December - January 2023


December - January 2023

Chronic wounds, also known as hard-to-heal wounds, have a significant economic and health burden globally. Hyaluronic acid has a number of advantageous properties in wound healing. These include properties of protection of stem cells, growth factors, and cytokines. Alligator tissues have been considered to have bacteriostatic and fungistatic properties. Chronic skin wounds are often infected with pathogenic microorganisms that may contribute to delayed wound healing. Antimicrobial-based advanced wound care products are widely used to treat microbially contaminated wounds. However, toxicology and wound healing studies have shown antimicrobials can delay wound healing and are cytotoxic at high levels. Furthermore, many of these products are not safe for the environment, as such there is a trend in the United States and Europe to utilize natural ingredients that are ecofriendly, reduce the bioburden, and accelerate wound healing. Since it has previously been suggested that American alligator-derived tissues exhibit bacteriostatic and fungistatic properties, we compared the bacteriostatic and fungistatic properties, biofilm eradication capability and cytotoxicity of American alligator-derived hyaluronic acid (aHA) with that of commercially available streptococcal-derived HA (sHA).
Introduction
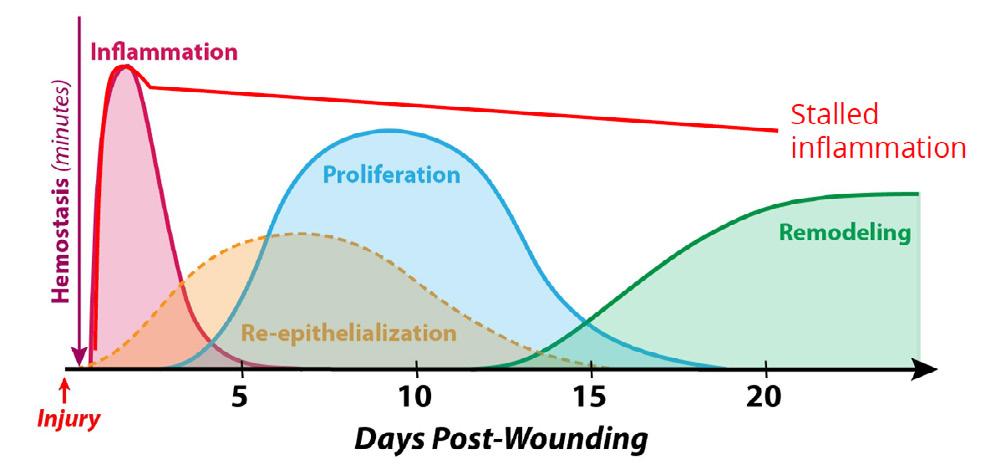
Worldwide, chronic wounds are a substantial burden to the quality of life (QoL) of the patients as well as a significant economic burden to our health care system with an estimated annual cost of $50B in the US alone.1,2 Nonhealing wounds are often stalled in the inflammatory phase (see Figure 1) and considered hard-to-heal or chronic wounds when healing is delayed in the phase of wound healing for greater that 4 weeks and up to more than three months.3 Some wounds remain chronic for years. Thus, the longer time it takes these wounds to heal provides a severe risk for a polymicrobial infection which can stall the wound even further in the inflammatory phase and is concomitant with a higher incidence of lower extremity amputation (LEA) and mortality.4
Hyaluronic acid matrices have been utilized in wound care to replenish and stabilize the extracellular matrix in a chronic wound bed.5,6 Hyaluronic acid has also been shown to protect stem cells, growth factors, and cytokines required for cell proliferation and angiogenesis.7,8,9 Additional evidence suggests that hyaluronic acid may also inhibit bacterial growth and could possibly bind host and bacterial proteases that are hyperactivated in the stalled inflammatory phase of a diabetic wound.10,11







Since Alligator tissues have been implicated as having bacteriostatic and fungistatic properties, our hypothesis was that this source of aHA may play a critical role in reducing the bioburden to promote chronic wound healing.12,13
 Ms Nikole Siegmund
Mr Ted Bundrick
Ms Nikole Siegmund
Mr Ted Bundrick
Figure 1: Normal wounds progress swiftly through the inflammatory phase to granulation tissue formation and the tissue remodeling phase of wound healing. Chronic wounds are often infected and exhibit an extended and hyperproliferative inflammatory phase (redline). Bacterial toxins, specifically proteases such as V8, Endo Glu-C in Staphylococcus aureus play an important role in delaying the healing process by hyperactivation of host metalloproteases (MMPs) and human neutrophil elastase causing matrix degradation, leading to senescent fibroblasts and recruitment macrophages stalling the inflammatory phenotype (M1).
antimicrobial resistance isolate bank: Candida auris (C. auris) AR-0383.
In vitro, we tested the effectives of aHA at microbial stasis with common bacterial and fungal pathogens including Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Enterococcus faecalis, Escherichia coli, Candida albicans, and Candida auris. In vivo, we utilized a delayed wound healing diabetic mouse model to characterize the wound healing parameters, gene expression and immunohistochemistry with a novel high molecular weight aHA matrix, named LacertaMatrix™, that activates NF-kB activation to quell the inflammatory phase and accelerate wound healing. Here we provide evidence to suggest that aHA can act on the inflammatory phase of wound healing either by:
1. Reducing the microbial bioburden
2. Through the quelling of the inflammatory phase through NF-kb signaling and macrophage plasticity
Chemicals, Antibodies, and Cell Culture
Microbes were either purchased from ATCC, CDC, or were clinical ID wound isolates from our clinical wound isolate library. Specifically, Staphylococcus aureus (S. aureus) ATCC 6538, Pseudomonas aeruginosa (P. aeruginosa)
ATCC 15692, Enterococcus faecalis (E. faecalis) ATCC11823, Escherichia coli (E. coli)
ATCC BAA 1427. Streptococcus pyogenes (S. pyogenes) UCO012-and Candida albicans (C. albicans) 509-8-2 clinical wound strains from our clinical wound isolate library. A Candida strain was purchased from CDC
Hyaluronic acid was purified using a method describe by Sanders.14 Briefly we utilized a 4-step process grinding, extraction, anion exchange chromatography, and tangential flow filtration. The ground alligator backstrap was provided by Lacerta Life Sciences for overnight extraction at 4°C in a buffer containing; Tris, EDTA, and SDS (10 mM Tris, 10mM EDTA, 0.1% SDS. The extract was pH adjusted to 6.2 with 3M Na Acetate buffer and then clarified by depth filtration using a combination 5.0 - 0.2 μm filters (Pall Life Sciences). Next, anion exchange chromatography (STIC PA, Sartorius) was utilized with a 50m M- 3M NaCl step gradient to purify the hyaluronic acid from proteins and nucleic acids. Most protein eluted in the flow through, and the low salt step gradient (500-800 mM), while the purified hyaluronic acid eluded in a 2-3M step gradient of NaCl. The hyaluronic acid was desalted and concentrated using a 100K MWCO tangential flow filtration membrane. We utilized the Purple Jelly Kit (Biocolor LTD) to quantitate the concentration of hyaluronic and a DNA kit (ThermoFisher) to measure the impurities of each prep.
Bacteria (S. aureus, P. aeruginosa, S. pyogenes, E. faecalis, E. coli) were cultured in Tryptic Soy Broth (TSB) at 37°C for overnight. C. auris and C. albicans were grown in Yeast ExtractPeptone-Dextrose (YPD) overnight at 30°C Microbe concentrations were determined the following morning in Phosphate Buffer Saline (PBS) dilutions, and then the cultures were diluted to 106 cells/mL. 100 μl of each bacteria was added in pentuplicate to a 96 well plate with different concentration of HA (Plate 1) and then incubated at 37°C. 100 μl of C. albicans or C. auris was added in pentuplicate to a separate plate with different concentrations of HA (Plate 2) and incubated at 30°C. Bacteriostatic and fungistatic properties were measured at 600nm and the background was subtracted from the media + HA alone controls at each concentration of HA. Two HA incubation concentrations at 3 and 1.5 mg/mL were used for S. aureus, S. pyogenes, E. faecalis and C.
was used for E. coli, P. aeruginosa, and C. auris.
We developed a method for forming hyaluronic acid matrices using serine benzyl ester and zero length crosslinkers N-Hydroxysuccinimide (NHS) and ethylene dichloride (EDC) as described previously (Sanders, 2019). Briefly the hyaluronic acid is diluted to 1.2 mg/mL and reacted with an equal amount w/w of serine benzyl ester and NHS follow by a 1-5fold molar excess of EDC. The material was mixed for 2 - 5 minutes with a high-speed mixer and then poured in metal trays lined with parchment paper to set overnight at 37°C with 30-35% relative humidity.14
85 male diabetic (BKS.Cg-Dock7m +/+ Leprdb/J) mice, 9 weeks old were purchased and received from Jackson Laboratories. We utilize male mice because they have a stronger disease phenotype and are profoundly obese, insulin resistant, and glucose intolerant.15,16
Animal wound healing studies were carried out at Woodland Biosciences and Alpha Preclinical at the Tufts Cummings School of Veterinary Medicine AAALAC-accredited laboratories in North Grafton, MA. The IACUC protocols for the study were G2019-96 and G2021-28.
Animal health was accessed daily to check for general health, mortality, and moribundity. Animals were housed two per cage in 100% PET plastic, BPA-free caging with corncob bedding (Innovive, San Diego, CA). Environmental controls included a 12-hour light-dark cycle (0700 - 1900) at 20 – 26°C and 30 - 70% relative humidity. Animals were provided water and rodent chow ad libitum.
All procedures were approved by the Tufts University Cummings School of Veterinary Medicine Institutional Animal Care & Use Committee. Mice were anesthetized by inhalation of isoflurane (1 - 4%) with oxygen (O2). Each animal received Buprenorphine-SR (1.0 mg/kg) subcutaneously one hour prior to wounding for analgesia.
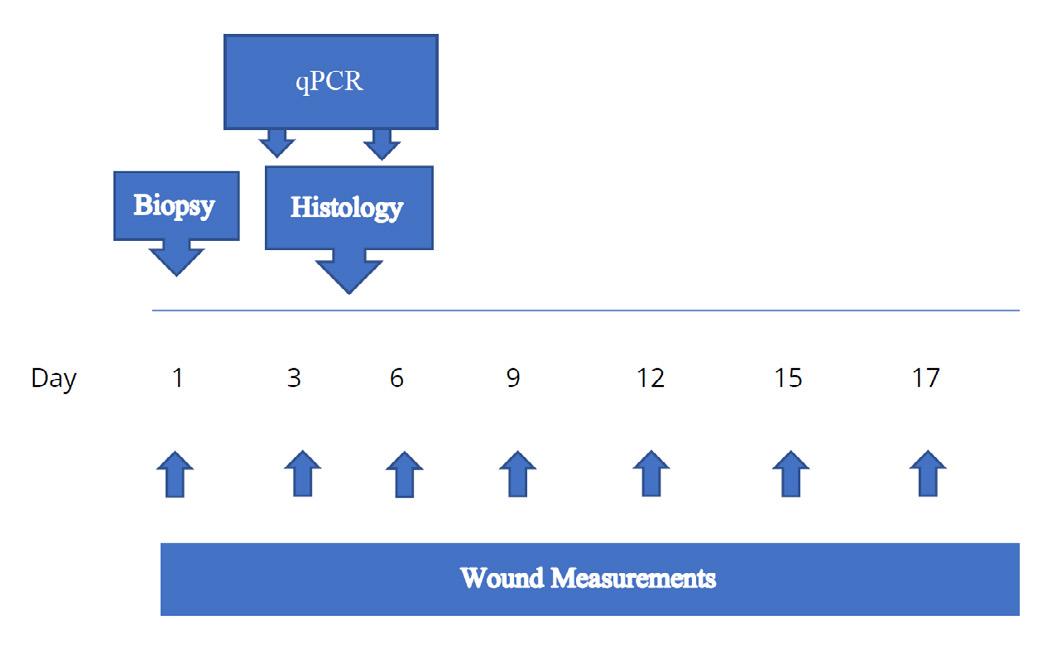
The dorsal skin was shaved and was cleaned with isopropanol wipes prior to wounding the skin with a sterile 8 mm punch biopsy. Animals were treated weekly with aHA or sHA and then each wound was covered with a sterile Tegaderm™ dressing. The Negative controls consisted of Tegaderm™ alone, and positive controls PDGF-β (10 μg/ml) and TGF-α (1 μg/ml) prepared in 0.5% hydroxypropyl methylcellulose (HPMC) and applied topically for the first seven (7) days of the study. Animals were anesthetized every three days post wound creation (Days 3, 6, 9, 12, 15, 18 and day 20 prior to termination). The Tegaderm™ film dressing was removed for taking images of the wounds measured with Tissue Analytics imaging software of the wound and a new Tegaderm™ film dressing applied. Topical treatments were applied on Days 0-6 for controls (anesthetized each day) and Days 0, 3, 6, 9, 12, 15, 18 for aHA and sHA treatments (Figure 2). Animals were observed daily from start through termination and bodyweights were recorded at least three times per week.
Wound healing was assessed over a 20-day period in terms of:
i. Initiation of neo-dermal repair responses
ii. Wound closure
Table 1: Genes expression, by qPCR, as performed on wounds harvested from study animals on day 6 of wound healing. Genes involved in the proliferative phase of wound healing were chosen and are listed along with the forward and reverse primers, and the thermal cycling parameters.
IL6
IRAK1
CXCL9
TACCACTTCACAAGTCGGAGGC CTGCAAGTGCATCATCGTTGTTC
CGACTGGCTGTGGACACCGATAC
CCTAGTGATAAGGAATGCACGATG
CCTGGGCTACTCCTCACACTTGATTC
CTAGGCAGGTTTGATCTCCGTTC
MIP2 CATCCAGAGCTTGAGTGTGACG GGCTTCAGGGTCAAGGCAAACT
NFκB-1
GAAATTCCTGATCCAGACAAAAAC ATCACTTCAATGGCCTCTGTGTAG
TNF-α GGTGCCTATGTCTCAGCCTCTT GCCATAGAACTGATGAGAGGGAG
NGF
KI-67
Arg1
GTTTTGCCAAGGACGCAGCTTTC GTTCTGCCTGTACGCCGATCAA
ATCATTGACCGCTCCTTTAGGT GCTCGCCTTGATGGTTCCT
AACACGGCAGTGGCTTTAACC GGTTTTCATGTGGCGCATTC
CD163 GGCTAGACGAAGTCATCTGCAC CTTCGTTGGTCAGCCTCAGAGA
GAPDH* CATCACTGCCACCCAGAAGACTG ATGCCAGTGAGCTTCCCGTTCAG
HPRT1* CTGGTGAAAAGGACCTCTCGAAG CCAGTTTCACTAATGACACAAACG
* Housekeeping Gene
Initiation of neo-dermal tissue formation were expressed as the number of wounds responding in each group at each time point. Wound closure was considered in both overall terms and in terms of its components, wound contraction and wound re-epithelialization. Wound closure (contraction and reepithelialization) was determined from digital images taken on Days 0, 3, 6, 9, 12, 15, 18, and 20. Images were taken before the treatment started. Any adverse effects of treatment were fully documented and managed by the preclinical site, according to IACUC guidelines, in consultation with the attending veterinarian, up to and including euthanasia.
Wound Healing measurements were taken every 3 days either with a digital caliper and/or Tissue Analytics software which allows the measurements of the wound volume as well as the color of the wound which complements the visual assessment of the wound bed.
To quantify the level of mRNA expression on Day 3 and Day 6 of the target genes with the four treatment groups, wounds were isolated and quickly incubated in tissue extraction buffer (Qiagen Catalogue number 76106) followed by snap freezing in liquid nitrogen, and storing at -80°C until processed for RNA extraction. Total
1. 50°C - 10 minutes
2. 95°C - 1 minute
3. 95°C - 10 seconds
4. 55°C - 30 seconds
5. Plate read
6. Repeat steps 3, 4, 5 35X
7. 4°C infinity
Total RNA was extracted by a Qiagen kit according to manufacturer’s instructions. About 2–5 μg of RNA was used for reverse transcription with the CFX384™ Real-Time PCR thermocycler. The resultant cDNA was used for qPCR with the SYBR Green qPCR mix (Bio-Rad) according to manufacturer’s instructions. qPCR was carried out using CFX384™ Real-Time PCR thermocycler (Bio-Rad Laboratories, Hercules, CA) with fluorescence detection and set of reagents iTaq Universal SYBR Green 1- Step Kit (BioRad #172-5151). The primers used for qPCR are found in Table 1 which includes the thermal cycling parameters. The CFX Maestro software 3.1 was used to quantitate the relative gene expression and the p values for statistical significance (day 3 and day 6).
The wound tissue was harvested on day 4, and were fixed in 4% paraformaldehyde in PHEM Buffer (60 mm PIPES, 25 mm HEPES, 10 mm EGTA, and 2 mm MgCl2 at pH 6.9) at 4°C for 24 hours. The samples were rinsed with PBS, followed successive dehydration steps with ethanol (70%, 90%, 100%), the tissue was embedded in paraffin, and sectioned at 5m for histopathology. Paraffin sections were dewaxed and were rehydrated by xylene and gradient alcohols and were stained with hematoxylin and eosin (H&E) for microscopic observation. Histology was performed by HistoWiz, Inc. (Brooklyn, NY) using standard operating procedures and fully automated
workflow. Multiplex immunohistochemistry was performed on a Bond Rx auto-stainer (Leica Biosystems, Nussloch, Germany) with heat-mediated epitope retrieval by using standard protocols. The primary antibody that was used is Arg1 (Cell Signaling Technologies, Danvers, MA; CAT# CST93668), at a dilution of 1/100. Wholeslide scanning (40x and 60x) was performed with an Aperio AT2 (Leica Biosystems, Nussloch, Germany). A secondary fluorescent antibody was used for the indirect immunofluorescence staining with Arg1.
Statistical analysis was performed using GraphPad Prism 9.2 (San Diego, CA) with the differences between the negative control, positive control and the treatment groups analyzed by ANOVA (p <0.05 considered as statistically significant). Results were expressed as mean +/- standard deviation.
This study assessed the bacteriostatic and fungistatic properties of hyaluronic acid derived from America Alligator (aHA) and a Streptococcus source (sHA) and the wound healing properties of LacertaMatrix™ a novel aHA derived matrix versus HyaloMatrix® derived from sHA.
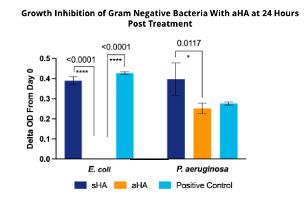
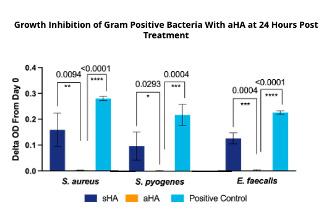
Our findings indicate that aHA is more effective at bacteriostatsis than sHA for both gram positive (+) and gram negative (-) bacteria (Figure 3 and 4). Low concentrations aHA were required for microbial stasis of Gram (+) bacteria and C. albicans
In contrast, C. auris, a multidrug resistant and emerging pathogen, was resistant to the fungistatic properties of aHA and Gram (-) bacteria and required a higher concentration of sHA (5 mg/mL) to be effective.
In a non-diabetic mouse, the phases of wound healing occur over a 18-20 day period. The overlapping wound healing process including hemostasis (0–several hours after injury), inflammation (1–3 days), proliferation (4–21 days) and tissue remodeling (21 days–1 year).
In contrast, in a diabetic mouse model the early stages of wound healing are profoundly
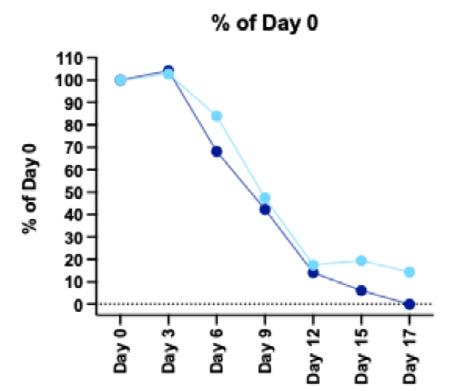
delayed in the inflammatory (1-6 days) and the proliferative (7-31 day) phases. Our findings indicate that LacertaMatrix™ quells the inflammatory phase by 3 days as judged by the reduced wound size and significant reduction in red color by days by 5-6 vs. the untreated control (p <0.05).
Over 24 hours. Bacteriostasis was proportional to the concentration of HA. In general, higher concentrations of aHA were required for treating gram negative bacteria (5 mg/mL) vs gram positive bacteria (1.5 mg/mL). Five (5) mg/mL of aHA totally inhibited the bacterial growth of the E. coli as compared to the streptococcalderived HA. Surprisingly, LacertaMatrix™ was less effective at inhibiting P. aeruginosa even at 5 mg/ ml and the streptococcal-derived HA enhanced the growth of the bacteria.
Figure 4: Fungal Growth Inhibition Over 24 hours. Fungistasis was profound at low concentrations (1.5 and 3 mg/mL) of aHA. Streptococcal-derived (sHA) also inhibited C. albicans but the growth inhibition was not as effective as aHA. p-values of statistical significance are all noted above the respective bars. Limited activity was seen against C. auris which is a hard pathogen to kill.

In addition to dramatic reduction in the inflammatory phase, hyaluronic acid treated animals completed healing by 17 days, as if they were normal animals (Figure 5).
These findings are quite different from the treatment of these diabetic wound with human amnion chorion matrices (HACM) which seem to only accelerate the proliferative and tissue remodeling phases of wound healing (Sanders et al., submitted).
H&E Staining indicates that the LacertaMatrix™ healed wound have a mature and repaired epidermal and dermal layer with marked tissue granulation but no apparent
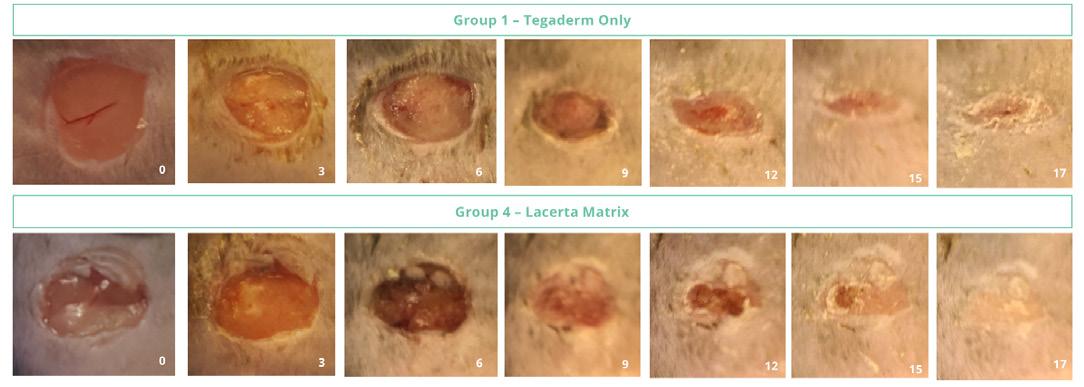
inflammation or necrosis. In contrast the negative control wounds had incomplete reepithelialization with a higher inflammatory response (not shown). The LacertaMatrix™ dressing derived from aHA had statistically significant healing vs. the untreated control (Figure 6).
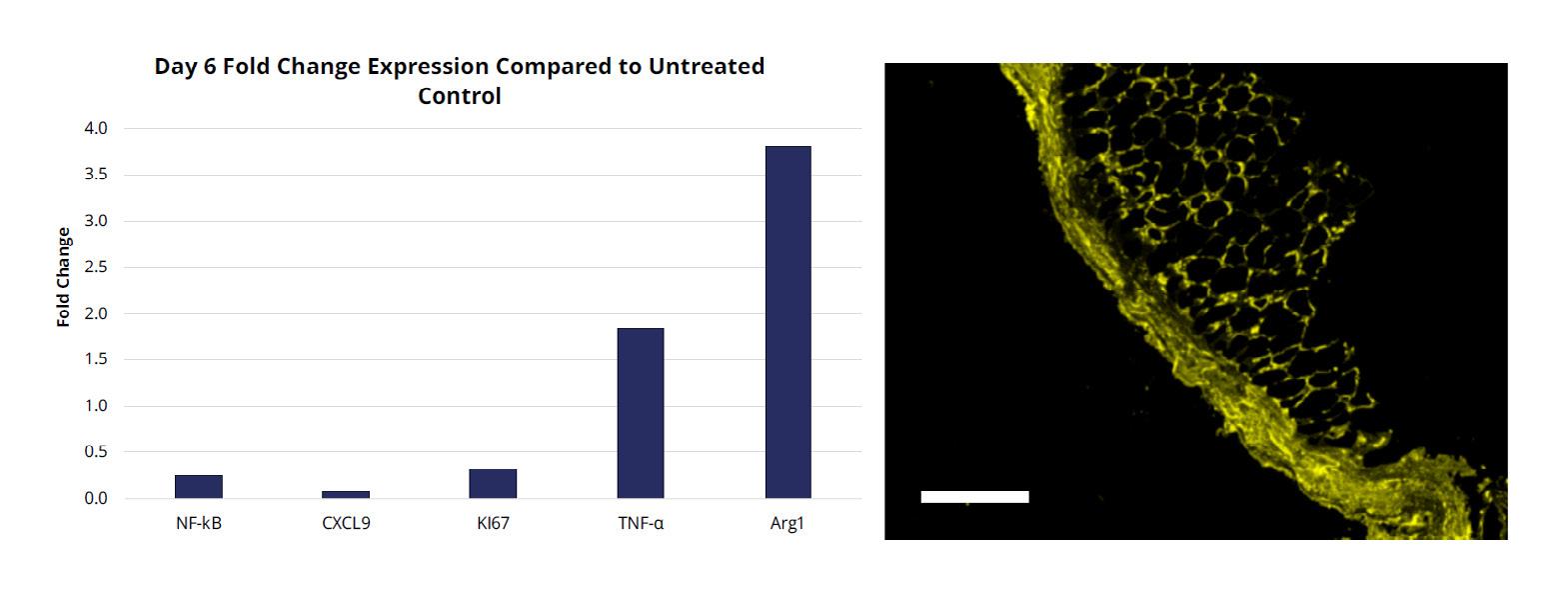
Based on the reduction in the inflammatory phase in wound healing, we wanted to examine gene expression levels on day 3 and Day 6 to study biomarkers that are thought to be involved in quelling the inflammatory phase and stimulating the early proliferative phase of wound healing (Figure 7


By day 3 there was an induction of transcription factor NF-kB but by day 6 it was dramatically attenuated. NF-kB is an important transcription factor that functions as a pivotal mediator of the inflammatory response in wound healing. Attenuation of NF-kB occurred at the same time as a reduction of CXC9 and K167 and the over expression of TNF-α and Arginase 1 (Arg1). Arg1 is a key effector and marker of anti-inflammatory M2a macrophages. M2a macrophages stimulate fibroblast proliferation and tissue repair. TNF-α produced by M2a macrophages promotes the formation of new extra cellular matrix (ECM) to accelerate tissue proliferation. Immunofluorescence also indicates that Arg1 has a higher level of staining than the untreated control which is consistent with what was observed by qPCR (Figure 8). This induction of Arg1 by day 6 is somewhat unanticipated since previous reports have shown that the transition of inflammatory M1 macrophages to the M2a anti-inflammatory macrophages usually occurs much later in the proliferative phase of wound healing (Sanders et al., submitted).17
Our findings indicate that the source of HA (aHA, Alligator vs. sHA Streptococcal) has profound differences in microbial stasis properties and to a lesser extent slightly improved wound healing in a diabetic mouse delayed healing model. By STIC PA anion exchange chromatography, we determined that aHA is more anionic and binds to a STIC PA anion exchange column more strongly with 2-3M NaCl elution profile, while sHA elutes from the same column at 500mM NaCl. The strong anionic character of the aHA likely is modulating the improved bacteriostatic and fungistatic properties (as described in Figures 3 and 4). Higher concentrations of aHA were required for gram negative bacteria, which makes sense because gram negative bacteria have three membranes (inner cytoplasmic membrane (CM), a peptide glycan wall (PGW), and a closely attached outer membrane (OM)). In contrast gram positive bacteria only have a CM and a PGW, while lack the outer membrane. Findings by Erickson et al., (2021)18 suggest that these bacteria are under a great amount of turgor pressure and we suspect that the aHA treated bacteria are experiencing osmotic shock due to the strong
anionic nature of the hyaluronic acid material which inhibits the growth at 37°C. In 2011, Kang et al. demonstrated that low molecular weight (1630 kDa) sHA had modest fungistatic properties and the activity was concentration dependent.19 However, there was only a 1020 hr inhibition that C. albicans was able to recover from by 24 hours. Our findings suggest that aHA is much more potent than sHA, even after 3 days of incubation. C. auris is a multidrug resistant and emerging pathogen in hospitals and we suspected that this organism would be more resistant to aHA, and our findings suggest that to be case. We observed a statistical reduction in OD600 for C. auris but it was modest compared to that of the C. albicans data. It is well understood that the transition from M1- to M2-like macrophage phenotypes is impaired in diabetic wounds resulting in increased accumulation of M1-like macrophage phenotypes (Figure 1). Our findings indicate that the novel hyaluronic acid matrix is able to quell the inflammatory phase of wound healing based on clinical signs, wound healing and gene expression (qPCR). Regulation of NF-kB is imperative for an appropriate inflammatory response.20,21 HA has been shown to modulate the inflammatory response through the proposed mechanism of binding CD44 to activate NFkB pro-inflammatory pathways or through activation of antiinflammatory pathways, dependent upon the molecular weight (MW) range of HA fragments.22,23 LacertaMatrix™ contains HA fragments in a range of MWs that is crosslinked into a high MW, therefore the HA present in this novel aHA-derived matrix may aid in correcting chronic inflammation while simultaneously contributing to cellular proliferation. In contrast low MW HA can be internalized by cells by a CD44 mediated endocytosis process which can exacerbate the inflammatory phase of wound healing.24 Our results suggest that LacertaMatrix™ has a dual role to quell the inflammatory phase through microbial stasis and while modulating anti-inflammatory pathways and macrophage plasticity (M1 to M2 switching) to aid in the closure of chronic wounds. These findings collectively suggests that LacertaMatrix™ may be a beneficial early intervention for chronic wounds stalled in the inflammatory state.
Disclosures
This project was funded by Lacerta Life Sciences.
1. Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, Car J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019 Jan;27(1):114-125. doi: 10.1111/wrr.12683. Epub 2018 Dec
2. PMID: 30362646.
2. Sen CK. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 2019 Feb 1;8(2):39-48. doi: 10.1089/wound.2019.0946. Epub 2019 Feb 13. PMID: 30809421; PMCID: PMC6389759.

3. Eriksson E, Liu PY, Schultz GS, Martins-Green MM, Tanaka R, Weir D, Gould LJ, Armstrong DG, Gibbons GW, Wolcott R, Olutoye OO, Kirsner RS, Gurtner GC. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022 Mar;30(2):156-171. doi: 10.1111/wrr.12994. Epub 2022 Feb 7. Erratum in: Wound Repair Regen. 2022 Jul;30(4):536. PMID: 35130362; PMCID: PMC9305950.
4. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020 Mar 24;13(1):16. doi: 10.1186/ s13047-020-00383-2. PMID: 32209136; PMCID: PMC7092527.
5. Caravaggi C, Grigoletto F, Scuderi N. Wound Bed Preparation With a Dermal Substitute (Hyalomatrix® PA) Facilitates Reepithelialization and Healing: Results of a Multicenter, Prospective, Observational Study on Complex Chronic Ulcers (The FAST Study). Wounds. 2011 Aug;23(8):228-35. PMID: 25879233.
6. Motolese A, Vignati F, Brambilla R, Cerati M, Passi A. Interaction between a regenerative matrix and wound bed in nonhealing ulcers: results with 16 cases. Biomed Res Int. 2013;2013:849321. doi: 10.1155/2013/849321. Epub 2013 Jul 18. PMID: 23971047; PMCID: PMC3732600.
7. Ren Y, Zhang H, Wang Y, Du B, Yang J, Liu L, Zhang Q. Hyaluronic Acid Hydrogel with Adjustable Stiffness for Mesenchymal Stem Cell 3D Culture via Related Molecular Mechanisms to Maintain Stemness and Induce Cartilage Differentiation. ACS Appl Bio Mater. 2021 Mar 15;4(3):2601-2613. doi: 10.1021/acsabm.0c01591. Epub 2021 Feb 15. PMID: 35014377.
8. Kim H, Kong WH, Seong KY, Sung DK, Jeong H, Kim JK, Yang SY, Hahn SK. Hyaluronate- Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules. 2016 Nov 14;17(11):3694-3705. doi: 10.1021/acs. biomac.6b01216. Epub 2016 Nov 3. PMID: 27775884.

9. Shahbazi MA, Sedighi M, Bauleth-Ramos T, Kant K, Correia A, Poursina N, Sarmento B, Hirvonen J, Santos HA. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega. 2018 Dec 27;3(12):18444- 18455. doi: 10.1021/acsomega.8b03182. Erratum in: ACS Omega. 2019 Mar 28;4(3):5931. PMID: 31458417; PMCID: PMC6711357.
10. Carlson GA, Dragoo JL, Samimi B, Bruckner DA, Bernard GW, Hedrick M, Benhaim P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun. 2004 Aug 20;321(2):472-8. doi: 10.1016/j.bbrc.2004.06.165. PMID: 15358200.
11. Gao Z, Yang X, Jones E, Bingham PA, Scrimshire A, Thornton PD, Tronci G. An injectable, self-healing and MMPinhibiting hyaluronic acid gel via iron coordination. Int J Biol Macromol. 2020 Dec 15;165(Pt B):2022-2029. doi: 10.1016/j. ijbiomac.2020.10.079. Epub 2020 Oct 17. PMID: 33080264.
12. Santana FL, Arenas I, Haney EF, Estrada K, Hancock REW, Corzo G. Identification of a crocodylian β-defensin variant from Alligator mississippiensis with antimicrobial and
antibiofilm activity. Peptides. 2021 Jul;141:170549. doi: 10.1016/j.peptides.2021.170549. Epub 2021 Apr 15. PMID: 33865931.
13. Barksdale SM, Hrifko EJ, van Hoek ML. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev Comp Immunol. 2017 May;70:135-144. doi: 10.1016/j.dci.2017.01.011. Epub 2017 Jan 13. PMID: 28089718.
14. Purification of reptilian hyaluronic acid and its use for soft and hard tissue repair. WO WO2021108790A1 Mitchell C. Sanders Lacerta Life Sciences, LLC Priority 2019-11-30 • Filed 2020-11-30 • Published 2021-06-03
15. Huynh P, Phie J, Krishna SM, Golledge J. Systematic review and meta-analysis of mouse models of diabetes-associated ulcers. BMJ Open Diabetes Res Care. 2020 May;8(1):e000982. doi: 10.1136/bmjdrc-2019-000982. PMID: 32467222; PMCID: PMC7259859.
16. Gilliver SC, Emmerson E, Campbell L, Chambon P, Hardman MJ, Ashcroft GS. 17betaestradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol. 2010 Jun;176(6):2707-21. doi: 10.2353/ajpath.2010.090432. Epub 2010 May 6. PMID: 20448060; PMCID: PMC2877833.
17. Sanders MC, S Balaji, WB Martin, N Siegmund, L Poland, MS Hanna, H Kaliada, S Littlejohn, and T Ganey, Protecting human amnion and chorion matrices (HACM) during processing: Performance enhancement in a Diabetic Mouse Model. Wound Repair and Regeneration (In Revision, Wound Repair and Regeneration).
18. Erickson HP. How Teichoic Acids Could Support a Periplasm in Gram-Positive Bacteria, and Let Cell Division Cheat Turgor Pressure. Front Microbiol. 2021 May 10;12:664704. doi: 10.3389/fmicb.2021.664704. PMID: 34040598; PMCID: PMC8141598.
19. Kang JH, Kim YY, Chang JY, Kho HS. Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral Dis. 2011 Sep;17(6):577-83. doi: 10.1111/j.1601-0825.2011.01807.x. Epub 2011 Apr 8. PMID: 21477181.
20. Müller S, Sindikubwabo F, Cañeque T, Lafon A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C, Charafe-Jauffret E, Durand A, Vallot C, Baulande S, Servant N, Rodriguez R. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5. Epub 2020 Aug 3. PMID: 32747755; PMCID: PMC7612580.
21. Jaskuła K, Sacharczuk M, Gaciong Z, Skiba DS. Cardiovascular Effects Mediated by HMMR and CD44. Mediators Inflamm. 2021 Jul 10;2021:4977209. doi: 10.1155/2021/4977209. PMID: 34335086; PMCID: PMC8286199.
22. Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183(5):2373- 2378. doi:10.1084/jem.183.5.2373
23. Pandey MS, Baggenstoss BA, Washburn J, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis (HARE) activates NF- B-mediated gene expression in response to 40-400- kDa, but not smaller or larger, hyaluronans. J Biol Chem. 2013;288(20):14068-14079. doi:10.1074/jbc.M112.442889
24. Müller S, Sindikubwabo F, Cañeque T, Lafon A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C, Charafe-Jauffret E, Durand A, Vallot C, Baulande S, Servant N, Rodriguez R. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5. Epub 2020 Aug 3. PMID: 32747755; PMCID: PMC7612580.