Profile: Nieves Gonzalo
page 12
Judith Hochman: ISCHEMIAEXTEND

page 18
Tricuspid valve intervention and intravascular imaging studies among “trials to watch” in 2023
TRIALS IN CORONARY IMAGING, transcatheter tricuspid valve interventions, transcatheter aortic valve implantation (TAVI) in small annuli, and further insights from the REVIVED-BCIS2 study are among the top trailed research coming in 2023 in the interventional cardiology space.

This is according to Mirvat Alasnag (King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia) and Roxana Mehran (Mount Sinai Hospital, New York, USA), who spoke to Cardiovascular News about the trials they are expecting to have the most impact throughout the year.


An analysis of Medicare claims data from more than 100,000 patients with multivessel coronary artery disease undergoing coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) has reopened debate on controversial guidelines concerning coronary revascularisation.
Analysis findings, involving data from the US Centers for Medicare and Medicaid Services, were presented by cardiac surgeon J Hunter Mehaffey (West Virginia University, Morgantown, USA) at the 59th annual meeting of The Society of Thoracic Surgeons (STS 2023; 21–23 January, San Diego, USA).
The research was instigated following the publication of guidelines from the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiovascular Angiography and Interventions (SCAI) in December 2021, which downgraded the indications for CABG from a class 1 recommendation to a class 2B in three-vessel coronary disease, a recommendation that left the cardiothoracic surgery world “shocked”, according to Mehaffey.
In a press release promoting the findings of the study, the STS—which pointedly declined to endorse the guidelines upon their publication in 2021—said that the guidelines rely heavily on data from the ISCHEMIA trial, which compared an initial invasive approach versus a
Consensus Update
conservative approach in patients with stable coronary artery disease. However, STS claims that the majority of patients in the ISCHEMIA trial were not representative of US patients undergoing CABG, and therefore the study did not fully represent the comparative benefits for patients who had multiple blockages in their coronary arteries.
The latest analysis, which was performed by a team of
Of the major cardiovascular society meetings coming up in 2023, the American College of Cardiology (ACC) annual meeting (4–6 March, New Orleans, USA) is the first out of the gate, with a programme that features several latebreaking trials namechecked by both Mehran and Alasnag.
Mehran highlights the release of results from the TRILUMINATE pivotal trial, the first major randomised trial evaluating the safety and efficacy of transcatheter tricuspid valve edgeto-edge repair (TEER) for severe tricuspid regurgitation (TR) as one to watch at the meeting. Trials in tricuspid interventions are of “pivotal” importance, Mehran comments, after significant previous focus has been placed on the aortic and mitral valves in the past. “New technologies are emerging that could take care of tricuspid valve regurgitation”, Mehran observed of the field in general.
TRILUMINATE is exploring the use of the Triclip (Abbott) system in patients in North America and Canada, and Mehran commented
Continued on page 2
Featured in this issue: www.cardiovascularnews.com February 2023 | Issue 68 7
the future of aortic
Ourania Preventza Multidisciplinary teams are
care
Vascular &
REGISTER NOW
APRIL 2023
IN PERSON AND VIRTUAL HILTON LONDON METROPOLE, UNITED KINGDOM C M Y CM MY CY CMY K ai16690416847_CX 2023 Ad Inhouse-225x54.pdf 1 21/11/2022 14:41:24
Endovascular
25–27
TUESDAY-THURSDAY
Continued on page 4
STS 2023
Medicare study reopens guideline debate around surgery and PCI in patients with multivessel coronary artery disease
Jim Lennon
CABG was associated with significantly improved longitudinal survival, with a nearly 60% reduction in all-cause mortality”
Continued from page 1
cardiac surgeons, cardiologists, and researchers at West Virginia University, included outcomes over a threeyear period, from 2018‒2020, capturing data from the US Centers for Medicare and Medicaid Services database for patients undergoing isolated CABG or multivessel PCI for acute coronary syndrome.
“We used one of the largest and most inclusive databases of patients hospitalised in the USA, including all patients over the age of 65 on Medicare,” said Mehaffey. “We performed a very robust statistical analysis including propensity score balancing to help ensure that the groups of patients who underwent stenting versus those who underwent bypass surgery were well matched and well balanced in order to compare their outcomes.”
The population included 104,127 patients with multivessel coronary disease, with more than 51,000 patients undergoing CABG and 52,000 undergoing PCI following the application of exclusion criteria.
According to Mehaffey, the analysis demonstrated a significantly lower hospital mortality for the patients who underwent CABG compared to those who underwent PCI. “CABG was associated with significantly improved longitudinal survival, with a nearly 60% reduction in all-cause mortality at only three years,” Mehaffey told delegates at the STS meeting, a finding that was met with a smattering of applause from members of the audience.
Additionally, the researchers found a marked reduction in both 30-day and three-year readmissions for myocardial infarction (MI). CABG patients were also significantly less likely to need any additional stenting or intervention on their coronary arteries during those three years, Mehaffey reported.
In discussion that followed the presentation at STS, speakers agreed upon the need to reappraise the recommendations within the ACC, AHA and SCAI guidelines based upon the analysis. Mehaffey commented that the findings underscore the importance of physicians “reading the fine print” when it comes to interpreting guidelines into their own practice. However, speaking to Cardiovascular News,
interventional cardiologist and clinical trialist David J Cohen (St Francis Hospital and Heart Center, Roslyn, USA) cautions against overplaying the results of the study, arguing that the use of observational data, rather than randomised trial data, limit the conclusions that can be drawn from the analysis.
Cohen questions the use of Medicare data as the basis for the study, commenting that these “provide very little in the way of granular information” such as angiographic complexity, SYNTAX score, left ventricular function, or other parameters—such as frailty—that may influence long term outcomes among these patients. “The simple answer is that the claims data are insufficient to truly risk adjust this comparison,” he says.

Further to this, Cohen suggests that the investigators have “failed to level the playing field” between the two therapies. “That can be seen in their risk-adjusted outcome curves that diverge in favour of bypass surgery almost immediately,” he says, which runs counter to trends seen in randomised trial data, which may take several years or more to emerge.
“If you look at the randomised trial data that support a survival benefit of bypass surgery over PCI in patients with three-vessel coronary disease the treatment effect is about a 20% relative risk reduction,” Cohen adds. “Here they found in their data almost a 60% relative risk reduction—another indication that there are major unmeasured differences between the two groups that have not adequately been accounted for in the analysis.”
Asked if he saw a route to moving this debate forward, Cohen commented that more randomised trial data are likely to be the only way to adequately inform the debate. “I wish that our colleagues would divert the time, energy and effort that they put into these types of study into designing and conducting randomised trials that would really inform the question,” he concludes.
n CX 2023: AORTIC AND PERIPHERAL ADVANCES
Cardiovascular News looks ahead to the 2023 Charing Cross (CX) Symposium (25–27 April, London) which features a comprehensive programme spanning advances in aortic techniques and technologies, as well as bringing together global experts to move towards consensus in revascularisation strategies for patients with chronic limb-threatening ischaemia (CLTI).

For more on this story go to page 8.
n TRICUSPID INTERVENTIONS IN THE SPOTLIGHT
One-year results from the TRISCEND study were delivered at PCR London Valves (27–29

November, London) by Stephan Windecker (Bern University Hospital, Bern, Switzerland) demonstrating favourable safety, efficacy, echocardiographic and quality of life outcomes in patients with symptomatic tricuspid regurgitation (TR) with the Evoque (Edwards Lifesciences) tricuspid valve replacement system. For more on this story go to page 15.
n ANTICOAGULATION POST-PCI IN STEMI PATIENTS
Bivalirudin is a safer and more effective anticoagulant than heparin for treating patients with ST-segment elevation myocardial infarction (STEMI) who undergo primary percutaneous coronary intervention (PCI), Gregg W Stone (Mount Sinai Hospital, New York, USA) and colleagues have reported.
For more on this story go to page 20.
Editor-in-chief: Simon Redwood
Publisher: Roger Greenhalgh
Content Director: Urmila Kerslake
Senior editor: Will Date will@bibamedical.com
Editorial contribution: Jamie Bell, Eva Malpass, Jocelyn Hudson
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: clientrelations@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Please contact the Cardiovascular News team with news or advertising queries
Tel: +44 (0)20 7736 8788
Published by: BIBA Publishing, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom
Tel: +44 (0) 20 7736 8788
BIBA Medical, North America, 155 North Wacker Drive Suite 4250, Chicago, IL 60606, United States
Tel: +1 708-770-7323
Printed by: Buxton Press
facebook.com/cardiovascularnews linkedin.com/company/cardiovascular-news
www.cardiovascularnews.com
Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address.
© BIBA Medical Ltd, 2023. All rights reserved.
Write to us!
If you have comments on this issue or suggestions for upcoming editions write to will@bibamedical.com
Make sure you get your copy of Next issue: May 2023
@cn_publishing
2 February 2023 | Issue68 News in brief The latest stories from the world of Cardiology
Top Stories
Medicare study reopens guideline debate around surgery and PCI in patients with multivessel coronary artery disease
STS 2023
If you look at the randomised trial data that support a survival benefit of bypass surgery over PCI in patients with three-vessel coronary artery disease, the treatment effect is about a 20% relative risk reduction”

Tricuspid valve intervention and intravascular imaging studies among “trials to watch” in 2023
Continued from page 1
that this “incredibly important” study, which is to be presented during a latebreaking trial session on day one of the ACC meeting, may possibly help to “improve and enhance” patient quality of life, as well as benefitting clinical outcomes, if early experience with the device is borne out in a randomised trial setting.
Another eagerly anticipated presentation at ACC 2023 will shed light on the effect of myocardial viability, percutaneous coronary intervention (PCI) and functional recovery as observed in the REVIVEDBCIS2 trial. The first data from the trial were released at the annual congress of the European Society of Cardiology (ESC 2022; 26–29 August, Barcelona, Spain), showing that PCI did not reduce all-cause mortality or heart failure hospitalisation in patients with severe left ventricular dysfunction and extensive coronary artery disease when compared to optimal medical therapy.
Alasnag recalls that the trial results sparked “intense discussions” at ESC 2022 following their initial presentation, and she said it will be “critical to assess the role of myocardial viability in the context of percutaneous revascularisation and functional recovery including clinical outcomes.”


“Teasing out the role of viability will be essential, particularly as we interpret these results in the context of other trials such as the STICH trial,” Alasnag tells Cardiovascular News, referencing the randomised trial comparing coronary artery bypass graft (CABG) surgery to medical therapy in patients with coronary disease and heart failure. Similarly, Mehran says that further findings from REVIVEDBCIS2 randomised clinical trial, will produce “enhanced data” on whether myocardial viability has a role in improving clinical outcomes in patients with heart failure due to coronary artery disease who have been treated with the best medical therapy versus PCI. The issue of myocardial viability is “key” in the context of ischaemic heart disease, said Mehran.
Outside of the ACC meeting, Alasnag identifies the RENOVATECOMPLEX-PCI trial, which is designed to investigate whether PCI under the guidance of intravascular ultrasound (IVUS) or optical coherence tomography (OCT) improves clinical outcomes compared with angiographyguided PCI in patients with complex lesions, as another to watch in 2023.
“This is a prospective, randomised, open label parallel trial where the choice of intravascular imaging devices such as IVUS or OCT are at the operator’s discretion,” explains Alasnag. “Use of intravascular imaging devices is permitted at any step of the PCI (pre-, during, and post-PCI, but intravascular imaging evaluation after
in six Chinese hospitals with 1,200 participants enrolled. Subjects are assigned to either the routine clinicallyindicated diagnostic care group or the CTA/CT-FFR care group.
Noting its comparability to recent studies in cardiovascular intervention, Alasnag notes: “[TARGET] would complement results of the previously published FORECAST trial conducted in the UK, where a strategy of FFR-CT compared to usual care of patients with stable chest pain did not lower costs or improve quality-of-life measures.”
Staying in the coronary space, Alasnag highlights the YELLOW III study as one to follow. The study aims to assess the effect of evolocumab on coronary plaque morphology in patients with stable coronary artery disease on maximally tolerated statin therapy. Using multi-modality intravascular imaging, gene expression analysis of peripheral blood mononuclear cells (PBMC) and transcriptomicbased machine learning algorithms, researchers are hoping to uncover the molecular mechanisms responsible for beneficial changes in atherosclerotic lesions of patients treated with evolocumab. This novel study can be ground-breaking in understanding plaque morphology changes in the context of molecular changes in patients treated with PCSK9 inhibitors, Mehran noted.
Valvular heart disease
“Continuing our quest to find less invasive management of patients with severe disease, particularly in ageing populations, is incredibly important,” comments Mehran, pinpointing the significance of trials relating to small
aortic annulus.
Mehran is a co-principal investigator in the SMART trial alongside global principal investigator Howard Herman (University of Pennsylvania, Pennsylvania, USA) and co-principal investigator Didier Tchétché (Clinique Pasteur Toulouse, Toulouse, France), which is expected to report results later this year. The trial is investigating safety and performance of the selfexpanding Evolut (Medtronic) versus the balloon-expandable Sapien (Edwards Lifesciences) TAVI systems in patients with a small aortic annulus and symptomatic severe native aortic stenosis.
“A large majority—up to 90% of these patients—as could be associated with small annulus, are women,” Mehran said, concluding the study will be one of the first leading female focused clinical trials.
Anticipating many other trials that are “on the horizon” in 2023, spanning across renal denervation, drug-coated balloons and artificial intelligence (AI), Mehran commented “the field is very bright.” She added: “2023 sparks interest in rebooting clinical trials and forums to reiterate how important evidence-based medicine is to the progression of interventional cardiology.”
“We are moving towards the future. What we have previously learnt and what we are going to learn in 2023, will make a huge impact when treating patients with cardiovascular disease, helping to promote the continued evolution of the interventional cardiology space—to improve health outcomes of our patients in a bigger and better way.”
stent implantation will be mandatory.”
With a focus on patients with stable coronary artery disease, Alasnag also pinpointed the TARGET trial, which aims to evaluate whether the availability of computed tomography angiography (CTA) and fractional flow reserve-computed tomography (FFR-CT) might effectively optimise the flow of clinical practice of stable chest pain. This is compared against the conventional clinical pathway in decision making, by avoiding the overuse of invasive procedures, reducing total expenditure and improving outcomes.
The randomised, open label, prospectively designed trial is running
Trials to watch
● TRILUMINATE – Comparing TriClip (Abbott) TEER with medical therapy for patients with severe TR, results from the pivotal trial are eagerly anticipated among structural heart specialists to reinforce interventional options for treating the tricuspid valve.

● REVIVED-BCIS2 – Primary results presented at ESC 2022, showing that PCI did not reduce all-cause mortality or heart failure hospitalisation in patients with severe left ventricular dysfunction, were among the biggest talking points of the year. Investigators follow up in 2023 with the presentation of findings of the effect of myocardial viability and functional recovery on clinical outcomes.
RENOVATE-COMPLEX-PCI
Investigators in Korea hope to offer some answers as to whether PCI under guidance of intravascular imaging devices (IVUS or OCT) improves clinical outcomes compared with
angiography-guided PCI in patients with complex lesions.
TARGET – Could the availability of CTA/ CT-FFR procedures avoid the overuse of invasive procedures in evaluating patients with stable chest pain? Over 1,200 patients have been enrolled in the TARGET study in a bid to answer this question.
SMART – This study aims to provide clinical evidence on the performance of self-expanding and balloon-expandable TAVI valves in patients with a small aortic annulus and symptomatic severe native aortic stenosis.
February 2023 | Issue68 4 Trials to Watch
What we have previously learnt and what we are going to learn in 2023 will make a huge impact when treating patients with cardiovascular disease”
Left: Mirvat Alasnag
Right: Roxana Mehran
Top: TriClip Above: Evolut





A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiac rhythm field Editorially independent Visit cardiacrhythmnews.com and click ‘Subscriptions’ for e-newsletter subscription Subscribe today Available in print and digital formats and through our social channels




*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Cardiovascular surgery
Multidisciplinary teams at the heart of new guidelines on aortic disease management
Closer ties between cardiologists, cardiac surgeons and vascular surgeons will be a hallmark of the future treatment of diseases of the aorta, a leading figure behind new guidelines for the diagnosis and management of aortic disease tells Cardiovascular News
Jointly published by the American College of Cardiology (ACC) and American Heart Association (AHA) in November 2022, the new guidelines are intended to support decision-making around diagnosis, screening, medical therapy, endovascular and surgical treatment, and long-term surveillance of patients with aortic disease.
Building upon an earlier version of the document, last updated in 2010, the new guideline incorporates latest evidence to reflect advances in care. Central among the latest additions is a focus on multidisciplinary aortic team care to determine appropriate timing and optimal medical, endovascular and surgical therapies.
“The important thing for these guidelines is the multidisciplinary approach that was not evident in prior guidelines,” Ourania Preventza (cardiac surgeon at The Texas Heart Institute and professor of surgery at Baylor College of Medicine, Houston, USA), vice-chair of the guideline writing committee tells Cardiovascular News. “There is a multidisciplinary aortic team that is determining the appropriate type of intervention and a shared decision-making approach between the patient and the providers.”

According to Preventza, this diversity of specialisms was embedded in the writing process from the offset, with the committee chaired by a cardiologist, Eric Isselbacher (Harvard Medical School, Massachusetts General Hospital, Boston, USA), alongside Preventza, a cardiac surgeon, and her fellow vice-chair, James Hamilton Black III (Johns Hopkins Medicine, Baltimore, USA), who is a vascular surgeon. Other spaces on the 26-strong writing committee were also taken, in addition to the cardiologists and to the cardiac and vascular surgeons, by cardiovascular anaesthesiologists, geneticists, and interventional radiologists.
“The thought process when we created this and assembled the committee was really to provide diverse perspectives. The only way to provide these diverse perspectives is when we include all these different specialties with specialist knowledge about therapy and diagnosis of patients with aortic disease,” Preventza adds.
Asked whether it was difficult to balance what may sometimes be differing schools of thought, Preventza comments that the writing committee was fundamentally led by evidence available to guide best practice, and says that the proof that they got this right is in the fact that the document has been endorsed by a number of societies in different fields, including the American Association for Thoracic Surgery (AATS), the Society for Thoracic Surgery (STS), the Society for Vascular Surgery (SVS), the Society for Cardiovascular Angiography and Interventions (SCAI), the Society of Cardiovascular Anesthesiologists (SCA) and the Society of Interventional Radiology (SIR).
“The guidelines are there to give the physicians something to base their practice on, to help patients, and make sure that
Risk assessment tool “sets a high bar” when considering alternatives to surgery in mitral valve repair
A NEW RISK ASSESSMENT tool has been produced for assessing operative risk of mitral valve repair for primary mitral regurgitation (MR).
In an article published in The Annals of Thoracic Surgery and the Journal of the American College of Cardiology (JACC), cardiology and cardiothoracic surgical researchers analysed US data to assess outcomes and risk of mitral valve repair for primary MR.
the medical and cardiovascular community have the same or similar approach, which is safe and effective.”
Detailing what she sees as the formula for a multidisciplinary aortic team, Preventza says that there is no firm blueprint, rather the approach is guided by shared decision-making in the interest of the patient.
“It is really a collaboration between cardiology, vascular and cardiac [surgeons],” she comments. “For example, an abdominal aneurysm is very well treated by the vascular surgeons. Somebody with an abdominal aneurysm may also have a thoracic aneurysm, involving the ascending aorta, and this is something that has to be taken care of by a cardiac surgeon. Or, perhaps the annuli of the aortic valve or the ascending aorta is not at the size yet that needs intervention, so in this case the patient can be followed by cardiology.”
The patient-specific approach is important, she adds, commenting that it is not a one-size-fits-all approach.
Other important takeaways from the revamped document include new thresholds for surgical intervention for sporadic aortic root and ascending aortic aneurysms, an updated definition for rapid aneurysm growth rate, and adjusted recommendations on when to intervene in patients who are smaller or taller than average. These are in conjunction with new entries covering family screening to identify those at high-risk of aortic disease and consistency in the acquisition and reporting of computed tomography (CT) or magnetic resonance imaging (MRI) in the measurement of aortic size.
“The plan is that the guidelines are a living document, to be updated in the next three years,” adds Preventza. “Nobody has a crystal ball, but given how the field is evolving I think it is important for us to re-evaluate and see where we are.”
Important areas to watch will include the evolving field of genetics as it relates to aortic disease, developments in the evidence in the area of endovascular intervention, and research looking at sex and socioeconomic disparities in aortic disease. “These are things that as we evolve we hope to improve, with more inclusive studies, and with advancements in technology; all of which will help us and help our patients and that is why it is important that these guidelines will be updated,” she concludes.
Assessing information from The Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database, the team identified 53,462 patients who underwent planned surgical mitral valve repair for primary MR between 2014 and 2020.
The authors found that there was an increasing frequency of operations done in a minimally invasive fashion, including robotically, and that the rate of successful repair has reached over 90% in the USA. Researchers also found that risk of mortality after this surgery was rare across nearly all age ranges. These data were used to develop a novel risk model for predicting 30-day outcomes based on the patient’s health conditions, which formed the basis for a risk calculator to assist healthcare providers to estimate the risks for their patients, to be made available online shortly via the STS.
“We have two options to treat primary mitral valve regurgitation. The historical standard has been surgical repair, but we also have US Food and Drug Administration (FDA)-approved transcatheter devices for minimally invasive mitral valve repair that have encouraging results, particularly in high-risk patients with primary MR,” said Vinay Badhwar (West Virginia University, Morgantown, USA), lead author of the multidisciplinary study.
“Past perceptions of the risk of surgery and repair rates based on older risk models may have influenced the design of two clinical trials to explore transcatheter therapy in lower risk older individuals.
The finding of 90% successful surgical repair with less than 1% mortality now achieved in the USA sets the outcome bar fairly high when considering alternative therapies to surgery. We hope this information will help physicians and patients make more informed decisions regarding treatment, as well as to inform the optimal design of future trials in the field.”
Issue68 | February 2023 7
It is a collaboration between cardiology, vascular and cardiac [surgeons]”
Ourania Preventza
Cardiovascular-aortic community comes together at CX 2023
From cutting-edge aortic interventions to consensus on revascularisation strategies in the peripheral arteries, the 2023 edition of the Charing Cross (CX) Symposium comes at a crucial time in the calendar for the cardiac, aortic, vascular and endovascular communities.
The three-day CX Symposium—taking place 25–27 April—returns to the Hilton London Metropole in central London for its second consecutive year, with attendees also tuning in virtually from across the globe. It is anticipated that the event will welcome more than 2,500 in-person attendees, with over 1,000 remote participants.

CX continues its three-year cycle of raising vascular and endovascular controversies in order to challenge the available evidence and to be able to reach a consensus after discussion with an expert audience. Sessions will explore routes to consensus in all vascular domains, spanning aortic, peripheral, venous, acute stroke and vascular access, punctuated by CX debates, live and edited cases and workshop demonstrations.
Running over the three days, the comprehensive aortic programme opens with aortic techniques and technologies on day one, followed by a full day focused on the abdominal and juxtarenal aorta, and finishing with debate centring on the thoracic aorta.
The sessions bring together worldleading experts in management and treatment of aortic disease from
the cardiovascular, vascular and endovascular worlds, with faculty including Gustavo Oderich (University of Texas Health Science Center at Houston, Houston, USA), Joseph Bavaria (University of Pennsylvania,
in the arch and thoracoabdominal aorta, and for me personally, to learn from colleagues in Europe and the UK on what is going on there, and what I should change in my techniques.”
Philadelphia, USA), Maximilian Pichlmaier (Ludwig Maximilian University Munich, Munich, Germany) and Marco Virgilio Usai (St Franziskus-Hospital Muenster, Muenster, Germany).
Commenting ahead of the symposium, Oderich, a member of the CX Aortic Executive Board, said: “We are all excited to see the latest advances
HANDSON AORTIC COURSE
Highlights from day one include insights into advances in both open and endovascular thoracic aortic aneurysm (TAA) repair, options for the treatment of complex aortic pathologies, and a focus on the aortic dissection toolkit.
On rati des experum quiam quunt offic testotaturit rem harciis int, simusam quaero beatibus ium eatempos.
A podium first presentation from Kevin Mani (Uppsala University, Uppsala, Sweden) is among the highlights from the abdominal aortic session at the start of day two, exploring the benefits of statin treatment after aortic repair. The following juxtarenal aortic consensus update session includes discussion on the impact of the ETTAA (effective treatments for thoracic aortic aneurysms) and UK-COMPASS (UK complex aneurysm) studies results with reference to National Institute for Health and Care Excellence (NICE) guidelines.
Thursday’s thoracic aortic consensus sessions close off the aortic programme with a debate on the conservative management of aortic intramural haematoma, featuring Michel Makaroun (University of Pittsburgh, Pittsburgh, USA) and Jean Panneton (Eastern Virginia Medical School, Norfolk, USA).
Treatment strategies for patients with chronic limb-threatening ischaemia (CLTI) have been firmly in the spotlight following the release of the first results from BEST-CLI in November 2022. CX will provide a platform to move the conversation forward, with firstto-podium results of the BASIL 2 randomised trial—comparing a ‘vein bypass first’ or a ‘best endovascular first’ revascularisation strategy—forming the centrepiece of a peripheral trial consensus update, taking place on the first day of the CX symposium.
During the session, BASIL 2 chief investigator Andrew Bradbury (University of Birmingham, Birmingham, UK) will deliver the first results from the trial, in a session that also sees participation from BEST-CLI investigators Matthew Menard (Brigham and Women’s Hospital, Boston, USA)—providing an update on the trial—and Alik Farber (Boston Medical Center, Boston, USA), presenting BEST-CLI quality-of-life data.
In-person attendees at CX 2023 will have the opportunity to attend a hands-on aortic workshop, led by Alexander Zimmermann (University of Zurich, Zurich, Switzerland), looking at the full range of fenestrated and branched endografts included devices that are physician modified. The Aortic Workshop will provide attendees with the opportunity to expand their knowledge and to test and improve their skills in a practical session taking place on Wednesday, 26 April. Offering a deep dive into physician modified stent grafts, this interactive session will explore the theory behind the history and indications of physician-modified grafts, and the technique to create fenestrations and branches.

February 2023 | Issue68 8 Section Name 8
xxxx
Scan here to view SIGN UP NOW
We are all excited to see the latest advances in the arch and thoracoabdominal aorta”
CX 2023 returns to Hilton London Metropole
CX VASCULAR AND ENDOVASCULAR CONSENSUS UPDATE 2023
Aortic Programme Snapshot
Day 2:
Wednesday 26th April
Abdominal Aortic Aneurysm (AAA) Consensus Update
Session highlights:
● Podium First: Statin treatment after aortic repair saves lives, but dose doesn’t matter (Kevin Mani, Uppsala, Sweden)
Sponsored Education
Day 1:
Tuesday 25th April
Aortic Techniques & Technologies Consensus Update
Session highlights:
● Edited Case: TAMBE Thoracoabdominal 4 vessel system (Gustavo Oderich, Houston, United States)
● Edited Case: Guo's aortic arch reconstruction (Wei Guo, Beijing, China)
Sponsored Education
Aortic Techniques & Technologies
Consensus Update Continued
Session highlights:
● Edited Case: The use of laser fenestrations to aid in the endovascular treatment of chronic dissection TAAAs (Timothy Resch, Copenaghen, Denmark)
● Edited Case: Overcoming challenging anatomy with custom made Frozen Elephant Trunk (FET) device (Maximillian Pichlmaier, Munich, Germany)
Sponsored Education
Aortic Techniques & Technologies
Consensus Update Continued
Session highlights:
● Edited Case: Branched endovascular aneurysm repair (BEVAR) (Giovanni Pratesi, Rome, Italy)
● Edited Case: Treatment of thoracic pathologies involving the Aortic Arch at level of the left subclavian artery with TEVAR (Marco Virgilio Usai, Muenster, Germany)
Sponsored Education
Aortic Techniques & Technologies
Consensus Update Continued
Day 3:
Thursday 27th April
Thoracic Arch Consensus Update
Session highlights:
First results of endovascular treatment of type aortic dissection with a single side branch device (Joseph Bavaria, United States)
Thoracic Consensus Update
Session highlights:
Ruptured Abdominal Aortic Aneurysm, (RAAA) Consensus Update
Session highlights: Ruptured abdominal aortic aneurysm: how to decrease turn down rate and procedural mortality at the same time (Maarit Venermo, Helsinki, Finland)
Iliac Consensus Update
Session highlights: Predicting factors for graft occlusion and type III endoleaks after Iliac branched devices for hypogastric artery aneurysms (Afshin Assadian, Vienna, Austria)

Juxtarenal Aortic Consensus Update
Sponsored Education
Juxtarenal Aortic Consensus Update Continued
Session highlights: Impact of ETTAA and UKCOMPASS results with reference to NICE guidelines (Rao Vallabhaneni, Liverpool, United Kingdom)
● Podium First: Meta-analysis of comparative studies between self-and balloon-expandable bridging covered stents for BEVAR (Konstantinos Spanos, Larissa, Greece)
Sponsored Education
Juxtarenal Aortic Consensus Update Continued
Session highlights:
● Podium First: 8-year results of a bridging stent for FEVAR/BEVAR (Eric Verhoeven, Nuremberg, Germany)
Radiation Consensus Update
Session highlights: Edited Case: TAAA branch repair with electromagnetic guidance (Stéphan Haulon, Paris, France)
● Edited Case: Transcaval access for urgent EVAR in ‘no access cases’ (Tilo Kölbel, Hamburg, Germany)
Sponsored Education
Thoraco-abdominal Consensus Update
Session highlights:
Thoraco-abdominal and juxtarenal elective and emergency aortic aneurysms in females with an endovascular branched device (Fiona Rohlffs, Hamburg, Germany)
Sponsored Education
Conservative Option Consensus Update
Session highlights:
● CX Debate: More often than not, aortic intramural haematoma (IMH) can be managed conservatively (For: Michel Makaroun, Pittsburgh, United States, Against: Jean Panneton, Virginia, United States)
Thoracic Consensus Update Continued
Session highlight:
● Live Case: Thoraco-abdominal TAAA endovascular treatment
Sponsored Education
How To Do It @ CX: Open, Hybrid & Endo Management of Complex Post-Dissection Aortic Aneurysms
9
Issue68 | February 2023
A step forward in scoring balloon catheter design


A new coronary scoring balloon dilatation catheter—Naviscore (iVascular)—features a hybrid design combining the benefits of scoring and cutting balloons to turn the page on calcified lesions. Cardiovascular News explores the features of this innovative device, and details insights from its deployment in a first-in-man study.
To interventional cardiologists, coronary artery calcification is a well-known and widelyacknowledged challenge in the cath lab—a major marker for poor outcomes in patients and an added layer of complexity when it comes to performing percutaneous coronary intervention (PCI) procedures. According to figures published by Robert S Copeland-Halperin (Icahn School of Medicine at Mount Sinai, New York, USA) and colleagues in the journal Catheterization & Cardiovascular Interventions, as many as 10% of patients undergoing PCI present with moderately calcified lesions, whilst a further 8% have more extensive—severe— calcification. Copeland-Halperin et al found that among the risk factors for moderate or severe calcification were older age and more complex target lesions. Therefore, it is a reasonable assumption that, with an ageing Western population and a higher number of PCI procedures being performed in patients with wide-ranging comorbidities, the challenge of calcific lesions will persist or even become greater into the future.
With these factors in mind, it is important for
interventional cardiologists to have access to an array of tools with features that are tailored to address the demands posed by coronary calcium. Currently, options at hand include the use of mechanical devices, such as rotational atherectomy, orbital atherectomy or intravascular lithotripsy (IVL), as well as a less complex option—scoring and cutting balloons. Though commonly used for the treatment of coronary plaques, contemporary scoring balloons come with various limitations. “One common complaint about these balloons is that they are bulky and stiff devices, the complaint is that it is very difficult to cross a tight lesion,” Antoni Serra (Hospital Sant Pau, Barcelona, Spain) told attendees of Turin CTO & CHIP Live 2022 (4–6 May, Turin, Italy). According to Serra, the bulkiness and stiffness of the devices can make tracking through calcific and tortuous vessels challenging, as it is difficult to cross the lesion and difficult to recross once inflated.
Introducing Naviscore
“Can we improve this?” Serra asked the audience at Turin CTO & CHIP Live 2022, where he delivered
6x larger scoring surface than competitor
an emphatic answer: Yes we can! He then introduced Naviscore, a new coronary scoring balloon dilatation catheter from iVascular that looks to overcome these existing drawbacks through a unique construction and innovative hybrid design, which is intended to combine the favourable elements of both cutting and scoring balloons.
The device features a nitinol structure placed over a high-pressure balloon and has a rated burst pressure of 20atm, with an average burst pressure up to 26atm. The nitinol lattice is laser-cut for precision, with struts measuring 125 better navigability and a low perforation risk while modifying plaque. The tip of the device features a platinum-iridium ring that serves as a marker of

FIM NAVISCORE
■ Stenosis seen prior to Naviscore procedure: 81%
■ Stenosis seen post Naviscore procedure: 33%
■ Stenosis seen post-stenting: 7.5%
Optimal trackability
10 Advertorial February 2023 | Issue68 THIS ADVERTORIAL IS SPONSORED BY iVASCULAR
Antoni Serra
penetration of the scoring balloon in the calcified lesion, a feature that is designed to assist with its positioning.
The device boasts a nylon compensation tube in the shaft, which assists with re-wrapping after the balloons have been deflated—a unique feature that cannot be found on any other device currently on the market. Importantly, Naviscore incorporates an axial filament orientation designed to ensure greater cross capacity and 90-degree axial plaque modification. The axial distribution of filaments allows for more push against the calcific lesions, and enables easier navigation of the device through tortuous and calcified vessels as a result of its favourable crossing profile.
In addition, and in counterpoint to other scoring balloons currently available on the market which have a relatively small filament surface area in contact with the calcific vessel, the axial design of the Naviscore device enables a larger contact area with the vessel.
also has a smaller diameter, with balloons available in diameters from 1.5mm to 6mm and 6mm to 15mm in length, meaning that Naviscore can be deployed in smaller, tighter vessels than would previously have been open to scoring or cutting.
Real-world insights: FIM NAVISCORE study
Naviscore features
Rapid Exchange catheter (RX)
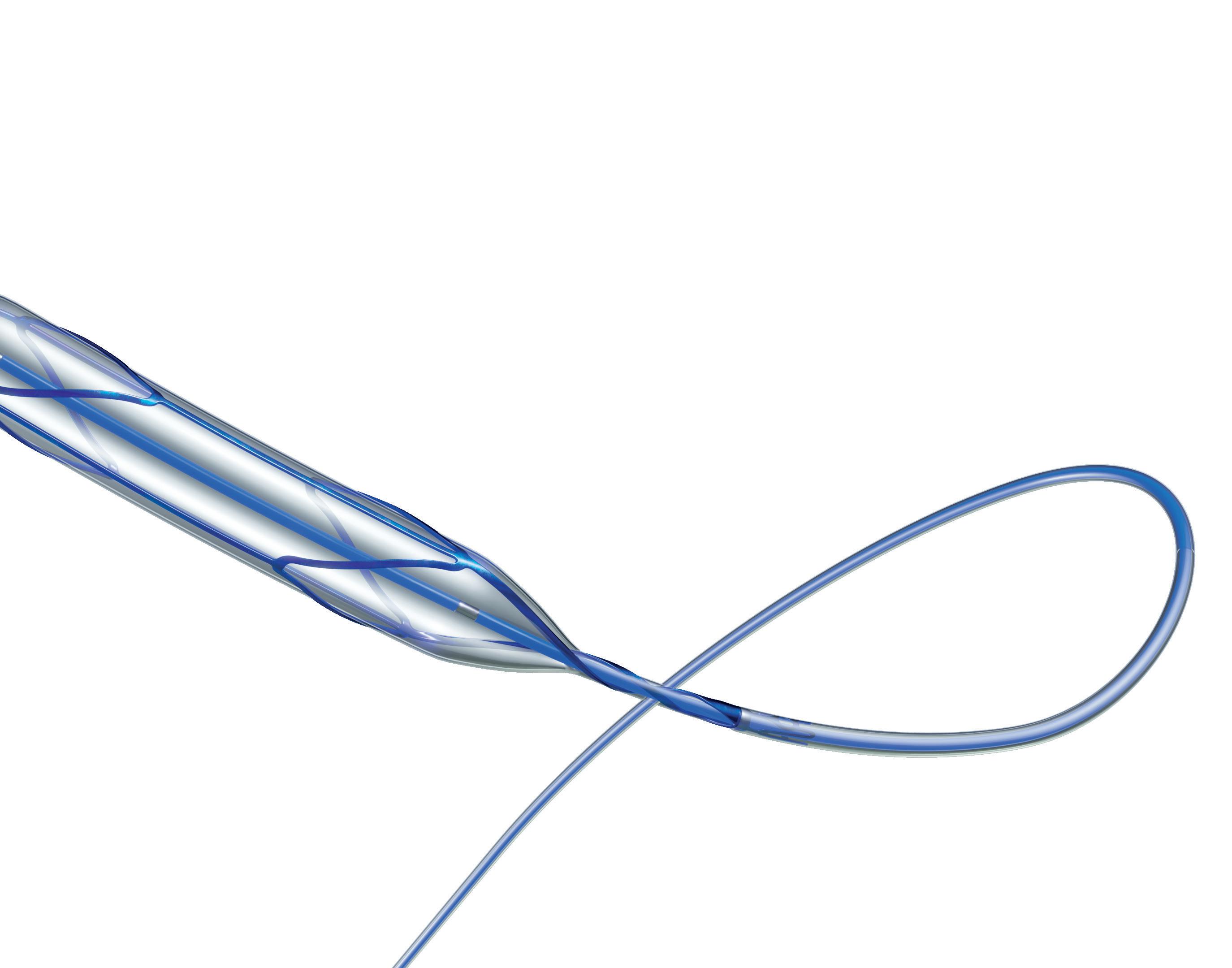
At Turin CTO & CHIP Live 2022 Serra detailed findings from the first-in-man, FIM NAVISCORE study, which gives the first real-world insights into the use of Naviscore from 10 centres in Spain and Portugal. The study included a total of 85 patients with moderate or severely calcified lesions in whom the Naviscore was intended for use as the first device in the PCI procedure. If this was unsuccessful, a small balloon could be used for predilatation. Each centre participating in the study carried out a minimum of five cases, having previously completed a handful of cases to gain experience with the device.
Guidewire compatibility: max. 0,014”
Nominal pressure (NP): 8atm
Rated burst pressure (RBP): 20atm
at crossing the lesion with Naviscore was successful. Of the 18 cases in which Naviscore was unable to cross at the first attempt, a small balloon was deployed, and following this, the Naviscore achieved successful crossing in 89% of the patients. Furthermore, in the 10 patients who were treated using an alternative to Naviscore at the first attempt— either rotational atherectomy or a small balloon—all 10 subsequently had the lesion successfully crossed using Naviscore.
Platinum iridium (PtIr)
radiopaque markers
Proprietary Hydrax plus hydrophilic coating
6Fr compatibility
FIM NAVISCORE
Outside of the requirement for calcified lesions, there were no exclusion criteria for the study. Serra
20 atm of RBP
Outlining the clinical results from the 85 cases, Serra told Turin CTO & CHIP Live 2022 attendees that, in the study, the device achieved a 94% procedural success rate comprising of a residual stenosis of <30% after stenting, no major complications and thrombolysis in myocardial infarction (TIMI) 3 flow. Additionally, angiography showed that the proportion of stenosis seen prior to the Naviscore procedure stood at 81%, falling to 33% post-Naviscore, and 7.5% post-stenting, suggesting, according to Serra, that Naviscore plays an important role in facilitating the opening of the
high-resistant semi-compliant balloon
76%
Successful lesion crossing in of patients at the first attempt
Excellent rewrap and recross capabilities
11 Advertorial Issue68 | February 2023
Naviscore should be the first-choice device for the treatment of these difficult and complex lesions thanks to its crossing capacity of
Nieves Gonzalo
Interventional cardiology is a rapidly evolving field, offering the opportunity to learn procedures with immediate and life-changing results for patients. This is what has attracted Nieves Gonzalo (Clinico San Carlos University Hospital, Madrid, Spain) to the specialism, and why she believes it will continue to draw in future generations of physicians. Recently appointed as a course director for EuroPCR, Gonzalo also serves as a deputy editor of EuroIntervention, and as a co-chair of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee. She tells Cardiovascular News about her career to date, research priorities, and the importance of representation.
Why did you choose to become a doctor and why, in particular, did you choose to specialise in interventional cardiology?
When I was in high school I loved biology and I was fascinated by the complexity of interactions that allow life to exist. Medicine was the perfect opportunity to continue learning about this and to apply this knowledge to improve lives. I decided to study cardiology because I found heart physiology enthralling and the specialty provided so many opportunities from diagnosis to treatment, and from the beginning, interventional cardiology was the choice for me. The possibility of performing a procedure that generates immediate results for the patient—for example primary percutaneous coronary intervention (PCI)—is extremely rewarding.
Who were the biggest influences on your early career?
I did my cardiology training at Hospital Clinico San Carlos (Madrid, Spain) and all of my colleagues in the interventional cardiology department were extremely supportive. The chief of interventional cardiology at that moment was a woman—Rosana Hernández— and she was a great inspiration. My mentor Javier Escaned, together with Manel Sabate, encouraged me to go abroad for a fellowship at the Thoraxcenter in Rotterdam (The Netherlands) with Patrick Serruys and this widened my view, helping me to incorporate research as a central aspect of my career. In Rotterdam, I worked very closely with Evelyn Regar to develop my thesis on optical coherence tomography (OCT) when the technique was starting out.

What has been the most important development in interventional cardiology during your career?
The most important developments in interventional cardiology during my career have been the incorporation of primary PCI (a lifesaving strategy), the invention of drug-eluting stents (DES) that have tremendously improved the long-term success of PCI, and the incorporation of intracoronary physiology and imaging techniques that have changed the way in which we perform PCI, providing a new insight into the pathophysiology of coronary heart disease. The other big development has been transcatheter aortic valve implantation (TAVI), another lifesaving procedure that has provided a new opportunity for many patients who did not have options before and is contributing to offer less invasive treatments for many others.
What has been the biggest technological disappointment?
The biggest disappointment for me was polymeric bioresorbable scaffolds. I worked intensively on the initial phase of the ABSORB studies during my
fellowship in Rotterdam and the initial results were promising. We were all thrilled with the possibility of treating atherosclerotic disease without the need for a permanent metallic cage, it was a pity when larger trials revealed significant problems that precluded their clinical application. I still hope, however, that the concept will be revived. There are so many potential advantages for the future, especially with an ageing population that will probably require repeated interventions throughout their lives.
What are your current research priorities?
My current research priorities are focused on treatment of complex coronary disease, a growing problem as we treat older patients with multiple comorbidities. In this regard, strategies to improve treatment of calcific lesions are going to be key to improve the long-term results of our interventions. I am also very interested in the treatment of restenosis, which is much more challenging in the context of DES, especially with the development of neoatherosclerosis. Another field of interest and research is the relation between physiology and atherosclerotic plaque morphology and the treatment of left main disease.
What are the key unanswered questions around the field that future research should prioritise?
There is currently a renewed interest in the search for high-risk plaques to be able to detect them before they rupture and generate an event. I think this is an important field where intracoronary imaging techniques and potentially non-invasive imaging technologies can provide new insights and help us move toward preventing events instead of treating them. The incorporation of artificial intelligence (AI) will probably be important, especially in plaque characterisation and development of new physiological indices derived from invasive and non-invasive technologies. PCI guidance based on physiology, and imaging and impact on future events as compared with other treatment strategies, is another important area to be addressed. The concept of leaving nothing behind when treating coronary disease—either with
bioresorbable devices or drug coated balloons—will also be relevant in future research.
What has been the most important paper published in the past year?
One of the most interesting papers published in the last year for me has been the long-term results of SYNTAX II. The paper demonstrated that applying a complete contemporary percutaneous revascularisation strategy incorporating heart team discussion, imaging and physiology to guide and optimise PCI can yield optimal clinical results in multivessel disease, making it competitive with more invasive strategies.
What are the current challenges facing women seeking to enter the interventional cardiology field, and how can these be overcome?
There are many challenges that women face when they
12 Interview
February 2023 | Issue68 PROFILE
alisonlang.com
Promoting a meritocratic system in which gender is not an issue will bring more women into the field and will help them to reach leading roles”
decide to pursue a career in interventional cardiology ranging from the lack of role models and support to concerns regarding pregnancy and reconciliation of family and work life. Big efforts have been made in recent years to generate guidance to overcome these barriers and promote the incorporation of women in leading positions and I am sure they will take effect in the next generation. Promoting a meritocratic system in which gender is not an issue will bring more women into the field and will help them to reach leading roles. Equal representation in research and education is also an important aspect that needs to be considered and properly promoted.
What can interventional cardiology do to better attract young physicians into the field?
The best way to attract young physicians is to show them how fascinating this specialty is. Interventional cardiology is in continuous evolution, meaning that you always keep learning and it is impossible to get bored. From a personal point of view, it is demanding but also really rewarding, offering you a lot of options to help patients. Promoting enthusiastic young colleagues and generating a good working environment for the future is a task for every one of us.
Looking back over your career, what has been your most memorable case?
There are many cases that have been memorable especially, for example, my first case of a patient with a left main occlusion who arrived in a critical situation and was able to survive and recover after primary PCI. There is another case that we performed recently that was also transformative for me. This involved a lady with advanced renal disease, severe peripheral disease with no conventional accesses, critical left main disease with extreme calcification and very severe left ventricular (LV) function. In this case, after careful planning with computed tomography (CT) and with the help of the vascular surgeon, we were able to perform the PCI with LV support through a surgical left subclavian access that was the only route available. For me it was an example of how teamwork is going to be critical in the future as we tackle more and more complex patients and disease.
Outside of medicine, what are your hobbies and interests?
I like nature and outdoor activities. Taking care of my garden is especially relaxing for me. Also, doing exercise is essential to keep me happy. I especially enjoy spending time with my children and share with them my love for music and books.

Appointments and training
Director Consultant
Interventional Cardiologist, Clinico San Carlos University Hospital, Madrid, Spain
Fellowship, Thoraxcenter, Erasmus University Rotterdam, Rotterdam, The Netherlands
Doctor of Medicine, Universidad Autónoma de Madrid, Madrid, Spain
Memberships
European Society of Cardiology (ESC)
European Association of Percutaneous Cardiovascular Interventions (EAPCI)
Research
RIBS IV and V OCT sub-study
VISTA study
Appointments
Deputy Editor, EuroIntervention
EuroPCR Course Director
Co-chair, EAPCI Scientific Documents and Initiatives Committee)
Fact file 13 Interview Issue68 | February 2023
A wealth of data underscore treatment choices for coronary DCBs

The modern field of percutaneous coronary intervention (PCI) has come to be dominated by drug-eluting stents (DES), but there are many instances in which “leaving nothing behind” in the vessel is an attractive prospect. Proponents of this approach, including Tuomas Rissanen (North Karelia Central Hospital, Joensuu, Finland), believe that using a drug-coated balloon (DCB) is an effective alternative to the stent that offers a range of benefits in selected patients.

UNDERPINNING THIS BELIEF IS A WEALTH of clinical data, including from registry-based studies, and randomised trials that inform treatment in a wide set of clinical and anatomical scenarios. Among the leading devices in the field is the paclitaxel and iopromide-coated SeQuent® Please product family (B. Braun)—widely acknowledged as the bestinvestigated DCB in the market—which has clinical evidence from more than 110 published studies, totalling more than 25,000 patients, and stretching back almost 15 years since its entry into the market.
In this interview, Rissanen discusses the clinical indications that are most suited to the use of a DCB, the studies that have been most impactful to the field, and how having a proven device backed by a wide set of data is key.

“The fundamental difference to a stent is that no foreign body material is left in the artery that may cause later problems such as stent thrombosis,” Rissanen explains, discussing what separates PCI with a DCB to stent implantation. In simple terms, the DCB delivers an antirestenotic agent directly to the lesion, usually either paclitaxel or sirolimus mixed with an excipient helping to move the drug from the balloon catheter to the vessel wall.
“Stents are very useful when the treated vessel needs mechanical support due to collapse of the vessel or significant flow-limiting dissection—in fact, stents were developed for these two purposes,” comments Rissanen. “However, when the predilatation result is good and there are no problems that would need a metallic implant, then it is best just to use a drug coated balloon catheter delivering an antirestenotic drug,” he advises.
“An advanced interventional cardiologist should absolutely have both tools in the toolbox,” Rissanen comments, pointing out that the DCB and DES offer complementary characteristics.
Predominantly the DCB has been seen as a tool for treating in-stent restenosis, where it carries a class I recommendation with evidence type A in guidelines from the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS), based largely on studies performed with the SeQuent paclitaxel and iopromide coating, including the PACCOATH ISR I and II or PEPCAD trials. However, more recently there has been an increase in interest in the use of DCBs in de novo lesions, where the therapy has been shown to be safe and effective in numerous scenarios.
“Depending on the patient and lesion characteristics, and most importantly, on the result of the predilatation of the lesion, the method that is best for the patient should be chosen,” says Rissanen. Typically, an adequate vessel preparation involves achieving less than 30% stenosis, no flow-






limiting dissection, and a fractional flow reserve (FFR) of >0.8.
Rissanen offers the view that this is particularly appealing among patients that are at high-risk of bleeding, as the DCB allows the clinician to stop antithrombotic medication within a month after the procedure in stable coronary artery disease patients, without an increase in event rates. Besides DCBonly usage in standard de novo lesions, he notes that there are also anatomic scenarios for DCB-only PCI, including, for example, in complex bifurcations, where the side branch, main branch, or both branches, can be treated using a DCB.
“The advantages of DCB-only therapy over stenting in this situation are that the side branches that are in the treatment area are not that easily occluded,” Rissanen adds. “Also, the lack of metallic implant allows later positive remodelling of the vessel and of course eliminates the risk of stent thrombosis.” Additionally, in recent years this technology is gaining more traction in complex and diffuse coronary artery disease being used alongside stents to reduce the overall stent-burden.
Turning to the data supporting the use of DCB in coronary lesions, Rissanen identifies two studies— BASKET-SMALL-2 and DEBUT, both of which used SeQuent Please/NEO DCBs—as being the standout studies to have led the field. BASKET-SMALL-2, results of which were published in The Lancet in
novo coronary lesions in patients with high-bleeding risk using DCB or a bare metal stent, results, published in The Lancet in 2019, showed that the DCB was superior to the stent against a primary outcome of MACE at nine months. A total of 208 patients were enrolled in the trial, with MACE having occurred in one patient (1%) in the DCB group versus 15 (14%) in the stent group.



The accumulation of data continues and according to Rissanen, of the more recent studies to influence thinking on DCBs is the 2020 publication of a metaanalysis in the Journal of the American College of Cardiology (JACC), authored by Bruno Scheller (University of Saarland, Homburg/Saar, Germany) is fundamental. This took data from 26 randomised controlled trials, including 4,590 patients, looking at paclitaxel-coated devices, and reached the conclusion that the use of paclitaxel DCBs for treatment of coronary artery disease is safe in the long term.
Outside of these landmark trials, Rissanen also points to a wealth of further research that has shaped perceptions on DCB in diverse patient populations. Among them is PEPCAD-NSTEMI, a trial that addressed DCB-only therapy in non-ST-elevation myocardial infarction (NSTEMI) patients, finding that treatment of coronary de novo lesions with DCB was non-inferior to stenting with bare metal or drugeluting stents. This was underlined in a sub-group analysis of the BASKET-SMALL 2 trial, where noninferiority to DES in acute coronary syndrome (ACS) patients, including around half with NSTEMI, was shown. Rissanen also highlights the REVELATION study, addressing a DCB-only approach in STEMI using FFR measurement as a surrogate marker. Do these data translate into clinical practice, and should it inform decision-making when it comes to device selection? Yes, according to Rissanen. “The SeQuent Please DCB has shown consistently good clinical results in many registry-based studies and randomised clinical trials,” he comments. “These trials involve different clinical and anatomical
2018, is an open-label randomised non-inferiority trial investigating the use of DCB in small vessels, comparing 758 patients with lesions <3mm in diameter receiving either a DCB or a DES. Results of the trial demonstrated the non-inferiority of the DCB versus DES, with major adverse cardiovascular events (MACE) shown to be comparable between the two groups (7.5% for the DCB group vs. 7.3% for the DES cohort). Rissanen notes that three-year followup from the trial, published in 2020, has shown consistent and durable results, while a sub-group analysis on high-bleeding-risk patients, in particular, has highlighted the benefit of DCB versus stenting.
In DEBUT, a single-blind, randomised, noninferiority trial, examining the treatment of de
scenarios and cover also more complex situations such as bifurcations, calcified lesions and even chronic total occlusions (CTOs).
“SeQuent Please DCB currently has the most clinical data to show its safety and efficacy. This is important as the patient population in the cath lab nowadays is very complex. You can trust the device that you are using in these circumstances and convince also your patient that he or she is receiving the best available treatment.”
Summing up why he chooses only to use the devices that have the best available evidence, Rissanen concludes: “In our centre we use devices based on the proven clinical data. It is both costeffective and best for the patient.”
14 Advertorial February 2023 | Issue68 THIS ADVERTORIAL IS SPONSORED BY B. BRAUN MELSUNGEN AG
dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh al Published studies 110+ Published Studies 25,000+ Documented Patients Countries 20+
Tuomas
Rissanen SeQuent® Please/NEO bbraun.com/dcb
Structural Heart Interventions
prior pacemaker or implantable cardioverter defibrillator. The Evoque implant procedure was performed successfully in 94.4% of patients, with a relatively “short” implant time of 71.6 minutes needed on average per patient. Discharge to home was achieved in 91.1% of patients, with the length of hospital stay averaging three days.
Turning to the clinical results, Windecker detailed that cardiovascular mortality, which stood at 1.7% at 30 days, totalled 9.4% at one year. Further to this, no incidence of myocardial infarction (MI) was reported, there was a low rate of stroke (0.6% at 30 days and 1.3% at one year). Nonelective tricuspid valve reinterventions were observed in 4% of patients at one year and severe bleeding was 16.9% at one month and 25.5% at one year.
TRISCEND trial reports oneyear outcomes in patients undergoing transcatheter tricuspid valve implantation
One-year results from the TRISCEND study, investigating the Evoque (Edwards Lifesciences) tricuspid valve replacement system, show favourable safety, efficacy, echocardiographic and quality of life outcomes in patients with symptomatic tricuspid regurgitation (TR), investigators have reported.
Stephan Windecker (Bern University Hospital, Bern, Switzerland) presented the trial’s clinical, functional and echocardiographic outcomes at 12 months in a late-breaking trial session at PCR London Valves (27–29 November, London, UK), following on from encouraging results at both 30 days and six months.

TRISCEND is a prospective, multicentre, single-arm study, that has enrolled 176 patients with symptomatic, moderate or greater primary or secondary tricuspid regurgitation despite medical therapy at 20 sites across the USA, Canada, France and Switzerland.
In his presentation, Windecker detailed that the Evoque transcatheter valve replacement system consists of a selfexpanding nitinol frame, with a pericardial bovine valve and fabric skirt, delivered through a 28Fr transfemoral delivery system, inserted transvenously.
Summarising the patient characteristics, Windecker described the trial’s population as elderly, with a mean age of 78.7, largely female (71%), and noted that these patients had frequent comorbidities and three quarters of the patients were highly symptomatic with New York Heart Association class III‒IV. Additionally, 92% of patients had atrial fibrillation (AF), 22% ascites, and 32% of patients had had
There were no major cardiac structural complications and the composite major adverse event rate was 18.6% at 30 days and 30.2% at one year, Windecker reported. Pacemakers were implanted in 13.3% of patients within 30 days, but there was no further implant beyond 30 days. Furthermore, the results showed a 90.1% rate of survival at one year, alongside an 88.4% rate of freedom from heart failure hospitalisation.
Windecker also then presented serial core lab assessment of TR at both 30 days and one year versus baseline. He commented: “What you note is that the vast majority of patients that had severe, massive or torrential TR, in nearly 90% of cases at baseline was turned into an outcome where there was no, or only mild tricuspid regurgitation in 98% of patients. Of note, these changes were durable at all times of follow-up: 30 days, six months and one year, and there was the absence of any severe or more tricuspid regurgitation.
Outlining the echocardiographic results, which were adjudicated by core lab at one year, Windecker commented that there was evidence of right ventricular (RV) remodelling after Evoque implant, demonstrated by a change in mean RV end-diastolic mid diameter from 41.4mm at baseline to 35mm at one year and inferior vena cava (IVC) diameter was reduced from 27.6mm at baseline to 20.4mm at one year.
On functional and quality of life changes, Windecker commented that prior to the intervention, most of the patients were highly symptomatic, but after the intervention, 93% were oligosymptomatic with New York Heart Association (NYHA) classification I or II. “You see a remarkable difference in Kansas City Cardiomyopathy Questionnaire (KCCQ) score where a mean score of 46 increased to 17.7 with a change of 26,” noted Windecker.
“To give you an impression, any change of more than 20 is considered large or a very large improvement in these patients, and there was also a highly significant difference in the improvements of six-minute walk test of 56 metres.”
Summarising the message from the presentation, Windecker said the findings demonstrate “favourable survival and freedom from heart failure hospitalisation”.
“The core lab-adjudicated rates of TR reduction were remarkable, where 98% of patients had either no or only mild tricuspid regurgitation,” he added. “There was echocardiographic evidence of right ventricular remodelling and there were also significant clinically meaningful improvements in heart failure symptoms, and very beneficial development as it relates to quality-of-life measures, KCCQ score and the six-minute walk test.”
No sex difference seen in outcomes for transcatheter tricuspid valve intervention
TRANSCATHETER TRICUSPID VALVE intervention is associated with similar outcomes in both women and men, research published in the European Heart Journal indicates. Investigators also concluded that patients undergoing transcatheter therapy benefitted from increased one-year survival compared to those receiving solely medical therapy, regardless of their sex.
Authored by Andrea Scotti, Augustin Coisne (both Montefiore Health System, New York, USA) and colleagues, the research sought to offer clarity on whether sex has an impact on characteristics and outcomes in patients with significant tricuspid regurgitation (TR) undergoing transcatheter tricuspid valve intervention.
“In our study, we showed that after transcatheter tricuspid valve intervention, clinical outcomes are similar in both women and men, with one-year survival rates of 81% and 78% respectively,” Scotti, Coisne et al write. “Similarly, the survival benefit of transcatheter tricuspid valve intervention over medical therapy was significant irrespective of sex.”
Using the TriValve registry—an international, multicentre, retrospective, multidevice registry evaluating tricuspid valve intervention—the researchers assessed data from 556 patients who underwent transcatheter tricuspid valve intervention at 24 centres between 2016–2021. Patients undergoing transcatheter therapy were compared to a control cohort of 2,072 medically managed patients with severe or greater TR, diagnosed at the Montefiore–Einstein Center for Heart and Vascular Care during 2015–2018.
The investigators report that after transcatheter therapy, there was no difference between women and men in one-year freedom from all-cause mortality (p=0.56), nor in heart failure hospitalisation (p=0.36), New York Heart Association (NYHA) Functional Classes II and IV (p=0.17), and TR severity >2+ at last follow-up. Multivariable Cox-regression weighted by inverse probability of treatment weighting (IPTW) showed improved one-year survival after transcatheter compared with medical therapy alone in both women (adjusted hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.23–0.83) and men (adjusted HR 0.42, 95% CI 0.18–0.89, p=0.03).
Discussing the findings, the authors highlight that the baseline characteristics between women and men were not markedly different. “This may explain the discrepancies with surgical series, where women were at much higher risk compared with the male candidates,” the study team writes, adding that this stresses the importance of timely referral and management of tricuspid valve disease.
15 Structural Heart Interventions Issue68 | February 2023
There was echocardiographic evidence of right ventricular remodelling and there were also significant clinically meaningful improvements in heart failure symptoms, and very beneficial development as it relates to quality-of-life measures, KCCQ score and the six-minute walk test”
Stephan Windecker (left)
Data point to lower rates of paravalvular leak with Acurate neo2 TAVI valve
The addition of an annular sealing skirt, among other design changes in the Acurate neo2 transcatheter aortic valve implantation (TAVI) system (Boston Scientific), has contributed to an improvement in rates of paravalvular leak (PVL) compared to a previous generation of the device, findings from a postmarket clinical follow-up study presented at PCR London Valves (27–29 November, London, UK) suggest.
This was among the talking points raised by Won-Keun Kim (Kerckhoff Heart Center, Bad Nauheim, Germany) in his presentation of the 30-day results from the Acurate neo2 postmarket study, during a late-breaking trial session at the PCR London Valves meeting. Findings of the postmarket study were also published in EuroIntervention
The Acurate neo2 aortic valve system received CE mark and was released to the European market in 2020. The device builds upon the earlier-generation Acurate neo platform, CE marked in 2014, and features annular sealing technology designed to conform to irregular, calcified anatomies and further minimise PVL.
“The Achilles’ heel has been a relatively high rate of PVL, around 10% in the SCOPE I and II studies,” said Kim of the earlier-generation device, in his PCR London Valves presentation. Data presented at EuroPCR 2021 (18–20 May, virtual) from the Early neo2 and ITAL-neo registries had demonstrated positive procedural performance, including low rates of PVL and permanent pacemaker implementation (PPI). Kim explained that the purpose of the postmarket study was to generate clinical data in routine practice out to one year.
Investigators enrolled 250 patients with severe aortic stenosis at 18 sites across Europe, assessing a primary safety endpoint of all-cause mortality,
which occurred in 0.8% of patients. Of the two patients who died, one was due to bleeding after the procedure and another patient experienced valve embolisation and coronary obstruction due to unsuccessful TAV-in-TAV, Kim detailed.
The data also demonstrated that no patients experienced greater than moderate PVL, 1.9% experienced moderate PVL and 18.9% experienced mild PVL. Other notable findings from the study included a low 6.5% rate of new pacemaker implantation 30 days post-procedure, with no incidence of disabling stroke or acute kidney injury.
The study also assessed a primary imaging endpoint to assess hypoattenuated leaflet thickening (HALT) after the procedure, reporting a HALT rate of 24.5% at 30 days post-procedure.
“There was a good haemodynamic improvement and low gradients at 30 days, very low pacemaker rates at 30 days and we have a greater improvement in PVL than observed with prior generation Acurate neo,” said Kim of the study’s main findings. “Ninety-eight percent of patients had mild or no/ trace PVL at 30 days and no patients had more than moderate PVL,” he summarised.
Kim’s presentation prompted discussion among panellists at the PCR London Valves late-breaking trial session to consider the potential impact of the design changes from the earlier

iteration Acurate neo platform. “The addition of the skirt has made the valve better, but in practical terms how much better?” asked Antoinette Neylon (Hopital Prive Jacques Cartier, Massy, France), as well as asking Kim if this changes the type of anatomy that the technology could be used in.
Kim responded that he and colleagues have compared the performance of Acurate neo to Acurate neo2 looking at the amount of total calcification that can be mitigated by the skirt. “We have also found that it is not the total amount of calcification but more the distribution,” he commented. “So, eccentric calcification would be more critical to the Acurate, even with the skirt. You would be rather careful with very extreme calcification plus eccentric calcification. If it is just concentric and symmetric calcification I think you can use the Acurate neo2.”

Asked for his “bottom line” on the study findings, Kim was pushed on whether he believes the results show that Acurate neo2 is non-inferior or similar to other devices in the market. “You cannot say that there is one valve that is the best,” he remarked, adding: “It is a difficult question and very political. I would say we have to try to get the best
HOSPITAVI elucidates underlying causes of rehospitalisations among TAVI patients
A single-centre retrospective study of rehospitalisation after transcatheter aortic valve implantation (TAVI) has found that almost half of patients treated with TAVI were readmitted to hospital within one year.
THE ANALYSIS—WHICH WAS CONDUCTED at Rigshospitalet (Copenhagen, Denmark)—has shown that first post-procedural rehospitalisation was more often due to cardiovascular than non-cardiovascular causes. Arrhythmias accounted for approximately 30% of cardiovascular rehospitalisations.
Researchers studied all patients who underwent TAVI at the centre between 2016 and 2020, followed at patient level during the first year after their index admission. Medical records were reviewed to validate the cause of rehospitalisation and mortality. They studied 1,403 patients who had undergone TAVI from 2016 to 2020, with a median age of 80 years.
Findings of the study were presented by Pernille Baekke (Rigshospitalet, Copenhagen, Denmark) at
PCR London Valves, after their publication in the European Heart Journal
The study authors report that within the first postdischarge year, 48.6% of patients were rehospitalised at least once, of which 26.2% were of cardiovascular cause, compared to 22.4% of non-cardiovascular cause. The median length of stay for the first rehospitalisation was three days.
Digging into the causes of these readmissions, the researchers note that the most common cardiovascular causes were arrhythmias occurring in 28.1% of the rehospitalised patients. Of these cases, 40.6% required permanent pacemaker implantation. Heart failure (14.4%) and neurological events (14.4%) were other common
for each patient, and there are different valves with different characteristics.”
Commenting on the patients who may benefit most from the Acurate neo2 valve, Kim said: “I would prefer those patients with small anatomies, low to moderate rate of calcification with high risk of conduction disturbances and importance of coronary access.”
In a press release circulated by Boston Scientific following the presentation of the results, Lars Søndergaard, (Rigshospitalet, Copenhagen, Denmark) also highlighted the change in annular sealing technology and its contribution to the lower rate of PVL with the new generation device.
“With this foundational dataset, we now have postmarket surveillance results that validate the use of the currentgeneration Acurate neo2 valve for the management of patients with severe aortic stenosis,” Søndergaard was quoted as saying. “The data suggest that the annular sealing technology minimises leakage around the valve—providing greater improvement in PVL than observed with the prior-generation Acurate neo valve— all while maintaining single-digit permanent pacemaker rates, which contributes to better long-term patient outcomes.”
cardiovascular causes of rehospitalisation.
The most common non-cardiovascular causes were infectious diseases (18.3%), with pneumonia accounting for 40.4% of these readmissions. The first rehospitalisation was elective in 14.8% of the admissions, primarily due to percutaneous left atrial appendage closure (LAAC), cancer or osteoarthritis.
“[HOSPITAVI] clearly demonstrated that there is potential to bring down the rehospitalisation rates and improve outcomes for patients. We also saw that a lot of patients were hospitalised due to an observational cause, indicating that these patients might need a safety net after discharge to be more secure in their own home,“ the study author, Baekke, told Cardiovascular News
The researchers conclude that further work to explore if the rate and causes of hospitalisations differ in the general population is needed and to establish a risk model to predict patients at high risk of rehospitalisation.
16 Structural Heart Interventions February 2023 | Issue68
We now have postmarket surveillance results that validate the use of the current-generation Acurate neo2 valve for the management of patients with severe aortic stenosis”
0.8% 0% 18.9% all-cause mortality at 30 days greater than moderate PVL mild PVL 1.9% moderate PVL
Won-Keun Kim
Pernille Baekke
Registry data point to a declining complication rate in primary mitral regurgitation patients
The largest dedicated registry on mitral transcatheter edge-to-edge repair (M-TEER) in primary mitral regurgitation (MR) has shown a constant decline in complication rates, which has corresponded with a gradual decline in the proportion of residual MR and overall improvements in outcomes over time.
THESE WERE THE HEADLINE CONCLUSIONS from the presentation of preliminary results of the PRIME-MR registry, presented by Benedikt Koell (University Heart Center Hamburg, Hamburg, Germany) at PCR London Valves (27–29 November, London, UK) in a late-breaking trial and innovation session.

To date, Koell said, the EVEREST II trial and large registry studies of mitral valve edge-to-edge repair have either focused upon primary or secondary mitral regurgitation, Koell said. However, he noted that since the publication of results from the COAPT and MITRA-FR trials, “scientific focus has shifted
Tendyne exhibits
towards secondary MR,” commenting that there is a lack of data on primary MR for TEER patients.
In order to bridge this gap, Koell and colleagues initiated PRIME-MR, an international, retrospective registry, collecting data from 24 high-volume TEER centres in Europe, the USA and Canada. In total, the registry has collected data from 2,363 patients treated between 2008 and 2022.
Koell’s presentation focused on temporal trends for M-TEER, focusing on safety endpoints according to the Mitral Valve Academic Research Consortium (M-VARC) and all-cause mortality. In total, 361 patents were excluded from the analysis due to missing follow-up. The remaining 2,002 patients were subdivided into four groups based upon the timing of their procedure, with 276 taken from 2008–2014, 384 from 2014–2016, 822 from 2017–2019, and 530 from 2020–2022.
Koell noted that the registry included an elderly patient cohort, with the main reason for patients being denied surgery being advanced age, followed by comorbidities, frailty, and previous heart surgery.
Most patients (83.7%) presented with isolated primary MR, while a smaller proportion (16.3%) had mixed MR, albeit with primary MR as the leading cause. Looking at the main MR pathology, Koell noted that there was a relatively even split between patients with prolapse (44%) and flail (49.8%). Implanted devices included MitraClip (Abbott) generations one through four, as well as Pascal (Edwards Lifesciences)
P10 and ACE.
Koell described the patients treated as an “older, symptomatic cohort”, with an average age of 81 years across the 14-year duration of the study. Looking at the different time intervals, Koell noted that, interestingly, the study saw a decline in symptomatic patients, detailing that the proportion of patients in New York Heart Association (NYHA) class III/ IV stood at 86.2% among the patients treated between 2008–2013, 86.4% in 2014–2016, 76.4% in 2017–2019 and
“sustainable”
reductions in mitral regurgitation at one year
One-year follow-up data from the Tendyne European experience registry, which were presented during a late-breaking clinical trial session at PCR London Valves, point towards “low” rates of allcause and cardiovascular mortality, investigators have reported, as well as noting that the transcatheter treatment exhibits a durable clinical benefit, with a sustained reduction in mitral regurgitation (MR).
Tendyne (Abbott) is the first and only commercially available transcatheter mitral valve replacement (TMVR), having received the CE mark in January 2020 for the management of patients with mitral regurgitation (MR) who are not suitable for mitral valve surgery or percutaneous edge-to-edge repair.
Michaela Hell (University Medical Centre Mainz, Mainz, Germany) presented the latest results from the European registry—known as TENDER—reporting data from 195 patients across 31 sites throughout Europe to have implanted the device commercially between January 2020 and December 2021. TMVR offers a treatment option for patients with high surgical risk, said Hell of the therapy,
adding: “We have very promising results from the early feasibility studies, with very low mortality rates.”
Detailing the baseline patient data for the patient population, Hell reported that patients had a mean EuroScore II of 7.7, with a prior rate of hospitalisation for heart failure of 68%, and 81% of patients were in New York Heart Association (NYHA) functional class III/IV at baseline. In terms of MR aetiology, 40% had primary MR, 39% secondary, and 21% were classified as “mixed”. Severe calcification was found in 11% of patients.
Significantly, the investigators included patients who were treated for an off-label indication—totalling 33% (65) of the procedures—with the predominant reasons for off-label use
67% in 2020–2022. The data also point to a decline in Society of Thoracic Surgeons (STS) Score over time, spanning 5.7±4.5 in 2008–2013, 5.1±4 in 2014–2016, 4.8±3.5 in 2017–2019 and 4.8±3.9 in 2020–2022.
Turning to the safety endpoints, Koell reported that there was a decline in safety endpoints and reintervention rates from 2008 through to 2022, with no M-VARC safety endpoints reported in 45.8% of patients between 2008–2013, 43.3% between 2014–2016, 58% in 2017–2019, and 69.5% in 2020–2022. Reintervention rates within 12 months of the procedure stood at 4.8% in 2008–2013, 1.8% for the 2014–2016 period, 1.7% for 2017–2019, and 2.3% for 2020–2022.
Detailing the M-VARC safety endpoints he noted there was a low procedural mortality, with about 1% over time, also a low procedural stroke rate of about 1.2%, but what is important, is that we see a constant decrease in minor bleeding, major bleeding and minor vascular complications over time,” Koell reported.
The data also demonstrated gradually improving rates of residual MR at discharge, with none or trace MR reported in 70.7% of patients in 2020–2022,
compared to 51.9% of patients at 2008–2013.
“This is the largest registry on M-TEER in primary MR patients, and in this first preliminary analysis, we see that the complication rates constantly decline, and more importantly that the proportion of residual MR also gradually declines and the overall outcomes improve,” Koell concluded.
including severe calcification or a prior mitral valve intervention or surgery.
Outcome data were available for 186 of the 195 patients treated, with Hell reporting a 29% all-cause mortality rate and 17% cardiovascular mortality rate at one year, compared to rates of 9% and 7% for the same figures respectively at 30 days. Breaking these figures down for patients who were treated in an on- or off-label context, Hell noted that all-cause mortality rates stood at 27% and cardiovascular mortality at 15% for on-label patients, alongside 33% all-cause mortality and 21% cardiovascular mortality at one-year among the off-label patients.
Turning to major adverse events, Hell reported that there was a reduction in heart failure hospitalisation from 66% at baseline to 29% at one year. Furthermore, the registry demonstrated a 1.6% overall rate of in-hospital disabling stroke, occurring exclusively in patients who received on-label treatment. Disabling stroke stood at 3% overall at one year, with an event rate of 1.1% in on-label patients and 7.3% in off-label patients.
Major bleeding—comprising Bleeding Academic Research Consortium (BARC) class 2, 3 or
5 bleeding—was reported in 14% of patients in hospital. At one year, 5.1% of patients required mitral valve reintervention or surgery, comprising 5.3% of on-label patients and 4.5% of off-label patients.
In terms of the reduction in MR seen among the patient cohort, Hell said that the registry data point toward “sustained improvement” at one year, with 99% of patients having MR grade 1 or 2 at one year. Improvements in NYHA functional class were also largely sustained out to the one-year timepoint, with 79% of patients remaining in NYHA class I or II. This was consistent across both on- and off-label patient cohorts, Hell detailed.
The data comprise “the largest series of commercial implants of a dedicated TMVR device,” Hell added, noting that the findings demonstrate “very similar low, one-year mortality rates, and even slightly lower severe mortality rates, compared to the previous early reports”. “We also showed a very durable clinical benefit, with an elimination of the MR and sustainability at one year. And, importantly of this registry, we can also say that there was no difference in survival outcomes of major adverse events between the on- and off-label indication groups,” Hell concluded.
17 Structural Heart Interventions Issue68 | February 2023
Tendyne valve device
This is the largest registry on M-TEER in primary MR patients, and in this first preliminary analysis, we see that the complication rates constantly decline”
Benedikt Koell
Extended follow-up of the ISCHEMIA-EXTEND trial has shown no difference in rates of all-cause mortality with an initial invasive strategy when compared to a conservative strategy in patients with chronic coronary disease and moderate or severe ischaemia.
JUDITH S HOCHMAN (NEW YORK University School of Medicine, New York, USA) presented this interim conclusion, taken at a median of 5.7 years, in a late-breaking trial session at the American Heart Association Scientific Sessions (AHA 2022; 5–7 November, Chicago, USA). The findings were simultaneously published in Circulation ISCHEMIA-EXTEND follows on from the ISCHEMIA trial, looking at the potential benefit in adding cardiac catheterisation and revascularisation— with either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery—to optimal medical therapy in stable
patients with at least moderate ischaemia on a stress test. Both patient groups in the study received guideline-directed medical therapy, with those in the conservative group treated invasively if symptoms worsened despite drug therapy or in the case of an emergency.
Hochman delivered the initial results of the trial at AHA 2019 (16–18 November, Philadelphia, USA), taken at a median of 3.2 years, and showing that the revascularisation strategy conferred no additional benefit when compared to the conservative approach.

Interim findings from the extended study presented at AHA 2022 included all 5,179 patients for whom data were collected in the initial trial, discounting
disease
USING MACHINE LEARNING AND CLINICAL data from electronic health records, researchers have constructed an in silico marker for coronary artery disease to better measure clinically important characterisations of the disease.
As detailed in a publication in The Lancet, the researchers hope that their model may lead to more targeted diagnosis and better disease management of coronary disease, and claim that their study is the first known research to map its characteristics on a spectrum. Previous studies have focused only on whether or not a patient has coronary disease. This and other common conditions exist on a spectrum of disease; each individual’s mix of risk factors and disease processes determines where they fall on the spectrum. However, most such studies break this disease spectrum into rigid classes of case (patient has disease) or control (patient does not have disease). This may result in missed diagnoses, inappropriate management, and poorer clinical outcomes, say the investigators.
“The information gained from this non-invasive
patients who had withdrawn consent after the initial phase of the study or declined participation in extended follow-up. In total, 557 deaths occurred from the start of the trial up to the end of the current study period which ran through to December 2021, roughly doubling the total of 289 deaths that had occurred after the initial study time point.
The objective of the long-term follow-up is to assess whether there are between-group differences and to increase precision around the treatment effect estimates for endpoints including all-cause mortality, cardiovascular [CV] mortality, and non-cardiovascular mortality, Hochman noted in her presentation at AHA 2022. Reporting the interim findings from the study, Hochman relayed that all-cause death occurred in 13.4% of patients in the conservative treatment group, compared to 12.7% in the invasive treatment group (hazard ratio [HR]: 1; 95% confidence interval [CI]: 0.85, 1.18; p=0.741 (log rank).
“For cardiovascular death, we had the hypothesis that spontaneous myocardial infarction (MI) reduction would lead to a reduction in cardiovascular death,” Hochman commented, before going on to note that this “appears to be the case,” with the rate of CV death standing at 8.6% in the conservative treatment arm and 6.4% in the invasive treatment arm (HR: 0.78, 95% CI: 0.63, 0.96; p=0.008). Finally, non-CV death stood at 4.4% in the conservative arm, and 5.6% in the invasive arm (HR: 1.44, 95% CI: 1.08, 1.91; p=0.016).
“Extended follow-up of the ISCHEMIA randomised trial over a median of 5.7 years demonstrated that an initial invasive strategy compared to an initial conservative strategy resulted
staging of disease could empower clinicians by more accurately assessing patient status and, therefore, inform the development of more targeted treatment plans,” says senior study author Ron Do (Icahn School of Medicine at Mount Sinai, New York, USA).
“Our model delineates coronary artery disease patient populations on a disease spectrum; this could provide more insights into disease progression and how those affected will respond to treatment. Having the ability to reveal distinct gradations of disease risk, atherosclerosis, and survival, for example, which may otherwise be missed with a conventional binary framework, is critical.”
In the retrospective study, the researchers trained the machine learning model, named in silico score for coronary artery disease (ISCAD), to measure coronary disease on a spectrum using more than 80,000 electronic health records from two large health system-based biobanks, the BioMe Biobank at the Mount Sinai Health System and the UK Biobank.
The model, which the researchers termed a “digital marker,” incorporated hundreds of different clinical features from the electronic health record, including vital signs, laboratory test results, medications, symptoms, and diagnoses, and compared it to both an existing clinical score for coronary disease, which uses only a small number of predetermined features, and a
in no difference in all-cause mortality, with nearly twice the number of deaths, lower risk of cardiovascular, but higher risk of non-cardiovascular mortality,” Hochman concluded. “These findings provide evidence for patients with chronic coronary disease and their physicians as they decide whether to add invasive management to guidelinedirected medical therapy.”
Summing up the findings after the presentation, Hochman said: “These longer-term results build on our original findings, which is that patients with daily or even weekly angina will likely see improved quality of life, but we have more robust data that an invasive approach is not going to prolong life.
“Although cardiovascular mortality was reduced, with an invasive approach it was offset by higher noncardiovascular mortality, so all-cause mortality is the same over time in both groups. Either strategy is acceptable, which is good news for patients, who have different preferences.”
Providing a commentary on the results at AHA 2022, Cecilia Bahit (INECO Neurociencias, Rosario, Argentina) said that the findings presented by Hochman appeared to be in line with prior data.

“When we look at the results and we put them into perspective, we see that the findings of all-cause mortality in the ISCHEMIA-EXTEND report are comparable to other previously recorded trials of revascularisation versus medical therapy,” she commented.
“During the extended follow-up investigators were able to identify more death, and were able to identify lower risk of cardiovascular death in the invasive strategy, and this was in benefit of an invasive strategy with a 21% relative risk reduction compared to the conservative arm.”
genetic score for coronary artery disease.
The 95,935 participants included participants of African, Hispanic/Latino, Asian, and European ethnicities, as well as a large share of women. Most clinical and machine learning studies on coronary disease have focused on white European ethnicity. Investigators found that the probabilities from the model accurately tracked the degree of coronary stenosis, mortality, and complications.
“Machine learning models like this could also benefit the healthcare industry at large by designing clinical trials based on appropriate patient stratification. It may also lead to more efficient data-driven individualised therapeutic strategies,” says lead author Iain S Forrest, (Icahn Mount Sinai, New York, USA). “Despite this progress, it is important to remember that physician and procedure-based diagnosis and management of coronary artery disease are not replaced by artificial intelligence, but rather potentially supported by ISCAD as another powerful tool in the clinician’s toolbox.”
Next, the investigators envision conducting a prospective large-scale study to further validate the clinical utility and actionability of ISCAD, including in other populations. They also plan to assess a more portable version of the model that can be used universally across health systems.
February 2023 | Issue68 18 Conference Coverage
Machine learning enables “clinically important” characterisation of coronary artery
Rates of allcause death align between invasive and conservative strategies in ISCHEMIAEXTEND
Judith S Hochman


The World-Leading Course in interventional cardiovascular medicine #EuroPCR REGISTER TODAY! Don’t miss this unique learning opportunity and the occasion to discuss diverse practical applications with colleagues from around the world! Until 7 March Early Fee! 16-19 May 2023 EuroPCR.com
BRIGHT-4 trial: Bivalirudin cuts 30-day mortality for STEMI patients undergoing PCI compared to heparin
BIVALIRUDIN IS A SAFER AND MORE effective anticoagulant than heparin for treating patients with ST-segment elevation myocardial infarction (STEMI) who undergo primary percutaneous coronary intervention (PCI), and can lower the risk of death or major bleeding by 31%.
These are key findings from a new study led by researchers from the Icahn School of Medicine at Mount Sinai (New York, USA). This is the first largescale clinical trial to compare the two anticoagulants most widely used after PCI, and shows bivalirudin given with a two- to four-hour high-dose infusion significantly reduces death, major bleeding, and thrombosis when compared with heparin, the researchers behind the study have said.
The results were presented in a late-breaking clinical trial at the American Heart Association Scientific Sessions (AHA 2022; 5–7 November, Chicago, USA) and published in The Lancet. This work could have broad implications, changing the treatment course for hundreds of thousands of patients across the world who experience a STEMI, the researchers claim.
“For the first time, this study identifies the best and safest treatment course for patients undergoing stenting to treat a STEMI heart attack,” said co-principal investigator Gregg W Stone (Mount Sinai Hospital, New York, USA). “Compared with heparin, bivalirudin plus a short infusion substantially improved the likelihood of surviving a STEMI and reduced the two most feared complications—major bleeding and stent thrombosis.”

In the BRIGHT-4 trial, patients with STEMI underwent primary PCI,
which requires anticoagulant therapy.
The most common anticoagulant used during primary PCI is heparin, but its use can lead to ischaemic and haemorrhagic complications. Heparin and bivalirudin have been compared in six prior large randomised trials in patients with STEMI, but in those studies, they were administered with varying regimens and background therapies. This new research evaluates the two most widely used regimens of heparin and bivalirudin, which have never been directly compared with each other in an adequately sized trial.
Stone along with Yaling Han (Shenyang Northern Hospital, Shenyang, China) led a team of researchers to analyse 6,106 patients enrolled in the study across 87 sites in China between February 2019 and April 2022. All underwent primary PCI for STEMI treatment, nearly all with a procedure that used the radial artery in the wrist to target the blocked heart artery. Patients were randomised to receive the two most widely used regimens of heparin and bivalirudin, which prior studies have shown to be the safest and most effective. One group received heparin alone, administered intravenously. The other group received bivalirudin intravenously, followed by a high-dose infusion for two to four hours after
Investigators followed patients for 30 days after the procedure. The primary goal of the study was to compare the occurrence of allcause mortality or major bleeding.
Researchers found that 4.4% of patients treated with heparin died or had a major bleed within 30 days, compared to 3.1% of patients treated with bivalirudin.
Overall, the bivalirudin
Depression increases likelihood of non-adherence to medical therapy post-PCI
Patients with depression are 10–20% less likely to adhere to guideline-directed medical therapy after percutaneous coronary intervention (PCI), research published in JAMA Network Open has shown.
ACCORDING TO MATTHEW E
Lapa (University of Pittsburgh School of Medicine, Pittsburgh, USA) and colleagues, who conducted the research, the recognition of depression among patients undergoing PCI may facilitate targeted interventions to address medical adherence and improve secondary cardiovascular disease prevention.
Lapa et al conducted a retrospective cohort study of patients undergoing PCI from January 2014 to December 2019, using data from the Optum Clinformatics DataMart database. The study team identified a diagnosis of depression as occurring during the 12 months of enrolment prior to PCI, or within six months after the procedure. Medication adherence was assessed with the proportion of days covered (PDC), calculated as the ratio of the number of days a medication is available
and the number of follow-up observation days. Twelve-month adherence was categorised as adequate (PDC ≥80% to <90%) or optimal (PDC ≥90%).
A total of 124,443 patients were included in the assessment, with a mean age of 69.3 years, 41,430 of whom (33.3%) were female. Of this population, 20,711 patients (16.6%) had a diagnosis of depression.
The researchers found that those with depression were significantly less likely to obtain adequate 12-month adherence to antiplatelets (odds ratio [OR], 0.80; 95% confidence interval [CI] 0.77–0.85), β-blockers (OR, 0.84; 95 CI, 0.80–0.88), and statins (OR, 0.88; 95% CI, 0.85–0.93) than those without depression. There was no association between depression and adherence to renin-angiotensin-aldosterone system inhibitors (OR, 0.93; 95% CI, 0.85–1).
group had a 31% reduction in the rate of death or major bleeding compared with patients in the heparin group—a highly statistically significant reduction.
The researchers then looked at the specific incidence of death alone and major bleeding alone between the groups. They found that deaths were reduced from 3.9% in heparin-treated patients to 3% in bivalirudintreated patients. Severe bleeding was also reduced from 0.8% in the heparin group to 0.2% in the bivalirudin group. Both differences were statistically significant.
They further analysed the rate of stent thrombosis, finding that this was also lower in the bivalirudin group at 0.4% compared with 1.1% in the heparin group.
“These results are dramatic,” Stone said. “The simple decision to use bivalirudin during primary PCI in patients with heart attacks, which is now generic and thus inexpensive, can save hundreds of thousands of lives per year and prevent major bleeding and stent thrombosis compared with heparin.”
The BRIGHT-4 trial was an investigator sponsored and organised trial, funded by the Chinese Society of Cardiology Foundation with a research grant from Jiangsu Hengrui Pharmaceuticals.
Those with depression had similarly decreased likelihood of optimal 12-month adherence to antiplatelets, β-blockers, and statins as well as renin-angiotensin-aldosterone system inhibitors (OR, 0.87; 95% CI, 0.82–0.94), the researchers report.

“These results indicate the critical importance of strategies to address depression as part of secondary cardiovascular disease prevention,” Lapa et al write. “The American Heart Association has advised depression screening for all patients with coronary disease given the adverse contribution of depression to cardiac prognosis. However, depression commonly goes unrecognised and undertreated in clinical settings.
“Either due to resistance from patients to report their depressive symptoms or poor screening practices, our findings of depression negatively affecting medication adherence could be underestimated. We advocate for multidisciplinary interventions to
ensure frequent follow-ups, prescription reminders, and counselling to target challenges to medical adherence.”
The study’s authors note that their research has several limitations, chiefly that it only included individuals with insurance, the potential for misclassification of depression status due to reliance on claims data, the use of records from prescription fills to determine adherence, and finally the potential for residual confounding from unmeasured variables that may be associated with depression and adherence.
“This cohort study of healthcare claims found an association between depression and likelihood of adequate or optimal 12-month adherence to essential guideline-directed medical therapy agents following PCI,” the study concludes. “Further studies are needed to determine whether treatment of depression may improve medication adherence as well as how such treatment improves secondary prevention of cardiovascular disease.”
February 2023 | Issue68 20 Coronary Interventions
The simple decision to use bivalirudin during primary PCI in patients with heart attacks, which is now generic and thus inexpensive, can save hundreds of thousands of lives per year and prevent major bleeding and stent thrombosis compared with heparin”
Gregg W Stone
Patients with depression 10–20% less likely to adhere to guideline-directed medical therapy after PCI
Otsuka Medical Devices and ReCor Medical submit premarket approval of Paradise ultrasound renal denervation system
Otsuka Medical Devices and ReCor Medical have announced the filing of the premarket approval (PMA) application to the US Food and Drug Administration (FDA) for the Paradise ultrasound renal denervation system (uRDN) system in the treatment of uncontrolled hypertension.
The Paradise uRDN system is designed to reduce sympathetic nerve activity by denervating nerves which surround the renal arteries with the goal of reducing blood pressure, and uses a combination of ultrasound energy to denervate the renal nerves and a waterfilled balloon to protect the renal artery. The system employs an interventional procedure in which a catheter is placed in each of the main renal arteries, following which two to three sevensecond ultrasound emissions are delivered to denervate the surrounding renal nerves, thereby reducing blood pressure.
Since 2009, ReCor has been focused on developing and testing the Paradise system to treat hypertension safely and effectively. ReCor has three global, independently powered, sham-controlled randomised clinical trials of the Paradise system in more than 500 patients with uncontrolled hypertension: RADIANCE-HTN SOLO, RADIANCE-HTN TRIO and RADIANCE II. Each RADIANCE trial met its prespecified primary efficacy endpoint of blood pressure reduction, with positive safety.
RADIANCE II is the US FDA’s investigational device exemption (IDE) pivotal trial. In September 2022, ReCor and Otsuka Medical Devices announced that the trial successfully reached its primary efficacy endpoint. Results showed a reduction in daytime systolic ambulatory blood pressure of -7.9 mmHg in those treated with uRDN and a difference between uRDN and sham of -6.3 mmHg (p <0.0001). The results from the all three RADIANCE clinical trials have been included in the
submission for approval to the FDA.
The Paradise system bears the CE mark for the treatment of hypertension in Europe and is an investigational device in the USA and Japan.
First US procedure performed using ShortCut leaflet splitting device
Pi-Cardia has announced that the first patient in the USA has been successfully treated with ShortCut—a dedicated device designed to split the leaflets of a pre-existing valve to enable safe transcatheter aortic valve implantation (TAVI) in patients at risk for coronary obstruction.
Pi-Cardia received Investigational Device Exemption (IDE) approval from the US Food and Drug Administration (FDA) to commence the ShortCut Pivotal Study in July 2022 and multiple patients have already been treated successfully outside the USA.
The ShortCut procedure was performed at Morristown Medical Center (Morristown, USA), by Philippe Généreux, Jim Slater, Leo Marcoff, and Robert Kipperman, in collaboration with Benjamin Seguy (Hospital Haut Leveque, Bordeaux, France).
“We successfully treated this patient with a degenerated valve who was at risk of coronary obstruction after TAVI with the ShortCut device,” said Généreux. “The targeted leaflet was effectively split within just a few minutes, allowing for safe implantation of the TAVI valve. ShortCut is a real game-changer enabling TAVI treatment in younger patients who will likely need multiple interventions over the course of their lives.”
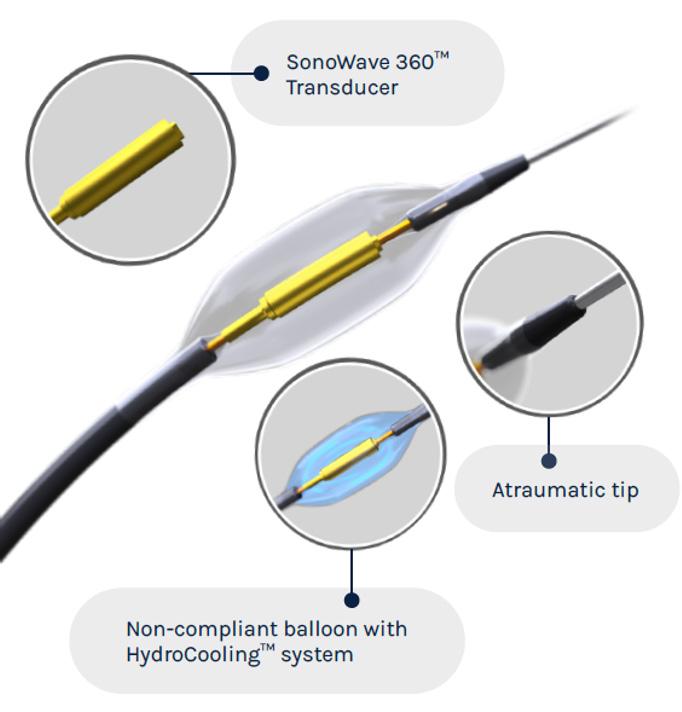

indications; as well as occlusive disease of the superficial femoral artery (SFA); and coronary in-stent restenosis.
Enrolment of the SELUTION SLR coronary de novo study will begin in the USA within the next few months.
This will complement the experience that the company has already gained with the SELUTION DeNOVO trial in Europe.
More than 800 patients of the 3,326 planned have been enrolled in this coronary randomised controlled study comparing Selution SLR versus any limus drug-eluting stent (DES). The study is powered to demonstrate superiority of Selution SLR drugeluting balloon (DEB) over DES in coronary de novo artery disease.
“Treatment of de novo coronary arteries with drug-eluting balloons is a breakthrough in revascularisation of coronary artery disease. The SELUTION SLR coronary de novo study is the first of its kind in the USA and will provide important data on the efficacy and safety of sirolimuseluting balloon as a viable alternative to drug-eluting stent, leaving nothing behind post-percutaneous coronary intervention (PCI) and eliminating in-stent restenosis and related complications,” said Ron Waksman (MedStar Heart and Vascular Institute, Washington DC, USA), chairman of the MedAlliance Coronary Study Steering Committee.
Selution SLR was awarded CE mark approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020.
US FDA approves use of Navitor TAVI valve in highrisk aortic stenosis patients
The US Food and Drug Administration (FDA) has approved the Navitor (Abbott) transcatheter aortic valve implantation (TAVI) system for the treatment of severe aortic stenosis among patients who are at high or extreme risk for open-heart surgery.
system, which features a slim design to accommodate different patient anatomies and small vessels for stable, predictable and accurate valve delivery and placement.
Michael Dale, senior vice president of Abbott’s structural heart business, said: “Navitor is the first TAVI system to offer optimal haemodynamics in all valve sizes while also preserving options for lifetime disease management, an important consideration for physicians and patients when selecting a TAVI solution.”
MedAlliance’s Selution SLR receives coronary de novo IDE approval
Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its pivotal clinical trial for the treatment of coronary de novo lesions.
This comes less than eight months after the company received its first IDE approval for Selution SLR in the treatment of below-the-knee (BTK)
“Abbott’s Navitor device features advancements to help doctors safely and effectively treat patients with aortic stenosis, including a design that reduces the backflow of blood around the valve that is often a complication following TAVI procedures,” said Michael Reardon (Houston Methodist Hospital, Houston, USA) who served as principal investigator for the study that led to FDA approval. “The innovative Navitor system also offers physicians stable and accurate device placement, even in challenging patient anatomies.”
Navitor features a fabric cuff— NaviSeal—to reduce or eliminate paravalvular leak (PVL), and Abbott describes the device as the only selfexpanding TAVI system with leaflets within the native valve; a feature intended to improve access to coronary arteries to facilitate future procedures for treating coronary artery disease.
The Navitor device is implanted using Abbott’s FlexNav delivery
CroíValve reports successful first-in-human implants of Duo tricuspid coaptation valve system
CroíValve has announced the successful first-in-human implants of its Duo tricuspid coaptation valve technology for the treatment of tricuspid regurgitation as part of its TANDEM I study in Poland.

The procedures were performed at the National Institute of Cardiology (Warsaw, Poland) by Adam Witkowski and Maciej Dąbrowski and the Medical University of Silesia (Katowice, Poland) by Wojciech Wojakowski.
The Duo system consists of a coaptation valve implant that works in tandem with the native tricuspid valve to restore valve function. The device is secured using a novel anchor system which leaves the frail right heart chamber and native valve apparatus untouched. The implant procedure uses standard imaging and is suitable for a broad patient cohort, including those who are challenging to treat with other valve repair and replacement technologies, the company said in a press release.
“The Duo system is unique in providing a solution that can effectively treat the dilated right heart anatomy that accompanies tricuspid regurgitation, while avoiding contact with the right heart to maintain normal cardiac motion,” commented Witkowski. “After 30 days, our patient has already experienced a transformative improvement in symptoms, highlighted by the Kansas City Cardiomyopathy Questionnaire (KCCQ) Quality of Life survey and a reduction from New York Heart Association (NYHA) III to NYHA II and significant improvement in six-minute walk test. In addition, the device continues to exhibit excellent efficacy.”
21 Product News Issue68 | February 2023 Market Watch
Implanting team at the National Institute of Cardiology in Warsaw with Adam Witkowski and Maciej Dabrowsk
Selution SLR
Paradise Catheter
Clinical News
Industry News
effectiveness and qualitative outcomes.
Analysis revealed that two patients (3.3%) developed early discharge after transradial stenting of coronary arteries Study (EASY) grade I/Bleeding Academic Research Consortium (BARC) type I haematomas at the device access site. As a measure of effectiveness, a haemodynamic effect was observed after each spike delivery in 54 patients (90%).
Pilot study of wire pacing sleeve yields positive results in TAVI and PCI procedures

A first-in-human trial using a purpose-built device to provide direct wire pacing without the need for a temporary venous pacemaker during transcatheter aortic valve implantation (TAVI) and complex percutaneous coronary intervention (PCI) has found that the device was safe and effective.
According to Electroducer, the company behind the Electroducer Sleeve, the device is an electroconductive device made of highly innovative material which is intended to transmit, in a safe way for the patient, the electrical signal from an external pacemaker to the heart through the guidewire.
Results of the first-in-man study were published in the journal EuroIntervention. Researchers from four French medical centres—the Clinique Pasteur in Toulouse, the Cardiovascular Institute in Grenoble, the Médipôle Lyon-Villeurbanne Hospital in Villeurbanne and the Jacques Cartier Private Hospital in Massy—enrolled 60 patients in the study, 39 underwent TAVI, and 21 underwent PCI.
The primary endpoint was an analysis of a safety outcome, defined as the occurrence of haematomas or bleeding complications at the device vascular access site. Secondary endpoints included analyses of
Conference calendar
25–28 February
Cardiovascular Research Technologies (CRT)
Washington DC, USA crtmeeting.org
4–6 March
American College of Cardiology (ACC)
2023 Scientific Session New Orleans, USA pcronline.com
16–18 March
Houston Aortic Symposium Houston, USA houstonaorticsymposium.com
Analyses of other secondary endpoints showed that two patients (6.3%) presented asymptomatic radial artery occlusion.
Jérôme Wintzer-Wekehind
(Cardiovascular Institute, Grenoble, France) principal investigator on the study, said: “As well as offering benefits for patients, this device is simple and universal—these advantages mean there will certainly be widespread uptake and usage.”
According to a press release from Electroducer, the Electroducer Sleeve is expected to reach the US market in 2023, followed by the European market in 2024, once it receives US Food and Drug Administration (FDA) authorisation and CE marking respectively.
DurAVR heart valve system gets green light from US FDA for early feasibility study
Anteris Technologies has announced that the US Food and Drug Administration (FDA) has conditionally approved its DurAVR transcatheter heart valve system for an investigational device exemption (IDE) application to commence an early feasibility study (EFS).
DurAVR is a balloon-expandable, 3D single-piece aortic valve, which is shaped to mimic the native human valve.
The EFS study will evaluate the safety and feasibility of the device in the treatment of subjects with symptomatic severe native aortic
stenosis. The FDA concluded the company provided adequate data to support the initiation of a clinical study in the USA, Anteris Technologies said in a press release. The EFS will enrol 15 subjects at seven heart valve centres of excellence within the USA. It is anticipated the study will commence in early 2023, paving the way for a pivotal, registration aortic stenosis trial in the first half of 2024.
The primary and key secondary endpoints of this trial include safety and device feasibility assessments such as success of implantation at the anatomically accurate position, and haemodynamic performance assessments including effective orifice area (EOA), mean gradient, aortic regurgitation, paravalvular leak (PVL) and Doppler Velocity Index (DVI). Patient outcomes such as stroke, myocardial infarction, life-threatening bleeds, and all-cause mortality are to be reported at 30 days, three months, and one-year post implantation.
The FDA has categorised DurAVR in this study as a Centers for Medicare & Medicaid Services (CMS) category B device, which permits the device to be sold during the study pending CMS approval.
“I am pleased and eager to begin the DurAVR transcatheter heart valve EFS to further evaluate this promising novel technology. The single piece, native-shape valve design of the DurAVR transcatheter heart valve represents an advancement to existing heart valve technologies. I am excited to see the potential of the DurAVR transcatheter heart valve in treating patients suffering from severe aortic stenosis,” stated Michael Reardon (Houston Methodist Hospital, Houston, USA), study chair for the DurAVR EFS.
The DurAVR THV System is limited for investigational use only.
First
in
patient randomised
RESPONDER-HF trial of
Corvia
atrial shunt
Corvia Medical has announced that the first patient has been randomised in RESPONDER-HF, a global
confirmatory trial of the Corvia atrial shunt in heart failure patients with preserved (HFpEF) or mildly reduced (HFmrEF) ejection fraction.
The first patient was enrolled and randomised by interventional cardiologist Scott Lilly and heart failure cardiologist Rami Kahwash (both Ohio State University Wexner Medical Center, Columbus, USA).
RESPONDER-HF is a randomised, sham-controlled trial including up to 260 patients from 60 centres across the USA, Europe, and Australia. The trial will evaluate the efficacy of the Corvia Atrial Shunt to reduce HF hospitalisations and improve quality of life (QoL). Sanjiv Shah (Bluhm Cardiovascular Institute, Chicago, USA) and and Martin Leon (Columbia University Irving Medical Center, New York, USA) serve as lead investigators for the study.
The RESPONDER-HF confirmatory trial builds on scientific data and progressive learnings from REDUCE LAP-HF II, the largest randomised controlled trial of a device-based therapy for HFpEF patients. As published in Circulation, REDUCE LAP-HF II is the only study of an implantable therapeutic device to show clinical benefit in this population. Within the large responder group, representing 50% of study patients, treatment with the Corvia Atrial Shunt resulted in a 45% reduction in HF events and a 55% greater improvement in QoL compared to sham control.
“We are committed to demonstrating the potential benefit of atrial shunt therapy and anticipate RESPONDERHF will validate the REDUCE LAP-HF II responder group findings, which correspond to two-thirds of people with HFpEF, or 2 million people in the USA alone,” commented Leon. Shah further added: “The RESPONDER-HF trial will not only continue to advance our scientific understanding of shunting in HFpEF, but also has the potential to change the treatment paradigm, and in doing so, move us one step closer to precision medicine in heart failure.”
20–22 March
Technology and Heart Failure Therapeutics (THT) Boston, USA tht2023.crfconnect.com
25–27 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
6–9 May
American Association for Thoracic Surgery (AATS) Annual Meeting Los Angeles, USA events.aats.org/am23
16–19 May
EuroPCR 2023 Paris, France pcronline.com
18–20 May
Society for Cardiovascular Angiography and Interventions (SCAI)
2023 Scientific Sessions Phoenix, USA scai.org/education-and-events
7–10 June
The Structural Heart Summit (TVT) Phoenix, USA tvt.crfconnect.com
25–28 August
European Society of Cardiology (ESC) Congress
Amsterdam, The Netherlands escardio.org
4–7 October
European Association for CardioThoracic Surgery (EACTS) Annual Meeting
Vienna, Austria eacts.org/annual-meeting/
23–26 October
TCT 2023
San Francisco, USA tct2023.crfconnect.com
22
February 2023 | Issue68 Market Watch
A TAVI procedure with wire
pacing
Corvia atrial shunt












*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Great just got better
Onyx Frontier DES inherits the same stent platform, clinical data, and indications you’ve come to rely on with Resolute Onyx™ DES. What makes it even better is its delivery system resulting in best-in-class with a lower crossing profile.2
deliverable
See more at medtronic.com/ OnyxFrontierGlobal
†Stent delivery system updates were implemented on the 2.0–4.0 mm Onyx Frontier DES diameters.
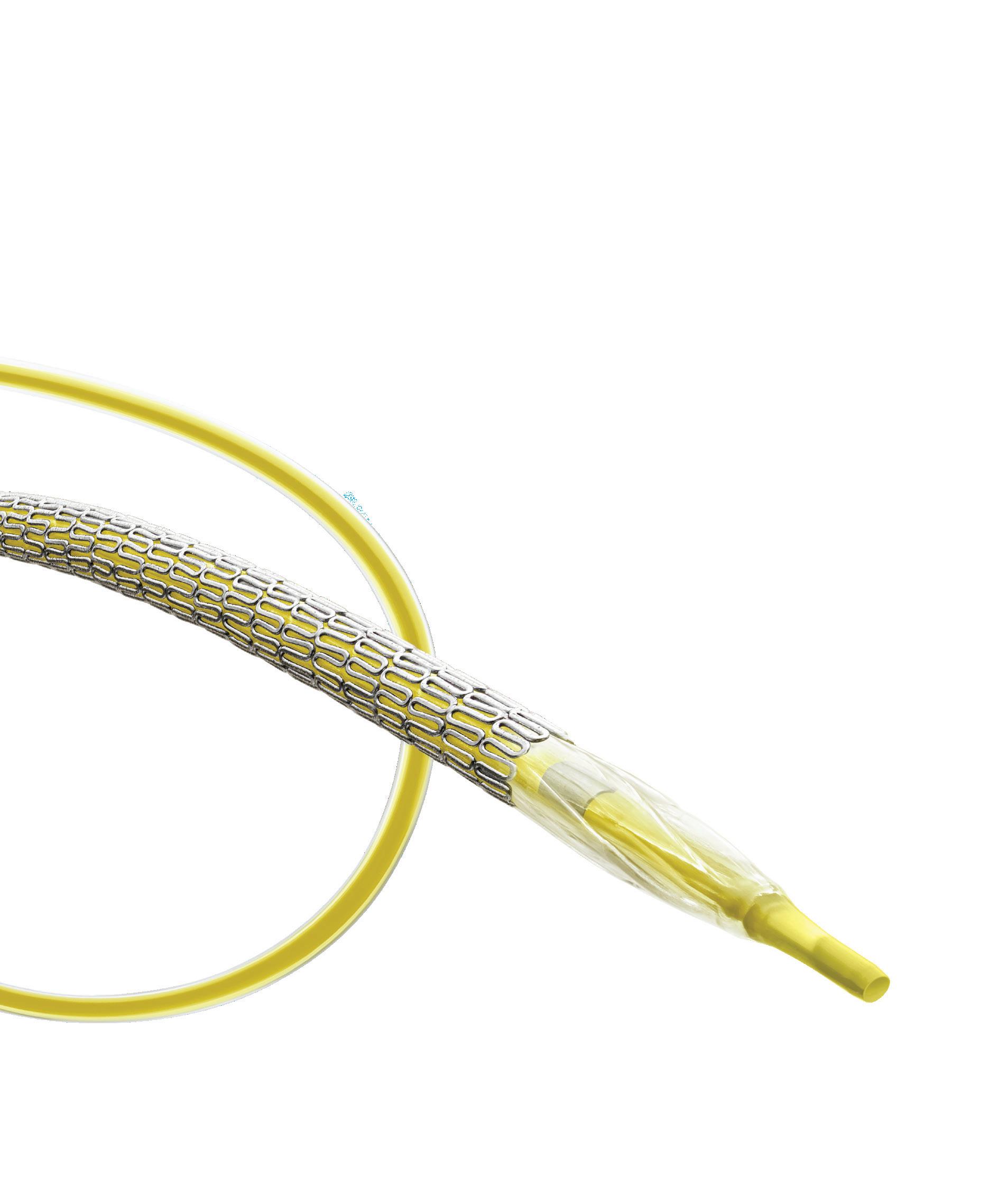
1 Based on bench test data on file at Medtronic. May not be indicative of clinical performance. N = 7 DES of each tested: Onyx Frontier DES, Resolute Onyx DES, Orsiro®* Mission DES, XIENCE Sierra™* DES, XIENCE Skypoint™* DES, SYNERGY™* DES, SYNERGY™* XD DES, Ultimaster™* Tansei™* DES.
2 Based on bench test data on file at Medtronic. May not be indicative of clinical performance. N = 5 DES of each tested: Onyx Frontier DES, Orsiro
Mission DES, Resolute Onyx DES, XIENCE Skypoint DES, SYNERGY DES, Ultimaster Tansei DES.
Europe
Medtronic Intl. Trading SARL
Tel: 41.21.802.7000
medtronic.com/OnyxFrontierGlobal
Asia Pacific Medtronic Intl. Ltd. Tel: 65.6436.5000
Latin America
Medtronic USA, Inc.
Tel: 786.709.4200
UC202211208 ML ©2022 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. For distribution only in markets where the Onyx Frontier coronary stent has been approved. Not for distribution in the USA, Canada, Japan, or France. 08/2022
Onyx Frontier™
DES





























































































