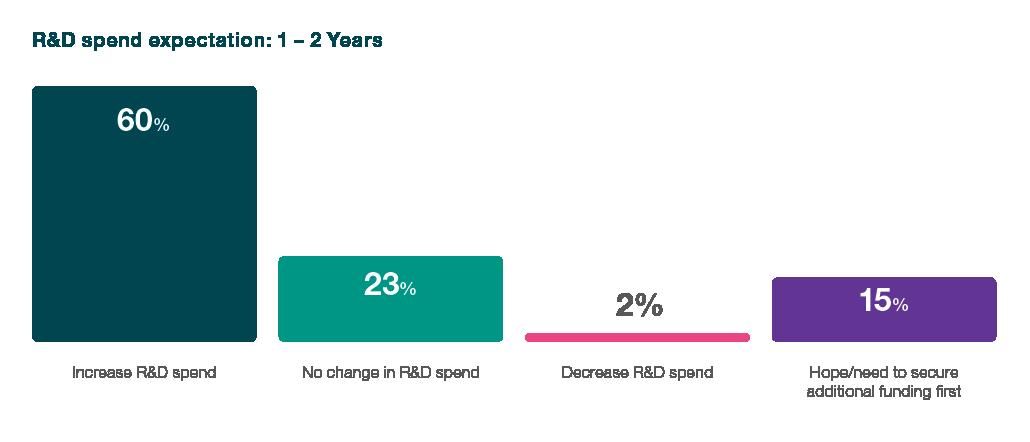Considerations on the Development and Manufacturing of Generic Peptides
Patients Are Waiting: Speeding Time to Treatment in Rare Disease
Biotech Sector Survey: From Investment Confidence to Strategic Collaborations
Using Predictive Compliance Risk Analysis to Avoid FDA Penalties
Sponsor Company:


www.international-biopharma.com Volume 7 Issue 1 Peer Reviewed
Stimulate your cell proliferation and productivity with Recombinant Insulin
Grow your cells with Recombinant Insulin
Are you working with biomanufacturing of therapeutic proteins or regenerative medicine and require highquality, animal-free, and reliable ingredients for your cell culture media formulation?
Recombinant Insulin from Novo Nordisk Pharmatech is produced by the world’s largest insulin manufacturer, Novo Nordisk. It is specifically manufactured for use in cell culture media, supported by a dedicated team of insulin experts, and securely supplied around the globe.
Learn more about our Recombinant Insulin and request a sample at novonordiskpharmatech.com

II INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
DIRECTOR: Mark A. Barker
INTERNATIONAL MEDIA DIRECTOR: Anthony Stewart anthony@senglobalcoms.com
EDITORIAL MANAGER: Beatriz Romao beatriz@senglobalcoms.com
DESIGN DIRECTOR: Jana Sukenikova www.fanahshapeless.com
FINANCE DEPARTMENT: Akash Sharma accounts@senglobal.co.uk
RESEARCH & CIRCULATION: Jessica Chapman info@senglobalcoms.com
COVER IMAGE: iStockphoto ©
PUBLISHED BY: Senglobal ltd.
Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0)20 4541 7569
Email: info@senglobalcoms.com www.international-biopharma.com
All rights reserved. No part of this publication may be reproduced, duplicated, stored in any retrieval system or transmitted in any form by any means without prior written permission of the Publishers.
The next issue of IBI will be published in Summer 2024. ISSN No.International Biopharmaceutical Industry ISSN 1755-4578.
06 Biotech Sector Survey: From Investment Confidence to Strategic Collaborations
In the rapidly evolving landscape, ICON’s recently published biotech sector survey provides a roadmap through the intricacies of investment confidence, funding dynamics, and the pivotal role of strategic collaborations. The survey, conducted on ICON’s behalf by Citeline, included 133 respondents, predominantly located in Europe and North America, from small pharmaceutical and biotech companies, mid-size pharmaceutical and biotech companies, to large biotech or venture capital organisations. Dr. Chris Smyth, President, ICON Biotech explores the imperative of partnerships, delving into their role in funding strategies, and the increasing trend of pharma collaborations, even at the preclinical stage.
12 Extractables and Leachables for Inhaled Medicines
The structured approaches encouraged by the FDA, EMA, and other regulatory bodies require additional upfront resources compared to a more traditional approach. Performing an initial assessment of materials used in the product and developing a more thorough understanding of products earlier in their development allows more appropriate experiments on the higher-risk aspects rather than generic approaches covering all materials. Paul Hardman at Broughton, outlines the best way to optimise your extractables and leachables strategy.
RESEARCH / INNOVATION / DEVELOPMENT
20
Using Predictive Compliance Risk Analysis to Avoid FDA Penalties
In the complex landscape of global regulations, particularly within healthcare and pharmaceutical sectors, a robust compliance strategy is essential for maintaining the integrity of businesses amidst rigorous regulatory requirements. Organisations need to be both flexible and resilient, adept at navigating and anticipating the multifaceted and ever-changing aspects of global compliance standards. These standards are often stringently enforced by regulatory bodies, with the U.S. Food and Drug Administration (FDA) being one of the most prominent and rigorous. In this article,
responsible for any loss or damage incurred. This publication is protected by copyright.

INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 1 www.international-biopharma.com
Senglobal ltd. Volume 7 Issue 1 – Spring 2024
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held
2024
& COMPLIANCE
04 Foreword REGULATORY
Contents
Hannah Crystal T. Jurolan and Joe Kalina at Xybion explain how companies can avoid FDA penalties using predictive compliance risk analysis.
THERAPEUTICS
28 Patients are Waiting: Speeding Time to Treatment in Rare Disease
Companies are overcoming challenges to develop, launch, and educate on new rare disease medicines faster. Rare diseases are no longer rare. Every year, people’s lives are upended by a diagnosis of one of 6,000-8,000 identified rare conditions, which collectively affect one in 17 people. Because a majority are genetic and appear early, more than half of these patients are children, and many are not expected to reach their fifth birthday. Chris Moore at Veeva Europe, explains the way to improve the treatment of rare disease.
MANUFACTURING & PROCESSING
30 Elevating Biomanufacturing Efficiency with N-1 Perfusion Technology
In the dynamic world of biomanufacturing, the quest for improved efficiency and productivity continues to shape industry advancements. Among these innovations, the emergence of N-1 perfusion technology stands out as a pivotal milestone, offering a practical pathway to enhance production processes and boost overall efficiency. Sridevi Khambhampaty of Syngene outlines how this strategic approach aims to maximise the output of manufacturing facilities and streamline the timelines of production.
32 Considerations on the Development and Manufacturing of Generic Peptides
The development of generic versions of peptides such as Semaglutide, the drug substance inside Novo Nordisk’s blockbuster drugs like Ozempic®, Rybelsus® or Wegovy®, or Tirzepatide, the API in Eli Lilly’s Mounjaro®, has emerged as a significant area of interest and opportunity. However, generic companies face critical decisions regarding the choice between recombinant and synthetic semaglutide. The path chosen can significantly impact regulatory approval, safety considerations, and market competitiveness. Rafael Antunes at Aurisco Pharmaceutical outlines some considerations on the development and manufacturing of generic peptides.
TECHNOLOGY
42 Ensuring Image Integrity When Reviewing Western Blots
Western blots are frequently included in scientific papers to report results when analysing proteins in a sample. However, research shows that Western blot images are a common source of integrity issues, the publication of which can be harmful to journals. Here, Dr. Dror Kolodkin Gal at Proofig AI, explores the challenges editors and publishers face when reviewing Western blot images and suggests how to identify issues more effectively before publication.
APPLICATION NOTES
05 Simple Analysis of Impurities in Oligonucleotide Therapeutics Using a Single Quadrupole Mass Spectrometer
Oligonucleotide therapeutics have attracted attention in recent years as a new modality for drug discovery because they can be used to create disease-specific therapeutic agents and can be
designed easily by chemical synthesis. Shimadzu will analyse the level of Impurities in Oligonucleotide Therapeutics Using a Single Quadrupole Mass Spectrometer.
16 A Guide to Understanding and Performing all Appropriate Validation Steps When Adopting a New Endotoxin Testing Reagent
The adaptation of in-house endotoxin testing for a pharmaceutical or medical device manufacturer can be a daunting task. Many small-volume manufacturers find themselves in one of two common situations. Either they are utilising a contract testing organisation (CTO) and paying a great deal of costs, or they have adopted a solution that is set up by the manufacturer with much of the validation services outsourced. Timothy Francis at FujiFilm Wako Chemicals shows us a guide to understand and perform all appropriate validation steps when adopting a new endotoxin testing reagent.
24 Optimising Pharmaceutical Processes: A Guide to Lyophilisation Cycle Development
Lyophilisation, commonly known as freeze-drying, is a critical unit operation in the pharmaceutical industry used to preserve and stabilise both small and large-molecule drug products and biologics, including monoclonal antibodies, vaccines and peptides. Lyophilisation is a process that involves freezing a liquid drug product and then removing the frozen solvent via sublimation, providing a stable solid matrix of drug product and other excipients. Lyophilisation cycle development is not only a science, but an art; each drug product that comes into the laboratory presents unique challenges, and the design of a cycle requires an understanding of individual chemistry, characteristics, and interaction to yield a high-quality product in every cycle. While there are a myriad of tools and techniques to perform, the below is an overall guide to the lyophilization process, and some of the steps needed for success. Matt Bourassa at PCI Pharma Services guides us to lyophilisation cycle development.
36 5,000L Single-use Bioreactors: The Next Generation in Biologics Manufacturing
The Thermo Fisher Scientific HyPerforma™ DynaDrive™ Single-Use Bioreactor line is suited for volumes ranging from 50L to 5,000L. Optimised for modern cell culture processes in a scalable, ergonomic design, the platform allows intensified, flexible manufacturing, enhanced by high-power input per volume, and better volumetric mass transfer performance. This article highlights the features and benefits of the DynaDrive™ SUB platform and explains how any new or existing facility can leverage the platform to achieve expected development and manufacturing objectives. From pre-clinical trials through commercialisation, manufacturers and companies looking to outsource their biologics can use the DynaDrive™ SUB to gain maximum efficiency and flexibility across a wide range of processes, cell lines, and molecules.
39 Enhancing Process Flexibility with Automated Filling
During the production and filling of highly complex biopharmaceuticals in cell and gene therapy, precise flow measurement and accurate air bubble detection play a crucial role. Flow meters and air bubble detectors ensure consistently high product quality to provide patients with effective and safe drugs. Using the example of the RoSS.FILL platform, developed by the Austrian company Single Use Support, ultrasound specialist. Nico Polley at SONOTEC explains how non-contact clamp-on flow meters and air bubble detectors can significantly increase the accuracy of automatic filling.
2 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Contents
MISSION
#OneStopShop for stability and release testing

Since 1995 A&M STABTEST has been a household name for highest quality analytical services “Made in Germany” Our 340 strong team of dedicated specialists and stateof- the - art equipment get your projects done on time and in budget. We are your #OneStopShop solution for:
• Stability and release testing of small molecule and biological drug substances and drug products
• Analytical development and method validation
• mRNA -LNP ready methods acc. to draft USP guideline
• Dedicated cell-based potency assay group
• ICH- stability storage and logistics service



• Laboratory dedicated to inhaled drug testing (DPI, MDI, Nebulizer)
• GMP compliant LC -MS service for extractable and leachable testing, protein characterization, identification of unknowns and quantification of impurities and excipients
• Functional testing of container closure systems, prefilled syringes and injection devices (CCIT, force measurements)
• Analysis of ATMPs


A&M STABTEST; Kopernikusstrasse 6; 501026 Bergheim; Germany
#CONTACT www.am-labor.de anfragen.BM@am-labor.de

Although this is the first issue of the year, we are already well into 2024. And of course, I am far too late for a review of last year, but before we look forward and into this is issue of IBI, please just let me shine the light on last year’s FDA list of Novel Drug Approvals. In 2023 the pharmaceutical industry received FDA´s approval for 55 new molecular entities (NME), the second highest NME approval since 2015. I personally think this is remarkable for two reasons. First, how efficient the life science and pharma industry are in translating new scientific discoveries into live saving or at least live improving products. Second, the community behind transforming scientific discoveries into new drugs should be highlighted as a role model for a globalized society. In my role at A&M STABTEST I see many different companied from startup to big pharma and I am always amazed by the many different nationalities, cultural backgrounds, female/male working together to discover and develop great things. Looking at the state of the world today and the trend to a “My-Nation-First” mentality, I think we should highlight and openly celebrate what we can achieve, if we work together rather than cut ourselves of form others, only because they look different, come from a different cultural background or want to live their lives differently.
Looking at 2024 NME approvals, we already have 6 novel drugs (as of March 22nd, 2024) approved., already very good! New drugs need to be produced to make them available to the patients that need them. This issue of IBI again host different articled offering solutions and guidance for production challenges.
First of, is an interesting article by Rafael Antunes at Aurisco Pharmaceutical exploring the differences between recombinant production versus chemical synthesis of generic peptides and the implications for regulatory approval of a generic product produced by different means.
Thermo Fisher Sientific showcases their HyPerforma™ DynaDrive™ Single-Use Bioreactor, which offers great flexibility from 50 to 5.000 liters. Ideal for scale up from early phase demand to commercial scale production.
IBI – Editorial Advisory Board
• Ashok K. Ghone, PhD, VP, Global Services MakroCare, USA
• Bakhyt Sarymsakova – Head of Department of International Cooperation, National Research Center of MCH, Astana, Kazakhstan
• Catherine Lund, Vice Chairman, OnQ Consulting
• Cellia K. Habita, President & CEO, Arianne Corporation
• Chris Tait, Life Science Account Manager, CHUBB Insurance Company of Europe
• Deborah A. Komlos, Senior Medical & Regulatory Writer, Clarivate Analytics
• Elizabeth Moench, President and CEO of Bioclinica – Patient Recruitment & Retention
• Francis Crawley, Executive Director of the Good Clinical Practice Alliance – Europe (GCPA) and a World Health Organisation (WHO) Expert in ethics
• Hermann Schulz, MD, Founder, PresseKontext
• Jim James DeSantihas, Chief Executive Officer, PharmaVigilant
Lyophilization, it the process is setup well, is a great means to formulate biopharmaceuticals and small molecules alike. Lyophilized products can often be stored at higher temperatures, e.g., at 2–8°C or room temperature rather than frozen at -20°C or even lower. This decreases costs for warehousing and shipmen, but also increases availability of the drugs to places with less developed infrastructure. Matt Bourassa at PCI Pharma Services, gives a comprehensive guide to the techniques of lyophilization.
Timothy Francis at FujiFilm Wako Chemicals giving advice on what to consider and how to validate Endotoxin test when implementing new reagents.
Patients all over the word trust that the drugs they are receiving are foremost save and efficacious. This trust is built on the fact that pharmaceutical companies and their supplier have to adhere to a complex set of rules and regulations which are rigorously controlled and enforced by national and international regulatory bodies. As most pharmaceutical companies act globally, they have to adhere to the national regulations of the markets they are operating in, which can be quite different. Hannah Crystal T. Jurolan and Joe Kalina at Xybion showcase how a predictive compliance risk analysis can be applied to navigate the different changing regulation and avoid regulatory findings.
Data integrity in scientific publications is an important as researchers need to be trust that the information conveyed is accurate and untampered. Dr. Dror Kolodkin Gal at Proofig AI, explores the challenges editors and publishers face when reviewing Western blot images and suggests how to identify issues before publication.
Dr. Steven A. Watt, CBDO (Chief Business Development Officer) at A&M STABTEST GmbH

• Jeffrey W. Sherman, Chief Medical Officer and Senior Vice President, IDM Pharma.
• Lorna. M. Graham, BSc Hons, MSc, Director, Project Management, Worldwide Clinical Trials
• Mark Goldberg, Chief Operating Officer, PAREXEL International Corporation
• Maha Al-Farhan, Chair of the GCC Chapter of the ACRP
• Rick Turner, Senior Scientific Director, Quintiles Cardiac Safety Services & Affiliate Clinical Associate Professor, University of Florida College of Pharmacy
• Robert Reekie, Snr. Executive Vice President Operations, Europe, Asia-Pacific at PharmaNet Development Group
• Stanley Tam, General Manager, Eurofins MEDINET (Singapore, Shanghai)
• Stefan Astrom, Founder and CEO of Astrom Research International HB
• Steve Heath, Head of EMEA – Medidata Solutions, Inc
4 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Foreword

Application Note
Simple Analysis of Impurities in Oligonucleotide Therapeutics Using a Single Quadrupole Mass Spectrometer
Oligonucleotide therapeutics have attracted attention in recent years as a new modality for drug discovery because they can be used to create disease-specific therapeutic agents and can be designed easily by chemical synthesis.
Typically, they are composed of oligonucleotides with about a dozen to several dozen bases (including modified bases). However, the development of analytical methods for quality assurance and standardization is still in progress. Quality control requires analysing impurities, such as by-products, unreacted residues, and degradation products, in addition to the principal components. HPLC-UV is commonly used for purity confirmation, but if impurities are detected, they must be checked to confirm whether they are known impurities or not. Mass spectrometry, which provides molecular weight information, is a valuable analytical tool in such cases. This application note describes an analysis of oligonucleotides and related impurities using an inert UHPLC system and a

single quadrupole mass spectrometer. Oligonucleotides and related impurities can be easily analysed using a Nexera XS inert UHPLC system and a LCMS-2050 single quadrupole mass spectrometer.
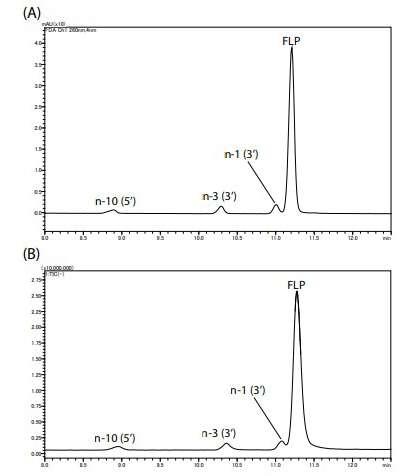
Figure 2 shows the UV (260 nm) and TIC chromatograms of the model oligonucleotides. Peaks were confirmed in the order of n10 (5’), n-3 (3’), n-1 (3’), and FLP. The mass spectra of impurities and FLP are shown in Figure 3. Multiply-charged ions (3 to 11 charges) were detected.
Marketing Communication Europe, Shimadzu Europa GmbH
Albert-Hahn-Str. 6–10, D-47269 Duisburg, Germany
Tel.: +49 (0)203-7687410
Email: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 5 www.international-biopharma.com BIOPHARMACEUTICAL 5
Figure 3 – Mass Spectra of Impurities and FLP
Figure 2 – Chromatograms of Model Oligonucleotide (A) UV Chromatogram, (B) TIC Chromatogram
Figure 1
Regulatory & Compliance
Biotech Sector Survey: From Investment Confidence to Strategic Collaborations
In the rapidly evolving landscape, ICON’s recently published biotech sector survey provides a roadmap through the intricacies of investment confidence, funding dynamics, and the pivotal role of strategic collaborations. The survey, conducted on ICON’s behalf by Citeline, included 133 respondents, predominantly located in Europe and North America, from small pharmaceutical and biotech companies, mid-size pharmaceutical and biotech companies, to large biotech or venture capital organisations.
A compelling narrative unfolds as we dissect the survey results, revealing the industry's resilience and determination despite funding challenges. We explore the imperative of partnerships, delving into their role in funding strategies, and the increasing trend of pharma collaborations, even at the preclinical stage.
Key Survey Findings: Stats and Implications
R&D Spending Expectations
The heartbeat of the biotech innovation lies in its Research and Development (R&D) endeavours, and the survey paints a vivid picture of the sector's commitment to pushing the boundaries despite financial headwinds. 60% of survey respondents expect to increase their R&D spending over the next one to two years, a testament to the resilience that permeates the industry. This statistic is a powerful indicator of the industry's faith in the transformative potential of its research initiatives and the impact these endeavours can have on shaping the future of healthcare.
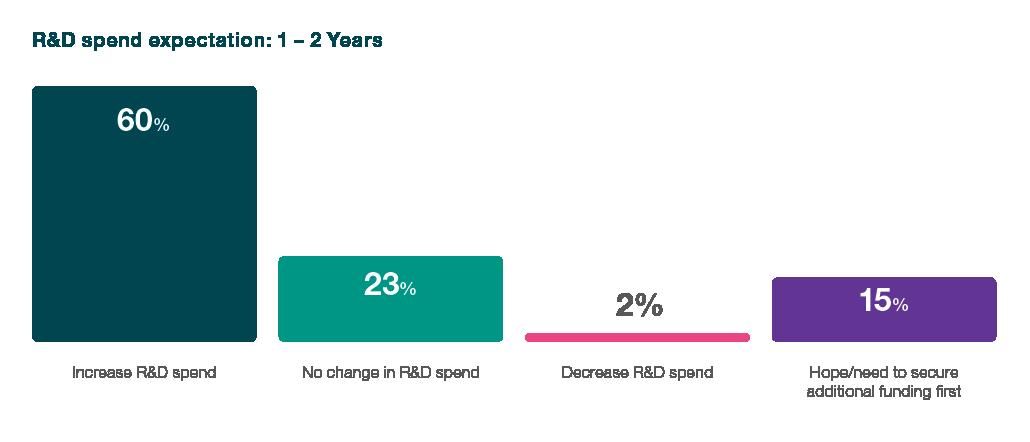
Despite the funding challenges that have become an intrinsic part of the biotech landscape, the overwhelming majority expressing an intent to boost R&D spending sends a powerful signal. It signifies a collective determination to forge ahead with novel research, undeterred by the financial uncertainties. This optimistic outlook not only underscores the resilience of biotech professionals but also speaks to a broader narrative of belief in the value and impact of the biotech innovation on global health.
These statistics symbolise a commitment to progress and a belief in the industry's ability to overcome obstacles. The biotech sector is not merely weathering the storm; it is actively
charting a course toward a future where innovative solutions can address some of the most pressing challenges in healthcare. As the industry braces for an uptick in R&D spending, it positions itself ready and able to unlock new frontiers in science and medicine.
Challenges in the Funding Landscape
In the complex interplay between innovation and financial viability, the survey unearths critical insights into the challenges embedded within the biotech funding landscape. A significant 47% of respondents identify the rising cost of capital as a substantial influencer. This finding underscores the industry's acute awareness of its financial pressures, with the cost of capital emerging as a pivotal factor shaping future operations. As biotechs navigate this upward trajectory in capital costs, strategic financial planning becomes paramount to sustaining momentum in drug development.
Furthermore, the survey reveals that 35% of respondents consider cost management a barrier to innovation. This finding adds a nuanced layer to the funding landscape, highlighting the delicate balance between financial prudence and fostering innovation. The industry's drive to innovate and bring transformative therapies to market is apparent. Yet, the challenges posed by cost management warrant carefully calibrating financial strategies to ensure sustained creativity and progress.
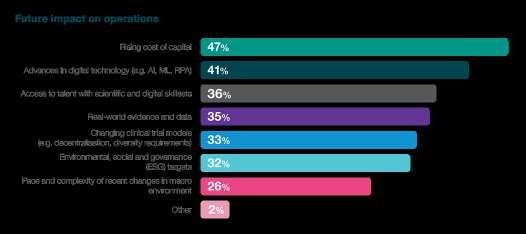
The implication of these findings is significant – the industry is at a crossroads where the imperative to innovate collides with the realities of financial constraints. Biotechs must navigate a delicate dance, strategically allocating resources to foster innovation while managing costs effectively. This challenge underscores the importance of creative financial models, strategic partnerships, and efficient operational practices to ensure that the flame of innovation continues to burn brightly. In essence, these survey findings highlight the financial tightrope biotech companies walk, emphasising the critical need for thoughtful financial management in pursuing groundbreaking advancements.
Investment Confidence and Product Success
In an operating environment where financial milestones intersect with the promise of product success, the survey findings point to a strong level of confidence within the
6 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
PEER REVIEWED


Driving development and connecting commercialization of sterile and lyophilized drug products, we are dedicated to your success in bringing life-changing therapies to patients.
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 7 www.pci.com talkfuture@pci.com Your sterile drug product, our world. OUR END-TO-END BIOLOGIC SOLUTIONS INCLUDE:
Formulation & Lyophilization Cycle Development
Lyophilization and Sterile Fill-Finish Manufacturing
Aseptic Robotic Technologies
Analytical Support
Clinical & Commercial Labeling & Packaging
Refrigerated/Frozen Storage & Distribution
Lyophilization
• Sterile
•
•
•
•
•
and Sterile Manufacturing
Regulatory & Compliance
biotech industry. An overwhelming 93% of respondents express confidence in meeting their investment milestones, showcasing a robust belief in the industry's ability to secure the necessary financial backing for their endeavours. This high level of confidence signals optimism and a tangible assurance that the biotech sector can navigate the intricate landscape of investment despite the challenges posed by funding difficulties.
Moreover, an impressive 87% of respondents express confidence in the success of their products. Despite the complexities of clinical trials, financial uncertainties, and the evolving healthcare landscape, biotech companies are resolute in their conviction that their products will meet and exceed expectations.
Clinical Trials Landscape
32% of respondents cited clinical trials as the greatest challenge for their organisation, with 51% identifying phase 3 as the most challenging period. Clinical trials topped the list of challenges, beating securing funding, which was cited as the top problem by just 14% of respondents, despite the current financing situation, emphasising the complexity and intricacies of this pivotal stage.
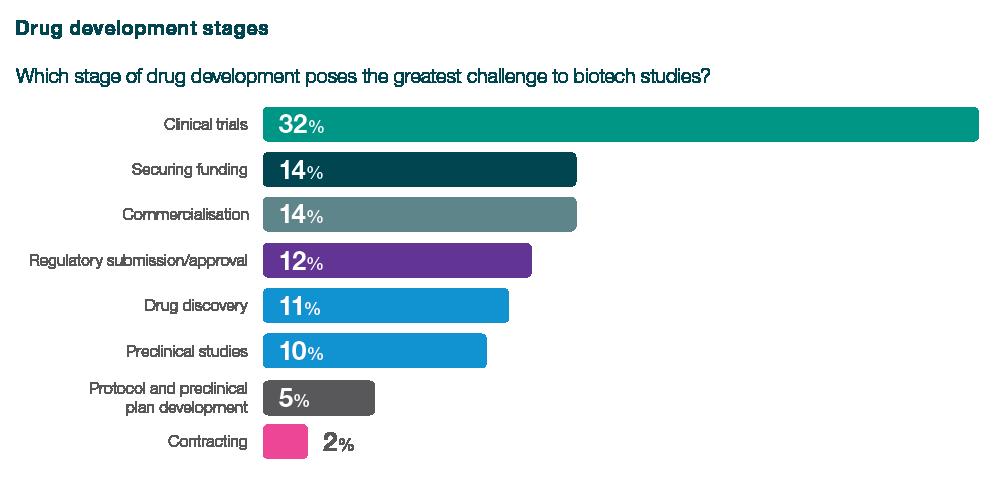
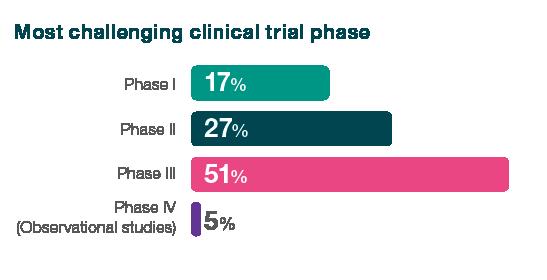
When asked about the clinical trial challenges they face, respondents said clinical trials are extremely costly, require significant expertise and coordination, are the most time-consuming element, and that candidates willing to volunteer for clinical studies are hard to find. Furthermore, they also cited difficulties due to differing laws in different countries.
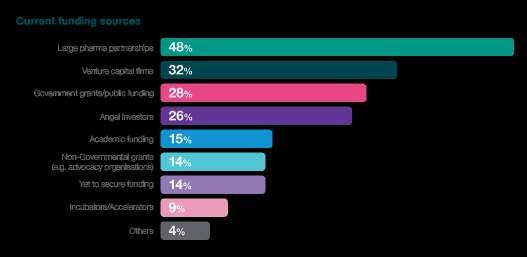

Partnering
The prominence of partnerships emerges as a strategic response to these challenges. A substantial 48% of respondents reveal that they are actively partnering with large pharmaceutical firms, signalling a recognition of the collaborative power established industry players bring. This strategic alignment with large pharma firms injects financial stability and provides access to invaluable expertise, resources, and an established infrastructure, potentially streamlining the clinical trial process.
The strategic role of partnerships in clinical trials is indispensable. In an environment where challenges are acknowledged, partnerships with large pharma firms and specialised CROs become tactical manoeuvres and strategic imperatives. These collaborations stand as a testament to the industry's recognition that the complexities of clinical trials demand a collaborative approach. They are committed to leveraging shared expertise, resources, and capabilities, thereby enhancing the efficiency, quality, and, ultimately, the success of clinical development endeavours. In essence, the clinical trials landscape outlined by the survey findings underscores the pivotal role of partnerships in navigating the intricate journey from research to regulatory approval.
Additionally, 41% of respondents express a preference for medium to large CROs when it comes to clinical development. This preference underscores the industry's reliance on specialised service providers, emphasising the value of working with CROs with a global presence and comprehensive service offerings. It also suggests that strategic collaborations with CROs play a crucial role in managing the intricacies of clinical trials, leveraging their expertise to optimise trial design, patient recruitment, and data management.
8 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1





INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 9 www.international-biopharma.com
Regulatory & Compliance

Looking Forward
When examining the findings of ICON's biotech sector survey, a comprehensive narrative emerges, painting a vivid portrait of an industry that survives and adapts amidst funding challenges. From R&D spending expectations to navigating the intricacies of the funding landscape and clinical trials, a resilient spirit permeates the biotech sector. Despite financial headwinds, the survey illuminates an industry-wide commitment to advancing innovation, as evidenced by a resounding 60% of respondents who anticipated increases in R&D spending.
The challenges embedded in the funding landscape, particularly the rising cost of capital and the delicate balance required for cost management, underscore the financial tightrope biotechs navigate. Yet, these challenges are not insurmountable. The industry acknowledges the imperative to innovate and is compelled to allocate resources, fostering creativity while managing costs effectively and strategically. The survey findings point to this financial balancing act, emphasising the critical need for thoughtful financial management to propel groundbreaking advancements.
Strategic partnerships emerge as a linchpin in overcoming challenges in the clinical trial landscape. A substantial 48% actively partner with large pharmaceutical firms, recognising the collaborative power they bring. Furthermore, 41% prefer medium-large CROs for clinical development, highlighting the industry's reliance on specialised service providers. These partnerships go beyond tactical manoeuvres; they are strategic
imperatives, reflecting an industry-wide commitment to collaborative approaches that enhance efficiency, quality, and the ultimate success of clinical development.
The survey outlines the challenges biotech organisations face and paints a portrait of an industry that is adapting to address them. The transformative potential of strategic partnerships, the unwavering confidence in innovation, and the commitment to navigating financial complexities can support biotech companies in their important work of driving innovative and scientific advancements of much needed medicines.

Dr. Chris Smyth
Dr. Chris Smyth is President of ICON Biotech. Dr. Smyth has 30 years' operational and therapeutic experience, most notably in biotech, MedTech and oncology. Prior to ICON, he spent 10 years in IQVIA Biotech, where he held EVP Oncology and Chief Operating Officer roles before assuming global leadership as President of the division. Dr. Smyth also previously led global clinical operations for 8 years at an oncology biotech company, Antisoma. Dr. Smyth holds a Bachelor of Science degree in Biochemistry from the University of Kent at Canterbury, a PhD in Reproductive Biology from the University of Edinburgh Medical School, and an MBA from Henley Management College.
10 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
MICROBIAL

CONTRACT DEVELOPMENT AND MANUFACTURING OF BIOPHARMACEUTICALS
Richter-Helm is a Germany-based GMP manufacturer specialized in products derived from bacteria and yeasts, with a proven 30-year track record.
Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide have already benefited from our commitment to good manufacturing practice and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Richter-Helm consistently works to the highest standards of pharmaceutical quality.
Contact us
+49 40 55290-801 www.richter-helm.eu
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 11 www.international-biopharma.com
MORE ABOUT OUR SERVICES AND CAPABILITIES
LEARN
PRODUCTION? ARE YOU LOOKING FOR EXPERTS IN
Extractables and Leachables for Inhaled Medicines How risk assessments can improve E&L strategies
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) are encouraging a more structured approach to product development, such as using Quality by Design (QbD) principles.
The structured approaches encouraged by the FDA, EMA, and other regulatory bodies require additional upfront resources compared to a more traditional approach. Performing an initial assessment of materials used in the product and developing a more thorough understanding of products earlier in their development allows more appropriate experiments on the higher-risk aspects rather than generic approaches covering all materials.
Benefits of Performing a Risk Assessment
Extractables and leachables (E&L) risk assessments are valuable processes that can identify and highlight the risks of potential leachables from both the container closure system and the manufacturing processes. The risk assessments also include the level of risk that leachables might present to user safety and product quality.
By performing a risk assessment, manufacturers can better understand the product, whether a medical device or the container closure system, and the manufacturing processes. Identifying risks and scoring them based on that understanding allows for subsequent E&L studies to be more focused. Therefore, decisions around the E&L aspects of the project can be more appropriate.
Risk Assessment Lifecycle
Quality risk management is a systematic process. The lifecycle can be split into Assessment, Control, and Review stages, and there should be regular communication with stakeholders throughout the lifecycle. A typical quality risk management process is described in ICH Q9 with a flowchart, and it is a good place to start when designing a strategy. An initial assessment can be performed based on previously mentioned tables and decision trees from the FDA and EMA guidelines. This can inform stakeholders early in the assessment about the studies that might be required for the project.
The first step of the process is the Risk Assessment, which is further divided into a series of sections:
• Risk Identification involves identifying the parameters that might affect the leachables by reviewing and brainstorming processes and materials.
• Risk Analysis collates information to understand identified failure modes.
• Risk Evaluation uses information gathered during the Risk Analysis to score the failure modes.
• Risk Control covers where the risks are either accepted or
Inhaled Medicines
In the UK, pressurised metered dose inhalers (pMDIs) are a commonly prescribed treatment for the 5.4 million people with asthma and the 1.2 million with chronic obstructive pulmonary disease (COPD). A 2019 study found that based on a sample of 85 patients, switching from dry powder inhalers (DPIs) to pMDIs was associated with decreased asthma exacerbations and improved asthma control. Despite alternatives such as DPIs being available, pMDIs still represent the foundation of asthma control in the UK.
pMDIs consist of a drug formulation (in suspension or solution) with a closure that delivers the required dosage efficiently and consistently. A pMDI usually consists of a pressurised canister that contains the active substance and propellant and is capped with a metering valve, along with a plastic holder consisting of the actuator, mouthpiece, expansion chamber, and mouthpiece.
Compared with other pharmaceutical products, pMDIs have a much greater risk of the packaging impacting drug delivery. The formulation includes the API in a hydrofluorocarbon (HFC) liquified gas, which acts as an effective solvent for leaching. Furthermore, pMDIs often consist of multiple materials and plastic components with a range of polymerisation catalysts, antioxidants, pigments, and slip agents used in their manufacture that may leach, all of which may carry varying toxicological risks.
experimental studies are planned based on mitigating risk appropriately.
• A Risk Review reassesses the failure modes and re-scores them following any risk mitigations. Risk review can also be part of lifecycle management.
Extractables Studies
Having identified the risk parameters, manufacturers can design their E&L studies accordingly. Extractable studies are chemical analyses that expose a sample of the container closure system or part of the manufacturing system to selected solvents and conditions that either simulate the product formulation or aggressively extract from (but do not destroy) the material to inform the potential for leachables under normal use. Extractable studies can help assess the risk of leachables in the finished product and help design the leachables studies.
Extractable studies are intended to identify potential leachables from a material. Several solvents are used to extract substances from components; the range of solvents should span a range of polarities and pH that may be observed in the finished product.
Solvents commonly used are water, isopropyl alcohol (IPA), and hexane to span solvent polarity. These can also be
12 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Regulatory & Compliance
Global CRO: Pioneering Your Journey From Discovery to Delivery

• Central Lab: Kitting, Logistics, and Biostorage With Virtual Sample Inventory Management
• Biospecimens: Curated Inventory and Analysis With Advanced Biobanking
• Preclinical: Advanced Cell-Based Assays and Target Validation
• Genomics: Single-Cell to Multiplex Studies
• Bioanalytics: Immunogenicity and PK Testing
• Immune Monitoring: Comprehensive Cell Phenotyping and Profiling
• Tissue and Liquid Biopsy: Rare Cell and CTC Isolation and Analysis
• Clinical Trials: Design, Strategy, and Full-Spectrum Support
• Diagnostics and CDx: Regulatory Consulting, Companion Dx, and NGS
• Data Sciences: Biometrics and Biostatistics via QuartzBio® precisionformedicine.com
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 13 www.international-biopharma.com
Data Sciences Biospecimens, Biostorage, Sample Protection IV, Transfusion Administration Clinical Trial Support Commercialization Circultaing Tumor Cells (CTCs) Protein Molecule DNA RNA Assays Flow Cytometry Sample Collection Sample Collection II Sample Processing Data Solutions Online Management, Tracking, and Reporting Biobanking Regulatory Global CRO Manufactiring Specialty Labs Central Lab Services Data Sciences Biospecimens, Biostorage, Sample Protection IV, Transfusion Administration Liquid Biopsy Tissue Custom Biospecimen Collections Circultaing Tumor Cells (CTCs) Protein Molecule DNA RNA Sample Collection Sample Processing Solutions Cells Clinical Site Training Global Shipping and Logistics Local Logistics Supply Chain Management Online Management, Tracking, and Reporting Global CRO Manufactiring Specialty Labs Central Lab Companion Diagnostic Blood, biofluids and derivatives Kitting, Custom Kit Production Liquid Biopsy Tissue Custom Biospecimen Collections Cells Clinical Site Training Global Shipping and Logistics Local Logistics Supply Chain Management Global CRO Manufactiring Specialty Labs Central Lab Services Companion Diagnostic Blood, biofluids and derivatives Kitting, Custom Kit Production Liquid Biopsy Tissue Custom Biospecimen Collections Cells Clinical Site Training Global Shipping and Logistics Local Logistics Supply Chain Management Global CRO Manufactiring Specialty Labs Central Lab Services Data Sciences Biospecimens, Biostorage, Sample Protection Companion Diagnostic Blood, biofluids and derivatives Kitting, Custom Kit Production Liquid Biopsy Tissue Custom Biospecimen Collections Circultaing Tumor Cells (CTCs) Protein Molecule Sample Collection Cells Clinical Site Training Global Shipping and Logistics Local Logistics Supply Chain Management Online Management, Tracking, and Reporting Global CRO Manufactiring Specialty Labs Central Lab Services Data Sciences Biospecimens, Biostorage, Sample Protection Companion Diagnostic Blood, biofluids and derivatives Kitting, Custom Kit Production Liquid Biopsy Tissue Custom Biospecimen Collections Circultaing Tumor Cells (CTCs) Protein Molecule Sample Collection Cells Clinical Site Training Global Shipping and Logistics Local Logistics Supply Chain Management Online Management, Tracking, and Reporting Biospecimens Specialty Labs Companion Diagnostics Clinical Trials Central Lab Services Data Sciences
Regulatory & Compliance
modified to span a relevant pH range. Solvents that simulate your product's formulation can also be used.
It should be noted that some regulatory agencies recommend three solvents, e.g., polar, non-polar, and semi-polar. Standard extraction techniques typically considered are reflux, sonication, autoclave, and solvent soaking; each has advantages and limitations. When choosing the extraction technique, consideration should be given to the objectives of any extractable study, i.e., aggressively extracting compounds from the sample without degradation of compounds extracted or damage to the material. Characterisation of the extract solutions is principally carried out using mass spectrometry techniques because of their sensitivity, selectivity, and low sample requirement; examples are headspace GC-MS for volatile species, direct injection GC-MS for semi-volatile species, LC-MS for non-volatile species and ICP-MS for elemental species.
Every effort should be made to identify extractable compounds above a previously determined analytical evaluation threshold (AET) using spectral libraries, test mixes, and trained analysts' expert knowledge and experience. Guidance such as USP <1663> and ISO 10993-12 are good places to start when designing extractable studies, and the risk assessment process allows studies to focus on the highest-risk areas. Following an extractables study, a toxicological assessment of the observed extractable species is required to inform whether any species must be targeted in long-term leachables studies due to their toxicity risk.
Leachable Studies
Leachables studies are chemical analyses of the finished product. Leachable studies can detect the release of compounds that are either washed from the surfaces of the container closure system or manufacturing equipment, or migrate from materials into the product under normal storage conditions.
Leachables are a potential risk to product quality and patient safety. Leachables studies are often run as part of a long-term stability program to observe the levels of migrating species over the product's intended shelf life. Depending on the product being tested, the sample matrix can introduce complexity to a leachable study compared to an extractables study, impacting both sample preparation and ease of analysis.
Formulation excipients such as flavours or drug substances can interfere with detecting the often trace level leachables, affecting the method sensitivity requirements. Simulant formulations, which simulate the final formulation's physical properties but may omit certain ingredients to aid the analysis process, are an option if sample preparation techniques cannot reduce interference. However, the use of simulant formulations must be robustly justified.
Storage of samples is typically based on ICH Q1A stability guidelines, and methods used can be targeted to analytes of concern, as determined by Toxicologists. Targeted methods must be validated as fully quantified or limit test methods (to ICH Q2), with limit test methods being justified if confidence exists that leachables will not be observed above a certain level. Extractable studies are routinely performed using clean solvents. Therefore, when product formulation is introduced in leachable studies, unforeseen leachables may occur due to the action of
formulation ingredients or the interaction of these ingredients with previously observed potential leachables; this may result in leachable compounds being observed in the leachables studies that were not observed during extractables studies. These new leachables would not be detected if only specific targeted methods were employed in leachables studies.
As methods for targeted analysis are commonly based on the screening methods used for the extractables study, it is good practice and recommended that screening capability be retained during leachables studies.
Risk Review
A toxicological review of the data generated during E&L experiments is essential to the E&L risk assessment. A toxicological review of the materials and ingredients contributes significantly to the quality of the risk assessment.
Toxicological experts' contributions include assessing the materials and ingredients before the risk identification process and advising appropriate sensitivity levels for analytical methods to inform the leachables study design. Following any leachables studies, the Toxicologists assess the leachables compounds and levels observed and play a critical role in informing the group whether the risk has been reduced or possibly increased due to results.
Regulatory agencies have shown an increased requirement to show aspects of QbD when developing new products. E&L risk assessments demonstrate knowledge of the product and its manufacturing process, contributing to applying QbD principles during a product's development. They also focus efforts on E&L projects, which can be expensive when performed inefficiently. By following the risk assessment process, only required studies are performed, and these studies can be designed to de-risk several failure modes.
Working with a science or regulatory consultancy can help you design and implement an effective E and L risk assessment. Broughton has extensive experience of developing tailored E and L studies that combine technical and analytical expertise with integrated toxicological consultancy and regulatory compliance. To find out more and to speak to one of our scientific experts, visit the Broughton website at www.broughton-group.com.

Paul Hardman
Paul Hardman is Managing Consultant, Chemistry at Broughton. He has 10 years of experience in developing inhaled pharmaceuticals; across API particle engineering, formulation development and scale up, and device design including interaction with the formulation to enable products to target either systemic absorption or local effect in the lung. He is passionate about product quality and understanding a product's chemistry, from product design to its intended function, how the chemistry changes over time, and assessing any special features. Paul is currently leading studies related to product chemistry across a wide range of consumer and medicinal products at Broughton.
14 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1

Application Note
A Guide to Understanding and Performing all Appropriate Validation Steps When Adopting a New Endotoxin Testing Reagent
Limitations of Common Endotoxin Testing Solutions
The adaptation of in-house endotoxin testing for a pharmaceutical or medical device manufacturer can be a daunting task. Many small-volume manufacturers find themselves in one of two common situations. Either they are utilising a contract testing organisation (CTO) and paying a great deal of costs, or they have adopted a solution that is set up by the manufacturer with much of the validation services outsourced.
A potential limitation of these methods is cost. Utilising a CTO is appealing to low-volume manufacturers. However, it can be a limitation to expansion as testing costs increase with volume. A system that does not allow versatility in reagent usage can lock the client into manufacturers’ costs and price increases.
However, an even bigger drawback to both situations is the lack of testing control and feedback that the user receives for each method. The disadvantage to the convenience of a contract testing service is the increase in time to results as well as greater limitations on the frequency of testing monitoring. In-house testing can provide results in an hour or less if needed. This nearly real-time feedback on the quality of the manufacturing process is invaluable to the trend monitoring of the manufacturing process.
Dangers of an Improperly Performed Analytical Methods Validation
Real-time feedback can be provided by seemingly attractive solutions. Some solutions utilise outsourced verification data provided by the manufacturer. This provides the convenience of having preparatory tests being done offsite – outsourcing much of the validation. However, a great limitation of this method is that the routine testing takes place in a different location with different analysts, accessories, and accompanying instruments, which may not fully support the verification test requirements stated in USP chapter 85: “To assure the precision or validity of the turbidimetric and chromogenic techniques, preparatory tests are conducted to verify that the criteria for the standard curve are valid and that the sample solution does not interfere with the test. Validation for the test method is required when conditions that are likely to influence the test result change.” Although an outsourced standard curve may technically check the requirements, it assumes that changing “conditions that are likely to influence the test result” is not a factor between the different locations, personnel, equipment, and accessories in and with which the validation testing and the routine testing takes place.
Although routine test monitoring includes the PPC and recovery rates at the testing location, the concern about the offsite verification data is not unfounded. Why would the
USP require suitability verification if redundant information is obtained during routine testing? The information obtained during routine testing is not for validation but to monitor daily test conditions. A validation performed in the same conditions as the actual test, including location and analyst, is imperative when setting up in-house testing.
Invalid results in routine testing presume an accurately validated test method to reveal issues in reagent preparation, contamination, and instrument failure. However, repeated failure of routine results can be caused by improperly determined testing parameters found using data obtained away from the in-house testing location. Being caught in this situation can cause undue stress on the clients as they face regular actionable results to investigate, retest, and report. The challenge of changing testing methods does not present itself as a feasible solution because much time and money are invested in the test method. However, this leads to the crucial blunder of passing off systematic test method errors such as random user mistakes or environmental contamination.
The solution: the user needs to perform the verification correctly onsite. What may feel like an unattainable goal due to the complexity of process validation, however, is simplified by the simple fact that the test is already considered to be validated. All that is required of a user is to verify that the already validated endotoxin test is suitable for the onsite products. The purpose of this document is to provide the user with the correct regulatory citations and guidance needed to give confidence in understanding the requirements of validation for the Bacterial Endotoxin Test according to USP compendial methods and advice, and FDA guidance.
Process Validation
Page 4 of the FDA’s “Guidance for Industry: Process Validation: General Principles and Practices” states, “Process validation is defined as the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering a quality product.” When a pharmaceutical or medical device manufacturer thinks of “validation,” this all-encompassing definition is what comes to mind. The somewhat vague definition is what allows the scope of process validation to apply to every area of the drug or device manufacturing process. However, the document does provide a clean division of process validation into two distinct categories. Page 10 states, “Process validation includes facility validation and process performance validation.”
Facility Validation
Facility validation will fall completely out of the scope of Bacterial Endotoxin Testing. The Bacterial Endotoxin Test only has a few direct, basic facility requirements such as climate control and adequate shelter from direct sunlight. However, a site that is manufacturing a product to endotoxin-free standards
16 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1

will need facility validation. Page 10 of the FDA document states, “Proper design of a manufacturing facility is required under part 211, subpart C, of the cGMP regulations on Buildings and Facilities. It is essential that activities performed to assure proper facility design and commissioning precede PPQ.”
Although not dealt with directly in BET validation, it is crucial that this portion of process validation is completed before the Process Performance Validation (PPQ), which includes endotoxin testing, proceeds. However, a client that is looking to implement the BET as a new test or a replacement of a previous technique should not need to change anything regarding their existing Facility Validation.
Process Performance Validation/Qualification (PPQ)
The Bacterial Endotoxin Test is one of the pieces that make up the PPQ. Page 11 of the FDA guidance on process validation explains that PPQ is the general name given to the combined validation of “the actual facility, utilities, equipment (each now qualified), and the trained personnel with the commercial manufacturing process, control procedures, and components to produce commercial batches. A successful PPQ will confirm the process design and demonstrate that the commercial manufacturing process performs as expected.” One can think of the PPQ as the aggregate of all method validations needed for the quality control checks in the manufacturing process. On page 13, the guidance explains that PPQ includes “the validation of analytical methods used in measuring the process, in-process materials, and the product.” Of course, one of these analytical methods is the Bacterial Endotoxin Test.
Analytical Method Validation (Non-compendial Analytical Procedures)
The manufacturer must provide a method of validation for every test they are using. The requirements for this validation are now outlined in the FDA guidance on “Analytical Procedures and
Methods Validation for Drugs and Biologics.” Page 7: “Analytical method validation is the process of demonstrating that an analytical procedure is suitable for its intended purpose. The methodology and objective of the analytical procedures should be clearly defined and understood before initiating validation studies.” The document provides a detailed description of potential objectives and tests to perform to ensure an analytical method is performing to its intended purpose. USP 1225 parallels these requirements: “Validation of an analytical procedure is the process by which it is established, by laboratory studies, that the performance characteristics of the procedure meet the requirements for the intended analytical applications.”
It is at this point that many users of the Bacterial Endotoxin Test misunderstand the requirements for adopting or converting to a new test reagent. Because they either outsource the entire test itself or portions of the validation for in-house testing, they believe that adopting a new in-house test reagent and equipment will require them to perform a completely new Analytical Method Validation. However, because the Bacterial Endotoxin Test is a compendial method outlined in USP 85, the FDA considers the Bacterial Endotoxin Test to be an already validated test method. As a result, users do not need to perform an Analytical Method Validation but can proceed with a Compendial Analytical Verification.
Compendial Analytical Verification/Verification of Compendial Procedures
When either adopting a new or pre-existing reagent in a compendial procedure, this step of Compendial Analytical Verification is what is required of the user. The FDA document states, “The suitability of an analytical procedure (e.g., USP/ NF, the Official Methods of Analysis of AOAC International, or other recognized standard references) should be verified
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 17 www.international-biopharma.com 17
Application Note

under actual conditions of use. Information to demonstrate that USP/NF analytical procedures are suitable for the drug product or substance should be included in the submission and generated under a verification protocol.” There is no need to validate the method itself, to determine its adequacy for the test, or even to perform a comparison study with an alreadyexisting method (although that is often valuable for internal evaluation).
The requirements are simply to verify the suitability of the method for the specific drug under actual conditions of use.
The USP chapter 1225 details what this compendial verification may look like, “Verification requirements should be based on an assessment of the complexity of both the procedure and the material to which the procedure is applied... Only those characteristics that are considered to be appropriate for the verification of the particular method need to be evaluated.” Although this may seem ambiguous, leaving the question open of how much of the Analytical Methods Validation steps need to be adopted for the BET test verification, the USP clearly outlines what is needed. USP chapter 1085 specifies that for

the Bacterial Endotoxin Test, this verification of compendial procedures includes analyst qualification, consumable qualification, equipment and instrument qualification, and method suitability qualification.
Analyst Qualification, Consumable Qualification, Equipment, and Instrument Qualification
These three portions of the BET verification are the preparatory tests needed to be in place before the method suitability qualification. The following from USP 1085 regarding analyst qualification, “training for performing any BET involves demonstration of acceptable proficiency for both sample preparation and assay method(s).” For consumable qualification, “use apparatus that is shown to be free of detectable endotoxin and does not interfere in the test.” For equipment and instrument qualification, “all instrumentation and equipment used in the performance of an LAL test... should be qualified using proper scientific standards and according to approved protocols. Incubating plate or tube readers should reference a user requirement specification (URS), an installation qualification (IQ), an operational qualification (OQ), and a performance qualification (PQ).”

18 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Application Note
Application Note







When providing proof of these qualifications, calibration certificates, CoA’s, and IQ/OQ/PQ documents are attached to the method of suitability testing for the product.
Method Suitability Testing
Finally, the actual verification required for a new user of the Bacterial Endotoxin Test is Method Suitability Testing. This is found in both USP 85 and expanded on in USP 1085 and includes the calculation of the endotoxin limit and MVD, the assurance of the standard curve test, and the interfering factors test. It is this process that is outlined in the following attachments. The new client has the assurance that the Bacteria Endotoxin Test is already considered to be validated when using an FDA-licensed reagent such as PYROSTARTM ES-F. They also have the assurance that these considerations found in USP 85 are all that need to be addressed when either adopting a new test method or switching test methods.
If a user is concerned that switching between gel clot reagent to a quantitative reagent or switching manufacturers in reagent or instrument will require them to perform a PPQ since one of their analytical methods has changed, then they can be assured by the words in the opening paragraph of USP 85 that from a regulatory standpoint, nothing has changed in terms of their Analytical Methods Validation: “There are three techniques for this test... Proceed by any of the three techniques for the test.”



Conclusion and Introduction to the Following Documents
If you are considering changing your LAL reagent or technique due to failed results with a previously outsourced validation or to adopt a better BET technique, you have the assurance that the FDA and the USP consider this a simple change in the reagent. If you are considering adopting the LAL reagent for BET testing, you have the assurance that the FDA and USP already consider the method you are adopting as compendial. Once this is understood, your confidence can arise that you are following a fully compliant and compendial method. To aid in your risk assessment and preparation for performing the appropriate suitability assessment based on the USP and FDA requirements, FUJIFLM Wako provides a full range of endotoxin-specific LAL reagents. FUJIFILM Wako can support your validation by providing example procedures and reports that can then be used as a part of the submission of a successfully validated Bacterial Endotoxin Test.
REFERENCES
1. FDA. Guidance for Industry: Process Validation: General Principles and Practices
2. FDA. Analytical Procedures and Methods Validation for Drugs and Biologics
3. USP <1225> Validation of Compendial Procedures.
4. USP <1226> Verification of Compendial Procedures.
5. USP <1085> Guidelines on the Endotoxins Test
6. USP <85> Bacterial Endotoxins Test

Timothy Francis
Timothy Francis is the Senior Technical Specialist for the LAL Division of FUJIFILM Wako Chemicals U.S.A. Corporation. He comes into the Technical Specialist role with 5 years of experience teaching the natural sciences at a college level. He is proficient at taking the complex, technical aspects of a topic and breaking them down into clear, understandable pieces that all connect back to the big picture. He draws upon this experience to provide professional technical support and training for the PYROSTAR™ line and to help you with your technical needs.
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 19 www.international-biopharma.com 19
Using Predictive Compliance Risk Analysis to Avoid FDA Penalties
In the complex landscape of global regulations, particularly within healthcare and pharmaceutical sectors, a robust compliance strategy is essential for maintaining the integrity of businesses amidst rigorous regulatory requirements. Organisations need to be both flexible and resilient, adept at navigating and anticipating the multifaceted and ever-changing aspects of global compliance standards. These standards are often stringently enforced by regulatory bodies, with the U.S. Food and Drug Administration (FDA) being one of the most prominent and rigorous. Insights into developing a strategic framework for identifying and mitigating compliance risks is critical in today's dynamic business landscape, where violations of regulations can lead to severe repercussions, far beyond minor setbacks, potentially jeopardizing the future of regulated businesses.
The Challenge of Disparate Quality Systems
In the intricate world of healthcare and pharmaceuticals, ensuring regulatory compliance across a complex network of operations is paramount.
The integration of diverse and complex quality systems, often resulting from mergers and acquisitions, poses significant challenges for effective risk management in the pharmaceutical and healthcare industries. These fragmented systems can create inconsistencies in the compliance infrastructure, leading to vulnerabilities that are challenging to detect and more challenging to rectify. This fragmented approach to quality system integration can undermine the overall effectiveness of compliance efforts, exposing organisations to regulatory risks and potentially eroding public confidence in their commitment to safety and quality.
Uniting Global Quality Management Under Predictive Compliance
In response to the challenges posed by disparate quality management systems, enterprises in the healthcare and pharmaceutical sectors are increasingly adopting predictive compliance risk analysis. This advanced approach offers a cohesive and strategic method for managing regulatory obligations without the need for extensive overhauls of existing systems.
Predictive compliance goes beyond merely reducing the likelihood of non-compliance; it enhances operational efficiency and refines the precision of internal audits. By centralising oversight across various locations and implementing forwardlooking measures, organisations can achieve a compliance framework that is both robust and flexible, ensuring resilience in the face of regulatory audits.
The Role of Predictive Risk Analysis in Compliance
Predictive risk analysis represents a transformative approach
to managing compliance obligations. Far from being merely an assemblage of advanced technological terms, predictive compliance risk analysis signifies a fundamental shift in strategy – from a reactive stance, addressing compliance issues as they arise, to a proactive one, aimed at foreseeing and mitigating potential breaches before they materialise.
This proactive methodology is grounded in the application of data analytics, machine learning, and other predictive technologies to analyse vast arrays of data for early signs of potential compliance risks. By harnessing these insights, organisations can transition from traditional, often cumbersome compliance models to more dynamic, real-time monitoring and response frameworks. This not only enhances the ability to maintain continuous compliance across various regulatory environments but also significantly reduces the likelihood of infractions that can lead to financial penalties, reputational damage, and operational disruptions.
Moreover, the adoption of predictive risk analysis in compliance processes redefines the benchmarks for regulatory adherence. It establishes a new norm that is not only anticipatory but also exceedingly precise, enabling organisations to tailor their compliance strategies to the specific risks and requirements of their operational contexts. This level of specificity and foresight in compliance management fosters a culture of compliance that is embedded within the operational fabric of the organisation, rather than being an external imposition.
In essence, predictive risk analysis in compliance is not just about avoiding penalties; it's about instilling a forward-thinking ethos that permeates every layer of an organisation's operations. It underscores the importance of strategic risk management as a cornerstone of sustainable business practices, ensuring that organisations are not merely reacting to regulatory landscapes but are actively shaping their journey through informed, data-driven decisions.
Key Components of Predictive Compliance Risk Analysis
Predictive compliance represents a sophisticated convergence of multiple disciplines, transforming traditional compliance frameworks into proactive, resilient systems. This advanced approach to compliance management is founded on a strategic amalgamation of data collection, analytics, and focused risk mitigation efforts.
• Establishing a Robust Data Foundation: The efficacy of predictive compliance begins with the meticulous collection of relevant data. This foundational step involves identifying and aggregating critical information sources, ranging from internal audit findings to historical regulatory inspection outcomes. The objective is to streamline the data acquisition process to ensure that it is both comprehensive and efficient, avoiding the pitfalls of data overload or irrelevant data capture.
20 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1 Research / Innovation / Development
PEER REVIEWED
UniSafe® Platform
Delivering the future with safety and simplicity
Developed around UniSafe®, our platform meets the needs of you, our partner, and your varying patients’ needs both now and in the future.

Proven, on market, in patient use

Fully industrialised and ready to supply

Coming soon
Want to know more?

Coming soon
Visit ompharmaservices.com/ibi-march2024 or email pharmaservices@owenmumford.com
UniSafe® is a registered trademark of Owen Mumford Ltd. ©
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 21 www.international-biopharma.com
A spring-free, passive safety device for 2.25mL pre-filled syringes, designed for simple assembly and use.
2024 OMPS/ibi/ad/ob/0324/7
A spring-free, passive safety device for 1mL pre-filled syringes, designed for simple assembly and use.
A reusable companion auto-injector for UniSafe® 1mL.
A reusable connected companion auto-injector for UniSafe® 1mL.
• Harnessing Analytical Insights: Once data is collected, the next step is to transform this raw information into meaningful insights. This transformation is achieved through the application of advanced analytics, utilising tools and platforms that employ statistical models, machine learning algorithms, artificial intelligence, and data visualisation techniques. The focus here is on interpreting the underlying patterns and indicators within the data that could signify potential compliance risks.
• Strategic Risk Identification and Mitigation: The true value of predictive compliance lies in its ability to proactively identify risks and prioritise them based on their severity and probability to occur. This requires a discerning approach to data analysis, one that goes beyond mere aggregation to a nuanced understanding of the data's implications in inspections. By focusing on key risk areas in an automated predictive compliance solution, organisations can allocate their resources more effectively, concentrating their efforts on mitigating the most significant compliance risks.
Benefits of Predictive Compliance Risk Analysis
The implementation of predictive compliance risk analysis offers a comprehensive array of benefits, underpinning a philosophy where preemptive action is invariably more effective and less costly than remedial measures. These benefits extend beyond mere regulatory adherence, enhancing operational efficiency, credibility, and financial health of organisations.
The essence of predictive compliance lies in its capacity to anticipate and mitigate regulatory risks before they escalate into significant issues. This proactive approach provides
organisations with a critical advantage – time. Time to identify, assess, and address potential compliance gaps before they result in severe regulatory repercussions.
The traditional approach to compliance – often characterised by a scattered allocation of resources in response to emerging issues – can be both inefficient and ineffective. Predictive compliance introduces a paradigm shift towards strategic resource allocation. By leveraging data-driven insights to pinpoint areas of highest risk, organisations can concentrate their efforts and resources more judiciously. This targeted approach enhances the efficacy of compliance programs and contributes to overall operational efficiency, reducing wasteful expenditure and focusing on areas that yield the highest return on investment.
An organisation's compliance framework is a critical component of its corporate integrity and reputation. Predictive compliance strengthens this framework by embedding a culture of continuous improvement and vigilance. Beyond evading penalties, predictive compliance is about embodying a commitment to the highest standards of regulatory compliance and ethical conduct.
Implementing Predictive Risk Analysis in Your Organisation
The integration of predictive risk analysis into an organisation's compliance framework is a sophisticated process that unfolds progressively, requiring meticulous planning and execution.
Assessing Organisational Readiness and Establishing Realistic Expectations
The initial phase in adopting predictive risk analysis involves

22 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1 Research / Innovation / Development
Research / Innovation / Development

a thorough assessment of the organisation's current state and readiness for such a transformative initiative. This crucial step encompasses evaluating existing compliance frameworks, technological infrastructure, and the organisational culture towards risk management. It also involves setting realistic expectations and aligning them with the capabilities and potential impact of predictive risk analysis. This stage is about ensuring a solid foundation, both in terms of infrastructure and mindset, to support the subsequent steps of implementation.
Selecting and Implementing Appropriate Technology
Technology serves as the backbone of predictive risk analysis, facilitating the collection, analysis, and interpretation of vast datasets to identify potential compliance risks. This step involves a comprehensive review of available technological solutions, including data analytics platforms, machine learning tools, and compliance management systems. The selection process should prioritise technologies that align with the organisation's specific requirements, scalability, and integration capabilities, ensuring they complement existing systems and processes.
Conducting a Pilot Program
Before fully integrating predictive risk analysis across an
organisation, conducting a pilot program is advisable. This controlled approach allows for the testing of processes, technologies, and team dynamics on a smaller scale, providing valuable insights into the system's effectiveness and areas for improvement. The pilot phase is instrumental in identifying potential challenges and refining the approach before broader implementation, thereby minimising the risk of large-scale issues and ensuring a smoother transition to predictive compliance models.
Embracing Predictive Compliance: A Strategic Imperative
In an era marked by rapid regulatory changes and heightened expectations for corporate accountability, the ability to foresee and address compliance risks proactively is more than just an operational benefit – it's a critical differentiator that can set the foundation for sustainable growth and innovation.
In regulated business environments, the transition to predictive compliance is not just a strategic move; it's a visionary one. It signifies a shift from reactive problem solving to a forward-thinking mindset that values preparedness, operational integrity, and adaptability. For enterprises ready to embark on this journey, predictive compliance offers a path to meeting the regulatory demands of today and thriving in the regulatory landscapes of tomorrow.

Hannah Crystal T. Jurolan
Hannah Crystal T. Jurolan has carved a niche for herself in the intersection of life sciences and technology and is currently the Assistant Marketing Manager at Xybion. With a robust background in biosystems engineering, her expertise bridges the gap between intricate biological systems and cuttingedge technological solutions. Over her three-year tenure at Xybion, Hannah has been instrumental in shaping the marketing strategies for Laboratory Information Management Systems (LIMS), leveraging her technical acumen to inform laboratory leaders of the latest LIMS technologies.
Email: hjurolan@xybion.com

Joe Kalina
Joe Kalina, MBA has distinguished himself as a leading figure in global LIMS marketing, spearheading the development of innovative new systems, notably a LIMS designed for managing COVID-19 tests. His comprehensive expertise spans across all areas of marketing including SEO, branding, demand generation, web development, and more. During his four-year tenure as Director of Marketing at Xybion Digital, Joe expanded the horizons of SaaS marketing and LIMS technology, transforming Xybion LIMS into an award-winning brand and co-authoring several industry guides with Hannah Jurolan that equip forward-thinking scientific leaders with the knowledge to harness the latest advancements in modern laboratory technology.
Email: jkalina@xybion.com
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 23 www.international-biopharma.com
Application Note

Optimising Pharmaceutical Processes: A Guide to Lyophilisation Cycle Development
Lyophilisation, commonly known as freeze-drying, is a critical unit operation in the pharmaceutical industry used to preserve and stabilise both small and large molecule drug products and biologics, including monoclonal antibodies, vaccines and peptides. Lyophilisation is a process that involves freezing a liquid drug product and then removing the frozen solvent via sublimation, providing a stable solid matrix of drug product and other excipients. This method is particularly suitable for heat-sensitive molecules, as it dramatically mitigates hydrolysis degradation found in liquid product, is more product-sensitive and practical than other drying methods, and avoids the difficulties of multi-component powder filling.
Biopharmaceutical companies have increasingly favoured lyophilisation for the formulation of their pharmaceutical products. Primarily, the driving factors leading to the increased use of lyophilisation is the ability to stabilise the drug product and excipients in a solid matrix, increasing the shelf life of the product. This, along with the removal of solvents, has a positive impact on storage and distribution requirements. For instance, many lyophilised drug products experience an increase in thermal stability and no longer require frozen storage. This provides a more cost effective, lower risk, and efficient way to optimise storage and distribution. This is particularly beneficial for drug products that are shipped to countries with tropical climates or lower infrastructure, where temperature may affect the stability of a product, and cold chain storage may not be available.
As companies continue to pioneer new molecules and treatments, it is clear that the stability of these molecules has increasingly become a detrimental factor upon every iteration, and that lyophilisation is the pathway to a solution. At PCI, we believe lyophilisation cycle development is not only a science, but an art; each drug product that comes into the laboratory presents unique challenges, and the design of a cycle requires an understanding of individual chemistry, characteristics, and interaction to yield a high quality product in every cycle. While there are a myriad of tools and techniques to perform, the below is an overall guide to the lyophilisation process, and some of the steps needed for success.
The Lyophilisation Cycle
Lyophilisation involves a series of steps to achieve optimal product stability and quality. While there are individual intricacies within these steps, they can be broadly categorised into three phases: freezing, primary drying, and secondary drying.
1. Thermal Treatment (Freezing) Phase
The first step in lyophilisation is the initial freezing and subsequent thermodynamic arrangement of the product, known as thermal treatment. Thermal treatment is a simple yet crucial
step to ensure full nucleation of the solvent and generate uniform frozen matrix to prepare the product for sublimation. Controlled freezing rates, along with annealing and process knowledge of super cooling effects, are often employed to achieve uniform ice crystal distribution. Newer technologies are also providing the ability to nucleate on demand, further increasing product uniformity across lyophiliser shelves, and is a highlight in future lyophilisation technology.
At the beginning of the lyophilisation process, products must be formulated in such a way that they are suitable to undergo thermal treatment. This often involves the inclusion of cryoprotectants such as saccharides and polyols to protect the product during freezing. Cryoprotectants help maintain the structural integrity of the product by protecting drug substance molecules against drying stresses and, in the case of biologics, help maintain conformation and prevent agglomeration. Bulking agents may also be added to the formulation to ensure a stable and elegant cake post lyophilisation.
2. Primary Drying Phase – Sublimation
Following thermal treatment, the most crucial aspect of the lyophilisation process begins – primary drying. Primary drying is the namesake thermodynamic process in freeze-drying and, in turn, the most critical to understand. Initially, a vacuum is applied at a very low temperature (the final freeze temperature) and set to control; for most processes this is typically between 30–300 mTorr. Once the vacuum is controlled, the shelf temperature is gradually increased to provide heat to the frozen product. Once the vapour pressure of the frozen solvent surpasses the pressure of the lyophiliser, sublimation begins. The temperature and pressure set points during primary drying, along with the times required, are critical to achieving efficient sublimation without compromising product quality. PAT tools such as Pirani gauge monitoring and product temperature monitoring, thermal characterisation analytics (mDSC, FDM) and technical prowess of analysis (e.g. understanding of product resistivity, evaporative cooling, and thermal treatment impact) are just some of processes that the PCI development teams use to strike the delicate balance between drying time and subsequent product quality. The process is strictly monitored while solvent undergoes sublimation, and upon completion, will have the appearance of a successfully lyophilised product.
3. Secondary Drying Phase – Desorption
Once primary drying is successfully complete, the process has typically removed between 90–95% of the solvent and produced a physically stable lyophilised matrix. There is one problem, however; there is often remaining solvent that is bound between crystals that cannot be fully removed from the energy input of sublimation alone. The final phase, secondary drying, involves further removal of the residual moisture in the lyophilised product by increasing the temperature and removing bound solvent via desorption. The temperature and rate of drying are primarily limited by the stability of the active pharmaceutical
24 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
ingredient (API) or bulk drug substance (BDS), so care must be taken to prevent degradation of the product. Monitoring residual moisture content is crucial during this phase, and critical to map and understand.
While there are a plethora of other characteristics and intermediary phases which need to be analysed and gauged throughout the process, successful design of the three phases above should yield an acceptably lyophilised product that can withstand the stresses, pathways, and time to get towards the most critical person in the process – the patient.
Critical Success Factors in Lyophilisation
A successful lyophilisation cycle can maintain the Critical Quality Attributes (CQAs) of the product throughout the product lifecycle with minimum time and energy consumption. Below are some critical success factors:
1. Product Formulation
The composition of the product, including excipients and the choice of cryoprotectants and bulking agents, significantly influences lyophilisation cycle development and the overall efficiency of the cycle. The product formulary must be designed with the lyophilisation process in mind, and any changes to the formulary must be heavily scrutinised against each phase of the lyophilisation process to ensure quality is maintained.
2. Drying Chamber Design
The design of the lyophilisation chamber affects the efficiency of the process. Factors such as chamber design (internal vs. external condensing), shelf temperature uniformity, chamber pressure control, and condenser capacity are critical in achieving consistent and reliable lyophilisation cycles. Ideally, chambers are rigorously modelled so that vial-to-vial variability is minimised.
3. Vacuum Level and Pressure Control
Proper vacuum levels and pressure control during the primary drying phase are essential for efficient sublimation. Monitoring and adjustment of these parameters ensure the removal of water vapour without compromising the structural integrity of the product.
4. Temperature Control
Precise temperature control throughout the lyophilisation cycle is vital. Both freezing and drying temperatures must be carefully monitored and controlled to prevent product collapse, degradation, or formation of analogous products.
5. Monitoring and Control Systems
Advanced monitoring and control systems are integral to lyophilisation cycle development. Real-time monitoring of critical parameters, such as shelf temperature and pressure, and PATs such as Pirani gauges, mass spectrophotometers, and state-of-the-art monitoring software, ensure process consistency and product quality.
Lyophilisation
Cycle Development Process
Lyophilisation cycle development is a meticulous and multifaceted task that requires careful consideration of various parameters to ensure product quality, efficacy, and stability is designed into the product during development. The development of an optimal lyophilisation cycle involves several steps:
Application Note
1. Formulation Design
The product’s formulation must be carefully designed to ensure that it is suitable for lyophilisation as the composition of the product, including buffers, excipients, and the choice of cryoprotectants, will significantly influence cycle development. The drug product formulation therefore must be optimised to ensure product stability and maintain the desired characteristics throughout the freezing and drying process.
2. Thermal Characterisation
Cycle development begins with understanding of what critical process parameters (CPPs) we typically see in the lyophilisation process, and leverage that understanding by ensuring a formulation is physically suited to undergo lyophilisation at the designed CPPs. Thermal characterisation utilises techniques such as Modulated Differential Scanning Calorimetry (mDSC) and Freeze Drug Microscopy (FDM) to establish the critical temperatures of the product, i.e. the temperatures in which the product undergoes a thermodynamic change in state via glass transition, recrystallisation, and eutectic melt. Even a qualitative change of state observed via FDM (collapse onset) is crucial to the characterisation of the product. Once established, the focus is placed back on the lyophilisation cycle parameters, and temperature and vacuum levels are recommended to ensure product quality and prevent failure.
3. Cycle Design and Optimisation
The cycle’s parameters, including freezing rate, shelf temperature, and vacuum pressure, are determined based on the product’s characteristics and stability requirements. Guided by Quality by Design (QbD) principles, cycle design is fine-tuned through a series of experiments to achieve an overall successful design space and range in which the lyophiliser parameters can operate with success. Lyophilisation cycle parameters are optimised for multiple factors such as a low residual moisture, cake appearance, reconstitution, low degradation, and total run time. Optimising the cycle for total run time can lead to cost efficiencies over the lifecycle of a product.
4. Cycle Validation
The optimum lyophilisation cycle is then validated to ensure reproducibility, consistency, and robustness. This step is essential for scalability and to meet regulatory standards.
The Future of Lyophilisation and Choosing a CDMO Partner
As the number of biologic molecules in the drug development pipeline increases, more and more products will stand to benefit from lyophilisation, many of which may not be commercially viable without lyophilisation. As noted in the LyoHub 2023 Annual Report, in the past decade submissions for lyophilised drugs approvals have increased by an average 15%. From 2012 to 2022, a total of 336 lyophilised drugs were approved which constitute 59% of the total fillings since 1954. In 2022, the US FDA approved 32 lyophilised drug products of which oncology and infectious diseases represented 82% of approvals equally.1
As a leading global CDMO, PCI Pharma Services is an expert and innovator in lyophilisation and offers one of the largest lyophilisation capacities in the industry. With over 25 years of experience we have the scientific expertise, global facilities, and scalable equipment to help our clients achieve success. We are uniquely positioned to develop lyophilisation cycles
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 25 www.international-biopharma.com 25

from the beginning or to optimise existing cycles, providing a commercially desirable yet economical process. Having developed over 675 lyophilisation cycles, client partners rely on us to achieve their Quality Target Product Profile and deliver life-changing therapies to patients.
CLIENT CASE STUDY
Client Challenge
A small US-based biotech company approached PCI Pharma Services seeking support for their labile monoclonal antibody drug product. They were still in the preclinical development phase and had determined that the drug product, in its current form, would not be suitable for Phase I clinical trials due to stability issues. They sought an experienced CDMO with scientific expertise to aid further formulation development and a partner with scalable sterile fill-finish and lyophilisation solutions to support not only their early phase clinical studies but also future late phase trials and ultimately commercialisation.
Process and Solution
PCI worked with the client to develop a strategic three part plan
to address not only their short-term objective of advancing their product into the clinic but also to plan for the later stage clinical trial programs. The three part development plan consisted of:
1. An initial quick start lyophilisation program to provide a limited study to conserve the client’s valuable bulk drug substance, expedite time, and reduce costs. Note: This phase appropriate solution would produce a lyophilised product suitable for Phase I trials, although further development would be required if the product advanced in the clinic.
2. A formulation development program to enhance overall liquid stability.
3. A fully optimised lyophilisation cycle to support long-term stability suitable for late stage clinical trials and future commercial manufacturing.
Part One
To address the client’s short-term needs of initiating Phase I trials with a lyophilised presentation of their drug product, PCI developed a plan to analyse the thermal properties of the products’ current liquid formulation, with the aim to design a cycle without the use of additional compounds. Using mDSC and
26 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Application Note
FDM the development team successfully defined and understood the physical characteristics of the formulated product and gained confidence that even in the current state, a cycle could be generated. This helped to guide a limited, data-based study using just two lyophilisation runs in the drug product’s current formulation, limiting drug quantity and providing a sufficient design space in which to operate. PCI was able to develop a preliminary lyophilisation cycle suitable for Phase 1 GMP clinical trial manufacturing within an accelerated timeframe of just a few weeks.
Part Two
Following the completion of the GMP clinical trial manufacturing campaign to provide supplies for their First in Human (FIH) clinical trial, the second stage of the program was initiated for future clinical trial supply. During this phase it was agreed upon with the client to undertake a development program for the drug formulation prior to further development of the lyophilisation cycle to help gain stability and mitigate elongated cycle times. To maximise the formulation development process, PCI formulation development scientists reviewed all pre-formulary data available and developed a plan to evaluate a small number of excipients to further enhance the molecule’s stability and assess a number of cryoprotectants with success of a lyophilised product in mind. A parallel development path was used to evaluate short-term stability of both the liquid and lyophilised presentations of the drug product.
With a view to the long-term cGMP manufacturing costs of the molecule, the various liquid formulations were screened prior to performing further development of the lyophilisation cycle. Developing a robust liquid formulation prior to final lyophilisation development served several purposes; primarily, having the most stable liquid formulation is beneficial if there are any delays during GMP product manufacturing, or as the program progresses and larger scale batches are needed. The additional liquid stability is required while the product is sterile filtered and filled into vials.
Additionally, as lyophilised drug products are more expensive (often 50 to 70% higher) to manufacture than GMP liquid fill/ finish production, developing a more stable liquid formulation of the finished drug product was deemed worth the additional investment in both development costs and time over the lifecycle of the product due to significant future cost savings that could be achieved. If the product has adequate stability as a liquid presentation, it may be worthwhile to pursue that pathway and during the development phase, the lyophilised dosage stability would then be used as a backup in the case of the liquid formulation is not supporting the required stability.
Part Three
In this case, the stability data did not support the case for a liquid presentation and a lyophilisation development and optimization program was initiated. The candidate formulation with the best stability data was selected for further lyophilisation development.
The selected formulation was reanalysed using mDSC and FDM to understand the thermal profile of the product and subsequently, a series of iterative lyophilisation development
Application Note
runs were conducted and evaluated against the agreed upon Critical Quality Attributes (CQA), including cake appearance, reconstitution time, moisture content, and assayed using HPLC. As the lyophilisation cycle parameters were nearing completion, a cycle optimisation program was initiated. During the optimisation phase of the program, a ‘sample thief’ was used to collect numerous samples during the secondary drying to map moisture content and residual solvents in real-time. This establish an efficient secondary drying cycle and fully characterised the design space of acceptable lyophilisation process parameters. This provided for the development of a robust and efficient lyophilisation cycle, saving significant long-term manufacturing costs.
To ensure a commercially robust lyophilisation process, reduce the risk of collapse and safeguard that the finished product consistently met the finished product CQAs at release, PCI’s expert Process Development team continued to characterise the formulation by performing “Intentional Collapse Studies” (ICS) during the final stages of lyophilisation development. This intentional collapse of the product allowed the team to fully understand the potential points of failure and verified that macro-collapse does occur in the final vial presentation where the FDM and DSC testing data indicated.
Throughout the course of the client’s clinical development journey, our experienced process development team maintained focus on the future and planned for long-term success, delivering a robust lyophilisation cycle with parameters that were transferable to any large-scale clinical/commercial freeze-dryer to support commercialisation.
At PCI, together with providing science driven, flexible scalable solutions, delivering best-in-class services efficiently and effectively, we are committed to meeting the dynamic needs of our client’s drug product journey. We immerse ourselves in every client project, working in partnership to provide collaborative, creative and tailored approaches to deliver upon our purpose of bringing life-changing therapies and patients.
REFERENCES
1. Pharmahub – Resources: LyoHUB 2023 Annual Report

Matt Bourassa
Matt Bourassa is the Manager of the Process Development group at PCI's Bedford, NH facility. During his fourteen years in the CDMO space, Matt has developed more than 30 robust and scalable lyophilisation cycles across a wide array of parenteral drug products, medical devices, and diagnostics. As an inaugural member of the Process Development team, Matt now manages highly skilled scientists in the same group, leveraging his process knowledge and technical prowess to inform scientists and clients alike, from small scale preclinical tests to late-stage characterisation and aseptic fill-finish. Matt received his B.S. in Chemical Engineering from the University of Massachusetts.
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 27 www.international-biopharma.com 27
Patients Are Waiting: Speeding Time to Treatment in Rare Disease
Rare diseases are no longer rare. Every year, people’s lives are upended by a diagnosis of one of 6,000–8,000 identified rare conditions, which collectively affect one in 17 people. Because a majority are genetic and appear early, more than half of these patients are children, and many are not expected to reach their fifth birthday.
For patients left waiting, the stakes for new medicines are high. Yet, less than five percent of rare diseases have at least one approved treatment. Even when effective therapies and medicines exist, reaching the right patients in need is challenging. Survey data depicts a long and emotionally gruelling journey for those awaiting a diagnosis. For adults, it can take up to five years, and half will receive a misdiagnosis.1
Rare diseases challenge traditional ways of doing business. They require the industry to better identify target patient populations for trials and then keep them engaged during the course of the study, even across geographies. Once a medicine is approved, it is crucial to seamlessly transition from trials to treatment given the smaller patient cohort, as the physicians who conduct the trials often become prescribers.
The good news is that the industry is making great progress in how medicines for rare diseases are developed and brought to market. From delivering better site support during clinical studies to greater connectivity between medical and commercial teams, companies are breaking down historic silos and cutting the time to treatment. Along with richer healthcare data and effective medical education for physicians on symptoms and treatment options, these advances will help those undergoing complex patient journeys.
Greater Focus on Patient Experience During Studies
Over half of orphan drug trials are eventually discontinued or fail to publish results after completion.2 Often, studies can’t recruit or lead to inconclusive results. When a trial gets off the ground, sites have to invest significant time in keeping a small number of participants engaged.
Removing excessive system and process complexity will improve their efforts. For years, sites have voiced their concerns about the multiple disconnected tools they must navigate just to keep a study going. As one site leader explains, navigating unintuitive technology absorbs time from trial activities, and can make them feel like they’re asking too much from patients: “The technology shouldn’t be the trial itself, it should support the trial. It’s taking time away from what we want to do, which is taking care of our patients.” Simplifying the technology

experience and reducing the admin burden will better meet sites’ and patients’ needs.
The knock-on effects could include better participant retention during rare disease trials. This may widen patient access to life-enhancing new treatments. Reflecting on her own experiences as a rare disease patient, Helen Shaw, co-founder of the virtual site VCTC, observes: “I see how hard it is to take part in a clinical trial. But patients do want that opportunity to be offered something that they wouldn't get in their standard care, whether additional MRIs or new medicines.”
Data Unlocks Effective Commercial Execution
Data is core to understanding where the greatest patient needs are ahead of a launch. ADVANZ PHARMA is a global biopharma company focused on specialty, hospital, and rare disease medicines. Andy Eeckhout, head of CRM and digital solutions for global commercial excellence, explains: “It’s crucial to take a patient perspective. This means using real-time data to understand the patient journey and identify the two or three most influential key opinion leaders (KOLs) per country, so we can best communicate the product across markets.”
An added complexity is that rare diseases often involve multiple specialties. Medical science liaison (MSL) teams have to get up to speed quickly on complex science before meetings with relevant experts – while also being responsible for other (more mainstream) disease areas. “Our customer-facing teams need to be agile communicators and effectively switch to a more patient-oriented, in-depth scientific discussion than with generics,” Eeckhout explains. Access to scientific resources and activity data in one place leads to higher-quality conversations. “Pre-call planning is crucial for MSLs before and after launch. The more data they can find, including on past interactions, the better,” Eeckhout adds.
Depending on the disease, there could be a long gap before a patient presents with potential symptoms, at which point HCPs will want timely access to experts and resources. Smoother handovers between field medical and sales ensure HCPs can find answers quickly and connect to MSLs if needed. ADVANZ, for example, has launched a pre-launch module in
28 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1 Therapeutics
Companies are overcoming challenges to develop, launch, and educate on new rare disease medicines faster.
its CRM so that market access, medical, and commercial teams can share information compliantly and effectively. Eeckhout notes: “Physicians need a direct line to the industry so they know who to contact when they have questions. Medical and commercial teams need to talk to each other and remain agile across customer conversations.”
Highly personalised content for HCPs is also key when targeting diseases with small patient populations. To be effective, marketing teams need clarity on the most impactful content to recommend for field visits across markets. For example, Recordati Rare Disease uses data analytics on its global repository of promotional and medical engagement tools, so it can support content use across a portfolio of 17 rare disease medicines of focus (and a growing global footprint). As its head of marketing and customer engagement, Gordon Daniels, notes, “How we engage with HCPs is critical. We need to know what percentage of our content is being developed and relevant to support different HCPs, whose patients rely on them for their rare disease diagnosis and management.”
Defy the Odds, Then Beat Them
Scientific breakthroughs that could bring hope to rare disease patients and their families come from organisations willing to embark on a long and risky trial-to-treatment pathway.
Companies are reducing the time to treatment through better support for sites, freeing them up to focus on patient recruitment and retention during trials. Once a drug is approved,



Therapeutics
connected technology and deep data can facilitate collaboration between functions that have not historically worked together so they can identify, engage, and provide medical education to relevant HCPs and KOLs.
Every rare disease patient faces a daunting journey. As one biotech leader points out: “Their diagnosis is with them every day. Often, they can’t pronounce the condition, and don’t know anyone else who has it.” Life sciences will do its part to help these patients defy the odds – and eventually beat them.

Chris Moore
As president of Veeva Europe, Chris Moore is responsible for growing the business in the region. A 30+ year veteran of the life sciences industry, Chris started his career at ICI Pharmaceuticals (now AstraZeneca). Most recently, Chris was the lead partner for life sciences for Europe, the Middle East, and Africa at EY. Chris holds a Bachelor of Science degree in information technology from the University of Salford.

Media and Communications
IPI
Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.
www.international-pharma.com
JCS
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.
www.journalforclinicalstudies.com
IAHJ
Peer Reviewed, IAHJ looks into the entire outsourcing mana-gement of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.
www.international-animalhealth.com
IBI
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.
www.international-biopharma.com
PNP


Pharma Nature Positive, is a platform for all stakeholders in this industry to influence decision making by regulators, governments, investors and other service providers to achieve Nature Net Positive Results. This journal will enable pharma the ability to choose the right services to attain this goal.
www.pharmanaturepositive.com
senglobalcoms.com
Manufacturing & Processing
Elevating Biomanufacturing Efficiency with N-1 Perfusion Technology
In the dynamic world of biomanufacturing, the quest for improved efficiency and productivity continues to shape industry advancements. Among these innovations, the emergence of N-1 perfusion technology stands out as a pivotal milestone, offering a practical pathway to enhance production processes and boost overall efficiency.
N-1 perfusion operates on a simple yet powerful principle known as process intensification. This strategic approach aims to maximise the output of manufacturing facilities and streamline the timelines of production. While continuous processing has garnered attention for its efficiency gains, the traditional fed-batch culture remains a fundamental method in stable protein production using mammalian cell culture. In this context, techniques to intensify fed-batch processes, such as N-1 perfusion, present exciting opportunities for the advancement of biomanufacturing practices.
The appeal of N-1 perfusion lies in its ability to support high cell densities with sustained exponential growth and viability. This capability extends beyond the production phase to include the critical aspect of seed train intensification – a fundamental process in bioprocessing. By enabling a significant increase in cell density, N-1 perfusion offers a way to streamline operations by reducing the size or number of required seed reactors, thereby optimising facility space and investment costs. At the heart of this strategy is the concept of seeding the fed-batch production bioreactor with substantially higher cell densities from the N-1 seed culture. This strategic shift in the early growth phase of production paves the way for streamlined timelines without compromising the essential growth and yield profiles of the final product.
In this article, we delve into the practical applications of N-1 perfusion technology, showcasing its potential to transform biomanufacturing processes. Through real-world examples and data, we demonstrate how N-1 perfusion enables facilities to achieve higher productivity and improved efficiency – all while optimising resource utilisation and operational costs. Let's now break down the discussion into key aspects of N-1 perfusion technology, exploring its suitability for various processes and products, considerations for implementation, its rising adoption in the industry, and future directions.
Suitable
Processes and Products for N-1 Perfusion
N-1 (seed) intensification through perfusion technology proves highly beneficial where the goal is to enhance productivity without major alterations to the production process. The essence of N-1 perfusion lies in increasing the cell density in the pre-production bioreactor, denoted as N-1 (N being the production bioreactor). This increase in cell density is achieved more effectively using perfusion technology compared to routine batch modes. This intensification strategy is applicable
to a wide range of cell lines and products, provided the cell line can achieve high densities while maintaining viability.
Processes or Products Not Ideal for N-1 Perfusion
In the realm of biologics, most things are not a one-size-fits-all solution. While N-1 perfusion can theoretically be applied to any process, a critical cost-benefit analysis is key. The potential efficiency gains of N-1 perfusion must be weighed against the costs of goods, initial capital investments for equipment, site maintenance, and process development. Furthermore, the enhancement in titer must align with efficient downstream processes to improve overall facility throughput and reduce the cost per gram of product manufacturing.
Increasing Adoption of N-1 Perfusion
Interest in N-1 perfusion has been steadily growing over the years, especially among manufacturers facing specific challenges in meeting market demands. The allure of quick entry into clinical trials, coupled with established knowledge and platform processes such as fed-batch, often prompts the initial choice. Subsequently, the evaluation of intensification options occurs as product requirements become clearer through clinical studies. Notably, N-1 perfusion can be integrated into existing Fed-Batch processes with minimal modifications, avoiding a complete overhaul of infrastructure. However, maintaining product quality attributes remains paramount to successful adoption.
N-1 Perfusion in Continuous Bioreactors
N-1 perfusion primarily serves as a seed-stage intensification method and may not directly translate to continuous production batch operations. Continuous manufacturing scenarios are better suited for N perfusion.
Impact on Reactor Scales and Productivity
Intensification through N-1 perfusion results in a 2-3-fold improvement in titer, enabling significantly higher throughput

30 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Manufacturing & Processing
from existing production bioreactors. A 2kL reactor, for instance, can achieve the productivity equivalent of a 4kL or 6kL reactor through this method.
Product Purity in Perfused Systems
The final product's purity remains comparable to that of traditional fed-batch processes, despite continuous by-product removal in perfusion systems.
Future Developments
Future advancements in N-1 perfusion may involve the integration of Process Analytical Technology (PAT) tools and enhanced downstream processing. The combination of these technologies could further amplify the advantages of N-1 perfusion, improving efficiency and product quality.
Clinical Phase Considerations
N-1 perfusion technology is versatile and can be implemented at any phase of product clinical trials. While Phase 3 is a common starting point, early adoption prior to Phase 1 offers earlier benefits and reduces the risk of future process changes.
Adoption in CDMOs
Not all CDMOs currently offer N-1 perfusion due to its nuanced nature. Developing the optimal media combination to sustain higher cell densities and boost productivity requires specific optimization with individual cell line platforms.
Syngene's Success with N-1 Perfusion
Syngene has showcased the effectiveness of N-1 perfusion with multiple monoclonal antibodies, achieving titer improvements of 2-3 folds compared to conventional fed-batch processes. This has resulted in impressive titers of up to 12 g/L.
Conclusion
The rise of N-1 perfusion technology marks a significant step forward in biomanufacturing, offering a practical and efficient approach to improve productivity and product quality. Through this discussion, we have explored the various ways in which N-1 perfusion enhances production processes and


streamlines operations. The core benefit of N-1 perfusion lies in its ability to intensify the seed train process by supporting high-density cell cultures. This means quicker production timelines, reduced facility costs, and increased efficiency without compromising the quality of the final product. Looking ahead, the potential for N-1 perfusion technology is vast. Its integration with emerging tools like Process Analytical Technology (PAT) and continuous downstream processing promises even greater efficiency gains and innovation in biomanufacturing. As exemplified by the success stories of Syngene and other industry pioneers, N-1 perfusion stands as a testament to the continual advancement of bioprocessing techniques. With further research and adoption, N-1 perfusion is poised to reshape the biopharmaceutical landscape, setting new standards for productivity and quality.

Sridevi Khambhampaty
Sridevi holds an MSc in Biotechnology and a doctorate in Biological Sciences. She completed her postdoctoral fellowship at Stanford University School of Medicine, focusing on cell biology. With over 20 years of experience, Sridevi has led bioanalytical and quality control departments, focusing on assessing protein therapeutics through in vitro and in vivo methods, including functional characterisation, pharmacokinetics, pharmacodynamics and immunogenicity evaluations. Furthermore, Sridevi has managed teams responsible for conducting lot release bioassays and validating analytical methods, encompassing areas like HPLC, biochemistry, cell-based bioassay and microbiology. She also oversaw stability studies within these roles. Sridevi's career includes leadership positions at Dr Reddy's Laboratories and Intas Pharmaceuticals Ltd. Currently serving as Vice President at Syngene International Limited, she oversees biopharmaceutical production. In this role, Sridevi is responsible for optimising processes, ensuring regulatory compliance, and driving innovation in manufacturing.
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 31 www.international-biopharma.com
Manufacturing & Processing
Considerations on the Development and Manufacturing of Generic Peptides
The development of generic versions of peptides such as Semaglutide, the drug substance inside Novo Nordisk’s blockbuster drugs like Ozempic®, Rybelsus® or Wegovy®, or Tirzepatide, the API in Eli Lilly’s Mounjaro®, has emerged as a significant area of interest and opportunity. However, generic companies face critical decisions regarding the choice between recombinant and synthetic semaglutide. The path chosen can significantly impact regulatory approval, safety considerations, and market competitiveness.
The Importance of Generic Competition
GLP-1 agonist drugs were developed to treat Type II Diabetes but became blockbusters after getting approved or prescribed off-label for weight loss. An analysis of electronic health records shared with CNN by Epic Records estimates that around 1.7% of all Americans have been prescribed a semaglutide medication in 2023 alone, reflecting a nearly 40-times increase over the past 5 years. In a Fierce Pharma article published in September 2023, J.P. Morgan’s market projection for GLP-1 drugs was of $71 billion by 2032, with Novo and Eli Lilly each accounting for 45% of its sales. Pfizer’s CEO Albert Bourla, Ph.D., estimated that the market could reach $90 billion by 2031.
The demand for semaglutide is growing faster than Novo’s efforts to build up capacity, creating shortages in the supplychain and negatively impacting access to Type II Diabetes patients. As of May 2023, Ozempic® and Wegovy® are both listed on FDA’s Drug Shortages list. To make it worse, FDA issued a form 483 after inspecting Novo’s Clayton facility between July 6 and July 13, 2023, flagging manufacturing shortfalls at the Danish drugmaker’s production plant in North Carolina, where Semaglutide API is manufactured for Rybelsus®. In December 2023, the European Medicines Agency issued an announcement on the shortage of Ozempic explaining that “Increased demand for Ozempic coupled with capacity constraints at some of the manufacturing sites have led to shortages. Although the company is taking mitigating measures, the shortage is expected to worsen in December 2023 and continue throughout 2024. It is uncertain when supplies will be sufficient to fully meet current demand.” In January 2024, in the UK, the NHS England and the Department for Health and Social Care issued a National Patient Safety Alert to address supply issues with GLP-1 RA medication, stating that “The global shortage in supply is partly due to a surge in off-label prescriptions of the drug semaglutide being issued for weight loss, which is exceeding supply.”
Seven out of 10 adults and three out of 10 children in the United States are overweight or obese, according to the Centers for Disease Control and Prevention. Total annual medical costs for obese adults are an average of $1,861 higher than medical costs for people with healthy weight. That amount increases to $3,097 for a severely obese adult. By 2035, half of the world’s population – about four billion people – will meet

the definitions of being overweight or obese, according to an estimate from the World Obesity Federation. While weight management and sustainable weight loss could drive significant health savings and improved health outcomes, quantifiable results will likely take time.
In the US, without insurance, the average monthly cost of a Mounjaro® (Tirzepatide) prescription to treat Type 2 diabetes is between $1,000 to $1,200. The cost for Rybelsus® oral tablet 3 mg is around $1,029 for a supply of 30 tablets, while a supply of 2 milliliters subcutaneous solution of Wegovy® is around $1,430 and of $1,029 per 1.5 milliliters of Ozempic®. About 60% of adults who are trying to lose weight, and 25% of those who aren’t currently trying to lose weight, would be interested in trying a safe and effective weight-loss prescription drug. However, only 16% would be interested in the drug if it was not covered by insurance or available as an affordable generic.

The Good – The key Novo Nordisk patent covering the original recombinant Semaglutide expires in most countries in 2026, while Lilly’s Tirzepatide key patent ensures protection from generics until May 2036, giving it another decade of protection. After 2026 the market opens up for generic semaglutide manufacturers to offer bioequivalent alternatives, offering cost savings, increased access to essential therapies, reducing shortages and reducing the risk of illegal and sub-standard/ fake drug sales.
32 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
PEER REVIEWED
Manufacturing & Processing
The Bad – When a drug is in shortage, compounders may be able to prepare a compounded version of that drug if they meet certain requirements in the Federal Food, Drug, and Cosmetic (FD&C) Act. However, FDA has received reports that in some cases, compounders may be using salt forms of semaglutide, including semaglutide sodium and semaglutide acetate. The salt forms are different active ingredients than is used the approved drugs, which contain the base form of semaglutide. The agency is not aware of any basis for compounding using the salt forms that would meet the FD&C requirements for types of active ingredients that can be compounded.
The Ugly – When a high value drug is in shortage, criminals take advantage and start selling life-threatening counterfeit versions through the internet and other illegal distribution channels, sometimes even infiltrating the legitimate supplychain. In December 2023, the USFDA found counterfeit Ozempic (semaglutide) injection 1 mg in the legitimate U.S. drug supply chain and has seized thousands of units of the product. The agency advised wholesalers, retail pharmacies, health care practitioners and patients to check the product they have received and not distribute, use, or sell products labeled with lot number NAR0074 and serial number 430834149057. (for more information visit https://www.fda.gov/drugs/ drug-safety-and-availability/fda-warns-consumers-not-usecounterfeit-ozempic-semaglutide-found-us-drug-supply-chain).
The Hope – While ANDA full generic versions of the oral drug need to wait further for patent extension expiry dates, the availability of a generic drug substance has the potential to address shortages and immediately opens door to 505(b)2 super-generics, creating opportunities for generic innovation.
Understanding the Differences Between Recombinant and Synthetic Peptides
Glucagon-Like Peptide 1 (GLP-1) agonists, such as Semaglutide, Tirzepatide, Liraglutide or Exenatide, can help regulate blood sugar, slow digestion, and decrease appetite. They can be synthesized through recombinant DNA technology (recombinant peptides) or by solid-phase synthesis (synthetic peptides).
Solid-phase Peptide Synthesis (SPPS) is a widely used method for the chemical synthesis of peptides. In SPPS, the peptide chain is assembled step by step on a solid support matrix, typically a resin bead. The process begins with the attachment of the C-terminal amino acid to the resin, followed by sequential addition of protected amino acids. Each amino acid is activated with coupling reagents to facilitate its attachment to the growing peptide chain. After each coupling step, the unreacted amino groups are capped to prevent unwanted side reactions. Cleavage of the fully assembled peptide from the resin and removal of side-chain protecting groups yield the desired peptide product. Historically, dichloromethane (DCM) was used as a solvent for solid phase synthesis as the kinetics of amino acid activation and amine coupling were much more favorable. However, solubility concerns, particularly for Fmoc-protected amino acids limited the utility of the solvent. Nowadays, dimethylformamide (DMF), N,N-dimethylacetamide (DMA) and N-methylpyrolidone (NMP) are the three principal solvents for both microwave assisted and room temperature solid phase peptide synthesis.
Recombinant peptide synthesis involves the production of peptides through genetic engineering techniques, typically utilising host organisms such as bacteria, yeast, or mammalian cells. These organisms are cultivated in aqueous growth media containing nutrients, salts, and other essential components required for cell growth and protein expression. The recombinant peptides are produced intracellularly or secreted into the culture medium, where they can be harvested and purified through various downstream processes. Therefore, while organic solvents may be used in purification steps such as chromatography, their use in the actual manufacturing process is minimal compared to chemical peptide synthesis methods, making it much more sustainable. In the recombinant process, genes encoding the desired peptide sequence are inserted into the host organism's genome, leading to the production of recombinant proteins, which are then cleaved to yield the target peptide. Recombinant peptide synthesis offers several advantages, including precise control over peptide sequence, high purity, and scalability. Furthermore, this method allows for the incorporation of post-translational modifications and facilitates the production of peptides that may be challenging to synthesize using traditional chemical methods.

INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 33 www.international-biopharma.com
Manufacturing & Processing
Regulatory Considerations
Regulatory agencies are issuing guidance on the development and manufacturing requirements for approval and market entry of generic peptides. Some generic companies may be enticed to expedite their development process by leveraging the USFDA’s Guidance for Industry on “ANDAs for Certain Highly Purified Synthetic Peptide Drug Products That Refer to Listed Drugs of rDNA Origin.” This guidance offers a waiver for clinical validation using the fully synthetic pathway.
However, it's essential to recognise the potential challenges associated with using the waiver when filling an ANDA with a significantly different fully synthetic peptide route. Those using the ‘shortcut’ may expect to receive a thick Complete Response Letter (CRL) requesting evidence that new specified peptiderelated impurities, even if present in concentrations below 0.5% of the drug substance, do not compromise the safety or effectiveness compared to the recombinant peptide RLD. Such evidence typically requires extensive biological evaluations, as outlined in the “Sameness Evaluations in an ANDA – Active Ingredients" Draft Guidance for Industry.
The use of generic recombinant peptides offers a compelling alternative, reducing risk by mirroring the manufacturing approach used by innovators. By adopting the appropriate

recombinant process for their generic peptide, companies benefit from a demonstrated similar impurity profile, thus reducing the risk of immunogenic reactions stemming from newly specified peptide-related impurities.
Patient and Healthcare Providers Perspectives
Type II Diabetes patients and other patients in need of weight loss treatment look forward to the end of the drug shortage. However, both patients and healthcare providers may express concerns about switching between recombinant originator and fully synthetic generic products, particularly in chronic or life-threatening conditions where treatment consistency is paramount. Therefore, the availability of affordable generic recombinant peptides reduces questions about safety, efficacy, and therapeutic interchangeability. Like in any other drug in the market, robust pharmacovigilance and post-marketing surveillance mechanisms are essential to monitor and address any potential safety issues associated with generic substitutions.
Health Economics and Sustainability
The adoption of generic recombinant peptides has significant implications for health economics and affordability as well as for sustainability. Generic competition has the potential to reduce healthcare expenditures, particularly in disease areas with high treatment costs. However, the realisation of cost savings depends on factors such as market dynamics, pricing strategies, insurance plans and reimbursement policies, and patient access
34 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Manufacturing & Processing
pathways. Policymakers and healthcare stakeholders must strike a balance between promoting competition and ensuring economically sustainable access to essential therapies.
From an environmental sustainability perspective, a recombinant process offers significant environmental advantages over a fully synthetic process, often replacing tens of chemical reactions with huge volumes of organic solvents and toxic chemical reagents that end up as waste streams by a single step of aqueous media fermentation. As such, generic pharma companies with sustainability goals can only take the recombinant process pathway.
Conclusion
The development and approval of generic versions of peptides such as Semaglutide and Tirzepatide is a focus and evolving aspect of today’s pharmaceutical industry. While generic competition holds promise in enhancing affordability, expanding patient access, and stimulating innovation, it also presents challenges related to regulatory requirements, market dynamics, patient safety, health economics and sustainability. A balanced approach that prioritises scientific rigour, regulatory oversight, patient-centricity, and healthcare sustainability is essential to realise the full potential of generic recombinant peptides while safeguarding public health and promoting therapeutic innovation. As the landscape continues to evolve, collaboration between stakeholders across the healthcare ecosystem will be critical in navigating the complexities and opportunities associated with generic peptides. Embracing the right strategic approaches to
generic development will be paramount in driving success and facilitating access to affordable, high-quality and sustainable therapeutics for patients worldwide. Selecting regulatory savvy and cGMP inspected manufacturers to produce the API with the appropriate recombinant process is key to success.

Rafael Antunes
Rafael Antunes is the Vice President of Business Development of Aurisco Pharmaceutical in Europe. He has over 20 years of Pharma Industry Experience, working in International European and Chinese, Generic and CDMO companies. Broad experience in multiple roles in Process Chemistry R&D, Tech-Transfer, Scale-up, Pilot-Scale and Commercial cGMP Manufacturing, Procurement, Management, Product Development and Licensing and Business Development. Strong Scientific and Business Management backgrounds and relevant HSE, Quality and Regulatory experience. Has been a qualified cGMP and ISO14001 auditor and participated as industry representative in RX360 and EFCG (European Fine Chemicals Group – a pharma industry CDMO lobby group, member of the broader European Chemical Industry Council (CEFIC). It has represented the EFCG at USFDA GDUFA II implementation meetings and Regulatory Science meetings. Championed the adoption of Science Based targets (SBTi), Ecovadis Sustainability ranking and joined the Sustainable Procurement Pledge (SPP).

INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 35 www.international-biopharma.com
5,000L
Single-use Bioreactors:
The Next Generation in Biologics Manufacturing
The Thermo Fisher Scientific HyPerforma™ DynaDrive™ Single-Use Bioreactor line is suited for volumes ranging from 50L to 5,000L. Optimised for modern cell culture processes in a scalable, ergonomic design, the platform allows intensified, flexible manufacturing, enhanced by high-power input per volume, and better volumetric mass transfer performance.
This whitepaper highlights features and benefits of the DynaDrive™ SUB platform and explains how any new or existing facility can leverage the platform to achieve expected development and manufacturing objectives. From pre-clinical trials through commercialisation, manufactures and companies looking to outsource their biologics can use the DynaDrive™ SUB to gain maximum efficiency and flexibility across a wide range of processes, cell lines, and molecules.
As scientific advancement in biopharmaceuticals continues to rise, so will the market. By 2030, total biologics revenues are expected to be worth $450 billion – up from the $285 billion biologics revenues seen in 2020. This rapid and continual growth has created a demand for therapeutics, expanding indications for biologics, and the growing portfolios of biosimilars. While these projections are good news for patients and for makers of therapies, growth always comes with new challenges. In this case, the industry must learn how to deploy efficient, flexible manufacturing technologies that can respond to many variables. These variables include the proliferation of new biologics, rapid shifts in annual volumetric requirements, and improvements in cell culture strategies.
A new generation of single-use bioreactors (SUBs), built to deliver high-volume performance, addresses many of these issues. This innovation outperforms existing 2,000L SUBs that
were once the only cost-effective alternative to stainless steel bioreactors.
Redesigned for Higher kLa Through Better Mixing Performance
Most SUBs with legacy designs have a single agitator attached to the top or bottom of the bag. Neither design mixes to a high degree of homogeneity, which can lead to product gradients and quality issues. The DynaDrive™ SUB, however, has a redesigned agitator shaft that spans the entire length of the bag – this new design contributes significantly to the technology’s reliable turndown ratio of up to 20:1.
Prior to the introduction of DynaDrive™ SUB, most turndown ratios on SUBs ranged from 2:1 to 5:1. Fast forward to today’s needs, many modern processes use higher producing cell lines and greater sophistication in process design. The need for greater oxygen transfer and mixing has become even more critical in process development.
One way to improve the oxygen transfer rate (OTR) is to increase agitation, thereby distributing gases more efficiently in a bioreactor. Many traditional SUBs, particularly at larger scales, are excessively challenged in terms of OTR for intensified processes and higher viable cell densities, making scale-up unfeasible.

36 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Application Note
The DynaDrive™ SUB helps overcome such limitations and improves processes in several ways. The increased efficiency of the drive train allows lower RPM setpoints while maintaining P/V ratio. The lower RPM correlates with lower mechanical shear stress experienced by the cells. Multiple impellers provide significantly more power in high-demand applications.
Bags are securely fastened at the top and bottom of the bioreactor and multiple impellers are distributed throughout the bag to achieve optimal mixing with lower gas consumption. The enhanced laser-drilled hole sparger in the DynaDrive™ SUB minimises gas entrance velocity, reduces foam formation, and is scalable from small-to large-scale bioreactors for a wide variety of processes.
The state-of-the-art design offers best-in-class performance with mixing power of greater than 80 W/m3 and a mass transfer rate of up to 40 hr-1. Biomanufacturers gain freedom to manage processes without losing productivity or product quality as they migrate from bench-scale bioreactors into large-scale manufacturing.

Smarter Ergonomics and Greater Operating Efficiency
The DynaDrive™ SUB has flexible drive trains that allow smaller packaging, simpler material airlock transfers, and quicker set up in the suite. A fully open door allows operators access to the internal bioreactor space for easier set up and take down.
The 3,000L and 5,000L DynaDrive™ SUBs have semiautomated loading systems that give operators the ability to load and unload bioprocess containers quickly with less risk of damage to the container. Installation can be completed in less than 45 minutes for the 5000L vessel, which is 25% faster than 2000L bioreactors. Fewer connections inside the bioreactor reduce labour costs and lower the risk of contamination at each connection.
Enabling Intensified Seed-train Strategy
In a traditional scale-up, cells expand from their initial vials through a variety of intermediate vessels to generate a cell mass that’s sufficient for a production run. Scaling up this way typically requires different vessels at each n-stage operation. Each switch from one vessel to another adds time and expense to set up and fill a new shake flask, rocker bag, or stirred tank reactor. And each transfer and manipulation of the cells adds the risk of contamination or improper handling, which can affect optimal cell behaviour.
Application Note
With a higher turndown ratio than ever before, the DynaDrive™ SUB allows users to consolidate multiple scale-up steps in a single vessel.
The DynaDrive™ SUB, with a maximum vessel volume of 50L, can be operated at a 10:1 turndown ratio and scale up can be accomplished in the same vessel – starting at 5L and ending at 50L. With a turndown ratio of up to 20:1 in the 500L and 5,000L SUBs, there can be starting volumes of 25L and 250L for each reactor respectively.
Staying in one bioreactor vessel and increasing volume as cells grow offers the following benefits:
• Reduces overall labour associated with cell-culture.
• Lowers consumable costs.
• Trims time in suite, enabling greater suite throughput and faster cycle time.
Higher Batch Volumes, Lower Operating Cost, and Reduced Capital Investment
DynaDrive™ SUB technology (5,000L) delivers mid-range economies of scale with less upfront capital and lower long-term operational costs. When a product’s success in the market is unproven, choosing mid-range 5,000L SUBs help biologics manufacturers avoid scaling up by moving processes to larger facilities, where they will be subject to transfer and validation activities. Manufacturers retain greater flexibility for processing multiple products in the same space – they can also process low-demand products that don’t meet minimum volumes requirements imposed by stainless-steel CMOs.

When considering a bioreactor for scaling and production, phase-appropriate demand of kilograms product should be tied to phase-appropriate volumes and batch count as described in Figure 1. For most Phase I activity, 50L SUBs (~0.75 kg required at P-I) provide sufficient batch-count to allow for continued CMC development exercises.
Similarly, 500L SUBs are well sized for Phase II (~ 3-4 kg at P-II). While enough for Phase III (~30 kg at P-III), 2000L reactors are limited in commercial scale-out possibilities. Comparatively,
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 37 www.international-biopharma.com 37
Application Note

5000L reactors meet demand at Phase 3 and can readily scale out to even the largest commercial manufacturing demands. The high turndown ratio of the DynaDrive™ SUBs further helps to reduce capital investment.
DynaDrive™ SUBs gives new sites and retrofitted facilities a wider range of volume alternatives with better operating efficiency and less time in suite, while retaining the benefits of SUB technology. One 5,000L DynaDrive™ SUB, for example, reduces the cost of consumables and labour by 50% and produces two and a half times the volume of a 2,000L bioreactor in the same time and footprint.
Existing sites with ceiling height restrictions can replace 2,000L vessels with DynaDrive™ SUB 3,000L and leave controllers in place, improving efficiency by 50% without investing additional capital in new facilities. New facilities can build higher ceilings and achieve two and a half times the output of traditional 2,000L SUBs when they choose 5,000L DynaDrive™ SUB technology.
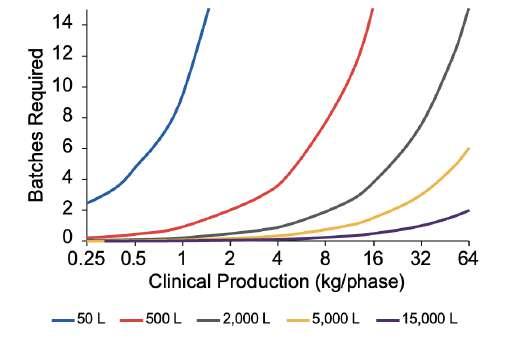
Benefits for Facility or Upstream Suite Construction
The 5,000L DynaDrive™ SUB also provides biomanufacturers with several operational efficiencies when compared to 2,000L SUBs or stainless-steel bioreactors:
• Eliminating clean-up and validation costs when switching from one product to another in a stainless- steel system.
• Meeting high-titer product demands and pipelines in a more versatile mid- to small-capacity facility.
• Avoiding long periods of low use and low efficiency when product pipelines aren’t large or broad enough to support
stainless-steel economies of scale.
• Responding to changing production volumes due to shifts in the clinical pipeline, such as targeted drugs for orphan diseases and precision medicine with lower volumetric requirements vs. blockbuster biologics.
Conclusion
With strong application data, showing cell growth and viabilities across all scales and cell densities, the DynaDrive™ SUB platform is a trusted solution that brings flexible manufacturing to modern cell culture processes.
Learn more about how to leverage DynaDrive™ S.U.B.s for better Cost of Goods Sold and Net Present Cost results in our special report, Updating the Economics of Biologics Manufacturing with 5,000L Single-Use Bioreactors.
For more information, visit:
Patheon.com/contactus (https://www.patheon.com/us/en/ about-us/contact-us/contact-form.html)
Patheon.com/biologics (https://www.patheon.com/us/en/ our-capabilities/large-molecule/biologics.html)
Thermofisher.com/dynadrive
Thermofisher.com/sut
Thermofisher.com/flexiblecontainment
The state-of-the-art design offers best-in-class performance with mixing power of greater than 80 W/m3 and a mass transfer rate of up to 40 hr-1.
38 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Application Note
Enhancing Process Flexibility with Automated Filling
During the production and filling of highly complex biopharmaceuticals in cell and gene therapy, precise flow measurement and accurate air bubble detection play a crucial role. Flow meters and air bubble detectors ensure consistently high product quality to provide patients with effective and safe drugs. Using the example of the RoSS. FILL platform, developed by the Austrian company Single Use Support, ultrasound specialist SONOTEC explains how non-contact clamp-on flow meters and air bubble detectors can significantly increase the accuracy of automatic filling.
Challenges in Aseptic Filling
Accurate and efficient filling of biopharmaceuticals in single-use bags, bottles, or vials is crucial. Even the smallest deviations in the filling volume can have a huge impact on the production process, or finally on the patient‘s therapy.
For example, if a liquid needs to be frozen for transport, overfilling or an inconsistent volume of single-use bags seriously increases the risk of bag leakage and product loss. In most cases, it is absolutely important that a patient receives exactly the same volume of medication per injection.
Operational errors in aliquotation processes often occur due to manual intervention or limitations. The major challenges in aseptic filling are:
• Accuracy
• Consistency
• Flexibility
• Scalability

By implementing automated filling solutions with leading flow measurement technology, product and material loss, human errors, as well as the risk of contamination can be significantly reduced, while process flexibility can be substantially increased.
Solution Provider: Single Use Support
Innovative automated fluid management solutions provided by the company Single Use Support set a new standard in biopharmaceutical manufacturing.
The company offers specific expertise and fast solutions in processes regarding cell and gene therapies (CGT) but also commercial bulk drug substance handling and vaccine manufacturing transfers, and drug productions. With its expertise and advanced bioprocessing solutions, Single Use Support has become the market leader in the accurate filling of very small volumes in single-use bags.
Highest Accuracy for Better Operator‘s Control
With its fill & filtration platform RoSS.FILL, Single Use Support offers unlimited scalability for aseptic filling from laboratory to commercial production. The modular platform is designed for filling multiple smaller and larger volumes between 1 mL and 1000 L into single-use bags or bioprocessing containers.
For low-volume dispensing at a commercial scale, accuracy and repeatability are essential. To achieve a filling accuracy range of even less than ± 1 mL with the RoSS.FILL platform, Single Use Support decided to implement SONOTEC‘s SONOFLOW CO.55 V3.0 ultrasonic flow meters. The sensors are easily clamped on the tubing and measured without any

INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 39 www.international-biopharma.com INTERNATIONAL BIOPHARMACEUTICAL 39
SONOFLOW CO.55 V3.0 flow meters installed in the RoSS.FILL CGT system, Sources: SONOTEC, Single Use Support
Application Note
contact and therefore without any risk of contamination or leakage.
Designed with a special focus on low-flow applications, SONOFLOW CO.55 V3.0 clamp-on flow meters combine excellent measurement accuracy over a wide flow range and highest clamp-to-clamp repeatability. Hence, the noncontact sensor is perfectly suited for single-use environments with regular tubing replacements.
With the smallest footprint in the market, the compact and reliable flow meter with built-in electronics has been easily integrated into the RoSS.FILL platform. All non-gravimetric small-volume filling lines are equipped with an ultrasonic SONOFLOW CO.55 V3.0 sensor to precisely measure and subsequently control the dispensed fluid. Thus, Single Use Support guarantees exact filling in bags from laboratory to commercial production.
Unlimited Scalability and Flexibility
In biopharmaceutical manufacturing, it is crucial to maintain accuracy while also acknowledging the importance of flexibility. If bag sizes or batch volumes vary due to scale-up or scale-down requirements, the filling system must do so accordingly. The modular RoSS.FILL platform can be adapted to the customer‘s needs: increase or lower the batch volume or bag size per rack. The highly accurate SONOFLOW CO.55 V3.0 flow sensor ensures the configured filling volume in the single-use bags or bioprocessing containers.
Advanced Process Safety with Bubble Detection
To ensure stable and safe filling processes, proactive and well-considered fluid management is necessary. With the integrated bubble detectors of the SONOCHECK ABD06 series, Single Use Support guarantees airless filling of biopharmaceuticals in single-use bags or containers. The non-contact clamp-on SONOCHECK ABD06 sensor detects air bubbles via ultrasound in the tubing.
Hence, RoSS.FILL users benefit from advanced process safety, flexibility, and scalability with accurate and airless volume dosing.
Air Bubble Detection in Biopharmaceutical Manufacturing and Aliquotation
Air bubble detectors have become an integral part of medical technology. In biotechnology, they are increasingly being used: From process development to the production and filling of highly complex drugs. Subsequently, they play an important role in ensuring consistently high product quality and efficient production processes.
In biopharmaceutical manufacturing, accuracy is key. The presence of air bubbles can significantly affect the quality of the final product. If they remain undetected, even the smallest air bubbles can change the dosing accuracy, e.g., when filling very small volumes, and – in the worst case – can affect the effectiveness of the medication.
Aliquoting, the precise measuring and dispensing of liquid volumes, is a critical step in bioprocess technology. This is where the importance of air bubble detection becomes
even clearer. Accurate aliquoting ensures the consistency and adherence to stringent quality standards of each pharmaceutical batch. Air bubble detection alerts as soon as an air bubble is detected and allows for quick adjustments in the process to save resources and production capacity.
Ultrasonic air bubble detection is based on non-invasive measurement technology: Through the walls of flexible and rigid plastic pipes using the transmission principle. The sensors’ clamp-on architecture enables non-contact measurement directly on the tubing, which only needs to be inserted into the measuring channel of the sensor. Two transducers mounted on opposite sides of the liquid-filled tubing act as transmitter and receiver. The transmitter generates an ultrasonic wave that moves through the tubing perpendicular to the liquid and reaches the receiver on the opposite side of the tubing. As the acoustic impedance of air is around 3500 times lower than that of water, the reflection coefficient at the water-air interface is correspondingly high and a large proportion of the sound energy is reflected. In addition, ultrasonic waves are significantly more attenuated when propagating in air than in water. Both phenomena lead to a reduction in the amplitude of the receiver signal.
As the acoustic transmission through the tubing is highly dependent on the ambient conditions, SONOTEC has implemented a patented closed loop control algorithm in the SONOCHECK ABD06 series, for example, to counteract these effects and enable dynamic adjustment of the sensor to ensure constant bubble sensitivity. In doing so, bubbles above a certain predefined size are reliably detected without being influenced by the tube setting, ambient humidity, temperature changes or possible tubing displacement. The built-in microcontroller enables intelligent and dynamic adjustment of the ultrasonic properties to ensure reproducible results. The sensor can detect bubbles down to 0.3 µl and does not need to be calibrated.
Resume
Highly accurate and reliable flow measurement and air bubble detection have become indispensable technologies in the field of bioprocess and biopharmaceutical manufacturing. Their role in ensuring accuracy, consistency and compliance emphasizes their importance as critical process parameters (CPP) for the production of high-quality medicines that ultimately benefit patients worldwide.
SONOTEC
Founded in 1991, SONOTEC GmbH is one of the world's leading product and solution specialist for innovative measurement technologies. With about 200 employees and a modern corporate structure comprising three independent business units – Non-Invasive Fluid Monitoring, Preventive Maintenance and Ultrasonic Transducers – the technology leader operates its global sales activities from the Halle (Saale) based German headquarter. The distributed portfolio includes customized ultrasonic transducers and sensors as well as testing devices and measurement solutions for a variety of different industries.
40 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
40 INTERNATIONAL BIOPHARMACEUTICAL Spring 2024 Volume Issue 1
Technical Background
ENGINEERING PRINCIPLE OF ULTRASONIC FLOW METERS
Ultrasonic transducers are the heart of any ultrasonic flow sensor. They consist of piezoelectric ceramics or composites that expand or contract when a DC voltage is applied, depending on the sign of the voltage (inverse piezoelectric effect).
By applying an alternating voltage, the piezoelectric expands and contracts periodically and emits a sound wave corresponding to the excitation frequency. This sound wave is sent out as a pulsating ultrasonic beam from an excitation transducer and is detected by a receiving transducer. The signal is evaluated electronically and output via various signal outputs (digital + analogue).
Transit-Time Technology
There are different ways how ultrasonic signals can be utilised to calculate flow rates. SONOTEC’s SONOFLOW sensors work on the basis of transit-time technology.
With this method, the transit-times with and against the flow direction of a medium are measured with high precision by time-to-digital converters. In the direction of flow, the transit-time of an ultrasonic wave is shorter than against the flow. The time difference combined with geometrical information of the tubing allows to determine the flow rate and volume.
This method causes neither a pressure drop in the tube nor a risk of leaks, as it can be applied in a completely non-invasive and non-intrusive manner. When appropriately calibrated, transit-time can work on almost all liquids, independent of viscosity, density, color or electromagnetic properties of the fluids. Ions and particulate matter are not required to calculate the measurement. Additionally, the contactless measurement method does not cause any wear or tear for the sensor. Thus, the clamp-on ultrasonic sensors are maintenance-free.
Application Note
Technical Background
MEASUREMENT PRINCIPLE OF SONOCHECK ABD06 BUBBLE DETECTORS
SONOCHECK ABD06 non-contact clamp-on bubble detectors incorporate intelligent ultrasonic transmission technology. The presence of air bubbles and obstructions is detected by means of dynamic amplitude monitoring.
Ultrasound waves are mechanical waves and are thus subject to the laws of their physics. Depending on the sound impedance of the adjacent media, reflection and transmission take place at the interfaces. If the impedance differences of the adjacent media are small, a transition takes place. At larger differences, the sound wave is reflected and does not penetrate the adjacent media.
When an air bubble passes the sensor channel, the signal level drops. Hence, the higher the drop of the signal level, the larger the bubble size.

Nico Polley
Nico Polley, born in 1980, is Product Manager of the non-contact flow meter series SONOFLOW CO.55 at SONOTEC GmbH. In the Business Unit “Non-Invasive Fluid Monitoring”, his main focus is the development and marketing of the ultrasonic clamp-on sensors in the global bioprocessing market. He holds a diploma in economics from the Martin-Luther-University Halle-Wittenberg. Before he joined SONOTEC, Nico Polley worked for KME, which is one the world’s largest manufacturers of semi-finished copper and copper alloy products. At KME, he was Team Leader Internal Sales before he got appointed Product Manager for strip products.
Email: sonotec@sonotec.de Web: www.sonotec.eu
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 41 www.international-biopharma.com INTERNATIONAL BIOPHARMACEUTICAL 41
Transit-Time Principle for Non-Contact Flow Measurement, Source: SONOTEC
Transmission Principle for Air Bubble Detection, Source: SONOTEC
Ensuring Image Integrity When Reviewing Western Blots Overcoming the Challenges Facing Editors and Publishers
Western blots are frequently included in scientific papers to report results when analysing proteins in a sample. However, research shows that Western blot images are a common source of integrity issues, the publication of which can be harmful to journals. Here, Dr Dror Kolodkin Gal, founder of image checking tool, Proofig AI, explores the challenges editors and publishers face when reviewing Western blot images and suggests how to more effectively identify issues before publication.
Western blotting is commonly used in laboratories around the world to detect proteins and gauge their expression levels. A search for ‘Western blot’ on PubMed returns over 400,000 results, 20,000 of which are from 2022.1 However, according to leading image data integrity analyst Jana Christopher, MA, the percentage of manuscripts flagged for image-related problems, including issues with Western blots, ranges from 20 per cent to 35 per cent.2
With potentially far-reaching consequences for any journals publishing such papers, and for the scientific literature as a whole, identifying and resolving these issues pre-publication is crucial.
Risks to Publishers
The most serious consequence of publishing an article containing image integrity issues is retraction. Arguably, retractions have the biggest impact on the authors' careers and reputations, but the journal can also pay a price.
The process might begin with a reader privately writing to the author’s institution to confess concerns about an article, or more publicly posting an anonymous comment on a platform such as Retraction Watch 3 or PubPeer. 4 The journal may then have to investigate, possibly in conjunction with other parties, such as the author's research institution, funding agencies or regulatory bodies. This process of investigating the results and the cause of the problem can take years.5 Multiple retractions could also lead to journals being delisted from indexes6 or closed.7
In addition, a retraction can damage the journal's reputation for publishing high-quality research, and reputational damage occurs during an investigation whether allegations turn out to be valid or not. This can lead to a loss of trust among readers, authors, and funding agencies. It can also make it more difficult for the journal to attract top-quality manuscripts in the future.
Retracting an article can also be expensive for the journal. It can involve the cost of notifying readers, authors, and funding agencies, as well as the cost of re-evaluating other articles by the same authors. In some cases, the journal can become
embroiled in legal battles or be forced to pay damages to the affected parties.
Where Issues Arise
People reporting instances of image issues often assume that the only reason for altering an image like a Western blot is to falsify results or fraudulently increase chances of publication, but most issues are honest mistakes.
In my experience of working with researchers to check images in their manuscripts before submission, around one in four manuscripts includes at least one image integrity issue. Most of these issues are mistakes, like duplications, but even if a mistake is made innocently or because of disorganisation, it can still impact the conclusions of the research and be very problematic.
While rare, some alterations are a deliberate attempt by cheats to mislead the reader and increase the chances of publishing a manuscript. “Western blots are easy to manipulate in an unsophisticated way,” said Chris Graf, Research Integrity Director at Springer Nature. “Researchers or paper mills might do that to beautify the data… or to fake it in the first place.8”
Volume of Submissions
The sheer number of submissions journals receive makes ensuring integrity and proactively identifying these issues a challenge. Many journals receive far more articles than they can publish. Science, for example, received over 10,000 submissions in 2022 and accepted just 6.1 per cent of them.9
Say a manuscript contains images of 50 Western bands. Reviewing those 50 bands for simple duplications requires 2,500 separate side-by-side comparisons. Manuscripts often contain dozens of subimages, sometimes hundreds, so just reviewing the figures for duplication errors requires a substantial time investment, let alone reviewing the study’s content or checking for less obvious image issues. Editors, reviewers and integrity teams therefore face a significant challenge in thoroughly examining this volume of work.
The challenge is compounded by the delicate patterns and subtle variations in band intensities, which often make Western blots look very similar to one another when reviewing by eye.
Proactive checks before publication offer significant benefits to journals and editors compared to reactive investigations. Streamlining checks prior to publication helps prevent problematic figures entering the literature, saves time and money and protects a journal’s reputation, but doing this manually is often unsustainable.
Automation
Using AI tools can significantly improve pre-publication image integrity checks by screening images and flagging any that require more detailed attention by a human reviewer.
42 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1 Technology
micro EDS
Plumb appliances into the AstellBio Micro EDS to ensure their wastewater is sterile before disposal
Liquid waste drains into a 25L holding tank
A sensor sends an alert if the holding tank is full
A self-cleaning ball valve automatically allows 9L at a time into the sterilisation tank
A biowell allows any sterilisation cycle to be validated
A heating element raises the temperature of the liquid under pressure to 132°C, sterilising it
A drain cooler ensures sterile waste leaving the AstellBio Micro EDS is at temperatures lower than 60°C

Upgrade to the AstellBio Sink

with an intergrated basin for an instant liquid waste disposal and sterilisation point
automatic liquid waste autoclaves
AstellBio.com
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 43 www.international-biopharma.com
UPTOCONTAINMENTLEVEL 3
L I QUIDBIOHAZARDDISPOSA L
for
Level 3
•
• Perfect
Containment Level 3 and Biosafety
environments
3
Sterlise liquid waste up to hazard group
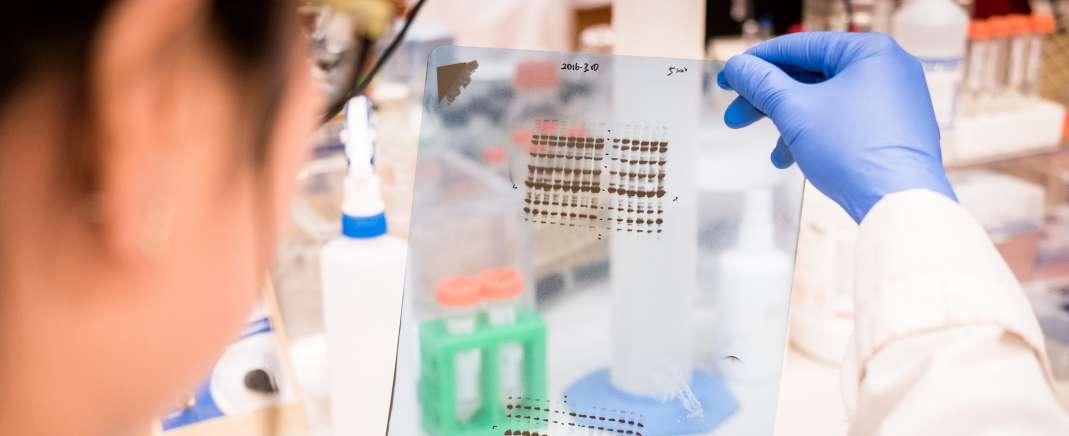
Once images are uploaded to the platform, the tool scans them all, checking each first against itself and then all other images. AI tools can identify a range of issues, including duplication, rotation, flipping, cropping, splicing, and manipulations including deletion and insertion of bands. Any potential issues are then highlighted and flagged for the user, who can then investigate the tool’s findings and make a final decision about whether or not an issue has occurred.
As well as reducing the time image checks take by selecting images most likely to contain issues, AI tools can also improve the investigation process. By using advanced filters to highlight anomalies that are invisible to the human eye, AI tools help catch errors that reviewers might have otherwise overlooked. This can enhance the overall accuracy of the image integrity assessment.
In a trial of image checking tool, Proofig AI, by the American Association for Cancer Research (AACR), tool-assisted review of manuscripts that had been provisionally accepted for publication identified more than twice the number of issues in just over half the time compared to manual review alone.10 Such tools help editors and reviewers work more efficiently and allocate more time to other critical aspects of the peer-review process.
“There’s the opportunity to use technology to interrogate [Western blot] images and to identify the overlapping regions within the image, the scrub marks that have been left by the use of an eraser tool in Photoshop, for example, and we’re able to spot the fact an image has been manipulated,” added Graf.6
These tools are not designed to replace human reviewers or to pass judgement. They offer time savings and efficiency improvements by highlighting potentially problematic images for editors to check in more depth.
While researchers are responsible for submitting work that conforms to the highest standards of scientific rigour, the probability of including problematic figures in journal publications is extremely high without prior examination using specialised software. Implementing AI tools for pre-publication
image analysis can assist journals in upholding their credibility, conserving resources and safeguarding the integrity of scientific literature.
REFERENCES
1. https://pubmed.ncbi.nlm.nih.gov/?term=western+blot
2. UKRIO. Expert interview with Jana Christopher, MA, Image Data Integrity Analyst. [accessed January 21, 2024]. https://ukrio. org/ukrio-resources/expert-interviews/jana-christopher-imageintegrity-analyst/
3. https://retractionwatch.com/
4. https://pubpeer.com/
5. https://doi.org/10.1111/anae.14414
6. https://retractionwatch.com/2023/03/21/nearly-20-hindawijournals-delisted-from-leading-index-amid-concerns-of-papermillactivity/
7. https://retractionwatch.com/2023/05/02/hindawi-shuttering-fourjournals-overrun-by-paper-mills/
8. https://www.researchinformation.info/feature/how-do-weimprove-peer-review
9. https://www.science.org/content/page/journal-metrics
10. https://peerreviewcongress.org/abstract/use-of-an-artificialintelligence-based-tool-for-detecting-image-duplication-priorto-manuscript-acceptance/

Dr. Dror Kolodkin Gal
Dr. Dror Kolodkin-Gal, Ph.D. is is a virologist and life sciences researcher that specialises in new ex-vivo explant models to help understand disease progression and treatments. During his research, he became familiar with the issues surrounding image duplication and image errors in scientific publications. Dror co-founded image check software provider Proofig to help colleagues avoid unnecessary reputational damage and the financial implications of an investigation into their careers, as well as academy institutions and publishing houses. Dror and his teams created Proofig’s artificial intelligence algorithms to help detect image duplication and manipulations in scientific papers. The software checks papers prior to submission and publication, preventing unexpected rejections and helping to improve article quality and credibility.
44 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Technology
INSPIRING SUSTAINABLE CONNECTIONS

10 - 14 June 2024
Frankfurt am Main, Germany
#ACHEMA24
World Forum and Leading Show for the Process Industries
ACHEMA is the hotspot for industry experts, decision-makers and solution providers. Experience unseen technology, collaborate cross-industry and connect yourself worldwide to make an impact.
Are you ready? Join now!
Genesis 2023 Review
For over two decades, the Genesis conference in London has provided an excellent opportunity to bring together key stakeholders and thought leaders from the Life Sciences sector, allowing them to assess recent trends and discuss the potential influences, challenges, and opportunities on the horizon.
A diverse array of 305 attendees, representing 208 companies, convened alongside 38 speakers and 22 exhibitors. The programme delved into key issues including Investment Trends; Accessing Patient Data; Business Models for Growth; Aligning Health-span, Life Span and Sustainability for Affordable Longevity; A Patient’s Perspective on Modern Illnesses and Hot Dealmaking Areas for 2024; and The Dealmaker’s Anatomy.
The discussions at Genesis vary each year, reflecting the evolving landscape of biomedical research and development and its associated financing. Kicking off the programme by reflecting on the Winners & Losers of the previous year, expertly curated by veteran industry commentator Mike Ward, always sets a strong foundation.
Under the moderation of Eleanor Malone, Editor-in-Chief of Commercial Insights at Citeline, the ‘Bio-Dollar Briefing’ panel shared reasons for optimism regarding bio-financing in Life Sciences, along with the challenges that remain to be overcome.
Dianne Lee, CEO of DLRC, steered the ‘Buy, Borrow or Grow-Your-Own?’ panel through a discussion on how growing Life Science companies make the decision of when to grow their internal team, out-source to expert providers or acquire new capacity through M&A.
The Genesis stage welcomed the five companies that were the finalists for the BioNewsRoundAward, NRG Therapeutics, Quadram Institute, Etcembly, Astex Pharmaceuticals, Maxion and PrecisionLife, to pitch why they believe they deserved to win the award. We were delighted to present the BioNewsRoundAward to Steve Gardner, CEO of PrecisionLife for their strategic ALS partnership with LifeArc.
TV presenter and actress Alexandra Gray shared her healthcare challenges with a rare disorder and how she has


managed her condition to date despite a protracted diagnosis and limited available treatments. Alex voiced what she, as a patient, would like to see from the life sciences industry.
Victoria English, Co-Founder & Editor of MedNous, together with an esteemed panel, tackled the key challenges of how to leverage the growing availability of data from multiple sources to inform, innovate and improve the R&D process, leading to better medicines.
A panel of leading Pharma partnering leaders, chaired by Sue Charles, the Founder and Director of Charles Consultants, explored which therapeutic areas and treatment modalities will likely be attracting their attention in the coming year. Accepting there is a comprehensive and broad spectrum of interests across the industry exemplified by the panel mentioning a range of their own deals, each picked out key messages for those seeking to partner assets and technology platforms.
Led by Healthcare Journalist Lisa Urquhart, one of the day's most diverse and stimulating panel discussions brought together opinion leaders from biopharma, precision nutrition, technology transfer, and innovation ecosystems. The discussion revolved around the potential opportunities and challenges in aligning prevention and cure approaches to foster affordable longevity across populations.
The final panel of the day, led by Mike Ward, explored the characteristics, influences, and role models crucial for successful collaborations that form ‘The Anatomy of a Dealmaker’. Leveraging his extensive experience, Mike facilitated the conversations aimed at understanding the dynamics shaping transformative transactions in the sector.
Should you wish to watch the recordings of the Keynote Sessions and Innovation Workshops, these can be found on the One Nucleus YouTube channel. Look out too for the Genesis 2023 Review publication, being released on 18 March 2024. Interested in joining us for Genesis 2024? Sign up now, and we look forward to meeting you there!
www.genesisconference.com
46 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Events Preview & Review
The panel ‘Buy, Borrow or Grow-Your-Own,’ moderated by Dianne Lee, CEO of DLRC
Jasmin Bannister, One Nucleus introduces the BioNewsRound Awards
Events Preview & Review
Drug Delivery to the Lungs 2024
DDL2024 will be delivered live at the Edinburgh International Conference Centre (EICC) and virtually for those unable to travel to Scotland, on Wednesday 11th, Thursday 12th and Friday 13th December 2024.
Celebrating its 35th year, the Drug Delivery to the Lungs Conference will again look to showcase the latest research in the area of inhaled drug delivery. Alongside the talks, exhibition and posters, there will be the usual convivial hospitality and networking opportunities for which the conference is renowned, with a complimentary Gala Dinner for all delegates in the spectacular National Museum of Scotland, Edinburgh.
DDL2023 Highlights – Last year the 34th Drug Delivery to the Lungs Conference welcomed over 900 delegates in person at the EICC in Edinburgh, with an additional 120 virtual attendees joining online. The Association of Inhalation Toxicologists held a morning workshop on ‘Pre-clinical development – Back to Basics‘, which attracted over 150 delegates ahead of the main conference on Wednesday morning.
Notable Achievements – Congratulations were extended to Olivia Merkel, Ludwig Maximilian University of Munich, who was awarded the DDL2023 Emerging Scientist title. Olivia opened the conference on Thursday morning with her talk on ‘What’s in the future of inhaled RNA?’. Olivia was presented with a cheque for £1,000 and a Commemorative Award.
The winner of the DDL2023 Pat Burnell New Investigator Award was Brunella Grassiri, University of Pisa. Her presentation on ‘Development of pulmonary formulations with gallium siderophores against Aspergillus fumigatus lung infections’ won her the acclaimed prize.
The abstracts and presentations from DDL2023 can be found on the DDL website.
Looking forward to DDL2024 – DDL2024 provides a balance of coverage across key areas of aerosol science and the development of inhaled medicines, e.g. CMC, clinical, device development, to appeal to both academia and industry, with a single conference stream meaning no difficult decisions as to which presentation to attend.
Nobel Prize Winner – On Friday morning there will be a presentation from Morten Meldal. Morten won the Nobel Prize in Chemistry 2022 relating to ‘the development of click chemistry and bioorthogonal chemistry’.
The conference programme will include talks from invited speakers as well as those who have submitted papers to be considered for podium presentations. The topics for consideration this year include Advances in Inhalation
Technology, Biologics to Target the Lungs, Inhalation across the ages, Transition to new Propellants and a session dedicated to Nasal.
As a “not for profit” organisation, we would like to extend our thanks to our valued conference sponsors, without whom we would not be able to bring you the great value, high quality DDL conference experience. Registration costs will continue to be held at a level that enables attendance across the breadth of industry, with attendance from academia actively encouraged, to facilitate cross fertilisation of knowledge across the sector. Students will continue to enjoy complimentary registration, providing the next generation of aerosol and drug delivery researchers an extra-ordinary opportunity to engage with world-renowned scientists from across the aerosol and respiratory delivery field, to promote their own work, and to gain experience participating in a premier conference in their research field.
DDL are proud to recognise and encourage new talent through our awards and activities:
• The Pat Burnell Young Investigator Competition. Named in honour of one of the founders of the Aerosol Society, shortlisted applicants for this prestigious award will have the opportunity to deliver a live presentation at DDL2024, with the winner being announced during the conference.
• The DDL Emerging Scientist Award. Entries are encouraged from researchers across the globe working in academia, industry, public service or other scientific establishments. The winner of this award will have demonstrated significant scientific accomplishment or innovation during the early stages their career in inhalation science (within 15 years from achieving their PhD). The winner will be announced and give a keynote presentation describing their research achievements at the conference.
• The DDL Career Development Grant. This flexible grant is intended to support new and emerging scientists by funding projects which contribute to a scientist’s career development. The grant can support individuals from public, private and university organisations.
• The New Researcher Network (NRN). Following their successful launch in 2018, the conference will once again host onsite networking events organised by the Network. The aim of the NRN is to develop a broad community of early career scientists and facilitate discussion surrounding research ideas/challenges, exchanges, joint projects and personal and professional development. New and early career researchers from both academia and industry, as well as PhD students, are encouraged to participate in the NRN, which has expanded to include a networking LinkedIn group.
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 47 www.international-biopharma.com
48 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1

+ Co-located with Automate Europe 2024
22 - 23 May 2024 | Basel, Switzerland
• 2-day Event
• In-Person Congress & Exhibition

Attendees of Discovery Europe 2024 also have access to Automate Europe 2024
Day One
• Track 1: Identification & Validation of Novel Targets
• Track 2: Identification & Validation - Targeted Protein Degradation
• Track 3: Advanced Screening Approaches & Enabling Technologies
• Track 4: Advances In Medicinal Chemistry, Drug Design
• Track 5: Therapeutic Strategies, Enabling Technologies & Biomarker Development




Day Two
• Track 1: Emerging Modalities of Drug Discovery- Targeted Protein Degradation
• Track 2: Animal Models for Disease, Organ Modelling -Organoid based Discovery & Organ On Chip development
• Track 3: Molecular Drug Design & Hit Finding/ Optimisation
• Track 4: Drug Discovery for Neurodegenerative Diseases
Companies Represented Include




What To Expect
• 350+ leading pharma, biotech & academic delegates
• 35+ hours of presentations, discussions & interactive content at both events
For any queries please contact:
marketing@oxfordglobal.com




• 9 hours of networking breaks, including speed networking & refreshments
• 240 pre-arranged 1-2-1 meetings, facilitating business growth
Scan the QR code to visit the Oxford Global website
INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 49 www.international-biopharma.com

The Essential Antibody and Protein Engineering Summit MAY 13-17, 2024 | BOSTON, MA + VIRTUAL NEW VENUE: OMNI BOSTON HOTEL AT THE SEAPORT Organized by PEGSummit.com 20 CONFERENCE TRACKS 125+ EXHIBITORS 3 PLENARY SESSIONS 2,400+ GLOBAL PARTICIPANTS 350+ EXPERT SPEAKERS



londonbiotechshow.com info@londonbiotechshow.com +44 (0)208 242 6566 Unlocking The Potential of Biotechnology For Revolutionising Medical & Healthcare Sectors Globally Orginised by Business Media 8 - 9 May 2024 Olympia West
Page 45
Page 48
Page 48
Page 3
Page 35
Page 9
Page 43
Page 15
Page 49
Achema 2024
Advances in Cell-Based Screening in Drug Discovery 2024
AI in Drug Discovery
A&M STABTEST
Aurisco Pharmaceutical Company Ltd.
Autoscribe Informatics UK
Astell Bio
Collaborative Drug Discovery Inc.
Discovery Europe 2024
BC FUJIFILM Wako Chemicals USA
Page 51
London Biotechnology Show
IFC Novo Nordisk Pharmatech A/S
Page 21
Page 7
Page 50
Page 13
Page 11
Page 29
IBC
Owen Mumford Ltd.
PCI Pharma Services
PEGSummit Boston
Precision for Medicine
Richter-Helm Biologics GmbH & Co. Kg
Senglobal Ltd
UPC Cambridge

52 INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY Spring 2024 Volume 7 Issue 1
Subscribe today at www.international-biopharma.com or email info@senglobalcoms.com
hope
the biopharmaceutical industry
is also now
on social
Follow us on:
Ad Index
I
this journal guides you progressively, through the maze of activities and changes taking place in
IBI
active
media.
www.facebook.com/Biopharmaceuticalmedia www.plus.google.com/biopharmaceuticalmedia www.twitter.com/biopharmace www.biopharmaceuticalmedia.tumblr.com/






INTERNATIONAL BIOPHARMACEUTICAL INDUSTRY 53 www.international-biopharma.com ––is
• • •
UPC’s
The future of synthetic endotoxin detection.

PYROSTAR ™
Recombinant Endotoxin Detection Reagent
Limulus Amebocyte Lysate
PYROSTAR™ Neo is a new endotoxin detection reagent that mirrors nature but is developed by recombinant technology.
FUJIFILM Wako Chemicals U.S.A. Corp.
© FUJIFILM Wako Chemicals U.S.A. Corp. - 2023
www.wakopyrostar.com ~ wkuspyrostarinfo@fujifilm.com