March - April 2023

Decellularized Fish Skin Technology
Kerecis® Marigen™
Introduction
What is Kerecis® Marigen™?
■ It is intact decellularized fish skin for the management of chronic wounds such as diabetic wounds, pressure ulcers, vascular ulcers, and draining wounds commonly treated in the private office and wound care centers. The fish skin sheets contain fat, protein, elastin, glycans and other natural skin elements and is supplied in multiple shapes and sizes

■ It is made from Atlantic cod that has been decellularized and sterilized with ethylene oxide
■ It forms a scaffold to which epithelial cells can attach and promote healing1
■ This graft is often used for cases with limited donor skin availability
■ The graft is rich in omega-3 fatty acids which are antiinflammatory and help with the healing of chronic wounds2

Keywords
■ Kerecis® Marigen™
■ Regenerative healing
■ Burns
■ Fish-skin graft (FSG)
■ Wound repair
■ Chronic wounds
How Kerecis® Marigen™ Works:
■ Debride the wound to remove necrotic tissue
■ Irrigate the wound to remove debris and exudates.
■ Fit the product roughly to the size of the wound and pre-hydrate in saline solution
■ Apply the product to the lightly bleeding wound bed; ensure that the sheet does not overlap the wound edges
■ More than one product may be necessary for complete coverage;�� overlap product edges slightly
■ Apply a non-adherent wound dressing
■ Inspect the wound every two days. The duration between inspections may be extended up to seven days as healing progresses
■ Insert a new product into the wound if the previously applied product has been absorbed and is no longer visible
■ Change the cover dressing as needed to maintain a moist, clean wound
64 Wound Masterclass - Vol 2 - March 2023
Masterclass
GUIDES
This Masterclass Guide is a concise overview of the use of a fish skin graft (FSG) product in wound management, and on how to incorporate this into your practice.
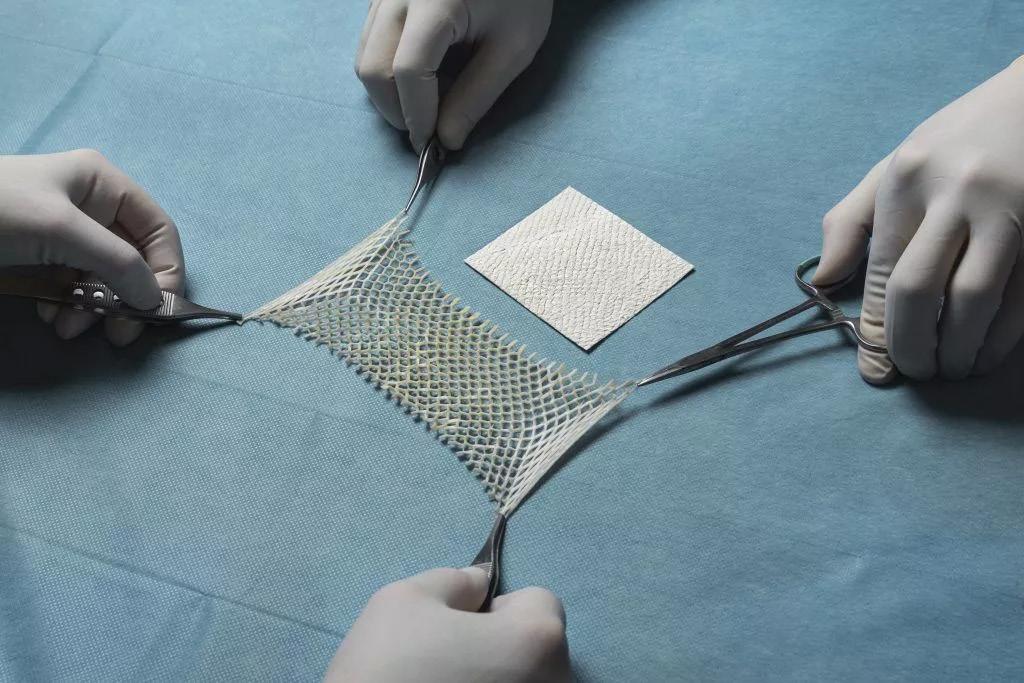
Figure 2: 2a: MariGen™ Standard. 2b: MariGen™ Micro.
2a 2b
Figure 1: MariGen™ Expanse.
Decellularized Fish Skin Technology
Kerecis® Marigen™
What Types of Wounds Are Suitable?
■ Partial and full thickness wounds
■ Pressure ulcers
■ Chronic vascular ulcers
■ Diabetic ulcers
■ Trauma wounds, including abrasions, lacerations and skin tears
■ Surgical wounds (donor site/ grafts, post-Mohs surgery, post-laster surgery, podiatric, wound dehiscence
■ Draining wounds
Contraindications
■ This product should not be used in people with allergies or sensitivities to fish material
Product Image Description Uses
MariGen™ Standard
MariGen™ Micro
MariGen™ Expanse
FSG in the form of sheets Thin sheets for most wound types


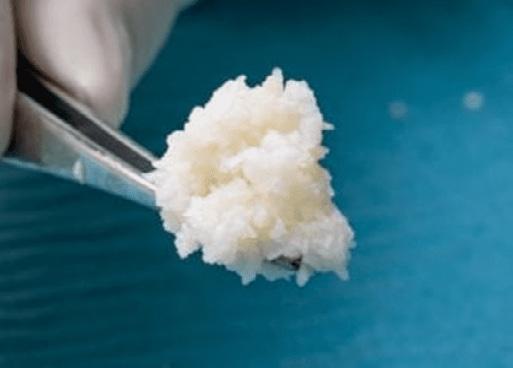
Fragmented fish skin Uneven, irregular wound surfaces
2:1 pre-meshed intact FSG Can expand and cover wounds 100cm2 and larger
Wound Masterclass - Vol 2 - March 2023 65
Masterclass
GUIDES
Figure 1: MariGen™ product range.
Decellularized Fish Skin Technology
Kerecis® Marigen™
Evidence
Kerecis® Marigen™ has had numerous trials supporting its efficacy, safety and cost-effectiveness.
Efficacy
■ Kirsner et al. performed a doubleblind, prospective, randomized clinical trial comparing the efficacy of FSG with dehydrated human amnion/ chorion membrane allograft (dHACM) in the treatment of full-thickness wounds3
■ The study had 85 healthy volunteers aged between 19 and 51 years. Full thickness wounds were created using punch biopsies
■ Acute wounds treated with FSG healed faster than wounds treated with dHACM, with a p-value of 0.0014
■ Similar results were obtained in another randomized control trial that compared FSG with porcine smallintestine submucosa
■ Kerecis® has demonstrated its proficiency in creating lipidcontaining tissue matrices from fish skin, and it has been shown that the material is safe, non-toxic and structurally sound
Infection
■ There is no risk of viral or prion disease transmission between humans and Atlantic cod
■ Fish skin does not need the same level of processing as mammalian tissue does5
■ Less processing of the tissue helps it retain a structure similar to human skin as most of the lipids can remain in the tissues6
Costs
■ Winters et al. conducted a retrospective comparative cohort study to compare the cost of FSG versus standard of care for the treatment of diabetic foot ulcers (DFUs)
■ The study showed that the fish skin treatment could result in lower costs, more wounds healing (83.2% vs. 63.4%), fewer amputations (4.6% vs. 6.9%), and a higher quality of life (0.676 vs. 0.605 quality-adjusted life year (QALY) than the standard of care7
“Fish skin grafts show promising results in treating diabetic foot ulcers (DFUs), venous leg ulcers…. many uses in clinical applications of the future.”
Fiakos et al.7
“Acellular fish skin xenografts may represent an effective, low-cost alternative in treatment of superficial- and partial-thickness burns.”
Luze et al.8
“Management with fish skin has shown faster granulation rates in burn wounds for skin grafting, resulting in improved patient outcomes with no documented infections.”
Reda et al.9
66 Wound Masterclass - Vol 2 - March 2023
Masterclass GUIDES
Decellularized Fish Skin Technology
Kerecis® Marigen™
Key Points
■ Available in various shapes and sizes
■ Cost-effective
■ Can be applied over any viable tissue
■ Safe and effective
References
1. Biazar, E, Heidari Keshel, S, Rezaei Tavirani, M, Kamalvand, M. Healing effect of acellular fish skin with plasma rich in growth factor on full-thickness skin defects. Int Wound J. 2022; 19( 8): 21542162. doi:10.1111/iwj.13821
2. Magnusson, S. et al. (2016) Acceleration of Wound Healing Through Utilization of Fish Skin Containing Omega-3 Fatty Acids, Hmpgloballearningnetwork.com. Today’s Wound Clinic. Available at https://www.hmpgloballearningnetwork.com/site/twc/articles/acceleration-wound-healing-through-utilization-fish-skin-containing-omega-3-fatty-acids (Accessed: March 30, 2023)
3. Kirsner, R.S., Margolis, D.J., Baldursson, B.T., Petursdottir, K., Davidsson, O.B., Weir, D. and Lantis, J.C., II (2020), Fish skin grafts compared to human amnion/chorion membrane allografts: A double-blind, prospective, randomized clinical trial of acute wound healing. Wound Rep Reg, 28: 75-80. https://doi.org/10.1111/wrr.12761
4. Baldursson BT, Kjartansson H, Konrádsdóttir F, Gudnason P, Sigurjonsson GF, Lund SH. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015 Mar;14(1):37-43. doi: 10.1177/1534734615573661. Epub 2015 Mar 9. PMID: 25759413.
5. Magnusson, S., Baldursson, BT., Kjartansson, H., Rolfsson, O., Sigurjonsson, GF., Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care, Military Medicine, Volume 182, Issue suppl_1, March 2017, Pages 383–388, https://doi.org/10.7205/MILMED-D-16-00142
6. Fiakos, Gabriella & Kuang, Zeming & Lo, Evan. (2020). Improved skin regeneration with acellular fish skin grafts. Engineered Regeneration. 1. 95-101. 10.1016/j.engreg.2020.09.002.
7. Winters C, Kirsner RS, Margolis DJ, Lantis JC. Cost Effectiveness of Fish Skin Grafts Versus Standard of Care on Wound Healing of Chronic Diabetic Foot Ulcers: A Retrospective Comparative Cohort Study. Wounds. 2020 Oct;32(10):283-290. PMID: 33370245.
8. Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz LP. The Use of Acellular Fish Skin Grafts in Burn Wound Management-A Systematic Review. Medicina (Kaunas). 2022 Jul 9;58(7):912. doi: 10.3390/medicina58070912. PMID: 35888631; PMCID: PMC9323726.
9. Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Mil Med. 2023 Feb 15:usad028. doi: 10.1093/milmed/usad028. Epub ahead of print. PMID: 36794813.

Masterclass GUIDES
Masterclass Guide: Decellularized Fish Skin Technology: Kerecis® Marigen™ Wound Masterclass. Volume 2. No 4. March 2023 How to Cite this Article Wound Masterclass - Vol 2 - March 2023 67 Useful Links Use your device to scan this QR code for more information about Kerecis® Marigen™ Click here to visit the Kerecis® website Sponsored By Kerecis®. All production resources provided by Kerecis® © Copyright. Wound Masterclass. 2023









