




On behalf of Tactile Medical, we want to introduce you to our airway clearance therapy, the AffloVest, a Mobile Mechanical HFCWO airway clearance therapy vest. It was designed to mimic the Gold Standard of airway clearance therapy, manual chest physiotherapy (CPT). The AffloVest is an important business growth opportunity for your company.
As you may know or have heard, there are many good reasons to market and sell the AffloVest (E0483 reimbursement code) such as:
• The highest 13-month capped rentals in respiratory
• Excellent return on investment
• New proven technology
• New revenue opportunity with existing patient base
Tactile Medical is now expanding our efforts in the respiratory market. Per a recent study, in Chronic Respiratory Disease dated March 2017, the current bronchiectasis market is $3-$5 billion (340,000-522,00 patients) that grows $700 million dollars annually (70,000 new patients per year). To put in perspective, there are only 1.5 million oxygen patients. However, Bronchiectasis is highly underdiagnosed because we must ask the doctors the right questions to learn about their respiratory patients with multiple chest infections treated with antibiotics each year.

• AffloVest Overview /701989-001C
• Welcome to AffloVest 7702073-001B
• VGM letter of designation
• VGM Financing 702511-001
Cystic Fibrosis, neuromuscular conditions and bronchiectasis are among the 67 ICD-10 codes covered for the AffloVest.
MEDICARE REQUIREMENTS FOR CYSTIC FIBROSIS AND NEUROMUSCULAR CONDITIONS:
Physicians order that includes: AffloVest prescription, qualifying diagnosis, chart notes to support the diagnosis, and well-documented failure of standard treatments to adequately mobilize retained secretions.
MEDICARE REQUIREMENTS FOR BRONCHIECTASIS:
Required: CT scan confirming diagnosis of bronchiectasis.
AND
Required: Daily productive cough for at least 6 continuous months.
OR
Frequent (i.e., more than 2/year) exacerbations requiring antibiotic therapy.
AND
Required: Well-documented failure of other standard treatments (flutter valve, percussion, postural drainage, breathing techniques) to adequately mobilize retained secretions.

There are over 67 ICD-10 codes that are covered for the AffloVest. E04893 Medicare allowable reimbursement is now $14,845.32 as a 13month capped rental for 2023. It is also commonly covered by commercial insurance. The national average for HFCWO is approximately $10,000 but do note this rate and coverage criteria can vary by payer.
REFERENCE DOCUMENTS:
• Medicare requirements /702272-001B
• ICD-10 Card 702072-001B
There are an enormous number of patients at your existing oxygen and NIV call points. Calling on the same doctors that prescribe oxygen E1390. This includes Family Practice, Internal Medicine, Physician Assistants, Geriatric etc. These doctors are looking for ways to keep patients in their clinics and can prescribe the AffloVest for their symptomatic patients with qualifying diagnoses.

Each of these clinics can have hundreds of respiratory patients with secretion issues, that they may think only have COPD but may additionally have bronchiectasis or other qualifying diagnoses instead. Bronchiectasis is easily diagnosed with a CT scan, and confirmation of persistent productive cough OR patient having multiple chest infections each year.
The typical symptoms for chronic respiratory diseases such as COPD, chronic bronchitis or asthma are similar and can have overlap.
• 45% of COPD patients with a chronic cough have episodes of acute bronchitis/ pneumonia1
• 42% of COPD patients may have bronchiectasis2
• The majority of COPD patients have hyperinflation of the lungs, resulting in diaphragmatic dysfunction3,4 and an ineffective productive cough leading to secretion mobilization issues.
Section Three: How to source new patients from your referring MDs
Simply ask your doctors about their respiratory patients who have daily productive cough that does not seem to go away or multiple chest infections per year requiring the use of antibiotics to treat them. These patients may have bronchiectasis or an additional qualifying diagnosis, not just COPD.
Using the questionnaire for at-risk respiratory patients (reference), formulate questions around the symptoms to identify the need for AffloVest.

Typical bronchiectasis symptoms and other covered diagnosis include:
• Chronic productive cough
• Mucus retention or plugging
• Frequent lung infections or pneumonias
• Recurring use of antibiotics for chest infections
To help facilitate follow-up with the patient once they have been setup, a follow-up questionnaire (reference) is available. This is helpful to ensure patient adherence and identify any issues that may require troubleshooting, minimizing the chance for equipment returns. In addition, the patient follow-up form gives you another opportunity to have a patient centered call with the physician, and keep airway clearance front of mind for the provider, so they will look for other patients that may need an AffloVest.
REFERENCE DOCUMENTS:
• At Risk Respiratory Questionnaire/ Screener 702270-001
• Follow Up Questionnaire 702445A
AffloVest, an airway clearance therapy, is the first battery-operated High Frequency Chest Wall Oscillation (HFCWO) therapy that lets patients with bronchiectasis, cystic fibrosis, and neuromuscular diseases receive state-of-the-art airway clearance therapy on the go.
Featuring patented Direct Dynamic Oscillation™, AffloVest has eight mechanical oscillating motors scientifically engineered to target all five lobes of the lungs, front and back, for fully mobile use. With no bulky hoses or generators, the AffloVest is engineered to encourage treatment adherence by allowing patients to experience an improved quality of life during airway clearance treatments.
The AffloVest offers the following advantages for your chronic respiratory patients:
COMPREHENSIVE TREATMENT
• Targets all five lobes of the lungs from the front and back
COMFORT
• AffloVest mobilizes secretions through high frequency chest wall oscillation and is engineered for comfort and to be less restrictive than older vest systems that require tubes and a compressor
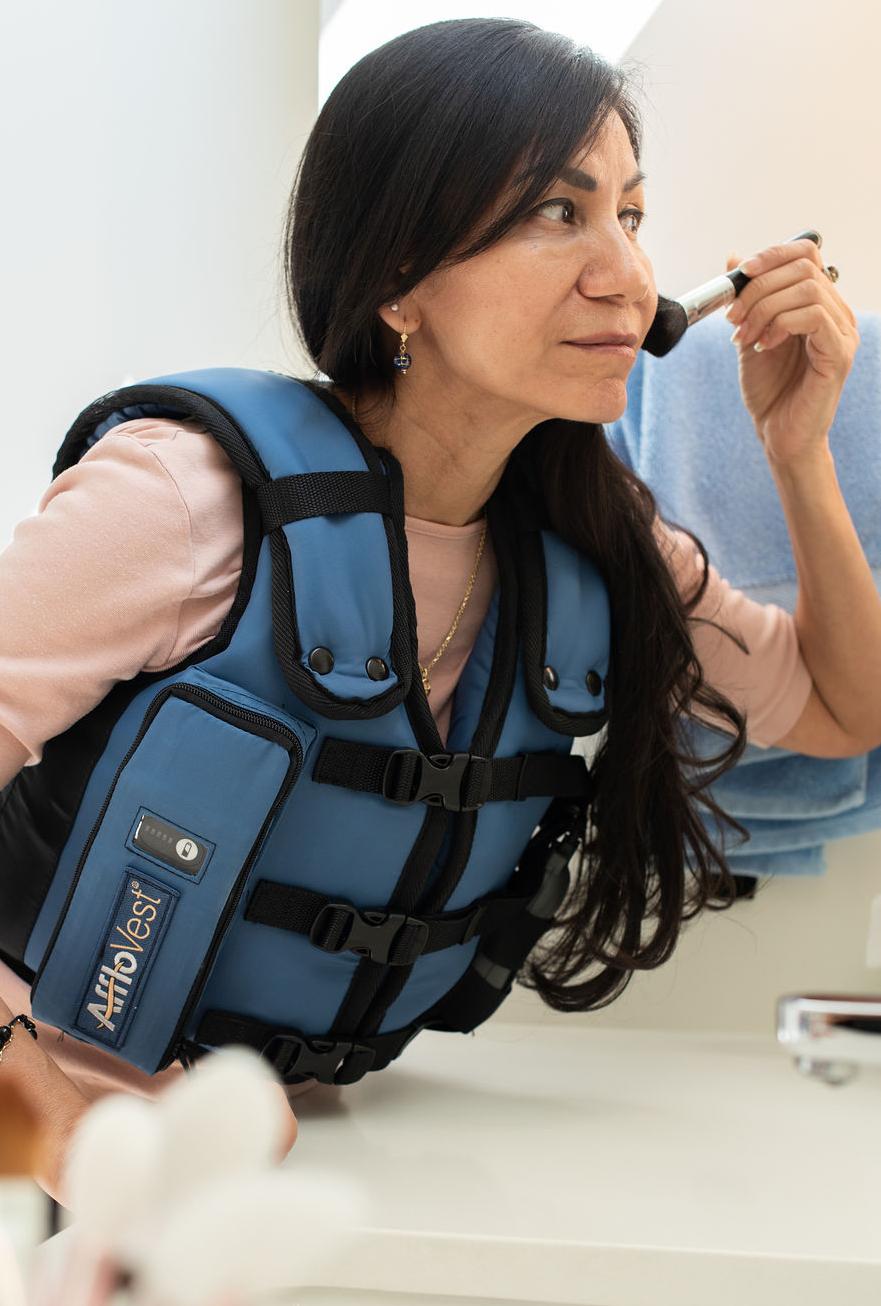
MOBILITY
• Battery powered and fully mobile during use so patients can stay active even during treatment
ACCURATE AND CUSTOM FIT
• Available in eight sizes accommodating chest circumferences from 18” to 75” for a custom fit. Weight range of vest is 5.1-9 0 pounds
WARRANTY
• The AffloVest is covered by a 5 year limited warranty. See the User Manual for full details and limitations.
USER MANUAL:
REFERENCE DOCUMENTS:
• AffloVest User Manual
One of the aspects prescribers and patients like about the AffloVest is that it is provided to them by a local DME company. The AffloVest Patient Training Guide is designed to help you facilitate a successful new patient set up.

REFERENCE DOCUMENTS:
• Patient training guide 021023-2
• Quick Start Guide 702288-001B
Lets say you’ve read through Section One, done you’re homework, and determined that the HFCWO device is right for your business. Before you begin developing your HFCWO program, there are still a few things you need to lay the groundwork. Follow the steps below to get started.
The first thing you need to do to get started is let CMS know you are entering the HFCWO device market. This simply requires you to updated your CMS 855S form (See example 2) include:
• Head to www.cms.gov/Medicare/CMSForms/Downloads/cms855s.pdf
• Go to Section 3: Products/Accreditation Information

• Find part D. Products and Services Furnished by This Supplier
• Check box labeled High Frequency Chest Wall Oscillation (HFCWO) Devices and/or Suppliers
• Check the box labeled Respiratory Assist Devices
• Return the form to CMS
That’s it. Once CMS processes the update, they’ll know that you will be submitting HFCWO devices and supplies for reimbursement.
Next, you’ll need to contact your accrediting body and inform them you’re taking referrals for HFCWO. They’ll go over the next steps and any requirements there may be, such as policies and procedures, and other forms.
If you’re in the market for one, VGM Partners with the Healthcare Quality Association on Accreditation (HQAA). HQAA makes the accreditation process easy for HME providers. They even pair your organization through the entire process.
Tactile Medical offers many resources for you including training to help you drive demand and educate the prescriber/patient community.
• IMPACT resources: Physician and patient education tool sets to facilitate disease state and airway clearance education (nonbranded for clinic use)
• Tactile Knowledge Center for continuing education courses
• INSIGHT Online E-learning platform for your sales, clinical and billing teams onsite
• Informative videos that explain bronchiectasis and the AffloVest to the patient population
• New literature and resource kit specific to bronchiectasis
• Email marketing and social media campaigns directed at bronchiectasis patients and clinicians
• Sales and marketing training programs for your staff. The AffloVest team will help train you and work in the field with your sales team.
For more detailed support and training please contact your local AffloVest representative.

REFERENCE DOCUMENTS:
• For contacts call AffloVest customer service: 800-575-1900
• Impact-BE.com
• education.tactilemedical.com/
• INSIGHT Quick Start Guide 702273-001
