MEDTRONIC CHIEF TECHNOLOGY AND INNOVATION OFFICER KEN WASHINGTON AND ENDOSCOPY CHIEF AI OFFICER HA HONG
Medtronic IS USING HOW







MARCH 2024 www.medicaldesignandoutsourcing.com THE BIGGEST ORTHO STORIES FROM AAOS 2024 P.38
P.44
P.28
ABBOTT BETS ON BALLOONS IN THE PULSED FIELD ABLATION BATTLE
















MEDTRONIC CHIEF TECHNOLOGY AND INNOVATION OFFICER KEN WASHINGTON AND ENDOSCOPY CHIEF AI OFFICER HA HONG
Medtronic IS USING HOW







MARCH 2024 www.medicaldesignandoutsourcing.com THE BIGGEST ORTHO STORIES FROM AAOS 2024 P.38
P.44
P.28
ABBOTT BETS ON BALLOONS IN THE PULSED FIELD ABLATION BATTLE



Premier Nitinol Solutions for Your Next MedTech Breakthrough Resonetics is your key to unlocking new dimensions of design freedom and reliability. Trust us to shape the future of your projects with excellence. Scan the QR code to dive into the world of nitinol prototyping and commercial manufacturing from Resonetics. Learn more at Resonetics.com | 1.800.759.3330 | 1.603.886.4233 | sales@resonetics.com Laser Cutting Shape Setting Electropolishing Braiding Nitinol Capabilities


Thermoplastic Injection Molding
Thermoplastic Injection Molding
Liquid Silicone Rubber (LSR) Molding
Liquid Silicone Rubber (LSR) Molding
White Room/Cleanroom
White Room/Cleanroom
Test Specimen Molding
Test Specimen Molding
In-house Tooling
In-house Tooling
SPI Class 101 and 102
SPI Class 101 and 102
Production Molds
Production Molds
PSN Labs can be your manufacturing partner for all injection molded components. Our team of scientists and engineers engage seamlessly across the full development process to ensure all aspects of a product have been evaluated from design through manufacturing to mitigate the risks associated with launching a product to market.
PSN Labs can be your manufacturing partner for all injection molded components. Our team of scientists and engineers engage seamlessly across the full development process to ensure all aspects of a product have been evaluated from design through manufacturing to mitigate the risks associated with launching a product to market.






www.PSNLabs.com
www.PSNLabs.com
Info@PSNLabs.com
Info@PSNLabs.com
ISO 9001:2015 ISO/IEC 17025:2017 ISO 13485:2016 CERTIFICATIONS YOUR FULL - SERVICE ENGINEERING
PARTNER FULL-SCALE PRODUCTION DESIGN TESTING RODUCTION
ISO 9001:2015 ISO/IEC 17025:2017 ISO 13485:2016 CERTIFICATIONS YOUR FULL - SERVICE ENGINEERING PARTNER
FULL-SCALE PRODUCTION DESIGN TESTING PRODUCTION

Seize the AI opportunity
Less than a year into his new role as Medtronic’s chief technology and innovation officer, Ken Washington was presenting on artificial intelligence to leaders of the company’s operating units. One of the GMs stopped him and asked for help making sense of all the buzzwords and acronyms.
Perhaps you know the feeling. It’s hard to grasp how AI seems to be everywhere, with advanced computing power making sense of vast datasets. It’s in the voice assistants on our smartphones, the streaming services on our various screens, mapping systems in our cars, the chatbots who respond when we need customer service, and online services ranging from e-commerce to social media and online banking.
It can even help bring you the latest intelligence in medtech m aterials and manufacturing in Medical Design & Outsourcing . While we’ll never use AI to write our stories, AI powers the voice recognition service I use to rapidly turn audio recordings of interviews into written transcripts (though I always check every fact and quote I use against the recording for validation). Ironically, AI is even in the software I use to check our contributions for plagiarism and AI-generated content.
And now it’s in medtech. As an enabling technology, AI is rapidly finding new medical device applications, including imaging, moni toring, screening and surgical assistance.
With so much hype around AI, it’s helpful to learn from those who have put these technologies to work in a way that saves lives. It’s hard to come up with a better example than Medtronic’s GI Genius, which flags trouble spots that might otherwise go undetected during colonoscopies. Medtronic is using AI in many other ways, so this issue includes insights and advice from Medtronic leaders like Washington to help device developers of all sizes seize the opportunity.





“While AI technology has been around for a long time, the modern-day version of AI … has only been with us for one to two years,” Washington said. “And so we have to spend the time and take the effort to teach ourselves and to teach others about the importance of these technologies.”
“The future is bright, and we’re just getting started,” he continued.
As always, I hope you enjoy this edition of Medical Design & Outsourcing — and thanks for reading.


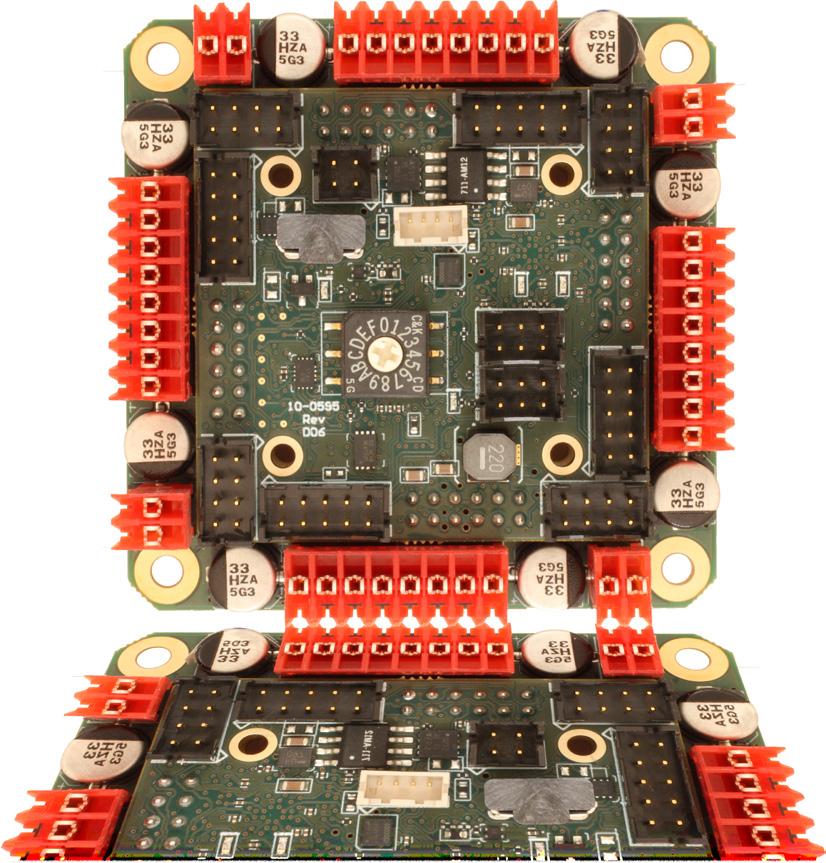
HERE’S WHAT WE SEE - brushed or bldc motors - 5 amps per axis - 16 analog inputs - 16 on/off drivers
home and limit in - live tech support - made in the USA WWW.ALLMOTION.COM (510) 471-4000 30097 Ahern Avenue Union City, CA 94587 Technical Support (408) 460-1345 See the EZQUAD SERVO in action! 2.25” 4 AXIS SERVO from NEW! 2 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
-




















































































































HOW
IS USING

MATERIALS:
ROBOTICS:
How Noah Medical’s robotic Galaxy system goes deep into the lungs
TUBING: Abbott bets on balloons in the pulsed field ablation battle




Major orthopedic device developers and manufacturers revealed new technologies at the annual show, while a study raised questions about robotic knee surgery.
38 medicaldesignandoutsourcing.com • March 2024 • Vol. 10 No. 2 CONTENTS DEPARTMENTS 02 09 12 16 20 24 28 44 48 THE BIGGEST ORTHO STORIES FROM AAOS 2024
6 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
MEDTRONIC
AI
Martha, Ken Washington and Ha Hong discuss Medtronic AI products and offer strategies, insights and advice for other device developers. ON THE COVER 32 HERE’S WHAT WE SEE: Seize the AI opportunity 3D PRINTING: New skin-safe materials for additive manufacturing will advance medical device design MANUFACTURING: The rundown on dip manufacturing for medical devices MOLDING: Advancing medical wearable devices through electrically conductive silicone molding
Geoff
What is a self-lubricating liquid silicone rubber?
preview:
favorite
additions
AD INDEX 38
DEVICETALKS: DeviceTalks Boston
Our
panels and new
to this year’s show



Precision Control Solutions
Designing efficient systems involves much more than simply understanding a few basic principles. There is a true art to balancing the specific requirements of an application in order to achieve the desired goals in the best possible way. Help us understand the unique needs of your application and together, we’ll develop something that surpasses what any of us could have done alone.
Contact your distributor to learn more, or visit clippard.com to request a free catalog and capabilities brochure.

•
•

•
•

•
•
•
•
•
•
•
•
877-245-6247 CINCINNATI • BRUSSELS • SHANGHAI
Electronic Valves
Proportional Valves
Isolation Valves
Precision Regulators
Toggle & Stem Valves
Needle Valves
Electronic Pressure Controllers
Pneumatic Assemblies
Special Manifold Designs
Pneumatic Circuit Design
Cylinders
Fittings, Hose & Tubing



GUILLAUME BAILLIARD is the president of healthcare at Formlabs, where he is focused on building out the healthcare unit to better serve the needs of medical device firms, clinicians and patients using the company’s 3D printing solutions. In this role, he brings more than 25 years of experience in the healthcare market, previously serving in leadership roles in early-stage innovative companies and at GE HealthCare, after having started his career designing medical devices at Genzyme Surgical.
DAN SANCHEZ is a product manager at Trelleborg Healthcare & Medical, where he helps medical device designers realize product concepts through engineered manufacturing solutions and design for manufacturability support. He holds a Bachelor of Science in mechanical engineering from California Polytechnic State University, San Luis Obispo, and has worked in silicone manufacturing for the healthcare and medical industry since 1998.

ALEX SANTAYANA, an applications




CONTRIBUTORS
NESNIDAL
WATCH devicetalks.com DeviceTalks Tuesdays is a weekly virtual event that bringsthe insights and energy of our in-person events to your desktop. Each week, the editorial team at Device Talks will organize discussions and workshops for Medtech companies who want to ensure their teams are atop of this changing world. Topics include: • Innovation & Finance • Prototype & Product Development • Manufacturing & Sourcing • Regulatory, Reimbursement & Market Development • New Tools and Technology TUESDAYS
BAILLIARD
New skin-safe materials for additive manufacturing will advance medical device design
New materials open the applications for using 3D printing for personalized medical devices.


Additive manufacturing will make personalized medical devices affordable. As 3D printers have become more accessible and manufacturers innovate new materials for medical applications, the industry is delivering patient-specific orthotics, prosthetics, dental devices and more. But adoption has not yet hit an inflection point, and material science innovation of new skin-safe and biocompatible materials will spark an increase in personalized healthcare via 3D printing. Medical device makers and facilities must understand the potential of these new materials and incorporate them into device design.
3D printing in medical applications
Aside from printing metal for implants, there are two main types of 3D printing relevant for medical applications: Stereolithography (SLA) and Selective Laser Sintering (SLS). SLA has dominated the healthcare industry for its ability to produce high-accuracy, isotropic, and watertight prototypes and end-use
This is a skull with a cranial implant prototype designed by BTech Innovation. The long, curved part is an airway device designed by VIDA Medical Devices. The triangular part is a drug delivery device (inhaler) prototype designed by Sandoz Device Development Centre (formerly Coalesce). The larger threaded part is an arthroscopic cannula, and the smaller threaded part is an interference screw.
The parts were printed in BioMed Black Resin and BioMed White Resin.
Photo courtesy of Formlabs
parts with fine features and smooth surface finish. In addition, biocompatible materials are already available for SLA 3D printers, enabling medical device designers and manufacturers to create custom devices that improve care with end-use and surgical devices. Designed to meet the need for sterilization and skin-contact, these materials are available in several colors — such as black, white, clear, or amber — which are validated for different uses, including long-term skin or short-term mucosal membrane contact.
With its performance and range of available materials, SLA 3D printing has historically been the go-to for medical device makers.
Now, SLS 3D printing is rising. This method uses a high-power laser to sinter small particles of polymer powder into a solid structure based on a 3D model. This makes it a popular choice for manufacturers in other industries because of its low cost per part with high productivity, ideal for rapid prototyping and creating end-use parts. >>
www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 9
Guillaume Bailliard Formlabs

New skin-safe materials for SLS printers will enable the medical industry to reap the benefits of this 3D printing type.
Before coming to market, these 3D printing materials are tested to meet ISO standards for irritation and skin sensitization so they can be used in custom-made medical applications, such as prosthetics, medical appliances, bolus devices, sports equipment, orthotics, wearable accessories and more. The ISO standards represent a biological and clinical evaluation of materials’ safety, and they provide material preparation instructions for medical device applications with skin contact. There are several different standards, durations, and endpoints for different skin exposures, such as in silico or in vitro methods, so it’s important that device engineers, facilities, and manufacturers understand uses for each material — and, by extension, 3D printing methods — for their application.
applications ensures that facilities and manufacturers choose the right path for point-of-care 3D printing, prototyping and end-use patientspecific part creation.
New materials enabling innovation
There’s a direct correlation between material innovation and the medical adoption of 3D printing. All new medical technologies must comply with regulatory standards to ensure patient safety, and
“Developing new medical-grade materials is critical to expanding access to point-of-care manufacturing for healthcare professionals.”
With SLA and SLS 3D printing for medical applications, device makers have more options for custom-made parts for medical care. Understanding the properties of these medical materials and the capabilities of various 3D printing
additive manufacturing leaders are beholden to these standards. The 3D printing industry creates materials with medical and dental requirements to enable innovation and new applications for 3D printing. For example, dental labs and practices use a mix of materials to create custom crowns and bridges, implants, dentures and more — easily and at a lower cost.
Developing new medical-grade materials is critical to expanding access to point-of-care manufacturing for healthcare professionals. The manufacturing process for healthcare materials includes ISO 13485 certified and FDA-registered facilities, and testing for ISO 10993 and USP biocompatibility and sterilization compatibility. Even with many SLA and SLS materials on the market, continued research and development supports personalized healthcare adoption.
When new materials are developed with safety and healthcare applications in mind, additive manufacturing innovation and adoption can advance. Not only is this increasing the opportunities for personalized healthcare, it can also set the stage for a more agile medical supply chain while improving surgery, trainee education, and patient engagement for years to come.
What’s next for 3D printing innovation
A variety of 3D printing workflows and materials for medical applications will move healthcare forward, equipping researchers, surgeons, radiologists, device engineers, dentists and orthodontists with the tools to provide patients with personalized, precision healthcare.
3D PRINTING
These 3D-printed device components and wearables were printed on the Fuse 1+ in TPU.
10 Medical Design & Outsourcing 3 • 2024
Photo courtesy of Formlabs
In addition to providing patientspecific devices and new medical device innovations at a lower cost, these materials can also help reduce risk and increase agility in the medical supply chain while improving surgery, trainee education and patient engagement for years to come.
For example, the pandemic sparked a rare, fast response in the industry as healthcare facilities innovated and collaborated to respond and deliver urgent patient care. Early 3D printing adopters were using the technology to create patient-specific surgical guides and anatomical models, advanced prosthetics, medical devices and more. When nasal swab shortages hit, existing 3D printers and a skin-
by USF Health, Northwell Health, and Tampa General Hospital, and printed on Formlabs 3D printers. This quick action was possible through the collaboration and pre-existing materials for medical applications that were ready for use.
This nimble response shows how healthcare leaders can utilize medical 3D printing and these materials to create more nimble supply chains to be ready for the next crisis. It’s a case study of how global healthcare facilities can collaborate to save lives.
To move forward, medical device designers and manufacturers must embrace 3D printing and educate themselves about the technologies and materials available for healthcare applications. As new
Beyond improving patient care, material innovation and growing 3D printing adoption in healthcare will enable providers, facilities, and manufacturers to become nimbler and respond to crises and patient needs faster.

cadenceinc.com /arc-florida | 800.252.3371 COMPLEX MIM PARTS FOR VARIOUS SURGICAL APPLICATIONS Micro MIM Complex Geometries Thin Wall Capabilities Learn More: Customizable Feedstock Visit us at MD&M West Booth #1801
The rundown on dip manufacturing for medical devices
Dip molding and dip coating each have advantages and drawbacks for medical device manufacturing.



Dip molding and coating involve immersing an object into liquid silicone and either forming or coating a component.
These methods can contribute to the success of a medical device project when correctly specified for an application. Component and contract manufacturers that understand customer requirements and align their manufacturing capabilities to them will have the greatest success with these dipping techniques.
Dip molding for medical devices
Dip molding involves submerging a mandrel, or a geometric form, into silicone. The silicone then cures to a solid before the mandrel and silicone shell are separated. The mandrel is used again for the next batch, and the silicone shell is used in a finished medical device.
Applications include balloons, breather bags, probe covers, tissue expanders and syringe covers.
Advantages of dip molding
Dip molding with silicone is valuable to customers because cured silicone takes the exact form of the mandrel.
Many variables can be controlled during dip molding, including the
number of dip coats, heating and movement of the mandrel, component thickness and mechanical properties, depending on material choice. By focusing on these factors, manufacturers can create an engineered solution to meet a customer’s specific requirements.
Dip molding provides excellent flexibility as it allows for a wide variety of shapes, sizes and wall thicknesses. Additionally, it enables quick prototyping of mandrils and prototypes resulting in short lead times. The prototyping process is cost-effective due to a relatively simple setup.
Disadvantages of dip molding
Achieving tight tolerances can be difficult with dip molding. Even though varying wall thicknesses offer flexibility, thin-walled dip-molded shells make ensuring accurate measurements complicated, especially when separating the shell from the mandrel.
Designs for dip molding must consider how the shell will be removed from the mandrel without tearing or distorting it, and how the hollow shell will be trimmed or covered in secondary processes.
(continued on page 14)
12 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
MANUFACTURING

MANUFACTURING
(continued from page 12)
Finally, even though dip molding can be quick to prototype, it can be relatively time-consuming and expensive compared to other production processes, so it is best used when the advantages outweigh any disadvantages.
Dip coating for medical devices
Dip coating involves immersing a component substrate into a liquid silicone, which adheres to the substrate as it cures.
To illustrate this, consider the dip coating process of a metal electrode scalpel used for electrosurgery. The silicone acts as a nonstick coating, and its flexibility allows the surgeon to manipulate the electrode to the preferred shape. Biocompatibility of the silicone is crucial for the safety of the patient in this invasive procedure. Other fluorinated coatings have been used on electrosurgical scalpels, but such coatings are outperformed by silicone



Dip-molded breast implant shells
Photo courtesy of Trelleborg


because they are not flexible and have poor biocompatibility.
Dip coating usually requires an adhesive bond between the coating and the substrate, which must be completely clean. Material selection of the coating plays an important role in adhesion. For example, some silicones can be formulated to bond with specific substrates while others may require better adhesion through surface preparation of the substrate. Silicone dispersions
are typically solvent-based, so a solvent evaporation step is often needed before a component is cured in an oven.
After cooling, several secondary operations can be performed to the dipped part to get it ready for the finished device. For example, electrode scalpels might receive an additional layer for electrical insulation on the undipped portion. In addition, a contract manufacturing partner can kit, package, or assemble dip-coated parts to a customer’s specifications.







14 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
Best in Class, High Performing Components for Your Medical Devices Drawing from our IN-DEPTH EXPERIENCE in the medical device eld, our in-house wiredrawing, wire-forming, coating, torque and assembly technologies provide a BROAD RANGE of OPTIONS for your device. CDMO / CMO Services Minimally Invasive Device Solutions Co-development Opportunities Access, Delivery, & Retrieval Systems Wire & Catheter Based Devices Contract Manufacturing Vascular Access Devices Guidewires, Therapeutic & Diagnostic Braided & Coiled Catheter Shafts Class 8 Clean Room TUBING & BRAIDING COILS ISO 13485 • 9001 Product Design & Development / Contract Manufacturing of Finished Devices Filmecc USA, Inc. | 3002 Dow Avenue, Suite 216 | Tustin, CA 92780 | Phone: 949.756.8252 | lmecc-us.com MACHINING & ASSEMBLY + COATINGS Asahi_2023-MDO_printer.pdf 1 7/12/2023 3:39:01 PM
Advantages of dip coating
The application of dip coating offers several advantages. For instance, when applied to electrosurgical scalpels, it reduces sticking and the accumulation of charred tissue, facilitating easier cleaning. This leads to a reduced frequency of blade changes and shorter surgery times. Dip coating also plays a significant role in enhancing patient comfort by minimizing friction and pain during procedures involving hypodermic needles and scalpels.
Dip coating can improve the grip on surgical instrument handles and jaws while also providing enhanced biocompatibility when encapsulating sensors. Another benefit is that dip coating can be efficiently scaled up for high-volume production, depending on the size and complexity of the instruments. Finally, quick production of dip-coated prototypes can contribute to shorter development lead times.
Disadvantages of dip coating
The disadvantages of dip coating include challenges in achieving uniform thickness on complex substrates, the need for secondary processes for inaccessible areas, and the careful management of volatile solvents for environmental and safety concerns.
Achieving a uniform thickness by dip coating is impractical for component substrates that include geometric
features like pockets, corners, and edges. Gripping of a substrate to control dipping can restrict coverage, requiring a secondary process for uncoated areas.
Finally, polymers used for dipping are often mixed with high concentrations of volatile solvents that must be controlled to ensure environmental health and safety.
Conclusion
Dip molding and dip coating are two processes that offer unique advantages and considerations for medical device production.
Successful implementation relies on effective collaboration between original equipment manufacturers and their component and contract manufacturing partners. By seeking knowledgeable partners who comprehend unique requirements and possess suitable capabilities, custom-engineered solutions can achieve optimal results in medical device manufacturing.

Medical Design & Outsourcing 15
Advancing medical wearable devices through electrically conductive silicone molding
Electrically conductive silicones combine the stability, biocompatibility and comfort of a silicone elastomer with increased conductivity from conductive additives.
 Michael Nesnidal ProMed Molded Products
Michael Nesnidal ProMed Molded Products

Wearable technology made its debut in the mainstream population in the late ‘70s with a wearable calculator that mimicked a wristwatch. Since then, the technology behind electronic devices worn on the body has expanded into a wide range of applications including smart watches that monitor heart rates, virtual reality headsets for entertainment, sensing devices to measure blood-glucose levels, and skin patches with sensors that can transmit a wearer’s vitals wirelessly.
Within the medical device industry, wearable devices, or wearables, are a growing segment due to the amount of real time data they can collect and supply. Many aspects of this technology have been adopted into medical devices to aid in patient monitoring and compliance, or immediate communication between
patient and practitioner. In the latest wave of innovation, device manufacturers are beginning to look for ways to deliver active therapy through wearable devices. ProMed has partnered with several innovative medical OEMs to address the challenges of developing wearable devices that deliver heat or electric therapies to the patient by using electrically conductive (EC) silicone elastomers. From mouthguards to treat periodontitis in a non-surgical manner to wristbands that reduce tremors associated with Parkinson’s disease, ProMed has developed a custom manufacturing approach for each application to successfully reach the commercial phase of their device. This article will take a look at the approach taken and challenges that were overcome in the development cycle.
(continued on page 18)
16 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
MOLDING


THE POWER OF SMOOTH FLOW
KNF further expands its Smooth Flow series, with the introduction of FP 7 and FP 25. These new liquid pumps deliver adjustable flow rates from 15 – 70 ml/min and 50 – 250 ml/min, respectively. Both pumps produce up to 1 bar (14.5 psi). High pressure versions achieve up to 6 bar (87 psi). All versions feature:
• Very low pulsation for efficient flow, reduced noise/vibration, and reduced system stress
• Self-priming, even at low motor speeds
• Options including materials, connections, mounts, motors, and boxer configurations. Ideal applications include medical equipment, inkjet printing, 3D printing, fuel cells, and solvent handling.

Learn more at knf.com/en/us/stories-events/news/article/fp7-fp25
A TEST PUMP: knf.com/shop
ORDER
(continued from page 16)
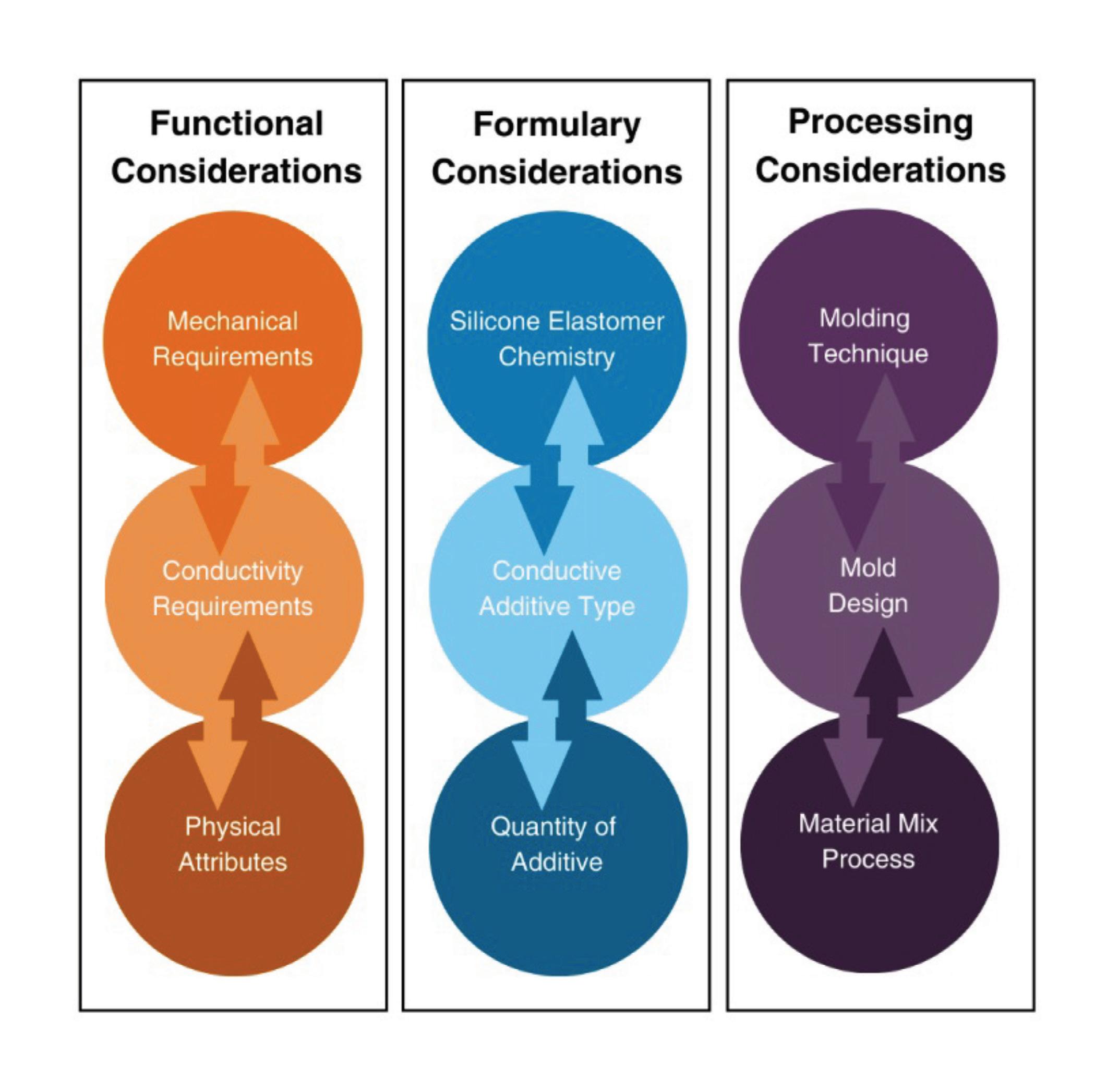
Electrically conductive silicones are a novel material which combines the stability, biocompatibility, and patient comfort of a silicone elastomer with increased conductivity from conductive additives. The challenge of working with EC silicones lies in selecting the right combination and balance of silicone formulation, conductive additive type and loading level, and molding method and tool design to produce a component that meets the requirements of the application from a mechanical and conductive standpoint.
Silicone formula selection
There are three common silicone elastomer formulations used in medical molding, high consistency rubber (HCR), liquid silicone rubber (LSR), and room temperature vulcanizing (RTV) materials. HCR materials are a high viscosity formulation that is typically compression or transfer molded. LSR and RTV formulations have lower viscosities that are commonly injection molded. The initial selection of
a base silicone material for an application is driven by application requirements such as strength, flexibility, or hardness needed for the device to perform or feel as the designer intends. The molding style dictated by the formulation of silicone elastomer will have a significant impact on the manufacturing method adopted when working with an EC silicone.
Conductive additive selection and loading level
Silicone elastomers on their own are insulative and require a critical concentration, or percolation threshold, to exhibit electrically or thermally conductive characteristics. At the percolation threshold there are sufficient additive-toadditive connections within the silicone matrix to transmit heat or electrical charge. To ensure the conductive performance is uniform across a molded silicone component, it’s critical to have homogenous additive dispersion and proper additive-silicone adhesion. Carbon fiber (CF) and carbon nanotubes (CNT) are proven additive choices for modifying the
Electrically conductive silicone molding considerations
Image courtesy of ProMed
electrical properties of silicone, though CNTs require a lower weight percentage (wt%) added to reach the percolation threshold. The wt% required to achieve the necessary conductive performance is important as the mechanical attributes of the silicone will degrade as the wt% of additive is increased.
Molding method and tool design
Between injection and compression molding processes, silicone elastomers may experience very different forces and temperatures which impact the conductive performance of an EC silicone. Specifically, the shear force imparted onto the elastomer as it is injected into the mold directly correlates with the percolation threshold for the conductive additive of choice. As the shear force increases, so will the percolation threshold and in turn the surface resistivity of the molded elastomer will also increase. Understanding how to minimize the shear effects through mold design and process optimization is critical to the development of a conductive silicone component. Between injection and compression molding processes, there is a greater amount of shear force introduced in an injection process, whereas compression molding yields a very isotropic product due to the lower shear required. Though compression molding produces more consistent product, the process is not favorable for over-molding applications. In such applications, it’s necessary to mitigate the shear force of an injection molding process through the tool design which may include adjustments to the style or size of gate used.
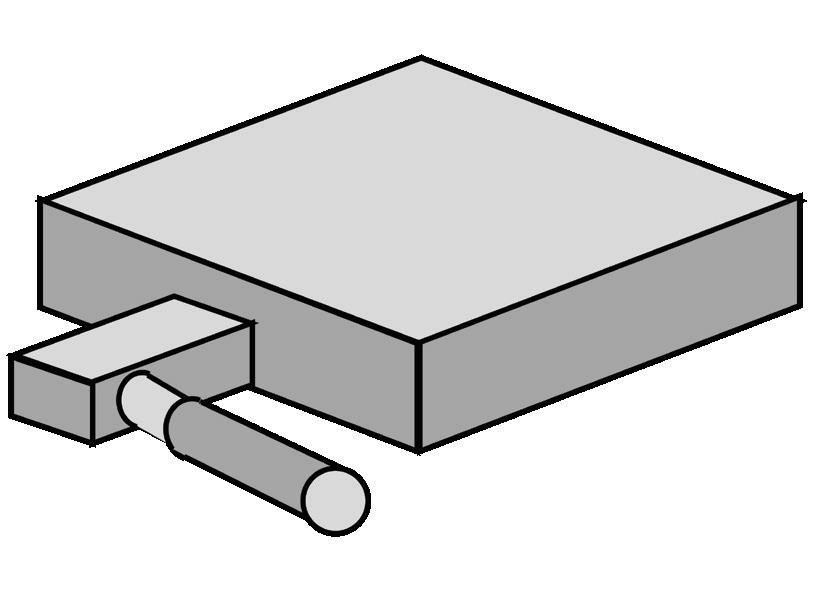
To assess the impacts of the three primary factors in molding with EC silicones, ProMed designed and injection molded four styles of silicone pad to test resistivity and resistivity uniformity across elastomer type, additive type, and gate style. Gate styles assessed were a tab gate with rectangular cross-section and narrow diameter of 0.009 in., and body gate with circular cross section and diameter of 0.040 in. The sample sets outlined in the table on the next page were made and tested in triplicate.
18 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com MOLDING

SILICONE ELASTOMER
CONDUCTIVE ADDITIVE
GATE STYLE
Sample A RTV- 60shA CF Tab
Sample B LSR- 65shA CNT Tab
Sample C RTV- 60shA CF Body
Sample D LSR- 65shA CNT Body
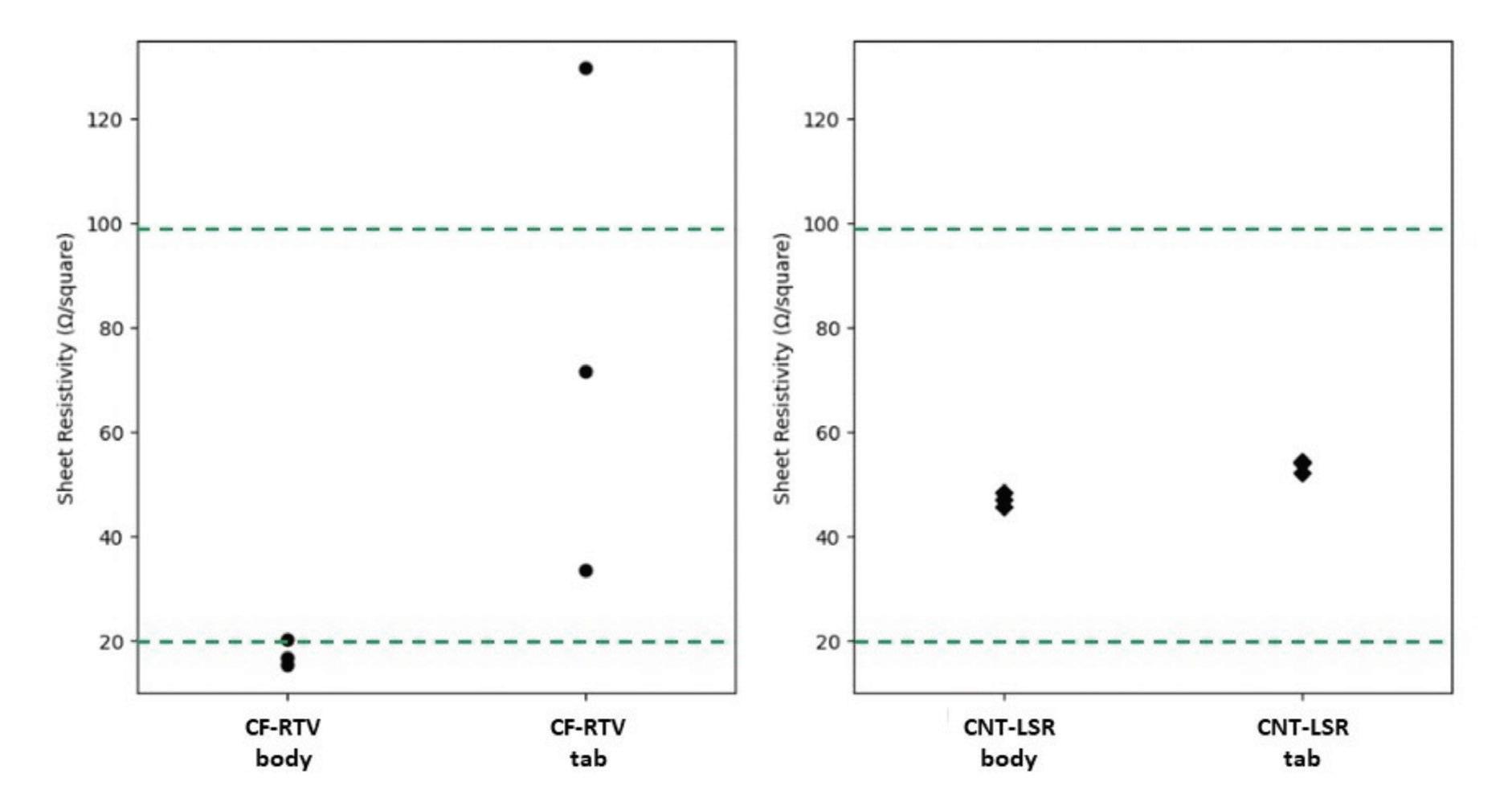
As indicated in the chart below, the average measured sheet resistivities for LSR with CNT added are very consistent and within a targeted range of 20-100 Ω/Square for both gate styles. A slight increase in resistivity is seen in the tab gated sample which is attributed to the increased shear force exerted though
Sheet resistivity measurements for injection molded EC silicone: Sheet resistivity was measured from three samples injection molded using CF-RTV and CNT-LSR silicones. In both cases, identical samples were molded using tab and body gates. Gate dimensions were consistent with typical designs for production molds. The target resistivity range for typical skin applications is indicated by the dashed lines.
Gate orientation for molding study: direct body gate (left) and tab gate (right)
chart above are reflected in the measured uniformity from the surface maps. The variability in the average resistivity for the RTV with CF additive samples is better understood considering the increased local resistivity along the left side of map A which correlates to the tab gate location. The impact of gate design and size is significantly more pronounced when working with the larger CF additive in an EC silicone elastomer.
Conclusion
the more constricted gate diameter. Samples made with RTV elastomer and a CF additive exhibited greater variability in resistivity for both gate designs, with a significantly wider spread with the tab gated sample. Similar to the LSR based samples, the resistivity rose in the tab gated RTV sample due to the shear force associated with the gate style. Because the CF additive has a larger particle size than CNTs, the influence of gate design is much greater.
The effect of the gate on surface resistivity is more clearly understood through sheet resistivity maps shown in the illustration below. Note, the false color scale is logarithmic and for tab gated samples, the gate would be located at the left hand side of the map. The consistent averages for the LSR with CNT additive samples observed in the
Understanding the impact and interaction of elastomer selection, conductive additive material selection, and molding process parameters is critical to manufacturing with EC silicone materials for wearable device applications.
It is crucial for the end device designer and manufacturer to work closely together to performance requirements and potential constraints to determine the best path forward to develop consistent conductive componentry.
In order to avoid costly mistakes or project delays, taking an iterative approach in the early stages of a project is recommended to find the right combination for success in a specific therapeutic device.

www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 19
Image courtesy of ProMed
Sheet resistivity maps for CF-RTV (left) and CNT-LSR (right) injection molded samples
Image courtesy of ProMed
 Alex Santayana
NuSil — a brand of Avantor
Alex Santayana
NuSil — a brand of Avantor
What is a self-lubricating liquid silicone rubber?
This medical-grade material can solve manufacturing challenges and increase medical device performance.
From well-established biocompatibility to robust performance characteristics, silicone offers medical device manufacturers a unique engineered material ideal for these applications.
One type, self-lubricating liquid silicone rubbers (LSRs), provides an excellent medical-grade material that can solve manufacturing challenges and increase device performance.
These elastomers contain a built-in lubricant that, over time, elutes to the molded part’s surface. For medical-grade LSRs, this lubricant offers many of the same properties as the elastomer, such as inertness or biocompatibility. In addition, the lubricant can be formulated so it won’t cause swelling when it contacts other silicone elastomers, a process that can create dimensional or even mechanical challenges.
Self-lubricating LSRs are available with varying lubricant levels and can be formulated to meet regulatory guidelines for short- and long-term implant use. These formulations are available offthe-shelf but can also be customized for specific manufacturing or end-user needs.
From innovative new cancer therapies to pioneering diabetes care, selflubricating LSRs may be the right choice to efficiently manufacture medical devices that make a life-changing difference in patients’ lives.

The characteristics of self-lubricating LSRs make them ideal for minimizing friction at component interfaces to enhance the performance of medical devices, including wearable devices. In addition to components like O-rings and connector gaskets, these elastomers are used in dynamic seals, slit valves, membrane septa and duck-bill valves.
For example, a self-lubricating LSR can be used on a silicone septum in an insulin delivery device to protect the needle, improve reliability and enable consistent performance. The elastomers have also been used in needle-free injection ports to deliver cancer treatments and other life-saving therapies. In these applications, a self-lubricating LSR allows manifolds to move more consistently and smoothly than a typical LSR.
What advantages do self-lubricating LSRs offer medical device designers?
Reduce time and labor: These elastomers eliminate the need for an extra processing step, like dipping or spraying the component with a lubricant, saving assembly time. In addition, self-lubricating LSRs can improve assembly in applications where sliding components tend to bind.
Create more reliable performance: Because the lubrication elutes from within, the article’s surface is coated with an even amount of oil. This eliminates the potential for inconsistency that can stem from imprecise or uneven applications, such as undercut or shadowed features that are incompletely lubricated during manufacturing.
(continued on page 22)
MATERIALS
Photo courtesy of Avantor

MATERIALS
(continued from page 20)
Reduce break-free force: Self-lubricating LSRs reduce the force required to overcome initial resistance to motion, a factor important in an application like when a needle must pierce the skin, remove a blood sample and then retract. By facilitating consistent insertion, the selflubricating LSR helps make the needle more reliable.
Resist blocking: Over time, when elastomers contact each other or other materials like metal, they can develop a degree of adhesion. Since self-lubricating LSRs resist blocking, they can help maintain component performance.
Prevent healing: A silicone component with a slit valve opening can begin to repair itself. Some types of sterilization, particularly gamma, can also initiate healing in silicone membranes. Self-lubricating LSRs will not heal, allowing the valve or other component to function as intended.






22 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
Self-lubricating liquid silicone rubbers (LSRs) provide an excellent medical-grade material that can solve manufacturing challenges and increase device performance. Photo courtesy of Avantor MagConnect® Medical Device Magnetic Safety Latching Connectors MagConnect® • Magnetic latching • Up to 20, 3 Amp Contacts MagConnectHV® • Magnetic latching • High Voltage Power • Data Contacts Visit www.onanon.com or email sales@onanon.com for more information FiberPlus® • Magnetic latching • Multi channel fiber Optic • Power Contacts • Data Contacts ➢ Low-Cost Disposable or Reusable ➢ Customizable ➢ Design Application Specific Medical Connectors ➢ Medical Cable Assemblies ➢ SmartConnect® integrated electronics ➢ Build to print assembly FDA Registered ISO-13485 Certified UL ZPFW2 Certified
How can medical device engineers choose the right self-lubricating LSR?
Factor in shrinkage: As self-lubricating LSRs elute oil, the rubber component shrinks over time. Engineers will typically factor in molding shrinkage to normal LSRs, but the self-lubricating LSR has a different shrink factor. This is an important consideration when designing a seal that requires critical tolerances, such as an O-ring that relies on highly controlled profiles and geometries.
Consider sterilization: Typically, selflubricating LSRs are a good fit for applications that require radiation sterilization. These elastomers resist the blocking, sticking and healing that radiation can trigger in plastics and typical rubbers. If the application requires dry heat sterilization, be aware that the process can cause self-lubricating LSRs to elute additional oil, causing more shrinkage than would be expected from a traditional silicone.
Understand the risk of migration:
During manufacturing, workers who touch oil from this type of LSR risk spreading it to other surfaces. Mitigate this risk by wearing gloves when handling — and regular cleaning. Migration can also impact device performance. For example, the oil from a self-lubricating LSR used on a seal could migrate and interfere with subsequent adhesive processes. In those types of applications, an appropriate, medical-grade silicone grease or a dry lubricious coating may be a more suitable solution.
Consider fouling: The use of selflubricating LSRs may require occasional mold cleaning because of their potential to foul tiny features like venting. During the tooling design phase, it’s important to factor in very small features that could be clogged by fouling.
Material selection is essential when designing the next breakthrough. Whether you’re developing an innovative new cancer therapy or pioneering diabetes care, medicalgrade self-lubricating LSRs may be the right choice to help you efficiently manufacture medical devices that make a life-changing difference in patients’ lives.

Harness our leading-edge vacuum technology . . . because lives depend on it.


Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.
For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.


Medical Design & Outsourcing 23
Annealing • Stress Relieving • Aging • Brazing • Solution Treat and Age (STA) Harden and Temper • Diffusion Bonding Medical Vacuum Heat Treating Services Eastern PA • Western PA • California • South Carolina • Michigan 1-855-WE-HEAT-IT solaratm.com


How Noah Medical’s robotic Galaxy system goes deep into the lungs
oah Medical’s Galaxy system for lung biopsy uses a robotic bronchoscope to reach and sample for suspected cancers deep in a patient’s lungs.
But the brightest star in the Galaxy system isn’t that disposable, robotic scope, but rather Noah Medical’s tool-inlesion tomosynthesis (TiLT) technology, designed to help surgeons retrieve samples that will provide a definitive answer from the pathology lab.
Medical Design & Outsourcing spoke with Noah Medical VP of Engineering John Shen to learn more about how the system works, how it was developed, and potential applications of the technology.
“Robotic systems are hellishly complex,” Shen said. “There are many, many, many systems or components that in their own right are complex devices, and they all need to be working together in harmony at the same time for everything to be going right. It’s software, it’s firmware, it’s hardware, it’s single-use
devices, it’s capital equipment. … A lot of the challenges are at the system level and making sure that you have very, very good system requirements and that everything is designed to work together.”
Noah Medical’s engineering challenges Shen credited a focused effort by the Noah Medical team to develop a singleuse, robotic bronchoscope that can reach six, seven or even eight generations into the periphery of the lungs.
“I couldn’t even tell you how many different designs we had … to get a scope [with] the right balance between cost, performance, manufacturability and all the rest. [How] we tackled that was a tight iterative loop between design, engineering and our clinical team so we could very rapidly develop versions of the scope, try them clinically, assess them against a set of metrics that we had developed and then iterate and continue to change. That’s what got us to the design that we have today.”
(continued on page 26)
24 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
ROBOTICS
Noah Medical’s Galaxy robotic bronchoscopy system has advanced imaging for real-time location updates of potentially cancerous lesions.
Image courtesy of Noah Medical
SNAP-IN
Field-wireable and overmoulded
Degree of protection IP67 (mated)
Versions with colour-coding
Flange parts with solder/dip solder contacts



www.binder-usa.com

“Robotic systems are hellishly complex. There are many, many, many systems or components that in their own right are complex devices, and they all need to be working together in harmony at the same time for everything to be going right.”
(continued from page 24)
The scope has a camera and a single LED light integrated into a tip module. The scope’s 2.1 mm working channel is sized to accommodate any off-the-shelf biopsy tool.

“If you look to the tip of this bronchoscope and a bronchoscope from any other manufacturer, they’ll probably look fairly similar,” Shen said. “The real trick is in the design of the scope itself: the bending section, the insertion tube and everything else. There’s a significant amount of work that goes into the robotic control calibration and robotic control of that scope to make it operate and feel like a good experience for the physician. … You have to kind of balance all these factors. There are times when you really want the scope to be flexible, and there are times where you really want it to be stiff so you can push it around a bend, and you can’t have both of those things at the same time, so you have to arrive at the right compromises, and that’s really where a lot of the design work went.”
After the robotic scope navigates to the approximate location of the lesion, Noah Medical’s proprietary TiLT technology helps the surgeon confirm that their biopsy tool is in the right place. The TiLT targeting mode displays the location of the lesion based on a computed tomography (CT) scan before the operation and real-time C-arm fluoroscopy during the procedure.
“Because you’ve done a TiLT sweep, you know exactly where it is, and then you can put your needle in it and do a biopsy under augmented fluoro that actually draws an augmented reality display of [the lesion’s location] even if you can’t see the lesion,” Shen said.
The TiLT board consists of metal beads arranged in a pattern underneath the patient, providing a reference to help provide 3D positioning from 2D imaging.
“In the end, it is an imageprocessing challenge. We’re writing algorithms to pull a 3D image out of the stack of 2D images, and as the algorithms get more advanced and as we try and drive up the quality,

26 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
ROBOTICS
The Noah Medical Galaxy system’s robotic bronchoscope
Photo courtesy of Noah Medical
Noah Medical’s robotic bronchoscope attaches to this robotic arm.
Photo courtesy of Noah Medical
you have all of your standard kinds of image processing algorithms that you might apply,” Shen said. “And then you can get into more exotic things, using AI or machine learning techniques to try and infer data to make the picture look more realistic and a better experience for the physician.”
Shen said work continues on image processing, but at this point, the technology allows physicians to target lesions quickly, safely and effectively. Noah Medical just released first-in-human study data showing 100% navigation success and tool-inlesion confirmation, with a diagnostic yield between 89.5% and 94.7%.
“We’re really looking to see how far we can push this. … So far, the results are encouraging, but we’re just at the beginning of the journey,” Shen said.
To accelerate speed to market and focus on innovating new technology to compete, Noah Medical used off-the-shelf components such as the robotic arm.
“The TiLT technology is really the big differentiator of the system,” Shen said. “It’s a software feature that relies on a few hardware elements, but a lot of the rest of it — even the robotics itself — is all secondary. All of those things are just getting you to where you’re in the area for a TiLT sweep and figuring out exactly where the lesion is. … If you were to take apart our system, you would find a good mix off-the-shelf stuff. Where there’s true value to be added is our custom technology, both hardware and software.”
What’s next for Noah Medical
The FDA cleared Noah Medical’s Galaxy system in March 2023. In April, the device developer said it raised $150 million in Series B funding to meet demand. In May, Noah Medical announced the first use of the system in the U.S.
Healthcare systems that are using Galaxy include the University of Chicago, Inova Fairfax in Virginia, and Prisma Health in South Carolina.
Another system using Noah Medical’s Galaxy system — CHI Memorial — performed the system’s 500th use in the U.S. in January 2024. Chatanooga, Tennesee-based CHI is the largest user by volume of the competing Intuitive Ion system, Shen said.

“It’s certainly exciting that they’re giving us a chance to show what we have,” he said.
Noah Medical’s technology could one day be used for other indications in the lungs and elsewhere in the body.
“Having just launched Galaxy in this indication, this is really our main focus, but the foundational technology is certainly applicable in a lot of other areas. … We are focused on endoluminal applications, so any kind of structure where you have a lumen and either a diagnostic or therapeutic application is certainly in play. Within the lung space itself, there are plenty of other procedures where this could be applicable. In the future, people are looking at therapeutics for lung cancer. That’s a little bit in the future, but certainly something that is exciting.”
“From a technology and innovation perspective, look for the continued expansion of artificial intelligence (AI) in robotics, the use of innovative new materials in robotic construction, ever-smaller footprint robotic systems, and surgery in the Metaverse.”
www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 27
This illustration depicts Noah Medical’s Galaxy system taking a biopsy of a lesion.
Illustration courtesy of Noah Medical
Go to wtwh.me/noah2024 for Noah Medical CEO Jian Zhang’s 2024 medical robotics outlook.

Abbott bets on balloons in the pulsed field ablation battle
The Abbott Volt aims to beat first-generation pulsed field ablation systems with its balloon-in-basket design and other features.
— the first of its kind approved by the FDA — or the Farawave catheter in Boston Scientific’s Farapulse PFA system.

The Abbott Volt’s balloon-in-basket is designed to support the efficient deployment of energy into the tissue during cardiac ablation to treat atrial fibrillation (AFib). That energy creates lesions, scarring the heart tissue to block the irregular electrical signals that disrupt the heart’s normal rhythm.
Abbott said the catheter’s design improves the accuracy, quality and efficiency of ablation to minimize the number of applications needed to treat a patient, as well as potential side effects.
“We have data showing if you use a balloon versus a basket without the balloon it makes a difference [in]
lesion depths by 20% to 30%,” said Dr. Christopher Piorkowski, the chief medical
To further maximize efficient energy designed the device with electrodes that only face outward and splines that are flat, not round.
Based on Abbott’s bench and animal testing data, the Volt design can drive chronic lesion depths of 6 to 8 mm, Piorkowski said, calling those depths “very sufficient for the left atrium and pulmonary vein isolation.”
At the same time, the balloon acts as an insulator for the blood inside the patient’s beating heart, reducing thermal effects such as bubble formation. Piorkowski said the Volt also uses less voltage for ablation than competing PFA devices, reducing the risk of thermal effects. (He declined to say how much voltage Abbott’s system uses or to quantify the difference.)
(continued on page 30)
28 & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
The Abbott Volt pulsed field ablation catheter features a balloon-in-basket design and eight electrode splines. Image courtesy of Abbott

Interpower® Protection: IEC 60320
C14 Seal Kits Combat Dust & Water
Interpower received an IP54 rating from a UL test report for its newly approved Face Seal and Plug Seal kits that are manufactured exclusively for Interpower’s IEC 60320 C14 inlet and molded C13 connector. The IP54 rating (solid particle ingress level 5, liquid ingress level 4), prevents corrosive dust and solid particles from reaching the terminals, and liquids from short-circuiting the terminals. The UL Ratings were conducted Under the IEC 60529 standard. The IP54-rated Seal Kits come in four corresponding part numbers:

The Face Kit: Available in two options: C14 Screw-mount Quick Disconnect Kit, Part #83050000; and the Solder Tab Kit, part #83050010. These kits include a moistureresistant polyurethane insert sealing the base of the C14 inlet and the face of the C13 connector after tightening the connector lock.

The Plug Kit: Comprised of a front seal made of flexible PVC with a lower durometer rating that plugs the front of the inlet to protect the terminals. Part #83050020 for the Quick Disconnect Kit, and part #83050030 for the Solder Tabs Kit.
INTERPOWER | P.O. Box 115 | 100 Interpower Ave | Oskaloosa, IA 52577 | Toll-Free Phone: (800) 662-2290 | Toll-Free Fax: (800) 645-5360 | info@interpower.com Order Online! www.interpower.com Business Hours: 7 a.m.–5 p.m. CST ® ®
(continued from page 28)
The balloon helps stabilize the basket inside the pulmonary vein, where the device’s round shape allows a physician to place the catheter at the ablation target site, apply energy, and rotate the device slightly to achieve a spline offset for a second energy application. In tests, physicians are using three to four applications per treatment.
“That is way below everything that is currently being done with PFA in the clinical field,” he said.
Measuring tissue contact during PFA
Another unique element of the Abbott Volt PFA system is a tissue contact assessment feature, Piorkowski said. Because PFA energy delivery depends on tissue contact, it’s important for the
electrodes




TUBING
The Abbott Volt PFA System can tell a physician whether the catheter’s
Visit www.confluentmedical.com for more information or email Nitinol@confluentmedical.com Confluent is proud to provide customers with the most reliable supply chain delivering the broadest level of Medical Nitinol services in mill products, hollows, tubes, wires, and components. Learn more at www.Nitinol.com
Mill
Products Nitinol Hollows Nitinol Tubing Nitinol Wire Nitinol Components
Abbott engineers took an impedance-based measurement that the company invented for previous ablation catheters and applied it to each of the Volt’s electrodes.
“We immediately saw the benefit in bench testing [and] also in the clinical field,” Piorkowski said. “In PFA, we have enabled the system to perform such contact assessment not on a single electrode, but on all eight splines of the device.”
What’s next for the Abbott Volt PFA system
Abbott recently announced that physicians had performed the first procedures in a global clinical trial of the Volt PFA system. The company said it hopes to win investigational device exemption (IDE) from the FDA for a U.S. clinical trial in the first half of 2024.
Because PFA is relatively new, the FDA and other regulators are learning about it along with the medtech industry, taking what they learn from publications, conferences and presentations and bringing their questions to device developers like Abbott during clinical trial design.
“Bubble formation was something in the beginning of the PFA era that was little talked about,” Piorkowski said. “When we designed our clinical trial — and we have a lot of pre-sub meetings, for instance, with FDA where we talk about the trial design and what they feel is important and how we should address it — this topic of bubble formation came up and we had to address it in preclinical testing and also in the trial design.”
The IDE trial will include patients with paroxysmal (intermittent) AFib and patients with persistent AFib. If successful, that study could lead to the Abbott Volt PFA system’s FDA approval and product launch as early as 2026, though Abbott has not yet shared a timeline for approval or commercialization.
“There are obviously risks down the road, [but] we try to be as fast as possible. … We see excitement on the enrollment side. These trials enroll really fast,” Piorkowski said. “Where we stand right now, we’ve treated 30 patients successfully in in Australia. These patients go through an intermediate assessment of safety and efficacy, and this data will be critical for the discussion of when we can start our IDE study.”











It’s good to know where you come from. At Nitto Kohki, we come from a line of engineers and businessmen dedicated to exceptional manufacturing execution. In our line of vacuum and pressure pumps, this is seen in the unique linear-piston design, where one moving part resolves to exceptional reliability, low vibration and noise, high energy efficiency, and long, consistent performance life (>10,000 hrs.) for a host of critical medical applications. It’s why the medical device industry has specied Nitto pumps for over sixty years— the quality in our DNA. Find out how you can use it to your advantage.



FIND OUT WHY YOU SHOULD KNOW NITTO: 800.843.6336 | NITTOKOHKI.COM/PUMPS QUALITY IS IN OURS Medical Design & Outsourcing 31

& Outsourcing 3 • 2024
Photo by Hardy Wilson for Medical Design & Outsourcing
Dr. Salomón Zebede prepares to operate using Medtronic’s Hugo robotic-assisted surgery system at Pacifica Salud Hospital in Panama. The hospital also uses Medtronic’s AIpowered Touch Surgery Enterprise product.

Medtronic AI products are catching colon cancer, assisting surgeons and finding new uses across the varying technologies developed by the world’s largest medical device manufacturer.
GEOFF MARTHA, KEN WASHINGTON AND HA HONG DISCUSS MEDTRONIC AI PRODUCTS AND OFFER STRATEGIES, INSIGHTS AND ADVICE FOR OTHER DEVICE DEVELOPERS.
JIM HAMMERAND MANAGING EDITOR
In recent interviews, Medtronic executives discussed how artificial intelligence is leading to new or improved devices. They also offered insights that can help device designers and engineers across the medtech industry take advantage of the rapidly advancing technology.
“We’re harnessing the power of AI today for use in clinical decision support, creating new indications, and delivering personalized treatments,” Medtronic Chair and CEO Geoff Martha said at the 2024 J.P. Morgan Healthcare Conference. “... I believe we’re uniquely positioned to advance AI and medtech.”
Medtech is one of the most exciting fields for applying AI because “it directly affects the patient’s life,” Medtronic Endoscopy Chief Artificial Intelligence Officer Ha Hong said in an interview with Medical Design & Outsourcing.
“The good news is you don’t have to invent the science of AI. That’s a solved problem,” Medtronic Chief Technology and Innovation Officer Ken Washington said in the same interview with Hong. “So what we have to do is organize the data and make it easier for our engineers and our scientists who design and do the R&D in our business units and our operating units to create the next generation of products that are going to be powered by these AI technologies.”
(continued on page 35)
3 • 2024 Medical Design & Outsourcing 33
AI
Photo courtesy of Medtronic
MEDTRONIC AND

(continued from page 33)
Medtronic AI products
Medtronic Endoscopy’s AI-powered GI Genius is “putting our GI business on the map,” Martha said. The deep learning technology flags potential polyps during colonoscopies, an innovation to a mature procedure where about 26% of polyps previously went undetected, according to clinical data cited by Medtronic.
“In the real world, we’re finding 50% are missed that the AI is picking up without any false positives,” Martha said. “It’s really phenomenal.”
Martha envisions GI Genius becoming the standard in colonoscopy, and said Medtronic is expanding the platform to enable additional AI applications for gastrointestinal procedures.
GI Genius is Medtronic’s bestknown artificial intelligence product, but Martha highlighted four others. For example, Medtronic’s AI-enabled AiBLE (pronounced “able”) digital ecosystem for neurosurgery helps surgeons plan, perform and analyze spinal and cranial procedures.
Medtronic Diabetes uses machine learning for automatic insulin delivery in the MiniMed 780G System, which features a Meal Detection Technology algorithm that Martha called “the secret sauce.”
Medtronic’s Touch Surgery Enterprise uses AI to process and annotate videos of procedures for immediate review by the surgeon on their phone, helping to improve training and compliance.
And the Medtronic LINQ insertable cardiac monitor uses the device developer’s AccuRhythm AI to better detect atrial fibrillation (AFib).
“The unlock here was AI,” Martha said. “We were picking up all the AFib and then some. [Physicians] didn’t want any false positives. They’re looking for 100% sensitivity and specificity, and the unlock was AI [to] eliminate those false positives.”
Medtronic’s AI insights and advice
Those Medtronic AI products “are just the tip of the iceberg,” said Washington, who joined Medtronic in June 2023 after serving as VP and GM of consumer robotics at Amazon, CTO at Ford Motor Co. and a VP at Lockheed Martin Space. >>

MEDTRONIC AND AI Medical Design & Outsourcing 35
At Amazon, Washington said he learned that robotics isn’t just about mechatronics.
“A successful robot solution has to have a mature and evolving and upgradable software stack,” he said. “That’s where the overlap between our AI strategy and our robotic strategy comes into play.”
Medtronic has a digital technology group in London designing AI technologies for surgical robotics systems, Washington said. Those features would provide surgeons with insights and guidance that could make them more efficient and effective, improving patient outcomes and reducing costs. These systems include Medtronic’s Hugo soft tissue robotics platform and the Mazor robotic guidance system for spine surgeries, though Washington declined to offer details.
“We have a strategy to continue to make our Hugo robot smarter and better and have additional instruments and end effectors as well as additional intelligence
and integration with other aspects of the operating room environment,” he said. “[And the Mazor] technology is going to only get smarter and better. We have a very rich roadmap for providing additional intelligence capabilities.”
As AI technology advances, their building blocks and tools are becoming more standardized for use by designers and engineers at device developers.
“Begin with a clear identification of clinical benefits that we can provide,” Hong said. “Start from there, and then apply all those really nice tools that are ready to be used.”
“The standardization and the data streaming and the common architecting of common building blocks is happening everywhere, not just within Medtronic,” he continued. “We are aware of this big trend, and we want to make our R&D process more streamlined and more efficient, taking those ready-made tools and really focusing on the area where we can provide our biggest value” with clinical,

“The good news is you don’t have to invent the science of AI. That’s a solved problem.”
manufacturing, and regulatory expertise.
Most AI-enabled medtech products cleared, approved or authorized by the FDA are based on deep learning, which is a kind of machine learning. The simplest tools are often best when it comes to development, validation and passing regulatory scrutiny, Hong said.
“When there’s a problem, it’s much easier to find the problem and fix the issue. ... If there are two technologies that achieve the same clinical benefit, you better go with a simpler model, because it has less moving parts that can go haywire.”
When to outsource AI and when to build it in-house
Like most outsourcing decisions in medtech, Medtronic doesn’t want to reproduce existing technologies, opting to instead buy or partner with a company with the right expertise.
“Sometimes you want to be able to enter the market quickly, and so it makes sense for us to partner

36 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
AND AI
MEDTRONIC
Medtronic SVP and Chief Technology and Innovation Officer Ken Washington
Photo by Hardy Wilson for Medical Design & Outsourcing
Medtronic Endoscopy Chief AI Officer Ha Hong
Photo by Hardy Wilson for Medical Design & Outsourcing
to accelerate our ability to get to a clinical outcome very quickly,” Washington said, citing Medtronic’s GI Genius partnership with Cosmo Pharmaceuticals subsidiary Cosmo Intelligent Medical Devices.
“AI technology — like what Cosmo had developed with the GI Genius underlying technology — we pair that with our understanding of the GI market and the GI clinical needs to enter the sales channel and deploy that product. Now we’re leveraging that to move into other therapies,” Washington said. “But in future technologies where there are additional therapies, we may choose to build that on our own. So it depends on a combination of desired speed, complexity of the technology, and when there’s a product that’s already mature enough that we could just leverage.”
When finding and vetting outsourcing partners, Washington said it’s important to take “time to get outside the walls of your own company.”
“Spend time with the broader technical community, understand how the startup community works, have a network of technology thought leaders that you can leverage for feedback,” he said. “Pressure test technologies that are presented to you to sift out what’s real versus what’s hype, to sift out what’s mature versus what’s so earlystage that it’s not a good risk to take.”
“At the end of the day, we work in a regulated industry, and we welcome that because it’s all about patient safety” Washington continued. “So if you can’t pass the test of the regulatory requirements, then it’s not going to work for us. ... There are over 700 FDAapproved, AI-enabled therapies — they already have a formula for how they evaluate and approve medtech devices and therapies. We feel good about that. Our therapies and our innovations will go through that same process for approval in the U.S. and the equivalent in other regions. And so anybody looking to use AI or collaborate with a third party or a supplier should look at it through that same kind of lens.”

“We’re harnessing the power of AI today for use in clinical decision support, creating new indications, and delivering personalized treatments ... I believe we’re uniquely positioned to advance AI and medtech.”

www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 37
Go to wtwh.me/medtronicai for more, including AI basics from Washington and AI implementation tips from Hong. Medtronic CEO and Chair Geoff Martha Sterilization resistant Fast curing system Passes ISO 10993-5 Paste consistency Resists up to 400°F Electrically insulative One Part Adhesive MasterSil 910Med Hackensack, NJ 07601, USA ∙ +1.201.343.8983 ∙ main@masterbond.com www.masterbond.com USP Class VI Approved Silicone

MAJOR ORTHOPEDIC DEVICE DEVELOPERS AND MANUFACTURERS REVEALED NEW TECHNOLOGIES
AT THE ANNUAL SHOW, WHILE A STUDY RAISED QUESTIONS ABOUT ROBOTIC KNEE SURGERY.
38 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
AAOS 2024

Stryker, Smith+Nephew, Osso VR and more made important announcements at the American Academy of Orthopaedic Surgeons' annual meeting (AAOS 2024) Feb. 12–16 in San Francisco. Here are some highlights on the latest in ortho technology from AAOS 2024:
CHRIS NEWMARKER EXECUTIVE EDITOR
SEAN WHOOLEY ASSOCIATE EDITOR
A new app for Stryker’s Mako
The world’s largest orthopedic device company says its Mako surgical robot
for ortho procedures continues to drive growth. Stryker ended 2023 with 60% of its knee replacement procedures and 34% of its hip replacements in the U.S. performed using the robotic system. Now, Stryker says it wants to further extend a surgeon’s Mako SmartRobotics experience in and beyond the operating room. The company used AAOS 2024 to highlight its myMako app for Apple Vision Pro and iPhone. >>
www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 39 3 • 2024
(ABOVE) Osso VR describes Hand Control as a more intuitive alternative to the standard controls, particularly advantageous in situations where enhanced dexterity is required. Image courtesy of Osso VR
Stryker also unveiled its Triathlon Hinge at AAOS 2024. Stryker said the new knee system reduces the procedural steps of a Triathlon revisionto-Hinge conversion during surgery. Image courtesy of Stryker
When used on Apple Vision Pro — Apple's new virtual reality headset — myMako allows surgeons to visualize and review patients’ Mako surgical


OUR CHAT WITH AAOS PITCH WINNER CYTEXORTHO
CytexOrtho CEO Bradley Estes discussed the company's technology during the Feb. 23 episode of our DeviceTalks Weekly podcast. Listen here:
wtwh.me/cytexortho
CYTEXORTHO’S IMPLANT FOR NATURAL JOINT RESTORATION
AAOS 2024 saw Durham, North Carolina–based CytexOrtho winning the inaugural OrthoPitch Technology Competition. The pre-clinical stage medical device company's ReNew Hip implant is designed to restore the joint naturally rather than replace it. It's a potential solution for active people with early hip disease who are too young for a total hip replacement, according to AAOS.
CytexOrtho founder and CEO Bradley Estes explained during the pitch competition that surgeons remove only the damaged tissue, replacing it with the implant. The implant restores the joint to its proper form and contour. The implant slowly

CytexOrtho says this woven textile on the top of its bioresorbable implant mimics cartilage. Image courtesy of CytexOrtho
absorbs into the body while cells move into gaps in the implant's layers and form functional tissue.
The device has FDA Breakthrough Device designation. The company is completing a phase 1 clinical trial and is seeking funding to start a phase 2 trial.
“The ReNew Hip implant is comprised of two very special components — one is a 3D-woven textile and the other is a high-precision, Tru3D printed component,” Estes said during the pitch event. “The integration of these two components gives us an implant that not only recreates the form and contour of a healthy articular joint surface but also recreates the function of articular cartilage and bone.”
40 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
AAOS 2024
A ‘GLIMPSE INTO THE FUTURE’ FOR SMITH+NEPHEW’S CORI SURGICAL ROBOT
Smith+Nephew's Cori surgical system for knee and hip surgeries includes a 3D intraoperative imaging system and an advanced robotic sculpting tool. The tool's spinning burr automatically stops when the surgeon is outside an area digitally “painted” beforehand.
At AAOS 2024, the British medtech giant showcased Personalized Planning (powered by AI) and the RI.Insights data visualization platform, which the company unveiled last year. The AI-powered software tools guide surgeons as they set patientcustomized implant starting positions.
The company is seeking an additional FDA 510(k) clearance for an image-agnostic solution to further aid in personalizing Cori roboticassisted surgery.


2024 was also the official kickoff for the full U.S. launch of S+N's Aetos shoulder system, which received FDA clearance last year. Aetos has since then secured an additional clearance for use with the company's AtlasPlan 3D planning software and patient-specific instrumentation for total shoulder arthroplasty.
Smith+Nephew designed Aetos to restore patients’ range of motion and help minimize arthritic shoulder pain. The system features the Aetos Meta Stem, which the company designed to maximize stability, preserve bone, and maintain patient anatomy.
(continued on page 43)
The Smith+Nephew Aetos shoulder system is indicated for anatomic and reverse total shoulder arthroplasty. Image courtesy of Smith+Nephew
www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 41 AAOS 2024
SMITH+NEPHEW FULLY LAUNCHES ITS AETOS S HOULDER SYSTEM
AAOS
The Cori system with Personalized Planning and RI.Insights. Image courtesy of Smith+Nephew










This inaugural event provides a unique platform for manufacturers to engage with industry leaders, technology experts, and peers who are navigating the complexities of digital transformation. Participants will gain invaluable insights into strategies, emerging technologies, and best practices that are reshaping the manufacturing landscape. From implementing Industry 4.0 solutions to leveraging data analytics and smart technologies, the forum delves into strategies that can redefine manufacturing processes and elevate operational excellence.


• Connect with manufacturers, engineers, and industry leaders


• Gain insight on digital transformation trends, technologies, and strategies

• Learn from experts who are now digitally transforming their manufacturing



• Hear and share lessons learned with manufactures across industries







Sponsorship opportunities are available for future Digital Transformation Forum programs. For more information, contact Colleen Sepich. 857.260.1360 | csepich@wtwhmedia.com
SUMMIT & EXPO
(continued from page 41)
OS S O VR ANNOUNCES CONTROLLER-FRE E OPTION FOR ITS S URGICAL TRAINING VR
Osso VR used AAOS 2024 to demonstrate its Hand Control, a controller-free option for its virtualreality-based surgical training system. The Hand Control feature uses cameras in the headset to track a user’s hand and figure movements within virtual reality. It leverages Meta’s latest hand-tracking APIs.
According to San Francisco–based Osso VR, Hand Control supports all standard controller interactions. Users can choose between hands-free gestures and traditional controllers.

Study: Surgical robots don’t improve knee surgery revision rates
Robotically assisted total knee arthroplasty has skyrocketed in popularity, but a new registry data analysis raises some questions about the benefits for knee surgery patients.
The analysis of American Joint Replacement Registry (AJRR) data found revision rates were similar in conventional and robotic-assisted cementless TKA at two years post-operatively. The study also found that the odds of revision due to infection or mechanical loosening were not significantly different between the two methods.
”A lot of single surgeon studies show there is improved precision with roboticassisted TKA,” Dr. Lucas Nikkel, assistant professor of orthopedic surgery at Johns Hopkins Medicine, said in a news release about the study. ”Some studies suggest there may be improved early recovery or less damage to soft tissue. One of the challenges with evaluating this is that many previous studies had significant financial conflicts of interest with the authors. We wanted to evaluate these questions using a registry study to eliminate the potential confounding factors to understand if there is a difference when this technology is applied to a surgery.”
Studying the results of robotic knee surgery matters as the field becomes a big business. Ortho device giant Stryker
has been grabbing market share amid the rising popularity of its Mako robots. Stryker ended 2023 with 60% of its knee replacement procedures and 34% of its hip replacements in the U.S. performed using the robotic system. The percentages were 40% of knees and nearly 20% of hips worldwide. In recent years, competitors including Johnson & Johnson’s DePuy Synthes business, Zimmer Biomet, and Smith+Nephew have entered the market with their own robotic systems.
The researchers acknowledged some limitations to the new knee surgery study. National registries such as AJRR rely on the accuracy of data submitted. About 60% of the TKAs on the AJRR register did not report whether or not robotic assistance was used. In addition, the study did not include younger patients.
An AAOS spokesperson said the research has not been to a peerreviewed journal yet, but will be at some point in the future.
The new study identified 9,220 cementless TKAs, nearly half of which were performed with robotic assistance. The results — which used a multivariable, mixed-effects logistic regression model with controlling for cofounders — included:
• The odds of all-cause revision at two years following surgery were similar between the conventional and
robotic-assisted cohorts (odds ratio 0.8, 95% CI .05 to 1.3; p=0.4).
• The odds of revision due to infection were similar between the two groups (OR 1.47 95% CI 0.8 to 2.6; p=0.19).
• Mechanical loosing was not significantly different between the cohorts (OR 3.2, 95% CI 0.8-12; p=0.09).
”Utilizing patients over age 65, we expected them to have higher failure rates as the potential for biologic fixation may be slightly lower,” Nikkel said.
”We found there was no significant differences in the risk of needing another operation within the first two years after surgery with a robotic-assisted or manual technique. This is significant in this population as the likelihood of an early failure is pretty much the same whether robotic assistance is used or not. Some patients desire a robotic-assisted TKA because they’ve heard it is better, but we’ve shown that there isn’t a true benefit in terms of the likelihood of needing another surgery in the early period.”
Go to wtwh.me/aaos2024 for more from the American Academy of Orthopaedic Surgeons' annual meeting.
www.medicaldesignandoutsourcing.com 3 • 2024 Medical Design & Outsourcing 43 AAOS 2024



Our favorite panels and new additions to this year’s show
Our team picks favorites from our upcoming show, including some new additions to the program.
Ienjoy reading bookstore staff recommendations. It’s nice to see how people in the know like to spend their time.
But if you asked me to pick my favorite panels on the agenda for DeviceTalks Boston, taking place May 1–2, 2024 at the Boston Convention & Exhibition Center, I couldn’t do it.
In fact, I won’t do it. Sorry, dear reader, I’ve put too much into this agenda to pick one favorite session. Plus, I owe neutrality to our speakers
— amazing professionals willing to give up two days to help me create something. That’s a covenant that I cannot violate by selecting a favorite.
But I can put our editorial team on the spot to pick their favorites. And they’re definitely in the know when it comes to medical devices. Here are the panels they picked:
“Is the U.S. going to see a British Invasion in surgical robotics?” asked Executive Editor Chris Newmarker about his pick, a panel of leaders from
U.K.-based CMR Surgical discussing how they’re transforming surgery with Versius, bringing robotic assisted surgery to patients worldwide.
“AI’s potential for disrupting the medical device industry is undeniable,” said DeviceTalks Managing Editor Kayleen Brown, who picked our panel featuring Medtronic and Boston Scientific leaders describing how medtech companies are laying the foundation for AI and future tech.
(continued on page 46)
44 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com BOSTON 2024





Sponsorship opportunities are available for future DeviceTalks and Robotics programs. For more information, contact Colleen Sepich. 857.260.1360 | csepich@wtwhmedia.com
SUMMIT & EXPO
Medtech conversations to inspire
BOSTON 2024
(continued from page 44)
Senior Editor Danielle Kirsh picked another Medtronic presentation: How Medtronic Structural Heart & Aortic is working to improve access and equity to life-saving technologies. Kirsh picked this panel for its “insights into innovative approaches that could significantly impact patient outcomes and reduce disparities in cardiovascular care and healthcare.”
Managing Editor Jim Hammerand picked our panel on new approaches to solving the sterilization challenge, given the industry’s need to ramp up sterilization methods beyond ethylene oxide (EtO). “With new rules and regulations looming for EtO sterilization, medical device developers are moving to alternatives — and it hasn’t been easy,” he said.
The Innovation Forum
Last year, we welcomed the MedTech Innovator All-Stars, two dozen topnotch medical device startups that had previously gone through MTI’s accelerator program. With CEO Paul Grand leading the presentations, these medtech entrepreneurs worked to secure the capital they needed to keep their companies moving forward.
This year, we’ll build on that momentum. We’re moving the MedTech Innovator program to our biggest room, which seats hundreds of people and is normally reserved for keynote interviews. The room will be filled with presentations, an investor panel, and a discussion between experienced medtech CEOs.
I’ll have special codes for startups and VCs, so reach out to me via email or LinkedIn.

Finally, Associate Editor Sean Whooley said he’s most excited for our panel on where sensors and other new tech will drive the orthopedics industry. “Canary Medical (with Zimmer Biomet) and Stryker are incorporating sensors, AI and more into orthopedic devices,” he said. “This session will offer insights into how those types of technologies could drive the orthopedics industry forward.”
While I won’t pick a favorite panel, here are a few of my favorite things that we’re adding to the DeviceTalks Boston program in 2024.
More networking
Over the previous two years, we’ve watched the networking ecosystem grow around DeviceTalks Boston.
It started with some sponsors holding cocktail receptions. Last year, MedTech Innovator held an off-site social gathering for its alumni.
This year we’re partnering with industry leaders to create unique opportunities to find people more easily.
We’ll host a MedtechWomen Talks networking breakfast on May 2. Kayleen Brown has done a fantastic


job creating the MedtechWomen Talks podcast series, so we’re bringing that partnership to live by inviting industry leaders to get an early start on Day 2.
In addition to that onsite session, we’ll be partnering on another networking session in the neighborhood. On April 30, Medtronic will host an exclusive networking opportunity at the headquarters of its surgical robotics business in Boston’s Seaport District, just a few blocks from the conference.
Look for more details soon on how DeviceTalks Boston attendees can take advantage of this unique opportunity to see more of Medtronic’s Hugo roboticassisted surgery system. People who have registered will hear about it first, so if you’re interested you should register. Don’t wait.
Expanded expo floor
One of the most important things I learned since taking this job in 2019 is that good device development requires a solid team. If you’re lucky to have that team in your company, great. But if you need to bring some help in from the outside, you can find the right help at DeviceTalks Boston.
I don’t talk enough about the immense talent you’ll fi nd on our exhibit fl oor, which will be full of people with the skills and knowledge to fi nd solutions to your most intractable problems.
While meeting experts who are exhibiting, you also might enjoy our new Engineering Forum, where we’ll be inviting technical experts to deliver their latest research.
You can also enjoy live podcast interviews — including MedtechWomen Talks — conducted in our new studio.
Wow.
I’m clearly excited about the entire agenda. We’ll be diving deep into surgical robotics, neuro, cardiovascular, sustainability, and of course, R&D and engineering. Our expert sponsors are ready to deliver workshops that can guide you around — or over — the biggest roadblocks.
So can I pick a favorite? Sorry, no. But join us at DeviceTalks Boston and tell us yours.
46 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com

Jim’s Pick: MAY 1, 2024
New Approaches to Solving the the Sterilization Challenge

Canary Medical CEO and President Bill Hunter
Sean’s Pick: MAY 1, 2024
Where Sensors and other New Tech Will Drive the Orthopedics Industry

Boston Scientific Director of Digital Health Platforms and Products Sandra Nagale
Kayleen’s Pick: MAY 2, 2024
How Medtech Companies Are Laying the Foundation for AI, Future Tech

CMR Surgical CEO Supratim Bose
Chris’ Pick: MAY 2, 2024
Transforming surgery with Versius: bringing robotic assisted surgery to patients worldwide

Medtronic SVP and Structural Heart & Aortic President Nina Goodheart
Danielle’s Pick: MAY 2, 2024
How Medtronic Structural Heart & Aortic Is Working to Improve Access and Equity to Life-saving Technologies










POWERED BY
Ryan
Jami
Ashley
Mary
Jim



48 Medical Design & Outsourcing 3 • 2024 www.medicaldesignandoutsourcing.com
Ashdown 216.316.6691 rashdown@wtwhmedia.com
Brownlee 224.760.1055 jbrownlee@wtwhmedia.com
N. Burk 737.615.8452 aburk@wtwhmedia.com
Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Dempsey 216.387.1916 jdempsey@wtwhmedia.com SALES Mike Francesconi mfrancesconi@wtwhmedia.com 630.488.9029 Courtney Nagle cseel@wtwhmedia.com 440.523.1685 Jim Powers jpowers@wtwhmedia.com 312.925.7793 Brian Toole 267.290.2386 btoole@wtwhmedia.com CEO Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844 EVP Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609 LEADERSHIP TEAM VP of Sales Mike Emich memich@wtwhmedia.com 508.446.1823 CFO Ken Gradman kgradman@wtwhmedia.com 773.680.5955 AD INDEX AllMotion ....................................................................... 2 Altech Corporation cover/corner, 4, 5 binder USA 25 Cadence 11 Clippard 7 Confluent Medical Technologies 30 Eagle Stainless Tube & Fabrication, Inc. 35 Filmecc USA, Inc, subsidiary of Asahi Intecc USA 14 Interpower 29 KNF Neuberger .......................................................... 17 LEMO USA, Inc. 21 Master Bond 37 Ming Tai Industrial Co., Ltd. 34 New England Tubing Technologies back cover Nitto Kohki U.S.A. Inc. 31 Novotechnik 15 Onanan 22 PSN Labs 1 PTI Engineering cover Resonetics inside front cover Sigmatron 13 Solar Atmospheres 23 Tegra Medical .................................... inside back cover









Since 1898, we've partnered with the most innovative companies around the world to solve complex challenges and advance technology.
As industry leaders, you can depend on our decades of experience, unrivaled reliability, and custom cable solutions that power technology from coast to coast and around the globe.





NO BOUNDARIES, JUST CONNECTIONS
CABLE FROM COAST
COAST Manufacturing Custom Wire & Cable Solutions Since 1898 603-838-6624 • newenglandwire.com Lisbon, New Hampshire USA
CUSTOM
TO


















































































































































































































 Michael Nesnidal ProMed Molded Products
Michael Nesnidal ProMed Molded Products









 Alex Santayana
NuSil — a brand of Avantor
Alex Santayana
NuSil — a brand of Avantor





















































































































