Haemodiafiltration mortality risk found lower than haemodialysis
The publication in The New England Journal of Medicine (NEJM) of new research on haemodiafiltration comes following a series of inconclusive prior studies. Lead author Peter J Blankestijn (University Medical Center Utrecht, Utrecht, The Netherlands) and colleagues note that previous trials and study-level meta-analyses have not resolved the “uncertainty” around the therapy’s ability to provide better outcomes for end-stage kidney disease (ESKD) patients, despite differences in practice across continents. Their pragmatic, open-label, randomised, controlled trial (RCT) now offers further clarity on haemodiafiltration’s survival benefit compared with conventional haemodialysis.


IN FOUR PREVIOUS RCTS COMPARing the two treatments, only one—that of Francisco Maduell (University of Barcelona, Barcelona, Spain) et al in the Journal of the American Society of Nephrology (JASN) in 2013—found a statistically significant survival advantage for haemodiafiltration, Blankestijn et al note. These trials also saw the raising of “concerns about attrition” by “observers in the scientific and nephrology communities”, the authors state.
With the need for more data in mind, the study authors designed the CONVINCE trial, in which 1,360 patients underwent randomisation at 61 centres across eight European countries. Of these, 683 patients were assigned to a group receiving highdose haemodiafiltration while 677 received high-flux haemodialysis.

All patients were aged ≥18 years and had a kidney failure diagnosis, and were candidates for high-dose haemodiafiltration, meaning “a convection volume of ≥23l in postdilution mode per session”. Among the exclusion criteria were “severe non-adherence” to dialysis and prescriptions as well as a life expectancy of less than three months, previous haemodiafiltration less than 90 days before screening and expectation of kidney transplant within six months of screening.
The trial’s primary outcome was identified as all-cause mortality, while secondary outcomes included cause-specific mortal-
Continued on page 2
A renal disease diagnosis is “a shock at any age”, and its “huge impact” on the lives of patients and their families is something that takes time to process. Doctors should engage better with patients and help to talk them through the experience—that was the message from Vivian Papageorgiou, a patient representative at the recent Endo Vascular Access (EVA) Meeting (23–24 June, Patras, Greece) who spoke to Nicholas Inston (Queen Elizabeth Hospital Birmingham, Birmingham, UK) to describe her experience of kidney disease and the host of treatment modalities she has undergone. The patient experience was also painted vividly by Phil West in conversation with Ounali Jaffer (Royal London Hospital, London, UK).
Papageorgiou’s journey with kidney disease, which has encompassed pre-dialysis care, dialysis, and multiple kidney transplantations, began at age 23 with a diagnosis of IgA nephropathy. Drawing on that experience, Papageorgiou, asked what could have improved it, said: “As patients, we understand that doctors have little to no time to sit down and connect individually with each and every patient, but I do believe that just a few moments of their time would have a very positive impact on the patient’s outlook and mental wellbeing during this tumultuous time.
“Firstly, [it would help] to take the time to explain to them exactly what is happening to their bodies and why—should there be a clear diagnosis—and then to be presented with their possible treatment options in a way that would best suit each patient given their background and lifestyle.”
She added that “graphs and statistics are black and white, but you need to talk to patients about how best to fit
a treatment into their lives. Connect mentally with patients, because what they are going through is shocking—every time. It is not easy for anyone,” she said.
Speaking elsewhere to Renal Interventions, Papageorgiou expanded on her personal experience. “The fact that it happened at a very young age made it especially difficult for me to process. Everything had to happen very quickly to ‘fix it’ […] and when you are not given that time, it affects you mentally.



“I wish medicine could find a better way to do dialysis—to give [patients] fewer restrictions, let them be able to travel, to just make them feel better in between sessions. It is a lifeline, but what kind of life is that?”
On her experience at EVA, she was upbeat. “Coming in, I was not exactly sure how [the physicians attending] would feel about me being sentimental and talking about the mental impact. But I am glad that I did—I see that the response was great, and I hope that it does help them in the long term to help others feel more understood.”
Another patient representative who featured as a speaker at EVA 2023 was West, who spoke to Jaffer on his 27-year experience living with chronic kidney disease (CKD). After an initial IgA nephropathy diagnosis in 1996, he said, he received a kidney transplant that year. He has also undergone both in-centre and home haemodialysis.
West highlighted the comorbidities associated with his diagnosis and treatment, including heart disease and a triple heart bypass, skin cancer from immunosuppressants, and bone disease from calcification breakdown. Jaffer noted that, given the frequent focus on kidney disease itself, these accompanying conditions were sometimes apportioned less consideration.
“It is a long journey,” West said. Echoing Papageorgiou’s comments on CKD’s mental impact, he said: “Not only [because of] those physical conditions, but the mental aspects as well. It can be very tiring—there is a lot to do, particularly if you do home dialysis, for you and for your family. I have a fantastic support team behind me, but the thing that stands out for me now is the mental factor. I would like, if possible, to see more support and understanding for this part of the disease.”

In this
issue: FDA update lowers red flag on paclitaxel page 4 ESKD Summit summarised page 6
September 2023 Issue 09 www.renalinterventions.net Follow Renal Interventions on all our social media platforms for the latest news, insight and events in kidney care
Profile: Kate Steiner page 14
EVA evaluated page 24
“Take time to connect”: Patient perspectives and mental impact in the spotlight
Phil West
on
2
Vivian Papageorgiou
Continued
page
Continued from page 1
Jaffer concurred, adding that it is particularly important for physicians to understand how kidney disease “consumes your life”. He described the difficulties for patients of learning how to “carry on” and integrate the condition and its treatment into the rest of their life, and asked West what more physicians could do to make the patient interaction and pathway easier.

In an echo of Papageorgiou’s message, West suggested: “Get to know your patient. We appreciate that you are all very busy, but that personal connection [is important]. We are all different, with different backgrounds, but if you can get to know your patient and they can get to know you, in a professional manner, it can only make the journey easier for both.”
The doctor’s perspective was similar, with Jaffer concluding that building relationships is also beneficial for physicians. When that connection is established, he stated finally, “we learn a lot more”.
Continued from page 1
ity, composite fatal and nonfatal cardiovascular events, kidney transplantation and recurrent hospitalisation from any cause. The authors used Cox proportional hazard models for the estimation of hazard ratios, and they describe patient demographic characteristics as “well-balanced at baseline”.
On the primary outcome, all-cause death occurred in 118 (17.3%) of those in the haemodiafiltration group (7.13 events per 100 patientyears) and 148 (21.9%) in the haemodialysis group (9.19 events per 100 patient-years; hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.65–0.93; p=0.005). Blankestijn et al detail also that 68 (25.6%) of the 266 deaths were attributed to cardiovascular disease, 26 (9.8%) to COVID-19, and 56 (21.1%) to other infections.
A history of cardiovascular events was associated with similar rates of mortality in both groups, but for those without this history the risk of death was lower in the haemodiafiltration group (HR 0.58; 95% CI, 0.42–0.79). Similarly, those in the haemodiafiltration group experienced a lower mortality risk if they had no history of diabetes milletus (HR 0.65; 95% CI, 0.48–0.87).
On the secondary outcomes, these were similar for the two groups apart from on infection-related death. On this measure, which included deaths related to COVID-19, the haemodiafiltration group was again at lower risk (HR 0.69; 95% CI, 0.49–0.96).
“Our trial differs from previous studies in that we enrolled patients who were likely candidates for high-dose haemodiafiltration nearly all the time,” Blankestijn et al explain. “We did not identify an association between failure to achieve the high-dose target and any particular patient characteristic or vascular access type. Thus, our trial results support the evidence that highdose haemodiafiltration can result in a clinically important survival benefit.” The randomised, controlled nature of the study also means that it avoids “confounding according to indication”, the authors argue.
be congratulated on performing a rigorous, large-scale trial during the COVID-19 pandemic, it identifies the “major limitation” with CONVINCE that patients “were selected from within the populations of the participating centre”.
The working group note also that “the characteristics of the total potentially available study population [were] not collected for logistical and organisational reasons”, and they argue that this “preliminary selection of patients” explains the high rates of both recruitment and arteriovenous fistulas (AVFs), as well as the low dropout rate.
“Investigators may have selected somewhat younger patients who were less frail and had fewer comorbidities,” the editorial suggests, “so that they could achieve the target convection volume. Thus, the CONVINCE population is not truly representative of the ‘usual’ population of adults receiving in-centre dialysis in Europe.”
The editorial also states that significantly lower mortality was only found for patients with AVFs rather than grafts or catheters “despite wellmatched groups”. Its authors also say that, when outcomes are stratified by convection volume, it could be the case that lower convection volumes “may also achieve beneficial effects, or that there may be a ceiling effect beyond which no further benefit is seen”.

All-cause mortality rate...
in haemodiafiltration group: 17.3%
in haemodialysis group: 21.9%
On the question of their findings’ applicability in the clinical setting, they suggest that their trial design may have yielded a population healthier than the average dialysis population in Europe and the USA, and they suggest that the absolute survival advantage within the haemodiafiltration group “may have varied between individual patients”.
Despite these limitations, the authors conclude that, when looked at alongside other trials and large observational studies, their results “indicate that the safety of haemodiafiltration was acceptable, provided that hygienic and microbiologic rules are fully respected.”
Do the findings CONVINCE?
The trial is said to be an effort to be an “end of discussion” RCT in an editorial published in Nephrology Dialysis Transplantation by the EuDial Working Group of the European Renal Association (ERA), the writing of which was led by Rukshana Shroff (Great Ormond Street Hospital and Institute of Child Health, London, UK). Despite stating that its authors “must
Editor-in-chief: Nicholas Inston | Editorial Board: Ziv Haskal, Stephen Hohmann, Robert Jones
Speaking to Renal Interventions on the study and the editorial, EuDial Working Group chair Carlo Basile (Miulli General Hospital, Acquaviva delle Fonti, Italy) said: “Currently, the uptake of haemodiafiltration in clinical practice is highly variable, and it is not available even in many high-income countries. In addition to monetary costs, the sustainability and environmental burden of dialysis therapy is already substantial.”
He also highlighted the environmental impact of shifting to haemodiafiltration, with sustainability already a developing focus in haemodialysis:
“The widespread adoption of online haemodiafiltration would require considerable improvements to infrastructure in some settings, in particular the provision of ‘ultrapure’ water. The production of such ultrapure water has an important climate impact: the production of an extra 23 litres of ultrapure water per dialysis session (required for some haemodiafiltration machines) would require 66 litres of water per session or 10,300 extra litres of water per patient per year (a 17% increase).
Basile made the case that improved personalisation of dialysis treatment, based on the benefits of haemodiafiltration shown in “select subgroups” in CONVINCE would “offer a compromise between improving survival and reducing the climate impact of dialysis”.
CONVINCE remains “a milestone in dialysis research”, Basile stated. Despite drawbacks, he and the working group conclude, it is the “first convincing evidence” for the survival advantages of haemodiafiltration.
EuDial
Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake | Head of Global News: Sean Langer | Editor: Benjamin Roche benjamin@bibamedical.com
Editorial contribution: Jocelyn Hudson, Will Date, Clare Tierney, Éva Malpass, Jamie Bell and Bryan Kay | Design: Terry Hawes, Wes Mitchell
Advertising: Melanie Stevenson melanie@bibamedical.com
Subscriptions:
Renal Interventions
September 2023 – Issue9 Introduction 2
@Renal_Ints linkedin.com/company/renal-interventions/
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 | BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Micropress Printers Ltd. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved. If you have comments on this issue or suggestions for upcoming editions, write to urmila@bibamedical.com
subscriptions@bibamedical.com | News or advertising queries: Tel: +44 (0)20 7736 8788
Haemodiafiltration mortality risk found lower than haemodialysis
“Take time to connect”: Patient perspectives and mental impact in the spotlight
“Graphs and statistics are black and white, but you need to talk to patients about how best to fit a treatment into their lives. Connect mentally with patients, because what they are going through is shocking— every time.
Vivian Papageorgiou
Carlo Basile
Peter Blankestijn
“The CONVINCE population is not truly representative of the ‘usual’ population of adults receiving incentre dialysis in Europe.”
Working Group

In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicated that the risk of mortality associated with paclitaxel-coated devices to treat peripheral arterial disease (PAD) is no longer supported based on data and analyses.
This update signals a lowering of the red flag raised in a 2019 letter from the Administration—published in response to a meta-analysis that indicated a late mortality signal—warning that treatment of PAD with paclitaxel-coated balloons and paclitaxel-eluting stents was “potentially associated with increased mortality”.
Alongside the letter, the US FDA has updated its recommendations for healthcare providers regarding the use of paclitaxel-coated balloons and stents for PAD. As well as removing reference to the possibility of increased mortality with these devices, the amended guidance softens the language around the monitoring of patients who
ELUDIA drug-eluting stent trial proffers “promising” results
Drug-eluting stents have been examined in a variety of recent studies for the treatment of arteriovenous fistula (AVF) stenosis and failure, from 2018 research by Scott Trerotola (University of Pennsylvania, Philadelphia, USA) and colleagues to that of Narayan Karunanithy (Guy’s and St Thomas’ NHS Trust, London, UK) et al in 2021. Now, findings from the ELUDIA study into the efficacy of Eluvia polymer-coated low-dose paclitaxel-eluting stents for stenosis treatment have been published in the Journal of Vascular Access (JVA), with its authors describing them as “promising long-term results”.
THE STUDY, LED BY KONSTANTINOS
Katsanos (University of Patras, Patras, Greece) enrolled 23 patients across three tertiary hospitals in Greece and Singapore. The researchers designated a primary outcome of long-term patency of the treated lesions and fistula circuits as “successful stent placement with resumption of uninterrupted haemodialysis and without

have been treated with paclitaxel-coated stents and balloons, stating that healthcare providers should continue ‘routine’ rather than ‘close’ monitoring of these patients, as had previously been stated.
The safety of paclitaxel—used in peripheral interventions to prevent restenosis—was called into question by data put forward in 2018 by Konstantinos Katsanos (University of Patras, Patras, Greece) et al that pointed to an increased risk of death at two and five years following the use of paclitaxel-coated balloons and paclitaxel-eluting stents in the femoropopliteal artery.
The FDA responded, notifying healthcare providers in early 2019 about a late mortality signal in patients treated for PAD in the femoropopliteal artery with paclitaxel-coated balloons and paclitaxel-eluting stents. Their most recent update on the topic, prior to that shared on 11 July 2023, was posted in August 2019.
In its new update, the FDA notes that “additional data from the pivotal randomised controlled trials (RCTs) has become available,” and that the Administration has worked with device manufacturers and external stakeholders to develop the protocol and analysis plan for new data generation.

The FDA references the fact that device manufacturers collaborated in an updated meta-analysis, which included “additional studies, more complete vital status information, and longerterm follow-up compared to prior studies”. Patient follow-up in these studies ranged from two to five years, the Administration notes, and led it to conclude that the updated RCT meta-analysis “does not indicate that the use of paclitaxel-coated devices is associated with a late mortality signal”.
Furthermore, the FDA states that it also reviewed additional analysis of the risk for late mortality, including the SWEDEPAD trial interim analysis, the VOYAGER PAD study, the German BARMER Health Insurance study, the US Veterans Health Administration study and the Medicare SAFE-PAD study. “None of these studies, with mean or median follow-up ranging from 1.7 to 3.5 years, found a risk for late mortality associated with paclitaxel-coated devices,” the FDA communicates, adding that longer-term follow-up in several of these studies is ongoing.
significant vascular restenosis (50% diameter stenosis threshold) or other secondary interventions during follow-up”. Failure of the AVF was defined according to the criteria of the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline.
A total of 23 patients received the stent, with the majority (12) having a brachiocephalic AVF, eight a radiocephalic, and three a transposed brachiobasilic native AVF. The mean age of the AVFs at the time of failure was 33.9±20.4 months, while the treated lesions varied in location: 12 of the included stenoses were in the juxta-anastomotic segment, nine in the outflow veins, and two were cephalic arch lesions, with a mean diameter stenosis of 86±8%.
The median follow-up period was 20 months. Katsanos et al observed during that period that 18 out of the 23 stents remained patent, indicating a cumulative patency rate of 78.3%. A KaplanMeier analysis revealed that the estimated primary patency of the ELUVIA stents was 80.6% at two years. The corresponding fistula circuit demonstrated a primary patency rate of 65.1%.
Katsanos and colleagues conclude that these results are “promising”—but that this is only an observational study. Randomised controlled trials on a much larger scale are required, they say, to more fully examine these stents’ use for access stenosis.
Cumulative 20-month primary patency of ELUVIA stent: 78.3%
Estimated patency at two years: 80.6%
4 September 2023 – Issue9 Paclitaxel
Long-awaited US FDA update finds data do not support excess mortality risk for paclitaxelcoated devices
“None of these studies, with mean or median follow-up ranging from 1.7 to 3.5 years, found a risk for late mortality associated with paclitaxelcoated devices.”
FDA
Konstantinos Katsanos
DCB superior to plain balloon for stent graft stenosis in Taiwanese RCT
Recent studies have evaluated drug-coated balloon (DCB) treatments’ ability to help maintain arteriovenous fistula (AVF) patency for haemodialysis patients “with mixed results”, according to Mu-Yang Hsieh (National Yang Ming Chiao Tung University, Hsinchu, Taiwan). However, he and co-authors including corresponding author Chih-Cheng Wu (National Taiwan University Hospital Hsin-Chu Branch, Hsinchu, Taiwan) contend that these studies have excluded stenoses that involve stent grafts—a subject to which they have now turned their attention in a new randomised controlled trial (RCT).


Their research is published in the European Journal of Vascular and Endovascular Surgery and compares the advantages of DCB treatment with those of conventional angioplasty in patients with stent grafts. The authors contend that intimal hyperplasia recurs at the edges of stent grafts in many of those patients who receive one, something which they note may be limited by paclitaxel, “the most commonly used drug in DCBs”.
With that in mind, the authors designed a single-centre, prospective, randomised controlled trial enrolling 40 patients with stent grafts, half of whom were assigned to a paclitaxel DCB arm and half of whom received plain balloon angioplasty. The authors note that the primary inclusion criterion was stent graft implantation in a patient with dysfunctional AVF or AV graft
(AVG), with dysfunction designated as their having at least one clinical indicator as defined by the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline.
The authors further detail that study patients underwent fistulography in order to assess whether they had stent graft stenosis >50% within the access circuit, while they state that the DCB chosen was an angioplasty balloon with a 2 μg/mm2 paclitaxel-based coating, 135cm shaft length, 4–8mm diameter, and 40–100mm length.
Describing the procedure, they note that in the DCB group the target lesion was dilated to nominal pressure for 120 seconds, before the DCB was inflated to
a nominal pressure, with the diameter “required to equal that of the initial dilatation balloons”. In the control group, angioplasty with a pre-dilatation balloon was performed again for 120 seconds.
Citing the “greater statistical power for a continuous endpoint” in a small RCT, Hsieh et al chose late luminal loss of the target lesion as their primary endpoint, and they evaluated minimal luminal diameter (MLD) at zero and six months using angiography. Secondary endpoints included target lesion primary patency and access circuit primary patency at six months, with the former defined as “no clinically driven reintervention on the target lesion” while access circuit patency “ended with any intervention on the access circuit”.
In total, angiographic follow-up was performed in 18 patients from each group. The late loss of MLD in the DCB group was found to be 1.82±1.83mm, which the authors describe as “significantly lower” than the mean late loss of 3.63±1.08mm in the control group (p=0.001). The six-month target lesion primary patency interval for the DCB group was 159±49 days and 93±67 days for the conventional angioplasty group (p=0.008). Hsieh et al state that these results
“demonstrated that drug-coated balloons prevented late luminal loss of stent-graft stenosis and potential superior primary target lesion patency
over conventional balloons”.
“With the increasing use of stent grafts, stenosis of the stent graft edge will become a common problem,” they add, making reference to the results of the RENOVA study. It is with this problem that they contextualise their own finding that “the absolute risk difference between the two groups [in our trial] was larger than that observed in DCB trials for AVFs”. They also make the conclusion that the edge of a stent graft is a location of particular risk for restenosis given their data—something they suggest has not been addressed previously.
Turning to the limitations of their study, they describe it as “substantially underpowered for binary outcomes”, and they call for a “much larger trial” to validate their results. They also say that the trial was too small to designate clinical endpoints. Nevertheless, they conclude that convention angioplasty is not a “durable” solution to stent graft stenosis. DCBs, they say, offer a potential solution to the problem of patency.
ESVS vessel diameter guidelines do not improve outcomes in new RCT
The European Society for Vascular Surgery (ESVS) guidelines on vascular access recommend minimal diameters of 2mm in the forearm and 3mm in the upper arm in vessels selected for the creation of an arteriovenous fistula (AVF). Whether this recommendation leads to better AVF-related outcomes, however, has been called into question by the publication of secondary analysis data from the Shunt Simulation study, led by Letty V Van Vliet (Maastricht University Medical Centre, Maastricht, The Netherlands) in the Journal of Vascular Access (JVA).
VAN VLIET ET AL MAKE THE CASE THAT the minimal blood vessel diameters considered appropriate for AVF creation “vary between dialysis units”. And it is not only individual units—there is variation even between the recommendations of different international guidelines, they say, pointing to the Kidney Disease Outcomes Quality Initiative (KDOQI) 2019 update, which offers only “a weak recommendation to use blood vessels smaller than 2mm only after critical assessment of their quality,” in divergence from the ESVS.
The Shunt Simulation study is uniquely positioned to provide meaningful data on the issue, Van Vliet et al say, given that all patients enrolled were operated on before the ESVS guidelines’ publication in June 2018. This, they suggest, eliminates the possibility of bias “resulting from deliberate deviation from published guidelines”.

The study was a randomised controlled trial (RCT) assessing the accuracy of a personalised computational model in predicting postoperative fistula flow. This secondary analysis, meanwhile, compared outcomes of patients treated as part of the study either in accordance with the ESVS recommendations or outside of them.
The latter group was defined as when the smallest segment of the artery or vein of the AVF was smaller than the 2mm for forearm and 3mm for upper arm fistulas.
Fistula maturation was assessed using duplex ultrasound, and was defined as a flow of >500ml/ min and a vein diameter of >4mm at six weeks post-surgery. A fistula was defined as functional if it was “cannulated with two needles for six sessions of haemodialysis at the prescribed access circuit flow within thirty days”. Predialysis patients were considered to have a functional access when the fistula could be used without first needing a central venous catheter upon dialysis initiation.
Of the patients who received fistulas in the study, 160 were available for analysis at one year post-fistula creation. A total of 72 (45%) of those patients received an AVF created outside of the clinical guidelines, with the remaining 88 (55%) doing so within them.
Van Vliet et al found that 82% of fistulas created within guideline recommendations had reached maturation by six weeks, compared with 71% of the fistulas with smaller vessel diameters. They also performed a multivariable regression analysis which found that guideline concordance in AVF creation was “not associated with improvements in obtaining a timely functional vascular access” (odds ratio [OR] 0.77, 95% confidence interval [CI]: 0.38–1.56) or in decreasing the rate of access-related interventions.
During the maturation process, the authors say, the outside-the-guideline vessels increased in size, becoming as large as the fistulas created in agreement with the guidelines. They do note, however, that only 52% of the subset of forearm fistulas with blood vessel diameters smaller than 2mm became functional.
Reviewing the evidence beyond their study,
Maturation rate at six weeks within guidelines: 82%

Maturation rate with smaller diameters: 71%
the authors state: “The scientific evidence indicates that although the chance of successful fistula maturation increases with greater blood vessel diameters, there is no strict diameter cut-off that consistently differentiates between fistulas with good and bad clinical outcomes.” On their study’s contribution to that evidence, Van Vliet et al suggest finally that their results support the assertion that “clinical decision-making should be guided by an individual approach” which considers other factors, including blood vessel quality, other access options, and patient preferences, “instead of by strictly adhering to diameter cut-off values”.
5 Issue9 – September 2023 AVF
“Clinical decisionmaking should be guided by an individual approach.”
Van Vliet et al
Chih-Cheng Wu
Mu-Yang Hsieh
“The absolute risk difference between the two groups [in our trial] was larger than that observed in DCB trials for AVFs.”
Letty V Van Vliet
This advertorial is sponsored by BD
ESKD Summit 2023: What matters to patients gains momentum with input from multidisciplinary experts

Focusing on the disease through the patient’s lens and establishing well-functioning multidisciplinary teams to potentially enhance both the patient’s journey and clinical outcomes got top billing at BD’s second EMEA End-Stage Kidney Disease (ESKD) Summit (9–10 May 2023, Milan, Italy). With a multispecialty faculty comprising 22 leading voices, including nephrologists, vascular and transplant surgeons, plus interventional radiologists (with a combined knowledge of over 400 years), selected from 12 countries, BD’s second edition of “The Changing Face of a Dialysis Patient” showed commitment to a new format of scientific peer-to-peer communication, characterised by open interaction and vibrant discussion. More than 30 presentations including challenging cases, 14 hands-on workshops and the participation of 200 attendees marked the event.
Scientific directors of the summit, interventional radiologist Fabrizio Fanelli (Florence, Italy) and vascular access and transplant surgeon Nicholas Inston (Birmingham, UK), welcomed delegates to a programme that encouraged placing patients at the heart of care in dialysis access creation, restoration and maintenance.
Disease overview and patient journey
Charting the various difficult terrains of end-stage kidney disease (ESKD) in the renal patient pathway, Inston said each person’s course depends on age, comorbidities, “events” and interventions that were influenced by decisions made by clinicians, and importantly, by patients. Even simple clinical decisions can have a disproportionate, and sometimes uncaptured, impact on a patient’s
life, he underscored. “When a fistula does not work, clinicians may simply plan to create another one, but this might have major disruption on a patient’s life and can potentially create a very negative loop for patients as they have likely gone through six to 12 months of other procedures to get to this point,” he said.
Based on his clinical experience, Inston referenced the concept of “much more tailored” minimally disruptive medicine (MDM). In this context, “the appropriate focus of treatment is the situation and the individual tackled as a whole”. MDM aims to reduce workload and individualise treatment, taking into account the effects of, for instance, “12 hours of dialysis per week, transport, maintenance interventions, clinical appointments and possible surveillance on a person’s life and calendar”.
Is there a case for rebooting questionnaires?
Inston kickstarted a discussion of wide-ranging quality of life issues with questions such as: “Is measuring patients’ quality of life via questionnaires of any use? Are they representative or do they misguide us? Do we add to the patients’ workload with more surveys/questions/studies? Are we considering what we can do to tailor care to the patient and reduce workload? And can minimally disruptive medicine be applied to dialysis care?”
Daniel Gallego, a patient advocate and president of the European Kidney Patient’s Federation, has been a kidney patient since 1993, undergoing haemodialysis since 1995. To Inston’s points, he responded: “Twenty years of in-centre haemodialysis is different from home haemodialysis or peritoneal dialysis. Health questionnaires have been decided by clinical professionals and they do not include components on self-esteem, physical appearance, or things that really matter to us. […] Many aspects that might improve the life or quality of life of patients are not really captured in the outcomes. […] And sometimes these are misguiding us.” He called for a redesign of the questions asked and the management of information gathered so that the right decisions for patients can be made. Gallego also placed the spotlight on including the perspective of care-partners and families who influence the quality of life experiences for patients.
Responding to a question from the audience on whether a patient can influence the final decision on treatment modality, Gallego pressed on to say that with enough information and involvement at every stage, yes, patients can be responsible for both the choice and consequences of that choice for their access.
Külli Kuningas, lead renal research nurse, Queen Elizabeth Hospitals, Birmingham, UK, then outlined the “Our Arteriovenous Fistula (AVF) Lives” project, a patient-led ethnographic research undertaking run by the hospital to capture more in-depth patient perspectives on living with vascular access for dialysis. Relying on primary source material in the form of video diaries and the filming of
September 2023 – Issue9 6 Advertorial
The multidisciplinary panel discussions were characterised by lively interaction
patients in their own environments without the use of questionnaires, researchers aim to analyse how patients describe their conditions and what is important to them, to capture a repository of “true patient perspective”.
Gallego stressed that patient-reported outcomes were received usually from articulate, perhaps educated respondents who have the luxury of time. A critical component will be to validate these responses so that they become applicable to patients who might have poor language skills, come from populations with different cultures and languages and to those who might never answer questionnaires. He also addressed the discussion point of disparities in guidelines by stating that a consensus was needed, without bias, on the right treatment. This sparked the question: should care be “guideline-driven” or “tailored to the individual patient”? “We need to adapt, because every single patient is unique,” said Gallego.


The ensuing panel discussion on harnessing multidisciplinary expertise into the care of patients with kidney disease painted the picture of a changing paradigm. In “the old days”, and according to the panel’s experience, the “thinkers” were the nephrologists and the “doers” interventionalists and surgeons. With nephrologists also embracing interventional skills, there is a merging of the “thinkers” and “doers” responsible for a patient’s access, but this varies considerably based on local expertise, the panel said. According to their experience, there was acceptance that the nephrologists and nurses were ‘closer’ to the kidney patient’s journey and pivotal in coordinating care within multidisciplinary teams. Longstanding relationships with the patient, underpinned by trust and time were also iden tified as central to the patient’s experience of care. The panel agreed that a close connection in the discussion between patients, nephrologists and vascular access teams was core to good patient care. “More than the specialties of clinicians, communication is the key, and having one person in charge, potentially a nurse or dialysis access coordinator, who communicates in simple language along the whole journey with patients, is vital,” offered Gallego.
Ounali Jaffer (interventional radiologist, London, UK), Fien Gryffroy (vascular surgeon, Antwerp, Belgium), Gürkan Sengölge (interventional nephrologist, Vienna, Austria) and Ioannis Griveas (nephrologist, Athens, Greece) contributed to the discussion.
Looks as well as books
Panos Kitrou (interventional radiologist, Patras, Greece) detailed the importance of the Haemodialysis Arteriovenous Access Cosmesis Scale (AVACS), which is a new measure for vascular access. While clinical decision-making is often based on the twin-pillars of safety and effectiveness, the impact that vascular access has on the patient is also very important, he said.

Research has shown that lumps, bulging and related tortuosity, “mega access”, access-related scarring and number of scars, and the noticeability of the vascular access have been identified as some issues that play on patients’ minds.
Kitrou noted that there is a paucity of reports about the effect of AV access cosmesis on patients’ quality of life, dialysis experience, and decision-making about choice of vascular access type and creation.



“The cosmetic effect has an influence on patients’ psychology and therefore on the type of vascular access selected,” Kitrou posited.
“Cosmesis in the context of access is defined as the preservation of, or change in, limb physical appearance after AV access creation. It is one of many important factors to be considered in choosing vascular access within the context of the patient’s ESKD Life-Plan. By creating a scoring instrument that allows stratification of cosmesis of AV accesses, factors that affect cosmesis can be assessed,” he informed delegates. “In doing so, both clinicians and patients will be better informed to make access decisions in line with a patient’s priorities,” he concluded.
New guidelines signal change


Narayan Karunanithy (interventional radiologist, London, UK) and José Ibeas (interventional nephrologist, Barcelona, Spain) then delved into the updates on the most recent guidelines.
The former unpicked the latest UK Kidney Association (UKKA) guidelines to point out that these guidelines, from the oldest continuously active nephrology society in the world, are not funded by any external organisation, commercial company or charity.




The recent haemodialysis access guideline update marks a considerable shift in how the organisation recommends that clinicians approach choices in vascular access for patients and does away with the idea of targets to place patient choice at the heart of vascular access creation and care. The team behind the guidelines have said it is a pragmatic guideline that had active representation from a variety of specialties but also three active patient representatives. “The guideline emphasises different patient characteristics rather than just saying: ‘This is the best access for you,” Renal Interventions was informed.
Karunanithy noted the endovascular-first approach to access dysfunction and that the additional value of covered stents and drug-coated balloons was discussed.






Despite high-quality data in many areas, evidence gaps still exist, he pointed out.

Ibeas then turned to the most recent guidelines from the European Renal Association (ERA), European Society for Vascular Surgery (ESVS), Kidney Disease Outcomes Quality Initiative (KDOQI) and El Grupo Español Multidisciplinar del Acceso Vascular (GEMAV) to distil the areas of commonality and dissonance in their recommendations.
“According to my experience, evidence-based medicine is subject to the influence of several factors, that is, the methodology, perspective of the specialty, geographical area or health management system; and the values and circumstances of the patient […] and to their interpretation,” he said.

“[…] the most important point is the effort for consensus on the one hand and generating evidence in the areas of conflict on the other. When reading the guidelines, it is convenient to understand how each recommendation in each guide is justified. In this way, the clinician can generate his own critical judgment and contribute to the implementation of the guidelines in his environment in the most rational way. That can benefit the ultimate recipient of the guidelines, the patient,” Ibeas said.
Continued on page 8
Issue9 – September 2023 7 Advertorial This advertorial is sponsored by BD
Yousof Al Zahrani
Joseph Touma
Fabrizio Fanelli Nicholas Inston Daniel Gallego Külli Kuningas
Panos Kitrou Ounali Jaffer Gürkan Sengölge
Ioannis Griveas Narayan Karunanithy Tobias Steinke
Luka Novosel Katarzyna Kolasa Noha Guzaiz Jamal Al Koteesh
Lean, clean and controlled: New tools for healthcare

Fanelli then brought to the fore an award-nominated project at the Careggi University Hospital, Florence, Italy, that applied lean management concepts to the patient’s journey. This approach seeks to define the process and the problem; measure performance; analyse the process for issues and root causes; improve by determining and implementing actions; and exercise control to maintain the improvement. When applied to endovascular procedures, lean management resulted in improved capacity and productivity; reduced procedure time; lowered operator stress; improved quality and reduced hospital stay.
“Lean healthcare is used in a growing number of hospitals to increase efficiency and quality of care. In healthcare organisations, it attempts to empower staff to generate continuous improvement through incremental but regular improvements in work processes. Periodic, detailed and specific feedback is extremely important to decrease complication rates and increase the service quality,” he shared.
Innovative highlights
Within the endoAVF creation session, Matteo Tozzi (vascular surgeon, Varese, Italy) presented a novel case report of Wavelinq endoAVF creation using carbon dioxide (C02) contrast.
Detailing the clinical benefit of preserving residual renal function (RRF) in dialysis patients, Tozzi commented that those with residual renal function had better control of serum potassium and sodium, acid-base status and volemia.
Outlining his goals for the case, Tozzi explained that he planned distal injection through a drug-coated balloon or plain balloon to use C02-viscosity wisely and enable a faster procedure with reduced risk and complexity.

His take-home message from this innovative approach to imaging: There was no damage on kidney residual function in chronic kidney disease patients and no damage on kidney residual function in kidney replacement therapy patients. “Non-iodinated contrast can be used in the implementation of [an] endovascular approach for vascular access creation,“ he submitted.
Tobias Steinke (vascular surgeon, Düsseldorf, Germany) then doubled down on the reasons for pursuing an endovascular creation strategy. He stated: “EndoAVF systems are designed to be less invasive and maybe to last longer; reduce the rate of primary failure and enable more medical specialties to participate in the creation of AVF, which can be relevant in areas underserved by vascular surgery.”
“Based on my experience, attempts to optimise perioperative haemodynamic conditions, regional blocks and endovascular technique modifications are worthwhile to improve fistula outcome and have the potential to increase the number of functional endoAVFs,” he underlined.
Yousof Al Zahrani (interventional radiologist, Riyadh, Saudi Arabia) then detailed the simplicity of his experience from learning to mastering WavelinQ in a hospital
setting that caters for 2,100 beds with the expertise of 12 interventional radiologists, four neuroradiologists, two paediatric interventional radiologists and six fellows.
In pursuing an endoAVF creation programme, the team has performed 32 percutaneous procedures, with the majority of patients undergoing a WavelinQ procedure under regional anaesthesia.
“Procedural success was 85%, with successful cannulation achieved in 88% with a time to cannulation of four to eight weeks. There were no device-related complications and reinterventions were needed in five patients (18%),” he detailed.
In a session examining restoration of dysfunctional AVF, Jose M Abadal, president of the Spanish Interventional Radiology society (SERVEI; Madrid, Spain) shared his approach to percutaneous angioplasty (PTA) balloon selection based on the type of stenosis. He exhorted that the degree of stenosis was not enough of a measure, but that the type of stenosis is vital in determining the choice of device. In his centre, choice rests on a combination of medical evidence, cost containment and a clinical practice that is heavily reliant on Doppler ultrasound use. He reminded delegates that the technique of PTA is still variable, quipping “standardisation is clearly reserved for cardiologists”. Abadal stated that plain balloon angioplasty is the first choice followed by a double whammy of angioplasty then drug-eluting balloon. Ultra-high pressure balloons are the first choice and in case of recoil either these or scoring balloons are applied, with stent grafts only selected if there is a clear indication. “Ultrasound is mandatory for sizing and new Doppler ultrasound criteria will be used to determine technical success,” he noted.
Joseph Touma (vascular surgeon, Paris, France) spoke on his centre’s experience of drug-coated balloon in fistula maintenance and Luka Novosel (interventional radiologist, Zagreb, Croatia) updated on the AVF cohort of the Lutonix AV Global registry, drawing out the patient and procedural variables of the study.
In the maintenance session for dysfunctional AVF, Griveas described the access life circuit that begins with vascular access stenosis. “Drug-releasing balloons can be a useful therapeutic option for patients with AVF stenosis due to accelerated endothelial hyperplasia. The use of paclitaxel-coated balloons helps reduce the risk of restenosis of arteriovenous anastomoses and is a safe, minimally invasive and immediate solution to AVF
management,” he quoted from a paper published by his group in Nephrology Dialysis Transplantation in 2020.
Jaffer opened the second day’s plenary with a general introduction around the pathology of lesions making the point that “veins are not arteries”. It is important to drill-down into why stenoses occur, he said. “Lesion characteristics can be complex. A UK expert consensus approach for managing symptomatic arteriovenous fistula (AVF) stenosis in haemodialysis patients, published in CardioVascular and Interventional Radiology in 2021, recommends that if a patient requires four or more interventions for the same stenosis in a 12-month period (or three or more in a six-month period), a multidisciplinary meeting and patient discussion on access options should be triggered. For de novo stenosis, plain balloon or high-pressure balloon angioplasty are acceptable first interventions. Bare metal stents have a place only for central region stenosis and if there is recurrence between three and 12 months, consider a drug-coated balloon,” he reported.
This was followed by Kitrou presenting on endovascular treatment of dysfunctional AVF including an algorithm option and Jamal Al Koteesh (interventional radiologist, Al Ain, United Arab Emirates) presenting cases of complex AVF lesions.
Karunanithy then described to delegates why covered stents are key to haemodialysis access salvage.
Katarzyna Kolasa (health economist, Warsaw, Poland) presented findings from a literature review to examine whether covered stents are a cost-effective choice to treat AVF stenosis in haemodialysis patients.
Setting out that only 35% of haemodialysis patients remain alive after five years of treatment, Kolasa pointed out that these survival rates remain worse than those for many common cancers.
AVF is the preferred access choice, and vascular access-related complications and interventions account for almost one-third of hospital admissions among patients receiving dialysis, she said.
To mitigate against the high economic burden associated with AVF stenosis, early consideration of additional procedures along with angioplasty in the course of treatment and postponing additional procedures until it is absolutely necessary have the greatest potential to produce long-term savings, she commented.
Identifying the clinical evidence required for budget holders to ensure funding of covered stents for AVF stenosis, Kolasa said real-world data confirming that the covered stents lower the risk of reintervention, economic modelling data showing the same findings and more such data verifying long-term outcomes for patients would be valuable tools.
“As the outcomes of haemodialysis patients are variably monitored, there is a need for country-specific data collection efforts to inform reimbursement decisions in order to improve access to treatment,” she said.
Inston and Fanelli summed up the discussions when they told Renal Interventions: “The ESKD summit brings together the multidisciplinary team to consider more than just procedures and devices. It approaches treatments as more than just isolated procedures, and consciously reframes these in the context of a patient’s journey. This journey may have one clinician at the centre or have multiple inputs into treatments, but it is one that has a profound impact on each individual patient and their families. We feel that the summit achieved this vision by using a patient-centred approach.”
Disclosures: Please consult Becton, Dickinson and Company product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use. The opinions and clinical experiences presented herein are for informational purposes only. The results from this case report may not be predictive for all patients. Individual results may vary depending on a variety of patient specific attributes. The clinicians have been compensated by Becton, Dickinson and Company to participate in this advertorial.
BD Switzerland Sarl, Terre Bonne Park – A4, Route De Crassier, 17, 1262 Eysins, Vaud. Switzerland, Tel: +41 21 556 30 00. Fax: +41 44 722 5370. bd.com. BD, the BD logo and Aspirex trademarks are the property of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved. Straub has joined BD. All rights reserved. BD-94938
September 2023 – Issue9 8 Advertorial BD advertorial cont...
Interventional radiologist Shagran Binkhamis (Riyadh, Saudi Arabia) leads a hands-on session
2797 0344 0483
Vascular surgeon Robert Shahverdyan (Hamburg, Germany) leads a hands-on session
Conventional haemodialysis (CHD) consists of three four-hour sessions a week, but a more regular daily haemodialysis (DHD) regimen of six three-hour sessions a week can improve cardiovascular health in chronic kidney disease (CKD) patients. This is according to recent research by Steven G Achinger (University of South Florida, Tampa, USA) and Juan Carlos Ayus (University of California Irvine, Irvine, USA).
THE WORK WAS PUBLISHED IN Hemodialysis International, and its text sketches out a previous gap in the literature—that though DHD “leads to improvements in left ventricular hypertrophy and mineral metabolism at one-year follow-up”, there is “no information from prospective studies” on the cardiovascular effects in the longer term.
Looking to help fill that gap, Achinger and Ayus carried out a four-year prospective cohort study comparing the cardiovascular outcomes of 26 patients receiving DHD and 51 receiving CHD. At four years, 15 DHD and 26 CHD patients completed follow-up, and the authors took measurements of left ventricular mass index (LVMI), blood pressure, haemoglobin and mineral metabolism markers.
EndoAVF maturation rates “similar” to surgical but bolstered by reinterventions
Published in the Journal of Vascular Surgery (JVS), new research by Theodore H Yuo (University of Pittsburgh Medical Center, Pittsburgh, USA) and others has drawn a favourable comparison on maturation rates between endovascular arteriovenous fistulas (endoAVF) and surgical AVF (sAVF). Finding them comparable, the study does, however, suggest further interventions and patient selection may play a role in elevating endoAVFs’ maturation rates.

The study authors explain in their abstract that their text reports “our experience with [endoAVF] in comparison to a contemporaneous sAVF group”. The single-centre study was a retrospective one, examining data from 51 recipients of endoAVF procedures alongside those from 51 randomly selected sAVF patients during the period 2018–2022. Outcomes of interest included procedural success rate, number of maturation procedures required and fistula maturation rates. Maturation was defined as successful use of the fistula for haemodialysis in those receiving that treatment or, if a patient was not on haemodialysis, then it was designated as when >500 ml/min superficial venous outflow was recorded in endoAVF, or according to clinical criteria in sAVF.
Procedural success was achieved for 98% (50) of the endoAVF patients, though there was a higher rate of reintervention in these patients in the categories of fistula angioplasty (60% vs. 29% for sAVF; p=0.002), ligation (24% vs. 2%; p=0.001) and embolisation of competing outflow veins (22% vs. 2%; p=0.002). Those who received a surgical fistula, meanwhile, underwent planned transpositions more frequently (39% vs. 6%; p<0.001).
With all maturation interventions combined, there was a higher overall rate of maturation procedures for the endoAVF cohort, though the authors note that this was not statistically significant (76% vs. 53%; p=0.692).
However, they also set out that the exclusion of planned second-stage transpositions yields a statistically significant higher rate of maturation procedures in this group (74% vs. 24%; p<0.001). Yuo and colleagues also looked at catheter removal, with 15 patients (58%) undergoing it in the endoAVF group and 18 (45%) in the sAVF group (p=0.314). It was additionally found that endoAVF patients were more often male (78% vs. 57%; p=0.033) and were less likely to have congestive heart failure (10% vs. 43%; p<0.001).
Yuo et al state finally that endoAVF maturation rates “appear to be similar” to those of sAVF but, based on their interpretation of their data, caveat that endoAVF’s rates “may be related to higher intensity of maturation procedures and patient selection”. In light of their findings, the researchers note: “An analysis of appropriately matched patients will assist in elucidating the possible role of [endoAVF] vis-a-vis sAVF.”
Steven G Achinger



There was found to be a “significantly lower” rate of systolic and diastolic blood pressures in the DHD group (128mmHg, 95% confidence interval [CI], 111–143) compared to the CHD group (148mmHg, 95% CI, 137–158; p<0.05). There was also an association between DHD and a lower proportion of patients taking any anti-hypertensive drug, with 50% of DHD patients taking one vs. 80% with CHD (p<0.05). DHD also demonstrated “improved attainment of mineral metabolism goals for phosphorus” (adjusted hazard ratio [aHR] 3.6, p=0.002) and calcium × phosphorus product (aHR 3.66, p=0.001) at four-year follow-up in comparison to CHD, while there was a “non-significant trend toward lower LVMI in the DHD group”. Haemoglobin improvements, meanwhile, were shown to persist at four-year follow-up.
In their conclusion, the authors state that “DHD is associated with long-term improvements in key cardiovascular risk factors”, noting the differences between the two groups in blood pressure, mineral metabolism and anaemia while also highlighting the “trends toward improved LVMI” that they uncovered.
Brachiocephalic AVFs not superior to radiocephalic for tunnelled catheter patients
The results of a new study in the Annals of Vascular Surgery suggest that patients who also have a tunnelled dialysis catheter (TDC) do not significantly benefit from receiving a brachiocephalic fistula (BCF) compared with a radiocephalic fistula (RCF).
FIRST AUTHOR LENEE PLAUCHE AND CORREsponding author Jeffrey J Siracuse (both Boston University Chobanian and Avedisian School of Medicine, Boston, USA) are part of the team behind the study. Their text makes reference to the work of Jonathan Misskey (Vancouver General Hospital, Vancouver, Canada) et al in 2018, published in the Journal of Vascular Surgery, which found that RCFs “demonstrated lower patency and maturation” compared with BCFs for patients aged over 65 years, as well as that they were “an independent predictor of secondary patency loss”.
Studies such as Misskey et al ’s lead Plauche and colleagues to suggest that BCFs “have been reported to have higher maturation and patency”. They also note that “more distal creation is encouraged”, while they posit that this can cause delays in the creation of permanent vascular access “and ultimately TDC removal”. Plauche et al’s aim “was to assess short-term outcomes after BCF and RCF creation for patients with concurrent TDCs”, with a view to establishing whether an initial brachiocephalic access may “minimise TDC dependence” and potentially improve outcomes.
For their study, the authors performed a retrospective analysis of data from the Vascular Quality Initiative (VQI) haemodialysis registry from the period 2011–2018. Among the characteristics assessed were patient demograph-
ics, comorbidities, and access type, as well as short-term outcomes such as occlusion, reintervention and access used for dialysis.
Of 2,359 patients with a TDC, 1,389 (58.9%) received a BCF while 970 (41.1%) received an RCF. The average patient age was 59 years while 62.8% were male. The authors state: “Compared with RCF, those with BCF were more often older, of female sex, obese, non-independently ambulatory, [more likely to] have commercial insurance, diabetes, coronary artery disease, chronic obstructive pulmonary disease, be on anticoagulation, and have a cephalic vein diameter of ≥3mm (all p<0.05).”
In a Kaplan-Meier analysis of outcomes at one year, primary patency for BCF was 45% to RCF’s 41.3% (p=0.88), while primary assisted patency was 86.7% for BCF compared with 86.9% for RCF (p=0.64). Among the BCF patients, 51.1% did not require reintervention compared to 46.3% for RCF patients (p=0.44). Survival was 81.3% for BCF and 84.9% for RCF (p=0.02).
A multivariable analysis found BCF was “comparable” to RCF on primary patency loss (hazard ratio [HR] 1.11, 95% confidence interval [CI] 0.91–1.36, p=0.316), primary assisted patency loss (HR 1.11 95% CI 0.72–1.29, p=0.66) and reintervention rates (HR 1.01, 95% CI 0.81–1.27, p=0.92). At three months, the type of access used was “similar but trending towards RCF being used more often” (odds ratio [OR] 0.7, 95% CI 0.49–1, p=0.05).
Plauche et al’s concluding claim is that BCFs do not demonstrate statistically significant superior fistula maturation and patency rates relative to RCF for patients that also have a TDC. “Creation of radial access, when possible,” they add, “does not prolong TDC dependence.”
Issue9 – September 2023 9 AVF
Daily dialysis reduces cardiovascular risk compared with three times weekly
“Daily haemodialysis is associated with longterm improvements in cardiovascular risk”
Theodore H Yuo
Jeffrey J Siracuse
“[EndoAVF’s maturation rates] may be related to higher intensity of maturation procedures”
This advertorial is sponsored by Merit Medical
Example procedural outcome: treatment of Bond’s patient with a left brachiocephalic AVF that presented with a stenosis at the cephalic arch with aneurysmal segment.
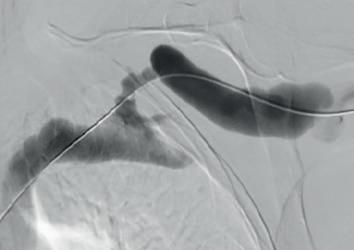

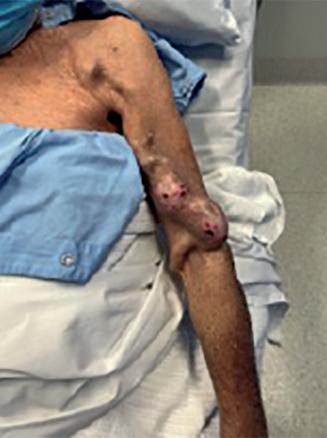
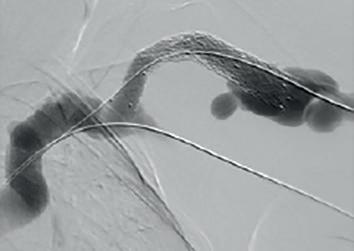
(A) Fistulogram taken preintervention and (B) after deployment of WRAPSODY to treat. (C) Fluoroscopy of treated segment at 18 months (D) and patient’s arm at time of presentation
WRAPSODY CellImpermeable Endoprosthesis™:
Maintaining an arteriovenous fistula/graft (AVF/AVG) is essential for long-term vascular access. However, over time, complications such as stenoses and occlusions can develop and may be life-threatening if not adequately addressed. Endovascular solutions to overcome such complications have traditionally encompassed percutaneous transluminal angioplasty (PTA) and/or the insertion of bare metal stents. However, low patency rates often necessitate multiple reinterventions. Recently, a novel selfexpanding endoprosthesis, the Wrapsody Cell-Impermeable Endoprosthesis (CIE; Merit Medical), was developed to improve access to the dialysis outflow circuit. Speaking to Renal Interventions, Robert Jones, an interventional radiologist at Queen Elizabeth Hospital Birmingham (Birmingham, UK), and Richard Bond, a vascular surgeon at Fiona Stanley Hospital (Perth, Australia) detail their experience with the device.
Bond was the first in the world to commercially implant Wrapsody CIE three years ago. “The first patient I remember well,” he says. “She had a left brachiocephalic fistula that had been put in place in 2014, and it had been working in the two years before I saw her in 2019. The main problem was that she was getting a lot of pain when the AVF was used for haemodialysis, and it was taking five to six hours to dialyse.” He adds that she “experienced bleeding from the fistula following dialysis.”
Bond explains that she was diagnosed with a particularly tight cephalic arch stenosis. Despite four previous treatment attempts by the radiology team rapid restenosis of her cephalic arch occurred each time. With her referral to Bond and the renal team, the initial treatment plan was to ligate the fistula. However, Bond opted for the Wrapsody CIE instead.
When asked why he chose Wrapsody CIE, he notes that, due to the anatomical anomaly present in this patient, no other endoprosthesis would have yielded a positive outcome. In addition, he indicates that the device’s design, specifically its tactile feel, made it a good treatment option. For Bond, the whole process was “very, very intuitive” and use of the device was “very straightforward”. As for the patient, he notes that she tolerated the procedure quite well: “The next day, the patient was achieving all of her dialysis
goals, and her pain was resolved overnight. She is due for her three-year angiogram this month— she has not had any issues with dialysis.”
Jones echoes Bond’s suggestion that Wrapsody is straightforward to use. “It tracks very well through the vessel, over the wire and through the stenosis. On deployment the device remains stable, and the trigger delivery system is very responsive.”
On the device’s design, Jones highlights its unique tri-layer configuration, with its polytetrafluoroethylene (PTFE) inner layer, its cell-impermeable layer, and expanded PTFE (ePTFE) outer layer that allows it to be embedded into the vessel wall. He also cites its optimised radial strength and relative compression resistance.

“For me,” he says, “one of the most interesting design features is the softened end-rows. These are designed in a scalloped fashion with the aim of reducing stress at the interface between the edge of the stent and the normal adjacent vessel, which will hopefully reduce the incidence of edge stenosis—the Achilles’ heel of stent grafts.”
“It is also fairly unique in that there are larger diameter devices in its portfolio, including 14mm and 16mm devices, something not currently offered by other manufacturers. This will allow us to treat patients with central or thoracic vein stenosis, where larger diameter devices are often required for safe and effective treatment.”
Jones became more aware of the device and its potential after participating in its first-in-man study as part of the data monitoring committee. “My interest in the research related to this device stems from the concept that it has great potential for overcoming many of the shortcomings of stent grafts.” The first-in-man study yielded a target lesion primary patency rate of 84.6% and access circuit primary patency rate of 65.9% for Wrapsody at twelve months’ follow-up. These results, Jones says, told us that Wrapsody is “safe and effective for treating vascular access outflow stenosis”.1
He has since taken on the position of co-principal investigator in the WAVE study, a pivotal trial designed to enrol 244 patients with AVFs and 113 patients with AVGs across a maximum of 50 clinical sites globally. Patients in the AVF cohort are randomised to either the Wrapsody CIE or PTA, after an initial successful pre-dilatation. The planned enrolment of 357 patients is a “good number for a clinical trial of this nature”, Jones states.


Regarding the need for further research, Jones notes that WAVE is not the only study investigating the performance of this device and also underway is the WRAP global registry, aimed at expanding the safety and efficacy data on the device based on real-world experience. The registry has a 500-patient target globally, with enrolment having begun in June 2022. The prospective, multicentre, post-market observational study will also include peripheral cases in both AVF and AVG patients, as well as a thoracic central vein cohort. Patients will be followed for two years. The last patient follow-up visit is expected in 2026.
Since his first implantation three years ago, Bond has treated approximately 25 patients with the device. Yet he states that, in the beginning, he was less excited. “When I was first shown the device and its features explained to me, it was clearly a flexible but robust device,” he explains. However, regarding the cell-impermeable layer he “was a little sceptical” about its ability to stop in-device neointimal hyperplasia.
“What has really been obvious over the years, however, is that it really does do that,” he says. Reflecting on his experience with the Wrapsody CIE in his current practice, he concludes: “It has all of the strengths of any current covered stents, but it also has new features—and it is these that make it effective in places that other options have not been in the past.”
In particular, Bond believes the device’s clinical benefits will be particularly evident in the cephalic arch and central veins: “These are areas that are lacking in any good, suitable treatment option. There, it is really exciting, with a nice combination of flexibility and strength for the angles involved in those vessels.”
1. Gilbert J, et al; First Clinical Results of the Merit WRAPSODY™ Cell-Impermeable Endoprosthesis for Treatment of Access Circuit Stenosis in Haemodialysis Patients CardioVascular and Interventional Radiology (2021). https://doi. org/10.1007/s00270-021-02953-8
September 2023 – Issue9 10 Advertorial
“It has all of the strengths of any current covered stents, but it also has new features—and it is these that make it effective in places that other options have not been in the past.”
Robert Jones
Richard Bond
A B C D
The next generation of covered stent
Empowering patients and deconstructing hierarchies key to building a successful multidisciplinary vascular access team
Renal Interventions reflects some unique perspectives and uncomfortable learnings from Yinhui-Lim Hartono, a resident new to the field of dialysis access, and Kathleen Rickert, a vascular access nurse, both from the vascular access center at Asklepios Klinik Barmbek (Hamburg, Germany). Along with Frida Fondelius, a dialysis nurse and vascular access coordinator at Skåne University Hospital (Malmö, Sweden), the trio elaborates on involving patients more and ironing out hierarchies that contribute to particular challenges, ahead of the 5th Hamburg Dialysis Access Symposium (8–9 September, Hamburg, Germany), a meeting directed by Robert Shahverdyan (Hamburg, Germany). Shahverdyan was instrumental in convening these interviews to shed light on how to improve multidisciplinary vascular access teams.
HARTONO IS KEEN TO FOREFRONT THE complexity and sometimes “overwhelming” number of things to learn as a new member of the access team. “Being new to the field, I am surprised at the widespread unawareness of the complexity of vascular access. It is a journey of life-long learning as the dialysis access field is constantly evolving with new technologies and advancements,” she says.
Fondelius shares a different slant as a coordinator. She believes the role is a pivotal one for the staff and vitally, for providing broad and reliable information to patients. “I help build and keep the team together and promote collaboration between the nephrology unit and the vascular surgery unit. This leads me to suggest that the role of a vascular access coordinator is a must— above all as a support for the nurses and doctors and for the patients, especially those who have several vascular access problems.
“Whenever there is need for a new access to be established, we always highlight the option of home dialysis, although we are in the vascular access meeting. For every patient we handle in the access meeting, we start our presentations considering why the patient is not suitable for peritoneal dialysis,” she explains.
Rickert, for her part, details that her experiences as a vascular access nurse and a leading renal nurse in the dialysis ward “is as a role model, empowering me to work as a specialist”. She shares the precision needed to assess the s of patients. “Practicing physical examination correctly and fostering the fistula postoperatively are vital and I have started to give lessons to healthcare professionals on the nephrology and dialysis ward at our hospital and other clinics on these topics. My role as an interface among the dialysis ward, the nephrology ward, the vascular access centre and the nephrological ambulance helps to bring together an interdisciplinary team equipped with optimised processes and forges a good relationship among the relevant stakeholders for best patient care,” she avers.
“With my acquired knowledge, I empower nurses to widen their perspectives beyond just sticking needles into fistulas. They learn about a 360-degree approach to a created fistula, its haemodynamics and flow dynamics. Moreover, I underline how important our role is to be a nurse for our patients with focus on physical examination, cannulation and advising patients regarding self-care. Awareness and patient-centricity for all caregivers is what I would like to aim for,” she adds.
Multidisciplinary teams: common goals, listening and trusting Fondelius overturns the idea of individualism. “In a multidisciplinary team, you must be prepared to listen to and trust others. If all members contribute to the discussion and to the same goal, we can find the best solution for this patient here and now.”
But she also sinks the myth that this is easy. “Some people are strong personalities and individualists. I think Sweden is an ‘equal’ country, so I have had no occasion to think that I have met any challenges just because I am a woman,” she adds.

Rickert, while emphasising that multidisciplinary teamwork is necessary to deliver the best patient care, also acknowledges that it is “hard to implement”. “We are all humans and people with feelings who are confronted daily with hierarchy especially in the business and system of delivering medicine.” She pushes to intensify the notion that: “In working with others, we should take words not personally, but objectively and accept when someone else knows more than ourselves. We must treat everybody with respect and have the willingness to understand the perspectives of others. This is mandatory in my opinion for everyone in the multidisciplinary team to reach common goals,” she says.
Hartono closes out the issue with the words: “Communication, understanding what each member is trying to achieve through their work, and interdisciplinary exchange are most important.”
Listening more to patients
Fondelius makes a strong case for learning from patients. “I have never met a patient who does not want to tell their story. If we are prepared to listen, we can learn a lot. Listen more to the patients—and include them even more in the multidisciplinary discussion. We can plan as much as we want, but if we do not include the patient, the outcome will be limited,” she urges.
Rickert echoes her views. “I would like to empower patients to take more care of themselves. Enabling selfresponsibility often results in more and better quality of life. To improve the patient’s kidney journey, teaching physical self-examination of their own fistula, selfcannulation and home dialysis are the milestones of that journey. Patients must be informed of these issues far before their first treatment.”
Ultrasound-guided cannulation

Rickert alludes to the shift enabled by technological refinement in imaging. “When I started to work at the dialysis unit, I knew nothing about vascular access. I just knew about sticking needles into the fistula and taking them off four hours later. When I had trouble during the treatment, I got stressed, tried to reposition the needles and eventually sought help. In 2018, I learned ultrasoundguided cannulation, my personal gamechanger, and have had no issues with access complications any longer, with the end and most important result being: satisfied patients.
“ In situ ultrasound-guided cannulation means to guide the needle safely in the middle of the vessel under ultrasonic observation. In my opinion every healthcare professional working in the field of dialysis should be trained in point-of-care-ultrasound, or POCUS, cannulation,” she states.
Hierarchy casts a shadow
Rickert recounts the noteworthy challenges she has faced, relating that she too, sees these as “less about being ‘a woman’, and more about being ‘a nurse’ in a team”.
“We talk about hierarchies and associated conflicts. When nurses (who are often women) can possess greater experience but the doctor ‘takes the lead’, this contributes to imbalances in the structure of clinical practice. Often, mandates are not given according to knowledge and experience.” That can threaten to stymie best practices and “that has been a very hard lesson to learn for me,” she says.
Hartono essentially agrees but traces an ongoing challenge: “Women do face certain challenges in the medical field due to gender bias, as well as unconscious bias or stereotypes that persist in the medical field, which is also seen among (older) patients. I have had a lot of experiences with this,” she says, noting another challenge is balancing work responsibilities with family commitments.
Rickert then tracks back to “the overarching goal” and her “driving force” which is to deliver the best possible care to patients. A recalibration of ‘authority figures’, is what she seeks and states she will not stand down until a more uniform “hierarchy” is achieved so that nurses can meet caregivers and stakeholders at eye level. “My role is that of a well-respected member of the multidisciplinary clinical team,” she avers.
Things to look forward to in Hamburg:
Fondelius lines up renal registries as being crucial requirements for quality measurements in renal care. “I will demonstrate how the Swedish Vascular Access Registry works and how we use it in our clinical everyday work in Malmö,” she says.


Hartono would like to bring fresh eyes to the basics, starting with ultrasound mapping for all accesses to aid decision making for the right access for the right patient at the right time. She also hopes to reach out to residents and trainees to bring them closer to the field of dialysis access.
Rickert reiterates her view that POCUScannulation should be advised as a standard for cannulation in the European Guidelines: “It results in enhanced patient care and satisfaction and staff, and for longer patency rates and fewer complications of arteriovenous fistulas.”

Issue9 – September 2023 11 Vascular Access
Frida Fondelius
Kathleen Rickert
Yinhui-Lim Hartono
Severe obesity linked to lower AVF maturation rates

Researchers behind a new study published in the Annals of Vascular Surgery, among them lead author Laura Anderson and corresponding author Benjamin Brooke (both University of Utah Health, Salt Lake City, USA), have found that severe obesity risks a greater likelihood of maturation failure in the arteriovenous fistulas (AVFs) of patients looking to start dialysis.
Their work cites a 2008 Kidney International-published meta-analysis by Y Wang (Johns Hopkins University, Baltimore, USA) et al, which suggested that 33% of all US cases of end-stage kidney disease (ESKD) are associated with obesity and metabolic syndrome. Some research, they add, has found higher AVF patency rates for obese patients, while other studies cite higher rates of failure and stenosis. They aimed to clarify the effect of a body mass index (BMI) ≥35, “the most severe classes of obesity”, to help enable better clinical decision-making.
They conducted a retrospective cohort study, analysing the data from patients who received either a radiocephalic, brachiocephalic, or brachiobasilic AVF at a single centre during the period 2016–2019. The primary outcome was functional maturation following AVF creation, defined as use of the AVF with two needles for 75% of dialysis sessions in a continuous four-week period. Secondary outcomes included AVF depth and diameter, as well as flow volume.
A total of 426 AVF procedures were performed during the study period, with 202 available for analysis following the exclusion criteria. Among these were 49 (24%) with radiocephalic, 87 (43%) with brachiocephalic, and 66 (33%) with brachiobasilic AVFs. Stratification by BMI yielded 53 patients (26%) in the severely obese group and 149 (74%) in the remaining categories.
The authors found a “significantly lower rate of functional maturation” in the severely obese group (58% severe obesity vs. 80% non-severe obesity; p=0.002).
A subanalysis by fistula type found that differences in primary and assisted maturation between obesity groups on brachiocephalic AVFs were significant, but were not so for those with radiocephalic and brachiobasilic AVFs. On the secondary outcomes, outflow average vein depth was “significantly increased” in severely obese patients compared to the other groups, with the difference marked across all three types of AVF (p<0.05 for all comparisons). Multivariable logistic regression analyses, which controlled for a variety of other factors including sex, age, smoking status, and AVF type, found that severely obese patients were 62% less likely to achieve functional maturation than AVFs in patients with BMI ≤34.9 (odds ratio [OR] 0.38; 95% confidence interval [CI] 0.18-0.78, p=0.009).
Weighing up the findings
The data suggest that obesity is of greatest concern in fistula maturation for severely obese patients with a brachiocephalic fistula, the authors say in their discussion, adding that it can also impact brachiobasilic AVFs “even after transposition”. Drawing attention to the “obesity paradox”, the finding of some studies that patients with higher BMIs have better survival rates with ESKD, sometimes attributed to “nutritional preserve and protection against wasting in the setting of acute illness”, they argue that their results indicate that this does not extend to fistula maturation. For treatment of severely obese patients with an AVF who cannot be cannulated, Anderson et al note that transposition and superficialisation of the fistula are established options.
Outlining some limitations, including the retrospective cohort design of their study and its focus on functional maturation outcomes rather than primary or secondary patency, the authors nevertheless conclude that their results support the claim that BMI ≥35 reduces functional maturation rates. “These data,” they state finally, “can help guide decision-making when planning AVF placement in severely obese patients, which should include scheduling adjuvant superficialisation procedures on appropriate patients.”
Weight gain in long dialysis intervals heightens mortality risk
A new retrospective cohort analysis published in Hemodialysis International has found that dialysis patients who gain weight in long intervals between dialysis sessions risk elevated mortality rates and quicker decline in kidney function. The authors of the study, including corresponding author Ramy M Hanna (University of California Irvine, Orange, USA), were led by Yoshikazu Miyasato (Kumamoto University Graduate School of Medical Sciences, Kumamoto, Japan).
MIYASATO AND COLLEAGUES outline in their introduction that interdialytic weight gain (IDWG) “is crucial in the association between long interdialytic intervals and mortality” in patients undergoing haemodialysis. Identifying a gap in the existing literature, they argue that there has yet to be a full analysis of IDWG’s effect on residual kidney function (RKF). Defined as IDWG “in the two-day break between dialysis sessions”, IDWG during long haemodialysis intervals (IDWGL) and its relationship with both mortality and RKF decline were the focus of the study.


Researchers including lead Muhammad Asghar and corresponding author Andrew D Rule (both Mayo Clinic, Rochester, USA) have published the results of a study comparing the risk assessment of chronic kidney disease (CKD) progression using age-based thresholds for nephrosclerosis against a single young adult threshold. The study in the Journal of the American Society of Nephrology (JASN) aimed to determine which approach is more effective in identifying clinically relevant CKD.
ASGHAR ET AL CARRIED OUT MORPHOMETRIC analyses of kidney biopsy images from a total of 4,697 participants, including 3,020 living kidney donors, 1,363 patients with kidney tumours, and 314 patients with native kidney disease. The researchers measured the percentage of globally sclerotic glomeruli (GSG), percentage of interstitial fibrosis and tubular atrophy (IFTA), and IFTA foci density in the kidney biopsy samples.

Normotensive living kidney donors were used to establish young-age thresholds for individuals aged 18–29 years. They also determined age-based 95th percentile thresholds, roughly categorised by decade, for comparison. Asghar et al then carried out a comparison of age-adjusted risk of progressive CKD, which they defined as kidney failure or a 40% decline in esti-
mated glomerular filtration rate (eGFR), among patients with kidney tumours and patients with kidney disease in groups that they designated as “normal compared with young”, “normal for age but abnormal compared with young”, and “abnormal for age”.
The authors found that the 95th percentiles for %GSG, %IFTA, and IFTA foci density varied across different age groups, ranging from 1.7–16%, 0.18–6.5%, and 8.2–59.3% per cm2, respectively, from the youngest group (18–29 years) to the oldest group (≥70 years).
The risk of progressive CKD did not differ significantly between individuals with nephrosclerosis that was considered “normal compared with young” and those considered “normal for age but abnormal compared with young.” However, the risk of progressive CKD was “significantly higher” when %GSG, %IFTA, or IFTA foci density were “abnormal” compared to when normal in both patients who underwent a kidney biopsy to determine the cause of their kidney disease and patients who had a kidney removed due to a tumour.
“Given that increased risk of progressive CKD occurs only when nephrosclerosis is abnormal for age,” Asghar et al conclude, age-based thresholds are recommended as a more accurate method for the identification of “clinically relevant CKD” compared to a single young-age threshold.
The retrospective study saw the authors analyse data from haemodialysis patients in the USA during the period 2007–2011. Seven degrees of IDWGL (0% to <1%, 1% to <2%, 2% to <3%, 3% to <4%, 4% to <5%, 5% to <6%, and ≥6%) were examined alongside mortality rates, using Cox regression models, as well as in relation to rapid decline of renal urea clearance (KRU), with the latter analysed with logistic regression models. Mortality was examined in 35,225 patients and rapid KRU decline in 6,425 patients.
“Higher IDWGL categories were linked to increased risk of adverse outcomes,” the authors explain. Miyasato et al note that they also analysed the continuous relationships between IDWGL and study outcomes using restricted cubic spline analyses.
“When IDWGL exceeded 2%,” they add, “the hazard ratios of mortality and the odds ratios of rapid KRU decline continuously increased.” Reviewing the findings in their discussion, the authors state that higher IDWGL was associated with both elevated mortality risk and rapid KRU decline, with greater adverse outcome risk with an IDWGL level above 2%. “Therefore,” they conclude, “IDWGL may be utilised as a risk parameter for mortality and RFK decline.”

September 2023 – Issue9 12 Risk Factors
Andrew D Rule
Yoshikazu Miyasato
Benjamin Brooke
Age-based nephrosclerosis threshold better predicts CKD progression
Some US kidney waitlist candidates passed over for less urgent candidates
A new study by Columbia University Irving Medical Centre (CUIMC; New York, USA) researchers has found that some US centres routinely skip their highestranking waitlisted candidates to give donor kidneys to a lower-ranked patient. This practice, known as “list diving,” occurs with little oversight and transparency, harming some patients and possibly contributing to disparities in organ transplantation and discard of donor organs, according to a CUIMC press release.
“It’s an open secret that some transplant centres regularly apply their own criteria for matching donor kidneys to eligible patients,” states lead author Sumit Mohan (Vagelos College of Physicians and Surgeons, New York, USA), “but no one has examined if this practice is widespread.”

In the study, published in JAMA Network Open, Mohan and colleagues analysed approximately 6,000 transplant candidates and 4,700 transplants at 11 centres between 2015 and 2019. Each centre was geographically isolated, meaning that it could decline the offer of a kidney without losing it to another centre, but still accept the organ for a lower-priority patient.
The study found that most kidneys offered to these centres (68%) were not placed with the top-ranked candidates on the waiting list and instead went to candidates further down the centre’s list. Most often, centres cited concerns
ChatGPT underperforms on clinical kidney disease questions
Research published in Kidney International Reports has found that the ChatGPT artificial intelligence (AI) language model (OpenAI) fails to consistently provide accurate answers to clinical questions in the treatment of glomerular disease. Though it recently achieved an “impressive” score on the United States Medical Licensing Examination, it still has “a long path to go” on before it is a useful tool for nephrologists, say a team of authors led by Jing Miao (Mayo Clinic, Rochester, USA).

THE AUTHORS FOCUSED THEIR attentions on ChatGPT’s answers to questions regarding glomerular disease for the reason that, they say, its effective management demands “a deep understanding of renal anatomy, physiology, pathology, and various treatment options” across a wide range of disciplines. They suggest that this more specific topic within the field of nephrology is the best way to assess the model’s capacity to provide useful clinical information.
Taking questions from the question bank of the Nephrology Self-Assessment Program (NephSAP), which “is a review of the literature over the last several years in different
about organ quality when declining the offer for the top-ranked candidate. According to the researchers, declined offers may be rooted in the centres’ belief that the top-ranked patient is likely to receive better quality organ offers within a reasonable timeframe, and that shorter wait times for lower-priority candidates would offset the lower organ quality.
“For example, if you have a 25-year-old at the top of the list and the centre is offered a kidney from a 75-year-old donor, the centre might decline that offer, believing it’s better suited for an older candidate lower down on the list,” Mohan says. However, the new study suggests other factors at play, as only 44% of the highest-quality kidneys were placed with the highest-ranked candidates.
“It seems that transplant centres often overlook their top candidates and there are many organ declines that we don’t have a good explanation for,” Mohan says.
Kidneys not offered to top-ranked candidates on waiting list:
68%
Ethics of list diving
There are several issues with list diving, according to the researchers. Although skipped candidates may receive a future offer, and it may even be a higher-quality organ, it is not uncommon for patients to die while waitlisted or deteriorate to the point that they are de-listed after having had offers declined on their behalf without their knowledge. In addition, declined offers create inefficiencies that add to the time that an organ is kept on ice, compromising organ viability, the press release argues. At present, one in four viable donated kidneys are discarded, at great cost to patients and to society given that kidney transplantation is “the most cost-effective therapy for end-stage kidney disease (ESKD)”.
Another concern Mohan outlines is that organs are typically declined on behalf of patients: “Patients are rarely involved in the decision-making process and transplant centres do not currently inform patients when an organ offer is declined on their behalf. We should give patients more say in the process and have them participate in shared decision making.”
List diving may contribute to disparities in access to transplantation, the authors suggest. They state that other studies have demonstrated that organ declines can be subjective, with differences reported based on the recipient’s race and other factors, such as obesity.
The system could benefit from improved allocation algorithms that more precisely match specific organs to recipients, the researchers say, and more transparency in the way organs are allocated.
“All told, list diving undermines the intended objective design of the allocation system in a manner that is shrouded from both patients and regulatory oversight,” Mohan says. “And it risks undermining the trust that patients and donor families have in a fair and equitable system.”
ance was higher for incorrect answers (82% compared with 67%). These rates, the authors explain, fall far short of the 75% passing rate for NephSAP and the 76% rate for KSAP. The 74% concordance rate, they say, suggests “limited repeatability”, though they add that the more recent paid version of the model, ChatGPT 4.0, may achieve greater consistency.
domains of nephrology”, the authors also used those on glomerular disease found in the Kidney Self-Assessment Program (KSAP) of the American Society of Nephrology (ASN), which is more “geared for preparation for the American Board of Internal Medicine (ABIM) nephrology board exam”. These they then presented to the free version of ChatGPT, GPT 3.5, first in its 14 March iteration and then, in a second run of questions, its 23 March version. Accuracy was compared between these two versions, and the concordance of their answers recorded.
Miao et al detail that, on the five NephSAP glomerular disease question banks
with a total of 150 questions, ChatGPT achieved a score of 45% on its first run and 41% on its second. Concordance between the two sets of answers was 73%. Next, the authors presented ChatGPT with 33 questions on glomerular disease from the KSAP question banks. On these, the model achieved 42% and 39% on the first and second runs respectively, with a 76% concordance rate.
In total, 183 questions on glomerular disease were presented, with 45% accuracy on ChatGPT’s first run and 41% on its second run answers, with concordance between its two sets of responses of 74%. The authors make the point that concord-
Miao et al voice concerns about the technology’s potential use in the American Board of Internal Medicine (ABIM) longitudinal knowledge assessment (LKA), which is taken at home as part of the renewal of the Nephrology Board Certificate. Steps should be taken to ensure ChatGPT is not utilised for this examination, they say. The model is also limited by an inability to engage with medical images, which comprise a significant portion of examinations, and the authors conclude that “further [research] and training are needed to improve its accuracy and repeatability in real-world clinical situations, particularly in processing medical images.”
Issue9 – September 2023 13 Waitlisting
“[List diving] risks undermining the trust that patients and donor families have in a fair and equitable system.”
“Further [research] and training are needed to improve its accuracy and repeatability in real-world clinical situations, particularly in processing medical images.”
Sumit Mohan
Q&A
Kate Steiner
From choosing her specialty to competing in dressage, interventional radiology (IR) lead at East and North Herts NHS Trust (Stevenage, UK) Kate Steiner speaks to Renal Interventions on her career in IR, her inspirations in and outside of the field, and how she spends the moments between cases. Vascular Access Society of Great Britain and Ireland (VASBI) treasurer and co-chair of the Charing Cross (CX) Symposium, she offers her perspectives on drug-coated balloons (DCBs), IR provision, and more.
What drew you to a career in medicine and in interventional radiology (IR)?
During my junior doctor training, I spent time as a neurology senior house officer (SHO) and then as a senior clinical fellow in intensive care at the Royal Free Hospital (London, UK), which is where I also trained as a medical student. Prior to my intensive care job, I had completed my Membership of the Royal College of Physicians (MRCP) and was spending this time working out what speciality would suit. Initially, I had wanted to pursue a career in neurology. I had always had an interest in neuroscience and I had completed an intercalated BSc in psychology as part of my medical degree. However, throughout my training I had always been very good at and enjoyed procedures such as central line insertion, arterial line insertion, and lumbar puncture—and I could cannulate the proverbial stone. While reading What Colour Is Your Parachute?, trying to work out what my next step in medicine would be, I went into an interventional radiologist’s office to ask advice on a difficult patient, and we got chatting about my career. He reminded me how much I had enjoyed the weekly neuroradiology teaching, and that I had an aptitude for interpreting imaging. He asked if I had considered a career in radiology. Radiology and IR were not something I had had any exposure to. I then spent some time in the radiology department and have never looked back! When I was in my second year of radiology training, I was taken aside by one of the interventional radiology fellows who had spotted I had an ability for IR, and she encouraged me to pursue it as a career. IR offers patients a minimally invasive treatment option that usually only required conscious sedation and can treat life threatening conditions such as acute haemorrhage. As a trainee, you see the high levels of patient satisfaction and how a couple of inexpensive coils and a sheath can save a life. It was clear to me that IR was a rewarding and exciting speci-
ality to be part of, where advances in technology were leading to improvements in patient care.
Do you have a mentor who has helped and inspired you in your career?
I have a mentor: she is an expert on women’s economic empowerment, with a special interest in women in global supply chains, and has a Membership of the Order of the British Empire (MBE). Having a mentor whose work is outside of medicine has provided me with a fresh perspective on many tricky issues and some useful tools to help me address them. Later in my career, I have unfortunately encountered people whose worldview has somewhat stereotypical opinions of women. Having a mentor has encouraged and enabled me to look past these attitudes and behaviours, and to continue to “lean in” and pursue my career goals.
Is there a particular case that has stayed with you during your career?
There is not a particular case that has stayed with me during my career, but there are many patients. The patients that we treat for arteriovenous (AV) access dysfunction and dialysis access often come back for repeat diagnostic ultrasound and for repeat endovascular procedures. Over time, you get to know each other well and a doctor-patient relationship develops. This makes AV access one of the most rewarding specialities to be part of.
Are there any recent developments in IR and kidney care that most excite you?
I think that endovascular arteriovenous fistulas (endoAVFs) have been the most innovative advance in dialysis access for many years. The other development which I believe will have a major positive impact on patient care is the publication of the Kidney Disease Outcomes Quality Initiative (KDOQI) 2019 guidelines. The individu-
alised End Stage Kidney Disease Lifeplan (ESDL, focusing on “right access, at the right time, for the right patient, for the right reasons”, is a patient-centred approach. This should lead to improvements in quality of care.
How do you think access to IR care can be improved for kidney patients in the UK?
The first step to improving access to IR care in the UK is taking a patient-centred, multidisciplinary approach. Where multidisciplinary teams which include interventional radiologists work together, this results in better quality care where patients will be offered a wider choice of treatment options. New emerging technology tends to be adopted in larger centres with interested interventional radiologists, while smaller centres may not have the time, resources or managerial support available to do this. Centralisation of lower volume procedures may mean that patients at smaller units can access speciality services at larger centres. At present, patients are not always referred onwards.
What is your perspective on the existing endoAVF options?
The safety and efficacy of the current devices has been demonstrated in the published trial data. The existing endoAVF devices in my opinion offer additional access options for patients which confers certain advantages, especially to younger patients or those patients who benefit from not having a high-flow upper arm AVF. At my institution, we have an endoAVF programme using the Ellipsys (Medtronic) device. Patients are very well-informed about their options, and even before starting the programme we had patients asking for an endoAVF. Patients sometimes prefer the cosmetic appearance of the endoAVF compared with a surgical fistula. They also hear from other patients that they have had a good experience with their endoAVF, which can often be cannulated early at around the four week mark. We have some patients who have refused a surgical fistula (sAVF), accepted an endoAVF, and are now dialysing via a functional AVF, and their catheter has been removed.
What is your current appraisal of drugcoated balloons (DCBs) and where they are appropriate?
In the IN.PACT AV access study, we now have a high-quality prospective, multicentre, randomised controlled trial (RCT) demonstrating the benefit of DBCs in AV access. However, the evidence from other trials and publications is mixed. I use DCBs in my practice, and have seen the benefit of less frequent repeat interventions for patients. However, I do not believe it is cost effective and best practice to use them for every lesion in every patient. Some patients will go a long time between interventions with effective plain-balloon angioplasty. Predicting who will respond well to plain-balloon angioplasty and who will benefit from DCB use is difficult. In my practice, I will almost certainly use a DCB for a recurrent lesion where there is intimal hyperplasia seen on ultrasound. I believe that in the future we will adopt a more patient-centred approach, with algorithms for treatment based on lesion appearance on b-mode ultrasound, lesion location, previous interventions, and perhaps biochemical/lesion markers which we do not yet fully understand.
Fact file
Current appointments: Consultant interventional radiologist and interventional radiology clinical lead: Lister Hospital East and North Herts NHS Trust and Hertfordshire and West Essex Vascular Network (Stevenage, UK)
Clinical Pathways
Workstream Chair: East of England Imaging Network
Education:
2002: Membership of the Royal College of Physicians
2008: Fellowship of the Royal College of Radiologists
2010–12: Subspeciality fellowship in interventional radiology: Royal Free Hospital (London, UK)
Honours (selected):
1997: Glaxo-Wellcome Blackwell book prize for clinical skill and knowledge
1999: Bunzl prize in psychiatry
2000: Runner-Up in the Royal Free Art Society ART 2000 exhibition
2009: British Society of Interventional Radiology (BSIR) 2009 award for the best oral presentation by a trainee (BSIR 2009 Brighton)
2017–present day: Vascular Access Society of Britain and Ireland (VASBI) council member, 2022 elected treasurer
2023: Co-chair of the Charing Cross (CX) International Symposium: Vascular Access Masterclass with Nicholas Inston. Executive Board member for the Charing Cross Vascular Symposium
Profile 14 September 2023 – Issue9
Illustration by: Andy Watt / NB Illustration
How do you feel your work with VASBI fits into the landscape of vascular access?
I think it is an exciting time for VASBI. I hope that our work as a council will help lead to better quality care for dialysis patients. The VASBI meeting showcases new technologies and advances in dialysis care. One of the aspects of the VASBI meeting which I have always found particularly useful in shaping my own practice is the examples of good clinical practice using existing technology and resources available to most centres which are shared both on and off the podium. VASBI is a truly multidisciplinary council and meeting, which I think allows us all to learn from and disseminate knowledge across co-dependent specialities in AV access. Research is now playing a more central role in the council agenda, and research specifically aimed at addressing key issues in dialysis access is essential to obtain the evidence needed to guide best practice.
What is your philosophy within medicine?
My philosophy within medicine is to take a patient-centred approach. I believe that improvements in patient pathways and protocols, as
well as resulting in better quality, safer care for patients, also leads to improvements in staff satisfaction and working conditions. We have seen this clearly with the build of our interventional radiology day case unit. One of my pet peeves is where silo working leads to conflicts between clinical specialities, either at a clinical, managerial or financial level, and “turf wars”—usually centred on limited financial resources or self-interest—lead to poor-quality patient care. My work as the chair of the clinical pathways workstream with the East of England E2 Imaging Network is to establish agreed uniform patient pathways and protocols across the network to improve patient experience and care. One of the most rewarding aspects of this role is learning from centres where there is good practice, and seeing this practice gradually adopted across other trusts within the network.
What are your interests outside of medicine? I love to spend my free time with my husband, my son and family. I am a keen equestrian—I have a beautiful Lusitano mare, we have fun together and compete in dressage. When I am not at work or at home I will be at the stables!

Profile 15 Issue9 – September 2023
“Centralisation of lower volume procedures may mean that patients at smaller units can access speciality services at larger centres. At present, patients are not always referred onwards. “
Cognitive therapy found to cut dialysis side effect burden
A new patient-guided intervention using telemedicine to treat the side effects of dialysis including fatigue and depression has delivered “results that persist for several months after the intervention ends” in a randomised clinical trial published in JAMA Internal Medicine by researchers at the University of Pittsburgh (Pittsburgh, USA) and University of New Mexico (Albuquerque, USA).

“Our patients and their caregivers report pain, fatigue and depression as major issues related to dialysis that affect their participation in life,” said lead author Manisha Jhamb (University of Pittsburgh, Pittsburgh, USA). “It is the nephrology community’s responsibility to study interventions that address patients’ mental health and quality of life concerns; otherwise, we are failing our patients. This study is a step in that direction.” At a recent US National Institutes of Health workshop, patient stakeholders “emphasised that post-dialysis fatigue is a serious concern”, a press release details.

Jhamb and her colleagues developed the Technology Assisted Stepped Collaborative Care (TACcare) trial to test whether 12 weekly sessions of cognitive behavioural therapy delivered via telemedicine either while patients receive their dialysis or at home could improve symptoms. To

ensure the results weren’t simply an effect of the added attention from the tele-sessions, they had a comparison group of patients receive weekly health education via telemedicine.
Jhamb and colleagues recruited a diverse pool of 160 participants from Pennsylvania and New Mexico who were receiving dialysis and had clinically significant levels of fatigue, pain or depression. They averaged 58 years of age; 28% were Black, 13% American Indian and 18% Hispanic.
The intervention allows the patient to set goals and tailor the care to address their specific concerns. For example, if the patient is concerned about pain, they can focus on psychotherapies to manage that pain and also receive medications.
“It was initially surprising that very few participants wanted medication for their symptoms—they were much more focused on the behavioural therapy,” Jhamb said. “But when you think about it, it makes sense: these patients
Improvement in energy levels with TACcare: 6%
Improvement in pain severity with TACcare: 10%
Peritoneal dialysis patients should be alerted to risk of drain pain, say researchers
In a research letter submitted to the editor of the American Journal of Kidney Diseases (AJKD), investigators including lead Zeenia Aga and corresponding author Jeffrey Perl (both St Michael’s Hospital, Toronto, Canada) argue that drain pain is a complication of peritoneal dialysis (PD) that demands more attention from clinicians. Writing as part of the team behind the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS), Aga et al sought to establish both the prevalence and the causes of the problem.
THE AUTHORS OF THE RESEARCH LETTER outline that drain pain’s impact on patients’ quality of life demands the development of “avoidance and management strategies”. This, they suggest, will be made easier by understanding the risk factors with which it is associated.
For their study, they identified 1,630 PD patients across 121 centres in five countries who had filled out a questionnaire on drain pain. Patients were asked if they experienced drain pain in the preceding week, and if so, were then asked to rate their pain on a Likert scale. Severe pain was defined as a score of >5/10 on the scale. Using multivariable logistic regression, the authors then specified patient and treatment risk factors associated with the complication.
In total, 461 (28%) of the 1,626 patients whose answers to the questionnaire were used reported experienc-
ing drain pain, while 35% of these described the pain as severe. There was geographical variation in reported rates, with the complication most prevalent in the UK (22/47 UK responses; 47%) and least so in the USA (77/409 US responses; 19%).
Patients aged 75 or over were found to have lower rates of drain pain than those aged 60–74 years (adjusted odds ratio [AOR] 0.58; 95% confidence interval [CI] 0.39–0.85), as were males compared with females (AOR 0.61; 95% CI 0.48–0.78). Aga et al also detail that incident patients who received PD (<3 months vs. >3 years) had higher odds of experiencing drain pain (AOR 2.64; 95% CI 1.74–4.00), as did those who had polycystic kidney disease (PKD; AOR 3.23; 95% CI 1.72–6.10).
The authors note a trend towards less drain pain with straight-tipped catheters vs. coiled (AOR 0.76; 95% CI 0.50–1.16), as well as with laparoscopic compared with open surgical or percutaneous catheter insertion (AOR 0.72, 95% CI 0.50–1.06).
They also found that automated PD was more strongly associated with drain pain than continuous ambulatory PD (AOR 1.61, 95% CI 1.20–2.17), but also with “less severe pain”—though only 39% of automated PD patients with PD received tidal prescriptions. Neither a history of abdominal surgery nor constipation were associated with any increased rate of drain pain, the authors add.
“The increased drain pain observed in PKD and females may relate to enlarged polycystic kidneys and a uterus ‘crowding’ the peritoneal space, pushing the PD catheter against sensitive tissues as seen in urinary retention and colonic distension,” the authors speculate towards the end of the letter.
On incident PD patients’ higher risk of drain pain, they suggest that “they need time to acclimatise to PD and PD catheter positions may change over time”. The lower rates of drain pain seen with laparoscopic insertion may be down to “direct visual inspection” and the “optimisation of PD catheter position”, they add.
are taking 10 or more medications per day. They do not want another pill.”
Compared to those who received educational materials, patients who received TACcare had a 6% improvement in energy levels and a 10% improvement in pain severity, both sustained for six months following therapy. They also had an improvement in depression symptoms, but it was “very small”, something Jhamb partially attributes to only a small number of participants initially having clinically significant depression.
She added: “These results are really promising and in the range seen by similar interventions for cancer patients. Until now, analgesic medications for pain and recommendations for better sleep and exercise to address fatigue have been the main suggestions we have been able to offer our patients, and they have not been satisfactory.”
The diversity of study participants also indicates that the results are generalisable to broad populations, the press release adds. Additionally, the successful use of telemedicine with rural participants in New Mexico provides an opportunity to offer TACcare to patients who might otherwise not have access to these specialised behavioural therapy services.
Jhamb says the next steps involve a cost-benefit analysis so that US policymakers and health insurers can consider mandating and paying for the intervention, as well as trials to test if “booster” sessions could prolong the results beyond six months.
Offering suggestions to clinicians, Aga et al suggest finally that “use of continuous ambulatory PD, tidal automated PD, straight PD catheters, and laparoscopic catheter insertion carefully considering pelvic PD catheter position” may all serve to mitigate drain pain.
More broadly, they say that “the possibility of drain pain should be discussed with patients” before initiation of PD—particularly those in more at-risk groups such as women and PKD patients. “This work,” the authors conclude, “informs the need for a validated drain pain questionnaire to support innovations in PD catheter design, implantation technique, and automated PD technology.”
Speaking to Renal Interventions, Aga said: “We hope that our research helps kidney care practitioners identify patients who have a high risk of drain pain to ensure these patients are appropriately counselled prior to initiation of peritoneal dialysis, and to consider implementation of protective strategies such as ambulatory PD prescriptions or tidal automated PD prescriptions to limit their risk of discomfort.
“In the future, with more practitioners adopting the approach of incremental peritoneal dialysis—which often lends itself to ambulatory PD prescriptions—it will be interesting to see if there is an associated reduction in drain pain frequency in incident PD patients. Further research on variations in drain pain by PD technology may help to further guide novel strategies to reduce the incidence of drain pain.”
16 September 2023 – Issue9 Dialysis Side Effects
Manisha Jhamb
Jeffrey Perl
Zeenia Aga
Ali Kordzadeh
ration, early-onset stenosis, and thrombosis of AVFs. Despite this, a reliable and prompt protocol has yet to be established. This, coupled with the identification of different inflammatory biomarkers, has become a pivotal point of discussion amongst access providers and patients.
This has sparked the question: “What if a continuous, dynamic, wearable, and patient-focused surveillance program could be implemented?” One that could eliminate the need for frequent hospital visits and instead be compatible with smartwatches or home devices for immediate identification and response to any complications.
Innovation in dialysis access: From vision to reality
The global burden of kidney disease has made dialysis a critical lifeline for millions, and the need for safer, more reliable, and more efficient access and its management has never been greater. The intersection of technology and healthcare has been a catalyst for innovation in recent years, and dialysis access stands at a crossroads of innovation and transformation. As we look to the horizon, it’s clear that we’re on the precipice of a new era that promises unparalleled advancements from innovative bioengineered grafts, to sophisticated wearable monitoring devices and new trends that will impact the way we approach dialysis access.
The development of cutting-edge technologies, techniques and artificial intelligence is set to transform this crucial aspect of patient care, promising a greater precision, improved patient safety, and fewer complications. These advancements are not confined to the distant future; they are within our grasp now, transforming the way we perceive, monitor, plan, perform and provide access. A dive into the potential of these innovations casts a spotlight on how they may revolutionise the landscape of dialysis access in the years to come.
Since the initiation of intermittent peritoneal dialysis in the 1940s, our journey in renal care has altered significantly. Currently, we stand on the threshold of a new epoch characterised by 3D-printed, automated peritoneal dialysis (PD) through a dynamic interface reinforced by the precision of artificial intelligence and robotics. This consequential evolution is enhanced by cutting-edge optical chemical sensors, facilitating non-invasive, dynamic assessments of peritoneal protein loss in end-stage kidney disease (ESKD), and initiating precise dialysis even during sleep.
Home pulse pressure monitoring has developed as a vital prognostic tool, precisely predicting mortality and cardiovascular events in PD patients. The addition of far-infrared therapy, meanwhile, offers potential improvements in PD by reducing the incidence of peritonitis. Their incorporation with point-of-care, self-driven, portable ultrasound and individualised peritoneal solutions through telemedicine is a testament to the strides that have been taken so far.
In addition to these trends, significant progress in vascular catheter design, novel materials, and the application of innovative coatings have significantly enhanced patient care. The diversification of central venous catheter design into step, split, symmetric and self-centring-tip with
superior biocompatibility, continues to cater for unique and dynamic clinical circumstances. The use of high-quality imaging data predicts and plans the optimal catheter placement and foresee potential complications, thereby enhancing procedural accuracy and safety. Improvements in real-time flow assessment at the catheter tip using microsensors provides immediate evaluation of central venous catheter (CVC) malfunction, limiting those complications that were once frequent.
In the realm of haemodialysis, arteriovenous fistulas (AVF) continue to be the gold standard for haemodialysis due to their durability, affordability and reliability. Annually, 5,000–6,000 patients in the UK and around 9.7 million globally require haemodialysis, a figure predicted to double by 2030. Their failure could have substantial psychological implications reducing quality-adjusted of life years (QALY).
Frequent interventions, recurrent hospital visits, failures in dialysis, and the implementation of interim modalities are directly associated with increases in all-cause mortality. For those of average-to-high socioeconomic status, life expectancy ranges between 5–7 years, forecasted to reach 9 years. These patients will generally require a new AVF every 2–3 years, with each typically necessitating two or more interventions to maintain their patency. Regrettably, this only applies to 40–60% of those AVFs that successfully mature and are subject to surveillance.
Endovascular AVF (endoAVF) techniques represent another profound leap in the field of haemodialysis access. These innovative, minimally invasive procedures have emerged as a robust alternative to traditional open surgeries, marking a significant shift in the landscape of renal healthcare. Groundbreaking devices are at the forefront of this transformation, harnessing advanced image guidance technology for the precise creation of AVFs. The ramifications of these technological advancements are substantial: reduced hospital stay durations, accelerated patient recovery, and significantly decreased risk of infection. Consequently, the integration of endoAVF creation represents an important advancement in renal care, fostering improved patient outcomes and greater healthcare efficiency.
In the last decade, a considerable amount of research has been conducted to identify the optimal investigative modalities and their timing for detection of failed or delayed functional matu-
The integration of non-invasive diagnostic techniques such as infrared, acoustic, optical stereoscopy, and duplex ultrasound sensors, along with measurements of flow velocity, pulse wave velocity, vascular resistance, and compliance has made such an idea plausible. The driven data from these innovations are able to identify patterns and trends that may indicate AVF malfunction, allowing timely intervention. Furthermore, assimilation of inflammatory biomarkers in such algorithms has expanded the horizon of remote patient care in dialysis—eliminating the vast psychological, clinical and cost burdens.
Incorporation of these technologies into wearables, gadgets or smart patches is here. It is paving the way for a world of minimum patient contact with maximum clinical outcome. In the last few years, we have witnessed such breakthroughs, where a simple adhesive patch has the ability to detect various biomarkers without the need for repeated blood tests.
Indeed, the current advancements in wearable technology have reached a stage where a single wearable watch can utilise signal amplitude, venous calibre, and pulsation data to detect the possibility of stenosis. Through sophisticated algorithms, this information can be filtered, calibrated, and adjusted to accurately assess blood flow, facilitating the prediction of successful maturation in vascular access for haemodialysis. This breakthrough represents a significant step forward in the field of haemodialysis access management, as it enables early detection and intervention, ultimately leading to improved patient outcomes and a more efficient and effective approach to dialysis care. As wearable devices and artificial intelligence continue to evolve, the future of haemodialysis access holds significant potential for enhancing patient care and transforming the landscape of renal therapy. Collaboration between medical teams, engineers and patients is crucial for advancing such technologies in haemodialysis access. Collaboration with patients and caregivers, meanwhile, is equally vital, and involving them in the design and testing process ensures that the solutions developed truly meet the needs and preferences of those who will use them. Patient feedback is invaluable in refining wearable devices and algorithms to be user-friendly, reliable, and well-integrated into their daily lives.
Ali Kordzadeh is a consultant vascular and access surgeon at Mid and South Essex NHS Foundation Trust (UK) and a visiting professor at Anglia Ruskin University (Chelmsford, UK).

The author stated no relevant disclosures.
References:
Available online at: www.renalinterventions.net/ innovation-in-dialysis-access-from-vision-to-reality/
17 Access Innovations Issue9 – September 2023
Point of View
“The integration of non-invasive diagnostic techniques and wearable technology holds promise for identifying access malfunction, enabling timely intervention and eliminating psychological, clinical, and cost burdens.”

Metabolic syndrome changes associated with kidney graft failure
A new study in Nephrology Dialysis Transplantation has examined the relationship between metabolic disorder (MetS) and adverse outcomes in recipients of kidney transplants. The national prospective cohort study was designed by a team led by Yu Ho Lee (Cha University, Seongnam, South Korea) and which featured corresponding author Hyeon Seok Hwang (Kyung Lee University Medical Center, Seoul, South Korea).
THIS WORK FOLLOWS A 2019 STUDY in Transplantation which also probed the condition in kidney transplant patients. First author Winnie Chan (Queen Elizabeth Hospital, Birmingham, UK) and others suggested in the conclusion of that report that dietary interventions, including a cut to fructose intake and a systemic endotoxaemia, were “plausible targets” for improving this population’s metabolic health, but they did not investigate the graft loss risk associated with MetS.
Lee and colleagues’ study looked at data from a total of 4,187 kidney transplant recipients registered in a Korean database during the period 2014–2020. MetS was designated as the presence of at least three components of the syndrome, while patients were categorised into four groups according to their pre- and post-transplant MetS status: MetS-free, MetS-developed, MetS-recovered, and MetS-persistent. The primary outcomes analysed were death-censored graft loss and a composite of cardiovascular events and death.
Among recipients without pre-transplant MetS, it was found that 19.6% (419 of 2,135) developed MetS after transplantation. Those with pre-transplant MetS, 38.7% (794 of 2,052) no longer had the condition following transplantation. Among the four groups, the MetS-developed group exhibited the worst graft survival rate, while the MetS-persistent group had a poorer composite event-free survival rate.
Compared to the MetS-free group, the MetS development was associated with a significantly higher risk of graft loss (adjusted hazard ratio [aHR], 2.35; 95% confidence interval [CI], 1.17–4.98). This risk “increased with the number of dysfunctional MetS components”, the authors note, while the MetS-persistent group faced increased risks of cardiovascular events and death (aHR, 2.46; 95% CI, 1.12-5.63). Changes in the number of dysfunctional MetS components did not affect this risk, however.
Lee et al argue in their conclusion that “kidney transplantation significantly alters the metabolic status” of patients. Post-transplantation development of MetS was associated with higher graft loss risk, while “persistent MetS exposure before and after transplantation increased the likelihood of cardiovascular events and negatively impacted patient survival”.
Earlier
transplantation show little benefit for young kidney patients
Published in the Clinical Journal of the American Society of Nephrology (CJASN), a new report has examined the timing of initiation of kidney replacement therapy (KRT), including both dialysis and transplantation, in children and young adults. The findings of the study, which was led by Nicholas Larkins (Perth Children’s Hospital, Nedlands, Australia), suggest that younger patients initiating KRT earlier do not achieve significantly better outcomes.

Larkins and colleagues make the claim that there is little in the way of evidence for deciding when to begin KRT in children, arguing that there are “no randomised trials” on the question. In that vein, the authors of this study “sought to define trends and predictors” of estimated glomerular filtration rate (eGFR) at initiation of KRT and its relationship with rates of patient survival. They also examined variation in clinical practice across centres.
The study drew on data for 2,274 children and young adults (1–25 years) in the Australia and New Zealand Dialysis and Transplant Registry, and its authors detail that they developed estimates of the association between eGFR upon beginning KRT for these patients and several covariates using quantile regression. Among them was its association with patient survival, which was analysed using Cox regression, while clinical practice variation was quantified through use of logistic regression which cate-
gorised eGFR around a value of 10ml/min/1.73m2

The authors state that they found “no association” between eGFR at therapy initiation and survival (hazard ratio [HR] 1.01 per ml/min/1.73m2; 95% confidence interval [CI]: 0.98 to 1.04). They make the claim that 6% of the total variance in the likelihood of initiating KRT earlier was down to centre variation, a rate which “rose to over 10% when comparing paediatric centres alone”. They add that the median eGFR of the patients included increased from 7ml/min/1.73m2 to 9ml/min/1.73m2 over the study period, and the 90th centile from 11ml/min/1.73m2 to 17ml/min/1.73m2
“The effect of era on median eGFR was modified by modality,” they explain, and they detail that there was a greater increase in the cases of pre-emptive kidney transplant (1ml/min/1.73m2 per 5 years; 95% CI: 0.6–1.5) and peritoneal dialysis (0.7ml/min/1.73m2 per 5 years; 95% CI: 0.4–0.9) compared to haemodialysis (0.1ml/ min/1.73m2 per 5 years; 95% CI: -0.1 to 0.3).
From these data, Larkins et al conclude that younger children and young adults initiated KRT “progressively […] earlier”, and that the effect was particularly notable in those children who received a pre-emptive transplant or peritoneal dialysis—and that much of the differences in clinical practice were simply down to centre variation. They note, however, that earlier initiation of transplant and dialysis, whether peritoneal or haemodialysis, did not produce statistically significantly better survival outcomes for these patients.
A team of researchers led by Shaifali Sandal (McGill University Health Centre, Montreal, Canada) has shared the results of a study evaluating a new risk model for predicting “inferior patient and graft outcomes” in those who receive a kidney transplant. The study, published in Kidney360, aimed to overcome what its authors describe as the limitations of using delayed graft function (DGF) alone as a measure of graft function and prognosis, despite its use as “an important endpoint in clinical trials”.

EXAMINING DATA FROM 792 ADULT KIDNEY
-only deceased donor transplant recipients between 1996 and 2016, the risk model stratified patients by looking at renal function recovery <100% and their 90-day estimated glomerular filtration rate (eGFR)—they note that 24.5% of patients experienced DGF, while 40.5% had renal function recovery below 100%, and 6.9% had an eGFR <30 at 90 days post-transplant. The authors then used Cox proportional hazards models “to assess the independent relationship between the exposure and death-censored graft failure (DCGF) and mortality”.
The median follow-up period for the study was 7.3 years. During this time, Sandal and colleagues observed that the rate of death-censored graft failure (DCGF) was 18.7%, while mortality occurred in 25.1% of recipients.
Analysed alongside a group judged as having no risk of inferior outcomes according to their criteria, the team found that kidney recipients demonstrated “an incremental increase in risk of DCGF” in a low-risk group (adjusted hazard ratio [aHR]=1.53, 95% confidence interval [CI]:1.03–2.27), a moderate-risk group (aHR=2.84, 95% CI:1.68–4.79) and a high-risk group (aHR=15.46, 95% CI:8.04–29.71).
When comparing the risk model’s predictive abilities to DGF alone, Sandal and her team found when using a “hierarchical approach” that “each additional exposure in the risk model predicted the risk of DCGF better than DGF alone”. The authors also state that 100 random bootstrap replications “supported the internal validity of the risk model”. On validity, they add that an external validation cohort who were evaluated as being at lower DCGF risk found “similar non-significant trends”.
The risk model, Sandal and colleagues argue, offers more “incremental assessment” of the risk of adverse outcomes than DGF on its own. “This,” they conclude, “can help advance the field of risk assessment in transplantation and inform therapeutic decision-making in patients at the highest spectrum of inferior outcomes.”
19 Issue9 – September 2023 Graft Function
dialysis,
New kidney graft function risk model promises more accurate prognosis
Nicholas Larkins
“This can help advance the field of risk assessment in transplantation and inform therapeutic decision-making.”
Shaifali Sandal
UKKW 2023: NHS renal service programme emphasises earlier intervention, home dialysis
A campaign to reshape kidney care within the National Health Service (NHS) in England was the subject of a centrepiece session at the recent UK Kidney Week event in Wales (5–7 June, Newport, UK), with a host of speakers setting out the ambitions of the Renal Services Transformation Programme (RSTP). The multi-agency programme is led by Ahmad Saleem Ullah and Smeeta Sinha (Salford Royal NHS Foundation Trust, Salford, UK), national clinical director for renal services in NHS England. Both were in attendance to outline the project, which has just completed its design phase.
Speaking to introduce both the programme and the session, Richard Fluck (Royal Derby Hospital, Derby, UK), national clinical director of the Internal Medicine Programme of Care at NHS England and clinical senior responsible officer for the programme, said that its aim is to build a service model “that moves towards a learning system” and “a structure that allows us to continually improve and develop our service”.
Expanding on this, Fluck stated: “To do that, the system needs to join up the care pathway for individuals and tackle unwarranted variation. We also need to look at population health and not just provider delivery.”
The core principle behind the RSTP is to expand clinician thinking across systems to a “whole pathway approach”, one which creates the maximum number of opportunities for early intervention. Fluck highlighted the concept of “vertical integration” of care, meaning the consideration of all of a patient’s needs at a particular stage rather than limiting the scope of their care to their kidney disease. He gave the example of frailty, something which must be managed alongside renal disease for many older patients, and he also noted that patients with cardiovascular disease, for instance, need to be treated in a way that limits their cardiovascular risk.

Before giving the floor to the next speaker, Fluck said the programme “has provided a roadmap for delegation of services”, allowing local NHS England services to find their own tailored solutions to their unique challenges. With the programme’s design complete, he concluded that 2023 will be the year to deliver it in practice and support implementation through renal clinical networks.
Next up came Sinha, who started by outlining the collaboration that allowed for the development of the RSTP. “The renal community has always had a multidisciplinary approach to the way we deliver care,” she stated. “This programme is no different—we have engaged with the charitable sector and other people within NHS England, and we have been going to various committees to highlight how important renal services are.”
Sinha explained that part of the programme included regional renal clinical networks, which help each region to deliver the its aims. Some of
those aims, she suggested, could be “quick wins” that could be accomplished with relative ease, among them an expansion of access to home haemodialysis.
More broadly, the programme’s “toolkit”— detailed later in the presentation—highlights the need for more preventative care, particularly in the areas of chronic kidney disease (CKD) and acute kidney injury (AKI). One of the challenges in CKD care in the NHS that Sinha outlined was the “significant variation in the services offered […] and outcomes achieved” for patients. Early detection, foundational to the toolkit, is crucial to improving the situation, she said.
Cost is another major health system challenge. Highlighting the work of another presence at UKKW, Kidney Research UK, Sinha pointed to their recently published report on the health economics of kidney disease to 2033. This report, she noted, suggests that previous estimates of the cost of kidney disease to the UK healthcare system of £2 billion were a considerable estimate—with the real cost estimated now to be £6.4 billion. This represents a cost that could be mitigated by service improvement. The same report, Sinha said, suggests that implementation of improvements like those recommended in the RSTP could prevent 10,000 premature deaths by 2033.
With the focus of the session turning to the resources the programme is launching, Sinha handed over the spotlight to Saleem Ullah. He began by explaining that the “drive” of the programme was to identify what kind of environment is required for systems to improve the quality of renal care. RSTP, he explained, was developed in response to a need to transform renal care and incorporated recommendations outlined in a report on renal services from the Getting It Right First Time (GIRFT) team, and looks to improve care through, in particular, better collection and integration of data.
With that in mind, the programme is launching two products. First is the toolkit, which comprises a collection of case studies, alongside principles for best practice that can be explored through self-assessment questionnaires and help systems with driving improvement. “It is not a mandated document or a commissioning guideline, or even national clinical guidance that we expect systems to adhere to,” Saleem Ullah
specified—it is designed to provide supporting information on all elements of the renal clinical pathway and cross cutting themes, and is not meant to be read in its entirety.
The toolkit is broken up into two sections, first a short-read summary which “summarises key messages we are trying to get across to drive transformation”, Saleem Ullah explained, and second an interactive section containing more details including actions that could be taken achieve transformation principles, self-assessment questionnaires, patient stories and links to other resources. He recommends that it is used in collaboration by a whole renal care system, rather than alone as an individual. It includes research and information from a variety of charities such as Kidney Care UK and the National Kidney Federation and amounts to a “one-stop shop” of information and resources in the area. The dashboard, launched alongside the toolkit, has been developed following an engagement process similar to developing the toolkit and provides information and data to clinicians to inform their decision-making, Saleem Ullah divulged.
Estimated cost of renal care to UK health system: £6.4 billion Report improvements lives that could be saved by 2030: 100,000
With the details sketched out, Sinha then retook the stage. She explained a little more on the philosophy behind the project—how it aims to look at CKD across a patient’s whole experience of the disease, rather than only involving renal services as the disease progresses. Further, she outlined the principles at the heart of the RSTP’s approach. The development of integrated CKD clinics, as well as shared decision making with patients, are prime among them. They also include greater focus on patient monitoring and risk reduction, as well as the integration of CKD dashboards and coding—“because we know that by coding people, their outcomes are better, as it helps with their treatment”.
Later speakers at the session included Nitin Kolhe (University Hospitals of Derby and Burton NHS Foundation Trust, Derby, UK), who focused on reforms to the treatment, management and prevention of AKI. The AKI section of the programme calls for improved identification of those patients at risk, as well as “timely intervention” and better post-discharge care.
In the penultimate part of the session, Nicholas Torpey (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) weighed in on the kidney transplantation first approach. Its key principles, he outlined, include a reduction in inequities in transplant and waiting times across NHS units. The aim is optimisation of the use of each donor organ, achieved in part through the minimisation of cold ischaemic time. Following Torpey, Saleem Ullah returned to cap the session, calling for clinicians to adopt the same multidisciplinary, co-operative approach used in the development of the programme in their own implementation of its recommendations.
20 September 2023 – Issue9 UKKW
Week
UK Kidney
Richard Fluck at UKKW
“The system needs to join up the care pathway for individuals and tackle unwarranted variation.”
UKKW 2023: Welsh research highlights patient reluctance towards home dialysis
Why do more patients not choose haemodialysis at home? That was the question Leah McLaughlin (Bangor University, Bangor, UK) sought to answer for the audience at UK Kidney Week 2023 (UKKW 2023; 5–7 June, Newport, UK) with a presentation on recent research that formed part of a broader session on unique kidney care insights from Welsh researchers and clinicians.
THE DIALYSIS OPTIONS AND CHOICES
Study, McLaughlin outlined, was a coproductive qualitative study “underpinned by the Making Good Decisions in Collaboration shared deci sion model”. It employed semi-structured inter views with a purposive sample from the period of February 2019 through to January 2020. The full paper is published in BMJ Open by McLaugh lin and others including first author Jane Noyes (Bangor University).
Participants numbered 95, including 39 healthcare professionals, 37 patients, and 19 family members across five Welsh kidney services. Coproduction was supported by 18 professionals, 18 patients, and eight family
members. The study team routinely collected Welsh National Health Service (NHS) data as part of the research.
“Many patients are asymptomatic, and those with symptoms did not always attribute it to their kidney,” McLaughlin said as she explored the findings of the process. She added that many patients “catastrophised future treatment”, particularly dialysis, which they feared would transform them into “a burden”. This, in turn, complicated decision-making around how dialysis would be performed.

“Most people’s image of dialysis was in a hospital […] Conversations about switching to a homebased option were met negatively, and families were rarely included in early discussions,” McLaughlin explained. Patients also expressed a sense of being overwhelmed by the number of choices with which they were presented before initiating dialysis, with “little or no opportunities to follow up on their concerns”.
The perspective of healthcare professionals differed. They agreed that home dialysis was often not prioritised, but cited “embedded culture”, noting that staffing pressure made it difficult to find the “motivation for change” that would lead to better provision of home haemodialysis. Though it is prioritised in the hierarchy of treatments, many suggested there were, in reality, “challenges in delivering the home agenda consistently”.
In-centre dialysis proved to maintain a host of advantages over home dialysis for some patients. Not least among the concerns around home dialysis that they expressed were fears that self-administering the treatment might lead to mistakes causing disfigurement or infections, as well as potential social isolation. There were also concerns from families, “not always initially
NHS study cuts haemodialysis carbon footprint with disinfection cycle reforms
One talk at UKKW 2023 moved the conference conversation onto the subject of sustainability, with Venkata Gullapudi (Leeds Teaching Hospitals NHS Trust, Leeds, UK) putting nephrology’s carbon footprint under the microscope with the presentation of an abstract reviewing the emissions and wastage resulting from one centre’s haemodialysis practices—and the effectiveness of changing them.
GULLAPUDI BEGAN BY SKETCHING OUT some background on the impact of haemodialysis, noting that the therapy imposes costs on the environment via its “usage of vast amount[s] of medical consumables, water and electricity”. In numbers, that translates to an estimated 3.8 tonnes of carbon dioxide-equivalent emissions (CO2e) produced annually secondary to haemodialysis provision for each patient, according to literature cited by Gullapudi. This, she suggests, “highlights the need for tackling carbon hotspots in our practices” in order to achieve the English National Health Service (NHS) goal of achieving net zero carbon emissions by 2040.
Across Leeds’ NHS trust, she outlined that there are 120 dialysis stations serving a population of 550 patients in eight units in 2022. That, she calculated, works out to 85,800 dialysis sessions per year. During each dialysis session, Gullapudi stated, approximately around 350–400l of raw water is utilised. Of this, around one to two thirds are discarded during the process of purifying the water.
She set out the process of preparing the stations for patients, with dialysis machines rinsed/disinfected (based on the unit protocol) and lined with new set following one dialysis session ready for the next. These lines are primed with dialysis fluid prior to commencement of dialysis—though, once primed, “there can be a period when dialysis machines continually run while waiting for patients to arrive to the unit”, during which time they consume significant amounts of electricity and dialysis fluid.
The researchers identified a set of areas with scope for improvement in reducing wastage. Some of the measures they implemented include reducing the idle running time of the dialysis machine by priming the machine when patient arrives or by dropping the rate of dialysis fluid flow to minimum whilst waiting for the patients, as well as reducing the number of disinfection cycles. They also describe “being creative with the storage facility usage in dialysis units which might allow reduction in the number of deliveries of consumables”. This latter consideration,
recognised”, about becoming carers, and the fear of “turning their house into a hospital”. Home dialysis was not always viewed as providing greater lifestyle benefit, McLaughlin said. What recommendations should be made, McLaughlin asked, in light of these findings?
More carefully tailored shared decision-making should be implemented, she suggested, paying particular attention to patients who may prefer a different option to home dialysis. Patients’ preconceived or mistaken ideas should be “gently challenged”, while healthcare professionals need “up-to-date knowledge” to offer the best advice. This is in light of reports from interviewed professionals that they were frustrated by patients suddenly changing their choice of modality following years of differing advice.
Alongside McLaughlin’s presentation, other insights from the Shared Learning from Wales session included details on a clinical peer review programme in kidney services in Wales as a cost-effective means of improving care. Other presentations touched on digital integration in NHS kidney care as well as the Wales Kidney Research Unit’s work to involve patients in research.
they state, led to their cutting their units’ weekly pharmacy deliveries to fortnightly.
“To understand the impact of these proposed changes,” Gullapudi explained, “we collected data on water consumption, acid power and water usage during each minute of haemodialysis machine run time and with each disinfection/ rinse cycle. We also collected information about the average idle waiting times of primed haemodialysis machine for patient to arrive.”
The researchers calculated carbon emissions of the above consumables using the resources provided by the centre for sustainable healthcare team. They implemented their measures in one satellite unit initially. This led to what Gullapudi described as a “huge amount of power and water saved”, amounting to 2,575.74Kwh of energy and 282.26m3 of water, alongside a 3,508.60l acid consumption reduction.
The impact of implementing similar changes across the entire Leeds haemodialysis service is predicted by the researchers to reduce acid consumption over a year by more than 30,000 KWh of power, 3387m3 of water and 42,000l of acid; this amounts to reduction in CO2 emissions of 14,546.03kg/CO2e/year. They say this could save the trust £29,526.94 over the twelve-month period. Gullapudi adds that future reforms could include the recycling of acid canisters.
Tonnes of CO2equivalent emmissions per dialysis patient:
3.8
“We are trying to engage our regional renal teams to adopt similar changes by rolling out this work,” she concluded, highlighting that small changes can have a big impact on sustainability in a dialysis unit. On concerns around reducing disinfection, she stated that “we have not come across increased blood-borne virus infections [...] during this period, which was reassuring”.
21 Issue9 – September 2023 UKKW UK Kidney Week
“[This] highlights the need for tackling carbon hotspots in our practices”
“Conversations about switching to home-based option were met negatively, and families were rarely included in early discussions”
Leah McLaughlin

Volume flow
vascular
Normal function of a vascular access, either an arteriovenous fistula (AVF) or an arteriovenous graft (AVG), is a prerequisite for end-stage kidney disease (ESKD) patients undergoing haemodialysis. Complications such as stenosis within the circuit leading to dysfunction or finally thrombosis are urgent situations that not only require immediate treatment but also compromise the longevity of the vascular access itself.
The decision to intervene in a vascular access is based on clinical criteria of a stenosis within the vascular access circuit which are verified angiographically based on anatomical criteria (>50% stenosis). Percutaneous vascular access treatment is recommended by both Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (2019) and the European Society for Vascular Surgery (ESVS) guidelines (2018) as a valid option for treatment improving its survival.1, 2 The different percutaneous options include percutaneous transluminal angioplasty (PTA), paclitaxel-coated balloons (PCB), scoring balloons (SB), cutting balloons (CB), bare metal stents (BMS) and covered stent (CS) placement. Nonetheless, any improvement provided is susceptible to restenosis.
What is more, the procedural outcomes of endovascular procedures are quantified by anatomical criteria based on angiographic or duplex ultrasound (US) images on the principle of <30% residual stenosis at the end of the procedure. The latter does not take into consideration the important haemodynamic component of vascular access functionality which is the volume flow (VF) measurement (measurement of blood flow velocity employing Doppler techniques in combination with calculation of the approximate cross-sectional vessel area) at the level of the brachial artery, which is the feeding vessel, in the case of upper limb circuits. Additionally, the KDOQI guidelines have de-emphasised the need for ultrasound surveillance and the introduction of haemodynamic criteria in vascular access surveillance. This decision was based on previous studies that used indirect flow measurement with optodilutional and ultrasound dilution methods, performed intradialytically, requiring line reversal. These methods are time-consuming, demanding expensive equipment, and flow measurement results cannot be compared directly between different devices.
Over the last few years, the evolution and
increased presence of ultrasound devices in dialysis clinics, nephrology, surgery departments, and cath labs, made ultrasound examination easily available and accessible. What is more, ultrasound examination is a fast and reproducible procedure. Notably, VF measurements are part of the ultrasound software nowadays. Haemodynamic variables already play a crucial role in other vascular beds. Fractional flow reserve (FFR) is an established assessment method in coronary artery disease and peak systolic velocity ratio (PSVR) in peripheral arterial disease. Hence, the use of ultrasound should not be restricted to surveillance. Although anatomical considerations are important (degree of stenosis), the introduction of haemodynamic endpoints could further add to our decision-making process. A benchmark VF value of a normal functioning vascular access is important to function as a benchmark value for every vascular access. Based on this value physicians can evaluate VF decrease and theoretically pre-emptively intervene. More importantly, by measuring VF intra-procedurally, a decision can be made on whether further dilatation or scaffold implantation is needed in a procedure.
Although a VF of 600ml/min is suggested as a haemodynamic criterion for maturation, it is known that, when functional, AVFs and AVGs behave differently and need different VF thresholds to perform prescribed haemodialysis. Many investigators have used different VF cut-off levels between the two circuit types to discern stenosis. Driven by observational data, a VF cut-off of <700–800ml/min has been recommended to instigate more imaging studies and potential intervention in AVGs compared to a VF cut-off of <400ml/min in case of AVFs.3 Other studies have also proposed different VF thresholds for diagnosis of flow-limiting stenoses depending on level of anatomy. Namely, investigators have proposed a VF threshold of <600ml/min for forearm and <900ml/min for upper-arm AVFs. Still, there is no definitive recommended VF threshold for intervention in the current literature with reported VF rates ranging from 350 to 800ml/min.4, 5
Assessing the evidence
A recent meta-analysis by Hatem Ali et al , published after the guidelines were available, and including 10 studies and 1,430 patients, concluded that “haemodialysis access surveillance using access blood flow monitoring can
reduce the risk of access thrombosis for patients with AV-fistulas, but this is not the case with AV-grafts”.6 On the other hand, no suggestion or recommendation has been provided on the need for a benchmark VF for every vascular access. In other words, the need for a baseline VF measurement that will be used to evaluate not only if a vascular access is functional/dysfunctional in the future, but also the outcomes of endovascular procedures.
To add to that, none of the guidelines explore or recommend the intra-procedural utilisation of VF measurements as a haemodynamic endpoint or in the process of decision-making. A recent study by Spiliopoulos et al investigated the sequential intraprocedural VF measurements using percutaneous DUS, to provide a physiological assessment of AVF PTA outcomes. In this pilot prospective study of 20 patients, intraprocedural VF measurements were well correlated with balloon angioplasty diameters and six-month late lumen loss, while VF-guided angioplasty optimised immediate PTA outcomes. The authors reported that VF increase of approximal 150% compared to baseline could be used as a physiological endpoint of PTA with satisfactory sensitivity (66.7%) and specificity (85.7%).7
Given the increasing interest in VF’s role in vascular access a decision was made by the meeting directors, Dimitrios Karnabatidis, Konstantinos Katsanos and myself (all University of Patras, Patras, Greece), as well as Stavros Spiliopoulos (University of Athens, Athens, Greece), to create a Delphi consensus (EVAluating VOlume FLOw measurements in Vascular Access—the EVA VOFLOVA consensus) at the last EVA Meeting (23–24 June, Patras, Greece). The concept was to explore the opinion of global vascular access experts and hopefully come to a consensus on this interesting and important topic.
The consensus questions are focused and divided into three different categories (chapters), namely the VF surveillance of vascular access that no previous intervention has taken place (chapter 1), post-procedural VF surveillance (chapter 3) and the utility of VF measurements intra-procedurally as a tool for decision making (chapter 2). The questionnaire applies for both AVFs and AVGs. Given the fact that evidence from properly powered randomised trials is missing, this consensus could work as a trigger for further research. In the era of tailored medicine, VF measurements may provide an additional tool to better understand and treat vascular access with an aim to improve healthcare provided to haemodialysis patients.
Panos Kitrou is a consultant interventional radiologist at Patras University Hospital (Patras, Greece).

References:
1. Lok CE, Huber TS, Lee T, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis. 2020;75(4 Suppl 2):S1-S164.
2. Schmidli J, Widmer MK, Basile C, et al. Editor’s Choice— Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(6):757-818.
3. Bandyk DF. Interpretation of duplex ultrasound dialysis access testing. Semin Vasc Surg. 2013;26(2-3):120-6.
4. Yii E, Lee L, Polkinghorne K, et al. Optimal flow volume measurements in forearm versus arm arteriovenous fistulas. Nephrology (Carlton). 2023;28(3):175-80.
5. Polkinghorne KR, Lau KK, Saunder A, et al. Does monthly native arteriovenous fistula blood-flow surveillance detect significant stenosis--a randomized controlled trial. Nephrol Dial Transplant. 2006;21(9):2498-506.
6. Ali H, Mohamed MM, Baharani J. Effects of haemodialysis access surveillance on reducing risk of haemodialysis access thrombosis: A meta-analysis of randomized studies. Hemodial Int. 2021.
7. Spiliopoulos S, Giannikouris IE, Katsanos K, et al. VOLume flow assistance for optimizing outcomes of dysfunctional autologous arteriovenous fistula Angioplasty: the VOLA Pilot Study. Eur Radiol. 2022;32(1):368-76.
The author stated no relevant disclosures.
Vascular Access 23 Issue9 – September 2023
Point of View
measurements in
access: Is it time for meaningful hemodynamic endpoints and variables?
Panos Kitrou
“[We aimed] to explore the opinion of global vascular access experts and hopefully come to a consensus.”
EVA meeting unveils new data and global perspectives to boost vascular access practice
The third EVA Meeting (23–24 June, Patras, Greece) sought to build on international collaboration and science, one of the stated aims of the meeting directors, Dimitrios Karnabatidis, Konstantinos Katsanos and Panos Kitrou (University of Patras, Patras, Greece). With the programme carefully constructed to build on the needs emerging from previous editions of the meeting, it reflected new clinical data, documented the sharp rise in use of ultrasound in vascular access management, and touched on harnessing augmented reality and artificial intelligence (AI) in the field. A session set aside to ‘the way ahead’ in vascular access brought a flavour of what the future holds into the present.
Datapoints and designs
In a 1st@EVA presentation, Gürkan Sengölge (Medical University of Vienna, Vienna, Austria) elaborated on a single-centre experience of the inside-out technique, which is a novel approach to facilitate upper body central venous access in patients with venous obstructions or other conditions that preclude access by conventional methods. Merit Medical recently acquired the Surfacer inside-out access catheter system from Bluegrass Vascular Technologies.
Sengölge set the scene to explain how his centre, which serves over 900 dialysis patients and more than 800 predialysis patients per year, has observed an increase in difficult central venous access cases. The team was therefore looking for “a tool and strategy to preserve vascular real estate as well as to gain safe vascular access in patients with end-stage vascular access”.
Selecting from 103 patients with a thoracic central venous occlusion, 97 patients were eligible for the procedure. Accounting for repeat procedures, there were 105 inside-out procedures performed and 104 central venous catheters successfully placed with no procedure based complications. In eight patients HeRO graft (Merit Medical) became an option in combination with Surfacer and was placed in the same session or after bridging.
“Always right, never subclavian was implemented as our centre strategy. In 67 patients who were running out of vessels, with rapidly diminishing access options, central venous catheters were quickly and safely implanted. Importantly, significant vascular real estate preservation in patients facing long-term access needs was achieved because thoracic central veins on the left side that are more prone to stenosis or occlusions were not used,” he said.
Kitrou set the context for Renal Interventions: “This is a unique device and procedure that offers a crucial bail-out option to no-option patients. Learning from the extensive experience of Sengölge, who has performed over 100 cases with the Surfacer is of paramount importance.”
Raphaël Coscas (Ambroise Paré Hospital and Paris-Saclay University, Paris, France) also presented the ABISS trial comparison with other studies in another 1st@ EVA. He underlined that in the ABISS trial, the primary endpoint of six-month primary patency was not met in the intention-to-treat analysis. It was however, met in the prespecified per-protocol analysis where the drug-coated balloon (DCB; Lutonix, BD) was superior to plain balloon angioplasty at three, six and 12 months. He compared post-hoc analyses based on analysis methods used in other large DCB trials to show “that different methods of analysis yield different conclusions”.
“DCB demonstrates a positive effect over plain balloon angioplasty in terms of reinterventions, restenosis and dialysis circuit patency at three months and for arteriovenous fistula (AVF) flow at six months. This benefit is lost during follow-up,” he clarified via video link.
Kitrou noted: “Inconsistency of endpoints in studies is
always an obstacle when trying to understand the results. Comparing studies by providing common endpoints is as important as the results themselves. To that end, this presentation is of extremely high value.”
Resounding favour for ultrasound
Jose Abadal (Hospital Universitario Severo Ochoa, Madrid, Spain) set out a robust case for the use of Doppler ultrasound before, during and after intervention.
“Doppler ultrasound should be performed before any endovascular treatment. Only ultrasound will determine the haemodynamic significance of a stenosis, and even the existence of stenosis; it characterises the type of stenosis, and it has treatment implications. Sometimes exovascular causes of stenosis are diagnosed. “When it comes to intraprocedural use, during intervention, ultrasound is my first image guidance choice because it is mandatory for real sizing of devices and allows us to be more precise during the intervention,” he said.
Technical success is interlinked with clinical success and should rest on adopting new endpoints that take into account haemodynamics, commented Abadal. When relying solely on anatomic endpoints of successful angioplasty (<30% residual stenosis on angiography), up to 20–30% of patients fail to show an increase in blood flow afterwards in dialysis, he explained.
“Previous angioplasty studies that do not consider haemodynamics as endpoints are half-blind. Guidelines should incorporate doppler criteria like access volume flow (VF), peak systolic velocity (PSV) and minimal luminal diameter beside angiography and of course patients’ clinic visits, to decide whether to treat or not a stenosis,” he said.
The haemodynamic component of vascular access functionality, which is the volume flow (VF) measurement (measurement of blood flow velocity employing Doppler techniques in combination with calculation of the approximate cross-sectional vessel area) turned into a major talking point at the EVA meeting.
Stavros Spiliopoulos (National and Kapodistrian University of Athens, Athens, Greece) raised the point that
intraprocedural VF can be used to quantify and optimise endovascular dialysis access treatment outcomes. Is this the ultimate tool, he rhetorically enquired. “Volume flow helps to determine the haemodynamic significance of lesions; baseline VF is a novel functional outcome of endovascular treatment that can impact surveillance. It is also a great intraprocedural decision-making tool that has changed my practice, and we need a randomised controlled trial to compare it to standard assessment,” he said.
VOLA II, a prospective, multicentre study of 100 AVFs revealed post-procedural VF is an independent predictor of patency, and has been submitted for publication. VF gain of 413ml/min is the optimal cut-off point for predicting improved patency (p=0.0014), Spiliopoulos stated, reiterating that as witnessed already in cardiology, “it is time to move over from anatomical to physiological” endpoints. “It became apparent from the previous EVA meeting that ultrasound is a valuable tool and needs further utilisation in vascular access. The session tried to provide evidence in the field and combined with the DELPHI initiative is expected to lead to an important and valuable discussion,” commented Kitrou.
Any conclusions on central venous stenosis and occlusions?
“Central venous stenosis and occlusion is an integral part of everyday practice for physicians treating haemodialysis patients both with catheters and vascular access. So, knowing how to access and treat these lesions, and being aware of the techniques and the device available, is a must. Even more important is to be aware of the potential adverse events and complications which, in this anatomic region, can be very serious. This is why, the majority of the “Morbidity and Mortality” cases in the corresponding session were on central veins. Discussing these rare but important complications, learning from problems other peers face, helps awareness and also to avoid falling into similar traps,” Kitrou said.
Jackie Ho Pei (National University Heart Centre, Singapore) presented on the histopathologic and haemodynamic background of central venous disease and provided her tips on crossing the lesion. Kitrou further reported on treatment options, prioritising that high-pressure balloon angioplasty remains the optimal treatment, with DCB perhaps offering an extra benefit. Bare metal stents should be avoided, but this remains a question mark for dedicated venous stents, he suggested. “Covered stents provide a benefit but remain a bail-out option. We need more dedicated prospective trials on the adjunctive treatments,” Kitrou noted.
Dheeraj Rajan (University of Toronto, Toronto, Canada) presented a case on ‘a not-so-routine’ superior vena cava angioplasty and Bart Dolmatch (Palo Alto, USA) unspooled a case detailing ‘a series of unfortunate events’ in the Morbidity and Mortality session.
Can putting AI (and machine learning) in play keep treatments precise and costs at bay?
Sparking a recurring current discussion on augmented reality, AI and machine learning in medicine, Jose Ibeas (Parc Taulí University Hospital, Barcelona, Spain), noted that evidence-based medicine is beginning to be replaced by data-driven medicine, and that “vascular access is not going to run away from it.”
“We are immersed in a paradigm shift and should be aware of it. […] Vascular access, both due to the diversity of variables involved and the complexity of its interpretation, becomes an ideal field for the use of AI. The results of the first studies are promising, but a validation process is required. We are in a phase in which […] the terrible speed of its progress requires necessary training both to be able to carry out research, to interpret the results and apply them,” he said.
The director of the Program for Promotion & Development of AI in the Catalan Health System, deconstructed that “essentially, AI consists of mathematical models that look for patterns in data and generate algorithms that learn on their own. By analysing lots of patient data and looking for patterns, AI learns to find which groups are most at risk of disease, as well as predict a patient’s outcome or response to treatment. AI allows us to know the evolution of a disease or the response of a patient to a treatment, but only a few people are aware of the opportunities and dangers inherent in its use. In fact, as with any medical device, this type of tool should be validated before its use, just like a new drug with prior clinical trials,” he said.

September 2023 – Issue9 24 EVA Coverage
L–R: Dimitrios Karnabatidis, Panos Kitrou and Konstantinos Katsanos
Saravanan Balamuthusamy (PPG Healthcare, Fort Worth, USA) who took to the podium to discuss financial realities of vascular access management, presented on harnessing machine learning (ML) as a tool towards cost-ef fectiveness. “Predictive analytics utilising machine learn ing can have numerous applications in AVF access. These include prediction of the likelihood of a successful AVF with an approximate maturation period, which would be time to cannulation. It may also help in identifying patients who might be candidates for successful endovascular AVF (endoAVF) creation, as well as in predicting patients for an early arteriovenous graft (AVG) as opposed to “fistula first”. Predicting risk for access dysfunction due to inflow versus outflow issues is also a possibility, as is estimating the need for reintervention in patients with arteriovenous access stenosis. Lastly, it may help in calculating the cost of arteri ovenous access creation and maintenance at a patient and group level,” he previously wrote in Renal Interventions

Kitrou said: “This new era coming from advancements in other scientific fields is very exciting and it is important to start understanding and hopefully implementing these advancements in the near future in vascular access with a view to provide better care for dialysis patients.”
Here comes the future
Monnie Wasse (Rush University Medical Center, Chicago, USA) issued a futuristic outlook on new generation covered grafts for vascular access, first outlining the broad clinical frameworks that prompt teams to consider using these devices. These begin with taking into account the patient’s life plan. Then, “If patients have been on dialysis with prolonged CVC [central venous catheter] exposure; have had previous failed AVF(s); limited vasculature for AVF creation or if patient survival is estimated to be between one and two years [AVG use may be considered appropriate].”
With regards to materials, there are synthetic nonbiodegradable materials such as expanded polytetraflorethylene (ePTFE/Gore-Tex), polyurethane and dacron in use for the covering. Xenografts, that are chemically fixed and do not remodel, repopulate with host cells or heal. These include bovine carotid artery and bovine mesenteric vein, Wasse explained, quoting from Lawson et al’s 2021 paper in Nature Reviews Nephrology. She then dwelt on the aXess graft (Xeltis), a novel, restorative, bioabsorbable implant made of electrospun supramolecular polymers. Previous coverage from Renal Interventions reveals that the device essentially allows the body to heal itself. Immediately after implantation, it acts as a functional haemodialysis AVG and, over time, provides a mechanical and structural scaffold for cell ingrowth propelled by the body’s natural healing process. The result is a living vessel that enables immediate cannulation, potentially better patency rates, lower bleeding times after needle removal, and lower access site infection rates, compared to commercially available AVGs.
The investigational haemodialysis graft—the InnAVasc Graft (IAVG)—which is designed to be used immediately post-implant and was in 2022 acquired by Gore also came in for a mention by Wasse, as did the InterGraft (Phraxis), implantable device that provides immediate access to dialysis and helps to prevent repeated access surgeries. This transcatheter delivered venous anastomotic connector offers a unique, minimally invasive approach to vascular access.
Further research is also underway including those like the European Union-funded Telegraft project that is investigating a “promising solution for patients who do not have direct access to haemodialysis infrastructures”. The project proposes to “develop a smart AVG that by biomimicry and drug-eluting properties minimises the risk of thrombosis and infection and also allows blood flow monitoring and early detection of inflammation and infection. It essentially serves as a telemonitoring system to allow early minimally invasive prevention of serious complications” per the Europa website.
Wasse then turned to tissue engineered dialysis AVGs that offer strong, and immunologically inert options to provide an alternative to autologous vessels. “Decellularised vessels mimic the vasculature,” she said, pointing to evidence acquired with the Human Acellular Vessel (HAV) graft (Humacyte) that has long-term phase two data showing no infections of the HAV during a five-year follow-up period, with the device being well tolerated and non-immunogenic. Recently published data on TRUE AVC (Vascudyne) a tissue-engineered vascular conduit also came in for some airtime. A recently published study demonstrates initial safety, mechanical durability and
lack of immune response with TRUE AVC. It also shows potential as an off-the-shelf blood vessel conduit for clinical use. A first-in-human, five patient study, demonstrated no infections or aneurysms at 26-week follow-up and 80% primary patency; and 100% secondary patency.
“An off-the-shelf, completely biological acellular vascular conduit, able to repopulate with the recipient’s own cells and become a living vessel in vivo, may provide long-term functional patency and low complication rates similar to the native fistula, while avoiding maturation failures and early thrombosis,” the authors Ebner et al, with Wasse as senior author on the research team, reported in the Journal of Vascular Access.
Wasse told EVA delegates that while challenges relating to commercial scaling, cost, and regulatory approval remain, bioengineered AVGs could represent the future.



“Studies to date show safety, non-immunogenicity, very low infection rates and good functionality for dialysis. Ongoing evaluation of cellular and decellularised devices support their use in patients at high risk of AVF non-maturation or access infection. For haemodialysis, the technology offers lower infection than synthetic AVGs and earlier use than AVF,” she said.
Dolmatch (Palo Alto Medical Foundation and professor emeritus UT Southwestern Medical Center, Dallas, USA) then examined “next generation stents” in ‘the way ahead’ session clarifying that there is currently just one study of a bare metal stent (Supera, Abbott) in enrolment phase, with the primary outcome measure being the number of patients with primary patency of juxta-anatostomic stenosis of the treatment site at three months. He
called into question outcome measures with short periods of follow-up. Moving on to drug-eluting stents (DES), while stating that “an anti-proliferative coating might be a very good idea”, he referenced the scarce published data including a small study with the Eluvia stent (Boston Scientific) that does signal positively, before stepping over to covered stent usage, which is backed by data from multicentre randomised controlled trials such as the REVISE and RENOVA trials (the former used Viabahn [WL Gore] and the latter the Flair graft [BD] as well as the AVeVA and AVeNEW trials (which used Covera, BD). “The Achilles’ heel of covered stents is edge restenosis, the dominant cause of covered stent restenosis,” Dolmatch said. Advances such as the Wrapsody cell impermeable endoprosthesis (Merit Medical), which is being assessed in the US Investigational Device Exemption (IDE) WAVE trial and has recently completed enrolment are underway, he noted.
“Bare metal stents are rarely used in arteriovenous access due to in-stent restenosis. There is scant data on DES. [At this point] We cannot tell. Covered stents have revolutionised AV access intervention. Even though these devices are superior to percutaneous transluminal angioplasty in many instances, there is room for improvement. New stent and graft technology is on the horizon,” he continued.
Kitrou concluded his commentary with: “An important part of our science is to know not only what is available, but also what we expect to see in the future. I think every meeting should have a session like this one, where physicians can see what is happening in the background, so that we can discuss what is not widely known yet.”
Issue9 – September 2023 25 EVA Coverage
Jackie Ho Pei
Saravanan Balamuthusamy
Bart Dolmatch
Monnie Wasse
Post-transplant blood transfusion associated with worse kidney graft outcomes
The “urgent development of transplant-specific anaemia guidelines” and a need for “rigorous patient blood management (PBM)” for those who receive kidney transplants—these are among the recommendations from authors of new research published in Frontiers in Nephrology on the prevalence of blood transfusion in UK transplant centres, and its association with transplant outcomes.
The authors, including first author Sevda Hassan (Imperial College London, London, UK), argue that PBM is a proven and World Health Organization (WHO)-approved approach to limiting “inappropriate” blood transfusions, which leads to both better outcomes and lower costs. They note that kidney transplant recipients represent a unique challenge in PBM, given their high rates of anaemia as well as factors exacerbating the condition, such as the frequent use of immunosuppressants to facilitate positive graft outcomes.
Despite this, they say, “guidance on post-transplant anaemia management frequently defaults to replicate protocols in the non-dialysis chronic kidney disease (CKD) population”. Citing a lack of evidence on the risks of transfusion in kidney recipients, they note a wide range in transfusion rates for this patient population of 18.1–74.6%.
The implementation of PBM to prevent inappropriate transfusions—which may lead transplant recipients to develop human leukocyte antigen (HLA) antibodies that could contribute to graft failure—demands data on “who is
being transfused, why they are being transfused and the impact, if any, that transfusion has on outcomes”, the authors add.
With the collaboration of the UK National Health Service Blood and Transplant Service (NHSBT), the British Transplant Society (BTS) and the HLA matched Red Cell Working Group, the study took place across four UK centres. From a total of 720 kidney transplant patients, 221 (30.7%) received blood transfusion, 214 (29.7%) of whom received red blood cell transfusion. The remaining patients were not transfused.
Patient survival at 12 months post-transplant was found to be inferior in the transfused group at 93.5% (95% confidence interval [CI] 88.3–96.5) compared with 99.3% (95% CI 97.7–99.8) in the non-transfused group (p<0.0001). Patients who were transfused experienced a lower rate of graft survival of 89% (95% CI 83.7–92.6) than the 97.2% rate of non-transfused patients (95% CI 95.3–98.4; p<0.0001). Renal transplant function was also found inferior in the transplanted group.
The authors performed risk-adjusted Cox proportional hazard model analysis which showed that transfusion was associated with infe
rior one-year patient survival (hazard ratio [HR] 7.94 [2.08–30.27]; p=0.002), allograft survival (HR: 3.33 [1.65–6.71]; p=0.0008) and inferior allograft function.
“It is the first couple of weeks [after transplantation] where efforts to optimise anaemia management need to be prioritised in the first instance,” the authors suggest in their discussion. They add that “adequate iron therapy in the pre-transplant setting is of the utmost importance” as a further recommendation, and say it is “imperative to investigate, where indicated, occult causes of blood loss and iron deficiency” as part of investigations of causes of potential anaemia prior to transplantation.
Hassan et al go on to say that “the need for blood transfusions will never be eradicated”, and that, though they did not study medium- and long-term consequences of transfusion in transplant patients, the area warrants more study. The data from their study also suggest that, in the UK, non-white ethnicity is a risk factor for transfusion, and given that non-white patients experience inferior allograft survival, “this again supports the call for research in this area”, they add.
“PBM applied to general surgical procedures has been shown to reduce the need for transfusions and improve patient outcomes by lowering morbidity and mortality,” they conclude, also stating that blood transfusions remain “common” despite association with inferior graft outcomes. Noting a “call to action” to implement PBM by the WHO, they identify a need for transplant-specific anaemia guidelines as well as further research on the connection between transfusion and transplant failure.
Corresponding author Michelle Willicombe (Imperial College London) noted that the work of the study was undertaken prior to the untimely passing of Sevda Hassan.

ASPEN trial finds roxadustat effective for anaemia in dialysis patients
New research has been published in Hemodialysis International which, say its authors, “enhances understanding” of how haemodialysis centres can utilise oral roxadustat. The anaemia drug is licensed for use in the European Union (EU), the UK, and other markets, but has not been approved for use in chronic kidney disease (CKD) patients with anaemia by the US Food and Drug Administration (FDA). The results of the ASPEN trial, led by Steven Fishbane (Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Great Neck, USA), indicate that the drug can be used safely and effectively for maintaining haemoglobin levels in patients receiving haemodialysis.
ANAEMIA OCCURS IN 90% OF PATIENTS with dialysis-dependent (DD)-CKD, state Fishbane et al, which they say exposes them to a heightened risk of all-cause and cardiovascular mortality, as well as of hospitalisation and CKD progression. They also note its impact on patients’ quality of life.
The study authors set out that it is “important” to assess the drug’s safety and efficacy in the realworld setting of haemodialysis organisations, as well as its “operational characteristics […] particularly given its oral mode of administration and dose titration algorithm”. The authors also point to a need for effective anaemia of CKD treatments that “can be administered in the home setting”.
Their assessment of the drug took the form of an open-label, single-arm study enrolling a total of 283 kidney failure patients on dialysis, 236 of whom completed a main 24-week course of treatment, while 216 continued into an extension period. There was also an initial six-week screening period, while the authors also note that follow-up was four weeks.
The authors identified two primary efficacy endpoints: the proportion of patients with mean haemoglobin (Hb) ≥10g/dL averaged over
weeks 16–24 of treatment, as well as the mean Hb change from baseline to the average over weeks 16–24. Among several exploratory endpoints were the proportion of patients with mean Hb in a target range of 10–12g/dL, and the proportion of patients with Hb >12g/dL and >13g/dL at any time during the treatment period. Safety was measured according to the incidence of treatment-emergent adverse events (TEAEs) and treatment-emergent serious adverse events (TESAEs).
Fishbane and colleagues found an 83.7% proportion (95% confidence interval [CI] 78.9–88.6) of patients had mean Hb ≥10g/dL during weeks 16–24, compared with 78.7% at base -
line. Subgroup analyses by sex, race, and other characteristics found results “consistent with the results for the overall population”. On the secondary endpoints, the proportion of patients with mean Hb within the 10–12g/dL target range during weeks 16–24 was 67.0% (95% CI 61.2–72.5), while the proportion of patients with >12.0g/dL was 47.9% (95% CI 41.9–53.9), and of patients with >13.0g/dL 12.4% (95% CI 8.8–16.8).
On safety, 180 (63.6%) patients reported TEAEs. Of these, 18 (6.4%) were considered either “related or possibly related to the study drug”. Reported TESAEs included gastrointestinal haemorrhage and respiratory failure, which occurred in four patients each. “Except for two patients who had markedly elevated bilirubin levels,” the authors detail, “potentially clinically significant laboratory abnormalities were consistent with the study population of patients with CKD”.
The greater number of patients who maintained Hb ≥10g/dL by weeks 16–24 than baseline “suggests that patients were successfully treated with roxadustat as demonstrated by maintaining Hb and treatment adherence”. Dose increases, which were mandated by the study protocol, were carried out by dialysis nursing staff, “further supporting the operational feasibility of oral roxadustat in an in-centre, community-based, haemodialysis clinic setting”.
Limitations are noted by the authors, including the fact that most enrolled patients were users of erythropoiesis-stimulating agents (ESAs), the existing standard of care for anaemia in CKD, as well as the single-arm, unblinded nature of the study, which “limits any direct comparisons between roxadustat and ESAs in this patient population”. Nevertheless, Fishbane et al conclude, their study demonstrates that roxadustat “effectively” treats anaemia in CKD patients “in large, community-based dialysis organisations”.
26 September 2023 – Issue9 Anaemia
Sevda Hassan
“The study demonstrates that roxadustat effectively treats anaemia in CKD patients in large, community-based dialysis organisations.”
NICE recommends finerenone for CKD in diabetic patients
The UK’s National Institute for Health and Care Excellence (NICE) has published new technology appraisal guidance on the use of finerenone in the treatment of chronic kidney disease (CKD) patients with type 2 diabetes. The guidance recommends the drug, which follows on from its acceptance for use in the National Health Service (NHS) in Scotland by the Scottish Medicines Consortium (SMC) in late 2022. The NICE announcement has been shared by Bayer, who produce the drug under the name Kerendia.
SETTING OUT THE CONDITIONS UNDER which the drug should be prescribed, the NICE guidance recommends it for type 2 diabetes patients with stage 3 or 4 CKD with albuminuria. However, it stipulates that this should only be done “as an add-on to optimised standard care”. This care, it explains, should include
Transplanting the “untransplantable”: French guidelines open doors for imlifidase
A “major breakthrough in kidney transplantation”—that is how imlifidase has been described in new French consensus guidelines on the drug’s use that have been published in Transplant International. The publication follows the 2020 conditional marketing authorisation of imlifidase by the European Medicine Agency (EMA), and represents an effort to build a “common framework” that establishes its benefits and tolerance in real-world care.
The working group behind the guidelines, led by Lionel Couzi (Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France), was convened by the French Society of Transplantation (SFT), the French-speaking Society of Nephrology, Dialysis and Transplantation (SFNDT) and the French Society of Histocompatibility and Immunogenetics (SFHI). The group was composed of 12 transplant nephrologists and four immunologists, including two human leukocyte antigen (HLA) experts. They aimed to create consistency in practice through a set of recommendations on “patient selection, choice of antibody characteristics, treatment, and follow-up”.
The guidelines come just over a year after the publication in July 2022 of the UK National Institute for Health and Care Excellence (NICE) set out their own imlifidase guidance, which recommended the drug for those awaiting transplant who are highly sensitised to HLA. Those guidelines state that “people who are highly sensitised wait longer for a suitable donor kidney, and imlifidase can improve access to kidney transplantation”.
“the highest tolerated licensed doses” of angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs), as well as of sodium-glucose cotransporter 2 (SGLT2) inhibitors. The patient should also have an estimated glomerular filtration rate (eGFR) of 25ml/ min/1.73m2 or more.
“This recommendation is not intended to affect treatment with finerenone that was started in the NHS before this guidance was published,” its authors add. They state further that those already being treated outside of this guidance before its publication may continue “until they and their NHS clinician consider it appropriate to stop”.
Those authors, including chair Charles Crawley (Cambridge University Hospitals NHS Trust, Cambridge, UK), explain: “The clinical evidence suggests that finerenone improves kidney function and helps to slow the worsening of the disease compared with placebo.” They note that this applies both with and without SGLT2 inhib-

Required eGFR for finerenone: ≥25ml/ min/ 1.73m2
itors, with which there has not yet been a direct comparative study. This leads the authors to state that finerenone cannot yet be recommended “instead of them”. On cost-effectiveness, they add that “estimates are uncertain” but fall within the bounds of acceptable cost for NHS use.
At the heart of the recommendation is the guideline team’s view that “there is an unmet need for treatment options for CKD associated with type 2 diabetes”. This is due to a “residual risk” of continued progression of the disease—as well as complications such as peripheral vascular disease—for this patient population even when receiving current standard care. The existing options fall short of satisfying their needs, the authors suggest, particularly in those patients where SGLT2 inhibitors are not considered appropriate.
The discussion of the guideline committee made reference to the FIDELIO-DKD study results published in The New England Journal of Medicine (NEJM) in 2020. That trial was a phase 3 randomised, double-blind, placebo-controlled, multicentre clinical trial of 5,734 adult patients with type 2 diabetes and CKD, randomised 1:1 to receive either oral finerenone or placebo, while additional evidence is also cited from the FIGARO trial and FIDELITY pooled results analysis.
The NICE recommendation means finerenone “should be available for use” as part of NHS treatment within three months of its 23 March 2023 publication date—a limit which has been passed as of 23 June 2023.
They also state that it is particularly important to select a donor profile that is at lower risk for delayed graft function, given their high antibody-mediated rejection rate (AMR). On the matter of associated therapies, it adds that patients must be given imlifidase as a single dose (0.25mg/kg, IV in 15 minutes) prior to transplantation after a premedication with glucocorticoids and antihistamines.
Other points of note in the guidelines include a recommendation against the use of imlifidase for transplantation in patients with a history of more than two kidney transplants. Patients themselves should also be made aware of the full implications of desensitisation, how it is performed, as well as both the expected benefits and risks.
The authors note that donor-specific antibodies (DSA) have been found to rebound “frequently with an increased risk of AMR” following transplantation with imlifidase. With a mind to limiting this risk, they also recommend “to only delist those with a single antigen flow bead (SAFB) mean fluorescence intensity (MFI) below 5,000” after a 1:10 dilution (one Lambda assay) (2D).
The guidelines build on two studies referenced by the French working group. Among these was a phase 2 study, in which the primary outcome of conversion of baseline positive cytotoxicity crossmatch (XM) to negative within 24 hours after imlifidase treatment occurred in 17 (89.5%) of 19 patients.
“Living-donor transplantation options are often limited, and most highly sensitised patients are transplanted with a deceased donor,” Couzi et al outline. They explain that imlifidase has been indicated in France to replace previous desensitisation treatment options for patients with a positive crossmatch against an HLA-incompatible deceased donor, and state that the EMA considers the drug to meet an unmet need for highly sensitised patients—though they note that “additional data on long-term graft function and survival are required”.
The new French guidelines recommend that patients selected for desensitisation with imlifidase have calculated panel reactive antibodies (cPRA) ≥98%, be ≤65 years of age, have spent ≥3 years on the waiting list, and be at a low risk of biopsy-related complications.
In their conclusion, Couzi et al describe imlifidase as “the first treatment authorised in our field since belatacept more than 10 years ago”, and state that it “could allow transplanting patients so far considered untransplantable”. They call their guidelines a “partly subjective […] international effort”, but say finally that more data from clinical trials will, in future, help in their revision and refinement.
27 Issue9 – September 2023
Pharmaceuticals
Lionel Couzi
“There is an unmet need for treatment options for CKD associated with type 2 diabetes.”
“[Imlifidase is] the first treatment authorised in our field since belatacept more than 10 years ago”, and state that it “could allow transplanting patients so far considered untransplantable.”
Clinical and Industry News
Quanta announces enrolment completion in home dialysis study
Quanta Dialysis Technologies has announced that it has completed enrolment of its Home Run study for at-home haemodialysis. The Home Run study is a prospective, multicentre, openlabel trial to assess the efficacy and safety of the Quanta dialysis system for home haemodialysis. Results are expected to be announced in the second half of 2023.
“People with end-stage kidney disease (ESKD) on haemodialysis are currently limited as to options available. Although frequent home haemodialysis is proven to deliver superior outcomes, for many, in-centre dialysis treatment three times a week is the only viable option,” said Quanta chief medical officer, Paul Komenda. “We hope that US Food and Drug Administration (FDA) clearance of an additional home haemodialysis device will contribute to more patients having access to regular care in their home environment. Enrolment completion is the first step forward in a potential new alternative for people with this life-threatening disease.”
The Home Run study, a press release details, is establishing non-inferiority in safety and efficacy when the Quanta dialysis system is used in the selfcare home environment with a care partner compared to a haemodialysis facility. Following enrolment, study participants begin haemodialysis treatments using the system on a prescription of four-hour treatments, three times per week for a minimum of four weeks. During this time, the press release adds, “both patients and their caregivers will undergo extensive training and competency sign off on all aspects of safely administering haemodialysis treatment in the home”. Upon completion of training and a one-week transition period, participants will perform home treatment four times per week for three-and-a-half hours per treatment for eight weeks.
a press release. In April 2023, Xeltis shared “highly-encouraging” six-month data from its first-in-human (FIH) aXess vascular graft trial. An ongoing pivotal trial for aXess is enrolling patients across Europe. Moreover, the EIC Accelerator funding will be used to advance Xeltis’ coronary artery bypass graft (CABG) programme.

Svetoslava Georgivea, chair of the EIC Fund Board said: “It is the ambition of the EIC Fund to invest in European companies that develop cutting-edge technologies with high impact. Xeltis, with its restorative medical device technology is an excellent example of such a company, which the EIC Fund invests in to support their potential to scale and grow their business.”
Data on Humacyte human acellular vessel published
Humacyte, a clinical-stage biotechnology platform company developing “universally implantable, bioengineered human tissue at commercial scale”, has announced in a press release the publication of controlled in vitro studies in the Journal of Vascular Surgery—Vascular Science describing the scientific basis for the “significantly lower rates of infection” observed in a clinical trial of the investigational human acellular vessel (HAV) compared to synthetic expanded polytetrafluoroethylene (ePTFE) grafts.
The in vitro study results show, the release details, that the HAV had more host cell infiltration than ePTFE grafts, and that the biocompatibility of the HAV supported neutrophil viability and function, each of which may explain the HAV’s superior resistance to bacterial infection versus ePTFE grafts observed in the clinical trial.
The publication, titled Biological Mechanisms of Infection Resistance in Tissue Engineered Blood Vessels
Compared to Synthetic ePTFE Grafts, combined histological evaluation of the HAV and ePTFE explants from both preclinical and clinical studies as well as in vitro experiments assessing the viability and function of human neutrophils on these materials.
Merit WRAPSODY cell-impermeable endoprosthesis (CIE) to percutaneous transluminal angioplasty (PTA) for treatment of stenosis/occlusion in the venous outflow circuit in patients undergoing haemodialysis.
Creation and maintenance of an arteriovenous fistula or graft (AVF/AVG) to achieve long-term vascular access is required for patients undergoing haemodialysis. However, progressive stenosis and/or occlusion of blood vessels where the AVF and AVG are located can prevent delivery of haemodialysis, which can have lifethreatening consequences. WRAPSODY was developed to help physicians treat patients with stenosis/occlusion in the vessels used for haemodialysis.
The WAVE study enrolled 244 patients with AVFs and 113 patients with AVGs across sites in Brazil, Canada, the UK, and the USA. Merit intends to collect safety and efficacy outcomes throughout the study follow-up period. Merit anticipates filing primary outcomes with the US Food and Drug Administration (FDA) for premarket approval (PMA) after six months postenrolment completion. The company intends to follow patients enrolled in the WAVE study for 24 months following completion of enrolment.
Enrolment completed in SAVE trial of Selution SLR for AVF treatment
MedAlliance has announced completion of patient enrolment in the SAVE clinical trial with the Selution Sustained Limus Release (SLR) 018 drug-eluting balloon (DEB) for the treatment of failed arteriovenous fistulas (AVFs) in renal dialysis patients. Selution SLR is a “novel sirolimus-eluting balloon that provides a controlled sustained release of drug, similar to a drug-eluting stent (DES)”, states a press release.

SAVE is a prospective multicentre, single-blinded, randomised controlled trial. A total of 84 patients have been randomised to either standard highpressure balloon angioplasty followed by local application of Selution SLR or high-pressure balloon angioplasty with no further lesion treatment. Subjects have been recruited at three sites in Europe and Singapore and are being followed up to 24 months. Endpoints of the study are primary patency at six months with angiographic follow-up and freedom from serious adverse events at 30 days. Major secondary endpoints at six months are clinical success, freedom from serious adverse events, late lumen loss and binary vessel restenosis.
Xeltis secures additional €12.5 million from European Innovation Council
Xeltis has announced the closing of an additional €12.5 million in funding from the European Innovation Council (EIC) Fund, set up by the European Commission. The recent extension from EIC and the closure of the Series D2 financing round announced in February 2023, brings the total amount raised to €44.5 million.
The additional funding will support the continued clinical development of Xeltis’ “transformative implants”, says
Neutrophils are a critical cell type for host defence against infection, the release explains. Data analysed from a comparative clinical trial demonstrated that the HAV had a significantly lower infection rate than ePTFE grafts.
Wrapsody (WAVE) pivotal study completes enrolment
Merit Medical has announced that it has completed enrolment in its Wrapsody Arteriovenous Access Efficacy (WAVE) pivotal study. Merit’s WAVE study is a prospective, randomised, controlled, multicentre study comparing the
“We very much look forward to the analysis of the primary endpoint of this important trial at six months, as this is the first prospective randomised trial of a sirolimus-coated balloon in AVF patients with angiographic follow-up. Furthermore, we have also measured fistula volume flow rates, which is another key index of failing or maintained fistula function. I thank MedAlliance for initiating a trial in this difficult patient population,” commented principal investigator (PI) Konstantinos Katsanos (Patras University Hospital, Patras, Greece).
US kidney organisations voice issues with screening programme
The American Society of Nephrology (ASN) and the US National Kidney
Foundation (NKF) have expressed concern that the United States Preventive Services Task Force (USPSTF) Final Research Agenda for chronic kidney disease (CKD) screening “continues to focus on screening only of asymptomatic, low-risk individuals” in a press release.
“By examining this issue from such a broad purview”, it states, “the USPSTF risks overlooking or diluting the significant evidence that supports screening for at-risk populations.”
The organisations also say they are concerned that the USPSTF overstates current clinical practice guidelines for screening in hypertensive populations, which list albuminuria testing as optional. They add that only approximately 40% of adults with diabetes receive albuminuria screening each year, illustrating the “urgent need” to evaluate benefits of screening among adults with diabetes, for whom annual albuminuria testing is recommended by clinical practice guidelines.
“Far too many high-risk individuals, many from historically disadvantaged and marginalised groups with burdensome social determinants of health and limited or inconsistent access to quality healthcare, have undiagnosed CKD and therefore are untreated for their elevated risk of cardiovascular disease, kidney failure, and death,” the release adds.
“While we are confident that the evidence will ultimately demonstrate the value of CKD screening, we urge USPSTF to expand its approach so that we can have a greater impact on increasing diagnosis of those with, or at most at risk for CKD.”
Pathfinder Medical and Imperial College London awarded Innovate UK Smart grant to improve dialysis treatment
Pathfinder Medical has been awarded a Smart grant for a £1.1 million (US$1.4 million) project by Innovate UK, the UK’s innovation agency. A press release details that the grant will support Pathfinder’s novel project to develop a minimally invasive arteriovenous fistula (AVF) at the wrist for patients with renal failure who require dialysis. The project is to be conducted in collaboration with James Moore from Imperial College London (London, UK).
Pathfinder details that its ePath catheter technology is an advanced electronic guidance system that is designed to facilitate the minimally invasive connection of an artery and a vein at the level of the wrist. According to the company, this endovascular AVF (endoAVF) procedure has the potential to develop a high-flow venous return that has similar performance characteristics to surgically created AVFs but without requiring a surgical procedure.
The company anticipates that this technological advance may provide a safe, reliable, and cost-effective method for dialysis AVF creation in the estimated 3.4 million patients that require haemodialysis annually. In addition, it states that the technology may be utilised to access vessels in other anatomical locations and has potential applications in many other interventional procedures which currently require extensive imaging to co-locate blood vessels.
Latest News 28 September 2023 – Issue9
Clinical and Industry News
offering, which includes Wrapsody cell-impermeable endoprosthesis, Haemodialysis Reliable Outflow (HeRO) graft, and the Surfacer system devices.
First enrolment in wearable peritoneal dialysis device trial
AWAK Technologies and Singapore General Hospital (SGH; Singapore) have announced the launch of a pre-pivotal clinical trial with the enrolment of their first subject to study the safety and efficacy of an “improved” Automated Wearable Artificial Kidney Peritoneal Dialysis (AWAK PD) device, according to a press release.
AWAK PD is a wearable and “ultraportable” peritoneal dialysis (PD) system that allows patients with endstage kidney disease (ESKD) to have dialysis on the go. It has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA).

The single-site, prospective, singlearm study is a follow-up on AWAK’s first-in-human study, which reported no serious adverse events. The team of SGH researchers is now recruiting subjects to participate in this pre-pivotal clinical trial.
AWAK CEO Suresha Venkataraya said: “Entering the pre-pivotal trial with the enhanced version of our device signifies a significant step in our mission to revolutionise the dialysis industry. The invaluable insights and feedback from the initial first-in-human trial have been vital in refining and enhancing our product. We remain committed to redefining kidney care by bringing disruptive products to improve the quality of life of people with kidney disease. We thank our board of directors, investors, and clinical partner, SGH, for their unwavering support throughout our innovation journey.”
Invizius announces ‘angry blood’ haemodialysis study and recruits 300th patient
Invizius, a biotechnology company developing second generation therapies for a range of complementdriven autoimmune and inflammatory disorders, has announced that it has recruited its 300th patient to its ‘angry blood’ study, CompAct-HD. This, it says, is a “significant milestone” in its “novel kinetic study” to identify patients with elevated complement responses during haemodialysis, what it calls ‘angry blood’, who may have a high risk of serious cardiovascular complications, an Invizius press release notes.
That release goes on to state that angry blood occurs in ±20% of patients undergoing haemodialysis, making them more susceptible to heart disease, risk of stroke, damage to blood vessels and a very poor patient prognosis. During haemodialysis, blood interacts with the biomaterials of the haemodialysis circuit. This interaction activates the complement system, which plays a key role in the innate immune response but, when dysregulated, contributes to the development of numerous diseases.
Speaking exclusively to Renal Interventions, Invizius CEO Richard Boyd said: “We think this is the largest, most comprehensive study ever of how patients’ immune responses to the haemodialysis procedure vary, and what that means for their cardiovascular health and outcomes generally. Recent research links high levels of immune activation during dialysis [angry blood] to significantly higher cardiovascular event risk.
“This study builds on that finding and seeks to verify the link between the origin of the immune response (activation of the complement system on the surfaces of the extracorporeal circuit) and the acute/chronic inflammation that is the harbinger or driver of so many life-limiting conditions suffered by patients. By linking this to outcomes, the study could provide the justification for intervention in the subset of patients with angry blood.”
ProKidney announces new vice president of clinical development
ProKidney, a late clinical-stage cellular therapeutics company focused on chronic kidney disease (CKD), has announced the appointment of Bruce Culleton as executive vice president of clinical development and commercialisation. Culleton, who will report to CEO Tim Bertram, joins ProKidney after more than two decades in industry and academia with a primary focus on kidney health.

Bertram said: “I am thrilled to welcome Bruce to the ProKidney team. His extensive experience in the identification and management of CKD and development of novel solutions for CKD patients will be invaluable as we continue advancing the development of React toward a potential commercial launch. I look forward to working closely with Bruce to optimally position each of our ongoing clinical studies for successful outcomes and preparing for ProKidney’s anticipated shift to a commercial organisation.”
Merit Medical announces series of dialysis catheter acquisitions
Merit Medical Systems has announced that it has completed the acquisition of a portfolio of dialysis catheter products and the BioSentry biopsy tract sealant system.
“We are selectively investing to expand our product portfolio in key strategic markets that leverage our existing commercial footprint,” said Fred P Lampropoulos, Merit’s chairman and CEO. “These acquisitions strengthen our position in the dialysis and biopsy markets, and expand the foundation of our growing specialty dialysis device
“Many dialysis patients rely on these solutions to receive vital therapies. Combining this broad portfolio of interventional solutions within Merit will allow us to leverage our physician relationships and commercial infrastructure to serve more patients in the multi-billion dialysis market.”
Posoleucel safe and effective for BK infection in kidney transplant recipients, new data suggest
AlloVir, a late-clinical stage allogeneic T cell immunotherapy company, has announced the presentation of final results from a Phase 2 study of posoleucel, an investigational, allogeneic, off-the-shelf, multi-virusspecific T cell (VST) therapy, being studied for the treatment of BK viraemia in adult kidney transplant recipients.
The findings, presented at the American Transplant Congress (ATC 2023; 3–7 June, San Diego, USA) during a late-breaking oral abstract session, support the safety and antiviral activity of posoleucel in adult kidney transplant recipients with BK virus (BKV) infection. Currently, an AlloVir press release states, there are no “effective treatment options for BKV infection”. Topline data were shared earlier this year.
Novel peripherally-inserted catheter found superior to conventional options

Medical device company Access Vascular has announced the publication of a peer-reviewed study of its HydroPICC peripherally inserted central catheter (PICC) in the Journal of Materials Science: Materials in Medicine. The retrospective study found that HydroPICC “significantly reduced” clot formation and failures, compared with conventional polyurethane PICCs, a press release states.
The release goes on to say that, of the estimated 2.7 million PICCs placed in US patients each year, roughly 95% are made from polyurethane, with occlusion rates ranging from 7.4 to 35%—meaning that there are hundreds of thousands of polyurethane-related PICC occlusions each year in the USA alone.
This retrospective study investigated whether the HydroPICC, constructed of a novel hydrophilic biomaterial with Access Vascular’s Mimix technology, reduced thrombotic catheter occlusions compared to polyurethane devices in 121 patients who received a PICC as part of their medical care.
The study results, the release details, demonstrated no occlusions or replacements in the 60 HydroPICC insertions, while 13 catheter occlusions were reported in the 61 polyurethane insertions catheter group and eight outright PICC replacements (13%).
“Reducing clot formation in PICCs helps to reduce delays in treatment, unscheduled catheter replacements, the risk of infection, and overall total cost of medical care,” said James Biggins, cofounder and CEO of Access Vascular. “If we conservatively project out the cost savings on just replacement devices seen in this study, there could easily be an annual savings of US$80 million in the USA—and, most importantly, greatly improve the standard of care for patients.”
Renalytix granted US FDA De Novo authorisation for CKD test
Renalytix has announced that the US Food and Drug Administration (FDA) has granted De Novo marketing authorisation for its KidneyIntelX.dkd prognostic test for CKD. This, the company says in a press release “affirms KidneyIntelX as a first-in-class, artificial intelligence enabled prognostic testing platform to guide care management” for adults with type 2 diabetes and early-stage diabetic CKD. It adds that it believes FDA authorisation will lead to increasing test adoption, informing clinical guidelines, expanding insurance coverage, and pursuing additional international regulatory approvals.
“Meeting the rigorous safety, clinical and analytical validation, and scientific data requirements of an FDA review, from breakthrough device designation to De Novo marketing authorisation, is a landmark event for healthcare providers and patients with diabetic kidney disease,” says James McCullough, CEO of Renalytix. “With this approval a new class, ‘prognostic test for assessment of chronic kidney disease progression’, has been established by the FDA, providing a roadmap for future expansion of KidneyIntelX into new indications and products.”
Xenotransplantation company wins KidneyX award for geneticallyengineered donor pig Makana Therapeutics has announced that it has been awarded an Artificial Kidney Phase II Prize from KidneyX to continue development of its genetically engineered donor pigs for use in kidney transplantation. The Kidney Innovation Accelerator or KidneyX is a public-private partnership between the US Department of Health and Human Services (HHS) and the American Society of Nephrology (ASN) to “accelerate innovation” in the prevention, diagnosis, and treatment of kidney diseases.
Makana, a press release states, is working to solve the organ shortage crisis by making genetically modified pigs for use as organ donors for human recipients. Makana’s “triple knockout” (TKO) pig is a combination of three xenoantigen gene knockouts in the pig that “effectively camouflage the cross-species grafts from the human recipient’s immune system”.
“Innovation is urgently needed,” said Matt Tector, chief scientific officer at Makana. “Through this prize competition, KidneyX is seeking to advance a field that has seen little progress in more than 60 years. The current standard of care for renal failure is a kidney transplant, but the supply of organs only addresses a small fraction of the need. Xenotransplantation could potentially save many lives. We are honoured to have been chosen as a KidneyX Phase II Prize winner.”
Latest News 29
Issue9 – September 2023
HydroPICC
Finerenone to be trialled for CKD in type 1 diabetes patients
Bayer has announced the initiation of FINE-ONE, a global, multicentre, randomised, placebo-controlled, doubleblind, parallel-group Phase III study to evaluate the efficacy and safety of a new investigational use of finerenone versus placebo in adults with chronic kidney disease (CKD) and type 1 diabetes. The primary objective of the study is to demonstrate efficacy of finerenone over placebo in reducing urine albumin to creatinine ratio (UACR) over six months. Finerenone is marketed as Kerendia and approved for the treatment of adults with CKD associated with type 2 diabetes (T2D) in more than 70 countries worldwide, including the USA. CKD affects up to 40% of people with type 1 diabetes, states a Bayer press release, while “the prevalence of CKD due to type 1 diabetes increased by 58.2% from 1990 to 2007 and by 21.7% from 2007 to 2017”.
“Apart from diabetes and hypertension management, there are currently limited treatment options to slow kidney disease progression in people with CKD and type 1 diabetes,” said Janet McGill (Washington University, St Louis, USA), co-chair of the study’s executive committee. “Despite progress in risk reduction in type 2 diabetes, CKD in type 1 diabetes remains understudied, leaving a huge unmet need for this population. New strategies are needed to slow their rate of decline in kidney function, which is why this important study comes as welcome news for people with CKD and type 1 diabetes and the clinical community alike.”
Empagliflozin approved in the EU for CKD
The European Commission (EC) has approved Jardiance (empagliflozin) for the treatment of adults with CKD, Boehringer Ingelheim and Eli Lilly and Company have announced. The approval, states a press release, “has the potential to advance the standard of care” for more than 47 million people in the EU living with CKD, and to “help relieve burden on healthcare systems by reducing the risk of all-cause hospitalisation” for people with CKD.

With existing indications in type 2 diabetes and heart failure, the release says, empagliflozin “could help manage the risks of cardio-renalmetabolic conditions, which are often interconnected”. Cardio-renal-metabolic conditions affect over one billion people worldwide.
“We celebrate this significant milestone in the field of chronic kidney disease. CKD is a silent killer and prevention and early detection are crucial
in the general population,” said Daniel Gallego, president of the European Kidney Patients Federation. “This new treatment option has the potential to further improve the management of cardio-renal-metabolic syndrome and renal disease, offering renewed hope and improved quality of life for countless individuals living with CKD worldwide.”
Imlifidase receives provisional approval in Australia for kidney transplant desensitisation
Hansa Biopharma has announced that the Australian Therapeutic Goods Administration (TGA) has provisionally approved Idefirix (imlifidase) as a desensitisation treatment for highly sensitised patients prior to kidney transplantation from both living and deceased donors. The provisional approval has a duration of two years and was based on data from Hansa’s phase 2 studies, which included highly sensitised patients who received a kidney from either a living (17%) or deceased donor (83%) following desensitisation treatment with imlifidase.

Søren Tulstrup, president and CEO of Hansa Biopharma said: “With nearly 28% of kidney transplant candidates in Australia considered highly sensitised, the approval of Idefirix represents an important innovation in kidney transplantation care for patients and clinicians. Hansa applauds the TGA for being the first regulatory body to approve the use of Idefirix in transplants from both living and deceased donors, thus ensuring comprehensive access for highly sensitised patients in Australia to this important therapy.”
US survey shows lack of kidney patient awareness of minimally invasive AVF options
A survey recently conducted by Medtronic and the US National Kidney Foundation (NKF) found that patients undergoing haemodialysis lack awareness about minimally invasive treatment options for creation of an arteriovenous fistula (AVF). The survey of more than 400 dialysis patients and care partners also showed patients have a high level of trust for their physicians, presenting an opportunity for greater patient-physician dialogue around treatment options for candidate patients.
“Blood access is one of the greatest challenges faced by people treated with haemodialysis both at home or in a clinic,” said Joseph Vassalotti, chief medical officer for the NKF in a press release. “Haemodialysis access complications can include infection and mechanical complications that are related to frequent use, typically three times weekly. A mature AVF as a haemodialysis access generally has lower complication rates than haemodialysis catheters.”
Nearly all patients surveyed (about 95%) reported having had surgery to create their fistula. Less than 4% of patients reported they received a newer minimally invasive AVF creation procedure. Minimally invasive AVF
procedures have been available for approximately five years. The survey revealed patients’ top concerns when speaking to their physician about a haemodialysis access procedure, including long-term maintenance and upkeep (45%), effectiveness of the fistula (42%), and fear of complications (30%).
Phase 1 study of kidney transplant rejection drug initiated
Tonix Pharmaceuticals has announced the initiation of a Phase 1 single ascending dose escalation study of TNX-1500 (Fc-modified humanised antiCD40L monoclonal antibody or mAb), a drug aimed at preventing rejection of transplanted kidneys, in healthy volunteers. The primary objectives of the study are to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous (IV) TNX-1500.
TNX-1500 is in development for the prevention of kidney transplant rejection “and other potential transplant and autoimmune disorder indications”, a company press release notes. It adds that recent animal studies indicate that TNX-1500 prevents organ rejection and preserves graft function either as a single agent or in combination with other drugs. Eligible participants enrolled in the Phase 1 study will be evaluated regularly over a 120-day period after dosing. Target enrolment is 36 participants. Initiation of this first-in-human study is intended to support dosing in a planned Phase 2 trial in kidney transplant recipients.
Quanta receives US FDA 510(k) clearance for dialysis system
Quanta Dialysis Technologies has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for an expanded indication of the Quanta dialysis system, a “compact and easy-to-use” haemodialysis device, for two modalities of continuous renal replacement therapy (CRRT): continuous venovenous haemodialysis (CVVHD) and slow continuous ultrafiltration (SCUF).
Under the new 510(k), the Quanta system is the “only dialysis device FDA-cleared to provide intermittent haemodialysis (IHD), sustained low efficiency dialysis (SLED) or bagless CRRT which creates dialysate on demand—all in a single machine”, states a press release.
The Quanta dialysis system was originally designed, the release adds, to serve the more than two million people with end-stage kidney disease (ESKD) worldwide who receive treatment with dialysis or a kidney transplant. The latest addition of its Trinal Kidney Therapy (TKT) software provides a treatment solution for critically ill patients diagnosed with acute kidney injury (AKI) who require dialysis. It features dialysate flow rates from 50 to 500mL/min and treatment times up to 24 hours of
continuous delivery.
“This clearance is a true game-changer for acute care settings,” said Quanta CEO, Alejandro Galindo. “Hospitals are often constrained with limited space and nursing staff. The Quanta dialysis system with TKT software provides an all-in-one solution for hospitals with an intensive care unit (ICU) looking to reduce their device footprint, maximise their operational efficiencies, reduce burden on nurses and substantially lower consumables expenses.”
Remdesivir
supplemental drug application for CKD patients approved by US FDA Gilead Sciences has announced that the US Food and Drug Administration (FDA) has approved a supplemental new drug application (sNDA) for the use of Veklury (remdesivir) in COVID-19 patients with severe renal impairment, including those on dialysis. With this approval, Veklury is now the first and only approved antiviral COVID-19 treatment that can be used across all stages of renal disease. The US approval comes on the heels of the European Commission decision to extend the approved use of Veklury to treat COVID-19 in people with severe renal impairment, including those on dialysis, which was adopted on 26 June 2023.
“Patients with advanced CKD and end-stage kidney disease (ESKD) are at high risk for severe COVID-19 with hospitalisation and mortality rates remaining high, even for those who are vaccinated. With limited clinical trial information for COVID-19 patients with advanced CKD and ESKD, few antiviral treatment options currently exist for this population,” said Meghan Sise (Massachusetts General Hospital, Boston, USA). “This latest update to the prescribing information for remdesivir now includes patients with advanced CKD and ESKD and this is an important advance for a population that remains highly vulnerable to the impacts of COVID-19.”
Health Canada approves IceCure Medical’s ProSense cryoablation system
IceCure Medical, developer of the ProSense system, a minimally-invasive cryoablation technology that destroys tumours by freezing as an alternative to surgical tumour removal, has announced that Health Canada, the Canadian government’s regulatory agency, has approved the system, its disposable cryoprobes, and introducers as cryosurgical tools.
The approval comes for indications including tumours, allowing the ablation of benign and malignant tumours of the lung, liver, kidneys, and musculoskeletal system, and benign tumours of the breast. It is also indicated for general surgery, palliative intervention, and other surgeries. IceCure’s cryoablation system currently has regulatory approval for various indications in 15 countries, including in the USA, China and European nations.
Latest News 30 Clinical and Industry News September 2023 – Issue9
Quanta dialysis system
Conference Calendar
08–09 September
5th Hamburg Dialysis Access Symposium Hamburg, Germany asklepios.com/hamburg/barmbek/ aerzte/fortbildung/shunt-symposium
09–13 September
Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Annual Congress

Copenhagen, Denmark cirsecongress.cirse.org/about/promo-material
17–20 September
European Society for Organ Transplantation (ESOT) Congress
Athens, Greece esot.org/esot-events/esot-congress-2023
28–29 September
Vascular Access Society of Britain and Ireland (VASBI) Annual Scientific Meeting
Birmingham, UK vasbi.org.uk/vasbi-2023
14–17 October
51st EDTNA/ERCA International Conference
Vilnius, Lithuania edtnaerca.org/conferences/ conferences-vilnius-2023
26–28 October
Controveries in Dialysis Access (CiDA) 2023
Atlanta, USA dialysiscontroversies.org
30 October–02 November




Vascular InterVentional Advances (VIVA) Las Vegas, USA viva-foundation.org/viva-programming


02–05 November
American Society of Nephrology (ASN) Kidney Week Philadelphia, USA asn-online.org/education/kidneyweek/ archives/future.aspx
08–10 November
British Society of Interventional Radiology (BSIR) Annual Meeting Newport, UK bsir.org/meetings/bsir-asm-2023
Ziv Haskal is a professor of radiology, and an interventional radiologist and interventional oncologist, at the University of Virginia School of Medicine in Charlottesville, USA.




Issue9 – September 2023 31 Conference Calendar *Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the field of renal disease management Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit renalinterventions.net and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Meet our editorial board
Nicholas Inston Chairman of the Editorial Board
Nicholas Inston is a transplant and vascular access surgeon, and the clinical service lead for renal surgery, at Queen Elizabeth Hospital in Birmingham, UK.
Ziv Haskal Board Member
Stephen Hohmann Board Member
Stephen Hohmann is a vascular and general surgeon at the Texas Vascular Associates clinic in Dallas, USA.
Robert Jones
Board Member
Robert Jones is an interventional radiologist at Queen Elizabeth Hospital in Birmingham, UK.









































































































