17 minute read
Moving Ahead with Intelligent Virtual Clinical Trials
Fuelled by the current COVID-19 pandemic, and the considerable volume of data generated during a clinical trial, Artificial intelligence (AI) has provided the much-needed impetus for transforming clinical trials into the virtual sphere for greater efficiency. From patient recruitment, protocol design, trial monitoring, to identifying the effect of blood thinners on virtual patients with irregular heartbeats, leveraging AI has helped life-saving drugs reach the market sooner.
Ayaaz Hussain Khan, Global Head Generics, Navitas Life Sciences (a TAKE Solutions Enterprise)
Advertisement
Healthcare today faces extraordinary challenges posed by the COVID-19 pandemic along with a rise in chronic disease burden worldwide, an aging population, and the growth of the middle-class Asian population. According to the World Health Organization (WHO 1), by 2020, an estimated three-quarters of all deaths globally would be due to chronic diseases. These stressors have helped facilitate innovations in conducting clinical trials in a bid to curb rising costs and reduce the time needed to conduct them. Simultaneously, there have been significant breakthroughs in science and technology, enabling intelligent clinical trial solutions.
A recent report by Researchand Markets stated that the global market for e-Clinical Trial technologies 2, catalysed by the COVID-19 pandemic, is an estimated US$5.4 Billion(2020), with an expected rise to US$ 9.9 Billion by 2027. The U.S e-Clinical Trial Technologies market is at an estimated US$1.6 billion this year, while the market size in China is expected to be US$1.7 billion by the year 2027. Among regional markets, China will be one of the fastest-growing, followed by Australia, India, and South Korea, with an estimated Asia-Pacific market value at US$1.1 Billion by 2027.
AI in Clinical Trials
Data provided by ClinicalTrials.gov shows that there was a reduction in the number
2. https://www.businesswire.com/news/ home/20200908005697/en/Global-e-ClinicalTrial-Technologies-Market-Trajectory-Analytics2012-2019-2020-2027---ResearchAndMarkets.com of new trials between January and May 2020. Moreover, Michael Lauer from the US National Institutes of Health stated that nearly 80% of non-COVID trials 3 were either stopped or interrupted. Investigative sites had to resort to ingenuity and flexibility during the subsequent period of recovery from June to July, with prior investment on the right technology aiding in risk mitigation. For instance, AI and machine learning (ML) platforms were leveraged by a global clinical research organisation to run six COVID-19 clinical trials using remote monitoring practices, while banking on vast experience and in-depth infectious disease expertise, that resulted in milestones reached ahead of time.
Furthermore, the advent of digital solutions in clinical trial management and conduct has improved transparency, with an onus on delivering better healthcare. Consumers or patients have access to a wide range of information, and, with this dissemination of information, there is increased expectancy. This has initiated a need for rethinking clinical trials to maximise benefits. There has been a significant shift towards embracing the incredible advantages of data analytics, along with digital models of engagement, to forge clinical trials that cater to the current demands.
Significant strides in incorporating digital health solutions began a few years
3. https://www.thelancet.com/journals/lancet/article/ PIIS0140-6736(20)31787-6/fulltext
ago, more as experimental solutions or as support for certain sections of clinical trials. Such investments have paved the way for hybrid clinical trials that are guiding forces for successful and efficiently run clinical trials.
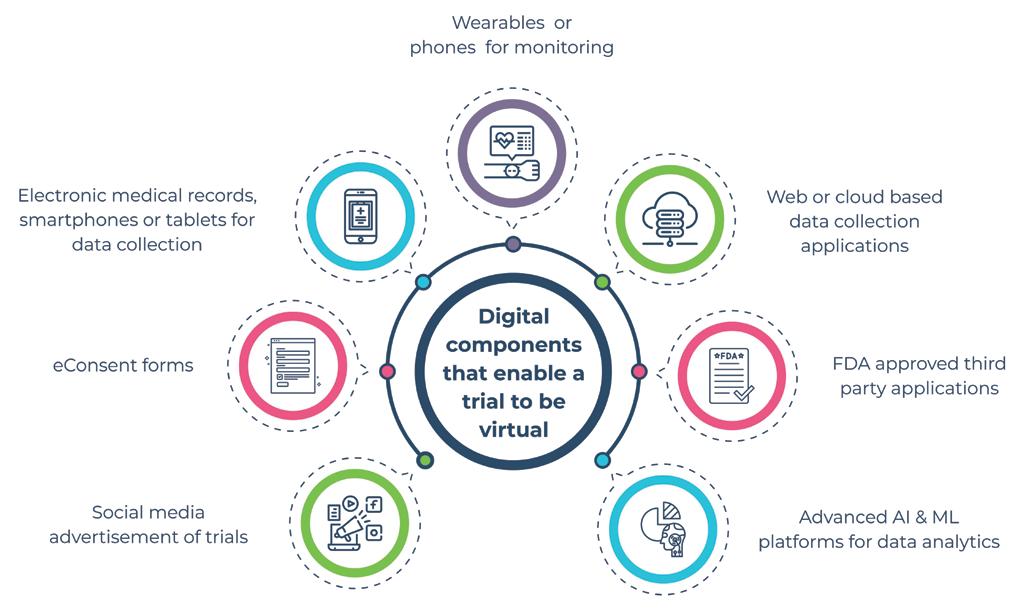
Key steps in a virtual clinical trial
A virtual clinical trial harnesses the power of technology to improve patient recruitment, retention, collection of data, and analysis. They support efficient trials as they tap into digital technologies, like apps, monitoring devices, and online social engagement platforms to conduct each stage of the clinical trial. This includes enhanced support for recruitment, informed consent, patient counselling, measuring clinical endpoints, and in determining adverse reactions. 1) Patient recruitment: Clinical protocol development is the first step in a clinical trial. With the multitude of data sources, like imaging health, genomics, and patient-reported outcomes, there has been a shift in trial protocols to meet regulatory requirements. Multiple reasons could hinder patient recruitment, including involving populations that are hard to reach, people with disabilities who cannot come to the trial centres, lack of awareness about the trials, and the financial burden on the study population if there is a need for frequent trips to the research centres.
Leveraging digital methods to facilitate recruitment has been in use since even before the current dictates of the pandemic. Encouragingly, such trends have been the reason for optimism, with clinical trial announcements popular over social media channels. Patients don’t have to travel to sites to sign up for the study; instead, they could send in e-consent forms. Technology reaches patients who would be most suitable for the study, ensuring that they participated with minimal travel to the site, significantly increasing patient participation and retention during clinical research studies. Such innovative solutions ensured that the first patient for a safety and efficacy trial for a COVID-19 drug product was recruited within 26 days of the study being commissioned.
To ensure seamless collaboration, detailed and uniform training for effective management and use of digital tools for protocol adherence, recruitment, sample management, PD/Issue Handling, data entry and cleaning requirements, good source documentation, regulations etc., need to be provided. 2) Digital health data collection: After patients are recruited for a study, it is important to collect data during the clinical research study process. There are multiple ways to collect this data using digital tools. Demographic and medical health data, patient activity, and physiological parameters, patient-reported outcomes, along with images, can now be collected using electronic medical records, smartphones, or tablets.
There has been a dramatic shift towards digital monitoring and biomarkers. This refers to objective measures of behavioural, pathologic, physiologic, anatomic, social, and patient self-assessment, which can be obtained remotely
using digital technology and used as a means of evaluation. Examples of such technology include wearable activity trackers or phones with sensors to detect cardiogenic chest wall vibrations as a means of identifying heart failure or heart rhythm. Other examples include sensors to detect sweat for glucose, lactate, electrolytes, or sensors placed in braces to determine structural health markers for knee joint injury. Statistical analysis may be used to identify the effect of blood thinners on virtual patients with irregular heartbeats.
Digital technologies have also helped in collating data that couldn’t be collected through traditional means before. A case in point is the use of unique applications available in smartphones. Patients need to perform a set of tasks indicated in these apps, which is used to detect signs of Parkinson’s.
Regulatory authorities have also been closely monitoring such advances in technology and data collection. The Food and Drug Administration (FDA) approved the use of the Apple watch in detecting heart rhythm abnormalities, like in atrial fibrillation, that use optical sensors for photoplethysmography and electrodes for electrocardiography. e-Source may be used as the primary source of patient data capture, except for informed consent form and laboratory data. The data from e-source may be synchronised with electronic data capture, so there is reduced data input required from sites. 3) Safety monitoring in virtual trials: The fast pace of digital technology movement into routine processes in a clinical trial has helped in significantly improving efficiency, reducing time and cost for sponsors. New tools are now being paired with traditional biomarker assessments to enhance safety and validation.
A considerable advantage of using digital tools in safety monitoring is that there is continuous data collection that can be used to detect infrequent events or even to identify situations that may not occur during a study visit. In a bid to better monitor clinical trials, artificial intelligence, and machine learningpowered digital platforms have been in use for many years to identify potential risks. Such platforms facilitate near realtime data insights that promote faster detection of events and reporting, which has a considerable impact on clinical trial timelines and cost.
One of the critical factors in incorporating digital tools in virtual trials is meeting safety and regulatory standards. 4) Data Security: To develop intelligent clinical trials that do not require constant human monitoring but are dependent on advancements in technology, certain challenges need to be addressed. There is an increased need to tighten data security during collection, transmission, and analysis. There is a heightened risk of breach of data regarding the location that could affect the study participants.
To overcome this, the FDA has put forward specific guidelines, like conveying information collected by the digital tools to all stakeholders. Medical device certification has been developed as a measure to control data breach.
There is a need to understand the regulations that govern the use of data within specific geographical regions and the changes in rules that may exist in other areas. There is reduced dependency on physical sites in virtual trials, and

patients may be enrolled from different cities or even countries. The rules that govern data collection, use, and inclusion in studies vary from one state to another and from one country to another. It is important to stay abreast of the latest in the field to ensure successful clinical research study outcomes.
The use of wearables and smartphones in data collection provides a continuous stream of information that is transferred to study investigators using web-based applications. This type of data transfer may be at risk of a security breach. The FDA is developing a risk-based approach in better regulating such third-party applications. Wearables provided by Apple and Fitbit have the necessary certificate as they follow the FDA's guidelines.
There have been warnings issued by the FDA against the use of certain insulin pumps and an implantable cardiac device as there were vulnerabilities in the pump that could result in tampering of the device by unauthorised people. The use of blockchain technology may be one potential solution to ensure data privacy and security. Though such vulnerabilities exist outside the clinical trial sphere, it is essential to use the right technology to ensure data security.
Virtual trials have a compelling advantage over traditional clinical trials when the technology is developed following guidelines. 5) Data analytics: Flexible, extensible, and scalable clinical trials can be carried out only with the support of effective data analysis. For example, an AI and ML powered platform enables study investigators to connect remotely and access data from clinical trials in near real-time. Such emerging technology helps automate processes and mapping data, with advanced analytic methods applied to manage multiple facets of clinical trials.
Such artificial intelligence and machine learning platforms help augment data extraction and in computational phenotyping that enhance efforts towards successful clinical trial outcomes.
6) Optimising trial methods: There are multiple ways in which virtual resources support clinical trials optimisation. A method of intervention optimisation, called micro-randomised trials, involves identifying factors like dose and timing that can be managed better using reminders. Such engagement strategies are best suited for patient recruitment, enrolling and retention. The personalisation of the clinical trial process helps in enhanced patient participation in clinical trials.
Optimisation of clinical trials can extend to other aspects of the trial as well. In time, it will help in building robust systems that greatly enhance the conduct of biomedical research.
The traditional clinical trials or the in-site system may not be effective during the current unprecedented times, however, the benefits of virtual clinical trials extend beyond the purview of the pandemic. There has been an increased dependency on innovative and intelligent solutions in managing clinical trials over the past two decades.
Societal interaction that has largely been based on face to face interaction is now slowly moving to the virtual space. Though this transition has been slow in the clinical trial sphere, many tools are now being embraced to re-engineer clinical research. Robust methods to track the progress of clinical trials using AI powered platforms, or remote monitoring using e-Source, with intelligent trial management using the trend output from such digital platforms have significantly elevated the conduct of clinical trials.
Large volumes of data from clinical trials can now be cleaned from the time of the first person enrolled in the study till the end of the study. Digital tools can also be used for periodic medical data review and to streamline clinical trials, with faster decisions taken on the drug development process.
Improved efficiencies throughout the clinical trial process will aid in reducing time and costs. The use of the right digital health solutions allowed rapid site activation within 20 days, faster recruitment of patients and comprehensive trial oversight for a safety and efficacy drug trial on moderate to severe COVID-19 patients. The innumerable benefits when using digital tools to support clinical research reinforces the need to incorporate new forms of technology which act as critical enablers to achieve better coordination and in simplifying processes between the various stakeholders and ecosystems in a clinical trial.
Despite the current COVID-19 times of difficulty and uncertainty, clinical trial conduct experienced a ray of hope demonstrating real feasibility of virtual clinical trials. Virtual clinical trials have demonstrated the ability to improve access, reduce participation burdens, and enable the collection of robust and secure data, among other benefits with the support of digital tools. To stay at the forefront of innovation, it is imperative that organisations understand critical healthcare needs, evaluate their capabilities and make necessary plans to deliver virtual clinical trials to patients.

AUTHOR BIO
Khan is an authority in the BA/BE domain of the generic drug industry. He brings with him a rich experience of conducting over 1000 Bioavailability/Bioequivalence studies. He is associated with Ecron Acunova (now under the banner of Navitas Life Sciences) for the past 10 years and is the Global Head of Generics. He has been a gold medallist in his academics.
Why Cell and Gene Contract Manufacturers Must Embrace Digitisation?

Rachit Jain, Global Cell and Gene Lead Software, Körber Business Area Pharma
The cell and gene industry is growing at a staggering 30 per cent CAGR and is estimated to reach US$14 bn by 2025. A number of cell, gene and stem cell therapy sponsors currently have novel drug substances and products and many rely on Contract Development Manufacturing Organizations (CDMO) to produce them with adherence to stringent regulatory cGMP conditions.
Cell and gene manufacturing for both autologous (one to one) and allogenic (one to many) treatments face difficult issues such as: a complex supply chain, variability on patient and cellular level, cell expansion count and a tight scheduling of lot disposition process. This complexity affects quality, compliance and accountability in the entire vein-to-vein process for critically ill patients.
Electronic batch recording becomes vital
Cell and gene companies are emerging from years of laboratory scale development through preclinical tests and pivotal clinical trials to ultimately reaching
FDA approval and commercialisation. Digital solutions such as a robust Electronic Batch Recording system (EBR) are vital. Given that the raw material for these processes are actual patient cells extracted in a clinic, a failed batch is devastating and could mean loss of a patient’s life. A failed batch due to poor paper record handling must be avoided at all costs.
A crucial component in reducing Cost of Goods Sold (COGS) in developing cell, gene and stem cell therapies is through increased efficiency of the manufacturing process. Automation provides a suitable option to address this challenge. Those manufacturers who have become early adopters of digitisation, automation and continuous improvement are reaping huge benefits. They have minimised the use of paper, automatised the calculations, sped the design of manufacturing batch records, reduced cGMP deviations and more quickly released manufacturing lots based on review by exception, which align with right first time initiatives.
Automated processes are a good starting point

Cell therapy manufacturers rate manufacturing process stability and scalability as their top two considerations throughout clinical trials. They equally rank process variability, scale-up ease and reduced cycle time as motivation for automating both their manufacturing processes and supply chain. In this vein, a CDMO that is already a fully automated facility with EBR capability can help achieve these goals.
The detrimental impact of Sars-Cov-2 has challenged cell therapy sponsors with a stoppage of clinical trials and supply chain hurdles. In the manufacturing domain, both in-house and contract manufacturers have implemented strict guidelines to ensure employee safety. Manufacturers, who had embraced automation, quickly rebounded to get systems back online to meet delivery deadlines. In addition, they had a reduced risk of contamination as fewer hands touch paper or the final product, thereby drastically reducing the four-eyes-principle steps.
Cell therapy sponsors select CDMOs
Cell therapy sponsors have two options for how to manufacture: build an internal manufacturing capability or employ a CDMO/CMO. Investing in their own manufacturing facility would develop internal expertise, optimise processes, control manufacturing capacity, and potentially save money in the long run, if the drug commercialises.
However, utilising CDMOs/CMOs can help provide flexibility in capacity planning, reduce commitments to evolving technology platforms, and reduce initial investments. CDMOs/CMOs have existing cGMP facilities designed to comply with regulatory authorities. More importantly, they have a skilled workforce who can execute the process, manufacture the product and deliver it to hospitals and clinics. Some cell therapy sponsors employ a regional manufacturing model and use CDMOs/ CMOs to manufacture their products in various geographical regions. Concurrently, smaller scale
sponsors are utilising co-working or collaborative spaces such as The Center for Breakthrough Innovation (King of Prussia, PA, USA) and the Cell and Gene Catapult (Stevenage, UK) for meeting their development and manufacturing needs.
Paper-based processes are inefficient and error-prone
Sponsors’ demands are growing and complex, which have many CDMOs/CMOs pushing to become leaner and digital. To be agile enough to meet sponsor requirements of patient delivery deadlines and frequent schedule changes, an Electronic Batch Record solution should be a minimum prerequisite. Paper-based manufacturing systems are too slow and cumbersome in the cell therapy world. They lack the ease for tracking of data and continuous improvement.
For instance, given the volume of different manufacturing processes in the cell, gene and stem cell space, contract manufacturers must continuously manage customers’ work instructions and Standard Operating Procedures (SOPs) which change regularly. Operator training, manufacturing recipes and product specifications also vary and need to be updated frequently. Accurately and safely managing all of these changing documents via paper-based records is incredibly challenging and inefficient, with significant risk for harmful errors, delays and a lack of transparency.
In conclusion, CDMOs/CMOs must embrace
digitisation. A cloud-hosted digital electronic batch records solution coupled with a flexible scheduling solution and integration to other supply chain and IT systems is the ‘need of the hour’ today. With an EBR, cell therapy sponsors can monitor the CDMO/CMO process right from their desk. The most important compliance parameters of chain of identity, chain of custody, chain of condition and limited time horizon for critically ill patients can be monitored right at one’s fingertips. Any events, delays or changes can be addressed immediately.
With some upfront initial effort, CDMOs/CMOs will save a lot of effort downstream when data is integrated across the enterprise and quality and production teams are relieved of an enormous documentation burden. Companies who have adopted EBR have reported an 83 per cent decrease in data input errors and more than 87 per cent decrease in review time after production completes by embracing the review by exception paradigm. Rachit Jain is Global Cell and Gene Lead Software at Körber Business Area Pharma. In his role, he is focused on helping clients build necessary digital ecosystems to support blockbuster individualised therapies (e.g. immunotherapy and regenerative therapies) to tackle rare diseases. Through his work in cell and gene and consulting, he has developed a unique understanding of the synergies between the software solutions of Körber Business Area Pharma and the challenges facing the cell and gene therapy industry today. Rachit has a background in Process Engineering and holds an MBA from an Ivy League Business School.

About Körber
Körber is an international technology group with about 10,000 employees, more than 100 locations worldwide and a common goal: We turn entrepreneurial thinking into customer success and shape the technological change. In the Business Areas Digital, Pharma, Supply Chain, Tissue and Tobacco, we offer products, solutions and services that inspire.
At the Körber Business Area Pharma we are delivering the difference along the pharma value chain with our unique portfolio of integrated solutions. With our software solutions we help drug manufacturers to digitise their pharma, biotech and cell & gene factories. The software product Werum PAS-X MES is recognised as the world’s leading Manufacturing Execution System for the pharma & biotech industry. Our data analytics and AI solutions accelerate product commercialisation and uncover hidden business value.
www.koerber-pharma.com










