





















































One of the world’s largest medical device manufacturers was concerned about wireless connectivity issues that can commonly occur in shielded MRI suites, so we developed a customized hybrid foot switch solution that featured cabled backup where wireless signals could not be transmitted.
Other Steute advantages:
Leadership in robotic applications – larger selection (1 to 4 pedal designs), variation of contact configurations and actuator types and applicable global certification.

Large selection of standard options – Optional auxiliary pushbuttons, fixed or collapsible braces, housing colors, cable and connector types, and contact type and configurations. Our renowned product sample program – We can get you a sample, for the most part, in a week or less.
Contact us today and let us create the perfect solution for you. More to the point, your customers will appreciate you made the switch to Steute!




































































EDITORIAL
TUESDAYS
DIGITAL MARKETING
WEB DEV/DIGITAL OPERATIONS




DeviceTalks Tuesdays is a weekly virtual event that brings the insights and energy of our in-person events to your desktop.


Each DeviceTalks Tuesday will kick off with a quick briefing from the editors of MassDevice and Medical Design and Outsourcing. These presentations will give attendees insights on what trends will be moving medtech in the days to come.
Be sure to check back frequently as new live events continue to be added.

Executive Editor Chris Newmarker cnewmarker@wtwhmedia.com @newmarker
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Pharma Editor Brian Buntz bbuntz@wtwhmedia.com
Associate Editor Sean Whooley swhooley@wtwhmedia.com @SeanWhooleyWTWH
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
Managing EditorDeviceTalks Kayleen Brown kbrown@wtwhmedia.com
Senior Vice President Courtney Nagle cseel@wtwhmedia.com 440.523.1685
CREATIVE SERVICES

VP, Creative Director Matthew Claney mclaney@wtwhmedia.com @wtwh_designer

Senior Art Director Allison Washko awashko@wtwhmedia.com @wtwh_allison
Senior Graphic Designer Mariel Evans mevans@wtwhmedia.com @wtwh_mariel
AUDIENCE DEVELOPMENT Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com
Web Development Manager B. David Miyares dmiyares@wtwhmedia.com @wtwh_webdave
Digital Media Manager Patrick Curran pcurran@wtwhmedia.com @wtwhseopatrick
Front End Developer

Melissa Annand mannand@wtwhmedia.com

Software Engineer David Bozentka dbozentka@wtwhmedia.com
Web Dev./Digital Production Elise Ondak eondak@wtwhmedia.com


PRODUCTION SERVICES

Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com
Customer Service Rep Trinidy Longgood tlonggood@wtwhmedia.com
Digital Production Manager Reggie Hall rhall@wtwhmedia.com
Digital Production Specialist Nicole Johnson njohnson@wtwhmedia.com
Digital Production / Marketing Designer Samantha King sking@wtwhmedia.com


Marketing Graphic Designer Hannah Bragg hbragg@wtwhmedia.com
VP, Digital Marketing Virginia Goulding vgoulding@wtwhmedia.com @wtwh_virginia



Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com @WTWH_Taylor
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com @WTWH_Matt
Webinar Manager Matt Boblett mboblett@wtwhmedia.com
Webinar Coordinator Halle Sibly hkirsh@wtwhmedia.com
Webinar Coordinator Emira Wininger emira@wtwhmedia.com
EVENTS
Events Manager Jen Osborne josborne@wtwhmedia.com @wtwh_jen
Events Manager Brittany Belko bbelko@wtwhmedia.com
Event Marketing Specialist Olivia Zemanek ozemanek@wtwhmedia.com
VIDEO SERVICES


Videographer Garrett McCafferty gmccafferty@wtwhmedia.com

Videographer Kara Singleton ksingleton@wtwhmedia.com
FINANCE Controller Brian Korsberg bkorsberg@wtwhmedia.com
Accounts Receivable Jamila Milton jmilton@wtwhmedia.com

devicetalks.com





PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development.
We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory.
Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.
PATIENT SAFETY IS THE #1 PRIORITY
• Work directly with our SMEs
• Customized programs to fit your device
• ISO 10993/ISO 18562 Testing
• Toxicological/Biocompatibility Risk Assessments
• FDA 510(k) Submission guidance
PSN Labs has the experts to isolate confounding variables and provide clear analysis to guide the commercialization of existing and new medical devices.



www.psnlabs.com

DESIGN AND DEVELOPMENT WITH BIOCOMPATIBILITY IN MIND
Kyree Miller recalls the day his heart stopped beating.
“I remember the entire room going white,” he said. “And I actually turned over on my side and I said, ‘Tell my mom I love her.’”


A couple of weeks later, the heart failure patient — who was only in his 20s at the time — received his first left ventricular assist device (LVAD) implant while he waited for a heart transplant. One year passed, then two, then three. Finally, after surviving on LVAD technology for seven years, his new heart came.
“When you get your transplant, there’s a whole new energy that you get. ... But I can honestly say there was a whole new energy that I got when I had my LVAD,” Miller said.
He shared his story at DeviceTalks Boston in May, joined by Abbott and Brigham and Women’s Hospital offi cials who described the challenge of expanding access to LVADs for heart failure patients who need more time.
“We know that this technology can save lives,” Miller said in a conversation led by Executive Editor Chris Newmarker, who wrote this edition’s cover story. “We know that the LVAD can bridge to transplant. We have this technology available. So let’s use it. Let’s get it out there.”
DeviceTalks Boston coverage from our team doesn’t end there. IP experts at the show offered advice for startups looking for an exit in a “brutal” funding environment, medical device manufacturers shared tips and red flags for selecting contract manufacturers, ZimVie discussed how their device for pediatric scoliosis came to be, and Medtronic dove into nitinol applications for heart devices.
Jim Hammerand | Managing Editor | Medical Design & Outsourcing | jhammerand@wtwhmedia.com |









Capping it all off is new DeviceTalks Managing Editor Kayleen Brown, who rounds up the most notable quotes from the show in her Medical Design & Outsourcing debut this month. Look for more from Brown in print and online in the months ahead as we gear up for our DeviceTalks West show Oct. 18–19 in Santa Clara, California.
This edition of MDO also features supplier innovations, ranging from a new method of medical device sterilization to sustainable device coatings and 3D printing technology.

And make sure to check out our continuing coverage of hot topics in medtech with Newmarker’s feature on AI breakthroughs and a roundup of diabetes tech startups by Associate Editor Sean Whooley.

As always, I hope you enjoy this edition of Medical Design & Outsourcing — and thanks for reading.

“We know that this technology can save lives.”












HERE’S WHAT WE SEE: Life-saving LVADs, supplier innovations and AI breakthroughs
DRUG DELIVERY: 11 diabetes tech startups you need to know



IP ISSUES: How medtech IP can help startups navigate a ‘brutal’ environment

MANUFACTURING: Tips and red flags to help you select a medtech contract manufacturer

ORTHOPEDICS: The ZimVie Tether helps kids with scoliosis — if they can get it in time



STERILIZATION: How Phiex could revolutionize medical device sterilization


SUSTAINABILITY: Sustainable device coatings are replacing ‘forever chemicals’





TUBING TALKS: How Medtronic uses nitinol to improve the structure and effectiveness of heart devices




DEVICETALKS: They said it at DeviceTalks















LVADS SAVE LIVES: SO WHY AREN’T MORE AVAILABLE?
Abbott says its HeartMate 3 LVAD is clinically proven to extend life by five years or more — but thousands of people in the U.S. still die of heart failure within a year.
50



AI BREAKTHROUGHS IN MEDTECH: 7 WAYS TO ENHANCE HEALTHCARE
Whether it’s OpenAI’s ChatGPT or Microsoft’s new Bing, 2023 is the year when generative artifi cial intelligence entered the popular consciousness.
WHAT’S NEW IN 3D PRINTING: MEDICAL DEVICES, RESEARCH, INNOVATION, AUTOMATION AND PARTNERSHIPS


Device developers, healthcare providers and 3D printing technology suppliers are collaborating to improve device design, product manufacturing and patient care.











Designing efficient systems involves much more than simply understanding a few basic principles. There is a true art to balancing the specific requirements of an application in order to achieve the desired goals in the best possible way. Help us understand the unique needs of your application and together, we’ll develop something that surpasses what any of us could have done alone.
Contact
 Whooley Associate Editor
Whooley Associate Editor

The diabetes technology space is always innovating, from continuous glucose monitoring to insulin pumps and the artificial pancreas.
Companies are looking for all kinds of ways to make their mark in the global fight against diabetes, with new offerings in drug delivery devices, digital health, software, coaching and more. Here are just 11 examples to keep an eye on:
1. DiabetesWise




Stanford, California-based DiabetesWise provides a free, unbranded, datadriven online resource that provides comprehensive tools to explore diabetes devices with regulatory approval. The Helmsley Charitable Trust funds the company with no financial support from device manufacturers.


In April 2022, the company launched its DiabetesWise Pro website to help providers make informed decisions on diabetes devices in the clinical practice or at the point of care. Providers can

identify the best available diabetes device on the market for their patients and the necessary steps for ordering the device based on insurance type.

Earlier this year, DiabetesWise launched its advanced Prescription Tool for the DiabetesWise Pro website. This tool provides an up-to-date guide for ordering devices. It offers an integrated platform that allows for both prescription and ordering support all in one place.
2. Diatech Diabetes
Memphis, Tennessee-based Diatech




Diabetes is the developer of SmartFusion infusion monitoring software. It detects insulin delivery failure and offers insights on how infusion performance affects diabetes management.
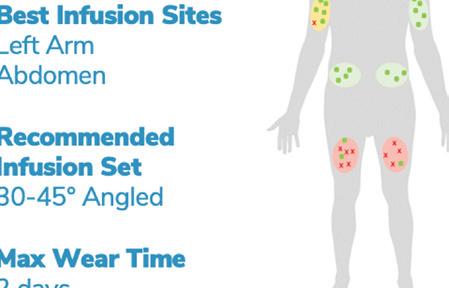





Pumps can only detect occlusion failures that prevent insulin delivery. However, if the system still delivers insulin but the user never receives it, the pump’s occlusion alarm may not detect it.
(continued on page 12)

to
Startups are developing smaller and safer syringes, infusion monitoring software and other tools to take on the diabetes epidemic.





(continued from page 10)
These common failures can lead to hospitalizations or death.
The SmartFusion algorithm helps to detect leaks, kinks, partial occlusions, infusion into damaged sites and dislodgements. Diatech Diabetes designed it specifically for insulin pumps to detect infusion failures they don’t currently detect themselves. The algorithm analyzes the data from the pump’s infusion mechanism to determine whether infusion was successful or not. It utilizes real-time data analysis with machine learning. The algorithm can also predict the likelihood of site failure based on historical data, helping to pick an optimal infusion site.
Users can connect Glaice’s platform to any continuous glucose monitor (CGM) and automatically transmit blood sugar data. They can choose their favorite exercise to receive tailored plans with science-based recommendations for safety. Glaice says its platform gives users control of their lifestyle and glucose, making their own choices rather than allowing their disease to do so.





4. GluCare
Dubai-based GluCare offers personalized care through a combination of digital therapeutics and a human metabolic health platform. It’s comprised of continuous metabolic monitoring, wearable technology, data analytics and expert support. According to GluCare, patients experience a 2.14% HbA1c reduction in just 90 days. They also report less use of medication. After a year of management, GluCare says, patients (on average) eliminated diabetes-specific medication.
5. HEAL.med
6. Medtech Concept
Delaware-based Medtech Concept develops a range of medical products for people with diabetes. Its diabetes management system combines digital health software with a market-tested, miniature therapeutic delivery device. The company aims to provide real improvements in the control of diabetes. Medtech Concept’s MiniPen2 addresses real-world needs of diabetics. The company designed it as an alternative to large and cumbersome insulin devices. The miniaturized delivery system’s lower price makes it available to a wider population compared to current auto-injectors.
MiniPen2 also has a new safety feature to reduce the spread of viruses and infections. After the syringe is filled with insulin, the plunger automatically locks into the barrel to prevent the syringe from being reused.




7. Open MedLabs





Bangalore-based Open MedLabs launched out of the Indian Institute of Science (IISc) to design and develop medical devices for diabetes and other conditions in resource-constrained settings.


Glaice seeks to combine medtech knowledge, healthcare domain knowledge, venturing expertise, software, AI and more in its platform to help people manage diabetes and other chronic diseases. The company’s app provides personalized exercise support for people with type 1 diabetes based on medical research and user data.
































Leicester, U.K.–based HEAL.med launched its Deapp 2.0 this year. HEAL. med’s education platform, which offers diabetes education, is used in hospitals across the U.K.
The diabetes education application aims to ensure access to high-quality, effective education for all children diagnosed with type 1 diabetes. As a nonprofi t, HEAL.med works closely with the NHS, Diabetes UK and medtech companies to deliver its education offerings. The platform includes videos, games and more featuring animations and props.
By leveraging open-source communities, the company aims to maximize the return on investment for the healthcare system. This comes through lower device and service costs. Multiple entities can operate and test these devices independently.
Open MedLabs believes it can potentially create a community around device designs, leading to contributions from independent innovators. Opensource designs in resource-constrained settings like India can offer patients access to cutting-edge technologies at affordable prices, too.
8. Orange Biomed
Seoul, South Korea–based Orange Biomed developed its flagship OBM rapid A1c point-of-care blood testing device to help people manage their diabetes through HbA1c testing.

The company points to a lack of at-home monitoring for diabetic patients with the accuracy and precision of laboratory devices, and designed OBM rapid A1c for both accuracy and precision at that high level.
Replica Health considers itself a search and recommendation engine for the user’s metabolism. It uses a diabetic’s data to model how their metabolism reacts to different foods and activities. Replica Health integrates previously unused data and applies machine learning to provide personalized information about carbohydrate breakdown. This helps turn mealtime guesses into data-driven decision-making.
The company also links CGM, insulin pump, Apple Health and location data. It creates a new model for understanding, analyzing and searching metabolic history. Replica Health says its avenues for exploring data through time, location and labels are like “Yelp for your metabolism.” The platform also utilizes an automated
event algorithm to track routines, reducing the time needed to log data.
10. Ryse Health
Ryse Health, which has offices in Baltimore and Arlington, Virginia, provides tech-enabled, office-based and virtual care to type 2 diabetes patients.
Ryse Health’s platform includes a CGM and a custom app. Together, these synthesize data, support selfmanagement and facilitate communication between the user and a care team of health coaches, endocrinologists, registered dieticians, certified diabetes care and education specialists and licensed clinical social workers.
In early clinical results, Ryse said it saw a two-point drop in patients’ A1C numbers for those starting at an A1C of greater than 8%. It also observed a 36% decrease in elevated blood sugars for all patients after the first 60 days of the program.
The company raised $3.4 million in seed funding in April 2022 and added another $6.5 million in March 2023.
11. SmartStart Health
U.K.-based SmartStart Health wants to make it easier to use CGMs with its SmartStart CGM education and onboarding app. SmartStart says its platform offers simplicity, engagement, relevance, fun and is both efficient on time and costs.
Near the beginning of the COVID-19 pandemic, some patients in the U.K. had to wait months to start using a CGM because in-person training was on hold and no virtual options existed. Using the principles of German CGM education program Spectrum, SmartStartHealth founder and CEO Melissa Holloway wanted to create a digital adaptation. The company also wanted to take advantage of the expanding evidence base for CGM use in people with type 2 diabetes.
With support from Switzerland’s Diabetes Center Berne, SmartStart has its proof-of-concept development underway.
There’s no sugar-coating the state of venture capital investment for young medical device companies that are looking for funding while building
“It’s brutal out there,” said venture capitalist Jeremy Sohn, managing general partner at P74 Ventures. “... Is it completely bleak? Absolutely not. There’s a lot of money out there, billions and billions if not trillions of dollars sitting on the sidelines. There absolutely is money to be taken. You’ve just got to be creative” to close a financing.
Sohn was speaking in early May at a DeviceTalks
Greenberg Traurig patent attorneys David Dykeman and Roman Fayerberg, as well as Luis Barros, an MIT lecturer and managing partner of Leading Business Ventures.
“It’s actually a great time to be starting a company,” Dykeman said. “In the public markets, there aren’t exits, but you’re not exiting a public market for two or three years. Now’s the time to build your company, build your relationships and get your house in order so when that window opens, you’re ready to jump through it.”
Medtech IP is a critical consideration in that process. Patents can help device companies secure new investments, control costs to buy more time, or sell their technology to a strategic buyer.
‘It is tough out there’
“It is tough out there, but the good management teams with protected technology going after a big unmet need
are still closing rounds,” Dykeman said. “It might not be at the valuation they want [or] the exact terms they want. It might take twice as long as they thought it would. But there is money being put to work. It’s much more a buyer’s market for the VCs and investors.”
He advised medtech founders to have many plans for funding, and warned “no matter how much a VC likes you, there’s a lot of smoke right now in the VC community.”
VC funds are holding off on financing for early-stage companies to keep the cash flowing to their existing investments, but may not be transparent about it, he said. Instead, “they’ll do the dance with you, they’ll take you all the way through” the diligence process and then drag their feet in the final stages.
“IP due diligence is usually one of the last things because they have to hire lawyers to do that,” Dykeman said. “A lot of the other market analysis they can do in-house, but getting them to actually fund and write that check, we’ve seen a number of clients that have gone all the way down the road and then at the last minute, ‘Oh, the partnership didn’t approve the investment.’”
(continued on page 16)

How medtech IP can help a startup navigate a ‘brutal’ environment
Now is the time for startups to build medtech IP for a future funding or exit, experts said at DeviceTalks Boston.Illustration courtesy of Adobe Stock
Jim Hammerand Managing Editor
“It’s actually a great time to be starting a company ... Now’s the time to build your company, build your relationships and get your house in order so when that window opens, you’re ready to jump through it.”


(continued from page 14)
“And you really look at it, and they haven’t done many early-stage investments. They’re keeping their powder to invest in their existing portfolio companies because there are no IPOs, there are no exits. Even if you think you’ve got the best VC or the best investor in the world, the money’s not in the bank until the check clears.”
Hunker down — but don’t stop building, and don’t stay quiet “Hunker down, reduce your costs, make sure you’ve got two years of runway, if not more, and forget about thinking about valuation,” Sohn said. “There’s not a CEO or leadership team out there right now in an honest moment that won’t tell you that’s absolutely the playbook for at least the next 18 months.”
Reviewing your IP portfolio can yield savings or even bring in revenue if you can find assets, applications or even issued patents to abandon, license or sell, Fayerberg said.

“If you have a good patent portfolio and you can show that it can generate revenue and dividends — it doesn’t have to be part of your core business, [just] something you might have — that’s not a bad way to monetize” through licensing or selling to a third party.
Communication remains paramount, especially in tough times for funding. Engaging early and often is the best way to win over investors.
“You have to show that you have traction and you have revenue,” Barros said. And milestones matter. Investors want to hear about continued progress toward product development milestones, customer milestones or revenue milestones.


“We have a lot of companies that approach us, and they’re painting a story, which is great, but what we want to see is that you’re making progress against that story,” Sohn said.
Protect your medtech IP Investors or potential buyers are also measuring the strength of your medtech IP and how well it protects your product from current or future competitors.
protection differs from country to country.
“IP is important. It’s part of your story, it’s explaining your technology,” Fayerberg said. “Everybody wants innovative proprietary technology. And the way you build the IP, you have to make sure it’s protected properly. ... Understanding what you have, what you need to do to protect it, and where to protect it is very important. It’s going to become part of your story, derisking your company and making it more attractive to investors or partners.”
Only a small percentage of deals are funded through venture capital, Barros said, and medtech founders should consider other sources of funding, especially today. Search out family offices, angel investing groups, non-dilutive alternatives, government grants, low-cost venture debt and other financing sources, even if that means looking beyond the borders of your state or country.
“That’s an easy way to save money because obviously there are fees that you pay to get the patents,” he said. “But then once patents issue, they have maintenance fees and things like that, and as your portfolio grows it becomes a big part of the spend. You have to see which patents are still relevant. Some might not cover the product, might still be good defensive patents, but you might also have a part of the portfolio that’s no longer relevant.”
Reviewing your medtech IP could also lead to new streams of recurring revenue, Barros said.
“Protect your IP,” Sohn said. “Make sure that it’s real. You want to give people reasons to lean in, and you don’t want to give reasons to not lean in and to walk away. Sometimes it means giving up a little bit more of your company to another partner. Sometimes it means spending a little bit more in already tight times, but it’s money well spent.”
Building a U.S. patent portfolio is expensive, and the costs increase significantly when filing patent applications in other countries. Be strategic about patents: Understand where your potential buyers and investors are and how medtech IP
“What I’m seeing is a lot of creativity in terms of entrepreneurs thinking about where are their markets, where they’re going to grow, and then strategically anchoring — it can be in multiple geographies, multiple continents — but always going for those geographies that they think will enhance their chances of success,” Barros said.
“In Massachusetts, for example, you’ll find that if you got an SBIR grant, you can probably match it with a state grant, and you can probably take that and apply it to the next-stage grant,” he continued.
“And you can probably then look at a place like Singapore, which has Enterprise Singapore, and you can match funds. ... And don’t forget all the stimulus programs that are coming from the government [including the Inflation Reduction Act]. All those programs actually do add up, and they’re very meaningful amounts of capital. So as an entrepreneur, think about where you want to build your company.”
“Hunker down, reduce your costs, make sure you’ve got two years of runway, if not more, and forget about thinking about valuation.”Greenberg Traurig patent attorney Roman Fayerberg






In particular, Barros recommended the states of Massachusetts, California, Minnesota, North Carolina, Texas, New York and the cities of Philadelphia and Chicago.


“The main thing is they want to create jobs,” he said. “They want to create new companies, they want them to innovate. And the most important thing: We have a lot of unmet medical needs.”

Sohn mentioned China as an opening market with a huge patient population, relatively decent reimbursement, and medical trends similar to the U.S.
“It’s one of these markets that’s so large and so consistent now with the U.S. around specific therapeutic areas, particularly those age-related diseases, you can’t ignore China,” he said. “To the extent that you’re looking for alternative ways of being able to either grow or leverage financing alternatives — partnering, venturing, joint venturing, raising capital, creating other mechanisms to be able to raise capital — to enter into the Chinese market may be an option. That tends to force you to be more thoughtful around your IP.” >>
“IP is important. It’s part of your story, it’s explaining your technology. Everybody wants innovative proprietary technology. And the way you build the IP, you have to make sure it’s protected properly.
... Understanding what you have, what you need to do to protect it, and where to protect it is very important.”
Dykeman said a client recently licensed patents to a partner in China to develop products for the China market.
“They were going to get into China eventually, but it was probably five to 10 years down the road,” he said. “But they were able to monetize that now to fund their U.S. R&D development.”
With IPOs rarer, mergers and acquisitions (M&A) — selling to a larger company — will be the most common way for founders to exit, but timing is important. Sell early or partner with a potential buyer, and they’ll fund development of your technology.
“But if you don’t get that early window, it could be a long haul,” Dykeman said. “Then you have to prove commercialization, you have to get your FDA approval, you have to show your sales ramp, and then they’ll pay a premium for you.”
However, major OEMs want to see foreign patents when negotiating with acquisition targets.
“If you want to get acquired by a J&J or a Medtronic and you don’t have a patent in China or Japan, they’re going to beat you up on valuation,” Dykeman said. “We’ve seen that in diligence: ‘We love your technology, but China’s the biggest market in the world, and you have no IP there.’ So then they beat you up on valuation, and the CEOs wish they had spent that extra $10,000 or $20,000 to get a patent in China.”
Larger medtech manufacturers can also help through development partnerships. A device developer usually considers multiple potential indications, but focuses on the most promising first. One medtech company found a way to develop two products for different indications simultaneously, Dykeman said.
“They found a commercial partner, one of the bigger medical device companies, that’s going to fund that development to the tune of what they were looking to raise in a VC round,” he said. “They ended up kind of spinning that
out, forming a separate subsidiary, but they’ve now got enough money to keep that engine moving and have two products in development at the same time.”
Creative financing solutions like these will help medtech developers move forward and prepare for better times ahead.
“Tomorrow’s [outlook] is always better than today,” Fayerberg said. “Think strategically. This market will pass, and things will get better.”



Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.

“Tomorrow’s [outlook] is always better than today. Think strategically. This market will pass, and things will get better.”


Compact KNF micro gas diaphragm pumps offer an ef cient and durable solution for environmental and industrial hygiene monitoring, inkjet printing, medical device and diagnostics, analytical instruments, and more. With ow rates ranging from 0.05 – 24 l/min, vacuum deeper than 29.6 inHg pressure up to 43.5 psig, and linear ow vs. speed, these products are well-suited for portable and handheld applications. Options include various materials, con gurations, and motors to balance required performance, lifetime, controllability, and cost.
Learn more at knf.com/en/us/micro-diaphragm-pumps


Afew years ago, Vivasure Medical faced an emergency that “nearly shut our company down,” cofounder Gerard Brett said.


“We picked a supplier in good faith — it looked like they had what it took to do the job for us,” he said. “We were working away at developing a part of our product, and we got a phone call with 24 hours’ notice to say the sheriff is going to put a lock on the door of that company.”
The supplier was about 4,500 miles from Vivasure’s Galway, Ireland headquarters where Brett serves as chief operating officer of the maker of advanced polymer implants and delivery systems.

“We literally put people on a plane, hired what looked like the CIA, we had
black Suburbans, and we backed up to the back of that facility at 2 a.m. and took our stuff out: equipment and materials,” Brett said. “At 6 a.m., it was locked.”
That’s what’s at stake when picking contract manufacturers, Brett said while presenting on a DeviceTalks Boston panel in May with MedAccred Operations Manager Justin McCabe and Spectrum Plastics Interventional & Surgical Technologies Sales VP Paul Melnychuck.
They offered tips, advice and red flags for medical device developers seeking outsourcing partnerships with contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs).






(continued on page 22)














The AxiForce tubeaxial fan series is ideal for keeping consistent, optimized temperature in control units for automation and other highly-modern technologies. Due to its variable installation, high cooling capacity in the smallest of spaces, and interactive integration into the device logic, it has already proven to be indispensable in modern automation.


(continued from page 20)
Getting started
“Ask first of all: What do you want, why do you want it and can you afford it?” Brett said. “Depending on the state of your company’s finances, depending on the state of development of your technology, what you need in a vendor from concept through design through development could be considerably different than what you need for post-clinical and commercialization.”
Device developers know their contract manufacturing partners will need the usual certifications and accreditations for devices to survive regulatory scrutiny. That’s just a starting point to dig deeper, said McCabe, whose organization works with major OEMs such as Medtronic, Johnson & Johnson, Stryker and Philips to vet suppliers.
“Don’t take those certifications and accreditations at face value,” McCabe said. “One of the things that MedAccred has built into our process is we require suppliers have a valid quality system in place before we’ll come in and conduct our audit. That
may be ISO 13485, it might be 9001. Then we come in and do our deep-dive technical assessment of that supplier’s capability. We see that ISO cert as an inch deep and a mile wide across an organization.”



While a contract manufacturer might have a certification that training programs are in place, MedAccred evaluates how effective those programs are for various capabilities and the people doing the work.




“Our auditors are talking to those operators, looking at the equipment on the shop floor, making sure that quality system is effective for that particular process and any standards that are utilized in that process,” McCabe said.
Melnychuck had good advice of his own: If your plan is to eventually sell your technology to a major device manufacturer, consider partnering with a contract manufacturer that’s already on the approved supplier list (ASL) for the companies you’d like to be acquired by.
“This has a lot of benefits. First of all, at a company like ours, our quality
management system is shaped by our customers’ QMS systems. And so we, to the extent possible, resemble their own systems,” he said. “Secondly, if you’re on the ASL, that saves probably six months — maybe more — of due diligence time to get on the ASL. There are business considerations, sometimes contract negotiations, and there are quality concerns. If you partner up front with a firm that’s on the ASL of three of the top five targets, you have a better chance of being able to slip that in. That time for a startup is valuable. That’s market access time, that’s worth money that I would say should add and contribute positively to the exit valuation of your firm.”

And always keep your options open with multiple plans for critical requirements and critical material.
“Do that as part of the process of setting up a vendor,” Brett said. “Otherwise, you’re walking in reasonably blinkered, and you can get into trouble that you can never anticipate.”
(continued on page 24)
Excellence that precisely ts your needs.
Get exactly the level of support you need for your Class I, Class II and Class III medical devices and components.

Get access to unparalleled expertise for standalone or integrated capabilities, including:
• Assembly and packaging
• Bioresorbable medical devices
• Injection molding
• Liquid silicone molding
• Micromolding
• Micro-MIM
• Precision machining
• Process validation
• Product development
• Prototyping
• Secondary operations
(continued from page 22)
Look for signs of trouble when you’re talking with CMOs and CDMOs even from the earliest stages.
“Be wary of empty promises,” Melnychuck said, warning that manufacturing is at or near capacity due to a lack of investment in the COVID-19 pandemic.
“Everyone’s busy, and if someone [says], ‘We can start Monday morning,’ I would really probe that because if there’s capacity available, you are paying for that because it means it was idle,” he continued. “Vet, do your due diligence and really be patient. And if that means the lead time is going to be a little longer before someone can start your project, whether it’s on the engineering design development side or commence manufacturing or transfer to manufacturing, I think it’s better to play the long game and work with a strong and viable partner that’s also efficient and is going to probably price that efficiency into your product as opposed to one that maybe has that idle capacity that you’re going to pay for in the long run.”
Remember that you’re looking for a long-term partnership that will benefit both of your businesses.
“If they don’t want to be part of the game with you and they’re doing it just because they’d like to get some more money, you’re in a danger zone,” Brett said. “You need to be sure that they want
Consider the size of a contract manufacturer against your own needs. The largest contract manufacturers might have more financial stability than smaller shops with fewer customers, but those smaller shops may be more responsive or even more knowledgeable in a particular area of specialization.

“When the pressure comes on, will you get the priority you need?” Brett asked.
“And how do you know you’re going to
Local suppliers are not only easier to vet before a partnership, but easier to drop into for check-ins, face time and in case of emergencies.
“You must touch, you’ve got to go visit, you’ve got to go see, you’ve really got to peel back,” Brett said.
“And this is where Justin’s organization (MedAccred) really comes into play because as a startup, sometimes you just don’t have the bandwidth or people to do that. But you need to. You need to take the time because the amount of aggravation and challenges and pain and sleepless nights that you’re going to avoid will be well worth it.”
to work with you just as much as you want to work with them. You need to make sure that they’ve got the financial stability.”
Beware of hidden costs, unanswered questions and a lack of detail in the proposed partnership, especially when it comes to more comprehensive projects.
“The concept development cost, the setting up a manufacturing process, characterizing the process, qualifying the process, getting into clinical studies, and then going into commercial, each one of those comes with a pretty hefty ticket,” Brett said. “Understand it all from start to finish before you make the jump.”
get that attention? How do you know it’s all going to work for you? Setting your expectations clearly up front, understanding what you want to do, understanding what they can do, and understanding that you can work together is vitally important.”
Supplier size by itself isn’t a guarantee of better service, price or speed. For more niche services, you may have limited to smaller suppliers — but one of them might just happen to be the best in the industry for your particular project.
Communication between both sides is crucial for success, and a big part of that is making sure there’s chemistry between the engineering teams and management teams before you work together.
“If you don’t, it doesn’t matter how good they are, you’re going to be challenged and it may or may not work,” Brett said. “Ensure you’ve got single points of contact. Don’t have 10 people from one company speaking to 10 people from the other. You’re going to get spaghetti junction communication. ... We pick the best communicator, the best negotiator to be that person.”
(continued on page 26)
“I think it’s better to play the long game and work with a strong and viable partner that’s also efficient and is going to probably price that efficiency into your product as opposed to one that maybe has that idle capacity that you’re going to pay for in the long run.”
• Creating value, stability, and consistent outcomes for the production life of your project
• Unrivaled cross-functional support team for NPD projects
• Delivering valuable DFM insight from manufacturing professionals
• Precision tolerance protoyping, pivoting and adjusting when necessary
• Advanced metrology and inspection capabilities, and data collection that create real learning opportunities throughout the NPD process


(continued from page 24)
Don’t overlook intellectual property in these conversations. After agreeing on your IP strategy internally — including up through your board and investors — you’ll need to be clear with your CMO or CDMO about who will have ownership.

Having all these conversations up front will provide a good foundation for building long-lasting relationships where there are no secrets and no surprises. It takes time, effort and trust.
“We have fi ve absolutely critical components,” Brett said. “I’m good friends with the CEO of each one of those companies and can pick the phone up, we have dinner together when we meet, and it works, and we don’t go poking each other and say, ‘Can you help me because I’m two days late on one part?’ But when something needs to happen, it can happen. It all comes back to relationship management, and if you’re not genuine, it’ll come apart very quickly.”

 Operations Manager Justin McCabe
founder and Chief Operating Officer Gerard Brett
Interventional & Surgical Technologies Sales VP Paul Melnychuck
Operations Manager Justin McCabe
founder and Chief Operating Officer Gerard Brett
Interventional & Surgical Technologies Sales VP Paul Melnychuck
“It all comes back to relationship management, and if you’re not genuine, it’ll come apart very quickly.”



 Jim Hammerand Managing Editor
Jim Hammerand Managing Editor
The ZimVie Tether is a groundbreaking system for treating adolescent idiopathic scoliosis, developed with the help of surgeons who saw an opportunity to improve the lives of their pediatric patients.

But for every patient, the clock is ticking, as children are only eligible if they have enough growth ahead of them for the technology to make a difference. And all too often, insurance companies can delay the treatment so long that patients are no longer eligible if and when insurers approve the procedure.
ZimVie SVP and Global Spine President Rebecca Whitney spoke about the system’s development and commercialization with ZimVie Spine Global R&D Director Ryan Watson at DeviceTalks Boston in May.
“We’ve worked for years to make sure that we have the right options available for these kids because it is a special patient population,” Whitney said. “… When you talk about kids who are typically aged 10 to 15, we are hopefully improving their lives for years and years to come. We take it very seriously, and it is something that all of us are very passionate about.”
What is adolescent idiopathic scoliosis?
Adolescent idiopathic scoliosis is a curvature of the spine affecting 2-3% of the
population. While diagnoses are evenly split between boys and girls, girls are eight times more likely to have curvature severe enough to require treatment.
“Idiopathic is basically code for ‘we don’t know.’ It’s not really clear as to why this happens, what creates the phenomenon,” Whitney said.
The condition can be lived with, but causes issues as it progresses, including aesthetic issues and potentially lifethreatening heart and lung compression. Traditional standards of care have had varying degrees of effectiveness.
“The first line of defense is a waitand-see approach because a lot of times these curves don’t progress to the point where they do need intervention,” Whitney said. “If they do, bracing is usually the first order of treatment. Bracing can be effective, but the challenge with bracing is these kids are required to wear a brace from 12 to 20 hours a day.”
Bracing can range from uncomfortable to painful, and the biggest reason why it fails is because patients don’t want to wear them. If bracing doesn’t work, the historical standard of care is fusion.
“Posterior spinal fusion is very effective at straightening out the spine,” Whitney said.
(continued on page 30)

Before spinning off from Zimmer Biomet, ZimVie developed the Tether device with surgeons who saw an opportunity to improve the lives of their pediatric patients.
The ZimVie Tether helps kids with scoliosis — if they can get it in timeThe ZimVie Tether system is FDA approved for adolescent idiopathic scoliosis under a humanitarian device exemption. Image courtesy of ZimVie

It’s good to know where you come from. At Nitto Kohki, we come from a line of engineers and businessmen dedicated to exceptional manufacturing execution. In our line of vacuum and pressure pumps, this is seen in the unique linear-piston design, where one moving part resolves to exceptional reliability, low vibration and noise, high energy efficiency, and long, consistent performance life (>10,000 hrs.) for a host of critical medical applications. It’s why the medical device industry has specied Nitto pumps for over sixty years— the quality in our DNA. Find out how you can use it to your advantage.
(continued from page 28)
“It does that part very well, but it’s also very invasive. … You’ve got screws and rods placed all up and down the spine, and then you’re fusing that spine in place. It fixes the curvature of the spine, but it comes with long-term, potential limitations as the patient goes on to grow and continue with their active lifestyle.”
Vertebral body tethering for adolescent idiopathic scoliosis
A less-invasive alternative to fusing for adolescent idiopathic scoliosis is motionpreserving vertebral body tethering.

“Instead of fusing the back all up and down, you have these anchor points, and you’re able to use basically a shoestring of sorts. And as the patient grows, that spine is able to straighten out without having to fuse the spine in place,” Whitney said. “We believe that for patients who are indicated, this is a really great solution, and it offers the benefits of fusion while also preserving lifestyle. And for the most part, these kids are very active and looking to get back to a very healthy level of a lifestyle.”
The procedure entails putting screws into the convex (curved) side of the spine, coming in laterally through the side of the patient – through their ribs or over their spine — and then putting the cord between the screws.
“On the operating table, the surgeon [can] tension that cord and pull the spine straight,” Watson said. “Now, they don’t get all of the correction on the table, because this is kind of the beauty of this product and what we’re after: It’s called growth modulation.”
The surgeon might be able to achieve 50–80% of the curvature correction in the operating room. Beyond that, the tether helps hold the convex side of the spine and allows the other side to grow and straighten over time.
“It’s a pretty phenomenal way to take advantage of these patients’ own growth,” Watson said. “Diagnostic tools are used with X-ray for an assessment of how much growth those patients still have to ensure that they’re the right candidate, because this fantastic technology is not necessarily the solution for every patient with these conditions. But for those that have the right parameters, it is really pretty special.”









The screw-and-cord approach dated back to the ‘90s, when what was then




Zimmer developed the Dynesys system for posterior spinal fixation. While Dynesys wasn’t designed for adolescent idiopathic scoliosis, surgeons realized the technology had potential. They adapted it for lateral placement to perform the corrections, collecting data and monitoring patients along the way.
“In 2013, we the company see this begin to take off, and we say, ‘You know what? We want to participate in this. We want to help create this and make it more readily available to these patients,’” Watson said.
As Zimmer considered the time and cost of clinical trials to take the technology to market for adolescent idiopathic scoliosis, surgeons continued on their own based on the results they were seeing.
“In 2015, the FDA began to see this being more prevalent and said, ‘OK, you need to stop this usage.’ But fortunately, so much good data was collected by the early surgeons, and the case was made. … They wanted to take part in making this available, so they showed up to the table, we worked with them and got a humanitarian use device designation,” Watson said.


Zimmer merged with Biomet in 2015. The FDA approved Zimmer Biomet’s Tether system under the humanitarian device exemption (HDE) pathway in 2019. It was the first approval of a humanitarian use device in spinal pediatrics in 15 years.
ZimVie — which spun out of Zimmer Biomet in 2022 — was the first company in the U.S. with HDE approval for vertebral body tethering to correct pediatric scoliosis. In 2019, ApiFix (now owned by OrthoPediatrics) received HDE approval for a rod-based growth modulation system. In May 2023, Globus Medical’s Reflect Scoliosis Correction
System won HDE approval for progressive idiopathic scoliosis in pediatric patients.

The Tether system had shorter screws for smaller patients and other minor changes from Dynesys, but the team was careful not to change too much at the risk of invalidating the data surgeons generated and collected in early cases.
Machinists and test engineers
prototyped 42 different instruments, 144 anchors and more than 400 screws over six months, Watson said. Then came computer simulations to test the concepts before cutting the metal, followed by testing 242 cords and conducting 281 mechanical tests on test frames. The team soon eclipsed 19,000 testing hours.
The ZimVie R&D team uses spine simulators that can put devices and systems through a series of motions. “I’ve got five or six machines running as we speak that are running around the clock,” Watson said.
He credited collaboration across the company and beyond for bringing the Tether system to market.

“It’s a tremendous amount of effort, testing and development that goes into this,” Watson said. “It’s not just the engineers, it’s the surgeons that had the vision partnering with the engineering team, the cross-functional members, and then the company that’s willing to do it.”
“This is a relatively small market and a lot of work,” Watson said. “We do it because it matters.”
Getting ZimVie’s Tether to more patients Only one in 10 children who are indicated for the Tether system are treated with it, Whitney said. “It’s relatively new, and like any adoption life cycle curve, we’re in that early stage.”
ZimVie is striving to increase adoption with surgeon training, patient awareness, and pushing through insurance barriers.
The company has an eight-station cadaver training facility in Colorado for surgeons, and also connects them with leaders in the fi eld. To reach patients, the company funds marketing aimed at parents including a website, myscoliosis.com, and promotes Facebook groups with resources for patients and parents.
“We don’t want to bias any of that conversation, but we do support their efforts because we know that it can be a really challenging time when you have a child who was diagnosed and you’re trying to seek out all the different information that’s out there,” Whitney said.
On the insurance side, ZimVie is constantly lobbying insurers to cover the procedure.
“We had a big win last summer when Anthem Blue Cross Blue Shield deemed the Tether as medically necessary. And we think that would be the first domino to follow, which is really encouraging,” she said. “But the reality is a lot of these kids get stuck in this constant denial and approval process with the insurers.”
That can often delay care, sometimes long enough that children have grown too much in the meantime for the system to help. But ZimVie is on track to receive a Category 1 Current Procedural Terminology code in January 2024 to significantly increase access for patients.
“Developing a market is a long game and takes commitment and a lot of resources and investment. We’re doing it because we firmly believe it’s the right thing to do,” Whitney said. “As we continue to develop this and we see more and more kids receiving access, it is the fuel and the passion that keeps all of us continuing to march forward.”
“When you talk about kids who are typically aged 10 to 15, we are hopefully improving their lives for years and years to come. We take it very seriously, and it is something that all of us are very passionate about.”Jim Hammerand Managing Editor



Phiex is working with Medtronic and other medical device manufacturers on chlorine dioxide packaging sterilization as an alternative to ethylene oxide (EtO).
edTech Innovator winner Phiex is working with medical device manufacturers on an alternative to ethylene oxide (EtO) sterilization.
The new method uses dry chlorine dioxide gas generated inside the medical device’s product packaging. The powder turns into a microbe-destroying gas when exposed to light, Phiex co-founder and CEO CL Tian said in an interview with Medical Design & Outsourcing
Medical device manufacturers can either integrate the powder into their product packaging or as a secondary pouch. And so far, most devices that can be sterilized with EtO can be sterilized with the Phiex process, Tian said.

EtO is the leading sterilization method for medical devices. Manufacturers and contract sterilization firms use EtO on more than 20 billion medical devices every year, or approximately half of all devices that require sterilization. But the medtech industry and FDA are exploring alternatives to EtO due to safety concerns about sterilization facility emissions. And industry association AdvaMed has warned that EtO sterilization capacity is already maxed out.
“It’s clear that EtO’s going to have to go away. It’s just not going to be tolerated … and I just think this is the best alternative,” said retired
Medtronic Chief Medical and Scientific Officer Dr. Rick Kuntz, who recently joined Phiex as an advisor.
Kuntz will familiarize Phiex with Medtronic’s product lines and leverage his network to help the startup build relationships within the medtech industry.
“When I met CL, they had already been connecting with material scientists at Medtronic that actually worked in my group,” Kuntz said.
Chlorine dioxide gas has been EPAregistered as a sterilant since 1988. It’s already used to sterilize medical devices in airtight chambers and is also used for food and water sterilization. The gas has not been linked to cancer or birth defects.
(continued on page 34)
Phiex’s medical device packaging sterilizes medical devices by releasing a dry chlorine dioxide gas when exposed to ambient, broad-spectrum light. The gas seeks the bound water in the cell wall of bacteria and essentially oxidizes the cell membrane.
Illustration courtesy of Phiex


At OKAY Industries, we believe in making happily ever after possible, partnering with medical device OEMs to develop innovative solutions that save and improve lives. Our early-stage engineering support, combined with fully integrated and automated capabilities, allows us to manufacture components with complex geometries, tight tolerances, and accuracy that doctors and surgeons rely on. Time after time, we deliver excellence from prototype to production.
What we manufacture is Part of Something Greater. Together, let’s engineer happier tomorrows. Visit okayind.com.


(continued from page 32)

Tian characterizes Phiex’s inpackaging sterilization as a gentle process, with no need for humidity, temperature or pressure preconditioning. Generating chlorine dioxide gas from a solid-state powder allows Phiex to control the concentration of gas needed to sterilize a particular device inside its packaging.

“We have a proprietary, dry state powder that we mix directly into the packaging,” she said. “When it is exposed to ambient light, there’s a reaction, and we generate what we call a micro-atmosphere of chlorine dioxide gas. … It has excellent material compatibility, and that’s been a major concern for the OEMs with any alternate sterilization modality.”
wrap and high barrier foils and films.

The global leader you trust for custom healthcare packaging solutions including pouches, bags, lids, film roll stock, flow wrap and high barrier foils and films.


And we continue to provide a full range of rigid packaging you can count on from concept to the point-of-use. Learn more about our custom designed trays, blisters, automation trays, die cards, performance packaging, sealers and lab validation services.



For more information, contact us: Email: info@nelipak.com | Phone: +1.401.946.2699
The Phiex approach can also be used as an alternative to radiation or heat sterilization methods, which can discolor or damage medical device materials such as silicone, polyurethanes, polyethylene, polyolefi ns and other sensitive polymers.






“We haven’t seen a material impact in our studies with our customers. There’s no change in mechanical properties, molecular weight, tensile strengths or coloration,” Tian said. “And that’s even with us exposing it to much higher concentrations of our dry — dry being the operative word — chlorine dioxide than we would expect in an actual commercial setting.”
Another advantage over radiation sterilization is that Phiex’s sterilization process doesn’t damage electronics. That’s helpful for device developers who are integrating batteries and electronics for the fi rst time into a device that could previously be sterilized with gamma rays, for example.

Radiation may remain the method of choice for manufacturers that need to sterilize inside airtight compartments where EtO, chlorine dioxide and other gases can’t reach. And while radiation and EtO sterilization need sterilization chambers and large facilities with expensive equipment like particle accelerators, those methods may still make sense for bulk sterilization of pallets of hardy devices.
“Where we play well is at the device level, sterilizing one or a couple inline,” Tian said. … “It’s a completely different way of thinking about how we sterilize.”



How Phiex works with manufacturers Phiex is currently developing customdeveloped packaging material to manufacturers for their production lines, but Tian declined to name customers.
“The business case for moving to a modality like ours — inline packagingbased sterilization modality — is so attractive from a strategic advantage perspective that even if we wanted to, our customers have been quite purposely hush-hush about working with us,” she said.

“Most OEM folks talk about the amount of time wasted with complications and issues that occur with sterilization, both from producing commercial products and also from taking novel products from the bench through the R&D process,” Tian said. “There’s a lot of interest in having a more sustainable and better-for-business sterilization modality.”
What’s next for Phiex?
Tian explained how the technology works with customers using a hypothetical Class II, FDA-cleared 510(k) device.
“You assemble it in your manufacturing line, put it together and place it into your primary packaging, which usually has a layer that is gas permeable if it’s currently sterilized with ethylene oxide,” Tian said. “Let’s just say that customer wants to use a secondary packaging, meaning after they place it in their primary and seal it, they can place some number into the secondary pouch, seal that, and start to sterilize it. After the cycle time is up, they can open it, place it into their paperboard carton, into their boxes, and then assuming they release the batch according to their release protocol, that box is now ready to go off to their point of distribution or their end customer.”
That process, Tian said, is weeks faster than sterilizing through third-party vendors, where it might take days or weeks just to make enough products to efficiently ship to a contract sterilizing facility. Depending on how backed up the contract sterilizer is, there could be more delays until the products are sterilized and returned to the device manufacturing plant or sent to a distribution center.
Phiex customers are in various stages of adoption, ranging from submitting updated filings with the FDA to implementing the technology into their manufacturing lines. The first products sterilized with Phiex packaging could reach endusers in late 2023 or early 2024. Things are moving fast for the Boston-based startup. Phiex had what Tian would only say was a “very positive interaction with the FDA” last year. Then the company won the $350,000 grand prize from the 2022 MedTech Innovator program, which Tian said was “hands down the best thing we’ve ever done.”
“There’s a lot of programs out there,” she said of the global medtech accelerator. “What makes this unique is the people in the program. They’re some of the top leaders in medtech, they’re so giving and willing to work with each of the portfolio companies — not just us — making introductions, working on the business, sharing best practices in a really open, generous way. One thing that we have in scarcity as startups is time, so any way to learn from what’s worked before, learn from successes rather than our own experience, is so invaluable. And I’ve really appreciated that community that [CEO Paul Grand] and the team have cultivated.”
What does the future hold for Phiex?
“If you had asked me where I thought we would be this time last year, I couldn’t have imagined that we’ve gotten this far and this much unsolicited industry interest and engagement with the major OEMs and major contract manufacturers,” she said. “I try to stay out of the business of predicting, because I have a suspicion that we’re going to go at a much faster clip than we’re conservatively planning.”
Retired Medtronic Chief Medical and Scientific Officer Dr. Rick Kuntz
“Most OEM folks talk about the amount of time wasted with complications and issues that occur with sterilization ... There’s a lot of interest in having a more sustainable and better-forbusiness sterilization modality.”
Sustainable coatings are replacing “forever chemicals” in plastic medical devices like these coated needles. Image courtesy of Surface Solutions Group

Group
Fluoropolymers are getting a bad name. For more than a decade, perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) used to manufacture polytetrafluoroethylene (PTFE) coatings for the medical device industry have been highly scrutinized by regulatory agencies including the EPA and the EU Medical Device Regulation (MDR).
In 2010, the EPA recommended the elimination of PFOA in all PTFE coatings by 2015, including those used for medical devices. PTFE coatings are used for guidewires, mandrels, hypotubes, coil wires and needles. Since then, PTFE manufacturing companies have turned to short-chain perfluoroalkyl and polyfluoroalkyl substances (PFAS) as an alternative, which seemed like an
effective substitute — until it wasn’t. There are thousands of chemicals that fall under the PFAS chemical bracket. PFAS are labeled as “forever chemicals” because they are widely used and break down very slowly in both the body and the environment. These alternative PFAS chemicals are under scrutiny with approaching deadlines to be handed down by EU regulators for elimination of their use as well.
Promisingly, research and development efforts have eliminated not only PFOA but also PFOS. Through similar research and best available technology, there will be replacements for the PFAS that are used in manufacturing PTFEs, making solid headway toward more sustainable functional PTFE coatings.
(continued on page 38)

’Forever chemical’ replacements are on the way thanks to sustainable coating research and collaboration.George Osterhout Surface Solutions
COUNT ON INDUSTRY-LEADING PRECISION & ACCURACY WHEN THERE'S ZERO ROOM FOR ERROR.



(continued from page 36)
The long history of PTFE PTFE was discovered by accident in 1938, the by-product of another project. That was DuPont’s very first Teflon, which helped to revolutionize manufacturing. The molecule PTFE is inert and is not water-soluble. At 0.05µ to 0.10µ, it has an extremely low coefficient of friction, making it ideal for heightening the performance of many products, from cookware to containers, pipes, automotive parts, and machinery.
For the medical device industry, PTFE has reduced the dynamic efforts required for maneuvering various guidewires through small vessels and arteries in the body to perform life-saving procedures. But it is the chemicals used to make PTFE that are now deemed undesirable.
The problem with completely eliminating PTFE in the coating is that medical devices, and so many other products, cannot effectively function to the standards we have come to expect. Fluoropolymers, or PTFEs, are used in millions of products we use every day. Without them, or a sustainable alternative, medical device efficacy and patient outcomes could be set back years. Hence, it is quite a wake-up call for the designers and engineers who must remake the devices to be compliant.
Because of the recent concerns about PFAS being forever chemicals and the EU looking to eliminate use in the near future, there is the potential for the EPA restrictions to follow the EU.
This has created a significant change for medical device companies under pressure to develop or source new sustainable replacement coatings that perform as well as the restricted counterparts. The device manufacturers are seeking more sustainable PTFE coatings, along with alternatives to PTFE.
This is quite a role reversal for medical device designers and engineers who traditionally drove the use of PTFE coatings. Instead, they find themselves in reactive mode, and are experiencing increased involvement of regulatory departments and validation teams in the replacement
of legacy PTFE coatings. The designers and engineers are searching for coatings that meet or exceed current regulatory requirements and those that they anticipate will be handed down in the future.
As a result of the changing regulations, major players in the market are significantly shifting their product strategies.
3M has chosen to completely cease making PFAS by 2025, while PPG has stopped making coatings for medical device use. Chemours has stepped up to the challenge and has committed to eliminate at least 99% of all PFAS used in the processing of PTFE by 2030.

Two other coatings manufacturers are also pushing forward, using established scientific data to reformulate existing coatings and create new ones to reduce the use of restricted chemicals while meeting product performance standards. For them, it is imperative that they relentlessly pursue viable solutions and attempt to fill the alltoo-critical supply chain gaps left by others.
These companies have developed water-based, low friction, biocompatible medical device coatings that are free from any PFOS, PFOA, solvent, and hex chrome compounds. They’re also REACH and RoHS compliant.
One company, Cavero Coatings, offers a coating that contains as much as 60% binders and pigments, with the balance being PTFE.
Another company has joined forces with functional coating technology company Surface Solutions Group (SSG) to create SSG’s GlideMed coating, which contains no PTFE. Our R&D teams have worked together to test and validate this coating to provide the needed, reliable data for design engineers. Independent test results indicate that its lubricity, durability, and adhesion properties are proving it to be a good, sustainable alternative to traditional PTFE coatings.
Throughout the medical device industry there are varying degrees of success as chemists and engineers collaborate and navigate the possibilities.
Still, there is some rough terrain ahead for everyone who uses PTFE. They will need to adapt, as they have through the years, to comply with ever-changing regulations. Fortunately, with continued research and innovation, the future holds much promise for creating more sustainable, compliant coating solutions.
George Osterhout is the president at Surface Solutions Group (SSG) and a career veteran of the coating industry. SSG develops and applies functional medical device coating technologies, offering 13 proven medical coating categories, which SSG says gives its medical device partners the widest range of coatings available through a single supplier.
Strengthen your innovation with our rapid prototyping and clinical know-how to take you seamlessly from concept to production









































Get to market faster by utilizing our platform technologies and market-ready products that provide differentiated performance and portfolio expansion

















































Extend your reach through our global R&D and manufacturing capabilities to consistently achieve design requirements, quality standards, and on-time delivery








































































emphasizing the need to visualize their unique properties and thickness.
Rather than relying solely on “textbook depictions,” designing medical tubing that accommodates the true characteristics of this delicate anatomy is crucial for optimal device performance.
“The ventricular anatomy is incredibly complex,” Laske said during a presentation at DeviceTalks Boston in May. “Everyone has a slightly different anatomy, and their conduction system is slightly different. Please consider that when designing devices.”


By acknowledging these differences, engineers can develop tubing solutions that seamlessly integrate with the diverse ventricular anatomy, ultimately enhancing device performance and patient outcomes.
The heart is often one of the most underappreciated aspects of human anatomy, and its atrial appendages are often overlooked even in cardiac anatomy.
These delicate and astonishingly thin structures are challenges for medical device developers.



Some intricate structures within the heart are so thin that a business card can be read through them, said Tim Laske, VP of research and business development for the cardiac ablation solutions business at Medtronic. When observing the heart in a surgical space, it becomes evident that atrial appendages carry blood,
Composed of an atomic mixture of nickel and titanium, nitinol possesses distinct properties that make it a preferred choice for medical tubing. In the context of tubing design, two particularly interesting properties come to light.
First, nitinol has excellent thermal connectivity, boasting effi cient conductivity that proves invaluable when used as an electrode. This property ensures optimal heat dissipation, enhancing the performance of devices utilizing nitinol tubing as an electrode component.
(continued on page 42)








(continued from page 40)
Secondly, nitinol demonstrates exceptional elasticity, making it an ideal material for applications that require high flexibility without the risk of kinking. Nitinol tubing can endure significant deformation without experiencing plastic deformation, making it particularly well-suited for guidewires and other critical areas where flexibility and shape retention are paramount.
transition temperature. This characteristic enables the customization and adaptation of tubing to suit various anatomical requirements, leading to enhanced patient comfort and procedural success.
Superelasticity, on the other hand, allows nitinol to withstand substantial strain and stress while retaining its original shape. This property is particularly advantageous in medical devices that undergo significant mechanical loads, ensuring longevity, reliability and safety.
“This shape change is important if you’re using it for the shape memory phenomenon,” Laske said.

Medical tubing made from nitinol offers several advantages. Its biocompatibility and biostability make it suitable for introduction into the body, while its flexibility minimizes trauma and reduces the risk of damage. Moreover, nitinol’s ability to undergo substantial size changes, from a small initial form to a larger expanded state within the body, enhances its versatility and applicability in various medical procedures, most notably less-invasive catheter delivery of cardiovascular implants.
Processing nitinol typically involves drawing it into wire for applications like guidewires or embolic coils. However, laser cutting from tubing is the more common approach.
Other processes such as electropolishing ensure a smooth surface finish. Smoothness is crucial in preventing the formation of titanium nitride occlusions, which can lead to fractures.
When selecting a nitinol vendor, it is essential to work with reputable sources and inspect the material for any occlusions that may compromise its performance, Laske said.
valves non-surgically, Laske said. In these procedures, arteries and veins are used as “superhighways” to access the heart. When using a nitinol stent in this application, engineers should consider the durability once placed in the heart and how small the device can be compressed for minimal impact on the vasculature. It also has to crimp down inside a sheath to access the heart and then spring into place at the proper location in the heart.
Medtronic uses nitinol in a range of devices.

“It’s not only more compliant and flexible than stainless steel but it can be deformed to a much greater extent without plastic deformation. Hence, it’s used in guidewires and other areas where you need something highly flexible that won’t kink,” Laske said.
Nitinol’s unique properties extend beyond its thermal connectivity and elasticity. Two notable phenotypes associated with nitinol are shape memory and super elasticity.
Shape memory allows nitinol to be heat-treated into a specific shape and then deformed. However, nitinol returns to its original shape upon reheating past its
“Anyone that’s worked with nitinol, you know your enemy is titanium nitride occlusions. You can get titanium nitride crystals, which can be pretty large, and those will nucleate fractures. So when you’re choosing your nitinol vendor, make sure you’ve got a very reputable house and then when you’re inspecting it, look for these occlusions and be sure that they don’t exist if it’s in a highfatigue-state environment.”
One of the more widely known applications of nitinol is in transcatheter heart valves, which are designed to replace heart
Medtronic uses self-expanding nitinol for the frame of its Harmony transcatheter pulmonary value, with nitinol wires handsewn together in zigzags.

Nitinol also allowed Medtronic’s Micra leadless pacemaker to be implanted inside the heart.

“It had a battery life of about six months, which was not enough to place inside the heart,” Laske said. “As battery technology evolved and with clever use of nitinol, the device can now be placed inside the heart as a permanent pacemaker.”
Micra has FlexFix nitinol tines that come out of the device’s sheath in the body to engage with the tissue and secure the device in place. If a physician needs to recapture the device, it can still be pulled back with the nitinol tines in place.

Medtronic also uses nitinol in its Arctic Front Advance cryoablation platform, Laske said. That system uses nitrous oxide to freeze the pulmonary veins to kill the diseased tissue circumferentially around the cardiac veins.
“This wouldn’t seem like it’s an obvious place to use nitinol,” Laske said. “But there are two different components.”

First, the Achieve Advance mapping catheter must navigate the pulmonary veins. Laske said the device needs to be able to travel in a straight line to the veins, then deploy and be manipulated while still able to return to its original shape.

The injection tube that the nitrous oxide runs through is also made of nitinol and can adapt to the shape of the pulmonary vein. Liquid nitrous oxide is then pumped down the nitinol tube and injected at the proper site.
Medtronic’s Affera and PulseSelect mapping and ablation systems use nitinol in their components as well. The Affera Sphere-9 catheter has a superelastic



Solutions VP of Research and Business Development



Tim Laske
nitinol mesh so it can be compressed into a small catheter and expand within the body, then compressing again to exit.
“[The device] needs to come out of a small catheter and go into a large configuration. It has good electrical conductivity, so it can be used as an electrode. It has well-understood biocompatibility and we know how it behaves inside the body into the bloodstream,” Laske said.
Medtronic's PulseSelect pulsed
field ablation catheter
Image courtesy of Medtronic
DDL has over 30 years of experience navigating complex testing standards and regulations for medical device and pharma products. Our reliable quality, responsive attention, and on-schedule completion for packaging, product and materials testing secure confidence in performance and safety while achieving regulatory compliance.

•
•
•
•


Abbott says its HeartMate 3 LVAD is clinically proven to extend life by five years or more — but thousands of people in the U.S. still die of heart failure within a year.
Kyree Miller, a 30-year-old college student, navigated seven long years of heart failure with the assistance of an Abbott HeartMate left ventricular assist device (LVAD).

“Before I got my LVAD, there was no way I was getting on a plane. Before I got my LVAD, there was no way I was going back to work. And I was able to do both of those things — and more. To be at Killington [ski resort] with an LVAD, I never thought it would have happened, but it did,” Miller told a group of medtech insiders during our annual DeviceTalks Boston show in May.
In August 2022, Miller received a new heart.

“Having the LVAD was crucial in bridging the gap before the heart transplant,” he said. “Without it, I don’t think I would have been here to receive the transplant.”
Miller was fortunate to live in Boston, a city that’s one of the nation’s top healthcare and research hubs. He was near top-notch hospitals with access to LVADS such as Brigham and Women’s Hospital, Beth Israel Deaconess Medical Center and Massachusetts General Hospital. Cardiologists at top hospitals knew what LVADs could do.
“Being here with the big centers a stone’s throw away was a big help,” Miller said. (continued on page 46)


 Former LVAD patient Kyree Miller holds an Abbott HeartMate 3 at DeviceTalks Boston in May.
Photo by Jeff Pinette for Medical Design & Outsourcing
Former LVAD patient Kyree Miller holds an Abbott HeartMate 3 at DeviceTalks Boston in May.
Photo by Jeff Pinette for Medical Design & Outsourcing
(continued from page 44)
His story highlights a challenge that Abbott officials are fighting to answer.
The company’s research has shown that its HeartMate 3 pump can extend the lives of advanced heart patients by at least five years. However, Abbott estimates the U.S. has 15,000 advanced heart failure patients who are managed with inotropic therapies alone; they have a projected median lifespan under a year.
“Kyree is lucky to be in a place where he can get access, was referred, and had people who made the right referral based upon the disease that he had. And the benefits are obvious,” said Dr. Robert Higgins, president of Brigham and Women’s Hospital and EVP of Mass General Brigham.
“The challenge that we're thinking about is that not everyone has a cardiologist, or access to advanced heart failure therapies,” Higgins continued. “And in particular, [minorities] and women don't necessarily have access to advanced heart failure therapies, which include LVADs, as well as the modern technologies — transplant being one of them.”
Miller later said: “As a gay Black man, the healthcare system can't be one-size-fits-all, either. And in some ways, it feels like it is.”
Implanted through open heart surgery, a left ventricular assist
device pumps blood from the lower chambers of the heart to the rest of the body. It can provide a crucial bridge to keep someone like Miller alive until a donor heart is available for transplant. Even when a person isn’t eligible for a transplant, it can sometimes still provide years of extra life.
The transformative power of LVADs in the realm of advanced heart failure therapy cannot be overstated. Abbott’s HeartMate
devices became the sole LVAD technology available in the U.S. when Medtronic stopped sales of its HVAD in June 2021 due to mounting issues with the platform. Abbott’s latest generation HeartMate 3 is smaller than a baseball. Its Full MagLev Flow Technology utilizes a fully magnetically levitated, self-centering rotor that does not require hydrodynamic or mechanical bearings. The result, according to the company, is gentle blood handling to minimize complications and hemocompatibility-related adverse events.
There are over 50,000 patients who have been implanted with Abbott’s HeartMate II or HeartMate 3, and at least 1,000 of those who received the HeartMate II have lived for over 10 years with LVAD support, according Dr. Robert Kormos, division VP of global medical affairs for Abbott’s heart failure tech.
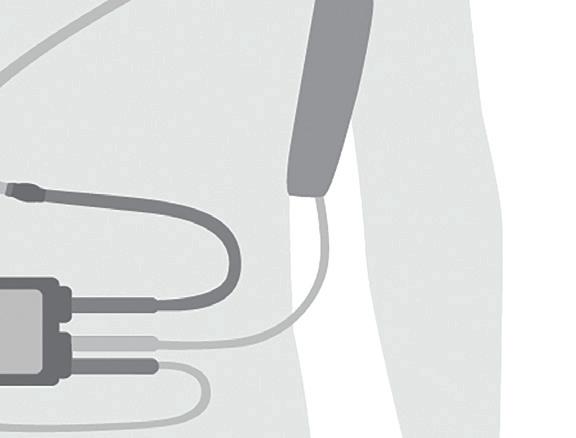




Abbott has some ideas about how to help Even a multibillion-dollar company such as Abbott can’t reform the U.S. healthcare system’s care disparity on its own, but company offi cials think there are things they can do to improve the situation.
They’re working on smaller pumps. And Kevin Bourque — VP of R&D at Abbott — thinks better education could clear up a misunderstanding among some doctors that a HeartMate 3 won’t fit in smaller patients, including women and children.
(continued on page 48)






Despite global material shortages and port lockdowns, Interpower® North American and international hospital-grade cords have resumed their industry-unique 1-week lead-times. Our hospital-grade cords provide the correct country-specific amperages and voltages for medical devices such as portable CT Scanners and X-ray machines, medical-grade treadmills, and ECMO machines.




When designing, manufacturing, and maintaining hospital-grade products worldwide, it’s vital to know the medical requirements of the country of export—select hospitals in Australia, Canada, Denmark, Japan, and the United States have proprietary requirements.

Our North American and international hospitalgrade cords never leave our facility without thorough examinations—they are rigorously tested to surpass UL 817 (18.2.4.1) and C22.2 No. 21-14 Interpower cords and components are rigorously tested. “We test more than the standards require,” Interpower Product Development Manager Ron Barnett said. “We do so because it lends better reliability to our design.”

• World-class customer service 7 a.m. to 5 p.m. CST
• In-stock cords ship the same day

• No minimum orders
• Value-added options such as colors, lengths, labeling and packaging
(continued from page 46)


A recent study found it’s the size of the heart that matters more than the size of the patient, he said.
“A couple of years ago, we got a pediatric approval for our HeartMate 3 devices that are being put into children as young as 7 or 8 years old,” Bourque said. “... There certainly is some room to go to get into truly small patients, truly small chests. But that's not the whole trick, being smaller. I think we need to be physiologically responsive as well. So we're thinking about how do we make these devices not just be life-savers, but life-optimizers. With all of those strategies in play, I think we can start to reduce the bridge, approaching the value that transplant can bring. We're kind of on the precipice of doing that.”
In general, better education and dissemination of heart failure treatment guidelines among physicians would help, according to Higgins.
“I think education is going to be critically important to help physicians understand not only that there are at-risk populations, women and persons of color, but everybody benefits from an informed provider who can identify heart failure at its earliest stages and get patients into a comprehensive therapy,” Higgins said.
Kormos suspects the medical community may be too focused on treating symptoms while neglecting the underlying cause. “The problem with that is that by the time a patient says, ‘I don't feel good anymore,’ it's too late.”






Access to better cardiac diagnostics and monitoring could catch heart problems earlier so people have time to find out about LVADs as an option.
“We have a pulmonary pressure monitor that we put in people, the CardioMEMS, which is a small dime-size sensor that goes into the pulmonary artery and can measure changes in your



























“Before I got my LVAD, there was no way I was getting on a plane. Before I got my LVAD, there was no way I was going back to work. And I was able to do both of those things — and more.”Cleanroom Molding Cleanroom Assembly Cleanroom Packaging
pulmonary pressure. We know that with heart failure, that goes up, and as it goes up, your right heart starts to fail,” Kormos said.
According to recent studies, CardioMEMS can nearly halve heart failure hospitalizations and significantly reduce the risk of death.
Miller still has a CardioMEMS inside him.
“I don't necessarily use it, obviously, as much as I used to,” he
said. “But every once in a while just to do a check in, we still actually use it just to make sure that things are going in the right direction.”
If Miller hadn’t gotten into a cuttingedge hospital system he wouldn’t have had access to CardioMEMS or an LVAD — and he wouldn’t be around today, Higgins said. “People are dying waiting for that type of technology.”



Kormos said Abbott and other
major medtech companies have been working on more inclusive clinical trials and designing devices to serve more diverse populations.
All of Abbott’s efforts matter because LVADs not only keep people alive, Bourque said, but they enable them to lead productive lives.
Said Bourque: “Every year that Kyree lives is a valuable thing. You’ve returned to society.”
Image courtesy of the Centers for Disease Control and Prevention

In the medtech space, it seems as though every company is seeking ways to incorporate some form of AI into the digital features of their products and services.
So what is artificial intelligence good at when it comes to advancing medtech and healthcare in general? Here are seven recent examples:
1. Helping physicians quickly identify medical problems
Interest is growing in artificial intelligence that can help radiologists, gastroenterologists and others better spot potential diseases in the countless images they examine each day.

For example, GI Genius is an AIassisted colonoscopy tool designed to help detect polyps that may lead to colorectal cancer. Medtronic is the exclusive worldwide distributor of the technology, which was developed and manufactured by Cosmo Pharmaceuticals.
(continued on page 52)
The experts in design, development, and large-scale manufacturing of interventional catheter-based devices and implants.
The experts in design, development, and large-scale manufacturing of interventional catheter-based devices and implants.












POLYMER TUBING
POLYMER TUBING

FILMCAST PTFE
FILMCAST PTFE




NITINOL COMPONENTS
NITINOL COMPONENTS









NITINOL TUBING
PARTNERING WITH YOU EVERY STEP OF THE WAY
PARTNERING WITH YOU EVERY STEP OF THE WAY
Learn more about our offerings at www.confluentmedical.com or email sales@confluentmedical.com for a custom quote.
Learn more about our offerings at www.confluentmedical.com or email sales@confluentmedical.com for a custom quote.



NITINOL TUBING


CATHETER COMPONENTS
CATHETER COMPONENTS
BALLON CATHETERS
BALLON CATHETERS
BIOMEDICAL TEXTILES
BIOMEDICAL TEXTILES


(continued from page 50)
Giovanni Di Napoli, president of the Gastrointestinal business at Medtronic, showed some GI Genius footage at our DeviceTalks West event in Santa Clara, California, this past October. Historically, gastroenterologists detected fewer lesions while performing colonoscopies as their day progressed from morning through the afternoon. GI Genius’ AIbased enhancements help physicians stay focused by placing green boxes around areas that may need extra scrutiny during the diagnostic imaging procedure.
GI Genius could soon get even better. Medtronic has partnered with Nvidia to enhance the tool's AI capabilities, allowing third-party developers to train and validate their AI models using the GI Genius AI Access platform. This technology has the potential to improve patient outcomes and reduce medical variability, while transforming the way healthcare approaches the early detection of colorectal cancer.
In other recent news, Viz.ai secured FDA clearance for its artificial intelligencepowered algorithm designed to detect and triage care for suspected abdominal aortic aneurysms (AAAs). Viz AAA automatically analyzes computed tomography angiography (CTA) scans for suspected AAAs. It alerts clinicians, displaying patient scans on their mobile devices for real-time communication among specialists. Jayme Strauss, chief clinical officer at Viz.ai, thinks Viz AAA will enable care teams to prevent aortic rupture and other catastrophic aortic emergencies by increasing surveillance. Viz.ai plans to continue expanding its healthcare offerings.

Meanwhile, HeartBeam recently secured a pivotal patent related to artificial intelligence capabilities for its AIMIGo system — a personal, portable vector electrocardiogram (VECG) system. HeartBeam is now partnering with Samsung to boost cardiac diagnostics.
It is hard these days to find a digital health or health monitoring company that is not talking about incorporating artificial intelligence into its offerings. AI has the potential to enable more discreet sensor arrays. The result is that doctors and patients can gain insights from the monitoring with minimal hassle. For example, Sensydia, which has undertaken multiple clinical studies, recently completed enrollment in a 225-subject development study for its AI-powered Cardiac Performance System (CPS). The Los Angeles–based company designed the noninvasive CPS device to analyze heart sound and provide physicians with a full cardiac assessment of patients in under 5 minutes. The CPS device may enable earlier detection and improved therapy
guidance for heart failure and pulmonary hypertension patients.
The CPS uses ultra-sensitive biosensors for the rapid, noninvasive measurement of a number of cardiac metrics, such as ejection fraction, cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure. Traditionally, patients need echocardiography and invasive catheterization to obtain these measurements, according to the company.

Sensydia announced in early May that it has raised an additional $8 million from an expanded syndicate of investors. The company plans to submit to the FDA in 2024 for prioritized review as part of the agency's Breakthrough Devices Program. Meanwhile, Boston-based Biofourmis has AI-driven software to collect and analyze patient data in real-time to identify shifts that require proactive interventions. It partnered with Japanese pharma giant Chugai to objectively measure pain in women with endometriosis. Their strategy is to use a biosensor and an AI-based algorithm utilizing Biofourmis' Biovitals.

Advanced AI algorithms hold promise to better enable people to use myoelectric control — involving discreet electric signals from their muscles — to control robotic prosthetics.
For example, Ottobock has its Myo Plus pattern recognition system for trans-radial users. Myo Plus is meant to allow the system to adjust to natural movements versus requiring the user to adapt to the system.



Sensydia's AI-powered Cardiac Performance System (above) uses ultra-sensitive biosensors for the rapid, noninvasive measurement of a number of cardiac metrics. Traditionally, patients need echocardiography and invasive catheterization (top) to obtain these measurements, according to the company. Image courtesy of Sensydia

4. Better visualization during surgery
Activ Surgical announced in January that it completed the first AI-enabled surgery using its ActivSight Intelligent Light product. Designed to provide enhanced visualization and real-time, on-demand surgical insights in the operating room, the ActivSight model attaches to laparoscopic and robotic systems and integrates with standard monitors. The first AI-enabled ActivSight surgery took place on Dec. 22, 2022, at the Ohio State University Wexner Medical Center. Dr. Matthew Kalady performed a laparoscopic left colectomy using the colorectal AI mode within ActivSight. The company says its goal is for every surgical imaging system to deliver intelligent information, reducing surgical complication rates. >>
LEMO and REDEL Push/Pull Connectors encompass a variety of plastic and metal (with EMC shielding) solutions that offer high reliability for patient monitoring and critical care medical and surgical applications.



FEATURES
• Available in IP50, through IP68 versions
• Multiple color options


• Medically sterilizable
• Lightweight
• Reliable
• 360° EMC shielding (metal versions)
Artificial intelligence’s effect on medtech was a question that came up continually during our DeviceTalks Boston show in May.
Here is what some of the top influencers in the industry had to say:
Boston Scientific CEO Mike Mahoney


“I'll give you some practical applications.
… We have manufacturing plants around the world, and we have great quality systems, and we have great quality engineers who inspect everything, and we have a zillion microscopes looking at every little product that we have all over the world.
Our team is leveraging AI capabilities for visualization inspection rather than the human eye constantly doing that with the mistakes that are inherent and scrapping products and so forth. … We're seeing cost productivity and better quality by just leveraging AI in our visualization inspection in our plants. So it's still at its infancy, but if you think of a large company where you're spending over 3%, probably 3.5% of sales and quality and X percent of that's within that visualization area, it's a great practical application that's going create less scrap, safer products. …
“You have variance of doctor skills all over the world. If you have that AI algorithm, just like they have with diagnostic imaging, you can raise the bar in terms of quality and potentially speed programs. You're seeing AI used all over the place. You see it used in our diagnostics business in CRM where we have these two-week Holter monitors. Your heart beats 100,000 times a day and you have thousands of patients that you're monitoring. And you can't hire 8,000 people in Gillette Stadium to read all these EKGs. So you leverage AI algorithms to actually read the EKGs for you, and they flag kind of red, yellow, green as to which ones to look at for the human inspection. I think you'll see it improve quality, as I mentioned. You'll
see it in our training and our marketing capabilities. And I think you're going see some amazing AI capabilities typically around the diagnostic area that’ll be married with our implants.”
BD CEO Tom Polen

“We're doing work with AI both to help us simplify our organization and … using AI to automate internal processes or using AI to optimize our manufacturing systems and cost positions, playing it in those scenarios. But also, we've been using AI for quite some time in product technology. ..
[We have] an AI platform that's really taking off that we have developed in partnership with Microsoft using their AI experts. And this is around looking at behaviors within a hospital of clinicians and how they interact with our devices across different parameters to identify who could be diverting narcotics in the hospital — and help make sure that we get those patients help and prevent harm to patients. … We’ve got a lot of other products with AI in the pipeline, and we see it both making BD stronger as a company in how we operate and also making products better for our customers.”
Dr. Nitin Goyal, orthopedic surgeon and chief science, technology and innovation officer at Zimmer Biomet “In my space, if you come in with knee arthritis, what determines whether you should have surgery? Today it's based on kind of who you see versus a real data driven thing. It’s, ‘Well, I have this much pain.’ Is that enough pain to have surgery? Your doctor thought it was at that time, but maybe if you see someone else they will tell you a different answer. And I'm not saying that that independence shouldn't exist, but I wonder if you could add more data to that story. So maybe even before you come to see >>
“While using one of ActivSight’s intraoperative visual overlays, the dyefree ActivPerfusion Mode, I was able to clearly see key critical structures in the surgical site and tissue perfusion in real-time,” Kalady said in a news release. “With the press of a button, the colorectal AI mode was enabled, removing distractions of background signals from non-bowel tissue and clearly focusing on perfusion to the colon. There was a clear difference in visualization during AI mode.”
Later, study results released from OSU found that that in 17.5% of cases surgeons changed an intraoperative decision based on additional information provided by ActivSight’s advanced visualization.
5. Improved data collection from medical settings
Artificial intelligence could help with surgical data collection. A case in point is Proprio, which is rolling its AI-powered surgical navigation system into operating rooms to collect data. The idea is that the data will ultimately help surgeons improve how they perform procedures. The Seattle-based startup said it has placed its Paradigm visualization and navigation system in several U.S. operating rooms to capture surgical data that will be useful for accelerating the system’s development.
Proprio announced FDA clearance of the Paradigm system in late April.
6. Making better sense of patient data
GE HealthCare last year unveiled its Edison Digital Health Platform, an AIpowered, vendor-agnostic hosting and data aggregation platform. The medtech giant said the aim of the platform is to enable the easy deployment of clinical, workflow, analytics, and AI tools, connecting devices and other data sources into an aggregated clinical data layer. The open and published interfaces on Edison support the integration of healthcare providers and third-party developers' applications into existing workflows. GE HealthCare had plans to integrate and deploy its own apps onto the platform. A company official said the goal was for clinicians to have actionable insights at their fingertips to help better serve their patients.

7. The ability to crack tough scientific problems — and enable countless innovations
DeepMind, which has the same parent company as Google, has an artificial intelligence system called AlphaFold that cracked an extremely difficult scientific problem. It predicted the structure of more than 200 million proteins, which represent almost every known cataloged protein. AlphaFold is capable of making it possible to search for the 3D structure of proteins almost as easily as doing a keyword Google search. It has also made the data freely available to users for any purpose. Applications of the AI system include accelerating the discovery of drugs for neglected diseases, combating antibiotic resistance, studying the nuclear pore complex, developing a novel malaria vaccine, shedding light on genetic variation, gauging the impact of rotavirus on gastroenteritis, and contributing to drug development for cancer and neurological disease.

that provider, they already know that you should be seeing that provider. So maybe you just need to see your [primary care provider], maybe you need to see a physician assistant. Maybe you don't need even to be evaluated by a surgeon, because that's not the level of problem.”
Kevin Bourque, divisional VP of research and development at Abbott “Abbott’s health tech solutions focus on greater access to healthcare where and when people need it via connected platforms. Smaller, smarter devices can reduce the burden on health systems by increasing efficiency, and ultimately AI and machine learning could identify patterns to help predict and reduce serious health issues through earlier intervention.”
Joe Mullings, CEO of The Mullings Group

“If you look at AI as it goes to optimization of current workflow, that's where your low-hanging fruit is. And I think the device companies that are going to be billion-dollar exits are going to be more focused on: ‘What's existing today? How do I optimize it? How do I address the labor issue? How do I make the clinicians job a little easier, drop their cognitive load? And how do I work with what's existing already in the ecosystem?’ Because that's where Medtronic and J&J and Stryker and Edwards will spend billions of dollars in acquisitions that protect the moat they already have — [and] supercharges it.”

Robert Cohen, president of digital, robotics, and enabling technologies at Stryker


“It’s remarkable what some of these small companies have done with ultrasound imaging, MRI, CT scans, and the insights they’ve got with artificial intelligence. Companies — like Stryker, doesn’t matter how you grew, been around for 80 years — if you’re slow, you’re going to lose. You have to look at product development in completely different ways. …
“With AI and with data access, preoperatively we can say, 'OK, you're a 60-year-old male, your BMI is 39, your arthritis has been around for 15 years, this is your pain threshold, we've got a CT scan, this is your bone mass density — oh, and by the way, you have two millimeters of bone worn away because you had an arthritic condition you articulated and guess what: You're also a diabetic. We never had that data on the patient before. We just normalized everybody into the same patient. Now, with AI and access to electronic health records data in ways we never had before, we can determine better what Stryker implant's best for that patient and where should that implant go in that specific patient. Is that patient safe for outpatient surgery or should that patient stay in the hospital one day or two or three days — now there's an economic component to it — and when should that person go back to work? What does success look like for that patient? That's super exciting. I think you'll see AI contributing to those use cases. We're mapping out all the procedures where Stryker equipment participates in the operating room, looking at the continuum of care and all the data elements, capturing all the data elements, and then looking at what we can do for predictive analytics to actually benefit healthcare and provide more information to the surgeon.”
Jeffery Alvarez, chief strategy officer of Moon Surgical
“What I would love to see in the near future is the use of generative AI to shorten the cycle time with the FDA. … What are the right questions to be asking? How do you need to perceive this technology? What are the right comparisons to make? What are the right tests to look at? If we could shorten that cycle time for innovation to get to the market, it would just be so much better. It'll also reduce the overhead to making design changes or evolving products so that we can develop all these unique AI applications faster.”
“With the press of a button, the colorectal AI mode was enabled, removing distractions of background signals from non-bowel tissue and clearly focusing on perfusion to the colon. There was a clear difference in visualization during AI mode.”
3Dprinting is helping more patients than ever before through personalized medical devices, faster and cheaper prototyping and more affordable manufacturing. Recent developments include research into tissue and organ regeneration, lightning-fast responses to supply chain shortages, wearables that improve patient treatment, and major investments by device manufacturers.

Device developers, healthcare providers and 3D printing technology suppliers are collaborating to improve device design, product manufacturing and patient care.

Here are some 3D printing advances that show what the future may hold. Go to wtwh.me/3dprinting2023 for links to more information about these projects, partnerships and innovations.
Breast tissue regeneration
3D printer manufacturer Stratasys and bioink developer CollPlant have launched a collaboration to take tissue and organ bioprinting to an industrial level. Their first initiative is focused on CollPlant’s
regenerative breast implants, which are being developed to regenerate a breast cancer patient’s natural tissue without triggering an immune response.
CollPlant said its bioprinted regenerative tissue formed connective tissue and neovascular networks in a preclinical, largeanimal study that had no adverse events. CollPlants said it plans to follow up this year with another large-animal study using commercial-size implants ahead of human studies and product commercialization.
“The combined, pioneering technologies of both companies will streamline the development and production process so that we have the most efficient means to produce our regenerative breast implants and other potential tissues and organs,” CollPlant CEO Yehiel Tal said.
“We believe that our rhCollagen-based regenerative implant has the potential to overcome the challenges of existing breast procedures that use silicone implants or autologous fat tissue transfer.” >>
A 3D-printed nasopharyngeal swab patented by the University of South Florida helped healthcare providers manage a global shortage of testing swabs in the COVID-19 pandemic.
The team developed the 3D-printed prototype in one week in March 2020 and shared the design for free with organizations, leading to the production of more than 100 million swabs. That act of innovative altruism won the Patents for Humanity prize from the U.S. Patent and Trademark Offi ce.
“This recognition by the U.S. Patent and Trademark Offi ce validates both the tremendous power of academic medicine, especially during a crisis, and the values and commitment these teams have for contributing to the greater good,” said Dr. Charles Lockwood, EVP for USF Health.
Anatomical models
3D printing is allowing device developers and surgeons to build personalized models of organs, bones and other bodily structures. It’s possible to use the models for training, education, pre-operative planning or to test new and improved device prototypes.

MIT engineers are 3D printing individualized heart replicas to test how a particular valve implant will affect the performance of a specific patient’s heart.

“We’re not only printing the heart’s anatomy, but also replicating its mechanics and physiology,” MIT mechanical engineering professor Ellen Roche said. “That’s the part that we get excited about.”
Meanwhile, Stratasys and Ricoh are partnering on 3D-printed, personalized anatomic models for clinical settings. The on-demand models provide specific visualizations of a patient’s anatomy to allow practitioners to plan and practice complex surgeries and improve communication between medical staff, the patient and families.
Researchers in Switzerland are working on a 3D-printable ink that can mineralize to form a high-strength, lightweight bio-composite that mimics the structure and properties of bone.
This “BactoInk” contains bacteria that trigger the production of calcium carbonate (CaCO3) without the need for the high temperatures necessary for manufacturing ceramics.
Researchers published a method for 3D-printing an ink that contains calcium carbonate-producing bacteria. The 3D-printed mineralized bio-composite is unprecedently strong, light, and environmentally friendly, with a range of applications from art to biomedicine.
And last year, Stryker opened a 156,000 ft² facility for 3D printing in Ireland. It’s the latest investment in the field by the world’s largest orthopedics company, which has been working with additive manufacturing technology for more than 20 years. 3D printing metal joint replacements, for example, allows Stryker to make more porous implant surfaces for better bone fixation.
Mighty Oak Medical uses HP Jet Fusion 5200 3D printers to make personalized surgical guides for the placement of pedicle screws in spinal surgeries. The medical device developer uses the same 3D printers to make patientspecific bone models for each surgery.
3D-printed face masks are helping pediatric burn patients heal better with personalized treatments.
The new technology also eliminates a potentially traumatic step for burn patients, who previously needed to endure warm plaster bandages to make masks of their faces.
“3D printing is something we’ve dreamed of for quite a long time,” said Christophe Debat, GM of Romans Ferrari pediatric rehabilitation center in France. “We had imagined that a 3D printer would allow us to produce a mask based on the scanned fi le without ever having to touch the patient.”
The team has treated more than 100 patients using a Formlabs Fuse 1 selective laser sintering (SLS) printer.
University of Hawaii researchers are working on a 3D-printed, wearable sweat sensor for health monitoring.
“3D printing enables an entirely new design mode for wearable sweat sensors by allowing us to create fluidic networks and features with unprecedented complexity,” Department of Mechanical Engineering Assistant Professor Tyler Ray said. “With the ‘sweatainer,’ we are utilizing 3D printing to showcase the vast opportunities this approach enables for accessible, innovative and cost-effective prototyping of advanced wearable sweat devices.”
Formlabs this year launched its Automation Ecosystem for manufacturers that want to expand from a single 3D printer to a scalable fleet.
Formlabs said the new offering can increase productivity and reduce materials costs and packaging waste.
“These solutions will enable companies such as dental labs, service bureaus, and internal job shops to ramp up production without increasing labor requirements, or expensive capital investment, making 3D printing for production more cost-effective,” Formlabs Chief Product Officer Dávid Lakatos said.


First FDA clearance for Stratasys













Stratasys won FDA clearance this year for its TrueDent 3D printing resin for making dentures.



The resin is the company’s first FDAcleared medical device, developed for printing exclusively with the Stratasys J5 DentaJet 3D printer and GrabCAD Print software platform.


“This new solution will be transformative for the dental industry, and we believe it will help our customers signifi cantly reduce the








time and cost of producing dentures and temporaries,” Stratasys Dental VP Ronen Lebi said at the time.

Stratasys and Desktop Metal plan to merge in a $1.8 billion deal by the end of 2023. Both companies serve medical device developers and the broader healthcare market.
The companies estimate they’ll have $1.1 billion in 2025 revenue after the combination. The combined companies will have more than 800 scientists and engineers.
“With attractive positions across complementary product offerings, including aerospace, automotive, consumer products, healthcare and dental, as well as one of the largest and most experienced R&D teams, industryleading go-to-market infrastructure and a robust balance sheet, the combined company will be committed to delivering ongoing innovation while providing







outstanding service to customers,” Stratasys CEO Yoav Zeif said.















































In April, Stratasys completed its acquisition of Covestro’s additive manufacturing materials business, covering approximately 60 additive manufacturing materials and an IP portfolio with hundreds of patents and patents pending. The deal also included R&D facilities and activities, a global development and sales team in Europe, the U.S. and Asia.




“With this acquisition, we’re not just expanding our materials portfolio for our broad array of 3D printing technologies — we’re also paving the way for more new innovations,” Zeif said at the time. “Additionally, our growing team of in-house materials experts will be in a stronger position to collaborate with our materials ecosystem partners. Together, we’ll be able to address more applications faster, pushing the boundaries of what’s possible in additive manufacturing.”






Bidding farewell to DeviceTalks
Boston 2023, we look back at an exceptional two-day medical device conference teeming with insights from over 100 top industry leaders. These experts unfolded many complexities of the medtech industry in more than 35 sessions, walking attendees from the medical device product development continuum through the latest medical innovations and strategies to tackle regulatory challenges, prototyping, manufacturing, product launches and more.
Between the high-profile keynote interviews with Boston Scientific Chair and CEO Mike Mahoney, Johnson & Johnson MedTech Global Head of Robotics R&D Martin Buehler, and Becton Dickinson Chair, CEO and President Tom Polen, plus the much-anticipated panel discussions between some of the industry’s top leaders, attendees left the live event armed with actionable insights, a broadened perspective and a panoramic view of the industry’s future.
Here are some of the most memorable insights from our live event captured by our on-the-ground editors. Go to wtwh.me/dtb2023 for full DeviceTalks Boston coverage.
 MIKE MAHONEY Chair and CEO BOSTON SCIENTIFIC
MIKE MAHONEY Chair and CEO BOSTON SCIENTIFIC

Polen shared some insights into his journey to the top of Becton Dickinson (BD). “I never thought of being CEO until near the end of the process.” He advised people looking to advance their careers: “Just focus on getting things done.”
The Mullings Group VP & Senior Partner Holly Scott said there’s hope despite recent layoffs in the medtech industry: “Hiring follows money. … The No. 1 accelerator for success is people.”

Mahoney told a packed crowd that Boston Scientific has succeeded because it focuses on its people culture first, then on innovation. “You have to be restless to have culture, talent and innovation.”
BD developed one of the first rapid COVID tests in the world. Polen said the company quickly identified three things it wanted to achieve for COVID when the pandemic was brewing: Help people get diagnosed, help people get well, and help people get vaccinated.
Polen also discussed how the pandemic drove BD’s diagnostic division to new heights. “We had to scale up diagnostics from making 9
They said it at DeviceTalks Boston
Medical device industry leaders from Boston Scientific, Abbott, Medtronic, Philips, Stryker and more met in Boston to share lessons learned and their perspectives on industry trends, device design and medtech innovation.
million diagnostic tests a year to 12 million a month.”









Spectrum Plastics VP of Sales for Interventional & Surgical Technologies Paul Melnychuck advised startups seeking a contract manufacturer to “be wary of empty promises. The reality is our industry, the manufacturing side of things is at or near capacity. … It’s better to play the long game.”
Materials are a core challenge for manufacturing, and understanding toxicology and the effect of chemistry on the human body should be considered when designing devices. “Building into biocompatibility is critical,” said PSN Labs VP Matthew Heidecker.









Kathryn Unger, VP of Environmental, Social and Governance (ESG) at Boston Scientific, had advice for companies that want to launch their own ESG function or get some traction.


“The patient is and must be at the center of everything we do. We’re constantly trying to ensure that we have the absolute best patient outcome, from a risk-to-the-




patient perspective, period. That has to be our guiding principle, right? However, that’s not an excuse to not improve the design of our medical devices. … There has to be product stewardship that starts before you get to the manufacturing piece. And that design needs to be circular and consider the full life cycle,” she said, later continuing, “It’s not having to convince investors or having to convince our leadership. It’s our customers pulling and us trying to keep up.”



Truveta Chief Medical Officer Ryan Ahern highlighted the potential of data and AI in improving clinical trials. “Historically, finding the right data, criteria and populations for clinical trials is very time-consuming. We’re trying to build a platform that can accelerate these processes by getting data at your fingertips faster.”
Stryker Endoscopy VP of Global Marketing Caitlin Clark emphasized the importance of data for meaningful AI in medtech. “You can’t reach true AI if you don’t have data coming in.” >>

Osso VR co-founder and CEO Justin Barad thinks there’s a medtech innovation bottleneck when it comes to ensuring health providers are using new technology to produce better results. “How do we get more people to use these technologies, and how do we get them to do it more correctly?”


Boston Scientific SVP and President of Urology Meghan Scanlon addressed innovation in medtech: “You have to create the evidence, the awareness and the buzz over time.” Adding that companies have to do it authentically, Scanlon later said, “Start with the data.”

Sagentia Innovation Medical Managing Partner Duncan Smith spoke on the potential for medtech innovation to contribute to a sustainable future. “Just by good, solid system engineering, you can have a radical impact on sustainability.” As a first step, “you have to choose a goal that’s meaningful for your organization.”
The biggest change in musculoskeletal since COVID is the transition to the ambulatory surgery center, said Zimmer Biomet Chief Science, Technology and Innovation Officer Nitin Goyal. “We’re talking about 45% growth over the next five years.”

FundamentalVR co-founder and CEO Richard Vincent spoke on the benefits of their virtual reality (VR) solution for surgical training. “We’ve seen that VR has been proven to accelerate learning, and haptic VR has been proven to accelerate skill transfer and acquisition.” VR can achieve significant logistic time and cost savings, he said: “It allows us to continue to provide opportunities to learn.”
While pulsed-field ablation is a trending topic in medtech, treating atrial fibrillation (AFib) with gold-standard methods can be a long process. Boston Scientific’s Mahoney thinks AFib treatment could become a 45-minute outpatient procedure within a few years because of continued innovation in interventional surgery. “The interventional space is a great benefit in the long term for any company,” said Mahoney. “We try to disrupt surgery.”






Polen thinks the shift toward more biologics would create more opportunities for BD. His company continues to look for acquisitions in “very specific areas” that complement what it is doing.





Mahoney laid out his strategy for continued growth at Boston Scientific: “We will focus on R&D, M&A and venture bets.”












MedTech Innovator CEO Paul Grand discussed the challenges of raising capital for small companies, “When you’re at a small company, you’re always thinking about your next round of capital. What milestones do you need to achieve to unlock the next round of investors? You always want someone who has a plan.”
Referencing raising capital in the current economic climate, Grand said, “We’re not recession-proof, but we’re resilient. The money is out there. Funds that are only for medtech are looking for great investments.”
“Surgeons knew the dissatisfaction wasn’t due to the design but where the implant went — personalized medicine. The robot was a way to get to that.” Stryker Digital, Robotics, and Enabling Technology President Robert Cohen said on the tipping point for establishing proof and support for surgical robotics, specifically with Stryker’s Mako Total Knee offering. Speaking to Stryker’s experience with Mako, Cohen said the market will follow if you address true clinical needs.
Vicarious Surgical CEO Adam Sachs discussed the untapped potential in surgical robotics, saying there’s only a 3.2% penetration of procedures addressed by legacy robots. “In the U.S., the vast majority of surgeries are done manually. This is an incredible opportunity.”
Johnson & Johnson MedTech Global Head of Robotics R&D Martin Buehler shared why J&J wants to make a difference with its surgical robotics program. “The unmet need in global healthcare is staggering. To address this, we’d have to supply 143 million additional surgeries a year. In short, more care is needed. More surgery is needed. All with fewer doctors. It’s going to be a challenge.”
Speaking to the future of surgical robotics, Buehler said, “As technologists, we want to combine areas, so surgeons become flexible pilots — ‘super surgeons.’ Then surgery gets to a place where surgical outcomes are as safe, predictable and consistent as what people expect from air travel.”
These quotes represent only a fraction of the insights and predictions shared at DeviceTalks Boston 2023. Don’t miss the opportunity to learn from industry leaders, innovators, engineers and hundreds of other medtech insiders at our next live event, DeviceTalks West, in Santa Clara, California Oct. 18-19. Reserve your spot at west.devicetalks.com.











Filmcast PTFE has historically been limited to a maximum diameter of around 0.100”, which restricted the option for larger French size aspiration catheters. Confluent was able to break through that threshold and currently offers PTFE ID sizes from 0.010” to 0.137” and up to 0.155” on a limited basis with lead times of 4 weeks or less. development provides customers with more options for thin-walled, flexible PTFE liners at these larger diameter sizes.



This latest
Compared to Ram Extruded PTFE, Filmcast PTFE can be manufactured to hold thinner wall thicknesses and tighter tolerances. Since Filmcast PTFE is coated over a mandrel, it is extremely well suited for the addition of a thermoplastic strike layer to promote adhesion.
www.confluentmedical.com


sales@confluentmedical.com

Nelipak® is a leading global manufacturer of rigid and flexible packaging solutions for medical device, diagnostic, pharmaceutical drug delivery and other demanding applications. To support the development of innovative sustainable packaging solutions, Nelipak offers in-house design, prototyping, tooling, simulation, validation, laboratory, and other value-added services as well as a line of tray sealing equipment. With 1,400 employees and 11 sites globally, including 6 sites in the Americas (US, Costa Rica, Puerto Rico) and 5 sites in Europe (Ireland, Netherlands, UK), Nelipak is committed to delivering superior quality, service, and customer experience through world-class cleanroom manufacturing; advancing sustainability in packaging development; and applying advanced simulation capabilities to optimize product design.



Ryan Ashdown 216.316.6691 rashdown@wtwhmedia.com
Mary Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Jami Brownlee 224.760.1055 jbrownlee@wtwhmedia.com
Ashley N. Burk 737.615.8452 aburk@wtwhmedia.com
LEADERSHIP TEAM
CEO Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844 @SMMcCafferty
Jim Dempsey 216.387.1916 jdempsey@wtwhmedia.com
Mike Francesconi mfrancesconi@wtwhmedia.com 630.488.9029
Courtney Nagle cseel@wtwhmedia.com 440.523.1685 @wtwh_CSeel Jim Powers jpowers@wtwhmedia.com 312.925.7793 @jpowers_media




Brian Toole 267.290.2386 btoole@wtwhmedia.com
VP of Sales Mike Emich memich@wtwhmedia.com 508.446.1823 @wtwh_memich
EVP Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609 @mmatheson























Why do medical professionals have so much confidence in our devices, sets and kits? It starts with five decades of contract manufacturing. The pharmaceutical industry relies on us to build custom kits with all the devices necessary to dispense and administer your drug. Our product portfolio contains hundreds of standard components plus capabilities including design, regulatory, manufacturing, packaging and sterilization. Think of us as your best, most direct route to market. After all, our devices and capabilities do more than simply meet expectations. They deliver confidence. Always have. Always will.