

What is the Role of Wireless


Electroceutical Dressings for Treatment of Venous Leg Ulcers
Editorial Summary
Introduction
Millions of Americans are afflicted with painful, open, draining ulcers on their lower extremities. Venous leg ulcers (VLUs), cause significant clinical and economic burden to the health care system and society.1,2 It is not uncommon for clinicians to see patients who have suffered for years with VLUs. VLUs are the result of chronic venous insufficiency, a malady caused by an abnormality of the veins in the lower extremity.
Venous Insufficiency
The lower extremity venous system is composed of both a superficial and deep system connected by an elaborate series of perforating veins.2,3 Under normal conditions, valves within these veins direct blood from the superficial into the deep system, which in turn carries the blood back towards the heart. The flow in the deep system is directly impacted by the pumping action of the musculature in the legs during physical activity. A host of illnesses and disease states can directly affect the anatomic function of the venous system. Deep vein thrombosis, for example, damages the valves in the veins of the deep and superficial systems. Pregnancy increases the risk of chronic venous insufficiency and VLUs: functional and structural changes occur in the venous system secondary to elevated hormone levels and damage caused by elevated pressure on the inferior vena cava by the enlarging fetus.4 In addition, there is a hereditary predisposition toward valvular dysfunction eventually leading to the development of VLU. Obesity and sedentary lifestyle can also contribute to the
development of venous insufficiency.
The common etiology of venous insufficiency is the reversal of blood flow from the deep to the superficial venous system.2,3 This reversal of flow leads to pooling of the blood and fluid in the legs. The patient may experience swelling or edema in the lower extremities as a first sign of disease. Over time, hallmark trophic changes in the tissues appear: hyper-pigmentation, venous stasis dermatitis; hemosiderin deposits, loss of hair; thickened nails, atrophy blanch, and lipodermatosclerosis. As a result of these changes, skin breakdown can occur, resulting in ulceration. Finally, VLUs typically occur in the medial gaiter region of the lower leg. This corresponds to the position of the perforating veins connecting the superficial and deep systems.
What Causes Skin Breakdown?
Several competing theories aim to describe the progression from chronic venous hypertension to skin breakdown and ulceration. A popular hypothesis is that hypertension in the venous system leads to the development of a pericapillary fibrin cuff, that forms a barrier to oxygen diffusion; the skin and subcutaneous tissue become hypoxic and subsequently ulcerate.5,6 The final common pathway for all the theories is tissue ischemia: another theory suggests that white cells plug the capillaries causing tissue hypoxia.6 A more recent theory of ulcer pathogenesis suggests that increased inflammation, brought on by a cycle of chronic ischemia-reperfusion, leads to skin breakdown.7 Neutrophils, activated by repeated ischemiareperfusion, release oxygen-derived free radicals
(ROS). The ROS stimulate the formation of capillary cuffs that impair oxygenation and trap more neutrophils, creating a vicious cycle of inflammation. The repeated activation of this cascade eventually overwhelms the body’s compensatory capacity and the balance tips in the favor of tissue destruction.8 In many cases the immediate cause of the ulceration is a traumatic event; however, healing is disrupted by one or a combination of factors described above.8 Correction of the underlying venous hypertension is the crux of treatment for VLUs, but compression alone fails to allow for optimization of the wound healing environment. Even with advanced wound care, many VLUs fall short of achieving complete wound resolution.
What Is the Role of a Wireless Electroceutical Device?
The author/ investigator hypothesized that the addition of a wireless electroceutical dressing (WED) to standard of care could further support wound healing in chronic VLU patients. The WED harnesses V.Dox Technology (Vomaris Wound Care, Inc., Tempe, AZ), mimics the electric potential found in the skin. When skin is wounded the physiologic electric field is disrupted. Clinical evidence has shown that application of low levels of exogenous electricity can support the body’s natural electrical gradient, contribute to cell migration, and encourage wound healing.9 The contact layer of the WED is composed of elemental silver and elemental zinc, in a dot-matrix pattern on a polyester substrate. In the presence of a conductive medium such as wound exudate, sterile saline, water or a wound hydrogel, silver and zinc ions are activated and the dot-matrix pattern creates a microcell battery. Low-level electric fields are generated at the surface of the dressing. Electricity is generated via a redox reaction. This mechanism of action is dissimilar to the ‘release of ions’ seen in traditional silver dressings. The voltage
between the dots is measured to be 0.2 - 1.0 V when in contact with wound fluid. These levels are non-hazardous and support the natural skin current.10 Biofilm is believed to be one of the most common causes of wound chronicity. It has been estimated that up to 90% of chronic wounds have biofilm bacteria.11 The WED has also been shown to effectively disrupt biofilm bacteria.12 VLUs are heavily exudative and are plagued by adherent biofilm formation at the wound base. Therefore, it would stand to reason that reducing biofilm bacteria with the use of the WED dressing would support more rapid wound healing. The purpose of this study was to determine the effects of the WED on wound healing and modulating biofilm, as detected by qPCR wound cultures, to support wound healing in chronic venous leg ulcers.
Materials and Methods
This was a prospective, randomized (1:1), open label, single center pilot study to investigate healing with WED dressing in venous leg ulcers (VLU) within a 12 week time frame. The study was planned to enroll 20 patients (10 in each group). A treatment group (WED and Multi-Level Compression Therapy (MLCT), was compared to a standard of care (SOC) group (silver alginate and MLCT). The primary endpoint was wound surface area percent reduction. A secondary endpoint was to determine disruption of biofilm causing bacteria within VLUs due to the different wound treatments.
All participants included in this pilot study were ≥18 years of age, and had a non-healing venous leg ulcer present for ≥4 weeks that had failed ≥1 wound care treatments. Following the IRB approved protocol, written informed consent from the subjects was obtained by the PI prior to performance of wound assessments or data collection. Only one wound per patient was selected by the Principal Investigator (PI) as the study (target) wound. If multiple wounds
“Correction of the underlying venous hypertension is the crux of treatment for VLUs, but compression alone fails to allow for optimization of the wound healing environment. Even with advanced wound care, many VLUs fall short of achieving complete wound resolution.”Wireless Electroceutical Dressings
(years)
females
(years)
Control Group (n=2)
were present, the largest that met eligibility criteria was selected. A review of inclusion and exclusion criteria was then performed; if deemed eligible, participants were enrolled in the Screening Phase of the study.
The study was divided into a Screening Phase and a Treatment Phase that spanned 12 weeks. Participants whose wound closed during the study or completed the Treatment Phase, but their wound did not heal, were considered as having completed the study.
Once subjects successfully completed the informed consent process, they entered into the Screening Phase of the study. Standard wound measurements and photographs were obtained using the eKare device (eKare Inc., Fairfax, VA). During the Screening Phase, all wounds were treated with inert alginate as the primary dressing and multilayer compression bandage therapy (MLCT), for the entire 14 day run-in period. The Screening Phase consisted of 2 wound assessments spaced 7 days (+3 days) apart to determine patient eligibility. At each screening visit the wound was measured and photographed via the eKare device. After completion of the Screening Phase, if the target ulcer had not decreased in size by ≥20% and the subject was able to tolerate MLCT, they then advanced to randomization.
The subjects were randomized via envelop system. Both groups were seen weekly during the 12 week Treatment Phase for dressing changes, wound assessments, wound photos and measurements. qPCR wound cultures (MicroGenDX, Lubbock, TX) were collected from both cohorts at weeks 1, 4 and 8, to determine bacterial cell abundance, and taxonomic composition.
Descriptive statistics were expressed as mean and standard deviation (SD). Mean, standard deviation, or percentage change/ reduction were calculated for primary outcomes.
Results
As a consequence of the global COVID-19 pandemic, in-person research was temporarily suspended at the author/ investigator’s institution, the grant funding for this project was not renewed and enrollment in this study was prematurely closed. A total of 6 patients were enrolled in the study prior to the work stoppage. Two in the standard of care arm (silver alginate and MLCT) and 4 in the treatment arm (WED and MLCT), completed the study. Patients’ demographics and baseline characteristics are listed in Table 1. Patients in the treatment group were noted to be older with the majority
Wireless
being diabetics (3 out of 4).
Safety
All patients receiving the WED compression tolerated the treatment well. There were no reported adverse events. Subjects experienced no pain, irritation, or maceration associated with the use of the WED product. No adverse events were reported in the SOC group either.
Primary Outcomes
One wound from each group completely closed at week 9 (SOC group), and week 10 (Treatment group). Average wound surface area in each group was reduced more than 60% by week 8, and 98% by week 12. Figure 1 illustrates wound surface measurement at baseline, week 4, 8 and 12 for all patients in both groups. Average wound surface area and wound reduction in both groups are listed in Table 2. It is worth noting that the baseline wound surface area (8.08 ± 1.81 cm2) in the SOC group is less than half the size of that in the Treatment group (17.45 ± 4.78 cm2). At week 12, all remaining wounds in both arms reached a significant reduction (97%) with average surface area of less than 0.5 cm2.
With the exception of patients #10 and #12 (Treatment group), all patients demonstrated significant improvement in their wound reduction by week 4 as noted in Figure 1. patient #12’s wound reduced significantly by week 8 while patient #10 reached a significant reduction at week 12.
Treatment Group
Patient #10 was a 67 year-old African American male with a history of gastric bypass (2018), PVD, NIIDM, hypertension, hyperlipidemia, peripheral neuropathy, edema, varicose veins,
xerosis, and baseline wound size of 14.8 cm2 This patient also showed wound culture of a dominant campylobacter ureolyticus at week 4, which decreased slightly later with E. colipresence at week 8 (it was below detection level at week 4). At week 12, wound surface area was 1.3 cm2.
Patient #12 was a 65 year-old Caucasian male with a history of NIDDM, hyperlipidemia, COPD, edema, PVD, neuropathy, Hep C and a baseline wound size of 22.04 cm2. This patient showed wound culture of a dominant staph aureus at week 4, which decreased significantly later at week 8. At week 12, wound surface area was 0.2 cm2.
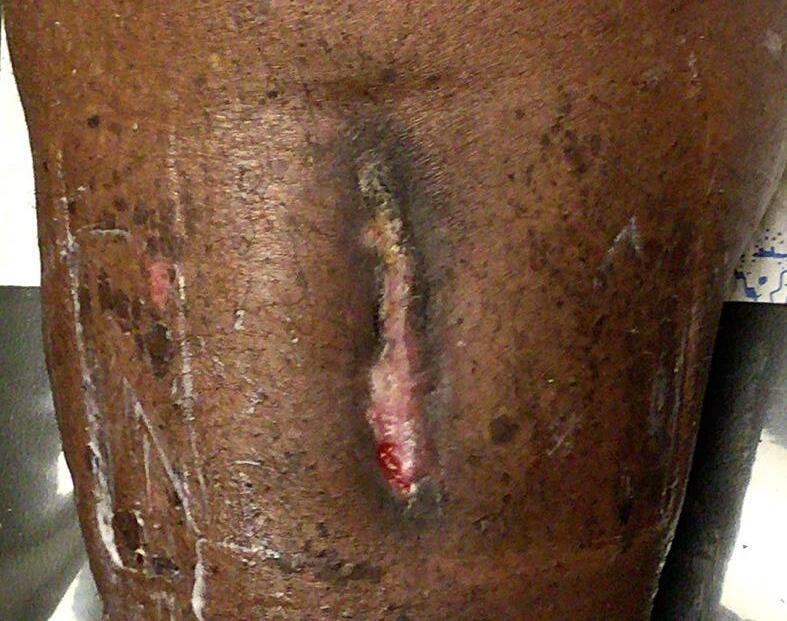
Patient #9 was a 57 year-old African American female with a history of NIDDM, hypertension, PVD and a baseline wound size of 20.88 cm2 in the right lower leg with a duration of 3 months. This wound failed prior therapies including



Wireless
Dressings to Treat Venous Leg Ulcers
Prisma, Alginate and compression bandages. At week 10 the wound completely closed. Figure 2 shows photos of the wound at baseline, week 4, week 8, and healing at week 10.
Patient #3 was a 76 year-old Caucasian male with a history of Vit D deficiency, CHF, hypertension, PVD, A-fib, Psoriasis and a baseline wound size of 12.09 cm2. At week 12 the wound surface area was 0.09 cm2.
SOC Group
Patient #7 was a 61-year-old African American male with a history of Gout, Chronic venous insufficiency, edema, anemia, CAD, hypertension, hyperlipidemia, and a baseline wound size of 9.36 cm2. At week 9 the wound completely closed.
Patient #6 was a 56 year-old Hispanic female with a history of PVD, edema and a baseline wound size of 6.8 cm2. At week 12, wound surface area was 0.35 cm2
Secondary Outcomes
Wound swab samples were collected from all patients at week 1, 4 and 8 and were analyzed by an independent lab to assess bacterial alfa diversity (species richness) and estimated bacterial cells abundance in each sample for each patient. Estimated bacterial cell abundance for each patient at each visit are presented in Figure 3. Bacterial species noted at each sample for each patient are presented in Figure 4.
There was no significant correlation between estimated bacterial cells with each collection visit (Kruskal-Wallis test; x2x2 = 13.10, p = 0.52). There was also no significant correlation between estimated bacterial cells and treatment groups (Kruskal-Wallis test; x2x2 = 13.81, p = 0.46).
Multiple bacterial species observed in all samples in both groups show how the diversity of wound microbiota differed between each treatment group and at each visit (Figure 4). Although the data only represents the 6 patients analyzed and no statistically significant differences were observed in this study, wound surface area and microbial species diversity decreased concurrently in both groups over the 8 week study period. This observed trend,
Figure 3: Plot depicts the log q value of each treatment group over the completed study period. Coloring has been applied to indicate each treatment group and lines connect dots associated to the same patient and wound. The horizontal dashed line represents the cutoff for the qPCR analysis. Values below the 4.5 threshold did not pass the limit of detection (Ct = 30) and can be treated as zeros.

which could be due to the small number of samples, contradicts previous research on wound healing which showed that decreasing wound diversity has a significant negative linear relationship with healing rate, suggesting further research is needed.13
Discussion
The main objective of this pilot study was to evaluate healing with the WED dressing and MLCT in venous leg ulcers (VLU), to gain knowledge of its performance for our clinical practice and possible further research. As noted above, due to COVID-19, the project was stopped after enrolling 6 patients. One wound from each group healed at week 9 and 10, and the remaining four wounds were reduced significantly (97%) from baseline at week 12.
Several authors reported and discussed VLUs healing rates and average monthly wounds reduction to predict wounds closure. In a retrospective review of 1323 patients enrolled in 6 prospective clinical studies, Rajhathy et al.14 identified 777 patients who were treated with compression and advanced wound dressings for VLUs, with a baseline wound surface area average of 9.8 cm2.
The authors reported wound closure in 328 (42.2%) patients by 3 months or 65.8% by 6 months, with an average monthly wound size reduction of 30%. Others suggested wound size
reduction by 20 - 40% in the first 4 weeks is predictive of complete closure by 6 months in participants treated with high compression.15,16
We find this data very helpful for clinicians to anticipate healing and recognize progress in wound size reduction at 4 weeks. In our patients, we noticed wound size reduction in the treatment group was 30% at week 4. By the end of study at 12 weeks, 97% of wound size reduction was achieved.
Polymerase chain reaction (PCR) is a chemical reaction harnessed to detect and identify trace bits of DNA, whether from a virus or bacteria to study the organism or diagnose an infection.17 Real-time PCR has been shown to be capable of detecting both live and dead bacterial DNA regardless of the inactivation procedure.18 The results of one study showed that direct qPCR resulted in an overestimation of up to 10 times of the amount of cells in the samples compared to viable counts, due to detection of DNA from dead cells.19 Therefore, it is possible that while bacteria were still detected on sampling visits, this was not representative of only live bacterial levels. In addition, variance in technique, while collecting the culture swab throughout the
Wireless Electroceutical Dressings to Treat Venous Leg
study, could have resulted in higher bacterial loads. For example, greater pressure applied while sampling or more twists of the swab could result in a larger bacterial concentration.20
The COVID-19 outbreak posed an unprecedented challenge to many aspects of academic life. Cancellations and postponement of scientific events, including national and international conferences, symposiums, workshops, in-person training programs and classes, as well as scientific research. In many fields, if research was not directly related to the pandemic it was abandoned or displaced, as was the case with this project.
Pilot studies remain important for the development of new ideas and the expansion of future agenda items, for the progress of science and scientific communities. VLU healing rates are often prolonged with only 40 - 60% on average healed within 12 - 24 weeks, and once healed, 75% develop a recurrence within 3 weeks.13,19 At least 60% of VLUs become chronic.21 Hence, it is the author’s contention that although there were not enough patients participating in this study, the results are encouraging and there is opportunity

to expand on the basic concepts presented in this report. For example, piggybacking off the information gained from this pilot study, the author/ investigator initiated a 5 patient case series in her out-patient clinical practice. All patients had baseline positive fluorescence wound images, indicating pathologic levels of surface bacteria were present (≥104 CF). These patients were then treated with the WED and a secondary moisture managing dressing. Dressings were changed every other day by the patient or caregiver. wound images were then obtained one week later to determine if there was evidence of changes in bacterial contamination based on fluorescence images. All 5 wounds in this small case series showed a marked decrease in bacterial fluorescence, with just one week of WED therapy, as noted by lack of fluorescence on the images. It was concluded that the WED did decrease harmful bacteria levels initially present in the wounds of varying etiologies to less than 104 CFU. Large randomized clinical trials are needed to validate the findings of this small case series.
Limitations
This was designed as an investigational pilot study. Although randomization is considered a strength, due to unforeseen circumstances brought on by the global pandemic, the study was limited due to the small sample size. Therefore, it is difficult to make widespread conclusions based on these study results alone.
Conclusion
The present study demonstrated that use of the WED was well tolerated in this patient population and may support wound healing in chronic venous leg ulcers. More research is needed. Real-time detection of bacteria through fluorescence imaging technology may better illustrate how the WED can help to manage bioburden and support an ideal environment for wound healing. Future studies should look
to incorporate larger sample sizes and greater durations of observation to affirm this limited pilot investigation.
Ackowledgements
This study was supported in part through a grant from the Podiatry Foundation and with the donation of the WED from Vomaris Wound Care, Inc. The authors would also like to recognize the MicrogenDX team (Nichola Sanford, PhD; Craig Tipton, and Jacob Ancira) for their assistance with the bacterial data analysis, and Stacey Coe, MS, CCRP for her help with data collection for this study.
References
1. Ballard JL, Bergan JJ, editors. Chronic venous insufficiency. Diagnosis and treatment. London: Springer-Verlag, 2000.
2. Negus D. Leg ulcers: A practical approach to management. 2nd ed. Oxford: Butterworth Heinemann, 1995.
3. Olivencia JF. Pathophysiology of venous ulcers: surgical implications, review and update. Dermatol Surg 199: 25:880-885
4. Ginekol Pol. Risk factors for the development of venous insufficiency of the lower limbs during pregnancy—part 1. Review Article 2012 Dec; 83(12):939-42.
5. Browse NL. Venous ulceration. BMJ 1983; 286:1920-1922.
6. Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. BMJ 1988;296:1726-1727.
7. Coleridge Smith PD. Causes of venous ulceration-a new hypothesis. Br Med J (Clin Res Ed) 1988; 296(6638):1726-7.
8. Falanga V. The “trap” hypothesis of the venous ulceration. Lancet 1993; 341:1006-1008.
9. Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1-26. doi: 10.1016/s0070-2153(03)58001-2. PMID: 14711011.
10. Banerjee J, Ghatak PD, Roy S, et al.; Improvement of Human Keratinocyte Migration by a Redox Active Bioelectric Dressing. PLoS ONE. 2014 Mar 3;9(3):e89239.
11. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of wound care. 2017;26(1):20-25.
12. Barki KG, Das A, Dixith S, Ghatak PD, Mathew-Steiner S, Schwab E, Khanna S, Wozniak DJ, Roy S, Sen CK. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann Surg. 2019 Apr;269(4):756-766.
13. Tipton CD, Wolcott RD, Sanford NE, Miller C, Pathak G, Silzer TK, et al. (2020) Patient genetics is linked to chronic wound microbiome composition and healing. PLoSPathog 16(6):e1008511.
14. Rajhathy EM, Murray HD, Roberge VA, Woo KY. Healing Rates of Venous Leg Ulcers Managed With Compression Therapy. J Wound Ostomy Continence Nurs. 2020 Sep/ Oct;47(5):477-483
15. Sibbald G, Woo K, Ayello EA. Increased bacterial burden and in¬fection: the story of NERDS and STONES. Adv Skin Wound Care. 2006;19;447-461.
16. Benigni J, Lazareth I, Parpex P, et al. Efficacy, safety and acceptability of a new two-layer bandage system for venous leg ulcers. J Wound Care. 2007;16(9):385-390.
17. Maurin M. Real-time PCR as a diagnostic tool for bacterial diseases. Expert Rev Mol Diagn. 2012 Sep;12(7):731-54. doi: 10.1586/erm.12.53. PMID: 23153240.
18. Wolffs P, Norling B, Rådström P. Risk assessment of false-positive quantitative real-time PCR results in food, due to detection of DNA originating from dead cells. J Microbiol Methods. 2005 Mar;60(3):315-23. doi: 10.1016/j.mimet.2004.10.003
19. Griffith C. Surface sampling and the detection of contamination. Handbook of Hygiene Control in the Food Industry. Elsevier; 2016:673-696
20. Abbade LP, Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449–56.
21. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560–82.
