A REPORT ON DISEASE DIAGNOSIS OF WHITE SPOT SYNDROME (WSSV) SURVEILLANCE PROGRAMME
AlluVenkataMadhuri,D.SrinivasaRao,LavanyaSaranu,RaghavendraRaoMV
1DepartmentofBiotechnology,AcharyaNagarjunaUniversity,Guntur,AP,India.
2ApolloInstituteofMedicalsciencesandresearch,Hyderabad,TS,India.

ABSTRACT
Indiaisendowedwithawidediversityofwaterresources,whichcanprovidescopeforhighlydevelopedfisheriessector Thesectorprovidesnutritionandlivelihood securityforalargenumberofpeopleandalsosourceofforeignexchange.Withrapidshiftfromcapturetoculturefisheries,thesectorneedssystematicinterventionon aquatichealthproblems.WhiteSpotSyndrome(WSSV)isprevalentinL.vannameibutdiagnosiscanhelpfarmersavoidandreducethespreadofWSSVtoother ponds.Theearlyharvestjustbyseeingthewhitespotsoncarapacecanbeavoidedbyimplementingthesediagnostictechniques.Significantproportionofbrackish waterfarmsareadoptingpoly-cultureBiologicalSamplesScreenedandCulture.Asamplesizeof51werescreenedforninepathogensanddiseasesweredetectedand revealedarethreediseasespathogennamely,IHHNV,WSSVandEHP Theappearanceofwhitespotsonshrimpispredominant,causingpanictofarmersandoften resorttoemergencyharvestandtosavewhatevertheycan.Thepresentworkhelpsindifferentialdiagnosisofwhitespotsatthelaboratorylevelandconfirmatory diagnosisbyusingrapidtestkitslikerapiddotorShrimple.Ontheotherhand,itisnecessarythatthefarmersshouldmaintainstrictbiosecuritymeasurestopreventthe spreadofthediseasestotheotherspecies.
INTRODUCTION:
India is endowed with a wide diversity of water resources, which can provide scopeforhighlydevelopedfisheriessector.Ithasalongcoastlineof8118KM andahuge1.9lakhKMareaofinlandwaterresourceswithawaterspreadareaof 7.4lakhhectares.Thesectorprovidesnutritionandlivelihoodsecurityforalarge numberofpeopleandalsosourceofforeignexchange.
Asotheranimals,aquaticanimalsarealsopronetodiseasesandneedinterventionandsurveillance.Thisisneededforimprovingtradewithothercountriesas alsoforprotectingourresources.Withrapidshiftfromcapturetoculturefisheries,thesectorneedssystematicinterventiononaquatichealthproblems.Asmore and more demand for fish is growing at a rapid rate. To meet this growing demand,productionsystemhastobemovetosemiintensiveandintensivefrom traditionalsystem.Thismovewillresultinfrequentdiseases. Tomeetthissituation,developmentofhealthsurveillancesystembecomesimportant.
MATERIALANDMETHOD:
SampleSize:
Atotalsampleof51samplesfrombrackishwatercoveringNelloredistrictswere collectedfromvariousfarmersofAndhraPradesh.
Samplecollection:
The survey was covered from different sources namely, hatcheries, nurseries, rearingpondsandgrow-outponds.Thenumberofgrow-outpondsweremoreas therearenotseparatedfromnurseryandrearingponds.Seedisthemajorsource forimpactondiseaseoutbreak.Itissignificanttonotethatasignificantproportionofbrackishwaterfarmsarealsoadoptingpoly-culture.
BiologicalSamplesScreenedandPathogensDetected:
Shrimpiscountedasoneof largestseafoodcommodityaccountsto17%.PenaeusmonodonandLitpaneous vannamei aremajorspeciesdominatedintheculturearea,itcontributingnearly 75%ofproductioninaquaculturesectorandthesetwoarealsorepresentunder invertebrateasasubstantialfoodanimal(FAO,2009,Televisory2020).
Amongthefisheryproductstradedinternationally
Shrimpismajorproductinmarineexports.Itiscultureinanextentofabout21 thousandhectaresanditsproductionisestimatedto3.75lakhMTduring201920 AndhraPradeshisthemajorcontributorofshrimpinthecountrywithashare of 58.7 per cent. But its share in area is only 31.2 per cent. Thus, the state achievedhighproductivityinshrimpproduction.Thus,AndhraPradeshstandsat thetopinbothproductionandproductivity
AndhraPradeshisknownforshrimpproductionandthestatecontributes60per centofallIndiashrimpproduction.
WhiteSpotSyndrome(WSSV)Thoughveryfewculturefarmsarepermitted forcultureofL.vannameiinIndiawithstrictbiosecurityconditionsandsupplying the healthy seed from specific pathogen brood stock, still there are disease outbreakssuchasWhiteSpotSyndrome(WSSV)inmostoftheculturedfarms. Henceanattempthasbeenmadeinthepresentstudytoconfirmwhetherreally theWSSVisprevalentin L.vannameiornotandtoidentifyasuitableandeasy methodofdiagnosisforthefarmers usingtheavailabletechniqueslike,molecular diagnostics Polymerase Chain Reaction (PCR) and isothermal PCR), histological (Histopathology using stained sections of hematoxylin and eosin and rapid gill staining), immunodiagnostics (Rapid dot kit and Shrimple kit), microscopicandclinicalobservations(DifferentialMorphologyofwhitespots and the variations in the morphology of white spot between juveniles and sub adults).Theearlyandaccuratediagnosisofthesediseasesmayhelpfarmersto avoidandspreadofWSSVtootherponds,canreducethemassmortality Also, theearlyharvestwithoutWSSVinfectionjustbyseeingthewhitespotsoncarapacecanbeavoidedbyimplementingthesediagnostictechniques(M.A.Badhul Haqetal.,2016)
Itisfoundthatthreediseasesnamely,IHHNV,WSSVandEHPweredominant disease. IHHNVis the most predominant disease found in 61.4 per cent of the cases,followedbyWSSVfoundin38.6percentandnewdiseaseEHPin20.3percentofthecases.TheresultsindicatethatWSSVisdominantduringthisperiod.
Method:
PCRmethodsforWSSVandIHHNVwasfollowedtheproceduresofIQ2000 KITmethod.IsothermalPCRforWSSVwasperformeraspertheprocedureof IQscreenkitmanufacturedbyFramingIntelligenceTech.Corp.Taiwan.
PhysicalObservation:
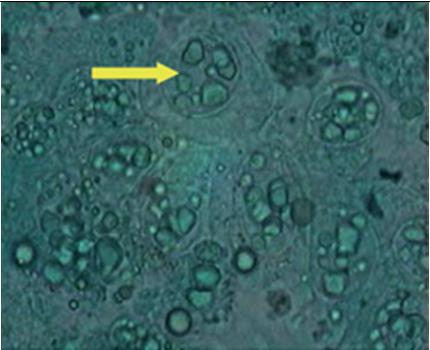
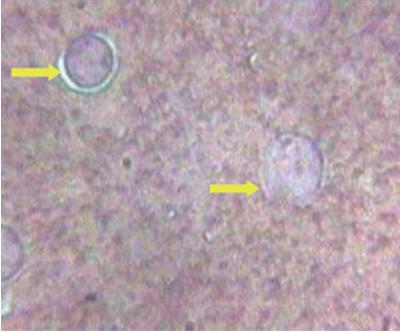
TheprevalenceofWSSVinfectioninexoticspecies,L.vannameiinspiteofits SpecificPathogenFree(SPF)statusofbroodstockandhighhealthpostlarvae. UnlikeP monodon,inL.vannameiwhitespotsarenotvisibleexternallydueto thewhitecolouroftheshrimp(Figs.1and2).Inadditiontothatwhitespotsare presentinalmostallthecasesofmortalitymightbeduetotheprevalenceofother diseaseslikevibriosis(Fig.3),inhealthyshrimps(Fig.4),moultedshells(Fig.5) andthusmakingdifficulttotakedecisionofharvesting(Earlyharvestmaypreventthespreadofthevirustotheotherpondsofthatfarmandamongotherfarms andcanalsoreducetheloss).Inseveralcasesitwasobservedthattheharvesting hasbeenperformedwithoutWSSVoutbreakbyjustobservingthewhitespotson thecarapaceandevenduringthemortalitybecauseofotherproblems.
Inadditiontoabove,thesignsandsymptomslikeanorexia,rednessofthebody (Fig.1),antennaecut(Fig.6),surfacingofshrimp,cannibalism(Fig.7),oedema inthecephalicregion(Fig.8),preandpostmoultdeathwerealsoobserved.However,thesesymptomsarenotcommoninallthecasesofWSSVdisease,similar signswerealsoreportedinotherdiseases(Lightneretal.,2006)Table1,making it difficult to take a decision. Hence, an attempt has been made with different available confirmative diagnostic procedures to evaluate and choose the best techniquesasgivenbelow
a. Polymerasechainreaction
b. IsothermalPCR
c. Histology
d. Rapidgillstainingtechnique
e. Rapiddotkit
f. Shrimplekit
g. Morphologyofwhitespotsdeveloped
a. PolymeraseChainReaction:
Theshrimps(10samples)suspectedtobeinfectedwithWSSVhaving external symptoms of white spots and mortality were analyzed, and foundthatonly5samplesarepositivebyPCRandIscreenisothermal PCR system (Fig.13). This clearly evidences that all the white spots observedinculturedshrimpsarenotcausedbyWSSV Eventheponds, whicharepositivebyPCRandisothermalPCR,survivedformorethan onemonthwithoutanyvisiblesymptomsofmorbidityandmortalityby implementing the effective management practices. Thus the PCR and IsothermalPCRtechniquesareverymuchusefulforearlydiagnosisof the disease and to prevent the spread of the virus to other ponds and other culture areas. In one particular case of our study, the post larvae wereobservedpositiveforWSSwithin5daysofstockingandsurvived for35dayswithoutanysignsandsymptomsofWSSV,thiscouldbepossiblebytheefficientpondmanagementpractices.Thisstudyconforms thePCRandisothermalPCRcanbeusedtodetectanddifferentiatemortalitycausedbyWSSV
juveniles (Fig. 21) was different from sub adults and lacking central holebuttheradiatinglineswerepresent.
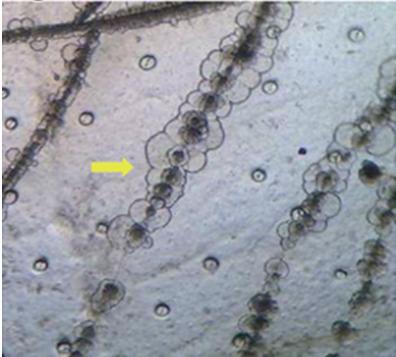
Exuviaoftheshrimpfromthehealthypondswerecollected,storedin thesamepondwaterandbroughttothelaboratory Themorphologyof whitespotpresentinalmostallthemoultedshellofhealthypondswithoutcausingmortalitywasdifferentwithoneortwohomocentricrings anddarkenedcenterwithfloralstructure(Figs.16and22)andresemblesthemorphologyofdescribedbyWangetal.(2000)inP monodon caused by the Bacillusspp. It is a well-accepted fact that WSSV is endemictoIndia.Duringthepresentstudy,ithasbeenobservedthatdue tolackofbiosecurityatthefarmlevel,likecrabfencing,birdfencing, pumpingwaterdirectlyfromcreakswithouttreatmentfordisinfection andfiltrationtopreventtheentryofcarriersarethemainreasonssuspectedforthepresentoutbreakofWSSV
b.
Histographyofgill:
The same samples used for PCR were fixed in Davidson fixative and processedforhistopathologyusinghematoxilinandeosinstains.5samplesshowthecharacteristicintranuclearcowdrytypeinclusionbodies asshowninfig.9confirmingWSSVinfection.Thisstudyistimeconsumingprocedure,requiresminimum5daystimeforgettingfinalresult andsometimesevenmortalitymaystartbeforegettingthefinalresult, as the incubation time is less than 5 days for some virulent strains of WSSV (OIE, 2009). The histopathological symptoms of early stages WSSV resembles the histopathology of IHHNV in formation of eosinophilicintranuclearcowdryatypeinclusionbodies(OIE,2009)
c. RapidGillstainingtechnique:
Thegillsfromthemoribundshrimpswerecollectedandprocessedfor rapidgillstainingoutoftensamplesonlythreewereshowedhypertrophied nuclei and it is also a characteristic feature of WSSV infection (Fig.10).Thistechniqueiscosteffectivebutitrequiresmorelaboratorysupport.
d. RapidDotkit:
Rapiddotkitisrelativelyhighsensitivethanshrimplekitanditisable todetecttheinfectionatleast15daysinadvancebeforetheonsetofmortality Basedontheintensityofthecolordevelopmentviralloadcanbe assessedanditisrelativelyeasyandeconomical,atatimefoursamples canbeanalyzedatpondsidewithin10minutes.(Fig11)
e. Shrimplekit:
ShrimplekitisalesssensitivetechniquethanPCR,isothermalPCRand rapiddotkit.Itcandetecttheinfectionthreetofourdaysinadvanceand very good tool for making a harvest decision. The procedure is relativelyeasyandkitislittlebitexpensivethantherapiddotkit.(Fig12) illustrates presence of red band at point T confirmation WSSV infection.Theadvantagesanddisadvantagesofthedifferentkitsusedinthe presentexperimentalstudyweredepictedinthetable-2.
f. Chromatophore
identification:
ThepleopodsoftheinfetedshrimpswithWSSVwereobservedunder the microscope for observing the chromatophores, they were first turnedtoyellow(Fig.14),andthecolourgraduallyturnedtored(Fig. 49),indicatingtheprealenceofviralstressintheculturedshrimpsand thispatternofchangeincolourcanbetakenasanindicator
g. MicroscopicidentificationofWhitespots:
During the culture practice, in most cases the farmers rely on white spotsexhibitingontheexoskeletonofshrimpasthespecificdiagnosis symptom for this most dreadedWSD and resort to an emergency harvestwithoutknowingtheactualcause.Hence,itisaimedtostudythe morphologyofwhitespotscausedbydifferentreasonstocomparethe etiologies.The samples with visible white spots on the carapace were observedunderthemicroscope,thedifferentmorphologicalcharacteristicsasfollows.ThecarapaceoftheL.vannameithosearepositiveby shrimplekitforWSSVwasobservedundermicroscope.ThemorphologyofwhitespotcausedbyWSSVinL.vannameiiswithaholeinthe centerandradiatinglines(Figs.17and18)isdifferentfromthewhite spot morphology of P.monodon with dense melanized dots (Fig. 19).OnlyonecaseofshrimpmortalitysuspectedwithVibrioinfection wasreportedduringtheresearchwork.Thewhitespotsthatwerepresent on carapace were observed under microscope for morphological studies.ThemorphologycausedbyWSSVwasdifferentfromthemorphologyofwhitespotcausedbyvibriospp.(Fig.20).Thismorphology ofthewhitespotcausedbyVibrioresemblestheonereportedbyCyrille Goarantetal.(2000)inL.stylirostriscausedbybacteria.Thecarapace ofP vannameijuvenileswithconfirmedWSSVinfectionwasobserved for morphology The morphology of white spot caused by WSSV in
Inaparticularcaseduringthepresentinvestigation,itwasobservedthat P monodon shrimp entered the L. vannamei pond prior to stocking due tothelackoffiltrationandfornottreatingthewaterinreservoirwithdisinfectants for eradicating the carriers for white spot virus. White spot disease first affected the P monodon and later spread to L. vannamei (Fig. 23). From the fig. 23 it was clearly evident that the size of P monodon is been bigger than the L. vannamei,so it indicates that P monodon entered the pond before stocking the target species and first mortalityhasbeenstartedwithP monodon.Thewhitespotsareclearly visibleonthepeeledcarapaceoftheP monodon,whosemorphologyis almostsimilartothemorphologyofwhitespot(Wangetal.,2000).
Table1:signsandsymptomsobservedinwhitespotsyndrome
Sign Noofcases OtherProblemwithsamesign
Redness 15/20cases Lowdo,vibrio,stress AntennaeCut 10/20cases Vibriosis
WhiteSpotsontheCuticle 20/20cases Bacterialspot Surfacing 10/20cases Lowdo Cannibalism 18/20cases Alldiseases Oedemaincephalicregion 2/20cases Lowdo Preandpostmoltdeath 10/20cases Ammoniatoxicity,DOdeficiency Anorexia 15/20cases Allcasesofstress
Table2:ComparisonofdifferentdiagnosticproceduresforWSSV
Diagnosticprocedure Advantages Disadvantages
PCR Highlysensitive Requiressophisticatedlab IsothermalPCR Highlysensitive RequiresIscreenoven Histology Confirmatory Requiteslab/time consuming
RapidgillStaining Confirmatory Requiteslabsupport Rapiddot Goodforharvesting decision Storageat4⁰Cand
ShrimpleKit Goodforharvesting decision Relativelyexpensive
RESULTANDDISCUSSIONS:
Observedthatthefollowingfactorsfortheoccurrenceofdiseaseoutbreak: (i) Poorqualityofseed (ii) Poorwaterqualityandmanagement (iii) Fluctuationsintheenvironmentalconditionsliketemperature.,release oftoxicgasesetc. (iv) Poorpondmanagementbetweencrops
(v) Inadequatebio-securitymeasuresandhighstockingdensities
(vi) Horizontaltransmissionofpathogensthroughcarriers
CONCLUSION:
Itisdesirabletoestablishrelationshipsbetweensoil,water,seedandfeedquality parametersandchancesofdiseaseoutbreak.
1. Alarge number of samples were screened for nine pathogens and diseases were detected and revealed are three diseases pathogen namely, IHHNV,WSSVand EHP It may be concluded thatWSSVare highly prevalentdiseasesduringthephase.
2. Itissuggestedthatthestatedepartmentsshouldrecognizesomeofthe private diagnostic laboratories with adequate facilities and bringing themintothefoldofregulardiseasesurveillanceprogramformonitoringdiseaseoutbreaksinthestateofAndhraPradesh.
3. Capacity building of all staff involved in disease surveillance programme and periodical feedback from the farming community, proper data management, reporting mechanism and follow up actions areimportanttostrengthenthediseasemanagement.
4. Thehighgrowthintherecentperiodisaccompaniedbyintensification and adoption new seed, feed and production technologies. With high demandintheexportmarket,productionisalsoshiftingtowardsshrimp andotherfinfishfromcarp.Thesechangeshavealsocontributedtofrequentoccurrenceofdiseases.Exportofanylivestockproducthastosatisfy OIE andWTO obligations.As per these standards, every country hastosubmitaquarterlyreporttoOIEaboutoccurrenceofdiseases.
5. It is envisaged that outbreak of white spot disease in exotic species L.vannamei.Theappearanceofwhitespotsonshrimpcausingpanicto farmersandoftenresorttoemergencyharvestandtosavewhateverthey can.However,thereweresomeincidences,farmersavoidedtheemergencyharvestandgotsuccessfulcrop.Ingeneral,theWhitespotscould beduetotheinfectionofWSSV,Vibriospp.andBacillusspecies.The presentworkhelpsindifferentialdiagnosisofwhitespotsatthelaboratorylevelandconfirmatorydiagnosisbyusingrapidtestkitslikerapid dot or Shrimple. On the other hand, it is necessary that the farmers shouldmaintainstrictbiosecuritymeasurestopreventthespreadofthe diseasestotheotherspecies
REFERENCES:
I. N.Kalaimani,T Ravisankar*,N.Chakravarthy,S.Raja,T C.SantiagoandA. G.PonniahCentralInstituteofBrackishwaterAquaculture,75SanthomeHigh Road,RajaAnnamalaiPuram,Chennai-600028,India.EconomicLossesdue toDiseaseIncidencesinShrimpFarmsofIndia.FisheryTechnology50(2013): 80–86
Ii. Tandel, G M , John, K Riji, George, M Rosalind, Jeyaseelan, M J Prince.2017.CurrentstatusofviraldiseasesinIndianshrimpaquaculture.Acta virologica61:131–137,2017.)
III. Tripathy S, Sahoo PK, Kumari JongW, GangnonngiwW, et al. (2017) EmergenceoftilapialakevirusinThailandandanalternativesemi-nestedRT-PCR fordetection.Aquaculture476:111-118.
IV Ramasamy P, Brennan G, Jayakumar R (1995) A record and prevalence of Monodonbaculovirusfrompost-larvaMishraBK,SarangiN(2006)Multiplex RT-PCRdetectionandsequencecomparisonofvirusesMrNVandXSVassociated with white tail disease in Macrobrachium rosenbergii.Aquaculture 258: 134-139.
V V Jagadeesan,P EzhilPraveena,T Bhuvaneswari,K.P JithendranAndS.K. Otta (2018) Screening of Penaeus vannamei Boone, 1931 collected from east coastofIndiaformonodonbaculovirus(MBV)andhepatopancreaticparvovirus(HPV).
VI. Naresh Kumar Dewangan, Gopalakrishnan Ayyaru, Raja Kuzhanthaivel, Somasundaram Thirugnanasambandan, Gary G Martin, Kannan Daniel, Rajkumar Singh Ramakrishna, Incidence of simultaneous infection of infectious hypodermal and haematopoietic necrosis virus (IHHNV) and white spot syndromevirus(WSSV)inLitopenaeusvannamei20March2017,Pages1-7
VII. Multiple infections caused by white spot syndrome virus and Enterocytozoon hepatopenaeiinpond-rearedPenaeusvannameiinIndiaandmultiplexPCRfor theirsimultaneousdetection
VIII. ChenSN,ChangPS,KouGH(1992)InfectionrouteanderadicationofPenaeus monodon baculovirus (MBV) in larval giant tiger prawns, Penaeus monodon. In:FulksW,MainKL(eds.).DiseasesofculturedpenaeidshrimpinAsiaand theUnitedStates,TheOceanicInstitute,Honolulu,Hawaii,USA.Pgno:177184.
IX. Algarswami K (1995) Status report on shrimp disease outbreak in coastal aquaculturefarmsontheeastcoastofIndiaduring19941995,fortheTechnical Committee,GovernmentofIndia,MinistryofAgriculture,NewDelhi,India.
X. Hasson KW, Lightner DV, Poulos BT, Redman RM, White BL, et al. (1995) Taura syndrome in Penaeus vannamei: demonstration of a viral etiology Dis AquatOrg23:115-126.
XI. LightnerDV,RedmanRM,PoulosBT,NunanLM,MariJL,etal.(1997)Riskof spreadofpenaeidshrimpvirusesintheAmericasbytheinternationalmovement ofliveandfrozenshrimp.RevSciTech16:146-160.
XII. LightnerDV,RedmanRM(1985)Aparvo-likevirusdiseaseofpenaeidshrimp. JInvertebrPathol45:47-53.
XIII. Lightner DV, Redman RM, Bell TA (1983) Infectious hypodermal and hematopoieticnecrosis,anewlyrecognizedvirusdiseaseofpenaeidshrimp.J InvertebrPathol42:62-70.
XIV OIE (World Organisation for Animal Health) (2007) Infectious Myonecosis. OIEAquaticAnimalDiseaseCards,Paris,France.
XV PantojaCR,LightnerDV,PoulosBT,NunanL,TangKFJ,etal.(2008)Paper PresentedonOverviewofDiseasesandHealthManagementIssuesRelatedto FarmedShrimp,OIEReferenceLaboratoryforShrimpDiseasesDepartmentof VeterinaryScience&Microbiology,UniversityofArizona,Tucson,USA.
XVI. MishraSS(2000)ShrimpViruses:Theirpathogenicity,diagnosisandcontrol. In:AdvancesinAquaculture,NatarajanP,JayaprakashV(eds.).Departmentof AquaticBiologyandFisheries,UniversityofKerala,Thiruvanthapuram.Pgno: 239-246.
XVII. KiatpathomchaiW,JaroenramW,ArunrutN,GangnonngiwW,Boonyawiwat V (2008) Experimental infections reveal that common Thai crustaceans are potential carriers for spread of exoticTaura syndrome virus. DisAquat Organ 79:183-190.
XVIII. Mohan CV, Bhatta R (2002) Social and economic impacts of aquatic animal healthproblemsonaquacultureinIndia.In:ArthurJR,PhillipsMJ,Subasinghe RP,ReantasoMB,MacRaeIH(eds.).PrimaryAquaticAnimalHealthCarein Rural,Small-Scale,AquacultureDevelopment,FAOFishTech.Pgno:63-75.
XIX. MohantyBR,SahooPK(2007)Edwardsiellosisinfish:abriefreview JBiosci 32:1331-1344.
XX. MoriK,NakaiT,MurogaK,ArimotoM,MushiakeK,etal.(1992)Propertiesof anewvirusbelongingtonodaviridaefoundinlarvalstripedjack(Pseudocaranx dentex)withnervousnecrosis.Virology187:368-371.
XXI. MPEDA(2016)State-wiseaquacultureproductivity:Areautilizedandproduction of Tiger Shrimp during 2015-16, The Marine Products Export Development Authority, Ministry of Commerce & Industry, Government of India, Kochi,Kerala.
XXII. Otta SK, Karunasagar I, Karunasagar I (2003) Detection of Monodon Baculo Virus (MBV) andWhite Spot SyndromeVirus (WSSV) in apparently healthy PenaeusmonodonfromIndiabypolymerasechainreaction.Aquaculture220: 59-69.
XXIII. QianD,ShiZ,ZhangS,CaoZ,LiuW,etal.(2003)ExtraSmallVirus-likeparticles(XSV)andNodavirusassociatedwithwhitishmusclediseaseinthegiant freshwaterprawn,Macrobrachiumrosenbergii.JFishDis26:521-527.
XXIV MishraSS,SwainP,RakeshD,PaniKC,SarkarS(2017)InvestigationofMass mortalityinDerjangReservoirinAngulDistrict,Odisha,India,duringApril2017.ReportsubmittedtoICAR-CentralInstituteofFreshwaterAquaculture, Bhubaneswar,India.
XXV Mohan CV, Bhatta R (2002) Social and economic impacts of aquatic animal healthproblemsonaquacultureinIndia.In:ArthurJR,PhillipsMJ,Subasinghe RP,ReantasoMB,MacRaeIH(eds.).PrimaryAquaticAnimalHealthCarein Rural,Small-Scale,AquacultureDevelopment,FAOFishTech.Pgno:63-75.
XXVI. Rajendran KV (2017) Health Management and Biosecurity in shrimp aquacultureinIndia-areview.In:ProceedingsofInternationalSymposiumon aquaticAnimal Health and Epidemiology for sustainableAsianAquaculture, ICAR-NationalBureauofFishGeneticResources,Lucknow,India.Pgno:1921.
XXVII. DongHT,SiriroobS,MeemettaW,SantimanawlPenaeusmonodon,inMardas, India.Aquaculture130:129-135.
XXVIII. LuY,TapayLM,BrockJA,LohPC(1994)InfectionoftheYellowheadBaculolike Virus (YBV) in two species of penaeid shrimp, Penaeus stylirostris (Stimpson)andPenaeusvannamei(Boone).JFishDis17:649-656.
XXIX. SheelaRR,MuralimanoharB,SundarrajA,SelvarajD,ChidambaramP,etal. (1998)InfectiousHypodermalandHaematopoieticNecrosisVirus(IHHNV)in culturedPenaeusmonodoninTamilNadu.India,IndianJFish.45:183-186.
XXX. Shike H, DharAK, Burns JC, Shimizu C, Jousset FX, et al. (2000) Infectious HypodermalandHematopoieticNecrosisVirus(IHHNV)ofshrimpisrelated tomosquitobrevidensoviruses.Virology277:167-177.
XXXI. FlegelTW,SriurairatanaS(1994)Shrimphealthmanagement:anenvironmentalapproach.In:SubasingheRP,ShariffM(eds.).Diseasesinaquaculture:The CurrentIssues.MalaysianFisheriesSocietyPublicationNo.8.KualaLumpur, UniversityPertanianMalaysia,Malayasia.Pgno:1-48
XXXII. Rathore G, Kumar G, SwaminathanTR, Swain P(2012) Koi HerpesVirus:A ReviewandRiskAssessmentofIndianAquaculture.IndianJVirol23:124-133.
XXXIII. SiddickA, Girigan G, Mishra CS, Oliver King EDI, Goddard E (2014) Home gardens and fish ponds for nourishment and empowerment. IDRC Stories of Change.
XXXIV Manivannan S, Otta SK, Karunasagar I, Karunasagar I (2002) Multiple viral infection in Penaeus monodon shrimp postlarvae in an Indian hatchery Dis AquatOrg48:233-236.
XXXV UmeshaKR,UmaA,OttaSK,KarunasagarI,KarunasagarI(2003)Detection byPCRofHepatopancreaticParvovirus(HPV)andothervirusesinhatcheryrearedPenaeusmonodonpostlarvae.DisAquatOrg57:141-145.
XXXVI. OIE (World Organisation for Animal Health) (2007) Infectious Myonecosis. OIEAquaticAnimalDiseaseCards,Paris,France.
XXXVII. Sahul Hameed AS, Abdul Majeed S, Vimal S, Madan N, Rajkumar T, et al. (2017) Studies on the occurrence of infectious myonecrosis virus in pondrearedLitopenaeusvannamei(Boone,1931)inIndia.JFishDis40:1823-1830.
XXXVIII. NitiChuchird,RattiyakornInthusai,ChalorLimsuwanandTemdoungSomsiri (2009),AquacultureBusinessResearchCenter,FacultyofFisheriesKasetsart University, Bangkok 10900, Thailand .E-mail address : ffisntc@ku.ac.th, the causesofwhitefeces
XXXIX. water, world aquaculture society OIE (2009). Diseases Listed by the OIE. In: Aquatic Animal Health Code. OIE (World Organisation for Animal Health), Paris.
XL. Oliver,L.M.andFisher,W S.(1995).Comparativeformandfunctionofoyster Crassostrea virginica hemocytes from Chesapeake Bay (Virginia) and ApalachiocolaBay(Florida).DiseasesofAquaticOrganisms22,217-225.
XLI. Paterson,W D.andKeith,I.R.(1992).Diseaseanddefensemechanismsofthe Americanlobster,Homarusamericanus.In:Shariff,M.,Subasinghe,R.P and Arthur,J.R.(editors).DiseasesinAsianaquaculture.I.ProceedingsoftheFirst SymposiumonDiseasesinAsianAquaculture,Bali,pp.81-88.