










































































































Our diverse technical experience and rapid-turn mindset adapt to changing project demands.
With deep expertise in complex catheter-based delivery systems—as well as electrophysiology, cryotherapy, and implants—our dedicated team of engineers can tackle engineering and manufacturing challenges at all phases of development. Together, we’ll help you advance your solution from concept to market, rapidly and cost-effectively, with the design documentation necessary for regulatory approval.


To learn more, visit resonetics.com/agile-product-development






























































EDITORIAL
Editor in Chief Chris Newmarker cnewmarker@wtwhmedia.com
WEB DEV/DIGITAL OPERATIONS
DIGITAL MARKETING
VP, Operations Virginia Goulding vgoulding@wtwhmedia.com


















Web Development Manager B. David Miyares dmiyares@wtwhmedia.com













Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Digital Media Manager Patrick Curran pcurran@wtwhmedia.com
Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com












HELP US HONOR THE COMPANIES THAT HAVE PROVIDED THE MOST LEADERSHIP IN THE MEDICAL TECHNOLOGY INDUSTRY.



Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Front End Developer Melissa Annand mannand@wtwhmedia.com
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com

































Editor in Chief, R&D World Brian Buntz bbuntz@wtwhmedia.com
Software Engineer David Bozentka dbozentka@wtwhmedia.com
Webinar Manager Matt Boblett mboblett@wtwhmedia.com



























Associate Editor Sean Whooley swhooley@wtwhmedia.com










Web Dev./Digital Production Elise Ondak eondak@wtwhmedia.com
Webinar Coordinator Halle Sibly hkirsh@wtwhmedia.com

PRODUCTION SERVICES
Webinar Coordinator Emira Wininger emira@wtwhmedia.com









The medical device industry just wrapped up another year with more blockbuster mergers grabbing headlines around the world and new, innovative technologies emerging seemingly every day.


DIGITAL MAGAZINE OF THE YEAR HONORABLE MENTION

















Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com




This level of success wouldn’t be possible without the innovation, ingenuity and determination of the people who drive it: leaders. These individuals and companies are working for the growth of the entire medical device industry.






















The future of medtech will build on the foundation of today’s efforts and Medical Design & Outsourcing would like to acknowledge such achievements.

We think they deserve recognition from you, too. Vote online for one or more of the companies listed through October.








NATIONAL GOLD FOR REGULAR DEPARTMENT
NATIONAL GOLD FOR OPENING FEATURE SPREAD DESIGN
Managing EditorDeviceTalks Kayleen Brown kbrown@wtwhmedia.com
Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
EVENTS

Director of Events Jen Osborne josborne@wtwhmedia.com
NATIONAL SILVER FOR COMPANY PROFILE
NATIONAL BRONZE FOR PUBLICATION DESIGN
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com

REGIONAL GOLD FOR OPENING FEATURE SPREAD DESIGN


REGIONAL GOLD FOR REGULAR DEPARTMENT

REGIONAL GOLD FOR COMPANY PROFILE
REGIONAL SILVER FOR SPECIAL ISSUE
REGIONAL SILVER FOR PUBLICATION DESIGN

REGIONAL SILVER FOR FEATURE ARTICLE DESIGN





Senior Vice President Courtney Nagle cseel@wtwhmedia.com 440.523.1685
CREATIVE SERVICES
VP, Creative Director Matthew Claney mclaney@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com
Digital Production Manager Reggie Hall rhall@wtwhmedia.com
Events Manager Brittany Belko bbelko@wtwhmedia.com
VIDEO SERVICES
Videographer Cole Kistler cole@wtwhmedia.com
FINANCE

REGIONAL BRONZE FOR DIVERSITY, EQUITY AND INCLUSION
REGIONAL BRONZE FOR ENTERPRISE NEWS STORY



Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com
Digital Design Manager Samantha Goodrich sgoodrich@wtwhmedia.com
Senior Digital Designer Hannah Bragg hbragg@wtwhmedia.com
Controller Brian Korsberg bkorsberg@wtwhmedia.com
Accounts Receivable Jamila Milton jmilton@wtwhmedia.com






LEADERSHIP TEAM
EVP







CEO Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844
CFO Ken Gradman kgradman@wtwhmedia.com 773.680.5955
Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609






- brushed or bldc motors
- 5 amps per axis
- 16 analog inputs
- 16 on/off drivers
- home and limit in
- live tech support
- made in the USA

egadeals meant massive moves and new names on our 2024 Medtech Big 100 ranking of the world’s largest medical device companies.
The Medtech Big 100 is bigger than ever with a record-high $474.8 billion in sales for the 100 companies. And that figure is growing faster than the year before, partly a reflection of continued stabilization from COVID-19 pandemic shocks to suppliers and customers.
But M&A and spinoffs are another factor in our list’s accelerating growth, and we expect they’ll continue to be in the years ahead.
For example, Johnson & Johnson MedTech’s revenue jumped 11% from the year before, with nearly half of those gains coming from its Abiomed acquisition. And our latest ranking doesn’t yet account for J&J’s purchase of Shockwave Medical, which climbed 11 spots in its final year as an independent organization.
J&J MedTech hasn’t yet surpassed Medtronic to take the No. 1 spot, though it’s getting closer and closer. But J&J’s growth pushed it past Medtronic by another measure, taking the No. 1 spot on our ranking of the top R&D spenders.
This year’s Medtech Big 100 report includes company details, our aggregate analysis of the ranking, rankings by total employment and R&D spending as a share of revenue, and a look at five companies that almost made the cut.
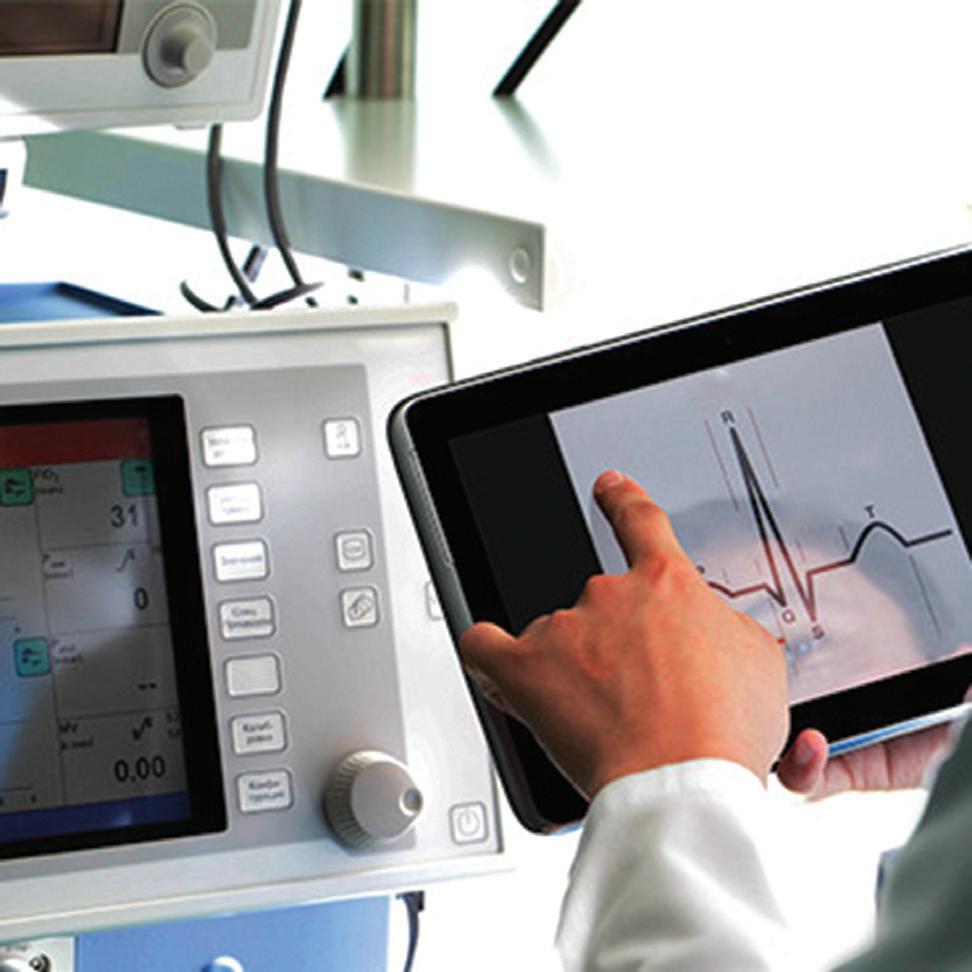
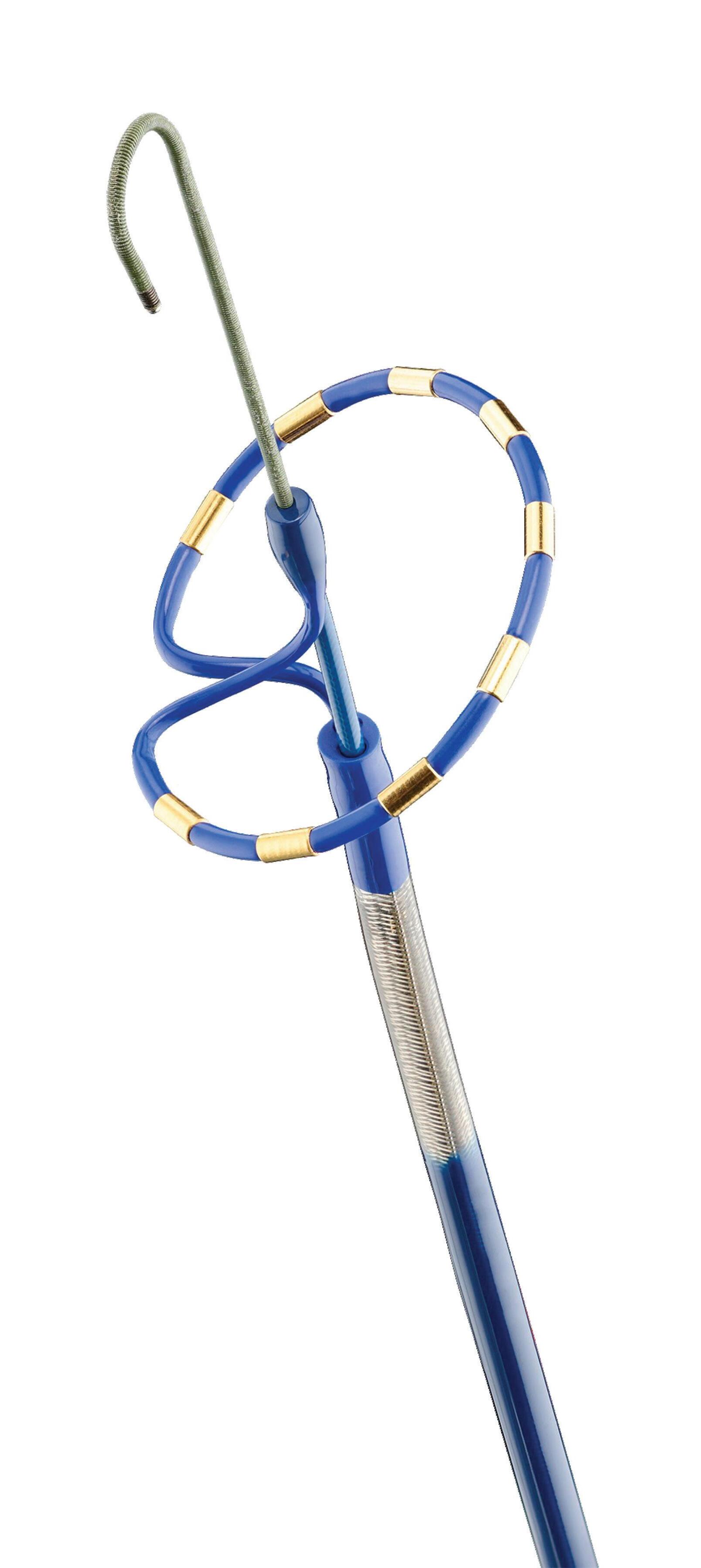
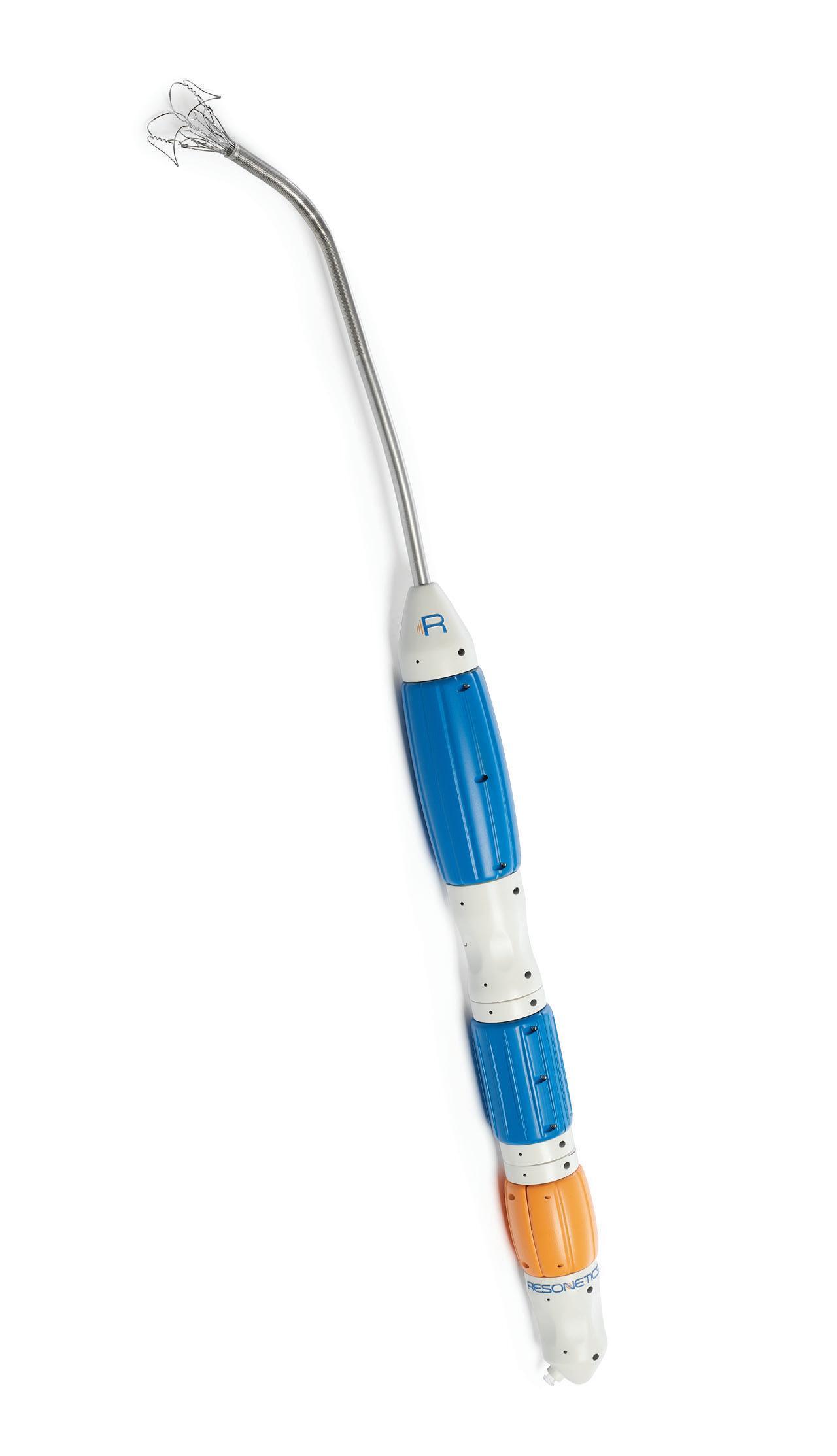
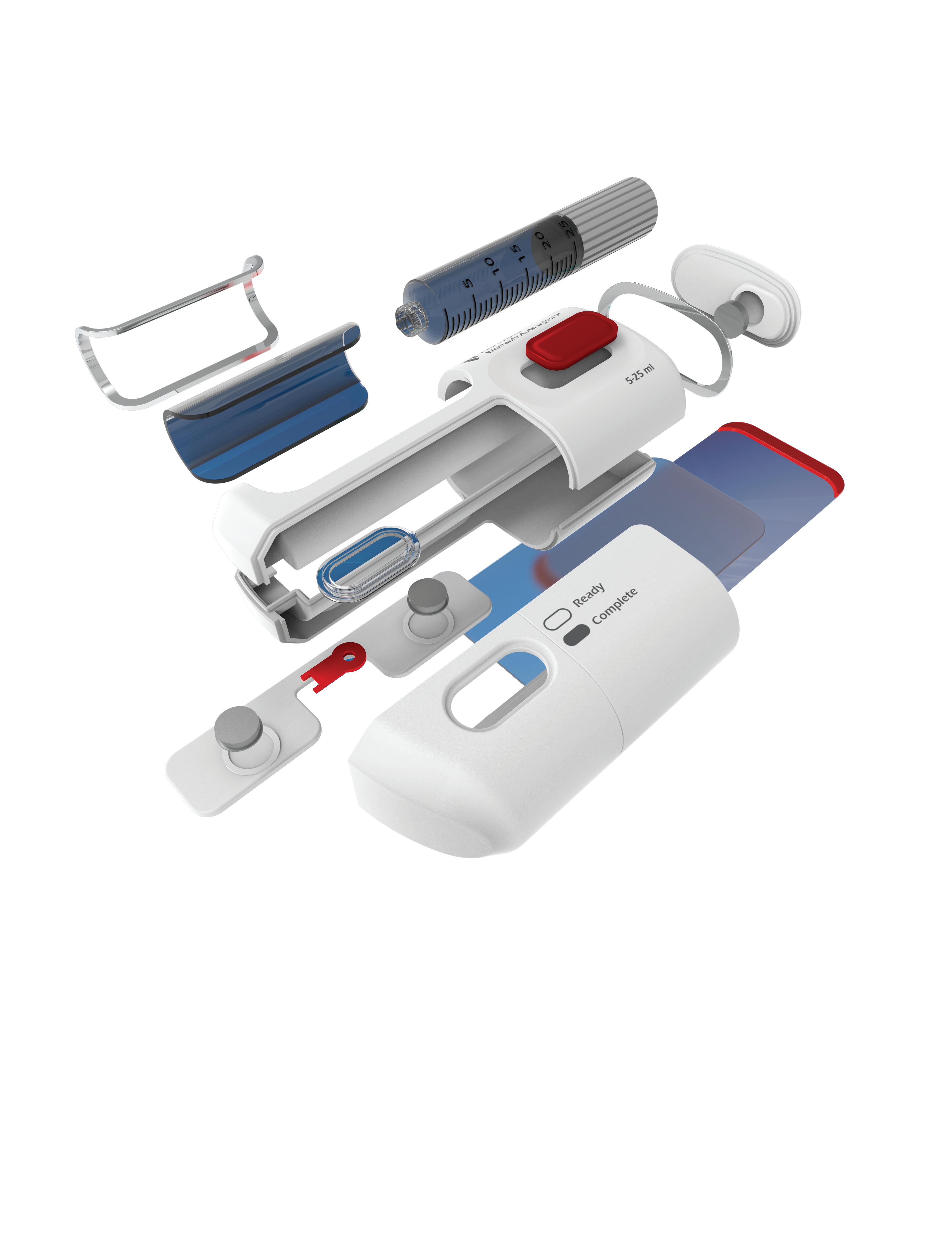
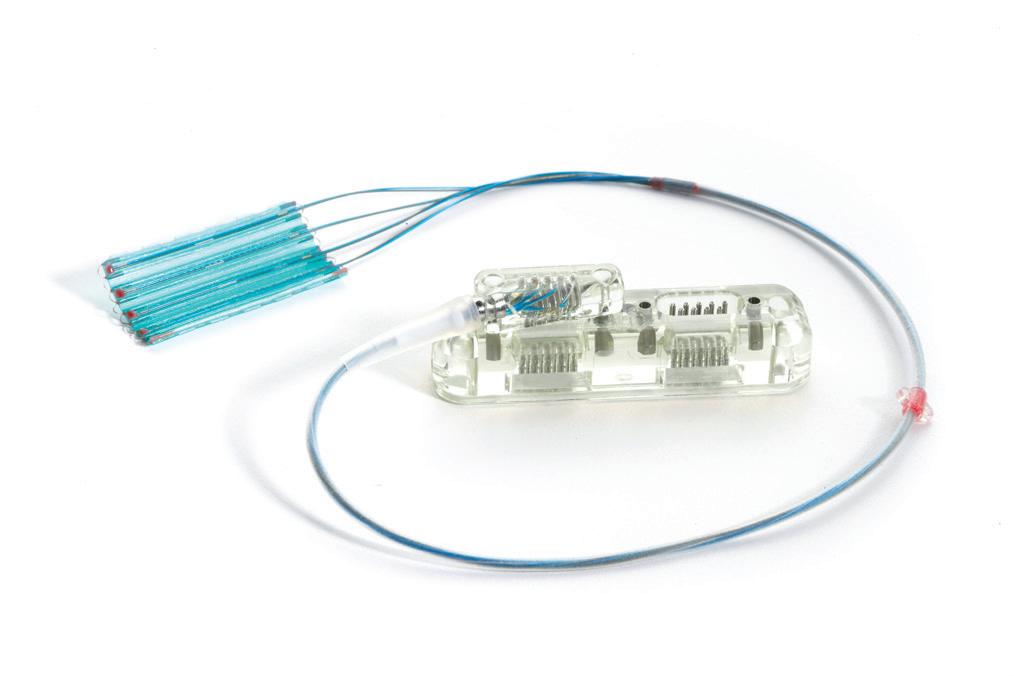
We’ve also got coverage of the technology coming out of the biggest of the big and companies that made big moves up our ranking. Medtronic, J&J MedTech, Abbott and Boston Scientific are all developing pulsed field ablation systems for minimally invasive catheter treatments of atrial fibrillation. For this month’s cover story, we interviewed experts at each of those Big 100 companies about the various shapes of their catheter designs and the advantages they offer.
In another feature, we look at the challenge Shockwave — and now J&J MedTech — faces in explaining how its Neovasc Reducer works in the coronary implant’s second bid for FDA approval.
Our new Nitinol department digs into how Abbott switched to the uniquely useful alloy to anchor its MitraClip and TriClip transcatheter edge-to-edge repair (TEER) systems for heart valves.
ResMed’s been climbing our Medtech Big 100 rankings, and they’re hoping their new AirFit F40 continuous positive airway pressure (CPAP) mask will give them another push. In our Product Design department, ResMed officials discuss the device’s new features and development challenges.
Philips explains in a contribution for our Product Development department how it collaborated with the University of Zurich on new technology to reduce anesthesiology-related mistakes due to cognitive overload in the operating room.
Intuitive — which just broke into the top 20 of our Medtech Big 100 ranking — offers commercial launch lessons for other device developers from the limited release of its new da Vinci 5 in our Surgical Robotics department.



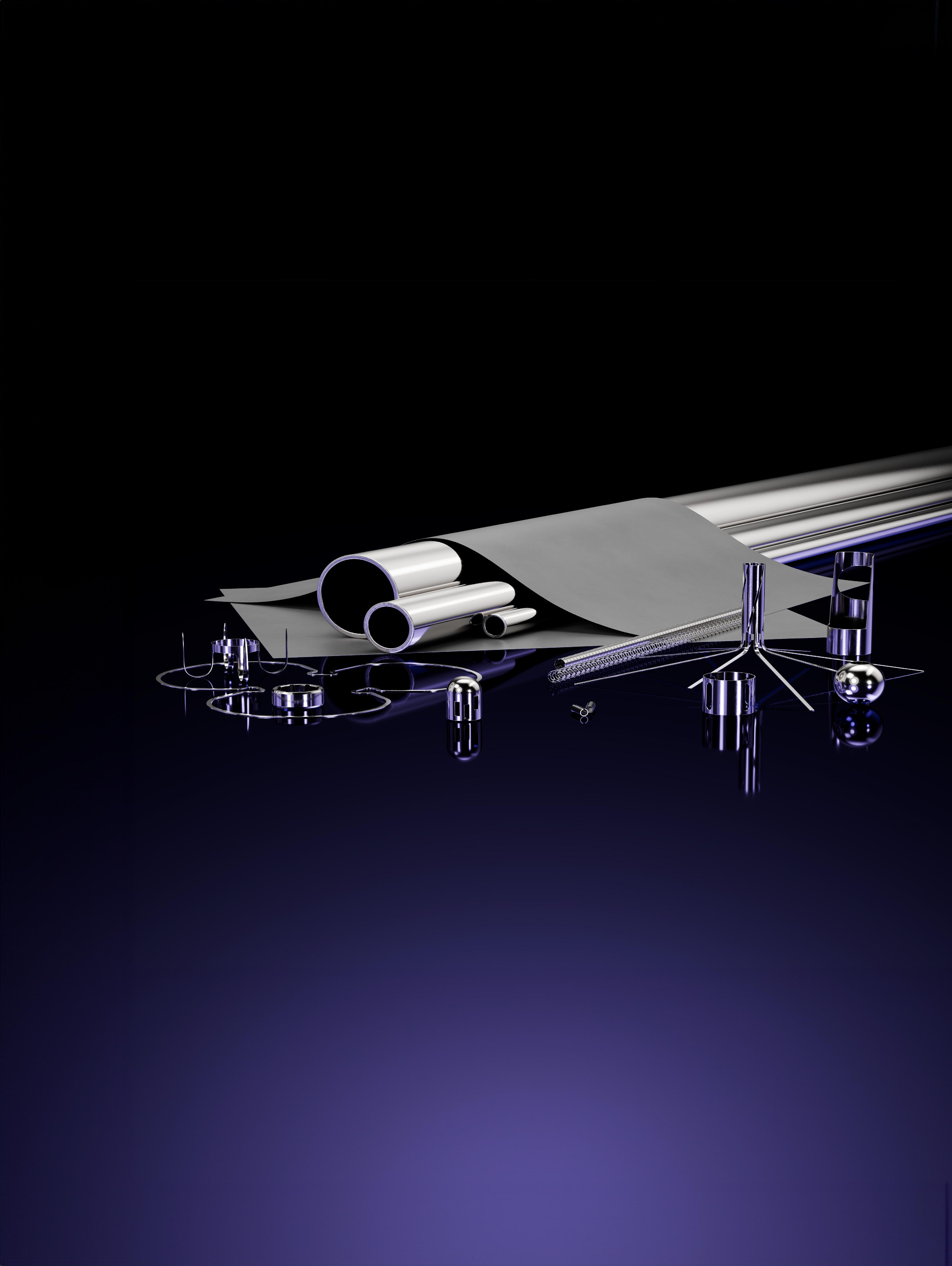
Medtronic Endoscopy leaders, meanwhile, picked three technologies that they say will be key for advancing the field in our Tubing department.
And it’s still not too late to meet our team in Santa Clara, California for DeviceTalks West on Oct. 16-17. This issue concludes with a preview of our show, which as always will feature Medtech Big 100 companies and smaller device developers that will likely make our ranking someday, either under their own flag or after an acquisition by a Medtech Big 100 buyer.
As always, I hope you enjoy this issue of Medical Design & Outsourcing — and thanks for reading.


HERE’S WHAT WE SEE:
The 2024 Medtech Big 100: Mergers, acquisitions and spinoffs — oh my!
NITINOL:
Nitinol grips prevent slips in Abbott’s heart valve clips
PRODUCT DESIGN:
How ResMed designed its new AirFit F40 CPAP mask
PRODUCT DEVELOPMENT:
Addressing cognitive overload to improve patient safety in the OR
SURGICAL ROBOTICS:

What Intuitive’s limited da Vinci 5 launch can teach other device developers
TUBING:
Medtronic leaders pick three technologies that are key for the future of endoscopy
MEDTECH BIG 100:
Our latest list ranks the giants of medtech manufacturing by annual revenue, R&D spending and employee headcount. SPECIAL ISSUE


DEVICETALKS:
6 critical medtech areas of focus at DeviceTalks West 2024

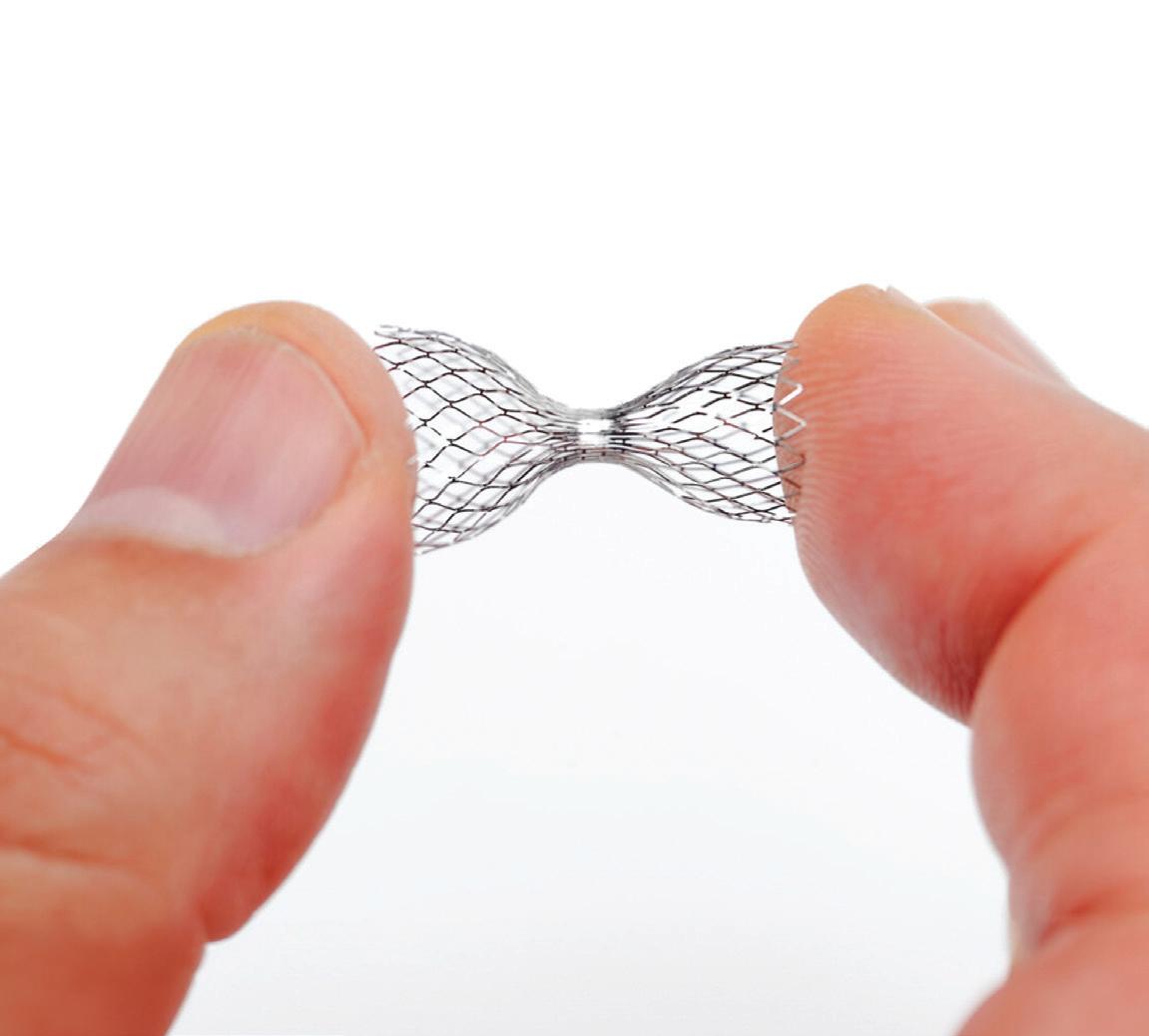
Experts from Medtech Big 100 device developers — Medtronic, Johnson & Johnson MedTech, Abbott and Boston Scientific — discuss the various shapes of their pulsed field ablation (PFA) catheters.
SHOCKWAVE CRACK THE CODE FOR FDA APPROVAL OF ITS REDUCER IMPLANT?
After buying the Neovasc Reducer, J&J Medtech’s Shockwave plans another attempt at FDA approval — and to explain how the implant treats refractory angina.

• Resolution ≤5 mV
• Accuracy ±0.25% of full scale
• Real-time adjustable PID control
• Integrated 0 to 10 VDC, 4-20 mA signal, or 3.3 VDC serial communication
• 0 to 10 VDC feedback pressure monitor
• Virtually silent
• No integral bleed required
• Multiple pressure ranges from vacuum to 150 psig
• 2.7 to 65 l/min flow control



The future of proportional control has arrived— and it’s digital. The Clippard Cordis is a revolutionary microcontroller primed for escape velocity from a proportional control market that has grown stagnant.
With unparalleled performance and flexibility not possible with current analog proportional controllers, the Cordis makes everything from calibration, to sensor variety, to future development opportunities more accessible and less complicated.
Contact your distributor today to learn more about how the Cordis can provide precise, real-time control for your application, or visit clippard.com to request more information.



Each year, Medical Design & Outsourcing collects thousands of data points to rank and analyze the largest medical device companies in the world, including publicly traded companies and privately held firms.
The Medtech Big 100 includes annual revenue, R&D spending, headcount, CEOs and key leaders, headquarters locations and descriptions of each company — or medical device business units within larger companies such as Abbott and Johnson & Johnson, which have significant pharma businesses.
We gather the data from regulatory disclosures filed with the U.S. Securities and Exchange Commission and annual reports from foreign and privately held firms. For many companies, we include data they share with us — straight from the CEO, in one case. This year we also opened an application process for companies to nominate themselves for inclusion among the 100 largest medtech companies.
A majority of the companies on the list have fiscal years that operate on standard calendar years, but some have fiscal years for which we’ve collected data as recently as April 2024.
We used fiscal 2023 data for companies with fiscal years that end in June or September such as Accuray, Asahi Intecc, BD, Cardinal Health, Carl Zeiss, Cochlear, Electromed, Embecta, Hologic, ResMed and Siemens Healthineers because their annual reports don’t come out before production time.
Please note that companies that report financial results in foreign currencies have been converted to U.S. dollars using standardized Federal Reserve rates for the revenue and R&D rankings.
Medtech Big 100 movers and shakers Mergers, acquisitions and spinoffs were the key trends in this year’s Medtech Big 100. Many companies near the bottom of our list in previous years — such as Silk Road Medical and Axonics — have struck
deals to be acquired by top 20 medtech companies. M&A also consolidated the revenue of companies that combined, such as the Globus Medical and NuVasive merger that moved Globus up our ranking.
We also saw spinoffs, including Solventum, fornerly 3M Health Care and now in its first year as a publicly-traded company. Zimmer Biomet spinoff ZimVie dropped down our list when it divested its spine and bone healing businesses to become a pure-play dental company — but the spine and bone healing businesses made their Medtech Big 100 debut as Highridge Medical.
One of the biggest stories in medtech — the ongoing Philips Respironics ventilator and CPAP recalls — pushed Royal Philips down a spot and allowed other companies to move up our list: Inspire Medical Systems grew its obstructive sleep apnea implant business significantly, while CPAP developer ResMed also increased its rank.
– Manging Editor Jim Hammerand and Senior Editor Danielle Kirsh (continued on page 14)




ACOPOS 6D allows you to move products freely through an open manufacturing space –unbound by the limits of one-dimensional production flow. Magnetic levitation provides six degrees of freedom for unprecedented processing density on a fraction of the floorspace.
No contact, no noise, no wear
Micron-precise positioning
11 shuttle sizes
Widest payload range on market
Most advanced planar software platform on the market
br-automation.com
PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development.
We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory.


Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.
• Specialized team of SMEs
• ISO 10993 and ISO 18562 series
• Toxicological Risk Assessment
• FDA Submission Assessment
PSN Labs has the experts to isolate confounding variables and provide clear analysis to guide the commercialization of existing and new medical devices.


from page 14)




Dublin, Ireland
(Operational HQ in Fridley, Minnesota) United States
$32,364,000,000
Fiscal year ended 4/26/2024
2023 rank: 1
R&D spend: $2,735,000,000
Employees: 100,716
CEO: Geoff Martha
www.medtronic.com
“Our top priority is restoring our earnings power — full stop,” MEDTRONIC Chair and CEO Geoff Martha said to start 2024. Over the following months, the world’s largest medtech company continued significant portfolio management moves, including the shuttering of its ventilators business. There was more discipline on headcount and expenses — including worldwide layoffs — and increased use of automation and digitization. Some high-ranking officials have left the company, too — including CFO Karen Parkhill, who resigned to take over as CFO at HP. At the same time, company leaders stressed that Medtronic is making strategic investments in research and development to boost future growth. They said in May that the company had achieved 130 product approvals in the last 12 months in key geographies. Recent wins include:
• The PulseSelect pulsed field ablation system becoming the first PFA system approved by the FDA to treat paroxysmal and persistent atrial fibrillation (AFib);
• FDA approval of the next-generation Evolut FX+ transcatheter aortic valve replacement (TAVR) system;
• FDA approval of its Percept RC deep brain stimulation system, which Medtronic described as the first DBS sensing-enabled, rechargeable device to treat movement disorders such as Parkinson’s disease;
• And the launch of the next-gen Micra AV2 and Micra VR2 leadless pacemakers, which were approved in 2023.
Medtronic is also seeking coverage and payments for its FDA-approved Simplicity Spyral renal denervation technology to treat hypertension. In August, it secured a CMS New Technology Add-on Payment (NTAP) win for the technology.
As of the writing of this profile in August, Medtronic was projecting 4–5% organic revenue growth in 2024. –CN
AI basics from Medtronic Chief Technology and Innovation Officer Ken Washington wtwh.me/Medtronic
New Brunswick, New Jersey United States
$30,400,000,000
Fiscal year ended 12/31/2023
2023 rank: 2
R&D spend: $3,122,000,000
Employees: Not available
CEO: Joaquin Duato, CEO; Tim Schmid, EVP and J&J MedTech worldwide chair
www.jnjmedtech.com
Now under the leadership of Johnson & Johnson veteran Tim Schmid after Ashley McEvoy’s announcement in October 2023 that she was resigning, JOHNSON & JOHNSON MEDTECH continues to grow. With its $13 billion acquisition of Shockwave Medical and its intravascular lithotripsy (IVL) technology completed in May, J&J’s medical device business could give Medtronic a run for the top spot in next year’s Medtech Big 100. On top of the Shockwave acquisition, recent developments at J&J MedTech include the unveiling of Polyphonic, its open and secure digital surgical ecosystem. Think data-source-agnostic software applications to deliver surgical insights. The first release included apps for surgical video, telepresence and planning. Watch for more artificial intelligence applications created through a partnership with Nvidia that the companies announced in March. Other recent J&J MedTech news includes:
• Its DePuy Synthes business has launched a spine surgery version of its Velys robot, created in collaboration with eCential Robotics.
• In April, the company sold its Ethicon business’ Acclarent ear, nose and throat treatment tech business to Integra LifeSciences for $275 million.
• J&J MedTech expects to submit its Ottava surgical robot for FDA investigational device exemption (IDE) in the second half of 2024.
• Its Biosense Webster business has submitted its AFib-treating Varipulse pulsed field ablation system for FDA approval.
J&J CEO Joaquin Duato spoke in July of the company having a “strong foundation for near and long-term growth.” –CN
$23,414,400,000
Fiscal year ended 9/30/2023 (€21,680,000,000)
2023 rank: 3
R&D spend: $2,015,280,000
Employees: 71,000
CEO: Bernd Montag
www.siemens-healthineers.com
SIEMENS HEALTHINEERS reported good progress in its third quarter despite ongoing order delays in China. The German medtech giant now expects 4.5–6.5% sales growth for fiscal 2024. “Varian and Diagnostics especially contributed to the strong operating performance,” CEO Bernd Montag said in late July. Recent innovations at the company include the FDA-cleared Syngo Virtual Cockpit, a platform that enables real-time collaboration between healthcare professionals across different locations. The FDA also cleared the Magnetom Cima.X 3 Tesla (3T) magnetic resonance imaging whole-body scanner. The company said that scanner has the strongest gradients ever for a whole-body scanner, which improve the visibility of smaller structures and accelerates image captures. Additionally, Siemens Healthineers announced in March that its selfdriving Ciartic Move mobile C-arm received an FDA clearance. Ciartic Move automates and accelerates imaging workflows in surgical environments Meanwhile, Varian in March announced clearance of its TrueBeam and Edge radiotherapy systems with HyperSight imaging. –CN
High voltage in the heart: PFA catheter design tips from Biosense Webster wtwh.me/JandJ










Northfield, Illinois United States
$23,200,000,000
Fiscal year ended 12/31/2023
2023 rank: 4
R&D spend: not available
Employees: 38,000
CEO: Jim Boyle
www.medline.com
MEDLINE revenue grew 9% in 2023. The privatelyheld medical supply manufacturer, distributor and services provider now operates in over 100 countries and territories, offering more than 335,000 medical products. Medline recently grew even more through its $950 million acquisition of Ecolab’s surgical solutions business. The deal gave Medline access to operating room equipment, Ecolab’s line of Microtek sterile operating room drapes and Ecolab’s fluid temperature management system.
“With this acquisition, we are eager to collaborate with healthcare providers and cutting-edge medical device companies to bring innovative solutions to the surgical suite,” Medline President and Chief Operating Officer Jim Pigott said. Medline recently spent $27 million to triple the size of its product testing and development lab in Mundelein, Illinois, to 74,000 ft². –CN
Portage, Michigan United States
$20,498,000,000
Fiscal year ended 12/31/2023
2023 rank: 6
R&D spend: $1,388,000,000
Employees: 52,000
CEO: Kevin Lobo
www.stryker.com
Still riding the success of its Mako robotic orthopedic surgery systems and digital surgery systems, STRYKER has gone on offense with M&A. Recent tuck-in acquisitions for the orthopedic and surgical tech giant include Artelon and its soft tissue fixation products for foot and ankle and sports medicine procedures, and Molli Surgical, which develops wire-free soft tissue localization technology for breast-conserving surgery. Stryker Chair and CEO Kevin Lobo recently indicated that the company has a very active deal pipeline for even more M&A. He said a soft-tissue surgical robotics play is a possibility, as well something in the neuromodulation space down the road. Notable updates include the myMako app for Apple Vision Pro, which enhances surgeons’ ability to visualize and plan surgeries. Stryker also introduced the Triathlon Hinge within its knee surgery offerings, aiming to simplify revision procedures Analysts expect Stryker to enjoy even more growth on shoulder and spine applications for Mako that are slated for later this year. –CN
Amsterdam Netherlands
$19,622,520,000
Fiscal year ended 12/31/2023
(€18,169,000,000)
2023 rank: 5
R&D spend: $2,041,200,000
Employees: 69,100
CEO: Roy Jakobs
www.philips.com
The fallout from PHILIPS’ massive, yearslong recall of CPAPs and other respiratory devices reached a critical point in 2024. In April, the Dutch medtech giant finalized a consent decree with the U.S. Department of Justice and FDA that provided a roadmap for resolving the Philips Respironics recall. Dr. Jeff Shuren, who was director of the FDA’s Center for Devices and Radiological Health (CDRH) at the time, said the agreement “marks the first time a device company is providing a remediation payment option for a recalled device under a consent decree.” Philips soon settled personal injury claims in the U.S. for $1.1 billion and is also paying at least $613 million to settle personal injury claims. By June, Philips announced the closure of its Respironics business’ Pittsburgh headquarters and elimination of hundreds of manufacturing jobs in the region. Exclude the impact of the Respironics recall, however, and Philips is still projecting 3–5% comparable sales growth in 2024. Said CEO Roy Jakobs: “We continue to focus on enhancing execution, improving endto-end supply chain resilience and increasing agility and productivity through simplifying our operating model. Patient safety and quality remains our number one priority.” –CN
How 3D printing and surgical robotics enable Stryker’s cementless knee implants wtwh.me/Stryker
Chicago, Illinois United States
$19,552,000,000
Fiscal year ended 12/31/2023
2023 rank: 7
R&D spend: $1,205,000,000
Employees: 51,000
CEO: Peter Arduini
www.gehealthcare.com
GE HEALTHCARE is facing headwinds in the market in China, which caused it in July to reduce its organic revenue growth projection to 1–2%, versus the previous projection of 4%. CEO Peter Arduini highlighted year-over-year sales growth and margin expansion in the second quarter: “We are pleased with our continued progress in advancing our margin goals, while continuing our investments for future growth.” GE HealthCare continues with new partnerships, products and tuck-in acquisitions, especially when it comes to medtech innovations enabled by artificial intelligence. Recent AI-related moves include:
• A joint development agreement with Volta Medical over AI-driven electrophysiology tech;
• Acquiring Intelligent Ultrasound Group’s clinical AI software business;
• The launch of the AI-enhanced Voluson Signature 20 and 18 ultrasound systems for women’s health imaging applications;
• A partnership with Biofourmis to use its FDAcleared, AI-guided algorithms to help deliver personalized healthcare and health monitoring in people’s homes.
In addition, GE HealthCare in May announced the launch of its “new era” of AI-enhanced oncology solutions, known as Revolution RT. –CN
Abbott Park, Illinois United States
$16,887,000,000
Fiscal year ended 12/31/2023
2023 rank: 10
R&D spend: not available
Employees: not available
CEO: Robert Ford, chair and CEO; Lisa Earnhardt, EVP medical devices
www.abbott.com
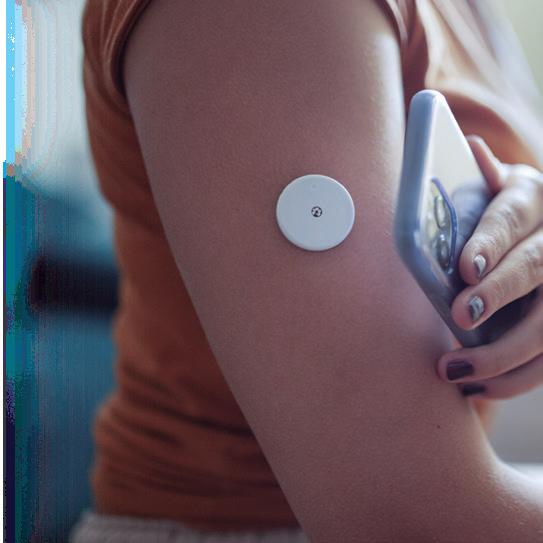
ABBOTT medical device segment sales were up more than 12% during the first half of 2024. Diabetes treatment tech — especially the Abbott FreeStyle Libre continuous glucose monitor (CGM) — played an important role fueling the growth. FreeStyle Libre sales totaled $1.6 billion in the second quarter alone, marking 18.4% growth year-over-year. A new partnership to combine FreeStyle Libre CGM technology with Medtronic’s automated insulin delivery systems could boost sales even more in the years ahead. In June, Abbott announced that it secured FDA clearance for two over-the-counter CGM systems, Lingo and Libre Rio. Electrophysiology is also an exciting space for Abbott. The company in June announced CE mark approval for its Aveir DR dualchamber leadless pacemaker system, nearly a year after Aveir won FDA approval. The company has seen recent launches and new indications for its Amplatzer Amulet, Navitor, and TriClip. When it comes to pulsed field ablation, Abbott continues to develop its Volt system, which has a balloon-in-basket design meant to enable efficient deployment of energy into the tissue during cardiac ablation to treat AFib. –CN
(medical segment)
Dublin, Ohio United States
$15,014,000,000
Fiscal year ended 6/30/2023
2023 rank: 8
R&D spend: not available
Employees: not available
CEO: Jason Hollar, CEO; Steve Mason, medical segment CEO
www.cardinalhealth.com
During CARDINAL HEALTH’S third-quarter earnings call on May 2, CEO Jason Hollar reported that the company’s Global Medical Products and Distribution (GMPD) business is seeing strong topline and bottom line performance, with growth accelerating as a turnaround plan accelerates. “GMPD’s quarter was overall consistent with our expectations and the team is already working hard on the continued ramp-up in Q4,” Hollar said. Cardinal Health said its U.S. Medical Products and Distribution Business won recognition from the HIRC Resiliency Badge program for its supply chain resiliency. –CN
Deerfield, Illinois United States
$14,813,000,000
Fiscal year ended 12/31/2023
2023 rank: 9
R&D spend: $667,000,000
Employees: 60,000
CEO: José Almeida
www.baxter.com
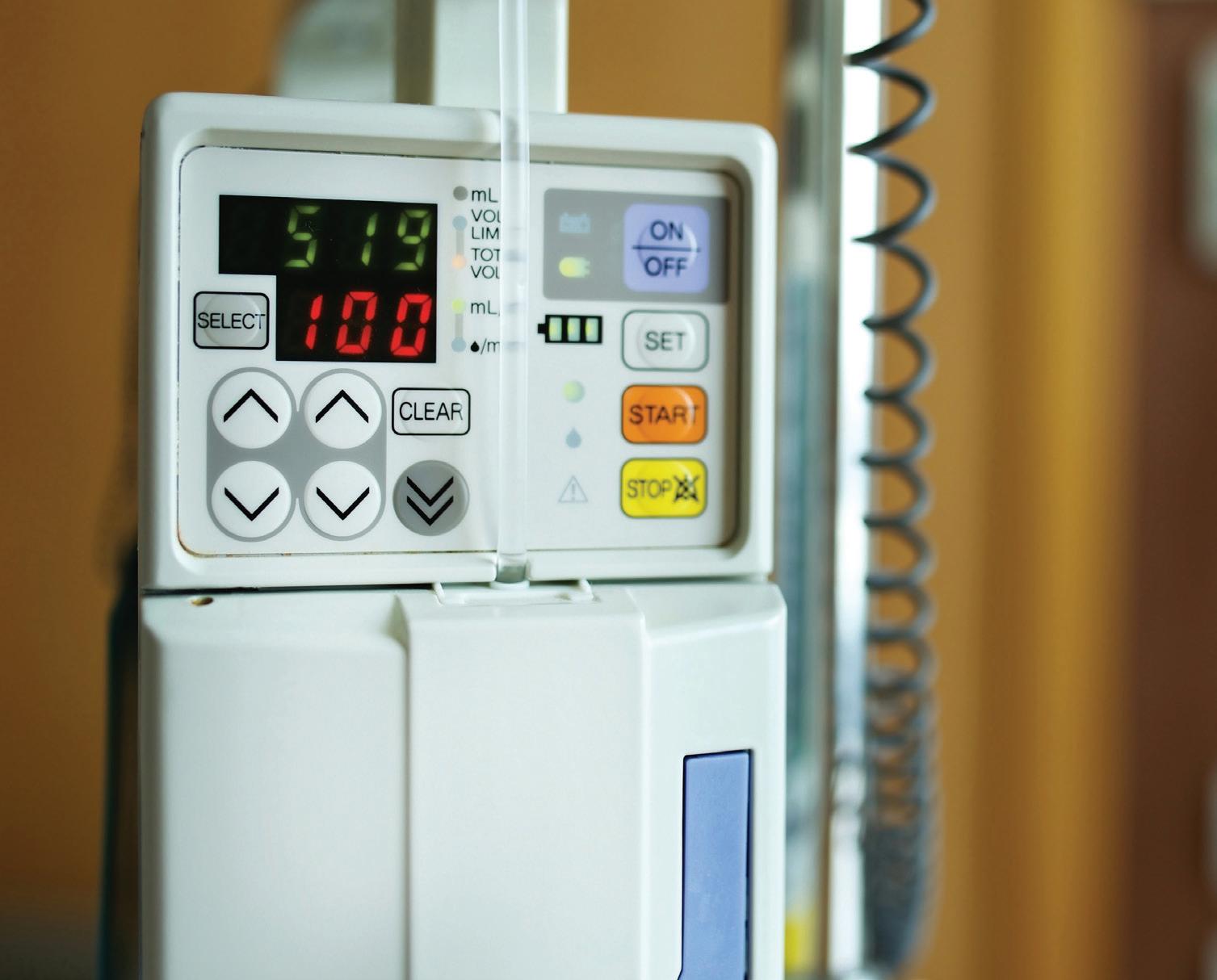
BAXTER continues to move forward with a separation of its Kidney Care business, which will be called Vantive. In mid-August it announced that it would sell Vantive to global investment firm Carlyle for $3.8 billion. The companies expect the transaction to close in late 2024 or early 2025, subject to customary approvals and closing conditions. “As a result of this proposed transaction, Baxter will emerge a more focused and more efficient company, better positioned to redefine healthcare delivery and advance innovation that benefits patients, customers and shareholders,” Baxter Chair and CEO José Almeida said. Meanwhile, the Baxter Medical Products & Therapies business has benefitted from positive pricing and demand. The second quarter also saw the first U.S. sales of the Novum IQ large-volume infusion pump with Dose IQ safety software. –CN
Harness our leading-edge vacuum technology . . . because lives depend on it.


Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.
For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.




11 13 14 12
Marlborough, Massachusetts United States
$14,240,000,000
Fiscal year ended 12/31/2023
2023 rank: 12
R&D spend: $1,414,000,000
Employees: 48,000
CEO: Michael Mahoney
www.bostonscientific.com
BOSTON SCIENTIFIC has been enjoying a banner year in 2024. As of mid-August, its stock was up more than 31% year-to-date, and the company used its second-quarter earnings report to once again raise its sales guidance. The cardiovascular, endoscopy, neuromodulation, and urology device company — which seeks to positively disrupt the general surgery space — now expects 13.5–14.5% sales growth for 2024. Recent highlights for Boston Scientific include more positive data for its Farapulse pulsed field ablation system for treating AFib. Boston Scientific announced in January that Farapulse won FDA approval to treat drug-refractory, recurrent, symptomatic, paroxysmal AFib. In July, it announced Farapulse approval in China. Boston Scientific also recently announced plans for its $1.16 billion acquisition of Silk Road Medical, which develops products designed to prevent stroke in patients with carotid artery disease. Boston Scientific is also in the process of acquiring Axonics and its neuromodulation systems for treating urinary and bowel dysfunction; the nearly $3.7 billion deal is presently expected to close during the second half of 2024 pending review by the U.S. Federal Trade Commission. Late in 2023, Boston Scientific made an initial upfront payment of $850 million to purchase Relievant Medsystems, enhancing its position in the chronic pain management market. –CN
(medical and interventional segments)
Franklin Lakes, New Jersey United States
$14,238,000,000
Fiscal year ended 9/30/2023
2023 rank: 11
R&D spend: not available
Employees: 50,000
CEO: Tom Polen; Mike Garrison, EVP and medical president; Richard Byrd, EVP and interventional president
www.bd.com
Market disruptions in China appear to be creating some headwinds for BD, but company officials boasted of continued strong performance overall during the company’s thirdquarter earnings announcement in early August. BD’s medical segment is enjoying increased market position in vascular access management and hypodermic products. Plus, medication management systems posted especially strong growth following a 510(k) clearance for its Alaris infusion pumps in 2023 that enabled U.S. distribution of the pumps to resume following a yearslong recall. Expect BD’s medical device industry footprint to grow even more now that it’s agreed to pay $4.2 billion to acquire Edwards Lifesciences‘ Critical Care business. Other important BD news includes its announcement early in 2024 that it would boost U.S. syringe production after the FDA issued warnings related to syringes made in China. –CN
Catheter design was key for the Boston Scientific Farapulse pulsed field ablation system wtwh.me/BostonScientific
Melville, New York United States
Mechanicsville, Virginia United States
$12,339,000,000
Fiscal year ended 12/31/2023
2023 rank: 13
R&D spend: not available
Employees: 25,000
CEO: Stanley Bergman
www.henryschein.com
HENRY SCHEIN’S stock is down more than 10% in 2024 as the major healthcare products and services distributor continues to manage the fallout from last year’s cyberattack. According to filings with the U.S. Securities and Exchange Commission, the company has paid $19 million for remediation efforts alone: $11 million in 2023 and $8 million during the first six months of 2024. The company said in its second-quarter filing with the SEC that residual effects of the cyberattack included decreased sales to episodic customers. The company has operations in 33 countries, with products and services sold to more than 1 million dental practices, laboratories, physician practices, ambulatory surgery centers, and more. Other major news included Henry Schein’s purchase earlier in 2024 of a majority stake in Santa Clarita, California–based TriMed, a manufacturer of orthopedic devices for the lower extremities (foot and ankle) and upper extremities (hand and wrist).–CN
$10,333,967,000
Fiscal year ended 12/31/2023 (Foreign currency revenue)
2023 rank: 14
R&D spend: $13,200,000
Employees: 22,200
CEO: Ed Pesicka
www.owens-minor.com
OWENS & MINOR in July announced it would spend $1.4 billion to acquire Rotech Healthcare Holdings, a privately held home-based care business that provides home medical equipment in the U.S. “Rotech squarely fits into our existing Patient Direct segment and directly aligns with the strategy we outlined last December during our Investor Day, supporting our expansion in the very large and fast-growing home-based care space,” Owens & Minor CEO Edward Pesicka said in a news release. As of mid-August, Owens & Minor expected full-year revenue in the $10.5–10.9 billion range. The company provides product manufacturing, distribution support and technology services in more than 90 countries. –CN and DK
Henry Schein’s cyberattack offers lessons for others wtwh.me/HenrySchein

Melsungen
Germany
$9,455,400,000
Fiscal year ended 12/31/2023 (€8,755,000,000)
2023 rank: 15
R&D spend: $523,368,000
Employees: 63,011
CEOs: Anna Maria Braun, CEO; Rob Albert, U.S. CEO
www.bbraun.com
With more than 300 subsidiaries in 64 countries, B. BRAUN MELSUNGEN manufactures over 5,000 medical devices and pharmaceutical products, which it supplements with an extensive range of services. B. Braun has three divisions: Hospital Care, Aesculap (surgical and interventional), and Avitum (chronic disease treatments).
Geneva Switzerland
Multiple generations of the Braun family have owned the company since its founding in the 19th century, with Anna Maria Braun presently serving as CEO. Despite what company officials described as a demanding environment, sales were up 3% and earnings were up more than 15% in 2023. The U.S. HQ is in Bethlehem, Pennsylvania.–CN 16 18
$9,400,000,000
Fiscal year ended 12/31/2023
2023 rank: 16
R&D spend: $828,000,000
Employees: 25,000
CEO: David Endicott
www.alcon.com
Formerly a subsidiary of pharmaceutical giant Novartis, ALCON spun out as a separate eye care business in 2019. The company was founded in 1945 by Robert Alexander and William Conner as a small pharmacy in Fort Worth, Texas, where its U.S. headquarters remain. In 2024, Alcon expanded its glaucoma portfolio with the acquisition of Belkin Vision and broadened its cataract offerings with FDA clearance for two of its Unity offerings. The company anticipates around $10 billion in sales for fiscal 2024. –SW
(previously 3M Health Care)
Maplewood, Minnesota United States
$8,197,000,000
Fiscal year ended 12/31/2023
2023 rank: 17
R&D spend: $758,000,000
Employees: 22,000
CEO: Bryan Hanson
www.solventum.com/
SOLVENTUM — formerly 3M Health Care — completed its spinoff from 3M in April, with its shares trading on the New York Stock Exchange under the symbol SOLV. The company operates in 38 countries, with more than 300 offices and facilities. Its business segments include medical surgical, dental, health information systems, and purification and filtration. In its first quarterly report as an independent company, Solventum’s second-quarter results beat the expectations of Wall Street analysts; the company upped its full-year revenue guidance to 0–1% growth. “As we continue to execute a complex transformation, we’re encouraged by our first financial results as an independent company and early ability to maintain business continuity,” said Solventum CEO Bryan Hanson, who left the corner office at Zimmer Biomet a year ago to lead the spinoff. “We are starting from a solid foundation and remain focused on addressing historical underperformance and spinrelated topics to unlock significant value creation over time.” –CN
Warsaw, Indiana United States
$7,394,200,000
Fiscal year ended 12/31/2023
2023 rank: 19
R&D spend: $458,700,000
Employees: 18,000
CEO: Ivan Tornos
www.zimmerbiomet.com
ZIMMER BIOMET continues to expand its orthopedic surgical robotics and digital surgery offerings to boost its competitiveness against rivals such as Stryker and Johnson & Johnson’s DePuy Synthes. In June, ZB announced a limited distribution agreement with Think Surgical for Think’s wireless, handheld TMINI miniature robotic system for total knee arthroplasty (TKA). Zimmer Biomet officials see TMINI complementing its flagship Rosa surgical robot portfolio with a handheld robotic option. Earlier in 2024, the FDA cleared the Rosa Shoulder robotic surgery system, making it the fourth application for ZB’s Rosa. On the M&A front, Zimmer Biomet recently announced a deal to acquire OrthoGrid Systems and its AI-driven surgical guidance systems for total hip replacement. –CN



Sunnyvale, California United States
$7,124,100,000
Fiscal year ended 12/31/2023
2023 rank: 22
R&D spend: $998,800,000
Employees: 13,676
CEO: Gary Guthart
www.intuitive.com
INTUITIVE widened the moat against the host of the companies — including giants Medtronic and Johnson & Johnson — that sought to challenge it in the soft-tissue surgical robotics space. It opened 2024 announcing that it submitted its next-gen da Vinci 5 system for FDA review. Two months later, it had da Vinci 5 clearance secured, with a limited launch phase expected to last into 2025. Analysts have expressed confidence that the new system will fuel future growth. As of the writing of this description in mid-August, Intuitive’s stock value was up more than 41% year-to-date. The da Vinci 5 joins Intuitive’s existing da Vinci robotic surgical system portfolio alongside the multiport X and Xi systems and the single-port SP. There is also Ion, Intuitive’s robotic-assisted platform for minimally invasive biopsy in the lung. Da Vinci 5 has more than 10,000 times the computing power of da Vinci Xi to enable new system capabilities and advanced digital experiences, now and in the future. The next-gen system also introduces Force Feedback technology and optional instruments that enable the system to measure subtle forces exerted on tissue during surgery and relay that feeling to surgeons. “We strive to provide customers with technology that meets their needs and solves important problems,” said Intuitive Chief Medical Officer Dr. Myriam Curet. –CN
(healthcare only)
Tokyo Japan
$6,940,208,583
Fiscal year ended 3/31/2024 (JP¥975,100,000,000)
2023 rank: 18
R&D spend: not available Employees: not available CEO: Teiichi Goto
www.fujifilm.com/us/en/ healthcare
Healthcare is the largest operating segment within FUJIFILM, responsible for nearly a third of the company’s revenue in fiscal 2024, with sales for the segment up 5% for the year. Fujifilm’s healthcare products include diagnostic equipment such as X-ray systems, endoscopes and ultrasound systems, and medical IT systems. Fujifilm Healthcare Americas is headquartered in Lexington, Massachusetts. Top Fujifilm healthcare news in 2024 included the company picking Brainlab to exclusively distribute its Arietta Precision ultrasound for neurosurgery applications in the U.S. The companies said Arietta Precision combined with Brainlab’s surgical navigation system “becomes a powerful intraoperative neurosurgery solution.” Fujifilm also won FDA clearance of its CAD Eye AI-powered detection system for endoscopic imaging, a system that could compete against Medtronic and Cosmo Intelligent Medical Devices’ GI Genius for colonoscopies. –CN and SW
The Intuitive da Vinci 5’s top design changes: ‘This is groundbreaking for robotic surgery’ wtwh.me/Intuitive
Tokyo Japan
$6,663,411,627
Fiscal year ended 3/31/2024 (JP¥936,210,000,000)
2023 rank: 20
R&D spend: $614,718,424
Employees: 28,838
CEO: Stefan Kaufmann
www.medical.olympusamerica.com
OLYMPUS medical devices primarily serve gastroenterology, general surgery, pulmonology, bronchoscopy, urology, gynecology, otolaryngology, bariatrics, orthopedics and anesthesiology. Olympus started 2024 by closing on its $370 million acquisition of Taewoong Medical, a Korean company known for its gastrointestinal metallic stents. Olympus officials say the acquisition significantly strengthens its portfolio in a critical business segment. –CN
Tokyo Japan
$6,561,297,821
Fiscal year ended 3/31/2024 (JP¥921,863,000,000)
2023 rank: 21
R&D spend: $491,529,899
Employees: 30,591
CEO: Hikaru Samejima
www.terumo.com
TERUMO’S wide range of products include interventional cardiology, blood transfusion and cell therapy. In May 2024, Terumo Cardiovascular announced FDA 510(k) clearance of its CDI OneView cardiopulmonary bypass surgery monitoring system. The nextgeneration system measures or displays up to 22 vital patient parameters, including oxygen delivery, cardiac index, area under the indexed oxygen delivery curve, oxygen extraction ratio and measured flow. –CN



Irvine, California United States
$6,004,800,000
Fiscal year ended 12/31/2023
2023 rank: 23
R&D spend: $1,071,800,000
Employees: 20,000
CEO: Bernard Zovighian
www.edwards.com
EDWARDS LIFESCIENCES won FDA approval of its Evoque tricuspid valve replacement system in February 2024 and then announced a series of M&A deals. First, it said in June it would sell its Critical Care business (which it previously planned to spin off) to BD for $4.2 billion. Edwards then announced plans to spend $1.2 billion to buy JenaValve Technology and Endotronix. The JenaValve Trilogy Heart Valve System for aortic regurgitation could win FDA approval in late 2025, while the FDA already approved the Endotronix Cordella implantable pulmonary artery pressure sensor in June, with a CMS national coverage determination slated for early 2025. Edwards is also acquiring mitral valve company Innovalve for $300 million and entered into a series of licensing and development deals with Affluent Medical involving Affluent’s Kalios adjustable mitral annulus and mitral valve technology. –CN
Watford, England United Kingdom
$5,549,000,000
Fiscal year ended 12/31/2023
2023 rank: 24
R&D spend: $339,000,000
Employees: 18,452
CEO: Deepak Nath
www.smith-nephew.com
SMITH+NEPHEW is the world’s fifth-largest orthopedic device company. In 2024, S+N introduced new artificial intelligence features for its Cori orthopedic surgical robotic system to boost personalization and efficiency of knee and hip arthroplasty procedures. Other top S+N news for the year included the full U.S. launch of its Aetos shoulder system with additional FDA clearances, plus FDA clearance of its new Catalystem primary hip system. –CN
Dublin, Ireland
(Operational HQ in Mentor, Ohio)
$5,138,701,000
Fiscal year ended 3/31/2024
2023 rank: 25
R&D spend: $103,679,000
Employees: 18,000
CEO: Daniel Carestio
www.steris.com
STERIS provides contract sterilization, infection-control equipment and consumables, testing and validation services, and medical devices such as surgical instruments and endoscopes. The company has three business segments: Healthcare, Applied Sterilization Technologies (AST) and Life Sciences. AST’s sterilization methods include ethylene oxide, gamma beam, electronic beam, X-ray and vaporized hydrogen peroxide. In May, the company sold its Dental segment and announced a restructuring plan, saying fewer than 300 positions would be eliminated. –JH
(healthcare products)
Bad Homburg Germany
$4,384,455,480
Fiscal year ended 12/31/2023 (€4,059,681,000)
2023 rank: 28
R&D spend: not available
Employees: not available
CEO: Helen Giza
www.freseniusmedicalcare.com
FRESENIUS MEDICAL CARE announced FDA clearance for its 5008X hemodialysis system in February 2024, marking a step toward bringing a new standard of care in dialysis therapy to the U.S. Fresenius said it would start U.S.-based clinical evaluations and user studies ahead of a broad launch in 2025. Fresenius also has long-term aspirations to make kidney disease care more personalized and precise. –CN and SW
San Diego, California United States
$4,289,002,810
Fiscal year ended 12/31/2023 (RMB¥34,930,000,000)
2023 rank: 27
R&D spend: $533,830,445
Employees: 18,044
CEO: Wu Hao
www.mindray.com
MINDRAY offers patient monitoring, anesthesia, and ultrasound solutions. The company launched its two-in-one TE Air wireless handheld ultrasound device with multi-device connectivity late in 2023 and expanded its U.S. non-invasive liver care offerings in January 2024. –SW
$4,222,993,000
Fiscal year ended 6/30/2023
2023 rank: 34
R&D spend: $287,642,000
Employees: 10,140
CEO: Michael Farrell
www.resmed.com
RESMED was founded in Australia in 1989 by Peter Farrell and has been under the leadership of his son, Mick Farrell, since 2013. The company specializes in cloudconnected respiratory devices, offering continuous positive airway pressure (CPAP) machines for sleep apnea and ventilators for conditions such as chronic obstructive pulmonary disease (COPD). With a major recall still mostly keeping Philips out of the market, ResMed’s revenue has grown by double-digit percentages in recent years. –CN











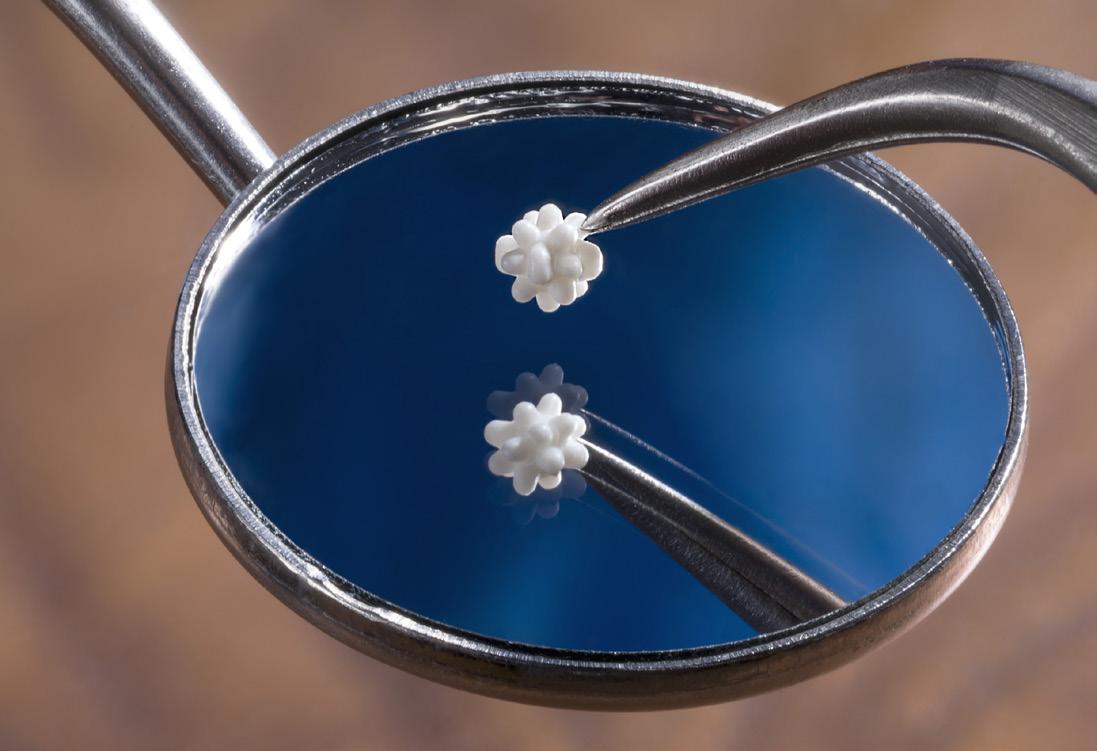
MTD’s advanced processes cater to the specific demands of these industries, from orthopedic and pharmaceuical to wearables and dental (as pictured above).
We ensure that vital components and devices meet the highest standards of precision, quality, and performance.
• Drug delivery devices with extremely tight tolerances and high-aspect ratio flow lengths
• Complex, intricate medical products that demand robust quality systems
• Bioabsorbable components that require consistent and minimal post-mold IV loss
• Overmolded components with ultra-thin walls encapsulating delicate substrates
LEARN MORE: mtdmicromolding.com
Stäfa
Switzerland
$4,037,177,204
Fiscal year ended 3/30/2024 (CHF3,627,000,000)
2023 rank: 30
R&D spend: $262,689,225
Employees: 18,151
CEO: Arnd Kaldowski
www.sonova.com
Founded in 1947, SONOVA specializes in developing hearing instruments, cochlear implants and a range of other hearing devices. The company’s product portfolio includes brands such as Phonak, Unitron, Hansaton and Advanced Bionics. –CN
Marlborough, Massachusetts
United States
$4,030,400,000
Fiscal year ended 9/30/2023
2023 rank: 26
R&D spend: $294,300,000
Employees: 6,990
CEO: Stephen MacMillan
www.hologic.com
HOLOGIC in February 2024 announced FDA clearance of its Genius digital diagnostics system, which uses AI to enhance the detection of pre-cancerous lesions and cervical cancer cells. The company said its the first FDAcleared digital cytology platform that combines deep learningbased AI with advanced volumetric imaging, marking an advancement in cervical cancer screening. In July 2024, Hologic paid $310 million to acquire Endomagnetics, which specializes in breast surgery localization and lymphatic tracing technology. –CN
Charlotte, North Carolina United States
$3,965,000,000
Fiscal year ended 12/31/2023
2023 rank: 29
R&D spend: $184,000,000
Employees: 15,000
CEO: Simon Campion www.dentsplysirona.com
DENTSPLY SIRONA manufactures and markets general dental supplies and devices, including CAD/CAM restoration systems (CEREC and inLab), dental restorative products, digital intra-oral, panoramic and 3D imaging systems, dental treatment centers, hand pieces, hygiene systems and dental specialty products for orthodontics, endodontics and implants. –DK
Ōtawara Japan
$3,941,634,205
Fiscal year ended 12/31/2023
(JP¥553,800,000,000)
2023 rank: 31
R&D spend: not available
Employees: not available
CEO: Fujio Mitarai, Chair and CEO; Toshio Takiguchi, president and CEO, Canon Medical Systems
global.medical.canon
CANON MEDICAL and Olympus in January 2024 announced a collaboration on endoscopic ultrasound technology. The partnership has Canon developing and manufacturing diagnostic ultrasound used in endoscopic ultrasonography, while Olympus handles sales and marketing efforts. The deal utilizes Canon’s Aplio i800 diagnostic EUS ultrasound and Olympus’ ultrasound endoscope. –SW and CN
San Diego, California United States
$3,622,300,000
Fiscal year ended 12/31/2023
2023 rank: 38
R&D spend: $505,800,000 Employees: 9,600
CEO: Kevin Sayer
www.dexcom.com
DEXCOM develops continuous glucose monitoring (CGM) technology. The company launched its latest-generation G7 in 2023. Dexcom expanded on G7 in 2024 by adding direct-to-watch capabilities with the Apple Watch. It also became the first company to bring an over-the-counter CGM to market with its Stelo system. –SW
Tempe Arizona
$3,862,260,000
Fiscal year ended 12/31/2023
2023 rank: 32
R&D spend: $346,830,000 Employees: 21,610
CEO: Joseph Hogan www.aligntech.com
ALIGN TECHNOLOGY is the creator of the Invisalign clear aligner system, offering an alternative to traditional metal braces. Established in 1997, the company disrupted the orthodontics market with its approach, combining digital treatment planning with advanced mass customization techniques. In 2024, the company announced its acquisition of Cubicure and its polymer additive manufacturing tech. Company officials said the deal expanded printing, materials and manufacturing capabilities for its 3D-printed product portfolio. –JH and CN
Tokyo Japan
Hoya (life care segment)
$3,772,410,126
Fiscal year ended 3/31/2024 (JP¥530,027,000,000)
2023 rank: 33
R&D spend: not available
Employees: not available
CEO: Eiichiro Ikeda
www.hoya.com/en/business/lifecare
HOYA Corp.’s life sciences division includes products such as eyeglasses, contact lenses, and intraocular lenses, which are crucial in eye care and ophthalmic surgery. Hoya also offers endoscopic systems and other advanced technology for minimally invasive treatments, as well as include prosthetic ceramic fillers, metallic implants, laparoscopic surgical instruments and automatic endoscope cleaning equipment. –CN
San Ramon, California United States
$3,593,200,000
Fiscal year ended 10/31/2023
2023 rank: 35
R&D spend: $137,400,000 Employees: 15,000
CEO: Albert White
www.coopercos.com
COOPER COS. operates through two main business divisions: CooperVision and CooperSurgical. CooperVision is a manufacturer of contact lenses, providing vision care products to millions of people in over 130 countries. CooperSurgical is focused on women's health, offering a portfolio of products and services that support fertility, diagnostics, and other aspects of women's healthcare. –CN
Humlebæk Denmark
$3,555,878,084
Fiscal year ended 9/30/2023
(DKK kr24,500,000,000)
2023 rank: 37
R&D spend: $126,560,232 Employees: 14,903
CEO: Kristian Villumsen
www.coloplast.us
COLOPLAST specializes in products and services for ostomy care, continence care, advanced wound care, interventional urology, and voice and respiratory care. The devicemaker is known for its ostomy bags, catheters, and advanced wound dressings. Coloplast has its U.S. headquarters in Minneapolis. –CN
Nipro (medical segment) Osaka Japan
$3,228,538,627
Fiscal year ended 3/31/2024 (JP¥453,610,000,000)
2023 rank: 36
R&D spend: $621,351,871 Employees: not available
CEO: Yoshihiko Sano
www.nipro.com
NIPRO offers medical devices across five areas: renal products, interventional catheter-delivered products, hospital products, cardiopulmonary products and enzymes. The company is one of the leading manufacturers of dialyzers globally. It also reports continuing results with artificial organs and in the new area of tissue engineering. –CN

Our range of Swiss Type CNC Machines and Fixed Headstock Automatic Lathes are ready to produce your most intricate part.
• 1mm to 38mm Bar Stock
• 5-axis machines with excellent cost performance to a high-end machine equipped with B axis and back spindle Y axis.
• LFV (low-frequency vibration) available on most models
• Barloaders “CAV” fully integrated through Machine Control
• Intuitive CITIZEN Controls offers ease of programming and operation
• Machining Solutions with Lifetime Customer Support
• 15 + models to choose from


• From simple single-spindle machines to twin-spindle, multi-turret turning centers covering the range right up to 80mm bar diameter.
• Accuracy, Rigidity and Durability
• LFV (low-frequency vibration) available on multiple models
• Large and accessible workspace
• On-machine program check
• Intuitive CITIZEN Controls offers ease of programming and operation
• Machining Solutions with Lifetime Customer Support
• 13 + models to choose from
• Fully integrated into the machine control for easier operation and total control from one single console
• Quick response between bar feeder and the machine sliding headstock
• A unique stabilizing mechanism: forces are evenly distributed, protecting the spindle from excess wear
• Automatic remnant retraction
• Hydrostatic oil support
• Quick channel change ( less than 35 seconds)
• One company to contact for parts and support
Smørum Denmark
$3,135,123,367
Fiscal year ended 12/31/2023
(DKK kr21,601,000,000)
2023 rank: 42
R&D spend: not available
Employees: not available
CEO: Søren Nielsen www.demant.com
Healthcare and audio technology company DEMANT’S has a history that stretches back 120 years to its founding in 1904. Demant provides hearing aids, hearing implants, hearing care products, diagnostic equipment and services to hearing care professionals and users in more than 130 countries. The majority of Demant A/S shares are held by the William Demant Foundation through the investment company William Demant Invest. Demant A/S is listed on Nasdaq Copenhagen. –CN
Gothenburg Sweden
$3,000,028,278
Fiscal year ended 12/31/2023
(31,827,000,000 SEK kr)
2023 rank: 40
R&D spend: $112,358,491
Employees: 11,739
CEO: Mattias Perjos
www.getinge.com
Wayne, Pennsylvania United States
$2,974,489,000
Fiscal year ended 12/31/2023
2023 rank: 41
R&D spend: $154,351,000
Employees: 14,500
CEO: Liam Kelly www.teleflex.com
BRUKER offers advanced scientific instruments and high-value analytical and diagnostic solutions that it says empower scientists to explore life and materials at molecular, cellular and microscopic levels. Its employees work at over 90 locations globally. German experimental physics professor Günther Laukien helped start the company in 1960, providing what the company says were the first high-resolution systems for analytical chemistry in the U.S. –CN 39
Founded in 1904, GETINGE provides ventilators, extracorporeal life support systems and other medical technologies. The company secured 2024 FDA clearances for its endoscopic vessel harvesting (EVH) solution and advanced clinical guidance support software. However, in May 2024 the FDA warned of “continued safety and quality concerns” related to its cardiovascular device recalls. –SW
TELEFLEX specializes in critical care and surgical technologies. Its portfolio spans vascular and interventional access, surgical, anesthesia, cardiac care, urology, emergency medicine and respiratory care. Highlights in 2024 included the launch of the UroLift 2 for treating benign prostatic hyperplasia (BPH) symptoms as an alternative to medications and major surgery. The company also initiated a serious balloon catheter recall related to reports of three deaths. –SW
Billerica, Massachusetts United States
$2,964,500,000
Fiscal year ended 12/31/2023
2023 rank: 44
R&D spend: $294,800,000
Employees: 9,707
CEO: Frank Laukien
www.bruker.com
Carl Zeiss (medical technology segment)
Jena Germany
$2,704,320,000
Fiscal year ended 9/30/2023 (€2,504,000,000)
2023 rank: See Page 14 footnote R&D spend: not available Employees: 7,736
CEO: Markus Weber, Carl Zeiss Meditec president and CEO and Zeiss Medical Technology segment head www.zeiss.com/meditec-ag/home.html
CARL ZEISS MEDITEC products and workflow solutions support the diagnosis and treatment of eye diseases. It also provides technology for visualizing minimally invasive surgical treatments. Carl Zeiss Meditec announced in April 2024 that it completed its $1 billion acquisition of the Dutch Ophthalmic Research Center (D.O.R.C.), which develops technology to address eye conditions such as retinal disorders, cataracts, glaucoma and refractive errors. –CN and SW
Lynge Denmark
$2,662,200,000
Fiscal year ended 9/30/2023 (€2,465,000,000)
2023 rank: 45
R&D spend: $193,320,000 Employees: 12,500
CEO: Jan Makela
www.wsa.com
WS AUDIOLOGY is a leader in the hearing aid industry, serving a global network of thousands of hearing care professionals. In addition to partnering with providers, the company connects directly with individuals affected by hearing loss through its consumerfacing businesses. –CN
43 44 45 46
Brea, California United States
$2,566,500,000
Fiscal year ended 12/31/2023
2023 rank: 43
R&D spend: $93,800,000 Employees: 12,700
CEO: Paul Keel www.envistaco.com
ENVISTA has a global family of more than 30 dental brands, including Nobel Biocare, Ormco, Dexis and Kerr. The company provides a comprehensive range of dental solutions, including dental implants, orthodontics and digital imaging technologies, catering to a wide spectrum of clinical needs. Envista’s products and technologies support the diagnosis, treatment and prevention of dental conditions, as well as enhancing the aesthetics of the human smile. –CN
Heidenheim Germany
$2,541,564,000
Fiscal year ended 12/31/2023 (€2,353,300,000)
2023 rank: 46
R&D spend: $91,908,000 Employees: 10,168
CEO: Britta Fünfstück www.hartmann.info
THE HARTMANN GROUP’S history goes back to the early 19th century, with a legacy of medical advances in wound care and skin health. The company focuses on areas such as wound care, incontinence management, and infection management. Hartmann has offices in more than 36 countries, and its network of distributors sell its products in more than 130 countries –CN
Milan Italy
$2,440,890,720
Fiscal year ended 12/31/2023
(€2,260,084,000)
2023 rank: 48
R&D spend: not available Employees: 20,300
CEO: Enrico Vita
www.amplifon.com
AMPLIFON is a major hearing aid provider. Its products and services include Ampli-easy, Ampli-mini, Ampli-connect, Ampli-energy, the Amplifon App, and Companion smart support. –CN
Bloomington, Indiana United States
$2,300,000,000
Fiscal year ended 12/31/2023
2023 rank: 50
R&D spend: not available Employees: 9,478
CEO: Carl Cook
www.cookmedical.com
COOK MEDICAL in July 2024 announced that it signed a letter of intent to sell its Reproductive Health business (Cook ART) to private equity firm Astorg. Cook Medical entered into this agreement nearly one year after a previous attempt to sell off these businesses to Cooper Cos. failed amid FTC scrutiny. Cook was able to sell select assets to Cooper for $300 million in late 2023. –CN
San Clemente, California United States
$2,259,126,000
Fiscal year ended 12/31/2023
2023 rank: 47
R&D spend: $85,344,000 Employees: 14,000
CEO: Vivek Jain
www.icumed.com
Syringe and infusion technology company ICU MEDICAL soared up the Big 100 ranks following its $2.35 billion acquisition of Smiths Medical in 2022. Smiths Medical provides syringe and ambulatory infusion devices, vascular access, and vital care products. With the addition, ICU Medical reported revenue of $2.3 billion in 2023. However, 2024 was rife with issues for the company, which closed multiple Smiths Medical plants and issued warnings on tracheostomy products, ventilators and syringe pump software. –SW
Basel Switzerland
$2,166,940,800
Fiscal year ended 12/31/2023 (CHF2,412,000,000)
2023 rank: 49
R&D spend: not available Employees: 11,000
CEO: Guillaume Daniellot
www.straumann.com
STRAUMANN is a global provider of tooth replacement and orthodontic products and services. Its brands include Anthogyr, ClearCorrect, Dental Wings, Medentika, Neodent, NUVO and Straumann, and it has other fully/ partly owned companies and partners. Straumann researches, develops, manufactures and supplies dental implants, instruments, CADCAM prosthetics, orthodontic aligners, biomaterials and digital tools. –CN





CDMO / CMO Services


Minimally Invasive Device Solutions
Co-development Opportunities
Access, Delivery, & Retrieval Systems
Wire & Catheter Based Devices
Contract Manufacturing
Vascular Access Devices
Guidewires, Therapeutic & Diagnostic
Braided & Coiled Catheter Shafts
Class 8 Clean Room


Reading, England United Kingdom
$2,142,000,000
Fiscal year ended 12/31/2023
2023 rank: 51
R&D spend: $104,000,000
Employees: 10,000
CEO: Karim Bitar
www.convatecgroup.com
CONVATEC provides technology for advanced wound care, ostomy care, continence and critical care and infusion care. It offers design and manufacturing services for infusion set technologies and insertion devices for insulin pump treatment. The company expects a 5–7% revenue increase in 2024. –SW
Lübeck Germany
$2,123,496,000
Fiscal year ended 12/31/2023 (€1,966,200,000)
2023 rank: 55
R&D spend: not available
Employees: not available
CEO: Stefan Dräger
www.draeger.com/en-us_us/hospital
DRÄGER’S medical division includes five business units: therapy (anesthesia devices, ventilators and thermoregulation equipment), hospital consumables and accessories, workplace infrastructure (supply units, lights and gas management systems), patient monitoring, and data business (software applications, system products and new services). –CN
Wilmington, Delaware United States
$1,707,197,000
Fiscal year ended 12/31/2023
2023 rank: 58
R&D spend: $75,331,000
Employees: 6,550
CEO: Matthew Trerotola
www.enovis.com
ENOVIS — the world’s sixth-largest orthopedic device company and parent company of the DJO brand — is getting larger as it integrates LimaCorporate and its patient-tailored, 3D-printed titanium implants for complex reconstructive surgeries. Enovis completed the $846 million acquisition in January 2024. Enovis is kicking off a multiyear cadence of new product introductions across its two business segments: Prevention and Recovery (P&R) and Recon. –CN
Acton, Massachusetts United States
$1,697,100,000
Fiscal year ended 12/31/2023
2023 rank: 61
R&D spend: $205,000,000
Employees: 3,000
CEO: Jim Hollingshead
www.insulet.com
INSULET develops wearable automated insulin delivery devices. Its flagship Omnipod patch pump is one of the leaders in the market, with the latest version — the Omnipod 5 — growing in popularity through the 2024 integration with the Dexcom G7 continuous glucose monitor (CGM). The company is seeking an expanded indication from the FDA for type 2 diabetes patients with a potential launch in 2025. –SW
Irvine, California United States
$2,048,100,000
Fiscal year ended 12/31/2023
2023 rank: 52
R&D spend: $175,200,000 Employees: 4,000
CEO: Joe Kiani
www.masimo.com
MASIMO’S fight with activist investor Politan Capital Managements continued as COO Bilal Muhsin threatened to quit if CEO and founder Joe Kiani is ousted. Pending litigation has delayed Masimo’s shareholders meeting until Sept. 19, 2024. Masimo said it received a bid for a majority stake in the consumer business that Masimo bought in 2022 — the deal that sparked the proxy fight. Meanwhile, Masimo continues to advance its monitoring technology, including the W1 watch. –SW
Plano, Texas United States
$1,596,673,000
Fiscal year ended 12/31/2023
2023 rank: 60 R&D spend: $63,771,000 Employees: 10,500
CEO: Joseph Dziedzic www.integer.net
INTEGER is one of the world’s largest medical device contract manufacturers, serving the cardiac rhythm management, neuromodulation, orthopedics, vascular, advanced surgical and portable medical markets. Integer’s stock price climbed more than 23% in 2024 as of mid-August. The company expects above-market sales growth of 9–11% for full year 2024. –CN
Stockholm Sweden
$1,707,905,626
Fiscal year ended 4/30/2024 (18,119,000,000 SEK kr)
2023 rank: 56
R&D spend: $132,341,713 Employees: 4,641
CEO: Gustaf Salford
www.elekta.com
ELEKTA develops precision radiation therapy devices and oncology software. In 2024, the company purchased Philips Healthcare’s Pinnacle Treatment Planning System (TPS) patent portfolio and expanded its radiation oncology software partnership with GE HealthCare. –CN
Tokyo Japan
$1,579,970,406
Fiscal year ended 3/31/2024 (JP¥221,986,000,000)
2023 rank: 57
R&D spend: $49,793,559 Employees: 5,891
CEO: Hirokazu Ogino www.nihonkohden.com
Yoshio Ogino founded NIHON KOHDEN in 1950 with a vision of bringing the power of electrical engineering to medicine. Over the decades, the company has been a pioneer in pulse oximetry, cerebral artery pressure meters, ECGs, heart monitors and fetal monitors. It operates subsidiaries in Irvine and Santa Ana, California; Guilford, Connecticut; Alachua, Florida; Charlottesville, Virginia; and Cambridge, Massachusetts. –CN








CGI Motion standard products are designed with customization in mind. Our team of experts will work with you on selecting the optimal base product and craft a unique solution to help di erentiate your product or application. So when you think customization, think standard CGI assemblies.

Connect with us today to explore what CGI Motion can do for you.






Audubon, Pennsylvania United States
$1,568,476,000
Fiscal year ended 12/31/2023
2023 rank: 71
R&D spend: $124,010,000 Employees: 5,000
CEO: Daniel Scavilla www.globusmedical.com
GLOBUS MEDICAL’S 2023 merger with NuVasive created the world’s seventh-largest orthopedic device company, but resulted in 157 layoffs at the former NuVasive headquarters at the start of 2024. In June 2024, Globus Medical took a big step forward in surgical robotics with FDA clearance of its Excelsius robot for total knee arthroplasty. However, the Excelsius system was at the center of an FDA warning letter in July 2024 that the company is working through. –SW
South Jordan, Utah
United States
$1,257,366,000
Fiscal year ended 12/31/2023
2023 rank: 64
R&D spend: $82,728,000
Employees: 6,950
CEO: Fred Lampropoulos
www.merit.com
As longtime MERIT MEDICAL founder and CEO Fred Lampropoulos prepares to retire in 2025, the business continues to plug along. In 2024, the company received FDA clearance for its surgical guidance system, partnered with Medtronic on its unipedicular, steerable balloon catheter, and expanded its endoscopy portfolio by purchasing the EndoGastric Solutions EsophyX Z+ device for gastroesophageal reflux disease. –SW
Princeton, New Jersey United States
$1,541,573,000
Fiscal year ended 12/31/2023
2023 rank: 59
R&D spend: $104,192,000 Employees: 3,946
CEO: Jan De Witte
www.integralife.com
Founded in 1989 after the acquisition of an engineered collagen technology platform designed to repair and regenerate tissue, INTEGRA LIFESCIENCES’ regenerative portfolio now includes surgical instruments, neurosurgical devices and advanced wound products. Its brands include AmnioExcel, Aurora, Bactiseal, BioD, CerebroFlo, CereLink Certas Plus, Codman, CUSA, Cytal, DuraGen, DuraSeal, DuraSorb, Gentrix, ICP Express, Integra, Licox, Mayfield, MediHoney, MicroFrance, MicroMatrix, NeuraGen, NeuraWrap, PriMatrix, SurgiMend, TCC-EZ, VersaTru — and now Acclarent, purchased in 2024 from Johnson & Johnson. –JH, DK and CN
Largo, Florida
United States
$1,244,744,000
Fiscal year ended 12/31/2023
2023 rank: 69
R&D spend: $52,602,000 Employees: 4,000
CEO: Curt Hartman
www.conmed.com
CONMED provides a wide range of surgical devices and equipment used in various medical specialties, including orthopedics, general surgery, gynecology, thoracic surgery and gastroenterology. –CN
Boston, Massachusetts United States
$1,309,055,000
Fiscal year ended 3/30/2024
2023 rank: 63
R&D spend: $54,435,000 Employees: 3,657
CEO: Christopher Simon www.haemonetics.com
HAEMONETICS technologies serve medical markets including blood and plasma component collection, the surgical suite and hospital transfusion. The company has been active in M&A, acquiring cardiologyfocused medical device company OpSens for $255 million in 2023 and esophagus-protecting devicemaker Attune Medical for $160 million in 2024. –CN and SW
London, England United Kingdom
$1,153,545,000
Fiscal year ended 12/31/2023
2023 rank: 72
R&D spend: $193,817,000 Employees: 2,900
CEO: Vladimir Makatsaria
www.livanova.com
Cardiac surgery and neuromodulation device company LIVANOVA has been under the leadership of Johnson & Johnson veteran Vladimir Makatsaria as CEO since February 2024. He was hired nearly a year after Damien McDonald resigned as CEO following some pipeline disappointments. During that interim period, the company decided to wind down its circulatory support unit to focus on its cardiopulmonary and neuromodulation businesses. –SW
Sydney Australia
$1,299,367,080
Fiscal year ended 6/30/2023 (AU$1,955,700,000)
2023 rank: 65
R&D spend: $162,711,560
Employees: 4,000
CEO: Dig Howitt
www.cochlear.com
Founded in Sydney in 1981, COCHLEAR develops ear implants to help people with hearing loss. The company says it has provided more than 750,000 implantable devices. –CN
Wilmington, Delaware (operational HQ in Parsippany, New Jersey) United States
$1,120,800,000
Fiscal year ended 9/30/2023
2023 rank: 66
R&D spend: $85,200,000 Employees: 2,200
CEO: Dev Kurdikar
www.embecta.com
BD diabetes spinoff EMBECTA submitted an open-loop, proprietary, disposable insulin patch pump for people with type 2 diabetes for FDA review in January 2024. It also has a closed-loop, automated version under development set to follow. In the meantime, Embecta looks like a potential acquisition target, as reports surfaced that the company enlisted advisers to look into a possible sale. –SW


Design | Engineering | Supply Chain | Manufacturing | After-Market
With Celestica’s engineering expertise, global manufacturing network, and stringent quality control processes, we help you design, develop, and deliver innovative medical devices that enable better patient outcomes.
Discover the Celestica Advantage:
ISO 13485 and FDA-registered sites
Extensive design and engineering expertise
Supply chain risk management and in-region supplier network
Leader in quality and regulatory compliance

Advanced manufacturing (automation and clean room finished goods production)

Auckland New Zealand
$1,070,602,040
Fiscal year ended 3/31/2024
(NZ$1,742,800,000)
2023 rank: 73
R&D spend: $121,754,260 Employees: 7,141
CEO: Lewis Gradon
www.fphcare.com/us
FISHER & PAYKEL HEALTHCARE designs and manufactures systems for acute and chronic respiratory care, surgery, and obstructive sleep apnea treatment. The company distributes its products in more than 120 countries. After two years of revenue decline, Fisher & Paykel boosted sales by 10% during its most recent fiscal year, moving up six spots on the Medtech Big 100. –CN
(healthcare segment)
Tokyo Japan
$988,611,396
Fiscal year ended 3/31/2024
(JP¥138,900,000,000)
2023 rank: 68
R&D spend: not available Employees: not available
CEO: Toshimitsu Taiko
healthcare.konicaminolta.us
KONICA MINOLTA Healthcare is a provider of medical diagnostic imaging and healthcare information technology, including X-ray, ultrasound and imaging management systems. The company’s Konica Minolta Healthcare Americas division is based in Wayne, New Jersey. –CN
$1,065,479,669
Fiscal year ended 3/31/2024
(JP¥149,700,000,000)
2023 rank: 67
R&D spend: not available Employees: not available
CEO: Junta Tsujinaga
www.omronhealthcare.com
OMRON is a major producer of home blood pressure monitors more than 50 years after introducing its first manual and manometer-type model, the HEM1. The company says it has continually advanced its technology to enhance accuracy and ease of use. Omron has sold over 350 million units across more than 110 countries. –CN
Ballerup Denmark
$987,227,866
Fiscal year ended 12/31/2023
(DKK kr6,802,000,000)
2023 rank: 75
R&D spend: $85,486,212 Employees: 4,349
CEO: Scott Davis, GN Hearing president
www.gn.com
Alameda, California United States
$1,058,522,000
Fiscal year ended 12/31/2023
2023 rank: 76
R&D spend: $84,423,000 Employees: 4,200
CEO: Adam Elsesser
www.penumbrainc.com
PENUMBRA was founded in 2004 by President, Chair and CEO Adam Elsesser and Dr. Arani Bose, who formerly served as chair, chief medical officer and chief innovator. Penumbra develops interventional devices for vascular and neuro therapies, including catheters, coils and clot retrievers. In 2024, Penumbra launched its Lightning Flash 2.0 computer-assisted vacuum thrombectomy (CAVT) system in the U.S. and its BMX81 and BMX96 neuro access catheters in Europe. –JH
$950,725,000
Fiscal year ended 12/31/2023
2023 rank: 77
R&D spend: $379,400,000 Employees: 8,230
CEO: Zhaohua Chang
www.microport.com
Tokyo Japan
$998,739,503
Fiscal year ended 3/31/2024
(JP¥140,323,000,000)
2023 rank: 70
R&D spend: not available Employees: not available
CEO: Kotaro Fukuda
www.fukuda.com
FUKUDA DENSHI is a worldwide manufacturer of electrocardiographs, patient monitors, and other advanced medical devices. Established in 1939, the company was a trailblazer in developing Japan’s first electrocardiogram (ECG) device. Its U.S. operations are headquartered in Redmond, Washington. –CN
Rancho Santa Margarita, California, United States
$815,000,000
Fiscal year ended 12/31/2023
2023 rank: 79
R&D spend: $156,000,000 Employees: 5,400
CEO: Said Hilal, CEO
www.appliedmedical.com
Founded in 1987, privately-held APPLIED MEDICAL RESOURCES provides technologies for minimally invasive and general surgery, as well as cardiac, vascular, urologic, colorectal, bariatric, obstetric and gynecologic specialties. It operates in more than 75 countries. –CN 71 72 73 74
GN HEARING develops and manufactures intelligent hearing technology as one of GN Store Nord’s two business units (the other is GN Audio). In 2024, GN Hearing launched a new line of Bluetooth-enabled hearing aids with an improved and more power-efficient way of wirelessly transmitting audio from one device to another. –SW
Founded in 1998 in a small office in ZJ Hi-Tech Park in Shanghai, MICROPORT has grown into a global medical device company. Its technologies can be found in a wide variety of medical fields, including orthopedics, cardiovascular intervention, cardiac rhythm management, electrophysiology, endovascular, and peripheral vascular intervention. –CN


Software plays a crucial role in the development, operation, and usage of medical devices. Qt's quality assurance solutions help ensure these devices meet the medical industry's rigorous standards for quality and safety.
Ensure your devices are safe, effective, secure and do what they do best: Save lives.

Miami Lakes, Florida United States
$790,545,000
Fiscal year ended 12/31/2023
2023 rank: not available
R&D spend: not available
Employees: 3,706
CEO: Scott Drake
www.cordis.com
Founded in 1957 and independent once again since its sale to private equity investors in 2021, CORDIS develops and manufactures medical devices for diagnostics and interventional procedures to treat coronary and peripheral vascular diseases. In 2023, the company expanded its business with the acquisition of MedAlliance and its drug-eluting balloon technology. In 2024, it picked up an FDA premarket approval for its Mynx Control venous vascular closure device (VCD). –SW
Reykjavík
Iceland
$786,000,000
Fiscal year ended 12/31/2023
2023 rank: 81
R&D spend: $38,142,000
Employees: 3,999
CEO: Sveinn Sölvason
www.ossur.com
San Diego, California United States
$747,718,000
Fiscal year ended 12/31/2023
2023 rank: # R&D spend: $169,667,000
Employees: 2,400
CEO: John Sheridan
www.tandemdiabetes.com
ORTHOFIX’S 2023 merger with SeaSpine made it the world’s eighthlargest orthopedic device company, with sales growing quarter after quarter and revenue expected to come in around $800 million in 2024. The company’s under new leadership after ousting former CEO Keith Valentine, CFO John Bostjancic and Chief Legal Officer Patrick Keran in late 2023. –SW 75 77 76 78
Santa Clara, California United States
$730,230,000
Fiscal year ended 12/31/2023
2023 rank: 88
R&D spend: $145,647,000 Employees: 1,468
CEO: Isaac Zacharias, president (former CEO Douglas Godshall exited when J&J closed its acquisition) www.shockwavemedical.com
SHOCKWAVE MEDICAL’S proprietary intravascular lithotripsy (IVL) tech delivers local sonic pressure waves to treat calcified plaque, fracturing calcium while reducing the risk of complications. Shockwave launched its new IVL catheter in October 2023 following FDA clearance. Johnson & Johnson purchased the company for $13.1 billion in May 2024, so this will be the company’s last year on the Medtech Big 100 as an independent company. Shockwave first made our list at No. 99 in 2022. –SW
ÖSSUR was founded in 1971 by Össur Kristinsson, an Icelandic prosthetist and an amputee who developed the Iceross liner, a silicone interface for prosthetic sockets. Over the decades, Össur’s bracing, support and prosthetics products have expanded to include the Unloader One, a dynamic brace to relieve the pain of knee osteoarthritis, and the Power Knee, the world’s first motor-powered prosthetic knee. Össur brands include CTi, Rebound, Miami J, Unloader, Iceross Pro-Flex, Proprio Foot and Rheo Knee. –CN
Ballerup Denmark
$693,033,382
Fiscal year ended 9/30/2023
(DKK kr4,775,000,000)
2023 rank: 83
R&D spend: not available Employees: 4,400
CEO: Britt Meelby Jensen
www.ambu.com
AMBU is a developer of single-use scopes for a range of applications. It is also active in the anesthesia and patient monitoring spaces. In July 2024, Ambu boosted its organic revenue growth projections for the year to 12-14%. –CN
(merged with SeaSpine)
Lewisville, Texas
United States
$746,641,000
Fiscal year ended 12/31/2023
2023 rank: 82
R&D spend: $80,231,000
Employees: 1,634
CEO: Massimo Calafiore
www.orthofix.com
TANDEM DIABETES CARE develops automated insulin delivery systems such as its flagship t:slim X2. It launched the Mobi miniature, durable pump in February 2024. Tandem completed significant CGM integrations in 2024 and plans to bring the Sigi patch pump acquired from AMF Medical to market. Tandem continues to register significant growth, projecting sales of nearly $900 million in 2024. –SW
Alpharetta, Georgia United States
$673,300,000
Fiscal year ended 12/31/2023
2023 rank: 78
R&D spend: $27,200,000 Employees: 3,771
CEO: Joseph Woody
www.avanos.com
AVANOS MEDICAL develops and manufactures medtech focused on two areas: digestive health (such as feeding tubes and feeding tech) and non-opioid pain management (such as surgical pain pumps, cold and compression therapy and RF ablation). –CN
Golden Valley, Minnesota United States
$624,799,000
Fiscal year ended 12/31/2023
2023 rank: 94
R&D spend: $116,536,000
Employees: 1,011
CEO: Tim Herbert
www.inspiresleep.com
INSPIRE’S pacemaker-like implant provides a sleep apnea alternative to continuous positive airway pressure (CPAP) machines in short supply due to the massive Philips Respironics recall. In 2024, Inspire secured a CE mark under the EU’s Medical Device Regulation and won FDA approval for its Inspire V therapy system, but also had a Class I recall of Inspire IV implantable pulse generators due to a manufacturing defect. Inspire expects sales to grow another 26–28% in 2024. –CN and JH

(medical segment)
Tokyo Japan
$596,440,857
Fiscal year ended 12/31/2023
(JP¥83,800,000,000)
2023 rank: 84
R&D spend: not available
Employees: not available
CEO: Toshihiko Kai, president and CEO; Masaru Yamamura, GM, medical division
www.nikkiso.com/products/medical
NIKKISO says it holds more than half of the market for dialysis machines in Japan and has a significant global presence. The company has diversified into blood purification devices, equipment for surgery, perioperative, and emergency care, and offers artificial pancreas technology for clinical use. –CN
Root Switzerland
$509,338,000
Fiscal year ended 12/31/2023
2023 rank: 85
R&D spend: $223,062,000 Employees: 1,453
CEO: Asaf Danziger
www.novocure.com
NOVOCURE specializes in developing therapies for difficult-to-treat tumors. The oncology company’s wearable tumor treating fields (TTFields) technology is FDA-approved for glioblastoma and mesothelioma. Novocure is studying brain and pancreatic cancer and plans to launch its next indication in non-small cell lung cancer. An ovarian cancer trial failed in 2023, but the company said it still yielded encouraging findings. Novocure has its U.S. headquarters in Portsmouth, New Hampshire and R&D operations in Haifa, Israel. –JH
(medical field segment)
Nagoya Japan
$559,088,570
Fiscal year ended 6/30/2023 (JP¥78,552,000,000)
2023 rank: 86
R&D spend: not available
Employees: not available
CEO: Masahiko Miyata
www.asahi-inteccusa-medical.com
ASAHI INTECC develops and manufactures medical devices for catheter-based treatments involving the cardiovascular, peripheral vascular, abdominal vascular and cerebrovascular systems. The company says its interventional guide wires and microcatheters are based on more than 40 years of wire innovation, and that its proprietary wire drawing, wire forming, torque and coating technologies enable products that provide physicians with precise control. –CN 2024
Irvine, California United States
$493,632,000
Fiscal year ended 12/31/2023
2023 rank: 96
R&D spend: $87,533,000 Employees: 1,300
CEO: Drew Hykes
www.inarimedical.com
INARI MEDICAL develops thrombectomy systems for treating venous diseases such as venous thromboembolism. Its devices include the ClotTriever for capturing and removing large clot burden from big vessels and FlowTriever for retrieval and aspiration when treating pulmonary embolism (PE). In 2023, Inari expanded its portfolio with its $415 million acquisition of LimFlow, which won FDA premarket approval the same year for its breakthrough Transcatheter Arterialization of Deep Veins (TADV) system to treat chronic limb-threatening ischemia (CLTI). –SW and JH 83 84 85 86 87 88 89 90
(eye care business)
Castel San Pietro Switzerland
$551,664,000
Fiscal year ended 12/31/2023 (€510,800,000)
2023 rank: 91
R&D spend: $21,130,200 Employees: 1,730
CEO: Francesco Siccardi
www.medacta.com
Established in 1999 in Switzerland, MEDACTA specializes in joint replacement, spine surgery and sports medicine. Beginning with minimally invasive procedures, the company has transitioned to offerings that are personalized for each patient. Medacta operates in more than 56 countries. –CN
San Francisco, California United States
$492,681,000
Fiscal year ended 12/31/2023
2023 rank: 93
R&D spend: $60,244,000 Employees: 2,000
CEO: Quentin Blackford
www.irhythmtech.com
Wearable cardiac monitor maker IRHYTHM began 2024 with a CE mark for its next-generation Zio ECG system and its Zeus AI algorithm, followed by a $661 million debt offering. However, despite progress remediating problems from an FDA warning letter received in May 2023, the company disclosed more FDA inspection issues in August. That same month, CFO Brice Bobzien submitted his resignation for personal reasons. Daniel Wilson starts as CFO on Aug. 31. –SW
Tokyo Japan
$535,031,648
Fiscal year ended 3/31/2024
(JP¥75,172,000,000)
2023 rank: 87
R&D spend: not available
Employees: not available
CEO: Takashi Eto
www.topconhealthcare.com
TOPCON HEALTHCARE offers a range of products and services to treat eye conditions. They include multimodal imaging, vendor-neutral data management and remote diagnostic technology. –CN
Carlsbad, California United States
$482,262,000
Fiscal year ended 12/31/2023
2023 rank: 98
R&D spend: $70,115,000 Employees: 839
CEO: Patrick Miles
www.atecspine.com
ALPHATEC — the world’s 10thlargest orthopedic device company — manufactures spinal implant and tissue technologies for spine surgeries. The company seeks to develop new approaches to treat the various pathologies to ultimately achieve the goals of spine surgery. In 2024, Alphatec launched EOS Insight, an end-to-end spine surgery platform powered by AI that delivers customized surgical plans, precise alignment measurements and rods pre-contoured for the long construct, all meant to increase confidence in the operating room. –SW

Hiroshima Japan
$464,711,413
Fiscal year ended 3/31/2024 (JP¥65,292,000,000)
2023 rank: 90
R&D spend: not available
Employees: 5,283
CEO: Ryuji Katsura www.jms.cc/english
Dr. Taro Tsuchiya founded JMS in the 1960s after identifying the need for disposable medical devices to reduce complications from blood transfusions. Today, JMS provides a wide range of products and services, including for infusion and transfusion, nutrition, dialysis, surgery, and blood/cell management. The company emphasizes its development approach, centering on real-world clinical needs and collaborating closely with healthcare professionals. –CN
91 92
(radiology solutions segment)
Mortsel Belgium
$459,000,000
Fiscal year ended 12/31/2023 (€425,000,000)
2023 rank: 89
R&D spend: not available
Employees: not available
CEO: Pascal Juéry
www.agfaradiologysolutions.com
AGFA-GEVAERT’S Radiology Solutions division specializes in analog and digital diagnostic imaging for clinicians in hospitals and imaging centers. Agfa's imaging equipment, combined with its Musica image processing software, focuses on productivity, safety and cost-effectiveness. New launches in 2023 included Smart Rotate for automatic image orientation correction, Zero Force Technology for improved ergonomics on the DR100s mobile X-ray, and XR Critical Scan for pointof-care alerts. –CN and JH
Palm Beach Gardens, Florida United States
$457,433,000
Fiscal year ended 12/31/2023
2023 rank: 74
R&D spend: $26,162,000 Employees: 2,600
CEO: Vafa Jamali
www.zimvie.com
At the end of 2023, ZIMVIE — created from the spine and dental units that spun out from Zimmer Biomet — announced that it planned to sell its spine business to H.I.G. Capital, establishing it as a pure-play dental company. About six months after closing the deal, ZimVie sale rumors started, with suggestions that South Korea–based dental implant maker Osstem was bidding for the company. –SW






Westwood, Massachusetts United States
$450,977,733
Fiscal year ended 12/31/2023
2023 rank: not available R&D spend: not available Employees: 2,946
CEO: Tom Testa
www.corza.com
CORZA MEDICAL manufactures surgical technologies ranging from reusable instruments to biologics and single-use tools designed to enhance patient eye care and vision outcomes for clinicians. Its products include Quill barbed sutures, Sharpoint Plus and Look surgical sutures, Sharpoint ophthalmic sutures, Katena reusable and Blink single-use ophthalmic instruments, and the TachoSil fibrin sealant patch. –SW










95 94 96
Madison, Wisconsin United States
$447,605,000
Fiscal year ended 6/30/2023
2023 rank: 92 R&D spend: $57,129,000 Employees: 1,024
CEO: Suzanne Winter
www.accuray.com
ACCURAY specializes in radiation therapy systems to deliver treatments including stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT). The company closed out 2023 with a 6% workforce reduction as part of a margin expansion plan. It picked up a big regulatory win in June 2024 as Chinese authorities approved its Precision treatment planning system (TPS), expanding access to care for cancer patients in China. –SW and JH



















Redwood City, California United States
$425,174,000
Fiscal year ended 12/31/2023
2023 rank: 95 R&D spend: $54,418,000 Employees: 1,215
CEO: Kevin Thornal
www.nevro.com
NEVRO provides pacemaker-like spinal cord stimulation systems for pain relief. The company started 2024 strong on the product front, with coverage wins for its painful diabetic neuropathy treatment and FDA clearance for a sacroiliac joint fusion device. However, with layoffs in January and questions over the company’s path to growth, the company announced in August that it’s looking at strategic options that could include partnerships, mergers or a sale. –SW



















Experienced CDMO with highly automated manufacturing capabilities.
• Machining
• Orthopedic Forging
• Laser Processing
• In-house Secondary Operations
Areas of Expertise:
• Advanced Surgical
• Robotic Assisted Surgery
• Orthopedic







• Women’s Health









Scan to learn more about our capabilities


A leader in the medical device industry with 30 years of experience. Our recognized brand reputation is one of excellence. Galt offers an extensive array of access products as single bulk product lines to private labeled sterilized kits. Servicing medical markets of Interventional Radiology, Interventional Cardiology, Vascular Surgery and IV Therapy.

View our portfolio…https://galtmedical.com/products/
(former ZimVie Spine and EBI Bone Healing businesses)
Westminster, Colorado United States
$409,181,000
Fiscal year ended 12/31/2023
2023 rank: not available R&D spend: $26,559,000 Employees: 713
CEO: Rebecca Whitney, Spine CEO; Glen Kashuba, EBI CEO
www.highridgemedical.com
HIGHRIDGE MEDICAL and EBI are portfolio companies of HIG Capital, which acquired ZimVie’s spine and
99 98
Mason, Ohio United States
$399,245,000
Fiscal year ended 12/31/2023
2023 rank: 99
R&D spend: $73,915,000 Employees: 1,200
CEO: Michael Carrel
www.atricure.com
ATRICURE develops devices for atrial fibrillation (AFib) and related conditions. AtriCure has carved out a strong niche in the surgical space,
Irvine, California United States
$366,379,000
Fiscal year ended 12/31/2023
2023 rank: 104 R&D spend: $34,886,000 Employees: 797
CEO: Raymond Cohen
www.axonics.com
AXONICS develops neuromodulation systems for treating urinary and bowel dysfunction. Its fourth-generation R20 rechargeable sacral neuromodulation
Kennesaw, Georgia United States
$354,004,000
Fiscal year ended 12/31/2023
2023 rank: 100
R&D spend: $28,707,000 Employees: 1,500
CEO: J. Patrick Mackin
www.artivion.com
ARTIVION (formerly known as CryoLife) develops devices for cardiac and vascular surgeons who specialize in treating patients with aortic









PDA Pre-filled Syringes & Injection Devices
PDA Pre-filled Syringes & Injection Devices
Oct. 22-23 / Booth #802 PODD
Oct. 22-23 / Booth #802 PODD
Oct. 28-29 / Booth #30
Oct. 28-29 / Booth #30

As the world’s biggest medical device companies get bigger, that makes it harder for companies to make our Medtech Big 100 ranking each year. The annual revenue needed to get onto the list at the No. 100 spot keeps climbing each year. This year's No. 100 company had annual revenue of $354 million, $40 million higher than last year’s No. 100 and nearly $118 million higher than the year before that.
However, companies that just miss making the Medtech Big 100 often find themselves on the list the following year. For example, this year’s No. 99 — Axonics — was No. 104 last year and now has a deal to be acquired by Boston Scientific, No. 12 on our list.
With that in mind, here are the companies that would have ranked Nos. 101 to 105 on this year's list.
— Senior Editor Danielle Kirsh
Aliso Viejo, California United States
$314,711,000
R&D spend: $138,768,000 Employees: 907
CEO: Thomas Burns
GLAUKOS develops ophthalmic medical devices for treating glaucoma, corneal disorders and retinal disease. The company also came close to making last year’s Medtech Big 100 list, ranking No. 101 with sales of $282.9 million. –DK
Barco (healthcare division)
Kortrijk Belgium
$308,763,360 (€285,892,000)
R&D spend: not available Employees: not available
CEO: An Steegen (Charles Beauduin will move from co-CEO to chair on Sept. 1, 2024)
BARCO’S healthcare division was No. 97 on our 2023 Medtech Big 100 at $358.8 million. It develops networked visualization technologies, including an array of medical displays and imaging systems. –DK
Andover, Massachusetts United States
$241,623,000
R&D spend: $36,055,000 Employees: 584
CEO: Waleed Hassanein
TRANSMEDICS develops organ transportation devices to preserve organs to be transplanted into endstage heart, lung and liver failure patients. The company says its Organ Care System platform is the “first and only multi-organ platform to leverage proprietary core technologies across multiple organs.” –DK
Sunnyvale, California United States
$177,134,000
R&D spend: $41,324,000 Employees: 474
CEO: Chas McKhann
SILK ROAD MEDICAL develops products designed to prevent stroke in carotid artery disease patients with a minimally invasive procedure called transcarotid artery revascularization (TCAR). The company struck a deal to be acquired by Boston Scientific for $1.16 billion in June 2024. –DK
Warsaw, Indiana United States
$148,732,000
R&D spend: $10,196,000 Employees: 247
CEO: David Bailey
ORTHOPEDIATRICS exclusively develops orthopedic devices for children. The company says it has more than 70 surgical systems serving three of the largest pediatric orthopedic market categories: trauma and deformity, scoliosis, and sports medicine/other procedures. –DK

THE WORLD'S LARGEST MEDICAL DEVICE COMPANIES ARE GETTING BIGGER WITH MULTIBILLION-DOLLAR DEALS THAT HELPED INCREASE MEDTECH BIG 100 SALES, R&D SPENDING AND EMPLOYMENT DESPITE WIDESPREAD LAYOFFS.
Awave of mergers and acquisitions made the world’s biggest medical device companies even bigger, with Medtech Big 100 sales, research and development (R&D) spending and headcounts all growing year over year.
Aggregate sales for the Medtech Big 100 — our annual ranking of the world’s largest device companies by revenue — grew nearly 5% to a record high of $474.8 billion.
That was a faster rate of growth than last year’s 3% and the third consecutive year of growth since the COVID-19 pandemic began, when total Medtech Big 100 sales dropped from 2019 to 2020. More than three-quarters of the companies on our list increased sales, and more than one-third reported double-digit growth.
The M&A activity — and some spinoffs — resulted in new names on our latest list. And the bar keeps getting higher. This year, Artivion is the last company on our list at No. 100 with $354 million in revenue. The aortic device developer took the same spot last year with just under $314 million in sales, which wouldn’t have been enough to make the cut this year.
Revenue gains driven by M&A
About three-fourths of the companies reported sales growth, with 33 companies experiencing double-digit growth. Companies leading in growth increased sales through M&A — like Globus Medical, which reported a 53% increase in revenue after merging with NuVasive — or struck deals to be acquired. >>
SINCE WE PUBLISHED OUR 2023 EDITION OF THE MEDTECH BIG 100, SEVERAL MEDICAL DEVICE COMPANIES ON THE LIST HAVE STRUCK BILLION-DOLLAR DEALS, INCLUDING JOHNSON & JOHNSON’S ACQUISITION OF SHOCKWAVE MEDICAL FOR $13.1 BILLION, BD’S ACQUISITION OF EDWARDS LIFESCIENCES’ CRITICAL CARE BUSINESS FOR $4.2 BILLION, AND BOSTON SCIENTIFIC’S ACQUISITION OF AXONICS FOR $3.7 BILLION. THESE DEALS AND THOSE LISTED IN THE FOLLOWING TABLE ARE EXPECTED TO BE INCLUDED IN THEIR NEXT FISCAL REPORTS (OUR 2025 MEDTECH BIG 100). HERE ARE THE LARGEST MERGERS AND ACQUISITIONS WE HAVE COVERED SINCE SEPTEMBER 2023.
Johnson & Johnson acquired Shockwave Medical
BD plans to acquire Edwards Lifesciences’ Critical Care business
Baxter plans to sell its kidney care business
Boston Scientific plans to acquire Axonics
Johnson & Johnson MedTech plans to acquire V-Wave
Owens & Minor plans to acquire Rotech Healthcare
Edwards plans to acquire JenaValve and Endotronix
billion
billion
Boston Scientific plans to acquire Silk Road Medical $1.16 billion
Carl Zeiss acquired Dutch Ophthalmic Research Center $1.07 billion
Boston Scientific acquired Relievant Medsystems
Enovis acquired LimaCorporate
$850 million
$840 million
Steris sold its dental segment $787.5 million
Surmodics plans to be acquired by private equity firm GTCR
$627 million
Inari Medical acquired LimFlow $415 million
Johnson & Johnson MedTech acquired Laminar $400 million
Alcon acquired Belkin Vision $385 million
ZimVie sold Spine and EBI businesses (now called Highridge Medical)
Getinge acquired Healthmark
Hologic acquired Endomagnetics
Edwards acquired Innovalve
Integra LifeSciences acquired Johnson & Johnson’s Acclarent
Haemonetics acquired OpSens
Smith+Nephew acquired CartiHeal
Integer acquired Pulse Technologies
Haemonetics acquired Attune Medical
Merit Medical acquired EndoGastric Solutions
Intravascular lithotripsy tech company Shockwave Medical and sacral neuromodulation tech company Axonics reported sales increases of 49% and 34%, respectively. The fast growth made them attractive acquisition targets. J&J MedTech bought Shockwave Medical for $13.1 billion in May 2024, opening up a spot for someone else on next year’s list. Meanwhile, Boston Scientific has a deal pending to buy Axonics for $3.7 billion.
At the other end of the spectrum, ZimVie became a pure-play dental company by selling off its spine business, decreasing its revenue from continuing operations by half. The spinoff became Highridge Medical, making its Medtech Big 100 debut as an independent company.
$375 million
$320 million
$310 million
$300 million
$275 million
$255 million
$180 million
$105 million
The second-largest revenue drop after ZimVie was due to another spinoff. Avanos Medical, which makes devices for pain management and chronic care that help reduce the use of opioids, reported an 18% sales drop after selling its respiratory health business to SunMed Group Holdings. Other spinoffs include 3M Health Care (now called Solventum) and Mozarc Medical, the kidney care company launched by Medtronic and DaVita. Baxter plans to sell its kidney care business (to be called Vantive) to Carlyle for $3.8 billion.
As companies get larger and more complex, they might realize they lack desired synergies while they look to (continued on page 56)


FOR THIS ANALYSIS, WE COLLECTED THE MOST RECENTLY AVAILABLE ANNUAL REVENUE FIGURES FROM BOTH PUBLIC AND PRIVATELY HELD MEDICAL DEVICE MANUFACTURERS AROUND THE GLOBE. NOTE THAT FISCAL YEARS FROM COMPANIES CAN VARY.
DUE TO THE VARYING STRENGTH OF THE U.S. DOLLAR, THE ANALYSIS USED FOREIGN CURRENCIES IN THE YEAR-OVERYEAR ANALYSES FOR COMPANIES THAT DON’T REPORT THEIR SALES AND R&D SPENDING IN U.S. DOLLARS.
(continued from page 54) invest in future growth programs, said Frank Jaskulke, the former Medical Alley Association VP of innovation who just joined Avio Medtech Consulting.
“The big companies haven't been spinning off their fastest-growing businesses. They tend to be spinning off ones that are more mature, and as a result often aren't getting the investment in R&D and other services to advance the technologies,” he said. “By making them independent, those companies can go get their own capital, be very focused, and likely deliver better returns and better innovation.”
Outside of M&A-driven growth, Inspire Medical Systems grew sales by 53% to $624.8 million in sales in 2023. Inspire’s neurostimulator implant treats obstructive sleep apnea, offering patients an alternative to continuous positive airway pressure (CPAP) therapy as Royal Philips exited the CPAP market following waves of recalls. Inspire spent 19% of its revenue on R&D while increasing its workforce by 34%.
Most Medtech Big 100 companies disclose R&D spending. Medtech Big 100 companies spent $28.9 billion on R&D in fiscal 2023, a $2.5 billion increase (10%) from our analysis last year. But that rate of growth is about half of what we found the year before.
Another metric we analyze is R&D spending as a percentage of a company's revenue. Among the Medtech Big 100 companies that disclose research spending, each company spent an average of 10% of revenue on R&D. The company spending the largest portion of its revenue on R&D was Novocure (44%), which is developing tumor treatment fields. Two dozen companies spent more than 10% of revenue on R&D.
Of the 74 companies that disclosed their R&D spending, Johnson & Johnson MedTech took the top spot with $3.1 billion. That figure — which does not include Shockwave R&D — equals 10% of J&J MedTech’s revenue, up from 9% the prior year. (continued on page 59)
(merged with NuVasive)
(merged with SeaSpine)
(former ZimVie Spine and
THIS RANKING EXCLUDES COMPANIES WITH NON-MEDICAL DEVICE OPERATIONS THAT DO NOT BREAK OUT R&D SPENDING BY DIVISION AND COMPANIES THAT DO NOT DISCLOSE R&D SPENDING. FOR MORE DETAILS, PLEASE REFER TO OUR RANKING METHODOLOGY ON PAGE 12 AND INDIVIDUAL COMPANY RANKINGS ON THE PAGES THAT FOLLOW.
(medical segment)
(merged with SeaSpine)
(previously 3M Health Care)
(continued from page 56)
Last year’s No. 1, Medtronic, only increased total R&D spending by 1%, while its R&D as a share of revenue remained relatively unchanged.
M&A offsets layoffs for Big 100 employment growth
Most of our Medtech Big 100 companies also disclose employee counts. These 83 companies reported a total of 1.25 million employees, up 6% from last year but still slightly lower than our 2022 count.
M&A again drove the largest employment gains, offsetting layoffs across the industry. For example, Globus Medical nearly doubled its headcount through its merger with NuVasive.
Shockwave Medical grew its workforce by 47% before its acquisition by Johnson & Johnson. Overall, 23 companies had doubledigit headcount growth.
MicroPort had the greatest headcount loss in fiscal 2023, employing 1,205 (13%) fewer people. Citing supply chain disruptions and the negative impact of hospital surgeries from intensified compliance controls, MicroPort “actively adjusted our business strategies and implemented various cost control measures” to focus on core products while reducing R&D and administrative spending, CEO and Chair Zhaohua Chang wrote in the annual report.
Other companies with double-digit workforce reductions included Royal Philips, GN Hearing and Cochlear, which all reported 11% drops.
Medtronic’s organization-wide layoffs made headlines in recent years, and while the company declined to quantify the cuts, its February 2024 employment was down 1% from 2022, the previous most recent time it offered a precise count.
“We're seeing investment and hiring trends that are very similar to 2019, which is actually good because we're now in a resumed cycle of healthy growth and expansion,” said Holly Scott, VP and partner at medtech search firm The Mullings Group. “The bigger organizations are doing what bigger organizations do after a fatty year. >>
HERE’S HOW MANY PEOPLE EACH COMPANY REPORTED AS EMPLOYEES, EITHER IN THEIR MOST RECENT ANNUAL REPORTS (FOR YEARS ENDING IN OR BEFORE APRIL 2024), OTHER FILINGS, THEIR WEBSITES, OR PROVIDED DIRECTLY TO MEDICAL DESIGN & OUTSOURCING.
They see the bottom fall out and realize they have to revert back to their core competency. … It is smart and it's necessary if [the strategics] want to continue to grow.”
Scott believes we’re past the worst of the layoffs from major device OEMs, “but we will continue to see the smaller, more subtle, nuanced layoffs.
“The big strategics are going to constantly have to adjust to what the new market looks like,” she said. “Commercialization in medtech, in hospitals, in ambulatory surgical centers, in home health is changing so dramatically that it's impossible to do projections that are entirely accurate. … But I do see a passionate push toward continuing, healthy growth in medical and healthcare, so with that, there's ample opportunity on both sides: on the innovative side as well as on the large, strategic side.”
“We’re
seeing investment and hiring trends that are very similar to 2019, which is actually good because we’re now in a resumed cycle of healthy growth and expansion. The bigger organizations are doing what bigger organizations do after a fatty year.”
Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.



Product Categories:
• Video Cables
• Patient Monitoring Cables
• Sensor Probe Cables
• RF Generator Cables
• Robotic Surgery System Cables
• Aesthetic Surgery Cables
• Single Use Cables




















Nitinol



itinol is a key material in the heart valve clips that Abbott designed for its TriClip and MitraClip transcatheter edge-toedge repair (TEER) systems.
Abbott designed the TriClip system (approved by the FDA in April 2024) for reducing tricuspid valve regurgitation using fourth-generation heart valve clips that Abbott originally developed for the MitraClip transcatheter mitral valve repair (TMVr) system more than a decade ago.
Nitinol grips prevent slips in Abbott’s heart valve clips

“Grippers, as the name suggests, are used for gripping the leaflets,” he said. “Those grippers have these elements called frictional elements. These are tiny protrusions on the grippers that engage with the leaflets and prevent them from slipping out. You insert the leaflets between the clip arms and the grippers and you drop the grippers. Now the grippers have gripped onto the onto the leaflets and then you close the clip arms. You have the leaflets well engaged and they won’t come out.”
“Grippers, as the name suggests, are used for gripping the leaflets. Those grippers have these elements called frictional elements. These are tiny protrusions on the grippers that engage with the leaflets and prevent them from slipping out.”
Abbott turned to nitinol as a crucial design change from the first generation of that clip to improve its grip on the heart valve’s thin, flexible leaflets, said Santosh Prabhu, divisional VP of product development for Abbott’s structural heart business.
Nitinol frictional elements are an essential component of the heart clip’s grippers, he explained in an interview with Medical Design & Outsourcing
Rows of frictional elements along both grippers of the clip’s arms secure the leaflets without perforating them. The fourth-generation MitraClip NT and NTW have four rows of frictional elements, while the larger fourth-generation MitraClip XT and XTW have six rows.
Abbott made the firstgeneration clip’s frictional elements out of Elgiloy, a brand of super-alloy consisting mostly of cobalt, chromium and nickel, with lesser amounts of molybdenum, manganese, silicon, carbon, beryllium, phosphorus, sulfur and iron. >>
But that alloy lacked an important property that has made nitinol (a nearly equiatomic alloy of nickel and titanium) so popular among medical device developers, Prabhu said.
Elgiloy “was not superelastic, and therefore it wouldn’t drop all the way down,” he said. “But that was one of the major changes that we made when we came up with the secondgeneration clip, which is the MitraClip NT. The TriClip product is derived from the MitraClip product — all versions of the TriClip product have had the nitinol grippers.”
The clips themselves are made of cobalt-chromium.
“Cobalt-chromium has very good strength for our applications, and at the same time is radiopaque, so it’s easier to visualize under fluoroscopy. It’s also very pliable for a metal, so you can shape it into different forms. The clip arms of the

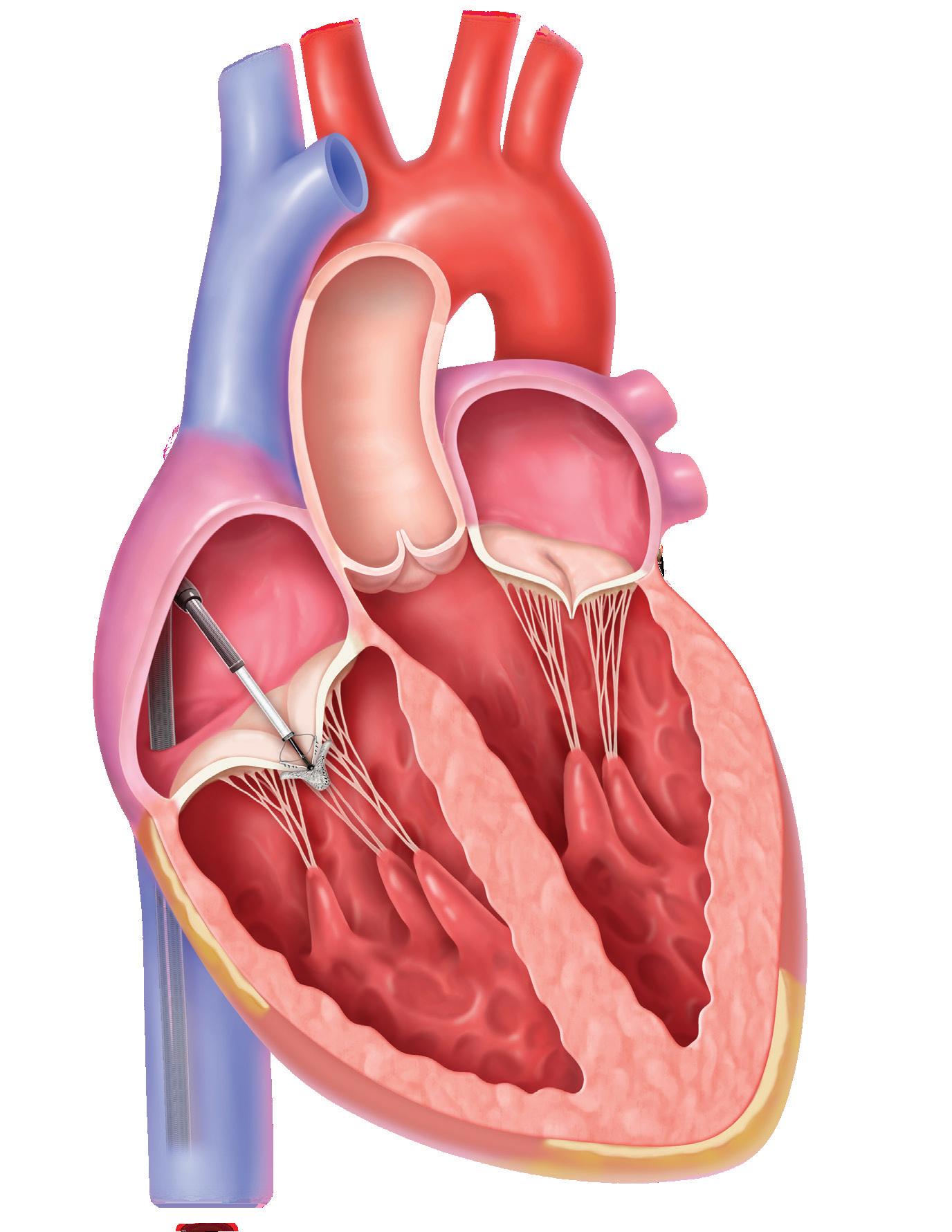

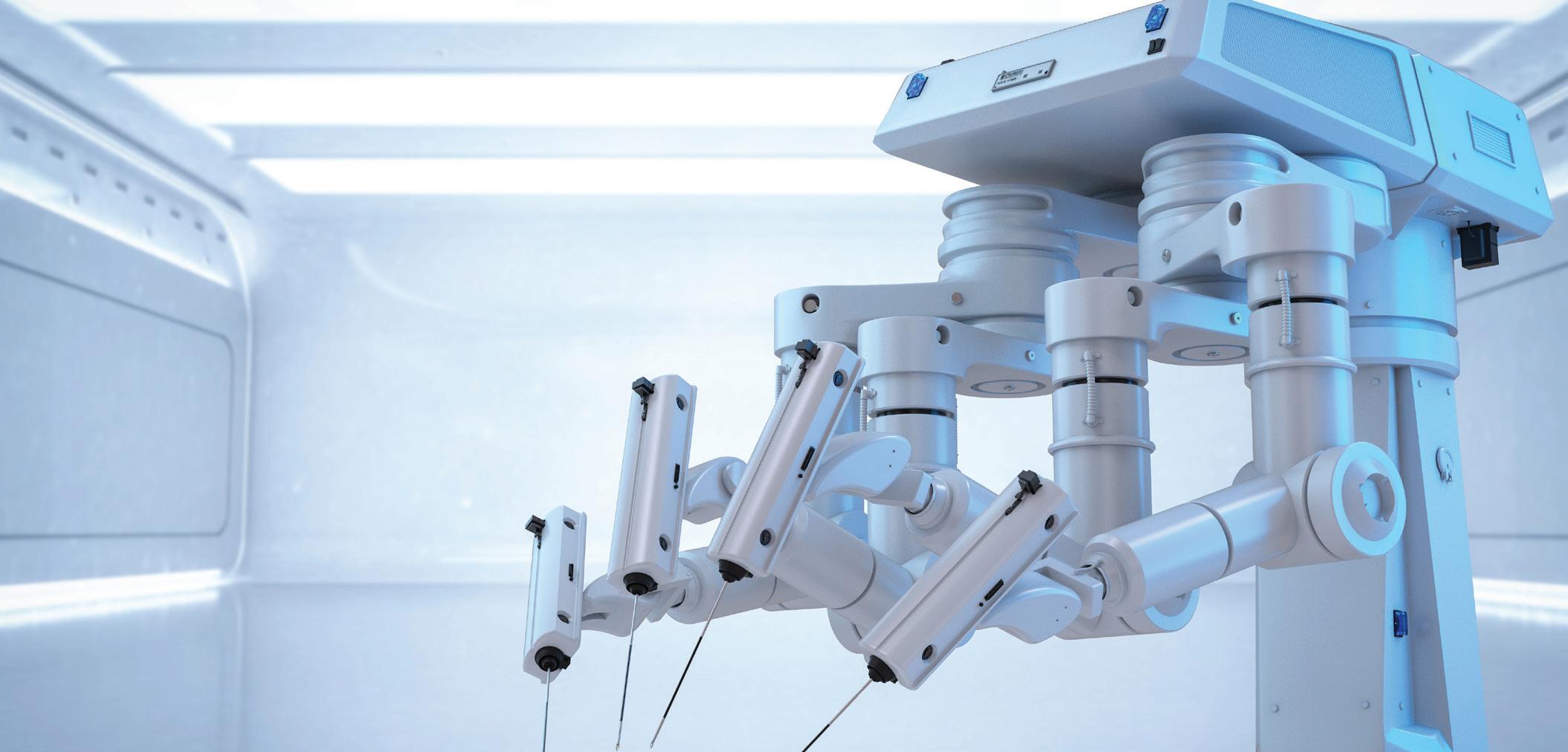
• Constant Force Springs
• Spiral Torsion Springs
• Power Springs
• Helical Springs
• Mechanical Assemblies
• Wire Forms
MitraClip are made of Elgiloy,” he said. “Nitinol, on the other hand, is used more for shape-memory or superelastic effects. It doesn’t undergo plastic deformation very easily. That’s where it lent itself in designing the grippers, because you have to close and open the clip arms multiple times during a procedure sometimes. Both during manufacturing and during the use of the product, you have to open and close the clip arms. Now the grippers have to open and close along with it, and you can’t afford to have it undergo plastic deformation because then it won’t go back to its original shape and drop down and engage with the leaflets. And that’s where nitinol came in.”
The clips are covered with
early stages to evaluate the effect of just a clip without any covering and then with the covering, and we found the healing response was better with with the covering,” Prabhu said.
Another important nitinol component is the leaf spring, which helps lock and unlock the clip.
“Again, it has to regain its original shape, and when you either lock the clip or unlock the clip, it has to deform and not undergo plastic deformation,” Prabhu said.
So why didn’t Abbott use nitinol for the gripper frictional elements to begin with? Their sharp configurations just couldn’t be manufactured with nitinol at the time, he said.
“Ten years later, laser technology was available to make some of those changes. [For] a lot of

Read more from our


Confluent is proud to provide customers with the most reliable supply chain delivering the broadest level of Medical Nitinol services in mill products, hollows, tubes, wires, and components Confluent now offers Nitinol Tubing within 14 weeks and Nitinol Wire within 20 weeks. Learn more at www.Nitinol.com

• CATHETERS

• TUBING & SYSTEMS
• MOLDED COMPONENTS
• CONDUCTIVE MATERIALS
• HYPOTUBES
• COATINGS

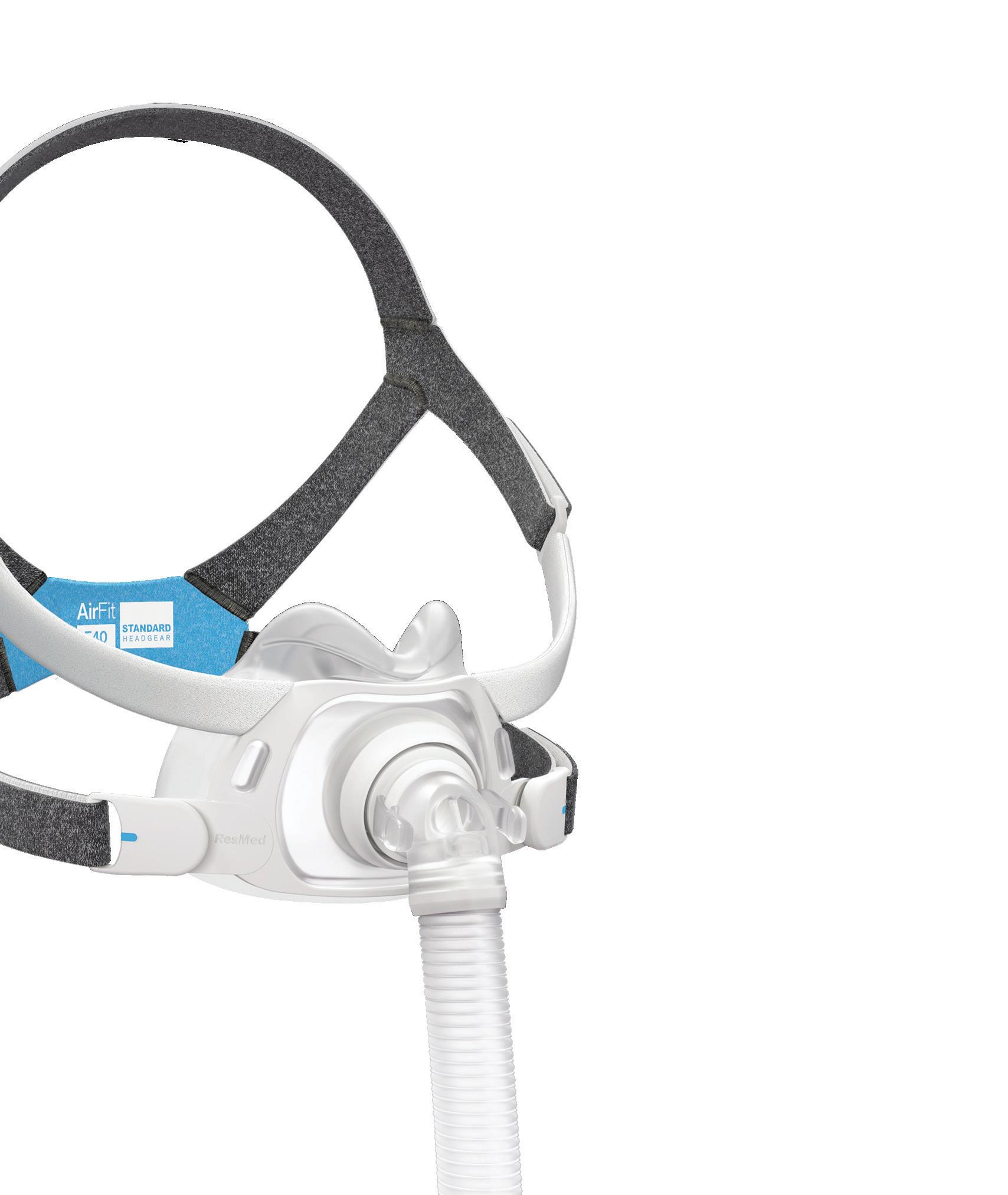
If you search FDA records for ResMed’s new AirFit F40 continuous positive airway pressure (CPAP) mask, you won’t find that name.
Instead, ResMed sought and won FDA 510(k) clearance for the AirFit F40 mask using the codename Oran Park after a raceway in Australia, where ResMed was founded.
ResMed is now headquartered in San Diego, but the engineering team still applies the names of Australian racetracks to the systems they develop.
“High performance is what we’re looking for,” ResMed Chief Product Officer Justin Leong said in an interview with Medical Design & Outsourcing. “And that’s this mask.”
The ResMed AirFit F40 mask
ResMed developed the AirFit F40 to offer the comfort of smaller masks without sacrificing performance. This could, in turn, help with sleep apnea therapy compliance for users of highpressure CPAP machines.
The mask is designed to provide pressure support in a more comfortable, low-profile way. ResMed wanted to make the mask better for those who sleep on their side — an estimated 50% of the population, Leong said — and patients who are claustrophobic or want the stability and seal of a universal-fit mask in a minimalist design.
ResMed’s key, Leong says, is the AdaptiSeal cushion. The soft silicone material helps to maintain a facial seal, even when the patient is moving around during sleep. >>
ResMed designed the AirFit F40 mask for CPAP side-sleepers, which the device developer estimates is about half of the population.
courtesy of ResMed


ResMed
Chief Product Officer
Justin Leong

Global Product Manager for Sleep
Leong said it’s the first cushion of its kind made out of fully soft silicone material, eliminating the hard plastic chassis commonly used in fullface sleep apnea masks. That rigid component helps maintain the shape of the cushion, but can cause discomfort if the mask is too tight. The AirFit F40’s new cushion allows the mask to “float” on the user’s face, Leong said.
easily disconnect the mask from the tube. The mask also has what Leong calls “simple headgear with four points to adjust, making it easier to remove than previous versions.
“Often, if you’re waking up in the middle of the night, you need to adjust your mask or take off your mask, so you want something really simple around your head,” Leong said. “We’ve redesigned that as well.”
ResMed AirFit F40 mask development challenges
Size was a key factor for ResMed to tackle when developing the new AirFit F40 mask. Face masks tend to be large, covering the mouth and nose. Along with the cushion, the frame was important, Leong said.
Lai Ying Ho, ResMed’s global product manager for sleep, said the challenge was to “design a mask with a cushion that is soft and seals well, [that] doesn’t collapse, and can be reliable and easy to manufacture.”
It was mainly a challenge of architecture, she said. With a different frame and an additional short tube compared to just an elbow in the previous version, the mask is flexible and sits differently than old frames, Leong said.
“The technical challenge is that these masks need to work under quite high pressure — that’s why
“We all have very different-shaped faces. To design something that fits most faces is very difficult. That’s where the clever engineering had to come in with the facial scanning.”
“The biggest thing we’ve done is make a small face mask that still performs well,” he said. “The way we’ve done that is a very innovative cushion that sits over your face. … It adapts to your face. That’s a really magical thing.”
In addition to the smaller, more comfortable features, the redesigned frame of the F40 sits “a bit differently” across the top of the face for clearer vision. Patients can even keep their glasses on while using the mask.
Another feature is a quickrelease tube that allows users to
usually they’re made with quite rigid material — and to make it keep in shape enough to seal but still be that comfortable, soft cushion,” Leong said.
“We all have very different-shaped faces,” he continued. “To design something that fits most faces is very difficult. That’s where the clever engineering had to come in with the facial scanning.”
(continued on page 70)
Learn how our dedicated First Step team and resources, paired with over 100 years of manufacturing expertise, pays off for our customers. When you need to deliver on your NPI/NPD project, First Step enables project teams to truly unleash innovation!
• 100+ machining centers & advanced applications engineers
• Next-level metrology and inspection capabilities
• Sound DFM input, rapid precision prototypes
• The broad capabilities of a vertically integrated manufacturer
Hobson & Motzer are highly skilled engineers, toolmakers, and quality professionals. We are makers of precision metal components with a remarkable depth of knowledge in med device, with a proven track record of adding value to some of the most challenging applications.
(continued from page 68
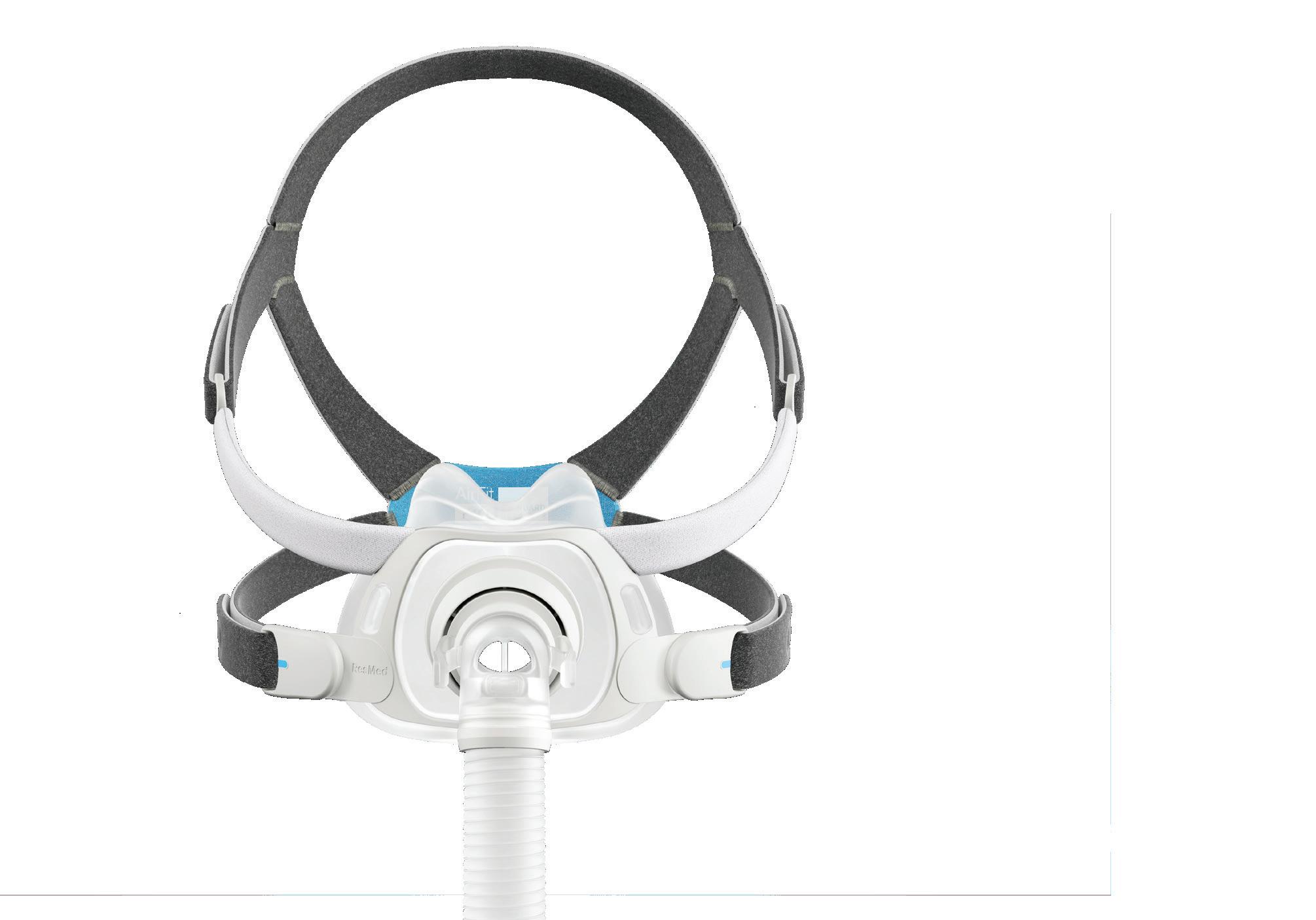
For the first time, ResMed used facial scans to kick off the design of a new mask, Ho said, collecting thousands of scans to find the right design that would fit as many faces as possible.
“During the development process, we do go back to patients to fit them in order to make sure that this is really fitting a wide variety of faces as we intended,” she said.
Taming the supply chain
Finally, given the long-running recall issues that knocked Philips Respironics out of the CPAP business, ResMed has expanded its share of the market. That increased demand came as the COVID-19 pandemic continued to disrupt supply chains.
“We’ve always had big manufacturing capacity around the world,” Leong said. “But sourcing materials has been challenging over the last few years.”
ResMed ramped up production while incorporating new materials in its updated mask. The company is one of the world’s largest buyers of the silicone used for the mask’s soft cushion, Leong said.
“We’ve had to spend time sourcing new suppliers and working with them over the years so that we have multiple suppliers,” he said. “… We’re in a much better situation today and we have good supply — touch wood.”





























We are the plastic manufactured solution with exceptional team-driven service that gives you the freedom to focus on your next product innovation. Whether you have a new device concept, an approved design, or a design transfer, we’ll work with you to provide the best injection molding solution, while keeping costs down. Our US-based facility offers: injection molding, injection blow molding, product design & development, R&D tooling, custom cleanroom assembly & packaging, Class 7 assembly, and Class 8 cleanroom manufacturing. ISO 13485:2016, & FDA 21CFR820.
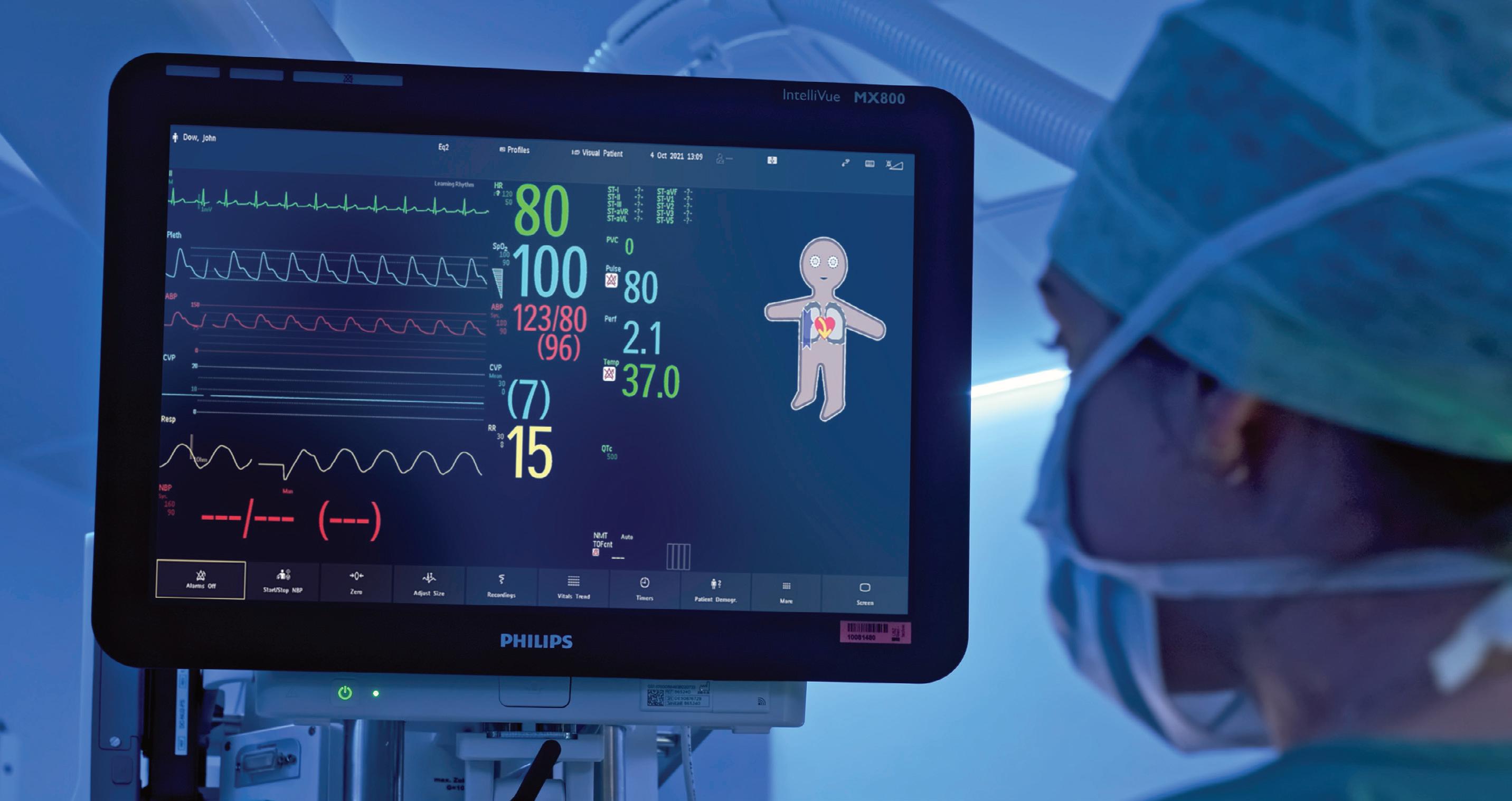

Information overload can impact patient care and safety, particularly in the operating room, where quick and accurate interpretation of a patient’s vital signs is crucial.
Philips and the University of Zurich developed a new way to display a patient’s vital signs in an effort to reduce anesthesiology-related mistakes due to cognitive overload.
Mark Holger-Konrad is head of medical and clinical hospital patient monitoring at Philips and previously held clinical leadership positions at PULSION Medical Systems SE and Getinge. He was a practicing anesthesiologist for more than 13 years and received his doctorate from Heidelberg University in Germany.
Time constraints, capacity limitations, and data overload contribute to poor situational awareness, which is the ability of clinicians to understand and effectively respond to the surrounding environment. In fact, poor situational awareness accounts for over 80% of anesthesia-related mistakes. This striking number shows the prevalence of data misinterpretation among clinicians and how it can lead to costly medical errors.
To address this challenge, the industry needed a new way to simplify how patient data was presented to anesthesiologists on patient monitors. Philips collaborated with clinical research partners at the University Hospital of Zurich to launch an innovative avatarbased patient monitoring solution
designed to reduce the impact of cognitive overload on decision-makers.
Informed by clinician feedback and research, the team demonstrated how adding a visual depiction of a patient’s health status improved clinical decisionmaking and enabled the delivery of quick care and life-saving, timely interventions. The creation process behind this innovation is a testament to the impact of a collaborative approach between designers and end-users to benefit clinicians and allow them to deliver care with confidence.
Alleviating cognitive overload through simple visual design
While visual representations of anesthesiology data existed, they were often centered around detailed human figures — frequently using a white male figure as the basis — that could be zoomed in to review specific data points. This information is all critical, >>
“Informed by clinician feedback and research, the team demonstrated how adding a visual depiction of a patient’s health status improved clinical decisionmaking and enabled the delivery of quick care and lifesaving, timely interventions.”
but this type of visual added complexity to what anesthesiologists were reviewing.
Instead, Philips and the University of Zurich set out to create a simple snapshot of a patient’s vitals and overall condition that would guide anesthesiologists’ assessment and allow them to quickly react to changes and focus their attention on the most critical patient information at the moment.
The Philips Visual Patient Avatar is represented through a basic silhouette that uses animations, colors, and shapes to demonstrate a patient’s vitals, such as oxygen levels, heart rate, and body temperature. This allows anesthesiologists and nurse anesthetists to recall and process critical data through a glance at the monitor, enabling better care decisions by minimizing distractions and confusion.
Using a simplified human shape with a neutral coloring instead of an anatomically correct figure also helps to remove medical assumptions

by eliminating sex and race from the visualization. Additionally, the Avatar’s design allows for the universal recognition of the visual cues that represent changes in a patient’s condition.
While doctors may be trained to deliver the same type of care, they do not always speak or understand the same language, including written text and even numbers. However, simple designs can be universally identifiable, reducing the need for interpretation and potential errors.
A clinical study found 73% of all vital sign information was correctly identified during the first use of the Avatar. Another clinical study found that users of the Avatar recalled almost double the number of vital signs after 3- and 10-second glances at the monitor. There was also an increase in the percentage of perceived vital signs by 57% when viewed for 10 seconds, and the perceived workload for the task decreased by 12%.
Cross-industry inspiration and clinical research partnerships for end usercentric solutions
When it comes to innovation, research has shown that bringing people from different industries together can be a source of radical novelty. The inspiration behind Visual Patient Avatar came from a source that may surprise you: the aviation industry.
In addition to being practicing anesthesiologists, the creators also happened to be pilots who were inspired by the synthetic vision used on a plane’s dashboard, specifically how it gave them more confidence to navigate poor weather and low-visibility situations. Spotting the similarities with the situational awareness challenges in the OR, the creators explored new opportunities and spent five years researching and developing the Avatar.
Developing an innovative solution requires collaboration and a willingness to evolve the design to meet the end-user’s needs. In the case of creating Visual Patient Avatar, we relied on a combination of clinical expertise with the right technology partner to bring it to life.
(continued on page 76)
Adva ncing t he d evelop ment a nd commercialis at ion of medical d evic es a nd diagnos t ic s .
– Compre he nsi ve re gulator y se r v ice s
– G lobal clinic al stud y de sign a nd exe cu tion
– D e dic ate d me dic al dev ice ex pe r ts
– Strate gic, multi-disciplina r y ope rational imple me ntation
Put t he pieces toget her wit h u s
I CO N p l c.c o m /d ev i c e s

(continued from page 74)
As part of the creation process, practicing clinicians were key stakeholders in the design phase as they had a firsthand view of the most pressing care-related problems, knew the challenges at hand and the priority in which they needed to be fixed. Additionally, they were aware of the frustrations in their daily jobs as anesthesiologists and cognizant of the challenging task of convincing other clinicians who rely on patient monitors that this simple visual would be impactful in alleviating cognitive overload.
The next stage in creating the avatar was ensuring the correct data was fed into accurate algorithms to deliver precise and actionable data to anesthesiologists. To do so, we had to consider key metrics such as perceptual performance, situational awareness, and qualitative indicators to test the effectiveness of the design.
Leveraging patient data and practical clinical experience, we fostered an effective partnership where the objective was the same — to develop an innovative solution that would meet the needs of the end users and their patients. Not only does this ensure that the solution will truly address their needs, but it will also keep patient safety at the forefront of their minds. If we continue to collaborate on technologies created with the patients and end-users in mind, we are doing our part to support healthcare in new and unique ways.
Novel ideas that solve some of the most critical problems can sometimes stem from unexpected places. Just like the synthetic vision on planes helped practicing anesthesiologists find a solution to alleviate cognitive overload, technology developers should continue to be inspired by the creations of other industries.
In the face of everyday challenges, healthcare technology manufacturers must pair with the right clinical research partners and prioritize creating devices and solutions that will truly benefit the clinician. Ultimately, focusing on patients and how to improve outcomes inspires healthcare innovation.
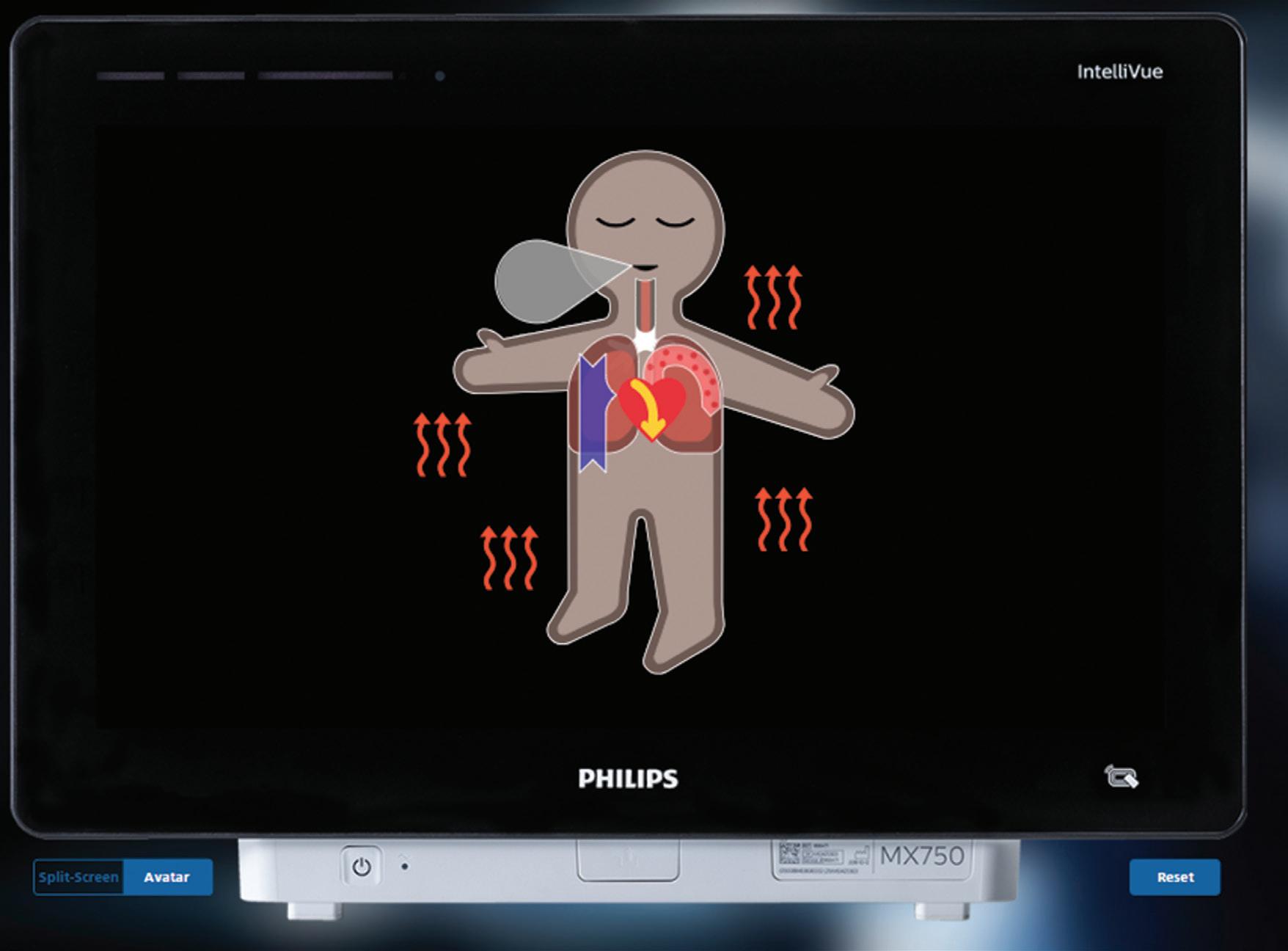
This Philips Visual Patient avatar displays critical information about a patient’s vital signs: for example, the red heat waves mean body temperature is high, the completely filled air bubble indicates end-tidal CO2 is normal, and closed eyes indicate brain activity below the lower threshold.
Image courtesy of Philips
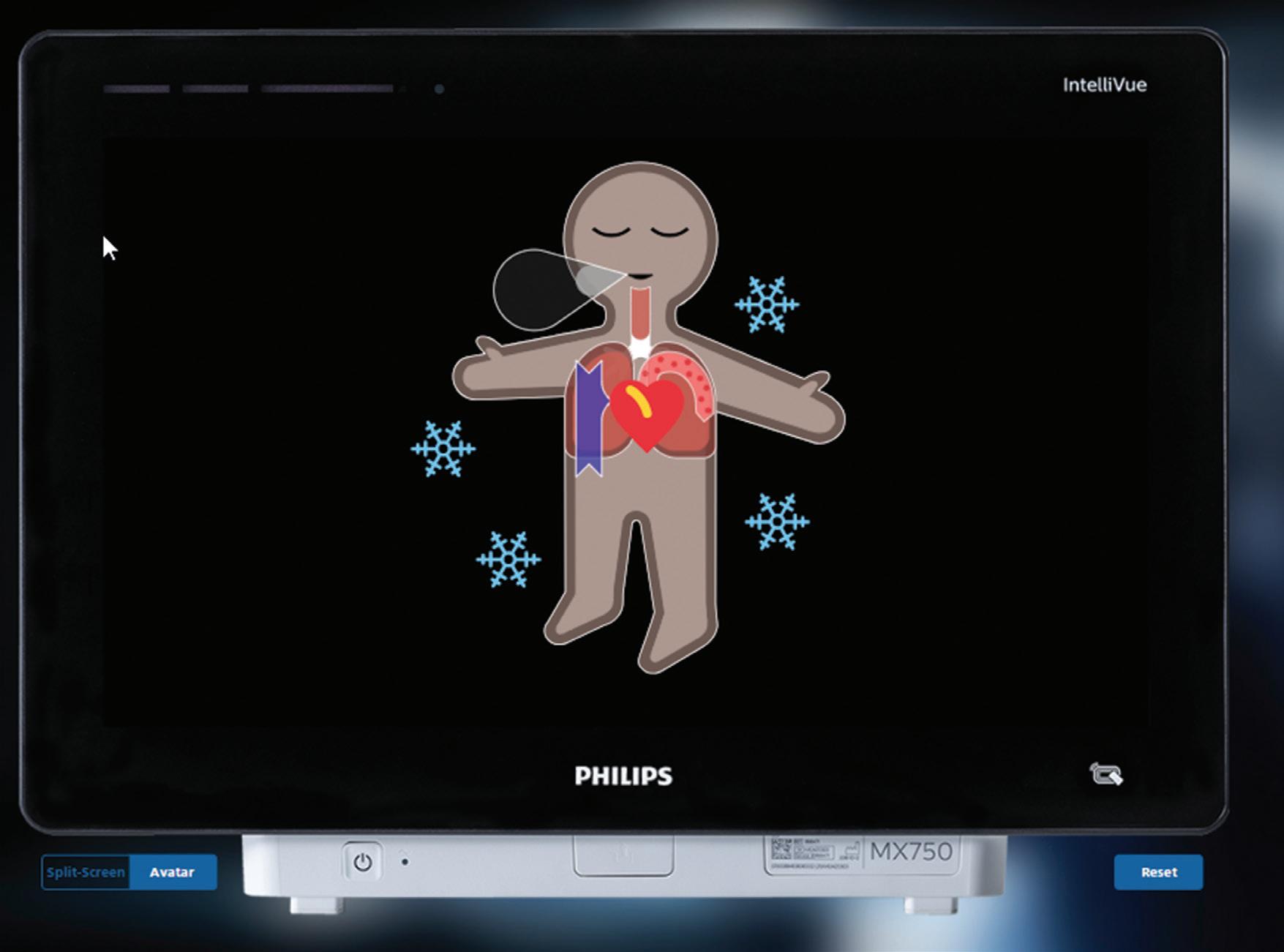
This Philips anesthesiology avatar displays critical information about a patient’s vital signs: for example, the snowflakes mean body temperature is low, the partially filled air bubble indicates low end-tidal CO2, the blue heart vein (indicating the vena cava) is filled to indicate normal central venous pressure, while the red heart vein measures cardiac output, showing the aorta as fully filled.
Image courtesy of Philips

Join Us at Booth #2612 October 16–17
We prioritize innovation, talent, and infrastructure. As a vertically integrated partner with over 75 years of expertise, we ensure excellence in everything from advanced engineering to effective project management and beyond. And with class 7 and 8 Cleanrooms, you can rely on us to have materials ready and available for the most exacting project requirements.
Expertise in Silicone and Thermoplastic
Elastomer Tubing (TPE) Molding & Extrusion
Nearshoring capacity available in Mexico & Poland

Expertise in Vascular Access with proprietary catheter tipping expertise and processes

To learn more about partnering with Flexan, visit Flexan.com or scan the QR code.



From components and finished devices to the next generation of medical technologies, now more than ever, you can count on us.




























Implantable Pulse Generator Systems

Suppor t Catheters
























Strengthen your innovation with our rapid prototyping and clinical know-how to take you seamlessly from concept to production






















Batteries & Capacitors Neuro Leads Aspiration & Access Catheters

Delivery & Retrieval Introducers Guidewires


















Get to market faster by utilizing our platform technologies and market-ready products that provide differentiated performance and portfolio expansion
Extend your reach through our global R&D and manufacturing capabilities to consistently achieve design requirements, quality standards, and on-time delivery





















Cardiac Leads
















Imaging & Sensing









Delivery Systems Electrophysiology Catheters















IWhat
limited da Vinci 5 launch can teach other device developers
Intuitive Surgical plans updates to the da Vinci 5 — including capabilities for surgeons using the console (pictured) — before fully launching the next-generation surgical robotics system.
ntuitive says it can’t make enough of its new da Vinci 5 surgical robotics system to meet customer demand.

But that’s part of the plan for what executives are calling a “measured rollout” so Intuitive can tweak the next-generation system based on feedback from early adoptors like those who partnered with the company on the DV5’s development.
“Really strong start. … It looks like a strong launch,” Wells Fargo analyst Larry Biegelsen said, citing the 70 placements of DV5 in the second quarter.
There are three main items on Intuitive’s checklist ahead of a full-scale DV5 launch, Intuitive executives said on their second-quarter earnings call, offering a blueprint for other device developers to take the same approach with their own product launches.
1. Expanding manufacturing capacity With DV5 systems accounting for nearly half of Intuitive’s U.S. system placements in the second quarter, the device manufacturer is ramping up production as part of the limited launch.
Intuitive CFO Jamie Samath said
DV5 placements will “increase modestly quarter to quarter” and “will be constrained through the first half of 2025.”
About half of the 550 employees Intuitive hired in the quarter were in manufacturing operations to help meet customer demand, Samath said.
“We are maturing our supply chain and our manufacturing capacity so that we’re able to get to the quality, to the cost, to the yields that we expect as we approach a broad launch,” Intuitive President Dave Rosa said.
2. Updating hardware and software Samath said Intuitive is planning a hardware and software update for da Vinci 5 in the second half of 2024.
“You have to organize how you do that build plan through the factory carefully for that update,” he said.
Some of the first updates include the integration of Intuitive Hub capabilities, allowing surgeons to access and manipulate anatomical 3D models while they’re in the surgeon console, Rosa said, as well as access to intraoperative video for replay. >>
Intuitive Surgical’s da Vinci
5 includes new features and equipment like a built-in insufflator.
Image courtesy of Intuitive Surgical

Intuitive Surgical CFO
Jamie Samath

“We’re trying to get that integrated into our systems and balancing the factory output,” Rosa later added, noting that more updates will come as a result of feedback from early DV5 users.
“Then if you look a little further out, some intraoperative technology building blocks that we’re working on [include] procedure step mapping and 3D-depth mapping that will use some AI and [machine learning] algorithms and leverage this compute power of da Vinci 5,” Rosa said. “Those things will set us for some more advanced features in the future.”
3. Gathering customer feedback
Other updates are coming as a result of requests and input from surgeons and healthcare organizations that are already using DV5, Intuitive executives said.
Intuitive Surgical President

Intuitive Surgical CEO
Gary Guthart

“As these placements happen, we’re listening to our customers and responding to their feedback,” Rosa said. “We’ll be releasing some software updates along the way here in order to respond to that feedback, so we want to get that in place before we get to launch.”
Rosa said customer feedback is indicating improvements in precision, imaging, ergonomics and integration — all of which add up to improve overall efficiency, and possibly the ability for surgeons to perform one more procedure per day.
“We launch new products thoughtfully, centered around outstanding customer experiences as our customers pursue operational and clinical excellence,” Rosa said. “We continue monitoring several key metrics across our measured rollout of da Vinci 5.”
Customer feedback is not only helping Intuitive polish the DV5’s performance and features before a full launch, but helping the company determine how much demand there is from surgeons and healthcare systems.
“In the early phase of a launch, you have a tendency to see early adopters look for the latest technology, and also the larger institutions, the large IDNs (integrated delivery networks),” Samath said. “You have to give it some time to see the full set of customers in terms of what that interest will be.”
Customer feedback also helps generate data and anecdotes in support of the DV5 system to generate more demand and convert those who are DV5 curious into customers.
“Many customers will want to evaluate da Vinci 5, they’ll want to see evidence grow over time in terms of the capability and feature set,” Samath said. “And of course, their desire for da Vinci 5 will depend on their their individual program and what they see the value of that, in part given the higher pricing or lease costs for da Vinci 5.”
“As we move through our measured launch, we’ll continue to have to underscore and reinforce the value that it brings and communicate that to executives and their teams,” Rosa later added.
Intuitive expects DV5 to be evaluated not by cost compared to other systems, but with the quadruple or quintuple aim in mind, CEO Gary Guthart said. (The quintuple aim refers to five goals: better patient outcomes, better patient experience, better care team experience, lower total cost of care, and equitable access to care.)
“We’re confident that we’re going to get there. I think we’re going to develop the data to support that perspective,” he said. “The apples-toapples price differences aren’t that great once you add the insufflator and some of the other things that are built in, and we’ve just got to justify that difference.”
“On first reflection and on case insights, these are powerful, foundational, new capabilities that are going into the market,” he later continued. “I think that’s really interesting, and we will work with leading customers to start evaluating where do they make sense and where don’t they. We, of course, believe there are going to be procedures and patient populations where they make a ton of sense and they generate real value. So early, it’s about data generation and research and feedback. And later, we’ll start to drive that through clinical publications and other things. … What you get today: You get precision, you get imaging, you get ergonomics, you get throughput, and there’s some things that in this platform, you get to participate in its research and development. And that will come later. And then it’s a core technology platform upon which we can build. … That set of sequences gives us some confidence to keep pushing hard.”
As medical devices have evolved, we have advanced our capabilities to meet your increasingly complex design requirements. Backed by nearly 80 years of proven manufacturing expertise and a laser focus on minimally invasive surgical instruments, MICRO delivers the highest
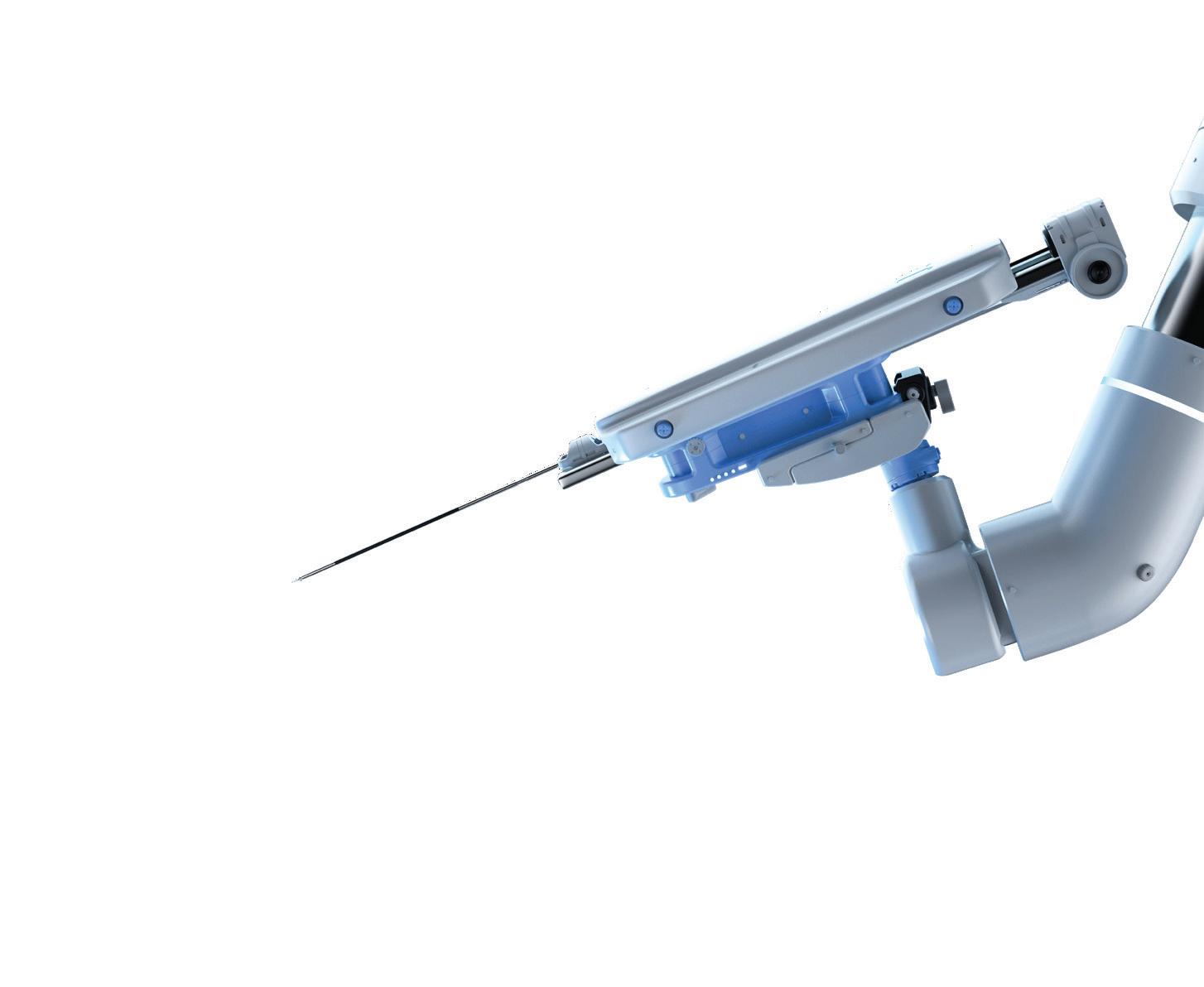






Medtronic leaders pick three technologies that are key for the future of endoscopy

ew Medtronic Endoscopy
President Raj Thomas and Medtronic Endoscopy Chief Medical Officer Dr. Austin Chiang say their business inside the world’s largest device company is betting on AI, robotics and connectivity as key technologies for the field’s future.
Medtronic Endoscopy has already demonstrated AI’s life-saving ability with their GI Genius system for colonoscopy, which uses deep learning to help physicians spot signs of colon cancer they otherwise might miss.

And Medtronic’s latest version of the PillCam capsule endoscopy kit takes a step forward in connectivity with a new adhesive, wearable link device that captures data from a swallowable PillCam inside a patient. That eliminates the need for patients to wear a bulky recorder and sensor belt — and means doctors don’t need to wait for that equipment to return before assigning it to another patient.
“The PillCam had been already approved for home ingestion and the vision — which currently as it is, isn’t entirely complete yet — is to save the patient from even having to go into the office at all,” Chiang said in an interview.
Asked by Medical Design & Outsourcing to identify key technologies for the future of endoscopy, here’s what Thomas and Chiang said (the following has been lightly edited for clarity and space):
1. Artificial intelligence
Thomas: “For us in endoscopy, it’s AI where we can improve patient outcomes by helping with detection or reducing human error. And I don’t mean that in a derogatory term, but instead like the second set of eyes that a physician has on whatever they’re doing. And then it’s just efficiency in the procedures for two aspects: the patient outcomes, but also being efficient in the procedures that start from the consent to the actual documentation. If you can be more efficient there or AI can help the physician there, then they’re freeing up time to actually focus on patient care versus documenting and all the different things.”
Medtronic Endoscopy Chief Medical Officer
Dr. Austin Chiang wearing the link device for the latest-generation PillCam (right) and the previous generation’s wearable recorder (left) Photo courtesy of Medtronic
“Within GI Genius, we have the AI Access platform,” Thomas later continued. “We’re working with third parties to bring apps with AI in the endoscopy space that will be developed and then added to our platform. Where we have been less specific but we need to get after is where else can AI apply within what we do: How does EndoFLIP (endoluminal functional lumen imaging probe) or Barrx or Bravo or PillCam fit into an AI ecosystem? That we are continuing to work on. We have highlevel plans, but that is in the forefront because I believe we have an advantage at the moment in our thinking around AI and we have the GI Genius — the box itself — that will support it. So how can we provide the extra value to our physicians and patients using AI? We do think about it a lot, and there will be more to come. AI Access is the closest thing to be talking about.”
Chiang: “I would echo everything Raj just said there about AI. … There’s still a lot of ways that not only AI, but also robotics, can help expand what we can do clinically. A lot of gastroenterology has been taking surgical procedures and making them less invasive. Both AI and robotics can help facilitate that and allow us to do things that we previously weren’t able to do and improve patient outcomes that way.”
Thomas: “The last one is device connectivity. With AI, how do you bring the data into the ecosystem, which helps with patient outcomes but also that efficiency? That’s how I’ve started to think about it from mostly an endoscopy standpoint, but there’s certainly applicability within med device.”

Chiang: “On connectivity, our newly FDA-cleared iteration for PillCam includes the link device and improves the patient experience. Connectivity can help with improving the whole patient experience around the care that they’re getting and the relationship with medical devices. That’s really important. Right now, Raj is very closely involved in this new movement that we have within Medtronic around patient centricity and involving patients at every step of the way of the innovation process and improving patient comfort and experience with the product. That aspect is probably very relevant to a lot of engineers who are in Medical Design & Outsourcing‘s audience in terms of human-centered design and ensuring that this isn’t just fulfilling a clinical need, but also helping with how the patient interacts with the product.”
DDL has over 30 years of experience navigating complex testing standards and regulations for medical device and pharma products. Our reliable quality, responsive attention, and on-schedule completion for packaging, product and materials testing secure confidence in performance and safety while achieving regulatory compliance.
TESTING EXPERTISE
• Packaging & Materials
• Medical Devices & Combination Products
• Dimensioning Services
• Consulting Services
Partner with us and gain a reliable testing expert at your side! Visit us at DDLtesting.com or call us at 1-800-229-4235
Minnesota | California | New Jersey
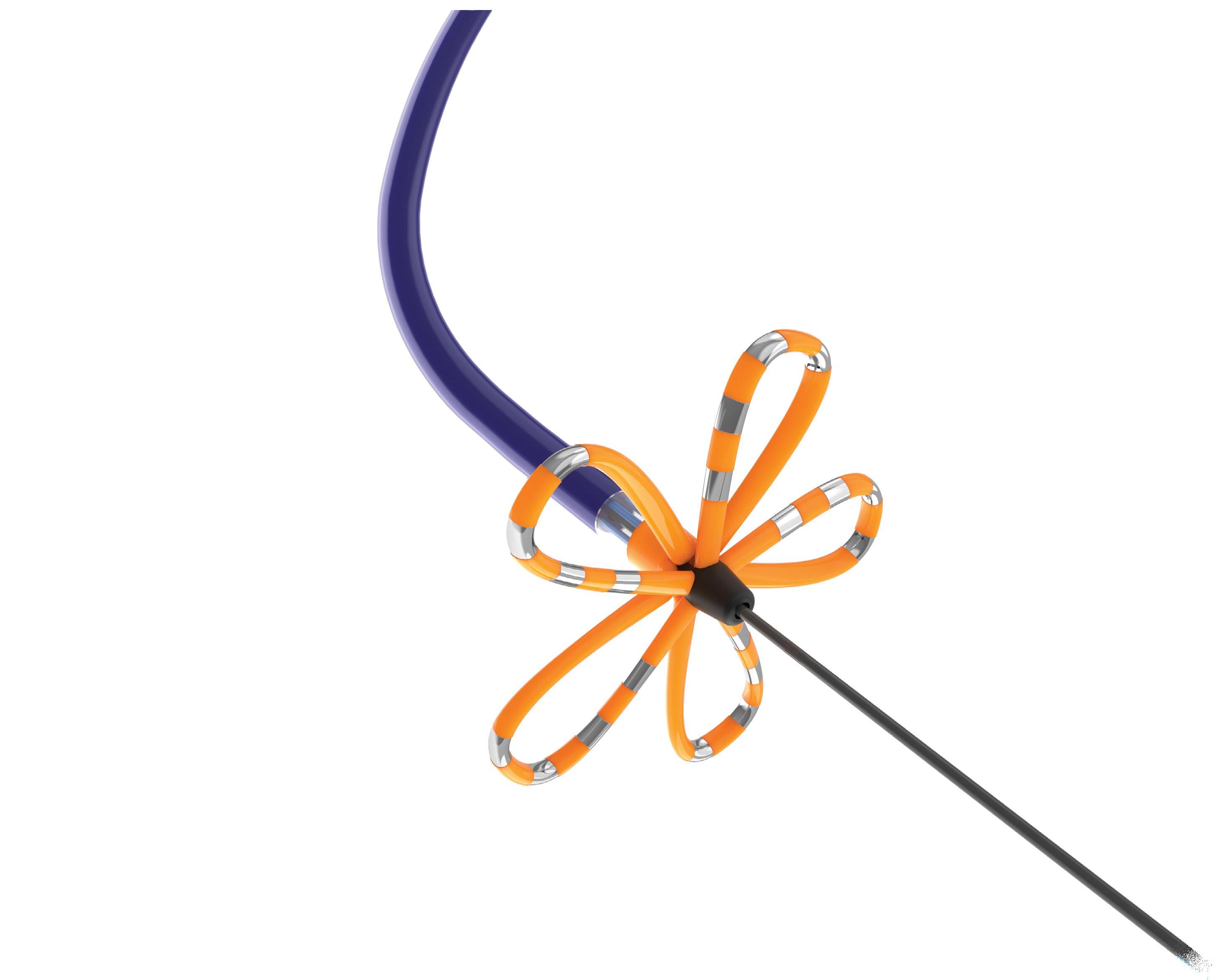

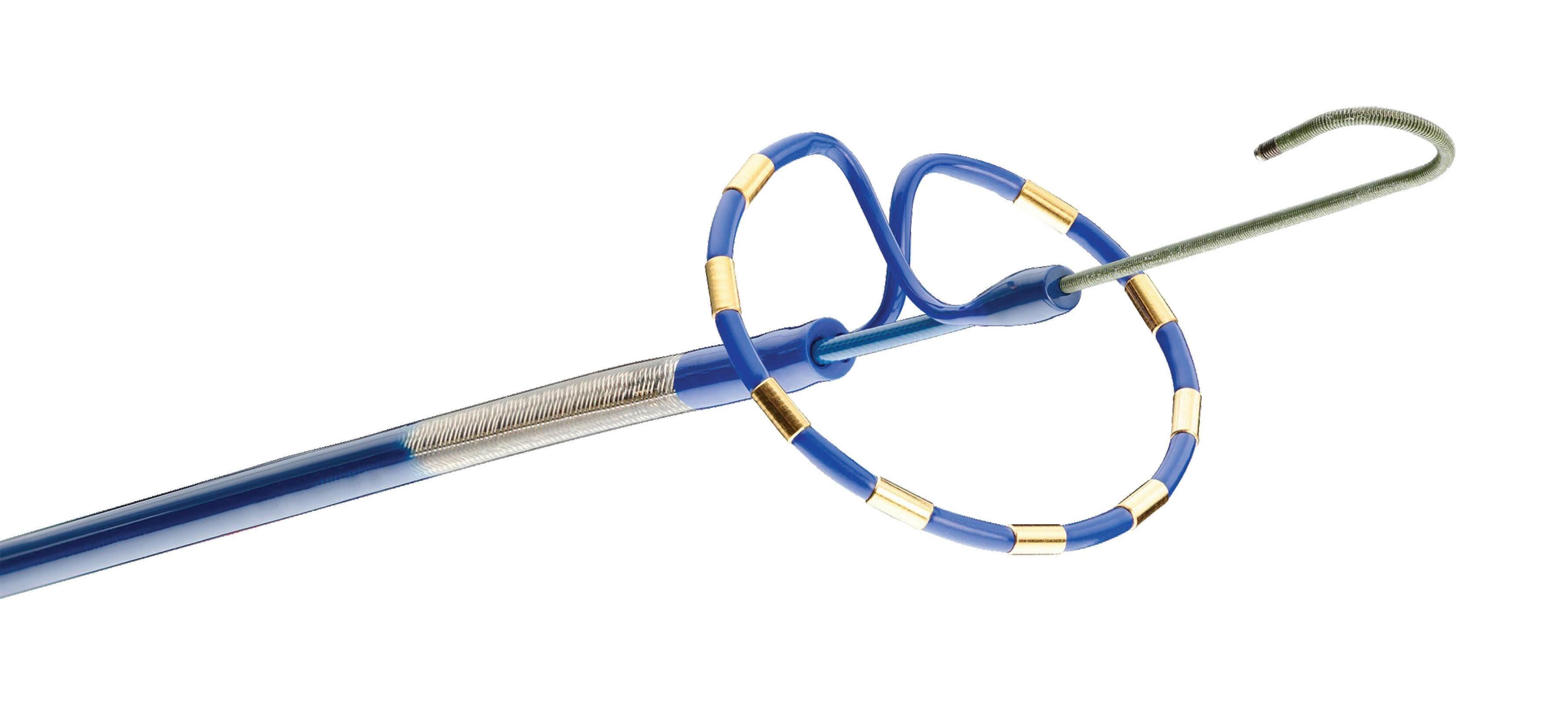

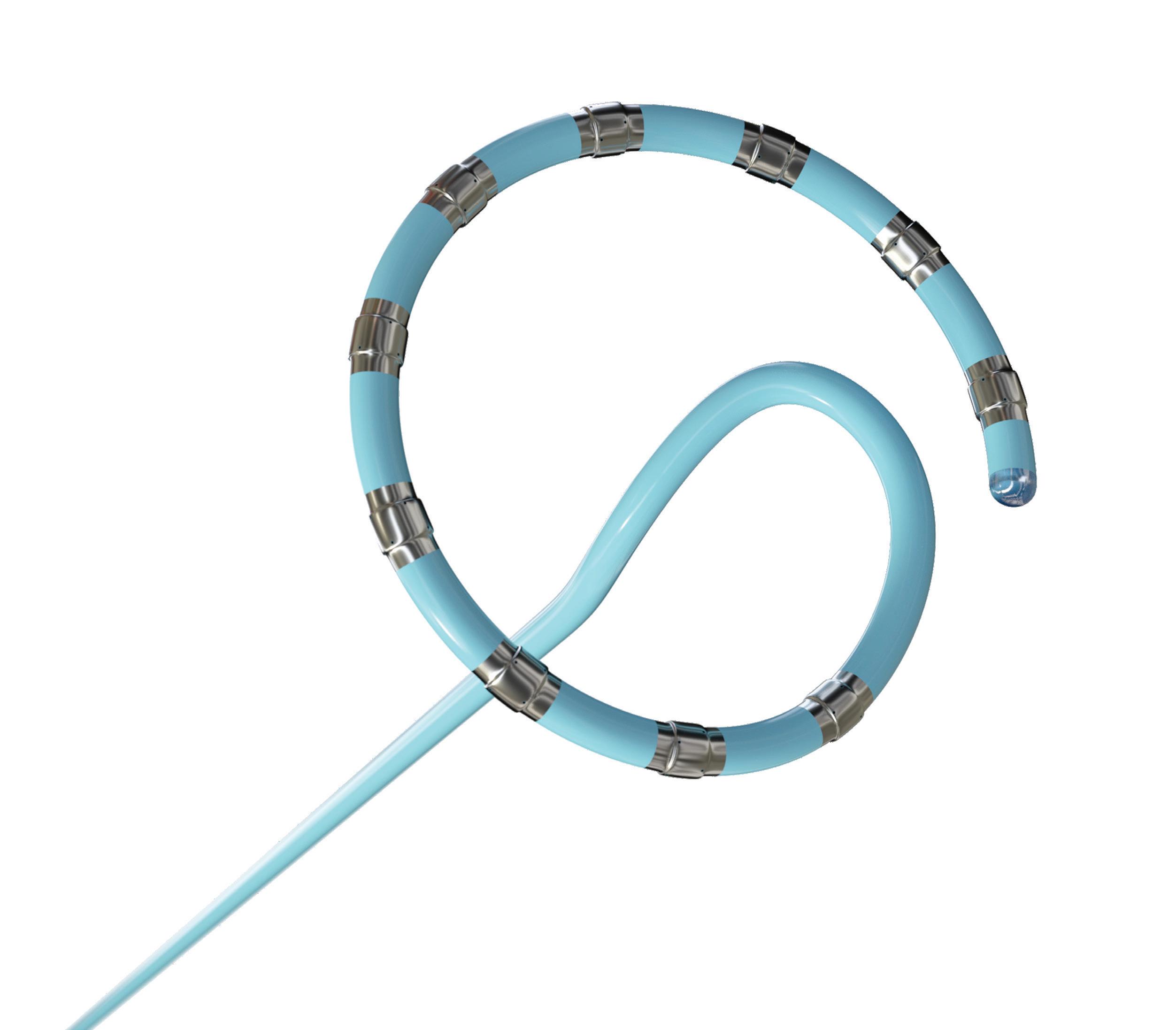

The Medtronic PulseSelect pulsed field ablation (PFA) system was first to win FDA approval to treat paroxysmal and persistent atrial fibrillation (AFIb).
Image courtesy of Medtronic



from flowers and loops to globes, baskets and balloons, there’s no standard shape for the pulsed field ablation (PFA) catheters coming out of Medtronic, Boston Scientific, Abbott and Johnson & Johnson MedTech’s Biosense Webster.
PFA’s tissue-selective energy kills cardiomyocytes (heart muscle cells) to block irregular signals that cause atrial fibrillation (AFib), but spares phrenic nerves and nerves in the esophagus.
These PFA catheter shapes offer unique abilities for cardiac ablation to treat AFib, said engineers and leaders at these companies — some of the world’s largest medical device manufacturers — in interviews with Medical Design & Outsourcing.
In late 2023, Medtronic’s PulseSelect became the first PFA system to win FDA approval for treating AFib. Medtronic tested other form factors — such as focal and linear catheters — before choosing the 25 mm loop shape for PulseSelect, said Tim Laske, VP of research and business development for Medtronic Cardiac Ablation Solutions.
“PulseSelect has a nitinol superstructure, which allows it to compress down [for delivery] through a 10-Fr catheter, and then it’s deployed in a very predictable shape,” Laske said.
The catheter also “has a 20-degree forward cant that gives you an indication of when you’re in contact with a tissue,” he said. “Then as you make contact, it also biases toward all of the electrodes touching the tissue, not just the ones that are in contact.”
PulseSelect’s loop shape allows for single-shot ablation, which is the delivery of energy across multiple parts of the pulmonary vein for a procedure that can be simpler and faster.
Medtronic is also advancing PFA catheters developed by Affera, which Medtronic bought in 2022.
Medtronic Affera Sphere-9 and Sphere 360 Affera’s Sphere-9 and Sphere-360 catheters both use their nitinol lattice as a single spherical electrode to deliver energy for ablation. When released from its introducer inside a patient’s heart, the Sphere-9 expands into a globe shape for focal ablation. >>
EXPERTS FROM MEDTECH BIG 100 DEVICE DEVELOPERS — MEDTRONIC, JOHNSON & JOHNSON MEDTECH, ABBOTT AND BOSTON SCIENTIFIC — DISCUSS THE VARIOUS SHAPES OF THEIR PULSED FIELD ABLATION (PFA) CATHETERS.
JIM HAMMERAND MANAGING EDITOR

Medtronic’s Affera Sphere-360 PFA catheter is adjustable, allowing it to take different shapes when deployed inside a patient. Image courtesy of Medtronic
The larger Sphere-360, however, is a single-shot catheter that’s adjustable for different shapes. That includes a sphere, a linear configuration, a pancake shape for a maximum diameter of 34 mm, or anything in between.
The Affera catheters’ nitinol lattices are both compliant, meaning they flex when forced against heart tissue, adjusting to the pulmonary vein’s shape for better contact and more effective ablation, Laske said.
“Nitinol is superelastic, and depending on how you design the struts, you can have more or less stiffness associated with that. So we want a catheter that will give you good contact with the tissue,” he said. “If you apply additional force, it’ll tend to just flatten a bit — particularly true with the Sphere-9 — and adjust to the pulmonary vein’s shape. The compliance gives you a little more forgiveness in ensuring that you have good, robust contact across the entire surface you’re trying to ablate.”
The FDA has not yet authorized Affera’s PFA catheters for treating AFib, though the Sphere-9 device is under review.
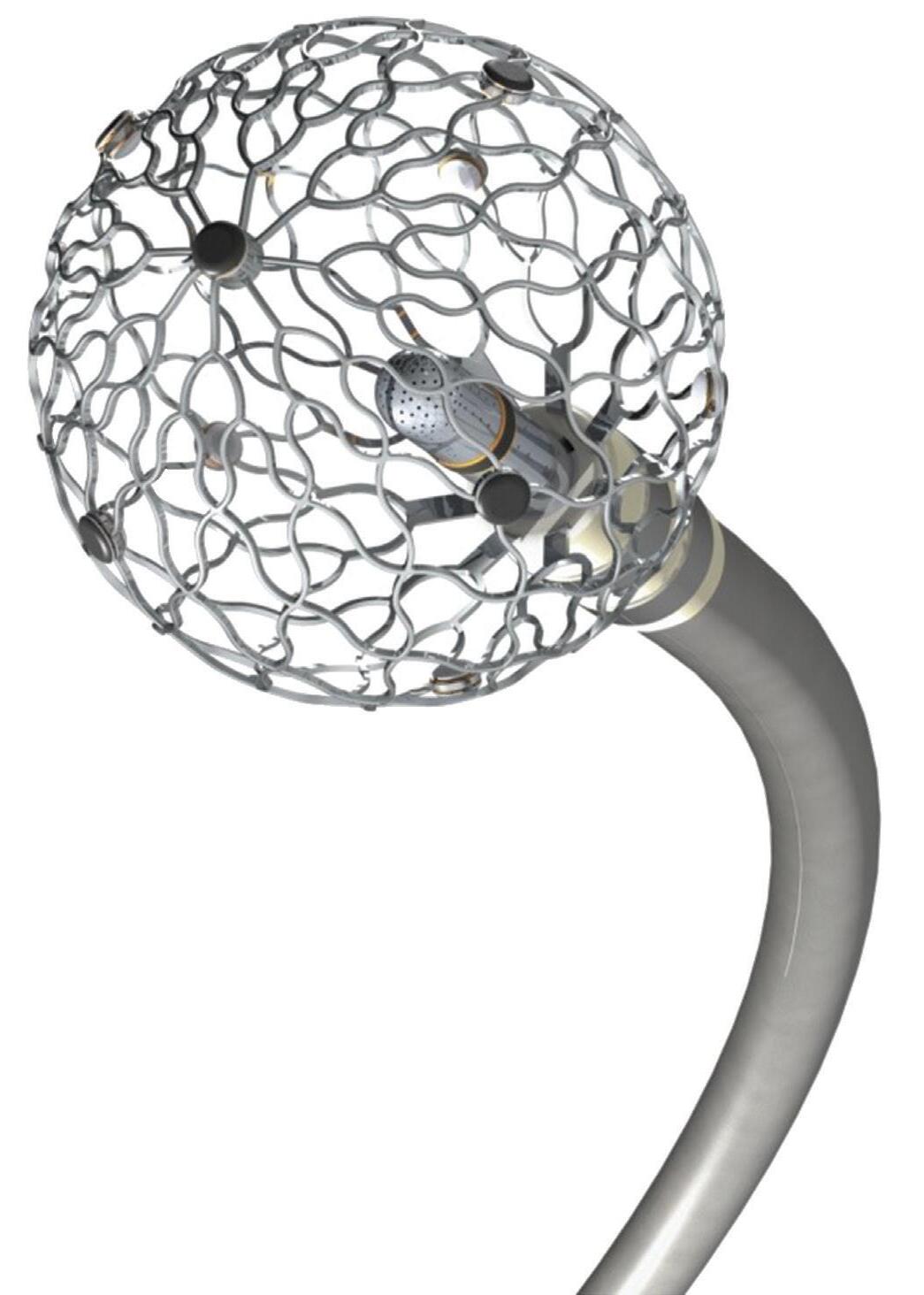
Read more about the design and features of the Affera catheters at wtwh.me/Sphere9 and wtwh.me/Sphere360

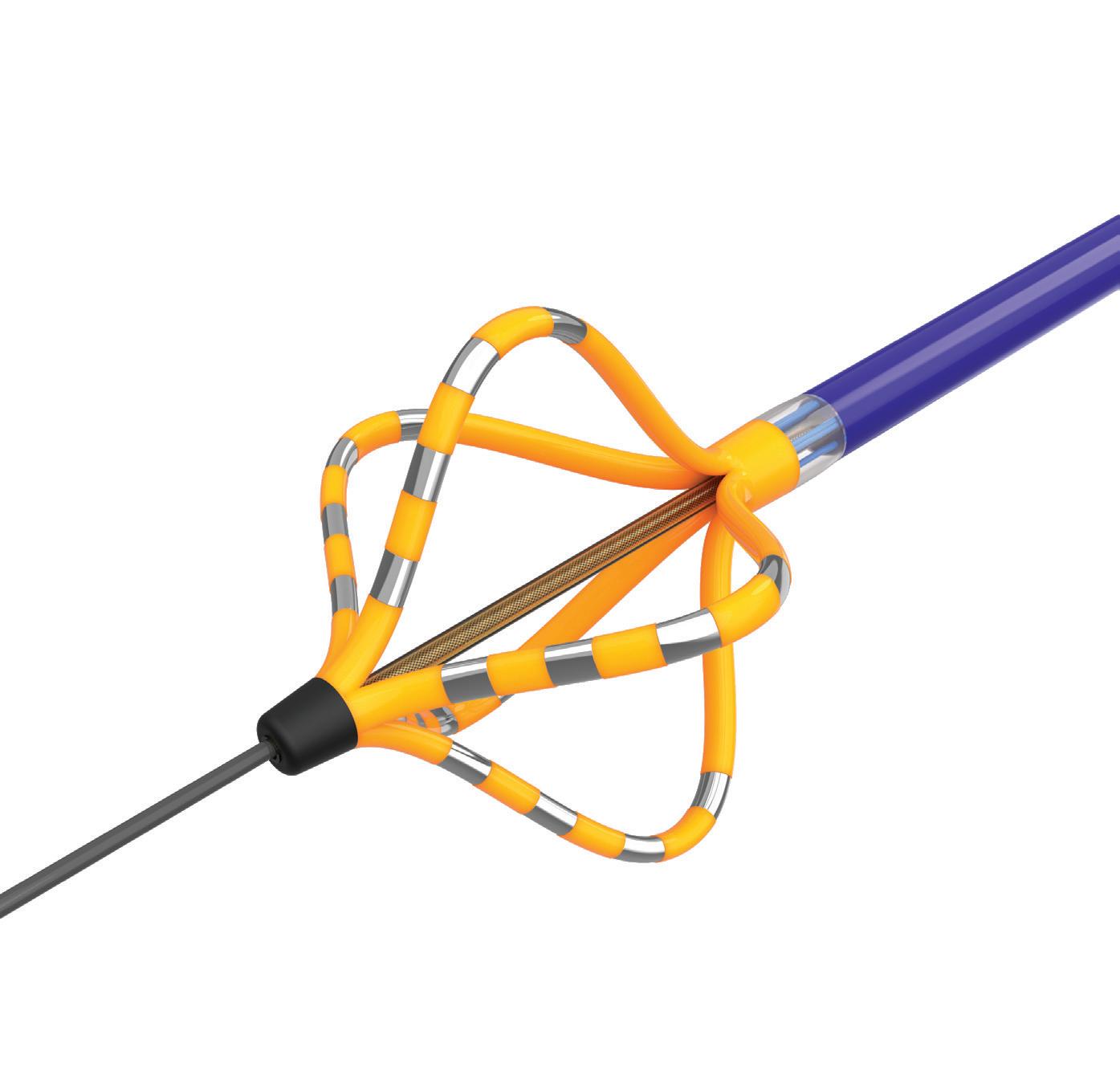
Boston Scientific Farawave
Boston Scientific was the second PFA catheter manufacturer to win FDA approval with its Farapulse system.
The Farapulse system’s Farawave PFA catheter can adapt to individual patient anatomies with its variable basket and flower shapes.
The single-shot catheter’s shape is also important — along with the waveform — in creating the shape of the electric field for tissue selectivity to kill cardiomyocytes but not nerves, Boston Scientific SVP and Global Chief Medical Officer Dr. Ken Stein said.
“If you can create the right shape of an electrical field — that gets back to catheter design — and if you provide just the right amount of energy — and that gets to things like the waveform — then you can tune it in a way that is relatively selective for heart tissue,” he said.
“Compliance gives you a little more forgiveness in ensuring that you have good, robust contact.”
Abbott Volt
Abbott designed its balloonin-basket Volt PFA catheter to support the efficient deployment of energy into the heart tissue, using electrodes that only face outward and insulated nitinol splines that are flat, not round. It’s a hybrid catheter that can be used for both single-shot and focal ablation.
Abbott said the investigational catheter’s design improves the accuracy, quality and efficiency of ablation to minimize the number of applications needed to treat a patient. The balloon acts as an insulator for the blood inside the patient’s beating heart, reducing the incidence of hemolysis and thermal effects such as bubble formation.
(continued on page 88)





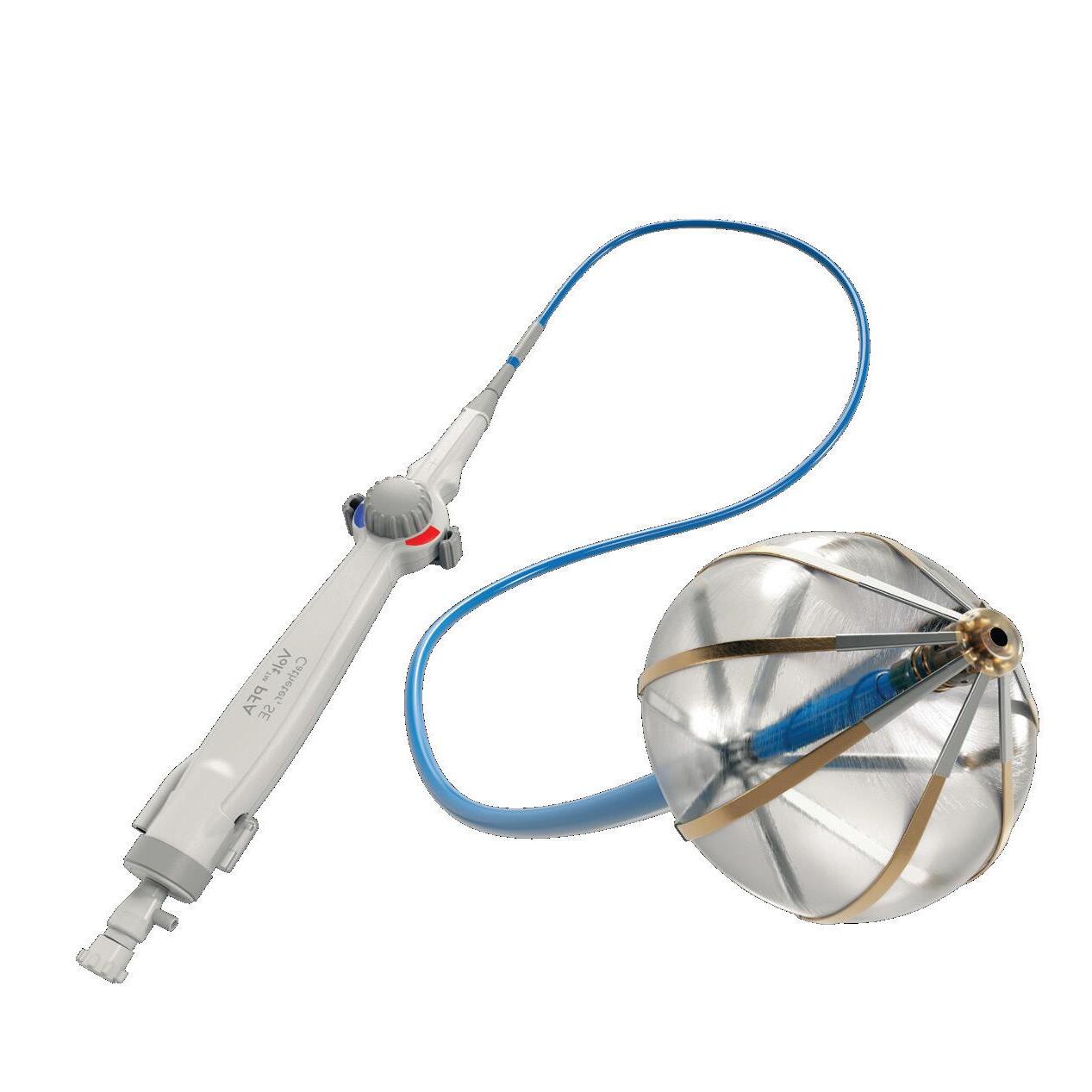
(continued from page 86)
The balloon also helps stabilize the basket inside the pulmonary vein, where the device’s round shape allows a physician to place the catheter at the ablation target site, apply energy, and rotate the device slightly to achieve a spline offset for a second energy application.
“We have data showing if you use a balloon versus a basket without the balloon it makes a difference [in] lesion depths by 20% to 30%,” said Dr. Christopher Piorkowski, the chief medical officer of Abbott’s electrophysiology division.
Abbott is testing the Volt system under an FDA investigational device exemption (IDE) and anticipates European approval in the form of a CE mark in 2025.
“We’ve invested heavily in our PFA portfolio, which you will start to see hit the market … definitely next year,” Abbott CEO Robert Ford said in July.
Johnson & Johnson MedTech
Biosense Webster Varipulse
Biosense Webster went with a loop shape for its Varipulse catheter, which can deliver single-shot therapy with all 10 electrodes, as well as more focused, segmental energy with just six electrodes to combine the best features of both types.
The pre-shaped Varipulse catheter is made of laser-cut nitinol, allowing it to curl into an adjustable, contractable loop to help ensure adequate tissue contact among varying cardiac anatomies, and making it easier to maneuver the catheter inside the cramped left atrium.
“It’s not limited to applying it in other areas of the heart, which might be some of the limitations of a flower configuration or a balloon configuration,” said Biosense Webster Senior Director of Medical Affairs Tushar Sharma. “In terms of electrode spacing … is it consistent, or is it changing? The loop-style catheter really allows us to keep that spacing consistent irrespective of the areas of the heart that we are applying the therapy to.”
Biosense Webster won a CE mark for the Varipulse catheter in February 2024 and filed for FDA approval in March.
— Associate Editor Sean Whooley contributed to this report.
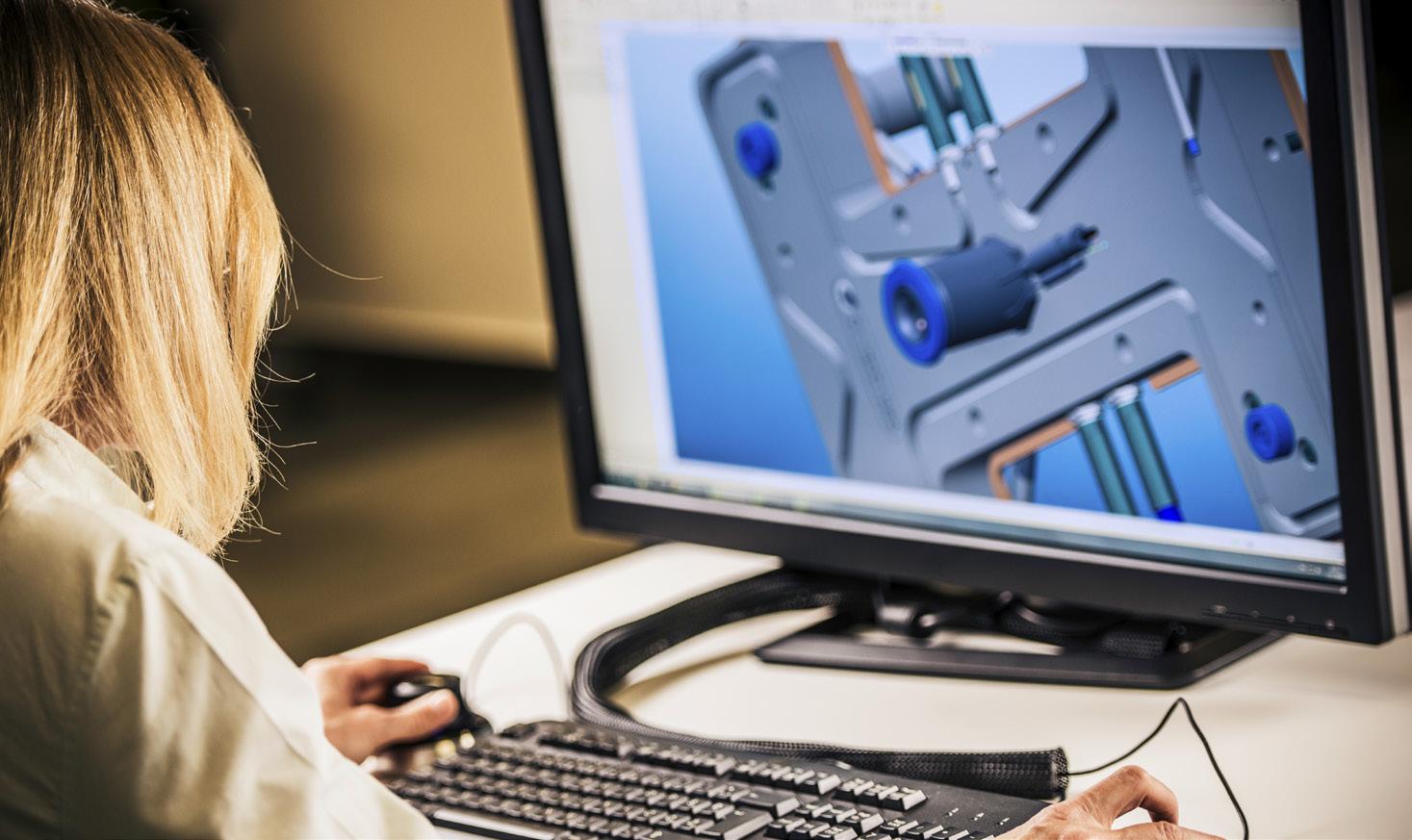
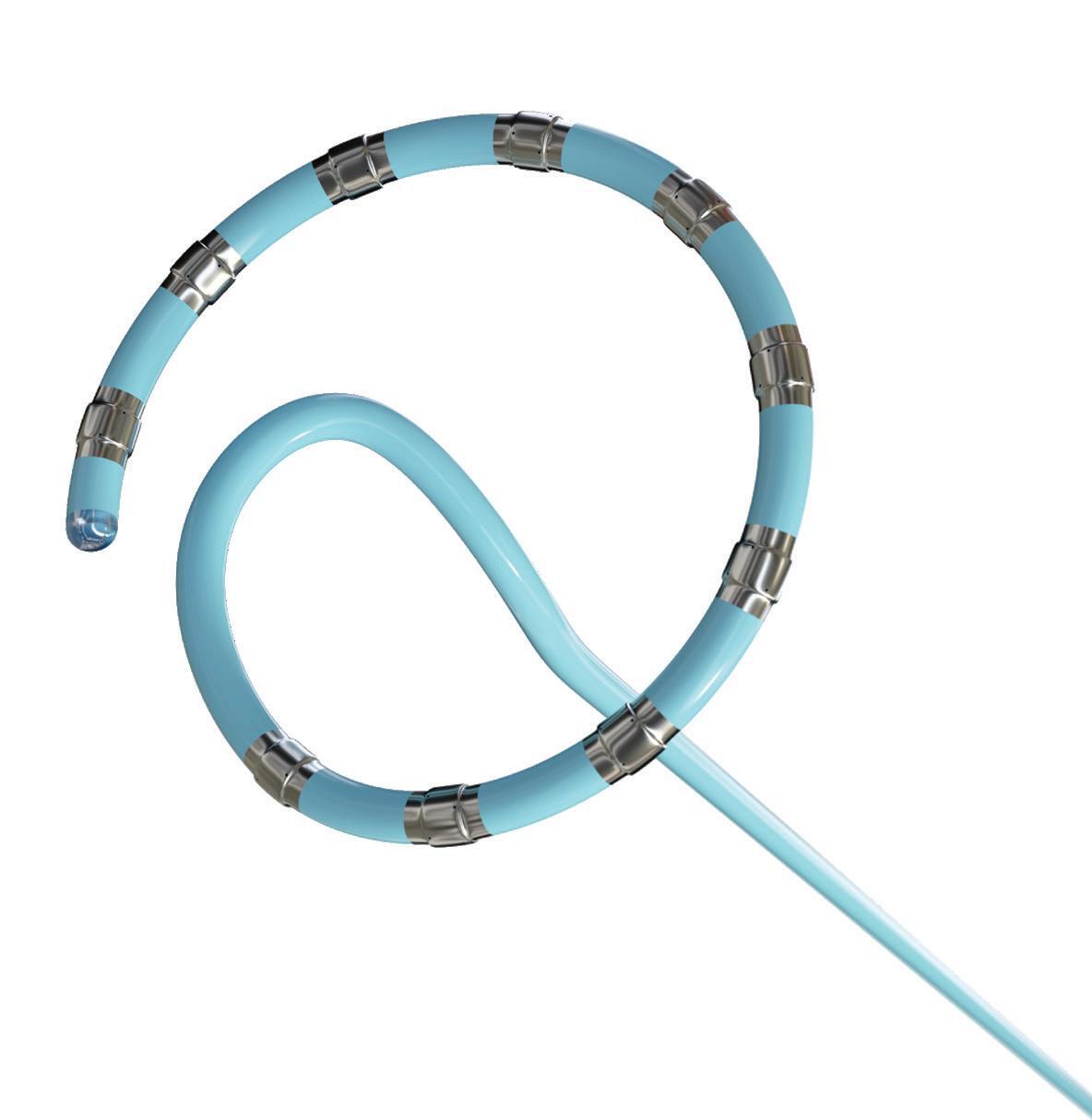
















Robust Interpower® hospital-grade cords are manufactured beyond minimum agency standards. Interpower NEMA and international hospital-grade cords provide correct amperages and voltages for medical devices—portable CT scanners, X-ray machines, medical-grade treadmills, and ECMO machines—essential machines demanding essential power. Interpower cords are hospital-grade torture tested!
When designing, manufacturing, and maintaining hospital-grade products used worldwide, it is vital to know the medical requirements of the country of export—select hospitals in Australia, Canada, Denmark, Japan, and the United States have proprietary requirements. Interpower hospital-grade cords surpass UL 817 (18.2.4.1) requirements, and the C22.2 No. 21-14 requirements for hospital-grade cords and cord sets through rigorous testing.
Our testing facility in Ames, Iowa, was designed to meet or exceed many of the testing criteria used at various test agencies worldwide. Also, every cord and component made in Oskaloosa and Lamoni is subjected to in-house testing and inspection after each phase of production via Interpower ’s strict quality control procedures and tortuous testing.
• 1-week lead-times
• Same-day shipping on in-stock products
• No minimum orders

MORE
JIM HAMMERAND MANAGING EDITOR
FDA APPROVAL — AND TO EXPLAIN HOW THE IMPLANT
It’s been three years since the FDA denied approval to the Neovasc Reducer system for treating refractory angina.
At the time, the device developer already had a CE mark on the catheter-delivered coronary implant and breakthrough device designation from the FDA, but struggled to explain exactly how it worked. That was one of the shortcomings identified in October 2020 by the Circulatory System Devices Panel of the Medical Devices Advisory Committee to the FDA when
nearly all of those experts said they were not convinced of the system’s effectiveness.
Now Shockwave Medical owns the technology through its 2023 acquisition of Neovasc. The intravascular lithotripsy (IVL) system developer — itself purchased by Johnson & Johnson for $13.1 billion in May 2024 — is conducting clinical trials to support Reducer’s next shot at FDA review, aiming for approval sometime around 2027.
Shockwave sees a $5 billion
market for Reducer, estimating that 500,000 patients in the U.S. and Europe have refractory angina with no obstructive coronary arteries (ANOCA) each year. The company believes the prevalence of refractory angina will increase due to “progressively longer life expectancy of patients.”

The Shockwave Reducer procedure Shockwave Medical is best known for its IVL systems that fracture arterial calcium before implantation of a vessel-expanding stent for blood flow restoration. >>
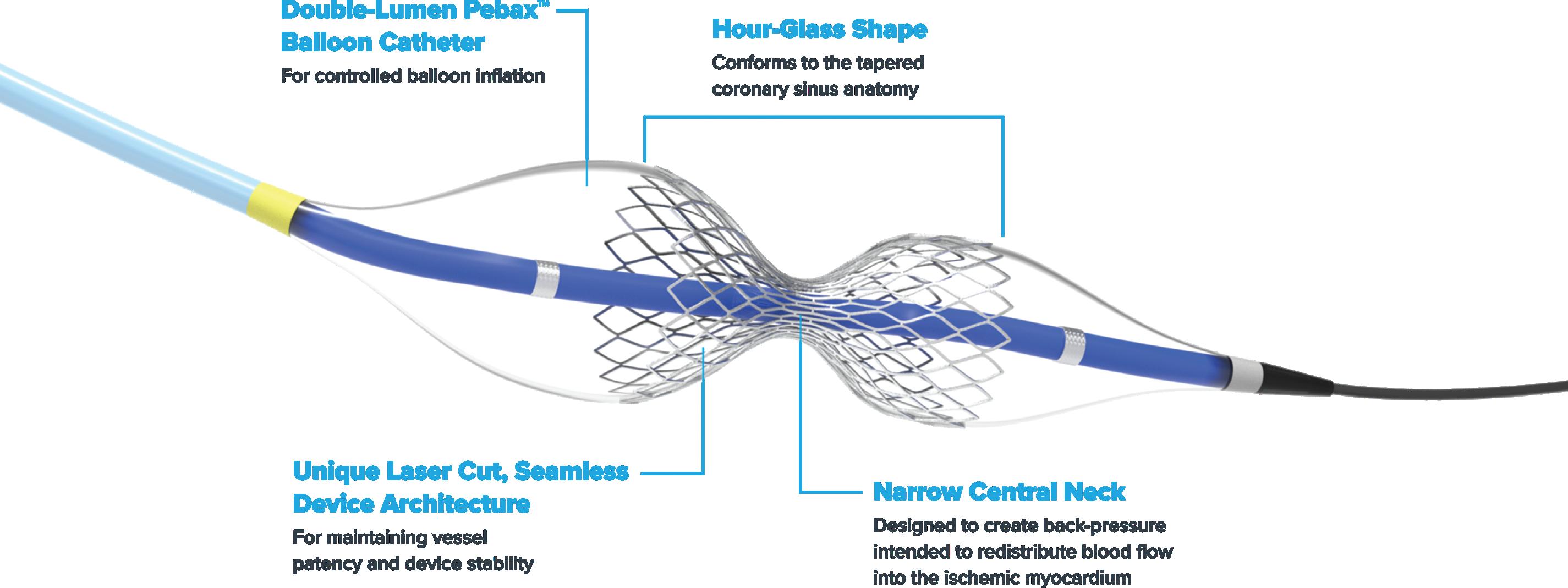
While the investigational Reducer system is also designed for revascularization, Shockwave says it does this by narrowing the heart’s largest vein, the coronary sinus, where deoxygenated blood drains into the right atrium.
The Reducer implant is an hourglass-shaped, bare metal device made of laser-cut stainless steel. This balloon-expandable implant is placed in the heart’s coronary sinus with a minimally invasive catheter procedure.
It starts with an ultrasound-guided catheter puncture in a patient’s jugular vein, first with a 6-Fr multipurpose angiographic (MPA) catheter sheath and then a 9-Fr delivery sheath, according to Dr. Hakim Benamer, a paid Shockwave consultant who discussed Reducer at Euro PCR, the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
This “mother-child” catheter technique allows for pressure monitoring with the MPA catheter sheath inside the delivery sheath. A contrast injection confirms positioning and appropriate oversizing of 1020% compared to the diameter of the coronary sinus before the balloon expands the Reducer device.
“The perfect landing zone is when you have a valve just after the angulation and before the distal branch of the sinus,” said Benamer, an interventional cardiologist at Hôpital Privé Jacques Cartier in France.
Shockwave says the implantation procedure requires minimal training and takes about 20 minutes once the 0.035-in. guidewire has accessed the coronary sinus.
How the Reducer limits flow to relieve angina symptoms
Understanding the mechanism of action of a drug or device can help physicians decide whether and how to use it to treat a patient. But it’s not always clear how drugs or devices work, said Dr. James Spratt, consultant cardiologist at St George’s University NHS Trust in London.
“Metformin is … probably the most prescribed drug in the world, which we know works really well for lots of things, but we still don’t really know how it does it,” Spratt said. “It’s not always clear how things work even though they have clear benefit.”
Spratt, who like Benamer is also a paid consultant to Shockwave, explained the Reducer’s proposed mechanism of action in another 2024 presentation at Euro PCR.
The Reducer implant’s hourglass shape was designed to conform to the
The investigational Shockwave Reducer is made of laser-cut stainless steel and delivered via a minimally invasive catheter procedure. Image courtesy of Shockwave
tapered anatomy of the coronary sinus, allowing for placement as long as the narrowest part of the vein is less than 13 mm in diameter.
The device’s narrow central neck throttles the flow of blood through the coronary sinus, creating back-pressure. Shockwave says that pressure redistributes blood into a patient’s ischemic myocardium (heart muscle), relieving the symptoms of refractory angina.
“We put in the Reducer [and] we increase the resistance to the venous draining, and like with any flow system that pressure is translated back to the venous system,” Spratt said. “… That reinflates the venous system and we get this kind of pressure transmission across the arterial side and we get reinflation of these compressed arterials.”
“This is the hard bit to get your head around, how the increase in venous pressure could lead to a





reduction in resistance to arterial flow,” he continued. “I think probably the closest medical equivalent is CPAP where you have compressed lungs, you put pressure into the lungs and you reinflate the lungs, therefore you get reduced resistance of flow.”
The back-pressure from the Reducer implant is designed to improve perfusion of the endocardium, helping to relieve ischemia and chest pain, shortness of breath and other debilitating symptoms, Shockwave says.
While Spratt said he himself is still improving his understanding of the complex physiology at play, the procedure itself doesn’t have much of a learning curve.
“I think you’re comfortable [after placing] five implants,” he said. “Having a background in complex PCI (percutaneous coronary intervention) is pretty helpful because we see those














patients in need and we also — I hope — understand the difference between futility and feasability and also the risk that some of these patients are [taking]. In my practice I would probably consider the Reducer in patients I wouldn’t have done five or 10 years ago, particularly postbypass patients with diffuse disease.”
“I also think we all need to take a step back and be a bit more realistic about what revascularization does achieve [and] the limits of revascularization,” he continued. “Physiology’s got a lot to teach us about what revascularization can achieve. Some humility in the field would be probably overdue.”
VIDEO: See how Shockwave’s Reducer implant works at wtwh.me/reducer




















Tom
Salemi | DeviceTalks Editorial Director |
DeviceTalks West 2024 in California will focus even more on connectivity, interventional tech, neurotech, structural heart, robotics and women’s health.
We’ve had great success with our redesigned DeviceTalks conferences over the past two years, drawing thousands of people and double-digit percentage increases to our events in Boston and Santa Clara, California.
But we can do better.
On Oct. 16-17 at the Santa Clara Convention Center, we’re building our DeviceTalks West agenda around the technologies that are driving the medtech industry on the West Coast and beyond. We’re still inviting the best engineering, manufacturing and operational speakers, but structuring our talks to focus on these critical areas.

Stryker Medsurg and Neurotech President Andy Pierce
1. Connected health
Medical devices will need to understand how to communicate with patients, physicians and each other. To explore this, we’ll have keynote interviews with Baxter EVP and Medical Product and Therapies President Heather Knight and Stryker Medsurg and Neurotechnology President Andy Pierce explaining how connected tools are built into new product releases. We’ll also have leaders from Biolinq, NVIDIA, Philips, Sovato and others revealing their use of connected technologies.

Abbott Vascular Division VP of R&D Richard Rapoza
2. Interventional technologies
More and more care can be delivered through a minimally invasive catheter. How? Penumbra CEO Adam Elsesser will share details about his company’s
growing interventional vascular and neurovascular portfolio. Medtronic SVP and Coronary and Renal Denervation SVP Jason Weidman will discuss the Symplicity Spyral system’s long journey to FDA approval. Abbott Vascular Division VP of R&D Richard Rapoza will unveil the latest innovations in bioresorbable technology. Kardium President Doug Goertzen will share details on its innovative tool to deliver pulsed field ablation.

structural heart business. Watch for more details on topics and speakers soon.
Noah Medical VP of Engineering
John Shen

Boston Scientific Neuromodulation VP and Chief Medical Officer Dr. Ray Baker
The neuro space is growing rapidly, particularly those tools using energy to create or block neural pathways. Boston Scientific Neuromodulation VP and Chief Medical Officer Dr. Ray Baker and Product Development and Operations VP Brian Donovan will break down the power behind the Relievant Intracept ablation procedure for vertebrogenic pain relief. In a different session, Kandu Health CEO Kirsten Carroll and Neurolutions CEO Leo Petrossian will show how an exoskeleton equipped with a brain-computer interface is helping stroke patients increase functional independence and accelerate motor recovery.

Director of R&D for Abbott Structural Heart
Ania Snell
The potential of this space is enormous. Abbott Structural Heart R&D Director Ania Snell will speak to Abbott’s portfolio. Meanwhile, Edwards Lifesciences leaders will share details on their aggressive plan to build out a
robotics. Endiatx CEO Torrey Smith will speak to the power and potential of the swallowable PillBot in a joint keynote for DeviceTalks West and our adjacent RoboBusiness meeting. Virtual Incision CEO John Murphy will introduce the marvels of the MIRA surgical robotic system, which has FDA de novo authorization for colon resection surgery. Moon Surgical Chief Technology Officer David Noonan will discuss the value of the FDA-cleared Maestro System. Meanwhile, attendees will learn more about Noah Surgical’s Galaxy System for lung cancer from Engineering VP John Shen. You’ll also hear from Intuitive leaders about the da Vinci 5 system’s clearance.

Abbott Global Clinical Affairs Divisional VP Jennifer Jones-McMeans
6. Women’s health
DeviceTalks Managing Editor Kayleen Brown is organizing a one-day track dedicated to women’s health. Speakers include Abbott Global Clinical Affairs Divisional VP Jennifer JonesMcMeans and Dr. Shirin Towfigh, CEO of Hexagon Health and president of Beverly Hills Hernia Center. Look for more speakers to be announced, including a keynote interview during our Women in Medtech luncheon.
Join us at DeviceTalks West to hear from these great speakers and more.


Ryan Ashdown 216.316.6691 rashdown@wtwhmedia.com
Jami Brownlee 224.760.1055 jbrownlee@wtwhmedia.com
Ashley N. Burk 737.615.8452 aburk@wtwhmedia.com
Mary Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Jim Dempsey 216.387.1916 jdempsey@wtwhmedia.com
Mike Francesconi mfrancesconi@wtwhmedia. com
630.488.9029
Courtney Nagle cseel@wtwhmedia.com 440.523.1685
Jim Powers jpowers@wtwhmedia.com 312.925.7793
Brian Toole 267.290.2386 btoole@wtwhmedia.com