
—WHAT AN ESTUARINE ECOLOGIST DOES AT THE UNC INSTITUTE OF MARINE SCIENCES— full story on page 76


—WHAT AN ESTUARINE ECOLOGIST DOES AT THE UNC INSTITUTE OF MARINE SCIENCES— full story on page 76





Check out all of our previous issues at issuu.com/uncsci. As the organization continues to grow, we would like to thank our Faculty Advisor, Dr. Lillian Zwemer, for her continued support and mentorship.












Mission Statement:
Founded in Spring 2008, Carolina Scientific serves to educate undergraduates by focusing on the exciting innovations in science and current research that are taking place at UNC-Chapel Hill. Carolina Scientific strives to provide a way for students to discover and express their knowledge of new scientific advances, to encourage students to explore and report on the latest scientific research at UNC-Chapel Hill, and to educate and inform readers while promoting interest in science and research.
Letter from the Editors:
In facing a future of uncertainty, the discovery of new knowledge at our university remains a comforting reminder of the transformative power of curiosity. As Editors-in-Chief, we have the privilege of witnessing firsthand the extraordinary work being carried out by our students, faculty, and collaborators across disciplines. This issue in particular, highlights the breadth and depth of intellectual inquiry taking place at our institution. From Astronomy to Zoology, each contribution exemplifies the rigor, creativity, and collaboration that define our scholarly community. Furthermore, none of this would be possible without our community. We extend our deepest gratitude to our researchers, to our writers and designers, to our magazine staff, and to our readers. To everyone who has continued to follow Carolina Scientific—thank you for making this issue a reality. As you explore the unique and engaging pages of this issue, we hope you’ll find inspiration, innovation, and a deeper appreciation for the science that connects us all.
- Sarah Giang & Isaac Hwang

Learn about an estuarine ecologist’s work on the impact of environmental stressors on coastal ecosystems at the UNC Institute of Marine Sciences.
Full story on page 76
Illustration by Tanisha Choudhury
carolina.scientific@gmail.com carolinascientific.org
instagram: @carolinascientific facebook.com/CarolinaScientific
Editors-in-Chiefs
Sarah Giang
Isaac Hwang
Design Editor Tanisha Choudhury
Copy Editor Corinne Drabenstott
Managing Editor Meitra Kazemi
Treasurer
Natalie Druffner
Secretary Reagan Gulledge
Publicity Chair Masha Dixon
Fundraising Chair Ria Patel
Associate Editors
Andrew Phan
Kruti Bhargav
Daniela Danilova
Sprihaa Kolanukuduru
Julia Boltz
Esha Agarwal
Online Content Manager Kirina Shah
Faculty Advisor Lillian Zwemer, Ph.D.
Staff Writers Copy Staff
Jacob Allred
Erin Atos
Brantley Aycock
Jack Blankenbaker
Nicole Branch
Ava Carlton
Anooshka
Deshpande
Emma Tang
Gargi Gole
Angelina Ho
Reiyah Jacobs
Shivank Kancharla
Joshua Kreuzer
Zenon Kuropas
Jinghan Li
Kameryn Lloyd
Lana Maizel
Mrunal Munshi
Hana Nakhle
Fiona Yeung
Neha Panda
Ryan Parsaee
Matthew Rodzen
Ruhi Saldanha
Julia Sallean
Sanjita Srinath
Skye Scoggins
Tanvi Sharma
Risha Solanki
Natalie Travis
Preston Szczesniak
Paige Twohill
Mytri Vunnam
Ellen Han
Gerald Ofosu
Sai Satvik Kolla
Sanjana Farmah
Anthony Yang
Olivia Gatto
Ambika Puri
Sara Boburka
Marley Boyer
Tanisha
Choudhury
Reem Fayyad
Prisha Gautam
Srinithi Gali
Gargi Gole
Lillian Guo
Cindy Lam
Clara Lord
Spoorthi Marada
Britney Munguia
Jacqueline
Nguyen
Neha Panda
Matthew Rodzen
Heidi Segars
Estella Monica
Abigail Wells
Sneha Adayapalam
Yasmine Ackall
Razmin Bari
Ana Barton
Nicholas Boyer
Sophia Blatchley
Megan Brantly
Amelia Bruns
Alin CamposMartinez
Dianne
Celemen
Jonah Ettore
Corinne
Drabenstott
Natalie Druffner
Grace Coolidge
Denise Coutinho
Juan Castillo
Julia Sallean
Cindy Lam
Raife Levy
Angela Liu
Alisa Luo
Navya
Maheshwari
Alacia McClary
Mckenzie Miller
Hana Nakhle
Kalasri
Narasimhan
Ryan Parsaee
Sajni Patel
Andy Pita
Shay Rooney
Khadeejah
Saleem
Gabriela
Santiago
Paige Strecker
Vina Senthil
Shivank
Kancharla
Gayatri
Venkatesan
Karen Zhu

Olivia
Predators or Prey: Inclusive Education in the Status Quo
Mrunal Munshi
From Recess to Responsibilities: A Deeper Look at Stress in Adolescents
Gerald
Health on Hold: Why College Degrees Don’t Always Equal Wellness
Jacob
Nicole
Ava
Anooshka
Beyond the Scale: Diet’s Role in Early Aging and Bone Density Loss
Mannah Patel
Menopause, Mood, and New Solutions
Skye
T(erminator): The MASTER Plan to Fight
Cracking the Code of Vascular Smooth Muscle Cells: From DNA to Protein
Anthony Yang
Spin it to Win it: Redefining Medical Imaging with Hyperpolarized Nuclear Spins
Fiona
Jack
Intuitive Paths: How Body Appreciation Supports Recovery Journeys
How We Fill in the Blanks
Gargi Gole
on the Brain: Studying Sex Differences in Alcohol Effects
Hana
How Do They Grow? Secrets of a Familiar Brain Cell
Matthew Rodzen
Qualitative Psychology: The Door to Discovery
Natalie Travis
Hybridization: How Genes “Hop” Between Species
Erin Atos
Too Hot to Handle: Global Warming’s Effects on Predator-Prey Interactions
Brantley Aycock
On the Destruction of Species
Zenon Kuropas
74 A Toast to Your Health: The Role of Kombucha in Metabolism
Julia Sallean
What an Estuarine Ecologist does at the UNC Institute of Marine Sciences on the North Carolina Coast
Preston Szczesniak
Chemistry and Biochemistry
Hopping to New Heights: Proton Transport in Designed Proteins
Shivank Kancharla
Electrode Materials and the Future of Sustainable Energy Storage
Joshua Kreuzer
82 Targeting lipid metabolism for novel therapeutics
Emma Tang
Point-of-Care AB Tests: A Dose of Confidence
Angelina Ho
Overcoming and going back into viral fear: using HIV for therapies
Jinghan Li
From Petri Dish to Production Curves: The Interdisciplinary Approach to Developing Gene Therapies
Ruhi Saldanha
To Maintain a Mouse: Research Pertaining to UNC’s Mutant Mouse Resource
Mytri Vunnam
Racing Binary Star Systems: Dwarf Stars, Supernovae, and Stellar Evolution
Ellen Han
Waving to the Mathematical Universe
Reiyah Jacobs
Exploring the Shape Dynamics of Nickel-64
Andy Pita

In a world where social media dominates the lives of millions, it’s easy to jump to conclusions about its effects, particularly on young people. But Dr. Kaitlyn Burnell, a research assistant professor at the University of North Carolina’s Department of Psychology and Neuroscience, argues that we may not understand it as well as we think. Backed by a PhD from the University of Texas at Dallas and postdoctoral work at Duke University, Dr. Burnell’s research delves into how digital technologies, especially social media, impact the mental health and development of adolescents. Despite widespread assumptions, she emphasizes a surprising lack of solid experimental research— particularly for those under 18—that leaves many unanswered questions about how these platforms shape wellbeing and body image.
By Olivia Gatto
“With body-neutral content, you are trying to take away that importance that’s being placed on appearance.”
those same types of people and get the support that those groups can provide in-person.” This is particularly valuable for individuals who may feel isolated or marginalized in their offline environments; social media can allow them to find communities that share similar experiences and offer support. Beyond social connectivity, social media can also serve as a tool for mood management, helping users unwind after stressful days, although more research is needed in this area. While social media’s effects are diverse, its potential to foster meaningful connections and provide emotional support is a key positive outcome for many users.
When it comes to social media, much of its impact depends on how individuals engage in social comparisons. Two key forms of comparison—upward and downward—can influence users in contrasting ways. Upward social comparisons occur when individuals compare themselves to those they perceive as better off—often leading to feelings of envy, jealousy, or inadequacy—which can negatively impact mental health. However, as Dr. Kaitlyn Burnell notes, “it’s not always negative. You could be inspired or experience admiration for people that you perceive to be better off than you, and that could enact positive behavioral change.” On the other hand, downward comparisons occur when individuals compare themselves to those they believe are worse off. Though this can boost self-esteem, it can also evoke more negative feelings of sympathy or pity. This nuanced dynamic underscores how social media’s influence is highly individual, with both risks and rewards depending on how users process these comparisons. While social media often gets criticized for its downsides, it also provides significant positive benefits, especially when it comes to social connection. One of the most well-documented advantages is its ability to help people stay connected when in-person interaction isn’t possible. As Dr. Kaitlyn Burnell highlights, “Being able to connect with certain groups that align with your identity can be helpful if you’re not able to access
One of the most controversial topics surrounding social media is whether parents should restrict or ban its use entirely for their children. Dr. Kaitlyn Burnell argues that such blanket restrictions might not be the most effective approach, emphasizing that “it depends on the kid” and that an abstinence-based strategy lacks sufficient research support. Burnell instead suggests an active mediation approach, where parents encourage open dialogue about online content and gradually adjust their restrictions as their children grow. This provides a balance between monitoring

Dr. Kaitlyn Burnell
and respecting a child’s need for autonomy, and offers a more tailored and thoughtful approach than simply banning social media outright.
Body image remains a significant issue for young girls, particularly as they navigate social media platforms that frequently showcase unrealistic beauty standards. On platforms like Instagram, users are often bombarded with highly filtered, idolized content that places a strong emphasis on physical appearance. Dr. Kaitlyn Burnell explains that even body positivity content—while promoting self-acceptance— still focuses on appearance, which can perpetuate the idea that looks are inherently important. She encourages bodyneutral content, expanding that “with body-neutral content, you are trying to take away that importance that’s being placed on appearance,” which may help reduce the negative effects arising from constant exposure to appearance-oriented content. By shifting the focus away from looks entirely, body neutrality may offer a healthier approach to managing body image on social media.
To navigate the complex effects of social media, individuals can benefit from actionable steps that prioritize selfreflection and self-compassion. Dr. Kaitlyn Burnell emphasizes that it’s crucial for people to understand how their own social media use affects their mental health. Take a step back and ask, “What is it I’m looking at and how is it making me feel?” This practice of mindfulness helps users assess whether certain posts evokes positive, negative, or neutral emotions and adjust their habits accordingly. In some cases, preventing exposure to harmful content in the first place can be a powerful tool. As Dr. Burnell notes, “trying to prevent exposure in the first place, but then also having cognitive strategies to be able to prepare yourself” in case of exposure can reduce negative impacts. For some, social media may serve as a harmless way to unwind,


while others might be following content that induces harmful comparisons or worsens body image concerns.
Practicing self-compassion, by acknowledging these emotions and learning to be kinder to ourselves, can help build resilience in the face of social media’s pressures. Ultimately, social media’s impact is deeply personal, and its influence varies based on how we engage with it. By becoming more mindful of the content we consume and how it makes us feel, we can regain control over our online experiences. In a world where scrolling through feeds is second nature, pausing to reflect on our mental and emotional responses allows us to use social media intentionally—transforming it from a potential source of harm into a tool for growth, connection, and self-awareness.
References
1. Interview with Dr. Kaitlyn Burnell
2. Burnell, K.; Fox, K. A.; Maheux, A. J.; Prinstein, M. J. Social Media Use and Mental Health: A Review of the Experimental Literature and Implications for Clinicians. Current Treatment Options in Psychiatry 2024. https://doi. org/10.1007/s40501-024-00311-2.
By Mrunal Munshi

Educational systems are increasingly called upon to embrace inclusivity as a foundational principle in transforming pedagogical methodologies and evaluative curricula. Conventional standardized assessments, particularly those utilized in high-stakes environments, often inadequately address the rich backstory of student experiences and cognitive profiles, leading to significant disparities in academic achievement and engagement. To cultivate equitable learning environments, especially within STEM disciplines where diversity remains critically lacking, it is crucial to reconceptualize definitions and metrics of academic success. In light of such discussions, Dr. Viji Sathy, a Professor of the Practice in the Department of Psychology and Neuroscience and the Director of the Townsend Program for Education Research, explores instilling inclusivity in multifaceted avenues to uplift education as an equalizer for underrepresented students.
Before diving into the future directions of the ever-changing manifestation that “inclusivity” takes in education, it is important to first understand the status quo’s use of psychometrics as a justification of fairness within evaluative frameworks.
While Dr. Sathy concedes that the current use of the ACT and SAT is justified to an extent, the most “vexing problem” test designers have to mindfully is the ability
By fostering a culture of inclusivity, we not only create pathways for marginalized voices to be heard and valued, but also dismantle systemic barriers, promote social cohesion, and pave the way for a more equitable society.
for a certain metric to be coachable, leading to the abuse rather than the use of such indicators of success. As a former researcher for the College Board, Dr. Sathy co-authored “A Historical View of Subgroup Performance Differences on the SAT Reasoning Test” which allowed the researchers to give credence to existing quantitative measures while also suggesting future research to conduct “an examination of the individual- and school-level factors contributing to academic performance discrepancies among measures is warranted” (Kobrin et al. 20). The observation is indicative
of a larger paradigm shift towards a holistic evaluation of student success. Dr. Sathy notes that college admission committees undergo rigorous selection processes as they attempt to address “inequities in how students navigate the summer” as it is a large aspect of academic achievement that is fundamentally shaped by students’ socioeconomic status.
Alternatively, pedagogy emphasizes the importance of equitable education by promoting inclusive and tailored curricula. Standardizing inclusivity allows for the equitable uplifting of those underrepresented in entire fields of study. Drawing upon the identity theory, it is important to first understand that “individuals interpret the responses of others to those interactions as approval or disapproval, and change or control their identities,” making the foundational development of such an identity crucial to students’ academic success (Atkins et al. 2). Research further asserts that “socioemotional and culturally relevant mentoring were strongly correlated with the development of research skills and independence, both key elements of scientific identity” (Atkins et al. 3). As such, the systemic inclusion of mentorbased programs in STEM departments
inherently embraces an inclusive approach to education. Communitybuilding in academic settings is traditionally held as a key pillar of success amongst top-ranked universities; the implementation of such programs would further aid in such efforts at a more grassroots level as each individual student is allowed to have interpersonal support systems established in a more accessible manner.
More specifically, inclusive practices become increasingly instrumental in shaping an academic identity amongst diverse competitive fields such as STEM. Specifically, Dr. Sathy notes that currently, STEM curricula largely follow a “sit and get” model rather than embracing a “scaffolded learning” approach which orients around active learning components. Such seemingly small differences accumulate over large, underrepresented student bodies, further exacerbating inequities in education. For example, Dr. Sathy and Dr. Kelly Hogan, the Associate Dean of Instructional Innovation at the University of North Carolina at Chapel Hill, write that many classes may have implicit “hidden curriculum” which professors increasingly mistake as a defining aspect of a “rigorous” course, allowing only certain students with a high level of “academic literacy” who have had exposure to a greater amount of academic resources and skill-building curricula which stresses problem-solving, critical thinking, and research writing (Hogan and Sathy). Essentially the term “rigor” suggests that “‘some students deserve to be here, and some don’t’” which may prompt students who come from underfunded educational programs to internalize such embedded biases, thus systemically instilling barriers in higher education and disadvantaging them during high-stakes stages of their academic careers. More recently, Dr. Sathy explored the inclusion of course-based undergraduate research experiences (CUREs) to account for such lack of equitable access to traditionally exclusive academic experiences. Sweeping systemic reforms that explicitly level the playing field not only reduces unnecessary competition but also allows for a generally researched-enriched graduating class.
The inclusivity crisis is simultaneous to underfunded programs, specifically in North Carolina. Underinvestment of
educational resources amongst K-12 educational programs is arguably the source of inequities that become further exacerbated in higher educational contexts. As such, conversations about inclusivity need to be disseminated to elementary educational settings through the conscious designing of a studentcentered curriculum. Conversations across various educational contexts effectively address the socioeconomic gaps that converge with educational gaps in the status quo. Broader implications of an inclusivity moment may have greater ripple effects on societal and economic hierarchies founded upon such strata. By fostering a culture of inclusivity, we not only create pathways for marginalized voices to be heard and valued, but also dismantle systemic barriers, promote social cohesion, and pave the way for a more equitable society.

1. Atkins, Kaitlyn et al. “‘Looking at Myself in the Future’: How Mentoring Shapes Scientific Identity for STEM Students from Underrepresented Groups.” International Journal of STEM Education, vol. 7, no. 42, 2020. https://doi. org/10.1186/s40594-020-00242-3.
2. Hogan, Kelly and Viji Sathy. “How an Inclusive Teaching Approach Helped Us Build a More Inclusive Curriculum for Our University.” The Association of College and University Educators, 27 Mar. 2017, acue.org/blog/inclusive-curriculum/.
3. Kobrin, Jennifer L et al. A Historical View of Subgroup Performance Differences on the SAT Reasoning Test™. College Board, 2007.

By Gerald Ofosu
Stress is a widespread experience that affects many individuals. For some, the effects of stress are silenced and unnoticeable. Others are heavily impacted daily, hoping their stress can be mitigated. Understanding stress is possible through extensive research and insights from personal experiences. Fortunately, professors nationwide, such as Dr. Aysenil Belger, are dedicated to achieving this understanding. While Dr. Belger boasts many accolades, she is most known as a Psychology Professor in the Psychiatry Department at the University of North Carolina at Chapel Hill. Dr. Belger also directs the Neurocognition and Imaging lab at UNC-Chapel Hill and is director of the Clinical Translational Core of the Carolina Institute for Neurodevelopment Disorders. Originally from Turkey, she moved to the United States in 1986, where she received her PhD from the University of Illinois. After graduating, she spent seven years at Yale as a faculty member before relocating to the Triangle to collaborate with Duke and Chapel Hill on her multimodal brain imaging projects. Her research utilizes advanced brain magnetic resonance imaging and electroencephalography techniques alongside autonomic

physiological sensors to explore biological mechanisms underlying neurodevelopmental disabilities, such as autism and Turner Syndrome. Additionally, she investigates the risk of psychopathology in adolescents, including schizophrenia, mood disorders, and substance use. Recently she has been focusing on stress as a precipitator of mental illness. Through her research, her current goal is to elaborate on the body’s overall response to stress, proclaiming, “When experiencing stress, numerous physiological response systems are activated simultaneously, so now we’re studying all the coordination across these responses with a particular attention to how the brain responds to stress, too” (Figure 1).1
Stress is the body’s response to various stimuli, such as a challenging class or sleeping through your alarm before a big event. The latter example describes a short-term stress response known as acute stress. In this phenomenon, the first prompt will be the brain realizing it has missed an alarm. Then, within the brain, a pathway of responses will be triggered. Starting in the prefrontal cortex, the amygdala will be activated,

Figure 2. Response vs. time graph representing the curveshaped reaction and recovery cycle that occurs during acute stress.
inducing a chain reaction. The amygdala will then activate the hypothalamus, a crucial communicator of stress response. Once the hypothalamus is activated, it signals the adrenal glands,

Figure 3. Graphical user interface of the Montreal Imaging Stress Task (MIST). From top to bottom, the figure shows the performance indicators (top arrow = average performance, bottom arrow = individual subject’s performance), the stress-inducing arithmetic task, the progress bar reflecting the time limit given, the text field for feedback, and the rotary dial for the response submission.
which play a significant role in the stress pathway. These glands release the hormones adrenaline and cortisol, which elicit rapid breathing, sweating, muscle tension, and a surge in energy. This acute stress response, otherwise known as fight-or-flight, is ideal for instantaneous stressors. The ideal nature is due to the “curve” phenomenon in which your body reacts quickly to stress and recovers. One of Dr. Belger’s most significant interests is to gain a deeper understanding of this “curve” (Figure 2).
Multiple studies were conducted to unravel the effects of stress on the mind and the brain. An early stress study by Dr. Belger. involved inducing stress in 98 children between the ages of 9 and 16. Once the children were put under stress, Dr. Belger and her research team had to implement methods to observe brain activity and the associations between regions of the brain activated during stress. The best way to view this activity was with an fMRI (functional magnetic resonance imaging) and stress-inducing scenarios. To induce stress, the participants of the study were instructed to complete the Montreal Imaging Stress Task, which was implemented to induce psychosocial stress associated with public evaluation of performance during using challenging puzzles and social situations. Once the tasks were completed, the results were evaluated (Figure 3).
The results revealed a strong coupling in the activation of Bilateral Amygdala and Hippocampus, reflecting activation in emotional and adaptive regions of the brain during stress. In contrast, activation between the insula and the Dorsal Anterior Cingulate regions, important for executive decision making and attentional salience , were reduced during stress, indicating that stress interferes with coordination of brain function associated with critical thinking, attention and decision making. Ultimately, the study highlighted specific parts of the brain that work together during stress and emphasized the significance of maintaining overall brain health for proper stress response.2
Dr. Belger’s latest study builds on previous research on acute stress by creating a multisystem stress response study. She examines adolescents aged 9 to 16 and monitors how relationships within the brain and linkages between peripheral physiological and central neural systems interact during stress to contribute to Anhedonia. She explains that, “Anhedonia, which is your inability to experience pleasure… is a key
component of depression and schizophrenia, and a contributor to suicidality.”1 The study evaluates two main body systems that are highly dependent on neural systems, such as the prefrontal cortex and limbic system. These body systems are a part of the autonomic nervous system (ANS), which controls involuntary body functions during stress, and the hypothalamic-pituitaryadrenal axis (HPA), which regulates stress hormones such as cortisol by directing signals from your brain to your glands. After the systems had been identified, the researchers developed a model that observed symptoms based on how the relationship between the HPA and ANS interacted with “profiles.” The profile associated with anhedonia exhibited high HPA activity and low ANS activity, which causes anxiety and depression. The profile associated with a healthy brain exhibited high parasympathetic nervous system activation within the ANS and moderate HPA activity (Figure 4). By interpreting these interactions, researchers can create treatments and preventative strategies to decrease the risk of anhedonia as children take on the stressors of adolescence.3
Through Dr. Belger’s groundbreaking research, it is possible to comprehend the complex manner of how stress impacts brain function and behavior and contributes to the onset of psychopathology in adolescents. Her research sheds light on the processes involved in acute stress, from the interactions occurring in the brain to the interconnecting neural and physiological responses to stress. The multifaceted relationship between stress, brain function, and mental health highlights the need for proactive strategies to support those currently affected by stress and foster resilience for future generations.

Figure 4. Conceptual Model of Multisystem Stress Response Biotypes: acute stress activates neural and physiological systems critical for the onset and regulation of adaptive stress responses.
1. Interview with Dr. Aysenil Belger 9/23/24
2. Pelletier-Baldelli, A., Corr, R., Campbell, A., Glier, S., Bizzell, J., & Belger, A. (2020). The impact of acute stress on dynamic neural circuits in adolescence: Relationships to psychosis-related symptomatology and development. Biological Psychiatry, 87(9), 800-810. https://doi.org/10.1016/j. biopsych.2020.01.023
3. Roubinov, D. S., & Belger, A. (2024). Multisystem stress response biotypes: A precision psychiatry approach to identifying and treating multidimensional risk factors for anhedonia in adolescence. Neuropsychopharmacology. https://doi. org/10.1038/s41386-024-01953-9


In Soviet Russia, abortion was not just a medical procedure, but a societal necessity. From the 1950s until the collapse of the Soviet Union, abortion was the primary means of fertility control for millions of women. This reality was not due to personal choice, but because the Soviet government failed to provide access to reliable contraceptives, as well as the authoritarian economy barring alternatives outside of government supply lines. Dr. Michele Rivkin-Fish, a medical anthropologist and expert on Russian reproductive health, has spent years studying how the Soviet healthcare system’s policies led to a culture where abortion was routine. Her research highlights how this system emerged, its impact on women’s health, and its lingering influence on modern Russian society, which has changed dramatically. Abortion became legal in the Soviet Union in 1955, and from that point, it became the main form of birth control.1 The legalization was a response to the high maternal mortality rates caused by unsafe and illegal abortions. Before 1955, women were resorting to dangerous methods to terminate pregnancies, often resulting in severe complications or death.2 Dr. Rivkin-Fish explains that the
By Ryan Parsaee
Soviet government legalized abortion primarily as a public health measure, not as a progressive statement on women’s reproductive rights. The procedures were undertaken through the dilation and curettage method, which involved scraping the uterine lining until the termination of pregnancy was complete.
“The state wanted women to have more children, but they didn’t provide the means for them to do so safely and responsibly.”
While hormonal contraceptives like the birth control pill were becoming widely used in the United States and Europe during the 1960s, Soviet health authorities refused to promote them, citing concerns about their safety.3 As a result, women had few alternatives. Condoms and intrauterine devices (IUDs) were scarce and of poor quality. According to Dr. Rivkin-Fish, “Contraceptives were considered a luxury by the Soviet planners. The production of goods like toiletries, body soap, and contraceptives was not prioritized, leaving many women with no effective way to prevent pregnancies.” This scarcity left women with little choice but to rely on abortion to control the number of children they had.
The Soviet healthcare system’s utilitarian approach to abortion gave little consideration to women’s comfort or moral implications. The procedure was widely available and covered by the health system in state-run clinics, which often operated under harsh conditions.1,3 Abortion clinics resembled assembly lines, where doctors would perform multiple procedures in quick succession. The procedure itself was done with minimal privacy or emotional support. As Dr. Rivkin-Fish recounts from her research, “Women typically underwent the procedure several times in their lives, with an average of three to four abortions per woman. Some experienced as many as ten to twelve abortions over their lifetimes.” Such repeated procedures could lead to long-term health complications,
especially when performed under less-than-ideal conditions. Abortion, while legally available, was not a celebrated choice.2 Soviet women did not have the same reproductive autonomy as women in other parts of the world. In fact, the Soviet government encouraged women to have more children to rebuild the population after the staggering number of losses of World War II.1,2 However, this pronatalist policy which encourages a higher birth rate, clashed with the harsh realities of Soviet life. Widespread poverty, lack of adequate housing, and insufficient healthcare resources meant that women were often forced to make difficult decisions about their reproductive health. “The state wanted women to have more children, but they didn’t provide the means for them to do so safely and responsibly,” Dr. Rivkin-Fish explains.1
Reliance on abortion as birth control persisted in the 1980s as the Soviet Union experienced economic decline.2 Even though abortion was legalized decades earlier, Soviet officials and healthcare planners still did little to improve access to modern contraceptives. By warning women that abortions were bad for their health, they focused on reducing abortion rates without addressing the underlying issue of contraception scarcity.1
After the collapse of the Soviet Union in 1991, Russian society began to transform in many ways, but abortion continued to play a central role in reproductive healthcare.1 Pharmaceutical companies introduced modern contraceptives like birth control pills and improved intrauterine device IUDs. A new non-governmental organization, the Russian Association of Family Planning, began teaching physicians how to prescribe them and counsel women about their use. Young people began using contraceptives eagerly, but cultural habits formed over decades made it difficult for many to shift away from abortion as their primary method of fertility control.
Additionally, the economic instability of the 1990s meant that healthcare access remained inconsistent, and many women could not afford new contraceptive options. Rivkin-Fish’s new book, Unmaking Russia’s Abortion Culture, explores the struggle for liberalizing social change in
reproductive rights that preceded the Federation’s delve into war and repression. Contraceptive use became very popular and routine. Abortion became stigmatized as a ‘barbaric’ form of birth control associated with the Soviet deprivation of proactive medical care.
Dr. Rivkin-Fish also notes that a growing conservative movement began to influence Russian reproductive politics after the fall of the Soviet Union. With support from Western anti-abortion groups, some factions in Russia pushed to reduce abortion rates through restrictions rather than expanding contraceptive access. In the current regime, clinics in several regions have began requiring women to obtain permission from their husbands before receiving an abortion; some doctors are even given monetary rewards for convincing women to continue their pregnancy rather than an abortion.
Today, abortion remains legal in Russia, but access to the procedure has become increasingly difficult in certain areas due to local restrictions and growing conservative pressure.
Dr. Rivkin-Fish’s reveals that Russia’s healthcare system is highly focused on promoting higher childbearing rates. Family planning services are no longer supported and women are treated as mothers-to-be, regardless of their plans and desires. The complicated history of abortion in Russia is a powerful example of how political, economic, and cultural factors shape reproductive rights. As Russian leaders continue to be preoccupied with increasing the birth rate, women’s reproductive rights continue to deteriorate as family planning services decay throughout the nation.

Figure 2. Soviet propaganda for pregnancies / maternal health. Courtesy of Wikimedia.
1. Interview with Michele Rivkin-Fish, Ph.D. 09/26/24
2. Karpov, V.; Kääriäinen, K. Sociol. Pract. 2005, 7, 13-33.
3. Temkina, A.; Rivkin-Fish, M. Soc. Theory Health 2020, 18, 340-357.
By Jacob Allred

Public health and sociological research has long viewed education as a social determinant of health that contributes to healthier and longer lifespans in the United States.2 With this said, new research questions this common belief, revealing that higher educational attainment does not always lead to better health outcomes.2 Dr. Lauren Gaydosh, Associate Professor of Sociology and Faculty Fellow at the Carolina Population Center, and her colleague, Dr. Kathleen Mullan Harris, have conducted pivotal research into the relationship between higher education and health outcomes. Dr. Gaydosh and Dr. Harris highlight that Black and Hispanic college graduates, specifically those who attended elite institutions, do not always benefit from improved health outcomes often associated with higher education. Unlike prior research on ethnoracial disparities and educational attainment, which focused heavily on self-reported health measures, Dr. Gaydosh’s study considers more objective measures of health using college graduate biomarker data. Specifically, it describes the role of varying college contexts in shaping the biomarker-measured physical health of college graduates of different demographic groups.2 Dr. Gaydosh’s

work uncovers the nuances behind this issue, raising important questions about how educational environments shape health outcomes and connect to racial and ethnic disparities.
Dr. Gaydosh’s study explores the critical issue surrounding why college graduates of certain demographic groups experience fewer health benefits from their educational attainment, and evaluates whether this varies between institutions. Dr. Gaydosh explains, “We were trying
to understand what it is about those types of environments that constrain the health benefits of educational attainment, particularly for Black and Hispanic adults, compared to White adults.”1 Using data from the National Longitudinal Study of Adolescent Health (Add Health), her research delves into the physical health disparities between White, Black, and Hispanic adults in the 24 to 32 age range. By using biological markers like blood pressure, cholesterol levels, triglycerides, and glycosylated hemoglobin, she and her colleagues measure the underlying health risks that often remain invisible at this age.2 The selected biomarkers represent functions across inflammatory, cardiovascular, and metabolic health that are associated with the stress response system.2 In this study, high-risk thresholds were defined for each biomarker according to the National Cholesterol Education Program Expert Panel guidelines, and a cardiometabolic risk score between 0 and 8 was created by summing the number of risk indicators across the markers.2 Among college graduates, levels of cardiometabolic risk were high at 2.52 despite their average age of 28, especially for Black and Hispanic college graduates compared to White graduates.2 White college graduates had
the lowest cardiometabolic risk at 2.42, Hispanic graduates had 2.76, and Black graduates had the highest at 3.16.2 As seen in Figure 2, a concerning average difference of .74 between White and Black graduates risk is shown, illustrating how one’s health may be connected to minority status within the college graduate demographic.
“...there are things involved with status attainment that themselves can be stressful, even if the status itself is ultimately a good thing.”
Additionally, it turns out that not all institutions provide the same health advantages or disadvantages. According to Dr. Gaydosh, attending elite colleges can be particularly detrimental to the health of minority students or graduates.1 The study found that for Black adults, attending elite colleges led to higher cardiometabolic risk scores.1 For Black college graduates, the associations for average and advantaged institution types were not significantly different relative to White graduates, however, degree completion from an elite institution is significantly associated with increased cardiometabolic risk relative to degree completion from a lower status institution.2 Furthermore, even after the study controlled for institution type and they allowed the effect of institution type to vary by ethnoracial group, Black college graduates experience significantly higher cardiometabolic risk.2 The research indicates that if Black graduates attended elite institutions at the same rate as White graduates, the gap in cardiometabolic risk would actually increase by nearly 4.5%.2 This finding suggests that prestigious institutions can increase stress for these students, leading to poorer health outcomes that emerge later in life. While educational attainment may lead to better socioeconomic status, the process of attaining the degree and the stressful social environments these students navigate lead to health deterioration. Dr. Gaydosh explains that “there are things involved with status attainment that themselves can be stressful, even if the status itself is ultimately a good thing.”1 Notably, the link between postsecondary institution types and adult cardiometabolic risk among minority college graduates is not explained by specific traits from the institutions, and the associations remain strong even when comparing students from the same high schools.2
The ability to translate educational attainment into tangible health benefits is not uniform. In general, more selective institutions with greater resources and more advantaged students are protective of health, yet this pattern masks underlying disparities when comparing ethnoracial groups.2 Differences across racial and ethnic groups due to factors such as racial discrimination, microaggressions, and a lack of social support for minority groups at certain elite institutions lead to increased stress that causes health disparities. Consequently, Black graduates of wealthy institutions are predicted to have similar cardiometabolic risk to their peers without a college degree.2 As Dr. Gaydosh and her colleagues continue to follow up on this research, the
main goal is to better understand how the college experiences of minority individuals will impact the long-term benefits of a degree. The research findings from this study highlight how upward mobility for ethnoracial minority individuals is complicated by the complex navigation of different school and work environments.

Figure 2. Cardiometabolic Risk Scores by College Completion for White, Black, and Hispanic Adults. Provided by Dr. Lauren Gaydosh and Dr. Kathleen Mullan Harris.
Dr. Gaydosh’s findings provide a foundation for understanding how educational attainment does not automatically translate into better health outcomes for everyone. The recent study raises significant questions about how college environments, racial inequalities, and stress combine to influence long-term health outcomes for U.S. college students and graduates. Looking to the future, this study contributes to our understanding of how health disparities can accumulate across the lifespan and provides a basis for addressing inequality, improving population health, and diminishing the role of institutions in maintaining disparities.
1. Interview with Dr. Lauren Gaydosh, Ph.D. 09/23/24.
2. Gaydosh, Lauren and Kathleen Mullan Harris. “Institutional Context Shapes the Physical Health of College Graduates Differently for US White, Black, and Hispanic Adults”. Demography. 61(3): 933-966. PMID: 38809598

By Nicole Branch
No country on Earth is untouched by human immunodeficiency virus (HIV). HIV destroys the body’s white blood cells, weakening a person’s ability to fight infection (Figure 1). This virus is currently transmitted in every country around the globe, having claimed roughly 42.3 million lives to date. Nearly 40 million people were living with HIV in 2023, 65% of this population residing in Africa. No cure for HIV exists–once a person is infected, they have HIV for life.1 However, it is treatable and preventable through medications.2
Of particular interest to Dr. Bonnie Shook-Sa is a set of prevention tools known as Pre-Exposure Prophylaxis (PrEP).
Dr. Shook-Sa holds a Bachelor’s in Mathematics and Political Science from Marshall University, a Master’s in Applied Statistics from Ohio State University, and a DrPH in Biostatistics from the University of North Carolina at Chapel Hill. Dr. ShookSa is currently an Associate Professor in UNC’s Department of Biostatistics and the Assistant Director of the UNC Center for AIDS Research. In collaboration with the study’s principal investigator Dr. Nora Rosenberg (UNC) and a team of graduate and postdoctoral students, Dr. Shook-Sa recently conducted an analysis using a machine learning model known as Lasso regression as a tool for identifying which women in various African countries were most at risk of HIV-1 infection. The team developed this novel risk-assessment tool to guide focused PrEP decision-making.3,4
In spite of wide-scale treatment availability, “HIV is still taking far too many lives,” according to Dr. Shook-Sa.5 Thus, it

is imperative to deliver PrEP to individuals at highest risk for HIV infection. To guide these efforts, Dr. ShookSa and her team set out to identify areas where PrEP distribution should be prioritized to produce the greatest possible impact.5 Her research team aggregated data from population-based HIV impact assessment surveys (PHIAs) conducted in 13 African countries and an additional two PHIA-like surveys. Dr. Shook-Sa’s team hoped to use the individual, partnership, and epidemiologic characteristics collected by these surveys to determine a relationship between these variables and recent HIV infections among African women. They restricted their data to women between 15 and 49 years old, as these women are of reproductive age with the potential to transmit HIV to infants during pregnancy (Figure 3). These data were pulled from random, probability-based surveys, meaning the participants were not selected from a biased pool of volunteers or solely from high-risk areas. The researchers could thus apply their eventual findings to all susceptible Dr. Shook-Sa

Figure 3. A map visualizing the share of the population (among people at least 15 years old) living with HIV that are women in 2021. Courtesy of Our World in Data (data compiled by World Bank).
women in the 15 countries.
Once the data were aggregated, Dr. Shook-Sa’s team fit a least absolute shrinkage and selection operator (Lasso) machine learning model to their data to predict HIV infection. Lasso uses a statistical method called regularization to determine the strongest set of predictive variables while avoiding overfitting (fitting the model so closely to the original data that it cannot predict new data).6 Given 28 possible predictor variables, the Lasso model narrowed to two characteristics for predicting recent HIV infections: 1) whether the woman lived in an area with high population viremia–the proportion of people infected with HIV but not on treatment–and 2) whether the woman reported a sexual partner living outside of her home. Unlike previous methods of determining HIV risk, which often involved a long list of invasive questions, the Lasso model only selected these two variables to identify the most at-risk women.
This Lasso regression tool returned the predicted probability of a particular woman becoming infected based on the two main risk factors. To emulate practical implementation of this risk assessment tool, the team developed risk thresholds to evaluate who was most in need of PrEP: anyone whose predicted probability was above this cutoff point was classified as high risk and anyone below it was low risk. This analysis revealed a tradeoff between reach and efficiency–as the predicted probability cut-off decreases, more women were considered in need of PrEP, so more infections would be averted, but women who are unlikely to contract HIV may be categorized as high-risk and receive PrEP unnecessarily. Alternatively, higher cut-offs recommend fewer women for PrEP, reducing costs but missing some women at risk of infection. Further demonstrating the efficiency trade-offs of this model, the number needed to treat (NNT)–the total number of women that would need to be treated to prevent one infection–varied significantly across the possible risk thresholds. With perfect prediction, NNT equals one, as atrisk women are perfectly identified; in this case, the NNT ranged from 39 to 96, meaning PrEP would need to be administered to
39 to 96 women to prevent one infection.
More data may be helpful in improving prediction and reducing the NNT. While the data set included over 200,000 women, the data was still sparse: according to Dr. Shook-Sa, “HIV is a huge problem, but having a recent HIV infection is still relatively rare. In terms of all the women in the population, a small percentage at any given time are getting infected.” Ultimately, given how representative the survey data was, this risk assessment tool is more generalizable and accurate than previous work done on this subject, making it a favorable model for developing PrEP distribution plans.4,5
Dr. Shook-Sa and her team intend to utilize a second ongoing set of PHIAs to conduct an external validation process for their published model, testing if their tool produces similar predictions with a different data set. Dr. Shook-Sa is eager to continue collaborating with Dr. Rosenberg, with the pair poised to publish a companion paper that uses similar methods to their original project to analyze risk factors for males.5 Due to the highly generalizable nature of their research, Dr. Shook-Sa and her collaborators expect that their findings will be used to guide decision-making related to PrEP. They anticipate policy makers employing this research to focus on particularly vulnerable geographic areas and groups. Using this innovative new tool, Dr. Shook-Sa is optimistic about a future of targeted PrEP access and a reduction in preventable HIV infections.
References
1. World Health Organization. 2024 Jul 22. HIV and AIDS. World Health Organization. https://www.who.int/newsroom/fact-sheets/detail/hiv-aids.
2. U.S. Department of Health & Human Services. 2023 Jan 13. What are HIV and AIDS? HIVgov. https://www.hiv.gov/ hiv-basics/overview/about-hiv-and-aids/what-are-hiv-andaids.
3. Bonnie Shook-Sa, DrPH - UNC Gillings School of Global Public Health. 2024 Jul 31. UNC Gillings School of Global Public Health. [accessed 2024 Oct 10]. https://sph.unc.edu/ adv_profile/bonnie-shook-sa-drph/.
4. Rosenberg N., Shook-Sa B., et al. 2024 Apr 24. A Human Immunodeficiency Virus Type 1 Risk Assessment Tool for Women Aged 15–49 Years in African Countries: A Pooled Analysis Across 15 Nationally Representative Surveys. Clinical Infectious Diseases. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae211/7657721.
5. Interview with Dr. Bonnie Shook-Sa (9/16/24)
6. IBM. 2024 Jan 18. What is lasso regression? | IBM. wwwibmcom. https://www.ibm.com/topics/lasso-regression.

By Ava Carlton
Approximately one out of every three women will be diagnosed with breast cancer each year, resulting in over three hundred thousand diagnoses.2 Researchers have been aiming to improve therapies and increase patient survival, and Dr. Michael Emanuele is no exception. Dr. Emanuele focuses primarily on breast cancer proliferation and the effects of treatment by examining the basic biology of cancer cells. He received his doctorate in Biochemistry and Molecular Genetics at the University of Virginia and later completed his postdoctoral work at Harvard College, acquiring a vast amount of knowledge on this subject. Now as an associate professor at the University of North Carolina at Chapel Hill, Dr. Emanuele works to understand the abnormalities of cancer cells and how they result in rapid growth.

Dr. Michael Emanuele
Cancer cells can avoid the body’s natural defense system and continue to proliferate. This behavior represents a malfunction in the cell cycle, the process cells use to replicate and divide, “cancer cells either have their brakes removed, or their gas pedal is constantly being pressed.”1 In 2015, the Food and Drug Admission approved the use of several drugs aimed at cancer treatments known as cyclin-dependent kinase
4 and 6 (CDK4/6) inhibitors. The drugs palbociclib, ribociclib, and abemaciclib have specifically been used to treat hormonereceptor-positive breast cancers. Their approval transformed breast cancer treatments. In hormone-positive-receptor breast cancers, enzymes become overactive and result in uncontrolled cell proliferation. CDK4/6 inhibitors disrupt these enzymes, slowing down the cell cycle. This is done through the ability of CDK4/6 inhibitors to slow down and even stop the transition between the G1 phase (when the cell prepares to divide), and the S phase (when DNA replication and repair occur).
A key part of his work involves studying the changes that occur within cancer cells after treatment—ranging from alterations in gene expression to modifications in the proteins and enzymes that drive cell proliferation. “We want to understand in comprehensive detail how these drugs reshape the way the cell looks at a molecular level”1. By mapping these changes, Dr. Emanuele aims to create a “molecular atlas” of how cancer cells behave when exposed to drugs. To identify which molecular pathways are disrupted by drugs like CDK4/6 inhibitors, it is vital to use such data to help notice this action.
While these drugs have proven revolutionary, some breast cancer cells are able to survive and proliferate despite treatment. This is known as drug resistance. According to Dr. Emanuele, resistance often emerges in response to targeted therapies, like CDK4/6 inhibitors. Currently, his lab is focused on identifying what makes certain breast cancer cells resistant to CDK4/6 inhibitors. “Still, we cannot predict who’s going to respond and who’s not going to respond. That really sort of shines a light on the fact that we don’t understand this quite

Figure 3. This image describes how CDK4/6 inhibitors affect the cell cycle.
as well as we think we do,” said Dr. Emanuele.1 He hopes to exploit vulnerabilities in resistant cancer cells and design new treatments that can target these weaknesses, “By becoming resistant here, you’ve now exposed some new vulnerability that we can now treat with a different drug or therapeutic.”1 Ultimately, Dr. Emanuele and his team aim to understand why these CDK 4/6 inhibitors are not working for all patients. Then, for the patients who do not see improvement outcome, they attempt to discover new therapies.
“We now know that when cells resist treatment, they expose new weaknesses, which we can target with different therapies.”
One of the most perplexing issues in breast cancer treatment is the ability of cancer cells to lie dormant for extended periods, only to reemerge years after treatment. As Dr. Emanuele explains, some cancer cells are essentially “out of the cell cycle,” meaning they aren’t actively proliferating, which makes them resistant to many therapies designed to target dividing cells. “There’s a lot of open questions here. What triggers these cells to reemerge after something like 10 years? We don’t know what the mechanism is that causes them to reenter the cell cycle and begin proliferating again.”1 Dr. Emanuele and his team aim to identify how breast cancer cells trigger their reentry into the cell cycle, and ultimately find ways to prevent them from reactivating. By targeting the mechanisms that drive this process, the hope is to develop more effective therapies that can eliminate these dormant cells before they become a threat again.
While Dr. Emanuele’s research is ongoing, the early results are promising. His team has begun to detect molecular changes in resistant cancer cells. They plan to investigate how these changes reveal new vulnerabilities, and their results have the potential to transform the advancement of cancer therapy.
“We now know that when cells resist treatment, they expose new weaknesses, which we can target with different therapies.”1 Knowing this may help doctors stay a step ahead of drug resistance. Overall, his study hopes to discover techniques for not just treating but preventing breast cancer from recurring. By understanding how cancer cells avoid natural processes and how these drugs enhance treatments, Dr. Emanuele’s research is advancing the larger endeavor to find a cure for breast cancer. Scientists across the globe are developing a foundation for the future of cancer treatments. All in all, breast cancer patients can be hopeful of a brighter future, and all women are looking towards a day when breast cancer is not a threat to their health.
References
1. Interview with Dr. Emanuele
2. Breast cancer statistics: How common is breast cancer?. Breast Cancer Statistics | How Common Is Breast Cancer? | American Cancer Society. (n.d.). https:// www.cancer.org/cancer/types/breast-cancer/about/howcommon-is-breast-cancer.h tml
By Britney Munguia Castillo
For the longest time, everyone thought nothing could live in the stomach due to its acidity. But 2005 Nobel Prize winner, Barry Marshall, proved them wrong by culturing the microbe Helicobacter pylori and eating it. Although he ended up giving himself gastritis, Marshall was able to show that H. pylori can colonize the stomach. Dr. Ian Carroll worked with this microbe while completing his dissertation in Ireland many years back. He found that it could inject molecules into the human epithelium, the tissue covering the internal and external surfaces of the body, and change the gut’s environment to allow it to thrive. Fascinated by the behavior of this bacterium, Dr. Carroll wanted to explore how microbes interact with their host and cause diseases, and eventually find targets for therapy. For the past 20 years, he has been searching for answers at the University of North Carolina at Chapel Hill’s Department of Nutrition.


Malnutrition is often thought of as not having enough to eat, but to Carroll, “malnutrition is bad nutrition… [and] can refer to individuals with obesity.” A diet high in fat and sugar, for example, will increase the risk of a “leaky gut.” The epithelium is held together by tight junction proteins, ones that get a little more permeable when interacting with high

Figure 1. The differences between a healthy and leaky gut, where tight junction proteins are loosen to let microorganisms into the bloodstream. Illustrated by Britney Munguia Castillo
amounts of fat and sugar. This permeability allows for harmful substances to pass through the intestinal wall; referring to the leaky part.6, 7 This enables more energy to be derived from digested food, leading to the weight gain seen in obese individuals. Dr. Carroll studied this system in bariatric surgery patients, noticing that, post-surgery, patients lost an immense amount of weight in a short period of time.2 Decreased food intake could not solely account for such numbers, concluding that gut microbiota played a significant role.
The gut digests and absorbs nutrients from ingested
food and excretes the waste. Over 100 trillion microbial cells make up the microbiota that help the gut carry out these functions.3 Imagine two friends both eat a 200-calorie KitKat bar. Person-A gained a pound, but their friend did not. Dr. Carroll and his team believe gut microbes interact with their host in a way that regulates the host’s ability to absorb calories. In this case, Person-A’s gut happens to absorb way more calories than their friend’s.
In a recent study, Dr. Carroll’s team tracked this calorie intake in bariatric surgery patients using…their poop. They would take a sample of their feces, dry it out, and combust it using a bomb calorimeter. A bomb calorimeter measures how much heat a substance releases when burned, which is expressed in calories. Returning to the KitKat example; knowing the bar contained 200 calories, if Person A’s feces were measured to have 20 calories, it could be estimated that their gut harvested 180 calories from the KitKat, hypothetically accounting for the pound they gained. The study found that before bariatric surgery, obese patients had a much higher capacity to harvest calories than after surgery.2 The diminished harvest allowed for more calories to flow out of the body, enabling patients to lose weight more easily. To tie the role of

Figure 2. The average weight of post-bariatric surgery shows a decrease over the course of 24 months.
gut microbiota to this weight loss, Grace, a graduate student at Dr. Carroll’s lab used a technique known as mediation analysis; a method that uses statistics to examine the relationships between independent and dependent variables. Grace looked at a handful of microbes and identified one particular microbe that was mediating the energy absorbance effect. They will have to prove that this specific microbe is responsible for the weight loss, which they hope to test in mice next.
Knowledge on microbes loosening junction proteins and causing weight gain can be applied to people dealing with anorexia nervosa. Anorexia nervosa patients restrict their food intake significantly. It has the second-highest mortality rate of any psychiatric disorder.5 During starvation, the gut becomes dysfunctional. The energy from food is no
longer being harvested—it goes straight through the body. Dr. Carroll seeks to find a way to fix a patient’s gut microbiota with leaky-gut-causing microbes. “Nobody has ever thought about applying it to anorexia nervosa,” he says. Dr. Carroll wants to do the physical treatment work in curing these patients. Although the gut has long-term curative properties, he believes that altering the microbiota can be much more efficient and less stressful for the patient. If the focus is not on restoring the energy-harvesting abilities of the gut, “you’re just wasting your time.” 1
Dr. Carroll credits Jeffery Gordon’s work, the “godfather of microbiota research”1, as the basis for his hypothesis. Gordon developed a microbiota supplement and took it to Bangladesh—a country where there is acute undernutrition.1 Gordon divided malnourished kids into two groups: one group received his microbiota recipe, and the other received standard, ready-to-eat therapeutic food that represented a calorically-dense diet.4 The children who took Gordon’s recipe consumed fewer calories but gained more weight than the control group. Dr. Carroll thinks Gordon’s recipe was restoring the gut ecosystem, enabling it to harvest energy again, and believes that this type of recipe-therapy may be applied at inpatient anorexia clinics.
To Dr. Carroll, “the gut is the center of the universe.”1 He wishes to have a meaningful impact on the scientific community by influencing clinical treatments. Though more research must be done, understanding the gut and its microbiota will enhance global health, lives, and communities.

1. Interview with Ian Carroll, PhD. 10/3/2024
2. Carroll, I.; Qian, Y.; Sorgen, A.; Steffen, K.; Heinberg, L.; Reed, K.; Malazarte, A.; Fodor, A. Res. Sq. 2024, 1. 3. Guinane, C. M.; & Cotter, P. D. 2013, 4, 295-308.
4. Saving lives with RUTF (ready-to-use therapeutic food) https://www.unicef.org/supply/stories/savinglives-rutf-ready-use-therapeutic-food#:~:text=RUTF%20 is%20the%20abbreviation%20for,or%20food%20of%20 adequate%20quality (accessed October 12th, 2024).
5. Enchi Dai: The Deadliest Mental Disorder - Anorexia Nervosa. https://balancedtx.com/blog/the-deadliestmental-disorder-anorexia-nervosa/#:~:text=Anorexia%20 Nervosa%20is%20the%20second,illness%2C%20only%20 behind%20opioid%20overdose. (accessed October 12th, 2024).
6. Shil, A., Olusanya, O., Ghufoor, Z., Forson, B., Marks, J., & Chichger, H. MDPI, 2024, 12, 1862.
7. Mishra, S. P., Wang, B., Jain, S., Ding, J., Rejeski, J., Furdui, C. M., Kitzman, D. W., Taraphder, S., Brechot, C., Kumar, A., et.al. 2023, 72, 1848-1865.

By Anooshka Deshpande


The gut microbiome hosts trillions of bacteria. Gut microbiota play a vital role in drug and nutrient metabolism, immunity, and maintaining the gut mucosal barrier. However, an imbalance between beneficial and harmful gut microbiota can lead to many gut conditions, seriously affecting other body regions as the gut interacts with the immune, endocrine, and nervous systems. Dr. Azcarate-Peril, a microbiologist and director of the Microbiome Core at the UNC School of Medicine, investigates how the gut microbiome’s composition can be regulated by prebiotics and probiotics. Her overarching goal is to devise prebiotic and probiotic interventions to prevent microbiota-health-related conditions.1
Prebiotics and probotics have a beneficial influence on the gut microbiome. Prebiotics, nondigestible fibers, serve as a food source for beneficial gut bacteria. They can be found in many plant-based sources such as bananas, whole grains, and beans. An oligosaccharide is a common prebiotic that contains chains of sugars linked by covalent bonds called glycosidic bonds. Gut bacteria break down these glycosidic bonds and ferment the oligosaccharide, releasing beneficial metabolites. Probiotics are beneficial bacteria and can be found in yogurts and fermented foods. Examples include bifidobacterium and Lactobacillus. Prebiotics facilitate the growth of favorable bacteria, and probiotics directly supply the gut with bacteria.1
Recently, scientists recognized that some probiotics, prebiotics and synbiotics benefit mental health and cognition through the gut-brain axis. They were termed “psychobiotics”. Many individuals suffer from mild cognitive impairment with advancing age. In mild cognitive impairment, an individual struggles with essential functions such as judgment and remembering tasks. Dr. Azcarate-Peril and her team assessed the impact of a beneficial gut bacterium called Lactobacillus rhamnosus (LGG) on cognition in a randomized, doubleblind clinical trial. LGG was used because it is one of the beststudied probiotics, is produced by reputable companies, and

is commercially available. The trial consisted of 169 adults who were 52 to 75 years old. The participants were split into two groups: the placebo and probiotic groups. The probiotic

Figure 2. The gut-brain axis. The gut brain axis is a group of nerves that connects the gut to the brain. Gut microbiota can impact cognition and mental health. Image courtesy of NIH.
group received LGG in the form of two capsules. The placebo group received microcrystalline cellulose, a substance that the gut microbiota cannot ferment, instead of LGG. An NIH questionnaire was used to assess the participants’ cognition. It consisted of questions that tested their attention and memory. Then, they received a cognitive performance score, determining whether they had intact or impaired cognition.2
Results indicated that the probiotic group, which received LGG, had better cognition than the placebo group. The gut microbiome composition of the participants was also analyzed after the trial. Several taxa that were found to be associated with mild cognitive impairment were Prevotella ruminicola, Bacteroides thetaiotaomicron, and Bacteroides xylanisolvens. These results are significant because they can be used to predict cognitive impairment and prevent it by changing diet and increasing probiotic intake.2 Dr. Azcarate-Peril found these results surprising; she did not expect to see an effect in the probiotic group due to individual variation in gut microbiome composition and due to the fact that humans have been feeding their gut microbiome with probiotics for millennia.1
Several limitations of this study include the small cohort size, the participants recruited, and the NIH questionnaire used. Most participants in clinical trials tend to be health conscious and have a healthy diet. As a result, the results of this trial may not apply to people with other types of diets. Additionally, the NIH questionnaire assigned the participants a score based on their cognitive performance, which could fluctuate depending on the reliability of the questionnaire.
In the future, Dr. Azcarate-Peril envisions herself developing prebiotics and probiotics for patients with gut microbiota-related conditions. She states that “personalized
nutrition, personalized medicine, and lifestyle choices are the future” and are necessary to maintain a healthy gut. She believes prevention is better than treatment and hopes to curb cognitive decline by adjusting one’s prebiotic and probiotic intake. Wouldn’t it be incredible if diet and lifestyle alone could slow down the memory loss accompanying age?
References:
1. Interview with Dr. Azcarate-Peril, Ph.D., 10/2/2024.
2. Aljumaah, M. R., Bhatia, U., Roach, J., Gunstad, J., & Azcarate Peril, M. A. (2022). The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clinical nutrition (Edinburgh, Scotland), (2022). 41(11), 2565–2576. https:// doi.org/10.1016/j.clnu.2022.09.012
By Oliver Ewy

According to the CDC, cancer is the second leading cause of death in the United States, only following heart disease.1 Many may know that cancer can be an incredibly difficult disease to treat and control, but they may not know that this difficulty comes from the fact that cancer cells are considered immortal. To researchers, this means that cancer cells can self-repair and replicate endlessly. In order to control the spread of cancer, one must control the rate of its replication.
Dr. Michael Jarstfer, an associate professor and the Assistant Dean for Graduate Education at the University of North Carolina at Chapel Hill’s Eshelman School of Pharmacy, works to resolve this exact problem. Dr. Jarstfer received his bachelor’s degree in biochemistry from Trinity University and his Ph.D. in chemistry from the University of Utah.2 During his time at Trinity, Dr. Jarstfer was introduced to the lab work and research that would lead to his current interests in cancer research. The tipping point would come when Dr. Jarstfer worked as a postdoctoral researcher at the University of Colorado Boulder studying telomerase.
Telomerase is the protein that rebuilds telomeres, the caps of DNA on the end of chromosomes that protect the DNA that codes for all of an organism’s genes.3 Cells need telomeres because each time a cell replicates, a small bit

of DNA does not get copied into the new cell, and when the DNA becomes too degraded, the cell will die (Figure 2). In normal adult cells, telomerase is inactive, but cancers require a telomere maintenance mechanism. As such, in many types of cancer, telomerase becomes highly active and allows cells to keep growing indefinitely. Cancers that grow because of reactivated telomerase are well-studied; however, a second less-understood telomere maintenance mechanism called the alternative lengthening of telomeres (ALT) pathway is just as important in learning how to treat different cancers. In the ALT pathway, telomeres are elongated without the use of telomerase instead using the processes normally associated with DNA repair, forcing researchers to rethink how they can study ALT-positive cancers.4
Cancerous cells have the same biological tool kits as the normal cells that they developed from.2 Though this allows scientists to study cancer cells in the same way they would study any other cell, it creates a unique challenge in developing treatments for the disease. Since cancer cells are almost exactly the same as the rest of our cells, cancer treatments have the potential to affect normal cells as well. According to Dr. Jarstfer, this problem is what makes the ALT pathway a promising line of study. Since ALT appears to be unique to cancerous cells, if he and his team can determine what allows the pathway to work in cancer cells, then he can identify targets and potential treatments to prevent continued telomere lengthening.
In its most recent study, the Jarstfer Lab, in collaboration with Dr. Samantha Pattenden’s lab, used a technique called high-throughput screening to identify potential drug targets.2 Dr. Jarstfer describes highthroughput screening as a method to perform hundreds to thousands of
individual tests all at once. For their study, the research team, led by then PhD student Merrill Froney, adjusted a previously used low-throughput method to be functional at a greater scale. In each well of the assay (Figure 3), a different reaction can occur that can later be tested for the presence of a certain molecule. This allows the team to test their samples efficiently and gather thousands of data points quickly. From the data that they collect, the team can see which drugs got a response from the samples and deserve further studying. Though the speed of highthroughput screening is enchanting to a researcher, it doesn’t come without its challenges. According to Dr. Jarstfer, one of the biggest challenges with his lab’s research is optimizing their experiments to get reliable data each time.2 He explains that it was initially difficult to gather reproducible data by following basic protocols, so they had to be extremely specific in each step of their process. From the initial steps of growing the sample cells, to the type and amount of chemicals they used, and to the length of time they replicated the cells’ DNA, each small step had to be optimized. If even one step was imprecise and threw off their data, all of the experiments would essentially be useless.
In their study, the lab looked at C-circles as a potential indicator for changes in ALT pathway function in response to various drugs.3 C-circles are small, circular pieces of DNA that are produced as byproducts of telomere extension and are uniquely associated with the ALT pathway.3,4 (Figure 4) Due to this association, C-circles were a prime option to study telomere function in ALT-positive cancers.2,3 In previous studies, an increase in the number of C-circles has been associated with ALT disruption, in which the number of C-circles tended to increase while other indicators decreased in number. As such,


pathway to work in cancer cells, then he can identify targets and potential treatments to prevent continued telomere lengthening.
In its most recent study, the Jarstfer Lab, in collaboration with Dr. Samantha Pattenden’s lab, used a technique called high-throughput screening to identify potential drug targets.2 Dr. Jarstfer describes highthroughput screening as a method to perform hundreds to thousands of individual tests all at once. For their study, the research team, led by then PhD student Merrill Froney, adjusted a previously used low-throughput method to be functional at a greater scale. In each well of the assay (Figure 3), a different reaction can occur that can later be tested for the presence of a certain molecule. This allows the team to test their samples efficiently and gather thousands of data points quickly. From the data that they collect, the team can see which drugs got a response from the samples and deserve further studying.
Though the speed of highthroughput screening is enchanting to a researcher, it doesn’t come without its challenges. According to Dr. Jarstfer, one of the biggest challenges with his lab’s

research is optimizing their experiments to get reliable data each time.2 He explains that it was initially difficult to gather reproducible data by following basic protocols, so they had to be extremely specific in each step of their process. From the initial steps of growing the sample cells, to the type and amount of chemicals they used, and to the length of time they replicated the cells’ DNA, each small step had to be optimized. If even one step was imprecise and threw off their data, all of the experiments would essentially be useless.
In their study, the lab looked at C-circles as a potential indicator for changes in ALT pathway function in response to various drugs.3 C-circles are small, circular pieces of DNA that are produced as byproducts of telomere extension and are uniquely associated with the ALT pathway.3,4 (Figure 4) Due to this association, C-circles were a prime option to study telomere function in ALTpositive cancers.2,3 In previous studies, an increase in the number of C-circles has been associated with ALT disruption, in which the number of C-circles tended to increase while other indicators decreased in number. As such, the researchers aimed to identify which drugs led to a change in C-circle levels.
Through their study, the Jarstfer lab was able to identify several compounds that changed the output of C-circles in treated cells.3 The researchers tested various drugs against two different types of cancers and recorded how the cells differed in C-circle output from cells that were not treated with the drugs. The Jarstfer lab found that some compounds did not change C-circle output at all, some affected one type of cell but not another, and some affected both cell types. To the research team, this data marked an incredible success.
Both the lab’s success in identifying compounds that affect C-circle output and their unique high-throughput screening method are beneficial to research on ALT pathway cancers.2 Since the team was
able to find compounds that alter C-circle output, they now have targets that can be further researched as treatments for ALT-positive cancers. The lab also used a screening method on a scale that had not been done previously. The fact that it was functional and accurate allows them to test different cell types rapidly, which is important given the information that some drugs may only affect certain cell types.
Continuing this vein of research is necessary for the understanding of the biology of ALT-positive cancers and what allows them to continue growing without telomerase.2 If researchers can understand the pathway, then they can figure out how to prevent the cancer from growing. Jarstfer hopes that the research his lab does and the techniques that they develop can contribute to the discovery of a target protein and a drug that can be used to treat ALT-positive cancers. Though research on this scale is still in its early stages, teams like the Jarstfer lab continue to make significant progress in advancing the field of cancer research with each study.
1. CDC: National Center for Health Statistics. “Leading Causes of Death”. 2024. 2. Interview with Michael Jarstfer, Ph.D. 9/03/2024
3. Froney, M.M.; Cook, C.R.; Cadiz, A.M.; Flinter, K.A.; Ledeboer, S.T.; Chan, B.; Burris, L.E.; Hardy, B.P.; Pearce, K.H.; Wardell, A.C.; et al. “A First-in-Class High-Throughput Screen to Discover Modulators of the Alternative Lengthening of Telomeres (ALT) Pathway”. ACS Pharmacol. Transl. Sci. 2024, 7, 2799–2819. https://pubs.acs. org/doi/10.1021/acsptsci.4c00251#.
4. Chen YY, Dagg R, Zhang Y, Lee JHY, Lu R, Martin La Rotta N, Sampl S, Korkut-Demirbaş M, Holzmann K, Lau LMS, Reddel RR, Henson JD. “The C-Circle Biomarker Is Secreted by Alternative-Lengthening-of-Telomeres Positive Cancer Cells inside Exosomes and Provides a Blood-Based Diagnostic for ALT Activity”. Cancers. 2021, 13, 5369. doi: 10.3390/cancers13215369.
By Sanjana Farmah

Image courtesy of the Edwards Lab at UNC-Chapel Hill
The heart is one of the first and most important organs to develop.1 Take a moment and put your hand on your heart. Do you feel it beat? The heart may be small, but it beats about 100,000 times a day!2 It’s truly magnificent, isn’t it? What if I told you that it started off as a small, linear tube? Unfortunately, in one percent of new babies, this small, linear tube does not properly turn into a functional heart.1 This condition is known as Congenital Heart Disease (CHD) and is one of the leading causes of infant mortality in the world. While the causes of CHD are not yet fully understood, depending on the structures it impacts in the heart, its severity can vary.3 CHD is a major health concern, yet the underlying cause in most cases is unknown.
Dr. Whitney Edwards, an Assistant Professor in Cell Biology and Physiology is the Principal Investigator of the Edwards Lab. In her lab, Dr. Edwards aims to understand the processes involved in embryonic heart development to see what exactly causes CHD to occur.

The heart first starts as a linear beating tube. The heart must then undergo key developmental events to form the major structures of the heart including the atria, ventricles, valves, and vessels. The heart is also made up of many cell types, including cardiomyocytes. All of these cell types must mature at the right time to ensure the intricate process of heart development occurs properly to create a fully functional heart. According to Dr Edwards, “[it is] kind of a miracle that all of those things happen in the majority of people”.1
Dr. Edwards found her love for developmental biology in a rather non-traditional way. She started her undergraduate career deciding between being a theater or a business major. She took a wide range of classes in her first year, including an introductory chemistry class. In that class, she fell in love with science, which prompted her to become a biochemistry major and participate in scientific research. After receiving her bachelor’s degree, she worked in the biomedical industry for a year. However, she wanted to conduct more exploratory research, so she decided to pursue her Ph.D. at the University of Illinois at Urbana-Champaign. Her research primarily focused on pituitary gland development and pituitary disorders. Dr. Edwards received her doctorate in Molecular and Integrative Physiology. She then moved to the University of North Carolina at Chapel Hill (UNC), where she was awarded the NIGMSfunded Seeding Postdoctoral Innovation in Research and Education (SPIRE) Fellowship
to conduct postdoctoral research. As a SPIRE fellow, Dr. Edwards worked in Dr. Frank Conlon’s lab. It was during this time that Dr. Edwards found her passion for the heart. When asked about the switch in organs of focus (from the pituitary gland to the heart), she says, “the complexity of what has to happen during heart development is what drives my interest in this”. In addition to the research she did as a SPIRE fellow, she was also able to also pursue her love for teaching. Dr. Edwards served as a visiting professor at North Carolina Agricultural and Technical State University, where she taught anatomy and physiology and developed a new course that focused on cardiac development and disease. The Edwards Lab started in 2023 and recently celebrated its oneyear anniversary.1
The Edwards Lab seeks to understand heart development from a rather unique perspective. They investigate how protein dynamics, protein interaction networks, and posttranslational modifications influence heart development and function. More recently, the lab began to investigate the role of protein-lipidation in heart


development. Protein-lipidation is the covalent attachment of specific lipid (fats) to a protein. While there are many forms of protein-lipidation, the Edwards Lab focuses on three types: prenylation, myristoylation, and palmitoylation. Each type is dependent on the lipid that modifies the protein. Numerous types of proteins that are critical in the cell require these modifications for their activity and localization. The Edwards Lab aims to identify which specific cardiac proteins are lipidated and how that can impact cardiomyocyte development and function in developing hearts.1
Using mouse models, quantitative mass spectrometry, primary subculture/ isolation of cells, and other tissue-based approaches, the Edwards Lab is working to uncover the role of protein lipidation in the heart. Currently, the lab has created several genetic mouse models with impaired protein lipidation in developing hearts. The mouse is an ideal model organism since the stages of the heart development are almost identical to that of an human. Additionally, while in humans, development occurs during embryonic weeks 4 – 8, the mouse heart develops between embryonic days 8.5 and 16.5. That can prove to be a challenge sometimes though since their small hearts also provide limited

amounts of tissue that can be analyzed in lab.1
While the research is still in the early stages, their findings suggest protein-lipidation is critical for cardiac morphogenesis. Cardiac morphogenesis is the process by which the small, beating linear tube becomes the mature heart after going through key developmental and maturation stages.4 For example, when protein prenylation was disrupted in the heart, the lab observed that the heart functioned poorly through three tell-tale signs. First the mice appeared to be very pale suggesting the heart did not have proper circulation. Secondly, they observed pericardial edema.1 The outermost layer of the heart is the pericardium, and it is surrounded by a sac. If that sac fills with fluid from nearby blood vessels, it can swell and be fatal if not taken care of quickly.5 Lastly, they observed that the heart had either altered or impaired beating. Poor heart function can lead to an either an altered or abnormal heartbeat. Additionally, if the damage is very severe, can be fatal. These signs suggest to the lab that not only do the mice hearts have impaired circulation and poor heart function, but that protein prenylation is vital for heart development. Therefore, if this form of protein-lipidation is altered, it may cause CHD.1
The Edwards Lab currently collaborates with Dr. Mark Distefano’s lab at the University of Minnesota. The Distefano Lab are experts in proteinlipidation. They aim to understand how these protein modifications can impact various types of cells and various diseases like cancer and Alzheimer’s. 6 They are a more chemistry-focused lab and have helped to develop the innovative chemical probes that the Edwards Lab uses to identify lipidated proteins in the developing heart.
Dr. Edwards is proud of how far she and her team have come in a short amount of time. As she puts it, “what we do is pretty special because it takes a lot”. With a clearer understanding of the heart, many developments can be made in treating one of the leading causes of infant mortality in the world! Additionally, this research may one day aid in developing new regenerative strategies for patients impacted by Congenital Heart Disease.1
1.Interview with Whitney Edwards, Ph.D. 10/08/24
2.Watson, S., Chang, L.. Five Amazing Facts About Heart Health and Heart Disease. WebMD.
3. Mayo Clinic Staff. (2024). Congenital Heart Disease. Mayo Clinic. 4. Jung, D., Bachmann, H., (2023). Protein Prenylation. Science Direct.
5. Wang, B., Dai, T., Sun, W. et al. (2021). Protein N-myristoylation: functions and mechanisms in control of innate immunity. Nature.Com.
6. Meoli, L., Günzel, D. (2020). Palitylation. Palmitoylation.
7. Mayo Clinic Staff. (2024). Edema. Mayo Clinic.
8. Cohen Lab. (2024). Cohen Lab. University of North Carolina at Chapel Hill. 9. Distefano Research Group (2024). Distefano Research Group. University of Minnesota.
By Sai Satvik Kolla

In the evolving landscape of health research, Dr. Erik Hanson, at the University of North Carolina at Chapel Hill (UNC), is at the forefront of investigating a pressing issue: the impact of sedentary (inactive) behavior on cardiometabolic disease (CMD) risk among young adults. Examples of CMD include heart attack, stroke, diabetes, and non-alcoholic fatty liver disease. Alarmingly, young adults today are more at risk for CMD development than ever before. Through the CONTEXTSB (Cardiometabolic Outcome Negation Through Earlyadulthood Context Specific Sedentary Behavior reduction) study, Dr. Hanson contributes to a critical area of public health, reshaping how we understand and address sedentary lifestyles in this vulnerable demographic.¹
Dr. Hanson’s journey to becoming a leading researcher in exercise physiology and sedentary behavior reflects a path of discovery and dedication. Initially a chemistry major with aspirations of attending medical school, Dr. Hanson discovered a more robust aptitude for biology. Dr. Hanson reflected, “I realized that my true interest lay in understanding biological processes rather than chemical reactions”.¹ This revelation led him to pursue a Master’s in Exercise Physiology at UNC, where he developed a passion for research and coaching.¹ A key turning point in Dr. Hanson’s career came in 2004 when he met Dr. Claudio Battaglini. Dr. Battaglini introduced Dr. Hanson to Exercise Oncology, a field that would significantly influence his future research¹. “Dr. Battaglini’s guidance was pivotal,” Dr. Hanson recalls.¹ “He helped me see the potential of exercise in improving cancer recovery and overall health”.¹ Following Dr. Hanson’s Masters, he pursued a PhD at the University of Maryland, where he furthered his expertise in exercise physiology.¹ After completing his PhD , he sought advanced postdoctoral training in Melbourne, Australia, renowned for its research in muscle physiology.¹ This experience solidified
his focus on the intersection of exercise and disease, guiding him back to UNC, where he could leverage its robust research environment to continue his work.¹
The CONTEXT-SB study represents Dr. Hanson’s groundbreaking effort to understand the impact of different sedentary behaviors on CMD risk among college-aged young adults (CB YA).² This research is crucial, given the rising prevalence of CMD among young adults, a trend exacerbated by sedentary lifestyles.² The study is a long-term observational study designed to recruit 500 individuals aged 18-24 years.² Participants will undergo two laboratory visits, spaced 12 months apart, where researchers will conduct comprehensive assessments.² These assessments include measuring arterial stiffness (healthier arteries are less stiff and vice versa), metabolic and inflammatory biomarkers (cholesterol levels and white blood cell counts), and body composition.² Additionally, participants will complete questionnaires on lifestyle behaviors and various levels of the socioecological model (SEM), which is used to determine the interactions between personal and environmental factors in a participant’s


life.² “The study employs advanced technologies, such as the Actigraph device to measure total sedentary time and the SleepScore Max device to assess sleep quality and movement patterns².” These tools will help capture a detailed picture of participants’ daily behaviors.²
The CONTEXT-SB study focuses on context-specific sedentary behaviors (CS-SB), such as television viewing, transportation, academic activities, and leisure computer use.² Dr. Hanson says, “Understanding which specific contexts are most harmful is key to developing targeted interventions”.¹ This approach is grounded in the observation that while total sedentary behavior is associated with CMD risk, certain contexts, such as prolonged television viewing- may have a more pronounced impact.² Research has shown that television viewing is particularly concerning because it often coincides with unhealthy behaviors like overeating.² “We need to look beyond just the amount of time spent sitting,” Dr. Hanson explains.¹ “It’s about understanding what people are doing while they’re sedentary and how those activities might influence their overall health”.¹
environment all shape sedentary behavior.¹ The study will explore how these factors interact and influence sedentary habits to inform more effective intervention strategies.²
“Socioecological factors can provide valuable insights into why certain behaviors persist.”
The CONTEXT-SB study is expected to yield several key outcomes.² It aims to determine whether certain types of CS-SB are more strongly associated with CMD risk than total sedentary behavior, assess whether the relationship between CS-SB and CMD risk is mediated by other lifestyle factors such as diet and physical activity, and identify socioecological predictors of CS-SB to help design targeted interventions.2 Dr. Hanson is optimistic about the potential impact: “By understanding the nuances of sedentary behavior and its various contexts, we can develop interventions that are not only effective but also tailored to the specific needs of young adults”.1
The study’s examination of socioecological predictors of CS-SB is another significant aspect.² The SEM examines how various socio-ecological predictors and factors at individual, interpersonal, and environmental levels influence sedentary behavior.² “Socioecological factors offer valuable insights into why certain behaviors persist,” Dr. Hanson notes.¹ For instance, personal attitudes, social norms, and the physical

1. A Breakdown of Behavior Patterns. Image courtesy of Dr. Aiden Chauntry.
The broader implications of Dr. Hanson’s research are significant.² As sedentary behavior continues to be a significant public health concern, particularly among young adults, studies like CONTEXT-SB provide critical insights that can drive effective interventions.² Dr. Hanson’s work contributes to a growing body of knowledge to improve health outcomes and prevent disease by addressing the complex relationship between sedentary behavior and CMD risk.2 His career trajectory and current research underscore a deep commitment to advancing our understanding of exercise science and sedentary behavior, serving as a testament to the importance of interdisciplinary research and its potential to address pressing health challenges.1,2 Through the CONTEXTSB study, Dr. Hanson and his team at UNC Chapel Hill are making significant strides toward a healthier future for this critical age group.2
References
1. Interview with Dr. Erik Hanson, 08/27/2024
2. Diana, J. C.; Chauntry, A. J.; Cowley, E.; Paterson, C.; Struder, J.; Pagan-Lasalle, P.; Meyer, M. L.; Lin, F.-C.; Moore, J. B.; Hanson, E. D.; Stoner, L. Protocol for a Study Investigating Context-Specific Sedentary Behaviors and Cardiometabolic Health in College-Based Young Adults (CONTEXT-SB). Preprints 2024, 26, 1-26. DOI: 10.21203/ rs.3.rs-4470004/v1.

By Kameryn Lloyd
Have you ever been so stressed you can barely function? Well, you and T-cells have something in common. Tumor-infiltrating lymphocytes (TILs) are a specific type of immune T cell whose job is to locate and kill cancer cells. However, these cells can become exhausted leading to less response to immunotherapies allowing for tumors to continue to grow. Dr Jessica Thaxton an associate professor in the Department of Cell Biology

Dr. Jessica Thaxton
and Physiology and her lab members are working together to target T-cell exhaustion, improving immunotherapy response and shrinking tumors.
All cells have a stress response which is a way to alert themselves that something is wrong or off-balance in the body. “It is just like when you become stressed.” Whether it be yoga or meditation. Every person has ways to cope with and relieve the stress in our lives and so do our immune cells. However in tumors, no matter how the immune cells try to respond and bring themselves back to their normal state, the stress can’t be relieved. This causes them to become exhausted and subsequently less effective at targeting and shrinking tumors. Similarly to a yoga instructor or a therapist, the goal of Dr. Thaxton’s lab is to bring these immune cells experiencing the highstress environment of tumors back to their normal homeostatic state.
Dr . Thaxton’s lab found that the main driver for the T-cell stress response was the Endoplasmic reticulum (ER). Her lab is separated into three groups all working towards targeting ER stress in different ways. One way is to target the molecules that alert the cell to the stress in the body. By tuning these molecules up and down they can limit
the amount of stress signaling the T-cells are receiving. However, these molecules can be difficult to target as they are important for other cells in the body. One of the methods they use to further this goal is RNA-seq. This technique allows the lab to examine all of the RNA sequences in a cell, which gives insight into the genetic components. The 2nd group has found that after the stress response starts there’s a drastic increase in metabolism in the T cell. Their goal is to find ways to get the cells more energy so that they can work harder and longer. One technique they are using to understand this is spectral flow cytometry. This is done by using metabolic dyes that become fluorescent tags for parts of the cell’s metabolism like glucose or fats. This allows them to compare the energy uptake in the TILs to other T-cells in the body. The third group has found that when the cells become stressed it causes the Endoplasmic Reticulum to change shape. Their goal is to target the ER structure in order to improve immune cell functioning. One method used to better understand ER structure is using imaging from a confocal microscope. This allows them to visualize the T-cells Endoplasmic Reticulum and better characterize the shape changes.
“My passion is to get the work we’re doing at the bench to directly translate to the bedside”. Dr. Thaxton’s end goal is to have her work become translational and help treat cancer patients. To accomplish this, her lab collaborates with clinicians. They work closely with multiple surgical oncologists to better compare if the stress responses seen in mouse models are also being seen in patients. Mouse models can also be improved by comparing the stress environment in the mice to the human sample. Working with patient samples will make it easier to bridge the gap between creating therapeutics that are equally as effective in mice and humans. Subsequently, the results from the collaboration have found that many of their discoveries found in mouse models do match patient tumors. However, there have been barriers that have made translation difficult. One of which is toxicity, it is challenging to find a unique drug target that does not cause harm to other cells and tissue. They have discovered one non-toxic target that has shown success. However, another barrier is finding pharmaceutical companies to source their therapeutics that are also prepared to enter clinical trials. These and many other obstacles continue even after discoveries in the lab are made.
“My passion is to get the work we’re doing at the bench to directly translate to the bedside.”
One of the things Dr Thaxton is excited about looking toward the future of her project is working with and continuing to mentor her lab members. She has seen how each member’s unique background and perspective have helped make the projects thrive. The lab is also working towards a future grant with her clinical partners that would allow them to have a project that solely focuses on using patient samples. Another project she is excited about is the non-toxic drug candidate discussed earlier and its possibilities in in-patient clinical testing. While research

can sound difficult Dr. Thaxton reminds undergrads to “always follow the science that you love” and not be intimidated as most research can be boiled down into simple questions. Dr. Thaxton’s questions on the relationship between T-cells and stress could transform cancer treatments and save patient’s lives.
1. Interview with Dr Jessica Thaxton 9/16/2024
2. Hunt EG, Hurst KE, Riesenberg BP, Kennedy AS, Gandy EJ, Andrews AM, Del Mar Alicea Pauneto C, Ball LE, Wallace ED, Gao P, et al. Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment. Cell Metabolism. 2024;36(5):969-983.e10. https:// doi.org/10.1016/j.cmet.2024.02.009. doi:10.1016/j.cmet.2024.02.009
3. Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8+ T cells. Cancer Immunology Research. 2019;7(3):476–486. https:// doi.org/10.1158/2326-6066.cir-18-0182. doi:10.1158/2326-6066.cir-18-0182
By Lana Maizel
In emergency medicine, every second counts—especially when addressing bodily trauma that requires prompt and effective wound healing. Hemorrhage, the medical term used to describe both internal and external bleeding, accounts for around 60,000 deaths in the United States every year and 1.5 million deaths worldwide. Coupled with the ongoing global blood shortage and the routine need for transfusions (the transfer of donated blood or blood components into a patient’s bloodstream) during surgeries, it is clear that current resources to address wound healing are insufficient.2 In short, the demand for blood exceeds the available supply, leaving patients without an effective method of preventing blood loss during critical moments.3
Enter Dr. Ashley Brown, Ph.D., a faculty scholar in the joint Department of Biomedical Engineering between North Carolina State University and the University of North Carolina at Chapel Hill. Dr. Brown’s research involves using platelets, a component found naturally in blood, to prevent further blood loss during these emergency situations. Platelets are small, microscopic fragments of cells that form the initial blood clot after someone is injured to stop the bleeding. They also play an essential role in wound healing after the initial clot is formed, and can be administered to patients via blood transfusions.
“If you have a situation where you need to get a blood transfusion, one of those blood components is going to be platelets. And so, what we’re trying to do is basically make synthetic platelets,” said Dr. Brown.4

Dr. Ashley Brown


tourniquets can be tight and constrictive, potentially leading to temporary or permanent tissue injury and even lasting nerve damage.5 More commonly, gauze and other physical barriers can also be placed at the site of the open wound to provide pressure and stop bleeding before a patient can receive a blood transfusion. Internal wounds, Dr. Brown says, are more complex to treat.4
“What we’re envisioning here is something that allows the patient to get from wherever their trauma situation is to the hospital, because there exists this very critical delay in many situations, like in rural locations.”
Synthetic platelets provide a promising new avenue for wound healing: they cause no tissue damage like tourniquets, are more effective than gauze, and are easier to transport than blood, which is in short supply.
“What we wanted to do was make a synthetic product that was easier to transport, could be stored for longer, wouldn’t have immune issues, and could be mass produced,” said Dr. Brown.4
Outside of blood transfusions, there are other preliminary techniques used to limit blood loss. Tourniquets, which are pieces of cloth or rubber tightly wrapped around a limb to compress blood flow, are an example of a technique used to control hemorrhaging before a patient can be admitted to a hospital. While effective,
Any type of wound, from a small papercut to a massive internal wound, follows the same four steps. The first step of wound healing is hemostasis — “hemo” meaning blood, and “stasis” meaning to stop. As the name implies, this step works to form blood clots that stop the bleeding, which begins happening seconds to minutes after the initial injury. After hemostasis and the formation of a blood clot to stop the bleeding, the next step is inflammation. During this phase, the wound swells as white blood cells combat potential infections. The third step, proliferation, involves blood cells

arriving at the site to build new tissue, with collagen serving as a scaffold to support this tissue. This stage marks the beginning of scar formation. Finally, in the remodeling phase, scar tissue matures as the collagen reorganizes and strengthens the scar. (Figure 2).
Dr. Brown’s research targets wound healing at its first stage: hemostasis. Platelets, which are found naturally in blood, immediately rush to the open wound, and, working in conjunction with other factors in the blood, cause the blood to coagulate (i.e., clot). More and more platelets form a barrier at the wound, and eventually other components of blood reinforce the wall that the platelets have created. (Figure 3).
Synthetic platelets, administered topically to external wounds or via injection to internal wounds, dramatically speed up this process. Because platelets are naturally found in the body, the risk of adverse side effects or rejection from additional platelets is minimal.
“I think one of the really coolest parts about the technology is that you can inject it and it can safely stop bleeding without causing adverse side effects,” said Dr. Brown.4
Current platelet solutions prepared by Dr. Brown can be stored for 3-5 days at room temperature. Given their long shelf life, Dr. Brown hopes that these solutions can become a vital addition to first aid kits.
“What we’re envisioning here is something that allows the patient to get from wherever their trauma situation is to the hospital, because there exists this very critical delay in many situations, like in rural locations,” said Dr. Brown.4
Nearly one-fifth of Americans live more than ten miles away from a hospital, and the time it takes for an ambulance to bring them to a hospital could be fatal.8 A 2014 study in Harris County, Texas, revealed that nearly half of all deaths due to hemorrhage were potentially preventable. Alarmingly, 35.8% of these fatalities occurred before patients reached a hospital, and 20.4% died within one hour of arrival at an acute care facility.
9 In such critical situations, having synthetic platelet solutions readily available for first responders could mean the difference between life and death for patients experiencing uncontrollable
bleeding.
With clinical trials within reach, Dr. Brown’s work could bridge the gap between injury and treatment, providing immediate aid in crisis situations where mere seconds could mean the difference between life and death.
1. Latif RK, Clifford SP, Baker JA, Lenhardt R, Haq MZ, Huang J, Farah I, Businger JR. Traumatic hemorrhage and chain of survival. Scandinavian Journal of Trauma Resuscitation and Emergency Medicine. 2023;31(1). https://doi.org/10.1186/ s13049-023-01088-8. doi:10.1186/s13049-023-01088-8.\
2. Tripathi, I., Raguveer, V., Raykar, N. “Blood Deserts” Face the Burden of Global Blood Deficits.
3. Manning FJ, Sparacino L. 1996. Blood supply fluctuations. Blood Donors and the Supply of Blood and Blood ProductsNCBI Bookshelf.
4. Interview with Ashley Brown, Ph.D. 9/27/24.
5. Masri BA, Eisen A, Duncan CP, McEwen JA. 2020. Tourniquet-induced nerve compression injuries are caused by high pressure levels and gradients – a review of the evidence to guide safe surgical, pre-hospital and blood flow restriction usage. BMC Biomedical Engineering. 2(1). doi:10.1186/s42490020-00041-5. https://doi.org/10.1186/s42490-020-00041-5.
6. Chester, D., Brown, A.C. The role of biophysical properties of provisional matrix proteins in wound repair, Matrix Biology, Volumes 60–61, 2017, Pages 124-140, ISSN 0945-053X, https:// doi.org/10.1016/j.matbio.2016.08.004.
7. “Lesson Explainer: The Mechanism of Blood Clotting Biology • Second Year of Secondary School.” Lesson Explainer: The Mechanism of Blood Clotting, Nagwa.
8. Geiger, A. How far Americans live from the closest hospital differs by community type. Pew Research Center. April 14, 2024. 9. Kalkwarf KJ, Drake SA, Yang Y, Thetford C, Myers L, Brock M, Wolf DA, Persse D, Wade CE, Holcomb JB. 2020. Bleeding to death in a big city: An analysis of all trauma deaths from hemorrhage in a metropolitan area during 1 year. Journal of Trauma and Acute Care Surgery. 89(4):716–722.
By Neha Panda

3. AAV-based vectors hold the potential for reliable, continuous effects.
Uveal melanoma (UVM) is a relatively rare but consistently deadly eye tumor most often affecting males over age sixty-five. Yet in Huntersville, NC, a recent rash of uveal melanoma appeared in over 20 women under the age of 30. This sudden, unusual incidence has garnered questions from families robbed of their loved ones about how incurable this disease truly is. The namesake of the Bower Lab, Dr. Jacquelyn Bower, reveals how her path as a scholar and researcher led to her interest in attempting to develop therapeutics to treat metastatic uveal melanoma.
capacity to pursue real solutions for real people, motivated by a refusal to allow “incurable” diseases to stay that way.1 In recent years, Dr. Bower’s work at the Carolina Eye Research Institute and the Lineberger Comprehensive Cancer Center surrounds the use of adenoassociated viruses (AAVs) as a means of targeting uveal melanoma’s weakest links.
Dr. Bower admires research’s capacity to pursue real solutions for real people, motivated by a refusal to allow “incurable” diseases to stay that way.
Since her childhood, Dr. Bower was drawn by the complexity, utility, and practicality of scientific research. Her fascination has only deepened through undergraduate research in molecular biology at the University of Notre Dame, graduate projects in cancer mechanisms at West Virginia University, and a post-doctorate in cell-signaling here at the University of North Carolina at Chapel Hill. She admires research’s
Though the most common ocular cancer in adults, uveal melanoma remains stubbornly unsolvable due to its tenacity. Cancers often originate from one-letter alterations in the correct sequence of a gene that affect regulations on cell division. This change drastically alters the gene’s activity; the DNA encourages the cell to proliferate at uncontrolled levels, forming a tumor. In uveal melanoma, the primary tumor in the eye can be treated with radiation therapies or complete removal of the diseased eye. However, around 50% of cases metastasize, typically spreading cancerous cells to the liver, where they can remain dormant for up to 15
years.2 This metastatic tumor is of acute concern: chemotherapy or surgery are rendered futile, and once the tumor begins to grow, the patient is left with less than a year to live. Each possible solution to metastatic UVM is shot down by a drawback. Cancerous tumors usually result from multiple instances of

Figure 1. siRNA molecules cunningly provoke a cancer-killing immune response. Figure courtesy of Wikimedia Commons.
mutations throughout multiple genes that disrupt various cell functions, including normal cell division. As a consequence, canonical approaches to gene therapy that target and replace the ‘broken’ gene would be ineffective. Additionally, the eye is immuneprivileged and tends to suppress its own immune response as a means of selfdefense; therefore, tumors originating from eye cells maintain resistance to immunotherapies at metastatic sites.3
Such was the intimidating battlefield that Dr. Bower and her team approached, armed with an unwavering mission and an innovative technique— the basis of the lab’s upcoming publication, “Allele-specific targeting of the GNAQQ209L mutant.” Dr. Bower learned that unlike most cancers, 80% to 90% of uveal melanoma cases can be traced to consistent mutations in genes called GNAQ or GNA11.4 Because the mutations are consistent, they are unusually targetable. Secondly, the tumor cells rely on that mutation to continue sending signals for the cell to grow, a dependence called classical oncogene addiction. Targeting gene products allows the team to “choke” the consistent flow of mutated signals that the tumor cells are dependent on to survive, effectively starving the tumor. Bower concluded, “Without that consistent oncogenic signaling, these cells will lose their ability to proliferate, and [they’ll] die.5” One final challenge remained in their design: the researchers needed to avoid off-target “choking” and preserve the signals keeping healthy cells alive.
The solution lies in a highly specific technique known as RNA interference. In a healthy, normal immune response, the body’s cells send short-interfering RNA (siRNA) to attack and degrade unwanted proteins and molecules. RNA interference therapy repurposes this natural process by introducing a specially designed siRNA that precisely targets products of the mutation in the GNAQ or GNA11 gene sequence causing UVM (Fig. 1).6 To administer the siRNA, an adeno-associated virus (AAV) will act as a vector, or a microscopic capsule, to store DNA until it is released within the cell.7 From there, it will utilize cellular machinery to continuously produce
the RNA molecule in the cell until the cell eventually dies.8 The siRNA therapy could be directly injected into the eye, but Bower also underscored IV infusions as uniquely capable of mitigating metastasis by reaching stray tumor cells circulating a patient’s bloodstream (Fig. 2).9 Thus far, the Bower Lab’s experiments and empirical evidence illustrate that delivery of these siRNAs to malignant cells in a dish successfully “choked out” or depleted the mutant forms of GNAQ in diseased cells while preserving healthy cells (Fig. 3).10
Though in its earliest stages, the Bower Lab’s therapy has checked most of the boxes and overcome the obstacles on the path to treating UVM. Post-publication, Dr. Bower and her lab hope to enter pre-clinical studies that prove the applicability of their siRNA gene therapy to tackle mutated cells in vivo, or in live animals. With luck, their efforts will someday produce a drug that can be administered over the course of a week yet offer patients hope of a life beyond metastatic uveal melanoma. When considering those taken too early by UVM, both in Huntersville and elsewhere, the significance of such a solution to families and patients is immeasurable, and the value of Dr. Bower’s research is quite clear.

1. Interview with Jacquelyn Bower, Ph.D. 09/17/24
2. Martine, J. J.; Shields, C. L.; Cebulla, C.M; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.; Carvajal, R.D.; Belfort, R.N.; 9, Jia, R.; Shields, J.A; et al. Uveal melanoma. Nat. Rev. Dis. Primers. 2020, 6(1). DOI: 10.1038/ s41572-020-0158-0.
3. Leonard-Murali, S., Bhaskarla, C., Yadav, G.S. et al. Uveal melanoma immunogenomics predict immunotherapy resistance and susceptibility. Nat. Commun. 2024, 15. DOI: 10.1038/ s41467-024-46906-4.
4. Bower, J.J.; McCall, T.F.; Sawyer, E.J.; Darnell, J.; Hirsch, M.L. Allele-specific depletion of GNAQQ209L via siRNA or a rAAV-shRNA vector induces selective toxicity in GNAQQ209L uveal melanoma cells. 2024.
5. Interview with Jacquelyn Bower, Ph.D. 09/17/24.
6. File:DdRNAi diagram.jpg. Wikipedia. https://commons.wikimedia. org/wiki/File:DdRNAi_diagram.jpg (accessed 2024-10-01).
7. Schwarz, D.S., Ding, H., Kennington, L., Moore, J.T., Schelter, J., Burchard, J., Linsley, P.S., Aronin, N., Xu, Z., Zamore, P.D. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genetics 2006, 2(9). DOI: 10.1371/journal. pgen.0020140
8. Li, C., Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev Genet. 2020, 21, 255–272. DOI: 10.1038/s41576-0190205-4
9. NIH Image Gallery. Gene therapy shows promise for treating Niemann-Pick disease type C1. https:// www.nih.gov/news-events/news-releases/gene-therapy-shows-promisetreating-niemann-pick-disease-type-c1 (accessed 2024-10-01).
10. Bower, J.J.; McCall, T.F.; Sawyer, E.J.; Darnell, J.; Hirsch, M.L. Allele-specific depletion of GNAQQ209L via siRNA or a rAAV-shRNA vector induces selective toxicity in GNAQQ209L uveal melanoma cells. 2024.

Weight up, health down. For most of us, the negative correlation between weight and obesity and overall health—cardiovascular, metabolic, mental—has been deeply ingrained in our understanding of our bodies. From youth into our later years, weight, obesity, and metabolic health often appear as the first and most convenient solutions to nearly all our healthcare concerns. For example, among the elderly, weight management and obesity have long served as the primary indicators of bone density and bone loss. However, recent research conducted at the University of North Carolina at Chapel Hill may fundamentally alter our reliance on these indicators for good.
By Mannah Patel
Endocrinology and Metabolism, Dr. Styner’s practice focuses on patients with endocrine disorders, diabetes, metabolic bone diseases, and osteoporosis. These clinical focuses have inspired Dr. Styner to gear her research towards the underlying causes of these conditions: bone and metabolic health. It was perhaps her passion towards translating her patient care experiences to applicable research that led Dr. Styner to her current research project. ¹
Among the elderly, weight management and obesity have long served as the primary indicators of bone density and bone loss.
Bone density declines over our lifetimes leading to conditions like osteoporosis that can significantly impact quality of life. Despite its reputation as a solid, steadfast tissue, bone is actually one of the most dynamic and adaptable tissues in the human body. Bones use wmolecules like calcium and collagen from our diet and fat/nutrient deposits as its primary ‘construction workers’, undergoing continuous structural remodeling and reconstruction throughout our lifetime.²,³ Unfortunately, the efficiency of these construction workers is not as reliable as we would like. There is existing research that highlights a gradual decrease in our bones’ reconstruction capabilities as we age.³ We also know that this decline is exacerbated by various factors such as hormonal changes, physical activity levels, and dietary habits.⁴ There are little to no answers, however, regarding how exactly the dietary and fat resources that our bones rely on either exacerbate or mitigate bone deterioration as we age.
Fortunately, clinician and researcher Dr. Maya Styner’s recent research at the University of North Carolina at Chapel Hill specifically addresses. As part of UNC’s Division of
Dr. Styner’s research sheds light on how dietary factors influence bone health, particularly in the context of age-related changes. Her study, titled “Diet-Stimulated Marrow Adiposity Fails to Worsen Early, Age-Related Bone Loss,” explores the relationship between diet-induced fat accumulation in bone marrow and its effects on bone density as we age.
Dr. Styner’s and her research group focus on marrow adiposity, the presence of fat cells within the bone marrow. Given the overall negative connotation of obesity and fat

Dr. Maya Styner

tissues in the context of metabolic health, an increase in marrow fat has been linked to negative outcomes for bone health. As the amount of fat in the bone marrow increases, not only is the bone marrow’s reconstructive function obstructed, but a physical increase in weight surrounding the bones also contributes to an increased risk of fractures. Interestingly, Dr. Styner’s findings may challenge all of this information and the fundamental ways in which we view the relationship between diet and aging.
The study used mice models to explore how different high-fat diets affected marrow adiposity and overall bone health. The control mice group was given a standard balanced diet while the experimental group was fed a high-fat diet. These mouse models, despite being far more accurate test subjects than detached tissue samples of cell vials, posed a challenge for Dr. Styner and team. The average American lifespan is 77.5 years, a stark contrast to the average labraised mouse’s lifespan of 2-3. ¹,⁵ Although mice’s bone density and tissue do work very similarly to that of humans, lining up and accurately comparing the aging processes of both species was a tedious, often confusing process.¹ However, with the help of advanced MRIs and computational imaging by research member Dr. Martin Styner, the comparison of the

mice’s bone loss and adiposity over time and across was, albeit painstakingly, made visually comprehensible and translatable to that in humans.
The experiment’s results came with both confirmations and surprises. The results displayed the expected trend of high-fat diets leading to significant increases in marrow adiposity. This was an intuitive confirmation of the hypothesis that dietary factors contribute to fat accumulation in bone marrow. Interestingly, however, this increase in marrow fat did not correlate with decreased bone density in the early stages of aging. In fact, the results indicated that bone density remained stable across any adipose increases and across aging processes.⁶ This suggests that, at least in the initial phases of aging, our bones can adapt against the potential negative impacts of increased marrow.¹ In some cases, Dr. Styner and her team suggest, higher levels of adiposity may even mitigate and reduce age-related decreases in bone density.
The team believes that other factors like physical activity and hormonal changes may be more involved in bone health than previous research may suggest. For example, most health campaigns directed toward the elderly and the aging emphasize cardiovascular health and weight loss or maintenance. However, these results showed that bone strength decreases per age and remains unaffected—perhaps even bettered—by adiposity. According to Dr. Styner, this may be a sign for a larger sociomedical emphasis on weight training, mobility, and fragility as people get older.¹ ⁶ These findings also merit further questions about how the body compensates for increased fat in the marrow and whether or not adipose deposits have the ability to enhance bone formation or reduce bone resorption rates as a way to maintain overall bone density in old age.
Overall, this project contributes to literature that challenges the longstanding villainization of dietary fat on our overall health. As researchers to explore the relationships between diet and bone metabolism, Dr. Styner’s work emphasizes the need for a nuanced understanding of the topic and what it means for all of us in the years ahead. As our understanding of bone health goes up, our reliance on weight indicators may, for the first time in healthcare history, be going down.
1. Osteoporosis: What You Need to Know as You Age.
2. Demontiero, O.; Vidal, C.; Duque, G. Ther. Adv. Musculoskelet. Dis. 2012, 4(2), 61–76.
3. Aging changes in the bones - muscles - joints. https:// www.mountsinai.org/health-library/special-topic/agingchanges-in-the-bones-muscles-joints. (accessed Month Day, Year)
4. Interview with Maya Styner, Ph.D. 09/24/2024
5. Life Expectancy. https://www.cdc.gov/nchs/fastats/ life-expectancy.htm. (accessed Month Day, Year)
6. McGrath, C.; Little-Letsinger, S. E.; Pagnotti, G. M.; Sen, B.; Xie, Z.; Uzer, G.; Uzer, G. B.; Zong, X.; Styner, M. A.; Rubin, J.; Styner, M. Obes. Facts 2024, 17 (2), 145–157.
By Skye Scoggins

Understanding menopause is crucial: this transitional phase brings heightened risks of mood disorders for women. This shift highlights the importance of ongoing research to provide effective solutions for mood disorder development. Leading this research is Dr. Susan Girdler, Director of the Stress and Health Research Program, within the University of North Carolina at Chapel Hill’s Department of Psychiatry.
Dr. Girdler received her Ph.D. in Experimental and Biological Psychology from UNC Chapel Hill. Her current research focuses on reproductive mood disorders, specifically how hormone sensitivity affects these disorders. In particular, she examines estradiol, a form of estrogen, the primary female sex hormone. Furthermore, her research aims to develop better interventions for women undergoing menopause.1

Dr. Susan Girdler
Menopause is defined as the end of menstruation, for 12 consecutive months. This indicates a decline in the ovaries’ production of hormones like estrogen, which is essential for regulating the menstrual cycle and maintaining reproductive health, and progesterone, which prepares the uterus for pregnancy and balances estrogen’s effects. The decline in hormone
“I came to really appreciate the influence of ovarian hormones on mood.”
production can lead to symptoms such as hot flashes, mood changes, and sleep disturbances. During this transition, there are significant fluctuations in estradiol levels, contributing to various physical and emotional challenges.2
Dr. Girdler’s earlier research explored how the menstrual cycle influences women’s cardiovascular stress reactivity, which is the heart’s response to stress. However, as she worked with participants during different phases of their menstrual cycles, she found a new passion. Dr. Girdler explains that during the early phases of the menstrual cycle, one participant displayed high functioning, stable mood, and strong engagement. However, in the premenstrual phase,
that same participant became agoraphobic.1 Agoraphobia is an anxiety disorder marked by fear or avoidance of places where escape feels difficult. This is just one example of the significant impact of ovarian hormones on mood, highlighting the need for more research in understanding women’s mental health across the many different phases and transitions of the menstrual cycle.
“I came to really appreciate the influence of ovarian hormones on mood,” said Dr. Girdler. 1
Dr. Girdler and her colleagues are now studying how fluctuations in estradiol during the menopausal transition, combined with very stressful life events (VSLEs), can increase depressive symptoms, and whether transdermal estradiol can help manage this risk. Transdermal estradiol, a form of estrogen therapy that delivers estradiol into the body through a topical patch. 2
Previous to Dr. Girdler’s research, no studies specifically tested whether hormone therapy can reduce depressive symptoms during perimenopause, the time shortly before menopause, and early postmenopause. The study aimed to evaluate the effectiveness of transdermal estradiol (TE) combined with intermittent micronized progesterone (IMP), a form of progesterone given in controlled doses at specific intervals, in preventing
depression in initially symptom-free women. Studying this combination is important because the two hormones work together to stabilize mood and hormonal imbalances.2
172 perimenopausal and early postmenopausal women aged 45 to 60 participated in this double-blind, placebo-controlled study, meaning that neither the participants nor the researchers knew who received the actual treatment and who received the placebo. They received either transdermal estradiol or a placebo for 12 months, along with oral progesterone or a placebo every three months. The primary outcome was the change in depressive symptoms, measured using the Center for Epidemiological Studies–Depression Scale (CES-D). This self-report questionnaire evaluates the frequency of depressive feelings and behaviors, with a score of 16 or higher indicating clinically significant depressive symptoms. Participants completed the CES-D at baseline and at months 1, 2, 4, 6, 8, 10, and 12. This allowed Dr. Girdler to assess the relationship between hormonal treatments and mental health outcomes in the participants.2
The results showed that women receiving the placebos were more likely to develop depressive symptoms than those on TE+IMP (32.3% vs.

1. Graphs show the treatment effects on high vs. low stress women over the course of a year. Courtsey of Gordon et al. 2018.
17.3%). The treatment was particularly effective for women in the early stages of menopause and for those who had experienced 2 or more stressful life events before the study. Overall, the study concluded that TE+IMP was more effective than placebo in preventing depression in perimenopausal and early postmenopausal women.2
Dr. Girdler emphasizes that a key finding of the study is that it is not only possible to treat women

Figure 2. Bar chart depicts the effects estradiol had on women with a score of 16 or greater on the CES-D. Courtsey of Gordon et al. 2018.
with current depression but also to potentially prevent the development of depression.1
Women in this stage of life often face intense stress from multiple fronts—empty nesting, changing family roles, caretaking, work status shifts, and marital or health challenges. With evidence that women who experience perimenopausal depression are more sensitive to changes in estradiol, the ability to intervene with hormone therapy could dramatically improve their quality of life. This study demonstrates that, for women exposed to stressful life events, depression later in life may be preventable. Furthermore, Dr. Girdler explains that the potential to prevent depression is critically important because it can subsequently reduce the risk of other comorbid mental illnesses and even physical health issues, such as cardiovascular disease.1
However, Girdler explains, “Replication is the cornerstone of science,” and it’s essential that these findings be reproduced.1
Dr. Girdler is now conducting research on the relationship between estrogen fluctuations and mood and how it may be moderated by the hypothalamic-pituitary-adrenal (HPA)
axis, the body’s major neuroendocrine system responsible for responding to stress. If she finds this is the case, it can help explain why stressful life events play such a significant role in the development of anxiety and depression.1
Dr. Girdler’s ongoing research continues to shed light on the relationship between hormones and mood, offering a hopeful future where

Figure 3. Graph depicts women in different phases of menopause and the effects of estradiol on women with a score of 16 or greater on the CES-D. Courtsey of Gordon et al. 2018.
depression during menopause may be preventable, improving the overall well-being of women during this critical time.1
References
1. Interview with Susan Girdler, Ph.D. 10/3/2024
2. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS. Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA Psychiatry. 2018 Feb 1;75(2):149-157. doi: 10.1001/jamapsychiatry.2017.3998.
3. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause. 2016 Mar;23(3):257-66. doi: 10.1097/ GME.0528.
By Tanvi Sharma

As the epidemic plows through the US, healthcare professionals and policymakers find themselves bewildered as they desperately attempt to find a solution. Nutrition is viewed as the way that food can influence bodily wellness. The body must receive the necessary nutrients as they are important for bodily tissues, energy levels, disease prevention, and more. Today, there are vast issues with nutrition across the globe. One such issue is the rise of obesity. It has been found that individuals who face obesity have been more susceptible to cardiovascular diseases, cancer, diabetes, and immunity, which is a huge issue that plagues the US. In fact, studies have shown in the US that the prevalence of adult obesity and severe obesity is projected to increase. Furthermore, the prevalence of obesity is not limited to the US, but also global. Data on levels of BMI over time show obesity increases not only in developed countries but in developing countries as well. Nearly a third of the world’s population is classified as overweight or obese.
Dr. Nancie MacIver, an associate professor of nutrition and pediatrics and the division chief of pediatric endocrinology at the University of North Carolina at Chapel Hill, has been specifically looking at how obesity can influence the immune system. Dr. MacIver found her love for pediatric endocrinology during a research project that examined immune cell metabolism. She initially looked at the hormone leptin, which is crucial in regulating energy balance, and from there,looked at nutrition and how it affects immunity. Then, she focused more on obesity and its effect on immunology. She now looks at improving immunity and its relation to obesity through her lab and works as a physician scientist.
Obesity is characterized as the abnormal or excessive

accumulation of fat in the human body, often quantified with a Body Mass Index greater than 30. While this may seem like a simple physical condition, the underlying biological processes it triggers are complex and far-reaching. Obesity can lead to changes such as decreased immunity, especially towards influenza because obesity can create metabolic changes in the circulating immune cells, specifically T cells. It has even been seen that those who suffer from obesity tend to have an increased risk of morbidity and even mortality from influenza. Dr. MacIver found that even with significant weight loss, the changes to the immune system appear to last, meaning that
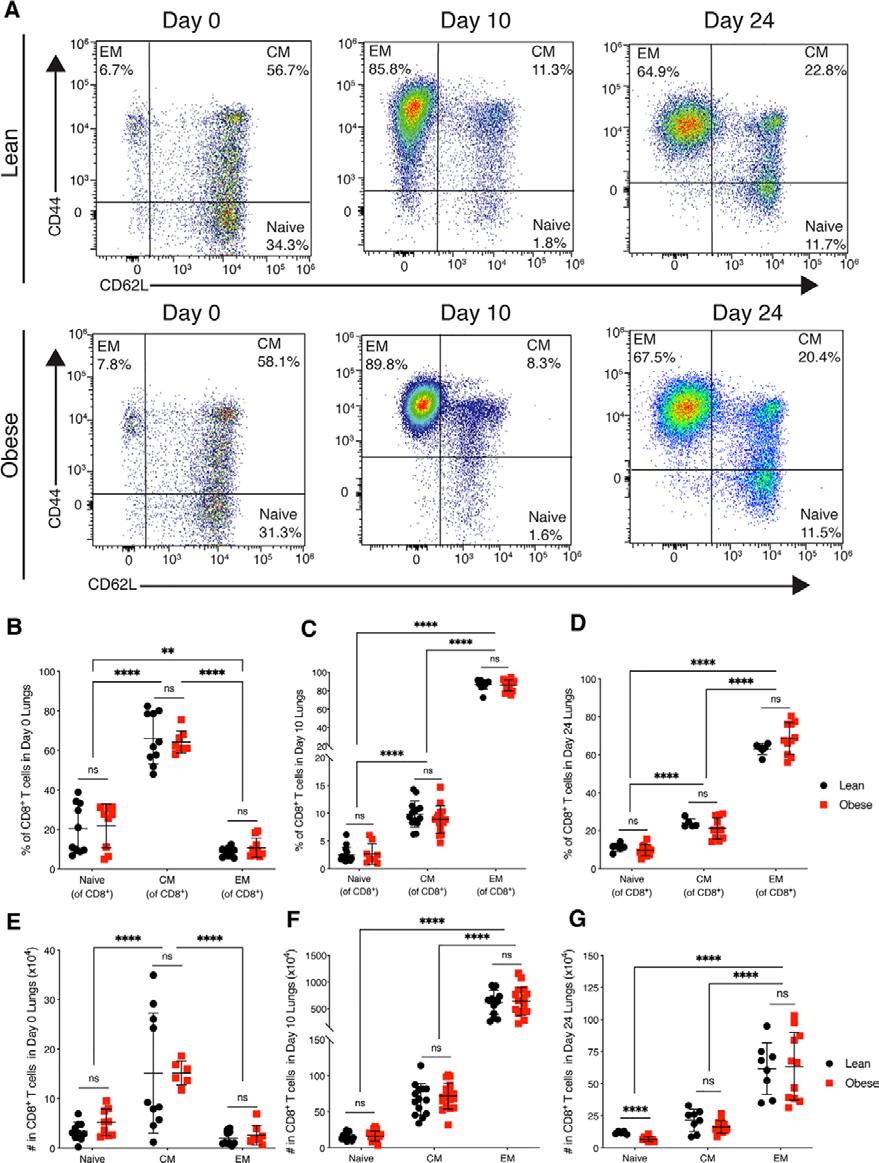

methods such as bariatric surgery will not help reverse the detriments on the immune system, a finding that surprised Dr. MacIver herself. This discovery challenges the assumption that weight loss alone is enough to restore immune health in obese individuals.
Furthermore, the MacIver Lab has examined different drugs, such as Metformin, commonly used to treat type 2 diabetes, and Semaglutide, also known as Ozempic, which is gaining popularity as a weight-loss drug for treating the adverse immune effects of obesity. For instance, in one project, she analyzed reverse abnormal T cell metabolism and restored T cell immunity (MacIver 2019). The lab primarily uses the mouse model to examine the effects of these different drugs. Past procedures include looking at splenic (spleen-related) CD8 T cells, cells that mediate the clearance of pathogens and establish immunological memory, and discovering that Metformin treatment of these cells alters their function by decreasing oxidative metabolism1. When addressing obstacles in the research process, Dr. MacIver has no shortage of them. One research obstacle she has faced is how observations observed in the mouse model may not align with the human model. She also emphasizes how a large amount of preliminary data needs to be collected because there is not much research on GLP 1 agonists, the class of drugs that can treat diabetes and obesity. To battle these roadblocks, she talked about the extensive collaboration she has implemented in this field, whether within her lab or with other researchers of different expertise, and how all the research requires the effort of numerous people and groups. Overall, the MacIver lab has done some amazing research and is on the path to doing so much more. In the future, one of her key interests is how obesity affects immunity against emerging viruses like COVID-19. Given the disproportionate impact of COVID-19 on individuals with obesity, understanding the immune mechanisms at
play is crucial for developing more effective treatments and preventive strategies. Furthermore, she wants to conduct more research into intermittent fasting, or in other words, time-restrictive eating, and its effects on the immune system and gut microbiome.
1. Green, W. D.; Al-Shaer, A. E.; Shi, Q.; Gowdy, K. M.; MacIver, N. J.; Milner, J. J.; Beck, M. A.; Shaikh, S. R. Metabolic and functional impairment of CD8+ T cells from the lungs of influenza-infected obese mice. J. Leukoc. Biol. 2021, 111 (1), 147–159. https://doi.org/10.1002/jlb.4a0120075rr.
2. Kiernan, K.; Nichols, A. G.; Alwarawrah, Y.; MacIver, N. J. Effects of T cell leptin signaling on systemic glucose tolerance and T cell responses in obesity. PLoS ONE 2023, 18 (6), e0286470. https://doi.org/10.1371/journal. pone.0286470.
3. Shaikh, S. R.; MacIver, N. J.; Beck, M. A. Obesity dysregulates the immune response to influenza infection and vaccination through metabolic and inflammatory mechanisms.
4. Annu. Rev. Nutr. 2022, 42 (1), 67–89. https://doi. org/10.1146/annurev-nutr-062320-115937.
5. Greene, E.; MacIver, N. J. Targeting T cell (oxidative) metabolism to improve immunity to viral infection in the context of obesity. Front. Immunol. 2022, 13. https://doi. org/10.3389/fimmu.2022.1025495.
By Sanjita Srinath

In the fight against cancer, breakthroughs in immunotherapy have sparked hope for treatments that go beyond traditional chemotherapy and radiation. Among the most promising is CAR-T therapy, where re-engineered immune cells become cancer’s worst nightmare. But what if we could make this already powerful treatment even stronger, faster, and more accessible?
Enter the MASTER implant—a cutting-edge innovation in material science that could redefine how scientists weaponize cells to defeat cancer from within, cheaper and quicker. Dr. Yevgeny Brudno, pictured in Figure 1, has been a key player in this endeavor from within Carolina’s community. Dr. Brudno received a Bachelor of Arts from the University of Pennsylvania in Chemistry and Biophysics in 2004 and went on to receive a PhD in Chemistry and Chemical Biology from Harvard University in 2010. He has been working in North Carolina with the Joint Department of Biomedical Engineering at the University of North Carolina at Chapel Hill and North Carolina State University for the past 7 years,working in drug delivery and now cell therapy. He is also currently an associate professor at the UNC Eshelman School of Pharmacy,
Division of Pharmacoengineering and Molecular Pharmaceutics. He is a selfdescribed “chemist by training” with a passion for material science and has seen this transfer into his work, where he and his lab currently investigate how they can use material science and chemistry to make innovative breakthroughs in

Dr. Yevgeny Brudno
the cell therapy space, specifically for cancer.
CAR-T cell therapy is a relatively new innovation in the field of cancer treatments, one that has shown incredible promise so far, but with room for improvement. CAR-T cell therapy can be best described in relation to The Terminator. Just as The
Terminator has one intention: to kill cells modified through CAR-T cell therapy have one intention: to kill.1 Our body produces B cells and T cells, two types of cells associated with the immune system. However, mutations can cause lymphoma to develop and turn “good” B cells into cancerous B cells, in a liquid cancer form, meaning the cancer is not concentrated in a solid body part and is much more fluid, due to lymphoma (and other blood cancers) being a blood cancer. This is where CAR-T comes into play by genetically modifying T cells to better detect cancerous cells, focusing on a specific antigen found on the cancerous cells.
However, CAR-T cell therapy is a very expensive, timely process costing between $400,000 and $1,000,000 USD per patient, due to it being a highly customized treatment plan. Whereas plans such as radiation and chemotherapy have broader access and can be formulaically applied to patients, CAR-T therapy requires extraction of the patient’s T cells, meticulously genetically engineering those cells, and then reinserting the newly modified T cells, now called CAR-T cells, into the patient. This is a process that can take up to 4 weeks.2 The development process up to 2 weeks is pictured in Figure 2.

Enter MASTER (Multifunctional Alginate Scaffold for T-cell Engineering and Release). Discovered accidentally by Dr. Pritha Agarwalla, PhD in Dr. Brudno’s lab in 2019, MASTER was developed in relation to a separate project, being used as a control group. However, through this, Dr. Agarwalla discovered that this sponge had the unique ability to quickly and easily modify T cells into CAR-T cells.1 This had immense implications. What was once a four-week process could be turned into a one- or two-day process by using sponges to process a patient’s T cells. This eliminates the need for painstaking human-done genetic engineering for the transformation of T cells into CAR-T cells.
The process involves extracting the patient’s white blood cells (T cells) and infusing them onto the MASTER sponge. As the sponge processes these cells, they are turned into CAR- T cells and the sponge is inserted into the body where it can stay alive for an extended period of time. This was tested in the mice and performed much more favorably to the more traditional CAR-T cell therapy1. Researchers observed that the traditional CAR-T cells were active in the mice body for a short time after infusion, approximately 50 days, despite its classification as a living drug, meaning that it has the ability to reproduce. In comparison, the CAR-T cells generated by MASTER had a lifespan closer to 100-150 days, giving the drug more potency and time to be effective.1 This genetic engineering process is “the hardest part of the process” and the development of MASTER would enable a much more efficient process to create these cells.1
Brudno, and the members of the lab, proceeded by using trial and error methods to better develop this accidental discovery. Recently, the lab has begun animal trials with mice in the lab. They hope to test MASTER with monkey species next and proceed to clinical trials afterwards.
“...the development of MASTER would enable a much more efficient process to create these cells.”
This discovery has the potential to make cancer treatments much more affordable and accessible for all patients across the globe, and there’s more to come. The Brudno lab is interested in investigating the potential effects of using similar technology to genetically modify diseases such as autoimmune diseases and sickle cell anemia, where specific cells can be modified through a sponge similar to MASTER to target specific areas. Ultimately, MASTER could be the key to curing cancer, affordably and efficiently.
Following the discovery of this sponge, Drs. Agarwalla,
1. Interview with Yevgeny Brudno,Ph.D., 12/30/2024
2. Pandit S, Agarwalla P, Song F, Jansson A, Dotti G, Brudno Y; Biomaterials 2024, Volume 308, https://doi.org/10.1016/j.biomaterials.2024.122580
By: Paige Twohill
When most people think of drug overdoses, they often picture illicit substances as the cause. Yet, one of the most dangerous culprits is found in nearly every household: acetaminophen, commonly found in well-known medicines such as Tylenol.1 Responsible for nearly half of all acute liver failure (ALF) cases in the U.S., acetaminophen overdose can silently turn a common pain reliever into a deadly threat. ALF is often difficult to treat due to the high level of damage already done to hepatocytes (liver cells), but new research is being conducted to discover potential new molecules that could act as therapeutics to treat this condition.
Dr. Katelyn Arnold, who earned a Ph.D. in Pharmaceutical Sciences here at the University of North Carolina at Chapel Hill (UNC-Chapel Hill), is currently an Assistant Professor on the Research Track at the UNC-Chapel Hill Eshelman School of Pharmacy (Figure 1). She also is the co-founder of Glyco Discoveries and a consultant for Glycan Therapeutics, both of which are biotech companies looking to commercialize Dr. Arnold’s therapeutic work. Dr. Arnold’s research focuses on using synthetic sulfated glycans to understand how these molecules function with the goal of using them as a therapeutic treatment for acute inflammatory conditions. Her research focuses on the high-mobility group box 1 (HMGB-1) protein that is normally inside cells and necessary for normal transcription and translation of proteins. When a cell dies, the HMGB-1 protein is released into the bloodstream, “and [HMGB-1] can get identified by immune cells as a foreign component because it is normally inside [the] cells and [the] immune system never
sees it.”2 The protein is now recognized by the immune cells as a damage-associated molecular pattern (DAMP) that causes a large influx of inflammation, leading to injuries such as liver failure, transplant injuries, and trauma. This inflammation can be quantified by the number of neutrophils (a type of white blood cell) infiltrating the injury site, such as the liver after an acetaminophen overdose. When inflammation occurs, neutrophil levels significantly increase.
“Actually, what we think we are doing is [supplementing] more HS that the cell is trying to use to protect itself…and in moments when the cell starts dying [HS] gets cleaved from the surface of the cell”.
The goal of Dr. Arnold’s research is to synthesize a molecule that could be used as a treatment to reduce inflammatory injury. Heparan sulfate (HS) is a naturally occurring glycan, or chain of single sugar molecules, located on the surface of many cells. HS is a type of molecule that binds to many proteins, with Dr. Arnold stating that “[m]any DAMPs, including HMGB1, are HS-binding proteins.”3 HMGB-1 will bind with HS, leading to a reduction in the inflammatory response as measured by a decrease in neutrophils (Figure 2). Based on Dr. Arnold’s results, she believes the administration of synthetic HS can supplement the endogenous source of HS, thereby reducing HMGB-1 levels below a threshold to prevent inflammatory injury.

Figure 1. A diagram showing how HS can bind to neutrophils and prevent DAMPs from producing a further inflammatory response. Image provided by Dr. Katelyn Arnold.
Figure 1. A diagram showing how HS can bind to neutrophils and prevent DAMPs from producing a further inflammatory response. Image provided by Dr. Katelyn Arnold.
One of the main focuses of Dr. Arnold’s research is the creation of synthetic HS using a unique method called chemoenzymatic synthesis. This technique utilizes enzymes to create the HS molecule (Figure 3). The advantage of this method is the ability to control the synthesis of HS and produce structurally defined oligosaccharides (shorter carbohydrate, or sugar, chains). Natural HS is a polysaccharide mixture (containing multiple sugars) of various carbohydrate chain lengths and structures, whereas synthetic HS are discrete molecular entities containing a distinct number of sugars to target the desired molecular properties. For example, natural HS is composed of polysaccharide structures ranging from a few sugars in length to structures over 100 sugars long. Synthetic HS structures commonly used in Dr. Arnold’s work are 12 sugars long (called 12-mer) and 18 sugars long (called 18-mer).
Synthetic HS is a possible new molecule able to successfully reduce neutrophil levels and inhibit HMGB-1 inflammation. Dr. Arnold says that “[her team] think[s] [they] are [supplementing] more HS that the cell is trying to use to protect itself…and in moments when the cell starts dying, [HS] gets cleaved from the surface of the cell”.2 If HS produced by the body cannot bind to HMGB-1 and inhibit the inflammatory effect of the HMGB-1 protein, the inflammation will increase and lead to organ injury.
Dr. Arnold utilizes a mouse model to test how synthetic HS like an 18-mer oligosaccharide could be used as a treatment for acetaminophen (APAP)-induced acute liver failure. In her experimental groups, Dr. Arnold initially injects APAP into

Figure 2. A drawing of a comparison of animal-produced heparan sulfate (HS) to synthetic HS. Image provide by Dr. Katelyn Arnold.
the mouse. After 24 hours, she injects 18-mer and collects the blood and tissues. She also has an injury control cohort (injection of APAP and no treatment of 18-mer) and noninjury control cohort (injection of saline and collection of tissue). It was found that the HS molecule that possessed only anti-inflammatory properties was most effective at treating APAP-induced ALF in the mouse liver by reducing neutrophil levels.4 Dr. Arnold is also currently experimenting with the use of synthesized HS containing either similar or a different combination of drug properties to apply to other acute conditions and determine its efficacy.
With the vast range of conditions that synthesized HS could treat, Dr. Arnold’s research is vital to finding new treatments for acute conditions like ALF to prevent loss of life. Dr. Arnold hopes to extend her research to chronic conditions involving the liver and other injuries. She is also interested in finding ways to understand the drug properties of synthetic HS molecule to find how it can be used as a therapeutic treatment. Her new analytical method of understanding the structures of HS produced by the body will further inform her research about the creation of synthetic HS. Dr. Arnold is contributing to a very important step in the drug discovery process that could help save countless lives by creating

Figure 3. A representation of the mouse model for treatment of synthetic HS in an IR injury to the liver during transplantation. Image provided by Dr. Katelyn Arnold.
opportunities for patients to get the treatment they need.

1. Acute liver failure - Symptoms and causes. Mayo Clinic. [accessed 2024 Oct 7]. https://www.mayoclinic.org/diseases-conditions/acute-liver-failure/symptoms-causes/ syc-20352863
2. Interview with Dr. Katelyn Arnold, Ph.D. 08/26/24.
3. Arnold K, Xu Y, Sparkenbaugh EM, Li M, Han X, Zhang Xing, Xia K, Piegore M, Zhang F, Zhang Xiaoxiao, et al. 2020. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Sci Transl Med. 12(535):eaav8075. doi:10.1126/scitranslmed. aav8075. [accessed 2024 Oct 8]. https://www.science.org/ doi/10.1126/scitranslmed.aav8075.
4. Arnold K, Xu Y, Liao Y-E, Cooley BC, Pawlinski R, Liu J. 2020. Synthetic anticoagulant heparan sulfate attenuates liver ischemia reperfusion injury. Sci Rep. 10(1):17187. doi:10.1038/s41598-020-74275-7. [accessed 2024 Oct 8]. https://www.nature.com/articles/s41598-020-74275-7.
By Anthony Yang

Today, the market price for a kidney in the United States is roughly $262,000. The third most expensive organ in the country, kidney transplants make up more than half of all organ transplants performed every year. Before reaching the stage of kidney transplant, patients can choose to use dialysis to remove toxic materials from their kidneys. These procedures require vascular access, which is primarily done through an arteriovenous graft (AVG) or arteriovenous fistula (AVF). Unfortunately, almost 50% of these procedures fail in the maturing stage due to neointimal hyperplasia or stenosis, leading to intense pain and other problems for patients. The primary cellular component within the vessels are vascular smooth muscle cells (VSMCs), that can differentiate into a variety of unique phenotypes with different functions. When these cells become damaged or dedifferentiated, such as during vascular access procedures, nearby cells migrate and begin proliferating at a fast rate. This rapid proliferation is the backbone for conditions such as neointimal
hyperplasia and stenosis. Dr. Gang Xi, a research associate professor at the University of North Carolina at Chapel Hill, is working to understand the pathways that regulate vSMC function under diseased conditions. His first projects were aimed

at examining the differences in protein and gene expression between arterial and venous VSMCs. With the results obtained, Dr. Xi hopes to analyze how spe -
cific growth factors and signal proteins interact under diseased conditions and how it regulates the differentiation, migration, and proliferation of venous and arterial vSMCs separately.
Dr. Gang Xi began his scientific journey at Zhejiang University in China, where he earned both his undergraduate B.S. and Masters of Science. Dr. Xi would then earn his Ph.D. in muscle biology at the University of Minnesota, before completing his fellowship at UNC Chapel Hill, where he continues his research. Originally focused on research in endocrinology, his strong background in cell biology and biochemistry made him a good candidate to be recruited by UNC Kidney Center. Throughout his career, Dr. Xi has worked on understanding how different signals affect protein interactions and pathways in VSMCs. Many researchers in the world of nephrology recently began noticing key differences in the functions and interactions between vein and artery SMCs. Thus, one of Dr. Xi’s first studies at the UNC Kidney Center was to characterize these differences.

Generally, differentiated smooth muscle cells are quiescent1, meaning they remain inactive, though their contractile structure allows them to still perform primary functions. However, when injury occurs, SMC phenotypic switching occurs and they begin to dedifferentiate, migrate, and proliferate quickly, leading to intimal hyperplasia, or thickening of the inner cell wall. During other experiments, Dr. Xi began to notice that venous SMCs migrated faster and proliferated more in response to stimuli such as injury or growth factors. Curious to investigate this observation, he hypothesized that venous smooth muscle cells have a higher ability to respond to growth factors or other stimuli as opposed to arterial cells.
In order to collect the data necessary to generate a conclusion, pig arterial and venous SMCs must first be cultured. Before collecting, the cells are introduced to differentiation media, containing 30 µl heparin for varying time periods. Then, the cells were harvested and visualized with either immunofluorescence (IF) staining or gel electrophoresis and western blotting, which revealed both location and quantity of certain proteins of interest, including fibronectin, MAPK, and IRS-1, among
others.2 The creation of a hyperglycemic or dedifferentiation milieu is an optional tool to further visualize how certain growth factors affect the activation and expression of relay or target proteins, including KLF-4 or p-53. Finally, RNA sequencing was used to determine which specific genes were more activated in arterial and venous SMCs.
As hypothesized, the results lined up with the original hypothesis. Protein level data revealed stark differences between pathway activations in arterial and venous SMCs. Interestingly, they also found that certain identical signal pathways in artery and vein cells were activated by distinct genes, discovered through RNA sequencing, though these results were not published. For example, venous SMCs had higher expression of fibronectin, which is involved in all celladhesive interactions such as migration and differentiation. These results are important because they may reveal an explanation to the varying susceptibilities of arteries and veins to develop vascular diseases. For Dr. Gang Xi, research in this field is about more than just curing the illness; instead, it’s imperative to continue research on these topics to reduce suffering in patients. According to Dr.
Xi, “almost 50% feel [pain] every time… if we can… block smooth muscle cell growth and migration, we can improve vascular access and reduce pain for those patients who need dialysis”.1 By working to publicize the differences in protein and gene expressions in venous and arterial vSMCs, Dr. Xi is one step closer to potentially developing relief for those patients.
Another interesting aspect of the dedifferentiation of vSMCs is the onset of calcification. Under uremic conditions, smooth muscle cells can differentiate into osteogenic, bone-type cells. In the late stages of kidney disease, stenosis and atherosclerosis cause intense calcification in the blood vessels, which leads to cardiovascular issues such as heart attacks. According to Dr. Xi, “Calcification is a huge problem, it forms a very tough layer at the surface of VSMCs. No drug can reverse that…”,1 thus analyzing the difference of calcification regulation in arterial and venous vSMCs is an important direction for Dr. Xi to take his research. As knowledge of arterial and venous VSMCs continue to grow, hopefully a biomedical cure can one day be discovered.
References
1. Interview with Dr. Gang Xi, Ph.D. 9/4/2024.
2. Arteaga EC, Wai C, Unimunkh Uriyanghai, Sudarsanam VA, Prabir Roy-Chaudhury, Xi G. 2023. Phenotypic Switch Study in Cultured Arterial and Venous SMCs: Messages for the Future Development of Novel Therapies for Vascular Access Dysfunction. Journal of the American Society of Nephrology. 34(11S):537–537. doi:https://doi. org/10.1681/asn.20233411s1537c. [accessed 2024 Oct 10]. https://journals. lww.com/jasn/citation/2023/11001/phenotypic_switch_study_in_cultured_arterial_and.1903.aspx.
3. Li L, Blumenthal DK, Terry CM, He Y, Carlson ML, Cheung AK. 2011. PDGF-induced proliferation in human arterial and venous smooth muscle cells: Molecular basis for differential effects of PDGF isoforms. Journal of Cellular Biochemistry. 112(1):289–298. doi:https:// doi.org/10.1002/jcb.22924. [accessed 2019 Mar 31]. https://www.ncbi.nlm. nih.gov/pmc/articles/PMC4454503/.

By Fiona Yeung
Lung cancer is the leading cause of cancer-related deaths globally, with 1 in 16 people diagnosed in their lifetime.1 Magnetic resonance imaging (MRI) procedures for diagnosis primarily detect hydrogen atoms from water and fat in organs. A problem stands: our lungs are air-filled structures, which leaves certain abnormalities virtually invisible in standard proton MRI.2 At the intersection between physics and medicine, the Branca Lab at the University of North Carolina at Chapel Hill says a technique called “hyperpolarization” may be our answer to revolutionizing diagnostic imaging.

Physicist Dr. Rosa Tamara Branca is a Professor in the Department of Physics and Astronomy and faculty member of the Biomedical Research Imaging Center at UNC Chapel Hill. Since joining UNC faculty in 2012, Dr. Branca has led her team in nuclear spin hyperpolarization, a subfield of MRI. This technique is capable of detecting atoms not normally observed due to low abundance in the target tissue. Through collaborations with physicians and investigators in the departments of Biomedical Research Imaging and Biomedical Engineering, the Branca Lab investigates the use of polarized gases in lung imaging.
used in MRI due to their abundance in the human body and their high level of responsiveness to magnetic fields in MRI scans. A hydrogen nucleus–a single proton– spins on its axis with a north and south pole, much like a magnet. When placed in an external magnetic field, such as an MRI scanner, protons are stimulated and start rotating rapidly. During the scanning, magnets in the walls of the scanner increase and decrease the strength of the magnetic field to the same frequency as the rotating protons. The protons absorb energy from the oscillating magnetic field, which causes a synchronization (known as a resonance).


from the field. The energy produced by hydrogen atoms is used to generate images with contrasting lightness and darkness for tissues with varying water and fat concentrations.3 Since the image is essentially a map of where water and fat are concentrated in tissue, there is little to see when imaging lungs.2
To improve atom detection, Dr. Branca’s research incorporates the use of xenon (Xe) hyperpolarization. Due to its unique properties, Xe has a large electron cloud that supports a large increase in nuclear spin polarization in a magnetic field. “The low concentration [of atoms] is overcome by increasing the nuclear spin polarization,” Dr. Branca says, “–which is a measurement of the number of nuclear spins that are aligned with the magnetic field.” The nuclear spins are detected by MRI; with increased spins comes greater signal intensity for imaging.2 Generally, the lowest energy state is most favorable and has a slightly higher population than other states; however, the excess lower energy population is what is usually detected. MRI normally detects the small excess of spins in the lower energy state and hyperpolarization can recover many of the discarded spins. Nuclear spin polarization puts all the states in the lower
energy state, which allows for detection even at very low spin concentrations since most of the signals are present.2
Still, there are many technical challenges while deploying this technique. One question posed is how long the atoms can stay in the lower energy state before returning to thermal equilibrium with random motion and orientation. “Some nuclear spins are hard to polarize because they go back to equilibrium too quickly,” Dr. Branca explains. “You can think of it like a lifetime, very similar to radioactive isotopes with short lifetimes.” Additionally, Dr. Branca notes the limitation of the instrumentations involved, especially since Xe hyperpolarization calls for a special laser that is costly.2
There are concerns with the radiation associated with present-day molecular imaging techniques, such as PET and CT scans. Medical professionals must weigh the harms and benefits of these imaging techniques on different patients. In some cases, visualizing glucose metabolism with PET scans can be greatly advantageous for diagnosing and evaluating patients with epilepsy or brain tumors. On the other hand, physicians may advise patients diagnosed with

cancer against undergoing such imaging procedures to scan for conditions not linked to cancer. When considering the risks of radiation exposure, many cancer patients forgo these procedures used to evaluate other health conditions.2
In that regard, the constraints would be eliminated if a non-invasive procedure with the diagnostic properties of PET scans was used instead. Advancing imaging with Xe hyperpolarized MRI is our potential solution. Furthermore, unlike previous molecular imaging methods mentioned, MRI does not expose patients to radiation. MRI would be accessible to cancer patients with higher vulnerability to radiation and safer for repeated use for any individual. Not to mention, we are able to look at the molecular byproduct with MRI, which is not possible with PET. PET only shows where molecules accumulate, whereas MRI reveals how molecules convert into metabolites. The imaging implications of hyperpolarization can be applied to organs beyond the lungs. With nuclear hyperpolarization, we can transform the future of medical detection and diagnosis.
1. Lung Cancer Research Foundation. 2023. Lung Cancer Facts. Lung Cancer Research Foundation. https://www.lungcancerresearchfoundation.org/lung-cancerfacts/.
2. Interview with Dr. Rosa Tamara Branca
3. How does MRI work? | The Dunedin Study - Dunedin Multidisciplinary Health & Development Research Unit. dunedinstudyotagoacnz. https:// dunedinstudy.otago.ac.nz/studies/newbrain-imaging-study-2/how-does-mriwork.
Image courtesy of GoodFon

Thirty to fifty percent of elderly individuals defined as “clinically normal” while alive end up meeting the diagnostic criteria for Alzheimer’s disease after death.1 Alzheimer’s disease is a neurodegenerative disease, meaning that it is caused by the progressive loss of neurons (the cells that send messages through the brain). It is a leading cause of dementia and the fifth-leading cause of death for Americans 65 and older.2
Yet, the severity of symptoms varies drastically across individuals. What causes one diseased brain to deteriorate while another, confronted with the same disease, maintains its functioning? Although past research has theoretically explored the brain’s general ability to withstand damage,3 there is still much uncertainty surrounding the process. Dr. Eran Dayan, recognizing the urgent need to quantify this process in the living brain, launched into his research of brain resilience.
Dr. Dayan, an Associate Professor at the University of North Carolina at Chapel Hill (UNC), primarily studies neurodegeneration and aging through a computational lens. Though this niche naturally fits his interests, his path to find it was anything but linear. Even while earning his doctorate in neuroscience, Dr. Dayan found himself spending time in the computer science department. “This was the first
By Jack Blankenbaker

significant exposure [for me] to how computational techniques can benefit neuroscience research.”4 This benefit has remained a driving force in his research to this day. Dr. Dayan’s PhD research explored the role of the basal ganglia, a brain region associated with Parkinson’s disease (a neurodegenerative disease that primarily impairs movement). Initially, this pursuit was led purely by his own interest in the brain region. However, this changed as the research led him to shadow a movement disorder neurologist for a year and half. In his words, it was “one of the most significant things that happened to me in my career.”4 Putting faces to the victims of neurodegeneration transformed Dr. Dayan’s view of neuroscience. He began to find purpose in the field, one
that was greater than just his personal interest. Drawn to research with direct applications to neurological disorders, he followed this purpose through his post-doc and fellowship at the National Institute of Neurological Disorders and Stroke (at the National Institutes of Health). Now, at UNC, most of Dr. Dayan’s research surrounds neurodegenerative disease. In particular, research on brain resilience has allowed him to combine his interests in both computational neuroscience and neurodegenerative disease into one pressing area of research.
Resilience, as defined by Dr. Dayan, is “the general property of the brain resisting perturbations.”4 Resilience can be in reference to both neurodegenerative disease and aging. The building blocks of resilience are nodes and edges. Brain regions can be defined as nodes, while edges are the connections between those regions (Figure 1). Perturbations make nodes and edges unusable, which is indicated with red circles and dashed lines in the figure, respectively. A more “redundant” network has more edges; thus, upon perturbation, it is more likely to retain efficient functioning compared to a less redundant network. (Although the word “redundancy” is typically used within this framework, redundancy and resilience go hand-in-hand.) Neurodegenerative
diseases are targeted attacks, disabling nodes central to a brain network’s connectivity. To quantify this in actual brains, Dr. Dayan’s lab mostly uses existing neuroimaging data, such as that from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)6,7 and the Parkinson’s Progression Markers Initiative (PPMI).8 The “connectedness”

Dr. Eran Dayan.
of two nodes in a brain is measured via data from fMRI, a brain imaging technique. A higher fMRI signal indicates increased brain activity in the region being measured. By analyzing the correlation in fMRI signals of two brain regions (nodes), the strength of their connection can be estimated (Figure 2). Redundancy can then be calculated with a “redundancy matrix,” or the sum of all direct and indirect connections between two nodes under a set length.6,8 The findings of Dr. Dayan’s lab repeatedly demonstrate the importance of redundancy, especially in aging. One study utilized a mathematical method formerly used to explain how financial systems handle crises to instead explain how the brain handles aging. It was found that across the brain, greater age was associated with less redundancy in highly interconnected parts of brain networks.5 This reduced redundancy has
consequences: the lab has found that redundancy within the hippocampus (a brain region primarily associated with memory and learning) protects against cognitive decline in aging.6
The purpose of Dr. Dayan’s lab is unwavering: to “stop, treat, [and] heal … neurodegeneration in the brain in any way we can.”
incorporating patients’ electronic health records, something Dr. Dayan has expressed interest in.
Amid these plans and the excitement they bring, the purpose of Dr. Dayan’s research is unwavering: to “stop, treat, [and] heal … neurodegeneration in the brain in any way we can.”4 Dr. Dayan has already greatly elucidated the importance of resilience and how it acts against neurodegenerative disease and aging, leading the world to a greater understanding of how brains are built to endure.

There are obvious limitations to studying resilience in living humans; for ethical and practical reasons, research often must be limited to observation. However, by using imaging data to create computational models of brain networks, attack simulations can be run to mimic neurodegenerative disease. Simulations performed by the lab have resulted in a promising insight: they can accurately predict whether individuals with neurodegenerative diseases will show cognitive decline.8 Predictive methods like these are vital, especially as new diseasemodifying immunotherapies make headlines, which are more effective the earlier they are implemented. AI, in conjunction with neuroimaging, can also help predict disease outcomes and identify therapy strategies for those with neurodegenerative diseases. Expansions beyond neuroimaging like these is the future path of Dr. Dayan’s research, specifically through the “fusion of imaging and non-imaging data with AI approaches.”4 The lab has explored this by training an AI model to recognize patterns in imaging data that humans may not be able to find. Specifically, the model found that atrophy (loss of neurons and their connections) in three brain regions—the hippocampus, fusiform, inferior temporal gyri—most strongly contributed to Alzheimer’s disease progression.7 In the future, models like these could lead to earlier diagnosis and better treatment planning for Alzheimer’s disease, especially when
1. Driscoll, I.; Troncoso, J. Asymptomatic Alzheimer’s Disease: A Prodrome or a State of Resilience? Curr. Alzheimer Res. 2011, 8 (4), 330-335. DOI: 10.2174/156720511795745348
2. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19 (4), 1598-1695. DOI: 10.1002/alz.13016
3. Stern, Y. JINS 2002, 8 (3), 448—460. DOI: 10.1017/S1355617702813248
4. Interview with Eran Dayan, PhD. 9/18/2024
5. Stanford, W.C.; Mucha, P.J.; Dayan, E. A robust core architecture of functional brain networks supports topological resilience and cognitive performance in middle- and old-aged adults. PNAS 2022, 119 (44). DOI: 10.1073/ pnas.2203682119
6. Langella, S.; Sadiq, M.U.; Mucha, P.J.; Giovanello, K.S.; Dayan, E. Lower functional hippocampal redundancy in mild cognitive impairment. Transl. Psychiatry 2021, 11, Article 61. DOI: 10.1038/ s41398-020-01166-w
7. Kwak, K.; Stanford, W.; Dayan, E. Identifying the regional substrates predictive of Alzheimer’s disease progression through a convolutional neural network model and occlusion. Hum. Brain Mapp. 2022, 43 (18), 5509-5519. DOI: 10.1002/hbm.26026
8. Cascone, A.D.; Langella, S.; Sklerov, M.; Dayan, E. Frontoparietal network resilience is associated with protection against cognitive decline in Parkinson’s disease. Commun. Biol. 2021, 4, Article 1021. DOI: 10.1038/s42003-021-02478-3
By Julia Ellington

Imagine a world where our relationship with food and our body image is defined not by restrictive diets or societal pressures, but by selfacceptance and intuitive choices. In the context of this vision, Dr. BardoneCone at the University of North Carolina at Chapel Hill is conducting groundbreaking research that examines how cultivating body appreciation and practicing intuitive eating can play a crucial role in the recovery process for individuals with eating disorders. Dr. Bardone-Cone authored “Body Appreciation and Intuitive Eating in Eating Disorder Recovery”, a work that highlights the transformative potential of these practices. The significance of this research is underscored by the pervasiveness of eating disorders, especially among young adults. The lifetime prevalence of eating disorders by age 40 is 14.3% for males and 19.7% for females, with the highest rates occurring around the age of 21. This context fuels Dr. Bardone-Cone’s ongoing work, as the need for effective, sustainable recovery strategies is urgent. Although there are misconceptions about eating disorders, Bardone-Cone asserts, “Eating disorders can affect anyone... a misconception
is that it just affects women or only certain kinds of women.” This study and its conclusions highlight the universal nature of eating disorders, underscoring the importance of recognizing and addressing these issues in all communities. By embracing these insights, society can foster a more inclusive understanding of disordered eating, encouraging compassion and support for everyone, regardless of background.
The study focuses on two important aspects of cognitive recovery: body appreciation and intuitive eating. At the time of its publication, research on eating disorders primarily centered on disordered eating behaviors and negative constructs like body shame and thin-ideal internalization, while little literature explored the positive aspects of body image and adaptive attitudes toward eating.
Body appreciation is characterized by having a positive view of one’s body, irrespective of physical appearance, weight, or shape, and respecting bodily needs. “Body appreciation is giving my body respect. Even if I think my body has some flaws, it’s fine,” says Dr. Bardone-Cone. Higher body appreciation is generally associated with lower consumption of appearance-
based media. Individuals can observe body appreciation in their lives through Dr. Bardone-Cone’s comparison of perceptions at different life stages. For instance, she notes, “When you were in early elementary school, what did you think about your body? In high school, how did that change?” This reflection illustrates how body appreciation is a multidimensional construct focused on acceptance and an adaptive mindset about appearance.
In a similar way, intuitive eating nurtures a positive relationship with the body, but focuses more specifically on the connection between eating habits and physical cues. Intuitive eating involves using physiological hunger cues and satiety to determine when to eat, rather than relying on situational or emotional cues. This approach encourages individuals to reconnect with their hunger and fullness signals, promoting a healthier relationship with food. By fostering self-acceptance and rejecting the diet mentality, intuitive eating helps people cultivate trust in their bodies, allowing them to enjoy a diverse range of foods without guilt or anxiety.
Dr. Bardone-Cone conducted this study with 66 women who had a history of eating disorders and 31 control
participants without such a history, surveying them to assess physical, behavioral, and cognitive recovery. The data obtained included factors such as BMI, engagement in binge eating, purging, or fasting in the past three months, and scores from the Eating Disorder Examination Questionnaire. Dr. Bardone-Cone emphasized that recovery is a personal journey and not uniform for everyone. However, for research purposes, a consistent definition is necessary across studies. For physical recovery, a BMI of 18.5 was used, aligning with the World Health Organization’s healthy weight minimum. Behaviorally, recovery was defined as no episodes of binge eating, purging, or fasting in the past three months. For cognitive recovery, the Eating Disorder Examination Questionnaire was utilized, examining subscales like dietary restraint and weight/shape concern. To measure cognitive recovery through body appreciation and intuitive eating, the research team employed the body
“Despite its flaws, I accept my body for what it is.”
appreciation scale and the intuitive eating scale. The body appreciation scale evaluates positive beliefs about one’s body, assessing the degree of respect and acceptance an individual has, regardless of appearance. Example items include, “Despite its flaws, I accept my body for what it is.”The intuitive eating scale assesses the inclination to follow physical hunger cues, with subscales such as unconditional permission to eat and reliance on hunger and satiety cues. After classification based on cognitive, physical, and behavioral recovery, 19 participants met the criteria for a current eating disorder, 28 met criteria for full recovery, and 11 met criteria for partial recovery. Analysis revealed that the fully recovered group did not significantly differ from controls, with both groups showing significantly higher levels of body appreciation and intuitive eating compared to the partially recovered or current eating disorder groups. The results excited Dr. Bardone-Cone because they indicated that intuitive
eating and body appreciation are related to the stability of recovery.
The study aimed to examine the relationship between body appreciation, intuitive eating, and eating disorder recovery. The results suggested that greater body appreciation is inversely associated with negative body image and disordered eating. Due to the relationship between recovery stability and these positive constructs, recovery should be reconceptualized as the presence of adaptive attitudes, not just the absence of eating disorder symptoms. A more comprehensive approach to assessing recovery—one that includes cognitive aspects—can lead to a lower risk of relapse and an improved quality of life.
Beyond clinical implications, body appreciation and intuitive eating can be relevant in everyone’s lives, whether individuals have an eating disorder or not. To better practice these principles in daily life, Dr. Bardone-Cone suggests focusing on “who you surround yourself with… Observe if negative body talk is happening. If friends are saying, ‘Oh my god, I’m so fat,’ don’t buy into that.” She encourages being the voice that challenges such comments, drawing attention to the larger culture that promotes these messages. Additionally, she advises minimizing comparisons and engaging in media literacy, emphasizing the importance of being a critical consumer of the media.
Ultimately, Dr. Bardone-Cone highlights the importance of selfcompassion. It’s natural to experience setbacks and to feel negatively about one’s body at times, especially given all the external messages. Remember to treat oneself with kindness.

References
1. Interview with Dr. Anna Bardone-Cone
2. Koller KA, Thompson KA, Miller AJ, Walsh EC, Bardone-Cone AM. Body appreciation and intuitive eating in eating disorder recovery. Int J Eat Disord. 2020 Aug;53(8):1261-1269. doi: 10.1002/eat.23238. Epub 2020 Feb 5. PMID: 32020677.
3. Ward, Z. J., Rodriguez, P., Wright, D. R., Austin, S. B., & Long, M. W. (2019). Estimation of Eating Disorders Prevalence by Age and Associations With Mortality in a Simulated Nationally Representative US Cohort. JAMA network open, 2(10), e1912925. https://doi.org/10.1001/jamanetworkopen.2019.12925
By Gargi Gole

In the United States, the average person speaks 16,000 words per day.1 This includes conversations with friends, coworkers, and family. Yet, amongst all of these conversations, people determine what to say depending on who they are talking to. They can comprehend what others say to them and simultaneously respond to information that they receive.
People know what to say and how to say it, but how is this possible? This is an ability unique to humans, called discourse processing.
Dr. Jennifer Arnold is a professor in the Department of Psychology and Neuroscience at the University of North Carolina at Chapel Hill. Her research is focused on psycholinguistics, the intersection of linguistics and cognitive processes, and on understanding the brain processes involved in language processing. Language processing is a function where input, what a speaker says, is interpreted. Dr. Arnold states, “language is a human capacity that doesn’t depend on the modality”. The human language processing system is not limited to just spoken language, but it also includes written and sign language. This is what makes language so unique. Her research at the UNC Language Processing Lab aims to understand how the human language system works, specifically focusing on pronoun comprehension. Pronoun comprehension is the cognitive process a person goes through when interpreting what the pronouns used by a speaker refer to based on the context of what is said. This is a process that occurs based on subconsciously observed patterns. These subconsciously observed patterns are known as statistical learning.
The brain is constantly active. People are constantly listening and learning, consciously and subconsciously, which has enabled them to have the unique ability to interpret ambiguity. Humans are repeatedly exposed to ambiguous
sentences, but often miss that ambiguity in sentences as their brains are trained to comprehend sentences by following a specific structure. Statistical learning results in people recognizing patterns in ambiguous sentences(the input), which creates cognitive biases. This allows humans to interpret ambiguous pronouns in sentences. Thus, when exposed to ambiguous sentences, statistical learning allows people to interpret pronouns according to a pre-made structure of how a sentence should be interpreted in their brains. Dr. Arnold has observed that these sentence structures typically associate the person mentioned in the sentence with an ambiguous

Figure 2. People hear others around them which influences how they interpret sentences. Image courtesy of Wikimedia Commons.
pronoun. An example given by Dr. Arnold says “Anna went hiking with Liz and she brought snacks”.3 Most people would associate Anna with being the person who brought food because it is a cognitive bias to assign the ambiguous pronoun “she” with the first person of the sentence.2
The basis of this statistical learning is exposure and experience. The more a person is exposed to a specific structure the more they will be primed to use it, creating a cognitive bias. Dr. Arnold’s lab seeks to discover whether these cognitive biases can be manipulated.
She hypothesized that statistical learning is a result of lifelong exposure and experience. She tested whether a new kind of exposure of structure which varied from previous experience had an effect on participants. This was done by exposing participants, all of whom were of whom were adults, over the age of 18 and had learned the English language before the age of 7, to a series of 32 non-ambiguous sentences, with specific pronouns (like he or she) that made it clear who was being referred to. Then they were exposed to a series of 12 ambiguous sentences, which did not include pronouns that clearly differentiated who was being referred to. Doing so she was exposing them to a specific structure that referred to the second person in a sentence. The use of this method, priming, is to create a new different cognitive bias in minds. In this case Dr. Arnold primed people to be more likely to choose the second person to associate with the ambiguous pronouns.
“...language is a human capacity that doesn’t depend on modality.”
Her hypothesis was proven to be correct when participants’ cognitive bias shifted towards the second person. While participants were exposed to different unambiguous sentence structures over a short period of time, the effects of these structural patterns were still observed.
The language system is a complex system that can be manipulated subconsciously through exposure and experience. People retain only a small amount of what they hear on a daily basis consciously, but retain much more subconsciously. As a result, the discourse processing system experiences changes as it is shaped by statistical learning. Between the ages of six and seven, children begin to develop these discourse processing systems to recognize structures and make inferences about ambiguous structures. Even adults whose discourse processing systems are developed due to exposure to language over their lifetime are still susceptible to statistical learning. Dr. Arnold’s research in the field of psycholinguistics has shown that humans are constantly learning and adapting.
As humans adapt, language follows. The language that is spoken now is not the same as the language spoken 100 years ago. The future of language is not set in stone, but rather it is developed based on current events and social changes. It is exposure that promotes statistical learning, and this exposure is based on what is going on in the world. For example, the increasingly common use of the singular “they” was a result of the increasing importance of gender identity.
This has increased the use of the singular “they”, in both written and spoken language, in the past decade and has an impact on statistical learning. The use of the singular “they” is becoming more similar to the use of the pronouns he and she. Statistical learning of the singular “they” is heightened through exposure through media as well. The importance and influence of media has increased exponentially over the past few years and has in turn influenced language. While the exact changes that language system will experience cannot be predicted, the trajectory can be observed through research studies conducted by linguists like Dr. Arnold.

1. Mehl MR, Vazire S, Ramirez-Esparza N, Slatcher RB, Pennebaker JW. 2007. Are Women Really More Talkative Than Men? Science. 317(5834):82–82. doi:https://doi.org/10.1126/science.1139940.
2. Langlois VJ, Zerkle SA, Arnold JE. 2023. Does referential expectation guide both linguistic and social constraints on pronoun comprehension? Journal of Memory and Language. 129:104401. doi:https://doi.org/10.1016/j. jml.2022.104401.
3. Ye Y, Arnold JE. 2023. Learning the statistics of pronoun reference: By word or by category? Cognition. 239:105546. doi:https://doi. org/10.1016/j.cognition.2023.105546. [accessed 2024 Oct 10]. https://www.sciencedirect.com/science/article/pii/ S0010027723001804?via%3Dihub.
4. Interview with Dr. Jennifer Arnold PhD. 09/20/24.

By Hana Nakhle
Studying the effects of alcohol presents a unique challenge. Unlike other substances such as nicotine, alcohol affects many different areas of the brain, making its effects broad and complex.1 As a result, our understanding of alcohol’s impact—especially in cases of Alcohol Use Disorder (AUD)—remains limited. AUD is a chronic disease characterized by the loss of control over the ability to drink moderately. Approximately 29.5 million people aged 12 and over in the U.S. suffer from AUD.2 Despite its high prevalence, AUD treatment options remain few. Further compli-
cating alcohol research is the frequent coexistence of AUD with psychiatric conditions like anxiety and depression. Understanding the effects of chronic alcohol use in both women and men is crucial not only for unraveling the mechanisms of Alcohol Use Disorder, but also for exploring alcohol’s relationship with mental health disorders, as these effects may differ between the sexes.
to chronic alcohol intake. Historically, biomedical research has predominantly focused on male subjects.1 This trend extends to AUD research, despite recent increases in alcohol use among females. As Dr. Herman points out, “If we want to improve treatment, it is important that we include both sexes in the population.”


One researcher trying to piece together the puzzle is Dr. Melissa Herman, an associate professor at the University of North Carolina at Chapel Hill. Dr. Herman first earned her B.S. in human physiology from Boston University, then developed an interest in studying stress and the brain while working at the Salk Institute in San Diego. Dr. Herman then pursued a PhD at Georgetown University where she focused on brain circuits before going into her postdoctoral work at the Scripps Research Institute. Today, Dr. Herman works at the Department of Pharmacology and Bowles Center for Alcohol Studies at UNC-Chapel Hill, where her lab investigates the effects of alcohol and other pharmacological substances on brain activity and behavior.
In particular, Dr. Herman’s lab investigates sex differences in response
In a recent paper, Dr. Herman’s lab explored sex differences in basic motivation and anxiety, chronic alcohol drinking, and activity of the amygdala in male and female mice. The amygdala is a region of the brain involved in processing emotions, such as anxiety, making it a key area to study when exploring the relationship between alcohol consumption and emotional states. The study aimed to determine whether internal factors in mice, such as anxiety or motivation, lead to increased alcohol consumption, or if external factors such as drinking itself change motivation and anxiety leading to even more alcohol consumption.3
To investigate this, they measured baseline anxiety levels in male and female mice using the Novelty-Suppressed Feeding test (NSF) where the mice were food-deprived and then placed in a new environment with a Fruit Loop. In an unfamiliar environment, mice can feel anxious and


hesitate to eat the Fruit Loop. The time it takes for a mouse to approach and eat the Fruit Loop reflects its anxiety-level with longer times indicating greater anxiety.1 When these mice were then allowed to consume alcohol, the study
“If we want to improve treatment, it is important that we include both sexes in the population.”
found no association between anxiety levels and alcohol consumption in either male or female mice, suggesting that pre-existing anxiety does not predict increased alcohol consumption.3 Additionally, after six weeks of voluntary alcohol drinking, there were no significant differences in anxiety levels between the alcohol-consuming and control groups, indicating that chronic
drinking does not affect anxiety-like behavior. Interestingly however, females consistently consumed more alcohol than males.
Another component measured was motivated behavior. After undergoing the NSF test, mice were put back in their home environment and were each given ten minutes to eat a pre-weighed amount of Fruit Loop. A mouse’s motivation to feed was measured based off the weight of Fruit Loop consumed. Results of the study showed that females ate more Fruit Loop, thereby corresponding to greater motivated feeding behavior compared to males, which may explain females’ higher alcohol consumption.3


Figure 1. (H) Depiction of NSF post-test to assess motivated feeding behavior. (I) Post-test food consumption results in males vs. females. Adapted from Magee et al.
The study also examined changes in amygdala neuronal activity in response to alcohol intake. Dr. Herman’s team measured levels of a protein called cFos (cellular Fos), which rise when brain cells are activated. Although no significant sex differences in cFos production were observed following alcohol consumption, female mice who consumed alcohol for a prolonged time—an 8-week period—exhibited lower cFos production in the amygdala compared to female mice that consumed only water.3 These results suggest that chronic alcohol intake
might reduce brain activity concerning emotion in females.
Overall, the research showed that, while pre-existing anxiety did not increase alcohol consumption, female mice consistently consumed more alcohol than male mice, and exhibited greater motivated feeding behavior, which could be linked to their higher alcohol intake. In addition, chronic alcohol intake did not significantly impact anxiety levels, but did reduce emotional brain activity in female mice, as indicated by lower cFos production in the amygdala. These findings provide valuable insights that can inform future research and treatment approaches for Alcohol Use Disorders in both men and women.
Looking ahead, Dr. Herman expresses a fascination in the challenges of alcohol research. She emphasizes, “[T]o get a complete understanding (of alcohol’s effects) we need a holistic approach.” This approach highlights the necessity of exploring not just the amygdala, but also the long-term effects of chronic alcohol intake on other brain regions. By broadening the scope of research by examining sex-specific differences and brain regions, researchers can gain a more comprehensive understanding of how alcohol impacts behavior and the brain—bringing researchers one step closer to combating AUD in the United States.
1. Interview with Dr. Herman, Ph.D. 9/16/24
2. Nehring, S. M.; Chen, R. J.; Freeman, A. M. Alcohol Use Disorder. https://www.ncbi.nlm.nih.gov/books/ NBK436003/ (accessed November 6th, 2024).
3. Magee, S. N.; Sereno, A. C.; Herman, M. A. Alcohol 2024, 120, 85–97.

by Ambika Puri
Do words matter? Can a simple label deter or impact behavior when it comes to alcohol consumption? Do we even know what we are putting in our body? The answers to these questions are always subject to interpretation, but in a recent article published, these questions are put to the test. Our wonderful university is privileged to have one such professor who wants to ensure that labels not only have meaning, but also can serve a purpose in making society better and safer. The genesis of this article is based on a recent conversation with her.
Marissa G. Hall is an Assistant Professor at the University of North Carolina at Chapel Hill Department of Health Behavior and serves as a member of the Lineberger Comprehensive Cancer Center. She is a triple Tar Heel, as she earned her bachelor’s, master’s, and her doctoral PhD all from the University of North Carolina. Dr. Hall has conducted extensive research in labeling, advertising, and marketing. As a result of her extensive work with regards to food and tobacco labeling, she and her collaborator decided to investigate alcohol warnings as this topic has not had much research conducted on it.
"Alcohol is a known carcinogen that causes cancer with regards to 7 types of cancer [and] most Americans do not know that alcohol causes cancer.”
impact of alcohol warnings. There is a lot of research regarding tobacco and labeling, but not on alcohol”.1 The rationale on this is because there has been a strong push over the last 30 years to determine the impact of tobacco on the health of humans. As Dr. Hall points out in our interview, the warning labels on alcohol products have not been updated in over 30 years. These warning labels also do not follow current best practices as they are located on the back of packaging and usually in tiny font, so it is difficult to read. The team then was prompted to dive into a more extensive study on the effectiveness of these warning labels and if they impact behavior. According to the abstract of the publication, “Health warnings about alcohol consumption could inform consumers and discourage alcohol consumption, but little is known about what topics these warnings should address. We sought to identify promising topics for alcohol warnings.”2 In other words, various messages were produced for the participants to review and then their potential reaction was captured. 2,522 participants were involved in this all-online
Dr. Hall recently served as a coauthor on a study published in 2024, Health harms that discourage alcohol consumption: A randomized experiment of warning messages. This study was a pilot study, or an initial study, to provide a platform for a longer-term project. As Dr. Hall explains, this project stemmed from conversations with her collaborator, Dr. Anna Grummon of Stanford University. They both have worked extensively in the field of product labeling, but as Dr Hall quoted; “they realized how little research has been done on the

Dr. Marissa G. Hall

experiment. Electronic consent was received, and random messages were then shown to the participants. These messages were all based on health harms associated with alcohol consumption. These included items “like alcohol can cause liver disease, alcohol can cause heart disease, and alcohol can cause cancer.” Participants then viewed random messages that rotated between 7 topics. 6 of these topics were warning topics, such as liver disease or a type of cancer, and the 1 controlled topic was regarding recycling or reselling. The participants then read two statements for each topic and then rated the two statements and chose which statement was more effective in preventing them from consuming or overconsuming the alcohol product.
The experiment results came from 2,522 adults. Of these participants, approximately 67% were White,12% African American, 6% Latino, and 5% Asian.2 The average age was 44.3 years old. The results determined that the 6 warning topics had the highest effectiveness than the control topic. This led to a very interesting point that Dr. Hall then presented. Dr. Hall then later explained that these topics were selected to help educate the participants about the dangers of alcohol. They wanted to improve the warnings on alcohol and make them more effective. She even quoted that “Alcohol is a known carcinogen that causes cancer with regards to 7 types of cancer and that most Americans do not know that alcohol causes cancer”.1 The cancer aspect was especially interesting to Dr. Hall and Dr. Grummon. Many Americans know that alcohol can lead to impaired driving and is harmful if you are pregnant. but the potential for cancer is not something that many Americans know. This is in great contrast to tobacco warning labels that are well documented and now made to be very clear, so many Americans know that tobacco can cause multiple forms of cancer. Dr. Hall and her team want to use this opportunity to educate Americans about the association between alcohol and cancer. As shown in Figure 3, 6 of the top 8 effective messages had to relate to various types of cancer. This helped to validate the initial thought process of this team that the cancer messages would resonate higher with most people. This was an important facet of this study as previous warnings did not mention this.
This online study is just the first of a potential three- or four-year project for Dr. Hall and her team and helped establish a baseline of which types of warning labels are the most impactful to help deter alcohol consumption. The next step in the project is to eventually have a more practical experiment in which subjects will be able to go to a control lab, which is a real physical facility where experiments and studies are conducted, with a container and then a label would be applied to it. The subject would then go home for the evening and any amount of alcohol consumed by them would be measured the next day. These labels would then be applied on a rotating basis and the effectiveness of the warning labels would then be measured.
This study is anticipated to serve as a national study in leading the way to changing how warning labels are placed on alcoholic products in the future. The hope of this study is to help lead the way in educating the public on additional negative impacts of alcohol consumption including various cancers and heart diseases. The overall goal is to educate society about the harms that can come from alcohol and to make these harms known in a much more open setting. The education of the public may be the single greatest outcome of this study. This study will help to show how various other health issues, such as cancer and heart disease can be attributed to alcoholic consumption. Over the last forty or so years, the efforts to show the harms of tobacco have been highly effective and many policy and legal changes have come from those efforts. The goal of this new study is to one day put the harms of alcohol consumption on the same level as tobacco and for the public at large to be able to know its impact.

2. This image shows which warning messages were most effective to the study participants. Provided by Dr. Hall’s Presentation.
1. Interview with Marissa G. Hall, PhD. 09/20/2024.
2. Grummon, A; Lee, C; Campos, A; Whitesell, C; Brewer, N; Lazard, A; Greenfield, T; Hall, M. Elsevier. 2024, 159. 1-9.
3. Hall, M. Health harms that discourage alcohol consumption: A randomized experiment. Alcohol Policy Conference, Alcohol Policy 20, 2024.
By Matthew Rodzen

Astrocytes are the most abundant cells in the human brain. They are vital to the homeostasis, development, and regulation of the central nervous system (CNS). These intricate and large cells were once dismissed as secondary to neurons, but current research is trying to elucidate the vital roles they play. Since no two astrocytes are exactly the same, they form very complex shapes interacting with neurons and even with one another. Understanding what gives rise to these morphological differences may be the key to understanding their complicated relationships with neurons and other cells in the CNS.

Astrocytes have the potential to unveil our brains’ underlying mechanisms and help us further understand this vastly complex organ.
the course of his research. “Most gene knockdowns at the time didn’t alter astrocyte morphologies very dramatically”.1 After finding Tre1 in Drosophila, he ventured out to find it’s analogue in zebrafish. Using a gene editing tools knowns as CRISPR, he was able to modify the zebrafish genome to elucidate this Tre1 analogue: S1pr1.
Dr. Jiakun Chen, PhD, a recent newcomer to the University of North Carolina at Chapel Hill, hopes to continue making monumental discoveries in the field of astrocyte research at this university. His passion for astrocyte research evolved out of his studies with glial cells in zebrafish. Glia are a category of cells other than neurons that carry out homeostatic processes. “At the time, researchers weren’t sure if there were astrocytes in zebrafish”.1 He took on this challenge and established that zebrafish did, in fact, have bona fide astrocytes that could also be used for research purposes. Dr. Chen collaborated with colleagues to discover Tre1, a gene that would change
It is a common strategy in genetics research to break some things (ie. make genomic edits) to find the function of their downstream proteins. Dr. Chen tried knocking out, or inactivating, both the Tre1 and S1pr1 genes to see what would happen when the proteins they encode are no longer produced. What Dr. Chen found was that fruit flies and zebrafish with Tre1 and S1pr1 knockout had significantly less complex astrocyte morphologies. That is, they had fewer and smaller processes extending from their cell bodies.

The process taken to reach Dr. Chen’s conclusions involves a variety of complex processes. First, fruit flies with the Tre1 knockdown were dissected. Their tiny CNS were removed with precision and prepared for imaging. This included staining the astrocytes and neurons with various antibodies to allow for a clearer understanding of their relationships to other cells under the microscope. These images were then processed to quantify the volume that astrocytes took up in the various strains. Zebrafish, however, are transparent, so their CNS can be imaged in vivo or while the organism is alive.
Astrocytes can also be imaged on the single-cell level. A single astrocyte expresses a fluorescent protein that makes it exhibit a certain color while those around it exhibit another. This allows the researcher to distinguish between astrocytes to perform comparisons. Dr. Chen found that Tre1 knockout led to simplified astrocyte morphologies that had less branching. Rac1, an important regulator in cell signaling and cytoskeletal structure, was also found to further simplify astrocyte branching, suggesting a possible relationship between the two genes. These diminished morphologies had behavioral effects on the organisms. Fruit flies were found to have significant climbing defects, and zebrafish had deficiencies in sensory processing and posture.
Many new emerging technologies in imaging are exciting in the field of astrocyte research. Dr. Chen is enthusiastic about the discoveries that may come with wholebrain imaging, a technique used to visualize cell activity in 3D. This advancement would allow for further quantification and let Dr. Chen see nuances in astrocyte morphologies and activities, empowering him to study new hypotheses regarding what is vital to their function.
Dr. Chen is surrounded by many wonderful colleagues who also conduct research in the world of neurobiology. For example, Dr. Hige studies the electrophysiology of neurons and its relation to their plasticity. Dr. Chen believes that collaborating with the Hige Lab will allow him to further understand how different astrocyte morphologies impact

neural circuity since astrocytes are also often connected to neural synapses. He is excited about the future because the field of astrocyte research is so new, and being surrounded by people who study related topics brings the possibility of making huge leaps in the field.
Astrocytes have the potential to unveil the brain’s underlying mechanisms and help further understand this vastly complex organ. It is an exciting time to be researching these cells, given that so much neuronal research has led to the development of techniques that can be applied to exactly this kind of research. By better understanding the development and morphologies of these unique and beautiful cells, we may come to understand our own brains better.

References
1. Interview with Jiakun Chen, Ph.D. 10/9/24

Adolescence, typically spanning ages ten through 19, is a period characterized by risk-taking behaviors—some enjoyable, others regrettable, and some capable of permanently altering motivated behaviors in adulthood. One significant risk during this developmental phase is alcohol consumption which can profoundly affect brain development, particularly in regions responsible for decision-making and behavioral flexibility. The prefrontal cortex (PFC), located at the front of the brain’s frontal lobe, plays a crucial role in higher cognitive functions such as decision-making, behavior regulation, and working memory.1 As the PFC matures, the capacity for sound, voluntary decision-making often relies on emotional stability—a balance that binge drinking can severely disrupt. To understand the consequences of adolescent binge drinking on voluntary behavior and learning mechanisms, the underlying brain changes must be explored.
Through her tenure, Dr. Donita Robinson has increasingly become interested in how alcohol exposure impacts the brain. Her desire to comprehend the broad consequences of alcohol use and its long-term effects on decision-making has fueled her research. Now, as the principal investigator in the Robinson Behavioral and Pharmacological Neurodynamics Lab at the University of North Carolina at Chapel Hill, she employs animal models to investigate the neurochemical changes associated with adolescent binge drinking and their impacts on motivated behavior in adulthood.

By Risha Solanki
The Robinson Lab has shown that alcohol exposure in adolescent rats leads to long-lasting changes into adulthood. For instance, imagine an adolescent who binge drinks during the critical period of prefrontal cortex development (roughly 12 to 25 years old) and then stops upon reaching adulthood. Neurocircuitry, involving brain regions such as the basal ganglia, plays a role in controlling actions by initiating voluntary movement and actively interacting with the PFC to manage behaviors and responses.2 Alcohol use can disrupt these neural circuits and Dr. Robinson aims to uncover the long-term consequences of these disruptions in brain function.
A central concept in Dr. Robinson’s research is behavioral flexibility—the ability to adapt behavior when circumstances change. Adolescents undergo significant brain development, especially in the PFC, which is essential for topdown control and conscious decision-making.1 Top-down control and conscious decision-making are both mechanisms that allow for the application of reasoning when making decisions. Although the brain’s wiring is established during adolescence, its efficiency is still developing and emotional events that impact adolescent mental health can disrupt this decision-making process.
In a recent paper, “Effects of Adolescent Intermittent Ethanol Exposure on Cortical Perineuronal Net and Parvalbumin Expression in Adulthood Mediate Behavioral Inflexibility,” Dr. Robinson and her team used animal models to mimic adolescent intermittent ethanol (AIE) exposure and examine the resulting neurochemical changes.3 Although these animal models do not perfectly replicate human behavior, they provide valuable insights into how similar alcohol exposures can lead to long-term behavioral changes and identify which brain regions are most affected. A primary technique for studying the effects of alcohol exposure is functional magnetic resonance imaging (fMRI), which allows researchers to observe brain connectivity by detecting changes in blood flow in living animals and understand interactions between different brain regions during specific behaviors.4 To explore any structural brain changes in greater detail, the lab sliced brain tissue to visualize perineuronal nets using antibodies, a laboratory method known as immunohistochemistry.
Dr. Robinson’s team aimed to measure the number and size of parvalbumin-expressing (PV+) interneurons and associated perineuronal nets (PNNs) within the cortex of female and male rats following AIE or control exposure and subsequent training on an attentional set-shift task (ASST). PNNs and PV+ interneurons are critical components of brain function, regulating brain activity and neural connection strength between brain regions. PNNs are matrix-like structures that stabilize neuron function by wrapping over

Figure 1. Representative immunohistological image showing perineuronal nets (green) and parvalbumin+ cells (red) from a rat. Provided by the Robinson Lab.
them, making them less plastic and more rigid (Figure 2). PV+ interneurons, inhibitory neurons primarily found in the cortex and hippocampus, also play an essential role.3 The research utilized ASSTs to explore behavioral flexibility.2 In these tasks, rats are challenged to shift behavioral responses to stimuli based on changing rules.
The results from the ASST revealed important distinctions between alcohol-exposed (AIE) and control rats across different task phases. The rats that underwent AIE exposure showed significantly more errors during the reversal of a learned stimulus response compared to the control group. This finding led the lab to look at the neurochemical changes present, particularly in the Anterior Insular Cortex (AIC), Infralimbic Cortex (IL), Prelimbic Cortex (PC), and Orbitofrontal Cortex (OFC). Immunohistochemistry showed no notable differences in PNN and PV+ count and size in the IL and PL. However, there was a notable increase in the total PNN count and a decrease in size on average for PV+ cells in the AIC.3 Additionally, the OFC experienced an increase in PNN size, but there was no change in the total PNN count.3 Next, Dr. Robinson’s team ran an analysis to see whether the change in neurochemistry led to the change in behavior. A mediation analysis revealed a connection with the neurochemistry in the AIC and alcohol induced reversal learning deficits.3 This
suggests that AIE exposure not only affects the cortex, but how. Ultimately, the lab’s findings indicated that AIE exposure increases PNNs in certain brain areas, which reduces the flexibility of the brain’s neural circuits.3
The study’s results were clear: adolescent intermittent ethanol exposure led to significant deficits in behavioral flexibility. While the animals could perform tasks involving learning, they struggled when required to change strategies—a difficulty known as perseveration, which is also seen in humans facing substance use disorders.2 A prior study from the lab found that the animals’ difficulty in switching behaviors correlated with altered brain connectivity.1 The fMRI showed that alcohol-exposed animals had reduced connectivity between key brain regions, such as the OFC, PC, and IL—all essential for decision-making and behavior regulation.1 These regions connect to the basal ganglia and hippocampus, structures that are crucial for learning and memory. Currently, the lab is investigating how increased PNN numbers in alcohol-exposed animals correlates with changes in functional connectivity MRI in brain regions related to decision-making and behavioral flexibility.
“Adolescent intermittent ethanol exposure led to significant deficits in behavioral flexibility.”
By examining the severity of adolescent binge drinking on brain development, Dr. Robinson’s research underscores the need for increased awareness about the risks associated with alcohol consumption during critical developmental periods. Understanding the neurochemical changes linked to alcohol exposure may lead to targeted intervention programs aimed at enhancing cognitive flexibility and decision-making skills in adolescents, ultimately helping to mitigate the adverse effects of early alcohol use. With a better understanding of how greatly binge drinking impacts behavioral flexibility, more personalized therapies can be built to support withdrawal symptoms and the several other consequences of adolescent binge drinking.
1. Gómez-A A; Dannenhoffer CA; Elton A; Lee S-H; Ban W; Shih Y-YI; Boettiger CA; Robinson DL. Front. Pharmacol. 2021, 12, 778884.
2. Interview with Dr. Donita Robinson, Associate Dean for Graduate Education at University of North Carolina at Chapel Hill. 9/20/24.
3. Sullivan EDK; Dannenhoffer CA; Sutherland EB; Vidrascu EM; Gómez-A A; Boettiger CA; Robinson DL. Alcohol: Clin. Exp. Res. 2024, 48(8), 1507-1518.
4. Broadwater MA; Lee S-H; Yu Y; Zhu H; Crews FT; Robinson DL; Shih Y-YI. Addict. Biol. 2018, 23(2), 810–823.
By Natalie Travis

Quantitative psychology is the combination of mathematical and statistical strategies to measure and model human behavior. It can be applied, most often in research and assessments, to all fields of psychology such as educational, social, or clinical. People encounter quantitative methods in their everyday lives. For example, grade point average (GPA) is a numerical reflection of academic performance. A 3.48 represents all of the hours of classes taken and the weight of each grade to measure academic performance. Dr. Patrick Curran and his co-host Dr. Greg Hancock run an entertaining and informative podcast called Quantitude on YouTube and Spotify where listeners can learn more about “all things quantitative, ranging from the relevant to the highly irrelevant.”
Dr. Patrick Curran’s interest in Quantitative Psychology, with a focus in Child Clinical Psychology, began as somewhat of an accident. While earning his PhD in Clinical Psychology from Arizona State University, he began to wonder how one measures and models behavior while accounting for all the factors that influence it. His curiosity led
him to pursue a postdoctoral fellowship in applied statistics at The University of California, Los Angeles. He served as director of the Thurstone Lab and the Quantitative Psychology program from 2008 to 2020. Now, Dr. Curran is in his 25th year at the University of North Carolina at Chapel Hill. In his research, Dr. Patrick Curran has taken on the formidable challenge of studying “the measurement and modeling of human behavior over time.”
Some behavioral traits are difficult to measure by self-reporting, especially in the case of children because they often have more difficulty communicating than adults. To combat this, psychologists will use multiple reporter data, gathering responses from other people around the subject to assess their behavior. Multiple reporter data can be challenging to analyze and understand because human behavior differs in different contexts and not everyone exhibits symptoms of the same underlying cause the same way. Thus, there are difficulties in accounting for variability when scoring, the process of assigning numerical values to data in order to interpret it, like how GPA is a numerical value that represents
academic performance.
Imagine a child named Peter is brought in for a psychological analysis because his parents are concerned about his mental health and they think he might have anxiety. In this case, Peter’s anxiety is called the Latent Trait, something that cannot be




Figure 2. This figure shows how the trifactor model analyzes information from multiple reporters. Provided by Dr. Patrick Curran.
directly measured but is discovered by a combination of behaviors. The psychologist then sends a questionnaire about his behavior to three reporters, his mother, babysitter, and teacher. The questionnaire contains 20 items and the reporters respond on a scale from one to five, “I strongly disagree” to “I strongly agree.” Once receiving the data, the psychologist could take an average by adding up each reporter’s answers and dividing them by 20 to present a score that is supposed to reflect the Latent Trait, Peter’s anxiety. However, this method does not fully reflect the complexities of human behavior because it makes two flawed assumptions.
The first is the assumption that all items in the assessment are equally related. For example, if one question is “Does Peter often seem worried or on edge?” and another is “Have you noticed Peter harm himself?”, an average of the data will represent the two questions as having the same weight but they tell very different things about Peter’s mental state. The first suggests that he might be thinking negative thoughts that are causing him to make worried expressions, and the second suggests that he may be a possible danger to himself.
The second assumption made by this method of scoring suggests that all
the questions will accurately reflect what Peter’s behavior suggests about his mental state. For example, one question is “Does Peter often look tired or have difficulty sleeping?,” and another is, “Does Peter often talk about being worried about things?.” Assumptions like these do not account for the fact that people can exhibit different behaviors for the same underlying reason. For example, some people with anxiety spend time worrying about things, others have trouble sleeping, and sometimes people have both. If Peter’s psychologist were to measure these scores as equal, they would not be getting a clear, wellrounded picture. If done with proper statistical tools, the psychologist should be able to account for this variance and get a full picture of Peter’s anxiety. Accounting for variability in multiple reporter data of human behavior is the challenge Dr. Curran and his colleagues have undertaken, leading to the creation of a new statistical tool: The Tri-Factor Model. The Tri-Factor Model, the original idea of Dr. Dan Bauer, allows psychologists to better interpret multiple reporter data by accounting for three main sources of variance, a Common factor, InformantSpecific Factors, and Residual Variance. The Common Factor in Peter’s case is his behavior, as all the reporters are
reporting on Peter’s behavior. The model assumes that his behavior is influenced by his anxiety, the Latent Trait. The Informant-Specific Factors represent differences in how reporters may describe Peter’s behavior. For example, the question “Does Peter pay attention?” will likely elicit different answers from his teacher or his parents. Peter may be attentive in class but seem spaced out and slow to answer questions at home. Thus, Peter’s behavior may be misunderstood if measured as one variable of “attention” without accounting for these differences. The model also accounts for Residual Error, random error that occurs that cannot be explained by the Common or Informant-Specific Factors. It is vital for clinical psychologists to interpret behavioral data accurately, and the TriFactor Model allows them to properly understand and treat their patients.
The term “Quantitative Psychology” can be daunting to many, but Dr. Curran believes it is not as scary as an introductory statistics class may make it seem as it is a creative and promising field with a lot to learn. As Dr. Curran and his team have done with the Tri-Factor Model, there are evergrowing ways to improve the way data is analyzed. By accounting for all possible variables, psychologists can better understand their patients. Nevertheless, there is a lot of work to be done and the field needs more inspired young people to chip in.
1. What is quantitative psychology?. Best Psychology Degrees | Your guide to top Psychology Degree Programs. . https://www.bestpsychologydegrees. com/faq/what-is- quantitative-psychology/(accessed 10/,10/,2024)
2. Interview with Dr. Patrick Curran
3. Bauer, D. J., Howard, A. L., Baldasaro, R. E., Curran, P. J., Hussong, A. M., Chassin, L., & Zucker, R. A. Psychological Methods 2013. A trifactor model for integrating ratings across multiple informants. Psychological methods, 18(4), 475–493. https://doi. org/10.1037/a0032475
4. https://stock.adobe.com/images/ sad-blond-boy-sitting-on-stairs-incorridor-of-school/273877419?prev_ url=detail

By: Erin Atos

Animal hybrids are known for their unique blend of features due to their unlikely parentage. Most animals tend to breed within their species, but the process of hybridization — the breeding of two different species — can lead to some intriguing offspring. Notable examples include the liger, bred from a lion and a tiger, and mule, a horse and donkey hybrid. What prevents these animal hybrids from being as prolific as their parent species is that most hybrids are sterile and cannot reproduce. This is not beneficial in terms of ecological fitness, which is an individual’s ability to reproduce. Nonetheless, there are some unique circumstances where hybridization occurs and appears to be advantageous. As species come into closer contact with each other, hybridization can be used as a tool to introduce genetic variation into small populations or increase fitness by sharing genes that allow hybrid generations to adapt to novel environments. Dr. Karin Pfennig, an ecologist and biology professor at the University of North Carolina at Chapel Hill, examines how behavior impacts biodiversity, ecology, and evolution. Through her
work with spadefoot toads, her lab studies the lesser-known advantages of hybridization and its genetic consequences.
Dr. Pfennig has a biology heavy background starting with her undergraduate degree in ecology, behavior, and evolution from the University of California at San Diego. Additionally, she earned her Ph.D. in Biology at the University of Illinois. As an ecologist, Dr. Pfennig is mainly interested in the impact of behavior on genetics and, consequently, evolution.
One such behavior that greatly impacts hybridization is adaptive mate choice, the process in which organisms select mates that would give their offspring the greatest chance of survival and reproduction. By looking closely at the breeding habits of the female plains spadefoot toad (Spea bombifrons) and the Mexican spadefoot toad (Spea multiplicata), Dr. Pfennig determined that when female plains spadefoot chose to mate with male Mexican spadefoot, their offsprings’ chances of survival increased.1
Despite their nomenclature, they are, in fact, frogs. Normally, the plains spadefoot would reproduce within their species. This behavior changes at the desert-like edge of their territory where the frogs are faced with a dilemma: shallow ponds that dry up before their tadpoles can mature. To investigate this, Dr. Pfennig’s team conducted lab experiments to witness the spadefoot frog’s approach firsthand.
Faced with undesirable pond options, the team discovered that a female plains spadefoot can evaluate the

water levels, and based on the water level, it can change their preference for the males they intend to mate with. The resulting hybridized spadefoot develops faster and matures before the pond dries.2 A second factor that contributed to mate choice was mate call. To test this, various hybrid tadpoles were reared in lab and compared for their body size, mass, and developmental phase. As seen in Figure 2., because males with slower mate calls, pulses, exhibited a positive correlation with tadpole fitness, slower pulse rates were the most accurate predictor of fitness.
In conjunction with tadpole rearing, behavioral experiments were conducted to observe how female spadefoot from different habitats reacted to the different mate calls. It was found that the spadefoot females did not differentiate between males with slow pulse rates versus fast pulse rates when they were accustomed to deeper water— an environment where hybridization would be less beneficial (P=0.15).1 When the spadefoot females were from an environment which benefitted hybridization, then they began to favor the males outside of their species with slower pulse rates that would result in fitter offspring (P=0.0026).1 In data analysis, p-values less than 0.05 are statistically significant values and are likely not due to chance. In this case, preference for slow mate calls was not statistically significant in deep water but was statistically significant in shallow water. This shows that the plains spadefoot toads were able to differentiate between males of a different species, and their different mating calls.
This benefit of hybridization comes with a caveat: sterility. At this time, the causes of sterility through hybridization are currently unknown and are still being investigated. While it may seem like hybridization works to the detriment of the resulting hybrid offspring, it is still more beneficial to produce a generation with reduced fertility than having no offspring at all.
Consequently, hybridization increases the amount of genetic information shared between populations, known as
gene flow, and promotes genetic variation. Greater amounts of genetic variation allow for a wide array of traits that may persist despite changes in environment, such as breeding in desert areas. How the genes have impacted the next generation of spadefoot is still being investigated. Some traits have yet to flow into the other species and remain distinct. One such trait that differs between the species is the measure of how exploratory an individual is.
Exploration describes how an organism moves through its environment, particularly in search of resources—such as food. This trait contributes to dispersal, or the movement to different habitats outside of its familiar range. The exploratory behavior of the hybrid spadefoot is still unknown in comparison to their parents. If these hybrid frogs were more prone to exploration, it is possible that they can expand their habitat and successfully adapt to new environments farther their point of origin.
In the context of evolutionary change, hybridization may potentially play an increasingly large role in natural selection and what traits pass on. Dr. Pfennig expresses that, “Hybridization is the source of much more ecological and evolutionary opportunity than we have historically realized”.2 However, this process must maintain a delicate balance. Hybridization has an equally likely chance of decreasing biodiversity by collapsing species via inviability, producing offspring unfit for survival, and infertility, an inability to conceive, replacing species, or even losing the distinct traits the differentiate species from one another.
“‘Hybridization is the source of much more ecological and evolutionary opportunity than we have historically realized.’”
The unexpected advantages of hybridization have given rise to a more fit generation of spadefoot toads. This phenomenon highlights the resilience of nature and its ability to promote survival and adaptability. As seen through the success of the hybrid spadefoot, it is evident that organisms and the changing environment can evolve in tandem and thrive in the face of biological adversity.
References
1. Interview with Dr. Pfennig
2. Chen C, Pfennig KS. 2020. Female toads engaging in adaptive hybridization prefer high-quality heterospecifics as mates. Science. 367(6484):1377–1379. doi:10.1126/science. aaz5109.
Image courtesy of Adobe Stock

By Brantley Aycock
ndustrial processes like burning fossil fuels for electricity, heat, and transportation generate greenhouse gasses. These gasses trap heat in the atmosphere. As a result of global warming, ocean temperatures rise. This warming affects ectothermic, or cold-blooded, animals that rely on their environment to regulate their body temperature. Many marine animals, like fish and invertebrates, are cold-blooded.
Marine ecologist Dr. John Bruno studies the effects of climate change on marine organisms. He earned his Ph.D. in Ecology and Evolutionary Biology at Brown University and is now a professor at the University of North Carolina’s Department of Biology. His work aims to relate the field of ecology to reverse engineering, especially as the focus of much research shifts to mitigating detrimental human impacts on marine life. “Time and again, it turns out you have to understand how the system works in order to repair it”.1 Ecology studies both how an organism is affected by its environment as well as how an organism affects its environment, which is why Dr. Bruno believes it is important to investigate the human contributions to climate change.1
It was his natural curiosity and love for marine life that inspired Dr. Bruno to become a marine ecologist.1 Growing up on an estuary in Florida, he loved camping and often participated in conservation efforts. Dr. Bruno attributes his continued passion for the field to his love for being outside, discovering the results of his data, and teaching students. Graduate students Isabel Silva and Esteban Agudo do the heavy-lifting in Dr. Bruno’s lab, which studies predator and prey relationships as well as the roles of foundation species, or species that provide a habitat for other organisms in the same ecosystem.1
Dr. Bruno has done research in the Caribbean, the
Galapagos, and, most recently, Little Cayman Island.1 Despite all of them harboring marine life, Dr. Bruno points out that these ecosystems are completely different from one another. While the shores of Belize lack large predators due to overfishing, the shores of the Galapagos house a productive ecosystem. Although the Galapagos are equatorial islands, their shores harbor cold water coming up from the Southern Ocean. Upwelling, the process of cold water currents rising from the bottom of the ocean, carries nutrients to the surface that help marine plants grow. More marine plants means more food for marine life all the way up the food chain, allowing predators to flourish. “Even from a boat, you can see the water is often darker because there’s more food in the water,” Dr. Bruno describes. “There’s whales jumping, there’s sea lions on the beach, there’s boobies diving into the water, there’s orcas.”1
Dr. Bruno’s research often surprises him.1 In one study, his team caught young sharks off the Gulf Coast and collected fecal samples before releasing them. By sequencing the DNA found in the sharks’ feces, the researchers were able to determine the sharks’ diets. They were found to be

unexpectedly diverse.1
Dr. Bruno’s recent research explores the temperature tolerance of marine life.1 On Little Cayman Island, he and his team collected ectothermic invertebrates and placed them in aquariums. They measured the organisms’ heart rate, respiratory rate, and other metabolic rates as they increased the temperature of the aquarium. Metabolic processes are the continuous chemical reactions that take place in an organism to maintain normal functioning.1 Knowing that heat is a carrier of energy, when cold-blooded organisms are exposed to higher temperatures, they gain energy and their metabolic processes speed up.1 Therefore, metabolic rates of the collected ectothermic organisms were found to initially increase, causing them to move faster. However, when the temperature increased beyond a point of toleration, the organisms slowed down and ultimately died.1
Dr. Bruno said the biggest takeaway from his research is the hidden effects of global warming.1 In the past, Dr. Bruno’s work focused on rates of coral bleaching, which is an easily observable effect like terrestrial wildfires and droughts. Coral is a foundation species, so coral loss affects the fish and other organisms that rely on the coral to be their home. Coral bleaching is such a visually drastic and widespread event that it can be seen from space. In contrast, Dr. Bruno’s current work focuses on the internal mechanisms of marine life that are affected by climate change.1

“There’s still so much to protect and preserve and to enjoy.”
As metabolic rates increase with temperature and marine life begins to move faster, predators eat more prey in an effort to keep up.1 As predators eat more, their prey runs out, leading to starvation. Even warm-blooded animals such as seabirds are affected. According to Dr. Bruno, these metabolic effects are more difficult to measure than coral bleaching, but starvation is one of the biggest ways climate change impacts marine species. He plans to continue his research on Little Cayman Island to further understand these impacts.1
Nutrients, human disturbances, and invasive species also contribute to the speeding up of marine ecosystems.2

Increased agricultural activity leads to more nutrients like phosphorus and nitrogen ending up in waterways. The smaller, faster organisms that feed on these nutrients then take up a greater proportion of energy, disrupting the balance of food webs. This can lead to a reduced food supply for larger animals. Human disturbances like fishing remove the older marine animals from the ecosystem, leaving the younger and faster generations. New invasive species, often weeds, become quickly growing competitors to other organisms in the ecosystem. As the metabolic rate of marine ecosystems accelerates, we will have to keep up to maintain our oceans as a resource.2
Despite his first-hand experience with the detriments of global warming, Dr. Bruno remains optimistic.1 He is inspired by the Sunrise Movement and other motivated young people, and he is encouraged by technological advances in clean energy. “There’s going to be a fair amount of loss, but I don’t think it means human extinction or that nothing’s worth saving anymore,” he said. “There’s still so much to protect and preserve and to enjoy.”1
References
1. Interview with Dr. John Bruno, Ph.D. 09/22/2024.
2. Johnston, E. L.; Clark, G. F.; Bruno, J.F. Climate Change Ecology. 2022.
By Zenon Kuropas

There are few places more well known in the world of biology than the Galapagos Islands. This is the location in which Charles Darwin visited and had his epiphany, later published as the theory of evolution in On the Origin of Species. It is also where a high number of species found nowhere else in the world live, including: 1,200 terrestrial invertebrates, 250 vascular plants, 86 species of fish, 28 birds, and 20 reptiles, among which are the famous Galapagos Giant Tortoise.1 But now, the Galapagos ecosystem that led to one of the greatest

scientific revelations of our time faces a looming threat — invasive species.
Dr. María de Lourdes Torres, a professor at the Universidad San Francisco de Quito in Ecuador, spearheads invasive species research, with recent work focusing on the Galapagos Islands in relation to trees. Dr. Torres earned a PhD in Biology with a focus on Plant Molecular Biology from the Free University in Berlin, Germany, where she started the biotechnology department at Universidad San Francisco de Quito (the only university in the world to have a campus on the Galapagos), and she has directed the Plant Biotechnology lab there for over a decade.
Dr. Torres’ most recent work focuses specifically on one type of tree that has become an increasing problem on the islands — Cedrela odorata, commonly known as Spanish Cedar. Spanish Cedar is native across South America, ranging from Mexico, along the Caribbean, and stretching into northern Argentina. On the mainland, over-exploitation of lumber has caused it to be classified as a vulnerable species. However, on the Galapagos islands it has become the second most invasive tree species threatening the ecosystem.
Invasive species, a globally investigated issue, are non-native species that have been introduced to regions and they can quickly upset the delicate balance of the established ecosystem, overrunning and outcompeting native organisms
and threatening extinction of many species. North Carolina residents may be well acquainted with kudzu, a creeping vine introduced from East Asia, which has since overrun much of the terrain. A similar effect is occurring in Galapagos in which Spanish Cedar is on track to destroy the ecosystem, even reducing one native genus of tree, Scalesia, to a miniscule 1% of its original population on the island.
Dr. Torres’ work aims at understanding genetic diversity and population dynamics, especially in the regions of Ecuador where she works. She studies the genome, comprising all the genetic information contained within organisms through DNA which dictates cells’ function and operations. DNA is made of thousands of genes, which determine every function of every cell. Understanding how genes vary across species and individuals within species (genetic diversity) helps to understand how groups within species may have evolved or how they currently interact with each other (population dynamics). Dr. Torres’ recent work has aimed at studying the genome of Spanish Cedar to investigate how the tree has spread across the islands. Understanding how species like Spanish Cedar spread can play a key role in containing further damage, especially since current measures of containment are

limited to digging up the harmful trees by hand. The objective of Dr. Torres’ work was understanding the evolution of the genome within Spanish Cedar. DNA naturally mutates accumulating small random changes over time as it is used and duplicated within each cell. Under this principle, a collection of organisms that have been in the same area for a long time is expected to have more differences in their DNA than a population that recently moved to a new area, due to the new population not having the necessary time to diversify their genome. Using this concept, Torres and her team can infer which of the Galapagos islands the Spanish Cedar trees first inhabited and which of the islands the trees spread to later.
The results of the research showed that the islands San Cristobal and/or Santa Cruz, the two easternmost islands and the closest to the Ecuadorian coastline, were the first islands exposed to Spanish Cedar seeds in the 1940’s or 50’s. From there, the species likely spread westward to Isabela and Floreana (Figure 2).2 Of course, as with any research targeting phylogenies — the change in organism’s genes over generations — there are degrees of uncertainty. However, any additional data that can be gleaned is helpful to restrict the further spread of the invasive tree, as well as being able to implement preventive measures for other invasive species in the future.
Of course, Torres’ work did not come without setbacks. To conduct research on the Galapagos islands, because it is a national park and heavily protected, permits are required. Torres describes the permit process as long, drastically slowing any attempt to conduct research such as hers on the islands. Additionally, materials can be harder to get in Ecuador. Dr. Torres specifically mentioned that obtaining primers, an important tool for the genetic analysis conducted in her work, was unpredictable. In the United States, primers can be bought and received almost overnight, while in Ecuador shipping often takes weeks or months. However, Torres and her team continue to work through these delays and conduct vital research on both the Galapagos and mainland Ecuador.
With the research that Dr. Torres and others have conducted perhaps there can be a slowing of the spread of Spanish Ceder, and a brighter future for the Galapagos ecosystem. The islands contain species that can’t be found anywhere else in the world and hold a position of neigh unparalleled importance in the biology community, not only for the research currently being undergone but also the research of the past, dating back to the days of Darwin and his great discovery.
1. Burdette, C. Galapagos Islands, off the coast of Ecuador. https://www.worldwildlife.org/ecoregions/nt1307 (accessed November 2nd, 2024)
2. Albuja-Quintana M.; Rivas-Torres, G.; Rojas López, K. E.; Asadobay, P.; Palacios Cuenca, W.; Vinueza, G.; & Torres, M. L. Ecol. Evol. 2024, 14, e11723
3. Interview with María de Lourdes Torres, PhD. 9/26/2024
By Julia Sallean

In modern day society, staying healthy isn’t easy. Heart disease has been the leading cause of death in the U.S for several decades, with more and more Americans falling victim to obesity, diabetes, and metabolic syndrome with each passing year. For many people, any recommendation on how to improve their lifestyle is a welcome one. While there can be no doubt that a healthy diet and exercise are incredibly important factors in maintaining overall health, research indicates that there’s also hope for improving health and metabolism through the use of a beverage called

Though it has experienced a surge in popularity recently, Kombucha has existed for at least two thousand years and originated in China during the Qin Dynasty. This slightly sweet beverage is a mixture of fermented sweet tea with bacteria and yeast cultures, and has long been associated with many health benefits. One of those benefits may be that Kombucha plays a role in assisting metabolism, although this claim had never been rigorously tested in a lab setting.
It was Rachel DuMez-Kornegay, a graduate student at the Dowen Lab at The University of North Carolina at Chapel Hill, who first decided to test the effect of Kombucha in C. elegans worms (Figure 1). Dr. Rob Dowen, who had previously studied plant-pathogen relationships while earning his PhD from the University of California San Diego, was also eager to return to his roots with host-pathogen interactions. C. elegans worms have a relatively simple digestive system, and the bacteria within Kombucha can colonize the intestinal lumen (Figure 2). This makes these bacterivores ideal candidates for studying metabolism. Furthermore, at the molecular level, the way that fats are stored and broken down in C. elegans is a highly conserved process across many species.
In order to make sure that C.
elegans worms raised on a mixture of KTMs (Kombucha Tea-associated microbes) exhibited normal eating habits, the feeding and developmental processes of these organisms were monitored.2 Since KTM-fed worms developed normally, were fertile, and exhibited similar swallowing rates while feeding compared to regular E. coli-fed worms, it was determined that these organisms could ingest Kombuchaassociated microbes exclusively in their diet without reducing caloric intake. Next, C. elegans worms were placed on a KTM “lawn”, or a small plate containing microbes found in any Kombucha brew (Figure 3). Their metabolism was then monitored through lipid staining, a process that stains the triglycerides

Figure 1. An electron scanning micrograph of microbes colonizing the gut of C.elegans. Image courtesy of the Dowen Lab
in lipid droplets that primarily store fat within the C. elegans’ intestine. By tracking triglyceride levels in this way, fat storage of these organisms can be accurately quantified.
Through lipid staining and biochemical analyses, the Dowen Lab discovered that C. elegans worms consuming Kombucha microbes exhibited an approximate 85%95% decrease in triglyceride levels as compared to organisms fed a normal diet.3 Additionally, both the size and abundance of lipid droplets in the intestine of these worms were significantly lowered as a result of Kombucha intake. This suggests that KTM consumption may stimulate a “fasting-like” state that enhances the breakdown of lipids such as triglycerides.
According to Rachel DuMez-Kornegay, “Preliminary results suggest that this fasting-like state likely leads to an increase in lifespan”, a result that could prove exciting with further research.3
The process by which Kombucha acts on these triglycerides is still not fully known, though current research indicates that the products produced during the fermentation process of Kombucha may play a role. Kombucha is created through the combination of two bacteria strains along with yeast,


which together ferment sugar from sweet tea into ethanol (Figure 4). This ethanol is then converted into acetic acid, a powerful metabolite that gives Kombucha its slight vinegary taste. Alongside a few other candidates, acetic acid is suspected to kick-start the metabolism process in a way that breaks down triglycerides. However, further research still needs to be done to identify the specific drivers in Kombucha that are responsible for triglyceride breakdown, a project that DuMez-Kornegay is currently pursuing. Kombucha may also interact with the genes responsible for lipid breakdown or the restriction of lipid accumulation. The Dowen Lab found that three genes that code for lipases—enzymes that break down fat—were upregulated in organisms that consumed KTMs. According to Dr. Dowen, the lab believes that Kombucha consumption creates “A signaling event that changes transcriptional programs that modulates the expression of these genes.” Together, these results indicate that Kombucha plays a vital role in the breakdown of triglycerides for C. elegans.
Although these results are certainly promising, the Dowen Lab cautions against assuming these effects will translate to humans. Further studies testing the effect of Kombucha on mammalian systems will need to be conducted in order to determine the precise interplay between the
consumption of Kombucha and human metabolism.4 However, the highly conserved nature of lipid breakdown in C. elegans across many other species indicates a potential for similar effects in humans. While the CDC cautions against drinking more than four ounces of Kombucha a day due to its acidic (and slightly alcoholic) nature, trying out this probiotic may prove beneficial to individuals who want to improve their overall digestive health. So, the next time you decide to listen to your gut, give Kombucha—and all its potential benefits—a try.
1. Interview with Rob Dowen.
2. Interview with Rachel DuMez-Kornegay
3. DuMez-Kornegay RN, Baker LS, Morris AJ, Whitney, Dowen RH. 2024. Kombucha Tea-associated microbes remodel host metabolic pathways to suppress lipid accumulation. PLOS genetics. 20(3):e1011003–e1011003. doi: https://doi.org/10.1371/journal. pgen.1011003.
4. Research Question Three: How do gut microbiota alter host metabolism and the aging process? - Rob Dowen Lab. 2023 Aug 15. Rob Dowen Lab. [accessed 2024 Oct 11]. https://robdowenlab.web.unc.edu/research-areas/research-question-three/.
By Preston Szczesniak

Have you ever wondered what kind of research an estuarine ecologist does? Along the coast of North Carolina at the UNC Institute of Marine Sciences, Dr. Joel Fodrie’s career helps to answer this question. Dr. Joel Fodrie is a faculty member of the Department of Earth, Marine, and Environmental Sciences at the University of North Carolina at Chapel Hill . He earned his B.A. in Biology with Highest Honors and his B.A. in History at the University of North Carolina at Chapel Hill in 1999. He then went on to earn his Ph.D. in Oceanography at the Scripps Institution of Oceanography at the University of California San Diego in 2006.
He is currently an estuarine ecologist studying populations and communities of aquatic species that live in estuarine habitats (coastal habitats with brackish water) on the coast of eastern NC. Specifically, he does this at the UNC Institute of Marine Sciences, which is a research institute owned by the University of North Carolina at Chapel Hill that is stationed on North Carolina’s coast in Morehead City that allows UNC students – both undergraduate and graduate - to pursue research projects in the marine sciences.
Dr. Joel Fodrie also studies how these aquatic species
are associated with the habitats that they live in. Specifically, he focuses on the connections between fisheries productivity

Figure 1. From 1955-1956 to 2013-2015, Dr. Joel Fodrie and his colleagues noticed that oyster populations decreased over time. Image courtesy of Tice-Lewis et al.
versus habitat availability and quality, the movement ecology of marine species, coastal marine-food web interactions, and the responses of populations and communities in these ecosystems to anthropogenic and natural stressors.
Currently, Dr. Joel Fodrie is studying a variety of topics in estuarine ecology at the Institute of Marine Sciences. Generally, he and his colleagues tend to “take a holistic view” of the various parts of the estuary since many parts of the estuary are heavily interconnected . In this context, his most recent research publications have involved conducting estuarine ecology research that involves studying environmental changes in shellfish communities. Specifically, he looked at how oysters are being affected by saltwater intrusion and water-quality deterioration due to anthropogenic factors such as the rise in sea levels, the modification of estuaries, and implementation of infrastructure development near estuaries
To explore these dynamics, he and his colleagues resampled oyster reefs in the Newport River Estuary from 20132015. From the collected data, they noticed that the Newport River Estuary has experienced increases in salinity which has ultimately resulted in degraded oyster communities, compared to oyster reefs from 1955-1956 (Figure 1). Furthermore, their research in this topic has also shown that pest and predatory species that degrade these oyster reefs have become even more present in these estuaries as salinities have increased. Because oyster populations in this estuary are deteriorating, this could have significant impacts on the economic viability of fisheries that harvest these organisms and other ecosystem services.
Additionally, some other recent research that Dr. Fodrie has led includes a recent publication that details how environmental stressors alter ecosystem dynamics such as predator-prey interactions . He and his students studied how anthropogenic (human-induced) environmental contaminants affected prey consumption by predators in estuarine ecosystems. He found that when estuarine

Figure 2. Dr. Joel Fodrie and his colleagues noticed that when primary and secondary consumers were exposed to environmental toxins, prey consumption decreased overall for all toxins and independently for each type of toxin. Image courtesy of Tice-Lewis et al.
ecosystems were exposed to anthropogenic environmental toxins, predators tended to consume less prey, altering the energy flow in the estuarine ecosystem. This was true not only for exposure to contaminants generally, but for exposure to each type of contaminant that they evaluated (Figure 2).
Dr. Joel Fodrie and his colleagues at the Institute of Marine Sciences ensure that the research they conduct off the coast of North Carolina can be easily applied to solve real-world problems, especially anthropogenic-induced estuarine degradation. He says that the research that he and his colleagues conduct “…have impacts at the state level and sometimes even broader levels even national and international.” Furthermore, the academic papers that he and his team publish allow them to help the state solve issues with estuarine degradation: “We’re working with people within the state to make those situations better.” Ultimately, Dr. Joel Fodrie and his team at the Institute of Marine Sciences hope that the research they conduct of NC coastal estuaries can be used to help solve issues these ecosystems are facing and subsequently promote conservation efforts.

Dr. Joel Fodrie
1. University of North Carolina at Chapel Hill. F. Joel Fodrie. https://emes.unc.edu/people-indiv/f-joel-fodrie/ (accessed October 10, 2024).
2. Interview with Dr. Joel Fodrie, Ph.D. 10/2/2024.
3. Tice-Lewis M, Zhang S, Redding G, Lindquist N, Rodriguez A, Fieseler C, Walker Q, Fodrie J. 2022. Coastal squeeze on temperate reefs: Long-term shifts in salinity, water quality, and oyster-associated communities. In: Ecological Society of America. Morehead City, North Carolina, United States.
4. Clance L, Ziegler S, Fodrie J. 2023. Contaminants disrupt aquatic food webs via decreased consumer efficiency. In: Science of The Total Environment. Morehead City, North Carolina, United States.
By Shivank Kancharla

Protons may be one of the smallest particles in the universe, but their movement within cells (especially across cell membranes) powers some of life’s most essential processes, from energy production and pH balance to nerve signaling.1 Inside cell membranes, protons typically move across proteins embedded in membranes, following carefully organized pathways that allow them to pass through exclusively while other ions like sodium and potassium are blocked from passing through. Selective movement is vital for maintaining the proper function of cells. Proton channels- the heart of this process- are specialized proteins that allow protons to hop along chains of hydrogen-bonded networks using what is known as the Grotthuss mechanism (Figure 2). In this process, protons move across hydrogen-bonded water molecules as if in a relay race. However, some parts of these channels are hydrophobic and

seem to block the formation of these hydrogen-bonding water molecules, making it difficult for protons to pass.

Figure 1. The figure above shows the nature of proton hopping. There is a net movement of protons in a given direction. Image courtesy Gabriel. Proton Hopping | ChemTalk. ChemTalk.
How, then, do protons navigate these water-repelling regions? Dr. Kratochvil, a biophysical chemist at the University of North Carolina at Chapel Hill, explores this question by designing minimalist proteins that mimic the natural proton channels in cells. Her lab’s work focuses on understanding how protons travel across membranes through computer simulations, protein design, and experimental validation. By engineering proteins de novo (from scratch), her team is gaining new insights into the mechanisms of proton transport, with potential applications in bioengineering and environmental science.
Dr. Kratochvil’s lab is pioneering the creation of de novo proteins to explore proton transport mechanisms. Traditional studies often rely on static models, but her lab emphasizes the importance of protein dynamics—the movements and fluctuations that drive function. “Proteins are super dynamic,” she explains. “Beyond just shape and structure, dynamics play a major role in protein function.” 2 Part of her lab is devoted to how protein and water dynamics can be leveraged to create functional protein designs. Her team primarily focuses on how proton channels define the Grotthuss mechanism in their conduction pathways. In natural proteins, hydrophobic regions appear to block this pathway, yet protons still manage to traverse these areas. “We took motifs we see in natural proteins and built a de novo protein around them.”2 Dr. Kratochvil hypothesized that transient water wires—temporary chains of water molecules— form in these hydrophobic regions, allowing protons to “hop” through.
To test this, her lab designed minimalist proton channels from scratch. Creating membrane protein designs, however, poses a unique challenge. Despite membrane proteins comprising 30% of the human genome, only about 1% of known proteins— around 1,700— are membrane proteins, which makes studying them challenging. However, using a bioinformatics approach, previous researchers have overcome these challenges. Using one of these designs
as a template and incorporating polar amino acids like glutamine at strategic positions within an otherwise hydrophobic pore (Figure 2), Dr. Kratochvil and coworkers were able to generate model proton channels.
Polar sites at glutamine attracted water molecules, which then formed a flickering water wire. In the presence of a proton or proton gradient, the polar site stabilized the proton within the protein, allowing for proper proton transport across the wire. The research combined computational simulations with experimental techniques. Using rational design, Dr. Kratochvil’s team creates new de novo proteins to test key hypotheses in proton transport. From molecular dynamics (MD) simulations powered by the GROMACS engine, they observed how their designed proteins behaved over time in a simulated environment that accounts for the physics of proteins, lipids, and water interactions. Such detailed computations were crucial for understanding whether water networks would form in the absence of a proton, which was not explicitly modeled.
“We took motifs we see in natural proteins and built a de novo
protein around them.”
To capture proton behavior, they collaborated with experts in reactive MD simulations, specifically those dealing with the energy landscape and proton hopping mechanisms. Together, they were able to define the water connectivity variable, which described how a network of water molecules could conduct protons. Although each of these reactive MD simulations took roughly one and a half years and was a major bottleneck, they allowed the group to develop a 2D energy plot showing how the polar mutations in the pores decreased the energy barriers for the formation of the proton-conductive water wires. Experimentally, Dr. Kratochvil and her team used lipidic cubic phase crystallography to determine the structures of their designed proteins and liposome assays to measure proton conduction. By creating vesicles with a high internal concentration of potassium and by using a potassium-selective transporter
to establish an electrochemical gradient, they could monitor proton influx using pH-sensitive fluorescent dyes. The team’s minimalist proteins successfully demonstrated proton conduction mediated only by transient water networks. Their work showed that even in completely hydrophobic pores, introducing

Figure 2. Image of hydrophobic and polar residues within a membrane-based proton channel. Image courtesy Dr. Kratochvil
a polar site allows protons to generate their water wire and move across. The research provided insight into how natural proteins achieve proton selectivity and opens avenues for engineering new materials.
Dr. Kratochvil reflected on what may be next for her lab: “We’re thinking about separation chemistry for the future of our research.”2 Dr. Kratochvil envisions broad applications for her work, particularly in environmental sustainability. She is excited by the potential of these proteins to selectively move water for water purification or desalination purposes. Protein-based membranes could help separate salt from water at a molecular level, offering a greener, more sustainable alternative to traditional synthetic materials used in desalination. The development of selective separations membrane technology could be applied to future global water scarcity crises while reducing the environmental impact of industrial processes. Her team is also exploring how to modulate proton selectivity and gating mechanisms to control when these channels open and close. By adjusting hydrophilicity and experimenting with different amino acid substitutions, they aim to improve proton conduction rates and develop proteins tailored for specific functions.
By dissecting and reassembling the fundamental motifs of natural proteins, Dr. Kratochvil’s research sheds light on the intricate dance of protons within cellular environments. Her minimalist designs not only validate the transient water wire hypothesis but also pave the way for innovative applications in bioengineering and materials science. As the dynamic world of proteins continues to be explored, her work stands as a testament to the power of combining computational modeling with experimental ingenuity.
References
1. Ives, H. E.; Rector, F. C. Proton Transport and Cell Function. Journal of Clinical Investigation 1984, 73 (2), 285–290. https://doi.org/10.1172/ jci111212.
2. Interview with Dr. Kratochvil, Ph.D. 10/04/24.
3. Kratochvil, H. T.; Watkins, L. C.; Mravic, M.; Thomaston, J. L.; Nicoludis, J. M.; Somberg, N. H.; Liu, L.; Hong, M.; Voth, G. A.; DeGrado, W. F. Transient Water Wires Mediate Selective Proton Transport in Designed Channel Proteins. Nature Chemistry 2023, 15 (7), 1012–1021. https://doi. org/10.1038/s41557-023-01210-4.
By Joshua Micah Kreuzer

When we think about sustainability and the pressing challenges humanity faces, clean energy production often takes center stage. However, even if we harness enough solar power to meet global demands, a crucial question remains: how do we efficiently store that energy for use when the sun sets? More specifically, what are the most effective and cost-efficient methods for storing clean energy while minimizing energy loss?
These are the critical issues that Dr. Megan Jackson, an electrochemist

at the University of North Carolina at Chapel Hill, is exploring in her research at the Jackson Lab. Dr. Jackson’s research journey began during her undergraduate studies at Caltech, where she developed a passion for electron transfer and inorganic chemistry. This fascination grew as she pursued her Ph.D. at MIT, ultimately guiding her to focus on sustainable energy storage in her current work at UNC-Chapel Hill. Her dedication to this field is a testament to the importance of understanding and innovating within energy systems. While it is easy to fixate on the statistics of energy produced from sustainable versus unsustainable sources, it is essential to remember that energy management is key to our daily lives. A complex network of energy storage is touch from simple tasks such as flipping a light switch or charging a phone. Energy storage can be compared to filling and emptying a water bottle: it is not simply about how much water is available, but also how well it can be filled and poured out without spilling. Effective energy storage is not only about keeping the lights on; it’s about the ability to capture and release power efficiently, especially with renewable sources like solar and wind. Without significant advancements in energy storage technology, even the
cleanest energy production methods will struggle to reach their full potential. As Dr. Jackson describes, “We already have solar power and we already have wind power, but one of the reasons that the grid isn’t 100% solar power and wind power is that the sun isn’t


always shining and the wind isn’t always blowing. So, we need to find ways to store that energy so that we can use it later.”2
At the Jackson Lab, the primary drive is on developing ground-up models to enhance how energy storage works. They are particularly interested in the materials that could comprise the batteries and fuel cells of the future—materials either within or at the surface of solid electrodes in which electrochemical reactions occur. Most modern energy storage systems, such as lithium-ion batteries, rely on reactions that happen at materials inside batteries to store and release energy. The interfaces between these materials and their surroundings are complex, and current electrochemical models lack a deep atomic level understanding of what is occurring at these interfaces.
only last longer but also charge more quickly, making them more practical for everyday use.
“If we can start to build molecular-level pictures of what’s happening at these complex interfaces, that will open up a whole new set of design principles.”
Dr. Jackson emphasizes the significance of understanding these tiny interactions at a molecular level, “If we can start to build molecular-level pictures of what’s happening at these complex interfaces, that will open up a whole new set of design principles.”2 By gaining insight into the minute details of chemical reactions, scientists can develop better materials that enhance battery efficiency and lifespan. This could lead to batteries that not
Despite being a relatively new lab, the Jackson Lab is already engaged in several exciting and groundbreaking projects. In the lab’s latest publication, lead author Devin Leimkuhl, a Ph.D. student in the Jackson Lab, introduces an innovative method for manipulating the surface chemistry of electrodes.1 This advancement improves the process of coating electrodes with metal oxides and emphasizes how small changes in the protocol for preparing the metal oxide film can cause large changes in coat quality. Changes that can improve the efficacy of the electrochemical reactions at these electrodes. While these findings are noteworthy on their own, the methodologies employed reveal the broader aspirations of the Jackson Lab and display why research like Dr. Jackson’s is so vital. By looking to reform our models of molecular level electronic interactions, it transcends mere incremental improvements and challenges us to rethink energy storage and usage at a fundamental level. As Dr. Jackson explains, “Chemistry provides this unique opportunity to understand things at this really detailed molecular level and then to use that understanding to control systems and make them
better.” 2
While the world increasingly shifts toward renewable energy sources, effective energy storage becomes critical in managing supply and demand. Expanding the basis for our electrochemical theory and models may prove instrumental in the creation of realistic sustainable energy grids. The insights gained from the Jackson Lab could pave the way for smarter, more resilient energy systems that can accommodate the fluctuations inherent in solar and wind energy production.
As Dr. Jackson and her team continue their pioneering work, they are poised to shape the next generation of materials and technologies that could revolutionize energy storage. In the coming years, the lessons learned in her lab may hold the key to a more sustainable, energy-efficient world.
References
1. Leimkuhl, D. P.; Donley, C. L.; Jackson, M. N. Controlling Nucleation Sites for Metal Oxide Film Growth on Glassy Carbon via Electrochemical Preoxidation. ACS Appl. Mater. Interfaces 2024, 16 (2), 2868–2876. https:// doi.org/10.1021/acsami.3c13417
2. Interview with Megan Jackson, Ph.D. 9/25/24

By Emma Tang
When one thinks of the four macromolecules, lipids probably aren’t the first thing that comes to mind. One thinks of proteins which are the basis for almost everything in the human body. One thinks of nucleic acids which are the building blocks of DNA and RNA. Or one thinks of carbohydrates with their prevalence in food and health. Lipids, however, often seem to go unnoticed. Lipids are just as important, performing a variety of important functions from storing energy to making hormones.
Cell membranes consist of a type of lipid known as phospholipids. These lipids have a hydrophilic (water-loving) head made up of a phosphate ion (Figure 1). Due to the structure of the phospholipid, the cell membrane plays a crucial role in cell signaling as many of the proteins attached to the phospholipids such as receptors receive signals from outside the cell and cause reactions that send information into the cell.
Professor Qisheng Zhang’s main research area is how these lipids function and how their disruption can cause diseases (Figure 2). Many diseases are characterized by lipid disruption. For instance, certain cancers are a result of incorrect levels of phospholipids. Alzheimer’s is also impacted by lipids as researchers have seen that people with Alzheimer’s have changed cholesterol metabolism innate immune response (cholesterol is a type of lipid). “[Lipids are] the place that we should go after because it’s so fundamental,” he says1. Therefore, his research specifically investigates how this entire dysfunction got started and specific enzyme inhibitors and activators that impact these processes.
One specific enzyme, Phospholipase C (PLC) is the current focus of some of his most recent projects. PLC is a phospholipid that is responsible for regulating signaling cascades that modulate embryonic development, cell migration, and immune response2. Two isoforms of this enzyme, PLC Gamma 1 and PLC


1. This image describes the structure of a phospholipid, the form of lipid found throughout the membranes. Image courtesy of Kim Christensen.
Gamma 2 have been found to play specific roles in cancer and Alzheimer’s respectively. Isoform is the name for different versions of the same enzyme where the functions are similar, but their amino acid sequence is different.
PLC Gamma 2 is found inside microglial cells, a type of immune cell. PLC Gamma 2’s specific role is to cleave the phospholipid PIP2 into IP3 and DAG3. These two compounds are secondary messengers or compounds that carry the signal further along the signaling cascade. Eventually, the signal carried by the secondary messengers causes the release of calcium which is what influences the microglial cell to function. A large population study found that there is a strong correlation for a protective mutant P522R in phospholipase C Gamma 2 that will protect people from late-onset Alzheimer’s diseases4 This is a gene variant that causes PLC Gamma 2 to activate which enhances the function of microglia to fight against Alzheimer’s. Researchers have found that people with the gene APOE4 homozygous (meaning they have two identical copies of this gene) will develop Alzheimer’s at some point in


Figure 4. High-throughput screen of selective, allosteric inhibitors of PLC-G1. Provided by Dr. Qisheng Zhang.
their lives. However, research has found that people with this gene and the P522R mutant will not develop Alzheimer’s in their lifetime (Figure 3). This protective mutant can completely bypass the effects of the APOE4 risk factor gene for Alzheimer’s disease. Dr. Zhang hopes to find a way to mimic this mutant for people with a completely regular PLC Gamma 2 where the enzyme is activated to protect against Alzheimer’s. Currently, Dr. Zhang states that they have a few compounds that look like they could successfully activate the enzyme and are hoping to move into the next phase of research where the compounds are implemented into animal models1
However, PLC’s other isoform, PLC Gamma 1, works in the opposite direction (Figure 4). PLC also impacts cell growth, migration, and apoptosis or cell death through the same DAG/ IP3 signaling cascade. While Dr. Zhang hopes to activate PLC Gamma 2, they hope to inactivate PLC Gamma 1. This is because previous research has shown that PLC Gamma 2 is often mutated in cancer patients, especially Adult T cell leukemia patients where up to 36% have mutations in that particular gene5. This causes the PLC Gamma 2 enzyme to have enhanced enzymatic activity. The goal is therefore to find a compound that can inhibit enzymes. Dr. Zhang’s lab has so far found some promising compounds that successfully inhibit the PLC Gamma 1 enzyme.
In his research, Dr. Zhang hopes to inhibit one isoform and activate the other. Therefore, he requires selectivity to ensure that the compounds he makes do not impact the other isoform. It could be possible that a compound that activates one isoform also activates the other. However, the two isoforms have unique enough properties that selectivity is not a problem for him. Gamma 2 is only found in some types of immune cells like microglia and neutrophils as well as hematopoietic cells while Gamma 2 is expressed in all cells. Dr. Zhang’s lab can take advantage of binding pockets (the site on the enzyme that activates it) unique to each isoform to differentiate between them. They can also take advantage of individual residues inside shared binding pockets and target them in a way that distinguishes the two PLCs.
To discover these compounds, Dr. Zhang’s lab uses many important methods, some of which they have developed themselves. Firstly, they use high throughput screening to find compounds that can bind to the enzyme. You can throw hundreds of thousands and even sometimes billions of different compounds at a target protein6. As long as one has a very sensitive readout, one can find which compound is the winner. Dr. Zhang describes it as a lottery system where one does not know going into it which compound will win but in the end, there will be a winner1. In conjunction with the high throughput
screening, Dr. Zhang’s lab also uses assays that track the enzyme modification2. Assays are a sensitive detection test that uses fluorescence to find and measure the compounds and their relationship with the enzyme. Additionally, fluorescence is used to analyze the outcomes of the enzymes (either activation or inhibition). His lab created two reporter molecules that can be used as enzyme substrates and fluorescence for detection1.
Dr. Zhang’s lab focuses on finding these hit compounds for the two PLC isoforms; however, the next step is animal models and eventually, if those work out, they will continue onto human studies and the development of their compounds into novel therapeutics.
So, the next time you think about macromolecules, maybe this time, lipids come to mind first. Phospholipase C, an enzyme that interacts with the phospholipids in cell membranes, is an important target for therapeutic research. Backed by studies that have found phospholipase C’s role in cancer and Alzheimer’s, Dr. Zhang develops compounds that can inhibit or activate the enzyme. More time is needed, but in the future, novel drugs can be produced using those compounds to help cure these two diseases.
1. Interview with Qisheng Zhang, PhD. 9/13/2024
2. Edhriz Siraliev-Perez, Jordan TB Stariha, Hoffmann RM, Brenda RS Temple, Zhang Q, Hajicek N, Jenkins ML, Burke JE, Sondek J. 2022 Jun 16. Dynamics of allosteric regulation of the phospholipase C-γ isozymes upon recruitment to membranes. eLife. [accessed 2024 Oct 10]. https://elifesciences. org/articles/77809.
3. Claes C, England WE, Danhash EP, Kiani Shabestari S, Jairaman A, Chadarevian JP, Hasselmann J, Tsai AP, Coburn MA, Sanchez J, et al. 2022 Feb 9. Alzheimer’s & Dementia. doi:https://doi.org/10.1002/alz.12577.
4. Andy Po-Yi Tsai, Dong C, Peter Bor-Chian Lin, Oblak AL, Viana G, Wang N, Hajicek N, Carr AJ, Lendy EK, Hahn O, et al. 2023. Genetic variants of phospholipase C-γ2 alter the phenotype and function of microglia and confer differential risk for Alzheimer’s disease. Immunity. 56(9):2121-2136.e6. doi:https://doi.org/10.1016/j.immuni.2023.08.008.
5. Chatterjee M, Ghosh A. 2023 Jan 1. Dysregulation of phospholipase C signaling pathway in breast and colorectal cancer: Association with progression and prognosis. Elsevier eBooks.:109–123. doi:https://doi.org/10.1016/b978-0-32395696-3.00004-1.
6. Pusterla T. 2019 Apr 10. High-throughput screening (HTS) | BMG LABTECH. wwwbmglabtechcom. https://www. bmglabtech.com/en/blog/high-throughput-screening/
By Angelina Ho

As the world grappled with the emerging COVID-19 pandemic in early 2020, researchers were racing against time to understand and combat the virus. Among them was Dr. Prem Lakshmanane, whose COVID-19 research journey commenced in March of that year, as early cases of the virus began to surface within China and the United States. Dr. Lakshmanane focused on developing diagnostic tests and other immunoassays to reduce virus transmission, understand antibody responses, and support the development of vaccine strategies.
his team uncertain about how quickly the virus would reach the United States. Recognizing the urgency of the situation, they began exploring the development of antibody tests that determine exposure to SARS-CoV-2.
“Our team instantly knew we wanted to develop a highly specific Antibody (Ab) test that would enable detection of individuals who have contracted the virus, regardless of the symptoms they experience,” said Dr. Lakshmanane, Principal Investigator and Associate Professor of the Department of Microbiology and Immunology.1 According to the National Library of

In the initial stages of the pandemic, the rapid spread of COVID-19 left Dr. Lakshmanane and
Medicine, antibodies are crucial barriers to viral infections.2 More specifically, individual immunity is

obtained by antibodies communicating with invading pathogens, allowing the body to protect and surveillance itself from future intruders. Since antibody-antigen interactions are crucial defense mechanisms for one’s health, Dr. Lakshmanane utilized the lab’s expertise in ongoing research on Arbovirus—a viral disease transmitted to humans or other vertebrates by arthropods, such as mosquitoes, ticks, or sandflies. Even amid challenges posed by university closures, Dr. Lakshmanane led a small research team that dedicated weekends and extra hours to this special project,
showcasing the commitment and resilience of researchers during the pandemic.
The team began their analysis by collecting early samples from hospitals—focusing on how antibodies could bind to specific antigens (Figure 2). To investigate the antibody responses, Dr. Lakshmanane conducted experiments in his BSL-2 lab (Biosafety Laboratory 2) in collaboration with Dr. Ralph Baric, whose lab performed SARS-CoV-2 experiments in a BSL-3 lab (Biosafety Laboratory 3). A BSL-2 lab enables researchers to work with infectious or pathogenic organisms causing moderate health hazards. In contrast, a BSL-3 lab has additional engineering and procedural controls to contain indigenous or exotic pathogens that can potentially induce lethal disease if inhaled. Therefore, Dr. Lakshmanane soon realized the need to establish a correlation between two types of antibody measurements used in different BSL settings (Figure 2).
In a BSL-2 lab, antibodies are first measured in a protein-coated assay plate—a specialized tool designed to capture and quantify antibodies by orientating their antigen-binding sites. The plates are coated with particular antigens that attract and bind to antibodies present in a sample. This process allows researchers to determine the concentration and type of antibody, indicating how well the immune system responds to an infection or vaccine. The second measurement performed in a BSL-3 lab involves neutralizing antibodies, which are specific antibodies that can block viruses from entering and infecting cells. The team’s collaborative efforts demonstrated a correlation between the magnitude of the antibodies to the receptor binding domain of the spike protein on SARS-CoV-2, and thus, the neutralization of the live virus. This relationship provides methods for developing a surrogate neutralization assay (assay that measures and detects neutralizing antibodies generated in response to COVID-19 vaccination) that can be performed in BSL2 laboratories. As the National Institute of Health asserts that the presence of neutralizing antibodies is significantly predictive of protective immunity, this high-impact study, published in Science Immunology, has become one of the most cited papers to date.3
“Dr. Lakshmanane led a small research team that dedicated weekends and extra hours to this special project, showcasing the commitment and resilience of researchers during the pandemic.”
booster shots by the Centers for Disease Control (CDC). However, a troubling trend emerged: individuals receiving boosters remained low despite these recommendations. To address this issue, Dr. Lakshmanane and his team worked on evaluating a simple, convenient Point-of-Care (POC) antibody test like the “At-Home Antigen” for detecting individuals with low Ab levels. If implemented, this new diagnostic test would inform individuals about inadequate immunity against ancestral and circulating strains of SARS-CoV-2. People with insufficient immunity may then use this information to decide on booster vaccinations. By detecting previous and current strains of SARS-CoV-2, this test could enhance vaccine uptake by inducing confidence and encouraging individuals to become vaccinated to protect those with waning or weakened immunity from emerging SARS-CoV-2 strains. “We are committed to developing new tests that educate individuals about their immunity, supporting strategies to build confidence in the COVID-19 vaccine,” said Dr. Lakshmanane.1
Dr. Lakshmanane aspires for his research to assist in identifying at-risk populations and reducing transmission rates. This foundational research aims to develop methodologies for diagnosing individuals effectively, which is a critical step in managing health crises. Antibody-antigen interaction investigations are vital for guiding public health interventions and tackling complex emerging virus threats. Consequently, the insights gained from his work will be instrumental in shaping effective responses to emerging diseases, paving the way for a healthier, more secure world and inspiring confidence in our ability to confront future challenges in infectious disease management.

Eventually, studies focused on a human cohort to assess the antibody response to vaccination. “As the SARSCoV-2 variants emerged, our research continued analyzing antibodies developed after vaccination against circulating SARS-CoV-2 variants,” said Dr. Lakshmanane.1 A significant finding revealed the heterogeneity, or difference, in antibody responses; not everyone developed a robust immune response to the vaccine. When evaluating the same human cohort six months later, Dr. Lakshmanane’s collaborative study revealed a concerning decline in antibody levels, coinciding with breakthrough infections against new viral variants. Many individuals lacked sufficient neutralizing antibodies, prompting recommendations for
Figure 2. Various types of results measuring the antibodyantigen interaction. Made by Allan Richard.
References
1. Interview with Prem Lakshmanane, PhD. 09/26/24.
2. Murin C.D.; Wilson I.A.; Ward A.B. 2019. Nat. Microbiol. 2019, 4,734-747.
3. Immordino P.; Pisciotta V.; Amodio E.; Bonura C.; Bonura F.; Cacioppo F.; Calamusa G.; Capra G.; Casuccio A.; De Grazia S.; et al. Vaccines (Basel). 2023, 11, 1702.
By Jinghan Li

The human immunodeficiency virus (HIV), something that terrified the world, the “irreversible time bomb,” “silent infection,” is notorious for killing CD4 helper T cells crucial for initiating the adaptive immune response. By inserting their genome, the guidebook to the cells’ function, into T cells, HIV turns T cells into virus factories which eventually release thousands of HIVs (Figure 1), dissembling the human immune system. However, with decades of hard work by researchers like Dr. Tal Kafri at the UNC Gene Therapy Center, HIV has been repurposed to cure unhealthy cells—a technique called gene therapy.

The human genome varies among individuals, making every one of us somehow different from each other. But these alternative forms of guidebooks sometimes cause disorders, known as genetic diseases. Sickle cell anemia is one such disease that can be caused by just one change (mutation) in the building blocks of the genome (nucleotides; there are ~3 billion in humans) that makes red blood cells deform into sickled shapes, obstruct blood vessels, and cause pain. Current solutions to genetic diseases only address the symptoms and not the primary cause, which will often require patients to take lifelong treatment that may cause side effects, decreased life quality, and early death. Gene therapy’s ability to deliver a “correct” guidebook or to edit the false guidebook, resolving the cause once and for all, is thus a great appeal. In 1990, the first clinical trial for gene therapy was approved on a 4-year-old patient with severe combined immunodeficiency, a disease that usually kills the victim in the first few years of their lives, and this kept her alive and healthy until today.1
The first success of gene therapy was the impetus for its further development. Viruses ranging from harmless AAVs to herpes viruses were made into vectors; gene therapy’s targets also expanded to more lifelong/fatal diseases ranging from blood clotting problems, thalassemia, blindness, neuron diseases, cancer, and immunodeficiency.2 Gene therapy is a
blooming field, a hope to treat many “incurable” diseases.
Dr. Kafri is one of the earliest researchers studying HIV1-based vectors for gene therapy.3 Unlike many other vectors, HIV1 integrates its genome into the cell’s genome, where it is maintained during the host cell’s replication (when vectors that do not integrate are lost, Figure 2); HIV1 can also integrate its genome into cells that are not dividing, which is most of our body cells. These properties make HIV1 a good suit to treat the rapidly dividing stem cells. The integration into the cell’s genome also enhances the expression of therapeutic genes. Furthermore, HIV1 vectors carry larger genetic cargo which can make more changes to the cells.
However, HIV1 vectors pose several risks, one of which is cancer development (oncogenesis). The precise location of vector integration is not controllable nor predictable, and integration in certain regions in the genome can alter gene expression, disrupting the cells and making them cancerous. Long terminal repeats (LTR), regions at the ends of the lentiviral vector genome that stimulate the expression of the parental HIV, may also alter the gene expression of the host cell. The internal sequence of the vector genome that stimulates therapeutic gene expression may further inadvertently stimulate unwanted gene expressions. During vector production, there are also chances that all genes encoding proteins for vector replication are
packaged into a single vector, making the vector capable of replicating inside the patient (known as replicationcompetent retrovirus, or RCR), causing diseases not only to the patient but also to the community.5 These impacts of gene therapy thus require people to be more cautious, limiting it to only treating fatal or lifelong diseases. Dr. Kafri, however, seeing the power of the delivery system of HIV1, devotes himself to pushing forward the efforts to improve its safety so its curing power could be used for more people. There are numerous measures taken to reduce such risks. In one piece, scientists, including Dr. Kafri, have discovered ways to disrupt the LTR so that it will not stimulate nearby cellular

Figure 2. Integrated genomes (blue) become a part of the cell’s chromosome (pink), while non-integrated genomes stay in the periphery. Courtesy of BioRender.
genes or mobilize the vector genome within the patients while still allowing the transcription of the vector genome during vector production. A stable packaging cell line system was also developed, where the host cells used to produce the vectors were improved to contain non-mobilizable genes encoding for proteins that the vector needs to replicate, preventing the possibility of RCR formation and making the production process more efficient.4
In parallel with vector (production) design, Dr. Kafri notes that vector safety concerns also stem from variations among patients. The diversity of the human genome guarantees that everyone receiving a vector will respond differently in some way, and testing the
vectors in different gene backgrounds may predict this variation before it happens in humans.
Dr. Kafri’s recent research Analysis of hepatic lentiviral vector transduction; implications for preclinical studies and clinical gene therapy protocols systematically tested the response to HIV1 vectors in 41 Collaborative Cross mice strains, a group of mice strains with different genetic compositions designed to mimic the genetic diversity among humans. It was found that between some strains there was a more than 100-fold difference in the amount of vector gene expression. The difference in the distribution of vector transduction across mice organs also varied among the strains, where in one strain the liver cells integrated 12 times more than did the spleen cells, while in another strain the liver cells only integrated half as the spleen did.6 Dr. Kafri remarks that these results, when translated to humans, may represent a huge difference where one patient has completely no response while another suffers from serious side effects. The reasons underlying these variations are only more nuanced. Sleep patterns due to genetic variations among mice strains were for the first time correlated with vector gene integration, and this pattern is further complicated by the interplay between environmental disturbances and the strains’ different responses towards these disturbances. This attribution to the seemingly irrelevant yet complex interplay between genetics and environment further illustrates the probable diversity in patients’ responses and the challenges in producing a universally safe protocol. Encouragingly, the study identified two mice strains related to response to HIV1 vectors, which can be used to study immune pathways and vector gene silencing, shedding light on the possibility of using genetic analysis for predicting patient response and customizing gene therapy treatments.5
As Dr. Kafri acknowledges, other weights are balancing these needs out, especially in the translational field made for producing solutions rather than explanations or guarantees. Countless lives are waiting for better treatment, and there is not much time for scientists
to linger on or look back. Dr. Kafri, however, has found his place at UNC where he had the perfect resources to go back to basic science, scrutinizing the basic scientific questions to build a reliable foundation for translational research, adding his contemplation and cautiousness to the gene therapy field, a

1. Wirth, T., Parker, N., & YläHerttuala, S. (2013). History of gene therapy. Gene, 525(2), 162-169.
2. Dunbar, C. E., High, K. A., Joung, J. K., Kohn, D. B., Ozawa, K., & Sadelain, M. (2018). Gene therapy comes of age. Science, 359(6372), eaan4672.
3. Kafri, T., Blömer, U., Peterson, D. A., Gage, F. H., & Verma, I. M. (1997). Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nature genetics, 17(3), 314-317.
4. Xu, K., Ma, H., McCown, T. J., Verma, I. M., & Kafri, T. (2001). Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Molecular therapy, 3(1), 97-104.
5. Interview with Tal Kafri, M.D., Ph.D. 9/17/2024.
6. Hu, P., Hao, Y., Tang, W., Diering, G. H., Zou, F., & Kafri, T. (2024). Analysis of hepatic lentiviral vector transduction; implications for preclinical studies and clinical gene therapy protocols. bioRxiv.
By Ruhi Saldanha

Your body has superpowers when it comes to fighting disease. This is because your body contains a code, and when this code works correctly, you are healthy. However, when there are issues with this code, diseases or other health problems may arise. We see many applications of this concept in vaccination, protein replacement therapies, and gene editing—the ability to leverage our bodies as medicines. Dr. Owen Fenton, Ph.D., wants to capitalize on this idea by delivering only the healthy parts of the code as a drug. Dr. Fenton is an Assistant Professor in the Molecular Pharmaceutics and Pharmacoengineering Division at the University of North Carolina at Chapel Hill’s Eshleman School of Pharmacy. Before joining UNC in 2022, he

completed his Ph.D. and postdoctoral studies at the Massachusetts Institute of Technology (MIT). Originally from Massachusetts, Dr. Fenton had always loved chemistry from a young age. At MIT, he got involved in classic synthetic chemistry and using total synthesis to make complex natural products, which ultimately stemmed his interest in pharmaceutical medicine. He ended up developing skillsets in RNA medicine, specifically mRNA-based nanoparticles. These lipid nanoparticles comprise the basis of Dr. Fenton’s research at UNC.
Daily, the Fenton group creates these new types of genetic medicines. Genetic medicines are often very delicate and fragile. This means they are very easy to degrade; often, this is one of the significant challenges when using them as a drug. What Dr. Fenton’s group focuses on is creating nanoparticles. These ultra-small vessels protect and stabilize reactive genetic drugs while directing where they must go inside the body. Due to different diseases originating in various organs and cell types, there is a need to figure out ways to direct these medicines to specific organs or cell populations of interest. The challenge also involves keeping them intact. To test this, the Fenton group makes and characterizes
nanomaterials, a tiny vehicle, for these gene therapies and then evaluates them on cells.2 Using cell cultures that take living cells outside the body, they treat them with their RNA nanomedicines to see how effective their gene therapy is in culture. Last, they work with these in drug living systems by evaluating them pre-clinically, usually in mice.2
They have a couple of specific disease targets: melanoma, leukemia, and triple-negative breast cancer. Further, they exert efforts in the pulmonary space, like certain types of lung cancer, cystic fibrosis, and asthma. These all sound like disparate diseases, but the benefit of gene therapy is that a singular idea can treat multiple types of diseases. Their therapies treat the root cause rather than just a symptom.

Dr Fenton’s team comes from backgrounds in interdisciplinary sciences. They combine biology, engineering, and chemistry expertise to develop their pharmaceuticals. Dr Fenton recognizes that “many of these next-generation medical approaches require different skill sets and expertise.”1 Because our bodies synergize all these disciplines, the group can make safer and more tailored medicines inside the body by combining these different backgrounds. It is similar to how we try to bucket knowledge and skill sets. Our bodies don’t know a chemistry textbook, so we must consider our bodies as a major system. The researchers want their genetic medicine to be in a specific location. This requires a lot of different skill sets. The particles that they make, the nanomedicines, must be able to go through the system. Think of these veins and arteries as pipes; they can draw inspiration from things like boiling gas, which an engineer is used to. Then, they want to think about the chemistry of these particles. The cells take up nanomedicines; they must interface with different receptors. When it comes to biology, a specific particle goes to a particular location and needs to be engineered particles so that the key fits the lock perfectly. Not only is that important for getting into the correct cell population, but it’s also essential to ensure the particle does not go to the
wrong place.
“Within medicine, these gene therapies aren’t just preventative; they can also be corrective.”
The Fenton group ultimately focuses on mRNA therapy within the gene therapy space, leveraging the body’s biological systems to become a factory. mRNA is the precursor to proteins. DNA gets transcribed into mRNA. And then, mRNA gets converted into proteins. So, they usually deliver mRNA as a gene payload. A cell is just a factory, just like a production facility. With this, you can draw inspiration from the classical manufacturer. In manufacturing, you need the right amount of product in time so somebody can purchase it. This is the same idea for a gene therapy payload. If they want a protein to help cure disease, they must deliver the right amount of RNA at the right time and dose. These are the exciting intersections across science and medicine. Drawing inspiration from classical manufacturing or business rates to generate enough of a product (the protein of interest) to cure diseases at the right time, Dr. Fenton’s lab exemplifies the advancement of science as interfacing between multiple skill sets because our body doesn’t utilize just one skill set. It combines physics, math, biology, chemistry, and more.2 COVID-19 quickly put a spotlight on these mRNA nanoparticle therapies. People have worked in the space for long periods and have built a lot of fundamental work, which has advanced the field significantly. When COVID hit, people were being physically injected

with an RNA-nanomedicine. Accredited mainly to the success of COVID-19 vaccines, there is a powerful impetus behind gene therapies in general. The vaccine was a type of preventative gene therapy. These mRNA therapies are preventative, but they develop the idea that your body could learn to protect itself before you get exposed to something. Within medicine, these gene therapies aren’t just preventative; they can also be corrective. There is a lot of impetus behind these, especially in clinical trials where scientists look at things that can cure different sorts of diseases that they historically could not before. Dr. Fenton hopes to convey that by creating and leveraging a multidisciplinary understanding to help us keep our superpowers.
1. Interview with Dr. Owen Fenton, Ph.D. 09/20/24.
2. Fenton, O; Olafson, K; Pillai, P; Mitchell, M. Advanced Materials. 2018, 30, 1705328.
By Mytri Vunnam

Mice are the most common model organisms used in the study of human genetics and disease, and for good reason. Mice are genetically similar to humans, sharing approximately 97.5% of their DNA. Furthermore, they are easy to genetically manipulate. With modern sequencing and genomic tools, mutant mice strains can be developed and bred to mimic nearly any human disease or physiological condition. Hundreds of these genetically modified mice are created every year by labs to study the manifestations of various conditions and diseases or to test the effects of specific genetic mutations.3 But what happens once the study is complete? Researchers can either choose to maintain the mutant mice strain in their lab, or they can get rid of the mice. However, getting rid of these designed animals represents a massive loss of time and money for the scientific community that was spent cultivating these mice strains.1 Here is where the Mutant Mouse Resource and Research Center (MMRRC) enters the picture. The MMRRC is a National Institutes of Health (NIH)-funded resource core built to maintain and distribute the mutant mice. There are four locations throughout the country: The Jackson Laboratory in Maine, the
University of California, Davis (UC Davis), the University of Missouri, and here at the University of North Carolina at Chapel Hill (UNC). Labs can submit their mutant mice to these centers, and the MMRRC will work to bio-archive the strains. They collect and cryopreserve the sperm, embryos, and even embryonic stem cells from these animals. If another study wants to use an existing strain for their research, the mouse can be rederived using the preserved sperm or embryos, skipping the lengthy design process. This essentially creates an archive of genetically modified mice that can repeatedly be pulled for use in scientific study.1
One of the hottest topics in genetics research is the examination of how environmental factors, such as diet, smoking, or stress, affect development. A leading goal of both the NIH and UNC’s MMRRC is to understand how these environmental exposures affect the sperm and embryos being collected from donor mice. Dr. Folami Ideraabdullah, an Associate Professor of UNC’s Departments of Genetics and Nutrition, has been working on the epigenome and developmental genetics for over a decade. She notably received her Ph.D. in Genetics and Molecular Biology from UNC. Her lab
focuses on how environmental factors, such as diet, affect the epigenome and can contribute to the development of metabolic and reproductive diseases. With the support of Terry Magnuson, the Principal Investigator of UNC’s MMRRC, the Ideraabdullah lab conducted a study to investigate how different standard laboratory chow diets and the timing of exposure to these diets affect the phenotype, microbiome, and epigenome of the mice consuming them. The mice donated to UNC’s MMRRC are reared on varying standard chow diets. While all these diets technically have “sufficient” nutrition, the specific amounts of macro- and micronutrients

Dr. Folami Ideraabdullah

Figure 1. Figure adopted from Knuth et al. (2024) showing a significant difference in PWAT weight between the different chow diet in the LE group but not DE group.
can vary greatly. For example, one type of standard chow diet can have double the amount of Vitamin D compared to another, and this variability can greatly affect the mice. The study was designed around the four most common diets that the donor institutes used: 5V5M, 5V0G, 5058, and 2920X.2
For this multigenerational study, six-week-old, genetically identical C57BL/6J mice were purchased from The Jackson Laboratory. The mice were equally divided across the four diet treatments for three weeks of chow diet acclimation. These mice made up the adult exposure (AE) group, as they were only exposed to the treatment diet during their adulthood. The mice were then bred, and their offspring were split into two groups, lifetime exposure (LE) and developmental exposure (DE). The LE mice were weaned onto the same diet as their parents, while the DE mice were weaned onto the 5V5M diet for the rest of their lifespan. Body weight, fat mass, lean mass, blood glucose level, crown-rump length, perigonadal white adipose tissue (PWAT) weight, and other measurements were taken from the groups.2
The first paper published from this study reported the phenotypic and microbiome variability observed between the four diets and the different timing of diet exposures. The study found a significant difference in body weight, fat mass, lean mass, and PWAT weight between the different diets within the LE group but not within the AE and DE groups. Since no significant changes were seen in the DE group, they recommended that “a normalization
diet post-weaning may be an effective way to reduce unwanted diet-induced phenotypic variability in mouse models.”2 Next, in collaboration with Craig Franklin and Aaron C. Ericsson of the University of Missouri, the study looked at the gut microbiome of the AE and LE mice. Interestingly, all the changes that were observed in the AE group overlapped with the changes observed in the LE group. The offspring mice had the same microbiome changes as the parent mice, as well as additional changes.1 The team concluded that diet exposure from conception to adulthood had a greater impact on the mouse phenotype and gut microbiome than exposure only in adulthood.2
The Ideraabdullah lab is currently working on the next phase of the study, focusing on diet and timing-induced epigenetic changes in mice sperm. Sperm samples from the LE and DE mice were bisulfite sequenced to measure the methylation levels of the samples. The lab is exploring the differences in methylation between the samples, which could potentially be diet-driven. However, working with genomewide sequencing and methylation data presents its own challenges. Sequencing generates incredibly large datasets, requiring extensive quality checks to make sure that data is wellfiltered, publishable, and reproducible for any future studies. The ultimate goal is to see if differences in these standard chow diets can induce significant epimutations in the male germline and whether these epimutations can be inherited and cause developmental changes in the mice’s offspring.
Eventually, Dr. Ideraabdullah would like to look at how these different diets and epimutations affect the embryo and embryonic development.1
The work from Dr. Ideraabdullah’s lab is vital in determining how differing diets, even those considered to be “sufficient” and “standard,” can affect the mouse phenotype and epigenome. Their work shows how the variability in diets used by different labs and research cores may affect the reproducibility of mice model studies and can help the MMRRC establish better recommendations for mice model studies, making it an important resource for the scientific community. One day, this exploration into how diet affects development in mice may help elucidate the same thing in their genetic counterparts, humans.
References
1. Interview with Dr. Folami Ideraabdullah, PhD, 10/04/2024
2. Knuth, Megan M et al. “Timing of standard chow exposure determines the variability of mouse phenotypic outcomes and gut microbiota profile.” bioRxiv : the preprint server for biology 2024.03.28.587032. 30 Mar. 2024, doi:10.1101/2024.03.28.587032. Preprint.
3. The Jackson Lab, What is a model mouse?, website, https://www.jax. org/why-the-mouse/model#:~:text=Mice%20can%20be%20genetically%20manipulated,and%20useful%20 disease%20research%20data.
By Ellen Han

Most astrophysical processes occur within the realm of thousands, millions, or billions of years. However, even amid these glacial processes, there are exceptions. Hot, dense dwarf stars locked in binary systems with each other can orbit at incredible speeds, crossing millions of miles in an astronomical blink of an eye, typically within a few hours or less—at the fastest, just five-and-a-half minutes.
These celestial peculiarities are the stars of Dr. Brad N. Barlow’s research group, the Evolved Stars Lab, which seeks to analyze evolved remnant dwarf stars and the unique conditions created by their stellar binaries—systems where two stars are gravitationally bound and locked in orbit around each other.

While Dr. Barlow is new to The University of North Carolina at Chapel Hill as a professor, he is a returning Tar Heel. After receiving his Ph.D. in astrophysics from UNC in 2011 and teaching at High Point University for just over a decade, he now plans to continue his research into evolved stars and their binary systems at the same campus where he first began his study of them. Understanding the unusual consequences that arise from these systems can help researchers better model astrophysical processes, including stellar evolution, stellar death, and supernova formation.
Several transformations occur during a single star’s lifetime. Stars shine due to nuclear fusion in their core, as lighter elements like hydrogen collide, releasing energy that allows a star to shine. Eventually, a star will morph into a comparatively cool white dwarf when no elements are left to fuse. Once our sun runs out of fuel, it too will follow the same trajectory. This process, in which stars about as large as the sun condense into Earth-sized objects, creates extremely dense white dwarf stars that are only able to resist collapsing under their own gravitational force if they fall under a certain mass limit, called the Chandrasekhar limit. Dr. Barlow’s research group focuses specifically on hot subluminous stars, a subcategory of stars commonly found
in orbit around a white dwarf, that share one trait that makes them particularly ideal candidates for study: virtually all of these stars require binaries for their formation.
Searching for these systems is akin to finding a needle in a haystack. The hunt begins with the European Space Agency’s GAIA space observatory. Launched in 2013, GAIA has cataloged millions of astronomical objects in the vast landscape of space, tracking their locations, movement, and brightness levels. From these data, just over 60,000 likely candidates have been identified. Researchers then take to NASA’s TESS spacecraft. Designed originally to monitor exoplanets, TESS also inadvertently monitors light fluctuations of stars over time, producing light curve data for candidate stars, which helps researchers identify the periodic brightness changes associated with binary systems. In collaboration with NASA, Dr. Barlow’s team has, to date, analyzed roughly one billion brightness measurements taken by TESS.
Finally, the team uses large telescopes, such as UNC’s SOAR to collect more precise brightness data. Spectrographic analysis, which reflects the electromagnetic radiation from an object, is then conducted on likely candidates. Like the wail of a siren on the street changing in pitch as it
approaches you, this data is subject to the Doppler effect, which shifts observed wavelengths of sound or light depending on the speed of the source and observer relative to each other. These fluctuations can then be examined to determine an object’s velocity, which allows researchers to calculate total mass—if this number is greater than the Chandrasekhar limit, a new system of interest has been successfully identified.
This search constitutes the bulk of the work required for Dr. Barlow’s research, but “the thrill of the chase” keeps the team going. Once the proverbial needles have been found, they can be analyzed and modeled to understand how binary interactions can shape the typical evolutionary track understood in isolated star systems.
One such subject of interest is Type Ia supernovae—the bright and powerful explosion of a dying white dwarf. When a white dwarf gains mass and exceeds the critical mass limit, it collapses under its own gravitational pressure, igniting a chain reaction within the star that results in a violent explosion that unleashes a shockwave of stellar matter into the surrounding space. As a white dwarf binary system orbits, the sheer velocities exhibited can prompt mass transfer between the stars, where some matter from the white dwarf’s companion star can be deposited and accumulated on the dwarf star’s surface. Dr. Barlow’s team is investigating the proposed double detonation of a white dwarf, where this layer of accumulated material detonates
and is sufficiently strong enough to also detonate the star’s core. This explosion, which would occur under the critical mass limit, theoretically results in an underluminous supernova. Through their exhaustive search, Dr. Barlow’s team has identified a binary system consisting of a white dwarf and a helium star which displays evidence that supports these theories of formation: modeling this system into the future predicts not only the double-detonation explosion of the white dwarf, but also the subsequent high-speed ejection of its companion from the galaxy itself.
The “thrill of the chase” is what keeps the team going.
stars but also of smaller objects, such as gas giants.
Looking towards the horizon, Dr. Barlow expects to delve deeper into his research The hurtling technological advancements in the past decades have presented researchers with huge quantities of data, and Dr. Barlow and the Evolved Stars Lab, like many other astrophysicists, are racing to comb through the data sets provided by new spacecrafts like GAIA and TESS. As the search continues, each new star system brings the team another step closer to discovering not only the secrets they may hold about stellar phenomena, but also their valuable insights into the very forces that created our own corner of the universe.

Figure 1. Render of HD265435, one such stellar binary system. With an orbital period of about 100 minutes, it is set to explode as a Type Ia supernova in 70 million years.⁶ Image courtesy of Keck Observatory.
Another of Dr. Barlow’s team’s research interests is star engulfment, in which a large star, such as a red giant, in a binary system with a dwarf star, will “consume” its smaller companion. Their extremely high orbital speeds mean that stars too close together will eventually share a “common envelope,” where their atmospheres begin to merge. Where it might be expected that the smaller star would be permanently swallowed by its much larger companion, many of the subluminous dwarf stars the lab focuses on are capable of surviving these extreme interactions, emerging from the common envelope unharmed. Working with researchers at the Tautenburg Observatory in Germany, Dr. Barlow has helped establish the Eclipsing Reflection Effect Binaries from Optical Surveys (EREBOS) project, which further analyzes systems where red giant engulfment occurs. One goal of this project determining whether there is a mass cutoff for engulfment survival. So far, EREBOS has found that even brown dwarfs, astronomical objects with sizes that lie between gas planets and white dwarves, can survive these interactions. Understanding the mechanics behind engulfment can help researchers build more accurate models, not just of
1. G.H.A. Roelofs; A. Rau; T.R. Marsh; D. Steeghs; P.J. Groot et al. The Astrophysical Journal Letters 2010, Vol. 711, No. 2.
2. R. Culpan; S. Geier; N. Reindl; I. Pelisoli; N. Gentile Fusillo et al. Astronomy & Astrophysics 2022, Vol. 662, Article 40.
3. Interview with Brad N. Barlow, PhD. 10/07/2024.
4. S. Geier; T. R. Marsh; B. Wang; B. Dunlap; B. N. Barlow et al. Astronomy & Astrophysics 2013, Vol. 554, Article 54.
5. V. Schaffenroth; B. N. Barlow; S. Geier; M. Vučković; D. Kilkenny et al. Astronomy & Astrophysics 2019, Vol. 630, Article 80.
6. I. Pelisoli; P. Neunteufel; S. Geier et al. Nature Astronomy 2021, Vol. 5, 1052-1061.
By Reiyah Jacobs

In 1908, the idea that space and time are intimately connected, coexisting in a four-dimensional continuum now known as spacetime, was introduced by Hermann Minkowski1. A result following from Einstein’s theory of special relativity, the spacetime fabric forms the background framework for wave equations, or mathematical equations describing the movement of waves such as light waves and sound waves. Different areas of the universe can be represented with different spacetime models, or metrics, and the properties of these different metrics

influence the behavior of waves within them. Additionally, within any particular model of spacetime, many different types of waves exist, some with more complicated behavior than others. For Dr. Jason Metcalfe, a Bowman and Gordon Gray Distinguished Term Professor and the chair of the Department of Mathematics at the University of North Carolina at Chapel Hill2, these are areas of significant interest: how do these different spacetime models affect the behavior of waves within them, and how long can certain types of waves last before growing dramatically to infinity or ceasing to exist?
Specifically, Dr. Metcalfe’s research examines the behaviors of nonlinear and dispersive wave equations. To put this into context, the simplest form of the wave equation represents a linear non-dispersive wave in one dimension, with linear in this case meaning the wave looks like a sine graph and nondispersive meaning the wave does not decay as it travels over time. Figure 1 shows a comparison between the appearances of linear and non-linear waves in one-dimensional space.
The behavior of a wave with non-linear structure is, in comparison, much less predictable, and the shape of a non-linear wave can vary greatly.
Furthermore, a dispersive wave decays and spreads out over time. To explain this, Dr. Metcalfe gives an example of dropping a pebble into a pool of still water. As the water ripples outwards, it models a dispersive wave. The first ripple, closest to the impact, creates the largest bump in the water; as the ripples move further away from the impact point, their effects become smaller.3
Wave equations that have nonlinear interactions are prone to reach singularities, points in time when the equation “blows up” (when solutions to the equation grow to infinity) or when solutions to the equation simply stop existing altogether. Some wave equations have solutions for a small period of time before stopping due to a singularity, while others have global solutions, or solutions that exist for all times. Some other wave equations are in-between, representing waves that exist for a long time but not necessarily forever. The amount of time for which a wave equation can exist before reaching a singularity is its lifespan. In his research, Dr. Metcalfe focuses on how non-linearity structures affect the lifespan of a wave equation.
“What sort of [non-linear] structures guarantee that the solutions will always exist... or how long the

Figure 1. The differences between the graphs of linear waves (sinusoidal) and nonlinear waves (skewed or asymmetric). Courtesy of Dr. Metcalfe.
solutions exist before something breaks down?” Dr. Metcalfe asks.3
To investigate this question, Dr. Metcalfe, put simply, does a lot of calculus. “The vast majority of it is integration by parts. We do lots of integration by parts, u-substitution… if this sounds like I do Calc 2 a lot, I do Calc 2 a lot,” he explains. “There are many more steps than you see in a calculus exam, but it really is just calculus.”3
“[Mathematics] feels like less of a human construct, and more of something that’s just there, waiting to be discovered.”
Together with collaborators Dr. Jacob Sterbenz from the University of California-San Diego and Dr. Daniel Tataru from the University of CaliforniaBerkeley, Dr. Metcalfe proved that local energy estimates, or specific ways to measure the dispersion in a wave, can be used to investigate wave equations in all spacetime models that do not “trap” waves in any particular region (in other words, in models in which waves can extend out to infinity).5 Dr. Metcalfe is among the first to have considered such methods for perturb geometries (more general models that have been altered from simpler ones), and this is an important result that will make it easier for mathematicians to analyze the behavior of wave equations in the future.
Some may wonder why this matters. As a theoretical mathematician, Dr. Metcalfe considers his major goal to be “advancing human knowledge”.3 In fact, many significant applications of pure mathematics do not appear until decades after the initial result is first published. Yet most of our technological and scientific advancements today were
built upon theoretical investigation. Beneath the structure of modern-day computers lies work stretching back to the 1800s, in fields such as Boolean algebra, number theory, and Fourier analysis.4,5 Euler’s formula, introduced around 1740, remains a fundamental concept in engineering disciplines.6 Currently, general relativistic principles used in Dr. Metcalfe’s work can also be found in GPS satellite operations, but even larger and greater applications might still be waiting in the future. For now, it is the simple pursuit of knowledge that makes Dr. Metcalfe’s research so interesting, and is what pushes him to continue his work each day. It is difficult for Dr. Metcalfe to predict specific areas of future investigation, as both his research and math itself are constantly evolving. As a mathematician becomes more experienced and their insight becomes more nuanced, more areas in mathematics to explore are realized, and the research process is never-ending. As such, Dr. Metcalfe’s work has extended across many topics, ranging from the study of the behavior of dispersive waves near black holes to his current focus on the lifespan of non-linear waves. “[Mathematics] feels like less of a human construct, and more of something that’s just there, waiting to be discovered,” Dr. Metcalfe describes.3 A recipient of the Carolina Women’s Leadership Council’s 2024 Faculty Mentoring Award7, Dr. Metcalfe has collaborated with many undergraduate students and even a current high school student, and he encourages undergraduate students with an interest in math to get involved. After all, according to Dr. Metcalfe, all you really need to be a mathematics researcher is to have a willingness “to get involved… and to be frustrated and uncomfortable”.3
1. Overduin J. GP-B — Einstein’s Spacetime. Stanford. https://einstein. stanford.edu/SPACETIME/spacetime2.html (accessed 2024 Oct 10).
2. Metcalfe, Jason - Department of Mathematics. https://math.unc.edu/ faculty-member/metcalfe-jason/ (accessed 2024 Oct 10).
3. Interview with Jason Metcalfe, Ph.D., 09/27/24.
4. Applications of Boolean Algebra: Claude Shannon and Circuit Design | Mathematical Association of America. https://old.maa.org/press/periodicals/ convergence/applications-of-boolean-algebra-claude-shannon-and-circuit-design (accessed 2024 Oct 21).
5. Interview with Jason Metcalfe, Ph.D., 10/22/24.
6. Block B-M, Mercorelli P. Front. Educ. 2014, 10, 1–7.
7. Shaping Futures: Jason Metcalfe Earns Coveted 2024 Faculty Mentoring Award - Department of Mathematics (2024). https://math.unc.edu/mathnew/shaping-futures-jason-metcalfe-earns-coveted-2024-faculty-mentoring-award/ (accessed 2024 Oct 21).
The next breakthrough in physics is hidden within the smallest building block of life: the atom. Studying the atom, scientists have discovered that heavier elements are more stable relative to each other, and others behave in unpredicted and unexpected ways. These differences cannot be accounted for with the current mathematical models for nucleon structure, and what is truly occurring at this beyond-microscopic level is slowly revealed to us through an intricate waltz of theoretical and experimental work, constantly revising and improving one another.
Dr. Robert Janssens heads an international collaboration dedicated towards exploring the behavior and qualities of nuclear structure across different elements. Dr. Janssens is the director of the Triangle University Nuclear Laboratory, a two-part building on Duke’s campus which houses some of the experimental technology vital for his work; he works in Chapel Hill’s Department of Physics and Astronomy, and is an Edward G. Bilpuch Distinguished Professor. He continues to work closely with the Argonne National Laboratory in Illinois, as he previously worked on the development of analytical devices such as Gammasphere.
Electrons within an atom can be thought of as arranging themselves in concentric rows pivoting about the
central nucleus. Similarly, it is theorized that stability within the nucleus is achieved by a shell-like organization parallel to that of electrons. To explain this shell structure, Dr. Janssens comments: “the nucleus is a system where shells are the key ingredient to build it up.”1 This theory gains credibility with the pronounced stability of ‘closedshell’ nuclei in comparison to nuclei which have ‘unclosed’ outer shells. Scientists have noted that nuclei with 2, 8, 20, 28, 50, or 82 protons and/ or neutrons—referred to as ‘magic numbers’—are particularly sound because of the large energy gap to the next available shell, which results in nucleons being more tightly bound (in terms of energy per nucleon) than nuclei of other orientations. Thinking of the nucleus as a building, it is logical that the strongest structures are those whose

Dr. Robert Janssens
bricks are packed tightly together (say, with cement), and consist of full rows, in comparison with something built with loose bricks piled irregularly.
As more and more subatomic particles congregate about one another, their structure and placement become crucial to retaining nuclear stability. As neutron amount increases, nuclei are aptly termed ‘neutron-rich.’ Recent studies of these neutron-rich nuclei have made clear that, on the super heavy scale, magic numbers disappear, and stability is reached with unexpected values.2 “Since we have been able to [look at superheavy nuclei], we realized that... magic numbers are not cast in stone,” Dr. Janssens says, “they can change as a function of the ratio between...protons and neutrons.”1 Dr. Janssens and his collaborators have been probing this phenomenon with varying isotopes, and have found exciting details about the subatomic system of nickel-64.
The presence of additional neutrons, by way of changing binding energy within the nucleus, impacts the shape of the nucleus itself. This shape is directly related to stability, as well as the orientation and ordering of nucleons (protons and neutrons) within the shells of the nucleus. While some are spherical, others are not: this discrepancy hints at subatomic interactions hitherto unknown to us.
To further probe the behavior of
superheavy nuclei, Dr. Janssens and his team devised an experiment to test preexisting models of these kinds of nuclei. The nickel-64 used in this experiment was populated by a two-neutron transfer from nickel-62; a process that transfers two of the neutrons from a projectile, in this case, from oxygen-18, to a target, nickel-62. Earlier calculations on deformations of the shape of nickel-64 had only suggested a single spherical orientation (see figure 2).3 To investigate this, highenergy excitation states in the nickel-64 were created by placing lead208 close to the nickel, but not sufficient for binding to occur. Even at energies below the repulsion bind, there is still electromagnetic interaction between these two elements, which is sufficient to excite the nickel. The gamma rays required to go from a ground state to this level of excitement in nickel-64 provides information on the shape of its nucleus. Using the γ -ray tracking array, GRETINA, in conjunction with the CHICO2 particle detector, both currently housed at the Argonne National Laboratory, it was possible to associate sparks of radiation energy with nickel-64’s nuclear shape (see figure 4). “The gamma rays we saw were the typical signature of a deformed nucleus, of a prolate nucleus,” comments Dr. Janssens, “that was the proof, that part of the stability [of nickel-64] comes from the fact that...nature can deform the nucleus.”1 The results determined that nickel-64 in fact had two coexisting

shapes that develop from its spherical ground state as energy is added to its nucleus: an elongated (prolate) and flattened (oblate) shape (see figure 3).4 This is particularly remarkable, as triple shape-coexistence in a stable nucleus
“So we can [go] back, they can refine the model, and we can try again. That’s the way we make progress.”
indicates profound changes in the way protons and neutrons can arrange themselves. Recent Monte Carlo shellmodel calculations confirm these results; a formidable power for theoretical modeling of complex nuclei that allows a benchmark for predictive power on other, more exotic elements. This rare phenomenon is theorized to originate from the action of the monopole tensor force, which effectively induces a weakened resistance in the nucleus to deformation in response to increasing single-particle energies.3 On the impact of these types of results, Dr. Janssens notes “Often, you come back [from experimentation], you have seen most of what [the theorists] want, but you have seen it differently, or you have seen other things in addition. So we can [go] back, they can refine the model, and we can try again. That’s the way we make progress.”1
others around the globe. Results have shown that these deformed states, as neutron count decreases, have excitation levels that rise higher and higher from the ground state; the interactions identified in other species may be too weak to even detect. The theoretical limit to how far an isotope of nickel can be pushed before these distinct deformed shapes disappear is thought to be nickel-62, however this is currently under analysis. Looking closely at these neutronrich nuclei has made it blatantly obvious that there is some force, acting between the protons and neutrons, causing these changes in state in response to nucleon count, that was previously unknown to science. This force may shed greater light on the mysterious workings of the nucleus; with respects to the stability of the nucleus, deeper knowledge and ability to predict this sort of structural behavior in atoms is vital to the hunt for element-120, the next great leap in filling out the periodic table. Dr. Janssens shares the hope of many experimental nuclear physicists: “we don’t know where the periodic table ends...There is no theory that tells you that that should be the end of the game.”1 As long as the periodic table continues, so will the exploration of superheavy nuclei.

Experiments on nickel-60, 66, 68, and 70 have since been completed, or are underway; both by Dr. Janssens’ group as well as
1. Interview with Robert F. Janssens, Ph.D. 09/18/24
2. Otsuka, A. Gade, O. Sorlin, T. Suzuki, and Y. Utsuno, Rev. Mod. Phys. 2020. 92, 015002
3. N. Mărginean et al. Shape Coexistence at Zero Spin in 64Ni Driven by the Monopole Tensor Interaction. Physical Review Letters, 2020. 125(10). https://doi.org/10.1103/physrevlett.125.102502
4. D. Little et al. Multistep Coulomb excitation of 64Ni: Shape coexistence and nature of low-spin excitations. Physical Review. 2022. C, 106(4). https://doi.org/10.1103/physrevc.106.044313

Sarah Giang Editor-in-Chief

Kazemi Managing Editor

Ria Patel Fundraising Chair


Isaac Hwang Editor-in-Chief

Natalie Druffner Treasurer

Kirina Shah Online Content Manager



Reagan Gulledge Secretary

Esha Agarwal Associate Editor



Dixon Publicity Chair

Kruti Bhargav Associate Editor

Are you interested in communicating science to a broad audience?
Do you want to engage in thought-provoking investigations?
Does your passion for the sciences extend into the world of research?
Do you want to combine your creative talents with your fascination with science? Carolina Scientific is always looking for staff writers, designers, and illustrators! If you are interested, please contact carolina.scientific@gmail.com Follow us on Instagram @carolinascientific Find us on Facebook facebook.com/CarolinaScientific




Follow us on Twitter @UNCSci Check out our website carolinascientific.org
“I was taught that the way of progress was neither swift nor easy.”
- Marie Curie


scıentific Carolina Fall 2024 | Volume 19 | Issue 1
This publication was funded at least in part by Student Fees which were appropriated and dispersed by the Student Government at UNC-Chapel Hill as well as the Carolina Parents Council.