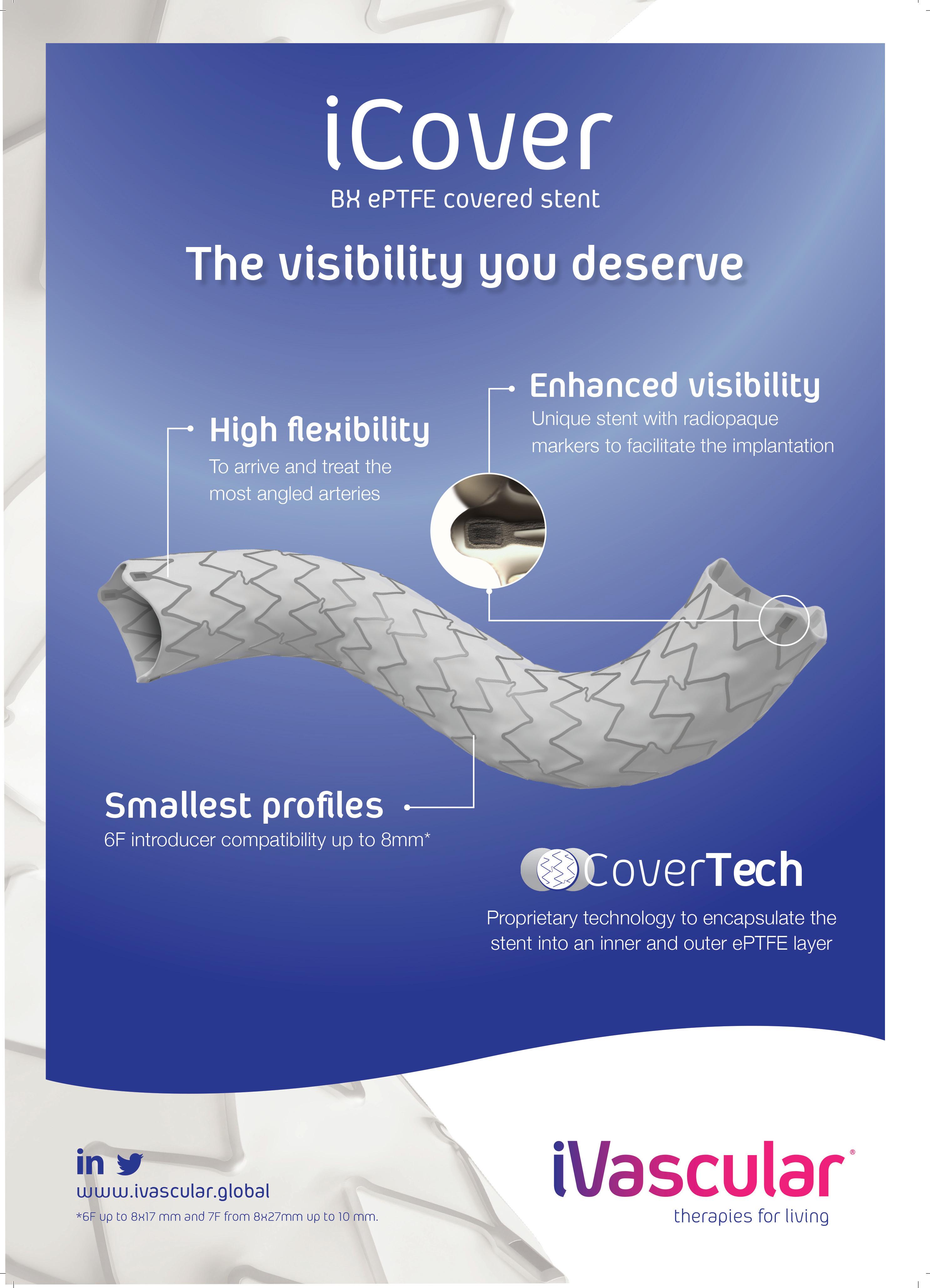
10
Non-invasive histotripsy performed in patient with primary solid renal tumour
Tze Min Wah, clinical lead for the interventional oncology programme at Leeds Teaching Hospitals NHS Trust (Leeds, UK), has performed the first treatment with histotripsy in the CAIN trial of the technology.


THE TRIAL IS A PHASE I PROSPECTIVE, multicentre study designed to evaluate the safety and technical success of histotripsy in targeting and destroying primary solid renal tumours completely non-invasively and without the need for incisions or needles.
The trial is named in honour of Charles Cain, former chair of biomedical engineering at the University of Michigan (Detroit, USA). Cain was a primary inventor and leader in the technology’s development, who passed away in March 2020.
Current kidney therapies such as partial nephrectomy, which is invasive, and the less invasive thermal ablation exhibit complications from bleeding and infection. While surgical intervention is the “gold standard” in removing kidney tumours, a non-invasive approach with histotripsy provides the potential to destroy targeted tissue without damaging non-targeted kidney tissue. Histotripsy’s purely mechanical mechanism of cellular destruction could preserve function of the kidney’s urine collecting system and eliminate certain complications seen with existing invasive approaches.
Wah commented: “I was delighted to lead the clinical team in carrying out this world-first kidney tumour treatment using histotripsy and [it was] a real privilege to have the trust of the patient and their family in translating this innovative technology into our clinic. The CAIN Trial represents a significant milestone for treatment of solid renal tumours with histotripsy as a ‘needle-less’ technology. It is a paradigm shift.”

The platform used in the CAIN trial also provides physicians with the ability to monitor the destruction of tissue under continuous real-time visualisation and control. The trial is expected to support a future expansion of the indication to include the destruction of kidney tissue.
Profile: Neghae Mawla page 12

Regenerative material found “safe and feasible” for haemodialysis access in first-in-human study
The advantages of autogenous fistulas to haemodialysis patients include long-term survival and low rates of complication. But failed or slow maturation as well as the risk of early thrombosis frequently “offset” these advantages and result in the use instead of a central venous catheter. Such “serious limitations”, write the authors of a new study published in the Journal of Vascular Access (JVA), demand the evaluation of alternative options.
Among those are biological regenerative conduits. Lead author Adrian Ebner (Sanatorio Italiano, Asuncion, Paraguay), alongside fellow researchers including Haimanot Wasse (Rush Medical College, Chicago, USA) and corresponding author Zeeshan Syedain (University of Minnesota, Twin Cities, USA), focused on the TRUE AVC (Vascudyne) tissue-engineered vascular conduit and examined whether it could demonstrate “functional patency, lack of infection, and a low adverse event rate”.
Ebner et al’s new study is among the first to focus on a novel tissue-engineered vascular conduit “cultured from completely biologic raw materials and allogeneic dermal cells”, which had previously been trialled in non-human primate models. That trial found no immune response, while “dialysis needle puncture sites demonstrated normal healing with no reduction in mechanical strength of the conduit”. This study, meanwhile, follows on from the work of Jeffrey H Lawson (Duke University, Durham, USA) and colleagues in a Lancet-published investigation from 2016, which found that a bioengineered human acellular vessel “seem[ed] to provide safe and functional haemodialysis access”, suggesting a potential step up from polytetrafluoroethylene arteriovenous grafts and their associated thrombosis and infection risks.
Building on this, Ebner and colleagues designed their first-in-human study to look at the outcomes from five patients who received an implant of the conduit “in an
initial evaluation of [its] clinical safety and performance”. Patients were selected if they were aged 18–75, diagnosed with end-stage kidney disease (ESKD)—although one selected was pre-ESKD—and either on dialysis or intended to initiate it within 12 weeks.
Primary efficacy endpoints of the study were primary patency, assisted primary patency, and secondary patency at 26 weeks, plus successful dialysis through to six months. Ultrasound and physical examination were used for assessment. Among the secondary endpoints was immune system response to the implant, while primary safety endpoints included freedom from clinically significant aneurysm and anastomotic bleeding.
The authors state that the conduit “handled well surgically, with properties similar to that of the native human vein”. Conduit flow following the procedure was “excellent in all cases,” meanwhile, “averaging 1098±388ml/min at week four and remaining stable through 1248±355ml/min at 26 weeks”. There was no significant change in surgical site healing compared to regular conduits, with no oedema or erythema found at four weeks. Primary patency at six months was found to be 80%, with secondary patency recorded at 100%. There was no infection in any of the patients, all of whom received dialysis, nor was there statistically significant change in the diameter of the conduit on ultrasound examination. The authors also note that “no specific IgG antibody response was seen in any patients”, and add that one patient required reintervention for thrombus removal at five months.

Ebner and colleagues say the results demonstrate “the initial and early safety of TRUE AVC”. Speaking exclusively to Renal Interventions, Syedain added: “Completely biological regenerative tissue conduits are designed to mimic the structure and function of natural blood vessels, potentially improving longevity and performance of haemodialysis grafts. This study demonstrates initial safety, mechanical durability and lack of immune response with TRUE AVC, and its potential as an off-the-shelf blood vessel conduit for clinical use.”
Looking forward, Ebner et al point out that larger cohort studies are planned to further investigate the safety and efficacy of TRUE AVC.
In this issue:
April 2023 Issue 07 www.renalinterventions.net
Renal Interventions on all our social media platforms for the latest news, insight and events in kidney care
Transplantation updates page
ASDIN: Programme highlights page 4 Saravanan Balamuthusamy on machine learning in dialysis access page 8
Follow
TRUE AVC
Tze Min Wah
Twitter debate highlights ongoing challenge facing interventional nephrology
A recent Twitter exchange cast light on the heated opinions of physicians providing interventional care for patients with kidney disease. In particular, questions were raised about which group—interventional radiologists or interventional nephrologists—were best placed to deliver this care, a continuing controversy which prompted a small but passionate wave of responses. Several physicians made the case for moving past “siloed” or “tribal” thinking so that these patients receive appropriate and timely care.
That debate centred on a thread in response to a Twitter post by interventional nephrologist
Neghae Mawla (Dallas Nephrology Associates, Dallas, USA) querying nephrologists on what they would do when the attempted removal of a catheter results in only half of the tube coming out. Responses to the post questioned whether the procedure would best be handled by interventional radiology (IR) or nephrology specialists.
This led to some discussion over the validity of interventional nephrology (IN) as a subspecialty, with the suggestion put forward by some that IN was born in response to reimbursement issues. Debate online continues, highlighting the ongoing battle for legitimacy in the eyes of some of their colleagues facing interventional nephrologists.
Speaking to Renal Interventions at the American Society of Diagnostic and Interventional Nephrology (ASDIN) 19th Annual Scientific Meeting (17–19 February, Orlando, USA) Brian Rifkin (Hattiesburg Clinic, Hattiesburg, USA) addressed criticisms of IN, saying: “As interventional nephrologists we talk about the whole patient, and not just their access. That might include end-of-life issues or peritoneal dialysis. This perspective means, as a nephrologist, I am patient-centred and not just procedure-centred. Anybody can do procedures—and the reality of interventional nephrology is that lots of different specialties overlap to do procedures in dialysis patients. The difference, in my opinion, is that we see the whole patient and take ownership of that relationship.”
Vascular surgeon Robert Shahverdyan (Asklepios Clinic Barmbek, Hamburg, Germany), who was among the respondents to the original Twitter thread in defence of IN, told Renal Interventions: “Personally, I was saddened to see disagreement online on this question of who is best-equipped to treat patients. Everyone would benefit from a cooler-headed debate.
“It is clear that we need to support each other as a medical community. This community takes care of increasing numbers of complex patients in the dialysis access field, and we need to avoid division and scepticism towards each other’s specialties. Only together we
Nicholas Inston
Interventional nephrology
can achieve the most important goal, which is helping our patients at a high level and in the best possible way.”
Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) also told this newspaper: “This debate has highlighted the advantages and the drawbacks of Twitter. The ability to immediately release information or comment is certainly an advantage, but the platform is also where medical data, which requires thoughtful and detailed consideration, is compressed for better or worse into short opinion bullets.
Though Twitter is often host to vigorous debate, this sometimes takes a form that might be better suited to the conference floor. Maybe we need to cool it—and to recall that some of the best research in haemodialysis access has been authored by interventional nephrologists. Every specialty has its bell curve of practitioners and personalities.”
Also speaking to Renal Interventions on the controversy was interventional radiologist Jeffrey Hull (Richmond Vascular Institute, Richmond, USA), who said: “The things people say and do speak more to who they are, their experiences, and their biases rather than to the situation at hand. Nephrologists face the needs of patients with renal disease all day every day and have the privilege and responsibility of the care of these patients. IN appears to be developing much like interventional cardiology did in the early days. They have worked with and learned from radiologists and surgeons to increasingly provide similar services and procedures including vascular access catheters, percutaneous peritoneal dialysis catheters and percutaneous arteriovenous fistulas.
“Throughout the world, the needs of dialysis patients are great and in large part unmet. The increasing knowledge, sophistication and skills that interventional nephrologists bring directly to their patients have been invaluable to patients requiring renal replacement therapy. The specialties providing healthcare are often competitive with each other, but all provide important ideas, skills and care to our patients and should be welcome. Friends—I am friends with all involved in this fracas—can agree to disagree, but I personally root for the advancement of renal care regardless of specialty or technology.”
Editor-in-chief: Nicholas Inston | Editorial Board: Ziv Haskal, Stephen Hohmann, Robert Jones


Given the controversy surrounding interventional nephrology (IN) and interventional radiology (IR), vascular access and transplant surgeon Nicholas Inston (Queen Elizabeth Hospital Birmingham, Birmingham, UK) writes to weigh in on how to get the best outcomes for patients.

WE ARE ALL ENTITLED TO OPINIONS, but is not the patient at the centre of all of this? Necessity is the mother of invention and IN would not have had the space to develop had there been no need for it.
The overall services that deliver care for patients needing dialysis access have evolved alongside each other naturally, rather than by design. There is no international blueprint for what is the best practice. In fact, for those criticising others a look in the mirror may be needed— clinical outcomes speak volumes.
Surely the obvious central advocate for a patient with kidney disease is an expert in this condition: a nephrologist. If that nephrologist is trained and delivers interventions with acceptable outcomes it is difficult to deny that this is an appropriate model of care. As with all areas of medicine, this will need to be within an integrated multidisciplinary team which at times will require surgeons, radiologists, dialysis specialists and many others to be closely involved.
Things occasionally do get complicated. IR did not develop, nor does it exist, in isolation. At times a surgeon will be needed to sort out a complication and vice versa, and we do not start to question the existence of IR (or surgery) when such events happen. Is it not time to move the discussion on and look at the real issues to which we can apply our energies? We need to focus on improving the patient’s experience, providing better quality and better value in access care, and increasing longevity of access whilst decreasing interventions.
Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake | Editor: Benjamin Roche benjamin@bibamedical.com | Design: Terry Hawes, Wes Mitchell
Editorial contribution: Jocelyn Hudson, Will Date, Clare Tierney, Eva Malpass, Jamie Bell and Bryan Kay
Advertising: Melanie Stevenson melanie@bibamedical.com Subscriptions:
Renal Interventions
April 2023 – Issue07 Introduction 2
@Renal_Ints linkedin.com/company/renal-interventions/
subscriptions@bibamedical.com | News or advertising queries: Tel: +44 (0)20 7736 8788 Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 | BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Micropress Printers Ltd. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved. If you have comments on this issue or suggestions for upcoming editions, write to benjamin@bibamedical.com
Editor’s letter
“an appropriate model of care”
Robert Shahverdyan Jeffrey Hull

RCTs only part of “montage” of evidence in vascular access
Randomised controlled trials (RCTs) are often considered the “gold standard” of evidence on which to base clinical decision-making, but, asked Theodore Saad (Sidney Kimmel Medical College, Philadelphia, USA), the first secretary and past-president of the American Society of Diagnostic and Interventional Nephrology (ASDIN), can they be “fool’s gold”? Receiving the society’s annual Gerald Beathard Award for “teaching excellence, scholarly activity, and clinical excellence” at its 19th Annual Scientific Meeting (17–19 February, Orlando, USA), his acceptance presentation turned the focus of the conference to the limitations of RCTs in vascular access, as well as the other evidence available to clinicians.
Opening by assuring the audience that he was “not here to diss RCTs”, Saad opined that it was among the proudest achievements of ASDIN that its members have participated in the organisation, execution, and publication of multiple important trials. “This is a massive accomplishment,” he stressed, before adding that, nevertheless, “RCTs are not the answer to everything.” He put it forward that personalised medicine offers a new paradigm, and quoted David S Jones and Scott Harris Podolsky (Harvard Medical School, Boston, USA) in a 2015 piece in The Lancet in saying that it “refocuses clinical attention away from the ‘typical’ patients analysed by RCTs and onto the idiosyncrasies, genetic or otherwise, of individual patients”.
Next, he compared two of his haemodialysis patients with arteriovenous grafts. The first was a patient with consistently low access flow of <300ml/min, severe outflow vein stenosis, and frequent dialysis hypotension on no anticoagulation— yet whose graft had never thrombosed. With this patient he contrasted a second with “rapid, repeated graft thrombosis” despite access flow >800ml/min, no significant stenosis of the access circuit, frequent dialysis hypotension, on full anticoagulation. Saad commented: “The second patient should not have been clotting while the first should have been clotting all the time—so there was something hugely different in the biology of these patients that I just did not understand.” Including either of these patients in an RCT studying an intervention such as stent-graft or drug-coated balloon angioplasty, would cause “some real muddling of the data,” he said.
Rather than a total reliance on RCT, then, there is a “montage” of evidence required in clinical decision-making, Saad argued: “We make the best decisions we can with various inputs—when we have a good RCT to inform a decision that is great, but most times we do not, and we have to take into account many other

factors which are hard to weight appropriately.” Those factors, he said, include case series, meta-analyses and even personal experience and anecdotes. Not least, he added, patients’ informed views must be considered.
Saad’s comments here highlight a need for good decision-making even when an RCT is not available, something made clear in a recently published systematic review published in the Journal of Vascular Access. This examines “the extent of variation in the planning and recruitment” of RCTs studying the “contentious area” of arteriovenous grafts (AVGs), and its “stark” findings highlight first that there have been only 31 such trials in 31
years, “the vast majority of which exhibited major limitations severe enough to undermine the results”. Its authors, led by David Kingsmore (Queen Elizabeth University Hospital, Glasgow, UK) call for higher-quality RCT and data which echo Saad’s counsel to consider a wider variety of evidence types when coming to a decision.

Making the case for other forms of scientific evidence, Saad turned to the example of Gerald A Beathard (University of Texas Medical Branch, Galveston, USA), after whom the Beathard award is named. With 579 publications listed on Google Scholar, Saad said he has “contributed an immeasurable amount” to nephrology “without necessarily publishing RCTs”—of that 579, only one publication is an RCT.
Saad recalled asking Beathard how he has been so prolific and was treated to a characteristic “Geraldism” in response. “It is my hobby, I enjoy writing papers,” Beathard said, “I do not play golf.” Saad punctuated his presentation with several other humorous but pointed quotes. When Beathard trained Saad in procedures, he gave him the following stipulation: “Ted, there are two ways to do this procedure: you can do it my way, or I can do it my way.”
The risks of putting too much emphasis on RCTs for a given clinical decision were also highlighted by Saad, who said such a lack can be “weaponised” in a way that prevents patients getting the best possible care. “We have to make the majority of our clinical decisions without them,” he contended. Saad referred listeners finally to a spoof on RCTs by Robert W Yeh (Harvard Medical School, Boston, USA) published in The BMJ investigating the effects of parachute use on “prevention of death and major trauma when jumping from aircraft”. Not every question, he concluded, can or should be answered by an RCT.
A glimpse of the stars
Elsewhere at ASDIN 2023, Renal Interventions spoke to Beathard himself on the state of nephrology, interventional or otherwise. The prominent researcher made the claim that the creation of vascular access has improved with more widespread training. “The advent of endovascular arteriovenous fistulas (endoAVFs) has me concerned that there is a tendency to over-utilise it,” Beathard said, “but over time I think that will take care of itself.”
A new focus on broader “life planning” for dialysis patients was something Beathard said he welcomed. “When we started, we fixed what was broken when it was broken. Fistula first was an important development in this because it increased people’s awareness of vascular access, leading to the new developments we have seen in training in the field. New ideas are entering the stage, too—the research around drug-eluting balloons (DEBs) is promising, if not quite exciting just yet.”
Asked if he thought the future of nephrology was interventional, he answered yes: “Subspecialties like these will attract new people into nephrology. There are many that did not exist even ten years ago, and it is because of those that young people are attracted to particular fields.”
Transplant nephrology and interventional nephrology he cited as examples, with ASDIN training ramping up for the latter. The challenge he outlined, however, is “to train the trainers,” with many programmes not having sufficient staff to administer them. His goal at the University of Texas Medical Branch is to meet that challenge, Beathard stated.
“If you can do procedures on your own patients that is a big advantage,” he explained. “General nephrologists today often know little about vascular access, and yet it is so important to dialysis, which is frequently a big part of their practice. They have relegated that to consultants who are sometimes not that well-trained or informed. For the nephrologist to take ownership of that is going to be extremely important.”
“Dialysis patients are very unique. A patient who has a need for a procedure is probably going to have a recurring need for one—having a nephrologist they have an ongoing relationship with, trained to provide that ongoing care, will be vital.” On training itself, he said that he now did some things very differently to when he started out in 1985—and even compared to a decade ago, particularly in areas such as excessive blood flow. “Some of those lessons I have learned from the people I am training!”
Finally, he turned to the subject of ASDIN itself, of which he is a founding member: “Meetings like ASDIN are important because I see how others do things there, even just in conversation—you learn as much in the hallway as you do in the presentations. You can read papers, but that personal contact is so important. You can ask questions that maybe no one else has. There is no substitute for that.”
“Zoom meetings are okay,” he said finally, coining a new Geraldism, “but it is like the movies: there is a big difference between seeing the stars on screen and seeing them in person.”
4 April 2023 – Issue 7 ASDIN ASDIN
“We make the best decisions we can with various inputs—when we have a good RCT to inform a decision that is great, but most times we do not.”
Theodore Saad
“Meetings like ASDIN are important because I see how others do things there, even just in conversation—you learn as much in the hallway as you do in the presentations.”
Gerald Beathard
HeRO or zero? An upper extremity alternative for haemodialysis access
The American Society of Diagnostic and Interventional Nephrology (ASDIN)’s 2023 meeting (17–19 February, Orlando, USA) saw its second day start with style, as Tze-Woei Tan (Keck School of Medicine of the University of Southern California, Los Angeles, USA) opened a session on controversies in access care with a comparison of the upper extremity Haemodialysis Reliable Outflow (HeRO) graft and lower extremity grafts.
A SELF-PROFESSED EARLY ADOPTER of the HeRO graft (Merit medical), Tan began by noting that the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines advise that, once a patient with more than one year life expectancy has exhausted other upper extremity graft options, their clinician should next consider either a HeRO graft, a chest wall graft or a lower extremity graft. “However,” argued Tan, “it doesn’t really tell us which of these steps to take.”
Looking at each option in turn, he turned first to lower extremity grafts. These, he said, are “pretty rare,” noting that they only comprised 1–5% of access procedures. He cited a Journal of Vascular Surgery (JVS) study comparing HeRO with lower-extremity grafts led by Samuel Steerman (Sentara Vascular Specialists, Norfolk, USA), which found better primary patency for lower extremity, though similar secondary patency for both. That means that though HeRO graft patients need more reintervention, their access can be kept open, Tan said. Infections were comparable.
Another study from 2016 in the JVS, however, found that the risk of thrombosis was “significantly lower in HeRO grafts”. A meta-analysis in the Journal of Vascular Access in 2018, meanwhile, found lower primary and secondary patency for HeRO but a lower rate of infection compared to lower extremity.
Summarising the findings, Tan called HeRO grafts a “reasonable” option for “patients without the option of upper extremity arteriovenous (AV) access due to central stenosis or occlusion”. While lower extremity grafts have consistently displayed “slightly superior patency”, he said, with HeRO requiring more reintervention, lower extremity grafts have similarly shown a higher infection and thrombosis risk.
“In my practice,” Tan concludes, “I decide whether to place thigh or HeRO grafts based on the patient’s anatomy. If they are younger, with longer life expectancy, I usually offer a fistula if possible— or, sometimes, a thigh graft. With a higher risk of infection, I go for HeRO—though they may need more reintervention.”
DCB evidence stokes demand for more research
The second day of the 2023 meeting of ASDIN played host to a packed afternoon session on new technologies for interventional nephrology. Amidst this came a presentation from Daniel Patel (Volusia Flagler Vascular Center, Daytona Beach, USA) on drug-coated balloon (DCB) use in arteriovenous fistula (AVF) stenosis.
Patel launched into a discussion of plain balloon and DCB angioplasty, noting that the former has a primary patency rate of only 50% after six months according to a 2019 systematic meta-analysis led by Ian Jun Yan Wee (National University of Singapore, Singapore). “Is there a better way to do what we do?” he asked. “Is there a physiological way rather than a mechanical way that we can get a better outcome here?”


Moving to focus on paclitaxel, he raised results from a study into its use with the Lutonix (BD) balloon published in 2018 in the Clinical Journal of the American Society of Nephrology (CJASN). This study, by Scott Trerotola (Perelman School of Medicine of the University of Pennsylvania, Philadelphia, Pennsylvania) et al, found that DCB angioplasty was equivalent in terms of safety with plain-balloon, but that it “did not meet the primary effectiveness end point at 180 days compared with conventional angioplasty”. It demonstrated no “no difference in access circuit patency” as well as “no lesion-specific benefit of DCB”.
Patel said “the story is not completely over for Lutonix”, drawing attention to the ABISS trial underway in France that has suggested “some improvement in Lutonix outcomes”, but that there was not yet meaningful data from this study to analyse. He also posed the question of whether paclitaxel-based DCBs could demonstrate better primary patency rates with higher doses. Initial concerns regarding risk of mortality with Pacl -
“Downright scary” distal radial artery cannulation illuminated
Rajeev Narayan (San Antonio Health Center, San Antonio, USA) has presented at ASDIN 2023 to give his tips and tricks on distal radial artery cannulation. “There are many things that are exciting in the field of nephrology,” said moderator of the session Anil Agarwal (Veterans Administration Central California Health Care System, Fresno, USA), introducing Narayan and his talk’s topic, “but some of them are downright scary”. Narayan aimed to shed some useful light on the procedure.
“BASICALLY,” NARAYAN SAID, “THE MORE YOU do something, the better you get at it—so this talk is mainly for those who do not do [distal radial artery cannulation] as much.” Beginning with indications, Narayan said that the procedure was appropriate for haemodynamic monitoring, as well as access for maturation of percutaneous arteriovenous fistula and dialysis access procedures.
He then moved on to assessing the safety of the procedure for a patient, something for which he emphasised the value of the complete superficial palmar arch (SPA)
itaxel doses have not been demonstrated multiple DCB dialysis access studies, Patel argued. More recent studies, he said, are beginning to examine the effects on animal models of higher doses of the drug in angioplasty than have previously been tried, up to 6μg compared to the 3–4μg of previous studies. These include a 2021 investigation by Ole Gemeinhardt (Humboldt-Universität zu Berlin, Berlin, Germany) et al
The IN.PACT Arteriovenous (AV) Access IDE study, published in The New England Journal of Medicine (NEJM) and carried out by Robert A Lookstein (Ichan School of Medicine at Mount Sinai, New York City, New York) et al, did also find that DCB was superior to plain-balloon angioplasty “for the treatment of stenotic lesions in dysfunctional haemodialysis arteriovenous fistulas during the six months after the procedure”. Its three-year data has also shown the benefits of “reduced interventions” while also demonstrating “improved patency” and “potential for cost savings” in comparison to treatment with plain balloon angioplasty. Sirolimus was also addressed in the course of Patel’s talk. Describing it as “effective in preventing arterial re-stenosis”, he cited the MATILDA trial published in the European Journal of Vascular and Endovascular Surgery (ESVS) and the ISABELLA trial cited in the Journal of Vascular Access (JVA). These prospective studies signalled efficacy of Sirolimus in treating dialysis access stenosis, Patel suggested, though he noted further studies are ongoing.
Finally, Patel asked: “are we there yet?” There remain many unanswered questions on DCBs, he argued, including particularly the advantages of a higher dose of paclitaxel. He also questioned whether “cutting balloon vessel prep” and employing larger-diameter balloons may be effective. He said data from the IN.PACT AV study were “compelling”, while DCBs may be“ideal for poor stent areas” like cannulation zones and vessel junctions.
and the deep palmar arch (DPA), making reference to a review by Marek Brzezinski (VA Medical Center, San Francisco, USA) et al from 2009. Using a modified Allen test (MAT), he advised clinicians to use the following instructions: “Make a fist for 30 seconds with occlusion of both radial and ulnar arteries. Open the hand and release ulnar artery.” Then, he said, if colour returns in 3–12 seconds, the patient will likely be able to tolerate radial artery occlusion. If the patient can demonstrate a complete palmar arch with ultrasound or Doppler, he added, this may be “better at predicting ischaemia to the hand with radial artery occlusion”. If the ulnar artery is not patent, he stressed, “I would avoid distal radial artery cannulation”.
Narayan urged his audience to remember “if nothing else” to cannulate above the level of the radial styloid. “We were all taught to cannulate at a 45 degree angle, but I do not really do that nowadays,” he added. “I come in at a much steeper angle nowadays and level off—I find that that is easier.” Another trick Narayan recommended was ulnar artery compression, which entailed using ultrasound “to localise and compress the ulnar artery, sometimes [found] in the upper forearm”. This can sometimes cause dilation of the distal radial artery “just enough to assist in cannulation”.
Narayan also suggested using a needle as a guide during cannulation, stating that it can be used “under ultrasound to push through clots or calcifications into a better part of the artery prior to advancing the wire”. He positied that cannulation should be performed “with haemostasis in mind”.
5 Issue 7 – April 2023 ASDIN ASDIN
Tze-Woei Tan
“Compelling”
Daniel Patel
It was not just presentation sessions that featured during the American Society of Diagnostic and Interventional Nephrology (ASDIN) 19th Annual Scientific Meeting (17–19 February, Orlando, USA). Among the several hands-on elements of the conference were tutorials on the “one-minute check” for dialysis access as well as a cannulation simulator.
Deborah Brouwer-Maier (Transonic, Ithaca, USA) spoke to Renal Interventions during the session to explain the utility of and rationale behind the one-minute check. She noted that it is the physical examination recommended by the Kidney Disease Outcomes Quality Initiative (KDOQI) for checking a vascular access. She outlined that it is a “look, listen and feel” standardised test initially used and published by Gerald Beathard (University of Texas Medical Branch, Galveston, USA), who has featured it as part of the core curriculum with ASDIN.
The check includes “an arm elevation test, where the fistula is raised to see if it collapses— if it doesn’t collapse, it indicates the area of
stenosis”. Also part of the check is “an augmentation test where you occlude to check the inflow”. Brouwer-Maier noted that it was a work group sponsored by the Fistula First Initiative that helped develop the method. It was designed to be performable by anyone from a clinician through to a dialysis technician nurse and even a caregiver. With that in mind, it was modelled on CPR checks that can be performed quickly and easily.
Brouwer-Maier made the case for the check: “When you talk to staff, most do not even take that minute to assess the access. Then, they cannulate the access and run into problems—if you do the one-minute check first, it reduces cannulation complications. You will know if the access is working, whether there is an aneurysm, an area at risk for a fatal access bleed, or an infection. You will feel the vessel and the thrill, listen to it and know whether there is a stenosis. It increases the chances of a successful cannulation in just one minute.”
Beyond educating at ASDIN, Brouwer-Maier said the aspiration was to take the check global. Centres without ultrasound may derive particular benefit, she suggested, though it also has a place in more fully equipped clinics. Cannulation mapping, demonstrated at the session by Forest Rawls (Emory Healthcare Dialysis Clinics, Atlanta, USA), was another way to assess the best approach to a given cannulation procedure.
Also featured during the Sunday hands-on sessions was a novel device for identifying what constitutes skilled cannulation. Joseph Ravi Singapogu (Clemson University, Clemson, USA), who led the team that developed the cannulation simulator, was on hand to explain how it allows the angle and pressure of a cannulation procedure to be evaluated in real time. “We have ‘sensor-ised’ cannulation: rather than the subjective opinion of a dialysis techni-

cian, the expertise will be measured by sensors,” Singapogu said. “The goal is to help improve skill—and if you cannot measure, how can you track skill progress?”
“The pressure is a really big one,” Brouwer-Maier added. “When I used to do “cannulation camp”—training with a fake arm—all I could do was try to figure out how tightly a trainee was holding the needle to understand how much pressure they were applying. The simulator is a way for the trainee themselves to know when the pressure or angle is wrong while they perform the procedure.”
Brouwer-Maier and Singapogu were united in saying that they hoped the simulator would end the use of patients as practice models. “If we were physicians,” Brouwer-Maier said, “that would not be happening.”
Singapogu stated in summary: “You are only as strong as your weakest link. Is cannulation that weakest link?” The simulator, he hopes, will make it easier to answer that question in the negative.
In one of the select presentations at the ASDIN 2023 meeting to receive a mid-lecture round of applause, Victoria Teodorescu, associate professor of vascular surgery at Emory University (Atlanta, USA), posed the question: should nephrologists create surgical arteriovenous fistulas (sAVFs)? The rapturous response came as she answered in the affirmative.
THIS RESOUNDING ANSWER, which began the presentation, “needs a little bit of explanation,” Teodorescu stipulated. She then drew up numbers illustrating just how many patients on dialysis and requiring access there are in the USA. Taking information from the United States Renal Data System Annual Data Report, she pointed out that the number of patients receiving in-centre haemodialysis in the USA almost doubled between 2000 and 2020 from around 250,000 to around 500,000. This raises the question, she suggested, of “who exactly is going to do the access surgery?” for this expanding patient population. The solution, she suggested, may lie with nephrologists.
Historically, the surgeries necessary for haemodialysis access have been done by general surgeons, Teodorescu said. This made sense because general surgery training included a lot of vascular education,
but more recent surgical training has seen this “drop off quite significantly, especially in arteriovenous (AV) access”, noting a 15-year decline (p=0.0218) according to the Accreditation Council for Graduate Medical Education (ACGME) Review Committee for Surgery. Teodorescu notes that the committee designates 10 dialysis accesses as the defined category minimum number for general surgery residents—something she says is “extraordinarily minimal experience”.
“Not all programs are created equal,” she continued, making use of the National Resident Report in the US for 2021–2022 to argue that “there are programmes where they do very little dialysis access”. Teodorescu said that, in 2022, 63 residents graduated from the 0+5 integrated vascular surgery training program, which she said was a “tiny, tiny number to do all of vascular surgery”— particularly when many showed a greater

interest in aneurysms and lower extremities than dialysis access. “Just because you have finished with this particular training does not mean you know a lot about dialysis access,” she posited.
“This is not to say that we do not finish our fellows with extremely broad interventional skills, including aortas and other lower extremity interventions— other cases requiring a high degree of catheter skills,” Teodorescu said, “but we do not really have any education in dialysis itself or what is happening in the unit. We have surgeons adept in the techniques of vascular surgery but not necessarily in renal failure.”
Next, Teodorescu turned to the ASDIN recommendations for training nephrologists to bridge the gap she identified. She concurred with them, particularly in their urging the improvement of competency-based education “in all aspects of dialysis, including questions on board exams”. Teodorescu stressed this point especially in light of the fact that, in her
view, future nephrologists will be charged with the responsibility for access in the dialysis unit.
The next recommendation with which she concurred was to ensure that fellows receive sufficient training in placing temporary dialysis catheters. Fellowships in interventional nephrology, she said, are also important. Access requires knowledge of dialysis, renal failure, and surgery, as well as intervention skills, she said: “We must develop a cadre of specialists who know everything about surgery, intervention, and what is going on in the dialysis unit—someone who knows why we might be having difficulty cannulating. We need the access specialist.”
This, she concluded, will pave the way for studies that will more effectively assess when surgery or intervention is the appropriate clinical decision. “We do not really have that right now,” Teodorescu remarked, “and until we each come out of our silos and know everything there is to know about everybody else’s field – nephrology, interventional nephrology and vascular surgery, we will not be serving our end-stage renal disease patients well.”
Speaking exclusively to Renal Interventions after the presentation, Teodorescu stated: “Providing the right access for the right patient requires a much broader set of skills than what any of our current training paradigms can provide. The difficult task of creating and maintaining dialysis access belongs in the hands of a new type of specialist, fully trained in surgical and interventional techniques as well as medical care of renal failure patients.”

6 April 2023 – Issue 7 ASDIN ASDIN
“One-minute check” and cannulation simulator promise improvements to dialysis access
“We need to come out of our silos”: Surgical education urged for nephrologists
Joseph Ravi Singapogu
Victoria Teodorescu
Tutorial at the ASDIN hands-on session
“Providing the right access for the right patient requires a much broader set of skills than what any of our current training paradigms provide.”
Victoria Teodorescu
Preservation or ligation? Post-transplant AV access best practice debated
A session on controversies in access care at the American Society of Diagnostic and Interventional Nephrology (ASDIN) 19th Annual Scientific Meeting (17–19 February, Orlando, USA) played host to a stimulating debate between Edgar Lerma (University of Illinois at Chicago, Chicago, USA) and Loay Salman (Albany Medical Center, Albany, USA), the question: should an asymptomatic high-flow arteriovenous fistula (AVF) be ligated or preserved in a patient who has received a transplant and ended dialysis?
Proposing preservation Salman started proceedings by laying out that transplantation remains the very best treatment for patients with end-stage renal disease (ESRD), but that a successful transplantation presents clinicians with the problem of whether to close their dialysis access. Setting out his stall as the pro-preservation speaker, Salman put forward that the evidence in favour of ligation for asymptomatic high-flow AVFs is not strong enough— particularly given the risks of complication the procedure presents.
The inconclusive nature of the evidence is suggested by a multi-national survey carried out by Bram M Voorzaat (Leiden University Medical Center, Leiden, The Netherlands) et al and published in The Journal of Vascular Access in 2019, Salman said. He pointed out that, among the 585 multidisciplinary respondents, it was found that, if an access had a “reasonable” flow of around 1000ml/min, there was a tendency to maintain it regardless of age. When the blood flow is more than 2500ml/min, however, there is a tendency to close regardless of age, as there also is with an ejection fraction of less than 30%. That study concludes that the “significant variability in preferences demonstrates that the current evidence is not convincing to recommend routine preservation or ligation”.
With this evidence base in mind, Salman presented the questions that he asks when faced with the issue of preservation or ligation. First among these is the status of the graft—Salman noted that many transplant patients will eventually end up back on dialysis, and that this means clinicians have a duty to ensure they do so in the best way possible.
Building his case, Salman asserted: “If you decide to ligate the access, there are certain risks you are exposing that patient to. Those include mortality with any necessary new access— patients who have already experienced graft failure have a worse mortality rate compared with those awaiting transplantation.
“There will be additional challenges around finding a new access, since some will already have been used, as well as the risk of maturation failure. These are all risks brought about just by ligating the previous access.”
His next question was simple: why ligate? The evidence does not show any proven benefit around patient mortality to ligation of access after a transplant, Salman argued, making reference to a 2019 Journal of Vascular Surgery study by Caitlin W Hicks (Johns Hopkins University School of Medicine, Baltimore, USA) et al that found little difference in mortality or graft failure risk with ligation.
Salman closed his argument by advising clinicians to “carefully weigh the benefits and risks of treating complications” against those of ligating an access, before stating clearly that, in the case of non-problematic arteriovenous (AV) access, ligation should not take place after transplant. He added that a randomised controlled trial (RCT) was needed to show its benefits before it could be routinely recommended—the COBALT study in England, he said, promises to provide the groundwork for a larger trial.
The case for ligation
Next up came Lerma, who introduced himself as “normally a nice guy” who “does not debate”. Nevertheless, he quickly began assembling his argument in favour of ligation, considering first how common cardiac complications are in chronic kidney disease (CKD) patients. He referred to a 2012 study in Nefrología that was led by Rocío Martínez-Gallardo (Hospital Infanta Cristina, Badajoz, Spain), which found that 17% of its 562 enrolled CKD patients developed at least one episode of pre-dialysis heart failure— one of the risk factors for which was the presence of AV access.
Another study in Clinical Transplantation, this one headed by Tabea Schier (Innsbruck Medical University, Innsbruck, Austria), found that the mean shunt flow among kidney transplant patients with heart failure symptoms who required closure was 2,197ml/min, compared with 851ml/min among patients who did not undergo shunt closure. The former number,
Lerma contended, “fits the criteria of high-flow AV access”. On top of this, Lerma referenced a further study in the European Heart Journal by Yogesh N V Reddy (Mayo Clinic, Rochester, USA) et al that found, in a retrospective study with a cohort of 137 patients with an AVF, that 43% of patients with no prior history developed incident heart failure.
“So we know high-output cardiac failure is a potential complication of high-flow AV access,” Lerma determined from this evidence. AV accesses with a blood flow >1.5l/min are at particular risk, he said, especially when created in the upper arm. He described a blood flow/ cardiac output ratio of >0.30 as a “valid screening tool to perform further cardiac testing”, and added that, “if no reversible cause for high-output heart failure is identified, a case can be made for flow reduction (banding) of the AV access”.
To progress his argument in favour of ligation, Lerma brought up two further studies. First was the 2019 Circulation-published study led by Nitesh N Rao (University of Adelaide, Adelaide, Australia) which concludes that “elective ligation of patent AVF in adults with stable kidney transplant function resulted in clinically significant reduction of left ventricular (LV) myocardial mass”. This is corroborated by the findings of the second study Lerma raised here, by Tania Salehi (University of Adelaide, Adelaide, Australia) et al, which suggest that the benefits of ligation on left ventricular mass (LVM) and LVM index “appear to persist in the long term”.

Summarising what he argued are ligation’s advantages, Lerma listed the LVM reduction found in the Salehi et al study, as well as the potential reduced cardiovascular risk, minimisation of risk of rupture and the cost. Finally, he spoke from experience: “Not all AV fistulas are created equal. Fistula closure should be done for stable kidney transplant patients with symptoms of overt cardiac failure, pulmonary hypotension or access-related complications such as aneurysms and steal syndrome.”
Addressing Salman’s arguments, Lerma said: “Regarding the risks, I feel that a patient-centric approach that includes patients’ preferences is more appropriate.” He said there may also be some benefits to a low-flow fistula patient keeping their fistula, but that those with flow >1l/min may have a propensity towards cardiac failure that justifies ligation. For patients who experience graft failure, he added, “banding may be in order”. He referred listeners lastly to a 2020 review in Advances in Kidney Disease and Health, to which he contributed, that proposes an algorithm for managing high-flow AV access.

Issue 7 – April 2023 7 ASDIN ASDIN
Loay Salman
Edgar Lerma
“Regarding the risks, I feel that a patientcentric approach that includes patients’ preferences is more appropriate.”
Edgar Lerma
Machine learning in AV access: The need for precision medicine in dialysis vascular access
Recent evidence suggests that machine learning algorithms promise to contribute to vascular access for dialysis in a variety of ways, not least through prediction of reintervention and the mitigation of costs.
By Saravanan Balamuthusamy
Predictive analytics with high levels of precision are in practice for weather forecasting every day, allowing 100,000 flights to take off and land safely all over the world, while financial forecasting models are established in the investment and banking community. Successfully developing similar mathematical models in healthcare is challenging, however, as the outcomes are driven by clinical variables which are hard to predict. Regression equations such as Framingham heart risk scores are well known within the medical community for risk assessment, but they lack real-time inputs, and cannot be precise to the risk portfolio of every patient. Machine learning is much more advanced than regression equations as it assimilates relevant data in real time which are also more pertinent to a single patient—or to a large cohort, as long as the outcomes are predefined.
Dialysis vascular access is a lifeline for haemodialysis patients until they get a kidney transplant. With better survival rates on dialysis, increasing kidney disease burden and fewer organs available for a kidney transplant, there is an increased need for the creation and maintenance of arteriovenous (AV) accesses that can withstand the test of time—that is, getting cannulated 312 times a year.1 Surveillance and monitoring technologies utilising wearables, needle reversal and transonics are highly focused on flow and pressure metrics to assess access function, but the upstream and downstream biological events that predispose a patient to access flow changes have already occurred before the clinician recognises that with screening and monitoring devices. A decrease in AV access flow is a pre-terminal event, hence there is a need for technology to identify patients who are at risk for developing upstream and downstream biological events that precipitate access flow compromise.2 Monitoring flow alone would be similar to a landing gear malfunction alarm at 30,000 feet as opposed to knowing the problem before takeoff.
Numerous factors including but not limited to age, sex, social determinants, ethnicity, comorbid conditions, lab parameters, genomics and anatomical dimensions determine why biological events occur in patients with AV accesses that lead to stenosis and, eventually, access failure. However, not all these variables have the same weight across all patients, and there is therefore a need for a precision medicine approach in dialysis vascular access. Application of precision medicine is often challenging due to scalability as workflows and data points (e.g. genomic lab tests) might not be available at all touchpoints of care. Machine learning in medicine requires access to data sources like electronic medical records (EMR), claims and image data. Numerous variables like demographics, labs and image data can be obtained from the EMR and cost data can be abstracted from claims. 3 Real-time information on access symptoms could be obtained from the patients if they use a mobile app geared towards access care. Wearables and flow surveillance devices would be helpful in identifying varia-
tions in flow and pressure in addition to recording vitals. However, an access biometric like flow could be just another variable, and it would not be sufficient in itself to predict emerging access dysfunction before damage occurs.
The application of machine learning in AV access could complement the end-stage kidney disease (ESKD) life plan recommended by the Kidney Disease Outcomes Quality Initiative (KDOQI).4 Predictive analytics utilising machine learning can have numerous applications in AV access. These include prediction of the likelihood of a successful AV fistula with an approximate maturation period, which would be time to cannulation. It may also help in identifying patients who might be candidates for successful endovascular AV fistula (endoAVF) creation, as well as in predicting patients for an early AV graft as opposed to “fistula first”. Predicting risk for access dysfunction due to inflow versus outflow issues is also a possibility, as is estimating the need for reintervention in patients with AV access stenosis. Lastly, it may help calculating the cost of AV access creation and maintenance at a patient and group level.
Cost is not a consequence in medicine—it is now an outcome based on which hospitals and physician groups are scored and paid. Therefore, understanding emerging risk of access dysfunction for maintaining and salvaging a patient’s lifeline alone is not sufficient; it is also essential to understand costs. We have recently published our outcomes on utilising machine learning analytics on predicting reinterventions, as well as its impact on AV access, utilising a data abstraction software (Plexus EMR) and machine learning analytics platform (OptMyCare Inc) to predict cost and outcomes in 200 patients. Treating physicians had visibility to the risk of reinterventions and the choice to use current standards of care including but not limited to stents, drugcoated balloons, flow reduction and other special balloons which they deemed fit for their scope of practice. Just like most other chronic conditions, 20% of the cohort contributed to over 50% of the cost in this study and there was a significant reduction in the need for thrombectomies, reinterventions and a US$1,000 cost reduction per patient per year after the machine learning analytics outputs were implemented.5
With increased scrutiny on cost and outcomes across value-based care entities in nephrology, there is an unmet and urgent need for utilising machine learning analytics in identifying the 20–30% of high-risk patients and appropriately intervening on them to improve outcomes, as well as to decrease hospitalisations and the cumulative cost of care.
Acknowledgements: Soumya Gopalakrishnan and Ayala Siddique for assisting with draft.
References available online at renalinterventions.net
Disclosures: Saravanan Balamuthusamy is chairman and CEO of OptMyCare.

Study points toward CKD patients who would most benefit from renal artery stenting
A new study probing which chronic kidney disease (CKD) patients would benefit most from renal artery stenting points toward those who experience a more rapid decline in renal function over six months prior to the procedure.
IT IS AN AREA THAT HAS BEEN A SOURCE OF GREAT frustration for vascular surgeon John Gregory Modrall (University of Texas Southwestern Medical School, Dallas, USA), ever since, in 2014, the CORAL (Cardiovascular outcomes in renal atherosclerotic lesions) trial established no benefit from renal artery stenting over medical therapy in patients with CKD. It created, the professor of surgery told Renal Interventions, “a state of therapeutic nihilism, if you will, in our specialty on how to manage people with renal artery stenosis, and either hypertension or CKD.” Why? The patients kept coming, he said, but vascular surgeons grew less certain about treatment.

So Modrall and colleagues set about identifying putative predictors to be able to select patients for renal artery stenting who are most likely to benefit. They dug into the Veterans Affairs Corporate Data Warehouse to find 695 patients who underwent renal stenting between 2000 and 2021, categorising them as “responders” if eGFR (estimated glomerular filtration rate) at 30 days or greater post-stenting increased 20% compared to pre-stenting. All others were “non-responders.”
The results were stark. Presented at the 2023 Southern Association for Vascular Surgery (SAVS) annual meeting (18–21 January, Rio Grande, Puerto Rico), they revealed that patients in CKD stages 3b and 4 (eGFR 15-44 mL/min/1.73m2) are the only sub-groups with a significant probability of improved renal function after renal stenting, with the rate of decline of preoperative eGFR over the months prior to stenting a powerful discriminator of patients who are most likely to benefit.
“Both of those are helpful to clinicians because we can then look at the patient’s history of where their renal function is currently when we see them in the office, and where it’s been for the last six–nine months,” Modrall explained, “and we can make a much more educated estimation of the probability of improved renal function when we stent them.”
“By the same token, on the flip side of that is, if a patient has, for instance, very flat renal function over six or nine months prior to stenting, we can tell the patient that the probability of improved renal function is so low that it doesn’t even merit the treatment. That’s a huge help to clinicians.”
The third—and negative—predictor, the research team uncovered in the study, is diabetes, Modrall continued. “This turned out to be an interesting finding that we have now seen in two successive studies with different datasets,” he said. “With many diabetic patients their kidney may already be too injured to benefit from stenting. While we’re not saying you shouldn’t stent those patients, we certainly would say you should be very circumspect and careful about choosing patients for stenting if they have diabetes.”
Importantly, Modrall pointed out, the predictors highlighted in the study are “putative,” or “candidate predictors,” that have not been validated in a prospective series. “The next step is to take the data from this study, combine it with two of our prior studies, and in doing so we will have close to 1,800 patients with renal artery stents,” he said. “That represents the single largest dataset of renal artery stenting patients in existence to my knowledge.”
8 April 2023 – Issue 7 Machine Learning
John Gregory Modrall
Saravanan Balamuthusamy
PAVE-2, paclitaxel and sirolimus-coated balloons for AVF
The range of options in drug-coated balloons (DCBs) for stenosis in arteriovenous fistulas (AVFs) demand comprehensive exploration. The PAVE-2 trial comes as the latest in a series of studies intended to assess the evidence and illuminate the space. By Michael
Robson and Narayan Karunanithy (King’s College London, London, UK)
Plain balloon fistuloplasty is an effective treatment for a dysfunctional arteriovenous fistula. However, restenosis is common, leading to a need for repeat intervention. To reduce this need, drug-coated balloons have been extensively studied to determine if they prolong the time to retinervention and provide benefit. The PAVE-2 trial, which promises to further illuminate the subject by assessing two types of DCB, will come as the latest in a line of trials exploring their efficacy.
Paclitaxel-coated balloons allow local delivery of paclitaxel to the site of stenosis. Paclitaxel is a drug which inhibits the cellular proliferation that leads to restenosis following fistuloplasty. Since 2015, there have been many studies published assessing their effectiveness in preserving patency following angioplasty of an AVF. There have been several systematic reviews and the general conclusion has been that there is no overall evidence of an effect for paclitaxel-coated balloons on target lesion primary patency rates. Many of the studies included in these meta-analyses had small sample sizes and there was considerable heterogeneity. There have been three multicentre, randomised controlled trials (RCTs) with more than 200 participants , which is significantly more participants than other studies, and it is useful to consider these in more detail.
The first was from Scott O Trerotola (Hospital of the University of Pennsylvania, Philadelphia, USA) et al with 285
participants and had a primary endpoint of target lesion patency at six months. There was no significant difference between groups treated with a paclitaxel-coated balloon compared with a control group receiving treatment with a non-coated balloon.1 A second study by Robert A Lookstein (Ichan School of Medicine at Mount Sinai, New York City, USA) et al with 330 participants, using the same binary primary endpoint but a different paclitaxel-coated balloon, did find a difference between groups.2
However, the National Institute for Health and Care Research (NIHR) funded the investigator-led Paclitaxel-assisted balloon angioplasty of venous stenosis in haemodialysis access (PAVE) trial with 212 participants, which failed to show an effect on time to end of target lesion primary patency after treatment with the Lutonix (BD) paclitaxel-coated balloon compared with a non-coated balloon. 3
The reason that the PAVE trial and the study by Trerotola et al did not show a benefit whereas the study by Lookstein et al did is not entirely clear. If the PAVE trial and the IN.PACT AV study are considered, there were differences in patient demography. In the PAVE
Older patients’ knowledge improved by multidisciplinary care
Chronic kidney disease (CKD) patients who are older or more vulnerable can achieve similar outcomes with their treatment, particularly on understanding of it and their condition, compared to those with less advanced disease through a combined “teambased approach” including “physicians, advanced practitioners, social workers, pharmacists and dietitians”. That is one conclusion of a recentlypublished study in Kidney Medicine
THOUGH MULTIDISCIPLINARY APPROACHES to CKD may require “more resources and potentially increase costs”, says first author Surekha Annadanam (University of Michigan, Ann Arbor, USA) and colleagues, previous studies have suggested that they may have a beneficial effect on patient outcomes. For their study, Annadanam et al focused on patient-centred outcomes, which they define as “outcomes that are individualised to the patient and their experiences”. They point to reports such as the one led by Hannah Tiu (Henderson Hospital, Henderson, USA) in Clinical Nephrology that suggest
trial, fistulas that had not been used for dialysis were included. It seems unlikely that these factors accounted for the differing results of the trials. Another possible explanation is that the two negative trials used the Lutonix balloon, whereas the Lookstein study used the IN.PACT AV balloon, which delivers a higher dose of paclitaxel. In summary, despite extensive study, experts in the field are uncertain about the benefit of paclitaxel-coated balloons for AVFs.
Sirolimus is another anti-proliferative drug that may be of benefit in this setting. Several strands of evidence suggest that sirolimus may be effective in preventing the neointimal hyperplasia that causes AVF restenosis. Firstly, sirolimus is an anti-proliferative drug and has been shown to inhibit both proliferation and migration in vascular smooth muscle cells in vitro and there is also supportive evidence from preclinical models of AVFs suggesting a benefit for sirolimus. Sirolimus-coated balloons have now been developed and are therefore an alternative to paclitaxel-coated balloons.
The MagicTouch (Concept Medical) balloon has been assessed in the single arm MATILDA study. 4 Target lesion primary patency rates at three, six and 12 months were encouraging at 98, 83 and 58%. Selution (MedAlliance) is another sirolimus-coated balloon that has been reported in the ISABELLA study5, which was also uncontrolled. Patency rates of 95, 72 and 44% were found. Although these results are promising, proper assessment requires an RCT. The MagicTouch balloon is being assessed in the IMPRESSION industry-sponsored trial of 170 patients at three sites in Singapore.6
The investigator-led Paclitaxel or sirolimus coated balloons for ArterioVEnous fistulas (PAVE-2) trial has been funded by NIHR. This will be a three-armed trial including over 600 patients from around the UK. Both
the IN.PACT AV and MagicTouch balloons will be compared to a control group, with a primary endpoint of time to end of treatment segment primary patency. Inclusion criteria will allow either one or two treatment segments, in contrast to PAVE where only one was allowed. The trial is currently in set-up, and we hope it will significantly advance our understanding of the efficacy of both paclitaxel and sirolimus coated balloons for AVFs.
Narayan Karunanithy is an honorary senior lecturer at the School of Biomedical Engineering and Imaging Sciences, King’s College London (London, UK).

Michael Robson is a reader in Nephrology in the Faculty of Life Sciences and Medicine, King’s College London.

Disclosures: Robson was the chief investigator for the PAVE trial. Karunanithy consults for BD, Medtronic, Boston Scientific and Penumbra.
References:
1. Trerotola, SO, Lawson J, Roy-Chaudhury P, Saad TF, Trial LAC. Drug Coated Balloon Angioplasty in Failing AV Fistulas: A Randomized Controlled Trial. Clin J Am Soc Nephro 2018;13(8):1215-24
2. Lookstein, RA, Haruguchi H, Ouriel K, Weinberg I, Lei L, Cihlar S et al. Drug-Coated Balloons for Dysfunctional Dialysis Arteriovenous Fistulas. N Engl J Med 2020;383(8):733-42
3. Karunanithy, N, Robinson EJ, Ahmad F, Burton JO, Calder F, Coles S et al. A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit during angioplasty of arteriovenous fistulas. Kidney Int 2021;
4. Tang, TY, Soon SXY, Yap CJQ, Chan SL, Choke ETC, Chong TT. Utility of Sirolimus Coated Balloons for Salvaging Dysfunctional Arteriovenous Fistulae: One Year Results From the MATILDA trial. Eur J Vasc Endovasc 2021;62(2):316-17
5. Tang, TY, Yap CJ, Soon SX, Tan RY, Pang SC, Patel A et al. Utility of the selution SLR sirolimus eluting balloon to rescue failing arterio-venous fistulas12 month results of the ISABELLA Registry from Singapore. Cvir Endovasc 2022;5(1):8
6. Pang, SC, Tan RY, Choke E, Ho J, Tay KH, Gogna A et al. SlroliMus coated angioplasty versus plain balloon angioplasty in the tREatment of dialySis access dysfunctION (IMPRESSION): study protocol for a randomized controlled trial. Trials 2021;22(1)
patients with kidney disease do not usually understand their treatment, nor do they get the best possible communication to help them understand it. Both studies were led overall by Julie Wright Nunes (University of Michigan Department of Internal Medicine, Ann Arbor, USA) and her research team and funded through the US National Institutes of Health (NIH)-affiliated National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The authors designed a study with several patient-centred outcomes, including “CKD-specific knowledge, kidney disease-related stress, overall health, and health status compared to one year ago”. Knowledge, measured through the kidney disease knowledge survey (KiKS), was the primary outcome.
In total, the study enrolled 245 patients with a mean age of 60 years. Of these, 168 (69%) received general nephrology care while 77 (31%) received multidisciplinary care. “Given the nature of the multidisciplinary care clinic,” the authors state, those in the latter group were on average older (64 years vs. 58 in the nephrology care group) and had more advanced CKD (66% with stage 4–5 vs. 36%).
The overall mean score for knowledge measured with KiKS was not significantly different between the multidisciplinary group and the general care group (66.7% and 67.0% respectively [p=0.89]). However, a greater percentage of those in the former group could correctly identify their own CKD stage versus those in the latter (48% vs. 34%).
The study authors note that patients seen in the multidisciplinary clinics specifically saw older patients with more advanced CKD. They argue that, given there is “a trend for older age […] to be associated with lower knowledge” (-0.1 confidence interval [CI], p=0.06), it is notable that this is levelled out after patients received multidisciplinary care—suggesting it helps older patients to match their younger counterparts in knowledge of their disease. The study also found levels of disease-specific stress varied not only across level of progression but also along lines of race, with African American patients found to have a higher level of stress (0.4 CI, p<0.01) compared to white patients.
Though multidisciplinary care was not found in this study to make significant improvements to stress level or knowledge generally, it can achieve outcomes on these metrics for more vulnerable patients that are comparable to those with less advanced CKD. “Focused, repetitive education”, they state, “may result in sustainable differences in specific areas of knowledge related to individual patients.”
9 Issue 7 – April 2023 DCBs
Michael Robson
Narayan Karunanithy
Transplantation
Higher mortality risk found for female recipients of male-donor transplant kidneys
A new Kidney International-published multinational cohort study has found that the excess risk of mortality for women who receive a kidney transplant is greater than for men when the donor is male—though not to a statistically significant degree if the donor is female. The differences were found for recipients aged 0–44 years and those over 60 years. The authors of the study, led by Amanda Vinson (Dalhousie University, Halifax, Canada), say their results “suggest that management may need to be modified to optimise transplant outcomes among females”
The introduction to the study draws attention to the increased risk of mortality for transplant recipients compared to the general population even given improvements in transplant care and graft survival. The authors make the claim that understanding sex-based differences in transplant recipient mortality can illuminate new ways to improve outcomes more generally, but that untangling such differences from the higher mortality rates of men in the general population means comparing excess mortality risk for those undergoing such procedures. Noting previous studies, such as the 2017 investigation by Fanny Lepeytre (University of Montreal, Montreal, Canada) et al that found immunologic factors to be driving sex-based differences in graft failure, they contend that differences in graft failure risk are the main cause of differences in mortality risk.
The study drew on three of the world’s largest transplant databases: the American Scientific Registry of Transplant Recipients (SRTR), the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA), and the international Collaborative Transplant Study (CTS) database. The SRTR and CTS data accounted for patients between 1 January 1988 and 31 December 2018, with the former followed to 1 June 2019 and the latter until 28 October 2020. The ANZDATA cohort included transplantations between 1 January 1988 and 31 December 2019, and was followed until 31 December 2019.
Setting out the study’s primary exposure as recipient sex, its authors also describe how they delineated between different combinations of donor and recipient sex. They also included “a donor-recipient sex combination by recipient current age interaction term in all models”, which they state allowed
Amanda Vinson
them to “account for the possibility that the association between excess mortality and recipient sex may differ by age”. It was an important element of the study, they argue, to recognise the variation in sex-related biological differences between men and women across age, owing to “sexual development and senescence”.
The primary outcome, meanwhile, was “overall mortality with observation censored at re-transplant, end of observation, or end of the study period”, with observation limited to a first transplant due to changes in donor characteristics with subsequent transplants. The SRTR included 243,371 patients (58.6% male), while the CTS included 209,340 (62.6% male) and ANZDATA 14,181 (62.3% male) who had a first kidney-only deceased donor transplant. The variables across these cohorts were harmonised, before the data of all 466,892 were combined.
The analysis found that, with a male kidney donor, female recipients 0–12 years (relative excess risk [RER] 1.54, 95% confidence interval [CI] 1.20–1.99), 13–24 years (RER 1.17, CI 1.01–1.34), 25–44 years (RER 1.11, CI 1.05–1.18) and 60 years and older (RER 1.05, CI 1.02–1.08) demonstrated greater excess mortality risks than same-age male recipients. With a female donor, meanwhile, the excess mortality risk for those >12 years was similar to when the donor was male, “but there were no significant differences in excess mortality rates in any age interval”.
Discussing their findings, Vinson et al note that they conflict with the earlier findings of a 2009 study led by S Joseph Kim (University of Toronto, Toronto, Canada) which suggested a survival advantage for female versus male kidney transplant recipients. Except for those aged 45–59 years, in Vinson’s study all ages demonstrated a greater risk of excess female
Immunosuppression optimisation “does not delay” transplant failure
The use of immunosuppressant drugs to facilitate allograft success in kidney transplant recipients “does not delay failure of renal transplants after development of donor-specific antibodies (DSAs)”. That is the conclusion of the OuTSMART trial, which has been published in eClinicalMedicine and coordinated by Dominic Stringer, corresponding author Anthony Dorling (King’s College London, London, UK) and colleagues.
KIDNEY TRANSPLANT RECIPIENTS CAN experience graft dysfunction, followed by graft failure, as a result of “chronic immune-mediated injury”, according to the OuTSMART authors, who outline the context and purpose of the investigation. These failures lead to tens of thousands of patients a year returning to dialysis after a transplant has failed, and have been linked to the development of antibodies (Abs) to
human leukocyte antigens (HLAs) in the transplant recipient.
DSAs have been associated by other studies with “a higher risk of graft loss compared to [antibodies] that are not donor-specific”. This has led to the use of immunosuppression as a strategy against “immune-mediated damage”.
The study authors set out to illuminate immunosuppression with a trial that took place across 13 UK transplant centres, where 2037 transplant patients were randomised, 1028 to unblinded care and 1009 to double-blinded care. Patients were grouped according to HLA Ab status, which was known at time of transplant for 91% of patients. Most recruited patients were taking tacrolimus (73%), MMF (67%) or prednisolone (55%) at randomisation, while 27% were taking all three and “baseline immunosuppression was balanced across groups”. The primary outcome of the study was time to graft failure in an intention to treat analysis, and was measured at 43
mortality, though they note that any sex-based differences were only statistically significant with a male donor.
Making reference to another study, this time a 2021 report by Nicole De La Mata (University of Sydney, Camperdown, Australia) utilising ANZDATA finding that “the number of life years lost by females is significantly greater than by males” undergoing kidney transplantation, Vinson et al argue that their work contributes to a growing evidence base backing the increased risk for female recipients of male-donor kidneys. Though women demonstrated higher graft loss rates, they note that they found differences in mortality even without loss of graft function, something they posit may be due to the greater risk of drug toxicity in women.

The authors outlined several limitations to the study, including missing data on cause of death in all three cohorts. They also state that “the unavailability of donor-to-recipient weight ratio in the CTS cohort may have resulted in biased pooled RER estimates”.
Vinson spoke exclusively to Renal Interventions to review the findings: “This study is important because it highlights the importance of considering sex-based differences in mortality risk in the context of the higher baseline mortality risk in males in the general population.
“Earlier studies that looked at mortality (but not excess mortality) have shown better survival in women post-transplant, but important information is lost; the bias towards higher mortality rates in males should be preserved among transplant recipients—unless there are sex differences in the effects of transplant, its treatment, or both.
“The fact excess mortality risk is higher post-transplant in female recipients of a male donor kidney—that is, there is less survival benefit with a transplant if you are a woman—is an important finding that requires further investigation.”
Amanda Vinson
Graft failures for DSA-positive patients
15–20%
Graft failures for negative patients
7%
months remotely due to the COVID-19 pandemic. From the cohort, 198 patients were found with DSA and 818 with non-DSA. DSA development, though not non-DSA development was found by the authors to be “predictive of graft failure”. The authors state that their results confirmed the prognostic value of monitoring DSA” but failed to show statistically significant change to graft failure rates with optimised oral immunosuppression, with “confirmatory 95% confidence intervals for hazard ratios that included the null value”. They found that 15–20% of grafts failed for DSA-positive patients, compared with 7% for negative patients, but for those undergoing immunosuppression optimisation “we failed to see an impact on time to graft failure”. While rejection was reduced by immunosuppression, this did not translate into decreased rates of transplant failure.
Speaking on the implications of their findings, Stringer et al state that the data “will impact significantly on how transplant centres around the world organise their post-transplant monitoring”. They make the case that it should also spark an international effort to find new approaches to the question of ensuring graft success.
10 April 2023 – Issue 7 Transplant Failure
“The bias towards higher mortality rates in males should be preserved among transplant recipients—unless there are sex differences in the effects of transplant, its treatment, or both.”
“Urgently deliver” pre-emptive living donor kidney transplantation “as the default”
Living-donor kidney transplantation should be “treatment of choice” over deceased-donor transplantation, owing to it being the “most secure way to achieve pre-emptive kidney transplantation”. That is the view of a group of doctors writing in Frontiers in Public Health, who argue that, given ongoing shortages of donor kidneys, deceased-donor transplantation may be a suboptimal solution that is more difficult to time appropriately, often occurring too early or too late relative to the onset of end-stage kidney disease (ESKD).
Living donor kidneys, the opinion piece’s authors argue, support improved outcomes as they frequently facilitate better graft survival rates due to their higher quality. They also benefit from “greater flexibility for transplantation” across blood type and immune system barriers, though the piece makes the case that the most important of all advantages to living donor donation is the ability to plan when transplantation will take place— which allows patients, crucially, to avoid dialysis and all its attendant “risks, complications and restrictions”.
These advantages have not been sufficient to raise the rates of pre-emptive living-donor kidney transplantation (PLDKT) beyond “disappointing” levels, the authors add. They ascribe this to the fact that it is not used as a quality indicator for kidney transplantation in a majority of countries, making what they suggest is an unflattering contrast with fields such as oncology, where preferred treatment options are based more closely on the latest evidence—and incentivised through strict targets.
In seeking to explain the low rates of PLDKT, they sketch out a few barriers to its wider use. These include “late referral, lack of cohesion, lack of education” as well as failure to provide sufficient financial support and infrastructure. However, there are also “theoretical drawbacks” that may have contributed, including patients potentially facing the risks associated with surgery and immunosuppression earlier than necessary—though too early is better than too late, according to lead author Frank Dor (Hammersmith Hospital, London, UK). Patients may also be less inclined to adhere to a course of immunosuppressants without having first “experienced the morbidity of dialysis”, though this, the piece’s authors say, has been disproven as a significant driver.
The authors of the report draw next on data from the UK Renal Registry 24th Annual Report, which show that only 17% of kidney replacement therapy starters are listed or receive living-donor transplantation before starting dialysis. In the period from April 2021 to March 2022, only 40% of kidneys received for transplantation were from living donors. This is lower than the rates in other European nations such as The Netherlands, where the authors note that in 2021 50% of kidney transplants came from living donors, of whom 44% were pre-emptive. The UK, they say, saw a median waiting time for chronic kidney
disease (CKD) patients from start of dialysis to deceased-donor transplantation of 1044 days for adults who were transplanted between April 2021 and March 2022.
“There is substantial mortality on dialysis,” they argue, “in addition to a negative impact on employment, societal participation, and quality of life, dialysis also leads to considerable healthcare costs. Patients face a significant risk of suspension from the waiting list, with associated increased mortality and worse graft outcome.”
The guidance on PLDKT they describe as “limited”, with the UK-based National Institute for Health and Clinical Excellence only encouraging the inclusion of living-donor transplantation “in the full informed discussion of options” faced by ESKD patients, with “pre-emptive living donor transplant [...] or pre-emptive listing for deceased donor transplantation to people considered eligible” sitting among a wider list of options. The authors of the Frontiers in Public Health piece, however, contend that such pre-emptive listing does not guarantee higher rates of pre-emptive transplantation. It is not only outcomes that they argue may be improved by an increase in these rates—PLDKT
April 2021–March 2022: kidneys transplanted from living donors
40%
is also a “cost-saving strategy” compared to non-pre-emptive transplantation. One study cited by the authors found cost savings of US$94,579 over a 20-year period, while it also avoids the costs associated with dialysis, for which they cite estimates of £20,660–£31,785 per patient per year. Dialysis vintage prior to transplantation, they add, is shown to negatively impact graft survival, and is a major risk factor for kidney transplant outcomes.
“Decreasing the number of patients starting dialysis by virtue of undergoing pre-emptive transplantation will reduce the need for dialysis capacity, allowing resources to be reallocated elsewhere,” the authors assert. “Preventing the burden of having dialysis three times a week may enable patients to continue to work and contribute to society.”
The authors’ enthusiasm for living donor-donation is caveated by reference to the risks, “albeit very low”, of complication during surgery, as well as the effects of having only one kidney, on donors. They experience a heightened relative risk of ESKD, though the absolute risk remains small, the authors state. Additionally, they argue that it is important to consider the “thorough workup” and yearly follow-up with donors that make up part of what constitute “very clear guidelines”.
The authors stake out the conclusive claim that it is “our duty, not only for the individual ESKD patient, but also from a public health perspective” to institute PLDKT “as the default” treatment for the condition, while also re-emphasising the need to use it as a quality indicator.
Speaking to Renal Interventions, Dor added that “a pre-emptive living donor transplant plan also gives patients control back over their lives, especially during times of turbulence and due to the extreme uncertainty of progressive kidney failure,” which he said remained undervalued.
“The benefits of PLDKT are massive,” he continued. “I don’t understand why this is not picked up on to a greater extent, and why dialysis is still set as the norm while a transplant from the deceased donor waiting list may or may not happen, and the happy few will have a living donor that will prevent them from having dialysis. Living donor kidney transplantation, especially pre-emptively, is not a luxury—it is the best option for people, but we are not collectively organising it in our current pathways. Time to change!”

11 Issue 7 – April 2023 Pre-Emptive Transplantation
Transplantation
“Living donor kidney transplantation, especially pre-emptively, is not a luxury—it is the best option, but we are not collectively organising it in our current pathways. Time to change!”
Frank Dor
Neghae Mawla
Neghae Mawla (Dallas Nephrology Associates, Dallas, USA) of the American Society of Diagnostic and Interventional Nephrology (ASDIN) is an interventional nephrologist (IN) and a passionate advocate for the specialty. He speaks to Renal Interventions to explore interventional nephrology’s unique position in dialysis access care, the technologies that most excite him, as well as his award-winning barbecue products.
How did you develop your interest in interventional nephrology?
I knew I would end up in nephrology early on, but it just so happened my nephrology fellowship—at St Louis University (St Louis, USA)—was also an interventional programme. Before that, I did not know it existed, but after I got my feet wet doing the technical side I thought, “this is really cool”. The more I did, the more I liked it. My pathway into the interventional side of nephrology was not planned.
What is your philosophy as a physician, particularly in the dialysis space?
My philosophy is about figuring out what you do best and focusing on it. Nephrology is so general, but I focused purely on intervention after some time in both general and interventional. I realised I am more suited for this pathway, these patients and this particular technology, where my skill can really be focused. Other physicians can do in-office or in-hospital better than I can—why do I need to keep them? I redirected my focus to the procedural element, including the creation and maintenance of endovascular arteriovenous fistula (endoAVF). I think I can provide that service in a very capable manner, even next to some surgeons that are in the field. I really worked on picking specific skills and trying to better them.
Is there a case that has stayed with you and, if so, why?
There is always that case where it clicks. Part of learning and doing my fellowship was follow-
ing step A, step B, and so on. It was actually a thrombectomy case where it finally clicked that “oh, I am doing this because of that”—all the reasons behind the steps became clear. I was no longer walking through the motion of a procedure but now finally understood how I approached that procedure. It is about learning to approach this particular patient. That is when the growth begins. Otherwise, you are just doing the same thing every day and it is not necessarily as impactful. I have to think about why I do what I do and what could be different next time. When you start thinking about these things is when they really start to shift.
What are your goals and what would you hope to see for IN as a specialty?
I would hope to see IN as the leader in access care not only from a technical perspective but also as the drivers of what happens with the patient and their access. The nephrology background—the understanding of dialysis and how it works—puts us at an advantage to guide what needs to happen with a graft or fistula. We could be the leaders that guide dialysis access care.
What are the most exciting technological advances bringing new life into your practice?
The biggest one for me is endoAVF, using both Ellipsys (Medtronic) and WavelinQ (BD) devices. I have been using them for several years and they have given me ways to serve patients in ways I have not been able to before. There are so many
other developments that are coming, too—the Surfacer device (Bluegrass Vascular) for catheters has a big role to play. Technology developers are finally showing interest in dialysis patients, and that is a good thing.
What would you say your unique learnings have been from your experience with endoAVF?
The learning curve for endoAVF has been about figuring out when I can make the anastomosis and which patient actually gets a good, working, viable fistula from it. There have been several cases where I have said, “I can make a connection, but is it going to be what you need for dialysis? Maybe not.” Identifying those patients that are better off with a surgical arteriovenous fistula (sAVF) is important. I know what I can do, but should I do it? The red flags are anatomical. My screening process has changed over the years, since someone may even be an anatomical candidate but functionally not.
What is the research question you would really like to see answered?
The biggest question we would all like to see an answer to is, “How does an endoAVF really compare to an sAVF?” It is neither the same anastomosis, nor is it the same location. Some will say you cannot compare an endoAVF to an sAVF because there are different vessels and physiology. The question on everyone’s mind is: are they functionally as good, or is one better? It may be a difficult question to answer.
How do you think endoAVF has impacted dialysis care, and what are your thoughts on central venous catheters?
I think the patient convenience factors, expansion of services and providers and shorter wait times for patients of endoAVF have impacted dialysis care. As far as central venous catheters go, for some patients, it is completely appropriate. It goes back to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines: it is about the right access for the right patient.
What do you do radically differently in your practice compared to 10 years ago?
The mindset is about always growing. Today I think about treatment in terms of the whole lifespan of the access, and how I can address it for a longer impact. There are things I may or may not do as I look forward. Earlier in my career it was about “this patient, today’s problem, fix it, go home”. Now, it is about thinking about beyond today.
What advice would you give to young physicians interested in dialysis care?
Dialysis care is a fascinating field with complex patients requiring a dedicated physician. That is what enticed me into nephrology in general. It is a complicated specialty with so many components that make you stop and think. The interventional side is similar but different. There is a technical component, but it is still very intellectual. Some think it is just a technical discipline, but there is a lot of intellectual thought that could go into dialysis access. My advice to young physicians is to come to the specialty both with a big heart and a big mind.
Fact file
Current appointments: Interventional nephrologist, Dallas Nephrology Associates, Dallas, USA
Education:
1996–2001: MD: University of Texas Medical School at Houston, Houston, USA
2001–04: Internship: St Louis University School of Medicine, St Louis, USA
2004–06: Fellowship: St Louis University School of Medicine, St Louis, USA
Honours (selected): Fellow, American Society of Diagnostic and Interventional Nephrology
Profile 12 April 2023 – Issue 7
Q&A
Illustration by: Andy Watt / NB Illustration
Along your journey in your career, are there any particular figures who have been mentors to you?
So many people have been influential in shaping who I have become, it is hard to name just one or two. From each attending and mentor, I try to walk away with one or two lessons that can change how I care for my patients. And I continue to do this with people I meet along my journey. Everyone can help me grow, as long as I look for an opportunity for growth.
What would you like endoAVF 2.0 to look like?
I have been very happy with the current devices and endoAVF outcomes. But it would be nice if we could expand locations next. A wrist or snuffbox endo-anastomosis is what I am looking forward to.
What are your interests outside of medicine?
One of my passions is barbecuing and grilling. I have a line of barbecue seasoning and rubs that I set up with a friend. Most of my time outside of work is outside with the grill. That keeps me entertained and everyone fed. We do a community barbecue on occasion and organise competitions where I compete and judge. We actually got our start in barbecue when we entered a city competition with our own recipe, which we won. The people there said we should market it as a product. We are casual competitors, but everyone liked it so we said, “let’s put it out there”. Our most popular flavour is Texas Tandoori. It is a nice way to disconnect from work and to ground me. The company, Halal BBQ Pitmasters, takes up a lot of time, and the rest is spent travelling with my family.

Profile 13 Issue 7 – April 2023
“It is about learning to approach this particular patient. That is when the growth begins. Otherwise, you are just doing the same thing every day and it is not necessarily as impactful.”
Organ Utilisation Group recommends “standardised patient pathways” for transplantation
A report has been issued on methods for improving organ provision by the Organ Utilisation Group (OUG), a UK Department of Health-established body dedicated to making “recommendations on how to maximise the potential for organ transplantation from living and deceased donors”. The review, which was limited to England’s health services, focused on the transplantation procedure from the offer of an organ through to post-transplant care.

The authors of the report drew on patient focus groups and online surveys in building their “evidence base”, while also performing visits to transplant centres and meetings with both UK-based and international “expert advisors”. A literature review was also integrated into the report.
The report’s primary finding was that patients described concerns that the quality of care they received was tied to where they lived, as well as “their socio-economic status and their ethnicity”. They are also given incomplete or even contradictory advice on transplant procedures, while some patients expressed feeling “lost in the system” when moving between different parts of the health service.
In response to these issues, the OUG recommends that patients being considered for transplantation, referral or listing should receive better advice and support, while there should be a renewed focus on providing “equal access” regardless of location or other factors such as ethnicity, “personal circumstances” or gender. To achieve this, the authors state, communication with patients must be “timely” while information about centres’ performance should be easily accessible.
The report’s second recommendation is for transplant centres to better integrate the feedback of patients into how they operate. This, it argues, can be achieved through regular meetings between patients and their clinical team, while patient-reported outcomes and experiences should be evaluated “with similar levels of focus and scrutiny” as clinical outcomes, with the measures themselves developed with patient input.
One of the report’s most notable recommendations was for the development of standardised patient pathways for each organ type, each with “well-defined timescales” for every part of the pathway. Decline meetings should be a “mandatory requirement”, while standards should be developed to prevent lack of theatre availability and other non-clinical reasons for organ procedures failing to go ahead. The report also recommends a “Clinical Lead for Utilisation who is responsible for data oversight and monitoring” in every unit. Regular meetings of units to discuss ways to improve organ provision, drawing on data, should also be performed.
The National Health Service (NHS) in England should also perform a “comprehensive review of cardiothoracic services” to ensure their sustainability and “resilience”, the authors argue. They add that the use of international benchmarking and patient outcome data, which is held by NHS Blood and Transplant (NHSBT), will make up an important part of that review’s evidence.
Moving on to discuss the NHS workforce, the OUG describes how a “lack of a clear workforce template” affects the consistency of care received by patients in different units. Transplant teams described their struggles with recruiting and maintaining staffing levels. Clinicians in
transplant units also deal with stress and even mental health issues as a result of the insufficient support with which they are provided. As a solution, the report proposes workforce planning toolkits, which would help allocate staffing to centres according to local demographic information including waiting list size and catchment area. These toolkits may incorporate algorithms used to support planning. Patients should receive psychological and social care support before and after a procedure.
Data was a theme highlighted later in the report, with the digitisation of paper-based records a particular focus. This digitised data should then be integrated as part of the decision-making in the standardised patient pathways recommended elsewhere in the report. This will also support the development of national multi-organ centres, which makes up another major recommendation of the report. This, the authors argue, will help introduce “new techniques into everyday clinical practice as rapidly as possible”, while also maximising organ availability. They should also be supplemented with a “national oversight system” for “assessment, perfusion and preservation”, which it is argued would be especially important for perfusion that starts or occurs in situ.
“National measurable outcomes must be defined and agreed”, the authors argue, as a
means of measuring the success of new organ initiatives. “Optimal” organ utilisation demands a more specific definition, while new means of educating patients and families should be developed to ensure they fully understand outcomes. In a similar vein, service specifications should be better defined, smoothing the relationship between NHSBT and commissioners, while a financial framework should be used to cost the patient pathway and assist modelling of future costs and demand.
Commenting in the OUG report, transplant surgeon and clinical lead for organ utilisation in kidney transplantation Nicholas Inston at the Queen Elizabeth Hospital Birmingham (Birmingham, UK) states: “A major concern is the transplant workforce and the current requirements to deliver a service over the next few years are unclear. To achieve sustainability and resilience in delivering transplantation will require planning—defining standards for staffing and facilities would be a valuable outcome from the OUG.” Inston spoke to Renal Interventions to add: “The OUG report sets out the structure and changes that will be required to implement the Organ Donation and Transplantation 2030: Meeting the Need strategy, which sets out a ten‐year vision for organ donation and transplantation in the UK.” This, he notes in the report, “will require improvements in efficiency and application of innovative solutions”.
Looking forward, the report authors say that putting their proposals into action will require the work of “a wide range of organisations”, including many NHS trusts as well as NHSBT. Finally, they say engagement with patients, families, carers and living donors, which underpinned the report, should be performed throughout the organ transplantation process.
April 2023 – Issue 7 14 Organ Utilisation Group Report
“To achieve sustainability and resilience in delivering transplantation will require planning—defining standards for staffing and facilities would be a valuable outcome from the OUG.”
Renal denervation “can reach new shores”
The publication of a consensus statement from the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) is among the final steps in the evaluation of renal denervation as a device-based treatment for hypertension. This is according to Felix Mahfoud (Saarland University Hospital, Homburg, Germany), a member of the expert committee behind the paper, and one of the foremost investigators of the technique. He discusses patient selection and future indications for the therapy with Renal Interventions.

What is the background to the ESC/ EAPCI consensus statement on renal denervation?
This consensus document was deemed necessary because a significant amount of new sham-controlled trial evidence has become available. In the 2018 guidelines of the ESC and the European Society of Hypertension (ESH), device-based hypertension treatment was graded with a class 3 recommendation, not to be used routinely in clinical practice. There was a sentence added to the statement that until further evidence regarding the safety and efficacy becomes available these devices should not be used outside of clinical trials.
We felt it was important to re-evaluate the evidence that has aggregated after publication of the guidelines, and by now we have five sham-controlled clinical trials that have indeed proven the efficacy and safety of renal denervation in the presence and absence of antihypertensive drugs.
What have the recent trials shown and how are these reflected in the consensus statement?
We know it works in patients with and without antihypertensive medication, but this is a very broad potential patient population. We felt it is also important to provide some guidance on where denervation may be used in clinical practice and felt this should be reserved as a treatment option for patients with so-called “resistant” hypertension, meaning despite treatment with three antihypertensive drugs, they still have uncontrolled blood pressure values, as confirmed by office and ambulatory blood pressure.
Despite the fact that we have evidence that this works in a very broad potential patient population, we nailed it down, first and foremost, to patients with resistant hypertension. There is another potential indication for renal denervation and that is in patients where drugs are not tolerated,
patients who are not willing or able to take antihypertensive drugs, and patients who express a preference to be treated with a device-based approach.
How significant a development is the consensus statement?
It is not the intent of the consensus statement to change people’s perception. The perception needs to be adapted according to the published trial evidence. This is really more to inform clinical practice.
What we did here is reach consensus on different statements. The challenge is who to treat within clinical practice, and this is the overall objective of such consensus statements. Which patients in which centres? How should the centres be trained? What are the potential complications of the procedure that interventionalists need to be informed about?
Are there “ideal” patients for renal denervation?

What we have to accept is that this is a treatment possibility for patients with uncontrolled blood pressure. It is not replacing drugs, it is not replacing lifestyle modification—this is another approach available in our armamentarium to lower blood pressure. But, it is not exclusive. It is not renal denervation or nothing. Most patients we treat have undergone lifestyle modification, but it was unsuccessful. They have been treated with several drugs, are still uncontrolled and have high blood pressure and have high cardiovascular risk. In these patients it is another treatment that may bring blood pressure down.
You have studied renal denervation in great detail, how excited are you by this treatment?
We started the scientific evaluation of this approach 15 years ago. It is among the very, very few device-based treatments that has been investigated against and has
Blood pressure successfully cut in RADIANCE II ultrasound
renal denervation trial
A new trial of endovascular ultrasound-based renal denervation technology has found that it reduced blood pressure in hypertensive patients at two months. The RADIANCE II study, published in The Journal of the American Medical Association (JAMA), was led by Michel Azizi (Université Paris Cité, Paris, France) and Ajay Kirtane (Columbia University Medical Centre, New York, USA). The international, multicentre, sham-controlled randomised clinical trial aimed to determine the procedure’s safety and efficacy “in the absence of the potentially confounding influence of antihypertensive medications”.
Azizi et al sketch out some background to the trial in their introduction, where they state that the widespread availability of pharmacotherapy for hypertension, which is at the core of existing treatment for the condition, has not prevented many patients from being inadequately treated. Endovascular renal denervation using radiofrequency or ultrasound technology for renal nerve ablation, they say, has been examined as an adjunct to pharmacotherapy and lifestyle change, but that earlier studies with the radiofrequency technology including the DENERHTN randomised controlled trial, also led by Azizi, and the Simplicity HTN3 trial reported “inconsistent results”. They also refer to the further RADIANCE-HTN SOLO trial using ultrasound renal denervation led by Azizi to place RADIANCE II in context.
RADIANCE II took place across 37 centres in the USA and 24 in Europe, enrolling a total of 1,038 patients of whom 224 met all criteria for randomisation. Of these 224, 150 were randomised to a group receiving ultrasound renal denervation and 74 to a sham procedure group. The mean age was 55 years, while 28.6% of patients were women. Antihypertensive medications were being taken
by 147 patients (65.6%) at the time of their enrolment, while the rest had previously received but ceased taking them. Those patients receiving renal denervation underwent a procedure of an average length of 77 minutes, compared with 44 minutes for the sham procedure. Bilateral ablation was successfully carried out in 148 of 150 patients (98.7%), with 30 (20%) of these having accessory renal artery ablations.
The primary efficacy outcome of the trial was the mean change in daytime ambulatory systolic blood pressure (SBP) at two months, with patients of both groups maintained off medications except for when blood pressure exceeded a certain level. There was also a primary safety outcome which was a composite of major adverse events including death, kidney failure, and major embolic, vascular, cardiovascular, cerebrovascular, and hypertensive events at 30 days and renal artery stenosis greater than 70% detected at six months.
Turning to the results, the study authors found that ultrasound renal denervation brought a greater reduction
beaten sham control. This is something we have to keep in mind. There has been a very rigorous evaluation of procedure, which is not available for other techniques that we are using every day. This is something we have to acknowledge, that we have a lot of clinical data, a lot of robust methodologically defined and properly designed studies conducted around the world and they have proven that this technology lowers blood pressure.
I am excited, of course, because it is about science, but this is probably among the last steps in the evaluation of this technology and this technique. We are working on a US Food and Drug Administration (FDA) submission so hopefully this will become available in the USA soon.
We are now looking into new indications such as heart failure, atrial fibrillation and ventricular tachycardia. We have registry data confirming that in those populations it is safe, and now we are heading off to new shores and among those are heart failure, certainly, and arrhythmias are very interesting also.
Where are the gaps in our knowledge regarding renal denervation?
Identification of responders is the unmet need, something that we are investigating in clinical studies to get further insights, and has never become available even for antihypertensive drugs. I am not sure if we will succeed in this with renal denervation, but we are still trying.
The second question is whether or not this blood pressure lowering brings improved outcomes. We know blood pressure as LDL [low-density lipoprotein] cholesterol is closely associated with cardiovascular morbidity and mortality, and when you lower thatl, it is believed that it lowers morbidity and mortality. I am pretty confident that this will translate into improved outcomes—but it has not yet been shown.
in daytime ambulatory SBP (mean, −7.9mmHg [standard deviation (SD), 11.6mmHg]) vs. the sham procedure (mean, −1.8mmHg [SD, 9.5mmHg]; baseline-adjusted between-group difference, −6.3mmHg [95% CI, −9.3 to −3.2mmHg], p<0.001), which the study authors reported to be consistent throughout the 24-hour circadian cycle. “Among seven secondary blood pressure outcomes,” Azizi et al also reported, “six were significantly improved with ultrasound renal denervation vs. the sham procedure.”
Discussing the findings, the authors note that patients in RADIANCE II had more severe hypertension than those in RADIANCE-HTN SOLO, while they also state that “the homogeneity of the blood pressure-lowering effect of ultrasound renal denervation, independent of the method of blood pressure measurement” is promising.
Azizi et al pointed to several limitations of RADIANCE II. The first noted was the limited period of follow-up of two months—though plans for further follow-up are mentioned. The trial was also “limited to those at low cardiovascular risk with an estimated glomerular filtration rate (eGFR) of 40 mL/ min/1.73m2 or greater and without significant comorbidities”, something that owed to the trial’s design, which required the withdrawal of blood pressure medications. Therefore, they suggest, the generalisability of the trial’s results to patients with the most severe hypertension could be limited—though its results are promising for those with hypertension which is resistant to existing treatments, as previously shown by Azizi et al in the RADIANCE TRIO trial.
The authors add that the effects of the procedure on any given individual patient may be difficult to predict as a result of the “variability in the prevailing state of sympathetic hyperactivity or variable renal nerve ablation”.
Issue 7 – April 2023 15 Renal Denervation
Michel Azizi
Felix Mahfoud
Over half of dialysis technicians report burnout in US survey
Work exhaustion and a lack of fulfilment are driving burnout among US dialysis patient care technicians (PCTs), according to a new survey whose results have been published in the American Journal of Kidney Diseases (AJKD).
The results corroborate those of a 2021 study in the Journal of Nursing Management by Xiaoyi Cao and Lin Chen (Sichuan University, Chengdu, China) which found that “higher levels of compassion fatigue and lower levels of resilience and work engagement” drive increased turnover in the dialysis workforce. The resilience of this workforce is a core concern of the recent Organ Utilisation Group report in the UK, while this AJKD study, led by Laura C Plantinga (Emory University, Atlanta, USA), makes clear the “imperative” to cut turnover. Plantinga and colleagues describe how “high professional fulfilment and low burnout” are necessary for “a stable dialysis workforce”.
To investigate the experiences of these technicians, the study authors designed a cross-sectional national US survey including PCTs from the National Association of Nephrology Technicians/ Technologists (NANT). Members of the association numbering 228 were surveyed in the period March-May 2022, with 42.6% aged 35–49, 83.9% female and 64.6% white. The exposures outlined by the authors included Likert-scale items of a range of 0–4 , with these “related to professional fulfilment and two domains of burnout (work
exhaustion and interpersonal disengagement)”, as well as “dichotomous items related to turnover intention”.
Setting out the study’s analytical approach, the authors explain: “Summary statistics (percentages, means, medians) were calculated for individual items and average domain scores. Burnout was defined by combined work exhaustion and interpersonal disengagement scores of ≥1.3 and professional fulfilment by a score ≥3.0.”
Of those who responded to the survey, almost three quarters (72.8%) worked ≥40 hours per week. The authors report that “work exhaustion, interpersonal disengagement, and professional fulfilment [median (interquartile range)] were 2.3 (1.3–3.0), 1.0 (0.3–1.8), and 2.6 (2.0–3.2), respectively”. Burnout was reported by 57.5% of respondents, while professional fulfilment was reported by only 37.3%. The survey also examined respondents’ views on the reasons for burnout, with 66.5% reporting salary-related issues and 64.0% supervisor support issues. Other points raised included a sense of doing purposeful work (54.5%), hours worked (52.9%) and respect from fellow dialysis staff (57.8%). Asked whether they planned to still be working as a
Home haemodialysis: The path to patient empowerment

Home dialysis can offer patients freedom that in-centre dialysis cannot, but it also improves their quality of life relative even to peritoneal dialysis—as well as providing the best outcomes. By
FOR DECADES, LIFE-PRESERVING dialysis treatment has been perceived and experienced by end-stage renal disease (ESRD) patients as complex, inflexible and disruptive to one’s life and activities. Patients who “crash” into maintenance dialysis in the acute setting in the USA are typically funnelled directly into an assigned weekly treatment schedule at a dialysis clinic or hospital. However, this long-held, disempowering, “one-size-fitsall” standard of care has been exposed to be unsustainable in a new age of patient-centred and user-friendly dialysis technology. Providers are finally overcoming long-held barriers to offering patients the ability to live life on their terms, with restored autonomy, agency and control, along with improved clinical outcomes.
Michael Aragon
cost, improving efficiency and demonstrating better outcomes. However, for most patients, this option has either been unavailable, inaccessible or too complex.
dialysis PCT in three years, only 52.6% said yes.
The authors describe the survey respondents as a “relatively engaged group of dialysis PCTs”, which they suggest makes it all the more “critical” that only around half intended to continue in the role. They note their survey has “limited generalisability to all US dialysis PCTs”, but that it appears nevertheless “imperative” to improve morale and cut turnover among this group.
Speaking exclusively to Renal Interventions, Plantinga reviewed the results: “While burnout and turnover are high among these critical frontline workers, our results highlight that there is hope to mitigate this worsening dialysis workforce crisis: dialysis patient care technicians still reported enjoying the intrinsic rewards of patient interactions (even in the setting of work exhaustion) and they also identified specific targets for intervention, including shorter work hours, lighter patient caseloads, more training, and greater respect and autonomy within the dialysis care team, which could improve working conditions and lessen burnout.”
Michael Aragon
Peritoneal dialysis (PD), more widely used than HHD, does offer improved quality of life for patients managing their own treatment in comparison to in-centre dialysis, but is not the long-term answer for most patients. Patients are burdened by needing to treat eight to 10 hours, seven days a week. Cardiovascular-related mortality is higher when compared to in-centre haemodialysis patients who have an arteriovenous fistula (AVF)1, half of patients will fail on PD after two years2 and half of PD patients are chronically fluid overloaded.3
Perceived barriers associated with implementing HHD successfully have come from the provider and patient side, but innovative new technology is helping to ease many of these fears and give both groups confidence. With prior technology, a low level of HHD education amongst providers, physician unfamiliarity with its unique prescribing of flow fraction and dialysate volume and the complexity of the device have impacted the ability to successfully send more patients home. Patients’ concerns include the burden of care they would take on themselves at home, the fear of unsuccessful treatment at home, and also not wanting to turn their home into a clinic-like setting.
Innovation is the key to alleviating these fears. As we look back through recent history, innovation has often facilitated solutions for some of the greatest healthcare challenges the world has faced. Advancements in cardiology, surgery, prosthetics and neurosurgery have improved care efficiency, outcomes and patient quality of life.
patient-centred technology facilitating personalisation of care that is safe, effective and easy to learn and manage9, and provides an enhanced patient experience. The way ahead is here: it’s home dialysis.
Disclosures: Michael Aragon is chief medical officer at Outset Medical.

References:
1. Perl J.Wald, R.McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol. 2011; 22:1113-21
2. CDRG analysis of USRDS data from 2020 report.
3. Evolution Over Time of Volume Status and PD-Related Practice Patterns in an Incident Peritoneal Dialysis Cohort. Wim Van Biesen, Christian Verger, James Heaf, et al. CJASN June 2019, 14 (6) 882-893; DOI: https://doi.org/10.2215/CJN.11590918
4. Jaber BL, Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM Study. Am J Kidney Dis. 2010
What is making this shift possible?
Something counterintuitive to the traditional in-clinic model: focus on the patient experience.
Home haemodialysis (HHD) has been used for decades on a small percentage of patients in developed nations throughout the world, allowing them to perform their own treatments at home, while reducing
What’s more, a growing body of clinical evidence is revealing impressive benefits of HHD versus in-centre haemodialysis. Findings include an improved patient quality of life, reduced post-dialysis recovery time4, lower drug burden5,6, lower mortality7 and lower risk of infection exposure. Nephrologists and dialysis nurses have even stated that they would choose HHD for themselves, over all other dialysis options.8
Dialysis, after decades of complexity and inflexibility, and fuelled by a rapidly expanding ESRD population, is primed and ready to follow suit. The patient voice for improved quality of life, empowerment and better overall care has never been louder. The pandemic has exposed the inefficiency and limitations of in-centre dialysis. Dialysis at home must become the standard for all. Not out of preference, but out of necessity. Technology innovation has once again provided a solution. The development of the Tablo Haemodialysis System, for instance, represents a new
5. Kotanko P, Effects of frequent hemodialysis on blood pressure: Results from the randomized frequent hemodialysis network trials. Hemodial Int. 2015
6. Daugirdas JT, Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;
7. Marshall, Mark R, Home Versus Facility Dialysis
8. Schiller B, Neitzer A, Doss S. Perceptions about renal replacement therapy among nephrology professionals. Nephrol News Issues. 2010 Sep;24(10):36
9. Plumb TJ, Alvarez L et al, Safety and efficacy of the Tablo hemodialysis system for in-center and home hemodialysis. Hemodial Int. 2019 Nov; 24(1):22-2
April 2023 – Issue 7 16 Dialysis
“While burnout and turnover are high among these critical frontline workers, our results highlight that there is hope to mitigate this worsening dialysis workforce crisis”
Laura C Plantinga
Clinical and Industry News
out the specifics behind the recall as well as the organisation’s advice on how to proceed for those affected.
Aortix pMCS pump provides “rapid” decongestion for patients with heart failure and renal impairment

Procyrion, a medical device company which aims to improve outcomes for patients with cardiac and renal impairment, has announced that use of its Aortix percutaneous mechanical circulatory support (pMCS) device led to rapid decongestion in a pilot study of patients admitted to the hospital with acute decompensated heart failure (ADHF) and worsening renal function, which is known as cardiorenal syndrome (CRS).


These patients, who were unresponsive to available medical therapy, demonstrated significant improvements in kidney function, cardiac function, and patient-reported assessment of shortness of breath at 30 days after treatment with the Aortix pump. The results from the CRS pilot study have been presented during the late-breaking clinical science session at the Technology and Heart Failure Therapeutics (THT) 2023 conference (20–22 March, Boston, USA).
Late-breaking data presented at THT showed substantial fluid loss and improvements in kidney and cardiac function at 30 days after Aortix pump therapy in patients admitted to the hospital with ADHF and worsening renal function.
“Patients admitted to the hospital with CRS are the most difficult to treat. They often have persistent congestion even after aggressive intravenous diuretic therapy, leading to very poor outcomes with high rates of mortality and rehospitalisation,” stated Jennifer A Cowger (Henry Ford Hospital, Detroit, USA). “After being unresponsive to available medical therapy for more than a week, patients who were treated with the Aortix pump showed a significant increase in urine output resulting in a large loss of excess fluid and improvements in kidney function present at 30 days, indicating the potential of the Aortix therapy to disrupt the harmful cycle of CRS.”
Medtronic recalls Mahurkar catheter due to “potential hub defect”

A catheter produced by Medtronic and used in haemodialysis has been recalled due to a defect that “may cause serious adverse health outcomes”.
An update on the US Food and Drug Administration (FDA) website has laid
The update describes a catheter hub defect in the Mahurkar acute dual lumen high-flow (13.5 French) haemodialysis catheter, which is also used for apheresis and infusion. It states that this may lead to “leaks across the catheters’ tubes”, which in the course of treatment could cause the mixing of arterial and venous blood, as well as “increased recirculation and poor dialysis” and even the development of clots in blood vessels. These complications could lead to the need for surgical removal of the catheter or bleeding. Medtronic advises that use of a faulty device could lead to “inadequate treatment, unintended radiation exposure, haemolysis, thrombus formation, embolism, delay to treatment, and potential infection”.
So far, there have been two reports of injuries related to the issue, though no reports of death. The release states that those receiving or administering care with the catheter may be affected, before it proceeds to cite a 7 December, 2022 letter from Medtronic for recommendations. For those with a device already in use, it says, clinicians should follow routine assessment procedures for “patency, function and efficacy” of the device, while also monitoring for movement of the catheter’s contents between its venous and arterial lumens. “It will not,” it is stressed, “present as an external leak or defect.” If the defect is detected, “clinical judgment” should be used to determine the “necessity and timing” of replacement.
Talaris Therapeutics announces restructuring and clinical trial discontinuations
Talaris Therapeutics has announced that it has completed a review of its business and programme prospects. Based on this review, Talaris has decided to discontinue its FREEDOM-1 and FREEDOM-2 clinical trials evaluating FCR001’s ability to induce durable tolerance in living donor kidney transplant recipients. This decision was primarily attributable to the pace of enrolment and the associated timeline to critical milestones. The company will continue to enrol its FREEDOM-3 Phase 2 clinical trial evaluating FCR001’s ability to induce tolerance in scleroderma.
The company has initiated a comprehensive review of strategic alternatives focused on maximising shareholder value, including possible business combinations and/or a divestiture of the company’s cell therapy chemistry, manufacturing and controls (CMC) capabilities. The company has not set a timetable for completion of this strategic review and does not intend to comment further on the status of this process unless or until its board of directors has approved a definitive course of action, or it is determined that other disclosure is appropriate. There can be no assurance that this strategic review will result in Talaris pursuing a transaction or that any transaction, if
pursued, will be completed on attractive terms.
In connection with the evaluation of strategic alternatives and in order to extend its resources, Talaris is implementing a restructuring plan that includes reducing its workforce by approximately one-third, with remaining employees primarily focused on maintaining the company’s cell therapy CMC capabilities and executing FREEDOM-3.
New anaemia options amid first US FDA approval for oral treatment
The US Food and Drug Administration (FDA) has approved Jesduvroq tablets (daprodustat, GlaxoSmithKline) as an oral treatment for anaemia caused by chronic kidney disease (CKD) for adults who have been receiving dialysis for at least four months. Jesduvroq is not approved for patients who are not on dialysis. Other FDA-approved treatments for this condition are injected into the blood or under the skin.
“With an oral drug option in addition to the FDA-approved injection options, adults with chronic kidney disease on dialysis now have multiple ways to treat their anaemia,” said Ann Farrell of the Center for Drug Evaluation and Research at the FDA. “This approval demonstrates the FDA’s commitment to helping bring a range of therapeutic options to patients with chronic diseases. Patients can consult with their healthcare providers to select the option that is most appropriate.”
Trial of histotripsy system for kidney tumours approved by US FDA
HistoSonics, the developer of a noninvasive platform and novel sonic beam therapy called histotripsy, has announced that the US Food and Drug Administration (FDA) has approved the company-sponsored #HOPE4KIDNEY trial, which is designed to evaluate the safety and technical success of the Edison system in targeting and destroying targeted primary renal tumours completely non-invasively, and without the need for incisions or needles. FDA approval of the #HOPE4KIDNEY investigational device exemption (IDE) trial comes after the company recently submitted results to the agency from its #HOPE4LIVER trials where the studies met both primary endpoints of safety and efficacy in destroying targeted liver tumours.
“We are very pleased with the FDA’s approval of our #HOPE4KIDNEY trial and appreciative of the agency’s prompt review process. This approval represents a substantial milestone for our company as we continue to expand histotripsy and its potential benefits into diseases that impact the lives of so many people,” commented Mike Blue, president and CEO of HistoSonics.
Blue added, “We are excited to expand on our experiences in successfully targeting and treating in the liver using our enhanced Edison platform that combines advanced imaging and targeting capabilities with real-time treatment monitoring. The kidney is a logical next application, as treating in the kidney has very similar procedural and anatomical considerations as the liver, and Edison was specifically designed to treat anywhere in the abdomen, as a starting
point. In addition, the prevalence of kidney disease remains high with many patients kept in active surveillance or watchful waiting.”
Donor kidney preservation technology “may enable us to salvage more organs”
Transplant experts at Hackensack University Medical Center have been the first in the USA to use new kidney preservation technology that can expand the window of time in which a kidney is viable—reducing the time it takes to receive a kidney transplant.
Now the team is evaluating this approach to see if it extends the lives of donated organs, and whether it can thus expand the number of donor kidneys that are viable for transplantation, helping to address the organ shortage in the USA. The technology, known as the Kidney Assist device (XVIVO), works by keeping a donated kidney warm, functioning, and supplied with nutrients outside of the body prior to transplant. Doctors use the experimental device to supply blood and oxygen to a human kidney at normal body temperature.
Alio receives second US FDA clearance
Alio, the medical technology company behind the SmartPatch device for chronic disease, has announced its latest US Food and Drug Administration (FDA) clearance. The company’s second clearance added three additional metrics to its remote patient monitoring platform—haematocrit, haemoglobin and potassium.
The platform consists of a SmartPatch, hub, and portal, which noninvasively capture clinical-grade metrics for care teams. Alio makes it possible for patients and their care teams to monitor their condition on an ongoing basis by issuing actionable notifications. With this clearance, the SmartPatch is cleared for use in monitoring key metrics including skin temperature, auscultation, heart rate, haematocrit, haemoglobin, and potassium.
While the technology can be deployed to monitor a number of chronic conditions, the company’s first area of focus is patients with end-stage kidney disease (ESKD). Until now, there has been little-to-no advancement in care management or innovative solutions for patients on dialysis and their care teams, an Alio press release states. Patients with ESKD, it adds, are at particularly heightened risk for hospitalisations due to complications like fluid overload, dyskalemia, and more. The current standard of care only monitors key metrics through a monthly blood draw whereas the Alio SmartPatch painlessly takes readings every few hours.
Latest News 17 Issue 7 – April 2023
Alio SmartPatch
Procyrion Aortix Pump therapy
Kidney Transplant Collaborative announces second phase of funding
The Kidney Transplant Collaborative (KTC) has received significant new funding that it says will “empower the organisation’s mission to reduce barriers in the transplant system with the ultimate goal of increasing the number of individuals able to receive a kidney transplant”. This new funding will enhance the KTC’s legislative efforts regarding living kidney donation and further support awarded grant projects that have achieved successful outcomes towards increasing kidney transplants.
A US national non-profit advocacy organisation, the KTC was founded in 2021 with the goal of reducing barriers for kidney patients, donors and families during the transplantation process. The KTC is funded by Ed and Penelope Peskowitz. Ed Peskowitz is a recipient of a kidney donation.
In February 2022, the KTC announced its inaugural grant recipients, who received funding for projects to improve the kidney transplantation process. This new funding will further support those projects that have shown potential in making a significant impact in increasing kidney transplants. The additional funding will provide the organisations with the ability to implement the projects regionally and nationally, saving more lives through kidney donations.
ASN and Home Dialysis University announce nephrology fellow education programme
The American Society of Nephrology (ASN) has announced a collaboration with the Home Dialysis University (HDU) to improve nephrology trainees’ knowledge, proficiency, and exposure to home dialysis therapies. Through this new collaboration, ASN will provide up to 30 scholarships for selected fellows to attend an in-person HDU fellows training course while, in partnership with HDU, ASN will launch a new 12-month virtual educational programme.
Home dialysis has been associated with lower cost and equal or better clinical outcomes than facility-based dialysis. It has been estimated that up to 85% of patients may be suitable to receive home dialysis. Yet, in 2019, only 12.6% of Medicare patients receiving dialysis underwent dialysis at home. Racial and ethnic minority populations have a disproportionately high risk of kidney failure. Despite this, minority populations are even less likely to be treated with home dialysis.
“Educating future nephrologists about home dialysis is foundational to ensuring all kidney failure patients have access to dialysis options that best fit their lives and needs”, said Michelle Josephson (University of Chicago, Chicago, USA), president of the ASN. “These are exciting times as ASN demonstrates its commitment to that effort by improving access to high quality home dialysis education for nephrology trainees. This collaboration between HDU and ASN represents a key initial step in that commitment.”
Enhanced ultrasound superior to MRI for diagnosing certain kidney tumours, study finds
Contrast-enhanced ultrasound (CEUS) is more accurate and reliable than magnetic resonance imaging (MRI) for examining certain kidney and liver nodules, according to two new studies published in the Journal of Ultrasound in Medicine (JUM) and highlighted by the International Contrast Ultrasound Society (ICUS).
An Ohio team reviewed clinical data over a 10-year period to assess the accuracy of CEUS diagnoses of benign kidney nodules in 341 patients. Their blinded analysis found that none of the CEUS diagnoses changed during that period.
“Our data confirm that when CEUS determines a kidney mass is benign, the mass is benign and no further follow up is needed—sparing the patient from unnecessary downstream tests, anxiety and costs,” according to Richard G Barr (Northeast Ohio Medical University, Rootstown, USA), the lead author of the kidney CEUS study.
“We also found that CEUS is a better and less expensive first-step evaluation for newly discovered liver nodules,” said Stephanie Wilson (University of Calgary, Calgary, Canada), principal investigator of the liver CEUS study. Wilson is also co-president of ICUS.
“CEUS found malignant or premalignant diagnoses that would likely have been missed or delayed if we had not used CEUS in these patients,” according to Wilson. This study shows that CEUS scans are at least equivalent if not superior to MRI for evaluating these liver lesions, and CEUS should be the first investigation for nodules found on surveillance for liver cancer, according to Wilson.
Interventional Systems announces endourology venture with surgical robot tech
Interventional Systems has announced a new venture—called LARC Robotics— aimed at using surgical robotics for percutaneous renal access.
Austria-based Interventional Systems said the unit simplifies percutaneous renal access in percutaneous nephrolithotomy (PCNL) procedures. This minimally invasive surgical technique helps to manage kidney stones.
The company said in a news release that Udo Nagele (Landeskrankenhaus Hall Hospital, Hall in Tirol, Austria) led the first surgeries at Landeskrankenhaus Hall. It plans to spend the coming months validating technical and clinical
claims in both the USA and Europe. Interventional Systems launched this venture just two weeks after a personnel change in its C-suite. New CEO Pedro Costa said the company launched LARC Robotics “after understanding the market need”. Its robot simplifies gaining renal access as it allows for targeting under real-time fluoroscopy. LARC Robotics intends to offer it in combination with a digital platform for visualising patient-specific, high-fidelity, 3D reconstructions of diagnostic scans. The platform also features a data and analytics module for postprocedural analysis.
EU ministers approve changes to MDR transition timetable
The European Union’s Council of Ministers has today adopted a resolution to extend the deadline for the certification of medical devices under the Medical Devices Regulation (MDR). Producers of medical devices will have until 31 December 2027 for higher risk devices and until 31 December 2028 for medium and lower risk devices to meet the legal requirements.
The extension of the transition period will be granted under certain conditions, ensuring that only devices that are safe and for which manufacturers have already started the certification procedure will benefit from the additional time.
The regulation—which changes the way that medical devices are certified for use in the European market—first came into effect in 2021 with an initial three-year transition period, having been delayed by one year in 2020 due to the onset of the COVID-19 pandemic. However, challenges in the implementation of the legislation led to concerns about a potential shortfall in the availability of certain devices, which prompted a rethink in the timetable for the regulation as proposed by the European Commission in December.

UK MHRA awarded £10 million to fast-track patient access to medical products
A total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)— an executive agency of the UK Department of Health and Social Care (DHSC)—to help bring innovative new medicines and medical technologies to UK patients more quickly, His Majesty’s (HM) Treasury has announced.
The funding will be used to accelerate routes for bringing innovative medical products developed in the UK onto the market, as well as those made and approved by other trusted regulatory partners globally.
Over the next two years, the MHRA will use the money to support development of a thorough but shortened process to speed up the
approval process for cutting-edge treatments developed in the UK with the greatest opportunity to meet the country’s healthcare priorities, such as cancer vaccines and artificial intelligence (AI)-based therapeutics for mental ill-health.
It will also support the establishment of an international recognition framework, allowing the MHRA to capitalise on the expertise and decision-making of trusted regulatory partners, and provide patients with fasttrack access to best-in-class medical products that have been approved in other countries.
Medtronic, DaVita launch Mozarc Medical, a new company aimed at accelerating kidney care innovation
Medtronic and DaVita have announced the launch of Mozarc Medical—an independent new company that, they state in a press release, is “committed to reshaping kidney health and driving patient-centred technology solutions”.
“Mozarc Medical’s focus will be on meaningful and innovative kidney health technologies that improve the overall patient experience and increase access to care globally,” said Ven Manda, CEO of Mozarc Medical. “At a time when patient preferences are evolving and inhome kidney care is on the rise, Mozarc Medical is uniquely positioned to better serve patients with kidney disease around the world.”
Central to the creation of Mozarc Medical, the press release adds, is its global workforce, which includes the former Medtronic Renal Care Solutions (RCS) business (now part of Mozarc Medical) and “other industryleading talent hired to advance the new company’s strategic mission”. In addition to Manda, a 28-year veteran of Medtronic, the former RCS leadership team has also transitioned to serve as Mozarc Medical’s leadership team.
Xeltis secures funding to progress clinical trials
Xeltis has raised €32 million in a Series D2 equity fundraise, backed by a syndicate of current and new investors, which the company says will enable it to progress its clinical programmes into pivotal trials. Investors include Grand Pharma, DaVita Venture Group, EQT Life Sciences, Invest-NL and others.
Xeltis’ proprietary endogenous tissue restoration (ETR) platform, states a press release, utilises “an advanced polymer-based material which triggers the body’s natural healing response to regenerate the patient’s own tissue around it, forming new, living and longlasting vessels and valves”.
The company’s most advanced program, aXess, is a vascular access graft for patients with chronic kidney disease (CKD) requiring haemodialysis. Xeltis is also pursuing clinical programmes in pulmonary valve replacement and coronary artery bypass grafts.
Alongside the equity fundraise, Xeltis and Grand Pharma have completed a license deal, covering Greater China, for aXess and other potential haemodialysis products developed under the same technology platform. Under the agreement, Grand Pharma will have exclusive rights to develop, produce and commercialise these products in Greater China.
Latest News 18 April 2023 – Issue 7
Clinical and Industry News LARC PCNL device
Conference Calendar
25–28 April
Charing Cross (CX) Symposium 2023 London, UK cxsymposium.com
26–29 April
13th International Congress of the Vascular Access Society (VAS) Porto, Portugal vas2023.com
19–21 May
Vascular Access Society of the Americas (VASA) Hands-On Practicum on Hemodialysis Access Houston, USA vasamd.org/events/2023-practicum
Meet our editorial board
Nicholas Inston
Chairman of the Editorial Board
Nicholas Inston is a transplant and vascular access surgeon, and the clinical service lead for renal surgery, at Queen Elizabeth Hospital in Birmingham, UK.



3–7 June
American Transplant Congress (ATC) San Diego, USA atcmeeting.org
5–7 June

UK Kidney Week (UKKW) Newport, UK ukkw.org
6–9 June
Leipzig Interventional Course (LINC) Leipzig, Germany leipzig-interventional-course.com
14–17 June
Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM23) National Harbor, USA vascular.org/vam-2023
15–18 June
60th European Renal Association (ERA) Congress Milan, Italy era-online.org/en/milan2023



23–24 June




Endo Vascular Access Meeting Patras, Greece evameeting.org
Ziv Haskal
Board Member
Ziv Haskal is a professor of radiology, and an interventional radiologist and interventional oncologist, at the University of Virginia School of Medicine in Charlottesville, USA.


Stephen Hohmann
Board Member
Stephen Hohmann is a vascular and general surgeon at the Texas Vascular Associates clinic in Dallas, USA.

Board Member
Robert Jones is an interventional radiologist at Queen Elizabeth Hospital in Birmingham, UK.

19 Issue 7 – April 2023 Conference Calendar *Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the field of renal disease management Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit renalinterventions.net and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** this issue: Wrapsody results dialysis care in the spotlight and the American Society of Nephrology nosing kidney disease. In its report, the NKF-ASN Task Force on Reassessing the the new estimated glomerular ltration creatinine equation that estimates kidney Tcommonly used biomarker kidney funcpatients and family members, medical students and other “This recommendation by the NKF-ASN task force an this new approach rapidly as possible that we can In the USA, more than 37 million adults have kidney work into three phases. The rst involved clarifying the asserting that race modi ers Bioarti cial device receives KidneyX award after reaching preclinical testing kidney device (iBAK) has moved closer tested the two essential components kidney functions, Prize, the team married these two units provide continuous renal replacement cial Kidney Prize—becoming one of six “The NKF and ASN urge all laboratories and healthcare systems nationwide to adopt this new approach as rapidly as possible so that we can move diagnosing kidney diseases that independent of race.” Paul Palevsky Renal community reckons with removal of race variable in kidney disease diagnosis
Robert Jones