Unifying IO globally: IASIOS reports first USaccredited centre

Interview: RSNA gold medallist Anne Roberts page 14
www.interventionalnews.com
PAIRS: IR training pathway roundtable
page 15


Profile: Alda Tam page 16


The International Accreditation System for Interventional Oncology Services (IASIOS) is the world’s first accreditation programme focused exclusively on standardising interventional oncology (IO) care, redefining the rapidly evolving field. Reporting record growth exceeding 150% in 2023, IASIOS recently announced their first accredited facility in the USA, a significant milestone which marked its expansion to a new and major corner of the international IO community.
“It’s accreditation and unification,” said Jack Jennings on behalf of the newly accredited Mallinckrodt Institute of Radiology (MIR) at Washington University in St Louis, USA. Embarking on their IASIOS accreditation process, Jennings and his team were driven by the opportunity to strengthen bonds with the physicians of IASIOS’ international network. This “alliance”, and mutual dedication to improving quality and safety in IO, is more relevant today than ever, Jennings conveyed, as the specialty does the groundwork for the “steep and rapid incline” of IO procedures in this booming faction of interventional radiology (IR).
SIR PREVIEW
Late-breaking analysis set to expose the ‘why and how’ of endovascular technical failure in CLTI patients












In a comment to Interventional News, chair of the IASIOS supervisory board Andreas Adam (Kings College London, London, UK) shared that the global nature of IASIOS is one of its greatest strengths, as it emphasises certain universal concepts such as IRs looking after their own patients. Remarking on the recent USA accreditation, Adam stated that MIR’s enrolment is a “major milestone” in IASIOS’ history. “The USA is the birthplace of IR and the largest provider of IR services. We are delighted to welcome the MIR, as its accreditation is a perfect demonstration of the universality of the principles on which IASIOS is founded.”
A world-first accreditation programme
IASIOS is the world’s first accreditation system focused solely on upholding quality assurance for minimally-invasive treatments for cancer. Due to the continued growth and
SET TO BE PRESENTED AT THE upcoming Society of Interventional Radiology (SIR) annual scientific meeting (23–28 March, Salt Lake City, USA), an anticipated latebreaking analysis of the BEST-CLI (Best endovascular versus best surgical therapy in patients with critical limb ischaemia) trial will expose the cause and significant impact that endovascular technical failure (ETF) had on patient outcomes. In doing so, the investigators hope to provide a granular, contextual understanding of why these failures happen in realworld practice.


Clockwise to centre: Liz Kenny, David Breen, Rodrigo Gobbo, Murat Dökdök, Mark Burgmans, Andreas Adam, Jack Jennings
recognition of IO as a key clinical discipline and the fourth pillar of cancer care, consensus has dictated that centres providing IO treatments must adhere to robust guidelines to ensure a universally high standard of care. During its conception, the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) lifted the current quality assurance standards for radiation oncology—developed by The Royal Australian and New Zealand College of Radiologists (RANZCR)—as a template which was well aligned with the practice of IO.
The fruits of this labour—the CIRSE Standards of Quality Assurance in Interventional Oncology—establishes a “gold standard” in patient care and safeguarding in IO. Yet, as RANZCR president at the time and current steering board member of IASIOS Liz Kenny (Royal Brisbane and Women’s Hospital, Herston, Queensland Australia) noted, IO is “still a
Continued on page 4
Published in 2022, the BESTCLI trial results found surgical intervention superior to endovascular revascularisation. Split into two cohorts based on the availability of suitable single segment greater saphenous vein (SSGSV) for bypass, BEST-CLI enrolees were randomised in a 1:1 fashion to either surgery with SSGSV bypass or endovascular treatment (cohort 1), or surgery with an alternate bypass conduit or endovascular treatment (cohort 2). Enrolment criteria required reasonable surgical risk and anatomy suitable for both bypass and endovascular treatment. The results showed that technical success was 98.3% vs. 84.7% for cohort 1, and 100% versus 80.6% in cohort 2, respectively.
Technical failure rates in both cohorts markedly contributed to the difference in outcome between surgery and endovascular outcomes in both groups, but the reason these failures occurred was unclear. This formed the basis for Richard Powell (Dartmouth Hitchcock Medical Center, Lebanon, USA) et al’s analysis which sought to evaluate the causes and impacts of ETF on outcomes.
Powell, speaking to Interventional News, explains that the BESTCLI findings offered a close-toaccurate representation of real-world endovascular treatment experience. “If you look at a lot of studies carried out by industry there are very low rates of
Continued on page 5
| Issue
March 2024
93
How accreditation can help IO evolve into a mainstream clinical discipline
Many years ago, when giving a talk on interventional oncology (IO), I expressed the hope that this promising and exciting branch of interventional radiology (IR) would become ‘the fourth pillar of cancer care’, alongside medical, surgical and radiation oncology. Since then, it has been heartening to hear that phrase used widely at various congresses around the world. But it has also been frustrating because that aspiration remains unrealised. We need to ask why IO is still not considered a mainstream discipline, and what we can do to help it to realise its potential.
The path to mainstream status would be easier if IR were a primary specialty alongside diagnostic radiology and radiation oncology, as it already is in the USA. There are efforts in several countries, including the UK and Ireland, towards that goal. But IO cannot wait until that has been achieved to put its house in order.
The greatest deficiency in the current pattern of practice of interventional oncologists is that most of them do not assume primary clinical responsibility for the patients they treat. Many fudge this issue, pointing to their ‘involvement’ in clinical decision making and the care of the patient. But this is insufficient to earn the respect of other oncologists. A true oncologist understands cancer and can discuss the options for the treatment of each patient on the basis of
clinical knowledge. Their expertise is judged not only on the basis of technical dexterity, but also on whether they have an intimate understanding of the cancer requiring treatment. Those IOs who tell themselves that it is acceptable to delegate the clinical care of their patients to the referring physician are ignoring the risks that this approach entails. Only they understand fully all the issues related to IO, and they are responsible not only for what they do but also for what they do not do.
In the early days of my IO practice, I used to receive referrals from all over the UK, as there were very few centres with the relevant expertise. One of my patients was an elderly lady from Newcastle, for whom the 275mile journey to London was very difficult. After ablating a tumour in her liver, I was pleased to see that the computed tomography (CT) the following morning showed no residual disease. When I told her that she would need another CT in three months, she was very concerned about the journey to London and asked me whether the study could be done in her local hospital and the report sent to the referring oncologist. Reluctantly, I agreed. The CT showed what the local radiologist, who had very limited experience with post-ablation imaging, considered to be equivocal findings. He issued a vague report which provided no guidance to the oncologist, who sent me the text and asked for my help. I requested the images, which showed a small, treatable local recurrence. By then the CT was old, so I arranged another one, and was very sad to see that, by then, the disease had become untreatable. Of course, this was all my fault. I led the patient down by agreeing to a follow-up arrangement that entailed significant risks.
IOs should aim to train and practise like surgical oncologists and radiation oncologists. They should understand the pathology and clinical features of the cancers they treat, and they should train formally and systematically in the techniques and equipment they use. They should run outpatient clinics like all other oncological disciplines, and they should follow up their own patients for as long as necessary to detect and treat recurrent disease.
The four pillars of modern oncology care

The nurses and radiographers who assist with procedures such as ablation and radioembolization should have the necessary skills. It would be unthinkable for a radiographer delivering radiation therapy not to be trained appropriately. Radiographers who assist interventional oncologists do not deliver treatment directly, but their skills contribute significantly to the efficacy and safety of IO procedures.

And yet, many interventional oncologists have walked into a CT room to do an ablation only to hear the radiographer say: “I’ve never seen one of these before”. This is unacceptable, and it is our responsibility to discuss this matter with the relevant professional organisations and encourage them to put in place appropriate training arrangements.
Continued on page 4
Editors-in-chief: Professor Robert Morgan, Professor Andreas Adam, Professor Brian Stainken
Publisher: Stephen Greenhalgh | Content director: Urmila Kerslake | Head of global news: Sean Langer
Editor: Éva Malpass | Editorial contribution: Jocelyn Hudson, Will Date, Bryan Kay, Jamie Bell and Adam Pearce
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Michael Broughton michael@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham
Printed by: Buxton Press. Reprint requests
n CMS CAROTID STENTING COVERAGE
EXPANSION:
In October 2023, the US Centers for Medicare & Medicaid Services (CMS) carotid artery stenting (CAS) confirmed coverage expansion. The decision made by the CMS removes several facility standards and approval requirements. Reflecting on this development, David Sacks (Reading Hospital, West Reading, USA) shares his scepticism over the scope and diligence of the CMS’ accreditation requirements, and the importance of maintaining quality of care.

For more on this story go to page 5.
n BIEN SOO TAN AWARDED SIT GOLD MEDAL:

Bien Soo Tan is a senior consultant at the Department of Vascular and Interventional Radiology (IR) at Singapore General Hospital (SGH). Ahead of his upcoming acceptance of the Society of Interventional Radiology (SIR) Gold Medal award at the 2024 annual congress, Tan speaks to Interventional News about his career achievements to date, developments at his centre and where he thinks IR will go next in the Asia-Pacific region.
For more on this story go to page 10.
n RUN YOUR OWN IR WORKSHOP:
Speaking on behalf of ‘IR Bites’—a group whose mission is to raise the baseline public understanding and level of education on interventional radiology (IR)—medical student Milindu Wickramarachchi details the group aims and the importance of elevating knowledge of IR, and outlines their pragmatic guide to running educational workshops that can be reproduced at any hospital or trust around the world.


For more on this story go to page 19.
If you have comments on this issue or suggestions for upcoming editions write to eva@bibamedical.com



March 2024 | Issue93 2 interventionalnews linkedin.com/company/interventional-news/ @in_publishing Subscribe
here
Road,
London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788
Drive, Suite
Chicago, IL 60606, United States Tel: +1 708-770-7323
Fulham,
BIBA Medical, North America, 155 North Wacker
4250,
correspondence
the newspaper should be addressed to the editor
and all
regarding
at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
EDITORIAL
NEWS IN BRIEF THE LATEST STORIES FROM THE INTERVENTIONAL WORLD
Medical Surgical Radiation Interventional 1 2 3 4
Adam
Andreas












merit.com Experience an at Merit Medical Headquarters EVENING OF INNOVATION ATTENDING SIR 2024? Join us for an evening of food and drinks, tour our manufacturing facilities, and hear from Fred Lampropoulos, President and CEO, as he discusses the future of IR medical devices. One night only! Secure your seat today. Tuesday, March 26, 2024 | 6:30 pm–9:00 pm Transportation Provided This event is not considered part of the SIR 2024 Annual Scientific Meeting as planned by the SIR Annual Scientific Meeting Committee.
How accreditation can help IO evolve into a mainstream clinical discipline
Continued from page 2
Some of the issues facing IO, such as the need for improvements in the training curriculum, are best addressed nationally. However, a lot can be done locally to raise standards. For example, a local programme can be put in place for the training of radiographers in the imaging techniques used to guide tumour ablation. Also, local managers can be asked for the resources needed to support IO outpatient clinics.
Accreditation of IO centres can help enormously with the efforts to raise standards of practice. And this is what IASIOS—the International Accreditation System for Interventional Oncology Services—is all about.
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) was urged to focus on the concept of quality assurance (QA) and its implications for IO by Liz Kenny (Royal Brisbane and Women’s Hospital, Brisbane, Australia). Kenny pointed out that QA is extremely important in daily practice in her specialty and argued that the same was true of IO, which has many similarities with radiation oncology. She encouraged CIRSE to focus on this important issue and explained that there would be great benefits for patients. She then asked the Royal Australian and New Zealand College of Radiologists (RANZCR) for permission to use its Standards of Practice in radiation oncology as a template for standards in IO, and helped CIRSE to produce them. The standards cover every aspect of the patient pathway and provide a basis for better training and practice in IO. They have been endorsed by 40 national and international societies, including the European Cancer Organisation, and are the basis for IASIOS. The accreditation scheme helps interventional radiologists engaged in cancer care to point out the need for better resources to those that fund the IO service in their hospital.
The IASIOS team at the CIRSE office, which was created by the CIRSE executive director, Daniel Waigl, and is led brilliantly by Mardis Karlsdottir, provides excellent support to IO centres applying for accreditation. The scheme has met with stunning success and now covers four continents, as the first centre in the USA, the Mallinckrodt Institute of Radiology, led by Jack Jennings, gained accreditation last autumn (see cover story).
Although IASIOS was created by CIRSE, it is truly international, and the standards on which it is based are universal. Interventional oncologists everywhere would greatly benefit from IASIOS membership, the ultimate aim of which is to make IO the true fourth pillar of cancer care.
PROFESSOR ANDREAS ADAM is an interventional radiologist at King’s College London, UK, and joint editor-in-chief of Interventional News.
Unifying IO globally: IASIOS reports first US-accredited centre
Continued from page 1
very young speciality and in many countries faces obstacles that other clinical disciplines have already overcome”.
For this reason, Kenny alongside CIRSE, spearheaded the development of the tiered accreditation system—IASIOS— to “distinguish between ‘core criteria’ i.e., those considered necessary to provide safe patient care, and ‘extended criteria’, which are more aspirational and considered the ideal”, which Kenny and IASIOS chief operations officer Mardis Karlsdottir, outlined in a recent article published in the Journal of Medical Imaging and Radiation Oncology.
The Standards of Quality Assurance in Interventional Oncology that form the foundation of IASIOS has since gained global support from over 40 national and international radiology and interventional radiology societies, drawing the discipline together in a “unified front”, Kenny and Mardis state, and this comes at an opportune moment.
In the house of IR—and an integral section of it—IO is embarking on a parallel path to greater recognition internationally, which is developing apace alongside novel innovations and technological advancements in the field. For Jennings, installing a “baseline accreditation” sets a background for quality and safety among the rapid growth of IO therapies, such as immunotherapy. “It’s great to have an overarching accreditation process to know that we’re not going rogue, or cowboy-esque; to bring greater uniformity, credibility and validity to the specialty on both sides of the pond,” Jennings explained.
“The truth is that [IO] has become a bit of an innovative Wild West,” said David Breen (University Hospital Southampton, Southampton, UK), resonating with Jennings while speaking on behalf of his IASIOS-accredited centre. IO sits within a “rapidly developing and complex therapeutic arena” he explained, stating that IASIOS, alongside CIRSE, has arrived at a critical time in the development of IO. “In a number of areas an individual’s cancer care pathway is set to become more complex, with increasingly significant contributions from IO. It’s important that IASIOS now begins to set agreed international standards and expectations of practising units. This will help IO develop in an organised fashion and realise its full potential.”
By surveying accredited facilities, recognition for IO varies geographically, but this remains a challenge which IASIOS intends to rectify across the globe. For Jennings in the USA, following the American Board of Medical Specialties (ABMS) recognition of IR as a primary specialty in 2012, quality and safety measures have “not been geared at [IO], which is seen as a subset of [IR’s] quality and safety”. In gaining IASIOS accreditation at his institution, Jennings hopes to lead the way for other interventional oncology facilities in the USA to follow suit, giving the specialty better footing—“a lot of people are talking about this” he said, suggesting that IASIOS accreditation has a “larger overall message” of awareness raising.
Connecting international centres
IASIOS accreditation for Murat Dökdök (Anadolu Medical Centre, Gebze, Turkey) has enabled his centre to look at IO procedures specifically, to “define the processes that are crucial in maintaining best medical practice”. As day-today interventional procedures are “complex to interpret”, he detailed, a clear benefit of IASIOS is its ability to “instil trust” and promote confidence for patients and physicians alike. Dökdök also expressed the importance of bolstering IO through IASIOS’ global network, noting that “collaboration among accredited centres from various countries can foster international research projects, joint training programmes and cross-border
healthcare initiatives”.
In The Netherlands, Mark Burgmans (Leiden University Medical Centre, Leiden, The Netherlands) agrees that IASIOS accreditation has made it “easier to reach out to other centres and share experiences” in order to “overcome hurdles” in the field. Yet, he maintained that the collection of robust data via randomised controlled trials in IO can be “difficult or impossible”. He furthered that real-world data and registries should be used to reinforce recognition for IO, as they have already found “convincing evidence of the efficacy and effectiveness of IO therapies”.
“Healthcare is facing tremendous challenges,” Burgmans continued, identifying rising staff shortages and financial constraints occurring amid increasing demand for care.
“IO is part of the solution—it offers effective therapies at lower costs and shorter hospital stays,” said Burgmans, advocating for “investment” in IO today, confident that this investment will return in the future, in part facilitated by IASIOS. “IASIOS will help to convince policy makers, health authorities, funding agencies, and board directors that IO is a safe bet”.
“We are close,” Breen added, “but healthcare commissioners and patients still need awareness of IO’s major, yet still potential, contribution to cancer treatment”. Concurrent to Burgmans, Breen believes that high-quality adoption of IO will be achieved through “highly-literate lobbying” with interventional oncologists placed on guideline panels. This is particularly crucial in the context of technological advancements, as Breen stated that IASIOS will bring “structure to IO provision before there is haphazard, poor-quality adoption by other services”.
It’s great to have an overarching accreditation process to know that we’re not going rogue, or cowboyesque; to bring greater uniformity, credibility and validity to the specialty on both sides of the pond.”
For Rodrigo Gobbo (Hospital Albert Einstein, São Paulo, Brazil) IASIOS has allowed of applying to become his centre to “stay abreast” of technological advancements, seeing their clinicians gain “comprehensive training” and maintain “consistent high-quality outcomes” for patients. “The accreditation acts as a guide for navigating the dynamic landscape of IO,” Gobbo said, underlining the adaptive nature of the IASIOS which ensures accredited centres “remain at the forefront of innovation to benefit both patients and clinicians alike”.
Gaining momentum
IASIOS’s global expansion is evident; with their most recent accreditation in the USA, sights are set on connecting more global facilities with their IO network. Championing excellence in oncological treatments, IASIOS’ objectives are “not just checkboxes” as chief operations officer Mardis conveyed, but rather “milestones in our collective journey towards excellence in IO”. “Our vision extends beyond accreditation, to a collaborative community that accelerates IO development and enhances global access to IO care. In 10 years, IASIOS aspires to be a central hub where IO practitioners contribute to and benefit from benchmarking, workshops and knowledge exchange, ensuring excellence in patient care on a global scale.”
IASIOS is no doubt gaining international momentum in its pursuit of a worldwide standard for the highest quality of care for patients with cancer. Creating a unified voice for the furtherment of IO, IASIOS continues to report a growing community with centres throughout Asia, Australia, Europe, the Middle East, North America, and South America.
4 March 2024 | Issue93 Cover Story
EDITORIAL COVER STORY continued
Late-breaking analysis set to expose the ‘why and how’ of endovascular technical failure in CLTI patients
Continued from page 1
technical failure, as lower-risk patients are often cherrypicked for enrolment meaning biased results,” Powell states. However, in extracting causal factors of ETF from the BEST-CLI data, Powell et al have drawn conclusions which will “[confirm] suspicions” about industry-run studies in this arena.
In their analysis, Powell and colleagues analysed the causes for ETF in both cohorts, the impact on major adverse limb events (MALE), above ankle amputation, death and major adverse cardiovascular events (MACE: defined as myocardial infarction, stroke and serious cardiovascular adverse events). ETF was defined as the inability to successfully complete the initial endovascular procedure.
“We approached this with an open mind,” details co-investigator John Kaufman (Oregon Health and Science University [OHSU] in Portland, USA). “We were interested that proximal SFA [superficial femoral artery]


occlusion, which was such an influential determinant, and that failure to cross the lesion was far more common than dissection, residual stenosis, or distal embolization as a failure mode.”
The investigators also analysed lesion location, which Powell highlights as a strength of their trial. In determining this, he hopes that granular predictions can be developed for patients that are less likely to achieve technical success, to map potential failure modes for each specific case.
Kaufman suspects there will be great interest in their findings set to be presented at highly-anticipated
We anticipate some difficult and maybe even some sceptical audience questions.”
CMS carotid stenting coverage expansion initiates concern over standard-of-care requirements and accreditation uptake
Dated 11 October 2023, the US Centers for Medicare & Medicaid Services (CMS) recently finalised its decision regarding National Coverage Determination (NCD) 20.7 for carotid artery stenting (CAS), confirming coverage expansion. Previously, patients were only eligible for CAS through clinical trials or if they were highsurgical-risk individuals. The decision made by the CMS removes several facility standards and approval requirements, as well as leaving coverage for any CAS procedure not described in the NCD to Medicare Administrative Contractor (MAC) discretion.
PREVIOUSLY, THE CMS required facilities to hold an accreditation certification as a condition of reimbursement for CAS procedures. This meant that centres must meet a minimum standard for a range of criteria, self-attesting quality in areas such as staffing, equipment status, device inventory and facility infrastructure. Sceptical about the scope and diligence of the CMS’ accreditation requirements, David Sacks (Reading Hospital, West Reading, USA), past president of the Intersocietal Accreditation Commission (IAC) CAS board and a representative of the Society of Interventional Radiology (SIR), worked with stakeholders from professional societies to develop the IAC Carotid Artery Stenting accreditation programme, which began accrediting CAS facilities in 2011.
“Accreditation has two main purposes,” said Sacks, “one is that it provides guidelines for facilities to do a good job, know what best practices are, and fulfil recommendations. Second, it provides some degree of assurance to patients and insurance companies
that patients are getting care that meets a particular benchmark—both of those have value”. Yet, he noted that some facilities see accreditation personified as “a policeman”, viewing the process as “unpleasant and usually not well-accepted”, rather than seeing accreditation as a means to provide “tools” to help organisations achieve better patient outcomes.
In the early days of CAS—around 2007—Sacks detailed that there was concern among specialties and societies that the procedure may be “abused” by providers with “unbridled enthusiasm” despite poor outcomes at that time.
“Many of us felt that accreditation could be a useful tool for reigning in some of this enthusiasm and allow patients to get good care. There was no accrediting organisation that focused on [CAS], so I organised multiple conference calls among various societies to see how we could move forward.”
Engaging multiple societies, Sacks and colleagues agreed that a multispecialty, multisociety accreditation programme would be effective. Representatives from the
SIR 2024 in Salt Lake City, USA, due to the significant influence ETF had on outcomes in the BEST-CLI dataset, and on real-world endovascular practice. He adds that their results will likely stimulate even more questions in an attempt to elucidate the reasons for ETF even further. “We anticipate some difficult and maybe even some sceptical audience questions at the SIR annual meeting” he speculates.
Taking this in stride, Kaufman views that this is all an essential part of science and important research, but there are questions that they will not be able to answer yet, such as the morphology of uncrossable occlusions that cause ETF. “We don’t yet know whether these are flush or heavily calcified, nor the specifics of the techniques used to attempt to cross the lesions. I think we all approach occlusions with a measure of respect, and these results support that.”
Ahead of the presentation of their results, USA, Kaufman hopes to shed some light on the context for why ETF happens in order to help provide guidance when evaluating patients, and point to areas of improvement and innovation in endovascular procedures. In using the BEST-CLI data for their current research, Powell adds that credit is due to the three principal investigators—Matthew Menard (Brigham and Women’s Hospital, Boston, USA), Kenneth Rosenfield (Massachusetts General Hospital, Boston, USA), and Alik Farber (Boston Medical Center, Boston, USA)—who accomplished a “huge undertaking” with a highly comorbid and sick patient population, which produced valuable, real-world data for the betterment of endovascular treatment.
American Academy of Neurology (AAN), American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/ CNS) Cerebrovascular Section, American Association of Physics in Medicine (AAPM), Neurocritical Care Society (NCS), Society for Vascular Medicine (SVM), Society for Vascular Surgery (SVS), the SIR, Society of NeuroInterventional Surgery (SNIS), and the Society of Vascular and Interventional Neurology (SVIN), and later joined by the Society of Cardiovascular Angiography and Interventions (SCAI) “took off their societal hats to work together” to create the programme. Sacks averred that their end product—the IAC CAS accreditation programme— upholds “good processes of care via a thorough history”, requiring facilities to provide detailed logs of CAS procedures and periodical case reviews.
Following the launch of the IAC CAS programme, Sacks stated how they had hoped the CMS would defer to them for CAS accreditation. “Medicare in the USA requires facilities to be accredited to be reimbursed for CAS, but their accreditation system was pretty much just attestation, stating ‘we do good work’ and Medicare saying ‘OK’. We were hopeful that they would defer to us, but they never did.”
clinical outcomes”, although the authors acknowledge the limitation of the study’s small sample size.
In Sacks et al’s discussion of their results, they aver that CMS-certified facilities “may not necessarily comply with process measures”, determining the value indicated by having an “external entity audit facilities” and provide “oversight to ensure best practices”.
In a separate article analysing cases submitted for accreditation 90% of CAS asymptomatic cases and 28% of symptomatic stenosis did not meet CMS requirements for stenosis severity. Accredited facilities were found to have had significantly better compliance. Sacks and colleagues subsequently communicated these results with the CMS, however CMS decided to remove several training requirements and quality assurance measures, allowing centres to selfmonitor procedural standards for CAS.

Aimed to reveal the distinction between IAC-accredited and nonaccredited facilities certified by the CMS with CAS best practices, Sacks et al conducted an exploratory study in 2019. Their results demonstrated that IACaccredited facilities are more likely to “follow best-practices, use quantitative tools to select appropriate patients, and quantitatively measure patient-centred
Casting a realistic eye toward the near future, Sacks stated that he is uncertain of whether facilities will choose to become accredited for CAS: “There could be many facilities who say ‘we are going to get into this and we want to be the best—what tools are available?’ Those folks might seek us out and apply for accreditation the same way people hire consultants to help them do a better job. Consultants aren’t mandatory, but they are a resource. We are hoping with the expected increase in the number of carotid artery stenting procedures and the number of facilities offering [CAS], that there will be an interest in using the IAC as a resource to improve and maintain quality of care.”
The IAC CAS accreditation is available both in the USA and internationally.
5 Issue93 | March 2024 Cover Story
David Sacks
Left to right: Richard Powell and John Kaufman
PREVIEW continued
SIR


UK MHRA update: Paclitaxelcoated device increased mortality risk is withdrawn for PAD
Following a review, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) has updated guidance on the use of paclitaxel-coated devices, stating that such devices can be considered for the treatment of peripheral arterial disease (PAD), including intermittent claudication and chronic limb-threatening ischaemia (CLTI).
THE MHRA HAD PREVIOUSLY issued a statement in April 2022 on the use of paclitaxel drug-coated-balloons or drug-eluting stents in patients with CLTI which stated that they should only be used in patients where ‘the benefits may outweigh the risks’. The parameters for use outlined that exposure should be kept to a minimum, which referred to using the lowest dose device available and avoiding/reducing repeated exposure to a device. Furthermore, the 2022 guidelines noted that paclitaxel devices should not be used in the routine treatment of patients with intermittent claudication due to the reported risk of longer-term increased mortality.
The updated guidance has been issued following a review of the most recent published literature. The Interim Devices Working Group (IDWG) advised that the new studies did not support a statistically significant increased risk of harm associated with the use of paclitaxel-coated devices
in patients with PAD, irrespective of disease type or severity.
In this update, the MHRA makes reference to the 2023 evaluation of numerous randomised controlled trials and real-world studies which compared paclitaxel-coated devices versus control devices in a patient-level pooled analysis. Led by Sahil A Parikh (Columbia University Irving Medical Center, New York, USA), the analysis included a total of 2,666 participants with a median follow-up of 4.9 years. Their results showed that no significant increase in deaths were observed for patients treated with paclitaxel-coated devices, providing reassurance to patients, physicians and regulators on the safety of said devices.
Subsequently, the MHRA has removed its previous restrictions on indication, dose and repeated exposure for paclitaxel-coated devices for both intermitted claudication and CLTI.
In a comment to Interventional News,
Janine Jolly, the MHRA deputy director of benefit/risk evaluation said: “We were one of the first regulators to establish a paclitaxel Expert Advisory Group to provide expert advice and, due to a lack of sufficient robust longterm data, we adopted a more cautionary approach.
 Robert Morgan
Robert Morgan
“We undertook a thorough assessment of the available published data, sought advice from the IDWG and invited experts, who agreed with the assessment conclusions that the new studies did not support a statistically significant increased risk of harm associated with the use of paclitaxel-coated devices when used to treat patients with PAD irrespective of disease type, severity, or another associated variable. We therefore amended our advice.”
Speaking to Interventional News, Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK) stated that the UK MHRA update “controversy and concern can finally be put to rest” over the use of paclitaxel devices in patients with lower limb peripheral arterial
Controversy and concern can finally be put to rest.”
disease. Morgan previously acted as corresponding author for the Cardiovascular Interventional Radiological Society of Europe (CIRSE) editorial which advocated for the benefits of paclitaxelcoated devices which was published in Cardiovascular Interventional Radiology (CVIR) in 2023.
“The guidance is quite specific that paclitaxel devices are safe to use and the UK MHRA have provided no instruction requiring these patients be followed up specifically to investigate mortality down the line.”
Morgan added that the UK MHRA are recommending that practitioners using paclitaxel devices for peripheral arterial procedures submit data to the National Vascular Registry (NVR) and the Outcomes and Registries Programme (ORP) for record of their use.
Reflecting on the entire overhaul of paclitaxel-coated devices sparked by Konstantinos Katsanos (Patras University Hospital, Rion, Greece) et al’s 2018 meta-analysis, Morgan believes that the process has exposed a “flaw” in the way that large trials seeking new devices are conducted. Although, Katsanos’ study highlighted an increased mortality signal that turned out to be false, Morgan believes that a positive effect gained from this will be greater focus on mortality in future studies.




7 Issue93 | March 2024 Guideline Update
for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**
*Available
‘A new narrative’: TACE in the age of Y90 and immunotherapy
Hotly debated across the Society of Interventional Oncology (SIO; 25–29 January 2024, Long Beach, USA) annual conference programme, speakers took to the stage to contest the survival of transarterial chemoembolization (TACE) in the age of yttrium-90 (Y90) transarterial radioembolization (TARE) and immunotherapy, their compelling arguments making some audience members “more confused and others happy”.
RIAD SALEM (NORTHWESTERN University, Chicago, USA) began by iterating that TACE will undoubtedly remain the global standard in terms of availability and feasibility. A better, “spicier” question, he posed, is what happens when both TACE and Y90 are widely available. “In reality TACE is already being replaced by Y90, based on curative data and therapeutic narratives,” Salem averred.
Despite his pro-Y90 position, Salem acknowledged that he is a “big fan” of TACE and has conducted the two largest TACE trials for hepatocellular carcinoma (HCC) in the USA—the first in 2010, and the second in 2016, evaluating response, toxicity and overall survival of TACE for HCC—alongside his mentor Michael Soulen (Abramson Cancer Center, Philadelphia, USA).
Yet, aside from his own contributions to the literature, Salem scrutinised the level of data that has solidified TACE in practice today, regarding the two seminal 2002 trials which found TACE to be beneficial in patients with limited disease and without portal vein thrombosis (PVT), when compared to no treatment in a small sample size randomised controlled trial (RCT). However, when compared to larger analyses of Y90, Salem highlighted that Y90 outperforms chemoembolization in four variables, namely: reproducibility/standardisation, precision, intentional threshold dosimetry, and clinical benefit.
A new narrative
Presenting data from individual cases of his colleagues and well-known trials to date, Salem attested to the “compelling” narrative that is being created by Y90, that provides high-risk patients with the same survival rates as low-risk patients, as well as improvements in quality-oflife measurements. “Who cares about quality of life?” he asked the audience. “Patients care about quality of life. They want to feel good, they want fewer treatments, they don’t want to feel sick”.
“You have the Swiss army knife of locoregional therapy. That’s the way I see it”, Salem continued, asserting that when reviewing the “scorecard”, Y90 far outperforms chemoembolization in areas such as downstaging, bridging, PVT management and
immune therapies, but lacks the global accessibility of chemoembolization due to the greater infrastructure needs of a Y90 programme.
With a focus on patient experience, Salem wrapped up his presentation with a frank appeal to the “pragmatists” in the audience. He called to those that “actually have to talk to patients and manage humans” in the hopes that his argument has resonated, while acknowledging the “purists”, who must have had “little-to-no interest” in his point of view. To the latter he remarked “I wish you well and good luck”, but concluded that he is optimistic about how Y90— building upon what has been learned through TACE—is creating new treatment paradigms to improve cancer care.
Key trials backing TACE
intent setting. Published in January 2024, he offered the updated analysis of the DOSISPHERE-01 trial which evaluated overall survival using Y90 microspheres in patients with inoperable locally advanced HCC. Principal investigator Etienne Garin (Rennes 2 University, Rennes, France) et al reported that, at long-term follow up, a meaningful improvement in overall survival was sustained after applying personalised dosimetry in patients who were successfully downstaged and resected, but consistent with prior studies that showed no survival benefit in unresectable patients.
Soulen then postulated on whether immunotherapy will change the landscape, considering the positive immunostimulatory effects of embolization using a large series of matched cohort studies from the 1970s concerning patients with Stage 4 renal cell carcinoma who had nephrectomy—“and there weren’t any good drugs back then”, he added.
Additionally, looking at the recent release of EMERALD-1 data, Soulen asserted that the benefits of combining


Next, moderator Sarah White (Medical College of Wisconsin, Wauwatosa, USA) invited Soulen to the podium to “slap his mentee” and present in favour of TACE’s survival in the age of radioembolization and immunotherapy.
Sparing no time, Soulen prefaced his presentation with a direct response to this question, and remarked: “Hell yeah, [TACE] is still the only evidence-based treatment in the space for unresectable hepatocellular carcinoma [HCC]”. Yet he spoke of the lack of a comprehensive regulatory pathway for procedures and for medical devices, which enter clinical practice without compelling evidence. “This is the Achilles’ heel of interventional radiology [IR]” Soulen said, in contrast to the highly regulated phase 3 trials involving thousands of patients that must be carried out for anti-cancer drugs to gain approval.
Referencing new data however, Soulen contended that there are level 1 data indicating that TACE works and level 1 data that Y90 does not in unresectable disease; notably the RCT of TACE +/- brivanib which prospectively analysed >500 TACE patients, the Japanese-Korean cooperative group study of TACE for HCC, and the Precision Italia trials, all of which showed two to three-year median overall survival after TACE versus the universally negative RCT’s for TARE.
Yet, he maintained that Y90 does have a valuable role in a curative
prescribing dose for complex tumours. Following Avritscher’s position on gaining better data, the panel were then invited to discuss; Salem first stated that new ways of collecting meaningful analyses must be devised, as RCTs—although ideal—are not constructive or representative of most of the procedures carried out in clinics. Developing this point, Soulen reiterated the difficulty they face in gaining data for both devices and drugs, and how making headway with the former is one of the “biggest challenge[s] in IR”. “We have no data because our devices don’t have to have data to be sold, and the companies won’t pay for trials because they don’t have to”.
With the information they have now, Soulen added, there will never have been a situation where TARE will replace TACE. As every patient is fundamentally different, individualised treatment is key. “It’s not a black and white decision in everyday life,” he contended, “there are other clinical factors besides their cancer and you’re going to initialise your care using whichever tool in your toolbox is suited to that so that [the patient] can have the best quality of life and outcomes can be improved”.

of TACE and immunotherapy are apparent. “We now have data showing better outcomes with the combination— so where’s the data for radiation and immunomodulation?” Posing this rhetorical question in front of a slide that read ‘crickets chirping’, Soulen finalised his claim, acknowledging that there are trials coming in this area with much still yet to be learned.
Mechanistic data are crucial With a focus on immunotherapy, Rony Avritscher (MD Anderson Cancer Center, Houston, USA) spoke next, and first pointed out the lack of mechanistic data offered by Salem and Soulen. He noted that collecting standardised data for interventional oncology (IO) therapies is one of the “biggest challenges” interventional oncologists face, due to the complex nature of their procedures and equipment, as well as the specialists needed to a dminister treatments.
Without crucial mechanistic and therapy standardisation, scalability for IO procedures will continue to be an area of “struggle” Avritscher claimed, placing the field at a “disadvantage”. In order to make these gains, the speaker stated that the development of tools which include innovative technologies such as artificial intelligence (AI) should be sought, with the capability of
Avritscher added that even different types of embolization procedures have “fundamentally different” effects on the human body which the community must seek to understand in order to pinpoint which patients would benefit from what combination of agents to specialise their care. Avritscher averred however that the only way this can be achieved on IO’s “turf” is to invest in basic science and translational studies looking at patient subsets and their molecular profiles.
The three speakers agreed that every treatment option to their disposal is valuable. To Soulen, these options give patients agency to select a route that best suits them in terms of quality of life: “TACE is quick and sick, Y90 is slow and glow. They work similarly well and some patients want the slow, gentle route, because they don’t want to be sick, but others say ‘No, I want this tumour dead tomorrow’ and will tolerate the subsequent sickness.”
Wrapping up, White asked the room to raise their hands if their institution offers and performs TACE or Y90, showing a visibly divided audience. Of the panel’s most salient conclusory points, consensus on TACE versus TARE may never be reached for good reason, so that case-by-case the most applicable treatment can be deployed for individual patient needs. Reaching agreement on this, Salem resolved that rather than focus being placed on one winning out over the other, attention should be paid to the new narrative that is being created by Y90 that is crucially expanding the dialogue for IO treatment.
8 March 2024 | Issue93 Conference Coverage
SIO
Left to right: Riad Salem, Michael Soulen and Rony Avritscher

Thierry de Baere promotes research and mentorship during SIO Wallace
Distinguished Lecture
“WE HAVE TO DO THIS. THE only way to improve patient care is by developing research—a cancer cure is a team effort. Nobody will cure cancer alone.” This was Thierry de Baere’s (Gustave Roussy, Villejuif, France) foundational message when giving the Sidney Wallace and Michael J Wallace Distinguished Lecture at the Society of Interventional Oncology (SIO) annual conference (25–29 January 2024, Long Beach, USA). Throughout his presentation he gave ample and persuasive reason to the critical nature of research in interventional oncology (IO) and the motivating influence of mentorship to not only encourage
trainees to engage with research, but to drive the specialty into the future.
At a meeting which champions education and training for early career interventional oncologists, de Baere aptly addressed trainees from the outset of his presentation, highlighting the importance of choosing an institution for which research is “a part of its DNA”. Yet for those interested in conducting research, de Baere warned against embarking on a blind pursuit of data, stating that a common bias is thinking that “because we are a hammer, every disease is a nail”.
“We must understand where we are going, what our research is,
and what we should do with our research,” de Baere told delegates at SIO. Searching for answers, de Baere told of how he took stock with his team, asking them to list the pros and cons of doing research. Consensus among them dictated that although research is rewarding and essential to advancing the field of IO, it demands time away from clinics, and with “no
support” and little experience, trainees inevitably hit a wall and have limited opportunities to get involved.
Mentorship, de Baere proffered, is a salve for the barriers to research for young interventional oncologists and stated that having a mentor doubles the likelihood that these individuals will conduct research. This is not only beneficial for those in training, but works reciprocally—“I just listen and learn, it’s coming back and forth”, de Baere told the audience. Importantly, the speaker also drew attention to the “complexity” women experience when seeking a mentor, and noted disparities still present in the field.
“Research is fun and we like to do it, but that is not the goal. We have to do it to improve patient care,” de Baere added, finally concluding that as IO as a specialty matures, interventionalists must understand that “practice must change if IO is to be a leader in future research”.
9 Issue93 | March 2024 Conference Coverage
Thierry de Baere
Striving for excellence: Bien Soo Tan awarded SIR 2024 Gold Medal
Bien Soo Tan is a senior consultant at the Department of Vascular and Interventional Radiology (IR) at Singapore General Hospital (SGH). Ahead of his upcoming acceptance of the Society of Interventional Radiology (SIR) Gold Medal award, Tan speaks to Interventional News about his achievements to date.
IN: Throughout your career you have won several awards and will now accept the SIR Gold Medal Award. What does winning these awards mean to you?
I am very honoured and humbled to have been presented with these prestigious awards over the course of my career so far. I view these awards as a testament to the good work and high standards of IR practised in my department in SGH, in my country, Singapore, and in the Asia-Pacific region. The practice of IR is a team game, and I would not have been able to make any significant contributions without the help of many colleagues in the various institutions and organisations who I have worked with. I would also like to acknowledge the key roles my many mentors have played in my career. These awards are also a reflection of their achievements.
IN: You are past president for the Asia-Pacific Society of Cardiovascular and Interventional
Pushing limits: ‘Extreme IR’ and upcoming SIR session to reveal cautionary cases and “unimaginable” successes
IN THE NEXT STEP OF ITS evolution, Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) tells Interventional News about the process of editing his book Extreme IR: Extraordinary Cases in Interventional Radiology and Endovascular Therapies. In curating a collection of cases that “[push] the limits of what people can do”, Extreme IR in its latest iteration captures the unique format of the original, internationally recognised event, and its later translation to monthly publications in the Journal of Vascular and Interventional Radiology (JVIR).
First embarked upon in 1998, Extreme IR consisted of a 16-mile hike punctuated by a layover lecture given halfway up the mountain on the Cascade Canyon Trail in the Grand Teton National Park, set against the backdrop
Radiology (APSCVIR) among other leadership positions. How important is outreach and education for IR in the Asia-Pacific region?
Today, the standard of IR in the Asia-Pacific region varies widely throughout. We have many centres of excellence across the region, but unfortunately even more regions where there is no access to IR expertise. Education is key to addressing disparity.
 Bien Soo Tan
Bien Soo Tan
The APSCVIR is striving to be the driving force for IR education in the Asia-Pacific region. I am privileged to be part of the APSCVIR outreach initiative. This initiative commenced in 2016 with in-person IR workshops where APSCVIR faculty volunteered to teach in multi-year IR educational programmes in countries of need. It is important that outreach programmes are not one-off events but continuous programmes working hand-in-hand with host member IR societies. The APSCVIR outreach programme unfortunately ground to a halt during the COVID-19 pandemic. However, we soon realised the potential of virtual education and established a series of IR webinars that achieved even greater reach.
Last year, we resumed our in-person outreach programmes, and the plan moving forward is to have a hybrid of in-person teaching in targeted countries while continuing virtual webinars, which reach a wider audience.
IN: You have played a pivotal role in the expansion and modernisation of SGH’s IR department. What has been a standout achievement for your centre?
The contributions towards building our hospital’s department of vascular and IR must be attributed to all IR staff at SGH, which includes our radiographers, nurses, ancillary and support staff, and doctors. We are very proud that today, we are a standalone IR department providing a comprehensive range of IR
of a glacial lake. “I had packaged the lecture consisting of 35mm slides and put them into a sealed envelope with those tiny plastic viewers,” Haskal described. Dubbed The Hike to CME [continuing medical education], requirements were stringent—“if you did not make the hike, you did not get the credit” Haskal said, which gave the course, which ran only twice, its “iconic” legacy.
In combination with extreme sports, Haskal’s Extreme IR courses sought to “create inspirational events and community”, he stated. When later translated in the JVIR as monthly segments and now via the Extreme IR book, Haskal’s goal is to put catalysing cases in front of trainees and practitioners alike to bolster and grow the field of interventional radiology (IR) through “excitement”.
Collecting cases for their “topicality and timeliness”, Haskal said that examples in the book tell of “difficulty and disasters” to build a “larger scope of awareness of these scenarios as cautionary notes”. He expanded that the importance of this may be most felt by individuals working in small practices alone or with few partners who might experience the “devastating mortal complications” of certain actions. For those practicing without others with whom these complications can be discussed, Haskal hopes his book will hold a space through which they can
services. We strive to be an IR centre of excellence, embracing both a high standard of clinical service as well as an academic practice. The strongest attribute we have is that despite being a large organisation, everyone pulls together to work as a team, in the interest of our patients. This is in keeping with our institution’s motto ‘Patients at the heart of all we do’.
IN: What is the current status of IR in South-East Asia? Where will IR go next in your region?
IR has grown tremendously in South-East Asia over the past decade, but there continues to be many regions and countries where access to IR services is not easily available.
I see continued growth in IR, with the clinical practice of IR being developed more robustly in this region. That means that radiologists have to care for and manage patients pre and post procedure, and not just be technicians performing procedures. I am optimistic that where IR is less developed, the timelines for catching up will be shortened. IR techniques are minimally invasive and work well for patients, and eventually patients will demand for the right to access IR services.
In the next five years, IR must embrace value-based care, as there is intense competition with increasing options in healthcare. For IR to grow, we must continue to collect the data and build the evidence for our techniques, as well as prove that our techniques are cost effective.
Education is key to addressing these disparities.”
“learn from [complications] and place them in perspective, to be able to soldier on in the face of sometimes personally devastating outcomes”.
Other cases—such as the one that is on the front cover—are of an “exotic and unusual” nature “pushing the limits of what people can do”, he explained. Aptly titled ‘Excalibur’, the front-cover case involved a crushed vertebral body, through which a vertebroplasty cannula was “hammered” and into which “cement” is poured to halt motion and cease pain. In this case however, Haskal pointed to the dramatically overshot rod, which penetrated far past the vertebral body, skimming past the abdominal aorta, the largest artery in the body, he commented.
“I remember saying to the presenter: ‘We are going to dine out on this [case] for a while’ and the audience just fell apart, there was so much nervous anxiety in the air. He had been called in to try and get this rod out for a partner that had somehow done this unimaginable thing, but he could not grab it with anything, and did not have a backup plan. So, they

go to the plumbers closet.”
Recalling the audience’s disbelief over the Excalibur case and its ultimate—although “unimaginable”— success, he noted that the story has been frequently brought up throughout the years. Haskal intends the book to mimic the relaxed-fit yet shocking case content of his sessions, but in the form of a “coffee-table book”, providing accessible, conversationprovoking cases in IR to engage the wider community.

Haskal will once again bring the rapid-fire Extreme IR session to the Society of Interventional Radiology (SIR) 2024 annual meeting (23–28 March, Salt Lake City, USA), continuing the “extreme” nature of sessions in “both case material and the drama of the event”. Reflecting on the history of Extreme IR and its evolution to date, he opined that the memories or events that “stick” are the “dramatic ones or those that happened under stress or tragedy”. With this format in mind, Haskal seeks to imbue the SIR 2024 session with the “undeniable adrenaline of an educational experience; seeing something and loving it, or witnessing a cautionary event that could hopefully impact healthcare and improve safety”.




10 March 2024 | Issue93 Interview
Ziv
Haskal is an interventional radiologist and creator of the Extreme IR session
A radial first approach puts patients first.
It provides a high standard of interventional care to patients, providers and hospital systems. With radial access you can:
• Reduce complications1
• Achieve faster ambulation and discharge2
• Optimize resources and cost savings 3 Deliver on the promise of minimally invasive solutions. Choose Radial First.



RADIAL. CHANGE THE COURSE OF CARE.



www.terumo-europe.com
References:
1. Valgimigli M et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet. 2018;392(10150):835-848.
2. Diego-Nieto A et al. Safety and feasibility of transradial access for percutaneous coronary intervention in chronic total occlusions. Rev Esp Cardiol (Engl Ed). 2022;S1885-5857(22)00140-2.



3. Mason PJ et al. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018;11(9):e000035.

IS1535GB0623MVI
Should all radiologists in training or interventional radiology fellows be taught how to handle their ideas and inventions?

Kieran Murphy
Point of View
I believe medicine is an art not a science. WH Auden said it was the art of wooing nature not the science of healing. We practice an artisanal craft, having become attuned to the patient’s needs and woes, using expensive complex technology to be minimally invasive. The devices and technologies we use today have been created by less than 3% of our colleagues. I know this because when I was running the annual Society of Interventional Radiology (SIR) meeting in 2010 I took the membership of the society—6,000 doctors—and cross-referenced their names with patent filings at the US and European patent offices. Of this survey, 447 people held 2,500 patents or patent filings.
Irepeated this work in 2020. I have longitudinal data on a cohort of inventors over time. We built social network maps of the inventors and identified those who invented during residency, fellowship and in their staff positions. Peak creativity for most interventional radiologists occurs during their fellowship. That’s when they perfect their skills, they work
FORS-powered
with mentors, they find their voice and culture as physicians, and they are challenged by impossible cases. It’s the impossible cases, which we do not decline to attempt to fix, that result in the creation of new devices and techniques.
The focus is traditionally on new device development, intellectual property, on royalties exits and initial
LumiGuide 3D imaging technology is rolled out at specialised centres
in Europe and USA
Andres Schanzer (UMass Memorial Health, Worcester, USA) has hailed it “one of the most exciting changes” seen in imaging during the course of his career. Philips’ LumiGuide “human GPS” technology— which uses light reflected along an optical fibre inside a guidewire to generate threedimensional (3D), high-resolution, colour images of devices inside a patient’s body in real-time powered by Fiber Optic RealShape (FORS)—is now available to specialised hospitals in Europe and the USA, the company has announced.
THE LUMIGUIDE SYSTEM ENABLES doctors to navigate through blood vessels during endovascular procedures using light, instead of X-ray, and was first used to treat patients in late 2023 at Maastricht University Medical Center in Maastricht, The Netherlands, closely followed by the University of Alabama at Birmingham in the USA.
public offerings (IPOs). That is misplaced. Let’s be clear that invention may be a technique not a device, and it may be shared by word of mouth and example for publication without there ever being any financial reward.
I interviewed many SIR inventors, and as in other fields, I found that the driving force for most was regret. People like us lie awake at night perseverating over bad outcomes and think of ways we could have done the procedure differently. Throughout medical history this is the case. Laser Greenfield developed his vena cava filter after a patient with multiple leg fractures had died from pulmonary embolism. He lamented about this in a bar with his friend who worked in the oil and gas industry. His friend told him of a filtering system used in pipes which Greenfield applied to the inferior vena cava. Harvey Cushing developed the intraoperative anaesthesia record after a patient he was sedating with chloroform died in his arms. Andreas Grüntzig, the inventor of the coronary angioplasty balloon, and many others were driven by grief from bad outcomes to develop better devices.
What became clear from analysis of the 2020 social network maps was that key institutions played a major role
Talented residents and fellows need to be nurtured, enabled and opportunities created for them.”
LumiGuide initially has been made available to aortic centres of excellence that perform complex aortic repairs in Europe and the USA, a Philips press release states. The radiation-free technology enables physicians to reduce their reliance on X-ray during complex aortic procedures that can take significantly more time, resulting in a higher radiation dose for patients and clinicians. LumiGuide can be used to see devices including off-the-shelf catheters from any angle and in multiple views, the company adds.
in enabling trainees to express their creativity through device development. These institutions are the classic ones, the inner-city hospitals of last resort, the major training programmes across the USA and Europe. Those inevitably are the locations or cultures that have developed the supports for this creativity. They can expedite and enable our trainees to succeed in new device development. The halos of those institutions’ open doors with industry that would be regrettably shut if you were training in a smaller, less globally relevant institution.
I developed my Murphy vertebroplasty needles while a fellow at the University of Geneva working with Professor Daniel Rufenacht. He told me I was going to be the vertebroplasty person. I didn’t want to do that but when I began to do those procedures I realised how inadequate the equipment was. Daniel opened the door for me with industry which gave me access to a catalogue of tools and devices. I was able to pick and choose and create better needles, better injectors and better cements for vertebroplasty. Great mentors create those opportunities. I try to do that for my trainees today. Some are incredibly talented. That talent is like the rare earth elements in the periodic table that creates the future of interventional radiology. Talented residents and fellows need to be nurtured, enabled and opportunities created for them. That is the critical part of our role as teachers.
Kieran Murphy is vice chair and vice chief of radiology and medical imaging at the University of Toronto, in Toronto, Canada.
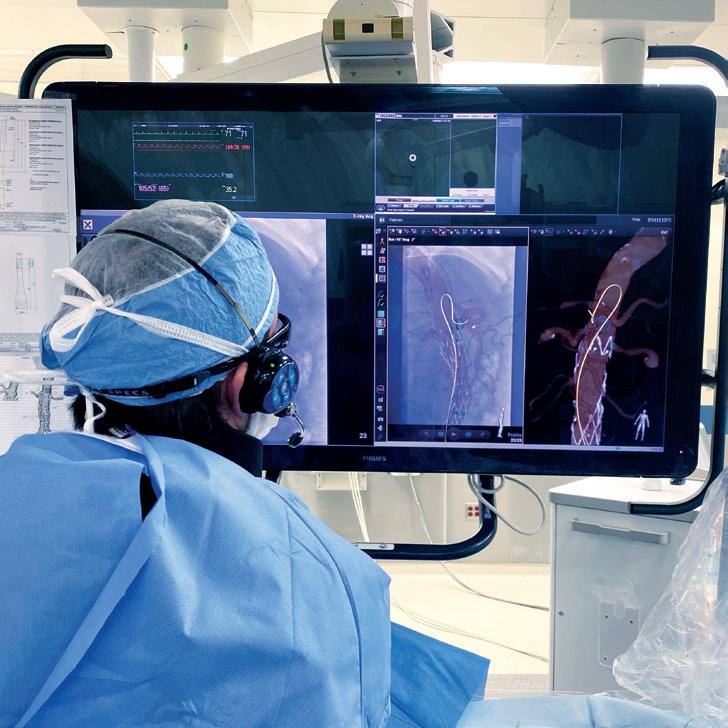
Following a limited release of FORS to nine aortic centres, more than 900 patients have undergone procedures using the technology, with one site conducting a historic cohort comparison showing a 37% reduction in complex aortic procedure time, and a 56% reduction in radiation exposure compared to X-ray.
As detailed in a presentation at the 2023 VEITHsymposium (14–18 November, New York, USA) by Joost van Herwaarden (University Medical Center Utrecht, Utecht, The Netherlands) the Limited Edition FORS technology—US Food and Drug
Administration (FDA) 501k-cleared in 2020— saw more than 800 completed cases by October of last year. The newly released LumiGuide includes workflow enhancements such as artificial intelligence (AI)based automatic registration, along with visualisation of a wider array of catheters, van Herwaarden pointed out Geert Willem Schurink (Maastricht University Medical Center, Maastricht, The Netherlands) who performed the first procedure with LumiGuide, said: “This artificial intelligence-based semi-automatic registration is very quick and accurate, even in the presence of stent grafts. Especially, if there is a need to re-register the device being guided in the patient’s body during the procedure, it is extremely helpful.”
12 March 2024 | Issue93 Point of View
REDUCTION IN RADIATION EXPOSURE COMPARED TO 56% X-RAY REDUCTION IN COMPLEX AORTIC PROCEDURE TIME 37% HAVE UNDERGONE PROCEDURES USING THE TECHNOLOGY 900PATIENTS Philips’ LumiGuide technology

NEW Adept Medical PRONE HEAD SUPPORT
A reusable and radiolucent head support device for managing your patient in the prone position

With a waterproof surface that is easy to disinfect between patients for multiple use, our latest device is here to bring you an alternative to current single-use solutions.
Made with viscoelastic foam to reduce pressure related injuries, our device conforms to the patient’s face for comfortable, yet supportive cushioning.
Compatible with most imaging tables and scanners, try our compact solution today.

AVAILABLE SOON Or ask us for a demo at SIR 2024 Salt Lake City Contact us today at adeptmedical.com |
More or less retired: RSNA gold medallist Anne Roberts reflects on her key career moments
With more than 35 years of tenure at the University of California, San Diego (UCSD) School of Medicine, Anne Roberts retired in 2022 and is a USCD distinguished professor of radiology, emeritus. Having held numerous leadership positions, the development of her self-titled catheter for uterine artery embolization, and now, her most recent gold medal from the Radiological Society of North America (RSNA), Interventional News interviewed Roberts to survey her key moments from a trailblazing career in interventional radiology (IR).
IN: You were previously awarded the Society of Interventional Radiology (SIR) Gold Medal in 2015, the American College of Radiology (ACR) Gold Medal in 2022, and now, the RSNA Gold Medal—what does winning these awards throughout your career mean to you?
I don’t think that anybody goes into their career with the idea that they’re going to win a gold medal of any kind, but the medals are a lovely recognition that I made some contributions to the field. In a way, [the awards] are all tied together because I had been involved in leadership positions with each society during times of development. For example, I had been president of the SIR and then was in leadership with the ACR when the idea of IR becoming a separate specialty arose, and I helped support this, despite the ACR not being initially too enthusiastic. The RSNA Gold Medal was probably also partially in recognition of my involvement in the push toward an IR primary specialty. At the end of the day, the main thing that awards do is say, well, you got involved, you helped, you did something, and some people recognised your work. I’m incredibly fortunate to be recognised since there are so many people who are involved in multiple societies that improve the specialty.
IN: Early in your career you took a sabbatical with the US Food and Drug Administration (FDA) and the Center for Devices and Radiological Health (CDRH). How did your experience influence your IR practice and why did you specifically become involved?
The reason I got involved with the US FDA was because I was eligible for a
about how IR devices weren’t being approved and that it was the FDA’s fault. This was a part of the reason I wanted to get involved, I was going to fix the FDA—talk about hubris. So, I got involved with the CDRH and it was my job to be a reviewer.
I discovered the reason devices weren’t getting through was because interventional radiologists were doing a very poor job of running their studies. They would enrol patients, some would be randomised but then the patients would be off protocol—it was sort of the Wild West, there just weren’t good data to support the FDA’s protocol. I think we’ve gotten better; we have learned a lot about running clinical trials, we’re certainly better than we were.
IN: You were at involved in advancing uterine fibroid embolization and the development of the Roberts Uterine Catheter (RUC)—what did this process look like?
When I was a fellow as Massachusetts General Hospital, Arthur Waltman took several of us to a medical device company to see the manufacturing process, and I watched the Birds Nest filter (Cook Medical) be made—by workers who were soldering the wires, making them all by hand—and I was blown away by that. I realised there was an industry out there that made things to specifications.
When uterine artery embolization came along, a lot of people were using the Waltman loop. I learnt the technique from Arthur but we were using a larger, thicker catheter, and it would tend to hold its shape. I had tried to do this with smaller catheters but the loop tended to unloop, and I couldn’t get it to do what I wanted—I guess that was one of my failures as one of Arthur’s trainees!
 Roberts
Roberts
accomplishment. I was very young when I became president and there weren’t many women in the society. Arina Van Breda (Sentara Northern Virginia Medical Center, Woodbridge, USA) was the first woman who was president in 1992–3 and she did a great job and was a terrific role model. This was a time of significant change, the society was opening up and some of the old guard weren’t too happy about that, but there were other members that knew this is where we should be going.
IN: You have been involved in several training programmes in Africa—what would you say are the priorities when it comes to making sure interventional radiologists-in-themaking receive the highest standard of training possible?
In Africa, training programmes can be difficult to set up due to the infrastructure of many of these countries. Electricity was a huge problem, for all kinds of medical equipment, but particularly for IR equipment—you can’t have the power suddenly going out. It’s also incredibly hard for them to access IR training, since it is so hands-on—the USA isn’t accepting short-term hands-on trainees from foreign countries, and it’s become difficult to find training in programmes in Europe and Asia.
What is needed is someone like me who’s retired—more or less—and put us someplace for several months at a time. In this way, the people in the community can become trained, and be trained on the problems that are present in their community, with the equipment they have available to them.
It’s important that we have people who have a desire for innovation and see IR as a place where this can be done.”
IN: What has been your experience as a woman in the field of interventional radiology?
So, I approached a manufacturer and said: I want a 5Fr catheter that has a long extra limb and I want it tapered down to a 4Fr so that it’s easier to get into the uterine artery. I want a marker put on the apex so that I can tell when I have got it over the bifurcation so it’s ready to reform. They made it and then they put my name on it.
I didn’t ask them to do that, and I don’t get anything for it. People have said ‘Oh boy, you could’ve made a lot of money’, all I wanted was for people to have something useful. It’s kind of sad that it’s become very difficult to get innovative things made, everybody is very risk averse and understandably so, but it has moved away from the individual being able to design something, and toward company development.
You have previously served as president of the SIR, vice-president of the ACR. Across these appointments what has been your greatest achievement?
The fact that I became president of SIR is probably my greatest
The great thing was at the beginning I never had to wait for a bathroom. Today, it’s unfortunate that many women are worried about going into IR, I think this is misplaced. There are great women in IR and I trained in a place where there were many women doing IR. I never felt that I couldn’t be an interventional radiologist, but there has been discrimination. I once called to apply for a private practice job and introduced myself, and this guy says to me ‘We’ve never hired a woman. We’ll never hire a woman and we’re not interested’. So, there were hurdles along the way.
IN: Where does IR go next? What one innovation do you see for the field in the next five years?
I’m not a very good prognosticator. Five to ten years ago if you spoke about radioembolization I would have said, what’s that? Things have changed a lot over my career, and will continue to change. I’m not smart enough to think that I know—or maybe I’m smart knowing that I don’t know.
Innovation has always driven IR, that’s what we do and it’s what drives the field forward. It’s important that we have people who have a desire for innovation and see IR as a place where this can be done. I don’t know where IR is going to go, but wherever it is, it’s going to be great.
14 March 2024 | Issue93 Interview
Anne
Roundtable sparks audience discussion on the pitfalls and successes in global IR training pathways
In a roundtable dedicated to comparing training pathways for interventional radiology (IR) in Saudi Arabia, the Middle East and North Africa (MENA), Europe and North America, discussion levelled on the challenges faced by many and heard first-hand experiences of trainees around the globe.
PRESENTING FIRST ON THE
Sunday of the Pan Arab Interventional Radiology (PAIRS) annual congress (10–13 February, 2024, Dubai, United Arab Emirates), Raman Uberoi (The John Radcliffe Hospital, Oxford, UK) elaborated that interventional radiologists are image-guided surgeons, and on the critical nature of IR assessment worldwide. He noted that in the past, interventional radiologists have no or very minimal exposure to IR in the early years of their training, and have no formal assessment in the quality of training they receive to confirm that they have achieved basic core competencies in IR. “Patients would be shocked if surgeons had no assessment in place to prove their core skills.”
In Europe, Uberoi’s work with the European Board of Interventional Radiology (EBIR) to consolidate
a comprehensive examination for training IRs was aimed at “evolving the way [interventional radiologists] work and train, focusing on the critical core skills that future interventionists will need”.
Abdulkader Alkenawi (King Abdulaziz Medical City, Jeddah, Saudi Arabia) explained that the bodies which determine the IR curriculum and examination are entirely separate in Saudi Arabia, but the parties frequently come together to ensure what trainees are being taught aligns with their assessment.
Although important, assessment can only take trainees so far, one audience member stated. He provided that his centre in Jordan sees very limited numbers of complex cases, and queried whether trainees would be accepted to facilities such as Alkenawi’s so that they may gain exposure to a broader

PAIRS session explores the efficacy of Botox in paediatric IR treatment
In a session dedicated to neonate, infant and paediatric interventional radiology (IR), Hisham Alshehri (King Abdulaziz University, Jeddah, Saudi Arabia) gave a talk at the Pan Arab Interventional Radiology (PAIRS) annual congress (10–13 February, 2024, Dubai, United Arab Emirates) on the wide range of paediatric conditions that botulinum toxins (Botox) can treat.
FIRST EXPLAINING ITS MECHANISM OF action, Alshehri detailed that Botox works by blocking the release of acetylcholine at the level of the neuromuscular junction, resulting in the relaxation of local muscles within two weeks of treatment. The injection effects typically last between three to
range of pathologies. To this, Alkenawi explained that due to Visa constraints and a consistently significant workload for their small IR team this is not yet a possibility, but hopes that in the future they may begin accepting trainees from other countries.
Mamoon H Al-Omari (King Abdullah University Hospital, Ar Ramtha, Jordan) then went on to describe how IR is still yet to be included within diagnostic radiology (DR) training today. At his hospital, those who are interested in IR can take one to two months elective training; however, he stressed the lack of exposure to IR for most trainees generally.
Sparking contetion, attendees and speakers turned to IR’s position within the house of DR. Saher Sabri (MedStar Georgetown University Hospital, Washington, DC, USA) asked the audience whether an IR-only residency would be attractive, including one/ two years IR training and a year or so training in DR, but would certify trainees in IR alone.
Taking stock of hands raised, Sabri concluded that most would prefer dual certification and when asked why, an audience member said that this is beneficial as trainees would have something to show for the significant portion of time spent training in DR.
Another audience member opined however, that if IR was selected from
the off, there would be more dedicated interventional radiologists who could create better recognition for the specialty. They stated that maintaining a qualification in DR when training in IR acts as a ‘safety net’, enabling trainees to have a field to fall back on, negatively affecting the growth of IR. Yet, another audience member viewed the ‘plan B’ quality of dual certification as positive, and explained that with limited jobs in the MENA region, having the ability to do both DR and IR procedures places interventionists in a better position to secure a place in a competitive job market.
“It’s not an easy path doing IR,” Alkenawi said, airing his concerns that IR has lost “too many battles” with other specialties to date, which Uberoi consolidated, noting the difficulties interventionists can encounter if they are “at the whim” of referring specialities. Sabri, however, in attempts to “uplift a little bit”, brought the discussion back to the positive practice models that are already in action for many centres, to which Alkenawi agreed that IR as a field has shown its capability to adapt to challenges.
Uberoi concluded that “the future is bright, but we must think in a radically different way. The next generation can hold the future of IR in their hands, which will mean [they] must grapple with these issues now, talk to [their] trainers and develop [themselves].”
six months.
The speaker detailed however that interventional radiologists should act with “careful consideration” when deciding dose for paediatric patients. “Botox affects children in a different way when compared with adults,” Alshehri explained, using hypertonia—a condition in which there is too much muscle tone which stiffens the arms, legs or neck, making them difficult to move—as an example. For patients with this condition, the underlying hypertonic disorder is superimposed onto two unique processes of childhood growth and development in contrast to adults.
Looking at appropriate dosage for paediatric patients, Alshehri noted that the total maximum body dose per visit must not exceed 12 units per kg or 400 units total, and the maximum volume per site should not exceed 0.5ml, except for certain cases that may require more.
Alshehri then discussed the niche indications that have shown to benefit from Botox treatment, such as upper and lower limb spasticity in children with cerebral palsy, congenital muscular torticollis, sialorrhea and brachial plexus palsy. The toxin can be used in all ages, but early research carried out by Barry Rawicki (Monash Medical Centre, Clayton, Australia) et al suggests a better response in children younger than eight years old.
Then, drawing the PAIRS 2024 audience’s attention to the current safety status of Botox treatment in paediatric patients, Alshehri detailed that serious side effects have been observed to date. These can include fever, muscle weakness, swallowing and respiratory difficulties, seizures, or death. Between 2006 and 2018 the US Food and Drug Administration (FDA) Adverse Event Reporting System recorded 51 cases of adverse reactions associated with Botox injections to children 17 years of age and younger.
However, he added that research in this area is still
in its infancy. A meta-analysis carried out by JeanSébastien Bourseul et al found that, in 437 children who received Botox injections for musculoskeletal conditions, no severe side effects were reported. In another study by the same author, they found that in 74 infants younger than two years old who received Botox for spasticity, there was a 3.6% incidence of mild side effects in patients younger than one year and a 6.5% incidence in infants between one and two years old.
Surveying these results, Alshehri pointed out that there is no international consensus for the use of Botox in paediatric patients younger than two years of age. Due to the safety and effectiveness of this treatment however, Botox can be used off-label as an adjuvant to physical therapy and surgery in moderate to severe cases.
He concluded that many of these musculoskeletal conditions come with permanent sequelae causing a “high level” of disability. Botox for the treatment of these paediatric patients offers a minimally invasive means of providing relief and preventing long-term disability. Concluding, he asserted that interventional radiologists should use tools such as ultrasound to increase the effectiveness and accuracy of Botox treatments, to crucially minimise adverse events.
There is no international consensus for the use of Botox in paediatric patients younger than two years of age.”
15 Issue93 | March 2024 Conference Coverage
PAIRS
ALDA TAM
Alda Tam is an interventional radiologist and professor at the University of Texas MD Anderson Cancer Center in Houston, USA, and is current president for the Society of Interventional Radiology (SIR). As an active and prolific researcher, Tam speaks to Interventional News about the trajectory of her career, maintaining her practice alongside her involvement with clinical trials, her development of clinical practice guidelines and more recent app development.
What attracted you to a career in interventional radiology (IR)?
Third year medical school came with the task of having to choose a future career and I had to decide whether I would be more interested in using my hands and operating (surgical specialty) or seeing patients and engaging in cerebral work (medical specialty). With an undergraduate degree in neuroscience and leaning towards surgery, I thought about neurosurgery and signed up for a summer research internship, complete with shadowing in the operating room. During one of the cases, I remember seeing brain tissue in the Yankauer suction and tubing and thought “I don’t know about this...maybe there’s something out there with more finesse?” During my radiology rotation, one of the faculty suggested I check out IR since they knew my affinity for surgery. It only took a few assignments in the IR suite to realise this was it for me; the complexities of surgery with the finesse I desired. That’s how I ended up entering the field.
Who were your mentors?
I completed my radiology residency at the University of Southern California (USC) and had an awesome team of IR mentors: Sue Hanks, Vicki Marx, Michael Katz (all Keck Medicine of USC, Los Angeles, USA), and Donald Harrell (Mammoth Hospital, Mammoth Lakes, USA). I witnessed a highfunctioning team delivering top notch care while having fun doing so, and thought that I could see myself in a career like that. During my fellowship at MD Anderson, I continued to find guidance from Marshall Hicks, Michael Wallace, Sanjay Gupta, and Steve McRae (all University of Texas MD Anderson Cancer Center, Housten, USA). They gave me my first job, and the rest is history. As my network has grown over time, there are many colleagues, friends, and peers—too many to name—who I continue to rely on for counsel and support. I only hope that I can serve in a similar manner to future interventional radiologists.
What led you to oncology? Was it always your intention to specialise there?
Becoming an interventional oncologist was pure serendipity! My husband is a medical oncologist and the years of training for internal medicine/oncology and radiology were not perfectly aligned. He was matched to Houston first and I joined him there after finishing my final year at USC. But I then finished my fellowship first and started my faculty position at MD Anderson while he was in his last year of training. Then we both ended up staying and we’ve been Houstonians for almost 20 years.
Could you describe a particularly memorable case of yours?
I’ll always remember my first case as a solo operator. I was the second year resident on-call and had been given the go-ahead to start the case by my attending, who was finishing up in the other room. Although it is what most interventional radiologists would consider a bread and butter case—a 65-year-old man in urosepsis, complete with hypotension and tachycardia, from ureteral obstruction related to stone disease, needing a nephrostomy tube—it was definitely an exciting yet nerve racking moment for me because once the patient was prepped and draped, we got word that there had been a hold up in the other room and my attending said, “start if you feel comfortable but if not, you can wait”. In all honesty, I would have preferred to wait, but the patient’s vital signs were not good and getting worse, so I made the decision to act and put in the nephrostomy tube. I will never forget the support of the seasoned radiologist technologist and nurse team who encouraged me and got me through the case. The patient defervesced after the pus in the collecting system was drained, was discharged out of the intensive care unit to a regular floor, and then subsequently home. As I realised what could be done with a needle and wire, this case became one of the moments that cemented my decision to specialise in IR.
What are some of the challenges which face interventional radiologists providing cancer treatment today?
Awareness and access. We continue to face the challenge of physicians and patients being unaware of the value IR can add to oncology care across the spectrum of diagnosis, local treatment, and palliation. That’s why it is key for us to emphasise the importance of IR research and IR-driven clinical trials to obtain the data that will permit the inclusion of our therapies into treatment guidelines. This would ensure that patients can be presented with all the available treatment options. We also have a challenge with patient access to IR. In the USA, approximately 30% of the country’s population do not have access to an interventional radiologist in their state (Ahmad Y et al). When you scale this concept, I suspect that there are many cancer patients globally who could benefit from the treatments that IR has to offer, if only their access to IR care could be improved.
Your largest body of work regards biopsies in oncology clinical trials. How important is biopsy acquisition?
This body of research has led to efforts to standardise biopsy acquisition within the National Cancer Institute Clinical Trials Network (ETCTN). The difference between a biopsy for clinical care and a biopsy for research purposes is that oftentimes, a patient
enrolled in a clinical trial that requires a research biopsy that may derive no direct benefit. Working at MD Anderson in the mid-2000s, we began to see an increase in the number of clinical trials that were incorporating research biopsies into their trial design and also realised that more tissue was usually needed to meet the goals of a research biopsy. This naturally brought up questions about how to optimise biopsy yield to inform trial endpoints while at the same time protecting patients from undue adverse events. Our collaborative group of medical oncologists, pathologists, and interventional radiologists at MD Anderson have performed research in this area.
FACT FILE
CURRENT APPOINTMENTS
Professor of interventional radiology, University of Texas MD Anderson Cancer Center, Houston, USA
Associate member faculty, MD Anderson University of Texas Houston Graduate School of Biomedical Sciences, Houston, USA
PREVIOUS APPOINTMENTS (SELECTED)
2016–2021: Member of the vascular/ interventional subcommittee of the scientific programme committee, Radiological Society of North America (RSNA)
2017–2018: President of the Western Angiographic & Interventional Society (WAIS)
2015–2018: Chair of the interventional radiology quality assurance committee
EDITORSHIPS (SELECTED)
Journal of Vascular and Interventional Radiology
Radiographics
Radiology
In one early study, we examined transparency, meaning how the risks and adverse events associated with biopsies in clinical trials are discussed within informed consent. We reviewed the IR database to identify all therapeutic clinical trials in which image-guided research biopsies were performed from 1 January, 2005, to 1 October, 2010. Among 57 clinical trials, 67% contained at least one mandatory biopsy. Most of the studies failed to convey the risks and benefits of research biopsies in study protocol and informed consent. We also evaluated how often the biopsy results of clinical trials are reported. We searched ClinicalTrials.gov for trials in oncology with completion dates between 1 January, 2000, and 1 January, 2015, with endpoint category terms including biopsy, biopsies, or tissue.
Among 301 trials, only 50.8% reported any biopsy-related results. In a similar analysis of 866 research biopsies performed across 46 clinical trials, 61% of trials did not report results from research biopsies. From an ethics perspective, questions arose about subjecting patients to unnecessary procedures with limited scientific gain and so the American Society of Clinical Oncology (ASCO) put together an ethical framework regarding the inclusion of biopsies in clinical trials. The ethical framework addresses three main areas: how to maximise the scientific utility associated with biopsies, how to minimise the participant risk, and how to improve oversight of the process. To help patients and clinical trialists determine whether a research biopsy fulfils the goal of having an overall favourable risk-benefit ratio—and therefore meets ethical justification—the framework explicitly defines the scientific contribution to be expected from a biopsy, categorises participant risk, and outlines when mandatory biopsy may be justified. As a result, the research led to the adoption of standard operation procedures for biopsy acquisition within the National Cancer Institute Clinical Trials Network (ETCTN). The idea is that by providing “best practice” guidance, we will minimise the number of patients who undergo unnecessary or nondiagnostic research biopsies as part of their participation in a clinical trial.
What do you hope to achieve as SIR president?
As SIR is a big organisation, I think it’s important that the president not necessarily have a personal agenda but rather, that the executive leadership works cohesively towards multi-year strategies that will drive forward initiatives for the society and specialty. As we begin to celebrate 50 years as a society in 2024, many of the initiatives in the past several years
16 Interview March 2024 | Issue93
PROFILE

have been focused on establishing a foundation to allow us to be able to compete as a primary medical specialty. Examples of this include the integration of the clinical specialty councils into the governance structure which allows for the voices of our key opinion leaders to be heard and participate in strategic decisionmaking; the variety of educational products, including the launch of SIR Edge, a new fall 2024 meeting offering; and the ongoing website and user digital experience update. I hope by the end of my time in leadership, I will have contributed to significant infrastructure changes that will set IR and SIR up for success in the coming years.
What was the rationale behind the creation of the SIR Guidelines app?
I became the standards division counsellor for the SIR in 2017, which is how I got involved in the creation of clinical practice guidelines. I’m really proud of the work that committee achieved because, together, we moved the Society to develop the National Academy of Medicine—compliant clinical practice guidelines—and SIR may very well
be the first radiology society in the world to do so. This is tremendously important because we’re still jockeying for position on where IR falls in medical treatment algorithms and the acceptance of IR therapies. For us to be viewed as legitimate and competitive as a primary specialty, we needed to put our data through the same methodological scrutiny that is considered the industry gold standard. This was a big step for us in terms of the maturation of the Society and the specialty.
Our first clinical practice guideline was on inferior vena cava filters in the treatment of patients with venous thromboembolic disease. When you read this guideline, you’ll see that it’s different in the sense that it’s questionbased with a literature search specific to that question and provides a recommendation that weighs out benefit and harm. Leading the Guidelines Committee was a professional and personal growth experience for me and one of the most rewarding leadership/volunteering experiences I’ve had.
I was also able to work with residents from University of California San Diego, San Diego, USA and some of our early career
“We continue to face the challenge of physicians and patients being unaware of the value IR can add to oncology care across the spectrum.”
section members to develop and launch the SIR Guidelines mobile app, which is now used worldwide.We have been downloaded in more than 130 countries and have over 17.5K users! The app gives clinicians point of care access to periprocedural anticoagulation and antibiotic management recommendations for each IR procedure. It returns the information a clinician needs to make a decision in about eight seconds. Ultimately, the guidelines app helps to disseminate information for practice guidance and can help with the adoption and adherence to evidenced-based recommendations.
What are your hobbies and interests outside of medicine?
I enjoy spending time with my family and travelling. We have been to many national parks and we like to hike—some of our more memorable hikes have been Angel’s Landing (Zion National Park) and Half Dome (Yosemite National Park). We are off to Banff this summer. I also like to read, particularly Japanese and Scandinavian murder mysteries, and continue to think about completing another half marathon.
17 Interview Issue93 | March 2024
alisonlang.com

May 16-19, 2024 Sheraton New York Times Square Hotel Gest2024.com #GEST2024 LEARN MORE


19 Workshop
The 13 Steps to Conduct an IR Bites Workshop:
Create a Poster: Use design tools like Canva to craft an engaging poster, facilitating effective promotion through various channels.
2 3
1
Book a Room and Set the Date: The first crucial step is to secure a venue and schedule the workshop, recognising that the date is pivotal for event progression.
Build Industry Contacts:
Attend IR events, connect with professionals, and leverage industry contacts to enhance workshop visibility.
5 4 Finalise the Agenda: Structure the workshop with a well-defined agenda, balancing informative talks with hands-on sessions.
6
Identify Speakers: Personally approach speakers, preferably two consultants and one registrar, to ensure diverse perspectives.
8
Implement Marketing Strategies: Employ diverse marketing strategies, including emails, newsletters, social media posts, and QR codes, following the “Jab, Jab, Jab, Right Hook” approach.
10
Plan the Venue Layout: Strategically plan the workshop space, accommodating seating arrangements and industry demo setups.
9
Secure Pizza Sponsorship: Engage industry representatives to sponsor refreshments, acknowledging the universal appeal of free pizza.
7 Streamline Event Registration: Utilise platforms like MedAll for seamless event registration, pre/post questions, and certificate distribution.
Coordinate Pizza Logistics: One week before the event, confirm pizza orders and ensure industry confirmations to avoid disappointment.
11
Collect Training Kit: Source training and expired kits from the IR department, photographing them for reference and display during the event.
12
Prepare for Dropouts: Anticipate lastminute changes by maintaining a reserve list of attendees, speakers, and industry representatives.
13
Execute the Event: Enjoy the workshop, savour the pizza, document attendance, and encourage immediate feedback using QR codes for a more impactful experience.
Researchers report new outcome data for pair of investigational carotid stenting systems
Results from two trials assessing the performance of investigational carotid artery stenting (CAS) systems—the Neuroguard (Contego Medical) and the CGuard (InspireMD)—were presented at VIVA 2023 (30 October–2 November, Las Vegas, USA).
Chris Metzger (OhioHealth, Columbus, USA) shared late-breaking 30-day results from the C-GUARDIANS US investigational device exemption (IDE) clinical trial evaluating the CGuard embolic prevention stent (EPS) system for stroke prevention.
C-GUARDIANS is a pivotal trial designed to evaluate the safety and efficacy of the CGuard carotid stent system when used to treat symptomatic and asymptomatic carotid artery stenosis in patients undergoing CAS and at a high risk for carotid endarterectomy (CEA).
From July 2021 to June 2023, 316 patients were prospectively enrolled in this single-arm CAS study, performed at 24 sites in the USA and Europe. The primary endpoint was a composite of either: incidence of major adverse events including death (all-cause mortality), any stroke or myocardial infarction (DSMI) through 30 days post-index procedure; or ipsilateral stroke from day 31 to day 365 postprocedure.
Metzger, who is principal investigator of the C-GUARDIANS trial, shared that stenting with the CGuard carotid stent system in patients with carotid artery stenosis and at high risk for CEA had a DSMI rate of 0.95%, from procedure through 30 days of follow-up.
“The follow-up data from C-GUARDIANS once again support the safety of the CGuard EPS stent, with its novel MicroNet technology, as reflected in the low rate of major adverse events observed through 30 days,” said Marvin Slosman, chief
executive officer of InspireMD, in a press release. “We believe the neuroprotective qualities of CGuard set it apart from competing stents on the market and should help accelerate the ongoing shift in carotid revascularisations from ‘surgery-first’ to an endovascular ‘stent-first’ approach. We look forward to reporting 12-month results as we continue to advance CGuard EPS toward potential FDA [Food and Drug Administration] approval in the first half of 2025.”
InspireMD anticipates reporting primary endpoint results from C-GUARDIANS—which may support a premarket approval (PMA) application—in the second half of 2024.
Late-breaking clinical results from the PERFORMANCE II carotid stent trial were also presented at VIVA 2023. PERFORMANCE II is a prospective, multicentre study evaluating the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) system in 305 patients at 40 clinical sites.
are deemed appropriate for intervention by delivering demonstrated best-in-class stroke protection.”
In the PERFORMANCE II study, the reported 30-day stroke rate was 1.31% in the intention-to-treat analysis and 0.98% in a per-protocol analysis, with no major strokes or contralateral strokes, and all patients returning to baseline neurologically within 30 days, according to a Contego press release. At one-year follow-up (all stroke within 30 days, and ipsilateral stroke between day 31 and 12 months), the reported stroke rate was 1.68% in the intention-to-treat analysis and 1.35% in a per-protocol analysis. No major strokes or neurological deaths occurred in the study.
The press release details that the Neuroguard IEP system leverages Contego’s clinically proven IEP technology—a unique platform with a micro-filter integrated on the delivery catheter, which is designed to deliver added safety during stent placement and balloon dilation. The micro-filter with 40µm pores captures the micro-emboli that other protection mechanisms do not, the release adds, giving physicians the procedural confidence that comes with advanced stroke protection in the treatment of their patients. The Neuroguard closed-cell stent also utilises FlexRing technology, providing optimised radial strength and flexibility while leveraging nitinol’s proven long-term material performance, Contego further claims.

“The one-year event rates in the PERFORMANCE II study are the lowest ever reported in any adequately powered, prospective, multicentre study of any type of carotid artery revascularisation, regardless of patient risk,” said William Gray (Main Line Health, Philadelphia, USA), co-national principal investigator of the trial. “These results can elevate the standard of care for patients with severe carotid artery disease who
No relationship found between DW-MRI lesions and long-term stroke risk following carotid revascularisation
A secondary analysis of existing clinical trial data has indicated that new ischaemic brain lesions detected via diffusion-weighted magnetic resonance imaging (DW-MRI) following carotid artery revascularisation—either with carotid artery stenting (CAS) or carotid endarterectomy (CEA)—do not appear to have a relationship with long-term stroke risks.
“THE RESULTS FROM OUR analysis do not support the role of ischaemic brain lesions discovered on [DW-MRI] after carotid revascularisation procedures as risk markers for long-term recurrent stroke or TIA [transient ischaemic attack],” Gert J de Borst (University Medical Center Utrecht, The Netherlands) et al write in the journal Stroke. “However, as new periprocedural [DW-MRI] lesions seem to be a marker for early recurrent cerebrovascular events, future randomised studies are needed to
evaluate whether the effect of treatment on these lesions corresponds to the effect of treatment on procedural stroke before surrogacy can be validated.”
The researchers initially posit that five-year follow-up findings from the randomised International Carotid Stenting Study (ICSS) indicate an increased risk of recurrent stroke or TIA following a CAS procedure, but that the trial failed to demonstrate any association between DW-MRI lesions and recurrent stroke/TIA risks following CEA.“The establishment
“The recent change in coverage by the Centers for Medicare & Medicaid Services (CMS) increases patient access to carotid artery treatment,” said Contego chief executive officer Ravish Sachar.

In addition to the PERFORMANCE II study—in which Neuroguard was placed via transfemoral or transradial access—the PERFORMANCE III study is currently enrolling patients to evaluate the same stent placed via direct transcarotid access.
“Our mission is to provide a new class of products which improve patient outcomes and procedural efficiency,” said Stacy Enxing Seng, operating partner at Lightstone Ventures and chairman of the board at Contego.
of the longer-term clinical relevance of the [DW-MRI] lesions after carotid revascularisation procedures is essential in the process of implementing a universally accepted surrogate outcome,” De Borst and colleagues note. “Today, thus far, there are no data describing adverse events in [DWMRI]-positive patients beyond five-year follow-up. Therefore, in this study, we intended to determine the long-term cerebrovascular outcome in [DWMRI]-positive compared with [DWMRI]-negative patients after carotid revascularisation procedures.”
The authors report that their secondary, observational, prospective cohort analysis included 162 patients with symptomatic carotid stenosis who were previously randomised to CAS or CEA in ICSS, and were subsequently included in the ICSS MRI substudy. They further relay that the primary composite clinical outcome of their analysis was the time to any stroke or TIA during follow-up. Patients with new DW-MRI lesions on post-treatment MRI scans (DWI+) were compared with patients without new lesions
(DWI–), De Borst et al add.
For the 162 patients included in the ICSS-MRI substudy between January 2004 and October 2008, 110 general practitioners (GPs) ultimately provided long-term follow-up data for the present study. Discussing the baseline characteristics of this patient population, De Borst et al note that those in the DWI+ group were more often treated with CAS, while total cholesterol at randomisation was higher in the DWI− group.
The authors state that the median follow-up time was 8.6 years, and that Kaplan-Meier cumulative incidence for the primary outcome after 12.5 years of follow-up was 35.3% in DWI+ patients and 31.1% in DWI− patients. With respective hazard ratios of 1.5 and 1.3, univariable and multivariable regression analyses did not show significant differences between these two patient cohorts, they add. Separate analyses relating to CAS and CEA showed that the higher primary outcome rate in DWI+ patients across the entire cohort was mainly caused by events in the CAS group.
20 March 2024 | Issue93 Vascular Updates VASCULAR
Chris Metzger
William Gray
One year on: Matthew Johnson reflects on the PRESERVE trial
“Is more research needed in the world of filters? Yes, but is more research needed to determine whether the class itself is safe? No.” Charting the recent history of inferior vena cava (IVC) filters through the PRESERVE trial (Predicting the safety and effectiveness of inferior vena cava filters), principal investigator Matthew Johnson (Indiana University, Indianapolis, USA) speaks to Interventional News on the ins and outs of filter removal.
“Hundreds of thousands of patients with venous thromboembolism [VTE] had filters placed, yet clearly there was an incidence of filter-related complications, such as breaks or perforations.” Johnson began by discussing the rationale behind PRESERVE, recounting the “maelstrom of concern” that was raised in 2010 following the US Food and Drug Administration (FDA) warning letter. The letter stated that all “implanting physicians and clinicians responsible for the ongoing care of patients with retrievable IVC filters should consider removing the filters as soon as protection from pulmonary embolism [PE] is no longer needed”.
Sparking “multidistrict litigation” against filter manufacturers in the USA, Johnson stated that there was a “huge lack of understanding” over the role of IVC filters as they relate to VTE. This, in part, may have been due to opaque definitions of VTE which, “for example, could be a sub-centimetre gastrocnemius
clot below the knee or the entire vena cava could be occluded causing massive symptoms”, he explained. “Our definitions made it difficult for us to evaluate things, because we weren’t exactly sure what the extent of disease was and how filters should be used in these populations.”
Primarily used to prevent deep vein blood clots from developing into PE, the patient population privy to this procedure is “incredibly variable” in Johnson’s experience. IVC filters are often placed in patients “immediately following trauma, as a preoperative measure in cancer patients, or for prophylaxis—which is another entirely complicated thing, placing a filter in a patient without VTE”.
In clinical practice, filters are most commonly placed in people who cannot be anticoagulated; randomised trials have sought to evaluate filter efficacy in patients who have received concomitant anticoagulation, because randomising patients who can be
anticoagulated to a filter or no treatment is unethical, Jonson explained. PRESERVE—a large multicentre prospective trial without a control group but with predetermined imaging and clinical outcome evaluations—was performed to obtain the best ethical data.
 Matthew Johnson
Matthew Johnson
Originally PRESERVE had been incited by a US FDA warning letter—“which scared people”, Johnson recalled—and which brought the Society of Interventional Radiology (SIR) and Society for Vascular Surgery (SVS) together, putting themselves forward to evaluate the safety and efficacy of IVC filters from the seven manufacturers who marketed filters in the USA.
Conducted at 54 sites in the USA between 10 October, 2015 and 31 March, 2019, Johnson et al identified 1,421 patients who had a filter implanted—1,019 of patients had existing deep vein thrombosis (DVT) or PE. Their results met primary safety and effectiveness endpoints, with investigators reporting that procedural adverse events were “uncommon” and there was a “low incidence” of clinically-significant PEs.
The investigators note that a “high rate” (44.5%) of filters were removed in this cohort and the reported low rate of adverse events was perhaps due to the “mandates for noting a plan for [IVC filter] removal at the time of placement”, accompanied by frequent follow-up.
Supplying a conclusory suggestion, Johnson et al believe that, when planning IVC filter placement, physicians should consider when the device should be removed, and patients should be followed closely in the interim.
“We should—all of us who place filters—view planning a patient’s treatment pathway as a central tenet of providing comprehensive, filterinclusive care. In today’s world you cannot place a filter and just leave it. That’s not good medicine,” Johnson said. Having demonstrated the correlation between retrieval planning and survival rate through PRESERVE, Johnson’s central takeaway is: “at the time of placement, have a plan for removal”, although he understands that that is “much easier said than done—people’s states change all the time, operation dates change, contraindications change”.
“We’re not going to do better than PRESERVE,” Johnson said, but more is yet to be done to “optimise patient care” in this population. “Newer devices are being developed, however more research is needed to evaluate a device that may be less likely to poke out of the vena cava, or is easier to remove, or may even dissolve,” said Johnson, who maintained that innovation for better outcomes in these patients will prevail through future research efforts.

21 Issue93 | March 2024 News Update

Programme Highlights
Peripheral REGISTER NOW CXSYMPOSIUM.COM CX CO-CHAIRS:
• BASIL-3 Podium First Presentation: Including clinical and health economic results
Tuesday, 23 April
• Inaugural Roger M Greenhalgh Late Breaking Trials Session:
Featuring podium first ATK and BTK trial results
Wednesday, 24 April
• Late Breaker:
BEST-CLI vs BASIL-2 outcomes when comparing apples with apples
Thursday, 25 April



CONTROVERSIES CHALLENGES CONSENSUS INNOVATION EDUCATION Controversies Update Vascular & Endovascular 23–25 APRIL 2024 TUESDAY-THURSDAY NEW VENUE, E xCeL LONDON, UNITED KINGDOM Peripheral Arterial Controversies Aortic Controversies Carotid & Acute Stroke Controversies Vascular Trauma Controversies Venous & Lymphatic Controversies Vascular Access Controversies The Hurting Leg Controversies Peripheral Arterial & CLTI CONTROVERSIES
45 YEAR LEGACY OF VASCULAR EDUCATION Arterial & CLTI Controversies


First-to-podium presentations spotlight aortic and peripheral controversies at CX 2024
The full line-up of Podium First and late-breaking trials to be presented at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) has been released, with highlights including first-in-the-world insights on lower limb revascularisation and acute aortic dissection.
The three-day CX Symposium comes from a new location, the ExCeL convention and exhibition centre in London’s Docklands, with attendees also tuning in virtually from across the globe. It is anticipated that the event will welcome more than 3,000 in-person attendees, with over 1,000 remote participants.
CX continues its three-year cycle of raising vascular and endovascular controversies to challenge the available evidence to be able to reach a consensus after discussion with an expert audience. Sessions across the three days will explore controversies in all vascular domains, spanning aortic, peripheral, venous, acute stroke and vascular access, punctuated by CX debates, live and edited cases, and workshop demonstrations.

The presentation of the long-awaited BASIL-2 (Bypass versus angioplasty for severe ischaemia of the leg) trial results was the undoubted talking point at CX 2023. The trial found that 63% of patients randomised to a vein bypass-first strategy of treatment underwent a major amputation or died during follow-up, compared to just 53% of those allotted to a best endovascularfirst approach.
BASIL-2 chief investigator Andrew Bradbury (University of Birmingham, Birmingham, UK) returns to the CX podium in 2024 to outline the next phase of the programme—BASIL-3—a multicentre randomised controlled trial of the clinical- and cost-effectiveness of drug-coated balloons (DCBs), drug-eluting stents, and
plain balloon angioplasty with bail-out bare metal stent revascularisation strategies for severe limb ischaemia secondary to femoropopliteal disease.
Bradbury will also feature alongside Matthew Menard (Brigham and Women’s Hospital, Boston, USA), an investigator in the BEST-CLI randomised controlled trial, as part of a late-breaking comparison of the trial’s findings alongside BASIL-2, subtitled “outcomes when comparing apples with apples”. In BEST-CLI it was shown that surgical bypass with adequate single-segment great saphenous vein (GSV) was a more effective revascularisation strategy for patients with chronic limb-threatening ischaemia (CLTI) who are deemed to be suitable for either an open or endovascular approach. The session will seek to reconcile important differences between the BASIL-2 and BEST-CLI trials and put the results into context.
Elsewhere, the CX 2024 peripheral programme features Podium First presentations from CX cochair and executive board member Andrew Holden (Auckland University School of Medicine, Auckland, New Zealand), offering a first look at the PREVISION first-in-human trial evaluating a novel sirolimus DCB; the first results of the SAMBA trial comparing a paclitaxel DCB to plain balloon angioplasty in the below-the-knee arteries, presented by CX executive board member Gunnar Tepe (Klinikum Rosenheim, Rosenheim, Germany); and final results of the DEEPER LIMUS trial, presented by Leyla Schweiger (Medical
University Graz, Graz, Austria).
The aortic programme at CX 2024 will bring forward new data to fuel the debate over controversies in the aortic space. Highlights from this section of the programme include a Podium First presentation from Arun Pherwani (University Hospitals of North Midlands, Stoke-on-Trent, UK), in which he will detail outcomes of aortic dissection in the UK.
Other Podium First highlights include real-world registry findings of low-profile thoracic endovascular aortic repair (TEVAR), delivered by Vincent Riambau (Hospital Clínic de Barcelona, Barcelona, Spain), and details of a phantom study examining whether operator position influences radiation dose from Fiona Rohlffs (University of Hamburg, Hamburg, Germany).
Peripheral Podium First Presentations:
● Podium First: BASIL-3 podium first presentation; Andrew Bradbury (Birmingham, United Kingdom), Tuesday, 23 April
● Late Breaker: BEST-CLI vs BASIL 2; outcomes when comparing apples with apples; Matthew Menard (Boston, United States) & Andrew Bradbury (Birmingham, United Kingdom), Thursday, 25 April
● Podium First: First look at the PREVISION FIH trial evaluating the novel sirolimus DCB catheter; Andrew Holden (Auckland, New Zealand) Wednesday, 24 April
● Podium First: First results of the SAMBA trial: A randomised trial comparing a paclitaxel DCB to POBA in BTK arteries; Gunnar Tepe (Rosenheim, Germany), Wednesday, 24 April
● Podium First: Final results of the DEEPER LIMUS trial; Leyla Schweiger (Graz, Austria), Wednesday, 24 April
Aortic Podium First Presentations:
● Podium First: Outcomes of acute aortic dissection in the UK; Arun Pherwani (Stoke-on-Trent, United Kingdom), Wednesday, 24 April
● Podium First: Low profile TEVAR durability in a realworld registry; Vincent Riambau (Barcelona, Spain), Wednesday, 24 April
● Podium First: Operator position influences radiation dose: A phantom study - Fiona Rohlffs (Hamburg, Germany), Thursday, 25 April
23 Issue93 | March 2024 CX 2024
The BASIL-2 trial was a highlight at CX 2023
Clinical News
Endovascular Engineering announces first patient enrolled in PE thrombectomy trial
Endovascular Engineering, a medical device company involved with clot removal technologies for venous thromboembolism (VTE), has announced the enrolment and treatment of the first patient in the pivotal phase of its ENGULF US clinical trial.
This IDE trial will further evaluate the safety and efficacy of the Hēlo pulmonary embolism (PE) thrombectomy system, a first-of-itskind technology, for the treatment of PE. This initial treatment, led by Malcolm Foster (Tennova Turkey Creek Medical Center, Farragut, USA) represents a step forward on the path to introducing the Hēlo PE system to the US market. Enrolment of the first patient in the ENGULF pivotal cohort follows the completion of the 25-patient feasibility phase of the study.
Sensome initiates trial assessing tissue microsensor technology in peripheral artery disease
Sensome has announced enrolment of the first patients into a feasibility clinical study using the Clotild smart guidewire in peripheral artery disease (PAD). Clotild was designated a breakthrough medical device for use in brain arteries by the US Food and Drug
Administration (FDA) in 2021.
The clinical trial, named SEPARATE, is designed to assess the artificial intelligence (AI)-powered Clotild sensor’s capability to detect various characteristics of blood vessel blockages in PAD patients. The first five patients have been enrolled at AZ Sint-Blasius Hospital in Dendermonde, Belgium, and preliminary results are anticipated in mid-2024. A key focus of the SEPARATE clinical trial— according to a Sensome press release— is to evaluate the Clotild sensor’s capacity in differentiating between soft and friable “fresh” clots, and organised “old” clots. This information empowers physicians to select the most suitable endovascular therapeutic approach, thereby mitigating complications, avoiding embolisation, and enhancing long-term treatment outcomes, the release adds.
Study of TIVUS renal denervation system for hypertension treatment completes enrolment
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the proprietary Therapeutic Intra-Vascular Ultrasound (TIVUS) system (SoniVie), has completed enrolment.
“Initial results from the ongoing REDUCED-1 study are encouraging and we continue to diligently follow the enrolled patients,” said Christian Spaulding, chief medical officer of SoniVie, in a press release announcing the study milestone.
The trial had two enrolment cohorts that were conducted under an identical protocol in the USA and in Israel. Twentyfive patients were enrolled in the USA and 15 patients were enrolled in Israel. All patients (N=40) are now in the follow-up phase of the study where primary efficacy (change in daytime systolic ambulatory blood pressure) will be analysed at three months and safety will be analysed at one month and 12 months follow-up respectively.
in getting the first patient-specific device for complex abdominal and extent IV thoracoabdominal aneurysms with fenestrations. The ZFEN+ device design aims to optimise patient needs,” said Oderich.
The ZFEN+ clinical study is being conducted under an investigational device exemption (IDE) from the US Food and Drug Administration (FDA).

Baird Medical announces first thyroid microwave ablation procedure in USA
Cook Medical reports first patient treated in ZFEN+ fenestrated endovascular graft study
Cook Medical has announced the first patient treated in the clinical study of the Zenith Fenestrated+ endovascular graft (ZFEN+) in the USA. The procedure was performed on 29 January 2024 at The University of Texas Health (UTHealth) in Houston, Texas, by Gustavo Oderich, global principal investigator.
“We are ecstatic at UTHealth Houston to have treated this first ZFEN+ patient. This is a step forward
Baird Medical Devices, a microwave ablation medical device developer and provider in China and the USA, has announced that it has performed its first thyroid microwave ablation procedure in the USA since obtaining registration clearance from the US Food and Drug Administration (FDA) on 22 November 2023.
The minimally invasive procedure was performed on 12 January 2024 at Utah Endocrinology Associates by interventional endocrinologist Alireza Falahati, marking an advancement in thyroid nodule management, a press release states. The emergent microwave ablation procedure for thyroid nodules offers patients an alternative to traditional excision surgery, enabling swifter recovery, a reduction of scar tissue in the neck and chest area, and greater efficiency for physicians.


24 Market Watch March 2024 | Issue93
Gustavo Oderich

This deep-dive meeting, hosted by the Society of Interventional Radiology (SIR), is tailored for advanced interventional radiologists seeking to expand their clinical care knowledge. SIR EDGE focuses specifically on clinical advancements, offering the opportunity to explore new devices and techniques across four compelling and sought-after topics in interventional radiology.
sirweb.link/EDGE2024

Peripheral vascular disease (PVD)
MSK embolization/pain management
Portal hypertension



1 MEETING | 3 DAYS | 4 TRACKS INFINITE POSSIBILITIES Oct. 17–19 Denver Seats are limited. Register today! Y-90/TARE
EmboCept® S degradable starch microspheres show high efficacy and good tolerability for TACE in liver tumours
Interventional radiologists Thomas Albrecht (Vivantes Clinic Neukölln, Berlin, Germany) and Roberto Iezzi (Policlinico Universitario Fondazione Agostino Gemelli, Rome, Italy) share their experience performing transarterial chemoembolization with degradable starch microspheres (DSM-TACE) using EmboCept S DSM 50 μm (Magle Group). Indicated for liver cancer treatment including hepatocellular carcinoma (HCC) and liver metastasis from colorectal cancer (mCRC), and lung tumours, they detail how this invaluable “tool in [their] toolbox” has expanded effective options for interventionists to provide better patient care.
EmboCept S DSM 50 μm is an adjuvant in the intra-arterial treatment of inoperable liver and lung tumours in combination with cytostatic agents. Using microspheres of roughly 50 μm in size and with a half-life of approximately 35 minutes, EmboCept S DSM 50 μm can be used for superselective treatments of single segments and selective targeting of one liver lobe to treat multifocal tumors.
EmboCept S DSM 50 μm in the landscape of TACE
EmboCept S DSM 50 μm DSM-TACE sits alongside drug-eluting bead transarterial chemoembolization (DEB-TACE), and conventional transarterial chemoembolization (cTACE), as a viable treatment option for patients with liver tumours. However, due to its unique degradable quality, EmboCept S DSM 50 μm can be used in a wider breadth of patients with varying levels of disease as Albrecht elaborates: “We use EmboCept S DSM 50 μm mainly in patients with multifocal or multisegmental disease, which is predominantly HCC in our practice, because you can treat patients with a high degree of efficacy and low toxicity.”
healthy liver, he outlines.
“When providing care for mCRC, we need to apply a lobar approach, which involves a total of two treatments per lobe—or four treatments if it’s a bilobar procedure—performed with a delay of two to three weeks. It’s essential that we have the ability to perform repeated treatments within a very short period of time.”
In Iezzi’s view, the possibility to combine EmboCept S DSM 50 μm with various drugs, such as doxorubicin or epirubicin, which are also commonly used, is invaluable. “We are experiencing great results and its potential to be combined with other drugs [opens] the door for the treatment of new indications. Not only HCC or mCRC, but also cholangiocarcinoma and other liver metastasis, from pulmonary or gastrointestinal to gynaecological tumours,” he says.


The degradable mechanism of action characteristic of EmboCept S DSM 50 μm allows for “transient” ischaemia, Albrecht states, detailing that this significantly decreases sides effects and improves the therapeutic effect. He adds that, during treatment, prolonged ischaemia has the potential to produce toxicity which can cause post-embolization syndrome and subsequently trigger loss of liver function. With EmboCept S DSM 50 μm however, the risk of these occurrences is “much less marked, if not minimal”, he says, providing a “much improved” safety profile, even when used in more extensive disease—“although we treat a larger proportion of the liver, it’s still better tolerated than other techniques”.
Treatment strategy
For Iezzi, the degradable characteristic of EmboCept S DSM 50 μm is also a notable benefit, enabling revascularisations “without concern over occlusion or collateralisation from a material point of view”. For mCRC, the ability to repeatedly perform treatment is key, as chemoembolization is often combined with irinotecan, which requires repeated activation by a
“It’s a local treatment option for patients with advanced multifocal disease,” Albrecht states, expanding on the potential application of EmboCept S DSM 50 μm for a range of indications. “These patients would often not be treated with other types of TACE and they are being treated with radioembolization less often,” he says. Causing “minimal toxicity”, Albrecht conveys that EmboCept S DSM 50 μm is an “extremely well-tolerated” treatment option with very few side effects, making the procedure tolerable to patients at a wider range of disease level.
Evidenced clinical performance of EmboCept S DSM 50 μm
Informing their assertions, Iezzi and Albrecht worked side-by-side to conduct the largest retrospective European multicentre study, which scrutinised the safety and efficacy of DSM-TACE for advanced stage HCC in patients for whom were ineligible for other standard therapies or for whom these had failed . Their results were published in 2021 in the journal Cancers
Led by Johannes M Ludwig (University DuisburgEssen, Essen, Germany), the investigators identified 121 patients from three European centres. To be treated with DSM-TACE, patients were required to have had unresectable HCC with specific inclusion criteria between institutions. In Berlin, patients with
a Barcelona Clinic Liver Cancer (BCLC) stage of C and D were deemed eligible; in Rome, patients with BCLC B refractory to TACE or stage C, those with a Child-Pugh class of A or B and those with an Eastern Cooperative Oncology Group (ECOG) status of between 0–1; in Essen, patients not suitable for ablation, transplantation, conventional TACE, radioembolization, or systemic therapy with kinase inhibitors, and an ECOG status of between 0–2.
They documented that the overall tumour burden in this population was high, with bilobar disease in 63.6%, >3 HCC nodules in 61.2%, and vascular invasion in 26.4%. Yet, their results showed that patients survived a median of 15.5 months with a median time of 9.5 months to tumour progression, and a 83.2% disease control rate. Additionally, they found that patients who survived longer had lesions of ≤10cm, the involvement of a single liver lobe, a lower Child-Pugh class and BCLC stage, and the absence of vascular invasion or of extrahepatic metastases.
In summary of their results, the investigators asserted that DSM-TACE using EmboCept S DSM 50 μm particles is a “veritable treatment option” for all unresectable HCC patients with high/diffuse tumour burden, “where other treatments fail or cannot be offered due to contraindications”.
The future of DSM-TACE with EmboCept S DSM 50 μm
For now, Albrecht says that he would like to see more interventionists using the technique, and to continue disseminating knowledge about its benefits to the wider community, with the approach still somewhat “unknown” in many countries. “For healthcare professionals, it’s an attractive option to be able to offer patients,” underlining how the effective treatment is also “relatively simple”.
Returning to the patient, Iezzi highlights that DSMTACE using EmboCept S DSM 50 μm represents another treatment option for those in whom options may be limited. “It’s characterised by a high level of comfort for the patient,” he continues, “and it could be combined with a transradial approach”. In Iezzi’s belief, the demonstrable efficacy and safety of EmboCept S DSM 50 μm in liver tumours can “open the door” to future “outpatient and ambulatory care. The patient could be dismissed from hospital six hours after treatment” he adds, with the aim of offering effective and comfortable treatment options for patients.

26 Advertorial March 2024 | Issue93
THIS
IS
BY SIRTEX
ADVERTORIAL
SPONSORED
Roberto Iezzi
Thomas Albrecht
Product News
Financing enables greater access for Serranator device
Cagent Vascular has announced a series C financing close in excess of US$30 million. US Venture Partners (USVP) led the round. Participation included new investor Blue Ridge Medical and existing investors, including Sectoral Asset Management.
“We are pleased with the significant investment from US Venture Partners and other new and existing investors.
To date, we estimate that over 10,000 Serranator PTA [percutaneous transluminal angioplasty] serration balloon catheters (Serranator) have been used to treat those suffering from peripheral arterial disease (PAD). This infusion of capital will increase our commercial reach, helping to provide greater access for healthcare providers and their patients,” stated Carol A Burns, CEO.
Efemoral Medical granted
Breakthrough Device designation
Efemoral Medical has announced that
Conference calendar
23–28 March
Society of Interventional Radiology Annual Scientific Meeting (SIR) Salt Lake City, USA www.sirmeeting.org
11–12 April
International Multidisciplinary Endovascular Forum Florence, Italy www.imendoforum.com
23–25 April
Charing Cross Symposium (CX) London, UK https://www.cxsymposium.com/
the US Food and Drug Administration (FDA) has granted its novel Efemoral vascular scaffold system (EVSS) Breakthrough Device status for the treatment of de novo or restenotic lesions of the infrapopliteal arteries in patients with chronic limb-threatening ischaemia (CLTI).
A press release states the EVSS offers a new approach to treating peripheral arterial occlusive disease by addressing the specific anatomic challenges and complex biomechanics of patients with athero-occlusive disease in the leg.
Gore’s Viabahn VBX stent graft receives FDA approval Gore has announced recent US Food and Drug Administration (FDA) approval of a lowerprofile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
“Our team is pleased to be the first commercial implanter of the new lower profile VBX stent graft,” said Darren Schneider (Penn Medicine, Philadelphia,
USA). “Combined with the flexibility, strength and deployment accuracy I’ve always trusted with the device, the new lower profile will enable me to treat most of my complex cases with a 6 or 7Fr device, reducing the risk of access complications while bringing trusted VBX stent graft outcomes to more of my patients.”
AngioDynamics announces US FDA 510(k) clearance of Auryon XL radial access catheter to treat PAD
AngioDynamics has announced that the US Food and Drug Administration (FDA) has cleared the Auryon XL catheter, a 225cm radial access catheter, for use with the Auryon atherectomy system in the treatment of peripheral arterial disease (PAD).

“Since its launch in September 2020, the Auryon atherectomy system, with its solid-state laser technology, has fundamentally changed patient treatment for PAD and quickly become an essential tool for providers and patients,” said Kimberly Nelson, senior director of Auryon global marketing at AngioDynamics. “Our entry into the radial-to-peripheral (R2P) space with Auryon XL is an important part of our focus on advancing the quality of
care delivery, and our commitment to meeting the needs of patients.”
Reflow Medical receives CE mark for Bare Temporary Spur stent system
Reflow Medical has announced it has received CE mark certification in the European Union for the Bare Temporary Spur stent system. The device is intended to treat de novo or restenotic lesions in the infrapopliteal arteries with a commercially available drug-coated balloon (DCB) to enhance drug absorption.
“The device performance and clinical study data for patients suffering from chronic limb-threatening ischaemia (CLTI) has been quite impressive,” said Thomas Zeller, who is chief of the Department of Angiology at University Heart Center Freiburg in Bad Krozingen, Germany. Zeller was a principal investigator in the DEEPER OUS clinical trial.
A Reflow Medical press release details that the Bare Temporary Spur stent system, followed by DCB treatment, reduces clinically driven target lesion revascularisation (CDTLR), improves wound healing, reduces recoil, and improves vessel patency through one year, compared to historical treatment outcomes with plain balloon angioplasty or a DCB alone.
28 April
5th Annual Endovascular Interventions Symposium Palm Springs, USA https://na.eventscloud.com/website/53519/
28 April–01 May
European Conference on Interventional Oncology (ECIO) Palma de Mallorca, Spain https://www.ecio.org/
16–19 May
Global Embolization Symposium Technologies (GEST) New York, USA www.thegestgroup.com
28–31 May
Leipzig Interventional Course (LINC) Leipzig, Germany https://www.leipzig-interventional-course. com/visitors/linc-2024/
14–15 June
Endo Vascular Access (EVA) Patras, Greece https://www.evameeting.org/
1–4 July
The European Conference on Embolotherapy (ET) Vienna, Austria https://www.etconference.org/
5–7 August
The Interventional Radiology Society of Australasia (IRSA) Christchurch, New Zealand https://irsa.com.au/annual-scientific-meetingasm-2024-save-the-date/
14–18 September
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE)
Lisbon, Portugal https://cirsecongress.cirse.org/







Sign up for a free print subscription* and e-newsletter subscription** www.interventionalnews.com Interventional News is a trusted, independent source of news and opinion in the interventional world. *Available for US and EU readers only ** Available worldwide 27 Market Watch Issue93 | March 2024
Serranator serration balloon catheter
Viabahn VBX stent graft
AI technology provides “exceptional”* capabilities for outpatient embolization treatment
“Our practice has embraced the concept of artificial intelligence [AI] as a tool that improves physician performance for better outcomes—placing patients at the centre of the equation,” shares Jayson S Brower, interventional radiologist and president of Inland Imaging in Spokane, USA. Translating treatment from a hospital setting to an office-based lab (OBL), Brower describes their unique setup using GE HealthCare’s Embo ASSIST AI software, which he claims streamlines procedures and ensures cost-effectiveness when treating increasingly complex cases in an outpatient interventional setting.
Opening their outpatient interventional radiology (IR) clinic during COVID-19, Brower and colleagues’ initial aims were to sustain treatment of immunosuppressed patients—often those attending for oncology procedures—while reducing exposure to illnesses found in hospitals. Hospitals in the USA, Brower points out, are “cash strapped”, meaning equipment can often be “outdated”. Shifting away from the traditional hospital setting, outpatient treatment centres, or OBLs, have become more common in the USA in recent years.
As Brower explains, “it has been a bit of a journey— we opened in 2019 after planning for about a year and half to two years prior to that. If you rewind all the way to 2017, there were maybe a handful of OBLs in the country—it was really a novel concept”.
hospital, emergencies arise, thereby delaying scheduled procedures.”
Opening their OBL to ensure patients can receive timely, efficient treatment, Brower was confident that patients were going to have “the same great quality [treatment] by our team, with significantly less cost to the system”, a benefit which is then passed on to the patient. “Those were the elements that really motivated us to start pushing this down the path,” he says.


As a champion for the OBL model, Brower believes that the translation of treatment to an outpatient environment is the “next best evolution in patient care, primarily due to the cost, quality and patient experience”, he asserts. “Outpatients can be seen on a timely basis—if we say we will treat you at 9am, we are going to treat you at 9am. Whereas, when you come to the
Brower and his team opted for the latest generation of imaging equipment, “opening the door for us to improve patient experience, with less radiation and overall improved patient experience” he says.
Choosing GE HealthCare’s “exceptional” equipment for their interventional suite, Brower and his team’s focus was on their “strong” concurrent alignment on AI as a tool that aims to improve patient care, which formed the foundations of their current collboration. Brower states that the incorporation of Embo ASSIST AI into their practice has proven to “tangibly benefit both the physician and the patient” in both diagnostic and interventional settings.
After setting up their outpatient interventional suite, Brower tells

Interventional News, their team’s focus on how best to integrate AI with their practice continued. “We invested in the software and software upgrades alongside the evolution of the technology, such as the Liver ASSIST Virtual Parenchyma [GE HealthCare] solution, which we have incorporated into our practice.”
In helping to reduce procedure times, Brower defines the “spill-over” benefits, such as reduced sedation and radiation, which also come with using the Embo ASSIST AI solution. However, his team will “continue to evaluate emerging AI tools to determine their value to patients”.
“There are many tools out there,” Brower explains, “but the question is does it bring value or is it just a flashy, ‘Hey, we can do this’, type of situation—it must bring value and then it must be applicable and reproducible.” Often performing liverdirected therapies such as Y90, Brower highlights the “highly reliable” Embo ASSIST AI, which has enabled his team to simplify procedural workflow and improve mapping via its three-dimensional (3D)-augmented image visualisation.
Facilitated by the software’s augmented imaging capabilities, Brower and his team are of the belief that AI will only “continue to improve and grow” as one of the “primary

The simple fact is AI will continue to play a major role in what we do across the board, but particularly in imaging—I would imagine that the role of AI is only going to increase within our specialty.”
Jayson Brower
tenets” on opening their OBL.
With this, Arnaud Marie, GE HealthCare CEO and general manager of interventional image-guided systems agrees, saying “nothing will be untouched by AI”. Speaking to Interventional News, Marie sets out the company’s three-fold intent with the development of Embo ASSIST AI.
First, by enabling augmented imaging to support embolization strategy planning, the technology allows users to simulate injection points,

28 Advertorial March 2024 | Issue93
Arnaud Marie
THIS ADVERTORIAL IS SPONSORED BY GE HEALTHCARE
Jayson Brower
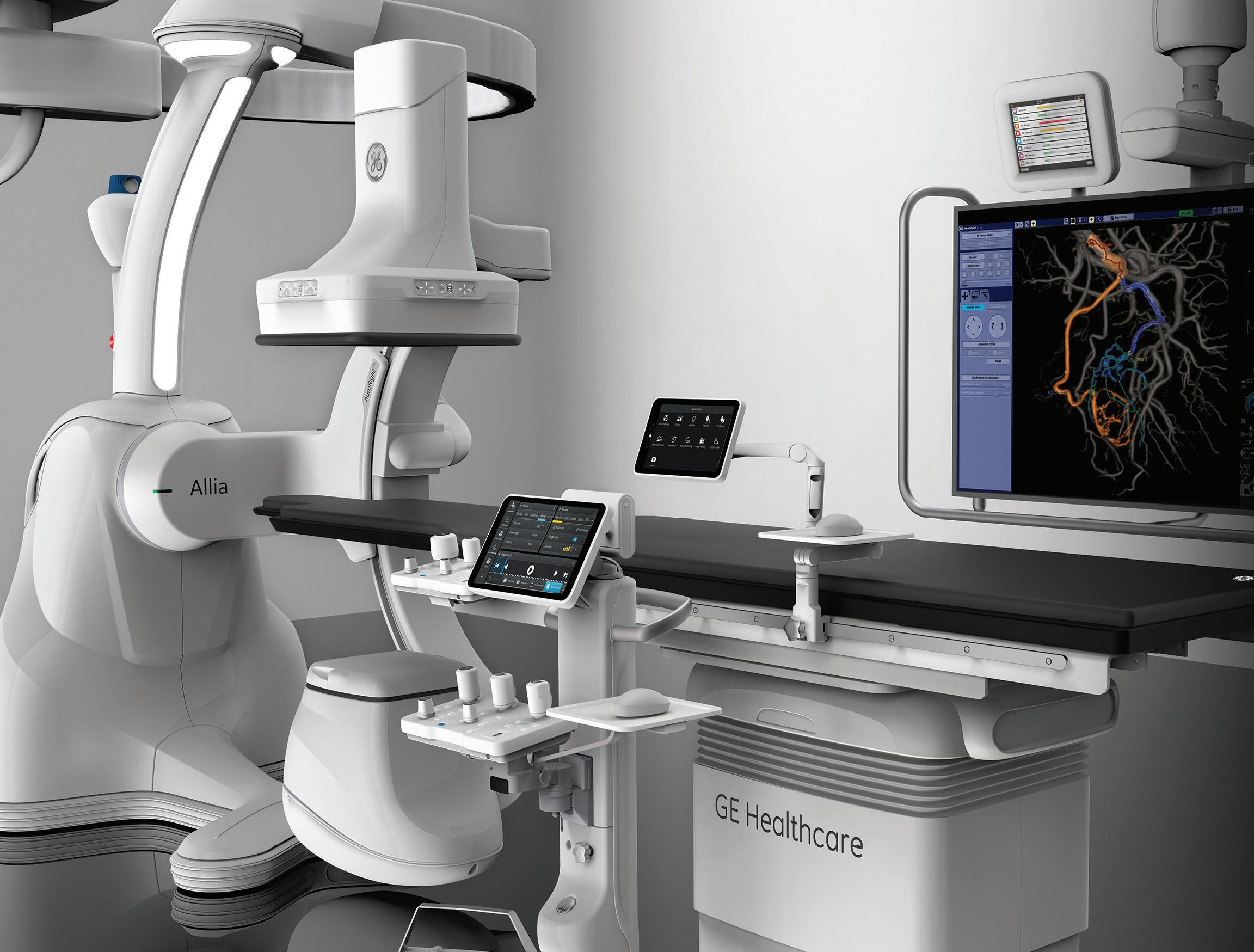
visualising arterial details. Designed to automatically track vessel pathways to anticipate navigation challenges, Marie asserts that interventional radiologists can “[define] the impact of any decision they make, to decide what treatments are best and to guide them during procedures”.
“The second element is to drive workflow efficiency—how can we eliminate steps during procedures? How can we make it as simple and as intuitive as possible?” Marie

expands that a key focus in developing the technology was to remove “unnecessary” procedural touchpoints or clicks. Embo ASSIST AI does this by exporting automatically segmented vessels and landmarks of potential embolization points for augmented 3D fusion guidance. Then, interventionists are able to overlay this information via live fluoroscopy on the frontal or lateral plane. Previously, several steps were necessary to segment vessels to remove pollution from the 3D model Marie describes. Now however, “there are no steps, there is almost nothing to do”, he says.
Marie then defines the third tenet that shaped the development of Embo ASSIST AI—to provide integrated patient care—and explains how the software brings together the “silos” which he states make up healthcare. By speeding up data consolidation, Marie hopes the AI technology can “link those different silos—from the doctor, the technician, the nurses—through information sharing, to revolutionise” the patient care pathway. Throughout this process, Marie states that the company’s overarching refrain was to ask: How can it be summarised?
How can it be accelerated? “We are all patients, or have been patients,” he states. “We want interventionists to have access to the best data and tools.”
Embo ASSIST AI’s imaging potential is of central importance to Brower, who describes the “community benefit” patients in Spokane have gained when attending their OBL clinic and imaging centre, which is operated with their joint venture partner Providence Healthcare.
“As imaging volumes continue to grow and have increased from 6 to 9% just through organic growth over the past year, then spin off from just diagnostic, to interventional, OBL practice should mirror that,” Brower says, highlighting their ability to consult and treat patients more efficiently today.
“Change is not easy,” Marie comments, commending OBLs like Brower’s that are successfully modifying their practice while “demonstrating how Embo ASSIST AI may enable a reduction of procedural time for interventionists, enabling them to solve problems that could not previously be solved”. Brower believes that their OBL has “removed some of the politics”, creating a space where
doctors from “any group, practice, or hospital setting can feel comfortable sending patients”. Although “biased”, Brower believes that they provide the “best patient care” in their market, working to ensure their “reputation and service stays intact—it all comes back to the patient”.
In light of increasing demands, Brower is in agreement with Marie, that “the simple fact is AI will continue to play a major role in what we do across the board, but particularly in imaging—I would imagine that the role of AI is only going to increase within our specialty”. Installing Embo ASSIST AI has been “pivotal” in getting their OBL to where it is now, Brower avers, “they have been fantastic, their equipment in my mind is exceptional—I am a firm believer that [OBL clinics] are the best way to treat patients”.
*Disclaimer: Jayson Brower is not a paid consultant for GE HealthCare. The statements made by Jayson Brower described here are based on his own opinions and on results that were achieved in his unique setting.
ASSIST
29 Advertorial Issue93 | March 2024
Embo
AI solution includes FlightPlan for embolization and requires AW workstation with Volume Viewer, Volume Viewer Innova, Vision 2, VesselIQ Xpress, Autobone Xpress. These applications are sold seperatley and may not be available in all countries.
Embo ASSIST AI helps interventional radiologists to develop embolization practice in OBLs
Industry News
IsomAb announces close of £7.5 million financing to accelerate lead candidate for treatment of PAD
IsomAb Ltd, a UK-based biotechnology company, has announced the closing of a £7.5 million (approximately US$9.4 million) seed financing round, led by Broadview Ventures, with further backing from existing investor, SCVC and participation from the MEIF Proof of Concept & Early Stage Fund, which is managed by Mercia Ventures and part of the Midlands Engine Investment Fund (MEIF).
value analysis
An analysis of data from the SPYRAL HTN-ON MED trial of the Symplicity Spyral (Medtronic) radiofrequency denervation system for the treatment of uncontrolled hypertension has found that the technique can be expected to provide “good value for money” as a therapeutic approach.

Founded in 2022, IsomAb is developing isoform-specific disease modifying antibody treatments for serious and life-threatening diseases with an initial focus on peripheral ischaemia. The seed funding round enables the company to advance the pre-clinical development of its lead antibody, ISM-001.
Jackie Turnbull, CEO of IsomAb, commented: “IsomAb’s lead programme targeting VEGF-A165b aims to treat peripheral arterial disease (PAD) in the large proportion of patients with concomitant metabolic syndrome and type 2 diabetes. We are delighted to have attracted a group of high-quality investors to support the early development of our novel programme toward CTA filing to enter the clinic.”
‘Get a Pulse on PAD’: Multisociety public awareness campaign launched The multi-society PAD Pulse Alliance is taking part in a peripheral arterial disease (PAD) public awareness campaign called “Get a Pulse on PAD”.
The group—comprised of the Society for Vascular Surgery (SVS), the Association of Black Cardiologists (ABC), the Society for Cardiovascular Angiography & Interventions (SCAI) and Society of Interventional Radiology (SIR)—aims to educate people on PAD risk factors and potential symptoms, as well as to encourage patients to advocate for their own health with their doctors.
The awareness campaign, taglined “Kick Off the Conversation,” kicked off as the PAD Pulse Alliance released survey data showing that 70% of Americans are unaware of the disease, contributing to 400 amputations performed each day in the USA.
The top risk factors for PAD are common chronic health conditions that disproportionately impact underserved communities. Yet among Black and Hispanic adults, nearly 80% report never having a doctor or healthcare provider discuss PAD with them.
Radiofrequency renal denervation meets cost efficiency threshold in
The analysis, authored by Andrew Sharp (University Hospital of Wales and Cardiff University, Cardiff, UK) et al and published in the European Heart Journal–Quality of Care & Clinical Outcomes, examined the cost-effectiveness of radiofrequency renal denervation for the treatment of the broader uncontrolled hypertension patient group in the setting of the UK healthcare system.
Recent US Food and Drug Administration (FDA) approval for the therapy and updated recommendations from the European Society of Hypertension (ESH) in its 2023 Management of Arterial Hypertension guidelines will “substantially increase global access to this therapeutic option”, the study’s authors write. These developments, they add, contribute to a growing interest in understanding the health-economic implications of radiofrequency renal denervation adoption.
InkSpace Imaging receives US FDA clearance for small body array MRI coil
InkSpace Imaging, a company engaged with diagnostic medical device technology, has announced it received US Food and Drug Administration (FDA) clearance for its next-generation small body array for the Siemens Healthineers Magnetom Skyra and Vida series 3T magnetic resonance imaging (MRI) scanners. This new medical device is part of a suite of MRI coils designed to revolutionise the patient experience while enhancing efficiency in MRI scans and clinical diagnosis.
Peter Fischer, CEO of InkSpace Imaging, stated: “The FDA clearance of InkSpace Imaging’s small body array marks a pivotal moment in MRI technology advancement. Our suite of

MR coils is a game-changer, offering total freedom in coil positioning and handling. What’s truly impressive is how our thin flexible coil contours to each patient’s body, offering comfort while enhancing image quality. It’s a win-win for patients and healthcare providers alike.”
Endologix Detour system
Mentice gains US FDA 510(k) clearance for Ankyras software
Mentice recently announced that Ankyras, the company’s clinical decision support application, has received 510(k) clearance from the US Food and Drug Administration (FDA). The approval of this product by the FDA demonstrates the safety and effectiveness of Ankyras, and confirms Mentice’s commitment to providing innovative and high-quality healthcare solutions, as per a company press release.
The software is designed to assist interventional neuroradiologists in selecting the most suitable flow diverter prior to the interventional treatment of intracranial aneurysms. Ankyras offers an “intuitive and precise solution” for clinical treatment planning, leading to more accurate predictions than traditional methods, the release adds.
SCAI, SIR, and SVS jointly publish proceedings from multispecialty peripheral IVUS roundtable Proceedings from an expert consensus roundtable that discussed the benefits of intravascular ultrasound (IVUS) in lower extremity revascularisation procedures were released in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI), Journal of Vascular and Interventional Radiology (JVIR), and Journal of Vascular Surgery: Vascular Insights.
The roundtable focused on the current challenges in diagnosing and treating lower extremity revascularisation, knowledge and data gaps, and the potential role of IVUS in addressing these challenges. Experts shared their insights and experiences from the fields of interventional cardiology, interventional radiology, and vascular surgery. The expert consensus meeting was convened by SCAI and co-sponsored by: American Vein and Lymphatic Society (AVLS), American Venous Forum (AVF), Society of Interventional Radiology (SIR), Society for Vascular Medicine (SVM), and Society for Vascular Surgery (SVS).
US Centers for Medicare & Medicaid Services grants Transitional Pass-Through payment for Endologix’s Detour system
Endologix recently announced that the US Centers for Medicare & Medicaid Services (CMS) has granted a Transitional Pass-Through (TPT) payment for the Detour system, effective since 1 January 2024.
The TPT payment was created to facilitate patient access for qualifying new medical technologies that substantially improve the diagnosis or treatment of Medicare beneficiaries. TPT will provide hospitals with additional device reimbursement when the Detour system is used for eligible cases in the hospital outpatient setting. Details on the TPT, code C1604, can be found in the January 2024 Update of the Hospital Outpatient Prospective Payment System (OPPS), accessible through CMS and Endologix.
Percutaneous transmural arterial bypass (PTAB) with the Detour system, a US Food and Drug Administration (FDA) Breakthrough Device, received premarket approval in June 2023. Endologix states that PTAB offers a novel approach to treating complex peripheral arterial disease (PAD).
GE HealthCare to acquire AI-imaging specialist
GE HealthCare has entered into an agreement to acquire MIM Software, a provider of artificial intelligence (AI)enabled image analysis and workflow tools across multiple care areas, including oncology, urology, neurology, and cardiology.
GE HealthCare expects to leverage MIM Software’s imaging analytics and digital workflow capabilities across various care areas to accelerate innovation and differentiate its solutions for the benefit of patients and healthcare systems around the world, the company says in a press release.
MIM Software’s portfolio of innovative imaging solutions provides a variety of beneficial features, including: the integration of diagnostic images from multiple modalities into treatment plans; automation to help reduce repetitive tasks and manual interventions; quantitation and advanced processing in diagnostic imaging and nuclear medicine to help determine therapy response; and a platform to assist with Theranostics imaging and dosimetry analysis for patients across their treament pathway and in the growing field of molecular imaging and radiation oncology.




30 Market Watch March 2024 | Issue93
Inkspace small body array MRI coil
REGISTER TODAY!
European Conference on Interventional Oncology LEADERS IN ONCOLOGIC INTERVENTIONS
ECIO 2024
April 28 - May 1 | Palma de Mallorca, ES
ECIO 2024 is just around the corner !
ECIO is home to the world’s biggest community of specialists for minimally invasive cancer care. It offers a wide array of sessions including more than 190 lectures on topic highlights such as kidney and adrenal treatment, immuno-oncology, paediatric IO, HCC treatment, and many more.



Browse the programme
ECIO 2024 will focus on a multi-disciplinary, minimally invasive approach to cancer care, with topic highlights including immuneoncology, paediatric IO, strategies for HCC treatment, a basic course on kidney and adrenal ablation, and much more
Register today !
Take advantage of the early fee and secure your spot today to join us in Palma de Mallorca.
Bring your MDT colleague for free !
The Collaborating Against Cancer Initiative supports multi-disciplinary teamwork by allowing all interventional radiologists/radiologists with a complete registration to take their non-radiologist colleague to ECIO 2024 free of charge.
Flight discount
Attendees of ECIO 2024 can enjoy reduced rates when traveling with the Lufthansa Group. Scan the QR code to get the discount code !
See you in Palma !
ECIO’s official travel partner, Kuoni Tumlare Congress, has secured a great number of hotel rooms at competitive prices and offers an easy-to-use booking tool for congress participants.
www.ecio.org





BioPearl™microspheres are indicated for embolization of blood vessels supplying primary-hypervascular tumours or metastases in the liver. Note: BioPearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician’s direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. BioPearl™ microspheres are compatible with doxorubicin, epirubicin, and idarubicin. BioPearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. BioPearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared. BioPearl Microspheres are not approved in the USA by the Food and Drug Administration. BioPearl™ microspheres are not approved in Canada. Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for additional information. ©2023 MicroVention, Inc. CE0297 Manufacturer: MicroVention Europe - 30 bis, rue du Vieil Abreuvoir - 78100 Saint-Germain-en-Laye - France - Tel: +33(0)1 39 21 77 46 Distributor: Terumo Europe N.V. - Interleuvenlaan 40 - 3001 Leuven - Belgium -Tel: +32 16 38 12 11 IS1026GB0224ITIV OPENING NEW TACE HORIZONS THE FIRST RESORBABLE DRUG ELUTING MICROSPHERES









































 Robert Morgan
Robert Morgan













 Bien Soo Tan
Bien Soo Tan

















 Roberts
Roberts







 Matthew Johnson
Matthew Johnson
















































