






























With 30+ years of experience, Resonetics’ expert engineers can process nitinol tubing with a wide range of sizes and a variety of finishes.
With a facility that processes the original melt and 2 facilities that process nitinol tubing, we can help you select the material for your next project and scale to meet your high-volume demands.

From small hypotubes for advanced interventional products, to large precision tubes for cardiovascular stents, we melt in-house and deliver tight tolerances.

Scan the QR code to discover the world of nitinol tubing and proprietary technology from Resonetics.

It’s hard to think of a product update with higher stakes for a device developer — and the medtech industry — than Intuitive Surgical’s da Vinci 5 robotic-assisted surgery system.
All eyes are on the world’s leading surgical robotics developer as it rolls out the next generation of its flagship system after winning FDA clearance. Will the new features and long list of design changes put even more distance between surgical robotics and conventional laparoscopy? Is Intuitive advancing its technology rapidly enough to maintain or expand its lead ahead of larger device manufacturers, maturing surgical robotics developers and fast-moving startups?
As we were putting the finishing touches on this issue of Medical Design & Outsourcing and our cover story on DV5, surgeons got their hands on the system at select hospitals and the 2024 Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) annual meeting. They shared positive feedback, including the observation that DV5’s design changes could shave 10 to 15 minutes off procedure times.
That’ll get the attention of doctors and hospital administrators looking for solutions to the critical healthcare staffing shortage. But there are many more surgical robotics developments coming this year, as DeviceTalks Editorial Director Tom Salemi writes in his DeviceTalks Boston 2024 column this month.
Meanwhile, DeviceTalks Managing Editor Kayleen Brown convened experts from Abbott and pediatric heart centers for tips on device design for the world’s smallest, most vulnerable patients. As many of our readers know from their own experience, these devices are uniquely challenging due to children’s smaller anatomy and growing, developing bodies — but solving these challenges is immensely rewarding.
Also in this issue of Medical Design & Outsourcing, Associate Editor Sean Whooley has the latest on diabetes care technology from the International Conference on Advanced Technologies & Treatments for Diabetes, including developments from Dexcom, Abbott, Roche Diabetes Care, Insulet, Medtronic and Know Labs.
Whooley has also been covering material research in hydrogels, offering three of the biggest advances that device developers need to know about. There’s more on materials in our Orthopedics department, where CytexOrtho’s CEO and co-founder shares how his startup makes absorbable hip implants to help younger patients delay or avoid full replacements.
For our standing Tubing department, I explore a renal denervation (RDN) system using alcohol to treat hypertension in the wake of Recor Medical and Medtronic’s RDN approvals using ultrasound and radiofrequency energy. And finally, in our Product Design and Development department, I spoke with Hologic about how they developed hardware and software for a de novo diagnostics system that could one day have applications beyond cervical cytology.
As always, I hope you enjoy this edition of Medical Design & Outsourcing

-
- made in the USA












































Jervaughn Hunter conducted research at the University of California San Diego establishing a hydrogel as a potential mitigation for right ventricle damage.
 Sean Whooley Associate Editor
Sean Whooley Associate Editor
Researchers and engineers from Harvard, MIT and the University of California San Diego are breaking new ground in their work with hydrogels.
ydrogel researchers have reported several advances in recent months that could yield new medical applications in pediatric heart condition treatment, regenerative medicine and optogenetics.
Here are three of the most exciting applications that could boost medical device designers’ and engineers’ product development efforts.
An injectable hydrogel could mitigate damage to the right ventricle of the heart with chronic pressure overload, according to researchers at the University of California San Diego, Georgia Institute of Technology and Emory University.
For this study, they used rodents, but a 2019 FDA-approved Phase 1 trial
demonstrated the hydrogel’s safety in human heart attack patients. As a result of the new preclinical study, the FDA approved an investigational new drug application (NDA). The teams at Emory and Georgia Tech hope to begin recruiting for a clinical trial investigating the treatment’s efficacy in newborns with hypoplastic left heart syndrome.
Children born with that condition have an underdeveloped, nonfunctional left ventricle. This disorder comprises less than 4% of congenital heart defects, but has only a 35% survival rate, causing 40% of newborn deaths associated with heart defects, the researchers said
Patients with this disorder face three open-heart surgeries before their fifth birthday to reroute oxygenated blood to the right ventricle. They eventually experience right ventricle failure and require a heart transplant.
“To the best of our knowledge, it’s the first time that an injectable biomaterial therapy has been evaluated to mitigate right ventricular heart failure,” said Jervaughn Hunter, who conducted the research at UCSD before joining BD as an engineer.
In the rodent study, injecting the hydrogel into the right ventricle improved function, allowed the heart to tolerate increased blood pressure and volume, and slowed tissue scarring and maladaptive muscle growth. Researchers hope the injection increases how long the heart functions, buying patients time to get on an adult heart transplant list.
“This isn’t a cure, but our goal is to prolong a patient’s life,” UCSD bioengineering professor Karen Christman said.
(continued on page 8)













(continued from page 6)
The hydrogel is made from cardiac extracellular matrix that is stripped of the cellular content through a cleansing process, dried and milled into powder form, then liquefied into a fluid that can be easily injected into the heart. At body temperature and pH, the liquid turns into a semi-solid, porous gel that encourages the patient’s cells to repopulate damaged cardiac tissue and improve heart function.
In preclinical trials, the researchers prepared the hydrogel with tissue from both the right and left ventricles of the pig hearts. Hydrogels from either side of the heart improved systolic function and reduced heart muscle growth and scarring while prompting arteriole formation and growth, but left-ventriclederived hydrogel was more effective.
The injected hydrogel also affected gene expression, specifically with pathways related to cardiac repair, including the development of the circulatory system, muscle structure and vasculature, plus the regulation of immune response and cellular response to oxygen-containing compounds.
2. Soft fibers could help test treatments for nerve-related pain MIT engineers developed soft, implantable hydrogel fibers that could help explore causes of and treatments for peripheral nerve disorders.
These flexible, stretchable fibers can deliver light to major nerves throughout the body. When the nerves are genetically manipulated to respond to light, the fibers can send pulses of light to the nerves to inhibit pain.
“Current devices used to study nerve disorders are made of stiff materials that constrain movement, so we can’t really study spinal cord injury and recovery if pain is involved,” said Siyuan Rao, who carried out part of the work as a postdoc at MIT. “Our fibers can adapt to natural motion and do their work while not limiting the motion of the subject. That can give us more precise information.”
Optogenetics is a technique that genetically modifies nerves to respond to light and is primarily employed in the brain, an area that lacks pain receptors and allows for the relatively
painless implantation of rigid devices. But the rigid devices can still damage neural tissues.
Researchers sought to expand the technique beyond the brain to identify the causes of peripheral nerve conditions and test therapies. However, they identified motion as the main hurdle to implementing the technique beyond the brain. These types of nerves experience constant pushing and pulling from the surrounding muscles and tissues. Rigid silicon devices in the periphery could constrain natural movement and potentially cause tissue damage.
Looking for an alternative, the team turned to soft, stretchable, transparent fiber made from hydrogel, tuning the ratio of polymers and water to create tiny, nanoscale crystals of polymers scattered throughout a gelatin-like solution. The fiber has a core layer and an outer shell cladding. Each layer has a specific, different refractive index and, put together, keeps light from escaping or scattering as it travels through the fiber.


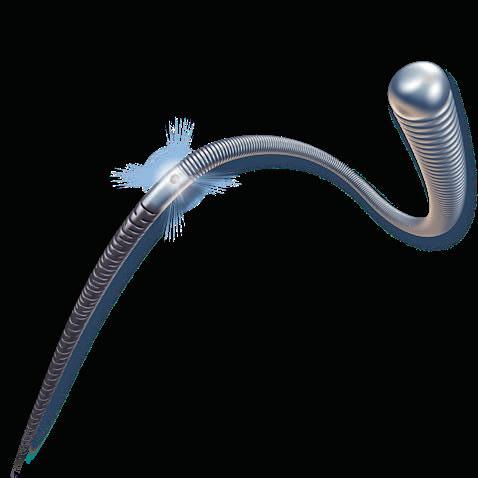








Testing mice who had their nerves genetically modified to respond to blue light or yellow light found blue light excited neural activity, while yellow inhibited it. Mice with the implanted fiber could run freely on a wheel, and after two months of wheel exercise the fiber remained resistant to fatigue and capable of transmitting light efficiently to trigger muscle contraction.
Using a yellow laser through the implanted fiber, the team found that mice were much less sensitive to pain than rodents that were not stimulated with light. The fibers significantly inhibited sciatic pain in those lightstimulated mice.
The fibers could offer a new tool to help identify the roots of pain and other peripheral nerve disorders.
“We are focusing on the fiber as a new neuroscience technology,” Liu said. “We hope to help dissect mechanisms underlying pain in the peripheral nervous system. With time, our technology may help identify novel mechanistic therapies for chronic pain and other debilitating conditions such as nerve degeneration or injury.”
3. Scientists develop new bonding method for biomedical hydrogels
Researchers at Harvard’s Wyss Institute and John A. Paulson School of Engineering and Applied Sciences created a method to bond layers of the same or different types of hydrogels and other polymeric materials for regenerative medicine.
The team developed a simple, versatile method to instantly and effectively bond those layers using a thin film of chitosan, a fibrous, sugar-based material from processed shellfish exoskeletons. They used their new approach to protectively cool tissues, seal vascular injuries and prevent unwanted surgical adhesions of internal body surfaces.
MIT engineers have designed a soft hydrogel optical fiber (shown illuminated) that stimulates peripheral nerves, and could help researchers in identifying the origins and treatments of nerve-related pain.
Image courtesy of MIT
“Chitosan films — with their abilities to effectively assemble, finetune, and protect hydrogels in the body and beyond — open numerous new opportunities to create devices for regenerative medicine and surgical care,” said founding Wyss Institute core faculty member David Mooney. “The speed, ease, and effectiveness with which they can be applied makes them highly versatile tools and components for in vivo assembly processes in often short time-windows during surgeries and the simple fabrication of complex biomaterial structures in manufacturing facilities.”
Former Wyss Research Associate Benjamin Freedman spearheaded the tough adhesives development with Mooney. They aimed to facilitate wound healing and tissue regeneration using stretchable hydrogels.
Chitosan films achieved rapid and strong bonding of hydrogels through chemical and physical interactions that are different from those involved in traditional hydrogel bonding methods. Rather than create new chemical bonds, chitosan’s sugar strands rapidly absorb water between hydrogel layers and entangle themselves with the polymer stands of hydrogels. That creates adhesive forces between hydrogels that exceed traditional approaches.
Tests showed the adhesives could wrap around cylindrical shapes like an injured finger as self-adhering bandages for improved wound care. This application also allowed the local cooling of the underlying skin, which could lead to alternative burn treatments. The team also modified the surfaces of hydrogels with thin
chitosan films and wrapped them around bowel, tendon and peripheral nerve tissue without bonding to the tissues themselves.
“This approach offers the possibility to effectively insulate tissues from each other during surgeries, which otherwise can form fibrotic adhesion with sometimes devastating consequences,” said Freedman. “Their prevention is an unmet clinical need that commercial technologies cannot adequately address yet.”
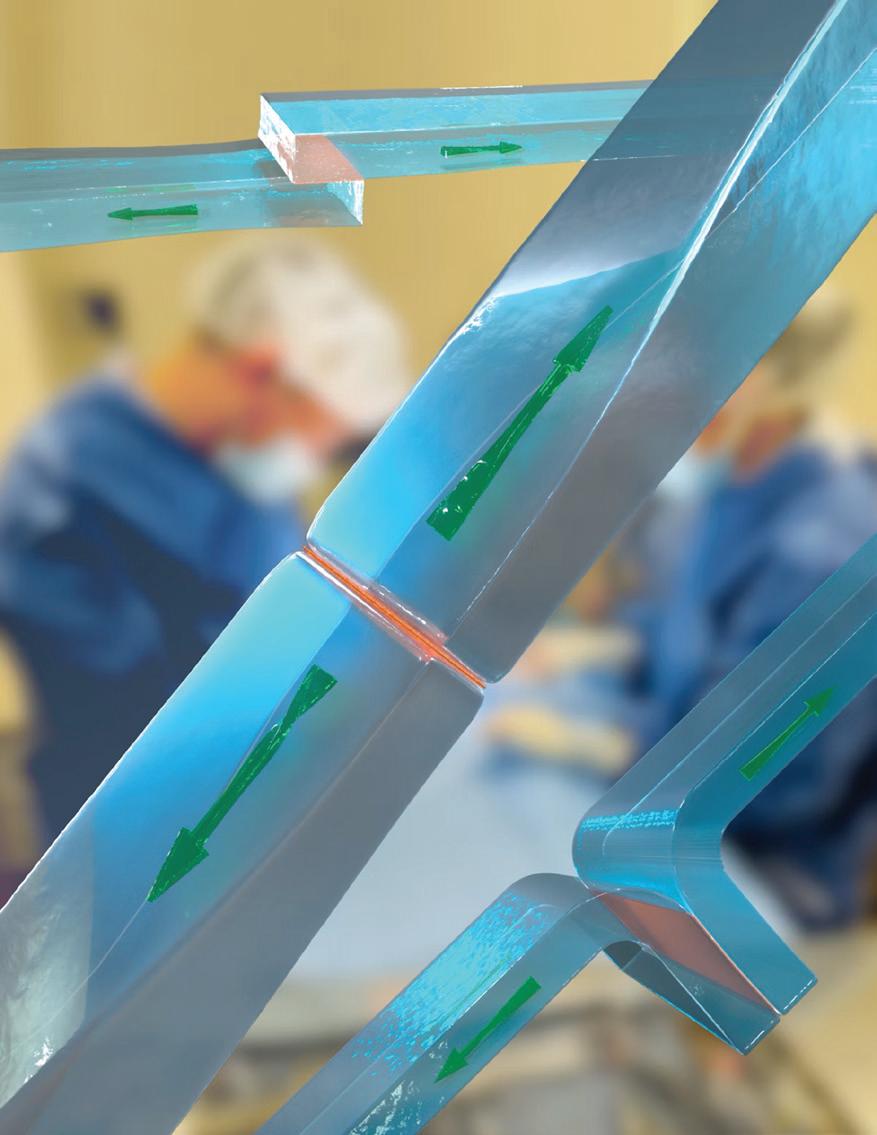
This illustration highlights how two hydrogels (shown in blue) can be bonded in different ways by thin chitosan films (shown in orange). The bonds that form are extraordinarily strong and can resist high tensions.
Image courtesy of Peter Allen, Ryan Allen, and James Weaver
Go to wtwh.me/hydrogels for more details, links to these studies and additional hydrogel coverage.
CytexOrtho shares the ‘secret sauce’ for its absorbable hip implants
CytexOrtho is using advanced 3D manufacturing techniques to make absorbable hip implants that could help younger patients delay or avoid hip replacements.


ytexOrtho is developing absorbable orthopedic implants with a unique manufacturing method to help a rapidly growing patient population.
The ReNew Hip implant developer won the inaugural OrthoPitch tech innovation competition at AAOS 2024, beating out more than 40 other nominees.
CytexOrtho co-founder and CEO Brad Estes said the startup has filed its FDA investigational device exemption (IDE) for a Phase 1 clinical trial in the U.S., Medical Design & Outsourcing after his recent DeviceTalks Weekly interview. The company plans to seek FDA premarket approval (PMA) after a larger Phase 2 pivotal trial.
CytexOrtho (incorporated as Cytex Therapeutics) designed the hip implants for people who are younger than traditional joint replacement candidates.
About half of all total hip replacements are for patients under the age of 65, Estes said, with a 40% growth rate for those procedures in patients ages 55 to 64.
There are also double-digit increases in total hip volumes for younger age groups: 25% for ages 45 to 54 and 14% for ages 35 to 44.
Many of those patients — and even some in their 20s — have pre-arthritic disease or less common conditions, with a total hip replacement likely within their next decade.
“Surgeons look at it and go, ‘I’ve got nothing for that patient right now.’ But they need help,” Estes said in an interview with DeviceTalks Editorial Director Tom Salemi and WTWH Media Life Sciences Editor in Chief Chris Newmarker. “One of our surgeons called
CytexOrtho uses a three-dimensional weaving process to make its ReNew Hip implants. Shown here is a close-up of the woven textiles. Image courtesy of CytexOrtho
it benign neglect — they’ll go into the joint, they will relieve whatever is causing the damage, but then they’re stuck with these lesions inside the joint that really don’t have any solutions. And we know that they’re going toward deterioration and degradation. This joint’s going to start falling apart and they’re on that fasttrack for a total hip replacement.”
“Those patients were never intended to get a hip replacement, and yet, that’s what’s driving this large market right now, and orthopedic surgeons can’t even keep up with the demand of it,” Estes later continued. “That’s a problem. This is a patient population that definitely needs a solution.”
CytexOrtho is developing its technology to reverse the symptoms of early to intermediate osteoarthritis and delay a total hip procedure for as long as possible, potentially sparing them another procedure to replace that total hip implant 15 or 20 years later.
“We’re basically targeting those patients who are too early for a hip replacement because they’re young, they’re active, and they’ll wear out their implant multiple times in their lifetime if they want to keep that active lifestyle,” Estes said. “We have technology that can basically restore the surface of their joint.”
(continued on page 12)

When you give your customers what they need, they’ll need you even more
Steute is unrivaled in its ability to customize foot switches to specific customer requirements
The needs of medical professionals (and the patients they treat) are changing as rapidly as the environments in which they work. No other foot switch supplier can match Steute’s ability to customize products to provide the best solutions when they are needed most.
All Steute foot switches are medical-grade and offer such custom options (with no tooling or NRE) as:
• Actuator styles
• Consoles/platforms
• Selectable actuating forces
• Strain reliefs
• Handles/foot rests
• Cable type/colors
• Console color and finish
• Wiring configuration
• IP-rating
• Connector styles
• Graphics
…and many more
Our team designs each product to optimize functionality, user-comfort, ease-of-use, aesthetic appearance, and is certified to meet all relevant standards and regulatory requirements regardless of how challenging the demand is.

CytexOrtho’s ReNew Hip implants act as cushioning within a patient’s hip joint and are fully absorbed by the body over two years as the patient’s normal tissue replaces it.
(continued from page 10)
To place the implant, a surgeon will first remove damaged tissue from the joint. Then the surgeon replaces that damaged joint tissue with the implant, offering a protective cushion that can be fully absorbed into the body after two years, leaving only the patient’s own tissue.
“We’re restoring both the form — which means the anatomy and the congruity of the joint — but we’re also restoring it functionally, and we’re doing it in a way that’s friendly to the biology in the joint,” Estes said. “People have done that before with with different types of products and different types of materials, but what we’re doing is in a way that is friendly to the cells and tissue development.”
The device is made of polycaprolactone (PCL), an absorbable polyester also used for sutures and bone plugs.
“It’s not super common in orthopedics, but it’s used and it’s got a good track record with FDA — it’s something they recognize and something they’re familiar with,” Estes said.
CytexOrtho uses 3D printing and a three-dimensional weaving process to make its ReNew Hip implants.
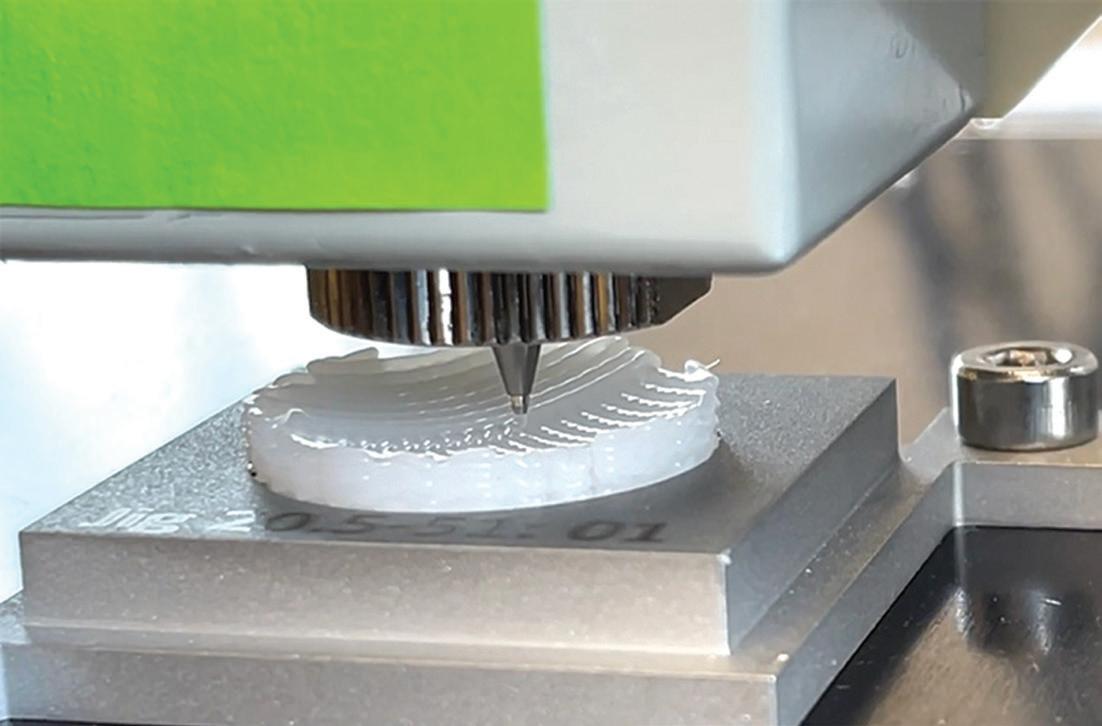
CytexOrtho tried other materials, including nonabsorbable materials, other biologics and silk, but ultimately went with PCL for its safety profile and biocompatibility.
and we’ve shown that as the implant absorbs, we don’t lose the form or the functionality of the joint.”
CytexOrtho’s ‘secret sauce’ CytexOrtho uses 3D printing for part of the implant, but Estes said a 3D weaving method is the company’s “secret sauce.”
“We’re actually weaving in three dimensions, if you can picture that. It’s really hard to imagine, but instead of just traditional X and Y basket-type weaving, we’re also weaving in that Z or third dimension as well,” he said. “What that does conceptually is by controlling the materials and the spacing in all three of those directions, you can start to dial in the mechanical properties of the tissues you’re trying to replace in your body.”
The weaving materials are multifilament yarns that are several hundred microns in diameter.
“We’ve controlled the sourcing of those and had to manufacture [them]. You can’t just go and order them from Amazon, so we had to figure out how to make those,” he said.
CytexOrtho designed its ReNew Hip implants to help patients delay or avoid total hip replacements.

“It absorbs so slowly, it gives the body ample time to make that functional tissue matrix in and around it as it absorbs,” Estes said.
“As cells come in, attach to the implant and then start to make tissue, we’ve shown this tissue to be functional in all our preclinical animal studies, and we’ve taken animal studies out to a year now, which is what FDA demands for clinical trials,” Estes said. “We’ve done that
Estes traces the concept back to a 2007 paper published in Nature Materials by his co-founders, Executive Chair Farshid Guilak and VP of Technology Frank Moutos.
“It showed that we could tune this structure to match the native properties of articular cartilage without any compromise of the ability to form tissue in the joint,” Estes said. “And that kind of started us out on our journey with that discovery. That’s kind of the hallmark feature of the implant.”
Advice for other medical device developers
“This journey is full of swamps and landmines and people shooting at you,” Estes said. “It’s extremely hard.”
CytexOrtho has funded its work through more than $19 million in National Institutes of Health (NIH) grant funding. While that means the technology went through thorough vetting and validation, “it’s also the absolutely slowest way to do product development,” Estes said.
It calls for a methodical, marathon approach with a focus on communication, grant-writing, thoughtful deliberation for every decision — and “incredible perseverance.”
“You have to know that you have the technology and your science is solid, but then you just have to keep hammering on it with NIH submissions and if the grant’s good and this technology is good, you’ll get it funded,” he said. “It’s an exercise in, ‘I’m going to be super diligent, super perseverant, and we’re going to get this funded, and I’m going to wait you out.’ We were just dogmatic in that approach.”



“It’s got to be your passion. … You just have to be willing to just persevere through solving tons of problems,” he later continued. “And if if you have a dream, and you have the solution for something and you want to make it happen, go for it.”























Hologic designed its Genius cytology technology for more efficient and accurate review of cervix cell samples — and there’s more to come.
Hologic



Go to wtwh.me/quickalpaca for Hologic VP Mike Quick’s explanation of medtech AI using alpacas and llamas.
ologic‘s Genius cytology system uses new scanning technology and artificial intelligence to flag cervical cancer cells and precancerous lesions.
Hologic won a de novo classification in January 2024 for its Genius Digital Diagnostics System and Genius Cervical AI algorithm for cervical cytology. Besides replacing Hologic’s ThinPrep Imaging System — used for the majority of cervical cancer screenings in the U.S. — the technology behind the Genius system could one day also help screen for other kinds of cancer like bladder cancer as well as infectious organisms, Hologic VP of R&D/Innovation Mike Medical Design & Outsourcing.
“It really has the potential of being applied to a variety of other disease states,” Quick said. “We’re excited about building out that roadmap and what that future state can look like. It really is more about a platform than it is as a single instrument that’s a replacement just for cervical cancer screening. … Candida, trichomonas, herpes can all be identified
on the Genius platform using the same AI-type algorithms.”
Taking pictures of microscopic slides for analysis is not a new idea. Quick — who trained and worked in cytology before joining Hologic in 2007 — said he has copies of some publications looking at image analysis for cytology dating back to the 1950s. In the past 15 years or so, however, there’s been greater adoption of the concept in pathology than cytology.
“When we first started looking at this at the start of this project in about 2015, what we found is that there were real technological limitations of why that space hadn’t evolved,” he said.
The fundamental difference is that pathology images use flat tissue sectioned with a razor blade on a microscope slide for very thin uniformity across the entire material. But with cytology, they’re whole cells in three dimensions on the microscope slide.
“You’re taking a much deeper thickness of the material, almost like the difference between a chest X-ray and a CT scan,” he said.
Quick initially hoped that Hologic could develop the AI image analysis component and use existing scanners, but his team found those scanners weren’t suited for three-dimensional cells.
“Either they couldn’t get images in focus because of the multiple plane issue, or if they could, they had to do it by essentially taking multiple pictures, 13 to 15 pictures at a time of a field, and then move down through the material, taking pictures one at a time,” Quick said. “What was reportedly the best-in-class scanner on the market took about an hour and five minutes to scan one slide using that approach, which we knew would never be a commercially viable option.”

So Hologic developed what it calls volumetric imaging to simultaneously capture multiple images for a 3D image before converting it into 2D. It’s a similar approach to what’s called focus stacking in photography, where a photographer
review of the patient sample.
“It’s the approach of analyzing 70,000 or 80,000 cells on a single patient sample and distilling that down to 30 tiles, individual cells or cell groups that are representative of the entire cell sample,” he said.
Hologic says the technology reduces false negatives of high-grade squamous intraepithelial and more severe lesions by 28% compared to microscopic review.
“We don’t want to miss anything, but the high grades are the lesions that are one step away from becoming cancer,” Quick said.

As a new technology, Hologic’s Genius system faced some common regulatory hurdles.
“Volumetric imaging — it’s new physics. It didn’t fit squarely into the boxes that the FDA had laid out in their guidance documents,” Quick said. “So how do you translate something that’s never been done before into the framework that the regulators are looking at?”
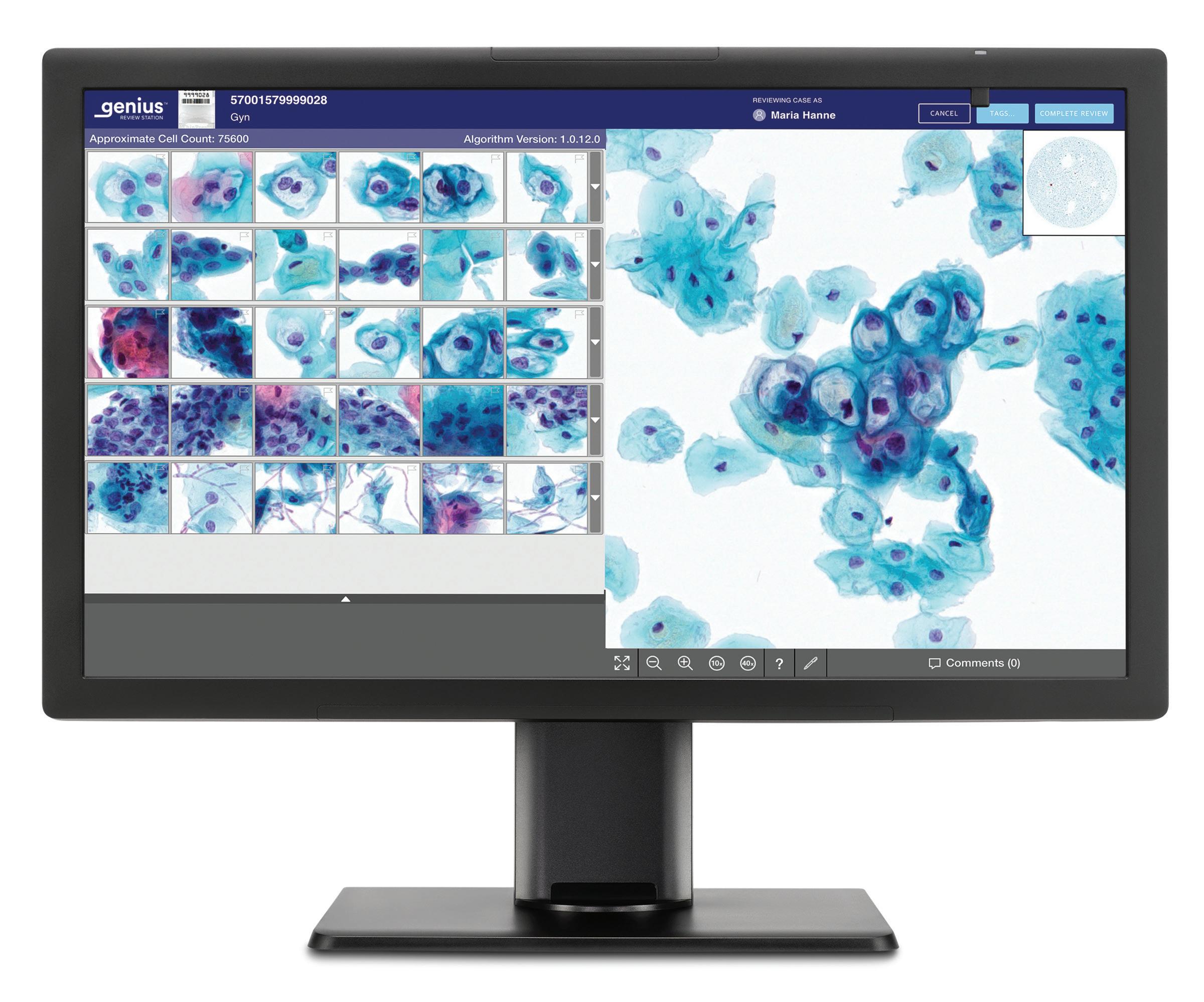
tens of thousands of images, Quick said, to provide more efficient and accurate
(TOP) Hologic’s Genius Digital Diagnostics System scanner
(MIDDLE) Specimen slides being loaded into Hologic’s Genius Digital Diagnostics System scanner
(BOTTOM) Cervical cells scanned and reviewed with Hologic’s Genius system Photos courtesy of Hologic
The Hologic team translated the technology for FDA reviewers through several informational meetings and then several pre-submission meetings.
“So much of that is about education,” Quick said. “The more that you can do on the front end — as laborious and time-consuming as it can be — it certainly needs to happen so that you get alignment when you’re aiming for a bit of a moving target, particularly in this space.”
Quick offered some final advice for other device developers and manufacturers.
“You’ve got to have the tenacity and belief to see it through to the end,” he said. “This was something we started pitching internally. It was the classic innovator’s dilemma of how do you invent and invest in something new when you’re the market leader in what you have today and need to vigorously defend what that is.”
“Building our internal advocates was as important as everything that we did externally to be able to move this into a project that our company invested in for the future,” he continued. “It’s been a long haul, but we definitely see the excitement and the enthusiasm, and literally have labs knocking down our door to talk about implementing it.”
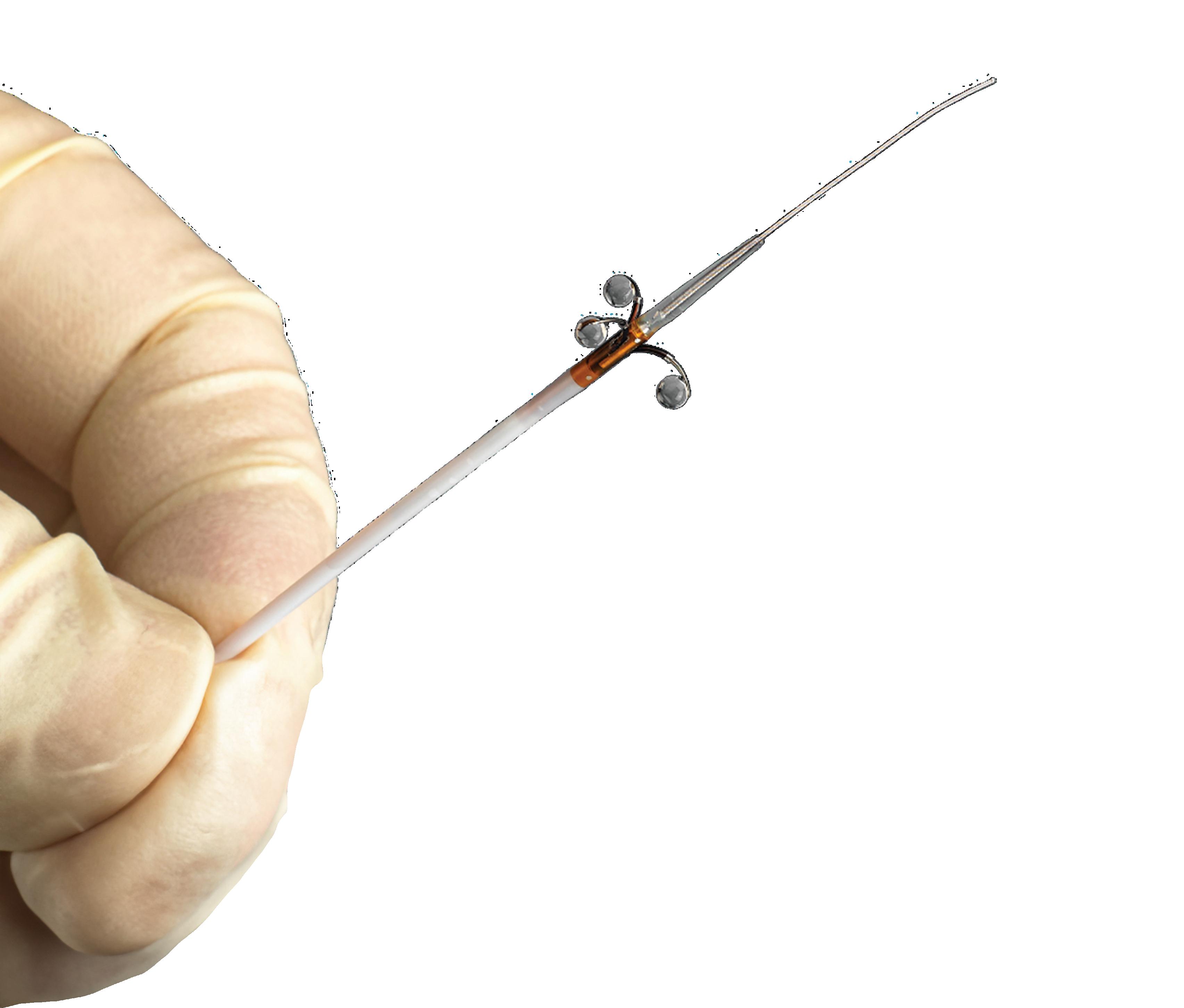
Alcohol could make renal denervation for hypertension faster and simpler
Ablative Solutions designed its Peregrine renal denervation catheter system with nitinol microneedles to ablate nerves with alcohol.

Ablative Solutions is developing the Peregrine renal denervation (RDN) system to treat hypertension. The company hopes to follow Recor Medical and Medtronic in winning FDA approval for the system.
“As a small company, it’s definitely better to be a fast follower than it is to be first to market,” Ablative Solutions CEO Kate Rumrill said in an interview. “I’m excited for Medtronic and Recor and their first year of sales and having these larger companies doing some of that early work as far as market awareness and market adoption.”
Medtronic’s Symplicity Spyral RDN system uses radiofrequency (RF) energy, while Recor’s Paradise system uses ultrasound. Peregrine doesn’t deliver energy at all, instead using alcohol as a neurolytic agent.
“There really isn’t anything special about the alcohol per se, in the sense that alcohol has been used to ablate nerves for decades in the periphery [as] nerve blocking agents,” Rumrill said.
What’s special about Ablative Solution’s system is the proprietary
Peregrine catheter system, designed to access the perivascular area around the renal arteries.
“Through that technology, we’re able to simply and in a reproducible way deliver that alcohol to the target site,” she said.
The system is named after the peregrine falcon — the world’s fastest animal — and the similarity between the raptor’s razor-sharp talons and the infusion catheter’s microneedles.
The Ablative Solutions Peregrine system’s design
The Peregrine system’s three nitinol microneedles deliver the alcohol when the single-use catheter is placed inside a patient’s renal artery.
Each microneedle has its own guide tube that deploys inside the artery, with all three pushing against the vessel wall to center the catheter within the vessel. Then the needles deploy for a clean shot through the vessel wall into the perivascular space, each delivering alcohol at precisely the desired depth for a total dose of 0.6 mL.
“We can treat vessels 3 to 7 mm in diameter,” Rumrill said. “Regardless of the size of the vessel, what these guide tubes do is ensure that you have contact with the inner lumen of the vessel wall. … It takes some of the guesswork out for the physician.”
The microneedles and guide tubes are radiopaque for fluoroscopic visibility to show the doctor the exact location of the catheter and guide tubes before deploying the microneedles. After delivering the alcohol, the physician retracts the needles and guide tubes back into the catheter, flushing with saline to ensure patency. Then it’s on to the patient’s other renal artery.
While it’s not yet clear whether alcohol RDN is safer or more effective than RF or ultrasound RDN, one advantage is system simplicity and cost. Because there’s no energy to be applied, an alcohol RDN system doesn’t need a generator.
“There’s no capital equipment costs, just a single-use catheter and a vial of alcohol,” Rumrill said. “For hospital system adoption, that capital equipment piece is taken off the table, which can help them immensely.”
The other advantage of Peregrine RDN is that it typically only requires one infusion per artery, though physicians can administer up to four infusions per patient if they wish to also denervate smaller, accessory arteries. Not all patients have accessory arteries, which come off the aorta either above or below the main artery. If those arteries are meaningful, treating them can lead to better patient outcomes.
“If it perfuses more than 20% of the blood flow to the kidney, we consider it meaningful,” Rumrill said. “In other words, if there’s more than 20% of the blood flow going to the kidney, likely there’s 20% of the nerves going to the kidney as well, so we choose to ablate those vessels.”
Less than a quarter of Peregrine patients have accessory arteries that call for additional infusions, Rumrill said, which means the average infusion per patient is around two. For comparison, some patients in Medtronic’s Symplicity Spyral RDN trials received dozens of ablations, while Recor’s Paradise system delivers four to six sonications per patient. >>

Harness our leading-edge vacuum technology . . . because lives depend on it.


Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.
For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.









Ablative Solutions is betting that the Peregrine system can get the job done faster and with less complexity than the competition.
“With shorter procedure time, you have less contrast used, less fluoro time, radiation — all of those things that are important to not only the patient, but everybody in the cath lab,” Rumrill said.
Peregrine’s path to approval
Unlike the RDN systems from Recor and Medtronic, Ablative Solutions’ Peregrine is regulated by the FDA as a combination product, which means they’re dealing with the FDA’s Center for Devices and Radiological Health as well as the agency’s Center for Drug Evaluation and Research.
The Peregrine catheter already has FDA 510(k) clearances for infusing diagnostic and therapeutic agents into the perivascular area of the peripheral vasculature. Ablative Solutions hopes to win FDA new drug application (NDA) approval for treating hypertension with alcohol RDN.
NDA approvals typically take 12 months, so Ablative Solutions is planning for approval and product launch in 2025.
Ablative Solutions is outsourcing manufacturing of the medical-grade alcohol used in its system to maintain some control over the quality and supply. She declined to name the partner, but offered some advice for device developers looking to partner with contract manufacturers.
“Start early and do your homework,” she said. “Regardless of who you select, in general whenever you’re working with a third-party supplier, make sure you have somebody in-house that has that expertise so they can interact and manage that relationship. For everything from clinical trial outsourcing to device manufacturing, every step of the way you still need to have somebody with that expertise internally to know are you on track and to be able to ask those questions of the supplier.”
“With shorter procedure time, you have less contrast used, less fluoro time, radiation — all of those things that are important to not only the patient, but everybody in the cath lab.”
The company reported in December that its pivotal trial hit its primary endpoint, finding a statistically significant difference in 24-hour ambulatory blood pressure between treatment and sham procedure at three months. Ablative Solutions will unblind the study at six months to allow patients in the sham group to receive the actual procedure. The trial will follow patients for a total of three years.
Wakefield, Massachusetts-based Ablative Solutions plans to explore other applications of alcohol RDN, but because it’s a small company — it has fewer than 50 employees — for now it’s focused on the first indication of hypertension.
“We outsource a lot, and we have some really great consultants that are a great part of the team as well,” Rumrill said.


Key considerations for OEMs in medical device design and manufacturing.
Recently, the role of medical Original Equipment Manufacturers (OEMs) in global healthcare has become increasingly pivotal. In order to reach customers quickly and cost-effectively, OEMs partner with Electronics Manufacturing Services (EMS) companies like Celestica to help bring their innovative medical devices to global markets. Kevin McFarlin, design engineering director at Celestica, explores how OEMs can leverage partnerships to scale production, integrate design and manufacturing insights, and manage supply chain risks to meet the urgent demands of the global healthcare industry.
MDO: WITH MEDICAL DEVICE DEVELOPMENT TIMES SHORTENING, HOW CAN OEMS ENSURE PRODUCTS REACH THE MARKET QUICKLY AND MEET INCREASED DEMAND?
McFarlin: OEMs should consider partnering with manufacturers that can scale to high volume. They need to be able to scale production to make products accessible to a global market. To do that, products need to be designed with the supply chain in mind.
OEMs that leverage manufacturing facilities with a qualified and localized supply chain in the same geography have a more robust distribution channel. They can reduce overhead costs from the raw materials coming into the manufacturing facility and the product distributed to end users. A manufacturing partner with a global presence can help them scale and manufacture those devices at a lower cost.
MDO: WHAT ARE THE KEY DESIGN CONSIDERATIONS MEDICAL OEMS SHOULD ADDRESS EARLY IN THE PRODUCT DESIGN PROCESS?
McFarlin: It's essential for OEMs to start with a clear understanding of both user and business needs while defining use conditions and product requirements in clear, quantifiable
terms. Then, teams should design for reliability by anticipating and discovering failure modes through analysis and testing, and eliminate failure modes through design. Additionally, the design process must consider manufacturability, ensuring that the device can be produced efficiently at the necessary scale and cost. Lastly, design with the supply chain in mind. Part of this includes conducting a thorough bill of materials analysis to identify and mitigate potential risks. Allowing for the proactive design of alternative components when necessary. These considerations help to ensure functionality and reliability of the medical device and its availability and affordability in the market.
MDO: WHY IS IT CRUCIAL TO INTEGRATE MANUFACTURING AND PROCESS ENGINEERS' INSIGHTS DURING THE PRODUCT DESIGN PHASE?
McFarlin: The manufacturing process should be considered part of product design. Experienced manufacturing engineers can fix assembly problems in the subsystems and components so when they're integrated into a larger system, they won’t contribute to a failure in the field. Following principles of robust design can help teams make more reliable products by eliminating opportunities for manufacturing defects caused by equipment variations and operator dependent processes. This allows the design team to address issues before they invest time, effort, and expense in the design verification of their product.
Customers partner with Celestica to help turn their innovations into reality. We help them achieve cost and delivery targets, ensure regulatory compliance, and manufacture at the desired scale to meet market and patient demand.
For more information on how OEMs can benefit from a strategic design and manufacturing partner, scan the QR code and read Celestica’s white paper.

Celestica’s engineering expertise, global manufacturing network and rigorous quality control processes help you design, develop and deliver innovative medical devices that enable better patient outcomes.
Discover the Celestica Advantage:
ISO 13485 and FDA-registered sites
Extensive design and engineering expertisee
Supply chain risk management and in-region supplier network
Leadership in quality and
Advanced manufacturing (automation and clean room finished goods production)
www.celestica.com/healthtech Celestica
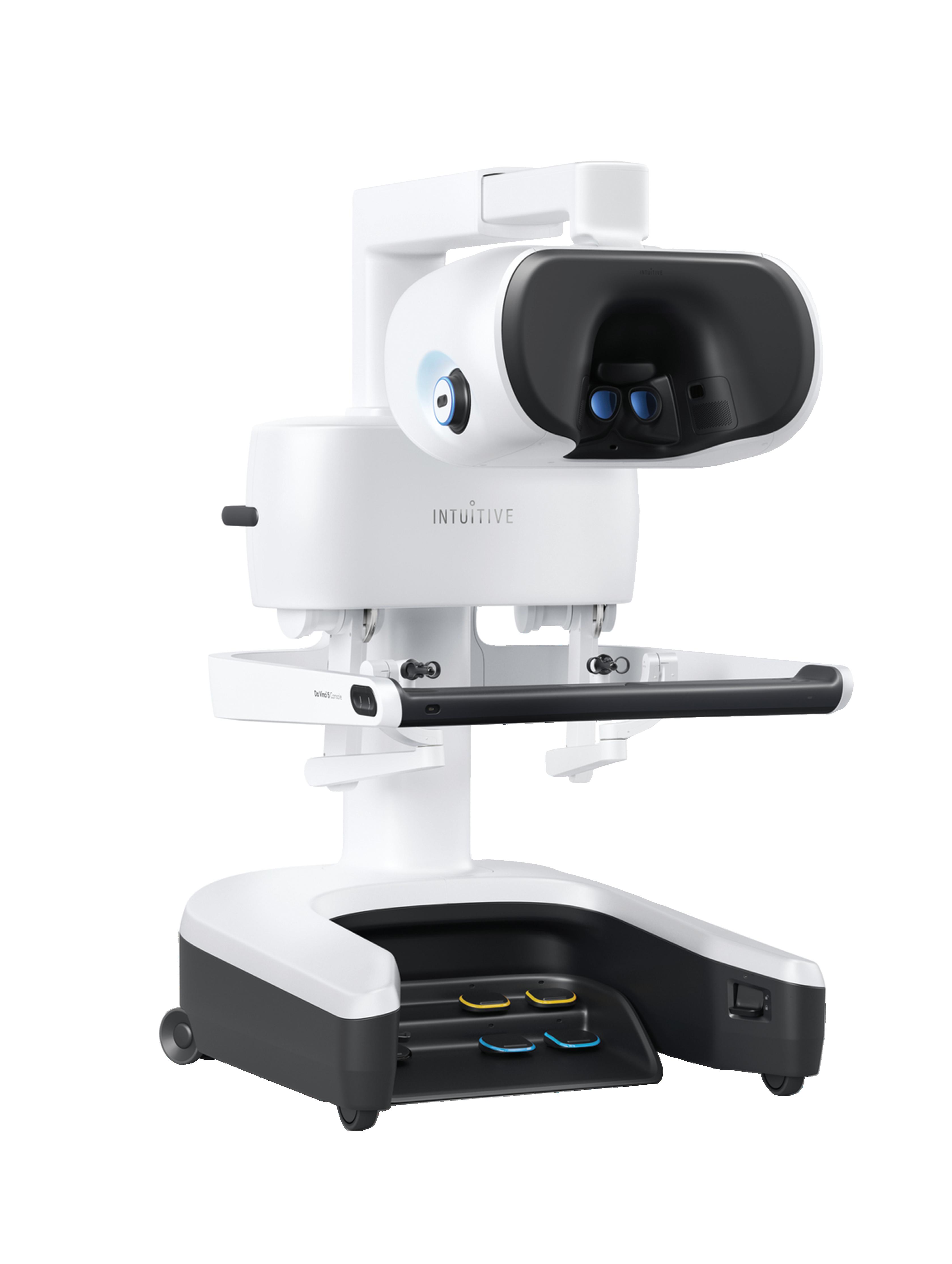
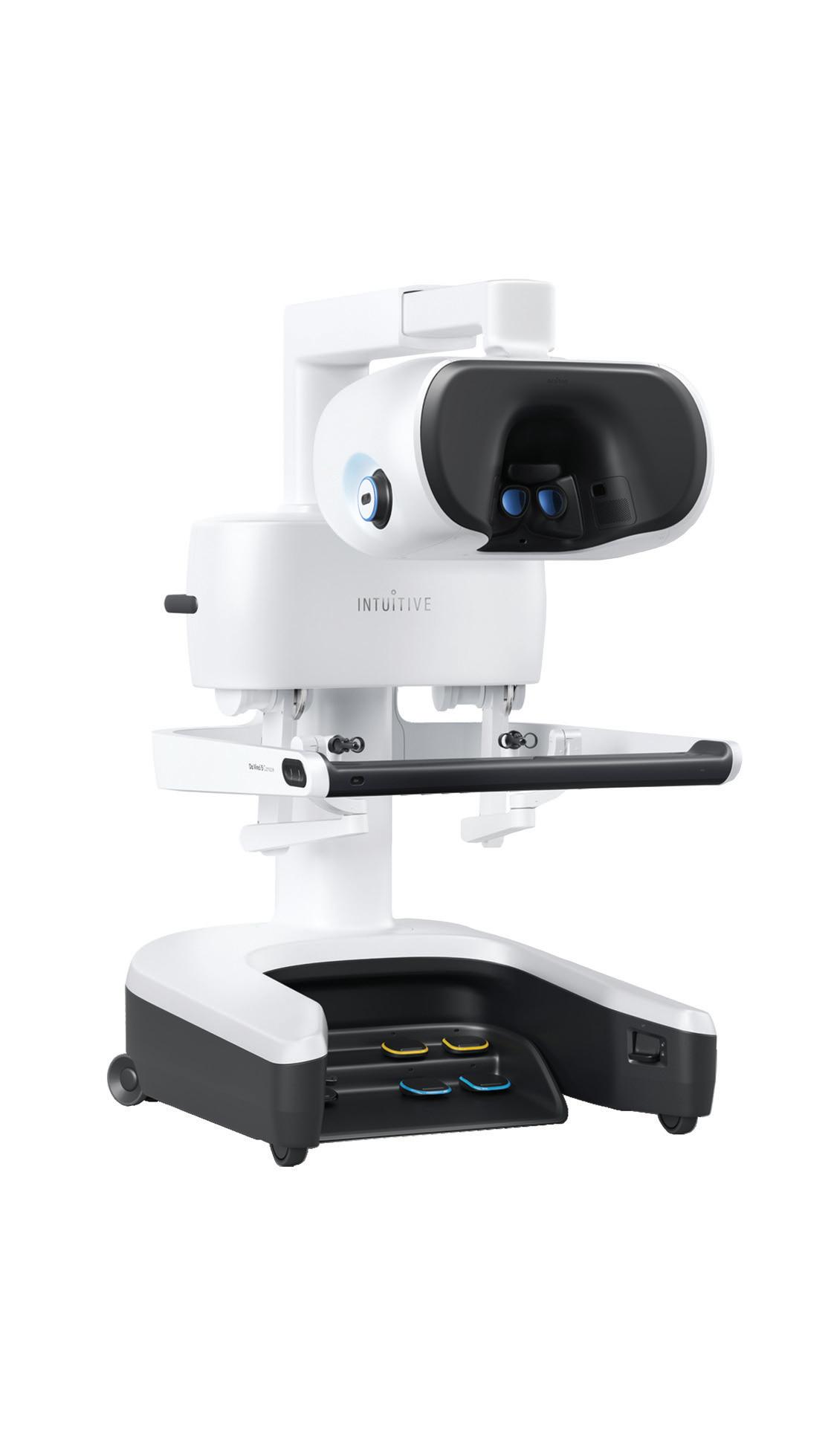
 The Intuitive Surgical da Vinci 5’s surgeon console Image courtesy of Intuitive Surgical
JIM HAMMERAND MANAGING EDITOR
The Intuitive Surgical da Vinci 5’s surgeon console Image courtesy of Intuitive Surgical
JIM HAMMERAND MANAGING EDITOR

‘This is groundbreaking for robotic surgery’
AS THE DOMINANT DEVELOPER OF SURGICAL ROBOTICS ROLLS OUT ITS NEXT-GENERATION SYSTEM, LEADERS OFFERED THEIR THOUGHTS ON DA VINCI 5’S MOST IMPORTANT DESIGN CHANGES AND NEW FEATURES.
Intuitive Surgical’s da Vinci 5 has more than 150 design enhancements and innovations since the surgical robotics developer’s fourth-generation systems, including one feature that an Intuitive leader described as “groundbreaking.” “Da Vinci 5 looks similar to [multiport predecessor da Vinci] Xi,” Intuitive President Dave Rosa said in a discussion of the upgrades and enhancements. “It built on Xi’s highly functional design, which has been used around the world in more than 7 million procedures.”
(continued on page 25)
YOUR FULL - SERVICE ENGINEERING PARTNER


Thermoplastic Injection Molding
Thermoplastic Injection Molding
Liquid Silicone Rubber (LSR) Molding
Liquid Silicone Rubber (LSR) Molding
White Room/Cleanroom
White Room/Cleanroom
Test Specimen Molding
Test Specimen Molding
In-house Tooling
In-house Tooling
SPI Class 101 and 102 Production Molds
SPI Class 101 and 102 Production Molds
PSN Labs can be your manufacturing partner for all injection molded components. Our team of scientists and engineers engage seamlessly across the full development process to ensure all aspects of a product have been evaluated from design through manufacturing to mitigate the risks associated with launching a product to market.
PSN Labs can be your manufacturing partner for all injection molded components. Our team of scientists and engineers engage seamlessly across the full development process to ensure all aspects of a product have been evaluated from design through manufacturing to mitigate the risks associated with launching a product to market.






www.PSNLabs.com
www.PSNLabs.com
Info@PSNLabs.com ISO
Info@PSNLabs.com ISO

(continued from page 23)
Rosa discussed da Vinci 5’s design along with Intuitive CEO Gary Guthart and Chief Medical Officer Dr. Myriam Curet.
“Da Vinci 5 takes surgical precision to a new level,” Rosa said. “The system is designed with next-generation surgeon controllers and patient-side manipulators with additional sensors. This combination translates to super-smooth, lowresistance and highly precise motion at both slow and high speeds. Unwanted tremor and vibration filtration is the best we’ve ever brought to market.”

“We believe that the ability to measure force during robotic surgery adds an important new data stream to surgical data science,” Curet continued. “Our insights engine will incorporate real-time surgical force measurement along with the surgical data Intuitive currently collects to build analytical insights for surgeons and care teams.”
The da Vinci 5’s 3D surgical imaging system is the “highest quality and most natural” ever developed by Intuitive, he said, and has additional capability for future generations of surgical endoscopes and vision software.
From a computing power standpoint, da Vinci 5 has 10,000 times more than the fourth-generation Xi system for integration with the My Intuitive app, Intuitive Hub video platform, SimNow virtual reality simulator and Case Insights, which lets surgeons review their surgeries with objective performance indicators and video of critical steps.

Intuitive
The extra computing power could support future features and integration such as better integration of preoperative images as Intuitive brings those into the surgical field, Rosa said.
“It sets us up for our innovation teams internally to make a lot of progress over the coming years,” he said.
William Blair analyst Brandon Vazquez spoke with surgeons who used the new da Vinci 5 system. In a research note from the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) 2024 meeting, he said the key improvements were in efficiency and haptic feedback.
“There is no singular feature we would call out for efficiency improvements,” Vazquez told his firm’s clients. “Rather, we would point to the culmination of 150-plus new features and UI improvements that doctors suggested could save up to 10 to 15 minutes per procedure.”
Intuitive’s ‘groundbreaking’ Force Feedback tech
Curet described Intuitive’s new Force Feedback technology on the da Vinci 5 as “groundbreaking for robotic surgery.” The system measures subtle forces exerted on tissue during surgery and relays that feeling back to surgeons, a feature that the device developer says is unique to the da Vinci 5 system.
“In preclinical testing, surgeons who used the Force Feedback feedback instruments on da Vinci 5 exerted significantly less force on tissue, which could translate into less tissue trauma during surgery when compared to da Vinci Xi,” she said. “With our customers, we intend to study how this could translate to realworld clinical and patient-reported outcomes as surgeons of all experience levels use this technology in a broad range of procedures.”
Da Vinci 5 can record interaction forces during a case when surgeons are using force-sensing instruments. The surgeon can choose to have that haptic feedback in their hands or to turn it off.
Overall, the FDA cleared da Vinci 5 for the same indications as the da Vinci Xi, except for cardiac and pediatric procedures. The Force Feedback needle driver is contraindicated for hysterectomy and myomectomy. While surgeons can still use instruments without Force Feedback, Intuitive is going to pursue the removal of those contraindications, Curet said.
“It’s very clear that there’s significant value in using the Force Feedback needle driver, so we believe that will be used in suturing steps of procedures, but there’s also significant use in retraction and getting that Force Feedback information on the force being applied to tissue.” Curet said. “… Some of that will depend on the surgeon and how much the surgeon wants to get that information during the procedure.”
Intuitive plans prospective and retrospective studies to generate clinical evidence on Force Feedback, with database studies to follow as more surgeons use the feature. Guthart expects a healthy debate among surgeons about Force Feedback, and predicts the feature will be more useful in some procedures than others.
“There will be some surgeons — particularly very experienced surgeons — who say, ‘I can see forces, I can use visual haptics’ … but even when we observe those highly experienced surgeons in lab, when Force Feedback is on versus off there’s a different amount of force that gets transmitted to the tissue,” Guthart said.
“Some of the study here will be exactly this issue of what is the difference in clinical outcome and patient experience when you turn Force Feedback on and >>
off,” he later continued. “It won’t be a matter of opinion — this is the thing that I love — you’ll get a chance to actually see what do you feel and what do you do. That data is the data we’re going to go out and collect over time, and I think it will be fascinating and transformative.”
User experience design and surgeon training
Curet also highlighted design improvements and innovations for increased surgeon autonomy and workflow efficiency to streamline operating room workflow and potentially save valuable time during certain procedures.
“During our clinical work and clinical trials, we saw preliminary evidence that procedures done with da Vinci 5 may take less time to complete compared to cases done with da Vinci Xi,” she said.
That’s based on what Intuitive said is aggregate, qualitative analysis of data from 53 first-in-human-use cases on da Vinci 5, including 23 surgeons at novice, intermediate and expert experience levels.
“Faster cases that don’t compromise patient safety allow for more efficient human use of precious human and capital resources at the hospital and should be well appreciated by our customers,” Curet continued.
Intuitive is starting to see growth in emergency and short-scheduled surgery volumes with da Vinci X and Xi, Guthart said. Da Vinci 5 could accelerate that trend if it proves easy to learn and delivers higher throughput over time.
“The ability for the surgeon to be more autonomous means he or she is less dependent on the expertise of the care team,” Curet said in a folllow-up. “The after-hours care teams have to do a larger breadth of procedures, so they may not know as much in-depth. I think that’s where da Vinci 5 can really bring value.”
Da Vinci 5 training for surgeons who are experienced with da Vinci Xi “is pretty rapid and actually can be done at the hospital,” said Curet, citing Intuitive’s IDE studies and preclinical study lab experience. ”For surgeons who are new to robotics who are going
to learn on DV5, they will follow our typical pathway that they would have followed for Xi, which is multilearning modality, multiday, multihour training pathway until they reach the ability to use the system independently.”
Intuitive’s da Vinci 5 ergonomics design
Intuitive also designed the da Vinci 5 system with ergonomic features to help surgeons make the most of every day.
Rosa said Intuitive’s design team “prioritized the often undervalued area of ergonomics” for surgeon comfort and stamina in the surgeon’s console, which “allows for a broad range of surgeon postures [and] less physical strain to the surgeon during the operation.”
To help surgeons be more productive on a daily basis and to lengthen their professional lifespans, Intuitive designed the surgeon’s console with musculoskeletal problems, repetitive motion issues and fatigue in mind. Intuitive also designed the latest system with easy-to-reach controls for the rest of the care team.
Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.




“We are all aware of the increasing need for surgeons around the world,” Curet said. “Intuitive continues our commitment to improving ergonomics with da Vinci 5 enhancements that could improve care team satisfaction and enable higher productivity during a single operative case and over a career.”
The latest surgeon console can fit different body types, including surgeons who are pregnant.
Intuitive included equipment on da Vinci 5 that had previously been purchased by hospitals as third-party add-ons, such as cameras, insufflators and electrosurgical generators.
The insufflator, for example, features integrated smoke evacuation with automatic sensing and actuation. However, even though the insufflator comes with da Vinci 5, customers can still use third-party tools with the new system.
Intuitive integrated those capabilities with da Vinci 5 for two reasons, Rosa said. The first is to add to the overall care team experience with better workflows and efficiencies. The second, he said, is because that equipment can generate or capture data that might be important to Intuitive’s efforts to deliver actionable insights and analytics to customers.
A big step forward
“Sophisticated technology tools well integrated into the operating room should be ubiquitous, and we think this is a step on that on that journey,” Guthart said. “I don’t think most surgeons care about the word robots, and I don’t think patients care about robots, nor should they. I think this is about how do you make really high-quality surgery ubiquitous, how do you make it easier for
care teams to become proficient quickly and in a data-driven way?”
“There’s an opportunity here, and one of the big steps forward in da Vinci 5 is really setting up surgical data science, the ability to have integrated, high-quality, synchronized data that allows computation plus sharp care teams to make really good decisions
“Faster cases that don’t compromise patient safety allow for more efficient human use of precious human and capital resources at the hospital and should be well appreciated by our customers.”
about what they’re doing and how to improve,” he continued. “And I think if we do that in a way that lowers the total cost to treat per patient episode so that it’s self-financing for the healthcare systems … that is the ethos behind da Vinci 5. I think it’s another step. It’s not the last step. It’s just the next step. And I think it’s going to get us there.”

• USP Class VI approved
• Elongation at break, 75°F 20-40%
• Elongation at break, 75°F 20-40%
• Dielectric strength, 75°F 450 volts/mil
• Dielectric strength, 75°F 450 volts/mil
• Complies with RoHS3 Directive (EU) 2015/863
• Complies with RoHS3 Directive (EU) 2015/863
 KAYLEEN BROWN MANAGING EDITOR, DEVICETALKS
KAYLEEN BROWN MANAGING EDITOR, DEVICETALKS
EXPERTS FROM ABBOTT, THE UC DAVIS PEDIATRIC HEART CENTER AND TEXAS CENTER FOR PEDIATRIC AND CONGENITAL HEART DISEASE
TACKLE THE CHALLENGES OF DESIGNING LIFE-SAVING DEVICES FOR THE WORLD’S TINIEST PATIENTS.
““What sets pediatric devices apart? It’s about giving children a chance at a full life,” said Dr. Lars Søndergaard, divisional VP of medical affairs and chief medical officer for Abbott’s Structural Heart division. “The solutions we design can’t just tackle the issue at hand — they need to enable normal development and stand the test of time across decades of life.”
Unlike the predictable anatomies of adults, young patients — some barely larger than the palm of a hand — are constantly changing, and those changes need to be accounted for in the long-term efficacy of the device. And though the challenges are substantial, the reward is immense. Successfully addressing an unmet need in pediatric care not only offers individual children a brighter future throughout their lives
(ABOVE) Abbott’s Amplatzer Piccolo — designed for catheter delivery inside an infant’s heart — is among the smallest pediatric devices ever made. Image courtesy of Abbott
but often has cascading benefits for treating adult patients.
At DeviceTalks West 2023, a panel featuring leading physicians and device innovators discussed the unique complexities and challenges of pediatric device development. Joining Søndergaard was Dr. Byron Holt, chief of pediatric cardiology at the Texas Center for Pediatric and Congenital Heart Disease, Dr. Frank Ing, professor of pediatrics, chief and co-director of the UC Davis Pediatric Heart Center, and Katy Fraser, global marketing manager with Abbott’s Heart Failure division.
(continued on page 30)


Abbott Structural Heart Division VP of Medical Affairs and Chief Medical Officer
Dr. Lars Søndergaard


UC Davis Pediatric Heart Center Co-director, Pediatric Cardiology Chief and Professor of Pediatrics
Dr. Frank Ing
(continued from page 28)
Challenge 1: Miniaturization with growth potential
One of the most critical hurdles is developing devices small enough to navigate pediatric blood vessels and anatomical structures without compromising efficacy. Continuous advances in catheter design and the increasing use of transcatheter methods make these intricate procedures significantly less invasive.
“We’re making devices small enough to go through little 4 French catheters,” said Holt, highlighting the current state-ofthe-art in catheter design.
“You have to think … can we do it less invasively? Their bodies are still growing. … [We] want something that can definitively address the problem without compromising normal patient growth and development.”
However, it’s not simply miniaturization; engineers must also address how to design for patient growth. Can a valve design accommodate a heart twice its size? Will that implant stretch in proportion to the patient’s developing stature? The challenge lies in creating devices that evolve in lockstep with unpredictable yet fundamental bodily changes.
“You’re talking about a wide range of body sizes. … You have to make sure that you can get it to the size of the adult,” Ing said.
Beyond these core devices, engineers must grapple with the rest of treatment. From bulky monitoring equipment to cumbersome support systems, how can these be made more child-friendly?
“Smaller is absolutely better” and allows kids to focus on simply being kids while technology works in the background, Fraser said.
A defining characteristic in treating pediatric patients is the long-term impact
of any implanted device. While durability is necessary for all medical devices, children present a unique challenge.
“You have to think … can we do it less invasively?” Holt said. “Their bodies are still growing. … [We] want something that can definitively address the problem without compromising normal patient growth and development.”
Adaptable devices hold the key for meeting these long-term demands.
“A successful pediatric device is dynamic, designed to evolve with the patient,” Ing said. “The challenge with many congenital issues is that you’re treating a baby when you know that rapid growth is on the horizon. Can we engineer devices that can be expanded over time, or are there biodegradable solutions that pave the way for healthier tissue to take over?”
The goal is to design for minimal interventions across a person’s lifespan, avoiding repeated surgeries whenever possible. Open, ongoing collaboration between engineers and physicians is vital. For effective solutions, engineers will benefit from an in-depth understanding of how tissues develop, the potential evolution of congenital heart defects, and the potential impact a device will have on the child’s development.
Challenge 3: Safety beyond standard precautions
Pediatric medicine demands heightened safety standards beyond simply addressing immediate complications. Engineers must meticulously consider potential impacts on brain development, lifelong radiation risks, and delicate vascular systems.
• Neurodevelopmental concerns: Repeated sedation is a concern for younger patients. “Every time we sedate a patient, we worry about neurodevelopment,” Holt said.
• Minimizing radiation risks: Pediatric patients have decades of life ahead, making cumulative radiation exposure particularly dangerous. Ing highlighted the need to find “ways to minimize radiation using non-radiation imaging systems to guide interventions.” MRI-guided technologies may be explored to address this need.
• Vascular preservation: As growing bodies may need multiple interventions, it’s vital to ensure the long-term integrity of blood vessels by avoiding obstructions or scarring that compromise later treatment access. Repeated procedures raise concerns about “losing those vessels … [so we need] ways to preserve access,” Ing said.
Pediatric patients should not be asked to live like those confined to a hospital bed. This necessitates innovation beyond smaller versions of adult devices and into design features that promote integration into active lives.
“These children … move. They do things,” Fraser said, and that requires pumps, tubing, and monitoring systems that flex and adjust naturally with a child’s normal activity. Furthermore, external solutions can’t exist in a vacuum.
“You have to take into consideration lifestyle,” Fraser said. “Beyond mobility, how can equipment support a child’s education, playtime with friends, and family support — key facets of normal development?”
Designers should consider intuitive use that limits burdens on parents and caretakers as well. This creates a ripple effect enhancing longterm outcomes for the child as their support system can readily engage with, maintain, and use the external devices without complex routines or specialized knowledge.
Pediatric cardiology offers immense potential for breakthroughs. The panel unveiled compelling directions that engineers should consider investing in.
• Collaboration is crucial: Holt advocated for the breaking down of silos in favor of a shared knowledge approach from the design phase to the market. In pediatrics, the FDA has shown increased receptiveness to streamlined collaboration and early dialogue can speed up beneficial breakthroughs.
• Hybrid OR growth: Hybrid procedures blending interventional
and surgical techniques will expand. Engineers developing specialized, smaller equipment and tools tailored for complex scenarios within this niche offer unique promise.
• MRI guidance: This radiation-free imaging alternative holds incredible safety advantages, but compatible tools are a limiting factor.
• The promise of bioabsorbables: Devices that serve immediate needs then disappear or self-evolve for growth remain tantalizing but difficult. A breakthrough here drastically reshapes long-term outcomes.
• Expanding the focus: Søndergaard reminds us not to overlook the mitral valve, the tricuspid valve and the pulmonary valve. These underserved anatomical targets represent major potential growth areas for both new and improved medical devices.







CATCH UP ON THE LATEST IN DIABETES TECH FROM DEXCOM, ABBOTT, ROCHE DIABETES CARE, INSULET, MEDTRONIC AND KNOW LABS.
Every year, the diabetes community comes together for the International Conference on Advanced Technologies & Treatments for Diabetes (ATTD).
In this year’s 17th installment, some of the biggest names shared new studies and technologies set to advance the diabetes space. Dexcom, Abbott, Medtronic and more contributed on their end, while some new technologies are coming to the fore as well.
Here are the biggest stories from this year’s ATTD conference in Florence, Italy.
Dexcom’s direct-to-watch feature
Dexcom announced at ATTD that it introduced a direct-to-Apple-Watch feature for its G7 continuous glucose monitor (CGM) users.
The company plans a phased launch for all G7 iOS users everywhere by the end of the second quarter of this year. This means G7 soon becomes the only CGM system that can connect directly to Apple Watch without needing to carry an iPhone. Those who use G7 can access this feature as soon as it launches in their country.
On top of the watch feature, Dexcom announced positive data backing its CGMs and their connectivity to automated insulin delivery (AID). The data includes results from the CGM with both the Tandem Diabetes Care t:slim X2 pump and Insulet Omnipod 5.
Additionally, the company launched Dexcom ONE+ in new geographies and has more data backing its technology.
(continued on page 34)

Our range of Swiss Type CNC Machines and Fixed Headstock Automatic Lathes are ready to produce your most intricate part.
• 1mm to 38mm Bar Stock
• 5-axis machines with excellent cost performance to a high-end machine equipped with B axis and back spindle Y axis.
• LFV (low-frequency vibration) available on most models
• Barloaders “CAV” fully integrated through Machine Control
• Intuitive CITIZEN Controls offers ease of programming and operation
• Machining Solutions with Lifetime Customer Support
• 15 + models to choose from


• From simple single-spindle machines to twin-spindle, multi-turret turning centers covering the range right up to 80mm bar diameter.
• Accuracy, Rigidity and Durability
• LFV (low-frequency vibration) available on multiple models
• Large and accessible workspace
• On-machine program check
• Intuitive CITIZEN Controls offers ease of programming and operation
• Machining Solutions with Lifetime Customer Support
• 13 + models to choose from
• Fully integrated into the machine control for easier operation and total control from one single console
• Quick response between bar feeder and the machine sliding headstock
• A unique stabilizing mechanism: forces are evenly distributed, protecting the spindle from excess wear
• Automatic remnant retraction
• Hydrostatic oil support
• Quick channel change ( less than 35 seconds)
• One company to contact for parts and support
(continued from page 32)
Dexcom launched ONE+ in Europe in February and has released the system in at least eight markets since that time, including Spain, Belgium and Poland.
While all this was going on, Dexcom also won the first FDA clearance for an over-the-counter CGM.
Abbott says CGM and GLP-1 combination can boost diabetes care GLP-1 receptor agonists like Ozempic and Wegovy provide therapy for diabetes and weight loss. This therapeutic class, a glucagon-like peptide 1, has proven to lead to improved blood sugar control and weight loss. The drug class continues to grow in popularity, with prescriptions for type 2 diabetes increasing.
Abbott says people with type 2 diabetes using GLP-1s with its FreeStyle Libre CGM had a greater improvement in HbA1c compared to GLP-1s alone.
“The data analyses confirm that people using GLP-1 medicines to manage their diabetes can achieve even better results when using it together with FreeStyle Libre technology.”
Last year, BTIG analysts spoke with an expert, determining the potential impact of GLP-1s on the diabetes market. Not long after, Abbott shared real-world data highlighting GLP-1s as a potential accelerator for its FreeStyle Libre CGM product family.
“The data analyses confirm that people using GLP-1 medicines to manage their diabetes can achieve even better results when using it together with FreeStyle Libre technology,” said Dr. Mahmood Kazemi, chief medical officer for Abbott’s diabetes care business.
Roche Diabetes Care unveils new CGM
At ATTD, Roche unveiled its Accu-Chek SmartGuide CGM offering.
Julien Boisdron, Roche Diabetes Care’s chief medical officer, called it “a solution more than a CGM” during the event. He explained that the system, comprised of a sensor and two applications, helps visualize data and make predictions as well.
Accu-Chek SmartGuide shares some similar properties to other CGMs on the market, like those made by Abbott and Dexcom. It features a 14day wear time, a one-step application process and watertight properties. However, this CGM requires initial calibration, which is different from some of the latest-generation systems.
Roche aims to bring the real-time CGM to both the type 1 and type 2 diabetes populations, including those on insulin therapy. Users can share their data with healthcare professionals and it has a differentiating predictive algorithm included.
The CGM remains an investigational device not yet authorized for sale. Analysts appeared unsure if there are enough differentiating factors to really challenge market leaders Abbott and Dexcom.
Insulet’s Omnipod 5 shows superiority to standard pump therapy
Results from a clinical trial pitting the Insulet Omnipod 5 automated insulin delivery system against non-automated insulin therapy demonstrated improved glycemic and patient-reported outcomes in type 1 diabetes with the wearable patch pump.
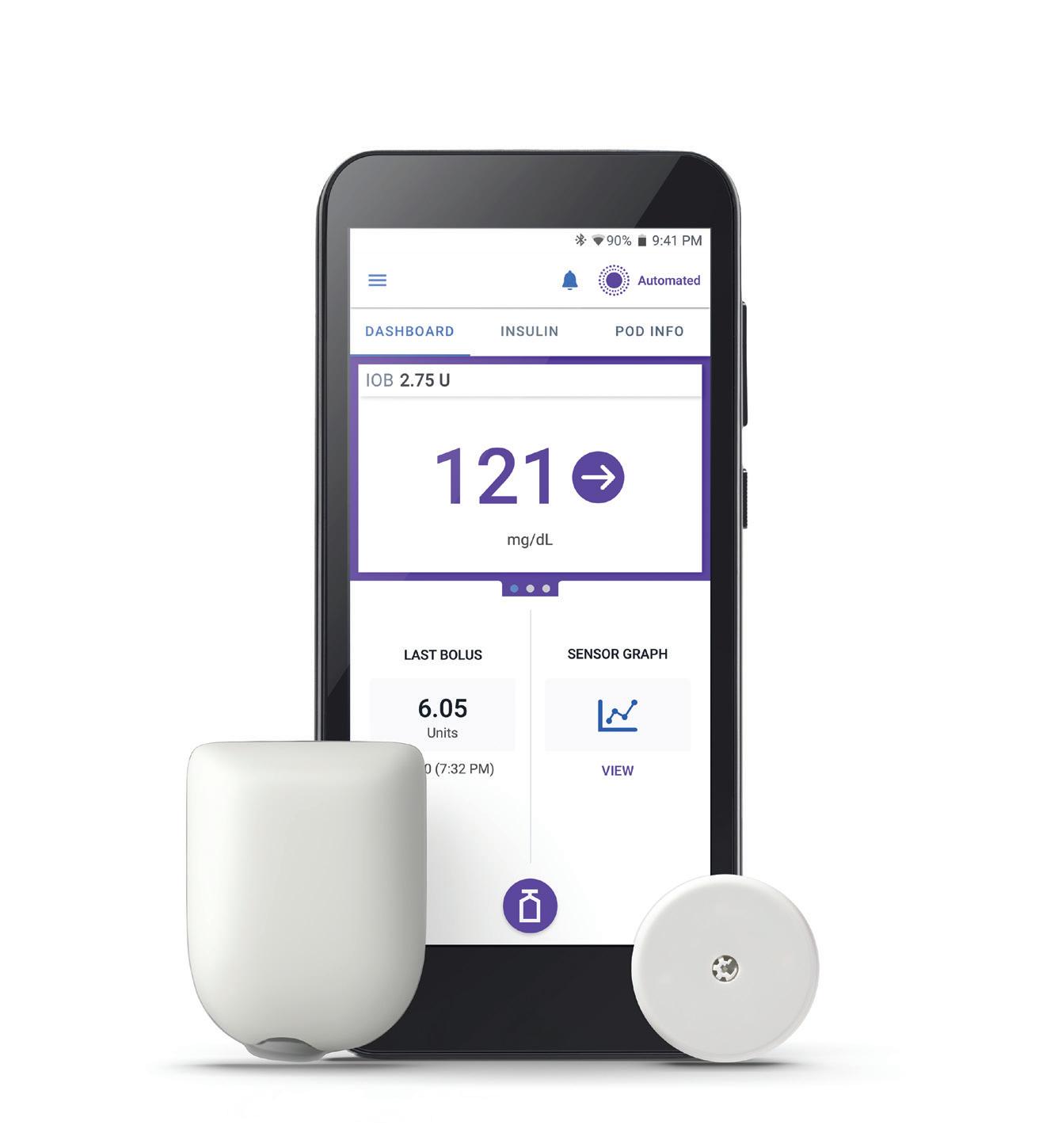
Omnipod 5 use led to a 17.5% improvement in time in range, decreased HbA1c and decreased time in hypoglycemia. It also led to a decreased mean glucose level in individuals with baseline HbA1c levels above the recommended target.
According to Insulet, the study met all primary and secondary endpoints. Exploratory endpoints showed no significant difference in the change in total daily dose from baseline or change in body mass index from baseline between Omnipod 5 and control.
Insulet also presented positive patient-reported results related to three key psychosocial measures. it included those measures — diabetes distress, hypoglycemia confidence and diabetes-related quality of life — as secondary endpoints.
Omnipod 5 use, as reported through individual surveys, resulted in both statistically significant and clinically meaningful improvements in each of these three measures compared to control.
Know Labs continues to progress its noninvasive CGM
Interim results presented by Know Labs at ATTD highlighted the accuracy of its noninvasive glucose monitoring sensor.
In February, the company unveiled KnowU, its wearable CGM set for FDA submission. KnowU’s sensor uses spectroscopy to direct electromagnetic energy through a substance or material. Through this, it can capture a unique molecular signature. The technology integrates into wearable, mobile or bench-top form factors.
Seattle-based Know Labs reported a mean absolute relative difference (MARD, a measure of accuracy) of 11.1%. Comparatively, market-leading minimally invasive CGMs from Dexcom and Abbott have demonstrated higher levels of accuracy. Dexcom’s G7 previously demonstrated a MARD of 8.2%, while Abbott’s FreeStyle Libre 3 came in at 7.6%.
However, Know Labs’ noninvasive offering remains an interesting prospect, given there are no FDA-cleared CGMs of this kind at present. Larry Ellingson, a Know Labs board member, highlighted the importance of this initial data.
“Achieving this level of accuracy in the first study using a blood reference device is truly remarkable,” Ellingson said. >>


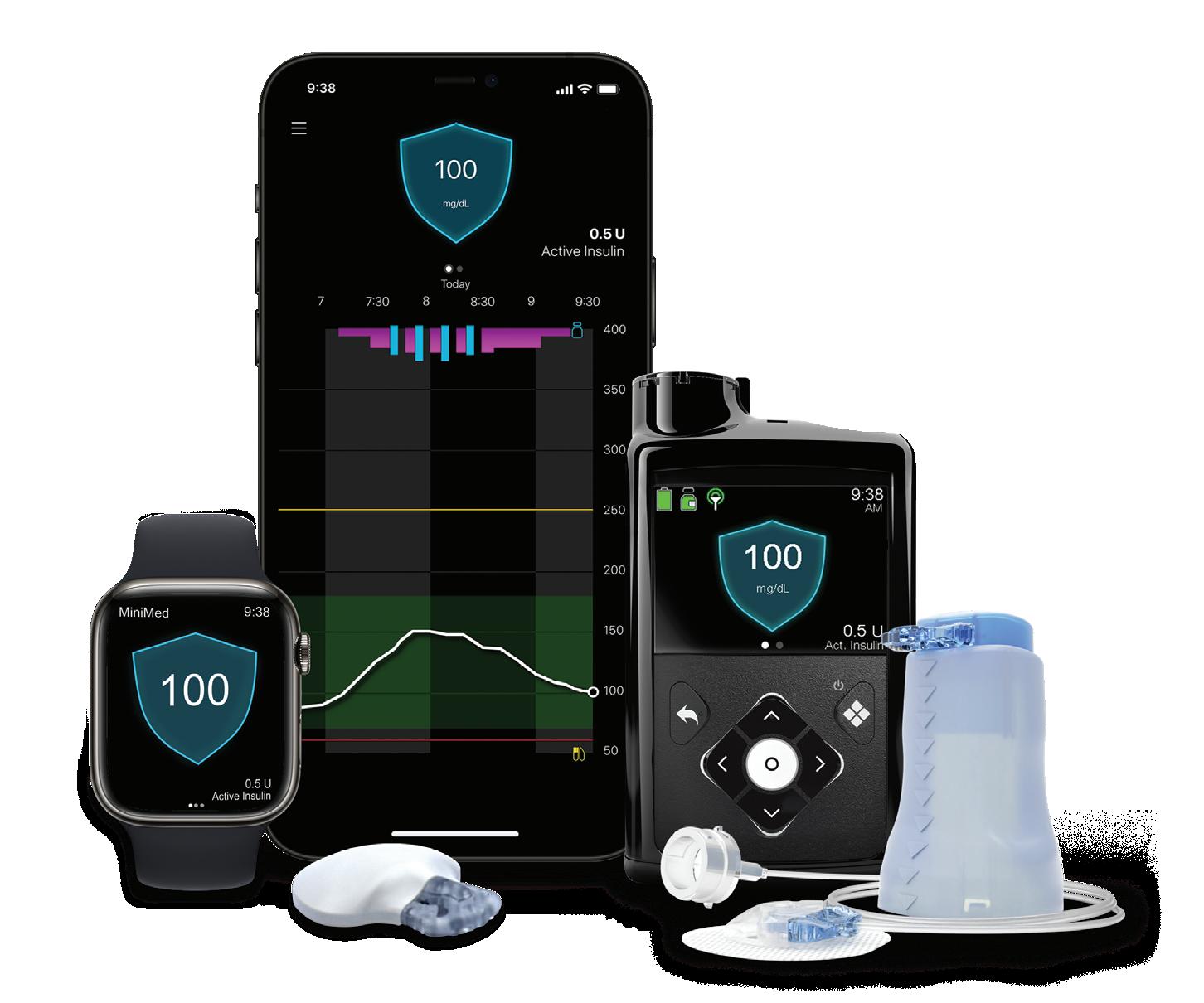
Medtronic’s MiniMed 780G is performing well all over the globe Data shared by Medtronic at ATTD included the largest set of data from early MiniMed 780G automated insulin delivery system users in the U.S. Results built upon three-year data from more than 100,000 real-world users who outperformed international time-in-range targets.
Medtronic’s MiniMed 780G, its latestgeneration automated insulin delivery system, won FDA approval in April 2023. The current iteration uses the latest Guardian 4 technology and requires no fingersticks while in “SmartGuard” mode.
New data presented by Medtronic aimed to evaluate MiniMed 780G’s ability to help users achieve time-intight-range (TITR) goals. This newer metric more closely mirrors the glucose levels of individuals without diabetes. The company defines it as the percentage of time a person spends in the glucose range of 70-140 mg/dL.
Results from Medtronic showed that users (13,461) achieved a TITR of greater than 56% when using recommended optimal settings. The company defines these optimal settings as 100 mg/dL set target with an active insulin time of two hours.
“In the absence of a cure, our goal is to relentlessly innovate therapies to help people maximize their health without adding burden, which our newest AID system has proven to do,” said Dr. Robert Vigersky, chief medical officer for Medtronic Diabetes.






























































5 things that will shape surgical robotics over the next decade
Surgical robots are smaller, smarter and being launched into space. 2024 is going to be a huge year — here’s why.

You’ll see a lot of surgical robotics talk at this year’s DeviceTalks Boston.
I believe 2024 will be remembered as the year when the surgical robotics sector — not just one company — planted both feet on the ground, slowly rose up and stood tall in the medical device industry.
Why? I’ll give you five reasons:

JOHN MURPHY
1. FDA approval of the da Vinci 5 The approval and slow release of the next-generation da Vinci 5 reasserted Intuitive’s dominance. It may have also changed the business.
In our DeviceTalks Weekly interview (available on our website or our DeviceTalks YouTube channel), Joe Mullings, chair and CEO of The Mullings Group (an executive search firm that’s done extensive work in the sector), says competitors building large surgical robotic units will have a difficult time keeping up with the da Vinci 5’s new features.
But the bigger impact may be a smaller footprint and new leasing arrangement that could open up markets in smaller health care facilities to Intuitive.
“Robotic-assisted-surgery-as-aservice is what Intuitive is pushing here,” Mullings said.
2. The emergence of smaller consoles Intuitive’s move isn’t likely to impede surgical robotics companies that have built — and are obtaining regulatory approval for — smaller systems that open up the market for surgical robotic systems.
On May 2, I’ll talk with the senior executive team at CMR Surgical — CEO Supratim Bose, CMO Mark Slack, and CTO Luke Hares — about their commercial plans for Versius, a modular and portable robot that can be moved from one operating room to another. It’s been used to perform more than 20,000 surgical cases across seven specialities.
Co-founders Slack and Hares “created a system that could be adapted to any operating room,” Bose said. “The hospitals, in terms of resources and investments overall for the life of this system, find Versius more of a value to them.”
Virtual Incision, earlier this year, grabbed national headlines by sending a version of its MIRA mobile surgical robotic system to the International Space Station. The publicity surrounding this news focused attention on the company’s true goal of making every operating room a surgical robotic suite.
In a DeviceTalks Weekly interview, Virtual Incision CEO John Murphy >>


Rajit Kamal
Sryker Group President for

“We believe we are a great robotics organization ... There’s some neat technology out there for sure.”
says his system’s robotic arm, which is fastened to the surgical table and doesn’t require a base console like other systems, will make surgical robotics possible for integrated delivery networks (IDNs) and ambulatory surgery centers (ASCs) that can’t currently afford or accommodate a standard surgical robotic system.
Similarly, Distalmotion is poised for success with its Dexter robot, which doesn’t require a dedicated room and has disposable instruments that don’t require sterilization after use. CEO Greg Roche, who will speak at DeviceTalks Boston, says the smaller robotic system puts the company in position to go where patients are seeking care.
3. The drive for remote connection Virtual Incision’s space excursion aside, surgical robotics companies recognize the importance of remote connection. At DeviceTalks Boston, we’ll have presentations from Intuitive and Medtronic focusing on the long-distance reach of their systems.
But we’re also seeing the emergence of an infrastructure built to support remote surgery. To some, 5G presents the conduit for steady connection. But serial entrepreneur Yulun Wang, who could be described as the founder of the surgical robotics sector, co-founded Sovato Health to develop a different network employing existing infrastructure of fiber optic cables.
Wang’s got a knack for getting ahead of trends. He founded Computer Motion in 1990, five years before the launch of Intuitive. The companies would merge 13 years later as a resolution of a patent dispute, freeing Wang to cofound telehealth company nTouch, which was acquired by Teladoc in 2020.
Wang and Sovato co-founder and CEO Cynthia Perazzo say patients are comfortable with remote care thanks in part to the pandemic. Regulators and hospitals also see the need for surgeons to extend their reach.
“A third of U.S. counties don’t have a single surgeon,” Perazzo said in a recent DeviceTalks Weekly interview.
On the technical side, Sovato execs say the current telecommunication network can handle the traffic. Wang recalls the world’s first transatlantic surgery performed (on a Computer Motion system) in 2000 ran on an ATM
line monitored by 15 engineers. Today, fiber optic cables are plentiful.
Finally, robotic surgery companies are building systems that can be controlled remotely. In a DeviceTalks Boston keynote, Intuitive EVP and Chief Digital Officer Brian Miller will cover many of da Vinci’s 5’s new features, including its potential for remote surgery.
At the close of the conference, Medtronic VP and GM of Surgical Robotics Rajit Kamal will demonstrate the remote functionality of Hugo RAS in a joint keynote for attendees of DeviceTalks Boston and our co-located Robotics Summit & Expo.
4. The power of AI
The ongoing adoption of artificial intelligence will certainly accelerate the development of surgical robotic systems. AI already has made a massive impact on the medical device industry.
Semiconductor chip giant NVIDIA added a tanker of gasoline to that fire at its 2024 GPU Technology Conference (GTC), launching close to two dozen new AI-powered, healthcare-focused tools and announcing partnerships with GE Healthcare and Johnson & Johnson. NVIDIA already had been working with Asensus Surgical and Medtronic.
Intuitive’s Miller and Medtronic’s Kamal both intend to cover AIpowered functionality in their DeviceTalks Boston talks.
5. Experienced competition emerging
The surgical robotics sector has been around enough to produce executives who have enjoyed successful outcomes. Now they’re back for more.
Former leaders from Auris Surgical, for example, are leading startups like Moon Surgical and Noah Medical.
Before becoming Distalmotion’s CEO, Roche served as global president for robotics and technology at Zimmer Biomet, where he led the successful global launch of the ROSA Robotic Knee System.
Quantum Surgical CEO Bertin Nahum led an earlier part of the ROSA story. Nahum founded one of the first successful surgical robotics companies, Medtech S.A., which was sold to Zimmer Biomet in 2016.
(continued on page 40)

Interpower® hospital-grade cords are manufactured to the highest hospital-grade standards. By providing the correct country-specific amperages and voltages, medical devices such as portable CT scanners, X-ray machines, medical-grade treadmills, and ECMO machines can run on reliable AC power when patients and hospital staff need it most.
When designing, manufacturing, and maintaining hospital-grade products worldwide, it is vital to know the medical requirements of the country of export—select hospitals in Australia, Denmark, and Sweden have proprietary requirements. Interpower hospital-grade cords surpass UL 817 (18.2.4.1) requirements, and the C22.2 No. 21-14 requirements for hospital-grade cords and cord sets. Every Interpower cord and component is subjected to Interpower’s strict quality control procedures and testing at every stage of manufacturing.
Add Cord Clips to hospital-grade cords to maximize patient and staff safety. The clip is molded at a convenient 4.75 inches behind the plug, and is made of a reinforced polypropylene resin to ensure consistency and optimal retention.
• 1-week manufacturing lead-times
• Same-day shipping on in-stock products
• Quick-change molding process


(continued from page 38)
Nahum will speak at DeviceTalks Boston about Quantum’s Epione system, an open robotic system that brings image-guided precision to minimally invasive cancer ablation.
Finally, Stryker, one of the more experienced players in surgical robotics and the leader in hard-tissue surgical robotics, could consider a move into soft tissue robotics. In an upcoming StrykerTalks podcast, Spencer Stiles, Stryker group president for orthopaedics and spine, answered the question about a move into softtissue this way.
“We believe we are a great robotics organization,” Stiles said. “We remain a passionate M&A company. And so you can imagine
continue to assess. There’s some neat technology out there for sure.”
Erik Todd, VP and GM of robotics and enabling technology at Stryker, will give an update on the MAKO System at DeviceTalks Boston.
So this is why we focused so much of DeviceTalks Boston on surgical robotics, but even I’m surprised at the pace at which the space is moving
If you had asked me in January, I’m not sure I would have said we’d see a new da Vinci cleared by the FDA, a surgical robotics system launched into space, and a mid-tier player like Karl Storz looking to change the game by buying a small, but tested, surgical robotics company like Asensus Surgical.
This is 2024. Almost anything can happen. Join us at DeviceTalks Boston to find out what’s next.








Since 1898, we've partnered with the most innovative companies around the world to solve complex challenges and advance technology.
As industry leaders, you can depend on our decades of experience, unrivaled reliability, and custom cable solutions that power technology from coast to coast and around the globe.




