
March 2024


March 2024
This comprehensive masterclass guide offers an in-depth look at the Mirragen® Bioactive Glass Wound Matrix (BGWM) designed to support wound closure. Discover insights into the product’s unique composition, mechanisms of action, and clinical applications. Discover suitable wound types for its use, master the proper usage techniques through step-by-step instructions and video, ensuring maximum efficacy and patient comfort. An evidence-based mini case series showcases the transformative power of this cutting-edge wound care solution in real-world clinical settings.
■ Mirragen® Glass Wound Matrix is a flexible and formable skin substitute that easily adapts to any wound bed. Made from natural elements, the porous fiber and microsphere structure absorbs wound exudate to maintain moisture balance.
■ Solely composed of bioabsorbable fibers and particles. It is a flexible and moldable wound matrix that can be easily customized to fit the wound bed. The fiber structure of Mirragen® Glass Wound Matrix allows it to absorb fluid from the wound and facilitate natural wound healing.
■ The material is biocompatible and will eventually be fully absorbed at the wound site. This resorption process is initiated by the exposure of the material to fluid at the wound site.


■ Bioactive glass matrix
■ Wound
■ Wound care
■ Moisture Management
■ Wounds
■ Wound Healing

■ Mirragen® Glass Wound Matrix is sterile and aseptic techniques should be used.
■ Prepare the wound bed using standard wound cleaning and debridement procedures.
■ Dry the periwound area.
■ Cut or shape Mirragen® Glass Wound Matrix to fit the size of the defect, directly in the wound bed, ensuring full coverage.
■ If applying Mirragen® Glass Wound Matrix to a dry wound, moisten the wound bed and/or the material with sterile saline to aid in maintaining a moist wound environment.
■ Use appropriate fixation as determined by wound location, size and depth.
■ Add a non-adherent layer to secure Mirragen® Glass Wound Matrix to the wound bed.
■ Place a suitable secondary dressing over the Mirragen® Glass Wound Matrix to manage the wound environment and protect the wound area. The secondary dressing should completely cover the Mirragen® matrix.
■ Reapplication of Mirragen® and all secondary dressings should be conducted according to standard wound care practices every 3 to 7 days. Heavily exudating wounds may require more frequent dressing changes.
■ As healing occurs, new tissue may grow into Mirragen® Bioactive Wound Matrix. This is desirable, DO NOT forcibly remove or debride areas of Mirragen® that are embedded or adhered to the wound site, these are 100% bioabsorbable and will eventually be absorbed at the wound site.
■ Cut or shape Mirragen® Advanced Wound Matrix to fit the size of the wound bed. Place the material directly in the wound, ensuring the entire wound bed is covered.
A
The
■ Venous ulcers
■ Pressure ulcers
■ Diabetic ulcers
■ Surgical wounds
■ Wound dehiscence
■ Trauma wounds
■ Calcitrant Wounds
■ Hard-to-Heal Wounds
■ Pyoderma Gangrenosum
During
The


Mirragen® Bioactive Glass Wound Matrix was applied weekly to the wound bed and affixed using a non-adherent silicone dressing, then wrapped with gauze.
At weekly reviews, the patient returned for wound check and reapplication.
The wound was not disturbed on subsequent visits and did not require any additional debridement.
Mirragen® was reapplied at each visit and secured in similar fashion.
The wound progressed towards closure after only six applications of Mirragen® within 42 days
The clinician was extremely impressed with how effective Mirragen® was at providing the right environment to support the body’s ability to heal this wound, particularly after other failed treatments.
The provider found dressing changes were much quicker and simpler compared to other skin substitutes and was impressed with how easy it was to apply Mirragen® to the wound bed.



The patient also had the following comorbidities, which contributed to delayed healing: diabetes, renal failure on dialysis, stroke, and peripheral vascular disease resulting in amputation.
Initially the complex wound measured 29 cm x 4 cm 116 sq cm with desiccated necrotic tendon and fascia.
The wound, necrotic tissue and tendon were debrided, and Mirragen® Bioactive Glass Wound Matrix was applied to the wound bed
Weekly reviews performed and no additional debridement required.
Mirragen® was applied once per week and after 18 weeks of Mirragen®, this complex and hard-to-heal wound is closing
Measuring 3 cm x 0.5 cm, 1.5 sq cm the wound was noticeably clean and improving at week 18 and mirragen® was discontinued and standard of care was administered until the wound closed.
The provider was satisfied with the support Mirragen® provided to the body’s natural healing process and ease of placement wound bed.
The
The


At week 1, the wound measured 8 cm x 8 cm x 2 cm, with a wound surface area of 64 sq cm
Mirragen® Bioactive Glass Wound Matrix ® was applied to the wound bed at weekly intervals.
At each visit, the wound was cleansed and debrided. Mirragen® was applied and covered with a non-adherent foam dressing.
Mirragen® was applied to the wound seven times over a 56 day period.
The wound progressed towards closure with seven applications of Mirragen® over 56 days.
The patient noticed early on that the odor from her wound had decreased significantly after just a couple of applications of Mirragen® and dressing changes were painless and comfortable.
This helped to ensure patient compliance with weekly visits to the clinic.
Mirragen®’s synthetic bioactive porous glass fibers, causes wicking of body fluids which aids in the placement of the product and once in the wound bed, mimics a fibrin clot, a well-documented healing process, allowing the body’s natural healing process to proceed.
contusion, from a offroad dirt bike accident with a delay in primary treatment of over 30 hours.
Pressure from the motorcycle caused compartment syndrome of the right leg
He underwent decompression surgery of his right leg. He was transferred intense physical rehab and continued wound care. The wound surface area was initially 112 sq cm




NPWT was used 3 times a week over exposed tendon with twice daily intensive physical therapy.
Mirragen® was used for tendon coverage and STSG was then performed.
Mirragen® was applied to the wound bed with contact layer and NPWT for 4 days post op.
Conversion to standard dressings occurred by Week 3 in this challenging wound resulting from acute spinal cord injury and multiple trauma to right lower extremity, due to healing progression.
All wounds, including both donor and skin graft sites, progressed towards closure in 3 weeks with 3 applications of mirragen® There was no evidence of scar contraction and there was improvement in the graft.
Mirragen® was selected because of its effectiveness at managing the wound environment, while supporting the body’s natural healing process and minimizing the need for multiple weekly dressing.
■ Atypical wounds
■ Undermined wounds
■ Skin graft donor sites
A 46-year-old
Past medical history included lower limb amputation due to PG. A non-healing wound was present for 6 years.
The dermatology team instigated steroids, STSG and a skin substitute, without success.
One further year of treatment with skin substitutes and multiple collagen products.


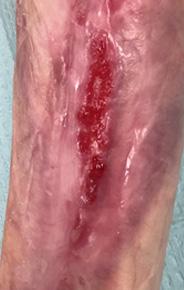

A plastic surgeon discussed other options including grafts or amputation.
Two weeks before scheduling the split thickness graft treatment commenced with Mirragen® Bioactive Glass Wound Matrix.
The wound was 8.2cm L x 5 cm W x 0.2 cm D, with a red, odorous, non-granulating, and painful wound bed and high exudate.
The wound was cleansed with normal saline, dried with gauze and Mirragen® was applied to the wound bed, covered with a nonadherent perforated dressing, and steristrips.
After the first two weeks, of Mirragen® the secondary dressing was decreased to one every 5 days, for exudate management.
A superabsorbent dressing with borders was applied
Wound progressed to closure at 18 weeks with applications of Mirragen® with a significant decrease in pain reported by patient.
The provider was able to clean the wound without pain and dressing changes were significantly faster
A significant decrease in product use because dressing changes reduced from 4 times a week to once a week.
On the last visit, the provider mentioned that the use of Mirragen® prevented an amputation on this patient.
The potential benefits and evidence supporting the use of Mirragen®, a novel bioactive glass wound matrix (BGWM) in the treatment of chronic, non-healing wounds is set out in the clinical evidence. The data, collected from various published retrospective case series, randomized controlled trials, and economic analyses, highlights the ability of BGWM to improve healing times, reduce costs, and streamline wound care practices.
■ In a randomized 12-week treatment after 2 weeks of screening, BGWM showed a favorable outcome in the treatment of patients with diabetic foot ulcers compared to standard of care (SOC) treatment.2
■ In a randomized trial of 40 patients receiving SOC or SOC plus BGWM for 12 weeks, a significantly higher proportion of wounds healed after 12 weeks of BGWM treatment at 70% compared to SOC alone (70% vs. 25%; adjusted P = .006).2
■ In a retrospective case series, five patients with 6 venous leg ulcers were treated all wounds healed after an average of 10.0 weeks (range: 3-27 weeks) during the use of BGWM, despite being present for an average of 15.1 weeks prior to initial BGWM application.7
■ A prospective pilot study involving three patients with a total of eight chronic, treatment-resistant pyoderma gangrenosum (PG) ulcers demonstrated that all previously non-healing wounds were returned to a healing trajectory following the introduction of BGWM therapy. 8
■ The application of BGWM resulted in the successful healing of all previously non-healing PG wounds, with patients experiencing significant pain reduction and no adverse events. 8
■ BGWM accelerates wound healing, with a wound closure rate of 6 weeks compared to 17 weeks with standard of care, reducing wound duration by 336 days. 2
■ In another case review, three patients with a mean age of 72 (range: 56-84) were treated for two surgical wounds and one radiation wound. All three wounds in this series had failed multiple previous advanced, costly therapies and surgeries with a mean wound duration of 13.2 months. Mean wound size volume at the start of BGWM was 1.9 cm³. All wounds healed after a mean of 8.1 weeks with the use of BGWM. 9
■ The result of a small case series suggests that BGWM has a success rate that exceeds the commonly reported industry standard of 50% and 70% of wounds healed; further, it could facilitate an earlier transfer of wound care patients into outpatient care, reduce healthcare costs, and improve quality of life. 10
■ Based on the physiologic properties of bioactive glasses at the wound interface, this innovative BGWM advanced wound matrix accelerates healing while minimizing the costs of care and improving outcomes.10
■ Mirragen® reduces time to heal by 336 days compared to standard of care. 12
■ Mirragen® has 2.8x faster closure rate than SOC12
■ In the case series of patients with venous leg ulcers, no systemic antibiotic therapy was required during the course of treatment with BGWM, suggesting a potential role for the matrix in managing wound bioburden and preventing secondary infections). This finding is noteworthy, given the concerns over antimicrobial resistance and the associated costs of antibiotic therapy. 7
■ The absence of systemic antibiotic therapy during the course of treatment with BGWM in patients with chronic PG ulcers highlights the potential role of the matrix in managing wound bioburden and preventing secondary infections, 8 which could lead to cost savings by avoiding the need for additional antibiotic treatment.
■ The ease of BGWM application and its ability to promote wound closure without requiring frequent dressing changes is advantageous from a clinical workflow perspective. 2 ,7 This streamlined approach to wound care may potentially reduce costs associated with frequent dressing changes and clinical visits.
■ Mirragen® results in 38% lower total costs of care over 40 weeks compared to standard of care 2,12
■ Mirragen® requires 53% fewer nursing/physician visits, contributing to a reduction in labor costs and enhanced cost-effectiveness 12
■ Mirragen® has 35% lower product costs due to a lower average application need, resulting in reduced overall product costs for healed wounds 12
■ Mirragen® leads to 78% lower unhealed wound costs over time, reducing long-term costs for unhealed wounds and saving an average of $8,377 per patient over 40 weeks 12
■ The estimated cost savings per patient between alternate advanced wound therapies and mirragen is $84,000 12
■ The total cost of care (TCOC) of using Mirragen®, compared to standard of care (SOC) for the treatment of diabetic foot ulcers over a 40 week period was modeled based on results from a 40 patient RCT 2,12
■ Results showed the TCOC for healed patients was $9,233 for Mirragen® versus $20,573 for SOC. The TCOC over the initial 12 week study was $6,925 per patient for Mirragen® and $5,143 for SOC. When including the cost for unhealed patients over 40 weeks, the total TCOC was $13,698 per patient for Mirragen® compared to $22,074 for SOC 12
■ The study concluded that use of Mirragen® for diabetic foot ulcers resulted in an estimated cost savings of $8,377 per patient over 40 weeks compared to standard of care. Mirragen® was found to be a cost-effective option for wound healing 2
■ An economic analysis found use of Mirragen® to be costeffective, with expected cost savings of $8,377 compared to standard care when evaluating total wound healing costs over 40 weeks. 2
■ Potential reduced need to utilise Negative Pressure Wound Therapy (NPWT) costing $119/ day. 12
■ A recent systematic review highlighted that the mean cost of treating diabetic foot ulcers (DFUs) surpasses $31,000, underscoring the economic burden associated with these wounds. 2
■ In a randomized controlled trial, Mirragen® doubled the wound area reduction as 12 weeks (79.4% vs. 36.5%) as compared to the standard of care (collagen alginate dressing)2
■ In the case series of patients with venous leg ulcers, compression wraps consisting of two layers were applied over the BGWM dressings and changed twice weekly at each study visit, along with inspection and reinforcement of the underlying BGWM application as needed. This protocol suggests that BGWM requires less frequent dressing changes compared to standard wound care practices. 7
■ The study involving patients with chronic PG ulcers mentioned that the BGWM was easy to apply and was used until complete wound closure was achieved for all ulcers 8, indicating a reduced need for frequent dressing changes.
■ The ability of BGWM to facilitate wound closure without requiring frequent dressing changes is mentioned as an advantage in the management of both venous leg ulcers and PG ulcers, 7 highlighting the potential of the matrix to streamline wound care and reduce the burden of frequent dressing changes on patients and healthcare providers.
■ These detailed findings, supported by the respective documents, underscore the potential benefits of using BGWM in the treatment of chronic, non-healing wounds, particularly in terms of accelerated healing times, cost reduction through decreased need for systemic antibiotics and frequent dressing changes, and a reduced frequency of dressing changes compared to standard wound care approaches.
■ Mirragen® Bioactive Glass Wound Matrix mimic the microstructure of a fibrin
■ Mirragen® Bioactive Glass Wound Matrix is biocompatible with
and bioresorbable. ■ Mirragen® Bioactive Glass Wound Matrix is free of toxic or irritant particles
■ Bioactive Wound Glass fiber absorbs wound fluid to maintain moisture balance
■ Material covers the wound to support a clean environment
■ Mirragen® Bioactive Glass Wound Matrix dissolves over time, eliminating the need to disrupt healing by removing material
■ Fibrous structure allows for gaseous exchange at the wound site
■ Bioactive Wound Glass fiber can conform to any wound shape
■ Mirragen® Bioactive Glass Wound Matrix is synthetic, eliminating the concern over potential disease transmission unlike biologic or cellular tissue-based products
■ Material is stable at room temperature and requires no special storage conditions
■ Mirragen® Bioactive Glass Wound Matrix has a long shelf life of 5 years, and can be stored at room temperature
■ No preparation steps required
■ No tissue tracking required
“I have used Mirragen® on one of my most difficult cases where other skin substitutes did not work. I am impressed by Mirragen®, and I have not been impressed in a long time.” Dr Laurentin Perez
Biodegradable and bioabsorbable
Made with natural body elements, Mirragen® dissolves in physiological fluid, so there’s no need to remove it.
Easy to place
Because it’s flexible, easy to use, and will attach to a moist surface, Mirragen® is ideal for challenging wounds, including those that require tunneling or undermining.
Proven safe and effective
Mirragen® has shown great clinical results for a variety of wound types, from easy to heal to complex. In a randomized controlled trial, no adverse events were observed.
