May
- June 2022


May

This Masterclass Guide is a concise overview aimed at exploring the use of a synthetic dermal matrix and how to incorporate this into your practice.

Keywords
■ Synthetic dermal matrix
■ Regenerative healing
■ NovoSorb® BTM
■ Biodegradable temporising matrix

■ NovoSorb® BTM (biodegradable temporising matrix), is a fully synthetic, biodegradable, biocompatible device used for the reconstruction of full or deep partial thickness wounds. It is intended to temporise dermal injuries, where the dermis has been decimated or lost, and to facilitate dermal repair by providing temporary wound closure and a scaffold for the generation of a neodermis
■ NovoSorb® BTM has a bilayer polyurethane design made of a sealing membrane and a 2mm matrix below. The sealing membrane physiologically closes the wound, provides a barrier to bacteria and limits moisture loss1,2
■ The fenestrated membrane allows for free drainage of excess fluid
■ The 2mm open cell matrix allows cellular infiltration and provides a model for reconstruction of the dermis1
■ The matrix is made from a polyurethane polymer, so does not feed bacteria. The benefit of this is that it can often be retained if infection is present3
■ Wound repair
■ Complex wounds
■ Reconstruction
■ Dermal matrix
Adhesive
A temporary, nonbiodegradable layer closes the wound, limiting moisture loss, while also serving as a barrier to outside bacteria1,2,5
A 2mm bioabsorbable open cell matrix allows for the infiltration of cellular materials and serves as a matrix to aid in the reconstruction of the deeper layers (dermis) of the skin4,5
How NovoSorb® BTM Works: The Ten Point Guide
1 Select the appropriate patient
2 Remove the old dressing
3 Under sterile conditions, the wound must be debrided to a healthy wound bed
4 Measure the soft tissue defect, measure NovoSorb® BTM and trim to the required size and shape
5 Secure with skin staples or sutures
6 Dressing changes as per regime, depending on clinical indication scenario
7 As NovoSorb® BTM integrates with the wound, its appearance will change. This integration usually takes up to three weeks
8 Once fully integrated into the wound bed, the sealing membrane can be removed, revealing a newly revascularized dermal layer (figure 3c)
9 A method of definitive skin closure can then be used, at the clinician’s discretion. For example, a split-skin graft (SSG).
10 Over time the polymer degrades and gradually disappears at approximately 18 months (figure 3d)





What Types of Wounds Are Suitable?
■ Full or deep partial thickness burns and wounds
■ Surgical and reconstructive wounds
■ Traumatic wounds
What Types of Wounds Are Not Suitable?
■ Overtly infected wounds
■ Wounds in which effective haemostasis has not been achieved

■ Undebrided wounds
BTM is applied to a wound once the unhealthy or dead tissue has been surgically removed. BTM temporarily closes the wound, which limits moisture loss2,5
Cellular migration into the matrix results in new blood vessel formation and collagen production throughout the matrix. This normally takes 2 to 3 weeks5
Once a clinician determines the tissue is fully integrated throughout the BTM, the sealing membrane can be removed, leaving a new vascularized dermal layer5
The method of closure will be chosen by the clinician depending on the wound (split-skin graft, moist wound healing dressings, etc). The matrix will retain its structure as it slowly deteriorates over time until fully absorbed at approximately 18 months5,6
NovoSorb® BTM has been the subject of numerous studies and trials, and is well evidenced for efficacy, resistance to infection and cost effectiveness.
■ In a study to explore the potential of NovoSorb® BTM in full-thickness burns patients, Greenwood et al. treated five participants between the ages of 18 and 70, with the device
■ The participants had between 20 and 50% Total Body Surface Area (TBSA) full-thickness burns
■ The BTM was secured using staples and dressed with dressing changes twice a week, with treatment culminating in a skin graft
■ All participants survived and recovered. The scarring in these participants was assessed after a period of six months from time of injury, with excellent results indicated by the scar assessment scales used3
■ Since this early study, there have been many reports by other authors on the clinical effectiveness of NovoSorb® BTM across a range of clinical indications7,8,9
■ The synthetic nature of this device gives it an obvious and significant advantage over biologicallyderived alternatives; the product does not feed bacteria because of this property and it does not have the same risk of immunorejection
■ Sreedharan et al. writes on the successful use of this product in the treatment of large wounds of high risk of infection, involving exposed tendons
■ “Over this (21 day) period, the BTM became gradually more hyperaemic and vascularised. Twenty-four days after implantation the BTM’s surface sealing layer was ‘delaminated’, revealing a vascularised matrix. The right leg anterior compartment tendons were no longer exposed. The wounds were then reconstructed...”8
■ Again, this device is synthetic and does not have a biological origin like most of the products which would be the natural choices for use in the treatment of similar wounds and reconstruction
■ The costs involved in producing the biologically-derived alternatives can be significant, and NovoSorb® BTM is therefore typically available at lower prices, or at least equivalent to cost effective alternatives10
“In each case, our experience of its application, evaluation of integration and split skin graft application, along with the management of complications such as sub-seal infection and haematoma, has steadily increased our confidence in its use.”
Greenwood et al., 2018
“We find the material safe, easy to use, monitor and delaminate. Graft loss over the BTM is extremely unusual.”
Greenwood et al., 2018
“In contrast to other dermal matrices where infection often leads to failure of the material, early infection was able to be successfully treated and the BTM salvaged with a combination of topical wound care and antibiotics.”
Solanki et al., 2020
■ Simple and easy application

■ Converts macro wounds to organised micro wounds
■ Designed to minimise contracture
■ Synthetic but biodegradable
■ Requires no preparation, besides cutting to size
■ Can be applied over any viable tissue, including complex wounds
■ No need for tissue tracking
■ Available in large sizes (up to 20x40cm)
■ Robust in the presence of infection
1. Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable temporising matrix against Integra(R) dermal regeneration template in a porcine wound model. J Burn Care Res. 2012; 33:163-73.
2. Dearman BL, Li A, Greenwood JE. Optimization of a polyurethane dermal matrix and experience with a polymer-based cultured composite skin. J Burn Care Res. 2014; 35(5): 437- 48.
3. Greenwood JE, Schmitt BJ, Wagstaff MJD. Experience with a synthetic bilayer Biodegradable Temporising Matrix in significant burn injury. Burns Open. 2018;2(1):17-34.
4. Wagstaff MJD, Schmitt B, Caplash Y, Greenwood JE. Free flap donor site reconstruction: A prospective case series using an optimized polyurethane temporising matrix. Eplasty. 2015; 15:231-48.
5. NovoSorb® BTM [Internet], Melbourne Australia: PolyNovo; [cited 8 June 2022]. Available from: https://polynovo.com/product-btm/
6. Wagstaff MJD, Schmitt BJ, Coghlan P, Finkemeyer JP, Caplash Y, Greenwood JE. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: a pilot study. ePlasty 2015; 15:102-18.
7. Solanki N, York B, Gao Y, Baker P, Wong She R. A consecutive case series of defects reconstructed using NovoSorb Biodegradable Temporising Matrix: Initial experience and early results. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2020;73(10):1845-1853.
8. Sreedharan S, Morrison E, Cleland H, Ricketts S, Bruscino-Raiola F. Biodegradable temporising matrix for necrotising soft tissue infections: a case report. Australasian Journal of Plastic Surgery. 2019;2(1):106-109
9. Wu SS, Wells M, Ascha M, Gatherwright J, Chepla K. Performance of biodegradable temporizing matrix vs collagen-chondroitin silicone bilayer dermal regeneration substitutes in soft tissue wound healing: a retrospective analysis. Wounds. 2022;34(4):106-115.
10. Health Technology Assessment. [Internet]. Adelaide, Australia: South Australian Policy Advisory Committee on Technology (SAPACT); [cited 19 May 2022]. Avavailble from: https://www.sahealth. sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/resources/health+technology+assessment+ hta+decision+summary+-+novosorb
11. Instructions for Use, NovoSorb BTM [Internet]. Melbourne Australia: PolyNovo; [cited 19 May 2022]. Available from : https://eifu.polynovo.com/
Useful Links
How
NovoSorb® BTM is a synthetic, bioabsorbable scaffold that enables generation of a vascularised neodermis, to provide a robust foundation for reconstruction over deep structures, including exposed bone and tendons.1,2
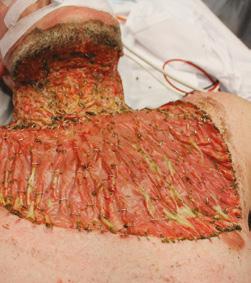
• Robust in the presence of infection3
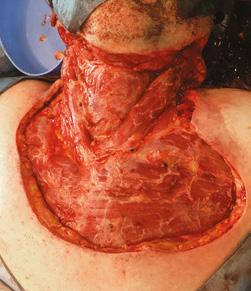
• Designed to minimise scarring and contracture4

Discover more: polynovo.com
Indicated for full or deep partial thickness burns, traumatic wounds, surgical and reconstructive wounds. Refer to the Instructions For Use for full device details. References:
 1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment