
March 2024

Steven Jung


March 2024

Steven Jung
This systematic review and economic evaluation assesses the health economics of a novel Bioactive Glass Wound Matrix (BGWM) in the management of chronic wounds. The primary objective is to evaluate the cost-effectiveness of BGWM compared to other treatment modalities, such as the standard of care (SOC), and identify areas where BGWM improves healthcare resource utilization and economic outcomes. The analysis included data from a randomized controlled trial on diabetic foot ulcers (DFUs) comparing BGWM to SOC, as well as multiple case series examining refractory wounds from various etiologies, including venous leg ulcers, pyoderma gangrenosum, radiation therapy, and chronic surgical wounds. The total cost of care associated with BGWM was compared to the costs of SOC and other therapies. The investigation revealed several statistically significant economic improvements, including reduced total cost of care across multiple study cohorts, decreased patient visit times, and lower secondary dressing costs. Additionally, the analysis found reduced rates of infection, improved neuropathy, pain reduction, and accelerated wound healing associated with BGWM treatment.
Chronic, non-healing wounds represent a major health burden and contribute substantial costs to healthcare systems. It is estimated that 2% of populations will develop a chronic wound during their lifetime, with 6.5 million Americans affected and costs in the billions per year in the United States alone.1 As such, wound care remains an area of high unmet need with opportunities for improved products and treatment approaches.

Advanced wound care strategies have explored the use of biologic products, including extracellular matrices derived from animal and human sources, as well as synthetically manufactured alternatives.2-4 However, many of these products are limited by the incorporation of unmatched allogeneic cells or harsh processing methods necessary for decellularization and pathogen inactivation, which can detrimentally impact the structural integrity and biological activity of the graft material.2 Clinical studies evaluating the efficacy of tissue-based products such as human amnion and chorion allografts, porcine urinary bladder matrices, porcine purified reconstituted bilayer matrices, fetal bovine collagen, and collagen hydrogels in the management of wounds have yielded variable outcomes.3,4 Time to apply product and storage conditions should also be considered. A common challenge across many of these trials is the lack of a well-defined, clinically relevant comparator group. For the subset of wounds refractory to conventional standard of care, bioactive glass wound matrices have emerged as a promising advanced wound care modality.5-9
Early evidence indicates these bioactive matrices may improve healing rates and reduce time to wound closure compared to standard dressings.5-8 Novel bioactive glass wound matrix (BGWM) (Mirragen®, Engineered Tissue Solutions) has been successfully utilized in a variety of clinical situations where other advanced wound care products have failed to achieve desired outcomes.5-9 Unlike certain other biologic products, bioactive glass matrices do not contain allogeneic cellular components and avoid harsh processing methods, potentially offering a more consistent and effective solution for recalcitrant wounds.8 Bioactive glass matrices, a pioneering advanced wound care option, promote healing by releasing ions that stimulate cellular migration and tissue regeneration, offering a promising solution for enhanced wound management.8 As reimbursement and adoption of new wound care products depends heavily on demonstrated economic value10-12, Health Economics Outcomes Research (HEOR) provides a crucial framework for evaluating the clinical and economic outcomes of BGWM.13
In the United States, the conservative annual cost of wound care is $28 billion, with the majority of these costs occurring in the outpatient setting.1 The average cost of treating a venous leg ulcer is estimated to be $4,000 to $5,000 per month.10
The mean cost per patient for diabetic foot ulcer treatment ranges from $10,000 to $20,000.11
“Comprehensive HEOR studies are vital to guide evidence-based decision making and ensure equitable access to advanced wound care modalities that meaningfully improve patient outcomes while providing economic value to the healthcare system.”
The average cost of treating a single fullthickness pressure ulcer is estimated to be $70,000.12 Approximately 15% of Medicare beneficiaries require treatment for at least one type of chronic wound or infection.1 These costs and statistics highlight the need for advanced wound care strategies to improve patient outcomes and reduce healthcare costs.
Factors Affecting Costs of Standard of Care
1. Rates and speed of wound closure
2. Reductions in adverse events
3. Impact on quality of life
4. Both direct and indirect costs across the continuum of care
Comparative effectiveness studies incorporating appropriate Standard Of Care (SOC) controls are necessary to quantify the incremental clinical and economic benefits of BGWM. Wound care Health Economics and Outcomes Research (HEOR) must also consider the specific patient populations and care settings most likely to benefit from advanced matrix products.
Factors that may significantly influence outcomes and costs include:
1. Wound etiology
2. Size
3. Duration
4. Comorbidities
5. Provider experience
Ultimately, the success of innovative wound care products like bioactive glass matrices will depend on:
Demonstrating superior clinical outcomes
• Reasonable cost-effectiveness compared to current standards of care
Comprehensive HEOR studies are vital to guide evidence-based decision making and ensure equitable access to advanced wound care modalities that meaningfully improve patient outcomes while providing economic value to the healthcare system.
What Do We Know about Bioactive Glass Matrix So Far?
Evidence Overview Methods
A number of data sources were considered to provide a comprehensive economic analysis of BGWM.
Table 1: Summary of the Referenced Clinical Work.
“The studies reviewed implemented a borate- based bioactive glass wound matrix BGWM for a range of chronic wound types, tailoring specific treatment protocols to individual patient groups. This approach ensured consistent application and monitoring, optimizing therapeutic outcomes across diverse clinical scenarios.”
Study 1: BGWM 40 patient Randomized Controlled Trial (RCT): A 40 patient RCT comparing the clinical and economic outcomes of BGWM versus SOC in Wagner 1 DFUs.8
Study 2: Case Series of six Chronic Venous Leg Ulcers (VLUs) treated with BGWM.6
Study 3: Case Series of eight Pyoderma Gangrenosum (PG) treated with BGWM.7
Study 4: Case series of three refractory wounds treated including longstanding surgical and radiation wounds treated with BGWM.5
Study 5: Case series of four chronic wounds with mixed etiologies including a pressure wound on the elbow and three DFUs treated with BGWM.13
Study 6: Health Economics Study: A study comparing the historical patient wound management costs with those after the implementation of BGWM in four DFUs.14
Study 7: Time study: A 20 patient study comparing the average patient visit time and potential for increased patient capacity and reimbursement when using BGWM versus SOC in a hospital outpatient department (HOPD) setting.
Existing published data cost estimates: Published studies reporting the average costs of treating venous leg ulcers, diabetic foot ulcers, and pressure ulcers and Standard-of-Care.10-12
The following types of economic outcomes were evaluated:
1. Total cost of care (TCOC) per healed patient
2. Average treatment cost per patient
3. Dressing costs and frequency of dressing changes
4. Patient visit time and potential for increased patient capacity and reimbursement
5. Comparison of costs to published data for treating different types of chronic wounds
Statistical Analysis
Statistical significance was assessed using twotailed t-tests for continuous variables, with a p-value < 0.05 considered significant.
Study Analysis
The studies reviewed implemented a boratebased bioactive glass wound matrix BGWM for a range of chronic wound types, tailoring specific treatment protocols to individual patient groups. This approach ensured consistent application and monitoring, optimizing therapeutic outcomes across diverse clinical scenarios. An economic model was designed to evaluate the total cost of care (TCOC) over a 40-week period. This model took into account various cost inputs including the prices of products, fees for nursing and physician visits, costs associated with offloading boots, and additional expenses for treating wounds that remained unhealed after 12 weeks.8
Study 1 was an RCT with strict enrollment criteria which included Wagner 1 DFU wounds of a certain size range, wounds that had existed
“For each study, wounds were cleansed with sterile normal saline solution and debrided once prior to initial BGWM application. BGWM was shaped to fit the size of the wound bed and pressed directly in contact with the wound, covering the entire wound area. A cover dressing was applied over BGWM to fixate the matrix to the wound surface, and a secondary dressing was then applied to assit with exudate management.”
for a minimum number of weeks in a chronic state, and a variety of other factors including exclusion of smokers or those with infections. During the run-in period, wounds that resolved more than 20% were excluded from the study. Randomization was blinded between the SOC and BGWM groups.
Study 2 was a case series of chronic VLU wounds that had been present for an average of 15 weeks and in a non-healing state. Patients had previously received a vascular assessment and three of the five patients required venous ablation procedures to improve venous insufficiency.
Study 3 was a case series of eight refractory PG wounds from three patients ranging from months to years in age. Each wound had failed several previous treatment plans including SOC, and a variety of advanced skin substitute products.
Study 4 was a case series of three patients with refractory wounds from surgical procedures and radiation therapy. These wounds were selected due to there refractory state.
Study 5 was a case series of four chronic wounds including three DFUs and a pressure wound located on patient’s elbow. Each wound was selected for this case series due to multiple failed therapies including SOC, surgery, NPWT and advanced skin substitutes. .
Study 6 was a case series of three DFUs and one VLU that also assessed the cost differential from historical treatment before BGWM, and the patient costs incurred during BGWM
treatment. Patients selected for this study had refractory wounds with at least one year in a wound care center prior to introduction of the BGWM.
Study 7 was a study evaluating the patient visit times comparing SOC therapy vs. BGWM. A total of 20 patient visits, 10 consecutive visits to each therapy group, were timed in an HOPD setting to look at overall patient visit efficiency.
For each study, wounds were cleansed with sterile normal saline solution and debrided once prior to initial BGWM application. BGWM was shaped to fit the size of the wound bed and pressed directly in contact with the wound, covering the entire wound area. A cover dressing was applied over BGWM to fixate the matrix to the wound surface, and a secondary dressing was then applied to assit with exudate management. Where indicated clinically, a two-layer compression dressing, or off-loading device, was utilized and changed per the provider’s preferred protocols.5-8 The BGWM was reapplied approximately weekly, based on clinical assessment, without further debridement or product removal.
In Study 1 the treatment phase considered the comprehensive costs associated with nursing and physician care, the products used, and the treatments applied to unhealed wounds up to 40 weeks post-enrollment.8
“These findings suggest positive outcomes for using BGWM in treating chronic wounds, particularly DFUs, potentially improving healing rates and reducing healthcare costs.8 Total dressing costs with BGWM compared to SOC were also statistically less (p < 0.05).”
Study 6 meticulously recorded the costs associated with previous treatments and compared them to the costs incurred during BGWM therapy. The cost analysis included dressings and debridement but excluded skin grafts, cellular and tissue-based product costs, hospitalization, antibiotics, or pain medication costs.14
Healing validation as implicitly based on clinical outcomes observed during treatment, with a focus on wound closure and wound closure rates.
Results
A randomized trial of 40 patients receiving SOC or BGWM for up to 12 weeks, the initial healing was assessed by complete epithelialization without drainage, with final healing evaluated by a panel of three blinded plastic surgeon wound experts. After 12 weeks, 70% of wounds in the BGWM group were healed compared to 25% in the SOC group, with the percent area reduction at 6 weeks (PAR) being significantly higher in the BGWM group (79%, SD: 48) compared to the SOC
group (37%, SD: 66). Moreover, individuals undergoing BGWM treatment had odds of healing that were over 11 times greater than those solely receiving SOC. Consequently, the finding that the difference in healing rates between treatment groups in this study (45%) is noteworthy. This finding is especially significant considering the effectiveness of bioactive glasses in similar populations and the severity of diabetic foot ulcer (DFU) wounds, as reported in various randomized controlled trials (RCTs) exploring different advanced wound care treatments.6 The BGWM demonstrated a 2.8x faster closure rate and a reduced time to heal compared to SOC (p < 0.001) which was statistically significant.
These findings suggest positive outcomes for using BGWM in treating chronic wounds, particularly DFUs, potentially improving healing rates and reducing healthcare costs.8 Total dressing costs with BGWM compared to SOC were also statistically less (p < 0.05).
No adverse events (AEs) related to infection of the index ulcer were reported in the BGWM group, while five such infections occurred in the SOC group. BGWM has previously shown in-vitro antimicrobial properties that may contribute to preventing infection.9,15
BGWM treatment led to a significant reduction in Semmes-Weinstein score compared to SOC alone (P = .008). In 40% of BGWM subjects a positive increase in sensation around the wound was noted.
The study’s findings underscore the efficacy of BGWM in accelerating wound healing compared to SOC. The data suggest that
“In summary, while BGWM’s initial costs may appear higher than the SOC, its clinical effectiveness and resource efficiency results in a reduction in long- term care costs. This analysis underscores the importance of considering both the direct costs and the clinical outcomes when assessing the economic impact of new medical treatments.”
BGWM not only enhances the rate of wound closure but also significantly reduces the healing time for patients. This is a critical factor in the management of DFUs, as prolonged wound openness increases the risk of infections and other complications, leading to a higher burden on patients and healthcare systems alike.
5. Comparison to Published Costs of Standard of Care
Utilizing the RCT data, an established economic model was employed to evaluate the total cost of care (TCOC) during the study period for the Standard of Care (SOC) and BGWM. The TCOC provides a comprehensive look at the financial impact of each treatment method, incorporating all costs associated with patient care over the study period. For the SOC, the total cost of care for the study period was $102,864, which, when broken down to a per-patient basis, reveals a cost of $5,143. This figure encapsulates the aggregate of both healed and unhealed patient costs divided by the total number of patients. In contrast, BGWM total cost of care was higher, at $138,502 for the study period, with an individual patient cost averaging $6,925. Although there is a higher initial cost compared to SOC, long term costs are lower. 8
Delving deeper into the cost-effectiveness of the treatments, the TCOC was analyzed for patients who healed during the study. The cost for a healed patient under SOC was $20,572. BGWM, however, presented a more favorable outcome, with the cost for a healed patient being substantially lower at $9,233. This notable difference indicates that while BGWM’s upfront costs may seem higher, the treatment is more cost-effective in the long run, particularly when it leads to successful healing. Importantly, the BGWM Total Cost of Care (TCOC) per healed patient (US$ 9,233) is lower than the previously published costs for treating diabetic foot ulcers ($10,000 to $20,000).8
A critical aspect to consider with HEOR in wound care is the cost associated when wounds do not heal. When evaluating the cost of unhealed wounds in the RCT, the unhealed wounds in the SOC cohort cost of $88,169, which is greater than the BGWM cost of unhealed wounds in this series ($63,924). This difference is pivotal in the overall cost savings achieved with BGWM, which were highlighted as significantly lower TCOC (p=0.001) and an average savings of $8,377 per patient over the period.
In summary, while BGWM’s initial costs may appear higher than the SOC, its clinical effectiveness and resource efficiency results in a reduction in long- term care costs. This analysis underscores the importance of considering both the direct costs and the clinical outcomes when assessing the economic impact of new medical treatments.
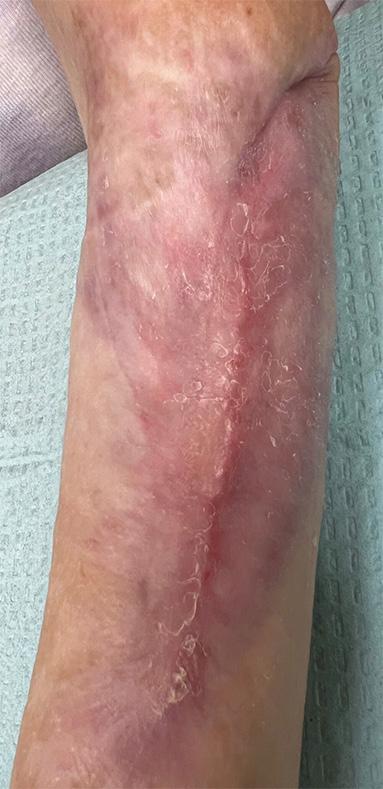
Study 2 focuses on venous leg ulcers in geriatric patients, featuring five patients with an average age of 80.3 years (ranging from 68 to 87), representing an elderly cohort with significant comorbidities affecting wound healing.
The mean initial ulcer size was 13.5 cm2, indicating that many wounds were large. BGWM facilitated healing even for the largest ulcer (56 cm2), suggesting effectiveness across a range of sizes and severity.6
An example of a VLU from this case series before and after treatment with BGWM in shown in Figure 1.
“The ease of BGWM application and ability to use until full closure without frequent dressing changes, alongside the lack of need for systemic antibiotic therapy from the real world data trials, supports clinical efficacy.”
2. Healing Time
A median healing time of 10 weeks compares favorably to expected healing times with standard of care.6
3. Pain Reduction
Significant pain reduction was reported by patients within just 2-3 dressing changes, from a mean of 5.8/10 to 0/10. An improved patient quality of life and treatment compliance was also noted.
4. Clinical Workflow
The ease of BGWM application and ability to use until full closure without frequent dressing changes, alongside the lack of need for systemic antibiotic therapy from the real world data trials, supports clinical efficacy.

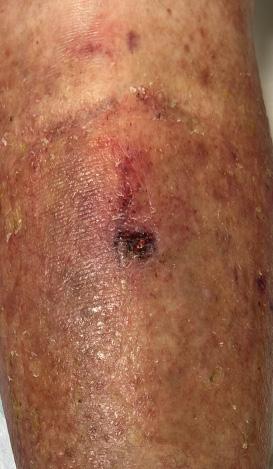
Study 3: Patient Case Series on Pyoderma Gangerosum
1. Patient Profile
Study 3 covers three patients with a total of eight PG ulcers, which were complex and challenging wounds associated with an inflammatory skin disease.5 Each of these patients failed to respond from conventional therapies over an extended period of time. An example of one of the PG wounds before and during the healing progression with BGWM is shown in Figure 2.
2. Healing Time
The application of BGWM in this study resulted in healing trajectories for all previously non-healing wounds in patients with pyoderma gangerosum ulcers. The absence of systemic antibiotics during the continuum of care was noted and treatment with BGWM was highlighted as a means of managing wound bioburden and preventing secondary infections.7
3. Pain Reduction
All patients reported significant reductions in the pain levels associated with the PG ulcers.
4. Adverse Events
No adverse events were reported during the BGWM therapy.
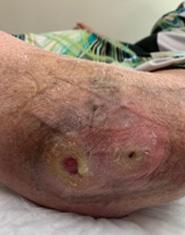
Figure 2a: This patient had refractory Pyoderma Gangrenosum, which had not responded to treatment.
2a
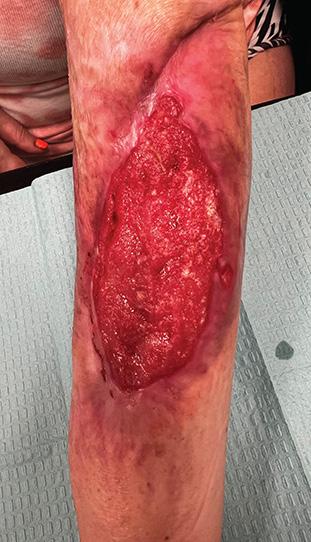
Figure 2b: Post-application, day 1. Wound shows signs of product integration.
2b
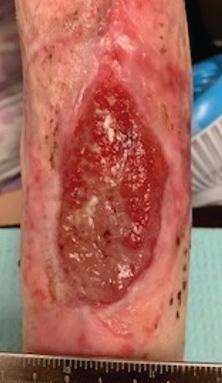
Figure 2c: Post-application, 3 weeks. The defect on close monitoring has decreased in size.
2c
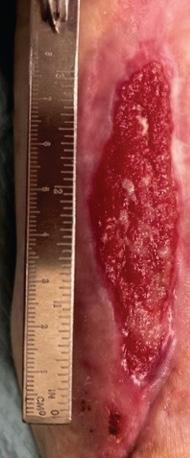
Figure 2d: Post-application, 4 weeks. The wound has fully healed.
2d
1. Patient Profile
Study 4 explores treating three complex refractory wounds comprising two surgical and one radiation-induced wound in a patient group averaging 72 years of age (ranging from 56 to 84) with BGWM. The wounds resulted from varied medical interventions and conditions, indicating a mature demographic facing severe healing challenges.5 These wounds were notably challenging, having not responded to numerous advanced and expensive treatments and surgeries previously.
2. Healing Time
On average, these wounds had remained open for 13.2 months before the intervention with BGWM. All wounds experienced healing within an average duration of 8.1 weeks following the application of BGWM.
3. Advantages of BGWM Therapy
The advantages of BGWM include full wound closure, rapid pain relief, avoidance of systemic antibiotics, and streamlined dressing changes.5
1. Patient Profiles
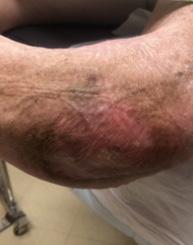
Patient 1 presented with a chronic left elbow wound following two operative incision and drainage procedures for an infected olecranon bursa with osteomyelitis and exposed bone. The wound had persisted for 212 days prior to initiating BGWM treatment. Due to the chronicity and severity of the wound, the patient had significant tissue loss and bone exposure. Additionally, the patient had multiple comorbidities, including poorly controlled diabetes, chronic hemodialysis dependence, a pacemaker, upper extremity arterio-venous graft, multiple sclerosis, and a seizure disorder.
Patient 2 had a chronic left diabetic foot ulcer that had persisted for 420 days prior to BGWM treatment initiation. The patient presented with poorly controlled diabetes and peripheral vascular disease, contributing to the chronicity and severity of the wound. Previous treatments included two failed operations for surgical closure, as well as prior use of Integra and MicroMatrix. The wound exhibited significant tissue loss and necrotic debris.
Patient 3 presented with a chronic diabetic ulcer on her left leg that had been present for 540 days prior to initiation of BGWM therapy.
Study 4: Patient Case Series on Refractory Wounds Study 5: Patient Case Series Comparing Previous Treatment Costs to Costs Associated with BGWM“During the course of treatment with the BGWM, no adverse events were reported in any of the four patients. None of the patients in the case series experienced ulcer recurrence during the follow-up period after complete wound closure.”
The wound was complicated by bone exposure and osteomyelitis, and the patient had a history of a prior below-the-knee amputation of her right leg due to peripheral vascular disease. Previous treatments included prolonged use of various wound care products, negativepressure wound therapy, hyperbaric oxygen, and multiple courses of antibiotics. The wound exhibited extensive tissue loss, necrotic debris, and signs of infection.
Patient 4 had a chronic foot wound following a left-sided transmetatarsal amputation. The wound had persisted for longer than 6 months and was complicated by osteomyelitis. The patient had poorly controlled diabetes and peripheral vascular disease. Previous treatments included multiple failed operations for surgical closure, as well as prior use of advanced modalities. The wound exhibited tissue loss, exposed bone, and signs of infection.13
Following initiation of BGWM treatment, Patient 1’s wound achieved complete closure 56 days after the first application of BGWM. The wound closure process was monitored closely during outpatient follow-up visits, with reapplication of the BGWM matrix as needed based on wound appearance and healing progress. Throughout the treatment period, the wound demonstrated a consistent reduction in size, eventually leading to full closure. The area reduction rate was calculated as [initial wound area - final wound area] / initial wound area * 100%.
Patient 2 healed completely 42 days after the initial application of BGWM. The wound closure process was rapid, indicating a positive response to BGWM treatment. Throughout the treatment period, the wound exhibited progressive reduction in size, with a notable decrease in wound area observed during follow-up visits.
Patient 3, the chronic diabetic ulcer on the left leg achieved complete closure 66 days after initiation of BGWM treatment. Despite the complexity of the wound, including bone exposure and osteomyelitis, BGWM therapy facilitated significant wound healing progress. Throughout the treatment course, the wound area decreased steadily, indicating ongoing tissue regeneration and closure. The area reduction rate was calculated to evaluate the rate of wound healing and assess the effectiveness of BGWM in promoting tissue regeneration.
Patient 4’s chronic foot wound healed completely 56 days after the initiation of BGWM treatment. Despite the presence of osteomyelitis and tissue loss, BGWM therapy led to substantial wound closure within a relatively short time-frame. Throughout the treatment period, the wound area decreased progressively, reflecting ongoing tissue regeneration and wound healing. The area reduction rate was calculated to monitor the rate of wound closure and evaluate the efficacy of BGWM in promoting tissue repair.
During the course of treatment with the BGWM, no adverse events were reported in any of the four patients. None of the patients in the case series experienced ulcer recurrence during the follow-up period after complete wound closure.13
Prior to initiating the BGWM treatment, the average estimated cost of prior wound interventions was approximately $87,750 per patient. However, with the application of BGWM treatment, the
“When comparing the estimated costs of prior treatments to the cost of closure using BGWM, a hypothetical reduction in treatment costs of $84,186 per patient was observed compared to SOC (p < 0.001).13 Additionally, the average reduction in wound duration was approximately 336 days per patient.”
average cost of wound closure reduced significantly to $3,564 per patient.13 Furthermore, when comparing the estimated costs of prior treatments to the cost of closure using BGWM, a hypothetical reduction in treatment costs of $84,186 per patient was observed compared to SOC (p < 0.001).13 Additionally, the average reduction in wound duration was approximately 336 days per patient.
Study 6: Patient Case Series Reducing Treatment Costs in Hard-To-Heal Wounds with BGWM
1. Patient Profiles
Patient 1 was a 60-year-old female who presented with two DFUs that she had endured for eight years (~2900 days). A progression of the healing from before BGWM treatment through complete closure is shown in Figure 3. Patient 2 was a 68-year-old male who had a DFU that had been present for 1.5 years (~550 days).
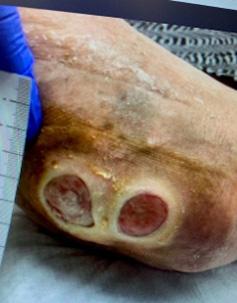
Patient 3 was a 39 year-old female with a 1 year old (~365 days) recalcitrant VLU which had been refractory to other advanced modalities.
Patient 1 saw her first ulcer healed after 9 applications of BGWM over a period of 15 weeks. The second ulcer healed following 13 applications of BGWM over 20 weeks. Patient 2 saw his ulcer respond positively to the BGWM treatment, closing after 7 applications within a seven-week timeframe. Patient 3 saw their wound close after four applications over an 11 week time period.14
3. Economic Impact
Patient 1 TCOC with BGWM therapy (total for both wounds) was $18,113, an 80% decrease from the $89,568 TCOC prior to BGWM. Patient 2 TCOC with BGWM therapy was $9,506, a 50% decrease from the $19,080 TCOC prior to BGWM. Patient 3 TCOC with BGWM therapy was $5,432, a 50% decrease from the $10,816 TCOC prior to BGWM.14
“Although not directly assessed in the RCT or the case series real world data, the BGWM ability to continue to work and promote healing despite patient noncompliances such as missed appointments is an additional economic benefit that could lead to further cost savings and improved healing rates in real world settings.”
Study 7: Time Savings and Increased Patient Capacity
The time study revealed that BGWM could save 19 minutes per patient visit (26 minutes vs. 45 minutes) compared to SOC (p < 0.001) at HOPD Wound Clinic. Extrapolating this time savings over the course of an outpatient clinic day, this could equate to an extra 8 patients to be treated per room per day, (18.4 patients with BGWM vs. 10.6 patients). In a busy clinic with 3-6 rooms, the potential additional reimbursement, if a skin substitute is applied, could range from $42,000 to $84,000 per day. In a real-world scenario, where approximately 10-15% of wounds use skin substitutes, the potential additional reimbursement related to increased office throughput and patient volume could result in $4,200 to $8,400 per day. These findings suggest that using BGWM in HOPD settings could result in increased reimbursement and cost savings, improved outcomes, presenting a positive economic story for the product.
Study 1: The strengths lie in its robust trial design, ensuring reliability in the findings, supported by adequate statistical power. Additionally, the standardized approach to standard of care (SOC) and adherence to CONSORT guidelines for reporting enhance the study’s credibility. However, weaknesses include the absence of investigator blinding, which could introduce bias. The trial’s results indicate a significant enhancement in wound healing for Wagner 1 diabetic foot ulcers (DFUs) upon the addition of BGWM to standard of care. This improvement bears positive implications for both infection prevention and neuropathy management. Nonetheless, further investigations are suggested to support these findings further, particularly in more complex wound scenarios where the efficacy of BGWM might vary.
Studies 2-6: The strengths in these case studies are that the cases are real-world complex and refractory wounds of multiple etiologies including PG. While there was no control arm in these studies, all of these wounds previously failed a combination of SOC and or advanced wound therapies including skin substitutes and skin grafts. The weaknesses to these case series include that fact that they include are relatively small numbers of wounds and no control group. The investigators were not blinded to the therapy which could introduce bias and there was no randomization to a comparator group. Promising pilot results that support further investigation in larger controlled trials.
Study 7: The strength of this study is that it recorded data from 10 consecutive patient visits of both the BGWM and SOC. The randomness of the patient variables such as patient mobility, wound location, and normal daily occurrences in an HOPD were consistent for both groups. The investigators were not blinded to the therapy, so this could have introduced bias.
Although not directly assessed in the RCT or the case series real world data, the BGWM ability to continue to work and promote healing despite patient non-compliances such as missed appointments is an additional economic benefit that could lead to further cost savings and improved healing rates in real world settings.
Chronic, non-healing wounds are a substantial financial burden, and treatments that can reduce this burden while promoting healing represent a significant advancement in wound care. The utilization of BGWM therapy in the treatment of chronic and refractory wounds is a significant advancement in the field of wound care. These therapies, grounded in the physiologic properties of BGWM at the wound
interface, have demonstrated the capacity to accelerate healing while concurrently minimizing the costs associated with patient care such as reducing average visit times, reducing the average treatment costs per patient, and improving patient outcomes. In addition, the resource efficiencies associated with BGWM usage could allow for higher daily patient volumes, which would allow more patients to be treated.
The significant difference in TCOC between BGWM and SOC, particularly for healed patients, is noteworthy. The BGWM model of care, requiring fewer applications and less intensive nursing visits, not only aligns with the clinical benefits of accelerated healing but also with economic efficiencies. These findings are crucial for payers, providers, and patients, demonstrating that the BGWM upfront costs are offset by its overall cost-effectiveness, particularly in a healthcare environment increasingly focused on value-based care.
Leveraging state-of-the-art technologies like BGWMs that target the underlying dysregulation in healing pathways represents a powerful strategy to accelerate wound closure and reduce morbidity. While further work remains to establish definitive evidence-based guidelines, BGWMs are emerging as an exciting solution to reframe the paradigm of chronic and refractory wound care for the 21st century.
References
1. Nussbaum, S. R., Carter, M. J., Fife, C. E., DaVanzo, J., Haught, R., Nusgart, M., & Cartwright, D. (2018). An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value in Health, 21(1), 27-32. https://doi.org/10.1016/j. jval.2017.07.007
2. Chattha, A., Naeger, T., Whyte, W., Bonias, P., Huddleston, E., Wu, L. C., & Eberlin, C. T. (2020). Decellularized human placenta-derived extracellular matrix: an update on its use in regenerative medicine. International Wound Journal, 17(3), 600-610. https://doi.org/10.1111/iwj.13322
3. Vowden, P., & Vowden, K. (2017). Wound dressings: principles and practice. Surgery, 35(9), 489-494. https://doi. org/10.1016/j.mpsur.2017.06.005
4. Cazzell, S. M. (2017). Challenging Clinical Scenarios: Evaluating the Safety and Efficacy of Available Biologic Products for Venous Leg Ulcers. International Wound Journal, 14(S3), 23-31. https://doi.org/10.1111/ iwj.12767
5. Castillo-Garcia E, Thuy Nguyen P. Complex Refractory Wounds: How to Overcome Treatment Recalcitrance and Restore the Healing Trajectory Using Innovative Bioactive Glass. Wound Masterclass. Volume 3. March 2024.
6 Johnson ML, Armstrong DG, Ortega W. How Can Novel Bioactive Glass Wound Matrix Optimise Hard-to-Heal Venous Leg Ulcers in Geriatric Patients with Multiple Comorbidities? Wound Masterclass. Volume 3. March 2024.
7 Laurentin LA, Buck DW, Rivera D. Can Bioactive Glass Matrix be Used to Facilitate Pain Reduction and Healing for Patients with Pyoderma Gangerosum Ulcers? Wound Masterclass. Volume 3. March 2024.
8 Armstrong DG, Orgill DP, Galiano RD, et al. A multi- centre, single-blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2021;1-11. doi:10.1111/iwj.13675
Dr Donald W. Buck II; Director of Plastic Surgery Mercy South (St. Louis, MO), Plastic Surgeon, Specialist in Aesthetic and Reconstructive Surgery of the Face, Breast, Body, and Lipedema, Chief Medical Officer of ETS LLC
Dr Steven B. Jung PhD; Inventor of BGWM Technology and Chief Technology Officer of ETS LLC
9. Jung S, Schultz G, Mafiz AI, Bevels E, Jaskula K, Brownell K, Lantz E, Strickland A. Antimicrobial effects of a borate-based bioactive glass wound matrix on wound- relevant pathogens. J Wound Care. 2023 Dec 7;32(12):763. https://doi.org/10.12968/jowc.2023.32.12.763
10. Ma, H., O’Donnell, T. F., Jr, Rosen, N. A., & Iafrati, M. D. (2014). The real cost of treating venous ulcers in a contemporary vascular practice. Journal of Vascular Surgery: Venous and Lymphatic Disorders, 2(4), 355-361. https://doi. org/10.1016/j.jvsv.2014.04.006
11. Mutluoglu, M., Sivrioglu, A. K., Eroglu, M., Uzun, G., Turhan, V., Ay, H., & Lipsky, B. A. (2013). The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scandinavian Journal of Infectious Diseases, 45(7), 497-503. https://doi.org/10.3109/00365548.2013.765589)
12. Brem, H., Maggi, J., Nierman, D., Rolnitzky, L., Bell, D., Rennert, R., Golinko, M., Yan, A., Lyder, C., & Vladeck, B. (2010). High cost of stage IV pressure ulcers. American Journal of Surgery, 200(4), 473-477. https://doi. org/10.1016/j. amjsurg.2009.12.021)
13. Buck, D.W., II, MD, FACS. Innovative Bioactive Glass Fiber Technology Accelerates Wound Healing and Minimizes Costs: A Case Series. Adv Skin Wound Care. 2020;33(3):1-6.
14. Rathinasamy P, Beckford J.C., How to Reduce Treatment Costs for Hard-toHeal Wounds: The Bioactive Glass Wound Matrix. Wound Masterclass. Volume 3.March 2024
15. Jung S., Day T., Boone T., Buziak B., Amin O., (2019). Anti-biofilm Activity of Two Novel, Borate Based, Bioactive Glass Wound Dressings. Biomedical Glasses, 5(1) 67-75. https://doi.org/10.1515/bglass-2019-0006