34 minute read
COVER STORY
KEVLAR The Super Tough Fibre!
Vaibhav Gaikwad
Advertisement
Abstract In today’s fast growing market, apart from general apparel clothing, some special applications are expected from textile fibers. Such fibers are used specifically for protective clothing and thus require certain high performance properties. This generation of fibers has been recently developed in the 20 th century and are called high performance fibers. A class of these fibers have very high tenacity and high modulus which are used in applications such as bullet proof jackets, whereas another class of fibers would have high thermal or chemical resistance which can be used as flame resistant fabrics etc. High performance fibers include polymeric fibers such as aramids, aromatic copolyesters, extended chain flexible polyolefin etc.; carbon fiber; glass fibers; ceramic fibers and metallic fibers. High performance polymeric fibers being used for high mechanical properties should be highly oriented, linear aliphatic or aromatic molecules since flexible chains would give low melting polymers and thus low thermal resistance. On the other hand, carbon fibers are planar graphite structures with outstanding mechanical properties. Inorganic fibers have a three dimensional structure compared to a one dimensional and two dimensional structure of polymeric materials and carbon fibers respectively. They have very good mechanical properties but are brit-
D.K.T.E Society’s Textile and Engineering Institute, Ichalkaranj,India tle and have the highest thermal resistance. However, carbon fibers as well as inorganic fibers (except glass fibers) are very expensive. In the following sections, particularly, polymeric fibers would be discussed in detail. Although in the past there were some research works had done and papers also been published, but this paper just simplifies the things as well as highlight some superb features of the Kevlar fiber. However this is not a research article rather than a review article. Keywords: history, chemical composition, production, properties, uses Introduction Kevlar fibers are para aramid fibers rather than meta aramid structure of Nomex. The monomers normally used for the production of the polymer for Kevlar fibers are p-phenylene diamine and terephthaloyl chloride. In Kevlar, the para-substitution of the monomers allows the benzene rings to lie centrally along the molecular axis. Due to this arrangement, greater number of intermolecular bonds and a stronger and a more thermally and chemically resistant fiber is formed than that for Nomex. These fibers have high tensile strength, high tensile modulus and high heat resistance because of the highly oriented rigid molecular structure. Kevlar is about five times lighter than steel in terms of the same tensile strength. This high strength Kevlar is produced by a special spinning process called the Liquid crystal spinning. Now, there exists a series of first, second and third generations ofpara-aramids. For example, Kevlar HT which has 20% higher tenacity and Kevlar HM which has 40% higher modulus than the original Kevlar 29 are largely used in the composite and the aerospace industries. History Poly-paraphenylene terephthalamide (K29) – branded Kevlar – was invented by Polish- American chemist Stephanie Kwolek while working for DuPont, in anticipation of a gasoline shortage. In 1964, her group began searching for a new lightweight strong fiber to use for light, but strong tires. The polymers she had been working with at the time, poly-p- phenyleneterephthalate and polybenzamide, formed liquid crystal while in solution, something unique to those polymers at the time. The solution was "cloudy, opalescent upon being stirred, and of low viscosity" and usually was thrown away. However, Kwolek persuaded the technician, Charles Smullen, who ran the spinneret, to test her solution, and was amazed to find that the fiber did not break, unlike nylon. Her supervisor and her laboratory director understood the significance of her discovery and a new field ofpolymer chemistry quickly arose. By 1971, modern Kevlar was introduced. However, Kwolek was not very involved in developing the applications of Kevlar. Kevlar 149 was invented by Dr. Ja-
COVER STORY cob Lahijani of Dupont in the 1980s. Chemical Composition of Kevlar Fibre The chemical composition of Kevlar is poly para-phenyleneterephthalamide (PPD-T) and it is more properly known as a para-aramid. It is oriented para-substituted aromatic units. Aramids belong to the family of nylons. Common nylons, such as nylon 6, 6 do not have very good structural properties, so the para-aramid distinction is important. Aramid fibers like Nomex or Kevlar, however, are ring compounds based on the structure of benzene as opposed to linear compounds used to make nylon. The aramid ring gives Kevlar thermal stability, while the para structure gives it high strength and modulus. Kevlar is made from a condensation reaction of para-phenylene diamine and terephthaloyl (PPD-T) chloride. The resultant aromatic polyamide contains aromatic and amide groups which makes them rigid rod like polymers. The rigid rod like structure results in a high glass transition temperature and poor solubility; which makes fabrication of these polymers, by conventional drawing techniques, difficult. Instead, they are melt spun from liquid crystalline polymer solutions as described later. The Kevlar fiber is an array of molecules oriented parallel to each other like a package of uncooked spaghetti. This orderly, untangled arrangement of molecules is described as a crystalline structure. Crystallinity is obtained by spinning PPD-T solutions, which involves extruding the molten polymer solution and drawing in the flow direction. Kevlar can acquire a high degree of alignment of long, straight polymer chains parallel to the fiber axis. The structure exhibits anisotropic properties, with higher strength and modulus in the fiber longitudinal direction than in the axial direction. The extruded material also possesses a febrile structure. This structure results in poor shear and compression properties for aramid composites. Hydrogen bonds form between the polar amide groups on adjacent chains and they hold the individual Kevlar polymer chains together. Hydrogen bonds form between the polar amide groups
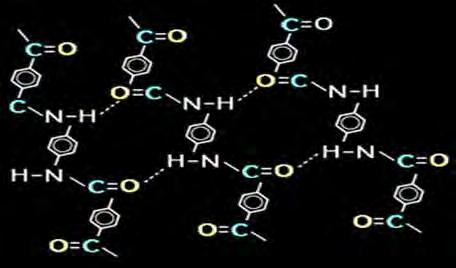
The reaction of 1, 4-phenylenediamine (para-phenylenediamine) with terephthaloyl chloride yielding Kevlar Kevlar (poly paraphenylene terephthalamide) production is expensive because of the difficulties arising from using concentrated sulfuric acid, needed to keep the water-insoluble polymer in solution during its synthesis and spinning. Grades of Kevlar There are three grades of Kevlar available: Kevlar 29, Kevlar 49, and Kevlar 149. Tensile modulus is a function of molecular orientation. As a spun fiber, Kevlar 29 (a high toughness variant) has a modulus of 62 GPA (9 Mpsi). Heat treatment under tension increases the crystalline orientation. The resulting fiber, Kevlar 49, has a modulus of 131 GPA. Several variant grades of Kevlar are available: Kevlar K-29 – in industrial applications, such as cables, asbestos replacement, tires, and brake linings. Kevlar K49 – high modulus used in
cable and rope products. Kevlar K100 – colored version of Kevlar Kevlar K119 – higher-elongation, flexible and more fatigue resistant Kevlar K129 – higher tenacity for ballistic applications Kevlar K149 – highest tenacity for ballistic, armor, and aerospace applications Kevlar AP – 15% higher tensile strength than K-29 Kevlar XP – lighter weight resin and KM2 plus fiber combination Kevlar KM2 – enhanced ballistic resistance for armor applications Properties of Kevlar Fibre Kevlar is a very strong fiber. In fact, it is the strongest textile fiber available today. It has tenacity in the range 22-26 gpd with a breaking
elongation of 2.5-4.4%. The fiber is cylindrical in the longitudinal microscopic view and circular in cross-sectional view. It is a dense fiber with density of 1.44-1.47g/cc. Similar to Nomex, it has average moisture regain of about 4.3% in standard atmosphere. Due to the aromatic structure of the polymer, Kevlar is difficult to ignite. Para aramids generally have high glass transition temperatures nearing 370°C and do not melt or burn easily, but carbonise above 425°C. Regarding the chemical properties of the fiber, the fiber is unaffected by most acids under normal conditions and has good resistance to alkalis and solvents, but not resistant to bleaches. It also has excellent resistance to mildew and aging. All aramid fibers are however prone to photodegradation and need protection against the sun when used out of doors. Applications of Kevlar Cryogenics - Kevlar is often used in the field of cryogenics for its low thermal conductivity and high strength relative to other materials for suspension purposes. It is most often used to suspend a paramagnetic salt enclosure from a superconducting magnet mandrel in order to minimize any heat leaks to the paramagnetic material. It is also used as a thermal standoff or structural support where low heat leaks are desired. Personal protection Armor - Kevlar is a well-known component of personal armor such as combat helmets, ballistic face masks, and ballistic vests. The PASGT helmet and vest used by United States military forces, use Kevlar as a key component in their construction. Other military uses include bulletproof face masks and spall liners used to protect the crew of armoured fighting vehicles. Nimitzclass aircraft carriers use Kevlar reinforcement in vital areas. Civilian applications include high heat resistance uniforms worn by firefighters, body armour worn by police officers, security, and police tactical Pieces of a Kevlar helmet used to help absorb the blast of a grenade

Kevlar is used to manufacture gloves, sleeves, jackets, chaps and other articles of clothing designed to protect users from cuts, abrasions and heat. Kevlar-based protective gear is often considerably lighter and thinner than equivalent gear made of more traditional materials. It is used for motorcycle safety clothing, especially in the areas featuring padding such as shoulders and elbows. In fencing it is used in the protective jackets, breeches, plastrons and the bib of the masks. It is increasingly being used in the peto, the padded covering which protects the picadors' horses in the bullring. Speed skaters also frequently wear an under-layer of Kevlar fabric to prevent potential wounds from skates in the event of a fall or collision. Sports Shoes - In 2013, Nike used Kevlar in shoes for the first time. It launched the Elite II Series, with enhancements to its earlier version of basketball shoes by using Kevlar in the anterior as well as the shoe laces. This was done to decrease the elasticity of the tip of the shoe in contrast to nylon used conventionally. As Kevlar expanded by about 1% against nylon which expanded by about 30%. Shoes in this range included LeBron, HyperDunk and Zoom Kobe VII. However these shoes were launched at a price range much higher than average cost of basketball shoes. It was also used in the laces for the Adidas F50 adiZero Prime football boot. Sports Equipment In kyudo or Japanese archery, it may be used as an alternative to more expensive hemp for bow strings. It is one of the main materials used for paraglide suspension lines. It is used as an inner lining for some bicycle tires to prevent punctures. In table tennis, plies of Kevlar are added to custom ply blades, or paddles, in order to increase bounce and reduce weight. Tennis racquets are sometimes strung with Kevlar. It is used in sails for high performance racing boats. Kevlar is a very popular material for racing canoes. Cycle tires - Several companies, including Continental AG, manufacture cycle tires with Kevlar to protect against punctures. Foldingbead bicycle tires, use Kevlar as a bead in place of steel for weight reduction and strength. A side effect of the folding bead is a reduction in shelf and floor space needed to display cycle tires in a retail environment, as they are folded and placed in small boxes. Music Audio equipment - Kevlar has also been found to have useful acoustic properties for loudspeaker cones, specifically for bass and mid-range drive units. Additionally, Kevlar has been used as a strength member in fiber optic cables such as the ones used for audio data transmissions. Bowed string instruments - Kevlar can be used as an acoustic core on bows for string instruments. Kevlar provides strength, flexibility, and stability for the bow users. To date, the only manufacturer of this type of bow is Coda Bow. Kevlar is also presently used as a material for tailcords (a.k.a. tailpiece adjusters), which connect the tailpiece to the endpin of bowed string instru-

ments. Drumheads - Kevlar is sometimes used as a material on marching snare drums. It allows for an extremely high amount of tension, resulting in a cleaner sound. There is usually a resin poured onto the Kevlar to make the head airtight, and a nylon top layer to provide a flat striking surface. This is one of the primary types of marching snare drum heads. Woodwind reeds - Kevlar is used in the woodwind reeds of Fibracell. The material of these reeds is a composite of aerospace materials designed to duplicate the way nature constructs cane reed. Very stiff but sound absorbing Kevlar fibers are suspended in a lightweight resin formulation. Motor vehicles Chassis and bodywork - Kevlar is sometimes used in structural components of cars, especially highvalue performance cars such as the Ferrari F40. Brakes -The chopped fiber has been used as a replacement for asbestos in brake pads. Indeed, aramids release a lower level of airborne fibres than asbestos brakes. Other uses Fire dancing - Wicks for fire dancing props are made of composite materials with Kevlar in them. Kevlar by itself does not absorb fuel very well, so it is blended with other materials such as fiberglass or cotton. Kevlar's high heat resistance allows the wicks to be reused many times.
Fire poi on a beach in San Francisco Frying pans - Kevlar is sometimes used as a substitute for Teflon in some non-stick frying pans. Rope, cable, sheath- Kevlar mooring line. The fiber is used in rope and in cable, where the fibers are kept parallel within a polyethylene sleeve. The cables have been used in suspension bridges such as the bridge at Aberfeldy in Scotland. They have also been used to stabilize cracking concrete cooling towers by circumferential application followed by tensioning to close the cracks. Kevlar is widely used as a protective outer sheath for optical fiber cable, as its strength protects the cable from damage and kinking. When used in this application it is commonly known by the trademarked name Parafil. Building construction - A retractable roof of over 60,000 square feet (5,575 square metres) of Kevlar was a key part of the design of Montreal Olympic stadium for the 1976 Summer Olympics. It was spectacularly unsuccessful, as it was completed 10 years late and replaced just 10 years later in May 1998 after a series of problems. Expansion joints and hoses - Kevlar can be found as a reinforcing layer in rubber bellows expansion joints and rubber hoses, for use in high temperature applications and for its high strength. It is also found as a braid layer used on the outside of hose assemblies, to add protection against sharp objects. Particle physics - A thin Kevlar window has been used by the NA48 experiment at CERN to separate a vacuum vessel from a vessel at nearly atmospheric pressure, both 192 cm in diameter. The window has provided vacuum tightness combined with reasonably small amount (only 0.3% to 0.4% of radiation length) of material. Smartphones - The Motorola RAZR Family, the Motorola Droid Maxx, OnePlus 2, and Pocophone F1 have a Kevlar backplate, chosen over other materials such as carbon fiber due to its resilience and lack of interference with signal transmission. Marine current turbines and wind turbines - The Kevlar fiber/epoxy matrix composite materials can be used in marine current turbines (MCT) or wind turbines due to their high specific strength and light weight compared to other fibers. Electricity generation - Kevlar was used by scientists at Georgia Institute of Technology as a base textile for an experiment in electricity-producing clothing. This was done by weaving zinc oxide nanowires into the fabric. If successful, the new fabric will generate about 80 milliwatts per square meter. Conclusion Kevlar is mainly used for two reasons and both for performance as I is lightweight and easy to integrate. A thin blanket can serve as structural reinforcement or ballistic protection, everywhere from seismic shear walls to bank counters. Fibres sprinkled into carbon composites can cut weight and boost strength. The grades Kevlar 49 and 149 are the lightest and most robust; Kevlar 29 is comparable in potency to glass fibre, but weighs less. The fact is, Kevlar is still expensive costs need to come down. References

1. Mera, Hiroshi; Takata, Tadahiko (2000). "HighPerformance Fibers". Ullmann's Encyclopedia of Industrial
Chemistry. doi:10.1002/14356007.a13_001. ISBN 978-3527306732.
2. Jump up to:a b "What is Kevlar". DuPont. Archived from the original on 2007-03-20. Retrieved 2007-03-28.
3. "Wholly aromatic carbocyclic polycarbonamide fiber having orientation... - US 3819587 A - IP.com". ip.com.
4. Tatsuya Hongū, Glyn O. Phillips, New Fibers, Ellis Horwood, 1990, p. 22.
5. J. K. Fink, Handbook of Engineering and Specialty Thermoplastics: Polyolefins and Styrenics, Scrivener Publishing, 2010, p. 35.
6. Jump up to:a b c "Inventing Modern America: Insight — Stephanie Kwolek". Lemelson-MIT program. Retrieved May 24, 2009.
8. Quinn, Jim. "I was able to be Creative and work as hard as I wanted". American Heritage Publishing. Archived from the original on December
2, 2008. Retrieved May 24, 2009.
9. https://digital.hagley.org/VID_2011320_B05_ ID01
10. How Kevlar® works: a simple introduction. Explainthatstuff.com (2009-12-07). Retrieved on 2012-05-26.
11.http://www.matweb.com/search/datasheettext.aspx?matguid=706f16a3a8be4682845 71dd36bbdea35
12. https://www.researchgate.net/publication/279740540_Determination_of_Fracture_ Behavior_under_Biaxial_Loading_of_Kevlar_149
13. Kevlar K-29 AP Technical Data Sheet – Dupont
14. Kevlar XP – Dupont
15. Kevlar KM2 Technical Description. dupont. com. Retrieved on 2012-05-26.
16. Yousif, Emad; Haddad, Raghad (2013-08-23). "Photodegradation and photostabilization of polymers, especially polystyrene:
review". SpringerPlus. 2: 398. doi:10.1186/21931801-2-398. ISSN 2193-1801. PMC 4320144. PMID 25674392.
17. Quintanilla, J. (1990). "Microstructure and properties of random heterogeneous materials : a review of theoretical results". Polymer
Engineering and Science. 39 (3): 559–585. doi:10.1002/pen.11446.
18. Michael C. Petty, Molecular electronics: from principles to practice, John Wiley & Sons, 2007, p. 310
19. KEVLAR Technical Guide. dupont.com. Retrieved on 2012-05-26.
20. Jump up to:a b Body Armor Made with Kevlar. (2005-0604). DuPont the Miracles of Science. Retrieved November 4, 2011
21. Kevlar – DuPont Personal Protection. dupont.com. Retrieved on 2012-05-26.
22. Genzini, Luigi. "Kyudo – the way of the bow ; The art of shooting the traditional Japanese bow according to the Heki Insai Ha School" 23. Pagen, Dennis (1990), Paragliding Flight: Walking on Air, Pagen Books, p. 9, ISBN 978-0936310-09-1
24. "Nike Basketball's ELITE Series 2.0 Rises Above the Rest". Nike News. March 20, 2013. Retrieved April 16, 2017.
25. "SafetySystem Breaker". www.continentaltires.com. Retrieved 2019-02-25.
26. Tom Ritchey
27. Audio speaker use. Audioholics.com (200907-23). Retrieved on 2012-05-26.
28. Welcome to Kevlar. (2005-06-04). DuPont the Miracles of Science. Retrieved November 4, 2011
29. Carbon fiber bows for violin, viola, cello and bass Archived 2011-11-10 at the Wayback Machine. CodaBow. Retrieved on 2012-05-26.
30. Carbon fiber bows for violin, viola, cello and bass Archived 2012-03-09 at the Wayback Machine. CodaBow. Retrieved on 2012-05-26.
31. Tailpieces and Tailcords Archived 2012-1123 at the Wayback Machine Aitchison Mnatzaganian cello makers, restorers and dealers. Retrieved
on 2012-12-17.
32. "Falam® Slam". Remo. Retrieved 11 December 2019.
33. "FibraCell Website".
34. "The story of the Ferrari F40 – by its creators". 2017-07-21.
35. "Superstar Kevlar compound disc brake pads review". BikeRadar. Retrieved 2016-10-23.
36. Jaffrey, S.A.M.T; Rood, A.P.; Scott, R.M. (1992). "Fibrous dust release from asbestos substitutes in friction products". The Annals of
Occupational Hygiene. 36 (2): 173–81. doi:10.1093/annhyg/36.2.173. ISSN 0003-4878. PMID 1530232.
37. M.Rubinstein, R.H.Colby, Polymer Physics, Oxford University Press, p337
38. Burgoyne, C. J. (1987-03-01). "Structural use of parafil ropes". Construction and Building Materials. 1 (1): 3–13. doi:10.1016/0950-
0618(87)90053-5. ISSN 0950-0618.
COVER STORY 12 39. Fabric Produces Electricity As You Wear It. Scientific American (2008-02-22). Retrieved on 2012-05-26.
40. Roof of the Montreal Olympic Stadium at Structurae
41. Clem's Baseball ~ Olympic Stadium. Andrewclem.com. Retrieved on 2012-05-26.
42. Shepherd, Robert; Stokes, Adam; Nunes, Rui; Whitesides, George (October 2013). "Soft Machines That are Resistant to Puncture and That Self Seal" (PDF). Advanced Materials. 25(46): 6709–6713. doi:10.1002/adma.201303175. PMID 24123311.
43. Gong (Ed), RH (2011). Specialist Yarn and Fabric Structures: Developments and Applications. Woodhead Publishing. p. 349. ISBN 9781845697570.
44. Meyer, Bruce (November 9, 2015). "Unaflex adding space, capacity at S.C. plant". Rubber & Plastics News.
45. Droid RAZR. (2011-10-11). Motorola Mobility. Retrieved November 4, 2011
46. Wang, Jifeng; Norbert Müller (December 2011). "Numerical investigation on composite material marine current turbine using CFD". Central European Journal of Engineering. 1 (4): 334–340. Bibcode:2011CEJE....1..334W. doi:10.2478/s13531-011-0033-6.
47. Kadolph, Sara J. Anna L. Langford. Textiles, Ninth Edition. Pearson Education, Inc 2002. Upper Saddle River, NJ
48. D. Tanner; J. A. Fitzgerald; B. R. Phillips (1989). "The Kevlar Story – an Advanced Materials Case Study". Angewandte Chemie
International Edition in English. 28 (5): 649–654. doi:10.1002/anie.198906491.
49. E. E. Magat (1980). "Fibers from Extended Chain Aromatic Polyamides, New Fibers and Their Composites". Philosophical Transactions of
the Royal Society A. 294 (1411): 463–472. Bibcode:1980RSPTA.294..463M. doi:10.1098/ rsta.1980.0055. JSTOR 36370. S2CID 121588983.
50. Ronald V. Joven. Manufacturing Kevlar panels by thermo-curing process. Los Andes University, 2007. Bogotá, Colombia.
https://www.physics.ncsu.edu/stxm/science/ kevlar/kevlar.html
Clothing from Chiengora (Dog) Fibres
Dr. N. N. Mahapatra
Eco-friendly fibers means more than a label. In this modern age of technology and quick results, there are always a few people who are keeping alive the old ways of doing things. Maybe it is a hobby, maybe it is a passion, or maybe it is a family tradition, but they learn and carry on a slower way of producing. Many are organic, but all of them take more time and care to create products with less impact on the environment. There are communities of hand-spinners all over the world, making natural fiber yarn from raw materials. Small, sustainable farms and ranches are providing a lot of eco-friendly fibers that create handspun slow yarn. Animal fibers are natural fibers that consist largely of particular proteins. Instances are silk, hair/fur (including wool) and feathers. The animal fibers used most commonly, both in the manufacturing world as well as by the hand spinners, are wool from domestic sheep and silk. Also very popular are alpaca fiber and mohair from Angora goats. Unusual fibers such as Angora wool from rabbits and Chiengora from dogs also exist, but are rarely used for mass production. Not all animal fibers have the same properties and even within a species, the fiber is not consistent. Merino is a very soft, fine wool, while Costwold is coarser, and yet both merino and Cotswold are types of sheep. This comparison can be viewed on the microscopic level, Business Head (Dyes) Shree Pushkar Chemicals & Fertilisers Ltd.
comparing the diameter and structure of the fiber. With animal fibers, and natural fibers in general, the individual fibers look different, whereas all synthetic fibers look the same. This provides an easy way to differentiate between natural and synthetic fibers under a microscope. Chiengora (pronounced she-angora) refers to yarn spun from dog hair. Chien is the French word for dog, and gora is derived from “angora” the soft fur of a rabbit. Spinning dog hair is not a new art form. The spinning of dog hair is an ancient art form dating back to prehistoric Scandinavia and in textiles from the Navajo Indians of North America. It was the main fiber spun on the North American continent before the Spaniards introduced sheep. The hair of dogs is known as Chiengora fibre, which has been used in the textile industry since long. The best hairs for this application are from ‘Northern’ breeds, such as Newfoundlands, Chow Chows, Samoyed, Norwegian Elkhounds, and the like. In modern times it is rarely used. In general it is only used by hand spinners with pet dogs. Chiengora is spun from the undercoat of a double coated breed. The average Labrador or Dalmation will not have the right kind of fur to be spun into yarn. The fur needs to be soft, woolly and relatively long in order to work for the spinner. Some breeds which can be combed out to make dog wool yarn are: · Newfoundland · Bernese · Spitz · Golden retriever · Great Pyrenees To collect the fur, one must brush it out of the coat. Shaving the dog

will not work, as you will have to separate the long, fine, glossy outer hairs from the undercoat. Far too much work! Instead, use a slicker brush or comb, brush out the undercoat, and collect it carefully from the brush. Collected dog wool must not be washed, unless one is a professional fiber artist with experience in preparing fleeces. Dog fur felts (mats) very easily if it is washed it incorrectly. Instead, gather it into a bag and store it carefully. If you are not a spinner, you can ask at your local spinning guild or yarn store to find a local spinner who is willing to spin the dog fur into yarn for you. Be sure that the spinner has experience in working with dog fur, because it requires more care than sheep’s wool. Dog fur when spun into yarn does not smell like dog, any more than wool yarn smells like sheep. When the spinner prepares the fur, they will remove the oils which cause that “doggy” smell. Most spinners will blend the dog fur with sheep’s wool (often merino) in order to compensate for some faults with chiengora. Unlike wool, dog wool does not have “bounce,” which means that a garment made from chiengora will tend to get stretched out. Blending the chiengora with merino fibers will help your garment keep its shape. Chiengora is a delightful yarn, with a halo and a soft feel similar to that of angora or mohair. It has much more loft and insulation ability than sheep fibers, which makes it surprisingly warm – far warmer than wool. Something to keep in mind when you’re planning a garment! The Chiengora Spinning Process Step 1. Raw fiber is received and is immediately weighed and catalogued. Step 2. Fiber is then prepared for cleaning. It is initially inspected by hand. To reduce cost, you are encouraged to inspect your fiber at home and remove any sticks or debris before shipment of dirty fiber. Step 3. Fiber is washed and deodorized using specialized methods, which prevent the occurrence of natural felting. Step 4. Once dry, the clean fiber is collected and carded using a manually operated drum carder in preparation for spinning. Step 5. A spinning wheel is used to hand spin the carded fiber to a selected thickness. Two strands are spun and then plied together, producing 2-ply yarn. Step 6. A skein of yarn is made using a yarn swift. The skeins are washed, weighted and hung to dry. Step 7. Now the fibers are ready for its intended purpose! A roving is a long and narrow bundle of fibre. It is usually used to spin woollen yarn. A roving can be created by carding the fibre, and it is then drawn into long strips. Because it is carded, the fibres are not parallel, though drawing it into strips may line the fibres up a bit. Roving is similar to sliver. Because roving has been created by carding, the fibres are less parallel than topcombed and are not of uniform length. Carded rovings look fluffier than combed top, which looks smooth and has a high lustre. The fibres in combed top tend to be of a fairly uniform length due to the method of preparation. Pencil roving is a type of roving that has been drawn until it is the size of a fat pencil. It can be used by spinners with minimal drafting. Knitters also use pencil roving, similar to Lopi style yarns, or when making a thrummed item. Regular roving can also be used in thrummed knitting. A rolag is a roll of fibre generally used to spin woollen yarn. A rolag is created by first carding the fibre, using handcards, and then by gently rolling the fibre off the cards. If properly prepared, a rolag will be uniform in width, distributing the fibres evenly. The Chiengora Weaving Process Weaving is a textile art in which two distinct sets of yarns are interlaced to create fabric. It can be a versatile and practical option when determining how to use your Chiengora. Woven Chiengora can be used to create scarves, wraps, blankets, throw pillows, and fabric for sewing projects. Step 1. Wool is measured using a warping mill to determine the warp, or size of any given weaving project. Step 2. That wool is then threaded through the floor loom in preparation for hand spun Chiengora to be incorporated. Step 3. Once the wool is secured in place, Chiengora is woven across the width of the warp to create an interlacing fabric. Step 4. When the weaving is completed, the Chiengora fabric is removed and the ends are tied off. Properties of Chiengora Fibres Chiengora is up to 80% warmer than wool and sheds water well. The fiber is not elastic like wool. Chiengora tends to fluff with use, creating a halo effect. It has a similar appearance to angora and is luxuriously soft. It is warm, even in frigid temperatures. Often Chiengora is blended with wool during the carding process. This blend has some give to it, which is preferable while knitting. It is also often blended with wool in order to create a yarn with less heat insulation. It is warm, even in frigid temperatures. Chiengora is waterproof, durable and also incredibly soft and fluffy – perfect for clothing and accessories next to skin. Occasionally people with a strong sense of smell complain that Chiengora never loses its doggy odor, but it has been found that a good washing removes any trace of the original wearer. Chiengora can be very hot when used by itself, so it is best mixed with sheeps wool. Wool adds durabilitiy, elasticity, and breathablity to dog wool fabric. When wet, it can smell a little funny, but any fiber smells different and stronger when wet. Many common varieties of dog have a soft fuzzy undercoat which is suitable for spinning into luxury yarn. It maintains a “halo” of fuzz around it after being spun, similar to mo-
COVER STORY hair or angora rabbit. Once knitted or woven, the halo covers the fabric surface. It is a fine alternative to faux fur or real fur as it is a natural fiber but no animal was harmed for the product. In fact, healthier happier dogs will produce better wool! Chiengora is rarely used as a commercially produced fiber, but occasionally a designer will commission some specialty pieces utilizing it. Natural protein fibers, such as wool, mohair, and silk, currently used in textile production can be very costly. Although non-traditional, a protein fiber, such as chiengora (dog hair), can prove to be a cheaper, environmentally friendly, and suitable substitute. However, very little information on the properties of these fibers can be found in the literature. As per the research the physical and mechanical properties of hair combed from 18 dog breeds were measured and compared to those of traditional animal hair fibers. Unwashed dog hair was collected, bagged and labeled by professional pet groomers. Results show that length, linear density, tenacity, strain, and elastic modulus of chiengora fibers are all similar to those of traditional protein fibers. Results also show that hairs from some breeds may be suitable for short-staple or long-staple processing. Uses of Chiengora Fibres Woven Chiengora can be used to create scarves, wraps, blankets, throw pillows, and fabric for sewing projects, Home Decors, Hats, Mitts, Scarves.
Effect of Chitosan concentration on 100% cotton fabric to study antimicrobial property
Mrs. Supriya Shirhatti, Mr. Satish Patil
Department of textiles, DKTES Textile Engineering Institute
Acrustaceans (shrimp, crab and other extent, after which increase does shellfish) Chitosan is obtained from not make a difference in the antimibstract Chitin by a deacetylation process. Chitin, the polysaccharide polymer crobial activity. Keywords: Chitosan, E. coli, S. auThe inherent properties of the textile fibres provide room for the growth of micro-organisms. Besides, the structure of the substrates and the chemical processes may induce the growth of microbes. In recent years, several technologies have been developed for modifying cotton and cotton blends as multi-functional textiles. Surface modification of cotton fabrics can impart wrinkle free finishes, selfcleaning properties, anti-microbial activity, UV protection, and flame retardancy. Self-cleaning features include Chitosan finish. Chitosan is a polysaccharide polymer containing more than 5,000 glucosamine and acetyglucosamine units with molecular weights of over one million Dalton’s. Chitin is found in fungi, arthropods and marine invertebrates. Commercially, Chitin is derived from the exo-skeletons of from which chitosan is derived, is a cellulose-like polymer consisting mainly of unbranched chains of N-acetyl-D-glucosamine.Chitosan can be used in water processing. Chitosan is the richest source of antimicrobial compounds. This study compares the antibacterial activity of cotton fabric treated with Chitosan in different concentrations. With increase in the concentration of Chitosan, increases upto certain reus, antimicrobial. Introduction Cotton is most important cellulose fiber in textiles. Many finishes are applied on these cotton and cotton blends. The inherent properties of the textile fibres provide room for the growth of micro- organisms. Besides, the structure of the substrates and the chemical processes may induce the growth of microbes. Humid and warm environment still aggravate the problem. Infestation by microbes cause cross infection by pathogens and develop odour, when the fabric is worn next to skin. In addition,
the staining and loss of the performance properties of textile substrates are the results of microbial attack. With a view to protect the wearer and the textile substrate itself antimicrobial finish is applied to the textile materials. Antimicrobial textile products continue to increase in popular demand for fresh smelling, skin friendly and high-performance fabrics. Modern performance fabrics are required in much specialist application, sports textile is one example. This need to exhibit high degrees of performance in terms of longevity and durability by imparting antimicrobial properties to the fabrics. These properties can be improved as well as increase the comforts as hygiene factor making them more pleasant to wear. Odour can be neutralized and skin problems caused by microbial growth reduced thus emphasizing the ‘hygienic’ nature of the treated product. Microbes are the tiniest creatures not seen by the naked eye. They include a variety of micro- organisms like Bacteria, Fungi, Algae and Viruses. Bacteria are unicellular organisms which grow rapidly under warmth and moisture. Further, sub-divisions in the bacteria family are Gram positive (Staphylococcus aureus), Gram negative (E-Coli), spore bearing or non-spore bearing type. Some specific types of bacteria are pathogenic and cause cross infection. Fungi, molds or mildew are complex organisms with slow growth rate. They stain the fabric and deteriorate the performance properties of the fabrics. Growing awareness towards health and hygiene has increased the demand of bioactive textiles. A durable finish is potentially effective means of controlling micro-organism on to textiles. In the last few decades, wide
Materials and Methods
2.1 Materials -The cotton fabric with following specifications was used in the study: Table 2.2. Chemicals Used for the study


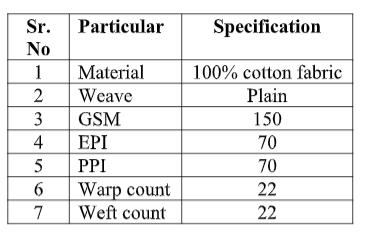
2.1.1. Chemicals used in the study:

2.2 Experimental Method

2.2.1 Desizing- 100 % cotton fabric treated with 3-5 gpl cellulase enzyme desizing to remove size paste. Desizing of cotton fiber was carried as follows:

Table 2.3. Chemicals used for desizing
In combined scouring and bleaching of cotton, the scouring process is accelerated in presence of H2O2 and less time is required to achieve good absorbency by the material. The process of combined scouring and bleaching was carried using Hydrogen peroxide (H2O2 ) and Sodium Hydroxide (NaOH). After Completion of this process washing and draining of fabric was carried out. The process of combined scouring and bleaching is carried out in alkaline pH which is maintained by Sodium Carbonate (Na2CO3 ). pH of combine scouring and bleaching was 9-11. The combine scouring and bleaching was carried out as follows:
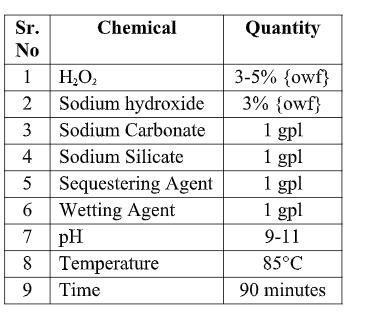
2.2.3 Preparation of chitosan and application Chitosan was dissolved in 2% aqueous acetic acid solution. The fabric was first immersed in the pad bath for 10 min. padded up to 80±5% wet pickup on weight of fiber [O.W.F.], dried on pin frames at 100ᵒ C for 5 minutes. Cured at 180ᵒC for 2 minutes, washed and dried. Samples were cured at 180ᵒC for a period of 2 minutes. 2.2.4 Treatment of Chitosan on Fabric Table 2.4. Chemicals used for Combined scouring and bleaching Different concentrations of solutions (3gpl, 4gpl, 5gpl, 6gpl, 7gpl, 8gpl, 9gpl 10gpl, 11gpl, 12gpl, 13gpl, 14gpl, 15gpl) keeping 65% expression, were prepared by dissolving chitosan overnight at room temperature. The pre-scoured and bleached cotton fabric was padded using pad-dry-cure method. The fabric samples were then dried at 80-85ᵒC to maintain the residual moisture content 8–10%.

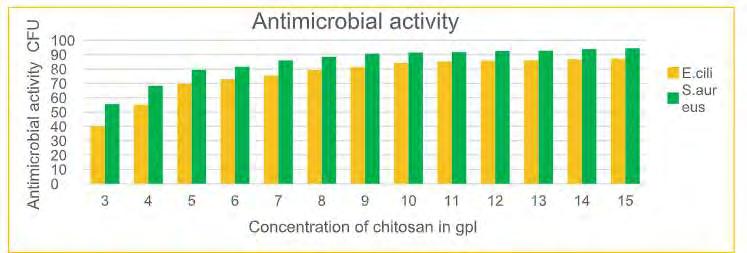
Table 2.5. Concentration of chitosan 2.3 Testing and Analysis 2.3.1 Antimicrobial Activity Anti-microbial testing was done by AATCC test method 100:2004 for the quantitative assessment of the antibacterial effectiveness of the antimicrobial agents against Gram positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli). Circular swatches of 4.8 ± 0.1 cm in diameter were cut from the test fabric. The cut pieces were stacked in 250 ml wide mouth glass jar with a screw cap followed by sterilization at 121°C for 15 mins. Then 0.5 ml of the bacterial solution was added to the swatches so that whole of it is absorbed by each swatch. The jar was kept for 24 hours in the incubator at 37°C. After ehich, 50 ml of sterilized saline water was added to each jar followed by 15 mins. shaking in the shaker. Further, three serial dilutions were done by taking 100 ml in 900 ml of saline water in Eppendorf micro test tubes. Nutrient agar plates were made and 100 ml of this diluted bacterial solution was inoculated into the agar plate and left in the incubator at 37°C. After 24 hours, the number of bacterial CFU of the bacteria formed on the agar plate were counted. Untreated cotton sample was used as the control sample every time. 2.3.2 Tensile Strength (ASTM D 5035) Prior to the test, specimens were conditioned to moisture equilibrium in the standard atmosphere of 65 % relative humidity, 27 ± 2 0 C temperature. Samples (fabric strip) were cut by using the given template. Threads were ravelled from both side of the sample to exactly 5cm width. Clamp was set on testing machine at a distance of 20 cms and strength indicating pointer to zero position. Sample was clamped between two jaws, with some length of fabric extending beyond the jaws at each end. Sample was elongated at a constant rate of 300 mm/min till rupture. Breaking load in Kgf was noted. Same procedure was repeated for all samples. 2.3.3 Measurement of Bending Length Prior to the test, specimens were conditioned to the standard atmospheric conditions. Samples were cut using the given template. Threads were ravelled from both side of the sample to exactly 1 inch x 6 inches. The sample was placed on the bending length track and made to slide in forward direction till incline angle become 41.5 0 . The bending length was noted. 3. Results and Discussion 3.1. Antimicrobial activity

Table 3.1. Antimicrobial activity
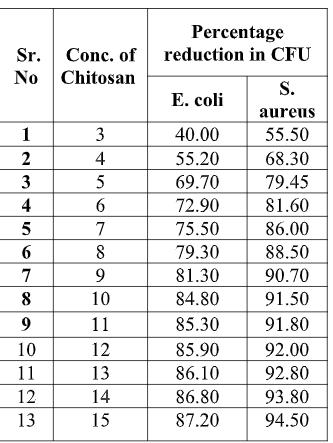
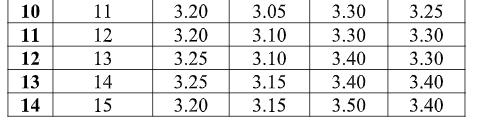
19 COVER STORY Fig. 3.1. Antimicrobial activity As shown in Fig. 3.1, chitosan gives good anti-microbial activity when concentration increases from 3 to 10 gpl. With increase in concentration of chitosan antimicrobial activity increases. After 10 gpl there is no remarkable improvement in the antimicrobial activity. The maximum antimicrobial activity is archived at 15 gpl concentration, which is 87.20 % for E. coli and 94.50% for s. aureus. The effect may increase with increase in concentration of chitosan, but increase in concentration decrease the penetration of chitosan. With increase in concentration of solution and temperature of curing antimicrobial activity increases. This is due to increase in the quantity of chitosan. 3.2. Tensile strength Table 3.2. Tensile strength of given Treated fabric (in kg/f)
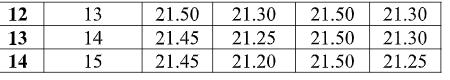
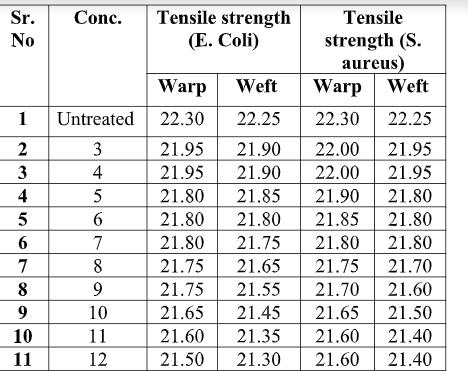
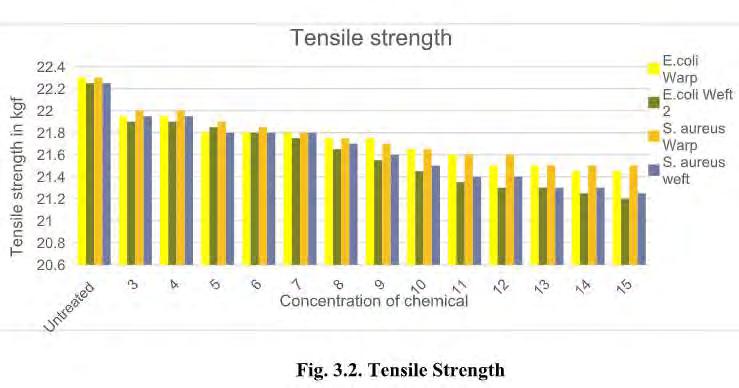
As shown Fig 3.2, with increasing concentration of Chitosan, tensile strength decreases. But decrease in tensile strength is not remarkable. 2 – 3 % tensile strength decreases in both warp and weft direction. This is due to breaking of hydrogen bonds and decrease in air permeability of fabric. 3.3. Bending Length of treated fabric Table 3.3. Blending length after application of Chitosan 3.3. Bending Length of treated fabric
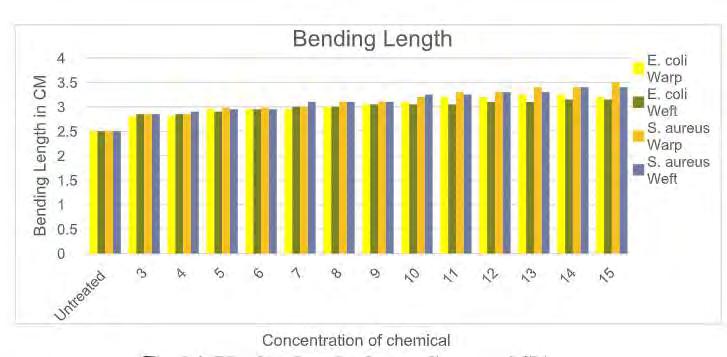
Fig 3.3 Blending length after application of chitosan
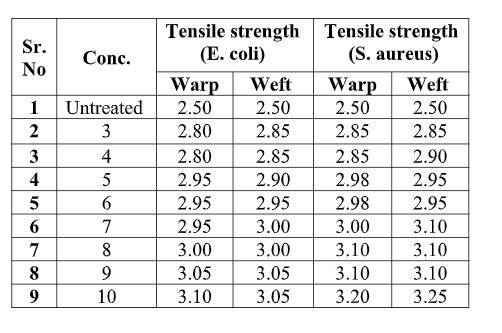
Table 3.3. Blending length after application of Chitosan