EDITORIAL STAFF


































EDITORIAL STAFF
EDITORIAL
Executive Editor Chris Newmarker cnewmarker@wtwhmedia.com @newmarker
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Pharma Editor Brian Buntz bbuntz@wtwhmedia.com
Associate Editor Sean Whooley swhooley@wtwhmedia.com @SeanWhooleyWTWH
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
VP Lifesciences Mary Ann Cooke mcooke@wtwhmedia.com 781.710.4659


CREATIVE SERVICES
VP of Creative Services Mark Rook mrook@wtwhmedia.com @wtwh_graphics
Senior Art Director Matthew Claney mclaney@wtwhmedia.com @wtwh_designer
DeviceTalks Tuesdays is a weekly virtual event that brings the insights and energy of our in-person events to your desktop.


Each DeviceTalks Tuesday will kick off with a quick briefing from the editors of MassDevice and Medical Design and Outsourcing. These presentations will give attendees insights on what trends will be moving medtech in the days to come.
Be sure to check back frequently as new live events continue to be added.

Senior Graphic Designer Allison Washko awashko@wtwhmedia.com @wtwh_allison
Graphic Designer Mariel Evans mevans@wtwhmedia.com @wtwh_mariel
AUDIENCE DEVELOPMENT Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com
WEB DEV/DIGITAL OPERATIONS









Web Development Manager B. David Miyares dmiyares@wtwhmedia.com @wtwh_webdave
Digital Media Manager Patrick Curran pcurran@wtwhmedia.com @wtwhseopatrick
Front End Developer Melissa Annand mannand@wtwhmedia.com
Software Engineer David Bozentka dbozentka@wtwhmedia.com
Web Dev./Digital Production Elise Ondak eondak@wtwhmedia.com


PRODUCTION SERVICES

Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com

Customer Service Rep Trinidy Longgood tlonggood@wtwhmedia.com
Digital Production Manager Reggie Hall rhall@wtwhmedia.com
Digital Production Specialist Nicole Johnson njohnson@wtwhmedia.com
Digital Production / Marketing Designer Samantha King sking@wtwhmedia.com


Marketing Graphic Designer Hannah Bragg hbragg@wtwhmedia.com
DIGITAL MARKETING
VP, Digital Marketing Virginia Goulding vgoulding@wtwhmedia.com @wtwh_virginia



Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com @WTWH_Taylor
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com @WTWH_Matt
Webinar Manager Matt Boblett mboblett@wtwhmedia.com
Webinar Coordinator Halle Sibly hkirsh@wtwhmedia.com
Webinar Coordinator Kim Dorsey kdorsey@wtwhmedia.com
EVENTS

Events Manager Jen Osborne josborne@wtwhmedia.com @wtwh_jen
Events Manager Brittany Belko bbelko@wtwhmedia.com
Event Marketing Specialist Olivia Zemanek ozemanek@wtwhmedia.com
VIDEO SERVICES


Videographer Garrett McCafferty gmccafferty@wtwhmedia.com
Videographer Kara Singleton ksingleton@wtwhmedia.com

FINANCE Controller Brian Korsberg bkorsberg@wtwhmedia.com
Accounts Receivable Jamila Milton jmilton@wtwhmedia.com
devicetalks.com



















The AxiForce tubeaxial fan series is ideal for keeping consistent, optimized temperature in control units for automation and other highly-modern technologies. Due to its variable installation, high cooling capacity in the smallest of spaces, and interactive integration into the device logic, it has already proven to be indispensable in modern automation.



































































































































































































































































































































- brushed or bldc motors

- 5 amps per axis

- 16 analog inputs


- 16 on/off drivers
- home and limit in
- live tech support
- made in the USA
The FDA has cleared or approved more than 500 devices enabled by artifi cial intelligence (AI) or machine learning, and the list keeps getting longer.
Medtech AI is helping physicians diagnose, treat and monitor patients like never before. Sometimes the software works as a medical device all on its own. But the real fun starts when device developers pair software with other enabling technologies like robotics, miniaturization, 3D printing and advanced materials.
For example, take Stryker, the world’s largest orthopedics company. Two years ago, Stryker launched a digital, robotics and enabling technologies group focused on cutting-edge technologies. In this edition, group leader Robert Cohen discusses how his team is utilizing pioneering technologies in new ways across the business, including AI, 3D printing, and of course, surgical robotics.
And at Penumbra, Interventional President Sandra Lesenfants says her company’s intelligent algorithms are powering new aspiration catheter designs to clear blood clots more quickly and safely than competing devices. With three smart-sucking products launching this year, Penumbra predicts it will break $1 billion in annual sales for the first time.
Sometimes new devices facilitate new procedures. Apollo Endosurgery’s OverStitch and ESG devices are the first cleared by FDA for endoscopic sleeve gastroplasty, a stomach-shrinking procedure for weight loss that’s performed endoscopically. That caught the attention of Boston Scientific, which is expected to close on its $615 million acquisition of Apollo Endosurgery in the coming months.
Contributions from expert authors in this edition include a primer on electromagnetic navigation sensors, advantages and challenges of onshoring medtech manufacturing operations, the use of styrenics in next-generation medical devices, applications of energy for medical ablation and electroporation, and advice on designing empathy into digital health apps.
 Jim Hammerand | Managing Editor | Medical Design & Outsourcing | jhammerand@wtwhmedia.com |
Jim Hammerand | Managing Editor | Medical Design & Outsourcing | jhammerand@wtwhmedia.com |






Our own Associate Editor Sean Whooley outlines what to expect on the diabetes front in the rest of 2023, including new regulatory clearances, device launches and drug updates. Speaking of drugs, Pharma Editor Brian Buntz reports on gene therapy developer Genascence and its quest to fight osteoarthritis. Local injections of the drug could help patients avoid surgery, rehab and lingering symptoms — and save healthcare systems big bucks.
DeviceTalks Editorial Director Tom Salemi is gearing up for our big show in Boston this May. Make sure to check out his preview of DeviceTalks Boston speakers featuring engineers from Boston Scientific, Abbott and Stryker.

Finally, as we enter our fourth year battling COVID-19 and it remains stubbornly seated as the third leading cause of death, Dr. Philip Adamson — the chief medical officer for Abbott’s heart failure business — shares what we’ve learned about the disease and how medical devices can help.
As we learn more about the mechanisms of the virus and its long-term effects, who knows how enabling technologies like AI can empower device and drug developers to finally beat back COVID and prepare for whatever pandemic comes next.
I hope this edition of Medical Design & Outsourcing leaves you better enabled to design new and improved medical devices. Thanks so much for reading.


Nova Primary addresses the needs of glucose device manufacturers and researchers for an accurate, easy-touse, blood glucose reference analyzer to replace the discontinued YSI STAT PLUS 2300. The Nova Primary glucose reference analyzer state-of-the-art features include:

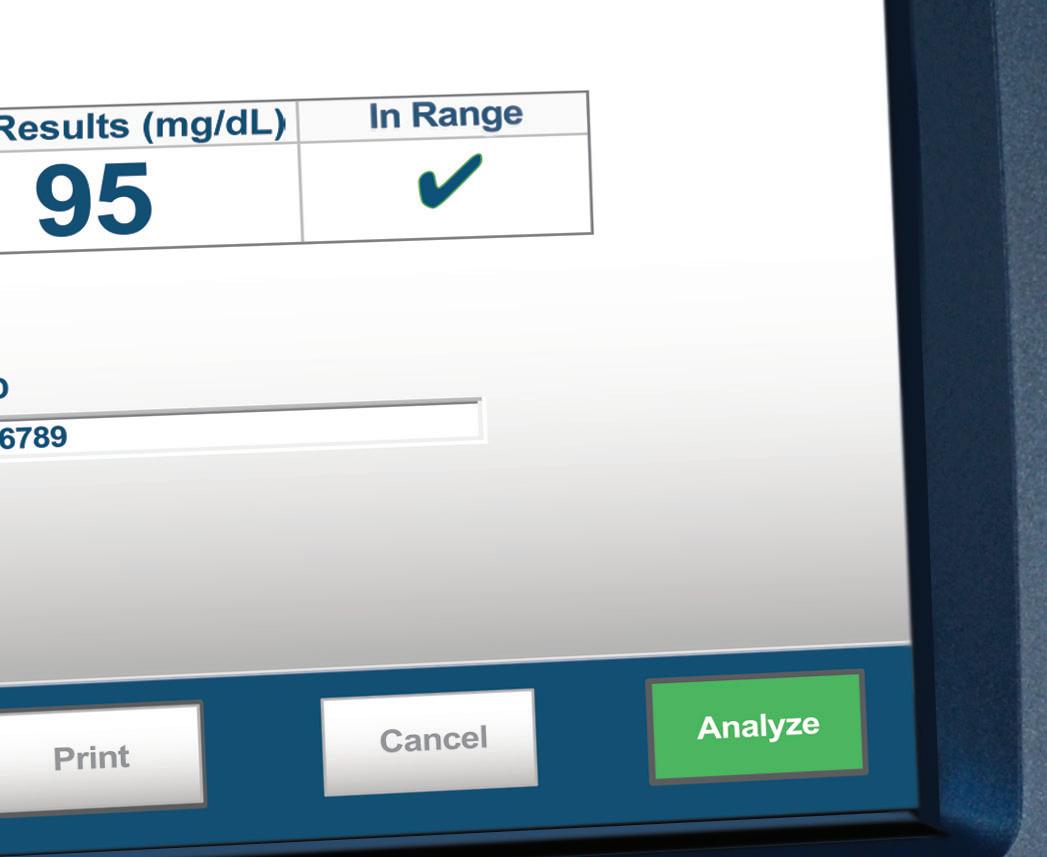

An accurate Nova glucose sensor




25 µL whole blood sample
Automatic hematocrit measurement and correction for plasma equivalent glucose results
Simple, color touchscreen operation




Comprehensive data storage and connectivity
FDA cleared

DAVID BOSCH is an electromagnetic engineering research scientist at Intricon. Focused exclusively on medical technology, Intricon is a contract development and manufacturing partner to industry-leading medical device companies around the world.

















REX CHEKAL is a principal product designer at TXI (formerly Table XI), a product innovation firm based on one big idea in three small words: tech done right. Since 2002, TXI has partnered with Fortune 100 companies, startups in Singapore and Tokyo, industry leaders in London and Los Angeles, and mission-driven nonprofits in its hometown of Chicago.
DANIEL FRIEDRICHS leads development engineering efforts at Minnetronix Medical for commercialization of surgical energy devices including in vivo electroporation systems and other energy-based medical, surgical, and drug-delivery therapies. He is named on more than 35 patents and has worked with an extensive network of clients seeking to commercialize a wide array of technologies.



RALPH TRICOMI is the director of market development for Web Industries, overseeing the market development and marketing activities for the Medical, Aerospace, Personal & Home Care and Industrial business units. He previously held business development positions at Bose Corp. and Ford Motor Co.


ALEXANDER SILVESTRE is the global director of healthcare at INEOS Styrolution. He is responsible for setting strategic and commercial direction and develops and maintains the company’s global policy to establish guidelines related to their products for pharmaceutical applications and use in medical devices.


 TRICOMI SILVESTRE CHEKAL
TRICOMI SILVESTRE CHEKAL



































HERE’S WHAT WE SEE: Incredible enabling technologies CONTRIBUTORS

COMPONENTS: Understanding the design of electromagnetic navigation technology







DRUG DELIVERY: What to expect in diabetes care in 2023

MANUFACTURING: How CMOs can simplify medical device onshoring


MATERIALS:
3 reasons to use styrenics in next-generation medical devices
























ORTHOPEDICS: Stryker on steroids












PHARMA: Genascence believes gene therapy can transform the treatment of knee osteoarthritis





















PRODUCT DESIGN AND DEVELOPMENT: Applications of electrical energy in medicine: RF ablation, pulsedfield ablation and electroporation
SOFTWARE: How to show empathy through digital health apps
TUBING TALKS: What’s so special about Apollo Endosurgery’s stomach-shrinking weight loss tech?




DEVICETALKS:















52
HOW PENUMBRA’S SMART-SUCKING ALGORITHMS AND CATHETERS SPEED UP CLOT REMOVAL

Penumbra developed algorithms and new catheters to remove blood clots from patients faster and minimize blood loss.










WHAT ABBOTT’S LEARNED ABOUT COVID-19 AND CARDIO DEVICES
Dr. Philip Adamson, CMO of Abbott’s heart failure business, expects higher rates of heart and lung failure in the years ahead.






Designing efficient systems involves much more than simply understanding a few basic principles. There is a true art to balancing the specific requirements of an application in order to achieve the desired goals in the best possible way. Help us understand the unique needs of your application and together, we’ll develop something that surpasses what any of us could have done alone.
Contact



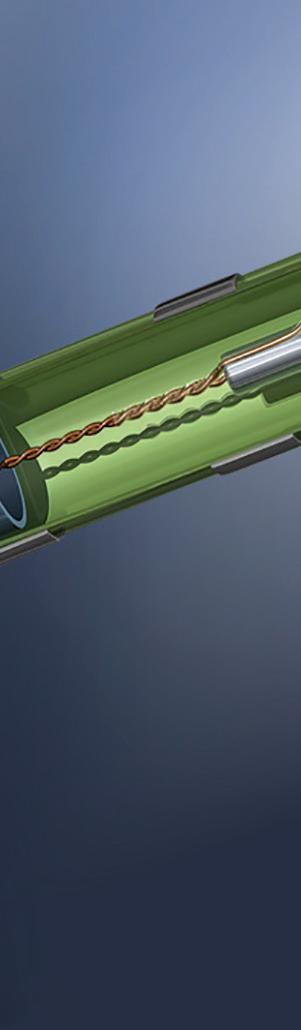


ince its inception in the 1990s to widespread adoption by the late 2000s, electromagnetic navigation (EMN) has emerged as the clear choice for surgical navigation and has been widely adopted in the fields of interventional bronchoscopy, urology, neurosurgery and cardiology.
A properly designed EMN system has several advantages. It can localize with the precision of optical tracking without the need for a line of sight. It offers the convenience of fluoroscopy for intrapatient visualization without the application of ionizing radiation. And it does not expose the patient to energy fields that any more harmful than ultrasound. Unlike alternative navigation technologies that rely upon backscattered radiation, EMN utilizes a passive measurement scheme. The surgically relevant region is saturated in a spatially inhomogeneous magnetic field which effectively serves as an invisible and biosafe x-y-z coordinate grid. Miniature sensors within this effective grid detect and transmit information regarding their precise location, which is then processed by an external computer system.


Since EMN only records the point locations of electromagnetic (EM) sensors, it is often used in conjunction with other visualization systems. Within clinical applications, the location of the sensor (generally placed within an interventional device) is often graphically superimposed upon 3D pre-operational scans of the patient. In this way, it’s possible to realize real-time visualization of the interventional device within the anatomy of a patient.
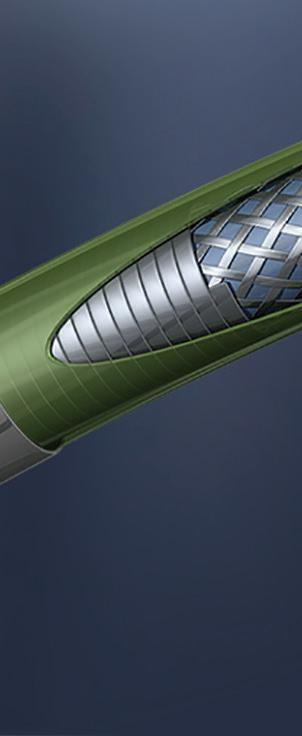
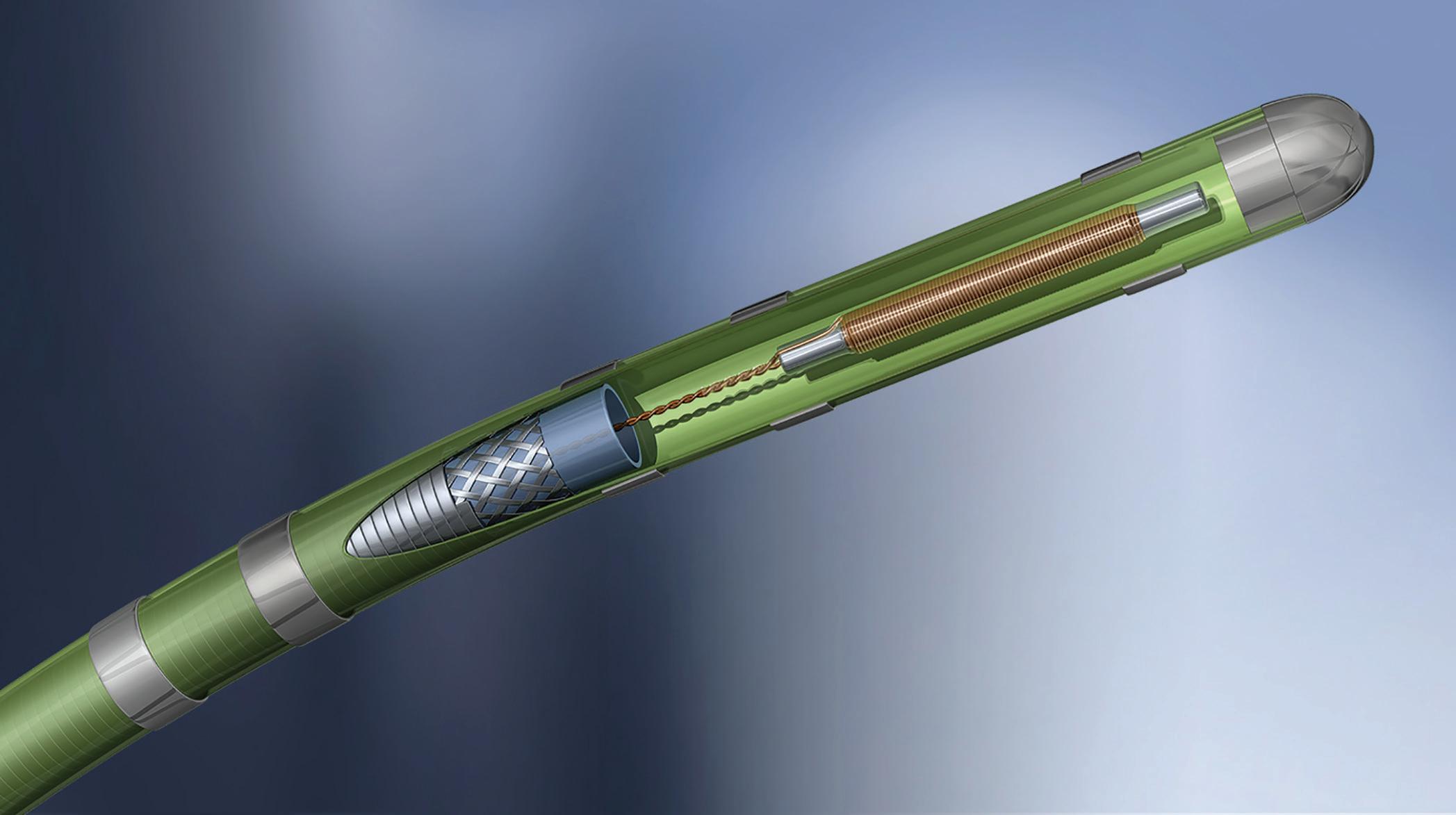


EMN relies on localizing a sensor with respect to a reference magnetic field. The magnetic field is provided by a calibrated field generator, which projects a field that is inhomogeneous in space and of known geometry. The EMN sensor must (indirectly) record the field at the location in which it is placed, which in turn can be translated into positional information. Several kinds of EMN sensors exist — for example, fluxgate and wireless — but wired induction sensors have received the most widespread adoption in interventional clinical applications. (Fluxgate sensors have not been widely adopted into clinical applications due to the size and relative complexity of

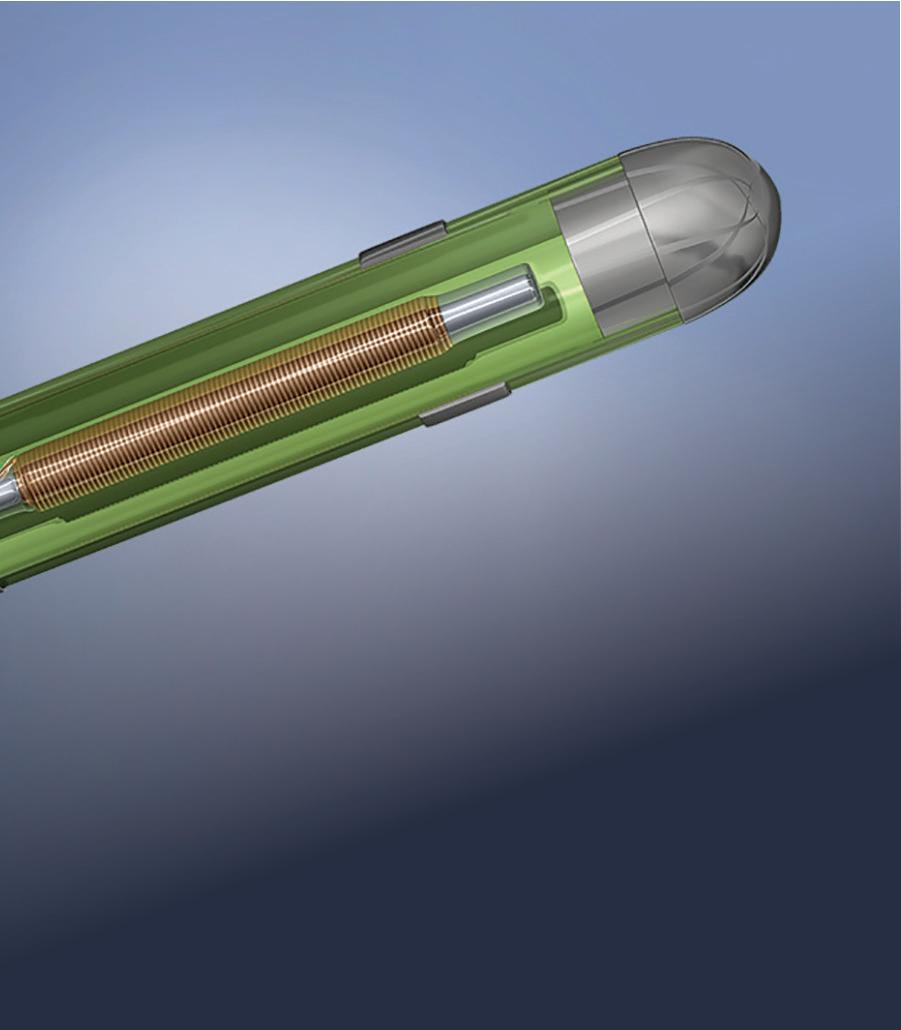
As EMN becomes the top choice for surgical navigation, sensor and design considerations are critical.David Bosch Intricon An example of an embedded electromagnetic (EM) sensor in a catheter tip Image courtesy of Intricon
the device. And wireless sensors utilize sensors that must function simultaneously as receivers and emitters. The bulky size of these sensors, coupled with the size and complexity of the external drive system, limits surgical applications.)
Wired induction sensors consist of one or more windings wound around a solid or hollow core and leaded out to a twisted wire pair for signal transmission. An electrical signal can be induced by virtue of Faraday’s law, which implies that an electromotive force (or voltage) will be generated in any closed path that is subjected to a varying magnetic field. In this case, the closed path is contributed by the wire wrappings of the induction sensor, which also serves as a conduit for current flow.

For the sake of logical continuity and mathematical aesthetics, the full form of Faraday’s law is described as follows:
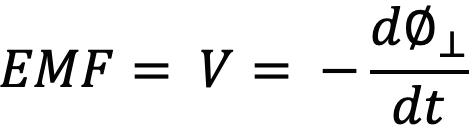
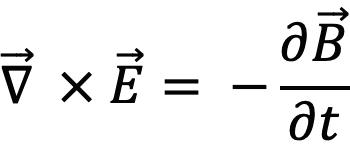
which can be reduced to the following form by Stokes theorem:
Intricon manufactures sensors compatible with commercial AC magnetic field generator systems such as those from Northern Digital Inc. (NDI), Quadrant Scientific, Radwave Technologies, and Polyhemus, as well as custom systems developed by various OEM medical device companies and their designers. These systems provide an AC magnetic field map in space via arranged field coils. Whereas the specific field coil arrangements and localization methods are proprietary, all of the systems share some general principles of operation. Because magnetic field magnitude decays by the cube of the distance from any field coil, it’s possible to use the intensity of the magnetic field (and induced voltage) within a sensor as a proxy for the relative distance between the sensor and any field coil. Different methods exist for full 3D localization, generally involving multiple spatially separated field coils each running at unique frequencies to achieve triangulation. Triaxial field generators utilize at least three mutually orthogonal field coils, whereas planar designs rely on high density of parallel or near-parallel field coils to achieve uniqueness in the reference field. Intricon coils are compatible with either localization scheme and can be customized to perform within custom localization volumes.
applications, it is beneficial to maximize the voltage induced in the coil to maximize the signal-to-noise ratio and increase localization precision. The pertinent term is sensitivity, defined as the voltage amplitude generated by a coil when subjected to a unit AC magnetic field of standard frequency.

Sensitivity increase can be most easily achieved by either increasing the number of winds — which effectively increases the effective cross-sectional area through which the magnetic field traverses — or by directly increasing the diameter of the sensor core. However, there are limitations to these methods. An unrestricted increase of physical sensor dimensions is rarely compatible with the tight working channel of most interventional devices, so alternative methods of boosting coil sensitivity are necessary.
The seamless integration of EM sensors within interventional devices allows for optimal performance. Medical device manufacturers typically need to partner with a design and manufacturing expert specialized in electromagnetic and physical design who can deliver fulllength devices (complete “tip-to-handle” solutions) for desired electromagnetic and clinical performance.


where Ø is the magnetic flux component that is perpendicular to windings of the induction sensor and flux is loosely defined as the product of the magnetic field and cross-sectional area.
The goal of any custom EMN sensor is to provide the ultimate signal integrity within the smallest possible envelope size. For practical EM sensor
The design process must incorporate a keen awareness of magnetic components within the finished device. Such design acumen is critically important to device performance because magnetic components distort the reference field provided by field generators, thus reducing localization precision. The mitigation of magnetic interference is nontrivial, given the number of metallic components that exist in modern interventional devices. While there are numerous advantages of EMN components in interventional devices, it takes careful optimization of EM sensors within a device to achieve maximal performance.
One-size-fi ts-all solutions rarely refl ect the complexities of a particular clinical application, nor do they automatically provide optimal performance within modern interventional devices. Therefore, medical device manufacturers need to carefully select a development and manufacturing partner with experience in both sensor and full-device design who understands the uniqueness of each customer, device and EMN application.
Throughout 2022, we saw a wide range of advancements in diabetes technology. We saw launches for next-generation technologies, exciting clinical trial results, and rumored spinouts and acquisitions.
There’s plenty to look forward to in the diabetes space in the months ahead. Here are a few things worth keeping an eye on.
In October, the Centers for Medicare and Medicaid Services (CMS) published a new local coverage determination (LCD). That LCD modified coverage criteria for continuous glucose monitors (CGMs). The modification includes people with diabetes who receive insulin treatment or have a history of problematic hypoglycemia.
Analysts suggested at the time that the decision was a win for CGM makers, including Abbott and Dexcom.
BTIG analyst Marie Thibault said the decision could benefit an estimated 3 million U.S. basal-only patients.
“We expect potential inclusion in the fi nal determination will catalyze increased CGM adoption in this patient population, benefi ting both [Abbott] and [Dexcom],” she wrote before the decision was fi nalized.
Dexcom is “really excited” by the potential expanded coverage,



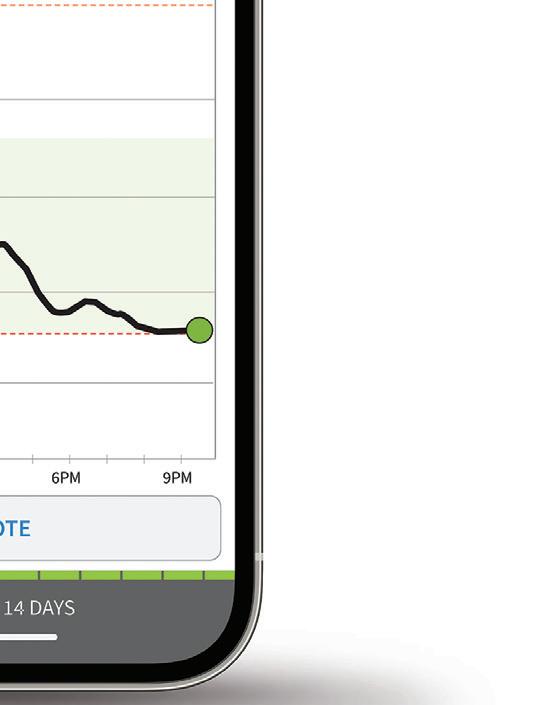
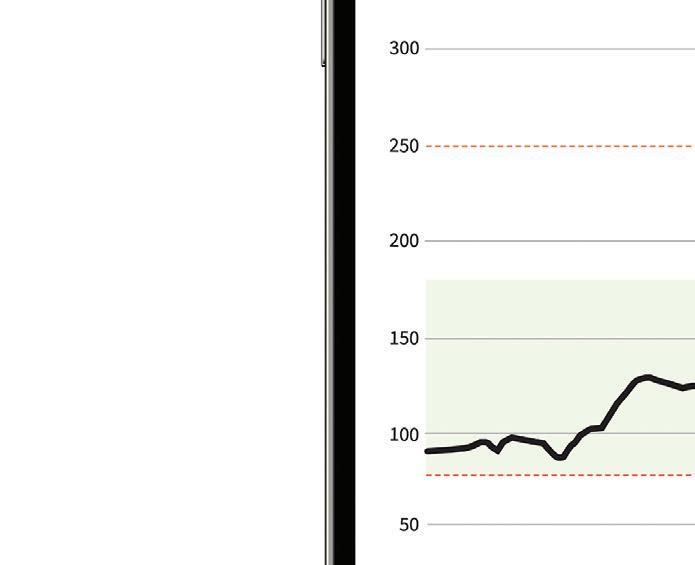
Dexcom CTO Jake Leach said in an interview with Medical Design & Outsourcing sister site Drug Delivery Business News before the decision.

“We see that as just another validation of how CGM can really benefit people with diabetes,” Leach said.
“This is a whole new group of folks that haven’t had access to it before,” he continued. “I think we’re going to really see some great outcomes as that population starts to use CGM more regularly. We’re really excited about G7 servicing that population when it becomes available. ... We’ll be ready.”
On March 2, CMS published the final LCD, confirming the reimbursement win for CGM makers. New, expanded coverage of CGMs under Medicare goes into effect in April.
In 2022, a slew of next-generation technologies cleared major regulatory hurdles. That includes the Dexcom G7, Abbott FreeStyle Libre 3, Insulet Omnipod 5 and the Senseonics Eversense E3.
(continued on page 16)
After a banner year for diabetes technology in 2022, there’s still plenty to look forward to this year.
Sean Whooley Associate EditorPhoto courtesy of Abbott


















(continued from page 14)
Given the general timelines for these technologies, it’s unlikely we’ll see the coming generations this year or even for a few years. That doesn’t mean these companies will stop innovating in the meantime.
Insulet continues to expand its insulin delivery platform, developing a basal-only pod for individuals with type 2 diabetes. The company said in November that it intends to submit for FDA 510(k) clearance “soon.” Expect updates on that timeline in 2023, with potential clearance on the horizon.

Senseonics’ Eversense E3 offers a 180day wear time, and the company already has plans to double that. It said in November that it completed enrollment of a pivotal trial for a 365-day sensor configuration. The company also submitted an IDE for enrollment of a pediatric cohort. While analysts expect a regulatory nod to come


in 2024, we could see data for the 365-day CGM in the interim sometime in 2023. Dexcom CEO Kevin Sayer recently told Drug Delivery Business News that the company has major technology expansion plans. That includes moving beyond diabetes to expand CGM use across a number of areas in health. The company plans to move to monitoring during pregnancy and in the hospital setting, among other spaces. Sayer also said Dexcom wants to extend the life of its sensor. It currently has a 10-day wear time, but Sayer wants to increase that to 15 days.

In 2022, Medtronic CEO Geoff Martha cited uncertainty over approval timing for the company’s next-generation platforms. That includes the MiniMed 780G insulin pump and Guardian 4 CGM sensor. A December 2021 FDA warning letter highlighted inadequacies in specific medical device quality system requirements at its Diabetes business’ Northridge, California, facility. Martha said on the company’s Q3 2022 earnings call that it achieved 90% of the FDA’s action items related to the warning.

 Abbott’s FreeStyle Libre 3 sensor
Abbott’s FreeStyle Libre 3 sensor
In September, the law fi rm of Kessler Topaz Meltzer & Check announced a lawsuit fi led in the U.S. District Court for Minnesota. It claimed securities fraud over how Medtronic disclosed its insulin pump problems.
That same month, Medtronic identifi ed a potential cybersecurity issue through internal testing with its MiniMed 600 series insulin pumps (MiniMed 630G and 670G). Affected devices also include the Guardian Link 3 transmitter, Contour Next Link 2.4 blood glucose monitor and the CareLink USB.
Still, what 2023 holds for Medtronic remains a mystery. Data for the nextgeneration MiniMed 780G with Guardian 4 has been positive. The company also launched a 7-day extended infusion set for insulin delivery in 2022.


Some of the big hitters in diabetes launched new products in 2022. Look for other companies to rise to the challenge in 2023.
In December, Korea-based EOFlow submitted a 510(k) application to the FDA for its EOPatch wearable, disposable insulin pump. Shortly after, EOFlow partnered with Diabeloop to commercialize EOPatch in Europe.



























Tandem Diabetes Care had a busy 2022 that could result in a prosperous 2023. The company dipped into the M&A market, acquiring infusion set developer Capillary Biomedical in July. Just before the end of the year, Tandem made another move, acquiring AMF Medical, maker of the Sigi patch pump for insulin delivery. That deal closed on Jan. 23.


Beta Bionics’ timeline for the iLet bionic pancreas system remains unclear, but the company is preparing for a launch. It recently hired Dr. Steven Russell as its new CMO with an eye on the commercial release of iLet. Watch for further developments from Eli Lilly and Ypsomed on the insulin pump front. Lilly broke off a partnership in pursuit of its own development program for pump technology. However, Ypsomed still aims to find partners for potential U.S. commercialization. It already has an automated insulin delivery system available in Europe through a collaboration with Abbott and CamDiab.
Embecta, the BD diabetes spinoff, also has a product under development. Analysts were hopeful to learn more at the end of the year about the company’s insulin patch pump, but Embecta has remained tight-lipped. More updates may come this year. >>
The Senseonics Eversense E3 CGM received FDA clearance this year for a 180-day duration, doubling its previous wear time.




Image courtesy of Senseonics

Diabetes patients are scrambling to find Ozempic due to its rise in popularity as an off-label weight loss drug. Novo Nordisk’s Ozempic is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus. It first received FDA clearance in 2017. The drug has been on the FDA’s shortage list since August. The shortage will persist barring an increase in production, curtailing of demand or the rise of an Ozempic alternative.

Could Oramed’s oral insulin be that alternative? Not for those with type 2 diabetes — for now, at least. Oramed recently said its Phase 3 trial missed its endpoints, leading the drugmaker to consider discontinuing development of the therapeutic for type 2 patients.
Hope remains for the drug in other diabetes indications. The ORMD-0801 oral insulin capsule is currently under evaluation in a second pivotal phase 3 trial. The company recently inked a deal to commercialize the oral insulin treatment in South Korea. It also picked up a new patent for its oral diabetes treatment in January.
“This is a whole new group of folks that haven’t had access to it before. I think we’re going to really see some great outcomes as that population starts to use CGM more regularly.”
PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development.
We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory.
Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.

• Specialized team of SMEs
• ISO 10993 and ISO 18562 series
• Toxicological Risk Assessment
• FDA Submission Assessment
PSN Labs has the experts to isolate confounding variables and provide clear analysis to guide the commercialization of existing and new medical devices.



Medical device manufacturers currently face unprecedented challenges to production and supply chain continuity. Successful onshoring — or reshoring, as it’s sometimes called — can mitigate these risks.


High on the list are the threats of continued global economic shutdowns due to the COVID pandemic, newly emerging pandemics and other global health crises, and geopolitical conflict and instability around the world.
Manufacturers are also dealing with rising transportation and energy costs,
Medical device manufacturers can benefit from the technical capabilities, adaptability and medicalsector knowledge of experienced contract manufacturing organizations.



labor shortages affecting logistics, manufacturing and services, supply and logistics bottlenecks continuing to plague all sectors of the economy, and ongoing effects of weather and climate instability on transportation, energy production, agriculture and input materials production.
Onshoring strategies involve tapping regional supply sources and relocating essential production tasks closer to a manufacturer’s home base. The process calls for due diligence, planning at the highest management
How CMOs can simplify medical device
Onshoring — also known as reshoring — can help medtech manufacturers reduce their exposure to a host of new and evolving challenges.Photo courtesy of Web Industries
level, and resources that are not always part of the core competence of an original equipment manufacturer (OEM). Expert outside assistance often offers the fastest and least disruptive way to bring production close to home.
For example, contract manufacturing organizations (CMOs) can handle changes and upgrades to tasks ranging from regulatory compliance and materials sourcing to production and shipping. They typically have or can easily acquire the technical resources, manufacturing equipment and space, and fl exible business culture needed to manage a manufacturer’s onshoring initiative.

Some background: Offshore sourcing strategies emphasizing lean manufacturing and low-cost-country sourcing solutions have traditionally taken precedence over risk mitigation.
This approach was feasible when supply chains were predictable and stable. But the strategy removed resilience from supply chains and made them more vulnerable to certain external market forces.
A single adverse event overseas could shut off critical materials or
CMOs offer highly automated medical manufacturing solutions, including reel-to-reel reagent deposition and lamination platforms. Photo courtesy of Web Industries



components, slowing production. In the wake of recent supply problems, medical device manufacturers are increasingly reassessing their supply strategies.
Onshoring empowers manufacturers to balance cost efficiency and disruption risks better. It typically requires the involvement of top management, application engineering, compliance, quality control, logistics and supply chain management. Quality personnel, for example, must check that quality standards are maintained during the transfer and manufacturing transition.
A CMO’s design and manufacturing engineering staff are an additional source of support. Their consultative services concerning designing for manufacturability and scale often reduce the number of components in a medical device manufacturer’s products without affecting performance.
In a globalized world, total onshoring of all supply sources and production tasks is usually impossible. In its place are compromise strategies such as multiregional sourcing, which balances domestic sourcing with sourcing from foreign locations to hedge against disruptive events and ensure continuity of supply and production.
Another example is near-shoring, which involves relocating production to domestic sites and nearby countries with a reputation for political stability. Yet another is a substitution strategy, whereby manufacturing disrupted at
one factory can be quickly channeled to production facilities elsewhere.
The primary onshoring challenges are enabling the transfer of technology from overseas and optimizing automation in new domestic facilities. These include hiring and training technical staff and ramping up the labor force to meet production requirements. CMOs are structured to address these tasks.
Consider the process of onshoring lateral flow immunoassay (LFI) test device production. LFI diagnostic test manufacturers can team with an experienced medical sector CMO to expertly oversee projects from start to finish, including planning, technical transfer, process validation and commercial-scale production. During planning, the CMO will consult with the manufacturer to determine staffing needs and equipment and infrastructure requirements.
In a typical technical transfer scenario, the OEM shifts its chemistries and reagents to the CMO’s biochemistry labs and manufacturing platforms, where technical specifications are developed and finalized. Then, critical machines are installed and undergo validation.
Close collaboration among supply partners is essential to realizing onshoring’s full potential.
Medical manufacturers and CMOs, for example, can conduct joint risk assessments of supplier companies in the newly formed network. This covers risks spotted upstream from the supplier, such as those related to the supplier’s raw material providers. Risk assessments might also include supplier audits, interviews with supply managers and reviews of manufacturing qualifications.
In addition, medical device manufacturers and CMOs can further integrate their enterprise resource planning (ERP) systems to exchange data in real time. This enables sharing of accurate demand and forecasting information between partners, eliminating time-consuming manual data entry. The integration can drive cost efficiencies and help supply partners deal with staffing and material shortages.
When selecting a CMO, look for a proven track record in the medical device sector. Qualifications should include adherence to industry standards, FDA certifications and GMP protocols.
Styrenics and plastics in general have a well-established position as key raw materials within the healthcare industry.




Now, more than ever, in the face of evolving technology and human health, supply-chain reliability, and a clearer focus on sustainability, design engineers are fi nding value in styrenics as foundational building blocks for undertaking projects to refi ne existing applications and/or in the development of next-generation medical devices.

Styrenic materials are ideal for an abundance of diverse medical applications thanks to their physical attributes, ease of processing in a manufacturing setting, and ability to comply with many regulatory guidelines.
For example, acrylonitrile butadiene styrene (ABS) is a dominant resin in some of the most common medical devices, such as inhalers, injection pens, and portable insulindelivery devices. Since these items
are frequently carried outside the home or hospital, they need to be aesthetically pleasing, lightweight and demonstrate excellent chemical resistance, all while meeting a variety of physical properties. ABS is a fantastic fit.
Other examples include polystyrene, often used in labware and home-testing kits. Styrene butadiene copolymer (SBC), styrene acrylonitrile resin (SAN), and other transparent styrenic products can be found in applications ranging from medical packaging and IV components to fluid containers and syringe bodies.
Medical design engineers need to consider the patient end-use, understand the possible interaction with medicinal drugs, and ensure materials are fully vetted for safe use when the potential for contact with drugs or bodily fluids exists. Whether for transparent fluid containers, drip chambers in IV sets, or medical packaging, styrenic materials meet a complex range of requirements and continue to be a material of choice by designers.
Styrenics is a common material used in a diversity of medical applications across the entire healthcare industry, and there are many good reasons why.A mixture of various enhanced and transparent specialty resins Image courtesy of INEOS Styrolution
When it comes to material selection for medical devices, in addition to technical and regulatory specifications, original equipment manufacturers (OEMs) are now finding it necessary to assess supply-chain reliability.
Medical design engineers have expanded their evaluation of materials and are not necessarily limiting themselves to the one or two products they have historically chosen.
In addition to the technical and regulatory concerns, material selection will also include whether a material is available from more than one facility, in multiple regions, and whether it is still a cost-effective solution for patient care. The reliable availability of styrenic materials ensures efficiency in the intricate manufacturing process of medical devices.

Raw material suppliers are increasingly focused on reducing product carbon footprint for themselves and their customers.
As the leading global styrenics supplier, INEOS Styrolution has made a strong commitment to supporting sustainability objectives and a plastics circular economy. Efforts are underway to implement new programs such as the use of bio-attributable feedstocks, developing chemical recycling technologies where PCR materials can be converted back to virgin styrene monomer, and implementation of mechanical recycling programs.
Our parent company, INEOS, has set a global corporate objective to be carbon neutral by 2050 and has engaged with a number of the largest medical OEMs to see how these programs can help drive their own downstream sustainability projects.
Medical-design engineers are bound by a profound matrix of requirements within the medical spectrum, notably the traceability and regulatory needs of critical patient-care applications.
With their immense versatility, established prevalence within healthcare applications and sustainability potential, styrenic materials are helping to innovate the next generation of medical devices into the future.
tryker is tapping health data and artificial intelligence (AI) to improve surgical robotics outcomes.







Robert Cohen, president of Digital, Robotics and Enabling Technologies at Stryker, recently discussed his mission with DeviceTalks Editorial Director Tom Salemi.

Stryker created the Digital, Robotics and Enabling Technologies group in 2021 to “stay laser-focused on the technology,” Cohen said.
“What’s going on in the world of digital, what’s going on in the world of health records, what’s going on in the world of product security? How do we assure there’s no bias in algorithm
development? We’re looking at local regulations as well as managing the R&D groups that are doing the robotics, the software, the navigation units for cranial ENT,” he said.
Stryker is working on ways that data collection and analysis can help surgeons personalize procedures for individual patients to improve outcomes. Stryker can pull health information from government sources, partners and unique data sets off its own equipment. The company wants to then find ways to use that data to better inform orthopedic device design and implantation, delivering software upgrades on its digital systems and portals for surgeons. Using electronic health record data in that way can benefit the entire orthopedics company, not just separate divisions.

“If you got a total hip, if you got a total knee, if you got lower spine, if you got foot and ankle, even if you’ve got shoulder, one of the success criteria is mobility. Another success criteria is readmission, reoperation. Those are all the same, which bridges three different or four different divisions,” Cohen said.
(continued on page 26)

Stryker President of Digital, Robotics and Enabling Technologies
Robert Cohen
on steroids
Robert Cohen explains how enabling technology will supercharge surgical robotics.
Jim Hammerand Managing Editor
































Stryker’s Mako surgical robotics system was designed to provide more predictable outcomes.

(continued from page 24)
“What we try to do is consolidate that so Stryker can innovate and Stryker can accelerate, and we want to do this fast, and we want to be a leader.”
Supercharging the Mako surgical robotics system
Cohen sees big potential for its Mako system in the next five years. The surgical robotics platform was focused on knee procedures when Stryker acquired Mako Surgical in 2013.
“We’re working on a total shoulder program. There are no robots in total shoulders right now. When that gets released, that runway is so large. There are so many different things we could do in shoulders with the existing Mako platform. So that’s a new application to an existing platform that’s super exciting. That will solve clinical issues. Then Mako spine — there’s a lot of different spine robots out there, but there’s nothing like Mako right now,” he said.
Combining the Mako surgical robotics system with Stryker’s imaging and power
instrument systems will let Stryker “stand alone” in the space. Cohen said. He also expects continued implant improvements through 3D printing and other technologies. All of these changes open up possibilities in joint replacements, such as smaller incisions, better placement options, shorter surgeries with less pain and faster recoveries.
Caution with AI
Cohen worries about the dangers of trendy AI products like ChatGPT, which are sometimes offering biased and inaccurate information.
Stryker’s data collection and analysis could give surgeons a uniquely useful source of information to consider while improving outcomes, safety, quality of care and effi ciency.
“If we could look at things like the comorbidities of someone, the characteristics of a patient and further identify them as a unique individual, then we can pool that and have the surgeon compare that to others of like on a large database and get some insights,” he said.
“Or in the operating room — This knee is too loose this way and this way. Before we do any cuts, what’s the planning?
Maybe we can have some automated algorithms that’ll give the surgeon all the mathematical formulas in the background and spit out an optimal plan based on the input provided by the surgeon. That’s as far as we’re going for a period of time.”
(continued on page 29)
“We’re working on a total shoulder program. There are no robots in total shoulders right now. When that gets released, that runway is so large. There are so many different things we could do in shoulders with the existing Mako platform.”Image courtesy of Stryker







Compact KNF micro gas diaphragm pumps offer an ef cient and durable solution for environmental and industrial hygiene monitoring, inkjet printing, medical device and diagnostics, analytical instruments, and more. With ow rates ranging from 0.05 – 24 l/min, vacuum deeper than 29.6 inHg pressure up to 43.5 psig, and linear ow vs. speed, these products are well-suited for portable and handheld applications. Options include various materials, con gurations, and motors to balance required performance, lifetime, controllability, and cost.
Learn more at knf.com/en/us/micro-diaphragm-pumps


(continued from page 26) Move fast or die Stryker’s move-fast strategy includes partnerships with large tech companies, digital companies and entities such as hospital systems and universities that have access to data and talent.


Stryker’s buying technology to hit the market faster than it could by coming up with it internally. In the operating room, for example, Stryker in 2021 acquired Gauss Surgical, which developed technology to monitor blood loss in real time using computer vision and AI. The Gauss group is now part of Cohen’s group to leverage across the entire organization.

“It shows you at Stryker, we’re going to get this stuff and put it on steroids, and we’re going to go quick. … It doesn’t matter how big Stryker is,” he said. “It doesn’t matter how long Stryker has been around. A slow company will fail.”

Listen to the whole conversation with Cohen at wtwh.me/CohenDT.


The Gauss Triton system for monitoring blood loss in operating rooms uses AI and computer vision to analyze used surgical sponges.



Image courtesy of Stryker





















Osteoarthritis (OA), a degenerative joint disease affecting the knee and other joints, affects hundreds of millions of people worldwide.
Genascence is developing a gene therapy known as GNSC-001 for OA of the knee. A potent inhibitor of interleukin-1 (IL-1) signaling, GNSC-001 uses a recombinant adeno-associated virus (AAV) vector with a coding sequence for interleukin-1 receptor antagonist (IL-1Ra).

The Palo Alto, California–based startup believes GNSC-001 could offer durable inhibition of IL-1 after a single injection.


“Gene therapy has been a big passion of mine,” Genascence CEO Tom Chalberg said in an interview. “And osteoarthritis is one of the few areas of medicine that hasn’t enjoyed any benefit of the biologics revolution.”
Traditional knee OA treatment includes non-surgical strategies such as weight management, physical therapy and medications to reduce pain and inflammation. Physicians may treat more advanced knee OA with surgical options such as osteotomy or joint replacement.

“We’re poised to be the first gene therapy in osteoarthritis,” Chalberg said.


Genascence plans to begin a phase 1b study of GNSC-001 involving approximately 50 patients. In May 2021, the company announced that GNSC-001 was safe and well tolerated in a phase 1 single-arm, open-label, dose-escalation clinical trial of the gene therapy involving nine patients with knee OA.
One year later, Genascence announced it raised $10.5 million in Series A financing.

(continued on page 32)

Brian Buntz | Pharma Editor |
The drug candidate shows potential as a disease-modifying treatment for osteoarthritis in preclinical studies.Osteoarthritis affects about 24% of adults in the U.S., or 58.5 million people, according to the CDC. The knee is one of the areas most frequently affected by the condition. Image by SciePro via Adobe Stock
“If successful, Genascence’s novel gene therapy approach could usher in a step-change in how we treat OA and other musculoskeletal diseases, which are among the leading causes of disability in California and around the world.”

(continued from page 30)
‘No larger unmet need’

Part of the company’s vision is to extend the domain of gene therapy beyond rare disease to areas with major unmet need. “And there is no larger unmet need than osteoarthritis,” Chalberg said.

Genascence plans to optimize the potential cost-benefit ratio of GNSC-001 by enabling local delivery.
“We call it ‘focal delivery’ rather than systemic delivery,” Chalberg said. “That is an important approach in major diseases because it has a much lower cost of manufacturing, which makes it possible to bring this to market at a reasonable price.”
According to one estimate, OA costs the healthcare system $140 billion annually.
Although Tylenol, NSAIDs or steroids can help with symptoms, a central challenge in treating OA is that little can be done to reduce its progression. Joint replacement surgery can help many patients, but even in good cases, rehabilitation can take multiple months.
In addition, postoperative swelling and pain can persist for some time after a knee replacement. Approximately 15% of patients who underwent knee replacement surgery have residual moderate to severe pain that can last for up to five years after the procedure, according to an article in the Journal of Orthopedics and Orthopedic Surgery
The potent inflammatory cytokine IL-1 plays a central role in the pathophysiology and development of OA. It destroys cartilage and prevents new cartilage formation. The original
“We’re poised to be the first gene therapy in osteoarthritis.”PHARMA
name for IL-1, catabolin, referenced its catabolic effects. Increasing amounts of IL-1 can amplify OA symptoms.
“Patients with high levels of IL-1 have worse pain, dysfunction and faster disease progression than patients with lower levels,” Chalberg said. “The good news is that you can block IL-1 with relatively modest levels of an inhibitor.”
Inhibiting IL-1 is not a novel goal. Several large double-blind, randomized controlled clinical studies targeting IL-1 have failed.

South Korea–based Kolon TissueGene is exploring a cell and gene therapy for osteoarthritis known as Invossa (TissueGene-C). In early 2022, TrialSpark announced that it acquired global rights to sprifermin, a recombinant form of human fibroblast growth factor 18 under

(CIRM) President and CEO

 Dr. Maria Millan
Dr. Maria Millan

investigation for OA, from Merck KGaA, Darmstadt, Germany.

In 2016, Vericel won FDA approval for MACI, a product based on a patient’s cartilage cells. MACI is the first FDAapproved autologous cellularized scaffold to repair cartilage defects in the knee.
The academic founders of Genascence considered a strategy to deliver IL-1 blockade locally to the joint without the need for frequent intraarticular injections.
“That was basically the inception of GNSC-001, which is a recombinant adeno-associated viral vector that carries IL-1RN, a transgene that expresses Interleukin-1 receptor antagonist (IL-1Ra),” said Annahita Keravala, co-founder and chief scientific officer of Genascence. >>
When IL-1 attaches to its receptor IL-1R1, the IL-1 accessory protein (IL-1RAcP) is activated, triggering pathways causing infl ammation. If IL-1Ra attaches to IL-1R1 instead, it prevents the receptor from forming a complex with IL-1RAcP, blocking the signaling of IL-1 and stopping infl ammatory cascade.
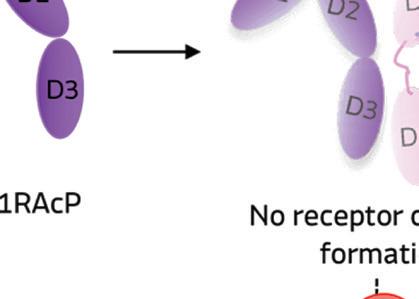
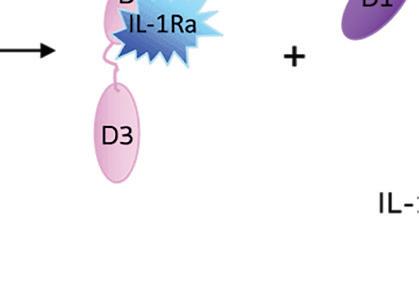
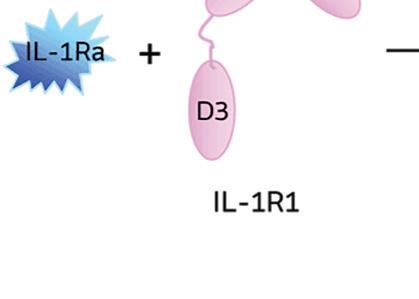
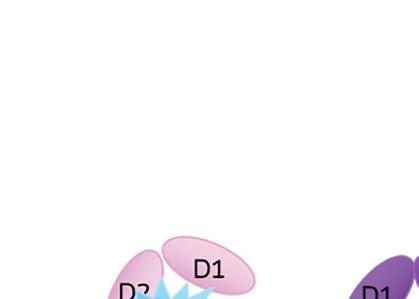
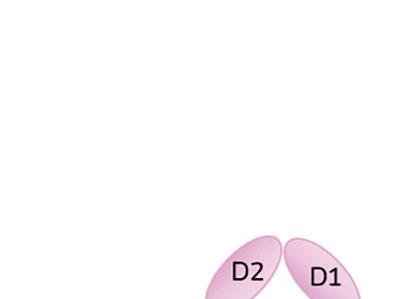

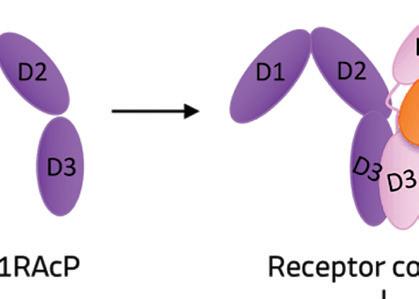
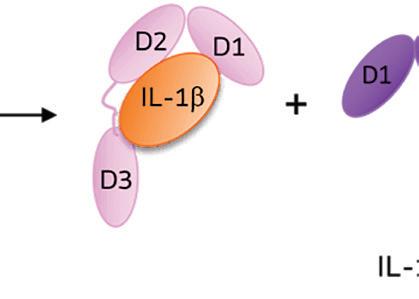
Illustration courtesy of Genascence
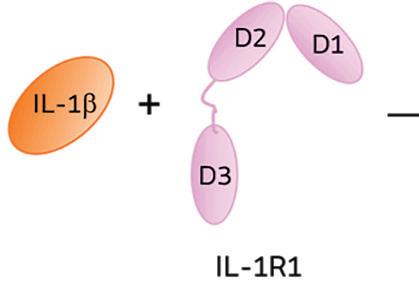
IL-1Ra is a potent inhibitor of IL-1. It binds to the same receptor as IL-1 and prevents the receptor from binding to its accessory protein and forming a reactive receptor.
“So when the receptor complex is not formed, IL-1 is unable to bind to it, thus muting the inflammatory effects of the cytokine,” Keravala said.
A little goes a long way
A small amount of IL-1RA has a potent effect in inhibiting IL-1.
“We believe that GNSC-001 is transformative for OA mainly because it is a single injection intra-articularly performed without any special device,” Keravala said.
The company envisions a physician administering the therapy with a standard 3 ml syringe.
Genascence is confident that a single administration of GNSC-001 could be effective because most gene therapies developed to date
show that a single injection of an AVmediated transgene can lead to stable expression for years.
In its preclinical research on horses, Genascence saw expression for 12 months in most animals — and 18 months in some cases.
Genascence says its AAV serotype not only transduces the synovial sites — the cells that line the joint capsule — but can transduce chondrocytes, the cells that make up the cartilage.
In that acute study in horses, the company saw an improvement in movement and MRI scores at three months. Genascence then initiated a 12-month study where each arm had 11 horses. The study showed long-term expression of IL-1Ra.
“There was some variability, but every horse [in the treatment arm] had levels of IL-1Ra that were higher than baseline,” Keravala said.
The treated animals also showed improvements in movement, MRI scores
and disease-modifying osteoarthritis drug (DMOAD) scores.
In February 2023, the company announced $11.6 million in funding from the California Institute for Regenerative Medicine (CIRM) to back the phase 1b clinical trial and support manufacturing activities.
The phase 1b study will test the safety and pharmacodynamics of GNSC001 at two dose levels and test its effect on knee OA symptoms, biomarkers and disease progression.
“We applaud the Genascence team for tackling one of the most prevalent degenerative diseases,” CIRM President and CEO Dr. Maria Millan said when announcing the four-year award. “If successful, Genascence’s novel gene therapy approach could usher in a stepchange in how we treat OA and other musculoskeletal diseases, which are among the leading causes of disability in California and around the world.”


Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.

• Miniaturization Experts—Micro molded components and assemblies with micro features, tight tolerances, and/or thin walls from 0.001” (25 µ)

• All In-House—Micro 3D Printing, Micro Tooling, Micro Molding, Micro Automated Assembly, and CT Scanning
• Experienced—Over 30 years as a trusted micro solutions provider to the medical and pharma markets





• Risk Mitigation—Quick-turn prototyping, DfM, DfA, and DfX for early detection with over 1,000 successful projects
• Fully Automated—Simple to complex automated assemblies achieving <1 micron positional accuracy
• Materials Expertise—All thermoplastic, long-term implantable, bioresorbable, API, silicone, and fluoropolymer resins


































While modern medicine is awash in high-tech electronics — from large surgical robots to tiny implanted sensors — there are several applications where electrical energy is directly applied to a patient to ablate tissue, convey drugs, or achieve other clinical effects.
Given the obvious concerns with applying electricity to humans, developing these tools safely and effectively is crucial.
This article briefly reviews these applications of electrical energy in medicine. It also looks at new opportunities to refine existing technology such as radiofrequency (RF) ablation, or realize radical improvements using new energy modalities such as pulsed-field ablation (PFA).
Use of energy to ablate diseased or disordered tissue can be affected using thermal means including RF, microwave and ultrasonic sources of heat.
RF is the most mature and prevalent technology, owing to its low cost, procedural simplicity, compatibility with minimally invasive techniques and well-understood performance. In RF ablation, high-frequency electrical current is conducted through an ablation target such as a tumor or nerve, causing heating and eventual cell death. The high frequency allows it to pass through tissue with minimal stimulation of muscle.
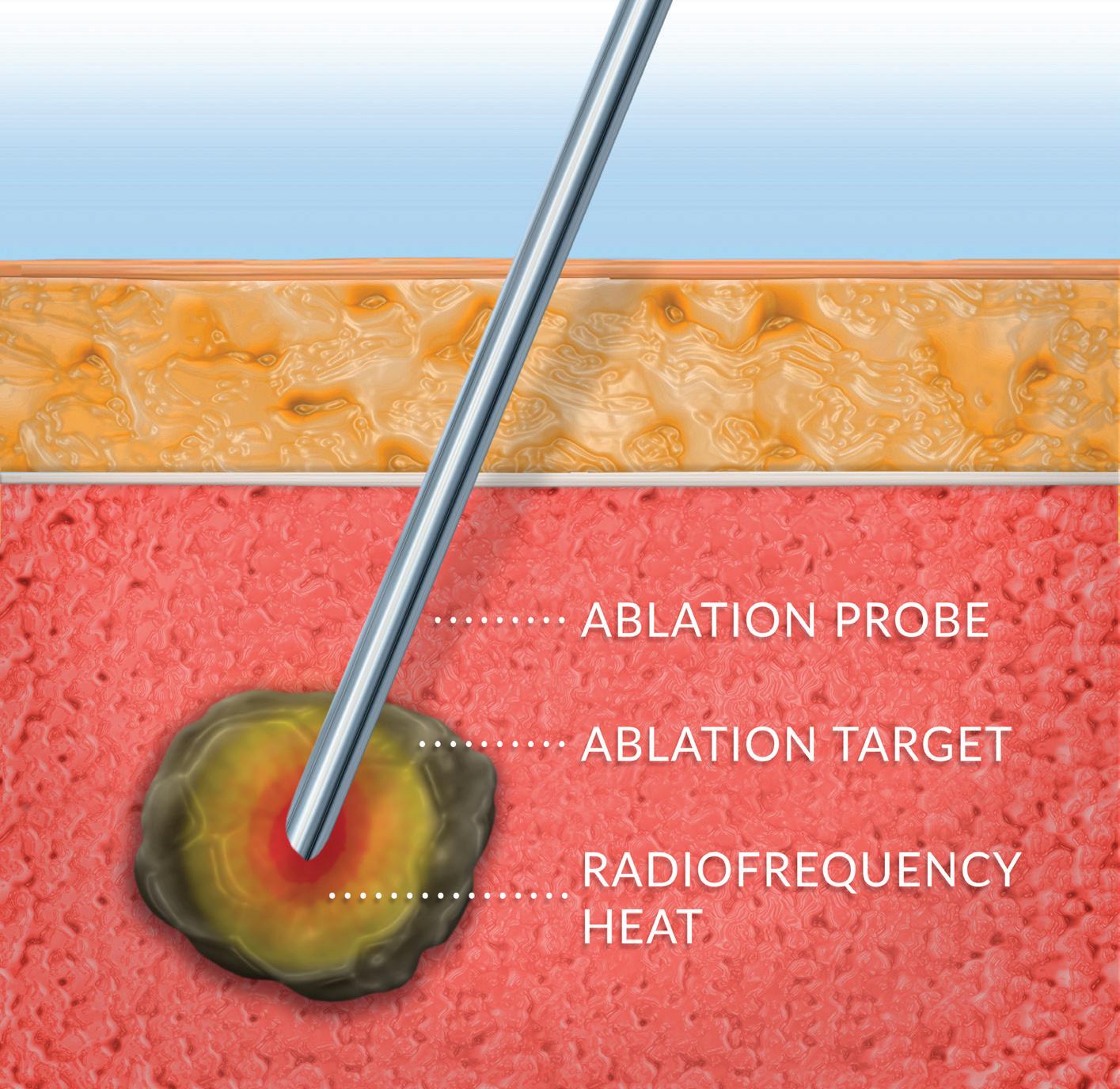
Modern RF ablation systems employ high-precision control systems which optimize ablation volume with minimal procedural time and can be tailored to specific clinical needs.

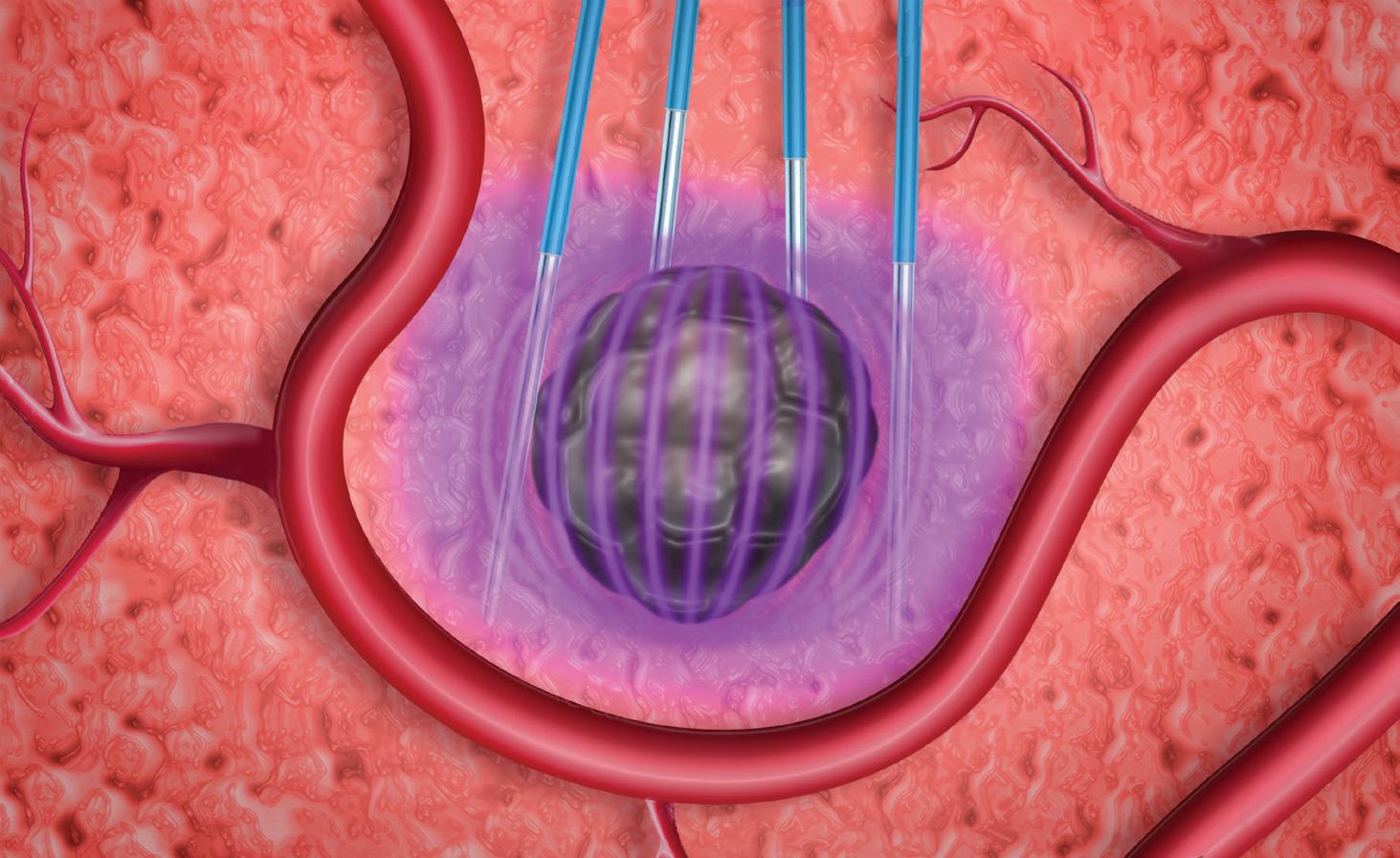
PFA is the use of high-strength electric fields to ablate cells through a nonthermal mechanism. Specifically, PFA degrades cellular membranes in an irreversible process leading to cell death.
(continued on page 38)
Applications of electrical energy in medicine: RF ablation, pulsedfield ablation and electroporation
Learn about new and promising applications of energy-based medical systems and the challenges of developing them.
Learn how our dedicated First Step team and resources, paired with over 100 years of manufacturing expertise, pays off for our customers. When you need to deliver on your NPI/NPD project, First Step enables project teams to truly unleash innovation!
• 100+ machining centers & advanced applications engineers
• Next-level metrology and inspection capabilities
• Sound DFM input, rapid precision prototypes
• The broad capabilities of a vertically integrated manufacturer
Hobson & Motzer are highly skilled engineers, toolmakers, and quality professionals. We are makers of precision metal components with a remarkable depth of knowledge in med device, with a proven track record of adding value to some of the most challenging applications.
(continued from page 36)
PFA differs from RF in that it is tissueselective, non-thermal and conducive to pre-planning.
Tissue-selective: Different tissue types have markedly different susceptibility to the electric fields employed in PFA, and this can permit tissue type-selective ablation. Cardiac electrophysiology applications are benefiting from this selectivity by utilizing PFA to ablate cardiomyocytes (which are highly susceptible to PFA) while avoiding unwanted collateral damage to the nearby smooth muscle of the esophagus (which is poorly susceptible to PFA). This selectivity may potentially provide significant reduction in morbidity for prostate and pancreatic cancer compared to RF or other treatments.
Non-thermal: While thermal ablation causes tissue necrosis and a subsequent inflammatory response, PFA causes cell death through apoptosis, which is the natural mechanism of cellular clearance and is not inflammatory. Patients thus benefit from quicker post-procedure recovery.
Conducive to pre-planning: Thermal ablation is difficult to pre-plan, as thermal properties of tissue — “heat sinks” from nearby blood vessels, “insulators” from nearby structures, and non-homogeneity of the ablation target — vary so significantly as to make pre-planning difficult or intractable. The electric fieldmediated mechanism of PFA, however, is affected by a smaller, less variable set of parameters, allowing for meaningful
pre-planning and control of energy delivery. Future applications of PFA are likely to include a patient-specific energy prescription derived from pre-procedural imaging and therapy simulation.

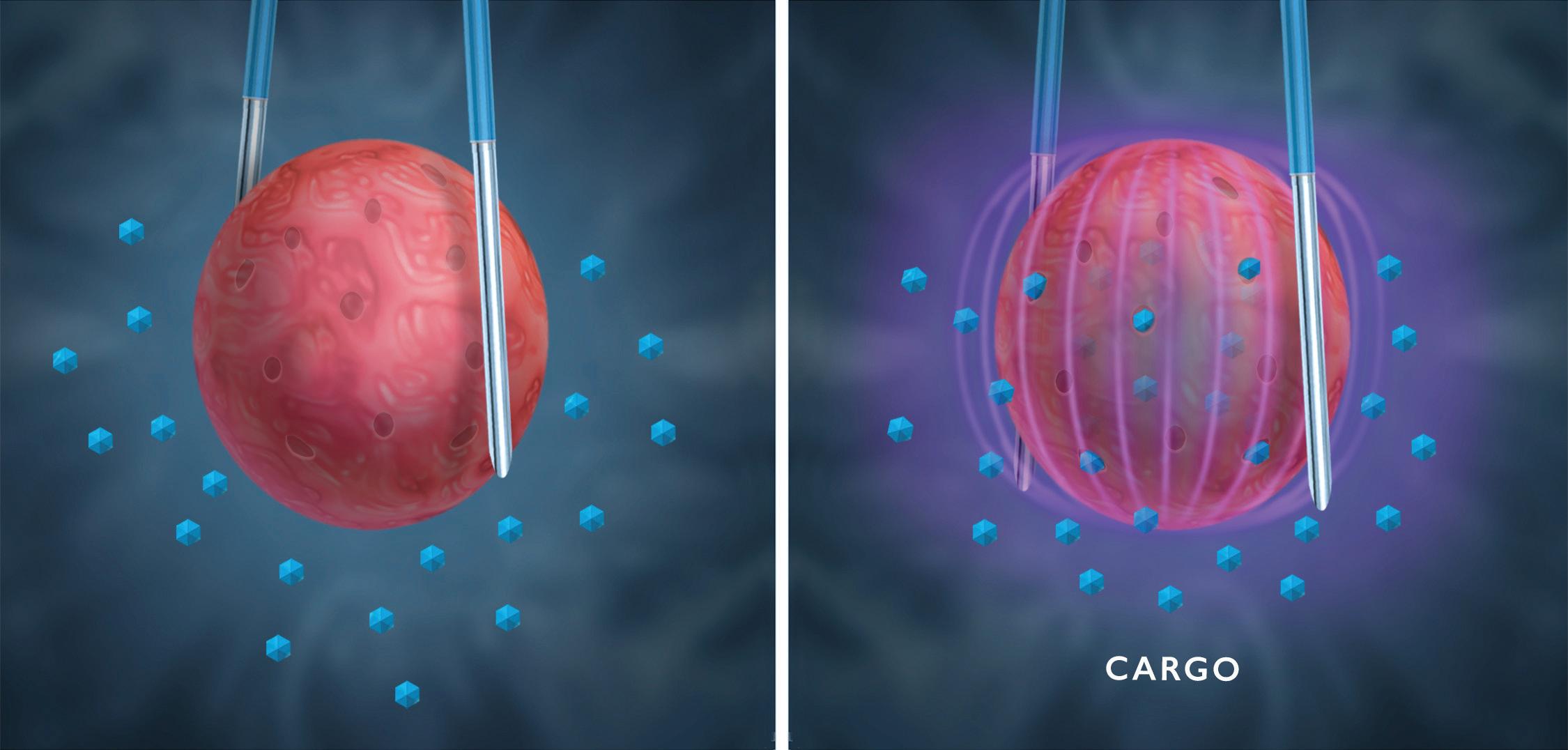
While PFA is an ablation tool, it also has a second act. By reducing the strength of the electric field, those same electric pulses can trigger a transient permeability in cell walls allowing injection of drugs, genes or other “cargo,” a technique variously called electroporation, electropermeabilization, or electrochemotherapy.
By whatever name it goes, this is a macro-scale physical technique for modifying a cellular-scale biological process. Present clinical applications include conveying large-molecule drugs, DNA vaccines and delivery of customized gene therapies.
Use of electroporation to deliver such a drug turns the device into a combination product, triggering higher levels of regulatory concerns and scrutiny. In development of such a system, most important is a clear understanding of the interaction between the tissue and electric field and the boundary between reversible electroporation, irreversible electroporation (pulsed-field ablation) and thermal ablation.
For companies developing energy-based therapies, there are many considerations during the early planning stages:
Pulsed-field ablation also uses electric pulses to trigger transient permeability of a cell to allow for drug delivery. This is a reversible process called electroporation. Illustration courtesy of Minnetronix
Translating experience with RF systems to PFA systems: Despite similar foundational architecture between RF and PFA, achieving the desired clinical outcomes is not trivial and can require many iterations.
That said, experience in RF design provides a solid foundation for developing PFA systems. There are architectural similarities, as well as similarities in measuring clinical outputs that can be adapted. In setting out to develop a PFA system, having resources or partners with extensive RF experience can help limit pitfalls.
Design customization, avoiding platform entanglements: It is tempting to use a platform architecture for the capital hardware and focus development on the disposables or instruments.
An off-the-shelf platform may give you speed to market for the first device, but will hinder expansion and generational improvements. Being tied to a third-party platform can also entangle a developer in licensing agreements and hinders lifecycle planning.
In a marketplace that will likely become as dynamic as any technology space in its early years, it behooves companies to develop their own capital systems for maximum flexibility expanding their portfolio.
Complete product lifecycle integration: It is surprising how many firms continue to plan development of complex systems like PFA programs in a piecemeal fashion. A typical system consists of several primary subsystems, each consisting of complex components and subassemblies.
A program of this magnitude needs to consider not just the immediate concerns of proof of concept to prototype, but how that prototype will translate through the process to commercialization.


In pulsed-field ablation, electronic fields degrade cells to death in an irreversible process without heat.
Illustration courtesy of Minnetronix
Adoption of digital health tech has exploded during the pandemic, but the story is different for digital health apps.
Accenture reports that just 18 percent of patients used a digital health app in 2021, a major drop from previous years. One reason for lagging adoption? A lack of empathy in design. Using a digital health app can be a highly personal experience, and patients want an app that makes them feel cared for. With an empathy-first approach to app development, digital health leaders

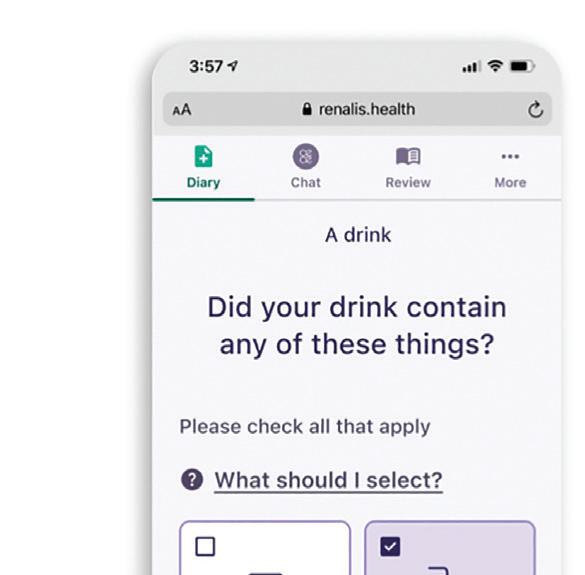
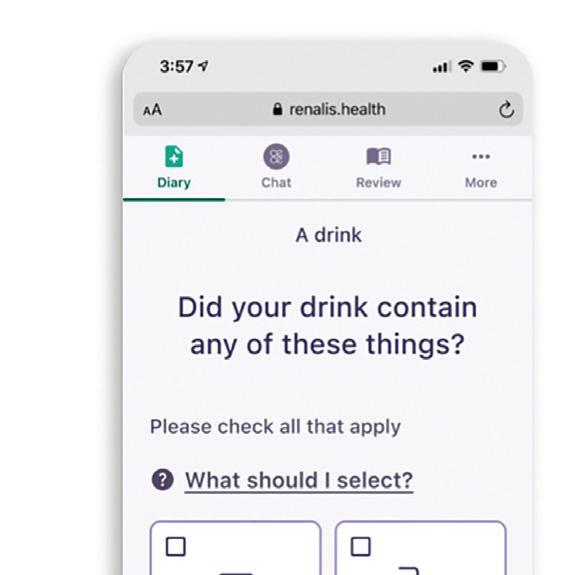

can boost app adoption, which helps patients adhere to treatment protocols and leads to better health outcomes.

Many digital health apps have two user groups: patients and providers. To design with empathy, it’s important to understand each user group’s unique needs.
This app for overactive bladder encourages patients for participating in their treatment. Image courtesy of TXI
Empathetic app design encourages patients to stick to a treatment plan, empowers them to participate in their own care and improves their health.
In many cases, the needs of patients and providers align. But digital health companies can’t assume they know what users want. The result could be a product that’s unsatisfying to use — or worse, an experience that’s emotionally harmful.
Instead of leading with assumptions, it’s important to interview users so you can understand their needs. During these interviews, listen to what users are saying as well as what they aren’t saying.
To illustrate, here’s an example from our own product design. We worked with digital therapeutics company Renalis on a digital health solution for patients with overactive bladder (OAB). When we interviewed patients, many said their condition wasn’t a big deal before sharing story after story about OAB’s impact on their daily life.
These interviews showed us two things. First, OAB is a serious and disruptive condition for these patients. And second, the embarrassment people often feel about their symptoms can cause them to downplay that impact. When designing Renalis’ digital health app, we needed to be sensitive to both these concerns.
When we spoke to providers, we learned they most valued actionable, easy-to-read patient data and apps that inspired adherence.
The takeaway for digital health leaders is that deep user research means reading between the lines so you can fully understand user behavior and emotions.

For many patients, it’s sometimes easier to communicate about their health digitally versus speaking with their provider face to face. But in-app communication can still be an emotional experience, especially for patients with stigmatized health conditions.
To encourage app adoption and treatment adherence, it’s important to ensure digital communication is clear, open, and sensitive. In my experience, tools like in-app chatbots can achieve this.
For example, in our work with Renalis, we found that patients responded well to a chatbot that: >>
Harness
Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.


For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.





















































• Has a name — This helps humanize the chatbot. Renalis named theirs “CeCe.” Users are more likely to feel like they’re communicating with someone they can trust.






























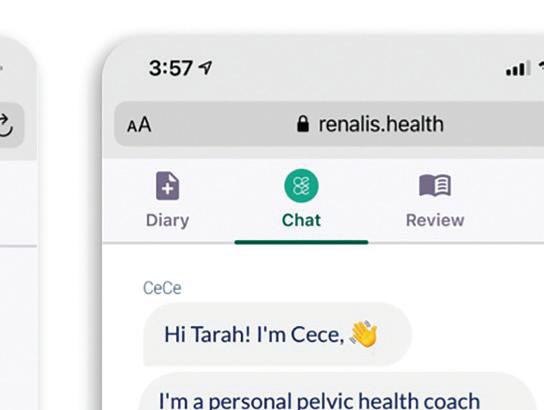
• Exists as a top-level app feature Patients should be able to easily engage with the chatbot without sifting through menus.





• Uses plain language instead of medical jargon — Complex, technical language can be confusing and alienating.







• Uses gender-inclusive language — This makes the app feel more welcoming to women and nonbinary individuals who are often underrepresented in medical research.





Alongside chatbots, digital health apps can also use stories and data to help chip away at the shame and embarrassment patients may have around their conditions.

(continued on page 44)

 Renalis named their digital health app’s chatbot “CeCe.”
Image courtesy of TXI
Renalis named their digital health app’s chatbot “CeCe.”
Image courtesy of TXI

(continued from page 42)
For example, when patients enter a bladder leak in the Renalis app, it offers context such as, “Did you know 35% of users have leaks after similar activities?”
Timing matters a lot. Sharing stories when patients are at their most vulnerable means the stories function as extra support in the form of empathetic communication. What’s more, each story shows users that their experience is more common than they thought, which helps them develop empathy for themselves over time.
A clunky interface can make any app hard to use. But with digital health apps, a poor UI can have serious consequences for users.
For patients, using the app can become an additional stressor on top of existing health challenges, which can hurt adoption and any benefits the app is supposed to offer. For providers, a hardto-navigate app can steal valuable time from their packed workdays.
Poor UIs are often a byproduct of insufficient user testing or untested feature stuffing. The result is a user experience that’s more of a burden than a benefit. To design with empathy, digital health leaders
should prioritize simple app design that’s useful for patients and providers.
On the patient side, the right design often includes features like clean lines, friendly icons, a mixture of images and text, and metrics visualized over time, like pain levels tracked over weeks or months. For providers, it may help to provide customizable, one-sheet charts alongside clearly highlighted correlations and trends.
The most useful design, though, is the one that users tell you works best. Make sure to get user feedback on every mockup and prototype so you can ensure you’re headed in the right direction.
When digital health leaders design with user needs in mind, they can smooth the path to adoption.
Empathetic app design keeps user needs front and center. For patients, the right app can make a tangible difference in treatment outcomes.
A friendly, easy-to-use app can encourage patients to stick to a treatment plan. With consistent app usage, they’ll feel empowered to participate in their own care – and improve their outcomes as a result.
This digital health app for overactive bladder patients makes it easy for them to input metrics used to personalize their treatment.
courtesy of TXI
“Timing matters a lot. Sharing stories when patients are at their most vulnerable means the stories function as extra support in the form of empathetic communication.”
Image


President of the African Ophthalmology Council and the first African to serve as a trustee Board Member of Ophthalmology at the American Academy of Ophthalmology.


Extend your medical business trip to South Africa and unearth the latest medical trends at the World Congress of the International Health Economics Association in Cape Town (8 - 12 July 2023). Learn more about the medical industry in South Africa, while experiencing our flavourful cuisine, breath-taking sights, and wonderful sense of togetherness. Exceptional for medical events. Phenomenal for you. Let’s meet in South Africa.

Arrive Focused. Leave Inspired.


Scan the QR code to learn more


Apollo Endosurgery — set to be purchased by Boston Scientific for $615 million in the coming months — develops new devices and minimally invasive procedures for weight loss.
The procedure is called endoscopic sleeve gastroplasty (ESG), and the device that made it possible is the OverStitch endoscopic suturing system.
Austin, Texas–based Apollo Endosurgery first won 510(k) clearance for OverStitch in 2008, with successive clearances for improved designs over the years. Most recently, in July the FDA granted de novo clearance for Apollo Endosurgery’s Apollo ESG, Apollo ESG Sx, Apollo REVISE and Apollo REVISE Sx systems. They’re the first FDA-authorized devices for ESG and endoscopic bariatric revision procedures.
Medical Design & Outsourcing spoke with Apollo Endosurgery Chief Medical Officer Dr. Christopher “CJ” Gostout and R&D VP Tom Neudeck to learn more about the new devices and procedures.


“We [have] the only intralumenal suture system that does full-thickness running and interrupted stitching,” Neudeck said. “There’s nothing else out there.”
“There is no competition” that provides consistent, full-thickness suture placement, Gostout added. This conversation has been lightly edited for space and clarity.
MDO: What sort of patients is this ESG procedure meant for?
Gostout: It’s a very viable, scalable procedure for weight loss that fits very nicely in the spectrum of opportunities to help guide obese patients throughout their life of weight loss. It fills the gap between standard weight loss efforts with diet and exercise, pharmacotherapy and traditional surgery. Our ESG system can be used within the BMI range of 30-50.
MDO: How does the ESG procedure work?

Gostout: It’s basically looking at the stomach as a relatively flaccid organ and then creating a series of folds, very similar to when you open the drapes in your living room.
(continued on page 48)
“We [have] the only intralumenal suture system that does full-thickness running and interrupted stitching. There’s nothing else out there.”

(continued from page 46)
We’re creating a series of folds in the stomach called plications that concentrically reduce the volume of the stomach. The procedure avoids two areas of the stomach: the very top of the stomach (the fundus) and the very bottom of the stomach (the antrum). The plications are stacked along the length of the stomach, foreshortening it while forming a channel that’s about the size of your thumb running down the length of the stomach, with the exception of the fundus and the antrum. The antrum is avoided because it’s tremendously muscular. In the process of digesting your food, it’s responsible for grinding and emptying food from the stomach. To try to have sutures hold up in that region is really challenging. The fundus of the stomach is avoided because it’s extremely thin. … The procedure works in two ways. The plications reduce the volume of the stomach, which means you can’t eat the same volume per meal that
you had prior to the procedure. The other effect is that the exclusion of the fundus causes a delay in gastric emptying. So now, not only can you not eat that much, but what you do eat takes a long time to eventually exit the stomach. That gives you this prolonged sense of fullness. … It takes an hour-and-a-half to two hours longer to empty the stomach.
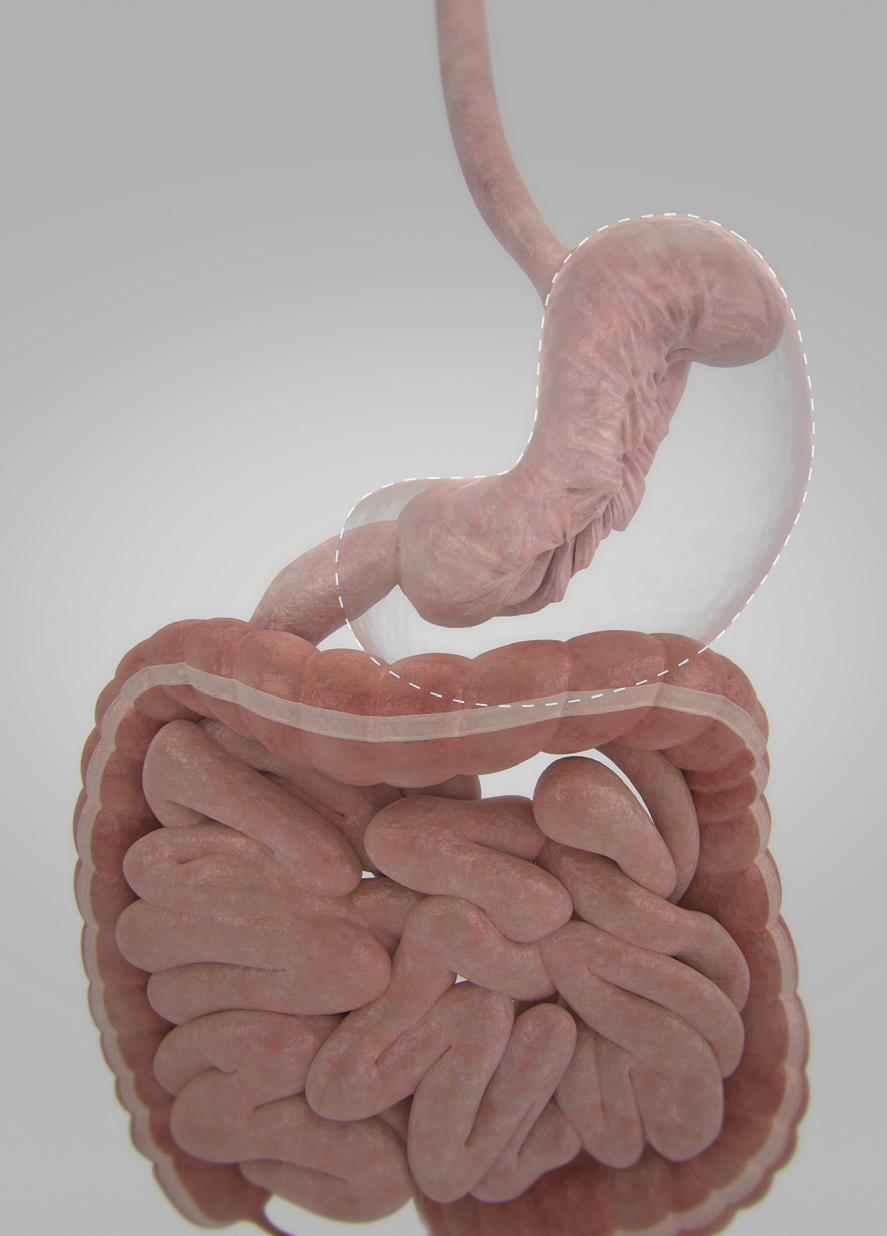
MDO: How much weight do patients lose after ESG?
Gostout: It doesn’t compare to the sleeve gastrectomy by any means. Patients will not lose the same amount of weight. They should be expected to experience total body weight loss ranging from 15% to 18% or more. There will be significant improvement in associated comorbidities such as type 2 diabetes, hypertension, and metabolic syndrome. A patient can get the benefits of a surgical procedure — not as much dramatic weight loss, but significant weight loss — and have improvement in some comorbidities with a safer, lower-risk procedure.
MDO: How did Apollo Endosurgery develop the OverStitch device?
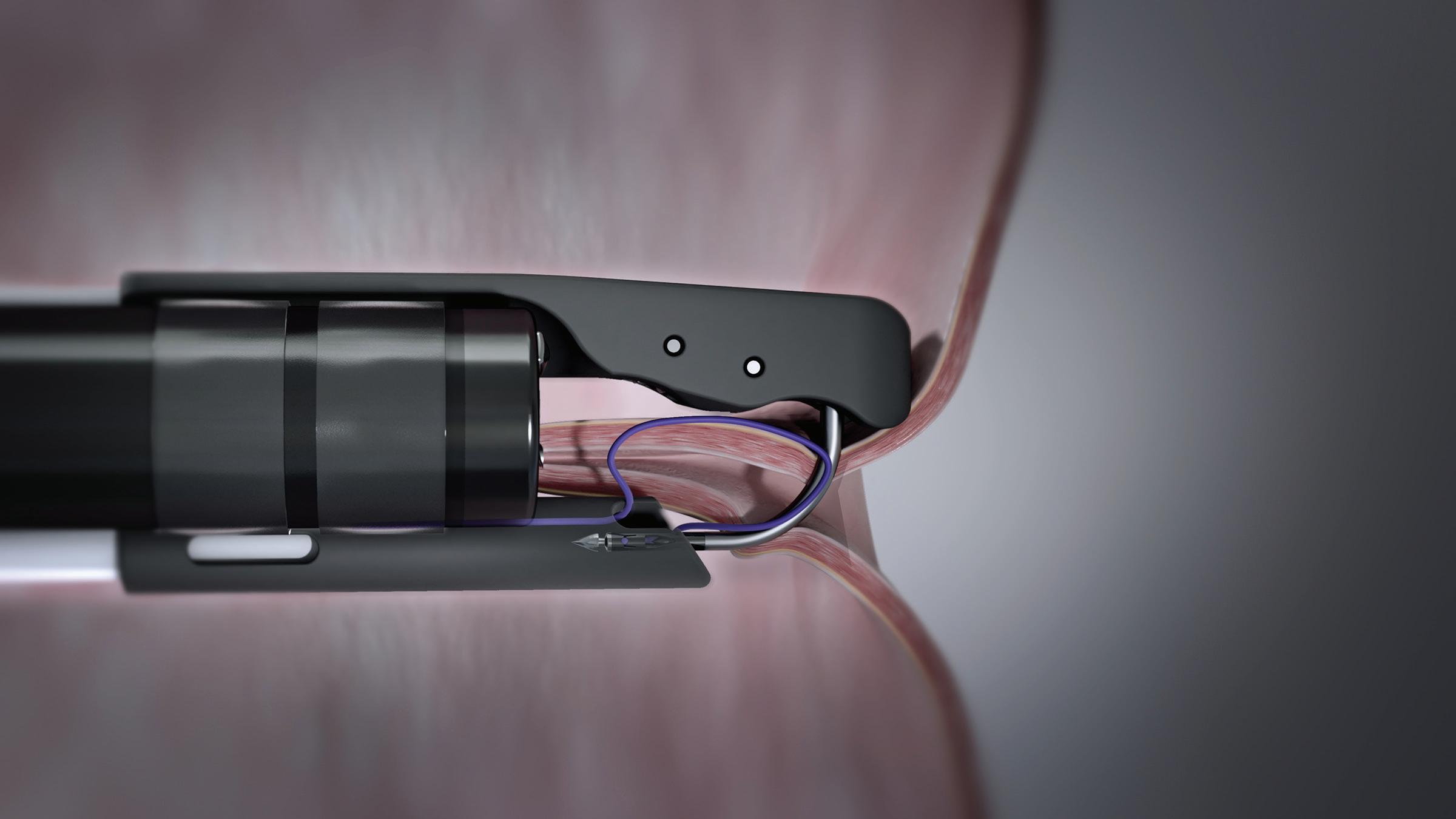
Neudeck: Apollo started as a natural orifice translumenal endoscopic surgery (NOTES) company. NOTES was a novel concept and was the big carrot for people who invested in Apollo. For a NOTES procedure, you needed a series of tools, you needed safe endoscopic access to the peritoneal cavity. Apollo developed a port that was inserted into the stomach endoscopically.
You then made an incision somewhere in the stomach in a favorable area depending on the procedure you want to perform. You then placed the sterile port through the opening and inflated two balloons on either side of the stomach wall. This secured the port and created a water-tight seal to have access to the abdominal cavity without any external incisions. You then needed different endoscopic tools to perform a surgical procedure, an examination or a biopsy. At the end of a NOTES procedure you always have to close the access hole left in the stomach. The intent of the OverStitch suturing device was to be able to perform this closure procedure safely and effectively by applying full-thickness suturing. We started off with a Gen 0 device, a pretty clunky device, mostly machined components with some 3D-printed parts that we tested in explant tissue and animal labs. After more design iterations, this morphed into what we called our Gen 1 device which did full-thickness suturing. We knew we couldn’t commercialize that Gen 1 device. It had too many machined components and was too expensive to manufacture. At that point, all manufacturing was done in-house. Commercially, it wouldn’t have been a viable solution. However, because endolumenal suturing did not exist at the time — we were the first ones in the world to accomplish this — the Gen 1 device enabled us to get some initial clinical data. We built a small amount of devices. I think we built a total of 300 for a small group of advanced gastroenterologists, the people at the top of their profession. They had access to that
Gen 1 device. Being an endoscopic suturing tool, you can do a lot more with it than just closing holes. It was a very advanced technology in the toolbox for endoscopists who always had the desire to use endoscopic access not just for diagnostics but for therapeutic treatments. You want to help the patient after you diagnose the issue, you want to treat rather than writing a report and sending the patient to somebody else for further treatment. People then very quickly developed a number of procedures: fistula closures, oversewing defects and stent fixation. When endolumenal stents get placed in the GI tract — for example, the esophagus — they all have one thing in common. They all have a tendency to migrate, because your GI tract is designed to pass things in one direction. Now GIs had access to a suture tool and were able to stitch stents in place, which greatly reduced the likelihood of dislodgement and migration. Once physicians had access to this novel technology, new therapeutic procedures were developed pretty quickly.
MDO: How did you make the turn toward commercialization?




























Neudeck: We received additional funding at that point and split our resources. One part of the business supported our clinical work with our Gen 1 device. And the majority of the R&D people focused on developing our Gen 2 platform,








taking into consideration the learnings and user feedback from the field. The second generation of Overstitch is the device which is commercially available today. We launched it just over 10 years ago. And the first ESG procedure was done with the Gen 2 device by Dr. Gostout in 2012. >>


It’s good to know where you come from. At Nitto Kohki, we come from a line of engineers and businessmen dedicated to exceptional manufacturing execution. In our line of vacuum and pressure pumps, this is seen in the unique linear-piston design, where one moving part resolves to exceptional reliability, low vibration and noise, high energy efficiency, and long, consistent performance life (>10,000 hrs.) for a host of critical medical applications. It’s why the medical device industry has specied Nitto pumps for over sixty years— the quality in our DNA. Find out how you can use it to your advantage.
MDO: That’s when you outsourced?










Neudeck: Yes, we switched from doing everything in-house to a fully outsourced supply chain. We collaborated with some good component suppliers, and worked with a contract manufacturer to develop a robust assembly process and component inspections and launched Overstitch Gen2. As volumes increased, we made a number of iterations on the manufacturing process to improve scalability. But in essence, the device we are selling today remained unchanged. About four years ago, we launched a new endoscopic suture platform called Sx. This device is scope agnostic — Gen 2 only works with specific endoscopes — and is compatible with most single-channel endoscopes that GIs and surgeons have access to and enables intralumenal full-thickness suturing.
MDO: How important is the OverStich device’s curved needle arm?



Gostout: Traditional suturing employs a curved needle. Every attempted suturing device functions as a sewing machine using straight needles. The Apollo Group wanted a curved needle to add the versatility of being able to place a stitch wherever you want as opposed to having to align tissue into a sewing machine. The OverStitch device needle tip has a T-tag design to the needle which fits into a curved driver arm. That curved arm simulates the ergonomics of hand suturing. That’s the beauty of our device, the curved needle arm.
Neudeck: In the stomach, one risk when placing a full-thickness suture is to inadvertently stitch through a sensitive organ on the outside. There are two ways the Overstitch procedure mitigates this risk. We use a tissue approximation tool called Tissue Helix with our suture system to pull the tissue into the suture aperture of the device before you place your stitch. It’s a corkscrew-type device that gets drilled into the tissue where you want to place your stitch. By pulling tissue towards the device instead of pushing the system against the stomach, you effectively mitigating the risk of accidentally suturing into an outside structure. Once you have the tissue pulled into the device, the curved needle ensures that your needle trajection is away from outside organs as opposite from a straight needle. With the combination of curved needle and Tissue Helix, we have never had any issues with stitching into the spleen or other sensitive organ.

Free more shelving space with Interpower’s blanket and scheduled ordering for North American 5-15, 5-20, 6-15, and 6-20 hospital-grade cord sets, and international hospital-grade cord sets. Scheduled orders allow customers to set predetermined schedules, e.g., order 1,000 and have 250 delivered quarterly, monthly—whenever you need them. Blanket orders allow customers to lock in a price and have their cord sets shipped when you need them throughout the year.
Our North American and international hospitalgrade cords never leave our facility without thorough examinations—they are rigorously tested to surpass UL
817 (18.2.4.1) and C22.2 No. 21-14 requirements for hospital-grade power cords and cord sets—and VDE requirements. “We test more than the standards require,” Interpower Product Development Manager Ron Barnett said. “We do so because it lends better reliability to our design.”

• World-class customer service 7 a.m. to 6 p.m. CST
• In-stock cords and components ship same day


• No minimum orders
• Value-added services such labeling, packaging, lengths and colors
Penumbra developed algorithms and new catheters to remove blood clots from patients faster and minimize blood loss.
JIM HAMMERAND MANAGING EDITORBlood loss is a big problem when using aspiration catheters to remove blood clots from patients’ veins and arteries.

“You suck out the clot, you also suck out the blood,” said Sandra Lesenfants, president of the interventional business at Penumbra.
Algorithms developed by Penumbra’s software team for the company’s continuous aspiration catheters are a key part of the solution, she said in an interview with Medical Design & Outsourcing.



Penumbra’s newest product, the Lightning Flash mechanical thrombectomy system, uses the company’s Lightning intelligent aspiration technology and adds a new dual clot detection feature.





“There is nothing equivalent on the








market,” Lesenfants said. “The power of aspiration and therefore the speed of aspiration is the way we can quickly and completely now remove the clots in a very short timeframe.”


And there’s more in the pipeline as Alameda, California-based Penumbra aims to break $1 billion in annual revenue for the first time this year. The company plans to launch two other computer-aided aspiration products in 2023: the Lightning Bolt system for arterial clots in March and the Thunderbolt system for ischemic stroke in the second half of the year.
Penumbra’s Lightning Flash Penumbra designed the Lightning Flash mechanical thrombectomy system to remove venous and pulmonary clots faster and more safely than previous devices.
Cleared by the FDA in late 2022, Lightning Flash builds on Penumbra’s previously launched computer-aided Lightning intelligent aspiration technology for differentiating between clot and blood. The latest version now has two algorithms — one for pressure and one for flow — for improved clot removal speed while still mitigating blood loss.








Penumbra’s proprietary software is embedded in the catheter and paired with dual pressure sensors to detect whether the catheter is sucking up clot as desired or whether it’s draining blood from the patient. The software controls a valve in the tubing for constant or intermittent suction.

“We measure the pressure and based on the pressure difference we can tell whether we are in clot or in blood,” Lesenfants said. “We have only one valve, which opens and closes something like 12 times in a second. It’s so rapid, it’s almost real-time. So we know immediately when we’re in clot, we know immediately when we are in blood, and therefore when we’re in clot, you can open [the valve].”
The system maintains constant suction when it senses the catheter is ingesting clot and automatically switches to intermittent aspiration when sensing open blood fl ow to minimize blood loss. >>










“It’s so rapid, it’s almost real-time. So we know immediately when we’re in clot, we know immediately when we are in blood, and therefore when we’re in clot, you can open [the valve].”Penumbra’s Lightning Flash system includes a catheter kit that attaches to the Penumbra Engine pump. Image courtesy of Penumbra
also uses the intelligent aspiration software
high-power aspiration catheters called Cat 7 and Cat 12 that were launched in 2021. The laser-cut, reinforced hypotube catheters have a soft, atraumatic tip to minimize damage to blood vessels.
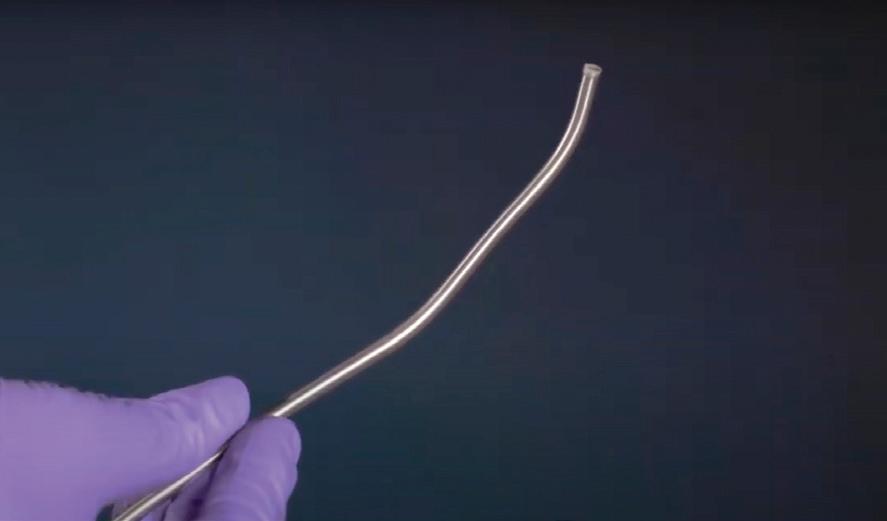
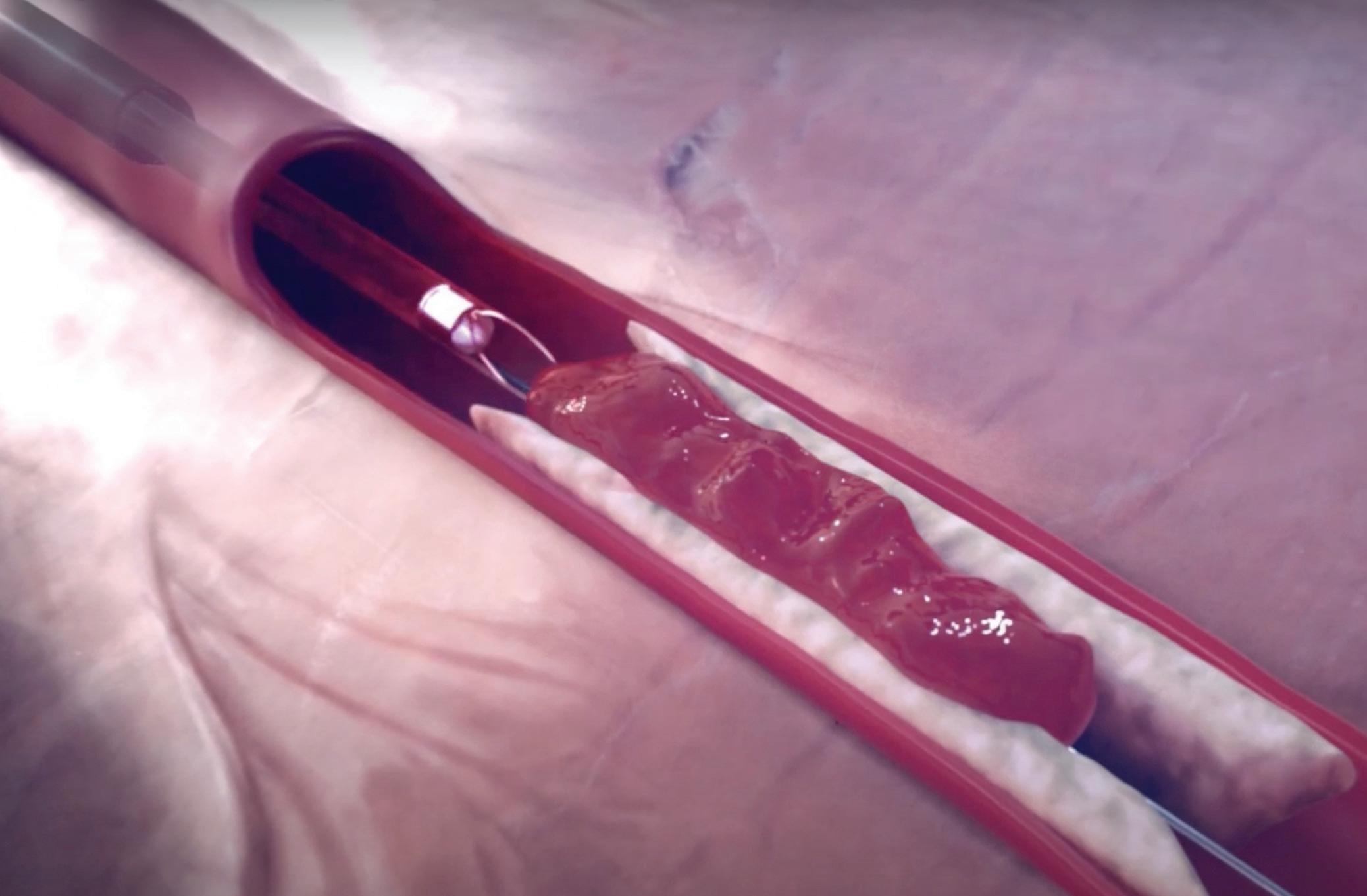
“We love the aspiration profile because it is the most atraumatic, and you want to preserve the vessel and structure. That’s very important,” Lesenfants said.
The main challenges when were how to measure the to maximize clot extraction
going to drive a new paradigm because we can take out more clot, we suck out less vein thrombosis patient in six minutes.
While the size of that clot might be an outlier, the removal time isn’t, with average Lightning Flash clot removal
taking about seven minutes, she said.
“When you design or innovate a new technology, you put all these assumptions together, you see it working on the model, you have animal data, but it’s always hard to translate it to a clinical use and what it’s going to look like in patients. … The power of aspiration here, the speed, the ease of use, the safety profile and all these things are well beyond our expectations.”
Lightning Bolt and Thunderbolt
Penumbra designed Lightning Flash for veins and pulmonary arteries, while Lightning Bolt is meant for the unique properties of arterial clots. Clots on the arterial side are usually more fibrous and more difficult to ingest for removal.
disrupt the friction between the clot and Penumbra expects the Lightning Bolt to win FDA clearance and launch
Penumbra’s Thunderbolt mechanical trial
Once the system senses clot engagement, by late March. That’s right around when thrombectomy system trial for strokes completion date, with the study to be
Like Lightning Bolt, the Thunderbolt system also uses the modulation algorithm to disrupt friction between the clot and catheter tip. The system uses Penumbra’s RED reperfusion catheters.
Penumbra’s high-performance
Penumbra’s not relying on software alone. Its engineers designed low-profile,





































The company’s MaxID (maximum inner diameter) technology gives the catheters a low profile to improve safety as well as trackability and torqueability, while offering an inner diameter size (or lumen) similar to large-bore catheters.

Penumbra aimed to minimize the thickness of the catheter, delivering Cat 7 with an inner lumen of 2.1 mm and a 7 FR (2.33 mm) outer diameter. For comparison, the predecessor Cat 8 has an inner lumen of 2.2 mm and an 8 FR (2.7 mm) outer diameter.




















































Cat 7 also has what Penumbra said is a uniquely angled catheter tip for a 28.3 mm circumferential sweep to help remove clots stuck to the vessel wall.

The Lightning Bolt system uses a Cat 7 catheter. The Lightning Flash system uses a larger 16 FR catheter, but it is similarly a laser-cut hypotube with an atraumatic, angled tip and MaxID technology to improve aspiration and navigation.
The Lightning Flash catheter’s flexible, low-profile design improves how well it can travel from one leg to the other in a deep vein thrombosis patient, or across the heart to treat pulmonary embolisms.
“The problem that we see with some of the current technologies is that these catheters are so big and so stiff, so rigid … so we designed a brand-new catheter that can track extremely well, cross the right side of the heart, go into the pulmonary artery,” Lesenfants said. “It’s so much easier, it’s softer, it’s more trackable.”
TOP: Penumbra designed its catheters to balance strength, flexibility and size.

BOTTOM: Penumbra makes mechanical thrombectomy systems that use sensors and algorithms to detect whether the aspiration catheter is sucking up a clot or blood.

Image courtesy of Penumbra
LEMO and REDEL Push/Pull Connectors encompass a variety of plastic and metal (with EMC shielding) solutions that offer high reliability for patient monitoring and critical care medical and surgical applications. FEATURES



•
•
•
•
•















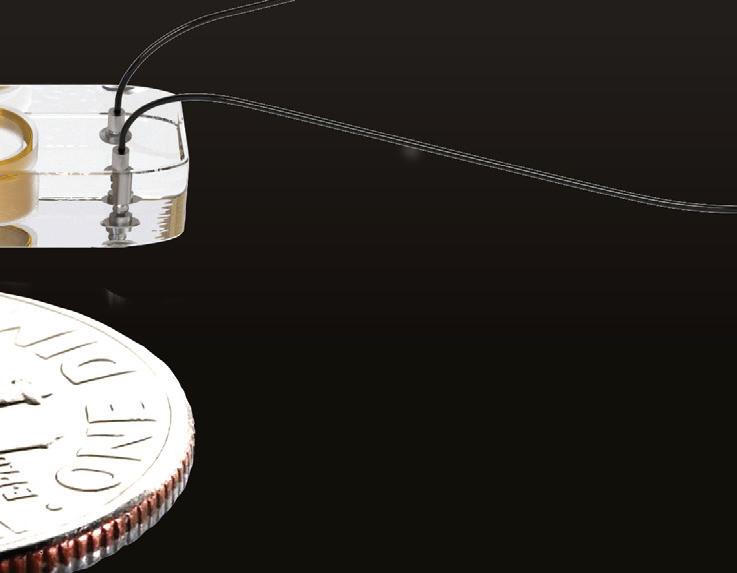


 JIM HAMMERAND MANAGING EDITOR
JIM HAMMERAND MANAGING EDITOR
Three years into the COVID-19 pandemic, we know more than ever about the SARS-CoV-2 virus and how it ravages the human body. What remains to be seen is how the virus — and our immune system’s response to it — will affect our health long after infection, even in mild cases. This once-in-a-century pandemic has already killed millions across the globe and could leave hundreds of millions more with chronic conditions.
“Not only is the viral infection bad for some people, but the body’s subsequent reaction to the viral illness in many people is remarkable. I personally have never seen anything like it,” said Dr. Philip Adamson, chief medical officer of Abbott’s heart failure business.
“I’ve lived through and trained through the AIDS epidemic and learned a lot about viral pathophysiology, but what this does in certain people is amazing. … Every organ is susceptible.”






and Dr. Philip Adamson, CMO of Abbo ’s heart failure business, expects higher rates of heart and lung failure in the years ahead.Abbott’s CardioMEMS implantable heart monitor with a dime for scale. Photo courtesy of Abbott
In interviews with Medical Design & Outsourcing over the past year, Adamson discussed what we know about COVID’s potential long-term cardiovascular impacts, the medical devices and components likely to be in greater demand in the decades ahead, and how medtech engineers should think about design in our new reality.
The following conversation has been lightly edited for space and clarity.
MDO: What are you seeing with COVID?
Adamson: What we call myocarditis associated with COVID-19 severe disease is somewhat rare, but the incidental damage due to the inflammatory response is pretty common. Patients come in many times with elevated levels of troponin — a marker of heart damage — but it’s not in a pattern that would reflect true overt focus on the heart as the centerpiece of the inflammatory response. It’s almost like an innocent bystander. Then the lungs. Lung circulation — the interface where oxygen and carbon dioxide exchange in the lungs, intricately woven with the heart —is where I think we see a lot of the ill effects of respiratory damage as a result of this illness. That’s probably one of the major areas in the future where we’re going to see residua. Those who survive severe disease very clearly have lung damage that decreases
Abbott’s CentriMag System is an acute circulatory support system approved for 30-day LVAD, RVAD and BiVAD support and used for extracorporeal membrane oxygenation in critical COVID-19 patients. Photo courtesy of Abbott

the effectiveness of the exchange of gases in lung circulation. As a result, you develop this new concept of lung failure, and it leads to a knee-jerk reaction to put a tube down the throat and breathe for the patient. But the breathing response and drive is probably not the problem. The problem is when you get air into the lungs, it can’t get into the bloodstream. We have now recognized the role of extracorporeal membrane oxygenation (ECMO), which you can think of as dialysis for the lung. Unfortunately, ECMO machines aren’t everywhere, and it is a fairly labor-intensive process right now, but as we move forward, we all anticipate the development of longerterm interstitial lung disease resulting from the inflammation of the acute illness. That interstitial lung disease tends to be progressive, and over time we believe that there will be an increase in the number of people with end-stage lung failure that would require some level of therapy, whether it be ECMO longer-term or transplant. Those are issues that we are grappling with now. Providing that lung dialysis, the ECMO process, in a way that can support a patient long-term is where we need to be when this result of the pandemic starts to hit clinical reality.
MDO: What have you learned about COVID-19 in the past year?
Adamson: We have more of a handle on the mechanisms, and that’s where I think we are beginning to have hope for clarity. In just about every disease that we are able to treat, we develop those treatments because we understand the fundamental mechanism of the disease. The receptor that this virus utilizes to get into the cell is part of the system we call the renin-angiotensin-aldosterone system. It’s the angiotensin II receptor. That’s really all over the body, but mostly expressed in the lungs, so that’s why it focuses on lung damage when it sets up an infection. But the process, we’ve now learned, is there are direct and indirect effects on the heart and blood vessels. The indirect effects of the cytokine storm and the intense inflammation that occurs typically in some people with this disease and syndrome are well defined now through multiple different inflammatory mediators that actually could be targets for therapy in the future. But that inflammatory mediation is maybe more exaggerated in people with preexisting cardiovascular inflammatory states like metabolic syndrome or other syndromes that would cause this underlying, >>
“We have more of a handle on the mechanisms, and that’s where I think we are beginning to have hope for clarity. In just about every disease that we are able to treat, we develop those treatments because we understand the fundamental mechanism of the disease.”
low-burn inflammatory state that we’re aware of. Clearly, those who have cardiovascular disease seem to have a higher chance of severe disease when they get COVID-19. What we were worried about early on is that we can fairly commonly see and detect injury to the heart during a severe COVID infection. When patients are having COVID and feeling bad but not going to the hospital, nobody tests those folks for heart inflammation. But when the moderate to severe disease that lends itself to hospitalization occurs, the cardiac community has been interested to see how that’s mediated, how many people get it, is it only found in moderate to severe disease? We typically make inferred diagnoses in cardiovascular disease. So if we measure a blood component that is a biomarker like troponin that is only in the bloodstream measurably when the heart’s damaged, or we measure a protein called B-type natriuretic protein — which really is only in the circulation in higher levels when the heart has stress on its filling — we then take those essential measurements and make diagnoses or inferred diagnoses from that. We find that troponin I (a significant marker of myocardial injury or heart muscle injury) is elevated in many people with moderate or severe disease. We thought that the total inflammation plus the direct effect on the heart might cause a marked increase in myocarditis,
which is a global inflammation of the heart muscle itself, and it is associated with other viral infections. That’s not been what we see in these infections. But there’s clear evidence that in about 20% of people there’s an elevation in troponin, which would be a diagnostic criterion for a myocardial injury or myocardial infarction. And that then leads to a higher risk of death. It’s pretty clear actuarially that if a moderate or severe COVID syndrome is associated with an elevation of troponin then the prognosis is much poorer. It is associated with higher risk of death through a variety of blood vessel and heart muscle abnormalities that can cause cardiovascular disability as well as death.
MDO: We initially thought of COVID-19 as a respiratory disease, but is it more helpful to think of it as a vascular disease in that it touches everything the vascular system touches down to the smallest capillaries?

Adamson: That’s a great question, and it turns out that it can be better understood when you understand the angiotensin receptor that’s involved in the transmission of this disease. The virus binds to this natural receptor to get into the cell, and in that process gets into the membrane and then it sets up all the inflammation, etc. The lung’s alveolar cells are really studded with these receptors, so that’s where the virus finds the opportunity into the cell. It makes sense then that most of the symptoms and developments as a result of COVID-19 syndrome are focused on the lungs. That being said, the angiotensin system is one of the most important regulatory systems for the cardiovascular system, both the heart and the blood vessels. Those receptors are everywhere, and they’re available when you inhale the virus. When it becomes systemic, then you do get direct effects on the blood vessels, on the heart, on the coagulation sequencing in the blood vessels, primarily probably stimulated by disruptions in the endothelial cells that line the inside of the blood vessels. The body doesn’t like disruptions in that. When it sees those kinds of breaks in the endothelium, it has to fix them, and it starts that fix with the coagulation system. That can deplete the system of its first responders, which then causes this concept of disseminated intervascular coagulation in which you get this cascade of coagulation. And that’s most apparent in the smaller blood vessels, especially those that are going from the heart to the lungs, the capillaries in the pulmonary circulation, and that just obstructs the ability to get oxygen then into the bloodstream. Then the oxygen level drops, acid-base levels change, electrolytes change, and now you have not only systemic inflammation, but you have all the substrate for injury to the heart. That would include direct muscle injury, direct production of arrhythmias, blood clots in small vessels. Deep vein thrombosis and pulmonary emboli are very common. It’s a cascading effect, a combination of both direct effects


on the heart and the blood vessels, and an indirect effect of this massive inflammation that can occur along with the oxygenation, etc. It is complex, but on the other hand, it kind of makes sense if you think of it as starting with this receptor that is found in a lot of places, and every place it’s found has a potential of being impacted by the disease. And so far, it’s shown great success in doing that.
MDO: What role can medical devices play in COVID-19 prevention and treatment?
Adamson: The most important are supportive devices when severe disease occurs. In my mind, it is very clear that ECMO is the most effective way to save a life. Early on, it was thought that when ECMO was applied, people tended to die. What we learned is that there was too much time before it was applied. It was applied too late. ECMO was this sort of temporizing
medical device intervention that saves lives. We need broader availability of that therapy if people are going to survive this disease.
MDO: What cardio products will be in greater demand as we get a few years out from the initial onset of COVID?
Adamson: We’ve talked about lung failure. I believe heart failure likely will go up as well. There’s this long-term idea that what we call nonischemic dilated cardiomyopathy — where the heart’s not damaged by blockages in the heart arteries, it just gets bigger and gets weaker over time — is probably based on the immune system chronically, slowly damaging the heart over time. And that’s probably from a sensitization of some people’s immune system to troponin C, what we measure to diagnose heart attacks. It’s really only found in the heart muscle, so if
the body sees that circulating, it could interpret that as an antigen that it needs to attack. That immune response slowly damages the heart over time. That can cause a dilated cardiomyopathy of unknown etiology. I’m not an expert in the future, but I do see that there is an increased probability we will see more heart failure. As that progresses to end stages, as that needs to be monitored, at Abbott many of our portfolios like MitraClip, CardioMEMS, CentriMag and HeartMate 3 will be called into action. Ten years ago, the American Heart Association suggested by 2030 we’ll see a doubling of heart failure prevalence. This is only going to make it even more prevalent, and all of those standard therapies that we have for people with heart failure will be in bigger demand.
Go to wtwh.me/AbbottCovid for more from our conversations with Adamson.









We love engineers here at DeviceTalks.




It’s obvious why. Engineers are the straw that stirs medtech’s drink (apologies to Reggie Jackson). Nothing happens — financing, manufacturing, approval, help for patients — without a well-conceived product.

And engineers often transcend their typical design roles. They grow into business leaders, technology evangelists, startup CEOs, and yes, some stay to lead ground-breaking research and development groups.
So I’m thrilled to be bringing engineers of all types into the agenda of DeviceTalks Boston. We’ll have engineers from Abbott, ZimVie, Philips, Boston Scientific, Stryker and many other companies on hand to talk about being an engineer.







But we’ll also hear from engineers who have followed their careers into other parts of the medical device industry.

In this column, we’ll walk you through some of their career highlights

as told in interviews on our DeviceTalks Weekly podcast. We’ll explain their roles at the upcoming DeviceTalks Boston conference happening May 10-11 at the Boston Convention & Exhibition Center.

















The business leader Megan Scanlon, the president of urology at Boston Scientific, calls herself a “recovering mechanical engineer” after leading medical device businesses for years.
Scanlon will lead a discussion at DeviceTalks Boston about Boston Scientifi c’s LithoVue Elite Single-Use Digital Flexible Ureteroscope System, which employs a sensor capable of monitoring intrarenal pressure (IRP) in real-time during ureteroscopy procedures to manage kidney stones.



Scanlon started her engineering career at Gillette Co. designing razors, “which was a pretty remarkable job.” But she left the company to secure a master’s degree in engineering and her master of business administration.


(continued on page 62)




We love engineers of all types — here are just a few you’ll meet at DeviceTalks Boston.






























































devicetalks.com/podcast/



















Robert Cohen has his eyes on the future of orthopedic surgery, but he brings a wealth of knowledge about its past as well. Over his career, Cohen has had a role in materials, 3D-printing, and began working with MAKO Surgical several years before its acquisition by Stryker in 2013. Now Cohen leads the team that will guide Stryker’s digital strategy in the future.
devicetalks.com/strykertalks/



(continued from page 60)




“That was really the springboard for me to change industries,” she said. She joined Johnson & Johnson in marketing, operations and finance roles before moving to Boston Scientific. Now she leads a $1.7 billion business.



What role does her engineering training play in running a medical device business? “I will never, ever completely put my engineering skills behind me,” Scanlon says. “The nice thing about medical devices is it’s very technical, both the biomechanics of the procedures that we’re doing, as well as the biomechanics of how the devices work. So my learning curve to kind of understand the technical aspects of these procedures, of these devices and of what surgeons are trying to accomplish was quite quick.”
Go to wtwh.me/ScanlonDT to hear Scanlon’s entire DeviceTalks Weekly Podcast interview.
























The technology evangelist Robert Cohen, president of digital, robotics and enabling technologies at Stryker, caught the biomedical engineering bug early on while attending the New Jersey Institute of Technology. An engineering professor of his was assisting a surgeon in developing a total knee prosthesis.
The professor and surgeon would later advise him on his master’s degree thesis. Fueled by that early interest, Cohen co-founded and sold two medical device companies and helped spark the surgical robotics revolution at Mako Surgical.
Today, Cohen leads Stryker’s campaign to connect robotics, digital tools, AI and data science into a seamless therapeutic system. He’ll be part of a May 11 keynote panel featuring other surgical robotics executives and leaders who discuss “Where Robotics, Data, and Digital Can Take the OR.”


How did Cohen position himself for the broader responsibilities of leading Stryker’s charge into the digital OR? Cohen points to his early days at orthopedic startups where “you were jack of all trades.” In his first startup, Cohen worked with product development, research and development, clinical research, manufacturing, marketing and regulatory.
“Back then,” he recalled. “We were not allowed to design a product if we couldn’t sell the product. So we had to be able to speak and articulate the merits, features and





BOSTON SCIENTIFIC AND ZENFLOW ARE BRINGING CRITICAL NEW TOOLS TO HELP UROLOGISTS TREATThe urology specialty faces a critical moment, with 10 urologists ready to retire for every new urologist entering the field. That puts the onus on medical device companies to develop faster, safer and more e cacious ways to break down kidney stones, shrink enlarged prostates and handle other critical treatments.
benefits of the products to surgeons.”

Cohen says forcing engineers to think as a marketing professional — or a surgeon — might is critical to designing the best product.
“When you have the surgeon in front of you, it becomes very obvious that what may be important to me may not be important to the surgeon,” Cohen said. “Or the way I may say. It may not be the way a surgeon really needs to hear it. This allows you to become a more effective communicator. That experience in talking to customers and sales reps and marketing people, I am hugely appreciative of because it really honed me into the person I am today.”
Go to wtwh.me/CohenDT to hear Cohen’s entire StrykerTalks Interview.



















The R&D leader
Kevin Bourque is an engineer’s engineer. Bourque targeted the medical device industry right out of college, applying to companies in the Boston area.

“I was focused on medtech and once I got in I got the bug,” Bourque said. “A lot of engineers who are working in medical devices have to admit to themselves that they’re stuck [in the industry] for life because it’s so rewarding.”
Bourque started at an MRI manufacturer, but has spent most of his career developing ventricular pump technology. Following a series of acquisitions he now leads research and development of Abbott’s HeartMate LVAD.
“One of the nice parts of hanging around in one spot for so long is you get to see the evolution” of a technology, Bourque says.

Go to wtwh.me/BourqueDT to hear Bourque’s interview.




In the LVAD’s early days, engineers wondered if they could actually pump blood. Now that Bourque and other engineers have helped answer that question, he’s joining a panel that will ask deep questions about healthcare equity. He’ll sit on a panel featuring Abbott executives and a former Heartmate user who has transitioned off the device thanks to a heart donation.
Abbott’s “Innovative Options to Treat Heart Failure Should Leave No Patient Behind” panel takes place at 2 p.m. on May 10.





DeviceTalks Boston is the place where engineers can not only improve their craft — think digital twins, high-powered sensors and other cool tech — but also understand how their work fits into the bigger healthcare picture. (And beyond, as DeviceTalks attendees can attend sessions at our Healthcare Robotics Engineering Forum and Robotics Summit & Expo.)
Finally, we’ll also host conversations about how engineers and other critical medtech makers can build their own success.
We love engineers at DeviceTalks. We hope it shows.





devicetalks.com/podcast/







EXPLORING HEARTMATE, CARDIOMEMS AND THE REST OF ABBOTT’S TOOLBOX TO HELP HEART FAILURE PATIENTSIn this episode, we’ll talk with Kevin Bourque, vice president of research and development, and Robert Kormos, division VP, global a airs of heart failure, about the advances of Heartmate 3. Both also detail how Abbott is working to help children su ering from heart failure.




SALES







Ryan Ashdown 216.316.6691 rashdown@wtwhmedia.com
Mary Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Courtney Nagle cseel@wtwhmedia.com 440.523.1685 @wtwh_CSeel


Jami Brownlee 224.760.1055 jbrownlee@wtwhmedia.com
Ashley N. Burk 737.615.8452 aburk@wtwhmedia.com

LEADERSHIP TEAM
CEO Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844 @SMMcCafferty


Jim Dempsey 216.387.1916 jdempsey@wtwhmedia.com

Mike Francesconi mfrancesconi@wtwhmedia.com 630.488.9029


Jim Powers jpowers@wtwhmedia.com 312.925.7793 @jpowers_media

Brian Toole 267.290.2386 btoole@wtwhmedia.com
VP of Sales Mike Emich memich@wtwhmedia.com 508.446.1823 @wtwh_memich

EVP Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609 @mmatheson












Why do medical professionals have so much confidence in our devices, sets and kits? It starts with five decades of contract manufacturing. The pharmaceutical industry relies on us to build custom kits with all the devices necessary to dispense and administer your drug. Our product portfolio contains hundreds of standard components plus capabilities including design, regulatory, manufacturing, packaging and sterilization. Think of us as your best, most direct route to market. After all, our devices and capabilities do more than simply meet expectations. They deliver confidence. Always have. Always will.