March - April 2023


March - April 2023





Lower extremity lymphedema is an underrecognized and undermanaged clinical condition. Educational endeavors at the medical school level continue to lag behind the progressive and robust research and peer reviewed publication rate present over the past decade. The resulting inadequate medical student and care provider education regarding the lymphatic vasculature, clinical pathophysiology and available treatment modalities impairs both the delivery of care and the prevention of progression and poor outcomes.1


Too often unrecognized, the correct diagnosis of lymphedema is essential for appropriate clinical management.2,3 Often undermanaged, or potentially mismanaged through the liberal use of diuretics, the result can lead to secondary unintended consequences and result in progressive adipose deposition, tissue fibrosis, increasing limb volume, heaviness, functional difficulties, increased susceptibility to recurrent episodes of cellulitis, and overall higher healthcare utilization. These associated comorbidities carry significant tangible and intangible burdens for patients, families, and the medical community alike. Due to the propagated medical education gaps, significant diagnostic barriers exist, resulting in unacceptable treatment initiation and management.4 The diagnostic gap also impairs determination of true epidemiologic analysis of lymphedema prevalence.5 Performing accurate epidemiologic analysis, integration of medical school and health provider education, and patient-level education will increase all facets of care for the patient with lymphedema. This ultimately will improve payor education, coverage, and a realized societal cost saving and demographic health improvement.

Traditional and dogmatic medical education continues to propagate the now antiquated understanding that 90% of interstitial fluid is reabsorbed in the venous capillary.6 The perpetuation of the dogmatic ‘Classical Starling Curve’ only impedes good clinical care for the patient with lower extremity edema. The sum of all the Starling forces, including capillary lumen hydrostatic and tissue osmotic, is now recognized to be a continuous net filtration force. This is now effectively characterized as the Revised Starling Principle, which not only illustrates the continuous net filtration force of the lymphatics, but also highlights the importance of the glycocalyx (GCX) in facilitating the movement of fluid and particles as seen in Figure 1.
 Dr Michael Shao
Dr M. Mark Melin
Dr Michael Shao
Dr M. Mark Melin
Providing care and proper management for patients with lymphedema within an up to date, evidence-based algorithms will improve clinical outcomes, maximize cost effective management, and potentially decrease related complications. This is a prudent task as lymphedema causes significant physical and psychosocial morbidity, and when left untreated can lead to recurrent infections, hospitalization, limb deformity, employment difficulties, and reduced quality of life.7,8,9 The aim of this article is to describe the anatomical and physiological basis for lymph accumulation, discuss the importance of the correct clinical diagnosis of lower extremity lymphedema, and emphasize the critical need for a multidisciplinary approach to management to obtain effective edema reduction and maintenance for improved patient quality of life.

As an integral component of the circulation, the lymphatic vasculature serves to maintain tissue fluid homeostasis and mediate the local and regional immune response.

Lymphatic endothelial cells have their origin in the cardinal vein of the 3 - 4 week embryo, thereby linking the venous and lymphatic system genetically and phenotypically together.10 The lymphatic system is a closed network originating within the interstitial tissues, as lymphatic buds have low resistance to the passage of fluid, hydrophilic molecules, cells, bacteria, and viruses from the interstitial space into the lumen of the lymphatics.11 A critical component of dermal lymphatic functionality are the anchoring filaments, composed of type VII collagen. The regular occurrence and extensive presence of anchoring filaments (Figure 2) aids to bind the outer lymphatic endothelial cell surface to surrounding collagen and connective tissues. Structurally, this facilitates the transfer of applied mechanical forces from the dermis to the lymphatics thereby separating lymphatic endothelial cells. The inter-endothelial cell gaps subsequently allow flow into the lymphatic vasculature lumen which allows for the clearance of interstitial fluid.12 The reabsorption of lymph fluid from the interstitium into the lymphatic vasculature occurs in part due to the subatmospheric environment of tissue beds.13,14,15 This concept

“As an integral component of the circulation, the lymphatic vasculature serves to maintain tissue fluid homeostasis and mediate the local and regional immune response.”
The lymphatic segment between lymphatic valves is termed a lymphangion and is considered the functional unit of the lymphatics.20
is known as the ‘suction theory’ of lymph reabsorption and can be considered as a constant ‘pull’ of fluid from interstitial spaces into the lymphatic vasculature.16 The ‘pull’ of fluid into the lymphatic system is maintained by flowmediated dilation which has been shown to be disrupted in animal models following lymphatic injury.17


The lymphatic segment between lymphatic valves is termed a lymphangion and is considered the functional unit of the lymphatics (Figure 3).18,19,20 The lymphatic vasculature contains elements of both striated, cardiac-like and smooth muscle cells that result in lymphangion contractility. Intraluminal shear forces result in the production of nitric oxide (NO) from lymphatic endothelial cells, a positive feedback to lymphangion contractility, and also contribute to the immune responsiveness of the lymphatic vasculature.6,21,22,23,24

The anatomical construction of the lymphatic and venous system is clinically important for understanding, preventing, and managing lower extremity lymphedema. The lymphatic system is structurally comprised of lymphatic
capillaries, precollectors, superficial lymphatic collectors, and deep lymphatic collectors (Figure 4).25
“The anatomical construction of the lymphatic and venous system is clinically important for understanding, preventing, and managing lower extremity lymphedema.”
“In lymphedema, there is disruption of the normal ‘lymphosome’ arrangement as lymph begins to flow backwards. This concept, known as ‘dermal backflow’, can be visualized with intradermal injection of a tracer material.”

The lower extremity lymphatic system is divided into the ventromedial and dorsolateral lymphatic bundles, with the first draining lymph nodes of the ventromedial bundle located in the groin.26 Kubik and Manestar’s work in 1995 was an early triumph in highlighting this important topographical alignment of the lymphatic and venous systems of the ventromedial bundle. They found that only the great saphenous vein is overcrossed by lymph collectors, which can be easily damaged with surgical interventions near the saphenous opening.27 This is also true at the dorsum of the foot, where lymphatic collectors lie superficially within the corium of the supramalleolar region.27 More recently, Suami and Scaglioni’s cadaveric work has led to the discovery of ‘lymphosomes’, which is an anatomical mapping connecting local lymphatic vessels to regional lymph nodes (Figure 5).25
Their work highlights the anatomical and physiological doctrine of the lymphatics (Figure 6), as follows: 25
1. Superficial lymphatics travel in the straight path towards its corresponding lymph node

2. No perforating lymphatic vessels connect the superficial and deep lymphatics
3. Superficial lymphatics do not overlap and are arranged in plane
In lymphedema, there is disruption of the normal ‘lymphosome’ arrangement as lymph begins to flow backwards. This concept, known as ‘dermal backflow’, can be visualized with intradermal injection of a tracer material. Dermal backflow is prevented in healthy lymphatics in part by the arrangement of bicuspid valves. Mutations in the genes for lymphatic valve development have been implicated in primary lymphedema and more recently in the development of cancer related lymphedema.28 Iyer et al. found that
when there is decreased lymph flow, there is reduced vascular endothelial-cadherin signaling required for lymphatic valve maintenance.28 This results in loss of lymphatic valves and further reduction in lymph flow leading to dermal backflow and stagnant lymph.
Vital to the ability of lymphatic endothelial cells to maintain fluid homeostasis, immune modulation, and anti-inflammatory capabilities is the lymphatic endothelial glycocalyx (GCX). The GCX is a negatively charged, polysaccharide brush-like layer, attached to endothelial cells lining the entirety of the arterial, venous, and lymphatic systems.29 The GCX composition consists of glycoproteins and proteoglycan ‘backbones’ which attach variable glycosaminoglycan chains. At least five types of glycosaminoglycan (GAG) chains have been identified, including heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate, and hyaluronic acid. Variation exists in the proteoglycan core proteins with regards to size, the glycosaminoglycan chains that are attached, and cell membrane ‘bound’ status. Other proteoglycans (mimecan, perlecan, biglycan) are attached after being secreted from cytosol bodies, and are subsequently assembled as GAG modified chains leading to soluble aspects of proteoglycans which diffuse into the blood stream.
The functions of the GCX are:
1. Modulate inflammatory responses through cytokine binding
2. Act as 'quenchers' of oxygen free radicals and maintenance of nitric oxide availability
3. Maintain anticoagulant components that contribute to the thromboresistant nature of healthy endothelium
4. Prevent leukocyte and platelet adhesion
5. Improve rheology of red blood cells in the microvasculature
6. Acts as a mechano-sensor and transducer responding to shear stress resulting in the production of nitric oxide (NO) and other cytokines30,31,32
7. Regulates vascular molecular and biofluid permeability, thereby preventing reabsorption of interstitial fluid into the venules29,31,32,33,34,35,36
Consequently, both venous and arterial hypertension have been associated with glycocalyx alterations, shedding, and microcirculatory dysfunction, thereby inhibiting these important functions of the GCX.38,39
The lymphatic system is integrally connected to the functional immune response. Unfortunately, lymphatic vessels are often thought of as a mere passageway to the lymphoid tissues instead of an integral component of immune activation. Lymphatic endothelial cells exhibit important immunomodulatory roles, with circulating dendritic and T cells, and exhibit both MHC I and MHC II molecules for antigen processing and presentation.40 In addition, lymphatic vessels maintain the appropriate gradients for cytokine and chemokine signaling, aid in lymphocyte rolling, maintain fluid balance of tissue and vasculature, and facilitate pathogen clearance.33,41,42 In states of high inflammation, lymphatic endothelial cells can coordinate immunosuppression by decreasing dendritic cell maturation.43 In a recent model study, high inflammatory states were found to initiate shedding of GCX components, thereby allowing for rapid drainage of inflamed tissue beds without subsequent neutrophil recruitment.44 The integrity of the GCX is therefore vital for modulating inflammation and facilitating the immune responses of the lymphatic system.
As we age so does our lymphatic system; aging-associated changes to the lymphatic system include decreased collecting vessels, decreased collateral conduits within local lymphatic arcades, aneurism-like formations, impaired contractile function, decreased nitric oxide production, and significant loss of the GCX.45 The age-associated loss of the GCX layer includes a reduction in both the size and continuity of this protective layer and the production of pro-inflammatory cytokines, causing increased lymphatic permeability.46
Endothelial nitric oxide synthase (eNOS) and subsequent nitric oxide (NO) production are essential to maintain native lymphatic pumping and increase the diastolic filling of lymphangions.47 As we age, there is a decrease in eNOS activity and a subsequent increase in inducible NO synthase (iNOS), leading to an increased lymphatic vessel load without the ability of the lymphatic system to adapt its pumping capabilities (Figure 7).46
Of interest, similar alterations to eNOS have been found in obesity-induced endothelial dysfunction due to direct and indirect alterations in regulatory microRNA expression.48 This results in decreased NO bioavailability and subsequently decreased endothelial vasodilatory capacity. As approximately onethird of our population is affected by obesity, and with the aging population, the clinical utility of evaluating microRNA gene regulation may hold promising therapeutic value.48,49

The recognition of decreased post-venule reabsorption of interstitial fluid as a result of the permeability function of the GCX resulted in a paradigm shift of a new and profound relevance of the lymphatic system, due to the modification of the dogmatic Starling curve. In 1896, E.H. Starling, working in the Physiological Laboratory of Guy’s Hospital, developed the Classic Starling Curve. The Classic Starling curve evaluated luminal hydrostatic pressures and the balance with tissue osmotic pressures.50 Based upon his data, Starling proposed that~90% of fluid shed into the interstitium is
reabsorbed into the venule, with the remaining 10% being collected by the lymphatic system. For decades, the dogmatic Starling curve relegated the lymphatics to a minor vascular system component, only of major significance in clinical lymphedema and surgical procedures gone awry that resulted in quality of life altering large vessel lymphatic leaks. The origins of the modification of the Starling curve, recognizing that all interstitial fluid is returned to the central venous system via the lymphatic system, and the recognition of the GCX to be a major determinant of vascular permeability, began in 1987 with the work of Michel and Phillips, demonstrating only transient venule reabsorption of interstitial lymphatic fluid.51
Further details were defined by Michel and Levick in 2004, with the introduction of the fully revised Starling principle recognizing the GCX layer of endothelial cells direct impact on reabsorption of interstitial lymphatic fluid into the lumen of venules. This resulted in the revised Starling Principle application to the treatment of lower extremity edema, expressly advocating for no diuretic use.52
Further investigation has characterized the
“The recognition of decreased post-venule reabsorption of interstitial fluid as a result of the permeability function of the GCX resulted in a paradigm shift of a new and profound relevance of the lymphatic system, due to the modification of the dogmatic Starling curve.”Figure 7: NO-dependent regulatory mechanisms in aged lymphatic vessels.46
7a:In adult
lymphatic vessels, the enhanced eNOS activity mediates the transient inhibition of contraction frequency to adapt the load by the increase of imposed flow.
7b:In
aged lympathic vessels, increased iNOS level renders the contractile parameters unchanged in response to increased imposed flow.
GCX as a semipermeable layer coating the endoluminal side of the capillary wall, recognizing the lymphatic system as the dominant force responsible for interstitial fluid homeostasis, and promoting interest in the molecular and genetic underpinnings of lymphatic dysfunction.42
In 2014, Drs. Mortimer and Rockson brought the Modified Starling Curve to international attention and clinical applicability, by recognizing that edema develops when the capillary and venule filtration rate exceeds lymph drainage either because the microfiltration rate is too high, lymphatic flow is impaired, or both exist; hence, all chronic edema indicates an inadequacy or failure of lymphatic drainage. Therefore, a clinical approach to subcutaneous edema of the extremities should begin with a consideration and assessment of lymphatic function to evaluate whether primary lymphatic impairment exists, or if lymphatic circulation is normal but overloaded by elevated microvascular filtration.6
The GCX is now thoroughly recognized in peer reviewed literature as critical to endothelial cells, micro/macro vascular and lymphatic function, and bioreactivity. Pioneering work by Bjork and Hettrick led to one of the first connections tying the intricacies of the GCX to the functional lymphatic system.53 Pathophysiologic conditions are associated with variable alterations at the local, regional and systemic levels, resulting in GCX thinning, shedding, and restoration. GCX dysfunction is directly correlated with endothelial cell dysfunction; such conditions include acute and chronic hyperglycemia in diabetes, events resulting in ischemic/ reperfusion injury, trauma, sepsis, tobacco use, arterial hypertension and venous hypertension.39,38 Persistent venous hypertension is thought to alter microvascular shear forces, resulting in shedding of the GCX, permitting leukocyte adhesion and migration through the endothelial cell lining, triggering
an inflammatory cascade that contributes to the pathophysiology of venous changes noted in chronic venous disease.38 Chronic venous insufficiency is an important secondary cause of lymphedema, a condition known as phlebolymphedema. Phlebolymphedema has been reported to be the predominant cause of lower extremity lymphedema, in a 440 patient cohort treated in a cancer-affiliated physical therapy clinic.54
Lymphedema is the result of a loss of the finely tuned balance of microvascular tissue fluid production and recovery through the lymphatic vasculature, chronic inflammation, and loss of integrity of the lymphatic endothelial cell GCX.6,33,41,53,55,56
The etiology of lymphedema is generally described as primary or secondary. Primary lymphedema is due to a genetic mutation, resulting in abnormal lymphatic vascular development causing either a structural or functional abnormality that impairs proper drainage of lymphatic fluid; primary lymphedema is further categorized as congenital (identification based upon abnormalities identified shortly after birth), praecox (abnormalities identified most often during teenage years or early adulthood) or tarda (typically occurring after age 35).6 It is important to note that genetic alterations resulting in clinical lymphedema can manifest and present later in life, though this can be a point of confusion and a missed opportunity to correctly diagnose the patient.6 If an accurate phenotypic profile is performed, the correct diagnosis of primary lymphedema can be established, despite the absence of the genetic identification.57 Due to some forms of primary lymphedema presenting later in life, this can hinder the ability of a clinician to accurately differentiate primary vs secondary etiology; as such, the presence of potentiating secondary
“The GCX is now thoroughly recognized in peer reviewed literature as critical to endothelial cells, micro/macro vascular and lymphatic function, and bioreactivity.”
“Accurate clinical staging of lymphedema is also critical for treatment, documentation, and to gain appropriate insurance coverage to support patient participation in the treatment plan.”
factors associated with secondary lymphedema, such as chronic venous insufficiency, obesity, lipedema, trauma, cellulitis, surgery, and cancer must be considered, but also the possibility of primary etiology should not be mutually excluded. An accurate diagnosis through genetic and phenotypic evaluation and an understanding of the complete disease process will result in the most appropriate treatment algorithm.
Accurate clinical staging of lymphedema is also critical for treatment, documentation, and to
The 2016 International Society of Lymphology classifies 4 stages:
Stage 0: Latent or subclinical; no evidence of swelling; subjective symptoms.
Stage I: Early accumulation of fluid; usually pitting; subsides with elevation.
Stage II: Swelling rarely reduced with elevation; pitting still present in early stage II, whereas pitting is absent in later stages as fibrosis and fat deposition begin.
Stage III: Lymphostatic elephantiasis; nonpitting with trophic skin changes, further deposition of fat and fibrosis, and warty overgrowths. 58
gain appropriate insurance coverage to support patient participation in the treatment plan. Due to the challenges associated with accurately diagnosing lymphedema, clinicians should carefully consider the differential diagnosis. Patients can be broadly segregated by those with unilateral asymmetric or bilateral leg edema, and by acuity of onset. For patients presenting with acute onset unilateral leg edema, it is imperative to consider deep vein thrombosis (DVT) and evaluate using duplex ultrasonography. If DVT has been excluded, patients should be evaluated for musculoskeletal injury or cellulitis, which should be evident based on history and physical exam findings.
Antidepressants
Antihypertensives
Antivirals
Specific medications
Monoamine oxidase inhibitors, trazodone
Beta-adrenergic blockers, calcium channel blockers, clonidine (Catapres), hydralazine, methyldopa, minoxidil
Acyclovir (Zovirax)
Chemotherapeutics Cyclophosphamide, cyclosporine (Sandimmune), cytosine arabinoside, mithramycin
Cytokines Granulocyte colonystimulating factor, granulocyte-macrophase colony-stimulating factor, interferon alfa, interleukin-2, interleukin-4
Hormones Androgen, corticosteroids, estrogen, progesterone, testosterone
Nonsteroidal antiinflammatory drugs
Celecoxib (Celebrex), ibuprofen
For patients presenting with bilateral leg edema, the differential diagnosis includes medicationinduced edema, acute heart failure, end-stage renal disease, and bilateral DVT. Common medication-induced edema is often forgotten within the differential, yet well described in the literature. Clinicians should closely examine medication lists as a significant proportion of patients now take antihypertensive medications commonly associated with edema (Table 1).59
The Wells score or Modified Wells score may be used to calculate clinical suspicion for DVT.60 If clinical suspicion is moderate or high, duplex ultrasound should be done to evaluate for DVT. Patients with a history of dyspnea, orthopnea, or paroxysmal nocturnal dyspnea; or signs of tachypnea, tachycardia, rales, or jugular venous distention, should undergo echocardiography to evaluate for acute heart failure. Urinalysis or urine dipstick can screen for the presence of proteinuria, and if present,
quantitative urine protein-to-creatinine ratio and serum albumin should be obtained.
For patients presenting with unilateral leg edema, the differential diagnosis includes chronic venous insufficiency, chronic lymphedema, Baker’s cyst, MayThurner syndrome, pelvic tumor, complex regional pain syndrome, syndromic limb hypertrophy (Klippel-Trenaunay syndrome and Proteus syndrome), and poor calf contractility (radiculopathy, stroke). Duplex ultrasonography can be helpful to identify chronic venous insufficiency and Baker’s cyst. Complex regional pain syndrome and chronic lymphedema can generally be diagnosed based on clinical features in the history and physical examination. Complex regional pain syndrome usually presents with skin hyperesthesia, allodynia, and/ or temperature or skin color asymmetry, 4 - 6 weeks following trauma. Syndromic limb hypertrophy is typically associated with other clinical features, such as capillary malformations (port wine stains), in the case of Klippel-Trenaunay syndrome. Primary lymphedema patients may report positive family history of lymphedema or early onset lymphedema (congenital lymphedema or lymphedema praecox); secondary lymphedema patients may report a history of cancer, inguinal or pelvic lymphadenectomy, radiation therapy, infection, obesity, chronic venous insufficiency, or inflammatory disorders.
As noted above, the early phases of lymphedema present with soft tissue infiltration which clinically resembles the edema of venous insufficiency; however over time, the inflammatory milieu of chronic lymphedema induces vascular and connective tissue changes
within the dermis and subcutis. The clinical and microscopic cutaneous findings of chronic lymphedema can be both benign/ reactive and malignant, as lymphedema is a known risk factor for skin and soft tissue malignancies.61
The benign, reactive cutaneous changes associated with chronic lymphedema include erythema, skin thickening and fibrotic, scarlike changes with hyperkeratosis, and a distinctive ‘cobblestone’ pattern referred to as ‘lymphostasisverrucosa cutis’, or ‘elephantiasis nostrasverrucosa’ (ENV).62 ENV presents as a diffusely infiltrated, typically hyperpigmented, firm plaque of the distal lower extremities, with a verruous, ‘pebbly’ surface and hyperkeratosis, which is sometimes described as ‘mossy’ in appearance.62 Microscopically, early lymphedema results in ectatic, thin-walled or slitlike small vascular channels within the upper dermis. The histopathologic changes of chronic lymphedema, including biopsies of ENV, include reactive (pseudoepitheliomatous) epidermal hyperplasia, dilated dermal lymphatic channels (which can be highlighted by immunostains such as D2-40/LYVE-1), and pronounced, scarlike fibrosis of the dermis and subcutis.63 These chronic fibrotic changes are believed to result from the induction of fibroblast proliferation by protein-rich lymph fluid.62
Chronic lymphedema is a significant risk factor for angiosarcoma (AS); this phenomenon is referred to as Stewart-Treves syndrome, and was first described in 1948.61 Stewart-Treves is classically associated with lymphedema resulting from breast cancer surgery and lymphadenectomy, but has also been observed in chronic lower extremity lymphedema. The clinical presentation typically involves rapidly-expanding purpuric plaques, nodules and tumors, commonly with ulceration. The microscopic features of AS include extensive replacement of dermal connective tissue by irregular and anastomosing vascular channels,
“Chronic lymphedema is a significant risk factor for angiosarcoma (AS).”
which dissect between collagen fibers, encircle normal dermal vessels, and adnexal structures, and exhibit endothelial cell atypia and multlayering. Chronic lymphedema is also a risk factor for Kaposi’s sarcoma (KS), especially in patients from areas with human herpesvirus-8 (HHV-8) endemicity.61 Cutaneous KS presents with purpuric, purplehued papules and plaques, and microscopically demonstrates slitlike vascular channels with spindled endothelial cells, stromal fibrosis and a lymphoplasmacytic infiltrate.
After other etiologies for generalized edema have been excluded, the diagnosis of chronic lymphedema is usually established based on typical clinical features found by thorough history and physical examination:
• Slowly progressive edema affecting one or both lower extremities
• History of surgery, lymph node dissection, or radiation therapy
• Non-pitting edema is suggestive, although pitting edema may be present in early-stage lymphedema until further deposition of fat and fibrosis causes the characteristic non-pitting presentation
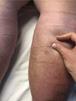
• Positive Stemmer sign: inability to pinch the skinfold at the base of the second toe (Figure 8)64
• Positive Bjork Bow Tie Test: gently pinched skin when lifted and rolled is thickened, less pliable, less able to be pinched and lifted off, and produce limited 'bow tie' of wrinkles (Figure 9)65
• Bier spots are the presence of multiple irregular white macules along extensor surfaces and have been associated with lower extremity lymphedema66
• Characteristic skin changes: hypertrophic nodules, hyperkeratotic, verrucous and vesicular skin lesions
• Dorsal hump and squaring of toes: this distinguishes lymphedema from lipedema which often spares the foot and toes. Lipedema can be further distinguished from lymphedema due to its characteristic adiposity distribution from hips to ankles while sparing the feet in a distinct 'step off' appearance.67
• 'Ski-jump' nails evident by anup-turned concavity can be clinically useful as a phenotypic manifestation of primary lymphedema68
Imaging modalities for chronic lymphedema include lymphoscintigraphy and indocyanine green lymphangiography, but are not usually necessary and are generally reserved for diagnostic dilemmas. Duplex ultrasound (DUS) has become a practical first-line modality for the evaluation of lymphedema, due to its availability and low-cost. DUS not only provides diagnostic value, but has also been shown to allow for classifying edema severity and monitoring response to treatment.69 DUS can be used to differentiate dependent edema from secondary lower extremity edema, by visualization of subcutaneous echogenicity and echo-free space.70 DUS can also aid in differentiating lipidema from lymphedema, as lipidema characteristically demonstrates normal dermal thickness and echogenicity, whereas lymphedema often demonstrates increased dermal thickness and reduced echogenicity.71 Newer modalities, although not available currently in the United States, is the use of magnetic resonance lymphography (MRL). MRL has been shown to identify superficial lymphatic vessels down to the 0.5 mm level, with a high sensitivity and specificity for illustrating abnormal lymphatics and drainage patterns.72 Similarly, the use of multifrequency bioimpedance analysis has been shown to be reliable and reproducible for accurately documenting the presence of lymphedema in a quick, cost-effective manner.73,74
Although there is currently no cure for lymphedema, it is a manageable disease by implementing the components of Complete Decongestive Physiotherapy (CDPT). The objective of CDPT is to achieve a reduction in limb volume and improve the integrity and quality of the skin. This is attained through a two-phase approach: phase one is the intensive or decongestion phase that is clinician-driven, and phase two is the maintenance phase that is patient-driven. The intensive phase is best performed daily (or as frequently as possible), until maximal volume reduction is achieved. Once the lymphedematous limb has plateaued and is no longer achieving a reduction in volume, the patient is transitioned into the maintenance phase, which is continued for life.


The components of CDPT are implemented and facilitated by a trained lymphedema specialist during the intensive phase and include:
1. Meticulous skin and nail care to the affected areas
2. Manual lymphatic drainage (MLD), which is a gentle manual technique that redirects lymph flow


3. Multilayer, short-strech compression bandaging during active decongestion, and over-the-counter or custom garments once the limb has decongested

4. Therapeutic exercise to enchance and promote lympathic pumping mirrors the effects of MLD, with patient reports of ease of use and comfort, without an increase in pain scores75
Additionally, during the maintenance phase, the utilization of intermittent pneumatic compression (IPC) may be employed as an adjunctive device to help maintain fluid reduction.
Of note is the growing understanding of the effects of manual lymph drainage beyond the reduction of fluid volume. Emerging evidence is highlighting the impact MLD has on soft tissue remodeling, and the resolution and prevention of soft tissue dysfunction. To appreciate this, it is important to understand the inter-relationship between the lymphatic and integumentary systems.
9a: Negative Bjork ‘bow tie’ test.
9b: Positive Bjork ‘bow tie’ test.
9c: ‘Bow tie’ of wrinkles in negative test.65
When lymphatic fluid is stagnant, a pathohistological state of chronic inflammation results.76 This chronic inflammatory state induces a cascade of events that results in connective tissue proliferation and excess collagen tissue formation, culminating in the thickened, fibrotic skin and wart-like projections (papillomatosis and verrucous) commonly seen with chronic lymphedema.77 Further, disorders of the lymph system, whether systemic (macro-lymphedema) or localized (micro-lymphedema), produce cutaneous regions susceptible to infection, inflammation and carcinogenesis.77 The chronic inflammation resulting from lymphedema creates a region of cutaneous immune deficiency, or a localized skin barrier failure; the associated abnormalities are called lymphostatic dermopathy, which is the failure of the skin as an immune organ.61,77,78 Areas of lymphostatic dermopathy are often associated with the presence of chronic wounds and chronic infections.
Manual lymph drainage helps to re-route stagnant lymph fluid to functional lymphatics, thereby mobilizing the fluid and reducing limb volume. This is coupled with the fact that mobilizing the stagnant lymph breaks the chronic inflammatory cycle and the deleterious integumentary sequelae that ensues. A growing body of evidence is shedding light on the positive effects MLD has on many structures and conditions. Although beyond the scope
“Manuallymph drainage helps to re-route stagnant lymph fluid to functional lymphatics, thereby mobilizing the
fluid and reducing limb volume.”Figure 8: 8a: True-negative Stemmer sign (examiner is able to pinch the skin in a patient without lymphedema). 8b: True-positive Stemmer sign (skin is unable to be pinched in an individual with lymphedema).
of this article, the following highlights the current evidence in support of MLD: the evidence supports MLD for influencing soft tissue remodeling;79,80 synergistic effects when combined with exercise;81 positive effects on the veins and venous ulceration;82,83,84,85,86 improving orthopedic outcomes such as total knee replacements;87,88,89 and MLD is beneficial for other diagnoses such as orthopedic injuries,90 neurological diseases, autoimmune diseases, autism, when combined with Proprioception Neuromuscular Facilitation.91,92,93,94,95 The benefits of MLD have been assessed using near-infrared fluorescence imaging evaluating pre- and post-therapy sessions, and illustrating improved contractile lymphatic function.96
It is well understood that lymphedema can be a complex and challenging condition to manage. This is further evidenced by a report that lymphedema patients seen in an outpatient wound clinic had an average of 7.3 comorbidities and took 8.4 concomitant medications.7 To assist healthcare providers in managing these complex patients, the Lower Extremity Lymphedema Complexity Score (LLCS) has been created.97
The tool is meant to help clinicians identify the various contributing factors and complexities of patients presenting with lymphedema and skin impairments. This could help drive proper examination and differential diagnosis, treatment planning, as well as utilization of proper resources. The LLCS contains 12 domains: comorbidities (referring to any copresenting condition that impacts overall health), limb edema, tissue texture, scars, skin integrity, skin changes, fat disorders (lipedema), BMI, mobility, activities of daily living (ADLs), pain/ discomfort and the Lymphedema Life Impact Scale (LLIS, used with permission from the author).
The 12 established domains reflect common presentations ranging in complexity that can contribute to lymphedema severity and complications. Each domain is to be scored based upon the complexity of the condition, ranging from nominal complexity (0), mild complexity (1), moderate complexity (2), severe complexity (3) or extreme complexity (4). If a patient has bilateral lymphedema, the score is based on the most complex or involved limb. Descriptions under each domain and section are provided for additional clarity. A
cumulative score will indicate if the patient presents with mild, moderate, severe or extreme complexity.
The next phase in the development of the LLCS is to create algorithms of suggested interventions and/ or referrals based upon standards of practice and evidence-based medicine. Though not prescriptive, the algorithms are meant to provide guidance and suggestions for healthcare providers as to how best manage the common associated complications and underlying pathologies widespread in this patient population.
Intermittent pneumatic compression pumps (IPCs) are available as simple (leg sleeves) and advanced (leg sleeves with an abdominal component). In addition, available IPCs have varying mechanisms of action, including push and squeeze, push-squeeze-pull, and pull. Push and squeeze IPC devices are typically high pressure and show a dose-dependent relationship between pressure and total fluid flow rates.98 At times, this can be beneficial as patients with later stage lymphedema often have epidermal and dermal thickening and fibrotic changes often requiring more force to move fluid.99 Consequently, high flow and pressure rates can also lead to collateral channel formation lacking lymphatic endothelial lining, therefore lacking the clinical benefits of the glycocalyx. Push-squeeze-pull IPC devices often cycle between pressures while completely deflating between compression phases. Pull IPC devices most closely mimic the suction theory and MLD by facilitating the subatmospheric environment of tissue beds and allowing interstitial fluid to be drawn into the lymphatic vasculature. The goal with IPC devices and conventional therapy on fluid redistribution is to promote lymphangiogenesisvia stimulation of local mediators and promote native lymphatic function.100,101,102
The push-squeeze-pull mechanism commonly seen in advanced IPC devices have been evaluated with Near InfraRed Florescence Imaging (NIRFLI) to effectively move lymphatic fluid proximally, similar in manner in which NIRFLI demonstrated the effectiveness of MLD.103,104,105
The purpose of the abdominal component in advanced IPC devices is to ‘prime’ the receiving abdominal and upper thigh lymphatics,
creating receptive capacitance as the pump ‘cycles’ through the abdominal chambers, initially to clear the underlying lymphatic vasculature with subsequent initiation of the leg sleeve chambers propagating distally from the ankles to the thighs. The advanced pumps mimic the cycling treatments of MLD therapy, maximizing extremity treatment.
For patients deemed to be appropriate candidates for an IPC, outcomes are best when used in conjunction with a physical medicine and rehabilitation/ vascular/ lymphatic specialist, and/ or a certified lymphedema therapist (CLT), providing treatments and education; MLD, CDPT, stretching, exercise, nutrition, and skin care remain critical elements of daily treatment. Also, because patients with lymphedema may present in various clinical settings, it is prudent for education awareness on the appropriate referral and management options that exist.
Multiple benefits of IPC devices are recognized, including patient access to use on a daily basis, utilization in locations where access to healthcare may be limited, and peer reviewed data demonstrating improved medical outcomes associated with decreasing overall medical resource utilization.106,107,108 In addition, advanced pneumatic compression devices have been shown to have significant economic and quality of life benefits when compared to conservative treatment. Advanced IPC use has been shown to reduce phlebolymphedema and phlebolymphedema sequelae related costs annually by 69%, including 59% reduction in hospitalizations, 82% lower inpatient costs, 55% lower outpatient costs, and reduction in physical and occupational therapy costs.108 In addition to the economic benefit, utilization of IPC can also lead to improved quality of life for patients.
Multiple over the counter medications tout a venotonic or lymphotonic effect; regarding many components present in over the counter medications, quality peer reviewed publications are limited. Flavonoids are a phenolic plant derived compound found in over 8000 different forms, with multiple biological properties109 and are among the most studied medications for management of lymphedema associated with chronic venous insufficiency. The specific flavonoids, hesperidin and diosmin, were evaluated in a 2017 meta-analysis, identifying moderate quality evidence demonstrating benefit for management of venous ulcerations and edema (phlebolymphedema).110 Diosmin, as a component of a hybrid medication, was found to improve outcomes when used in conjunction with CDT as compared to CDT alone.111 Diosmin formulations are commercially available in the U.S., including Vasculera’s diosmiplex, a proprietary micronized blend of purified diosmin glycoside and alkyline granules. Use of the FDA regulated Diosmiplex has been shown to have therapeutic value, although initially mislabeled in a recent publication in Wounds 2020 that has been since corrected.112
Micronized purified flavonoid fraction (MPFF) has been shown to decrease the inflammatory cascade and leukocyte-endothelial activation, thereby discouraging edema formation.113 MPFF consists of 90% diosmin and 10% hesperidin and supplementary flavonoids, and comes from extracts of rutaceaeaurantiae, a type of small orange that is micronized for improved bioavailability.113,114 MPFF has not been associated with known major side effects, while simultaneously showing significant improvement in quality of life in patients with chronic venous insufficiency, and accelerated healing of leg ulcers.113,115,116,117 Similarly,
“Micronized purified flavonoid fraction (MPFF) has been shown to decrease the inflammatory cascade and leukocyte-endothelial activation, thereby discouraging edema formation.”
selenium has been noted to have a positive impact on lymphedema symptoms in a recent literature review.118 Often considered for patients with lymphedema undergoing long-distance travel or flight is the use of pinus pinaster bark extract (PBE), which goes by the tradename Pycnogenol. PBE possesses vasorelaxant activity, and enhances microcirculation through increasing capillary permeability. PBE has been shown to be effective in improving leg heaviness, subcutaneous edema, and venous pressure in patients with chronic venous insufficiency.119
Patients should also be educated in skin care, including use of a ceramide based hydrating cream to maintain dermal integrity and hydration, in order to decrease micro-cracks that leave the patient susceptible to episodes of cellulitis and erysipelas. As lymphatic dysfunction progresses, the immune function of the lymphatics is also impaired, thereby increasing patient risk of infection. Immediate access to antibiotics via an up to date prescription is generally recommended to minimize the intensity and duration of an episode of cellulitis. For patients with recurrent cellulitic episodes, chronic prophylaxis may be the best management option to prevent persistent demise of already compromised lymphatics. It is well established that with every additional cellulitic episode there is further damage to the lymphatic system, causing a degree of secondary lymphedema.120,121 This creates a cyclical problem, as the already compromised lymphatics are susceptible to infection, which in return potentiates further lymphatic damage.122 It should be noted that there is no current recommended diet for the management of lymphedema; some patients are instructed to limit water or protein intake, however this is not supported by the literature.123,124
Current medical and clinical education regarding the anatomical and pathophysiological state of lymphedema have far lagged behind the rapidly advancing field of lymphatic medicine. Consequently, the accurate and evidencedbased approach to lymphedema assessment and management are not unanimously and universally employed. This paper seeks to outline the basic fundamental structure of the lymphatic vasculature and its importance in lymphatic function. As patients with
lymphedema often present in a myriad of different clinical settings, it is important to deploy accurate clinical assessment and readily offer best practice management options. These management options should include meticulous skin and nail care, manual lymphatic drainage, compression, therapeutic exercise, and review of pharmacologic options. As chronic lymphedema will likely require chronic management, the use and application of IPC devices is also discussed. The use of IPC devices as adjuncts to traditional therapy can be used to decrease lymphedema related complications and improve patient quality of life. In addition, IPC devices have the ability to be used in regions where limited lymphedema therapists or specialists exist, thereby continuing to provide patients with lymphatic stimulation. The goal of MLD or IPC devices is to stimulate the underlying lymphatic system, to redistribute stagnant lymph as outlined by the revised starling curve; by redistributing stagnant lymph back into circulation the not uncommon lymphedema sequelae of recurrent infection, ulceration, deformity, and dermal/ epidermal skin changes can be minimized.
1. Vuong, D.; Nguyen, M.; Piller, N. Medical education: A deficiency or a disgrace. J. Lymphoedema 2011, 6, 44–49.
2. Maclellan RA, Couto RA, Sullivan JE, Grant FD, Slavin SA, Greene AK. Management of Primary and Secondary Lymphedema: Analysis of 225 Referrals to a Center. Ann Plast Surg. 2015 Aug;75(2):197-200. doi: 10.1097/SAP.0000000000000022. PMID: 24691335.
3. Wang W, Keast D. Prevalence and characteristics of lymphedema at a wound-care clinic. J Wound Care. 2016;25(4):S11-S15.
4. Son A, O’Donnell TF Jr, Izhakoff J, Gaebler JA, Niecko T, Iafrati MA. Lymphedemaassociated comorbidities and treatment gap. J Vasc Surg Venous Lymphat Disord. 2019 Sep;7(5):724-730. doi: 10.1016/j.jvsv.2019.02.015. Epub 2019 Jun 24. PMID: 31248833.
5. Rockson, Stanley G., and Kahealani K. Rivera. 2008. “Estimating the Population Burden of Lymphedema.” Annals of the New York Academy of Sciences 1131: 147–54. https://doi.org/10.1196/annals.1413.014.
6. Mortimer, Peter S., and Stanley G. Rockson. 2014. “New Developments in Clinical Aspects of Lymphatic Disease.” The Journal of Clinical Investigation 124 (3): 915–21. https://doi.org/10.1172/JCI71608.
7. Wang W, Keast D. Prevalence and characteristics of lymphedema at a wound-care clinic. J Wound Care. 2016;25(4):S11-S15
8. Szuba, A., and S.G. Rockson. 1998. “Lymphedema: Classification, Diagnosis and Therapy.” Vascular Medicine 3 (2): 145–56. https://doi. org/10.1191/135886398674160447.
9. Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J. 2005 Mar;2(1):47-62. doi: 10.1111/j.17424801.2005.00066.x. PMID: 16722853; PMCID: PMC7951421.
10. Srinivasan, R. Sathish, Miriam E. Dillard, Oleg V. Lagutin, Fu-Jung Lin, Sophia Tsai, Ming-Jer Tsai, Igor M. Samokhvalov, and Guillermo Oliver. 2007. “Lineage Tracing Demonstrates the Venous Origin of the Mammalian Lymphatic Vasculature.” Genes & Development 21 (19): 2422–32. https://doi.org/10.1101/gad.1588407.
11. Negrini, Daniela, and Andrea Moriondo. 2011. “Lymphatic Anatomy and Biomechanics: Biomechanics of Initial Lymphatics.” The Journal of Physiology 589 (12): 2927–34. https://doi.org/10.1113/jphysiol.2011.206672. “(PDF) Role of Flavonoids as Wound Healing Agent.” n.d. ResearchGate. Accessed April 26, 2020. http://dx.doi.org/10.5772/intechopen.79179.
12. Leak, L. V., and J. F. Burke. 1968. “ULTRASTRUCTURAL STUDIES ON THE LYMPHATIC ANCHORING FILAMENTS.” The Journal of Cell Biology 36 (1): 129–49. https://doi.org/10.1083/jcb.36.1.129.
13. Clough, G. &Smaje, L. H. Simultaneous measurement of pressure in the interstitium and the terminal lymphatics of the cat mesentery. The Journal of Physiology283, 457–468 (1978).
14. Granger, H. J., Lane, G. A., Barnes, G. E. & Lewis, R. E. Dynamics and control of transmicrovascular fluid exchange. Edema8, 189–224 (1984).
15. Hogan, R. D. The initial lymphatics and interstitial fluid pressure. In “Tissue Fluid Pressure and Composition” (A. R. Hargens, ed.). Williams and Wilkins, Baltimore., 155–163 (1981).
16. Guyton, A. C. & Coleman, T. G. Regulation of interstitial fluid volume and pressure. Annals of the New York Academy of Sciences150, 537–547, https://doi. org/10.1111/j.1749-6632.1968.tb14705.x (1968).
17. Nelson TS, Nepiyushchikh Z, Hooks JST, Razavi MS, Lewis T, Clement CC, Thoresen M, Cribb MT, Ross MK, Gleason RL Jr, Santambrogio L, Peroni JF, Dixon
JB. Lymphatic remodelling in response to lymphatic injury in the hind limbs of sheep.
Nat Biomed Eng. 2020 Jun;4(6):649-661. doi: 10.1038/s41551-019-0493-1. Epub 2019
Dec 23. PMID: 31873209; PMCID: PMC7549051.
18. Borisov, A. V. 1997. “[The theory of the design of the lymphangion].” Morfologiia (Saint Petersburg, Russia) 112 (5): 7–17.
19. Gashev, A. A. 1991. “[The mechanism of the formation of a reverse fluid filling in the lymphangions].” Fiziologicheskiizhurnal SSSR imeni I. M. Sechenova 77 (7): 63–69.
20. Hansen, Kirk C., Angelo D’Alessandro, Cristina C. Clement, and Laura Santambrogio. 2015. “Lymph Formation, Composition and Circulation: A Proteomics Perspective.” International Immunology 27 (5): 219–27. https://doi.org/10.1093/intimm/dxv012.

