CX continues looking forward after 45 years of leading vascular education, innovation and evidence


Welcome to the 2023 edition of the Charing Cross (CX) International Symposium. After the overwhelming success of the first ever combined in-person and virtual symposium in 2022, CX continues looking forward to offer world-leading education and evidence on the pressing issues in the vascular and endovascular space in what is now its 45th year.
As an attendee, you can expect a comprehensive programme across all vascular domains including peripheral arterial, aortic, venous and lymphatic, vascular access, acute stroke, vascular trauma and—making its debut in 2023—the wide field of renal interventions.

This year’s programme highlights include a consensus update on key topics in the vascular and endovascular space—from revascularisation strategies for patients with chronic limb-threatening ischaemia (CLTI), to treatment options for superficial venous disease.

“If you have an interest in and are managing patients with vascular disease, there is something for you at CX,” CX founder and chairman Roger Greenhalgh (London, United Kingdom) tells CX Daily News, highlighting the multidisciplinary participation at the meeting. Eighteen Podium First presentations, over 38 live and edited cases, and two CX debates punctuate the three-day agenda of over 300 podium presentations to offer a comprehensive educational experience. The pillars of CX—Education, Innovation, and Evidence—will take centre stage across three rooms, Kensington 1, Kensington 2, and Admiral. In the long-established CX style, the meeting will champion a global audience and dedicate a significant proportion of time to audience participation, including discussion and
polling, while attendees can also expect a series of activities beyond the main programme, including workshop demonstrations and the CX exhibition.

Reflecting its ongoing evolution, CX welcomes three new cochairs from across the globe to the leadership team for 2023. Dittmar Böckler (Heidelberg, Germany), Andrew Holden (Auckland, New Zealand) and Erin Murphy (Charlotte, United States) are working alongside Greenhalgh to deliver the CX programme. “We are covering the globe and all vascular subjects,” he says of the new leadership team. “There will be every opportunity for the CX concept to continue if that is considered to be worthwhile.”
The centrepiece highlight of this year’s programme is the BASIL-2 (Bypass versus angioplasty in severe ischaemia of the leg-2) Podium First presentation, taking place during a Randomised Control Trial Update Session in Kensington 1 beginning at 11:10 this morning (Day One). “At the moment of speaking, publication of the results

CX 2023 OVERVIEW DAY ONE Peripheral Arterial & Acute Limb Ischaemia & Randomised Control Trial Update Tuesday, 08:00–13:00 Kensington 1
Renal Interventions Consensus Aortic Consensus Vascular Access Consensus Peripheral Arterial Consensus Peripheral Arterial Consensus VASCULAR Join our global online vascular community CXVASCULAR.COM Education, News and Insights for the global vascular community SIGN UP AT C M Y CM MY CY CMY K CXVASCULAR-APRIL-2023-225X58.pdf 1 07/04/2023 15:40:00 Join the community #CX2023 CX Vascular.com Tuesday 25 April 2023 Continued on page 2
If you have an interest in and are managing patients with vascular disease, there is something for you at CX.”
Peripheral Arterial & Chronic Limb Threatening Ischaemia (CLTI) Tuesday, 13:30–18:00 Kensington 1 Aortic Techniques & Technologies Tuesday, 08:00–18:00 Kensington 2 Vascular Access Tuesday, 08:00–13:00 Admiral Renal Interventions Tuesday, 13:30–18:00 Admiral
Clockwise from top left: Dittmar Böckler, Andrew Holden, Roger Greenhalgh and Erin Murphy
Continued from page 1
will take place in The Lancet, which is a huge moment for CX,” says Greenhalgh. “We have had 45 years of a very global CX, and tradition continues.”
BASIL-2 chief investigator Andrew Bradbury (Birmingham, United Kingdom) and other members of the trial group will deliver the findings, after which attendees in London and remote participants will have the opportunity to pose questions. A roundtable discussion is planned to include invited commentary from Eleni Whatley (US Food and Drug Administration [FDA], Silver Spring, United States) and UK Secretary of State for Health Steve Barclay (London, United Kingdom).
Randomised trial update
Alongside BASIL-2, attendees to this morning’s Randomised Control Trial Update session can expect to see new data from the BEST-CLI (Best endovascular versus best surgical therapy in patients with critical limb ischaemia) trial, with Matthew Menard (Boston, United States) due to give a clinical trial update and an insight into the quality of life data from the study. The aortic programme will feature five Podium First presentations, including one by Kevin Mani (Uppsala, Sweden) on statin treatment (see page 20 for details), and the vascular access masterclass will see Holden, CX co-chair and executive board member, present a threeyear data subanalysis from the IN.PACT AV Access
study (see page 8 for details).
CX attendees have the opportunity to test and improve their practical skills in a series of workshops at hands-on stations showcasing techniques and technologies across all three days of the meeting. In a dedicated Workshop Wing, attendees will be able visit the Vascular Access Workshop on Tuesday 25 April, the Aortic Workshop on Wednesday 26 April and the Venous and Hurting Leg Workshop on Thursday 27 April.
The CX International Symposium has a global audience and every year sees participation from around the world. There will be a dedicated CX meets Latin America session at this year’s meeting, with presenters from Brazil and Argentina sharing their research with the global CX audience (see page 10 for more details). The CX Innovation Showcase returns for 2023 on Day Three (Thursday 27 April), bringing to the fore an array of cutting-edge technologies and techniques. The CX audience can expect to see presentations on topics ranging from the power of intelligent mapping for complex endovascular aneurysm repair (EVAR), to wearable devices for non-invasive, remote monitoring and classification of access patency and flow characteristics in haemodialysis patients. The session will close with the annual Dragon’s Den-style competition and announcement of the winner of this year’s £1,000 CX Innovation prize.
Play your part in the discussion
Community engagement is central to CX, and audience participation is actively encouraged, with much of the programme dedicated to debate, discussion and audience polling. To take part during symposium sessions, you can submit your questions in-person, online at mycxonline.com, or via the dedicated MyCX mobile app, available through the Apple App Store or on Google Play. The app can also be used to vote during the polling sessions. To do so, download and open the app on a compatible device and choose the relevant live session page: Kensington 1, 2 or Admiral room.
CX in numbers:
THIS YEAR MARKS THE 45TH ANNIVERSARY OF THE CX SYMPOSIUM AND ITS WORLD-CLASS, GLOBAL BRAND OF VASCULAR EDUCATION CX 2023 will aim to reach consensus on pressing issues in multiple vascular domains, including peripheral arterial, aortic, venous, vascular access, acute stroke, vascular trauma and—for the first time—the wide field of renal interventions. Delegates can expect:
● 300+ presentations
● Three parallel streams of world-class education, innovation and evidence
● Three full-day vascular access, venous and hurting leg, and aortic workshops
● 38+ live and edited cases
● 170+ expert faculty members
● Two deep-dive roundtable discussions Join the CX community and experience a unique brand of vascular education #CX2023
Get CX at your fingertips: Access year-round education, news and insights through the CX Vascular platform
The CX Symposium is a standout fixture in the vascular education calendar, but the global vascular community’s appetite for high-quality knowledge-sharing and insights continues year-round.

CUE THE LAUNCH OF CX
Vascular—bringing the trusted CX brand of learning into the forefront of the digital age. CX Vascular is an online hub for the global vascular community. By signing up for free membership to the platform, users can access a suite of educational resources, access a vast library of content from the CX and CX
Aortic symposia back catalogue, and get up-to-the-minute news and updates from partner channels 365 days a year.
CX Vascular members will also be able to network with their peers to share expertise and experience through an instant messaging system, or join one of several dedicated speciality groups, which include tailored community areas
serving each vascular domain. And, CX 2023 will see the launch of CX Vascular Live, the channel’s flagship live talk show discussing the latest advances in the vascular field, featuring well-known and up-andcoming faces from the global vascular community. The first edition of CX Vascular Live will be broadcast live at CX 2023 from the CX Live studio (Richmond Mezzanine), featuring interviews, discussion and insight from CX faculty on a number of programme highlights.

By signing up to the CX Vascular
channel, members will have unlimited access to CX Vascular Live, as well as a host of other exclusive content featuring thought leaders in the space.
Tuesday 25 April 2023 2 FIRST EDITION #CX2023 Welcome to CX 2023
CX continues looking forward after 45 years of leading vascular education, innovation and evidence
Register now Visit CXVascular.com or scan the QR code in this article to join today.
Peripheral Arterial Consensus
CX 2023 highlight: The BASIL-2 trial
Data and discussion on revascularisation treatment strategies for patients with chronic limb-threatening ischaemia (CLTI) will take centre stage at the CX 2023 Consensus update, with results from the BASIL-2 (Bypass versus angioplasty in severe ischaemia of the leg- 2) randomised controlled trial (RCT) to be presented for the first time. Chief investigator Andrew Bradbury (Birmingham, United Kingdom) speaks to CX Daily News about the background, context and significance of the trial ahead of this year’s meeting.
BRADBURY NOTES THAT BASIL-2 HAS ITS origins in the original BASIL-1 trial, the short-term results of which were published in The Lancet in 2005. BASIL-1 triallists randomised (1999–2003) patients with severe limb ischaemia, mainly due to femoropopliteal disease, to either a plain balloon angioplasty-first or a bypass surgery-first revascularisation strategy. With “fairly limited followup,” Bradbury recalls, there did not seem to be much of a difference in the primary outcome of amputationfree survival. There was, however, a suggestion that the data from both groups were “beginning to diverge,” which prompted the team to follow the patients up for longer. Reporting the key finding from these later outcomes, Bradbury summarises that “people randomised in BASIL-1, and who were likely to live for more than two years, and who had a good vein, were best served by having a vein bypass first rather than a plain balloon angioplasty first”.
Speaking on his motivation for starting the BASIL-2 trial, Bradbury recollects how it became clear there were a number of gaps in peripheral arterial disease (PAD) research during his involvement in the UK National Institute for Health and Care Excellence (NICE) guideline expert group on PAD. One such gap had to do with infrapopliteal, or below-the-knee, disease, and Bradbury notes that NICE made a research recommendation to undertake an RCT to compare a ‘vein bypass-first’ with a ‘best endovascular treatment-first’ strategy for people who required an infrapopliteal procedure. This is what BASIL-2 endeavoured to achieve.

“Endovascular techniques and technologies for lower limb revascularisation are very different now from what they were 15–20 years ago when we did BASIL-1,” Bradbury remarks, noting for example better guidewires, better balloons, more skilful entry and retrograde cannulation to name just a few developments. As a result, the team are keen to see how the two treatment modalities compare in this new treatment landscape.
In addition, the question at the centre of BASIL-2 represents a “massive global problem” and therefore an important one to solve, Bradbury comments. He elaborates: “I have had the opportunity to visit

hospitals in many different countries, and vascular wards wherever you go are essentially full of people with CLTI.” Bradbury explains that BASIL-2 is his and his team’s “attempted contribution” to try and improve the evidence base for the treatment of these patients who are “very challenging” and often “very poorly,” with multiple comorbidities.
BASIL-2 is a superiority trial, Bradbury explains, noting that the hypothesis the investigators started with was that vein bypass would be superior due to the fact that BASIL-1 “seemed to show that vein bypass had advantages over endovascular intervention”. He adds that it is a pragmatic trial, and thus surgeons and interventional radiologists are permitted to use their preferred techniques and equipment, with the primary outcome being amputation-free survival (time to major—abovethe-ankle—amputation, or death from any cause, whichever occurs first).
Considering the BASIL-2 trial in its wider context of randomised data in this space, Bradbury notes that the investigators have been in “friendly dialogue” with the BEST-CLI team “from the getgo”. Results of the BEST-CLI trial were presented late last year, with the headline finding being that surgical bypass with adequate single-segment great saphenous vein is a more effective revascularisation strategy for patients with CLTI who are deemed to be suitable for either an open or endovascular approach.
Bradbury notes that there are a number of differences between the two trials, highlighting for example that BASIL-2 included a different group of patients.
“Only about 40% of [BEST-CLI]
patients have a below-the-knee intervention,” he says. “We are focused in BASIL-2 on below the knee, and [BEST-CLI] did not pre-specify an only below-theknee analysis.” While BEST-CLI is “not exclusively a femoropopliteal trial,” it has similarities to BASIL-1 in that it is more of a femoropopliteal trial with or without infrapopliteal disease, whereas BASIL-2 is specifically looking at infrapopliteal revascularisation, Bradbury explains.
BASIL-2 and BEST-CLI are both RCTs, however Bradbury is keen to stress the limitations of this high level of evidence. “An RCT is not a GPS, it is more like a wobbly compass near the North Pole, and all it can try and do is push you in a certain direction of travel,” he comments. He adds that while both the BASIL-2 and BEST-CLI teams are “huge enthusiasts” for RCTs, this type of research must still be scrutinised.
Looking ahead to CX, Bradbury expresses his excitement at the prospect of presenting the BASIL-2 data for the first time at what he describes as “the big UK vascular and endovascular meeting”. “I am really looking forward to seeing everyone at CX 2023 and it is going to be a great meeting. It is a pleasure and a privilege for us to be part of it and have the opportunity to present our trial data for the first time.”
During the CX session, Bradbury will deliver the results of the BASIL-2 trial, with co-investigators Catherine Moakes, Gareth Bate and Matthew Popplewell (all Birmingham, United Kingdom) and Lewis Meecham (Cardiff, United Kingdom) set to present on the journey from BASIL-1 to BASIL-2, methodology, study limitations and future work, among other topics.
Attendees in London and remote participants will have the opportunity to pose questions to the BASIL-2 investigators.

A roundtable discussion is planned to include invited commentary from the US Food and Drug Administration (FDA) and UK Secretary of State for Health.
Bradbury hopes that once the audience hear the results, as well as the limitations, which “every RCT has,” they will “go away and reflect” on the data and think about them “in the context of their own practice, their own healthcare system and come to a decision as to whether these new data are going to influence their practice, or not, as the case may be”.
4 Tuesday 25 April 2023 FIRST EDITION #CX2023 BASIL-2 Trial
Join the conversation at CX 2023
Expanding the possibilities of EVAR with ultra-low profile design

• Up to 60o infrarenal angle

• Double-sealing stent

• Trimodular design

• Helical leg extensions design


• Ultra-low profile
• Leave-behind sheath
The most complete portfolio for EVAR





Come visit us at booth F19

Every AAA patient deserves the most appropriate treatment... ... and we have the right solution for each of them.
©2023 Lombard Medical. All rights reserved
First-time data presentations to feature across all three days of symposium
Once again, this year’s Charing Cross Symposium will play host to a series of first-time data presentations across the full spectrum of vascular care—from much-anticipated findings of the BASIL-2 trial, to new studies in the vascular access and juxtarenal aortic spaces.

In a morning session on day one of CX 2023 (Tuesday 25 April), Sean Lyden (Cleveland, United States) is set to deliver primary endpoint results from STRIDE, a trial assessing thrombectomy via the Indigo aspiration system (Penumbra) in acute limb ischaemia patients.

Another podium-first presentation will follow this, with data being disclosed from the BASIL-2 study—a multicentre randomised controlled trial intent on comparing clinical outcomes, and cost effectiveness, with ‘vein bypass-first’ and ‘best endovascular-first’ revascularisation strategies in patients with severe limb ischaemia.
Subsequently, in a session anchored by CX chair Roger Greenhalgh (London, United Kingdom), two further podium-first talks will see Matthew Menard (Boston, United States) deliver a clinical trial update and new quality-of-life data from the BEST-CLI study.
Audience participation as well as roundtable discussions moderated by Andrew Holden (Auckland City Hospital, Auckland, New Zealand) and Dittmar Böckler (Heidelberg, Germany) will follow.
As is the case each year at CX, vascular access represents a key part of the conference’s programme in 2023. One podium-first presentation will see Holden take to the stage himself to deliver three-year subanalysis data from the IN.PACT AV Access trial evaluating the IN.PACT AV Access drugcoated balloon (Medtronic) in kidney patients with stenosed arteriovenous fistulas.
After this, another even more novel device in the vascular access space—the restorative Axess graft (Xeltis) for haemodialysis—will feature in a first-time presentation from Frans Moll (Utrecht, Netherlands).


The programme’s focus will shift towards
abdominal aortic aneurysms (AAAs) on day two; firstly, with a podium-first presentation from Kevin Mani (Uppsala, Sweden) entitled “Statin treatment after aortic repair saves lives, but dose [does not] matter”. Later on in the same morning session, 10year primary AAA outcomes from ENGAGE OUS—a real-world registry analysing the Endurant stent graft system (Medtronic)—will be delivered by Hence Verhagen (Rotterdam, Netherlands).
During the afternoon’s juxtarenal aortic-dedicated sessions, the first of three late-breaking talks will be given by Eric Verhoeven (Nuremberg, Germany). Verhoeven is set to deliver eight-year results seen with a bridging stent for fenestrated/branched endovascular aortic repair (F/BEVAR) cases.




Following this, findings from the CoBaGI study assessing covered versus bare-metal stents in chronic atherosclerotic gastrointestinal ischaemia patients will be disclosed by Luke Terlouw (Rotterdam, Netherlands), and the results of a meta-analysis of comparative studies between self- and balloon-expandable bridging covered stents for BEVAR will be the subject of a podium-first presentation from Konstantinos Spanos (Larissa, Greece).
A single podium-first talk is set to be given on the third and final day of CX 2023 (Thursday 27 April), with Andrew Holden delivering first-in-human experiences with the SutureTight device (Vesteck)—an innovation intended to eliminate migration in EVAR procedures.
Tuesday 25 April 2023 6 FIRST EDITION #CX2023 First-to-Podium Presentations TERUMO AORTIC SYMPOSIUM Tuesday 25th April 2023 • 12:30 - 13:00 • Kensington 2 Raising Clinical Efficacy through Technological Advancements: Fenestrated TREO Eric
Kakkhee
Giovanni
Vincente
Fenestrated
is a Gamechanger Florian
Austria Experience from Radiological Perspective Advancing Fenestrated Horizons Fenestrated TREO Visit our website for more information on use, indications, contraindications, warnings/precautions and availability within your market. Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs. Custom made devices are not available in the US and availability is subject to local regulatory approval. OUS_0247-A Feb23 • PM-06701 To find out more, visit: terumoaortic.com/fenestrated-treo
Ducasse Moderator
Yeung Technical Advantages of Fenestrated TREO
Pratesi Clinical Feedback: Genova Case Series with Fenestrated TREO
Riambau Why
TREO
Wolf Fenestrated TREO:
Venous & Lymphatic Consensus
CX 2023 seeks consensus in “rapidly” progressing venous field
The CX 2023 venous and lymphatic programme is set to be a “highlight” of this year’s meeting, CX co-chair and executive board member Erin Murphy (Charlotte, United States) tells CX Daily News. Manj Gohel (Cambridge, United Kingdom), also an executive board member, adds that there have been “important new guidelines, important new trials, and lots of advances in the deep, superficial and pelvic venous spaces” since last year’s meeting, all of which will provide the basis for the 2023 programme.
ON WEDNESDAY 26 APRIL, MURPHY will anchor a whole day of venous sessions in Kensington 2, starting with the superficial and lymphatic programme in the morning, followed by deep venous presentations and discussion after lunch. Gohel will moderate alongside executive board members Stephen Black (London, United Kingdom) and Armando Mansilha (Porto, Portugal) throughout the course of the day.
This year’s programme will not only highlight progress in the space, but also areas in which there is a need to “drive movement,” Murphy details, adding that the importance of comprehensive care will be a key underlying theme. “As venous interventionalists, we need to focus on treating all aspects of venolymphatic disease,” she remarks.
Superficial venous and lymphatic programme highlights
On Wednesday morning, presentations, edited cases and discussion will home in on the management of superficial venous disease. Traditionally, Murphy explains, superficial disease was treated with surgical stripping and phlebectomy. She notes that nowadays, however, treatment is becoming increasingly advanced in this space, with options such as thermal closures, medical adhesive closures, and foam now the standard of care.

Commenting on the use of foam as a closure tool, Murphy believes this is an area in which there is not yet true consensus. While the technique is used widely in the United States for ulcer-bed sclerotherapy, Murphy states that there may be a higher risk of deep vein thrombosis (DVT) when it is
used as a primary closure tool. There is still room for this treatment modality to be “optimised,” she believes.
At CX 2023, discussion will focus on which of these treatment options for superficial venous disease—thermal closures, medical adhesive closures, or foam options—might be better suited to certain patient groups and certain clinical scenarios.
In this part of the programme, Gohel will deliver a SPECTRUM study programme update for patients with chronic venous disease. Murphy predicts that this study will show positive results for medical adhesive closure—data that would “support the direction in which the field is going”. However, she says, “we will have to wait and see.”
Elsewhere on the superficial venous and lymphatic programme, Gohel will speak on an optimal strategy for superficial venous ablation in patients with C6 disease and Mansilha on the role of venoactive medications in patients with swollen legs and venous leg ulceration. In addition, Karoliina Halmesmaki (Helsinki, Finland) will give a presentation on long-term saphenous vein occlusion rates in mechanochemical venous thermoablation.
Addressing some broader topics in the superficial venous and lymphatic space, Steve Elias (Englewood, United States) will highlight the difference between US and European venous guidelines and Mitchell Silver (Columbus, United States) the areas where consensus is lacking in the management of venous disease. Marianne de Maeseneer (Rotterdam, Netherlands) will outline some tips for success and longevity as a venous specialist.
This part of the programme also features edited cases, including one from Efthymios Avgerinos (Pittsburgh, United States) on multimodal superficial intervention for venous ulceration.
Deep venous programme highlights
This year’s deep venous programme will take centre stage on Wednesday afternoon, with incompetence of the deep venous system within the lower extremities and the pelvis set to feature. “We traditionally did open surgery for these patients,” Murphy recalls, noting however that open reconstructions are “very complex surgically” and are associated with a relatively high rate of eventual failure and thrombosis. She notes that there are new technologies available in this space that are “really encouraging”. CX 2023 will look at how these technologies might impact the future of treatment in the space.
Venous stenting will be another topic on the Deep Venous programme agenda, with a presentation by Gerard O’Sullivan (Galway, Ireland) on how to avoid migration and another by Houman Jalaie (Aachen, Germany) on defining patients who should not undergo stenting. In this part of the programme, Murphy is set to provide insight into the US venous stent investigational device exemption (IDE) trials, outlining both trends and disparities.
Elsewhere on the programme, Ramon Varcoe (Sydney, Australia) will deliver the latest outcome data for endovascular valve creation for deep venous reflux and Kush Desai (Chicago, United States) on acceptable indications for inferior vena cava (IVC) filters in 2023. The presentations will be interspersed by a series of edited cases, including one from Black on reintervention for chronically occluded venous stents.
The venous field is making “huge leaps and bounds”
According to Murphy, the venous field is progressing “very rapidly” on a yearly basis. “I think the CX programme committee has worked very hard to make a comprehensive and up-to-date programme highlighting what we know now and where we are going as a field.”
The programme is becoming more international every year, Murphy comments, with interest expanding due to recognition that it is “truly a valueadd to the venous space”. She adds “I think [the CX 2023 venous and lymphatic programme] is a great update for anybody who is in the venous space and who is interested in what progress we are making.”
Tuesday 25 April 2023 FIRST EDITION #CX2023 7 Wednesday Highlight
As venous interventionalists, we need to focus on treating all aspects of veno-lymphatic disease.”
Erin Murphy
Renal Interventions Consensus
Renal interventions CX session programme promises nephrology newsflashes
The question of the optimal dialysis therapy will take centre stage at CX 2023’s dedicated renal interventions session, the first of its kind at the symposium. It comes following the addition of Kate Steiner (Stevenage, United Kingdom) to the CX executive board, who will also be an anchor of the session alongside Nicholas Inston (Birmingham, United Kingdom).
ROBERT SHAHVERDYAN (HAMBURG, Germany) will kick off the early part of the session, moderated by Ounali Jaffer (London, United Kingdom), with a presentation on a new device for endovascular arteriovenous fistula (endoAVF) creation, before Andrew Holden (Auckland, New Zealand) reappraises percutaneous transluminal angioplasty (PTA). Though it is an “older technology”, Holden will put forward recommendations on the best approach to the procedure and outline its potential for “newer outcomes” as a treatment for arteriovenous (AV) access dysfunction. Matthew Gibson (Reading, United Kingdom) follows this up with a talk on thrombosis in AV access.

Cephalic arch lesions will be examined by Matteo Tozzi (Varese, Italy), who promises to provide an “algorithm for PTA, covered stents, and drug-coated balloons” for use in their management. Cephalic arch stenosis, meanwhile, will be the focus of Samuel
Vascular Access Consensus
Walker’s (Birmingham, United Kingdom) talk. He will put forward ideas on how best to treat the condition with stent grafts while interrogating to what extent “the position matters”.
Stenting and when it is appropriate will be the subject of the proceeding talk from Andrew Wigham (Oxford, United Kingdom), before Alexandros Mallios (Paris, France) makes the case for the prioritisation of outcomes in renal care. Offering another perspective will be Jeremy Crane (London, United Kingdom), fighting the corner of costeffectiveness as the most important metric in the space.
Crane will then step up to moderate the next phase of the session, where Frank Dor (London, United Kingdom) will probe the subject of peritoneal dialysis to clarify whether it represents “an optimal home therapy”. Another development that may enable dialysis away from
From sirolimus to SONAR: A CX vascular access masterclass preview

Among the sessions at CX 2023 will be the vascular access masterclass running from 08:00 to 13:00 on the first day of the conference. Anchored by Nicholas Inston (Birmingham, United Kingdom) and Kate Steiner (Stevenage, United Kingdom), it will also feature the latter as both moderator and, in the session’s first presentation, speaker, on patient selection algorithms for endovascular procedures.
FOLLOWING STEINER IS
Ounali Jaffer (London, United Kingdom), who expands on these themes by exploring how to add endovascular arteriovenous fistula (endoAVF) creation to dialysis practices. Alexandros Mallios (Paris, France) adds to the conversation on fistula creation by examining novel approaches, the use of devices, as well as new sites and indications. Also focusing on fistulas is Simon Hogan (London, United Kingdom), speaking on electric field-guided minimally-invasive fistula creation.
Evidence takes centre stage next, with Jaffer returning to discuss whether there is “too much evidence” in the drug-
eluting balloon (DCB) space. David Kingsmore (Glasgow, United Kingdom) asks then “is evidence reliable?”, setting out some of the lessons that can be learned from arteriovenous grafts (AVGs). Narayan Karunanithy follows this by outlining a “need for more evidence” in the context of the PAVE2 trial on DCBs, before Daniel Patel (Daytona Beach, United States) explores arteriovenous (AV) revision with a covered stent in an edited case study.
“Do new definitions create new data?” asks Tobias Steinke (Düsseldorf, Germany) in the next phase of the session, his question coming in the context of further examination of endoAVF. Jonathan de Siqueira (Leeds,
the clinical setting is implantable continuous dialysis haemofiltration technology, the theme of Dirk Hentschel’s (Boston, United States) talk immediately after Dor’s. An outline of the N-PATH international interventional nephrology training programme will follow from Georgia Georgopoulous (Patras, Greece). The penultimate part of the session is a dedicated renal artery management masterclass, where Andrew Garnham (Wolverhampton, United Kingdom) will talk training for vascular surgeons aimed at preparing them to treat renal patients. Robert Jones (Birmingham, United Kingdom) will review renal artery aneurysms from his perspective as an interventional radiologist, while David Kingsmore (Glasgow, United Kingdom) will serve up the surgical angle. Turning to kidney transplantation, Andrew Willis (Birmingham, United Kingdom) will talk on the vascular issues associated with the procedure. Rounding out the renal interventions programme will be a set of quick-fire five-minute abstract presentations. These will cover subjects from the treatment of intragraft stenosis in haemodialysis grafts in the case of Chai-Hock Chua’s (Taipei, Taiwan) talk, to research comparing drug-coated balloons with plain-balloon angioplasty in the final presentation of the session from Yinhui-Lim Hartono (Hamburg, Germany). These final presentations, like each of the others in the session, will come with a short audience participation and question component.
Stay up to date
The Renal Interventions newspaper, recently launched by BIBA Medical, offers a yearround digital and print accompaniment to this new CX stream. Pick up the latest issue at CX.
United Kingdom) then looks to the future, weighing up the priorities in vascular access research. Reviewing existing research next is Charmaine Lok (Toronto, Canada), averring that “the evidence has been analysed” and setting out a vascular access treatment algorithm that incorporates patient choice. Robert Shahverdyan offers after this another edited case covering both fistula creation and illuminating conversation with an experienced interventionalist.
Following the mid-session coffee break, Andrew Holden (Auckland, New Zealand) will provide a Podium First insight into the three-year subanalysis data from the IN.PACT AV Access trial, building on his presentation at CX 2022 of data from the trial. The ACCess study follows, with Emma Aitken (Glasgow, United Kingdom) arguing the importance of anaesthesia. ACCESS 2, after Aitken, sees Rajesh Sivaprakasam (London, United Kingdom) survey sirolimus for fistula maturation. Subsequent is the SONAR trial, an update on which is provided courtesy of Gavin Pettigrew (Cambridge, United Kingdom), and which is intended to shed light on the ability of ultrasound to reliably predict AVF failure in chronic kidney disease patients.
Another trial follows— the ABISS trial comparing DCB with plain-balloon
angioplasty, with Raphaël Coscas (Paris, France) presenting. Dirk Hentschel (Boston, United States), meanwhile, offers his “experience and indications” in central venous access with an access catheter system before Surendra Shenoy (St Louis, United States) sets out results from a first-in-man trial of vein dilation pre-fistula formation. Sivaprakasam returns too to discuss patient monitoring devices, and Ellen Dillavou (Raleigh, United States) vaunts external support as a means of achieving “better outcomes and cost-effectiveness”. Frans Moll (Utrecht, Netherlands) provides a further Podium First with “a new kind of graft”, while Matteo Tozzi gives a “histological analysis of intensive needling”. The final presentation in the masterclass comes once more from David Kingsmore, who puts forward “surveillance and procedural considerations” in AVG stenosis.
8 Tuesday 25 April 2023 FIRST EDITION #CX2023 Today’s Highlights
Nicholas Inston
Advanta V12
balloon expandable covered stent

Right from the start Still going strong
Advanta V12 is a balloon expandable, fully encapsulated PTFE stent with first to market covered balloon expandable stent technology that has served more than 700,000 patients. Known for its precision and predictability – the versatile Advanta V12 has been meeting the needs of surgeons and patients for 20 years and is the only durable solution backed up by decades of real-world evidence and more than 550 publications.
getinge.com Getinge, and are trademarks or registered trademarks of Getinge AB, its subsidiaries, or affiliates in the United States or other countries. Copyright 2023 Atrium Medical Corp. All rights reserved. PN011929 Rev AA 03/2023
Join the discussion
The CX community thrives on the participation of its members. At CX 2023, there are multiple opportunities for you to get involved in the discourse, with a significant proportion of the programme dedicated to debate, discussion and polling.
To take part during symposium sessions, you can submit your questions in-person, online at mycxonline.com, or via the dedicated MyCX mobile app available through the Apple App Store or on Google Play. You can also use the app to vote during the polling sessions. To vote and send questions, click on the CX Symposium 2023 event on your preferred platform, and choose the relevant live session page: Kensington 1, 2 or Admiral room.
The Hurting Leg Consensus
Competition hopes to raise public awareness of ‘hurting leg’ significance
Earlier this year, students and trainees involved in caring for vascular patients from all over the world were invited to create an infographic and/or infomercial intended to educate members of the public about chronic limbthreatening ischaemia (CLTI) and encourage patients to present to their general practitioner.
This was for a new competition designed by the Rouleaux Club—the United Kingdom’s national vascular trainee society—in association with CX 2023 and BIBA Medical, to encourage early detection and treatment of ‘hurting legs’.
Attendees at CX 2023 will be able to view the top five infomercials and infographics, and vote for their preferred one. The winners of this new “innovative and exciting” competition, as described by Rouleaux Club president Leanna Erete (London, United Kingdom), will be announced during this year’s CLTI and hurting leg consensus session. The prizes are £1,000 for the infomercial and £500 for the infographic.
Other highlights of this year’s CLTI and hurting leg programme include ‘Best of abstracts’ from rising stars in the vascular field, and a roundtable discussion led by Naseer Ahmad (Manchester, United Kingdom) on the possibility of using existing screening programmes like aneurysm and breast screening to opportunistically identify ‘the hurting leg’.
CX meets Latin America 2023
ON THE MORNING OF THE FIRST DAY OF THIS year’s Charing Cross International Symposium, CX meets Latin America seeks to connect the global community of vascular physicians through consensus discussion, exploration of international innovations, and open dialogue and evaluation from audience members. The session taking place on Tuesday 25 April will be chaired by notable vascular and endovascular surgeon Arno Von Ristow (Rio de Janeiro, Brazil), who will be joined by Bruno Freitas (Leipzig, Germany), Frans Moll (Utrecht, and Sophie Renton (London, United Kingdom). This session will speakers from Brazil and Argentina, who will be sharing their with the global CX audience attending both in-person and
Beginning the session Bruno Freitas will present on complex limb-threatening ischaemia (CLTI) disease, ‘opening the toolbox’ to discuss technical tips to overcome challenging scenarios for vascular surgeons. Audience participation and discussion will directly follow after each presentation, allowing attendees to pose questions and engage in conversation centred around the research.
Closely following Freitas, Tony Furuie (Sao Paulo, Brazil) will give a case report on ipsilateral iliac branch repair using a looped wire, and give details on the precannulated gate technique to do so. Subsequent to Furuie, Mariana Castelli (Buenos Aires, Argentina) will provide insight into predictors of type IIIa endoleak after endovascular aneurysm repair (EVAR) with anatomic fixation endograft.
Chairman of this year’s CX meets Latin America, Ristow, will go on to discuss balloon expandable endograft in aortoiliac occlusions and subsequently open a dialogue with the audience to expand on the research provided. After, Castelli will again take to the podium to present on fenestrated endovascular aortic repair (FEVAR) with physician modified endograft after previous failed EVAR within an emergent setting, building on his extensive research within this field and later allowing for audience discussion on the topic.
Closing the session and the first day of this years’ CX Symposium, Freitas will present for a second time, speaking on intramural haematoma and penetrating aortic ulcer, providing current evidence and contemporary technical insights.

To finish, Ristow, moderators and speakers will commence a roundtable discussion to elucidate research further, analyse and debate the presented data, and provide consensus on innovative techniques and treatments on a global scale.

10 Tuesday 25 April 2023 FIRST EDITION #CX2023 Programme Highlights
The session takes place 10:00–12:00 in Mezzanine 10
CX 2023 brings day-long workshops, including new aortic course

Following last year’s successful, post-pandemic return to hands-on education, CX 2023 will again be offering attendees the chance to test and improve their practical skills. The workshops will cover the areas of vascular access, aortic, and venous, and run across the symposium’s three days, with longer opening hours than the 2022 workshops. Delegates can get up close and hands-on with new devices, guided by demonstrators who will be walking through techniques for the various devices on show. There will also be a brand new aortic course on physician-modified fenestrated and branched endovascular aneurysm repair (F/BEVAR) procedures, for which attendees will need to preregister by emailing aleksandra@bibamedical.com.
THE WORKSHOP ZONE, WHERE ALL hands-on sessions take place, is located in the East Wing of the hotel (level -2) and will therefore be open to all. Visitors can move from station to station as they wish, as within the exhibition, during the workshop opening hours of 8am–6pm on Tuesday, Wednesday and Thursday.
Tuesday starts off with the Vascular Access Workshop, which will allow attendees to test and improve their practical skills at hands-on stations showcasing vascular access techniques and technologies. The novel devices on show will feature among them systems for endovascular fistula creation and a wireless ultrasound probe. The demonstrators for the workshop will include Gavin Corrigan (Dublin, Ireland), John McCafferty (Edinburgh, United Kingdom), and Daniela Romero (Edinburgh, United Kingdom), all of them ready to share their insights.
Day 2—Wednesday—will feature the Aortic
Workshop, including a new course on physicianmodified BEVAR and FEVAR procedures, as well as other aortic techniques and technologies. There are two sessions during which this F/BEVAR course will run—the morning slot (9.30am–12pm), and the afternoon session (2–4.30pm). Instructed by Alexander Zimmermann and Benedikt Reutersberg (both Zürich, Switzerland), attendees of the course will learn the correct indication for a physicianmodified F/BEVAR, how to use CT angiography as a basis for the preparation of a physician-modified F/BEVAR, and how to carry out the CT angiography-informed F/BEVAR preparation.
The technologies available for hands-on trial and demonstration within the wider workshop, for which there is no preregistration required, include off-
the-shelf solutions for challenging proximal sealing zones, an intravascular lithotripsy device, and an ‘intelligent’ mapping system to aid in endovascular surgery planning and guidance. These will be demonstrated by Giacomo Isernia (Perugia, Italy), Adam Jones (London, United Kingdom), and Amber Gislason-Lee (Leeds, United Kingdom), among others.
On Thursday, the third day of the symposium, the Venous and Hurting Leg Workshop will take place, which will give participants the chance to try out venous and hurting leg techniques and technologies, as well as seeing them in action showcased by the demonstrators. The workshop will also include a programme of presentations and discussions running throughout the day, with CX faculty speakers.
Attendees will have the opportunity to expand their knowledge of superficial venous disease and lymphoedema, and deep venous disease, while hearing about speakers’ challenging cases and what they wish they had known earlier about treating patients in the venous space. The workshop technologies on show will include devices for clot retrieval and management, an intravascular ultrasound system, and various closure devices. The demonstrators for these technologies will include Journa Gaëtan (Loos, France), Enric Roche (Barcelona, Spain), and Emma Wilton (Oxford, United Kingdom).
Workshop sessions will offer handson learning

The largest prospective study of interventional treatment of high-risk PE
>90% Reduction in High-risk PE Mortality*
*
>90% reduction in high-risk PE in-hospital all-cause mortality vs. other contemporary treatments. Context arm patients were treated with systemic thrombolysis (68.9%), anticoagulation alone (23.0%), CDT (6.6%) or surgical thrombectomy (1.6%). Source: Outcomes In High-risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy: Results From The FLAME Study presented at ACC March 2023 by Dr. Mitchell J. Silver
The FlowTriever® Retrieval/Aspiration System is indicated for (1) the non-surgical removal of emboli and thrombi from blood vessels; and (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The FlowTriever system is intended for use in the peripheral vasculature and for the treatment of pulmonary embolism. Indications, Contraindications, warnings and instructions for use can be found in the product labeling supplied with each device.

Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
500 Patients enrolled
* ≥75% thrombus removal assessed by an independent core laboratory. In-hospital and 30-day Outcomes from the Fully-enrolled Multicenter Prospective CLOUT Registry presented by
Tuesday 25 April 2023 FIRST EDITION 11
Dr. David Dexter at WEINS 2022 The ClotTriever® Thrombectomy System is indicated for the non-surgical removal of thrombi and emboli from blood vessels, and for injection, infusion, and/or aspiration of contrast media and other fluids into or from a blood vessel, for use in the lower peripheral vasculature, including Deep Vein Thrombosis. Indications, Contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. All trademarks are property of their respective owners. Inari Medical, Inc. headquarters: 6001 Oak Canyon, Suite 100 | Irvine CA 92618 Inari Medical Europe GmbH, a subsidiary of Inari Medical, Inc. | Messeplatz 10 | 4058 Basel, CH ClotTriever® System Clinical Registry Study >90% Complete or Near Complete Thrombus Removal* n=486 Standout performance. Unmatched outcomes. Visit us at Booth A15
WORKSHOP PREVIEW



Tuesday 25 April 2023 12 FIRST EDITION #CX2023 Floor Plan LIFT LIFT2 LIFT3 LIFT4 RECEPTIONMAIN LOBBYAND MAIN ENTRANCE MAIN ENTRANCE BOWBAR BOWBAR SEMI-PRIVATE WEST WING LOBBY HOXTONROOM SPEAKER READY ROOM ORGANISERS OFFICE TYBURNMARKET TYBURN BAR TYBURN RESTAURANT HYBRID MEETING ROOMS HYBRID MEETING ROOMS HYBRID MEETING ROOMS KENSINGTON 2 KENSINGTON 1 CATERING CATERING A27 A29 A23 A21 B24 A25 D38 B36 B38 C36 C32 B28 B32 C28 D24 E40 D40 E38 D38 E36 D36 E32 D28 D32 F33 F27 F25 F19 D20 B20 A19 A15 F23 G12 G13 G14 G11 G10 A30 F35 MEZZANINE BALCONY 26 SQM 28 SQM WORKSHOPS & Abstracts ADMIRAL PLENARY 60 SQM
Day 1: Tuesday 25 April
Kensington 1
Kensington 2
Admiral
Peripheral Arterial & Acute Limb Ischaemia & Randomised Control Trial Update
Anchor: Roger Greenhalgh (London, United Kingdom)
Peripheral Arterial Consensus Update
Time: 08:00–13:00
Moderators: Andrew Holden, Auckland, New Zealand
Dittmar Böckler, Heidelberg, Germany
Sponsored Education
Time: 09:40–10:10
Acute Limb Ischaemia
Consensus Update
Time: 10:30–10:40
Moderator: Gunnar Tepe, Rosenheim, Germany
Sponsored Education
Time: 10:40–11:10
Randomised Control Trial
Consensus Update
Time: 11:10–13:00
Moderators: Andrew Holden
Dittmar Böckler
Peripheral Arterial & Chronic Limb Threatening Ischaemia (CLTI)
Potential Advances Consensus Update
Time: 13:30 –15:20
Anchor: Roger Greenhalgh
Moderators: Andrew Holden
Dittmar Böckler
Sponsored Education
Time: 15:20–15:50
Peripheral Arterial Consensus Update
Continued
Time: 16:10–16:50
Anchor: Dittmar Böckler
Moderators: Gunnar Tepe
Marianne Brodmann, Graz, Austria
Chronic Limb-Threatening Ischaemia & Hurting Leg Consensus Update
Time: 16:50–18:00
Anchor: Andrew Holden
Moderators: Gunnar Tepe
Peter Schneider, San Francisco, United States
Aortic Techniques & Technologies
Aortic Techniques & Technologies
Consensus Update: Thoracic Arch
Time: 08:00–09:40
Anchor: Tilo Kölbel, Hamburg, Germany
Moderators: Roberto Chiesa, Milan, Italy
Gustavo Oderich, Houston, United States
Sponsored Education
Time: 09:40–10:10
Aortic Techniques & Technologies
Consensus Update: Thoraco-abdominal
Time: 10:30–12:30
Anchor: Tilo Kölbel
Moderators: Gustavo Oderich
Roberto Chiesa
Sponsored Education
Time: 12:30–13:00
Aortic Techniques & Technologies
Consensus Update: Thoraco-abdominal
Continued
Time: 13:30–14:30
Anchor: Tilo Kölbel
Moderators: Roberto Chiesa
Gustavo Oderich
Aortic Techniques & Technologies
Consensus Update: Thoracic Dissection
Time: 14:30–15:20
Anchor: Tilo Kölbel
Moderators: Roberto Chiesa
Gustavo Oderich
Sponsored Education
Time: 15:20–15:50
Aortic Techniques & Technologies
Consensus Update
Time: 16:10–18:00
Anchor: Tilo Kölbel
Moderator: Gustavo Oderich
Vascular Access
Vascular Access CX Masterclass
Time: 08:00–09:30
Anchor: Nicholas Inston, Birmingham, United Kingdom
Moderator: Kate Steiner, Stevenage, United Kingdom
Vascular Access CX Masterclass
Continued
Time: 09:30–10:10
Anchor: Kate Steiner
Moderator: Narayan Karunanithy, London, United Kingdom
Sponsored Education
Time: 10:30–11:00
Vascular Access CX Masterclass Continued
Time: 11:00–13:00
Anchor: Nicholas Inston
Moderator: Alexandros Mallios, Paris, France
Sponsored Education
Time: 13:30–14:00
Renal Interventions
Renal Interventions
Time: 14:00–15:20
Anchor: Kate Steiner
Moderator: Ounali Jaffer, London, United Kingdom
Renal Interventions Continued
Time: 15:20–15:50
Anchor: Nicholas Inston, Birmingham, United Kingdom
Moderator: Jeremy Crane, London, United Kingdom
Masterclass: Managing the Renal Artery
Time: 16:10–17:00
Anchor: Kate Steiner
Moderator: Frank Dor, London, United Kingdom
Vascular Access: Best of Abstracts
Time: 17:00–18:00
Anchor: Kate Steiner
Moderator: Frank Dor
13 Tuesday 25 April 2023 FIRST EDITION #CX2023 Programme
AT-A-GLANCE GUIDE
All
Key: Peripheral Aortic Vascular Access Renal Interventions
times are London time BST (British Summer Time), which is GMT+1. The organisers reserve the right to alter timings if necessary.
CX 2023 exhibitors and major sponsors A-Z
PLATINUM SPONSORS
■ Gore & Associates D24 & Hoxton Lounge
With more than 50 million medical devices implanted over the course of more than 45 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. Gore is joined in service with clinicians and through this collaboration we are improving lives. www.goremedical.com
■ Medtronic B24
We lead global healthcare technology, boldly attacking the most challenging problems. Our Mission—to alleviate pain, restore health, and extend life—unites a global team of 90,000+ people, and our technologies transform the lives of two people every second, every hour, every day. Expect more from us.
Medtronic. Engineering the extraordinary. www.medtronic.com
GOLD SPONSORS
■ Concept Medical
D28
Concept Medical, Inc., headquartered in Tampa, United States, specialises in developing drug-delivery platform technology and products for the treatment of coronary and peripheral arterial disease (PAD). Concept Medical has developed sirolimus-coated balloon with the help of its proprietary Nanolute technology like the Magic Touch percutaneous transluminal angioplasty (PTA) for superficial femoral artery (SFA) and below the knee (BTK) and Magic Touch arteriovenous fistula (AVF) for dysfunctional AVF/ arteriovenous graft (AVG). Concept Medical has recently been granted IDE approval for BTK indication for its Magic Touch PTA.
Visit Concept Medical at booth #D28 and watch the scientific session on 27 April (3:20–3:50pm) to know more about the sirolimus-coated balloon potential in PAD treatment.
www.conceptmedical.com
■ Cook
C28
better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees and our communities. Visit our website to find out more, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
www.cookmedical.com
■ Terumo Aortic B28
At Terumo Aortic, we understand that no two aortas are alike. We are 100% focused on the aorta, from the arch to the iliacs. With our comprehensive portfolio of surgical, endovascular, hybrid and custom solutions, we help you address your patients’ unique challenges—so no patient is left behind.
www.terumoaortic.com
SILVER SPONSORS
■ Artivion D20
Artivion, Inc. is a medical device company focused on developing simple, elegant solutions that address cardiac and vascular surgeons’ most difficult challenges in treating patients with aortic diseases. Artivion’s four major product groups include: aortic stents, stent grafts, surgical sealants, mechanical heart valves, and implantable cardiac/vascular human tissues.
www.artivion.com
■ Bentley B20
Bentley’s passion is the development, manufacturing and distribution of innovative implants for minimalinvasive treatments of vascular diseases. Since market launch in 2012 we rapidly expanded worldwide. Thanks to our international network of exclusive distribution partners we are represented in more than 80 countries—in some we are already market leader.
www.bentley.global
■ Getinge F23
10,000 people worldwide and the products are sold in more than 135 countries. www.getinge.com/int/insights/ events/exhibitions/2023/cx
■ Inari Medical A15
Inari Medical, Inc. is a medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally invasive, novel catheterbased mechanical thrombectomy devices—FlowTriever (pulmonary embolism [PE]) and ClotTriever (deep vein thrombosis [DVT]). The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism (VTE): DVT and PE. www.inarimedical.com/int
■ Endovastec F19
Lombard Medical Limited is focused solely on the minimally invasive treatment of aortic disease.
In partnership with MicroPort Endovastec, we can provide a broad product portfolio covering endovascular aneurysm repair (EVAR), thoracic endovascular aortic repair (TEVAR) and fenestrated EVAR (FEVAR), adding even more treatment options to the physician’s armamentarium.
To find out more, visit us in the Exhibition Hall.
www.lombardmedical.com
■ Penumbra A19
Penumbra, Inc., headquartered in Alameda, United States, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical conditions in markets with significant unmet need. Penumbra supports healthcare providers, hospitals and clinics in more than 100 countries.
www.penumbrainc.com
■ Shockwave Medical E32
EXHIBITORS
■ AOTI D32
AOTI’s Nexa NPWT and TWO2 topical wound oxygen therapy are designed to increase access and compliance via in-home therapy. TWO2 is clinically proven to heal chronic wounds, reducing hospitalisations and amputations, leading to significant clinical, quality-of-life and cost saving benefits.
www.aotinc.net
■ BD B32
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. BD helps customers enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to healthcare.
www.eu.bd.com/emea-peripheralinterventions
■ BIBA MedTech Insights
Ground floor
BIBA MedTech Insights is a leading provider of market analysis services. We serve medical professionals and organisations in the medical device industry worldwide. Our research products include quarterly monitors, customised research, and tailored services.
www.bibamedtech.com
■ Boston Scientific F33
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare.
www.bostonscientific.eu
■ Cordis C32
Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver
With a firm belief that every person and community should have access to the best possible care, Getinge provides hospitals and life science institutions with products and solutions aiming to improve clinical results and optimise workflows. The offering includes products and solutions for intensive care, cardiovascular procedures, operating rooms, sterile reprocessing and life science. Getinge employs over
Shockwave Medical is a company focused on developing and commercialising products intended to transform the way calcified cardiovascular disease is treated. We aim to establish a new standard of care for medical device treatment of atherosclerotic cardiovascular disease through our differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which we refer to as ‘Intravascular Lithotripsy.’
www.shockwavemedical.com
Cordis is a worldwide leader in the development and manufacture of interventional vascular technology, with a reputation for clinical acumen, training and services. For more than 60 years, Cordis has delivered revolutionary products to treat millions of patients.
www.cordis.com/emea/home
■ Cydar D38
Cydar Medical is a global cloud-based software company that provides an integrated solution for planning, navigation and review of surgical procedures using the power of artificial intelligence (AI) to augment a clinician’s decision making.
www.cydarmedical.com
Tuesday 25 April 2023 14 FIRST EDITION #CX2023 Exhibitors
AT-A-GLANCE GUIDE
CX 2023 exhibitors and major sponsors A-Z contd.
■ CX Vascular
Ground Floor
CX Vascular is an online community site that delivers curated news and content, year-round education and key insights into the vascular landscape. It will also feature live talk shows and offer access to a state-of-the-art production studio in London, United Kingdom. We would welcome you to discuss partnering opportunities to produce roundtables, episodes, podcasts and more. Join the global CX Vascular community at www.cxvascular.com
■ CX Vascular Live Studio
Level 2
The CX Vascular Live Studio makes professional, compelling medical videos with editorial input from experienced medical journalists and a high-end production team in a bespoke studio environment. Contact nathalie@bibamedical.com for more information.
www.cxvascular.com
■ Dendrite Clinical Systems
G11
Vascular surgery databases, registries, MDT, clinical workflow and e-PROMs systems. Clinical data analysis, audit, research, benchmarking. www.e-dendrite.com
■ Frontier Bio
G10
We are pushing the boundaries of tissue engineering to combat cardiovascular disease and improve the development pathway for vascular medical devices.
Our innovative technology enables the growth of living blood vessels in vitro, in various shapes and sizes, for applications in surgery and in device testing as alternatives to animals. www.frontierbio.com
■ iVascular
B36
iVascular is a fast-growing company founded in 2010 in Barcelona, Spain, with the aim of developing medical devices and therapies to improve patients’ quality of life. It is empowering the value of technology and innovation in the vascular field. Nowadays, iVascular has fulfilled the quality standards of more than 70 countries. www.ivascular.global
■ LeMaitre Vascular
F25
LeMaitre is a leading global provider of devices for the treatment of cardiovascular and peripheral vascular disease and for arteriovenous (AV) access. We manufacture and market disposable and implantable devices for vascular and cardiac surgeons. Our
diversified product portfolio consists of well-known brand name products like the LeMaitre Valvulotomes, XenoSure biologic patches and Omniflow II vascular prosthesis.
www.lemaitre.com
London academic vascular units, with the support of three clinical trials units across the United Kingdom. We are planning to host a number of surveys, workshops, and trial development focus groups over the next seven months, online and face-to-face. Based on this information, we will design two ambitious future trials in the relevant vascular disease areas. www.nihr.ac.uk/explore-nihr/ funding-programmes/healthtechnology-assessment.htm
with different devices designs. Digital twin technique is particularly relevant in planning complex and standard endovascular aneurysm repair, where clinical outcomes are uncertain. predisurge.com
■ Scanlan International A25
■
LifeTech Scientific F27
Established in 1999, LifeTech Scientific Corporation (Stock Code: 1302.HK) is committed to the R&D, manufacture, and sales of minimally invasive interventional medical devices for cardio-cerebrovascular and peripheral vascular diseases. The company provides patients with innovative device solutions in the treatment of structural heart diseases, peripheral vascular diseases, and bradycardia, and the company also expands its business scope in respiratory interventional business, neurointerventional business and interventional oncology business. The company has over 1,500 highquality patents been filed, and the sales network has penetrated more than 100 countries and regions around the world.
www.lifetechmed.com/en
■ LSO Medical D40
LSO Medical is a French company specialising in the design and manufacture of Vascular Lasers for over 20 years. Serving patients is a privilege, for which we require the highest standards of quality and ethics. LSO Medical improves the quality of life of patients, by constantly optimising existing treatments.
www.lsomedical.com
■ neoLaser G13
NeoLaser is a world leader in design and manufacturing of surgical laser systems. Specifically, NeoLaser is at the forefront of endovenous laser ablation (EVLA) technology, offering the most advanced platform in the world for EVLA treatments, including the new NeoV1940 system and the revolutionary Infinite Ring fibre family for optimal safety and efficacy.
www.neo-laser.com
■ NIHR Platform TRIALS TT5
The National Institute for Health and Care Research (NIHR) Health Technology Assessment Programme recently funded two ambitious research projects to develop adaptive platform trials, which will assess new vascular treatments and technologies. The two projects are named PAEDIS and VEIN; they will focus on peripheral arterial disease and venous disease, respectively. The projects are led by the Leicester and Imperial College
■ optimed E38
As a German manufacturer for self-expanding nitinol stents with a patient-centric mindset, we stand for high quality and innovation in stents. Our deep expert knowledge in vascular surgery and great partnerships with surgeons worldwide help us to create unique products such as life-saving ductus stents for infants or special venous stents, like the Sinus-Obliquus. Our latest addition to the wide portfolio is the Tentos 4F, a COF-adjusted arterial stent for infrapopliteal and superficial femoral artery applications, with the largest stent variety in the 4F segment. www.optimed.com
■ Philips D36
At Philips, we look beyond technology to the experiences of consumers, patients, providers and caregivers across the health continuum—from healthy living and prevention to diagnosis, treatment and home care. We unlock insights leading to innovative solutions that address the quadruple aim: improved patient experience, better health outcomes, improved staff experience, and lower cost of care. With leading research, design and innovation capabilities, we partner with our customers to transform the delivery of healthcare. www.philips.com
■ Pie / 3mensio Medical Imaging B38
Pie Medical Imaging is simplifying clinicians’ daily practice with the user friendly 3mensio vascular preoperative planning software; developed specifically for endovascular aneurysm repair (EVAR), thoracic endovascular aortic repair (TEVAR) and fenestrated EVAR (FEVAR) interventions. We cordially invite you to our booth during Charing Cross to test-drive 3mensio yourself.
www.piemedicalimaging.com
■ PrediSurge G12
PrediSurge develops innovative predictive software solutions for cardiovascular interventions. Based on pre-operative imaging, our technology enables the creation of patient-specific digital twins used to simulate different endovascular interventions strategies,
Celebrating over 100 years and our journey continues... The highest quality surgical instruments designed and manufactured by the Scanlan Family since 1921. Experience the Scanlan difference at our exhibit A25.
www.scanlaninternational.com
■ SCITECH C36
Scitech Medical is a minimally invasive medical device company that was founded over 20 years ago and is currently present in more than 45 countries. Its 6,950m2 state-of-the-art CE 13485 certified facility is located in Brazil. Currently the company develops a wide portfolio of products for peripheral vascular, interventional cardiology etc.
www.scitechmed.com
■ Shape Memory Medical D38
Shape Memory Medical is reshaping clinical success through the science of smart polymer. Smart polymer upgrades device performance and redefines embolization possibilities. Our conformable smart polymer delivers unparalleled volume, returns imaging clarity, and promotes healing as the material absorbs. We continue to drive a cross-specialty portfolio to meet procedural demands.
www.shapemem.com
■ STARmed E40
The global leader in the thyroid radiofrequency (RF) ablation.
www.starmed4u.com
■ Teleflex E36
Teleflex is a global provider of medical technologies designed to improve the health and quality of people’s lives. We apply purpose driven innovation—a relentless pursuit of identifying unmet clinical needs—to benefit patients and healthcare providers. Our portfolio is diverse, with solutions in the fields of vascular and interventional access, surgical, anaesthesia, cardiac care, urology, emergency medicine and respiratory care. Over 14,000 Teleflex employees worldwide are united in the understanding that what we do every day makes a difference.
www.teleflex.com/emea/en
■ The University of Edinburgh
F35
Study part-time for a Master of Surgery degree in Vascular & Endovascular Surgery completely online and gain extensive knowledge of the specialty.
15 Tuesday 25 April 2023 FIRST EDITION #CX2023 Exhibitors
CX 2023 exhibitors and
major sponsors A-Z contd.
Improve your clinical decision-making skills through learning how to evaluate and apply evidence in your practice. Provides structured learning for those preparing for board exams. Contact chm.info@ed.ac.uk. edin.ac/3kNwa5e
■ VARIXIO
A27
VB Devices develops unique medical devices that resolve practical clinical needs in vascular medicine. It was founded in Barcelona, Spain, in 2016 by vascular surgeon Enric Roche and biomedical entrepreneur Federico Grego. VB Devices’ main product is Varixio, an automated foam-preparation system for use in the treatment of varicose veins with sclerotherapy. The flexibility and versatility Varixio brings to foam sclerotherapy can greatly expand the applicability of this minimally invasive technique, making it possible to treat varicose veins of all sizes. www.varixio.com
■ Veryan Medical
A23
Veryan Medical, an Otsuka Medical Devices company, is committed to transforming the lives of patients suffering from peripheral vascular disease. The BioMimics 3D stent has a unique three-dimensional helical shape, designed to impart natural curvature to the femoropopliteal artery to promote swirling flow and elevate wall shear that are patency-protective. www.veryanmed.com
■ Wisepress Medical Bookshop
A30
Wisepress.com, Europe’s leading conference bookseller, attends around 200 conferences every year. We have an extensive range of books and journals relevant to the themes of CX available at our booth. We also have a comprehensive range of STM titles available on our online bookshop. Follow us on Twitter @WisepressBooks. www.wisepress.com
■ Ziehm Imaging
A21
Since 1972, Ziehm Imaging has produced technologies that enhance imaging and streamline clinical workflows. Our devices’ exceptional image quality and flexibility in the operating room serve as an important basis for treatment success.
Ziehm Imaging is specialised in the development and manufacture of mobile C-arms. ziehm.com/en/home.html
CX 2022: Up close and personal
Reunited and refreshed: The global vascular community came together in London in-person for CX 2022 for the first time since the start of the pandemic last April. Here are a selection of images capturing those three days of education, evidence and innovation.


16 Tuesday 25 April 2023 FIRST EDITION #CX2023
AT-A-GLANCE GUIDE








17 Tuesday 25 April 2023 FIRST EDITION #CX2023
“We have had 45 years of a very global CX, and the tradition continues.”
Experts debate TCAR versus stenting in Acute Stroke session
08:00–10:10 Wednesday 26 April
Admiral
At this year’s CX Symposium, experts will gather to discuss the most pressing issues in the field of acute stroke. Anchor Maarit Venermo (Helsinki, Finland) along with moderators Alexander Zimmermann (Zurich, Switzerland) and Domenico Valenti (London, United Kingdom) will oversee the session on the second day of CX 2023. The first item on the programme is a CX Debate, during which Peter Schneider (San Francisco, United States) and Valenti will respectively argue for and against the motion that transcarotid artery revascularisation (TCAR) is better than percutaneous carotid stenting.
Among the podium presentations in the session, Venermo is set to speak on the aetiology and treatment patterns of ruptured extracranial carotid artery aneurysms, as well as on whether carotid endarterectomy is safe immediately after thrombolysis and the consequences of delay. Zimmermann is due to present on identification of baroreceptors responsible for mechanotransduction in the human aortic arch, and Valenti on simultaneous mechanical thrombectomy and carotid artery stenting for severe internal carotid artery stenosis. The latter will outline indication risks and current evidence. Additionally, Alun Davies (London, United Kingdom) will argue that NASCET (North America symptomatic carotid endarterectomy trial) and ECST (European carotid surgery trial) need to be reconducted, and Michael Stoner (Rochester, United States) will speak on how calcified plaque volume predicts haemodynamic restenosis and also on the use of flow-based protection to treat tandem supra-aortic branch stenoses.
In keeping with CX style, the session will feature audience participation throughout and close with a panel discussion.
Patient-centric innovation that stands the test of time
Vascular Trauma Consensus
Comprehensive vascular trauma programme offers consensus update
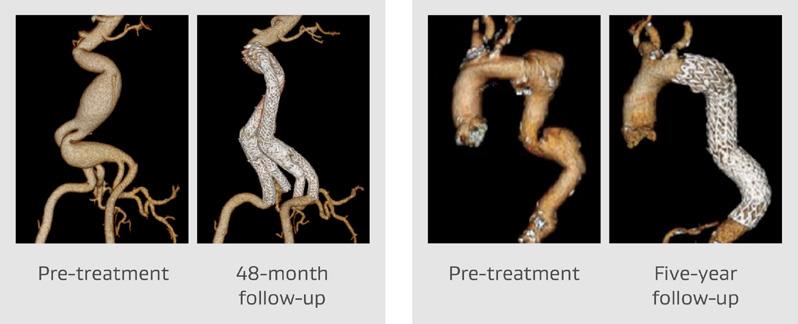

11:00–13:00 Wednesday 26 April
Admiral
This year’s Vascular Trauma programme at CX will hone in on a number of key topics in the field, namely endovascular trauma, extremity vascular trauma and limb salvage, and cerebrovascular trauma. At the end of the session, the CX audience can expect half an hour of presentations on the best vascular trauma abstracts.
Anchor Ross Davenport (London, United Kingdom) and moderator Christopher Aylwin (London, United Kingdom), both executive board members, will oversee a series of podium presentations, as well as audience participation and discussion, all in the interest of reaching consensus and moving the subject forward.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) will be a central focus of the endovascular trauma section, with presentations including those from Robert Lendrum (London, United Kingdom) on refining the use of REBOA for major vessel injury in trauma and Megan Foley (Dublin, Ireland) on limb complications of REBOA.
Moving the focus to extremity vascular trauma and limb salvage, Anna Sharrock (London, United Kingdom) will offer CX attendees an update on prediction models of limb salvage, and Simon Glasgow (Harrow, United Kingdom) on decision-making in complex extremity vascular trauma, among other presentations.
The cerebrovascular trauma section will cover blunt injuries, specifically thresholds for imaging, diagnosis and monitoring, as well as the management of these injuries and penetrating carotid and vertebral injuries.
At the end of the session, in a ‘Best of Abstracts’ section, the audience will hear about a 10-year experience in a major trauma centre, pointing to opportunities for training in emergency open approaches.
Even though it is celebrating its aortic portfolio reaching its 25-year milestone, Gore believes that this is only the beginning when it comes to being an aortic ally. The GORE® EXCLUDER® AAA Endoprosthesis and the GORE® TAG® Device family are commemorating 25 years of commercial availability and the procedural innovations they have inspired along the way.
Twenty-five years of patient impact and durability is worth celebrating in the life of any medical device. And, when a device reaches this milestone, it is celebrating more than just 25 years in the treatment landscape.
The EXCLUDER® device and the TAG® Conformable Device have been used to treat more than 675,000 patients worldwide and have become the most studied thoracic aortic aneurysm repair (TEVAR) and endovascular aortic aneurysm repair (EVAR) devices according to company-sponsored trials and registries shown on ClinicalTrials.gov of all currently available stent grafts. Contributing to the advancement of aortic treatment with over 1,500 peer-reviewed publications, this is a testament to the device’s legacy—making a difference for physicians and their patients.
The durability of this device can be attributed to its material quality and design. Both devices feature a nitinol stent, sutureless stent-to-graft attachment, and proprietary expanded polytetrafluoroethylene (ePTFE) film layers.

“The EXCLUDER® Device has been on the market helping patients for well over two decades—a truly

remarkable accomplishment and the longest stretch in the industry,” says Michel Makaroun, chief of the Division of Vascular Surgery at the University of Pittsburgh Medical Center (Pittsburgh, United States) who has also been an investigator in all EXCLUDER® Device clinical studies to date.

“The close collaboration between medical community and manufacturer has allowed for numerous innovations and improvements along the way, providing more accurate deployment and better outcomes.”
“I have much admired the ongoing family interest in the business and how it has continued in the way that Gore intended it,” says Roger Greenhalgh (London, United Kingdom), the chairman of the Charing Cross (CX) Symposium, of Gore’s history of innovation and research. “The company is known for supporting high-quality medical education.”
Over the last two decades, in collaboration with passionate healthcare providers, Gore has continued to develop and study additional solutions within its aortic portfolio, receiving approvals for larger trunk and contralateral limb diameters, and iliac branch devices. As well, the next generations of both devices are conformable and feature the GORE® ACTIVE CONTROL System, allowing physicians more treatment options to consider for their patient’s anatomy.
Tuesday 25 April 2023 18 FIRST EDITION #CX2023 Tomorrow’s Highlights
Acute Stroke Consensus
THIS ADVERTORIAL IS SPONSORED BY GORE
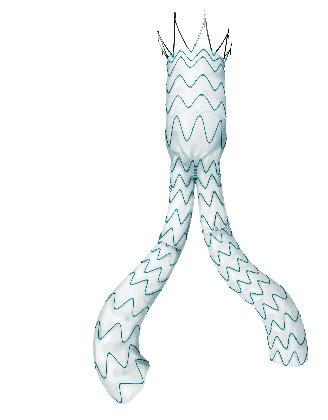
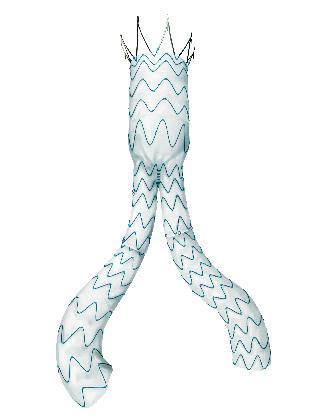
Pre-implant, four- and five-year follow-up showing the conformability of the GORE® EXCLUDER® and CTAG® devices
For more information on Gore’s Aortic Ally, scan the QR code to the left
Michel Makaroun
Gore® EXCLUDER®





























*As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface. Learn more at goremedical.com/eu/peripheral/av-access MAKING A DIFFERENCE IN THE LIVES OF HEMODIALYSIS PATIENTS GORE AV ACCESS SOLUTIONS W. L. Gore & Associates, Inc. goremedical.com Asia Pacific +65 6733 2882 Australia/New Zealand 1800 680 424 Europe 00800 6334 4673 United States Flagstaff, AZ 86004 800 437 8181 928 779 2771 Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. Products listed may not be available in all markets. GORE, GORE-TEX, Together, improving life, ACUSEAL, INTERING, PROPATEN, VIABAHN and designs are trademarks of W. L. Gore & Associates. © 2023 W. L. Gore & Associates GmbH 23862750-EN JANUARY 2023 GORE® ACUSEAL Vascular Graft
INTERING® Vascular Graft
PROPATEN® Vascular Graft
STRETCH Vascular Graft
GORE®
GORE®
GORE-TEX®
Surface*
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive
Edited cases and imaging at the forefront of aortic techniques and technologies sessions

The latest advances in imaging technologies for use in endovascular aortic repair form a key part of the Aortic Techniques and Technologies programme, spanning a full day of sessions in Kensington 2 on Tuesday 25 April—with a particular focus on edited cases to bring discussion to life.
ANCHORED BY TILO KÖLBEL (HAMBURG, Germany), alongside moderators Roberto Chiesa (Milan, Italy), and Gustavo Oderich (Houston, United States), the CX community will strive towards consensus on the latest developments in the aortic field. Presentation and discussion will feature world-leading experts in management and treatment of aortic disease from the cardiovascular, vascular and endovascular worlds, with faculty including
Maximilian Pichlmaier (Munich, Germany), Marco Virgilio Usai (Münster, Germany), Ross Milner (Chicago, United States) and Joseph Coselli (Houston, United States).
“At this year’s CX Techniques and Technologies, we will focus even more on edited cases to showcase the newest and the latest technologies in endovascular aortic repair,” Kölbel tells CX Daily News. Among the programme highlights picked out by Kölbel are first-in-Europe insights from the GIANT study, a prospective, multicentre study on the safety and efficacy of a modular inner branch stent graft system. This will come in an edited aortic arch reconstruction case from Wei Guo (Beijing, China) who has led the development of the offthe-shelf modular double inner branch stent-graft system. Guo’s presentation is the first on the programme on Tuesday
Kölbel also calls attention to an edited case from Jean Panneton (Virginia, United States) in which he will be offering an update on in situ
CX 2023 heralds the multidisciplinary approach

Alexander Zimmerman (Zürich, Switzerland), who is instructing the upcoming aortic course (Wednesday 26 April), emphasised the importance of continuous education for the practising surgeon in an interview with CX Daily News. During the discussion, he advocated for a multidisciplinary approach to treating and managing the aorta.
ACCORDING TO ZIMMERMANN, the CX Symposium as a whole encourages taking a multidisciplinary approach—”Not only are there aortic specialists like [me] but all of the different vascular specialties come together to talk about the different developments and technologies in our field.” He continued, pointing out that “not everyone is doing just aortic or peripheral arteries, but all of us are doing different tasks so it is a great opportunity to become aware of the evolutions […] in our specialty.” The idea of sharing knowledge
and skills in the vascular space in a cross-disciplinary manner bears relevance to the Wednesday aortic workshop sessions that Zimmermann is instructing for the reason that it is important, as he said, to maintain awareness of the different developments occurring within surgical and endovascular technologies—these aortic sessions centre around how to use physician-modified F/BEVAR stent grafts. “This is really unique,” Zimmermann opined during the interview—“I have never heard of a hands-on course before on the topic.”
laser fenestration during thoracic endovascular aortic repair (TEVAR), an innovative method to revascularise arch branches for diverse thoracic aortic diseases. “This is specifically interesting because Jean has been the pioneer of this technique and has the world’s greatest experience with in situ laser fenestration,” says Kölbel of the programme highlight, which also comes on Tuesday morning.
Further to these individual programme highlights, Kölbel says that the deployment of imaging technologies will be an important thread running through the session. “We are all moving towards better visualisation of what we do when we do endovascular aortic repair and to new, exciting devices—electromagnetic visualisation as well as laser-guided visualisation of catheters and wires will be shown,” he comments. On the imaging topic, Milner offers attendees a “method to success” for transcaval TEVAR with the use of intravascular ultrasound (IVUS).
New device technologies also feature prominently, with Nikolaos Tsilimparis (Munich, Germany) delivering an edited case offering insights on first experiences with a new covered stent, and Kak Khee Young (Amsterdam, Netherlands) reporting on overcoming clinical challenges through technological advancements with a new fenestrated device. Elsewhere, Michele Antonello (Padua, Italy) presents outcomes of a novel off-the-shelf preloaded inner branch endograft for the treatment of complex aortic pathologies in the Italian Branched Registry (INBREED).
Also on the programme, Weiguo Fu (Shanghai, China) will share multicentre clinical trial results from China on a thoracic aortic stent based on petticoat technology, Germano Melissano (Milan, Italy) will speak on open conversion after endovascular aneurysm repair (EVAR)—a technique he considers the best option for a safe procedure—and Florian Elger (Göttingen, Germany) will highlight the importance of respecting the fenestrated and branched EVAR (F/BEVAR) boundaries in clinical practice.
“We have excellent data on [physician-modified] stent grafts—[they seem] the way to go. If you are familiar with treating patients with fenestrated grafts or chimney grafts, it will only be a small step to implement the technique with physician-modified stent grafts into your daily routine,” Zimmermann went on to assure potential course participants, in the conversation with CX Daily News. “This is something we want to bring to you—how you measure the aorta, how you plan the grafts, how you do the modifications, and how you implant the stent graft in the patient,” he asserted.
Working together in the patient interest
In another interview, this time with CX Chairman Roger Greenhalgh (London, United Kingdom), Zimmerman reinforced the importance of taking interdisciplinary approach to treating patients—“I think the multidisciplinary approach is key […] you can learn so much from an interventional radiologist in terms of techniques or how to address a problem”.

Other presentations during the
CX Symposium on the subject of (multidisciplinary) approaches to aortic aneurysm repair include an edited case on Tuesday 25 April demonstrating the use of laser fenestrations to aid in the endovascular treatment of chronic dissection thoracoabdominal aortic aneurysms (TAAAs). Presented by Timothy Resch (Copenhagen, Denmark) in Kensington 2, attendees will be able to observe this case from 15:00 to 15:10 as part of the day-long Aortic Techniques & Technologies programme.
There will also be a live case of TAAA endovascular treatment by Martin Austermann (Münster, Germany).
Part of the Thoracic Aortic programme on Thursday 27 April, delegates will be able to watch the case in Kensington 1 between 11:20 and 12:10. ‘Frailty, comorbidities and quality of life: A multidisciplinary approach to patient selection for aortic aneurysm repair’ will be presented by Bijan Modarai (London, United Kingdom) later on that day, also in Kensington 1, between 14:20 and 14:25.
20 Tuesday 25 April 2023 FIRST EDITION #CX2023 Aortic Consensus
Aortic Consensus
Tilo Kölbel (left) with Gustavo Oderich





LEARN SHARE & SHAPE Join your global community to the future of patient care worldwide 16-19 May 2023 #EuroPCR Paris The World-Leading Course in interventional cardiovascular medicine
Tuesday 25 April: Satellite symposia
Kensington 1 — 09:40–10:10
Tools and techniques to improve PTA outcomes
Moderator: Michael Lichtenberg, Arnsberg, Germany
09:40–09:47
Why I use IVUS for PAD procedures
Speaker: Ashish Patel, London, United Kingdom
09:47–09:54
How atherectomy improves PTA results
Speaker: Michael Lichtenberg, Arnsberg, Germany
09:54–10:01
The importance of dissection, detection and treatment
Speaker: Peter Schneider, San Francisco, United States
10:01–10:10
Audience participation & discussion
Kensington 2 — 12:30–12:50
Raising clinical efficacy through technological advancements:

Fenestrated Treo
Moderator: Eric Ducasse, Bordeaux, France
12:30–12:35
Technical advantages of fenestrated Treo
Speaker: Kak Khee Yeung, Amsterdam, Netherlands
12:35–12:40
Clinical feedback: Genova case series with fenestrated Treo
Speaker: Giovanni Pratesi, Roma, Italy
12:40–12:45
Why fenestrated Treo is a gamechanger
Speaker: Vincent Riambau, Barcelona, Spain
12:45–12:50
Fenestrated Treo: Austria experience from radiological perspective
Speaker: Florian Wolf, Vienna, Austria
Kensington 2 — 09:40–10:10
Nectero sponsored education

09:40–09:42

Introduction
Speaker: Michael Jaff, Boston, United States
09:42–09:47
Small and mid-sized abdominal aortic aneurysm management: What we know from screening programmes and incidental aneurysm detection
Speaker: Janet Powell, London, United Kingdom
09:47–09:52

Current rationale and experience with treating small and mid-sized aneurysms: Aetiologies, presentations, management approaches
Speaker: John Curci, St. Louis, United States
09:52–09:57
The Nectero clinical experience to date and future research/study plans
Speaker: Andrew Holden, Auckland, New Zealand
09:57 – 10:10
Panel discussion and Q&A
Admiral — 13:30–14:00
Vascular closure device in EVAR setting: MANTA gamechanger
13:30–13:52
Vascular closure device in EVAR setting: MANTA gamechanger
Speaker: Ozan Yazar, Heerlen, Netherlands
13:52–14:00
Audience participation & discussion
Admiral — 10:30–11:00
Long-term evidence: Setting a new standard for AVF creation and maintenance?
10:30–10:35
Introduction
Speaker: Alexandros Mallios, Paris, France
10:35–10:42
Experience with Ellipsys system fistulas: Long-term data and long-term use
Speaker: Alexandros Mallios, Paris, France
10:42–10:48
DCB evidence through three years: What it means for my algorithm Andrew Holden, Auckland, New Zealand
10:48 – 11:00
Discussion & questions
Kensington 2 — 15:20–15:50
A long history of aortic devices

conformability and impact on clinical outcomes
15:20–15:30
Does conformability have an impact on the outcomes of endovascular repair of Type B aortic dissection?
Speaker: Michele Antonello, Padua, Italy
15:30–15:40
Does conformability have an impact on the outcomes of endovascular repair of AAA with angulated infrarenal necks?
Speaker: Giovanni Pratesi, Roma, Italy
15:40–15:50
Does conformability have an impact on the outcomes of endovascular repair of iliac limbs performance?
Speaker: Jan Heyligers, Tilburg, Netherlands
Kensington 1 — 10:40–11:10
The concept of arterial clot removal: Realworld data and latest innovations

Moderator: Jos van den Berg, Lugano, Switzerland
10:40–10:45
Introduction
Speaker: Jos van den Berg, Lugano, Switzerland
10:45–10:52
High limb salvage rate and low mortality: Thirty-day data from STRIDE study
Speaker: Sean Lyden, Cleveland, United States
10:52–10:59
Penumbra Lightning system: The next horizon for arterial clot management


Speaker: Gianmarco de Donato, Siena, Italy
10:59 – 11:10
Panel discussion and Q&A
Tuesday 25 April 2023 22 FIRST EDITION
C M Y CM MY CY CMY K
#CX2023
SATELLITE PREVIEW All times are London time BST (British Summer Time), which is GMT+1. The organisers reserve the right to alter timings if necessary.
CLASSICAL OPEN AND ENDOVASCULAR SOLUTIONS


CARDIAC, VASCULAR AND ENDOVASCULAR AORTIC ADVANCES

HOLD THE DATES
MONDAY–WEDNESDAY

2–4 OCTOBER 2023
IN PERSON AND VIRTUAL ANDAZ VIENNA AM BELVEDERE, VIENNA, AUSTRIA









AORTIC
cxaortic.com
VIENNA





Join the CX Vascular platform today to connect and engage with the global vascular community, share expertise and experiences, and stay up-to-date with the latest education and news in the vascular world. Members will also have access to exclusive content including live discussions on the latest advances in the vascular field and interviews with key thought leaders in the space. Register now Education, News, Insights Join the community at https://cxvascular.com


















































































































