Suggested Pathway for Treatment
Organ SSI Symptoms
Seek Urgent Medical Attention - Consider Sepsis in the following settings:
• Involve any part of the anatomy other than the incision during surgery; CRP— C-reactive protein; WBC—white blood count
• Purulent drainage from a drain placed through the skin into the organ or body space
• Organ or body space abscess diagnosed by radiological or histopathological examination
• Evidence of infection involving the organ or body space seen on direct examination during surgery
• Post-operative fever
• Positive result of blood cultures, deep tissue biopsies, surgical sampling or pathological blood test findings (see deep SSI column)
High Risk of SSI
•
•
•
•
•
•
•
No Wound Dehiscence
Apply a Leukomed® Sorbact® dressing
aseptic, non-touch technique, monitor for further signs and symptoms of infection and treat accordingly
YES NO
•
Wound Review
Wound Dehiscence
Wound Dehiscence
• Subcutaneous layer exposed, fascia not visible
Make urgent referral to Plastic Surgery and Tissue Viability, upload a wound photo. Apply a Cutimed® Sorbact® ribbon dressing and a Mepilex Border using aseptic, non-touch technique, monitor for further signs and symptoms of infection and treat accordingly
Pathway to Optimizing C-Section Wound Healing Wound Masterclass - Vol 1 - September 2022 47
Apply Leukomed® Sorbact® following wound closure
Use Leukomed® Sorbact® dressings for up to 5 days after the procedure
for signs and symptoms of infection
Monitor
if saturated
wound
required due to risk of
Change dressing
or
check
infection
Single
wound
Apply PICO™
Use NPWT System following
closure in theatre
Once applied as per instructions for use, ensure device is operating correctly
Monitor for signs and symptoms of infection
Change dressing if saturated or wound check required due to risk of infection
Monitor dressing for amount of ‘staining‘ - leave in place until staining has reached the ‘port‘, or for up to 7 days, whichever is sooner
a wound swab
- No further dressing
of
as per
Are there superficial signs of infection and/ or dehiscence? For ALL of the above take
Leave open
required Assess level
dehiscence
below
Dermal layer only involved; no visible subcutaneous fat
a
® Sorbact® ribbon
with
using aseptic, non-touch
monitor for further signs and symptoms of
and treat accordingly. If
the
or
the TVN
Apply
Cutimed
dressing
a Mepilex Border
technique,
infection
on next wound review
wound has not improved
has deteriorated send a referral to Plastic Surgery and
team
using
What Is Leukomed® Sorbact®?
Leukomed® Sorbact® is a sterile single-use dressing developed for use in closed surgical wounds. It offers a dual effect of binding bacteria at the wound bed and protecting the wound from external contamination.
A transparent, showerproof, polyurethane film adhesive outer layer protects and keeps the wound moist, and covers an absorbent pad with a green contact layer coated with dialkylcarbamoyl chloride (DACC), (a fatty acid derivative), which irreversibly binds the bacteria (Figure 1).
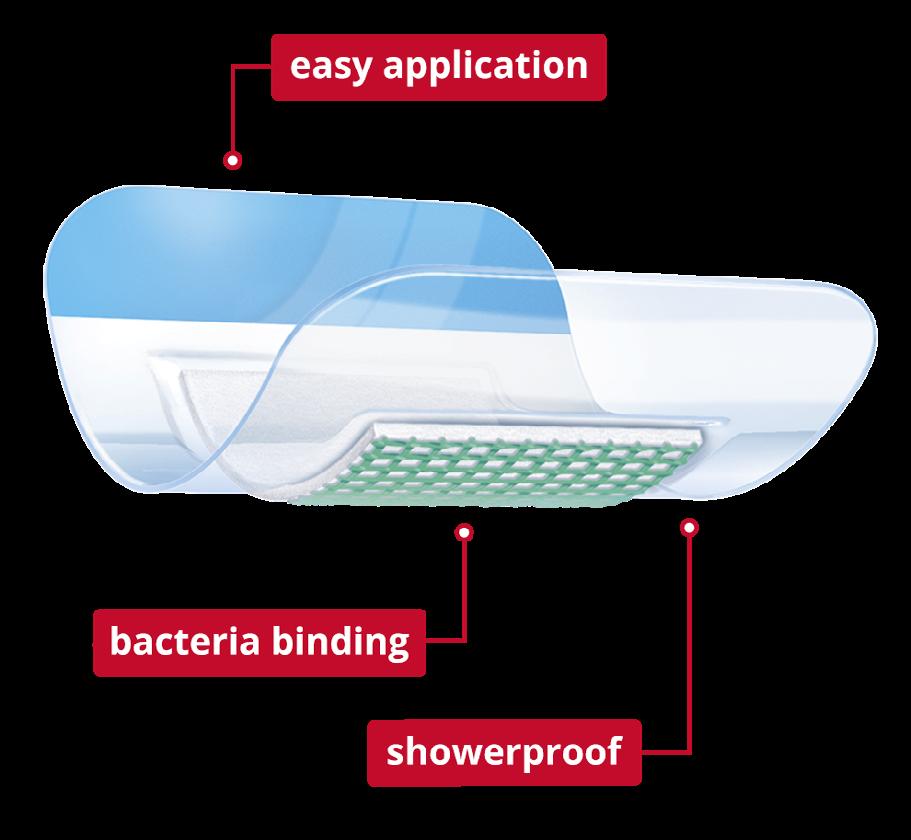
The dressing utilizes a unique technology by irreversibly binding the bacteria and is proven to prevent surgical site infections (SSI).
Overall savings were realised in terms of lower SSI and reduced readmission rates, and consequently a better patient experience was achieved in a trust that incorporated Leukomed® Sorbact® in place of the standard dressings in a maternity unit.6
Evidence for Leukomed® Sorbact®
Leukomed® Sorbact® can reduce the rate of surgical wound infections, antibiotic usage and associated hospital admissions.
Efficacy
Stanirowski et al. conducted a randomized, single-blinded controlled study which assessed the efficacy of Leukomed® Sorbact® in preventing SSI in women after a C-section. The participants were randomly assigned to two groups, one given standard surgical dressings and the other group given Leukomed® Sorbact® dressings. The authors note a decreased rate of SSI in the group given Leukomed® Sorbact® dressings.7

A meta-analysis of efficacy of DACCimpregnated dressings found reductions of SSI risk (RR 0.33 [95% CI 0.14–0.77; p = 0.01]), compared to other types of advanced dressings (silver-impregnated dressings, copperinpregnated dressings and chlorhexidine gluconate dressings).8
Costs
Reduced costs in the group treated with Leukomed® Sorbact®, due to reduced need for further treatment resulting from SSI.7 The NICE (The National Institute for Health and Care Excellence) guidance for Leukomed® Sorbact® further supports the cost effectiveness, efficacy and safety in use of Leukomed® Sorbact® dressings, to prevent SSI.5
• 65% reduction in SSI risk in post-C-section patients7
Pathway to Optimizing C-Section Wound Healing 48 Wound Masterclass - Vol 1 - September 2022
Click Here For our Masterclass Guide to Leukomed® Sorbact®
Figure 1: Leukomed® Sorbact®
What Is PICO™ 7?
Evidence supports the case for adopting PICO negative pressure wound dressings for closed surgical incisions in the NHS. They are associated with fewer surgical site infections and seromas compared with standard wound dressings in a specified group. PICO negative pressure wound dressings should be considered as an option for closed surgical incisions in people who are at high risk of developing surgical site infections.
PICO 7 (Figure 2) provides negative pressure wound therapy (NPWT) which draws out excess fluid from a wound and protects the injured area. The wound dressing protects the wound from bacteria to ultimately help promote healing. It consists of a battery-operated NPWT pump connected to an absorbent gentle adhesive dressing. The dressing is applied to the wound and extra strips are placed over the outside edge to help hold the dressing in place.

The dressing may be left in place for up to seven days depending on the amount of fluid drawn from the wound. This will depend on the size, type, drainage amount and position of the wound.
An AIRLOCK◊ Technology layer distributes pressure evenly and consistently for up to 7 days, with optimal fluid management to help reduce the potential for maceration.9
Evidence for PICO™ 7
Efficacy
A systematic literature review and meta-analysis conducted by Saunders, et al. report reductions in surgical site complications, risks and hospital stay length, including:
• Reduction of 63% In SSI risk with PICO sNPWT compared with standard care
• Reduction of 30% in dehiscence risk with PICO sNPWT compared with standard care
• Reduction of 77% in seroma risk with PICO sNPWT compared with standard care
• 1.75 day reduction in length of hospital stay seen with PICO sNPWT compared with conventional dressings10
NICE Guidance demonstrates that PICO◊ sNPWT provides better outcomes than standard care for preventing surgical site complications in high-risk patients with closed surgical incisions, with similar overall cost.
PICO◊ sNPWT reduced the incidence of SSIs by 50% in women with ≥30 BMI following C-sections compared with standard dressing (p=0.007).11
In an RCT of 876 women undergoing C-section with pre-pregnancy BMI ≥≥ 30, PICO◊ sNPWT significantly reduced the relative risk of SSIs by 50% compared with standard dressings (p=0.007).11
Costs
The evidence reporting on the prophylactic use of PICO demonstrates clinical and cost benefits in patient populations with a prepregnancy BMI of ≥30.
It was also estimated to be cost saving compared with standard dressings in women with prepregnancy BMI ≥35kg/m2, being associated with low hospital readmission rate (0.8%).12
Figure 2: PICO™ 7
Pathway to Optimizing C-Section Wound Healing Wound Masterclass - Vol 1 - September 2022 4 Wound Masterclass - Vol 1 - September 2022 49
Discussion
A review of the rates of C-Section SSIs in your unit is essential to ascertain the reason for any increase in accepted rates. Assessment of wound cultures and the review of microbiology may reveal that wounds were not being infected by the same microorganisms. The presence of the types of microorganisms may elucidate the reason for the different sources of infection. Giving the wound the best chance to heal before potential exposure to microorganisms is vital.
Often times, dressings applied to the wound in theatre are only left in place for 24 hours before being removed. This leaves the wound at an increased risk is developing an infection, having only been closed 24 hours prior. Successful healing needs to have taken place before a dressing is removed.
Conclusion
In conclusion, consideration of incorporating Leukomed® Sorbact®, and the PICO system, into the SSI C-Section management pathway has seen many benefits; reduction of SSIs, reduction in readmittance due to an SSI; potential reduction in antibiotic usage, protecting the wound from potential organisms whilst it is at its most vulnerable, and potential cost savings due to a reduction in SSIs.
The next step would be to complete an audit on our C-Section SSI figures when the pathway has been in place for six months; this will give a deeper analysis of the aforementioned benefits.
References
1. Wound Infection In Clinical Practice: Principles of best practice. International Wound Infection Institute. Wounds International. 2022.
2. Siddiqui AR and Bernstein JM. Clin Dermatol, 2010; 28(5): 519-26.
3. Eberlein T. Critical colonisation and local infection - Current therapy by use of polihexanide.
https://lohmann-rauscher.co.uk/downloads/clinical-evidence/SXP010-T-EberleinCriticalcolonisation-and-local-infect.pdf, 2009.
4. Dumville JC et al. Cochrane Database Syst Rev, 2016; 12(12): CD003091.
5. NICE. 2021. Leukomed Sorbact for preventing surgical site infection. NICE National Institute for Health and Care Excellence. MTG55
6. Magro, M. Towards Value-Based Healthcare: Reducing Surgical Site Infections PostCaesarean Section. 2022.
7. Stanirowski PJ, Bizoń M, Cendrowski K, Sawicki W. Randomized Controlled Trial Evaluating Dialkylcarbamoyl Chloride Impregnated Dressings for the Prevention of Surgical Site Infections in Adult Women Undergoing Cesarean Section. Surg Infect (Larchmt). 2016 Aug;17(4):427-35. doi: 10.1089/sur.2015.223. Epub 2016 Feb 18. PMID: 26891115; PMCID: PMC4960475.
8. Wijetunge S, Hill R, Katie Morris R, Hodgetts Morton V. Advanced dressings for the prevention of surgical site infection in women post-caesarean section: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021 Dec;267:226-233. doi: 10.1016/j. ejogrb.2021.11.014. Epub 2021 Nov 11. PMID: 34826671.
9. Smith & Nephew. PICO™ 7. Patient home care information. PDF. Available from: https:// www.smith-nephew.com/global/anz/awm/pico/sn14083%20-%20pico7%20patient%20info%20 booklet.secure.pdf. 2022.
10. Saunders C, Buzza K, Nherera L. 2019. A single use negative pressure system reduces surgical site complications compared with conventional dressings in closed surgical incisions: a systematic literature review with meta-analysis. Poster presented at the European Wound Management Association annual meeting, June 5-7, 2019, Gothenburg, Sweden.
11. Hyldig N, Vinter CA, Kruse M, Mogensen O, Bille C, Sorensen JA, Lamont RF, Wu C, Heidemann LN, Ibsen MH, Laursen JB. Prophylactic incisional negative pressure wound therapy reduces the risk of surgical site infection after caesarean section in obese women: a pragmatic randomised clinical trial. BJOG: An International Journal of Obstetrics & Gynaecology. 2019 Apr;126(5):628-35.
12. Norman G, Goh EL, Dumville JC, Shi C, Liu Z, Chiverton L, Stankiewicz M, Reid A. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020 Jun 15;6(6):CD009261. doi: 10.1002/14651858.CD009261.pub6. Update in: Cochrane Database Syst Rev. 2022 Apr 26;4:CD009261. PMID: 32542647; PMCID: PMC7389520.
Pathway to Optimizing C-Section Wound Healing 50 Wound Masterclass - Vol 1 - September 2022




