ENDOLUMIK’S















A NEW FDA PROGRAM TO GIVE SAFER MEDICAL DEVICES A PUSH HAS ITS FIRST PRODUCT CLEARANCE.



ENDOLUMIK’S















A NEW FDA PROGRAM TO GIVE SAFER MEDICAL DEVICES A PUSH HAS ITS FIRST PRODUCT CLEARANCE.


Prototyping support with dedicated resources and experts in micro manufacturing. Test your knowledge of the MedTech industry and Resonetics’ capabilities with this crossword.

Across
1. used to show doctors where a device is in the body
5. a part used in the development process
7. type of sensor that uses light
8. the leader in advanced engineering, product development, prototyping, & micromanufacturing
12. moves small amounts of liquid, commonly used in diagnostic devices
13. brand of off-the-shelf batteries from Resonetics
14. network of labs dedicated to quick-turn prototyping and process development
15. number of Lightspeed Labs at Resonetics
Down
2 laser process used to remove small amounts of material
3. type of grinding process
4. machining process using photo resist and acid
6. shape memory alloy made from nickel titanium
9 machining process using wire and electricity to cut parts
10. highspeed laser that needs limited post-processing
11. type of laser process used to connect 2 metals






































Canon Virginia, Inc. is uniquely qualified to be your medical device manufacturing partner. From incorporating advanced technologies to clean room production at scale, see how our full-service offerings can help you solve your manufacturing challenges at cvi.canon.com/mfg.



EDITORIAL STAFF
EDITORIAL
Executive Editor Chris Newmarker cnewmarker@wtwhmedia.com @newmarker
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Pharma Editor Brian Buntz bbuntz@wtwhmedia.com
Associate Editor Sean Whooley swhooley@wtwhmedia.com @SeanWhooleyWTWH
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
Senior Vice President Courtney Nagle cseel@wtwhmedia.com 440.523.1685
CREATIVE SERVICES
VP of Creative Services Mark Rook mrook@wtwhmedia.com @wtwh_graphics
Senior Art Director Matthew Claney mclaney@wtwhmedia.com @wtwh_designer
DeviceTalks Tuesdays is a weekly virtual event that brings the insights and energy of our in-person events to your desktop.

Each DeviceTalks Tuesday will kick off with a quick briefing from the editors of MassDevice and Medical Design and Outsourcing. These presentations will give attendees insights on what trends will be moving medtech in the days to come.
Be sure to check back frequently as new live events continue to be added.

Art Director Allison Washko awashko@wtwhmedia.com @wtwh_allison
Senior Graphic Designer Mariel Evans mevans@wtwhmedia.com @wtwh_mariel
AUDIENCE DEVELOPMENT Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com
WEB DEV/DIGITAL OPERATIONS







Web Development Manager B. David Miyares dmiyares@wtwhmedia.com @wtwh_webdave
Digital Media Manager Patrick Curran pcurran@wtwhmedia.com @wtwhseopatrick
Front End Developer



Melissa Annand mannand@wtwhmedia.com

Software Engineer David Bozentka dbozentka@wtwhmedia.com
Web Dev./Digital Production







Elise Ondak eondak@wtwhmedia.com
PRODUCTION SERVICES

Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com
Customer Service Rep Trinidy Longgood tlonggood@wtwhmedia.com
Digital Production Manager Reggie Hall rhall@wtwhmedia.com
Digital Production Specialist Nicole Johnson njohnson@wtwhmedia.com
Digital Production / Marketing Designer Samantha King sking@wtwhmedia.com


Marketing Graphic Designer Hannah Bragg hbragg@wtwhmedia.com
DIGITAL MARKETING
VP, Digital Marketing Virginia Goulding vgoulding@wtwhmedia.com @wtwh_virginia



Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com @WTWH_Taylor

Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com @WTWH_Matt
Webinar Manager Matt Boblett mboblett@wtwhmedia.com
Webinar Coordinator Halle Sibly hkirsh@wtwhmedia.com
Webinar Coordinator Kim Dorsey kdorsey@wtwhmedia.com
EVENTS

Events Manager Jen Osborne josborne@wtwhmedia.com @wtwh_jen
Events Manager Brittany Belko bbelko@wtwhmedia.com
Event Marketing Specialist Olivia Zemanek ozemanek@wtwhmedia.com
VIDEO SERVICES



Videographer Garrett McCafferty gmccafferty@wtwhmedia.com
Videographer Kara Singleton ksingleton@wtwhmedia.com

FINANCE Controller Brian Korsberg bkorsberg@wtwhmedia.com
Accounts Receivable Jamila Milton jmilton@wtwhmedia.com

One of the world’s largest medical device manufacturers was concerned about wireless connectivity issues that can commonly occur in shielded MRI suites, so we developed a customized hybrid foot switch solution that featured cabled backup where wireless signals could not be transmitted.
Other Steute advantages:
Leadership in robotic applications – larger selection (1 to 4 pedal designs), variation of contact configurations and actuator types and applicable global certification.

Large selection of standard options – Optional auxiliary pushbuttons, fixed or collapsible braces, housing colors, cable and connector types, and contact type and configurations. Our renowned product sample program – We can get you a sample, for the most part, in a week or less.
Contact us today and let us create the perfect solution for you. More to the point, your customers will appreciate you made the switch to Steute!


High in the Wind River mountains of present-day Wyoming, the inhabitants of a remote alpine village — perhaps the oldest in North America — may have used fresh rawhide soaked in water as a splint to immobilize fractured bones thousands of years ago. Before them, the ancient Egyptians used tree bark and linens, and native tribes of South Australia used thick clay. They would all no doubt be amazed by the modern practice of orthopedics on display at this year’s American Academy of Orthopaedic Surgeons (AAOS) annual meeting — after they recovered from the shock of the scintillating sights of Las Vegas.
In this edition of Medical Design & Outsourcing, Executive Editor Chris Newmarker rounds up the biggest launches, updates and findings at AAOS, including news from Stryker, Zimmer Biomet, Smith+Nephew, Orthofix and more. And in our Orthopedics department, I spoke with former Medtronic Spine VP of R&D Tommy Carls about his new endeavor, the Proprio system for spinal procedure visualization. The technology is already observing in operating rooms and could one day be used for joint procedures.
Contributions from expert authors in this edition cover the use of transducer arrays to disrupt solid tumor cell division, digitization in medtech manufacturing, and hurdles standing between device developers and patients. Sadly, this edition also includes a final contribution from Sean Hägen, the late founder of user research and product design firm BlackHägen.
 Jim Hammerand | Managing Editor | Medical Design & Outsourcing | jhammerand@wtwhmedia.com |
Jim Hammerand | Managing Editor | Medical Design & Outsourcing | jhammerand@wtwhmedia.com |












Also in this edition, Pharma Editor Brian Buntz explores 10 promising new therapies that could make a big difference for patients with high blood pressure, cancer, Alzheimer’s and other conditions. Senior Editor Danielle Kirsh has the latest on a 3D-printed pumping heart replica, while Associate Editor Sean Whooley reports on a surgical robot prototype that might one day bioprint inside the human body to repair defects and wounds.
In Software, I spoke with the CEO of heart failure app developer Cordio Medical for tips on making software as a medical device (SaMD) products — and it all starts before you write a single line of code. I also spoke with the co-founders of Endolumik after their device won the fi rst product clearance through a new FDA program meant to get safer products to patients faster. They and their regulatory consultant shared advice on working with the FDA through the agency’s new process, which offers several key benefi ts for device developers.
And fi nally, DeviceTalks Editorial Director Tom Salemi offers an extensive preview of DeviceTalks Boston. Make sure to visit us online May 10 and 11 during the show and after for full coverage from our entire team.
I hope you enjoy this edition of Medical Design & Outsourcing As always, thanks for reading.










Designed to your specifications.
• Available in 1, 2 and 3 pedal designs

• Robust and slip resistant


• High quality materials, such as Aluminum cast alloy and reinforced polyamide











• Environmental protection level up to IP X8 per IEC/EN 60529


































• IEC 60601-1 certi cation





























• Easily cleaned and hygienic
• Ergonomic design with intuitive actuation
• Custom colors, markings, circuits, cable types and lengths available
























 Heavy Base Model
Two Pedal Medical Foot Switch with Lockable Carrying Handle
Heavy Base Model
Two Pedal Medical Foot Switch with Lockable Carrying Handle





















































































































































































































































































































































































BILL DOYLE, executive chair of Novocure, is committed to advancing the company’s patient-forward mission of extending survival in some of the most aggressive forms of cancer through the development and commercialization of its innovative therapy, Tumor Treating Fields. For more than 30 years, Doyle has dedicated his career to expanding patient access to innovative medical technologies.








SEAN HÄGEN was the late founder and principal and director of research & synthesis at BlackHägen Design, an R&D consultancy focused on medical device innovation. He had over 30 years of research and production development experience and served in the management of user research and insight translation phases of product development for BlackHägen’s clients in medical device and other industries. Hägen died Feb. 25 after a brief illness; read about his legacy at wtwh.me/hagen.



DAWN IRONS is director of product management at MasterControl and has over 20 years of life sciences experience in consulting, implementation services, and product management for manufacturing execution systems (MES), enterprise content management (ECM), and data visualization solutions. She has a Bachelor of Science in biochemistry and molecular biology from Penn State University and a Master of Science in business intelligence and data analytics from St. Joseph’s University.
























































HALEY SCHWARTZ started Catalyze Healthcare as a passionate commercialization expert with over 10 years of customer-facing experience in medtech and a knowledgeable leader in driving technology adoption, exceeding business goals and advocating for sustainable process improvement. Catalyze Healthcare works with medical device companies struggling to commercialize in the U.S. by focusing on developing marketing plans, launch plans, go-to-market strategies, competitive analysis, pricing models and more for successful product launches.



HERE’S WHAT WE SEE: Orthopedic device tech advances CONTRIBUTORS
COMPONENTS: Leveraging biophysics to fight the most aggressive forms of cancer




MANUFACTURING: Reduce inefficiency in medtech manufacturing with digitally connected data










ORTHOPEDICS:



Proprio’s OR navigation system could improve orthopedic surgeries


PACKAGING: Improve medical device packaging with human factors engineering
PHARMA: 10 novel therapies set to shape the landscape of medicine


PRODUCT DESIGN AND DEVELOPMENT: 3 reasons why your medical device won’t make it to a patient


RESEARCH: This 3D-printed heart replica pumps blood like a real heart
SOFTWARE: SaMD development lessons from Cordio’s voice AI heart failure app
TUBING TALKS:
Surgical robotic arm could 3D bioprint inside the human body

DEVICETALKS:









54 ORTHO HOT TOPICS: THE TOP ORTHOPEDIC DEVICE NEWS OUT OF AAOS 2023




The 2023 American Academy of Orthopaedic Surgeons annual meeting drew top medical and medtech talent to Las Vegas.


PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development.
We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory.
Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.

• Specialized team of SMEs
• ISO 10993 and ISO 18562 series
• Toxicological Risk Assessment
• FDA Submission Assessment
PSN Labs has the experts to isolate confounding variables and provide clear analysis to guide the commercialization of existing and new medical devices.




For more than 20 years, Novocure has been at the forefront of innovation, pioneering biophysical cancer treatments. With the invention and development of Tumor Treating Fields therapy, Novocure is striving to extend survival in some of the most aggressive forms of cancer.


In modern medicine, cancer treatment has relied on combinations of surgery, ionizing radiation, and drugs — each of which can come with challenging side effects. In 2011, the FDA approved a first-of-its-kind medical device for recurrent glioblastoma that changed cancer therapy expectations with its novel mechanism of action for killing cancer cells.

Yoram Palti, professor emeritus of physiology and biophysics at the Israel Institute of Technology (Technion), founded Novocure in 2000 to leverage his expertise in electrophysiology and create a new way to treat solid tumor cancers. His goal was to destroy cancer
Novocure developed the Optune wearable device to deliver Tumor Treating Fields, electric fields that slow or stop cancer cells from dividing.
cells and spare healthy tissue, improving therapeutic efficacy while avoiding many of the life-altering side effects of existing cancer therapies.
Palti built a laboratory in his basement to test his hypothesis that electric fields could kill cancer cells.
In his lab, Palti proved the biophysical cell death pathway and invented the treatment he called Tumor Treating Fields. Tumor Treating Fields are intermediate frequency, low intensity alternating electric fields that disrupt cancer cell division and kill cancer cells when applied to tissue in specific ways.
An innovative, patient-forward approach Tumor Treating Fields are delivered to patients via a wearable device developed and marketed by Novocure. The device is designed for continuous at-home use, allowing patients who are battling aggressive cancers to receive therapy to extend survival.



(continued on page 16)


Leveraging biophysics to fight the most aggressive forms of cancer
An innovative device harnesses the power of electric fields to disrupt solid tumor cell division.Photo courtesy of Novocure
Learn how our dedicated First Step team and resources, paired with over 100 years of manufacturing expertise, pays off for our customers. When you need to deliver on your NPI/NPD project, First Step enables project teams to truly unleash innovation!
• 100+ machining centers & advanced applications engineers

• Next-level metrology and inspection capabilities
• Sound DFM input, rapid precision prototypes
• The broad capabilities of a vertically integrated manufacturer
Hobson & Motzer are highly skilled engineers, toolmakers, and quality professionals. We are makers of precision metal components with a remarkable depth of knowledge in med device, with a proven track record of adding value to some of the most challenging applications.

(continued from page 14)
Designed to be used with best standard-of-care pharmacotherapies, Tumor Treating Fields therapy is approved by the FDA as a first-line treatment for adults with newly diagnosed glioblastoma. This is a particularly aggressive brain cancer. Those diagnosed with the disease typically live only 12 to 18 months.
In a randomized, phase 3 clinical trial of 695 patients with newly diagnosed glioblastoma, Tumor Treating Fields therapy extended survival by five months on average. Five-year survival increased from 4% to 13% on average. Mild to moderate skin irritation was the most common device-related side effect.

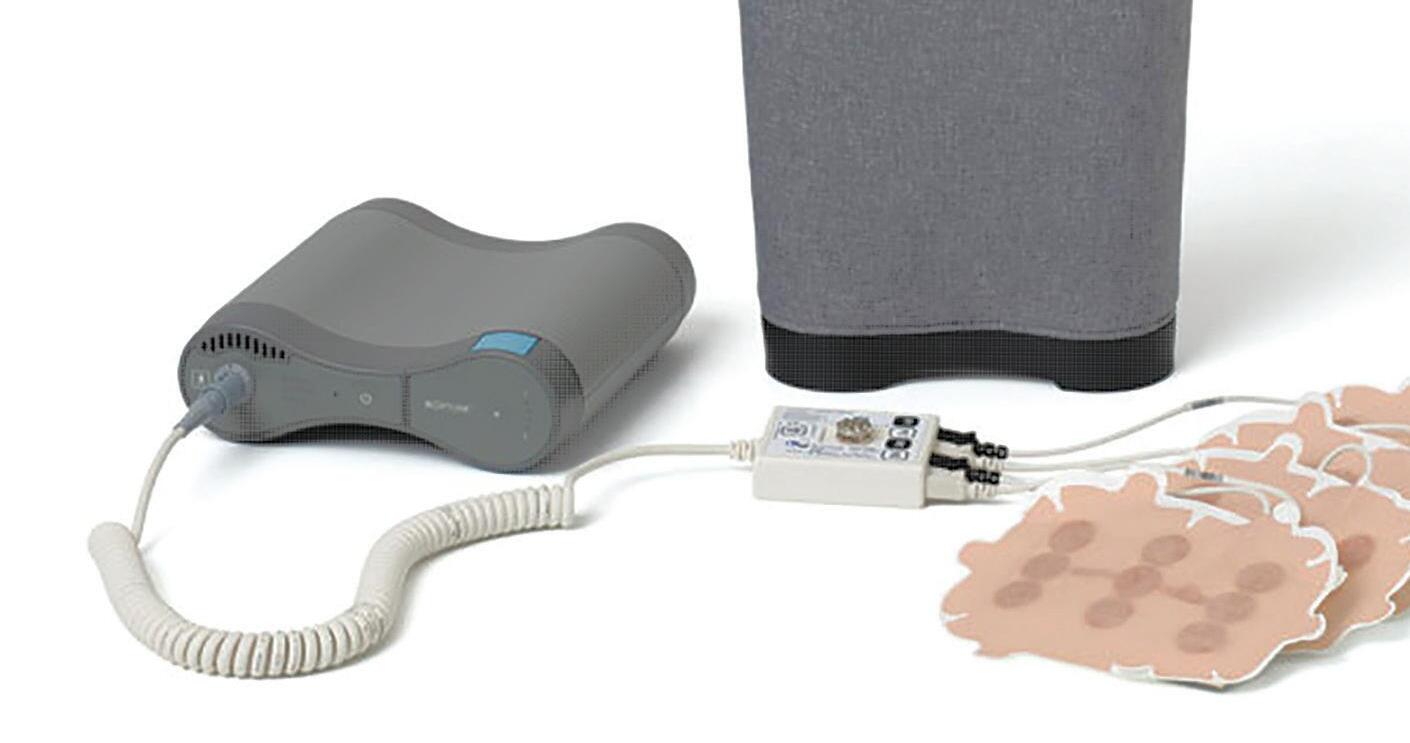
Novocure’s Tumor Treating Fields delivery device is comprised of a durable, battery-operated electric field generator and disposable transducer arrays. Providing this therapy to cancer patients would be impossible without Novocure’s global supply chain, managed from our Root, Switzerland facility.




Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.

system includes a batteryoperated electric field generator and disposable transducer arrays.


Manufacturing partners in Israel and Austria assemble the transducer array circuits are at high volumes using automated lines. Transducer arrays contain critical ceramic elements that were originally developed for sonar systems. The ceramic elements and all other critical components are manufactured by multiple sources to ensure supply continuity. The transducer array circuits are converted into finished medical products and sterilized by multiple manufacturing partners.




True innovation never comes without challenges. The effectiveness of Tumor Treating Fields therapy correlates with the amount of time energy is delivered to the tumor. Patients derive the best outcomes when they receive 18 or more hours of therapy per day. To provide this benefit, Novocure developed a first-of-its-kind, wearable medical device capable of continuously delivering electric fields to a patient’s tumor.

A key challenge was ensuring our devices were robust enough to be used in a patient’s home 24/7. The fi eld generator delivers more than 20 watts of power continuously. That’s signifi cantly more power than most home-use devices, medical or otherwise. Novocure designed proprietary circuits to deliver power effi ciently (greater than 90%) while allowing wearability so patients can go about their mobile lives while receiving therapy.
A second challenge was ensuring our state-of-the-art medical devices were user-friendly and our patients were equipped with the resources to receive continuous therapy as they engaged in the activities of daily life. Early on, we learned that an accessible, hands-on patient support team was essential, so we built a one-of-a-kind medical device patient training and support network. Led by our team of fi eld-based device specialists, Novocure’s support staff is available to patients and caregivers around the clock.
Novocure’s patient-forward approach includes a direct-to-patient distribution model. After receiving a prescription from an oncologist, a Novocure device support specialist hand-delivers devices to patients in their homes and provides in-depth training to patients and their caregivers.
Having invested more than two decades in research and development and treated more than 27,000 cancer patients across the globe so far, we are proud of our innovative work at Novocure. We are committed to improving our systems to further enhance effi cacy and advancing our therapy for use in a broad spectrum of solid tumors, including non-small cell lung, ovarian and pancreatic cancers. While we have come a long way since Yoram Palti invented Tumor Treating Fields, we are confident that we are only at the beginning of our journey of striving to extend survival in some of the most aggressive forms of cancer.
In an increasingly data-driven manufacturing environment, medical technology manufacturers must be able to make data-driven, informed decisions about the state of their business.

Yet paper and disconnected systems are creating an offline data gap that makes it difficult to track production and nearly impossible to make informed decisions.
Three issues are driving a data gap in manufacturing. The first is the continued reliance on paper processes. When manufacturers rely on paper-based processes, cumulative manual errors turn into poor data that advances through the production process and slows everything down. That means more deviations,

production bottlenecks, downtime, wasted resources, and delays in quality review and product release.
The second is data trapped in disconnected systems. When manufacturers work in disconnected systems, they’re relying either on paper or manually tracking and transcribing data from one system to another. Disconnected systems create a data gap that reduces visibility into production processes and raises the risk of data integrity issues, increasing inefficiency and waste.
The third is organizations’ approach to digitization. Even when manufacturers have digitized and are collecting data, (continued on page 20)
Reduce inefficiency in medtech manufacturing with digitally connected data
In medical device and diagnostics manufacturing, closing the data gap is key to reducing inefficiency, waste, and unnecessary cost.
Dawn Irons MasterControlPhoto courtesy of MasterControl
“So much data is captured on paper or in a way that makes it hard for companies to analyze, contextualize and make business decisions that improve efficiency.”
At Pulse Technologies, we’ll produce your component or assembly at any quantity with speed, quality and integrity. As engineers, we’ll act as an extension of your team to develop validation processes, test products and design for manufacturability. As problem solvers, we’ll collaborate and innovate to answer your toughest challenges, anticipate your future needs—and stop at nothing to create your industry edge.




(continued from page 18) many aren’t at a level of data maturity to leverage advanced analytics to connect the dots between data points and improve decision-making.
So much data is captured on paper or in a way that makes it hard for companies to analyze, contextualize and make business decisions that improve efficiency.
Digitizing manufacturing with a data-first mindset is the key to improving decisionmaking and efficiency in production.
Embracing a modern manufacturing execution system (MES) solution with fully electronic device history records (eDHRs) makes manufacturers’ data accessible. Digitally connecting the MES solution and eDHRs with core systems, such as an enterprise resource planning (ERP) system, connects data sources, processes, and people for a holistic view of data. This way, manufacturers can capture and share
real-time production data across systems and departments seamlessly and eliminate data integrity issues before they spread. One factor that manufacturers often overlook when digitizing is considering the intelligence they wish to gain. For example, if understanding on-time release metrics is a goal but the manufacturer isn’t tracking the target release date, how will they adequately track this measure? For this reason, in the process of digitizing, manufacturers must keep a data-first mindset in their MES configurations. Keeping a data-first mindset may entail not only ensuring a single source of truth for lists of values and ensuring best practices and standards for configuration, but also understanding the data questions that the manufacturer wants to answer. Ensuring that data is captured in a consistent, unambiguous manner allows data to be more readily comparable and used. Common lists of values and consistent best configuration practices ensure users who build their own
dashboards do so in a concise manner that will reduce the risk of multiple people creating the same report/dashboard and getting different results. Ensuring the right data captures are built will ensure the most pressing questions are answered.
Implementing a modern MES solution with a data-first mindset will bring offline data online by collecting production data digitally and directly at the source. This automates time-consuming and error-prone manual processes such as DHR data entry, signature gathering, and tracking standard operating procedures (SOPs), training records, and quality events, ensuring high data integrity throughout the entire data life cycle. Data now provides value because manufacturers can connect the dots in new ways to unlock what the data means for operations and react to reduce inefficiency and waste. Real-time data tracking enables









work-in-progress (WIP) visibility and traceability into the status of lines, lots, and operator performance. If something doesn’t look right, the manufacturing supervisor can drill deeper into specific production runs to determine which lots are impacted, which unit procedures or operations are impacted, and more.
The next step is using the data to forecast and process trends. This allows manufacturing to become more proactive, entering the predictive phase of analytics. No longer is it just the manufacturing supervisor looking at the day-to-day production data. Leadership can analyze trends and make data-driven, evidencebased business decisions.
From there, organizations can grow with advanced analytics to further evolve into the prescriptive phase of analytics, where data can recommend actions to prevent ineffi ciencies. Finally, the data becomes truly intelligent by proactively recommending ways to improve effi ciencies.
While manufacturers can expect longterm benefi ts from digital transformation efforts, just starting to digitize unlocks value for manufacturers, including:
• Risk reduction – De-risk processes with visibility of fully connected data across systems;
• Efficiency gains – Use current data as the foundation for future process improvements;
• And cost avoidance – Understand where material loss and rework are occurring.

Manufacturers now generate more data than ever. But in-process visibility into all that data is severely limited when so much of the data is trapped in paper and disparate systems.
To begin making the data work for them and close the offl ine data gap, manufacturers must remove the paper from manufacturing and shift their digitization approach to focus on the data they want to analyze.
Once these foundations are in place, medtech manufacturers must start analyzing by looking at current processes to see how to de-risk, build efficiencies, and reduce waste.
Proprio is rolling its AI-powered surgical navigation system into operating rooms to collect data that will ultimately help surgeons improve how they perform procedures.
The Seattle-based startup said it has placed its Paradigm system in several U.S. operating rooms to capture surgical data that will be useful for accelerating the system’s development.

“Our data-informed platform allows all members of the surgical team access to the right information, at the right time, in the right environment,” Proprio co-founder and CEO Gabriel Jones said in a statement. “By passively capturing data in the background of surgery, we understand and quantify surgery better and are positioned to create the most data-rich platform for surgeons.”
For now, the system’s sensors are only watching and recording in select hospitals, but Proprio will activate the surgical navigation capabilities after getting a green light from the FDA. The company said it expects a commercial release in 2024.
The system uses light field computer vision and AI to help surgeons visualize the patient’s anatomy and surgical space in three dimensions without radiation. Sensors and four cameras in different positions monitor the procedure in realtime, stitching the different views together to help a surgeon visualize from different angles and around obstructions.
(continued on page 24)
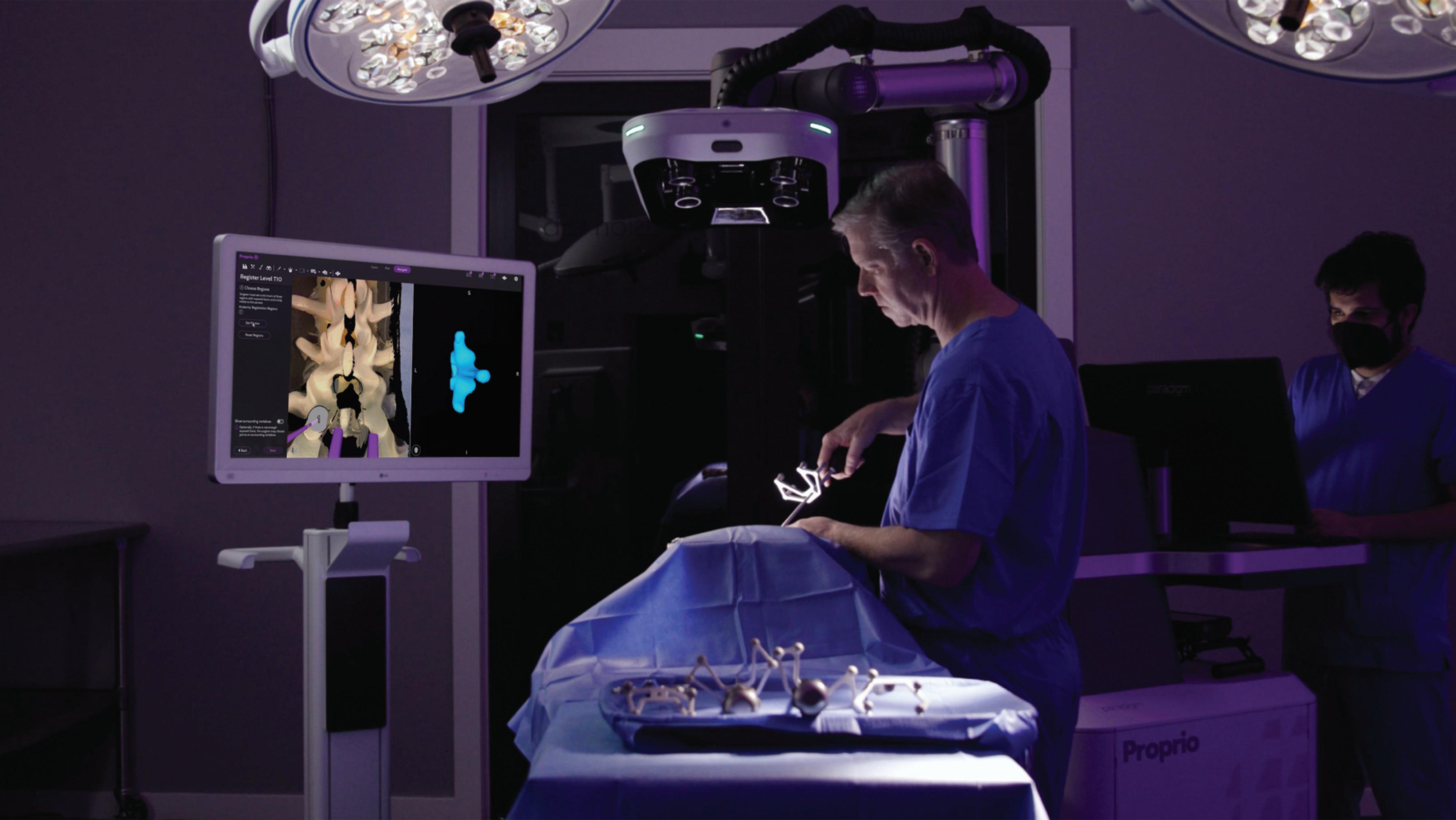
The AI-powered system uses light-field computer vision to help surgeons visualize a patient’s anatomy and surgical space in three dimensions without radiation.Proprio designed its Paradigm system to help surgeons better visualize spinal surgeries. Photo courtesy of Proprio








(continued from page 22)
“Five years ago, computing power was not enough to handle this much information that quickly, so that’s probably one of the biggest leaps forward,” said Tommy Carls, VP of product management and marketing.

Carls, who was once VP of R&D for Medtronic Spine and now explores how to apply Proprio’s technology to specific orthopedic procedures, explained the Proprio technology in an interview with Medical Design & Outsourcing.
Proprio currently has the system focused on navigating spinal procedures. It will allow surgeons to digitally map and visualize the surgery site in the operating room, and then take the information outside of the OR for post-op analysis. The system can monitor which implants are used, the length of the total procedure and specific portions, and peer deeper into the body than a surgeon can easily see.
“We can see down very small corridors. I liken it to, say, a 22 mm hole,
if you will,” Carls said. “We can see down that because we are projecting infrared light as well. It can see pretty far. It doesn’t have to be a completely wideopen procedure. We only need a pinkie fingernail’s surface area to register the locations of this anatomy.”
The system can also update anatomy as bones move in real-time to help surgeons position vertebral bodies.
What’s ahead for Proprio
There’s also potential for computers to analyze and utilize the surgical data in the operating room in real-time, Carls said, creating something like a digital twin of the surgical event.
“Your mind could go wild on what you could do if you had all that information in real-time digitized where a system could be applying AI and machine learning and bringing it right back in the OR in realtime,” he said. “That’s kind of the magic of light field. It allows us to live between two different worlds: the world that is
“By passively capturing data in the background of surgery, we understand and quantify surgery better and are positioned to create the most data-rich platform for surgeons.”



 Tommy Carls
Tommy Carls

physically in front of the surgeon as they’re trying to accomplish some sort of task, and then the digital world that can be overlaid or presented to them in a way that they previously have not had access to.”
For example, suppose a surgeon is placing a pedicle screw, and the system detects a deviance from prior successful surgeries. In that case, the system might one day be able to display a warning sign.

“Because we’ve digitized the entire scene — the anatomy, the soft tissue, everything there — and we are also understanding the exact location of the instrument or implant that the surgeon is placing into the spine, we can do a boundary analysis on what happens if these two cross and provide a warning if you’re one or two millimeters away, before they make that critical error,” Carls said.
Proprio could eventually expand the technology beyond spinal surgeries to other orthopedic procedures such as hips, knees, ankles, wrists and so on. And further developments could integrate different imaging modalities together, combining hard bone imagery from CT scans with soft tissue imagery from MRI scans.
“That’s computing power a couple more notches above, but we would be able to help with soft tissue procedures as well as hard tissue procedures,” Carls said. “I think all of that is possible.”
Human factors engineering (HFE) determines human behavior, abilities, limitations and other characteristics of medical device users and is utilized in the design of medical devices. It involves mechanical and software-driven user interfaces (UI), systems, tasks, instructional documentation, packaging, labeling and user training.
The movement to develop more innovative and advanced technology devices for in-home and the clinical environment makes device packaging more instrumental in improving the user experience and clinical outcomes. And you can expect more packaging innovations in the future, such as 2-D barcodes with unique numbering/ serialization, UV identification codes, holograms and hidden text printed using security or magnetic ink to safeguard against counterfeiting.
While the early focus of HFE tends to be more product-design-intense, it also defines how the device can be packaged safely and efficiently for end-users and validation by regulatory authorities.

Requirements from agencies like the FDA consider safe packaging and labeling a significant part of the user interface. Regulators require a validation process demonstrating safe and effective use. Thus, the packaging is a principal design consideration, and HFE principles ensure user acceptance and successful validation.

Medical device packaging influences user satisfaction and significantly impacts user safety. HFE harmonizes ease of use and safety for an optimal user experience and a positive clinical outcome for both the patient and the clinician.

Packaging and labeling are diffi cult to separate since labeling is often seen as part of the packaging, and both are medical device user interfaces. Typically, medical device packaging incorporates labeling information as part of the package, such as equipment, control, directions for use and maintenance manuals. The displays on CRTs and other electronic display panels are also considered labeling if they provide instructions, prompts, cautions or parameter identifi cation information.
In applying HFE, the mechanical and industrial design of the packaging can have a tremendous impact on usability from both a risk management and ease of use perspective. Packaging can deliver a positive experience for intended access by using an intuitive sequence aligned with a purposeful workflow. The packaging can accentuate labeling of criticality through the physical arrangement of the device in the packaging and associated labeling. Additionally, labeling can minimize contamination by directing users to remove surgical instruments from packaging in the purest way into the sterile field.
(continued on page 28)
Sean Hägen BlackHägenMedical device packaging is increasingly critical as care shifts to homes and product developers make new advances.Photo courtesy of Adobe Stock
• Miniaturization Experts—Micro molded components and assemblies with micro features, tight tolerances, and/or thin walls from 0.001” (25 µ)

• All In-House—Micro 3D Printing, Micro Tooling, Micro Molding, Micro Automated Assembly, and CT Scanning
• Experienced—Over 30 years as a trusted micro solutions provider to the medical and pharma markets





• Risk Mitigation—Quick-turn prototyping, DfM, DfA, and DfX for early detection with over 1,000 successful projects
• Fully Automated—Simple to complex automated assemblies achieving <1 micron positional accuracy
• Materials Expertise—All thermoplastic, long-term implantable, bioresorbable, API, silicone, and fluoropolymer resins


































(continued from page 26)
In quality assurance (QA) programs, manufacturers must incorporate several elements that relate to labeling to meet the good manufacturing practice (GMP) requirements of quality system regulations. It must ensure that labeling meets the GMP device master record requirements with respect to legibility, adhesion and placement, and ensure that the correct labeling is always issued and properly applied.
Medical device packaging influences
Understanding and addressing how packaging can influence device preparation and deployment is important due to the potential for use in different environments. For example, medical device usage at home is growing exponentially. The home medical device market could reach $57.1 billion in 2028 with a compound annual growth rate (CAGR) of 7% from 2021 to 2028, according to Insight Partners. It makes sense that the $29.8 billion medical
device packaging market would follow with a CAGR of 6.4% from 2020 to 2028, according to Grand View Research.
Looking at the many aspects of the packaging design of medical devices, it’s clear that HFE can help direct packaging development processes beyond conducting usability after design development. For instance, when the HFE team analyzes the workflow for care delivery using task analysis, it assesses the user’s interaction with the packaging, including all its layers (primary and secondary packaging), which is one of the initial tasks in the analysis.
The value of task analysis
HFE task analysis often reveals design packaging opportunities to enhance workflow efficiency by designing functionality that does more than protect the device during shipping and storage.
Medical device manufacturers use HFE task analysis to outline the device kit contents and their purpose as well as how to deliver safer passage for a device from its packaging to the sterile field. It prioritizes the device kit contents in the order of use. The task analysis provides a staging area for device usage and deployment that enables better access to labeling (like a quick reference guide), more efficient storage and better access to stored packaging.
The task analysis typically will not increase the recurring production cost of the packaging. Adding features to a thermoformed tray to organize and stage the contents may increase design and testing time, but the packaging production cost stays the same if the overall dimensions of the tray have not changed.
Start packaging and labeling HFE earlier To alleviate stress and minimize risk and costly surprises, factor medical device packaging and labeling early in the development process. Prioritizing packaging and sterilization at the onset of the design process facilitates commercialization and can accelerate time to market.
Packaging design and development used to happen during the last few stages of the medical device development process. But now, using HFE early on when designing the packaging system and selecting the appropriate materials, stress is alleviated during the commercial launch.
A packaging design process started early will not only create a more effective product but will reduce timeline risks and any cost surprises of the packaging materials.

“The movement to develop more innovative and advanced technology devices for in-home and the clinical environment makes device packaging more instrumental in improving the user experience and clinical outcomes.”





The pharma industry is developing a wave of promising therapies that could treat a range of diseases, including cancer, Alzheimer's and hemophilia, with monoclonal antibodies leading the way.

 Brian Buntz | Pharma Editor |
Brian Buntz | Pharma Editor |


An array of novel therapies could significantly impact the treatment of several diseases.



From hypertension to cancer, Alzheimer’s to hemophilia, these promising therapies could change the lives of millions worldwide.
About one-third of the novel therapies on this list are monoclonal antibodies. Gene therapies, antivirals and other drug types are also included.
1. Aprocitentan Indication: Difficult-to-treat hypertension
Estimated peak sales: $2.5 billion (per Jeffries)
The investigational hypertension drug aprocitentan from Idorsia Pharmaceuticals is a dual endothelin receptor antagonist. By blocking the peptide endothelin, the drug can block the constriction of blood vessels, causing blood vessels to relax and leading to a decrease in blood pressure.
In 2017, Idorsia entered into a collaboration agreement with Janssen to develop the drug candidate for treatmentresistant hypertension. In January 2022, Idorsia submitted for European authorization to market aprocitentan for treatment-resistant hypertension. Idorsia filed for FDA approval at the end of 2022.

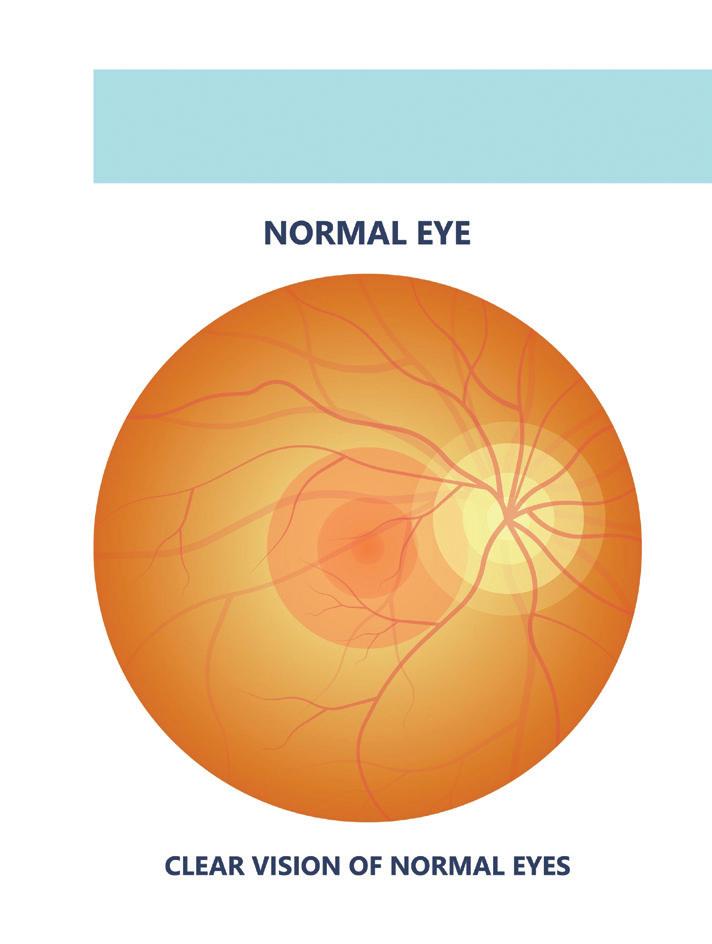
2. Aflibercept (high-dose)
Indications: wAMD, DME, diabetic retinopathy
Sales (2022): $9.6 billion (per Evaluate)
Regeneron submitted a biologics license application to cover high-dose aflibercept (Eylea) for wet age-related macular degeneration (wAMD), diabetic macular edema (DME) and diabetic retinopathy in December 2022. The company used a
A diverse range of groundbreaking treatments have the potential to influence the future of healthcare.Photo illustration by kwanchaift/Adobe Stock
Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, represents a promising new treatment option for moderate-tosevere plaque psoriasis.
priority review voucher when submitting the filing. On Feb. 23, Regeneron announced that the agency accepted the priority review covering an 8 mg dose of aflibercept for the above-mentioned conditions. The agency set a target action date of June 27, 2023.
The drug is already one of the bestselling pharmaceuticals on the market. Analysts are upbeat about the prospects of high-dose aflibercept, which “knocks it out of the park,” according to an SVB Securities analyst. “Regeneron could reap big rewards from high-dose Eylea,” said market research and consulting firm Evaluate.


3. Deucravacitinib Indications: Plaque psoriasis
Projected sales: $2.12 billion by 2027 (per Clarivate)
Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 inhibitor from Bristol-Myers Squibb for moderate-tosevere plaque psoriasis. With a trade name of Sotyktu, the drug scored FDA approval in September 2022. The drug works by inhibiting the activity of the TYK2 protein, which is involved in inflammation regulation.
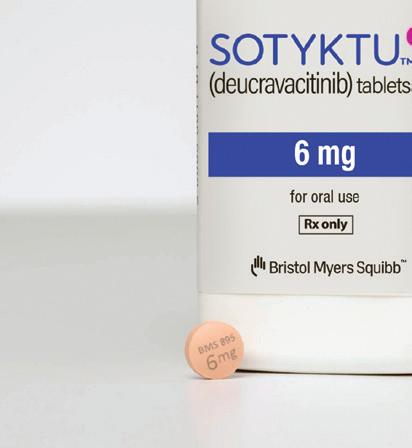
In January 2023, Bristol-Myers Squibb announced that the European Union’s Committee for Medicinal Products for Human Use (CHMP) backed deucravacitinib for adults with moderateto-severe plaque psoriasis.
Clarivate notes that deucravacitinib’s oral administration, efficacy and safety profile could set it apart from injectable erythropoiesis-stimulating agents used to treat plaque psoriasis.

4. Donanemab Indication: Alzheimer’s Estimated peak sales: $4.8 billion (per UBS)
Lilly’s Alzheimer’s monoclonal antibody, donanemab, could receive traditional approval. In January 2023, FDA sent Lilly a complete response letter for the accelerated approval submission for the drug over concerns about the limited number of patients with at least 12 months of drug exposure data included in the submission.
Donanemab works by targeting amyloid plaques in the brain that are associated with Alzheimer’s disease. A growing number of novel therapies with a similar mode of action that target amyloid are set to be available for Alzheimer’s in the near future. Their efficacy remains controversial for the time being, however. FDA approved the first such antibody, aducanumab, in 2021. >>
Macular degeneration, a leading cause of blindness, could see an advance in treatment with the introduction of high-dose aflibercept. Illustration by rumruay/Adobe Stock

5. Efanesoctocog alfa






Indication: Hemophilia A




















Estimated peak sales: €2.3 billion (per Barclays)





























In February, FDA approved Altuviiio (efanesoctocog alfa), a novel, high-sustained factor VIII replacement therapy for hemophilia A from Sanofi and Sobi. The FDA signed off on its use for routine prophylaxis and on-demand treatment to control bleeding episodes and in perioperative management for patients with the bleeding disorder. FDA had granted the drug Fast Track, Orphan Drug and Breakthrough Therapy designations. In addition, the agency evaluated the therapy under Priority Review. Altuviiio can offer hemophilia A patients more days with close-to-normal factor VIII activity levels than older therapies.






6. Epcoritamab












Indication: Lymphoma

Estimated peak sales: $2.75 billion






The FDA accepted AbbVie’s application for the priority review of epcoritamab, an investigational bispecific antibody that would treat adults with relapsed/refractory large B-cell lymphoma. Meanwhile, the European Medicines Agency accepted AbbVie’s Marketing Authorization Application for epcoritamab for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL), a major subtype of large B-cell lymphoma. Data from the EPCORE NHL-1 Phase 2 trial supports the applications. EPCORE NHL-1 assessed the safety and preliminary effectiveness of epcoritamab in adults with relapsed or refractory NHL. AbbVie is developing the drug in partnership with Genmab.


















Hemophilia A patients could see improved quality of life with the approval of efanesoctocog alfa, a novel, high-sustained factor VIII replacement therapy.Illustration by denisismagilov/ Adobe Stock
7. Etrasimod
Indications: Ulcerative colitis and other inflammatory disorders
Estimated peak sales: $3 billion (consensus estimate)
Pfizer’s S1P inhibitor has potential for various conditions, including ulcerative colitis, eosinophilic esophagitis, alopecia areata, Crohn’s disease and atopic dermatitis.

The signaling molecule S1P plays a role in regulating immune cell trafficking and function. Blocking the receptor could put the brakes on immune cells’ travel from lymph nodes to the bloodstream and target organs. That functionality enables it to curb inflammation and tissue damage.
8. Lecanemab
Indication: Alzheimer’s
Estimated peak sales: $9 billion (consensus estimate)
At the beginning of 2023, the FDA granted accelerated approval for Leqembi (lecanemab), a medication for Alzheimer’s developed by Eisai and Biogen. Despite being expected to outsell Aduhelm, the drug has met with a mixed response from the medical community and researchers owing to its steep cost, limited effectiveness, and potential side effects. The yearly cost for lecanemab is set at $26,500.
9. Nirsevimab
Indication: To prevent RSV
Estimated peak sales: $3 billion (per Jefferies)
AstraZeneca’s and Sanofi’s Beyfortus (nirsevimab) is a monoclonal antibody intended to prevent respiratory syncytial virus (RSV) infection in young children. The antibody targets the RSV fusion (F) protein, which the virus uses to enter and infect cells in the respiratory tract. In November 2022, European regulatory authorities approved nirsevimab in infants. Jefferies estimates that nirsevimab will have peak sales of $3 billion, while Evaluate projected peak sales of $700 million in 2026.
10. SRP-9001
Indication: Duchenne muscular dystrophy

Estimated peak sales: $3.6 billion (per SVB Securities)
Sarepta Therapeutics partnered with Roche to develop the gene therapy product SRP-9001 (delandistrogene moxeparvovec)
for Duchenne muscular dystrophy (DMD). Currently, corticosteroids are the standard of care for treating the rare genetic disorder DMD. Unfortunately, the prognosis for the condition tends to be poor, and its symptom burden is high, including progressive muscle weakness, problems walking, frequent falls and learning disabilities.
Mutations in the dystrophin gene are responsible for the condition. SRP-9001 deploys an adeno-associated virus serotype rh74 (AAVrh74) to shuttle a functional copy of the missing or defective dystrophin gene to muscle cells. The drug thus has the potential to restore the expression of functional dystrophin, which is essential for maintaining muscle integrity and preventing muscle damage. Accordingly, FDA has handed SRP-9001 Fast Track designation as well as Orphan Drug designation. FDA designates roughly one-third of novel therapies as Fast Track.
Learn about 10 more promising drugs at wtwh.me/noveltherapies.

Medical devices don’t come to life in a standard operating procedure.
Medical device innovation and commercialization is a complex, orchestrated symphony, and clinical needs, product testing and compliant scalability are the key instruments.

In this article, we explore each of these areas and how you can optimize each to harmonize the process and get your product to patients.
1. Clinical customer misalignment
naysayers who believe “what we’re doing is good enough.”
Haley Schwartz Catalyze Healthcare
Many companies fight — and lose — the battle to fundamentally align a brilliant clinical idea and true clinical requirements for widespread adoption.
For example, commoditized products such as cannulae, syringes and blood pressure cuffs are subject to the forces of distributor and group purchasing organization (GPO) contracts. On the other hand, breakthrough products are inherently subject to skepticism, long lead times to generate clinical evidence, and
Innovating and driving new product adoption in this environment is challenging. To cut through the noise, you must design a product that convinces physicians it addresses an unmet clinical need that existing products do not. The product must also scale in manufacturing, eliminate the potential for user error, increase safety and be adoptable at all levels of clinical acumen to broaden patient reach.
Here’s how to do all of that and more. You’ll need to speak with endusers to leverage their treasure troves of knowledge. Create a deep understanding of your product’s safety, quality and user experience by gathering key insights. The feedback should center around product features, customer service, device longevity, digital integration, supply chain redundancies and continuity into the home, if applicable.
(continued on page 37)



























































































Enjoy more storage space and peace of mind with Interpower’s scheduled ordering for North American (NEMA 5-15, 5-20, 6-15, and 6-20) and international hospital-grade cord sets. Scheduled orders allow customers to lock in prices with predetermined schedules, such as ordering 1,000 cords and having 250 delivered quarterly or monthly—what you need when you need it.
Our hospital-grade cords ship straight from the factory. Want your cords hanked, coiled, tied, bagged and boxed? Need 1-D or QR barcodes for easier warehousing? Customize cord lengths, colors, labeling and packaging for country-specific cords ready to use out of the box. Instock cords ship same day!
Interpower cords and components are continually tested. “We test more than the standards require,” Interpower Product Development Manager Ron Barnett said. “We do so because it lends better reliability to our design.”

• World-class customer service 7 a.m. to 6 p.m. CST
• In-stock cords ship the same day
• No minimum orders
• Value-added options such as colors, lengths, labeling and packaging
• Value-added services such labeling, packaging, lengths and colors
(continued from page 34)
To reach these stakeholders and collect their insights, partner with sales and marketing teams. Deploy questions and surveys at a regular cadence to stay relevant with market demand shifts, competitor evolution and new product introductions. Make sure to use that knowledge, because the time of clinicians (and your commercial partners) is valuable. Respecting their input will allow for an ongoing relationship as your product pipeline evolves.
Is the juice worth the squeeze? Today’s healthcare environment is more dynamic than ever, so if you are not asking these questions, you can be sure your competition is.
2. Limiting device testing to the bench Medical device companies often decline to take testing beyond the bench in an effort to get to market with less firefighting. But testing beyond the bench is one of the best investments a device developer can make.
For patients and physicians alike, quality of life has replaced patient survival as a key metric of success. Medical devices today are measured on the contributions to reduce time, save money and improve outcomes.
With a new product, there is always an eagerness to launch full bore and limit beta testing. Bad idea. Work with your leadership team to define a robust yet short pilot with the product. Animal labs are a great place to start. Time in the hospital is best.
Bench testing may pass with flying colors, but issues can quickly surface when devices are in the clinical environment for the first time. It can be argued that SOPs and a test method validation — TMV, making sure the test you are running indeed tests the thing you want to test — is enough to justify bench testing and circumvent field testing.
What could go wrong? Imagine a small, clear part that is visible enough in a lab, but its transparency makes it easy to lose in a surgical field of blue drapes. Worst case: The clear piece unknowingly falls into a patient’s open cavity and isn’t retrieved, leading to clinical complications.
And while all IT connections may work well in the lab, wait until the hospital’s security system disallows your code on their network. That could mean your product is dependent on the hospital’s IT
infrastructure and cannot be implemented. Another example: If testing products on pigs or other animals that are not reflective of all patients’ skin tones, malfunctions may not be revealed in testing if the product’s performance varies based on different patient pigmentation. Get out there and go beyond your application failure mode and effects analysis that speculates all the ways your product could fail in the clinical environment.
First to market is an extreme advantage when introducing a new product or revolutionary therapy. Likewise, being first to market can threaten clinical adoption when a product that does not perform well is introduced too quickly. The negative impact here affects both the technology’s potential in the marketplace and the reputation of the manufacturer.


Once the right product has been developed and appropriately tested, it’s time to mitigate against an inconsistent global launch. Quality and testing demands differ across international markets to satisfy local regulatory requirements. The FDA and European Union Medical Device Regulations (EU MDR) hold testing to high, albeit different standards. Similarly, standards in Japan, China and rest of the world differ for the same product, creating considerable hurdles the manufacturer must overcome to commercialize and scale globally.
Consider reviewing these nuances as testing protocols are being developed to ensure fewer surprises are later uncovered and impede your product from getting to a patient. Organizations such as the Regulatory Affairs Professional Society (RAPS) can help you develop a baseline understanding allowing for a more educated discussion with your internal regulatory partners. While this adds a little more work for you, remember your regulatory partners are often swamped. Have a targeted, informed list of questions that you need answered for the next iteration of your device to maximize your product development efforts.
Patients are urgently awaiting your next medical device innovation to brighten their lives, just as an eager audience awaits hearing your symphony. With these strategies in mind, what will you do to make the orchestra play even better?

Engineers at the Massachusetts Institute of Technology have developed a 3D-printed heart replica that pumps and looks like a human heart.
MIT engineers designed the soft robotic models to be patient-specific, which could help clinicians determine the best implant for an individual. It’s possible to control the soft and flexible replica’s actions to mimic a patient’s blood-pumping ability.

To make the device, researchers converted medical images of a patient’s heart into a three-dimensional computer model. Researchers can then 3D print the model using polymer-based ink. The resulting model is a soft, flexible shell that is the exact shape of a patient’s heart. The researchers can also use the method to print a patient’s aorta.
The 3D-printed heart’s pumping action is mimicked using sleeves similar to blood pressure cuffs that wrap around a printed heart and
aorta. The underside of each sleeve resembles precisely patterned bubble wrap. When the sleeve is connected to a pneumatic system, researchers can tune the outfl owing air to rhythmically infl ate the sleeve’s bubbles and contract the heart.
Researchers can also inflate another sleeve surrounding a printed aorta to constrict the vessel. They can tune it to mimic aortic stenosis, a condition in which the aortic valve narrows and causes the heart to exert more energy to pump blood through the body.
“All hearts are different,” said study co-author Luca Rosalia, a graduate student in the MIT-Harvard Program in Health Sciences and Technology. “There are massive variations, especially when patients are sick. The advantage of our system is that we can recreate not just the form of a patient’s heart, but also its function in both physiology and disease.”
(continued on page 40)
MIT engineers hope to help doctors tailor treatments to a patient’s specific heart anatomy and function with a custom robotic heart.MIT engineers developed this robotic heart model that can be 3D-printed for each individual patient. Photo courtesy of Melanie Gonick/MIT
“All hearts are different. There are massive variations, especially when patients are sick. The advantage of our system is that we can recreate not just the form of a patient’s heart, but also its function in both physiology and disease.”
The Italian Trade Agency, in cooperation with UCIMU-SISTEMI PER PRODURRE, is pleased to announce this one-day forum



































YOU ARE INVITED TO JOIN US TO LEARN AND NETWORK WITH:
















Leading Italian suppliers to industry and how each contributes to a more scalable, resilient, and secure manufacturing environment.
Scalable, resilient, and secure manufacturing principles are important for businesses to ensure that they can handle growth or changes. These principles can be applied to build greater resilience into supply chains, structure organizations and decision processes for resilience, build resilient and scalable machinery and technologies, and build more secure and resilient next-gen U.S. supply chains and manufacturing ecosystems.
MORE INFO & REGISTRATION: www.italtradeusa.com/vitruv-2023
Thought leaders delivering Manufacturing Market Outlook and Smart Manufacturing Trends presentations.






(continued from page






























In one study, the researchers found that for each 3D-printed heart, they could accurately recreate the same heart-pumping pressures and flows that were previously measured in each respective patient.

“Being able to match the patients’ flows and pressures was very encouraging,” said MIT mechanical engineering professor Ellen Roche, another study co-author. “We’re not only printing the heart’s anatomy, but also replicating its mechanics and physiology. That’s the part that we get excited about.”

The MIT research team wanted to replicate some of the interventions that some patients underwent to see if the printed heart and vessel responded similarly. For example, some patients received valve implants to widen the aorta, so Roche and her colleagues implanted similar valves in the printed aortas modeled after each patient.

When they activated the printed heart to pump, they observed that the implanted valve produced similarly improved fl ows as in actual patients following their surgical implants.
In continuing research, the MIT researchers used an actuated printed heart to compare implants of different sizes to see which would result in the best fit and flow.
“Patients would get their imaging done, which they do anyway, and we would use that to make this system, ideally within the day,” said co-author Christopher Nguyen, a researcher based out of the Cleveland Clinic in Cleveland, Ohio. “Once it’s up and running, clinicians could test different valve types and sizes and see which works best, then use that to implant.”
Ultimately, Roche and the researchers suggest that patient-specific replicas could help develop and identify ideal treatments for individuals with unique and challenging cardiac geometries.
“Designing inclusively for a large range of anatomies, and testing interventions across this range, may increase the addressable target population for minimally invasive procedures,” Roche said.


The research was published in the journal Science Robotics and was supported, in part, by the National Science Foundation, the National Institutes of Health and the National Heart Lung Blood Institute.
Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.


For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.







 MIT graduate student
Luca Rosalia
MIT graduate student
Luca Rosalia
epeat after me: My dad is a big chef. Speaking that short sentence and a few others like it into a smartphone once a day helps identify deteriorating heart failure patients at risk of declining, Cordio Medical CEO Tamir Tal said.

Israel-based Cordio developed its software as a medical device (SaMD) product, the artificial intelligence (AI) voice app HearO, after a cardiologist noticed he could hear congestion in a declining patient’s voice. HearO compares a patient’s voice against baseline recordings to detect lung fluid build-up and alert their care team.

“The holy grail of the entire [heart failure] monitoring industry is to find a solution that will help a patient know in advance when they’re deteriorating so they can change their diuretics and hopefully avoid hospitalization,” Tal said in an interview.
The Israel-based company is conducting a pivotal study at 30 sites in the U.S. with hopes of winning FDA approval in early 2024. The company already has FDA breakthrough device designation and a CE mark in Europe.
If the company wins FDA approval, Tal said Cordio plans to pursue chronic obstructive pulmonary disease (COPD) and even bipolar disorder.
Until then, Medical Design & Outsourcing asked Tal for a few SaMD development lessons Cordio learned through the development of HearO.
Start with a good business model.
“Even before you develop the first code, before you write the first script, you need to understand how you’re going to sell it in the market,” Tal said. “Five or seven years ago in digital health, you could raise money very easily. Now it’s not the case.”
(continued on page 44)
There’s an easier way to manufacture your complex medical device.

Here’s why. You can streamline operations, save money and reduce program risk. Our ISO-13485-certified and FDA-registered facilities deliver quality you can trust. Transfer your device to a ready, willing and completely capable partner.
To learn more, visit Cirtecmed.com

(continued from page 42)
Research your niche — heart failure, in Cordio’s case — to learn how to get a reimbursement code, the best way to win regulatory approval, and the proper indication for patients first.
“Only then do you want to develop a system,” Tal said.
Cordio’s targeting heart failure, in part, because those patients frequently return to the hospital for lengthy, expensive stays. COPD and bipolar patients are also often commonly readmitted. Early warning to adjust medication for those patients could avoid hospital stays and cut costs for health insurers.
Deliver binary results. Your device might be collecting huge volumes of data, but doctors are already overwhelmed with data points as they’re trying to make decisions, Tal said. Make it easier for them by offering simple, clearly defined operative decisions.
“One of the biggest lessons that I think that people should learn is that whatever you do in digital health, the results should be binary,” Tal said. “Physicians don’t want to be bombarded with information.”

Understand the difference between software engineers and biomedical engineers.
The leaders of an SaMD developer often need to bring engineers from different worlds together to successfully develop a medical device. That means
“Software people think they can do anything, because everything is a code. Eventually, you’ll crack it if you’re good enough,” Tal said. “But in SaMD, you’re stuck because there are some things you’re not allowed to do. And you need to work with clinical information and regulatory, which is complicated. It’s a lot of work for CEOs and CTOs to connect all those teams into one team that can work nicely and create a device.”
Don’t forget about patients as users in SaMD development.
Developers of medical devices like implants or surgical tools are wise to keep doctors and payers in mind as device users. But SaMD development often includes a third user to think about: patients, who might be using a software device like Cordio’s on their personal smartphone.
“Most digital health devices are monitoring. That is what we do,” he said. “We use sensors in the mobile device, in our case a microphone that is very available to the patient. So it’s a little bit different.”
The Cardio system keeps it short and simple for patients, asking them to say five sentences into the microphone. They’re short sentences — “The puppy sat on the ship” is another example — and the process takes only about 30 seconds. The app handles the rest, sending the recordings to the cloud for almost instantaneous analysis.
they must understand the difference between biomedical engineers and software engineers.
Biomedical engineers have the training and experience to understand physicians, regulatory constraints and the quality assurance needed for medical devices.
“Even if you’re developing a robot or something that’s very sophisticated from a technology point of view, they understand the universe they’re living in,” Tal said.
But the limitations of medical device development can be a tough reality for algorithm developers to accept.
And one day, instead of telling a practitioner that a patient needs attention, HearO might notify the patient first so they can adjust their own medication as a diabetes patient would with a glucometer.
“I think most digital health devices will not be in the hospital,” Tal said. “They’ll be part of something that’s around you and gives you information. And if that first tier of support doesn’t work, then it goes to the physician, giving them the binary sort of information he needs to decide to call you for a visit.”

“The holy grail of the entire [heart failure] monitoring industry is to find a solution that will help a patient know in advance when they’re deteriorating so they can change their diuretics and hopefully avoid hospitalization.”Photo courtesy of Cordio Medical
For 125 years, New England Wire Technologies has manufactured the most technologically advanced wire and cable in the industry. Our 400,000 square foot, state-of-the-art facility, located in Lisbon, NH, houses manufacturing, R&D, quality/testing, tooling/machine fabrication and office space. With over 400 employees, we operate 3 shifts per day, five days a week. As an ISO9001 registered company, we specialize in technical cable solutions, short lead times, and unparalleled customer service.
For 125 years, New England Wire Technologies has manufactured the most technologically advanced wire and cable in the industry. Our 400,000 square foot, state-of-the-art facility, located in Lisbon, NH, houses manufacturing, R&D, quality/testing, tooling/machine fabrication and office space. With over 400 employees, we operate 3 shifts per day, five days a week. As an ISO9001 registered company, we specialize in technical cable solutions, short lead times, and unparalleled customer service.

New England Wire Technologies
130 North Main Street
Lisbon, NH 03585
www.newenglandwire.com
www.newenglandwire.com
Founded in 1898 in response to the birth of the communication age, today New England Wire is advancing innovation in several industries including medical device and electronics, aerospace, defense, robotics/automation, power generation, and alternative energy. Our capabilities include custom Litz wire, miniature, micro-miniature, single and multiconductor cables, low-noise, high temperature, coaxial and hybrid cables. Since the 1960’s, we have provided cabling and insulating services to the superconductor industry where our prducts are integrated into most of the major accelerator projects, ore separator magnets, NMR magnets, and superconducting magnetic energy storage (SMES) magnets.
Founded in 1898 in response to the birth of the communication age, today New England Wire is advancing innovation in several industries including medical device and electronics, aerospace, defense, robotics/automation, power generation, and alternative energy. Our capabilities include custom Litz wire, miniature, micro-miniature, single and multiconductor cables, low-noise, high temperature, coaxial and hybrid cables. Since the 1960’s, we have provided cabling and insulating services to the superconductor industry where our prducts are integrated into most of the major accelerator projects, ore separator magnets, NMR magnets, and superconducting magnetic energy storage (SMES) magnets.
Through our creative design and development expertise, our engineering staff specializes in product development, concurrent engineering, design for manufacturability, and quick-turn prototyping. Our on-site manufacturing processes include wire drawing, plating, braiding, cabling, insulating, and extrusion services. And, since all of our proprietary equipment and tooling is designed and built in-house, we have complete control while also offering quick modifications when needed. Through true vertical integration of the manufacturing processes, our customers’ design-to-market curve is the shortest in the industry.
Through our creative design and development expertise, our engineering staff specializes in product development, concurrent engineering, design for manufacturability, and quick-turn prototyping. Our on-site manufacturing processes include wire drawing, plating, braiding, cabling, insulating, and extrusion services. And, since all of our proprietary equipment and tooling is designed and built in-house, we have complete control while also offering quick modifications when needed. Through true vertical integration of the manufacturing processes, our customers’ design-to-market curve is the shortest in the industry.
New England Wire Technologies is committed to being the premier custom cable manufacturer of choice for customers in existing and emerging worldwide specialty cable markets. We help our customers dream beyond today’s technology and achieve the impossible!
New England Wire Technologies is committed to being the premier custom cable manufacturer of choice for customers in existing and emerging worldwide specialty cable markets. We help our customers dream beyond today’s technology and achieve the impossible!

A new bioprinting method can go into the body just like an endoscope with the potential for precise 3D wound reconstruction.

 Sean Whooley Associate Editor
Sean Whooley Associate Editor

Engineers in Australia say they have developed a miniature robotic arm for 3D printing biomaterial directly on human organs.

University of New South Wales (UNSW) Sydney researchers developed the device as a flexible, soft robotic arm for 3D bioprinting. This process fabricates biomedical parts from “bioink” to construct natural, tissue-like structures. Predominantly used for research purposes, the process normally requires the use of large 3D printing machines to produce cellular structures outside the body.
Thanh Nho Do — who leads the UNSW Medical Robotics Lab — and PhD student Mai Thanh Thai collaborated with other UNSW researchers to develop a new bioprinting method. Professor Nigel Lovell, Hoang-Phuong Phan and Associate Professor Jelena RnjakKovacina collaborated on the research, published in Advanced Science. The proof-of-concept device is called F3DB and can go into the body just like an endoscope. It directly delivers multilayered biomaterials onto the surface of internal organs and tissues. F3DB features a highly maneuverable swivel head that prints the bioink. It
attaches to the end of a long, flexible, snake-like robotic arm. The researchers say it can all be controlled externally. Within five to seven years, the engineers feel that — with further development — medical professionals could use their technology to access hardto-reach areas inside the body through small skin incisions or natural orifices. The engineers already performed early testing inside an artificial colon. They also 3D printed a variety of materials with different shapes on the surface of a pig’s kidney.





Do explained that the approach addresses existing 3D bioprinters’ limitations, such as surface mismatches and structural damage.
(continued on page 48)


Surgical robotic arm could

(continued from page 46)
He said it offers the potential for precise 3D wound reconstruction, including gastric wall injuries or colon damage and disease.


With a flexible body, the prototype can 3D print all kinds of biomaterials in confined and hard-to-reach spaces.



“Existing 3D bioprinting techniques require biomaterials to be made outside the body, and implanting that into a person would usually require large open-fi eld open surgery, which increases infection risks,” said Do. “Our fl exible 3D bioprinter means biomaterials can be directly delivered into the target tissue or organs with a minimally invasive approach.”


The smallest F3DB prototype they produced has a diameter similar to commercial therapeutic endoscopes, approximately 11-13 mm. The device is small enough for insertion into a human gastrointestinal tract. The researchers say they could scale it even smaller for future uses.

confined spaces inside the body.”
Features of the soft robotics device
The F3DB device includes a threeaxis printing head directly mounted

head features soft artificial muscles for movement in three directions. According to the engineers, this works very similarly to conventional desktop 3D printers. The soft robotic arm uses hydraulics to bend and twist. The engineers can fabricate it at any length and finely tune its stiffness with various elastic tubes and fabrics. According to the researchers, they can program the nozzle to print pre-determined shapes. It can also operate manually for

more complex or undetermined bioprinting when required. The team introduced a machine learning-based controller to aid the printing process.
To demonstrate feasibility, the UNSW team tested the cell viability of living biomaterial after the system printed it. Experiments demonstrated no effect on the cells related to the process. Most cells were alive after printing and continued to grow for the next seven days. The researchers observed four times as many cells one week after printing.


The researchers say F3DB could be an allin-one endoscopic surgical platform for a variety of functions. These include surgery for removing certain cancers, such as colorectal cancer. According to the UNSW team, the printing head can operate as a type of “electric scalpel” to mark and cut away cancerous lesions.
Users can also direct water through the nozzle to simultaneously clean any blood and excess tissue from the site. Immediate 3D printing of biomaterial while the robotic arm remains in place can promote faster healing, too. The researchers say they demonstrated these multi-functional attributes on a pig intestine.
“Compared to the existing endoscopic surgical tools, the developed F3DB was designed as an all-in-one endoscopic tool that avoids the use of changeable tools, which are normally associated with longer procedural time and infection risks,” Mai Thanh Thai said.


For its next steps, the team plans in vivo testing on living animals. They received a provisional patent for the technology and also aim to introduce additional features. These could include an integrated camera and a real-time scanning system. The real-time scanning would reconstruct the 3D tomography of the moving tissue inside the body.

“Our flexible 3D bioprinter means biomaterials can be directly delivered into the target tissue or organs with a minimally invasive approach.”

























ndolumik's story started a few years ago as the FDA sounded the alarm over the risks of internal surgical staplers.
In 2019, the federal agency warned healthcare providers that it had received more than 41,000 medical device reports (MDRs) related to surgical staplers and staples for internal use from 2011 to 2018. Those MDRs tallied more than 32,000 malfunctions, at least 9,000 serious injuries and 366 deaths.
Two years later, the FDA increased the risk level associated with the staplers, reclassifying the devices from Class I to Class II and subjecting them to premarket review and special controls.
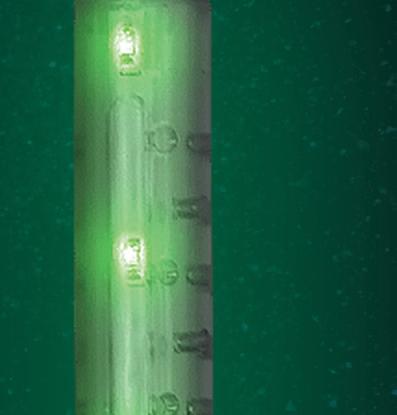
Bariatric surgeon Dr. Nova Szoka had already been working on a device that could prevent some of those injuries. She envisioned an illuminated gastric calibration tube that would shine near-infrared light through stomach tissue and fat. This camera-visible fluorescence would make it easier to see the tube inside a patient, lessening the risk of a surgeon perforating the stomach or stapling the tube to the stomach during bariatric procedures.
In 2020, Szoka founded Endolumik with Mara McFadden, now CEO of the Morgantown, West Virginia-based device developer. In 2022, they submitted their device to the FDA under the new Safer Technologies Program (STeP) and this year won the agency's fi rst STeP authorization.








"Safety was always the most important thing," Szoka told Medical Design & Outsourcing.



As Szoka and McFadden got started at Endolumik in 2020, the FDA prepared to launch the STeP initiative. The FDA based STeP on the Breakthrough Devices Program, which offers streamlined reviews for devices designed to treat life-threatening or irreversibly debilitating diseases or conditions. >>



STeP also offers accelerated review times, but for devices that treat or diagnose less serious diseases or conditions than the breakthrough program. The FDA's goal for both programs is to get safer, better devices to patients faster than the agency's traditional pathways alone.
"Advancements in medical devices that are ineligible for the Breakthrough Devices Program but offer a significant safety advantage in treating and/or
diagnosing less serious diseases or conditions can also provide an important public health benefit," the FDA said when launching STeP in 2021. "... Efforts to improve safety are directly related to improving overall clinical benefits and may also help patients experience fewer serious adverse events."
Medical device developers can ask to join the voluntary program through a Q-Submission. Then, they'll work with the FDA to chart the best path to marketing
in depth how their device improves safety, McFadden said.
"It's non-trivial to demonstrate that you are safer," she said. "You have to show at least one of four different factors. You can either reduce the occurrence of a known adverse event, reduce the occurrence of a known failure mode, reduce the occurrence of a known user hazard or user error, or improve the safety of another device. You really have to do your homework and be ready to speak in depth in terms of how you're going to have an impact on safety."
"McFadden recommended getting help from a regulatory consultant for the STeP request and 510(k) submission. Endolumik used Nilo Medical Consulting Group, led by former FDA reviewer Michael Nilo.
"The process for novel Class II medical devices is not always as straightforward as it may seem with the 510(k) program," Nilo said. "FDA does a great job of setting up programs for innovative, safer devices that can help developers get better access to the review teams and encourage collaboration. I'd advise developers to look into all the programs and give it a shot. They are free to apply to, and the worst outcome is an introduction to the review team that you'll be working with."
authorization, whether that be premarket approval, de novo or 510(k).
The FDA accepted 14 STeP requests out of 30 applications last year, according to the Center for Devices and Radiological Health's annual report.
Taking the big STeP Endolumik's founders were sure they should take the 510(k) pathway, and in a Q-Submission meeting with the FDA in 2022, both sides decided the device was a good fit for STeP.
"We found them to be really responsive in terms of timely, interactive review," McFadden said in an interview. "Sometimes you hear horror stories of submissions just sitting for the full 90 days before you hear much of anything, which was not our experience. They got back to us pretty quickly, and we had the opportunity to interactively go back and forth."
Medical device developers should clearly understand STeP's eligibility requirements and be prepared to discuss
Endolumik's contract manufacturer, Virtec Medical, was indispensable, McFadden said. The biggest challenge in designing the device was balancing fl exibility and rigidity.
Too stiff, and the device couldn't safely navigate through the throat and might punch a hole through the esophagus or stomach. Too soft, and the device would "buckle like a spaghetti noodle" before reaching the stomach, McFadden said.



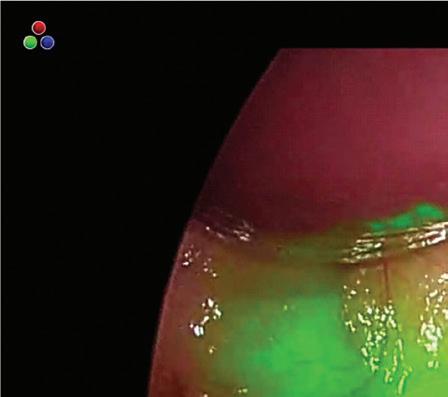
The trial-and-error process started with the first 3D printed prototypes in pig and cadaver testing.
"Holding the balance of the functional dimensions we needed in terms of the outer diameter and the size of the electrical lumen and getting the cross-section correct to get the mechanical properties we wanted — buckling and kink resistance and that sort of thing — took more iteration than

“Advancements in medical devices that are ineligible for the Breakthrough Devices Program but offer a significant safety advantage in treating and/or diagnosing less serious diseases or conditions can also provide an important public health benefit.”
I would've guessed from the fi rst go," McFadden said. "That was one of our biggest unanticipated challenges."
The tube is made of a PVC-like material, with one lumen for the nearinfrared LEDs and another to provide suction so surgeons can remove stomach juices as needed. To determine the right material and dimensions, the team went beyond durometer testing of existing gastric tubes to fi nd a "Goldilocks-perfect" balance.



"Our engineering team really broke down different characteristics of this device: kink pressure, buckle pressure, twisting pressure — basically quantifying all those things and then dialing it in," she said.
At the final cadaver lab, the team invited some surgical residents to give it a try.

"Once I was able to see some trainees — who had never used a device before — safely introduce it, that's the point where I felt we were ready to jump to a humangrade device in a clinical trial."


Endolumik's clinical trial has treated 22 patients so far without any adverse results, Szoka said.


What's next for Endolumik
Endolumik's florescent gastric tube is a single-use device. One goal for future improvements is to design for sustainability, including improved recyclability.

And while McFadden declined to go into detail about other possibilities for the company, she referenced the

growing number of near-infrared cameras in operating rooms that could give surgeons better visibility with Endolumik devices of the future.
"It's really prompted us to start thinking long and hard about where else in the world of minimally invasive surgery are there hazards created by not having a great line of sight to a tool," McFadden said. "We defi nitely have one other product in the R&D pipeline that we're really excited about, that we think is solving an equal, if not greater, pain point in the anatomy."
"We think it's the beginning of a digital surgical tool revolution that we're being able to exploit the tools that are in the OR today, the towers that are in the OR today, to give surgeons advanced visuals," she continued. "And we're really excited to be part of what's next as even more digital surgical tools come on the market, and to see what the next generation of cameras can do."

“We think it’s the beginning of a digital surgical tool revolution... And we’re really excited to be part of what’s next.”Endolumik cofounders Dr. Nova Szoka (left) and CEO Mara McFadden Photo courtesy of Endolumik














The 2023 American Academy of Orthopaedic
Surgeons annual meeting drew top medical and medtech talent to Las Vegas.
Stryker’s nextgen Mako total robotic knee surgery system and more AI integration for Zimmer Biomet’s ZBEdge surgical platform topped the news at this year’s AAOS 2023. Nearly 20,000 people attended the event, which ran March 7–11. Here is a roundup of the major news from the show.
Stryker launches Mako Total Knee 2.0 Stryker unveiled its Mako Total Knee 2.0 surgical robotics platform. Informed by more than 500,000 procedures, Stryker said it designed the next-gen version of the robotic knee
surgery system to provide an elevated user experience with customizable workflows and other key features. New features include a digital tensioner for assessing knee stability intraoperatively during total knee arthroplasty — without the need for additional instrumentation.
Stryker began a limited release of Mako Total Knee 2.0 in August 2022. It plans to continue a phased rollout in 2023, starting with the U.S. before launching globally in select markets.
“Over the last six years, we’ve gathered key feedback from our customers and incorporated those fi ndings into the development of Mako Total Knee 2.0 — refl ecting our ongoing commitment to our customers and their patients,” said Don Payerle, president of Stryker’s joint replacement division. >>
Zimmer Biomet announces enhancements to its ZBEdge platform
Zimmer Biomet announced that it plans more AI integration and other enhancements to its ZBEdge Dynamic Intelligence platform for hip and knee surgeries.
The improvements include greater integration of ZB’s WalkAI, artificial intelligence that can predict which patients will have lower gait speed 90 days after hip or knee surgery.





Warsaw, Indiana–based Zimmer Biomet designed its ZBEdge Dynamic Intelligence platform to integrate its digital, robotic and implant technologies. It connects and collects objective data throughout the entire episode of care. The company aims for ZBEdge Dynamic Intelligence to transform data into insights. The goal is to elevate the standard of care.
“Nearly two years following its introduction, ZBEdge Dynamic Intelligence continues to deliver innovations with breakthrough technologies designed to enable smarter decision-making, more efficient care and optimized user experiences,” said Ivan Tornos, COO at Zimmer Biomet.
Stryker said it designed the next-gen Mako Total Knee 2.0 to have an elevated user experience with customizable workflows and other key features.
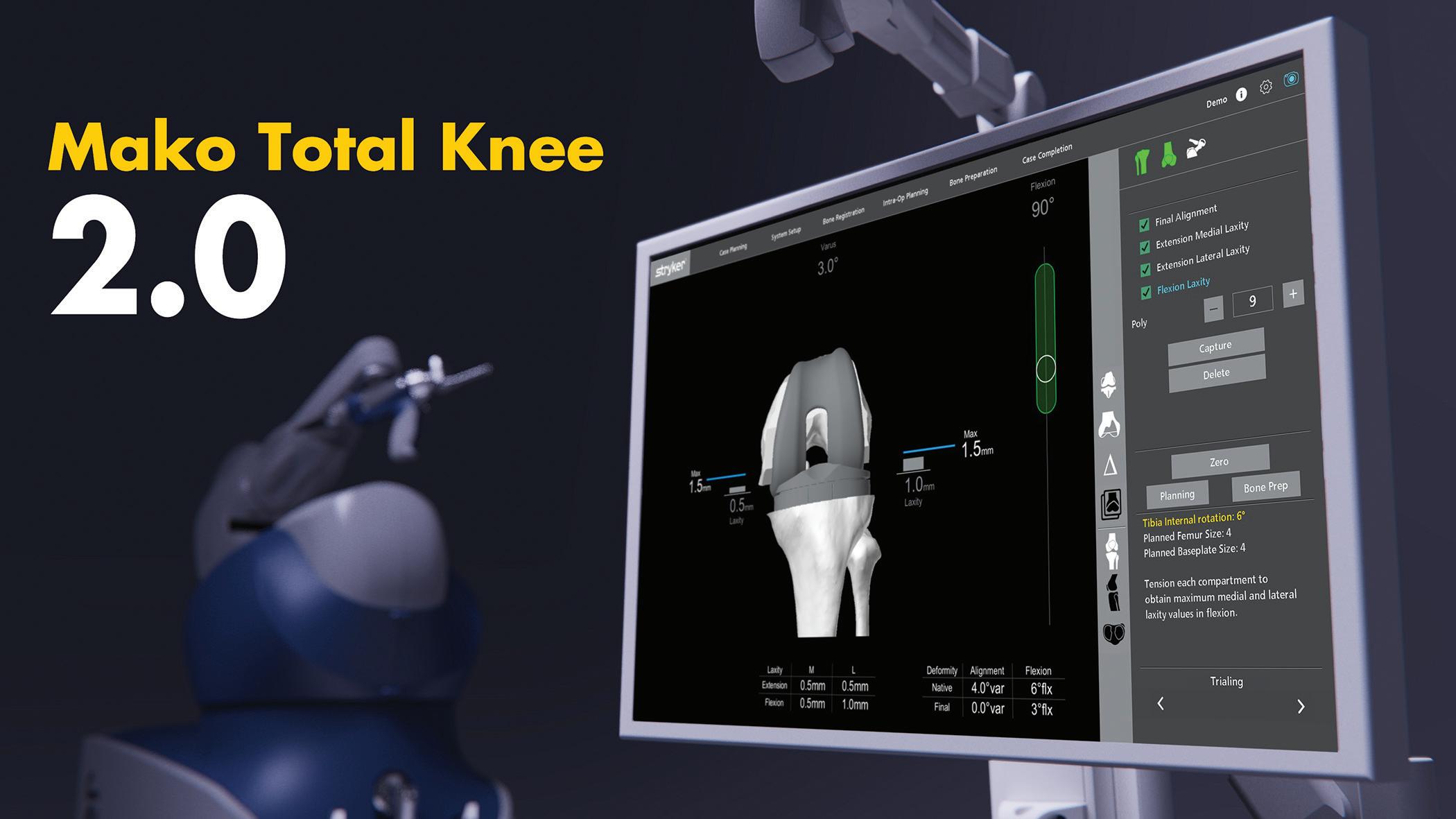
Image courtesy of Stryker
Smith+Nephew data backs its patientspecific knee surgery tools Smith+Nephew published a systemic literature review that backs its Visionaire patient-specifi c instrumentation for knee surgeries.


The research was published online by the Archives of Orthopaedic and Trauma Surgery. It included a meta-analysis of 25 relevant studies that compared Visionaireenabled total knee arthroplasty (TKA) with conventional instrumentation-enabled TKA. The analysis demonstrated improvements in alignment accuracy, efficiency in surgical procedures, and reduction in length of hospital stay in comparison with conventional instrumentation, London-based Smith+Nephew said.

People are enthusiastic about getting joint replacements on an outpatient basis Most people who underwent a total joint arthroplasty on an outpatient basis at an academic medical center (AMC) would do it again.
That’s according to research presented at AAOS 2023. The study backs up the trend of more orthopedic surgeries that don’t require hospital stays.
Lead author Soham Ghoshal, a medical student at Harvard Medical School, said academic medical centers historically haven’t done the procedures on a sameday basis. Showing that people want the procedures on an outpatient basis — and that they’re safe with similar outcomes — could guide the academic medical centers to make a shift, Goshal said.
The study included a total of 281 total joint arthroplasty (hip or knee) and unicondylar knee arthroplasty procedures performed on an outpatient basis at AMCs. Cumulatively, 94.6% of patients said they would undergo their procedure again, and 92.7% would choose a sameday discharge again. The mean time to discharge was 5.4 hours. Only nine patients needed readmission.
Orthofix announced that it surpassed 5,000 device implants for its Fitbone TAA intramedullary limb-lengthening system.
With more than 20 years of clinical history, the device demonstrated safety and effectiveness in limb lengthening and deformity correction in adults and children, according to Orthofix. It acquired the Fitbone intramedullary lengthening nail from Wittenstein SE in March 2020.

The fully implantable system corrects leg length and deformity discrepancies through a minimally invasive implantation procedure. Fitbone features a motorized intramedullary nail, a subcutaneously placed receiver and an external control set. This enables the patient to manage the distraction phase at home. Once the patient completes their treatment, they have the nail and receiver removed.
Younger total hip arthroplasty patients have low revision rates at eight years Surgeons are providing more total hip arthroplasty (THA) procedures for people under 65. The concern has been that the younger patients historically
had poor long-term outcomes associated with implant failure.

However, a recent study presented at AAOS 2023 drew on American Joint Replacement Registry data to show that only 1% of sampled patients needed revision surgery for THA within an eightyear period. The study also found higher THA revision rates among Black patients when compared to white patients.

“The demand for total hip replacements is expected to increase 174% from 2005 to 2030, and 28% of the 572,000 THA procedures performed annually are in patients under the age of 55,” said lead author David Cieremans, a medical student at Philadelphia College of Osteopathic Medicine.

“When a young and active patient is exploring THA, it’s important for their surgeon to understand the expected life span of the implant to help make informed decisions. The AJRR provided an incredible resource for our team to obtain robust data on hip replacements, along with a standardized metric to analyze surgical trends and patient outcomes from around the country,” Cieremans said.
Orthofix’s Fitbone system corrects leg length and deformity discrepancies through a minimally invasive implantation procedure. Image courtesy of Orthofix
Philadelphia College of Osteopathic Medicine student David Cieremans
































Medtech faces many challenges, but every challenge presents an opportunity — and DeviceTalks Boston will focus on making the best of them.
Editor’s note: This issue of Medical Design & Outsourcing may reach some print edition readers after DeviceTalks Boston. Visit us online for coverage during and after the conference.

Leaders like to say every challenge presents an opportunity. It sounds uplifting and offers the benefits of being at least partially true, in life and in medtech.
It’s fair to say the medical device industry is staring at a vast sea of opportunity. Our nation’s healthcare system is under enormous stress. Our financial systems are showing signs of a different type of strain, and medical device companies of all sizes are being forced to make cuts to programs and, unfortunately, employees.


The challenges presenting all these opportunities might hurt in the short



term. But, as attendees of DeviceTalks Boston will learn, leaders are emerging to guide the medical device sector out of the darkness.







We’ll examine many challenges

May 10–11 at the Boston Convention and Exhibition Center. Fortunately, we’ll have experts on hand to help you find the opportunities as well.

Finding sustained growth: Medical device companies of all sizes are getting drained by rising interest rates, the strong U.S. dollar, persistent supply chain disruptions and more, making it difficult to sustain growth.


Opportunity: No doubt, this will be a question posed to many speakers, but I’m looking forward to speaking with Boston Scientific CEO Michael Mahoney in particular.



(continued on page 60)






































VP of Research & Business Development, Cardiac Ablation Solutions; MEDTRONIC



















devicetalks.com/podcast/




















(continued from page 58)


In his decade-plus at the helm, the company’s market cap has grown 10X. I’ll ask Mahoney how Boston Scientific will sustain growth. In my podcast interview with him last year, he said he drew a lot of lessons from co-founder Peter Nicholas, who passed away last year. “He was an optimistic person who did things the right way,” Mahoney said. “Always looking forward and never worried about celebrating the past. [He was] just looking for new opportunities constantly.”

Workforce: Both global medical device companies and smaller startups are feeling pressure from larger economic pressures, forcing them to cut payroll and delay projects.
Opportunity: This disruption creates an opportunity for medical device professionals looking to redirect their careers. Our opening panel will bring together investors, engineers and other experts to give medtech professionals a sense of the roles and opportunities facing engineers at medical device startups. Participating firms include MedTech Innovator, The Mullings Group, and Viant Medical. Our colleagues at the Healthcare Robotics Engineering
Forum — which runs concurrently with DeviceTalks Boston — will co-host a robotics industry job fair organized by industry group MassRobotics. Finally, our concluding panel discussion will include me, Life Sciences Executive Editor Chris Newmarker, and Joe Mullings, CEO of the Mullings Group, to examine where our industry is headed.
Engineering and innovation challenges
Competitive ablation field: Cardiac ablation is becoming an increasingly competitive field with entrenched and emerging players.






Opportunity: Medtronic last year paid $1 billion for Affera’s advanced mapping and ablation technology. The company’s Sphere-9 catheter in March secured CE Mark approval. (It’s currently undergoing a pivotal clinical trial in the U.S.) In this presentation, Tim Laske— Medtronic’s VP of Research & Business Development, Cardiac Ablation Solutions — will share how the company is leveraging the unique flexibility and durability of nitinol to construct the Sphere-9 catheter, potentially giving the company a leg up in a competitive space.
Designing pediatric devices: Designing surgical implants for pediatric patients is diffi cult.
Opportunity: Cracking the code for pediatric implants can open new markets. ZimVie recently secured a string of reimbursement approvals for its Tether Verterbral Body Tethering System, a non-fusion spinal device intended for treatment of idiopathic scoliosis. In this panel, Rebecca Whitney, global president of spine, and Ryan Watson, director of R&D, will detail the work that went into the design, construction and commercial rollout of this novel device.
SVP, Global Spine President; ZIMVIE
R&D Director; ZIMVIE SPINE






Incremental innovation: Medical device companies selling new technologies and tools face increased scrutiny from hospital technology committees. Hospitals are in crisis mode and have less time to onboard new tools, so any new tech must be essential.
Opportunity: More than ever, engineers, product developers and clinical leaders at medical device companies must find a way to develop critical “need-to-have” functions in their new products. Orthopedic surgeon Dr. Nitin Goyal, Zimmer Biomet’s chief science, technology and innovation officer, will discuss how the orthopedics giant looks to cut through resistance by incorporating critical customer needs into its innovation process.

Sensor overload: Sensors are small and effective enough to find a home on many medical devices, but when does their addition make sense?
Opportunity: Boston Scientific sees a viable use in urology. Meghan Scanlon, president of Urology, will lead a discussion about the company’s LithoVue Elite SingleUse Digital Flexible Ureteroscope System, which is the first system with sensors capable of monitoring intrarenal pressure during procedures. The FDA issued 510(k) clearance for the device in February. Kristin LaRocca, SVP, sales, will be on hand to explain the market opportunities.


Risks with longer medical device lifespans: Medical device makers building implantable devices to treat chronic diseases need to account for two important factors. First, materials used to make medical devices degrade over time, sometimes becoming toxic to the patients they’re built to help. Second, neurostimulators and other implantable devices requiring batteries need power sources with long lives or wireless rechargeability, or else the patient likely faces repeated surgical procedures. Opportunity: Two presentations at DeviceTalks Boston will examine what it takes to build medical devices with longer lives. PSN Labs will look at how supply chain disruptions have forced device makers to use less optimal materials, increasing the risk of non-biocompatibility over time. PSN will offer possible solutions. Meanwhile, Resonant Link, which is collaborating with major medical companies, will examine advancements in wireless power that will lead to devices that last longer and create a competitive advantage. >>
Chief Science, Technology and Innovation Officer; ZIMMER BIOMET




President, Urology; BOSTON SCIENTIFIC





devicetalks.com/podcast/















Chief Strategy Officer;






Healthcare workers: Hospitals and other healthcare settings are facing shortages of physicians, nurses and other critical staff.
Opportunity: In a keynote interview, Becton Dickinson CEO Tom Polen will lay out a plan for growth building off a sequence of acquisitions over the past two years that has


the company loaded with tech — including digital and robotics tools. In a DeviceTalks Weekly interview, Polen saw automation of pharmacies and lab testing as a sizable growth opportunity for BD.

The next day, Jeffrey Alvarez, chief strategy offi cer of Moon Surgical, joins a panel of digital health, robotics and imaging leaders to discuss the future of the operating room. Moon Surgical’s Maestro robot — which can hold surgical tools in place — is designed to replace surgical assistants in the OR.
Role of diagnostics: The pandemic elevated the essential role of point-ofcare testing.

Opportunity: Dave Hickey, EVP and president of the life sciences segment at BD, will review the shifting role of diagnostics in healthcare over the past three years. Hickey led BD’s Integrated Diagnostic Systems unit during the COVID-19 surge in 2020 and 2021. BD is on track to expand its portfolio of at-home diagnostics, putting more healthcare in the hands of patients.

Streamlining training and clinical testing: Medical training and clinical testing are both arduous processes that require enormous time and resources.
Opportunity: We’ll commit a significant portion of our Day 1 agenda to virtual technologies that simulate a healthcare workplace setting or the human body itself. FundamentalVR, a healthcare technology company using VR, haptics (the sense of touch), and machine learning, will demonstrate the value of these technologies in training. According to FundamentalVR, its tools can be used to create long-distance training platforms for medical device and pharmaceutical companies as well as healthcare systems. Meanwhile, Dassault Systèmes is taking virtual simulation technology to the patient level. In a presentation that immediately follows, the company will explain the power of virtual twins, which are dynamic, digital representations of patients. Companies using virtual twins can simulate and test treatments on virtual cohorts when in development and use streams of real-world data fed from sensors to the virtual twin when used in clinical trials and ongoing care. These insights allow medtech organizations to bring better products to market more efficiently.






Medical sales: Medical device sales teams are under increasing scrutiny as being an expensive part of bringing new products to market.
Opportunity: DeviceTalks Boston will feature solutions from two different companies — AcuityMD and Integrity Solutions — that offer medical device companies new tools, data and approaches to build new markets more effectively. AcuityMD is offering access


















to a trove of reimbursement data that can help sales teams prioritize sales targets. Integrity Solutions, meanwhile, will provide a comprehensive, actionable roadmap for enlisting clinicians into the customer-facing sales teams.

Environmental, social and governance challenges







Sustainability: Medical devices can produce a profoundly positive effect on individuals needing care, but they create enormous pressure on waste streams once they’re outdated and discarded. Opportunity: Medical device makers can avoid this growing catastrophe by building sustainability into their designs. In two different sessions, leaders Canon Virginia, Gradian Health Systems and Sagentia Innovation will examine how medical devices can be made with a view toward global health.
Healthcare equity: How can we ensure life-saving medical technology reaches everyone who needs it?





Opportunity: Medical device companies must continue to push for equitable access to their products. For example, Abbott’s HeartMate 3 left ventricular assist devices (LVADs) are clinically proven to extend life to five years and beyond, yet the company estimates 15,000 advanced heart failure patients have median lifespans of less than a year. To address this disparity, Abbott leaders assembled a panel featuring Kevin Bourque, VP of R&D for HeartMate 3, a left ventricular assist device, and Dr. Robert Kormos, division VP, Global Medical Affairs, Heart Failure. Abbott also enlisted Dr. Robert S.D. Higgins, MSHA, president of Brigham and Women’s Hospital and EVP, Mass General Brigham, as well as a patient who has transitioned off the HeartMate 3 and onto a transplanted heart.







These are just some of the topics we’ll be addressing at DeviceTalks Boston on May 10-11. Whatever challenge you’re facing, we think you’ll find the support you need at the meeting. Register at devicetalks. com. See you in Boston!









SALES











Ryan Ashdown 216.316.6691 rashdown@wtwhmedia.com
Mary Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Courtney Nagle cseel@wtwhmedia.com 440.523.1685 @wtwh_CSeel




Jami Brownlee 224.760.1055 jbrownlee@wtwhmedia.com
Ashley N. Burk 737.615.8452 aburk@wtwhmedia.com

LEADERSHIP TEAM
CEO Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844 @SMMcCafferty


Jim Dempsey 216.387.1916 jdempsey@wtwhmedia.com

Mike Francesconi mfrancesconi@wtwhmedia.com 630.488.9029





Jim Powers jpowers@wtwhmedia.com 312.925.7793 @jpowers_media

Brian Toole 267.290.2386 btoole@wtwhmedia.com
VP of Sales Mike Emich memich@wtwhmedia.com 508.446.1823 @wtwh_memich

EVP Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609 @mmatheson
































It starts with decades of product development expertise. Our vast catalog of standard components can be customized to generate endless possibilities for sets and kits. We put in place processes for ensuring stringent quality levels, managing volumes of documentation and tracking all project details. Add in a full suite of capabilities, and you have a turnkey solution that’s more like a partnership for creating confidence.