidity, gap geometry and surface chemical condition. Capillary bridges increase the cohesive forces that bind a tablet together, and like Van der Waals, they can also be detrimental by increasing the adhesion forces between the granule and the punch tip faces, causing sticking. This is why it’s important to control
the environment in all areas of tablet production and reduce humidity.
The third cohesive force is electrostatic. This is when tribocharging occurs between contacting materials. It forms static electricity and is evident when the granule is very dry. The resulting force can be fairly strong and long-ranged, creating cohesive and adhesive forces. The environment is the biggest influence on adhesive forces causing sticking between the formulation and punch tip faces. Temperature can affect some active pharmaceutical ingredients (APIs). A good example of this is Ibuprofen, which has a very low melting point. For this reason, it is important to control the temperature in any area where the tabletting process takes place. Keeping the temperature at a constant low level will reduce sticking.
Humidity is also a problem. Moisture within a tablet can strengthen the adhesive force. This occurs with an increase in capillary action between the tooling surface and the granule. Capillary bridges form, causing high adhesion areas, creating sticking. Moisture can enter the process during wet granulation or from excess humidity in the compression chamber in a non-environmentally controlled area – the latter can even affect direct compression formulations.
Deformation Mechanics
Deformation Mechanics are another adhesive force that can cause sticking. The physical properties of the granule being compressed are either elastic or plastic. When under compression, a particle will either remain deformed or return to its original shape. Formulations with more time dependent consolidation behaviour will need a longer dwell time to form strong bonds between the particles. Extended dwell time at main compression is, therefore, more important for formulations with predominantly elastic deformation behaviour, rather than brittle fracture behaviour. In these instances, extended dwell flat tooling will enable a suitable compression dwell time for a formulation without the disadvantage of slowing the press.
Sticky Granule: Solution
Overcoming sticky granule problems can be a complex task. A good starting point is to optimise the environment in which tablet compression takes place and areas where tooling and granule are stored with the correct temperature and humidity controls.
Consider your tablet tool coating. Choosing an anti-stick coating is essential, as conductive or non-conductive tool coatings or treatments can also influence cohesive and adhesive forces.
Specialised punch and die coatings can have a huge impact on the productivity of tablet manufacture. When used in conjunction with high-quality tooling steel, they allow for better tableting efficiency and output.
Surface Finish: Problem
The morphology or surface roughness of a punch tip can affect tablet production. While there is the perception that polishing the punch tip face to a mirror finish will prevent sticking, this is not the case. Research on the interaction

of different surface textures has shown that the standard specification for tablet punch tip faces can include a range of surfaces, which interact with formulations in different ways.
The required standard surface for a punch tip is between 0.1 to 0.025Ra (roughness average). This variance can have a huge effect on sticking. Each coating’s surface condition works differently because of the surface roughness. In order to prevent sticking, it’s important to understand the effect on the interaction between granule and surface finish.


The punch surface can be determined in a number of ways, such as:
• Measuring the surface roughness after polishing using optical surface profilometry
• Using scanning electron microscopy to study the structure of the steels and coatings
• Measuring adhesion forces to create a nanoscale map of the surface.
By investigating the surface condition, the correct coating can be applied.
Tool maintenance should also be addressed. Assessing and polishing tooling are crucial steps to ensure that any tooling defects can be dealt with early. Automated polishing using a MF polishing machine is the best method to ensure a suitable surface will be maintained. This also repairs small areas of damage and abrasion that occur during production.



By restoring the optimum finish of the tooling, problems like sticking or picking can be prevented.
Insufficient tablet compaction is another cause of sticking. In a tablet with decreased hardness, the cohesive force can be less than the adhesive force to the tooling surface. This is a common problem with effervescent tablets as they require a lower hardness to help increase the dissolution rate. Tablet design also has an influence on sticking. A typical indicator is when sticking is found around a tablet’s core. However, it can occur in other parts of the tablet as well.
The principal reason for insufficient tablet compaction is an uneven distribution of compaction forces across the tablet. This is usually due to the shape of the tablet and how concave it is. With a concave tablet profile, the core can be lower in hardness, causing sticking to the centre of the punch face. This occurs because a concave punch tip profile compresses

the formulation around the edge of the tablet more than in the centre due to the tablet profile.
Specially designed tooling with an elliptical head is one way to increase the dwell time, helping the granule to consolidate during compaction without automatically increasing tablet hardness. Dwell time is defined as the amount of time each individual punch head flat is in contact with the compression roller of a rotary tablet press when the compression force applied to form the tablet is above 90% of its peak value.
Adding either a coating or a polymer insert will also help to reduce the adhesive force.
A tablet design with a flatter profile and changing from a deep single radius to a double radius, reduces the soft area


in the centre of the tablet with the lowest cohesive force. This results in an even tablet hardness, helping to decrease sticking issues by increasing the cohesive forces.
Changing the profile to a double radius can also be beneficial because an even coating thickness is easier to achieve and branding or required markings like break-lines can be accommodated.
Sticking is detrimental to tablet production. The knockon effect is costly as tablet press downtime and reduced productivity is inevitable. Sticking must be resolved in order to mass produce quality tablets. There can be many reasons for the cause of sticking from Van der Waals forces to capillary action associated with high moisture content, or conversely, from static electricity generated in very dry conditions. Because the physical properties of any sticky formulation are unique, there is no one-size-fits-all anti-stick solution.
It is important to contact an experienced tooling manufacturer who understands the science behind tablet production and can introduce innovative solutions to tabletting problems, therefore, maximising production capabilities.

Since joining I Holland in 2004 Rob has been instrumental in the development of I Holland's PharmaCote® range of surface treatments and coatings for tablet compression tooling designed to improve properties such as wear resistance, corrosion resistance and antistick characteristics. He was also part of the Eurostandard steering committee and responsible for I Holland's registration to ISO 9001:2008. Rob holds multiple patents linked to solid dose manufacture. Rob also co-ordinates I Holland's close collaboration with various respected.






OBC Alltech
Page 31 CPHI 2024
IBC Trilogy Writing & Consulting Gmbh
Page 15 Autoscribe Informatics
Page 3 Moredun Scientific
Page 37 Natoli Engineering
Page 29 Nipro
Page 13 Nordson
Page 5 PCI Pharma Services
IFC Royal GD
Page 18–21 Vetoquinol
Page 27 Senglobal Ltd
Subscribe today at www.international-animalhealth.com or email info@senglobalcoms.com

www.twitter.com/AHMJournal www.facebook.com/Animal-Health-Media www.plus.google.com/+Animalhealthmediajournal www.animalhealthmedia.tumblr.com/



