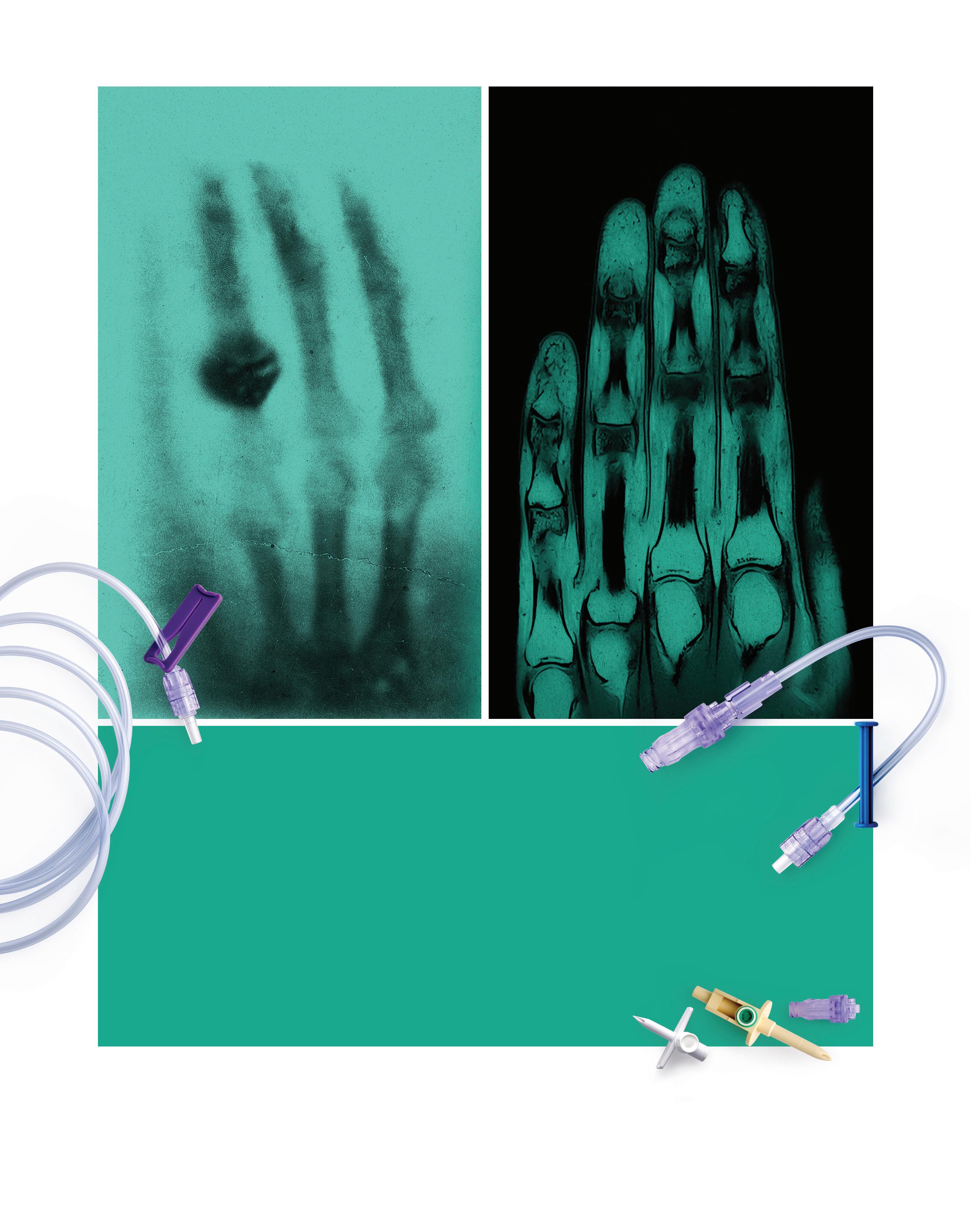
With off-the-shelf materials in the most common sizes, our Quick Turn Catheter Shafts put functional parts in your hands faster.
Our LCT reinforced Quick Turn Catheter Shaft offering streamlines your prototyping process, enabling you to bring your innovations to market faster. With a focus on precision and efficiency, we help compress your lead times by delivering superior catheter shafts in as little as two weeks. Simply provide us with your size specifications and application details, and we’ll handle the rest.
Scan to learn more about the Quick Turn Catheter Shaft.



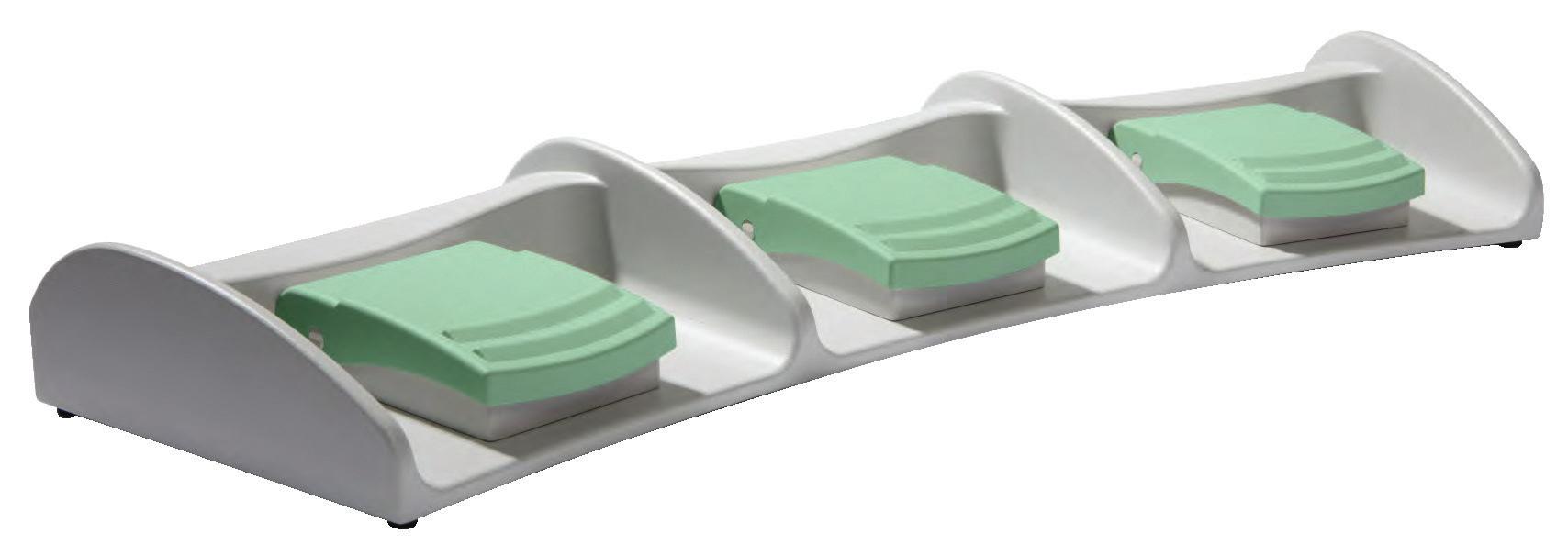
Designed to your specifications.

• Available in 1, 2 and 3 pedal designs
• Robust and slip resistant
• High quality materials, such as Aluminum cast alloy and reinforced polyamide
• Environmental protection level up to IP X8 per IEC/EN 60529
• IEC 60601-1 certi cation
• Easily cleaned and hygienic
• Ergonomic design with intuitive actuation

• Custom colors, markings, circuits, cable types and lengths available





Compact, one pedal foot switch Standard Features
• Housing and Pedal Cover: Polyamide protection upto IP X8
• Protection Level: IP 43 and IP 65
• Switch Insert: Snap action microswitch, cCSAus approved
• Electrical Rating: 5A/125-250V AC
• Contact Types: Momentary, maintained, or two stage momentary
• Circuit: SPDT-SB, DPDT-SB, 2x SPDT-SB
• Strain Relief: PP Cable Gland
• With or Without Cable
• Cable: Multi conductor, PVC, SJT, 2 m (6 ft. - 6 in.)
• Temperature Range: -20°C up to +80°C (-4°F up to +176°F)
• Protection Level: up to IP68
• Switch Insert:
- Gold plated contacts for low current applications
Electrical Rating: 0.1A/125V AC
- Silver-Nickel plated contacts
Electrical Rating: 10.1A, (1/4HP)/125-250V AC
• Metal Top Guard or Heavy Metal Base
• Strain Relief: Per customer speci cations
• Cable: Per customer speci cations
• Connectors: Per customer speci cations







Certification to the latest medical standards:
• 60601-1 General requirements for safety
• 60601-1-6 Usability
• 60601-2-2 HF surgical equipment
• 60601-2-22 Laser equipment: Surgical / Cosmetic / Therapeutic / Diagnostic
• 60601-2-43 X-ray equipment






NATIONAL
NATIONAL SILVER FOR COMPANY PROFILE
NATIONAL BRONZE FOR PUBLICATION DESIGN
REGIONAL GOLD FOR OPENING FEATURE SPREAD DESIGN





REGIONAL GOLD FOR REGULAR DEPARTMENT
REGIONAL GOLD FOR COMPANY PROFILE
REGIONAL SILVER FOR SPECIAL ISSUE
REGIONAL SILVER FOR PUBLICATION DESIGN
REGIONAL SILVER FOR FEATURE ARTICLE DESIGN
REGIONAL BRONZE FOR DIVERSITY, EQUITY AND INCLUSION
REGIONAL BRONZE FOR ENTERPRISE NEWS STORY
EDITORIAL
Editor in Chief Chris Newmarker cnewmarker@wtwhmedia.com
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Editor in ChiefR&D World Brian Buntz bbuntz@wtwhmedia.com
Associate Editor Sean Whooley swhooley@wtwhmedia.com
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
Managing EditorDeviceTalks Kayleen Brown kbrown@wtwhmedia.com


CREATIVE SERVICES
VP, Creative Director Matthew Claney mclaney@wtwhmedia.com
AUDIENCE DEVELOPMENT
Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com
PRODUCTION SERVICES
Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com

VP, Operations Virginia Goulding vgoulding@wtwhmedia.com
Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com
LEADERSHIP TEAM
CEO & Co-Founder Scott McCafferty smccafferty@wtwhmedia.com 310.279.3844
Senior Vice President Courtney Nagle cseel@wtwhmedia.com 440.523.1685











Design for Access, Says Abbott’s Lisa Earnhardt, Medtech’s Most Powerful Woman ........................................... 24
“Innovation is core to who we are,” Abbott Medical Devices Group President Lisa Earnhardt says in an interview covering device design, customer needs and diversity in medtech.
Gender Parity in Medtech Leadership Remains Elusive
The medical device industry’s efforts to achieve gender parity in top leadership have stalled.
As Cardiovascular Deaths Soar for Women,
Nina Goodheart and the
Structural Heart and Aortic President Nina Goodheart shares how Medtronic seeks to save more lives through its SMART trial and development of gender-sensitive medical devices..




Designing efficient systems involves much more than simply understanding a few basic principles. There is a true art to balancing the specific requirements of an application in order to achieve the desired goals in the best possible way. Help us understand the unique needs of your application and together, we’ll develop something that surpasses what any of us could have done alone.
Contact your distributor to learn more, or visit clippard.com to request a free catalog and capabilities brochure.




Succeeding in the rapidly evolving medical device manufacturing industry requires deep expertise to deliver absolute quality, precision and speed, while reducing overall costs. It also requires investing in new and next-gen technologies.
For nearly 60 years, we’ve met – and continue to meet – virtually every contract manufacturing challenge accepted from design through delivery.
You also can rely on our impeccable track record for regulatory compliance, meeting deadlines and on-time delivery.

Bring your medical devices to market with confidence.
Visit donatellemedical.com to learn more.


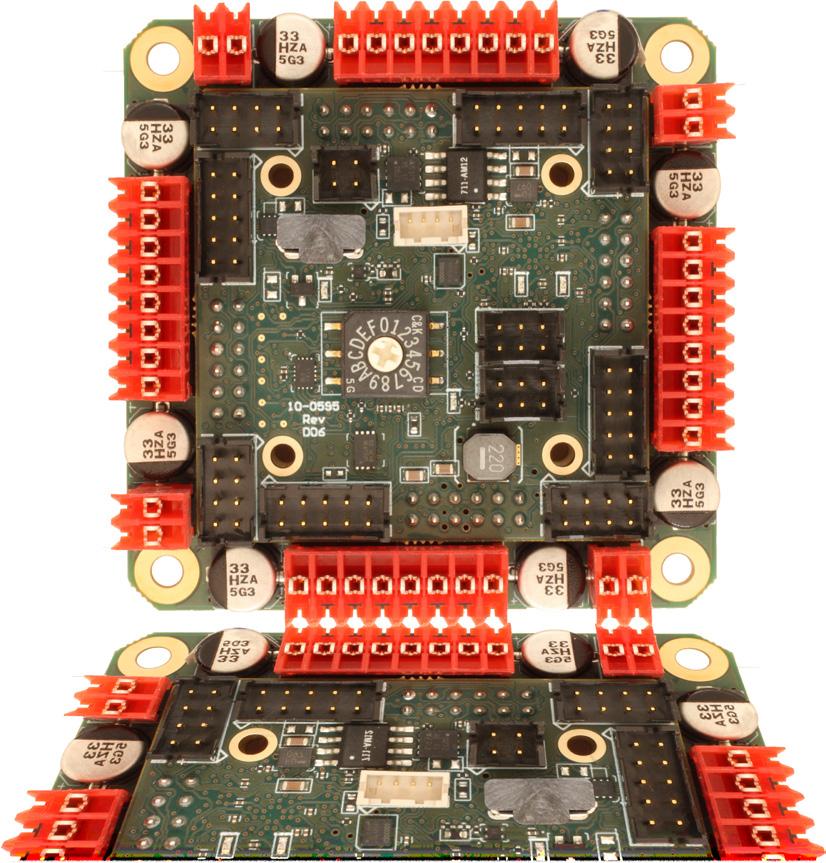
- brushed or bldc motors
- 5 amps per axis
- 16 analog inputs
- 16 on/off drivers
- home and limit in
- live tech support
- made in the USA

As we publish another edition of Women in Medtech, it’s clear that innovation and diversity are inextricably linked in shaping the future of healthcare.
One of the most important lessons I’ve learned in the seven years producing this special Women in Medtech edition is the value of persistence. When we started focusing on gender equity in medtech leadership, the data was not always easy to find, and the stories were often harder to tell.
Yet, year after year, we’ve seen incremental progress in diversity at the top levels of the industry. Our annual analysis shows that women now hold 24.4% of top leadership roles, a slight increase from last year. However, much work remains to ensure that executive teams are as diverse as their patient populations.
As women like Abbott EVP and Medical Devices Group President Lisa Earnhardt break barriers and set new standards for leadership, we’re reminded that change — though slow — comes from consistent effort and dedication.
“It’s imperative for us as an industry to increase the number of women in medtech,” Earnhardt said in an interview for this issue’s cover story. “We collectively are underrepresented. Half the world is female, so we need to be more representative of the customers we serve.”
For Earnhardt, a key takeaway learned early on was the value of understanding the customer. This perspective continues to guide her leadership at Abbott, where patient-centric design has driven innovations like the FreeStyle Libre, a continuous glucose monitor that democratizes diabetes care with affordability and ease of use. The earlier failure of a more complicated product taught her team an invaluable lesson: Simplicity and accessibility are the cornerstones of impactful healthcare solutions. Read more about lessons learned in Earnhardt’s interview with Managing Editor Jim Hammerand in our cover story.





This edition also features interviews with other influential women in the medtech industry, including Medtronic SVP and Structural Heart and Aortic President Nina Goodheart. Their insights highlight the importance of diverse perspectives in driving the innovation necessary to meet today’s healthcare challenges.
The lasting sentiment from leaders in the industry is that diverse C-suites are a prerequisite for innovation. We hope these stories inspire continued progress in both technology and leadership, as the medtech industry strives for more inclusive innovation.

Porex medical-grade porous components deliver precise, reliable, and consistent performance. Custom engineered for your medical & diagnostic devices, our solutions solve your toughest design challenges, enhancing product accuracy and reliability.











Here are nine tips for improving patient outcomes, function and materials when 3D printing O&P devices.

















printing is revolutionizing the orthotics and prosthetics (O&P) industry, enabling the creation of highly customized medical devices tailored to individual patients. Additive manufacturing technology is poised to do far more by offering significant improvements in patient outcomes and device functionality.
For O&P companies to harness the potential of 3D printing, they need strategies that optimize patient outcomes, improve device functionality and maximize 3D-printed material strength, reliability and durability.
Patient outcome improvements
1. Plan in advance: Effective 3D printing begins with meticulous planning. Gone are the days when designers and engineers could get away with applying traditional manufacturing methods to 3D printing and call it good. Force-fitting old ways into new technology will not work. To be effective, it’s important to adopt a 3D mindset and take a unique approach while planning for each project.





Software such as Leopoly’s LeoEngine can help facilitate the automation of various design and fabrication steps, enhancing the efficiency and scalability of device designs.
Developers of the Arize Orthotic Solution, an end-to-end digital orthotic service for podiatrists and orthotists, used design automation to reduce lab review time. Automation can significantly improve manufacturing and delivery times for less complex medical devices such as foot orthoses or ankle-foot orthoses (AFOs).

Computational design, which uses algorithms to optimize and automate processes, can also significantly reduce the time and effort of creating custom devices. Smith Optics uses computational design to efficiently create custom-fit ski goggles from facial scans.
2. Think customization from the start: Customization is what 3D printing is all about when it comes to delivering better patient outcomes in O&P. Rather than initially thinking about ways to mass produce artificial ankles, knees, >>
feet, insoles, or spinal, wrist and hand supports, better patient outcomes are achieved by identifying ways to tailor 3D-printed devices to the needs and anatomy of specific patients or patient groups. If those groups are large enough, designs can be extended to accommodate the needs of those patient segments.
3. Integrate smart features: Incorporating smart features into 3D-printed devices can further enhance patient care. Embedded sensors and connectivity options will eventually enable proactive remote monitoring and datadriven insights to improve patient comfort and track O&P device performance, whether it might be about to fail, how the patient’s anatomy is interacting with it, how much pressure it’s withstanding each day and even how active the patient has been since wearing the device. This builtin feedback loop allows for improvements in each generation of a device to be constructed using real world data.
Functional improvements
4. Embrace design for additive manufacturing (DfAM): DfAM principles are critical to optimizing geometries for improved functionality. These are not attributed to a single individual or group, but notable contributors to their development include academic institutions, industry leaders and organizations such as the Additive Manufacturing Users Group (AMUG) and standards bodies like ASTM International. Plenty of work has gone into these best practices for optimizing 3D printing design processes, making them useful for the many traditional designers and engineers whose expertise is primarily in CNC machining and injection molding rather than 3D printing. While there is no single set of DfAM principles, they generally involve part consolidation, build packing optimization, design freedom and biomimicry. Look into comprehensive training courses to bring designers and engineers up-to-speed on DfAM.
5. Conduct iterative prototyping: Iterative prototyping and testing is vital to refining the functional aspects of 3D-printed devices. This process allows designers to make incremental improvements based on real-world

feedback and performance data. By continuously iterating designs, manufacturers can enhance device performance and address specific patient needs more effectively.
6. Incorporate complex structures: Exploring and incorporating complex structures into device design can address functional requirements in innovative ways, particularly for those that exhibit meta-material properties and can behave in both characteristic and useful ways based on the material’s sub-structure. Indeed, small adjustments to reduce weight, disperse heat, or manage pressure distribution can improve a device while creating revenue opportunities.
Click Medical recently secured HCPCS Code L5783 certification from the Centers for Medicare & Medicaid Services for insurance coverage and reimbursement eligibility.
Material improvements
7. Understand material attributes: When imagining 3D-printed devices, a designer needs a working knowledge of material types, their capabilities and limitations, and general availability before getting started. It’s a mistake to assume materials used in traditional manufacturing will be just as applicable to additive manufacturing. Common 3D printing materials fall into three categories: plastics/ polymers, metals and ceramics. Each offers varying durability, reliability, elasticity, heat resistance, biocompatibility and sustainability. You’ll need to select materials to support specific projects rather than hastily buying good-enough options that may be more affordable or have fared well on prior manufacturing jobs. HP offers a guide to 3D printing materials.
8. Enhance material properties: Enhanced or specialized materials knowledge should also be part of a device designer’s toolkit. Chemical vapor smoothing, for example, is a post-processing method that uses a combination of heat and solvent vapor to improve the mechanical properties of parts and products. Materials with high abrasion resistance, like ESTANE 3D TPU M95A, can also be useful for jigs and fixtures that undergo thousands of cycles. Multi Jet Fusion (MFJ) technology needs less heating power than selective laser sintering (SLS) systems and can improve material properties while reducing waste. Finally, biocompatible materials like polylactic acid (PLA) or BioMed Amber Resin can ensure patient safety and compliance with FDA regulations.
9. Research novel materials: Novel materials may be essential for addressing specific medical needs. MJF technology
often uses PA11 or PA12 polyamide plastics, which are sometimes compared to sheet-source polypropylene found in traditional manufacturing plants but are not all that close. Designers need to understand the differences and choose the right material for the specific job at hand. Collaborating with application engineers and designers can help designers study and understand the interplay of materials, agents and designs for projects. By leveraging novel materials, design teams can integrate and consolidate functions into a single part, such as adding claps, latches, or other adjustments directly into the printed part.
3D printing is poised to revolutionize O&P by enabling highly customized, patient-specific devices with intricate geometries and optimized functionalities. Careful planning,
meticulous design and strategic material choices will be needed to guide this transformation. By harnessing the power of automation, personalized solutions, smart integrations and cutting-edge DfAM principles, designers can reshape patient outcomes, refine device functionality and raise materials usefulness with unprecedented precision and payoffs for years to come.
Melanie Shelton is a software and digital workflow product manager at HP Inc. with nearly two decades of experience in the orthotics and prosthetics (O&P) industry, including establishing and overseeing distributorships for orthotic fabrication equipment across Europe, the Middle East and Southeast Asia. She previously worked at Orthotic Holdings Inc., where she helped clinicians with selection, fitting and provision of lower limb devices.






CDMO / CMO Services


Minimally Invasive Device Solutions
Co-development Opportunities
Access, Delivery, & Retrieval Systems
Wire & Catheter Based Devices
Contract Manufacturing
Vascular Access Devices
Guidewires, Therapeutic & Diagnostic
Braided & Coiled Catheter Shafts
Class 8 Clean Room



Boston University researchers explain how advanced MRI coils and textile metamaterials could make
ith over 100 million magnetic resonance imaging (MRI) scans performed annually worldwide, including 40 million in the U.S., MRI remains a cornerstone of modern medicine due to its safety and noninvasiveness.
Now, wireless and wearable coils, along with textile metamaterials, are emerging as game-changers. These advanced coils manipulate electromagnetic fields to enhance imaging performance, offering a promising, cost-effective upgrade to traditional MRI systems.
The challenge
MRI technology has traditionally relied on rigid, anatomy-specific RF coils, hardwired to a direct RF feed, to ensure high imaging sensitivity. While effective, these coils are bulky and expensive due to their numerous nonmagnetic electronic components, feed boards, cable traps, and adapters. These bulky, rigid coils increase
patient discomfort and complicate handling during imaging procedures. Additionally, imaging centers often need 5 to 7 different coils for various anatomical regions, driving up costs.
The emergence of metamaterials — artificially constructed materials composed of sub-wavelength unit cells — has opened new possibilities for enhancing the signal-to-noise ratio (SNR) of MRI systems through wireless, cost-effective solutions. By precisely tailoring electromagnetic waves in the near-field region, metamaterials can significantly improve SNR as auxiliary devices working with the body coil of MRI, improving image resolution and acquisition efficiency.
Despite this promise, previous metamaterial designs have faced slow clinical adoption due to lower sensitivity compared to state-of-theart receive coils and incompatibility with common clinical sequences. Their rigid, bulky structures also limit both sensitivity and patient comfort. >>
To overcome these limitations, we have developed an innovative solution using off-the-shelf coaxial cables. These wearable coils cost less than $100 per unit and maximize sensitivity by closely conforming to anatomical regions, achieving a SNR comparable to or even surpassing that of advanced MRI coils.
Coaxial cables revolutionize resonator design
Coaxial cables, known for their duallayered internal conductors, have long been a staple in telecommunications, delivering efficient signal transmission with minimal losses and robust protection against external interference. Now, these cables are transforming MRI technology.
In an innovative leap, we have leveraged the superior properties of coaxial cables to develop coaxially shielded loop resonators. By strategically adding welding connections between the inner and outer conductors, we created substantial structural capacitance, enabling the necessary resonance capacity despite the coil’s compact size relative to the RF wavelength in an MRI system.
Unlike traditional resonators that rely on single conductive wires, these coaxially shielded resonators depend solely on their geometric properties and total length, eliminating the need for lumped elements along the cable. This design reduces losses and enhances the quality factor, significantly improving MRI performance.
One of the major advantages of this innovation is its ability to overcome size constraints. The integration of multiple welding connections allows for flexible

Form-fitting coils and textile metamaterials for wearable imaging
The proximity of MRI coils to the target anatomy is crucial for optimal magnetic coupling, signal reception, depth of penetration, and image quality. Leveraging the flexibility of coaxial cables and the cable-free design of coaxially shielded resonators, we have developed form-fitting coils that can be bent, twisted, and shaped to conform to the 3D contours of the body.
resonator sizing while maintaining the desired resonance frequency, enabling customization for specific anatomical sites.
Traditionally, MRI coils have been hardwired to the scanner, lacking wireless functionality. To address this, we introduced intelligent self-adaptivity to the resonators. By leveraging the rectifying effect and bistable nonlinear behavior of PN junctions in diodes, these resonators selectively amplify the magnetic field, preventing interference with the RF transmission field and ensuring patient safety.
Our research further explores the operational principles of these resonators. By manipulating the electric field distribution, we aim to achieve frequency tunability, ensuring precise resonance alignment with MRI systems. This investigation into coaxially shielded resonators with self-adaptivity and frequency tunability offers advanced and efficient solutions for wireless and wearable imaging.
Supported by 3D-printed scaffolds, these coils can be easily attached to extremities such as fingers, wrists, or ankles without additional tools or fasteners, simplifying coil positioning during MRI scans. By placing these wireless coils close to the anatomical site, the form-fitting design effectively prevents the signal from dissipating into the surrounding environment, resulting in a significant gain in SNR.
Additionally, the wireless standalone coils weigh only 35 grams, a substantial reduction compared to several kilograms for commercial surface coils. Their lightweight, cable-free design reduces positioning constraints, enhances patient comfort, and requires fewer adjustments, offering a streamlined workflow that increases productivity while maintaining excellent image quality. This groundbreaking work is detailed in the June 12 issues of Science Advances.
While these form-fitting coils enhance imaging capabilities for smaller anatomical regions, clinical MRI often requires visualization of larger areas, such as the knee or spine. To address

this, we propose the development of metamaterials featuring an array of coaxially shielded resonators inductively coupled to function collectively for signal acquisition. Using computeraided embroidery, we integrate these metamaterials onto a textile substrate, creating a wearable configuration that conforms closely to patient anatomies, enhancing sensitivity. Weighing only 50 grams, including the housing fabric, these metamaterials improve patient comfort during MRI procedures.
Compared to previous designs, our metamaterials offer substantial SNR gains due to superior magnetic response and minimized electric dipole moments. MRI images acquired with this metamaterial using various pulse sequences achieve an SNR comparable to or surpassing that of a state-of-the-art 16-channel knee coil. This research is detailed in the Aug. 1 issue of Advanced Materials.
Our objective is to develop advanced MRI coils that seamlessly integrate into routine healthcare settings and existing clinical workflows. This innovation promises to enhance patient care and streamline MRI procedures, making high-quality imaging more accessible and efficient.
To achieve this, we will be working closely with clinical research partners, gathering valuable insights and understanding the specific needs of targeted diseases to refine the wearable imaging technology.
Looking ahead, we plan to partner with industry collaborators to pave the way for the large-scale commercialization of wearable coils and metamaterials for MRI applications, marking a significant step forward in the evolution of MRI technology.
Xin Zhang is a distinguished professor of engineering at Boston University. Her recent research focuses on metamaterials for applications in clinical medical imaging, photonics and optics, as well as acoustic silencing and noise reduction.
Ke Wu is a postdoctoral researcher at the Photonics Center at Boston University. He specializes in integrating metamaterials and RF electronics to engineer mechanically adaptive devices and systems, with the aim of advancing magnetic resonance imaging technologies.
Xia Zhu is a PhD researcher at the Department of Mechanical Engineering at Boston University. He specializes in developing metamaterials and RF components to engineer wireless devices and systems, with the aim of improving magnetic resonance imaging technologies.

Cutting-edge technology in Filmcast PTFE liners that exceed conventional standards with the industry's shortest lead times
PTFE lead times of 2-4 weeks
REACH Compliant Polyimide
Braided and Coiled Reinforced Shafts
PTFE ID sizes from 0.010" to 0.155"

Whether a 1-foot NEMA 5-15 hospital-grade cord with an IEC C13 terminating the other end, or a 50-foot NEMA 6-20 with an IEC 60320 C19 terminating the opposite end, accessory power possibilities abound—especially if multiple hospital machines need to use the same accessory power strip.
Interpower cords and components are manufactured in accordance with Interpower’s product quality plan: hipot testing, continuity testing, and ground testing until surpassing all worldwide agency standards. With 1-week lead times on U.S. orders, and same day shipping on over 2 million parts in stock, you can afford to keep inventories low.
North American and Japanese hospital-grade plugs and receptacles bear the green dot, signifying the plugs have passed the rigorous UL 817 Abrupt Removal Test (UL 817, 18.2.4.1) and C22.2 No. 21-14 requirements for hospital-grade cords. Other countries using hospital-grade cords such as Australia and Denmark, have proprietary requirements. Many countries use standard cords for their hospitals, which require less testing.

• 1-week U.S. lead times
• Same-day shipping on in-stock products
• Quick-change molding process
Understanding the EPA’s final NESHAP ruling for commercial EtO sterilizers
An FTIR gas analysis expert offers a comprehensive overview of regulatory guidelines and the monitoring solutions available for long-term resiliency.

For years, the Environmental Protection Agency has been looking for ways to regulate the use of ethylene oxide (EtO) — a difficult-to-measure gas that is widely used to sterilize medical devices and some food products — as it’s been found to have carcinogenic properties.
In March 2024, the EPA made its final amendments to the National Emission Standards for Hazardous Air Pollutants (NESHAP) for EtO commercial sterilizers, which will require that facilities significantly reduce emissions, employ systems for continuous monitoring, and regularly report monitoring data to the agency. The goal is to achieve over 90% reduction in EtO emissions nationwide.
To comply with the EPA’s NESHAP guidelines and mitigate disruption to the medical device supply chain, it’s critically important for sterilizing
facilities to thoroughly understand which emissions sources are being regulated, the applicable compliance standards and parameters for the size of facility and operational use of EtO, the variables in the timeline to come into compliance, the types of continuous emissions monitoring technologies available for long term resiliency, and the performance metrics as required by the NESHAP.
The EPA’s final rule requires owners and operators to use continuous monitoring to demonstrate compliance at several emissions sources: sterilization chamber vents (SCV), aeration room vents (ARV), chamber exhaust vents (CEV) and room air emissions. The SCV evacuates EtO following sterilization, fumigation, >>
and any subsequent gas washes before the chamber door is opened, while the ARV evacuates EtO-laden air from the aeration room or chamber that is used to facilitate off-gassing of the sterile product.
Some facilities may need to introduce monitoring technologies at emissions sources that were previously unregulated, such as building leaks or room air emissions, and at CEVs. Room air emissions include emissions resulting from indoor EtO storage, dispensing, vacuum pump operation, and pre- and post-aeration handling of sterilized material. Each source will require continuous emissions monitoring and quarterly reporting, including electronic reporting and technical revisions. Commercial sterilizing facilities will also need to ensure compliance at each source during periods of startup, shutdown, or malfunction so there is continuous protection.
As required by the final ruling, commercial sterilizers must adopt technology that allows for real-time and continuous monitoring of EtO. The EPA also set standards for compliance based on facility size and operational use of EtO. Facilities that use more than 100 lb of EtO per year must use a continuous emissions monitoring system (CEMS) to demonstrate compliance. Facilities that use less than 100 lb of EtO per year will have the option to use CEMS or performance testing and parametric monitoring to demonstrate compliance.
While the EPA’s NESHAP requires that 10 parts per billion by volume (ppbv) be consistently demonstrated and replicated across a wide range of emissions profiles, there are cutting-edge analytical technologies that allow users to achieve single-digital ppbv detection limits. Owners and operators of the facilities will need CEMS to stay compliant.
Techniques such as gas chromatography (GC) have been used historically, but GC cannot achieve the sensitivity required by the updated NESHAP, and operating GC technology can be costly and often requires experienced workers to maintain the analyzer. Today, there are optically enhanced Fourier-transform infrared (OE-FTIR) spectroscopy
solutions which can be engineered to meet the standards for EtO emissions testing at ultra-low detection limits, allowing both novice and experienced operators to get fast and accurate data. For commercial sterilizers who need to comply with the EPA’s NESHAP, fully automated FTIR CEMS have provided reliable continuous emissions monitoring without calibrations.
While not explicitly required by the NEHSAP, facilities are encouraged to implement redundant backup monitoring systems, which are CEMS installed to be on standby in case the primary system can’t provide reliable data. A CEMS with multi-channel sampling capabilities as the redundant system can be a cost-effective way to allow for future facility expansion and prepare for potential amendments to the NESHAP’s downtime requirements.
required to demonstrate 99.99% percent emission reduction by the pollution control device. Most facilities will need to monitor at the inlet and outlet of their pollution control device to determine percent reduction. This is an important consideration when evaluating the suitability of an EtO CEMS because inlet concentrations are significantly higher than at the outlet, so the CEMS must be capable of analyzing EtO over a wide concentration range. Monitoring technologies like OE-FTIR are cost-effective because the same analyzer can be used to measure higher concentrations of EtO at the inlet and low ppb levels at the outlet.
As required by the final ruling, commercial sterilizers must adopt technology that allows for real-time and continuous monitoring of EtO. The EPA also set standards for compliance based on facility size and operational use of EtO.
Understanding performance metrics and compliance standards based on facility size and operational output When choosing a CEMS, facilities should consider the performance metrics for CEMS devices, including level of detection (LOD), calibration drift and measurement error (ME). The EPA chose these metrics to meet the new Performance Specification 19 (PS-19) used to evaluate the acceptability of an EtO CEMS. A source that demonstrates its CEMS meeting the criteria of PS-19 may use the system to continuously monitor EtO under any regulation or permit that requires compliance with this specification. When choosing a CEMS technology, an owner or operator should ensure that it meets PS-19. Emission standards vary depending on the source and quantity of EtO used, but all are based on percent emission reduction by the control device rather than the mass of EtO emitted. Existing and new sterilization chamber vents using at least 30 tons of EtO per year are
The timeline for facilities to come into initial compliance has some variation. At a high level, facilities will have two to three years to comply and produce an initial validation report, but the steps to compliance are broken down by emissions source. Facilities should work with technology vendors for retrofits to their existing control devices, implementing new controls and procuring CEMS and other equipment needed according to the NESHAP. Any new facility designs will be required to meet EPA’s current emissions standards. Once the initial compliance timeframe is completed, facilities will have an additional 180 days to demonstrate compliance with a validation report.
It’s important to note that the EPA issued a separate proposed interim decision to reduce sterilization worker exposure to EtO under a federal pesticide control law, which is expected to be finalized in October 2024. This ruling may also impact facility design decisions.
The EPA is constantly reviewing the evolving needs of industry and improving technologies and applications, conducting health and risk assessments of pollutants, and making amendments to proposed regulations. As owners and operators work to bring their facilities into compliance, they should consider solutions that will provide long-term resiliency for EtO monitoring. Innovative technologies such as OE-FTIR are highly effective for demonstrating compliance with EtO emission reduction standards, and installing redundant monitoring systems can be a cost-effective solution to mitigate future downtime with expansion or new regulations.
Kelly McPartland —applications manager with Thermo Fisher Scientific, Gas Analysis Solutions — is an expert in FTIR gas analysis, associated method development and data validation. As a senior-level technical liaison for gas analysis customers, McPartland has been a key architect and manager for data acquisition gas analysis software and also works closely with state and federal regulatory agencies to develop quality assurance plans and test methods for new technologies.
As medical devices have evolved, we have advanced our capabilities to meet your increasingly complex design requirements. Backed by nearly 80 years of proven manufacturing expertise and a laser focus on minimally invasive surgical instruments, MICRO delivers the highest






JIM HAMMERAND MANAGING EDITOR

“INNOVATION IS CORE TO WHO WE ARE,”
ABBOTT MEDICAL DEVICES GROUP PRESIDENT
LISA EARNHARDT SAYS IN AN INTERVIEW COVERING DEVICE DESIGN, CUSTOMER NEEDS AND DIVERSITY IN MEDTECH.
Lisa Earnhardt still remembers an important takeaway from the frontline of her first product launch.
The Abbott EVP — who’s group president of the world’s eighth-largest medical device business — was working at Dairy Queen in the summer of 1985 as it debuted the Blizzard. She recalled stressful moments turning the frozen desserts upside down in front of the customer, proving they were so thick they wouldn’t fall out of the container.
“It was the first job I had where I had customers that I recognized,” Earnhardt said in an interview with Medical Design & Outsourcing. “At the end of the day, the customer is always right. Understanding what it means to thrill a customer was a great learning [experience].”
It’s a lesson medtech’s most powerful woman is reiterating as Abbott launches new and improved devices across its portfolio, including innovative systems for cardiac rhythm management, electrophysiology, structural heart, heart failure, vascular therapies, diabetes care, and neuromodulation.
All are meant to get the device, drug, diagnostics and nutritional products manufacturer to its goal of treating 3 billion people around the world by 2030, a 50% increase from Abbott’s 2 billion patients in 2020 when the company set the target. >>

Abbott launched the FreeStyle Libre in 2014, eliminating the need for fingersticks. Abbott designed its FreeStyle Libre products (including the latest-generation sensor held here by Lisa Earnhardt) for automated manufacturing.
In 2023, Abbott medical device sales grew 14% to $16.9 billion, helping it rise from No. 10 to No. 8 in MDO’s latest Medtech Big 100 ranking of the world’s largest device companies.
“We truly are in a transformational moment in the medtech industry broadly, and at Abbott we are leaders in this transformation, helping people with some of the most chronic, prevalent diseases like diabetes, cardiovascular care, chronic pain, movement disorders, some of the most challenging conditions,” Earnhardt said. “… Innovation is core to who we are.”
“We truly are in a transformational moment in the medtech industry broadly, and at Abbott we are leaders in this transformation, helping people with some of the most chronic, prevalent diseases like diabetes, cardiovascular care, chronic pain, movement disorders, some of the most challenging conditions. … Innovation is core to who we are.”
Earnhardt’s medtech career
After her first jobs detasseling corn in rural Illinois and slinging soft serve, Earnhardt earned a degree in industrial engineering from Stanford and then an MBA from Northwestern.
She was a healthcare consultant before joining Guidant in 1996, where she worked for medtech veteran Maria Sainz, succeeding her as Guidant’s cardiac surgery president following Boston Scientific’s 2006 acquisition of the company. A unique blend of attributes makes Earnhardt a strong leader from several perspectives, Sainz said.
“She has this incredible balance of intellect, humanity, leadership and drive,” Sainz told MDO. “… She’s a very caring human being, someone with a special touch, someone that will make sure those who matter feel that she cares, that she is there for you.”
Earnhardt left Boston Scientific in 2008 to be president and CEO of Intersect ENT. She led the implant developer for 11 years and stepped down to join Abbott in 2019, three years before Medtronic bought Intersect ENT for $1.1 billion.
Abbott hired Earnhardt to lead the medical devices business after promoting her predecessor, Robert Ford (now Abbott’s CEO and chair), to serve as president and chief operating officer. The medical device business was and still is the company’s largest segment, bringing in 42% of Abbott’s total revenue in 2023.
“The world needs more leaders like Lisa at the helm of businesses,” former Johnson & Johnson MedTech Worldwide Chair Ashley McEvoy said in an interview, describing her former competitor as “fair and fierce.”

“What I’ve always appreciated about Lisa is her deep belief that innovation’s best years are ahead to improve patient care, and she’s very collaborative to advance the health of the industry,” McEvoy said. “… Lisa is a beautiful ambassador of the industry, because she’s got street cred on engineering, innovation, building cultures, being a good collaborator, getting performance and always being in service of patients.”
While many other leaders of the world’s largest device businesses hold MBAs, Earnhardt’s background as an engineer is unusual among that cohort.

“Engineering is all about problemsolving, and it taught me a methodical way to think about problem-solving,” Earnhardt said. “In medtech, we’re solving some of the most challenging problems we’re faced with in terms of healthcare, and we’re doing it through technology.”
As she sees it, that starts with understanding the needs of the customer — or the consumer, in the case of diabetes patients who manage their own care with increasingly affordable and user-friendly wearable sensors and pumps.
“I always say, ‘God gave you two eyes, two ears and one mouth for a reason.’ It’s listening and observing or watching what’s working — or more importantly, what’s not working — and what we can do to add value,” Earnhardt said.
(continued on page 28)

Ashley McEvoy
PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development.
We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory.


Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.
• Specialized team of SMEs
• ISO 10993 and ISO 18562 series
• Toxicological Risk Assessment
• FDA Submission Assessment
PSN Labs has the experts to isolate confounding variables and provide clear analysis to guide the commercialization of existing and new medical devices.



(continued from page 26)
Expanding healthcare access through device design
The global diabetes epidemic is one of Abbott’s biggest opportunities to reach more patients, with more than half a billion people who could benefit from glucose monitoring.
About 6 million patients use Abbott’s FreeStyle Libre continuous glucose monitoring (CGM) platform, but Earnhardt said a previous product’s “complete failure” paved the way.
To cut manufacturing costs at scale, Abbott designed its FreeStyle Libre products for high-speed, automated fabrication and assembly while investing hundreds of millions of dollars to upgrade and expand its manufacturing operations, including a new factory in Ireland.
Abbott designers joined the transmitter and receiver for a one-piece sensor that’s more efficient to make and easier to use, for example, and for the latest-generation FreeStyle Libre
Earnhardt said. “… It’s set up very intentionally from the very beginning of the development process.”
Now, Abbott’s launching Lingo, an over-the-counter, wearable CGM sensor for real-time glucose readings. The goal is to use machine learning algorithms and Abbott’s trove of more than 50 billion hours of glucose data to help users predict how their activities affect blood sugar levels so they can make appropriate changes.
“This is a powerful example of not just how healthcare and consumer
“What I’ve always appreciated about Lisa is her deep belief that innovation’s best years are ahead to improve patient care, and she’s very collaborative to advance the health of the industry. … Lisa is a beautiful ambassador of the industry, because she’s got street cred on engineering, innovation, building cultures, being a good collaborator, getting performance and always being in service of patients.”
“It was complicated to use, it didn’t have the impact we had thought, and the team went back and soul-searched,” Earnhardt said. “[They] came forward with some principles to have an impact on the way that people manage their diabetes: We need to make it easy to use, we need to make it affordable and accessible to the millions of people who can benefit.”
3 designed a one-piece applicator instead of two pieces. All these efforts paid off with a device that sells for half the price of competing sensors.
“We worked in partnership with a number of external vendors on the automation piece, and it’s been a game-changer for us as a business and a game-changer for the field of diabetes,”
technology are coming together, it’s an amazing example of how we’re able to move to what I truly consider healthcare, and it’s going to be an amazing example of how we can leverage the power of AI,” Earnhardt said, displaying a Lingo sensor on her own arm. (continued on page 30)











Rely on the proven experience of ARCH to support your medical manufacturing and supply chain needs. With an expansive footprint of state-of-the-art facilities across the nation, our technologies and processes are second to none. We offer expertise in orthopedic, spinal, dental, surgical robotics, and medical device production in a diverse range of materials.
Additive manufacturing of orthopedic devices
MedTorque silicone-handled instruments
Precision grinding of surgical cutting instruments
gSource® precision surgical instruments
Surgical implants and instruments manufacturing
Full range of validated processes
FDA registered and ISO 13485 certified
a part We have in that




(continued from page 28)
Another Abbott product tapping AI is its Ultreon intravascular imaging software, which uses optical coherence tomography to help interventional cardiologists visualize plaque inside a patient’s artery for more efficient and accurate stent placement.
“You wouldn’t necessarily think about that as innovating for access,” Earnhardt said, “but to the extent that we can democratize procedures and make it easier and more predictable for more clinicians to be able to utilize the technology in a more efficient fashion, that has global impact.”
She called out other innovations like the AVEIR dual-chamber leadless pacemakers, the Abbott Volt pulsed field ablation system for cardiac ablation, the bioabsorbable Esprit BTK drug-eluting scaffold for peripheral artery disease, and the Eterna rechargeable spinal cord stimulation system for pain relief.
“We just announced we’re launching a trial for treatment-resistant depression utilizing deep brain stimulation (DBS),” she said. “That’s a huge unmet need, a very difficult condition. We think there’s a really compelling application of DBS for that. The list goes on and on.”
Another opportunity to reach more patients is to increase gender, racial and ethnic diversity within the medtech industry and Abbott, where women make up 46% of the company’s global workforce and 42% of its managers.
“At Abbott, I’m surrounded by talented women [but] we still have work to do,” Earnhardt said. “It’s ensuring you’re recruiting the right talent, developing the talent, retaining the talent, even recruiting and developing the talent before they’re ready to be hired at a company like Abbott. We have a program starting at the high school level focusing in particular on girls and minorities in STEM, and part of that is making sure we have the right pipeline of talent and they get interested in an industry like medtech.”
Asked for advice for other women in medtech, she encouraged them to always keep learning and challenging themselves, and to trust in themselves and their experience.
“Sometimes that self-doubt comes in, probably more so with women. … That self-doubt voice needs to be put aside,” Earnhardt said. “You are where you are because you’ve demonstrated your abilities. You’ve demonstrated your potential.”
And asked for advice more broadly for everyone in medtech, she said time with customers is time well spent, whether that be informal ride alongs and observations in clinical settings, or more structured activities like market research, focus groups and advisory boards.
“Hearing directly from patients who are impacted [is] incredibly motivational, not just understanding what the needs are in the mind, but feeling it in the heart,” Earnhardt said.
“Oftentimes the interactions we have — whether it be our commercial
organizations or our clinical teams and our R&D engineers — tend to be with the clinicians or the physicians using our products, and those are incredibly beneficial and make a huge difference,” she later continued. “But … it’s amazing when you hear from [patients] how we might be able to make their lives easier or provide more reassurance or give them more information so they can control their own health. It’s powerful.”
Finally, she emphasized the importance of “being authentic to who you are.”
“It helps build trust,” she said. “Trust is the foundation for all relationships. It’s the foundation for strong collaboration, for building world-class teams. Work is hard enough, so being yourself is the easiest thing to do.”

Abbott employees in the testing stage of the Esprit BTK stent’s development Photo courtesy of Abbott



















Diversity in corporate leadership was a hot-button issue just a few years ago, particularly amid social movements and public calls for racial and gender equity in 2020. Many medical device companies appeared to make strides by hiring diversity leaders and championing the need for change at the executive level.
“Studies show that when women are in leadership roles, companies benefit from more diverse perspectives, fostering innovation and more effective decision-making,” said Lisa Jacobs, co-founder of Stripes and president of North America for eCential Robotics.
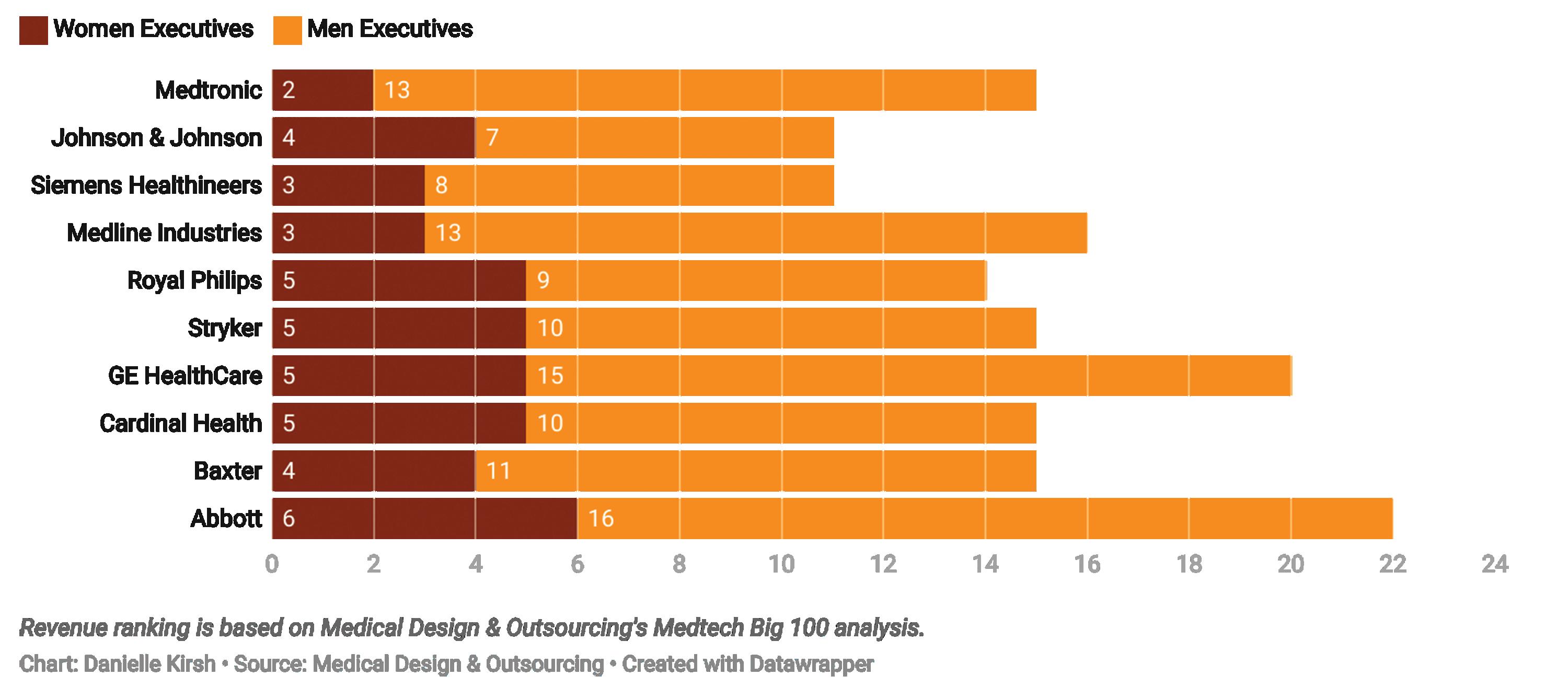
The number of women in top executive roles (in our analysis, those listed on corporate leadership web pages) rose just 1 percentage point to 21% in 2021, but then climbed another 2 percentage points to 23% in 2022 and increased to 23.6% in 2023.
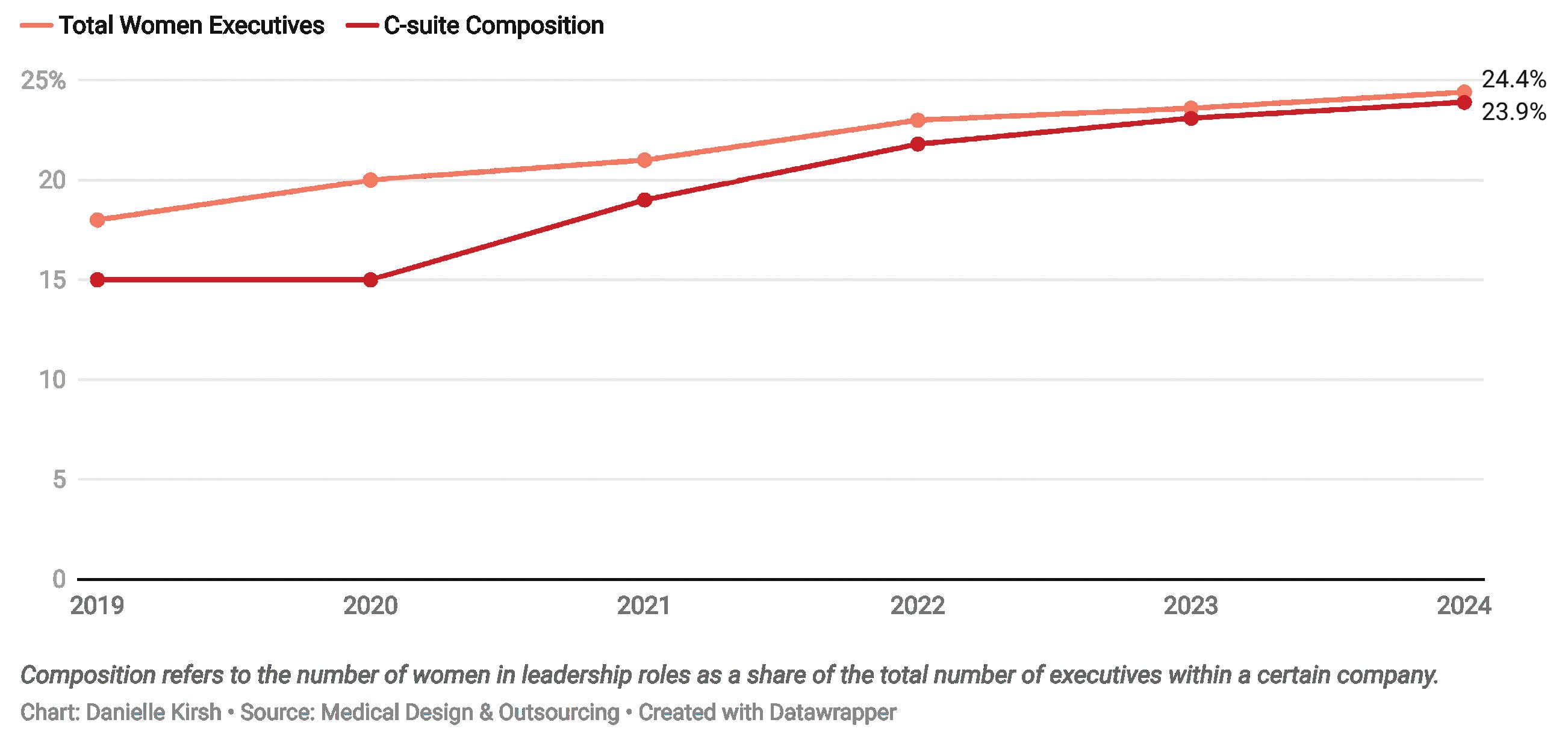
However, as the spotlight on diversity has dimmed, so apparently have the efforts to push for more inclusion in the top leadership ranks, particularly for women. Women now hold 24.4% of all leadership positions at the largest medical device companies in the world.
The average composition within each medtech company increased to 23.9% from 23.1% last year.
Composition refers to the number of women in leadership roles as a
share of the total number of top executives within a company. The rise in average composition means companies are slowly creating more gender-diverse executive teams.
These modest improvements from the previous year underscore the persistent gender gap in executive medtech roles. These leaders shape the direction and decision-making of companies responsible for saving and improving patient lives, so it’s crucial to diversify the executive ranks to be more representative of the patient population, according to Nada Hanafi, co-founder of MedTech Color, a nonprofit organization that aims to advance the representation of underrepresented ethnic minority groups. >>

“It’s not reflective of the patients we’re serving, and that’s why women’s health and focus is so behind,” Hanafi told Medical Design & Outsourcing. “There’s an opportunity to do much better here. … Challenge the industry. If you’re not intentional about who you’re hiring, you’re not leading or leaning into wanting better health outcomes.”
The data for 2024 shows that despite years of pledges to improve, women continue to be underrepresented in executive positions across top medtech firms. Last year, the industry lost its most powerful leader with the resignation of former J&J MedTech Worldwide Chair Ashley McEvoy, who was succeeded by Tim Schmid.
“Industries that understand their customer base outperform the S&P. We have blind spots. We need to pay attention to who is making the buying decisions — often it’s women,” McEvoy said at the MedExec Women conference in April. “Let’s look at our patient population and ask: What can medtech do to better represent the patient population within our companies, and on our boards? … It’s not equitable yet. Smart companies are sticking by diversity, equity and inclusion until we get equity. Public companies have to perform. We
need a talent base that reflects our customer or patient base.”
The state of women in medtech leadership
Our analysis uses job titles from corporate website leadership pages. We excluded 22 of the companies on the Medtech Big 100 (our ranking of the world’s largest medical device businesses) from this analysis because they did not list executives on their websites or provide information to determine gender, such as pronouns or executive portraits.
In 2024, women held just 24.4% of executive positions across 78 of the largest medical device companies, with 217 women among 889 executives. This figure is a slight increase from previous years but far from parity.
On average, medtech companies had three women in leadership roles, but there are stark differences between companies. Leading the pack, Ambu has achieved 50% female leadership, and Getinge (44.4%) and Insulet (42.9%) are also close to achieving parity in leadership.
In 2024, three companies — Demant, Medacta, and Alphatec — reported no women in top leadership roles. This is an improvement from the seven companies
This chart shows the C-suite composition at the largest revenue-generating medtech companies in the world.
Six years of data show that the growth in the percentage of women in executive roles is slowing down.
with no female executives in top roles in 2023 and 14 in 2022.
Britt Meelby Jensen at Ambu, Suzanne Winter at Accuray, Ashley Cordova at Novocure, Anna Maria Braun at B. Braun Melsungen, An Steegen at Barco, Britta Fünfstück at Paul Hartmann, and Helen Giza at Fresenius Medical Care are among the few women leading top medtech companies.
The overall list of women medtech CEOs remains relatively unchanged from the year prior, aside from Cordova becoming CEO of Novocure this year and former GN Hearing CEO Gitte Pugholm Aabo stepping down.
Just under 9% of companies in our analysis have women CEOs, indicating that the medtech industry isn’t much different from overall corporate America. According to Fortune, 10.4% of CEOs on the 2024 Fortune 500 list are women, unchanged from 2023.
How to improve diversity in medtech
There are medtech organizations and networking groups that aim to strengthen the presence of women leaders. MedtechWomen wants to advance the representation of underrepresented ethnic minority groups in the industry. MedTech
Color boosts representation in the medtech industry. MedExecWomen also empowers women to improve gender parity in medtech leadership. Stripes is a networking group that empowers women to take on leadership roles.
“Organizations like Stripes are playing a pivotal role in advancing women in leadership within the medical device industry,” said Jacobs, who is one of Stripe’s co-founders. “By providing leadership development programs and mentorship opportunities, Stripes and similar organizations are helping to create a more dynamic and inclusive industry, where women can drive the future of healthcare innovation.”
McEvoy, who now sits on the board at Proctor & Gamble, said change can start with a single individual.
“We can each contribute to increasing diversity by thinking about whether we are perpetuating biases through our choices and even the words we use,” she said. “More of us need to recognize and advocate for a diverse team. Be self-aware, then change how you behave. When you are in a position of power, challenge biases and model what it takes to ensure a merit-based approach to growth.”

Design, develop and manufacture custom medical device components and finished devices.
Continuous glucose monitors (CGMs) and applicators
Insulin patch pumps and traditional insulin pumps
Automated external defibrillators (AEDs) and wearable defibrillators
Robotic surgical systems and accessories
Electrophysiolog y catheters and systems

Intravascular ultrasound systems (IVUS) catheters and consoles

KAYLEEN M. BROWN MANAGING EDITOR – DEVICETALKS

STRUCTURAL HEART AND AORTIC PRESIDENT NINA GOODHEART SHARES HOW MEDTRONIC SEEKS TO SAVE MORE LIVES THROUGH ITS SMART TRIAL AND DEVELOPMENT OF GENDER-SENSITIVE MEDICAL DEVICES.
The exclusion of women from clinical trials for decades has resulted in a staggering lack of data and understanding about their health.
This historical oversight has had profound consequences on the treatment and diagnosis of cardiovascular disease in women, Medtronic SVP and Structural Heart and Aortic President Nina Goodheart said in an interview at DeviceTalks Boston 2024.
“We are facing a crisis in women’s health, and it’s imperative that we use every tool at our disposal to address it,” she said.
>>
Historically, cardiovascular care focused predominantly on men, leading to a systemic exclusion of women in research and treatment. This bias was cemented in the 1970s after the thalidomide crisis, which prompted the FDA to ban the inclusion of women of childbearing age in early-phase clinical trials. Even preclinical studies were primarily conducted on male animals to avoid the complexities of female hormones.
“For about 20 years or so, there were no studies of women,” Goodheart said. It wasn’t until the 1990s that pioneering cardiologist Dr. Nanette Wenger pushed the National Institutes of Health to mandate the inclusion of women in clinical trials. The American Heart Association’s Go Red for Women campaign in the early 2000s further increased awareness, leading to a decrease in female cardiovascular deaths. However, recent data shows a renewed uptick in fatalities among women.
Women often present with different, less recognizable symptoms, leading to delayed diagnoses and missed opportunities for life-saving treatment. And physicians don’t take women and their symptoms as seriously as they do for male patients, Goodheart said.
“We’ve got to do better taking care of 50% of the population,” she said.
Goodheart and Medtronic are trying to rectify the imbalance and save more lives. To close the gap, they’re using findings from a clinical trial and real-world evidence (RWE) to develop gender-sensitive medical devices.
One of the biggest barriers to improving women’s cardiovascular outcomes is their reluctance to participate in clinical trials. This is due to a lack of representation in trial design, mistrust of predominantly male clinical environments, and societal factors that discourage women from seeking care.
“If we want better outcomes for women, we need to make trials more inclusive and address the systemic issues that keep women out,” Goodheart said.
Medtronic is partnering with associations, physicians, and even competitors to make trials more inclusive. Medtronic has also introduced patient education initiatives to increase
“If we want better outcomes for women, we need to make trials more inclusive and address the systemic issues that keep women out.”
trust and awareness, helping more women understand the importance of trial participation and encouraging them to seek care earlier
“It’s not just about getting more women to participate,” Goodheart said. “It’s about designing trials that take into account the real-life experiences of women and other underrepresented groups.”
Traditional clinical trials have historically been conducted in controlled environments with limited diversity, making it difficult to assess how devices work for a broad population.
The Small Annuli Randomized to Evolut or Sapien (SMART) trial is different. It’s an international study led by Medtronic, designed
Goodheart described the trial as “bringing together engineers, physicians, and researchers to generate data that are inclusive of the female population.”
“We’re not just looking at gender,” she said. “We’re also considering other demographics like age, ethnicity, and lifestyle factors. This gives our engineers a deeper understanding of how to design devices that perform optimally for all patients, not just the average male.”
Medtronic’s engineers have taken these insights to heart, developing new cardiac valves and stents specifically for women, whose arteries tend to be smaller than men’s. This miniaturization requires meticulous precision without compromising effectiveness, an ongoing challenge that Medtronic is actively addressing, Goodheart said.
The trial also sheds light on the
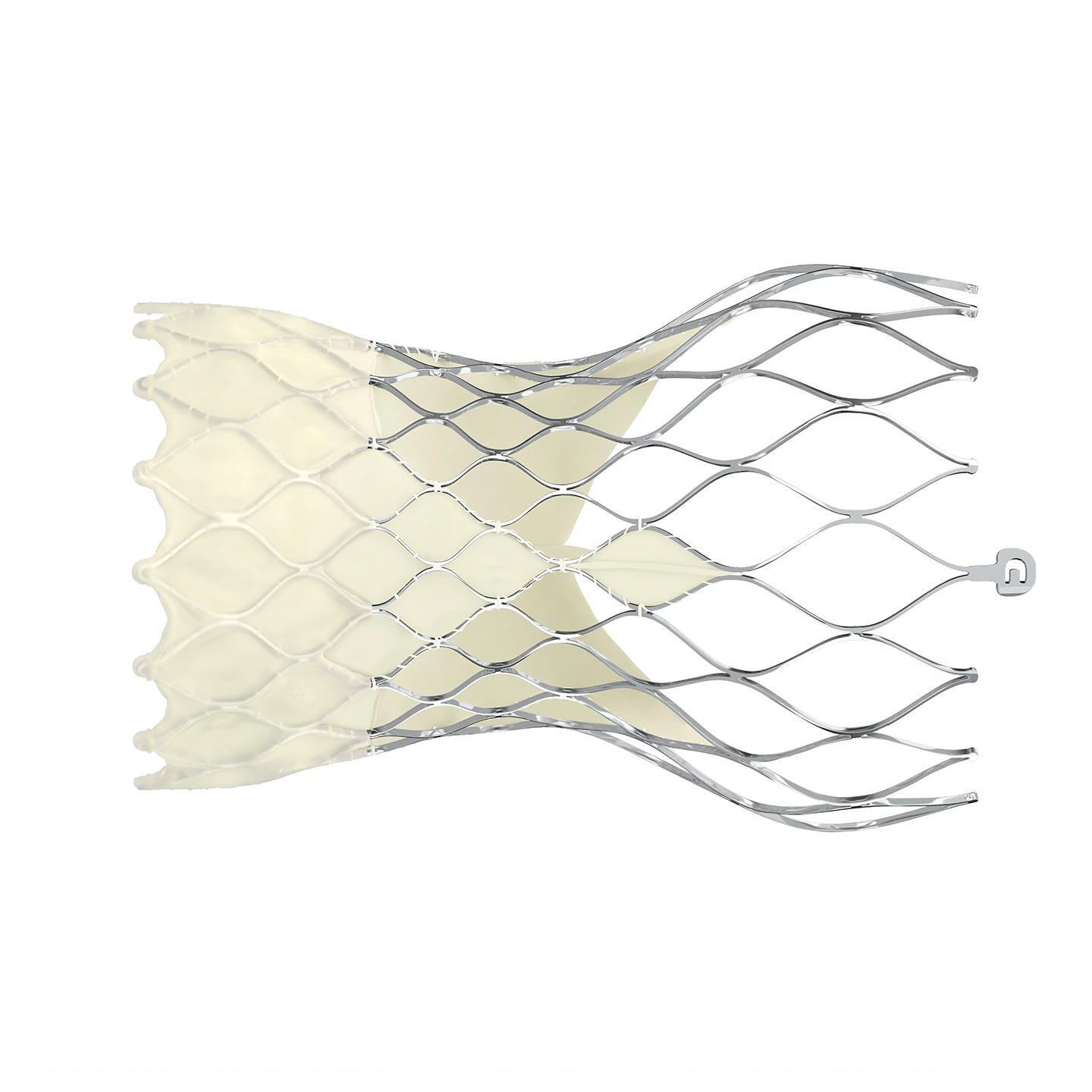
The Medtronic Evolut Pro has a self-expanding nitinol frame. Image courtesy of Medtronic

From components and finished devices to the next generation of medical technologies, now more than ever, you can count on us.




























Implantable Pulse Generator Systems

Suppor t Catheters
























Strengthen your innovation with our rapid prototyping and clinical know-how to take you seamlessly from concept to production






















Batteries & Capacitors Neuro Leads Aspiration & Access Catheters

Delivery & Retrieval Introducers Guidewires


















Get to market faster by utilizing our platform technologies and market-ready products that provide differentiated performance and portfolio expansion
Extend your reach through our global R&D and manufacturing capabilities to consistently achieve design requirements, quality standards, and on-time delivery





















Cardiac Leads
















Imaging & Sensing









Delivery Systems Electrophysiology Catheters














(continued from page 38)
How RWE is driving change in medical device engineering
One of the most impactful aspects of the SMART trial is its use of RWE, Goodheart said.
“Traditional clinical trials are often limited in scope and don’t capture the full range of patient experiences,” she said. “RWE allows us to see how devices perform outside of controlled conditions, which is crucial for understanding how they will work in the real world.”
For engineers, the use of RWE means they can design with confidence.
“We’ve been able to identify gender-specific trends in device performance, allowing us to make real-time adjustments that improve outcomes for women,” Goodheart said.
For example, heart valves engineered with data from RWE are now
being designed to accommodate the unique physiological needs of female patients, like those differences in artery size and tissue elasticity
And because women’s bodies change over time due to factors like pregnancy and menopause, which can affect how devices perform, it’s essential to develop more adaptable devices.
“Our goal is to create devices that can adjust to these changes, offering women better outcomes no matter where they are in life,” she said.
Beyond individual device adjustments, RWE is guiding broader engineering strategies. Medtronic is using data from the SMART trial to develop more durable materials for heart valves for longer durability.
“Women live longer than men on average, which means their devices need to last longer
and perform reliably over time,” Goodheart said.
This insight has led to the development of more robust valves that not only reduce the need for future surgeries but also improve patient outcomes and overall healthcare costs.
Other major device developers are also leveraging RWE to make their devices more inclusive. This shift toward data-driven design is setting a new standard prioritizing patient diversity and long-term outcomes.
“RWE is more than just data,” Goodheart said. “It’s a tool for innovation.”

























General Manager and Senior VP of Quality & Regulatory ARCH Medical Solutions
BS Biomedical Engineering
Tulane University
MS Engineering
Purdue University

Dawn Frazer is Senior Vice President of Quality & Regulatory at ARCH Medical Solutions (AMS) and serves as the General Manager of ARCH Medical Solutions - Seabrook facility. Over her 35+ years in the medical device industry, she has gained specialized expertise in all aspects of manufacturing, quality, and regulatory.
Rather than gaining experience by changing places of employment, in contrast, Dawn has expanded her knowledge by growing from within. She has held positions in two OEM businesses and one contract manufacturing company, all of which produced medical devices.
She started her career in Research and Development, stepped into Quality and Regulatory positions, migrated to Operations, and finally landed at General Manager. While some might say that the changing areas of responsibility can make one a “jack of all trades, master of none”, in Dawn’s case, it has given her an extensive knowledge base and the ability to look at problems as part of the big picture.
Under Dawn’s leadership, AMS Seabrook has optimized its organization structure, improved supervisory capabilities by promoting from within, implemented live business metrics and enhanced continuous improvement initiatives leading to a sizeable increase in the company’s EBIDTA and revenue per employee metrics.

What initially attracted you to the MedTech industry? How has your perspective on the industry evolved over the course of your career?
As a young adult making decisions about my future career, I was drawn to the idea of becoming a doctor. I entered Biomedical Engineering School at Tulane due to the offering of an accelerated program. After meeting some medical students and residents while at Tulane, I decided that I was not well suited to be a medical professional. This was in part due to my intolerance for “blood” and second, due to my observation of the “crazy” hours worked by medical students. Instead, I turned to a Master’s degree in Engineering. This change led to my first job as a Biomedical Engineer at a division of Medtronic that designed and manufactured ECG / TENS / Defibrillation electrodes and associated cables. Working in R&D on patient products seemed to be the next best thing to direct patient contact.
As I entered the work force in the mid-1980s, the medical device industry was far less controlled and far less broad than it is today. The FDA had authority over Good Manufacturing Practices (GMP) for medical devices. Design Control requirements were written into the 1996 Quality System Regulation (QSR), which became effective on June 1, 1997 and then regulated Design Control requirements for medical devices. Now, in 2024, we see the FDA issuing the Quality Management System Regulation (QMSR) to harmonize the FDA’s cGMP regulatory framework with that used by other regulatory authorities. This latest move by the FDA works to promote consistency between international and US requirements by referencing the international standards specific for medical device quality management systems set by International Organization for Standardization (ISO), ISO 13485:2016.
I still enjoy the ability to produce products to serve patients in need. The increasing requirements that have come over the decades make this endeavor reliant on cost effective processes to be able to meet both the regulatory and business pressures.
As technology continues to advance rapidly, how do you think MedTech engineers can stay ahead of the curve and ensure they are equipped with the necessary skills and knowledge?
As devices become more complex with new features and tighter tolerances, it is crucial to prioritize training of the manufacturing labor force. Dedicating adequate time to train employees allows MedTech engineers to stay updated with current industry needs and necessary skills. This includes a high-level understanding of manufacturing equipment and the programming of machines. Once employees achieve the required level of expertise within their specialized areas, opportunities arise to expand their knowledge across other areas of the business. For instance, attending trade shows is one way that our engineers at AMS Seabrook stay up to date with new innovations in the industry.
For the rest of Dawn Frazer’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech

Shane Wood is the Director of Technology for polymers (filmcast, extrusion, complex catheter manufacturing, and balloons) for Confluent Medical Technologies.
She has approximately 19 years of experience in medical device manufacturing, and, in that time, has led production and new product introduction engineering teams utilizing filmcast, extrusion, and catheter assembly technologies. Prior to medical device manufacturing, she held engineering positions in the semiconductor and optical fiber industries. Shane holds undergraduate degrees in chemistry/ math and chemical engineering from Agnes Scott College and the Georgia Institute of Technology. She has a Master of Engineering Degree from North Carolina State and a Master of Biomedical Engineering from Colorado State University. Shane also holds a graduate certificate in Regulatory Affairs from the University of Georgia and is a Certified Six Sigma Blackbelt.
She lives in Chattanooga, TN with her wife and two awesome kids. In her spare time, she enjoys hands-on projects and artistic welding.

perspective on the industry evolved over the course of your career?
I was originally interested in the medtech/pharmaceutical industry because I wanted the opportunity to build a long-term career in a stable manufacturing engineering environment. At that time, I had seen, firsthand, the impact of sustained volatility in the semiconductor and optical fiber markets. Thankfully, over the last 19 years, the medtech industry has given me the opportunity to build a career while improving lives and doing work that matters.
As a seasoned professional in the industry, how do you envision the future of medtech? What emerging technologies or trends do you believe will have the most significant impact?
The future of medtech will be driven by those willing to innovate and consistently challenge the status quo. The trend toward decreasing size (thinner walls, tighter tolerances) without sacrificing device performance will continue to have a significant impact in the coming years.
From your experience, what are some of the key challenges that medtech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
I think there are a number of trends that will challenge engineers in the medical device industry in the coming years. These trends include increasing regulatory scrutiny of materials (for example pigments, PFAS, solvent regulation, etc), the tendency toward increased performance while decreasing device size, and sustained cost pressure. In most of these cases, innovation is key. Innovation can help solve the first two and, in most cases, help minimize the downward pressure on cost.
How do you think diversity and inclusion can contribute to the advancement of medtech engineering? How do you foster a culture of innovation within your team or organization?
Research shows that more diverse ideas consistently lead to better overall results. Teams with diverse backgrounds and perspectives bring a wider range of ideas, which lead to more creative and effective solutions to problems. Innovation is a core value at Confluent. As leaders, we help foster that culture by supporting an environment where we are not afraid to fail. Learning through failure is a necessary part of a culture of continuous improvement and innovation.
For the rest of Shane Wood’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech
Product Development
Engineering Supervisor
Cretex Medical | QTS
Bachelor’s Degree in Chemical Engineering
Saint Louis University, Baguio, Philippines

Rona Gungon is a Product Development Engineering Supervisor with over 17 years of experience in the medical device packaging industry, including the designation of Certified Packaging Professional (CPP).
Rona’s career in the medical device packaging sector has been marked by a series of progressive roles, including Quality Assurance, Customer Quality Liaison, and Project Engineering. Her experience and insightful understanding of the industry’s complexities enables her to now lead a team of Product Development engineers.
Before transitioning to the medical device packaging industry, Rona spent over four years in the semiconductor industry as a Senior Failure Analysis Engineer. This role honed her problemsolving skills and provided a strong foundation for her subsequent career in packaging engineering.
Rona holds a bachelor’s degree in chemical engineering from Saint Louis University, Baguio, Philippines.

What initially attracted you to the medtech industry? How has your perspective on the industry evolved over the course of your career?
When I moved to Minnesota, a major medtech hub, it was natural for me to look at changing careers. My mother, who was the recipient of a life-saving medical device, has also inspired me to continue working in this field.
I’ve found the medtech industry to be a hotbed of innovation and advancement. I’ve seen new technologies emerge that are saving lives, and improving the quality of life, for patients around the world.
As a seasoned professional in the industry, how do you envision the future of medtech? What emerging technologies or trends do you believe will have the most significant impact?
I think the integration of artificial intelligence (AI) into med tech will have the most significant impact. With the increasing use of robotic-assisted systems, there is significant potential for integrating AI into these technologies.
From your experience, what are some of the key challenges that medtech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
The direction of the industry is increased integration of AI and digital health solutions with existing medical devices and systems. The technical aspects of those changes alone present significant challenges for engineers. Additionally, these technologies will drive greater regulatory complexity.
Engineers must stay up to date with both the technology, and the everevolving state of global regulations, to ensure that safety standards, patient privacy and data security concerns are correctly addressed.
As technology continues to advance rapidly, how do you think medtech engineers can stay ahead of the curve and ensure they are equipped with the necessary skills and knowledge?
I think continuous learning and professional development are crucial to staying ahead of the curve. This can be achieved by attending industry conferences and seminars to learn from experts and discover new technologies. While ensuring technical skills stay sharp, it is also very important to invest in soft skills, such as communication and adaptability. Being open to change is vital for success in our constantly evo lving field.
For the rest of Rona Gungon’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech

her parents sort plastic parts for their manufacturing business, Value Plastics. In 1987, that experience inspired her to purchase Eldon James, a small shop with a few molding machines. Since then, she has built the Colorado-based business into an ISO 9001 and ISO 13485 certified, world-class manufacturer of 6,000-plus proprietary and customized tubing and fluid-handling solutions designed for pharmaceutical, medical device, life sciences, bioprocess, biomedical, and other critical-use applications. Along the way, in 2001, she became the sole owner. In March 2024, Marcia spearheaded Eldon James’ sixth expansion with a new 106,000-squarefoot facility that adds manufacturing and clean room availability. From the start, family has played an important role in the business. For years Marcia’s three children, now grown, held summer jobs and contributed to special projects. And her husband, William, joined the business nearly 20 years ago where he continues to serve as Eldon James’ vice president.

on the industry evolved over the course of your career?
In growing and diversifying the business, I noticed that the systems used by pharmaceutical companies to produce vaccines, antibiotics, medical tests, intravenous fluids, and other medical liquids were highly sophisticated. But traditional tubing connections for these systems remained relatively simple. So, there was a clear opportunity to support this underserved aspect of the industry with innovative new tubing connection products.
Working with medical and pharmaceutical companies has only deepened my belief that they deserve a world-class product that won’t compromise health and safety standards for the patient, environment or our communities.
From your experience, what are some of the key challenges that medtech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
We are seeing a cultural shift as the medtech industry matures. In the past, there was more of a mindset that cost was no obstacle, whether introducing new products or expanding production to meet demand. That’s changing in the wake of greater global competition and economic pressures. There’s more business pressure to introduce efficiencies into the manufacturing equipment and processes used to produce medical devices and pharmaceuticals—without compromising quality and safety. And, at the same time, there is the move toward greater environmental stewardship.
The medtech professionals who thrive in this new business reality will be those who consider all of these factors; commit themselves to continuous education on new processes, design practices and materials; and take time to listen to the real-world experiences of customers, employees and patients.
Considering the increasing importance of interdisciplinary collaboration, how do you think medtech engineers can effectively collaborate with professionals from other fields, such as medicine and computer science, to drive innovation in the industry? Interdisciplinary collaboration should start first within your own organization. There is so much that each of us can learn. I encourage employees to cross-train wherever possible and ask questions. Real innovation comes not just from collaborating with other technical professionals in medicine and science. It also comes from hearing what customers want and need, what they like and dislike, and how their lives would improve if something we take for granted could be done differently. Customers have been some of our greatest sources of inspiration for creating new product lines that completely reimagine how medical and pharmaceutical systems connect.
For the rest of Marcia Coulson’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech
BA Computer Science and Economics (First Class Honors) University College Cork, Ireland

Pauline Oakes is currently Vice President, Operations, EMEA/APAC region for Integer. In this role, she oversees all operations in the region spanning four manufacturing plants and responsibility for approximately 2,000 associates. She joined Integer in 2019 and brings more than 27 years of medical device industry experience to the company.

Prior to joining Integer, Pauline held various roles of increasing responsibility in areas spanning site leadership, operations, supply chain and IT with companies including Becton Dickinson, Bard, Clearstream Technologies and AngioDynamics. Throughout her career, she has honed her change management, cross-functional leadership, strategy development and continuous improvement skills through involvement in a varied range of corporate development phases, from start-ups, buy-outs, launches, and acquisitions, each requiring different leadership skills and approaches.
Pauline holds a bachelor’s degree in computer science and economics from University College Cork in Ireland. She is also a member of the governing board of South East Technological University and a Greentech HQ board member.

What initially attracted you to the medtech industry? How has your perspective on the industry evolved over the course of your career?
As a young professional, I initially joined the medical device industry in an IT role with a startup after working as an IT programmer for a number of years. My job was to set up IT systems to support the business. In working with various functions across the business to achieve this objective, over time I learned about the complexity of designing and manufacturing medical device products and the impact they can have on people’s lives. It became very apparent how critical it is to have excellent safety, quality, and of course top talent on your team to be successful in the medtech industry.
As a seasoned professional in the industry, how do you envision the future of medtech? What emerging technologies or trends do you believe will have the most significant impact?
Over the years, it has been amazing to see the progression of products and advances in procedures that have enabled more patients to get treated successfully. It truly reinforces the importance of innovation. When you are on the ground working in the industry day in and day out, you have the opportunity to witness ways everyone can contribute to genuine progress in the treatment of complex healthcare challenges.
In the future, I think we will see more collaboration between medical device, technology and robotics companies as they leverage new and emerging areas like AI and robotics to treat patients and maximize cost efficiencies in their manufacturing processes.
From your experience, what are some of the key challenges that medtech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
Perhaps the biggest challenge I see in the coming years is the ability to keep up with the pace of change in the medtech industry, from both a procedural and regulatory perspective. Additionally, offsetting significant inflationary pressures with automation, achieving efficiency improvements and building robust supply chains to ensure continuity of supply will remain a challenge.
Engineers can prepare for these challenges by keeping abreast of advancements in the industry by attending trade shows, watching clinical procedures, working with the appropriate partners to assess automation opportunities, and partnering with supply chain teams in the early design phase to ensure security of supply with dual sourcing strategies.
For the rest of Pauline Oakes’ insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech

Engineering Manager
Medical Device Components
BS Industrial Chemistry
Chemical Sciences Faculty at Autonomous University of Chihuahua, Mexico
Bachloer of Engineering in Chemical Engineering
Chemical Sciences Faculty at Autonomous University of Chihuahua, Mexico MBA
CETYS University in Mexicali, Mexico


What initially attracted you to the medtech industry? How has your perspective on the industry evolved over the course of your career?
Being in an industry with a lot of speed on engineering advances, is a great market to develop creativity skills with a purpose that is beneficial for the society. As the industry expands, technology evolves, and companies diversify their portfolios, this industry is the perfect environment for a challenge seeker personality.
Ser una industria con una gran velocidad en avances tecnológicos, un gran mercado para desarrollar habilidades creativas con un propósito benéfico para la sociedad. De la misma forma que esta industria crece, la tecnología evoluciona por lo que la diversificación de portafolio en este mercado es el ambiente perfecto para un buscador de grandes retos.
As a seasoned professional in the industry, how do you envision the future of medtech? What emerging technologies or trends do you believe will have the most significant impact?
Process technologies will imply cleaner and shorter processes, those where our materials will require less temporary additions to them, this will result in better products with less chemical interactions, higher engineering challenges and continued research. Energy technology will lead the material transformation processes.
La tecnología en un futuro implicara procesos más rápidos y limpios, esos en los que nuestros materiales necesitarán menos aditivos temporales, esto tendrá como resultado mejores productos con menos exposiciones a químicos nocivos, retos ingenieriles y tecnológicos, así como investigaciones más complejas.
From your experience, what are some of the key challenges that medtech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
Continued self development is key in this industry, we should keep up with medical innovations accordingly, we as engineers should be aware of the growth in medical advances as well as applications that will result in technology innovations as well.
La educación continua es clave en esta industria, nosotros como ingenieros debemos mantenernos al día con innovaciones, avances y aplicaciones médicas que por ende resultarán en innovaciones tecnológicas.
For the rest of Maya Aguilar’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech
Senior Manager, Commercial Excellence in R&D
Phillips Medisize
Bachelor’s in economics and Language ; MS Supply Chain Management ; Executive MBA
Copenhagen Business School

Reporting to the vice president of Global Innovation and Development, Gitte Pfeffer heads up a global commercial excellence unit within the disciplines of cost estimation and business analytics, customer contracts and negotiations, and strategic sourcing. Her team supports the business across Pharma, MedTech, Diagnostics and Commercial segments.

She has more than 20 years of leadership and international business experience working with suppliers, partners and co-workers across the US, Europe, the Middle East and Asia, primarily in the pharma industry. Her leadership style is energizing, caring and result-driven. She has a distinct skill within negotiations and business understanding and has driven several organizational changes and started up new business areas.
Pfeffer’s professional career has taken her in the direction of leadership, project management, strategic sourcing, leadership development, consulting, change management, sales, business development, strategy development, alliance management, contract negotiations and organizational turnarounds, to name a few. Pfeffer has held various positions ranging from Lead Negotiator to equity partner in a consulting house to Executive Management support. Prior to joining Phillips Medisize in 2022, she was Senior Director in Device R&D at Novo Nordisk A/S in Denmark.

What initially attracted you to the medtech industry?
It was pure coincidence. I have a commercial background specializing in Strategic Sourcing. I worked for many years at a pharma company that was a frontrunner in terms of applying a strategic approach to how companies procure goods and services from their suppliers. I was fortunate to take on several different roles in the company, and at one point I was appointed head of Strategic Sourcing for Devices and Needles. I immediately felt passionately about devices and the role they can play if they are designed to enable patients to not feel limited by their illness.
How has your perspective on the industry evolved over the course of your career?
The industry is very broad, but in the part, I am involved in, I see an industry that has evolved and has a more patient-centric approach. Patients do not want a vial and syringe with a needle as thick as a straw or receive treatment that requires them to live a life full of limitations. Today, you need to provide patients with treatment solutions that allow them to live better lives, without limitations. Patients want to be in control and take charge of their life and health. Medtech has become an important enabler for that.
In your opinion, what areas or applications within medtech have the greatest potential for growth and innovation? Why do you consider them promising?
Any type of device related to measuring and tracking health data and providing feedback will have the greatest potential. Longevity is becoming a phenomenon in the broader population and that links to many different areas, including physical health, mental health and physical appearance, which all have an almost unlimited potential of related areas for growth. A healthy population is good business.
As technology continues to advance rapidly, how do you think medtech engineers can stay ahead of the curve and ensure they are equipped with the necessary skills and knowledge?
Staying ahead of the curve is challenging but keeping up with the development is possible. Stay curious and updated on the latest trends, and encourage your people to upgrade their skills. My experience from working with highly skilled professionals is that they naturally seek new knowledge and opportunities for learning and growing. If that is not possible, they seek opportunities elsewhere.
For the rest of Gitte Pfeffer’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech

and emergency medicine. She earned her bachelor’s degree in chemistry from Allegheny College and has accumulated a wealth of experience across various fields, including food manufacturing quality assurance and emergency medical services.
Bobbie’s career is marked by her expertise in ISO 18562 Particulate and VOC testing, highlighting her commitment to maintaining the highest standards in medical technology. In her previous roles in food manufacturing, she worked closely with regulatory agencies such as the FDA and USDA, demonstrating her ability to navigate complex compliance requirements. Her experience in emergency medicine and emergency transport has also given her a unique perspective on rapid response and critical care.

on the industry evolved over the course of your career?
What initially attracted me to the medical field, and eventually to the MedTech industry, was the opportunity to make a meaningful impact on people’s lives. As a college student working in the emergency department, I quickly realized that the patients I interacted with were often in distress, whether their situation was life-threatening or not. In those moments, I had the chance to positively influence their emotional and physical wellbeing. As I transitioned from the medical field into food manufacturing and then entered MedTech, I found that I could continue to make that same kind of impact, albeit in a different way. My journey through the MedTech industry has taken me through both the application side and the device safety side, giving me a well-rounded perspective on the field. While my view has evolved, the core mission remains the same: ensuring patient safety and wellbeing.
As a seasoned professional in the industry, how do you envision the future of MedTech? What emerging technologies or trends do you believe will have the most significant impact?
Beyond her professional achievements, Bobbie is a passionate advocate for women’s empowerment. She actively participates in and presents at various summits focused on advancing women in the workforce. Additionally, she engages with local school districts, delivering educational seminars on MedTech to inspire and inform future generations.
Bobbie’s community involvement extends to her role in the volunteer fire department, reflecting her dedication to public service and emergency preparedness. She is also focused on advancing her career with aspirations of becoming a toxicologist and is currently working towards obtaining her EMT certification.
MedTech is ever evolving, with regulatory agencies like the FDA becoming increasingly knowledgeable about medical device advancements and adapting their regulations accordingly. In recent years, we have witnessed significant changes in the medical device and pharmaceutical testing industries, leading to more stringent standards. I believe that the current trend, where guidelines are interpreted differently among manufacturers and testing facilities, will be curtailed, giving way to more strategic and consistently enforced regulations. We are already seeing this shift in areas like ISO 10993-17 toxicological risk assessments.
From your experience, what are some of the key challenges that MedTech engineers are likely to face in the coming years? How can professionals in the industry prepare for and navigate these challenges effectively?
MedTech engineers consistently face the challenge of working with new technologies and novel medical devices. While the rules and guidelines remain consistent, the customization required for these innovative solutions demands an outside-the-box approach. Adapting to the evolving landscape is essential for MedTech professionals, especially for engineers with years of experience. It is easy to fall into familiar patterns but clinging to old habits can be a limiting factor in this industry. Leveraging past knowledge is valuable, but being open to change and being flexible in your approach is crucial for success in this dynamic field.
For the rest of Bobbie Heisler’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech
BS Mechanical Engineering
Worcester Polytechnic Institute
MS Engineering Management
Tufts University

Andrea Nicolaisen is an accomplished medical device quality and regulatory executive with a strong foundation in product development. Currently serving as the Vice President of Quality at Resonetics, a leader in advanced manufacturing for the medical device industry, she ensures regulatory compliance, quality systems management, and continuous improvement initiatives, all of which contribute to delivering exceptional products and services to clients in the medical device field.
Andrea has experience leading product development efforts for a range of complex technologies, including electro-mechanical PDT light systems, software-driven medical devices, dental implant systems, orthobiologics, regenerative products, glucose meters (IVD), and implantable left ventricle assist devices (LVADs).
She has a proven track record of leading multi-site quality organizations to achieve global regulatory compliance and maintain key certifications. Her expertise includes FDA 21 CFR Part 820 (cGMPs), 21 CFR 1271, ISO 13485:2016, ISO 14971, MDSAP, MDR, AATB, and IEC 60601-1 standards. Her commitment to quality and innovation has made her a trusted leader in the industry.

What initially attracted you to the medtech industry? How has your perspective on the industry evolved over the course of your career?
I’ve always had a deep passion for healthcare, which initially drew me toward the medical industry. In high school, I focused heavily on math and the sciences, but at the time, I wasn’t fully aware of the possibilities that engineering could offer within the medical device space. I assumed my path would lean more toward a clinical healthcare role. However, during my undergraduate studies, everything changed when I secured an internship at a large medical device manufacturer.
That experience opened my eyes to the incredible intersection between engineering and healthcare, and I realized how much innovation was possible in this field. The opportunity to apply engineering principles to improve patient care was a pivotal moment for me, and it set me on the path to a career in medical devices.
Over the course of my career, my perspective has evolved as I’ve witnessed the rapid pace of medical device innovation. The integration of emerging technologies today like artificial intelligence (AI), machine learning, and precision manufacturing has been remarkable, and it’s exciting to see how these advancements are pushing forward the boundaries of healthcare. Today, more than ever, the focus is on improving patient outcomes—whether through faster recovery times, enhanced/lifesaving decisionmaking through advanced analytics, or improving quality of life with new life sustaining and life supporting products. I’m proud to be part of an industry that is continuously evolving to make a meaningful impact on patients’ lives.
As technology continues to advance rapidly, how do you think medtech engineers can stay ahead of the curve and ensure they are equipped with the necessary skills and knowledge?
Keeping up with technological advancements is essential, and engineers must prioritize continuous learning to stay ahead of the curve. Staying informed about emerging technologies, industry trends, and regulatory changes is crucial for navigating the evolving medical device landscape. Developing cross-disciplinary knowledge is equally important, as the synergies between fields like AI, robotics, and healthcare are expanding rapidly.
Collaboration plays a key role in this process. Engaging with clinicians, regulatory experts, and fellow engineers fosters a deeper understanding of current trends and challenges, allowing engineers to anticipate market needs and innovate effectively. By embracing ongoing learning and collaboration, MedTech engineers can remain at the forefront of industry advancements.
For the rest of Andrea Nicolaisen’s insights, visit www.medicaldesignandoutsourcing.com/women-in-medtech