14 minute read
Special alloys changing how sulfuric acid plants are built and operated
By: Nelson Clark, Joanes Barros, Matheus Sanchez, Bruno Ferraro, Gabriel Murakami, Lucas Camargo and Paulo Portilho, Clark Solutions, Brazil
Since the first industrial sulfuric acid plants were built in the early 1900s, very little change occurred in the materials used to handle the harsh conditions of the acid producing environment. More recently, the development and increased availability of special alloys has dramatically simplified the design and construction of the plants— small, skid-mounted plants can now be built and shipped to customers for installation with minimal field work and assembly.
old school construction
The contact process was first patented in 1831, and the process variant for applying vanadium catalyst was developed by BASF in 1913. Until the late 20th century the materials of construction applied in sulfuric acid plants changed very little. But in the later years of the last century, the development of new alloys and special materials as well as a reduction in their costs led to innovation throughout the contact section of acid plants.
oxidation converters
SO2 oxidation converters are the core of any sulfuric acid facility. As a general rule, they are large, cylindrical, vertical vessels (though Clark Solutions has used horizontal vessels on small skid mounted plants.) They house 3, 4, or 5 catalytic beds where the SO2 to SO3 oxidation reaction takes place when the gases contact vanadium-based catalyst at reaction temperatures that range from 380-650 degrees C (7151200 degrees F).
The high temperatures associated with the presence of a small amount of water and a sulfur trioxide rich atmosphere make the converter environment extremely aggressive when temperatures fall below the dew point or there are steam/water leaks. Historically, the approach to handling the gases in these converters has been the use of a refractory brick lined carbon steel vessel shell coupled with high temperature resistant cast iron internals (support grids and posts).
Although construction can be excellent and long-lasting, carbon steel and cast iron have some disadvantages. The mechanical resistance of carbon steel is strongly affected in the temperature range above 500 degrees C (900 degrees F). Cast iron may suffer deformation in temperatures above 650 degrees C (1200 degrees F) (although some cast iron, such as Meehanite™, can withstand higher temperatures).
The industry and designers always aim to increase production with the smallest plant possible. This drove designs to increase SO2 concentration in the reactor and thus increase operating temperatures. First pass bottoms operating above 620 degrees C (1150 degrees F) that were unusual in the mid 1900s became the new standard. Some plants, at the expense of fast catalyst aging, would operate at 650 degrees C (1200 degrees F) continuously.
The prevalence of these shifts became possible with the popularization of different grades of stainless steels. The carbon steel and cast iron construction has slowly but steadily been replaced by special 304 stainless steel grades, more resistant to the thermal stress and corrosion than the carbon steel.
The new material has also led the industry to change converter design. While in the past the first catalytic pass had to be placed on top of the reactor due to thermal stress and increases in pressure drop increase, stainless steel construction (not as affected by thermal stress as carbon steel) allowed the first pass to be positioned wherever it made more sense to the designer. Many designers chose to install the first catalytic bed at the bottom of the reactor to simplify. Also, superheaters could be located on the ground level, which saved on ducting and supports.
The heavy duty high temperature resistant cast iron castings used for catalyst support and internals of the converter have also been replaced by special grades of 304 or 321 stainless steel, the latter in the hotter areas.
With proper design, the new materials of construction allowed the refractory brick to be partially or completely eliminated, depending on process conditions, making the vessels cheaper and lighter than prior versions.
gas-gas heat exchangers
Another traditional piece of equipment in double absorption plants that has benefited from improved and more accessible materials is the gas-gas heat exchanger.
Heat exchangers cool the gases prior to entering the interpass absorption tower while at the same time re-heating the cold gases exiting the absorption tower. In the hot side of the exchanger temperatures and SO2/SO3 laden gas are the challenges; on the cold side acid mist and SO3 slippage are potential problems. In a way, the same problems afflicting the converter affect hot gas-gas heat exchangers.
Temperatures that could surpass 500 degrees C (900 degrees F) and the SO3 laden gas requires that hot heat exchangers use high temperature resistant materials of construction. An early solution was metallized carbon steel, a strategy to make the base material bear the hot and harsh conditions. The metallization process is extremely difficult, though, and if done improperly can actually shorten tube life.
What happened with hot gas-gas heat exchangers has its parallel in cold gas-gas exchangers. For nearly one century gas-gas heat exchangers have been built in plain carbon steel. The material selection is perfect and should last a very long time with regular design operating conditions of the exchanger. The only problem is that actual operation does not always go by the book.
When engineers design a plant, they choose materials that operate at the design conditions. The problem with these designs is the non-expected operating conditions: low capacity operation, poor air/gas drying performance, unexpected mist carryover from the interpass absorption, improper SO3 absorption, and water or steam leakage.
When one of these conditions exist, the cold exchangers are pushed beyond their design limits. Hot, strong, and corrosive acid will completely change the dynamics of corrosion. When this happens, the consequences are the same: accelerated corrosion, sulfate formation and pressure drop build up, gas leaks, increased emissions, reduced capacity, and earlier than expected plant shut down. This is why cold gas-gas heat exchangers are among the most frequent maintenance items and shutdown drivers in a double-absorption plant.
The answer? Stainless steel construction in gas-gas heat exchangers increases equipment life, reduces corrosion and sulfate formation in upset conditions, and saves money on cleaning and maintenance. When properly designed and operated, stainless steel exchangers last longer and will pay for the extra cost on a “total cost of ownership” basis.
strong acid piping
For more than a century hot strong sulfuric acid piping was designed and built using cast iron piping and connections. Cast iron grades changed from place to place, from country to country. Some places use 250# class piping and fittings to provide extra wall thickness for corrosion. Conventional cast iron fittings and gravity cast parts have chaplets to separate the molds—another weak point that in many situations is the starting point of a leak.
In the end, the corrosion resistance of cast iron allied to the thick walls has for a long time been the only option to strong acid piping despite the natural shortfalls. Thus, the development of special alloys and steels, such as Clark solutions CSX™ family of high silicon stainless steels, was very welcome.
Special alloys are designed to operate with corrosion rates below 0.02-0.04 mm/year (1-2 mils/year), while even the best cast irons will show corrosion rates at average transport velocities in the range of 0.15-0.30 mm/year (5-10 mils/year). The thick walls guarantee a long lifetime, at expense of substantial iron being captured by the acid.
As an example, while some of the most frequently used cast irons have wall thicknesses as high as 22 mm (0.9 in), CSX piping uses wall thicknesses of 4 mm (0.2 in) or 6 mm (0.3 in) while still providing 20 or more years of service.
The thinner walls make special alloy piping lighter, but this is not the only advantage. Cast iron piping is generally operated with acid at velocities of 1.0-2.0 m/s. Corrosion rates on cast iron increase with transport velocity. CSX and special alloy piping are normally designed for around 3 m/s for long runs and 5 m/s for short runs. The special alloys’ corrosion rates are not sensitive to transport velocity, so the design is limited only by acceptable pressure drop.
Another advantage is welding capability. Welded lines substantially reduce the number of flanges used, which significantly reduces the risk of leaks at flanged connections. Even better, in the event of a leak or failure special alloy lines can be locally welded. No cranes, no replacement of large parts, no new gasketing or tightening. This saves a huge amount of time and energy when compared to cast iron lines, which may cost one or two days of production loss.
In Brazil, where Clark Solutions designed and replaced cast iron pipelines with CSX, the customer reported gains in plant operation and up to 60-80% less downtime compared to prior operation.
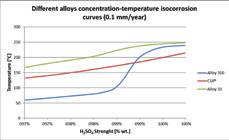
Corrosion of different alloys.
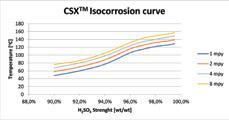
CSX™ Isocorrosion curve.
drying, absorption towers & pump tanks
Drying and absorbing towers and tanks face the harshest conditions in an acid plant. As a result, it is this equipment that industry experts have the most disagreement about. The traditional approach to preventing corrosion is to build a bricklined carbon steel vessel to avoid direct contact of acid with the metal.
Every technology vendor has its special recipe. The basic concept around surface protection of this equipment is the installation of a resin or polymeric material, a rubber, an asphaltic mastic, or a special resin in contact with the carbon steel shell sometimes followed by an adhesive PTFE film over which potassium or sodium mortar is applied with acid resistant brick.
Lining a carbon steel vessel to operate with hot strong acid is a work of art. It requires extremely skilled installers and attention to detail. Properly done, a good lining system may last as long as 35-40 years. Skilled masons are becoming more scarce and expensive; and quality work takes time.
In addition, resins and polymers are not all the same—their resistance to acid varies. Sometimes shelf life affects performance. Improper mixing or installation may leave weak spots where acid attack will initiate.
Bricks and mortars are permeable—it is a question of time when acid will contact the liners. If the installation was not properly done or if the materials were not properly chosen, the lining may last for just a few years.
Brick-lined towers and tanks are extremely heavy, requiring extremely strong foundations and in most cases requiring the lining service to be constructed in place.
New materials eliminate the need for lining, PTFE, and brick. Towers and tanks, just like piping, can be in direct contact with the hot and strong acid.
Special alloys including Clark Solutions’ CSX or Outotec’s SX are the premium option for metal tower construction. Special grades of 310 stainless steel, such as Clark Solutions 310M can be used with good performance and long life in 98.0-98.5% strong acid and in heat recovery towers, where acid can be as hot as 240 degrees C and at concentrations as high as 99.5% in heat recovery systems, such as in Clark Solutions Safehr® .
Lighter metal towers can quickly replace brick-lined towers, without requiring new foundations. In some replacement situations, eliminating the brick lining while keeping the outside diameter the same can increase cross-sectional area, which can mean a substantial capacity gain.
Of course, nothing is perfect. Alloy towers do require proper concentration and temperature controls. While a shortterm concentration or temperature excursion may be tolerated and won’t lead to substantial corrosion, long-term excursions may accelerate corrosion and lead towers to an early failure.

acid distributors
As in piping, improved alloys also took over acid distributor manufacturing. Cast iron distributors corrode, which means sulfate in the tower and iron in circulating acid. Sulfate on top of the packing may increase pressure drop and in extreme cases compromise drying or absorption efficiency.
Alloy distributors are currently built in different forms and designs, and are lighter weight, simpler to install, and less prone to corrosion. They also allow more design flexibility. Old standard irrigation densities used in cast iron distributor equipped towers are usually around 10 to 15 distribution points per square meter. This calls for increased packing heights to guarantee proper liquid distribution across the total height of the ceramic packing.
Special alloy distributors can be made of smaller parts that allow a much richer liquid distribution. Clark Solutions has built distributors with up to 500 points/ square meter for some special applications. For sulfuric acid plants, given the nature of the service and the packing used, distribution densities with special alloy will usually vary from 25 to 50 points per square meter.
The 2-5 times denser irrigation allows substantial reduction in packing heights, not only shortening tower heights but also reducing pressure drop across packing or allowing the design of smaller towers running at the same pressure drop.

acid coolers
The first acid coolers used in the sulfuric acid industry were cast iron construction. Huge installations used very large areas where water would wet the external surface of the hot cast iron tube banks while hot acid was flowing inside. The water cooled the tubes by evaporation and convection, and the tubes cooled the acid flowing inside.
The long lengths added a lot of iron to the product acid. This additional iron can be a problem, depending on the industry. And cast iron coolers, like piping, also used dozens and dozens of flanges and connection points, each a potential leak source.
The first attempt to replace the cast iron coolers started in the early 1980s with the introduction of anodically protected (AP) acid coolers. These shell and tube heat exchangers, with acid flowing on the shell and water in the tubes, operate with an impressed current that guarantees that the metal exposed to the flowing acid is always on a passive region.
One of the few disadvantages of the anodically protected acid coolers is that the corrosion protection film formed by the passivation current film is temperature sensitive. An increase in the temperature may cause the metal to move from a passive state to an active state, accelerating corrosion. Luckily this is a rather unusual occurrence, especially when trained operators are in charge.
The development of new alloys brought a myriad of options to the sulfuric acid producer. Shell and tube heat exchangers do not need anodic protection anymore. They can be built out of CSX, 310M, Alloy 3033, and others. Any of these are adequate for some process conditions.
Alloyed shell and tube heat exchangers give the operator the same benefits of anodically protected acid coolers with the advantage of not requiring the instrumentation and controls needed by AP acid coolers. Kept and operated properly, alloy shell and tube coolers will have a long service life with little maintenance.
But the shell and tube were not the only heat exchangers to benefit from new alloys. Gasketed, semi-welded, and fully welded plate and block exchangers can also be made of special alloys, such as Hastelloy D-205 or Alloy 33. Plate exchangers are smaller and cheaper than shell and tube exchangers.
However, 0.3 mm distance between 0.5 mm thick plates require good quality water treatment as well as an acid with little or no dissolved silica or other materials and ions that may foul, clog, or precipitate corrosion on the plates.
Alloy plate coolers also work fine at temperatures as high as 150-160 degrees C in 98-98.5% acid and without issues maintaining cooling water temperature or flow. Both shell & tube and plate exchangers in stainless steel construction are much more forgiving in this regard compared to AP coolers.
More recently Clark Solutions and Alfa Laval developed a fully welded 310M block exchanger designed to handle high temperatures and concentrations of the Safehr® heat recovery system. The newly designed 310M Compabloc® systems have now been in service at 99.0+% acid and 220-240 degrees C with no sign of corrosion and no loss of performance.

Cast iron acid coolers. CSX shell and tube strong acid cooler.

Conclusion
All the improvements provided by the development and application of new materials in the sulfuric acid industry combined to simplify, reduce weight, and increase capacity of the new plants. These features allowed plants to be built faster, at lower costs, and with less field labor.

Shop manufactured modular acid plants require minimal field work.
As materials science progresses, we expect even more improvements that will add to the simplification and reliability of new and future sulfuric acid plants. Plants are getting lighter, more energy efficient, and more reliable, and will continue to do so.
For more information, please visit www.clarksolutions.com. q










