




THE EUROPEAN SOCIETY of Cardiology (ESC) recently published its 2024 guidelines for the management of peripheral arterial and aortic diseases (PAAD), evaluating these vascular conditions together as part of the same cardiovascular system.
A debate at this year’s European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland) centred on innovation in vascular surgery, with experts considering whether it will ‘change everything’ in the field. While Matt Thompson, chief executive officer of Endologix, underscored the inherently innovative nature of vascular surgery and the inevitability of technological advancement— he urged specialists to “safely adopt new technologies or become irrelevant”—vascular surgeon Jon Boyle (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) championed a more cautious, patient safetyfocused approach, highlighting past instances of innovation having caused harm.
Thompson began by arguing that vascular surgery is, at its core, an innovative specialty. He cited several historical examples of disruptive advancements in the field, from John Hunter ligating peripheral aneurysms over 200 years ago, to the advent of open surgery and the subsequent endovascular revolution, all of which ushered in new and distinctive eras of
treatment and fundamentally changed the vascular surgery landscape.
In the ongoing endovascular era, Thompson put forward, several innovations—including bioresorbable stents and non-invasive devices—have refined, or are set to refine, longstanding techniques and technologies. Indeed, the presenter said, there is “still room” for endovascular innovation despite the largely unchanging nature of its fundamental building blocks. However, these innovations will not, in Thompson’s view, ‘change everything’ in vascular surgery. Instead, he argued that the next paradigm shift in the field will be defined by the accelerated pace of technological change that is happening in the wider world.
Thompson argued that the pace of technological advancement in society has already led to “enormous” changes in how people live their lives. “It took 2.4 million years for humans to learn to control fire, but only 66 years to go from the first flight to humans landing on the moon,” he said.
The presenter continued that technology can change the world in ways that are unimaginable until they happen, noting, for example, that “switching on an electric light would have been unimaginable for our medieval ancestors”. This is only compounded by the accelerating pace at which technology is
There’s going to be some technological advance that completely changes our practice”
— Matt Thompson
The guidelines are aimed at cardiologists but were coordinated for alignment with guidelines for surgeons by the European Association for Cardio-Thoracic Surgery (EACTS) and endorsed by the European Reference Network on Rare Multisystemic Vascular Diseases (VASCERN) and the European Society of Vascular Medicine (ESVM).
“These updated guidelines have been introduced now due to significant advancements and shifts in our understanding and management of aortic and peripheral arterial diseases (PAD), including new treatment modalities, since the last guidelines were published in 2014 and 2017, respectively,” says ESC guidelines co-chair Jose Fernando Rodriguez Palomares (University Hospital Vall d’Hebron, Barcelona, Spain).
“The decision to integrate these guidelines is based on several key factors. The aorta and peripheral arteries are integral components of the same arterial system. Disorders in one part of this system often have implications for the other,” adds co-chair Lucia Mazzolai (Lausanne University Hospital, Lausanne, Switzerland. “Combining the guidelines provides consistent and standardised recommendations for the management of arterial diseases
Continued on page 4
Dear fellow vascular specialists, We are now well into the autumn/fall congress season and look forward to the Vascular Interventional Advances (VIVA) and VEITHsymposium meetings in November, to name but two of them.
September was remarkable for the European Society for Vascular Surgery (ESVS) congress in Kraków, Poland, and the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) congress in Lisbon, Portugal. Both meetings were very well attended and provided excellent scientific fare.
With regard to the ESVS, I would like to congratulate my vascular surgical colleague, Ian Loftus, for an excellent year as ESVS president, who completed his term in Kraków.
Looking inside interesting articles, which we anticipate will catch your attention. We lead with coverage of a debate at the ESVS meeting between Matt Thompson and Jon Boyle on the topic of whether “innovation will change everything in vascular surgery”. The second lead story focuses on the new European Society of Cardiology (ESC) guidelines that combine peripheral arterial and aortic diseases for the first time.
In the aortic space, there is an article entitled “Aortic dissection toolkit sees progress, but specialists warned against complacency”. There is also coverage of the three aortic dissection trials—EARNEST, IMPROVE-AD and SUNDAY—including a comment piece from Firas Mussa and a question and answer feature with Tim Resch.
Also in the aortic space, there is an article that reports on a paper recently published in the Therapy (JEVT in the elderly?” There is another interesting article that looks at the use of deep learning artificial intelligence (AI) techniques for the detection of endoleak after endovascular aneurysm repair (EVAR) based on non-contrast computed tomography (CT). Finally, there is coverage of the just-held CX Aortic Live symposium in Vienna, Austria, which featured a series of live cases.
Regarding peripheral arterial disease (PAD), there is an article on standardising the description of endovascular lower-limb interventions for PAD in clinical trials—the Endo-STAR framework, focused on a paper from the recent ESVS Prize Session—and a report of a study recently published in the Journal of Vascular Surgery (JVS), which suggested that social support is associated with better PAD health outcomes.
outcomes of a randomised clinical trial of endothermal ablation versus conventional surgery for great saphenous varicose veins; and a report of Merit Medical’s Wrapsody WAVE trial that demonstrated superior patency versus standard of care in arteriovenous (AV) fistula patients.
This issue’s profile highlights Ramon Varcoe’s career and there is also an interview with Jennifer Jongen on radiation protection and pregnancy in vascular surgery and interventional radiology.

I hope that you agree that there is something to interest everybody in this issue.

Other interesting articles in this issue include a report of a debate at ESVS that suggested the two-week carotid endarterectomy (CEA) target for symptomatic carotid patients is outdated; an article that reports the 10-year


ROB MORGAN is professor of interventional radiology at St George’s University Hospitals NHS Foundation Trust (London, UK) and the president of the British Society of Interventional Radiology.
THE LATEST STORIES FROM THE VASCULAR WORLD
n EVAR IN THE ELDERLY:


A large meta-analysis of elective endovascular aneurysm repair (EVAR) outcomes by age, recently published in the Journal of Endovascular Therapy (JEVT), has shown that three-to-fiveyear survival is very low in octogenarians. Based on this key finding, authors Dominic PJ Howard, Sebastian Vaughan-Burleigh and colleagues (Oxford, UK) conclude: “Our findings challenge the notion of routine intervention in elderly patients, and support very careful selection for elective EVAR.”
For more on this story go to page 8.
n CAROTID ENDARTERECTOMY:
Findings from a retrospective analysis provide evidence that— for symptomatic carotid artery stenosis patients—endarterectomy “remains a useful and relevant intervention in the era of improving medical therapy”. Sashini Iddawela (London, UK) delivered these data at the recent European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland).

For more on this story go to page 12.
n VASCULAR ACCESS:

Positive six-month findings from the randomised arteriovenous (AV) fistula arm of Merit Medical’s Wrapsody arteriovenous access efficacy (WAVE) pivotal trial have been revealed. The data were shown at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (14–18 September, Lisbon, Portugal) during a FIRST@ CIRSE presentation. Wrapsody is a cell-impermeable endoprosthesis which is intended to extend long-term vessel patency in dialysis patients.
For more on this story go to page 22.
Editors-in-chief: Robert Morgan (European Edition) and Ross Milner (North American Edition) | Publisher: Stephen Greenhalgh
Rob Morgan (European Edition) and Ross Milner (North American Edition)
Content director: Urmila Kerslake | Global commercial director: Sean Langer
Publisher: Stephen Greenhalgh | Content director: Urmila Kerslake | Global sales director: Sean Langer Kay and
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Editorial contribution: George Barker, Jamie Bell, Will Date, Bryan Kay, Éva Malpass | Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788
BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
VascularNews linkedin.com/company/Vascular-news @VascularNews
Scan the QR code to subscribe
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
Continued from page 1
changing, he added.
Giving some examples of the technological advancements that are currently happening outside of vascular surgery, Thompson referenced the mRNA vaccines that were ushered in during the COVID-19 era at a pace that had before seemed inconceivable, to name just one.
So, the presenter asked, what do technological advancements in the wider world mean for vascular surgery? At present, he said, it is hard to know. He stressed that the only certainty is the inevitability of change.
“There’s going to be some technological advance that completely changes our practice,” Thompson posited, listing wearable technology, personalised and regenerative medicine, and artificial intelligence (AI) and machine learning as just three technologies that have practice-changing potential. Thompson had a clear message here: adopt—safely—or be left behind.
“I’d submit to you that in a few years’ time, or a few generations’ time, we will have the same approach looking at the current practice of vascular surgery today and thinking it was about as barbaric as our medieval ancestors,” Thompson said in his conclusion. “I certainly don’t want that for me or my patients.”
“We’ve got to be careful how we innovate”
Putting forward a counter argument, Boyle stressed the importance of striking a balance between innovation and patient safety. In several instances of innovation, Boyle argued, “we’ve actually got it wrong—we’ve innovated, and patients have come to harm.” Boyle’s presentation highlighted a number of devices and techniques used over the last 20 to 30 years that have
harmed rather than benefitted patients.
First, Boyle referenced data from the landmark EVAR-1 trial, noting that patients who were randomised to endovascular aneurysm repair (EVAR) “had far greater numbers of interventions out to 14 years” than the patients who received open repair.
Acknowledging the age of the EVAR-1 data as a weakness of his argument, Boyle subsequently moved on to newer EVAR evidence. Specifically, the presenter spoke on results from the VQI VISION study regarding the AFX graft (Endologix). The 2022 data, which illuminate reintervention and rupture rates with an early iteration of the device in the USA, were published by Goodney et al in the British Medical Journal
“When we fix an aneurysm, we’re trying to do it to prevent rupture, but the early AFX graft had a near 40% reintervention rate at eight years and a 10% rupture rate at 10 years,” Boyle informed the ESVS audience, summarising the VQI VISION data. “So, 10% of the patients treated with this device ended up rupturing their aneurysm.”
In light of these data, Boyle remarked: “We’re fixing aneurysms, but we’re not really fixing them.”
The presenter also shared similar findings with regard to fenestrated repair. He commented bluntly that “fenestration means twice the likelihood of being dead at three years,” according to data from Vallabhaneni
Evidence-based, tried and tested techniques often result in better patient outcomes”
— Jon Boyle
et al’s UK-COMPASS study, published in 2024 in the European Journal of Vascular and Endovascular Surgery (EJVES).
Boyle subsequently presented data on peripheral arterial disease (PAD), citing a growing body of evidence showing that early intervention for claudication leads to worse outcomes.
Programme directors Tilo Kölbel (University Heart Center Hamburg, Hamburg, Germany) and Aung Oo (St Bartholomew’s Hospital and Barts Heart Centre, London, UK) recently welcomed a global, multidisciplinary audience to CX Aortic Live (7–8 October, Vienna, Austria) for two days of live case transmissions, edited cases and podium presentations on the latest cardiac, vascular and endovascular aortic advances. Here, Vascular News presents a snapshot of meeting highlights.
Live case: Open repair of AAA in Marfan syndrome
One of the highlights of the day two agenda was a live case—one of 18 across the programme—from CX Symposium co-chair Dittmar Böckler (University Hospital of Heidelberg, Heidelberg, Germany) and team, who presented a live open repair of an abdominal aortic aneurysm (AAA) in a young patient with Marfan syndrome. “Treatment of young patients with connective tissue disease is always a challenge,” Böckler told Vascular
News, emphasising that the age of the patients requiring intervention necessitates “optimal treatment with the longest durability we can obtain”.
Endo-Bentall: Podium presentation and edited case exhibit ‘final frontier’ for endovascular repair
Mehrdad Ghoreishi (Miami Cardiac and Vascular Institute, Miami, USA) delivered a podium presentation on the Endo-Bentall technique, which was described in the title of his talk as the
Referencing an Australian study from Golledge et al, published in 2018 in the British Journal of Surgery, the presenter shared that patients with intermittent claudication who were treated early with endovascular intervention had a five-year amputation rate of 6.2% compared to 0.7% for those who received best medical therapy and exercise. “Early intervention for intermittent claudication with new devices led to a significantly higher amputation rate in this patient cohort,” he summarised.
The story is similar regarding carotid stenting, Boyle pointed out. “We’ve pretty much stopped doing carotid stenting in the UK,” he said, sharing that only around 250 such cases are conducted in the country every year. “The reason for that,” he explained, “is that for symptomatic disease, your relative risk of stroke and death following carotid stenting is 1.7 compared to carotid endarterectomy.”
Towards the close of his presentation, Boyle went back to Endologix, this time focusing on Nellix. “We got our fingers burnt with this device,” the presenter acknowledged. He recalled a sense of optimism in the early stages of using this device. “We all felt at the start this was a device that may change practice,” Boyle remembered. In the end, however, the presenter stressed that “a lot of patients came to harm as a result of being treated with this device”.
Sharing some data on the device from his centre in Cambridge—specifically from a 2021 EJVES paper by Singh et al—the presenter noted that, at six-year follow-up, only 32% of patients still had freedom from device failure and there were “quite a few ruptured aneurysms”.
In his conclusion, Boyle reiterated his central argument that “we’ve got to get the balance right— we need to innovate, but we need to do it in an environment where we can ensure our patients are safe”. The presenter continued: “Evidence-based, tried and tested techniques often result in better patient outcomes.”
A show of hands following the debate revealed a close result, with ESVS president Ian Loftus (St George’s University Hospitals NHS Foundation Trust, London, UK) declaring a narrow win for Thompson.
‘final frontier’ for endovascular repair. The presenter shared some case examples, with one of his key conclusions being that the pathology should determine whether to use twostage or single-stage devices. Ghoreishi also underscored the importance of a multidisciplinary approach, saying that a cardiac surgeon, vascular surgeon and interventional cardiologist all need to be present for the procedure.
Following Ghoreishi’s talk, Thomas Gandet (University of Montpellier, Montpellier, France) shared an edited case on the Endo-Bentall technique for acute type A aortic dissection (TAAD).
Risk stratification for TAAA patients: “Know your own limits”
In a podium presentation on risk stratification for thoracoabdominal aortic aneurysm (TAAA) patients, Tara Mastracci (Barts Health NHS Trust, London, UK) advised CX Aortic Live attendees to “know your own—and your technology’s—limits,” among other recommendations.
Mastracci first addressed physiologic risk, focusing on ‘prehab’. The

presenter opined that “having a prehab programme in your thoracoabdominal programme is really important,” going on to share some positive preliminary data from Barts. She relayed that objective measures like the six-minute walk test and sit-to-stand test help quantify progress for these patients in a cost-effective way.
Subsequently, Mastracci spoke on anatomic risk. Here the presenter encouraged delegates to take their time deploying devices accurately. Doing so, she remarked, “not only makes the case easier but also improves the longterm outcomes”.
On-demand content is available to registrants at www.mycxonline.com.
as a whole. This ensures that patients receive cohesive and coordinated care across different vascular conditions, reducing fragmentation and improving overall treatment outcomes.”
An ESC press release notes that PAAD is estimated to affect around 113 million people aged 40 and over globally, of whom nearly half (43%) are in low- and middle-income countries. The global prevalence is 1.5% and increases with age, affecting 15–20% of those aged 70 years and over and 20–30% of those aged 80 years and older. Prevalence increased by 72% from 1990 to 2019, despite the global population growing by only 45%.
The authors say the most important recommendations in the new 2024 guidelines are those addressing the chronic nature of PAAD, the importance of screening, and the necessity of comprehensive treatment strategies—and awareness that this is a chronic disease that needs lifetime follow-up.
age, the presence of cardiovascular risk factors, family history and/or presence of syndromic features. PAAD diagnosis can be easily achieved with a non-interventional vascular test/ imaging,” says Rodriguez Palomares. The guidelines highlight that optimal pharmacological treatment (antithrombotic, lipid-lowering, antihypertensive, antidiabetic) and emphasis on exercise and lifestyle changes are mandatory and effective in reducing burden of disease. Patients with PAAD have a very high cardiovascular risk and require optimal management
The aorta and peripheral arteries are integral components of the same arterial system. Disorders in one part of this system often have implications for the other”
— Lucia Mazzolai
Prevalence of peripheral arterial and aortic diseases increased by 72% from 1990 to 2019, despite the global population growing by only 45%
Peripheral arterial and aortic diseases increased by

comprises chronic diseases requiring continued attention.
cardiologists, and a multidisciplinary team. Women often present with atypical or asymptomatic disease, warranting special attention during screening. Exercise and lifestyle changes are crucial before considering interventional management in chronic PAAD.” New ESC guidelines combine peripheral arterial and aortic diseases for first time
“A significant proportion of patients are asymptomatic and therefore PAAD screening is crucial, based on
of risk factors such as hypertension, hyperlipidaemia, and diabetes to prevent serious complications.
Finally, the authors emphasise gender aspects and that PAAD
They conclude: “PAAD is a chronic disease necessitating lifelong follow-up by vascular specialists,
Following the publication earlier this month of a research letter on radiation protection and pregnancy in vascular surgery and interventional radiology, Vascular News caught up with lead author Jennifer Jongen (Amsterdam, The Netherlands) at the 2024 European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland) to discuss the findings.
JONGEN AND COLLEAGUES’ LETTER, which features in the European Journal of Vascular and Endovascular Surgery (EJVES), follows the publication last year of the ESVS’ clinical practice guidelines on radiation safety.
At the ESVS annual meeting in 2022, Bijan Modarai (London, UK) had presented the guidelines for the first time. Modarai was co-chair of the guideline writing committee alongside Stéphan Haulon (Paris, France).
The document, published in the EJVES, includes a chapter on pregnancy and radiation exposure with one recommendation, as defined by law: “A welldefined pathway must exist at each institution for pregnant employees to declare their pregnancy in order to manage subsequent occupational radiation exposures”.
Jongen explained that the research letter was based on the premise that pregnant women “have difficulties
finding their place in the guidelines” and “have a tendency to work around radiation while they are pregnant”.
The research set out to illuminate the radiation safety landscape for pregnant women in The Netherlands specifically, and whether there is room for improvement.
Jongen and colleagues sent out a questionnaire to vascular surgeons and interventional radiologists and found that 20% of vascular surgeons who responded had postponed getting pregnant because of their training programme, with the figure being similar among interventional radiologists.
“Based on our results,” Jongen commented, “we should have a better handling of radiation and at least have better guidelines on how to use it.” Jongen also highlighted a one-size-fits-all approach to personal protective equipment (PPE) for radiation safety, which she noted disadvantages women—and

especially pregnant women—in particular. She cited women often having to improvise with the available equipment, for example with two lead aprons, despite this not providing adequate protection.
“It’s a subject that we need to talk about,” Jongen said, sharing one of her key messages from the research.
She continued: “Hopefully, these results demonstrate the importance of this subject, improve standardisation of radiation practice in The Netherlands, and address the need for better education regarding radiation during pregnancy as well as better personal protective equipment.”





Firas Mussa (Houston, USA) urges specialists to put biases aside when it comes to treatment options for uncomplicated type B aortic dissection (TBAD).
SURGEONS IN GENERAL AND vascular surgeons in particular have not been at the forefront of generating evidence. We have debated about asymptomatic carotid disease, claudication, and most recently on what to do with patients with uncomplicated TBAD without doing much research on any of them. Some are even debating the definition of uncomplicated TBAD and offer a stent graft to almost all
comers. None of these debates have been based on high-quality data. Besides biases and/or conflicts, I am not sure how to characterise the craze to push for one treatment over the other.
In 2023, the US National Heart, Lung, and Blood Institute (NHLBI) funded the IMPROVE-AD trial. This was the culmination of a decade-long effort built on clinical equipoise and led by a team that has a track record
in clinical trials. The first patient was enrolled in April 2024 with lukewarm enthusiasm from sites. The hurdles are not too dissimilar to those faced by other NHLBI-funded trials in the past but unique to this disease is the widespread belief that we have all the data we need to offer stents to almost all patients. The risk to science is real, especially with societal guidelines adopting a copy/paste approach to other published documents, upgrading the level of recommendation (from 2b to 2a) without new evidence. In other words, guidelines have lost credibility to independently evaluate data with nuance and/or accuracy.
To move forward, investigators from around the world have the moral obligation to patients and the scientific responsibility to their respective societies to do better. We all need to put our biases aside, move more towards the centre and enrol patients in clinical trials. The traditional rate of one out of four patients approached/consented is still true, but not attempting is unacceptable. We are privileged to care for our patients and offering what is in their best interest is expected.
When it comes to uncomplicated TBAD, we have two treatment modalities that are ‘good enough’.
A dedicated toolkit has made it safer to be an aortic dissection patient in England today than it was before the project’s 2022 launch, but there is still work to be done, Rachel Bell (Freeman Hospital, Newcastle, UK) informed attendees at the inaugural Interdisciplinary Aortic Dissection Symposium (IADS; 6 September, London, UK).
BELL WAS DELIVERING AN UPDATE ON THE National Health Service (NHS) England Acute Aortic Dissection Toolkit—designed to improve care for emergency acute aortic dissection patients.
The toolkit, which represents a collaboration between several specialties, has a core set of seven key principles, which Bell listed as regional governance, coordination through multidisciplinary teams, regional rotas with a single point of contact, timely image transfer, safe interhospital patient transfer, specialist treatment for all acute aortic dissections, and a regional educational programme.
Bell, an aortic specialist in the North East of England and Yorkshire, noted that the project has aimed from the outset to recognise regional differences across England. “London is full of specialists who deal with aortic dissection,” the presenter told the London gathering, “but as you move north, that degree of specialisation is less concentrated.” To address this, Bell detailed that the toolkit recognises seven distinct regions, each with their own vascular surgery and cardiac surgery leads.
Reflecting on one of the standout successes of the project to date, Bell stressed that there has been a “healthy national dialogue” around the toolkit’s key principles. “It’s been incredibly powerful getting people in a room and talking about the problems they have in their own regions,” she said, noting that representatives from the seven regions are now meeting on a monthly basis.
More specifically with regard to progress, the
presenter reported that the North West, South of England and London now have published rotas for aortic dissection specialists and that the East of England has funded an aortic dissection coordinator.
In the North East, Bell shared that the team is about to launch a new regional picture archiving and communication system (PACS). As a result, Accident and Emergency computed tomography (CT) scans will be available immediately, which Bell remarked will be “enormously helpful”. Similarly, in the South East, the team is testing a cross-regional image transfer system.
However, we do not know which is best, and among many other questions; we do not know the impact of ‘high-risk’ features, ideal timing of intervention, length of coverage, what patients want and at what cost. Therefore, enrolling in the ongoing trials—IMPROVE-AD, EARNEST and SUNDAY—is in the best interest of all patients, present and future.
Firas Mussa is professor of surgery at The University of Texas Health Science Center at Houston (UTHealth Houston) in the USA, and principal investigator and co-chair of the executive committee for the IMPROVE-AD trial.
Enrolling in the ongoing trials—IMPROVEAD, EARNEST and SUNDAY—is in the best interest of all patients, present and future”
difficulties of data collection overall but particularly for type B aortic dissection (TBAD) patients. This is because most TBADs are treated medically initially, and procedures are much easier to count.
Bell shared that data from the National Clinical Improvement Programme (NCIP) reveal a “poor” three-year mortality rate amongst those patients treated medically for TBADs, reaching 32%. She noted that this may represent an opportunity to improve medical treatment and surgical intervention for TBAD under follow-up and that this will need to be tackled in the ‘elective’ pathway.

Additionally, the presenter detailed that several aortic centres have started using referral management systems—like Patient Pass and Referapatient—to ensure that the new pathways are followed and patients are referred rapidly to the correct centres.
“Lots of regions have done really well,” Bell summarised, highlighting the South of England—led by vascular surgeon Marcus Brooks (North Bristol NHS Trust, Bristol, UK) and cardiac surgeons Jonathan Hyde (Royal Sussex County Hospital, Brighton, UK) and George Krasopoulos (Oxford University Hospitals NHS Foundation Trust, Oxford, UK)—as “best in class” thanks to the team’s development of a supraregional standard operating procedure document.
Bell also touched on some areas for growth. The presenter highlighted, for example, the ongoing
In addition, Bell noted ongoing problems with image transfer and safe interhospital transfer. Here, the presenter referenced notable regional disparities. “As you get higher and higher up the country, there’s less support for appropriate interhospital transfer,” she said, specifying that in the North East the interhospital transfer is not provided 24/7. Finally, Bell highlighted future opportunities around research. She mentioned in particular the UK-specific EARNEST trial, which is set to commence recruitment in March 2025. The study, Bell explained, “hopefully will answer some questions about what we do with those patients who are initially treated medically but we think may benefit from endovascular intervention in the subacute phase, and whether that will make a difference to their longer-term mortality.”
Concluding her presentation, Bell was keen to stress that the work is far from done. “Please don’t become complacent—we’ve got lots to improve, and we’ve still got the elective pathway to tackle,” she said. All this, Bell pointed out, against the backdrop of a rising incidence of aortic dissection.
However, the presenter also highlighted the “significant progress” made over the last two years, reiterating in particular the positive impact of communication.
“I think the big message is don’t underestimate the power of talking and raising awareness nationally— it’s really made a difference.”
The inaugural Interdisciplinary Aortic Dissection Symposium (IADS; 6 September, London, UK) highlighted three trials— EARNEST, IMPROVE-AD and SUNDAY—all of which are set to provide some much-needed data on treatment options for uncomplicated type B aortic dissection (TBAD). Here, Tim Resch (University of Copenhagen, Copenhagen, Denmark) gives an update on SUNDAY, detailing enrolment progress and the challenges of conducting a randomised trial.
What is the SUNDAY trial?
The SUNDAY trial is a clinical study investigating the best treatment approach for patients with uncomplicated TBAD. It is a randomised, open-label trial conducted across the Nordic countries, including Denmark, Sweden, Norway, Finland, and Iceland.
The trial compares two treatment strategies for uncomplicated TBAD patients: standard medical therapy (SMT) alone versus SMT combined with thoracic endovascular aortic repair (TEVAR). SMT typically involves controlling blood pressure and providing pain relief, with β-blockers as first-line treatment.
What data are you hoping to gain from the trial?
The primary aim of the SUNDAY trial is to determine whether the addition of TEVAR improves fiveyear survival rates compared to SMT alone. Secondary outcomes include aortic-related mortality, neurological events, quality of life, reinterventions,
and healthcare costs. The trial aims to enrol around 554 patients and follow them for up to five years. The trial design is pragmatic, meaning it tries to reflect real-world practices by allowing flexibility in the type of TEVAR procedure performed and SMT management based on local protocols.
This study seeks to address a crucial question: does early intervention with TEVAR offer significant survival benefits in uncomplicated cases of TBAD? Previous research suggested potential benefits, but larger, randomised trials like SUNDAY are needed to provide definitive answers.
How is enrolment going?
The SUNDAY trial has initiated all 21 major vascular centers in the Nordics and enrolled 60 patients to date.
There are some significant challenges with running a large, multicentre randomised controlled trial (RCT). So far, we have encountered a number of subjects that present with pathologies which are not typical for textbook aortic dissections and thus pose challenges
Hybrid aortic arch: “We believe there is still a role for a versatile multiplebranched arch option”
MID-TERM RESULTS FROM an analysis of a series of hybrid aortic arch reconstructions utilising overlapping single thoracic branch endoprostheses in a dual-branch configuration have shown the technique to be safe and versatile.
Delivered at the 2024 Western Vascular Society (WVS) annual meeting in (7–10 September, Colorado Springs, USA), the paper was winner of the WVS Rapid Fire Competition.
Presenter Evan R Brownie, a vascular surgeon at Intermountain Health in Murray, USA, characterised use of the procedure following the commercial launch and subsequent widespread use of the Gore Tag thoracic branch endoprosthesis (TBE). Despite this, said Brownie, “we believe there is still a role for a versatile multiple-branched arch option.”
to inclusion. The inclusion period is relatively long after symptoms occurrence and this might lead to selection bias if criteria are not adhered to and the intent of the trial followed. Being a strategy-based trial (like the IMPROVE trial for ruptured abdominal aortic aneurysm [AAA]) it is important that investigator truly attempt to include and randomise patients before evaluating the applicability of a TEVAR procedure or the trial might be biased towards “cherry picking” only anatomically appealing candidates which might skew the trial in favour

of TEVAR. The strategy-based setup will likely lead to a number of crossover cases post randomisation but will obviously add the benefit of identifying what the applicability of TEVAR actually is.
Tim Resch
We are also discovering as we move along some of the weaknesses in the medical system when it comes to caring and surveilling dissection patients after conservative management. Many patients remain inadequately medicated for hypertension and are often managed by multiple specialties, creating therapeutic challenges.
The UK National Institute of Health Research (NIHR)-funded EARNEST randomised controlled trial aims to recruit the first patients from March 2025, Colin Bicknell (Imperial College London, London, UK) tells Vascular News
Bicknell, pictured here at IADS, explains that EARNEST will address the question of whether thoracic endovascular aortic repair (TEVAR) in the subacute phase after uncomplicated type B aortic dissection (TBAD) in addition to best medical therapy (BMT) compared to BMT and surveillance confers a long-term advantage and whether this practice is cost effective. He notes that 470 patients with uncomplicated TBAD will be recruited in vascular centres throughout the UK.
“The results are expected to inform international guidelines and change practice in the UK and abroad,” Bicknell comments.
Brownie and colleagues performed their first 49 TBE deployments in 44 patients from October 2022 to December 2023, five of which involved the dual-TBE configuration in question. Median follow-up is six months, and up to 12 months.
“All of these patients had at least one prior sternotomy with an existing ascending aortic stent graft, some sort of systolic heart failure and one of the five patients had a prior recurrent laryngeal nerve injury at the time of their index procedure, henceforth why—even though we do an equal number of zone 0 deployments off a single branch—we are trying to minimise risk for this patient population and the discrepancies that can be seen with a single branch perfusing all of the great vessels,” he explained.
Three of the five underwent a dual brachiocephalic configuration with a left carotid to subclavian transposition through a left cervical incision, Brownie said. The other two had a left common carotid to right common carotid transposition, he added, explaining that “we do a separate arteriotomy in the right carotid artery to maintain cerebral perfusion throughout the case.”
For the endovascular portion of the procedure, he continued, “we obtained bilateral percutaneous femoral and bilateral percutaneous arm access. We do a zone 2 thoracic branch stent
An appropriate trial design and regulatory application could bridge the gap until we have a dedicated arch graft available”
graft, put in an oversized Viabahn stent graft [Gore Medical] in position, then extend proximally with our zone 0 stent graft and deploy it. So, we are gaining the overlap seal between the devices as well as the parallel seal zone just to maintain perfusion to the subclavian limb.”
Procedures were typically staged, Brownie said, with the transposition performed two days prior to the endovascular repair. Following transposition, there were no strokes, cranial nerve injuries or early thrombosis, he revealed. Similarly, following endovascular repair, there were no cases of spinal cord, renal or bowel ischaemia. Furthermore, one patient was returned to the operating room for an access site complication, one had a stroke, and there were no type I or II endoleaks, nor gutter leaks. Sixty percent of these patients have already demonstrated sac regression, Brownie added.
“An appropriate trial design and regulatory application could bridge the gap until we have a dedicated arch graft available,” he concluded. “And, of course, this will largely be dependent on compensation and reimbursement for such procedures.”
A large meta-analysis of elective endovascular aneurysm repair (EVAR) outcomes by age has shown that three-to-five-year survival is very low in octogenarians, prompting researchers to caution against routine intervention in these patients.
DOMINIC PJ HOWARD, Sebastian Vaughan-Burleigh and colleagues (Oxford, UK) write in the Journal of Endovascular Therapy (JEVT) that elective repair for abdominal aortic aneurysm (AAA)—a condition known to increase in prevalence with age—is commonly performed in octogenarians. However, while previous trials have confirmed elective EVAR to be an effective intervention for AAA, the authors note that the data are limited for elderly patients due to them often being excluded from randomised trials.
To evaluate the safety and outcomes of elective EVAR in elderly patients, the researchers performed a systematic review and meta-analysis of studies reporting risk of complications and death in relation to age. They included observational studies and, if outcome rates or raw data were provided, interventional arms of randomised trials.
Howard et al identified 38 eligible


studies from 9,060 citations, including eight national and five international registries, 25 retrospective studies, and their own prospective cohort. The analysis included 208,997 non-octogenarians and 84,589 octogenarians in total.
The authors report in JEVT that 30day mortality post-elective EVAR—the study’s primary outcome—was higher in octogenarians than in younger patients.
Another key finding from the study was that linear regression demonstrated a 0.83% increase in 30-day mortality for every 10-year age increase above
60 years old.
In addition, the researchers write that mortality for octogenarians increased “significantly” during follow-up, with Howard and colleagues sharing figures of 11.35%, 22.8%, 32%, 47.53%, and 51.08% at one-through-five-year follow-up, respectively.
Finally, the authors reveal that 30day complication rates after elective EVAR were higher in octogenarians than in younger patients.
“We have performed the largest meta-analysis of elective EVAR outcomes stratified by age to date,” Howard and colleagues state, going on to summarise that, while octogenarians experience “higher but acceptable” perioperative morbidity and mortality compared to younger patients, threeto-five-year survival is “very low” in older patients.
The authors recognise that there are certain limitations to their study, including the “increasingly infrequent”
reporting of mortality data beyond 30 days, the “inconsistent” reporting of data outside mortality across studies. Despite these limitations, Howard et al stress that many patients in the study came from registries, which they note are “generally considered most representative of underlying populations”.
Based on their findings, the authors conclude: “Our findings challenge the notion of routine intervention in elderly patients, and support very careful selection for elective EVAR.”
They continue that many octogenarians with ‘peri-threshold’ AAAs, i.e. those less than 6cm in diameter, “may derive no benefit from EVAR” due to limited three-tofive-year survival and the low risk of aneurysm rupture that is associated with conservative management. “An adjusted threshold for intervention in octogenarians may be warranted,” they assert.
MORTALITY FOR OCTOGENARIANS INCREASED “SIGNIFICANTLY” DURING FOLLOW-UP
Use of AI in endoleak detection deemed “feasible” with “potentially significant” implications for patients and health systems
A recently published retrospective study has shown that a deep learning model based on non-contrast computed tomography (CT) can detect endoleak after endovascular aneurysm repair (EVAR) “with high sensitivity”.
Writing in CardioVascular and Interventional Radiology (CVIR), Qingqi Yang, Jinglang Hu (both Sun Yat-Sen University, Guangzhou, China) and colleagues share that their study involved 245 patients who underwent EVAR between September 2016 and December 2022, with all patients undergoing both non-enhanced and enhanced followup CT. The researchers note that the presence of endoleak was evaluated based on CT angiography (CTA) and radiology reports.
Yang et al detail in their methods section that, first, the aneurysm sac was segmented, and radiomic features were extracted on noncontrast CT, after which statistical analysis was conducted to investigate differences in shape and density characteristics between aneurysm sacs with and without endoleak. Subsequently, a deep learning model was trained to generate predicted segmentation of the endoleak, and a binary decision was made based on whether the model produced a segmentation to detect the presence of endoleak. “The absence of a predicted segmentation indicated no endoleak, while the presence of a predicted
segmentation indicated endoleak,” the authors write.
Finally, Yang and colleagues add that the performance of the model was evaluated by comparing the predicted segmentation with the reference segmentation obtained from CTA, with model performance assessed using metrics such as dice similarity coefficient, sensitivity, specificity, and the area under the curve (AUC).
In CVIR, the authors relay that their study finally included 85 patients with endoleak and 82 patients without endoleak. “Compared to patients without endoleak, patients with endoleak had higher CT values and greater dispersion,” they report, noting that the AUC in the validation group was 0.951, dice similarity coefficient was 0.814, sensitivity was 0.877, and specificity was 0.884.
In their, Yang et al highlight several limitations to their study. These include its retrospective nature and the fact that the team used imaging data from the past seven years, which the researchers acknowledge “may introduce variations due to differences in imaging equipment,” among others.
The authors conclude that the deep learning model
constructed using non-contrast CT “can accurately detect endoleaks”. However, they also stress that “multicentre studies should be conducted to evaluate the diagnostic robustness of the algorithm, and the feasibility of using non-contrast CT for endoleak satisfaction should be explored to further refine the model”.
In an associated commentary, Robert Morgan (St George’s University of London, London, UK) writes that, despite the limitations of the research, the data presented “confirm that the use of AI [artificial intelligence] in the detection of endoleaks is feasible”.
Morgan goes on to say that the data also “confirm that endoleaks can be detected using deep learning techniques even in the absence of intravenous contrast medium,” which he highlights as a “very interesting piece of news”.
Whilst acknowledging that further work is required to assess the applicability of these
Multicentre studies should be conducted to evaluate the diagnostic robustness of the algorithm”
techniques to a wider population, Morgan states that “if the results are replicable, the implications for patients and health systems post-EVAR are potentially significant, removing the need for contrast medium in CT follow-up in many cases”.






Pilot testing has shown that a new framework—dubbed Endo-STAR—can be used to describe and standardise endovascular interventions for peripheral arterial disease (PAD) within a randomised controlled trial (RCT) protocol and monitor adherence to the protocol over the course of a trial. Ewa M Zywicka (University of Bristol Medical School, Bristol, UK) shared this conclusion during the Prize Session at the recent European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland).
ZYWICKA BEGAN BY NOTING THAT
rigorous evaluation of novel lower-limb endovascular interventions is “critically important” to ensure adoption of effective technologies and rejection of those that are ineffective or harmful.
The presenter continued that poor reporting of RCTs limits the evaluation of technologies for lower-limb endovascular interventions, highlighting a recent study in which more than half of RCTs did not adequately describe interventions, and almost 80% did not report any form of standardisation.
The aim of Zywicka and colleagues’ present study, therefore, was to develop a specific framework for describing and standardising endovascular lower-limb interventions within clinical trials.
Zywicka explained to the ESVS audience that the Endo-STAR framework was developed using qualitative research methodology and the Enhancing the Quality and Transparency of Health Research (EQUATOR) methodological framework. The presenter added that trial reports and associated protocols identified in a recent systematic review of endovascular infrainguinal lower-limb RCTs for PAD provided the data for developing the preliminary framework.
Going into more detail about the research methods used, Zywicka detailed that a framework approach to thematic analysis of qualitative data was employed to code and categorise text into steps and components of endovascular infrainguinal interventions.
Subsequently, focus groups were conducted
with key international stakeholders such as clinical practitioners—namely vascular surgeons, interventional radiologists, angiologists and cardiologists—trialists, industry representatives and journal editors to refine the framework and consider clarity and feasibility issues. Zywicka noted that the framework was updated based on this feedback, and
We expect that actually being able to know and clearly describe what was done in a trial can be used to easily compare trials and even potentially compare results in the long term as well”
a consensus between stakeholders was reached via a modified Delphi-style questionnaire.
Finally, Zywicka shared, the framework was refined through cognitive interviews with trialists to test the real-world feasibility of using the Endo-STAR framework in contemporary endovascular RCTs. An online version of the Endo-STAR framework
Patients with peripheral arterial disease (PAD) reporting lower levels of social support experience worse health outcomes, a new Yale-led study finds.
SOCIAL SUPPORT IS THOUGHT to bolster cardiovascular health by facilitating health-promoting behaviours and acting as a buffer against the impacts of stress on the heart. A team led by Santiago Callegari of the Yale Department of Internal Medicine (Yale University, New Haven, USA) used questionnaires to assess perceived social support (ENRICHD Social Support Inventory), PAD-specific health status (Peripheral Artery Disease Questionnaire), and general health status (EuroQOL Visual Analog Scale) for 949 patients at baseline and 12 months later.
For the 18.2% of respondents reporting low social support, researchers found scores more than seven points lower on average on both the Peripheral Artery Disease Questionnaire and the EuroQOL Visual Analog Scale, indicating significantly poorer outcomes in PAD-specific and general health metrics. The association between low social support and poorer outcomes remained strong even when adjusting for factors including stress, depression, and socioeconomic status.
The research team says their work highlights the importance of psychosocial factors, like social
Twenty-four key stakeholders participated in three focus groups, contributing to the refinement of the framework
was developed to facilitate implementation and dissemination.
At ESVS, Zywicka informed attendees that the preliminary framework was developed after including data from 112 RCTs evaluating endovascular infrainguinal interventions for PAD.
Zywicka relayed that 24 key stakeholders participated in three focus groups, contributing to the refinement of the framework, and all 24 participants took part in the consensus questionnaire. After the first round of the questionnaire, the presenter continued, an agreement above 85% was reached for each framework section. Ten trialists involved in contemporary endovascular trials took part in pilottesting the framework.
Ultimately, Zywicka reported, the Endo-STAR framework was structured into six main sections: 1) expertise of the professional delivering the intervention, 2) setting/location of the intervention, 3) anaesthesia provided for the intervention, 4) imaging used at the time of the intervention, 5) intervention components: access, crossing the lesion, treating the lesion (including specific steps and details related to all currently available endovascular devices) and closure of the artery, and 6) pharmacological interventions.
In discussion time following Zywicka’s talk, jury member Jon Boyle (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) asked how the framework might help in terms of monitoring long-term outcomes of endovascular interventions, to which the presenter responded: “What we are aiming to achieve with this framework is to know what has been done in a trial […] we expect that actually being able to know and clearly describe what was done in a trial can be used to easily compare trials and even potentially compare results in the long term as well.”
support and depression, in the treatment of cardiovascular disease.
“The focus has been on specific
This study shows that it is time to see patients with PAD in a multidimensional way, such that a multidisciplinary team needs to get involved in their management”
devices to open blockages or do bypasses,” says corresponding author Carlos Mena-Hurtado, associate professor of medicine (cardiology) at Yale. “This study shows that it is time to see patients with PAD in a multidimensional way, such that a multidisciplinary team needs to get involved in their management.”
The study was published online ahead of print in the Journal of Vascular Surgery

Carlos Mena-Hurtado


www.ivascular.global
NEW STUDY DATA SUGGEST that the interventional treatment of symptomatic carotid artery disease may result in an improved rate of age-adjusted overall survival compared to medical management. However, the same study—presented at this year’s European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland)—also indicates a relatively low future ipsilateral stroke risk among patients who were not offered carotid intervention, highlighting medical management’s importance as a standalone strategy in patients deemed to be ‘high risk’ if treated via intervention. Findings from the study in question were delivered by Eric T A Lim (Waikato Hospital, Hamilton, New Zealand), on behalf of Manar Khashram (Waikato Hospital, Hamilton, New Zealand) and other investigators.
“There is Level 1 evidence around the management of symptomatic carotid artery stenosis, with superiority of intervention compared to medical therapy for stroke prevention,” Lim and colleagues state in their abstract.
“While, long term, the efficacy of carotid intervention has been clearly
documented, the natural history on symptomatic patients with ipsilateral carotid stenosis has not been well described in the contemporary era— when best medical therapy has evolved. With an ageing population and increase in frailty, information on medical management alone should be discussed with patients to assist in clinical decision-making.”
On this basis, Lim et al’s prospective, observational study—undertaken in a single vascular tertiary referral centre in New Zealand—aimed to assess the outcomes of symptomatic carotid artery stenosis cases that were managed nonoperatively. The study was carried out from June 2019 to January 2024, with all symptomatic carotid artery disease referrals at the centre being included, but cases with no carotid imaging or in which the patient subsequently underwent carotid surgery within six weeks of referral being excluded. Lim and colleagues performed Kaplan-Meier analyses to assess ipsilateral strokefree and age-adjusted Cox proportional hazard models, and ultimately determine all-cause survival.
During the study period, a total of 280 patients were referred with symptomatic
relevant’
carotid disease. The majority of these patients (63.7%) underwent carotid endarterectomy (CEA), while 1.3% underwent carotid artery stenting (CAS) and 35% were managed medically. In the 84 patients managed medically—or ‘conservatively’—the median age was 73.6 years. Lim et al found that the three most common reasons for selecting conservative management were the patient being below the threshold for the consideration of intervention (43.2%); internal carotid artery (ICA) occlusion/ near occlusion (25%); and poor neurological recovery post-stroke (11.4%).
the investigators report. However, there were five patients (6%) who developed a further ipsilateral stroke or transient ischaemic attack (TIA) following conservative management during the follow-up period. The majority of patients (65.5%) were commonly managed with dual antiplatelet therapy (DAPT) and statins, Lim et al relay.

Out of the 10 patients with poor neurological recovery post-stroke, only two went on to receive a delayed carotid intervention,
Larger cohorts are warranted to assess the longterm outcomes comparing surgical intervention to non-operative management in symptomatic carotid artery disease”
Findings from a retrospective analysis presented at ESVS 2024 provide evidence that—for symptomatic carotid artery stenosis patients—endarterectomy “remains a useful and relevant intervention in the era of improving medical therapy”.
DELIVERING THESE DATA AT ESVS 2024, Sashini Iddawela (University College London Hospital NHS Trust, London, UK) initially noted that urgent carotid endarterectomy (CEA) is currently the first-line recommendation for symptomatic, significant carotid artery stenosis. There is also speculation, however, that presentation with symptomatic stenosis could be substantially reduced thanks to today’s advancements in optimal medical therapy and anti-major cardiovascular event (antiMACE) medications.
As such, Iddawela and her colleague Daryll Baker—also of the University College London Hospital NHS Trust—undertook a study in an effort to determine whether or not patients undergoing CEA were already on optimal medical therapy prior to their index admission. They performed a retrospective analysis of patients receiving urgent CEA following development of a stroke or transient ischaemic attack (TIA) at a regional hub in Northwest London between 2021 and 2023,
collecting data on these patients’ index presentation of symptomatic stenosis features at a healthcare facility.
The researchers recorded data on comorbidities and medication history— including antiplatelets, anticoagulants, antihypertensives, and diabetic control. Medications at discharge were also recorded as well as any reasons for alteration. Proportions of patients on antiplatelets, antihypertensives and statins— both pre- and post-CEA—were compared using the chi-squared test. Optimal medical therapy was defined via the standard set by ESVS 2023 guidelines for asymptomatic stenosis: antiplatelets and statins as a minimum, with an antihypertensive added on where appropriate. A total of 124 patients were consecutively analysed. Hypertension and hyperlipidaemia were found to be the most common pre-CEA morbidities recorded (64% and 42%, respectively), followed
According to the investigators, patients who underwent carotid intervention had a 0.25 (0.17–0.5) hazard ratio of survival compared to patients managed conservatively— when adjusted for age. The overall survival rates at one and four years were 88% and 57%, respectively, and corresponding ipsilateral stroke-free survival rates at both one and four years were 97% in the medically managed patient group.
“These data support [the finding] that patients who were not offered intervention for symptomatic carotid artery disease, and were managed medically, had lower age-adjusted overall survival compared to patients undergoing carotid intervention,” Lim and colleagues conclude. “However, the risk of future ipsilateral stroke was relatively low compared to the landmark trials, highlighting the importance of medical therapy as a standalone strategy for those patients deemed high risk for carotid intervention. Further, larger cohorts are warranted to assess the long-term outcomes comparing surgical intervention to non-operative management in symptomatic carotid artery disease.”
by diabetes mellitus (36%) and ischaemic heart disease (23%). In addition, the majority of patients were on an antihypertensive agent or statin at index presentation (64% and 60%, respectively), and a 36% rate of antiplatelet use was also demonstrated across the cohort—with 77% of these patients being on aspirin monotherapy and the remainder being on clopidogrel/ticagrelor/prasugrel.

Overall, 36 patients (29%) were on a combination of an antiplatelet, antihypertensive and statin during their index presentation with symptomatic carotid stenosis. According to the researchers, there was no significant difference between the proportion of patients on antiplatelets, antihypertensives or statins pre- versus post-CEA. However, patients with ischaemic heart disease, diabetes or hypertension were observed as being significantly more likely to be on an antiplatelet (odds ratio [OR], 1.9; p<0.01), statin (OR, 1.25; p<0.01) or antihypertensive (OR, 1.76; p<0.01) at the point of their first presentation to a healthcare facility.
The researchers concluded that patients undergoing CEA are generally multimorbid and are also more likely to be on cardiovascular risk-modifying therapy at their index presentation with symptomatic carotid artery stenosis. And, on this basis, they aver that—even within the context of recent improvements in medical therapy for carotid disease—CEA should still be considered a useful and relevant intervention in these patients.



Gianluca Faggioli (University of Bologna, Bologna, Italy) and Gert J de Borst (UMC Utrecht, Utrecht, The Netherlands) went headto-head at the recent European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland) to debate a motion that the two-week carotid endarterectomy (CEA) target for symptomatic patients is ‘outdated’ and that evidence-based treatment is now achievable.
FAGGIOLI’S MAIN ARGUMENT, opening the debate, was that both clinical and anatomic factors should be used to determine the timing of intervention rather than a set threshold.
The presenter first addressed the origin of the two-week CEA target. “It’s come from the fact that the risk of recurrent stroke usually happens within 15 days,” he informed ESVS attendees.
Early intervention, Faggioli continued, is supported by a paper from Rantner et al, in which performing CEA within seven days is shown to reduce the risk of subsequent stroke.
However, Faggioli stressed that to fully answer the question at hand, it is important to understand that neurological symptoms in symptomatic patients are highly varied and must be categorised into stable and unstable, with stable symptoms being far more common.
Citing a review of the literature on patients with stable symptoms, Faggioli relayed that stroke and death rates among patients operated within 48 hours actually appear to be higher than those in patients operated later on, but not significantly so.
Regarding transient ischaemic attack
(TIA), the presenter continued, the literature and the guidelines suggest ascertaining the cause of the TIA before intervening. “After that,” he said, “you can intervene without a significant recommendation regarding the timing of the intervention”.
Faggioli also noted that intervening within 48 hours after a stroke incurs a far greater risk of complication than intervening later on.
However, the presenter stressed that there is a wide variety of data on this topic, adding also that in patients with more severe strokes and extended ischaemic lesions, the risks associated with intervening quickly “are very much increased”.
“We have to consider not only the presence of the cerebral lesions but also their size and the clinical appearance of these strokes,” Faggioli emphasised.
Furthermore, the presenter noted that there is a “significant” difference in outcomes for patients with large ischaemic lesions treated before and after four weeks.
Finally, in patients with more significant ischaemic lesions, Faggioli continued, European guidelines recommend deferring carotid
intervention, while the US guidelines advise against treating these patients.
“Symptomatic carotid patients can present with a variety of clinical and anatomical situations,” Faggioli said in his conclusion, emphasising that these must be central to any treatment decision. “Timing of revascularisation should be decided according to both clinical and anatomical factors.”
De Borst was in agreement that the two-week threshold is ‘outdated’.
Where his argument differed from Faggioli’s related to the available evidence, with the presenter highlighting its heterogeneity.
“First of all,” de Borst began, “I think it’s crucial that we discuss stable symptoms—that’s where the greatest benefit can be achieved.”
With this in mind, the presenter gave an overview of the available literature, starting with the oldest. He highlighted a high natural-cause risk apparent here. “In the past,” he explained, “relatively early surgery was considered to be associated with very high perioperative risk, and that was why CEA was delayed on purpose—to six weeks or more.”
This changed with an analysis from Rothwell et al, de Borst noted. “Not
Within studies there’s a very heterogeneous definition of the index event— this should be standardised so we can speak the same language”
— Gert J de Borst
The Society for Vascular Surgery Vascular Quality Initiative (SVS VQI) clinical registry recently announced the Carotid Care Quality Champion recognition programme.
THE PROGRAMME HONOURS
healthcare organisations across the USA dedicated to improving the safety and effectiveness of vascular care through participation in the VQI’s carotid artery stenting and carotid endarterectomy registries.
Today, select facilities across the country have been recognised as Carotid Care Quality Champions. These organisations engage the SVS VQI infrastructure to monitor the quality of care for their vascular patients and help them comply with the latest societal and governmental guidelines, including the change in carotid artery stenting coverage reflected in the Centers for Medicare and Medicaid Services’ (CMS) recent national coverage decision (NCD) 20.7.
As part of the CMS NCD 20.7 reimbursement modifications, the organisation requires centres
to establish a quality monitoring programme and maintain institutional and physician standards. Registry participation provides a robust and userfriendly approach to CMS compliance. A press release notes that the SVS VQI carotid artery stent registry is the gold standard for developing a carotid quality assurance programme, with registry participation enabling centres to ensure the safety of their patients and adhere to the CMS coverage decision.
“We are proud to launch the Carotid Care Quality Champion programme to honour organisations across the country that are demonstrating their commitment to exceptional patient care by maintaining a robust quality programme,” says Jens Eldrup-Jorgensen (Maine Medical Center, Portland, USA), SVS Patient Safety Organization (PSO) medical director. “By
everybody is aware where this 14-day threshold actually came from—it’s from this analysis,” he pointed out.
Here de Borst remarked: “If Rothwell would have chosen 10 days or 20 days, all of us would have been discussing that time frame.” On the two-week threshold, he continued: “It’s absolutely an arbitrary threshold.” De Borst commented that “it’s not bad to do a surgery at 15 days from symptoms—it’s only bad if you do surgery at 15 days and you have a very high perioperative stroke risk, and then you’re not providing any benefit to your patients”.
Since the Rothwell analysis, de Borst continued, a meta-analysis from Sweden of 12 studies including more than 230,000 patients—which the presenter noted “caused a lot of noise in our field”—revealed “very high and alarming” stroke and mortality rates resulting from operating within the first two days post-index operation. “This is astonishing, this should not be true,” he said. Following this meta-analysis, de Borst noted that several subsequent studies “showed a completely different picture”.
Referencing four randomised controlled trials on symptomatic patients, de Borst highlighted that stroke and death rates both within and beyond seven days show that surgery is safe in both time frames, while rates with stenting are “alarmingly high”.
Citing some of the most recent data, de Borst put forward a central component of his argument: “In my view, the most critical point is that within studies there’s a very heterogeneous definition of the index event—this should be standardised so we can speak the same language.”
Here he reiterated the “arbitrary” nature of the two-week cut-off, aligning with the view put forward by Faggioli, while going on to stress that “there’s still no strong evidence to change policy today”.
harnessing the power of data with benchmarked reports from over 900 centres, these centres have access to the best resources available for quality assurance and focused quality improvement efforts.”
Since the SVS VQI’s inception, clinical data gathered from participating centres has dramatically impacted patient care, leading to scientific discoveries that have changed the way in which care is delivered. Participating centres include academic medical centres, teaching hospitals, community hospitals and office-based labs.
A press release details that VQI participation offers centres the ability to:
● Track provider and centre performance and compare to national benchmarks
● Identify areas for quality improvement, including patient outcomes and length of stay
● Participate in a robust quality improvement programme, with access to quality improvement toolkits, quality charters, webinars and one-on-one mentoring for members
● Network with other participating centres to review outcomes data, foster collaboration and spark further clinical innovation
Matteo Tozzi (Varese, Italy) recently spoke to Vascular News about the role of drug-coated balloons (DCBs) in vascular access.
The Aperto® over-the-wire (OTW) DCB from Cardionovum—which is available in lengths of 20, 40 and 60mm and diameters from 4 up to 12mm—was developed specifically to solve unmet clinical needs in the treatment of haemodialysis access stenosis and recanalisation of arteriovenous fistula shunt grafts.
Tozzi, who is a full professor of vascular surgery at the University of Insubria, started using the Aperto in his vascular access practice over 10 years ago and is planning to soon publish his clinical experience in a study called Aperto 600.
Today, Tozzi explained, there is a pressing need for a device such as the Aperto. Haemodialysis patients now survive far longer than they used to, and therefore the key goal at present is to maintain patency of the access circuit to ensure that it can be used for several procedures over a long period of time.
“This goal has been achieved,” Tozzi reported, attributing this result to the Aperto. In his clinical practice, Tozzi shared that patients have a patent vascular access circuit without restenosis at one year after DCB treatment in the vast majority of cases.
Previously, Tozzi said, by way of comparison,
treatment being extended, with the average time in his centre now being eight months and only 6% of patients undergoing two or more procedures over the course of a year. “This is important for the patients, because it means less time in hospital,”
Tozzi commented.


treatment was limited to percutaneous transluminal angioplasty (PTA) alone, which at his centre resulted in no patients having a patent vascular access circuit at one year. The results with DCB, however, are “incredible”.
Tozzi was keen to stress that DCB is not the “final cure” for intimal hyperplasia. However, he underlined the importance of focusing on what the treatment option does offer in this regard. Significantly, he said, Aperto results in the mean time from first to second
While the efficacy of DCBs has been proven to be superior to that of PTA for these patients, there are limited data on their economic benefit. To rectify this, Tozzi noted that he has started a collaboration that aims to assess the cost benefit of DCBs in the vascular access failure population using the data coming from his Aperto 600 study combined with data from a metaanalysis of the available literature. The study looked at PTA alone compared to PTA with DCB.
He described the preliminary results from this economic analysis as positive, citing less time in hospital and less money spent on procedures, leaving more space in hospital to treat new patients and providing an opportunity to reduce waiting lists. “It is important to have the correct allocation of money and resources, so that we can treat more patients with less money,” he remarked.
Finally, Tozzi looked ahead to future guidelines regarding vascular access in haemodialysis patients. He highlighted the fact that the evidence base for DCBs continues to grow, and that the scientific community is waiting for this treatment option to be added to existing recommendations.
Tozzi concluded that, in his opinion, DCBs should now be viewed as a “first-line” treatment for stenosis and vascular access failure in haemodialysis patients.
Disclaimer:
The efficacy and safety of these products have not been confirmed by US Food and Drug Administration (FDA)-approved research.

Following his presentation last year of 12-month results from the “landmark” LIFE-BTK randomised controlled trial, Ramon Varcoe (Sydney, Australia) speaks to Vascular News about this research as part of his wider career in vascular surgery so far. The vascular surgeon at Prince of Wales Hospital and full professor at the University of New South Wales also considers some of the biggest challenges currently facing the specialty, details his hobbies and interests outside of medicine, and reflects on how past experience in professional athletics has influenced his current work.
Why did you choose to pursue a career in medicine and what drew you to vascular surgery?
I come from a family with no history in medicine, so it surprised everyone when I chose that path at a young age. It’s hard to articulate why I was so strongly called to this career; sometimes, you just know things deep within.
Initially, I was drawn to psychiatry, which still fascinates me, but I ultimately prefer hands-on work, visualising anatomy in three dimensions, and solving problems. Vascular surgery, with its complex operations and the need to manage major blood vessels during critical moments, really appealed to me. At that time, the field was undergoing significant changes, shifting from traditional open surgery to an endovascular approach. I saw this as a perfect opportunity to be part of that transformation, where creativity and academic innovation were essential for advancing the specialty. I thrive in creative environments and have a passion for the scientific process.
Who were your career mentors and what was the best advice that they gave you?
I’ve had so many influential experiences that it’s tough to choose just a few. My earliest exposure to vascular surgery was with a pioneer in open surgery and transplantation in Sydney, John Frawley. He was a largerthan-life figure with a wealth of stories and an incredibly creative approach to surgery that was truly infectious. His inspiration shaped my early career.
In Adelaide, John Anderson was one of the early endovascular pioneers who demonstrated what’s possible when you think outside the box. At Westmead in Sydney, Professor John Fletcher became my first academic mentor, guiding me toward a lifelong passion for inquiry and a career in academia.
Geoff White, an endovascular aneurysm repair (EVAR) pioneer, and I collaborated on several conferences before his passing. The VERVE Symposium reflects much of what I learned while working alongside him. More recently, I’ve been fortunate to be mentored by Drs Michael Jaff and Peter Schneider, two giants in our field. They have both supported me and imparted invaluable lessons as my career has evolved.
What have been some of the most important developments in vascular surgery over the course of your career so far?
When I began my career in vascular surgery, it was primarily an open surgical specialty. EVAR was just emerging, and vascular
surgeons rarely performed peripheral endovascular interventions, especially complex ones. We had large, dedicated vascular wards filled with dozens of inpatients, many facing piecemeal amputations that often led to belowknee or above-knee amputations. Our intensive care units (ICUs) were crowded with vascular patients, and large-scale abdominal surgeries, particularly open abdominal aortic aneurysm (AAA) repairs, were routine, both elective and emergency.
That landscape has changed dramatically. We now admit only a fraction of those patients, and major amputations are rare at our centre. We often feel out of place in the ICU since we’re there so infrequently. The endovascular revolution has significantly improved patient care. Now, over 90% of my patients are likely to receive an endovascular-first procedure, with very few needing open surgery. This shift is beneficial for both patients and the specialty as a whole.
What are the biggest challenges currently facing vascular surgery?
I believe the biggest challenge we face now is how to manage the future of open vascular surgery. There are several key issues to consider. First, how do we ensure high-quality training for our trainees when open surgeries are becoming less common? Procedures like open abdominal aortic repairs, thoracoabdominal repairs, and distal bypass surgeries come to mind as complex operations that require a high level of technical skill. Unfortunately, these surgeries are rarely performed in most contemporary practices, and when they are, they often represent the most challenging cases. This makes training increasingly difficult.
Moreover, senior surgeons may feel less confident in supervising these procedures if it’s been a while since they last performed them. Over time, this could lead to newly graduated surgeons having less experience, further exacerbating the issue.
I’m also noticing a trend where vascular surgeons are positioning themselves as either endovascular or open surgery advocates. My perspective is that we need a balanced approach in our practice. Both endovascular and open techniques are valuable, and each may be the best treatment option for different patients at different times. I see them as complementary skills that every vascular unit should strive to provide at the highest standard.
At TCT 2023 you presented results from the LIFE-BTK trial, which were simultaneously published in The New
Consultant vascular surgeon, Prince of Wales Hospital (Sydney, Australia)
Full professor, vascular surgery, University of New South Wales (Sydney, Australia)
Course director, VERVE Symposium
QUALIFICATIONS
1997: Bachelor of Medicine, Bachelor of Surgery, University of Adelaide (Adelaide, Australia)
2004: Master of surgery, University of Sydney (Sydney, Australia)
2007: Fellow of the Royal Australasian College of Surgeons, vascular surgery
2016: Doctorate of Philosophy, University of New South Wales
2021: Master of Medicine (Clinical Epidemiology), University of Sydney
MEMBERSHIPS (SELECTED)
Full member, ANZ Society of Vascular Surgery
Full member, International Society of Endovascular Specialists
Full member, International Society on Thrombosis and Haemostasis
Full member, International Society for Vascular Surgery
England Journal of Medicine (NEJM)
What are your reflections on this trial one year on and what is next in the space?
This is one of the landmark randomised controlled trials in our field for several reasons. The endovascular treatment of infrapopliteal peripheral arterial disease has struggled with durability, and recent trials aimed at demonstrating that new technologies could provide better patency than simple angioplasty have largely failed. However, LIFE-BTK demonstrated a 30% risk difference in the primary endpoint, favouring the Esprit drug-eluting resorbable scaffold [Abbott] over angioplasty. This success led to US Food and Drug Administration (FDA) approval and subsequent commercial availability in the USA and parts of Asia, with more regions expected to follow. This development has the potential to revolutionise treatment options for patients with chronic limb-threatening ischaemia. Twelve months later, we’re preparing to present the 24-month results at Vascular Interventional Advances (VIVA; 3–6 November). We will also share several related studies, including an economic analysis, a diversity, equity, and inclusion assessment, and an anatomical analysis using the Global Limb Anatomic Stating System (GLASS).
Additionally, several fast followers are emerging, with new scaffolds currently being evaluated in various phases of clinical trials, both above and below the knee. This has become an exciting area to watch and is definitely worth following.
You have been the lead investigator on several other trials. Are there any that have surprised you in their findings? Our initial research on the previous generation of drug-eluting bioresorbable scaffolds revealed patency results in tibial arteries that surprised us all. This work paved the way for the development of the Esprit scaffold, which we utilised in the LIFE-BTK trial. The outcomes of that trial astonished even me. Despite my over 10 years of experience with these devices, I never anticipated a 30% difference in the primary efficacy endpoint. This finding highlights the effectiveness of these devices in the infrapopliteal circulation.
You are founder and course director of the VERVE Symposium—how has this meeting evolved since its first iteration 12 years ago?
VERVE has matured significantly over the past 12 years. What began as a small gathering of around 100 people at Coogee Beach has now evolved into the largest vascular and

endovascular congress in the region. We draw faculty and delegates from across the globe, showcasing over 300 presentations and featuring more than 15 live cases. The event also has a vibrant social aspect, with a popular harbour ferry tour and a gala dinner with live music that everyone is welcome to enjoy.
Could you outline one of your most memorable cases?
Although my practice now primarily emphasises endovascular techniques, I still have a passion for performing major open surgeries. I particularly enjoy open thoracoabdominal repairs and continue to use this approach for select younger patients. I recently completed one on a man in his late 30s under hypothermic arrest conditions. The procedure took 15 hours, but he was discharged within 14 days and has had an excellent outcome.
What advice would you give to someone looking to start a career in medicine? There are two quotes that resonate with me
when reflecting on a career in medicine, and I believe they offer valuable advice for anyone considering this path.
The first pertains to choosing the right professional direction:
“Find a job you love, and you will never have to work a day in your life.” — Confucius.
The second emphasises the importance of service, which is a core principle behind pursuing a career in health:
“Service. Let this word accompany you throughout your life. May it guide you as you seek your purpose and responsibilities in the world. Remember it when you’re tempted to forget or overlook it. It may not always be a comfortable companion, but it will always be a faithful one, leading you to happiness regardless of your experiences. Keep this word not just on your lips, but in your heart. Let it teach you to do good simply and humbly.” — Albert Schweitzer.
You had a previous career in professional athletics—how has this influenced your career in vascular surgery?
“Vascular surgery, with its complex operations and the need to manage major blood vessels during critical moments, really appealed to me”
Professional sports impart valuable lessons, such as the importance of hard work and dedication, the pursuit of excellence, and the drive to compete at your best every day. They teach you how to turn failure into immediate learning opportunities for future growth, which is essential for continual improvement. Additionally, sports highlight the significance of camaraderie and relationships. My experience as an athlete has greatly influenced my career, making me a better surgeon.
What are your hobbies and interests outside of medicine?
I have a passion for food and wine, especially when dining out while traveling in different countries. I also enjoy cooking at home and collecting wine to age in my cellar.
Water activities are a big part of my life; I love scuba diving, sailing, boating, and deepsea game fishing. Additionally, I collect art, which decorates both my office and home. Recently, I’ve taken up road cycling, which I enjoy around Sydney and during my travels abroad.
Complex peripheral chronic total occlusions (CTOs) represent a daily challenge for interventionalists, necessitating a standardised approach for optimal patient outcomes. A team of experts has developed such a protocol—published in 2023 in JACC: Cardiovascular Interventions. Interventional vascular specialist Andrej Schmidt (University Hospital Leipzig, Leipzig, Germany), one of the co-authors of the algorithm, has extensive experience in this field. The BeBack crossing and re-entry catheter is bringing a change to this algorithm in Schmidt’s practice, adding simplicity with its multitool function and giving the option to either stay intraluminal or go subintimal with a re-entry.
Integrating the BeBack before advanced techniques
Schmidt notes that his centre is one of the largest in Germany for the endovascular treatment of peripheral arterial occlusive disease and receives a high number of complex cases. He describes the BeBack as a “reliable” crossing and re-entry device for the treatment of such cases, noting its “slim yet stable” design.
Schmidt uses the device first and foremost as a crossing tool, and secondly as a bailout option for reentry when going subintimal. “We try harder now to stay intraluminal and BeBack is one of the tools that we use for that,” he states. For example, the device can be used in cases of calcified CTOs where wire passage with catheters and CTO wires will not work. While these are “really selected cases,” Schmidt says, they are becoming more common.
For long CTOs, especially if they are complex, calcified lesions, Schmidt remarks that it does not
CTO crossing algorithm
make sense to stay intraluminal. In these situations, he says, the BeBack can be used as a re-entry device.
Summarising his approach, Schmidt shares the following guidance for using the BeBack prior to the advanced technique stage of the algorithm: for short occlusions, stay intraluminal and use it as a crossing device; for long occlusions, go subintimal and use it as a re-entry device.
According to Schmidt, the algorithm can be expanded depending on the morphology and size of the occlusion in question, with the BeBack often coming into play. “Once you get into the situation where you have to go retrograde,” he says, “then the BeBack can be incorporated into a more detailed algorithm.”
Schmidt opines that the BeBack has changed the CTO crossing algorithm for the better, making interventions simpler, quicker, and more reliable. For example, Schmidt notes that the device is easier to use for re-entry than earlier options. As a result, he
In their 2023 JACC: Cardiovascular Interventions paper, Schmidt and colleagues set out a step-by-step guide to crossing CTOs.
1. The authors first advise choosing an optimal access site, evaluating the CTO by computed tomography (CT) angiography, and evaluating the proximal/distal cap morphology and other angiographic parameters predicting feasibility of antegrade passage.
2. As a next step, Schmidt et al suggest opting for an antegrade approach initially and then, in the event of antegrade failure, attempting retrograde access. The authors recommend changing strategy to an advanced technique and/or re-entry device in the event of failure of both antegrade and retrograde access.
3 Subsequently, in the event of success, Schmidt et al encourage proceeding with either treatment from antegrade (if antegrade was used initially) or wire externalisation and treatment from antegrade (if retrograde was used initially). In the event of failure—specifically if no progress has been made after three hours or in the instance of high radiation or contrast dosage, patient discomfort or vessel-specific complications—the authors advise stopping the procedure and instead changing course to a conservative treatment strategy or planning either redo or open surgery.
“By establishing the algorithm in the daily routine of endovascular specialists, improvements in vessel and patientspecific outcomes are anticipated,” Schmidt et al write. The authors go on to state that “future research and continued collaboration between experts is warranted”.

says, “it can be used in a much larger variety of cases”.
He continues that the device has shortened procedure times in the event of antegrade and retrograde failure. “If we cannot go through using either an antegrade or a retrograde approach, we can go directly to one balloon from one side and the BeBack from the other side,” he explains, comparing this to having to use two balloons when the device is not part of the procedure. In addition, due to the 4Fr size of the device, Schmidt details that it can be taken through tibial sheaths in complex retrograde-access cases, “which has made the intervention faster and more reliable”.
Listing the indications for the BeBack, Schmidt shares that he has used the device in superficial femoral artery (SFA), below-the-knee (BTK) and venous lesions, as well as intraluminally in the iliacs. On the latter, he comments: “With re-entry devices we hesitated in the past because in the iliacs we don’t want to puncture in the wrong direction. Using the BeBack we now feel more comfortable, because it’s slimmer than other re-entry devices.” He continues that using the device in this morphology makes for a “more elegant” procedure.
Schmidt has considerable experience using the BeBack in iliac arteries. In fact, his first experience with the device was during the treatment of a CTO of the common iliac artery in an abdominal aneurysm patient. “We failed to get through the CTO coming from retrograde, cross-over and antegrade using an arm-access,” he recalls. “Nothing worked, until eventually we used the BeBack catheter via the retrograde approach. With this, device passage through the CTO back into the aorta succeeded immediately. This experience was an eye-opener for us with regard to the success of this catheter.”




• BeBack with 2.9 F or 4.0 F for retrograde, antegrade and crossover approach
• Needle out of the tip in adjustable length and direction
• The effective low profile catheter for support, crossing and re-entry

During a satellite symposium session dedicated to real cases and trial outcomes which was held at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (14–18 September, Lisbon Portugal), experts presented compelling data from large registries and randomised controlled trials (RCTs) which suggest that the Eluvia drug-eluting stent (DES, Boston Scientific) should be preferred when treating the superficial femoral artery (SFA).
The Münster registry was led by Giovanni Torsello (St. Franziskus Hospital, Munster, Germany)—his son, Giovanni Federico Torsello (University Medical Center Göttingen, Göttingen, Germany) presented his research in his absence at the CIRSE annual congress.1 Torsello began by stating that the goal of real-world registries is to generate data which can be extrapolated to daily practice, considering all of the variables that are encountered day-to-day. To reflect this, the Münster registry assessed the performance of the Eluvia DES in long and complex femoropopliteal lesions, and assessed consecutive patients treated between 2016–2018 for stent primary patency.

his team often opt for upfront stenting of calcified occlusions paired with PTA to expand the DES. He added that short balloons should be used to exert more force in the segments which are heavily calcified and stenosed and promoted the use of a DES in this setting to benefit from lower rates of restenosis.
Taking what has been learnt and placing it within the context of the Münster registry, Torsello shared that the Eluvia DES showed 71% primary patency at two years, which increased
to 78% if the entire lesion is covered by the DES, underlining its successful deployment in long lesions.
As published in the journal CardioVascular and Interventional Radiology, the primary patency rate for the Eluvia DES was 65% at five years, with a clinically driven target lesion revascularisation (CDTLR) and major amputation rate of 79% (Figure 2) and 96%, respectively.
Sustained high patency rates
“What we have seen is that you can successfully treat the SFA and downgrade patients’ disease with the Eluvia DES. What is important is a sustained high patency rate which we have achieved here, with results which are not unlike that of bypass but by endovascular means—it’s amazing to see,” Torsello commented.
Overall, he stated that the Eluvia DES offers a good bailout strategy in cases with a sub-optimal vessel preparation result such as dissection or recoil, or in complex lesions. In light of their long-term registry data, Torsello stated that he looks forward to the RCT data to come in this arena which he believes will bear out their outcomes and bolster Eluvia within the procedural standard.
The criteria for using the Eluvia DES, Torsello noted, was to treat recoil post-percutaneous transluminal angioplasty (PTA) or flow-limiting dissection post-PTA, labelling the registry the “bailout Eluvia DES study”. The likelihood of needing a bailout stent increases due to lesion length, chronic total occlusion (CTO) and calcification.
In the Münster all-comer cohort, 130 patients were enrolled totalling 137 lesions with a mean length of 194mm; 21% of included patients had a Rutherford classification of 5–6; 67% were identified as having a PACSS classification of 3 or 4; and 74% of patients had total occlusion of the SFA. Patients with TransAtlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) A/B lesions and those with in-stent restenosis were excluded.
Key lessons from the Münster registry Using a selection of cases which exemplify the Eluvia DES’s efficacy in a range of scenarios, Torsello pulled out key lessons from his experience treating long CTOs and displayed an exemplar case to depict this (Figure 1). He stated that a DES is necessary in these patients, as “if you don’t use a scaffold, you will not have mechanical stability— and if you use a scaffold without eluting an antirestenotic drug, you will see in-stent restenosis”.
“What about calcium?” Torsello asked, moving on to show the “more difficult” calcification cases, which pose the added challenge of drug uptake and impaired expansion. He stated that, in these cases,
What is important is a sustained high patency rate which we have achieved here, results which are not unlike bypass but by endovascular means —it’s amazing to see”

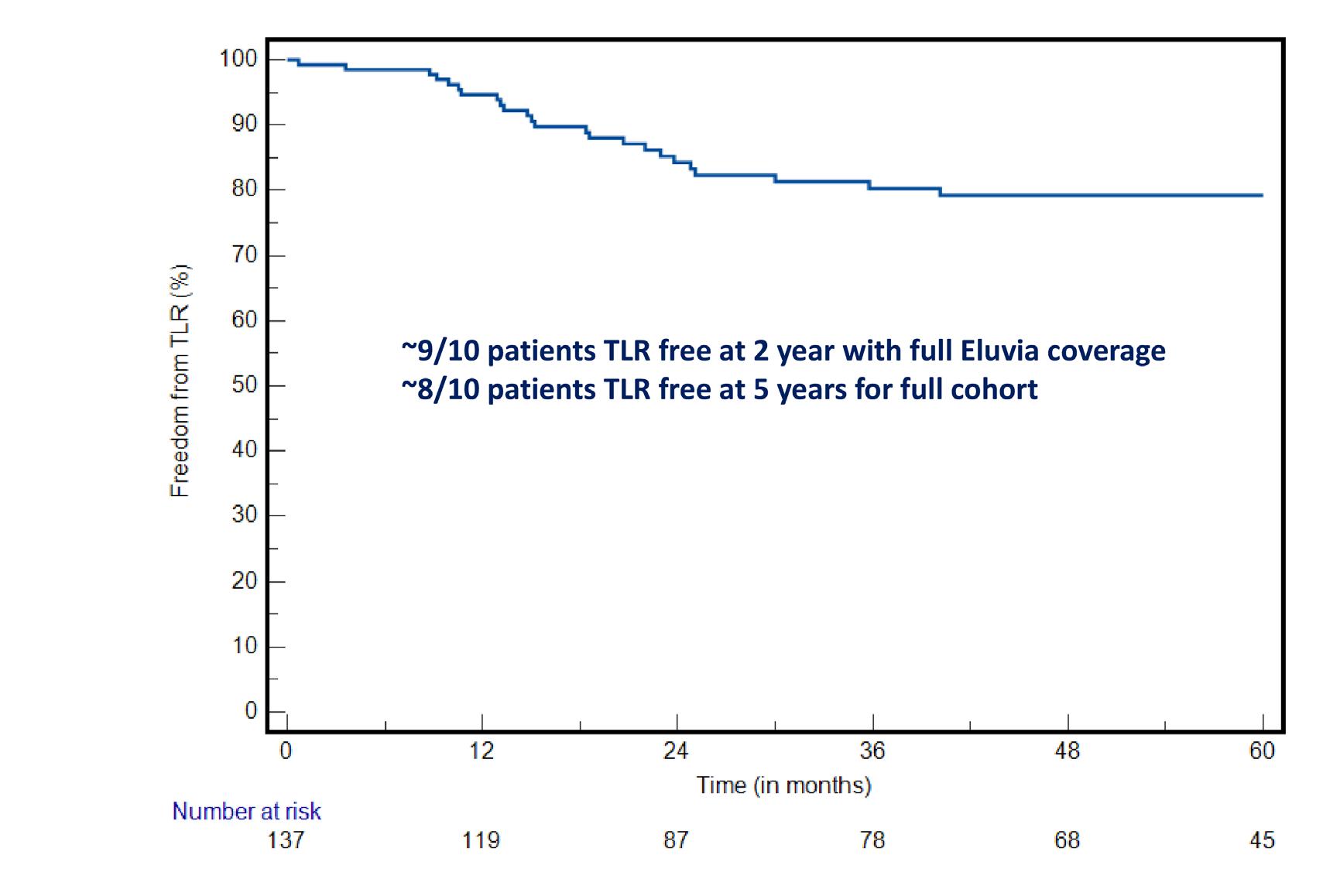
“Truly long lesions have been underrepresented in randomised controlled trials [RCTs], so there were no definitive data on primary treatment strategy or decision making—the SPORTS trial was born out of limitation of current data on TASC C and D lesions for endovascular intervention”, were the opening statements of Gunnar Tepe (Klinikum Rosenheim, Rosenheim, Germany) who also presented during the satellite symposium session, sharing insights from the SPORTS RCT using the Eluvia drug-eluting stent (DES) in complex lesions.2
Initially, Tepe brought attention to the EMINENT and IMPERIAL (Boston Scientific) RCTs, which each showed superior primary patency when using the Eluvia DES in the superficial femoral artery (SFA).
The EMINENT trial—the largest RCT to compare drug-eluting to self-expanding bare metal stents— randomised 508 patients, and reported that Eluvia demonstrated superiority over bare metal stents, with a statistically significant primary patency of 85.4% versus 76.3% through to one year. The IMPERIAL trial, involving 465 randomised patients, compared the Eluvia DES to Zilver PTX (Cook Medical). At one year, the Eluvia device demonstrated a primary patency rate of 86.8%, compared to 77.5% for Zilver PTX. The longer sustained drug release from the Eluvia system was highlighted as a key differentiator.
SPORTS data updates DES patency
Tepe then situated the SPORTS RCT within the arena of other Eluvia trials, commenting that IMPERIAL and EMINENT were “early” explorations and concerned short lesions which minimised its broader application. Since then, these data have been updated with those gained from later trials, such as IMPERIAL Long and SPORTS, which have demonstrated the pertinency of the Eluvia DES in complex lesions while maintaining high patency rates.
The SPORTS RCT was a prospective, randomised, multicentre trial which compared the angiographic and clinical outcomes of TASC C/D lesions in the SFA after treatment with the Eluvia DES and the SeQuent Please OTW (BBraun) drug-coated balloon (DCB), or a bare nitinol stent. Tepe—the trial’s principal investigator—and colleagues enrolled 224 patients with a mean lesion length of 228mm and a Rutherford classification of 2–4; 69% of patients were identified as having a PACSS classification of 3/4 and 76% had total occlusion of the SFA.
At one year, concerning angiographic stenosis, the data revealed superior results for the Eluvia DES (Figure 3) when compared to the bare nitinol stent. The SeQuent DCB was non-inferior to the bare nitinol stent. Of their secondary endpoints, Tepe noted that late lumen loss at 12 months “differed significantly” between treatment groups, however the least loss was reported in the Eluvia DES group.
In his response to Brodmann’s question, Torsello emphasised post-interventional medication and the lack of uniformity in its usage. He stated that, although studies such as VOYAGER have provided data on this, its takeaways “don’t apply to every patient”. Additionally, Torsello stated that research concerning the profunda femoris artery would be interesting, which is an area often overlooked in studies concerning the common femoral artery.
In conclusion, the Münster registry and SPORTS RCT presented at CIRSE provide compelling evidence for the sustained efficacy of the Eluvia DES in treating long, complex SFA lesions. The high primary patency rates displayed in these trials underscore the effectiveness of this endovascular approach.
Figure 3: One-year follow up angiography. Left leg treated with Eluvia randomised in the SPORTS RCT, right leg treated at a later stage with DCB. Left leg shows no neointimal formation unlike the right DCB-treated leg.
This, Tepe stated, is due to the price of target lesion revascularisation (TLR) which can cost more than 22,000 euro per procedure.3
He concluded his talk by asserting that further analysis is underway to investigate the “astonishing” number of TLRs and failures in the DCB group, to create core-lab adjudicated “failure modes” for each treatment strategy.
Which gaps should future data fill?

Tepe situated the SPORTS RCT among others that have investigated the use of nondrug-eluting stents, showing that patency rates decrease when these are used in longer lesions. “With the Eluvia DES we have a really good patency even in those long, hard-to-treat lesions,” he continued.
“The SPORTS RCT provided new data on primary strategies in TASC C/D lesions and confirmed the Eluvia DES as superior to operator ‘best choice’ bare metal stenting for full lesion coverage,” Tepe stated. He described how the use of a DCB with or without bailout spot stenting as a strategy was non-inferior to full lesion bare metal stenting, however long bare metal stent restenosis remains “problematic”.
Following Tepe’s talk, session moderator Marianne Brodmann (Medical University of Graz, Graz, Austria) asked the presenters what data is still missing concerning treatment of the SFA and what is needed now to guide daily practice and build a treatment algorithm. Tepe highlighted vessel preparation and the lack of data there, and noted that data to determine which device is superior in this setting would be beneficial, but conduction a study on this would be “very difficult”.
Sabine Steiner (Medical University Leipzig, Leipzig, Germany)—who also presented insights from the BEST SFA trial cases during the session—answered next, agreeing that the heterogeneity of SFA lesions makes conducting clinical trials in this space near impossible. “We need extremely good registry data with core-lab adjudication from angiogram. This is a prerequisite, because we know what investigators are reporting is in part ‘wishful thinking’,” Steiner said. She stated that intravascular ultrasound (IVUS) might assist in this area in the future, to “truly see what has an impact on our outcomes”.
The SPORTS RCT provided new data on primary strategies in TASC C/D lesions and confirmed the Eluvia DES as superior to operator ‘best choice’ bare metal stenting for full lesion coverage”
The Eluvia DES has demonstrated its value as a reliable bailout strategy in challenging cases, particularly where vessel preparation is suboptimal. Looking ahead, further research, including further RCTs and improved real-world registry data, will be crucial in addressing current challenges, such as restenosis in bare metal stents and high TLR rates in DCB treatments.
References 1. Muenster: Stavroulakis, Konstantinos & Torsello, Giovanni & Bosiers, Michel & Argyriou, Angeliki& Tsilimparis, Nikolaos & Bisdas, Theodosios. (2021). 2-Year Outcomes of the Eluvia Drug-Eluting Stent for the Treatment of Complex Femoropopliteal Lesions. JACC: Cardiovascular Interventions. 14. 692-701.10.1016/j.jcin.2021.01.026.
2. Tepe, G. SPORTS Trial: Drug Eluting Stent or Primary Bare Nitinol Stent Application versus Drug Coated Balloons in Long SFA Lesions. Presented at TCT 24 Oct 2023.
3. Saratzis et al. COST Analysis of Target Lesion Revascularisation in Patients With Femoropopliteal In-Stent Restenosis or Occlusion: The COSTLY-TLR Study. Eur J Vasc Endovasc Surg. 2024 Feb 6:S10785884(24)00160-6. doi: 10.1016/j.ejvs.2024.02.001. Epubahead of print. PMID: 38331163.
Merit Medical has announced positive six-month findings from the randomised arteriovenous (AV) fistula arm of its Wrapsody arteriovenous access efficacy (WAVE) pivotal trial. The data were shown at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (14–18 September, Lisbon, Portugal) during a FIRST@CIRSE presentation.
WRAPSODY IS A CELLimpermeable endoprosthesis which is intended to extend long-term vessel patency in dialysis patients.
The AV fistula arm of the WAVE trial enrolled 245 patients at 43 sites. Patients were randomised 1:1 to Wrapsody or percutaneous transluminal angioplasty (PTA).
Merit shares in a press release that target lesion primary patency in patients treated with Wrapsody was 27 percentage points higher than patients in the PTA cohort (89.8% vs. 62.8%, p<0.0001). The proportion of patients who experienced an adverse event was similar between cohorts.
“The superiority of the six-month efficacy data is compelling and provides clinicians the chance to evaluate how Wrapsody can help us
prolong the vascular access of our patients. Wrapsody should be the new standard of care for these patients,” said Mahmood K Razavi (St Joseph Heart and Vascular Center, Orange, USA), co-principal investigator of the WAVE trial.
“Meeting with colleagues at CIRSE to discuss the AV fistula arm of the WAVE study was the first of what we hope to be many productive discussions,” said Robert G Jones (Queen Elizabeth Hospital Birmingham, Birmingham, UK), coprincipal investigator of the WAVE study. “The potential for Wrapsody to help us safely extend vascular access for our patients is a vital component of care.”
“The data release of positive findings from the randomised AV fistula arm
of the WAVE study marks a major milestone for Merit,” said Fred P Lampropoulos, Merit’s chairman and chief executive officer (CEO). “This is an important next step in our continued efforts to seek ways to improve care for patients requiring haemodialysis treatment.”
Merit advises in a press release that the Wrapsody cell-impermeable endoprosthesis is not approved or available for commercial distribution in the USA and may not be approved or available for sale or use in other countries. In the USA, the device is being used under an investigational device exemption (IDE) from the US Food and Drug Administration (FDA).
The company notes that findings
from the WAVE study expand on results from the first-in-human study (WRAPSODY FIRST) and support the premarket approval (PMA) application to the US FDA for commercial use in the USA. The Wrapsody device is available in Brazil and in the European Union.
Wrapsody should be the new standard of care for these patients”
— Mahmood K Razavi

the next
At the Controversies in Dialysis Access (CiDA) 2024 meeting (3–5 October, Washington D.C., USA), Robert Shahverdyan (Hamburg, Germany) gave a presentation sharing the one-year results of the VENOS-1 first-in-human study, which examined the Velocity (Venova Medical) percutaneous arteriovenous fistula (pAVF).
BEGINNING BY OUTLINING THE background of pAVFs, Shahverdyan pointed out that, whilst there are currently two US Food and Drug Administration (FDA) approved devices available— WavelinQ (BD) and Ellipsys (Medtronic)—the lack of worldwide adoption was, in his opinion, a “missed opportunity” that has led to decreased usage of pAVFs over the past couple of years.
After outlining his algorithm for the use of pAVFs and sharing some of his own experiences and results with their use from the last six years, Shahverdyan conceded that the main challenges with pAVFs are the issues with cannulation and a high rate of reinterventions (such as flow directing procedures and/or juxtaanastomotic stenosis angioplasties), which consecutively increase the costs for pAVFs.
Following on from this, he stated that what was needed from the next generation of pAVFs was a
revaluation of cost-effectiveness, fewer interventions after pAVF creation, and an improved procedural efficiency without compromising on efficacy.
A device that he feels fulfils this brief is Velocity—the “generation 2.0 pAVF”. Shahverdyan stated that it is able to replicate surgical AVF anatomy, via its optimal geometry that allows for ideal flow conditions to support fast maturation, and a low incidence of anastomotic stenosis thanks to the endothelium being shielded from turbulence, inflammation or thermal injury.
The first-in-human trial for this device, VENOS-1, commenced in 2023, with enrolment being completed by October of the
same year. It consisted of two cohorts; with the last cohort of 10 patients treated by the updated and final design, and now completed the final one-year follow-up visits. All 10 procedures were technically successful, with all of them being performed under regional anaesthesia. There were no serious adverse events at six weeks, as well as no instances of hand ischaemia.
What was interesting, according to Shahverdyan, is that the ultrasound findings showed that there was a 1:1 relationship between arterial flow and superficial venous flow, confirming a targeted AVF flow, and that 100% of the pAVFs matured within six weeks, with no re-interventions needed to reach this endpoint.
The trial results also demonstrated 80% of primary patency at one year, with only two reinterventions for the entire cohort in the first year, including a single angioplasty for a juxtaanastomotic stenosis at day 242.

With that, Shahverdyan concluded that the Velocity pAVF system demonstrates the least inflammatory way of creating an AVF with the implant shielding the juxtaanastomotic segment from turbulence and developing a stenosis, improving maturation rates, adding that a VENOS-2 US-based early feasibility study is planned to start end of 2024.
Robert Shahverdyan

Scan to learn more or visit laminatemedical com/vasQ

VasQ is intended for use as an external support for upper extremity arteriovenous fistulas created for vascular access by means of vascular surgery Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events available at https://laminatemedical com/eIFU


A new deep vein thrombosis (DVT) diagnostic pathway incorporating non-expert artificial intelligence (AI)-guided compression ultrasound could reduce workload and costs for healthcare systems while providing a quicker diagnosis and improving patient care.
Avgerinos (Athens, Greece) shared this key conclusion from a pilot study of the ThinkSono guidance system (ThinkSono) at the European Society for Vascular Surgery (ESVS) annual meeting (24–27 September, Kraków, Poland).
“The diagnosis of DVT in a tertiary setting is part of a complicated clinical pathway including clinical probability scoring, D-dimer testing and a full duplex scan, often leading to long waiting times,” Avgerinos—who is co-director of the Clinic of Vascular and Endovascular Surgery at Athens Medical Group and visiting professor of vascular surgery at the University of Athens in Greece—told Vascular News ahead of the ESVS meeting. He added that the need to have trained radiologists or sonographers available 24/7 incurs high costs and represents a “suboptimal use of resources”.
duplex. “It is an AI-based software that you can install on your cell phone or tablet and will guide a non-expert on how to perform a compression ultrasound using a wireless probe,” he continued.
In their pilot study of the ThinkSono system, Avgerinos and colleagues set out to evaluate its clinical utilisation in realworld practice—having previously demonstrated its ability to generate valid images—hoping to show that the system could spare the need for standard venous duplex.
The researchers prospectively enrolled 53 patients who were suspected to have a DVT. These patients underwent a ThinkSono scan, performed by a nurse or resident in the emergency room, with the resulting images then reviewed remotely by the on-call radiologist. D-dimer testing was also performed as a safety measure.
89% of the scans were negative for DVT. The team managed to discharge one-third of the patients without performing any standard duplex scan, which Avgerinos commented represents significant cost and time savings.
Six out of eight suspected DVTs were confirmed on standard duplex to be real, with the ThinkSono technology not missing a diagnosis. “Interestingly enough,” Avgerinos added, “all positive D-dimer testing patients who had a negative ThinkSono scan were also eventually negative, indicating D-dimer has a very low specificity and is not really a powerful testing method to rule in DVT.”
Avgerinos continued that it took on average six and a half minutes in total for scan and review. The median length of time between scan and review was 30 minutes, although there was a wide variation in this figure.
According to Avgerinos, this “reflects unoptimised hospital logistics—mainly the availability of the qualified clinician to review the data”. He clarified that the expert clinicians who took part in the pilot study were volunteers who had various other clinical lab duties, going on to suggest that careful resource planning or the use of dedicated reviewers could help to maximise the efficiency of the AI-assisted pathway. Avgerinos summarised: “Our study
demonstrated that in DVT diagnostic workflow, using AI and remote expert review could safely decrease the need for a full duplex ultrasound and even D-dimer testing for a significant proportion of the patients who are suspected to have a DVT in the emergency room.”
Looking ahead, he noted that a large trial is needed to validate the results of this pilot study, and that clinical commercialisation will soon launch in the UK and Germany, while a US Food and Drug Administration (FDA) trial is underway in the USA.
Finally, Avgerinos considered the wider application of this technology. While the pilot study focused on tertiary centres, he hinted at its potential future use in community hospitals and ambulatory centres. “Not a lot of hospitals have radiologists who can perform standard duplex scans 24/7,” he said. “With this technology, patients would not need to travel to the reference centres. A nurse at the community hospital, a nurse at the GP’s office or at the ambulatory centre could perform the scan and an expert could review it remotely from the main hospital. The application eventually expands to any remote or rural underserved area, essentially democratising ultrasound—anybody can do it, anywhere.”
The ThinkSono system, Avgerinos explained, is based on the point-of-care ultrasound concept and eliminates the need for an expert operator and the absolute need to perform a standard
Avgerinos shared that all 53 scans generated were of appropriate quality to be read by the radiologist. “This is a great milestone for an AI technology in the hands of non-experts,” he told Vascular News, going on to reveal that
The application eventually expands to any remote or rural underserved area, essentially democratising ultrasound—anybody can do it, anywhere”
Ten-year data from the randomised HELP trial show that, while both endothermal ablation and conventional surgery are effective treatments for great saphenous varicose veins at 10 years, the former was associated with superior clinical and quality-of-life (QoL) outcomes. These findings were recently published in the British Journal of Surgery (BJS).
AUTHORS ABDURAHEEM H MOHAMED (Hull York Medical School, Hull, UK) and colleagues write that both endothermal ablation and surgical ligation and stripping, i.e. surgery, are both effective treatment for varicose veins, improving QoL up to five years. However, they note that few data are available on long-term outcomes.
The aim of the HELP trial, Mohamed et al state, was to evaluate the outcomes of both endothermal ablation and surgery 10 years after interventions in a randomised controlled trial (RCT).
Previously, the authors share, their RCT demonstrated that endothermal ablation is associated with superior postprocedural QoL, more rapid recovery, and lower rates of early clinical recurrence.
Their most recent analysis reports outcomes out to 10 years.
In their methods section, Mohamed and colleagues detail that patients with symptomatic varicose veins owing to unilateral great saphenous vein reflux were randomised to either endovenous laser ablation (EVLA) or surgery. They specify that outcomes at 10 years included clinical recurrence and QoL.
The investigators report that they obtained data for 206 of a total of 280 patients (73.6%) at 10 years.
In BJS, Mohamed et al reveal that both the EVLA and surgery groups retained significant QoL improvement compared with pretreatment levels, as assessed by the Aberdeen Varicose Vein Questionnaire (AVVQ), Short Form 36 (SF-36) and
EQ-5D, with a p value of less than 0.001. They continue that clinical disease progression from baseline was observed in only 10.7% of patients and that the clinical recurrence rate was lower in the EVLA group (37 vs. 59%; p=0.005), with the number needed to treat with EVLA to avoid one clinical recurrence within 10 years being five. This, the authors write, was associated with significantly higher (better) generic QoL scores with EVLA in several SF-36 domains, including bodily pain—median 84 (interquartile range [IQR], 51–100) vs. 62 (IQR, 41–84); p=0.009)—and general health, at 77 (IQR, 62–87) vs. 67 (IQR, 52–82); p=0.017. In addition, AVVQ scores in the EVLA group were also lower (better), at 3.1 (IQR, 0–7.7) vs. 6.3 (IQR, 0.7–13.3); p=0.029).
The investigators obtained data for 206 out of 280 patients (73.6%) at 10 years
206 out of 280 patients 10 YEARS at
73.6%
A new paper that drills into the impact of a novel bioprosthetic venous valve replacement by CEAP (Clinical, Etiological, Anatomical and Pathophysiological) classification shows sustained clinical improvement regardless of grading, with patients classed as C4b exhibiting greater resolution of symptoms and quicker ulcer healing within the first three months after implantation than those with more advanced disease.
THE FINDINGS EMERGED DURING THE 2024 Midwestern Vascular Surgical Society (MVSS) annual meeting (12–14 September; Chicago, USA) in the latest analysis of the SAVVE (Surgical Antireflux Venous Valve Endoprosthesis) multicentre pivotal trial assessing the safety and efficacy of the VenoValve (enVVeno Medical) in patients with chronic venous insufficiency.
The fully enrolled trial—made up of 75 patients across 21 sites in the USA—measures disease in CEAP categories C4b, C4c, C5 and C6. The paper
presented at MVSS 2024 sought to elucidate how the valve affects disease severity, pain levels, quality of life and ulcer healing at the point of enrollment out to 12-month follow-up.
C6 patients comprised 50% of the study population. “What was interesting to see here was that 70% of the patients who were enrolled in the study had a non-healing ulcer for more than a year or so,” Raghu Motaganahalli (Indiana University, Indianapolis, USA), a SAVVE trial principal investigator, told MVSS 2024. He reported primary and secondary patency rates for the valve of 87% and 96%, respectively, adding that there were four reinterventions and no procedural-related deaths or device-related infections. Benefits from device implantation in terms of venous reflux time were seen after three months and significantly decreased, he added.
In terms of clinical improvement by CEAP classification, Motaganahalli reported improvements in revised Venous Clinical Severity Score (rVCSS) across CEAP categories at three- and six-month follow-up. Compared to the baseline, he said there was an overall 9-point gain in rVCSS at 12-month follow-up after valve implantation. Visual Analogue Score (VAS) for pain assessment improved by almost 50% at one year, Motaganahalli continued, and quality of life, as measured by the VEINES-QOL/ Sym instrument, also “significantly improved” across the CEAP spectrum.
Motaganahalli also noted a decrease in the use and need for compression stockings in tandem with the increase in patient quality of life. Meanwhile, ulcer area was reduced from 18.16cm2 at baseline to 3cm2 “It was also interesting to see that if a patient has an ulcer for less than three months, they have a 100% resolution of the ulceration over time,”

Raghu Motaganahalli
Motaganahalli added. Concluding, he said: “For patients with chronic venous insufficiency, the VenoValve was a novel treatment option. It has shown improvement by rVCSS, we have seen a reduction in pain as evidenced by VAS, ulcers heal, limb swelling is improved by the VEINES-QOL/Sym score, and all improvements are shown within the first three months of the clinical period.”
During discussion, Motaganahalli highlighted how the paper helps draw attention to optimal intervention points. “By the time you transition from a C4b, or a C4c, to a C6 category, you have a lot of damage in those patients,” he said. “That is exactly the purpose of this paper: to see where to intervene and when to intervene. In fact, the C4b category patients had more resolution of their symptoms. Ulcers heal much more quickly in that group of patients—they were the ones to improve within the three-month window—as opposed to much more advanced disease.”
What was interesting to see here was that 70% of the patients who were enrolled in the study had a non-healing ulcer for more than a year or so”

OCTOBER 17-19, 2024

Thursday, October 17 14:35 - 14:55 Meet the Expert: Tips & Tricks in venous stenting


InspireMD receives IDE approval from US FDA for CGUARDIANS II pivotal trial
InspireMD has announced that the US Food and Drug Administration (FDA) approval of the company’s investigational device exemption (IDE) application to initiate the CGUARDIANS II pivotal study of its CGuard Prime 80cm carotid stent system during transcarotid artery revascularisation (TCAR) procedures.
In February 2024, InspireMD announced that professor of surgery and radiology Patrick Geraghty (Washington University School of Medicine, St. Louis, USA) and programme director and chief of vascular surgery Patrick Muck (Good Samaritan Hospital, Cincinnati, USA) have agreed to act as lead investigators for the trial.
Marvin Slosman, chief executive officer of InspireMD, stated: “The approval of our CGUARDIANS II IDE is an important milestone and a significant step forward in our mission to serve the broadest range of physician and patient needs with a comprehensive set of tools that can deliver our best-in-class carotid stent system, CGuard Prime, for both carotid artery stenting and TCAR procedures. The CGUARDIANS II study is intended to facilitate approval of the use of CGuard Prime in an optimised TCAR version and indication.”
“In parallel, we continue to advance development of our comprehensive next generation TCAR Neuroprotection System, SwitchGuard NPS. Each of these initiatives helps pave the way, once approved, for us to initiate commercial sales and strive for market leadership in the USA. Our mission to improve stroke prevention and carotid disease management with our CGuard platforms continues as we build our company toward US expansion and global success. Additionally, as we previously announced, we are thrilled to have Geraghty and Muck as coprincipal investigators for the study, as well as a world class group of investigators committed to the trial’s success,” Slosman concluded.
iVascular announces first data from BARISTA trial of Restorer BMS in iliac artery treatment iVascular has announced the first clinical outcomes of the Restorer bare metal stent (BMS) in iliac artery treatment.
The data were presented earlier this summer at the Leipzig Interventional Course (LINC 2024; 28–31 May, Leipzig, Germany) by Lieven Maene (OLV Hospital, Aalst, Belgium), principal investigator of the study. This presentation highlighted interim results from the BARISTA trial, a study evaluating the efficacy and safety of the Restorer in treating aortoiliac lesions.
A press release reports that the

BARISTA trial has now successfully completed patient enrolment, involving a total of 200 participants. This is a prospective, multicentre, nonrandomised, single-arm observational study designed to assess the long-term (24-month) safety and efficacy of Restorer in treating aortoiliac lesions, classified under TASC A, B, C, and D.
The interim analysis presented at LINC focuses on data from the first 67 patients with follow-up to 12 months. This first cohort included mainly TASC A and B lesions (86.6% combined) but 76.1% of all the lesions showed moderate to severe calcifications.
iVascular shares that Restorer demonstrates “outstanding” efficacy at 12 months in:
Primary endpoint success: The study achieved primary patency of 96.9%, freedom from target lesion revascularisation (fTLR) of 98.5%, and 100% freedom from major amputation at 12 months.
Ankle brachial index improvement: There was a significant mean increase of +0.24 in the ankle brachial index at 12 months compared to baseline.
Maene stated: “The interim results confirm the safety and efficacy of the Restorer stent in the treatment of iliac arteries, even with a high percentage of calcified lesions.”
He also highlighted Restorer’s benefits, such as its “excellent” trackability due to the low profiles (6Fr for all diameters) and flexibility to reach the lesion and achieve optimal wall apposition.
Twelve-month STRIDE data show high limb salvage rate and quality of life improvement
One-year limb salvage and quality of life (QoL) data from the STRIDE study, showing that frontline use of Penumbra’s Indigo system for patients with lower extremity acute limb ischaemia (LE-ALI) is safe and effective, has been published in the Journal of Vascular Surgery. Results were presented at the Vascular Annual Meeting (VAM 2024; 19–22 June, Chicago, USA).
One-year outcomes include: 88.5% target limb salvage rate and 12% mortality rate at 365 days, and overall improvement in QoL, as measured by the VascuQoL-6 questionnaire, from baseline to one year follow-up.
The latest results build on evidence from the initial publication of 30-day STRIDE data, in which the primary
endpoint, target limb salvage, was achieved in 98% of patients. The Indigo system was able to achieve these high rates of limb salvage and low rate of mortality with median device time of 22 minutes.
“The latest STRIDE data continue to demonstrate the use of Penumbra’s Indigo aspiration system to address LE-ALI results in excellent outcomes, including high target limb salvage rates and quality of life,” said Thomas Maldonado (New York University of Langone Health, New York, USA), national principal investigator of the STRIDE study. “The data is promising in that it underscores the significant benefits of continuous aspiration to remove thrombus and should be considered a frontline treatment for LE-ALI.”
STRIDE was a multicentre, prospective, single-arm, observational study that enrolled 119 patients across 16 sites in the USA and Europe. The study, which completed follow-up in October 2023, was designed to assess safety and efficacy of mechanical thrombectomy using the Indigo aspiration system in patients with LE-ALI. Secondary endpoints at 365 days included target limb salvage and mortality. Additionally, the VascuQoL-6 questionnaire, developed for evaluating patient-centred QoL outcomes for peripheral arterial disease, was assessed at baseline and follow-up through 365 days.
the device compared to using an APCD, a press release reports. These results are consistent with prior studies’ findings associated with Dayspring for the treatment of lymphoedema.
“The introduction of Dayspring represents a clinically differentiated and therapeutically distinct advancement in the treatment of lower extremity lymphoedema,” said Michael Barfield (University of Tennessee Health Science Center, Nashville, USA), lead investigator. “Patients participating in the TEAYS study expressed a strong preference for the Dayspring treatment over advanced pneumatic compression devices. The ability for patients to remain mobile while receiving effective treatment is a game-changer in improving adherence and overall quality of life.”
Key findings from the TEAYS study include:
● Efficacy: Patients using Dayspring experienced a mean limb volume reduction of 369.9mL, significantly greater than the 83.1mL reduction observed in the APCD treatment arm.
● Quality of life: Significant improvements were noted in the overall Lymphedema Quality of Life Questionnaire (LYMQOL) scores for Dayspring users, with a mean improvement of 1.01 compared to 0.17 for APCD users.
Key functional sub-scores such as symptoms, appearance, and function also favoured Dayspring.
ALI, a sudden lack of blood flow to a limb, is associated with a high risk of amputation and death. Early diagnosis and intervention are crucial to improving patient outcomes and preserving limb function. Penumbra’s Indigo aspiration system is a minimally invasive continuous aspiration device that is designed to quickly remove emboli and thrombi from vessels of the peripheral arterial and venous systems.
Koya Medical announces full results from TEAYS study of Dayspring device
Koya Medical recently announced that full results from the TEAYS clinical study have been published in the Journal of Vascular Surgery (JVS).
The data highlight superior efficacy and improved quality of life for patients suffering from lower extremity lymphoedema with Dayspring, a novel nonpneumatic compression device (NPCD), compared to advanced pneumatic compression devices (APCDs).
Dayspring met the study’s primary and secondary outcome measures and showed a broad range of superior clinical benefits for patients using
● Adherence: Dayspring users demonstrated an adherence rate of 81%, significantly higher than the 56% adherence observed in the APCD group.
● Safety: No device-related adverse events were reported for either treatment, highlighting the safety of Dayspring.
The TEAYS (Treatment effectiveness of a non-pneumatic compression device versus an advanced pneumatic compression device for lower extremity lymphoedema swelling) study is Koya Medical’s eighth clinical study evaluating Dayspring for the treatment of lymphoedema since its US Food and Drug Administration (FDA) clearance in 2021. The TEAYS study was a prospective, multicentre, randomised, single-crossover clinical trial conducted across nine sites in the USA. It involved 71 patients with confirmed lower extremity lymphoedema, comparing the treatment effectiveness and patient adherence between Dayspring and APCDs.


LEIPZIG INTERVENTIONAL COURSE 28–30 January 2025 | Leipzig, Germany Don’t miss the most innovative endovascular meeting in the world.
Join us back in January for this premier event filled with record breaking live cases from several global centers, insight into the latest field developments, and in-depth discussions on hot topics with key opinion leaders.


and subscribe to our newsletter to stay informed: www.leipzig-interventional-course.com
Contego Medical’s Neuroguard IEP system receives US FDA approval for carotid revascularisation
Contego Medical has announced the US Food and Drug Administration’s (FDA) premarket approval (PMA) of the Neuroguard IEP system for carotid revascularisation.
The company shares in a press release that clinical studies of the Neuroguard IEP system, including the PERFORMANCE I trial and the PERFORMANCE II investigational device exemption (IDE) trial, have consistently recorded unprecedented low event rates—zero major strokes, zero neurologic deaths, and zero stent thrombosis at 30 days and one year.
William Gray (Main Line Health, Wynnewood, USA), conational principal investigator of PERFORMANCE II, said: “FDA approval confirms the results of the clinical studies. The innovative Neuroguard IEP system performs exceptionally well with the lowest one-year stroke rates ever shown for any type of carotid revascularisation, thereby establishing a new standard of care for meaningfully reducing the risk of procedural and long-term stroke among patients with carotid artery disease.”
The Neuroguard IEP system utilises Contego Medical’s Integrated Embolic Protection (IEP) technology, featuring a 40-micron filter integrated on a 6 French delivery catheter. The size of the integrated filter can be adjusted by the operator to custom fit each patient’s unique anatomy, and the pores on the filter are three to four times smaller than those on traditional filters, the company claims, improving protection against stroke and cognitive impairment. According to Conteo Medical, the newest generation closed cell stent is highly conformable and shows remarkable short- and longterm durability, with no thromboses, no clinically driven target lesion revascularisation, and very low restenosis rates at one year.
Cook Medical creates custommade aortic device for fenestrated FET procedure Cook Medical recently announced that it has created a custom-made device for the frozen elephant trunk (FET) aortic procedure.
A press release details that, earlier this year, cardiothoracic surgeon François Dagenais (Quebec City, Canada) approached Cook Medical. One of his patients had severe complex aortic arch disease. He explained that he needed to perform a FET procedure on the patient but wondered if it could be done with some innovative and different techniques. He asked if Cook could extend to the descending thoracic aorta and include a unique fenestration for the left subclavian artery (LSA).
Cook Medical advises that a FET procedure is a hybrid technique that combines surgically replacing the aortic arch while simultaneously stenting the descending thoracic aorta. Patients requiring FET procedures often have complex aortic arch disease. One of the significant benefits of using an FET procedure, the company notes, is that it enables the physician to complete the aortic repair in a single operation instead of multiple operations.

According to Cook Medical, a FET procedure had never before been performed with a custom-made device that had a fenestration. Adding a fenestration in the aortic graft removed the need for surgical intervention for the LSA vessel, allowing for a smooth endovascular LSA stenting and a streamlined, more minimally invasive procedure.
collaborating with physicians to address the unmet needs of their patients.”
Surmodics receives US FDA 510(k) clearance for Pounce XL thrombectomy system
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy system is indicated for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in vessels 5.5–10mm in diameter, making it suitable for iliac, femoral, and other arteries within this range. The Pounce XL thrombectomy system dramatically increases the size range of the Pounce platform, which also includes the thrombectomy system, indicated for 3.5–6mm peripheral arteries, and the Pounce Low Profile (LP) thrombectomy system, indicated for 2–4mm peripheral arteries. The Pounce thrombectomy system and Pounce LP thrombectomy system were introduced in 2021 and 2024, respectively.
“Securing FDA clearance for Pounce XL is a major step forward in Surmodics’ pursuit of a complete mechanical thrombectomy solution for all peripheral arteries, notably critically ischemic lower extremity vessels,” said Gary Maharaj, president and chief executive officer of Surmodics.
“The Pounce thrombectomy platform has already demonstrated its performance as a rapid, efficient solution for the removal of both acute and chronic thrombi and emboli in peripheral arteries without the use of thrombolytics. The addition of the Pounce XL thrombectomy system to our Pounce thrombectomy platform demonstrates our commitment to setting the pace and direction of innovation in this critical space.”
Philips LumiGuide
the 1000th patient treated milestone using FORS since its first clinical use in 2020.
A press release notes that the introduction of a longer FORS-enabled LumiGuide navigation wire enables US clinicians to visualise a broader range of catheters and expands usage of this technology to patients in the USA treated in centres equipped with LumiGuide.
“The new enhanced Philips LumiGuide navigation guidewire represents a significant leap forward in endovascular surgery, offering unprecedented 3D visualisation and precision during complex procedures,” said Timaran. “As the first to use this guidewire for a complex aortic repair, I experienced firsthand its potential to revolutionise how we approach minimally invasive vascular interventions. The patient who underwent this groundbreaking procedure is doing well, further validating the efficacy of this innovative technology.”
Philips shares that LumiGuide— powered by the company’s FORS technology—allows clinicians to see their guidewires and catheters in 3D and colour as they manipulate them inside the patient’s body, from any angle, in real-time, and with minimal radiation. The company adds that its FORS technology simplifies navigation in tortuous vessels.
“This FDA approval is a huge step forward for Contego and for patient care. The Neuroguard IEP system transforms how we approach patients with carotid artery disease by addressing the specific threats of microembolisation while simultaneously reducing procedural steps, ensuring patients receive the highest level of protection throughout the procedure,” said Ravish Sachar, Contego Medical’s chief executive officer and founder. “The ongoing PERFORMANCE III trial is evaluating the Neuroguard IEP system with a nextgeneration direct transcarotid access and protection system, TCAR-IEP, to demonstrate advanced stroke protection regardless of vascular approach.”
“We performed the world’s first procedure using a fenestrated frozen elephant trunk device. This unique case was designed through Cook Medical’s custom-made device programme. During FET procedures, the LSA can be challenging to access surgically. Using an endovascular technique to revascularise the LSA through a pre-designed fenestration within the FET graft facilitates the procedure, decreases operative time and minimises the complication rate of the FET procedure,” Dagenais commented.
He continued: “The development of this unique FET device is an example of collaboration between clinicians and the expertise of Cook Medical in fenestrated graft technology. Many thanks to Cook Medical for your support; one more step forward in our journey of innovation for the best interest of patient care.”
“As Cook Medical continue innovating in the aortic space, our custom-made devices are an area in which we are prioritising resources” said Johnny LeBlanc, director of Cook’s aortic product management team. “We are committed to
Maharaj added: “Critically ischaemic peripheral arteries often have older, organised clots that resist catheter-directed thrombolysis and aspiration thrombectomy. The Pounce thrombectomy platform allows physicians to rapidly restore blood flow regardless of clot morphology, which has the potential to reduce the need for follow-up procedures and additional thrombolytic therapy requiring intensive-care-unit admission.”
Philips announces US FDA approval for enhanced LumiGuide guidewire, marks 1000th patient treated with FORS
Royal Philips recently announced the introduction of the 160cm US Food and Drug Administration (FDA)-approved version of its LumiGuide endovascular navigation wire. This enhanced LumiGuide guidewire, which utilises the company’s Fiber Optic RealShape (FORS) technology, was used for the first time on a patient by Carlos Timaran (UT Southwestern Medical Center, Dallas, USA). He used the technology during a complex aortic aneurysm repair operation, marking
Using this advanced technology, research published in the Journal of Vascular Surgery and from Philips shows that complex cases such as aortic repair procedures can be done 37% faster and use 70% less X-ray imaging during the process.
Providing greater reach and enhanced catheter loading possibilities than the company’s existing 120cm (47 inch) guidewire, Philips claims that its new 160cm (63 inch) LumiGuide wire enables US physicians to experience 3D device guidance with more catheters than before, enabling use of the technology for more patients and procedures.
“LumiGuide unlocks the colour visualisation of wires, catheters, and patient anatomies in 3D from any angle, including simultaneous angles to generate ‘virtual biplane’ images. Combined with device navigation viewed from angles physically unachievable using conventional C-arm systems, it has already been shown to improve workflows, reduce procedure times, and decrease patient and staff radiation dose,” said Atul Gupta, chief medical officer for diagnosis and treatment at Philips and a practicing interventional radiologist.


Boston Scientific completes acquisition of Silk Road Medical
Boston Scientific announced the close of its acquisition of Silk Road Medical, the company behind transcarotid artery revascularisation (TCAR).
“The integration of the TCAR platform into our portfolio means we can offer a treatment option for patients suffering from carotid artery disease that can reduce the risk of stroke and lead to improved patient outcomes,” said Cat Jennings, president of vascular peripheral interventions at Boston Scientific.
Boston Scientific revealed on 18 June that it had entered into a definitive agreement to acquire Silk Road.
InterVene announces US$13 million Series A financing to advance Recana system through US market clearance
InterVene recently announced the closing of its US$13 million Series A financing round. The financing was co-led by new investor Treo Ventures and existing investor RiverVest Venture Partners. In conjunction with this financing, Brad Vale, Treo Ventures founding general partner, has joined InterVene’s board of directors.
A press release notes that proceeds from the round will fund the final
2–3 November
The VEINS (Venous Endovascular Interventional Strategies) 2024 Las Vegas, USA viva-foundation.org/ veins-programming
3–6 November Vascular Interventional Advances (VIVA) Las Vegas, USA viva-foundation.org/ viva-programming

development of the company’s Recana catheter system, initiation of clinical cases, regulatory submission, and market clearance in the USA. In addition, proceeds will support preparation for initial commercialisation and postmarket clinical research.
“RiverVest and Treo Ventures have strong track records in emerging endovascular fields and InterVene is thrilled to have their support as Recana reaches its final phases of development,” said InterVene chief executive officer Jeff Elkins.
“We are excited to work with these seasoned, accomplished teams on such a significant opportunity to help physicians and their patients with venous conditions.”
InterVene designed Recana to recanalise and restore patency in chronically obstructed deep veins and venous stents, particularly when recanalisation capabilities are
6–8 November British Society of Interventional Radiology (BSIR) annual scientific meeting 2024 Brighton, UK bsir.org/events/bsir-asm
7–9 November VERVE Symposium Sydney, Australia vervesymposium.com
limited or when long-term chronic stent maintenance is necessary. The company states that, utilising standard fluoroscopy/intravascular ultrasound (IVUS)-guided interventional techniques, Recana can be easily incorporated by vascular specialists already performing deep vein thrombosis (DVT) treatment and deep venous stenting.
“With the dramatic growth of venous interventions, DVT thrombectomy, and venous stents, experience has made us more aware of the causes and often life-long impact of deep venous obstruction and occlusion as well as a need for reinterventions in many patients,” noted Stephen Black, venous surgery physician at King’s College London (London, UK), consultant vascular surgeon at Guy’s and St Thomas’ Hospital (London, UK) and InterVene advisor.
He continued: “This is especially prevalent before and after treatment in post-thrombotic patients and the challenging problem of in-stent restenosis. Recana will be a valuable tool for recanalisation in these settings and offers new treatment options for patients requiring ongoing care.”
Endovascular Engineering appoints Dan Rose as chief executive officer Endovascular Engineering (E2), which specialises in the advancement of clot removal technologies for venous
19–23 November VEITHsymposium New York, USA veithsymposium.org/index.php
27–29 November
The Vascular Societies’ annual scientific meeting (VSASM) Brighton, UK vascularsociety.org.uk/ annual-meeting
5–6 December 13th Munich Vascular Conference (MAC) 2024 Munich, Germany mac-conference.com
11–13 December Aortic Surgery “Peripheral & Venous” | How To Do It (HDTI) 2024 Milan, Italy aorticsurgery.it
thromboembolism (VTE), has named Dan Rose as chief executive officer (CEO). Former CEO and founding team member Mike Rosenthal will continue as chief operating officer and remain on the company’s board of directors. He will also continue in his role as founder and general partner at Inventure Group, where E2 was incubated.
Most recently, Rose served as CEO of LimFlow, a company dedicated to limb salvage for patients with chronic limb-threatening ischemia (CLTI). He joined LimFlow as the first employee and successfully guided the company through its acquisition by Inari Medical. A press release reports that Rose assembled a “world-class” team that developed and commercialised a “practice-changing” therapy for the treatment of late-stage CLTI, which resulted in a US Food and Drug Administration (FDA) approval and a publication in The New England Journal of Medicine
Previously, Rose served as vice president and general manager Europe for Direct Flow Medical, and led commercial and clinical activities at Sequana Medical. Earlier in his career, he held successive commercial leadership roles at Medtronic in Europe. Rose has a BA and MA from the University of Virginia (Charlottesville, USA) and an MBA from its Darden School of Business.
12–14 December Paris Vascular Insights (PVI) Paris, France paris-vascular-insights.com
2025 16–19 February
VENOUS2025: American Venous Forum (AVF) annual meeting Atlanta, USA venousforum.org/education/ annual-meeting

















When you hold someone’s life in your hands, you need to trust your aortic technology. So choose endovascular solutions that have helped over 600,000 patients and are substantiated in peer-reviewed journals. We’re your aortic ally.
Learn more, visit gmd.cm/aortic-outcomes
